- Search Menu
- Sign in through your institution
- Advance articles
- Darwin Reviews
- Special Issues
- Expert View
- Flowering Newsletter Reviews
- Technical Innovations
- Editor's Choice
- Virtual Issues
- Community Resources
- Reasons to submit
- Author Guidelines
- Peer Reviewers
- Submission Site
- Open Access
- About Journal of Experimental Botany
- About the Society for Experimental Biology
- Editorial Board
- Advertising and Corporate Services
- Journals Career Network
- Permissions
- Self-Archiving Policy
- Dispatch Dates
- Journal metrics
- Journals on Oxford Academic
- Books on Oxford Academic


Article Contents
Introduction, features of deficit irrigation, deficit irrigation and water productivity, deficit irrigation for biomass production, deficit irrigation in annual crops, deficit irrigation in fruit trees and vines.
- < Previous
Deficit irrigation for reducing agricultural water use
- Article contents
- Figures & tables
- Supplementary Data
Elias Fereres, María Auxiliadora Soriano, Deficit irrigation for reducing agricultural water use, Journal of Experimental Botany , Volume 58, Issue 2, January 2007, Pages 147–159, https://doi.org/10.1093/jxb/erl165
- Permissions Icon Permissions
At present and more so in the future, irrigated agriculture will take place under water scarcity. Insufficient water supply for irrigation will be the norm rather than the exception, and irrigation management will shift from emphasizing production per unit area towards maximizing the production per unit of water consumed, the water productivity. To cope with scarce supplies, deficit irrigation, defined as the application of water below full crop-water requirements (evapotranspiration), is an important tool to achieve the goal of reducing irrigation water use. While deficit irrigation is widely practised over millions of hectares for a number of reasons—from inadequate network design to excessive irrigation expansion relative to catchment supplies—it has not received sufficient attention in research. Its use in reducing water consumption for biomass production, and for irrigation of annual and perennial crops is reviewed here. There is potential for improving water productivity in many field crops and there is sufficient information for defining the best deficit irrigation strategy for many situations. One conclusion is that the level of irrigation supply under deficit irrigation should be relatively high in most cases, one that permits achieving 60–100% of full evapotranspiration. Several cases on the successful use of regulated deficit irrigation (RDI) in fruit trees and vines are reviewed, showing that RDI not only increases water productivity, but also farmers’ profits. Research linking the physiological basis of these responses to the design of RDI strategies is likely to have a significant impact in increasing its adoption in water-limited areas.
Forecasts of water withdrawals on a global scale predict sharp increases in future demand to meet the needs of the urban, industrial, and environmental sectors. This is due to the fact than more than one billion people do not yet have access to running water or sanitary facilities, and also to insufficient attention being paid, until now, to meet the water requirements of natural ecosystems. Given that the single biggest water problem worldwide is scarcity ( Jury and Vaux, 2005 ) there is significant uncertainty about what the level of water supply will be for future generations.
Irrigated agriculture is the primary user of diverted water globally, reaching a proportion that exceeds 70–80% of the total in the arid and semi-arid zones. It is therefore not surprising that irrigated agriculture is perceived in those areas as the primary source of water, especially in emergency drought situations. Currently, irrigated agriculture is caught between two perceptions that are contradictory; some perceive that agriculture is highly inefficient by growing ‘water-guzzling crops’ ( Postel et al. , 1996 ), while others emphasize that irrigation is essential for the production of sufficient food in the future, given the anticipated increases in food demand due to world population growth and changes in diets ( Dyson, 1999 ). Globally, food production from irrigation represents >40% of the total and uses only about 17% of the land area devoted to food production ( Fereres and Connor, 2004 ). Nevertheless, irrigated agriculture is still practised in many areas in the world with complete disregard to basic principles of resource conservation and sustainability. Therefore, irrigation water management in an era of water scarcity will have to be carried out most efficiently, aiming at saving water and at maximizing its productivity.
Irrigation is applied to avoid water deficits that reduce crop production. The process of crop water use has two main components: one due to evaporation losses from the soil and the crop, usually called evapotranspiration (ET), and the other that includes all the losses resulting from the distribution of water to the land. All irrigation waters contain salts and, as water evaporates, salts concentrate in the soil profile and must be displaced below the root zone before they reach a concentration that limits crop production. Salt leaching is achieved by the movement of water applied in excess of ET. Thus, some of the water losses are unavoidable and are needed to maintain the salt balance; however, they can be minimized with efficient irrigation methods and by appropriate management. Reducing ET without a penalty in crop production is much more difficult, however, because evaporation from crop canopies is tightly coupled with the assimilation of carbon ( Tanner and Sinclair, 1983 ; Monteith, 1990 ; Steduto et al. , 2006 ). A water supply constraint that decreases transpiration below the rate dictated by the evaporative demand of the environment is paralleled by a reduction in biomass production. Given the high costs of irrigation development, until now the paradigmatic irrigation strategy has been to supply irrigated areas with sufficient water so that the crops transpire at their maximum potential and the full ET requirements are met throughout the season. This approach is increasingly challenged by segments of society in regions where water is scarce, because of both the large amounts of water required by irrigation and the negative effects that such diversions and use have on nature. Thus, a strategic change in irrigation management is taking place, one that limits the supply available for irrigation to what is left after all other sectors of higher priority satisfy their needs. Under such situations, farmers often receive water allocations below the maximum ET needs, and either have to concentrate the supply over a smaller land area or have to irrigate the total area with levels below full ET.
The application of water below the ET requirements is termed deficit irrigation (DI). Irrigation supply under DI is reduced relative to that needed to meet maximum ET ( English, 1990 ). Therefore, water demand for irrigation can be reduced and the water saved can be diverted for alternative uses. Even though DI is simply a technique aimed at the optimization of economic output when water is limited, the reduction in the supply for irrigation to an area imposes many adjustments in the agricultural system. Thus, DI practices are multifaceted, inducing changes at the technical, socio-economical, and institutional levels. Nevertheless, the focus of this paper is on providing further understanding of the DI concept for biological scientists interested in the relationships between plants and water, leading to the broader issues that govern the optimization of a limited supply of water in crop production. Loveys et al. (2004) have proposed a number of physiological approaches to enhance irrigation practices under limited water conditions. Hopefully, the application of research conducted at the various levels of biological organization, that is, from molecular to whole plant physiology ( Sinclair and Purcell, 2005 ), will offer new avenues for involving plant biologists in the improvement of DI practices in the future.
In the humid and sub-humid zones, irrigation has been used for some time to supplement rainfall as a tactical measure during drought spells to stabilize production. This practice has been called supplemental irrigation ( Cabelguenne et al. , 1995 ; Debaeke and Aboudrare, 2004 ) and, although it uses limited amounts of water due to the relatively high rainfall levels, the goal is to achieve maximum yields and to eliminate yield fluctuations caused by water deficits. Furthermore, supplemental irrigation in humid climates has often been advocated as more efficient than irrigation in the arid zones, because the lower water vapour deficits of the humid zones lead to higher transpiration efficiency than in the arid zones ( Tanner and Sinclair, 1983 ). More recently, the term supplemental irrigation has been used in arid zones to define the practice of applying small amounts of irrigation water to winter crops that are normally grown under rain-fed conditions ( Oweis et al. , 1998 ). In this case, this is a form of DI, as maximum yields are not sought. Thus, the terms deficit or supplemental irrigation are not interchangeable, and each DI situation should be defined in terms of the level of water supply in relation to maximum crop ET. One consequence of reducing irrigation water use by DI is the greater risk of increased soil salinity due to reduced leaching, and its impact on the sustainability of the irrigation ( Schoups et al. , 2005 ).
To quantify the level of DI it is first necessary to define the full crop ET requirements. Fortunately, since Penman (1948) developed the combination approach to calculate ET, research on crop water requirements has produced several reliable methods for its calculation. At present, the Penman–Monteith equation ( Monteith and Unsworth, 1990 ; Allen et al. , 1998 ) is the established method for determining the ET of the major herbaceous crops with sufficient precision for management purposes. There is, however, more uncertainty when using the same approach to determine the ET requirements of tree crops and vines ( Fereres and Goldhamer, 1990 ; Dragoni et al. , 2004 ; Testi et al. , 2006 ).
When irrigation is applied at rates below the ET, the crop extracts water from the soil reservoir to compensate for the deficit. Two situations may then develop. In one case, if sufficient water is stored in the soil and transpiration is not limited by soil water, even though the volume of irrigation water is reduced, the consumptive use (ET) is unaffected. However, if the soil water supply is insufficient to meet the crop demand, growth and transpiration are reduced, and DI induces an ET reduction below its maximum potential. The difference between the two situations has important implications at the basin scale ( Fereres et al. , 2003 ). In the first case, DI does not induce net water savings and yields should not be affected. If the stored soil water that was extracted is replenished by seasonal rainfall, the DI practice is sustainable and has the advantage of reducing irrigation water use. In the second case, both water use and consumption (ET) are reduced by DI but yields may be negatively affected. The challenge of quantifying the ET reduction effected by DI (net water savings) remains, as direct measurements are complex ( Burba and Verma, 2005 ), and the models used to estimate the actual ET of stressed canopies are still quite empirical (Burba and Verma, 2006).
In many world areas, irrigation delivery at the farm outlet is less than what is required. The high costs of irrigation and its benefits offer a justification to expand the networks beyond reasonable limits in order to reach the highest possible number of farmers. This approach has been used in many countries and has led to chronic DI ( Trimmer, 1990 ). Sometimes, the cropping intensity used in the original design becomes obsolete due to marketing reasons, and another of higher intensity and thus of greater water demand is adopted. Inadequate estimation of the crop water requirements in project design is another reason for insufficient network capacity. Finally, in drought periods, irrigated agriculture has the lowest priority and the delivery from irrigation networks may be drastically curtailed. In most of the cases described above, the farmers are at the mercy of the delivery agencies and there is very little margin for them to manage the limited supply efficiently ( Tyagi et al. , 2005 ). In particular, drought periods represent a threat to the sustainability of irrigation, not only because water supply is restricted, but also because of the uncertainty in determining when it will be available. Because of chronic water scarcity, in some areas inadequate irrigation supply is becoming the norm rather than the exception, as in Andalusia, Spain, where during the period between 1980 and 1995 in the Guadalquivir Valley, only in four years was there a normal irrigation supply ( Fereres and Ceña, 1997 ). When the supply is restricted, farmers are often faced with having to use DI to achieve the highest possible returns. Even though the economics of DI are relatively straightforward ( English, 1990 ), the reality is that there are many engineering, social, institutional, and cultural issues that determine the distribution and the management of irrigation water. Furthermore, in any attempt to optimize water use for irrigation, there is significant uncertainty in the anticipated results and, often, the alternatives that anticipate higher net returns also have higher risks ( English et al. , 2002 ). To reduce uncertainty and risk, computer models that simulate irrigation performance ( Lorite et al. , 2005 ), together with social research, can aid in assisting water managers to optimize a limited supply of irrigation water. Nevertheless, until now there has been little or no flexibility in most collective networks to manage irrigation with the degree of precision needed in optimal DI programmes, where controlling the timing of application is essential for avoiding the detrimental effects of stress.
Contrary to the rigid delivery schedules experienced by farmers located in many collective networks, those that have access to water supply on demand or can irrigate directly from groundwater sources, have the capability of managing water with much more flexibility. The ability to adjust the timing and amount of irrigation makes it possible to design first and then to manage and control the best possible DI programme when supply is restricted. The use of permanent, pressurized irrigation systems also makes it possible for small amounts at frequent intervals to be applied, providing an additional tool for stress management. It is therefore possible in water-limited situations, if sufficient knowledge exists, to manage DI optimally with the objective of maintaining or even increasing farmers’ profits while reducing irrigation water use.
When water supplies are limiting, the farmer's goal should be to maximize net income per unit water used rather than per land unit. Recently, emphasis has been placed on the concept of water productivity (WP), defined here either as the yield or net income per unit of water used in ET ( Kijne et al. , 2003 ). WP increases under DI, relative to its value under full irrigation, as shown experimentally for many crops ( Zwart and Bastiaansen, 2004 ; Fan et al. , 2005 ).
There are several reasons for the increase in WP under DI. Figure 1 presents the generalized relationship between yield and irrigation water for an annual crop. Small irrigation amounts increase crop ET, more or less linearly up to a point where the relationship becomes curvilinear because part of the water applied is not used in ET and is lost ( Fig. 1 ). At one point (I M ; Fig. 1 ), yield reaches its maximum value and additional amounts of irrigation do not increase it any further. The location of that point is not easily defined and thus, when water is not limited or is cheap, irrigation is applied in excess to avoid the risk of a yield penalty. The amount of water needed to ensure maximum yields depends on the uniformity of irrigation. In the simulation of Fereres et al. (1993) , the seasonal irrigation depth required for maximum yield increased from 1.3 I M to 2.0 I M , when the coefficient of uniformity decreased from 90% to 70%. Under low uniformity, irrigation efficiency decreases and water losses are high. By contrast, in DI the level of water application is less than I M and the losses are of much less magnitude ( Fig. 1 ). Thus, under the situation depicted in the Fig. 1 , the WP of irrigation water under DI must be higher than that under full irrigation. Another, more realistic way of illustrating the fact that WP is higher under DI, is by displaying the distribution of irrigation water over a field in two dimensions ( Fig. 2 ). Because water cannot be applied with perfect uniformity, variations in applied water over the field are ranked and plotted against the fraction of the area ( Fig. 2 ). The depth of water is normalized against the required depth, X R , needed to refill the soil water deficit ( Losada et al. , 1990 ; Mantovani et al. , 1995 ). Under full irrigation, the linear distribution of applied water intercepts X R at the 0.5 fraction of the total area. Thus, half of the field is over-irrigated and the other half has a deficit, the slope of the line being indicative of the distribution uniformity of the application method. Under DI, the depth of application is less than X R and, in the case of Fig. 2 , all of the applied water remains in the root zone and may be used in ET. Evidently, in the case of DI in Fig. 2 , the whole field has some soil water deficit after irrigation and there will be areas with a level of deficit that may be detrimental for production. The DI line of Fig. 2 emphasizes the need to have irrigation systems of high application uniformity under DI (the lowest possible slope) to limit the level of deficit in the areas of the field that receive the lowest depths. It is also evident from Fig. 2 that the WP of irrigation water under DI must be higher than that under full irrigation.
Generalized relationships between applied irrigation water, ET, and crop grain yield. I W indicates the point beyond which the productivity of irrigation water starts to decrease, and I M indicates the point beyond which yield does not increase any further with additional water application.
Distribution of irrigation depth, X, as a function of fractional irrigated area. Hypothesized relationships, resulting from the spatial distribution of irrigation water over a field, between the depth of water applied (X, normalized with respect to the required depth to refill the soil water deficit) as a function of the fraction of the area irrigated for full and deficit irrigation. Note that under full irrigation, 50% of the area receives water in excess of the required depth, X R , needed to refill the root zone.
In addition to the factors associated with the disposition of irrigation water, WP is also affected by the yield response to irrigation. Yield responses to irrigation and to ET deficits have been studied empirically for decades ( Stewart and Hagan, 1973 ; Vaux and Pruitt, 1983 ; Stewart and Nielsen, 1990 ; Howell, 2001 ). It turned out that it is not only biomass production that is linearly related to transpiration, but the yield of many crops is also linearly related to ET, as shown in Fig. 1 . The design of a DI programme must be based on knowledge of this response but the exact characteristics of the response function are not known in advance. Also, the response varies with location, stress patterns, cultivar, planting dates, and other factors. In particular, many crops have different sensitivities to water stress at various stages of development, and the DI programme must be designed to manage the stress so that yield decline is minimized. However, when the yield decline, in relative terms, is less than the ET decrease, WP under DI increases relative to that under full irrigation. Nevertheless, from the standpoint of the farmer, the objective is not WP per se , but net income, low risk, and other issues related to the sustainability of irrigation are more important. Knowledge of the crop response to DI is essential to achieve such objectives when water is limited.
The close link between biomass production and water use makes it difficult to use DI when the objective is the production of total biomass. Nevertheless, one major irrigated crop in the arid zones that is grown for its biomass, alfalfa, has been the subject of many studies aimed at reducing its water consumption. Alfalfa WP is relatively low and its ET is quite high; in the western states of the USA, ET normally ranges from 900 mm to 1200 mm, but it can reach 1800 mm in desert areas ( Grismer, 2001 ). A reduction in alfalfa transpiration due to water deficits is associated with a decrease in biomass production and there seems to be little opportunity to reduce its consumptive use ( Sammis, 1981 ). However, because evaporative demand changes throughout the season, it may be possible to limit or eliminate irrigation in the months of high evaporative demand and to produce alfalfa in periods of low evaporative demand. Figure 3 presents the monthly ET requirements to produce 1 ton of alfalfa at Cordoba in Spain. In spring and autumn, the consumptive use is about half of what evaporates in the peak summer months. Thus, if irrigation is reduced during summer, the WP of alfalfa would increase ( Tayfur et al. , 1995 ). One limitation is that the longevity of the stand may be affected by summer water deficits ( Ottman et al. , 1996 ) and that may be related to the pattern of accumulation of root reserves ( Rapoport and Travis, 1984 ) and to their role in regrowth following water deficits. This is one area where research in plant physiology can aid in determining the minimum irrigation levels during summer that would be required for optimal alfalfa DI.
Average monthly consumptive use (ET) requirements for producing 1 metric ton of alfalfa at Cordoba, Spain. Biomass was calculated with a simple model that used transpiration efficiency values, obtained by Asseng and Hsiao (2000) in Davis, CA, USA, the long-term average consumptive use of alfalfa at Cordoba, Spain, and a correction for root dry matter estimated from Rapoport and Travis (1984) .
Harvestable yield of annual crops is normally a fraction of the biomass produced ( Evans, 1993 ). Water deficits, by affecting growth, development, and carbon assimilation, reduce the yield of most annual crops ( Hsiao and Bradford, 1983 ). The reduction in yield by water deficits is caused by a decrease in biomass production and/or by a decrease in the fraction of biomass that is harvested, termed the harvest index (HI). It should be noted that here reference is only made to above-ground biomass production. This is because, in most studies, information on roots is scant, given the difficulties in quantifying root biomass under field conditions. Past research has shown that the response to water deficits very much depends on the pattern of stress imposed ( Dorenboos and Kassam, 1979 ). In one pattern that has been frequently used, the water deficit increases progressively as the season advances due to a combination of the uniform application of a reduced amount and the depletion of the soil water reserve. This pattern, hereafter called sustained deficit irrigation (SDI), allows for water stress to develop slowly and for the plants to adapt to the water deficits, in soils with significant water storage capacity. Under an SDI regime, the differential sensitivity of expansive growth and photosynthesis to water deficits ( Hsiao, 1973 ) leads to reduced biomass production under moderate water stress due to a reduction in canopy size and in radiation interception. However, dry matter partitioning is usually not affected and the HI is maintained. As the water stress increases in severity, though, there could be direct effects on the HI in many determinate crops, particularly when the post-anthesis fraction of total transpiration is too low ( Fischer, 1979 ).
The response to SDI described above has been documented extensively in the major field crops and Fig. 4 exemplifies the response of maize, wheat, and sunflower. As biomass production (B) is reduced, the HI stays constant until it starts to decrease, in the case of Fig. 4 , at about 60% of maximum biomass. That declining point varies in different reports and it can be less or more depending on the rate of development of water deficits, in turn determined by the root-zone water storage capacity and the evaporative demand. Deficit irrigation in this case should be designed within a domain where the HI is conserved at its maximum value; that is, at irrigation targets that produce at least 60% of maximum biomass. That SDI regimes should be designed at relatively high levels of irrigation supply has been verified in numerous experiments summarized in wheat by Musick et al. (1994) , by many recent DI experiments in China, primarily with maize and wheat ( Li et al. , 2005 ; Zhang et al. , 2005 ), and by Oweis and co-workers in the Middle East working with grain legumes ( Oweis et al. , 2004, 2005 ).
Relationship between harvest index (HI R ) as a function of biomass production (B R ) in response to water deficits. Both are expressed relative to the values observed under full irrigation and all were obtained in experiments conducted under sustained DI. The maize data are from Farré and Faci (2006) , the sunflower data from Soriano et al. (2002) , and the wheat data from two 4-year experiments reported by Ilbeyi et al. (2006) .
There are a few major crops where the HI response differs from that of Fig. 4 . Figure 5 presents the HI–B relationship for grain sorghum and for two cotton cultivars under SDI. When sorghum is subjected to mild-to-moderate stress, its HI increases above that of full irrigation, in particular in deep, open soils such as the Yolo loam in Davis, CA, USA ( Hsiao et al. , 1976 ). As stress increases in severity, HI is conserved until it starts to decline at levels below 0.4 B R ( Fig. 5 ). In the case of cotton, an indeterminate crop, the HI of one cultivar (Coker-310; Fig. 5 ) increases significantly over a wide range of water deficits, while the HI of another cultivar (Jaen) does not vary much with water deficits. The two varieties differed in maturity date; Jaen in the environment where it was grown was able to complete the process of fruit development in all water treatments, while the maturation of Coker-310 fruits was enhanced by water deficits relative to full irrigation ( Orgaz et al. , 1992 ). Because cotton cultivars are chosen to maximize potential yield, SDI is an excellent tool to match the water supply available to the maturity date of a given cultivar. Thus, in the cases of crops such as sorghum and cotton ( Fig. 5 ), DI can and should be used to achieve maximum WP and profits by growing the crop at ET levels below its maximum potential.
Relationship between harvest index (HI R ) as a function of biomass production (B R ) in response to water deficits for sorghum (closed circles) and cotton (open and crossed squares) under SDI regimes. The sorghum data are from Farré and Faci (2006) and Faci and Fereres (1980) . The open squares are for the cotton cv. Coker-310, and the crossed squares for cv. Jaen. The cotton data were originally reported by Orgaz et al. (1992) .
The differential sensitivity of crop yield to water deficits at different developmental stages has been a classic topic of research ( Taylor et al. , 1983 ). Figure 6 shows, for maize and sunflower, the responses to pre- and post-anthesis deficits, relative to the response to SDI in the HI–B plot. The well-known response to post-anthesis stress is negative and should be avoided by appropriate irrigation scheduling. The increase in HI in response to pre-anthesis stress of Fig. 6 , similar to that shown in cotton and sorghum under SDI ( Fig. 5 ), offers an opportunity to use DI to achieve higher WP and profits at ET levels below the maximum. One practical limitation is that stress during the vegetative phase reduces leaf area, and that such a reduction can have an effect on the partitioning of ET into evaporation and transpiration, favouring evaporation and negating some of the potential improvement in WP. Perhaps a change in planting patterns could overcome this limitation by using smaller plants and increasing planting density, although the duration of the vegetative phase is quite short in intensive production systems and thus the potential ET reduction in this phase may be limited.
Relationship between the harvest index (HI R ) as a function of biomass production (B R ) in response to pre- and post-anthesis water deficits for maize (circles) and sunflower (triangles). The maize data are from Farré (1998) and from NeSmith and Ritchie (1992 a , b ), and the sunflower data are from Soriano et al. (2002) . The dashed line is the same as that depicted in Fig. 4 for SDI regimes. (DPre, DPost, DFl: water deficits during pre- and post-anthesis and at flowering, respectively.)
The basis for designing DI strategies lies on the response of the HI to the watering regime. Thus, it would be desirable to have a model that could predict the HI response to water supply. Sadras and Connor (1991) have proposed a model for predicting the HI of sunflower and other determinate crops as a function of post-anthesis transpiration. The model calculates HI, corrected for biomass composition, as a function of the fraction the transpiration during post-anthesis and also normalized for the vapour pressure deficit of that period. In that model, HI is nearly constant until the normalized fraction of post-anthesis transpiration does not decline below about 0.2. Data from two sunflower experiments conducted at Cordoba were fitted to the model of Sadras and Connor (1991) and the resulting curve is plotted in Fig. 7 . The results of Fereres and Soriano ( Soriano, 2001 ), obtained under SDI, do not fit their relationship ( Fig. 7 ), perhaps because the experiments of Fereres and Soriano were conducted in open, deep soils and those of Sadras and Connor (1991) were done in pots. Also, it was found that the model is very sensitive to small changes in the date of anthesis, as a few days could displace the curve significantly. Furthermore, when the model was tested with data of other treatments that included one with N limitation ( Fig. 8 ), the curve obtained for the SDI treatment did not fit the data from other treatments, as there were substantial differences in HI for the same fraction of post-anthesis transpiration ( Fig. 8 ). It appears that there are no simple answers to modelling HI, at least when the imposed water stress patterns differ from those in SDI regimes. However, if the best DI strategy is to impose a sustained deficit throughout the season, the assumption of a constant HI over a range of mild-to-moderate water deficits is supported by strong experimental evidence in most of the major crop plants, as discussed above.
Harvest index (HI PV ) as a function of post-anthesis transpiration fraction (fT VPD ) for sunflower, standardized with respect to the production value (PV=amount of biomass produced per unit of hexose substrate; Penning de Vries et al. , 1974 ) of biomass and to the vapour pressure deficit (VPD), respectively. The dashed line represents Sadras and Connor (1991) model: HI PV =fT VPD /[1–( a – b fT VPD )]; ( a =0.91; b =1.63); a and b were obtained following the derivation of Sadras and Connor (1991) but using original data obtained in Cordoba in two sunflower experiments under an SDI regime. The data used were: closed circles and triangles, from an SDI regime measured in a summer experiment reported by Soriano (2001) . Squares are from an SDI regime under high N, measured in a spring experiment in 1985 ( Álvarez, 1987 ). The continuous line represents the best fit to all the Cordoba experimental data (HI PV =0.116 ln(fT VPD )+0.608; r 2 =0.89).
Harvest index (HI PV ) as a function of post-anthesis transpiration fraction (fT VPD ). Closed triangles are from a DI regime that had pre-anthesis deficits while open triangles are from another DI regime in the same experiment under post-anthesis deficits, as reported by Soriano (2001) . Squares are from an SDI regime under N limitation (no N fertilizer applied) measured in the 1985 experiment of Álvarez (1987) . The two lines are the same as those depicted in Fig. 7 .
Deficit irrigation so far has had significantly more success in tree crops and vines than in field crops for a number of reasons ( Fereres et al. , 2003 ). First, economic return in tree crops is often associated with factors such as crop quality, not directly related to biomass production and water use. The yield-determining processes in many fruit trees are not sensitive to water deprivation at some developmental stages ( Uriu and Magness, 1967 ; Johnson and Handley, 2000 ). Because of their high WP, tree crops and vines can afford high-frequency, micro-irrigation systems that are ideally suited for controlling water application and thus for stress management ( Fereres and Goldhamer, 1990 ). From the standpoint of water conservation, a given reduction in water supply to trees and vine canopies will be translated into a greater decrease in transpiration than in field crops, leading to more net water savings. This is because tall, rough canopies are better coupled to the atmosphere than the short, smooth canopies of field crops ( Jarvis and McNaughton, 1986 ), and a reduction in stomatal conductance is scaled up to a greater extent in the canopies of tree crops and vines.
Traditionally, fruit tree irrigation recommendations allowed for some stress development ( Veihmeyer, 1972 ) and there has been awareness of the benefits of water stress in some aspects of fruit production such as fruit quality for a long time ( Uriu and Magness, 1967 ). The imposition of water stress at certain developmental periods could therefore benefit yield and quality in fruit tree and vine production. The concept of regulated deficit irrigation (RDI) was first proposed by Chalmers et al. (1981) and Mitchell and Chalmers (1982) to control vegetative growth in peach orchards, and they found that savings in irrigation water could be realized without reducing yield. Even though similar results were reported for pears ( Mitchell et al. , 1989 ), RDI was found not to be as successful in other environments ( Girona et al. , 1993 ). Nevertheless, experiments with RDI have been successful in many fruit and nut tree species such as almond ( Goldhamer et al. , 2000 ), pistachio ( Goldhamer and Beede, 2004 ), citrus ( Domingo et al. , 1996 ; González-Altozano and Castel, 1999 ; Goldhamer and Salinas, 2000 ), apple ( Ebel et al. , 1995 ), apricot ( Ruiz-Sánchez et al. , 2000 ), wine grapes ( Bravdo and Naor, 1996 ; McCarthy et al. , 2002 ), and olive ( Moriana et al. , 2003 ), almost always with positive results. Thus, there is sufficient evidence at present that supplying the full ET requirements to tree crops and vines may not be the best irrigation strategy in many situations ( Fereres and Evans, 2006 ).
Regardless of the type of irrigation programme used, there is a need to develop scientific irrigation scheduling procedures ( Fereres, 1996 ). In particular, if DI is used, monitoring the soil or plant water status is even more critical for minimizing risk, given the uncertainties in determining the exact water requirements. In the case of fruit trees, because of the complexity of monitoring the root-zone water status under localized irrigation, plant-based methods for detecting water deficits have important advantages ( Fereres and Goldhamer, 1990 ). Jones (2004) has recently reviewed the recent advances in plant-based methods for irrigation scheduling and has thoroughly described the many options currently available. One of the major limitations of currently established methods in fruit trees such as the measurement of stem water potential (SWP) is the high labour requirements of the monitoring process. Alternative methods to using SWP that can be automated, such as the use of dendrometry ( Goldhamer and Fereres, 2001 ), have relatively high variability ( Intrigliolo and Castel, 2004 ; Naor et al. , 2006 ) and, thus, require additional research to reduce uncertainty in their use before they can be recommended for adoption. The use of infrared thermometry and thermal imaging may be a very promising option for stress monitoring in trees and vines ( Jones, 2004 ), as shown in a recent report on stress detection in olive trees from infrared imagery ( Sepulcre-Cantó et al. , 2006 ).
The mechanisms responsible for the lack of yield decline under RDI have been explored ( Chalmers et al. , 1986 ; Girona et al. , 1993 ). The obvious explanation is that high sensitivity of expansive growth of the aerial parts to water deficits must affect the partitioning of assimilated carbon, as photosynthesis is unaffected by mild water deficits. It has been shown that root growth is favoured under water deficits ( Sharp and Davies, 1979 ; Hsiao and Xu, 2000 ), and partitioning to fruit growth must also be unaffected ( Gucci and Minchin, 2002 ). More research is needed to elucidate the basis for observed responses, in view of the interactions between water stress and crop load ( Naor et al. , 1999 ). One feature of the yield response of tree crops to ET deficits is that, contrary to the linearity observed in annual crops ( Fig. 1 ), the response appears to be curvilinear ( Moriana et al. , 2003 ). This means that WP is highest at low levels of water application and that DI is the appropriate irrigation strategy.
Another developmental period when water deficits may be applied safely is between harvest and leaf fall. Johnson et al. (1992) found that, in peach, relatively severe water deficits may be imposed during that period, although severe stress increased the number of double fruits and other fruit-shape disorders the following year. The RDI response is very dependent on the timing and degree of severity of the water deficits, as well as on crop load ( Marsal and Girona, 1997 ). There are significant differences among species, however. For instance, Goldhamer et al. (2006) proved that in almond trees an SDI regime is the least detrimental to yield. The results of this study, summarized in Fig. 9 , indicate that for the same level of applied water, yields were less affected under SDI than under two RDI regimes that biased the water deficits, either pre- or post-harvest ( Fig. 9 ). The treatment with post-harvest stress had a significant decline in fruit number due to carry-over effects, with a reduction in the number of fruiting buds the following year ( Goldhamer et al. , 2006 ). By contrast, fruiting density was enhanced above the control in the pre-harvest RDI treatment, although tree canopy and nut sizes were reduced ( Goldhamer et al. , 2006 ).
Response of almond yield to three deficit irrigation regimes. Average results of a 4-year experiment conducted in California where three different DI regimes were applied. Drawn from data of Goldhamer et al. (2006) .
One limitation of many studies on RDI is that comparisons among treatments are often not fair because the amount of applied water in the different DI treatments is not the same. A long-term experiment has been conducted on a peach farm located on deep alluvial soil near Cordoba where SDI was compared with RDI, using the same amount seasonally. The RDI regime concentrated the application of water to the period of rapid expansion of fruit growth (Stage III), while the SDI applied the water throughout the irrigation season. In both DI treatments, water application was about two-thirds that of the control. Figure 10 presents the evolution of SWP for the three treatments during the fourth experimental year (2005). In RDI, SWP declined in early summer to values about twice those of the control and the SDI treatment. Recovery of SWP in RDI following irrigation was rapid, reaching control values in <1 week, while the SWP of SDI declined during Stage III to values that were 0.3 MPa lower than in the other two treatments. Following harvest, irrigation was interrupted in RDI but continued in the other two treatments. Yield response, shown in Table 1 , indicates that the RDI treatment had the same yield and fruit size as the control despite its lower water status, while the SDI had a 10% decrease in yield and 15% reduction in fruit size that were statistically significant. This was despite the fact that the value of SWP integrated over the season was more negative in RDI than in the other two treatments ( Table 1 ). Nevertheless, during the period of rapid fruit growth, the absolute value of SWP of RDI was less than that of SDI ( Table 1 ). From this experiment it can be concluded that, for the same amount of applied water, RDI is advantageous over SDI in peach production.
Stem water potential (MPa, integrated over the irrigation season and the RDI irrigation period, see Fig. 10 ), yield (t ha −1 ), and fruit volume (cm 3 ) in three irrigation treatments (RDI, SDI, and full irrigation) in the fourth year (2005) of a peach experiment near Cordoba, Spain
| Treatment | Stem water potential (MPa) integrated over | Yield (t ha ) | Fruit volume (cm ) | |
| Season | RDI irrigation period | |||
| RDI | −125.6 | −34.7 | 48.1 a | 178 a |
| SDI | −108.1 | −39.2 | 43.8 b | 155 b |
| FI | −86.7 | −31.2 | 49.2 a | 171 a |
| Treatment | Stem water potential (MPa) integrated over | Yield (t ha ) | Fruit volume (cm ) | |
| Season | RDI irrigation period | |||
| RDI | −125.6 | −34.7 | 48.1 a | 178 a |
| SDI | −108.1 | −39.2 | 43.8 b | 155 b |
| FI | −86.7 | −31.2 | 49.2 a | 171 a |
Means followed by a different letter (within a column) are significantly different at the 0.05 probability level according to LSD.
Irrigation season (1 May to mid-September).
Seasonal patterns of stem water potential (SWP, MPa) of peach trees in response to the irrigation treatments (RDI, SDI, and full irrigation) during the fourth experimental year (2005); fruit growth stages (I, II, and III) are shown and the arrow H indicates harvest date. Error bars indicate ±standard error.
Experience that full irrigation is not the best strategy abounds in many perennial horticultural crops, but in none is it more evident than in wine grapes. The quality of wine in semi-arid areas is strongly associated by enologists with water stress ( Williams and Matthews, 1990 ) to the point that, as an example, irrigation of vineyards was forbidden by law in Spain until 1996. Nevertheless, the benefits of RDI to the yield and quality of wine grapes have been clearly demonstrated relative to rain-fed production ( Girona et al. , 2006 ). Among the techniques used for imposing RDI on wine grapes is one that alternates drip irrigation about every 2 weeks on either side of the vine row; this is called partial root drying (PRD) ( Dry and Loveys, 1998 ). The PRD technique has its foundation in the root-to-shoot signalling that regulates the plant response to drying soil ( Davies and Zhang, 1991 ; Dodd, 2005 ). Shoot physiological processes are affected by root signalling, including leaf expansion ( Passioura, 1988 ). The control of vegetative growth is of paramount importance in the production of high-quality wine grapes ( Loveys et al. , 2004 ), and it has been shown that PRD controls canopy growth and is advantageous over full irrigation in wine production ( McCarthy et al. , 2002 ). There have been commercial applications of PRD and the system has already been tested in vineyards located in many environments ( Dos Santos et al. , 2003 ; Girona et al. , 2006 ).
The PRD technique has also been tested in other crops, notably fruit trees. While positive results have been reported ( Kang et al. , 2000 ), it appears that, when meaningful comparisons under field conditions have been carried out that have avoided the interactions between the amount and the mode of placement of irrigation water, PRD has not improved the crop response over an RDI regime that applied the same amount of water, as shown in peach ( Goldhamer et al. , 2002 ), apple ( Leib et al. , 2006 ), and olive ( Wahbi et al. , 2005 ), among others. Nevertheless, the PRD is a useful water application technique that, by reducing the number of emission points that wet the soil at one time, alters the partitioning between evaporation and transpiration. The reduction in evaporation under PRD relative to an RDI regime that has twice the number of emitters, increases the WP of a limited supply of water. The alternate wetting in PRD reduces drainage losses relative to a regime that always wets the same side of the plant row ( Kang et al. , 2000 ). Another factor that needs exploration is the observation that the alternate wetting and drying of PRD promotes root growth ( Mingo et al. , 2004 ). If this is confirmed in fruit trees, the recovery following stress periods could be enhanced by PRD. For instance, the time for recovery of SWP in the RDI treatment in Fig. 10 was about 1 week, and any shortening of that period would have had a positive influence on fruit expansion rate, and probably on fruit size.
Today, irrigation is the largest single consumer on the planet. Competition for water from other sectors will force irrigation to operate under water scarcity. Deficit irrigation, by reducing irrigation water use, can aid in coping with situations where supply is restricted. In field crops, a well-designed DI regime can optimize WP over an area when full irrigation is not possible. In many horticultural crops, RDI has been shown to improve not only WP but farmers’ net income as well. It would be important to investigate the basis for the positive responses to water deficits observed in the cases where RDI is beneficial. While DI can be used as a tactical measure to reduce irrigation water use when supplies are limited by droughts or other factors, it is not known whether it can be used over long time periods. It is imperative to investigate the sustainability of DI via long-term experiments and modelling efforts to determine to what extent it can contribute to the permanent reduction of irrigation water use.
We acknowledge the support of grants from INIA (RTA02-070) and the European Commission DIMAS project (INCO-CT-2004-509087), and the skilled technical assistance of C Ruz in the peach experiment.
Google Scholar
Google Preview
| Month: | Total Views: |
|---|---|
| December 2016 | 1 |
| January 2017 | 111 |
| February 2017 | 166 |
| March 2017 | 242 |
| April 2017 | 171 |
| May 2017 | 217 |
| June 2017 | 164 |
| July 2017 | 81 |
| August 2017 | 123 |
| September 2017 | 146 |
| October 2017 | 170 |
| November 2017 | 228 |
| December 2017 | 768 |
| January 2018 | 624 |
| February 2018 | 606 |
| March 2018 | 718 |
| April 2018 | 590 |
| May 2018 | 485 |
| June 2018 | 447 |
| July 2018 | 430 |
| August 2018 | 507 |
| September 2018 | 453 |
| October 2018 | 559 |
| November 2018 | 635 |
| December 2018 | 990 |
| January 2019 | 546 |
| February 2019 | 606 |
| March 2019 | 747 |
| April 2019 | 746 |
| May 2019 | 835 |
| June 2019 | 595 |
| July 2019 | 670 |
| August 2019 | 642 |
| September 2019 | 660 |
| October 2019 | 457 |
| November 2019 | 475 |
| December 2019 | 383 |
| January 2020 | 425 |
| February 2020 | 335 |
| March 2020 | 380 |
| April 2020 | 525 |
| May 2020 | 285 |
| June 2020 | 399 |
| July 2020 | 391 |
| August 2020 | 364 |
| September 2020 | 463 |
| October 2020 | 542 |
| November 2020 | 447 |
| December 2020 | 499 |
| January 2021 | 378 |
| February 2021 | 429 |
| March 2021 | 555 |
| April 2021 | 537 |
| May 2021 | 482 |
| June 2021 | 408 |
| July 2021 | 377 |
| August 2021 | 377 |
| September 2021 | 442 |
| October 2021 | 449 |
| November 2021 | 433 |
| December 2021 | 465 |
| January 2022 | 471 |
| February 2022 | 416 |
| March 2022 | 528 |
| April 2022 | 508 |
| May 2022 | 525 |
| June 2022 | 454 |
| July 2022 | 449 |
| August 2022 | 462 |
| September 2022 | 389 |
| October 2022 | 448 |
| November 2022 | 436 |
| December 2022 | 409 |
| January 2023 | 468 |
| February 2023 | 379 |
| March 2023 | 439 |
| April 2023 | 425 |
| May 2023 | 440 |
| June 2023 | 321 |
| July 2023 | 258 |
| August 2023 | 307 |
| September 2023 | 387 |
| October 2023 | 416 |
| November 2023 | 372 |
| December 2023 | 377 |
| January 2024 | 400 |
| February 2024 | 438 |
| March 2024 | 510 |
| April 2024 | 424 |
| May 2024 | 405 |
| June 2024 | 407 |
| July 2024 | 399 |
| August 2024 | 350 |
Email alerts
Citing articles via.
- Recommend to your Library
Affiliations
- Online ISSN 1460-2431
- Print ISSN 0022-0957
- Copyright © 2024 Society for Experimental Biology
- About Oxford Academic
- Publish journals with us
- University press partners
- What we publish
- New features
- Open access
- Institutional account management
- Rights and permissions
- Get help with access
- Accessibility
- Advertising
- Media enquiries
- Oxford University Press
- Oxford Languages
- University of Oxford
Oxford University Press is a department of the University of Oxford. It furthers the University's objective of excellence in research, scholarship, and education by publishing worldwide
- Copyright © 2024 Oxford University Press
- Cookie settings
- Cookie policy
- Privacy policy
- Legal notice
This Feature Is Available To Subscribers Only
Sign In or Create an Account
This PDF is available to Subscribers Only
For full access to this pdf, sign in to an existing account, or purchase an annual subscription.

