Log in using your username and password
- Search More Search for this keyword Advanced search
- Latest content
- Global health
- BMJ Journals More You are viewing from: Google Indexer

You are here
- Volume 13, Issue 8
- Clinical course of a 66-year-old man with an acute ischaemic stroke in the setting of a COVID-19 infection
- Article Text
- Article info
- Citation Tools
- Rapid Responses
- Article metrics
- http://orcid.org/0000-0002-7441-6952 Saajan Basi 1 , 2 ,
- Mohammad Hamdan 1 and
- Shuja Punekar 1
- 1 Department of Stroke and Acute Medicine , King's Mill Hospital , Sutton-in-Ashfield , UK
- 2 Department of Acute Medicine , University Hospitals of Derby and Burton , Derby , UK
- Correspondence to Dr Saajan Basi; saajan.basi{at}nhs.net
A 66-year-old man was admitted to hospital with a right frontal cerebral infarct producing left-sided weakness and a deterioration in his speech pattern. The cerebral infarct was confirmed with CT imaging. The only evidence of respiratory symptoms on admission was a 2 L oxygen requirement, maintaining oxygen saturations between 88% and 92%. In a matter of hours this patient developed a greater oxygen requirement, alongside reduced levels of consciousness. A positive COVID-19 throat swab, in addition to bilateral pneumonia on chest X-ray and lymphopaenia in his blood tests, confirmed a diagnosis of COVID-19 pneumonia. A proactive decision was made involving the patients’ family, ward and intensive care healthcare staff, to not escalate care above a ward-based ceiling of care. The patient died 5 days following admission under the palliative care provided by the medical team.
- respiratory medicine
- infectious diseases
- global health
This is an open access article distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/ .
https://doi.org/10.1136/bcr-2020-235920
Statistics from Altmetric.com
Request permissions.
If you wish to reuse any or all of this article please use the link below which will take you to the Copyright Clearance Center’s RightsLink service. You will be able to get a quick price and instant permission to reuse the content in many different ways.
SARS-CoV-2 (Severe Acute Respiratory Syndrome Coronavirus 2) is a new strain of coronavirus that is thought to have originated in December 2019 in Wuhan, China. In a matter of months, it has erupted from non-existence to perhaps the greatest challenge to healthcare in modern times, grinding most societies globally to a sudden halt. Consequently, the study and research into SARS-CoV-2 is invaluable. Although coronaviruses are common, SARS-CoV-2 appears to be considerably more contagious. The WHO figures into the 2003 SARS-CoV-1 outbreak, from November 2002 to July 2003, indicate a total of 8439 confirmed cases globally. 1 In comparison, during a period of 4 months from December 2019 to July 2020, the number of global cases of COVID-19 reached 10 357 662, increasing exponentially, illustrating how much more contagious SARS-CoV-2 has been. 2
Previous literature has indicated infections, and influenza-like illness have been associated with an overall increase in the odds of stroke development. 3 There appears to be a growing correlation between COVID-19 positive patients presenting to hospital with ischaemic stroke; however, studies investigating this are in progress, with new data emerging daily. This patient report comments on and further characterises the link between COVID-19 pneumonia and the development of ischaemic stroke. At the time of this patients’ admission, there were 95 positive cases from 604 COVID-19 tests conducted in the local community, with a predicted population of 108 000. 4 Only 4 days later, when this patient died, the figure increased to 172 positive cases (81% increase), illustrating the rapid escalation towards the peak of the pandemic, and widespread transmission within the local community ( figure 1 ). As more cases of ischaemic stroke in COVID-19 pneumonia patients arise, the recognition and understanding of its presentation and aetiology can be deciphered. Considering the virulence of SARS-CoV-2 it is crucial as a global healthcare community, we develop this understanding, in order to intervene and reduce significant morbidity and mortality in stroke patients.
- Download figure
- Open in new tab
- Download powerpoint
A graph showing the number of patients with COVID-19 in the hospital and in the community over time.
Case presentation
A 66-year-old man presented to the hospital with signs of left-sided weakness. The patient had a background of chronic obstructive pulmonary disease (COPD), atrial fibrillation and had one previous ischaemic stroke, producing left-sided haemiparesis, which had completely resolved. He was a non-smoker and lived in a house. The patient was found slumped over on the sofa at home on 1 April 2020, by a relative at approximately 01:00, having been seen to have no acute medical illness at 22:00. The patients’ relative initially described disorientation and agitation with weakness noted in the left upper limb and dysarthria. At the time of presentation, neither the patient nor his relative identified any history of fever, cough, shortness of breath, loss of taste, smell or any other symptoms; however, the patient did have a prior admission 9 days earlier with shortness of breath.
The vague nature of symptoms, entwined with considerable concern over approaching the hospital, due to the risk of contracting COVID-19, created a delay in the patients’ attendance to the accident and emergency department. His primary survey conducted at 09:20 on 1 April 2020 demonstrated a patent airway, with spontaneous breathing and good perfusion. His Glasgow Coma Scale (GCS) score was 15 (a score of 15 is the highest level of consciousness), his blood glucose was 7.2, and he did not exhibit any signs of trauma. His abbreviated mental test score was 7 out of 10, indicating a degree of altered cognition. An ECG demonstrated atrial fibrillation with a normal heart rate. His admission weight measured 107 kg. At 09:57 the patient required 2 L of nasal cannula oxygen to maintain his oxygen saturations between 88% and 92%. He started to develop agitation associated with an increased respiratory rate at 36 breaths per minute. On auscultation of his chest, he demonstrated widespread coarse crepitation and bilateral wheeze. Throughout he was haemodynamically stable, with a systolic blood pressure between 143 mm Hg and 144 mm Hg and heart rate between 86 beats/min and 95 beats/min. From a neurological standpoint, he had a mild left facial droop, 2/5 power in both lower limbs, 2/5 power in his left upper limb and 5/5 power in his right upper limb. Tone in his left upper limb had increased. This patient was suspected of having COVID-19 pneumonia alongside an ischaemic stroke.
Investigations
A CT of his brain conducted at 11:38 on 1 April 2020 ( figure 2 ) illustrated an ill-defined hypodensity in the right frontal lobe medially, with sulcal effacement and loss of grey-white matter. This was highly likely to represent acute anterior cerebral artery territory infarction. Furthermore an oval low-density area in the right cerebellar hemisphere, that was also suspicious of an acute infarction. These vascular territories did not entirely correlate with his clinical picture, as limb weakness is not as prominent in anterior cerebral artery territory ischaemia. Therefore this left-sided weakness may have been an amalgamation of residual weakness from his previous stroke, in addition to his acute cerebral infarction. An erect AP chest X-ray with portable equipment ( figure 3 ) conducted on the same day demonstrated patchy peripheral consolidation bilaterally, with no evidence of significant pleural effusion. The pattern of lung involvement raised suspicion of COVID-19 infection, which at this stage was thought to have provoked the acute cerebral infarct. Clinically significant blood results from 1 April 2020 demonstrated a raised C-reactive protein (CRP) at 215 mg/L (normal 0–5 mg/L) and lymphopaenia at 0.5×10 9 (normal 1×10 9 to 3×10 9 ). Other routine blood results are provided in table 1 .
CT imaging of this patients’ brain demonstrating a wedge-shaped infarction of the anterior cerebral artery territory.
Chest X-ray demonstrating the bilateral COVID-19 pneumonia of this patient on admission.
- View inline
Clinical biochemistry and haematology blood results of the patient
Interestingly the patient, in this case, was clinically assessed in the accident and emergency department on 23 March 2020, 9 days prior to admission, with symptoms of shortness of breath. His blood results from this day showed a CRP of 22 mg/L and a greater lymphopaenia at 0.3×10 9 . He had a chest X-ray ( figure 4 ), which indicated mild radiopacification in the left mid zone. He was initially treated with intravenous co-amoxiclav and ciprofloxacin. The following day he had minimal symptoms (CURB 65 score 1 for being over 65 years). Given improving blood results (declining CRP), he was discharged home with a course of oral amoxicillin and clarithromycin. As national governmental restrictions due to COVID-19 had not been formally announced until 23 March 2020, and inconsistencies regarding personal protective equipment training and usage existed during the earlier stages of this rapidly evolving pandemic, it is possible that this patient contracted COVID-19 within the local community, or during his prior hospital admission. It could be argued that the patient had early COVID-19 signs and symptoms, having presented with shortness of breath, lymphopaenia, and having had subtle infective chest X-ray changes. The patient explained he developed a stagnant productive cough, which began 5 days prior to his attendance to hospital on 23 March 2020. He responded to antibiotics, making a full recovery following 7 days of treatment. This information does not assimilate with the typical features of a COVID-19 infection. A diagnosis of community-acquired pneumonia or infective exacerbation of COPD seem more likely. However, given the high incidence of COVID-19 infections during this patients’ illness, an exposure and early COVID-19 illness, prior to the 23 March 2020, cannot be completely ruled out.
Chest X-ray conducted on prior admission illustrating mild radiopacification in the left mid zone.
On the current admission, this patient was managed with nasal cannula oxygen at 2 L. By the end of the day, this had progressed to a venturi mask, requiring 8 L of oxygen to maintain oxygen saturation. He had also become increasingly drowsy and confused, his GCS declined from 15 to 12. However, the patient was still haemodynamically stable, as he had been in the morning. An arterial blood gas demonstrated a respiratory alkalosis (pH 7.55, pCO 2 3.1, pO 2 6.7 and HCO 3 24.9, lactate 1.8, base excess 0.5). He was commenced on intravenous co-amoxiclav and ciprofloxacin, to treat a potential exacerbation of COPD. This patient had a COVID-19 throat swab on 1 April 2020. Before the result of this swab, an early discussion was held with the intensive care unit staff, who decided at 17:00 on 1 April 2020 that given the patients presentation, rapid deterioration, comorbidities and likely COVID-19 diagnosis he would not be for escalation to the intensive care unit, and if he were to deteriorate further the end of life pathway would be most appropriate. The discussion was reiterated to the patients’ family, who were in agreement with this. Although he had evidence of an ischaemic stroke on CT of his brain, it was agreed by all clinicians that intervention for this was not as much of a priority as providing optimal palliative care, therefore, a minimally invasive method of treatment was advocated by the stroke team. The patient was given 300 mg of aspirin and was not a candidate for fibrinolysis.
Outcome and follow-up
The following day, before the throat swab result, had appeared the patient deteriorated further, requiring 15 L of oxygen through a non-rebreather face mask at 60% FiO 2 to maintain his oxygen saturation, at a maximum of 88% overnight. At this point, he was unresponsive to voice, with a GCS of 5. Although, he was still haemodynamically stable, with a blood pressure of 126/74 mm Hg and a heart rate of 98 beats/min. His respiratory rate was 30 breaths/min. His worsening respiratory condition, combined with his declining level of consciousness made it impossible to clinically assess progression of the neurological deficit generated by his cerebral infarction. Moreover, the patient was declining sharply while receiving the maximal ward-based treatment available. The senior respiratory physician overseeing the patients’ care decided that a palliative approach was in this his best interest, which was agreed on by all parties. The respiratory team completed the ‘recognising dying’ documentation, which signified that priorities of care had shifted from curative treatment to palliative care. Although the palliative team was not formally involved in the care of the patient, the patient received comfort measures without further attempts at supporting oxygenation, or conduction of regular clinical observations. The COVID-19 throat swab confirmed a positive result on 2 April 2020. The patient was treated by the medical team under jurisdiction of the hospital palliative care team. This included the prescribing of anticipatory medications and a syringe driver, which was established on 3 April 2020. His antibiotic treatment, non-essential medication and intravenous fluid treatment were discontinued. His comatose condition persisted throughout the admission. Once the patients’ GCS was 5, it did not improve. The patient was pronounced dead by doctors at 08:40 on 5 April 2020.
SARS-CoV-2 is a type of coronavirus that was first reported to have caused pneumonia-like infection in humans on 3 December 2019. 5 As a group, coronaviruses are a common cause of upper and lower respiratory tract infections (especially in children) and have been researched extensively since they were first characterised in the 1960s. 6 To date, there are seven coronaviruses that are known to cause infection in humans, including SARS-CoV-1, the first known zoonotic coronavirus outbreak in November 2002. 7 Coronavirus infections pass through communities during the winter months, causing small outbreaks in local communities, that do not cause significant mortality or morbidity.
SARS-CoV-2 strain of coronavirus is classed as a zoonotic coronavirus, meaning the virus pathogen is transmitted from non-humans to cause disease in humans. However the rapid spread of SARS-CoV-2 indicates human to human transmission is present. From previous research on the transmission of coronaviruses and that of SARS-CoV-2 it can be inferred that SARS-CoV-2 spreads via respiratory droplets, either from direct inhalation, or indirectly touching surfaces with the virus and exposing the eyes, nose or mouth. 8 Common signs and symptoms of the COVID-19 infection identified in patients include high fevers, severe fatigue, dry cough, acute breathing difficulties, bilateral pneumonia on radiological imaging and lymphopaenia. 9 Most of these features were identified in this case study. The significance of COVID-19 is illustrated by the speed of its global spread and the potential to cause severe clinical presentations, which as of April 2020 can only be treated symptomatically. In Italy, as of mid-March 2020, it was reported that 12% of the entire COVID-19 positive population and 16% of all hospitalised patients had an admission to the intensive care unit. 10
The patient, in this case, illustrates the clinical relevance of understanding COVID-19, as he presented with an ischaemic stroke underlined by minimal respiratory symptoms, which progressed expeditiously, resulting in acute respiratory distress syndrome and subsequent death.
Our case is an example of a new and ever-evolving clinical correlation, between patients who present with a radiological confirmed ischaemic stroke and severe COVID-19 pneumonia. As of April 2020, no comprehensive data of the relationship between ischaemic stroke and COVID-19 has been published, however early retrospective case series from three hospitals in Wuhan, China have indicated that up to 36% of COVID-19 patients had neurological manifestations, including stroke. 11 These studies have not yet undergone peer review, but they tell us a great deal about the relationship between COVID-19 and ischaemic stroke, and have been used to influence the American Heart Associations ‘Temporary Emergency Guidance to US Stroke Centres During the COVID-19 Pandemic’. 12
The relationship between similar coronaviruses and other viruses, such as influenza in the development of ischaemic stroke has previously been researched and provide a basis for further investigation, into the prominence of COVID-19 and its relation to ischaemic stroke. 3 Studies of SARS-CoV-2 indicate its receptor-binding region for entry into the host cell is the same as ACE2, which is present on endothelial cells throughout the body. It may be the case that SARS-CoV-2 alters the conventional ability of ACE2 to protect endothelial function in blood vessels, promoting atherosclerotic plaque displacement by producing an inflammatory response, thus increasing the risk of ischaemic stroke development. 13
Other hypothesised reasons for stroke development in COVID-19 patients are the development of hypercoagulability, as a result of critical illness or new onset of arrhythmias, caused by severe infection. Some case studies in Wuhan described immense inflammatory responses to COVID-19, including elevated acute phase reactants, such as CRP and D-dimer. Raised D-dimers are a non-specific marker of a prothrombotic state and have been associated with greater morbidity and mortality relating to stroke and other neurological features. 14
Arrhythmias such as atrial fibrillation had been identified in 17% of 138 COVID-19 patients, in a study conducted in Wuhan, China. 15 In this report, the patient was known to have atrial fibrillation and was treated with rivaroxaban. The acute inflammatory state COVID-19 is known to produce had the potential to create a prothrombotic environment, culminating in an ischaemic stroke.
Some early case studies produced in Wuhan describe patients in the sixth decade of life that had not been previously noted to have antiphospholipid antibodies, contain the antibodies in blood results. They are antibodies signify antiphospholipid syndrome; a prothrombotic condition. 16 This raises the hypothesis concerning the ability of COVID-19 to evoke the creation of these antibodies and potentiate thrombotic events, such as ischaemic stroke.
No peer-reviewed studies on the effects of COVID-19 and mechanism of stroke are published as of April 2020; therefore, it is difficult to evidence a specific reason as to why COVID-19 patients are developing neurological signs. It is suspected that a mixture of the factors mentioned above influence the development of ischaemic stroke.
If we delve further into this patients’ comorbid state exclusive to COVID-19 infection, it can be argued that this patient was already at a relatively higher risk of stroke development compared with the general population. The fact this patient had previously had an ischaemic stroke illustrates a prior susceptibility. This patient had a known background of hypertension and atrial fibrillation, which as mentioned previously, can influence blood clot or plaque propagation in the development of an acute ischaemic event. 15 Although the patient was prescribed rivaroxaban as an anticoagulant, true consistent compliance to rivaroxaban or other medications such as amlodipine, clopidogrel, candesartan and atorvastatin cannot be confirmed; all of which can contribute to the reduction of influential factors in the development of ischaemic stroke. Furthermore, the fear of contracting COVID-19, in addition to his vague symptoms, unlike his prior ischaemic stroke, which demonstrated dense left-sided haemiparesis, led to a delay in presentation to hospital. This made treatment options like fibrinolysis unachievable, although it can be argued that if he was already infected with COVID-19, he would have still developed life-threatening COVID-19 pneumonia, regardless of whether he underwent fibrinolysis. It is therefore important to consider that if this patient did not contract COVID-19 pneumonia, he still had many risk factors that made him prone to ischaemic stroke formation. Thus, we must consider whether similar patients would suffer from ischaemic stroke, regardless of COVID-19 infection and whether COVID-19 impacts on the severity of the stroke as an entity.
Having said this, the management of these patients is dependent on the likelihood of a positive outcome from the COVID-19 infection. Establishing the ceiling of care is crucial, as it prevents incredibly unwell or unfit patients’ from going through futile treatments, ensuring respect and dignity in death, if this is the likely outcome. It also allows for the provision of limited or intensive resources, such as intensive care beds or endotracheal intubation during the COVID-19 pandemic, to those who are assessed by the multidisciplinary team to benefit the most from their use. The way to establish this ceiling of care is through an early multidisciplinary discussion. In this case, the patient did not convey his wishes regarding his care to the medical team or his family; therefore it was decided among intensive care specialists, respiratory physicians, stroke physicians and the patients’ relatives. The patient was discussed with the intensive care team, who decided that as the patient sustained two acute life-threatening illnesses simultaneously and had rapidly deteriorated, ward-based care with a view to palliate if the further deterioration was in the patients’ best interests. These decisions were not easy to make, especially as it was on the first day of presentation. This decision was made in the context of the patients’ comorbidities, including COPD, the patients’ age, and the availability of intensive care beds during the steep rise in intensive care admissions, in the midst of the COVID-19 pandemic ( figure 1 ). Furthermore, the patients’ rapid and permanent decline in GCS, entwined with the severe stroke on CT imaging of the brain made it more unlikely that significant and permanent recovery could be achieved from mechanical intubation, especially as the damage caused by the stroke could not be significantly reversed. As hospitals manage patients with COVID-19 in many parts of the world, there may be tension between the need to provide higher levels of care for an individual patient and the need to preserve finite resources to maximise the benefits for most patients. This patient presented during a steep rise in intensive care admissions, which may have influenced the early decision not to treat the patient in an intensive care setting. Retrospective studies from Wuhan investigating mortality in patients with multiple organ failure, in the setting of COVID-19, requiring intubation have demonstrated mortality can be up to 61.5%. 17 The mortality risk is even higher in those over 65 years of age with respiratory comorbidities, indicating why this patient was unlikely to survive an admission to the intensive care unit. 18
Regularly updating the patients’ family ensured cooperation, empathy and sympathy. The patients’ stroke was not seen as a priority given the severity of his COVID-19 pneumonia, therefore the least invasive, but most appropriate treatment was provided for his stroke. The British Association of Stroke Physicians advocate this approach and also request the notification to their organisation of COVID-19-related stroke cases, in the UK. 19
Learning points
SARS-CoV-2 (Severe Acute Respiratory Syndrome Coronavirus 2) is one of seven known coronaviruses that commonly cause upper and lower respiratory tract infections. It is the cause of the 2019–2020 global coronavirus pandemic.
The significance of COVID-19 is illustrated by the rapid speed of its spread globally and the potential to cause severe clinical presentations, such as ischaemic stroke.
Early retrospective data has indicated that up to 36% of COVID-19 patients had neurological manifestations, including stroke.
Potential mechanisms behind stroke in COVID-19 patients include a plethora of hypercoagulability secondary to critical illness and systemic inflammation, the development of arrhythmia, alteration to the vascular endothelium resulting in atherosclerotic plaque displacement and dehydration.
It is vital that effective, open communication between the multidisciplinary team, patient and patients relatives is conducted early in order to firmly establish the most appropriate ceiling of care for the patient.
- Cannine M , et al
- Wunderink RG
- van Doremalen N ,
- Bushmaker T ,
- Morris DH , et al
- Wang X-G , et al
- Grasselli G ,
- Pesenti A ,
- Wang M , et al
- American Stroke Assocation, 2020
- Zhang Y-H ,
- Zhang Y-huan ,
- Dong X-F , et al
- Li X , et al
- Hu C , et al
- Zhang S , et al
- Jiang B , et al
- Xu J , et al
- British Association of Stroke Physicians
Contributors SB was involved in the collecting of information for the case, the initial written draft of the case and researching existing data on acute stroke and COVID-19. He also edited drafts of the report. MH was involved in reviewing and editing drafts of the report and contributing new data. SP oversaw the conduction of the project and contributed addition research papers.
Funding The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests None declared.
Patient consent for publication Next of kin consent obtained.
Provenance and peer review Not commissioned; externally peer reviewed.
Read the full text or download the PDF:
We have a new app!
Take the Access library with you wherever you go—easy access to books, videos, images, podcasts, personalized features, and more.
Download the Access App here: iOS and Android . Learn more here!
- Remote Access
- Save figures into PowerPoint
- Download tables as PDFs

