-->

  Short explanationLong explanation.
 Wind-up spinner toy Microwave light bulb Traveling flame Screaming dry ice Dry ice in a balloon Special: Dry ice color change Dry ice smoking soap bubble snake Dry ice giant crystal ball bubble Dry ice in water Rainbow milk Gummy bear osmosis Floating ping pong ball Rotating Earth Special: Colored fire Special: Fire bubbles Water cycle in a jar Egg drop challenge Taking the pulse Orange candle Glass bottle xylophone Warped spacetime Homemade rainbow Water implosion Warm and cold plates Plastic bag kite Tamed lightning Yeast and a balloon Forever boiling bottle Moon on a pen Moon in a box Inexhaustible bottle Crystal egg geode Magic ice cut Leaf pigments chromatography Heavy smoke Popsicle stick bridge Micrometeorites Special: Fire tornado Special: Whoosh bottle Dancing water marbles Brownian motion Flying static ring Water thermometer String telephone Special: Dust explosion Disappearing styrofoam Special: Burning money Special: Burning towel Salt water purifier Fish dissection Hovering soap bubble Homemade sailboat Water mass meeting Plastic bag and pencils Water sucking bottle Water sucking glass Mentos and coke Aristotle's illusion Spinning spiral snake Imploding soda can Carbon dioxide extuingisher Plastic bag parachute Dental impression Impact craters Rolling static soda can Static paper ghost Color changing flower Upside down glass Shrinking chip bag Solar system model Strawberry DNA Electric motor Flashy electric motor Bouncing soap bubbles Toilet paper roll maraca Cloud in a bottle 1 Cloud in a bottle 2 Balloon rocket Water whistle Homemade yogurt Special: Screaming gummy bear Homemade compass Trash airplane Tea bag rocket Balancing soda can Lung volume test Fireproof balloon Baking powder popper Expanding space Straw propeller Wooden cutlery Levitating match Human reflexes Electromagnet Soil layers Straw potato Straw rocket launcher Water bowls Straw duck call Solar eclipse Silo of salt Balloon skewer Newspaper tower Heavy paper Rubber chicken bone Homemade marble run Drops on a coin Cartesian diverContent of website.   Fireproof BalloonBalloons and flames don't mix, unless you add a little water to conduct heat. Print this Experiment  Common sense tells you that it’s impossible to boil water in a paper bag, but this classic parlor trick was a favorite of Victorian magicians. The real difficulty in performing this effect is making it look harder than it is! As you might imagine, the secret lies in yet another amazing property of water – its ability to conduct heat. Instead of using a paper bag, this modern day version of the demonstration uses an ordinary balloon, some water, and a candle. It’s a combination that’s guaranteed to make people stand back. Experiment VideosHere's What You'll NeedSafety glasses, let's try it.  Blow up a balloon just as you normally would and tie it off.  Light a candle and place it in the middle of the table.  Put on your safety glasses because it’s time to pop the balloon. Hold the balloon a foot or two over the top of the flame and slowly move the balloon closer and closer to the flame until it pops. You’ll notice that the flame doesn’t have to even touch the balloon and the balloon pops. Let’s just say you had to prove what you already know.  Let’s repeat the experiment, but this time the bottom of the balloon will have a layer of water inside. Fill the balloon to the top with water—it probably holds a few ounces (that’s 60 mL for you scientists out there)—and then blow it up with air. If you accidentally let go of the balloon before you tie it off, you’ll spray yourself, and your friends will love it. Just tie off the balloon and get ready for the next step.  Hold the water-filled balloon at the top while you slowly lower it over the candle and watch as people start to run. Everyone knows that it’s going to pop, but for some strange reason it doesn’t. If you’re very brave, you can actually allow the flame to touch the bottom of the balloon, but it still doesn’t pop.  Remove the balloon from the heat and carefully examine the soot on the bottom. Yes, there’s soot, yet the balloon didn’t pop. Before reading the explanation, try to figure out why the layer of water kept the balloon from popping. How Does It WorkWater is a great substance for soaking up heat. The thin balloon allows the heat to pass through very quickly and warm the water. As the water closest to the flame heats up, it begins to rise and cooler water replaces it at the bottom of the balloon. This cooler water then soaks up more heat and the process repeats itself. In fact, the exchange of water happens so often that it keeps the balloon from popping . . . until the heat of the flame is greater than the water’s ability to conduct heat away from the thin balloon and the balloon pops. But watch out! If you turn the balloon so that the candle flame is close to the side of the water balloon, the balloon will pop because the water is not conducting the heat away from the surface of the balloon. At least the water will help put out the fire! The soot on the bottom of the balloon is actually carbon. The carbon was deposited on the balloon by the flame, and the balloon itself remains undamaged. Safety InformationWARNING! This science activity uses matches, which means you need to find a very cool supervising adult to help with this experiment. Real-World ApplicationUsing water to control heat is a valuable process. Special superabsorbent polymer foams are currently being used by firefighters as a way to help protect homes from being consumed by a raging forest fire. The water-absorbing polymer foam is similar to the superabsorbent polymer found in a baby diaper. The foam is applied like shaving cream to the outside of the house. As the fire burns closer and closer to the home, the water-filled foam absorbs heat energy from the fire and buys firefighters some extra time as they try to fight the flames with water. Your body even uses water to control heat. When you exercise, your body produces sweat in an attempt to regulate your temperature so you don’t get overheated. As the sweat evaporates, it takes heat energy with it, leaving cooler skin behind. Related Experiments Fire Bubbles - The Methane MambaWhen is the last time you made bubbles catch on fire? Probably never . . . but then again, this is the teachers-only section of […]  Fire TornadoWhen we picture a tornado, most of us imagine a whirling column of air poking down from the clouds. But this tornado-like effect is not […]  CO2 Fire ExtinguisherCarbon dioxide (CO2) is a gas that humans interact with every day. For instance, you exhale it from your lungs. Drive a vehicle and it’s in the engine […]  Holiday Fire - RedEveryone likes to cuddle up next to a nice warm fire, but we couldn’t possibly settle for any normal fire. Why not make a more […]  Burning MoneyDo you have money burning a hole in your pocket? It’s probably not a wise idea to soak a $20 bill in a flammable liquid […]  Blubber GloveThe animals of the Arctic and Antarctic circles spend their lives surviving subfreezing air temperatures and frigid water. Their secret is blubber, a thick layer […] Browse more experiments by concept: Why Balloon With Water Does Not Burst? (Fireproof Balloon Experiment)
In this article, lets explore how balloon with water does not burst when exposed to fire. This Fireproof Balloon Experiment can help kids to learn how heat gets conducted through different materials. This is a great activity to teach all about thermal conductivity. In this simple science activity, children can hold the balloon over the fire without popping it. Fireproof Balloon ExperimentThe experiment helps kids to understand more about the difference between theremal conductivity of water and air. In addition, they also learn about the transfer of heat in air vs. water.  Things we need3) Any heat source that emits fire like candle, matches, or lighter 4) Safety Goggles (optional)  That’s it! Just collect these materials and get ready to experience the mind-blowing science of balloons, water, and heat. Before we land on the experiment directions, let us discuss a few questions that help to awaken your child’s predictions on the activity! 1) What happens to the balloon when brought closer to the fire? 2) What is the role of water inside the balloon? 3) Why the balloon with water does not burst over a hot flame? 4) What is more conductive between water and air? 5) Define the black soot formed on the balloon after the activity. Simple Step by Step Instructions to make The Fireproof BalloonStep-1: As a first step, blow up the balloon in the same way as you regularly do and give it a tight knot at its mouth part. Step-2: In the second step, light up the candle and place it on the experiment table. And bring the inflated balloon as closer as possible over the flame. The moment the balloon experiences the heat, it pops out.  Note: Do not forget to direct your child to put on their safety glasses and hand gloves as they are dealing with fire. Repeat the same experiment but this time using a little bit of water. 