Deficit Irrigation
A Remedy for Water Scarcity
- © 2020
- Samiha Ouda 0 ,
- Abd El-Hafeez Zohry 1 ,
- Tahany Noreldin 2
Water Requirements and Field Irrigation Research Department, Soils, Water and Environment Research Institute, Agricultural Research Center, Giza, Egypt
You can also search for this author in PubMed Google Scholar
Crop Intensification Research Department Field Crops Research Institute, Agricultural Research Center, Giza, Egypt
- Provides an in-depth review of deficit irrigation and its role in dealing with water security
- Quantifies the effect of deficit irrigation application on four crop types
- Examines climate change studies in Egypt on crops production and cultivation
6929 Accesses
23 Citations
This is a preview of subscription content, log in via an institution to check access.
Access this book
Subscribe and save.
- Get 10 units per month
- Download Article/Chapter or eBook
- 1 Unit = 1 Article or 1 Chapter
- Cancel anytime
- Available as EPUB and PDF
- Read on any device
- Instant download
- Own it forever
- Compact, lightweight edition
- Dispatched in 3 to 5 business days
- Free shipping worldwide - see info
- Durable hardcover edition
Tax calculation will be finalised at checkout
Other ways to access
Licence this eBook for your library
Institutional subscriptions
About this book
This book focuses on proving that deficit irrigation could play an important role in increasing food production in times of water scarcity. Although the application of deficit irrigation can involve loss in crop productivity, it still secures water to be use in cultivating more lands and producing more food. The following questions are discussed and the authors offer solutions to these problems:
Will the production, on a national level, resulting from these new added areas compensate yield losses attained by application of deficit irrigation?
Is it possible to use deficit irrigation practice to reduce the applied irrigation water to certain crops that have a surplus in their production, and direct this saved water to cultivate new areas with crops have low self-sufficiency ratios?
This book will appeal to students and researchers involved with water scarcity and food security.
Similar content being viewed by others

Deficit Irrigation: An Optimization Strategy for a Sustainable Agriculture

Crop Choices with Limiting Water Supplies: Deficit Irrigation and Sensitive Crop Growth Stages
Adapting irrigated agriculture to drought in the san joaquin valley of california.
- Climate Change and Climate Change Impacts
- Deficit Irrigation Techniques
- Food Gaps and Insecurity
- Irrigation Water Management
- Water Productivity and Management
- Wheat Self-sufficiency Ratio
- water quality and water pollution
- climate change impacts
- water policy
Table of contents (8 chapters)
Front matter, water scarcity leads to food insecurity.
- Samiha Ouda, Abd El-Hafeez Zohry
Deficit Irrigation and Water Conservation
Samiha Ouda, Tahany Noreldin
Egypt Faces Water Deficiency, and Food Insufficiency
- Abd El-Hafeez Zohry, Samiha Ouda
Field Crops and Deficit Irrigation in Egypt
- Samiha Ouda, Tahany Noreldin, Abd El-Hafeez Zohry
Vegetable Crops and Deficit Irrigation in Egypt
Wheat insufficiency and deficit irrigation, climate change assessment in egypt: a review, climate change and wheat self-sufficiency, authors and affiliations.
Abd El-Hafeez Zohry
About the authors
Professor Samiha Ouda had her BSc. from Cairo University in 1982 (Agricultural Economy Department), Egypt and her MSc from Ain Shams University in 1993 (Agronomy Department), Egypt. She had her PhD in 1998 from Iowa State University, USA (Crop Physiology and management). She had her professor appointment in 2009. Prof. Ouda have been working in the Agricultural Research Center in Water Requirements and Field Irrigation Research Department; Soils Water and Environment Institute; in Egypt for 28 years. She won three prizes: two of them local and the third one from International Commission on Irrigation and Drainage (ICID-CIID) in 2015. She published 88 research papers, 40 book chapters and 4 books on irrigation water management, modeling, crop simulation, agroclimatology, climate change impacts on crops and its water requirements. She supervised 4 Master and PhD theses on simulation models and climate change.
Professor Abd El-Hafeez Zohry have been working in the Agricultural Research Center for 28 years in Crops Intensification Research Department; Field Crops Research Institute; Agricultural Research Center in Egypt. He had his BSc from El-Minia University in Egypt in 1987 (General Agriculture Department) and his MSc from Al-Azhar University in 1990 in Egypt (Agronomy Department). He had his PhD in 1994 from Al-Azhar University (Crop Physiology and Production). He had his professor appointment in 2006. He published 39 research papers, 27 book chapters, 2 books and 4 extension bulletins on intensive cropping, and crop rotations. He supervised one MSc and one PhDs theses on intensive cropping. He won water innovation prize from International Commission on Irrigation and Drainage (ICID-CIID) in 2015.
Dr. Tahany Noreldin obtained her BSc, MSc. and PhD from College of Agriculture, Cairo University, Egypt (Agronomy Department) in 1998, 2005 and 2010, respectively. She have been working in Water Requirements and Field Irrigation Research Department; Soils Water and Environment Institute; Agricultural Research Center in Egypt for 7 years. She had her associated professor appointment in 2018. She published her MSc and PhD theses in two books. She contributed in authoring 2 books, 5 book chapters and 34 research papers on irrigation water management, modeling, crop simulation, agroclimatology, and climate change.
Bibliographic Information
Book Title : Deficit Irrigation
Book Subtitle : A Remedy for Water Scarcity
Authors : Samiha Ouda, Abd El-Hafeez Zohry, Tahany Noreldin
DOI : https://doi.org/10.1007/978-3-030-35586-9
Publisher : Springer Cham
eBook Packages : Earth and Environmental Science , Earth and Environmental Science (R0)
Copyright Information : Springer Nature Switzerland AG 2020
Hardcover ISBN : 978-3-030-35585-2 Published: 23 January 2020
Softcover ISBN : 978-3-030-35588-3 Published: 23 January 2021
eBook ISBN : 978-3-030-35586-9 Published: 22 January 2020
Edition Number : 1
Number of Pages : XIV, 196
Number of Illustrations : 4 b/w illustrations, 20 illustrations in colour
Topics : Waste Water Technology / Water Pollution Control / Water Management / Aquatic Pollution , Hydrology/Water Resources , Agriculture , Climate Change/Climate Change Impacts , Plant Sciences , Water Policy/Water Governance/Water Management
- Publish with us
Policies and ethics
- Find a journal
- Track your research
Click through the PLOS taxonomy to find articles in your field.
For more information about PLOS Subject Areas, click here .
Loading metrics
Open Access
Peer-reviewed
Research Article
Effect of climate change-induced water-deficit stress on long-term rice yield
Roles Conceptualization, Data curation, Formal analysis, Funding acquisition, Methodology, Project administration, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing
* E-mail: [email protected]
Affiliation Department of Agronomy, National Taiwan University, Taipei, Taiwan
Roles Data curation, Funding acquisition, Investigation
Affiliation Taichung District Agricultural Research and Extension Station, Council of Agriculture, Changhua, Taiwan
Roles Data curation
- Hungyen Chen,
- Yi-Chien Wu,
- Chia-Chi Cheng,
- Chih-Yung Teng

- Published: April 17, 2023
- https://doi.org/10.1371/journal.pone.0284290
- Reader Comments
The water requirements of crops should be investigated to improve the efficiency of water use in irrigated agriculture. The main objective of the study was to assess the effects of water deficit stress on rice yields throughout the major cropping seasons. We analyzed rice yield data from field experiments in Taiwan over the period 1925–2019 to evaluate the effects of water-deficit stress on the yield of 12 rice cultivars. Weather data, including air temperatures, humidity, wind speed, sunshine duration, and rainfall were used to compute the temporal trends of reference evapotranspiration and crop water status (CWS) during rice growth stages. A negative CWS value indicates that the crop is water deficient, and a smaller value represents a lower water level (greater water-deficit stress) in crop growth. The CWS on rice growth under the initial, crop development, reproductive, and maturity stages declined by 96.9, 58.9, 24.7, and 198.6 mm in the cool cropping season and declined by 63.7, 18.1, 8.6, and 3.8 mm in the warm cropping season during the 95 years. The decreasing trends in the CWSs were used to represent the increases in water-deficit stress. The total yield change related to water-deficit stress on the cultivars from 1925–1944, 1945–1983, and 1996–2019 under the initial, crop development, reproductive, and maturity stages are -56.1 to 37.0, -77.5 to -12.3, 11.2 to 19.8, and -146.4 to 39.1 kg ha -1 in the cool cropping season and -16.5 to 8.2, -12.9 to 8.1, -2.3 to 9.0, and -9.3 to 8.0 in the warm cropping season, respectively. Our results suggest that CWS may be a determining factor for rice to thrive during the developmental stage, but not the reproductive stage. In addition, the effect of water-deficit stress has increasingly affected the growth of rice in recent years.
Citation: Chen H, Wu Y-C, Cheng C-C, Teng C-Y (2023) Effect of climate change-induced water-deficit stress on long-term rice yield. PLoS ONE 18(4): e0284290. https://doi.org/10.1371/journal.pone.0284290
Editor: Josily Samuel, CRIDA: Central Research Institute for Dryland Agriculture, INDIA
Received: September 22, 2022; Accepted: March 28, 2023; Published: April 17, 2023
Copyright: © 2023 Chen et al. This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Data Availability: All relevant data are within the paper and its Supporting information files.
Funding: This work was supported by funding from the National Science and Technology Council, Taiwan (109-2313-B-002-027-MY3) and Taichung District Agricultural Research and Extension Station, Council of Agriculture, Executive Yuan, Taiwan (111a20-1) to HC. There was no additional external funding received for this study.
Competing interests: The authors have declared that no competing interests exist.
Introduction
Global climate change, including increased temperatures and fluctuating rainfall, has become a threat with a high potential to affect the water supply and agricultural sectors [ 1 – 3 ]. The increase in temperature and decrease in rainfall negatively affects the growth of plants because plants are subjected to temperature and water stress due to an increase in evapotranspiration [ 4 , 5 ]. The impact of climate change on the hydrological cycle, water balance, and runoff characteristics has emerged as a significant stressor at local and district levels, although there are uncertainties regarding the impacts of climate variability on water resources [ 6 , 7 ]. It is suggested that water availability and crop productivity will decrease significantly, and climate change will have an impact on irrigation water requirements and crop yield [ 8 , 9 ]. Crop yield change is expected due to the shifting growth phase and photosynthetic capacity, and increasing respiration and water requirements, which result from climate change [ 10 , 11 ]. To investigate the general effects of crop yield change on climate change, it is necessary to analyze long-term temporal variations between crop yields and climate variables [ 12 – 14 ].
To improve water-use efficiency in irrigated agriculture, it is important to study and understand crop water requirements. Evapotranspiration is a vital component when describing the hydrological cycle in ecological systems, estimating water balance, and determining water availability along with precipitation [ 15 , 16 ]. Reference evapotranspiration (ET 0 ) is a parameter of climatic conditions that has been widely investigated as an indicator of climate change [ 17 , 18 ]. Crop evapotranspiration (ET C ) is a variable for the optimization of irrigation water productivity and designing the schedule of irrigation in the implementation of agricultural water management [ 19 ] (Gong et al., 2020) and is highly influenced by irrigation water supply under different irrigation levels [ 20 , 21 ]. The Penman-Monteith (PM) model based on the Food and Agriculture Organization (FAO)-56 guidelines has served as a reference method because it produces the most accurate results compared to lysimetric measurements [ 22 , 23 ]. The PM model has been widely used for the estimation of daily or monthly ET 0 in different agro-climatic zones by many researchers for decades [ 22 , 24 , 25 ].
Rice is a semi-aquatic plant that depends on the rainfall and temperature of the cultivation area and hence, is heavily affected by climate change [ 5 , 26 ]. Severe effects of drought and high temperature on the growth and yield of rice due to insufficient water supply and improper scheduling of irrigation have been reported [ 27 , 28 ]. Some reports have revealed that rice yield may be affected by temperature and precipitation because of some physiological mechanisms [ 29 , 30 ]. Although many studies have revealed the impacts of climate change on crop production utilizing climate model projections of temperatures and rainfall [ 31 , 32 ], the number of studies that analyze the effect of water-deficit stress on rice yield using long-term field experimental data is limited.
Water deficit stress occurs when the amount of water required is greater than the amount of water available during a certain time. Our goal was to assess the effects of water deficit stress on rice yields throughout the major cropping seasons. In this study, we analyzed the yield data of 12 rice cultivars in cool and warm cropping seasons, separately, from field experiments conducted under irrigated conditions with optimal management at a research station in Taichung, Taiwan over the period 1925–2019. First, weather data, including average, maximum, and minimum temperatures, humidity, wind speed, and sunshine duration, collected at the research farm were used to compute the long-term temporal trends of reference evapotranspiration during the initial, crop development, reproductive, and maturity stages of rice growth during the 95 years. Second, the crop evapotranspiration of rice under the growth stages was calculated using the estimated reference evapotranspiration and crop coefficient of rice. Third, the crop water status during the growth stages was calculated using the estimated crop evapotranspiration and collected rainfall data. Fourth, long-term temporal trends in crop water status during the growth stages were deduced to reveal the temporal trend in water-deficit stress. Fifth, a multiple linear regression model was applied to evaluate the relationships between rice grain yield and water-deficit stress during the four growth stages. Finally, total yield changes computed from the regression coefficients for each growth stage over the periods 1925–1944, 1945–1983, and 1996–2019 were used separately to reveal the effects of water-deficit stress on rice yield and the temporal variations during the experimental period.
Materials and methods
Field experiment.
A field experiment on rice growth in two cropping seasons was conducted from 1925 to 2019 at the Taichung District Agricultural Research and Extension Station, Council of Agriculture, Executive Yuan, Taiwan (1925–1983: 24º09′ N 120º41′ E, altitude 77 m above mean sea level; 1996–2019: 24º00′ N 120º32′ E, altitude 19 m above mean sea level). The rice seeds were sown in the cool cropping season in mid-January, and the seeded area was dibbled either in February or March every year over the period 1925–2019 except for 1948–1951, 1985–1995, and 2014–2016. Rice from the cool cropping season was harvested either in June or July. The rice seeds were sown in the warm cropping season in June, and the area was dibbled either in July or August every year over the period 1925–2019, except for 1945, 1947–1951, 1985–1995, and 2013–2015. Rice in the warm cropping season was harvested either in October or November. The seedlings were transplanted into the fields by hand. The area of the plot for each cultivar was 27 m 2 . Continuous flooding irrigation to 5 cm above the soil surface was carried out in the field during the period between transplanting and drying. Re-irrigation was applied when the field water subsided to the soil surface. The grain yield was obtained by harvesting from all the hills in the plots (at a grain maturity rate of 98%), except for the side rows, and then measuring the grain weight. No field permits were required for this work at the research station which the authors are affiliated with.
Rice yield data
Twelve rice cultivars were used throughout the experimental period in cool and warm cropping seasons, separately. In cool cropping season, Nakamura (NM; 1925–1931), Taichung S2 (TCS2; 1925–1932), Baiker (BK; 1925–1944), Taichung S6 (TCS6; 1933–1944), Wugen (WG; 1925–1947, 1952–1976), Baimifun (BMF; 1945–1947, 1952–1976), Taichung 65 (TC65; 1930–1947, 1952–1983), Taichung 150 (TC150; 1945–1947, 1952–1983), Taiagro 67 (TA67; 1996–2013, 2017–2019), Taichung 189 (TC189; 1996–2013, 2017–2019), Taichung Indica 10 (TCI10; 1996–2013, 2017–2019), and Tai Japonica 9 (TJ9; 2000–2013, 2017–2019) were used. In warm cropping season, Nakamura (NM; 1925–1931), Taichung S2 (TCS2; 1925–1944), Jingou (JG; 1925–1944), Nyaoyao (NY; 1925–1944), Swanjian (SJ; 1946, 1952–1976), Sianlou (SL; 1946, 1952–1976), Taichung 65 (TC65; 1930–1944, 1946, 1952–1983), Taichung 150 (TC150; 1946, 1952–1983), Taiagro 67 (TA67; 1996–2012, 2016–2019), Taichung 189 (TC189; 1996–2012, 2016–2019), Taichung Indica 10 (TCI10; 1996–2012, 2016–2019), and Tai Japonica 9 (TJ9, 2000–2012, 2016–2019) were used.
For each cropping season, three groups of cultivars with overlapping cultivation periods were clustered together to calculate the group average value, which represents the effect of water stress on rice yield in each period. Based on the cultivation period among cultivars, four cultivars were included in the 1925–1944, 1945–1983, and 1996–2019 periods, separately.
Four distinct stages of rice growth were used for the analyses. For the cool cropping season, the initial stage was from March 1–31, the crop development stage was from April 1–30, the reproductive (mid-season) was from May 1–31, and the maturity (late season) stage was from June 1–30. For the warm cropping season, the initial stage was from August 1–31, the crop development stage was from September 1–30, the reproductive (mid-season) stage was from October 1–31, and the maturity (late season) stage was from November 1–30.
Weather data
A weather station was set up on the research station farm. The site is surrounded by field crops and the topography is flat. Daily weather data recording began on January 1, 1925. Meteorological instruments at the station included a solarimeter, glass thermometers for minimum and maximum temperatures, a psychrometer, and a thermo-hygrograph. The air temperature, humidity, wind speed, rainfall, and sunshine duration during the cropping seasons throughout the experimental period were used for the analyses. The average, minimum, and maximum temperatures, average relative humidity, and average wind speed (2 m above the soil surface) under the four growth stages for each year were calculated as the average of the daily values in the cool and warm cropping seasons, respectively. Rainfall and sunshine durations under the four growth stages for each year were calculated as the sum of the daily values in the two cropping seasons.
Statistical models
The Kc values for rice during the initial, crop development, reproductive (mid-season), and maturity (late season) stages were 1.15, 1.23, 1.14, and 1.02, respectively, as estimated by Tyagi et al. [ 35 ].
The value of the CWS represents the water status of crop growth under weather conditions. A negative CWS value indicates that the crop is water deficient, and a smaller value represents a lower water level (greater water-deficit stress) in crop growth. In cases where all the water needed for optimal crop growth is provided by rainfall, irrigation is not required, and the CWS equals zero. The CWS determined in this study was inspired by the formula for irrigation water need (IN) [ 36 ], IN = ET C + PERC + WL—PE. The value of the CWS equals the negative number of the value of IN.
The total rice yield change (kg ha -1 ) related to water stress under each growth stage was computed using the regression coefficients for water stress ( β CWS ) and the estimated change in crop water status (ΔCWS) throughout each cultivation period.
Long-term temporal variations in rice yield
In the cool cropping season, the yield of the four rice cultivars for the period 1925–1944 ranged between 1,530 and 8,055 kg ha -1 , with an average ± standard deviation (SD) of 4,236 ± 1,114 kg ha -1 . The yield of the four rice cultivars for the period 1945–1983 ranged between 2,550 and 8,215 kg ha -1 , with an average ± SD of 4,708 ± 774 kg ha -1 . The yield of the four rice cultivars during 1996–2019 ranged between 4,181 and 9,268 kg ha -1 , with an average ± SD of 6,523 ± 1,080 kg ha -1 ( Fig 1a–1d ). In the warm cropping season, the yield of the four rice cultivars for the period 1925–1944 ranged between 2,181 and 6,493 kg ha -1 , with an average ± standard deviation (SD) of 3,880 ± 873 kg ha -1 , the yield of the four rice cultivars for the period 1945–1983 ranged between 2,180 and 6,384 kg ha -1 , with an average ± SD of 4,149 ± 796 kg ha -1 , and the yield of the four rice cultivars for the period 1996–2019 ranged between 2,861 and 7,269 kg ha -1 , with an average ± SD of 4,704 ± 971 kg ha -1 ( Fig 1e–1h ).
- PPT PowerPoint slide
- PNG larger image
- TIFF original image
https://doi.org/10.1371/journal.pone.0284290.g001
Long-term temporal variations in reference evapotranspiration
In the cool cropping season, the ET 0 ranged between 2.3 to 4.7, 2.6 to 5.0, 3.4 to 5.7, and 3.4 to 5.8 mm day -1 under the initial, crop development, reproductive, and maturity stages, respectively ( Fig 2a–2d ). The average values ± SDs of ET 0 were 3.3 ± 0.5, 3.8 ± 0.4, 4.3 ± 0.5, and 4.4 ± 0.5 mm day -1 under the four growth stages, respectively ( Fig 2a–2d ). In the warm cropping season, the ET 0 ranged between 3.4–5.7, 3.6–5.9, 3.2–5.1, and 2.2–4.2 mm day -1 under the initial, crop development, reproductive, and maturity stages, respectively ( Fig 2e–2h ). The average values ± SDs of ET 0 were 4.6 ± 0.4, 4.3 ± 0.4, 3.8 ± 0.3, and 2.8 ± 0.3 mm day -1 under the four growth stages, respectively ( Fig 2e–2h ).
https://doi.org/10.1371/journal.pone.0284290.g002
Long-term temporal variations in crop water status
In the cool cropping season, the CWS on rice growth under initial, crop development, reproductive, and maturity stages ranged between -422.4 to -145.0, -428.9 to -42.0, -438.8 to 105.0, and -407.0 to 617.3 mm, respectively ( Fig 3a–3d ). The average values ± SDs of CWS were -327.2 ± 58.6, -323.1 ± 75.4, -263.9 ± 122.7, and -177.9 ± 195.1 mm under the four growth stages, respectively ( Fig 3a–3d ). In the warm cropping season, the CWS on rice growth under initial, crop development, reproductive, and maturity stages ranged between -455.7 to 333.8, -466.2 to 136.4, -435.2 to -260.1, and -416.3 to -244.8 mm, respectively ( Fig 3e–3h ). The average values ± SDs of WDS were -208.3 ± 173.4, -322.9 ± 123.5, -380.2 ± 24.9, and -366.2 ± 24.3 mm under the four growth stages, respectively ( Fig 3e–3h ). The CWS on rice growth under the initial, crop development, reproductive, and maturity stages declined by 96.9, 58.9, 24.7, and 198.6 mm in the cool cropping season, respectively ( Fig 3a–3d ), and declined by 63.7, 18.1, 8.6, and 3.8 mm in the warm cropping season, respectively ( Fig 3e–3h ) from 1925 to 2019.
Grey line represents the linear regression line. * represents p-value < 0.05.
https://doi.org/10.1371/journal.pone.0284290.g003
Effects of crop water status on rice yield
The long-term temporal variation in the estimated effects of the CWS on the grain yield of 12 rice cultivars in the cool and warm cropping seasons from 1925 to 2019 are shown in Fig 4 . A positive value of the regression coefficient reflects a coincident pattern between grain yield and CWS, and a negative value indicates an inverse response of grain yield to CWS. In the cool cropping season, the average regression coefficients under the initial and maturity stages revealed positive values in 1925–1944 and 1996–2019, but a negative average value over the period 1945–1983 ( Fig 4a and 4d ); the average regression coefficients under the crop development stage revealed all positive values and decreased throughout the experimental period ( Fig 4b ); and the average regression coefficients under the reproductive stage revealed all negative values and increased throughout the experimental period ( Fig 4c ). In the warm cropping season, the average regression coefficients under the initial stage revealed positive values in the periods 1925–1944 and 1945–1983, but a negative average value from 1996–2019 ( Fig 4e ). The average regression coefficients under the crop development and maturity stages revealed positive values in the periods 1925–1944 and 1996–2019, but a negative average value from 1945–1983 ( Fig 4f and 4h ). The average regression coefficients in the reproductive stage revealed a negative value from 1925–1944 and 1945–1983, but a positive value from 1996–2019, and increased throughout the experimental period ( Fig 4g ).
Filled circles represent the value of a cultivar. Open circles represent the average value of a group of cultivars having overlapped cultivation period. The length of the black line on the open circle represents the value of standard deviation. Horizontal dash lines separate the groups of cultivars having overlapped cultivation periods. In each panel, the upper, mid, and lower zone represent the periods of 1925–1944, 1945–1983, and 1996–2019, respectively.
https://doi.org/10.1371/journal.pone.0284290.g004
Yield changes responding to crop water status
The mean total yield change relating to the CWS on the cultivars in cool cropping seasons over the periods 1925–1944, 1945–1983, and 1996–2019 are -43.2, 37.0, and -56.1 kg ha -1 in the initial stage, respectively, -77.5, -39.0, and -12.3 kg ha -1 in the crop development stage 19.3, 19.8, and 11.2 kg ha -1 in the reproductive stage, and -146.4, 39.1, and -12.4 kg ha -1 in the maturity stage, respectively ( Table 1 ). The mean total yield change related to the CWS on the cultivars in the warm cropping seasons over the periods 1925–1944, 1945–1983, and 1996–2019 are -1.3, -16.5, and 8.2 kg ha -1 in the initial stage, -12.9, 8.1, and -0.4 kg ha -1 in the crop development stage, 9.0, 4.1, and -2.3 kg ha -1 in the reproductive stage, and -2.8, 8.0, and -9.3 kg ha -1 in the maturity stage, respectively ( Table 1 ).
https://doi.org/10.1371/journal.pone.0284290.t001
In most cultivated areas of Taiwan, two cropping seasons are maintained throughout the year. The cool cropping season starts in late February or early March (initial stage) and ends in late June (maturity stage), and the warm cropping season starts in late July or early August (initial stage) and ends in late November (maturity stage). The patterns of temperature variation are opposite in the two cropping seasons. The average temperature increases throughout the cool cropping season while the average temperature decreases throughout the warm cropping season. The patterns of variation in reference evapotranspiration under the four growth stages of rice were the opposite between the cool and warm cropping seasons ( Fig 2 ). Thus, to reveal the climatic effect, the rice yield response to climate variables needs to be analyzed separately in the cool and warm cropping seasons. The value for Qiu et al. [ 37 ] differentiates the changes in the seasonal crop evapotranspiration of rice in terms of growth duration under varying types of warming patterns using an evapotranspiration estimation model. The reference evapotranspiration increased throughout the cool cropping season, whereas it decreased throughout the warm cropping season ( Fig 2 ). The different patterns of temporal and spatial variation in the reference evapotranspiration and sensitivity coefficient responses to precipitation and temperature were investigated [ 38 , 39 ].
Long-term decreasing trends and negative values in crop water status were observed at all four growth stages in the two cropping seasons ( Fig 3 ). This result showed that increasing crop water deficiency led to greater water-deficit stress on rice growth. The decreased crop water status was due to the increased air temperature and decreased rainfall [ 1 ]. The water deficit is limiting the growth and productivity of crops and has been a major problem for crop production worldwide, especially in rain-fed agricultural areas [ 40 – 42 ]. In the cool cropping season, the decreasing trend of crop water status was severe during the initial and maturity stages and mild during the reproductive stage ( Fig 3 ). Compared with the cool cropping season, the decreasing trend in crop water status in the warm cropping season was relatively small under the four growth stages ( Fig 3 ). This result may be due to the greater temperature increase and rainfall decrease in the cool season as opposed to the warm season [ 43 ].
The rice yield changes related to the crop water status were negative during the rice development stage (except for the warm cropping season from 1945–1983). This result suggests that crop water may be a determining factor for rice growth during the development stage [ 22 , 44 ]. The rice yield changes related to the crop water status were positive during the rice reproductive stage (except for the warm cropping season over the period 1996–2019). This result suggests that crop water may not be a determining factor for rice growth during the reproductive stage [ 22 , 45 ]. In recent years, from 1996 to 2019, negative yield changes were observed under all four growth stages in the cool cropping season and under crop development, reproductive, and maturity stages in the warm cropping season. This result may suggest that water-deficit stress has had a greater effect on rice growth in recent years [ 46 , 47 ].
The values of crop water status under the four growth stages had little correlation with each other in both the cool (| r | ≤ 0.24) and warm (| r | ≤ 0.16) cropping seasons ( Table 2 ). The annual variations in the yields of the cultivars with overlapping cultivation periods were correlated with each other in the same groups ( Fig 3 ). The correlation coefficients of the yearly yields among the rice cultivar pairs with overlapping cultivation periods averaged 0.838, 0.605, and 0.665 in the cool cropping season during 1925–1944, 1945–1983, 1996–2019, respectively, and 0.716, 0.566, and 0.735 in the warm cropping season during 1925–1944, 1945–1983, 1996–2019, respectively. The correlations between the changes in crop water status under the four growth stages may make it difficult to separate the effects of different growth stages due to the co-linearity [ 48 ]. Although these problems have been discussed, the observations at our station showed low to little correlation among the values at different growth stages during the cropping seasons.
https://doi.org/10.1371/journal.pone.0284290.t002
The cultivars of japonica type rice in Taiwan are the only japonica type rice that can grow under relatively high temperatures and produce good-quality rice with a high yield [ 49 , 50 ]. Crops in tropical regions have been reported to be more sensitive to warming because their temperature is already close to their optimum temperature during the growing period [ 3 ]. In many regions, a slight increase in temperature with sufficient rainfall may have a positive effect on crops [ 51 ]. The lowland rice varieties were reported to be highly sensitive to soil drying, and their yields decline when the soil dries below saturation [ 52 ].
Data were collected from the same research station during the long-term experimental period. To obtain general results, the analysis of the crop yield response to global or national water-deficit stress should be extended. Data collected in different areas or on different temporal and spatial scales may result in different conclusions [ 53 , 54 ]. For example, up to 45% yield reductions of rice are expected by the end of this century due to climate change, including water deficit, in the countries in eastern Africa [ 55 ]. In Iran, it was reported that water deficit during vegetative, flowering and grain filling stages reduced mean grain yield by 21, 50 and 21% on average in comparison to control, respectively [ 56 ]. In this study, long-term temporal variation in the rice yield response to water-deficit stress was revealed, even though the rice cultivars varied throughout the study period. During an experimental period of over 90 years since 1925, it is impossible to maintain the crop yield experiments using the same cultivar and maintaining the same environmental and cultivational conditions consistently. It is also difficult to consider the factors that may affect the growth and production of crops, such as insects, diseases, and soil fertility [ 57 – 60 ], as well as human-induced effects, such as modern management, improving technology, and cultivator practices [ 26 , 58 ] for long-term observations. Crop evapotranspiration could be influenced by other factors, such as soil condition, canopy cover, and the fraction of leaf senescence; thus, the information of these coefficients may be considered for the calculation of crop evapotranspiration, if possible [ 19 , 22 ]. In addition, extreme climatic events, such as floods and heatwaves, may pose additional risks to crop production [ 61 ].
This study revealed the effect of water-deficit stress on rice yield in both cool and warm cropping seasons. The results provide long-term evidence of declining crop water status during the rice-growing seasons. The average values of ET 0 were estimated as 3.3–4.4 mm day -1 , and 2.8–4.6 mm day -1 in cool and warm cropping seasons, respectively, under the rice growth stages. The crop water status has decreased by 24.7–198.6 mm in the cool cropping season and 3.8–63.7 mm in the warm cropping season under the rice growth stages since 1925 and during the 95 years. Compared with the cool cropping season, the decreasing trend in crop water status in the warm cropping season was relatively slight under the four growth stages. The total water-deficit stress related yield change in the cultivars in the cool cropping season during 1925–1944, 1945–1983, and 1996–2019 were -56.1 to 37.0, -77.5 to -12.3, 11.2 to 19.8, and -146.4 to 39.1 kg ha -1 under the initial, crop development, reproductive, and maturity stages, respectively. The total yield change related to the CWS on the cultivars in the warm cropping season during 1925–1944, 1945–1983, and 1996–2019 are -16.5 to 8.2, -12.9 to 8.1, -2.3 to 9.0, and -9.3 to 8.0 kg ha -1 under the initial, crop development, reproductive, and maturity stages, respectively. Our results suggest that crop water may be a determining factor for rice growth during the developmental stage, but not during the reproductive stage. In addition, water-deficit stress has been increasingly affecting rice growth in recent years. To maintain high productivity and quality, our results on the effect of water-deficit stress on rice grain yield should be considered along with other adaptation strategies targeting agronomic efforts and breeding technologies.
Supporting information
S1 file. field experimental data..
https://doi.org/10.1371/journal.pone.0284290.s001
Acknowledgments
The authors wish to thank Dr. Jia-Ling Yang and other researchers in Taichung District Agricultural Research and Extension Station, Council of Agriculture, Taiwan who assisted in the field investigation and data collection.
- View Article
- Google Scholar
- PubMed/NCBI
- 22. Allen RG, Pereira LS, Raes D, Smith M. Crop Evapotranspiration. Guidelines for Computing Crop Water Requirements. FAO Irrigation and Drainage Paper 56. FAO, Rome, Italy. 1998.
- 34. FAO. ETo calculator. Land and water digital media series N 36. FAO, Roma, Italy. 2012.
- 49. Chang TC. Evolvement and background of rice culture in Taiwan. In: Chang TC (eds.) The history of development of rice culture in Taiwan, 9–18. Agriculture and Forestry Division, Taiwan Provincial Government Press, Taiwan. 1999.
Information
- Author Services
Initiatives
You are accessing a machine-readable page. In order to be human-readable, please install an RSS reader.
All articles published by MDPI are made immediately available worldwide under an open access license. No special permission is required to reuse all or part of the article published by MDPI, including figures and tables. For articles published under an open access Creative Common CC BY license, any part of the article may be reused without permission provided that the original article is clearly cited. For more information, please refer to https://www.mdpi.com/openaccess .
Feature papers represent the most advanced research with significant potential for high impact in the field. A Feature Paper should be a substantial original Article that involves several techniques or approaches, provides an outlook for future research directions and describes possible research applications.
Feature papers are submitted upon individual invitation or recommendation by the scientific editors and must receive positive feedback from the reviewers.
Editor’s Choice articles are based on recommendations by the scientific editors of MDPI journals from around the world. Editors select a small number of articles recently published in the journal that they believe will be particularly interesting to readers, or important in the respective research area. The aim is to provide a snapshot of some of the most exciting work published in the various research areas of the journal.
Original Submission Date Received: .
- Active Journals
- Find a Journal
- Proceedings Series
- For Authors
- For Reviewers
- For Editors
- For Librarians
- For Publishers
- For Societies
- For Conference Organizers
- Open Access Policy
- Institutional Open Access Program
- Special Issues Guidelines
- Editorial Process
- Research and Publication Ethics
- Article Processing Charges
- Testimonials
- Preprints.org
- SciProfiles
- Encyclopedia

