Disappearing Colors Experiment
Easy Bleach Project for Kids
FatCamera/Getty Images
- Projects & Experiments
- Chemical Laws
- Periodic Table
- Scientific Method
- Biochemistry
- Physical Chemistry
- Medical Chemistry
- Chemistry In Everyday Life
- Famous Chemists
- Activities for Kids
- Abbreviations & Acronyms
- Weather & Climate
- Ph.D., Biomedical Sciences, University of Tennessee at Knoxville
- B.A., Physics and Mathematics, Hastings College
Let kids see for themselves how bleach works with this easy disappearing colors experiment.

Disappearing Colors Project Materials
- food coloring
- household bleach
- glass or jar
- Fill a glass or jar about halfway full with water.
- Add a few drops of food coloring. Stir the liquid to make it colored.
- Add drops of bleach until the color starts to disappear. You can stir the contents of the glass if you like. Continue until the color is gone.
- Add a few drops of another color. What happens? The color doesn't spread out the same way as it did when coloring was added to pure water. It forms swirls, which may disappear if there is enough bleach in the water.
Why It Works
Bleach contains sodium hypochlorite, which is an oxidizer. It oxidizes or reacts with the chromophore or color molecules in food coloring. Although the pigment molecule remains, it's shape changes so that it can't absorb/reflect light the same way, so it loses its color as a result of the chemical reaction .
Safety Information
- Be careful to avoid spilling bleach on skin or clothes. Rinse any spills immediately with lots of water.
- Make sure young experimenters don't drink bleach or the contents of the glass. Diluted bleach is not particularly dangerous, but not good for you either!
- When you are done with the project, it's safe to dump the contents of the glass down the drain and to re-use the washed glass for food.
- 10 Fun Chemistry Demonstrations and Experiments
- How to Make Red Cabbage pH Paper
- Color Change Chemistry Experiments
- Bubble Rainbow Science Project
- How to Grow Crystals From Salt and Vinegar
- Fun Bubble Science Projects
- Make Potassium Chlorate from Bleach and Salt Substitute
- How to Make Disappearing Ink
- How to Make a Baking Soda Volcano
- Best Crystals for Beginners
- Homemade Dippin' Dots Liquid Nitrogen Ice Cream
- Metal Projects That Help You Explore Chemistry
- Fizzy Sparkling Lemonade Made With Science
- Make a Density Column
- How to Make Your Own Snow Globe
- Crystal Projects Photo Gallery
- All Adventures
- Original Adventures
- The New Adventures
- Collections & Bundles
- Study Guides
- Hats and T-Shirts
- Mack Meets History
- The Adventum Audio Adventure
- Creation Museum Adventure
- Novarupta Audio Adventure
- Gifts and Activities
- What are Audio Adventures?
- Learn about Jonathan Park
- Parents Blog
- Continue Shopping
- Your Cart is Empty

3 Fun Experiments to Try with your Kids + Powerful Bible Lessons
April 26, 2018

In this post we will be sharing 3 exciting object lessons that can not only be used for teaching Bible truths, but also as a fun science lesson. Object lessons are a enjoyable way to keep your child engaged while learning about the Bible and to help them commit the lesson to memory. These lessons will require minimal supplies (many you already have at home) and the steps are simple- but these fun “experiments” are sure to keep your child’s attention. Be prepared for them to ask you to do them over and over!
Cotton Balls in A Glass of Water
A bag of cotton balls
Clear drinking glass filled ⅔ of the way with water
Experiment:
Fill a cup or jar almost full of water.

Ask your child to guess how many cotton balls will fit in the almost full glass (most children will say “just a few”). Have them start to put the cotton balls in the glass, one by one, allowing for them to absorb the water. They will soon be surprised to see you can fit many (we were able to fit 31) cotton balls in the jar. Something they thought was impossible, ended up being possible.

Now to Him who is able to do exceedingly abundantly above all we ask or think, according to the power that works in us” (Ephesians 3:20)
We may look at something and think we have enough facts to prove it is impossible, like not being able to fit more of something in an almost full cup. Yet, God is able to to do anything and everything, even the things we think are impossible.
Science: The cotton balls are made of fiber from a cotton plant. If you were to look at this fiber (cellulose) under a microscope you would see that it is full of tiny air bubbles. When the cotton ball is placed in the water the air bubbles fill up with water. This redistributes the water instead of displacing it like most solid things (like a rock) would.
Illustrating Me, Sin, and Jesus
3 Clear plastic cups
Stickers / Tape or some other way to label the jars
Take one cup and label it “Me” (or Us works too). Fill this cup half full of waterTake the second cup and label it “Sin”. Mix a ratio of 90% water and 10% iodine. Take the third cup and label it “Jesus”. In this cup mix a ratio of 90% water and 10% bleach. The rest of the experiment is done during the lesson.

“He is the atoning sacrifice for our sins, and not only for ours but also for the sins of the whole world.” 1 John 2:2
Explain that Jesus’ sacrifice on the cross has freed us from our sins. Pour a little bit of the mixture from the “Sin” cup into the “Me” jar. Sin pulls us away from God and tarnishes us. This is symbolized by the brownish color that the water in our “Me” cup now has.

But, for those who believe in Jesus as our Savior, hope is not lost. When we ask for forgiveness our sins can be washed clean. Pour from the “Jesus” cup into the “Me” cup until the liquid is clear again.

When mixed with water the iodine water mixture turns the water slightly reddish brown. When the bleach/water mixture is added a chemical reaction occurs between the chlorine molecules in the bleach and the iodine molecules, turning the mixture clear.
Cans of Soda Illustrating Anger
2 cans of soda
Take both cans of soda and shake them up as hard as you can. Explain that the shaking is like your anger. Open one of the cans (make sure you are in a space where it is ok to make a mess) and watch the soda explode from the can. Set the other can of soda aside and wait about 5 minutes. When you come back to it and open it up. It should not explode or, at most, should only foam a small amount. Have your child note the differences when the two cans were opened.
Do not say, “I will repay evil”; wait for the Lord, and he will deliver you. Proverbs 20:22
Explain that even though everyone experiences anger, that how we handle our anger is what matters. When we react quickly in anger, before allowing ourselves time to calm down and think about the situation, we are setting ourselves up to sin. When we take time to walk away and calm down, we can make better decisions, and with the help of the Holy Spirit we can keep yourselves from sinning as a result of our anger.
When soda is canned, a gas called carbon dioxide is added to the liquid to create the bubbles. This is called carbonation. There is a small amount of carbon dioxide that takes up the free space at the top of the can. When the can is shaken, that carbon dioxide mixes with the liquid, becoming suspended. When the can is opened and the pressure released, the soda and carbon dioxide escape together creating the explosion. If you leave the can to sit after it has been shaken, the carbon dioxide begins to rise to the open space in the top of the can, leaving the normal amount of carbonation left in the liquid which is not enough to cause an explosion.
Leave a comment
Comments will be approved before showing up.
Also in Journal

Going Deeper with Noah’s Ark
July 19, 2018 1 Comment
View full article →

Why Do “Bad Things” Happen to “Good People”?
July 11, 2018

Say No to Distractions – Yes to Your Kids!
July 05, 2018
Join us and get a faith-building activity to share with your kids every week!
Recent Articles
- Going Deeper with Noah’s Ark July 19, 2018
- Why Do “Bad Things” Happen to “Good People”? July 11, 2018
- Say No to Distractions – Yes to Your Kids! July 05, 2018
- A Simple Approach to Sharing God's Truth June 28, 2018
- Seeking the Approval that Really Counts June 19, 2018
- The Great Author of Science June 05, 2018
- 10 Activities for a Christ-Centered Summer May 24, 2018
- Why Do God’s Children Look Different? May 15, 2018
- Teaching your Children to Pray like Jesus May 08, 2018
- Teaching Your Child what Obedience Really Means May 02, 2018
Customer Support
- Having Trouble Downloading?
- Returns and Exchanges
- Privacy and Terms
- Shipping Info
- Paracord Activity Kit Instructions
Let's Be Friends
Free newsletter.
Unlock Email-subscriber Online Offers, Biblical Evidence, and Fun Activities
© 2024 Jonathan Park . All Rights Reserved
- Become a Member

Clever Sin Object Lessons for Sunday School
For your elementary kids.
Sin is important to be able to explain to kids so they understand. Here are a couple fin or clever ideas for using everyday objects to explain what God's Word says about sin.

Share this with friends:
1. Severed Rope
This object lesson shows how sin separates us from our Heavenly Father.
Key Thought: Sin separates us from God.
Materials needed:.
- long piece of rope
Directions:
- Watch the video above to learn the "magic" trick
- Fold the rope in half, bring the middle of it to the top and use the scissors to cut it it half.
- Bring the ends down so you have two pieces
- Tie the top two pieces together.
- Magically slide the knot right off the rope!
Tip: Before doing this in class, take a minute to practice. It's not difficult, but you won't get it perfect the first time 🙂
Sin is when we disobey God's law - the rules He has made for all human beings.
And that sin is serious because it separates us from God.
And no matter how much good we do in the future, there is still no way to make up for doing wrong, for sinning.
That's why we are so thankful that God sent His son Jesus to the earth to die on the cross and pay the penalty for our sins.
ROMANS 5:12
" Sin came into the world because of what one man did, and with sin came death. This is why everyone must die—because everyone sinned. " (NCV)
Have you asked Jesus to save you so that you are no longer separated from God? Here's some help for leading a child to Christ .
2. The Sin Solution
This object lesson explains why Jesus died, and how that paid for our sin.
(That's not me in this video)..but this guy does do a great job!
Key Thought: Jesus defeated the power of sin.
- 3 clear containers
- Marker or labels for each container
- a towel for some light cleanup 🙂
- Fill the YOU container with water
- Fill the SIN container with water and iodine
- Fill the JESUS container with water and bleach
- Pour SIN into YOU
- Pour SIN into JESUS
- Pour JESUS into YOU
There are a number of different ways you can narrate this object lesson. The video above does a great job of presenting one of them.
A couple other ideas to consider:
- Once YOU is black consider pouring YOU back into JESUS to talk about giving all our sin to Jesus and asking Him to save us.
- The stronger the bleach mixture in the JESUS bottle the better.
- You can find iodine in the medical bandages and stuff at your local Walmart / Target / etc...
- I would use different sized containers like the video. It takes a fair amount of iodine to make a nice black color so a small SIN container works well
GALATIANS 1:4
" Jesus gave himself for our sins to free us from this evil world we live in, as God the Father planned. " (NCV)
Free Complete Lesson on Faith and Trusting in God
Includes songs, games, this object lesson , a fun lesson script , small group materials, take home sheet and more!

