
- HRP Staff Directory
- Office Hours
- Quality Improvement Project vs. Research
- Self Exempt & UROP
- Case Reports
- Commercial IRB Reliance Agreements
- National Cancer Institute Central IRB (CIRB) Independent Review Process
- UCI as the Reviewing IRB
- Submitting the Application
- Lead Researcher Eligibility
- Training & Education
- Ethical Guidelines, Regulations and Statutes
- Other Institutional Requirements
- Department of Defense Research Requirements
- Levels of Review
- Data Security
- Protected Health Information (HIPAA)
- European Union General Data Protection Regulation (EU GDPR)
- China’s Personal Information Protection Law
- Required Elements of Informed Consent
- Drafting the Informed Consent Form
- Consent and Non-English or Disabled Subjects
- Use Of Surrogate Consent In Research
- Vulnerable Populations
- Data and Safety Monitoring for Clinical Research
- Placebo-Controlled Studies
- Expanded Access to Unapproved Drugs or Biologics
- Right to Try: Unapproved Drugs or Biologics
- Use of Controlled Substances
- Expanded Access to Unapproved Medical Devices
- Humanitarian Use Devices
- Human Gene Transfer Research
- How To Register and Update Your Study
- Post-Review Responsibilities

Assessing Risks and Benefits
The IRB is responsible for evaluating the potential risks and weighing the probability of the risk occurring and the magnitude of harm that may result. It must then judge whether the anticipated benefit, either of new knowledge or of improved health for the research subjects, justifies inviting any person to undertake the risks.
Per DHHS and FDA regulations ( 45 CFR 46.111 and 21 CFR 56.111 ) two of the required criteria for granting IRB approval of research are:
- Risks to subjects are minimized by using procedures which are consistent with sound research design and which do not unnecessarily expose subjects to risk, and whenever appropriate, by using procedures already being performed on the subjects for diagnostic or treatment purposes.
- Risks to subjects are reasonable in relation to anticipated benefits, if any, to subjects, and the importance of the knowledge that may reasonably be expected to result. In evaluating risks and benefits, the IRB Committee will consider only those risks and benefits that may result from the research, as distinguished from risks and benefits of therapies subjects would receive even if not participating in the research.
- Benefit A valued or desired outcome; an advantage.
- Risk The probability of harm or injury (physical, psychological, social, or economic) occurring as a result of participation in a research study. Both the probability and magnitude of possible harm may vary from minimal to significant. Federal regulations define only "minimal risk."
- Minimal Risk A risk is minimal where the probability and magnitude of harm or discomfort anticipated in the proposed research are not greater, in and of themselves, than those ordinarily encountered in daily lives of the general population or during the performance of routine physical or psychological examinations or tests.
- Minimal Risk for Research involving Prisoners The definition of minimal risk for research involving prisoners differs somewhat from that given for non-institutionalized adults. Minimal risk is in this case is defined as, "the probability and magnitude of physical or psychological harm that is normally encountered in the daily lives, or in the routine medical, dental or psychological examinations of healthy persons."
There are two sources of confusion in the assessment of risks and benefits. One arises from the language employed in the discussion:
- "Risk" is a word expressing probabilities;
- "Benefits" is a word expressing a fact or state of affairs.
It is more accurate to speak as if both were in the realm of probability: i.e., risks and expected or anticipated benefits. Confusion also may arise because "risks" can refer to two quite different things:
- those chances that specific individuals are willing to undertake for some desired goal; or
- the conditions that make a situation harmful to a subject.
Researchers should provide detailed information in the IRB application about potential risks and benefits associated with the research, and provide information about the probability, magnitude and potential harms associated with each risk.
The IRB cannot approve research in which the risks are judged unreasonable in relation to the anticipated benefits. The IRB must:
- As applicable, evaluate the available clinical and nonclinical information on an investigational product to determine if the data is adequate to support the proposed clinical trial;
- Determine that the risks will be minimized to the extent possible [see below];
- Identify the probable benefits to be derived from the research;
- Determine that the risks are reasonable in relation to be benefits to subjects , if any, and the importance of the knowledge to be gained; and
- Assure that potential subjects will be provided with an accurate and fair description (during consent) of the risks or discomforts and the anticipated benefits.
The risks to which research subjects may be exposed have been classified as physical, psychological, social, and economic .
- Physical Harms Medical research often involves exposure to minor pain, discomfort, or injury from invasive medical procedures, or harm from possible side effects of drugs. All of these should be considered "risks" for purposes of IRB review. Some of the adverse effects that result from medical procedures or drugs can be permanent, but most are transient. Procedures commonly used in medical research usually result in no more than minor discomfort (e.g., temporary dizziness, the pain associated with venipuncture).Some medical research is designed only to measure more carefully the effects of therapeutic or diagnostic procedures applied in the course of caring for an illness. Such research may not entail any significant risks beyond those presented by medically indicated interventions. On the other hand, research designed to evaluate new drugs or procedures may present more than minimal risk, and, on occasion, can cause serious or disabling injuries.
- Psychological Harms Participation in research may result in undesired changes in thought processes and emotion (e.g., episodes of depression, confusion, or hallucination resulting from drugs, feelings of stress, guilt, and loss of self-esteem). These changes may be transitory, recurrent, or permanent. Most psychological risks are minimal or transitory, but some research has the potential for causing serious psychological harm.Stress and feelings of guilt or embarrassment may arise simply from thinking or talking about one's own behavior or attitudes on sensitive topics such as drug use, sexual preferences, selfishness, and violence. These feelings may be aroused when the subject is being interviewed or filling out a questionnaire. Stress may also be induced when the researchers manipulate the subjects' environment - as when "emergencies" or fake "assaults" are staged to observe how passersby respond. More frequently, however, is the possibility of psychological harm when behavioral research involves an element of deception.
- Is the invasion of privacy involved acceptable in light of the subjects' reasonable expectations of privacy in the situation under study;
- Is the research question of sufficient importance to justify the intrusion?
- The IRB must also consider whether the research design could be modified so that the study can be conducted without invading the privacy of the subjects.
- Note: Breach of confidentiality is sometimes confused with invasion of privacy, but it is really a different risk. Invasion of privacy concerns access to a person's body or behavior without consent; confidentiality of data concerns safeguarding information that has been given voluntarily by one person to another.
- Some research requires the use of a subject's hospital, school, or employment records. Access to such records for legitimate research purposes is generally acceptable, as long as the researcher protects the confidentiality of that information. However, it is important to recognize that a breach of confidentiality may result in psychological harm to individuals (in the form of embarrassment, guilt, stress, and so forth) or in social harm (see below).
- Social and Economic Harms Some invasions of privacy and breaches of confidentiality may result in embarrassment within one's business or social group, loss of employment, or criminal prosecution. Areas of particular sensitivity are information regarding alcohol or drug abuse, mental illness, illegal activities, and sexual behavior. Some social and behavioral research may yield information about individuals that could "label" or "stigmatize" the subjects. (e.g., as actual or potential delinquents or schizophrenics). Confidentiality safeguards must be strong in these instances. Participation in research may result in additional actual costs to individuals. Any anticipated costs to research participants should be described to prospective subjects during the consent process.
- Provide complete information in the protocol regarding the experimental design and the scientific rationale underlying the proposed research, including the results of previous animal and human studies.
- Assemble a research team with sufficient expertise and experience to conduct the research.
- Ensure that the projected sample size is sufficient to yield useful results.
- Collect data from standard-of-care procedures to avoid unnecessary risk, particularly for invasive or risky procedures (e.g., spinal taps, cardiac catheterization).
- Incorporate adequate safeguards into the research design such as an appropriate data safety monitoring plan, the presence of trained personnel who can respond to emergencies, and procedures to protect the confidentiality of the data (e.g., encryption, codes, and passwords).
- IRB-SBS Home
- Contact IRB-SBS
- IRB SBS Staff Directory
- IRB SBS Board Members
- About the IRB-SBS
- CITI Training
- Education Events
- Virginia IRB Consortium
- IRB-SBS Learning Shots
- HRPP Education & Training
- Student Support
- Access iProtocol
- Getting Started
- iProtocol Question Guide
- iProtocol Management
- Protocol Review Process
- Certificate of Confidentiality
- Deception and/or Withholding Information from a Participant
- Ethnographic Research
- IRB-SBS 101
- IRB-SBS Glossary
- Participant Pools
- Paying Participants
- Research in an Educational Setting
- Research in an International Setting and/or Location
- Risk-Sensitive Populations
- Student Researchers and Faculty Sponsors
- Study Funding and the IRB
Understanding Risk in Research
- Vulnerable Participants
- IRB-SBS PAM & Ed
- Federal Regulations
- Ethical Principals
- Partner Offices
- Determining Human Subjects Research
- Determining HSR or SBS

Assessing risk in a research study is one of the primary responsibilities of an IRB and one of its most controversial tasks. By nature, studying human beings is a complicated process because the subject matter itself is complicated. The level of risk can vary because of many factors including: the population included in the study, the situations encountered by the participants, and/ or the experience of the researcher or team. Two studies may appear similar but a few factors could make one inherently more risky than the other.
This section describes what a researcher needs to consider when developing a protocol as well as the risk analysis conducted by an IRB board member. This section does not cover ever scenario nor is it meant to be all inclusive; if you have a specific question about the risks in your study, please contact our office for further guidance.
Section Topics
The Essex website uses cookies. By continuing to browse the site you are consenting to their use. Please visit our cookie policy to find out which cookies we use and why. View cookie policy.
Research risk assessment
It's the responsibility of the principal investigators (PI) and researchers to identify reasonably foreseeable risks associated with their research and control the risks so far as is reasonably practicable.
All participants and research assistants have the right to expect protection from physical, psychological, social, legal and economic harm at all times during an investigation. Certain research may also present reputational, legal and / or economic risks to the University.
As part of the ethical approval process for research involving human participants you are required to identify potential risks associated with your research and the action you will take to mitigate risk. You may be asked to submit your risk assessment.
The risk assessment process is a careful examination of what could cause harm, who/what could be harmed and how. It will help you to determine what risk control measures are needed and whether you are doing enough.
Risk assessment responsibility
The PI and researchers need to take responsibility for all assessments associated with their projects. Occasionally you may need research workers or students to risk assess an aspect of the work and you will need to check the assessments are adequate and sign them off.
Risk assessors need to be competent and you’ll need to ensure they have adequate training and resource to do the assessments. There is risk assessment training available and help and advice help and advice help and advice from your Health and Safety adviser and safety specialists (for health and safety risks), or the REO Research Governance team for other risks. In some cases, the hazards are so unique to the research that the PI and their team might be the only people who know the work well enough to make valid judgements about the risk and justify their conclusions.
Risk assessment process
The risk assessment process is a careful examination of what could cause harm, who/what could be harmed and how. It will help you to determine what risk control measures are needed and whether you are doing enough.
To simplify the process you can use the health and safety risk assessment templates, risk estimation tool and guidance for all risks associated with your research project. Please refer to the research risk estimation guidance under how to carry out a risk assessment below to assist you.
Research risks
Typical risks that need to be considered as part of research ethics are:
- Social risks: disclosures that could affect participants standing in the community, in their family, and their job.
- Legal risks: activities that could result in the participant, researchers and / or University committing an offence; activities that might lead to a participant disclosing criminal activity to a researcher which would necessitate reporting to enforcement authorities; activities that could result in a civil claim for compensation.
- Economic harm: financial harm to participant, researcher and / or University through disclosure or other event.
- Reputational risk: damage to public perception of University or the University/researchers’ reputation in the eyes of funders, the research community and / or the general public.
- Safeguarding risks: Risk to young people, vulnerable adults and / or researcher from improper behaviour, abuse or exploitation. Risk to researcher of being in a comprising situation, in which there might be accusations of improper behaviour.
- Health and safety risks: risks of harm to health, physical injury or psychological harm to participants or the researcher. Further information on health and safety risks is given below.
Health and safety risks
The potential hazards and risks in research can be many and varied. You will need to be competent and familiar with the work or know where to obtain expert advice to ensure you have identified reasonably foreseeable risks. Here are some common research hazards and risks:
- Location hazards Location hazards Location hazards and risks are associated with where the research is carried out. For example: fire; visiting or working in participant’s homes; working in remote locations and in high crime areas; overseas travel; hot, cold or extreme weather conditions; working on or by water. Also hazardous work locations, such as construction sites, confined spaces, roofs or laboratories. For overseas travel, you will need to check country / city specific information, travel health requirements and consider emergency arrangements as part of your research planning, by following the University’s overseas travel health and safety standard .
- Activity hazards Activity hazards Activity hazards and risks associated with the tasks carried out. For example: potentially mentally harmful activities; distressing and stressful work and content; driving; tripping, or slipping; falling from height; physically demanding work; lifting, carrying, pushing and pulling loads; night time and weekend working.
- Machinery and equipment Machinery and equipment Machinery and equipment . For example: ergonomic hazards, including computer workstations and equipment; contact with electricity; contact with moving, rotating, ejecting or cutting parts in machinery and instruments; accidental release of energy from machines and instruments.
- Chemicals and other hazardous substances . The use, production, storage, waste, transportation and accidental release of chemicals and hazardous substances; flammable, dangerous and explosive substances; asphyxiating gases; allergens; biological agents, blood and blood products. You’ll need to gather information about the amount, frequency and duration of exposure and carry out a COSHH or DSEAR assessment which will inform whether you may need health surveillance for yourself and / or your research participants.
- Physical agents Physical agents Physical agents . For example: excessive noise exposure, hand-arm vibration and whole body vibration; ionising radiation; lasers; artificial optical radiation and electromagnetic fields. You’ll need to gather information about the amount, frequency and duration of exposure inform whether you may need health surveillance for yourself and / or your research participants.
When to carry out a risk assessment
Carrying out initial risk assessments as part of the planning process will help you identify whether existing resources and facilities are adequate to ensure risk control, or if the project needs to be altered accordingly. It will also help you to identify potential costs that need to be considered as part of the funding bid.
Once the project is approved, research specific risk assessments need to be carried out before work starts.
The research may need ethical approval if there is significant risk to participants, researchers or the University.
How to carry out a risk assessment
The University standard on risk assessments provides guidance, tips on getting it right, as well as resources and the forms to help you produce suitable and sufficient risk assessments and must be used.
- Risk assessment template (.dotx)
- Flow chart to research risk assessment (.pdf)
- Research risk assessment: Risk estimation tool (.pdf)
- Example of a Social Science research risk assessment (.pdf)
Refer to carrying out a risk assessment carrying out a risk assessment carrying out a risk assessment for step by step guidance.
Risk assessments must relate to the actual work and must be monitored by the PI. If there are significant changes to the activities, locations, equipment or substances used, the risk assessment will need to reviewed, updated and the old version archived. Risk assessments should also consider the end of projects, arrangements for waste disposal, equipment, controlled area decommission and emergencies.
Things to consider:
- The risks may be specialist in nature or general. Information can found from legislation, sector guidance, safety data sheets, manufacturers equipment information, research documents, forums and health and safety professionals.
- Practical research might involve less well-known hazards. Do you or your team have the expertise to assess the risk adequately? Do you know who to go to for expert advice?
- The capabilities, training, knowledge, skills and experience of the project team members. Are they competent or are there gaps?
- In fast changing research environments, is there a need to carry out dynamic risk assessments? Are they understood and recorded?
- The right personal protective equipment for the hazards identified and training in how to use it.
- Specific Occupational Health vaccinations, health surveillance and screening requirements identified and undertaken. With physical agents and substances you’ll need to make an informed decision about the amount, frequency and duration of exposure. If you need help with this contact Health and Safety.
- Associated activities: storage, transport/travel, cleaning, maintenance, foreseeable emergencies (eg spillages), decommissioning and disposal.
- The safe design, testing and maintenance of the facilities and equipment.
- Planned and preventative maintenance of general plant and specialist equipment.
These risk assessments relate to the actual work and must be monitored by the PI. If there are significant changes to the activities, locations, equipment or substances used, the risk assessment will need to reviewed, updated and the old version archived. Risk assessments should also consider the end of projects, arrangements for waste disposal, equipment and controlled area decommission and emergencies.
Training
If you would like training on completing a risk assessment, please book onto our Risk Assessment Essentials course via HR Organiser. If you are unable to access this, please email [email protected]
- Carrying out a risk assessment Carrying out a risk assessment Carrying out a risk assessment
- People especially at risk People especially at risk People especially at risk
- IOSH/USHA/UCEA guidance on managing health and safety in research (.pdf)
- Research governance: Ethical approval
- For enquiries contact your Student Services Hub
- University of Essex
- Wivenhoe Park
- Colchester CO4 3SQ
- Accessibility
- Privacy and Cookie Policy
The role of scientific research in risk assessment and risk management decisions
Affiliation.
- 1 Office of Health Research, U.S. Environmental Protection Agency, Washington, DC 20460.
- PMID: 1608626
- DOI: 10.1177/019459989210600604
Risk-based decisions are an integral part of societal efforts to protect the public from the harmful health effects of environmental pollution. Scientific information about the magnitude and extent of risks experienced by people and about the causes of those risks is a critical factor in setting priorities and choosing cost-effective mitigation strategies. To be effective in strengthening risk assessment and risk management decisions, research must focus on developing four types of predictive tools: (1) methods to screen and characterize toxicity; (2) biologically based dose-response models; (3) physiologically based pharmacokinetic models; and (4) integrated human exposure models. This approach is the key to reducing the uncertainties currently associated with many environmental health problems.
- Decision Support Techniques*
- Environmental Pollution
- Models, Theoretical
- Business Essentials
- Leadership & Management
- Credential of Leadership, Impact, and Management in Business (CLIMB)
- Entrepreneurship & Innovation
- Digital Transformation
- Finance & Accounting
- Business in Society
- For Organizations
- Support Portal
- Media Coverage
- Founding Donors
- Leadership Team

- Harvard Business School →
- HBS Online →
- Business Insights →
Business Insights
Harvard Business School Online's Business Insights Blog provides the career insights you need to achieve your goals and gain confidence in your business skills.
- Career Development
- Communication
- Decision-Making
- Earning Your MBA
- Negotiation
- News & Events
- Productivity
- Staff Spotlight
- Student Profiles
- Work-Life Balance
- AI Essentials for Business
- Alternative Investments
- Business Analytics
- Business Strategy
- Business and Climate Change
- Design Thinking and Innovation
- Digital Marketing Strategy
- Disruptive Strategy
- Economics for Managers
- Entrepreneurship Essentials
- Financial Accounting
- Global Business
- Launching Tech Ventures
- Leadership Principles
- Leadership, Ethics, and Corporate Accountability
- Leading Change and Organizational Renewal
- Leading with Finance
- Management Essentials
- Negotiation Mastery
- Organizational Leadership
- Power and Influence for Positive Impact
- Strategy Execution
- Sustainable Business Strategy
- Sustainable Investing
- Winning with Digital Platforms
What Is Risk Management & Why Is It Important?

- 24 Oct 2023
Businesses can’t operate without risk. Economic, technological, environmental, and competitive factors introduce obstacles that companies must not only manage but overcome.
According to PwC’s Global Risk Survey , organizations that embrace strategic risk management are five times more likely to deliver stakeholder confidence and better business outcomes and two times more likely to expect faster revenue growth.
If you want to enhance your job performance and identify and mitigate risk more effectively, here’s a breakdown of what risk management is and why it’s important.
Access your free e-book today.
What Is Risk Management?
Risk management is the systematic process of identifying, assessing, and mitigating threats or uncertainties that can affect your organization. It involves analyzing risks’ likelihood and impact, developing strategies to minimize harm, and monitoring measures’ effectiveness.
“Competing successfully in any industry involves some level of risk,” says Harvard Business School Professor Robert Simons, who teaches the online course Strategy Execution . “But high-performing businesses with high-pressure cultures are especially vulnerable. As a manager, you need to know how and why these risks arise and how to avoid them.”
According to Strategy Execution , strategic risk has three main causes:
- Pressures due to growth: This is often caused by an accelerated rate of expansion that makes staffing or industry knowledge gaps more harmful to your business.
- Pressures due to culture: While entrepreneurial risk-taking can come with rewards, executive resistance and internal competition can cause problems.
- Pressures due to information management: Since information is key to effective leadership , gaps in performance measures can result in decentralized decision-making.
These pressures can lead to several types of risk that you must manage or mitigate to avoid reputational, financial, or strategic failures. However, risks aren’t always obvious.
“I think one of the challenges firms face is the ability to properly identify their risks,” says HBS Professor Eugene Soltes in Strategy Execution .
Therefore, it’s crucial to pinpoint unexpected events or conditions that could significantly impede your organization’s business strategy .
Related: Business Strategy vs. Strategy Execution: Which Course Is Right for Me?
According to Strategy Execution , strategic risk comprises:
- Operations risk: This occurs when internal operational errors interrupt your products or services’ flow. For example, shipping tainted products can negatively affect food distribution companies.
- Asset impairment risk: When your company’s assets lose a significant portion of their current value because of a decreased likelihood of receiving future cash flows . For instance, losing property assets, like a manufacturing plant, due to a natural disaster.
- Competitive risk: Changes in the competitive environment can interrupt your organization’s ability to create value and differentiate its offerings—eventually leading to a significant loss in revenue.
- Franchise risk: When your organization’s value erodes because stakeholders lose confidence in its objectives. This primarily results from failing to control any of the strategic risk sources listed above.
Understanding these risks is essential to ensuring your organization’s long-term success. Here’s a deeper dive into why risk management is important.
4 Reasons Why Risk Management Is Important
1. protects organization’s reputation.
In many cases, effective risk management proactively protects your organization from incidents that can affect its reputation.
“Franchise risk is a concern for all businesses,“ Simons says in Strategy Execution . “However, it's especially pressing for businesses whose reputations depend on the trust of key constituents.”
For example, airlines are particularly susceptible to franchise risk because of unforeseen events, such as flight delays and cancellations caused by weather or mechanical failure. While such incidents are considered operational risks, they can be incredibly damaging.
