Thank you for visiting nature.com. You are using a browser version with limited support for CSS. To obtain the best experience, we recommend you use a more up to date browser (or turn off compatibility mode in Internet Explorer). In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript.
- View all journals
- My Account Login
- Explore content
- About the journal
- Publish with us
- Sign up for alerts
- Open access
- Published: 09 July 2022

Evaluation of water quality index and geochemical characteristics of surfacewater from Tawang India
- Nisha Gaur 1 ,
- Arpan Sarkar 1 ,
- Dhiraj Dutta 1 ,
- B. J. Gogoi 1 ,
- Rama Dubey 1 &
- Sanjai Kumar Dwivedi 1
Scientific Reports volume 12 , Article number: 11698 ( 2022 ) Cite this article
9590 Accesses
36 Citations
Metrics details
- Biogeochemistry
- Environmental sciences
In this study,the water samples were collected from 31 sites of Tawang, Arunachal Pradesh, India (North-Eastern Himalaya), during the winter season to check the suitability of water for drinking and irrigation purposes.The study scientifically demonstrates the estimation of Water quality index (WQI) andhydrogeochemical characteristics of surface water samples by utilizing multivariate statistical methods. The main water quality parameters considered for this study were TDS, conductivity, salinity, pH, hardness, cations and anions. WQI was calculated in order to find out the deviation in the water quality parameters particularly with respect to BIS permissible limits.The major influencing factors responsible for the variation in these parameters were derived by using Principal component analysis (PCA) and Correlation matrix.To check the suitability of water for drinking purpose, hydrogeochemical facies and rock water interaction was derived by using well established methods such as Piper Plot (determine water type), WQI (Quality monitoring), and saturation index (for mineral dissolution). The results revealed that the silicate weathering was the main ionic source in comparison to carbonate weathering which is due to the higher dissolution capacity of silicate minerals.The results of the scattered plot between (Ca 2+ + Mg 2+ )–(HCO 3 ˉ + SO 4 2 ˉ) versus (Na + + K + )–Clˉ (meq/L) highlighted thation exchange occurs between Mg 2+ and Ca 2+ ofsurface water with Na + and K + of rock /soil. This means that calcium ion was getting adsorbed, and sodium ion was getting released. The Ca 2+ –Mg 2+ –HCO 3 ˉ, Na + –HCO 3 ˉand Na + –Clˉ type of surface water suggested permanent and temporary hardness respectively in the studied region. The dominant cations of this study were Na + and Ca 2+ while the dominant anions were HCO 3 ˉ and SO 4 2 ˉ. In order to check the suitability of water sources for irrigation, parameters like, Magnesium hazard (MH), Total hardness (TH), Permeability Index (PI), Kelly Index (KI), Sodium adsorption rate (SAR), Sodium percentage (Na%), and Residual sodium carbonate (RSC) were determined. The results showed that 93% of the samples had PI score < 75, which indicates the suitability of the water for irrigation. Also the WQI calculation showed an average WQI value of 82.49, amongst which 61% samples were in the range of 0–50 being considered as good for drinking, while 39% were catageorised as unsuitable for drinking showing a value of > 50. Hence the above findings reveal that geogenic activities play a major role in influencing the water quality of Tawang region. Hence suitable water treatment technologies or methods might be used to eliminate thenon desirable elements and minerals present in surface water.
Similar content being viewed by others
Groundwater quality assessment using water quality index and principal component analysis in the Achnera block, Agra district, Uttar Pradesh, Northern India
Hydro-chemical characterization and irrigation suitability assessment of a tropical decaying river in India
Assessment of drinking water quality using Water Quality Index and synthetic pollution index in urban areas of mega city Lahore: a GIS-based approach
Introduction.
For all life forms present on earth water is a basic need fulfilled from variousnatural resources. It is desired that, the water being consumed should not contain any microbes or harmful chemicals thatcan cause damage to life. Due to industrialization, surface and groundwater has been contaminated with a widevariety of pollutants.Both natural (precipitation, the geography of the watershed, atmosphere, and geology) and anthropogenic (industrial activities, domestic and/or agricultural run-off) activities determine the chemical, physical and biological composition of surface and groundwater. Water contamination leads to deterioration of water quality whichthreatens the life present on earth as well as disturbs theeconomic advancement and social success 1 , 2 .