Nailed it! Check your email in about 60 seconds for all the free stuff.
3. Disappearing Sin
This object lesson reminds your elementary kids that Jesus can wash away their sin if they ask Him to!
Key Thought: Jesus washes away my sin.
- Paper Towel
- Permanent Marker
- Washable Markers
- With PERMANENT MAKER draw a heart on both sides of the towel
- Draw "sin" all over the heart on the inside of the fold with WASHABLE marker
- Gently lay the paper towel in the water and tap it to wash all the sin (the washable marker) away.
Our hearts are full of sin. (We believe in total depravity at BetterBible Teachers - more about our beliefs here .)
And although we pretend like there is nothing wrong sometimes we know that we don't even live up to OUR OWN standards - much less, God's standard.
And not obeying God, or living up to His standards is called sin.
The good news is that Jesus died for our sins - so that we can be washed clean!
This simply beautiful object lesson pictures this perfectly.
REVELATION 1:5
" and from Jesus Christ. Jesus is the faithful witness, the first among those raised from the dead. He is the ruler of the kings of the earth. He is the One who loves us, who made us free from our sins with the blood of his death. " (NCV)
4. Only One Way to Heaven
In this object lesson we are reminded that because of our sin, there is only ONE way to Heaven.
Key Thought: There is only one way to heaven.
- cardboard circle template (empty bathroom tissue roll works great)
- Show the cardboard with all 5 dots showing
- Flip it over to show 2 days
- Flip it over to show 4 dots (hand covering one)
- Flip it over to show 1 dot (hand covering one)
We like to think that sin "isn't that bad.
Or that...God will accept us for being "good enough."
But we know what sin really means..that it results in forever death - and there is only ONE way of "escape". And that's to accept Jesus as your personal Savior.
This object lesson works best when it is done quickly, so run it through a couple times in the mirror before you show it to your elementary Sunday School class.
" Jesus answered, “I am the way, and the truth, and the life. The only way to the Father is through me." " (NCV)
5. A Sin Free Life
In this easy sin object lesson we see that God forgives ALL of our sin - no matter what we have done.
Key Thought: God wants to forgive all of our sin.
- clear glass of water
- laminated paper printed back on one side, and red on the other
- a black cloth
- Show the glass of water with the black paper showing
- Cover the glass with the cloth and secretly spin it around to show the red side
- Remove the laminated paper and black cloth in one motion to reveal just clear water.
When God forgives us of our sin - it is complete. He covers our past and future sin with what He did on the cross.
Of course, that doesn't. mean we have permission to do whatever we want...no way! Asking Jesus to forgive us of our sin means entering into a relationship with Him where it is our desire to do what He says.
But what a wonderful truth to be reminded of...that when we are forgiven, we are COMPLETELY forgiven for everything we have done.
" But if we confess our sins, he will forgive our sins, because we can trust God to do what is right. He will cleanse us from all the wrongs we have done. " (NCV)
6. Gospel Airplane Object Lesson
In this paper object lesson we see that there is NOTHING we can do to be with God on our own.
Key Thought: Believing in Jesus allows us to go to Heaven.
- I'm not going to try to describe all the folds...just follow the directions in the video 🙂
Part origami, and part object lesson. This is a different object lesson that you don't need anything special for. Aside from understanding how to fold the paper this one is simple!
This object lesson is also a great, post-lesson craft. So you may want to demonstrate this during your lesson and then give everyone a piece of paper later so they can learn the process too!
Wouldn't it be cool if you heard from parents that kids were showing their parents and friends this sin object lesson??!!
" Lord, you are kind and forgiving and have great love for those who call to you. " (NCV)
7. Jesus Takes Aways Our Sin
This object lesson demonstrates the power of Jesus to take away our sin.
Key Thought: Jesus has power over our sin!
- Clear vase or jar
- Red food coloring
- penny (optional)
- Light the candle and put it on a plate of water
- Add the penny (if desired)...the plate can also represent us if you'd rather
- Cover the candle with the clear jar and watch the water get sucked up!
Tip: Practice this one to figure out the ideal amount of water, and the best size candle. You may have a little water left on the plate...you just don't want a lot of water left.
So what else can I say about sin, that hasn't already been covered on this page...lol
This a fun object lesson because it involves fire...which always makes things fun.
This one you need to see up close. You may want to gather your kids around the plate as you do this particular object lesson so they can get a great view.
And...hopefully ALL the boys don't stick their finger in the water 🙂
" The next day John saw Jesus coming toward him. John said, “Look, the Lamb of God, who takes away the sin of the world! " " (NCV)
8. Free from the Power of Sin
A slightly more complicated sin object lesson you'll love!
Key Thought: Jesus breaks the power of sin!
- a rubber band
- a little practice 🙂
- Watch the video demonstration...
Maybe you've read through the other object lessons here and thought...
" Nathan , I want you to challenge me a little!"
Well, then...no problem!
This one is going to require a little practice - and that's OK!!
Once you are comfortable with the "magic move" described in the video you'll have a wonderful demonstration of our life before Jesus - we are "dead in our trespasses and sins"
But Jesus has made us, ALIVE! We are free from the power of sin because of the blood of Jesus. Use this fun magic trick object lesson to demonstrate just that.
" Through Christ Jesus the law of the Spirit that brings life made you free from the law that brings sin and death. "
9. No More Sin
In this simple object lesson we see that Jesus removes and forgive us of all our sins.
Key Thought: Jesus removes our sin from us!
- Paper (flash paper is recommended)
- Put the paper into the small pot
- Light the paper on fire
- Show the empty pot!
This trick is as easy as "light flash paper on fire"
But sometimes it's the easiest tricks that leave the most impact! This is why I'm suggesting you use flash paper because it will make a big burst of fire and then disappear. Lighting regular old paper on fire will create smoke, will take longer, and potentially will leave ash on the floor.
Lastly... I would encourage you not to talk about our sin "disappearing" but rather - Jesus removing it or that Jesus took our sin and defeated it. I don't know that I see anywhere in Scripture the idea that our sin "disappears".
Either way - enjoy this lesson!
PSALMS 103:12
" He has taken our sins away from us as far as the east is from west. "
The BetterBibleTeachers Curriculum Library
An ever-growing collection of elementary teaching resources...( 250+ weeks of lessons and counting!)

Full Curriculum Access
Every member has immediate access to ALL of the resources.
Games Galore
Inside every lesson you'll find several games. Kids don't just learn by 'sitting and soaking'.
Image already added
Songs with motions
Every lesson includes links to songs with lyrics AND motions!
Print and Teach Ready
Using the materials is as easy as... Step 1: Print. Step 2: Teach!
Object Lessons
You'll find object lessons galore throughout all of my material. (I love them!!)
Words for the Week
Each lesson includes a lesson recap kids can take home to help remember what they've learned.
Complete Lesson Script
Every lesson includes a complete script to follow along with or inspire you.
Stand-Alone Lessons
Every member has access to a bunch of stand-alone lessons for those weeks when you don't want to start a new series.
Fun Activities
Since kids learn through play, each lesson includes easy to play games for both large and small groups.
Other Popular Posts:

Be Thankful Sunday School Lesson for Thanksgiving

28 Bible Verses for Kids

How to teach so kids remember what you taught them

Moses Crosses the Red Sea Kids Sunday School Lesson

How To Train Sunday School Teachers

10 Ways to Share the Gospel with Kids Easily
- Next »

Science Experiment: Jesus Washes Our Sins Away
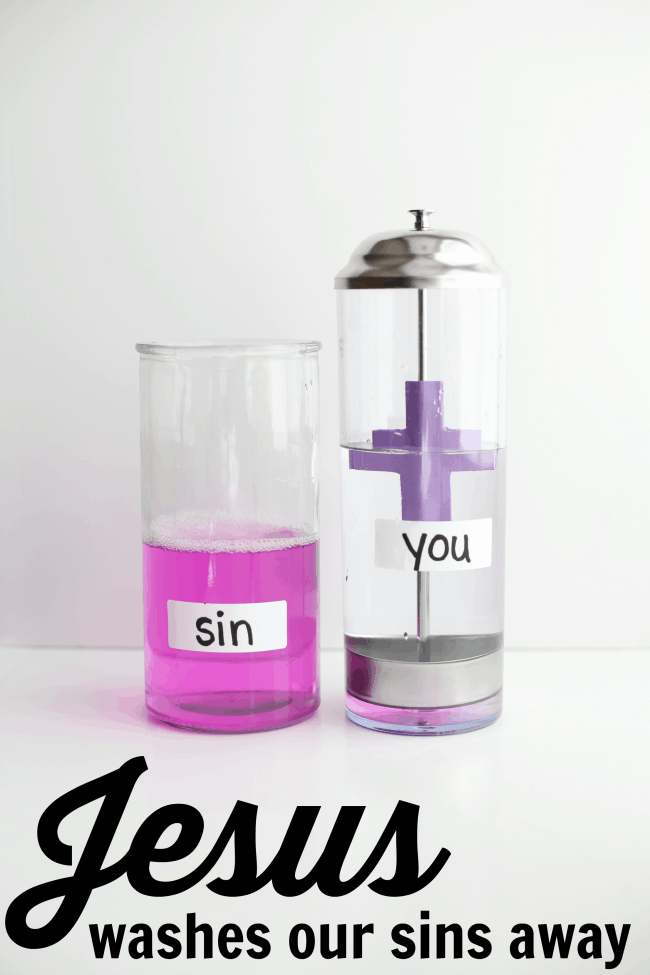
Last year in one of our Sunday School classes, one of the teachers showed us a demonstration very similar to this one. Not only did I think it was amazing from a science perspective, I also thought it was perfect for demonstrating the work of Jesus in our lives. After scouring the internet, I found this tutorial and purchased the necessary supplies…and then forgot about it until nearly a year later. :)
The experiment is simply a chemical reaction…but the demonstration is meant to show in a visual way what happens internally when we accept Jesus into our lives.
Science Experiment: Jesus Washes Our Sins Away
This object lesson is perfect for demonstrating the power of Jesus in our lives. All of us sin and we are tainted beyond repair, even when we try ourselves to fight off the “sin that so easily entangles.” It is only when we allow Jesus to come into our lives that we are cleansed and purified. Once Jesus lives in us, sin can no longer tarnish us in the eyes of God. The blood that Jesus shed on the cross washes us white as snow.
PLEASE NOTE : There are dangerous chemicals involved in this demonstration, so please use extreme caution and make sure to read the labels on each of the chemicals involved before attempting this at home (don’t let any of the chemicals come in contact with your skin, eyes, or be ingested). Do not let young children help with this demonstration .
Here’s what you’ll need for this demonstration:
1 glass jar Straw Dispenser Household Ammonia (found in the cleaning aisle) White Vinegar Bottle of Phenolphthalein (I ordered ours on Amazon ) Sticky-back Craft Foam in any color

1. Cut out 2 identical cross shapes from your sticky-backed craft foam.

2. Peel off the paper backing from the craft foam and stick the two sides together on the pole of the straw dispenser.

3. Fill the bottom of the straw dispenser with white vinegar.

4. Fill your straw dispenser about halfway with water and then add a squirt (or 5-6 drops) of your phenolphthalein .
Not pictured: Fill your other glass jar about 1/3 with water and add a splash of ammonia.

5. Use return address labels to label each jar: the jar with ammonia and water “sin” and the jar with phenolphthalein and water “you”.

For the demonstration: Pour the “sin” into the “you” jar and it will turn pink. Pour some of the pink back into the sin jar (in preparation for the next step).
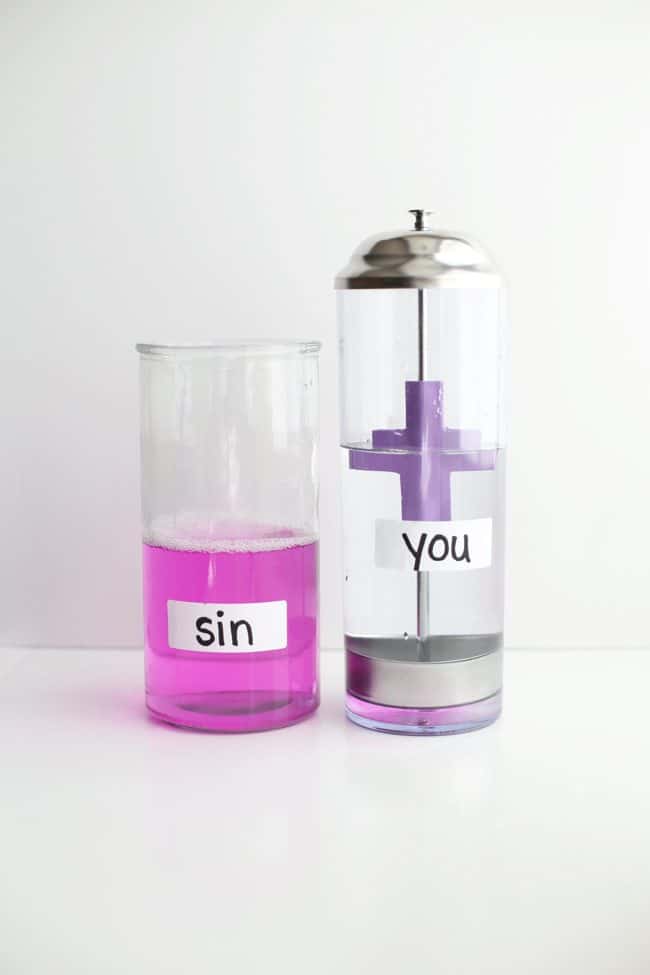
Carefully place the straw dispenser lid (with the vinegar in the bottom) into the “you” jar and watch as it changes back to clear.