In 2016, Delta Airlines experienced a national computer outage, resulting in over 2,000 flight cancellations. Delta not only lost an estimated $150 million but took a hit to its reputation as a reliable airline that prided itself on “canceling cancellations.”
While Delta bounced back, the incident illustrates how mitigating operational errors can make or break your organization.
2. Minimizes Losses
Most businesses create risk management teams to avoid major financial losses. Yet, various risks can still impact their bottom lines.
A Vault Platform study found that dealing with workplace misconduct cost U.S. businesses over $20 billion in 2021. In addition, Soltes says in Strategy Execution that corporate fines for misconduct have risen 40-fold in the U.S. over the last 20 years.
One way to mitigate financial losses related to employee misconduct is by implementing internal controls. According to Strategy Execution , internal controls are the policies and procedures designed to ensure reliable accounting information and safeguard company assets.
“Managers use internal controls to limit the opportunities employees have to expose the business to risk,” Simons says in the course.
One company that could have benefited from implementing internal controls is Volkswagen (VW). In 2015, VW whistle-blowers revealed that the company’s engineers deliberately manipulated diesel vehicles’ emissions data to make them appear more environmentally friendly.
This led to severe consequences, including regulatory penalties, expensive vehicle recalls, and legal settlements—all of which resulted in significant financial losses. By 2018, U.S. authorities had extracted $25 billion in fines, penalties, civil damages, and restitution from the company.
Had VW maintained more rigorous internal controls to ensure transparency, compliance, and proper oversight of its engineering practices, perhaps it could have detected—or even averted—the situation.
Related: What Are Business Ethics & Why Are They Important?
3. Encourages Innovation and Growth
Risk management isn’t just about avoiding negative outcomes. It can also be the catalyst that drives your organization’s innovation and growth.
“Risks may not be pleasant to think about, but they’re inevitable if you want to push your business to innovate and remain competitive,” Simons says in Strategy Execution .
According to PwC , 83 percent of companies’ business strategies focus on growth, despite risks and mixed economic signals. In Strategy Execution , Simons notes that competitive risk is a challenge you must constantly monitor and address.
“Any firm operating in a competitive market must focus its attention on changes in the external environment that could impair its ability to create value for its customers,” Simons says.
This requires incorporating boundary systems —explicit statements that define and communicate risks to avoid—to ensure internal controls don’t extinguish innovation.
“Boundary systems are essential levers in businesses to give people freedom,” Simons says. “In such circumstances, you don’t want to stifle innovation or entrepreneurial behavior by telling people how to do their jobs. And if you want to remain competitive, you’ll need to innovate and adapt.”

Netflix is an example of how risk management can inspire innovation. In the early 2000s, the company was primarily known for its DVD-by-mail rental service. With growing competition from video rental stores, Netflix went against the grain and introduced its streaming service. This changed the market, resulting in a booming industry nearly a decade later.
Netflix’s innovation didn’t stop there. Once the steaming services market became highly competitive, the company shifted once again to gain a competitive edge. It ventured into producing original content, which ultimately helped differentiate its platform and attract additional subscribers.
By offering more freedom within internal controls, you can encourage innovation and constant growth.
4. Enhances Decision-Making
Risk management also provides a structured framework for decision-making. This can be beneficial if your business is inclined toward risks that are difficult to manage.
By pulling data from existing control systems to develop hypothetical scenarios, you can discuss and debate strategies’ efficacy before executing them.
“Interactive control systems are the formal information systems managers use to personally involve themselves in the decision activities of subordinates,” Simons says in Strategy Execution . “Decision activities that relate to and impact strategic uncertainties.”
JPMorgan Chase, one of the most prominent financial institutions in the world, is particularly susceptible to cyber risks because it compiles vast amounts of sensitive customer data . According to PwC , cybersecurity is the number one business risk on managers’ minds, with 78 percent worried about more frequent or broader cyber attacks.
Using data science techniques like machine learning algorithms enables JPMorgan Chase’s leadership not only to detect and prevent cyber attacks but address and mitigate risk.

Start Managing Your Organization's Risk
Risk management is essential to business. While some risk is inevitable, your ability to identify and mitigate it can benefit your organization.
But you can’t plan for everything. According to the Harvard Business Review , some risks are so remote that no one could have imagined them. Some result from a perfect storm of incidents, while others materialize rapidly and on enormous scales.
By taking an online strategy course , you can build the knowledge and skills to identify strategic risks and ensure they don’t undermine your business. For example, through an interactive learning experience, Strategy Execution enables you to draw insights from real-world business examples and better understand how to approach risk management.
Do you want to mitigate your organization’s risks? Explore Strategy Execution —one of our online strategy courses —and download our free strategy e-book to gain the insights to build a successful strategy.

About the Author

An official website of the United States government
Here's how you know
Official websites use .gov A .gov website belongs to an official government organization in the United States.
Secure .gov websites use HTTPS A lock ( Lock Locked padlock ) or https:// means you’ve safely connected to the .gov website. Share sensitive information only on official, secure websites.

FHFA Insights Lessons Learned from Assessing Exposure to Climate-Related Risks
Climate Scenario Analysis is a tool for assessing exposure to climate-related risks under different future climate conditions. Preliminary analysis highlights the importance of resolving data and methodology gaps to enhance confidence in the results. It also reveals the sensitivity of results to modeling assumptions.
The safety and soundness of the U.S. housing finance system could be adversely affected by the risks associated with climate change, which can disrupt the financial system. 1 These risks pose significant costs on the housing finance system and could increase delinquencies, defaults, and foreclosures, especially if borrowers are underinformed or underinsured. 2 Additionally, climate-related risks could cause long-term harm to local economies, infrastructure, and housing prices that could lead to decreases in habitability and affordability. As such, it is critical for the Federal Housing Finance Agency (FHFA) to better understand how climate risk will affect its regulated entities (Fannie Mae, Freddie Mac, and the Federal Home Loan Banks) as part of its supervisory responsibility to ensure they fulfill their mission and operate in a safe and sound manner, while providing liquidity for housing finance and community investment.
Climate-related risks may be evaluated through two primary categories. The first is physical risk, represented by damage to property, infrastructure, and land due to severe weather events and lasting environmental changes. The second is transition risk posed by policy and technological changes to achieve a greener, low carbon economy that may cause stress to households, certain institutions, or business sectors.
Climate-related physical risks such as hurricanes, wildfires, flood, and sea level rise can directly damage housing stock and reduce property values. Further, borrowers with expenses incurred from weather events may have less disposable income in the event of a disruption to income or employment. Similarly, reduced coverage from insurance companies in high-risk areas could negatively impact local economies and infrastructure, thereby placing downward pressure on house values. These risks may be transmitted to FHFA’s regulated entities through an increased probability of mortgage default and higher loss severity, resulting in higher expected losses.
The transition to a low carbon economy will also spur a significant reshaping of existing policies, means of production, and technologies across regions and sectors. In the long-run, potential transition policies—such as requirements for increased energy efficiency features in homes—could yield consumers increased savings from reduced energy usage and energy bills, while lowering overall macroeconomic costs by helping mitigate the physical impacts of climate change. In the short to medium terms, however, transition policies may also introduce stress to certain sectors or regions.
To this end, assessing the climate risk exposure of FHFA’s regulated entities is a key part of the Agency’s climate-related objectives. 3 While FHFA and its regulated entities are broadly assessing exposure to different climate-related impacts, this blog focuses on climate-related flood risk. 4 While the work is in its early stages, there are several important lessons for evaluating how climate risk may impact the housing finance market. 5
Climate Scenario Analysis
One tool used by financial institutions and regulators to assess exposure to climate-related risks is Climate Scenario Analysis (CSA). 6 CSA relies on complex models—including natural hazard catastrophe models and integrated assessment models. While the former are used for physical risk analysis, the latter combine physical and social science models to forecast the impact of climate change on the economy under different climate scenarios. 7 These forward-looking scenarios can include a range of forecasts to allow for a better understanding of the uncertainties around the impact of climate change and the potential stress on financial entities. CSA methodologies are relatively new but are evolving rapidly. Catastrophe and integrated assessment models typically only produce estimates for damages to property, and their output subsequently need to be incorporated into financial models to assess losses. The figure below provides an example framework for CSA as applied toward physical risks.
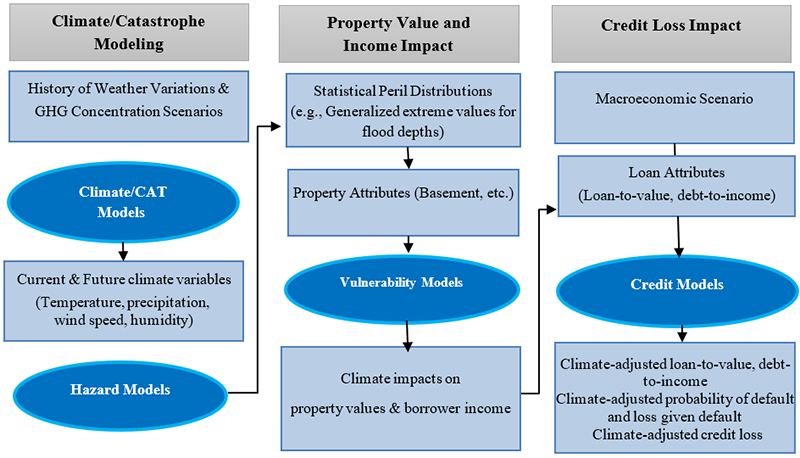
Source: Freddie Mac. Modified by FHFA
Lessons Learned
FHFA and its regulated entities performed exploratory CSA exercises to measure the regulated entities’ exposure to flood risk under a range of potential scenarios, methodologies, and assumptions. This provided FHFA with valuable insights into the regulated entities’ climate risk assessment capabilities, management practices, and challenges. The key observations are as follows:
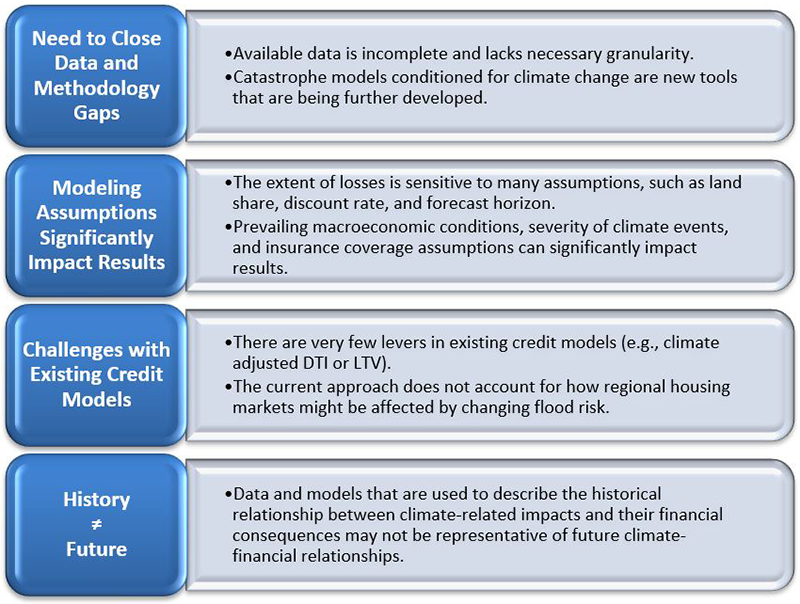
First, performing CSA analysis of flood risk highlighted the importance of resolving data and methodology gaps to enhance confidence in the analysis, given the reliance of current tools on assumptions that introduce significant uncertainty. The available data are also incomplete and lack sufficient granularity for catastrophe models to accurately assess losses at the property level. For example, in the specific case of flooding, predicted losses from flooding can vary with the presence of a basement. These expected losses can also vary significantly depending on the underlying flood model. Further, catastrophe models were designed and traditionally used for estimating average losses in the near-term. Thus, their ability to accurately estimate tail risks and long-term losses is uncertain.
Second, the magnitude of losses is sensitive to modeling assumptions. For example, the impact of climate-related flood risk changes substantially based on the availability and coverage of flood insurance, the predicted severity of the flood events, and the prevailing macroeconomic conditions.
Third, existing credit loss models have limited capabilities to adjust for climate risks. One lever is to allow for a change in property value by flood-related damages or changes in insurance costs. This leads to a recalculation of the loan-to-value ratio, which is a key variable used to predict loan performance. However, this change does not account for the impact of changes in land value, exiting insurers, migration to other areas, and decreased economic opportunities due to frequent events.
Fourth, the data and models that are used to describe the financial impact of climate-related events may not be accurate in predicting future impacts.
These insights highlight that no single risk assessment can capture the full impact of increased flood risk or the other climate-related physical and transition risks. CSA can help FHFA and its regulated entities understand the resilience of the regulated entities to different climate outcomes over varying time frames.
FHFA and its regulated entities have made significant progress in developing their understanding of climate risks and CSA, as well as acquiring some of the necessary data and methodological tools to perform CSA. The regulated entities will continue to work on assessing their exposure to climate risks as the data and methodology mature. FHFA will also further develop expertise and capabilities related to climate scenario analyses and will evaluate other ways to assess exposure to these risks while providing guidance to the regulated entities. The work undertaken in these early years will serve as building blocks for future CSA exercises.
Responsibilities of the Climate Change and ESG Assessing Exposure to Climate Change Working Group:
- Develop expertise on climate-related risk analysis including the underlying data and methodology.
- Perform outreach to other stakeholders in this space.
- Build capacity for FHFA to run climate-related scenario analysis.
Readers are encouraged to explore the FHFA Climate Change & ESG homepage for additional blogs and information related to climate risk.
1 The Financial Stability Oversight Council (FSOC) discusses the threats of climate related risks to U.S. financial stability in their Report on Climate-Related Financial Risk, 2021, https://home.treasury.gov/system/files/261/FSOC-Climate-Report.pdf .
2 These concerns were expressed by many relevant stakeholders in response to FHFA’s Request for Input: Synopsis of Climate and Natural Disaster Risk Management RFI Responses (fhfa.gov) .
3 See https://www.fhfa.gov/AboutUs/Reports/ReportDocuments/FHFA_StrategicPlan_2022-2026_Final.pdf and FHFA Annual Performance Plan for Fiscal Year 2024 .
4 NOAA reports that tropical cyclones are the costliest billion-dollar disaster. ( https://www.ncei.noaa.gov/access/monitoring/dyk/billions-calculations#:~:text=In%20short%2C%20tropical%20cyclones%20are,Price%20Index%20adjustment%20to%202024 ). The majority of these damages typically stem from flooding, and flooding is also expected to increase with climate change due to sea level rise and warmer atmospheres holding more water.
5 For information on how climate change might impact the housing finance sector beyond the scope of CSA, see FHFA’s Working Paper “When Climate Meets Real Estate” for an overview of the academic literature at the intersection of housing finance and climate change: https://www.fhfa.gov/PolicyProgramsResearch/Research/Pages/wp2305.aspx .
6 Central banks of the U.S., Europe and England have been pursuing climate scenario analysis. See, for example, participant instructions for the Federal Reserve Board: https://www.federalreserve.gov/publications/climate-scenario-analysis-exercise-instructions.htm .
7 The leading global resources on climate scenarios are the Network for Greening the Financial System (NGFS) on-line portal (see https://www.ngfs.net/ngfs-scenarios-portal/) and the Intergovernmental Panel on Climate Change (IPCC) (see https://www.ipcc.ch/).
Authored by: Caroline Hopkins Senior Economist, Division of Housing Mission and Goals
Authored by: Charles Hu Supervisory Financial Analyst, Division of Housing Mission and Goals
Authored by: Andrew Davenport Principal Economist, Division of Housing Mission and Goals
Authored by: Barry Carroll Senior Financial Analyst, Division of Housing Mission and Goals
Authored by: Stefan Szilagyi Supervisory Economist, Division of FHLBank Regulation
Editor: Varun Joshi Economist, Climate Change and ESG Branch, Division of Research and Statistics
Tagged: Source: FHFA; climate change; climate change and national disaster; Climate Risk
- Open access
- Published: 24 May 2024
Systematic review and meta-analysis of hepatitis E seroprevalence in Southeast Asia: a comprehensive assessment of epidemiological patterns
- Ulugbek Khudayberdievich Mirzaev 1 , 2 ,
- Serge Ouoba 1 , 3 ,
- Zayar Phyo 1 ,
- Chanroth Chhoung 1 ,
- Akuffo Golda Ataa 1 ,
- Aya Sugiyama 1 ,
- Tomoyuki Akita 1 &
- Junko Tanaka 1
BMC Infectious Diseases volume 24 , Article number: 525 ( 2024 ) Cite this article
197 Accesses
1 Altmetric
Metrics details
The burden of hepatitis E in Southeast Asia is substantial, influenced by its distinct socio-economic and environmental factors, as well as variations in healthcare systems. The aim of this study was to assess the pooled seroprevalence of hepatitis E across countries within the Southeast Asian region by the UN division.
The study analyzed 66 papers across PubMed, Web of Science, and Scopus databases, encompassing data from of 44,850 individuals focusing on anti-HEV seroprevalence. The investigation spanned nine countries, excluding Brunei and East Timor due to lack of data. The pooled prevalence of anti-HEV IgG was determined to be 21.03%, with the highest prevalence observed in Myanmar (33.46%) and the lowest in Malaysia (5.93%). IgM prevalence was highest in Indonesia (12.43%) and lowest in Malaysia (0.91%). The study stratified populations into high-risk (farm workers, chronic patients) and low-risk groups (general population, blood donors, pregnant women, hospital patients). It revealed a higher IgG—28.9%, IgM—4.42% prevalence in the former group, while the latter group exhibited figures of 17.86% and 3.15%, respectively, indicating occupational and health-related vulnerabilities to HEV.
A temporal analysis (1987–2023), indicated an upward trend in both IgG and IgM prevalence, suggesting an escalating HEV burden.
These findings contribute to a better understanding of HEV seroprevalence in Southeast Asia, shedding light on important public health implications and suggesting directions for further research and intervention strategies.
Research Question
Investigate the seroprevalence of hepatitis E virus (HEV) in Southeast Asian countries focusing on different patterns, timelines, and population cohorts.
Sporadic Transmission of IgG and IgM Prevalence:
• Pooled anti-HEV IgG prevalence: 21.03%
• Pooled anti-HEV IgM prevalence: 3.49%
Seroprevalence among specific groups:
High-risk group (farm workers and chronic patients):
• anti-HEV IgG: 28.9%
• anti-HEV IgM: 4.42%
Low-risk group (general population, blood donors, pregnant women, hospital patients):
• anti-HEV IgG: 17.86%
• anti-HEV IgM: 3.15%
Temporal Seroprevalence of HEV:
Anti-HEV IgG prevalence increased over decades (1987–1999; 2000–2010; 2011–2023): 12.47%, 18.43%, 29.17% as an anti-HEV IgM prevalence: 1.92%, 2.44%, 5.27%
Provides a comprehensive overview of HEV seroprevalence in Southeast Asia.
Highlights variation in seroprevalence among different population groups.
Reveals increasing trend in HEV seroprevalence over the years.
Distinguishes between sporadic and epidemic cases for a better understanding of transmission dynamics.
Peer Review reports
Introduction
Hepatitis E is a major global health concern caused by the hepatitis E virus (HEV), which is a small, nonenveloped, single-stranded, positive-sense RNA virus belonging to the Paslahepevirus genus in the Hepeviridae family. There are eight genotypes of HEV: HEV-1 and HEV-2 infect only humans, HEV-3, HEV-4, and HEV-7 infect both humans and animals, while HEV-5, HEV-6, and HEV-8 infect only animals [ 1 ].
HEV infections affect millions of people worldwide each year, resulting in a significant number of symptomatic cases and deaths. In 2015, the World Health Organization (WHO) reported approximately 44,000 deaths from hepatitis E, accounting for 3.3% of overall mortality attributed to viral hepatitis [ 2 ]. The primary mode of transmission for hepatitis E is through the fecal–oral route. Outbreaks of the disease are often associated with heavy rainfall and flooding [ 3 , 4 ]. Additionally, sporadic cases can occur due to poor sanitation, vertical transmission, blood transfusion or close contact with infected animals, which serve as hosts for the virus [ 5 ]. Southeast Asia carries a substantial burden of hepatitis E, influenced by its unique socio-economic and environmental factors as well as variations in healthcare systems. Understanding the seroprevalence of hepatitis E in this region is crucial for implementing targeted public health interventions and allocating resources. To achieve the effective control and prevention of HEV, it is required to address the waterborne transmission and considering the specific characteristics of each region. By taking these measures, healthcare authorities can work towards reducing the global impact of hepatitis E on public health. Systematic reviews and meta-analyses on hepatitis E play a crucial role in synthesizing and integrating existing research findings, providing comprehensive insights into the epidemiology, transmission, and burden of the disease, thereby aiding evidence-based decision-making and public health strategies [ 6 , 7 ].
Recent systematic reviews and meta-analysis conducted on hepatitis E have varied in their scope or were limited by a smaller number of source materials [ 8 , 9 ]. The objective of this study was to determine the pooled seroprevalence of hepatitis E in countries within Southeast Asia by aggregating findings from a multitude of primary studies conducted across the region.
To commence this systematic review and meta-analysis, we adhered to the Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA) guidelines and used the PRISMA assessment checklist [Supplementary Table 1 ]. The study included pertinent research conducted within the population of Southeast Asian countries, as outlined by the United Nations [ 10 ], and perform a meta-analysis on the seroprevalence of hepatitis E in this specific region.
PICOT assessment
In this systematic review and meta-analysis, the eligible population comprised individuals from the Southeast Asia region, irrespective of age, gender, ethnic characteristics, or specific chronic diseases. However, studies involving populations outside the designated countries, travelers, migrants, animal species studies, and those lacking clear descriptions of the study population were excluded.
Intervention and comparison
Intervention and comparison are not applicable to the prevalence studies.