Step-4: As a fourth step, bring the inflated balloon filled with water over the flame-like just 3-4 cm away from the fire. Whatever the angle you hold the balloon, you need to make sure that the water layer touching side of the balloon is upon the flame. Step-5: The final step is to observe the outcome! The inflated balloon with water inside does not pop out even if you make it touch the fire. This is the outcome you must witness after the experiment.  Note: If your balloon blows out even after filling with water, you are going wrong in holding the balloon in the right place. So, re-check the instructions and experiment to see our fire-resistant water balloon. Here is a small step to do, i.e., continue to hold the balloon over the flame. You will see a black soot formation on the balloon at the point of contact with the fire. To know why to continue reading to explore the science behind it. The Science behind the Fireproof Balloon ExperimentMaterials when exposed to flame/fire, they catch fire only when their temperature reaches above the flash point . Flash point is nothing but the temperature at which the material itself catches fires and burs on its own. When the balloon with water exposed to flame, the latex material gets heated up on one side. However since we have water on the other side of material, the heat gets absorbed by the water and the water temperature rises instead of the latex’s temperature. Water is a great conductor of heat and absorbs heat much better than air. That is the reason the water absorbs heat and changes its temperature when brought over the flame. On the other hand, the latex material of the balloon also supports the transfer of heat through and inside it. The heated water molecules have less density and travel upwards, i.e., towards the cooler side. In contrast, the space left by hot water molecules substituted by the cold water molecules. And these cold water molecules get heated up and travel upwards again. So this process inside the balloon continues as a cycle. As the cycle of exchanging water continues, it makes the balloon fire-resistant and does not allow the balloon to blow up! The balloon can hold the heat until the heat of a fire is greater than the water conductivity of heat. So, all the magic of resisting heat by the balloon lies in the heat conduction process by water. The soot formation at the end of the activity is from the flames coming out of the candle’s fire. That is nothing but carbon, the primary substance present in the heat flames. It forms as a layer of black coating on the balloon and keeps it undamaged. Here is a list of interesting ballon activities to try at home: Balloon Balance Experiment Balloon in Hot and Cold water Balloon in Bottle Experiment  Discussion (extension) Ideas after the experiment1) In what way, our demonstration relates the temperatures over ocean water and the coastal areas? 2) Will there be any change in the experiment results if you change the amount of water quantity inside the balloon? 3) Find out what the other liquids are the excellent conductors of heat! Perform the same experiment to learn the changes. 4) What is the reason behind the soot formation?  Leave a Reply Cancel ReplyYour email address will not be published. Required fields are marked * Name * Email * Add Comment * Save my name, email, and website in this browser for the next time I comment. Post Comment

The Unpoppable Balloon Science TrickApril 17, 2024 By Emma Vanstone Leave a Comment In this science activity, you can put a balloon into a candle flame without it popping! How is that possible? It’s all about heat transfer . A balloon filled with air will pop when it gets too close to a flame as the heat from the candle warms the balloon’s skin and weakens it. The trick to making an unpoppable balloon is to add water to it. The heat from the flame heats the water instead of the balloon, which then doesn’t burst. If you thought putting a skewer through a balloon was impressive, the unpoppable balloon science trick will blow your mind! You’ll needTwo balloons Safety goggles This activity requires adult supervision. 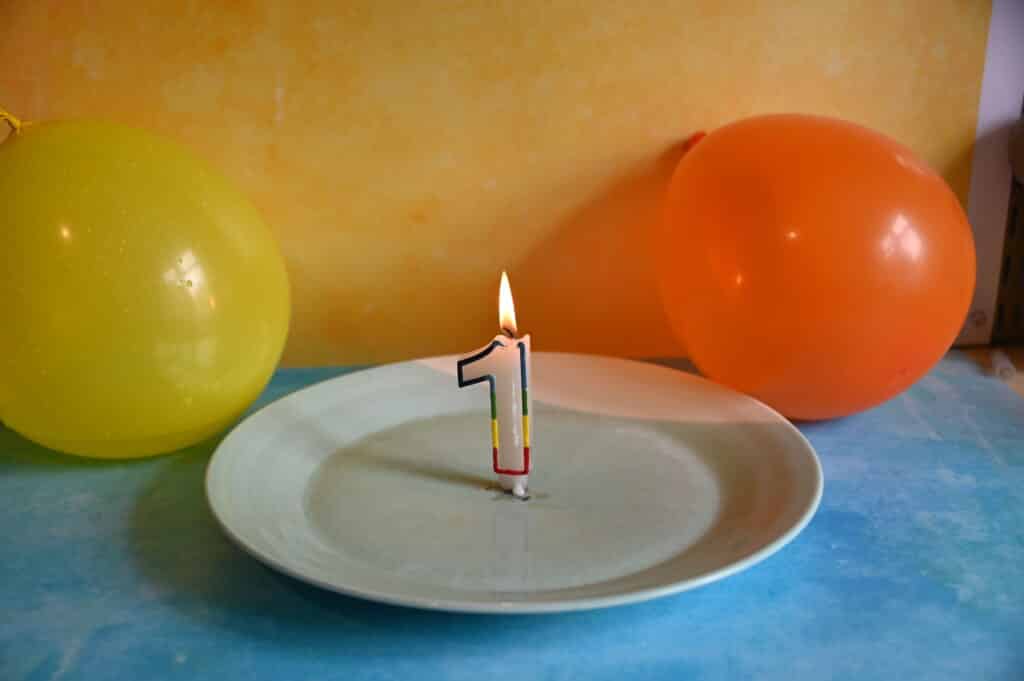 Unpoppable Balloon InstructionsPut safety goggles on. Light the candle and place it safely on a plate. A tea light candle works well. Blow up the first balloon and slowly lower it over the lit candle. The balloon will pop. 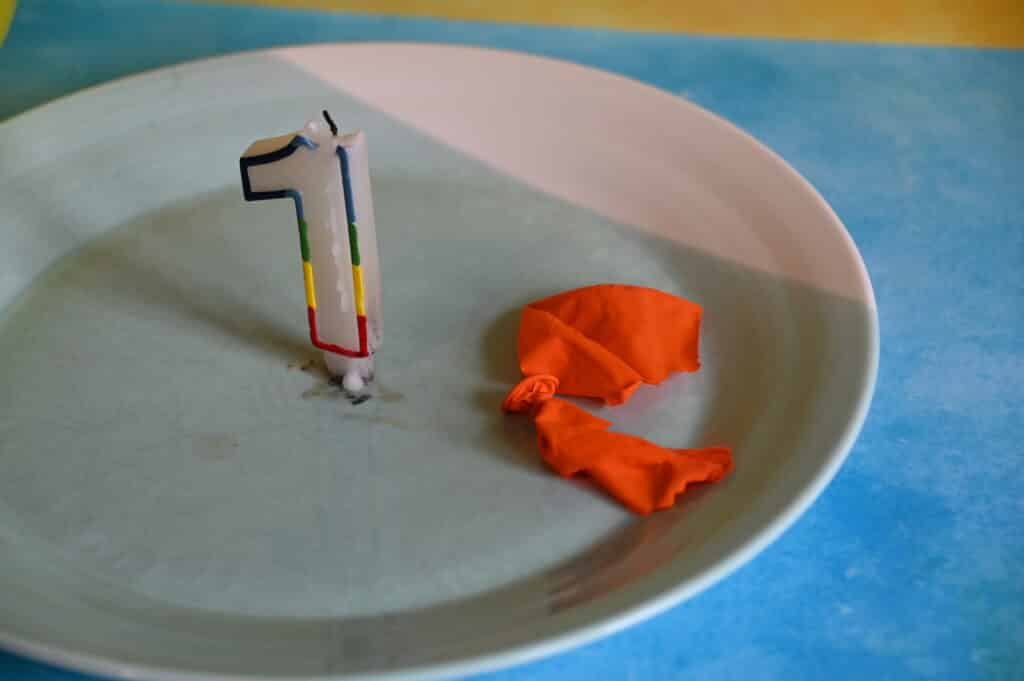 Half blow up the second balloon and add about 200ml of cold water. Blow it up to the same size as the first one. Lower the balloon over the candle again. The balloon should be able to touch the flame and not pop.  Why does the balloon containing water not pop?It’s all about heat transfer . When the first balloon moved near the candle, it popped because the heat from the candle weakened the balloon skin, which then burst. The balloon containing water didn’t pop, as the water absorbed the heat energy from the candle, not the balloon skin. Thanks to the water, the balloon didn’t get hot enough to burn. Water is much better at absorbing heat than air. The black substance on the bottom of the balloon containing water is carbon from the candle flame. Make it an investigation.To make this activity an investigation, think of a variable to test and a question to answer. Some ideas are:
More science experiments using a balloonFind out how to burst a balloon with an orange . Learn about burps with burping balloons . Design and build a balloon powered car . Make a balloon rocket with Science Bob! Discover how a hot air balloon works .  Last Updated on April 18, 2024 by Emma Vanstone Safety NoticeScience Sparks ( Wild Sparks Enterprises Ltd ) are not liable for the actions of activity of any person who uses the information in this resource or in any of the suggested further resources. Science Sparks assume no liability with regard to injuries or damage to property that may occur as a result of using the information and carrying out the practical activities contained in this resource or in any of the suggested further resources. These activities are designed to be carried out by children working with a parent, guardian or other appropriate adult. The adult involved is fully responsible for ensuring that the activities are carried out safely. Reader InteractionsLeave a reply cancel reply. Your email address will not be published. Required fields are marked *
Have 10% off on us on your first purchase - Use code NOW10 Free shipping for orders over $100 Available for dispatch within 2 days Free gift with purchase of over $100 Check out with Paypal and Afterpay Balloon survives the flameFollow FizzicsEd 150 Science Experiments: You will need:

 Light the bottom of the candle and allow the wax to melt. Then stick the candle onto the plate.  Fill a balloon with water and tie its end. Make sure that you keep the balloon fairly small to avoid stretching the rubber too much.  Light the candle and place the balloon over the flame so that the flame touches the balloon.  Slowly count to ten and then remove the balloon from the flame. It should not have popped.  Try the same experiment with a balloon filled with air. It should pop straight away. Or try holding the water balloon over the flame for a longer period of time. Will it eventually pop?  School science visits since 2004!– Curriculum-linked & award-winning incursions. – Over 40 primary & high school programs to choose from. – Designed by experienced educators. – Over 2 million students reached. – Face to face incursions & online programs available. – Early learning centre visits too! Online courses for teachers & parents– Help students learn how science really works  Get the Unit of Work on Heat Energy here!
Includes cross-curricular teaching ideas, student quizzes, a sample marking rubric, scope & sequences & more Why Does This Happen?You’ll find that you can run this for quite a while longer than the air-filled balloon purely because the water will continue to absorb the heat from the flame. So, how long could you run this? Well, it really depends on how much water you have in the balloon as well as the quality of the rubber balloon itself. Try varying the levels of the water in the balloon and see how long your experiment can last! BTW; whilst the water balloon survives the flame, the water inside the balloon will start to cycle around and around due to convection. Convection is the movement of either gases or liquids due to uneven heating causing density changes that drive movement. How does this convection work in the balloon then? – Warmer water rises due to the water expanding and becoming less dense than the surrounding colder water. – As the warmer water rises, the surrounding colder water moves underneath to replace the warm water. – Once the warmer water reaches the top of the balloon it is away from the flame… so it contracts as it cools down. This contraction makes this water denser and so this water moves down again. – In the meantime… the water that had moved near the candle flame also heats up and expands & rises… and the process keeps repeating as a continual circular motion of water within the balloon! Variables to testMore on variables here
 Learn more!From liquid nitrogen shows to hot & cold workshops , we’ve got your unit on properties of materials covered! Get in touch with FizzicsEd to find out how we can work with your class. Hot & Cold WorkshopYears 1 to 6 Maximum 30 students School workshop (NSW & VIC) 60 or 90 minutes Online Class Available STEM Full Day Accelerator - PrimaryDesigned from real classroom experiences, this modular day helps you create consistently effective science learning that directly address the new curriculum with easily accessible and cost-effective materials.  Be Amazing! How to teach science, the way primary kids love.Love science subscribe. Receive more lesson plans and fun science ideas. SCIENCE PARTIESCalendar of events.  HIGH SCHOOL Science@Home 4-Week Membership 12PM: March 2024Price: $50 - $900  PRIMARY Science@Home 4-Week Membership 2PM: March 2024 Light and Colour Online Workshop, Jan 18 PMLight and colour online workshop, jan 18 am.  Lego Robotics, Sydney Olympic Park Jan 2024 Creative Coding, Sydney Olympic Park Jan 2024 Creative Coding, Sydney Olympic Park July 11 2023Price: $100  Fizzics Education STEAM Day: Robots vs Dinosaurs, Lalor, Apr 14Price: $45 - $50 Creative Coding, Sydney Olympic Park April 14 2023Science@home after school 4-week membership: march 2023. Price: $40 - $1200 Leave a Reply Cancel replyYour email address will not be published. Required fields are marked * School Comments View AllFizzics Education curated a thoughtful and hands-on experience for the children, incorporating practical, skill-based learning activities and followed by a science presentation at the end of the event involving liquid nitrogen. This was delivered safely and effectively, capturing both the children and the parents for the duration of the presentation. Fizzics Education ran a show today at our school and it was wonderful. He was a great facilitator and the show was age appropriate and well done. I just wanted to pass on how much the staff and students really enjoyed it and how perfect it was to launch our science week activities. The students were enthralled, educated and entertained – a perfect trifecta! Thanks so much for presenting at our school on Monday. Our students enjoyed the show. Fizzics Education Awards
Free Chemistry Book! Sign-up to our newsletter and receive a free book! Female Accountant Apply HerePhysics teacher apply here, science teacher apply here, view all vacancies, join our team apply here. Send us an Email at [email protected] Phone Number: 1300 856 828Email: [email protected], address: unit 10/55 fourth ave blacktown, nsw 2148, australia.
Copyright 2024 Fizzics Education . All rights reserved. This website uses cookies to improve user experience. By using our website you consent to all cookies in accordance with our Cookie Policy . Get more science with our newsletter!Thank you for looking to subscribing to our newsletter 🙂 Through this service you’ll be first to know about the newest free experiments, science news and special offers. PLUS: Get a free Kitchen Chemistry Booklet with >20 experiments, how to use variables plus a handy template! Click the image to preview Please select an ebook!  Kids Edition  Parent Edition  Teacher Edition Please fill out the details below and an email will be sent to you. Once you get that just click on the link to confirm your subscription and you're all done! First Name * Last Name * Email Address * Phone Number Subscribe as a Teacher? Preschool Teacher Primary Teacher High School Teacher Vacation Care or Library Subscribe as a Parent? Enquiry FormExtra things, products that might interest you.  Rainbow Fireworks Glasses Magic Crystal Tree Science Kit Helicopter Spiral Top Fly Back Glider
Where are you located?
Location not listed?Which grade level are you teaching.
Which broad syllabus outcome you want to teach?What is the age range of the attendee?
General Enquiry FormCheck if you require a live online class. Subscribe for special offers & receive free resources? How did you hear about us?Choose a program * Choose from school show * * Please select a value! * Please add a value! Date required * Time required *
Noon Edition
 Give Now »  Choose which station to support! 