Attempt to pour sin into the “you” bottle with the cross still in it and it will change to clear, representing that sin can no longer taint us in the eyes of God…Jesus’ life was a sacrifice that cleanses us once and for all.
“But if we walk in the light, as he is in the light, we have fellowship with one another, and the blood of Jesus, his Son, purifies us from all sin.” -1 John 1:7
Check out more awesome Sunday School ideas here.
The Science Behind It : Phenolphthalein is an acid/base indicator that turns pink in the presence of bases. Ammonia is a base. When the ammonia and phenolphthalein mix, it creates a chemical reaction that turns the liquid pink. The vinegar in the base of the straw dispenser acts as an acid neutralizer and neutralizes the reaction, turning it clear again.
Related Posts

Celebrate the resurrection of Jesus with these 30+ Christ-Centered Easter Activities and Crafts for Kids!…

I've always been puzzled by the term "Good Friday". To the disciples and everyone…

As I have mentioned before, I volunteer to help write supplemental children's curriculum at…

Jesus Heals The Blind Man (John 9: 1-12) I filled in for the 2-3 year…
I received my first copy of Thriving Family magazine yesterday and was thrilled with everything inside.…

If you have a little one who is fascinated with colors and fireworks, this simple…
36 Comments
That is the most rediculous thing I have ever seen!!! What exactly are you trying to prove!!
I’m not trying to prove anything. It is simply an external demonstration of the internal work that happens when we accept Jesus into our lives.
The experiment isn’t meant to be scientific…just a demonstration for kids and adults alike. I’m sorry this isn’t a post that resonated with you. Have a great day!
This is FANTASTIC!! The concept of sin in our heart/soul making it filthy before a holy God is a difficult one for kiddos to grasp & this visual is perfect! The idea of something (our souls) being made clean by Christ is clearly illustrated here in way that will facilitate a question & answer time where the gospel can be explained again. Thank you for posting this!
Thanks so much! I appreciate your kind and supportive words!
It is literally titled “Science Experiment”. This is a religious demonstration. A science experiment has set steps to follow called the Scientific Method.
You are right, it should have been titled “demonstration” or “visual representation”. However, it does include a description of the science behind the actual experiment, which I also explained to my children. They know the illustration itself wasn’t supernatural, just a visual for the internal work that happens inside of us when we accept Jesus. Sort of like a modern day parable. :)
Thankyou. We can se this in Sweden too. As the Gospel….it’s spread in the world. Thanks for your god heart!
Well, what a load of tripe. When I see this kind of stuff, I often think of the quote that goes; intelligence is limited, but stupidity is boundless! Is is your obligation to teach your children to think, reason, and question everything, and not fill there fertile minds with such nonsense. Teach them facts not faith, reason not religion, and science not stupidity. The world is a wonderful place and could be better without the kind of garbage you have posted.
I’m sorry you found this post to be so offensive. My children are learning to think, reason, and question the world around them daily. They are smart and inquisitive. We are also teaching them that everything in our lives is framed through our relationship with God. The two are not mutually exclusive. We are not perfect parents by any stretch of the imagination and we fail daily, but we are doing our best to point them to the One who is perfect all the time.
I know this post obviously won’t change your mind about what you label as “garbage” and you certainly have the right to express your opinion. And surprisingly, I don’t mind being called a fool. :)
“But God chose what is foolish in the world to shame the wise; God chose what is weak in the world to shame the strong” -1 Corinthians 1:27
Jesus is the only way!!! I pray that all who don’t believe will have a change of heart and accept Jesus as their savior.
Why Joe Grimmitt, are you soo mean?
Probably you should call this NOT A SCIENCE EXPERIMENT: Pretending there is a need to wash away an imaginary friend from your life. Don’t be sorry you offended people, we get it a lot…its just so hard to grapple with people who believe in things like Santa Claus, the Tooth Fairy, and Jesus. We love you unconditionally and accept you in our hearts, but we’ve established a lot of science over the years and we’re trying to go forwards, not backwards in our established, objective observations of the world. Things like this tend to confuse people into thinking that something supernatural is occurring, when it is clearly not. Enjoy the SCIENCE that made your trick look magical and not attribute it to your lord and saviour, jesus.
You are right, it would more accurately be described as a visual representation or demonstration rather than “experiment”. Although, I did a “two for one” with my kids, explaining the science behind the reaction while also telling them that it is a great visual representation of the internal work that Jesus does inside of each of us when we choose to accept him. They did not think it was “supernatural” in the least…they helped me and saw the chemicals we used. But it does have a “wow” factor that causes us to think, which is what the post is intended for. I think of it as a modern-day parable. Jesus used agricultural metaphors to reach the people in his day, why can’t we use chemical reactions?
I really appreciate your respectful comment (minus the imaginary friend part, that was a bit disrespectful), even though you disagree with me and my beliefs. Jesus is truly the rock of my life–I can not and will not ever deny his transforming power inside of me. I am NOT sorry for offending people, I am sorry that this post OFFENDS someone. There is a difference–I will never apologize for sharing my beliefs, but I am sorry that people take such offense at those beliefs. It is my life’s goal is to help my children come to know Him while simultaneously showing His love to those around me. Have a wonderful day!
I love your respectful response! Keep strong to move the Kingdom forward!
Thank you!!!
Thank you Janae – this is great and we will be using in our Sunday School lesson this next term to represent as you say the internal work that Jesus does in our lives. I fail to understand some of the previous commentors – if you don’t agree with a belief system don’t enter articles and criticize and belittle other people because of your insecurities and disbelief. I hope you are not affected by their negativity and continue doing such a great job!!! Lots of love!!! Debbie and Shannon
This is a very helpful demonstration.
Is it possible to start with the “sin” jar already pink, so you do not need to pour some water back into the sin jar? I think it would help with the flow of the presentation. Because, I did it once already and kids asked, “Why did you pour back into sin?”
I saw someone perform this demonstration on youtube and they poured the pink back and forth a couple of times stating we try to rid ourselves of sin without any success. I thought that made it flow better.
Jenae, thank you so much for your post. I thoroughly enjoyed it and plan to use it in our Sunday School. Keep up your strong witness for our Lord and Savior Jesus Christ. I applaud your testimony in Him! I am one who “gets it”! Praise Him!
Thank you so much, Louise!
I stumbled across this today and plan to use for sure. Thank you and keep up the good work. Be blessed.
I’m reading this, this morning, and love it. I don’t mind being labeled a fool, or any other type of “label “that someone may feel capable of listing on us, but I think it is really neat. thank you, keep up the good work. God bless! lew combs Wilder, KY.
We did this at Awana’s last night. The kids — and the helpers — loved it. Such a great visual!!! Thank you!
Good Afternoon Ms. As a science educator and a Sunday school teacher. I found this activity relevant to the theme. Using science activity (i.e experiments) in a bible story, it helps the children visualize or imagine why Jesus sacrifice himself for us. I am happy that people like you do their best to share Jesus to everyone and creates ideas and crafts that engage children to learn more about God.
P.S. I hope that you do not listen to the negative comments here. Keep going coz’ you are a blessing to every children of God.
Thank you so much, Mitzi!
Hi, I am a student teacher on my final placement in a primary school setting. I just wanted to thank you so much for this amazing idea. I believe it will really help my class in understanding why Jesus died for us. I am awaiting an inspection, and I believe this will absolutely knock the socks off any inspector who comes into my classroom! I don’t understand why you have received so many negative comments. Personally, I am not a believer, but I still have to teach about God and Jesus. Other people’s opinions and beliefs do not influence my life, I would not get so angry at someone for sharing clever ways to portray their beliefs! Thank you again, you have really saved my bacon with this!
absolutely beautiful. some people will not understand… but we can keep praying for them..
Love this Object Lesson! Using this on Sunday for our Children’s church as we talk about sin and forgiveness!! Thank you!
I loved this visual. SO creative and a great option to another similar demo with colored water and bleach that I have done over the years. I think your response to negativity was classy and loving! Great job!
Thank you so much, Carla!!
Thank you for sharing this experiment and WONDERFUL OBJECT LESSON! I can’t wait to try this in school! I think it’s great for adults as well! Thanks again! (and don’t worry about the haters…pray for them)
This is a fantastic idea! Perfect for my Children’s Church kids. We are studying the life of Jesus now and this will fit perfectly into the lesson. Thanks so much for sharing.
Thank you for sharing your post. This is a wonderful demonstration for adults and children as well to grasp the concept of how we can become more Christlike through repentance.
What a great idea to use for Easter. Thank you for the idea.
I have been using this object lesson for the past 3 years on Easter Sunday. This is my absolute favorite illustration to use! There is something so powerful about such a simple object lesson. I truly appreciate you sharing this. I LOVE when the Bible comes alive to my students and this does such a great job in aiding with that. Thank you! Thank you! Thankyou!
Leave a Reply Cancel reply
Your email address will not be published. Required fields are marked *
Summer Research Program for Science Teachers
Michael J. O'Leary
Analysis of Bleach
In this experiment we will analyze and compare various laundry bleaches by testing their relative oxidizing powers. We will run two reactions. First we will use the bleach to oxidize iodide to iodine and then we will titrate the iodine to determine exactly how much was produced by the bleach. [ 9-12 Content Standard B - Chemical reactions]
Introduction
The active ingredient in most chlorine bleaches is sodium hypochlorite, NaOCl. The oxidizing action of hypochlorite ion, OCl-, kills germs and also decolorizes many stains and dyes. [ 9-12 Content Standard E - Understandings about science and technology] The quantity of hypochlorite ion in a sample of bleach can be determined by finding out how much iodine, I 2, it can produce by oxidizing iodide ion, I . The quantity of iodine produced is measured by titrating it with sodium thiosulfate, Na2S2O3, which converts the colored iodine back to colorless iodide ion.
The equations are:
Oxidation of iodide ion to iodine with bleach:
2H+ + OCl- + 2 I - I 2 + Cl- + H20
Titrating iodine with thiosulfate:
I 2 + 2S2032- 2 I + S4062-
________________________________________________________________
· CAUTION! UNDILUTED BLEACH AND HYDROCHLORIC ACID CAN CAUSE CHEMICAL BURNS AND RUIN YOUR CLOTHES. BLEACH IS ALSO IRRITATING TO THE EYES. [ Teaching Standard D - Ensure a safe working environment]
Obtain two burets and a 250 ml volumetric flask from the stockroom. One buret will be used for diluted bleach and the other for sodium thiosulfate.
(1) Measure out exactly about 25 ml of the liquid bleach assigned to you from the buret provided for unknowns. Collect this measured amount carefully from the buret in an Erlenmeyer flask or a beaker. Note the initial reading and the final reading on the unknown's buret to the hundredths of a ml (two decimal places).
(2) Pour this bleach into the clean (distilled water) but not necessarily dry 250 ml volumetric flask. Rinse your beaker or Erlenmeyer flask and anything else you used in the transfer (funnel, etc.) very thoroughly with distilled water and pour all the rinsings into the volumetric flask. Be sure that every last drop of the bleach you took gets into the volumetric.
(3) Fill the flask to the mark exactly with distilled water, using an eyedropper for the last bit if necessary. Stopper the flask and shake well to mix thoroughly.
(4) Prepare two burets by rinsing (three times) and filling (to near the zero mark), one buret with the diluted bleach solution, the other with 0.3000M sodium thiosulfate solution.
(5) Prepare a 100 or 150 ml Erlenmeyer flask with about 10 ml of a 10% KI solution in it; add about 10 ml of 2M H2504 to the flask and stir.
(6) Read the initial reading on the bleach buret and then run about 25 ml of the bleach solution into the Erlenmeyer with the acidified KI solution. Note the color change as iodine is formed Take the final reading on the bleach buret.
(7) Record the initial reading on the other buret and titrate the iodine in the Erlenmeyer flask with the sodium thiosulfate solution. The end point is the disappearance of the iodine color. To get a more sensitive endpoint, stop the titration when the red-brown iodine color is very faint but still visible. Add 2 ml of fresh 1% starch solution to the flask; a starch-iodine complex forms which has an intense blue color. Continue the titration until this blue color just disappears. Record the final buret reading. The endpoint is made more distinct by the addition of the starch solution, which combines with excess iodine to form the deeply colored complex. [ Content Standard Unifying Concepts - Change, constancy, and measurement]
I2 + starch (starch-12 complex)
red-brown deep blue
(8) Repeat steps (5), (6) and (7) in a second trial. Do the same for a third trial. Be sure you refill the burets before you start a trial.
Calculations
If the exact Molarity of the sodium thiosulfate is known, you can use the balanced equations in the introduction to determine the Molarity of the bleach for OCl- ion. Note that half as many moles of the OCl- ions are required as S2O32- ions in the reactions. If you can do these calculations, find the Molarity of the OCl- in your bleach for each case and average the three values. Otherwise, for each titration calculate the ml of thiosulfate used per ml of bleach solution and average the values.
Obtain other students' results for other brands of bleach different from yours. Compare your results for different brands in the class. Take into account the original dilution, if there are significant differences between different students' work. See Report Sheet. [ Teaching Standard B - Orchestrate scientific discourse]
PRELABORATORY ASSIGNMENT
NAME _________________________________________
LAB SECTION __________________ DATE _________________
(A) Oxidation (B) Oxidizing agent [ 9-12 Content Standard B - Chemical reactions]
(2) Balance the following oxidation-reduction equation
SnC I 2 + HC I O3 + HC I SnC I 4 + H2O
REPORT SHEET
NAME __________________________________
LAB SECTION ____________ DATE _______________
Estimate all buret readings to two decimal places.
Type of bleach you used _________________________________
TITRATION I 1 I 2 I 3 I
ml amount bleach dilute bleach thio- sulfate dilute bleach thio- sulfate dilute bleach thio- sulfate
Final Reading
Initial Reading
Volume Used
(1) If the exact Molarity of the thiosulfate is not known, go to (2), next page.
From the Molarity of thiosulfate calculate the number of moles of thiosulfate used in each of the three titrations, the number of moles of hypochlorite in the diluted bleach, and then the Molarity of the hypochlorite in the diluted bleach for the three trials. Do this and find an average value. Show one set of calculations (attach sheet).
Molarity of hypochlorite in diluted bleach
Titration 1 ____________ Titration 2 ________________
Titration 3 ____________ Average ________________
(2) Do not fill in this section unless you did not complete section (1)
This page is to be used only if you did not know the exact concentration of the thiosulfate.
Calculate the ml thiosulfate used per ml of diluted bleach in each of the three trials. Average the three values. Show one set of calculations (attach sheet).
ml thiosulfate per ml diluted bleach
Titration 1 __________ Titration 2 ______________
Titration 3 __________ Average ______________
(3) Give any comparisons you made with other students' bleach of a different brand from yours.
(4) Questions
(a) In step 2 of the procedure, why is it not necessary to use a completely dry volumetric flask? (b) In most titrations we add an indicator. Why is none used in this experiment? [ Teaching Standard B - Orchestrate scientific discourse]
Return to Chemistry Lesson Plans Menu
Subscribe to stay updated
She Loves Science
A Mom Inspiring with Science
Make Color Disappear with Science
May 10, 2017 by Tracy 1 Comment