Anti-HEV antibodies positivity either total antibodies or IgG or IgM among the Southeast Asian countries' population was assessed.
All studies conducted between 1987 and 2023 were included in this meta-analysis.
Search strategy
To conduct the data search, we utilized three databases, namely “PubMed”, “Scopus”, and “Web of Science”. The search terms comprised keywords related to the Hepatitis E virus, such as “Hepatitis E virus” OR “Hepatitis E” OR “HEV” AND names of each country “Brunei”, “Cambodia”, “Timor-Leste” OR “East-Timor”, “Laos” OR “Lao PDR”, “Indonesia”, “Malaysia”, “Myanmar” OR “Burma”, “Philippines”, “Singapore”, “Thailand”, “Vietnam” and “Southeast Asia”.
The search process in the databases finished on May 29 th , 2023, with two members of the study team conducting independent searches. Subsequently, the search results were unified. A grey literature search was performed from June 25 th to 30 th , 2023, by examining the references of review manuscripts and conference materials, along with using specific keywords in the Google Scholar database. Notably, during the gray literature search, additional studies from the Philippines that were initially missing in the first search were identified and included. Moreover, due to the diverse language expertise of the team, studies in Russian and French related to Cambodia and Vietnam were also considered for inclusion.
After applying the inclusion and exclusion criteria, each article selected for this systematic review (SR) was considered relevant. The quality assessment of each article was conducted using specific JBI critical appraisal instruments [ 11 ] [Supplementary Table 2 ].
Sporadic transmission of HEV infection
For the systematic review and meta-analysis of sporadic infection of HEV, we divided the study population into cohorts by countries, by risk of acquiring HEV—low and high risk. The low risk cohort included the general population (apparently healthy individuals, students, some ethnic populations, or individuals included in original studies as “general population”), blood donors, pregnant women, and hospital patients, while pig farmers, those with chronic hepatitis, HIV positive patients, and solid organ transplant patients in the high-risk group.
Lastly, we analyzed data in three decades—1987–1999, 2000–2010, and 2011–2023—to reveal seroprevalence rates over time.
Epidemic outbreaks of HEV infection
We separated epidemic outbreaks from sporadic cases due to distinct patterns and scale of transmission in epidemy. Epidemics are characterized by rapid and widespread transmission, affecting a large population within a short period and often following a specific pattern or route of propagation.
Statistical analysis
A meta-analysis of proportions was conducted using the 'meta' and 'metafor' packages in the R statistical software. To account for small proportions, the Freeman-Tukey double arcsine method was applied to transform the data. The Dersimonian and Laird method, which employs a random-effects model, was utilized for the meta-analysis, and the results were presented in a forest plot. Confidence intervals (CIs) for the proportions of individual studies were computed using the Clopper-Pearson method.
Heterogeneity was evaluated using the Cochran Q test and quantified by the I 2 index. Heterogeneity was considered significant if the p -value of the Cochran Q test was below 0.05.
For the assessment of publication bias, a funnel plot displaying the transformed proportions against the sample size was created. The symmetry of the plot was examined using the Egger test ( p < 0.1).
The initial search yielded 1641 articles, which covered 9 out of 11 Southeast Asia countries. We couldn't find any information on hepatitis E from Brunei. We excluded a study from East Timor because it focused on the wrong population (US Army troops). The final screening resulted in the selection of 57 relevant studies, and the grey literature search added 9 more papers that met our inclusion criteria (Fig. 1 ). Among 9 papers through a grey literature, two relevant studies from the Philippines [ 12 , 13 ], one each from Indonesia [ 14 ] and Lao PDR [ 15 ], one study covered both Vietnam and Cambodia [ 16 ], one study provided HEV seroepidemiology information for Myanmar, Thailand, and Vietnam [ 17 ], two studies reported in Russian [ 18 , 19 ] (from Vietnam) and one reported in French [ 16 ] (from Vietnam and Cambodia). In total, our analysis included 66 papers from which we extracted data. This involved a total of 44,850 individuals (Table 1 ).
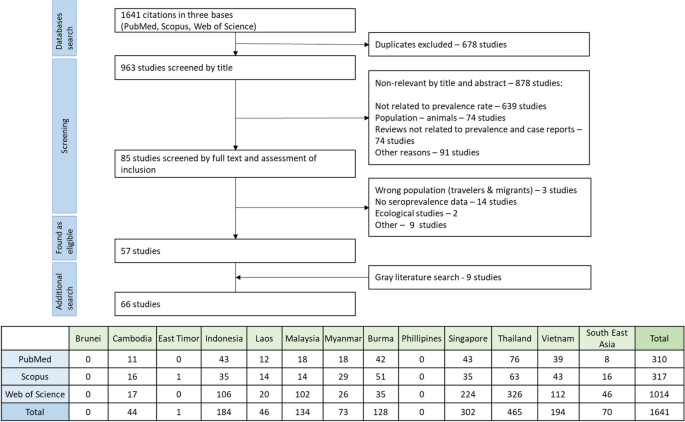
Flowchart of the identification, inclusion, and exclusion of the study. Table under flowchart informing about the studies which were found by the initial search in databases
Sporadic transmission IgG and IgM prevalence in Southeast Asian countries (excluding outbreak settings)
The sporadic cases involving 42,248 participants out of 44,850 participants (the remaining 2,602 people are considered in the “ Epidemic outbreaks ” section) from Southeast Asian countries the pooled prevalence of IgG was found to be 21.03%, while for IgM, it was 3.49% among 34,480 individuals who were tested (Fig. 2 ). Among these countries, Myanmar registered the highest pooled prevalence of IgG at 33.46%, while Malaysia had the lowest at 5.93%. For IgM prevalence, Indonesia had the highest rate at 12.43%, and Malaysia again had the lowest at 0.91% (Table 2 ) [Supplementary Figures 1 and 6 ].
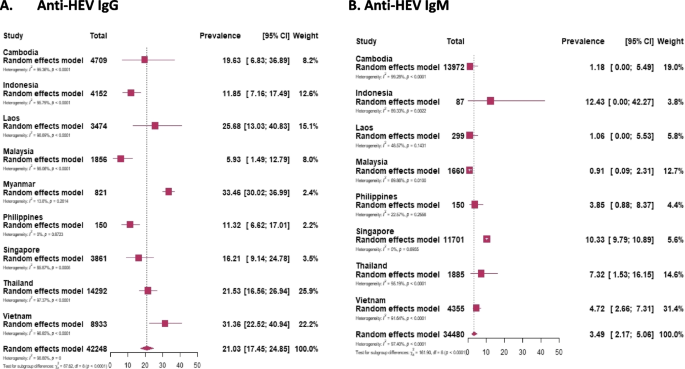
Forest plot of meta-analysis of the prevalence of anti-HEV IgG ( A ) and anti-HEV IgM ( B ) in Southeast Asian countries. The plot includes the number of study participants for each country
Seroprevalence among specific groups
High risk of acquiring hev.
The high-risk group, which included farm workers and chronic patients, demonstrated a pooled anti-HEV IgG prevalence of 28.9%, with IgM prevalence at 4.42% [Supplementary Figures 2 and 8 ].
Chronic patients
This group, comprising individuals with chronic liver disease, HIV infection, or solid organ transplantation, exhibited the highest prevalence of pooled IgG among all cohorts, standing at 29.2%. Additionally, IgM prevalence was 3.9% [Supplementary Figures 2 and 7 ].
Farm workers
Farm workers were divided into several subgroups based on exposure to animals (reservoirs of HEV), including pig or ruminant farmers, slaughterhouse workers, butchers, and meat retailers. Among this group, the highest IgG prevalence was observed at 28.4%, while the pooled IgM level was 6.21% [Supplementary Figures 2 and 7 ].
Low risk of acquiring HEV
The low-risk group, comprising the general population, blood donors, pregnant women, and hospital patients, exhibited anti-HEV IgG and IgM prevalence of 17.86% and 3.15%, respectively. [Supplementary Figures 2 and 9 ].
General population
The general population in Southeast Asian countries, represented by 22,571 individuals, showed a presence of IgG in 21.4% of them. IgM was tested in 10,304 participants, and 2.63% of acute infection cases were identified [Supplementary Figures 2 and 7 ].
Blood donors
Blood donors, as a selected subgroup of the general population, exhibit differences in health status, age, gender distribution, and representativeness, warranting separate assessment. Among blood donors in Southeast Asian countries, the pooled prevalence of IgG and IgM were found to be 11.77% and 0.83%, respectively [Supplementary Figures 2 and 7 ].
Pregnant women
Pregnant women considered a vulnerable group regarding disease consequences, demonstrated an anti-HEV IgG prevalence of 18.56% among 1,670 individuals included in the study. Furthermore, 1.54% of them tested positive for anti-HEV IgM [Supplementary Figures 2 and 7 ].
Hospital patients
A group of 18,792 patients who visited hospitals with clinical signs of acute infection, jaundice, high temperature, and elevated liver enzymes, showed anti-HEV IgG and IgM prevalence of 16.3% and 4.45%, respectively [Supplementary Figures 2 and 7 ].
Temporal seroprevalence of HEV
Given the studies' long duration, the data was presented by decades: 1987–1999, 2000–2010, and 2011–2023. The prevalence of IgG showed an upward trend over these decades, with rates of 12.47%, 18.43%, and 29.17%. Similarly, for IgM, the prevalence rates were 1.92%, 2.44%, and 5.27% for the first, second, and third decades, respectively (Fig. 3 ).
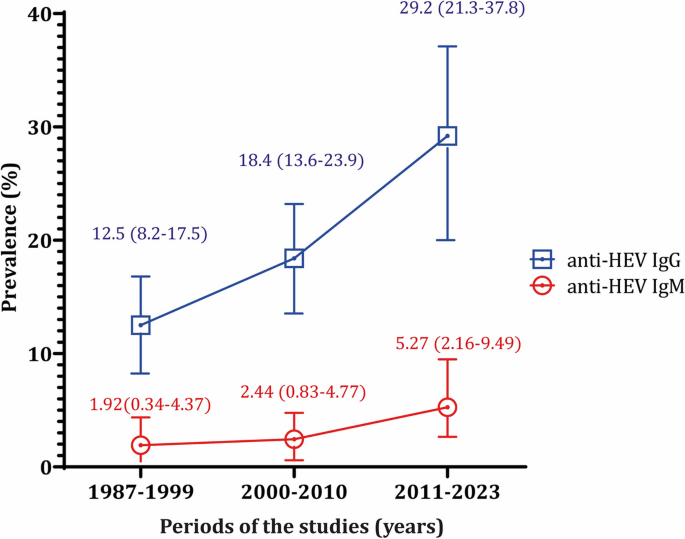
The prevalence of anti-HEV IgG and IgM in Southeast Asian countries throughout the decades
Evaluating the trend of seroprevalence over decades within the same population and country proved challenging due to the limited availability of research papers. Consequently, we assessed anti-HEV antibody prevalence over decades, considering population cohorts and individual countries.
In Fig. 4 , we can see that all population groups show a consistent increase in the prevalence of both IgG and IgM antibodies over the decades. Figure 5 , we analyze the prevalence of anti-HEV antibodies in different countries over time, except for Indonesia and Malaysia, where we observe an increase in prevalence.
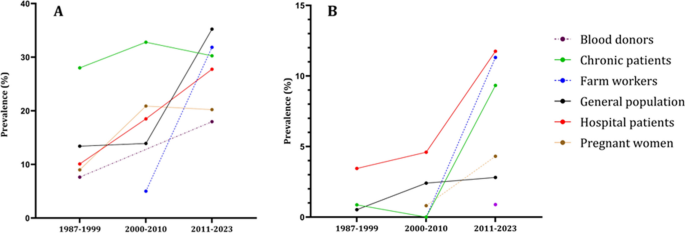
The epidemiological data regarding the occurrence of anti-HEV IgG ( A ) and anti-HEV IgM ( B ) antibodies within population cohorts across Southeast Asian nations divided by decades. The population cohorts delineated by the disrupted lines in the figure lack comprehensive data representation, as they provide information for only two out of three decades. Blood donors group has the anti-HEV IgM only for the last decade
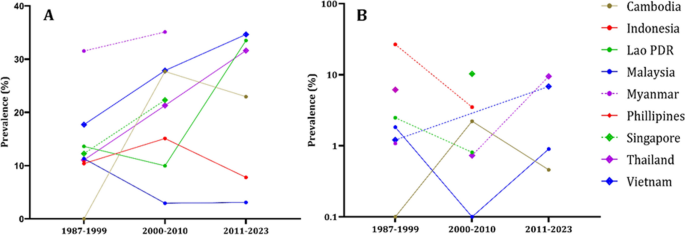
The epidemiological data regarding the occurrence of anti-HEV IgG ( A ) and anti-HEV IgM ( B ) antibodies within countries of Southeast Asia divided by decades. The countries delineated by the disrupted lines in the figure lack comprehensive data representation, as they provide information for only two out of three decades. Philippines has the anti-HEV IgG antibodies information only for the first decade. Philippines, Myanmar, Singapore have anti-HEV IgM information only for single decade
Some studies lacked information on the collection time of the samples [ 13 , 19 , 41 , 48 , 59 , 62 , 64 , 82 ]. In these studies, the pooled IgG and IgM prevalence was 26.5% and 4.75%, respectively [Supplementary Figures 3 , 4 , 5 , 10 , 11 , 12 ].
Epidemic outbreaks
We separated epidemic outbreaks from sporadic cases due to distinct patterns and scale of transmission in epidemy. Epidemics are characterized by rapid and widespread transmission, affecting a large population within a short period and often following a specific pattern or route of propagation. The outbreaks occurred between 1987 and 1998 in several Southeast Asian countries, namely Indonesia [ 31 , 33 , 34 ], Vietnam [ 77 ], and Myanmar [ 54 ] [Supplementary Figure 13 ]. These outbreak investigations involved a total of 2,602 individuals, with most participants from Indonesia (2,292 individuals). The studies were mainly conducted using a case–control design. Among the participants, 876 were considered controls, while 1,726 were classified as cases. The pooled prevalence of total anti-HEV immunoglobulins was estimated as 61.6% (95% CI 57.1–66) (Table 2 ).
Assessment of publication bias
We checked for publication bias using a funnel plot and Egger's test. Both the studies on anti-HEV IgG and IgM showed asymmetry with Egger's test indicating a p -value less than 0.001 for both cases (Fig. 6 ).
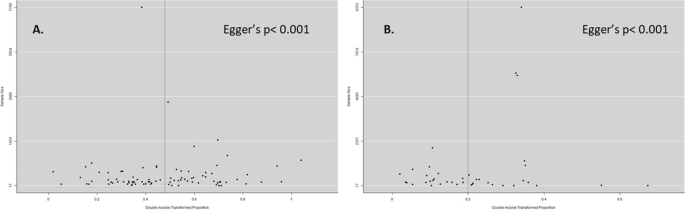
Funnel plot of anti-HEV IgG ( A ) and anti-HEV IgM prevalence. Double arcsine transformed proportion of individual studies is plotted against the sample size. The distribution of studies in the funnel plot revealed the presence of publication bias
A paper search yielded varying numbers of manuscripts from Southeast Asian countries. The Philippines had the fewest studies, while Thailand had the highest with 15 studies. No data was found for Brunei Darussalam and East Timor or Timor Leste on the human species.
The results of this study provide valuable insights into the seroprevalence of IgG and IgM antibodies against HEV in different populations across Southeast Asian countries. Understanding the prevalence of these antibodies is essential for assessing the burden of HEV infection and identifying high-risk groups.
The extensive analysis of anti-HEV IgG prevalence in this study covered a wide range of population groups in Southeast Asia, including the general population, blood donors, pregnant women, hospital patients, farm workers, and chronic patients. The results unveiled an overall pooled prevalence of 21.03%, indicating significant exposure to the Hepatitis E virus among individuals in the region at some point in their lives. Moreover, a consistent increase in IgG prevalence was observed over the years, with the highest prevalence occurring in the most recent decade (2011–2023). This suggests a progressive rise in HEV exposure within the region.
Upon examining the prevalence data across different decades and population cohorts, a uniform upward trend in HEV antibody prevalence became apparent across all groups. Several factors could be assessed as potential contributors to this trend:
Notably, the expanding population in Southeast Asian nations during this timeframe increased the number of individuals at risk of Hepatitis E infection.
The rapid urbanization, characterized by the migration from rural to urban areas, led to higher population density and conditions conducive to Hepatitis E virus transmission [ 84 ]. Access to clean drinking water and adequate sanitation facilities emerged as critical factors in preventing Hepatitis E. Regions with inadequate infrastructure, particularly in water and sanitation, faced an elevated risk due to contaminated water sources. Climate-related events, such as heavy rainfall and flooding, significantly impacted waterborne diseases like Hepatitis E. The increasing frequency and severity of such events emphasized the importance of considering climate-related factors in assessing prevalence trends [ 85 ]. Consumption of contaminated or undercooked meat, particularly pork, was identified as a source of Hepatitis E transmission. Changes in food consumption habits over time may have contributed to changes in seroprevalence [ 86 ]. Limited access to healthcare facilities in certain areas exacerbated the spread of Hepatitis E. Increased awareness together with advances in medical research and the establishment of robust surveillance systems likely improved the detection and reporting of Hepatitis E cases, contributing to the observed increase in seroprevalence [ 87 , 88 , 89 ]. These multifaceted factors have likely played a collective role in shaping the changing landscape of Hepatitis E seroprevalence in Southeast Asian nations over the past decades. The upward trend emphasizes the importance of continued monitoring, intervention, and public health measures to mitigate the spread of Hepatitis E in the region.
Among specific populations, pregnant women exhibited an IgG prevalence of 18.56%, indicating that a considerable number of pregnant individuals have been exposed to HEV. Pregnant women are particularly vulnerable to the consequences of HEV infection, as it can lead to severe outcomes for both the mother and the foetus.
Hospital patients with clinical signs of acute infection showed an IgG prevalence of 16.3%, suggesting that HEV is still a significant cause of acute hepatitis cases in the hospital setting. Similarly, farm workers, especially those exposed to animals (reservoirs of HEV), had a high prevalence of IgG (28.4%), highlighting the occupational risk associated with zoonotic transmission.
Chronic patients, including individuals with chronic liver disease, HIV infection, or solid organ transplantation, exhibited the highest pooled IgG prevalence among all cohorts at 29.2%. This finding underscores the importance of monitoring HEV infection in immunocompromised individuals, as they may develop chronic HEV infection, which can lead to severe liver complications.
The prevalence of IgM antibodies, which are indicative of recent or acute HEV infection, was lower overall compared to IgG. The general population showed an IgM prevalence of 2.63% among acute infection cases. Among hospital patients exhibiting clinical signs of acute infection, the prevalence of IgM antibodies indicative of recent or acute HEV infection was higher at 4.45%.
Farm workers, particularly those exposed to animals, demonstrated the highest IgM prevalence at 6.21%. This finding highlights the occupational risk of acquiring acute HEV infection in this population due to direct or indirect contact with infected animals.
The study also identified a high-risk group, consisting of farm workers and chronic patients, with a pooled IgG prevalence of 28.9% and an IgM prevalence of 4.42%. This group is particularly susceptible to HEV infection and requires targeted interventions to reduce transmission and prevent severe outcomes.
Overall, this study provides valuable data on the seroprevalence of HEV antibodies in different populations in Southeast Asian countries. It highlights the importance of continued surveillance and public health interventions to control HEV transmission, especially in vulnerable groups. Understanding the prevalence trends over time can aid in developing effective strategies for the prevention and management of HEV infections in the region. However, further research and studies are warranted to explore the underlying factors contributing to the observed seroprevalence trends and to design targeted interventions to reduce HEV transmission in specific populations. Among the countries of Southeast Asia Myanmar was the most for HEV infection, while Malaysia registered the lowest seroprevalence.
This study has some limitations that we should be aware of. We looked at studies in three languages (English, Russian, and French), but we couldn't find data from two out of the 11 countries. This means we might not have a complete picture of the disease's prevalence in the whole region.
The way we divided the groups based on occupation or status could be questioned. Different criteria might give us different results, so it's something we need to consider. Another challenge is that the study covers a long time from 1989 to 2023 by published research and involves many different countries. This makes it difficult to compare the results because the tests used, and the diagnostic abilities might have changed over time and vary across countries.
Despite these limitations, our study presents a detailed epidemiologic report of combined seroprevalence data for HEV in Southeast Asian countries following the UN division. It gives us a basic understanding of the disease's prevalence in the region and offers some insights into potential risk factors. However, to get a more accurate picture, future research should address these limitations and include data from all countries in the region. Furthermore, certain countries such as Myanmar and the Philippines have not reported HEV prevalence data since 2006 and 2015, respectively. The absence of recent HEV prevalence reports from certain countries raises concerns about the availability of up-to-date epidemiological data for assessing the current status of hepatitis E virus infections in these regions.
Our comprehensive analysis study involving Southeast Asian countries provides significant insights into the seroprevalence of hepatitis E virus (HEV) infection in this region and in various populations. The rates of anti-HEV antibodies observed among different groups, as well as the increasing trend in seroprevalence over decades, emphasize the dynamic nature of HEV transmission in the region. These findings contribute to a better understanding of HEV prevalence across countries, populations, and time periods in Southeast Asia, shedding light on important public health implications and suggesting directions for further research and intervention strategies.
Availability of data and materials
All data generated or analyzed during this study were included in this paper either in the results or supplementary information.
Abbreviations
Hepatitis E Virus
Preferred reporting items for systematic review and meta-analysis
Enzyme-Linked Immunosorbent Essay
Hepatitis E virus Immunoglobulin G
Hepatitis E Virus Immunoglobulin M
Smith DB, Izopet J, Nicot F, Simmonds P, Jameel S, Meng XJ, et al. Update: proposed reference sequences for subtypes of hepatitis E virus (species Orthohepevirus A). J Gen Virol. 2020 [cited 2023 Aug 3];101(7):692. Available from: /pmc/articles/PMC7660235/.
