
- High contrast
- Press Centre

Search UNICEF
What you need to know about covid-19 vaccines, answers to the most common questions about coronavirus vaccines..

- Available in:
Vaccines save millions of lives each year. The development of safe and effective COVID-19 vaccines are a crucial step in helping us get back to doing more of the things we enjoy with the people we love.
We’ve gathered the latest expert information to answer some of the most common questions about COVID-19 vaccines. Keep checking back as we will update this article as more information becomes available.
What are the benefits of getting vaccinated?
Vaccines save millions of lives each year and a COVID-19 vaccine could save yours. The COVID-19 vaccines are safe and effective, providing strong protection against serious illness and death. WHO reports that unvaccinated people have at least 10 times higher risk of death from COVID-19 than someone who has been vaccinated.
It is important to be vaccinated as soon as it’s your turn, even if you already had COVID-19. Getting vaccinated is a safer way for you to develop immunity from COVID-19 than getting infected.
The COVID-19 vaccines are highly effective, but no vaccine provides 100 per cent protection. Some people will still get ill from COVID-19 after vaccination or pass the virus onto someone else.
Therefore, it is important to continue practicing safety precautions to protect yourself and others, including avoiding crowded spaces, physical distancing, hand washing and wearing a mask.
Who should be vaccinated first?
Each country must identify priority populations, which WHO recommends are frontline health workers (to protect health systems) and those at highest risk of death due to COVID-19, such as older adults and people with certain medical conditions. Other essential workers, such as teachers and social workers, should then be prioritized, followed by additional groups as more vaccine doses become available.
The risk of severe illness from COVID-19 is very low amongst healthy children and adolescents, so unless they are part of a group at higher risk of severe COVID-19, it is less urgent to vaccinate them than these priority groups.
Children and adolescents who are at higher risk of developing severe illness from COVID-19, such as those with underlying illnesses, should be prioritized for COVID-19 vaccines.
When shouldn’t you be vaccinated against COVID-19?
If you have any questions about whether you should receive a COVID-19 vaccine, speak to your healthcare provider. At present, people with the following health conditions should not receive a COVID-19 vaccine to avoid any possible adverse effects:
- If you have a history of severe allergic reactions to any ingredients of a COVID-19 vaccine.
- If you are currently sick or experiencing symptoms of COVID-19 (although you can get vaccinated once you have recovered and your doctor has approved).
Should I get vaccinated if I already had COVID-19?
Yes, you should get vaccinated even if you’ve previously had COVID-19. While people who recover from COVID-19 may develop natural immunity to the virus, it is still not certain how long that immunity lasts or how well it protects you against COVID-19 reinfection. Vaccines offer more reliable protection, especially against severe illness and death. Vaccination policies after COVID-19 infection vary by country. Check with your health care provider on the recommendation where you live.
Which COVID-19 vaccine is best for me?
All WHO-approved vaccines have been shown to be highly effective at protecting you against severe illness and death from COVID-19. The best vaccine to get is the one most readily available to you.
You can find a list of those approved vaccines on WHO’s site .
Remember, if your vaccination involves two doses, it’s important to receive both to have the maximum protection.
How do COVID-19 vaccines work?
Vaccines work by mimicking an infectious agent – viruses, bacteria or other microorganisms that can cause a disease. This ‘teaches’ our immune system to rapidly and effectively respond against it.
Traditionally, vaccines have done this by introducing a weakened form of an infectious agent that allows our immune system to build a memory of it. This way, our immune system can quickly recognize and fight it before it makes us ill. That’s how some of the COVID-19 vaccines have been designed.
Other COVID-19 vaccines have been developed using new approaches, which are called messenger RNA, or mRNA, vaccines. Instead of introducing antigens (a substance that causes your immune system to produce antibodies), mRNA vaccines give our body the genetic code it needs to allow our immune system to produce the antigen itself. mRNA vaccine technology has been studied for several decades. They contain no live virus and do not interfere with human DNA.
For more information on how vaccines work, please visit WHO .
Are COVID-19 vaccines safe?
Yes, COVID-19 vaccines have been safely used to vaccinate billions of people. The COVID-19 vaccines were developed as rapidly as possible, but they had to go through rigorous testing in clinical trials to prove that they meet internationally agreed benchmarks for safety and effectiveness. Only if they meet these standards can a vaccine receive validation from WHO and national regulatory agencies.
UNICEF only procures and supplies COVID-19 vaccines that meet WHO’s established safety and efficacy criteria and that have received the required regulatory approval.
How were COVID-19 vaccines developed so quickly?
Scientists were able to develop safe effective vaccines in a relatively short amount of time due to a combination of factors that allowed them to scale up research and production without compromising safety:
- Because of the global pandemic, there was a larger sample size to study and tens of thousands of volunteers stepped forward
- Advancements in technology (like mRNA vaccines) that were years in the making
- Governments and other bodies came together to remove the obstacle of funding research and development
- Manufacturing of the vaccines occurred in parallel to the clinical trials to speed up production
Though they were developed quickly, all COVID-19 vaccines approved for use by the WHO are safe and effective.
What are the side effects of COVID-19 vaccines?
Vaccines are designed to give you immunity without the dangers of getting the disease. Not everyone does, but it’s common to experience some mild-to-moderate side effects that go away within a few days on their own.
Some of the mild-to-moderate side effects you may experience after vaccination include:
- Arm soreness at the injection site
- Muscle or joint aches
You can manage any side effects with rest, staying hydrated and taking medication to manage pain and fever, if needed.
If any symptoms continue for more than a few days then contact your healthcare provider for advice. More serious side effects are extremely rare, but if you experience a more severe reaction, then contact your healthcare provider immediately.
>> Read: What you need to know before, during and after receiving a COVID-19 vaccine
How do I find out more about a particular COVID-19 vaccine?
You can find out more about COVID-19 vaccines on WHO’s website .
Can I stop taking precautions after being vaccinated?
Keep taking precautions to protect yourself, family and friends if there is still COVID-19 in your area, even after getting vaccinated. The COVID-19 vaccines are highly effective against serious illness and death, but no vaccine is 100% effective.
The vaccines offer less protection against infection from the Omicron variant, which is now the dominant variant globally, but remain highly effective in preventing hospitalization, severe disease, and death. In addition to vaccination, it remains important to continue practicing safety precautions to protect yourself and others. These precautions include avoiding crowded spaces, physical distancing, hand washing, and wearing a mask (as per local policies).
Can I still get COVID-19 after I have been vaccinated? What are ‘breakthrough cases’?
A number of vaccinated people may get infected with COVID-19, which is called a breakthrough infection. In such cases, people are much more likely to only have milder symptoms. Vaccine protection against serious illness and death remains strong.
With more infectious virus variants such as Omicron, there have been more breakthrough infections. That’s why it's recommended to continue taking precautions such as avoiding crowded spaces, wearing a mask and washing your hands regularly, even if you are vaccinated.
And remember, it’s important to receive all of the recommended doses of vaccines to have the maximum protection.
How long does protection from COVID-19 vaccines last?
According to WHO, the effectiveness of COVID-19 vaccines wanes around 4-6 months after the primary series of vaccination has been completed. Taking a booster to strengthen your protection against serious disease is recommended if it is available to you.
Do the COVID-19 vaccines protect against variants?
The WHO-approved COVID-19 vaccines continue to be highly effective at preventing severe illness and death.
However, the vaccines offer less protection against infection from Omicron, which is the dominant variant globally. That's why it's important to get vaccinated and continue measures to reduce the spread of the virus – which helps to reduce the chances for the virus to mutate – including physical distancing, mask wearing, good ventilation, regular handwashing and seeking care early if you have symptoms.
Do I need to get a booster shot?
Booster doses play an important role in protecting against severe disease, hospitalization and death.
WHO recommends that you take all COVID-19 vaccine doses recommended to you by your health authority as soon as it is your turn, including a booster dose if recommended.
Booster shots should be given first to high priority groups. Data shows that a booster shot plays a significant role in boosting waning immunity and protecting against severe disease from highly transmissible variants like Omicron.
If available, an additional second booster shot is also recommended for some groups of people, 4-6 months after the first booster. That includes older people, those who have weakened immune systems, pregnant women and healthcare workers.
Check with your local health authorities for guidance and the availability of booster shots where you live.
What do we know about the bivalent COVID-19 booster doses that have been developed to target Omicron?
Bivalent COVID-19 booster shots have now been developed with both the original strain of the coronavirus and a strain of Omicron. These have been designed to better match the Omicron subvariants that have proven to be particularly transmissible. Lab studies have shown that these doses help you to mount a higher antibody response against Omicron. Both Moderna and Pfizer have developed these bivalent vaccines, and some countries have now approved their use.
Check with your local health authorities for information about the availability of these doses and who can get them where you live. And it’s important to note that the original COVID-19 vaccines continue to work very well and provide strong protection against severe illness from Omicron.
Can I receive different types of COVID-19 vaccines?
Yes, however, policies on mixing vaccines vary by country. Some countries have used different vaccines for the primary vaccine series and the booster. Check with your local health authorities for guidance where you live and speak with your healthcare provider if you have any questions on what is best for you.
I’m pregnant. Can I get vaccinated against COVID-19?
Yes, you can get vaccinated if you are pregnant. COVID-19 during pregnancy puts you at higher risk of becoming severely ill and of giving birth prematurely.
Many people around the world have been vaccinated against COVID-19 while pregnant or breastfeeding. No safety concerns have been identified for them or their babies. Getting vaccinated while pregnant helps to protect your baby. For more information about receiving a COVID-19 vaccination while pregnant, speak to your healthcare provider.
>> Read: Navigating pregnancy during the COVID-19 pandemic
I’m breastfeeding. Should I get vaccinated against COVID-19?
Yes, if you are breastfeeding you should take the vaccine as soon as it is available to you. It is very safe and there is no risk to the mother or baby. None of the current COVID-19 vaccines have live virus in them, so there is no risk of you transmitting COVID-19 to your baby through your breastmilk from the vaccine. In fact, the antibodies that you have after vaccination may go through the breast milk and help protect your baby. >> Read: Breastfeeding safely during the COVID-19 pandemic
Can COVID-19 vaccines affect fertility?
No, you may have seen false claims on social media, but there is no evidence that any vaccine, including COVID-19 vaccines, can affect fertility in women or men. You should get vaccinated if you are currently trying to become pregnant.
Could a COVID-19 vaccine disrupt my menstrual cycle?
Some people have reported experiencing a disruption to their menstrual cycle after getting vaccinated against COVID-19. Although data is still limited, research is ongoing into the impact of vaccines on menstrual cycles.
Speak to your healthcare provider if you have concerns or questions about your periods.
Should my child or teen get a COVID-19 vaccine?
An increasing number of vaccines have been approved for use in children. They’ve been made available after examining the data on the safety and efficacy of these vaccines, and millions of children have been safely vaccinated around the world. Some COVID-19 vaccines have been approved for children from the age of 6 months old. Check with your local health authorities on what vaccines are authorized and available for children and adolescents where you live.
Children and adolescents tend to have milder disease compared to adults, so unless they are part of a group at higher risk of severe COVID-19, it is less urgent to vaccinate them than older people, those with chronic health conditions and health workers.
Remind your children of the importance of us all taking precautions to protect each other, such as avoiding crowded spaces, physical distancing, hand washing and wearing a mask.
It is critical that children continue to receive the recommended childhood vaccines.
How do I talk to my kids about COVID-19 vaccines?
News about COVID-19 vaccines is flooding our daily lives and it is only natural that curious young minds will have questions – lots of them. Read our explainer article for help explaining what can be a complicated topic in simple and reassuring terms.
It’s important to note that from the millions of children that have so far been vaccinated against COVID-19 globally, we know that side effects are very rare. Just like adults, children and adolescents might experience mild symptoms after receiving a dose, such as a slight fever and body aches. But these symptoms typically last for just a day or two. The authorized vaccines for adolescents and children are very safe.
My friend or family member is against COVID-19 vaccines. How do I talk to them?
The development of safe and effective COVID-19 vaccines is a huge step forward in our global effort to end the pandemic. This is exciting news, but there are still some people who are skeptical or hesitant about COVID-19 vaccines. Chances are you know a person who falls into this category.
We spoke to Dr. Saad Omer, Director at the Yale Institute for Global Health, to get his tips on how to navigate these challenging conversations. >> Read the interview
How can I protect my family until we are all vaccinated?
Safe and effective vaccines are a game changer, but even once vaccinated we need to continue taking precautions for the time being to protect ourselves and others. Variants like Omicron have proven that although COVID-19 vaccines are very effective at preventing severe disease, they’re not enough to stop the spread of the virus alone. The most important thing you can do is reduce your risk of exposure to the virus. To protect yourself and your loved ones, make sure to:
- Wear a mask where physical distancing from others is not possible.
- Keep a physical distance from others in public places.
- Avoid poorly ventilated or crowded spaces.
- Open windows to improve ventilation indoors.
- Try and focus on outdoor activities if possible.
- Wash your hands regularly with soap and water or an alcohol-based hand rub.
If you or a family member has a fever, cough or difficulty breathing, seek medical care early and avoid mixing with other children and adults.
Can COVID-19 vaccines affect your DNA?
No, none of the COVID-19 vaccines affect or interact with your DNA in any way. Messenger RNA, or mRNA, vaccines teach the cells how to make a protein that triggers an immune response inside the body. This response produces antibodies which keep you protected against the virus. mRNA is different from DNA and only stays inside the cell for about 72 hours before degrading. However, it never enters the nucleus of the cell, where DNA is kept.
Do the COVID-19 vaccines contain any animal products in them?
No, none of the WHO-approved COVID-19 vaccines contain animal products.
I’ve seen inaccurate information online about COVID-19 vaccines. What should I do?
Sadly, there is a lot of inaccurate information online about the COVID-19 virus and vaccines. A lot of what we’re experiencing is new to all of us, so there may be some occasions where information is shared, in a non-malicious way, that turns out to be inaccurate.
Misinformation in a health crisis can spread paranoia, fear and stigmatization. It can also result in people being left unprotected or more vulnerable to the virus. Get verified facts and advice from trusted sources like your local health authority, the UN, UNICEF, WHO.
If you see content online that you believe to be false or misleading, you can help stop it spreading by reporting it to the social media platform.
What is COVAX?
COVAX is a global effort committed to the development, production and equitable distribution of vaccines around the world. No country will be safe from COVID-19 until all countries are protected.
There are 190 countries and territories engaged in the COVAX Facility, which account for over 90 per cent of the world’s population. Working with CEPI, GAVI, WHO and other partners, UNICEF is leading efforts to procure and supply COVID-19 vaccines on behalf of COVAX.
Learn more about COVAX .
This article was last updated on 25 October 2022. It will continue to be updated to reflect the latest information.
Related topics
More to explore, covid-19 response.
Resources and information about UNICEF’s response to the COVID-19 pandemic
How to talk to your children about COVID-19 vaccines
Tips for navigating the conversation
How to talk to friends and family about vaccines
Tips for handling tough conversations with your loved ones
COVAX information centre
UNICEF and partners led the largest vaccine procurement and supply operation in history
Thank you for visiting nature.com. You are using a browser version with limited support for CSS. To obtain the best experience, we recommend you use a more up to date browser (or turn off compatibility mode in Internet Explorer). In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript.
- View all journals
- My Account Login
- Explore content
- About the journal
- Publish with us
- Sign up for alerts
- Open access
- Published: 14 May 2021
Public attitudes toward COVID-19 vaccination: The role of vaccine attributes, incentives, and misinformation
- Sarah Kreps 1 ,
- Nabarun Dasgupta 2 ,
- John S. Brownstein 3 , 4 ,
- Yulin Hswen 5 &
- Douglas L. Kriner ORCID: orcid.org/0000-0002-9353-2334 1
npj Vaccines volume 6 , Article number: 73 ( 2021 ) Cite this article
19k Accesses
73 Citations
43 Altmetric
Metrics details
- Health care
- Translational research
While efficacious vaccines have been developed to inoculate against severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2; also known as COVID-19), public vaccine hesitancy could still undermine efforts to combat the pandemic. Employing a survey of 1096 adult Americans recruited via the Lucid platform, we examined the relationships between vaccine attributes, proposed policy interventions such as financial incentives, and misinformation on public vaccination preferences. Higher degrees of vaccine efficacy significantly increased individuals’ willingness to receive a COVID-19 vaccine, while a high incidence of minor side effects, a co-pay, and Emergency Use Authorization to fast-track the vaccine decreased willingness. The vaccine manufacturer had no influence on public willingness to vaccinate. We also found no evidence that belief in misinformation about COVID-19 treatments was positively associated with vaccine hesitancy. The findings have implications for public health strategies intending to increase levels of community vaccination.
Similar content being viewed by others