Article Menu
- Subscribe SciFeed
- Recommended Articles
- Google Scholar
- on Google Scholar
- Table of Contents
Find support for a specific problem in the support section of our website.
Please let us know what you think of our products and services.
Visit our dedicated information section to learn more about MDPI.
JSmol Viewer
Effect of water deficit on morphoagronomic and physiological traits of common bean genotypes with contrasting drought tolerance.

1. Introduction
2. material and methods, 2.1. plant material and experimental design, 2.2. induction and monitoring of water deficit, 2.3. morphoagronomic evaluations, 2.4. physiological evaluations, 2.5. data analyses, 3.1. morphoagronomic traits, 3.2. physiological traits, 3.3. correlation network, 3.4. heatmap representation, 4. discussion, 5. conclusions, author contributions, conflicts of interest.
- Beebe, S.E.; Rao, I.M.; Blair, M.W.; Acosta-Gallegos, J.A. Phenotyping common beans for adaptation to drought. Front. Physiol. 2013 , 4 , 1–20. [ Google Scholar ] [ CrossRef ] [ PubMed ] [ Green Version ]
- Ramirez-Cabral, N.Y.Z.; Kumar, L.; Taylor, S. Crop niche modeling projects major shifts in common bean growing areas. Agric. For. Meteorol. 2016 , 218 , 102–113. [ Google Scholar ] [ CrossRef ]
- Hummel, M.; Hallahan, B.F.; Brychkova, G.; Ramirez-Villegas, J.; Guwela, V.; Chataika, B.; Curley, E.; McKeown, P.C.; Morrison, L.; Talsma, E.F.; et al. Reduction in nutritional quality and growing area suitability of common bean under climate change induced drought stress in Africa. Sci. Rep. 2018 , 8 , 16187. [ Google Scholar ] [ CrossRef ]
- Mukeshimana, G.; Butare, L.; Cregan, P.B.; Blair, M.W.; Kelly, J.D. Quantitative trait loci associated with drought tolerance in common bean. Crop. Sci. 2004 , 54 , 923–938. [ Google Scholar ] [ CrossRef ] [ Green Version ]
- Assefa, T.; Mahama, A.A.; Brown, A.V.; Cannon, E.K.S.; Rubyogo, J.C.; Rao, I.M.; Blair, M.W.; Cannon, S.B. A review of breeding objectives, genomic resources, and marker-assisted methods in common bean ( Phaseolus vulgaris L.). Mol. Breed. 2019 , 39 , 1–23. [ Google Scholar ] [ CrossRef ] [ Green Version ]
- Asfaw, A.; Blair, M.W. Quantitative trait loci for rooting pattern traits of common beans grown under drought stress versus non stress conditions. Mol. Breed. 2012 , 30 , 681–695. [ Google Scholar ] [ CrossRef ]
- Hoyos-Villegas, V.; Song, Q.; Kelly, J.D. Genome-wide association analysis for drought tolerance and associated traits in common bean. Plant Gen. 2016 , 10 , 1–17. [ Google Scholar ] [ CrossRef ] [ Green Version ]
- Farooq, M.; Gogoi, N.; Barthakur, S.; Baroowa, B.; Bharadwaj, N.; Alghamdi, S.S.; Siddique, K.H.M. Drought stress in grain legumes during reproduction and grain filling. J. Agron. Crop. Sci. 2017 , 203 , 81–102. [ Google Scholar ] [ CrossRef ]
- Cortés, A.J.; Blair, M.W. Genotyping by sequencing and genome–environment associations in wild common bean predict widespread divergent adaptation to drought. Front. Plant Sci. 2018 , 9 , 1–13. [ Google Scholar ] [ CrossRef ] [ Green Version ]
- Chai, Q.; Gan, Y.; Zhao, C.; Xu, H.L.; Waskom, R.M.; Niu, Y.; Siddique, K.H. Regulated stress irrigation for crop production under drought stress. A review. Agron. Sust. Devel. 2016 , 36 , 1–21. [ Google Scholar ]
- Arruda, I.M.; Moda-Cirino, V.; Koltun, A.; dos Santos, O.J.A.P.; Moreira, R.S.; Moreira, A.F.P.; Gonçalves, L.S.A. Physiological, biochemical and morphoagronomic characterization of drought-tolerant and drought-sensitive bean genotypes under water stress. Physiol. Mol. Biol. Plants 2018 , 24 , 1059–1067. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Polania, J.; Poschenrieder, C.; Rao, I.; Beebe, S. Estimation of phenotypic variability in symbiotic nitrogen fixation ability of common bean under drought stress using 15N natural abundance in grain. Eur. J. Agron. 2016 , 79 , 66–73. [ Google Scholar ] [ CrossRef ] [ PubMed ] [ Green Version ]
- Manjeru, P.; Madanzi, T.; Makeredza, B.; Nciizah, A.; Sithole, M. Effect of Water Stress at Different Growth Stage on Components and Grain Yield of Common Bean ( Phaseolus vulgaris L.). Afr. Crop. Sci. Conf. Proc. 2007 , 8 , 299–303. [ Google Scholar ]
- Ambachew, D.; Mekbib, F.; Asfaw, A.; Beebe, S.E.; Blair, M.W. Trait associations in common bean genotypes grown under drought stress and field infestation by BSM bean fly. Crop. J. 2015 , 3 , 305–316. [ Google Scholar ] [ CrossRef ] [ Green Version ]
- Fleta-Soriano, E.; Munné-Bosch, S. Stress memory and the inevitable effects of drought: A physiological perspective. Front. Plant Sci. 2016 , 7 , 143. [ Google Scholar ] [ CrossRef ] [ Green Version ]
- Ye, H.; Roorkiwal, M.; Valliyodan, B.; Zhou, L.; Chen, P.; Varshney, R.K.; Nguyen, H.T. Genetic diversity of root system architecture in response to drought stress in grain legumes. J. Exp. Bot. 2018 , 69 , 3267–3277. [ Google Scholar ] [ CrossRef ] [ Green Version ]
- Arruda, I.M.; Moda-Cirino, V.; Koltun, A.; Zeffa, D.M.; Nagashima, G.T.; Gonçalves, L.S.A. Combining Ability for Agromorphological and Physiological Traits in Different Gene Pools of Common Bean Subjected to Water Stress. Agronomy 2019 , 9 , 371. [ Google Scholar ] [ CrossRef ] [ Green Version ]
- Polania, J.; Poschenrieder, C.; Rao, I.M.; Beebe, S.E. Root traits and their potential links to plant ideotypes to improve drought resistance in common bean. Theor. Exp. Plant Physiol. 2017 , 29 , 143–154. [ Google Scholar ] [ CrossRef ] [ Green Version ]
- Polania, J.; Rao, I.M.; Cajiao, C.; Grajales, M.; Rivera, M.; Velasquez, F.; Beebe, S.E. Shoot and root traits contribute to drought resistance in recombinant inbred lines of MD 23–24 × SEA 5 of common bean. Front. Plant Sci. 2017 , 8 , 296. [ Google Scholar ] [ CrossRef ]
- Rosales, M.A.; Ocampo, E.; Rodríguez-Valentín, R.; Olvera-Carrillo, Y.; Acosta-Gallegos, J.; Covarrubias, A.A. Physiological analysis of common bean (Phaseolus vulgaris L.) cultivars uncovers characteristics related to terminal drought resistance. Plant Physiol. Biochem. 2012 , 56 , 24–34. [ Google Scholar ] [ CrossRef ]
- Heinemann, A.B.; Ramirez-Villegas, J.; Souza, T.L.P.; Didonet, A.D.; di Stefano, J.G.; Boote, K.J.; Jarvis, A. Drought impact on rain fed common bean production areas in Brazil. Agric. For. Meteorol. 2016 , 225 , 57–74. [ Google Scholar ] [ CrossRef ] [ Green Version ]
- Darkwa, K.; Ambachew, D.; Mohammed, H.; Asfaw, A.; Blair, M.W. Evaluation of common bean ( Phaseolus vulgaris L.) genotypes for drought stress adaptation in Ethiopia. Crop. J. 2016 , 4 , 367–376. [ Google Scholar ] [ CrossRef ] [ Green Version ]
- Blum, A. Plant Breeding for Stress Environments ; CRC Press: Boca Raton, FL, USA, 2018; pp. 1–223. [ Google Scholar ]
- Moda-Cirino, V.; Oliari, L.; Lollato, M.A.; Fonseca Júnior, N.S. IAPAR 81—Common bean. Crop Breed. Appl. Biotechnol. 2001 , 1 , 203–204. [ Google Scholar ] [ CrossRef ]
- Aguiar, R.S.; Moda-Cirino, V.; Faria, R.T.; Vida, L.H.I. Avaliação de linhagens promissoras de feijoeiro ( Phaseolus vulgaris L.) tolerantes ao déficit hídrico. Semin. Cienc. Agrar. 2008 , 29 , 1–14. [ Google Scholar ] [ CrossRef ] [ Green Version ]
- Androciolli, L.G. Fenotipagem Para Tolerância a Seca Em Cultivares De Feijão. Master’s Thesis, Agronomic Institute of Paraná, Londrina, Brazil, 2007. [ Google Scholar ]
- Weatherley, P.E. Studies in the water relations of the cotton plant. I. The field measurement of water deficits in leaves. N. Phytol. 1950 , 49 , 81–87. [ Google Scholar ] [ CrossRef ]
- Bartlett, M.S. Properties of sufficiency and statistical tests. Proc. R. Soc. Lond. 1937 , 1670 , 268–282. [ Google Scholar ]
- Shapiro, S.S.; Wilks, M.B. An analysis of variance test for normality. Biometrika 1965 , 52 , 591–611. [ Google Scholar ] [ CrossRef ]
- Box, G.E.; Cox, D.R. An analysis of transformations. J. R. Stat. Soc. 1964 , 26 , 211–243. [ Google Scholar ] [ CrossRef ]
- Scott, A.J.; Knott, M. A cluster analysis method for grouping means in the analysis of variance. Biometrics 1974 , 30 , 507–512. [ Google Scholar ] [ CrossRef ] [ Green Version ]
- Ward, J.H. Hierarchical grouping to optimize an objective function. J. Am. Stat. Assoc. 1963 , 58 , 236–244. [ Google Scholar ] [ CrossRef ]
- Ferreira, E.B.; Cavalcanti, P.P.; Nogueira, D.A. ExpDes: An R package for ANOVA and experimental designs. Appl. Math. 2014 , 5 , 2952. [ Google Scholar ] [ CrossRef ] [ Green Version ]
- Fox, J.; Weisberg, S.; Adler, D.; Bates, D.; Baud-Bovy, G.; Ellison, S.; Heiberger, R. Package ‘Car’. Available online: https://cran.microsoft.com/snapshot/2017-06-17/web/packages/car/car.pdf (accessed on 10 January 2020).
- Epskamp, S.; Cramer, A.O.; Waldorp, L.J.; Schmittmann, V.D.; Borsboom, D. qgraph: Network visualizations of relationships in psychometric data. J. Stat. Softw. 2012 , 48 , 1–18. [ Google Scholar ] [ CrossRef ] [ Green Version ]
- Galili, T.; O’Callaghan, A.; Sidi, J.; Sievert, C. heatmaply: An R package for creating interactive cluster heatmaps for online publishing. Bioinformatics 2017 , 34 , 1600–1602. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Mazengo, K.D.; Tryphone, G.M.; Tarimo, A.J.P. Identification of drought selection indices of common bean ( Phaseolus vulgaris L.) genotypes in the Southern Highlands of Tanzania. Afr. J. Agric. Res. 2019 , 14 , 161–167. [ Google Scholar ]
- Terán, H.; Singh, S.P. Comparison of sources and lines selected for drought resistance in common bean. Crop. Sci. 2002 , 42 , 64–70. [ Google Scholar ] [ CrossRef ]
- Beebe, S.E.; Rao, I.M.; Cajiao, C.; Grajales, M. Selection for drought resistance in common bean also improves yield in phosphorus limited and favorable environments. Crop. Sci. 2008 , 48 , 582–592. [ Google Scholar ] [ CrossRef ]
- Oliveira-Neto, S.; Zeffa, D.M.; Sartori, M.M.P.; Moda-Cirino, V. Cultivars selection of Carioca beans type to be harvested in arid farmlands. IRRIGA 2017 , 22 , 775–788. [ Google Scholar ] [ CrossRef ]
- Asfaw, A.; Ambachew, D.; Shah, T.; Blair, M.W. Trait associations in diversity panels of the two common bean ( Phaseolus vulgaris L.) gene pools grown under well-watered and water-stress conditions. Front. Plant Sci. 2017 , 8 , 733. [ Google Scholar ] [ CrossRef ]
- Rao, I.; Beebe, S.; Polania, J.; Ricaurte, J.; Cajiao, C.; Garcia, R.; Rivera, M. Can tepary bean be a model for improvement of drought resistance in common bean? Afr. Crop. Sci. J. 2013 , 21 , 265–281. [ Google Scholar ]
- Barrios, A.N.; Hoogenboom, G.; Nesmith, D.S. Drought sress and the distribution of vegetative and reproductive traits of a bean cultivar. Sci. Agric. 2005 , 62 , 18–22. [ Google Scholar ] [ CrossRef ] [ Green Version ]
- Sponchiado, B.N.; White, J.W.; Castillo, J.A.; Jones, P.G. Root growth of four common bean cultivars in relation to drought tolerance in environments with contrasting soil types. Exp. Agric. 1989 , 25 , 249–257. [ Google Scholar ] [ CrossRef ]
- Moda-Cirino, V.; Gerage, A.C.; Riede, C.R.; Sera, G.H.; Takahashi, M.; Abbud, N.S.; Nazareno, N.R.X.; Araújo, P.M.; Auler, P.M.; Yamaoka, R.S.; et al. Plant breeding at Instituto Agronômico do Paraná –IAPAR. Crop. Breed. Appl. Biotechnol. 2012 , 2 , 25–30. [ Google Scholar ] [ CrossRef ] [ Green Version ]
- Rosales, M.A.; Cuellar-Ortiz, S.M.; de la Paz Arrieta-Montiel, M.; Acosta-Gallegos, J.; Covarrubias, A.A. Physiological traits related to terminal drought resistance in common bean ( Phaseolus vulgaris L.). J. Sci. Food Agric. 2013 , 93 , 324–331. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Polania, J.; Rao, I.M.; Cajiao, C.; Rivera, M.; Raatz, B.; Beebe, S.E. Physiological traits associated with drought resistance in Andean and Mesoamerican genotypes of common bean ( Phaseolus vulgaris L.). Euphytica 2016 , 210 , 17–29. [ Google Scholar ] [ CrossRef ]
- Tardieu, F.; Simonneau, T.; Muller, B. The physiological basis of drought tolerance in crop plants: A scenario-dependent probabilistic approach. Annu. Rev. Plant Biol. 2018 , 69 , 733–759. [ Google Scholar ] [ CrossRef ] [ PubMed ] [ Green Version ]
- Duan, H.; Chaszar, B.; Lewis, J.D.; Smith, R.A.; Huxman, T.E.; Tissue, D.T. CO 2 and temperature effects on morphological and physiological traits affecting risk of drought-induced mortality. Tree Physiol. 2018 , 38 , 1138–1151. [ Google Scholar ] [ CrossRef ] [ Green Version ]
- Murtaza, G.; Rasool, F.; Habib, R.; Javed, T.; Sardar, K.; Ayub, M.M.; Rasool, A. A review of morphological, physiological and biochemical responses of plants under drought stress conditions. Imp. J. Interdiscip. Res. 2016 , 2 , 1600–1606. [ Google Scholar ]
- Blankenagel, S.; Yang, Z.; Avramova, V.; Schön, C.C.; Grill, E. Generating plants with improved water use efficiency. Agronomy 2018 , 8 , 194. [ Google Scholar ] [ CrossRef ] [ Green Version ]
- Medrano, H.; Tomás, M.; Martorell, S.; Flexas, J.; Hernández, E.; Rosselló, J.; Bota, J. From leaf to whole-plant water use efficiency (WUE) in complex canopies: Limitations of leaf WUE as a selection target. Crop. J. 2015 , 3 , 220–228. [ Google Scholar ] [ CrossRef ] [ Green Version ]
- Moshelion, M.; Halperin, O.; Wallach, R.; Oren, R.A.M.; Way, D.A. Role of aquaporins in determining transpiration and photosynthesis in water-stressed plants: Crop water-use efficiency, growth and yield. Plant Cell Environ. 2015 , 38 , 1785–1793. [ Google Scholar ] [ CrossRef ]
- Liu, B.; Liang, J.; Tang, G.; Wang, X.; Liu, F.; Zhao, D. Drought stress effects on growth, water use efficiency, gas exchange and chlorophyll fluorescence of Juglans rootstocks. Sci. Hortic. 2019 , 250 , 230–235. [ Google Scholar ] [ CrossRef ]
- Briñez, B.; Perseguini, J.M.K.C.; Rosa, J.S.; Bassi, D.; Gonçalves, J.G.R.; Almeida, C.; Valdisser, P.A.M.R. Mapping QTLs for drought tolerance in a SEA 5 × AND 277 common bean cross with SSRs and SNP markers. Genet. Mol. Biol. 2017 , 40 , 813–823. [ Google Scholar ] [ CrossRef ] [ PubMed ] [ Green Version ]
- Diaz, L.M.; Ricaurte, J.; Tovar, E.; Cajiao, C.; Terán, H.; Grajales, M.; Raatz, B. QTL analyses for tolerance to abiotic stresses in a common bean ( Phaseolus vulgaris L.) population. PLoS ONE 2018 , 13 , e0202342. [ Google Scholar ] [ CrossRef ] [ PubMed ]
Click here to enlarge figure
| Cultivar | Origin | Market Group | Cycle (Days) | Growth Habit | Drought Reaction | Reference |
|---|---|---|---|---|---|---|
| BAT 477 | CIAT | Brown | 94 | III | Tolerant | [ ] |
| IAPAR 81 | IAPAR | ‘Carioca’ | 92 | III | Tolerant | [ ] |
| IAC Tybatã | IAC | ‘Carioca’ | 95 | II | Sensitive | [ ] |
| BRS Pontal | EMBRAPA | ‘Carioca’ | 95 | II | Sensitive | [ ] |
| Source of Variation | Mean Square of the Morphoagronomic Traits | |||||||
|---|---|---|---|---|---|---|---|---|
| SDB | RDB | RV | PP | TNG | NGP | GW | GY | |
| Genotype (G) | 24.2 ** | 5.2 ** | 23.1 ** | 104.0 ** | 3181.7 ** | 10.2 ** | 77.5 ** | 234.4 ** |
| Water deficit (W) | 54.8 ** | 12.2 ** | 41.6 ** | 94.1 ** | 2882.1 ** | 14.1 ** | 137.5 ** | 137.1 ** |
| G × W | 33.2 ** | 2.1 | 5.8 | 40.0 ** | 1372.9 ** | 0.9 | 5.5 | 63.3 ** |
| Error | 5.6 | 1.9 | 5.8 | 4.4 | 245.1 | 0.6 | 3.5 | 9.7 |
| Mean (control) | 25.3 | 22.7 | 24.96 | 130.3 | 5.2 | 9.3 | 5.4 | 16.3 |
| Mean (water deficit) | 19.3 | 20.3 | 16.9 | 84.5 | 5.0 | 6.0 | 3.5 | 12.4 |
| CV (%) | 16.5 | 31.5 | 31.4 | 10.1 | 14.6 | 14.5 | 8.7 | 13.9 |
| Source of Variation | Mean Square of Physiological Traits | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| A | E | g | iC | iWUE | F /F | RWC | CE | Lt | |
| Genotype (G) | 149.9 ** | 3.03 * | 0.28 | 0.65 ** | 133.6 ** | 2.39 ** | 768.2 ** | 558.0 ** | 1.69 |
| Water deficit (W) | 1867.4 ** | 81.88 ** | 4.98 ** | 5.54 ** | 32.2 * | 16.18 ** | 2902.8 ** | 123.9 ** | 102.51 ** |
| Time (T) | 691.2 ** | 28.90 ** | 6.63 ** | 2.04 ** | 213.7 * | 0.69 | 285.6 ** | 272.7 ** | 45.82 ** |
| G × W | 149.1 ** | 1.82 * | 0.07 | 0.55 ** | 151.8 ** | 2.38 * | 140.2 | 764.6 ** | 1.50 |
| W × T | 245.1 ** | 11.09 ** | 0.98 ** | 1.80 ** | 81.4 ** | 0.67 | 706.7 ** | 178.9 ** | 13.58 ** |
| G × T | 93.4 ** | 4.07 ** | 0.69 ** | 0.69 ** | 32.0 * | 0.33 | 107.2 | 126.6 ** | 2.10 |
| G × W × T | 40.3 ** | 1.93 * | 0.10 | 0.68 ** | 29.8 * | 0.47 | 117.7 | 91.1 ** | 0.92 |
| Error | 4.4 | 0.55 | 0.08 | 0.08 | 11.0 | 0.46 | 68.5 | 11.5 | 1.78 |
| Mean (control) | 16.4 | 3.6 | 0.29 | 220.1 | 70.2 | 0.80 | 80.9 | 223.7 | 29.0 |
| Mean (water deficit) | 10.1 | 2.3 | 0.19 | 109.9 | 73.3 | 0.78 | 73.1 | 115.8 | 30.4 |
| CV (%) | 15.7 | 25.1 | 36.3 | 27.3 | 32.1 | 5.8 | 10.7 | 4.8 | 5.5 |
Share and Cite
Godoy Androcioli, L.; Mariani Zeffa, D.; Soares Alves, D.; Pires Tomaz, J.; Moda-Cirino, V. Effect of Water Deficit on Morphoagronomic and Physiological Traits of Common Bean Genotypes with Contrasting Drought Tolerance. Water 2020 , 12 , 217. https://doi.org/10.3390/w12010217
Godoy Androcioli L, Mariani Zeffa D, Soares Alves D, Pires Tomaz J, Moda-Cirino V. Effect of Water Deficit on Morphoagronomic and Physiological Traits of Common Bean Genotypes with Contrasting Drought Tolerance. Water . 2020; 12(1):217. https://doi.org/10.3390/w12010217
Godoy Androcioli, Leonardo, Douglas Mariani Zeffa, Daniel Soares Alves, Juarez Pires Tomaz, and Vânia Moda-Cirino. 2020. "Effect of Water Deficit on Morphoagronomic and Physiological Traits of Common Bean Genotypes with Contrasting Drought Tolerance" Water 12, no. 1: 217. https://doi.org/10.3390/w12010217
Article Metrics
Article access statistics, further information, mdpi initiatives, follow mdpi.

Subscribe to receive issue release notifications and newsletters from MDPI journals
Thank you for visiting nature.com. You are using a browser version with limited support for CSS. To obtain the best experience, we recommend you use a more up to date browser (or turn off compatibility mode in Internet Explorer). In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript.
- View all journals
- Explore content
- About the journal
- Publish with us
- Sign up for alerts
- Open access
- Published: 19 June 2023
Responses of yield, quality and water use efficiency of potato grown under different drip irrigation and nitrogen levels
- Mustafa Akkamis 1 &
- Sevgi Caliskan 1
Scientific Reports volume 13 , Article number: 9911 ( 2023 ) Cite this article
2704 Accesses
15 Citations
Metrics details
- Plant sciences
Proper irrigation and fertilization are essential for achieve high tuber yield and quality in potato production. However, the high cost of these inputs necessitate optimization of their use to improve both water use efficiency and crop productivity. This study aimed to investigate the impact of irrigation and nitrogen fertilization on potato yield, quality and water use efficiency. The research included different drip irrigation treatments (100%, 66%, and 33% of field capacity) and nitrogen levels: 0 (N0), 100 (N1), 200 (N2), 300 (N3), 400 (N4) and 500 (N5) kg N ha −1 . The results indicated that potato yield and growth were more sensitive to irrigation treatment than nitrogen levels. Full irrigation with 300 kg N ha −1 produced the highest total tuber yield, while low irrigation treatments resulted in significantly lower yields. In contrast, the 66% field capacity irrigation treatment consistently had the highest water use efficiency in both years of the study. Furthermore, the study showed that the quality characteristics of the tubers were negatively impacted by full irrigation treatments compared to low irrigation. These findings suggest that with appropriate irrigation and nitrogen application, potatoes can be produced with acceptable yields while conserving water and minimizing nitrogen use. This research emphasizes the importance of optimizing inputs to improve water use efficiency and yield productivity while reducing water. As a result, obtaining useful information on crop management for farmers to make informed decisions may be possible by achieving optimal irrigation and nitrogen levels.
Similar content being viewed by others

Potato growth, photosynthesis, yield, and quality response to regulated deficit drip irrigation under film mulching in a cold and arid environment

Tuber yield and water efficiency of early potato varieties ( Solanum tuberosum L.) cultivated under various irrigation levels