Article CAS PubMed PubMed Central Google Scholar
Hepatitis E. Available from: https://www.who.int/news-room/fact-sheets/detail/hepatitis-e . Accessed 22 July 2023.
Viswanathan R. A review of the literature on the epidemiology of infectious hepatitis. Indian J Med Res. 1957;45:145–55.
CAS PubMed Google Scholar
Naik SR, Aggarwal R, Salunke PN, Mehrotra NN. A large waterborne viral hepatitis E epidemic in Kanpur, India. Bull World Health Organ. 1992 [cited 2023 Jul 20];70(5):597. Available from: /pmc/articles/PMC2393368/?report=abstract.
CAS PubMed PubMed Central Google Scholar
Aslan AT, Balaban HY. Hepatitis E virus: epidemiology, diagnosis, clinical manifestations, and treatment. World J Gastroenterol. 2020;26(37):5543–60.
Mulrow CD. Rationale for systematic reviews. BMJ. 1994 [cited 2023 Jul 20];309(6954):597–9. Available from: https://pubmed.ncbi.nlm.nih.gov/8086953/ .
Cook DJ, Mulrow CD, Haynes RB. Systematic reviews: synthesis of best evidence for clinical decisions. Ann Intern Med. 1997;126(5):376–80.
Article CAS PubMed Google Scholar
Wasuwanich P, Thawillarp S, Ingviya T, Karnsakul W. Hepatitis E in Southeast Asia. Siriraj Med J. 2020 [cited 2023 Jul 20];72(3):259–64. Available from: https://he02.tci-thaijo.org/index.php/sirirajmedj/article/view/240129 .
Article Google Scholar
Raji YE, Peck Toung O, Mohd N, Zamberi T, Sekawi B, MohdTaib N, et al. A systematic review of the epidemiology of hepatitis E virus infection in South – Eastern Asia. Virulence. 2021 [cited 2023 Jul 20];12(1):114. Available from: /pmc/articles/PMC7781573/.
South East Asia. Available from: https://www.unep.org/ozonaction/south-east-asia . Accessed 28 May 2023.
Chapter 5: Systematic reviews of prevalence and incidence - JBI Manual for Evidence Synthesis - JBI Global Wiki. [accessed 2023 May 20]. Available from: https://jbi-global-wiki.refined.site/space/MANUAL/4688607/Chapter+5%3A+Systematic+reviews+of+prevalence+and+incidence .
Gloriani-Barzaga N, Cabanban A, Graham RR, Florese RH. Hepatitis E virus infection diagnosed by serology: a report of cases at the San Lazaro Hospital Manila. Phil J Microbiol Infect Dis. 1997;26(4):169–72.
Google Scholar
Lorenzo AA, De Guzman TS, Su GLS. Detection of IgM and IgG antibodies against hepatitis E virus in donated blood bags from a national voluntary blood bank in Metro Manila Philippines. Asian Pac J Trop Dis. 2015;5(8):604–5.
Article CAS Google Scholar
Jennings GB, Lubis I, Listiyaningsih E, Burans JP, Hyams KC. Hepatitis E virus in Indonesia. Trans R Soc Trop Med Hyg. 1994 [cited 2023 Jul 24];88(1):57. Available from: https://pubmed.ncbi.nlm.nih.gov/8154003/ .
Pauly M, Muller CP, Black AP, Snoeck CJ. Intense human-animal interaction and limited capacity for the surveillance of zoonoses as drivers for hepatitis E virus infections among animals and humans in Lao PDR. Int J Infect Dis. 2016 [cited 2023 Jul 24];53:18. Available from: http://www.ijidonline.com/article/S1201971216312693/fulltext .
Buchy P, Monchy D, An TT, Srey CT, Tri DV, Son S, Glaziou P, Chien BT. Prévalence de marqueurs d’infection des hépatites virales A, B, C et E chez des patients ayant une hypertransaminasémie a Phnom Penh (Cambodge) et Nha Trang (Centre Vietnam) [Prevalence of hepatitis A, B, C and E virus markers among patients with elevated levels of Alanine aminotransferase and Aspartate aminotransferase in Phnom Penh (Cambodia) and Nha Trang (Central Vietnam)]. Bull Soc Pathol Exot. 2004;97(3):165–71.
Abe K, Li T, Ding X, Win KM, Shrestha PK, Quang VX, Ngoc TT, Taltavull TC, Smirnov AV, Uchaikin VF, Luengrojanakul P. International collaborative survey on epidemiology of hepatitis E virus in 11 countries. Southeast Asian J Trop Med Public Health. 2006;37(1):90–5.
PubMed Google Scholar
Lichnaia EV, Pham THG, Petrova OA, Tran TN, Bui TTN, Nguyen TT, et al. Hepatitis e virus seroprevalence in indigenous residents of the Hà Giang northern province of Vietnam. Russ J Infect Immun. 2021;11(4):692–700.
Ostankova YuV, Semenov AV, Valutite DE, Zueva EB, Serikova EN, Shchemelev AN, et al. Enteric viral hepatitis in the Socialist Republic of Vietnam (Southern Vietnam). Jurnal Infektologii. 2021;13(4):72–8. Available from: https://www.scopus.com/inward/record.uri?eid=2-s2.0-85123451810&doi=10.22625%2f2072-6732-2021-13-4-72-78&partnerID=40&md5=968b7e50231d40b12aded0e0da133936 .
Kasper MR, Blair PJ, Touch S, Sokhal B, Yasuda CY, Williams M, et al. Infectious etiologies of acute febrile illness among patients seeking health care in south-central Cambodia. Am J Trop Med Hyg. 2012;86(2):246–53. Available from: https://pubmed.ncbi.nlm.nih.gov/22302857/ .
Article PubMed PubMed Central Google Scholar
Nouhin J, Madec Y, Prak S, Ork M, Kerleguer A, Froehlich Y, et al. Declining hepatitis E virus antibody prevalence in Phnom Penh, Cambodia during 1996–2017. Epidemiol Infect. 2018;147:e26. Available from: https://pubmed.ncbi.nlm.nih.gov/30309396/ .
Nouhin J, Prak S, Madec Y, Barennes H, Weissel R, Hok K, et al. Hepatitis E virus antibody prevalence, RNA frequency, and genotype among blood donors in Cambodia (Southeast Asia). Transfusion (Paris). 2016;56(10):2597–601. Available from: https://pubmed.ncbi.nlm.nih.gov/27480100/ .
Nouhin J, Barennes H, Madec Y, Prak S, Hou SV, Kerleguer A, et al. Low frequency of acute hepatitis E virus (HEV) infections but high past HEV exposure in subjects from Cambodia with mild liver enzyme elevations, unexplained fever or immunodeficiency due to HIV-1 infection. J Clin Virol. 2015;71:22–7. Available from: https://pubmed.ncbi.nlm.nih.gov/26370310/ .
Article PubMed Google Scholar
Yamada H, Takahashi K, Lim O, Svay S, Chuon C, Hok S, et al. Hepatitis E Virus in Cambodia: prevalence among the general population and complete genome sequence of genotype 4. PLoS One. 2015;10:e0136903. Available from: https://pubmed.ncbi.nlm.nih.gov/26317620/ .
Chhour YM, Ruble G, Hong R, Minn K, Kdan Y, Sok T, et al. Hospital-based diagnosis of hemorrhagic fever, encephalitis, and hepatitis in Cambodian children. Emerg Infect Dis. 2002;8(5):485–9. Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2732496/pdf/01-0236-FinalR.pdf .
Utsumi T, Hayashi Y, Lusida MI, Amin M, Soetjipto, Hendra A, et al. Prevalence of hepatitis E virus among swine and humans in two different ethnic communities in Indonesia. Arch Virol. 2011;156(4):689–93. Available from: https://pubmed.ncbi.nlm.nih.gov/21191625/ .
Mizuo H, Suzuki K, Takikawa Y, Sugai Y, Tokita H, Akahane Y, et al. Polyphyletic strains of hepatitis E virus are responsible for sporadic cases of acute hepatitis in Japan. J Clin Microbiol. 2002 [cited 2023 Jul 24];40(9):3209. Available from: /pmc/articles/PMC130758/.
Achwan WA, Muttaqin Z, Zakaria E, Depamede SA, Mulyanto, Sumoharjo S, et al. Epidemiology of hepatitis B, C, and E viruses and human immunodeficiency virus infections in Tahuna, Sangihe-Talaud Archipelago Indonesia. Intervirology. 2007;50(6):408–11. Available from: https://pubmed.ncbi.nlm.nih.gov/18185013/ .
Surya IG, Kornia K, Suwardewa TG, Mulyanto, Tsuda F, Mishiro S. Serological markers of hepatitis B, C, and E viruses and human immunodeficiency virus type-1 infections in pregnant women in Bali Indonesia. J Med Virol. 2005;75(4):499–503. Available from: https://pubmed.ncbi.nlm.nih.gov/15714491/ .
Wibawa ID, Suryadarma IG, Mulyanto, Tsuda F, Matsumoto Y, Ninomiya M, et al. Identification of genotype 4 hepatitis E virus strains from a patient with acute hepatitis E and farm pigs in Bali Indonesia. J Med Virol. 2007;79(8):1138–46. Available from: https://pubmed.ncbi.nlm.nih.gov/17596841/ .
Sedyaningsih-Mamahit ER, Larasati RP, Laras K, Sidemen A, Sukri N, Sabaruddin N, et al. First documented outbreak of hepatitis E virus transmission in Java, Indonesia. Trans R Soc Trop Med Hyg. 2002;96(4):398–404. Available from: https://pubmed.ncbi.nlm.nih.gov/12497976/ .
Widasari DI, Yano Y, Utsumi T, Heriyanto DS, Anggorowati N, Rinonce HT, et al. Hepatitis E virus infection in two different regions of Indonesia with identification of swine HEV genotype 3. Microbiol Immunol. 2013;57(10):692–703. Available from: https://pubmed.ncbi.nlm.nih.gov/23865729/ .
Corwin A, Putri MP, Winarno J, Lubis I, Suparmanto S, Sumardiati A, et al. Epidemic and sporadic hepatitis E virus transmission in West Kalimantan (Borneo). Indonesia Am J Trop Med Hyg. 1997;57(1):62–5. Available from: https://pubmed.ncbi.nlm.nih.gov/9242320/ .
Corwin A, Jarot K, Lubis I, Nasution K, Suparmawo S, Sumardiati A, et al. Two years’ investigation of epidemic hepatitis E virus transmission in West Kalimantan (Borneo), Indonesia. Trans R Soc Trop Med Hyg. 1995 [cited 2023 Jul 20];89(3):262–5. Available from: https://pubmed.ncbi.nlm.nih.gov/7660427/ .
Wibawa ID, Muljono DH, Mulyanto, Suryadarma IG, Tsuda F, Takahashi M, et al. Prevalence of antibodies to hepatitis E virus among apparently healthy humans and pigs in Bali, Indonesia: identification of a pig infected with a genotype 4 hepatitis E virus. J Med Virol. 2004;73(1):38–44. Available from: https://pubmed.ncbi.nlm.nih.gov/15042646/ .
Goldsmith R, Yarbough PO, Reyes GR, Fry KE, Gabor KA, Kamel M, et al. Enzyme-linked immunosorbent assay for diagnosis of acute sporadic hepatitis E in Egyptian children. Lancet. 1992 [cited 2023 Jul 25];339(8789):328–31. Available from: https://pubmed.ncbi.nlm.nih.gov/1346411/ .
Bounlu K, Insisiengmay S, Vanthanouvong K, Saykham, Widjaja S, Iinuma K, et al. Acute jaundice in Vientiane, Lao People’s Democratic Republic. Clin Infect Dis. 1998;27(4):717–21. Available from: https://pubmed.ncbi.nlm.nih.gov/9798023/ .
Khounvisith V, Saysouligno S, Souvanlasy B, Billamay S, Mongkhoune S, Vongphachanh B, et al. Hepatitis B virus and other transfusion-transmissible infections in child blood recipients in Lao People’s Democratic Republic: a hospital-based study. Arch Dis Child. 2023;108(1):15–9. Available from: https://pubmed.ncbi.nlm.nih.gov/36344216/ .
Khounvisith V, Tritz S, Khenkha L, Phoutana V, Keosengthong A, Pommasichan S, et al. High circulation of hepatitis E virus in pigs and professionals exposed to pigs in Laos. Zoonoses Public Health. 2018;65(8):1020–6. Available from: https://pubmed.ncbi.nlm.nih.gov/30152201/ .
Tritz SE, Khounvisith V, Pommasichan S, Ninnasopha K, Keosengthong A, Phoutana V, et al. Evidence of increased hepatitis E virus exposure in Lao villagers with contact to ruminants. Zoonoses Public Health. 2018;65(6):690–701. Available from: https://pubmed.ncbi.nlm.nih.gov/29888475/ .
Bisayher S, Barennes H, Nicand E, Buisson Y. Seroprevalence and risk factors of hepatitis E among women of childbearing age in the Xieng Khouang province (Lao People’s Democratic Republic), a cross-sectional survey. Trans R Soc Trop Med Hyg. 2019;113(6):298–304. Available from: https://pubmed.ncbi.nlm.nih.gov/31034060/ .
Holt HR, Inthavong P, Khamlome B, Blaszak K, Keokamphe C, Somoulay V, et al. Endemicity of zoonotic diseases in pigs and humans in lowland and upland Lao PDR: identification of socio-cultural risk factors. PLoS Negl Trop Dis. 2016;10(4):e0003913. Available from: https://pubmed.ncbi.nlm.nih.gov/27070428/ .
Syhavong B, Rasachack B, Smythe L, Rolain JM, Roque-Afonso AM, Jenjaroen K, et al. The infective causes of hepatitis and jaundice amongst hospitalised patients in Vientiane, Laos. Trans R Soc Trop Med Hyg. 2010;104(7):475–83. Available from: https://pubmed.ncbi.nlm.nih.gov/20378138/ .
Chansamouth V, Thammasack S, Phetsouvanh R, Keoluangkot V, Moore CE, Blacksell SD, et al. The aetiologies and impact of fever in pregnant inpatients in Vientiane, Laos. PLoS Negl Trop Dis. 2016;10(4):e0004577.
Wong LP, Tay ST, Chua KH, Goh XT, Alias H, Zheng Z, et al. Serological evidence of Hepatitis E virus (HEV) infection among ruminant farmworkers: a retrospective study from Malaysia. Infect Drug Resist. 2022;15:5533–41. Available from: https://pubmed.ncbi.nlm.nih.gov/36164335/ .
Wong LP, Lee HY, Khor CS, Abdul-Jamil J, Alias H, Abu-Amin N, et al. The risk of transfusion-transmitted hepatitis E virus: evidence from seroprevalence screening of blood donations. Indian J Hematol Blood Transfus. 2022;38(1):145–52. Available from: https://pubmed.ncbi.nlm.nih.gov/33879981/ .
Wong LP, Alias H, Choy SH, Goh XT, Lee SC, Lim YAL, et al. The study of seroprevalence of hepatitis E virus and an investigation into the lifestyle behaviours of the aborigines in Malaysia. Zoonoses Public Health. 2020;67(3):263–70. Available from: https://pubmed.ncbi.nlm.nih.gov/31927794/ .
Ng KP, He J, Saw TL, Lyles CM. A seroprevalence study of viral hepatitis E infection in human immunodeficiency virus type 1 infected subjects in Malaysia. Med J Malaysia. 2000;55(1):58–64. Available from: https://pubmed.ncbi.nlm.nih.gov/11072492/ .
Hudu SA, Niazlin MT, Nordin SA, Harmal NS, Tan SS, Omar H, et al. Hepatitis E virus isolated from chronic hepatitis B patients in Malaysia: sequences analysis and genetic diversity suggest zoonotic origin. Alexandria J Med. 2018;54(4):487–94. Available from: https://www.tandfonline.com/doi/pdf/10.1016/j.ajme.2017.07.003 .
Anderson DA, Li F, Riddell M, Howard T, Seow HF, Torresi J, et al. ELISA for IgG-class antibody to hepatitis E virus based on a highly conserved, conformational epitope expressed in Escherichia coli. J Virol Methods. 1999 [cited 2023 Jul 25];81(1–2):131–42. Available from: https://pubmed.ncbi.nlm.nih.gov/10488771/ .
Seow HF, Mahomed NM, Mak JW, Riddell MA, Li F, Anderson DA. Seroprevalence of antibodies to hepatitis E virus in the normal blood donor population and two aboriginal communities in Malaysia. J Med Virol. 1999;59(2):164–8. Available from: https://pubmed.ncbi.nlm.nih.gov/10459151/ .
Saat Z, Sinniah M, Kin TL, Baharuddin R, Krishnasamy M. A four year review of acute viral hepatitis cases in the east coast of Peninsular Malaysia. Southeast Asian J Trop Med Public Health. 1999;30(1):106–9.
Li TC, Yamakawa Y, Suzuki K, Tatsumi M, Razak MA, Uchida T, et al. Expression and self-assembly of empty virus-like particles of hepatitis E virus. J Virol. 1997 [cited 2023 Jul 25];71(10):7207–13. Available from: https://pubmed.ncbi.nlm.nih.gov/9311793/ .
Uchida T, Aye TT, Ma X, Iida F, Shikata T, Ichikawa M, et al. An epidemic outbreak of hepatitis E in Yangon of Myanmar: antibody assay and animal transmission of the virus. Acta Pathol Jpn. 1993;43(3):94–8. Available from: https://pubmed.ncbi.nlm.nih.gov/8257479/ .
Nakai K, Win KM, Oo SS, Arakawa Y, Abe K. Molecular characteristic-based epidemiology of hepatitis B, C, and E viruses and GB virus C/hepatitis G virus in Myanmar. J Clin Microbiol. 2001;39(4):1536–9. Available from: https://pubmed.ncbi.nlm.nih.gov/11283083/ .
Chow WC, Ng HS, Lim GK, Oon CJ. Hepatitis E in Singapore–a seroprevalence study. Singapore Med J. 1996;37(6):579–81. Available from: https://pubmed.ncbi.nlm.nih.gov/9104052/ .
Wong CC, Thean SM, Ng Y, Kang JSL, Ng TY, Chau ML, et al. Seroepidemiology and genotyping of hepatitis E virus in Singapore reveal rise in number of cases and similarity of human strains to those detected in pig livers. Zoonoses Public Health. 2019 [cited 2023 Jul 24];66(7):773–82. Available from: https://pubmed.ncbi.nlm.nih.gov/31293095/ .
Tan LTC, Tan J, Ang LW, Chan KP, Chiew KT, Cutter J, et al. Epidemiology of acute hepatitis E in Singapore. J Infect. 2013 [cited 2023 Jul 24];66(5):453–9. Available from: https://pubmed.ncbi.nlm.nih.gov/23286967/ .
Pourpongporn P, Samransurp K, Rojanasang P, Wiwattanakul S, Srisurapanon S. The prevalence of anti-hepatitis E in occupational risk groups. J Med Assoc Thai. 2009;92:S38–42. Available from: https://pubmed.ncbi.nlm.nih.gov/19705545/ .
Siripanyaphinyo U, Boon-Long J, Louisirirotchanakul S, Takeda N, Chanmanee T, Srimee B, et al. Occurrence of hepatitis E virus infection in acute hepatitis in Thailand. J Med Virol. 2014;86(10):1730–5. Available from: https://pubmed.ncbi.nlm.nih.gov/24984976/ .
Poovorawan K, Jitmitrapab S, Treeprasertsuk S, Tangkijvanich P, Komolmitr P, Poovorawan Y. Acute hepatitis E in Thailand, 2009–2012. J Gastroenterol Hepatol. 2012;27:233.
Maneerat Y, Wilairatana P, Pongponratn E, Chaisri U, Puthavatana P, Snitbhan R, et al. Etiology of acute non-A, B, C hepatitis in Thai patients: preliminary study. Southeast Asian J Trop Med Public Health. 1996;27(4):844–6. Available from: https://pubmed.ncbi.nlm.nih.gov/9253895/ .
Sa-nguanmoo P, Posuwan N, Vichaiwattana P, Wutthiratkowit N, Owatanapanich S, Wasitthankasem R, et al. Swine is a possible source of hepatitis E virus infection by comparative study of hepatitis A and E seroprevalence in Thailand. PLoS One. 2015;10:e0126184. Available from: https://pubmed.ncbi.nlm.nih.gov/25927925/ .
Pilakasiri C, Gibbons RV, Jarman RG, Supyapoung S, Myint KSA. Hepatitis antibody profile of Royal Thai Army nursing students. Trop Med Int Health. 2009;14(6):609–11. Available from: https://onlinelibrary.wiley.com/doi/pdfdirect/10.1111/j.1365-3156.2009.02264.x?download=true .
Louisirirotchanakul S, Myint KS, Srimee B, Kanoksinsombat C, Khamboonruang C, Kunstadter P, et al. The prevalence of viral hepatitis among the Hmong people of northern Thailand. Southeast Asian J Trop Med Public Health. 2002;33(4):837–44. Available from: https://pubmed.ncbi.nlm.nih.gov/12757235/ .
Jupattanasin S, Chainuvati S, Chotiyaputta W, Chanmanee T, Supapueng O, Charoonruangrit U, et al. A nationwide survey of the seroprevalence of hepatitis E virus infections among blood donors in Thailand. Viral Immunol. 2019;32(7):302–7. Available from: https://pubmed.ncbi.nlm.nih.gov/31403386/ .
Hinjoy S, Nelson KE, Gibbons RV, Jarman RG, Mongkolsirichaikul D, Smithsuwan P, et al. A cross-sectional study of hepatitis E virus infection in healthy people directly exposed and unexposed to pigs in a rural community in northern Thailand. Zoonoses Public Health. 2013;60(8):555–62. Available from: https://pubmed.ncbi.nlm.nih.gov/23280251/ .