Measuring the impact of COVID-19 vaccine misinformation on vaccination intent in the UK and USA
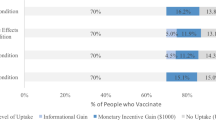
Vaccine hesitancy and monetary incentives
Providing normative information increases intentions to accept a COVID-19 vaccine
Introduction.
In less than a year, an array of vaccines was developed to bring an end to the SARS-CoV-2 pandemic. As impressive as the speed of development was the efficacy of vaccines such as Moderna and Pfizer, which are over 90%. Despite the growing availability and efficacy, however, vaccine hesitancy remains a potential impediment to widespread community uptake. While previous surveys indicate that overall levels of vaccine acceptance may be around 70% in the United States 1 , the case of Israel may offer a cautionary tale about self-reported preferences and vaccination in practice. Prospective studies 2 of vaccine acceptance in Israel showed that about 75% of the Israeli population would vaccinate, but Israel’s initial vaccination surge stalled around 42%. The government, which then augmented its vaccination efforts with incentive programs, attributed unexpected resistance to online misinformation 3 .
Research on vaccine hesitancy in the context of viruses such as influenza and measles, mumps, and rubella, suggests that misinformation surrounding vaccines is prevalent 4 , 5 . Emerging research on COVID-19 vaccine preferences, however, points to vaccine attributes as dominant determinants of attitudes toward vaccination. Higher efficacy is associated with greater likelihood of vaccinating 6 , 7 , whereas an FDA Emergency Use Authorization 6 or politicized approval timing 8 is associated with more hesitancy. Whether COVID-19 misinformation contributes to vaccine preferences or whether these attributes or policy interventions such as incentives play a larger role has not been studied. Further, while previous research has focused on a set of attributes that was relevant at one particular point in time, the evidence and context about the available vaccines has continued to shift in ways that could shape public willingness to accept the vaccine. For example, governments, employers, and economists have begun to think about or even devise ways to incentivize monetarily COVID-19 vaccine uptake, but researchers have not yet studied whether paying people to receive the COVID-19 vaccine would actually affect likely behavior. As supply problems wane and hesitancy becomes a limiting factor, understanding whether financial incentives can overcome hesitancy becomes a crucial question for public health. Further, as new vaccines such as Johnson and Johnson are authorized, knowing whether the vaccine manufacturer name elicits or deters interest in individuals is also important, as are the corresponding efficacy rates of different vaccines and the extent to which those affect vaccine preferences. The purpose of this study is to examine how information about vaccine attributes such as efficacy rates, the incidence of side effects, the nature of the governmental approval process, identity of the manufacturers, and policy interventions, including economic incentives, affect intention to vaccinate, and to examine the association between belief in an important category of misinformation—false claims concerning COVID-19 treatments—and willingness to vaccinate.
General characteristics of study population
Table 1 presents sample demographics, which largely reflect those of the US population as a whole. Of the 1335 US adults recruited for the study, a convenience sample of 1100 participants consented to begin the survey, and 1096 completed the full questionnaire. The sample was 51% female; 75% white; and had a median age of 43 with an interquartile range of 31–58. Comparisons of the sample demographics to those of other prominent social science surveys and U.S. Census figures are shown in Supplementary Table 1 .
Vaccination preferences
Each subject was asked to evaluate a series of seven hypothetical vaccines. For each hypothetical vaccine, our conjoint experiment randomly assigned values of five different vaccine attributes—efficacy, the incidence of minor side effects, government approval process, manufacturer, and cost/financial inducement. Descriptions of each attribute and the specific levels used in the experiment are summarized in Table 2 . After seeing the profile of each vaccine, the subject was asked whether she would choose to receive the vaccine described, or whether she would choose not to be vaccinated. Finally, subjects were asked to indicate how likely they would be to take the vaccine on a seven-point likert scale.
Across all choice sets, in 4419 cases (58%) subjects said they would choose the vaccine described in the profile rather than not being vaccinated. As shown in Fig. 1 , several characteristics of the vaccine significantly influenced willingness to vaccinate.
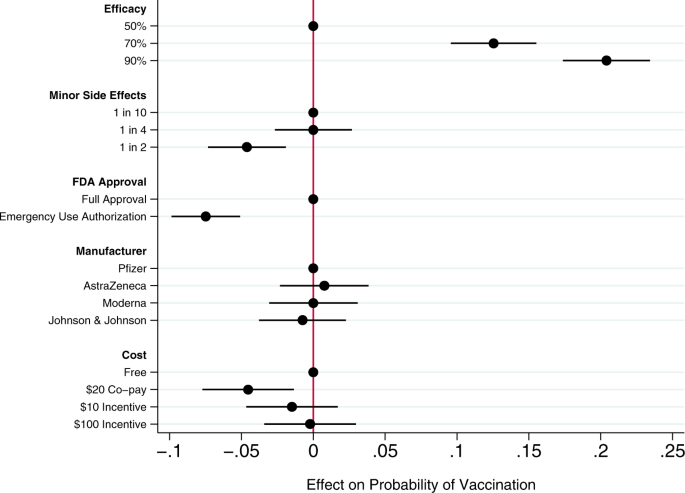
Circles present the estimated effect of each attribute level on the probability of a subject accepting vaccination from the attribute’s baseline level. Horizontal lines through points indicate 95% confidence intervals. Points without error bars denote the baseline value for each attribute. The average marginal component effects (AMCEs) are the regression coefficients reported in model 1 of Table 3 .
Efficacy had the largest effect on individual vaccine preferences. An efficacy rate of 90% increased uptake by about 20% relative to the baseline at 50% efficacy. Even a high incidence of minor side effects (1 in 2) had only a modest negative effect (about 5%) on willingness to vaccinate. Whether the vaccine went through full FDA approval or received an Emergency Use Authorization (EUA), an authority that allows the Food and Drug Administration mechanisms to accelerate the availability and use of treatments or medicines during medical emergencies 9 , significantly influenced willingness to vaccinate. An EUA decreased the likelihood of vaccination by 7% compared to a full FDA authorization; such a decline would translate into about 23 million Americans. While a $20 co-pay reduced the likelihood of vaccination relative to a no-cost baseline, financial incentives did not increase willingness to vaccinate. Lastly, the manufacturer had no effect on vaccination attitudes, despite the public pause of the AstraZeneca trial and prominence of Johnson & Johnson as a household name (our experiment was fielded before the pause in the administration of the Johnson & Johnson shot in the United States).
Model 2 of Table 3 presents an expanded model specification to investigate the association between misinformation and willingness to vaccinate. The primary additional independent variable of interest is a misinformation index that captures the extent to which each subject believes or rejects eight claims (five false; three true) about COVID-19 treatments. Additional analyses using alternate operationalizations of the misinformation index yield substantively similar results (Supplementary Table 4 ). This model also includes a number of demographic control variables, including indicators for political partisanship, gender, educational attainment, age, and race/ethnicity, all of which are also associated with belief in misinformation about the vaccine (Supplementary Table 2 ). Finally, the model also controls for subjects’ health insurance status, past experience vaccinating against seasonal influenza, attitudes toward the pharmaceutical industry, and beliefs about vaccine safety generally.
Greater levels of belief in misinformation about COVID-19 treatments were not associated with greater vaccine hesitancy. Instead, the relevant coefficient is positive and statistically significant, indicating that, all else being equal, individuals who scored higher on our index of misinformation about COVID-19 treatments were more willing to vaccinate than those who were less susceptible to believing false claims.
Strong beliefs that vaccines are safe generally was positively associated with willingness to accept a COVID-19 vaccine, as were past histories of frequent influenza vaccination and favorable attitudes toward the pharmaceutical industry. Women and older subjects were significantly less likely to report willingness to vaccinate than men and younger subjects, all else equal. Education was positively associated with willingness to vaccinate.
This research offers a comprehensive examination of attitudes toward COVID-19 vaccination, particularly the role of vaccine attributes, potential policy interventions, and misinformation. Several previous studies have analyzed the effects of vaccine characteristics on willingness to vaccinate, but the modal approach is to gauge willingness to accept a generic COVID-19 vaccine 10 , 11 . Large volumes of research show, however, that vaccine preferences hinge on specific vaccine attributes. Recent research considering the influence of attributes such as efficacy, side effects, and country of origin take a step toward understanding how properties affect individuals’ intentions to vaccinate 6 , 7 , 8 , 12 , 13 , but evidence about the attributes of actual vaccines, debates about how to promote vaccination within the population, and questions about the influence of misinformation have moved quickly 14 .
Our conjoint experiment therefore examined the influence of five vaccine attributes on vaccination willingness. The first category of attributes involved aspects of the vaccine itself. Since efficacy is one of the most common determinants of vaccine acceptance, we considered different levels of efficacy, 50%, 70%, and 90%, levels that are common in the literature 7 , 15 . Evidence from Phase III trials suggests that even the 90% efficacy level in our design, which is well above the 50% threshold from the FDA Guidance for minimal effectiveness for Emergency Use Authorization 16 , has been exceeded by both Pfizer’s and Moderna’s vaccines 17 , 18 . The 70% efficacy threshold is closer to the initial reports of the efficacy of the Johnson & Johnson vaccine, whose efficacy varied across regions 19 . Our analysis suggests that efficacy levels associated with recent mRNA vaccine trials increases public vaccine uptake by 20% over a baseline of a vaccine with 50% efficacy. A 70% efficacy rate increases public willingness to vaccinate by 13% over a baseline vaccine with 50% efficacy.
An additional set of epidemiological attributes consisted of the frequency of minor side effects. While severe side effects were plausible going into early clinical trials, evidence clearly suggests that minor side effects are more common, ranging from 10% to 100% of people vaccinated depending on the number of doses and the dose group (25–250 mcg) 20 . Since the 100 mcg dose was supported in Phase III trials 21 , we include the highest adverse event probability—approximating 60% as 1 in 2—and 1 in 10 as the lowest likelihood, approximating the number of people who experienced mild arthralgia 20 . Our findings suggest that a the prevalence of minor side effects associated with recent trials (i.e. a 1 in 2 chance), intention to vaccinate decreased by about 5% versus a 1 in 10 chance of minor side effects baseline. However, at a 25% rate of minor side effects, respondents did not indicate any lower likelihood of vaccination compared to the 10% baseline. Public communications about how to reduce well-known side effects, such as pain at the injection site, could contribute to improved acceptance of the vaccine, as it is unlikely that development of vaccine-related minor side effects will change.
We then considered the effect of EUA versus full FDA approval. The influenza H1N1 virus brought the process of EUA into public discourse 22 , and the COVID-19 virus has again raised the debate about whether and how to use EUA. Compared to recent studies also employing conjoint experimental designs that showed just a 3% decline in support conditional on EUA 6 , we found decreases in support of more than twice that with an EUA compared to full FDA approval. Statements made by the Trump administration promising an intensely rapid roll-out or isolated adverse events from vaccination in the UK may have exacerbated concerns about EUA versus full approval 8 , 23 , 24 , 25 . This negative effect is even greater among some subsets of the population. As shown in additional analyses reported in the Supplementary Information (Supplementary Fig. 5 ), the negative effects are greatest among those who believe vaccines are generally safe. Among those who believe vaccines generally are extremely safe, the EUA decreased willingness to vaccinate by 11%, all else equal. This suggests that outreach campaigns seeking to assure those troubled by the authorization process used for currently available vaccines should target their efforts on those who are generally predisposed to believe vaccines are safe.
Next, we compared receptiveness as a function of the manufacturer: Moderna, Pfizer, Johnson and Johnson, and AstraZeneca, all firms at advanced stages of vaccine development. Vaccine manufacturers in the US have not yet attempted to use trade names to differentiate their vaccines, instead relying on the association with manufacturer reputation. In other countries, vaccine brand names have been more intentionally publicized, such as Bharat Biotech’s Covaxin in India and Gamaleya Research Institute of Epidemiology and Microbiology Sputnik V in Russia. We found that manufacturer names had no impact on willingness to vaccinate. As with hepatitis and H. influenzae vaccines 26 , 27 , interchangeability has been an active topic of debate with coronavirus mRNA vaccines which require a second shot for full immunity. Our research suggests that at least as far as public receptiveness goes, interchangeability would not introduce concerns. We found no significant differences in vaccination uptake across any of the manufacturer treatments. Future research should investigate if a manufacturer preference develops as new evidence about efficacy and side effects becomes available, particularly depending on whether future booster shots, if needed, are deemed interchangeable with the initial vaccination.
Taking up the question of how cost and financial incentives shape behavior, we looked at paying and being paid to vaccinate. While existing research suggests that individuals are often willing to pay for vaccines 28 , 29 , some economists have proposed that the government pay individuals up to $1,000 to take the COVID-19 vaccine 30 . However, because a cost of $300 billion to vaccinate the population may be prohibitive, we posed a more modest $100 incentive. We also compared this with a $10 incentive, which previous studies suggest is sufficient for actions that do not require individuals to change behavior on a sustained basis 31 . While having to pay a $20 co-pay for the vaccine did deter individuals, the additional economic incentives had no positive effect although they did not discourage vaccination 32 . Consistent with past research 31 , 33 , further analysis shows that the negative effect of the $20 co-pay was concentrated among low-income earners (Supplementary Fig. 7 ). Financial incentives failed to increase vaccination willingness across income levels.
Our study also yields important insights into the relationship between one prominent category of COVID-19 misinformation and vaccination preferences. We find that susceptibility to misinformation about COVID-19 treatments—based on whether individuals can distinguish between factual and false information about efforts to combat COVID-19—is considerable. A quarter of subjects scored no higher on our misinformation index than random guessing or uniform abstention/unsure responses (for the full distribution, see Supplementary Fig. 2 ). However, subjects who scored higher on our misinformation index did not exhibit greater vaccination hesitancy. These subjects actually were more likely to believe in vaccine safety more generally and to accept a COVID-19 vaccine, all else being equal. These results run counter to recent findings of public opinion in France where greater conspiracy beliefs were negatively correlated with willingness to vaccinate against COVID-19 34 and in Korea where greater misinformation exposure and belief were negatively correlated with taking preventative actions 35 . Nevertheless, the results are robust to alternate operationalizations of belief in misinformation (i.e., constructing the index only using false claims, or measuring misinformation beliefs as the number of false claims believed: see Supplementary Table 4 ).
We recommend further study to understand the observed positive relationship between beliefs in COVID-19 misinformation about fake treatments and willingness to receive the COVID-19 vaccine. To be clear, we do not posit a causal relationship between the two. Rather, we suspect that belief in misinformation may be correlated with an omitted factor related to concerns about contracting COVID-19. For example, those who believe COVID-19 misinformation may have a higher perception of risk of COVID-19, and therefore be more willing to take a vaccine, all else equal 36 . Additional analyses reported in the Supplementary Information (Supplementary Fig. 6 ) show that the negative effect of an EUA on willingness to vaccinate was concentrated among those who scored low on the misinformation index. An EUA had little effect on the vaccination preferences of subjects most susceptible to misinformation. This pattern is consistent with the possibility that these subjects were more concerned with the disease and therefore more likely to vaccinate, regardless of the process through which the vaccine was brought to market.
We also observe that skepticism toward vaccines in general does not correlate perfectly with skepticism toward the COVID-19 vaccine. Therefore, it is important not to conflate people who are wary of the COVID-19 vaccine and those who are anti-vaccination, as even medically informed individuals may be hesitant because of the speed at which the COVID-19 vaccine was developed. For example, older people are more likely to believe vaccines are safe but less willing to receive the COVID-19 vaccine in our survey, perhaps following the high rates of vaccine skepticism among medical staff expressing concerns regarding the safety of a rapidly-developed vaccine 2 . This inverse relationship between age and willingness to vaccinate is also surprising. Most opinion surveys find older adults are more likely to vaccinate than younger adults 37 . However, most of these survey questions ask about willingness to take a generic vaccine. Two prior studies, both recruiting subjects from the Lucid platform and employing conjoint experiments to examine the effects of vaccine attributes on public willingness to vaccinate, also find greater vaccine hesitancy among older Americans 6 , 7 . Future research could explore whether these divergent results are a product of the characteristics of the sample or of the methodological design in which subjects have much more information about the vaccines when indicating their vaccination preferences.
An important limitation of our study is that it necessarily offers a snapshot in time, specifically prior to both the election and vaccine roll-out. We recommend further study to understand more how vaccine perceptions evolve both in terms of the perceived political ownership of the vaccine—now that President Biden is in office—and as evidence has emerged from the millions of people who have been vaccinated. Similarly, researchers should consider analyzing vaccine preferences in the context of online vaccine controversies that have been framed in terms of patient autonomy and right to refuse 38 , 39 . Vaccination mandates may evoke feelings of powerlessness, which may be exacerbated by misinformation about the vaccines themselves. Further, researchers should more fully consider how individual attributes such as political ideology and race intersect with vaccine preferences. Our study registered increased vaccine hesitancy among Blacks, but did not find that skepticism was directly related to misinformation. Perceptions and realities of race-based maltreatment could also be moderating factors worth exploring in future analyses 40 , 41 .
Overall, we found that the most important factor influencing vaccine preferences is vaccine efficacy, consistent with a number of previous studies about attitudes toward a range of vaccines 6 , 42 , 43 . Other attributes offer potential cautionary flags and opportunities for public outreach. The prospect of a 50% likelihood of mild side effects, consistent with the evidence about current COVID-19 vaccines being employed, dampens likelihood of uptake. Public health officials should reinforce the relatively mild nature of the side effects—pain at the injection site and fatigue being the most common 44 —and especially the temporary nature of these effects to assuage public concerns. Additionally, in considering policy interventions, public health authorities should recognize that a $20 co-pay will likely discourage uptake while financial incentives are unlikely to have a significant positive effect. Lastly, belief in misinformation about COVID-19 does not appear to be a strong predictor of vaccine hesitancy; belief in misinformation and willingness to vaccinate were positively correlated in our data. Future research should explore the possibility that exposure to and belief in misinformation is correlated with other factors associated with vaccine preferences.
Survey sample and procedures
This study was approved by the Cornell Institutional Review Board for Human Participant Research (protocol ID 2004009569). We conducted the study on October 29–30, 2020, prior to vaccine approval, which means we captured sentiments prospectively rather than based on information emerging from an ongoing vaccination campaign. We recruited a sample of 1096 adult Americans via the Lucid platform, which uses quota sampling to produce samples matched to the demographics of the U.S. population on age, gender, ethnicity, and geographic region. Research has shown that experimental effects observed in Lucid samples largely mirror those found using probability-based samples 45 . Supplementary Table 1 presents the demographics of our sample and comparisons to both the U.S. Census American Community Survey and the demographics of prominent social science surveys.
After providing informed consent on the first screen of the online survey, participants turned to a choice-based conjoint experiment that varied five attributes of the COVID-19 vaccine. Conjoint analyses are often used in marketing to research how different aspects of a product or service affect consumer choice. We build on public health studies that have analyzed the influence of vaccine characteristics on uptake within the population 42 , 46 .
Conjoint experiment
We first designed a choice-based conjoint experiment that allowed us to evaluate the relative influence of a range of vaccine attributes on respondents’ vaccine preferences. We examined five attributes summarized in Table 2 . Past research has shown that the first two attributes, efficacy and the incidence of side effects, are significant drivers of public preferences on a range of vaccines 47 , 48 , 49 , including COVID-19 6 , 7 , 13 , 50 . In this study, we increased the expected incidence of minor side effects from previous research 6 to reflect emerging evidence from Phase III trials. The third attribute, whether the vaccine received full FDA approval or an EUA, examines whether the speed of the approval process affects public vaccination preferences 6 . The fourth attribute, the manufacturer of the vaccine, allows us to examine whether the highly public pause in the AstraZeneca trial following an adverse event, and the significant differences in brand familiarity between smaller and less broadly known companies like Moderna and household name Johnson & Johnson affects public willingness to vaccinate. The fifth attribute examines the influence of a policy tool—offsetting the costs of vaccination or even incentivizing it financially—on public willingness to vaccinate.
Attribute levels and attribute order were randomly assigned across participants. A sample choice set is presented in Supplementary Fig. 1 . After viewing each profile individually, subjects were asked: “If you had to choose, would you choose to get this vaccine, or would you choose not to be vaccinated?” Subjects then made a binary choice, responding either that they “would choose to get this vaccine” or that they “would choose not to be vaccinated.” This is the dependent variable for the regression analyses in Table 3 . After making a binary choice to take the vaccine or not be vaccinated, we also asked subjects “how likely or unlikely would you be to get the vaccine described above?” Subjects indicated their vaccination preference on a seven-point scale ranging from “extremely likely” to “extremely unlikely.” Additional analyses using this ordinal dependent variable reported in Supplementary Table 3 yield substantively similar results to those presented in Table 3 .
To determine the effect of each attribute-level on willingness to vaccinate, we followed Hainmueller, Hopkins, and Yamamoto and employed an ordinary least squares (OLS) regression with standard errors clustered on respondent to estimate the average marginal component effects (AMCEs) for each attribute 51 . The AMCE represents the average difference in a subject choosing a vaccine when comparing two different attribute values—for example, 50% efficacy vs. 90% efficacy—averaged across all possible combinations of the other vaccine attribute values. The AMCEs are nonparametrically identified under a modest set of assumptions, many of which (such as randomization of attribute levels) are guaranteed by design. Model 1 in Table 3 estimates the AMCEs for each attribute. These AMCEs are illustrated in Fig. 1 .
Analyzing additional correlates of vaccine acceptance
To explore the association between respondents’ embrace of misinformation about COVID-19 treatments and vaccination willingness, the survey included an additional question battery. To measure the extent of belief in COVID-19 misinformation, we constructed a list of both accurate and inaccurate headlines about the coronavirus. We focused on treatments, relying on the World Health Organization’s list of myths, such as “Hand dryers are effective in killing the new coronavirus” and true headlines such as “Avoiding shaking hands can help limit the spread of the new coronavirus 52 .” Complete wording for each claim is provided in Supplementary Appendix 1 . Individuals read three true headlines and five myths, and then responded whether they believed each headline was true or false, or whether they were unsure. We coded responses to each headline so that an incorrect accuracy assessment yielded a 1; a correct accuracy assessment a -1; and a response of unsure was coded as 0. From this, we created an additive index of belief in misinformation that ranged from -8 to 8. The distribution of the misinformation index is presented in Supplementary Fig. 2 . A possible limitation of this measure is that because the survey was conducted online, some individuals could have searched for the answers to the questions before responding. However, the median misinformation index score for subjects in the top quartile in terms of time spent taking the survey was identical to the median for all other respondents. This may suggest that systematic searching for correct answers is unlikely.
To ensure that any association observed between belief in misinformation and willingness to vaccinate is not an artifact of how we operationalized susceptibility to misinformation, we also constructed two alternate measures of belief in misinformation. These measures are described in detail in the Supplementary Information (see Supplementary Figs. 3 and 4 ). Additional regression analyses using these alternate measures of misinformation beliefs yield substantively similar results (see Supplementary Table 4 ). Additional analyses examining whether belief in misinformation moderates the effect of efficacy and an FDA EUA on vaccine acceptance are presented in Supplementary Fig. 6 .
Finally, model 2 of Table 3 includes a range of additional control variables. Following past research, it includes a number of demographic variables, including indicator variables identifying subjects who identify as Democrats or Republicans; an indicator variable identifying females; a continuous variable measuring age (alternate analyses employing a categorical variable yield substantively similar results); an eight-point measure of educational attainment; and indicator variables identifying subjects who self-identify as Black or Latinx. Following previous research 6 , the model also controlled for three additional factors often associated with willingness to vaccinate: an indicator variable identifying whether each subject had health insurance; a variable measuring past frequency of influenza vaccination on a four-point scale ranging from “never” to “every year”; beliefs about the general safety of vaccines measured on a four-point scale ranging from “not at all safe” to “extremely safe”; and a measure of attitudes toward the pharmaceutical industry ranging from “very positive” to “very negative.”
Reporting summary
Further information on research design is available in the Nature Research Reporting Summary linked to this article.
Data availability
All data and statistical code to reproduce the tables and figures in the manuscript and Supplementary Information are published at the Harvard Dataverse via this link: 10.7910/DVN/ZYU6CO.
Hamel, L., Kirzinger, A., Munana, C. & Brodie, M. KFF COVID-19 Vaccine Monitor: December 2020 | KFF (2020).
Dror, A. A. et al. Vaccine hesitancy: the next challenge in the fight against COVID-19. Eur. J. Epidemiol. 35 , 775–779 (2020).
Article CAS PubMed Google Scholar
Scharf, I. & Ben Zion, I. As Vaccinations Lag, Israel Combats Online Misinformation. AP2 (21AD).
Nyhan, B. & Reifler, J. Does correcting myths about the flu vaccine work? An experimental evaluation of the effects of corrective information. Vaccine 33 , 459–464 (2015).
Article PubMed Google Scholar
Hussain, A., Ali, S., Ahmed, M. & Hussain, S. The anti-vaccination movement: a regression in modern medicine. Cureus 10 , e2919 (2018).
PubMed PubMed Central Google Scholar
Kreps, S. et al. Factors Associated With US Adults’ Likelihood of Accepting COVID-19 Vaccination. JAMA Netw. open 3 , (2020).
Motta, M. Can a COVID-19 vaccine live up to Americans’ expectations? A conjoint analysis of how vaccine characteristics influence vaccination intentions. Soc. Sci. Med . 113642, https://doi.org/10.1016/j.socscimed.2020.113642 (2021).
Bokemper, S. E., Huber, G. A., Gerber, A. S., James, E. K. & Omer, S. B. Timing of COVID-19 vaccine approval and endorsement by public figures. Vaccine , https://doi.org/10.1016/j.vaccine.2020.12.048 (2021).
Quinn, S. C., Jamison, A. M. & Freimuth, V. Communicating effectively about emergency use authorization and vaccines in the COVID-19 pandemic. Am. J. Public Health e1–e4 (2020).
Malik, A. A., McFadden, S. A. M., Elharake, J. & Omer, S. B. Determinants of COVID-19 vaccine acceptance in the US. EClinicalMedicine 26 , 100495 (2020).
Article PubMed PubMed Central Google Scholar
Fisher, K. A. et al. Attitudes toward a potential SARS-CoV-2 vaccine: a survey of U.S. adults. Ann. Intern. Med. 173 , 964–973 (2020).
Lazarus, J. V. et al . A global survey of potential acceptance of a COVID-19 vaccine. Nat. Med . 1–4 (2020).
Schwarzinger, M., Watson, V., Arwidson, P., Alla, F. & Luchini, S. COVID-19 vaccine hesitancy in a representative working-age population in France: a survey experiment based on vaccine characteristics. Lancet Public Heal ., https://doi.org/10.1016/s2468-2667(21)00012-8 (2021).
Wood, S. & Schulman, K. Beyond politics—promoting Covid-19 vaccination in the United States. N. Engl. J. Med . NEJMms2033790, https://doi.org/10.1056/NEJMms2033790 (2021).
Marshall, H. S., Chen, G., Clarke, M. & Ratcliffe, J. Adolescent, parent and societal preferences and willingness to pay for meningococcal B vaccine: a discrete choice experiment. Vaccine 34 , 671–677 (2016).
Administration, F. and D. Development and Licensure of Vaccines to Prevent COVID-19: Guidance for Industry . (2020).
Polack, F. P. et al. Safety and efficacy of the BNT162b2 mRNA Covid-19 vaccine. N. Engl. J. Med. 383 , 2603–2615 (2020).
Baden, L. R. et al. Efficacy and safety of the mRNA-1273 SARS-CoV-2 vaccine. N. Engl. J. Med ., https://doi.org/10.1056/nejmoa2035389 (2020).
Johnson & Johnson. Johnson & Johnson Announces Single-Shot Janssen COVID-19 Vaccine Candidate Met Primary Endpoints in Interim Analysis of its Phase 3 ENSEMBLE Trial. (2020). Available at: https://www.jnj.com/johnson-johnson-announces-single-shot-janssen-covid-19-vaccine-candidate-met-primary-endpoints-in-interim-analysis-of-its-phase-3-ensemble-trial . (Accessed 28 Feb 2021).
Jackson, L. A. et al. An mRNA vaccine against SARS-CoV-2—preliminary report. N. Engl. J. Med. 383 , 1920–1931 (2020).
Anderson, E. J. et al. Safety and immunogenicity of SARS-CoV-2 mRNA-1273 vaccine in older adults. N. Engl. J. Med. 383 , 2427–2438 (2020).
Quinn, S. C., Kumar, S., Freimuth, V. S., Kidwell, K. & Musa, D. Public willingness to take a vaccine or drug under emergency use authorization during the 2009 H1N1 pandemic. Biosecurity Bioterrorism 7 , 275–290 (2009).
Holden Thorp, H. A dangerous rush for vaccines. Science 369 , 885 (2020).
Limaye, R. J., Sauer, M. & Truelove, S. A. Politicizing public health: the powder keg of rushing COVID-19 vaccines. Hum. Vaccines Immunother ., https://doi.org/10.1080/21645515.2020.1846400 (2020).
Feuer, W. Trump Says ‘No President’s Ever Pushed’ the FDA Like Jim, Vaccine Coming ‘Very Shortly’. CNBC (2020).
Soysal, A., Gokçe, I., Pehlivan, T. & Bakir, M. Interchangeability of a hepatitis A vaccine second dose: Avaxim 80 following a first dose of Vaqta 25 or Havrix 720 in children in Turkey. Eur. J. Pediatr. 166 , 533–539 (2007).
Greenberg, D. P. & Feldman, S. Vaccine interchangeability. Clin. Pediatrics 42 , 93–99 (2003).
Article Google Scholar
Bishai, D., Brice, R., Girod, I., Saleh, A. & Ehreth, J. Conjoint analysis of French and German parents’ willingness to pay for meningococcal vaccine. PharmacoEconomics 25 , 143–154 (2007).
Cameron, M. P., Newman, P. A., Roungprakhon, S. & Scarpa, R. The marginal willingness-to-pay for attributes of a hypothetical HIV vaccine. Vaccine 31 , 3712–3717 (2013).
Litan, R. Want herd immunity? Pay people to take the vaccine. Brookings (2020).
Kane, R. L., Johnson, P. E., Town, R. J. & Butler, M. A structured review of the effect of economic incentives on consumers’ preventive behavior. Am. J. Preventive Med. 27 , 327–352 (2004).
Volpp, K. G., Loewenstein, G. & Buttenheim, A. M. Behaviorally informed strategies for a National COVID-19 vaccine promotion program. JAMA - J. Am. Med. Assoc. 325 , 125–126 (2020).
Google Scholar
Nowalk, M. P. et al. Improving influenza vaccination rates in the workplace. A randomized trial. Am. J. Prev. Med. 38 , 237–246 (2010).
Bertin, P., Nera, K. & Delouvée, S. Conspiracy beliefs, rejection of vaccination, and support for hydroxychloroquine: a conceptual replication-extension in the COVID-19 pandemic context. Front. Psychol. 11 , 2471 (2020).
Lee, J. J. et al. Associations between COVID-19 misinformation exposure and belief with COVID-19 knowledge and preventive behaviors: cross-sectional online study. J. Med. Internet Res. 22 , e22205 (2020).
Reyna, V. F. Risk perception and communication in vaccination decisions: a fuzzy-trace theory approach. Vaccine 30 , 3790–3797 (2012).
Lin, C., Tu, P. & Beitsch, L. M. Confidence and receptivity for covid‐19 vaccines: a rapid systematic review. Vaccines 9 , 1–32 (2021).
Broniatowski, D. A. et al. Facebook pages, the ‘Disneyland’ measles outbreak, and promotion of vaccine refusal as a civil right, 2009–2019. Am. J. Public Health 110 , S312–S318 (2020).
Moon, K., Riege, A., Gourdon-Kanhukamwe, A. & Vallée-Tourangeau, G. The Moderating Effect of Autonomy on Promotional Health Messages Encouraging Flu Vaccination Uptake Among Healthcare Professionals . (PsyArXiv, 2020), https://doi.org/10.31234/OSF.IO/AJV4Q .
Ferdinand, K. C., Nedunchezhian, S. & Reddy, T. K. The COVID-19 and influenza “Twindemic”: barriers to influenza vaccination and potential acceptance of SARS-CoV2 vaccination in African Americans. J. Natl. Med. Assoc. 112 , 681–687 (2020).
PubMed Google Scholar
Jaiswal, J., LoSchiavo, C. & Perlman, D. C. Disinformation, misinformation and inequality-driven mistrust in the time of COVID-19: lessons unlearned from AIDS denialism. AIDS Behav. 24 , 2776–2780 (2020).
Determann, D. et al. Acceptance of vaccinations in pandemic outbreaks: a discrete choice experiment. PLoS ONE 9 , e102505 (2014).
Simpson, C. R., Ritchie, L. D., Robertson, C., Sheikh, A. & McMenamin, J. Effectiveness of H1N1 vaccine for the prevention of pandemic influenza in Scotland, UK: A retrospective observational cohort study. Lancet Infect. Dis. 12 , 696–702 (2012).
Remmel, A. COVID vaccines and safety: what the research says. Nature 590 , 538–540 (2021).
Coppock, A. & McClellan, O. A. Validating the demographic, political, psychological, and experimental results obtained from a new source of online survey respondents. Res. Polit. 6 , 1–14 (2019).
de Bekker-Grob, E. W. et al. The impact of vaccination and patient characteristics on influenza vaccination uptake of elderly people: A discrete choice experiment. Vaccine 36 , 1467–1476 (2018).
Determann, D. et al. Public preferences for vaccination programmes during pandemics caused by pathogens transmitted through respiratory droplets–A discrete choice experiment in four European countries, 2013. Eurosurveillance 21 , 1–13 (2016).
de Bekker-Grob, E. W. et al. Girls’ preferences for HPV vaccination: A discrete choice experiment. Vaccine 28 , 6692–6697 (2010).
Guo, N., Zhang, G., Zhu, D., Wang, J. & Shi, L. The effects of convenience and quality on the demand for vaccination: results from a discrete choice experiment. Vaccine 35 , 2848–2854 (2017).
Kaplan, R. M. & Milstein, A. Influence of a COVID-19 vaccine’s effectiveness and safety profile on vaccination acceptance. Proc. Natl. Acad. Sci. USA 118 , 2021726118 (2021).
Hainmueller, J., Hopkins, D. J. & Yamamoto, T. Causal inference in conjoint analysis: Understanding multidimensional choices via stated preference experiments. Polit. Anal. 22 , 1–30 (2014).
Organization, W. H. Coronavirus disease (COVID-19) advice for the public: Mythbusters. (2020). Available at: https://www.who.int/emergencies/diseases/novel-coronavirus-2019/advice-for-public/myth-busters . (Accessed 14 Jan 2021).
Download references
Acknowledgements
S.K. and D.K. would like to thank the Cornell Atkinson Center for Sustainability for financial support.
Author information
Authors and affiliations.
Department of Government, Cornell University, Ithaca, NY, USA
Sarah Kreps & Douglas L. Kriner
Injury Prevention Research Center, University of North Carolina, Chapel Hill, NC, USA
Nabarun Dasgupta
Department of Pediatrics, Harvard Medical School, Boston, MA, USA
John S. Brownstein
Computational Epidemiology Lab, Boston Children’s Hospital, Boston, MA, USA
Epidemiology and Biostatistics, University of California, San Francisco, CA, USA
Yulin Hswen
You can also search for this author in PubMed Google Scholar
Contributions
S.K. and D.K. designed the experiment/survey instrument and conducted the statistical analysis. S.K., N.D., J.B., Y.H., and D.K. all contributed to the conceptual design of the research and to the writing of the paper.
Corresponding author
Correspondence to Douglas L. Kriner .
Ethics declarations
Competing interests.
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary information, reporting summary, rights and permissions.
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/ .
Reprints and permissions
About this article
Cite this article.
Kreps, S., Dasgupta, N., Brownstein, J.S. et al. Public attitudes toward COVID-19 vaccination: The role of vaccine attributes, incentives, and misinformation. npj Vaccines 6 , 73 (2021). https://doi.org/10.1038/s41541-021-00335-2
Download citation
Received : 25 January 2021
Accepted : 06 April 2021
Published : 14 May 2021
DOI : https://doi.org/10.1038/s41541-021-00335-2
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
This article is cited by
Progress with covid vaccine development and implementation.
- Richard W. Titball
- David I. Bernstein
- Alan D. T. Barrett
npj Vaccines (2024)
Knowledge, attitudes and demographic drivers for COVID-19 vaccine hesitancy in Malawi
- Yamikani Ndasauka
- Halima Sumayya Twabi
- Catherine Makhumula-Mtimuni
Scientific Reports (2024)
A synthesis of evidence for policy from behavioural science during COVID-19
- Kai Ruggeri
- Friederike Stock
- Robb Willer
Nature (2024)
COVID-19 Vaccine Acceptability and Financial Incentives among Unhoused People in Los Angeles County: a Three-Stage Field Survey
- Allison D. Rosen
- Jacqueline Beltran
- Chelsea L. Shover
Journal of Urban Health (2022)
The shot, the message, and the messenger: COVID-19 vaccine acceptance in Latin America
- Pablo Argote
- Elena Barham
- Oscar Pocasangre
npj Vaccines (2021)
Quick links
- Explore articles by subject
- Guide to authors
- Editorial policies
Sign up for the Nature Briefing: Translational Research newsletter — top stories in biotechnology, drug discovery and pharma.
- Program Finder
- Admissions Services
- Course Directory
- Academic Calendar
- Hybrid Campus
- Lecture Series
- Convocation
- Strategy and Development
- Implementation and Impact
- Integrity and Oversight
- In the School
- In the Field
- In Baltimore
- Resources for Practitioners
- Articles & News Releases
- In The News
- Statements & Announcements
- At a Glance
- Student Life
- Strategic Priorities
- Inclusion, Diversity, Anti-Racism, and Equity (IDARE)
- What is Public Health?
A Top Vaccine Expert Answers Important Questions About a COVID-19 Vaccine
The covid-19 vaccine is on track to become the fastest-developed vaccine in history. that doesn’t mean the process is skipping any critical steps..
Understanding what we know—and still don’t—about a vaccine for COVID-19 can help shed light on its safety and efficacy.
Ruth Karron, MD , is one of the top vaccine experts in the world, serving on vaccine committees for the CDC, the WHO, and the FDA. Karron, who leads the Center for Immunization Research at the Johns Hopkins Bloomberg School of Public Health, recently spoke with Josh Sharfstein and answered a list of important questions about the COVID-19 vaccine.
How close are we to a vaccine?
There are some very encouraging developments. We have a few vaccines now that will go into Phase 3 clinical trials, also known as efficacy trials. That means that those vaccines have passed certain goalposts in terms of initial evaluations of safety and immune response such that they can be evaluated in larger trials.
We know that these vaccines are promising, but we don’t yet know if they are going to work. That’s what the purpose of an efficacy trial is—as well as to provide a broader assessment of safety of the vaccine in a large number of people.
Tell me more about these efficacy trials. What do they actually entail?
They involve large numbers of people: In these particular trials for COVID vaccines, there are going to be about 30,000 people enrolled per trial. Individuals are given a vaccine, and then they are followed both to make sure that the side effects from the vaccine are acceptable and to see whether they develop a SARS-CoV-2 infection along with some symptoms.
These are placebo-controlled trials, meaning that some individuals will get a COVID vaccine and some will get a placebo. Then, the rates of disease will be compared in the people who got placebo and the people who got the vaccine to determine the efficacy of the vaccine.
How successful does a vaccine have to be in one of these studies for it to be considered effective?
Recently, the FDA issued guidance about the development of COVID vaccines. The guidance that they issued to vaccine manufacturers— this is a document that is available to the general public —is that a vaccine would need to be at least 50% effective. This means that an individual who was vaccinated would be 50% less likely to get COVID disease—or whatever the particular endpoint is that’s measured in the trial—than individuals that weren’t vaccinated.
This is a reasonable goal for a number of reasons. Typically, the more severe a disease is, the better chance a vaccine has of preventing that disease. So, a vaccine that’s 50% effective against mild COVID disease—which might be the endpoint that’s measured in a clinical trial, or any evidence of COVID infection with any symptom, which is how a lot of trials are designed—might be more effective against severe disease.
When you have a disease that’s as prevalent as COVID—and if we think about what the U.S. has experienced in the past several months in terms of severe disease and death—even if we were only able to cut those numbers in half, that would be a major achievement.
How long would a vaccine be effective for? If you get 50% effectiveness or more, that’s good news. But if it’s only effective for a few months, that’s not such good news.
Time will tell for that. The short answer is that we don’t yet know. Even for the data we have on the vaccine so far in smaller studies, we haven’t yet had the opportunity to follow individuals for very long. The very first people who got the very first vaccine were immunized in March and it’s only July. So, we don’t know very much about the durability of the immune response in people.
Our hope would be [that protection would last] at least a year or more and then people might need boosters.
It’s also possible that a vaccine might not entirely protect against mild disease. So you might actually experience mild disease and then have a boost in your immune response and not suffer severe disease. From a public health perspective, that would be completely acceptable. If we turned a severe disease not into “ no disease ” but into mild disease, that would be a real victory.
Let’s talk about safety. What are they looking for in a 30,000-person study to figure out whether a vaccine is considered safe enough to use?
Every person who is enrolled in the trial will complete information about the kinds of acute symptoms that you might expect following an infection. People will need to provide information about swelling, redness, tenderness around the injection site, fever, and any other symptoms they might experience in the three to seven days following vaccination.
More long term, people will be looking to make sure that when COVID disease is experienced, there’s not any evidence of more severe disease with vaccination [which is known as disease enhancement].
There was a lot of discussion as these vaccines were being developed of a concern about disease enhancement. This is based on some animal models—not with SARS-CoV-2 but with other coronaviruses. We haven’t seen any evidence of enhanced disease thus far and there are a number of scientific reasons why we don’t think it should occur with these vaccines. But, of course, it’s something we would still watch for very carefully just as with any other safety signal.
How should we think about the possibility of adverse effects that might come up after the period of the vaccine trial?
There are a couple of things to mention about that, and one is that individuals with these trials will be followed for a year or longer. It may be that a vaccine is either approved for emergency use or licensed before all of that long-term follow up is completed. Nevertheless, companies will be obligated to complete that follow up and report those results back to the FDA.
It’s important to enroll older adults in these studies. All of these large efficacy trials will be stratified so there will be some younger adults and some older adults enrolled.
In addition, it’s very likely—and this would not just happen with COVID vaccines, but whenever the FDA licenses vaccines—that there is an obligation for post-licensure assessments. If a COVID vaccine is licensed, the companies will work with the FDA to determine exactly what kind of post-licensure safety assessments will need to be done.
COVID affects certain populations more than others—particularly older adults and people with chronic illnesses. What do these studies need [in order] to address the question of whether a vaccine will be protective for them?
I also think it will be important to enroll older adults across an age span. A 65-year-old is not the same as an 85-year-old. Also, a healthy older adult is not the same as a frail older adult who might be living in a care facility.
We’ll need some information about diverse elderly populations in order to think about how to allocate vaccines. There may also be other alternatives for older adults if they don’t respond well to vaccines. There’s a lot of work going on on development of monoclonal antibodies [ learn more about lab-produced antibodies in a recent podcast episode with Arturo Casadevall ] as an alternative for groups that don’t respond well to vaccines such as elderly, frail adults.
Let’s say there are 30,000 patients in the study and only a few hundred who are over 80 years old. What can you learn about a relatively small population of much older adults that would be informative about that group?
We may not have a large enough number of people in that subgroup to directly look at efficacy of a vaccine. But we might have enough to look at the immune response—the antibody response, for example, of a vaccine.
If, in the course of these trials, we can determine a correlative protection—for example, a laboratory measure like a level of a particular kind of antibody that correlates with protection against COVID disease—we can at least look at the immune responses in that subset of very elderly and decide if they are the same or different than the younger groups’. If they are the same, we may be more comfortable making the leap to say that it’s likely those individuals will also be protected by the vaccine.
So, we will learn more from a vaccine trial than just whether or not a vaccine works. We’re going to find out, perhaps, what predicts whether the vaccine works. That information might help us understand—without having to do a whole new trial—who might be protected by a vaccine.
It’s certainly a hope.
The majority of vaccines that we use today don’t have such a marker of protection and they’re very effective. Just because we can’t detect a marker doesn’t mean that a vaccine is not effective. It means that we’re not smart enough to figure out what that marker should be.
We really hope that there will be such a marker of protection because then we can link that—and, in FDA speak, that’s called “bridging”—to another population where we can just look at that marker of immunity rather than doing a whole efficacy trial.
How should we think about the need for racial and ethnic diversity in these clinical trials?
It’s critically important that we have racial and ethnic diversity.
We know that COVID causes increased rates of severe disease in Latinx and Black populations and in Native American populations. We will certainly want to be able to offer these COVID vaccines to these high-risk populations and encourage their use. But we need to know how well these vaccines work in these populations—if different vaccines work differently—so that we can offer the most effective vaccines.
It would not be an understatement to say that there can be a measure of distrust from some communities that have experienced discrimination from the health care system. How does that play into vaccine research?
It’s really important to engage those communities in a number of ways. One way is to engage local leaders early in the process. Lay leaders and leaders of faith communities can have focus groups to find out what their concerns are and how those can be allayed.
I think a very important issue that has been raised by some people who might potentially volunteer for some of these trials has to do with eventual access. People want to have some sense that if they participate in a trial, not only might they have access to the vaccine at the end of that trial, but their families and their communities would, too. Ensuring access among these high risk and vulnerable communities is really critical.
A clear policy decision to make sure that a vaccine is widely available without charge might actually help with the studies to prove whether or not that vaccine is safe and effective?
That’s absolutely the case. It’s great that you brought up the “without charge” piece, too, because a vaccine that’s made available but costs something to the individual may not be used. Particularly for people who don’t have health insurance or people who are undocumented. It has to be broadly and freely available.
Let’s talk about other specific populations. One of those is pregnant women. We know that they can certainly get COVID-19 and that there are some signs that they can have a more severe course. How do you think about the issue of pregnant women in vaccine studies?
I’ve done some work in this area —particularly with Ruth Faden and Carleigh Krubiner in the Berman Institute of Bioethics —specifically related to ensuring that pregnant women are considered and included in vaccine development and implementation for vaccines against epidemic and pandemic diseases.
When thinking about trials, there needs to be a justification for excluding pregnant women from trials rather than a justification for including them. The justification often is—and certainly is the case with these early COVID vaccines—that we don’t know enough yet about the vaccine or the vaccine platform or the safety of the vaccine to do a study in pregnant people.
With the mRNA vaccine, for example, [the type of vaccine being considered for COVID-19] we don’t currently have a licensed mRNA vaccine. It’s a new platform and we’re just learning about the safety of that platform so it wouldn’t have been appropriate to include pregnant women in the early stage trials.
But these 30,000-person studies are going to be really big studies. They will certainly enroll people of child-bearing potential. And even though there’s what we call an exclusion criterion—women are not supposed to be pregnant at the time they are enrolled, and usually women of child-bearing potential will take a pregnancy test prior to enrollment and immunization—we know from previous experience that it’s quite likely that some women will become pregnant in the months immediately following immunization. It happens quite frequently. So, it’s important for companies and the government to anticipate that this will be the case and to think about how they will systematically collect data from women who do become pregnant during these trials.
It’s not that the data needs to be interpreted cautiously—because pregnant women aren’t being formally randomized and we don’t have that kind of trial design—but there are things that could be learned and it’s important to think now about how to collect those data. It’s also important to think about how pregnant women could be directly included in both trials and deployment later down the road.
What about young children who are less likely to get severe disease? Would your approach to clinical trials be different?
Yes. I think we need to learn a bit more about the epidemiology in children. Fortunately, children don’t seem to suffer from acute COVID disease at the rates that adults do. But we need to learn more about that and we also need to learn from our trials in adults before we make decisions about how and whether children will be included in vaccine trials.
Once we have a vaccine that has made it through these various stages and we’re ready to start immunizing people outside of a pure clinical trial, how close are we to really getting the benefit of the vaccine? How does all the work it takes to develop a vaccine compare to what comes next?
The best vaccine in the world won’t work if it isn’t used.
Use has two parts to it: One is availability and access, and the other part is acceptance.
We need to think about what kind of infrastructure we should be planning now for what we’re going to need to deliver this vaccine. We’ll set priorities; certainly not everyone is going to get a vaccine all at once. But certainly, over time we will expect that all adults will receive the vaccine and perhaps children. So we’ll need to have systems in place that can deliver the vaccine. At the same time, we need to make sure that the vaccine is acceptable. We need to communicate the importance of vaccination to the public and address their concerns so that we can not only be able to deliver vaccines, but have those be accepted by the public.
So, there’s a lot of work to be done. But this isn’t science fiction: We are really on a path to a vaccine for a brand new infectious disease.
Yes. If you think back to the fact that in January, we barely knew what this virus was, and here we are, seven months later, embarking on efficacy trials, it’s really a remarkable accomplishment. We have a lot to do yet, but in the time that we’re assessing the efficacy of these vaccines and making sure that they can be delivered to the public, people really need to stay safe and to do all the things we’ve been encouraging them to do all along.
But we are well on our way to developing vaccines not only for people in the U.S., but for people all over the world.
Public Health On Call
This conversation is excerpted from the July 31 episode of Public Health On Call.
Subscribe to Podcast
Related Content