Tawang, a small district in Arunachal Pradesh, Indialocated at an altitude of 4500 m is surrounded with high mountains, glaciers and lakes. The water requirement of local population is met from the surface water sources such as springs, lakes, ponds and rivers. At the mentioned altitude, the only propable sources of contamination are natural and geogenic activities. Few reports onwater quality of Eastern Himalayan region showed severe fecal contamination of surface water sources and the reservoir tank used by the community, leading to high health risk 3 , 4 . Additionally, the geological composition of sourrounding soil and rock-formation strongly affects the water quality, since the flowing and stagnant water comes in their close contact.Now a days hydrogeochemical studies are gaining momentum worldwide to ascertain the impact of geological strata on water quality of natural water bodies. The studies throw light on water rock interaction leading to presence of certain minerals in the water mainly derived from weathering of rocks and dissolution of minerals. The reported studies have highlighted the presence of silicates, carbonates, alkali and alkaline earth metals, heavy metals etc. based on the hydrogeochemical analysis of water sources by using different statistical approaches 5 . Hence the hydrogeochemical characterization of surface water demonstrates thelevel of minerals and ions contributing to aquifer's composition. Furthermore, the surface water is also susceptible to other forms of contamination arising from domestic activities. The constant decline of surface water quality not only affects the humans but also poses a serious threat to the ecosystems flourishing within it. Hence, to maintain the health of any water source, certain water quality indicators or parameters must be monitored regularly to keep the aquifer system performing at its best 6 , 7 . Regular monitoring of water quality is a crucial part for determining the baseline data which would help in identifying any existing problems, or any issues that could emerge in the future related to water quality.
Water quality is generally defined by its physicalapperance (colour, odour, taste), chemical (pH, turbidity, hardness, alkalinity, total solids, presence of metallic or non-metallic salts) and biological properties.Many researchers have proposed WQI in the form of a simple expression in order to represent the general quality of surface water as there are a variety of physical, chemical and biological water quality parameters 8 , 9 , 10 . It is a concise and comprehensive method to express the quality of water for different stages of usage and is commonly represented by a single number. WQI of any water sample is calculated by aggregating the values of different parameters that givesa single number which expresses the quality or contamination status of water. WQI not only can be used for the prediction of pollutants present in water but also compares the water qualities of different sources and hence decides the proper usage of water resources 11 , 12 . It is very difficult to comprehend a complex and large data matrix comprising a large number of parameters while calculating WQI. To overcome this problem and make the process less subjective, different multivariate techniques (Principal Component Analysis (PCA), factor analysis, etc.) helps in better interpretation of the results. Several parameters are monitored and analyzed simultaneously along with studying environmental issues. Ideally, PCA can reduce the dimensionality of the multivariate data set while maintaining its structure to the maximum extent. Hence, while dealing with environmental data PCA has often been used 13 .
In recent years Geographic Information System (GIS) technique is emerging as a powerful tool for storing, assessing, monitoring and displaying spatial dataof surface and groundwater quality. This tool is also effective in developing solution for water resource related problems, understanding the natural environmentand managing water resources on required scale.Additionally, for evaluation and analysis of spatial information of water resources, Inverse Distance Weighted (IDW) interpolation methodalong with GIS techniques has proved as a powerful tool. For transforming huge data sets to generate various spatial distribution maps and projections revealing trends, associations, and sources of contaminant it is an economically feasible and time-efficient technique 14 , 15 , 16 .
Therefore, in the present study, the physico-chemical and bacterial properties of surface water samples collected from different sites of Tawang were determined and compared with other related studies. Main emphasis was laid on interaction between physico-chemical parameters of water by using Principal component analysis (PCA) and correlation matrix. The selected parameters were normalized by using a z-score before PCA as it requires individual indicators to have a common unit. In the final step, the Pearson correlation analysis was done followed by water quality index calculation. These statistical analysis tools are important to understand the vital variables which affect the quality of water. For spatial evaluation of various surface water quality parameters, the GIS technique has been used.