The interacting effects of irrigation, sowing date and nitrogen on water status, protein and yield in pea ( Pisum sativum L.)
Introduction.
Irrigation and nitrogen management (N) are important factors affecting potato ( Solanum tuberosum L.) yield, quality, and net profit 1 . Potato yields are maximized when the soil moisture is consistently maintained at an optimal level and adequate nitrogen supply is provided 2 . A sufficient nitrogen is necessary for the high growth rate of potato plants, leading to increased tuber yield but decreased specific gravity. Insufficient nitrogen results in reduced leaf area and tuber size due to early leaf drop, while excessive nitrogen content in the soil leads to an increase in plant dry matter content and a decrease in the duration of tuber growth 3 , 4 , 5 , 6 , 7 , 8 .
Applying nitrogen at the right rate, time and place increases N efficiency. Potato need nitrogen most during the tuber growth period. Approximately 58–70% of N during the entire production period is taken at this stage of development 9 . When nitrogen is applied to the plant in the most appropriate form and amount, it has a positive effect on growth and plant development. However, excessive use of nitrogen negatively affects the resistance of the plant against diseases and pests. Due to low nitrogen in the tuber formation stage, drying of the tuber and old leaves occurs and therefore reduces tuber development.
The limited root system of potato requires the application of nitrogen fertilizers since the plant has a low utilization capacity for nitrogen. Therefore, effective management of irrigation and nitrogen fertilization is crucial for optimal growth and development of the crop, with due consideration for attaining maximum yield and quality of the harvest 10 . The water consumption of potato ranges from 500 to 700 mm depending on the climatic factors. To achieve high yields in potato, being an exceptionally moisture-sensitive crop, must maintain an available water content of not less than 65% 11 , 12 . During the period from the initiation of tuber formation to 15 days prior to harvest, the potato displays its greatest demand for water. In the absence of proper irrigation during this stage, the tubers may exhibit secondary growth. While irrigation promotes an increase in average tuber weight, it may not necessarily lead to a higher number of tubers per plant 13 .
The irrigation method utilized in potato cultivation varies based on the region and availability of water resources. In the potato-growing regions of Türkiye, sprinkler irrigation is the predominant method, although drip irrigation methods have also gained popularity 14 . Nitrogen can infiltrate under the root through irrigation and precipitation. Accordingly, fertilizers and chemicals that cannot be taken by the plant move underground with the water. Precipitation and irrigation are instrumental in determining the movement rate of such chemical through to the soil surface, which can be used to manage their submergence Therefore, controlled irrigation management is vital for regulation the transport of chemicals and nutrients. Proper application of irrigation method can also facilitate nitrogen uptake, thus minimizing potential seepage losses below the root zone 15 . Although the information in the literature on irrigation and nitrogen management is conflicting, tuber yield and quality are affected by N and irrigation applications. Proper management of nitrogen and water is necessary to achieve growth and marketable tuber. Incomplete irrigation creates differential effects on nutrient uptake, growth, and yield. Nitrogen can replace deficient water, and effective nitrogen management can mitigate yield loss due to the under-irrigation.
Two potential strategies to enhance water utilization efficiency in potato production are the implementation of appropriate irrigation scheduling and the utilization of drip irrigation. Effective nitrogen management can also significantly contribute to improved plant growth and yield 16 . Therefore, attention must be paid to N and water management for the potato to provide quality and marketable tubers. In many parts of the world, various studies were conducted on irrigation and fertilization of potato. The most limiting nutrient for potato growth, the need for nitrogen varies greatly with climate, soil, variety, irrigation, and cultural practices. Accordingly, the present study aims to investigate the effects of different irrigation and nitrogen fertilization levels on yield and quality of potato plants cultivated under drip irrigation conditions.
Materials and methods
Site description.
Field trials were conducted during the years 2021 and 2022 at research area (N37° 94’, E34° 96’) Faculty of Agricultural Sciences and Technologies, Nigde Ömer Halisdemir University, Türkiye. The experimental site is at an altitude of 1299 m above sea level and receives an average of 343 mm of precipitation annually. In both years, a rainfall recorder (Turkish State Meteorological Service Nigde Meteorology Station) was used to measure the precipitation during the growing season. At the sowing time, the soil bulk density of experimental field (0–40 cm) was 1.11 g cm −3 , field water capacity was 31%, soil pH value was 7.95, and the soil available nitrogen, phosphorus and potassium contents were 0.138%, 10.85 mg kg −1 , and 201.19 mg kg −1 , respectively.
Experimental design
In each year, the field experiments were conducted according to the split-plot design with 18 sub-plots replicated four times. The treatments were comprised of three irrigation water levels (100% = S100, 66% = S66, 33% = S33 field capacity) and six nitrogen levels (0 = N0, 100 = N1, 200 = N2, 300 = N3, 400 = N4, 500 = N5 kg ha −1 ). The nitrogen levels were randomized in main plots whereas the irrigation levels were randomized in sub-plots. ‘Agria’ was used as a variety in experiments during both the years. The disease-free seed tubers of this variety were obtained from Doga Seed Company, Nevsehir, Türkiye. The seeds were sown on the ridge tops with a sowing machine on May 13, 2021, and May 29, 2022, and were harvested on October 4, 2021, and on September 27, 2022 during the first and second year of study, respectively. The entire field (65.1 m × 29.4 m) was divided into four blocks (replications) and each block measured 65.1 m × 5.1 m. Among the blocks, an area measuring 3 m was kept unplanted to facilitate data recording and to prevent irrigation applications from affecting each other. Each block was divided into six main plots. The main plots were consisted of 12 rows and sub-plots consisted of four rows. Two rows between the main plots and one row between the sub-plots were kept unplanted. Experimental research on plants, field studies, collection of plant material and irrigation practices were carried out in accordance with the Standards of the Ministry of Agriculture of the Republic of Türkiye.
Irrigation management and crop water consumption
After planting potato seeds, drip irrigation systems were placed on the field. Immediately after planting, emitters at 30 cm intervals were placed in a drip tape with an emitter flow rate of 4 L h -1 . A system consisting of a screen filter, fertilizer tank, a valve and two pressure gauges was used to measure the irrigation amount and control the pressure. Irrigation started on May 13, 2021 and May 29, 2022. To measure the field capacity, soil samples were taken from 0–20 to 20–40 cm depths with a soil digger. Field capacity was measured as the amount of water retained in a saturated soil after 2–3 days of gravity drainage. Volumetric moisture content was calculated gravimetrically. The amount of irrigation water applied to each plot was calculated with the following Eq. ( 1 ) 17 .
where “Fc” is field capacity (31%), ‘’Sm’’ is soil moisture before irrigation (%), “R d ” is root depth (mm), “Pa” is plot area (m 2 ) and “Pw” is wetted soil percentage.
Water was applied when the soil moisture decreased by 30–40% of the field capacity, separate irrigation was applied to each nitrogen plot. To monitor the soil moisture content (%), soil samples were taken every 3–4 days from the full irrigation plot (100% Fc) of each nitrogen application, and the gravimetric method (g/g) soil moisture measurement was performed.
Crop water consumption (ETc, mm) was calculated at 15-day intervals for each nitrogen level using the soil water balance (Eq. 2 ) 18 .
where I is the irrigation water (mm), P is the rainfall (mm), ∆S is the change in soil water storage (mm 60 cm −1 ) and D is the deep percolation (mm), R is the runoff (mm). There was no Runoff as adequate weirs were provided. Deep percolation was accepted zero when soil moisture was less than field capacity. When soil moisture after irrigation or precipitation surpassed field capacity, deep percolation was evaluated as the difference between field capacity and soil moisture plus irrigation/precipitation 19 .
Water use efficiency (WUE)
WUE (kg mm −1 ha −1 ) were calculated using Eqs. ( 3 ) described by Hou et al. 20
where Y is the crop yield, ET is the evapotranspiration during the entire growth period.
Fertilization management
After the completion of the land preparation, fertilizers were applied. P 2 0 5 —125 kg ha −1 K 2 0—150 kg ha −1 were surface spread prior to planting. Likewise, half of the nitrogen dose was applied during planting and the remaining half was applied during tuber bulking. Planting was performed with a distance of 30 cm between plants and 70 cm between rows. Plant protection practices were carried out throughout the entire growing season. Potato seeds separated as seeds were sprayed before planting, with Thiamethoxam active ingredient, against pests after emergence. At the growth stage, fungicide against blight disease were also used as per requirements.
Data collection
Yield and growth parameters.
The growth parameters of plants in each replicated plot, including the number of tuber plant −1 , number of stems −1 , and height of plants (cm) were noted. Tubers in each plot were first classified, then counted and finally weighed. Classifications: Diameter greater than or equal to 45 mm—class 1; greater than 25 and less than 45 cm—class 2; Less than or equal to 25 mm—class 3. 1st, 2nd, and 3rd class yields of tubers were added and ton ha −1 weights were calculated.
Tuber quality parameters
At harvest, tuber dry matter (TDM) and specific gravity (SG) were measured each year on all treatments. TDM and SG of treatments were measured by Martin Lishmans’s digital potato hydrometer. TDM and SG were measured with approximately 2.5 kg of clean, raw tubers from each treatment. Starch concentration was calculated using the underwater weight of the tubers with Eq. ( 4 ) 21 .
Statistical analysis
All data were subjected to experimental design analysis of variance (ANOVA) to evaluate the effects of treatments on yield, growth components and tuber quality of potato. The SAS Institute (Version 9, Cary, NC, USA, 2002) was used to perform the analysis of variance. Comparison of the means was obtained using the least significant difference (LSD) at the 5% probability level.
Results and discussion
Meteorological parameters.
The mean monthly meteorological parameters for both years are presented in Fig. 1 . The maximum mean monthly temperature was observed during the tuber formation in 2021 and tuber expansion month in 2022. Precipitation levels during the 2021 growing season were lower compared to the subsequent season (2022), but not unevenly distributed, with majority of the rain occuring in June (Fig. 1 ). The total precipitation during the potato growing seasons in 2021 and 2022 was 82.20 mm and 177.60 mm, respectively (Fig. 1 ). Morever, lower temperature values prior to planting in 2022 resulted in a delay in the planting time.
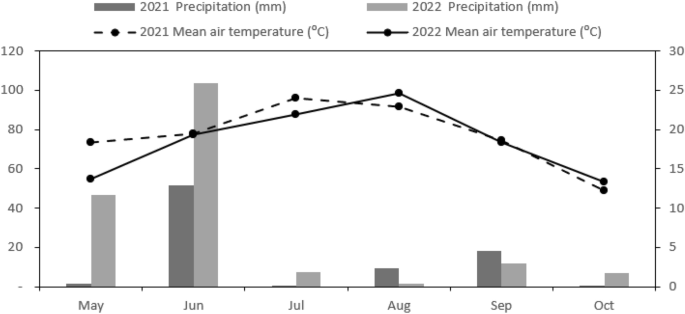
Mean air temperature and precipitation during the growing season of 2021–2022.
Irrigation water applied and crop water consumption
The total water application for each treatment and growing season is shown in Fig. 2 . The average total irrigation applied to the crop was 227.70, 280.90, and 335.70 mm for S33, S66, and S100 treatments in 2021, respectively and 160.10, 241.00, and 324.3 mm for S33, S66, and S100 treatments in 2022, respectively. The first-year irrigation amount was higher than the second year because the water deficit period was delayed in the first year. The seasonal crop water consumption (ETc) values determined are given in Table 1 . Seasonal average ET c values varied between 181.78 and 289.79 mm in 2021 and between 234.48 and 369.14 mm in 2022.
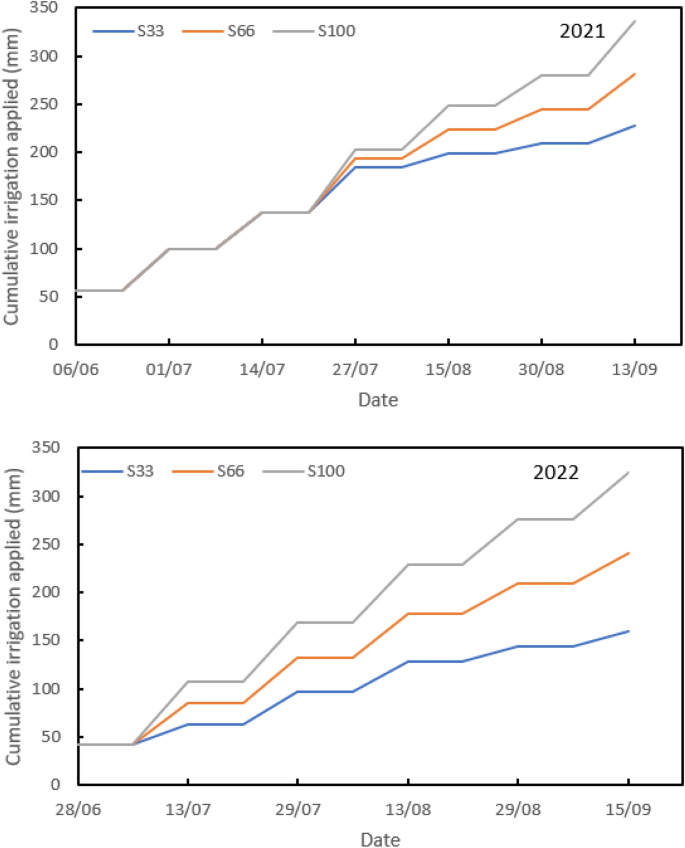
Water applied amount for each irrigation level during the years 2021 and 2022.
The potato tuber yields exhibited significant variation in response to different levels of nitrogen and drip irrigation, as indicated in Table 2 . The S100 irrigation level achieved highest tuber yield under all N levels. Notably average yield was more sensitive at irrigation levels than nitrogen levels. Gradual water deficit resulted in an average reduction in total yield of 14.9% in 2021 and 10.5% in 2022 with a reduction in irrigation water at the S66 level, whereas the application of S33, which represented a lower amount of water, led to a 37.2% decrease in potato yield in 2021 and 39.8% in 2022. The findings of Badr et al. 2 support this observation, as full irrigation resulted in the highest tuber yield under all nitrogen levels. Moreover, as the amount of irrigation water decreased, the total yield reduced by an average of 7.8% with a 20% reduction in irrigation water. Meanwhile, a decrease in potato yield by 27.3% and 44.6% was observed when 40% and 60% less water was applied, respectively. It was further noted that while the total yield increased up to the N3 level with an increase in the amount of nitrogen, it decreased beyond this point. The decrease in tuber yield beyond a certain level of nitrogen was attributed to the plant experiencing stress, ultimately leading to a decrease in yield.The application of irrigation and nitrogen fertilizer rates individually have demonstrated significant impacts on the growth attributes of potato crops, with a notable interaction effect between the two factors. It was observed that the maximum growth attributes of potato crops were achieved when irrigated with S100, while the minimum values of plant height were recorded in plots irrigated with S33, across both years. Similarly, an increase in nitrogen application rates was positively correlated with plant height. In particular, the highest plant height was recorded in response to N4 treatment in 2021 and N1 treatment in 2022 (Table 2 ). Notably, the response of plants to water scarcity in nitrogen fertilization presents a crucial factor in understanding how plants allocate their resources to aboveground and underground organs, thereby influencing their growth and development. Therefore, the association with plant height was observed to be slightly but consistently shorter in N0 plants. This observation aligns with findings reported by Wang et al. 22 where N-fertilization treatments were found to result in significantly higher plant heights in full irrigation treatments than in other irrigation treatments. Moreover, Kumar et al. 23 have similarly noted that plant height tends to increase with increasing N doses up to 180 kg N ha −1 . Furthermore, Yuan et al. 13 indicated that plant height was observed to increase proportionately with the increasing amount of irrigation from Ep0.25 to Ep1.25.
According to the results presented in Table 2 , there were no significant differences in the number of tubers per plant for nitrogen fertilization and irrigation in 2021. However, in 2022, there were significant differences observed. (Table 2 ). The number of tubers tended to increase with an increase in nitrogen content. Nevertheless, decreased under water-deficit conditions. In particular, the irrigation level of S100 in 2021 resulted in a higher number of tubers per plant, while the irrigation level of S66 in 2022 resulted in the highest number of tubers per plant. On the other hand, the number of tubers decreased under N0 in both years, with the highest tuber number obtained from the N5 treatment in 2021 and the N3 treatment in 2022. Onder et al. 11 reported that irrigation levels of 66% of full irrigation resulted in the highest number of tubers per plant. Mattar et al. 24 observed that the number of tubers per plant was highest with full irrigation. However, in contrast, Ghasemi et al. 25 and Fandika et al. 26 indicated that the effect of irrigation water on the number of tubers was not significant. Previous studies have found that water stress reduces the number of tubers per plant. Also, the lowest tuber number per plant was found in 0 kg ha −1 N application, and other treatments were in the same statistical group by Güler 27 . Similarly, Ahmed et al. 28 reported a reduced number of tubers per plant, and the lowest number was obtained when using low application rates of 130 and 180 kg N/fed.
The number of stems per plant varied significantly in response to different N fertilization and irrigation levels. When N applications were evaluated, the highest stem values per plant were obtained from N4 application in 2021 and from N0 application in 2022 (Table 2 ). In addition, the increase in the amount of nitrogen caused an increase in the number of stems in 2021 and a decrease in 2022. The reason is that stem number is not affected much by mineral nutrients. Stem numbers per plant were affected significantly by varied irrigation levels. The irrigation level of S100 resulted in a higher stem number per plant in 2021, while the irrigation level of S66 resulted in the highest number of stems per plant in 2022. Contrary to our research, Adhikari and Rana 29 and Kumar et al. 23 showed that the effect of various irrigation levels on the number of stems per hill was not significant. Factors such as the storage conditions of tubers, the number of viable sprouts during planting, sprout damage and growing conditions during planting, physiological age of the seed tuber and tuber size also affect the number of stems 30 .
The effects of nitrogen and irrigation on tuber dry matter (TDM), specific gravity (SG) and starch in both years are shown in Table 3 . The mean values determined a significant difference ( P < 0.01) of tuber TDM, SG and starch in irrigation and nitrogen levels in both years. Regarding the two-year analysis, the highest dry matter content was achieved with N2 treatment for nitrogen. The findings suggest that the amount of dry matter in irrigation levels tends to decrease with increasing irrigation. TDM was higher with deficit irrigation than with full irrigation in 2022. However, there was no change in 2021. Several research reports have observed a reduction in tuber dry matter with increasing N application rates 30 , 31 . Others have demonstrated that increasing N fertilization had no significant effect on tuber dry matter 32 . In the current study, an increasing trend in TDM was observed with increasing N treatments in 2021, while no significant changes were observed in 2022. It is possible that the higher amount of irrigation in the first year compared to the second year led to excessive nitrogen intake, which in turn contributed to the increase in TDM.
SG showed a tendency to decrease with increasing applied in 2022, with less irrigation water produced higher SG tubers. However, in 2021 SG was not affected by rising water levels. Over the two years, the highest SG was achieved with S100 for irrigation and N2 for nitrogen levels (Table 3 ). SG is an important quality factors for processing potato. There is a range of specific gravities that is considered optimal. Many factors such as climatic conditions and N fertilization affect tuber SG 2 . Rising of SG with increasing N application might be attributed to the increase in dry matter content, as there is high correlation between SG in tubers and dry matter. Furthermore, deficit irrigation after tuber initiation in the middle of the growing season creates tubers with reduced SG. Yuan et al. 13 reported that as applied water increased, SG tended to decrease. In addition. Alva et al. 1 explained that nitrogen increases SG but is not affected by irrigation.
The results revealed that increasing N application rate led to an increase in starch content, while no significant difference was observed in the irrigation regimes in 2021. However, in 2022, there was no significant difference in starch content between different nitrogen levels. It was also noted that starch accumulation was positively correlated with the amount of irrigation applied, and the highest starch content was observed in the S100 treatment for irrigation and N2 treatment for nitrogen levels. (Table 3 ). These results agree with previous studies indicating a positive correlation between water content and starch accumulation in potato tubers. Specifically, lower water availability has been associated with increased starch content, potentially due to reduced cell size resulting from water stress 22 , 33 . However, there are some contrasting reports suggesting that increased nitrogen fertilizer rates could lead to a reduction in starch content. Considering these findings, proper management of irrigation and nitrogen application is essential for maximizing starch accumulation in potato tubers 34 .
Water use efficiency
The water use efficiency (WUE) of potato crops was affected by different irrigation and nitrogen levels during the growing season in each year as shown in Figs. 3 and 4 . There was a significant difference between the years. Comparisons among mean values of the water levels treatments indicated that S66 had the highest mean value of WUE in both years, followed by S33, S100 and S100, S33 in 2021 and 2022, respectively. The effect of irrigation levels on WUE may depend on the level of water stress at different growth periods. In low water stress conditions, transpiration decreases more than photosynthesis under the condition of slight closure of stomata, and as a result WUE increases 35 .
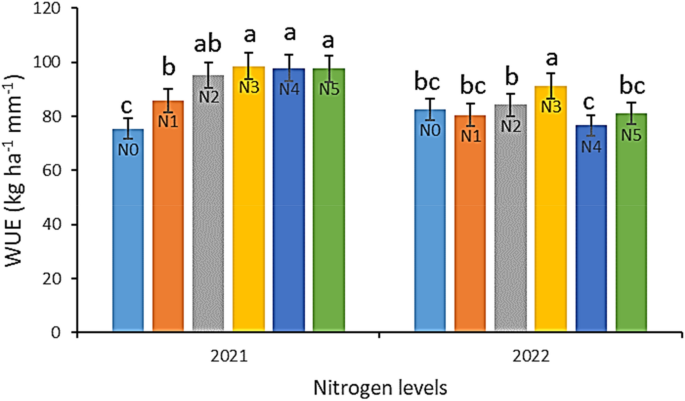
Effect of different nitrogen levels on water use efficiency of potato. Different letters are significantly different. (N0 = 0 kg ha −1 ; N1 = 100 kg ha −1 ; N2 = 200 kg ha −1 ; N3 = 300 kg ha −1 ; N4 = 400 kg ha −1 ; N5 = 500 kg ha −1 ).
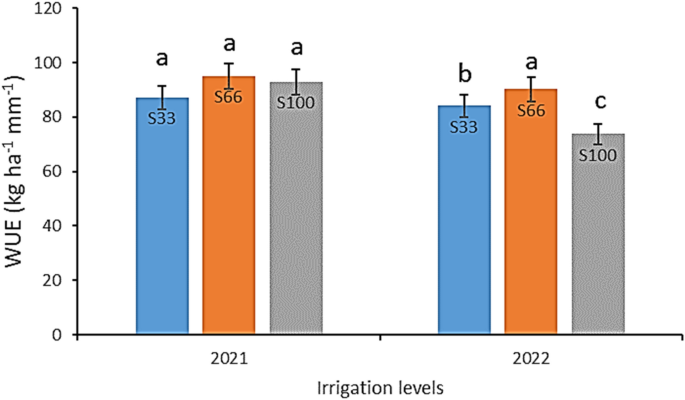
Effect of irrigation levels on water use efficiency of potato. Different letters are significantly different (S33 = 33% FC; S66 = 66% FC; S100 = 100% FC).
The nitrogen levels had a significant effect on WUE, with potato crops exhibiting an increase in WUE up to the N3 level, followed by a decrease in WUE after this threshold in both years (Fig. 3 ). Moreover, the findings suggest that WUE was positively correlated with irrigation levels, whereby an increase in water levels resulted in a concomitant increase in WUE. Under water-deficient conditions, WUE was found to be enhanced, which is in line with previous reports that have documented an increase in WUE relative to an increase in water stress 2 , 36 , 37 .
Relationship between tuber yield, irrigation levels and Etc
Linear regression analysis was utilized to determine the total amount of irrigation applied in tons per hectare. The relationship between potato tuber yield and applied water is presented in Fig. 5 , showing an increase in yield with increasing irrigation application. The linear regressions between irrigation applied and tuber yield were found to be significant. Moreover, significant linear relationships were also observed between potato tuber yield and ETc, as depicted in Fig. 5 . Previous research has indicated that potato yield responds linearly to the quantity of water applied 2 , 11 , 38 . Ünlü et al. 39 reported that depending on the irrigation regimes, evaporation and tuber yield were positively affected by nitrogen fertilizer. Badr et al. 2 indicated that the relationships between tuber yield and crop ET were linear. The relationship between potato yield and ET serves to elucidate the strength of the yield's linear increase with ET 40 , 41 . The aim of irrigation applications is to achieve maximum efficiency through optimal irrigation application and appropriate irrigation regimes 42 .
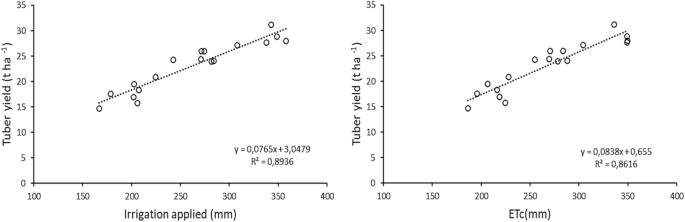
Relationship between tuber yield, irrigation applied and Etc of potato.
Conclusions
Effective management of irrigation and nitrogen is crucial for optimizing potato yield, quality, and water use efficiency. This study highlights that full irrigation with an application rate of 300 kg N/ha resulted in the highest tuber yield, indicating the importance of providing adequate water and nitrogen for optimal crop performance. However, it is important to note that excessive nitrogen levels can have detrimental effects on potato yield and quality. Therefore, careful attention to irrigation and nitrogen levels is necessary to achieve the desired outcomes. The findings of this study provide valuable insights for potato growers and agricultural practitioners. By implementing appropriate irrigation and nitrogen management strategies, farmers can maximize productivity and quality while minimizing the use of water resources. This is particularly relevant in regions where water scarcity and environmental concerns are significant challenges. Furthermore, this research emphasizes the need for sustainable crop management practices. Balancing the application of water and nitrogen is essential not only for achieving optimal yields but also for conserving water resources and minimizing nutrient losses. By adopting precise irrigation scheduling and optimizing nitrogen application rates, farmers can enhance water use efficiency and reduce potential negative environmental impacts. To further advance our understanding, future research should focus on assessing the long-term effects of different irrigation and nitrogen management strategies on potato crops. This would enable the development of more comprehensive guidelines and recommendations for growers to make informed decisions regarding irrigation and nitrogen application.
Data availability
The datasets generated and/or analysed during the current study are not publicly available due as it is part of the corresponding author’s doctoral thesis, and the other part of the study is in progress but are available from the corresponding author on reasonable request.
Alva, A. K., Moore, A. D. & Collins, H. P. Impact of deficit irrigation on tuber yield and quality of potato cultivars. J. Crop Improv. 26 (2), 211–227. https://doi.org/10.1080/15427528.2011.626891 (2012).
Article Google Scholar
Badr, M. A., El-Tohamy, W. A. & Zaghloul, A. M. Yield and water use efficiency of potato grown under different irrigation and nitrogen levels in an arid region. Agric. Water Manag. 110 , 9–15. https://doi.org/10.1016/j.agwat.2012.03.008 (2012).
Belanger, G., Walsh, J. R., Richards, J. E., Milburn, P. H. & Ziadi, N. Yield response of two potato cultivars to supplemental irrigation and N fertilization in New Brunswick. Am. J. Potato Res. 77 , 11–21. https://doi.org/10.1007/BF02853657 (2000).
Fontes, P. C., Braun, H., Busato, C. & Cecon, P. R. Economic optimum nitrogen fertilization rates and nitrogen fertilization rate effects on tuber characteristics of potato cultivars. Potato Res. 53 (3), 167–179. https://doi.org/10.1007/s11540-010-9160-3 (2010).
Goffart, J. P., Olivier, M. & Frankinet, M. Potato crop nitrogen status assessment to improve N fertilization management and efficiency: Past-present-future. Potato Res. 51 , 355–383. https://doi.org/10.1007/s11540-008-9118-x (2008).
Article CAS Google Scholar
Oliveira, C. A. S. Potato crop growth as affected by nitrogen and plant density. Pesqui. Agropecu. Bras. 35 , 939–950. https://doi.org/10.1590/S0100-204X2000000500011 (2000).
Rodrigues, M. A., Coutinho, J., Martins, F. & Arrobas, M. Quantitative sidedress nitrogen recommendations for potatoes based upon crop nutritional indices. Eur. J. Agron. 23 , 79–88. https://doi.org/10.1016/j.eja.2004.10.001 (2005).
Silva, M. C. C., Fontes, P. C. R. & Miranda, G. V. Modelos estatísticos para descrever a produtividade de batata em função da adubação nitrogenada (statistical models to describe the potato yield as a result of the nitrogen fertilization). Hortic. Bras. 25 , 360–364. https://doi.org/10.1590/S0102-05362007000300008 (2007).
Koch, M., Naumann, M., Pawelzik, E., Gransee, A. & Thie, H. The importance of nutrient management for potato production part I: Plant nutrition and yield. Potato Res. 63 , 97–119. https://doi.org/10.1007/s11540-019-09431-2 (2020).
Andrews, M., Raven, J. A. & Lea, P. J. Do plants need nitrate? The mechanisms by which nitrogen form affects plants. Ann. Appl. Biol. 163 , 147–199. https://doi.org/10.1111/aab.12045 (2013).
Onder, S., Caliskan, M. E., Onder, D. & Caliskan, S. Different irrigation methods and water stress effects on potato yield and yield components. Agric. Water Manag. 73 (1), 73–86. https://doi.org/10.1016/j.agwat.2004.09.023 (2005).
Tulucu, K. Irrigation of Special Plants (Cukurova University Faculty of Agriculture Publications, 2003).
Google Scholar
Yuan, B. Z., Nishiyama, S. & Kang, Y. Effects of different irrigation regimes on the growth and yield of drip-irrigated potato. Agric. Water Manag. 63 (3), 153–167. https://doi.org/10.1016/S0378-3774(03)00174-4 (2003).
Caliskan, S. & Demirel, U. Potato cultivation-irrigation. In Agriculture Agenda (Ed. Akınerdem, F.) 56–65 (Hurriyet Printing Press, 2018).
Alva, A. K. et al. Nitrogen and irrigation management practices to improve nitrogen uptake efficiency and minimize leaching losses. J. Crop Improv. 15 (2), 369–420. https://doi.org/10.1300/J411v15n02_11 (2006).
Albassam, B. A. Effect of nitrate nutrition on growth and nitrogen assimilation of pearl millet exposed to sodium chloride stress. J. Plant Nutr. 24 , 1325–1335. https://doi.org/10.1081/PLN-100106984 (2001).
James, L. G. Principles of Farm Irrigation Systems Design (John Wiley and Sons Limited, 1988).
Huang, Y. L., Chen, L. D., Fu, B. J., Huang, Z. L. & Gong, J. The wheat yields and water use efficiency in the Loess Plateau: Straw mulch and irrigation effects. Agric. Water Manag. 72 , 209–222. https://doi.org/10.1016/j.agwat.2004.09.012 (2005).
Kaur, A. et al. Interactive effects of nitrogen application and irrigation on water use, growth and tuber yield of potato under subsurface drip irrigation. Agronomy 13 (1), 11. https://doi.org/10.3390/agronomy13010011 (2022).
Hou, X., Li, R., He, W. & Ma, K. Effects of planting density on potato growth, yield, and water use efficiency during years with variable rainfall on the Loess Plateau, China. Agric. Water Manag. 230 , 1–9. https://doi.org/10.1016/j.agwat.2019.105982 (2020).
Haase, N. Estimation of dry matter and starch concentration in potatoes by determination of under-water weight and near infrared spectroscopy. Potato Res. 46 (3), 117–127. https://doi.org/10.1007/BF02736081 (2003).
Wang, X. et al. The effects of mulch and nitrogen fertilizer on the soil environment of crop plants. Adv. Agron. 153 , 121–173. https://doi.org/10.1016/bs.agron.2018.08.003 (2019).
Kumar, P., Pandey, S. K., Singh, S. V. & Kumar, D. Irrigation requirements of chipping potato cultivars under west-central Indian plains. Potato J. 34 (3–4), 193–198 (2007).
Mattar, M. A., Zin El-Abedin, T. K., Al-Ghobari, H. M., Alazba, A. A. & Elansary, H. O. Effects of different surface and subsurface drip irrigation levels on growth traits, tuber yield, and irrigation water use efficiency of potato crop. Irrig. Sci. 39 (4), 517–533. https://doi.org/10.1007/s00271-020-00715-x (2021).
Ghasemi, S. F., Hekmat, M. & Pourkhiz, E. Effect of under irrigation management on potato performance components. Int. J. Agric. Manag. Dev. 2 (2), 143–148 (2012).
Fandika, I. R., Kemp, P. D., Millner, J. P., Horne, D. & Roskruge, N. Irrigation and nitrogen effects on tuber yield and water use efficiency of heritage and modern potato cultivars. Agric. Water Manag. 170 , 148–157. https://doi.org/10.1016/j.agwat.2015.10.027 (2016).
Güler, S. Effects of nitrogen on yield and chlorophyll of potato ( Solanum tuberosum L.) cultivars. Bangladesh J. Bot. 38 (2), 163–169. https://doi.org/10.3329/bjb.v38i2.5141 (2009).
Ahmed, A., Abd El-Baky, M., Ghoname, A., Riad, G. & El-Abd, S. Potato tuber quality as affected by nitrogen form and rate. Middle East. Russ. J. Plant Sci. Biotechnol. 3 (1), 47–52 (2009).
Adhikari, R. C. & Rana, M. K. Effect of irrigation and potash levels on growth and yield of potato. J. Agric. Environ. 18 , 106–114. https://doi.org/10.3126/aej.v18i0.19895 (2017).
Zelalem, A., Tekalign, T. & Nigussie, D. Response of potato ( Solanum tuberosum L.) to different rates of nitrogen and phosphorus fertilization on vertisols at Debre Berhan, in the central highlands of Ethiopia. Afr. J. Plant Sci. 3 (2), 16–24 (2009).
CAS Google Scholar
Casa, R., Pieruccetti, F., Sgueglia, G. & Lo Cascio, B. Potato tuber quality improvement through nitrogen management optimization: Review of methodologies. Acta Hortic. 684 , 65–72 (2005).
Sharifi, M., Zebarth, B. J., Hajabbasi, M. A. & Kalbasi, M. Dry matter and nitrogen accumulation and root morphological characteristics of two clonal selections of Russet Norkotah potato as affected by nitrogen fertilization. J. Plant Nutr. 28 , 2243–2253. https://doi.org/10.1080/01904160500323552 (2005).
Zhang, Y. L. et al. Influence of different plastic film mulches and wetted soil percentages on potato grown under drip irrigation. Agric. Water Manag. 180 , 160–171. https://doi.org/10.1016/j.agwat.2016.11.018 (2017).
Lin, S., Sattelmacher, B., Kutzmutz, E., Muhling, K. H. & Dittert, K. X. Influence of nitrogen nutrition on tuber quality of potato with special reference to the pathway of nitrate transport into tubers. J. Plant Nutr. 27 , 341–350. https://doi.org/10.1081/PLN-120027658 (2007).
Cantore, V. et al. Yield and water use efficiency of early potato grown under different irrigation regimes. Int. J. Plant Prod. 8 , 409–428 (2014).
Ati, A. S., Iyada, A. D. & Najim, S. M. Water use efficiency of potato ( Solanum tuberosum L.) under different irrigation methods and potassium fertilizer rates. Ann. Agric. Sci. 57 (2), 99–103. https://doi.org/10.1016/j.aoas.2012.08.002 (2012).
Darwish, T. M., Atallah, T. W., Hajhasan, S. & Haidar, A. Nitrogen and water use efficiency of fertigated processing potato. Agric. Water Manag. 85 (1–2), 95–104. https://doi.org/10.1016/j.agwat.2006.03.012 (2006).
Ierna, A. & Mauromicale, G. Potato growth, yield and water productivity response to different irrigation and fertilization regimes. Agric. Water Manag. 201 , 21–26. https://doi.org/10.1016/j.agwat.2018.01.008 (2018).
Ünlü, M., Kanber, R., Şenyigit, U., Onaran, H. & Diker, K. Trickle and sprinkler irrigation of potato ( Solanum tuberosum L.) in the Middle Anatolian Region in Turkey. Agric. Water Manag. 79 (1), 43–71 (2006).
Badr, M. A., El-Tohamy, W. A., Salman, S. R. & Gruda, N. Yield and water use relationships of potato under different timing and severity of water stress. Agric. Water Manag. 271 , 1–8. https://doi.org/10.1016/j.agwat.2022.107793 (2022).
Camargo, D. C., Montoya, F., Ortega, J. F. & Córcoles, J. I. Potato yield and water use efficiency responses to irrigation in semiarid conditions. Agron. J. 107 , 2120–2131. https://doi.org/10.2134/agronj14.0572 (2015).
Waqas, M. S., Cheema, M. J. M., Hussain, S., Ullah, M. K. & Iqbal, M. M. Delayed irrigation: An approach to enhance crop water productivity and to investigate its effects on potato yield and growth parameters. Agric. Water Manag. 245 , 1–9. https://doi.org/10.1016/j.agwat.2020.106576 (2021).
Download references
Acknowledgements
This research was funded by the Scientific Research Project Office (BAP) of Nigde Omer Halisdemir University, grant number TGT 2021/8-BAGEP. We would like to thank Doğa Seed company for providing support for the supply of potato We thank Assoc. Prof. Khawar JABRAN for critical reading and valuable suggestions on the manuscript and Prof. Dr. Ali ÜNLÜKARA for support in experimental design and irrigation calculations. Mustafa AKKAMIŞ has scholarships from YÖK 100/2000 and Ayhan Şahenk Foundation. We would like to thank Niğde Ömer Halisdemir University, Higher Education Council and Ayhan Şahenk Foundation for their support.
Author information
Authors and affiliations.
Department of Plant Production and Technologies, Faculty of Agricultural Sciences and Technologies, Nigde Omer Halisdemir University, Nigde, Turkey
Mustafa Akkamis & Sevgi Caliskan
You can also search for this author in PubMed Google Scholar
Contributions
M.A. and S.C. planned the study and designed the experiments. M.A. performed the experiments. M.A. conducted data analysis. M.A. and S.C. wrote the paper. The authors read and approved the final manuscript.
Corresponding author
Correspondence to Mustafa Akkamis .
Ethics declarations
Competing interests.
The authors declare no competing interests.
Additional information
Publisher's note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.

Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ .
Reprints and permissions
About this article
Cite this article.
Akkamis, M., Caliskan, S. Responses of yield, quality and water use efficiency of potato grown under different drip irrigation and nitrogen levels. Sci Rep 13 , 9911 (2023). https://doi.org/10.1038/s41598-023-36934-3
Download citation
Received : 19 February 2023
Accepted : 13 June 2023
Published : 19 June 2023
DOI : https://doi.org/10.1038/s41598-023-36934-3
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
This article is cited by
Rejuvenating potato growth and yield in challenging semiarid and saline sandy cholistan: harnessing pgpb-coated n and p application strategies.
- Muhammad Wasim Haider
- Muhammad Nafees
- Kassem A. S. Mohammed
BMC Plant Biology (2024)
Whole-genome sequencing of tetraploid potato varieties reveals different strategies for drought tolerance
- Florian Schilling
- Christina Schumacher
- Renate Horn
Scientific Reports (2024)
Regulation effects of water and nitrogen on yield, water, and nitrogen use efficiency of wolfberry
- Guangping Qi
- Jianjun Wang
Journal of Arid Land (2024)
Reduced Late-Season Irrigation Improves Potato Quality, Often at the Expense of Yield and Economic Return
- Francisco Gonzalez T.
- Mark J. Pavek
- Zachary Holden
American Journal of Potato Research (2024)
Promoting excellence or discouraging mediocrity – a policy framework assessment for precision agriculture technologies adoption
- Georgios Kleftodimos
- Leonidas Sotirios Kyrgiakos
- George Vlontzos
Precision Agriculture (2024)
By submitting a comment you agree to abide by our Terms and Community Guidelines . If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.
Quick links
- Explore articles by subject
- Guide to authors
- Editorial policies
Sign up for the Nature Briefing newsletter — what matters in science, free to your inbox daily.