What to Know About COVID FLiRT Variants

Rotavirus the Leading Cause of Diarrheal Deaths Among Children Under 5, New Analysis Finds

Exploring Cancer’s Ancestral Links

Research Gaps Around Type 1 Diabetes

Outbreak Preparedness for All
Should COVID-19 vaccines be mandatory? Two experts discuss
Senior Research Fellow, Oxford Uehiro Centre for Practical Ethics, University of Oxford
NIHR Academic Clinical Fellow in Public Health Medicine, UCL
Disclosure statement
Alberto Giubilini receives funding from the Arts and Humanities Research Council/UK Research and Innovation (AHRC/UKRI) and has previously received funding from the Wellcome Trust.
Vageesh Jain is affiliated with Public Health England under an honorary contract as a speciality registrar.
University College London provides funding as a founding partner of The Conversation UK.
University of Oxford provides funding as a member of The Conversation UK.
View all partners

To be properly protective, COVID-19 vaccines need to be given to most people worldwide. Only through widespread vaccination will we reach herd immunity – where enough people are immune to stop the disease from spreading freely. To achieve this, some have suggested vaccines should be made compulsory , though the UK government has ruled this out . But with high rates of COVID-19 vaccine hesitancy in the UK and elsewhere , is this the right call? Here, two experts to make the case for and against mandatory COVID-19 vaccines.
Alberto Giubilini, Senior Research Fellow, Oxford Uehiro Centre for Practical Ethics, University of Oxford
COVID-19 vaccination should be mandatory – at least for certain groups. This means there would be penalties for failure to vaccinate, such as fines or limitations on freedom of movement.
The less burdensome it is for an individual to do something that prevents harm to others, and the greater the harm prevented, the stronger the ethical reason for mandating it.
Being vaccinated dramatically reduces the risk of seriously harming or killing others. Vaccines such as the Pfizer , AstraZeneca or Moderna ones with 90-95% efficacy at preventing people from getting sick are also likely to be effective at stopping the virus from spreading, though possibly to a lower degree. Such benefits would come at a very minimal cost to individuals.
Lockdown is mandatory. Exactly like mandatory vaccination, it protects vulnerable people from COVID-19. But, as I have argued in detail elsewhere, unlike mandatory vaccination, lockdown entails very large individual and societal costs. It is inconsistent to accept mandatory lockdown but reject mandatory vaccination. The latter can achieve a much greater good at a much smaller cost.
Also, mandatory vaccination ensures that the risks and burdens of reaching herd immunity are distributed evenly across the population. Because herd immunity benefits society collectively, it’s only fair that the responsibility of reaching it is shared evenly among society’s individual members.
Of course, we might achieve herd immunity through less restrictive alternatives than making vaccination mandatory – such as information campaigns to encourage people to be vaccinated. But even if we reach herd immunity, the higher the uptake of vaccines, the lower the risk of falling below the herd immunity threshold at a later time. We should do everything we can to prevent that emergency from happening – especially when the cost of doing so is low.
Fostering trust and driving uptake by making people more informed is a nice narrative, but it’s risky. Merely giving people information on vaccines does not always result in increased willingness to vaccinate and might actually lower confidence in vaccines. On the other hand, we’ve seen mandatory vaccination policies in Italy recently successfully boost vaccine uptake for other diseases.
Mandatory seatbelt policies have proven very successful in reducing deaths from car accidents, and are now widely endorsed despite the (very small) risks that seatbelts entail. We should see vaccines as seatbelts against COVID-19. In fact, as very special seatbelts, which protect ourselves and protect others.

Vageesh Jain, NIHR Academic Clinical Fellow in Public Health Medicine, UCL
Mandatory vaccination does not automatically increase vaccine uptake. An EU-funded project on epidemics and pandemics, which took place several years before COVID-19, found no evidence to support this notion. Looking at Baltic and Scandinavian countries, the project’s report noted that countries “where a vaccination is mandatory do not usually reach better coverage than neighbour or similar countries where there is no legal obligation”.
According to the Nuffield Council of Bioethics, mandatory vaccination may be justified for highly contagious and serious diseases. But although contagious, Public Health England does not classify COVID-19 as a high-consequence infectious disease due to its relatively low case fatality rate.
COVID-19 severity is strongly linked with age, dividing individual perceptions of vulnerability within populations. The death rate is estimated at 7.8% in people aged over 80, but at just 0.0016% in children aged nine and under. In a liberal democracy, forcing the vaccination of millions of young and healthy citizens who perceive themselves to be at an acceptably low risk from COVID-19 will be ethically disputed and is politically risky.
Public apprehensions for a novel vaccine produced at breakneck speed are wholly legitimate. A UK survey of 70,000 people found 49% were “very likely” to get a COVID-19 vaccine once available. US surveys are similar . This is not because the majority are anti-vaxxers.
Despite promising headlines, the trials and pharmaceutical processes surrounding them have not yet been scrutinised. With the first trials only beginning in April , there is limited data on long-term safety and efficacy. We don’t know how long immunity lasts for. None of the trials were designed to tell us if the vaccine prevents serious disease or virus transmission.
To disregard these ubiquitous concerns would be counterproductive. As a tool for combating anti-vaxxers – estimated at around 58 million globally and making up a small minority of those not getting vaccinated – mandatory vaccines are also problematic. The forces driving scientific and political populism are the same . Anti-vaxxers do not trust experts, industry and especially not the government. A government mandate will not just be met with unshakeable defiance, but will also be weaponised to recruit others to the anti-vaxxer cause.
In the early 1990s, polio was endemic in India , with between 500 and 1,000 children getting paralysed daily. By 2011, the virus was eliminated. This was not achieved through legislation. It was down to a consolidated effort to involve communities, target high-need groups, understand concerns, inform, educate, remove barriers, invest in local delivery systems and link with political and religious leaders.
Mandatory vaccination is rarely justified. The successful roll-out of novel COVID-19 vaccines will require time, communication and trust. We have come too far, too fast, to lose our nerve now.
- Mandatory vaccination
- Coronavirus
- Vaccine hesitancy
- Coronavirus insights

Compliance Lead

Lecturer / Senior Lecturer - Marketing

Assistant Editor - 1 year cadetship

Executive Dean, Faculty of Health

Lecturer/Senior Lecturer, Earth System Science (School of Science)
Persuasive Essay Guide
Persuasive Essay About Covid19
How to Write a Persuasive Essay About Covid19 | Examples & Tips
11 min read

People also read
A Comprehensive Guide to Writing an Effective Persuasive Essay
200+ Persuasive Essay Topics to Help You Out
Learn How to Create a Persuasive Essay Outline
30+ Free Persuasive Essay Examples To Get You Started
Read Excellent Examples of Persuasive Essay About Gun Control
Crafting a Convincing Persuasive Essay About Abortion
Learn to Write Persuasive Essay About Business With Examples and Tips
Check Out 12 Persuasive Essay About Online Education Examples
Persuasive Essay About Smoking - Making a Powerful Argument with Examples
Are you looking to write a persuasive essay about the Covid-19 pandemic?
Writing a compelling and informative essay about this global crisis can be challenging. It requires researching the latest information, understanding the facts, and presenting your argument persuasively.
But don’t worry! with some guidance from experts, you’ll be able to write an effective and persuasive essay about Covid-19.
In this blog post, we’ll outline the basics of writing a persuasive essay . We’ll provide clear examples, helpful tips, and essential information for crafting your own persuasive piece on Covid-19.
Read on to get started on your essay.
- 1. Steps to Write a Persuasive Essay About Covid-19
- 2. Examples of Persuasive Essay About Covid19
- 3. Examples of Persuasive Essay About Covid-19 Vaccine
- 4. Examples of Persuasive Essay About Covid-19 Integration
- 5. Examples of Argumentative Essay About Covid 19
- 6. Examples of Persuasive Speeches About Covid-19
- 7. Tips to Write a Persuasive Essay About Covid-19
- 8. Common Topics for a Persuasive Essay on COVID-19
Steps to Write a Persuasive Essay About Covid-19
Here are the steps to help you write a persuasive essay on this topic, along with an example essay:
Step 1: Choose a Specific Thesis Statement
Your thesis statement should clearly state your position on a specific aspect of COVID-19. It should be debatable and clear. For example:
Step 2: Research and Gather Information
Collect reliable and up-to-date information from reputable sources to support your thesis statement. This may include statistics, expert opinions, and scientific studies. For instance:
- COVID-19 vaccination effectiveness data
- Information on vaccine mandates in different countries
- Expert statements from health organizations like the WHO or CDC
Step 3: Outline Your Essay
Create a clear and organized outline to structure your essay. A persuasive essay typically follows this structure:
- Introduction
- Background Information
- Body Paragraphs (with supporting evidence)
- Counterarguments (addressing opposing views)
Step 4: Write the Introduction
In the introduction, grab your reader's attention and present your thesis statement. For example:
Step 5: Provide Background Information
Offer context and background information to help your readers understand the issue better. For instance:
Step 6: Develop Body Paragraphs
Each body paragraph should present a single point or piece of evidence that supports your thesis statement. Use clear topic sentences, evidence, and analysis. Here's an example:
Step 7: Address Counterarguments
Acknowledge opposing viewpoints and refute them with strong counterarguments. This demonstrates that you've considered different perspectives. For example:
Step 8: Write the Conclusion
Summarize your main points and restate your thesis statement in the conclusion. End with a strong call to action or thought-provoking statement. For instance:
Step 9: Revise and Proofread
Edit your essay for clarity, coherence, grammar, and spelling errors. Ensure that your argument flows logically.
Step 10: Cite Your Sources
Include proper citations and a bibliography page to give credit to your sources.
Remember to adjust your approach and arguments based on your target audience and the specific angle you want to take in your persuasive essay about COVID-19.