Chapter 7: 10 Real Cases on Transient Ischemic Attack and Stroke: Diagnosis, Management, and Follow-Up
Jeirym Miranda; Fareeha S. Alavi; Muhammad Saad
- Download Chapter PDF
Disclaimer: These citations have been automatically generated based on the information we have and it may not be 100% accurate. Please consult the latest official manual style if you have any questions regarding the format accuracy.
Download citation file:
- Search Book
Jump to a Section
Case review, case discussion, clinical symptoms.
- Radiologic Findings
- Full Chapter
- Supplementary Content
Case 1: Management of Acute Thrombotic Cerebrovascular Accident Post Recombinant Tissue Plasminogen Activator Therapy
A 59-year-old Hispanic man presented with right upper and lower extremity weakness, associated with facial drop and slurred speech starting 2 hours before the presentation. He denied visual disturbance, headache, chest pain, palpitations, dyspnea, dysphagia, fever, dizziness, loss of consciousness, bowel or urinary incontinence, or trauma. His medical history was significant for uncontrolled type 2 diabetes mellitus, hypertension, hyperlipidemia, and benign prostatic hypertrophy. Social history included cigarette smoking (1 pack per day for 20 years) and alcohol intake of 3 to 4 beers daily. Family history was not significant, and he did not remember his medications. In the emergency department, his vital signs were stable. His physical examination was remarkable for right-sided facial droop, dysarthria, and right-sided hemiplegia. The rest of the examination findings were insignificant. His National Institutes of Health Stroke Scale (NIHSS) score was calculated as 7. Initial CT angiogram of head and neck reported no acute intracranial findings. The neurology team was consulted, and intravenous recombinant tissue plasminogen activator (t-PA) was administered along with high-intensity statin therapy. The patient was admitted to the intensive care unit where his hemodynamics were monitored for 24 hours and later transferred to the telemetry unit. MRI of the head revealed an acute 1.7-cm infarct of the left periventricular white matter and posterior left basal ganglia. How would you manage this case?
This case scenario presents a patient with acute ischemic cerebrovascular accident (CVA) requiring intravenous t-PA. Diagnosis was based on clinical neurologic symptoms and an NIHSS score of 7 and was later confirmed by neuroimaging. He had multiple comorbidities, including hypertension, diabetes, dyslipidemia, and smoking history, which put him at a higher risk for developing cardiovascular disease. Because his symptoms started within 4.5 hours of presentation, he was deemed to be a candidate for thrombolytics. The eligibility time line is estimated either by self-report or last witness of baseline status.
Ischemic strokes are caused by an obstruction of a blood vessel, which irrigates the brain mainly secondary to the development of atherosclerotic changes, leading to cerebral thrombosis and embolism. Diagnosis is made based on presenting symptoms and CT/MRI of the head, and the treatment is focused on cerebral reperfusion based on eligibility criteria and timing of presentation.
Symptoms include alteration of sensorium, numbness, decreased motor strength, facial drop, dysarthria, ataxia, visual disturbance, dizziness, and headache.
Sign in or create a free Access profile below to access even more exclusive content.
With an Access profile, you can save and manage favorites from your personal dashboard, complete case quizzes, review Q&A, and take these feature on the go with our Access app.
Pop-up div Successfully Displayed
This div only appears when the trigger link is hovered over. Otherwise it is hidden from view.
Please Wait
- Open access
- Published: 06 September 2022
Stroke in young adults, stroke types and risk factors: a case control study
- Priscilla Namaganda 1 ,
- Jane Nakibuuka 2 ,
- Mark Kaddumukasa 3 &
- Elly Katabira 4
BMC Neurology volume 22 , Article number: 335 ( 2022 ) Cite this article
10k Accesses
13 Citations
Metrics details
Stroke is the second leading cause of death above the age of 60 years, and the fifth leading cause in people aged 15 to 59 years old as reported by the World Health Organization global burden of diseases. Stroke in the young is particularly tragic because of the potential to create long-term disability, burden on the victims, their families, and the community at large. Despite this, there is limited data on stroke in young adults, and its risk factors in Uganda. Therefore, we determined the frequency and risk factors for stroke among young adults at Mulago hospital.
A case control study was conducted among patients presenting consecutively to the general medical wards with stroke during the study period September 2015 to March 2016. A brain Computerized Tomography scan was performed to confirm stroke and classify the stroke subtype. Controls were patients that presented to the surgical outpatient clinic with minor surgical conditions, matched for age and sex. Social demographic, clinical and laboratory characteristics were assessed for both cases and controls. Descriptive statistics including frequencies, percentages, means, and standard deviation were used to describe the social demographics of case and controls as well as the stroke types for cases. To determine risk factors for stroke, a conditional logistic regression, which accounts for matching (e.g., age and sex), was applied. Odds ratio (with 95% confidence interval) was used as a measure for associations.
Among 51 patients with stroke, 39(76.5%) had ischemic stroke and 12(23.5%) had hemorrhagic stroke. The mean age was 36.8 years (SD 7.4) for stroke patients (cases) and 36.8 years (SD 6.9) for controls. Female patients predominated in both groups 56.9% in cases and 52.9% in controls. Risk factors noted were HIV infection, OR 3.57 (95% CI 1.16–10.96), elevated waist to hip ratio, OR 11.59(95% CI 1.98–68.24) and sickle cell disease, OR 4.68 (95% CI 1.11–19.70). This study found a protective effect of oral contraceptive use for stroke OR 0.27 95% CI 0.08–0.87. There was no association between stroke and hypertension, diabetes, and hyperlipidemia.
Among young adults with stroke, ischemic stroke predominated over hemorrhagic stroke. Risk factors for stroke were HIV infection, elevated waist to hip ratio and sickle cell disease.
Peer Review reports
Stroke is the second leading cause of death above the age of 60 years, and the fifth leading cause in people aged 15 to 59 years old as reported by the World Health Organization (WHO) global burden of diseases [ 1 ]. The severity of stroke in the young is relatively low in developed countries ranging from 2 -7% in Italy and USA respectively [ 2 , 3 ]. In Africa, on the other hand the prevalence of stroke among young adults is 12.9% in Nigeria [ 4 ], 31% in South Africa [ 5 ], 28.9% in Morocco [ 6 ]. The incidence of ischemic stroke in the young has been increasing globally over the last 2–3 decades. From the Danish National Patient Register, the incidence rates of first‐time hospitalizations for ischemic stroke and transient ischemic attack (TIA) in young adults have increased substantially since the mid 1990s while the incidences of hospitalizations for intracerebral hemorrhage and subarachnoid hemorrhage remained stable during the study period [ 7 ].
In Uganda, literature on stroke in young adults is limited however results of a study done among acute stroke patients admitted to the national referral hospital (Mulago hospital) showed a 30-day mortality of 43.8%. Out of 133 patients, 32 patients (25%) were less than 51 years old. Out of the 56 patients that died, 13 patients (23%) were less than 51 years [ 8 ].
Rapid western cultural adaption (sedentary lifestyle, deleterious health behavior like consumption of tobacco and alcohol and high fat/cholesterol diet) and Human immunodeficiency syndrome/ Acquired immunodeficiency syndrome (HIV/AIDS) that is highly prevalent in Africa has accelerated risk factors and increased the burden of stroke [ 9 ].
Most literature indicates that the traditional risk factors i.e., hypertension, diabetes mellitus and dyslipidemia are still the commonest risk factors with hypertension having the highest frequency. Other risk factors common to the young include smoking, excessive alcohol intake, illicit drug use, oral contraceptive use and migraine [ 10 ].
Although stroke is predominantly a disease of the middle age and the elderly, its occurrence in younger age groups is not rare. Stroke in young adults seems to be increasing and is particularly tragic because of the potential to create long-term disability, burden on the victims, their families, and the community at large such as Uganda. Despite the huge socioeconomic impact of stroke in this age group, there is a scarcity of data regarding stroke in young adults in sub-Saharan Africa including Uganda. Effective stroke prevention strategies in the young require comprehensive information on risk factors and possible causes. Although case reports and etiologic investigations of possible causes of stroke in the young have been identified especially in developed countries, there is limited data on risk factors in Africa Uganda inclusive. Information obtained from this study will fill the knowledge gap in this area of stroke in the young which will inform institutional strategies on prevention and management of stroke in this age group. This study, therefore, seeks to determine the frequency of stroke types and risk factors for this population.
The aims of the study were:
To determine the frequency of stroke types among young adults on the general medical wards in Mulago hospital between September 2015 and March 2016.
To determine the risk factors for stroke (i.e., ischemic, and hemorrhagic stroke) among young adults on the general medical wards in Mulago hospital between September and March 2016.
This was a case control study. Cases were defined as patients with a confirmed diagnosis of stroke by brain computerized tomography (CT) scan that met the inclusion criteria. Controls were defined as patients with minor surgical conditions that met the inclusion criteria. The study was carried out in Mulago hospital which is the national referral hospital in Uganda as well as the teaching hospital of Makerere University College of health sciences. It has a bed capacity of 1500 beds and has both inpatient wards, outpatient departments both for medical and surgical specialties. It has a radiological department with CT scan and highly trained personnel and a well-equipped laboratory. Cases were recruited consecutively from the medical wards specifically on the neurology ward of Mulago hospital. Patients on the neurology ward are managed by physicians that have had additional training in the management of neurological conditions.
Controls were recruited from general surgical outpatient departments from Mulago hospital. They were matched for age and sex. Eligible patients were patients aged 15–45 years, confirmed diagnosis of stroke on brain CT scan and with a written informed consent or assent for patients less than 18 years. These included patients with intracranial hemorrhages and ischemic stroke, none had subarachnoid hemorrhage. Patients were excluded if they were unconscious and with no valid surrogate (next of kin) and HIV positive with opportunistic infections. Patients eligible as control were, patient aged 15–45 years, minor surgical condition, written informed consent or assent for patients less than 18 years. Patients with features of stroke secondary to non-vascular causes like trauma, tumors were excluded as controls. For controls, we chose patients with minor surgical conditions because we wanted controls to be hospital patients but with non-medical conditions that could confound our findings. Such conditions included lacerations, hernias, lipomas, ingrown toenails, circumcision.
Based on the catchment area of Mulago, patients with minor surgical conditions are likely to have similar social economic status and come from similar neighborhoods as would health controls living in the catchment areas as patients with stroke.
The best alternative would have been healthy controls from the neighborhoods of the patients with stroke, but this would have been resource consuming.
The sample size was calculated assuming a prevalence of 62.2% of hypertension among the stroke patients as was indicated in a similar study among the young Thai adults in Bangkok, Thailand (Bandasak et al., 2011) [ 11 ]. We also assumed that the risk for stroke is higher among the hypertensive with an OR of 3. With this sample size, we were powered to detect associations with other risk factors like smoking (OR 2.6) [ 12 ], diabetes (OR 13.2 for black men and 22.1 for black women) [ 13 ].
With these assumptions, a sample size of 51 cases and 51 controls was found sufficient with 80% power and 0.05 level of significance.
Sampling procedure
All young patients admitted on the general medical wards suspected of having stroke were screened and brain CT scan done. Once a diagnosis of stroke was confirmed on CT scan, participants who consented to participate in the study were recruited consecutively, a standardized questionnaire administered by the research team for those patients able to communicate. For patients not able to communicate, consent and information were obtained through the care givers. Controls were selected from the general surgical outpatient clinic using consecutive sampling method. This was done after we had obtained all the cases. These were matched for age and sex until the sample size was accrued.
Information was collected on:
Social demographic characteristics i.e., age, sex, level of education, occupation, religion, history of smoking and alcohol consumption, history of illicit drug use, history of oral contraceptive use.
Clinical examination included general physical examination, blood pressure using a digital blood pressure machine. For patients who were too weak to sit up, blood pressure measurement was taken in supine position. For those able to sit, it was taken in the sitting position. The two blood pressure measurements were taken at an interval of 5 min and the average blood pressure recorded as the final blood pressure.
Physical measurements for the weight and hip were taken using a stretchable tape measure. Waist measurements were taken at the narrowest point-umbilicus and hip measurements at the widest point- buttocks. A waist to hip ratio was obtained and recorded on the questionnaire.
Blood was drawn for laboratory tests; high density lipoprotein, low density lipoprotein (HDL/LDL), fasting blood sugar, full blood count, Hb electrophoresis, prothrombin time/ international normalization ratio (PT/INR), HIV serology, Treponema pallidum hemagglutination (TPHA).
Other information obtained was history and family history of diabetes and hypertension.
The general surgical outpatient clinic runs every Tuesday, and Thursday in Old Mulago hospital Participants were identified at the surgical outpatient clinic. Those matching the age and sex of the cases were recruited, written consent/assent obtained, and questionnaire was administered by the PI. The procedure as explained above was followed for the controls.
Data collection
A pre-tested and standardized questionnaire was used as a data collection tool. The principal investigator administered the questionnaire to the participants in data collection. Data on socio demographics and past medical history was collected.
Results from imaging and laboratory investigations were also recorded into the questionnaire.
Data collected was double entered into the computer using EPI-DATA (version 3.1) software to minimize data entry errors. Data was then backed up and archived in both soft and hard copy to avoid losses. Confidentiality was ensured using code numbers instead of patients’ names. Questionnaires were stored in a lockable cabinet for safety.
Data analysis
Data was analyzed using STATA Version 12 (StataCorp. 2011. Stata Statistical Software: Release 12 . College Station, TX: StataCorp LP). Descriptive statistics were used to describe characteristics of the study participants and the stroke subtypes which included frequencies, percentages, means and standard deviation. To determine factors associated with stroke, a conditional logistic regression, which accounts for matching (e.g., age and sex), was applied. Odds ratio (with 95% confidence interval) was used as a measure for associations. Factors with p -values < 0.2 at a bi-variable analysis were entered into a multiple conditional logistic regression to obtain the adjusted estimates. Factors whose 95% confidence interval for the odds ratio that excludes a 1 or whose p -value < 0.05, were considered statistically significant at the adjusted level. Post-hoc power calculation was performed for the adjusted analysis to check if there was enough power to detect a difference between cases and controls.
Quality control
To ensure quality of results several measures were undertaken, these included:
The questionnaires were pre-tested and standardized before study commenced.
The research team administered the structured, pre- coded and pre-tested questionnaire to enrolled participants on a face-to-face basis and brain CT scans were done by competent and well-trained radiology technicians and interpretation done by a specialist radiologist at the Radiology Department of Mulago hospital.
The questionnaires were checked for completeness at the end of every interview. The two files were compared, and any discordance corrected against data recorded with the questionnaire. The data were then backed up.
Ethical consideration
Written informed consent/ assent was obtained from all participants or their parent/guardian or legal authorized representative to participate in the study. Ethical approval was obtained from Makerere University, school of medicine research and ethics committee (SOMREC) (reference number #REC REF 2015–105).
Confidentiality was ensured using code numbers instead of patients’ names. Questionnaires were stored in a lockable cabinet for safety.
Profile of the study
Enrollment of study participants was carried out between September 2015 to March 2016 in Mulago hospital. The patient flow diagram for cases and controls is as shown in Fig. 1 .
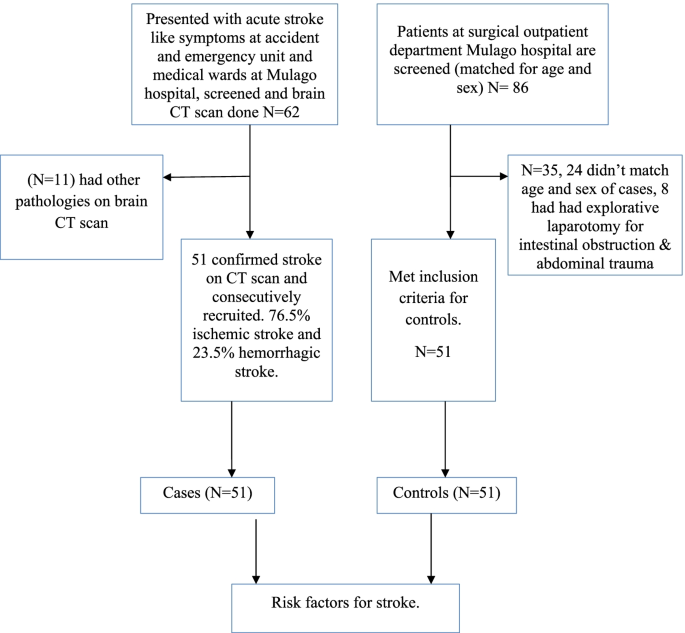
Patient flow diagram
Social demographic characteristics of the study population
A total of 51 cases aged 18 to 45 years and the same number of hospital control matched for age and sex were identified. The mean age of cases was 36.8 years (standard deviation (SD) 7.4) and the control was 36.8 years (SD 6.9). Females predominated in both groups with 56.9% in cases and 52.9% in controls. There was no significant difference in other baseline characteristics between cases and controls except in oral contraceptive use, waist to hip ratio, HIV status and sickle cell disease. Details of the social demographic characteristics are shown in Table 1 .
Clinical characteristics of the study participants
The mean fasting blood sugar was 6.6 (SD 3.9) for cases and 5.3 (SD 0.7) for controls. This was statistically significant with a p value of 0.015. Waist to hip ratio was also statistically significant with a p value of 0.007. Cases with an elevated wait to hip ratio were 14 (27.5%) and controls were 3 (5.9%). Table 2 shows the baseline clinical characteristics of the study participants.
Laboratory characteristics of the study participants
HIV serology and Hb electrophoresis were statistically significant with a p value of 0.076 and 0.023 respectively. 18 patients (35.3%) were reactive for HIV among cases and controls 10 (19.6%). 12 patients (23.5%) had abnormal Hb electrophoresis among cases controls 3 (5.9%). Table 3 shows the laboratory characteristics of the study participants.
Stroke types
Stroke types by social demographic characteristics of cases.
Among 62 patients, who had brain CT scan done, 11 patients had non stroke pathologies (4 had brain abscesses, 7 patients had ring enhancing lesions suggestive of toxoplasmosis). Among 51 patients with stroke confirmed on CT scan, the frequency of ischemic stroke was 76.5% and hemorrhagic stroke was 23.5%.
Most participants with ischemic or hemorrhagic stroke were in the age group 36–45 years. Females predominated in both ischemic and hemorrhagic stroke. Details of the social demographic characteristics by stroke types are shown in Table 4 .
Clinical and laboratory characteristics by stroke types
Majority of patients with hemorrhagic stroke were hypertensive (91.7%) compared to only 25.6% among patients with ischemic stroke. Details of the clinical and laboratory characteristics of the study participants by stroke subtypes are shown in Table 5 .
Risk factors for stroke at univariate analysis
Social demographic characteristics at univariate analysis.
Oral contraceptive use showed a significant difference with an unadjusted OR of 0.27 (95% CI 0.08–0.87) case subjects 23.3% and control subjects 56.5%. Belonging to other religion (seventh day advent, Pentecostal) was statistically significant with a p value of 0.009, OR 0.17. These findings are detailed in Table 6 below.
Clinical characteristics at univariate analysis
There was a significant difference in waist to hip ratio between cases (27.5%) and controls (5.9%), with unadjusted OR 6.85 (CI 1.70–27.62). HIV serology with an unadjusted OR of 2.64 (95% CI 1.03–6.82). Hb electrophoresis with an unadjusted OR of 4.31 (95% CI- 1.15–16.17). Fasting blood sugar with an unadjusted OR of 1.64 (95% CI 1.02–2.62). Details of the above findings are shown in Table 7 below.
Risk factors for stroke at multivariate analysis
At multivariate analysis, HIV serology (OR 3.72, 95% CI 1.16–10.96), waist to hip ratio (OR 11.26 95% CI 1.98–68.24) and sickle cell disease OR 4.78 95% CI 1.11–19.70) were independent risk factors for stroke in young adults. Table 8 shows risk factors at multivariate analysis. None of the patients with HIV met the definition of AIDS as defined by the occurrence of any of the more than 20 life-threatening cancers or “opportunistic infections”, by WHO.
This case–control study showed that the frequency of ischemic stroke was higher than that of hemorrhagic stroke in young Ugandan population. We showed that positive HIV serology, elevated waist to hip ratio and sickle cell disease were independent risk factors for stroke in this population.
This is consistent with several studies that have been done and found ischemic stroke to be more prevalent than hemorrhagic stroke. Studies done in Africa, in Libya reported 77% ischemic stroke and 23% hemorrhagic stroke (these included both intracerebral and subarachnoid hemorrhagic stroke) [ 14 ], in Morocco, 87.3% ischemic stroke and 12.7% hemorrhagic (study did not specify on the subtypes of hemorrhagic stroke) [ 6 ]. In a study from Bosnia and Herzegovina, Subarachnoid hemorrhage was more frequent in young adults compared with older patients (> 45 years of age) (22% vs. 3.5%), intracerebral hemorrhage (ICH) was similar in both groups (16.9% vs. 15.8%), but ischemic stroke (IS) was predominant stroke type in the older group (61% vs. 74%) [ 15 ]. On the other hand, studies focusing on all young stroke patients and including also subarachnoid hemorrhages have found much higher proportion of hemorrhagic strokes in younger vs. older individuals. Population-based studies have reported as low as 57% prevalence for ischemic stroke in those aged > 45, as reported by a recent narrative review [ 16 ]. This difference in occurrence of stroke subtypes could be due to the low prevalence of hypertension in this population in our setting given that hypertension has been reported to be the commonest risk factor for hemorrhagic stroke.
Most previous studies of HIV and stroke have been retrospective, but the prospective studies in Africa and East Africa have reported the importance of HIV as a risk factor for stroke [ 17 ]. A recently published study done in Malawi, with defined cases and population controls and 99% ascertainment of HIV status, reported HIV infection as an independent risk factor for stroke. This study further found that patients who had started standard HIV treatment in the previous six months had a higher risk of stroke (OR 15.6 95% CI 4.21–46.6). This was probably due to an immune reconstitution inflammatory syndrome (IRIS) like process [ 18 ]. A variety of mechanisms have been implicated in the association of HIV and stroke, these include HIV associated vasculopathy, vasculitis which causes abnormality of the intracranial or extracranial cerebral blood vessels and neoplastic involvement. Indirectly through cardioembolic, coagulopathy in association with protein C and protein S deficiency. Some infections are well established causes of stroke, such as Mycobacterium tuberculosi s , syphilis, and varicella zoster virus through increased susceptibility to acquisition or reactivation of these infections [ 19 , 20 ]. Combined antiretroviral therapy (cART) might unmask occult opportunistic infections that subsequently cause a stroke. This possibility should be considered in all patients who have had an acute stroke or have worsening of stroke symptoms after initiation of cART [ 21 ].
An elevated waist to hip ratio (WHR) was associated with 12 times increased risk of stroke among young adults in Mulago hospital compared to individuals with a normal waist to hip ratio. Abdominal obesity (measured as waist–hip ratio) is associated with an increased risk of myocardial infarction, stroke, and premature death [ 22 ]. This agrees with a few studies that have assessed the association of stroke with waist to hip ratio. Aaron et al. 1990, assessed the relation between body fat distribution, and the 2-year incidences of hypertension and stroke in a cohort of 41,837 women aged 55–69 years. Women who developed stroke were 2.1 (95% CI 1.5–2.9) times more likely to have an elevated ratio than those who did not [ 23 ]. Md Habib et al. 2011 assessed high waist to hip ratio as a risk factor for ischemic stroke for overall stroke and he found 64% of the ischemic stroke patient had abnormal WHR in Bangladesh [ 24 ]. Abdominal obesity measured with WHR was an independent risk factor for cryptogenic ischemic stroke (CIS) in young adults after rigorous adjustment for concomitant risk factors in the Revealing the Etiology, Triggers, and Outcome (SECRETO; NCT01934725) study, a prospective case–control study that included patients aged 18–49 years with a first ever CIS at 19 European university centers [ 25 ].
Sickle cell disease was also associated with increased risk of stroke among young adults in Mulago hospital. This agrees with several studies that have associated sickle cell disease with stroke. Ohene et al. 1998 assessed cerebrovascular accidents (CVA) in sickle cell disease, found the highest rates of prevalence of 4.01% and incidence of 0.61 per 100 patient-years. The incidence of hemorrhagic stroke was highest among patients aged 20 to 29 years [ 26 ].
In our study, the unadjusted OR for oral contraceptive use was 0.26 95% CI 0.08–0.87 with a p value of 0.028. This observation at the unadjusted level is significant but could be due to another variable which is a confounder to OC use such as higher socioeconomic status and better control of other possible risk factors.
In our study, we found no association between hypertension and stroke in young adults though it’s an independent risk factor for stroke in the older population. This finding is different from the multinational interstroke study which attributed most strokes among young adults in low- and middle-income countries to hypertension. In that study, only one fifth of the patients were from wealthier African countries where hypertension, diabetes and hypercholesterolemia are likely to occur with higher prevalence than in Mulago hospital [ 27 ]. Other studies have also reported the role of hypertension as a risk factor for stroke in young adults, low physical activity and hypertension were the most important risk factors, accounting for 59.7% and 27.1% of all strokes, respectively among a German nationwide case–control study based on patients enrolled in the SIFAP1 study (Stroke in Young Fabry Patients) 2007 to 2010 and controls from the population-based GEDA study (German Health Update) 2009 to 2010 [ 28 ]. A study that used population-based controls for hospitalized young patients with ischemic stroke demonstrated that independent risk factors for stroke were atrial fibrillation (OR 10.43; cardiovascular disease (OR, 8.01; type 1 diabetes mellitus (OR, 6.72; type 2 diabetes mellitus (OR, 2.31, low high‐density lipoprotein cholesterol (OR, 1.81; current smoking status (OR, 1.81; hypertension (OR, 1.43, and a family history of stroke (OR, 1.37) [ 29 ].
This finding could be explained by the high prevalence of hypertension in the general peri urban Ugandan population among young adults as reported by Kayima et al. 2015. He found a prevalence of 15% (95% CI 14.2 – 19.6%) % for young adults aged 18–44 years [ 30 ].
The study was conducted at Mulago hospital which is a national referral hospital in Uganda situated in central Uganda. Mulago hospital received patients both referred patients from all over Uganda and those from its catchment area. This is generally representative of the whole Ugandan population.
Uganda has a young population and with an HIV prevalence comparable to most countries in Sub-Saharan Africa, so the findings of this study are generalizable to other Sub-Saharan African populations.
Ischemic stroke is more prevalent than hemorrhagic stroke among young adults in Mulago hospital. Independent risk factors for stroke among young adults in Mulago hospital were HIV infection, elevated waist to hip ratio and sickle cell disease. Oral contraceptive use was found to be protective of stroke among young adults in Mulago hospital. There was no significant association between stroke among young adults and hypertension, diabetes, hyperlipidemia, smoking, alcohol use and illicit use.
Study limitations
The sample size was too small to detect all but the strongest associations with common exposures. When designing the study, we based on hypertension as a significant driver for strokes in this population based on other studies done to calculate the sample size, however based on our findings, hypertension was not a big driver of stroke in this population. Secondly the nature of stroke type associated with hypertension is hemorrhagic which were less common in this study. This was an unexpected finding and needs more evaluation.
Consecutive sampling methods has selection bias in which a variable that is associated with the outcome under investigation may occur more frequently or less in those sampled in this period as compared to the general population.
The use of a combined ischemic stroke and intracerebral hemorrhage group may have obscured relationships specific to one group, i.e., the risk factors for stroke were not stratified for type of stroke.
The best alternative for controls would have been healthy controls from the neighborhoods of the patients with stroke, but this would have been resource consuming hence the choice of hospital controls with different medical conditions from cases.
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Go AS, Mozaffarian D, Roger VL, Benjamin EJ, Berry JD, Borden WB, Turner MB. Heart disease and stroke statistics—2013 update: a report from the American Heart Association. Circulation. 2013;127(1):e6–245.
PubMed Google Scholar
Griffiths D, Sturm J. Epidemiology and etiology of young stroke. Stroke research and treatment, 2011. 2011.
Google Scholar
Bevan HS, Sharma K, Bradley W. Stroke in young adults. Stroke. 1990;21(3):382–6.
Article CAS Google Scholar
Mustapha AF, Sanya EO, Bello TO. Stroke among young adults at the LAUTECH Teaching Hospital, Osogbo, Nigeria. Nig Q J Hosp Med. 2012;22(3):177–80.
CAS PubMed Google Scholar
Hoffmann M. Stroke in the young: the multiethnic prospective Durban stroke data bank results. J Stroke Cerebrovasc Dis. 1998;7(6):404–13.
Chraa M, Louhab N, Kissani N. Stroke in young adults: about 128 cases. Pan Afr Med J. 2014;17(37).
Tibæk M, Dehlendorff C, Jørgensen HS, Forchhammer HB, Johnsen SP, Kammersgaard LP. Increasing incidence of hospitalization for stroke and transient ischemic attack in young adults: a registry-based study. J Am Heart Assoc. 2016;5(5):e003158. https://doi.org/10.1161/JAHA.115.003158 .
Article PubMed PubMed Central Google Scholar
Kwarisima L, Mukisa R, Nakibuuka J, Matovu S, Katabira E. Thirty-day stroke mortality and associated clinical and laboratory factors among adult stroke patients admitted at Mulago Hospital (Uganda). Afr J Neurol Sci. 2014;33(1):79–86.
Feigin VL, Lawes CM, Bennett DA, Anderson CS. Stroke epidemiology: a review of population-based studies of incidence, prevalence, and case-fatality in the late 20th century. Lancet Neurol. 2003;2(1):43–53.
Article Google Scholar
Katsnelson MJ, Della-Morte D, Rundek T. Stroke in the young. Period Biol. 2012;114(3):347–53.
Bandasak R, Narksawat K, Tangkanakul C, Chinvarun Y, Siri S. Association between hypertension and stroke among young Thai adults in Bangkok, Thailand. Southeast Asian J Trop Med Public Health. 2011;42(5):1241–8 PMID: 22299451.
Bhat VM, Cole JW, Sorkin JD, Wozniak MA, Malarcher AM, Giles WH, Stern BJ, Kittner SJ. Dose-response relationship between cigarette smoking and risk of ischemic stroke in young women. Stroke. 2008;39(9):2439–43. https://doi.org/10.1161/STROKEAHA.107.510073 . Epub 2008 Aug 14. PMID: 18703815; PMCID: PMC3564048.
Rohr J, Kittner S, Feeser B, Hebel JR, Whyte MG, Weinstein A, Sherwin R. Traditional risk factors and ischemic stroke in young adults: the Baltimore-Washington Cooperative Young Stroke Study. Arch Neurol. 1996;53(7):603–7.
Radhakrishnan K, Ashok PP, Sridharan R, Mousa ME. Stroke in the young: incidence and pattern in Benghazi. Libya. 1986;73(4):434–8.
CAS Google Scholar
Smajlović D, Salihović D, Ibrahimagić OĆ, Sinanović O. Characteristics of stroke in young adults in Tuzla Canton Bosnia and Herzegovina. Coll Antropol. 2013;37(2):515–9.
Tatlisumak, et al. Nontraumatic intracerebral haemorrhage in young adults. Nat Rev Neurol. 2018;14:237–50. https://doi.org/10.1038/nrneurol.2018.17 .
Article PubMed Google Scholar
O’Donnell M, Yusuf S. Risk factors for stroke in Tanzania. Lancet Glob Health. 2013;1:e241–2.
Benjamin LA, Corbett EL, Connor MD, et al. HIV, antiretroviral treatment, hypertension, and stroke in Malawian adults, a case control study. Neurology. 2016;86:324–33. https://doi.org/10.1212/WNL.0000000000002278 .
Article CAS PubMed PubMed Central Google Scholar
Benjamin LA, Bryer A, Emsley HCA, Khoo S, Solomon T, Connor MD. HIV infection and stroke: current perspectives and future directions. Lancet Neurol. 2012;11(10):878–90. https://doi.org/10.1016/S1474-4422(12)70205-3 .
Lammie GA, Hewlett RH, Schoeman JF, Donald PR. Tuberculous cerebrovascular disease: a review. J Infect. 2009;59:156–66.
Berger JR. AIDS and stroke risk. Lancet Neurol. 2004;3:206–7 [PubMed: 15039031].
Nejtek V, Talari D. Novel Risk Factors for TIA and Stroke in Young Adults. 2016.
Folsom AR, Prineas RJ, Kaye SA, Munger RG. Incidence of hypertension and stroke in relation to body fat distribution and other risk factors in older women. Stroke. 1990;21:701–6.
Khan MH, Chakraborty SK, Biswas RSR. High Waist to Hip Ratio as a Risk Factor for Ischemic Stroke Patients Admitted in a Tertiary Care Hospital. Bangladesh J Anat. 2011;9(1):30–4.
JJaakonmäki N, Zedde M, Sarkanen T, Martinez-Majander N, Tuohinen S, Sinisalo J, SECRETO Study Group. Obesity and the risk of cryptogenic ischemic stroke in young adults. J Stroke Cerebrovasc Dis. 2022;31(5):106380.
Rothman SM, Fulling KH, Nelson JS. Sickle cell anemia and central nervous system infarction: a neuropathological study. Ann Neurol. 1986;20:684–90.
O’Donnell MJ, Xavier D, Liu L, et al. Risk factors for ischemic and intracerebral hemorrhagic stroke in 22 countries (the INTERSTROKE study): a case-control study. Lancet. 2010;376:112–23.
Aigner A, Grittner U, Rolfs A, Norrving B, Siegerink B, Busch MA. Contribution of established stroke risk factors to the burden of stroke in young adults. Stroke. 2017;48(7):1744–51.
Kivioja R, Pietilä A, Martinez-Majander N, Gordin D, Havulinna AS, Salomaa V, Putaala J. Risk factors for Early-Onset ischemic stroke: a Case-Control study. J Am Heart Assoc. 2018;7(21):e009774.
Kayima J, Nankabirwa J, Sinabulya I, Nakibuuka J, Zhu X, Rahman M, Kamya MR. Determinants of hypertension in a young adult Ugandan population in epidemiological transition—the MEPI-CVD survey. BMC Public Health. 2015;15(1):1–9.
Download references
Acknowledgements
We acknowledge the patients of Mulago hospital who gave us consent to obtain this information.
This study was funded with funds from the MEPI-Neurology program under Makerere University. The funding project had no role in the design of the study and collection, analysis, and interpretation of data and no role in writing the manuscript.
Author information
Authors and affiliations.
Kiruddu National Referral Hospital, P.O. Box 6553, Kampala, Uganda
Priscilla Namaganda
Mulago National Referral Hospital, Mulago Hospital Complex, P.O. Box 7272, Kampala, Uganda
Jane Nakibuuka
Department of Medicine, School of Medicine, College of Health Sciences, Makerere University, Kampala, Uganda
Mark Kaddumukasa
Infectious Diseases Institute, Makerere University, Kampala, Uganda
Elly Katabira
You can also search for this author in PubMed Google Scholar
Contributions
PN– conception, design of work, acquisition, analysis, interpretation of data, drafted and substantively revised the manuscript, JN– analysis, interpretation of data, drafted and substantively revised the manuscript, MK – analysis, interpretation of data, drafted and substantively revised the manuscript, EK– design of work, acquisition, analysis, interpretation of data, drafted and substantively revised the manuscript. All authors read and approved the final manuscript.
Corresponding author
Correspondence to Priscilla Namaganda .
Ethics declarations
Ethics approval and consent to participate.
Written informed consent/ assent was obtained from all participants or their parent/guardian or legal authorized representative to participate in the study. Ethical approval was obtained from Makerere University, school of medicine research and ethics committee (SOMREC) (reference number #REC REF 2015–105). All methods and procedures were carried out in accordance with relevant guidelines and regulations.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ . The Creative Commons Public Domain Dedication waiver ( http://creativecommons.org/publicdomain/zero/1.0/ ) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
Reprints and permissions
About this article
Cite this article.
Namaganda, P., Nakibuuka, J., Kaddumukasa, M. et al. Stroke in young adults, stroke types and risk factors: a case control study. BMC Neurol 22 , 335 (2022). https://doi.org/10.1186/s12883-022-02853-5
Download citation
Received : 18 March 2022
Accepted : 23 August 2022
Published : 06 September 2022
DOI : https://doi.org/10.1186/s12883-022-02853-5
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Young adults
- Risk factors
BMC Neurology
ISSN: 1471-2377
- General enquiries: [email protected]

Case Presentation
Statement of ethics, conflict of interest statement, funding sources, author contributions, ischemic stroke in a 29-year-old patient with covid-19: a case report.
- Split-Screen
- Article contents
- Figures & tables
- Supplementary Data
- Peer Review
- Open the PDF for in another window
- Get Permissions
- Cite Icon Cite
- Search Site
Christian Avvantaggiato , Loredana Amoruso , Maria Pia Lo Muzio , Maria Assunta Mimmo , Michelina Delli Bergoli , Nicoletta Cinone , Luigi Santoro , Lucia Stuppiello , Antonio Turitto , Chiara Ciritella , Pietro Fiore , Andrea Santamato; Ischemic Stroke in a 29-Year-Old Patient with COVID-19: A Case Report. Case Rep Neurol 2 September 2021; 13 (2): 334–340. https://doi.org/10.1159/000515457
Download citation file:
- Ris (Zotero)
- Reference Manager
Increasing evidence reports a greater incidence of stroke among patients with Coronavirus disease 2019 (COVID-19) than the non-COVID-19 population and suggests that SARS-CoV-2 infection represents a risk factor for thromboembolic and acute ischemic stroke. Elderly people have higher risk factors associated with acute ischemic stroke or embolization vascular events, and advanced age is strongly associated with severe COVID-19 and death. We reported, instead, a case of an ischemic stroke in a young woman during her hospitalization for COVID-19-related pneumonia. A 29-year-old woman presented to the emergency department of our institution with progressive respiratory distress associated with a 2-day history of fever, nausea, and vomiting. The patient was transferred to the intensive care unit (ICU) where she underwent a tracheostomy for mechanical ventilation due to her severe clinical condition and her very low arterial partial pressure of oxygen. The nasopharyngeal swab test confirmed SARS-CoV-2 infection. Laboratory tests showed neutrophilic leucocytosis, a prolonged prothrombin time, and elevated D-dimer and fibrinogen levels. After 18 days, during her stay in the ICU after suspension of the medications used for sedation, left hemiplegia was reported. Central facial palsy on the left side, dysarthria, and facial drop were present, with complete paralysis of the ipsilateral upper and lower limbs. Computed tomography (CT) of the head and magnetic resonance imaging of the brain confirmed the presence of lesions in the right hemisphere affecting the territories of the anterior and middle cerebral arteries, consistent with ischemic stroke. Pulmonary and splenic infarcts were also found after CT of the chest. The age of the patient and the absence of serious concomitant cardiovascular diseases place the emphasis on the capacity of SARS-CoV-2 infection to be an independent cerebrovascular risk factor. Increased levels of D-dimer and positivity to β2-glycoprotein antibodies could confirm the theory of endothelial activation and hypercoagulability, but other mechanisms – still under discussion – should not be excluded.
Coronavirus disease 2019 (COVID-19), caused by the novel coronavirus SARS-CoV-2, is characterized by a wide range of symptoms, most of which cause acute respiratory distress syndrome [1, 2], associated with intensive care unit (ICU) admission and high mortality [3]. On March 11, 2020, the large global outbreak of the disease led the World Health Organization (WHO) to declare COVID-19 a pandemic, with 11,874,226 confirmed cases and 545,481 deaths worldwide (July 9, 2020) [4]. In many cases, the clinical manifestations of COVID-19 are characteristic of a mild disease that may, however, worsen to a critical lower respiratory infection [2]. At the onset of the disease, the most frequent symptoms are fever, dry cough, fatigue, and shortness of breath as the infection progresses may appear signs and symptoms of respiratory failure that require ICU admission [5, 6]. Although acute respiratory distress syndrome is the most important cause of ICU admission for COVID-19 patients, several studies have underlined the presence of neurological symptoms such as confusion, dizziness, impaired consciousness, ataxia, seizure, anosmia, ageusia, vision impairment, and stroke [7, 8]. In particular, the state of hypercoagulability in patients affected by COVID-19 favors the formation of small and/or large blood clots in multiple organs, including the brain, potentially leading to cerebrovascular disease (ischemic stroke but also intracranial hemorrhage) [9, 10 ].
We found an interesting case of stroke following a SARS-CoV-2 infection in a young patient. A 29-year-old woman, during her ICU hospitalization for COVID-19-related pneumonia, was diagnosed with ischemic stroke of the right hemisphere, without other cardiac/cerebrovascular risk factors except hypertension. The young age of the patient and the absence of higher cerebrovascular risk factors make the present case very interesting as it can help demonstrate that COVID-19 is an independent risk factor for acute ischemic stroke. In a case series of 214 patients with COVID-19 (mean [SD] age, 52.7 [15.5] years), neurologic symptoms were more common in patients with severe infection who were older than the others [ 11 ]. New-onset CVD was more common in COVID-19 patients who had underlying cerebrovascular risk factors, such as older age (>65 years) [ 12 ], and very few cases of stroke in patients younger than 50 years have been reported [ 12, 13 ]. Our case seems to be the only one younger than 30 years.
On the night between March 19 and 20, 2020, a 29-year-old woman was referred to our hospital “Policlinico Riuniti di Foggia” due to a progressive respiratory distress associated with a 2-day history of fever, nausea, and vomiting. At presentation, the heart rate was 128 bpm, the blood oxygen saturation measured by means of the pulse oximeter was 27%, the respiratory rate was 27 breaths per minute, and the blood pressure was 116/77 mm Hg. The arterial blood gas test showed a pH of 7.52, pO 2 20 mm Hg, and pCO 2 34 mm Hg. The patient was immediately transferred to the ICU where she underwent tracheostomy and endotracheal intubation for mechanical ventilation due to her severe clinical condition and deteriorated pulmonary gas exchange. The diagnosis of COVID-19 was confirmed by PCR on a nasopharyngeal swab.
The family medical history was normal, and the only known pre-existing medical conditions were polycystic ovary syndrome (diagnosed 3 years earlier), conversion disorder, and hypertension (both diagnosed 2 years earlier). Ramipril and nebivolol were prescribed for the high blood pressure treatment, and sertraline was prescribed for the conversion disorder treatment. Drug therapy adherence was inconstant. The patient had no history of diabetes, cardiac pathologies, strokes, transient ischemic attacks, thromboembolic, or other vascular pathologies.
Laboratory tests showed neutrophilic leukocytosis (white blood cell count 14.79 × 10 3 , neutrophil percentage 89.8%, and neutrophil count 13.29 × 10 3 ), a prolonged prothrombin time (15.3 s) with a slightly elevated international normalized ratio (1.38), and elevated D-dimer (6,912 ng/mL) and fibrinogen levels (766 mg/dL). Other findings are shown in Table 1 .
Laboratory test

This pharmacological therapy was set as follows: enoxaparin 6,000 U.I. once a day, piperacillin 4 g/tazobactam 0.5 g twice a day; Kaletra, a combination of lopinavir and ritonavir indicated for human immunodeficiency virus (HIV) infection treatment, 2 tablets twice a day; hydroxychloroquine 200 mg once a day; and furosemide 250 mg, calcium gluconate, and aminophylline 240 mg 3 times a day. No adverse events were reported.
On April 7, 2020, during her stay in the ICU and after suspension of the medications used for sedation, left hemiplegia was reported. The same day, the patient underwent a computed tomography examination of the head, which showed areas of hypodensity in the right hemisphere due to recent cerebral ischemia.
On April 16, 2020, the patient was oriented to time, place, and person. Central facial palsy on the left side, dysarthria, and facial drop were present, with complete paralysis of the ipsilateral upper and lower limbs. The power of all the muscles of the left limbs was grade 0 according to the Medical Research Council (MRC) scale. Deep tendon reflexes were reduced on the left upper limb but hyperactive on the ipsilateral lower limb, with a slight increase in the muscle tonus. The senses of touch, vibration, and pain were reduced on the left side of the face and body.
On the same day, the patient underwent magnetic resonance imaging (MRI) of the brain (Fig. 1 a), showing lesions on the right hemisphere affecting the territories of the anterior and middle cerebral arteries. On May 5, 2020, magnetic resonance angiography showed an early duplication of the sphenoidal segment of the right middle cerebral artery, the branches of which are irregular with rosary bead-like aspects (Fig. 1 d, e); on the same day, the second MRI (Fig. 1 b) confirmed the lesions. Computed tomography of the chest (Fig. 1 c) and abdomen (Fig. 1 f), performed 5 days after the MRI of the brain, showed not only multifocal bilateral ground-glass opacities but also a basal subpleural area of increased density within the left lung (4 × 4 × 3 cm), consistent with a pulmonary infarction. In addition, a vascular lesion, consistent with a splenic infarct, was found in the inferior pole of the spleen. Doppler echocardiography of the hearth showed regular right chambers and left atrium and a slightly hypertrophic left ventricle with normal size and kinetics (ejection fraction: 55%). The age of the patient and the absence of serious concomitant cardiovascular diseases place the emphasis on the capacity of SARS-CoV-2 infection to be an independent cerebrovascular risk factor.

Imaging. a April 16, 2020; MRI of the brain: lesions in the right hemisphere affecting the territories of the anterior and the middle cerebral arteries. b May 5, 2020; MRI of the brain: same lesions in the right hemisphere shown in the previous image. d , e May 5, 2020; MRA showed an early duplication of the sphenoidal segment of the right middle cerebral artery, the branches of which are irregular with rosary bead-like aspect and reduction of blood flow in the middle cerebral artery. c April 20, 2020; CT of the abdomen: vascular lesion, consistent with a splenic infarct, found in the inferior pole of the spleen. f April 20, 2020; CT of the chest: basal subpleural area of increased density within the left lung (4 × 4 × 3 cm), consistent with a pulmonary infarction. MRA, magnetic resonance angiography; CT, computed tomography; MRI, magnetic resonance imaging.
The pandemic outbreak of novel SARS-CoV-2 infection has caused great concern among the services and authorities responsible for public health due to not only the mortality rate but also the danger of filling up hospital capacities in terms of ICU beds and acute non-ICU beds. In this regard, the nonrespiratory complications of COVID-19 should also be taken into great consideration, especially those that threaten patients’ lives and extend hospitalization times. Stroke is one of these complications, since a greater incidence of stroke among patients with COVID-19 than the non-COVID-19 population has been reported, and a preliminary case-control study demonstrated that SARS-CoV-2 infection represents a risk factor for acute ischemic stroke [ 14 ].
We found that the reported case is extremely interesting, since the woman is only 29 years old and considering how stroke in a young patient without other known risk factors is uncommon. Not only elderly people have higher risk factors associated with acute ischemic stroke or embolization vascular events [ 15 ], but it is also true that advanced age is strongly associated with severe COVID-19 and death. The severity of the disease is directly linked to immune dysregulation, cytokine storm, and acute inflammation state, which in turn are more common in patients who present immunosenescence [6].
Inflammation plays an important role in the occurrence of cardiovascular and cerebrovascular diseases since it favors atherosclerosis and affects plaque stability [ 16 ]. The ischemic stroke of the 29-year-old woman does not appear to be imputable to emboli originating a pre-existing atheromatous plaque, both for the age of the patient and for the absence of plaques at the Doppler ultrasound study of the supra-aortic trunks.
Most likely, COVID-19-associated hypercoagulability and endothelial dysfunction are the causes of ischemic stroke, as suggested by other studies and case reports [ 10, 13, 17 ]. Although the mechanisms by which SARS-CoV-2 infection leads to hypercoagulability are still being studied, current knowledge suggests that cross talk between inflammation and thrombosis has a crucial role [ 18 ]. The release of inflammatory cytokines leads to the activation of epithelial cells, monocytes, and macrophages. Direct infection of endothelial cells through the ACE2 receptor also leads to endothelial activation and dysfunction, expression of tissue factor, and platelet activation and increased levels of VWF and FVIII, all of which contribute to thrombin generation and fibrin clot formation [ 17 ]. The 29-year-old patient showed an increased level of D-dimer, which is a degradation product of cross-linked fibrin, indicating a global activation of hemostasis and fibrinolysis and conforming to the hypothesis of COVID-19-associated hypercoagulability. Endothelial activation and hypercoagulability are also confirmed by positivity to β2 glycoprotein antibodies. Anticardiolipin antibody and/or β2 glycoprotein antibody positivity has been reported in a few studies [ 17, 19, 20 ]. In addition, widespread thrombosis in SARS-CoV-2 infection could also be caused by neutrophil extracellular traps (NETs). Neutrophilia [ 21 ] and an elevated neutrophil-lymphocyte ratio [ 22 ] have been reported by numerous studies as predictive of worse disease outcomes, and recently, the contribution of NETs in the pathophysiology of COVID-19 was reported [ 23 ]. Thrombogenic involvement of NETs has been described in various settings of thrombosis, including stroke, myocardial infarction, and deep vein thrombosis [ 24 ]. The high neutrophil count found in our case does not exclude the hypothesis that NETs are involved in the pathogenesis of ischemic stroke.
Ischemic stroke in young patients without pre-existing cerebrovascular risk factors is very unusual. In this regard, our case of an ischemic stroke, reported in a 29-year-old woman, is very interesting. Although it is not possible to determine precisely when the thromboembolic event occurred, our case of stroke during COVID-19-related pneumonia seems to confirm that COVID-19 is an independent risk factor for acute ischemic stroke. The mechanisms by which coronavirus disease leads to stroke are still under study, but it is clear that hypercoagulability and endothelial activation play a key role. Testing for SARS-CoV-2 infection should be considered for patients who develop neurologic symptoms, but it is equally important to monitor COVID-19 patients during their hospitalization to find any neurological sign or symptom in a timely manner. Our case suggests that discovering neurological deficits in sedated patients promptly can be very difficult; for this reason, sedation in mechanically ventilated patients has to be considered only if strictly necessary. Performing serial laboratory testing and waking up the patient as soon as clinical conditions allow are strategies that should be taken into account.
Written informed consent was obtained from the patient for publication of this case report and any accompanying images. A copy of the written consent is available for review by the editor-in-chief of this journal.
The authors certify that there is no conflict of interest with any financial organization regarding the material discussed in the manuscript.
No funding was received for the publication of this case report.
All authors agree with the contents of the manuscript and were fully involved in the study and preparation of the manuscript. All authors read and approved the final version of the manuscript. M.A. Mimmo, M.P. Lo Muzio, M. Delli Bergoli, and L. Amoruso collected the data. C. Avvantaggiato wrote the manuscript with support of N. Cinone, L. Santoro, and C. Ciritella. C. Avvantaggiato, A. Turitto, and L. Stuppiello researched and discussed the neurophysiological principles of this study. P. Fiore and A. Santamato supervised the project.