Do you need a few ideas on how to combine teaching science and teaching religion for Vacation Bible School? Have you volunteered to teach a religion class and need a bit of inspiration? I recently gave a presentation to my church mom’s group on how to enhance a children’s bible study using science. You should have seen all of us moms trying out experiments and telling Bible stories. It was a lot of fun. You should also check out this amazing book here for more inspiration.
This one is my absolute favorite and it is so simple to do. All you need are things that you already have in your kitchen. It is making color disappear to teach that Jesus washes away our sins AND I’m going to let you in on a little trick to give it a great wow factor!
Here’s what you need: 2 clear cups (one labeled “sin” and one labeled with a cross), food coloring, bleach, baking soda, and water
How you do it:
- Fill one glass about 3/4 full of water
- Stir in 1 tsp of baking soda
- Add food coloring
- Next pour about 1/4 cup of bleach in the colored water
Check this out:
What is the science?
The oxygen molecules in water (H20) will combine with the oxygen molecules in the bleach (NaClO) causing the food coloring to neutralize and disappear. The trick is by adding baking soda this chemical reaction occurs more quickly making it a perfect demonstration for a group of kids (and moms!)
What is the Bible Lesson?
This is an easy and impressive way to demonstrate 1 John 1:7 “But if we walk in the light as He is the light then we have fellowship with one another and the blood of his son Jesus cleanses us from all sin.”
I have just loved sharing these science bible activities with you. I think you could turn practically any experiment into a great lesson about our Creator. Enjoy this one! Its so much fun!
Reader Interactions
June 11, 2020 at 7:52 pm
Hi, how are you? Very interesting article, thank you for sharing. Actually what I would like to do is the complete opposite. I want discolored water to turn clear, without using bleach. I make homemade cleaning products. I see herbs and distilled water and vinegar, and it causes the water and vinegar to change color. I’m looking for something to make it clear again, but I do not want to use bleach. Do you know how to do that? I would really love your feedback. PS. I’m a Christian too. I used to teach kindergarten, and I would’ve loved to of been able to do something like this at that time. They would’ve loved it!
Leave a Reply Cancel reply
Your email address will not be published. Required fields are marked *
This site uses Akismet to reduce spam. Learn how your comment data is processed .
Subscribe to our newsletter to get updates on all our latest product releases, sales, and some free goodies!

|
|
The Blood of Jesus Christ, God's Son, Cleanses Us From All Sin
Have three medium-sized glasses (so you can clearly see the contents).
Label each glass with a little label at the top. One glass is marked SIN; one glass is marked MAN; and one glass is marked JESUS.
Prior to the lesson you should fill each of the glasses. The glass marked SIN should be filled with iodine. The glass marked MAN should be filled with water. The glass marked JESUS should be filled with bleach. Each glass should be filled with the same amount of liquid but should not be filled to the brim. Fill each glass 2/3 full or 3/4 full. The glasses with bleach and water will both look like they contain water. The iodine has a dark, dirty looking color.
Begin by talking about the "MAN" glass. The water looks very pure. When was man pure and clean? The only time was in the garden with Adam and Eve. Did their purity last very long? No, soon sin entered the world (using a spoon add some iodine to the "MAN" glass. So when we were born into the world, we did not begin pure but we started out with Adam's sin (add more iodine). But we are not only impure because of Adam's sin, but also because of the things we have done.
Sometimes we tell lies (add a little iodine). Sometimes we get angry at a brother or sister (add a little more iodine). Sometimes we say words we should not say, such as swear words (add a little more iodine). Sometimes we disobey our parents (add a little more iodine). Etc.
What can I do to make myself pure? What if I decide I'm going to try to live better and obey my parents and love my sister, etc.? But even if no more sin is added, I still have all the sins that were added previously? How can I get clean? What can wash away my sins? "Nothing but the blood of Jesus."
The Lord Jesus died on the cross so that we could be clean and pure. "Thou shalt call His Name Jesus for he shall save His people from their sins" (Matt. 1:21). He died so you could be pure, but what do you need to do in order to be pure? The Bible says that if we believe in Him we will receive forgiveness (remission) of sins (Acts 10:43).
When we believe on the Lord Jesus Christ and receive Him as our Saviour, watch what happens (pour a good amount of bleach into the dirty "MAN" glass. The sin will amazingly disappear (might need a little stirring). The children will be amazed to see the dirty water turn into pure water very quickly. God can make us pure and clean on the inside.
Now that we are saved, is it still possible for us to sin (1 John 1:8,10)? But when we sin now, it's quite different, because the blood of Jesus Christ, God's Son, keeps on cleansing us from all sin (1 John 1:7). So let's say you sin by getting angry at your sister. What happens then? [put a spoonful of iodine into the "MAN" glass, and as soon as it hits the liquid, it will turn clear and pure]
Even though God has cleansed us from all of the sins we commit now that we are saved, yet it is still important for us to do what? (1 John 1:9). You need to tell God that you have sinned, and also you need to tell your sister that you were wrong to get angry with her, and by doing this you will keep your relationship with God right and you will keep your relationship with your sister right.
Jesus Christ is the only way we can get pure and keep ourselves pure! He's a wonderful Saviour!
Return to More Object Lessons (Index Page)
| | |
Courageous Christian Father
A Christian Blog about the Bible, Theology, God, Jesus Christ, Christian Music, Christian Movies, Family, Cats, Odd Holidays and much more.
You, Sin and Christ – Simple Illustration
You, Sin and Christ – A Simple Illustration – a great way to show how the blood of Jesus Christ can wash us clean.
If we say we have no sin, we deceive ourselves and the truth is not in us. Jesus Christ was the atoning sacrifice for our sins. God placed upon Him the Sin of us all. So He can Justified the Sins of the Whole World
This video is a great illustration or analogy to show us how Jesus can wipe our slate clean. Sin has no bearing on us if we are Saved through Christ. He washes us white as snow. He takes the magic eraser you can say and whites the slate clean. Also, our sins, after we are saved are cast as far as the East is to the West.
Watch how when sin enters you it turns it, but watch how when Christ is poured into you that the sin is cleansed. Watch as how he tries to pour sin into Christ and how Christ won’t turn. That shows how Christ is without sin.
I saw this video on Facebook and wanted to share it, but the guy talking spoke a different language . I finally found this video where someone is speaking English.
Blog entries on sin:
- A License to Sin
- Camouflaged in Sin
- Kryptonite is Like Sin
- Quote about sin
This is just a few of the blog entries on sin at Courageous Christian Father .
You may also want to check out this blog post on forgiveness .
Forgiveness
Below are Scripture references to look up that go with this illustration :
- Psalm 103:11-12
- 1 John 1:8-10
- Romans 3:25
- 2 Corinthians 5:21
- Hebrews 9:22
Isaiah 1:18
Can you think of other scriptures that go with this illustration, I would love to hear from you.
Chemicals used in the You, Sin and Christ – Simple Illustration
After I posted this I had a person give me a comment asking what chemicals were used, so I went to look into it. I came across these, I am not totally sure, but this is what I found. One person said they do this experiment the key ingredient is Sodium Thiosulfate . But another person shared posted this, and it seems to make more sense and what I was thinking the ingredients were.
This is not mine, it was someone Else’s video I am sharing. I just know of the chemicals that were used. Please use caution when doing this too.
You = water Sin = water + iodine Christ = water + bleach
I am sure in lieu of iodine, you can use a dark colored soda/ coke or dark colored drink. You could also use water and dark food coloring. The key thing is the bleach. The bleach is what strips the color in this illustration.
Also safety first, make sure to wear safety glasses when performing this illustration.

Here are links to other people doing the same thing on YouTube.
- You sin christ (tu sin Cristo)
- The Power Christ Have on You and Sin
- A Science Experiment How Christ Can Wash Away Our Sins
I will try to add more as they pop up. But this is a list at this time. Check back to this post often! If you know of any that I can add or I should take off, please let me know.
If you liked this demonstration then you should check out this one on prayer: It’s All Clear .
Just Keep Praying Soon It Will All Be Clear
Click Share below and share on your favorite social network!
Remember you may know how Christ washes us clean, but someone who follows you or on your friend list may not. So share this post using social buttons below or copy posting URL.
This demonstration would be great for any Bible lessons on what Jesus done for us. Anything on sin and the forgiving power of the Precious blood of Jesus.
Again, I didn’t make this You, Sin and Christ – Simple Illustration video, etc. I am just sharing it on this blog!
Tu Sin Cristo | Vous Péché Christ
Check out Courageous Christian Father’s Wish list on Amazon where you can purchase and this items sent directly to him and they will be used for the ministry.
Subscribe To Courageous Christian Father!
Don’t miss any blog posts! Subscribe today! You can subscribe via WordPress or by entering your email! Thank you!
Enter your email address
Follow Courageous Christian Father on Social Media
Below are some examples of blog entries from all the blogs that I do. (Courageous Christian Father, Steve Sews Stuff , and SteveZ DesignZ ).
Recent Posts

Thank You For Reading Courageous Christian Father!
Thank you for reading. Please feel free to share and like this blog post.
Clipart: Unsplash , Pixabay , Pexels , Openverse , Adobe Express , Adobe Stock , FreePik , MetroCreative , and more. This site uses Amazon Affiliate Ads & amp ; Google Ads.
I did not do this video, this was a video I found while doing research online. I am simply sharing this video. I First published to share this on September 5, 2014. Last updated or republished on March 30, 2021.
About the Author
Steve Patterson
A Christian Blogger that enjoys blogging about the Bible, Theology, God, Jesus Christ, Christian Music, Family, Cats , Odd Holidays, sewing and much more. I have been blogging since 2004, however, I have been blogging on Courageous Christian Father since 2012. I enjoy listening to Christian Music. I am married with 1 daughter, 2 step-sons and a step daughter.
See author's posts
Sharing is Caring! Please Share!

- Share on Tumblr
Author: Steve Patterson
20 thoughts on “ you, sin and christ – simple illustration ”.
😉 RT : You, Sin and Christ – Simple Illustration http://t.co/hbginbfGea #bgbg2 #ccf #you #sin #Christ
This illustration is great. Can someone tell me what the liquids were that were used? I’d like to “perform” this for my class. Thanks.
I do not know, I would like to know too.
Researching this, I seen one say the key ingredient is Sodium Thiosulfate, but not sure about that. Another I saw, You = water Sin = water+iodine Christ = water+bleach
Heavy duty spray starch Bottle of iodine 10% povidone / iodine works fine. Sodium Sulfite (available from online chemical suppliers) Vinegar Phenolphthalein solution
Thanks for sharing.
I saw on one of the YouTube demonstrations of this illustration the formula: Sin = 80% water, 20% iodine; Christ = 80% water, 20% bleach; You = 100% water. Using these ratios, I tried it and it worked.
Thanks Scott I appreciate that.
I saw this not to long ago! How amazingly true it is! Thank You Jesus for dying for ALL our sins <3
It is neat demonstration. Would make a great presentation in a bible study, small group, Sunday school etc. I did research and found chemicals used too.
https://www.facebook.com/groups/817290018331781/?ref=ts&fref=ts
@Tammy Pickeral Curtis Amen, we have a lot to be thankful for, we didn’t deserve Jesus dying for us, but he loved us anyway. No greater love than one laying down their life for another. Thanks for the comment and God Bless.
@Amber That is a good verse thanks for sharing Amber.
what amount do you use with each liquid and what liquid do you use for you, sin, wine and Jesus? Do I need to add anything to any of the liquids, like water for instance?
I just found a place listed the chemicals used not how much, but I’ll try to find out.
Praise Jesus for washing us clean and for faultless!
Amen! Our slate was washed clean.
Thanks, as this reminds me of it is Christ poured into us, not us poured into Christ Take a empty pitcher, a bag of rice and a bag of walnuts Pour the bag of rice in the pitcher, then the bag of walnuts, does it fit,? The rice is God, the Walnuts are you The walnuts do not fit into the pitcher, the you Now if you pour in the walnuts first into the picture, then the rice, you see it all fits perfectly
So be filled Brother as you are filled Acts 17:28
Thank you for sharing the rice and walnut alalogy with us here. I like that. Have a great day and again thank you for stopping by and commenting. God Bless!
Feel free to share your comment! Thank you! Cancel reply
This site uses Akismet to reduce spam. Learn how your comment data is processed .