Paper Due? Why Suffer? That's our Job!
Examples of Persuasive Essay About Covid19
When writing a persuasive essay about the Covid-19 pandemic, it’s important to consider how you want to present your argument. To help you get started, here are some example essays for you to read:
Check out some more PDF examples below:
Persuasive Essay About Covid-19 Pandemic
Sample Of Persuasive Essay About Covid-19
Persuasive Essay About Covid-19 In The Philippines - Example
If you're in search of a compelling persuasive essay on business, don't miss out on our “ persuasive essay about business ” blog!
Examples of Persuasive Essay About Covid-19 Vaccine
Covid19 vaccines are one of the ways to prevent the spread of Covid-19, but they have been a source of controversy. Different sides argue about the benefits or dangers of the new vaccines. Whatever your point of view is, writing a persuasive essay about it is a good way of organizing your thoughts and persuading others.
A persuasive essay about the Covid-19 vaccine could consider the benefits of getting vaccinated as well as the potential side effects.
Below are some examples of persuasive essays on getting vaccinated for Covid-19.
Covid19 Vaccine Persuasive Essay
Persuasive Essay on Covid Vaccines
Interested in thought-provoking discussions on abortion? Read our persuasive essay about abortion blog to eplore arguments!
Examples of Persuasive Essay About Covid-19 Integration
Covid19 has drastically changed the way people interact in schools, markets, and workplaces. In short, it has affected all aspects of life. However, people have started to learn to live with Covid19.
Writing a persuasive essay about it shouldn't be stressful. Read the sample essay below to get idea for your own essay about Covid19 integration.
Persuasive Essay About Working From Home During Covid19
Searching for the topic of Online Education? Our persuasive essay about online education is a must-read.
Examples of Argumentative Essay About Covid 19
Covid-19 has been an ever-evolving issue, with new developments and discoveries being made on a daily basis.
Writing an argumentative essay about such an issue is both interesting and challenging. It allows you to evaluate different aspects of the pandemic, as well as consider potential solutions.
Here are some examples of argumentative essays on Covid19.
Argumentative Essay About Covid19 Sample
Argumentative Essay About Covid19 With Introduction Body and Conclusion
Looking for a persuasive take on the topic of smoking? You'll find it all related arguments in out Persuasive Essay About Smoking blog!
Examples of Persuasive Speeches About Covid-19
Do you need to prepare a speech about Covid19 and need examples? We have them for you!
Persuasive speeches about Covid-19 can provide the audience with valuable insights on how to best handle the pandemic. They can be used to advocate for specific changes in policies or simply raise awareness about the virus.
Check out some examples of persuasive speeches on Covid-19:
Persuasive Speech About Covid-19 Example
Persuasive Speech About Vaccine For Covid-19
You can also read persuasive essay examples on other topics to master your persuasive techniques!
Tips to Write a Persuasive Essay About Covid-19
Writing a persuasive essay about COVID-19 requires a thoughtful approach to present your arguments effectively.
Here are some tips to help you craft a compelling persuasive essay on this topic:
Choose a Specific Angle
Start by narrowing down your focus. COVID-19 is a broad topic, so selecting a specific aspect or issue related to it will make your essay more persuasive and manageable. For example, you could focus on vaccination, public health measures, the economic impact, or misinformation.
Provide Credible Sources
Support your arguments with credible sources such as scientific studies, government reports, and reputable news outlets. Reliable sources enhance the credibility of your essay.
Use Persuasive Language
Employ persuasive techniques, such as ethos (establishing credibility), pathos (appealing to emotions), and logos (using logic and evidence). Use vivid examples and anecdotes to make your points relatable.
Organize Your Essay
Structure your essay involves creating a persuasive essay outline and establishing a logical flow from one point to the next. Each paragraph should focus on a single point, and transitions between paragraphs should be smooth and logical.
Emphasize Benefits
Highlight the benefits of your proposed actions or viewpoints. Explain how your suggestions can improve public health, safety, or well-being. Make it clear why your audience should support your position.
Use Visuals -H3
Incorporate graphs, charts, and statistics when applicable. Visual aids can reinforce your arguments and make complex data more accessible to your readers.
Call to Action
End your essay with a strong call to action. Encourage your readers to take a specific step or consider your viewpoint. Make it clear what you want them to do or think after reading your essay.
Revise and Edit
Proofread your essay for grammar, spelling, and clarity. Make sure your arguments are well-structured and that your writing flows smoothly.
Seek Feedback
Have someone else read your essay to get feedback. They may offer valuable insights and help you identify areas where your persuasive techniques can be improved.
Tough Essay Due? Hire Tough Writers!
Common Topics for a Persuasive Essay on COVID-19
Here are some persuasive essay topics on COVID-19:
- The Importance of Vaccination Mandates for COVID-19 Control
- Balancing Public Health and Personal Freedom During a Pandemic
- The Economic Impact of Lockdowns vs. Public Health Benefits
- The Role of Misinformation in Fueling Vaccine Hesitancy
- Remote Learning vs. In-Person Education: What's Best for Students?
- The Ethics of Vaccine Distribution: Prioritizing Vulnerable Populations
- The Mental Health Crisis Amidst the COVID-19 Pandemic
- The Long-Term Effects of COVID-19 on Healthcare Systems
- Global Cooperation vs. Vaccine Nationalism in Fighting the Pandemic
- The Future of Telemedicine: Expanding Healthcare Access Post-COVID-19
In search of more inspiring topics for your next persuasive essay? Our persuasive essay topics blog has plenty of ideas!
To sum it up,
You have read good sample essays and got some helpful tips. You now have the tools you needed to write a persuasive essay about Covid-19. So don't let the doubts stop you, start writing!
If you need professional writing help, don't worry! We've got that for you as well.
MyPerfectWords.com is a professional persuasive essay writing service that can help you craft an excellent persuasive essay on Covid-19. Our experienced essay writer will create a well-structured, insightful paper in no time!
So don't hesitate and place your ' write my essay online ' request today!
Frequently Asked Questions
Are there any ethical considerations when writing a persuasive essay about covid-19.
Yes, there are ethical considerations when writing a persuasive essay about COVID-19. It's essential to ensure the information is accurate, not contribute to misinformation, and be sensitive to the pandemic's impact on individuals and communities. Additionally, respecting diverse viewpoints and emphasizing public health benefits can promote ethical communication.
What impact does COVID-19 have on society?
The impact of COVID-19 on society is far-reaching. It has led to job and economic losses, an increase in stress and mental health disorders, and changes in education systems. It has also had a negative effect on social interactions, as people have been asked to limit their contact with others.
Write Essay Within 60 Seconds!

Caleb S. has been providing writing services for over five years and has a Masters degree from Oxford University. He is an expert in his craft and takes great pride in helping students achieve their academic goals. Caleb is a dedicated professional who always puts his clients first.

Paper Due? Why Suffer? That’s our Job!
Keep reading

An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
Preview improvements coming to the PMC website in October 2024. Learn More or Try it out now .
- Advanced Search
- Journal List
- Springer Nature - PMC COVID-19 Collection

An Introduction to COVID-19
Simon james fong.
4 Department of Computer and Information Science, University of Macau, Taipa, Macau, China
Nilanjan Dey
5 Department of Information Technology, Techno International New Town, Kolkata, West Bengal India
Jyotismita Chaki
6 School of Information Technology and Engineering, Vellore Institute of Technology, Vellore, Tamil Nadu India
A novel coronavirus (CoV) named ‘2019-nCoV’ or ‘2019 novel coronavirus’ or ‘COVID-19’ by the World Health Organization (WHO) is in charge of the current outbreak of pneumonia that began at the beginning of December 2019 near in Wuhan City, Hubei Province, China [1–4]. COVID-19 is a pathogenic virus. From the phylogenetic analysis carried out with obtainable full genome sequences, bats occur to be the COVID-19 virus reservoir, but the intermediate host(s) has not been detected till now.