Furthermore, the hydrogeochemical facies and hydrogeochemical signatures such as ion exchange process, rock-water interaction, and dissolution were also studied for the analysis of chemical characteristics of surface water. Conventional graphical plots such as Magnesium hazard (MH), Total hardness (TH), Permeability Index (PI), Kelly Index (KI), Sodium adsorption rate (SAR), sodium percentage (Na%), and residual sodium carbonate (RSC) have been used to determine the various hydrogeochemical processes controlling the hydrochemical characteristics of water collected from the study area. Hence this study will throw some light on the untouched surface water sources in the Tawang area and thus can act as a baseline for water quality assessment in various other districts of Arunachal Pradesh and Northeastern region of India.The ultimate objective of the present study was to highlight the interaction between geogenic factors and water and correlate it with the water quality index, so that its suitabilitycan be ascertained for potability and irrigation purpose.
The Ecological hotspot of Tawangis in the Arunachal Pradesh (latitude 91° 33’ E to 92° 26’E; longitude 27° 29’N to 27°52’N) at an altitude of 1800–3300 m above mean sea level in Eastern Himalayan Region (Fig. 1 a and b). This place is famous for its 400-year-old biggest Buddhist monastery and important pilgrim center for the followers of Buddhism. It is also famous for its natural beauty which enchants the traveller. It was observed that the maximum number of tourists visit this place during the summer season due to pleasant weather. The people of the forward location of Tawang depend on natural water sources for their daily needs. Because of the tough terrain, it is very difficult to bring fresh water from other locations so there is a need to preserve these water bodies.
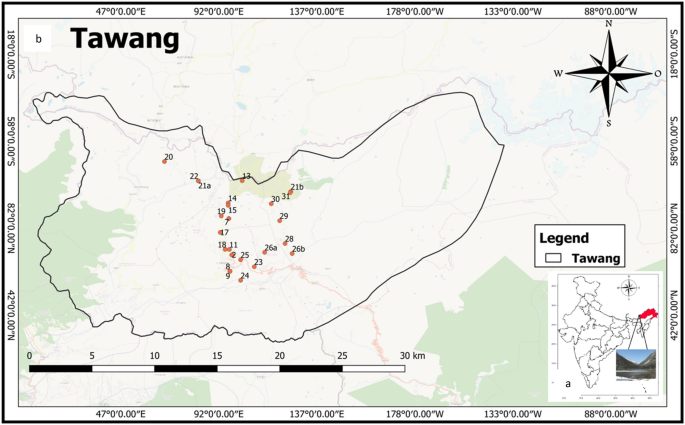
Location Map of Ecological hotspot of Tawang, Arunachal Pradesh, India. ( a ) Location of Tawang on Indian map ( b ) Sample collection sites. QGIS Software Desktop 3.18.3 ( https://qgis.org/en/site/forusers/download.html ).
During winters and the rainy season (because of landslides and heavy snowfall), the survival condition is very harsh and very difficult for local people to collect potable water from a nearby area. Therefore, this area was selected to check the water quality from different natural water sites of Tawang. As the population of this area is comparatively less and no anthropogenic activity was observed so thirty-one different natural freshwater sites alongthe forward location of Tawang, Arunachal Pradesh were chosen for sample collection to ascertain thesutability of water quality for drinking and irrigation.
Methodology
The water sampling from 31different locations of Tawang district, Arunachal Pradesh was done during the winter season (December 2020) as per the standard procedures of the American Public Health Association 17 . For the collection and analysis of various water variables, standard methods were followed 17 , 18 . With the help of a global positioning system (GPS) as shown in Fig. 1 b, the sampling locations were marked. All the plastic bottles were thoroughly washed and dried before sample collection and the bottles were rinsed with water sample to be collected at the time of collection. After sample collection proper labelling was done. The latitude, longitude, and altitude of all the sampling sites along with the source were recorded during the sample collection using a GPS system (Model: Garmin GPS 72H) as mentioned in Table 1 .