Getsuwan S, Pasomsub E, Yutthanakarnwikom P, Tongsook C, Butsriphum N, Tanpowpong P, et al. Seroprevalence of hepatitis E virus after pediatric liver transplantation. J Trop Pediatr. 2023;69(2):fmad011. Available from: https://pubmed.ncbi.nlm.nih.gov/36811578/ .
Gonwong S, Chuenchitra T, Khantapura P, Islam D, Sirisopana N, Mason CJ. Pork consumption and seroprevalence of hepatitis E virus, Thailand, 2007–2008. Emerg Infect Dis. 2014;20:1531–4. Available from: https://pubmed.ncbi.nlm.nih.gov/25148245/ .
Komolmit P, Oranrap V, Suksawatamnuay S, Thanapirom K, Sriphoosanaphan S, Srisoonthorn N, et al. Clinical significance of post-liver transplant hepatitis E seropositivity in high prevalence area of hepatitis E genotype 3: a prospective study. Sci Rep. 2020;10(1):7352. Available from: https://pubmed.ncbi.nlm.nih.gov/32355268/ .
Jutavijittum P, Jiviriyawat Y, Jiviriyawat W, Yousukh A, Hayashi S, Itakura H, et al. Seroprevalence of antibody to hepatitis E virus in voluntary blood donors in Northern Thailand. Trop Med. 2000;42(2):135–9. Available from: https://www.scopus.com/inward/record.uri?eid=2-s2.0-0033638431&partnerID=40&md5=12c324fd502945b8c8dc425274f7cad2 .
Boonyai A, Thongput A, Sisaeng T, Phumchan P, Horthongkham N, Kantakamalakul W, et al. Prevalence and clinical correlation of hepatitis E virus antibody in the patients’ serum samples from a tertiary care hospital in Thailand during 2015–2018. Virol J. 2021;18(1):145. Available from: https://pubmed.ncbi.nlm.nih.gov/34247642/ .
Huy PX, Chung DT, Linh DT, Hang NT, Rachakonda S, Pallerla SR, et al. Low prevalence of HEV infection and no associated risk of HEV transmission from mother to child among pregnant women in Vietnam. Pathogens. 2021;10(10):1340. Available from: https://pubmed.ncbi.nlm.nih.gov/34684289/ .
Hoan NX, Huy PX, Sy BT, Meyer CG, Son TV, Binh MT, et al. High hepatitis E virus (HEV) Positivity among domestic pigs and risk of HEV infection of individuals occupationally exposed to pigs and pork meat in Hanoi, Vietnam. Open Forum Infect Dis. 2019;6(9):ofz306. Available from: https://pubmed.ncbi.nlm.nih.gov/31660396/ .
Hoan NX, Tong HV, Hecht N, Sy BT, Marcinek P, Meyer CG, et al. Hepatitis E virus superinfection and clinical progression in hepatitis B patients. EBioMedicine. 2015;2(12):2080–6. Available from: https://pubmed.ncbi.nlm.nih.gov/26844288/ .
Hau CH, Hien TT, Tien NT, Khiem HB, Sac PK, Nhung VT, et al. Prevalence of enteric hepatitis A and E viruses in the Mekong River delta region of Vietnam. Am J Trop Med Hyg. 1999;60(2):277–80. Available from: https://pubmed.ncbi.nlm.nih.gov/10072151/ .
Corwin AL, Dai TC, Duc DD, Suu PI, Van NT, Ha LD, et al. Acute viral hepatitis in Hanoi, Viet Nam. Trans R Soc Trop Med Hyg. 1996;90(6):647–8. Available from: https://pubmed.ncbi.nlm.nih.gov/9015503/ .
Corwin AL, Khiem HB, Clayson ET, Pham KS, Vo TT, Vu TY, et al. A waterborne outbreak of hepatitis E virus transmission in southwestern Vietnam. Am J Trop Med Hyg. 1996;54(6):559–62. Available from: https://pubmed.ncbi.nlm.nih.gov/8686771/ .
Berto A, Pham HA, Thao TTN, Vy NHT, Caddy SL, Hiraide R, et al. Hepatitis E in southern Vietnam: seroepidemiology in humans and molecular epidemiology in pigs. Zoonoses Public Health. 2018 [cited 2023 Jul 24];65(1):43–50. Available from: https://onlinelibrary.wiley.com/doi/full/10.1111/zph.12364 .
Li TC, Zhang J, Shinzawa H, Ishibashi M, Sata M, Mast EE, et al. Empty virus-like particle-based enzyme-linked immunosorbent assay for antibodies to hepatitis E virus. J Med Virol. 2000;62(3):327–33.
Shimizu K, Hamaguchi S, Ngo CC, Li TC, Ando S, Yoshimatsu K, et al. Serological evidence of infection with rodent-borne hepatitis E virus HEV-C1 or antigenically related virus in humans. J Vet Med Sci. 2016 [cited 2023 Jul 24];78(11):1677–81. Available from: https://pubmed.ncbi.nlm.nih.gov/27499185/ .
Nghiem XH, Pham XH, Trinh VS, Dao PG, Mai TB, Dam TA, et al. HEV positivity in domesticated pigs and a relative risk of HEV zoonosis among occupationally exposed individuals in Vietnam. J Hepatol. 2018 [cited 2023 Jul 24];68:S186–7. Available from: https://www.researchgate.net/publication/324700980 .
Tran HTT, Ushijima H, Quang VX, Phuong N, Li TC, Hayashi S, et al. Prevalence of hepatitis virus types B through E and genotypic distribution of HBV and HCV in Ho Chi Minh City. Vietnam Hepatology Research. 2003 [cited 2023 Jul 24];26(4):275–80. Available from: https://pubmed.ncbi.nlm.nih.gov/12963426/ .
South-East Asia | Demographic Changes. Available from: https://www.population-trends-asiapacific.org/data/sea . Accessed May 18 2023.
Sentian J, Payus CM, Herman F, Kong VWY. Climate change scenarios over Southeast Asia. APN Sci Bull. 2022 [cited 2023 Sep 28];12(1):102–22. Available from: https://www.apn-gcr.org/bulletin/?p=1927 .
Lee T HJ. Southeast Asia’s growing meat demand and its implications for feedstuffs imports. Amber Waves: The Economics of Food, Farming, Natural Resources, and Rural America. 2019;(03). https://ideas.repec.org/a/ags/uersaw/302703.html . https://www.ers.usda.gov/amber-waves/2019/april/southeast-asia-s-growing-meat-demand-and-its-implications-forfeedstuffs-imports/ .
Rossi-Tamisier M, Moal V, Gerolami R, Colson P. Discrepancy between anti-hepatitis E virus immunoglobulin G prevalence assessed by two assays in kidney and liver transplant recipients. J Clin Virol. 2013 [cited 2023 Jul 27];56(1):62–4. Available from: https://pubmed.ncbi.nlm.nih.gov/23089569/ .
Wenzel JJ, Preiss J, Schemmerer M, Huber B, Jilg W. Test performance characteristics of Anti-HEV IgG assays strongly influence hepatitis E seroprevalence estimates. J Infect Dis. 2013 [cited 2023 Jul 27];207(3):497–500. Available from: https://pubmed.ncbi.nlm.nih.gov/23148290/ .
Chongsuvivatwong V, Phua KH, Yap MT, Pocock NS, Hashim JH, Chhem R, et al. Health and health-care systems in southeast Asia: diversity and transitions. Lancet. 2011;377(9763):429–37.
Download references
Acknowledgements
The authors would like to thank all researchers of the primary research included in this study.
This work was supported by Project Research Center for Epidemiology and Prevention of Viral Hepatitis and Hepatocellular Carcinoma, Hiroshima University led by Prof. Junko Tanaka (PI).
Author information
Authors and affiliations.
Department of Epidemiology, Infectious Disease Control and Prevention, Graduate School of Biomedical and Health Sciences, Hiroshima University, 1-2-3, Kasumi, Hiroshima, Minami, 734-8551, Japan
Ulugbek Khudayberdievich Mirzaev, Serge Ouoba, Ko Ko, Zayar Phyo, Chanroth Chhoung, Akuffo Golda Ataa, Aya Sugiyama, Tomoyuki Akita & Junko Tanaka
Department of Hepatology, Research Institute of Virology, Tashkent, Uzbekistan
Ulugbek Khudayberdievich Mirzaev
Unité de Recherche Clinique de Nanoro (URCN), Institut de Recherche en Sciences de La Santé (IRSS), Nanoro, Burkina Faso
Serge Ouoba
You can also search for this author in PubMed Google Scholar
Contributions
UM, TA, and JT conceptualized the study. UM and SO contributed to developing the study design and data acquisition. UM, CC, ZP, AG, SO, and JT analysed and interpreted the data. UM, KK, and AS drafted the manuscript. TA, AS, KK, SO, and JT contributed to the intellectual content of the manuscript. All authors read and approved the final manuscript. JT and TA shared the co-correspondence.
Corresponding authors
Correspondence to Tomoyuki Akita or Junko Tanaka .
Ethics declarations
Ethics approval and consent to participate.
Not applicable.
Consent for publication
Competing interests.
The authors declare no competing interests.
Additional information
Publisher’s note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Supplementary material 1., supplementary material 2., supplementary material 3., rights and permissions.
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ . The Creative Commons Public Domain Dedication waiver ( http://creativecommons.org/publicdomain/zero/1.0/ ) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
Reprints and permissions
About this article
Cite this article.
Mirzaev, U.K., Ouoba, S., Ko, K. et al. Systematic review and meta-analysis of hepatitis E seroprevalence in Southeast Asia: a comprehensive assessment of epidemiological patterns. BMC Infect Dis 24 , 525 (2024). https://doi.org/10.1186/s12879-024-09349-2
Download citation
Received : 30 October 2023
Accepted : 24 April 2024
Published : 24 May 2024
DOI : https://doi.org/10.1186/s12879-024-09349-2

Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Hepatitis E virus
- Southeast Asia
- Immunoglobulins
- Systematic review
- Meta-analysis
- Epidemiologic patterns
BMC Infectious Diseases
ISSN: 1471-2334
- General enquiries: [email protected]
ORIGINAL RESEARCH article
This article is part of the research topic.
Urban Energy System Planning, Operation, and Control with High Efficiency and Low Carbon Goals
Asset Risk Assessment and Management of Large-scale Electricity Enterprises under the Concept of Financial Sharing Provisionally Accepted

- 1 School of Economics and Management, North China Electric Power University, China
The final, formatted version of the article will be published soon.
The power grid is an important industry that is crucial to national security and economic development, and its importance in society continues to grow. As an emerging concept, financial sharing enables internal resource sharing and optimization, thereby improving the efficiency and effectiveness of asset management. This study investigates and analyzes the current situation of asset management in large-scale electricity enterprises in X Province, China, and proposes a comprehensive asset management strategy optimization plan based on the concept of financial sharing. The proposed plan integrates management models such as PDCA and designs an entire information management architecture to enhance resource utilization efficiency, reduce environmental pollution risks, and optimize asset allocation and operational decisions. In addition, it also utilizes the status of assets to assess the risks associated with fixed assets in the power grid. The results indicate that the asset risk assessment method under the concept of financial sharing can reduce power grid asset losses, effectively enhance the competitiveness and sustainable development capabilities of electricity enterprises.
Keywords: Financial sharing, Electricity enterprises, asset management, Risk Assessment, Strategy Optimization
Received: 10 May 2024; Accepted: 31 May 2024.
Copyright: © 2024 Bai and Yuan. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY) . The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
* Correspondence: Miss. Minyue Bai, School of Economics and Management, North China Electric Power University, Beijing, China
People also looked at
Fats and Cholesterol
When it comes to dietary fat, what matters most is the type of fat you eat. Contrary to past dietary advice promoting low-fat diets , newer research shows that healthy fats are necessary and beneficial for health.
- When food manufacturers reduce fat, they often replace it with carbohydrates from sugar, refined grains, or other starches. Our bodies digest these refined carbohydrates and starches very quickly, affecting blood sugar and insulin levels and possibly resulting in weight gain and disease. ( 1-3 )
- Findings from the Nurses’ Health Study ( 4 ) and the Health Professionals Follow-up Study ( 5 ) show that no link between the overall percentage of calories from fat and any important health outcome, including cancer, heart disease, and weight gain.
Rather than adopting a low-fat diet, it’s more important to focus on eating beneficial “good” fats and avoiding harmful “bad” fats. Fat is an important part of a healthy diet. Choose foods with “good” unsaturated fats, limit foods high in saturated fat, and avoid “bad” trans fat.
- “Good” unsaturated fats — Monounsaturated and polyunsaturated fats — lower disease risk. Foods high in good fats include vegetable oils (such as olive, canola, sunflower, soy, and corn), nuts, seeds, and fish.
- “Bad” fats — trans fats — increase disease risk, even when eaten in small quantities. Foods containing trans fats are primarily in processed foods made with trans fat from partially hydrogenated oil. Fortunately, trans fats have been eliminated from many of these foods.
- Saturated fats , while not as harmful as trans fats, by comparison with unsaturated fats negatively impact health and are best consumed in moderation. Foods containing large amounts of saturated fat include red meat, butter, cheese, and ice cream. Some plant-based fats like coconut oil and palm oil are also rich in saturated fat.
- When you cut back on foods like red meat and butter, replace them with fish, beans, nuts, and healthy oils instead of refined carbohydrates.
Read more about healthy fats in this “Ask the Expert” with HSPH’s Dr. Walter Willett and Amy Myrdal Miller, M.S., R.D., formerly of The Culinary Institute of America
1. Siri-Tarino, P.W., et al., Saturated fatty acids and risk of coronary heart disease: modulation by replacement nutrients. Curr Atheroscler Rep, 2010. 12(6): p. 384-90.
2. Hu, F.B., Are refined carbohydrates worse than saturated fat? Am J Clin Nutr, 2010. 91(6): p. 1541-2.
3. Jakobsen, M.U., et al., Intake of carbohydrates compared with intake of saturated fatty acids and risk of myocardial infarction: importance of the glycemic index. Am J Clin Nutr, 2010. 91(6): p. 1764-8.
4. Hu, F.B., et al., Dietary fat intake and the risk of coronary heart disease in women. N Engl J Med, 1997. 337(21): p. 1491-9.
5. Ascherio, A., et al., Dietary fat and risk of coronary heart disease in men: cohort follow up study in the United States. BMJ, 1996. 313(7049): p. 84-90.
6. Hu, F.B., J.E. Manson, and W.C. Willett, Types of dietary fat and risk of coronary heart disease: a critical review. J Am Coll Nutr, 2001. 20(1): p. 5-19.
Terms of Use
The contents of this website are for educational purposes and are not intended to offer personal medical advice. You should seek the advice of your physician or other qualified health provider with any questions you may have regarding a medical condition. Never disregard professional medical advice or delay in seeking it because of something you have read on this website. The Nutrition Source does not recommend or endorse any products.
An official website of the United States government
The .gov means it's official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you're on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
- Browse Titles
NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
Methods Guide for Effectiveness and Comparative Effectiveness Reviews [Internet]. Rockville (MD): Agency for Healthcare Research and Quality (US); 2008-.

Methods Guide for Effectiveness and Comparative Effectiveness Reviews [Internet].
Assessing the risk of bias in systematic reviews of health care interventions.
Investigators: Meera Viswanathan , Ph.D., Carrie D. Patnode , Ph.D., M.P.H., Nancy D. Berkman , Ph.D., Eric B. Bass , M.D., M.P.H., Stephanie Chang , M.D., M.P.H., Lisa Hartling , Ph.D., M. Hassan Murad , M.D., M.P.H., Jonathan R. Treadwell , Ph.D., and Robert L. Kane , M.D.
Published: December 13, 2017 .
- Structured Abstract
Risk-of-bias assessment is a central component of systematic reviews but little conclusive empirical evidence exists on the validity of such assessments. In the context of such uncertainty, we present pragmatic recommendations that can be applied consistently across review topics, promote transparency and reproducibility in processes, and address methodological advances in the risk-of-bias assessment.
Study Design:
Epidemiological study design principles; available empirical evidence, risk-of-bias tools, and guidance; and workgroup consensus
We developed recommendations for assessing the risk of bias of studies of health care interventions specific to framing the focus and scope of risk-of-bias assessment; selecting risk of bias categories; choosing assessment instruments; and conducting, analyzing, and presenting results of risk-of-bias assessments. Key recommendations include transparency and reproducibility of judgments, separating risk of bias from other constructs such as applicability and precision, and evaluating risk of bias per outcome. We recommend against certain past practices, such as focusing on reporting quality, relying solely on study design, or numerical quality scores, and automatically downgrading for industry sponsorship.
Conclusion:
Risk-of-bias assessment remains a challenging but essential step in systematic reviews. We presented standards to promote transparency of judgments.
The Agency for Healthcare Research and Quality (AHRQ), through its Evidence-based Practice Centers (EPCs), sponsors the development of evidence reports and technology assessments to assist public- and private-sector organizations in their efforts to improve the quality of health care in the United States. The reports and assessments provide organizations with comprehensive, science-based information on common, costly medical conditions and new health care technologies and strategies. The EPCs systematically review the relevant scientific literature on topics assigned to them by AHRQ and conduct additional analyses when appropriate prior to developing their reports and assessments.
Strong methodological approaches to systematic review improve the transparency, consistency, and scientific rigor of these reports. Through a collaborative effort of the Effective Health Care (EHC) Program, the Agency for Healthcare Research and Quality (AHRQ), the EHC Program Scientific Resource Center, and the AHRQ Evidence-based Practice Centers have developed a Methods Guide for Comparative Effectiveness Reviews. This Guide presents issues key to the development of Systematic Reviews and describes recommended approaches for addressing difficult, frequently encountered methodological issues.
The Methods Guide for Comparative Effectiveness Reviews is a living document, and will be updated as further empiric evidence develops and our understanding of better methods improves.
If you have comments on this Methods Guide paper, they may be sent by mail to the Task Order Officer named below at: Agency for Healthcare Research and Quality, 5600 Fishers Lane, Rockville, MD 20857, or by email to vog.shh.qrha@cpe .
- Gopal Khanna, M.B.A. Director Agency for Healthcare Research and Quality
- Stephanie Chang, M.D., M.P.H. Director Evidence-based Practice Center Program Center for Evidence and Practice Improvement Agency for Healthcare Research and Quality
- Arlene S. Bierman, M.D., M.S. Director Center for Evidence and Practice Improvement Agency for Healthcare Research and Quality
- Acknowledgments
The authors gratefully acknowledge the following individuals for their contributions to this project: Issa J. Dahabreh, M.D., M.S., Celia Fiordalisi, M.S., Makalapua Motu’apuaka, B.S., Robin Paynter, M.L.I.S., Edwin Reid, M.S., and Lyndzie Sardenga, B.S.
- Peer Reviewers
Prior to publication of the final evidence report, EPCs sought input from independent Peer Reviewers without financial conflicts of interest. However, the conclusions and synthesis of the scientific literature presented in this report does not necessarily represent the views of individual reviewers.
Peer Reviewers must disclose any financial conflicts of interest greater than $10,000 and any other relevant business or professional conflicts of interest. Because of their unique clinical or content expertise, individuals with potential non-financial conflicts may be retained. The Task Order Officer and the EPC work to balance, manage, or mitigate any potential non-financial conflicts of interest identified.
- Roger Chou, M.D., FACP Director, Pacific Northwest EPC Portland, OR
- Susanne Hempel, Ph.D. Co-Director, Southern California EPC RAND Corporation Santa Monica, CA
- Jennifer Lin, M.D., M.C.R. Director, Kaiser Permanente EPC Portland, OR
- Terri Pigott, Ph.D. Associate Provost for Research Loyola University Chicago Chicago, IL
- Gillian Sanders Schmidler, Ph.D. Director, Duke University EPC Durham, NC
- P. Lina Santaguida, Ph.D., M.Sc. Assistant Professor, McMaster University South Hamilton, ON
- Karen Schoelles, M.D., S.M., FACP Director, ECRI Institute-Penn Medicine EPC Plymouth Meeting, PA
- Jeffrey C. Valentine, Ph.D. Professor College of Education and Human Development University of Louisville Louisville, KY
- C. Michael White, Pharm.D., FCP, FCCP Director, University of Connecticut EPC Storrs, CT
- Key Recommendations
- Clearly separate assessing the risk of bias from other important and related activities such as assessing the degree of congruence between the research questions of a systematic review and designs of included studies, the precision of an effect estimate, and the applicability of the evidence.
- The methodology for assessing risk of bias should be transparent and reproducible. This requires the review’s protocol to include clear definitions of the types of biases that will be assessed and a priori decision rules for assigning the risk of bias for each individual study. New or changed processes developed over the course of the review should be documented clearly.
- Assess risk of bias based on study design-specific criteria and conduct rather than quality of reporting of methods and results. Poorly reported studies may be judged as unclear risk of bias.
- Allow for separate risk-of-bias ratings for each outcome to account for outcome-specific variations in potential types or extent of bias. For some studies, all outcomes may have the same sources of bias; for other studies, the sources of bias may vary by outcome.
- Use risk of bias assessments to explore heterogeneity of results, to interpret the estimate of effect through sensitivity analysis (quantitatively if studies can be pooled, qualitatively otherwise), and to grade the strength of evidence.
- Do not rely solely on study design label (e.g., randomized controlled trial [RCT] or cohort, case-control) as a proxy for assessment of risk of bias of individual studies.
- Reviewers who incorporate existing systematic reviews in new reviews or subgroup analyses from individual studies should evaluate the credibility of these sources of information.
- Select risk of bias categories as appropriate for the topic and study design because not all categories of bias matter equally for all topics and designs.
- When selecting risk of bias categories, consider bias arising in the randomization process or due to confounding; departures from intended interventions; missing data; measurement of outcomes; and selective outcome reporting in all studies. Additionally, biased participant selection and misclassification of interventions may influence results in nonrandomized or poorly randomized studies.
- Do not use poor or incomplete reporting, industry funding, or disclosed conflict of interest to rate an outcome or study as high risk of bias; do, however, report these issues transparently and consider their impact on bias.