A Brief History of the Coronavirus Outbreak
A novel coronavirus (CoV) named ‘2019-nCoV’ or ‘2019 novel coronavirus’ or ‘COVID-19’ by the World Health Organization (WHO) is in charge of the current outbreak of pneumonia that began at the beginning of December 2019 near in Wuhan City, Hubei Province, China [ 1 – 4 ]. COVID-19 is a pathogenic virus. From the phylogenetic analysis carried out with obtainable full genome sequences, bats occur to be the COVID-19 virus reservoir, but the intermediate host(s) has not been detected till now. Though three major areas of work already are ongoing in China to advise our awareness of the pathogenic origin of the outbreak. These include early inquiries of cases with symptoms occurring near in Wuhan during December 2019, ecological sampling from the Huanan Wholesale Seafood Market as well as other area markets, and the collection of detailed reports of the point of origin and type of wildlife species marketed on the Huanan market and the destination of those animals after the market has been closed [ 5 – 8 ].
Coronaviruses mostly cause gastrointestinal and respiratory tract infections and are inherently categorized into four major types: Gammacoronavirus, Deltacoronavirus, Betacoronavirus and Alphacoronavirus [ 9 – 11 ]. The first two types mainly infect birds, while the last two mostly infect mammals. Six types of human CoVs have been formally recognized. These comprise HCoVHKU1, HCoV-OC43, Middle East Respiratory Syndrome coronavirus (MERS-CoV), Severe Acute Respiratory Syndrome coronavirus (SARS-CoV) which is the type of the Betacoronavirus, HCoV229E and HCoV-NL63, which are the member of the Alphacoronavirus. Coronaviruses did not draw global concern until the 2003 SARS pandemic [ 12 – 14 ], preceded by the 2012 MERS [ 15 – 17 ] and most recently by the COVID-19 outbreaks. SARS-CoV and MERS-CoV are known to be extremely pathogenic and spread from bats to palm civets or dromedary camels and eventually to humans.
COVID-19 is spread by dust particles and fomites while close unsafe touch between the infector and the infected individual. Airborne distribution has not been recorded for COVID-19 and is not known to be a significant transmission engine based on empirical evidence; although it can be imagined if such aerosol-generating practices are carried out in medical facilities. Faecal spreading has been seen in certain patients, and the active virus has been reported in a small number of clinical studies [ 18 – 20 ]. Furthermore, the faecal-oral route does not seem to be a COVID-19 transmission engine; its function and relevance for COVID-19 need to be identified.
For about 18,738,58 laboratory-confirmed cases recorded as of 2nd week of April 2020, the maximum number of cases (77.8%) was between 30 and 69 years of age. Among the recorded cases, 21.6% are farmers or employees by profession, 51.1% are male and 77.0% are Hubei.
However, there are already many concerns regarding the latest coronavirus. Although it seems to be transferred to humans by animals, it is important to recognize individual animals and other sources, the path of transmission, the incubation cycle, and the features of the susceptible community and the survival rate. Nonetheless, very little clinical knowledge on COVID-19 disease is currently accessible and details on age span, the animal origin of the virus, incubation time, outbreak curve, viral spectroscopy, dissemination pathogenesis, autopsy observations, and any clinical responses to antivirals are lacking among the serious cases.
How Different and Deadly COVID-19 is Compared to Plagues in History
COVID-19 has reached to more than 150 nations, including China, and has caused WHO to call the disease a worldwide pandemic. By the time of 2nd week of April 2020, this COVID-19 cases exceeded 18,738,58, although more than 1,160,45 deaths were recorded worldwide and United States of America became the global epicentre of coronavirus. More than one-third of the COVID-19 instances are outside of China. Past pandemics that have existed in the past decade or so, like bird flu, swine flu, and SARS, it is hard to find out the comparison between those pandemics and this coronavirus. Following is a guide to compare coronavirus with such diseases and recent pandemics that have reformed the world community.
Coronavirus Versus Seasonal Influenza
Influenza, or seasonal flu, occurs globally every year–usually between December and February. It is impossible to determine the number of reports per year because it is not a reportable infection (so no need to be recorded to municipality), so often patients with minor symptoms do not go to a physician. Recent figures placed the Rate of Case Fatality at 0.1% [ 21 – 23 ].
There are approximately 3–5 million reports of serious influenza a year, and about 250,000–500,000 deaths globally. In most developed nations, the majority of deaths arise in persons over 65 years of age. Moreover, it is unsafe for pregnant mothers, children under 59 months of age and individuals with serious illnesses.
The annual vaccination eliminates infection and severe risks in most developing countries but is nevertheless a recognized yet uncomfortable aspect of the season.
In contrast to the seasonal influenza, coronavirus is not so common, has led to fewer cases till now, has a higher rate of case fatality and has no antidote.
Coronavirus Versus Bird Flu (H5N1 and H7N9)
Several cases of bird flu have existed over the years, with the most severe in 2013 and 2016. This is usually from two separate strains—H5N1 and H7N9 [ 24 – 26 ].
The H7N9 outbreak in 2016 accounted for one-third of all confirmed human cases but remained confined relative to both coronavirus and other pandemics/outbreak cases. After the first outbreak, about 1,233 laboratory-confirmed reports of bird flu have occurred. The disease has a Rate of Case Fatality of 20–40%.
Although the percentage is very high, the blowout from individual to individual is restricted, which, in effect, has minimized the number of related deaths. It is also impossible to monitor as birds do not necessarily expire from sickness.
In contrast to the bird flu, coronavirus becomes more common, travels more quickly through human to human interaction, has an inferior cardiothoracic ratio, resulting in further total fatalities and spread from the initial source.
Coronavirus Versus Ebola Epidemic
The Ebola epidemic of 2013 was primarily centred in 10 nations, including Sierra Leone, Guinea and Liberia have the greatest effects, but the extremely high Case Fatality Rate of 40% has created this as a significant problem for health professionals nationwide [ 27 – 29 ].
Around 2013 and 2016, there were about 28,646 suspicious incidents and about 11,323 fatalities, although these are expected to be overlooked. Those who survived from the original epidemic may still become sick months or even years later, because the infection may stay inactive for prolonged periods. Thankfully, a vaccination was launched in December 2016 and is perceived to be effective.
In contrast to the Ebola, coronavirus is more common globally, has caused in fewer fatalities, has a lesser case fatality rate, has no reported problems during treatment and after recovery, does not have an appropriate vaccination.
Coronavirus Versus Camel Flu (MERS)
Camel flu is a misnomer–though camels have MERS antibodies and may have been included in the transmission of the disease; it was originally transmitted to humans through bats [ 30 – 32 ]. Like Ebola, it infected only a limited number of nations, i.e. about 27, but about 858 fatalities from about 2,494 laboratory-confirmed reports suggested that it was a significant threat if no steps were taken in place to control it.
In contrast to the camel flu, coronavirus is more common globally, has occurred more fatalities, has a lesser case fatality rate, and spreads more easily among humans.
Coronavirus Versus Swine Flu (H1N1)
Swine flu is the same form of influenza that wiped 1.7% of the world population in 1918. This was deemed a pandemic again in June 2009 an approximately-21% of the global population infected by this [ 33 – 35 ].
Thankfully, the case fatality rate is substantially lower than in the last pandemic, with 0.1%–0.5% of events ending in death. About 18,500 of these fatalities have been laboratory-confirmed, but statistics range as high as 151,700–575,400 worldwide. 50–80% of severe occurrences have been reported in individuals with chronic illnesses like asthma, obesity, cardiovascular diseases and diabetes.
In contrast to the swine flu, coronavirus is not so common, has caused fewer fatalities, has more case fatality rate, has a longer growth time and less impact on young people.
Coronavirus Versus Severe Acute Respiratory Syndrome (SARS)
SARS was discovered in 2003 as it spread from bats to humans resulted in about 774 fatalities. By May there were eventually about 8,100 reports across 17 countries, with a 15% case fatality rate. The number is estimated to be closer to 9.6% as confirmed cases are counted, with 0.9% cardiothoracic ratio for people aged 20–29, rising to 28% for people aged 70–79. Similar to coronavirus, SARS had bad results for males than females in all age categories [ 36 – 38 ].
Coronavirus is more common relative to SARS, which ended in more overall fatalities, lower case fatality rate, the even higher case fatality rate in older ages, and poorer results for males.
Coronavirus Versus Hong Kong Flu (H3N2)
The Hong Kong flu pandemic erupted on 13 July 1968, with 1–4 million deaths globally by 1969. It was one of the greatest flu pandemics of the twentieth century, but thankfully the case fatality rate was smaller than the epidemic of 1918, resulting in fewer fatalities overall. That may have been attributed to the fact that citizens had generated immunity owing to a previous epidemic in 1957 and to better medical treatment [ 39 ].
In contrast to the Hong Kong flu, coronavirus is not so common, has caused in fewer fatalities and has a higher case fatality rate.
Coronavirus Versus Spanish Flu (H1N1)
The 1918 Spanish flu pandemic was one of the greatest occurrences of recorded history. During the first year of the pandemic, lifespan in the US dropped by 12 years, with more civilians killed than HIV/AIDS in 24 h [ 40 – 42 ].
Regardless of the name, the epidemic did not necessarily arise in Spain; wartime censors in Germany, the United States, the United Kingdom and France blocked news of the disease, but Spain did not, creating the misleading perception that more cases and fatalities had occurred relative to its neighbours
This strain of H1N1 eventually affected more than 500 million men, or 27% of the world’s population at the moment, and had deaths of between 40 and 50 million. At the end of 1920, 1.7% of the world’s people had expired of this illness, including an exceptionally high death rate for young adults aged between 20 and 40 years.
In contrast to the Spanish flu, coronavirus is not so common, has caused in fewer fatalities, has a higher case fatality rate, is more harmful to older ages and is less risky for individuals aged 20–40 years.
Coronavirus Versus Common Cold (Typically Rhinovirus)
Common cold is the most common illness impacting people—Typically, a person suffers from 2–3 colds each year and the average kid will catch 6–8 during the similar time span. Although there are more than 200 cold-associated virus types, infections are uncommon and fatalities are very rare and typically arise mainly in extremely old, extremely young or immunosuppressed cases [ 43 , 44 ].
In contrast to the common cold, coronavirus is not so prevalent, causes more fatalities, has more case fatality rate, is less infectious and is less likely to impact small children.
Reviews of Online Portals and Social Media for Epidemic Information Dissemination
As COVID-19 started to propagate across the globe, the outbreak contributed to a significant change in the broad technology platforms. Where they once declined to engage in the affairs of their systems, except though the possible danger to public safety became obvious, the advent of a novel coronavirus placed them in a different interventionist way of thought. Big tech firms and social media are taking concrete steps to guide users to relevant, credible details on the virus [ 45 – 48 ]. And some of the measures they’re doing proactively. Below are a few of them.
Facebook started adding a box in the news feed that led users to the Centers for Disease Control website regarding COVID-19. It reflects a significant departure from the company’s normal strategy of placing items in the News Feed. The purpose of the update, after all, is personalization—Facebook tries to give the posts you’re going to care about, whether it is because you’re connected with a person or like a post. In the virus package, Facebook has placed a remarkable algorithmic thumb on the scale, potentially pushing millions of people to accurate, authenticated knowledge from a reputable source.
Similar initiatives have been adopted by Twitter. Searching for COVID-19 will carry you to a page highlighting the latest reports from public health groups and credible national news outlets. The search also allows for common misspellings. Twitter has stated that although Russian-style initiatives to cause discontent by large-scale intelligence operations have not yet been observed, a zero-tolerance approach to network exploitation and all other attempts to exploit their service at this crucial juncture will be expected. The problem has the attention of the organization. It also offers promotional support to public service agencies and other non-profit groups.
Google has made a step in making it better for those who choose to operate or research from home, offering specialized streaming services to all paying G Suite customers. Google also confirmed that free access to ‘advanced’ Hangouts Meet apps will be rolled out to both G Suite and G Suite for Education clients worldwide through 1st July. It ensures that companies can hold meetings of up to 250 people, broadcast live to up to about 100,000 users within a single network, and archive and export meetings to Google Drive. Usually, Google pays an additional $13 per person per month for these services in comparison to G Suite’s ‘enterprise’ membership, which adds up to a total of about $25 per client each month.
Microsoft took a similar move, introducing the software ‘Chat Device’ to help public health and protection in the coronavirus epidemic, which enables collaborative collaboration via video and text messaging. There’s an aspect of self-interest in this. Tech firms are offering out their goods free of charge during periods of emergency for the same purpose as newspapers are reducing their paywalls: it’s nice to draw more paying consumers.
Pinterest, which has introduced much of the anti-misinformation strategies that Facebook and Twitter are already embracing, is now restricting the search results for ‘coronavirus’, ‘COVID-19’ and similar words for ‘internationally recognized health organizations’.
Google-owned YouTube, traditionally the most conspiratorial website, has recently introduced a connection to the World Health Organization virus epidemic page to the top of the search results. In the early days of the epidemic, BuzzFeed found famous coronavirus conspiratorial videos on YouTube—especially in India, where one ‘explain’ with a false interpretation of the sources of the disease racketeered 13 million views before YouTube deleted it. Yet in the United States, conspiratorial posts regarding the illness have failed to gain only 1 million views.
That’s not to suggest that misinformation doesn’t propagate on digital platforms—just as it travels through the broader Internet, even though interaction with friends and relatives. When there’s a site that appears to be under-performing in the global epidemic, it’s Facebook-owned WhatsApp, where the Washington Post reported ‘a torrent of disinformation’ in places like Nigeria, Indonesia, Peru, Pakistan and Ireland. Given the encrypted existence of the app, it is difficult to measure the severity of the problem. Misinformation is also spread in WhatsApp communities, where participation is restricted to about 250 individuals. Knowledge of one category may be readily exchanged with another; however, there is a considerable amount of complexity of rotating several groups to peddle affected healing remedies or propagate false rumours.
Preventative Measures and Policies Enforced by the World Health Organization (WHO) and Different Countries
Coronavirus is already an ongoing epidemic, so it is necessary to take precautions to minimize both the risk of being sick and the transmission of the disease.
WHO Advice [ 49 ]
- Wash hands regularly with alcohol-based hand wash or soap and water.
- Preserve contact space (at least 1 m/3 feet between you and someone who sneezes or coughs).
- Don’t touch your nose, head and ears.
- Cover your nose and mouth as you sneeze or cough, preferably with your bent elbow or tissue.
- Try to find early medical attention if you have fatigue, cough and trouble breathing.
- Take preventive precautions if you are in or have recently go to places where coronavirus spreads.
The first person believed to have become sick because of the latest virus was near in Wuhan on 1 December 2019. A formal warning of the epidemic was released on 31 December. The World Health Organization was informed of the epidemic on the same day. Through 7 January, the Chinese Government addressed the avoidance and regulation of COVID-19. A curfew was declared on 23 January to prohibit flying in and out of Wuhan. Private usage of cars has been banned in the region. Chinese New Year (25 January) festivities have been cancelled in many locations [ 50 ].
On 26 January, the Communist Party and the Government adopted more steps to contain the COVID-19 epidemic, including safety warnings for travellers and improvements to national holidays. The leading party has agreed to prolong the Spring Festival holiday to control the outbreak. Universities and schools across the world have already been locked down. Many steps have been taken by the Hong Kong and Macau governments, in particular concerning schools and colleges. Remote job initiatives have been placed in effect in many regions of China. Several immigration limits have been enforced.
Certain counties and cities outside Hubei also implemented travel limits. Public transit has been changed and museums in China have been partially removed. Some experts challenged the quality of the number of cases announced by the Chinese Government, which constantly modified the way coronavirus cases were recorded.
Italy, a member state of the European Union and a popular tourist attraction, entered the list of coronavirus-affected nations on 30 January, when two positive cases in COVID-19 were identified among Chinese tourists. Italy has the largest number of coronavirus infections both in Europe and outside of China [ 51 ].
Infections, originally limited to northern Italy, gradually spread to all other areas. Many other nations in Asia, Europe and the Americas have tracked their local cases to Italy. Several Italian travellers were even infected with coronavirus-positive in foreign nations.
Late in Italy, the most impacted coronavirus cities and counties are Lombardia, accompanied by Veneto, Emilia-Romagna, Marche and Piedmonte. Milan, the second most populated city in Italy, is situated in Lombardy. Other regions in Italy with coronavirus comprised Campania, Toscana, Liguria, Lazio, Sicilia, Friuli Venezia Giulia, Umbria, Puglia, Trento, Abruzzo, Calabria, Molise, Valle d’Aosta, Sardegna, Bolzano and Basilicata.
Italy ranks 19th of the top 30 nations getting high-risk coronavirus airline passengers in China, as per WorldPop’s provisional study of the spread of COVID-19.
The Italian State has taken steps like the inspection and termination of large cultural activities during the early days of the coronavirus epidemic and has gradually declared the closing of educational establishments and airport hygiene/disinfection initiatives.
The Italian National Institute of Health suggested social distancing and agreed that the broader community of the country’s elderly is a problem. In the meantime, several other nations, including the US, have recommended that travel to Italy should be avoided temporarily, unless necessary.
The Italian government has declared the closing (quarantine) of the impacted areas in the northern region of the nation so as not to spread to the rest of the world. Italy has declared the immediate suspension of all to-and-fro air travel with China following coronavirus discovery by a Chinese tourist to Italy. Italian airlines, like Ryan Air, have begun introducing protective steps and have begun calling for the declaration forms to be submitted by passengers flying to Poland, Slovakia and Lithuania.
The Italian government first declined to permit fans to compete in sporting activities until early April to prevent the potential transmission of coronavirus. The step ensured players of health and stopped event cancellations because of coronavirus fears. Two days of the declaration, the government cancelled all athletic activities owing to the emergence of the outbreak asking for an emergency. Sports activities in Veneto, Lombardy and Emilia-Romagna, which recorded coronavirus-positive infections, were confirmed to be temporarily suspended. Schools and colleges in Italy have also been forced to shut down.
Iran announced the first recorded cases of SARS-CoV-2 infection on 19 February when, as per the Medical Education and Ministry of Health, two persons died later that day. The Ministry of Islamic Culture and Guidance has declared the cancellation of all concerts and other cultural activities for one week. The Medical Education and Ministry of Health has also declared the closing of universities, higher education colleges and schools in many cities and regions. The Department of Sports and Culture has taken action to suspend athletic activities, including football matches [ 52 ].
On 2 March 2020, the government revealed plans to train about 300,000 troops and volunteers to fight the outbreak of the epidemic, and also send robots and water cannons to clean the cities. The State also developed an initiative and a webpage to counter the epidemic. On 9 March 2020, nearly 70,000 inmates were immediately released from jail owing to the epidemic, presumably to prevent the further dissemination of the disease inside jails. The Revolutionary Guards declared a campaign on 13 March 2020 to clear highways, stores and public areas in Iran. President Hassan Rouhani stated on 26 February 2020 that there were no arrangements to quarantine areas impacted by the epidemic and only persons should be quarantined. The temples of Shia in Qom stayed open to pilgrims.
South Korea
On 20 January, South Korea announced its first occurrence. There was a large rise in cases on 20 February, possibly due to the meeting in Daegu of a progressive faith community recognized as the Shincheonji Church of Christ. Any citizens believed that the hospital was propagating the disease. As of 22 February, 1,261 of the 9,336 members of the church registered symptoms. A petition was distributed calling for the abolition of the church. More than 2,000 verified cases were registered on 28 February, increasing to 3,150 on 29 February [ 53 ].
Several educational establishments have been partially closing down, including hundreds of kindergartens in Daegu and many primary schools in Seoul. As of 18 February, several South Korean colleges had confirmed intentions to delay the launch of the spring semester. That included 155 institutions deciding to postpone the start of the semester by two weeks until 16 March, and 22 institutions deciding to delay the start of the semester by one week until 9 March. Also, on 23 February 2020, all primary schools, kindergartens, middle schools and secondary schools were declared to postpone the start of the semester from 2 March to 9 March.
South Korea’s economy is expected to expand by 1.9%, down from 2.1%. The State has given 136.7 billion won funding to local councils. The State has also coordinated the purchase of masks and other sanitary supplies. Entertainment Company SM Entertainment is confirmed to have contributed five hundred million won in attempts to fight the disease.
In the kpop industry, the widespread dissemination of coronavirus within South Korea has contributed to the cancellation or postponement of concerts and other programmes for kpop activities inside and outside South Korea. For instance, circumstances such as the cancellation of the remaining Asian dates and the European leg for the Seventeen’s Ode To You Tour on 9 February 2020 and the cancellation of all Seoul dates for the BTS Soul Tour Map. As of 15 March, a maximum of 136 countries and regions provided entry restrictions and/or expired visas for passengers from South Korea.
The overall reported cases of coronavirus rose significantly in France on 12 March. The areas with reported cases include Paris, Amiens, Bordeaux and Eastern Haute-Savoie. The first coronaviral death happened in France on 15 February, marking it the first death in Europe. The second death of a 60-year-old French national in Paris was announced on 26 February [ 54 ].
On February 28, fashion designer Agnès B. (not to be mistaken with Agnès Buzyn) cancelled fashion shows at the Paris Fashion Week, expected to continue until 3 March. On a subsequent day, the Paris half-marathon, planned for Sunday 1 March with 44,000 entrants, was postponed as one of a series of steps declared by Health Minister Olivier Véran.
On 13 March, the Ligue de Football Professional disbanded Ligue 1 and Ligue 2 (France’s tier two professional divisions) permanently due to safety threats.
Germany has a popular Regional Pandemic Strategy detailing the roles and activities of the health care system participants in the case of a significant outbreak. Epidemic surveillance is carried out by the federal government, like the Robert Koch Center, and by the German governments. The German States have their preparations for an outbreak. The regional strategy for the treatment of the current coronavirus epidemic was expanded by March 2020. Four primary goals are contained in this plan: (1) to minimize mortality and morbidity; (2) to guarantee the safety of sick persons; (3) to protect vital health services and (4) to offer concise and reliable reports to decision-makers, the media and the public [ 55 ].
The programme has three phases that may potentially overlap: (1) isolation (situation of individual cases and clusters), (2) safety (situation of further dissemination of pathogens and suspected causes of infection), (3) prevention (situation of widespread infection). So far, Germany has not set up border controls or common health condition tests at airports. Instead, while at the isolation stage-health officials are concentrating on recognizing contact individuals that are subject to specific quarantine and are tracked and checked. Specific quarantine is regulated by municipal health authorities. By doing so, the officials are seeking to hold the chains of infection small, contributing to decreased clusters. At the safety stage, the policy should shift to prevent susceptible individuals from being harmed by direct action. By the end of the day, the prevention process should aim to prevent cycles of acute treatment to retain emergency facilities.
United States
The very first case of coronavirus in the United States was identified in Washington on 21 January 2020 by an individual who flew to Wuhan and returned to the United States. The second case was recorded in Illinois by another individual who had travelled to Wuhan. Some of the regions with reported novel coronavirus infections in the US are California, Arizona, Connecticut, Illinois, Texas, Wisconsin and Washington [ 56 ].
As the epidemic increased, requests for domestic air travel decreased dramatically. By 4 March, U.S. carriers, like United Airlines and JetBlue Airways, started growing their domestic flight schedules, providing generous unpaid leave to workers and suspending recruits.
A significant number of universities and colleges cancelled classes and reopened dormitories in response to the epidemic, like Cornell University, Harvard University and the University of South Carolina.
On 3 March 2020, the Federal Reserve reduced its goal interest rate from 1.75% to 1.25%, the biggest emergency rate cut following the 2008 global financial crash, in combat the effect of the recession on the American economy. In February 2020, US businesses, including Apple Inc. and Microsoft, started to reduce sales projections due to supply chain delays in China caused by the COVID-19.
The pandemic, together with the subsequent financial market collapse, also contributed to greater criticism of the crisis in the United States. Researchers disagree about when a recession is likely to take effect, with others suggesting that it is not unavoidable, while some claim that the world might already be in recession. On 3 March, Federal Reserve Chairman Jerome Powell reported a 0.5% (50 basis point) interest rate cut from the coronavirus in the context of the evolving threats to economic growth.
When ‘social distance’ penetrated the national lexicon, disaster response officials promoted the cancellation of broad events to slow down the risk of infection. Technical conferences like E3 2020, Apple Inc.’s Worldwide Developers Conference (WWDC), Google I/O, Facebook F8, and Cloud Next and Microsoft’s MVP Conference have been either having replaced or cancelled in-person events with internet streaming events.
On February 29, the American Physical Society postponed its annual March gathering, planned for March 2–6 in Denver, Colorado, even though most of the more than 11,000 physicist attendees already had arrived and engaged in the pre-conference day activities. On March 6, the annual South to Southwest (SXSW) seminar and festival planned to take place from March 13–22 in Austin, Texas, was postponed after the city council announced a local disaster and forced conferences to be shut down for the first time in 34 years.
Four of North America’s major professional sports leagues—the National Hockey League (NHL), National Basketball Association (NBA), Major League Soccer (MLS) and Major League Baseball (MLB) —jointly declared on March 9 that they would all limit the media access to player accommodations (such as locker rooms) to control probable exposure.
Emergency Funding to Fight the COVID-19
COVID-19 pandemic has become a common international concern. Different countries are donating funds to fight against it [ 57 – 60 ]. Some of them are mentioned here.
China has allocated about 110.48 billion yuan ($15.93 billion) in coronavirus-related funding.
Foreign Minister Mohammad Javad Zarif said that Iran has requested the International Monetary Fund (IMF) of about $5 billion in emergency funding to help to tackle the coronavirus epidemic that has struck the Islamic Republic hard.
President Donald Trump approved the Emergency Supplementary Budget Bill to support the US response to a novel coronavirus epidemic. The budget plan would include about $8.3 billion in discretionary funding to local health authorities to promote vaccine research for production. Trump originally requested just about $2 billion to combat the epidemic, but Congress quadrupled the number in its version of the bill. Mr. Trump formally announced a national emergency that he claimed it will give states and territories access to up to about $50 billion in federal funding to tackle the spread of the coronavirus outbreak.
California politicians approved a plan to donate about $1 billion on the state’s emergency medical responses as it readies hospitals to fight an expected attack of patients because of the COVID-19 pandemic. The plans, drawn up rapidly in reaction to the dramatic rise in reported cases of the virus, would include the requisite funds to establish two new hospitals in California, with the assumption that the state may not have the resources to take care of the rise in patients. The bill calls for an immediate response of about $500 million from the State General Fund, with an additional about $500 million possible if requested.
India committed about $10 million to the COVID-19 Emergency Fund and said it was setting up a rapid response team of physicians for the South Asian Association for Regional Cooperation (Saarc) countries.
South Korea unveiled an economic stimulus package of about 11.7 trillion won ($9.8 billion) to soften the effects of the biggest coronavirus epidemic outside China as attempts to curb the disease exacerbate supply shortages and drain demand. Of the 11,7 trillion won expected, about 3.2 trillion won would cover up the budget shortfall, while an additional fiscal infusion of about 8.5 trillion won. An estimated 10.3 trillion won in government bonds will be sold this year to fund the extra expenditure. About 2.3 trillion won will be distributed to medical establishments and would support quarantine operations, with another 3.0 trillion won heading to small and medium-sized companies unable to pay salaries to their employees and child care supports.
The Swedish Parliament announced a set of initiatives costing more than 300 billion Swedish crowns ($30.94 billion) to help the economy in the view of the coronavirus pandemic. The plan contained steps like the central government paying the entire expense of the company’s sick leave during April and May, and also the high cost of compulsory redundancies owing to the crisis.
In consideration of the developing scenario, an updating of this strategy is planned to take place before the end of March and will recognize considerably greater funding demands for the country response, R&D and WHO itself.
Artificial Intelligence, Data Science and Technological Solutions Against COVID-19
These days, Artificial Intelligence (AI) takes a major role in health care. Throughout a worldwide pandemic such as the COVID-19, technology, artificial intelligence and data analytics have been crucial in helping communities cope successfully with the epidemic [ 61 – 65 ]. Through the aid of data mining and analytical modelling, medical practitioners are willing to learn more about several diseases.
Public Health Surveillance
The biggest risk of coronavirus is the level of spreading. That’s why policymakers are introducing steps like quarantines around the world because they can’t adequately monitor local outbreaks. One of the simplest measures to identify ill patients through the study of CCTV images that are still around us and to locate and separate individuals that have serious signs of the disease and who have touched and disinfected the related surfaces. Smartphone applications are often used to keep a watch on people’s activities and to assess whether or not they have come in touch with an infected human.
Remote Biosignal Measurement
Many of the signs such as temperature or heartbeat are very essential to overlook and rely entirely on the visual image that may be misleading. However, of course, we can’t prevent someone from checking their blood pressure, heart or temperature. Also, several advances in computer vision can predict pulse and blood pressure based on facial skin examination. Besides, there are several advances in computer vision that can predict pulse and blood pressure based on facial skin examination.
Access to public records has contributed to the development of dashboards that constantly track the virus. Several companies are designing large data dashboards. Face recognition and infrared temperature monitoring technologies have been mounted in all major cities. Chinese AI companies including Hanwang Technology and SenseTime have reported having established a special facial recognition system that can correctly identify people even though they are covered.
IoT and Wearables
Measurements like pulse are much more natural and easier to obtain from tracking gadgets like activity trackers and smartwatches that nearly everybody has already. Some work suggests that the study of cardiac activity and its variations from the standard will reveal early signs of influenza and, in this case, coronavirus.
Chatbots and Communication
Apart from public screening, people’s knowledge and self-assessment may also be used to track their health. If you can check your temperature and pulse every day and monitor your coughs time-to-time, you can even submit that to your record. If the symptoms are too serious, either an algorithm or a doctor remotely may prescribe a person to stay home, take several other preventive measures, or recommend a visit from the doctor.
Al Jazeera announced that China Mobile had sent text messages to state media departments, telling them about the citizens who had been affected. The communications contained all the specifics of the person’s travel history.
Tencent runs WeChat, and via it, citizens can use free online health consultation services. Chatbots have already become important connectivity platforms for transport and tourism service providers to keep passengers up-to-date with the current transport protocols and disturbances.
Social Media and Open Data
There are several people who post their health diary with total strangers via Facebook or Twitter. Such data becomes helpful for more general research about how far the epidemic has progressed. For consumer knowledge, we may even evaluate the social network group to attempt to predict what specific networks are at risk of being viral.
Canadian company BlueDot analyses far more than just social network data: for instance, global activities of more than four billion passengers on international flights per year; animal, human and insect population data; satellite environment data and relevant knowledge from health professionals and journalists, across 100,000 news posts per day covering 65 languages. This strategy was so successful that the corporation was able to alert clients about coronavirus until the World Health Organization and the Centers for Disease Control and Prevention notified the public.
Automated Diagnostics
COVID-19 has brought up another healthcare issue today: it will not scale when the number of patients increases exponentially (actually stressed doctors are always doing worse) and the rate of false-negative diagnosis remains very high. Machine learning therapies don’t get bored and scale simply by growing computing forces.
Baidu, the Chinese Internet company, has made the Lineatrfold algorithm accessible to the outbreak-fighting teams, according to the MIT Technology Review. Unlike HIV, Ebola and Influenza, COVID-19 has just one strand of RNA and it can mutate easily. The algorithm is also simpler than other algorithms that help to determine the nature of the virus. Baidu has also developed software to efficiently track large populations. It has also developed an Ai-powered infrared device that can detect a difference in the body temperature of a human. This is currently being used in Beijing’s Qinghe Railway Station to classify possibly contaminated travellers where up to 200 individuals may be checked in one minute without affecting traffic movement, reports the MIT Review.
Singapore-based Veredus Laboratories, a supplier of revolutionary molecular diagnostic tools, has currently announced the launch of the VereCoV detector package, a compact Lab-on-Chip device able to detect MERS-CoV, SARS-CoV and COVID-19, i.e. Wuhan Coronavirus, in a single study.
The VereCoV identification package is focused on VereChip technology, a Lab-on-Chip device that incorporates two important molecular biological systems, Polymerase Chain Reaction (PCR) and a microarray, which will be able to classify and distinguish within 2 h MERS-CoV, SARS-CoV and COVID-19 with high precision and responsiveness.
This is not just the medical activities of healthcare facilities that are being charged, but also the corporate and financial departments when they cope with the increase in patients. Ant Financials’ blockchain technology helps speed-up the collection of reports and decreases the number of face-to-face encounters with patients and medical personnel.
Companies like the Israeli company Sonovia are aiming to provide healthcare systems and others with face masks manufactured from their anti-pathogenic, anti-bacterial cloth that depends on metal-oxide nanoparticles.
Drug Development Research
Aside from identifying and stopping the transmission of pathogens, the need to develop vaccinations on a scale is also needed. One of the crucial things to make that possible is to consider the origin and essence of the virus. Google’s DeepMind, with their expertise in protein folding research, has rendered a jump in identifying the protein structure of the virus and making it open-source.
BenevolentAI uses AI technologies to develop medicines that will combat the most dangerous diseases in the world and is also working to promote attempts to cure coronavirus, the first time the organization has based its product on infectious diseases. Within weeks of the epidemic, it used its analytical capability to recommend new medicines that might be beneficial.
Robots are not vulnerable to the infection, and they are used to conduct other activities, like cooking meals in hospitals, doubling up as waiters in hotels, spraying disinfectants and washing, selling rice and hand sanitizers, robots are on the front lines all over to deter coronavirus spread. Robots also conduct diagnostics and thermal imaging in several hospitals. Shenzhen-based firm Multicopter uses robotics to move surgical samples. UVD robots from Blue Ocean Robotics use ultraviolet light to destroy viruses and bacteria separately. In China, Pudu Technology has introduced its robots, which are usually used in the cooking industry, to more than 40 hospitals throughout the region. According to the Reuters article, a tiny robot named Little Peanut is distributing food to passengers who have been on a flight from Singapore to Hangzhou, China, and are presently being quarantined in a hotel.
Colour Coding
Using its advanced and vast public service monitoring network, the Chinese government has collaborated with software companies Alibaba and Tencent to establish a colour-coded health ranking scheme that monitors millions of citizens every day. The mobile device was first introduced in Hangzhou with the cooperation of Alibaba. This applies three colours to people—red, green or yellow—based on their transportation and medical records. Tencent also developed related applications in the manufacturing centre of Shenzhen.
The decision of whether an individual will be quarantined or permitted in public spaces is dependent on the colour code. Citizens will sign into the system using pay wallet systems such as Alibaba’s Alipay and Ant’s wallet. Just those citizens who have been issued a green colour code will be permitted to use the QR code in public spaces at metro stations, workplaces, and other public areas. Checkpoints are in most public areas where the body temperature and the code of individual are tested. This programme is being used by more than 200 Chinese communities and will eventually be expanded nationwide.
In some of the seriously infected regions where people remain at risk of contracting the infection, drones are used to rescue. One of the easiest and quickest ways to bring emergency supplies where they need to go while on an epidemic of disease is by drone transportation. Drones carry all surgical instruments and patient samples. This saves time, improves the pace of distribution and reduces the chance of contamination of medical samples. Drones often operate QR code placards that can be checked to record health records. There are also agricultural drones distributing disinfectants in the farmland. Drones, operated by facial recognition, are often used to warn people not to leave their homes and to chide them for not using face masks. Terra Drone uses its unmanned drones to move patient samples and vaccination content at reduced risk between the Xinchang County Disease Control Center and the People’s Hospital. Drones are often used to monitor public areas, document non-compliance with quarantine laws and thermal imaging.
Autonomous Vehicles
At a period of considerable uncertainty to medical professionals and the danger to people-to-people communication, automated vehicles are proving to be of tremendous benefit in the transport of vital products, such as medications and foodstuffs. Apollo, the Baidu Autonomous Vehicle Project, has joined hands with the Neolix self-driving company to distribute food and supplies to a big hospital in Beijing. Baidu Apollo has also provided its micro-car packages and automated cloud driving systems accessible free of charge to virus-fighting organizations.
Idriverplus, a Chinese self-driving organization that runs electrical street cleaning vehicles, is also part of the project. The company’s signature trucks are used to clean hospitals.
This chapter provides an introduction to the coronavirus outbreak (COVID-19). A brief history of this virus along with the symptoms are reported in this chapter. Then the comparison between COVID-19 and other plagues like seasonal influenza, bird flu (H5N1 and H7N9), Ebola epidemic, camel flu (MERS), swine flu (H1N1), severe acute respiratory syndrome, Hong Kong flu (H3N2), Spanish flu and the common cold are included in this chapter. Reviews of online portal and social media like Facebook, Twitter, Google, Microsoft, Pinterest, YouTube and WhatsApp concerning COVID-19 are reported in this chapter. Also, the preventive measures and policies enforced by WHO and different countries such as China, Italy, Iran, South Korea, France, Germany and the United States for COVID-19 are included in this chapter. Emergency funding provided by different countries to fight the COVID-19 is mentioned in this chapter. Lastly, artificial intelligence, data science and technological solutions like public health surveillance, remote biosignal measurement, IoT and wearables, chatbots and communication, social media and open data, automated diagnostics, drug development research, robotics, colour coding, drones and autonomous vehicles are included in this chapter.