Steve Sews Stuff

Top Posts & Pages
Random Post

CCF Community
Christian Blogs
Subscribe to blog via email.
Enter your email address to subscribe to this blog and receive notifications of new posts by email.
Email Address
Recept Post:
Stevez designz, follow on wordpress, true life church.

Audio Books Listened To:
Currently listening to:, bible gateway blogger grid (bg²) member.

On Top List

Free Christian Music

Free Printables

Free Gospel Tracts

Road Signs of the Bible

Donations help offset cost.

Simple One Time Donation
Just a simple donation can help with the funding for this blog ministry of Courageous Christian Father. Thanks and God Bless!
Faithful Bloggers Member

Copyright © 2004-2024 Courageous Christian Father
Design by ThemesDNA.com
Imagination Station will be OPEN on Labor Day
Monday, September 2 | 10-5

Iodine Clock Reaction

Try an at home version of this experiment using a few things you may have in your bathroom medicine cabinet. In may ways this experiment feels almost like magic. Two colorless liquids are mixed together and after a few moments the mixture turns a dark blue color. There are actually a couple of simple chemical reactions going on at the same time to make this “clock reaction” occur. This version of the classic “iodine clock reaction” uses safe household chemicals most people have on hand at home.
What you need:
- distilled water (tap water will work OK as well)
- a couple plastic cups
- 1000 mg vitamin C tablets
- tincture of iodine (2%)
- hydrogen peroxide (3%)
- liquid laundry starch
What to do:
- Make a vitamin C solution by crushing a 1000 mg vitamin C tablet and dissolving it in 2 oz of water. Label this as “vitamin C stock solution”.
- Combine 1 tsp of the vitamin C stock solution with 1 tsp of iodine and 2 oz of water. Label this “solution A”.
- Prepare “solution B” by adding 2 oz of water to 3 tsp of hydrogen peroxide and 1/2 tsp of liquid starch solution.
- Pour solution A into solution B, and pour the resulting solution back into the empty cup to mix them thoroughly. Keep pouring the liquid back and fourth between the cups.
What’s going on?
There are actually two chemical reactions going on at the same time when you combine the solutions. During these reactions two forms of iodine created – the elemental form and the ion form.
In Reaction # 1 iodide ions react with hydrogen peroxide to produce iodine element which is blue in the presence of starch. BUT, before that can actually happen, the Vitamin C quickly reacts and consumes the elemental iodine.
The net result, at least for part of the time is that the solution remains colorless with excess of iodide ions being present. Now after a short time as the reactions keep proceeding in this fashion, the Vitamin C gets gradually used up. Once the Vitamin C is used up, the solution turns blue, because now the iodine element and starch are present.
Safety Precautions
Be careful when working with the iodine – it stains, and it stains really well. Be very careful not to spill any of the solution.
Water and Wine – The story of bleach and iodine
I came across this video on Facebook recently, have a look, it’s only 40 seconds.
The thing has had over 300,000 shares! That’s pretty impressive. The christians who are impressed with the concept are falling over themselves with excitement.
The video is instructive, primarily for Sunday School teachers. Can you imagine showing this to children? You are clean and pure, then sin comes along, uninvited and pours itself all over you. The only way to get clean is to have christ in your life.
That is really a horrible message.
I made my own version, with a science label instead of sin. Have a look – it’s only 23 seconds, it’s not as convoluted as religion and gets to the point much quicker.
It’s had about 50,000 shares on Facebook so far. Still got a way to go!
I’ve had more negative feedback on my short 23 second video than I have on my 50 minutes on television! I can feel the christian love.
People feel entitled to message me to tell me about their god. I don’t mind that. I don’t feel obliged to engage with them in any deep conversation. I’m polite and respectful to them, but quite adamant about where I stand on religion. Here’s a couple of my favourite conversations:

If you want to read the full transcript then here is Jennepher Petitt and Matt Sidney .
This conversation was of particular fun:

Here’s the full story: Δημήτριος Δεσποτικός
Just in case you want to make your own science experiment, it the Science jar I’ve put water and a dash of bleach. The christ jar has water and enough iodine to make it reddish/brown. The You cup is only water.
Just remember, it’s a little bit of chemical reaction, nothing more. You don’t have to put labels on them!
Share this:
4 responses to “water and wine – the story of bleach and iodine”.
All a ‘god’ beaker would need in it is manure. Just saying.
Thank you for your brilliant work in talking with these people, it’s amazing how they take their bat and ball then run home once their ramblings got bounced back at them.
I had to have a chuckle at the one who said that they wouldn’t be a Christian if it wasn’t for prayer. For me, the journey went in the opposite direction, prayer and Bible study led me away from Christianity on my own journey. I was a bit too good at Bible study. I could see what they were trying to say, then see through it.
Now I have no issues with people believing what they like, and there are some good things in many religions (though often buried in dogma). For me, Christianity provided me with a good and familiar (as I had grown up in the Church of England) starting point for my own journey. I object to being told what I should believe. I’ve been on my own journey, so I’ve come to my own spiritual understanding in my own way, not had it handed down from a pulpit.
If Christianity works for someone, great! But religion is a personal thing, I don’t want to be forced into Christiasnity, Islam, Hinduism, Buddhism or any other religion, and I certainly won’t force anyone to follow my path.
I’ve had plenty of the same argument and it always ends with them getting angry because there’s absolutely no way they can win the argument. Just like Matt Sidney above, they feel degraded because they can’t defend their beliefs and then the true hatred religious devotion inspires comes out.
I’m convinced that most people who choose (or for those raised in religious families choose to stay in) a religion do so for (1) political reasons (good church-goin’ folk are more likely to be elected as community leaders, and that comes with real political power), (2) because they just wanna go with the flow and don’t give a shit one way or the other, or (3) they’re self-esteem is so low they have to be accepted by some group. Besides, it offers them an ability to feel superior to others with no effort other than proclaiming their believe in God or Jesus or Muhammad or Buddha (who was an atheist, by the way) or Thor or Zeus or Peter Pan.
What’s better to someone with low self-esteem than to be able to “acceptably” vent their anger on someone everybody in the gang, er, sorry, congregation, agrees deserves it?
Leave a Reply
Name (required)
Mail (will not be published) (required)
CAPTCHA Code *
Recent Posts
- Ongoing treatment brings a smile 😃
- In this together.
- Buttercup Creek
- Bottom to near normal
- Just be there
Categories
Archive .
- Entries (RSS)
- Comments (RSS)
With Google+ plugin by Geoff Janes
Redox Titration -Thiosulfate & Iodine ( Edexcel International A Level Chemistry )
Revision note.

Core Practical 13b: Thiosulfate & Iodine Titration
Iodine-thiosulfate titrations.
- A redox reaction occurs between iodine and thiosulfate ions:
2S 2 O 3 2– (aq) + I 2 (aq) → 2I – (aq) + S 4 O 6 2– (aq)
- The light brown/yellow colour of the iodine turns paler as it is converted to colourless iodide ions
- When the solution is a straw colour, starch is added to clarify the end point
- The solution turns blue/black until all the iodine reacts, at which point the colour disappears.
- This titration can be used to determine the concentration of an oxidising agent , which oxidises iodide ions to iodine molecules
- The amount of iodine is determined from titration against a known quantity of sodium thiosulfate solution
Worked example
Analysis of household bleach
Chlorate(I) ions, ClO - , are the active ingredient in many household bleaches.
10.0 cm 3 of bleach was made up to 250.0 cm 3 . 25.0 cm 3 of this solution had 10.0 cm 3 of 1.0 mol dm -3 potassium iodide and then acidified with 1.0 mol dm -3 hydrochloric acid.
ClO - (aq) + 2I - (aq) + 2H + (aq) → Cl - (aq) + I 2 (aq) + H 2 O (l)
This was titrated with 0.05 mol dm -3 sodium thiosulfate solution giving an average titre of 25.20 cm 3 .
2S 2 O 3 2- (aq) + I 2 (aq) → 2I - (aq) + S 4 O 6 2- (aq)
What is the concentration of chlorate(I) ions in the bleach?
Answer:
- Therefore, 1 : 2 ratio of ClO - (aq) : S 2 O 3 2- (aq)
- Number of moles of ClO - (aq) in 250.0 cm 3 = 6.30 x 10 -4 x 10 = 6.30 x 10 -3 moles
- 10 cm 3 bleach = 6.30 x 10 -3 moles of ClO - ions
- 1.0 dm 3 bleach = 0.630 moles of ClO - ions
- Therefore, the concentration of ClO - ions in the bleach is 0.630 mol dm -3
General sequence for redox titration calculations
- Write down the half equations for the oxidant and reductant
- Deduce the overall equation
- Calculate the number of moles of manganate(VII) or dichromate(VI) used
- Calculate the ratio of moles of oxidant to moles of reductant from the overall redox equation
- Calculate the number of moles in the sample solution of the reductant
- Calculate the number of moles in the original solution of reductant
- Determine either the concentration of the original solution or the percentage of reductant in a known quantity of sample
You've read 0 of your 10 free revision notes
Get unlimited access.
to absolutely everything:
- Downloadable PDFs
- Unlimited Revision Notes
- Topic Questions
- Past Papers
- Model Answers
- Videos (Maths and Science)
Join the 100,000 + Students that ❤️ Save My Exams
the (exam) results speak for themselves:
Did this page help you?
Author: Richard
Richard has taught Chemistry for over 15 years as well as working as a science tutor, examiner, content creator and author. He wasn’t the greatest at exams and only discovered how to revise in his final year at university. That knowledge made him want to help students learn how to revise, challenge them to think about what they actually know and hopefully succeed; so here he is, happily, at SME.

Not Sure What I Did Wrong
One of the Two-Minute Drills that I organized for my class on Monday evening involved a demonstration using water, food coloring, and bleach. You fill a clear glass with water (about half full), add a drop or two of food color to represent sin, stir, and then add bleach to make the color disappear, representing the effect of God’s grace. Many catechists and Sunday school teachers use this demonstration to show how grace dispels sin. Here are some examples:
http://connecting.nazarene.org/english/documents/centennial/ec/EC_Lesson_4.pdf
http://childrensministryvault.com/ministry-lessons-ideas-training/561/jesus-washes-away-our-sin/
I could swear that I’d done this demonstration before and the water immediately became clear after I poured in the bleach…but maybe I’m dreaming! I tried this at home before class on Monday and the bleach only made the colored water lighter and very slowly made the water clear. I tried various food colors: red, green, even black, but they all worked (or didn’t work) the exact same way. The same thing occurred in class…it was very un-dramatic.
I’m not sure if I did something wrong or if I’m thinking of some other demonstration that has a more immediate and dramatic effect. Any thoughts or suggestions?
- eighth grade
Related Articles