We apologize for the inconvenience...
To ensure we keep this website safe, please can you confirm you are a human by ticking the box below.
If you are unable to complete the above request please contact us using the below link, providing a screenshot of your experience.
https://ioppublishing.org/contacts/
- Research article
- Open access
- Published: 08 March 2023
Water intake, hydration status and 2-year changes in cognitive performance: a prospective cohort study
- Stephanie K. Nishi 1 , 2 , 3 , 4 , 5 ,
- Nancy Babio 1 , 2 , 3 ,
- Indira Paz-Graniel 1 , 2 , 3 ,
- Lluís Serra-Majem 3 , 6 ,
- Jesús Vioque 7 , 8 ,
- Montserrat Fitó 3 , 9 ,
- Dolores Corella 3 , 10 ,
- Xavier Pintó 3 , 11 , 12 ,
- Aurora Bueno-Cavanillas 7 , 13 ,
- Josep A. Tur 3 , 14 ,
- Laura Diez-Ricote 15 ,
- J. Alfredo Martinez 3 , 16 , 17 ,
- Carlos Gómez-Martínez 1 , 2 , 3 ,
- Andrés González-Botella 18 ,
- Olga Castañer 9 ,
- Andrea Alvarez-Sala 10 ,
- Cristina Montesdeoca-Mendoza 6 ,
- Marta Fanlo-Maresma 3 , 11 ,
- Naomi Cano-Ibáñez 7 , 13 , 19 ,
- Cristina Bouzas 3 , 14 ,
- Lidia Daimiel 3 , 15 , 20 ,
- María Ángeles Zulet 3 , 16 ,
- John L. Sievenpiper 4 , 5 , 21 , 22 , 23 , 24 ,
- Kelly L. Rodriguez 25 ,
- Zenaida Vázquez-Ruiz 3 , 26 &
- Jordi Salas-Salvadó 1 , 2 , 3
BMC Medicine volume 21 , Article number: 82 ( 2023 ) Cite this article
10k Accesses
12 Citations
143 Altmetric
Metrics details
Water intake and hydration status have been suggested to impact cognition; however, longitudinal evidence is limited and often inconsistent. This study aimed to longitudinally assess the association between hydration status and water intake based on current recommendations, with changes in cognition in an older Spanish population at high cardiovascular disease risk.
A prospective analysis was conducted of a cohort of 1957 adults (aged 55–75) with overweight/obesity (BMI between ≥ 27 and < 40 kg/m 2 ) and metabolic syndrome from the PREDIMED-Plus study. Participants had completed bloodwork and validated, semiquantitative beverage and food frequency questionnaires at baseline, as well as an extensive neuropsychological battery of 8 validated tests at baseline and 2 years of follow-up. Hydration status was determined by serum osmolarity calculation and categorized as < 295 mmol/L (hydrated), 295–299.9 mmol/L (impending dehydration), and ≥ 300 mmol/L (dehydrated). Water intake was assessed as total drinking water intake and total water intake from food and beverages and according to EFSA recommendations. Global cognitive function was determined as a composite z -score summarizing individual participant results from all neuropsychological tests. Multivariable linear regression models were fitted to assess the associations between baseline hydration status and fluid intake, continuously and categorically, with 2-year changes in cognitive performance.
The mean baseline daily total water intake was 2871 ± 676 mL/day (2889 ± 677 mL/day in men; 2854 ± 674 mL/day in women), and 80.2% of participants met the ESFA reference values for an adequate intake. Serum osmolarity (mean 298 ± 24 mmol/L, range 263 to 347 mmol/L) indicated that 56% of participants were physiologically dehydrated. Lower physiological hydration status (i.e., greater serum osmolarity) was associated with a greater decline in global cognitive function z -score over a 2-year period ( β : − 0.010; 95% CI − 0.017 to − 0.004, p -value = 0.002). No significant associations were observed between water intake from beverages and/or foods with 2-year changes in global cognitive function.
Conclusions
Reduced physiological hydration status was associated with greater reductions in global cognitive function over a 2-year period in older adults with metabolic syndrome and overweight or obesity. Future research assessing the impact of hydration on cognitive performance over a longer duration is needed.
Trial registration
International Standard Randomized Controlled Trial Registry, ISRCTN89898870. Retrospectively registered on 24 July 2014
Peer Review reports
Cognitive decline is an important public health concern given 55 million people have been diagnosed with dementia and almost 80 million people are projected to be affected by 2030 [ 1 ]. Cognitive function is particularly important, especially as the population ages, because it determines the maintenance of our independence, the performance of everyday activities, and the quality of life [ 1 ]. Cognitive decline and dementia have a diverse etiology, and effective treatment is still not available [ 2 ]. For this reason, prevention strategies targeting modifiable factors, such as nutritional habits and dietary intake to slow the development of cognitive impairment, remain a promising public health approach [ 2 ]. Evidence to date assessing individual dietary factors and dietary patterns in relation to cognitive health has been summarized in the Lancet Neurology [ 3 ], while there is information related to various macro- and micronutrients and patterns, evidence related to water appears limited.
Water intake is a nutritional habit that is often overlooked, yet it is considered essential for the optimal physiological function of the human body [ 4 ]. As the most abundant component of the human body, water intake and optimal hydration are considered key aspects for the proper functioning of organ systems, aiding, among others, in efficient digestion, elimination of toxins, energy production, thermoregulation, and joint lubrication, as well as a multitude of biochemical reactions [ 5 , 6 ]. Furthermore, proper hydration is thought to be important for optimal cognitive functioning as it plays a vital role in neural conductivity [ 7 ].
Dehydration occurs when the body loses more water than is taken in, whereas hypohydration refers to the state of water deficit; these conditions result in less-than-optimal hydration and may elicit adverse physiological consequences [ 8 ]. For the purposes of this paper, dehydration will be the term used to encompass the state of improper hydration due to unbalanced water loss or water deficit. In Europe, the percentage of the population reported to have inadequate water intake is estimated to vary from 5 to 35% [ 9 , 10 , 11 ]. Understanding water intake and hydration status is of particular importance in older adults as this population tends to be less likely to meet recommendations on water intake and is at greater risk for dehydration due to blunted sensitivity to thirst signals, lower body reserves due to reduced muscle mass, reduced ability to deal with heat stress, and use of medications or laxatives with diuretic effects [ 12 , 13 , 14 , 15 ]. Multiple health organizations and dietary guidelines have acknowledged that adequate hydration status is associated with the preservation of physical and mental functions and that water intake is the best way to achieve hydration [ 16 , 17 , 18 , 19 ].
To maintain adequate hydration status, several countries and public organizations have proposed water intake recommendations for the public [ 20 , 21 ]. These recommendations have been determined based on data from population studies and accounted for water from beverage and food sources [ 22 ]. For instance, the European Food Safety Authority (EFSA), based on data from population studies from 13 European countries, proposed the dietary reference values (DRV) for the adequate intake (AI) of water which increases with age up to 2.5 L and 2.0 L of water daily for men and women (aged 14 to 70 years), respectively [ 20 ]. Similarly, the Institute of Medicine (IOM) proposed increasing water intake levels with age up to 3.7 L/day and 2.7 L/day (aged 19 to 70 years) in men and women, respectively. Yet, the World Health Organization and other guidelines related to cognitive health do not currently include recommendations related to water intake or hydration status [ 23 , 24 , 25 ]. Further understanding of health behaviors, such as cognitive functioning, related to something as fundamental as water intake can have a substantial impact on public health.
Inadequate water intake and dehydration have been associated with existing signs of cognitive impairment among older adults living in long-term care facilities [ 26 , 27 , 28 , 29 ]. Moreover, acute studies have shown that dehydration and water supplementation affect mood and cognitive performance [ 30 ]. However, fluid and water intake has received limited attention in epidemiological studies, and the literature scarcely examines water intake as a predictor of cognitive performance among older adults. The few studies that have assessed hydration status as a potential predictor of cognitive function among community-dwelling older adults have been inconclusive [ 31 , 32 , 33 , 34 ]. Furthermore, to date, few studies have prospectively captured and examined the impact of water and hydration status on cognitive function over a multi-year period. Therefore, the objective of the present analyses was to prospectively investigate the relation between hydration status, water intake, and 2-year changes in cognitive performance in community-dwelling older adults with metabolic syndrome and overweight or obesity.
Study design
This prospective cohort study is based on data collected during the first 2 years of the PREDIMED-Plus (PREvención con DIeta MEDiterránea Plus) study. Briefly, the PREDIMED-Plus study is an ongoing randomized, parallel-group, 6-year multicenter, controlled trial designed to assess the effect of lifestyle interventions on the primary prevention of cardiovascular disease. The primary aim of the trial is to assess the effects of an intensive weight loss intervention based on an energy-reduced Mediterranean diet (MedDiet), physical activity promotion, and behavioral support (intervention group) compared to usual care and dietary counseling only with an energy-unrestricted MedDiet (control group) on the prevention of cardiovascular events. Details of the design and methods of PREDIMED-Plus have been previously described [ 35 , 36 ] and are available at https://www.predimedplus.com/ .
Ethics, consent, and permissions
The PREDIMED-Plus study protocol and procedures were approved by the Research Ethics Committees from each of the participating centers, and the study was registered with the International Standard Randomized Controlled Trial Registry (ISRCTN; ISRCTN89898870 ). All participants provided written informed consent.
Study participants
PREDIMED-Plus participants were recruited from 23 centers across Spain between September 2013 and December 2016. A total of 6874 adults met the eligibility criteria and were randomly allocated in a 1:1 ratio to either the intervention or the control group. Couples sharing the same household were randomized together, and the couple was used as a unit of randomization. Eligible participants were community-dwelling adults (aged 55 to 75 for men; 60 to 75 for women) with overweight or obesity (BMI: 27 to 40 kg/m 2 ) who met at least three criteria for metabolic syndrome (35), without previous cardiovascular events or diagnosed neurodegenerative diseases at baseline. All participants provided written informed consent.
The present longitudinal analysis involves a sub-study conducted in 10 of the 23 PREDIMED-Plus recruiting centers. Of the participants in the PREDIMED-Plus sub-study who had a completed validated 32-item Spanish fluid intake questionnaire, participants were excluded if they did not have a completed baseline Food Frequency Questionnaire (FFQ) or who reported implausible total energy intakes based on those proposed by Willet (≤ 500 and ≤ 3500 kcal/day in women and ≤ 800 and ≤ 4000 kcal/day in men) [ 37 ]. For the water and fluid intake analyses, using the interquartile range method (using a 1.5 multiplier for the first and third quartiles), participants with extreme intakes of fluid (daily fluid intakes for men < 188 mL or > 3862 mL and women < 263 mL or > 3539 mL) were excluded for the assessment of water and fluid intakes. Similarly, participants without blood sample values for urea, sodium, potassium, glucose, and serum osmolarity values < 100 mmol/L were also excluded from the analyses of hydration status and cognition. Furthermore, associations were tested for those participants who had completed the various cognitive tests.
Assessment of water and fluid intake
A validated, semi-quantitative 32-item Beverage Intake Assessment Questionnaire (BIAQ) [ 10 ] and a 143-item validated semi-quantitative FFQ (38) specifying usual portion sizes, were administered by trained dietitians to assess habitual fluid and dietary intakes, respectively. These two questionnaires have been validated within populations of older, Spanish individuals, which are analogous to the current study population, and both have been found to be reproducible with relative validity [ 10 , 38 ]. The BIAQ recorded the frequency of consumption of various beverage types during the month prior to the visit date. The average daily fluid intake from beverages was estimated from the servings of each type of beverage. The questionnaire items on beverages included: tap water, bottled water, natural fruit juices, bottled fruit juices, natural vegetable juices, bottled vegetable juices, whole milk, semi-skimmed milk, skimmed milk, drinking yogurt, milkshakes, vegetable drinks, soups, jellies and sorbets, soda, light/zero soda, espresso, coffee, tea, beer, non-alcoholic beer, wine, spirits, mixed alcoholic drinks, energy drinks, sports drinks, meal replacement shakes, and other beverages. The water and nutrient contents of the beverages were estimated mainly using the CESNID Food Composition Tables [ 39 ], complemented with data from the BEDCA Spanish Database of Food Composition [ 40 ].
The FFQ collected data on food intake based on the year prior to the visit according to nine possible frequency categories, which ranged from “never or almost never” to “> 6 portions/day” and based on the dietary guidelines for the Spanish population [ 41 ]. The information collected was converted into grams per day, multiplying portion sizes by consumption frequency and dividing the result by the period assessed. Ten food groups composed of vegetables, fruits, legumes, cereals, dairy beverages, meat and poultry, fats, nuts, fish/seafood, and other foods were determined to assess the contribution of foods to total water intake. Food groups and energy intake were estimated using Spanish food composition tables [ 42 , 43 ]. Drinking water intake, water intake from all fluids, total water intake, EFSA total fluid water intake (TFWI), and EFSA total water intake (TWI) were computed (descriptions summarized in Additional file 1 : Table S1). Drinking water intake was estimated based on tap and bottled water intakes based on BIAQ responses. Water intake from all fluids was computed from tap and bottled water, plus water from other beverages based on responses to the BIAQ. Total water intake encompassed water intake from all fluids in addition to water present in food sources based on responses to the FFQ. Water intake was further categorized based on established reference values. The EFSA recommendations for total water intake (EFSA TWI) for older adults (2.5 L/day and 2.0 L/day for men and women, respectively) in conditions of moderate environmental temperature and moderate physical activity [ 20 ] were used as reference values. Further categorizations were determined based on total water intake from fluids alone, based on EFSA recommendations (EFSA TFWI), where recommended levels for older adults are set to at least 2.0 L/day and 1.6 L/day for men and women, respectively [ 20 ].
Assessment of hydration status
Hydration status was estimated based on calculated serum osmolarity (SOSM), which is considered a more reliable biomarker of hydration status than urinary markers in older adults [ 44 ]. Fasting serum glucose, urea, sodium, and potassium were measured by standard methods. Blood urea nitrogen was determined from urea values using the conversion factor of 0.357 and reported in mmol/L. With all relevant serum analyte measures presented in mmol/L, SOSM was estimated using the following equation [ 45 ]:
Based on this equation, dehydration, impending dehydration, and hydrated statuses were defined as SOSM > 300, 295–300, and < 295 mmol/L, respectively [ 28 , 46 ].
Assessment of cognitive performance
A battery of 8 neuropsychological tests assessing different cognitive domains was administered at baseline and 2-year follow-up by trained staff to assess cognitive performance. The following tests were assessed: the Mini-Mental State Examination (MMSE), two Verbal Fluency Tests (VFTs), two Digit Span Tests (DSTs) of the Wechsler Adult Intelligence Scale-III (WAIS-III), the Clock Drawing Test (CDT), and two Trail Making Tests (TMTs).
Briefly, a Spanish-validated version of the MMSE questionnaire, a commonly used cognitive screening test, was used in the present analysis [ 47 ]. A higher MMSE score indicates better cognitive performance [ 48 ]. Verbal ability and executive function were evaluated using the VFTs, which consist of two parts: the semantic verbal fluency task-animal category version (VFT-a) and the phonemic verbal fluency task-letter “p” version (VFT-p) [ 49 ]. The DST of the WAIS-III Spanish version assessed attention and memory. The DST Forward Recall (DST-f) is representative of attention and short-term memory capacity, and the DST Backward Recall (DST-b) is considered as a test of working memory capacity [ 50 , 51 ]. The CDT-validated Spanish version was mainly used to evaluate visuospatial and visuo-constructive capacity [ 52 , 53 , 54 ]. Lastly, the TMT, another tool often used to assess executive function, consists of two parts. Part A (TMT-A) assessed attention and processing speed capacities, and part B (TMT-B) further examined cognitive flexibility [ 55 ]. All instruments included in the cognitive battery have been standardized for the Spanish population in the age range of the study population.
To assess overall cognitive function, a global cognitive function (GCF) score was determined as the main outcome measure, in addition to evaluating the individual neuropsychological tests (supplementary analyses). Raw scores at baseline and scores of changes at 2 years of follow-up for each individual cognitive assessment, as well as GCF, were standardized using the mean and standard deviation from the baseline measurements as normative data, creating z -scores [ 56 ]. GCF was calculated as a composite z -score of all 8 assessments, adding or subtracting each individual test value based on whether a higher score indicates higher or lower cognitive performance, respectively, using the formula:
Covariate assessment
The trained staff collected baseline socio-demographic (i.e., sex, age, education level, and civil status) and lifestyle (i.e., physical activity, total energy intake, alcohol intake, caffeine consumption [ 57 ], sleeping habits, and smoking status) related variables, as well as information about medication use, in face-to-face interviews using self-reported general questionnaires and a 143-item validated semi-quantitative FFQ for the dietary related variables [ 38 ], which were further estimated using Spanish food composition tables [ 42 , 43 ]. Leisure time physical activity was estimated using the validated Minnesota-REGICOR Short Physical Activity questionnaire [ 58 ]. These socio-demographic and lifestyle variables were considered as possible covariates because of reports that younger adults, women, individuals with higher educational attainment, married, more active, greater consumers, and non-smokers tend to consume higher amounts of fluids from beverages and foods and hence more likely to meet recommendations on water intake [ 59 , 60 ]. Alcohol was accounted for as a potential covariate as it may act as a diuretic at certain levels [ 61 ] as well as being associated with an elevated risk of dementia when consumed regularly [ 62 ]. Similarly, caffeinated beverages may have a mild diuretic effect [ 63 ], as well as may affect attention and alertness [ 64 ] and could be associated with reduced cognitive decline and dementia risk [ 65 ]. Sleeping habits have also been associated with cognitive health [ 66 ]. Anthropometric measures, including weight and height were measured by trained staff using calibrated scales and wall-mounted stadiometers, respectively. Body mass index (BMI), which may modify the relationship between water intake and hydration status [ 67 ], was calculated as weight in kilograms divided by height in meters squared. History of chronic disease (i.e., type 2 diabetes, hypertension, and hypercholesterolemia) was self-reported or collected from patient medical records and included as these conditions may cause fluid imbalance, cause dehydration, and have been associated with cognitive performance possibly leading to mild cognitive impairment [ 68 , 69 , 70 ]. Depressive symptomatology was evaluated using the Beck Depression Inventory-II (BDI-II), given the association observed with cognitive health [ 71 ] where depressive symptomatology risk was established as a score ≥ 14 [ 72 ].
Statistical analyses
For the present analyses, a prospective cohort study was conducted within the framework of the PREDIMED-Plus study using the database updated to December 22, 2020. Participants were categorized into quantiles based on baseline water intake (drinking water, water intake from all fluids, total water intake), recommended categories of water intake (EFSA TWI, EFSA, TFWI), and hydration status according to serum osmolarity. Baseline characteristics of participants for each category and quantile of water intake and hydration status were presented as numbers and percentages using Pearson’s chi-square test for categorical variables and means ± standard deviations or median (interquartile range [P25–P75]) using one-way ANOVA or Kruskal Wallis test for continuous variables, as appropriate.
Multivariable linear regression models were fitted to assess longitudinal associations comparing the 2-year change in cognitive function across baseline variables of hydration status and water intake and for meeting the EFSA recommendations for TWI and TFWI [ 20 ]. When analyses were performed with categorical variables, p for trend was calculated. The p for linear trend was calculated by assigning the median value of each category and modeling it as a continuous variable.
Multivariable linear regression models were adjusted for several potential confounders. Model 1 adjusted for age (years), sex (man or woman), intervention group, participating center size (< 100, 100 to < 150, 150 to < 200, ≥ 200 participants for hydration status and < 100, 100 to < 200, 200 to < 300, ≥ 300 participants for fluid-related analyses), respective baseline cognitive function score, and corrected for clusters (to account for couples living in the same household being randomized as a single unit). Model 2 was additionally adjusted for BMI (kg/m 2 ), educational level (primary, secondary, or college), civil status (single, divorced or separated, married, widower), smoking status (current, former, or never), and physical activity (METs/min/day). Model 3 was additionally adjusted for sleeping habits (hours of nighttime sleep), depressive symptomatology (yes/no), diabetes prevalence (yes/no), hypertension (yes/no), hypercholesterolemia (yes/no), total energy intake (kJ per day), alcohol consumption in g/day (and adding the quadratic term), and caffeine intake (g/day). To assess the linear trend, the median value of each category of exposure variables (hydration status and various assessments of water and fluid intake) was assigned to each participant and was modeled as continuous variables in linear regression models. The Bonferroni correction was used to correct for multiple comparisons and reduce the risk of a type 1 error.
Several stratified and sensitivity analyses were additionally performed to test the robustness of the findings. First, sex-stratified regression approaches were employed to examine the relationships between hydration status and these water and fluid intake categories and 2-year changes in global cognitive function. Sensitivity analyses were additionally performed, testing the addition of estimated glomerular filtration rate (eGFR), an indicator of renal function derived based on serum creatinine level, age, and sex [ 73 ], or dietary intake covariates (amount [g/day] of vegetables, fruits, legumes, grains, non-fluid dairy, meat, oils, fish, nuts, and pastries determined via the validated 143-item semi-quantitative FFQ [ 19 ]) to the multivariable models, but also after removal of participants with baseline MMSE < 24 (mild dementia and poorer) [ 74 ], or the removal of participants with extreme GCF z -scores at baseline (< 5% and > 95%).
The data were analyzed using the Stata 14 software program (StataCorp LP, TX, USA), and the results were originally considered statistically significant at a p -value (2-tailed) < 0.05, and after the Bonferroni correction, statistical significance was considered at a p -value (2-tailed) < 0.005.
A total of 1957 participants (mean age 65.0 ± 4.9 years and 50.5% women) were available for the assessment of water and fluid intake and 1192 participants for the assessment of hydration status after excluding missing values or implausible data (Additional file 1 : Fig. S1). Table 1 presents the baseline characteristics of the study population according to sex, water (tap and bottle) intake amount, and hydration status. The median (range) consumption of drinking water intake in men and women was 900 (0 to 3100) and 900 (0 to 2700) mL/day, respectively. Compared with participants in the group with the lowest drinking water intake (< 500 mL/day), those with the highest drinking water intake (1.8 to 3.1 L/day) were more likely to be younger ( p < 0.001), have a higher BMI ( p = 0.001), and have a lower alcohol intake ( p < 0.001). Compared to participants considered to be hydrated according to serum osmolarity status, participants considered to be dehydrated tended to be older ( p = 0.008), women ( p = 0.008), have type 2 diabetes ( p < 0.001), have depressive symptoms ( p = 0.025), and less likely to drink alcohol ( p = 0.002). Participants involved in the present analyses did not differ from the rest of the participants enrolled in the PREDIMED-Plus trial in terms of age, sex, BMI, and prevalence of obesity and type 2 diabetes ( p > 0.05 for all comparisons). Furthermore, 80% of participants (69% of men and 90% of women) met the EFSA fluid intake recommendations based on questionnaire responses, yet serum osmolarity values indicated over 50% of participants were physiologically dehydrated with only 10% of participants being considered physiologically hydrated based on serum osmolarity levels.
Figures 1 and 2 summarize the multivariable-adjusted β -coefficients (95% CIs) of water intake and hydration status categorically and continuously, respectively, with 2-year changes in GCF z -scores (full details from all models are presented in Additional file 1 : Tables S2-S4). Categorical analyses showed a non-significant trend towards participants considered to have a dehydrated status ( β : − 0.11; 95% CI: − 0.24 to 0.02; p for trend = 0.058) to have a greater decline in global cognitive function compared to those who were considered hydrated (Fig. 1 ). No significant associations were observed between the various classifications of water intake (i.e., drinking water, all fluids, water from beverage and food sources, and based on ESFA water and fluid recommendations) and 2-year changes in GCF in the multivariable-adjusted models. The results of the continuous linear regression analyses suggest the presence of a significant association between hydration status and global cognitive decline over a 2-year period ( β : − 0.10; 95% CI: − 0.02 to − 0.004; p = 0.002) (Fig. 2 ).
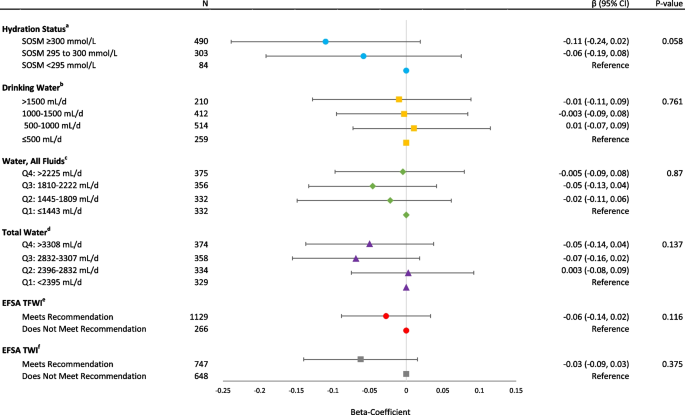
Hydration status, water and fluid intakes categorically with 2-year changes in global cognitive function ( z -scores). Data are presented as beta-coefficients and 95% CI. Multivariable linear regression models were adjusted for baseline covariates, including baseline GCF score, age (years), sex, intervention PREDIMED-Plus randomized group, and participating center (for hydration status: ≤ 100, 100 to < 150, 150 to < 200, > 200 participants; for fluid-related exposures: ≤ 100, 100 to < 200, 200 to < 300, > 300 participants), body mass index (kg/m 2 ), educational level (primary, secondary, or college), civil status (single, divorced or separated, married, widower), smoking habit (current, former, or never), physical activity (METs/min/day), sleep status (hours per day), depressive symptomatology (yes/no), diabetes prevalence (yes/no), hypertension (yes/no), hypercholesterolemia (yes/no), energy intake (kcal/day), alcohol consumption in g/day (and adding the quadratic term), and caffeine intake (mg/day). a Hydration status refers to serum osmolarity, where dehydration, impending dehydration, and hydrated statuses were defined as SOSM > 300, 295–300, and < 295 mmol/L, respectively. b Drinking water refers to tap and bottled water intakes. c Water, all fluids refers to tap and bottled water, plus water from other beverages and fluid food sources, such as soups and smoothies. d Total water refers to water from all fluids in addition to water present in food sources. e EFSA TFWI refers to the recommended levels of total fluid water intake for older adults at 2.0 L/day and 1.6 L/day for men and women, respectively. f EFSA TWI refers to the recommended levels of total water intake, from fluids and foods, for older adults at 2.5 L/day and 2.0 L/day for men and women, respectively. Abbreviations : EFSA, European Food Safety Authority; TFWI, total fluid water intake; TWI, total water intake; SOSM, serum osmolarity
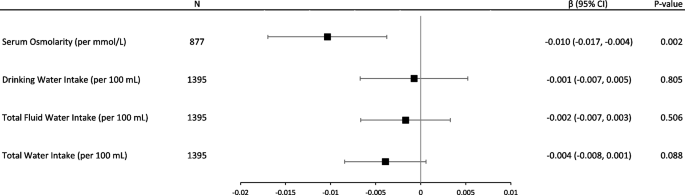
Hydration status, water and fluid intakes continuously with 2-year changes in global cognitive function (z-scores). Data are presented as beta-coefficients and 95% CI. Multivariable linear regression models were adjusted for baseline covariates, including baseline GCF score, age (years), sex, intervention PREDIMED-Plus randomized group, and participating center (≤ 100, 100 to < 150, 150 to < 200, > 200 participants), body mass index (kg/m 2 ), educational level (primary, secondary, or college), civil status (single, divorced or separated, married, widower), smoking habit (current, former, or never), physical activity (METs/min/day), sleep status (hours per day), depressive symptomatology (yes/no), diabetes prevalence (yes/no), hypertension (yes/no), hypercholesterolemia (yes/no), energy intake (kcal/day), alcohol consumption in grams/day (and adding the quadratic term), and caffeine intake (mg/day). Beta represents the changes in global cognitive function, expressed as z -scores, with each hydration or fluid intake component continuously. Bonferroni correction analyses have been run to correct for multiple comparisons and indicate a p < 0.005 is statistically significant
Additional file 1 : Tables S2-S4 show the unadjusted and multivariable-adjusted β -coefficients (95% CIs) of water and fluid intake (from both beverage and food sources, assessed individually and combined), as well as hydration status with changes in the global and individual assessments of cognitive function over a 2-year period. When each neuropsychological test was investigated separately, participants with the highest category of intake of drinking water (1.0 to 1.5 L/day) non-statistically significantly presented with a 0.17-point increase ( β : 0.17; 95% CI: 0.02 to 0.32; p for trend = 0.021) in DST-f z -score compared to those with the lowest water intake (< 0.5 L/day) over a 2-year period (Additional file 1 : Table S2). Total fluid intake showed similar findings where participants in the highest category of intake of total fluid water (2.5 L, range 2.2 to 3.4 L/day) presented with a 0.12-point increase ( β : 0.12; 95% CI: 0 to 0.24; p for trend = 0.041) in DST-f z -score compared to those with the lowest water intake (1.1 L, range 0.4 to 1.4 L/day) over a 2-year period (Additional file 1 : Table S2). No other associations in the multivariable-adjusted categorical or continuous analyses were observed. Furthermore, post hoc analyses of available data showed no significant differences in changes over time in the various fluid intake assessment variables (over a 2-year duration) or hydration status (over a 1-year duration) ( p > 0.005) among the participants.
Stratified analyses
When the analyses were stratified by sex, no changes in significance were observed with the associations between water and fluid intakes, in either categorical or continuous investigations, and global cognitive function. However, when hydration-related analyses were restricted to men or women participants, findings were attenuated when women only were assessed both categorically and continuously ( p > 0.005), whereas in men those with a SOSM ≥ 300 mmol/L, indicating a dehydrated status, showed a higher cognitive decline over the 2-year period compared to those hydrated ( β : − 0.20, 95% CI: − 0.36 to − 0.04, p = 0.012 and p -trend = 0.002), and the significance remained in the continuous analyses ( β : − 0.013, 95% CI: − 0.022 to − 0.004, p = 0.004) (Additional file 1 : Figs. S2-S3).
Sensitivity analyses
Regarding the sensitivity analyses, associations additionally adjusted for eGFR did not significantly modify the findings (Additional file 1 : Tables S5-S7). Moreover, associations additionally adjusted for intake of dietary variables, where applicable, including the amount (g/day) of vegetables, fruits, legumes, grains, non-fluid dairy, meat, oils, fish, nuts, and pastries did not significantly modify the findings (Additional file 1 : Tables S5-S7). Furthermore, the main results did not substantially change after the removal of extreme GCF baseline z -scores (< 5% and > 95%). Whereas following the removal of participants with a baseline MMSE score < 24 ( n = 51), the categorical association of hydration status with GCF became greater showing a more dehydrated status related to greater 2-year global cognitive decline compared to hydrated participants ( p -trend = 0.046), and the association of the continuous variables remained significant ( p = 0.002). The findings related to water and fluid intake did not substantially change with the removal of participants with a baseline MMSE score < 24 (Additional file 1 : Tables S5-S7).
To the best of our knowledge, this is the first multi-year prospective cohort study to assess the association between water intake (from fluid and food sources) and hydration status, with subsequent changes in cognitive performance in older Spanish adults with metabolic syndrome and overweight or obesity. In this large sample of older Spanish adults, poorer hydration status was associated with greater global cognitive decline over a 2-year period, particularly in men, whereas water intake, from fluid and/or food sources, and meeting related EFSA recommendations, was not associated with global cognitive function. Nonetheless, when results of cognitive function tests were considered independently, water intake (> 1.5 L/day compared to < 0.5 L/day from tap or bottled water and 2.2 to 4.4 L/day compared to < 1.4 L/day of water from all fluid sources) was related to better attention and short-term memory, as assessed by DST-f, over a 2-year period.
Despite the general acknowledgment that an appropriate level of fluid intake and hydration status is important for health, there have been limited investigations to date assessing the relationship between fluid intake or hydration status and cognitive function. Existing evidence suggests that good hydration status may be associated with better cognitive test results and that mild, induced dehydration can impair cognitive abilities [ 75 ], but findings are not consistent and there are only a few studies exploring the relationship of hydration status and hardly any assessing amount of water intake, with cognitive performance in older community-dwelling adults.
Of the few relevant and recent studies that have been conducted, one cross-sectional analysis of 2506 community-dwelling older American adults (aged ≥ 60 years) from the Nutrition and Health Examination Survey (NHANES) 2011–2014 cycles assessed both hydration status and water intake in relation to cognitive performance [ 34 ]. In comparison with the present findings, this study found women (but not men) considered to be hydrated, based on a SOSM level between 285 and 289 mmol/L, had better attention and processing speeds, based on a Digit Symbol Substitution test (DSST), than women not at optimal hydration [ 34 ]. These cross-sectional findings differ from the present observations where global cognitive function, but not individual tests related to attention and processing, was associated with hydration status. Whereas, correspondingly, water intake (but not hydration status) was positively associated with DST-f, which is similar to the DSST in that it is an indicator of attention as well as short-term memory capacity, and this was seen across all older adults (both men and women). Additionally, in the NHANES study, cognitive test scores were significantly lower among adults who failed to meet EFSA recommendations on adequate intake (AI) of water in bivariate analyses, yet this significance was attenuated in the multivariable analyses among both women and men. Yet, using the alternative AI of daily water intake of 1500 mL or more, which is comparable to the highest drinking water intake group in the present study, women scored higher on the Animal Fluency Test, a measure of verbal fluency and hence executive function, and DSST than women with intake levels below this amount, and findings among men trended in the same direction [ 34 ].
Similarly, hydration status has been associated with cognitive function in two cross-sectional studies of older community-dwelling adults by Suhr and colleagues [ 32 , 33 ]. First, Suhr et al. showed that in 28 healthy community-dwelling older adults (aged 50 to 82 years), a lower hydration status, determined in this study via total body water measured using the bioelectrical impedance method, was related to a decreased psychomotor processing speed, poorer attention, and memory [ 33 ]. A second cross-sectional study by Suhr et al. [ 32 ] conducted in 21 postmenopausal women (aged 50 to 78 years) reported a positive association between total body water, also measured by the bioelectrical impedance method, and working memory or memory skills.
Moreover, the possible relation between hydration and cognitive function shown by the present findings and demonstrated by the abovementioned cross-sectional studies in older adults align with acute heat- and exercise-induced dehydration studies in younger populations, as illustrated by a meta-analysis of 33 trials (pre-post or crossover design) of 413 adults free of disease (aged < 45 years). It should be noted that these studies assessed dehydration over a period of less than 72 h with 27 (81%) of the studies including only men participants, and 25 (76%) studies involving recreationally or highly athletic individuals. The authors concluded that despite variability among the included studies, dehydration impaired cognitive performance, particularly for tasks involving attention, executive function, and motor coordination when water deficits exceeded 2% body mass loss [ 76 ].
Conversely, a cross-sectional study conducted in Poland among 60 community-dwelling older adults (aged 60 to 93 years) found no significant relationship between cognitive performance, as assessed using the MMSE, TMT, and the Babcock Story Recall Test, and hydration status as assessed by urine specific gravity [ 31 ]. The discrepancy between the findings from this cross-sectional study and the present PREDIMED-Plus analyses might be because all participants in the cross-sectional study from Poland were considered to be adequately hydrated and hence the authors of that study could not assess the impact of a dehydrated state on cognition.
A noteworthy consideration when interpreting the literature and the main findings of the current study for practical use and in the determination of potential mechanisms of action is the distinction between water intake and water balance (related to hydration status) within the body. When homeostasis of fluids within the body is disrupted, modifying water intake may impact cognitive function, yet due to the dynamic complexity of body water regulation impacting hydration status may be dependent on individualized physiological water intake needs [ 8 ]. Thus, while the biological mechanism by which water intake and a hydrated status may reduce the risk of cognitive decline is unclear, evidence suggests that aspects related to hydration and fluid homeostasis or a lack thereof, such as hormone regulation and changes in brain structure, could be a key underlying factor.
Several mechanisms regulate water intake and output to maintain serum osmolarity, and hence hydration status, within a narrow range. Elevated blood osmolarity resulting in the secretion of antidiuretic hormone (ADH), also known as vasopressin or arginine vasopressin, a peptide hormone which acts primarily in the kidneys to increase water reabsorption, is one such mechanism that works to return osmolarity to baseline and preserve fluid balance [ 77 ]. In addition to its role in mediating the physiological functions related to water reabsorption and homeostasis, evidence has suggested that ADH participates in cognitive functioning [ 78 ] and that the associated cognitive modulations may further interplay with sex hormones [ 79 ]. Antidiuretic hormone may be influenced by the androgen sex hormone, which is generally more abundant in the brains of males than in females [ 80 ]. As a result, the impact of ADH on cognition could be greater in males [ 80 ].
Exercise- and heat-induced acute dehydration studies implicate possible modifications to the brain structure as another potential mechanism of action for an association between water intake, hydration status, and cognitive function. Evidence has proposed that acute dehydration can lead to a reduction in brain volume and subtle regional changes in brain morphology such as ventricular expansion, effects that may be reversed following acute rehydration [ 81 , 82 ]. Acute dehydration studies have further implicated hydration status in affecting cerebral hemodynamics and metabolism resulting in declines in cerebral blood flow and oxygen supply [ 83 , 84 ]. A lower vascular and neuronal oxygenation could potentially compromise the cerebral metabolic rate for oxygen, thereby contributing to reductions in cognitive performance [ 81 , 85 , 86 , 87 , 88 ]. Nonetheless, other potential unknown mechanisms cannot be disregarded.
There are several limitations and strengths of the present analyses that need to be acknowledged. The first notable limitation is that the results may not be generalizable to other populations since the participants are older Spanish individuals with metabolic syndrome and overweight or obesity. Second, measurement error and recall bias are possibilities given the use of questionnaires to estimate water and fluid intake and that these rely on responders’ memory which is a component of cognitive function. However, these questionnaires have been validated and determined as reliable methods of assessing long-term intake in the present study population [ 37 , 38 ]. Third, despite its longitudinal design, water and fluid intake and hydration status were only considered at baseline; however, as the questionnaires measure habitual beverage and food intake, and older adults are considered to have reasonably stable dietary habits [ 37 , 38 ], this is not expected to significantly impact the findings. Along these lines, the possible effect of seasonality on water intake and osmolarity was not considered a concern in the present analyses as the validation of the fluid questionnaire measurements included assessments at various points throughout the year (baseline vs. 6 months vs 1 year) with no significant differences observed in fluid consumption across the different time points assessed [ 10 ]. Hence, the finding of no difference between 6-month intervals, suggests no significant differences between opposing seasons (e.g., winter vs. summer; spring vs. fall). Furthermore, SOSM determination may not necessarily detect acute dehydration or rehydration immediately prior to the cognitive testing, and it is unknown whether observed elevated SOSMs were due to inadequate water intake, ADH abnormality, or other factors. While it is possible that the hydration status of some individuals was misclassified because serum osmolarity was estimated as opposed to being directly measured, the equation has been shown to predict directly measured serum osmolarity well in older adult men and women with and without diabetes or renal issues with a good diagnostic accuracy of dehydration and has been considered a gold standard for the identification of impending and current water-loss dehydration in older adults [ 44 , 45 , 89 , 90 , 91 ]. Lastly, a discrepancy was observed between the percentage of individuals that were considered to have met EFSA fluid intake recommendations and those considered to be dehydrated based on calculated osmolarity. This may have been due to the fact that the EFSA fluid intake recommendations are meant for individuals in good health [ 20 ]; whereas the present study population had overweight or obesity, and it has been shown that individuals with higher BMIs have higher water needs related to metabolic rate, body surface area, body weight, and water turnover rates related to higher energy requirements, greater food consumption, and higher metabolic production [ 92 ]. Strengths of the present analyses include the longitudinal, prospective design, the large sample size, the use of an extensive cognitive test battery, the use of validated questionnaires, and the robustness of the current findings due to the adjustment of relevant covariates.
Findings suggest that hydration status, specifically poorer hydration status, may be associated with a greater decline in global cognitive function in older adults with metabolic syndrome and overweight or obesity, particularly in men. Further prospective cohort studies and randomized clinical trials are required to confirm these results and to better understand the link between water and fluid intake, hydration status, and changes in cognitive performance to provide guidance for guidelines and public health.
Availability of data and materials
The dataset supporting the conclusions of this article is available upon request pending application and approval of the PREDIMED-Plus Steering Committee. There are restrictions on the availability of data for the PREDIMED-Plus trial, due to the signed consent agreements around data sharing, which only allow access to external researchers for studies following the project purposes. Requestors wishing to access the PREDIMED-Plus trial data used in this study can make a request to the PREDIMED-Plus trial Steering Committee chair: [email protected] . The request will then be passed to members of the PREDIMED-Plus Steering Committee for deliberation.
Abbreviations
Antidiuretic hormone
Adequate intake
Beck Depression Inventory-II
Base de Datos Española de Composición de Alimentos
Beverage Intake Assessment Questionnaire
Body mass index
El Centro de Enseñanza Superior de Nutrición y Dietética
Clock Drawing Test
Confidence interval
Dietary reference value
Digit Symbol Substitution Test
DST Backward Recall
DST Forward Recall
European Food Safety Authority
Estimated glomerular filtration rate
Food Frequency Questionnaire
Global cognitive function
Institute of Medicine
International Standard Randomized Controlled Trial Registry
Mediterranean diet
Metabolic equivalents
Mini-Mental State Examination
Nutrition and Health Examination Survey
PREvención con DIeta MEDiterránea
- Serum osmolarity
Total fluid water intake
Trail Making Test Section A
Trail Making Test Section B
Total water intake
Verbal Fluency Test Animals Category
Verbal Fluency Test Letter “p” Category
Wechsler Adult Intelligence Scale-III
Gauthier S, Rosa-Neto P, Morais JA, Webster C. World Alzheimer Report 2021: Journey through the diagnosis of dementia. London: Alzheimer´s Disease International; 2021.
Petersen RC, Lopez O, Armstrong MJ, Getchius TSD, Ganguli M, Gloss D, et al. Practice guideline update summary: mild cognitive impairment report of the guideline development, dissemination, and implementation. Neurology. 2018;90:126–35.
Article PubMed PubMed Central Google Scholar
Scarmeas N, Anastasiou CA, Yannakoulia M. Nutrition and prevention of cognitive impairment. Lancet Neurol. 2018;17:1006–15.
Article PubMed Google Scholar
Johnson EC, Adams WM. Water intake, body water regulation and health. Nutrients. 2020;12:702.
Lorenzo I, Serra-Prat M, Carlos YJ. The role of water homeostasis in muscle function and frailty: a review. Nutrients. 2019;11:1857–72.
Article CAS PubMed PubMed Central Google Scholar
Jéquier E, Constant F. Water as an essential nutrient: the physiological basis of hydration. Eur J Clin Nutr. 2010;64:115–23.
Kleiner SM. Water. J Am Diet Assoc. 1999;99:200–6.
Article CAS PubMed Google Scholar
Armstrong L, Johnson E. Water intake, water balance, and the elusive daily water requirement. Nutrients. 2018;10:1928.
Nissensohn M, Sánchez-Villegas A, Ortega RM, Aranceta-Bartrina J, Gil Á, González-Gross M, et al. Beverage consumption habits and association with total water and energy intakes in the Spanish population: findings of the ANIBES study. Nutrients. 2016;8:232–50.
Ferreira-Pêgo C, Nissensohn M, Kavouras SA, Babio N, Serra-Majem L, Águila AM, et al. Relative validity and repeatability in a Spanish population with metabolic syndrome from the PREDIMED-PLUS study. Nutrients. 2016;8:457–70.
Article Google Scholar
Nissensohn M, Sánchez-Villegas A, Galan P, Turrini A, Arnault N, Mistura L, et al. Beverage consumption habits among the European population: association with total water and energy intakes. Nutrients. 2017;9:383–96.
Rolls BJ, Phillips PA. Aging and disturbances of thirst and fluid balance. Nutr Rev. 2009;48:137–44.
Kenney WL, Tankersley CG, Newswanger DL, Hyde DE, Puhl SM, Turner NL. Age and hypohydration independently influence the peripheral vascular response to heat stress. J Appl Physiol. 1990;68:1902–8.
Begg DP. Disturbances of thirst and fluid balance associated with aging. Physiol Behav. 2017;178:28–34.
Hooper L, Bunn D, Jimoh FO, Fairweather-Tait SJ. Water-loss dehydration and aging. Mech Ageing Dev. 2014;136–137:50–8.
Lieberman HR. Hydration and cognition: a critical review and recommendations for future research. J Am Coll Nutr. 2007;26:555S–61S.
Murray B. Hydration and physical performance. J Am Coll Nutr. 2007;26:542S–8S.
AESAN Scienitific Committee, Alfredo Martínez Hernández J, Cámara Hurtado M, Maria Giner Pons R, González Fandos E, López García E, et al. Informe del Comité Científico de la Agencia Española de Seguridad Alimentaria y Nutrición (AESAN) de revisión y actualización de las Recomen-daciones Dietéticas Para la población española. Revista del Comité Científico de la AESAN. 2020;32:11–58.
Google Scholar
U.S. Department of Agriculture, U.S. Department of Health and Human Services. Dietary guidelines for Americans, 2020-2025. 9th ed; 2020.
EFSA Panel on Dietetic Products Nutrition and Allergies (NDA). Scientific opinion on dietary reference values for water. EFSA J. 2010;8:1459–507.
Institute of Medicine. Dietary reference intakes for water, potassium, sodium, chloride, and sulfate. Washington, D.C.: National Academies Press; 2005.
Gandy J. Water intake: validity of population assessment and recommendations. Eur J Nutr. 2015;54 Suppl 2:S11–6.
Risk reduction of cognitive decline and dementia: WHO guidelines. Geneva: World Health Organization; 2019. Licence: CC BY-NC-SA 3.0 IGO.
Volkert D, Chourdakis M, Faxen-Irving G, Frühwald T, Landi F, Suominen MH, et al. ESPEN guidelines on nutrition in dementia. Clin Nutr. 2015;34:1052–73.
National Institute for Health and Care Excellence (NICE). Dementia, disability and frailty in later life-mid-life approaches to delay or prevent onset. NICE guideline; 2015. p. 1–56. www.nice.org.uk/guidance/ng16
Armstrong-Esther CA, Browne KD, Armstrong-Esther DC, Sander L. The institutionalized elderly: dry to the bone! Int J Nurs Stud. 1996;33:619–28.
Marra MV, Simmons SF, Shotwell MS, Hudson A, Hollingsworth EK, Long E, et al. Elevated serum osmolality and total water deficit indicate impaired hydration status in residents of long-term care facilities regardless of low or high body mass index. J Acad Nutr Diet. 2016;116:828–36.
Hooper L, Bunn DK, Downing A, Jimoh FO, Groves J, Free C, et al. Which frail older people are dehydrated? The UK DRIE study. J Gerontol Ser A Biol Sci Med Sci. 2016;71:1341–7.
Lauriola M, Mangiacotti A, D’Onofrio G, Cascavilla L, Paris F, Paroni G, et al. Neurocognitive disorders and dehydration in older patients: clinical experience supports the hydromolecular hypothesis of dementia. Nutrients. 2018;10:562–76.
Majdi M, Hosseini F, Naghshi S, Djafarian K, Shab-Bidar S. Total and drinking water intake and risk of all-cause and cardiovascular mortality: a systematic review and dose-response meta-analysis of prospective cohort studies. Int J Clin Pract. 2021;75:e14878.
Bialecka-Dębek A, Pietruszka B. The association between hydration status and cognitive function among free-living elderly volunteers. Aging Clin Exp Res. 2019;31:695–703.
Suhr JA, Patterson SM, Austin AW, Heffner KL. The relation of hydration status to declarative memory and working memory in older adults. J Nutr Health Aging. 2010;14:840–3.
Suhr JA, Hall J, Patterson SM, Niinistö RT. The relation of hydration status to cognitive performance in healthy older adults. Int J Psychophysiol. 2004;53:121–5.
Bethancourt HJ, Kenney WL, Almeida DM, Rosinger AY. Cognitive performance in relation to hydration status and water intake among older adults, NHANES 2011–2014. Eur J Nutr. 2020;59:3133–48.
Martínez-González MA, Buil-Cosiales P, Corella D, Bulló M, Fitó M, Vioque J, et al. Cohort profile: design and methods of the PREDIMED-plus randomized trial. Int J Epidemiol. 2019;48:387–388o.
Salas-Salvadó J, Díaz-López A, Ruiz-Canela M, Basora J, Fitó M, Corella D, et al. Effect of a lifestyle intervention program with energy-restricted Mediterranean diet and exercise on weight loss and cardiovascular risk factors: one-year results of the PREDIMED-plus trial. Diabetes Care. 2019;42:777–88.
Willett W. Nutritional epidemiology, 3rd edn. Chapter 11. 3rd ed. New York: Oxford University Press; 2013. p. 306.
Fernández-Ballart JD, Piñol JL, Zazpe I, Corella D, Carrasco P, Toledo E, et al. Relative validity of a semi-quantitative food-frequency questionnaire in an elderly Mediterranean population of Spain. Br J Nutr. 2010;103:1808–16.
Palma I, Farra A, Cantós D. Tablas de composición de alimentos por medidas caseras de consumo habitual en España. Madrid: S.A. McGraw-Hill/Interamericana de España; 2008.
Book Google Scholar
RedBEDCA, AESAN. Base de Datos Española de Composición de Alimentos; 2006.
Grupo Colaborativo de la Sociedad Española de Nutrición Comunitaria (SENC), Aranceta Bartrina J, Arija Val V, Maíz Aldalur E, Martínez de la Victoria Muñoz E, Ortega Anta RM, et al. Dietary guidelines for the Spanish population (SENC, December 2016); the new graphic icon of healthy nutrition. Nutr Hosp. 2016;33(Suppl 8):1–48.
Moreiras O, Carbajal A, Cabrera L, Cuadrado C. Tablas de composición de alimentos Guía de prácticas [Food Composition Tables. Practice Guide]. 19th ed. Madrid: Piramide; 2018. p. 496.
Mataix Verdú J, García Diz L, Mañas Almendros M, Martinez de Vitoria E, Llopis González J. Tablas de composición de alimentos [Food composition tables]. 5th ed. Granada; Universidad de Granada; 2013.
Hooper L, Abdelhamid A, Attreed NJ, Campbell WW, Channell AM, Chassagne P, et al. Clinical symptoms, signs and tests for identification of impending and current water-loss dehydration in older people. Cochrane Database Syst Rev. 2015. https://doi.org/10.1002/14651858.CD009647.pub2 .
Khajuria A, Krahn J. Osmolality revisited - deriving and validating the best formula for calculated osmolality. Clin Biochem. 2005;38:514–9.
Hooper L, Bunn DK, Abdelhamid A, Gillings R, Jennings A, Maas K, et al. Water-loss (intracellular) dehydration assessed using urinary tests: how well do they work? Diagnostic accuracy in older people. Am J Clin Nutr. 2016;104:121–31.
Blesa R, Pujol M, Aguilar M, Santacruz P, Bertran-Serra I, Hernández G, et al. Clinical validity of the “mini-mental state” for Spanish speaking communities. Neuropsychologia. 2001;39:1150–7.
Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12:189–98.
Benton AL, Hamsher K, Sivan AB. Multilingual aphasia examination. 3rd ed. Iowa: AJA Associates; 1994.
Wechsler D. Wechsler adult intelligence scale-III. San Antonio: Psychological Corporation; 1997.
Rossi L, Neer C-R, Lopetegui S. Escala de inteligencia Para adultos de WECHSLER. WAIS-III Índice de comprensión verbal. Normas Para los subtests: Vocabulario, analogías e información, Para la ciudad de La Plata Edades: 16 a 24 AñosRevista de Psicología; 2008. p. 223–36.
Aprahamian I, Martinelli JE, Neri AL, Yassuda MS. O Teste do Desenho do Relógio: Revisão da acurácia no rastreamento de demência. Dementia e Neuropsychologia. 2009;3:74–80.
Paganini-Hill A, Clark LJ. Longitudinal assessment of cognitive function by clock drawing in older adults. Dement Geriatr Cogn Dis Extra. 2011;1:75–83.
Del Ser QT, García De Yébenes MJ, Sánchez Sánchez F, Frades Payo B, Rodríguez Laso Á, Bartolomé Martínez MP, et al. Evaluación cognitiva del anciano. Datos normativos de una muestra poblacional española de más de 70 años. Med Clin (Barc). 2004;122:727–40.
Llinàs-Reglà J, Vilalta-Franch J, López-Pousa S, Calvó-Perxas L, Torrents Rodas D, Garre-Olmo J. The trail making test: association with other neuropsychological measures and normative values for adults aged 55 years and older from a Spanish-speaking population-based sample. Assessment. 2017;24:183–96.
Brouwer-Brolsma EM, Dhonukshe-Rutten RAM, van Wijngaarden JP, van de Zwaluw NL, In’t Veld PH, Wins S, et al. Cognitive performance: a cross-sectional study on serum vitamin D and its interplay with glucose homeostasis in Dutch older adults. J Am Med Dir Assoc. 2015;16:621–7.
Paz-Graniel I, Babio N, Becerra-Tomás N, Toledo E, Camacho-Barcia L, Corella D, et al. Association between coffee consumption and total dietary caffeine intake with cognitive functioning: cross-sectional assessment in an elderly Mediterranean population. Eur J Nutr. 2021;60:2381–96.
Molina L, Sarmiento M, Peñafiel J, Donaire D, Garcia-Aymerich J, Gomez M, et al. Validation of the REGICOR short physical activity questionnaire for the adult population. PLoS One. 2017;12:e0168148.
Paz-Graniel I, Babio N, Serra-Majem L, Vioque J, Zomeño MD, Corella D, et al. Fluid and total water intake in a senior Mediterranean population at high cardiovascular risk: demographic and lifestyle determinants in the PREDIMED-plus study. Eur J Nutr. 2020;59:1595–606.
Drewnowski A, Rehm CD, Constant F. Water and beverage consumption among adults in the United States: cross-sectional study using data from NHANES 2005–2010. BMC Public Health. 2013;13:1068.
Polhuis KCMM, Wijnen AHC, Sierksma A, Calame W, Tieland M. The diuretic action of weak and strong alcoholic beverages in elderly men: a randomized diet-controlled crossover trial. Nutrients. 2017;9:660–73.
Xu W, Wang H, Wan Y, Tan C, Li J, Tan L, et al. Alcohol consumption and dementia risk: a dose–response meta-analysis of prospective studies. Eur J Epidemiol. 2017;32:31–42.
Zhang Y, Coca A, Casa DJ, Antonio J, Green JM, Bishop PA. Caffeine and diuresis during rest and exercise: a meta-analysis. J Sci Med Sport. 2015;18:569–74.
van den Berg B, de Jong M, Woldorff MG, Lorist MM. Caffeine boosts preparatory attention for reward-related stimulus information. J Cogn Neurosci. 2020;33:104–18.
Chen JQA, Scheltens P, Groot C, Ossenkoppele R. Associations between caffeine consumption, cognitive decline, and dementia: a systematic review. J Alzheimers Dis. 2020;78:1519–46.
Xu W, Tan CC, Zou JJ, Cao XP, Tan L. Sleep problems and risk of all-cause cognitive decline or dementia: an updated systematic review and meta-analysis. J Neurol Neurosurg Psychiatry. 2020;91:236–44.
Rosinger AY, Lawman HG, Akinbami LJ, Ogden CL. The role of obesity in the relation between total water intake and urine osmolality in US adults, 2009-2012. Am J Clin Nutr. 2016;104:1554–61.
You Y, Liu Z, Chen Y, Xu Y, Qin J, Guo S, et al. The prevalence of mild cognitive impairment in type 2 diabetes mellitus patients: a systematic review and meta-analysis. Acta Diabetol. 2021;58:671–85.
Iadecola C, Gottesman RF. Neurovascular and cognitive dysfunction in hypertension: epidemiology, pathobiology, and treatment. Circ Res. 2019;124:1025–44.
An Y, Zhang X, Wang Y, Wang Y, Liu W, Wang T, et al. Longitudinal and nonlinear relations of dietary and serum cholesterol in midlife with cognitive decline: results from EMCOA study. Mol Neurodegener. 2019;14:51–70.
John A, Patel U, Rusted J, Richards M, Gaysina D. Affective problems and decline in cognitive state in older adults: a systematic review and meta-analysis. Psychol Med. 2019;49:353–65.
Sanz J, Luis A, Carmelo P, Resumen V. Adaptación española del Inventario Para la Depresión de Beck-II (BDI-II): 2. Propiedades psicométricas en población general the Spanish adaptation of Beck’s depression inventory-II (BDI-II): 2. Psychometric properties in the general population. Clínica y Salud. 2003;14:249–80.
National Institute of Diabetes and Digestive and Kidney Diseases. Estimating glomerular filtration rate. https://www.niddk.nih.gov/health-information/professionals/clinical-tools-patient-management/kidney-disease/laboratory-evaluation/glomerular-filtration-rate/estimating . Accessed 1 Jun 2022.
Dementia Care Central, National Institute on Aging. Mini-Mental State Exam (MMSE) Alzheimer’s/Dementia Test: administration, accuracy and scoring. 2020. https://www.dementiacarecentral.com/mini-mental-state-exam/ . Accessed 5 Jul 2021.
Masento NA, Golightly M, Field DT, Butler LT, van Reekum CM. Effects of hydration status on cognitive performance and mood. Br J Nutr. 2014;111:1841–52.
Wittbrodt MT, Millard-Stafford M. Dehydration impairs cognitive performance: a meta-analysis. Med Sci Sports Exerc. 2018;50:2360–8.
Kanbay M, Yilmaz S, Dincer N, Ortiz A, Sag AA, Covic A, et al. Antidiuretic hormone and serum osmolarity physiology and related outcomes: what is old, what is new, and what is unknown? J Clin Endocrinol Metab. 2019;104:5406–20.
Insel TR. Translating oxytocin neuroscience to the clinic: a National Institute of Mental Health perspective. Biol Psychiatry. 2016;79:153–4.
Lu Q, Lai J, Du Y, Huang T, Prukpitikul P, Xu Y, et al. Sexual dimorphism of oxytocin and vasopressin in social cognition and behavior. Psychol Res Behav Manag. 2019;12:337–49.
Bluthe R, Gheusi G, Dantzer R. Gonadal steroids influence the involvement of arginine vasopressin in social recognition in mice. Psychoneuroendocrinology. 1993;18:323–35.
Kempton MJ, Ettinger U, Schmechtlg A, Winter EM, Smith L, McMorris T, et al. Effects of acute dehydration on brain morphology in healthy humans. Hum Brain Mapp. 2009;30:291–8.
Duning T, Kloska S, Steinstrater O, Kugel H, Heindel W, Knecht S. Dehydration confounds the assessment of brain atrophy. Neurology. 2005;64:548–50.
Trangmar S, Chiesa S, Kalsi K, Secher N, González-Alonso J. Hydration and the human brain circulation and metabolism. Nutr Hosp. 2015;1:10261.
Rasmussen P, Nybo L, Volianitis S, Møller K, Secher NH, Gjedde A. Cerebral oxygenation is reduced during hyperthermic exercise in humans. Acta Physiol. 2010;199:63–70.
Article CAS Google Scholar
Piil JF, Lundbye-Jensen J, Trangmar SJ, Nybo L. Performance in complex motor tasks deteriorates in hyperthermic humans. Temperature. 2017;4:420–8.
Ogoh S. Relationship between cognitive function and regulation of cerebral blood flow. J Physiol Sci. 2017;67:345–51.
Ogoh S, Tsukamoto H, Hirasawa A, Hasegawa H, Hirose N, Hashimoto T. The effect of changes in cerebral blood flow on cognitive function during exercise. Physiol Rep. 2014;2(9):e12163.
Claassen JAHR, Thijssen DHJ, Panerai RB, Faraci FM. Regulation of cerebral blood flow in humans: physiology and clinical implications of autoregulation. Physiol Rev. 2021;101:1487–559.
Hooper L, Abdelhamid A, Ali A, Bunn DK, Jennings A, John WG, et al. Diagnostic accuracy of calculated serum osmolarity to predict dehydration in older people: adding value to pathology laboratory reports. BMJ Open. 2015;5:e008846.
Lacey J, Corbett J, Forni L, Hooper L, Hughes F, Minto G, et al. A multidisciplinary consensus on dehydration: definitions, diagnostic methods and clinical implications. Ann Med. 2019;51:232–51.
Siervo M, Bunn D, Prado CM, Hooper L. Accuracy of prediction equations for serum osmolarity in frail older people with and without diabetes. Am J Clin Nutr. 2014;100:867–76.
Chang T, Ravi N, Plegue MA, Sonneville KR, Davis MM. Inadequate hydration, BMI, and obesity among US adults: NHANES 2009-2012. Ann Fam Med. 2016;14:320–4.
Download references
Acknowledgements
We thank all PREDIMED-Plus participants and investigators. CIBEROBN, CIBERESP, and CIBERDEM are initiatives of the Instituto de Salud Carlos III (ISCIII), Madrid, Spain. The Hojiblanca (Lucena, Spain) and Patrimonio Comunal Olivarero (Madrid, Spain) food companies donated extra virgin olive oil. The Almond Board of California (Modesto, CA), American Pistachio Growers (Fresno, CA), and Paramount Farms (Wonderful Company, LLC, Los Angeles, CA) donated nuts for the PREDIMED-Plus pilot study.
This work was supported by the official Spanish Institutions for funding scientific biomedical research, CIBER Fisiopatología de la Obesidad y Nutrición (CIBEROBN) and Instituto de Salud Carlos III (ISCIII), through the Fondo de Investigación para la Salud (FIS), which is co-funded by the European Regional Development Fund (six coordinated FIS projects leaded by JS-S and JVi, including the following projects: PI13/00673, PI13/00492, PI13/00272, PI13/01123, PI13/00462, PI13/00233, PI13/02184, PI13/00728, PI13/01090, PI13/01056, PI14/01722, PI14/00636, PI14/00618, PI14/00696, PI14/01206, PI14/01919, PI14/00853, PI14/01374, PI14/00972, PI14/00728, PI14/01471, PI16/00473, PI16/00662, PI16/01873, PI16/01094, PI16/00501, PI16/00533, PI16/00381, PI16/00366, PI16/01522, PI16/01120, PI17/00764, PI17/01183, PI17/00855, PI17/01347, PI17/00525, PI17/01827, PI17/00532, PI17/00215, PI17/01441, PI17/00508, PI17/01732, PI17/00926, PI19/00957, PI19/00386, PI19/00309, PI19/01032, PI19/00576, PI19/00017, PI19/01226, PI19/00781, PI19/01560, PI19/01332, PI20/01802, PI20/00138, PI20/01532, PI20/00456, PI20/00339, PI20/00557, PI20/00886, PI20/01158); the Especial Action Project entitled: Implementación y evaluación de una intervención intensiva sobre la actividad física Cohorte PREDIMED-Plus grant to JS-S; the European Research Council (Advanced Research Grant 2014–2019; agreement #340918) granted to MÁM-G.; the Recercaixa (number 2013ACUP00194) grant to JS-S; grants from the Consejería de Salud de la Junta de Andalucía (PI0458/2013, PS0358/2016, PI0137/2018); the PROMETEO 17/2017 and PROMETEO 21/2021 grant from the Generalitat Valenciana; the SEMERGEN grant; and the Juan de la Cierva-Incorporación research grant (IJC2019-042420-I) of the Spanish Ministry of Economy, Industry and Competitiveness and European Social Funds. This research was also partially funded by EU-H2020 Grants (Eat2beNICE/H2020-SFS-2016-2) and the Horizon 2020 PRIME study (Prevention and Remediation of Insulin Multimorbidity in Europe; grant agreement #847879). S.K.N. is supported by a postdoctoral fellowship from the Canadian Institutes of Health Research (CIHR, MFE-171207). CG-M is supported by a predoctoral grant from the University of Rovira I Virgili (2020PMF-PIPF-37). IP-G is supported by a predoctoral grant from Ministerio de Ciencia, Innovación y Universidades (FPU 17/01925). AA-S has received a post-doctoral grant (APOSTD/2020/164) from the Consellería de Innovación, Ciencia y Sociedad Digital, Generalitat Valenciana, Fondo Social Europeo-FSE. CB is supported by a Juan de la Cierva postdoctoral grant from Ministerio de Ciencia, Innovación y Universidades. JS-S, the senior author of this paper, was partially supported by ICREA under the ICREA Academia program. Publication of this work is supported by a conference award grant from Hydration for Health presented by the Danone Global Research & Innovation Center (#2022-08514) awarded to S.K.N. for being the conference attendee voted recipient of the Early Career Researcher Award. None of the funding sources took part in the design, collection, analysis, interpretation of the data; writing of the report; or the decision to submit the manuscript for publication.
Author information
Authors and affiliations.
Universitat Rovira i Virgili, Departament de Bioquímica i Biotecnologia, Unitat de Nutrició, Reus, Spain
Stephanie K. Nishi, Nancy Babio, Indira Paz-Graniel, Carlos Gómez-Martínez & Jordi Salas-Salvadó
Institut d’Investigació Sanitària Pere Virgili (IISPV), Reus, Spain
Centro de Investigación Biomédica en Red Fisiopatología de la Obesidad y la Nutrición (CIBEROBN), Institute of Health Carlos III, Madrid, Spain
Stephanie K. Nishi, Nancy Babio, Indira Paz-Graniel, Lluís Serra-Majem, Montserrat Fitó, Dolores Corella, Xavier Pintó, Josep A. Tur, J. Alfredo Martinez, Carlos Gómez-Martínez, Marta Fanlo-Maresma, Cristina Bouzas, Lidia Daimiel, María Ángeles Zulet, Zenaida Vázquez-Ruiz & Jordi Salas-Salvadó
Toronto 3D (Diet, Digestive Tract and Disease) Knowledge Synthesis and Clinical Trials Unit, Toronto, ON, Canada
Stephanie K. Nishi & John L. Sievenpiper
Clinical Nutrition and Risk Factor Modification Centre, St. Michael’s Hospital, Unity Health Toronto, Toronto, ON, Canada
Research Institute of Biomedical and Health Sciences (IUIBS), University of Las Palmas de Gran Canaria & Centro Hospitalario Universitario Insular Materno Infantil (CHUIMI), Canarian Health Service, Las Palmas de Gran Canaria, Spain
Lluís Serra-Majem & Cristina Montesdeoca-Mendoza
CIBER de Epidemiología y Salud Pública (CIBERESP), Instituto de Salud Carlos III (ISCIII), 28029, Madrid, Spain
Jesús Vioque, Aurora Bueno-Cavanillas & Naomi Cano-Ibáñez
Instituto de Investigación Sanitaria y Biomédica de Alicante. Universidad Miguel Hernández (ISABIAL-UMH), Alicante, Spain
Jesús Vioque
Unit of Cardiovascular Risk and Nutrition, Institut Hospital del Mar de Investigaciones Médicas Municipal d’Investigació Médica (IMIM), Barcelona, Spain
Montserrat Fitó & Olga Castañer
Department of Preventive Medicine, University of Valencia, Valencia, Spain
Dolores Corella & Andrea Alvarez-Sala
Lipids and Vascular Risk Unit, Internal Medicine, Hospital Universitario de Bellvitge-IDIBELL, Hospitalet de Llobregat, Barcelona, Spain
Xavier Pintó & Marta Fanlo-Maresma
School of Medicine, Universitat de Barcelona, 08907, Barcelona, Spain
Xavier Pintó
Department of Preventive Medicine and Public Health, University of Granada, Granada, Spain
Aurora Bueno-Cavanillas & Naomi Cano-Ibáñez
Research Group on Community Nutrition & Oxidative Stress, University of Balearic Islands, 07122, Palma de Mallorca, Spain
Josep A. Tur & Cristina Bouzas
Nutritional Control of the Epigenome Group, Precision Nutrition and Obesity Program, IMDEA Food, CEI UAM + CSIC, 28049, Madrid, Spain
Laura Diez-Ricote & Lidia Daimiel
Department of Nutrition, Food Sciences, and Physiology, Center for Nutrition Research, University of Navarra, IdiSNA, Pamplona, Spain
J. Alfredo Martinez & María Ángeles Zulet
Precision Nutrition and Cardiometabolic Health Program, IMDEA Food, CEI UAM + CSIC, Madrid, Spain
J. Alfredo Martinez
Centro de Salud Raval de Elche, Alicante, Spain
Andrés González-Botella
Instituto de Investigación Biosanitaria Granada, IBS-Granada, Granada, Spain
Naomi Cano-Ibáñez
Departamento de Ciencias Farmacéuticas y de la Salud, Facultad de Farmacia, Universidad San Pablo-CEU, CEU Universities, Urbanización Montepríncipe, Boadilla del Monte, 28660, Spain
Lidia Daimiel
Department of Nutritional Sciences, Faculty of Medicine, University of Toronto, Toronto, ON, Canada
John L. Sievenpiper
Department of Medicine, University of Toronto, Toronto, ON, Canada
Division of Endocrinology & Metabolism, St. Michael’s Hospital, Toronto, ON, Canada
Li Ka Shing Knowledge Institute, St. Michael’s Hospital, Toronto, ON, Canada
Departament of Occupational Risk Prevention, Virgen de la Arrixaca’s Hospital (HCUVA), Murcia, Spain
Kelly L. Rodriguez
Department of Preventive Medicine and Public Health, Instituto de Investigación Sanitaria de Navarra (IdiSNA), University of Navarra, Pamplona, Spain
Zenaida Vázquez-Ruiz
You can also search for this author in PubMed Google Scholar
Contributions
LSM, JV, MF, DC, XP, ABC, JAT, JAM, and JSS contributed to the study concept and design and data extraction from the participants from the PREDIMED-Plus study which provides the framework for the present prospective cohort analysis. SKN, NB, IPG, CGM, and JSS made substantial contributions to the conception of the present study. SKN performed the statistical analyses and initial interpretation of the data. NB, IPG, CGM, and JSS contributed to the review of the statistical analyses and interpretation of the data. SKN drafted the manuscript. All authors substantively reviewed the manuscript for important intellectual content and approved the final version to be published. All authors had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Corresponding authors
Correspondence to Stephanie K. Nishi or Nancy Babio .
Ethics declarations
Ethics approval and consent to participate.
The PREDIMED-Plus study protocol and procedures were approved by the Research Ethics Committees from each of the participating centers, and the study was registered with the International Standard Randomized Controlled Trial registry (ISRCTN; ISRCTN89898870 ). All participants provided written informed consent.
Consent for publication
Not applicable.
Competing interests
S.K.N. is a volunteer member of the not-for-profit group Plant-Based Canada and received an Early Career Researcher Award conference grant from Hydration for Health presented by Danone Global Research & Innovation Center. D.C. reported receiving grants from Instituto de Salud Carlos III. R.E. reported receiving grants from Instituto de Salud Carlos III, Uriach Laboratories, and Grand-Fountain Laboratories for clinical trial and personal fees from Brewers of Europe, Fundación Cerveza y Salud; Instituto Cervantes in Albuquerque, Milano, and Tokyo; Fundación Bosch y Gimpera; non-financial support from Wine and Culinary International Forum, ERAB (Belgium), and Sociedad Española de Nutrición; and fees of educational conferences from Pernaud Richart (Mexico) and Fundación Dieta Mediterránea (Spain). R.C. reported receiving fees of educational conferences from Fundación para la investigación del Vino y la Nutrición (Spain). J.L.S. has received research support from the Canadian Foundation for Innovation, Ontario Research Fund, Province of Ontario Ministry of Research and Innovation and Science, Canadian Institutes of health Research (CIHR), Diabetes Canada, American Society for Nutrition (ASN), International Nut and Dried Fruit Council (INC) Foundation, National Honey Board (US Department of Agriculture [USDA] honey “Checkoff” program), Institute for the Advancement of Food and Nutrition Sciences (IAFNS; formerly ILSI North America), Pulse Canada, Quaker Oats Center of Excellence, The United Soybean Board (USDA soy “Checkoff” program), The Tate and Lyle Nutritional Research Fund at the University of Toronto, The Glycemic Control and Cardiovascular Disease in Type 2 Diabetes Fund at the University of Toronto (a fund established by the Alberta Pulse Growers), The Plant Protein Fund at the University of Toronto (a fund which has received contributions from IFF), and The Nutrition Trialists Network Fund at the University of Toronto (a fund established by an inaugural donation from the Calorie Control Council). He has received food donations to support randomized controlled trials from the Almond Board of California, California Walnut Commission, Peanut Institute, Barilla, Unilever/Upfield, Unico/Primo, Loblaw Companies, Quaker, Kellogg Canada, WhiteWave Foods/Danone, Nutrartis, and Dairy Farmers of Canada. He has received travel support, speaker fees, and/or honoraria from ASN, Danone, Dairy Farmers of Canada, FoodMinds LLC, Nestlé, Abbott, General Mills, Nutrition Communications, International Food Information Council (IFIC), Calorie Control Council, International Sweeteners Association, and International Glutamate Technical Committee. He has or has had ad hoc consulting arrangements with Perkins Coie LLP, Tate & Lyle, Phynova, and Inquis Clinical Research. He is a former member of the European Fruit Juice Association Scientific Expert Panel and a former member of the Soy Nutrition Institute (SNI) Scientific Advisory Committee. He is on the Clinical Practice Guidelines Expert Committees of Diabetes Canada, European Association for the study of Diabetes (EASD), Canadian Cardiovascular Society (CCS), and Obesity Canada/Canadian Association of Bariatric Physicians and Surgeons. He serves or has served as an unpaid member of the Board of Trustees and an unpaid scientific advisor for the Carbohydrates Committee of IAFNS. He is a member of the International Carbohydrate Quality Consortium (ICQC), an Executive Board Member of the Diabetes and Nutrition Study Group (DNSG) of the EASD, and a Director of the Toronto 3D Knowledge Synthesis and Clinical Trials foundation. His spouse is an employee of AB InBev. J.S.-S. reported receiving research support from the Instituto de Salud Carlos III, Ministerio de Educación y Ciencia, the European Commission, and the USA National Institutes of Health; receiving consulting fees or travel expenses from Instituto Danone and Abbott Laboratories; receiving nonfinancial support from Patrimonio Comunal Olivarero, the Almond Board of California, and Pistachio Growers and Borges S.A; serving on the board of and receiving grant support through his institution from the International Nut and Dried Foundation and personal fees from Instituto Danone; and serving in the Board of Danone Institute International. The rest of the authors declared that they have no competing interests. The funders had no role in the design of the study; in the collection, analyses, or interpretation of the data; in the writing of the manuscript; or in the decision to publish the results.
Additional information
Publisher’s note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1: table s1..
Hydration and water intake definitions. Table S2. Associations between cognitive assessments and water and fluid intake exposures. Table S3. Associations between cognitive assessments and EFSA fluid intake related guidelines. Table S4. Associations between cognitive assessments and hydration status. Table S5. Sensitivity analysis in global cognitive function according to water and fluid intake related exposures Table S6. Sensitivity analysis in global cognitive function according to EFSA fluid intake related guidelines. Table S7. Sensitivity analysis in global cognitive function according to hydration status. Fig. S1. Flow diagram of participants. Fig. S2. Continuous sensitivity analysis by sex. Fig. S3. Categorical sensitivity analysis by sex.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ . The Creative Commons Public Domain Dedication waiver ( http://creativecommons.org/publicdomain/zero/1.0/ ) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
Reprints and permissions
About this article
Cite this article.
Nishi, S.K., Babio, N., Paz-Graniel, I. et al. Water intake, hydration status and 2-year changes in cognitive performance: a prospective cohort study. BMC Med 21 , 82 (2023). https://doi.org/10.1186/s12916-023-02771-4
Download citation
Received : 02 November 2022
Accepted : 06 February 2023
Published : 08 March 2023
DOI : https://doi.org/10.1186/s12916-023-02771-4
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Serum osmolality
- Cognitive function
- Cognitive performance
- PREDIMED-plus
BMC Medicine
ISSN: 1741-7015
- General enquiries: [email protected]
An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
Preview improvements coming to the PMC website in October 2024. Learn More or Try it out now .
- Advanced Search
- Journal List
- BMC Plant Biol
- PMC11351449