Skip to content
Questions and Answers about COVID-19 Vaccines
Vaccine education center.
On this page, you will find answers to some of the most common questions people are asking about COVID-19 disease and vaccines. Just click on the question of interest and the answer will appear below it.
Can't find what you're looking for?
- Check the “Archived COVID-19 Questions” page.
- Ask your COVID-19 vaccine questions here.
You can also find information related to COVID-19 in these additional resources:
- Printable Q&A, "COVID-19 vaccines: What you should know" English | Spanish | Japanese
- “Look at Each Vaccine: COVID-19 Vaccine” webpage
- Animations: “How COVID-19 Viral Vector Vaccines Work” and “How mRNA Vaccines Work.”
Do DNA fragments in COVID-19 mRNA vaccines cause harm?
The short answer to this question is no, but let’s look a bit more closely.
The quantity
It is important to realize that mRNA vaccines undergo several steps during production, including multiple purification steps. This means that the amount of DNA fragments remaining in a dose of vaccine is extremely small. In fact, the leftover amount is so small that it can only be measured in nanograms, which are 1 billionth (with a “b”) of a gram or 1/1,000,000,000 of a gram. Think of it like one snowflake among 1,000,000,000 — an inconsequential quantity that would escape our notice unless we were looking for it.
The biology
With this said, some people might still be concerned that any DNA fragments are in the vaccine at all. First, it is important to realize that we are exposed to DNA fragments all the time. Anytime we eat plants or animals, we consume DNA, so our bodies need to protect against damage from foreign DNA. And while it is true that when we consume these fragments, they do not necessarily enter our bloodstream, like those in an injection will, we can still be reassured that our cells are designed to protect our DNA. Here are three relevant examples of how cells protect our DNA:
- Cytoplasm – As the vaccine is processed, DNA fragments may find their way to the cytoplasm of a cell, but our cells contain enzymes and immune system mechanisms for detecting and destroying anything foreign, so even if the fragments end up in our cell, they are destroyed.
- Nucleus – In our cells, our own DNA is housed inside the nucleus. The nuclear membrane acts like a moat around a castle, such that only with the appropriate “clearances” can something enter the nucleus. In our cells, these clearances are controlled by “nuclear access signals,” which are not part of (or accessible to) the DNA fragments.
- DNA – Further, for DNA to be changed, certain enzymes must be present. One example is integrase. Without integrase, our own DNA will not “open” to allow another piece of DNA to be added to it. In the example of the DNA fragments in vaccines, integrase is not present, so change cannot occur.
Some have also suggested that because the mRNA vaccine is delivered in lipid particles, the aforementioned description is not accurate. However, this is also a misconception. While the lipid particles help deliver the mRNA into a cell, the vaccine components are taken into compartments called endosomes. Endosomes contain acids and enzymes that break down the lipids and most of the DNA fragments, so they are quickly destroyed.
Watch this video as Dr. Offit describes these safety mechanisms.
To find out more about DNA and other vaccine ingredients, check the “Vaccine Ingredients” section of our website .
Read more about the misinformation surrounding DNA fragments in Dr. Offit’s Substack, “Beyond the Noise.”
Last updated: April 24, 2024
Are the mRNA vaccines a type of gene therapy?
The short answer to this question is no. Check out the article in our April 2024 Parents PACK newsletter to find out why.
If young children do not get severely ill from COVID-19, why should I consider giving this vaccine to my child who is younger than 5 years of age?
As parents weigh the relative risk and benefits of getting their youngest children vaccinated against COVID-19, some wonder about the need for their child to get a relatively new vaccine when the disease doesn’t seem too bad in most children. Most healthcare providers agree that the benefits of vaccination outweigh the risks for our youngest family members:
- As of April 2024, about 2,000 children 17 years of age or younger have died from COVID-19. While this is a small number compared with the more than 1.1 million deaths in the U.S., for those families, their world will never be the same.
- Millions of children have been infected with the virus that causes COVID-19. Some of those children were hospitalized with severe disease or developed a condition called multi-inflammatory syndrome in children (MIS-C), which can damage organs and on rare occasions be deadly. Importantly, it appears that newer variants are less likely to cause MIS-C. Watch this video in which Dr. Offit discusses this trend.
- Like adults, some children who have had COVID-19, even mild cases, have experienced lingering symptoms, commonly referred to as “long COVID.” In younger children it may be difficult for them to express what they are feeling or experiencing, which can make this condition even more difficult to identify and address.
- Millions of vaccines have been administered safely to children at this point.
For a more detailed look at the considerations related to COVID-19 vaccination of children and a series of resources, check out the March 2023 issue of Parents PACK and this April 2023 article penned by Dr. Offit, the VEC’s director .
Watch this short video of Dr. Offit discussing why children should get the COVID-19 vaccine.
What COVID-19 vaccines are currently available in the U.S.?
The U.S. has three approved COVID-19 vaccines; however, the two that are most often used based on the current vaccine recommendations are those described as “mRNA vaccines.” Find out more about each vaccine:
- Pfizer mRNA bivalent vaccine – This vaccine contains mRNA to protect against the spike protein from the XBB.1.5 variant of SARS-CoV-2, the virus that causes COVID-19. The bivalent Pfizer vaccine that contained two spike proteins is no longer authorized for use in the U.S. Available in three different doses based on the age of the individual (3 micrograms per dose for 6 months to 4 years, 10 micrograms per dose for 5 to 11 years, and 30 micrograms per dose for 12 years and older).
- Moderna mRNA bivalent vaccine – This vaccine also contains mRNA to protect against the spike protein from the XBB.1.5 variant of SARS-CoV-2. Likewise, the bivalent Moderna vaccine is no longer authorized for use in the U.S. Two doses are available and are based on the age of the individual (25 micrograms for 6 months to 11 years and 50 micrograms for 12 years and older).
- Novavax protein-based vaccine – This vaccine only contains the spike protein from the XBB.1.5 variant. This vaccine is approved for those 12 years and older who did not previously get a COVID-19 vaccine. In this situation it should be administered as a two-dose series separated by three to eight weeks. As a booster dose, it can be given as a single dose to those 18 years and older.
A fourth vaccine, J&J/Janssen adenovirus-based vaccine , is no longer available in the U.S., but it is still used in other countries. This vaccine contains a replication-defective adenovirus that has been altered to include the gene (DNA) of the spike protein for the original SARS-CoV-2 virus. Because of the availability of other vaccines and due to the rare but serious side effects associated with this vaccine (i.e., Guillain-Barre syndrome (GBS) and thrombosis with thrombocytopenia syndrome (TTS)), and its monovalent formula, this vaccine was removed from the U.S. vaccine supply in the spring of 2023.
What is the Novavax vaccine and who can get it?
The Novavax COVID-19 vaccine uses a “tried and true” approach to inducing immunity. Specifically, the vaccine delivers the spike protein and an adjuvant, which is something that increases immune responses to the protein. It is given as two doses, separated by 3-8 weeks, to those 12 years of age and older. This technology is exactly the same as that used to make one of the influenza vaccines (FluBlok) and very similar to that used to make the hepatitis B and human papillomavirus vaccines.
To find out more about the Novavax vaccine, watch this video of VEC Director, Paul Offit, MD, who is on the FDA’s advisory committee and, therefore, reviewed the data presented during the advisory committee meeting.
Who should get a booster dose of COVID-19 vaccine?
The general rule of thumb related to CDC guidance is that individuals in the U.S. receive a single booster at least eight weeks after their last dose of COVID-19 vaccine. However, some nuance exists for different age groups and vaccines, as shown on this CDC reference table . As such, if you are not sure whether you need an additional dose, we recommend speaking to your healthcare provider.
Adults 65 years of age and older can get up to two doses of the 2023-2024 vaccine separated by at least four months.
Moderately or severely immune compromised individuals may require more doses than their age-matched, immune competent counterparts; however, the recommendations vary based on vaccination history and age, so it is important to talk to your healthcare provider about your or a family member’s specific needs. The recommendations for this group are summarized on these CDC reference tables .
My teen is a student-athlete and already had COVID-19, so does he need the COVID-19 vaccine? We are worried about myocarditis.
While myocarditis is rare, it is also real; so, we can understand why some parents may be hesitant to get their teens vaccinated. But it is important when making these decisions to realize that the choice not to vaccinate is also a choice to risk COVID-19, so let’s take a look.
Vaccination and myocarditis
By June 2022, almost 55 million doses of Pfizer’s mRNA vaccine were given to children and teens from 5 to 17 years of age. Of those, 635 cases of myocarditis were diagnosed. Most cases occurred in males, and the side effect was most likely to occur in the first seven days after the second dose, albeit a small number of cases occurred after receipt of the first dose or a booster dose.
When a group of 5- to 17-year-olds who experienced myocarditis after COVID-19 vaccination were followed to see how they did, about half were hospitalized and none died. Most of those who were hospitalized went home within three days. In addition, according to their cardiologists, three months after the event, more than 6 in 10 were fully recovered and an additional 2 in 10 had likely fully recovered, but tests were still outstanding.
COVID-19 disease and myocarditis
A review of the literature, published in August 2022, found that an individual is at least 7 times more likely to experience myocarditis resulting from a COVID-19 infection than from a COVID-19 vaccine.
These other considerations are important when deciding about COVID-19 vaccination of teens (or teen athletes):
Studies have shown that children younger than 5 years of age do not experience myocarditis following receipt of the COVID-19 mRNA vaccines, so vaccinating young children before the risk of myocarditis increases is one way to avoid this potential side effect. Importantly, immunization of our youngest population against COVID-19 has been extremely limited, so it is possible that over time, as more youngsters are vaccinated, we would identify a low risk for myocarditis in young children as well. However, it is also possible that we would see a greater risk from infection compared with vaccination. These are the types of information we need to continue working toward understanding when it comes to this disease.
For those at greater risk of this side effect, increasing the time between doses to at least eight weeks, appears to lessen the risk of this side effect.
While older individuals can experience myocarditis in the first week after vaccination, the risk is greatest for males between 12 and 39 years of age. The risk for females is lower than for males, but still can occur and is more prevalent between 12 and 29 years of age.
It is also important to realize that myocarditis following vaccination is short-lived and tends to resolve on its own, whereas myocarditis following an infection tends to be more severe.
- We are still learning about “long COVID,” the condition that causes people to experience symptoms well after their infection goes away. While we don’t yet know how often this occurs in younger people, it is clear that some young people suffer similar long-term consequences.
Watch this video to hear Dr. Paul Offit talk about COVID-19 vaccine and myocarditis in teens.
Last updated: September 20, 2023; reviewed April 24, 2024
Can someone with COVID-19 get the COVID-19 vaccine or booster?
In the U.S., the CDC recommends that anytime someone has a respiratory illness, they try to stay away from others until their symptoms start improving and they have not had a fever for at least 24 hours.
Likewise, while the CDC previously recommended delaying vaccination for patients who were treated with antibody-based therapies, data now demonstrate that the modest reduction in antibody responses seen in these patients does not warrant the delay. With this said, most recently infected individuals are still recommended to wait for about three months before getting vaccinated, so the antibodies introduced by treatment are unlikely to be problematic anyway.
Why are booster doses recommended?
The goal of vaccination is to prevent serious illness. This is achieved by generating immune memory cells, such as B cells and T cells. These cells are typically long-lived and reside in the bone marrow, bloodstream, and lymph glands to monitor for exposure to a pathogen. If the pathogen is detected, these memory cells quickly become activated and stimulate the immune response to efficiently fight the infection before the infection can get out of control and cause serious illness. In the case of COVID-19 mRNA vaccines, studies demonstrated that high levels of memory cells are generated, and as variants emerged, we saw that the levels of memory cells generated by both the mRNA (Pfizer and Moderna) and adenovirus-based (J&J/Janssen) vaccines were sufficient to prevent serious illness in most cases. As such, these findings would not warrant a booster dose.
However, a second goal of vaccination could be to prevent any level of illness, meaning that vaccinated people would not even experience mild or asymptomatic infection. To accomplish this, people need to have high levels of neutralizing antibodies circulating in their bloodstream. Neutralizing antibodies prevent the virus from attaching to and entering cells. Typically, neutralizing antibody levels fade over time. When this happens, a booster dose can stimulate the memory B and T cells to cause production of neutralizing antibodies, thereby increasing the level of detectable antibodies in the bloodstream and decreasing the chance for any level of illness for another brief period of time (a couple of months).
While prevention of any level of illness is a noble goal, historically, prevention of serious illness has been the goal of vaccination, particularly for respiratory infections, like COVID-19. These two goals have been at the heart of the scientific “debate” over the need for booster doses. In truth, prevention of serious illness is the only reasonable and attainable goal for a virus like SARS-CoV-2, which has a short incubation period.
Watch this video to hear Dr. Offit talk about the Fall 2023 COVID-19 recommendations.
Who is considered immune compromised when it comes to deciding about COVID-19 vaccines?
People should talk with their healthcare providers to determine whether they are considered moderately or severely immune compromised since each individual is unique. However, the CDC has provided some guidance that may help.
People typically considered moderately or severely immune compromised include the following:
- People currently being treated for cancers of the blood or organs (so-called “solid tumor” cancers)
- People with blood-related cancers, regardless of current treatment status, including those with chronic lymphocytic leukemia, non-Hodgkin lymphoma, multiple myeloma and acute leukemia
- People who received an organ transplant and take immunosuppressive medications to prevent rejection of the organ
- People who had a stem cell transplant or received CAR T-cell therapy less than 2 years ago or who are taking immunosuppressive medications
- People with conditions that are considered to cause permanent immune deficiency because the condition affects cells of their immune system, such as DiGeorge syndrome or Wiskott-Aldrich syndrome
- People infected with HIV whose infection is untreated or considered to be at an advanced stage
- High-dose corticosteroids (more than 20 mg prednisone or similar medications per day)
- Alkylating agents
- Antimetabolites
- Transplant-related immunosuppressive medications
- Cancer chemotherapeutic medications that are considered severely immunosuppressive (e.g., tumor-necrosis, or TNF, blockers)
- Biologic agents that suppress or modulate the immune response (e.g., B-cell depleting agents)
Some people 12 years of age and older in these groups may be eligible for ongoing protection through intravenous receipt of a monoclonal antibody product called Pemgarda. Treatments are required every three months. This product cannot be used for treating COVID-19. If you want to find out if you are eligible and could benefit from this product, speak to your healthcare provider who treats you for your immune-compromising condition.
People who should work with their healthcare provider to determine their need for additional doses include:
- People taking medications that make them uncertain whether they would be included in the list of individuals mentioned above
- People with immune-system-related conditions not specifically mentioned above
- People preparing to start one of the above-mentioned medications
People not considered to be in this category include:
- People who do not have compromised immunity.
- People without a spleen.
- People who had cancer but are no longer being treated.
- People with chronic conditions that do not involve the immune system or require treatment with high doses of corticosteroids, such as diabetes, asthma, COPD, kidney disease, heart conditions, sickle cell disease, among others. If you are not sure, check with your healthcare provider.
Can people get other vaccines at the same time as their COVID-19 vaccine?
Yes. The CDC has indicated that COVID-19 vaccine can be administered at the same visit as any other vaccines (including influenza and RSV vaccines as well as nirsevimab, the RSV monoclonal antibody prevention for infants).
One exception, however, is for people, particularly young males, who need both a COVID-19 and an orthopoxvirus (mpox or smallpox) vaccine. These people should consider waiting at least four weeks between receipt of the vaccines due to increased risk of myocarditis.
Vaccines given at the same visit should be given in different locations separated by at least one inch.
Watch this video to hear Dr. Hank Bernstein talk about getting the COVID-19 vaccine at the same time as other vaccines.
What is the difference between emergency use authorization and the normal process of vaccine approval?
The main difference between emergency use authorization, or EUA, and the normal process, which is via a biologic licensure application, or BLA, is how long data were collected prior to the vaccines being reviewed for use. So, when considered quite literally, the vaccines being used under EUA are no different than those that are used after the vaccines get full approval (BLA). The reason for the shortened timeline for COVID-19 vaccines was, of course, because of the pandemic. But, importantly, steps were not skipped to shorten the timeline, and at this point, these vaccines have been given safely to millions of people.
Were the COVID-19 vaccines approved by the FDA?
Even though the COVID-19 vaccines were initially released under Emergency Use Authorization (EUA), they were still approved by the Food and Drug Administration (FDA). The review process was the same, but because of the pandemic, the data could be submitted after a shorter period of participant follow-up than usual. However, even after submitting data (and getting an EUA), those studies continued.
Last updated: July 21, 2022; reviewed April 24, 2024
Is it safe for my teen to get the COVID-19 vaccine given the stories about myocarditis?
Cases of myocarditis, or inflammation of the heart, have been reported in a small number of people after receipt of the COVID-19 mRNA vaccine:
- The cases of myocarditis occur more often in boys and young men and more often after the second dose. Symptoms typically occur within 4 days after receipt of the dose. Recently immunized teens and young adults who experience chest pain or shortness of breath should be seen by a healthcare provider and report recent their vaccination.
- Myocarditis is somewhat common, particularly following viral infections. In fact, cases tend to occur more often in the spring due to viruses that circulate at this time of year (specifically, coxsackie B viruses). Typically, about 100-200 cases occur per million people per year.
- Available data suggest that the incidence of myocarditis following mRNA vaccines is about 1 to 10 per 100,000 vaccine recipients; however, this risk increases in males between 16 and 39 years of age to about 1 per 10,000 vaccine recipients. These numbers are lower in females. They are also lower than if people are infected with the virus that causes COVID-19, which increases the risk of myocarditis at least sevenfold.
- Parents and teens should watch for symptoms that may include chest pain, pressure, heart palpitations, difficulty breathing after exercise or lying down, or excessive sweating. One or more of these symptoms may also be accompanied by tiredness, stomach pain, dizziness, fainting, unexplained swelling, or coughing. If a recently vaccinated teen develops these symptoms or you are unsure, contact the child’s doctor or seek more immediate medical assistance if needed.
Find out more in this article from our Vaccine Update newsletter for healthcare providers.
Watch a video featuring one of our pediatric cardiologists, Dr. Matt Elias, discussing treating patients with myocarditis.
Last updated: April 26, 2023; reviewed April 24, 2024
Is it safe for my child to get the COVID-19 vaccine?
The mRNA vaccines are approved for those 6 months of age and older.
At this point, millions of children and teens have been safely vaccinated against COVID-19. The clinical trials in those 5 years of age and younger showed the vaccines to be safe and effective against severe disease. Moderna’s vaccine for the youngest children (6 months to 11 years of age) is one-half the dose (25 micrograms) of their vaccine for those 12 years and older (50 micrograms for 12 years and older). Pfizer’s vaccine for the youngest children (6 months to 4 years of age) is one-tenth the dose (3 micrograms) of their adult vaccine (30 micrograms for 12 and older). The Pfizer bivalent vaccine dose for 5- to 11-year-olds is one-third the adult dose (10 micrograms).
See more about the importance of vaccinating children against COVID-19:
- March 2023 issue of Parents PACK
- April 2023 article penned by Dr. Offit, the VEC’s director
- “Why Should Children Get the COVID-19 Vaccine?” (video featuring Dr. Offit)
If my child is near one of the cutoff ages for different doses (5 or 12 years of age), is it better to get them vaccinated or wait?
Since COVID-19 is still circulating and it takes several weeks for a person to be considered fully immunized, it is generally recommended to start the vaccination process with the vaccine the child is currently eligible to receive even if it is a lower dose.
If your child’s birthday occurs during the period between doses, the child will be offered the higher dose for their subsequent doses. Two exceptions are worth noting. First, if your child started with the Pfizer vaccine at age 4 and then turns 5, they will still be given the third dose of the vaccine for younger children (3 micrograms). Second, if your child is moderately or severely immune compromised and they transition from 11 to 12 years of age during their dosing, they may finish with the original doses or the doses for their age. Talk with your child’s healthcare provider if you feel your child might be in this situation.
What side effects will my child experience from the COVID-19 vaccine?
Side effects in children were similar to what has been found in other age groups, including pain at the injection site, fatigue, headache, fever, chills, muscle pain, or joint pain.
Even though a small number of cases of myocarditis, or heart inflammation, have been identified in teens and young adults, particularly in the 4 days after receipt of the second dose of the vaccine, this side effect has not been found in younger age groups, who receive lower doses. However, it is still important to monitor younger children for this potential side effect. Chest pain, shortness of breath, or related symptoms should be reported to a healthcare provider.
Other serious side effects have not been identified, nor have long-term effects. Find additional information:
- Long-term Side Effects of COVID-19 Vaccine? What We Know.
- Reproductive Health and COVID-19 Vaccines
Last updated: June 21, 2022; reviewed April 24, 2024
Can the COVID-19 vaccine affect puberty or fertility in my child?
No. The rumors related to COVID-19 vaccines affecting puberty or fertility are unfounded. The mRNA vaccines are processed near the injection site and activated immune system cells travel through the lymph system to nearby lymph nodes. In this manner, they are not traveling to other parts of the body. As such, there would not be a biological reason to expect that maturation or reproductive functionality of either males or females would be negatively affected by COVID-19 vaccination now or in years to follow. Importantly, due to reports of menstrual cycle changes following vaccination, studies have been, and continue to be, conducted. Studies to date have suggested about a one-day difference in menstrual cycles; however, further data are needed to understand this finding and these reports, particularly because many factors can affect the timing of an individual’s cycle. As such, analyzing the data carefully will be important. In addition, five large, national monitoring systems have not revealed any concerning findings related to miscarriage, stillbirth, preterm birth, birth defects, pregnancy or post-delivery complications or outcomes, infant or neonatal outcomes, menstrual irregularities or post-menopausal bleeding.
Watch this short video in which Dr. Paul Offit discusses COVID-19, the vaccines and infertility.
You can read more about fertility and COVID-19 vaccines in this Vaccine Update article.
If I got a COVID-19 vaccine in another country, can I get one in the U.S.?
Individuals vaccinated in another country are recommended to receive one dose of the 2023-2024 vaccine if they have not received an updated version approved by the FDA or WHO. The dose should be administered at least eight weeks after the most previous COVID-19 vaccine.
Young children (6 months to 4 years of age and people who are considered immune compromised should check with a healthcare provider, as the recommendations may vary somewhat for them based on U.S. recommendations for these groups.
To see which vaccines are approved by the WHO, check this webpage .
Last updated: September 20, 2023; reviewed April 24, 2024
Can I get the COVID-19 vaccine during my menstrual cycle?
Yes. Although minor changes (about one day in length) to the cycle have been observed, women do not need to schedule their COVID-19 vaccine around their menstrual cycle. The reasons for the changes are possibly the result of effects on specific types of immune system cells that are also present in the uterus or hormonal changes associated with the immune response.
Of note, the COVID-19 vaccine is not shed after vaccination, so being around recently vaccinated individuals would not be expected to affect someone’s cycle.
You can read more about menstruation and COVID-19 vaccines in this Vaccine Update article.
Last updated: December 23, 2022; reviewed April 24, 2024
Do the COVID-19 vaccines contain live virus?
The mRNA (Moderna and Pfizer) vaccines do not contain live virus. Each of these contain a single gene from the virus that causes COVID-19. The gene instructs our cells to make the protein, but no other proteins from the virus are made, so whole virus particles are never present. In this manner, people who were vaccinated cannot shed, or spread, the virus to other people as a result of vaccination. If, however, the individual subsequently becomes infected, they can spread the virus during the days before and early during their infection. Of note, the amount of virus shed by vaccinated people quickly decreases, so they generally shed less virus overall compared to unvaccinated, infected individuals. This is also the case for the J&J/Janssen vaccine; however, that version is no longer available in the U.S.
The Novavax vaccine does not contain live virus, either. It delivers the spike protein directly, rather than having our cells make the protein. As such, viral shedding does not occur following receipt of this version.
In this video, Dr. Paul Offit talks about the ingredients used in the COVID-19 mRNA vaccines.
Do the COVID-19 vaccines cause viral shedding?
Viral shedding occurs when a person is infected with a virus and whole viral particles produced during the infection are transmitted in the individual’s secretions. For viruses that infect the respiratory tract, like COVID-19, these particles are often found in secretions from the nose and mouth, such as saliva or mucus.
Some people wonder whether they can shed the virus as a result of vaccination. In the case of COVID-19 mRNA vaccines approved for use in the U.S., the short answer is no. The same is true of adenovirus-based vaccines (like J&J/Janssen) although this type of COVID-19 vaccine is no longer approved for use in the U.S. In both cases (mRNA- and adenovirus-based vaccines), only the gene for a single protein from the virus that causes COVID-19 – the spike protein – is introduced. As such, whole viral particles are never produced during vaccine processing. Indeed, people are not considered to be infected when they are vaccinated because the virus does not replicate in them. Further, the vaccines are processed near the site of injection, so the spike protein produced during processing would not be found in nasal or oral secretions. As such, they cannot “shed” the single protein either. Likewise, the Novavax vaccine, which delivers the spike protein directly, cannot result in viral shedding.
However, if vaccinated people are infected, the virus will replicate at low levels in their nasal or oral cavity before the immune system stops it. In this scenario, the individual can shed the virus beginning about two days before the start of symptoms and through the first three to four days after symptoms begin.
Read more about viral shedding in this Parents PACK article, “Viral Shedding and COVID-19 — What Can and Can’t Happen."
Watch this short video of Dr. Offit answering the question, “Can I spread coronavirus to others when I get the COVID-19 vaccine?”.
How do mRNA vaccines work?
People make mRNA all the time. In our cells, DNA in the nucleus is used to make mRNA, which is sent to the cytoplasm where it serves as a blueprint to make proteins. Most of the time, the proteins that are produced are needed to help our bodies function.
mRNA vaccines take advantage of this process by introducing the mRNA for an important protein from the virus that the vaccine is trying to protect against. In the case of COVID-19, the important protein is the spike protein of the SARS-CoV-2 virus. The mRNA that codes for the SARS-CoV-2 spike protein is delivered to our muscle cells, which make the protein. The protein is then processed by immune system cells, called dendritic cells, which express the spike protein on the cell surface, travel to a local lymph node, and stimulate other cells of the immune system (B cells) to make antibodies. These antibodies protect us, so that if we are exposed to SARS-CoV-2 in the future, our immune system is ready and we don’t get sick.
The vaccine is processed over a 1- to 2-week period after vaccination during which time the immune response develops. However, the mRNA only directs protein production in the cell for 1 to 3 days before it breaks down. Once it breaks down, the cell stops making the spike protein.
- See more about dendritic cells and the adaptive immune system in this animation.
- Watch this short video of Dr. Offit describing how mRNA vaccines work.
Last updated July 29, 2021; reviewed April 24, 2024
How do adenovirus vector vaccines work?
Although COVID-19 adenovirus-based vaccines are no longer used in the U.S., they remain in use in some other countries. These vaccines take advantage of a class of relatively harmless viruses, called adenoviruses. Some adenoviruses cause the common cold, but others can infect people without causing illness. To use these viruses for vaccine delivery, scientists choose types of adenovirus that do not cause illness and to which most people have not been exposed. They alter the virus by removing two of the genes that enable adenovirus to replicate in people, and they replace one of those genes with the one for the SARS-CoV-2 spike protein.
Like human cells, adenoviruses contain DNA as their genetic material. So, when an adenovirus vaccine is administered, it enters muscle cells where it releases the DNA that includes the gene for the spike protein, and the genetic material enters the nucleus of the cell. In the nucleus, the DNA is used to make messenger RNA (mRNA), which is released into the cytoplasm to serve as a blueprint for making proteins. The DNA from the viral vector, however, cannot insert into the cell’s DNA. The mRNA causes the SARS-CoV-2 protein to be produced. Specialized cells of the immune system, called dendritic cells, put pieces of the newly produced SARS-CoV-2 spike protein on their surface and travel to a draining lymph node where they stimulate other cells of the immune system; specifically, B cells that make antibodies, T cells that help B cells make antibodies, and other T cells that can kill virus-infected cells. Antibodies against the spike protein will now prevent the virus from causing an infection in the future.
Find out more about adenovirus vaccines in this Vaccine Update article, “Getting Familiar with COVID-19 Adenovirus-replication-deficient Vaccines.”
How does the protein-based vaccine (Novavax) work?
The Novavax COVID-19 vaccine delivers the SARS-CoV-2 spike protein into our muscle. Once in our muscle, immune system cells that circulate throughout our body recognize the protein as foreign and attack it. Specialized immune system cells, called dendritic cells, put pieces of the protein on their surface and travel to nearby lymph nodes to activate other parts of the immune system. It takes about 1 to 2 weeks for the vaccine to be processed. The result is immunologic memory cells that are specialized to recognize the viral spike protein in the event of a future encounter with the virus.
This process takes advantage of our adaptive immune system, which responds to foreign proteins every day. To find out more about this part of our immune system, watch this animation .
Watch this short video of Dr. Offit describing the Novavax COVID-19 vaccine.
How did the vaccine companies (e.g., Pfizer and Moderna) decide which mRNA to use?
In order for a virus to reproduce and cause infection, it must get into cells and take over the cellular machinery . Because viruses attach to cells using a particular protein on their surface , in this case the SARS-CoV-2 spike protein, scientists understood that blocking that attachment would be a direct way to prevent infection. One way to block this attachment is with antibodies that bind to the surface protein. As such, when the genome was published, scientists developing the nucleic acid or protein subunit vaccines (i.e., those that only used part of the virus) chose the gene for the spike protein, anticipating that this would be the most direct route to developing an effective vaccine. The 2023-2024 COVID-19 mRNA vaccines use mRNA for the spike protein from a newer variant of the virus (XBB.1.5), so that the antibodies our immune system produces more closely match the surface protein of the SARS-CoV-2 viruses currently circulating.
Last updated: September 21, 2023; reviewed April 24, 2024
Who should NOT get the COVID-19 vaccine?
Most people are able to get COVID-19 vaccine. But a few groups of people either should not get the vaccine or should get a particular version. Likewise, some individuals should consult with their doctor or follow special procedures.
People who should NOT get any COVID-19 vaccine:
- Those younger than 6 months of age.
- People currently or recently experiencing a COVID-19 infection; these people can get vaccinated once they have been without a fever for 24 hours and their primary symptoms have resolved although it is recommended that these individuals wait at least three months to be vaccinated so they develop a more robust immune response to the vaccine dose.
People who cannot get the mRNA vaccine (Pfizer or Moderna), but may be able to get the Novavax vaccine:
- Anyone with a previous severe allergic reaction (i.e., one that causes anaphylaxis, any reaction that causes swelling that affects the airway (i.e., tongue, uvula, or larynx), or diffuse rash that also involves respiratory surfaces, such as Stevens-Johnson Syndrome) to a COVID-19 mRNA vaccine dose or an mRNA vaccine component.
- Anyone with a known polyethylene glycol (PEG) allergy.
People who cannot get the protein-based vaccine (Novavax), but may be able to get the mRNA (Pfizer or Moderna) vaccine:
- Anyone with a previous severe allergic reaction (i.e., one that causes anaphylaxis), any reaction that causes swelling that affects the airway (i.e., tongue, uvula, or larynx), or diffuse rash that also involves respiratory surfaces, such as Stevens-Johnson Syndrome, to a COVID-19 protein-based vaccine (Novavax) dose or one of its components.
- Anyone with a known polysorbate allergy.
People who may get the vaccine after considering risks and benefits and/or consulting with their healthcare provider:
- Individuals with a history of a non-severe, immediate (within 4 hours) allergic reaction to a previous dose of COVID-19 vaccine. (These individuals should be observed for 30 minutes after receipt of the vaccine.)
- People who have a severe or immediate allergic reaction to one of the types of vaccines and for whom the cause of the reaction is unknown (i.e., which component caused the reaction) should consult an allergist or immunologist to determine whether the individual can get the other version. If they proceed, they should be vaccinated at a location with medical facilities and staff prepared to respond to medical emergencies.
- People who cannot get one type of COVID-19 vaccine may be able to get the other type.
- People who are moderately or severely ill (regardless of whether they have a fever) may delay vaccination until they feel better.
- People with a history of MIS-C or MIS-A should delay vaccination until at least 90 days after diagnosis and they experience a return of normal cardiac function and are considered clinically recovered.
- People who experienced myocarditis or pericarditis within 3 weeks of receipt of COVID-19 vaccine are typically advised not to get additional doses of any COVID-19 vaccine. In some instances, individuals and their healthcare providers may decide to proceed with an additional dose based on the risk-benefit assessment. In this situation, symptoms should have resolved and at least 8 weeks should have passed before any additional doses are administered. Note: This does not apply to people with history of myocarditis or pericarditis unrelated to COVID-19 vaccination (including from COVID-19 infection, prior to COVID-19 vaccination, or more than 3 weeks after COVID-19 vaccination), nor does it apply to people with a history of heart disease.
People who should follow special procedures
- Pregnant people who develop a fever after vaccination should take acetaminophen. (See more in the pregnancy-related questions lower on this page.)
- People treated with convalescent plasma should not receive measles- or varicella-containing vaccines until at least 7 months after receipt of the plasma.
- People with a known COVID-19 exposure can get vaccinated if they don’t have symptoms.
- People with a current infection should wait at least until symptoms resolve but may have a better immune response to the vaccine if they wait at least 3 months after start of symptoms.
Watch this video of Dr. Offit answering the question, “Who Should Not Get the COVID-19 mRNA Vaccines?”.
Where can I get the vaccine?
COVID-19 vaccines are generally widely available. As such, we recommend checking for vaccine at your provider’s office, local pharmacies, healthcare facilities, or mobile clinics. For children younger than 5 years of age, we recommend contacting your child’s healthcare provider or checking with clinics or pharmacies before going for vaccination as some may have certain age requirements for administering vaccines.
Last updated: Nov. 10, 2021; reviewed April 24, 2024
What are the side effects of the COVID-19 vaccine?
Common side effects are caused as part of the immune response to each vaccine.
mRNA vaccines: Older children and adults
- Muscle aches
Side effects occurred during the first week after vaccination but were most likely one or two days after receipt of the vaccine. During clinical trials, side effects were more frequent following the second dose and more likely to be experienced by younger, rather than older, adults. Although most people will not have significant side effects, some people may wish to schedule their vaccination, so that they will not need to call out of work the next day if they don’t feel well.
A small number of people who get the mRNA vaccine experience mild, short-lived inflammation of the heart, called myocarditis. About 1 to 10 of every 100,000 mRNA vaccine recipients experience this condition, but it is most likely in adults 39 years and younger and more often occurs in males. This condition tends to occur within 4 days of receipt of the second dose, but it can occur after any dose and up to several days after vaccination. Recently vaccinated individuals who experience chest pain or shortness of breath should seek medical care. This condition tends to resolve within 2-3 weeks and does not cause long-term heart damage. Importantly, COVID-19 infections can also cause myocarditis, and this tends to occur more frequently after infection compared with vaccination. (See “My teen is a student-athlete and already had COVID-19, so does he need the COVID-19 vaccine? We are worried about myocarditis.” on this page for more detailed information.)
mRNA vaccines: Children younger than 5 years of age
Young children who received either the Pfizer or Moderna mRNA vaccine commonly experienced:
- Pain, tenderness, and swelling near the injection site
- Irritability
- Decreased appetite
Older children in this age group, who are better able to communicate what they are feeling, sometimes also experienced headaches, chills, achiness or joint pain, and nausea or vomiting. These effects were somewhat more likely after receipt of the Moderna vaccine, which is a higher dose, but occurred infrequently overall.
Myocarditis was not detected in this age group, either in clinical trials or since the vaccines have been in use; however, because COVID-19 mRNA vaccines are a rare cause of myocarditis in older adolescents and young adults, it is possible that it could be observed in younger children. Experience with these vaccines in older children and adults suggest that the likelihood of myocarditis is significantly lower following vaccination compared with infection. Also, the doses given to this age group are even lower than those given to older children and adults. However, parents and care providers should still monitor their children in the days following vaccination and contact healthcare providers or seek emergency care should concerns arise.
Protein-based vaccine: Adults
The most common side effects from the protein-based vaccine (Novavax) are:
- Injection site pain and less often redness or swelling
A small number of cases of myocarditis have occurred in individuals who received this vaccine; however, additional data are necessary to determine the level of risk. Recently vaccinated individuals who have heart-related symptoms should seek medical care.
Adenovirus-based vaccine: Adults
Adenovirus-based vaccines are no longer available in the U.S., but they are still used in some other countries.
The most common side effects from the adenovirus vaccine (Johnson & Johnson/Janssen) are:
Side effects occurred during the first seven to eight days after vaccination but were most likely to occur one or two days after receipt of the vaccine. Side effects were more often experienced by younger, rather than older vaccine recipients.
Two rare, but potentially dangerous conditions, have been identified following receipt of the adenovirus-based vaccines, such as the J&J/Janssen version:
- Thrombosis with thrombocytopenia syndrome, or TTS, occurs in about 1-2 of every 1 million vaccine recipients and develops up to 3 weeks after getting vaccinated. Individuals between 18 and 64 years of age, both female and male, who got the J&J/Janssen vaccine have experienced this condition; however, women between the ages of 30 and 49 years of age are at the greatest risk. Anyone who got the J&J/Janssen vaccine less than 3 weeks ago should seek medical care if they develop severe headache, shortness of breath, severe abdominal pain, unexplained leg pain, easy bruising, or small red spots on the skin. Anyone seeking medical care with one or more of these symptoms should mention their recent receipt of the vaccine, so healthcare providers can order the appropriate diagnostic tests and treatments.
- Guillain-Barré syndrome, or GBS, occurs in about 1 of every 100,000 vaccine recipients, most often during the first 3 weeks after getting vaccinated. The condition has most often been identified in males between 50 and 64 years of age, but it can occur in females and those 65 years and older on occasion. While rare, most cases have required hospitalization and at least one person has died. Anyone who recently received an adenovirus-based COVID-19 vaccine and experiences muscle weakness or paralysis should seek medical treatment and inform the healthcare provider of the recent vaccination. It should also be noted that COVID-19 infection has been associated with GBS; so, natural infection with SARS-CoV-2 also appears to be a rare cause of GBS. Find out more about GBS in this Parents PACK article, “Guillain-Barré Syndrome (GBS) & Vaccines: The Risks and Recommendations.”
Can I take medicine for the side effects after I get the vaccine?
The CDC has indicated that you can take anti-fever or anti-inflammatory medications if necessary following COVID-19 vaccination, but it is important to know that doing so could diminish the level of immunity that develops. This is true anytime you take these types of medications, whether following vaccination or to treat illness. Generally speaking, the “symptoms” people experience following vaccination or during illness, such as fever, redness at the injection site, or fatigue, are caused by your immune system response. For example, fever is your body turning up its “thermostat” to make the immune system more efficient and the pathogen less efficient. For these reasons, if you are not very uncomfortable, it is better not to take these medications.
Some wonder how long they should wait after vaccination before taking these types of medicines, so their immune response is not affected. As a rule of thumb, the immune response following receipt of the mRNA vaccine develops over a week or two after vaccination and for the adenovirus vaccine, over the course of about four weeks, but the greatest chance of affecting your immune response would be in the first few days after receipt of the vaccine. Indeed, in the adenovirus vaccine studies, about 1 in 4 vaccine recipients took fever-reducing medication (antipyretics), and most people were still protected from severe disease and all were protected against hospitalization. Responses to the protein-based vaccine (Novavax) develop over a period of a couple of weeks, but side effects, like fever, are most likely in the first couple of days after receipt of the vaccine.
Find out more in this Parents PACK article, "Medications and COVID-19 Vaccines: What You Should Know."
If I don’t have side effects, does that mean the vaccine did not work?
Many people will get the vaccine and not experience side effects. This does not mean that the vaccine did not work for them. In the clinical trials side effects occurred at varying rates, for example only about 1 to 20 of every 100 people who received the mRNA vaccine had a fever, but we know that the mRNA vaccine worked for more than 90 of every 100 people.
Last updated: March 1, 2021; reviewed April 24, 2024
What are the expected long-term side effects of the vaccination for COVID-19?
- Most negative effects occur within 6 weeks of receiving a vaccine, which is why the FDA asked the companies to provide 8 weeks of safety data after the last dose.
- mRNA vaccines: The mRNA in the vaccine breaks down quickly because our cells need a way to stop mRNA from making too many proteins or too much of a single protein. But, even if for some reason our cells did not breakdown the vaccine mRNA, the mRNA stops making the protein within about a week, regardless of the body’s immune response to the protein. Read more about COVID-19 mRNA vaccines in this Parents PACK article, “Long-term Side Effects of COVID-19 Vaccine? What We Know.” Watch a short video of Dr. Paul Offit explaining why COVID-19 vaccines would not be expected to cause long-term side effects.
- Protein-based vaccines: The protein is processed within a few days.
Although no longer available in the U.S., it is worth mentioning that the DNA from adenovirus-based vaccines does not break down as quickly as mRNA. The DNA in the vaccine cannot alter our DNA because a gene for the enzyme integrase is not present. These vaccines are processed within about 4 weeks, so they would not be expected to cause any long-term effects either.
Should I stop taking my daily dose of aspirin before getting the COVID-19 vaccine?
If your daily dose of aspirin was prescribed by your physician following a stroke or heart attack, we recommend speaking to that doctor about whether to stop taking your medication for a day or two prior to vaccination. If, however, your daily dose of aspirin is because you have risk factors for a stroke or heart attack (such as high blood pressure or high levels of “bad” cholesterol) but have never had a stroke or heart attack, you should talk to your doctor about discontinuing the aspirin not only prior to your COVID-19 vaccine, but all together. The data show that while daily aspirin helps prevent second strokes or heart attacks, it does not help prevent first occurrences, even in people who are at increased risk. Our director, Dr. Paul Offit, carefully reviewed the data related to this topic for his book, Overkill: When Modern Medicine Goes Too Far .
Last updated: Jan. 24, 2022; reviewed April 24, 2024
What should I do if I took pain medicine before getting the COVID-19 vaccine?
While your initial immune response may have been lower, you will likely still have developed some immunity. Even if your immune response is somewhat lower overall, you are likely to develop sufficient levels of immunity to reduce your chance for infection. In addition, even if you were infected, you would be likely to experience disease that is less severe and of shorter duration.
Can additional doses of the COVID-19 vaccine be from a different company?
Previously unvaccinated children 6 months to 4 years of age and those 5 years and older who are unvaccinated or partially vaccinated AND moderately or severely immune compromised should get all doses of the same brand, except in certain situations. If you are in this group, talk to your healthcare provider to determine the recommendations for your situation.
Those 5 years and older who have completed their initial vaccine series according to the recommendations for their age and immune status can get any brand. For people who are not immune compromised, they are generally considered to have completed their initial series after at least one previous dose. For those who are severely or moderately immune compromised, they are generally considered to have completed their initial series after at least 3 doses of mRNA vaccine or 1 dose of adenovirus- or protein-based vaccine PLUS 1 or more doses of mRNA vaccine. If you are unsure of whether you can switch brands based on your vaccination history, talk to your healthcare provider to help determine the recommendation for your situation.
How long do I need to wait if I had or need to get a non-COVID-19 vaccine?
In most cases, individuals do not need to delay receipt of COVID-19 vaccine and other vaccines; however, if given during the same appointment, the vaccines should be administered in different locations (different arms or separated by at least one inch on the same arm).
The one exception is that people who need to get both an orthopoxvirus vaccine (mpox/smallpox) and a COVID-19 vaccine, particularly teen and young adult males, should consider waiting for 4 weeks between receipt of the two vaccines due to known or potential risks of myocarditis related to individual orthopox and COVID-19 mRNA and protein-based vaccines. However, if the individual is at risk for mpox due to an outbreak or exposure or at risk for severe COVID-19, they should not delay their vaccination given that they would be trading a real risk for a theoretical risk by delaying.
Watch this short video in which Dr. Hank Bernstein explains the benefits of receiving routine vaccines at the same time as the COVID-19 vaccine.
What is multisystem inflammatory syndrome (MIS-C or MIS-A)?
Multisystem inflammatory syndrome can occur in children (MIS-C) or adults (MIS-A). Development of symptoms typically occurs about 4 to 6 weeks after SARS-CoV-2 infection and can occur even in those who did not experience symptoms of COVID-19. Often multiple organs and body systems are involved, including effects on the gastrointestinal tract, heart, kidneys, skin, lungs, and eyes. Individuals with unexplained rash, vomiting or diarrhea, shortness of breath or chest pain or palpitations should seek medical care. Some people with MIS-C or MIS-A will require admission to intensive care and a small number may require mechanical ventilation.
Find out more about MIS-C and long COVID-19 in this video with one of CHOP’s infectious diseases pediatricians.
Watch this short video in which Dr. Offit discusses whether MIS-C after COVID-19 infection is going away.
Watch this short video in which Dr. Caroline Diorio explains the importance of getting kids vaccinated against COVID-19.
Last updated: December 30, 2022; reviewed April 24, 2024
What is long COVID?
Long COVID, also known as post-COVID conditions or long-term COVID, is characterized by long-lasting symptoms related to previous SARS-CoV-2 infection. Symptoms can last for weeks or months after viral clearance and resolution of the initial infection. Examples of the types of symptoms that affected individuals report include fatigue, difficulty thinking or concentrating (“brain fog”), headache, change in or loss of taste or smell, dizziness, heart palpitations, chest pain, shortness of breath, cough, joint or muscle pain, anxiety, depression, sleep problems, feelings like “pins and needles,” diarrhea or stomach pain, rash, changes in menstrual cycle, or fever. Symptoms sometimes appear or worsen after physical or mental activity. People, particularly those who experienced severe COVID-19 infections, may also develop new chronic conditions, such as diabetes, heart conditions or neurological conditions.
Scientists continue to research long COVID. Current theories about the causes include:
- Long-term SARS-CoV-2 replication or reactivation of other viruses that remain in the body from previous infections
- Changes to the immune system’s ability to self-regulate after infection with the virus
- Blood clots (specifically microclots) caused by infection in an array of body organs
- Damage to mitochondria, which are the energy factories in our cells
Watch this short video in which Dr. Offit discusses what we are learning about long COVID and how some of these possibilities would be resolved by different approaches to treatment.
Does a vaccinated person present a risk to an unvaccinated person?
Vaccinated people do not shed virus following vaccination. COVID-19 vaccines do not contain live viruses, nor do they cause production of whole viral particles. As such, there is no infectious virus to spread from a vaccinated person to someone else.
But a vaccinated person can still be infected and potentially spread the virus to others. If they do not have symptoms, they may spread the virus without even knowing they are infected. While vaccinated individuals who become infected can be a source of viral spread, they do not appear to spread as much virus as unvaccinated individuals who become infected because their immune response is able to respond to the infection more quickly – shortening the length of infection and, therefore, the amount of virus produced.
Read more, “Vaccinated or Unvaccinated: What You Should Know.”
What ingredients are in the COVID-19 mRNA vaccine?
The mRNA vaccines include:
- mRNA – The mRNA is for the spike protein of the XBB.1.5 strain of SARS-CoV-2, the virus that causes COVID-19.
- Lipids - These are molecules that are not able to dissolve in water. They protect the mRNA, so that it does not break down before it gets into our cells. These can be thought of as little “bubbles of fat,” which surround the mRNA like a protective wall. There are four different lipids in the Pfizer vaccine and three in the Moderna vaccine. One of the lipids in both vaccines is cholesterol. The lipids are the most likely components of the vaccine to cause allergic reactions.
- Salts and amines - The Pfizer vaccine contains four salts. One is table salt. The salts are used to keep the pH of the vaccine similar to that found in the body, so that the vaccine does not damage cells when it is administered. The Moderna vaccine also contains four chemicals to balance the pH, but two are in a class of organic compounds known as “amines” and two are acetic acid and its salt form, sodium acetate. Acetic acid is the main component of vinegar (other than water).
- Sugar – This ingredient is literally the same as that which you put in your coffee or on your cereal. It is used in both of the vaccines to help keep the “bubbles of fat” from sticking to each other or to the sides of the vaccine vial.
These are the only ingredients in the mRNA vaccines.
NOT in the COVID-19 mRNA vaccines:
- Animal Products
- Antibiotics
- Blood products
- Egg Proteins
- Fetal material
- Pork products
- Preservatives, like thimerosal
Note: The trace quantities of small DNA fragments, which are contained in several biologics, including other vaccines, are well within the levels established as safe by the FDA. To find out more about DNA fragments, see “Do DNA fragments in COVID-19 mRNA vaccines cause harm?” at the beginning of this page.
Watch this short video in which Dr. Paul Offit talks about the ingredients of COVID-19 mRNA vaccines.
What ingredients are in the COVID-19 adenovirus-based vaccine?
Adenovirus-based vaccines are no longer available in the U.S.; however, they are used in other countries.
The adenovirus vaccine includes:
- Adenovirus type 26 (Ad26) containing SARS-CoV-2 spike protein gene and altered so that it cannot replicate
- Stabilizers – Salts, alcohols, polysorbate 80, and hydrochloric acid
- Manufacturing by-products – amino acids
Because the adenovirus-based COVID-19 vaccine is grown in fetal cells and although the product is highly purified, remnants of the fetal cells may remain in the final product.
NOT in the COVID-19 adenovirus vaccines:
- Preservatives, like thimerosal
What ingredients are in the COVID-19 protein-based vaccine (Novavax)?
The protein-based vaccine includes:
- SARS-CoV-2 spike protein from the XBB.1.5 version of the virus
- An adjuvant derived from the soap bark tree (Quillaja saponaria), called Matrix-M
- Stabilizers – Salts (including table salt), polysorbate 80, and hydrochloric acid
NOT in the COVID-19 protein-based vaccine:
Do COVID-19 vaccines contain antibiotics?
No. COVID-19 vaccines do not contain antibiotics.
Watch this short video in which Dr. Hank Bernstein discusses which ingredients are and are not in the COVID-19 mRNA vaccines.
Can mRNA vaccines change the DNA of a person?
Since mRNA is active only in a cell’s cytoplasm and DNA is located in the nucleus, mRNA vaccines do not operate in the same cellular compartment that DNA is located.
Further, mRNA is quite unstable and remains in the cell cytoplasm for only a limited time (See “What stops the body from continuing to produce the COVID-19 spike protein after getting an mRNA vaccine?” below.) mRNA never enters the nucleus where the DNA is located, so it can’t alter DNA. For more details, see “Do DNA fragments in COVID-19 mRNA vaccines cause harm?” at the beginning of this page.
Watch this short video in which Dr. Paul Offit explains why it’s not possible for mRNA vaccines to alter a person’s DNA.
Can adenovirus-based vaccines change the DNA of a person?
Although adenovirus-based vaccines are no longer available in the U.S., they are still used in some other countries and some people in the U.S. received them previously, so it is useful to know that they cannot change a person’s DNA. Adenovirus-based vaccines contain DNA, which enters the nucleus of cells after vaccination, but the virus cannot replicate and the vaccine does not include a necessary enzyme, called integrase. Therefore, the vaccine cannot change a person’s DNA.
What stops the body from continuing to produce the COVID-19 spike protein after getting a COVID-19 mRNA or adenovirus-based vaccine?
Both the mRNA and adenovirus vaccines result in production of spike protein that results from mRNA blueprints. Because our cells are continuously producing proteins, they need a way to ensure that too many proteins do not accumulate in the cell. So, generally speaking, mRNA is always broken down fairly quickly. Even if for some reason our cells did not breakdown the vaccine mRNA, the mRNA stops making the protein within about a week, regardless of the body’s immune response to the protein. Once the mRNA is broken down, the blueprint is gone, so the cell can no longer continue to make spike proteins.
Likewise, while the adenovirus-based vaccine delivers DNA and the DNA lasts longer than mRNA, studies have shown that adenovirus-based DNA does not last longer than a few weeks.
Watch this short video in which Dr. Hank Bernstein explains how the mRNA from the COVID-19 vaccine is broken down and removed from the body.
For more details on the process by which spike protein production is limited, see the “mRNA vaccine” section of this article .
Last updated March 28, 2023; reviewed April 24, 2024
Will the spike protein from current vaccines cause an issue if there are future variants?
The spike protein does not remain in the body for an extended time, nor does it travel around the body. The only thing that remains after the vaccine is processed are antibodies and memory immune cells. To date, previous vaccination against COVID-19 has produced immunologic memory that remains effective against newer variants. However, 2023-2024 COVID-19 vaccines were updated for a newer variant, XBB.1.5.
Is it okay to donate blood after getting the COVID-19 vaccine?
Giving blood after getting the COVID-19 vaccine will not diminish the resulting immune response, which mostly builds in the lymph nodes near the injection site. Likewise, the American Red Cross (ARC) does not require a delay following vaccination with the vaccines currently approved for use in the U.S.; however, individuals must know which brand of vaccine they received and show the immunization card if possible. More details about blood donation are available on the ARC website.
Last updated: March 18, 2021; reviewed April 24, 2024
Are COVID-19 vaccines made in fetal cells?
The mRNA vaccines (those by Pfizer and Moderna) and the protein-based vaccine (Novavax) do not contain fetal cells.
But the adenovirus-based vaccines (no longer used in the U.S.), like Johnson & Johnson/Janssen’s, use cells originally isolated from fetal tissue (often referred to as fetal cells). These fetal cells are used to grow the vaccine virus.
To replicate, a virus needs to take over a cell’s machinery (See this animation ); however, the adenoviruses used in these vaccines have been altered, making them unable to complete the replication process. So, to make the vaccine, these altered viruses need to infect cells that have been changed in a way that allows the defective virus to reproduce. The special cells for this process were isolated decades ago from one of two terminated fetuses and later adapted for the adenovirus reproduction process. Neither of these are used to produce any existing vaccines grown in fetal cells :
- HEK-293 — This is a kidney cell line that was isolated from a terminated fetus in 1972.
- PER.C6 — This is a retinal cell line that was isolated from a terminated fetus in 1985.
These two cell lines have been maintained in the laboratory, and no additional fetuses are needed to produce adenovirus-vector vaccines.
In this short video, Dr. Paul Offit addresses fetal cells and COVID-19 vaccines.
You can find out more about the adenovirus-based vaccines and fetal cells in this Vaccine Update article .
Last updated September 21, 2023; reviewed April 24, 2024
How long will vaccine immunity last?
Discussions related to immunity following COVID-19 vaccination have been fraught with confusion and misinformation. To understand what we have learned from scientific studies, it is important to understand some basics related to immunity in general.
A quick look at immunity
When we are infected with a pathogen, our immune system responds by making B cells and T cells. Some of the B cells are short-lived and have the job of being antibody-producing factories that work to stop the infection. A small number of B cells are long-lived to form one part of our immunologic memory. Likewise, some T cells work to kill virus-infected cells to stop the infection and others serve as army generals, producing chemical signals to control the immune response (ramp it up or down as needed). And, as with B cells, a small number of T cells remain long after the infection to form another part of our immunologic memory.
The long-lasting B and T cells protect us against future infections. If the pathogen is detected, these cells reproduce, so that our immune system overcomes the infection more quickly than it did during the first infection. Whereas first infections can take about a week to control, our immune system is usually at full speed within 3 to 5 days during subsequent infections.
What we have learned about COVID-19 immunity
During a COVID-19 infection, our immune systems produce B cells, antibodies, T cells, chemical signals and immunologic memory — just as in other infections. Antibodies have been found to be important for stopping COVID-19 infections, so in the period of weeks to a few months after an infection or vaccination, lingering antibodies afford protection. But, antibodies are not long lived, so after a few months, we need to rely on immunologic memory. Memory B and T cells have been shown to be important for protection against future infections, particularly certain subsets of T cells. However, as described in the previous paragraph, immunologic memory takes a few days (3 to 5 days) to ramp up. So, during this period, the virus will reproduce in the upper respiratory tract, causing cold-like symptoms (e.g., nasal and sinus congestion, runny nose and coughing) for some and positioning the individual to spread the virus to others (even if they don’t have symptoms). Because of this gap period during which symptoms and transmission can occur, some feel the vaccine doesn’t work. However, the same situation is possible following infection or vaccination, and it will always be the case because of the biology of immunologic memory.
With this said, we have also learned that certain groups of people are at increased risk for severe disease if they are infected. This happens for two reasons — their immune system is not strong enough to overcome the infection by the time their immunologic memory ramps up or one or more components of their immunologic memory is lacking. For these people, help in the form of recently developed antibodies (e.g., vaccination during the part of the year when COVID-19 is circulating) or early treatment with antiviral medications (e.g., Paxlovid™) is most important. The people in these groups include those who are:
- Elderly (65 years and older)
- Moderately or severely immune compromised
- Diagnosed with a chronic disease, particularly heart, kidney, liver or lung disease, diabetes, and obesity, among others (See the CDC’s list here .)
A new monoclonal antibody product, called Pemgarda, is available for some eligible individuals who are at least 12 years of age and are considered moderately or severely immune compromised. Pemgarda is given intravenously every three months. To find out more about the product and who is eligible, talk to your healthcare provider.
In this short video, Dr. Hank Bernstein talks about how long COVID-19 vaccine immunity lasts.
If you had COVID-19, do you still need to get the vaccine?
People who had COVID-19 are recommended to get the vaccine about 3 to 4 months after they have recovered. Some studies have indicated two benefits:
- Vaccination more consistently produces protective immune responses than infection.
- Vaccination provides a wider breadth of protection based on the types of memory responses produced.
In addition, studies have suggested that “hybrid immunity,” that is immunity developed as a result of both infection and vaccination, provides better protection than either vaccination or infection alone.
Watch this video of Dr. Offit explaining why people previously infected with COVID-19 can benefit from receiving the vaccine.
Is a coronavirus vaccine necessary?
SARS-CoV-2 infections can be a minor hindrance or lead to severe disease or even death. Likewise, some people, including children, experience lingering symptoms, called “long COVID,” which is yet to be understood. This virus will continue to circulate in the United States and the world for decades if not longer. While hygiene measures, such as social distancing, handwashing, and wearing masks, offer some help, the best way to stop this virus is to generate SARS-CoV-2-specific immunity. At this point, most people have some immunity either from vaccination or from infection. However, two points are worth considering:
- Most of the individuals being hospitalized for COVID-19 at this point are either individuals who are in a high-risk group or individuals who are unvaccinated.
- Studies have shown that “hybrid immunity,” which is immunity generated when someone has had both vaccination and an infection, is likely superior to immunity generated from infection alone. As such, even people who were previously infected, can benefit from vaccination.
For more information, watch this short video of Dr. Paul Offit addressing “Why does it matter if I don’t get the COVID-19 vaccine?”
How long before a coronavirus vaccine takes effect?
The Pfizer mRNA vaccine requires one to three doses for those greater than 6 months old, depending on age. Protection against severe disease is greatest about two weeks after the last recommended dose. For those getting a single dose (ages 5 years and older), the working assumption is that these individuals were likely exposed to the virus in the community, so the dose of vaccine will enhance their immunologic memory. Unfortunately, we cannot be sure that these individuals were equally immune to begin with, so time will tell whether their immunity is as robust as it is for those who had multiple doses.
The Moderna mRNA vaccine requires one or two recommended doses depending on age. The same working assumption as described in the Pfizer vaccine paragraph above relates to single dose recipients here as well (those 5 years of age and older).
Currently, the broadest, longest-lasting immunity is induced by either three doses of an mRNA-containing vaccine or at least two doses plus a natural infection.
The protein-based vaccine (Novavax) requires two doses. Immunity will be most robust about two weeks after the second dose.
Do the variants affect vaccine effectiveness?
Current vaccines will protect most against severe disease and death independent of what variants are circulating; however, neutralizing antibodies that develop shortly after receipt of a dose will fade in a few months, so people can still develop mild illness. For some with immune conditions that affect their response to the vaccine or infection and for those of older age, immune protection is less robust, so they are more likely to benefit from an additional dose. See more details in the answer to “How long will vaccine immunity last?”
Can pregnant people get the COVID-19 vaccine?
With data from tens of thousands of pregnant people now in hand, no concerns have been identified and the vaccine works. Further, we now know that:
- Pregnant people are at higher risk for severe COVID-19 compared with those of the same age who are not pregnant.
- Vaccination during pregnancy also affords some protection to the baby in the months after delivery and before they are old enough to be vaccinated.
Pregnant people who get the COVID-19 vaccine should take acetaminophen if they develop a fever after vaccination, as fever during pregnancy can negatively affect a developing baby. Taking acetaminophen during pregnancy has been found to be safe.
In this short video, Dr. Hank Bernstein discusses COVID-19 vaccination during pregnancy.
You can read more about pregnancy and COVID-19 vaccines in this Vaccine Update article.
Drs. Paul Offit and Ripudaman Minhas discuss vaccines, pregnancy, development and autism in this video.
Last updated: April 27, 2023; reviewed April 24, 2024
Can I get the COVID-19 vaccine if I am breastfeeding?
Yes. COVID-19 is not transmitted through breast milk and vaccination has not caused a concern either.
In addition, breastfeeding does not need to be delayed for any period of time before or after vaccination.
Babies may benefit from antibodies or immune cells introduced through breast milk after the mother is vaccinated. This is called passive immunity.
Both the Academy of Breastfeeding Medicine and the American College of Obstetricians and Gynecologists support this approach.
Hear from Dr. Caroline Diorio, a pediatric oncologist, about the importance of getting the COVID-19 vaccine during pregnancy.
In this short video, Dr. Hank Bernstein discusses COVID-19 vaccination when breastfeeding.
You can read more about breastfeeding and COVID-19 vaccines in this Vaccine Update article.
Drs. Paul Offit and Amna Husain discuss vaccines and breastfeeding in this video.
Can I get the COVID-19 vaccine if I am trying to get pregnant?
Yes, people who are trying to get pregnant can get the COVID-19 vaccine. Likewise, vaccination can be finished during pregnancy, and it is important to do so since pregnancy increases one’s risk of being hospitalized and having preterm births if infected with COVID-19 before delivery.
Last updated: June 23, 2022; reviewed April 24, 2024
Should I delay getting pregnant if I got the COVID-19 vaccine?
No, you do not need to delay pregnancy. The COVID-19 vaccines do not present a cause for concern related to pregnancy.
Last updated: Jan. 25, 2022; reviewed April 24, 2024
Why was I told to wait a month after getting the COVID-19 vaccine before getting a mammogram?
Some people experience swelling of the lymph nodes under their vaccinated arm after getting the COVID-19 mRNA vaccine. Because this could be mistakenly identified as spread of breast cancer to lymph nodes, delaying the mammogram can prevent the chance of this happening.
Why was I asked if I recently received the COVID-19 vaccine on the questionnaire for my MRI?
People occasionally experience swelling of the lymph nodes near the vaccine injection site, which could interfere with interpreting the results of the MRI depending on what location is being imaged.
Last updated: Jan. 25, 2022; reviewed April 24, 2024
Is it necessary to wait to get blood work done after getting the COVID-19 vaccine?
Generally speaking, it would be recommended to wait about a week after getting the mRNA vaccine and a few weeks after getting the adenovirus-based vaccine before getting bloodwork. Delays are not likely to be needed after receipt of the protein-based vaccine. However, it would be better to inquire with the healthcare provider who ordered the bloodwork as they have the benefit of knowing the reason for the bloodwork, the type of tests ordered, and the patient’s medical history. As such, they will be in the best position to offer this guidance for each individual situation.
If I have an autoimmune or immune-compromising condition, can I be vaccinated?
Most people with immune-compromising conditions may get the COVID-19 vaccine as long as they do not have a severe allergy to a vaccine component (i.e., one that causes anaphylaxis or requires medical intervention).
However, it is recommended that individuals with compromised immune systems discuss their personal risks and benefits with a healthcare provider to determine whether to receive the vaccine or if they may need additional doses.
Knowing the potential for a lower immune response, if someone with an immune-compromising condition decides to get vaccinated, it will be important to get all recommended doses, depending on their condition. Some people may be eligible for ongoing treatment with a monoclonal antibody product called Pemgarda, and some may choose to continue practicing other public health measures during periods of high virus circulation.
Last updated: June 23, 2022; reviewed April 24, 2024
Can I get the COVID-19 vaccine if I had Guillain-Barré Syndrome (GBS)?
People with a history of Guillain-Barré Syndrome (GBS) can get the COVID-19 vaccine, as long as they do not have another condition that puts them among the people recommended against vaccination. A small number of cases of GBS (about 1 of 100,000 people) have been identified following receipt of the adenovirus-based COVID-19 vaccine (J&J/Janssen); however, this vaccine is no longer used in the U.S.
A note about GBS and influenza vaccines
Some people wonder if they can get the COVID-19 vaccine if they developed GBS following receipt of an influenza vaccine. Since COVID-19 and influenza (flu) vaccines are made differently, people with this history would not be expected to have an issue with COVID-19 vaccine. As such, they are still recommended to get COVID-19 vaccine.
Finally, many people are incorrectly told that if they had GBS, they cannot get a flu vaccine. However, most people with a history of GBS can get the flu vaccine. Only people who were diagnosed with GBS less than 6 weeks after receipt of influenza vaccine are considered to have a “precaution” for receipt of influenza vaccine, meaning that the patient and the healthcare provider should discuss the relative risks and benefits associated with getting the influenza vaccine. In fact, studies have shown that influenza disease presents a greater risk of GBS than influenza vaccination. Find out more:
- ”Vaccines and Guillain-Barré Syndrome” webpage
- Guillain-Barré Syndrome (GBS) & Vaccines: The Risks and Recommendations , September 14, 2021, Parents PACK newsletter
Can I still get vaccinated if I have a cold?
People with mild cold-like symptoms are not prevented from getting the COVID-19 vaccine. However, if they are not feeling well, their symptoms just started, or their symptoms are getting worse, they may want to delay vaccination until they feel better; otherwise, they might not be able to tell effects of illness from those of the vaccine. If they are uncertain, they should speak to their doctor, who has the benefit of their medical history and will be in the best position to help them weigh the potential pros and cons. This advice is similar for other vaccines as well.
If I am taking anticoagulants (blood thinners), can I get the COVID-19 vaccine?
Patients on blood thinners can get the COVID-19 vaccine. However, because the vaccine is given intramuscularly, the risk for bleeding is slightly greater for these individuals. As such, they should tell the healthcare provider administering the vaccine about their use of an anticoagulant. The vaccine itself does not increase the risk for this group of patients. The same advice is true for other vaccines that are injected as well.
Last updated: April 28, 2023; reviewed April 24, 2024
If I am currently taking antibiotics, can I get the COVID-19 vaccine?
As long as you are not still sick from your recent infection, you can get the COVID-19 vaccine even if you are taking an antibiotic. But, if you are still having symptoms, you should wait until you are feeling better, so that it is easier to tell if any new symptoms are from your infection or the vaccination.
Last updated: Sept. 28, 2021; reviewed April 24, 2024
If I am taking antivirals, can I get the COVID-19 vaccine?
You do not need to stop taking antiviral medication before vaccination. Because the COVID-19 vaccines being used in the U.S. do not rely on viral replication, antivirals should not affect development of the immune response. However, if you are still experiencing symptoms of the infection for which the antivirals were prescribed, you should wait until you are feeling better before getting the vaccine. This will allow you to distinguish symptoms from your infection with side effects from the vaccine.
If I am taking biologics, can I get the COVID-19 vaccine?
Taking biologics, like Humira, is not a reason to forgo COVID-19 vaccination as per CDC guidelines. However, patients taking these types of medication may wish to consult with their doctor to discuss the potential risks and benefits of getting the COVID-19 vaccine, given that these types of medications are often prescribed for individuals with immune-compromising conditions. As a result, there may be other considerations related to the potential risks and benefits of vaccination.
For general information about vaccines and biologics, check out this printable Q&A sheet.
Last updated: Jan. 25, 2021; reviewed April 24, 2024
How long should I wait to get the COVID-19 vaccine after getting a steroid injection or vice versa?
You should speak with your doctor to determine whether the quantity of steroids that you are receiving is suppressing your immune system. If so, you should hold off on receiving vaccines until the effect of the steroids has worn off.
Does the COVID-19 vaccine cross the blood-brain barrier?
It would not be expected that the COVID-19 vaccines would cross the blood-brain barrier (BBB) for a few reasons.
mRNA vaccines:
- Most of the protein that is made is bound to cells - The vaccine is injected into muscle, where mRNA from the vaccine causes production of COVID-19 spike protein. The protein (not the mRNA) is then processed by dendritic cells where pieces of the protein are put on the cell surface before the dendritic cell travels to the nearest lymph node and stimulates other cells of the immune system to make an immune response against the protein. This process is typical of our adaptive immune system, which you can find out more about in this animation , or you can watch this animation that describes how the mRNA vaccine is processed .
- Even if the protein left the cell whole (which it doesn’t), it is too large to cross the BBB.
Adenovirus vaccine (no longer used in the U.S.):
- As with the mRNA vaccine, pieces of the protein that result from vaccine processing are put on dendritic cells that travel to the nearest lymph node. See how the adenovirus-based vaccine is processed in this animation .
- The virus used to deliver the vaccine is also too large to cross the BBB.
Protein-based vaccines (e.g., Novavax) would not be expected to cross the BBB either as the proteins are too large.
Does the COVID-19 vaccine cause antibody-dependent enhancement (ADE)?
Antibody-dependent enhancement (ADE) occurs when the antibodies from a previous infection (or vaccination) help the virus gain access to cells rather than blocking access to cells. Getting an infection after vaccination does NOT provide evidence of ADE. These are two distinct immunologic phenomena.
ADE has not been identified as a concern related to SARS-CoV-2 infection or following COVID-19 vaccination. In fact, a body of evidence has suggested that ADE is not a concern:
- First, most people have been infected with other coronaviruses in their lifetime, and ADE has not been identified as a result of these infections.
- Second, in human studies, people previously infected with coronavirus were infected with different types of coronavirus, and they did not experience enhanced disease.
- Third, experimental animals vaccinated against SARS-CoV-2 did not develop enhanced disease when challenged, or infected, with the virus.
- Fourth, when people with COVID-19 received plasma containing SARS-CoV-2 antibodies, they did not experience enhanced disease.
- Finally, millions of people have been vaccinated against COVID-19. Some of them have subsequently been infected with SARS-CoV-2, and none of them have shown evidence of ADE.
Watch a short video in which Dr. Paul Offit explains why COVID-19 vaccines are unlikely to cause ADE.
Does the COVID-19 vaccine cause fertility issues?
Infertility has not been found to be an issue in women or men infected with or vaccinated against COVID-19.
Unfortunately, misinformation about fertility-related issues continues to circulate. These concerns take a few forms:
- Compromised fertility in the vaccine recipient – Some concerns related to a placental protein, called syncytin-1. This protein is associated with the placenta during pregnancy. Online claims early during the pandemic promoted a paper suggesting that a small number of similar amino acids in the spike protein and the placental protein would cause vaccine-induced antibodies to react against syncytin-1. Since human proteins are made using the same 20 amino acid building blocks, many proteins have short sections that are similar to one another. However, most of our antibodies do not cross-react with other proteins because a variety of other factors come into play. The most important of which is antibody specificity to the three-dimensional version of its target. As such, while a theoretical paper like the one previously mentioned can generate an interesting hypothesis, the idea requires clinical confirmation, which never materialized for this idea that unfortunately spread quickly and, quite frankly, unnecessarily scared people.
- Some concerns related to males, and whether the vaccines could decrease sperm count. While fever can cause a temporary decrease in sperm count, there is no biologically plausible reason to expect that the vaccines would cause any long-term effect on sperm count.
- Compromised fertility in individuals near someone who recently received COVID-19 vaccine – This misperception conflates two concepts: effects on fertility and viral shedding. As mentioned above, the vaccines do not affect fertility in the vaccinated person, so there would not be a reason to expect that they would affect someone else’s fertility. Second, it assumes that recently vaccinated individuals shed virus or spike protein. Neither of these occur. While these vaccines cause the body to generate spike protein, they do not cause production of whole virus particles, nor do parts of the vaccine migrate to the nasal cavity. As such, a recently vaccinated person does not shed any part of the virus and cannot, therefore, spread vaccine-related components to another person.
This Parents PACK article about vaccination of children 5 to 11 years of age also addresses fertility-related concerns.
Is there any hope that a vaccine will help people with lingering aftereffects from coronavirus?
Clinical studies are underway to determine whether antiviral medications might help ease the lingering effects of COVID-19 infection, and studies of vaccination have suggested that by decreasing the severity of infection, fewer people experience long-term symptoms. However, vaccination does not appear to help ease symptoms in people who are already suffering the lingering effects of an infection.
Does the COVID-19 vaccine contain blood products?
The COVID-19 vaccines available in the U.S. do not contain any blood products, including red blood cells, white blood cells or platelets.
Watch this short video in which Dr. Offit talks about the ingredients used in the COVID-19 mRNA vaccines.
Do COVID-19 vaccines contain a microchip?
COVID-19 vaccines do not contain microchips. This idea is based on a false narrative and misinformation campaign waged online. You can find out more about where this idea came from on snopes.com .
Last updated: Dec. 15, 2020; reviewed April 24, 2024
If my baby has had some of her vaccines, is she protected from COVID-19?
A baby’s other vaccines will not protect them from COVID-19.
If the baby is at least 6 months of age, she can receive the COVID-19 vaccine; however, she should not be considered immune until at least 2 weeks after receipt of her last dose.
COVID-19 video resources
This section of the page will house video resources and interviews related to COVID-19.
Vaccine Makers Project videos and animations The Vaccine Makers Project (VMP) is the classroom program of the Vaccine Education Center (VEC). VMP resources include a variety of science-based animations that show not only how COVID-19 vaccines work, but also how viruses take over our cells and how our immune systems work.
Talking about Vaccines with Dr. Paul Offit: COVID-19 This VEC playlist features several short videos in which Dr. Offit addresses common questions about COVID-19.
Talking about Vaccines with Dr. Hank Bernstein: COVID-19 This playlist features a series of short videos in which Dr. Hank Bernstein answers common questions about COVID-19 vaccines.
Materials in this section are updated as new information and vaccines become available. The Vaccine Education Center staff regularly reviews materials for accuracy.
You should not consider the information in this site to be specific, professional medical advice for your personal health or for your family's personal health. You should not use it to replace any relationship with a physician or other qualified healthcare professional. For medical concerns, including decisions about vaccinations, medications and other treatments, you should always consult your physician or, in serious cases, seek immediate assistance from emergency personnel.