Email alerts
Citing articles via, suggested reading.
- Online ISSN 1662-680X
INFORMATION
- Contact & Support
- Information & Downloads
- Rights & Permissions
- Terms & Conditions
- Catalogue & Pricing
- Policies & Information
- People & Organization
- Stay Up-to-Date
- Regional Offices
- Community Voice
SERVICES FOR
- Researchers
- Healthcare Professionals
- Patients & Supporters
- Health Sciences Industry
- Medical Societies
- Agents & Booksellers
Karger International
- S. Karger AG
- P.O Box, CH-4009 Basel (Switzerland)
- Allschwilerstrasse 10, CH-4055 Basel
- Tel: +41 61 306 11 11
- Fax: +41 61 306 12 34
- Contact: Front Office
- Experience Blog
- Privacy Policy
- Terms of Use
This Feature Is Available To Subscribers Only
Sign In or Create an Account
Want to create or adapt books like this? Learn more about how Pressbooks supports open publishing practices.
This case study presents a 68-year old “right-handed” African-American man named Randall Swanson. He has a history of hypertension, hyperlipidemia and a history of smoking one pack per day for the last 20 years. He is prescribed Atenolol for his HTN, and Simvastatin for Hyperlipidemia (but he has a history of not always taking his meds). His father had a history of hypertension and passed away from cancer 10 years ago. His mother has a history of diabetes and is still alive.
Randall was gardening with his wife on a relaxing Sunday afternoon. Out of nowhere, Randall fell to the ground. When his wife rushed to his side and asked how he was doing, he answered with garbled and incoherent speech. It was then that his wife noticed his face was drooping on the right side. His wife immediately called 911 and paramedics arrived within 6 minutes. Upon initial assessment, the paramedics reported that Randall appeared to be experiencing a stroke as he presented with right-sided facial droop and weakness and numbness on the right side of his body. Fortunately, Randall lived nearby a stroke center so he was transported to St. John’s Regional Medical Center within 17 minutes of paramedics arriving to his home.
Initial Managment
Upon arrival to the Emergency Department, the healthcare team was ready to work together to diagnose Randall. He was placed in bed with the HOB elevated to 30 degrees to decrease intracranial pressure and reduce any risks for aspiration. Randall’s wife remained at his side and provided the care team with his brief medical history which as previously mentioned, consists of hypertension, hyperlipidemia and smoking one pack per day for the last 20 years. He had no recent head trauma, never had a stroke, no prior surgeries, and no use of anticoagulation medications.
Physical Assessment
Upon first impression, Nurse Laura recognized that Randall was calm but looked apprehensive. When asked to state his name and date of birth, his speech sounded garbled at times and was very slow, but he could still be understood. He could not recall the month he was born in but he was alert and oriented to person, time, and situation. When asked to state where he was, he could not recall the word hospital. He simply pointed around the room while repeating “here.”
Further assessment revealed that his pupils were equal and reactive to light and that he presented with right-sided facial paralysis. Randall was able to follow commands but when asked to move his extremities, he could not lift his right arm and leg. He also reported that he could not feel the nurse touch his right arm and leg. Nurse Laura gathered the initial vital signs as follows: BP: 176/82, HR: 93, RR: 20, T:99.4, O2: 92% RA and a headache with pain of 3/10.
Doctor’s Orders
The doctor orders were quickly noted and included:
-2L O2 (to keep O2 >93%)
– 500 mL Bolus NS
– VS Q2h for the first 8 hrs.
-Draw labs for: CBC, INR, PT/INR, PTT, and Troponin
-Get an EKG
-Chest X ray
-Glucose check
-Obtain patient weight
-Perform a National Institute of Health Stroke Scale (also known as NIHSS) Q12h for the first 24 hours, then Q24h until he is discharged
-Notify pharmacy of potential t-PA preparation.
Nursing Actions
Nurse Laura started an 18 gauge IV in Randall’s left AC and started him on a bolus of 500 mL of NS. A blood sample was collected and quickly sent to the lab. Nurse Laura called the Emergency Department Tech to obtain a 12 lead EKG.
Pertinent Lab Results for Randall
The physician and the nurse review the labs:
WBC 7.3 x 10^9/L
RBC 4.6 x 10^12/L
Plt 200 x 10^9/L
LDL 179 mg/dL
HDL 43 mg/dL
Troponin <0.01 ng/mL
EKG and Chest X Ray Results
The EKG results and monitor revealed Randall was in normal sinus rhythm; CXR was negative for pulmonary or cardiac pathology
CT Scan and NIHSS Results
The NIH Stroke Scale was completed and demonstrated that Randall had significant neurological deficits with a score of 13. Within 20 minutes of arrival to the hospital, Randall had a CT-scan completed. Within 40 minutes of arrival to the hospital, the radiologist notified the ED physician that the CT-scan was negative for any active bleeding, ruling our hemorrhagic stroke.
The doctors consulted and diagnosed Randall with a thrombotic ischemic stroke and determined that that plan would include administering t-PA. Since Randall’s CT scan was negative for a bleed and since he met all of the inclusion criteria he was a candidate for t-PA. ( Some of the inclusion criteria includes that the last time the patient is seen normal must be within 3 hours, the CT scan has to be negative for bleeding, the patient must be 18 years or older, the doctor must make the diagnosis of an acute ischemic stroke, and the patient must continue to present with neurological deficits.)
Since the neurologist has recommended IV t-PA, the physicians went into Randall’s room and discussed what they found with him and his wife. Nurse Laura answered and addressed any remaining concerns or questions.
Administration
Randall and his wife decided to proceed with t-PA therapy as ordered, therefore Nurse Laura initiated the hospital’s t-PA protocol. A bolus of 6.73 mg of tPA was administered for 1 minute followed by an infusion of 60.59 mg over the course of 1 hour. ( This was determined by his weight of 74.8 kg). After the infusion was complete, Randall was transferred to the ICU for close observation. Upon reassessment of the patient, Randall still appeared to be displaying neurological deficits and his right-sided paralysis had not improved. His vital signs were assessed and noted as follows: BP: 149/79 HR: 90 RR: 18 T:98.9 O2: 97% 2L NC Pain: 2/10.
Randall’s wife was crying and he appeared very scared, so Nurse John tried to provide as much emotional support to them as possible. Nurse John paid close attention to Randall’s blood pressure since he could be at risk for hemorrhaging due to the medication. Randall was also continually assessed for any changes in neurological status and allergic reactions to the t-PA. Nurse John made sure that Stroke Core Measures were followed in order to enhance Randall’s outcome.
In the ICU, Randall’s neurological status improved greatly. Nurse Jan noted that while he still garbled speech and right-sided facial droop, he was now able to recall information such as his birthday and he could identify objects when asked. Randall was able to move his right arm and leg off the bed but he reported that he was still experiencing decreased sensation, right-sided weakness and he demonstrated drift in both extremities.
The nurse monitored Randall’s blood pressure and noted that it was higher than normal at 151/83. She realized this was an expected finding for a patient during a stroke but systolic pressure should be maintained at less than 185 to lower the risk of hemorrhage. His vitals remained stable and his NIHSS score decreased to an 8. Labs were drawn and were WNL with the exception of his LDL and HDL levels. His vital signs were noted as follows: BP 151/80 HR 92 RR 18 T 98.8 O2 97% RA Pain 0/10
The Doctor ordered Physical, Speech, and Occupational therapy, as well as a swallow test.
Swallowing Screen
Randall remained NPO since his arrival due to the risks associated with swallowing after a stroke. Nurse Jan performed a swallow test by giving Randall 3 ounces of water. On the first sip, Randall coughed and subsequently did not pass. Nurse Jan kept him NPO until the speech pathologist arrived to further evaluate Randall. Ultimately, the speech pathologist determined that with due caution, Randall could be put on a dysphagia diet that featured thickened liquids
Physical Therapy & Occupational Therapy
A physical therapist worked with Randall and helped him to carry out passive range of motion exercises. An occupational therapist also worked with Randall to evaluate how well he could perform tasks such as writing, getting dressed and bathing. It was important for these therapy measures to begin as soon as possible to increase the functional outcomes for Randall. Rehabilitation is an ongoing process that begins in the acute setting.
Day 3- third person
During Day 3, Randall’s last day in the ICU, Nurse Jessica performed his assessment. His vital signs remained stable and WNL as follows: BP: 135/79 HR: 90 RR: 18 T: 98.9 O2: 97% on RA, and Pain 0/10. His NIHSS dramatically decreased to a 2. Randall began showing signs of improved neurological status; he was able to follow commands appropriately and was alert and oriented x 4. The strength in his right arm and leg markedly improved. he was able to lift both his right arm and leg well and while he still reported feeling a little weakness and sensory loss, the drift in both extremities was absent.
Rehabilitation Therapies
Physical, speech, and occupational therapists continued to work with Randall. He was able to call for assistance and ambulate with a walker to the bathroom and back. He was able to clean his face with a washcloth, dress with minimal assistance, brush his teeth, and more. Randall continued to talk with slurred speech but he was able to enunciate with effort.
On day 4, Randall was transferred to the med-surg floor to continue progression. He continued to work with physical and occupational therapy and was able to perform most of his ADLs with little assistance. Randall could also ambulate 20 feet down the hall with the use of a walker.
Long-Term Rehabilitation and Ongoing Care
On day 5, Randall was discharged to a rehabilitation facility and continued to display daily improvement. The dysphagia that he previously was experiencing resolved and he was discharged home 1.5 weeks later. Luckily for Randall, his wife was there to witness his last known well time and she was able to notify first responders. They arrived quickly and he was able to receive t-PA in a timely manner. With the help of the interdisciplinary team consisting of nurses, therapists, doctors, and other personnel, Randall was put on the path to not only recover from the stroke but also to quickly regain function and quality of life very near to pre-stroke levels. It is now important that Randall continues to follow up with his primary doctor and his neurologist and that he adheres to his medication and physical therapy regimen.
Case Management
During Randall’s stay, Mary the case manager played a crucial role in Randall’s path to recovery. She determined that primary areas of concern included his history of medical noncompliance and unhealthy lifestyle. The case manager consulted with Dietary and requested that they provide Randall with education on a healthy diet regimen. She also provided him with smoking cessation information. Since Randall has been noncompliant with his medications, Mary determined that social services should consult with him to figure out what the reasons were behind his noncompliance. Social Services reported back to Mary that Randall stated that he didn’t really understand why he needed to take the medication. It was apparent that he had not been properly educated. Mary also needed to work with Randall’s insurance to ensure that he could go to the rehab facility as she knew this would greatly impact his ultimate outcome. Lastly, throughout his stay, the case manager provided Randall and his wife with resources on stroke educational materials. With the collaboration of nurses, education on the benefits of smoking cessation, medication adherence, lifestyle modifications, and stroke recognition was reiterated to the couple. After discharge, the case manager also checked up with Randall to make sure that he complied with his follow up appointments with the neurologist and physical and speech therapists,
- What risk factors contributed to Randall’s stroke?
- What types of contraindications could have prevented Randall from receiving t-PA?
- What factors attributed to Randall’s overall favorable outcome?
Nursing Case Studies by and for Student Nurses Copyright © by jaimehannans is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License , except where otherwise noted.
Share This Book
Learning together for better health using an evidence-based Learning Health System framework: a case study in stroke
Affiliations.
- 1 Monash Centre for Health Research and Implementation, 43-51 Kanooka Grove, Clayton, VIC, Australia. [email protected].
- 2 Monash Partners Academic Health Science Centre, 43-51 Kanooka Grove, Clayton, VIC, Australia. [email protected].
- 3 Stroke and Ageing Research, Department of Medicine, School of Clinical Sciences at Monash Health, Monash University, Level 2 Monash University Research, Victorian Heart Hospital, 631 Blackburn Rd, Clayton, VIC, Australia. [email protected].
- 4 Stroke Theme, The Florey Institute of Neuroscience and Mental Health, University of Melbourne, Heidelberg, VIC, Australia. [email protected].
- 5 Stroke and Ageing Research, Department of Medicine, School of Clinical Sciences at Monash Health, Monash University, Level 2 Monash University Research, Victorian Heart Hospital, 631 Blackburn Rd, Clayton, VIC, Australia.
- 6 Stroke Theme, The Florey Institute of Neuroscience and Mental Health, University of Melbourne, Heidelberg, VIC, Australia.
- 7 Department of Neurology, Melbourne Brain Centre, Royal Melbourne Hospital, Parkville, VIC, Australia.
- 8 Department of Medicine, Faculty of Medicine, Dentistry and Health Sciences, University of Melbourne, Victoria, Australia.
- 9 School of Health Sciences, Heart and Stroke Program, University of Newcastle, Hunter Medical Research Institute, University Drive, Callaghan, NSW, Australia.
- 10 Monash Partners Academic Health Science Centre, 43-51 Kanooka Grove, Clayton, VIC, Australia.
- 11 Monash Centre for Health Research and Implementation, 43-51 Kanooka Grove, Clayton, VIC, Australia.
- 12 School of Medicine and Dentistry, Griffith University, Birtinya, QLD, Australia.
- 13 Clinical Excellence Division, Queensland Health, Brisbane, Australia.
- 14 John Hunter Hospital, Hunter New England Local Health District and University of Newcastle, Sydney, NSW, Australia.
- 15 School of Nursing, Midwifery and Paramedicine, Australian Catholic University, Sydney, NSW, Australia.
- 16 Nursing Research Institute, St Vincent's Health Network Sydney and and Australian Catholic University, Sydney, NSW, Australia.
- 17 Stroke Foundation, Level 7, 461 Bourke St, Melbourne, VIC, Australia.
- PMID: 38750449
- PMCID: PMC11094907
- DOI: 10.1186/s12916-024-03416-w
Background: In the context of expanding digital health tools, the health system is ready for Learning Health System (LHS) models. These models, with proper governance and stakeholder engagement, enable the integration of digital infrastructure to provide feedback to all relevant parties including clinicians and consumers on performance against best practice standards, as well as fostering innovation and aligning healthcare with patient needs. The LHS literature primarily includes opinion or consensus-based frameworks and lacks validation or evidence of benefit. Our aim was to outline a rigorously codesigned, evidence-based LHS framework and present a national case study of an LHS-aligned national stroke program that has delivered clinical benefit.
Main text: Current core components of a LHS involve capturing evidence from communities and stakeholders (quadrant 1), integrating evidence from research findings (quadrant 2), leveraging evidence from data and practice (quadrant 3), and generating evidence from implementation (quadrant 4) for iterative system-level improvement. The Australian Stroke program was selected as the case study as it provides an exemplar of how an iterative LHS works in practice at a national level encompassing and integrating evidence from all four LHS quadrants. Using this case study, we demonstrate how to apply evidence-based processes to healthcare improvement and embed real-world research for optimising healthcare improvement. We emphasize the transition from research as an endpoint, to research as an enabler and a solution for impact in healthcare improvement.
Conclusions: The Australian Stroke program has nationally improved stroke care since 2007, showcasing the value of integrated LHS-aligned approaches for tangible impact on outcomes. This LHS case study is a practical example for other health conditions and settings to follow suit.
Keywords: Evidence-based medicine; Healthcare improvement; Learning Health System; Models of care; Person-centred care; Stroke.
© 2024. The Author(s).
- Evidence-Based Medicine
- Evidence-Based Practice / methods
- Learning Health System*
- Stroke* / therapy

Stroke Overview

What Is a Stroke?
A stroke, also known as a brain attack, occurs when blood flow to the brain is blocked or a blood vessel inside or on the surface of the brain bursts. A stroke is a serious medical emergency and requires immediate medical attention, just like a heart attack. Stroke ranks as the fourth leading killer in the United States. It is the most common cause of adult disability.
With stroke, the sooner treatment begins, the better. Knowing the signs of stroke and calling 911 immediately can help save the person from death or disability. Timely treatment can save brain cells and greatly reduce or even reverse the damage.
A stroke can happen blood vessel in the brain becomes fully or partly blocked or when a blood vessel bursts. When blood flow to the brain is stopped or reduced during a stroke, some brain cells die because they stop getting the oxygen and nutrients they need. Other brain cells die because they are damaged by swelling caused by the blockage or bleeding or by inflammation. Some brain cells die quickly but many stay in a compromised or weakened state for several hours. This allows for treatment of the stroke in many cases. Stroke causes permanent brain damage within minutes or hours. But early treatment can reduce disability and save lives.
Strokes can be prevented and treated. Making lifestyle changes and getting regular medical and prenatal care can help prevent stroke and significantly reduce the risk for other disorders such as dementia, heart disease, and diabetes. The stroke rate is rising in adults under the age of 49. While nearly three-quarters of strokes occur in people over 65, the risk about doubles each decade after age 55.
Common Effects of Stroke
The brain is nourished by a rich network of blood vessels. A blockage or bursting in one of these blood vessels can occur in any area of the brain. Since each brain region is responsible for different functions, the effects of stroke range from mild to severe depending on the type, severity, and location of the stroke. The effects of stroke may be temporary or permanent.
Problems with muscle movement (motor sensory impairment)
Damage to the part of the brain that controls balance and coordination can cause movement problems. A common problem after a stroke is weakness or paralysis (being unable to move). This may affect only the face, an arm, or a leg, or it may affect one entire side of the body. A person who has had a stroke may have problems with daily activities such as walking, dressing, eating, and using the bathroom. Slurred speech due to weakness or a lack of coordination of the muscles involved in speaking is called dysarthria and is a physical, not a language, problem. Dysarthria can arise from damage to either side of the brain. It is often associated with trouble swallowing (dysphagia).
Problems with cognition, thinking, or memory
Stroke may cause problems with thinking, awareness, attention, learning, judgment, and memory. Some people with stroke have a “neglect” syndrome, which means that they have no knowledge of one side of their body (usually the left side), or one side of the visual field, and are unaware of the problem. A person with stroke may be unaware of his or her surroundings, or may be unaware of the cognitive, emotional, and/or behavioral problems that resulted from the stroke. Some people will experience a permanent decline in cognitive function known as vascular cognitive impairment (VCI), which includes vascular dementia, but also refers to a gradual decline in mental function caused by multiple strokes over time. VCI appears to primarily affect the brain's executive function—the ability to plan activities. This could include anything from getting dressed in the morning to managing medications, finances, or negotiating a business deal. Controlling risk factors for stroke can reduce the risk of vascular cognitive impairment and dementia.
Problems with speaking or understanding speech
People who have had a stroke often have problems speaking or understanding spoken language. This is often accompanied by similar problems in reading and writing. In most people, language problems result from damage to the left hemisphere of the brain. Severe damage can result in a complete inability to speak or understand (called aphasia).
Problems with emotion
People with stroke may have difficulty controlling their emotions or may express inappropriate emotions in certain situations such as difficulty controlling crying or laughing. Post-stroke depression, which is common, is a serious medical problem that can hamper recovery and rehabilitation and may even lead to death by suicide. Post-stroke depression is treatable with medications and psychotherapy.
Problems with pain and sensation
After a stroke, some people may experience pain, uncomfortable numbness, or strange sensations. These sensations can be due to many factors, including damage to the sensory regions of the brain, stiff joints, spastic muscles, or a disabled limb. An uncommon type of pain resulting from stroke is called central stroke pain or central pain syndrome (CPS). CPS results from damage to an area of the brain called the thalamus, which is involved with sensory perception and movement. The pain is a mixture of sensations, including heat and cold, burning, tingling, numbness, sharp stabbing, and an underlying aching pain. It is intense in the area affected most by the stroke and is made worse by movement and temperature changes, especially cold temperatures. There are a few treatments or therapies to combat CPS that can be effective, but most pain medications provide little—if any--relief from these sensations.
Types of Stroke
There are two major kinds of stroke. About 80% of all strokes are ischemic strokes, which are caused by a blood clot that blocks a blood vessel or artery in the brain. About 20% are hemorrhagic strokes, which are caused by a blood vessel in the brain that breaks and bleeds into the brain.
Ischemic Stroke
An ischemic stroke occurs when the supply of blood to one or more regions of the brain is suddenly cut off or interrupted. Ischemic stroke is most commonly caused by a blood clot or debris (such as plaque—a mixture of fatty substances, including cholesterol) that blocks or plugs an artery in the brain. Arteries are the blood vessels that carry blood from the heart to the brain and body.
Blockages that cause ischemic strokes can come from three conditions:
- Thrombosis —A clot develops within a diseased blood vessel of the brain and grows large enough to impair blood flow.
- Embolism —A clot forms in another part of the body (such as the heart or a diseased artery in the chest or neck) and moves into a narrower artery in the neck or brain.
- Stenosis —An artery in the head or neck narrows. The most common cause of stenosis is atherosclerosis—a condition where deposits of plaque collect along the inside of arteries, causing a thickening and hardening and loss of elasticity in the walls of arteries. Atherosclerosis of heart vessels can also lead to a heart attack.
Ischemic stroke can also provoke inflammation, swelling (edema), and other processes that can continue to cause damage for hours to days after the initial stroke. In large ischemic strokes, the swelling can cause the pressure inside the skull to rise to dangerous levels.
Immediately after an ischemic stroke, the brain usually contains an irreversibly damaged core of tissue and an area of viable but at-risk tissue. Restoring normal blood flow—a process called reperfusion—is essential to rescuing the tissue that is still viable. The longer reperfusion is delayed, the more cells will die.
Hemorrhagic Stroke
In a healthy brain, neurons (brain cells) do not come into direct contact with blood. The blood-brain barrier, an elaborate meshwork of tightly fitting cells that form the inside layer of tiny blood vessels called capillaries, regulates which parts of the blood can pass through to the brain cells and what substances from neurons can pass into the bloodstream.
When an artery in the brain bursts, blood gushes into or around the brain, damaging the surrounding tissue. This is called a hemorrhagic stroke. The blood that enters the brain increases the pressure inside the skull (intracranial pressure), which can cause significant tissue damage. The mass of blood compresses the adjacent brain tissue, and toxic substances in the blood further injure the brain.
There are multiple types of hemorrhagic stroke, categorized by where the bleeding occurs:
- Subarachnoid hemorrhage involves rupture of a vessel on the surface of the brain and bleeding into the space between the brain and an envelope of tissue called the arachnoid layer.
- Intracerebral hemorrhage involves bleeding within the brain itself.
Transient Ischemic Attack (TIA)
A transient ischemic attack (TIA) is a temporary interruption of blood flow to the brain, often caused by a clot, which dissipates after a short time. After the blockage dissipates, stroke symptoms go away. Any stroke damage from a TIA is typically temporary and is not visible on brain imaging such as MRI. However, a TIA is an important warning sign that a larger, more serious stroke could come soon.
A TIA—sometimes incorrectly referred to as a mini-stroke—starts just like any other stroke. Generally, the symptoms or deficits begin to disappear in less than 20 minutes, and often go away within an hour. One type of TIA is caused by a narrowing of the carotid artery and causes occasional vision loss in one eye. People often describe this feeling “like a shade coming down over the eye” on the affected side.
TIAs often indicate a high risk for a more serious stroke and an underlying condition that requires medical help. About one in three people who have a TIA will have a stroke sometime in the future, with the majority of those occurring within a year of the TIA. Because TIAs last for only a few minutes, many people mistakenly ignore them. However, taking action can save a life. Calling 911 as soon as symptoms appear can make the difference in avoiding lifelong disability.
Stroke in Women
Some risk factors for stroke apply only to women, including pregnancy, childbirth, and menopause. These factors are tied to hormonal changes that affect women at different stages of life. In women of childbearing age, stroke risk is relatively low (with an annual incidence of one in 10,000). However, studies have shown that being pregnant increases that risk threefold.
Several factors contribute to the increased risk of stroke during pregnancy:
- Blood clotting proteins are naturally more active during pregnancy. In some cases, clots form in the brain's large draining veins, leading to stroke, headache, or seizure.
- Pregnancy-related stroke is more likely to occur in women who experience certain complications, such as infections or preeclampsia (high blood pressure with fluid retention), or who have other risk factors for stroke, such as high blood pressure or diabetes.
- Most strokes occur during the first few weeks after delivery. These strokes may be caused by a drop in blood volume or by the rapid hormonal changes that follow childbirth.

In the same way that hormonal changes during pregnancy and childbirth are associated with increased stroke risk, hormonal changes during menopause can also increase the risk of stroke. Although hormone replacement therapy (HRT) may help some symptoms of menopause, studies have shown that HRT may increase the risk of stroke, especially in women more than 10 years post-menopause. Estrogen patches used on the skin have not been found to increase the risk of stroke and may be a better option than estrogen pills for these women. More research is needed in this area. Women should talk with their doctor about their symptoms and risks to find appropriate treatments.
Children and Stroke
Children have several unique stroke risk factors. Children’s risk of stroke is highest during the perinatal period—which begins just before birth and ends a few weeks after. Boys and Black children are at a higher risk for stroke than other groups.
Infants and children who have a stroke usually experience the same symptoms of stroke as adults. But children are more likely to have other symptoms, including seizures, breathing problems, or loss of consciousness. It can be difficult to identify stroke in younger children.

Risk factors for childhood stroke include congenital (present at birth) heart problems, head trauma, and blood-clotting disorders. An important risk factor for Black children is sickle cell disease , which can cause narrowing of brain arteries.
The outcome of stroke in children is difficult to predict. Children who have a stroke generally recover better than adults with appropriate treatment and rehabilitation. Children’s brains have greater plasticity. Brain plasticity is the brain’s ability to reorganize, change, and adapt to deficits and injury, and to rewire itself to carry on necessary functions. Generally, outcomes are worse in children under age one and in those who lose consciousness or have seizures. A stroke that occurs during infancy or childhood can cause permanent disability.
Where can I find more information about stroke? Information may be available from the following organizations and resources: American Stroke Association Phone: 888-478-7653 Hazel K. Goddess Fund for Stroke Research in Women Brain Aneurysm Foundation Phone: 781-826-5556 or 888-272-4602 Heart Rhythm Society Phone: 202-464-3400 Child Neurology Foundation Phone: 612-928-6325 Joe Niekro Foundation Phone: 877-803-7650 Children's Hemiplegia and Stroke Association Phone: 817-492-4325 National Aphasia Association Phone: 212-267-2814 or 800-922-4622 Fibromuscular Dysplasia Society of America Phone: 216-834-2410 or 888-709-7089 YoungStroke, Inc. Phone: 843-248-9019 or 843-655-2835
Ohio State nav bar
The Ohio State University
- BuckeyeLink
- Find People
- Search Ohio State
Stroke case study
- Sanaya Batcho
- Kara Kitzmiller
- Emma Overman
Our reason for choosing this disorder
Stroke is the leading cause of disability in the United States. As advanced practice nurses, we anticipate caring for those impacted by strokes in many healthcare settings including emergency rooms, acute care, rehab settings, extended care facilities, and in primary care. Early diagnosis and treatment are imperative in the treatment of a stroke in order to minimize permanent deficits so it is important for advanced practice nurses to be proficient in recognizing clinical manifestations of a stroke. There are also many modifiable risk factors for strokes so advanced practice nurses need to be able to educate patients and families on potential lifestyle changes that can decrease stroke risk.
A .gov website belongs to an official government organization in the United States.
A lock ( ) or https:// means you've safely connected to the .gov website. Share sensitive information only on official, secure websites.
- Signs and Symptoms
- Risk Factors
- Stroke Facts
- Stroke Communications Toolkit
- About Heart Disease
- About High Blood Pressure
- About Cholesterol
About Stroke
- Stroke causes parts of the brain to become damaged or die.
- Quick treatment is critical for stroke.
- There are two types of stroke: ischemic and hemorrhagic.
- A transient ischemic attack is sometimes called a “mini-stroke."
A stroke, sometimes called a brain attack, occurs when something blocks blood supply to part of the brain or when a blood vessel in the brain bursts.
In either case, parts of the brain become damaged or die. A stroke can cause lasting brain damage, long-term disability, or even death.
Learn about the health conditions and lifestyle habits that can increase your risk for stroke .
What happens in the brain during a stroke?
The brain controls our movements, stores our memories, and is the source of our thoughts, emotions, and language. The brain also controls many functions of the body, like breathing and digestion.
To work properly, your brain needs oxygen. Your arteries deliver oxygen-rich blood to all parts of your brain. If something happens to block the flow of blood, brain cells start to die within minutes, because they can't get oxygen. This causes a stroke.
Learn more about the signs and symptoms of stroke.
There are two types of stroke:
- Ischemic stroke.
- Hemorrhagic stroke.
A transient ischemic attack (TIA) is sometimes called a "mini-stroke." It is different from the major types of stroke. Blood flow to the brain is blocked for only a short time—usually no more than 5 minutes. 1
Ischemic stroke
Most strokes are ischemic strokes. An ischemic stroke occurs when blood clots or other particles block the blood vessels to the brain.
Fatty deposits called plaque can also cause blockages by building up in the blood vessels.
Hemorrhagic stroke
A hemorrhagic stroke happens when an artery in the brain leaks blood or ruptures (breaks open). The leaked blood puts too much pressure on brain cells, which damages them.
High blood pressure and aneurysms—balloon-like bulges in an artery that can stretch and burst—are examples of conditions that can cause a hemorrhagic stroke.
Transient ischemic attack (TIA or “mini-stroke”)
TIAs are sometimes known as "warning strokes." It's important to know that:
- A TIA is a warning sign of a future stroke.
- A TIA is a medical emergency, just like a major stroke.
- Strokes and TIAs require emergency care. Call 9-1-1 right away if you feel symptoms of a stroke or see signs in another person.
- There is no way to know in the beginning whether symptoms are from a TIA or from a major type of stroke.
- Like ischemic strokes, blood clots often cause TIAs.
- More than a third of people who have a TIA and don't get treatment have a major stroke within 1 year. As many as 10% to 15% of people will have a major stroke within 3 months of a TIA. 1
Recognizing and treating TIAs can lower the risk of a major stroke. If you have a TIA, your health care team can find the cause and take steps to prevent a major stroke.
What CDC is doing
- High Blood Pressure
- Heart Disease
- Cholesterol
- Paul Coverdell National Acute Stroke Program
Million Hearts® and CDC Foundation
" Heart-Healthy Steps." This campaign encourages adults 55 and older to get back on track with the small steps: scheduling their medical appointments, getting active, and eating healthy, so they can get back to living big.
"Live to the Beat." This campaign focuses on empowering Black adults to pursue heart-healthy lifestyles on their own terms—finding what works best individually and consistently, as they live to their own beat.
- National, Heart, Lung, and Blood Institute: What Is a Stroke?
- National Institute of Neurological Disorders and Stroke (NINDS): Stroke Information Page
- NINDS Know Stroke Campaign
- NINDS Know Stroke Campaign Spanish Toolkit
- American Stroke Association. Transient ischemic attack (TIA). American Heart Association. Accessed January 29, 2024. https://www.stroke.org/en/about-stroke/types-of-stroke/tia-transient-ischemic-attack
Stroke is a leading cause of death in the United States and is a major cause of serious disability for adults. It is also preventable and treatable.
For Everyone
Public health.
Loading metrics
Open Access
Peer-reviewed
Research Article
The role of leptomeningeal collaterals in redistributing blood flow during stroke
Roles Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Project administration, Software, Validation, Visualization, Writing – original draft, Writing – review & editing
* E-mail: [email protected] (RE); [email protected] (FS)
Affiliation Institute of Fluid Dynamics, ETH Zurich, Zurich, Switzerland
Roles Data curation, Formal analysis, Investigation, Resources, Writing – review & editing
Affiliation Institute of Pharmacology and Toxicology, University of Zurich, Zurich, Switzerland
Affiliation Deptartment of Neurology, University Hospital Zurich and University of Zurich, Zurich, Switzerland
Roles Funding acquisition, Resources, Writing – review & editing
Roles Conceptualization, Funding acquisition, Resources, Supervision, Writing – review & editing
Roles Funding acquisition, Methodology, Resources, Writing – review & editing
Roles Conceptualization, Data curation, Formal analysis, Funding acquisition, Investigation, Methodology, Project administration, Resources, Software, Supervision, Validation, Writing – original draft, Writing – review & editing
Affiliations Institute of Fluid Dynamics, ETH Zurich, Zurich, Switzerland, Institute of Pharmacology and Toxicology, University of Zurich, Zurich, Switzerland, ARTORG Center for Biomedical Engineering Research, University of Bern, Bern, Switzerland
- Robert Epp,
- Chaim Glück,
- Nadine Felizitas Binder,
- Mohamad El Amki,
- Bruno Weber,
- Susanne Wegener,
- Patrick Jenny,
- Franca Schmid