- Choose risk-of-bias instruments that are based on epidemiological study design principles, established measurement properties (e.g., reliability, internal consistency) or empirical evidence (when available).
- Choose instruments that include items assessing specific concerns related to each of the risk of bias categories that pose threats to the accuracy of the effect estimate.
- Use processes to reduce uncertainty in individual judgments such as dual independent assessment of risk of bias with an unbiased reconciliation method. First-order assessments of risk of bias by machine-learning methods require secondary human review.
- Balance the competing considerations of simplicity of presentation and burden on the reader when presenting results of risk of bias assessments. An overall study or outcome-specific risk of bias rating alone, without supporting details, offers simplicity but lacks transparency. Provide enough detail to make the rationale for the assessment clear
- Consider both the direction and magnitude of possible bias on the effect estimate when possible, rather than leaving the burden to the reader.
- Avoid the presentation of risk of bias assessment solely as a numerical score; at minimum, consider sensitivity analyses of these scores.
- When summarizing the evidence, consider conducting sensitivity analyses to evaluate whether including studies with high or unclear risk of bias (overall or in specific categories) influences the estimate of effect or heterogeneity.
- Systematic reviewers who choose to exclude high risk-of-bias studies from their analysis should explain and justify the criteria used to identify excluded studies.
- Introduction
Assessing the risk of bias of studies included in the body of evidence is a foundational part of all systematic reviews. 1 , 2 It is distinct from other important and related activities of assessing the degree of the congruence of the research question with the study design and the applicability of the evidence. The specific use of risk-of-bias assessments can vary. Assessment of risk of bias (labeled as unclear, high, moderate, or low) are intended to help interpret findings and explain heterogeneity; in addition, EPC reviews use risk-of-bias assessments of individual studies in grading the strength of the body of evidence. Some EPC reviews may exclude studies assessed as high risk of bias.
Despite the importance of risk-of-bias assessments in systematic reviews, evidence on the validity of such assessments is available only for a few risk-of-bias categories. 3 – 5 Specifically, evidence suggests that effect sizes may be inaccurate when allocation is inappropriately concealed; random sequences are inadequately generated; and patients, clinicians, or outcome assessors (particularly for subjective outcomes) are not blinded. 4 , 6 The influence on estimates of effect can be inconsistent and difficult to predict for other bias categories such as confounding, fidelity to the protocol, and attrition bias, possibly because meta-epidemiological studies are inadequately powered. 5 In addition to concerns regarding the validity of such assessments, methodological studies have raised concerns about the limited reliability of risk-of-bias judgments. 7 , 8
We do not attempt, in this document, to address the underlying and important sources of uncertainty related to the validity or reliability of risk-of-bias assessment. This document updates the existing Agency for Healthcare Research and Quality (AHRQ) Evidence-based Practice Center (EPC) Methods Guide for Effectiveness and Comparative Effectiveness Reviews on assessing the risk of bias of individual studies. This update adds areas of guidance (e.g., evaluating subgroup analyses and including systematic reviews as evidence), modifies guidance to reflect new thinking (e.g., risk-of-bias categories), and offers guidance to promote clarity and consistency. As with other AHRQ methodological guidance, our intent is to present standards that can be applied consistently across EPCs and review topics, promote transparency and reproducibility in processes, and account for methodological changes in the systematic review process. These standards are based on epidemiological study design principles, available empirical evidence, or workgroup consensus. As greater evidence accumulates in this methodological area, our standards will continue to evolve. When possible, our guidance offers flexibility to account for the wide range of AHRQ EPC review topics and included study designs, but also offers parameters within which this flexibility can be applied.
In this guidance document, we define terms as appropriate for the EPC program, explore the potential overlap in different steps of the systematic review, and offer recommendations on the inclusion and exclusion of constructs that may apply to multiple steps of the systematic review process. This guidance applies to systematic reviews exploring the link between an intervention or exposure and outcome. (Reviewers focusing on diagnostic tests, 9 prognosis, 10 – 12 prevalence, or qualitative 13 analysis should also consult guidance specific to these topics.) Later sections of this guidance document provide advice on minimum design-specific criteria to evaluate risk of bias and the stages involved in assessing risk of bias. We conclude with guidance on summarizing risk of bias.
- Terminology
- “Likelihood.” The actual bias of a study is unknowable, because the true effect size is unknowable. Further, poor study reporting can make important aspects of study design and conduct unclear. A risk-of-bias assessment offers a qualitative judgment of likelihood of bias.
- “Inaccuracy.” A study can either overestimate or underestimate the true effect, and EPC reviewers should consider both possibilities.
- “Estimate.” This word places the focus of risk of bias on the study’s point estimate of the effect, not the precision of that point estimate. We discuss this in more detail in the next section (“Constructs Included and Excluded in Risk of Bias Assessment”).
- “Causal effect.” In assessing the efficacy or effectiveness of one intervention versus another or versus a control, a key goal is to assess the extent to which an observed outcome difference can be directly attributed to the treatment difference.
- “In that study.” This phrase is meant to exclude the concept of applicability from risk of bias. Whether the study results apply to other contexts is outside the scope of risk-of-bias assessment. 14 – 16
We use the phrase “risk of bias” rather than “quality assessment,” because the meaning of the term quality varies, depending on the source of the guidance. Quality has been defined as “the extent to which all aspects of a study’s design and conduct can be shown to protect against systematic bias, nonsystematic bias, and inferential error.” 17 The Grading of Recommendations Assessment, Development and Evaluation Working Group (GRADE) uses the term quality to refer to judgments based about the strength of the body of evidence. 18 The U.S. Preventive Services Task Force (USPSTF) equates quality with internal validity and classifies individual studies first according to a hierarchy of study design and then by individual criteria that vary by type of study. 19 Cochrane argues for wider use of the phrase “risk of bias” instead of “quality,” reasoning that “an emphasis on risk of bias overcomes ambiguity between the quality of reporting and the quality of the underlying research (although [this emphasis] does not overcome the problem of having to rely on reports to assess the underlying research).” 14
Because of inconsistency and potential misunderstanding in the use of the term “quality,” this guidance uses risk of bias as the preferred terminology. Assessing the risk of bias of a study can be thought of as assessing the risk that the results are skewed by bias in study design or execution. This assessment process should be tailored to the specific research and clinical context of the review. We recommend that EPCs define the terms selected in their systematic review protocols and describe the risk-of-bias categories included in the assessment.
In the remainder of this document, we refer to components of risk of bias as categories and elements within each category as criteria (or items, if we are referring specifically to a tool). Because ideas on risk-of-bias categories have evolved, the next section describes debated larger constructs that either continue or are no longer considered to be risk-of-bias categories.
- Constructs To Include and Exclude From Risk-of-Bias Assessment
- conduct of the study or internal validity,
- applicability or external validity,
- poor reporting of study design and conduct,
- selective reporting of outcomes,
- choice of outcome measures,
- design of included studies,
- fidelity to the intervention protocol, and
- conflict of interest in the conduct of the study.
The lack of agreement on what constructs to include in risk-of-bias assessment stems from two issues. First, no strong empirical evidence supports one approach over another; this gap leads to a proliferation of approaches based on the practices of different academic disciplines and the needs of different clinical topics. Second, in the absence of clear guidance on related components of systematic reviews (such as selection of evidence, 22 assessment of applicability, 23 or grading the strength of evidence 18 , 24 – 32 ), some review groups continue to use practices that have served well in the past.
In the absence of strong empirical evidence, methodological decisions in this guidance document rely on epidemiological study design principles. 1 Systematic reviewers have the responsibility to evaluate potential sources of bias and error if these concerns could plausibly influence study results; we include these concerns even if no empirical evidence exists that they influence study results.
The constructs selected in the assessment of risk of bias may differ because of the clinical topic, academic orientation of the reviewers, and guidelines by sponsoring organizations. In AHRQ-sponsored reviews, guidance and requirements for systematic reviews have reduced the variability in other related steps of the systematic review process and, therefore, allow for greater consistency in risk-of-bias assessment as well. Some constructs that EPCs may have considered part of risk-of-bias assessment in the past now overlap with or fall within other systematic review tasks. Table 1 illustrates which constructs to include for each systematic review task when reviews separately assess the risk of bias of individual studies, the strength of the body of evidence, and applicability of the findings for individual studies. Specific categories to consider when assessing risk of bias are noted separately below. Constructs wholly or partially excluded from risk-of-bias assessment continue to play an important role in the overall assessment of the evidence. The remainder of this section describes these constructs in greater detail and the rationale for including or excluding them in risk-of-bias assessments.
Addressing precision, applicability, and bias within a systematic review.
Precision refers to the degree of uncertainty surrounding an effect estimate with respect to a given outcome, based on the sufficiency of sample size and number of events. 24 Both GRADE 33 and AHRQ guidance on evaluating the strength of evidence 24 separate the evaluation of precision from that of the summary of risk of bias for a body of evidence (study limitations). Systematic reviews now routinely evaluate precision (through consideration of the optimal information size or required information size and confidence intervals around a summary effect size from pooled estimates) when grading the strength of the body of evidence. 24 Thus, the inclusion of precision as a construct under risk of bias would constitute double-counting limitations to the evidence from a single source. We recommend that AHRQ reviews exclude considerations of power and precision of the effect estimate when assessing the risk of bias .
Applicability
Applicability refers to the extent to which the effects observed in published studies are likely to reflect the expected results when a specific intervention is applied to the population of interest under “real-world” conditions. 34 Both GRADE 33 and AHRQ guidance on evaluating the strength of evidence 24 exclude considerations of applicability in risk-of-bias assessments of individual studies. We note, however, that some study features may be relevant to both risk of bias and applicability. Duration of follow-up is one such example: if duration of follow-up is different across comparison groups within a study, this difference could be a source of bias; the absolute duration of follow-up for the study would be relevant to the clinical context of interest and therefore the applicability of the study. Likewise, the study population may be considered within both risk of bias and applicability: if the populations are systematically different between comparison groups within a study (e.g., important baseline imbalances) this may be a source of bias; the population selected for the focus of the study (e.g., inclusion and exclusion criteria) would be a consideration of applicability. We recommend that reviewers clearly separate study features that may be potential sources of bias from those that are concerned with the applicability of the individual study to the intervention, population, and context of interest.
Poor or Inadequate Reporting
In theory, risk of bias focuses on the design and conduct of a study. In practice, assessing the risk of bias of a study depends on the availability of a clear and complete description of how the study was designed and conducted, and may require additional information by reviewing clinical trials registries or study protocols or reaching out to investigators. Although new standards seek to improve reporting of study design and conduct, 35 – 39 EPC review teams continue to need a practical approach to dealing with poor or inadequate reporting. Empirical studies suggest that unclear or poor reporting may not always reflect poor study conduct. 40
EPC reviews have varied in their treatment of reporting of study design and conduct. Some have elected to rate outcomes from poorly reported studies as having high risk of bias. Other EPCs have chosen to select an “unclear risk-of-bias” category for studies with missing or poorly reported information on which to base risk-of-bias judgments. In other cases, EPCs have judged that specific bias components, although poorly reported, have no material effect on overall risk of bias. We recommend that assessment of risk of bias focus primarily on the design and conduct of studies and not on the quality of reporting. However, we recognize that poor reporting can impede judgments of risk of bias. Therefore, we also recommend that EPCs clearly document inadequate reporting for all risk of bias domains. When reviews include meta-analyses, we recommend that systematic reviewers consider sensitivity analyses to assess the impact of including studies with poorly reported risk-of-bias components; when studies cannot be pooled, consider qualitative analyses.
Selective Outcome Reporting
Reporting bias occurs when the nature and direction of the results influences their dissemination. 1 Reporting bias includes bias in whether to publish or not (publication bias), when to publish (time lag bias), where to publish (location bias [selecting venues with greater or lesser ease of access depending on the direction of results]) and what to publish (selective outcome reporting). Many of these sources of bias are best addressed at the level of the body of evidence; patterns of bias may not be discernable at the level of the individual study. Selective outcome reporting, specifically, has 41 major implications for both the risk of bias of individual studies and the strength of the body of evidence 41 and can be discerned in some instances at the level of the individual study. Comparisons of the full protocol to published and unpublished results can help to flag studies that selectively report outcomes. In the absence of access to full protocols, 24 , 32 Guyatt et al. note that “[o]ne should suspect reporting bias if the study report fails to include results for a key outcome that one would expect to see in such a study or if composite outcomes are presented without the individual component outcomes.” 32 Note that selective outcome reporting includes selective reporting of planned analyses and selective reporting of results.
Methods continue to be developed for identifying and judging the risk of bias when results deviate from protocols in the timing or measurement of the outcome. No guidance currently exists on how to evaluate the risk of selective outcome reporting in older studies with no published protocols or whether to downgrade all evidence from a study where comparisons between protocols and results show clear evidence of selective outcome reporting for some outcomes. Even when access to protocols is available, the evaluation of selective outcome reporting may be required again at the level of the body of evidence. Selective outcome reporting across several studies within a body of evidence may result in downgrading the body of evidence. 32
Previous research has established the link between funding source and an array of consequential study decisions on design, conduct, and dissemination of results (sponsor bias). 42 – 44 Publication bias may be a pervasive problem in some bodies of evidence and should be evaluated when grading the body of evidence, as should time lag and location bias. 43 As methods on identifying and weighing the likely effect of selective outcome reporting and other reporting biases continue to be developed, this guidance will also require updating. We recommend considering the risk of selective outcome reporting for both individual studies and the body of evidence, particularly when a suspicion exists that forces such as sponsor bias may influence the reporting of analyses and results.
Choice of Outcome Measures
The use of valid and reliable outcome measures reduces the likelihood of bias in measuring outcomes. For example, some self-report measures may be rated as having a higher risk of bias than clinically observed outcomes in unblinded designs; at the same time, patient-reported outcomes may also be more applicable to the general population. In addition, use of different outcome measures for each study arm (e.g., electronic medical records for control arm versus questionnaires for intervention arm) constitute a source of measurement bias and should, therefore, be included in assessment of risk of bias. We recommend that assessment of risk of bias of individual studies include the evaluation of the validity and reliability of outcome measures overall, and differences in validity and reliability between study arms.
The validity and reliability measures across treatment arms are criteria for judging the risk-of-bias, but the choice of specific outcome measures should also be considered when judging the directness of the outcome and applicability of the study. Directness of outcomes (or comparisons) refers to whether the evidence directly links interventions to important health outcomes and is a key domain in assessing the strength of the body of evidence 24 or applicability. 34
Study Design
In general, stronger study designs will have lower risk of bias. Some designs possess inherent features (such as randomization and control arms) that reduce the risk of bias and increase the potential for causal inference, particularly when considering benefit of the intervention. Other study designs, often included in EPC reviews, have specific and inherent risks of biases that cannot be minimized. However, instead of equating risk of bias solely with study design, the bias represented by study design features may be considered at the overall strength of evidence level. For example, both AHRQ and GRADE approaches to evaluating the strength of evidence include study design and conduct (risk of bias) of individual studies as components needed to evaluate the body of evidence. The inherent limitations present in nonrandomized designs are factored in when grading the strength of evidence. EPCs generally give evidence derived from nonrandomized studies a lower starting grade and evidence from randomized controlled trials a high grade. They can then upgrade or downgrade the nonrandomized and randomized evidence based on the strength of evidence domains (i.e., risk of bias of individual studies, directness, consistency, precision, and additional domains if applicable). 24
We recommend that EPCs do not use study design labels (e.g. observational studies) as a proxy for assessment of risk of bias of individual studies. In other words, EPCs should not downgrade the risk of bias of individual studies based solely on the study design label but should use risk-of-bias categories or criteria that consider the role of the design element and the subsequent risk of bias. A study can be conducted well but still have some (if not serious) potential risk of bias because of underlying design flaws. 1
EPCs may consider whether to exclude evidence from study designs with limited ability to address causal inference, such as case studies and case series. Under such circumstances, our guidance is to consider the question of value to the review with regard to each study design type: “Will [case reports/case series, etc.] provide valid and useful information to address key questions?” Depending on the clinical question and the context, EPCs may judge that the information provides value or that the risk of bias from a particular study design may be unacceptably high. If such nonrandomized studies are included, we recommend that EPCs consider the risk of bias of individual studies, rather than applying a single common rating based on design without considering study-specific variations in design and conduct .
Fidelity to the Intervention Protocol
Failure of the study to maintain fidelity to the intervention protocol can bias performance; it is, therefore, a component of risk of bias assessment. We note, however, that the interpretation of fidelity may differ by clinical topic and the nature of the outcome evaluated. For instance, some behavioral interventions include “fluid” interventions; these involve interventions for which the protocol explicitly allows for modification based on patient needs or concomitant treatments. Such fluidity does not mean the interventions are implemented incorrectly, and an intention-to-treat analysis will capture the effect of the intervention as assigned. When interventions implement protocols that have minimal concordance with practice, the discrepancy may be considered an issue of applicability. This lack of concordance with practice does not, however, constitute risk of bias. When systematic reviewers are interested in the effect of starting and adhering to interventions (the per-protocol effect), deviations from the intervention protocol (including lower-than-expected adherence) can bias results. We recommend that EPCs account for the specific clinical and outcome considerations in determining and applying criteria about fidelity for assessment of risk of bias.
Conflict of Interest
Studies have found that conflicts of interest (financial and nonfinancial) can threaten the internal validity and applicability of primary studies and systematic reviews. 45 , 46 Conflicts of interest can arise from when investigators or funders of studies deploy strategies that influence the results such as (1) selecting specific designs and hypotheses—for example, choosing noninferiority rather than superiority approaches, 47 picking comparison drugs and doses, 47 choosing outcomes, 46 or using composite endpoints (e.g., mortality and quality of life) without presenting data on individual endpoints; 48 (2) selectively reporting outcomes—for example, reporting relative risk reduction rather than absolute risk reduction; selecting from multiple endpoints 47 or reporting on subscales of larger scales; reporting inappropriately developed categorical variables, based on selected cut-points in continuous measures; 49 (3) presenting results in a biased 48 or inadequate manner 49 and (4) failing to publish results, thereby contributing to publication bias. 50
EPCs can evaluate these pathways if and only if the relationship between the sponsor(s) and the author(s) is clearly documented; in some instances, such documentation may not be sufficient to judge the likelihood of conflict of interest (for example, authors may receive speaking fees from a third party that did not support the study in question). In other instances, the practice of ghost authoring (i.e., primary authors or substantial contributors are not identified) or guest authoring (i.e., one or more identified authors are not substantial contributors) 51 makes the actual contribution of the sponsor very difficult to discern. 52 , 53
Given these concerns, conflicts of interest should be considered when critically appraising the evidence because they may serve as an indirect marker of risk of bias. For several reasons, we caution against simple-to-follow rules such as equating industry sponsorship with high risk of bias. First, financial conflicts of interest are not limited to industry; nonprofit and government-sponsored studies may also have conflicts of interest. Researchers may have various financial or intellectual conflicts of interest by virtue of, for example, accepting speaking fees from many sources. 54 Second, financial conflict is not the only source of conflict of interest: other potential conflicts include personal, professional, or religious beliefs, desire for academic recognition, and so on. 45 Third, the multiple pathways by which conflicts of interest may influence studies are not all solely within the domain of assessment of risk of bias: several of these pathways fall under the purview of other systematic review tasks. For instance, concerns about the choice of designs, hypotheses, and outcomes relate as much or more to applicability than other aspects of reviews. Reviewers can and should consider the likely influence of conflicts of interest on selective outcome reporting for individual studies, but when these judgments may be limited by lack of access to full protocols, the assessment of selective outcome reporting may be more easily judged for the body of evidence than for individual studies.
Conflicts of interest may be particularly apparent in conclusions of studies. 55 Although of concern to the general reader, biased presentation or “spin” on results, if limited to the discussion and conclusion section of studies, should have no bearing on systematic review conclusions because systematic reviews should not rely solely on interpretation of data by study authors. Nonetheless, biased presentation of results may serve as a flag to evaluate the potential for risk of bias closely.
Internal validity and completeness of reporting constitute, then, the primary pathway by which conflicts of interest may influence the validity of study results that is entirely within the purview of assessment of risk of bias. We acknowledge that this pathway may not be the most important source of conflict of interest: as standards for conduct and reporting of studies become widespread and journals require that they be met, differences in internal validity and reporting between studies with and without inherent conflicts of interest will likely attenuate. In balancing these considerations with the primary responsibility of the systematic reviewer—objective and transparent synthesis and reporting of the evidence— we recommend: (1) at a minimum, EPCs should routinely report the source of each study’s funding (or the failure of the study to report such information); (2) EPCs should consider issues of selective outcome reporting at the individual study level and for the body of evidence; and (3) EPCs should conduct sensitivity analyses (quantitative or qualitative) for the body of evidence when they have reason to suspect that the source of funding or disclosed conflict of interest is influencing studies’ results. 47
- Stages in Assessing the Risk of Bias of Studies
International reporting standards require documentation of various stages in a systematic review. 56 – 58 We lay out recommended approaches to assessing risk of bias in five steps: protocol development, pilot testing and training, assessment of risk of bias, interpretation, and reporting. Table 2 describes the stages and specific steps in assessing the risk of bias of individual studies that contribute to transparency through careful documentation of decisions.
Stages in assessing the risk of bias of individual studies.
The plan for assessing risk of bias should be included within the protocol for the entire review . As prerequisites to developing the plan for assessment of risk of bias, EPCs must identify the important outcomes that need risk-of-bias assessment and other study descriptors or study data elements that are required to assess risk of bias in the systematic review protocol. Protocols must describe and justify what risk-of-bias categories and tools will be used and how the reviewers will incorporate risk of bias of individual studies in the synthesis of evidence.