Physiological traits, crop growth, and grain quality of quinoa in response to deficit irrigation and planting methods
Sayyed mohammad mirsafi.
1 Department of Water Engineering, School of Agriculture, Shiraz University, Shiraz, Iran
Ali Reza Sepaskhah
2 Drought Research Center, Shiraz University, Shiraz, Iran
Seyed Hamid Ahmadi
Associated data.
Data could be available by reasonable request.
Climate change has become a concern, emphasizing the need for the development of crops tolerant to drought. Therefore, this study is designed to explore the physiological characteristics of quinoa that enable it to thrive under drought and other extreme stress conditions by investigating the combined effects of irrigation water levels (100%, 75%, and 50% of quinoa's water requirements, WR as I1, I2 and I3) and different planting methods (basin, on-ridge, and in-furrow as P1, P2 and P3) on quinoa's physiological traits and gas exchange. Results showed that quinoa’s yield is lowest with on-ridge planting and highest in the in-furrow planting method. Notably, the seed protein concentrations in I2 and I3 did not significantly differ but they were 25% higher than those obtained in I1, which highlighted the possibility of using a more effective irrigation method without compromising the seed quality. On the other hand, protein yield (PY) was lowest in P2 (mean of I1 and I2 as 257 kg ha −1 ) and highest in P3 (mean of I1 and I2 as 394 kg ha −1 , 53% higher). Interestingly, PY values were not significantly different in I1 and I2, but they were lower significantly in I3 by 28%, 27% and 20% in P1, P2, and P3, respectively. Essential plant characteristics including plant height, stem diameter, and panicle number were 6.1–16.7%, 6.4–24.5%, and 18.4–36.5% lower, respectively, in I2 and I3 than those in I1. The highest Leaf Area Index (LAI) value (5.34) was recorded in the in-furrow planting and I1, while the lowest value was observed in the on-ridge planting method and I3 (3.47). In I3, leaf temperature increased by an average of 2.5–3 o C, particularly during the anthesis stage. The results also showed that at a similar leaf water potential (LWP) higher yield and dry matter were obtained in the in-furrow planting compared to those obtained in the basin and on-ridge planting methods. The highest stomatal conductance (gs) value was observed within the in-furrow planting method and full irrigation (I1P3), while the lowest values were obtained in the on-ridge and 50%WR (I3P2). Finally, photosynthesis rate (An) reduction with diminishing LWP was mild, providing insights into quinoa’s adaptability to drought. In conclusion, considering the thorough evaluation of all the measured parameters, the study suggests using the in-furrow planting method with a 75%WR as the best approach for growing quinoa in arid and semi-arid regions to enhance production and resource efficiency.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12870-024-05523-5.
Introduction
Plants are exposed to different environmental conditions such as different abiotic stresses including shortage of available water, salinity, and unfavorable temperature [ 1 ]. Climatic conditions in semiarid areas (limited precipitation and excessive evapotranspiration due to high temperatures) cause a negative water balance. Growing water demand from urban centers and industries, in addition to a more evaporative climate due to climate change, has reduced irrigation water availability for farmers [ 2 ]. Therefore, developing water-efficient cropping methods and adapting them to the impact of climate change is crucial. Different field management strategies have been reported to improve the performance of crops under extreme conditions, including appropriate planting methods [ 3 – 6 ], selecting appropriate planting density [ 7 , 8 ], and deficit irrigation. Deficit irrigation is considered an approach to address water scarcity in arid and semi-arid areas [ 9 – 11 ].
Under climate change, crops that have been grown for decades are losing their potential for yield, and are unable to bridge the food supply–demand gap . This alarming situation necessitates the development of drought-tolerant crops. Plants have different approaches to cope with water stress such as morpho-anatomical, physiological, and biochemical adjustments which aim to preserve their hydric status [ 12 ]. Plants’ tolerance to water stress mainly depends on plant genotype, stress intensity, and duration [ 13 , 14 ]. The quinoa crop might be a suitable alternative to grain crops that are commercially grown to meet the food demand in inappropriate environments . Quinoa ( Chenopodium quinoa Willd.) has gained increasing attention on a global scale since 2013 due to its greater protein content than other cereals and a better distribution of the necessary amino acids [ 15 ]. It is a C3 crop native to the Andean region, which is reported to be a potential crop for food security [ 16 ]. It is reported that quinoa has a low water requirement [ 17 , 18 ], on the contrary, there are studies that indicate the evapotranspiration of quinoa varies between 1100 and 1600 mm in arid and semi-arid areas [ 7 , 19 ].
Quinoa tolerance to water stress has been studied widely [ 20 – 25 ]. Quinoa deals with low water availability by tolerating water stress through a vigorous and deep root system [ 7 , 26 , 27 ], reducing transpiration, leaf area [ 27 ], and maintaining leaf turgor and closing of stomata [ 28 ].
The physiological indices including photosynthesis rate, stomatal conductance, intrinsic water use efficiency and transpiration reduced in response to a water shortage [ 29 ]. The most sensitive measure of water stress is the stomatal conductance, which is a common approach to plant studies in drought conditions that involves monitoring of gas exchange. Gas exchange estimates plant transpiration as a function of the leaf water status [ 30 ]. Leaf area index (LAI), the total one-sided area of photosynthetic tissue per unit horizontal ground surface area is one of the most important parameters as it regulates gas exchange processes such as photosynthesis [ 31 ] and evapotranspiration [ 32 ]. LAI can be determined by direct methods, which are time-consuming. Light extinction coefficient (K) is used to estimate the irradiance into the canopy and is an indicator of light penetration through the crop canopy. LAI and K are the key indicators of vegetation canopy characteristics. Accurate quantification of these characteristics is essential as it plays a crucial role in ecosystem studies on productivity, carbon cycles, nutrient allocation, and biological diversity [ 33 ].
Water scarcity affects agricultural development and productivity, as well as food security [ 34 ]. Therefore, there is a need to introduce new crops which can be adapted to the arid and semi-arid areas, which are facing severe drought. To effectively understand the growth and yield of crops, a greater understanding of the variables, which are influencing biomass is necessary. This will make it possible to measure productivity in various situations, which will improve understanding of constraints brought on by stress, canopy design, and leaf area dynamics [ 34 ]. Quinoa’s physiological adaptability, which enables it to thrive in drought and other extreme stress conditions, is an invaluable opportunity with enormous potential to cope with current and potential climate challenges [ 35 ]. However, it is necessary to investigate the effect of adverse environmental conditions on the growth and yield of quinoa, as a new crop, on a small scale before planting on a large scale.
Most of the cultivated areas in Iran are arid and semi-arid, that are short in water resources and groundwater depletion [ 36 ]. Therefore, appropriate irrigation water management such as deficit irrigation and in-furrow planting method should be used in field to reduce the irrigation water use and enhance its productivity [ 3 , 11 ]. Quinoa cultivation area in Iran was about 300 ha in 2016 and is increasing recently, with mean yield of 1850 kg ha −1 [ 37 , 38 ]. In addition, to increase crop production, preserve water supplies, and establish sustainable agricultural practices in semi-arid regions like Iran, deficit irrigation and planting methods in relation to quinoa physiology must be investigated as they offer important insights into how quinoa reacts to water scarcity and how to effectively manage this crop in difficult environmental circumstances. Thus, investigating the variation in quinoa physiological parameters, gas exchange, morph-physiological processes responsible for drought tolerance and light extinction coefficient is critical to cope with drought stress. So far, there are some reports on the physiological response of quinoa to drought in greenhouse experiments [ 29 , 35 ] or in field [ 39 , 40 ] but the variation of the effect of deficit irrigation combined with different planting method on quinoa has not been investigated. Thus, the aim of this study is to investigate the combined effects of irrigation water level and planting method on the physiological growth and gas exchange of quinoa.
Methods and materials
Site description.
This study was conducted at the Experimental Station, the School of Agriculture, Shiraz University, in a semi-arid region of the Bajgah area (29 ◦ 56’ N, 52 ◦ 02’ E and at 1810 m above the mean sea level) during 2017 and 2018 in south-western Iran. The physical and chemical characteristics of the field soil are listed in supplementary Table S1 . Climatic data (including air temperature and humidity, wind speed, and hours of sunshine) were obtained from a standard weather station near the experimental field. Supplementary Figure S1 shows the average daily minimum and maximum temperature and average relative humidity during the first and second growing seasons. The daily vapor pressure deficit for each growing season is shown in Supplementary Fig. S2a. Total precipitation during the growing season was 15.5 and 60 mm in the first and second year, respectively (Supplementary Fig. S2b). The modified Penman–Monteith equation for semi-arid environments in the study area reported by [ 41 ] was used to calculate the potential reference evapotranspiration (ET o ) (Supplementary Fig. S2b).
Experimental design and treatments
A split plot arrangement in a randomized complete block design with three replicates was used with three irrigation water levels [100% (I1). 75% (I2), and 50% (I3) of quinoa water requirement (WR)] as the main plot, and three planting methods [basin planting method (P1), on-ridge planting (P2), and in-furrow planting (P3)] as the subplot with three replications. Quinoa water requirement was determined according to increasing soil water content to field capacity in the root zone in 100%WR (I1). The used irrigation water levels are similar to those applied in previous studies for other crops (i.e., wheat and saffron) in the same study area [ 10 , 11 ]. Furthermore, the irrigation level of 100%WR (I1) was determined based on raising soil water content before irrigation event to soil field capacity; therefore, irrigation water level should not be less than 50%WR (I3) due to severe soil water stress. After deep plowing and leveling, plots of 1.5 m × 2.0 m were established. It might be claimed that the above- and below-ground growing conditions in this small plot would be interfered by its surrounding field, and any sampling during the growing season would also affect the crop growth. It should be mentioned that in this study, we have mainly focused on the non-destructive measurements obtained in the middle of plots. Furthermore, a buffer distance of 1.0 m was maintained between adjacent plots to prevent any adjacent plot interference. Also, guard plots were established around the experimental site to avoid possible interference by the surrounding fields.
Triple superphosphate at a rate of 50 kg P ha −1 was applied uniformly to all plots before planting. A schematic representation of the ridges and furrows for the basin, in-furrow, and on-ridge planting methods is shown in Supplementary Fig. S3.
Quinoa seeds (cv. Titicaca) were sown at 10–20 mm depth on May 5, 2017, and March 31, 2018. In the flat basin planting, the seed was sown at 30 cm row distances. For in-furrow planting, the seed was sown in rows 30 cm apart at the bottom of the furrows. For on-ridge planting, the seed was planted in rows 30 cm apart on top of the ridges. The seedlings were thinned to a planting density of 186,667 plants per hectare. During the growing seasons, urea was mixed with the topsoil layer at a rate of 75 kg N ha −1 and applied to the plots at the beginning of the vegetative and reproductive (flowering) stages (total rate of 150 kg N ha −1 ). Weeds were frequently removed manually.
Plots were irrigated regularly at 7-day intervals, increasing the water content of the soil to the soil field capacity in the root zone. The seven-day interval is consistent with the used irrigation schedule for quinoa experiments in the study region [ 42 ]. Soil water content was measured using the neutron scattering method before each irrigation event in three replicates at 10–30 cm, 30–60 cm, 60–90 cm, and 90–120 cm soil depth. Supplementary Figure S3 shows the location of the access tubes in each plot of the representative planting method. Soil water content in the first 10 cm of the soil layer was measured by gravimetric method. The following equation was used to determine the irrigation depth.
where, I is the irrigation water depth (m), θ Fci and θ i are the volumetric soil water content in layer i at field capacity (m 3 m − 3 ) and soil water content in layer i before each irrigation event, respectively (m 3 m − 3 ), Di is the depth of each layer (m), and n is the number of soil layers within the rooting zone. The following equation was used to determine the rooting depth during the growing season [ 43 ]:
In which R d , R dmax , and R dmin are the root depth (m), the maximum root depth (1.2 m) [ 7 ], and the seed sowing depth (0.05 m), respectively, D ag is the number of days after sowing date in which the plants were supposed to be irrigated. D tm is the number of days after sowing that root reaches the maximum depth (96 days).
For most crops, the amount of time between planting and physiological maturity is often a function of accumulated heat and growing degree days (GDD). Each crop has a certain quantity of GDD needed to grow and mature [ 44 ], The following equation is typically used to estimate daily GDD using the daily average air temperature:
in which, T a is the daily mean temperature, T c-min is the minimum required for growth and T c-max is the air temperature above which growth is limited. The corresponding values for quinoa are 4 °C and 35 °C, respectively.
The lengths of the entire growth cycle and each phenological stage of quinoa in the first and second growing seasons are shown in Supplementary Fig. S4 based on cumulative growing degree days (∑GDD). Additionally, the growth stages are displayed using the Biologische Bundesanstalt, Bundessortenamt and CHemical industry (BBCH) system [ 45 ]. This scale system was developed to meet the need for basic biological knowledge and to identify the key life stages of a plant as well as the phenological events of plants that are important to agriculture [ 45 ]. The length of the growth cycle was 16 days longer in the second growing season. Mean daily maximum temperature and mean air temperature were 3.8 °C and 2.4 °C higher, respectively, in the first growing season than those in the second growing season which led to higher ET o .
Growth and yield components
Quinoa crops were harvested on September 1, 2017 (first growing season) and August 13, 2018 (second growing season). The entire 3 m 2 plot was harvested for grain yield and shoot biomass. At the end of each growing season, grains were first cut from the stems to determine grain yield. To determine straw dry matter (leaves and stems), stems were cut from the soil surface and dried in an oven at 70 °C and weighed after 24 h. At the end of each growing season, the number of total productive branches per plant and the length of the main panicle (panicle placed at the top of the plant) were measured in 3 crops per plot, and the panicles were separated from the shoots. The quinoa grains were separated from the coatings by crushing and dried in an oven at 65 °C for 72 h to determine seed yield. Samples of the dried seeds were used to determine seed protein concentration (%) by multiplying the nitrogen concentration by 6.25. The nitrogen concentration of the seeds was determined by the Kejldahl method [ 46 ]. Seed yield was multiplied by seed protein concentration to determine protein yield. Quinoa height, stem diameter, and leaf area index, the total one-sided area of photosynthetic tissue per unit horizontal ground surface (LAI), were measured on 3 crops per plot at 37, 65, 80, 95, 110 days after sowing (DAS) in the first growing season and at 43, 72, 88, 105,115 (DAS) in the second growing season.
Partitioning coefficient (PC) is defined as the amount of dry matter required for seed production and it shows the ratio of the distribution of dry matter between foliage and seeds. To calculate PC, the intercept of the relationship between the grain yield and shoot dry matter (SDM), was deducted from the total dry matter, and divided by the seed weight.
Gas exchange measurements
A LCi analyzer (ADC BioScientific Ltd, UK.) was used to measure leaf surface temperature, net photosynthesis rate, stomata conductance, and transpiration rate under fair weather conditions at 11:00 a.m. 4 times in each growing season. These measurements were done during different phenology stages on 24, 52, 76, and 97 DAS in the first growing season and 32, 65 89, and 110 DAS in the second growing season corresponding to early vegetative to seed filling growth stages. Leaf water potential was measured using a pressure chamber (Soil Moisture Equip. Corp. Mod. 5100A, Santa Barbara, CA, USA) on 24, 40, 52, 64, 76, 88, 106 and 113 days after sowing in the first growing seasons at 11 o’clock. The corresponding days for the second growing season were 30, 44, 60, 74, 90, 104 and 119.
Assuming that the air within the stomata is saturated, the saturated vaper pressure in the leaf was calculated by the following equation [ 47 ]:
In which, e s is the saturated vapor pressure (kPa) and T is the leaf temperature ( o C). The ambient vapor pressure (e a ) in the free air was calculated using saturated air vapor pressure (e s ):
In which, RH is the relative humidity of the outside air. The following equation was used to calculate the vapor pressure deficit between the leaf and the air:
In which, VPD is the vapor pressure deficit (kPa).
Photosynthesis active radiation (PAR) and extinction coefficient
In the absence of abiotic stress, biomass development only depends on incoming PAR (photosynthetically active radiation) which changes according to latitude, season, sowing date, and plant phenology [ 48 ]. Therefore, PAR was determined to understand how it would change under abiotic stress and how it would affect crop growth. The light extinction coefficient (K) is a key indicator of the efficiency of interception of light penetrating through the canopy because of the gradual decrease in light intensity due to repeated attenuation by leaf elements [ 49 ]. To calculate the extinction coefficient, instantaneous solar radiation was measured at the top of the canopy and bottom of the canopy with an instrument, simultaneously with leaf surface and dry matter sampling using Solari- meter LI-190R with quantum sensor ( LI-COR Biosciences Ltd UK). The LI-190R measures the photosynthetically active radiation in µmol of photons m −2 s −1 .
Solar radiation was measured on each plot at 37, 65, 80, 95, and 110 days after planting in the first growing season and at 43, 72, 88, 105, and 115 days after planting in the second growing season between 11:00 and 13:00. For this purpose, two perpendicular measurements were taken on the top of the canopy and two measurements were taken on the bottom of the canopy (one measurement along the rows and one measurement perpendicular to the planting rows) on each plot. Knowing the leaf area index (LAI), the amount of PAR reaching the lower part of the canopy (PAR c ), and the amount of PAR reaching the upper part of the canopy (PAR o ), the light extinction coefficient (K) was determined based on the Beer-Lambert equation of light extinction [ 50 ] as:
To calculate the daily absorbed light, first, the daily solar radiation (I 0 ) reaching the top of the canopy was calculated based on latitude, season, day length, atmospheric transmission coefficient and the solar hour of the area as follows.
In which, R a is the extraterrestrial radiation in MJ m −2 d −1 ; n is the actual duration of sunshine in h; N is the maximum possible duration of sunshine in h and a s and b s are the coefficients of radiation function. Malek [ 51 ] found a s and b s equal to 0.31 and 0.55, respectively, in the study region with a latitude of 29° 83 ′ 50 ″ N; a longitude of 52° 83 ′ 50 ″ E and an elevation of 1810 m above sea level.
where, PAR a is the daily light absorbed by the canopy (MJ m −2 day −1 ), I 0 is the daily solar radiation in MJ m −2 d −1 , p is the reflection coefficient, K is the extinction coefficient which is determined by Eq. ( 7 ).
Statistical analysis
MSTAT-C statistical software [ 52 ] was used for statistical analysis of interaction effects between irrigation water level and planting methods each year. Analysis of variance (ANOVA) was performed using Duncan’s method to detect statistically significant differences between means ( p ≤ 0.05). Also, the effect of the year on different parameters was analyzed. If the effect of year was not significant ( p -value > 0.05), the data was pooled over the two growing seasons.
Crop growth
Growth components.
The variation of quinoa height during the growing seasons and at the end of the growing season under different irrigation water levels and planting methods are shown in Fig. 1 and Table 1 , respectively. The interaction effect of irrigation water level and planting method on the height at the end of the season was not significant; however, irrigation water level significantly affected plant height only at 50%WR (Table 1 ). Plant height was reduced by 6.1% and 16.7% at 75%WR and 50%WR, respectively, compared to that in 100%WR. Although the plant height in 100%WR was not different in the different planting methods; in 75%WR it was, on average, 13.8% higher in the basin and in-furrow planting methods in comparison with on-ridge planting methods.