Physiochemical parameters determination
After sample collection, the bottles were taken to the laboratory in an icebox to avoid any unusual change in water quality and stored at 4 °C for further analysis. Electrical conductivity, TDS, and salinity were analyzed with the help of EUTECH Instruments CD650 (Thermo Scientific, United State). A digital pH meter (EUTECH pH 610) was used to estimate pH. Turbidity meter (EUTECH TN 100) was used to determine turbidity and iron was estimated by using a UV–Vis spectrophotometer (Analytikjena SPECORD 205). The acid titration method was carried out for Bicarbonate analysis. Chloride, Nitrite, Flouride, Nitrate, and Sulfate were evaluated by using Ion Chromatography (Metrohm, 882 Compact IC plus, 858 Professional Sample Processor). Sodium, Potassium, Calcium, and Magnesium were analyzed by following the standard methods specified in IS-1500:2012 18 , 19 . All the analysis was done as per the mathods methods mentioned in APHA 20 .The Physiochemical properties and spatial distribution pattern of collected water samplesare shown in Fig. 2 a–q.
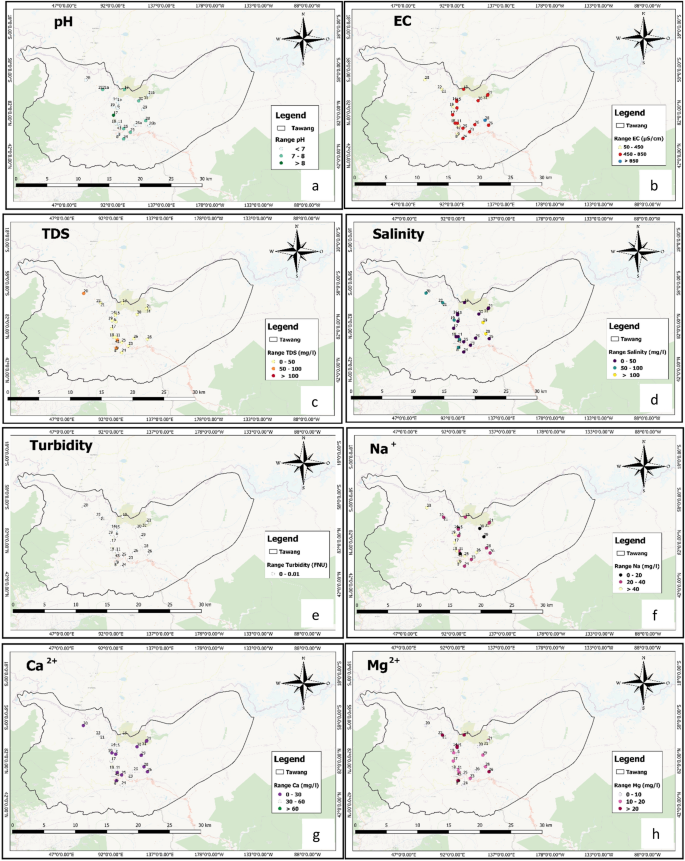
Spatial distribution map of ( a ) pH, ( b ) EC, ( c ) TDS, ( d ) Salinity, ( e ) Turbidity, ( f ) Sodium (Na + ), ( g ) Calcium (Ca 2+ ) and ( h ) Magnesium (Mg 2+ ). Spatial distribution map of ( i ) Potassium (K + ), ( j ) Iron (Fe 2+ ), ( k ) Chloride (Cl - ), ( l ) Bromide (Br - ), ( m ) Fluoride (F - ), ( n ) Nitrite (NO 2 - ), ( o ) Nitrate (NO 3 - ), ( p ) Phosphate (PO 4 3- ) and ( q ) Sulphate (SO 4 2- ) QGIS Software Desktop 3.18.3 ( https://qgis.org/en/site/forusers/download.html ).
Quality assurance and quality control (QA/QC)
Standard solutions were used for the pre-calibration of all the instruments used for all the analysis as per the company guidelines to maintain the accuracy and precision in observations. Before each in situ observation all the electrodes were properly washed with double distilled water. Conditioning of probes should be done in the sample before each use for best stabilization time. Freshely prepared buffer solutions of two different units were used for calibration of rinsed and dry pH probe. For each chemical analysis, a blank sample had been run for each chemical analysis and for each quality parameter at least three observations had been taken. Standard operating protocols were adopted for each instrument and chemical analysis with adequate safety throughout the study period. To get the accurate data/information the analytical grade chemicals and glasswares (Borocile, Merk Thermo Fishers) were used for chemical analysis. To get the linear calibration curves in Ion chromatography, the calibration was performed by running the replicate standards of different anions.