Indiana Public Media | WFIU - NPR | WTIU - PBS The Fireproof BalloonBy -->don glass -->, posted february 2, 2004.
Want to try an experiment involving water, fire, and a balloon? First you'll need to blow up a balloon. Next, light a candle. What do you think will happen when you put the flame under the balloon? It'll pop, of course. The flame will heat the rubber to the point where it becomes weak and can't hold up under the pressure from the air inside. But what happens when you add water to the mix? Put about a quarter cup of water in the balloon and blow it up. Then light another candle. When you place the flame under the balloon, the balloon won't pop! As long as the flame is beneath the water in the balloon, the water absorbs most of the heat and the rubber doesn't get very hot. Eventually the water will get hot enough to break the rubber. Water has a really large specific heat, meaning that it takes a lot of heat to make water boil, and even more heat to make it vaporize. In fact, it takes about 540 calories of heat to evaporate one gram of water. However, if you move the flame to another part of the balloon it will pop.  About A Moment of ScienceA Moment of Science is a daily audio podcast, public radio program and video series providing the scientific story behind some of life's most perplexing mysteries. Learn More » Science Fun Flameproof Balloon Easy Science ExperimentIn this fun and easy science experiment we are going to use science to create a flameproof balloon. Important: An adult’s assistance is required for this experiment as flames and matches are involved.
Instructions:
EXPLORE AWESOME SCIENCE EXPERIMENT VIDEOS! How it Works:The water absorbs the heat from the flame which momentarily allows the balloon to appear flameproof. The water will eventually get warm enough that is can no longer successfully absorb enough heat which will result in the balloon popping. Make This A Science Project:Try adding salt to the water. Try adding different amounts of water to the balloon. Try adding water beads to the balloon (Be sure to perform your experiment over a tray in case the balloon pops to keep the water beads from going everywhere!) EXPLORE TONS OF FUN AND EASY SCIENCE EXPERIMENTS! SUBSCRIBE AND NEVER MISS A NEW SCIENCE FUN VIDEO! previous experimentNext experiment. Fireproof BalloonsActivity length, chemical reactions states of matter, activity type, discrepant event (investigatable). In this activity, students can defy logic by putting flame to a balloon without popping it, thanks to the ability of water to conduct heat. Water has a high heat capacity . In other words, it takes a lot of heat and energy to change the temperature of water by 1 o C. The high heat capacity of water is due to the fact that it takes a lot of energy to separate water molecules (the physical bonds are very strong). Water has a heat capacity about four times that of air. This means that it takes about four times as much heat to raise the temperature of a balloon full of water than it would a similar sized balloon filled with air. As the water-filled balloon is put on the flame, the heat of the flame is easily absorbed through the balloon and into the water. The water directly above the hot spot rises, cools, and sinks again, carrying away the heat from the hot spot (this cycle is called a convection current ). In other words, the thin rubber surface that is being heated is cooled by the comparatively large volume of water above it. This cooling process continues until either all of the water in the balloon becomes too hot, or until a far more concentrated source of heat, such as a blowtorch, is applied to one small area on the balloon, When an air-filled balloon is placed in a flame, it bursts. Air is a relatively poor conductor of heat away from the thin layer of rubber. As a result, the rubber overheats and the physical bonds holding the rubber polymers together are broken. Describe the relationship between water’s heat capacity and thermal heating or cooling. Compare the thermal conductivity of air and water. Per Demo: a pair of safety glasses 2 round balloons matches or lighter a candle with candleholder 60 ml of water Key Questions
Other ResourcesScience World |Youtube| Demonstrate the Transfer of Heat Energy (using Hot & Cold Water) About the sticker Artist: Jeff Kulak Jeff is a senior graphic designer at Science World. His illustration work has been published in the Walrus, The National Post, Reader’s Digest and Chickadee Magazine. He loves to make music, ride bikes, and spend time in the forest. Comet Crisp T-Rex and Baby Artist: Michelle Yong Michelle is a designer with a focus on creating joyful digital experiences! She enjoys exploring the potential forms that an idea can express itself in and helping then take shape. Buddy the T-Rex Science Buddies Artist: Ty Dale From Canada, Ty was born in Vancouver, British Columbia in 1993. From his chaotic workspace he draws in several different illustrative styles with thick outlines, bold colours and quirky-child like drawings. Ty distils the world around him into its basic geometry, prompting us to look at the mundane in a different way. Western Dinosaur Time-Travel T-Rex Related ResourcesIn the activities that follow, students explore balloon properties and their use in demonstrating various scientific concepts. list of activities…, in these activities students explore the impressive force of air and learn how air pressure affects their daily lives., wonderful water, what are the physical and chemical properties of water that make it so unique and necessary for living things…, related school offerings.  Science SurprisesWe believe that now, more than ever, the world needs people who care about science. help us fund the future and next generation of problem solvers, wonder seekers, world changers and nerds.. Playing With Rain Explore the World Around You in Instagram Feed · Kids Science Experiments · Weather Science How to Fireproof a BalloonShare with your friends! This fun and simple science experiment only requires a few household materials along with some adult supervision to teach kids about the high heat capacity of water. Here is How to Fireproof a Balloon. Get more fun and simple Weather experiments for kids here! PIN THIS EXPERIMENT FOR LATER  This experiment is super fun, and should only take 10 minutes or less to gather up the supplies around your house and enjoy some eye-popping (and balloon-popping) excitement! Table of Contents This post may contain affiliate links. As an Amazon Associate, I earn from qualifying purchases. Supplies Needed:

Step 1: Blow up the first balloonUse your mouth, or you can use an inflation pump if you choose to fill one of your balloons with air. It doesn’t really matter how big or small the size of the balloon as long as you are consistent with the size for both balloons. One important thing to consider though is that the larger you blow up your balloons, the larger the explosion will be when the balloon pops! Once you have blown your balloon up to your desired size, be sure to tie if off so that it holds the air. Step 2: Light your lighterThis is where the adult supervision becomes very important as we will be playing with fire. If you are using a lighter, then you will need to hold the lighter steady with one hand under the balloon, while holding the balloon above the lighter with the other hand. You can also use a candle for your flame source. The benefit of using a candle is that it will free up your other hand from holding the lighter while giving you two hands to hold the balloon. Check out this Balloon and Candle Experiment to see this experiment done with a candle instead of a lighter. Step 3: Don’t forget your safety glasses!This is probably the most important step of the experiment because safety is always important! Now that we have a balloon aired up and a flame going, you will need to put your safety glasses/ safety goggles on. This will protect your eyes from any flying balloon pieces when the balloon explodes! I might even recommend putting your eye protection on at the very beginning of the experiment and not waiting until step 3. This will help you not forget about your safety once you are in the middle of all the fun! Step 4: See how close you can get your balloon to the flame before it popsNow that your balloon is aired up, your flame is burning, and your goggles are on, you are ready to see what happens when you heat your balloon up with the flame. Whether you are using a candle, or a long stem lighter, hold your balloon from the top and lower the bottom of the balloon closer to the flame below it.  The thin latex on the balloon will start to heat up and weaken due to the flame below it. Eventually the pressure inside the balloon will become high enough to burst the balloon before the flame even touches the bottom. No matter how prepared you are for it, this is the part that will always surprise you since there is no way to know exactly when the balloon will bust in your face (Another reason to wear eye protection)! Step 5: Fill the second balloon with water and air and try againNow that we know the balloon with just air will quickly pop when heated up from the flame, let’s see what happens when we add a little water inside the balloon. Before inflating your second balloon with air, simply pour a little bit of cool, or room temperature water into the deflated balloon. Using a measuring cup with a pouring spout, or small funnel will help with this step.  Once you have filled the balloon with the small amount of water that it will hold, go ahead and inflate it with air to about the same size as your first balloon and tie it off. Step 6: Lower the water-filled balloon towards the flameNow that we have a balloon filled with mostly air, and a little puddle of water in the bottom, go ahead and repeat step 4 with this new balloon and see what happens! Depending on how brave you are, you can actually lower the bottom of the balloon to touch the flame and it still should not pop! The water inside your balloon has made your balloon fireproof…for a little while anyway.  I tried this several times and depending on much much water is in your balloon and how large your balloon is will determine how long your balloon can resist popping against the flame. Step 7: Remove the balloon from the flame and examine the bottom of the balloonYour balloon might not have popped while holding it over the flame, but it will look a little bit different where the flame was in contact with the bottom of the balloon. You will notice some black soot on the bottom that appears to have burnt the balloon. Believe it or not the balloon is perfectly fine and not burnt or damaged. The black soot on the balloon is a result of the carbon deposited from the flame onto the balloon. You can actually take a wet paper towel and wipe most of it off your balloon and it will look perfectly normal again! Pretty cool huh!? Not lets dive into the science behind how the high heat capacity of water is what really keeps your balloon from popping over the flame! Why Does a Balloon Filled With Water Not Pop?Water has a much higher heat capacity than other materials such as rocks and dirt. This high heat capacity of the water in the bottom of the balloon quickly absorbs the heat from the flame and keeps the latex cooler. The water that is heated by the flame then rises to the top of the puddle and is replaced to cooler water that is then heated up and rises. This cycle continues to keep the balloon from popping until all of the water is heated. 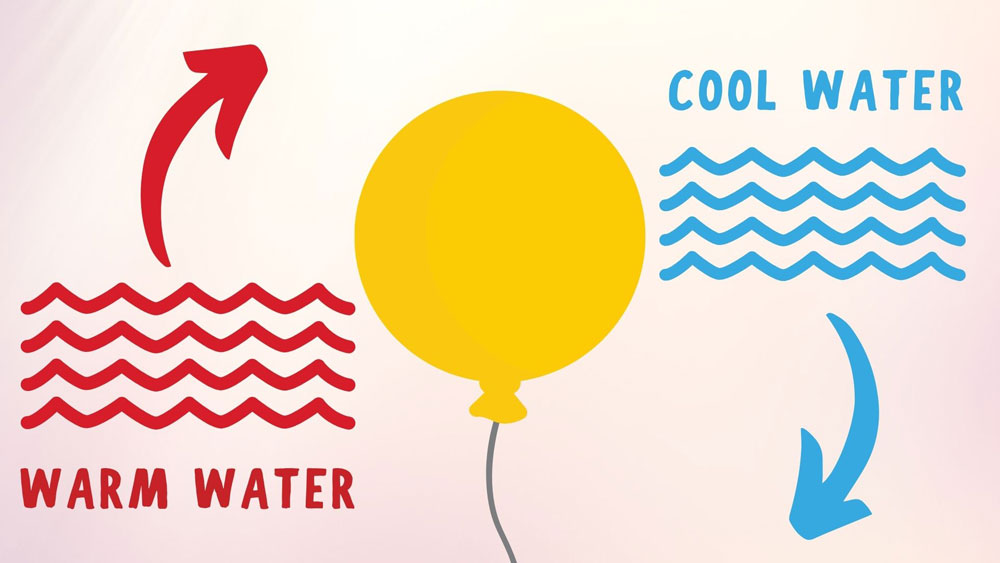 Try some variables:You can try different variables like different sizes of balloons, how much water is in your balloon, and how long you hold it over the flame to see if that changes how long your balloon remains fireproof. How Does the High Heat Capacity of Water Influence the Weather?Thanks to water’s incredible ability to absorb heat and energy, areas near large lakes and oceans are typically more temperate than areas surrounded by land. 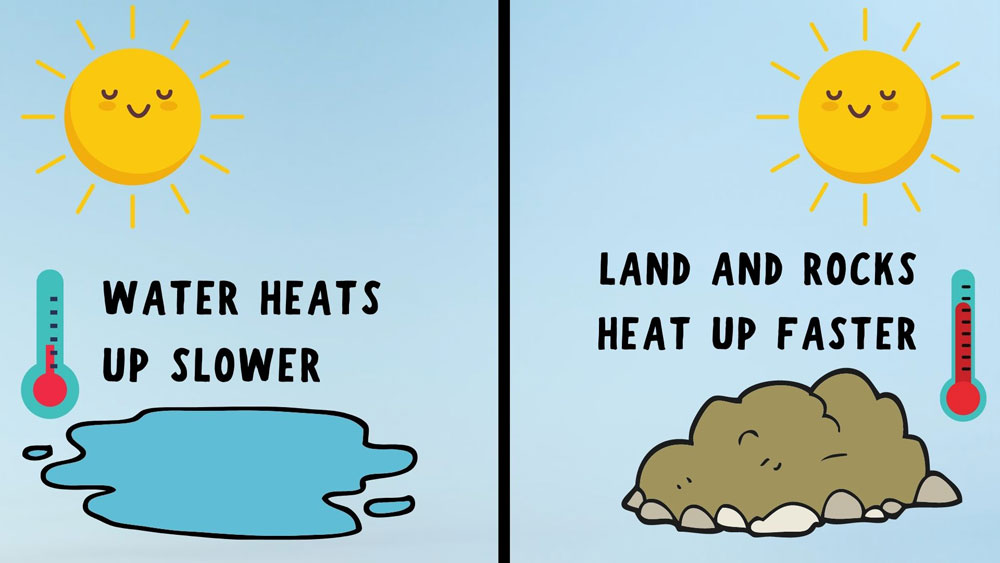 For example, a place like Honolulu, Hawaii that is surrounded by the ocean often times only sees daily temperature fluctuations between high and low temperatures of about 10°F. Meanwhile, areas like Phoenix, Arizona that are landlocked and away from large bodies of water can see vary large daily temperature fluctuations between the high and low temperatures of over 30°F.  More Easy Science Experiments for Kids:
Leave a Reply Cancel replyYour email address will not be published. Required fields are marked * Save my name, email, and website in this browser for the next time I comment. MORE ABOUT PLAYING WITH RAINLET’S CONNECTSearch for more ideas, create at your own risk. All content on this blog was created for inspiration and entertainment purposes. Creating anything with the suggested tools, products or methods, is under your own risk!
Balloon Thermal Experiment
Contributed by Yutao Zhou Introduction
1. First we light a candle 2. Then we find a balloon and fill it up with water. 3. After we fill it up with water and put the balloon directly above the fire, we will see that the balloon would get toasted but it would not pop. 4. We remove the water in the balloon and blow it up to the same size with air. 5. Place the balloon above the fire again, it will pop within a second. Physics Concepts and Questions Why does the very same balloon pop when there isn’t water in it? This is because the water inside the balloon absorbed the heat from the fire. Conclusions and Further Investigations
Recent Posts
Recent Comments
© 2024 UCSB Physics Circus. Built using WordPress and the Highlight Theme
The Invincible Balloon Experiment When a balloon goes up against a candle there seems like there can only be one winner! Think again, here's how to make your balloon invincible and learn a thing or two about conductivity too!  What Do I Need?