- Published: October 23, 2023
- https://doi.org/10.1371/journal.pcbi.1011496
- Reader Comments
Leptomeningeal collaterals (LMCs) connect the main cerebral arteries and provide alternative pathways for blood flow during ischaemic stroke. This is beneficial for reducing infarct size and reperfusion success after treatment. However, a better understanding of how LMCs affect blood flow distribution is indispensable to improve therapeutic strategies. Here, we present a novel in silico approach that incorporates case-specific in vivo data into a computational model to simulate blood flow in large semi-realistic microvascular networks from two different mouse strains, characterised by having many and almost no LMCs between middle and anterior cerebral artery (MCA, ACA) territories. This framework is unique because our simulations are directly aligned with in vivo data. Moreover, it allows us to analyse perfusion characteristics quantitatively across all vessel types and for networks with no, few and many LMCs. We show that the occlusion of the MCA directly caused a redistribution of blood that was characterised by increased flow in LMCs. Interestingly, the improved perfusion of MCA-sided microvessels after dilating LMCs came at the cost of a reduced blood supply in other brain areas. This effect was enhanced in regions close to the watershed line and when the number of LMCs was increased. Additional dilations of surface and penetrating arteries after stroke improved perfusion across the entire vasculature and partially recovered flow in the obstructed region, especially in networks with many LMCs, which further underlines the role of LMCs during stroke.
Author summary
Cerebral ischaemic strokes are a leading cause of death and disability worldwide. Among other factors, the outcome of stroke treatment is determined by the existence and extent of collateral flow paths, which sustain residual blood supply to the obstructed brain region. To improve therapeutic strategies and to reduce reperfusion injuries during treatment, an in-depth understanding of the role of collaterals for maintaining blood supply is indispensable. We performed numerical simulations to quantify how leptomeningeal collaterals impact blood flow redistribution in response to middle cerebral artery occlusion. Our studies have the unique feature that they are consistent with the topology of case-specific pial arterial networks from mouse brains and aligned with sparse in vivo blood flow measurements. This allows the valuable joint interpretation of numerical studies and in vivo experiments. We observed that maintaining perfusion to the obstructed region comes at the cost of reduced blood supply in other areas. Moreover, dilation of arterial vessels improved perfusion in the entire vasculature. Importantly, flow changes vary significantly across and even within vessel types, which underlines the benefits of numerical models with single vessel resolution. Taken together, our framework establishes a strong link between experimental and numerical studies necessary to advance our understanding of perfusion changes in response to stroke and after clot removal.
Citation: Epp R, Glück C, Binder NF, El Amki M, Weber B, Wegener S, et al. (2023) The role of leptomeningeal collaterals in redistributing blood flow during stroke. PLoS Comput Biol 19(10): e1011496. https://doi.org/10.1371/journal.pcbi.1011496
Editor: Timothy W. Secomb, University of Arizona, UNITED STATES
Received: December 30, 2022; Accepted: September 3, 2023; Published: October 23, 2023
Copyright: © 2023 Epp et al. This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Data Availability: All relevant data are within the paper and its Supporting Information files. The simulation framework microBlooM, which includes the blood flow model for constant haematocrit distributions, the vessel distensibility model and the inverse model, is available on github (release version v1.0.0, https://github.com/Franculino/microBlooM/releases/tag/57ba603 ). Additionally, the research data, including the raw simulation data, various scripts for generating the semi-realistic microvascular networks and the setup files for microBlooM, are stored in a permanent repository ( https://doi.org/10.3929/ethz-b-000634335 ).
Funding: RE, FS and PJ received funding from the Swiss National Science Foundation ( https://www.snf.ch ) (Grant No. 166707) and ETH Zürich ( https://ethz.ch ). FS received funding from the Swiss National Science Foundation (Grant No. 202192). BW received funding from the Swiss National Science Foundation (Grant No. 310030_182703). SW received funding from the Swiss National Science Foundation (Grant No. 310030_200703). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Competing interests: The authors have declared that no competing interests exist.
Introduction
Due to the limited energy storage of the brain, maintaining a robust oxygen and nutrient supply is crucial. During healthy conditions, the interconnected network of microvascular blood vessels [ 1 – 3 ] sustains blood flow to all brain areas and regulates flow in response to local changes in neuronal activity [ 4 – 6 ]. However, during ischaemic stroke blood supply to specific brain regions is reduced drastically by a clot obstructing large arterial vessels. This typically causes tissue damage, which often results in permanent disability or even death [ 7 ].
Leptomeningeal anastomoses or collaterals (LMCs) are blood vessels connecting branches of major feeding arteries at the cortical surface of the brain [ 8 , 9 ], e.g. the middle (MCA) and the anterior (ACA) cerebral arteries. Due to their relatively low flow velocity [ 10 ] and small vessel diameters, LMCs are often described as being “almost dormant” during healthy physiological conditions. However, during stroke LMCs dilate [ 11 – 14 ] and provide alternative routes for blood to partially maintain perfusion in under-supplied brain regions [ 7 ].
Current treatments for stroke include the removal of the clot by either thrombolysis with recombinant tissue plasminogen activator (rt-PA) or mechanical thrombectomy [ 7 ]. Among other factors, the outcome of stroke treatment is determined by the existence and extent of leptomeningeal collaterals of individual patients [ 7 ]. However, even after successful recanalisation of the occluded vessel, some brain regions may remain unperfused, e.g. due to microvascular obstructions by neutrophils [ 15 ], secondary occlusions caused by fragments of the original clot [ 16 ] or constrictions of downstream vessels [ 17 ]. In order to improve therapeutic strategies, an in-depth understanding of how LMCs redistribute flow during stroke across vessel types is essential [ 8 , 16 ].
Studies in mice showed that the diameter and number of LMCs vary for different strains [ 11 , 12 ], but also between individual animals [ 18 ]. This directly impacts the infarct volume and therewith the overall outcome after stroke [ 12 , 14 , 19 – 21 ]. Furthermore, it has been suggested that functional activation of the cortex can offer protection from stroke, possibly by re-routing flow over collateral flow paths [ 22 , 23 ]. While experimental studies generally agree that the presence of many large LMCs reduces the infarct size after stroke [ 12 , 14 , 19 , 20 ], relatively little is known on how LMCs precisely redistribute flow at the level of individual vessels and across the entire vasculature. Even though scanning speeds for two-photon microscopy (2PM) line scans are increasing [ 24 , 25 ], generally 2PM studies are limited to quantifying flow in a few vessels [ 13 , 26 – 28 ]. Moreover, data on perfusion changes in all vessel types, e.g. arteries and capillaries, and over the entire depth of the cortex is usually not available. Other measurement techniques such as functional magnetic resonance (fMRI) [ 29 , 30 ] or laser speckle contrast imaging (LSI) [ 15 , 31 ] provide results on a more global scale. However, their resolution is either very coarse (fMRI) or the results are difficult to interpret quantitatively (LSI) [ 31 ]. More-advanced high-resolution tomographic imaging methods [ 32 – 35 ] have the potential to quantify flow in large brain regions at single vessel resolution. While these methods are very promising for the future, they are not yet widely used in the field.
In contrast to in vivo studies, the numerical simulations employed here offer the advantage that flow values for the entire vasculature are obtained [ 36 – 39 ]. Furthermore, individual vessel diameters or even the network topology can be adjusted to evaluate the isolated impact of these changes on overall perfusion characteristics [ 40 – 44 ]. This is challenging in vivo , where localised vascular modifications may trigger a series of changes within large areas of the brain. Thus, in silico studies are a convenient tool to provide novel insights on the role of LMCs on flow redistribution during stroke across all vessel types.
To simulate blood flow, a network representation of the vasculature is required. Generally, the acquisition, segmentation and vectorisation of large realistic microvascular networks with thousands of vessels is challenging, especially if complete connectivity and accurate capillary diameters are required, as it is the case for blood flow simulations [ 3 , 36 , 37 ]. Nonetheless, recent advances in imaging and data processing allow the ex vivo mapping of the entire mouse brain vasculature [ 45 – 48 ]. However, these networks have not been used in simulation studies yet, and a direct comparison to in vivo flow measurements would be difficult. Alternatively, fully artificial or semi-realistic networks matching realistic characteristics can be used [ 21 , 49 , 50 ].
Here, we present a novel approach that allows us to generate large semi-realistic microvascular networks that are based on case-specific experimental data available from in vivo studies. More precisely, our framework combines incomplete pial arterial networks obtained from mice with realistic penetrating trees and an artificial capillary bed to obtain a network that mimics the realistic vasculature. By applying an inverse model the networks are tuned such that they are aligned with in vivo two-photon microscopy red blood cell (RBC) velocity measurements [ 15 , 38 , 51 ] from individual subjects and literature. These networks are then used for in silico experiments studying changes of blood flow distributions during stroke. While inverse modelling has previously been used to incorporate experimental data into blood flow simulations [ 52 – 58 ], to the best of our knowledge, our framework is the first that tunes network characteristics in large semi-realistic microvascular networks by incorporating sparse data from in vivo experiments at arterial level. Importantly, our framework is well-suited to handle both large parameter spaces and a large number of constraints. This strong connection between in vivo and in silico allows to reduce uncertainties and opens the door for novel interesting research questions.
In the current study we used our novel simulation framework to compare the flow fields during (1) baseline (Base), (2) MCA occlusion (MCAo), (3) after subsequent LMC dilation (MCAo & LMC-dil) and (4) after additionally dilating all arteries (MCAo & LMC/SA/DA-dil) for vascular networks derived from mice with (C57BL/6) and no LMCs (BALB/c). Additionally, we analysed the flow field in modified test cases by adding or removing LMCs. This approach allowed us to study the flow and pressure fields in the entire vasculature during stroke and LMC dilation. Based on that, we provide novel insight on the role of LMCs and comment on the impact of the number of LMCs for the overall perfusion.
Materials and methods
Ethics statement.
All animal experiments were approved by the local veterinary authorities in Zurich and conformed to the guidelines of the Swiss Animal Protection Law, Veterinary Office, Canton of Zurich (Act of Animal Protection 16 December 2005 and Animal Protection Ordinance 23 April 2008, animal welfare assurance numbers ZH165/19 and ZH224/15).
In vivo experiments in pial arteries
Our study builds on in vivo data from two different mouse strains characterised by either having many (C57BL/6) or close to no (BALB/c) LMCs between the MCA and ACA territories [ 11 , 12 , 20 ]. In vivo two-photon imaging was used to extract the topology and vessel lengths of four pial or surface artery (SA) networks (2x C57BL/6J (JAX: #000664), 2x BALB/cByJ (JAX: #001026)) located at the cortical surface in the whisker and hindlimb area of the cortex (cranial window size 3.5 x 3.5mm 2 , MCA-M4/M5 territory, S10 Fig ). Animal surgery for two-photon imaging and blood flow measurements were performed as described earlier [ 15 ]. Fig 1A shows a manually traced reconstruction of SAs located at the MCA-ACA watershed line (wsl). The SAs are fed by either MCA or ACA, and for C57BL/6 networks the downstream MCA and ACA sided SAs are connected by LMCs (yellow squares). All visible descending artery (DA) roots, i.e., locations where DAs branch off from the SAs to supply the capillary bed, are marked by red dots. Diameters and RBC velocities were measured by line-scans and were processed with a custom-designed image processing tool box for MATLAB (Cellular and Hemodynamic Image Processing Suite, CHIPS, [ 59 ]; R2014b; MathWorks). Vessel diameters at baseline were determined at full width half maximum from a Gaussian fitted intensity profile drawn perpendicular to the vessel axis for a subset of vessels in all networks. Additionally, baseline RBC velocities in selected vessels of one C57BL/6 and one BALB/c network were analysed with the Radon method [ 60 ] implemented in CHIPS (see S1 Table for the measurements of the network C57BL/6 I in Fig 1A ).
- PPT PowerPoint slide
- PNG larger image
- TIFF original image
(A) Experimentally acquired reconstruction of surface artery (SA) network from a mouse with LMCs (network C57BL/6 I ). The MCA and ACA sided SAs are fed by the respective main feeding arteries, and are connected by LMCs (yellow squares) across the watershed line (wsl). The numbers refer to diameter and RBC velocity measurements obtained in individual SAs ( S1 Table ). Red dots are used to mark the locations of descending artery (DA) root points. For the reconstructions and measurements of the other three networks, refer to S9 Fig , and S24 , S25 and S26 Tables, respectively. (B) Network representing SAs. The original topology is shown with red lines and the additionally added SAs to connect the sampled DA roots (orange dots) are indicated by orange lines. Furthermore, ascending vein (AV) roots (blue dots) are distributed around the DAs. (C) Hierarchical structure of the microvascular network consisting of SAs, DAs, AVs and capillaries (Cs). Additional SAs are added to connect the MCA and ACA sided SAs to a common inflow vertex, i.e., the circle of Willis (CoW). (D-G) Networks C57BL/6 I and C57BL/6 II with LMCs (D-E) and BALB/c I and BALB/c II without LMCs (F-G). The locations of MCA occlusion (MCAo) are indicated with red circles. The networks are located in the MCA-M4/M5 territory and the MCAo is at the MCA-M2 [ 61 ].
https://doi.org/10.1371/journal.pcbi.1011496.g001
Generation of semi-realistic microvascular networks
To study perfusion characteristics in the entire vasculature, we generated semi-realistic microvascular networks that are based on realistic pial topologies ( Fig 1A ) but also contain penetrating trees and capillaries. Within this manuscript we use the term descending artery (DA) and ascending vein (AV) to refer to all arterial and venule vessels below the cortical surface, i.e., this includes arterioles and venules. Detecting all DA roots in the area below the cortical window is difficult without acquiring complete z-stacks. Therefore, in a first step, we increased the number of DA roots to match experimental values as observed in high-resolution images of the vasculature obtained by light sheet microscopy [ 61 ] (Table A in S1 Appendix ). To attain this goal we sampled additional tree locations directly into the SA reconstruction (orange dots in Fig 1B ) and connected the novel DA roots to the nearest SAs by introducing new vessels (orange edges in Fig 1B ). See S1 Appendix for more details on the refinement algorithm for the pial vasculature. Additionally, root points of venous trees at the cortical surface, i.e., the AV roots, were added such that they are located around DA roots and mimic a rhombic lattice with a DA:AV ratio of 1:3 [ 3 ] (blue dots in Fig 1B ). Importantly, the resulting pial network ( Fig 1B ) is still characterised by the overall SA topology of the originally acquired network ( Fig 1A ). However, the density of DA roots is now consistent with mean values observed in high-resolution experiments. While in the current study a mean DA root density was prescribed for the entire network, the applied sampling approach would also allow to match a spatially varying distribution of penetrating trees, given such data is available from experimental studies.
The refined SA topology ( Fig 1B ) was used to generate large three-dimensional semi-realistic microvascular networks consisting of pial arteries (SAs), descending arteries (DAs), capillaries (Cs) and ascending veins (AVs) ( Fig 1C ). When available, in vivo diameter measurements were assigned to the refined SA topology ( S1 Table ). The remaining vessel diameters were estimated by interpolating between the available values. Note that the prescribed vessel diameters are only preliminary and their uncertainties will be reduced in a subsequent step by using inverse modelling. Additional pial arteries (SAs to CoW) were added to connect the MCA and ACA inflows to a common inflow vertex, which represents the vessel offspring at the circle of Willis (CoW). Subsequently, we added realistic penetrating trees (DAs, AVs) and connect them to the pial network at the DA and AV root points. The penetrating trees were sampled from a database previously obtained from the somatosensory cortex of the rat [ 36 , 62 ], and scaled by a factor of 2/3 to account for size differences of cortical thickness between mouse and rat [ 37 , 63 ]. Finally, a simplified artificial capillary bed (Cs) consisting of a stacked hexagonal network with uniform diameters, lengths and tortuosity was added mimicking the typical highly interconnected and mesh-like topology [ 2 , 3 , 48 , 64 ]. The values for capillary diameters (4 μm [ 3 ]), lengths (62 μm) and tortuosity (1.37 [ 3 , 48 , 65 ]) were chosen such that the overall length and volume densities of the vasculature are within the range observed in vivo (see S3 Table ). By connecting the leaf vertices of the penetrating trees to the respective closest capillaries, we obtained a full network which represents the typical hierarchical structure of realistic microvascular networks, where blood sequentially flows through SAs, DAs, Cs and AVs [ 64 ]. As such, our approach enables fast and straightforward generation of large microvascular networks, which are based on realistic pial vascular topologies and represent the overall characteristics of the in vivo microvasculature. More details on the validation of our semi-realistic networks are presented at a later stage.
Blood flow model
As commonly done in previous works by our group [ 37 , 38 , 42 – 44 ] and others [ 39 , 49 , 66 – 68 ], we represented the brain vasculature by a network consisting of edges and vertices, which correspond to individual blood vessels and connections of at least two blood vessels. Poiseuille’s law was used to compute blood flow rates, RBC velocities and pressures in the entire network. As our focus is not on local capillary perfusion characteristics [ 37 ] but on overall flow changes, we neglected the effects of phase-separation [ 69 ] and assumed a constant haematocrit of 0.3. The impact of haematocrit and vessel diameter on flow resistance is considered by the formulation of Pries et al. [ 70 ]. Please refer to S2 Appendix for a more detailed description of the blood flow model.
Pressure boundary conditions of 100 and 10 mmHg [ 37 ] were assigned to in- and outflow vertices, respectively, and kept constant for all simulations, i.e., at baseline, MCAo, MCAo & LMC-dil and MCAo & LMC/SA/DA-dil. The networks were constructed such that only one inflow vertex exists at the most upstream SA vessels at the circle of Willis (CoW in Fig 1C ). Outflow boundary conditions were assigned to all AV tree root vertices [ 37 ].
Tuning of vessel diameters to match experimental data
As described in the previous sections, the three-dimensional semi-realistic microvascular network ( Fig 1D ) mimics the topology and structure of the real vasculature and is consistent with case-specific pial networks from in vivo experiments. However, the uncertainties related to vessel diameters are still large. To reduce these uncertainties, additional data from in vivo measurements and literature were incorporated into the model.
(A) Network C57BL/6 I , where red edges represent SAs with in vivo RBC velocity measurements. Furthermore, purple and blue dots highlight the locations of DA and AV roots, where a range for the RBC velocities is prescribed. (B) Illustration of the two types of cost functions used in this work for prescribing target values (left) or ranges (right) for the RBC velocities. (C) RBC velocities in SAs, DAs and AVs before and after tuning. (D-E) Diameters (D) and RBC velocities (E) at the SAs after tuning the semi-realistic network (see also S11 Fig ).
https://doi.org/10.1371/journal.pcbi.1011496.g002
The comparison of diameter distributions before and after tuning ( S11(A)–S11(C) Fig ) shows that applying the inverse model mainly affected SAs. Changes of other vessel types were also observed, however, they were mainly located close to penetrating trees and in the upper layers of the networks ( S11(D)–S11(F) Fig ). Note that with our inverse modelling approach it is expected that the largest diameter changes are close to the measurements, because the cost function is minimised by adjusting the parameters with the highest sensitivity ( S3 Appendix ).
Fig 2C demonstrates that the velocities in SAs after tuning the vessel diameters agree well with the target values from the measurements. Furthermore, the velocities in DA and AV roots are now within the prescribed velocity ranges. This demonstrates that using an inverse model for optimizing the network is an effective tool to improve the overall quality and to obtain RBC velocity distributions that are consistent with given experimental data. The diameters and RBC velocities after tuning in SAs of C57BL/6 I are visualised in Fig 2D and 2E .
Validation of semi-realistic microvascular networks
The final semi-realistic microvascular networks of all four datasets are visualised in Fig 1D–1G . On average, the networks span an area of 28.75 mm 2 and consist of approximately 200’000 edges. A more detailed summary of network characteristics is available in S2 Table . We validated the networks by comparing different vessel and flow characteristics to literature values. Overall, the length and volume densities, as well as the mean diameters of SAs, DAs, Cs and AVs, are within physiological range in all four networks ( S3 Table ). The flow field in the vasculature is highly heterogeneous [ 37 , 64 , 74 ], which results in order of magnitude differences of RBC velocities in individual vessels. Generally, both the magnitudes ( S4 and S28 Tables) and heterogeneity ( S12 and S13 Figs) of velocities and RBC flow rates are captured well by our simulations and agree with experimental data from literature. Furthermore, our networks agree with reported values for cerebral blood flow ( S27 Table ) and wall shear stresses in different vessel types ( S29 Table ).
Adjustment of vessel diameters to represent the effects of MCA occlusion, LMC dilation and artery dilation
To mimic a stroke at the level of the MCA (MCAo) we constricted an edge segment with a length of 50 μm upstream to the pial network to 10% of its baseline diameter (MCAo in Fig 1D–1G ). This occlusion is at the MCA-M2 bifurcation [ 61 ] and induces at significant pressure drop. The semi-realistic microvascular networks described above are located further downstream in the M4/M5 area and of course are affected tremendously by the MCAo. The precise response of vessels in the M4/M5 area (both on the MCA and the neighboring ACA side) is still not fully understood and likely a mix of dilations and constrictions [ 17 , 27 , 28 ]. Further confounding factors are the precise time point after stroke and potential effects of anesthesia [ 75 ]. In any case, the alterations in diameter are likely a result of a multitude of response mechanisms, including some form of autoregulation, passive changes due to vessel elasticity and potentially additional pathological adaptations.
As our focus is on flow redistribution immediately after stroke and the effect of LMCs, we decided to limit vascular adaptations to three clearly defined states: 1) no diameter adaptations except MCAo, 2) MCAo and LMC dilation (MCAo & LMC-dil) and 3) MCAo & LMC-dil and SAs and DAs dilation (MCAo & LMC/SA/DA-dil). While state 1) & 2) represent the situation immediately after stroke, state 3) is intended to mimic simplified forms of either autoregulation or therapeutic intervention causing arteriole dilation by vasodilators [ 76 , 77 ] or stimulation [ 22 , 23 ].
The diameters of LMCs for the state MCAo & LMC-dil were derived from the in vivo experiments, when such data was available (C57BL/6 I in Fig 1A and S1 Table ). For all other scenarios, a uniform dilation factor of 1.7 was assumed, which corresponds to the median dilation factor of the C57BL/6 I network. For the state MCAo & LMC/SA/DA-dil, SAs and DAs were dilated by 10%, which is the average peak dilation observed in vivo for pial vessels during functional hyperaemia [ 64 ]. Note that models for autoregulation are available [ 78 , 79 ]. However, as autoregulation is likely impaired during stroke [ 80 ] we decided to work with a more general state that allows us to analyze how SA & DA dilations could counteract the flow and pressure reductions caused by the MCAo.
For all states we accounted for the elasticity of blood vessels by using a pressure-area relationship based on linear elastic theory [ 21 , 79 , 81 ] to estimate passive diameter changes due to pressure alterations in response to MCAo. Such pressure alterations are observed across large parts of the vasculature ( S6(A) and S6(C) Fig ). Interestingly, despite the large changes in pressure, the calculated constrictions due to vessel elasticity were generally below 10% ( S4 Fig ) and the elasticity of vessels only slightly affected the results compared to when all vessels were assumed rigid (see S21 Table , S22 and S23 Tables). For more details on the vessel elasticity model, please refer to S4 Appendix .
It is important to note that the defined post-stroke states are a strong simplification in comparison to the complex situation in vivo . However, at the same time, these clearly defined states offer the advantage that simulation results can be compared rigorously and that it is possible to comment on the isolated effects of specific alterations, e.g. LMC-dilation. This would be challenging for states including all post-stroke vasodynamics at once.
Variation of LMC densities
One of our key goals was to quantify the impact of the number of LMCs on flow redistribution after MCAo, MCAo & LMC-dil and MCAo & LMC/SA/DA-dil. As the overall network structure differs between individual data sets, it is difficult to directly compare networks with and without LMCs. Consequently, we exploited the benefits of in silico investigations and modified the network characteristics by removing selected or all LMCs from C57BL/6 networks, and analogously adding any desired number of LMCs to BALB/c networks. This allows us to create additional scenarios with varying LMC densities for all four networks. Subsequently, we analysed how the presence of many (100% LMC), few (50% LMC) or no (0% LMC) LMCs affects the overall perfusion changes in an isolated manner, i.e., without any other differences between the networks. For the C57BL/6 networks, the 50% LMC and 0% LMC scenarios were defined by randomly removing half or all existing LMCs, respectively ( S5(A) Fig ). Accordingly, LMCs were added to the BALB/c networks based on the LMC characteristics derived from the two C57BL/6 datasets (see S5 Fig for details).
With our simulations we studied how the occlusion of the MCA (MCAo), the subsequent dilation of LMCs (MCAo & LMC-dil) and the additional dilation of all arteries (MCAo & LMC/SA/DA-dil) affect the distribution of blood flow compared to baseline (Base). We first focus on results obtained for networks characterised by many LMCs (C57BL/6 I and C57BL/6 II ) and analysed the changes in blood flow rates in SAs, DAs and Cs. The results are then compared to networks with fewer or no LMCs, which provides further insights on the role of LMCs during stroke. To facilitate comparison, the results for MCAo & LMC/SA/DA-dil are depicted together with the other states. However, to focus on the impact of arterial dilations, the description of this state is addressed in a separate section, i.e., after describing perfusion changes at the level of SAs, DAs and Cs in response to MCAo and LMC-dil.
An occlusion of the MCA passively increases the flow rates in LMCs and ACA sided SAs
(A-B) Relative changes of blood flow rates in individual SAs of the network C57BL/6 I after MCAo compared to baseline (A) and after LMC-dil in comparison to MCAo only (B). (C) Classification of SAs and DAs into MCA and ACA sided vessels. Additionally, the SAs are classified into vessels located on a shortest path to LMCs (solid lines) and others (dashed lines). The grey shaded patches group DAs into different categories according to their planar distances to LMCs (spacing between differently coloured patches is 250 μm). The wsl describes the interface between MCA and ACA territory, which was extracted based on the Voronoi tessellation around all DA roots (Fig A panel B in S1 Appendix ). (D) Relative changes of blood flow rates in individual SAs of the network C57BL/6 I after dilating all arteries (MCAo & LMC/SA/DA-dil compared to MCAo & LMC-dil only). (E) Flow rates in individual SAs (grey lines) at baseline, MCAo, MCAo & LMC-dil and MCAo & LMC/SA/DA-dil for both C57BL/6 networks, according to their classification from panel (C) ( SAs on paths to LMCs in left panel, others in right panel). Boxplots and mean values (◇-symbol) are shown for each category.
https://doi.org/10.1371/journal.pcbi.1011496.g003
Dilating the LMCs subsequently to MCAo also increased the mean flow rates in ACA SAs on paths to LMCs ( Fig 3E left). This is consistent with the improved perfusion of LMCs, which after stroke were exclusively supplied by ACA sided vessels. In Other ACA SAs not on a path to LMCs, the dilation of LMCs caused a reduction in flow ( Fig 3E right), originating from the lower pressure level in ACA SAs ( S5 Table and S6 Fig ) and the resulting smaller pressure difference towards the capillary bed. Relative changes for C57BL/6 II are available in S5 and S6 Tables.
While the overall trends were comparable for both networks with LMCs ( S6 Table ), the precise value of the relative changes is affected by the network topology. Key factors for the resulting relative flow changes in response to MCAo and LMC-dil are the number of LMCs ( S2 Table ), the vessel connectivity and the size of the MCA and ACA region used in the in silico study ( S2 Table ).
LMC dilation after MCAo improves flow in MCA DAs and Cs, but causes a further reduction in ACA DAs and Cs
(A-B) Relative perfusion changes in DA trees of the network C57BL/6 I after MCAo compared to baseline (A) and after MCAo & LMC-dil in comparison to MCAo only (B). The feeding area of each DA tree is approximated by the Voronoi polygons given by the tessellation around all DA roots (consistent with Fig A panel B in S1 Appendix ). Each polygon is colour-coded based on the flow rate changes through the corresponding DA roots. Refer to S8(A) Fig for results after SA/DA-dil. (C) Top: Scatter plot with flow rate ratios “MCAo to baseline” (∘, dark colours) and “MCAo & LMC-dil to baseline” (△/▽, light colours) for the DAs of both C57BL/6 networks. △ and ▽ symbols are used to indicate whether the flow rate is increased or reduced after dilating the LMCs in comparison to MCAo only. The grey shading indicates the bins used to categorise the data points with respect to their distances to the LMCs according to Fig 3C . Bottom: Boxplots and mean values (◇-symbol) summarising the flow rate ratios for the different distance categories. Note that the flow rate ratios “MCAo & LMC/SA/DA-dil to baseline” are also shown here. (D) Flow rate ratios “MCAo to baseline”, “MCAo & LMC-dil to baseline” and “MCAo & LMC/SA/DA-dil to baseline” for Cs of both C57BL/6 networks.
https://doi.org/10.1371/journal.pcbi.1011496.g004
The overall trends for capillaries ( Fig 4D and S8 Table ) were generally comparable to the results in DAs. Nonetheless, the integral flow reduction after MCAo was slightly less pronounced in MCA sided Cs and slightly larger in ACA Cs in comparison to MCA and ACA sided DAs, respectively. This resulted in a smoother transition between the integral perfusion drops in MCA and ACA sided Cs close to LMCs, which is plausible because capillaries in the watershed area can be fed simultaneously by MCA and ACA DAs. Importantly, the response at the level of individual capillaries is very heterogeneous and flow increases, decreases and also reversals have been observed. This provides evidence for a significant redistribution of blood flow within the capillary bed. Moreover, the reduced drop in integral perfusion for MCA sided Cs close to the wsl showed that the high interconnectivity of the capillary bed offers some robustness during MCAo, which would be difficult to capture if flow changes were only analysed in DAs or individual Cs.
In summary, the partial recovery of flow in MCA sided DAs and Cs can be augmented by dilating LMCs. However, this comes at the cost of a reduced perfusion of ACA sided DAs and Cs, which likely however is not so severe that under-perfusion is to be expected for ACA sided vessels.
Dilation of arteries after stroke improves the perfusion of MCA and ACA sided vessels
Taken together, dilating arteries subsequent to stroke improved the perfusion of all vessel types and across the entire vasculature. This can be expected since larger vessel diameters automatically reduce the flow resistance, and thus affect pressures and flow rates throughout the network.
The impact of having many, few or no LMCs
The results presented above (Figs 3 and 4 ) focused on blood flow distributions after stroke in networks with LMCs. In the following, we wanted to understand the influence of LMCs on flow redistribution, and how flow characteristics changed in networks with few or without LMCs. To attain this goal, we vary the amount of LMCs in each of the four datasets to obtain three networks per dataset that are characterised by many, few or no LMCs (see Fig 5A for C57BL/6 I and S5(A)–S5(C) Fig for C57BL/6 II , BALB/c I and BALB/c II ). Since the number of LMCs is the only difference between the three networks of each dataset, the comparison of results provides direct insights on the role of LMCs during stroke.
(A) Top: Map of the network C57BL/6 I and visualisation of the three scenarios with many, few and no LMCs. For the 50% scenario, only the pink-coloured LMCs are kept. The grey shaded area highlights the region within 250 μm to LMCs. Bottom: Colour map for the results displayed in panels B-F. (B) Relative changes of mean flow rates in MCA and ACA SAs of individual networks (markers) and averaged over all datasets (bars). The SAs are classified into vessels on a shortest path to LMCs (top) and others (bottom) (see Fig 3C ). (C-D) Relative changes of integral flow rates computed over all MCA and ACA sided DAs (C) and for DAs located within 250 μm of LMCs only (D). The integral flow rate is defined as the sum of flow rates over all vessels of the respective vessel category ( S7 Table ). (E-F) Relative changes of integral flow rates in Cs in the entire network (E) and within 250 μm of LMCs (F).
https://doi.org/10.1371/journal.pcbi.1011496.g005
Pial arteries.
The pressure changes in SAs after MCAo, and the resulting pressure differences towards the capillaries, explain the impact of LMCs on Other MCA/ACA SAs ( Fig 5B bottom): The average reduction in mean pressure in MCA SAs due to MCAo was more severe with fewer LMCs ( S10 Table ). This resulted in lower flow rates in Other MCA SAs for approximately similar pressures in the capillary bed ( S9 Table ). The opposite trend was observed for Other ACA SAs after MCAo, where pressures ( S10 Table ) and flow rates ( S9 Table ) decreased more in networks with many LMCs. These pressure changes were amplified by dilating LMCs, which translated to larger flow changes in Other ACA SAs after MCAo & LMC-dil compared to MCAo. After dilating DAs and SAs, the pressure level slightly increased for most scenarios ( S17 Table ) compared to MCAo & LMC-dil ( S10 Table ). This generally improved the mean flow rates in both MCA and ACA sided SAs. While this trend is consistent for all numbers of LMCs ( S18 Table ), larger differences were observed for the cases with more LMCs.
In summary, a higher number of LMCs increased flow redistribution from the ACA to the MCA side. This is most apparent from the increased flow rates in ACA SAs on paths to LMCs and the flow reductions in other ACA SAs. These trends are comparable across the four datasets, even though the variability with respect to mean flow rate changes is relatively large. As previously stated, this is caused by topological differences across the four datasets, e.g. the number of MCA and ACA sided SAs, the number of LMCs and the DA tree density.
Descending arteries and capillaries.
Fig 5C shows the relative change of integral blood flow rates through all MCA and ACA sided DAs (see S11 and S19 Tables for a summary of averaged results). The differences between networks with different number of LMCs were qualitatively consistent with the results for Other MCA/ACA SAs . This is expected, since downstream of SAs blood eventually flows into DA trees and capillaries. A closer look at DAs within a planar distance of 250 μm to LMCs ( Fig 5D ) revealed that flow was reduced less in MCA DAs close to LMCs, especially in networks with 100% LMC or 50% LMC. The opposite behaviour was observed in ACA DAs, where flow dropped more close to LMCs and for a higher number of LMCs. This further confirms that LMCs redistribute blood from ACA DAs towards MCA DAs.
In line with the results in ACA sided SAs, we observed an increase in integral flow in ACA DAs close to LMCs for all four networks with 0% LMC ( Fig 5D ). As previously mentioned, this is caused by the reduced pressure in ACA sided Cs close to the wsl. Occasionally, this was also observed in networks with LMCs, predominantly in ACA DAs close to the watershed line connected to SAs that do not lead to LMCs ( Fig 4A and S3(A) Fig ).
The results for DAs can be directly translated to Cs, as seen in Fig 5E for the entire network and in Fig 5F for vessels within 250 μm of LMCs (see S12 and S20 Tables for averaged results). One major difference to DAs is that the integral flow rates in Cs decreased after MCAo for all networks and for all numbers of LMCs. This was the case for both MCA and ACA sided Cs, independently of whether all ( Fig 5E ) or only vessels close to the watershed line ( Fig 5F ) were analysed. Thus, importantly, the flow increase in some DAs close to the watershed line did not translate to an overall higher integral capillary perfusion, but likely only affected the capillaries proximal to the respective DAs. Even though the MCAo had a much larger effect on the flow reduction in MCA Cs compared to ACA Cs, the differences were smaller than in DAs. This is because the entire capillary bed is highly interconnected and there is no sharp boundary between capillaries that are only fed by MCA or ACA, respectively. Consequently, individual capillaries may receive flow from multiple DAs, located at the MCA, ACA or both sides. This leads to a smoother transition between observations for MCA and ACA Cs, if compared to DAs.
As for SAs, the precise level of flow rate change in DAs and Cs varied across datasets due to differences in topology. Nonetheless, we consistently observed that more LMCs and LMC dilation reduce the drop in perfusion in MCA DAs and Cs during stroke. For MCA sided capillaries the integral perfusion increased by +57.2% in C57BL/6 I , +32.5% in C57BL/6 II , +63.5% in BALB/c I and +88.0% in BALB/c II , if we compare the setup with 100% LMC and dilated LMCs to the case without LMCs. This goes hand in hand with a larger reduction in flow in ACA DAs and Cs for the case with many dilated LMCs (-10.9% in C57BL/6 I , -7.9% in C57BL/6 II , -15.5% in BALB/c I and -9.6% in BALB/c II ). Furthermore, dilating all arteries by 10% improved the perfusion of MCA sided DAs and Cs, and partially compensated the flow reductions in ACA sided vessels observed after LMC-dil. Taken together our results suggest that more LMCs and the dilation of LMCs is beneficial to maintain some perfusion during MCAo, especially close to the watershed line. While this redistribution of flow comes at the cost of a reduced perfusion on the ACA side, this drop is relatively small and likely does not cause any immediate tissue damage. Additional dilations of SAs and DAs help to further improve perfusion but rely on LMCs to distribute blood flow to the MCA side.
By performing blood flow simulations in large semi-realistic microvascular networks, we generate novel insights into the redistribution of blood flow in response to stroke. In networks with LMCs, we observed a pronounced increase in blood flow in all LMCs and a directed flow from ACA towards MCA sided SAs. It is noteworthy that these responses occurred without dilating any LMCs and directly resulted from the changed pressured field due to the occlusion of the MCA. Interestingly, the observed increase in flow in all LMCs and some ACA sided SAs after MCAo did not translate into a rise in integral perfusion of DAs and Cs, but to a redistribution of flow from ACA sided DAs to MCA sided DAs. This effect was most pronounced in networks with a large number of LMCs and was further enhanced by LMC dilation. It is also consistent with the experimentally [ 12 ] and numerically [ 21 ] observed smaller infarct volumes in networks with LMCs.
Additional dilation of all arteries after stroke increased flow rates in the entire vasculature, which is consistent with experimental studies showing that functional stimulation can protect the cortex after MCAo, likely by more efficiently re-routing flow via collateral vessels [ 22 , 23 ]. We also observed that the effect of artery dilation on recovering flow in MCA sided vessels is more pronounced in networks with many LMCs. Even more strikingly, our simulations suggest that the sole existence of LMCs has a larger effect on reducing the perfusion drop in MCA sided vessels than dilating arteries. This suggests that therapeutic vasodilations have potential to further increase perfusion after stroke, but therapeutic success likely strongly depends on the extent of LMCs. This aspect is also a probable explanation why clinical studies have been reporting different results [ 76 , 77 ].
Within our current set-up it is challenging to precisely asses to which extent LMC-dil and SA/DA-dil offer protection for the neural tissue in the stroke area. Even for MCAo & LMC/SA/DA-dil, perfusion in the capillary bed on the MCA side drops on average by -83.7%. While this is less than for MCAo in networks without LMCs (-90.9%), it still might lead to an energetic undersupply of neurons in the MCA area. A key factor to reduce tissue damage is certainly the time point at which perfusion can be re-established within the infarct area. In this regard, maintaining minimal perfusion to the infarct area might have the advantage that it could help to reduce secondary pathological alterations, such as capillary constrictions [ 82 – 84 ] or capillary stalls by neutrophils [ 15 ]. However, further in vivo and in silico studies will be necessary to quantify the protective capacity of LMCs and arterial dilations.
Another interesting observation is that the perfusion change in individual vessels in response to MCA occlusion, LMC dilation and artery dilation was highly heterogeneous. Differences were not only observed with respect to vessels types, but also with respect to the precise location of the vessel, e.g. if the SA is on a path to the LMCs or not. More precisely, at pial level an increase in perfusion predominantly occurred in ACA sided SAs located on a direct flow path from the ACA towards the LMCs. The flow rates in other ACA sided SAs remained approximately constant or decreased substantially in MCA sided SAs. At the level of MCA sided DAs and Cs we observed that vessels close to LMCs were generally affected less by the drop in perfusion than vessels further away. This is also interesting considering that recent work of our group revealed that reperfusion dynamics vary for the MCA-M3 and the MCA-M4/M5 territory [ 61 ]. While the precise origins of these differences are not yet fully understood, our current work already shows (on a even more local scale) that analysing flow changes at the level of SAs or in single DAs and Cs is not sufficient to obtain a complete picture of the perfusion change in the entire vasculature. This also highlights the necessity to account for the precise location of the vessel while interpreting both in vivo and in silico results and for the comparison of both. In this context, in silico approaches offer the advantage that flow changes can be analysed across all vessels and are not limited to a subset of vessels, as for example in two-photon microscopy.
Generally our observations are in agreement with experimental studies analysing perfusion changes during stroke in single LMCs [ 10 , 13 , 22 , 28 , 85 , 86 ] and in SAs, where flow reductions, reversals or even increases have been observed [ 26 , 27 ]. Also at the level of DAs [ 27 , 28 , 87 ] and Cs [ 87 ] a reduction in blood flow after stroke has been reported in in vivo experiments. Nonetheless, two important aspects have to be kept in mind if comparing our in silico data to in vivo experiments.
First, in the current study we only modelled how vessel diameters change passively after stroke, and additionally considered scenarios where LMCs and arteries were dilated to specified values. While this is an advantage to study the isolated effect of MCAo and LMCs and gives an impression on how perfusion changes immediately after stroke, it is also a strong simplification, because in vivo both active and passive constrictions and dilations are observed across SAs and DAs in response to stroke [ 15 , 26 – 28 ]. Furthermore, at the level of capillaries, constrictions [ 17 , 82 – 84 ] and blockages by neutrophils have been reported [ 15 ]. While it is evident that such alterations play a significant role for the resulting flow rate changes, their precise impact cannot be quantified until an in-depth description of all vasodynamics in response to stroke becomes available. For example, numerical models for autoregulation are available in literature. However, as autoregulation is impaired during stroke [ 80 ], it is currently unknown how these models would need to be adjusted in a post-stroke state. Nonetheless, incorporating such data into the presented in silico model is generally possible and would allow more refined studies on the redistribution of flow.
A second aspect that should be mentioned is that we focused on the analysis of relative flow rate changes. In in vivo experiments the measured quantity commonly is RBC velocity. For constant vessel diameters, the change in flow rate is identical to the change in velocity. However, if the vessel diameter is not constant the relative flow rate and RBC velocity change may differ. For example, in a dilated vessel the flow rate might increase, while the RBC velocities decreases. This has to be considered if comparing RBC velocity measurements against flow rate changes.
In the current study only few RBC velocity measurements and literature data were used. However, generally the inverse modelling approach allows to incorporate large numbers of in vivo measurements, e.g. from high-resolution tomographic imaging methods [ 32 – 35 ]. Moreover, the tuning of networks is not limited to velocity measurements, and other flow characteristics such as blood pressures, wall shear stresses or average flow rates measured on a coarser scale [ 43 ] could be added for tuning. This would further strengthen the link between in vivo experiment and in silico setup, and solidify the joint interpretation of results.
The presented simulation framework is not only versatile with respect to the type of incorporated measurements, but could also be employed to reduce uncertainties in fully realistic microvascular networks. For example, our inverse modelling approach could be used to improve diameter estimates in whole brain vascular reconstructions [ 45 – 48 ] based on reported structural and functional characteristics of the microvasculature, e.g. to make the networks consistent with observed flow rate distributions or vascular densities. In this context it is important to note, that inferring diameters of large networks based on limited experimental data inevitably results in ambiguous solutions, since multiple values of vessel diameters can match the prescribed data [ 43 , 72 , 73 ]. In the current study, we used a maximum allowable diameter change to reduce the ambiguity and validated the resulting networks with literature data. Alternatively, regularisation constraints could be applied to further reduce the space of possible solutions [ 72 ].
Besides addressing static scenarios, our approach could be extended to model reperfusion dynamics after clot removal. Possible applications would be to improve therapeutic interventions or strategies for clot removal and for avoiding reperfusion injuries [ 7 , 16 ]. In addition to the aforementioned in-depth characterisation of the vasodynamics after MCAo, such studies would require a model to describe the dissolution of the clot. Based on our current observations the dilation of LMCs, and possibly also other SAs, has the potential to increase perfusion in the territory affected by MCA occlusion. Thus, vasodilations at the level of SAs could be a therapeutic target point to reduce the size of the under-perfused territory. Nonetheless, it has to be kept in mind that the area of impact is likely limited to regions close to the watershed line and that the flow increase on the MCA side paritally comes at the cost of reduced blood supply on the ACA side. This also raises the interesting question if for an increasing number of LMCs we would observe a saturation effect regarding the flow redistribution or if the overall stroke outcome due to local reductions in blood supply would worsen.
Taken together, to the best of our knowledge, we present the first simulation study in large semi-realistic microvascular networks derived from case-specific pial networks and consistent with in vivo velocity measurements. This strong link between in silico and in vivo data is a key benefit of our novel simulation framework that certainly will also be beneficial for other study designs. Moreover, our in silico approach offers the advantage that networks can be altered systematically and resulting perfusion characteristics can be analysed quantitatively. This is crucial considering that the variability of the vascular topology is large across different mouse strains and even in individual animals [ 11 , 12 , 18 ]. As such, our in silico approach allows more robust conclusions on the role of specific microvascular characteristics, as for example the number of LMCs. Because of the strong connection to case-specific in vivo data it is ideally suited to be employed hand-in-hand with in vivo experiments and to amplify the amount of conclusion to be drawn.
Supporting information
S1 fig. relative changes of blood flow rates in sas..
Relative changes of blood flow rates in individual SAs of the network C57BL/6 II from Base to MCAo (A) and MCAo to MCAo & LMC-dil (B). The yellow squares indicate the locations of the LMCs. Supplement to Fig 3A and 3B . Refer to S7(A) Fig for results after LMC/SA/DA-dil.
https://doi.org/10.1371/journal.pcbi.1011496.s001
S2 Fig. Direction changes in response to MCAo.
Edges with direction changes (dark blue) in response to MCAo for the networks C57BL/6 I (A) and C57BL/6 II (B). The yellow squares indicate the locations of the LMCs. Supplement to Fig 3 .
https://doi.org/10.1371/journal.pcbi.1011496.s002
S3 Fig. Relative changes of blood flow rates in DAs.
Relative changes of blood flow rates in DAs of the network C57BL/6 II from Base to MCAo (A) and MCAo to MCAo & LMC-dil (B). Supplement to Fig 4A and 4B . Refer to S8 Fig for results after LMC/SA/DA-dil.
https://doi.org/10.1371/journal.pcbi.1011496.s003
S4 Fig. Relative changes of SA diameters in response to MCAo.
Relative changes of SA diameters in response to MCAo in the networks C57BL/6 I (A) and C57BL/6 II (B).
https://doi.org/10.1371/journal.pcbi.1011496.s004
S5 Fig. Maps of pial networks and visualisation of the three scenarios with many, few and no LMCs.
Maps of the pial networks C57BL/6 II (A), BALB/c I (B) and BALB/c II (C), and visualisation of the three scenarios with many, few and no LMCs. Supplement to Fig 5A . To define the number of added LMCs for the 100% LMC scenarios of the BALB/c datasets, the LMC density along the watershed line was computed from the two C57BL/6 datasets. The goal was then to obtain the same overall LMC density along the watershed line for the BALB/c networks. This was done with a sequential procedure by randomly selecting MCA DA vertices at the watershed line and connecting them to the closest DA vertices in the ACA territory (D). The sampled LMCs were then accepted or rejected based on two criteria derived from the C57BL/6 datasets: 1) The distance to already existing LMCs Δ c was larger than 310 μm and 2) the maximum LMC length l c was 1000 μm (E). As for the C57BL/6 networks, the 50% LMC scenario for BALB/c networks was defined by randomly removing LMCs.
https://doi.org/10.1371/journal.pcbi.1011496.s005
S6 Fig. Relative changes of pressures in SAs in response to MCAo and LMC-dil.
Relative changes of pressures in SAs of the network C57BL/6 I from Base to MCAo (A) and MCAo to MCAo & LMC-dil (B). The results for the network C57BL/6 II are shown in panels (C) and (D), respectively. Refer to S7(B) and S7(C) Fig for results after LMC/SA/DA-dil.
https://doi.org/10.1371/journal.pcbi.1011496.s006
S7 Fig. Relative changes of flow rates and pressures in SAs in response to LMC/SA/DA-dil.
(A) Relative changes of flow rates in SAs of the network C57BL/6 II from MCAo & LMC-dil to MCAo & LMC/SA/DA-dil. (B-C) The corresponding pressure changes are shown in panels (B) and (C) for both networks C57BL/6 I and C57BL/6 II , respectively.
https://doi.org/10.1371/journal.pcbi.1011496.s007
S8 Fig. Relative changes of blood flow rates in DAs after LMC/SA/DA-dil.
Relative changes of blood flow rates in DAs from MCAo & LMC-dil to MCAo & LMC/SA/DA-dil in the networks C57BL/6 I (A) and C57BL/6 II (B).
https://doi.org/10.1371/journal.pcbi.1011496.s008
S9 Fig. Reconstructions of surface artery networks.
Experimentally acquired reconstructions of the surface artery (SA) networks BALB/c I (A), BALB/c II (B) and C57BL/6 II (C). The numbers refer to diameter and RBC velocity measurements obtained in individual SAs ( S24 , S25 and S26 Tables).
https://doi.org/10.1371/journal.pcbi.1011496.s009
S10 Fig. In vivo two-photon images of the four datasets used in the current study.
In vivo two-photon images (top) and reconstructions of surface arteries (bottom) of the networks C57BL/6 I (A), C57BL/6 II (B), BALB/c I (C) and BALB/c II (D).
https://doi.org/10.1371/journal.pcbi.1011496.s010