Multivariate statistic methods
Kolmogorov–Smirnov (K–S) test with the SPSS 21.0 Pro software packages (SPSS Ins., Chicago., USA) ( https://www.ibm.com/support/pages/downloading-ibm-spss-statistics-21 ) was used to assess the normality of the water quality parameters. To compare the spatial and temporal variations of 15 water quality parameters measured in different sites of Tawang, descriptive statistical analysis including one-way Analysis of Variance (ANOVA) was performed. Tukey’s multiple range tests were performed to evaluate the large differences in mean values of water quality parameters. The Pearson’s correlation coefficient (r) was calculated to determine the correlation between variables. Principal Component Analysis (PCA)was used to analyze the spatial and temporal changes in water quality. In addition, PCA also extracts the pollution factor and recognizes pollution sources applied in water quality analysis. PCA also reduces the data sets and enables the formation of new factors. In this case, the parameters have been reduced from 15 to 13. The classification of factor loading is strong (> 0.75), moderate (0.75–0.50), and weak (0.50–0.30) relating to the absolute loading value. To consider the suitability of the data to run PCA, Kaiser–Meyer–Olkin (KMO) test was applied and if the value of the KMO test is below 0.5 it is unacceptable, between 0.5 to 0.7 is sufficient, and > 0.7 is considered as good. PAST Software 4.03 ( https://www.softpedia.com/get/Science-CAD/PAST.shtml ) was used for correlation analysis and principle component analysis. These analyses were applied as a complementary tool to describe water quality deterioration 8 , 9 , 21 , 22 .
Water quality index (WQI)
Several national and international organizations have formulated a huge number of water indices such as Weight Arithmetic Water Quality Index (WAWQI), National Sanitation Foundation Water Quality Index (NSFWQI), Canadian Council of Ministers of the Environment Water Quality Index (CCMEWQI), Oregon Water indices which are followed worldwide 23 , 24 .
Water quality index (WQI) is defined as “the grading technique that provides the combined effect of each of the water quality parameters on the overall water quality for human consumption” 25 . It is widely used to characterize the availability of potable water resources and their usefulness for domestic purposes. In the present study the weight arithmetic water quality index was determined as per the methods mentioned in the literature 26 . The mean values of analyzed 15 parameters (pH, turbidity, EC, TDS, Salinity, Clˉ, Brˉ, NO 2 ˉ, NO 3 ˉ, PO 4 3 ˉ, SO 4 2 ˉ, Na + , Ca 2+ , Mg 2+ and K + of 31 samples were included in the calculation. Depending on the water quality effect and its importance for human health, the average weight value (AW) between 1 and 5was assigned for each parameter. In the first step, the relative weight (RW) was calculated by using the Eq. ( 1 ):
The quality rating (Q i ) was calculated in the second step by dividing the measured parameters (C i ) to the permitted drinking water values (S i ) (as per WHO) and multiply by 100 as mentioned in Eq. ( 2 ):
In the third step, the Sub-indices (SI) were calculated by using Eq. ( 3 ), and WQI was calculated by using Eq. ( 4 ).
Based on the computed WQI the water was classified into five types: WQI 0–25 excellent, 26–50 good, 51–75 poor, 76–100 very poor, and > 100 unsuitable 27 , 28 .
Results and discussion
QGIS SoftwareDesktop 3.18.3 ( https://qgis.org/en/site/forusers/download.html ) was used to prepare the water quality map in this study based on the selected parameters as shown in Fig. 2 a–q. The physicochemical parameters and bacterial analysis results of collected 31 water samples from different locations of Tawangare discussed below. In this study for the reference purpose, permissible limit of different parameters studied were taken from Bureau of Indian Standards (BIS) 29 and World Health Organization (WHO) 19 .