 How Do I Do It?STEP1 - Make sure you've got an adult to help out. The first step is to light the candle. If you want to prove what you know will happen you can try lowering your balloon over the flame. WARNING: Your balloon will pretty quickly go pop! STEP2 - Let's make the balloon invincible. Fill your balloon up with water, then inflate it. This means there will be a small puddle of water in the bottom of the balloon. STEP3 - Slowly lower your balloon down on top of the flame of the candle. Hold it there, does it go pop? You can lower it right down and actually put the candle out with the balloon. Finally, make sure to have a look at the bottom of the balloon.  What’s Going On?Well, why does the balloon go pop when there is no water inside of it? It's really just the heat from the candle melting the outside of the balloon till it's so weak that it can't contain the pressure of the air that's inside of it! How does the water make a difference? That puddle of water inside of the balloon pulls the heat away from the surface of the balloon. This is called conduction. So, instead of the balloon skin getting so hot it melts, the heat is spread (or 'dissipated') into the water that's inside the balloon. That water slowly starts to get warmer but the balloon doesn't pop.  More Fun Please! - Experiment Like A Real Scientist!
Want A FREE Science Experiment Book?If you like fun science experiments then you'll love this FREE Science experiment book.... It's packed full of awesome experiments you can do at home with "stuff" you've already got. Your FREE Science experiment book is:
Click Here to download your FREE copy now!  Kids Party Coming Up?If you like fun science experiments then you’ll love the Dragons’ Den winning Sublime Science Party. Check price & availability in your area and grab your FREE Kids Party Survival Guide right now!  Home - About - Privacy Policy - Terms & Conditions - Facebook - Instagram - LinkedIn - Twitter - Sitemap - Blog - Contact Copyright 2008-2023 - Sublime Science - All Rights Reserved - Registered in England and Wales, Company Number 6680269. Registered Address: The Sublime Science Lab (Unit4) Fernleigh Business Park, Blaby Rd, Enderby, Leicester LE19 4AQ - Call: 0116 380 0750 You Just Couldn't Resist!There's some serious science behind why you just clicked! If you'd like more awesome science experiments then enter your email address below and grab your FREE science experiment book! You'll get your FREE copy of 'Don't Eat Your Slime', weekly 'Top Secret Science Experiment Printables' and occasional awesome offers. Happy experimenting! (enter your email address below to get your FREE science experiment book) YES, I Want My FREE Experiment Book!( You'll get your FREE copy of 'Don't Eat Your Slime', weekly 'Top Secret Science Experiment Printables' and occasional awesome offers. Happy experimenting!
Get 50% off your first box of Home Chef! 🥙 20 Balloon Experiments to Make Your Lessons Really PopSee what we did there?  There’s something about the sight of colorful balloons that just makes you feel a little excited, don’t you think? That’s why kids will go crazy for these balloon experiments, whether they’re building a balloon-powered boat or powering a light bulb with static electricity. Plus, balloons are inexpensive, so stock up at the dollar store and get ready to throw a science party! 1. Blow up a balloon … without blowingThis is one of those classic balloon experiments everyone remembers doing in school. Kids learn about chemical reactions by mixing acids and bases. They’re always amazed at the results! Learn more: Balloon Baking Soda Experiment  2. Design a balloon-powered carExplore the laws of motion and encourage creativity when you challenge students to design, build, and test their own balloon-powered cars. Bonus: Use only recycled materials to make this project green! ( Find more cool car activities for the classroom here. ) Learn more: Balloon-Powered Car Challenge  3. Skewer a balloon without popping itIf you do this one right, you’ll make kids’ eyes pop—but not the balloon! They’ll learn about the polymers that make balloons possible, and even a little bit about how to stay cool under pressure. Learn more: Balloon Skewer  4. Float a balloon-powered boatDiscover the power of air pressure and the third law of motion with this fun and inexpensive balloon experiment. Take this one outside on a sunny day and let kids splash away while they learn! Learn more: Balloon-Powered Sponge Boat  5. Create ice crystal explosionsFill balloons with water and leave them to freeze overnight. The next day, carefully cut open the balloons to reveal the beauty inside. Kids learn about crystallization and the expansion of water as it freezes. ( Get more science experiments involving ice and snow here. ) Learn more: Super Cool Melting Ice Experiment  6. Explore the science of swim bladdersJust how do fish manage to float without sinking or rising? Find out when you explore buoyancy with this swim bladder experiment using a glass bottle, balloon, and a few other basic materials. Learn more: How Fish Sink and Float  7. Assemble a heart pump modelAnatomy lessons literally come alive when you do balloon experiments like this one. This working heart model demonstrates how blood pumps through the valves and chambers. Learn more: DIY Heart Pump  8. Learn how lungs workYour students might be surprised to learn that lungs have no muscles to make them work. Instead, the contraction of the diaphragm pulls air in and forces it out. This clever model helps explain the process. Learn more: Lung Science Experiment  9. Blast off with a two-stage rocketThe rockets used for space flight generally have more than one stage to give them the extra boost they need. This experiment uses balloons to model a two-stage rocket launch, teaching kids about the laws of motion. Learn more: Two-Stage Balloon Rocket  10. Build a hovercraftIt’s not exactly the same model the military uses, but this simple hovercraft is a lot easier to build. An old CD and a balloon help demonstrate air pressure and friction in this simple experiment. Learn more: DIY Hovercraft  11. Parachute a water balloonWater balloon experiments make a big splash with kids! In this one, they’ll explore how air resistance slows a water balloon’s landing using a homemade parachute. Learn more: Water Balloon Skydiving  12. Sink or swim with water balloonsFill water balloons with a variety of different liquids like oil, salt water, and corn syrup, then float them in a bucket of water to learn about density and buoyancy. Learn more: Water Balloon Experiment  13. Perform the two balloons experimentYou have two balloons, one filled with more air than the other. When you open the valve between them, what will happen? The answer is almost certain to surprise you. Learn how it works in the video at the link below. Learn more: Air Pressure Experiment  14. Power a light bulb with static electricityOne of the first balloon experiments most kids try is rubbing a balloon on their hair to make their hair stand on end. The next step is to hold the balloon over a compact fluorescent light bulb (CFL) to see it glow from the static electricity. Wow! Learn more: Magic Light Bulb Balloon Science Experiment  15. Spin a penny round and roundIn this simple experiment, students use kinetic energy and centripetal force to spin a penny inside a balloon. They’ll want to try other objects too, so hold a contest to see which spins the longest. Learn more: The Spinning Penny  16. Fire up an air cannonDiscover the power of an air vortex with this easy DIY air cannon. To really understand how it works, use some incense to create visible smoke rings that will really impress your students. Learn more: Air Cannon Smoke Ring  17. Create a working water fountainSee the power of air pressure when you build a balloon-activated water fountain. You’ll only need simple supplies like a plastic bottle, straw, and putty. Learn more: Water Bottle Fountain  18. Explore the effects of hot and cold airThe concept of expansion and contraction of air can be hard to visualize. That’s where this experiment comes in to save the day. Watch the balloon expand and contract as the air around it changes temperature. Learn more: Exploring the Effects of Hot and Cold Air  19. Fireproof a balloonA balloon will obviously pop when touched to a hot flame, right? Not if you put some cold water in it first! Kids will be so amazed they won’t even realize they’re learning about the heat conductivity of water. Learn more: Fireproof balloon  20. Experiment with balloons and pushpinsA pin pops a balloon in no time flat, so what happens when you place a balloon on a table full of them? Once again, the answer won’t be quite what your students expect until you explain the science of distributed pressure. Learn more: Pinning a Balloon Have more balloon experiments to add to the list? Come and share in our We Are Teachers HELPLINE group on Facebook.Plus, check out our big list of easy science experiments .  You Might Also Like 40 Easy Kindergarten Science Experiments for Hands-On LearningEvery day brings a new discovery! Continue Reading Copyright © 2024. All rights reserved. 5335 Gate Parkway, Jacksonville, FL 32256
How to Make a Fireproof BalloonMost of us would have encountered a scenario, where the balloon we had tried to fill up gets exploded it comes in contact with a candle on a certain occasion. Fortunately, there is a way in basic physics where we would be able to prevent that from happening. To know how to do it, let us look at the fireproof balloon experiment given below.  Fireproof Balloon ExperimentWe know that balloons are made of rubber and are fragile. The balloon, when it comes in contact with fire, burns. A fire can weaken the rubber and cause it to burst. Water is a substance with high heat capacity. It acts against fire and helps from catching fire. Let us now have a look at the fireproof balloon experiment.