IMAGES
VIDEO
COMMENTS
Answer. Answer: A COVID‑19 vaccine is a vaccine intended to provide acquired immunity against severe acute respiratory syndrome coronavirus 2 (SARS‑CoV‑2), the virus causing coronavirus disease 2019 (COVID‑19). Prior to the COVID‑19 pandemic, there was an established body of knowledge about the structure and function of coronaviruses ...
Answer. Answer: The COVID-19 vaccine is super important. It helps stop the spread of the virus and keeps people safe. Scientists and doctors worked really hard to make the vaccine. They did tests to make sure it worked and didn't have any bad side effects. There are different types of vaccines, like the Pfizer and Moderna ones.
For some COVID-19 vaccines, two doses are required . It's important to get the second dose if the vaccine requires two doses. For vaccines that require two doses, the first dose presents antigens - proteins that stimulate the production of antibodies - to the immune system for the first time. Scientists call this priming the immune response.
العربية. 25 October 2022. Vaccines save millions of lives each year. The development of safe and effective COVID-19 vaccines are a crucial step in helping us get back to doing more of the things we enjoy with the people we love. We've gathered the latest expert information to answer some of the most common questions about COVID-19 ...
In the short term, a person who has had a COVID-19 vaccine may experience flu-like symptoms and other side effects, including: •pain at the injection site. •swelling at the injection site. •fatigue. •headache and muscle pain. •a fever. The side effects may be worse after the second dose of the vaccine because the body's immune ...
The COVID-19 pandemic has led the world to undertake the largest vaccination campaign in human history. In record time, unprecedented scientific and governmental efforts have resulted in the acquisition of immunizers utilizing different technologies (nucleotide acids, viral vectors, inactivated and protein-based vaccines).
Amid the staggering amount of suffering and death during this historic pandemic of COVID-19, a remarkable success story stands out. The development of several highly efficacious vaccines against a previously unknown viral pathogen, severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), in less than 1 year from the identification of the virus is unprecedented in the history of vaccinology.
Answer: Today marks the start of the World Immunization Week. The COVID-19 pandemic, while draws the world's attention to the vaccine, also reminds us of the importance of immunization, which saves millions of lives each year. WHO is working with partners all over the world to accelerate research and development of a safe and effective ...
Ahead of the COVID-19 vaccine rollout, health agencies in many countries issued guidance for phased vaccination campaigns, with health-care workers and residents of nursing homes and long-term ...
In the case of [COVID-19 vaccines], we had a lot of urgency and all the money was put up, up front. The companies didn't have to find the money for each stage—they were just able to just proceed from the safety study to the effectiveness study very quickly. This let the coronavirus vaccines go to the front of the line because of the urgency.
While efficacious vaccines have been developed to inoculate against severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2; also known as COVID-19), public vaccine hesitancy could still ...
In a human challenge vaccine study, healthy volunteers are given an experimental vaccine, and then deliberately exposed to the organism causing the disease to see if the vaccine works. Some scientists believe that this approach could accelerate COVID-19 vaccine development, in part because it would require far fewer volunteers than a typical study.
The COVID-19 vaccine is on track to become the fastest-developed vaccine in history. That doesn't mean the process is skipping any critical steps. Understanding what we know—and still don't—about a vaccine for COVID-19 can help shed light on its safety and efficacy.
Development. Pfizer's COVID-19 vaccine was the quickest vaccine to be developed, taking just about 7 months after its phase I/II trial took place in May 2020 for the FDA to allow for its emergency use in December 2020. 8 The previous record set by pharmaceutical company Merck took 4 years to develop the world's first effective vaccine against mumps in 1967.
Here, two experts to make the case for and against mandatory COVID-19 vaccines. Alberto Giubilini, Senior Research Fellow, Oxford Uehiro Centre for Practical Ethics, University of Oxford. COVID-19 ...
1. Safety and immunogenicity study of 2019-nCoV vaccine (mRNA-1273) for prophylaxis of SARS-CoV-2 infection (COVID-19) This clinical trial is designed to assess the safety, reactogenicity, and immunogenicity of mRNA-1273. It encodes for a full-length, prefusion stabilized spike (S) protein of SARS-CoV-2.
Examples of Persuasive Essay About Covid-19 Vaccine. Covid19 vaccines are one of the ways to prevent the spread of Covid-19, but they have been a source of controversy. Different sides argue about the benefits or dangers of the new vaccines. Whatever your point of view is, writing a persuasive essay about it is a good way of organizing your ...
Answer: A COVID‐19 vaccine is a vaccine intended to provide acquired immunity against severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2), the virus causing coronavirus disease 2019 (COVID‐19). The COVID-19 vaccines produce protection against the disease, as a result of developing an immune response to the SARS-Cov-2 virus.
The COVID-19 vaccination will help keep you from getting the virus. COVID-19 vaccines were evaluated in clinical trials and have been approved because those studies show that the vaccine significantly reduces the probability of contracting the virus. Based on what has been proved about vaccines for other diseases, the COVID-19 vaccine may help ...
A novel coronavirus (CoV) named '2019-nCoV' or '2019 novel coronavirus' or 'COVID-19' by the World Health Organization (WHO) is in charge of the current outbreak of pneumonia that began at the beginning of December 2019 near in Wuhan City, Hubei Province, China [1-4]. COVID-19 is a pathogenic virus. From the phylogenetic analysis ...
Answer: Researchers around the world are working hard on accelerating the development of vaccines and therapeutics for COVID-19. WHO has launched various working groups to accelerate various aspects of vaccine development. A call was made by 130 scientists, funders and manufacturers to help speed the availability of a vaccine against COVID-19.
Essay of covid 19 vaccine . Answer: The coronavirus disease 2019 (COVID-19) has turned into a global human tragedy and economic devastation. Governments have implemented lockdown measures, blocked international travel, and enforced other public containment measures to mitigate the virus morbidity and mortality.
The Novavax COVID-19 vaccine uses a "tried and true" approach to inducing immunity. Specifically, the vaccine delivers the spike protein and an adjuvant, which is something that increases immune responses to the protein. It is given as two doses, separated by 3-8 weeks, to those 12 years of age and older.