Water quality assessment using physio-chemical parameters
The nature of the geological materials through which the surface water flows and the quality of the recharge water defines the types and concentrations of natural contaminants. A wide range of compounds (calcium, magnesium, chloride, arsenate, fluoride, nitrate, iron, etc.) may be picked up by the water when it moves through sedimentary rocks and soils. Hence, the harmful effect of these natural pollutants depends on their type and concentration 12 . The descriptive statistics for the analyzed parameters are summarized in Table 2 . The results of the K-S test showed that pH, EC, salinity, Ca 2+ , Mg 2+ and Na + was normally distributed ( p > 0.05) while Clˉ, Brˉ, NO 2 ˉ, NO 3 ˉ, PO 4 3 ˉ, SO 4 2 ˉ, Fˉ, TDS and K + were not normally distributed ( p < 0.05).
The acidic or basic nature of any water body is reflected by its pHwhich is one of the most important operational water quality parameters. Since it controls the solubility of various metallic contaminants, it is considered as one of the important parameters of water quality. Discharge of industrial pollutants or human waste in the nearby vicinity, or biological activity are some of the reasonsfor fluctuations in the pH value of any water body. The physico-chemical parameters of water also show a change if the pH of any water body changes due to abovementioned reasons. There are possibilities of the formation of trihalomethanes (toxic) if the pH becomes very high. In the case of alkaline pH, the shifting in pH up over 7 was observed due to the presence of alkaline earth metals that interact with soluble CO forming carbonate and bicarbonates. In the present study, the value of pH from all sources was found to be in the range of 5.96 to 7.43. As per WHO, the permissible limit for pH in drinking water is 8.5 19 , hence the pH of collected water was found to be within this limit.
The presence of all dissolved solids in water represents TDS (mg/L),along with salinity and conductivity. Additionally, the dissolved organic matter and inorganic salts such as Ca 2+ , Mg 2+ Na + , K + , HCO 3 ˉ, Clˉ and SO 4 2 ˉ in water also contribute to TDS increment . Since change in pH affectsthesolubility of suspended matter,itstrongly affects TDS which may sometimes lead to precipitation of some of the dissolved solutes.In the present study, the TDS of the collected water sample varied from 13.85 mg/L to 140.8 mg/L (mean-50.163 mg/L)as represented in Fig. 3 . The desirable limit of TDS for drinking water is < 500 mg/L which indicates that the water is in good condition. Tawang water has potable water potential andissuitable for aquatic biota in terms of this parameter. The chief causes of TDS include agricultural operations, domestic runoff, soil contamination caused by leaching, and point source water pollution discharged by industrial or sewage treatment plants 30 .
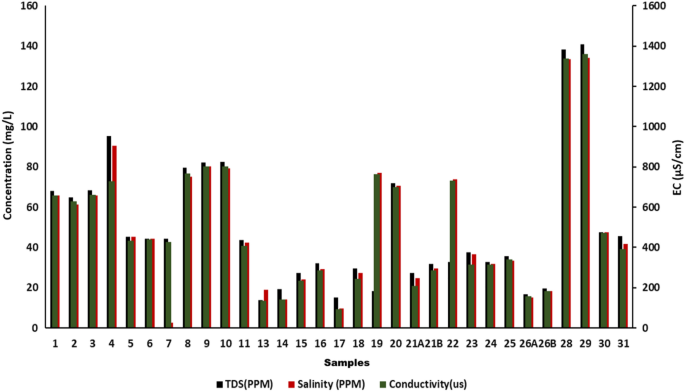
The physio-chemical parameters of 31 water samples collected from Tawang.
Electrical conductivity (EC) is the ability of water to conduct electrical current. The increase in the dissolved salt concentration increases the electrical conductivity of water and, therefore, it provides the general indication of water quality with respect to the amount of total dissolved solids in the form of cations and anions, their concentration and mobility, etc. The electrical conductivity varies with temperature as the solubility of salts which areresponsible for ionic composition is also affected by changes in temperature. Hence, in the present study, the electrical conductivity of the collected water samples was found to be in the range of 136.1 µS/cm to 1361 µS/cm as shown in Fig. 3 . The average EC value of Tawang samples collected from studied location was 525.13 µS/cm. Though this area is not near to dense urbanization still the EC value is slightly higher thanthepermissible limit (400 µS/cm) this might be due to the natural geological activities 31 .