S11 Fig. Vessel diameters before and after tuning.
https://doi.org/10.1371/journal.pcbi.1011496.s011
S12 Fig. RBC velocity distributions.
Histograms of RBC velocities u rbc [ mm / s ] in the networks C57BL/6 I (A), C57BL/6 II (B), BALB/c I (C) and BALB/c II (D), classified into the vessel types SAs (red), DAs (pink), Cs (grey) and AVs (blue). The corresponding mean and median values are shown with dashed and dashed-dotted lines, respectively. Capillaries at the border of the networks, i.e., with a distance >200 μm to any DA edge, were excluded from the analysis. However, in contrast to S4 Table , velocity values for all DA and AV edge segments were included into the analysis here. Exemplary RBC velocity distributions from in vivo measurements are for example available in the following references: [ 17 , 71 ] (DAs, AVs) and [ 17 , 71 , 74 , 88 – 91 ] (Cs).
https://doi.org/10.1371/journal.pcbi.1011496.s012
S13 Fig. RBC flow rate distributions.
Histograms of RBC flow rates q rbc [fl/s] in the networks C57BL/6 I (A), C57BL/6 II (B), BALB/c I (C) and BALB/c II (D), classified into the vessel types SAs (red), DAs (pink), Cs (grey) and AVs (blue). The corresponding mean and median values are shown with dashed and dashed-dotted lines, respectively. Capillaries at the border of the networks, i.e., with a distance >200 μm to any DA edge, were excluded from the analysis. However, in contrast to S28 Table , flow rate values for all DA and AV edge segments were included into the analysis here. Exemplary RBC flux distributions from in vivo measurements are for example available in the following references: [ 17 , 71 ] (DAs, AVs) and [ 17 , 71 , 74 , 88 , 89 , 91 ] (Cs).
https://doi.org/10.1371/journal.pcbi.1011496.s013
S1 Appendix. Refinement of surface artery network.
References of S1 Appendix : [ 3 , 18 , 61 , 92 – 94 ].
https://doi.org/10.1371/journal.pcbi.1011496.s014
S2 Appendix. Blood flow model details.
References of S2 Appendix : [ 37 , 39 , 70 , 95 ].
https://doi.org/10.1371/journal.pcbi.1011496.s015
S3 Appendix. Inverse model details.
References of S3 Appendix : [ 43 , 70 ].
https://doi.org/10.1371/journal.pcbi.1011496.s016
S4 Appendix. Vessel elasticity model details.
References of S4 Appendix : [ 21 , 79 , 81 , 96 , 97 ].
https://doi.org/10.1371/journal.pcbi.1011496.s017
S1 Table. In vivo two-photon microscopy diameter and RBC velocity measurements in surface arteries of network C57BL/6 I ( Fig 1A ) at baseline.
For LMCs, diameter measurements are additionally given for the state after MCAo & LMC-dil. “x” is used if no velocity or diameter measurement was obtained in the vessel. The measurements are grouped into MCA and ACA sided SAs, and LMCs. Refer to S24 , S25 and S26 Tables for measurements in other datasets.
https://doi.org/10.1371/journal.pcbi.1011496.s018
S2 Table. Characteristic parameters of the four networks (2x C57BL/6, 2x BALB/c) used in the present study.
https://doi.org/10.1371/journal.pcbi.1011496.s019
S3 Table. Validation of characteristic vascular parameters with literature data.
Mean ± standard deviation of diameters after tuning were calculated by considering all edge segments of the respective vessel type. Furthermore, the ranges of reported mean literature values are given in the last row. References for literature values: A Length density [ 3 , 65 , 98 , 99 ]; B Volume density [ 3 , 45 – 48 , 99 , 100 ]; C SAs [ 17 , 28 , 101 ]; D DAs [ 3 , 17 , 28 , 84 , 88 , 101 – 103 ]; E Cs [ 3 , 17 , 46 – 48 , 84 , 88 , 99 , 101 – 103 ]; F AVs [ 3 , 17 , 88 ].
https://doi.org/10.1371/journal.pcbi.1011496.s020
S4 Table. Validation of characteristic velocities in different vessel types of all four networks.
Mean ± standard deviation are given. Capillaries at the border of the networks, i.e., with a distance >200 μm to any DA edge, were excluded from the analysis. The average values for DAs and AVs refer to the segments of the penetrating trees closest to the cortical surface, i.e., the DA and AV root edges. The ranges of reported mean literature values are given in the last row. References for literature values: A SAs [ 17 , 26 , 27 , 101 ]; B DAs [ 17 , 37 , 92 , 101 , 102 , 104 , 105 ]; C Cs [ 17 , 37 , 74 , 88 – 91 , 101 – 103 ]; D AVs [ 17 , 71 ].
https://doi.org/10.1371/journal.pcbi.1011496.s021
S5 Table. Relative changes of mean pressure in response to MCAo and LMC-dil in MCA and ACA sided SAs of the datasets C57BL/6 I and C57BL/6 II .
https://doi.org/10.1371/journal.pcbi.1011496.s022
S6 Table. Relative changes of mean flow rate in response to MCAo and LMC-dil in SAs of the datasets C57BL/6 I and C57BL/6 II .
https://doi.org/10.1371/journal.pcbi.1011496.s023
S7 Table. Relative changes of integral flow rate after MCAo and LMC-dil in DAs of the datasets C57BL/6 I and C57BL/6 II .
https://doi.org/10.1371/journal.pcbi.1011496.s024
S8 Table. Relative changes of integral flow rate after MCAo and LMC-dil in Cs of the datasets C57BL/6 I and C57BL/6 II .
https://doi.org/10.1371/journal.pcbi.1011496.s025
S9 Table. Relative changes of mean flow rate in SAs (definition in S6 Table ) in response to MCAo and MCAo & LMC-dil for different number of LMCs.
〈…〉 is used to refer to average values of all four datasets. The results are consistent with the bars in Fig 5B . Refer to S18 Table for results after LMC/SA/DA-dil.
https://doi.org/10.1371/journal.pcbi.1011496.s026
S10 Table. Relative changes of mean pressure in SAs (definition in S5 Table ) in response to MCAo and MCAo & LMC-dil for different number of LMCs.
〈…〉 is used to refer to average values of all four datasets. Refer to S17 Table for results after LMC/SA/DA-dil.
https://doi.org/10.1371/journal.pcbi.1011496.s027
S11 Table. Relative changes of integral flow rate in DAs (definition in S7 Table ) in response to MCAo and MCA & LMC-dil for different number of LMCs.
〈…〉 is used to refer to average values computed over all four datasets. The results are consistent with the bars in Fig 5C and 5D . Refer to S19 Table for results after LMC/SA/DA-dil.
https://doi.org/10.1371/journal.pcbi.1011496.s028
S12 Table. Relative changes of integral flow rate in Cs (definition in S8 Table ) in response to MCAo and MCAo & LMC-dil for different number of LMCs.
〈…〉 is used to refer to average values computed over all four datasets. The results are consistent with the bars in Fig 5E and 5F . Refer to S20 Table for results after LMC/SA/DA-dil.
https://doi.org/10.1371/journal.pcbi.1011496.s029
S13 Table. Relative changes of mean pressure (definition in S5 Table ) in response to MCAo & LMC/SA/DA-dil in MCA and ACA sided SAs of the datasets C57BL/6 I and C57BL/6 II .
https://doi.org/10.1371/journal.pcbi.1011496.s030
S14 Table. Relative changes of mean flow rate (definition in S6 Table ) in response to MCAo & LMC/SA/DA-dil in SAs of the datasets C57BL/6 I and C57BL/6 II in comparison to Base and MCAo.
https://doi.org/10.1371/journal.pcbi.1011496.s031
S15 Table. Relative changes of integral flow rate (definition in S7 Table ) after MCAo & LMC/SA/DA-dil in DAs of the datasets C57BL/6 I and C57BL/6 II in comparison to Base and MCAo.
https://doi.org/10.1371/journal.pcbi.1011496.s032
S16 Table. Relative changes of integral flow rate (definition in S7 Table ) after MCAo & LMC/SA/DA-dil in Cs of the datasets C57BL/6 I and C57BL/6 II in comparison to Base and MCAo.
https://doi.org/10.1371/journal.pcbi.1011496.s033
S17 Table. Relative changes of mean pressure in SAs (definition in S5 Table ) in response to MCAo & LMC/SA/DA-dil for different number of LMCs.
〈…〉 is used to refer to average values computed over all four datasets.
https://doi.org/10.1371/journal.pcbi.1011496.s034
S18 Table. Relative changes of mean flow rate in SAs (definition in S6 Table ) in response to MCAo & LMC/SA/DA-dil for different number of LMCs in comparison to Base and MCAo.
https://doi.org/10.1371/journal.pcbi.1011496.s035
S19 Table. Relative changes of integral flow rate in DAs (definition in S7 Table ) in response to MCAo & LMC/SA/DA-dil for different number of LMCs.
https://doi.org/10.1371/journal.pcbi.1011496.s036
S20 Table. Relative changes of integral flow rate in Cs (definition in S7 Table ) in response to MCAo & LMC/SA/DA-dil for different number of LMCs.
https://doi.org/10.1371/journal.pcbi.1011496.s037
S21 Table. Comparison of relative changes of mean flow rates (definition in S6 Table ) in SAs after MCAo with and without taking the elasticity of blood vessels into account.
https://doi.org/10.1371/journal.pcbi.1011496.s038
S22 Table. Comparison of relative changes of integral flow rates (definition in S7 Table ) in DAs after MCAo with and without taking the elasticity of blood vessels into account.
https://doi.org/10.1371/journal.pcbi.1011496.s039
S23 Table. Comparison of relative changes of integral flow rates (definition in S7 Table ) in Cs after MCAo with and without taking the elasticity of blood vessels into account.
https://doi.org/10.1371/journal.pcbi.1011496.s040
S24 Table. In vivo two-photon microscopy diameter and RBC velocity measurements in pial arteries of network BALB/c I ( S9(A) Fig ) at baseline.
“x” is used if no velocity or diameter measurement was obtained in the vessel. The measurements are grouped into MCA and ACA sided SAs.
https://doi.org/10.1371/journal.pcbi.1011496.s041
S25 Table. In vivo two-photon microscopy diameter measurements in pial arteries of network C57BL/6 II ( S9(C) Fig ) at baseline.
The measurements are grouped into MCA and ACA sided SAs.
https://doi.org/10.1371/journal.pcbi.1011496.s042
S26 Table. In vivo two-photon microscopy diameter measurements in pial arteries of network BALB/c II ( S9(B) Fig ) at baseline.
https://doi.org/10.1371/journal.pcbi.1011496.s043
S27 Table. Comparison of simulated cerebral blood flow.
The average blood flow per surface area and tissue volume were determined based on the total blood flow that enters the network and the estimated surface area of the network, which was calculated from the Voronoi polygons shown in Fig A panel B in S1 Appendix . Based on the average blood flow per tissue volume, cerebral blood flow per mass was computed assuming a tissue density of 1046 kg/m 3 [ 106 ]. References for CBF literature values: A CBF [ 107 – 111 ].
https://doi.org/10.1371/journal.pcbi.1011496.s044
S28 Table. Validation of characteristic RBC flow rates in different vessel types of all four networks.
Mean ± standard deviation are given. Capillaries at the border of the networks, i.e., with a distance >200 μm to any DA edge, were excluded from the analysis. The average values for DAs and AVs refer to the segments of the penetrating trees closest to the cortical surface, i.e., the DA and AV root edges. The ranges of reported mean literature values are given in the last row. References for literature values: A DAs [ 17 , 37 , 71 , 92 ]; B Cs [ 17 , 37 , 71 , 88 , 89 , 91 ]; C AVs [ 17 , 71 ].
https://doi.org/10.1371/journal.pcbi.1011496.s045
S29 Table. Validation of wall shear stress (WSS) in different vessel types of all four networks.
https://doi.org/10.1371/journal.pcbi.1011496.s046
Acknowledgments
We thank Dr. med. Anna Maria Reuss for clearing and staining (iDISCO) of the brains as well as assisting with imaging. Moreover, we thank Chryso Lambride and Timo Koch for their support and discussions. We also thank Denise Mühlethaler and Gaia Lauper for performing preliminary simulations during an early stage of this project.
- View Article
- PubMed/NCBI
- Google Scholar
- 4. Mosso A. Über den Kreislauf des Blutes im menschlichen Gehirn. Leipzig: Veit; 1881.
- 54. Sunwoo J, Cornelius NR, Doerschuk PC, Schaffer CB. Estimating brain microvascular blood flows from partial two-photon microscopy data by computation with a circuit model. In: 2011 Annual International Conference of the IEEE Engineering in Medicine and Biology Society. IEEE; 2011. p. 174–177.
- 62. Reichold J. Cerebral blood flow modeling in realistic cortical microvascular networks; 2011.
- 75. Moeini M, Tabatabaei MS, Bélanger S, Avti P, Castonguay A, Pouliot P, et al. Effects of anesthesia on the cerebral capillary blood flow in young and old mice. In: Multiphoton Microscopy in the Biomedical Sciences XV. vol. 9329. SPIE; 2015. p. 199–204.
- Open access
- Published: 15 May 2024
Learning together for better health using an evidence-based Learning Health System framework: a case study in stroke
- Helena Teede 1 , 2 na1 ,
- Dominique A. Cadilhac 3 , 4 na1 ,
- Tara Purvis 3 ,
- Monique F. Kilkenny 3 , 4 ,
- Bruce C.V. Campbell 4 , 5 , 6 ,
- Coralie English 7 ,
- Alison Johnson 2 ,
- Emily Callander 1 ,
- Rohan S. Grimley 8 , 9 ,
- Christopher Levi 10 ,
- Sandy Middleton 11 , 12 ,
- Kelvin Hill 13 &
- Joanne Enticott ORCID: orcid.org/0000-0002-4480-5690 1
BMC Medicine volume 22 , Article number: 198 ( 2024 ) Cite this article
192 Accesses
1 Altmetric
Metrics details
In the context of expanding digital health tools, the health system is ready for Learning Health System (LHS) models. These models, with proper governance and stakeholder engagement, enable the integration of digital infrastructure to provide feedback to all relevant parties including clinicians and consumers on performance against best practice standards, as well as fostering innovation and aligning healthcare with patient needs. The LHS literature primarily includes opinion or consensus-based frameworks and lacks validation or evidence of benefit. Our aim was to outline a rigorously codesigned, evidence-based LHS framework and present a national case study of an LHS-aligned national stroke program that has delivered clinical benefit.
Current core components of a LHS involve capturing evidence from communities and stakeholders (quadrant 1), integrating evidence from research findings (quadrant 2), leveraging evidence from data and practice (quadrant 3), and generating evidence from implementation (quadrant 4) for iterative system-level improvement. The Australian Stroke program was selected as the case study as it provides an exemplar of how an iterative LHS works in practice at a national level encompassing and integrating evidence from all four LHS quadrants. Using this case study, we demonstrate how to apply evidence-based processes to healthcare improvement and embed real-world research for optimising healthcare improvement. We emphasize the transition from research as an endpoint, to research as an enabler and a solution for impact in healthcare improvement.
Conclusions
The Australian Stroke program has nationally improved stroke care since 2007, showcasing the value of integrated LHS-aligned approaches for tangible impact on outcomes. This LHS case study is a practical example for other health conditions and settings to follow suit.
Peer Review reports
Internationally, health systems are facing a crisis, driven by an ageing population, increasing complexity, multi-morbidity, rapidly advancing health technology and rising costs that threaten sustainability and mandate transformation and improvement [ 1 , 2 ]. Although research has generated solutions to healthcare challenges, and the advent of big data and digital health holds great promise, entrenched siloes and poor integration of knowledge generation, knowledge implementation and healthcare delivery between stakeholders, curtails momentum towards, and consistent attainment of, evidence-and value-based care [ 3 ]. This is compounded by the short supply of research and innovation leadership within the healthcare sector, and poorly integrated and often inaccessible health data systems, which have crippled the potential to deliver on digital-driven innovation [ 4 ]. Current approaches to healthcare improvement are also often isolated with limited sustainability, scale-up and impact [ 5 ].
Evidence suggests that integration and partnership across academic and healthcare delivery stakeholders are key to progress, including those with lived experience and their families (referred to here as consumers and community), diverse disciplines (both research and clinical), policy makers and funders. Utilization of evidence from research and evidence from practice including data from routine care, supported by implementation research, are key to sustainably embedding improvement and optimising health care and outcomes. A strategy to achieve this integration is through the Learning Health System (LHS) (Fig. 1 ) [ 2 , 6 , 7 , 8 ]. Although there are numerous publications on LHS approaches [ 9 , 10 , 11 , 12 ], many focus on research perspectives and data, most do not demonstrate tangible healthcare improvement or better health outcomes. [ 6 ]
Monash Learning Health System: The Learn Together for Better Health Framework developed by Monash Partners and Monash University (from Enticott et al. 2021 [ 7 ]). Four evidence quadrants: Q1 (orange) is evidence from stakeholders; Q2 (green) is evidence from research; Q3 (light blue) is evidence from data; and, Q4 (dark blue) is evidence from implementation and healthcare improvement
In developed nations, it has been estimated that 60% of care provided aligns with the evidence base, 30% is low value and 10% is potentially harmful [ 13 ]. In some areas, clinical advances have been rapid and research and evidence have paved the way for dramatic improvement in outcomes, mandating rapid implementation of evidence into healthcare (e.g. polio and COVID-19 vaccines). However, healthcare improvement is challenging and slow [ 5 ]. Health systems are highly complex in their design, networks and interacting components, and change is difficult to enact, sustain and scale up. [ 3 ] New effective strategies are needed to meet community needs and deliver evidence-based and value-based care, which reorients care from serving the provider, services and system, towards serving community needs, based on evidence and quality. It goes beyond cost to encompass patient and provider experience, quality care and outcomes, efficiency and sustainability [ 2 , 6 ].
The costs of stroke care are expected to rise rapidly in the next decades, unless improvements in stroke care to reduce the disabling effects of strokes can be successfully developed and implemented [ 14 ]. Here, we briefly describe the Monash LHS framework (Fig. 1 ) [ 2 , 6 , 7 ] and outline an exemplar case in order to demonstrate how to apply evidence-based processes to healthcare improvement and embed real-world research for optimising healthcare. The Australian LHS exemplar in stroke care has driven nationwide improvement in stroke care since 2007.
An evidence-based Learning Health System framework
In Australia, members of this author group (HT, AJ, JE) have rigorously co-developed an evidence-based LHS framework, known simply as the Monash LHS [ 7 ]. The Monash LHS was designed to support sustainable, iterative and continuous robust benefit of improved clinical outcomes. It was created with national engagement in order to be applicable to Australian settings. Through this rigorous approach, core LHS principles and components have been established (Fig. 1 ). Evidence shows that people/workforce, culture, standards, governance and resources were all key to an effective LHS [ 2 , 6 ]. Culture is vital including trust, transparency, partnership and co-design. Key processes include legally compliant data sharing, linkage and governance, resources, and infrastructure [ 4 ]. The Monash LHS integrates disparate and often siloed stakeholders, infrastructure and expertise to ‘Learn Together for Better Health’ [ 7 ] (Fig. 1 ). This integrates (i) evidence from community and stakeholders including priority areas and outcomes; (ii) evidence from research and guidelines; (iii) evidence from practice (from data) with advanced analytics and benchmarking; and (iv) evidence from implementation science and health economics. Importantly, it starts with the problem and priorities of key stakeholders including the community, health professionals and services and creates an iterative learning system to address these. The following case study was chosen as it is an exemplar of how a Monash LHS-aligned national stroke program has delivered clinical benefit.
Australian Stroke Learning Health System
Internationally, the application of LHS approaches in stroke has resulted in improved stroke care and outcomes [ 12 ]. For example, in Canada a sustained decrease in 30-day in-hospital mortality has been found commensurate with an increase in resources to establish the multifactorial stroke system intervention for stroke treatment and prevention [ 15 ]. Arguably, with rapid advances in evidence and in the context of an ageing population with high cost and care burden and substantive impacts on quality of life, stroke is an area with a need for rapid research translation into evidence-based and value-based healthcare improvement. However, a recent systematic review found that the existing literature had few comprehensive examples of LHS adoption [ 12 ]. Although healthcare improvement systems and approaches were described, less is known about patient-clinician and stakeholder engagement, governance and culture, or embedding of data informatics into everyday practice to inform and drive improvement [ 12 ]. For example, in a recent review of quality improvement collaborations, it was found that although clinical processes in stroke care are improved, their short-term nature means there is uncertainty about sustainability and impacts on patient outcomes [ 16 ]. Table 1 provides the main features of the Australian Stroke LHS based on the four core domains and eight elements of the Learning Together for Better Health Framework described in Fig. 1 . The features are further expanded on in the following sections.
Evidence from stakeholders (LHS quadrant 1, Fig. 1 )
Engagement, partners and priorities.
Within the stroke field, there have been various support mechanisms to facilitate an LHS approach including partnership and broad stakeholder engagement that includes clinical networks and policy makers from different jurisdictions. Since 2008, the Australian Stroke Coalition has been co-led by the Stroke Foundation, a charitable consumer advocacy organisation, and Stroke Society of Australasia a professional society with membership covering academics and multidisciplinary clinician networks, that are collectively working to improve stroke care ( https://australianstrokecoalition.org.au/ ). Surveys, focus groups and workshops have been used for identifying priorities from stakeholders. Recent agreed priorities have been to improve stroke care and strengthen the voice for stroke care at a national ( https://strokefoundation.org.au/ ) and international level ( https://www.world-stroke.org/news-and-blog/news/world-stroke-organization-tackle-gaps-in-access-to-quality-stroke-care ), as well as reduce duplication amongst stakeholders. This activity is built on a foundation and culture of research and innovation embedded within the stroke ‘community of practice’. Consumers, as people with lived experience of stroke are important members of the Australian Stroke Coalition, as well as representatives from different clinical colleges. Consumers also provide critical input to a range of LHS activities via the Stroke Foundation Consumer Council, Stroke Living Guidelines committees, and the Australian Stroke Clinical Registry (AuSCR) Steering Committee (described below).
Evidence from research (LHS quadrant 2, Fig. 1 )
Advancement of the evidence for stroke interventions and synthesis into clinical guidelines.
To implement best practice, it is crucial to distil the large volume of scientific and trial literature into actionable recommendations for clinicians to use in practice [ 24 ]. The first Australian clinical guidelines for acute stroke were produced in 2003 following the increasing evidence emerging for prevention interventions (e.g. carotid endarterectomy, blood pressure lowering), acute medical treatments (intravenous thrombolysis, aspirin within 48 h of ischemic stroke), and optimised hospital management (care in dedicated stroke units by a specialised and coordinated multidisciplinary team) [ 25 ]. Importantly, a number of the innovations were developed, researched and proven effective by key opinion leaders embedded in the Australian stroke care community. In 2005, the clinical guidelines for Stroke Rehabilitation and Recovery [ 26 ] were produced, with subsequent merged guidelines periodically updated. However, the traditional process of periodic guideline updates is challenging for end users when new research can render recommendations redundant and this lack of currency erodes stakeholder trust [ 27 ]. In response to this challenge the Stroke Foundation and Cochrane Australia entered a pioneering project to produce the first electronic ‘living’ guidelines globally [ 20 ]. Major shifts in the evidence for reperfusion therapies (e.g. extended time-window intravenous thrombolysis and endovascular clot retrieval), among other advances, were able to be converted into new recommendations, approved by the Australian National Health and Medical Research Council within a few months of publication. Feedback on this process confirmed the increased use and trust in the guidelines by clinicians. The process informed other living guidelines programs, including the successful COVID-19 clinical guidelines [ 28 ].
However, best practice clinical guideline recommendations are necessary but insufficient for healthcare improvement and nesting these within an LHS with stakeholder partnership, enables implementation via a range of proven methods, including audit and feedback strategies [ 29 ].
Evidence from data and practice (LHS quadrant 3, Fig. 1 )
Data systems and benchmarking : revealing the disparities in care between health services. A national system for standardized stroke data collection was established as the National Stroke Audit program in 2007 by the Stroke Foundation [ 30 ] following various state-level programs (e.g. New South Wales Audit) [ 31 ] to identify evidence-practice gaps and prioritise improvement efforts to increase access to stroke units and other acute treatments [ 32 ]. The Audit program alternates each year between acute (commencing in 2007) and rehabilitation in-patient services (commencing in 2008). The Audit program provides a ‘deep dive’ on the majority of recommendations in the clinical guidelines whereby participating hospitals provide audits of up to 40 consecutive patient medical records and respond to a survey about organizational resources to manage stroke. In 2009, the AuSCR was established to provide information on patients managed in acute hospitals based on a small subset of quality processes of care linked to benchmarked reports of performance (Fig. 2 ) [ 33 ]. In this way, the continuous collection of high-priority processes of stroke care could be regularly collected and reviewed to guide improvement to care [ 34 ]. Plus clinical quality registry programs within Australia have shown a meaningful return on investment attributed to enhanced survival, improvements in quality of life and avoided costs of treatment or hospital stay [ 35 ].
Example performance report from the Australian Stroke Clinical Registry: average door-to-needle time in providing intravenous thrombolysis by different hospitals in 2021 [ 36 ]. Each bar in the figure represents a single hospital
The Australian Stroke Coalition endorsed the creation of an integrated technological solution for collecting data through a single portal for multiple programs in 2013. In 2015, the Stroke Foundation, AuSCR consortium, and other relevant groups cooperated to design an integrated data management platform (the Australian Stroke Data Tool) to reduce duplication of effort for hospital staff in the collection of overlapping variables in the same patients [ 19 ]. Importantly, a national data dictionary then provided the common data definitions to facilitate standardized data capture. Another important feature of AuSCR is the collection of patient-reported outcome surveys between 90 and 180 days after stroke, and annual linkage with national death records to ascertain survival status [ 33 ]. To support a LHS approach, hospitals that participate in AuSCR have access to a range of real-time performance reports. In efforts to minimize the burden of data collection in the AuSCR, interoperability approaches to import data directly from hospital or state-level managed stroke databases have been established (Fig. 3 ); however, the application has been variable and 41% of hospitals still manually enter all their data.
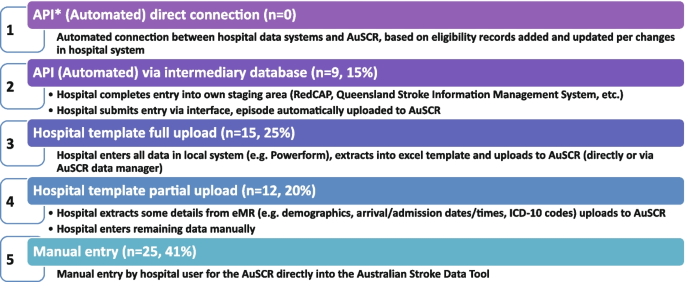
Current status of automated data importing solutions in the Australian Stroke Clinical Registry, 2022, with ‘ n ’ representing the number of hospitals. AuSCR, Australian Stroke Clinical Registry; AuSDaT, Australian Stroke Data Tool; API, Application Programming Interface; ICD, International Classification of Diseases; RedCAP, Research Electronic Data Capture; eMR, electronic medical records
For acute stroke care, the Australian Commission on Quality and Safety in Health Care facilitated the co-design (clinicians, academics, consumers) and publication of the national Acute Stroke Clinical Care Standard in 2015 [ 17 ], and subsequent review [ 18 ]. The indicator set for the Acute Stroke Standard then informed the expansion of the minimum dataset for AuSCR so that hospitals could routinely track their performance. The national Audit program enabled hospitals not involved in the AuSCR to assess their performance every two years against the Acute Stroke Standard. Complementing these efforts, the Stroke Foundation, working with the sector, developed the Acute and Rehabilitation Stroke Services Frameworks to outline the principles, essential elements, models of care and staffing recommendations for stroke services ( https://informme.org.au/guidelines/national-stroke-services-frameworks ). The Frameworks are intended to guide where stroke services should be developed, and monitor their uptake with the organizational survey component of the Audit program.
Evidence from implementation and healthcare improvement (LHS quadrant 4, Fig. 1 )
Research to better utilize and augment data from registries through linkage [ 37 , 38 , 39 , 40 ] and to ensure presentation of hospital or service level data are understood by clinicians has ensured advancement in the field for the Australian Stroke LHS [ 41 ]. Importantly, greater insights into whole patient journeys, before and after a stroke, can now enable exploration of value-based care. The LHS and stroke data platform have enabled focused and time-limited projects to create a better understanding of the quality of care in acute or rehabilitation settings [ 22 , 42 , 43 ]. Within stroke, all the elements of an LHS culminate into the ready availability of benchmarked performance data and support for implementation of strategies to address gaps in care.
Implementation research to grow the evidence base for effective improvement interventions has also been a key pillar in the Australian context. These include multi-component implementation interventions to achieve behaviour change for particular aspects of stroke care, [ 22 , 23 , 44 , 45 ] and real-world approaches to augmenting access to hyperacute interventions in stroke through the use of technology and telehealth [ 46 , 47 , 48 , 49 ]. The evidence from these studies feeds into the living guidelines program and the data collection systems, such as the Audit program or AuSCR, which are then amended to ensure data aligns to recommended care. For example, the use of ‘hyperacute aspirin within the first 48 h of ischemic stroke’ was modified to be ‘hyperacute antiplatelet…’ to incorporate new evidence that other medications or combinations are appropriate to use. Additionally, new datasets have been developed to align with evidence such as the Fever, Sugar, and Swallow variables [ 42 ]. Evidence on improvements in access to best practice care from the acute Audit program [ 50 ] and AuSCR is emerging [ 36 ]. For example, between 2007 and 2017, the odds of receiving intravenous thrombolysis after ischemic stroke increased by 16% 9OR 1.06 95% CI 1.13–1.18) and being managed in a stroke unit by 18% (OR 1.18 95% CI 1.17–1.20). Over this period, the median length of hospital stay for all patients decreased from 6.3 days in 2007 to 5.0 days in 2017 [ 51 ]. When considering the number of additional patients who would receive treatment in 2017 in comparison to 2007 it was estimated that without this additional treatment, over 17,000 healthy years of life would be lost in 2017 (17,786 disability-adjusted life years) [ 51 ]. There is evidence on the cost-effectiveness of different system-focussed strategies to augment treatment access for acute ischemic stroke (e.g. Victorian Stroke Telemedicine program [ 52 ] and Melbourne Mobile Stroke Unit ambulance [ 53 ]). Reciprocally, evidence from the national Rehabilitation Audit, where the LHS approach has been less complete or embedded, has shown fewer areas of healthcare improvement over time [ 51 , 54 ].
Within the field of stroke in Australia, there is indirect evidence that the collective efforts that align to establishing the components of a LHS have had an impact. Overall, the age-standardised rate of stroke events has reduced by 27% between 2001 and 2020, from 169 to 124 events per 100,000 population. Substantial declines in mortality rates have been reported since 1980. Commensurate with national clinical guidelines being updated in 2007 and the first National Stroke Audit being undertaken in 2007, the mortality rates for men (37.4 deaths per 100,000) and women (36.1 deaths per 100,0000 has declined to 23.8 and 23.9 per 100,000, respectively in 2021 [ 55 ].
Underpinning the LHS with the integration of the four quadrants of evidence from stakeholders, research and guidelines, practice and implementation, and core LHS principles have been addressed. Leadership and governance have been important, and programs have been established to augment workforce training and capacity building in best practice professional development. Medical practitioners are able to undertake courses and mentoring through the Australasian Stroke Academy ( http://www.strokeacademy.com.au/ ) while nurses (and other health professionals) can access teaching modules in stroke care from the Acute Stroke Nurses Education Network ( https://asnen.org/ ). The Association of Neurovascular Clinicians offers distance-accessible education and certification to develop stroke expertise for interdisciplinary professionals, including advanced stroke co-ordinator certification ( www.anvc.org ). Consumer initiative interventions are also used in the design of the AuSCR Public Summary Annual reports (available at https://auscr.com.au/about/annual-reports/ ) and consumer-related resources related to the Living Guidelines ( https://enableme.org.au/resources ).
The important success factors and lessons from stroke as a national exemplar LHS in Australia include leadership, culture, workforce and resources integrated with (1) established and broad partnerships across the academic-clinical sector divide and stakeholder engagement; (2) the living guidelines program; (3) national data infrastructure, including a national data dictionary that provides the common data framework to support standardized data capture; (4) various implementation strategies including benchmarking and feedback as well as engagement strategies targeting different levels of the health system; and (5) implementation and improvement research to advance stroke systems of care and reduce unwarranted variation in practice (Fig. 1 ). Priority opportunities now include the advancement of interoperability with electronic medical records as an area all clinical quality registry’s programs needs to be addressed, as well as providing more dynamic and interactive data dashboards tailored to the need of clinicians and health service executives.
There is a clear mandate to optimise healthcare improvement with big data offering major opportunities for change. However, we have lacked the approaches to capture evidence from the community and stakeholders, to integrate evidence from research, to capture and leverage data or evidence from practice and to generate and build on evidence from implementation using iterative system-level improvement. The LHS provides this opportunity and is shown to deliver impact. Here, we have outlined the process applied to generate an evidence-based LHS and provide a leading exemplar in stroke care. This highlights the value of moving from single-focus isolated approaches/initiatives to healthcare improvement and the benefit of integration to deliver demonstrable outcomes for our funders and key stakeholders — our community. This work provides insight into strategies that can both apply evidence-based processes to healthcare improvement as well as implementing evidence-based practices into care, moving beyond research as an endpoint, to research as an enabler, underpinning delivery of better healthcare.
Availability of data and materials
Not applicable
Abbreviations
Australian Stroke Clinical Registry
Confidence interval
- Learning Health System
World Health Organization. Delivering quality health services . OECD Publishing; 2018.
Enticott J, Braaf S, Johnson A, Jones A, Teede HJ. Leaders’ perspectives on learning health systems: A qualitative study. BMC Health Serv Res. 2020;20:1087.
Article PubMed PubMed Central Google Scholar
Melder A, Robinson T, McLoughlin I, Iedema R, Teede H. An overview of healthcare improvement: Unpacking the complexity for clinicians and managers in a learning health system. Intern Med J. 2020;50:1174–84.
Article PubMed Google Scholar
Alberto IRI, Alberto NRI, Ghosh AK, Jain B, Jayakumar S, Martinez-Martin N, et al. The impact of commercial health datasets on medical research and health-care algorithms. Lancet Digit Health. 2023;5:e288–94.
Article CAS PubMed PubMed Central Google Scholar
Dixon-Woods M. How to improve healthcare improvement—an essay by Mary Dixon-Woods. BMJ. 2019;367: l5514.
Enticott J, Johnson A, Teede H. Learning health systems using data to drive healthcare improvement and impact: A systematic review. BMC Health Serv Res. 2021;21:200.
Enticott JC, Melder A, Johnson A, Jones A, Shaw T, Keech W, et al. A learning health system framework to operationalize health data to improve quality care: An Australian perspective. Front Med (Lausanne). 2021;8:730021.
Dammery G, Ellis LA, Churruca K, Mahadeva J, Lopez F, Carrigan A, et al. The journey to a learning health system in primary care: A qualitative case study utilising an embedded research approach. BMC Prim Care. 2023;24:22.
Foley T, Horwitz L, Zahran R. The learning healthcare project: Realising the potential of learning health systems. 2021. Available from https://learninghealthcareproject.org/wp-content/uploads/2021/05/LHS2021report.pdf . Accessed Jan 2024.
Institute of Medicine. Best care at lower cost: The path to continuously learning health care in America. Washington: The National Academies Press; 2013.
Google Scholar
Zurynski Y, Smith CL, Vedovi A, Ellis LA, Knaggs G, Meulenbroeks I, et al. Mapping the learning health system: A scoping review of current evidence - a white paper. 2020:63
Cadilhac DA, Bravata DM, Bettger J, Mikulik R, Norrving B, Uvere E, et al. Stroke learning health systems: A topical narrative review with case examples. Stroke. 2023;54:1148–59.
Braithwaite J, Glasziou P, Westbrook J. The three numbers you need to know about healthcare: The 60–30-10 challenge. BMC Med. 2020;18:1–8.
Article Google Scholar
King D, Wittenberg R, Patel A, Quayyum Z, Berdunov V, Knapp M. The future incidence, prevalence and costs of stroke in the UK. Age Ageing. 2020;49:277–82.
Ganesh A, Lindsay P, Fang J, Kapral MK, Cote R, Joiner I, et al. Integrated systems of stroke care and reduction in 30-day mortality: A retrospective analysis. Neurology. 2016;86:898–904.
Lowther HJ, Harrison J, Hill JE, Gaskins NJ, Lazo KC, Clegg AJ, et al. The effectiveness of quality improvement collaboratives in improving stroke care and the facilitators and barriers to their implementation: A systematic review. Implement Sci. 2021;16:16.
Australian Commission on Safety and Quality in Health Care. Acute stroke clinical care standard. 2015. Available from https://www.safetyandquality.gov.au/our-work/clinical-care-standards/acute-stroke-clinical-care-standard . Accessed Jan 2024.
Australian Commission on Safety and Quality in Health Care. Acute stroke clinical care standard. Sydney: ACSQHC; 2019. Available from https://www.safetyandquality.gov.au/publications-and-resources/resource-library/acute-stroke-clinical-care-standard-evidence-sources . Accessed Jan 2024.
Ryan O, Ghuliani J, Grabsch B, Hill K, G CC, Breen S, et al. Development, implementation, and evaluation of the Australian Stroke Data Tool (AuSDaT): Comprehensive data capturing for multiple uses. Health Inf Manag. 2022:18333583221117184.
English C, Bayley M, Hill K, Langhorne P, Molag M, Ranta A, et al. Bringing stroke clinical guidelines to life. Int J Stroke. 2019;14:337–9.
English C, Hill K, Cadilhac DA, Hackett ML, Lannin NA, Middleton S, et al. Living clinical guidelines for stroke: Updates, challenges and opportunities. Med J Aust. 2022;216:510–4.
Cadilhac DA, Grimley R, Kilkenny MF, Andrew NE, Lannin NA, Hill K, et al. Multicenter, prospective, controlled, before-and-after, quality improvement study (Stroke123) of acute stroke care. Stroke. 2019;50:1525–30.
Cadilhac DA, Marion V, Andrew NE, Breen SJ, Grabsch B, Purvis T, et al. A stepped-wedge cluster-randomized trial to improve adherence to evidence-based practices for acute stroke management. Jt Comm J Qual Patient Saf. 2022.
Elliott J, Lawrence R, Minx JC, Oladapo OT, Ravaud P, Jeppesen BT, et al. Decision makers need constantly updated evidence synthesis. Nature. 2021;600:383–5.
Article CAS PubMed Google Scholar
National Stroke Foundation. National guidelines for acute stroke management. Melbourne: National Stroke Foundation; 2003.
National Stroke Foundation. Clinical guidelines for stroke rehabilitation and recovery. Melbourne: National Stroke Foundation; 2005.
Phan TG, Thrift A, Cadilhac D, Srikanth V. A plea for the use of systematic review methodology when writing guidelines and timely publication of guidelines. Intern Med J . 2012;42:1369–1371; author reply 1371–1362
Tendal B, Vogel JP, McDonald S, Norris S, Cumpston M, White H, et al. Weekly updates of national living evidence-based guidelines: Methods for the Australian living guidelines for care of people with COVID-19. J Clin Epidemiol. 2021;131:11–21.
Grimshaw JM, Eccles MP, Lavis JN, Hill SJ, Squires JE. Knowledge translation of research findings. Implement Sci. 2012;7:50.
Harris D, Cadilhac D, Hankey GJ, Hillier S, Kilkenny M, Lalor E. National stroke audit: The Australian experience. Clin Audit. 2010;2:25–31.
Cadilhac DA, Purvis T, Kilkenny MF, Longworth M, Mohr K, Pollack M, et al. Evaluation of rural stroke services: Does implementation of coordinators and pathways improve care in rural hospitals? Stroke. 2013;44:2848–53.
Cadilhac DA, Moss KM, Price CJ, Lannin NA, Lim JY, Anderson CS. Pathways to enhancing the quality of stroke care through national data monitoring systems for hospitals. Med J Aust. 2013;199:650–1.
Cadilhac DA, Lannin NA, Anderson CS, Levi CR, Faux S, Price C, et al. Protocol and pilot data for establishing the Australian Stroke Clinical Registry. Int J Stroke. 2010;5:217–26.
Ivers N, Jamtvedt G, Flottorp S, Young J, Odgaard-Jensen J, French S, et al. Audit and feedback: Effects on professional practice and healthcare outcomes. Cochrane Database Syst Rev . 2012
Australian Commission on Safety and Quality in Health Care. Economic evaluation of clinical quality registries. Final report. . 2016:79
Cadilhac DA, Dalli LL, Morrison J, Lester M, Paice K, Moss K, et al. The Australian Stroke Clinical Registry annual report 2021. Melbourne; 2022. Available from https://auscr.com.au/about/annual-reports/ . Accessed 6 May 2024.
Kilkenny MF, Kim J, Andrew NE, Sundararajan V, Thrift AG, Katzenellenbogen JM, et al. Maximising data value and avoiding data waste: A validation study in stroke research. Med J Aust. 2019;210:27–31.
Eliakundu AL, Smith K, Kilkenny MF, Kim J, Bagot KL, Andrew E, et al. Linking data from the Australian Stroke Clinical Registry with ambulance and emergency administrative data in Victoria. Inquiry. 2022;59:469580221102200.
PubMed Google Scholar
Andrew NE, Kim J, Cadilhac DA, Sundararajan V, Thrift AG, Churilov L, et al. Protocol for evaluation of enhanced models of primary care in the management of stroke and other chronic disease (PRECISE): A data linkage healthcare evaluation study. Int J Popul Data Sci. 2019;4:1097.
CAS PubMed PubMed Central Google Scholar
Mosalski S, Shiner CT, Lannin NA, Cadilhac DA, Faux SG, Kim J, et al. Increased relative functional gain and improved stroke outcomes: A linked registry study of the impact of rehabilitation. J Stroke Cerebrovasc Dis. 2021;30: 106015.
Ryan OF, Hancock SL, Marion V, Kelly P, Kilkenny MF, Clissold B, et al. Feedback of aggregate patient-reported outcomes (PROs) data to clinicians and hospital end users: Findings from an Australian codesign workshop process. BMJ Open. 2022;12:e055999.
Grimley RS, Rosbergen IC, Gustafsson L, Horton E, Green T, Cadigan G, et al. Dose and setting of rehabilitation received after stroke in Queensland, Australia: A prospective cohort study. Clin Rehabil. 2020;34:812–23.
Purvis T, Middleton S, Craig LE, Kilkenny MF, Dale S, Hill K, et al. Inclusion of a care bundle for fever, hyperglycaemia and swallow management in a national audit for acute stroke: Evidence of upscale and spread. Implement Sci. 2019;14:87.
Middleton S, McElduff P, Ward J, Grimshaw JM, Dale S, D’Este C, et al. Implementation of evidence-based treatment protocols to manage fever, hyperglycaemia, and swallowing dysfunction in acute stroke (QASC): A cluster randomised controlled trial. Lancet. 2011;378:1699–706.
Middleton S, Dale S, Cheung NW, Cadilhac DA, Grimshaw JM, Levi C, et al. Nurse-initiated acute stroke care in emergency departments. Stroke. 2019:STROKEAHA118020701.
Hood RJ, Maltby S, Keynes A, Kluge MG, Nalivaiko E, Ryan A, et al. Development and pilot implementation of TACTICS VR: A virtual reality-based stroke management workflow training application and training framework. Front Neurol. 2021;12:665808.
Bladin CF, Kim J, Bagot KL, Vu M, Moloczij N, Denisenko S, et al. Improving acute stroke care in regional hospitals: Clinical evaluation of the Victorian Stroke Telemedicine program. Med J Aust. 2020;212:371–7.
Bladin CF, Bagot KL, Vu M, Kim J, Bernard S, Smith K, et al. Real-world, feasibility study to investigate the use of a multidisciplinary app (Pulsara) to improve prehospital communication and timelines for acute stroke/STEMI care. BMJ Open. 2022;12:e052332.
Zhao H, Coote S, Easton D, Langenberg F, Stephenson M, Smith K, et al. Melbourne mobile stroke unit and reperfusion therapy: Greater clinical impact of thrombectomy than thrombolysis. Stroke. 2020;51:922–30.
Purvis T, Cadilhac DA, Hill K, Reyneke M, Olaiya MT, Dalli LL, et al. Twenty years of monitoring acute stroke care in Australia from the national stroke audit program (1999–2019): Achievements and areas of future focus. J Health Serv Res Policy. 2023.
Cadilhac DA, Purvis T, Reyneke M, Dalli LL, Kim J, Kilkenny MF. Evaluation of the national stroke audit program: 20-year report. Melbourne; 2019.
Kim J, Tan E, Gao L, Moodie M, Dewey HM, Bagot KL, et al. Cost-effectiveness of the Victorian Stroke Telemedicine program. Aust Health Rev. 2022;46:294–301.
Kim J, Easton D, Zhao H, Coote S, Sookram G, Smith K, et al. Economic evaluation of the Melbourne mobile stroke unit. Int J Stroke. 2021;16:466–75.
Stroke Foundation. National stroke audit – rehabilitation services report 2020. Melbourne; 2020.
Australian Institute of Health and Welfare. Heart, stroke and vascular disease: Australian facts. 2023. Webpage https://www.aihw.gov.au/reports/heart-stroke-vascular-diseases/hsvd-facts/contents/about (accessed Jan 2024).
Download references
Acknowledgements
The following authors hold National Health and Medical Research Council Research Fellowships: HT (#2009326), DAC (#1154273), SM (#1196352), MFK Future Leader Research Fellowship (National Heart Foundation #105737). The Funders of this work did not have any direct role in the design of the study, its execution, analyses, interpretation of the data, or decision to submit results for publication.
Author information
Helena Teede and Dominique A. Cadilhac contributed equally.
Authors and Affiliations
Monash Centre for Health Research and Implementation, 43-51 Kanooka Grove, Clayton, VIC, Australia
Helena Teede, Emily Callander & Joanne Enticott
Monash Partners Academic Health Science Centre, 43-51 Kanooka Grove, Clayton, VIC, Australia
Helena Teede & Alison Johnson
Stroke and Ageing Research, Department of Medicine, School of Clinical Sciences at Monash Health, Monash University, Level 2 Monash University Research, Victorian Heart Hospital, 631 Blackburn Rd, Clayton, VIC, Australia
Dominique A. Cadilhac, Tara Purvis & Monique F. Kilkenny
Stroke Theme, The Florey Institute of Neuroscience and Mental Health, University of Melbourne, Heidelberg, VIC, Australia
Dominique A. Cadilhac, Monique F. Kilkenny & Bruce C.V. Campbell
Department of Neurology, Melbourne Brain Centre, Royal Melbourne Hospital, Parkville, VIC, Australia
Bruce C.V. Campbell
Department of Medicine, Faculty of Medicine, Dentistry and Health Sciences, University of Melbourne, Victoria, Australia
School of Health Sciences, Heart and Stroke Program, University of Newcastle, Hunter Medical Research Institute, University Drive, Callaghan, NSW, Australia
Coralie English
School of Medicine and Dentistry, Griffith University, Birtinya, QLD, Australia
Rohan S. Grimley
Clinical Excellence Division, Queensland Health, Brisbane, Australia
John Hunter Hospital, Hunter New England Local Health District and University of Newcastle, Sydney, NSW, Australia
Christopher Levi
School of Nursing, Midwifery and Paramedicine, Australian Catholic University, Sydney, NSW, Australia
Sandy Middleton
Nursing Research Institute, St Vincent’s Health Network Sydney and and Australian Catholic University, Sydney, NSW, Australia
Stroke Foundation, Level 7, 461 Bourke St, Melbourne, VIC, Australia
Kelvin Hill
You can also search for this author in PubMed Google Scholar
Contributions
HT: conception, design and initial draft, developed the theoretical formalism for learning health system framework, approved the submitted version. DAC: conception, design and initial draft, provided essential literature and case study examples, approved the submitted version. TP: revised the manuscript critically for important intellectual content, approved the submitted version. MFK: revised the manuscript critically for important intellectual content, provided essential literature and case study examples, approved the submitted version. BC: revised the manuscript critically for important intellectual content, provided essential literature and case study examples, approved the submitted version. CE: revised the manuscript critically for important intellectual content, provided essential literature and case study examples, approved the submitted version. AJ: conception, design and initial draft, developed the theoretical formalism for learning health system framework, approved the submitted version. EC: revised the manuscript critically for important intellectual content, approved the submitted version. RSG: revised the manuscript critically for important intellectual content, provided essential literature and case study examples, approved the submitted version. CL: revised the manuscript critically for important intellectual content, provided essential literature and case study examples, approved the submitted version. SM: revised the manuscript critically for important intellectual content, provided essential literature and case study examples, approved the submitted version. KH: revised the manuscript critically for important intellectual content, provided essential literature and case study examples, approved the submitted version. JE: conception, design and initial draft, developed the theoretical formalism for learning health system framework, approved the submitted version. All authors read and approved the final manuscript.
Authors’ Twitter handles
@HelenaTeede
@DominiqueCad
@Coralie_English
@EmilyCallander
@EnticottJo
Corresponding authors
Correspondence to Helena Teede or Dominique A. Cadilhac .
Ethics declarations
Ethics approval and consent to participate, consent for publication, competing interests, additional information, publisher's note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ . The Creative Commons Public Domain Dedication waiver ( http://creativecommons.org/publicdomain/zero/1.0/ ) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
Reprints and permissions
About this article
Cite this article.
Teede, H., Cadilhac, D.A., Purvis, T. et al. Learning together for better health using an evidence-based Learning Health System framework: a case study in stroke. BMC Med 22 , 198 (2024). https://doi.org/10.1186/s12916-024-03416-w
Download citation
Received : 23 July 2023
Accepted : 30 April 2024
Published : 15 May 2024
DOI : https://doi.org/10.1186/s12916-024-03416-w
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Evidence-based medicine
- Person-centred care
- Models of care
- Healthcare improvement
BMC Medicine
ISSN: 1741-7015
- Submission enquiries: [email protected]
- General enquiries: [email protected]
An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
Preview improvements coming to the PMC website in October 2024. Learn More or Try it out now .
- Advanced Search
- Journal List
- Int J Mol Sci