Variation of quinoa height in different treatments (I1: 100%WR, I2: 75%WR and I3: 50%WR), and different planting methods in different days after sowing (DAS) (first column: 2017, second column: 2018). The dash lines indicate the growth stages as shown in Fig. S1
Table 1
Plant height (m), maximum stem diameter (mm) and maximum LAI of quinoa, panicle number, main panicle length (cm), seed protein concentration (%) and seed protein yield (kg ha −1 ) on average in two growing seasons
| 1.280 a | 1.240 a | 1.320 a | |
| 1.207 ab | 1.080 c | 1.230 ab | |
| 1.087 c | 1.077 c | 1.127 bc | |
| 14.5 a | 13.7 ab | 14.45 a | |
| 13.4 b | 12.9 b | 13.8 ab | |
| 11.3c | 10.6 c | 12.3 bc | |
| 5.02 ab | 4.88 b | 5.34 a | |
| 4.62 b | 4.18 c | 4.83 b | |
| 3.64cd | 3.47 d | 3.87 cd | |
| 21.8ab | 21.2ab | 24.8a | |
| 19.3bc | 17.3c | 20.7b | |
| 15.8d | 16.0cd | 17.8c | |
| 25.7b | 26.0ab | 30.9a | |
| 23.3b | 22.2bc | 23.9b | |
| 19.8c | 19.8c | 22.0bc | |
| 14.4cd | 13.3d | 16.1bc | |
| 16.7b | 16.7b | 19.5a | |
| 18.4ab | 17.5b | 20.5a | |
| 320.9b | 249.2c | 376.0ab | |
| 328.3b | 265.1c | 412.4a | |
| 230.5cd | 188.4d | 313.2b | |
a Means followed by the same letters in columns for each factor and each trait are not significantly different at 5% level of probability, using Duncan’s multiple range test
The planting method and irrigation water level affected the stem diameter significantly ( p -value < 0.05). The stem diameter was 9% smaller in the on-ridge planting method in comparison with that obtained in the in-furrow. It was also significantly affected by irrigation water level as it was reduced by 6.4% and 24.5% at 75%WR and 50%WR compared to that obtained at 100% WR (Table 1 , Fig. 2 ). Figures 1 and and2 2 show that the height and stem diameter of quinoa were not affected by irrigation water level during the first stages of crop growth as they had almost the same values in different treatments; however, after anthesis stage and in the beginning of seed filling stage both height and diameter were affected adversely.

Variation of quinoa stem diameter in different treatments in different days after sowing (DAS) on average in different treatments (I1: 100%WR, I2: 75%WR and I3: 50%WR), and different planting methods in different days after sowing (DAS) during two growing seasons (first column: 2017, Second column: 2018)..The dash lines indicate the growth stages as shown in Fig. S1
Number of panicles (NP) and length of panicles (LP) in different treatments are presented in Table 1 . The effect of year was not significant ( p -value > 0.05); therefore, the data is pooled over two growing seasons. NP in the in-furrow was 15.4% higher than those obtained in basin and on-ridge planting methods. Irrigation water level affected the NP significantly ( p -value < 0.05). Regardless of planting methods, 75%WR and 50%WR reduced NP by 18.4% and 36.5%, respectively in comparison to those obtained in 100% WR. The same trend was observed in LP variation. LP in the in-furrow was 19.7% higher than those obtained in the basin and on-ridge in 100% WR. The corresponding value for 50% WR was 10.9%. The main effect of irrigation water level was significant on LP ( p -value < 0.05) as it was reduced by 18.6% and 33.6% in 75% WR and 50%WR, respectively, compared to that obtained in 100% WR.
Leaf area index
The interaction effect of irrigation water levels and planting methods on maximum leaf area index (LAI max ) was statistically significant ( p -value < 0.05). The highest LAI (5.34) was observed in the in-furrow planting method under full irrigation (Table 1 ). Regardless of irrigation water level, LAI max in the in-furrow planting method was 5.7% and 12.2% higher than those obtained in the basin and on-ridge planting methods, respectively. Deficit irrigation significantly reduced LAI max in all planting methods ( p -value < 0.05), as it was reduced by 11.9% and 24.1% in 75%WR and 50%WR, respectively in comparison with that obtained in 100%WR. It should be noted that LAI max in 75%WR treatment was not significantly different in the basin and in-furrow planting, whereas it was lower significantly in the on-ridge planting (ORP). However, its value was statistically similar to that obtained in 100% WR in the basin planting. (Table 1 ).
Seasonal variation of leaf area index of quinoa grown in different treatments during the first and second growing seasons are shown in Fig. 3 . Maximum LAI occurred 80 days and 88 days after sowing in the seed filling growth stage, respectively in the first and second growing seasons. The difference in the obtained LAI in different planting methods is significant only in 100% WR treatment as the highest LAI values were obtained in the in-furrow planting method and the lowest values were observed in the on-ridge planting method. Deficit irrigation reduced the difference between LAI values in different planting methods.

Variation of quinoa leaf area index (LAI) in different treatments (I1: 100%WR, I2: 75%WR and I3: 50%WR), and different planting methods (P1: basin, P2: on-ridge, and P3: in-furrow) in different days after sowing (DAS) during two growing seasons (first column: 2017, Second column: 2018). The dash lines indicate the growth stages as shown in Fig. S1
Dry matter, grain yield and protein yield of quinoa
The interaction effect of irrigation water levels and planting methods on total dry matter (sum of shoot and grain yield, TDM) and grain yield of quinoa was significant ( p -value < 0.05). Results are shown in Fig. 4 and indicated that the irrigation regime of 75%WR reduced TDM by 3%, 11%, and 17% for the in-furrow, basin, and on-ridge planting methods, respectively, compared to 100% WR. The corresponding values for 50% WR were 11%, 21%, and 30%, respectively, which showed higher reduction.

Two years average of grain yield (kg ha-1) and Biomass (kg ha-1) in different treatments (I1: 100%WR, I2: 75%WR and I3: 50%WR), and different planting methods (P1: basin, P2: on-ridge, and P3: in-furrow)
The interaction effect of the planting methods and the irrigation water levels was significant for both protein yield and protein concentration ( p -value < 0.05). The percentages of seed protein concentration and calculated protein yields are shown in Table 1 . Statistical analysis showed that the interaction effect of the planting methods and irrigation levels was significant ( p -value < 0.05). The highest protein concentrations were obtained in 50%WR and in-furrow (20.5%), but the highest protein yield was observed at 75%WR and in-furrow (412.4 kg ha −1 ). Deficit irrigation increased protein concentration, as it was 21% and 29% higher in 50%WR than those in 75% and 100%WR, regardless of planting methods, respectively. In addition, taking planting methods into consideration, protein concentration was 15% higher in the in-furrow planting compared to the other planting methods, on average, of different irrigation water levels. Although protein concentration was not significantly different in 75%WR and 50%WR in all planting methods, the protein yield was lower significantly in 50%WR. On the other hand, protein yield in the in-furrow planting was 32% higher in 75%WR compared to 50%WR due to higher grain yield, indicating that the 75%WR in-furrow planting method is the optimal planting method.
Physiological traits
Leaf temperature.
Leaf temperature (Tl) was measured four times during each growing season. The mean Tl in early vegetative, vegetative, anthesis and maturity was 32.3, 34.6, 35.7, and 36.7 °C in the first growing season, respectively. The corresponding values for the second growing season were 31.6, 33.2, 34.9, and 36.7 °C, respectively. In the anthesis and maturity stages, Tl was 10.5% and 14.8% higher than that obtained in the early vegetative stage, respectively. Tl increased throughout the growing seasons regardless of different treatments (Fig. 5 ), which is mainly due to the increased air temperature (Fig. S1 ) especially in 75%WR and 50%WR treatments since the soil water content was not sufficient to support high transpiration rate. The quinoa seeds were sown earlier in the second growing season to avoid high air temperature during the anthesis and maturity stages; as a result, lower Tl in the second growing season was observed due to the lower air temperature (Fig. S1 ).

Variation of the leaf temperature (oC) in different irrigation water levels (I1:100%WR, I2:75%WR and I3:50%WR) and planting methods (P1: basin, P2: on-ridge and P3: in-furrow) in different days after sowing (DAS) in the first (2017) and second (2018) growing seasons
The main effect of irrigation water level was significant on Tl, and in 50%WR it was, on average, 1.7 and 1.0 °C higher than those obtained in 100%WR and 75%WR. In addition, Tl was 2.5–3 °C higher in the 50%WR in the anthesis stage in comparison to 100%WR and 75%WR irrigation levels. Also, higher Tl was observed in on-ridge planting methods in comparison to those obtained in the basin and in-furrow planting methods. The Tl values in the on-ridge planting were 1.3 and 0.8 °C higher in comparison to those obtained in the basin and in-furrow planting, respectively.
Leaf water potential
The leaf water potential (LWP) of quinoa was measured eight times during each growing season before each irrigation event (Fig. 6 ). LWP was the highest (-0.5 to -1.5 MPa) in the early growth stages and reduced gradually (-3.5 to -4.5 MPa) till late season. In both growing seasons, the highest reduction was observed in 50%WR especially during anthesis and seed filling stages (Fig. 6 ), which indicate the higher susceptibility of quinoa to extreme water stress during anthesis and seed filling. A decrease in yield was also reported when water stress was imposed at both the flowering and grain filling stages [ 27 , 53 , 54 ]. The mean value of LWP during the growing seasons is shown in Table 2 . Results showed that 75%WR and 50%WR reduced LWP by 12.5% and 47.4% in comparison to that obtained in 100%WR, respectively. Also, the main effect of planting methods on LWP was significant as it was 15.8% and 9.9% lower in the on-ridge planting in comparison to those obtained in the in-furrow and basin planting, respectively.