The assessment should include a minimum of two independent reviewers per study with an unbiased reconciliation method such as a third person serving as arbitrator. EPCs should anticipate having to review and revise assessment of risk-of-bias forms and instructions in response to problems arising in training and pilot testing. Although we recommend that risk-of-bias assessment be performed in duplicate, reviewers should be aware of recent software developments that may improve the efficiency of the process. A study by Marshall et al. (2014) 59 , 60 applied text-mining software to 2,200 full-text publications and their parent Cochrane reviews. The software analyzed textual patterns between full-text articles and the eventual risk-of-bias assessments of Cochrane authors (e.g., the occurrence of the phrase “sealed envelopes” in a full article is likely an accurate predictor of “low” risk of bias with respect to concealment of allocation). Although the software should not be used to completely replace reviewers (as it did make some erroneous predictions), other possible uses include the production of first-pass judgments (with subsequent human review), or the automation of text flagging to support reviewers’ risk-of-bias judgments. First order assessments of risk of bias by machine-learning require secondary human review.
Assessment of risk of bias should be consistent with the registered protocols of the reviews. The synthesis of the evidence should reflect the a priori plan in the protocol for incorporating risk of bias of individual studies in qualitative or quantitative analyses. EPCs should report the outcomes of all preplanned analyses that included risk-of-bias criteria regardless of statistical significance or the direction of the effect. Published reviews should also include justifications of all post hoc decisions to limit synthesis of included studies to a subset with common methodological or reporting attributes. When reviewers exclude high risk-of-bias studies from their analysis entirely without any sensitivity analyses, we recommend that reviewers explain their decision.
- Identifying, Selecting, and Assessing Categories of Risk of Bias
Identifying Categories of Risk of Bias
Different categories of bias are often described by a host of different terms and the same terms are sometimes used to refer to different categories of bias depending on the study design of interest. Here, we rely and expand on the newly developed ROBINS-I tool 61 to outline specific categories of risk of bias (termed “domains” in the ROBINS-I tool) for assessment in systematic reviews ( Table 3 ). We chose this tool because it offers a comprehensive array of bias categories that captures recent advances in epidemiological thinking. Despite the focus on assessing the risk of bias in nonrandomized studies (e.g., controlled nonrandomized clinical trials, prospective or retrospective cohort studies, and case-control studies) in the ROBINS-I tool, the core categories of risk of bias apply to randomized trials. The key additions relate to biases occurring before or at the start of the intervention. The categories outlined here specifically relate to designs that allow a causal interpretation of the effect of the intervention on outcomes and suggest a preliminary set of criteria for RCTs, nonrandomized cohort designs (nonrandomized controlled designs, prospective and retrospective cohorts with comparisons), and case-control studies. It excludes case studies, case series and cross-sectional studies, although some systematic reviews may choose to include information from such studies. If a study that claims to be an RCT is determined to be better classified as a nonrandomized study (e.g., due to major problems with “randomization”), reviewers may elect to classify the study as nonrandomized, and thus assess risk of bias based on criteria for nonrandomized studies.
Description of risk-of-bias categories and study design-specific assessment criteria for randomized and nonrandomized studies of interventions (adapted from ROBINS-I).
In the ROBINS-I taxonomy of bias, pre-intervention sources of bias arise from confounding and selection of participants into the study. Biases arising at the start of the intervention can occur when intervention status is misclassified (i.e., intervention groups are not clearly defined or recorded at the start of the intervention, classification of the intervention status is affected by knowledge of the outcome). Biases occurring after the initiation of the intervention may arise from departures in intended interventions, missing data, measurement of outcomes, and selective reporting. The authors propose evaluating potential sources of bias in a nonrandomized study against a “target” trial that avoids biases arising lack of randomization in assignment. A target trial is a hypothetical randomized controlled trial of the intervention; feasibility or ethics do not play a role in constructing such a hypothetical trial. 61
Selecting and Assessing Relevant Categories of Bias For a Review
Determining the risks of bias that are most salient or that require special consideration is often dependent on the focus of the clinical topic being reviewed. For example, in the table below, biases arising from departures from intended interventions are particularly relevant for outcomes for which the exposure of interest is starting and adhering to interventions. 61 Reviewers should determine a priori whether the intervention of interest is assignment to the intervention at baseline, or assignment and adherence to the assigned intervention. Prespecification of outcomes (as it relates to bias in reporting results) is another example that requires topic- or outcome-specific evaluation. For example, prespecification of benefits within a study is entirely appropriate and expected, regardless of study design. The prespecification of particular harms , however, may not be possible for all topics; in these cases, data from observational studies may offer the first opportunity to identify unexpected outcomes. Likewise, for review topics in search of evidence on rare long-term outcomes, requiring prespecification would be inappropriate. Another example of a criterion requiring topic-specific evaluation is the expected attrition rate. Differential or overall attrition because of nonresponse, dropping out, loss to follow-up, and exclusion of participants can introduce bias when missing outcome data are related to both exposure and outcome. Reviewers of topics that focus on short-term clinical outcomes may expect a low rate of attrition. We note that with attrition rate in particular, no empirical standard exists across all topics for demarcating a high risk of bias from a lower risk of bias; these standards are often set within clinical topics. Some criteria included in Table 3 , particularly intention-to-treat, have been interpreted in a variety of ways. The Cochrane Handbook of Systematic Reviews offers a more detailed description of intention to treat. 1
Reviewing the risk of bias within individual studies often begins by looking at a study as a whole for potential biases (e.g., valid randomization and allocation procedures, confounding) and then focusing on risks that might occur at an outcome-specific level as not all sources of bias will influence all outcomes measured in a study in the same degree or direction. For instance, biases in the measurement of outcomes (e.g., blinding of outcome assessors) and biases due to missing data may be different for each outcome of interest. That is, blinding of outcome assessors may be particularly important for self-reported measures that are interviewer-administered but may not be a central risk for objectively-measured clinical outcomes. Likewise, in cases of high attrition within a study or for particular outcomes, the appropriateness and effect of procedures to account for missing data (e.g., baseline or last observation carried forward methods) should be considered at an outcome-specific level.
Table 3 is not intended to be used as an instrument. We recommend selecting the most important categories of bias for the outcome(s) and topic at hand. No checklist can replace a thoughtful consideration of all relevant issues. A hypothetical consideration of a target trial can help identify the most important risk-of-bias considerations. 61 In particular, in relation to assessing non-randomized studies, a combination of methods and topical expertise will be necessary to anticipate the most important sources of bias, assess risk of bias, and interpret the effect of potential sources of bias on estimates of effect.
- Tools for Assessing Risk of Bias
- were specifically designed for use in systematic review,
- are specific to the study designs being evaluated,
- show transparency in how assessments are made by providing explicit support for each assessment,
- specifically address items related to risk-of-bias categories,
- are, at minimum, based on theory and are preferably based on empirical evidence that risk-of-bias categories are associated with biased effect estimates or have reasonable face validity, and
- avoid the presentation of risk-of-bias assessment solely as a numerical score (or, if numerically scored, conduct sensitivity analyses of these scores at minimum).
- Direction and Magnitude of Bias
Reviewers should consider both the direction and magnitude of possible bias on the effect estimate in arriving at a risk of bias rating. Regarding direction, reviewers should be careful not to assume that all study biases result in overestimation of effect sizes. As defined earlier, bias is any mis-estimation of an effect size, and both underestimation and overestimation are problematic for decision makers. Although the task of considering the direction and magnitude of bias can be challenging, it helps reviewers judge whether the potential bias is consequential or should be ignored because it is unlikely to materially alter results. It also helps reviewers judge whether deficiencies noted for different areas of bias are related. For example, baseline imbalances in observational studies that have no relationship with the outcome may not be consequential.
The likely direction of bias depends on the risk-of-bias category being considered as well as specific considerations within that category. In the case of confounding—as described by ROBINS-I (“pre-intervention prognostic factor that predicts whether an individual receives one or the other intervention of interest”)—effect size is often overestimated, and a classic case is “confounding-by-indication,” since patients with different medical indications would have had different outcomes regardless of treatment. In the category of missing data, on the other hand, the direction of bias depends on whose data are missing and why they are missing. If one treatment group had a larger rate of missing quality-of-life data and the reason for missing data was that those patients were cured and felt no reason to attend follow-up appointments, then the available data are biased against the group with the larger rate of missing data. But if the reason for missing data was deteriorating health (e.g., did not feel well enough to attend follow-up appointments), the available data are biased in favor of the group with more missing data.
Further complicating matters is the possibility of different biases cancelling each other out. If a study has two clear biases but they appear to work in opposite directions, reviewers may infer that the effect size estimate may be fairly accurate. This inference depends on numerous assumptions, including (1) that the reviewer has correctly judged the direction of bias in both cases; (2) that the two biases have similar magnitude; and (3) that the reviewer has correctly judged that no other biases play an important role. All three of these are subjective judgments. Thus, the claim of “cancelling out,” while theoretically possible, would require strong consensus within a review team.
Regarding the magnitude of bias, an ideal scenario is when one can use existing research to quantify the risk of bias of each effect size estimate, and then adjust the estimates accordingly (“bias adjustment”). Rarely will a review team have the necessary evidence and resources to support this endeavor. Despite the likely lack of empirical evidence for quantitatively adjusting estimates to account for bias, we believe that considerations of magnitude of bias matter. We also note, however, that current review processes entail several implicit judgments about the magnitude of bias. For example, when reviewers decide which risk-of-bias items to use, they are attempting to capture the biases that have the largest influence on effect size. Also, some risk-of-bias items use numerical thresholds (e.g., did at least 85% of enrolled patients provide data to the time point of interest?), and studies meeting that threshold are considered to have no bias for that item. Our recommendation, then, is to consider the implications of the risk of bias carefully rather than in a formulaic fashion. Such an effort will help focus reviewers on consequential sources of bias. It will also help understand how different sources of bias might be related.
- Assessing the Credibility of Subgroup Analyses
Systematic reviewers routinely consider benefits and harms in specified subpopulations or other subgroups (e.g., by specific route of administration of a drug). Subgroup analyses can help to improve understanding of factors that contribute to heterogeneity of study results. A misleading subgroup analysis may not fit the classic description of bias or confounding, but can have the same effect by providing evidence users with incorrect conclusions. Therefore, when systematic reviewers report or synthesize subgroup analyses, they should inform readers of their assessment of the credibility (trustworthiness) of inferences derived from such analyses.
Studies rated as having a high risk of bias for the main analysis of benefits or harms will also likely have a high risk of bias for subgroup analysis. However, studies with low risk of bias for their overall analysis of benefits or harms may not necessarily have credible subgroup analysis. In fact, empiric evaluation shows that the credibility of subgroup effects, even when overall claims are strong, is usually low. 67
Assessing the credibility of subgroup analyses in primary studies requires paying attention to issues such as whether: (1) chance can explain the apparent subgroup effect (i.e., an interaction test can be conducted to demonstrate whether the difference in effect size between subgroups is less likely to be caused by chance); (2) the subgroup effect is consistently observed in several studies; (3) the subgroup hypothesis is one of a small number of hypotheses developed a priori with a specified direction; (4) there is strong preexisting biological rationale for the effect; and (5) the evidence supporting the subgroup effect is observed within studies (as opposed to only being observed in comparisons across studies; which is less credible). 68 There is no specific tool or checklist that has been validated for assessing the credibility of subgroup analysis although criteria have been proposed for preventive clinical services 69 and for randomized controlled trials. 70
In addition to challenges that relate to spurious subgroup effects that are demonstrated to be statistically significant (but may not be credible), there are other challenges that relate to the fact that subgroup analyses are usually underpowered. 69 Therefore, a statistically nonsignificant subgroup interaction cannot rule out a true interaction.
- Assessing the Risk of Bias for Harms
Although harms are almost always included as an outcome in intervention studies that requires a risk-of-bias assessment, the manner of capturing and reporting harms is significantly different from the outcomes of benefit. Harms are defined as the “totality of possible adverse consequences of any intervention, therapy or medical test; they are the direct opposite of benefits, against which they must be compared.” 71 For a detailed explanation of terms associated with harms please refer to the AHRQ Methods Guide on harms. 72 Decisionmakers need to consider the balance between the harms and benefits of the treatment. Empirical evidence across diverse medical fields indicates that reporting of safety information receives much less attention than the positive efficacy outcomes. 73 , 74 Bias for harms from observational studies continue to be a major concern. Design and analytic choices can substantially alter results. 75
Because the type, timing, and severity of some harms are not anticipated—especially for rare events—many studies do not specify exact protocols to actively capture events. Standardized instruments used to systematically collect information on harms are often not included in the study methods. Study investigators may assume that patients will know when an adverse event has occurred, accurately recall the details of the event, and then “spontaneously” report this at the next outcome assessment. Thus, harms are often measured using passive methods that are poorly detailed, resulting in potential for selective outcome reporting, misclassification, and failure to capture significant events. Although some types of harms can be anticipated (e.g., pharmacokinetics of a drug intervention may identify body systems likely to be affected) that include both common (e.g., headache) and rare conditions (e.g., stroke), harms may also occur in body systems that are not necessarily linked to the intervention from a biologic or epidemiologic perspective. In such instances, an important issue is establishing an association between the event and the intervention. The primary study may have established a separate committee to evaluate association between the harm and the putative treatment; blinding is not possible in such evaluations. Similarly, evaluating the potential for selective outcome reporting bias is complex when considering harms. Some events may be unpredictable or they occur so infrequently relative to other milder effects that they are not typically reported. Given the possible or even probable unevenness in evaluating harms and benefits in most intervention studies, reviewers may elect to evaluate the risk of bias for benefits and harms in different ways. We recommend that EPCs be explicit about whether they plan to apply the same methods for risk of bias to both benefits and harms and justify the choice of methods.
- Assessing the Credibility of Existing Systematic Reviews
This guide focuses on assessing risk of bias of primary studies; however, it is becoming more common to use existing systematic reviews in evidence synthesis products. There are two main approaches to using systematic reviews. First, if there are systematic reviews on the interventions (or topics) of interest, reviewers may choose to conduct an overview of reviews. Overviews are defined by Cochrane as knowledge synthesis products that bring together “multiple systematic reviews addressing a set of related interventions, conditions, population, or outcomes.” 76 In overviews, “the unit of searching, inclusion and data analysis is the systematic review.” 76 Second, systematic reviews may be integrated into de novo reviews, i.e., parts of the systematic review(s) may be used as a basis for information in a new systematic review. 77 , 78 For example, the list of included studies may be used as a starting point for a new systematic review, with additional searching that builds upon the search in the existing review. Other parts of an existing systematic review may also be used, such as risk-of-bias assessments, data extraction, and/or data analyses conducted by those who produced the original systematic review. More details on integrating systematic reviews can be found in another EPC Methods Guide. 77 , 78
When conducting an overview of reviews, it is important to assess the credibility of the included systematic reviews, as well as evaluate the procedures for and document the results of risk-of-bias assessments of the included studies. Likewise, when considering whether or not to integrate systematic review results into de novo reviews, it may also be important to assess their credibility to guide decisions about whether to use elements of the review (i.e., what confidence do we have in the methodological rigor with which the review was conducted) and to report on the risk of bias if elements are used and reported in a de novo review (i.e., informing the reader about the methodological rigor of the information that has been incorporated).
Several tools have been developed to determine how trustworthy systematic reviews are; these tools have used variable terms including “risk of bias” and “methodological quality.” The term “credibility” was suggested to replace “risk of bias” when dealing with determining how trustworthy the review process was. 79 , 80 The rationale for this differentiation is that a very well conducted systematic review of poorly conducted trials can produce biased estimates but the review itself may have been well done. Conversely, a review with a poor search strategy may lead to estimates that do not represent the totality of evidence, yet, the estimates are not necessarily biased towards one particular direction (overestimation or underestimation of the treatment effect). Therefore, the credibility of the process of a systematic review can be defined as the extent to which its design and conduct are likely to have protected against misleading results. 79 Credibility may be undermined by inappropriate eligibility criteria, inadequate literature search, or failure to optimally synthesize results. On the other hand, the term “risk of bias” remains as a descriptor of possible bias in individual studies or a body of studies.
Several tools are available to assess the credibility of systematic reviews; although some without much uptake. 81 – 84 The more commonly used tool, developed in 2007, is the Assessing the Methodological Quality of Systematic Reviews Evaluations (AMSTAR) tool. The developers of the original AMSTAR tool are currently working on modifying the tool. 85 A second more recent tool is ROBIS, Risk of Bias in Systematic Reviews, which was released in 2015. 86 ROBIS focuses on risk of bias as opposed to the rigor of the process of a systematic review, which is the focus of AMSTAR. 87
In addition to the above tools, there are at least two reporting guidelines for systematic reviews: Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) and Meta-analysis of Observational Studies in Epidemiology (MOOSE). 57 , 88 Both are available at www.equator-network.org , along with variations/extensions and guidelines for other types of reviews (e.g., meta-narrative reviews and realist syntheses). These may provide a proxy for methodological quality/risk of bias/credibility and an indication of the extent or comprehensiveness of reporting. a
- Reporting the Risk of Bias
During the protocol phase, reviewers should decide on the best approach for reporting the results of the risk-of-bias assessments. The approach used to summarize risk-of-bias assessments should balance considerations of simplicity of presentation and burden on the reader. Risk-of-bias results of individual studies can be reported using a composite or a components approach. In a composite approach, systematic reviewers combine the results of category-specific risk-of-bias assessments to produce a single overall assessment. This assessment often results in a judgement of low, moderate, high, or unclear risk-of-bias. Because a study’s risk-of-bias category or “rating” can be different for different outcomes, review teams may opt to record the overall assessments by outcome. Alternatively, if the risk-of-bias assessments were generally uniform across outcomes, an overall study-level risk-of-bias rating could be generated for the study as a whole that can be applied to all outcomes.
Although creating a summary risk-of-bias judgment for each study or outcome may be a necessary step for strength of evidence judgment, such a summary runs the risk of ignoring or overweighting important sources of bias. In a components approach, reviewers report the risk-of-bias assessment for each study for each bias category or even each item. Previous research has demonstrated that empirical evidence of bias differed across individual categories rather than overall risk of bias. 89 Reviewers may use meta-analyses to examine the association between risk-of-bias categories or items and treatment effect with subgroup analyses or meta-regression. 90 – 92
An approach that relies solely on presentation of judgment on the components (categories or items) alone, however, devolves the burden of effort of interpretation of a study’s risk of bias from the systematic reviewer to the readers. Therefore, we suggest that reviewers carefully consider composite (outcome- or study-specific) summary risk-of-bias judgements as well as component (category)-specific assessments. When presenting the results, reviewers should focus on the elements of risk of bias of greatest relevance to understanding and interpreting the evidence.
Transparency is important so that users can understand how final assessments were assigned. Transparency also helps to ensure that risk-of-bias results can be reproduced and assures that the same process was used for all included studies. In applying the same rules across all outcomes to ensure consistency, there is a danger, however, in being too formulaic and insensitive to the specific clinical context of the outcome. For example, if an outcome is unaffected by blinding, then the unconsidered use of a blinding “rule” (e.g., studies must be blinded to be categorized as low risk of bias) would be inappropriate for that outcome. Thus, we recommend careful consideration of the clinical context as reviewers strive for good transparency. The presentation of risk-of-bias assessments should be done in a way that allows readers not only to determine whether each type of bias is present, absent, or unknown for each study, but also the most likely direction and magnitude of bias when bias is likely to be present (when possible).
Again, we recommend that, in aiming for transparency and reproducibility, EPC reviewers use a set of specific rules for assigning risk-of-bias “ratings”. These rules should take the form of declarative statements that indicate any judgments or weighting that was applied to specific risk-of-bias items or domains. Though the use of quantitative scales is a way to employ a transparent set of results, any weighting system, whether qualitative or quantitative, must be recognized as subjective and arbitrary, and different reviewers may choose to use different weighting methods. Consequently, we believe that reviewers should avoid attributing unwarranted precision (such as a score of 3.42) to ratings or creating subcategories or ambiguous language such as “in the middle of the fair range.”
Assessment of risk of bias is a key step in conducting systematic reviews that informs many other steps and decisions made within the review. It also plays an important role in the final assessment of the strength of the evidence. The centrality of assessment of risk of bias to the entire systematic review task requires that assessment processes be based on theoretical principles at minimum, and sound empirical evidence when possible. In assessing the risk of bias of studies, EPCs should prioritize transparency of judgment through careful documentation of processes and decisions.
- Critical Appraisal Skills Program (CASP) systematic review checklist ( http://www .casp-uk.net/checklists )
- Health Evidence Quality Assessment Tool (HE-QAT) ( http://www .healthevidence .org/documents/our-appraisal-tools /QA _tool&dictionary_18 .Mar.2013.pdf )
- JBI (Joanna Briggs Institute) critical appraisal instrument for Systematic reviews and Research Syntheses ( http://joannabriggs .org /assets/docs/jbc/operations /criticalAppraisalForms /JBC_Form_CritAp_SRsRs.pdf )
- National Institute for Health and Care Excellence (NICE) systematic reviews and meta-analyses methodology checklist ( https://www .nice.org .uk/process/pmg10/chapter /appendix-b-methodology-checklist-systematic-reviews-and-meta-analyses )
- Scottish Intercollegiate Guidelines Network (SIGN) Systematic Reviews and Meta-Analysis Checklist ( http://www .sign.ac.uk /checklists-and-notes.html ).
This report is based on research conducted by the Agency for Healthcare Research and Quality (AHRQ) Evidence-based Practice Centers’ Methods Workgroup. The findings and conclusions in this document are those of the authors, who are responsible for its contents; the findings and conclusions do not necessarily represent the views of AHRQ. Therefore, no statement in this report should be construed as an official position of AHRQ or of the U.S. Department of Health and Human Services.
None of the investigators have any affiliations or financial involvement that conflicts with the material presented in this report.
This research was funded through contracts from the Agency for Healthcare Research and Quality to the following Evidence-based Practice Centers: RTI (290-2015-00011-I), University of Alberta (290-2015-00001-I), ECRI-Penn (290-2015-00005-I), The Johns Hopkins University (290-2015-00006-I), Brown University (290-2015-00002-I), Mayo Clinic (290-2015-00013-I), Minnesota University (290-2015-00008-I), and Kaiser Permanente Center for Health Research (290-2015-00007-I).