Racism: Are We Cultivating a Patch of Weeds in Our Garden?
Recently after Sunday Mass, my wife and I were chatting with our pastor outside of the church. He drew our attention to one of the gardens along the side of the church, pointed at a patch of green plants and said, “I’ve been meaning to ask someone, are these weeds? Should I be pulling them?” Joanne and I, with our limited knowledge of gardening, were not quite sure ourselves. Some weeds are easy to spot. […]
A Mixed Bag
So last evening’s class was kind of a mixed bag. The main focus was on the “Ten Commandments Two-Minute Drills” that I wrote about yesterday. Here’s the scoop: All ten students were present and in good spirits. During opening prayer, one of the boys prayed for Brian Campbell, Blackhawks’ defenseman, who suffered a broken collar bone and cracked ribs as a result of a vicious hit by Alex Ovechkin on Sunday. I thought that was […]
Trust in the Goodness of God
Tonight’s the night! And I’m not referring to the great 1975 Neil Young song of the same title! Tonight is my first religious ed session for the 2009-10 catechetical year! May the “wind” of the Holy Spirit be at my sails! Here’s my plan: The theme (BIG idea) of this first session is: “We can trust in the goodness of God the Father” I plan to meet and greet the young people at the door […]
49 Comments
I use this with my reconciliation prep kids – with the verse from Isaiah – ‘your sins are scarlet and I will make them white as snow.’
Two things: ratio is really important. The smallest drop of food coloring you can imagine. (A little goes a long way.)
Second, the largest amount of bleach you can use. I try to make it at least fifty-fifty water to bleach. I use a large clear bowl, so one tumbler of water, a tiny drop of food coloring and a tumbler of bleach.
However, it doesn’t get clear instantly. It is sort of pinkish and then clears. At least for me.
Cathy, I believe you were the one who first told me about this a few years ago! I guess I’m dreaming about it vanishing instantly.
I did the experiment with stamp pad ink,it cleared immediately…..then I tried the food colouring and had the same problem you had. So ink is best!
Thanks, Annelene, this is very helpful!
Use iodine instead of food coloring for immediate results.😁
I found that I needed to use a drop of the coloring and about two ounces of bleach to make it work. I also stirred the solution and it cleared up with 3o seconds and became clearer as it stood.
Cheryl, thanks…it sounds like the smallest of drops of food color is required.
I haven’t ever tried to dramatize this in class, but I love props. Offhand this seems like an intuitive way to do it….I haven’t come up with anything else after reflecting for nearly 30 seconds!
Give it a try and let me know how it works!
We’ve done this several times for penance services. I agree with lots of bleach to minimal food coloring – in addition – when it hasn’t worked as quickly as we’d like our pastor relates that sometimes it takes a little while for grace to work in our lives – because we have to let it! And beware of reds – that takes the longest to clear up
Thanks Jean!
I did something similar for a Vacation Bible school a few years back but used an Oxciclean solution instead of bleach. It gave that instant color change you might be looking for.
Theresa, another interesting option…thanks!
It works instantly if you use drops of iodine instead of food coloring. I had the same experience of the food coloring taking a while to change – and never quite getting truly clear. The iodine, however, works instantly and is much more effective.
Kristin, that’s fascinating! I’ver never heard that before. I’ll have to try it. Thanks!
So you are saying the bleach, iodine, and regular tap water work the best for this? Looking forward to trying this!
Tried this today with the beach & iodine. Took a while to perfect! Colorless iodine & bleach actually turn the water black! It’s all about the ratios; less iodine more bleach.
I did this today twice. First time it worked well but second time I had more of iodine which took long time when mixed with bleach.
Wow, everybody does this but me; I’m further behind the curve than I’d imagined.
Different strokes…
A similar illustration (technically referred to as turning “water into wine”) achieves an instant change from clear to red, then back to clear. Go to http://chemistry.about.com/od/chemistryhowtoguide/ht/waterwine.htm . It requires two basic chemicals that you might have to order online, but they’re available at sciencecompany.com for under $4 each, and will last for quite a while. This illustration connects most obviously to Jesus changing water to wine, but can also be tied to forgiveness of sins.
Leonard, as Mr. Spock would say, “Fascinating!” Before long I’ll be wearing a lab coat when teaching religious ed! 🙂
Joe: I buy disappearing ink! I also write the steps of reconciliation on large card stock (Contrition, Confession, Absolution, Penance), the last card is God’s Forgiveness. My kids are to stand on Contrition and recognize their sins and name a few (I disobeyed my parents…) as I drop in the ink for each sin. They can see their sins in the cup. I do this quickly because I don’t want the ink to disappear to fast! Then I cover the clear class cup with a cloth because we can’t actually see our sins just it’s effects. Then they walk through the steps and when they get to the God’s Forgiveness card the cloth is removed and the water is clear. They love it!!
Mary Ellen, this just keeps getting better and better! How creative!
We’re on the third day of VBS (Go Fish program). Am doing the lesson demo re forgiveness. Put 10 drops iodine tincture (representing the 10 commandments and our failure to follow) into 4 cups water. Water became a little yellowish – murky. Then poured 1/2 cup bleach into the murky water. The water turned inky black ! Since the idea was for the water to ‘come clean’, representing God’s forgiveness, this would not have worked. Thank God I was at our kitchen table and not in the front of class ! Ouch. Am working with your other ideas with food coloring and so on. Thanks !! (I’ve got about 6 hours befor the lesson, so plenty of time to get it right, huh ????!!!!)
John, let us know what you figured out!
I’m teaching the same lesson tomorrow, and I looked up the experiment. Its a good thing I found this! My 4+5 year olds would have been so confused!
what I eneded up doing is a little more labor intensive, and requires a little more discipline on the part of the kids, but it ended up being a clear and quick demo of the concept of forgiveness. Now that I think about it, you probably could adopt this to other lessons. Anyway – this is what I did: 1) With grey chalk,draw cross on plain white paper towel , place clear glass bowl on paper towel so that cross shows through bottom of bowl. I did this on food tray on floor so kids coul look though bowl and clear water and see cross under neath.. 2)(this is where the discipline comes in) – have kids gather round on floor so they can see the cross trought water under bowl. 3) add 4-5 drops of red food coloring and mix – explained this as original sin – we can still sort of see the cross , but its ‘cloudy’ 4) add 4-5 drops of green food coloring to reddish water , one drop as a sin is mentioned by one of the kids. The drop will swirl and ‘blacken’ the water but will discipate slowly enough to talk about how its getting harder to see the cross. After 4-5 drops swirl into red water, mix water well. Cross will not be visible throuh blackness of water, like our sins completely blocked our view of the cross. 5)Use a 1 C Pyrex measuring ‘glass’ for this step. Have a mix of 1/4 C vinegar and 3/4 C bleach ready in 1 C measuring cup with spout. As you pour this mixture into the black water, talk about forgiveness because Jesus died on the cross so we could see Him and God once againg. Water will clear almost immediately. Cross becomes visible again. Did this on a food tray on the floor so kids could look throught bowl and see cross. Worked well, and was quick enough for us to stay on schedule. PTL ! Things went well.
John, you’re a real trooper for seeing this through…thanks for sharing your results and your “recipe” with us!
Your Welcome. Sorry about the ‘long windedness’ of my comment. Comes from doing task analysis in special ed settings. Ttfn, God bless, and de colores !!!!!!!!!!!!! IHL, John Reinhart
PS: A part of this VBS was for kids to learn how to locate verses in the bible. Location of books, etc. Time really didn’t allow for this. And then the bibles the kids were using were NOT kid oriented re size of letters, clear labeling of books , chapters and verses . I’m thinking that when kids are expected to find verses etc. in such a short time, that all this needs to be taken into account long BEFORE VBS. Others wise we loose the message to mechanics.
John, isn’t it amazing how many “minor” details are involved in making sure that things go effectively?
ADD VINEGAR TO THE WATER WITH THE FOOD COLORING FIRST!!!! I spent half a Sunday trying to figure this out because I’d seen it before too, and it didn’t work over and over with bleach, at least not quickly like you said, but the vinegar made magic I tell you! I found the suggestion on sugardoodle.net. Maybe somehow it could represent how Christ is necessary for repentance to work, that you can’t become clean again without the power of His atonement!!
Thanks Kalia! It’s funny how figuring this out can become an obsession for us!
Oh, oops, I see you already figured that out, still it’s cool isn’t it!
Use a mason jar and fill it about a quarter full of water. Add your food coloring drops as needed. then use another mason jar (these work the best because you can put lids on them and have them pre prepared for the lesson) about 1/2 to 3/4 full of a mixture of bleach and white distilled vinegar. Stir with a spoon. It disappears within seconds. I don’t remember the combination of vinegar and bleach but I don’t ever remember it being too specific – half half maybe.
I was having the same problem – just a side note – some one said to add vinegar. While this will help speed the process, those two chemicals combined make a toxic gas! No object lesson is worth it!
Hello! I found your page by googling for easter ideas. This is a way of doing it: Fill a glass of water, and pour half of it into another glass. Stirr 2-3 teaspoons of sodium thiosulphate into one of the glasses. Add approximately one third bottle with 2% iodine alcohol into the other glass. The content then becomes brown. Compare with sin and Jesus as the clear water. Pour the “dirty” water up in the clean and everything becomes clean.
Hey guys. I have tried this several times but can’t get it to work. What type of iodine do I need? And when you say bleach, are you referring to hydrogen peroxide, and if so what strength. Any help would be very useful. Really hoping to do this on easter Sunday with my Sunday school class. Thanks
Hi Andrew. I recommend that you read through the entire thread of comments above…some folks seem to have figured it out!
Try using iodine. it sometimes works for me when I used it in sessions at camp. I never had problems with it and never use color dye because it never seems to work for me. sorry this is a late comment I just saw your thing.hope you use this solution in your next session.
kind of feel stupid now just saw your above comments you want to use the strongest type of iodine. and you want to use the bleach you would use to wash some clothing.
I do this with my kids, but I use iodine and it clears up immediately.
EAIEST WAY TO MAKE IT WORK FAST… USE BAKING SODA. It will make bleach clear up the food coloring. No vinegar, iodine, etc. needed. It may slightly cloud the water, but the reaction is notably faster with a quick stir. Add it to any, preferrably all of the liquids in use. The more you use tge faster the reaction. It’s a catalyst: makes chemical reactions faster. If your liquid doesn’t clear up in 15 seconds with stirring add more soda and a little more bleach to your solution.
Thanks Mr. Mormon!
when you add bleach into water with coloring food, it mixes and fades. Why doens’t food coloring mix when we add it to water with bleach on it?
I did the experiment according to the bleach/vinegar method without knowing that this creates chlorine gas and now my lungs have steadily been acting up since yesterday (when I did the experiment). It feels like I got full on bronchitis in a matter of 12 hours. DO NOT MIX BLEACH AND VINEGAR. You can do a better job with Water, Iodine and Sodium Sulphate and it’s much safer since it does not release chlorine gas. Also, you should especially not do this while kids hover their heads over the bowls.
Use iodine instead of food coloring! It clears instantly when you put in the bleach.
When I did this, I changed it up with great success. I had 3 beakers and one was marked you, one was marked Jesus and one was marked the world. Each beaker had what appeared to be clear water. I explained how you (and I) are born in sin and poured red food coloring into the beaker marked you and how you and I are continually adding to our sin til I had a decently dark shade of red. I explained how Jesus died for our sins and when we believe our sins are removed and cited how we all need Jesus. I poured Jesus into you and the water instantly became clear. But I also explained that Jesus didn’t die just for you, but the whole world and poured some into the world (which was a deep red color) and it instantly cleared. The secret? Jesus had a tablespoon of Iron Out in the water (undetectable by sight or smell). You must dispose of this immediately as Iron Out is toxic.
I have done this many times — use iodine instead of food coloring.
Leave a Reply Cancel reply
Your email address will not be published.
Save my name, email, and website in this browser for the next time I comment.
Copyright © 2006-2024 Loyola Press. All rights reserved. Any copying, redistribution, or retransmission of the contents of this service without the express written consent of Loyola Press is expressly prohibited.
Your browser is not supported
Sorry but it looks as if your browser is out of date. To get the best experience using our site we recommend that you upgrade or switch browsers.
Find a solution
- Skip to main content
- Skip to navigation

- Back to parent navigation item
- Primary teacher
- Secondary/FE teacher
- Early career or student teacher
- Higher education
- Curriculum support
- Literacy in science teaching
- Periodic table
- Interactive periodic table
- Climate change and sustainability
- Resources shop
- Collections
- Remote teaching support
- Starters for ten
- Screen experiments
- Assessment for learning
- Microscale chemistry
- Faces of chemistry
- Classic chemistry experiments
- Nuffield practical collection
- Anecdotes for chemistry teachers
- On this day in chemistry
- Global experiments
- PhET interactive simulations
- Chemistry vignettes
- Context and problem based learning
- Journal of the month
- Chemistry and art
- Art analysis
- Pigments and colours
- Ancient art: today's technology
- Psychology and art theory
- Art and archaeology
- Artists as chemists
- The physics of restoration and conservation
- Ancient Egyptian art
- Ancient Greek art
- Ancient Roman art
- Classic chemistry demonstrations
- In search of solutions
- In search of more solutions
- Creative problem-solving in chemistry
- Solar spark
- Chemistry for non-specialists
- Health and safety in higher education
- Analytical chemistry introductions
- Exhibition chemistry
- Introductory maths for higher education
- Commercial skills for chemists
- Kitchen chemistry
- Journals how to guides
- Chemistry in health
- Chemistry in sport
- Chemistry in your cupboard
- Chocolate chemistry
- Adnoddau addysgu cemeg Cymraeg
- The chemistry of fireworks
- Festive chemistry
- Education in Chemistry
- Teach Chemistry
- On-demand online
- Live online
- Selected PD articles
- PD for primary teachers
- PD for secondary teachers
- What we offer
- Chartered Science Teacher (CSciTeach)
- Teacher mentoring
- UK Chemistry Olympiad
- Who can enter?
- How does it work?
- Resources and past papers
- Top of the Bench
- Schools' Analyst
- Regional support
- Education coordinators
- RSC Yusuf Hamied Inspirational Science Programme
- RSC Education News
- Supporting teacher training
- Interest groups