From the above observation, you might have seen that it is quite difficult to prevent the balloon from exploding unless you fill it with water.
Conclusion :
NOTE: Perform the fireproof balloon experiment with proper guidance and safety measures. Fireproof Balloon Experiment Application
Through this fireproof balloon experiment, you would see how the subject of science is so fascinating as it has a lot of unexplored areas yet to be discovered. Hence, projects like these would provide the impetus for you to probe more into the domain of science. Stay tuned with BYJU’S to learn more. Have a look at various interesting science experiments:
Frequently Asked Questions – FAQsWhen the balloon is in contact with the flame, the black colour deposition on the balloon is seen. what is it known as, state true or false: water is a good absorber of heat., what happens when the air-filled balloon is placed over a flame, state true or false: the rubber of the balloon without water becomes hotter than the rubber of the balloon with water., what happens when a water-filled balloon is placed overa a flame.  Put your understanding of this concept to test by answering a few MCQs. Click Start Quiz to begin! Select the correct answer and click on the "Finish" button Check your score and explanations at the end of the quiz Visit BYJU'S for all Physics related queries and study materials Your result is as below Request OTP on Voice Call
Leave a Comment Cancel replyYour Mobile number and Email id will not be published. Required fields are marked * Post My Comment Register with BYJU'S & Download Free PDFsRegister with byju's & watch live videos.
| |||||||||||||||||||||||||||||
Getting the facts right
| Besides pedagogy or psychology, it can be also relevant just to get the facts right. The Water-Candle experiment is an illustrative example. It is a situation where many different effects play together and where it is hard to figure out, which ones really matter. My own perspective about this experiment has shifted several times and comments of some who wrote me added valuable insight. Please look also towards the end of this page, where some interesting links are added and information like why the great Lavoisier himself replaced this experiment as it appeared to be too subtle. |
| Cover a burning candle with a pitcher so that the candle is in an air-tight room sealed by the water at the ground. | After some time, the candle dims and goes out. Just before the candle dies, the water level rises to almost 1/10 th of pitcher height. No air bubbles are seen. The water level stays up for many few minutes more. |
| : oxygen O and C H react. The burning produces water H O and carbon dioxide C O . For n=1, we balance the equation as follows: + C H = C O + 2 H O |
| There are two different effects. Both a chemical and a physical reasoning are needed to explain what we can see. Both and matter. The initial cancellation effect can confuse the observer. plays a role when the chemical equations are balanced. |
Photos of the experiment
An exhibit of explanations
What do we learn, appendix: the chemical equation for general n.
| O + x C H = y C O + z H O |
| (1+3 n) O + 2 C H = 2 n C O + (2+2n) H O |
Appendix: the ideal gas equation
| p V = N k T |
Added March 20, 2011
Added September 26, 2011
| What is happening in this experiment? When we ignite the candle, the hydrocarbon reacts with oxygen (in excess) to produce carbon dioxide and water. The burning sets an air current which gives dome shape to candle flame and it helps to get complete combustion at the bottom and the outer surface of the flame. The hot air and products of combustion rise up above the flame. As soon as the gas jar comes over the flame, the hot gases moving upward enter the jar and air inside the jar expands pushing some of the air out of the jar. This process goes unnoticed. As soon as the jar touches the water, the burning occurs in a closed environment. Further pressing the jar into water helps to retain the air in jar which is less in quantity than at room temperature and pressure. However, due to thermal expansion, the pressure is higher than atmospheric pressure which is balanced by pressure from the water. The burning of hydrocarbon in the jar produces about 30% more molecules of carbon dioxide and water than the molecules of oxygen consumed in the reaction (see below the title expected chemical reaction). The increased heat and number of molecules increases the pressure in side as a result if not careful some bubbles of gas will escape from the jar. Over the time the oxygen in the jar is reduced and conditions for burning are changed. Burning under reduced oxygen may not produce carbon dioxide rather carbon monoxide (very little). When the candle is put out, the temperature decreases followed by also a decrease in pressure due to condensation of water vapour and decreased quantity of air due to thermal expansion during the process of placing the jar on the candle. The overall situation is a decrease in pressure inside the jar as compared to atmospheric pressure. Therefore, despite water is heavier that air, it is pulled into the jar. How much water rises as a result of dissolving of carbon dioxide? Very little practically negligible during 30 - 40 minutes, the time the experiment usually takes for performing in a classroom situation. If the number of candles is increased in the jar, the heat produced is more therefore more air is likely to escape from the jar due to thermal expansion during the process of pacing the jar over them. Therefore, more water will rise in the jar with more candles. The nature and quantity of the products will depend upon the composition of candle material. However, it is assumed that combustion of saturated hydrocarbons is taking place during burning. C H (s) + (1.5n+0.5) O (g) = n CO (g) + (n+1) H O(g) For n=1, two moles of oxygen reacts with a mole of CH to produce three moles of product molecules. Assuming that supply of methane was controlled and it is stopped as soon as the flame is put out, otherwise there will be an explosion. The number of moles of the product molecules is 1.50 times that of oxygen. As n increases, the multiple factor decreases from 1.50 and approaches 1.0 at n = ? For n=30 (a typical paraffin wax), the factor will be 1.34. The overall understanding of the experiment is that all the oxygen is not used up (I have rested the presence of oxygen after the candle is put out in our laboratory using yellow phosphorus) and the consumption of oxygen does not create empty space rather the number of product molecules in the jar increases over that of the consumed oxygen. Thus giving rise to an increase in overall pressure in the jar (see above equation). Moreover, almost equal number of molecules of CO and H2 are produced. A quick rise of water in the jar after the candle is extinguished is mainly due to a decrease in pressure as a result of a decrease in amount of air in the jar due to thermal expansion during the process of placing the jar on the candles, bubbles escaping (if any) through the water and may be the condensation of the water vapour. The amount of condensation of water will depend upon the temperature difference between initial and final temperature of the air in the jar. Since air is above water, therefore saturated water vapour pressure is considered in the beginning of the experiment. Increase in temperature, during the candle burning, will make air unsaturated to accommodate additional water vapours especially produced as a product of burning. A decrease in temperature over time after the candle is off to the initial temperature will help water vapour to condense. This condensation will decrease the pressure inside the jar and will help water rise in the jar. The amount of water vapours condensed during a small change of temperature as usually occurs in this experiment may even be small to notice. The amount of CO dissolved in water is minimal in the 30-40 minutes during which experiment is conducted. |
Added November 20, 2011
| : theoretically, if you assume that the candles will burn up all the oxygen in the container, and assume the room is completely air tight and assume that both water and air incompressible, it does not matter. You will have the same water level at the end in both setups after the candles have burned out and the situation cooled down. In real experiments, there are differences but they depend on the actual experiment: |
Added January 23, 2012
| Simo Tolvanan from Helsinki kindly informed me about the . explains things very well and also contains much history and references. This paper makes the story again interesting. It points to the fascinating story of Lavoisier, who first realized that the total does not change during this process and who noticed that only a fraction of the oxygen reacts before the candle goes out by demonstrating that a mouse still can breath afterwards. The authors of the article provide also The classical is compatible what is seen by everybody else and which matches the . The experiments demonstrate only a one percent increase. The authors conclude that bubbling and hot air trapping are responsible for the rising water. The setup for and the experiments are very different. In the later case, the candle burning is violent and the container is very long. Heavier CO (which the ignition already produces in the first moments) can kill the candle before much of the oxygen is out. |