The information in this report is intended to help health care decisionmakers—patients and clinicians, health system leaders, and policy makers, among others—make well-informed decisions and thereby improve the quality of health care services. This report is not intended to be a substitute for the application of clinical judgment. Anyone who makes decisions concerning the provision of clinical care should consider this report in the same way as any medical reference and in conjunction with all other pertinent information (i.e., in the context of available resources and circumstances presented by individual patients).
This report is made available to the public under the terms of a licensing agreement between the author and the Agency for Healthcare Research and Quality. This report may be used and reprinted without permission except those copyrighted materials that are clearly noted in the report. Further reproduction of those copyrighted materials is prohibited without the express permission of copyright holders.
AHRQ or U.S. Department of Health and Human Services endorsement of any derivative products that may be developed from this report, such as clinical practice guidelines, other quality enhancement tools, or reimbursement or coverage policies may not be stated or implied. Persons using assistive technology may not be able to fully access information in this report. For assistance, contact vog.shh.qrha@cpe .
Suggested citation: Viswanathan M, Patnode C, Berkman ND, Bass EB, Chang S, Hartling L, Murad HM, Treadwell JR, Kane RL. Assessing the Risk of Bias in Systematic Reviews of Health Care Interventions. Methods Guide for Comparative Effectiveness Reviews. (Prepared by the Scientific Resource Center under Contract No. 290-2012-0004-C). AHRQ Publication No. 17(18)-EHC036-EF. Rockville, MD: Agency for Healthcare Research and Quality; December 2017. Posted final reports are located on the Effective Health Care Program search page . DOI: https://doi .org/10.23970 /AHRQEPCMETHGUIDE2 .
Prepared by: Scientific Resource Center, Portland, OR
- Cite this Page Viswanathan M, Patnode CD, Berkman ND, et al. Assessing the Risk of Bias in Systematic Reviews of Health Care Interventions. 2017 Dec 13. In: Methods Guide for Effectiveness and Comparative Effectiveness Reviews [Internet]. Rockville (MD): Agency for Healthcare Research and Quality (US); 2008-.
- PDF version of this page (423K)
In this Page
Other titles in these collections.
- AHRQ Methods for Effective Health Care
- Health Services/Technology Assessment Text (HSTAT)
Related information
- PMC PubMed Central citations
- PubMed Links to PubMed
Similar articles in PubMed
- Recommendations for assessing the risk of bias in systematic reviews of health-care interventions. [J Clin Epidemiol. 2018] Recommendations for assessing the risk of bias in systematic reviews of health-care interventions. Viswanathan M, Patnode CD, Berkman ND, Bass EB, Chang S, Hartling L, Murad MH, Treadwell JR, Kane RL. J Clin Epidemiol. 2018 May; 97:26-34. Epub 2017 Dec 14.
- Review Assessing the Risk of Bias of Individual Studies in Systematic Reviews of Health Care Interventions. [Methods Guide for Effectivenes...] Review Assessing the Risk of Bias of Individual Studies in Systematic Reviews of Health Care Interventions. Viswanathan M, Ansari MT, Berkman ND, Chang S, Hartling L, McPheeters M, Santaguida PL, Shamliyan T, Singh K, Tsertsvadze A, et al. Methods Guide for Effectiveness and Comparative Effectiveness Reviews. 2008
- The future of Cochrane Neonatal. [Early Hum Dev. 2020] The future of Cochrane Neonatal. Soll RF, Ovelman C, McGuire W. Early Hum Dev. 2020 Nov; 150:105191. Epub 2020 Sep 12.
- Folic acid supplementation and malaria susceptibility and severity among people taking antifolate antimalarial drugs in endemic areas. [Cochrane Database Syst Rev. 2022] Folic acid supplementation and malaria susceptibility and severity among people taking antifolate antimalarial drugs in endemic areas. Crider K, Williams J, Qi YP, Gutman J, Yeung L, Mai C, Finkelstain J, Mehta S, Pons-Duran C, Menéndez C, et al. Cochrane Database Syst Rev. 2022 Feb 1; 2(2022). Epub 2022 Feb 1.
- Review Evaluating non-randomised intervention studies. [Health Technol Assess. 2003] Review Evaluating non-randomised intervention studies. Deeks JJ, Dinnes J, D'Amico R, Sowden AJ, Sakarovitch C, Song F, Petticrew M, Altman DG, International Stroke Trial Collaborative Group, European Carotid Surgery Trial Collaborative Group. Health Technol Assess. 2003; 7(27):iii-x, 1-173.
Recent Activity
- Assessing the Risk of Bias in Systematic Reviews of Health Care Interventions - ... Assessing the Risk of Bias in Systematic Reviews of Health Care Interventions - Methods Guide for Effectiveness and Comparative Effectiveness Reviews
Your browsing activity is empty.
Activity recording is turned off.
Turn recording back on
Connect with NLM
National Library of Medicine 8600 Rockville Pike Bethesda, MD 20894
Web Policies FOIA HHS Vulnerability Disclosure
Help Accessibility Careers
- Go back to Main Menu
- Client Log In
- MSCI Client Support Site
- Barra PortfolioManager
- MSCI ESG Manager
- MSCI ESG Direct
- Global Index Lens
- MSCI Real Assets Analytics Portal
- RiskManager 3
- CreditManager
- RiskManager 4
- Index Monitor
- MSCI Datscha
- MSCI Real Capital Analytics
- Total Plan/Caissa
- MSCI Fabric
- MSCI Carbon Markets

Navigation Menu
- Our Clients
Insights on MSCI One
Institutional client designed indexes (icdis), total portfolio footprinting, esg trends to watch, factor models, visualizing investment data.
- Our Solutions
- Go back to Our Solutions
- Analytics Overview
- Crowding Solutions
- Fixed Income Analytics
- Managed Solutions
- Multi-asset Class Factor Models
- Portfolio Management
- Quantitative Investment Solutions
- Regulatory Solutions
- Risk Insights
- Climate Investing
- Climate Investing Overview
Implied Temperature Rise
Trends 2024.
- Biodiversity
- Carbon Markets
- Climate Lab Enterprise
- Real Estate Climate Solutions
- Sustainable Investing
- Sustainable Investing Overview
ESG and Climate Funds in Focus
What is esg, role of capital in the net-zero revolution.
- Sustainability Reporting Services
- Factor Investing
- Factor Investing Overview
MSCI Japan Equity Factor Model
- Equity Factor Models
- Factor Indexes
- Indexes Overview
Index Education
Msci climate action corporate bond indexes.
- Client-Designed
- Direct Indexing
- Fixed Income
- Private Real Assets
Thematic Exposure Standard
- Go back to Indexes
- Resources Overview
MSCI Indexes Underlying Exchange Traded Products
- Communications
- Equity Factsheets
- Derivatives
- Methodology
- Performance
- Private Capital
- Private Capital Overview
Global Private Capital Performance Review
- Total Plan (formerly Caissa)
- Carbon Footprinting
- Private Capital Indexes
- Private Company Data Connect
- Real Assets
- Real Assets Overview
2024 Trends to Watch in Real Assets
- Index Intel
- Portfolio Services
- Property Intel
- Private Real Assets Indexes
- Real Capital Analytics
- Research & Insights
- Go back to Research & Insights
- Research & Insights Overview
- Multi-Asset Class
- Real Estate
- Sustainability
- Events Overview
Capital for Climate Action Conference
- Data Explorer
- Developer Community
- Technology and Data
2022 Annual Report
- Go back to Who We Are
- Corporate Responsibility
- Corporate Responsibility Overview
- Enabling Sustainable Investing
- Environmental Sustainability
- Governance Practices
- Social Practices
- Sustainability Reports and Policies
- Diversity, Equity and Inclusion
Henry A. Fernandez
- Recognition
Main Search
Esg rating hero banner, esg ratings.
Measuring a company’s resilience to long-term, financially relevant ESG risks
Social Sharing
Esg rating intro para, what is an msci esg rating.
MSCI ESG Ratings aim to measure a company’s management of financially relevant ESG risks and opportunities. We use a rules-based methodology to identify industry leaders and laggards according to their exposure to ESG risks and how well they manage those risks relative to peers. Our ESG Ratings range from leader (AAA, AA), average (A, BBB, BB) to laggard (B, CCC). We also rate equity and fixed income securities, loans, mutual funds, ETFs and countries.
ESG Ratings video
How do MSCI ESG Ratings work? What are significant ESG risks? What does a poor rating look like? How can you use them?
Download Transcript (PDF, 120 KB) (opens in a new tab)
ESG ratings
Download brochure (PDF, 1.08 MB) (opens in a new tab)
How do MSCI ESG Ratings work?
How does msci esg ratings work.
ESG risks and opportunities can vary by industry and company. Our MSCI ESG Ratings model identifies the ESG risks, (what we call Key Issues), that are most material to a GICS® sub-industry or sector. With over 13 years of live track history we have been able to examine and refine our model to identify the E, S, and G Key Issues which are most material to an industry.
View our Key Issues framework | ESG Methodologies (opens in a new tab) | What MSCI’s ESG Ratings are and are not
ESG Ratings module
A company lagging its industry based on its high exposure and failure to manage significant ESG risks
A company with a mixed or unexceptional track record of managing the most significant ESG risks and opportunities relative to industry peers
A company leading its industry in managing the most significant ESG risks and opportunities
Explore our ESG transparency tools
Contact sales
Explore our ESG Transparency Tools content - part 1
Explore the Implied Temperature Rise, Decarbonization Targets, MSCI ESG Rating and Key ESG Issues of over 2,900 companies.
Explore E, S & G Key Issues by GICS® sub-industry or sector and their contribution to companies' ESG Ratings.
Example: Explore the data metrics and sources used to determine the MSCI ESG Rating of a US-based producer of paper products.
Explore our ESG Transparency Tools content - part 2
ESG Fund Ratings aim to measure the resilience of mutual funds and ETFs to long term risks and opportunities.
Explore ESG and climate metrics for all MSCI equity, fixed income and blended indexes regulated by the EU.
ESG ratings Tabs
Integrating esg ratings into the investment process: key features.
A growing body of client, industry and MSCI research has shown the value of integrating MSCI ESG Ratings to manage and mitigate risks and identify opportunities. We are proud to work with over 1,700 clients worldwide that help inform and improve our ESG Research, including our ESG Ratings methodology and coverage. Investor clients use MSCI ESG Ratings as follows.
Fundamental / quant analyses
Portfolio construction / risk management, benchmarking / index-based product development, disclosure and reporting for regulators and stakeholders, engagement & thought leadership.
- Stock analysis
- ESG Ratings used for security selection or within systematic strategies
- ESG Factor in quant model- identify long term trends and arbitrage opportunities
- Adjust discounted cashflow models
- Identify leaders and laggards to support construction
- Use ratings and underlying scores to inform asset allocation
- Stress testing, and risk and performance attribution analysis
- ESG as a Factor in Global Equity Models
- MSCI ESG Ratings are used in many of MSCI’s 1,500 equity and fixed indexes
- Select policy or performance benchmark
- Develop Exchange-Traded-Funds and other index-based products
- Make regulatory disclosures
- Report to clients & stakeholders
- Demonstrate ESG transparency and leadership
- Engage companies and external stakeholders
- Provide transparency through client reporting
- Conduct thematic or industry research
ESG rating Key benefits
Key product features:.
We rate over 8,500 companies (14,000 issuers including subsidiaries) and more than 680,000 equity and fixed income securities globally (as of October 2020), collecting thousands of data points for each company.
MSCI ESG Research Experience and Leadership
Msci esg research experience and leadership.
- We have over 40 years 2 of experience measuring and modelling ESG performance of companies. We are recognized as a ‘Gold Standard data provider’3 and voted 'Best Firm for SRI research' and ‘Best Firm for Corporate Governance research' for the last four years 3
- We were the first ESG provider to assess companies based on industry materiality, dating back to 1999. Only dataset with live history (13+ years) demonstrating economic relevance
- Objective rules based ESG ratings, with an average 45% of data, 5 coming from alternative data sources, utilizing AI tech to extract and verify unstructured data
- First ESG ratings provider to measure and embed companies’ ESG risk exposure 4
ESG Ratings Related Content
Related content, .rel-cont-head{ font-size: 31px important; line-height: 38px important; } sustainable investing.
Companies with strong MSCI ESG Ratings profiles may be better positioned for future challenges and experience fewer instances of bribery, corruption and fraud. Learn how our sustainability solutions can provide insights into risks and opportunities.
Climate and Net-Zero Solutions
To empower investors to analyze and report on their portfolios’ exposures to transition and physical climate risk. 1 .
Sustainable Finance
ESG and climate regulation and disclosure resource center for institutional investors, managers and advisors.
ESG ratings footnotes
MSCI ESG Research LLC. is a Registered Investment Adviser under the Investment Adviser Act of 1940. The most recent SEC Form ADV filing, including Form ADV Part 2A, is available on the U.S. SEC’s website at www.adviserinfo.sec.gov (opens in a new tab) .
MIFID2/MIFIR notice: MSCI ESG Research LLC does not distribute or act as an intermediary for financial instruments or structured deposits, nor does it deal on its own account, provide execution services for others or manage client accounts. No MSCI ESG Research product or service supports, promotes or is intended to support or promote any such activity. MSCI ESG Research is an independent provider of ESG data, reports and ratings based on published methodologies and available to clients on a subscription basis.
ESG ADV 2A (PDF, 354 KB) (opens in a new tab) ESG ADV 2B (brochure supplement) (PDF, 232 KB) (opens in a new tab)
1 GICS®, the global industry classification standard jointly developed by MSCI Inc. and S&P Global.
2 Through our legacy companies KLD, Innovest, IRRC, and GMI Ratings.
3 Deep Data Delivery Standard http://www.deepdata.ai/
4 Through our legacy companies KLD, Innovest, IRRC, and GMI Ratings. Origins of MSCI ESG Ratings established in 1999. Produced time series data since 2007.
5 Source: MSCI ESG Research 2,434 constituents of the MSCI ACWI Index as of November 30, 2017.
UtmAnalytics

IMAGES
VIDEO
COMMENTS
The above list covers issues ranging from important features of risk assessment to overall aspects concerning risk management and governance. It can obviously be extended. One example to add is the link between sustainability and risk, which is an emerging research topic; see e.g. Fahimnia et al. (2015) and Giannakis and Papadopoulos (2016). 7.
LEARNING OBJECTIVES. After reading this article you will be able to: • explain the concept of risk as it applies to clinical practice • recognise that risk assessment is much more than gathering and interpreting information to make judgements about future harm • understand the wider psychological, interactional and system influences on risk assessment in a way that informs better practice.
research. Minimizing Risk . Risks, even when unavoidable, can be reduced or managed. Precautions, safeguards, and alternatives can be incorporated into the research activity to reduce the probability of harm or limit its severity or duration. An important aspect of risk assessment is the nature and type of planned protections to minimize the
This session will. Discuss the importance of research risks for compliance officers. Delve into how research risks affect approval, IRB review, consent, and indemnifications. Describe how risk can be minimized using preliminary risk assessments. 3.
1. Introduction. Recent research has recognized a continuous spread of fundamental issues in health risk assessment (HRA), as well as a poor, or at least unclear, link between HRA results and (risk management) decision-making [1,2,3,4,5].Some studies expressed concern about inconsistent practices that are drifting away from the definition and generally approved process of HRA [6,7,8,9], while ...
Introduction. Risk is an essential part of everyday life and risks are unavoidable in any complex program. 1 A common definition of risk is "the chance of something happening that will have an impact on the achievement of the stated organizational objectives". 2 Risk management is defined in the literature as "all the activities connected with hazard identification, assessment, selection ...
Background. Research ethics committees (RECs) are tasked to do a risk-benefit assessment of proposed research with human subjects for at least two reasons: to verify the scientific/social validity of the research since an unscientific research is also an unethical research; and to ensure that the risks that the participants are exposed to are necessary, justified, and minimized [].
Introduction. The concept of risk and risk assessments has a long history. More than 2400 years ago the Athenians offered their capacity of. assessing risk before making decisions ( Bernstein ...
Benefit A valued or desired outcome; an advantage. Risk The probability of harm or injury (physical, psychological, social, or economic) occurring as a result of participation in a research study. Both the probability and magnitude of possible harm may vary from minimal to significant. Federal regulations define only "minimal risk."
Deployment of healthcare risk management has traditionally focused on the important role of patient safety and the reduction of medical errors that jeopardize an organization's ability to achieve its mission and protect against financial liability. But with the expanding role of healthcare technologies, increased cybersecurity concerns, the fast pace of medical science, and the industry's ...
Understanding Risk in Research. Assessing risk in a research study is one of the primary responsibilities of an IRB and one of its most controversial tasks. By nature, studying human beings is a complicated process because the subject matter itself is complicated. The level of risk can vary because of many factors including: the population ...
A risk assessment determines the likelihood, consequences and tolerances of possible incidents. "Risk assessment is an inherent part of a broader risk management strategy to introduce control measures to eliminate or reduce any potential risk- related consequences." 1 The main purpose of risk assessment is to avoid negative consequences related to risk or to evaluate possible opportunities.
Risk assessment process. The risk assessment process is a careful examination of what could cause harm, who/what could be harmed and how. It will help you to determine what risk control measures are needed and whether you are doing enough. To simplify the process you can use the health and safety risk assessment templates, risk estimation tool ...
Risk management is a process of evaluating risks and developing strategies for managing them. Citation 5 The risk management process comprises five steps, which are preparation, risk identification, risk assessment, risk control, and record-keeping and review. Citation 6 Identification of risks is the most critical step in the whole process.
Abstract. Risk-based decisions are an integral part of societal efforts to protect the public from the harmful health effects of environmental pollution. Scientific information about the magnitude and extent of risks experienced by people and about the causes of those risks is a critical factor in setting priorities and choosing cost-effective ...
The role of stakeholders in risk assessment. Risk assessment has a long tradition in many areas, such as insurance, finance, medicine, engineering, psychology, and anthropology (Renn, 2008). Approaches vary widely in these fields, depending on the data available. Risk assessment in insurance and medicine only works where sufficient quantitative ...
This paper, therefore, reports the design process for—and content of—a risk assessment framework (RAF). The RAF aims to guide healthcare staff on risk assessment as well as to address current challenges by learning from prescribed good risk assessment practice and the experience of healthcare staff (e.g. doctors, nurses and managers).
Conducting a Risk Assessment . A risk assessment can be a valuable tool to help your unit identify, evaluate and prioritize its risks in order to improve decision-making and resource allocation. Harvard's Institutional Risk Management (IRM) program recommends the following process for c onducting risk assessments. We are here to consult with
Risk assessment is a straightforward and structured method of ensuring the risks to the health, safety and wellbeing of employees (and others) are suitably eliminated, reduced or controlled. The main purpose of risk assessments are: To identify health and safety hazards and evaluate the risks presented within the workplace.
4 Reasons Why Risk Management Is Important. 1. Protects Organization's Reputation. In many cases, effective risk management proactively protects your organization from incidents that can affect its reputation. "Franchise risk is a concern for all businesses," Simons says in Strategy Execution. "However, it's especially pressing for ...
This study developed an integrated risk assessment approach of MALs for heavy metals in agricultural soil. This approach is a four-step process from data collection, scenario assumptions, risk assessment, and threshold derivation. Fig. 1 provides a schematic of this approach and the details of the process were described below.
Climate Scenario Analysis is a tool for assessing exposure to climate-related risks under different future climate conditions. Preliminary analysis highlights the importance of resolving data and methodology gaps to enhance confidence in the results. It also reveals the sensitivity of results to modeling assumptions. Background The safety and soundness of the U.S. housing finance system could ...
The study underscores the critical necessity for nuanced TBI risk assessment tailored to age and gender, emphasizing the importance of age-specific protective strategies in managing TBIs across diverse demographics. Future research employing individual modeling techniques and exploring a wider age spectrum holds promise in refining our ...
Dr. Charles Haas, Drexel University, a member of the symposium planning committee, summarized the standard risk assessment process. The major steps in risk assessment were first articulated in a National Research Council report titled Risk Assessment in the Federal Government: Managing the Process (NRC, 1983), otherwise known as the "Red Book." This report has been updated several times ...
To commence this systematic review and meta-analysis, we adhered to the Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA) guidelines and used the PRISMA assessment checklist [Supplementary Table 1].The study included pertinent research conducted within the population of Southeast Asian countries, as outlined by the United Nations [], and perform a meta-analysis on the ...
The power grid is an important industry that is crucial to national security and economic development, and its importance in society continues to grow. As an emerging concept, financial sharing enables internal resource sharing and optimization, thereby improving the efficiency and effectiveness of asset management. This study investigates and analyzes the current situation of asset management ...
Fats and Cholesterol. When it comes to dietary fat, what matters most is the type of fat you eat. Contrary to past dietary advice promoting low-fat diets, newer research shows that healthy fats are necessary and beneficial for health. When food manufacturers reduce fat, they often replace it with carbohydrates from sugar, refined grains, or ...
Risk-of-bias assessment is a central component of systematic reviews but little conclusive empirical evidence exists on the validity of such assessments. In the context of such uncertainty, we present pragmatic recommendations that can be applied consistently across review topics, promote transparency and reproducibility in processes, and address methodological advances in the risk-of-bias ...
Objective rules based ESG ratings, with an average 45% of data, 5 coming from alternative data sources, utilizing AI tech to extract and verify unstructured data. First ESG ratings provider to measure and embed companies' ESG risk exposure 4. MSCI ESG Research LLC. is a Registered Investment Adviser under the Investment Adviser Act of 1940.
It is increasingly important to effectively predict the failure of HVDC transmission lines caused by wildfire disasters. On the basis of comprehensively considering the distribution of fire points, the characteristics of wildfire propagation, and the failure factors of the transmission line, a method for calculating the probability of failure in HVDC transmission lines during wildfire ...