Pathophysiology and Treatment of Stroke: Present Status and Future Perspectives
Stroke is the second leading cause of death and a major contributor to disability worldwide. The prevalence of stroke is highest in developing countries, with ischemic stroke being the most common type. Considerable progress has been made in our understanding of the pathophysiology of stroke and the underlying mechanisms leading to ischemic insult. Stroke therapy primarily focuses on restoring blood flow to the brain and treating stroke-induced neurological damage. Lack of success in recent clinical trials has led to significant refinement of animal models, focus-driven study design and use of new technologies in stroke research. Simultaneously, despite progress in stroke management, post-stroke care exerts a substantial impact on families, the healthcare system and the economy. Improvements in pre-clinical and clinical care are likely to underpin successful stroke treatment, recovery, rehabilitation and prevention. In this review, we focus on the pathophysiology of stroke, major advances in the identification of therapeutic targets and recent trends in stroke research.
1. Introduction
Stroke is a neurological disorder characterized by blockage of blood vessels. Clots form in the brain and interrupt blood flow, clogging arteries and causing blood vessels to break, leading to bleeding. Rupture of the arteries leading to the brain during stroke results in the sudden death of brain cells owing to a lack of oxygen. Stroke can also lead to depression and dementia.
Until the International Classification of Disease 11 (ICD-11) was released in 2018, stroke was classified as a disease of the blood vessels. Under the previous ICD coding rationale, clinical data generated from stroke patients were included as part of the cardiovascular diseases chapter, greatly misrepresenting the severity and specific disease burden of stroke. Due to this misclassification within the ICD, stroke patients and researchers did not benefit from government support or grant funding directed towards neurological disease. After prolonged advocacy from a group of clinicians, the true nature and significance of stroke was acknowledged in the ICD-11; stroke was re-categorized into the neurological chapter [ 1 ]. The reclassification of stroke as a neurological disorder has led to more accurate documentation of data and statistical analysis, supporting improvements in acute healthcare and acquisition of research funding for stroke.
2. Epidemiology of Stroke
Stroke is the second leading cause of death globally. It affects roughly 13.7 million people and kills around 5.5 million annually. Approximately 87% of strokes are ischemic infarctions, a prevalence which increased substantially between 1990 and 2016, attributed to decreased mortality and improved clinical interventions. Primary (first-time) hemorrhages comprise the majority of strokes, with secondary (second-time) hemorrhages constituting an estimated 10–25% [ 2 , 3 ]. The incidence of stroke doubled in low-and-middle income countries over 1990–2016 but declined by 42% in high-income countries over the same period. According to the Global Burden of Disease Study (GBD), although the prevalence of stroke has decreased, the age of those affected, their sex and their geographic location mean that the socio-economic burden of stroke has increased over time [ 3 ].
Age-specific stroke : The incidence of stroke increases with age, doubling after the age of 55 years. However, in an alarming trend, strokes in people aged 20–54 years increased from 12.9% to 18.6% of all cases globally between 1990 and 2016. Nevertheless, age-standardized attributable death rates decreased by 36.2% over the same period [ 3 , 4 , 5 ]. The highest reported stroke incidence is in China, where it affects an estimated 331–378 individuals per 100,000 life years. The second-highest rate is in eastern Europe (181–218 per 100,000 life years) and the lowest in Latin America (85–100 per 100,000 life years) [ 3 ].
Gender-specific stroke : The occurrence of stroke in men and women also depends on age. It is higher at younger ages in women, whereas incidence increases slightly with older age in men. The higher risk for stroke in women is due to factors related to pregnancy, such as preeclampsia, contraceptive use and hormonal therapy, as well as migraine with aura. Atrial fibrillation increases stroke risk in women over 75 years by 20%. Based on the National Institutes of Health Stroke Scale (0 = no stroke, 1–4 = minor stroke, 5–15 = moderate stroke, 15–20 = moderate/severe stroke, 21–42 = severe stroke), mean stroke severity was estimated at 10 for women and 8.2 for men. Both brain infarction and intracerebral hemorrhage (ICH) are common in men, but cardioembolic stroke, a more severe form of stroke, is more prevalent among women. The fatality rate for stroke is also higher among women [ 5 , 6 , 7 ]. Women live longer than men, which is one reason for their higher incidence of stroke; another important concern is women’s delay in accepting help for ongoing symptoms [ 8 ]. For men, the most common causes of stroke are tobacco smoking, excessive alcohol consumption, myocardial infarction and arterial disorders [ 9 ].
Geographic and racial variation : As noted earlier, stroke incidence varies considerably across the globe. A global population-based study of the prevalence of stroke and related risks examined demography, behavior, physical characteristics, medical history and laboratory reports, and revealed the contribution of exposure to air pollution and particulate matter to stroke mortality [ 10 ]. Another population-based study, conducted in north-eastern China, is thought to be broadly representative of the disease situation in developing countries. It found hypertension to be a statistically significant risk for stroke, specifically ischemic stroke [ 11 ]. A study conducted in the United States (US) also identified hypertension as a major cause of stroke and described geographical variation in symptomatic intensity in stroke sufferers. Insufficient physical activity, poor food habits and nicotine and alcohol consumption were considered added risks [ 12 ]. Differences in exposure to environmental pollutants, such as lead and cadmium, also influenced stroke incidences across regions. This study also revealed differences in stroke incidence between non-Hispanic white and black populations aged 40–50 years [ 13 ].
Socioeconomic variation : There is a strong inverse relationship between stroke and socioeconomic status, attributable to inadequate hospital facilities and post-stroke care among low-income populations [ 14 ]. A case study conducted in the US showed that people with high financial status had better stroke treatment options than deprived individuals [ 15 ]. A study in China linked low income and lack of health insurance to prevention of secondary stroke attack [ 16 ]. Research conducted in Austria associated level of education with take-up of treatments such as echocardiography and speech therapy; however, there was no difference in administration of thrombolysis, occupational therapy, physiotherapy or stroke care for secondary attack by socioeconomic status [ 17 ]. Similarly, in the Scottish healthcare system, basic treatments like thrombolysis were provided irrespective of the economic status of patients [ 18 ].
3. Pathophysiology of Stroke
Stroke is defined as an abrupt neurological outburst caused by impaired perfusion through the blood vessels to the brain. It is important to understand the neurovascular anatomy to study the clinical manifestation of the stroke. The blood flow to the brain is managed by two internal carotids anteriorly and two vertebral arteries posteriorly (the circle of Willis). Ischemic stroke is caused by deficient blood and oxygen supply to the brain; hemorrhagic stroke is caused by bleeding or leaky blood vessels.
Ischemic occlusions contribute to around 85% of casualties in stroke patients, with the remainder due to intracerebral bleeding. Ischemic occlusion generates thrombotic and embolic conditions in the brain [ 19 ]. In thrombosis, the blood flow is affected by narrowing of vessels due to atherosclerosis. The build-up of plaque will eventually constrict the vascular chamber and form clots, causing thrombotic stroke. In an embolic stroke, decreased blood flow to the brain region causes an embolism; the blood flow to the brain reduces, causing severe stress and untimely cell death (necrosis). Necrosis is followed by disruption of the plasma membrane, organelle swelling and leaking of cellular contents into extracellular space [ 20 ], and loss of neuronal function. Other key events contributing to stroke pathology are inflammation, energy failure, loss of homeostasis, acidosis, increased intracellular calcium levels, excitotoxicity, free radical-mediated toxicity, cytokine-mediated cytotoxicity, complement activation, impairment of the blood–brain barrier, activation of glial cells, oxidative stress and infiltration of leukocytes [ 21 , 22 , 23 , 24 , 25 ].
Hemorrhagic stroke accounts for approximately 10–15% of all strokes and has a high mortality rate. In this condition, stress in the brain tissue and internal injury cause blood vessels to rupture. It produces toxic effects in the vascular system, resulting in infarction [ 26 ]. It is classified into intracerebral and subarachnoid hemorrhage. In ICH, blood vessels rupture and cause abnormal accumulation of blood within the brain. The main reasons for ICH are hypertension, disrupted vasculature, excessive use of anticoagulants and thrombolytic agents. In subarachnoid hemorrhage, blood accumulates in the subarachnoid space of the brain due to a head injury or cerebral aneurysm ( Figure 1 ) [ 27 , 28 ].

Molecular mechanism of stroke.
4. Risk Factors for Stroke
As noted earlier, the risk of stroke increases with age and doubles over the age of 55 years in both men and women. Risk is increased further when an individual has an existing medical condition like hypertension, coronary artery disease or hyperlipidemia. Nearly 60% of strokes are in patients with a history of transient ischemic attack (TIA). Some of the risk factors for stroke are modifiable, and some are non-modifiable ( Figure 2 ).