Variation of mean water potential [LWP (MPa)] in different treatments (I1: 100%WR, I2: 75%WR and I3: 50%WR), and different planting methods in different days after sowing (DAS) during two growing seasons (first column: 2017, Second column: 2018). The dash lines indicate the growth stages as shown in Fig. 1
Table 2
Mean values of leaf water potential [LWP (MPa)], Leaf temperature (Tl), photosynthesis rate [An (µmol m −2 s −1 )], stomatal conductance [gs (mol m −2 s − 1)], intrinsic water use efficiency [An/gs (µmol mol −1 )], transpiration rate [Tr (mol m −2 s −1 )] and transpiration efficiency (An/Tr (g kg −1 ) at different irrigation water levels and planting methods on average in two growing seasons
| Parameter | LWP (MPa) | Tl ( C) | An (µmolm s ) | gs (molm s ) | An/gs (µmolmol ) | Tr (mmolm s ) | An/Tr (g kg ) |
|---|---|---|---|---|---|---|---|
| Irrigation level | |||||||
| I1 (100%WR) | -2.08a | 33.6b | 22.83a | 0.46a | 48.48b | 4.67a | 5.10a |
| I2 ( 75%WR) | -2.34b | 34.3ab | 20.71ab | 0.40b | 48.50b | 4.46ab | 4.64ab |
| I3 ( 50%WR) | -3.07c | 35.7a | 18.06b | 0.32c | 58.75a | 3.97b | 4.53b |
| Planting method | |||||||
| P1 (Basin) | -2.46a | 34.5a | 21.02a | 0.43a | 51.7a | 4.36a | 4.82a |
| P2 (On-ridge) | -2.70b | 35.1a | 19.62a | 0.38b | 52.1a | 4.19a | 4.67a |
| P3 (In-furrow) | -2.30a | 33.8a | 20.98a | 0.41ab | 54.2a | 4.55a | 4.58a |
Photosynthesis rate and stomatal conductance
The photosynthesis rate (An) of quinoa was measured four times during the first and second growing seasons. The variation of An during each growing season is shown in Fig. 7 and the mean values are shown in Table 2 . The main effect of irrigation water level was significant on An as it was reduced, on average, by 7.1% and 18.3% in 75%WR and 50%WR in comparison with those obtained in 100%WR, respectively. In addition, the An values (pooled over the whole season, on average) were decreased by 8.1% in the on-ridge planting in two growing seasons.

Variation of photosynthesis rate [An (µmol m − 2 s − 1)] and stomatal conductance [gs (mol m − 2 s − 1)] and transpiration rate [Tr (mol m − 2 s − 1)] in different irrigation water levels (I1:100%WR, I2:75%WR and I3:50%WR) and planting methods (P1: basin, P2: on-ridge and P3: in-furrow) in the first(2017) and second growing seasons(2018)
The variation of stomatal conductance (gs) of quinoa was measured four times before the irrigation events during both growing seasons. As shown in Fig. 7 , stomatal conductance was reduced from the highest values (0.5–0.8 mol m −2 s −1 ) in the early growing season to the minimum values (0.1–0.3 mol m −2 s −1 ) in the late season. The lower values of gs obtained in the late season may be due to the higher ambient temperature as both plant-specific characteristics, and the surrounding environment affect stomatal conductance. In the second growing season, quinoa was sown 35 days earlier than the first growing season to avoid very high air temperatures, especially in the mid-season. On average in two growing seasons, the highest value of gs (0.72 mol m −2 s −1 ) was observed in the in-furrow planting when it was fully irrigated (I1P3), and the lowest value (0.13 mol m −2 s −1 ) was obtained in the on-ridge planting and 50%WR (I3P2) as it was 21.6% and 29.3% lower than those obtained in 100%WR and 75%WR, respectively. Although the average gs values were higher in the in-furrow planting method, the main effect of the planting method was not significant on average value of gs. The main effect of irrigation water level on stomatal conductance was significant (p-value < 0.05) in 50%WR irrigation level (Table 2 ).
The main effect of irrigation water level and planting method on the intrinsic water use efficiency (An/gs) was investigated (Table 2 ). There was no significant difference in the An/gs values in different planting methods, however, 50%WR significantly increased the An/gs in both growing seasons. Leaf transpiration rates were also measured 4 times (on the same dates with An and gs) during the growing seasons (Fig. 7 ). Leaf transpiration rate (Tr) was 17.6% higher in 100%WR in comparison to that obtained in 50%WR, respectively. Furthermore, Tr was 4.5% and 8.6% higher in the in-furrow in comparison to basin and on-ridge planting methods, respectively (Table 2 ).
Light extinction coefficient
The fraction of transmitted radiation vs. leaf area index for quinoa, on average in two growing, is shown in Fig. 8 , as the effect of year was not significant (p-value > 0.05) on K values. The slope of the fitted line to the logarithm of the ratio of transmitted light against the leaf area index (LAI) indicates the light extinction coefficient (K). The K values were higher in the in-furrow planting in comparison to the basin and on-ridge planting. On the other hand, irrigation water level significantly affected K values (Table 3 ). Pooled over planting method, K values were 0.63, 0.54 and 0.43 in 100%WR, 75%WR and 50%WR, respectively, which was 16.8% and 46.4% higher in 100%WR in comparison to those obtained in 75%WR and 50%WR, respectively. Also, the in-furrow planting increased the K by 8.4% and 10.8% in comparison to basin and on-ridge planting methods, respectively, when it was fully irrigated.

Fraction of transmitted radiation vs. leaf area index in different Irrigation water levels (I1:100%WR, I2:75%WR and I3:50%WR) and planting methods in two growing seasons
Table 3
Extinction coefficient for different irrigation water levels and planting methods on average in two growing seasons
| I1 (100%WR) | 0.604 | 0.617 | 0.669 |
| I2 ( 75%WR) | 0.556 | 0.558 | 0.504 |
| I3 ( 50%WR) | 0.429 | 0.413 | 0.449 |
Crop growth components and yield
Soil water stress is one of the abiotic stresses that plants encounter during their life cycle [ 55 ]. It poses a serious threat to various aspect of plant development including plant growth, yield, survival, and productivity [ 56 – 58 ]. In the current study, we observed a reduction of 11% in plant height of quinoa across the reduced water levels, while reduction in height ranged between 10 and 13% in basin, in-furrow and on-ridge planting, regardless of time. A reduction in growth parameters, grain yield and yield components as a result of limiting soil water content from complete irrigation to deficit irrigation and its effects on plant growth has been well established in the literature [ 59 , 60 ]. Soil water stress negatively affect the plant physiological mechanisms that aid in water and nutrient uptake, thus severely affecting the cell growth and division [ 61 , 62 ]. Also, the decrease in plant growth under deficit irrigation could be attributed to decrease in water and nutrient uptake as well as a decrease in stomatal conductance, which results in reduced photosynthesis [ 63 ].
Quinoa height, stem diameter and leaf area index were found to be significantly (p-value < 0.05) affected by deficit irrigation. This is consistent with previous research [ 64 , 65 ], indicating that disruption in cell division and cell elongation processes is a direct effect of water stress leading to the reduction in plant height and leaf area of plants. Semerci et al. [ 66 ] observed a significant decline in total growth, including shoot height, biomass, and leaf number, during drought stress associated with reduced turgor pressure, which led to growth retardation in the plant. Also, it is reported that quinoa avoids water stress primarily by developing a longer root, intense root system, leaf dropping and reduced leaf area [ 67 , 68 ]. Furthermore, the application of in-furrow planting treatments resulted in high leaf area index values, which may have contributed to lower evaporation from the soil surface.
Water scarcity disrupts the plants’ water balance by lowering the soil’s water potential, which seriously affects the plants’ water potential. Consequently, the immediate reaction of all plants under drought stress is to reduce transpiration by closing the stomatal opening [ 69 ]. In response to the drought stress, the quinoa plant maintains its turgor by accumulating a variety of inorganic ions [ 70 ], which results in decreased leaf osmotic potential. In addition, drought escape, or tolerance is mainly achieved through low osmotic potential and tissue elasticity [ 35 ].
The relationship of the grain yield and the total dry matter with LWP in different planting methods is shown in Fig. 9 a and b. The equations of the fitted lines are shown in Table 4 ,

The relation of total dry matter and grain yield with the average leaf water potential of quinoa in different planting methods (P1: basin, P2: on-ridge and P3: in-furrow) on average in two growing seasons
Table 4
Relationships between Toral dry matter (TDM) and grain yield (GY) and leaf water potential (LWP)
| Number | Planting | Equation | R | SE | value |
|---|---|---|---|---|---|
| 11 | In-furrow | TDM = -2.63(-LWP) + 14.35 | 0.99 | 0.04 | 0.024 |
| 12 | On-ridge | TDM = -2.55(-LWP) + 13.58 | 0.97 | 0.01 | 0.005 |
| 13 | Basin | TDM = -2.38(-LWP) + 13.63 | 0.99 | 0.003 | 0.0015 |
| 14 | In-furrow | GY = -1.06(-LWP) + 4.34 | 0.99 | 0.02 | 0.024 |
| 15 | On-ridge | GY = -0.85 (-LWP) + 3.79 | 0.99 | 0.02 | 0.005 |
| 16 | Basin | GY = -0.76 (-LWP) + 3.74 | 0.99 | 0.007 | 0.0015 |
a R 2 is coefficient of determination, SE Is standard error
in which, TDM is the total dry matter (Mg ha −1 ), GY (Mg ha −1 ) and LWP is the average leaf water potential (MPa). Our results provided further insights into how dry matter buildup interacts at a certain LWP threshold in different planting methods. The higher slope of the fitted line in the in-furrow planting showed that a higher amount of yield and dry matter was obtained in a specific LWP in comparison to those obtained in the basin and on-ridge planting indicating that less water stress was imposed to the crop in the in-furrow in comparison to on-ridge planting. The lower plant temperature in the in-furrow planting method can result in lower plant respiration which leads to higher grain and dry matter yield. Li et al. [ 71 ] reported that high respiration may be the primary contributor to yield losses in high temperatures. Also, Fig. 10 illustrates the relationship between leaf surface temperature and LWP. Results depict that as LWP decreased the leaf surface temperature increased owing to decreased transpiration or the loss of water vapor via the leaf stomata, that results in less cooling of the leaf surfac.

The relationship between Leaf surface temperature and leaf water potential (LWP) of quinoa on average in two growing seasons
We analyzed the combined effect of irrigation water level and planting methods on the yield and dry matter of quinoa. In this study, the grain yield varied within the range of 1.05 Mg ha −1 and 2.5 Mg ha −1 , which is consistent with the findings of Algosaibi et al. [ 24 ] and Delgado et al. [ 72 ]. Yield reduction was mainly due to drought stress which was imposed on the crop. Our result showed that deficit irrigation can significantly reduce quinoa yield and dry matter especially in 50%WR, which is consistent with the results of Al-Naggar et al. [ 73 ] and in contrast with the results of Pulvento et al. [ 74 ] and Razzaghi et al. [ 75 ], which reported that deficit irrigation does not affect quinoa yield and growth significantly. The contrast may be due to different climate conditions (lower temperature) in their study region and the region of the current study. Bertero [ 76 ] also challenges the notion that quinoa reaches high yields with low water availability by analyzing several kinds of literatures on quinoa yield and reporting that the highest efficiency of quinoa is from temperate climates. Greater yield loss results from the interaction of the stresses of heat and drought than from either stress alone. Hinojosa [ 77 ] reported that quinoa is sensitive to the combination of heat and drought. Geerts et al. [ 54 ] reported that by applying 50% of the required irrigation water depth, the quinoa yield can be stabilized between the range of 1.2–2.0 Mg ha −1 . In addition, the TDM performance of quinoa under water stress in the in-furrow planting was better than the other two planting methods. Furthermore, TDM production of quinoa was not much affected under water stress conditions proportional to the imposed water stress. The relationship between quinoa grain yield and shoot dry matter was obtained as follows:
In which, SY is the grain yield and SDM is the shoot dry matter (kg ha −1 ). The intercept of the relationship between the grain yield and SDM showed that about 1143 kg ha −1 of shoot dry matter is required before seed production begins. The process in which the assimilates move from source organs to sink organs (i.e., seeds) is called the partitioning of dry matter [ 78 ] that is an important variable to consider when assessing adaptability to abiotic stress [ 79 ]. In this study the dry matter partitioning coefficient (PC) for seed was 11.4%, 11.5% and 9.4% in 100%WR, 75%WR and 50%WR, respectively. Thus, 75%WR did not reduce PC for seed, however, it was reduced by 18.2% in 50%WR. Therefore, quinoa is susceptive to severe water stress.
Quinoa crop is drought resistant; nevertheless, their performance is reduced under water stress in deficit irrigation [ 80 ]. The effect of deficit irrigation on quinoa performance depends on the degree of water stress and it also influenced by other environmental factors [ 81 ]. Results revealed that deficit irrigation reduces quinoa growth and yield in the current investigation, and our results are in agreement with previous studies [ 81 – 85 ].
There are several methods to conserve the soil water and increase the crop growth [ 86 ], whereas in-furrow planting is one of these methods [ 59 ]. By in-furrow planting, where the canopy cover shades the soil surface in the furrow, it reduces the soil surface evaporation and increases the crop transpiration and crop growth [ 11 ]. Besides reduction in evaporation, in-furrow planting increases the soil temperature in winter and decreases it in summer, that enhances the soil environmental condition for root and crop growth [ 11 ]. The reduction of dry matter (Fig. 4 ) was more associated with a significant decrease in stem diameter rather than LAI (Table 1 ), which is in agreement with that reported by Hejnak et al. [ 87 ].
Photosynthesis, which is regarded as an invariably important process, is extremely vulnerable to drought stress and is the first process that is affected by deficit irrigation [ 88 ]. Drought-induced decreases in photosynthetic capacity have been widely reported in the literature, because of reduced stomatal conductance and defective photosynthetic machinery [ 89 ]. In response to drought stress the plants lower their transpiration by closing stomatal openings. Stomatal openings regulate CO 2 and water in the plants. Stomatal closure reduces water loss; however, it lowers CO 2 absorption [ 90 ], which is an essential element of photosynthesis, resulting in carbon deficiency, which affects many other mechanisms [ 91 ]. There are many studies that reported that drought stress affects physiological parameters and gas exchange of plants. Ali et al. [ 92 ] reported a decline in photosynthetic rate, transpiration rate, stomatal conductance, and intercellular CO 2 . Yang et al. [ 63 ] observed stomatal conductance reduction and enhanced leaf water potential in quinoa plants. They stated that the decrease in stomatal conductance can be attributable to the increasing ABA concentration in leaves as under abiotic stresses especially drought stress. It signals the plant to close its stomata to conserve water. The relationship between stomatal conductance and transpiration rate with leaf water potential are determined and shown in Fig. 11 a and b and Table 5 , in which, gs is the stomatal conductance (mol m −2 s −1 ), Tr is the leaf transpiration rate (mmol m −2 s −1 ) and LWP is the leaf water potential (MPa). Comparing the slope of relationship between gs and LWP (-0.133) and Tr and LWP (-0.69) showed that transpiration rate is more sensitive to the variation in LWP. The value of gs at LWP equal to zero (0.69 mol m −2 s −1 ) is the highest gs that can be obtained. The relationship between photosynthesis rate and stomatal conductance was also determined (Fig. 12 ). In early growth stages of crops under water stress, reduced stomatal conductance, and lowered transpiration rate more than it does the intercellular CO 2 concentration, which is the driving force for photosynthesis [ 93 ]. Previous studies suggested nonlinear relationship between An and gs [ 93 , 94 ]; therefore, an exponential equation was fitted to the data and is shown in Table 5 , in which, An is the photosynthesis rate (µmol m −2 s −1 ) and gs is the stomatal conductance (mol m −2 s −1 ). In Fig. 1 2, 0.45 mol m −2 s −1 can be considered as the turning point of the fitted line, which indicated that water productivity can be increased at mild water stress because of the non-linear relationship between An and gs and the fact that An is less sensitive to water stress than gs [ 93 ]. For water-scarcity adaptation, the intrinsic water use efficiency (An/gs) is regarded as a crucial factor [ 29 ]. In our study, no variations in An/gs values amongst planting methods were found to be statistically significant; nevertheless, it was observed that An/gs values in 50%WR were greater than those obtained in 100% and 75%WR. The An/gs determination is based on gas exchange measurements made all at once which are unable to provide accurate variations in An/gs [ 95 ].

Relationship between a : stomatal conductance (gs) and b : transpiration rate (Tr) with leaf water potential (LWP) measured during two growing seasons
Table 5
Relationships between total dry matter (TDM) and photosynthesis rate (An), stomatal conductance (g s ) and leaf water potential (LWP), leaf transpiration (Tr) and LWP, An and g s , and An and LWP
| Number | Equation | R | SE | n | value |
|---|---|---|---|---|---|
| 17 | gs = -0.133(-LWP) + 0.72 | 0.68 | 0.0001 | 18 | 0.0001 |
| 18 | Tr = -0.69(-LWP) + 6.09 | 0.68 | 0.0001 | 18 | 0.0001 |
| 19 | An = 29.08 (1- e ) | 0.66 | - | - | - |
| 20 | TDM = 367.3 An | 0.97 | 0.0001 | 18 | 0.0001 |
| 21 | A = -3.3451(-LWP) + 29.05 | 0.91 | 0.0001 | 18 | 0.0001 |
a R 2 is coefficient of determination, SE is standard error, n is number of data

Relationship between stomatal conductance (gs) and photosynthesis rate (An) measured during two growing seasons
Figure 12 shows the relationship between stomatal conductance and photosynthesis with the difference between air temperature (Ta) and leaf temperature (Tl). The negative Ta-Tl is related to treatments exposed to severe water stress, where there was not enough water to help the plant to reduce the leaves temperature, and it was related to the mid and late season, when the air temperature exceeded 35 °C degrees. A reduction in stomatal conductance, an increase in photosynthesis, and a greater differential between air and leaf temperatures were related to high temperatures [ 96 ]. According to Fig. 13 , the highest An, gs and Tr values occurred when the air temperature was 3–5°C higher than the leaf temperature. In addition, the relation between the difference between air and leaf temperature (ΔT) with leaf water potential (LWP) is shown in Fig. 14 , which showed that ΔT is equal to zero when the LWP is -3.1 MPa. According to Fig. 14 , when the LWP decreased to less than -3.1 MPa, the leaf temperature increased even to be higher than the air temperature as there was not enough water to help the crop cool down.

The variation of a : stomatal conductance (gs) and b : photosynthesis rate (An) and c : transpiration rate (Tr) with the difference between air temperature (Ta) and leaf temperature (Tl)

The relationship of the difference between air and leaf temperature with leaf water potential (-LWP) on average during two growing seasons
Also, the relationship between total dry matter and photosynthesis rate was determined and is shown in Table 5 , in which, TDM is the total dry matter (kg ha −1 ) and An is the photosynthesis rate (µmol m −2 s 1 ). Results showed that there is a positive relationship between the seasonal mean photosynthesis rate and end-of-season dry matter. Thus, higher photosynthesis rates contributed to higher dry matter in both seasons. The relationship between the photosynthesis rate (A n ) and leaf water potential (LWP) was also determined and is shown in Table 5 , in which, An is the photosynthesis rate (µmol m −2 s −1 ) and LWP is the leaf water potential (MPa). Equation (21) in Table 5 shows that An was reduced with a decrease in leaf water potential; however, the rate of decline has not been very sharp. Also, the intercept of the equation shows that the highest photosynthesis rate at LWP equal to zero, is 29 µmol m −2 s −1 .
LAI regulates gas exchange processes such as photosynthesis [ 31 ], evapotranspiration [ 32 ]. LAI depends on species, developmental stage, prevailing site conditions, seasonality, and management practices [ 97 ]. Under water stress conditions, inhibiting leaf growth improves water balance and stress tolerance by reducing water loss to ensure plant survival [ 98 ]. LAI can be determined by direct methods which are time-consuming. The higher dry matter and leaf area index in the in-furrow is the result of higher photosynthesis rate. The value of An had the highest amount at the beginning of the growing seasons and was reduced to the lowest in the late season. This may be due to the increase in air temperature at the end of the growing season.
Under high irrigation water level with high soil water condition the gas exchange parameters were higher, and this increase was in agreement with that reported by Hejnak et al. [ 87 ]. Furthermore, this increase in gas exchange parameters resulted in increase in crop yield. Lu and Zeiger [ 99 ] reported that the higher yield of cotton was associated with stomatal conductance (g s ), where Levi et al. [ 100 ] found no relation between cotton yield and g s . Our results for quinoa were supported by the earlier findings under the well-watered conditions. However, it was not supported by the later finding due to different cultivars of cotton.
By decrease in irrigation level (100%WR to 50%WR) the reduction in An of quinoa was greater than g s (Table 2 ); therefore, the An/g s was higher. However, decrease in irrigation level from 100%WR to 75%WR resulted no reduction in An/g s that is the reason for 75%WR and in-furrow planting to be optimal treatments. This also is supported by leaf water use efficiency (An/T r ) in Table 3 . Also, results of Hejnak et al. [ 87 ] for cotton indicated the enhanced tolerance to deficit irrigation was correlated with the g s trait and efficiency of An. Furthermore, they showed that the most noticeable decrease in irrigation water level induced the gas exchange parameters, An, g s and T r .
Higher An in the in-furrow planting led to higher dry matter and LAI for saffron in comparison with that obtained in the basin planting [ 101 , 102 ] due to appropriate soil water condition as a result of reduced soil surface evaporation. Furthermore, An is highly sensitive to severe deficit irrigation (soil water stress) [ 101 , 103 , 104 ]. However, in the current study on quinoa, 50%WR reduced the An by 21% in comparison with that obtained in 100%WR. This may be due to the fact that most of the quinoa water requirement is provided by irrigation water, which is reduced in 50%WR deficit irrigation. Also, results for quinoa showed higher An that led to higher leaf dry matter, that is in agreement with those reported by Echarte et al. [ 105 ]. The negative relationship between An and leaf water potential (LWP) [Eq. (21) in Table 5 ] was also determined. Similarly, Renau-Morata et al. [ 106 ] reported that high An was maintained by supplying water by root in higher LWP.
The value of g s for quinoa was reduced in deficit irrigation compared to 100%WR similar as reported for saffron by Dastranj and Sepaskhah [ 101 ]. Its value was also higher in the in-furrow planting compared to the basin planting similar as reported for saffron by Dastranj and Sepaskhah [ 101 ]. Only deficit irrigation of 50%WR reduced leaf transpiration (T r ) for quinoa (Table 2 ). Furthermore, the in-furrow planting increased T r and leaf water use efficiency (An/T r ) compared to the basin planting. These findings support the in-furrow planting and 75%WR irrigation as the optimal treatment for quinoa to be recommended in field irrigation management for quinoa.
At present, quinoa farmers in Iran do not use this field irrigation management. Common irrigation scheduling is four surface irrigation events with irrigation efficiency of 50% in semi-arid region in four different growth stages as: (i) Germination, (ii) Vegetative, (iii) flowering initiation, (iv) Grain filling [ 107 ]. Therefore, farmers can apply irrigation water depth as 75%WR at four different growth stages and save irrigation water.
Light extinction coefficient (K), which is a valuable metric for assessing light penetration through crop canopy, can be used to estimate LAI. It can also provide insights into quinoa's photosynthetic potential. The value of K is related to the leaf inclination angle, leaf arrangement and LAI. In the current study, K values varied between 0.41 to 0.67. This is consistent with the findings of Ruiz and Bertero [ 108 ], which reported that K varied between 0.52 and 0.74 for different planting densities. Comparatively, quinoa’s extinction coefficients were moderate when comparing to other crops such as sunflower (0.82, [ 109 ], barley (0.4–0.46, [ 110 ], sorghum and corn (0.4, [ 111 ]). The decrease in K in the 75%WR and 50%WR treatments in comparison to 100%WR may be attributed to the change in leaf’s angle because of the wilting and dropping of the leaves as a result of deficit irrigation and plant water stress. The negative correlation between K and LAI may be since the increase of LAI in the growing season is usually associated with the change in canopy architecture, such as foliage density, stem length, and clumping intensity. The amount of water needed to support normal plant development at any stage varies not only on the soil’s water status but also on the environment around the plants as well as their individual characteristics [ 112 ]. The relation between the water stress coefficient (presented in [ 59 ]) and the extinction coefficient was determined (Fig. 15 ). Results showed that K increases as the Ks increases. Thus, water stress reduced leaf area growth resulting in decreased PAR interception, which in turn results in decreased K leading to decreased biomass production and yield.

The relationship between Extinction coefficient and water stress coefficient
A prominent abiotic stress that plants experience during their life cycle is drought stress, which poses a serious threat to plant productivity, yield, growth, and survival. To establish the optimal strategy for quinoa cultivation, we investigated how different planting techniques and irrigation water levels affected the production, physiological characteristics, and gas exchange of quinoa in a dry and semi-arid region.
Our research demonstrated that drought stress has a substantial effect on quinoa cultivation, emphasizing the need of using optimal planting procedures and irrigation strategies. The results revealed that the highest protein yield was obtained in 75%WR combined by in-furrow planting while the highest grain yield was observed in in-furrow planting method in 100%WR, which highlighted the possibility of using more effective irrigation method without compromising the seed quality. It is important to note that yield reduction can be primarily attributed to the imposed drought stress. Furthermore, the in-furrow planting exhibited higher leaf water potential, indicating better water availability for the crop compared to the other planting methods. On the other hand, the leaf temperature values in the on-ridge planting were higher in comparison to those obtained in the basin and in-furrow planting methods. Under water stress condition, the leaf growth was decreased to minimize the water loss to ensure plant survival; however, photosynthesis was the first process that was affected by deficit irrigation. However, photosynthesis rate (An) reduction with diminishing LWP was mild which provided insights to quinoa’s adaptability to drought. The extinction coefficient for quinoa was found to be intermediate compared to other crops and it was decreased when exposed to the deficit irrigation. The order of grain yield and dry matter reduction in irrigation levels was 100%WR and 75%WR < 50%WR, and the order of planting methods were in-furrow < basin < on-ridge planting; therefore, the in-furrow and 75%WR is preferrable. Furthermore, the 75%WR and in-furrow planting is optimal for protein yield. To sum up, the on-ridge planting method is not suggested for quinoa cultivation and the in-furrow planting method with 75%WR proved to be the best treatment in terms of yield and physiological traits of quinoa in the study area.
Acknowledgements
This research was supported in part by the Research Project funded by grant no. 02-GR-AGR-42 of Shiraz University Research Council, and Drought Research Center, Center of Excellence for On-Farm Water Management and Iran national Science foundation (INSF).
Abbreviations
| ABA | Abscisic acid |
| An | Photosynthesis rate |
| DAS | Days after sowing |
| ETo | Potential reference evapotranspiration |
| ETc | Standard crop evapotranspiration |
| GDD | Growing degree days |
| Gs | Stomatal conductance |
| GY | Grain yield |
| I1, I2, and I3 | 100% WR, 75%WR, and 50%WR |
| K | Light extinction coefficient |
| LAI | Leaf area index |
| LAI | Maximum leaf area index |
| LP | Length of leaf |
| LWP | Leaf water potential |
| NP | Number of panicles |
| P1, P2, and P3 | Basin, on-ridge, and furrow planting |
| PAR | Photosynthetically active radiation |
| PC | Partitioning coefficient |
| PY | Protein yield |
| SDM | Shoot dry matter |
| T | Air temperature |
| TDM | Total dry matter |
| Tl | Leaf temperature |
| Tr | Leaf transpiration rate |
| WR | Water requirement |
Authors’ contributions
Conceptualization: A.R.S. Data curation: S.M. M. Formal analysis: S.M.M., and A.R. S. Funding acquisition: A.R.S. Investigation: S.M.M., and A.R. S. Methodology: S.M. M., and A.R.S. Project administration: A.R.S. Resources: A.R.S. Supervision: A.R.S. Validation: A.R.S., and S.H.A. Visualization: S.M.M., A.R.S., and S.H.A. Writing—original draft: S.M.M., and A.R.S. Writing – Review & editing: S.M.M., A.R.S., and S.H.A.
Availability of data and materials
Declarations.
The Quinoa seeds have been collected from the former experiments of the third author Seyed Hamid Ahmadi that had fulfilled and published the associated articles. The references to these experiments and articles are:
Razzaghi et al., (2011) Water Relations and Transpiration of Quinoa (Chenopodium quinoa Willd.) Under Salinity and Soil Drying, Journal of Agronomy and Crop Science 197(5): 348–360.
Razzaghi et al., (2012) Effects of Salinity and Soil–Drying on Radiation Use Efficiency, Water Productivity and Yield of Quinoa (Chenopodium quinoa Willd.), Journal of Agronomy and Crop Science 198(3): 173–184.
Not applicable.
The authors declare no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.

IMAGES
VIDEO
COMMENTS
Global-to-basin impacts. We calculate both physical water scarcity (Fig. 1B) and its economic impact (Fig. 1C) over the 21st century for 235 river basins for each of the 3000 global change ...
Water Resources Research is an AGU hydrology journal publishing original research articles and commentaries on hydrology, water resources, and the social sciences of water. ... (2008, 2013) and use water deficit as an impact metric defined as the difference between water demand and water allocated (see section 2.1.4 ... In this paper we measure ...
Water consumption may also facilitate weight management (15,17). Water deficits can impact physical performance (25,38), and recent research suggests that cognitive performance may also be impacted (4,13,20-22,35,36). This article will address water balance, hydration assessment, and the effect of water balance on cognitive performance.
Vapor pressure deficit is the difference between the amount of water vapor that air can hold and the amount of vapor in the air, whereas climatic water deficit measures climatic water demand unmet by soil moisture supply (Grossiord et al., 2020; Seager et al., 2015; Stephenson, 1990). It is the supply and demand imbalance quantified in the CWD ...
In the first two decades of the 21st century, drought severely affected 79 global cities (Zhang et al., 2019). Global urban population (GUP) facing water scarcity is projected to increase from 933 million (33% of GUP) in 2016 to 1.693-2.373 billion people (which will represent from 35 to 51% of GUP) in 2050.
Water Resources Research is an AGU hydrology journal publishing original research articles and commentaries on hydrology, water resources, and the social sciences of water. ... In this paper, the approximation of ΔTWS was calculated by the following method according to S. Wang, ... The water deficit occurred mainly in April, May, June, July ...
Soil water content also decreases with the increase of vegetation planting years, which may lead to or aggregate soil desiccation (Jia et al., 2017, Liu et al., 2018, Huang et al., 2018). Our research also showed that the long-term planting of S. japonica has caused a serious water deficit in the 0-160 cm soil layer of the forestland ...
Deficit irrigation and water productivity. When water supplies are limiting, the farmer's goal should be to maximize net income per unit water used rather than per land unit. Recently, emphasis has been placed on the concept of water productivity (WP), defined here either as the yield or net income per unit of water used in ET (Kijne et al., 2003).
Climate change is increasingly impacting the water deficit over the world. Because of drought and the high pressure of the rising human population, water is becoming a scarce and expensive commodity, especially in developing countries. The identification of crops presenting a higher acclimation to drought stress is thus an important objective in agriculture. The present investigation aimed to ...
RESEARCH PAPER. The legacy of water deficit on populations having experienced negative hydraulic safety margin. Marta Benito Garzón, Corresponding Author. ... The aim was to examine whether recent mortality can be explained by hydraulic failure linked to water deficit. Location. Western Europe. Time period. 1986-2014.
Deficit Irrigation and Water Conservation. Samiha Ouda, Tahany Noreldin; Pages 15-27. Download chapter PDF ... She published 88 research papers, 40 book chapters and 4 books on irrigation water management, modeling, crop simulation, agroclimatology, climate change impacts on crops and its water requirements. She supervised 4 Master and PhD ...
In general, provision of water is beneficial in those with a water deficit, but little research supports the notion that additional water in adequately hydrated individuals confers any benefit. ... Lamb DR, editors. Youth, exercise, and sport: Symposium: Papers and discussions; 1989; Indianapolis: Benchmark; 1989. pp. 335-367. [Google Scholar ...
The water requirements of crops should be investigated to improve the efficiency of water use in irrigated agriculture. The main objective of the study was to assess the effects of water deficit stress on rice yields throughout the major cropping seasons. We analyzed rice yield data from field experiments in Taiwan over the period 1925-2019 to evaluate the effects of water-deficit stress on ...
Water deficit is considered one of the most limiting factors of the common bean. Understanding the adaptation mechanisms of the crop to this stress is fundamental for the development of drought-tolerant cultivars. In this sense, the objective of this study was to analyze the influence of water deficit on physiological and morphoagronomic traits of common bean genotypes with contrasting drought ...
Fig. 4 highlights the differences between the two different weights exemplified in this paper; in particular, we can notice that the fractional weight emphasizes the effect of plant water stress on the water content dynamics: this can also be deduced by observing different plant water deficit index in Fig. 5.. Download : Download high-res image (295KB)
intensity and duration, when applied alone or in combination (Bhadula et al., 1998). The. acclimation capacity of the plant depends on the presence of a certain buffer propert y, i.e. a. given ...
Gradual water deficit resulted in an average reduction in total yield of 14.9% in 2021 and 10.5% in 2022 with a reduction in irrigation water at the S66 level, whereas the application of S33 ...
The main component of the overall water deficit for Egypt originates from the intrinsic water gap between the internal demand and the presently available renewable water supply. ... This research is funded under support from the Zumberge Research and Innovation Fund of the University of Southern California allocated to the Arid Climates and ...
For the purposes of this paper, dehydration will be the term used to encompass the state of improper hydration due to unbalanced water loss or water deficit. In Europe, the percentage of the population reported to have inadequate water intake is estimated to vary from 5 to 35% [9,10,11].
Research Full Length Research Paper Impact of water deficit on growth attributes and yields of banana cultivars and hybrids K. Krishna Surendar1*, V. Rajendran1, D. Durga Devi2, P. Jeyakumar2, I. Ravi3 and K. Velayudham4 1Vanavarayar Institute of Agriculture, Pollachi, India.
Climate change has become a concern, emphasizing the need for the development of crops tolerant to drought. Therefore, this study is designed to explore the physiological characteristics of quinoa that enable it to thrive under drought and other extreme stress conditions by investigating the combined effects of irrigation water levels (100%, 75%, and 50% of quinoa's water requirements, WR as ...
Nitrogen (N) uptake is regulated by water availability, and a water deficit can limit crop N responses by reducing N uptake and utilization. The complex and multifaceted interplay between water availability and the crop N response makes it difficult to predict and quantify the effect of water deficit on crop N status.
The impact of water deficit and bioinoculants on soil microbial activity (fluorescein diacetate hydrolysis) was also evaluated. Moderate and severe water deficit negatively affected soil microbial activity, as well as, maize growth, by reducing plants' shoot biomass and increasing root/shoot ratio at 60 and 40% of WHC.
Water scarcity within the world may be an enormous threat to living beings. Potable water can be produced by renewable energy using solar desalination. In the quest for efficient water desalination methods, researchers have turned to solar stills as a promising solution.
Water deficit has a significant impact on the quality and yield of crops. Canopy leaf water content is an important indicator of water deficit in plant tissues (Li et al., 2022a). Timely and accurate acquisition of crop canopy water content (CWC) information is crucial for precision irrigation in arid regions, enhancing water use efficiency and ...