Since salinity indicates the presence of dissolved salts, it also gives the information of water TDS and conductivity, as these three parameters are interrelated. In addition, it is a key factor limiting biota distribution. If the quantities of dissolved salts in the natural water increase it may lead to severe health issues like high blood pressure (BP) or hypertension leading way to cardiovascular diseases (CVD) 12 . In the present study as shown in Fig. 3 , the minimum salinity value was 9.51 mg/L and maximum 134.2 mg/L while the average value was 50.037 mg/L. A statistically significant difference was found between the samples ( p < 0.05). Because of fewer human activities in this area, the water is found to be within the permissible limit.
Ions such as Clˉ and SO 4 2 ˉ in water also affect the salinity. The highest Clˉlevel was recordedat site 11 (17.766 mg/L) and the highest SO 4 2 ˉ was measured 887.54 mg/L for site 1. The results confirm that the anthropogenic activities and hot water springs present in these locations adequatelyimpact the salinity. The Clˉ and SO 4 2 ˉparameters did not show any significant difference between locations. The average value of Clˉ and SO 4 2 ˉ in the present study was 3.36 mg/L and 37.104 mg/L respectively. The Clˉion,andSO 4 2 ˉ ion present in the Tawang region were in permissible limit except for site 1(above permissible limit). In practice salinity is mostly the result of the following major inorganic ions such as Na + , Ca 2+ , Mg 2+ , K + , Clˉ, SO 4 2 ˉ , etc. Water quality is also decreased by increasing the nitrogenous and phosphorous compounds in it. The increase in these compounds might be due to the excessive use of fertilizers in farming which are mixed with the river stream by runoff due to rainy climate.This event is known as primary contamination which may lead to eutrophication giving rise to secondary contamination, threatening the biodiversity in water 12 . The highest NO 3 ˉ was recorded to be 32.15 mg/L for site 9 while the highest NO 2 ˉ was measured at sight 6 (8.86 mg/L). These two ions in 31 sites of Tawangwere in the permissible limit (50 mg/L)as per WHO. However, the PO 4 3 ˉvalues in sites 2, 26B, and 30 were 378.9,88.39 and 322.88 respectively which is above the permissible limit (40 mg/L). The fluoride ions in the Tawang region in some sites (3, 16, 23, 24, 26A, and 28) were above the permissible limit (1.5 mg/L). This might be due to fluoride-bearing minerals present in rocks and sediments. In addition, the use of pesticides in agriculture might be increasing the fluoride concentration in these sites. Table 3 shows the values of ions present in 31samples from different locations which were identified by ion chromatography.
Turbidity is the easiest measure of water quality for any human as it shows how clean or cloudy water is. Several factors affect the turbidity of any water body, and it is caused by particles (clay, silt, phytoplanktons algae, fine organic and organic matter, inorganic matter, and other microscopic organisms, etc.) which are suspended or dissolved in water that scatter light making it appear cloudy. The presence of many suspended solids indicates the high turbidity which reduces the aesthetic quality of any water source 32 . Additionally, turbidity also increases the cost of the water treatment process from different industries such as food processing, pharmaceutical, etc. The main sources of turbidity are natural (erosion from uplands, stream channel movement, etc.) or anthropogenic activities (rock blasting or digging also cause erosion). Turbidity may not be intrinsically harmful, but it interferes with disinfection during water treatment and provides a medium for microbial growth. This leads to serious consequences such as nausea, cramps, diarrhea, etc. 8 , 9 , 21 . In the present study, the water samples collected from Tawang, India showedaturbidity value of 0. This might be due to weather conditions as the samples were collected during the winter season. During this season the average rainfall in the Tawang area is 53.3 mm which is very little as compared to average rainfall during the rainy season (1723 mm). Moreover, in this season the soil leaching is very much negligible and mostly water sources in frozen condition.