| January 27 2012: the bubbling effect. Here is an illustration why many teachers report bubbles. If you place the pitcher flat on then bubbles escape initially. One can avoid this by tilting the glass first. We just want initially to have the same level of water and the same pressure inside and outside. The experiment starts then. | |
| Candle experiment done carefully so that initially the water level inside is close to the water level outside. | Bubbles which escape. |
Added February 5, 2013
| had a great idea to modify the experiment. He wrote: The stoichiometry for coal is different than for paraffin. In the case of only carbon, one has and one would indeed expect that the volume would stay the same. Since the pressure decreases afterwards, this could indicate that indeed some air has gone out when the heat has expanded the inside. After cooling, the plastic wrap collapses. Peter Dureen again: I think this is more indication that some hot air has left the container before it started to cool down. I have repeated the experiments also with different type of containers and seen also some air, as other teachers have observed too. Faraday had been a fantastic experimenter and assisted as a chemist before for a long time. Lavoisier was definitely a great pioneer in this context. |
Added January 21, 2014
| sent the following interesting thoughts: . What do you think of this? This is a pretty good simplification. It defuses well the myth that the oxygen is burned away. The reason why the myth persists because the rise of water matches the amount of oxygen in the air. again: O produced in burning the candle wick was wet and hard to relight. Thus I decided that there was no way that Lavoisier could have learned much from this particular experiment. So I managed to locate . Note that he abandons the candle and water experiment as having potential flaws. He moves to mercury instead, and lights the candle after the jar is in place. What he ends up on is this: "In the middle of a glass stand, was placed a small wax candle; and on the top of the wick was fixed a small piece of Kunckel's phosphorus. The stand was then placed in a basin of mercury and covered with a jar. I made a piece of iron wire red hot then passed it through the mercury set fire to the little piece of phosphorous and by this means the candle was lighted." What he found was that the heated air initially pushed the mercury down, but when everything had cooled, there was a tiny loss in the volume of air, 1/300th the volume. But then he reacted the air with a CO absorber and the volume was reduced by 1/10. In other words he claims that the total volume was virtually unchanged, but (assuming air is 1/5 oxygen) about 1/2 the oxygen was converted into CO (with an unspecified amount turned into water. He may not have realized water was a byproduct yet). The combustion of paraffin is C H + 38 O => 25 CO + 26 H O. Depending on what fraction of the water remains as vapor, one goes from 38n moles to between 25n and 51n moles of CO +H O of vapor (with the rest in condensed H2O). Now it could be by chance that the C O+H O vapor happened to be near 38n, but that would be just chance. In your opinion, what fraction of the H O condenses? This should depend on the temperature and the humidity already present in the room. If we believe the account of Lavoisier, it could indeed be that things pretty much balances out when done as described. This makes the experiment so interesting. There are various effects which play a role: physical like temporary heating and cooling as well as condensation as well as chemical due to the reaction of paraffin with stochiometric computations which depending on the type of paraffin is used. The experiment depends on the size of the container, the surrounding temperature, air humidity present as well as on the experimenter (lightening the candle, allowing air to escape initially for example through bubbles or due to the expansion while removing the lightener). |
Added March 30, 2021
| My answer: |
Added January 28, 2024
| My answer: |

COMMENTS
The "Fireproof Balloon" is a simple and safe inquiry-based demonstration that challenges the students to explain why the instructor-filled balloon does not pop over a lit candle while student-filled balloons do. The 25 mL of water placed in the instructor's balloon prior to the demonstration will absorb sufficient heat from the candle ...
The nonflammable balloon. Hold it over a candle flame and it won't pop. This is an experiment about water's ability to absorb heat.
Light a candle and place it in the middle of the table. Put on your safety glasses because it's time to pop the balloon. Hold the balloon a foot or two over the top of the flame and slowly move the balloon closer and closer to the flame until it pops. You'll notice that the flame doesn't have to even touch the balloon and the balloon pops.
Learn how to make a fireproof balloon using this thermal conductivity experiment at home. Teach your kids about difference between thermal conductivity of different materials.
Instead of using a paper bag, this modern day version of the demonstration uses an ordinary balloon, some water and a candle. It's a combination that's guaranteed to make people stand back.
A balloon filled with air will pop when it gets too close to a flame as the heat from the candle warms the balloon's skin and weakens it. The trick to making an unpoppable balloon is to add water to it. The heat from the flame heats the water instead of the balloon, which then doesn't burst.
When the balloon is placed above the burning candle, the heat from the candle flame is absorbed by water inside the balloon and keeps the balloon from bursting out.
1. Light the bottom of the candle and allow the wax to melt. Then stick the candle onto the plate. 2. Fill a balloon with water and tie its end. Make sure that you keep the balloon fairly small to avoid stretching the rubber too much. 3. Light the candle and place the balloon over the flame so that the flame touches the balloon. 4.
Want to try an experiment involving water, fire, and a balloon? Learn about "The Fireproof Balloon" on this Moment of Science.
In this fun and easy science experiment we are going to use science to create a flameproof balloon. Important: An adult's assistance is required for this experiment as flames and matches are involved. Materials: 2 balloons Matches or lighter Cold water Tea light or small candle Instructions: Blow up one of the balloons and tie off the end. Do not blow up the other balloon at this time. Have an ...
Science Experiment for kids! REQUIREMENTS :- 2 Balloons Water Candle Kitchen Lighter WHAT TO DO :- Fill one of the balloons with air and the other with a little water in it.
Fire proof balloon. What you need: Step 1: Pour the water into your balloon so that the balloon overflows with water. Step 2: Blow the balloon up and tie it. Step 3: Light a candle or get something that gives off a small flame. Hover the balloon above the flame, you can even let the balloon touch the flame.
Add about 60 ml of water to the second balloon and then blow it up to the same size as the first balloon. As before, slowly lower the balloon over the candle flame.
This fun and simple science experiment only requires a few household materials along with some adult supervision to teach kids about the high heat capacity of water. Here is How to Fireproof a Balloon.
1. First we light a candle. 2. Then we find a balloon and fill it up with water. 3. After we fill it up with water and put the balloon directly above the fire, we will see that the balloon would get toasted but it would not pop. 4. We remove the water in the balloon and blow it up to the same size with air. 5.
Discover How to Put A Candle Out with a Balloon! Dragons Den Approved. Check it out and grab your FREE Science Experiment Book Now!
There's something about the sight of colorful balloons that just makes you feel a little excited, don't you think? That's why kids will go crazy for these balloon experiments, whether they're building a balloon-powered boat or powering a light bulb with static electricity. Plus, balloons are inexpensive, so stock up at the dollar store and get ready to throw a science party!
Get the full course at: http://www.MathTutorDVD.comIn this cool science experiment, we show how you can put a balloon directly into a candle flame and the ba...
Learn how to make a fireproof balloon and conduct a fireproof balloon experiment. Also, learn the real-world application of fireproof balloon experiments at BYJU'S - The Learning App!.
For this experiment you will need: two round balloons, not inflated. several matches. water. Inflate one of the balloons and tie it closed. Place 60 milliliters (¼ cup) of water in the other balloon, and then inflate it and tie it shut. Light a match and hold it under the first balloon. Allow the flame to touch the balloon.
Experiment: Cover a burning candle with a pitcher so that the candle is in an air-tight room sealed by the water at the ground.
In this cool science experiment, I test the difference between an air-filled balloon and a water-filled balloon when held over a candle flame. Watch as the a...
Fill one balloon with water, but not overly filled, and tie it off. Blow the other one up with breath and tie it off. The demonstration is quite simple. When the candle is brought beneath the "air only" balloon, it will almost immediately pop. When the candle is placed under the water filled balloon, the water in the balloon dissipates the ...