- More navigation items
Estimating the concentration of bleach
In association with Nuffield Foundation
- No comments
Compare the chlorine content and concentration of sodium hypochlorite in different bleaches in this class practical
In this experiment, students add excess hydrogen peroxide to measured samples of household bleach, collecting and measuring the volume of oxygen produced. They then use this data to compare the chlorine content of different bleaches, and calculate the concentration of sodium chlorate(I) (sodium hypochlorite).
This experiment can be done as a demonstration as well as a class practical (with suitable students). Younger students can compare the relative concentration or ‘value’ of different bleaches. Intermediate students could determine concentration and revise redox reactions and half-equations. Advanced students could make use of oxidation numbers to balance equations, consider electrode potentials for the reactions, and also meet hydrogen peroxide behaving as a reducing agent.
A few commercial bleaches in their containers, with prices, can be placed on a suitable tray, each with a 10 cm 3 syringe and 250 cm 3 beaker – both labelled – into which small samples of the bleach can be placed. Students can measure 5 cm 3 of each bleach into their side-arm flask for each experiment. Small samples of the hydrogen peroxide solution could be collected in a 100 cm 3 beaker.
Students should have no access to acid solutions. Bleaches liberate toxic chlorine gas on contact with acids.
Depending on the apparatus used and the number of bleaches investigated, the practical could be completed in 15–45 minutes.
- Eye protection
- Conical side-arm flask, 250 cm 3
- Bung, with single hole to fit 10 cm 3 syringe nozzle
- Plastic syringe, to deliver 10 cm 3 (see note 5 below)
- Plastic syringe, to deliver 5 cm 3 (see note 6)
- Delivery tube (see diagram below)
- Rubber tubing, short length
- Water trough or washing-up bowl
- Measuring cylinder, 100 cm 3
- Measuring cylinder, 25 cm 3
- Beaker, 100 cm 3
- Clamp and stand, x2
- Deionised or distilled water, 25 cm 3
- A variety of household bleach solutions (IRRITANT) (see note 3 below)
- Hydrogen peroxide, ‘20 volume’ solution (IRRITANT at this concentration), 10 cm 3 per bleach solution
Health, safety and technical notes
- Read our standard health and safety guidance.
- Wear eye protection throughout.
- Household bleach solutions (containing sodium chlorate(I) / sodium hypochlorite) (unlikely to be CORROSIVE but may be IRRITANT) – see CLEAPSS Hazcard HC089 . Commercial household bleaches usually contain about 5% sodium chlorate(I). Some bleaches also contain detergents and thickening agents, which may cause excessive frothing in this experiment. Note that nowadays some commercially available bleaches do not contain any chlorine and are based on peroxy-compounds. They should not be used here.
- Hydrogen peroxide solution, H 2 O 2 (aq), (IRRITANT at concentration used) – see CLEAPSS Hazcard HC050 and CLEAPSS Recipe Book RB045.
- Plastic syringes are used to measure and deliver a known volume of hydrogen peroxide solution, and their nozzles should fit tightly into the hole in the flask bung.
- Plastic syringes can be used to measure 5 cm 3 of bleach solution, but volumetric pipettes with safety fillers could be used instead.
Splashes of bleach and hydrogen peroxide should be washed off immediately with plenty of water.
- Use a plastic syringe to measure out 5 cm 3 of the first bleach into the flask. If a ‘thick’ bleach is used, add approximately 25 cm 3 deionised water. Swirl to ensure complete mixing.
- Discuss the fact that this dilution does not change the amount of bleach put into the flask, but does enable proper mixing to take place and ensure the reaction has goes to completion.
- Half fill the trough with water. Submerge the 100 cm 3 measuring cylinder and fill it with water, invert under water and clamp it in position.
- Attach the delivery tube to the side arm flask and arrange the rest of the apparatus as shown in the diagram below.
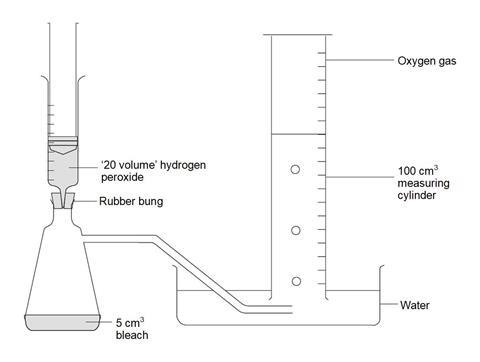
Source: Royal Society of Chemistry
How to set up the equipment required to estimate the concentration of different bleaches, using hydrogen peroxide as a reducing agent
- Measure 10 cm 3 hydrogen peroxide solution into a clean plastic syringe, attach it to the bung and gently empty the contents into the flask. Leave the syringe in place.
- Carefully swirl the contents of the conical flask and collect the gas liberated.
- Continue until no further reaction is seen. Measure and record the final volume.
- Carefully disconnect the delivery tube from the flask.
- Discard the solution into the sink. Flush away with plenty of water. Rinse the flask thoroughly. Ensure any splashes of bleach are washed off skin immediately and swabbed off benches.
- If time allows, repeat the experiment with the same bleach to obtain three results and take an average.
- Repeat the experiment with different bleaches.
Teaching notes
Here hydrogen peroxide behaves as reducing agent with another more powerful oxidising agent. (More advanced students may expect it to behave as an oxidising agent.)
Half-equation:
H 2 O 2 → O 2 + 2H + + 2e -
Chlorate(I) ions are an oxidising agent.
OCl – + 2e – → O 2– + Cl –
Overall equation showing the change of oxidation state of chlorine:
H 2 O 2 (aq) + NaOCl(aq) → H 2 O(l) + NaCl(aq) + O 2 (g)
The maximum volume of gas recorded in the measuring cylinder should have 10 cm 3 deducted from it to compensate for the injection of 10 cm 3 hydrogen peroxide solution into the flask.
Direct comparison of volume of oxygen collected in the measuring cylinder compares the effectiveness of the bleaches for younger students.
’Value’ can be compared by dividing the volume of oxygen liberated by the cost of 5 cm 3 of bleach. This extra step is well within the grasp of most introductory students
Calculation of concentration (in g NaOCl per dm 3 ) for more advanced students:
- (V–10) cm 3 is the volume of oxygen liberated.
- (V–10)/24000 is the number of moles of oxygen gas.
- (V–10)/24000 is the number of moles of NaOCl in 5 cm 3 bleach (see equations above).
- (V–10)/24000 x 1000/5 is the number of moles of bleach in a 1 dm 3 bottle of bleach.
- ((V–10)/24000 x 1000/5) x 74.5 is the mass of NaOCl in 1 dm 3 of bleach solution.
Additional information
This is a resource from the Practical Chemistry project , developed by the Nuffield Foundation and the Royal Society of Chemistry. This collection of over 200 practical activities demonstrates a wide range of chemical concepts and processes. Each activity contains comprehensive information for teachers and technicians, including full technical notes and step-by-step procedures. Practical Chemistry activities accompany Practical Physics and Practical Biology .
© Nuffield Foundation and the Royal Society of Chemistry
- 14-16 years
- 16-18 years
- Practical experiments
- Demonstrations
- Redox chemistry
- Quantitative chemistry and stoichiometry
Specification
- The reaction of chlorine with cold, dilute, aqueous NaOH and uses of the solution formed.
- 12 iii. the disproportionation reaction of chlorine with cold, dilute aqueous sodium hydroxide to form bleach
- e) explanation of the term disproportionation as oxidation and reduction of the same element, illustrated by: the reaction of chlorine with water as used in water treatment; the reaction of chlorine with cold, dilute aqueous sodium hydroxide, as used to…
Related articles

4 ways to teach redox in terms of electrons
2024-07-03T05:06:00Z By Kristy Turner
Use these teacher-tested approaches to help learners gain a deeper understanding of redox reactions

Mastering titration apparatus
2024-05-07T08:38:00Z By Kristy Turner
Use this poster, fact sheet and classroom activity to show learners the names and uses of equipment they’ll encounter in this practical

Crime-busting chemical analysis
2024-02-26T05:00:00Z By Kit Chapman
From dog detectives to AI, discover the cutting-edge advances in forensic science
No comments yet
Only registered users can comment on this article., more experiments.

‘Gold’ coins on a microscale | 14–16 years
By Dorothy Warren and Sandrine Bouchelkia
Practical experiment where learners produce ‘gold’ coins by electroplating a copper coin with zinc, includes follow-up worksheet

Practical potions microscale | 11–14 years
By Kirsty Patterson
Observe chemical changes in this microscale experiment with a spooky twist.