Risk factors associated with stroke.
4.1. Non-Modifiable Risk Factors
These include age, sex, ethnicity, TIA and hereditary characteristics. In the US in 2005, the average age of incidence of stroke was 69.2 years [ 2 , 29 , 30 ]. Recent research has indicated that people aged 20–54 years are at increasing risk of stroke, probably due to pre-existing secondary factors [ 31 ]. Women are at equal or greater risk of stroke than men, irrespective of age [ 32 ]. US research shows that Hispanic and black populations are at higher risk of stroke than white populations; notably, the incidence of hemorrhagic stroke is significantly higher in black people than in age-matched white populations [ 33 , 34 , 35 ].
Transient ischemic attack is classified as a mini stroke; the underlying mechanism is the same as for full-blown stroke. In TIA, the blood supply to part of the brain is blocked temporarily. It acts as a warning sign before the actual event, providing an opportunity to change lifestyle and commence medications to reduce the chance of stroke [ 36 , 37 ].
Genetics contribute to both modifiable and non-modifiable risk factors for stroke. Genetic risk is proportional to the age, sex and race of the individual [ 38 , 39 ], but a multitude of genetic mechanisms can increase the risk of stroke. Firstly, a parental or family history of stroke increases the chance of an individual developing this neurological disorder. Secondly, a rare single gene mutation can contribute to pathophysiology in which stroke is the primary clinical manifestation, such as in cerebral autosomal dominant arteriopathy. Thirdly, stroke can be one of many after-effects of multiple syndromes caused by genetic mutation, such as sickle cell anemia. Fourthly, some common genetic variants are associated with increased stroke risk, such as genetic polymorphism in 9p21 [ 40 ]. A genome-wide association study of stroke showed high heritability (around 40%) for large blood vessel disease, and low heritability (16.7%) for small vessel disorders. Recent evidence suggests that studying heritability will improve the understanding of stroke sub-types, improve patient management and enable earlier and more efficient prognosis [ 5 , 41 ].
4.2. Modifiable Risk Factors
These are of paramount importance, because timely and appropriate medical intervention can reduce the risk of stroke in susceptible individuals. The major modifiable risk factors for stroke are hypertension, diabetes, lack of physical exercise, alcohol and drug abuse, cholesterol, diet management and genetics.
Hypertension : It is one of the predominant risk factors for stroke. In one study, a blood pressure (BP) of at least 160/90 mmHg and a history of hypertension were considered equally important predispositions for stroke, with 54% of the stroke-affected population having these characteristics [ 42 , 43 ]. BP and prevalence of stroke are correlated in both hypertensive and normal individuals. A study reported that a 5–6 mm Hg reduction in BP lowered the relative risk of stroke by 42% [ 44 ]. Randomized trials of interventions to reduce hypertension in people aged 60+ have shown similar results, lowering the incidences of symptoms of stroke by 36% and 42%, respectively [ 45 , 46 ].
Diabetes : It doubles the risk of ischemic stroke and confers an approximately 20% higher mortality rate. Moreover, the prognosis for diabetic individuals after a stroke is worse than for non-diabetic patients, including higher rates of severe disability and slower recovery [ 47 , 48 ]. Tight regulation of glycemic levels alone is ineffective; medical intervention plus behavioral modifications could help decrease the severity of stroke for diabetic individuals [ 49 ].
Atrial fibrillation (AF) : AF is an important risk factor for stroke, increasing risk two- to five-fold depending upon the age of the individual concerned [ 50 ]. It contributes to 15% of all strokes and produces more severe disability and higher mortality than non-AF-related strokes [ 51 ]. Research has shown that in AF, decreased blood flow in the left atrium causes thrombolysis and embolism in the brain. However, recent studies have contradicted this finding, citing poor evidence of sequential timing of incidence of AF and stroke, and noting that in some patients the occurrence of AF is recorded only after a stroke. In other instances, individuals harboring genetic mutations specific to AF can be affected by stroke long before the onset of AF [ 52 , 53 ]. Therefore, we need better methods of monitoring the heart rhythms that are associated with the vascular risk factors of AF and thromboembolism.
Hyperlipidemia : It is a major contributor to coronary heart disease, but its relationship to stroke is complicated. Total cholesterol is associated with risk of stroke, whereas high-density lipoprotein (HDL) decreases stroke incidence [ 54 , 55 , 56 ]. Therefore, evaluation of lipid profile enables estimation of the risk of stroke. In one study, low levels of HDL (<0.90 mmol/L), high levels of total triglyceride (>2.30 mmol/L) and hypertension were associated with a two-fold increase in the risk of stroke-related death in the population [ 55 ].
Alcohol and drug abuse : The relationship between stroke risk and alcohol intake follows a curvilinear pattern, with the risk related to the amount of alcohol consumed daily. Low to moderate consumption of alcohol (≤2 standard drinks daily for men and ≤1 for women) reduces stroke risk, whereas high intake increases it. In contrast, even low consumption of alcohol escalates the risk of hemorrhagic stroke [ 57 , 58 , 59 ]. Regular use of illegitimate substances such as cocaine, heroin, phencyclidine (PCP), lysergic acid diethylamide (LSD), cannabis/marijuana or amphetamines is related to increased risk of all subtypes of strokes [ 60 ]. Illicit drug use is a common predisposing factor for stroke among individuals aged below 35 years. US research showed that the proportion of illicit drug users among stroke patients aged 15–44 years was six times higher than among age-matched patients admitted with other serious conditions [ 61 ]. However, there is no strong evidence to confirm these findings, and the relationship between these drugs and stroke is anecdotal [ 62 ].
Smoking : Tobacco smoking is directly linked to increased risk of stroke. An average smoker has twice the chance of suffering from a stroke of a non-smoker. Smoking contributes to 15% of stroke-related mortality. Research suggests that an individual who stops smoking reduces the relative risk of stroke, while prolonged second-hand smoking confers a 30% elevation in the risk of stroke [ 63 , 64 , 65 ].
Insufficient physical inactivity and poor diet are associated with increased risk for stroke. Lack of exercise increases the chances of stroke attack in an individual. Insufficient physical activity is also linked to other health issues like high BP, obesity and diabetes, all conditions related to high stroke incidence [ 66 , 67 ]. Poor diet influences the risk of stroke, contributing to hypertension, hyperlipidemia, obesity and diabetes. Certain dietary components are well known to heighten risk; for example, excessive salt intake is linked to high hypertension and stroke. Conversely, a diet high in fruit and vegetables (notably, the Mediterranean diet) has been shown to decrease the risk of stroke [ 68 , 69 , 70 , 71 , 72 ].
5. Animal Models of Stroke
Animal models usually used for research include induced, spontaneous, negative and orphan models. In the induced model, a disease condition is induced in the animal with a view to studying the effects, whereas in the spontaneous model, an animal is selected with a similar disease state naturally present in the model. Negative animal models are used to study the resistance mechanisms underlying a particular disease condition. Orphan models are deployed to understand the pathology of a newly characterized disease in human subjects [ 73 , 74 ].
Many animal models have been developed to study the pathophysiology associated with stroke; they offer several advantages over studying stroke in humans or in vitro. The nature of stroke in humans is unpredictable, with diverse clinical manifestation and localization, whereas animal models are highly predictable and reproducible. Pathophysiological investigation often requires direct access to brain tissue, which is possible with animal models but not in humans. Moreover, current imaging techniques are unable to characterize events occurring within the first few minutes of a stroke. Finally, some aspects of stroke, such as vasculature and perfusion, cannot be studied in in vitro models [ 75 ]. Different stroke models used in animals are described in the session below ( Table 1 ).
Advantages and disadvantages of the stroke models.
The intraluminal suture MCAo model : The middle cerebral artery (MCA) is vulnerable to ischemic insult and occlusion in humans, accounting for 70% of stroke-related disability. This disease model has been widely studied in rat and mouse models, with more than 2600 experiments conducted [ 76 , 77 ]. The MCAo procedure is minimally invasive; it involves occlusion of the carotid artery by insertion of a suture until it interrupts blood flow to the MCA. This procedure is applied for time periods such as 60 or 90 min or permanently, to induce infarction, and has a success rate of 88–100% in rats and mice [ 78 ]. The most commonly used animal for studying pre-clinical stroke is the Sprague–Dawley rat, which has a small infarct volume [ 79 ]. In mice, C57BL/6 and SV129 are commonly used to introduce MCA infarction. The reproducibility of the technique depends on a multitude of factors, such as the animal strain, suture diameter, body weight and age. The advantage of this model is that it mimics the human ischemic stroke and displays similar penumbra [ 80 ]. The MCAo model is appropriate for reproducing ischemic stroke and associated clinical manifestations such as neuronal cell death, cerebral inflammation and blood–brain barrier damage [ 75 ].
Craniectomy model : This model uses a surgical procedure for inducing occlusion in the artery. In this technique, a neurological deficit can be induced in mice by electrocoagulation causing permanent insult or a microaneurysm until blood flow is interrupted. Alternatively, three-vessel occlusion is used, reducing the blood flow and resulting in damaged tissue. The infarct volume differs depending on whether the occlusion is permanent or transient [ 81 , 82 , 83 ]. A study conducted in neonatal P14–P18 rats mimicked pediatric stroke in a younger human population; a 3-h occlusion was performed to induce lesions affecting 40–50% of the brain [ 84 ]. Similarly, in P7 rats, oedema formation was observed in the MCA, followed by microglial infiltration. The P12 CB-17 is another animal model used for stroke research, mainly due to low variability in occlusion insult to the brain [ 85 ]. The other advantages of this model include reproducible infarct size and neurofunctional deficits, reduced mortality and visual ratification. The CB-17 model was successfully used to reproduce cerebral infarction and long-term survival rate, and to study ischemic reperfusion. Researchers showed that reperfusion supports neuron survival, rescues vascular phenotypes and is associated with functional recovery after stroke [ 86 ].
The Levine–Rice model : It involves histological examination and behavioral tests in rat pups, and it is used to study neonatal hypoxic-ischemic stroke [ 87 ]. In this model, a unilateral ligation is followed by reperfusion and recovery. Later, the animal is placed in a hypoxic chamber to understand neonatal stroke pathophysiology as well as regenerative and rehabilitative therapeutic possibilities. P7 rat animal models are commonly used to study the clinical manifestations of hypoxic-ischemic injury [ 88 , 89 , 90 ].
Photo-thrombosis model : This model is based on photo-oxidation of the vasculature leading to lesion formation in the cortex and striatum. In this method, the skull is irradiated with a photoactive dye that causes endothelial damage, intraparenchymal vessel aggregation and platelet stimulation in the affected area. It is injected intraperitoneally in mice and intravenously in rats [ 91 ]. This model is highly reproducible, with a low mortality rate and no surgery. The pathophysiology of this method is slightly different to that seen in human stroke due to little collateral blood flow or formation of ischemic penumbra. However, recent researchers modified the photothrombotic ischemia model to include hypoperfusion in an attempt to mimic penumbra. It has also been deployed in freely moving mice to evaluate the development of motor cortex ischemia and motor deficits. This model permits assessment of the ongoing infarction and improves our understanding of the neuronal insult and repair process [ 92 , 93 ].
Endothelin-1 model: Endothelin-1 (ET-1) : ET-1 is a small peptide molecule produced by smooth muscle cells and the endothelium. It is a paracrine factor that restricts the vascular system through cell-specific receptors. Ischemic lesion is induced by stereotaxic injection of ET-1 directly into the exposed MCA in the intracerebral or cortex region [ 94 ]. ET-1 administration was observed to cause 70–90% reduction in cerebral blood flow, followed by reperfusion [ 95 ]. This technique is minimally invasive, has a low death rate and can be applied to deep and superficial brain regions. It is appropriate for long-term lesion studies, and the lesion size can be controlled by regulating ET-1 concentration, which is critical for reproducibility [ 95 ]. ET-1 is expressed by both neurons and astrocytes, which may decrease the stringency of interpretation of neuronal dysfunction in stroke [ 96 ]. A study in juvenile P21 rats used ET-1 to induce focal lesion in the striatum [ 97 ]. Similarly, aged P12 and P25 rats showed neuronal damage and lesion formation after injection of ET-1 into the hippocampus [ 98 ].
The embolic stroke model : It includes microsphere, macrosphere and thromboembolic models. The microsphere model involves introduction of spheres of diameter 20–50 μm into the circulatory system using a microcatheter to form multifocal infarcts [ 99 ]. Macrospheres are 100–400 μm in diameter and introduced into the intracerebral artery (ICA) to produce reproducible lesions in the MCA [ 100 ]. In the thromboembolic model, thrombin is directly injected to form clots in the ICA or MCA. The volume of the infarct depends upon the size of the clot formed [ 101 ]. This model closely resembles the type of stroke seen in humans. Prior study of clots induced by this model in mice have showed that they are mainly comprised of polymerized fibrin with few cells and platelets present, and 75% of clots exhibit platelet/fibrin build-up and deposition of neutrophils, monocytes and erythrocytes [ 102 ].
Neurorehabilitation in animal models : Various rehabilitative devices and forced training strategies have been deployed in stroke-affected animals to study neurological behavior. Robotic and electric devices have also been developed for training purposes in animal models to evaluate the functionality and effectiveness of the rehabilitation process. Similarly, forced exercise regimes, such as running on a treadmill or task-oriented motor training, are used to study rehabilitation scope in humans. Housing environments that provide social, motor and sensory stimuli and support cell engraftment, creating a more realistic approximation of human treatment, can be tested using animal models [ 103 , 104 , 105 ].
Animal models in biomaterial testing : Animal models have been well characterized for the study of brain tissues via brain atlases ( http://www.med.harvard.edu/AANLIB/ , https://portal.brain-map.org ) for the required species. Stereotaxic techniques are utilized to introduce biomaterials or cells into particular coordinates of the target tissue. Microlesions can be studied precisely, and targeted localization can be confirmed using magnetic resonance imaging (MRI)-based lesion cartography [ 106 , 107 , 108 ].
6. Prevention and Treatment Strategies for Stroke
Stroke prevention involves modifying risk factors within a population or individuals, while stroke management depends on treating its pathophysiology. Despite an enormous amount of research into stroke over the last two decades, no simple means of treating or preventing all the clinical causes of stroke has been established. The overall direction of current stroke research is to generate novel therapies that modulate factors leading to primary and secondary stroke. Recent and current strategies for stroke prevention and treatment are discussed below ( Figure 3 ).

Stroke therapy. This represents the overall process to manage the incidence of stroke.
Excitotoxicity : Neuronal death is a key manifestation of stroke. A key reason for this phenomenon is neuronal depolarization and inability to maintain membrane potential within the cell. This process is mediated by glutamate receptors N-methyl-D-aspartate (NMDA) and α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA), which were among the first neuroprotective agents tested in stroke prevention. However, the untimely release of glutamate overpowers the system that removes glutamate from the cell and causes abnormal release of NMDA and AMPA molecules, leading to uninhibited calcium influx and protein damage. As a result, these agents have not been shown to reduce neuronal death in human subjects. Targeting the molecular pathways downstream of excitotoxicity signaling, rather than directly targeting glutamatergic signaling, might reduce the side effects of the process [ 109 , 110 ].
Gamma aminobutyric acid (GABA) agonists : Clomethiazole is a GABA agonist that has been tested for its ability to improve stroke symptoms in patients, but failed to reduce the toxicity induced by the glutamate receptor [ 111 ].
Sodium (Na + ) channel blockers : Na + channel blockers have been used as neuroprotective agents in various animal models of stroke. They prevent neuronal death and reduce white matter damage. Many voltage-gated Na + channel blockers have been tested in clinical trials, but most have proved to be ineffective [ 112 ]. Mexiletine is a neuroprotectant and Na + channel blocker that proved effective in grey and white matter ischemic stroke, though further evaluation is required to confirm its role [ 113 ]. Lubeluzole was shown to reduce mortality in stroke in initial clinical trials, but successive trials failed to reproduce similar outcomes. Similarly, sipatrigine is a Na + and Ca 2+ channel blocker which failed in a Phase II clinical trial in stroke patients. Amiodarone was shown to aggravate brain injury due to defective transportation and accumulation of Na + ions in the brain after stroke [ 114 ].
Calcium (Ca 2+ ) channel blockers : Voltage-dependent Ca 2+ ion channel blockers have been shown to decrease the ischemic insult in animal models of brain injury. The Ca 2+ ion chelator DP-b99 proved efficient and safe in Phase I and II clinical trials when administered to stroke patients. Similarly, Phase II trials significantly improved clinical symptoms in stroke patients treated within 12 h of onset [ 115 ]. In another study, Ca 2+ channel blockers reduced the risk of stroke by 13.5% in comparison to diuretics and β-blockers [ 116 ].
Antioxidants : Reactive oxygen species produced in the normal brain are balanced by antioxidants generated in a responsive mechanism. However, in the ischemic stroke model, excess production of free radicals and inactivation of detoxifying agents cause redox disequilibrium. This phenomenon leads to oxidative stress, followed by neuronal injury. Therefore, antioxidants are employed in treatment of acute stroke to inhibit or scavenge free radical production and degrade free radicals in the system. In one study, antioxidant AEOL 10,150 (manganese (III) meso-tetrakis (di-N-ethylimidazole) porphyrin) effectively regulated the gene expression profiles specific to inflammation and stress response to decrease the ischemic damage and reperfusion in stroke patients [ 117 ]. In another, deferoxamine was shown to regulate the expression of hypoxia-inducible factor-1, a transcriptional factor regulated by oxygen levels, which in turn switched on other genes like vascular endothelial growth factor and erythropoietin. This mechanism, studied in an animal stroke model, proved beneficial in reducing lesion size and improving sensorimotor capabilities [ 118 , 119 ]. Similarly, NXY-059 compound acts as a scavenger to eliminate free radicals and decrease neurological deficits. The Stroke-Acute-Ischemic-NXY-Treatment-I (SAINT) clinical trial showed the efficacy and safety of NXY-059, but SAINT II failed to reproduce the positive effect of this drug in stroke patients [ 120 , 121 ]. In another study, researchers employed intravenous injection of antioxidants directly into mice brains to understand the benefits of route of administration. This method reduced neurological defects, but had minimal influence on brain damage [ 122 ].
6.1. Reperfusion
The intravenous thrombolytics (IVT) : The IVT treatment paradigm was originally developed to treat coronary thrombolysis but was found to be effective in treating stroke patients. The efficiency of thrombolytic drugs depends on factors including the age of the clot, the specificity of the thrombolytic agent for fibrin and the presence and half-life of neutralizing antibodies [ 123 ]. The drugs used in IVT treatment aim to promote fibrinolysin formation, which catalyzes the dissolution of the clot blocking the cerebral vessel. The most effective IVT drug, recombinant tissue plasminogen activator (rt-PA, or alteplase), was developed from research conducted by the US National Institute of Neurological Disorders and Stroke (NINDS) [ 124 ]. However, European Cooperative Acute Stroke Study (ECASS and ECASS II) researchers were unable to reproduce NINDS’ results. Later, it was found that this drug was effective in reducing clot diameter in stroke patients within three hours of incidence. The Safe Implementation of Thrombolysis in Stroke Monitoring Study (SITS-MOST) confirmed the efficacy and safety of alteplase within the designated time frame [ 125 ]. Another category of thrombolytics, consisting of fibrin and non-fibrin drugs, is used for treatment of stroke symptoms. Fibrin activators like alteplase, reteplase and tenecteplase convert plasminogen to plasmin directly, whereas non-fibrin activators like the drugs streptokinase and staphylokinase do so indirectly [ 123 ].
Intra-arterial thrombolysis (IAT) : IAT is another approach designed to combat acute stroke. This treatment is most effective in the first six hours of onset of MCA occlusion, and requires experienced clinicians and angiographic techniques [ 115 ]. Prolyse in Acute Cerebral Thromboembolism II (PROACT II) and Middle Cerebral Artery Embolism Local Fibrinolytic Intervention (MELT) were randomized clinical trials (RCTs) undertaken to test the efficacy and safety of a recombinant pro-urokinase drug [ 126 , 127 ], but did not produce any data useful for stroke treatment. Thrombolytics and glycoprotein IIb/IIIa antagonists were combined in two small clinical trials; this approach was helpful in treating atherosclerotic occlusions but less effective for cardioembolism [ 128 , 129 ]. The Interventional Management of Stroke (IMS) III trial tested IVT and IAT together to assess the benefits of combining rapid administration of therapy (IVT) and a superior recanalization methodology for faster relief (IAT) [ 130 ]. The IMS III trial was fruitful with bridging therapy (combination of IVT and IAT) as compared to IVT alone. There was an increase of 69.6% in the recanalization rate using bridging therapy in stroke patients [ 131 , 132 ].
Fibrinogen-depleting agents : Research has found a strong correlation between high fibrinogen levels in stroke patients and poor diagnosis for clinical outcomes. Fibrinogen-depleting agents decrease blood plasma levels of fibrinogen, hence reduce blood thickness and increase blood flow. They also remove the blood clot in the artery and restore blood flow in the affected regions of the brain. However, although some RCTs of defibrinogen therapy identified beneficial effects of fibrinogen-depleting agents in stroke patients, others failed to show positive effects on clinical outcomes after stroke [ 133 ]. Moreover, some studies reported bleeding after treatment with defibrinogen agents. Ancrod is a defibrinogenating agent derived from snake venom that has been studied for its ability to treat ischemic stroke within three hours of onset [ 134 ]. The European Stroke Treatment with Ancrod Trial (ESTAT) concluded that controlled administration of ancrod at 70 mg/dL fibrinogen was efficacious and safe, and achieved lower prevalence of ICH than observed at lower fibrinogen levels [ 135 ].
6.2. Others
Antihypertensive therapy : Hypertension is a risk factor for stroke. There are many reasons for high BP in stroke, including a history of hypertension, acute neuroendocrine stimulation, increased intracranial pressure, stress linked to hospital admission and intermittent painful spells [ 136 ]. Correct treatment of high BP during stroke is uncertain due to contradictory outcomes of clinical studies. Some research shows positive correlations between high BP and stroke-related mortality, hematoma expansion or intracerebral damage, suggesting that high BP should be treated. In other studies, low BP levels led to tissue perfusion and increased lesion size, thereby worsening the clinical outcome [ 137 , 138 ]. The multi-center Acute Candesartan Cilexetil Therapy in Stroke Survivors (ACCESS) Phase II study proved that taking medication (candesartan) for BP during stroke was safe, with no orchestrated cerebrovascular events reported due to hypotension. Similar research has been performed with antihypertensive drugs, such as the Continue Or Stop post Stroke Antihypertensives Collaborative Study (COSSACS) to study the efficacy of antihypertensive therapy in stroke; the Control of Hypertension and Hypotension Immediately Post Stroke (CHHIPS) study, designed to determine the cut-off value for BP during an attack; and the Scandinavian Candesartan Acute Stroke Trial (SCAST), which aimed to measure the effectiveness of the drug candesartan on stroke and cardiovascular disease [ 115 , 139 ]. In the COSSACS study, continuing antihypertensive drugs for a two-week period produced no extra harm as compared to stopping it and might be associated with reduced two-week mortality in patients with ischemic stroke [ 140 ]. The CHHIPS study demonstrated that a relatively moderate reduction in blood pressure lowered the mortality rate [ 141 ], whereas the SCAST study suggested that a careful BP-lowering treatment was associated with a higher risk of poor clinical outcome [ 142 ].
Glucose management : Hyperglycemia (elevated blood glucose) is common in stroke patients, so targeting blood glucose levels is an efficient stroke management strategy. Hyperglycemia > 6.0 mmol/L (108 mg/dL) is observed in most stroke patients; it initiates lipid peroxidation and cell lysis in compromised tissue, leading to stroke complications. An experimental study conducted in a rat model of collagenase-induced ICH found that hyperglycemia worsens edema formation and increases cell death, accelerating the course of ischemic injury. Increased blood glucose level is also associated with progression of infarction, reduced recanalization and poor clinical outcome [ 143 ]. Continuous glucose monitoring systems have been deployed to reduce stroke-related risks in both diabetic and non-diabetic stroke patients [ 144 ].
Antiplatelet therapy : This therapy is used for acute ischemic stroke management and for prevention of stroke incidence. It is also vital in controlling non-cardioembolic ischemic stroke and TIA. Antiplatelet agents like aspirin, clopidogrel and ticagrelor are the most widely used drugs administered to stroke sufferers within the first few days of attack [ 145 ]. Dual antiplatelet therapy, which involves a combination of clopidogrel, prasugrel or ticagrelor with aspirin, has become popular; many studies have tested the efficacy and safety of this dual therapy. It has been claimed that clopidogrel and aspirin combination therapy is most beneficial if introduced within 24 h of stroke and continued for 4–12 weeks [ 146 ].
Stem cell therapy : It offers promising therapeutic opportunities, safety and efficacy to stroke patients. Research on embryonic stem cells, mesenchymal cells and induced pluripotent stem cells has assessed their potential for tissue regeneration, maintenance, migration and proliferation, rewiring of neural circuitry and physical and behavioral rejuvenation [ 147 ]. Recently, a new type of mesenchymal stem cells (MSCs), called multilineage differentiating stress-enduring (Muse) cells, has been found in connective tissue. These cells offer great regenerative capacity and have been tested as a stroke treatment. After intravenous transplantation of Muse cells in a mouse model, they were found to engraft into the damaged host tissue and differentiate to provide functional recovery in the host [ 148 ]. Neovascularization is another mode of action of cell therapies in stroke; studies conducted in vitro and in vivo have shown that transplanted cells promote angiogenesis [ 149 , 150 ]. Furthermore, multiple stroke studies have reported that MSCs stimulate neurogenesis; this was confirmed in human embryonic neural stem cells using BrdU-labelling [ 151 , 152 ]. Stem cell therapy enhances the proliferation of neural stem cells and neuritogenesis [ 153 ]. Careful experimental design and clinical trials of stem cell therapies are likely to usher in a new era of treatment for stroke by promoting neurogenesis, rebuilding neural networks and boosting axonal growth and synaptogenesis.
Neural repair : This is an alternative therapy to neuroprotection. It is used to rejuvenate the tissue when the damage is already done and is therefore not time-bound but is most effective when administered 24 h after stroke attack. Many animal models have been used in attempts to stimulate neurogenesis and initiate the neuronal repair process [ 154 ]. Neural repair utilizes stem cell therapy to initiate repair mechanisms through cell integration into the wound or use of neurotrophic factors to block neuronal growth inhibitors. These cells may be channeled to any injured region to facilitate greater synaptic connectivity. Clinical trials using neural stem cells have proven beneficial in stroke patients. However, trials of myelin-associated glycoprotein, neurite outgrowth inhibitor (NOGO) proteins and chondroitin sulphate proteoglycans have shown these agents to be insufficiently effective; more clinical trials are required to increase treatment efficacy [ 155 ]. Biological intrusions may foster regeneration of newer cells, improve axonal guidance and enhance neural circuitry. Pharmacological and immunological interventions may target receptors to provide signaling cues for regeneration or block inhibitory factors in stroke-affected regions of the brain [ 156 ].
Rehabilitation : Stroke can leave individuals with short- and long-term disabilities. Daily activities like walking and toileting are often affected, and sensorimotor and visual impairment are common. Rehabilitation aims to reinforce the functional independence of people affected by stroke [ 157 ]. It includes working with patients and families to provide supportive services and post-stroke guidance after 48 h of stroke attack in stable patients. Stroke rehabilitation may involve physical, occupational, speech and/or cognitive therapy. It is designed to assist patients to recover problem-solving skills, access social and psychological support, improve their mobility and achieve independent living. Rehabilitation may also include neurobiological tasks designed to lessen the impact of cognitive dysfunction and induce synaptic plasticity, as well as long-term potentiation [ 158 , 159 ]. Neuromodulators play a vital role in triggering expression of specific genes that promote axon regeneration, dendritic spine development, synapse formation and cell replacement therapy. Task-oriented approaches, like arm training and walking, help stroke patients to manage their physical disability, and visual computer-assisted gaming activities have been used to enhance visuomotor neuronal plasticity [ 160 ].
7. Trends in Stroke Research
The incidence of stroke-related emergencies has decreased substantially over recent years due to improved understanding of the pathophysiology of stroke and identification of new drugs designed to treat the multitude of possible targets. Technological advancements like telestroke [ 161 ] and mobile stroke [ 162 ] units have reduced mortality and morbidity. Therefore, stroke management systems should include post-stroke care facilities on top of existing primary care and access to occupational, speech or any physical therapy following hospital discharge. Hospitals should develop standardized policies to handle emergencies in a timely fashion to avoid casualties and prevent secondary stroke [ 163 ]. Recently, the role of physiotherapists has emerged as an important aspect of post-stroke care management. Physiotherapists have initiated clinical trials of stroke recovery processes and rehabilitation therapy sessions. One ongoing study includes a strategy to manage disability by improving mobility using treadmill exercise, electromechanical device therapy and circuit class therapy [ 164 , 165 ]. Stroke Recovery and Rehabilitation Roundtables bring physiotherapists and other experts together to recommend research directions and produce guidance for the post-stroke healthcare system. Optimized delivery of stroke care systems and access to rehabilitation services are the future of healthcare for stroke [ 166 ].
Animal models used in stroke research reflect only a portion of the consequences of the condition in human subjects. Moreover, experiments conducted within a single laboratory are often constrained in terms of their research output. In vivo animal models of stroke should include aged populations to maximize their relevance, but most recent studies involve young and adult animals. Stroke studies should be conducted in both male and female subjects to exclude gender bias, and should take account of other confounders like hypertension, diabetes and obesity. All these issues make stroke research complex and expensive, and imply that it should be carried out collaboratively, across multiple labs. Ideally, an international multicenter platform for clinical trials would be established to increase the validity of research outcomes with respect to efficacy, safety, translational value, dose–response relationships and proof-of-principle. This strategy will help to overcome the current hurdles in transforming laboratory data into therapeutics for stroke.
Advancements in stem cell technologies and genomics have led to regenerative therapy to rebuild neural networks and repair damaged neurons due to ischemic insult [ 167 , 168 ]. The WIP1 gene is a regulator of Wnt signaling and a promising target for drug development. Studies in mice models showed that knockdown of WIP1 downregulates the stroke functional recovery process after injury, and that the presence of this gene regulates neurogenesis through activation of β-Catenin/Wnt signaling [ 169 ]. Similarly, NB-3 (contactin-6) plays a vital role in neuroprotection, as shown by knockdown of NB-3 in mice after stroke attack. NB-3-deficient mice had increased brain damage after MCAo, which also affected neurite outgrowth and neuronal survival rate. NB-3 is believed to have therapeutic benefits for ischemic insult [ 170 ]. Therefore, WIP1 and NB-3 are promising candidates for future drug trials. This is a vast field, and more research must be conducted in the coming years to enable the development of therapeutic drugs.
Numerous natural compounds have proven to be beneficial for stroke prevention and treatment. They can be synthesized at a lower cost than synthetic compounds and offer competitive efficacy and safety. Honokiol is a natural product that showed neuroprotective effects in animal models, and appears to have a role in reducing oxidative stress and inhibiting inflammatory responses [ 171 ]. Gastrodin, a compound extracted from Gastrodia elata , is a promising candidate in stroke treatment. In a mouse model, it improved neurogenesis and activated β-Catenin-dependent Wnt signaling to provide neuroprotection after ischemic insult. It also has antioxidative effects which protects the neural progenitor cells from neuron functional impairment. Gastrodin’s safety has been proved in clinical trials, hence it is an option for stroke management in the coming years [ 172 ].
The Utstein methodology is a process of standardizing and reporting research on out-of-hospital stroke and defining the essential elements of management tools. Its growing popularity led to the establishment of the Global Resuscitation Alliance (GRA), an organization that governs best practices. The primary aim of GRA is to facilitate stroke care from pre-hospital admission to rehabilitation and recovery. It has developed 10 guidelines to ensure smooth transitioning of services during and after attack. It has implemented a stroke registry, public awareness and educational programs, promoted techniques for early stroke recognition by first responders, sought to optimize prehospital and in-hospital stroke care, advocated the use of advanced neuroimaging techniques and promoted a culture of excellence. The Utstein community has developed comprehensive plans to improve early diagnosis and treatment of stroke patients globally [ 173 ].
Future clinical trials should aim not only to determine the efficacy and safety of drugs but to characterize recovery and clinical outcomes. Clinical trials of pharmacological therapies for post-stroke recovery should adhere to the following guidelines [ 174 ]. Patients should be enrolled within two weeks of stroke whenever possible. Studies should include sampling from a multicenter platform and include global scale criteria for data analysis. The underlying mechanism of action of the tested drugs on target molecules should be thoroughly understood. Secondary measurements like day-to-day progress of recovery, length of rehabilitation, treatment endpoint analysis and any other compounding factors should also be recorded. Overall, research on stoke management has advanced rapidly in recent years and is certain to make additional valuable discoveries through the application of new technologies in hypothesis-driven clinical trials.
8. Translational Challenges for the Current Stroke Therapeutic Strategies
Stroke research has seen fundamental advancements over recent years. The improvements in the selection of animal models, imaging techniques and methodological progress have led to immense drug targets and therapeutic interventions. In spite of this, the subsequent clinical trials failed to prove pre-clinical outcomes. Recanalization therapy showed some promising results in the clinical trials but only a small section of stroke patients benefited from this treatment [ 175 ]. Hence, the translational potential of stroke research is still under-investigated.
The key challenges that hinder the smooth transition of pre-clinical research into successful drugs include relevant endpoint selection, confounding diseases models like hypertension and diabetes, modelling age and gender effects in stroke patients, development of medical devices, investigating medical conditions that co-exist during stroke incidence, reproducibility of pre-clinical stroke research data and modelling functional and behavioral outcome [ 176 , 177 , 178 ]. Multiple causality of the stroke occurrence is another problem that is often over-looked. Homogeneity in stroke models to exhibit the broad spectrum of stroke pathophysiology associated with ischemic lesions or cortical or intracerebral damage is critical. Therefore, stroke animal models that target specific causes of stroke should be included. Latent interaction between comorbidities and stroke treatment should be identified to increase the safety and efficacy of the clinical outcome [ 179 ]. Short-term experimental trials often result in failed therapeutic development due to false-negative outcomes in the clinical settings [ 180 ]. Understanding the functional and behavioral output which might mislead true recovery is problematic in clinical trials wherein animal models have greater ability to mask the functional benefits [ 181 ]. This affects the affecting translational capability of the research. Adapting a combined approach to model recovery and rehabilitation is also important for successful transition.
One of the other problems with the clinical trials for stroke is the lack of efficient data management. The impact of large data generated from numerous clinical experiments is over-whelming and there should be a standardized system to manage such data. Moreover, these data should be deposited into a public data repository for easy access.
Industry and academic corroborations in stroke research are critical to improve the translational value [ 182 ]. A consensus between industry and academic interests is vital for successful transition. The industry collaborations are mostly monetary driven and have time constraints which might compromise the pre-clinical study protocol design, appropriate sample sizes and overestimation of treatment effects. IP protection and publication of research data may discord between these groups. A multicenter approach, long-term collaborations, effective project management, use of advanced methodologies and establishment of functional endpoints will probably advance the translational roadblocks in stroke research [ 183 ].
9. Conclusions
Stroke is the second leading cause of death and contributor to disability worldwide and has significant economic costs. Thus, more effective therapeutic interventions and improved post-stroke management are global health priorities. The last 25 years of stroke research has brought considerable progress with respect to animal experimental models, therapeutic drugs, clinical trials and post-stroke rehabilitation studies, but large gaps of knowledge about stroke treatment remain. Despite our increased understanding of stroke pathophysiology and the large number of studies targeting multiple pathways causing stroke, the inability to translate research into clinical settings has significantly hampered advances in stroke research. Most research has focused on restoring blood flow to the brain and minimizing neuronal deficits after ischemic insult. The major challenges for stroke investigators are to characterize the key mechanisms underlying therapies, generate reproducible data, perform multicenter pre-clinical trials and increase the translational value of their data before proceeding to clinical studies.
Author Contributions
Conceptualization, D.K.; writing—original draft preparation, D.K.; writing—review and editing, Z.X.; funding acquisition, Z.X. All authors have read and agreed to the published version of the manuscript.
This research and The APC was funded by Apex Biotech Research.
Conflicts of Interest
The authors declare no conflict of interest.
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
- Pay My Bill
- Get a Quote
- Call 800.227.4527
- Fax 510.318.6700
- MIEC 6250 Claremont Avenue Oakland, CA 94618
- Report a Claim
- Member Services
- Patient Safety
- Apply for Coverage
Misinterpretation of CT Scan Delays Stroke Diagnosis
Published on: May 17, 2024
This case study was put together by our partners at CRICO and was written by Katherine Zigmont, Clinical Program Specialist
Description
A misinterpretation of a stat CT scan result and a lack of communication between providers delays an unresponsive woman’s stroke diagnosis.
Clinical Events
A 54-year-old female was brought to the emergency department (ED) in the late afternoon after being found unresponsive at work. The ED provider noted that the patient presented as lethargic, stuporous, and aphasic. A stat CT scan was performed, which the radiologist read as negative for a stroke. In the evening, the family asked for a neurology consult as they thought the patient had had a stroke. They were told the CT scan was negative for a stroke and that a neurologist would see the patient in the morning because no one was available after hours.
Later that night, the ED provider documented that the patient was presenting with a left-sided droop, weakness, and neglect. The patient then had a Computed Tomography Angiography (CTA) scan, which a radiologist read but did not record the results. The radiologist reportedly informed the tech the CTA scan was sub-optimal and not diagnostic, but they did not mention any findings or follow up on the results with any providers.
The patient was admitted to the floor the next morning. The patient’s care was transitioned to a hospitalist who noted that the patient’s condition remained unchanged and ordered a neurology consult. The neurologist ordered an MRI which was read as a large stroke. Later that evening, the patient became unresponsive and their right pupil was fixed and dilated. They were placed on a ventilator, transferred to a higher level of care, and passed away the following day.
The original CT scan was later re-read with marked abnormality in the right hemisphere with loss of gray-white matter in the parietal area consistent with an acute middle cerebral artery infarct. When the CTA scan was reevaluated, it showed loss of blood flow consistent with a right middle artery infarct and developing edema.
The family sued the radiologist and the emergency medicine provider for a delay in diagnosis of acute middle cerebral artery infarct.
Disposition
The case was settled for more than $500,000.
Analysis The radiologist’s misinterpretation of the CT scan was a primary contributing factor to the patient’s outcome. When faced with a physical assessment that does not align with the test result, a discussion between the radiologist and the ED provider could have been key to resolving any confusion. The ED provider failed to appreciate signs and symptoms of a stroke. Despite having initial concerns about the patient having a stroke, the ED provider developed confirmation bias after receiving the negative CT scan result and did not pursue reasons for the patient’s condition. It was not until the patient’s condition worsened that they ordered a CTA, but because the radiologist did not communicate the test issues, it confirmed the ED provider’s bias that the patient was not having a stroke. The radiologist failed to follow up with the ED provider about the test results. The lack of communication between the radiologist and the ED provider about any results or issues with the CTA further influenced the ED provider’s initial assessment. Weekend and off-shift resources were not available. If a neurologist was either in the hospital or on-call, the patient might have been able to receive tPA in a timely manner. An MRI was only obtained once a neurologist saw the patient the next day.
Discussion Questions
- What processes are in place to ensure follow-up of test results between providers?
- How do you reconcile a patient’s presenting symptoms that don’t align with test results?
- What is the competency of this radiologist given the stroke was easy to see when it was re-read?
If you would like to view the original case study, click here .
If you would like to download a PDF copy of this case study, click here .
If you would like to view other CRICO case studies, click here .
Crico’s national database of medical professional liability (MPL) cases is a robust patient safety learning engine, built for making better data-informed decisions that can help save lives. rmf.harvard.edu/ or call 877.763.2742
Quick Links
- Knowledge Library
- Events & Seminars
- MIEC Wellness
For Policyholders
- Annual Report
- Report A Claim
Log in using your username and password
- Search More Search for this keyword Advanced search
- Latest content
- Current issue
- For authors
- BMJ Journals More You are viewing from: Google Indexer
You are here
- Online First
- The pEGASUS-HPC stent system for intracranial arterial stenosis: a single-center case series
- Article Text
- Article info
- Citation Tools
- Rapid Responses
- Article metrics
- http://orcid.org/0000-0003-2725-7685 Daniel Pielenz 1 ,
- Joachim Klisch 1 , 2 ,
- http://orcid.org/0000-0002-2677-8780 David Fiorella 3 ,
- Matthias Gawlitza 4 ,
- Andreas Steinbrecher 5 ,
- Elke Leinisch 5 ,
- Elmar Lobsien 5 ,
- Karl-Titus Hoffmann 4 ,
- http://orcid.org/0000-0002-3936-0981 Donald Lobsien 1
- 1 Department of Neuroradiology , Helios Hospital Erfurt , Erfurt , Thüringen , Germany
- 2 Department of Neuroradiology , Helios Vogtland Hospital Plauen , Plauen , Sachsen , Germany
- 3 Department of Neurosurgery , Stony Brook University , Stony Brook , New York , USA
- 4 University Hospital Leipzig Institute for Neuroradiology , Leipzig , Sachsen , Germany
- 5 Department of Neurology , Helios Hospital Erfurt , Erfurt , Thüringen , Germany
- Correspondence to Daniel Pielenz, Neuroradiology, Helios Hospital Erfurt, Erfurt, Thüringen, Germany; daniel.pielenz{at}helios-gesundheit.de
Background Intracranial arterial stenting is a technique for the treatment of symptomatic stenosis. In this single-center retrospective case series we evaluated a novel low profile laser-cut stent with an antithrombogenic hydrophilic polymer coating (pEGASUS-HPC, Phenox GmbH, Bochum, Germany) for the treatment of intracranial stenosis in the setting of acute ischemic stroke and elective cases.
Methods All patients treated with pEGASUS-HPC for one or more intracranial arterial stenoses at our institution were retrospectively included. Clinical, imaging and procedural parameters as well as clinical and imaging follow-up data were collected.
Results We performed 43 interventions in 41 patients with 42 stenoses in our neurovascular center between August 2021 and February 2024. Twenty-one patients (51.2%) were female and the mean±SD age was 71±10.8 years. Thirty-seven (86.1%) procedures were performed in the setting of endovascular acute ischemic stroke treatment. Technical or procedural complications occurred in seven patients (16.3%), six in the thrombectomy group and one in the elective group. One stent-related hemorrhagic complication (subarachnoid hemorrhage) occurred in emergency cases and symptomatic intracerebral hemorrhage occurred in one patient treated in an elective setting. Overall stenosis reduction following pEGASUS-HPC stent implantation was 53.0±18.0%. On follow-up imaging, which was available for 16 patients (37.2%) after an average of 32±58.6 days, 62.5% of the stents were patent.
Conclusion Our single-center case series demonstrates the feasibility of using the pEGASUS-HPC stent system, especially in emergency situations when thrombectomy fails.
- angiography
- atherosclerosis
Data availability statement
All data relevant to the study are included in the article or uploaded as supplementary information.
https://doi.org/10.1136/jnis-2024-021737
Statistics from Altmetric.com
Request permissions.
If you wish to reuse any or all of this article please use the link below which will take you to the Copyright Clearance Center’s RightsLink service. You will be able to get a quick price and instant permission to reuse the content in many different ways.
WHAT IS ALREADY KNOWN ON THIS TOPIC
Intracranial arterial stenting for the treatment of symptomatic intracranial stenoses is controversial but emerges as a valuable option in the setting of acute stroke thrombectomy. The pEGASUS-HPC is a novel laser-cut self-expandable stent with antithrombogenic polymer coating, potentially reducing the risk of local thrombotic complications.
WHAT THIS STUDY ADDS
In our study, the novel pEGASUS-HPC stent showed a high level of procedural effectiveness.
HOW THIS STUDY MIGHT AFFECT RESEARCH, PRACTICE OR POLICY
Bail-out stenting using this stent in failed thrombectomy cases is a valuable treatment option as well as treatment of intracranial stenosis with recurrent symptoms under best medical treatment.
Introduction
Intracranial arterial stenosis poses a significant challenge in the treatment of acute ischemic stroke. While stenting is mostly used as a secondary therapy in elective cases after exhausting best medical treatment options, it remains a subject of ongoing debate as a bail-out therapy in acute stroke recanalization. Emerging data suggest potential benefits for ‘rescue’ or ‘bail-out stenting’ in certain acute cases. 1–4 The pEGASUS-HPC stent (Phenox GmbH, Bochum, Germany) is a novel low-profile, self-expanding laser-cut stent designed for intracranial aneurysm and stenosis treatment. It is available with the HPC coating that reduces thrombogenicity. 5
We present a retrospective single-center series documenting our initial experience with the pEGASUS-HPC stent system for the treatment of intracranial arterial stenosis in both emergency and elective settings.
Materials and methods
Study design.
The database of our neurovascular center was retrospectively screened and all patients and procedures fulfilling the following criteria were included in the study:
Patients (age ≥18 years) treated with the pEGASUS-HPC stent for intracranial arterial stenosis.
Conditions potentially leading to a luminal stenosis (ie, atherosclerotic, dissection, wall-adherent thrombus as bail-out in unsuccessful thrombectomy, unknown origin) of any location, grade or clinical presentation.
Patients undergoing an initial treatment or a retreatment of the index lesion.
Patients with or without acute demarcated ischemic infarction on pre-interventional imaging.
Balloon angioplasty prior to or after delivering the stent was optional.
All types of balloons for pre- or post-dilatation were allowed.
All types of access material (ie, guiding catheters, aspiration catheters, microcatheters and microguidewires).
All types of antiplatelet protocols before, during and after the procedure.
We defined the following exclusion criteria:
Acute intracranial hemorrhage.
Additional stenting of stenosis adjacent to treated aneurysm.
Treatment of cerebral vasospasms following aneurysmal subarachnoid hemorrhage.
The patients were categorized as elective cases if the stenosis was known and the treatment was planned and prepared several days or weeks before the treatment. These were all patients with recurrent neurological symptoms despite best medical treatment. Emergency cases were all patients with acute ischemic stroke with large vessel occlusion and a luminal narrowing discovered after mechanical thrombectomy.
Data collection
We documented demographic data for each patient, stenosis grade according to the WASID method, 6 location of the stenosis, and pre-, peri-, and post-interventional antiplatelet therapy. Additionally, we recorded peri-procedural, post-procedural, and delayed complications, as well as clinical and anatomical imaging outcomes.
The stenosis diameter in relation to the adjacent normal vessel diameter was assessed both before and after implantation of the pEGASUS-HPC stent, including any additional balloon dilatation if performed. Successful treatment was defined as a reduction of the initial stenosis by ≥50%.
A thorough evaluation of all procedures entailed documenting and analyzing all materials used, procedural complications and their management, as well as the type of anesthesia administered. Subjective impressions of the operators were also recorded, categorized as ‘pushing through the microcatheter’ (satisfactory/unsatisfactory) and ‘positioning of the stent’ (satisfactory/unsatisfactory).
Revascularization rates of the stenosis were assessed based on the percentage of residual stenosis and stenosis improvement at the conclusion of the procedure and during follow-up, if available. All available follow-up assessments, including digital subtraction angiography (DSA), magnetic resonance angiography (MRA) and CT angiography (CTA), were included. Early follow-ups were conducted based on individual clinical circumstances post-treatment.
Stent patency was graded using a 4-stage scale:
No stenosis.
≤50% stenosis.
>50% stenosis.
Occlusion, assessed at the conclusion of the procedure and during all available follow-ups.
Statistical analysis
All data were collected anonymously in an Excel spreadsheet. Image evaluations were conducted at our neurovascular center using our routine PACS (Picture Archiving and Communication System).
Demographic data, baseline and follow-up data as well as procedural characteristics were summarized and reported as mean±SD and range for continuous variables. Categorical data were summarized as numbers and percentages.
Basic demographics
From August 2021 to November 2023 we identified 41 patients in whom we treated 42 stenoses. Among them, 21 patients (51.2%) were female and the mean±SD age was 71±10.8 years.
Five patients underwent elective treatment, accounting for a total of six interventions (13.9%). The remaining 37 patients (86.1%) received treatment in the context of mechanical thrombectomy. During the same period we performed 826 thrombectomies resulting in intracranial bail-out stenting in emergency cases using the pEGASUS-HPC in 4.5% of all thrombectomies. This is in line with recent trends in endovascular therapies. 7 8
Before stent implantation the stenosis grade in relation to the normal adjacent vessel diameter averaged 87.8±9.9%. Following stent implantation, the residual stenosis measured 34.5±15.8% in emergency cases and 29.2±18.1% in elective cases ( table 1 ).
- View inline
Basic demographics, clinical and angiographic characteristics and outcome
The majority of stenoses were located in the anterior circulation (74.4%), with the posterior circulation accounting for the remaining 25.6%. Predominantly, stenoses were found in the middle cerebral artery (M1: 46.5%; M2: 9.3%), followed by the internal carotid artery (ICA, C2: 7.0%; C3 and C5 each 0%; C4 and C6 each 4.7%, C7: 16.3%, according to Bouthillier classification 9 ). Stenoses were also observed in the vertebral artery (14.0%) and basilar artery (9.3%). In six emergency cases (14.0%) dissection was diagnosed as the underlying cause, with an additional five patients (3 emergency, 2 elective) presenting with suspected dissection.
All patients underwent general anesthesia. Intra-arterial blood pressure measurements were performed primarily through femoral access sheaths during the intervention and via peripheral access (mostly radial artery) post-interventionally. The systolic arterial pressure threshold was set at 140 mmHg and efforts were made to avoid blood pressure spikes.
Endovascular treatment
For the treatment of 42 stenoses a total of 50 pEGASUS-HPC stents were used: 46 (92.0%) were implanted and four (8.0% of all stents) were discarded. To mitigate the risk of secondary dislocation and maximize the utilization of the stent’s radial force, pEGASUS-HPC stents were intentionally oversized, with a mean oversizing of 26.1±37.3% compared with the parent vessel diameter.
In three patients two stents were implanted during the same session: one instance involved a slight dislocation of the first stent necessitating the placement of an additional stent while, in another case, two stents were used to cover a long stenotic segment. Furthermore, one patient with bilateral high-grade V4 stenosis was successfully treated in two separate interventions.
In five cases (13.2%) a transition from a balloon-expandable stent to the pEGASUS-HPC stent occurred due to the inability to deliver the stent to the target lesion. Balloon angioplasty was performed in 41 interventions (95.3%), with the NeuroSpeed balloon (Acandis) being the most frequently used (55.8%). For stent deployment, the NeuroSpeed balloon catheter was employed in 51.2% of interventions followed by Excelsior SL10 (Stryker, Fremont, California, USA) in 34.9%. In all four cases where the pEGASUS-HPC stent was discarded, a NeuroSpeed balloon was used as the delivery microcatheter. This was due to dislocation caused by friction in three interventions, while insurmountable friction prevented the delivery of the stent in one patient. In these instances, the Excelsior SL10 microcatheter was used for the successful deployment of the second pEGASUS-HPC stent. In dilatation, we deliberately chose balloons that were undersized compared with the estimated vessel diameter in 97.5% of cases to mitigate the risk of high-grade dissection and vessel perforation.
For the first balloon the undersizing averaged 23.9±22.1%, with diameters ranging from 1.5 to 4.0 mm and lengths from 8 to 20 mm. If a second balloon was employed, the mean undersizing was 16.2±22.7%, using the same lengths. Pre-dilatation alone was used in 28 cases (58.1%), while pre- and post-dilatation were conducted in 13 cases (30.2%). Post-dilatation alone was performed in two cases (4.7%). In two cases (4.7%) angioplasty was not used as these were suspected dissection cases.
Figures 1 and 2 show examples of pre- and post-dilatation and pre-dilatation only, respectively. All angioplasty balloons used were uncoated and none were drug-eluting. Thrombectomy before stent implantation was carried out in 37 cases (86.1%), typically using the SAVE technique. 10 The mean number of thrombectomy maneuvers was 3.6±3.4, with a maximum of 16 thrombectomy maneuvers recorded in one intervention. The criteria for implementing bail-out stenting in cases of unsuccessful thrombectomy were the identification of high-grade stenosis in the previously occluded vessel, or the presence of a stenosis exhibiting low and/or turbulent flow, or a tendency toward early reocclusion. Regarding the infarct core, there was no specific quantitative threshold to avoid stenting. Systemic thrombolysis before intervention was administered in 12 patients, constituting 32.4% of patients with acute stroke, and the mean dose administered was 58.4±17.8 mg.
- Download figure
- Open in new tab
- Download powerpoint
Patient with acute ischemic stroke postoperatively (tumor nephrectomy). (A) Initial complete occlusion of left M1. Partial leptomeningeal collateralization from left anterior cerebral artery (ACA). (B) Proximal M1 stenosis after two thrombectomies using the SAVE technique. Possible wall-adherent plaque (note the fuzzy margin of the distal stenosis). (C) Pre-dilatation using a NeuroSpeed balloon catheter (2.0×8.0 mm) after 500 mg aspirin IV and 2500 IE heparin IV. (D) Minor residual stenosis following pEGASUS-HPC stent implantation (3.5×15 mm). (E) Post-dilatation using same NeuroSpeed balloon catheter (2.0×8.0 mm). (F) Final run (modified Treatment in Cerebral Ischemia grade 2c). ACA collaterals are diminished due to normal middle cerebral artery flow.
Patient with acute ischemic stroke. (A) Initial occlusion of distal right M1 despite Actilyse IV, aplasia of right A1. (B) High-grade M2 stenosis (superior trunk) after three thrombectomies using the SAVE technique. (C) Pre-dilatation using a Maverick balloon catheter (1.5×20 mm). (D) Positioning of pEGASUS-HPC stent (3.5×15 mm) from superior trunk of M2 to distal M1 following 500 mg aspirin IV. (E) Control run, no post-dilatation necessary. (F) Final run, complete revascularization (modified Treatment in Cerebral Ischemia grade 3).
Antiplatelet protocols
In all elective cases, patients received 100 mg aspirin orally and 75 mg clopidogrel orally before the procedure, along with 250 mg aspirin and 5000 IU heparin IV during the procedure. After the procedure, patients were prescribed 100 mg aspirin orally and 75 mg clopidogrel orally for 6–12 months. Antiplatelet function tests using the multiplate test (Roche Diagnostics, Mannheim, Germany) were conducted for all patients on the day before the procedure, with all patients having a satisfactory response to aspirin and clopidogrel.
In emergency interventions the antiplatelet protocol varied, largely due to pre-existing medications administered for other conditions. Details concerning the individual antiplatelet protocols used are summarized in table 2 .
Antiplatelet/anticoagulant protocols and thrombolytica
Technical outcome
In the final DSA run, all implanted pEGASUS-HPC stents were found to be patent. The mean±SD initial stenosis overall was 87.8±9.9% and, after stenting, the residual stenosis was 34.8±15.6% resulting in a mean stenosis improvement of 53.0±18.0%. In emergency interventions the stenosis improvement was 52.6±17.3%, while in elective interventions it was 58.1±21.7%.
At the first follow-up, which was available for 16 patients (37.2%) after a mean of 32.1 days, imaging modalities included 43.8% DSA, 31.2% CTA, and 25.0% MRA. Among these, no in-stent stenosis was observed in seven patients (43.7% of available follow-ups), moderate restenosis was found in three patients (18.7%), and severe stenosis in two patients (12.5%). Additionally, one stent was occluded (6.2%). In one case residual stenosis showed further improvement at the first follow-up and, in another case, a stent was impossible to evaluate by CTA due to beam hardening artifacts.
Clinical outcome
The modified Rankin Scale (mRS) was used to assess all patients at hospital discharge. Overall, the mRS score improved slightly from pre-intervention (mean 4.2) to post-intervention (mean 3.9), with a slightly more pronounced difference observed in emergency patients (pre-intervention mean 4.5, post-intervention mean 4.1). In elective patients, the mean mRS score of 2.7 showed no change. Improvement in the mRS score was recorded in 19 patients, with a good clinical outcome (mRS 0–2) achieved in nine patients overall (20.9%), including seven emergency interventions (19.9%).
Of the 37 emergency patients, 11 (25.6%) died with four developing sepsis, one experiencing massive abdominal bleeding and hemorrhagic shock, one suffering cardiogenic shock, and five encountering basilar thrombosis with vertebrobasilar infarction. No death was directly related to stent implantation. One death (due to hemorrhagic shock) was almost certainly caused by femoral access site bleeding after intra-/post-procedural tirofiban infusion.
Intra-procedural technical and clinical complications
Revascularization of the occluded vessel or improvement of stenosis was achieved in all cases with a reduction of the initial stenosis by ≥50%. No intra-procedural hemorrhagic complications or directly pEGASUS-related complications occurred in elective cases. However, in one patient (16.7%) dissection distal to the stenosis occurred after pre-dilatation. In response, a flow diverter (p64 MW-HPC 350-21, Phenox) was implanted and the remaining intervention proceeded without further incident.
In emergency interventions, seven cases (18.9%) experienced major intra-procedural complications. In two cases, emboli occurred distally to the stent. In the first case it was managed with microwire fragmentation and continued tirofiban infusion, achieving an overall mTICI 2c recanalization. Unfortunately, the patient’s neurological deficit did not improve post-intervention. Additionally, the patient suffered from severe urinary tract infection and died 4 days after the intervention due to combined severe acute ischemic infarction (National Institutes of Health Stroke Scale (NIHSS) score of 19 pre-intervention) and sepsis. In the second case an embolism distal to the stenosis occurred immediately after pre-dilatation and was treated with aspiration thrombectomy and tirofiban infusion, resulting in partial recanalization. However, the patient died the following day.
In a patient in their 80s with acute left-sided distal ICA occlusion, an acute in-stent thrombosis occurred in the M1 segment. Additionally, the patient suffered from a high-grade stenosis in the distal cervical segment of the ipsilateral ICA. Both conditions were addressed in the same intervention with a balloon-expandable stent (Multilink Vision 4×15, Boston Scientific) deployed to treat the high-grade stenosis. The in-stent thrombosis of the pEGASUS-HPC stent was managed with tirofiban and angioplasty, resulting in patency of the stent and a final mTICI 2c result. The patient’s condition slightly improved from a mRS score of 5 to 4 and a NIHSS score from 20 to 16.
Three (8.1%) iatrogenic dissections occurred, all in emergency cases. Two were caused by failed positioning of balloon-mounted stents and one was caused by pre-dilatation. These patients were successfully treated and experienced both angiographic and clinical improvement with mRS scores improving from 4 to 1, 4 to 3, and 5 to 4.
Additionally, one (2.7%) extracranial paravasation in nuchal tissue adjacent to the distal V3 segment of the vertebral artery occurred due to failed positioning of a balloon-expandable stent (PRO-Kinetic Energy 3×9, Biotronik SE & Co. KG, Berlin, Germany). The vessel was reconstructed with two flow diverters (2× p64 MW HPC 4.0×24 mm, Phenox).
Post-procedural complications
Post-procedural complications related to the pEGASUS-HPC stent system occurred in three (8.1%) patients undergoing an emergency intervention, consisting of one hemorrhagic and two ischemic events. In one emergency patient treated with two stents due to slight dislocation of the first stent, delayed post-procedural loading with clopidogrel resulted in subtotal occlusion of the stent within less than 24 hours despite successful initial revascularization. This led to aspiration thrombectomy and tirofiban bolus infusion, but the patient suffered partial MCA infarction and neurological deterioration. Another patient with M1 occlusion experienced post-procedural subarachnoid hemorrhage under continuous tirofiban infusion and died 6 days later due to additional complications. The third patient treated for an M1 occlusion due to an underlying high-grade stenosis developed high-grade in-stent stenosis, partial MCA infarction, three-valve endocarditis with sepsis, and non-occlusive mesenteric ischemia and died 3 days after the stent procedure.
In three (50.0%) of the elective interventions, post-procedural stent-related complications occurred. One patient treated for high-grade stenosis of the C7 segment of the left ICA with pEGASUS-HPC stentand with short-term postinterventional tirofiban perfusor experienced post-procedural symptomatic intracerebral hemorrhage (sICH) with motoric aphasia. Another patient with high-grade ICA stenosis underwent successful revascularization with the pEGASUS stent but required an additional flow diverter for the treatment of iatrogenic dissection distal to the stenosis. The third patient with bilateral high-grade distal V4 stenosis and elective stenting of the left-sided stenosis experienced rotating vertigo, nausea, and gait ataxia, possibly correlated with a small pontine infarction observed on MRI 7 days after stent implantation.
In this retrospective observational single-center study we present our experience with the pEGASUS-HPC stent system. Revascularization achieved success rates of 100%, defined as an improvement in the stenosis grade of 50% or more. Subsequent occlusion after initial treatment was observed in three of the 50 stents deployed. The majority of interventions consisted of futile thrombectomies (87.8%), with a minority comprising elective interventions for symptomatic stenosis (12.2%).
The findings of our study are noteworthy in light of the Stenting and Aggressive Medical Management for Preventing Recurrent Stroke in Intracranial Stenosis (SAMMPRIS) trial, which reported a higher incidence of stroke or death in the stenting+best medical treatment group (14.7%) compared with the best medical treatment group (5.8%). 11 Since 2009 there have been significant technological advancements in intracranial stent systems. For example, the Wingspan stent used in the SAMMPRIS trial is a relatively complex stent that requires larger delivery catheters than the pEGASUS-HPC (0.027 inches vs 0.0165 inches). This difference in catheter size reflects improvements in stent design and delivery systems, allowing for more minimally invasive procedures with smaller access vessels. Such advancements contribute to enhanced safety and efficacy profiles of newer generation stents like the pEGASUS-HPC. This stent system received CE (Conformité Européenne) mark clearance in the European Union in 2021 for the treatment of aneurysms and intracranial stenosis. It is coated with a glycan-based hydrophilic multilayer polymer coating (HPC) that is a mere 10 nm thick and covalently bound to the stent material, thus offering a less thrombogenic stent surface. 5 12–14 This surface alteration mimics the biological properties of the glycocalyx present on the endothelium, contributing to improved biocompatibility and reduced thrombogenicity of the stent. 12 The coating does not compromise the mechanical properties of the pEGASUS-HPC stent. In vivo studies have shown no inflammatory response or intima hyperplasia. 14 15 The coating has been shown to reduce thrombogeneity of the coated devices and promising results were reported of the use of flow diverters coated with HPC and mono antiplatelet therapy 16 .
One of the primary competitors to the pEGASUS-HPC stent in terms of construction similarities is the Neuroform Atlas Stent (Stryker, Portage, USA). Although the Atlas stent is not certified for the treatment of intracranial stenosis according to IFU, intracranial stenosis treatment with a good safety profile using the Atlas stent was recently published. 17 However, we chose the pEGASUS-HPC mainly because it is certified for stenosis treatment and also because it is available with antithrombotic coating. While there is no comprehensive quantitative comparison of the radial force of intracranial stents available in the published literature, it is estimated that the radial force of the pEGASUS-HPC stent lies above that of the Atlas stent but below that of the Wingspan stent.
Other reasons for stent selection may be anatomical reasons. Residual stenosis may be less pronounced with balloon-expandable stents, 18 but in elongated vessels it can be impossible to push them to the desired destination. In five (13.2%) patients a primarily selected balloon-expandable stent could not be placed at the site of stenosis. Recent studies have highlighted the benefits of modern stent and catheter materials. 19–21
NeuroSpeed double-lumen PTA balloons were used in 55.8% of cases, effectively eliminating the need for further probing or exchange maneuvers. This approach demonstrates the importance of selecting appropriate tools and techniques to optimize procedural outcomes when dealing with challenging stent designs. 22 However, in four cases (8.0%) the pEGASUS-HPC stent had to be discarded because of friction and dislocation of the NeuroSpeed catheter, a problem not encountered with single-lumen catheters. The reasons were not clear, since deployment of the stents worked well in most of the cases and might be due to anatomical reasons.
Our study included patients with considerable heterogeneity in baseline characteristics including medical comorbidities, premedication, blood pressure, body mass index, demographics, and history of smoking. All patients treated with the pEGASUS-HPC stent for intracranial arterial stenosis were included, which also comprised individuals with a high likelihood of poor outcomes. Many interventions were conducted in the setting of severe acute ischemic stroke, resulting in complex scenarios. Therefore, a certain degree of negative selection bias is likely, as evidenced by the mean number of thrombectomy procedures of 3.6±3.4. When excluding these patients from the analysis and focusing solely on those with a pre-stroke mRS score ranging from 0 to 2, it was observed that 50% experienced an improved mRS, a finding consistent with other recent studies demonstrating favorable outcomes in rescue stenting scenarios. 7 23–25
Moreover, 67.0% of all treated patients were transferred from smaller primary stroke centers or, in some cases, from peripheral hospitals lacking neurological care units. This situation often resulted in prolonged transfer times for these patients. 26 Additionally, higher NIHSS and mRS scores are strong predictors of poor functional outcome. 27 28
All elective interventions were conducted following a dual antiplatelet protocol. However, there was considerable heterogeneity in emergency stentings (see table 2 ), with 32.4% pretreated with recombinant tissue plasminogen activators. Intra-procedurally, aspirin was used in 62.2% of cases while tirofiban was used in 56.8% (with some overlap of the two medications). The rate of intracranial hemorrhage (5.4% in failed thrombectomy) was not higher than in current studies on bail-out stenting in failed thrombectomy 7 24 or in studies evaluating the risk of sICH for thrombectomy in patients on different antiplatelet or anticoagulation therapy. 29 sICH rates were also below the rates of the intervention group in SAMMPRIS. 11
Our study has limitations inherent to its retrospective observational design, including a relatively small sample size and a short follow-up period. Most post-procedural mRS values were assessed at the time of hospital discharge (with a mean±SD of 12.3±9.5 days) and the 90-day mRS scores could potentially be significantly lower.
This single-center case series demonstrates the safety and viability of the HPC-coated pEGASUS stent system, especially in emergency situations when thrombectomy fails. Further randomized controlled trials are needed to evaluate the suspected additional benefit of the biomimetic HPC coating in emergency and elective endovascular treatment of intracranial arterial stenosis and to appraise the best stent design for the diverse clinical scenarios.
Ethics statements
Patient consent for publication.
Not applicable.
Ethics approval
Ethics approval for the study was provided by the Ethics Committee of the Association of Medical Doctors of the state of Thuringia. Verbal confirmation of the Ethics Committee was given on March 6, 2024 (reference number: 22748/2024/6). Patient informed consent was waived due to completely anonymized data collection and analysis. This report adheres to the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement guidelines.
- Ong NY , et al
- Doheim MF ,
- Al-Bayati AR ,
- Bhatt NR , et al
- Pan Y , et al
- Qureshi AI ,
- Ma X , et al
- Lenz-Habijan T ,
- Peters M , et al
- Samuels OB ,
- Joseph GJ ,
- Lynn MJ , et al
- Stracke CP ,
- Fiehler J , et al
- Alexander MJ ,
- Menaker SA , et al
- Bouthillier A ,
- van Loveren HR ,
- Kabbasch C , et al
- Derdeyn CP ,
- Chimowitz MI ,
- Aguilar Pérez M , et al
- Gawlitza M ,
- Kaiser DPO , et al
- Bannewitz C , et al
- Hellstern V ,
- Aguilar Pérez M ,
- Henkes E , et al
- Cao W , et al
- Kwak JK , et al
- Mohammaden MH ,
- Nogueira RG ,
- Tekle W , et al
- Ma N , et al
- Kyselyova AA ,
- Frölich AM ,
- Bester M , et al
- Li C , et al
- Pérez-García C ,
- Gómez-Escalonilla C ,
- Rosati S , et al
- Ifergan H ,
- Dargazanli C ,
- Ben Hassen W , et al
- Psychogios M-N ,
- Schlemm E , et al
- Wouters A ,
- Thijs V , et al
- Kitano T , et al
- Becerril-Gaitan A ,
- Hunt A , et al
Contributors DP: guarantor, data collection, study design, data analysis, writing of the manuscript and revisions. JK: data collection, careful revision of the manuscript. ELe and ELo: careful revision of the manuscript, data collection. DF, AS, K-TH: careful revision of the manuscript. DL: data collection, study design, careful revision of the manuscript.
Funding The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests DP: Phenox – travel expenses. DF: Medtronic, Cerenovous, Microvention, Penumbra, Stryker, Balt USA, Seimens, Mentice, Neurogami, Rapid.AI, Rapid Medical, Q’apel Medical, Arsenal Medical, Phenox, Scientia, NVMed, Perfuze, Vesalio – consulting fees; Medtronic, Cerenovous, Microvention, Penumbra, Stryker, Balt USA, Q’apel Medical – speaker honoraria; Medtronic, Cerenovous, Microvention, Penumbra, Stryker, Balt USA, Seimens, Mentice, Neurogami, Rapid.AI, Rapid Medical, Q’apel Medical, Arsenal Medical, Phenox, Scientia, NVMed, Perfuze, Vesalio – travel expenses; Scientia, MENTICE, Neurogami, NVMed, Perfuze – leadership role; Scientia, Perfuze, NVMED, Mentice, Neurogami – stock options. JK: Phenox travel expenses, speaker honoraria; Phenox, Microvention – consulting fees (money paid to institution). MG: Phenox – speaker honoraria, consulting fees; Microvention – speaker honoraria, consulting fees; Balt – consulting fees; Simq GmbH – Scientific advisory board member. AS, EL, EL: none. KT-H: Bayer – speaking honoraria, advisory fees. DL: Phenox – travel expenses, speaker honoraria (money paid to institution).
Provenance and peer review Not commissioned; externally peer reviewed.
Read the full text or download the PDF:

IMAGES
VIDEO
COMMENTS
CASE 1. A 20 year old man with no past medical history presented to a primary stroke center with sudden left sided weakness and imbalance followed by decreased level of consciousness. Head CT showed no hemorrhage, no acute ischemic changes, and a hyper-dense basilar artery. CT angiography showed a mid-basilar occlusion.
Some case studies in Wuhan described immense inflammatory responses to COVID-19, including elevated acute phase reactants, such as CRP and D-dimer. Raised D-dimers are a non-specific marker of a prothrombotic state and have been associated with greater morbidity and mortality relating to stroke and other neurological features. 14
Read chapter 7 of Patient Management in the Telemetry/Cardiac Step-Down Unit: A Case-Based Approach online now, exclusively on AccessMedicine. AccessMedicine is a subscription-based resource from McGraw Hill that features trusted medical content from the best minds in medicine.
Dr. Schwamm: The hospital-based response to acute stroke in this case began with the administration of intravenous t-PA, which according to established guidelines, 9 is a class 1, level of ...
In the WAKE-UP (Efficacy and Safety of MRI-based Thrombolysis in Wake-Up Stroke) trial, 503 patients with a time of onset of disabling acute ischemic stroke that was unclear, but greater than 4.5 ...
In one study, the five most frequent stroke mimics were seizure, syncope, sepsis, migraine and brain tumours 12; detailed reviews can be found in Practical Neurology. 13 14. ... Insufficient data, case-by-case risk decision with obstetric team. Unruptured intracranial aneurysm: Small (<10 mm) unsecured unruptured intracranial aneurysm should ...
In Uganda, literature on stroke in young adults is limited however results of a study done among acute stroke patients admitted to the national referral hospital (Mulago hospital) showed a 30-day mortality of 43.8%. Out of 133 patients, 32 patients (25%) were less than 51 years old. Out of the 56 patients that died, 13 patients (23%) were less ...
Other studies have also reported the role of hypertension as a risk factor for stroke in young adults, low physical activity and hypertension were the most important risk factors, accounting for 59.7% and 27.1% of all strokes, respectively among a German nationwide case-control study based on patients enrolled in the SIFAP1 study (Stroke in ...
Miranda J, Moscoso M, Yan L, Diez-Canseco F, Málaga G, Garcia H and Ovbiagele B (2017) Addressing post-stroke care in rural areas with Peru as a case study. Placing emphasis on evidence-based pragmatism , Journal of the Neurological Sciences , 10.1016/j.jns.2017.02.027 , 375 , (309-315) , Online publication date: 1-Apr-2017 .
Stroke is one of these complications, since a greater incidence of stroke among patients with COVID-19 than the non-COVID-19 population has been reported, and a preliminary case-control study demonstrated that SARS-CoV-2 infection represents a risk factor for acute ischemic stroke .
IVT has been demonstrated to improve outcomes in patients with acute ischemic stroke within the first 4.5-h. 14, 15, 20-22 In 1996, the Food and Drug Administration (FDA) approved intravenous-tPA (IV-tPA) for treating acute ischemic stroke, based on successful outcomes in the National Institute of Neurological Disorders and Stroke study. 23, 24 ...
ISBN -521-67367-4, $72.00. Scope: This is a multiauthored book encompassing 60 common and uncommon cases related to stroke. Each vignette introduces a short history and salient features of the neurologic examination. Most of the cases include CT, MRI, angiography, transcranial Doppler, or cerebral perfusion scanning.
This case study presents a 68-year old "right-handed" African-American man named Randall Swanson. He has a history of hypertension, hyperlipidemia and a history of smoking one pack per day for the last 20 years. ... the doctor must make the diagnosis of an acute ischemic stroke, and the patient must continue to present with neurological ...
For this study, a stroke‐free control population, frequency matched for age (5‐year bands) and sex, was randomly selected from the southern Finland FINRISK survey participants from years 1997, 2002, and 2007, that is, from the same time period and the same geographic region as the case population.
Life after stroke support provides a lifeline to people affected by stroke, improves quality of life for both stroke survivors and carers, and means people can live well beyond stroke. ... Our case studies. Michael Pursey. Former sports coach and teaching assistant Michael Pursey, from Wales, expected to be in a wheelchair forever after having ...
Patient Case Presentation. Image courtesy of uofmhealthblogs.org. D.B. is a 72 year old African American female who presented to the ED with complaints of headache, altered mental status as evidenced by confusion and lethargy, slurred speech, right sided weakness, and a facial droop. Symptoms were first noted when patient woke up from a nap ...
The Australian Stroke program has nationally improved stroke care since 2007, showcasing the value of integrated LHS-aligned approaches for tangible impact on outcomes. This LHS case study is a practical example for other health conditions and settings to follow suit.
A PFO has been demonstrated in 10%-26% of healthy adults. 14 In young patients who have had a cryptogenic stroke, however, the prevalence is thought to be much higher, for example, 40% in one study. 15 It is thought that a PFO allows microemboli to pass into the systemic circulation leading to a stroke.
A stroke, also known as a brain attack, occurs when blood flow to the brain is blocked or a blood vessel inside or on the surface of the brain bursts. A stroke is a serious medical emergency and requires immediate medical attention, just like a heart attack. Stroke ranks as the fourth leading killer in the United States.
Stroke case study Creators. Sanaya Batcho; Kara Kitzmiller; Emma Overman; Our reason for choosing this disorder. Stroke is the leading cause of disability in the United States. As advanced practice nurses, we anticipate caring for those impacted by strokes in many healthcare settings including emergency rooms, acute care, rehab settings ...
A stroke, sometimes called a brain attack, occurs when something blocks blood supply to part of the brain or when a blood vessel in the brain bursts. In either case, parts of the brain become damaged or die. A stroke can cause lasting brain damage, long-term disability, or even death. Learn about the health conditions and lifestyle habits that ...
Author summary Cerebral ischaemic strokes are a leading cause of death and disability worldwide. Among other factors, the outcome of stroke treatment is determined by the existence and extent of collateral flow paths, which sustain residual blood supply to the obstructed brain region. To improve therapeutic strategies and to reduce reperfusion injuries during treatment, an in-depth ...
Some case studies in Wuhan described immense inflammatory responses to COVID-19, including elevated acute phase reactants, such as CRP and D-dimer. Raised D-dimers are a non-specific marker of a prothrombotic state and have been associated with greater morbidity and mortality relating to stroke and other neurological features. 14
The Australian Stroke program was selected as the case study as it provides an exemplar of how an iterative LHS works in practice at a national level encompassing and integrating evidence from all four LHS quadrants. Using this case study, we demonstrate how to apply evidence-based processes to healthcare improvement and embed real-world ...
Patients with initial stroke or transient ischemic attack (TIA) are at high risk for further strokes, death or cardiovascular events. Even the first-ever stroke is associated with a high chance of disability and need for assistance. The risk of long-term health care demands increases with each ...
A case study conducted in the US showed that people with high financial status had better stroke treatment options than deprived individuals . ... Stroke studies should be conducted in both male and female subjects to exclude gender bias, and should take account of other confounders like hypertension, diabetes and obesity. ...
Crico's national database of medical professional liability (MPL) cases is a robust patient safety learning engine, built for making better data-informed decisions that can help save lives. rmf.harvard.edu/ or call 877.763.2742. This case study was put together by our partners at CRICO and was written by Katherine Zigmont, Clinical Program ...
Objectives—Metallic elements and fibrin clot properties have been linked to stroke. We examined metallic and nonmetallic elements, fibrin clot lysis time (CLT), and maximum absorbance (Absmax) in relation to ischemic stroke. Design—A case-control study of ischemic stroke patients vs. healthy individuals. Subjects and Methods—Plasma and serum were collected from 260 ischemic stroke ...
Background Intracranial arterial stenting is a technique for the treatment of symptomatic stenosis. In this single-center retrospective case series we evaluated a novel low profile laser-cut stent with an antithrombogenic hydrophilic polymer coating (pEGASUS-HPC, Phenox GmbH, Bochum, Germany) for the treatment of intracranial stenosis in the setting of acute ischemic stroke and elective cases.