Antibacterial properties of the halogens | 14–18 years
By Kristy Turner
Use this practical to investigate how solutions of the halogens inhibit the growth of bacteria and which is most effective
- Contributors
- Email alerts
Site powered by Webvision Cloud
- Summer School
- Experiments
- Reset Search
- communication
- Computer Science
- Creative Art Projects
- Creative Arts Projects
- Engineering & Technology
- environment
- lesson plan
- Maths & Stats
- Photography
- Science Communication
Level of Education
- Post Secondary
Recommended Age
Time Required
~10 minutes
- ~20 minutes
- ~30 minutes
- ~45 minutes
- 1 day or more
Number of people
- 100 – 200 €
Supervision
Vanishing Colours
Meta Description
Learning Objectives
To learn about how we perceive colours and why different objects have different colours.
To grasp the concept of how bleach interacts with dyes and pigments.
To realise and understand why bleach affects individual colours differently.
Chlorine bleach
Bleach that has a chloride compounds as the active ingredient.
Chromophore
Part of the dye molecule which absorbs wavelengths in the visible light region and is therefore responsible for the molecule’s colouration.
A dissolved organic substance which gives off a colour.
Hydrogen peroxide
A liquid used for bleaching due to its strong oxidizing properties.
Oxidising bleach/agent
A substance that causes another substance to be oxidised while the agent itself is reduced by gaining electrons or gaining hydrogen atoms.
Oxygen bleach
This is considered to be safer to use than chlorine bleach, however, it is not able to remove colour from certain clothing.
Reducing bleach/agent
A substance that causes another substance to be reduced while the agent itself is oxidised by losing electrons or losing hydrogen atoms.
Sodium hypochlorite
A compound that when dissolved in water forms liquid bleach.
Fill six separate cups with equal amounts of water (about 200 mL each).
Add two drops of yellow food colouring in one of the glass cups and mix the solution. Repeat this procedure with blue and red food colouring in separate cups (total of 3 glass cups).
In the fourth cup, add one drop of red food colouring, and another drop of yellow food colouring. Mix the solution.
In the fifth cup, add one drop of blue food colouring, and another drop of yellow food colouring. Mix the solution.
In the sixth cup, add two drops of yellow food colouring. Mix the solution and mark this as the ‘CONTROL’.
Add three drops of bleach to the coloured water in cups 1-5 and mix the water using a wooden stick. (Do NOT add bleach to the cup marked ‘CONTROL’.)
Observe the colour changes.
Different coloured food colouring can be used. One can make use of different colours of food colouring which would still give a colourless solution on addition of bleach.
The glass cups can easily be replaced by see through plastic cups.
- An adult should handle the bleach. Wear nitrile gloves and avoid direct skin contact as this could result into severe skin burns . Also avoid contact with eyes as it can cause serious eye damage.
- Help keep our environment clean by reducing waste, reusing materials, and recycling whenever possible!
Imagine observing a football field filled with several football from above. You can see that each team within the field is forming a circle to represent the dye structure. All of a sudden it started to rain (representing the bleach) and some of the players forming the circle structure started leaving to seek shelter. As it continued to rain, more teams broke the circle and left the field.
You have obviously seen a christmas tree and undoubtedly you observed that it has colourful christmas lights.
Now, lets imagine that we have our own christmas light made up of the three main colours, blue, red and yellow. However, there is something special with our Christmas light. The thickness of the glass making up the bulbs is different according to the colour. That is, the yellow bulbs are made up of very thin glass, the red bulbs have a slightly thicker glass and the blue bulbs are made up of the thickest glass. We left the Christmas tree with our special light outside. All of a sudden it started to hail, hitting the bulbs. The yellow bulbs, having the thinnest glass broke first, followed by the red coloured bulbs. The Christmas lights left were mainly blue, but with further hail, even the blue bulbs got broken. This left the Christmas tree without any coloured light. The left the Christmas tree without any lights just as the water was left without any colours on addition of bleach.
On addition of bleach, the different coloured solutions take different amount of time to become colourless. Why?
Different dyes have particular molecular structures that react differently with bleach.
What happens if more bleach is added?
The solutions turn colourless in a shorter time.
Why is it that addition of bleach causes fading instead of immediate loss of colour?
The numerous dye structures making up the colour of the solution are broken down gradually and not all at once.
Knowing that brown is made up of red, yellow, and blue which colour do you expect to remain after the addition of bleach?
Colour changes observed are brown to purple to blue.
What would you expect to happen if some bleach droplets end up on your clothes?
White stains would form as the dye of the clothes is broken down causing discoloration.
Bleach is a common household colourless liquid that is an excellent cleaning agent. It is often used to whiten clothing, to remove stains, and as a sanitizer for the bathroom.
There are different types of bleach . The most common type of bleach consists of a solution of sodium hypochlorite which gives it oxidising abilities (It is the hypochlorite anion that give the bleach its oxidising property.) Oxidising bleaches, oxidise the organic dye or pigment, that is providing the solution with colour, by breaking its chemical bonds.
Mainly, a chemical change occurs in the structure of the chromophore of the organic substance, preventing it from absorbing any visible light. This results for the amount of dye molecules producing the colour of the solution to decrease . Consequently, one first observes fading of the colour until eventually, all of the dye structures are broken down, resulting in a completely colourless solution. An important observation is that different colours require different quantities of bleach to lose colour. An indication that different dyes have different structures that react at a different rate with bleach. In fact, an orange solution initially turns into a red colour as all the yellow dye s oxidised first.
Different types of bleach consist of different components . For example, chlorine bleach usually contains sodium hypochlorite, whilst oxygen bleach contains hydrogen peroxide. The majority of the bleach are oxidizing agents, however, bleach can also consist of components with reducing properties instead. In this experiment, one makes use of a chlorine bleach, thus, with oxidizing properties. In such a case, the bleach breaks down the chemical bonds of the chromophore of the dye molecule.
The chromophore is that part of the dye molecule which is responsible for absorbing wavelength in the visible light region, resulting for the molecule to depict colour; but what exactly is colour? The colours we are able to detect by the human eye are part of the visible light. The visible spectrum of light include R ed, O range, Y ellow, G reen, B lue, I ndigo and V iolet or as commonly abbreviated as ROYGBIV. Every colour has a particular wavelength that the chromophore absorbs and the remaining reflected colours are observed. Depending on the structure of the chromophore molecule, some wavelengths (colours) are absorbed more strongly. Example if a substance has a red colour, then it means that red wavelength is reflected (not absorbed) while all the other colours are absorbed.
Red, Yellow and Blue are the three colours that do not change into a different colour on addition of bleach (unlike green, orange and purple). To simplify matters, it can be stated that red, yellow and blue are considered as primary colours, since such colours cannot be created through mixing of other colours. To explore further in this topic read the following article .
An oxidising bleach removes electrons from the chromophore , resulting in an alteration in the chemical structure of the chromophore. As a result, the dye molecule either contains a chromophore that absorbs outside the visible spectrum range or it no longer contains a chromophore. For that reason, the colour is no longer observed.
The colour change observed is not an immediate one but rather a relatively slow process where the solution first fades and then turns colourless. This occurs because the dye molecules are scattered through the volume of water, and bleach does not react with all the dye molecules at once. The intensity of the colour decreases as the amount of molecules that have reacted with bleach increase. Hence, the molecules absorbing light decrease. It is important to understand that this happens not because the molecules themselves are fading.
Finally, the other type of bleach, which was not used in this experiment, are reducing bleaches . Reducing bleach acts by reducing the double bond of the dye molecule to single bonds. Again, the molecules will no longer be able to absorb visible light resulting in a colourless solution.
Application Though bleach might be considered as something hazardous and to be used with caution, it has multiple applications. Bleach is used as a herbicide to effectively kill harmful plants if their growth can interfere with the growth of the desired plants. It can also be used as a pesticide or insect repellent to eradicate undesired pests.
It is also a very common household cleaning agent to disinfect as it is able to kill a wide range of bacteria and other microbes.
Bleach is also useful in industry for paper bleaching . Paper bleaching is the chemical process whereby wood pulp is treated in order to whiten the colour of the pulp. It is most commonly used to produce paper. Previously, chlorine based bleaching was used but nowadays there is a shift to chlorine free bleaching to minimise harm to the environment.
Research Research on mice has discovered that skin damage can be impeded by dilute bleach solution. The research is considering clinical trials in humans so as to provide a safe and inexpensive way to treating wound healing.
- Add more bleach to the orange and the green dye and observe what occurs. Which of the two had a faster rate of fading? Why do you think there is this difference?
- Prepare a violet dye by mixing together one drop of red and one drop of blue food colouring. Add water to the dyes to prepare the violet solution. Add bleach to the solution and swirl whilst observing the colour changes.
- Fill two cups and fill one with cold water and the other one with an equal volume of warm water (add about 200 mL of water). Add three drops of blue food colouring in each and mix to have a blue solution. Now add dropwise equal quantities of bleach to each cup. What is occurring different in the two cups?
Preparation: 3 minutes
Conducting: 5 minutes
Clean Up: 3 minutes
Number of People
1 participant
6 Glass cups
Disposable pipettes
Red, blue and yellow food colouring
Wooden stick
Contributors
What is Bleach and How Does It Work?
Battling Stains with Bleach
What are Chromophores & Auxochromes? – Definitions & Types
Why are red, yellow, and blue the primary colors in painting but computer screens use red, green and blue?
Is is Safe to Drink Bleach?
Additional Content
12 Smart Ways to Use Bleach
Bleach Use in Wastewater Treatment
How Bleach is Made
Kinetics Studies of the Bleaching of Food Dyes
Textile Bleaching
Cite this Experiment
Spiteri, C., Fenech Salerno, B., Sammut, D., & Mercieca, R. (2017, September 29). Vanishing Colours. Retrieved from http://steamexperiments.com/experiment/vanishing-colours/
First published: September 29, 2017 Last modified: October 30, 2017

Similar Experiments
Hydrogen balloon.
Store in a balloon the light (Hydrogen) gas produced from a chemical reaction. This gas will combust in the presence of oxygen, causing a small explosion to occur.
Seven-Layer Density Column WIP
Experiment with the science of density by stacking different liquids in order to make a colourful tower.
Sculpting with milk
A very easy experiment to create hard shapes simply by curdling milk with ethanoic acid.
Extraction of DNA from Strawberries
To study DNA, scientists just like you have to first extract it from the cells. This experiment allows you to extract DNA from strawberries and investigate it.
Leave a Reply Cancel Reply
You must be logged in to post a comment.

COMMENTS
Procedure. Fill a glass or jar about halfway full with water. Add a few drops of food coloring. Stir the liquid to make it colored. Add drops of bleach until the color starts to disappear. You can stir the contents of the glass if you like. Continue until the color is gone. Add a few drops of another color.
Iodine. Bleach. Water. Stickers / Tape or some other way to label the jars. Experiment: Take one cup and label it "Me" (or Us works too). Fill this cup half full of waterTake the second cup and label it "Sin". Mix a ratio of 90% water and 10% iodine. Take the third cup and label it "Jesus".
Learn how much iodine you can apply to the water and still counteract it with bleach, and the opposite as well. It's a great, simple visual to accent your talk on forgiveness. If you do it right, you should be able to make the water dark (sinful) and then clear (forgiven), and back to dark, back to clear several times over in one session.
About Press Copyright Contact us Creators Advertise Developers Terms Privacy Policy & Safety How YouTube works Test new features NFL Sunday Ticket Press Copyright ...
3. Slowly pour liquid bleach into the water jug. The water should become clear again. This illustrates the cleansing power of Jesus. The amount of bleach required will depend on the concentration of the bleach you use, and how much food coloring has been added.
The stronger the bleach mixture in the JESUS bottle the better. You can find iodine in the medical bandages and stuff at your local Walmart / Target / etc... I would use different sized containers like the video. It takes a fair amount of iodine to make a nice black color so a small SIN container works well
Mixing Iodine and Bleach J0603 Objectives/Goals I wanted to know the exact ratio of iodine to bleach to make 100mL of water stay clear. What is the ... if anyone else or I decide to redo this experiment, I recommend that they drop the bleach at a constant speed, not reducing or increasing it to see if the liquid still gets darker. ...
Cut out 2 identical cross shapes from your sticky-backed craft foam. 2. Peel off the paper backing from the craft foam and stick the two sides together on the pole of the straw dispenser. 3. Fill the bottom of the straw dispenser with white vinegar. 4. Fill your straw dispenser about halfway with water and then add a squirt (or 5-6 drops) of ...
Analysis of Bleach. In this experiment we will analyze and compare various laundry bleachesby testing their relative oxidizing powers. We will run two reactions. First we willuse the bleach to oxidize iodide to iodine and then we will titrate the iodine todetermine exactly how much was produced by the bleach. [ 9-12 Content Standard B ...
safe. One uses household bleach and the other uses tincture of iodine. Bleach method: If the water is clear, mix 1/8 teaspoon of unscented, liquid chlorine bleach with one gallon (same size as two-2-liter drink bottles!) of water. If the water is cloudy or colored, mix 1/4 teaspoon of unscented, liquid chlorine bleach with one gallon (same size as
How you do it: Fill one glass about 3/4 full of water. Stir in 1 tsp of baking soda. Add food coloring. Next pour about 1/4 cup of bleach in the colored water. Check this out: What is the science? The oxygen molecules in water (H20) will combine with the oxygen molecules in the bleach (NaClO) causing the food coloring to neutralize and disappear.
The glass marked JESUS should be filled with bleach. Each glass should be filled with the same amount of liquid but should not be filled to the brim. Fill each glass 2/3 full or 3/4 full. The glasses with bleach and water will both look like they contain water. The iodine has a dark, dirty looking color. Begin by talking about the "MAN" glass.
Watch on. I saw on one of the YouTube demonstrations of this illustration the formula: Sin = 80% water, 20% iodine; Christ = 80% water, 20% bleach; You = 100% water. Using these ratios, I tried it and it worked. It is neat demonstration. Would make a great presentation in a bible study, small group, Sunday school etc.
Iodine Clock Reaction. Try an at home version of this experiment using a few things you may have in your bathroom medicine cabinet. In may ways this experiment feels almost like magic. Two colorless liquids are mixed together and after a few moments the mixture turns a dark blue color. There are actually a couple of simple chemical reactions ...
The iodine clock reaction is a favorite demonstration reaction in chemistry classes that usually requires toxic or hazardous chemicals. During the reaction, two clear liquids are mixed, resulting in another clear liquid. After some time, the solution suddenly turns dark blue. The reaction is called a clock reaction because the amount of time ...
The extent of reaction decreases down Group 17. With iodine it is so small that the acidic and bleaching properties of the solution are not seen in this experiment. In the displacement reactions chlorine displaces both bromine and iodine from their compounds and bromine displaces iodine. For example: Cl 2 (aq) + 2KI(aq) → I 2 (aq) + 2KCl(aq)
Here's the full story: Δημήτριος Δεσποτικός. Just in case you want to make your own science experiment, it the Science jar I've put water and a dash of bleach. The christ jar has water and enough iodine to make it reddish/brown. The You cup is only water. Just remember, it's a little bit of chemical reaction, nothing more.
A redox reaction occurs between iodine and thiosulfate ions: 2S2O32- (aq) + I2 (aq) → 2I-(aq) + S4O62- (aq) The light brown/yellow colour of the iodine turns paler as it is converted to colourless iodide ions. When the solution is a straw colour, starch is added to clarify the end point. The solution turns blue/black until all the ...
Prepare a paste by adding about 2 g of soluble starch to 30 mL of deionized water. The starch serves as the indicator for titrations involving iodine. Boil about 1 liter of water in a 1.5- or 2-L beaker and add the paste. Stir the solution while continuing to heat until the solution is completely transparent.
One of the Two-Minute Drills that I organized for my class on Monday evening involved a demonstration using water, food coloring, and bleach. You fill a clear glass with water (about half full), add a drop or two of food color to represent sin, stir, and then add bleach to make the color disappear, representing the effect of God's grace. Many catechists and Sunday school teachers use this ...
Potato. Cooked chicken. Place a small piece of each of the foods to be tested on the plastic sheet. Place a small potassium iodide crystal on top of the piece of food. Add one drop of bleach solution (sodium hypochlorite solution) and allow it to run over both crystal and food. If an intense blue-black colour is seen, the food contains starch.
Use a plastic syringe to measure out 5 cm 3 of the first bleach into the flask. If a 'thick' bleach is used, add approximately 25 cm 3 deionised water. Swirl to ensure complete mixing. Discuss the fact that this dilution does not change the amount of bleach put into the flask, but does enable proper mixing to take place and ensure the reaction has goes to completion.
For example, chlorine bleach usually contains sodium hypochlorite, whilst oxygen bleach contains hydrogen peroxide. The majority of the bleach are oxidizing agents, however, bleach can also consist of components with reducing properties instead. In this experiment, one makes use of a chlorine bleach, thus, with oxidizing properties.