The dissolved polyvalent metallic ions from sedimentary rocks, seepage, and runoff from soils are the principal natural sources of hardness in water. Hard water is very dangerous to human health as it causes many diseases such as osteoporosis, nephrolithiasis, hypertension, stroke, etc. It occurs mainly due to the salts of magnesium and calcium. Though these are the common essential mineral constituents of food,their excess intake can cause various abovementioned diseases. Hardness in water is expressed in terms of milligrams of calcium carbonates equivalent per liter 12 , 33 . Water containing calcium carbonate below 60 mg/L is considered as soft water while containing 60–120 mg/Lis moderately hard and having 120–180 mg/Lis hard, and more than 180 mg/Lis considered to bevery hard water. Therefore, in the present study, the calcium was in the range between 13 mg/L and 58 mg/L and magnesium was in the range between 2 mg/L and 32 mg/L as mentioned in Fig. 4 . According to these results, the water of the Tawang area is soft as the values comply with national and international drinking water standards such as BIS-IS-2500 (2012), WHO, and EU 18 , 19 , 29 . The mean values of Ca 2+ (33.13 ± 12.06 mg/L) and Mg 2+ (15.53 ± 8.93 mg/L) in the study area are within the permissible limit.
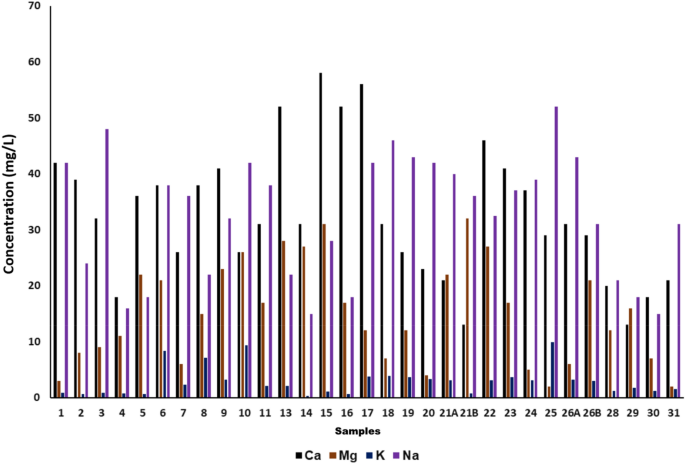
Calcium (Ca 2+ ), Magnesium (Mg 2+ ), Sodium (Na + ) and Potassium (K + ) of 31 water samples collected from Tawang.
One of the most abundant elements of the earth’s crust is iron. Natural or anthropogenic sources can be the cause of iron contamination in water. The iron contaminated water not only leads to staining of laundry and utensils but also reacts with tea and coffee producing black color. Iron is an essential element and structural component of hemoglobin, myoglobin,several enzymesetc, and its deficiency leads to anemia and in severe cases loss of well-being. In addition, its overdose in humans can cause severe health problems such as liver cancer, diabetes, cirrhosis of the liver, heart disease, infertility, etc. The high concentration of iron in water changes its color, taste, odor, and also corrodes water pipelines 12 , 33 . In the present study ironcontamination of the collected water samples was found to be in the range of the permissible limit.
Principle component analysis
Principle component analysis (PCA) is used to reduce the dimensions of multivariate datasets. While trying to reduce the input data dimensions, PCA retains the maximum informative value of the input data sets. PCA decreases the number of dimensions that are not correlated and summarizes the information that is dispersed in several dimensions. PCA discards the redundant and highly correlated parameters and selects the independent variables. Additionally, it also identifies the variance in terms of a small number of new pseudo-variable (Principle component) within a huge dataset of correlated variables 9 , 34 .
Kaiser–Meyer–Olkin (KMO) and Bartlett’s tests of Sphericity have been performed to examine the suitability of the present dataset for PCA. Sampling adequacy is measured by KMO which indicates that the proportion of variance is caused by underlying Principal Components 22 , 35 . A value closer to 1 generally indicates that the data sets may be used for PCA and in this study KMO value is 0.547. If the KMO value < 0.5 then the data sets will not be useful for PCA. To examine whether the correlation matrix is an identity matrix, Bartlett’s test of Sphericity was used. All variables become unrelated making the PCA model inappropriate and unsuitable statistical tool for advanced data analysis if the correlation matrix is an identity matrix. The Null Hypothesis of Bartlett’s test assumes that there is no scope for dimensionality reduction (if the correlation matrix is the identity matrix). In the present work, the significance level (0.000) is less than 0.05 which rejects the Null Hypothesis. Therefore, it means that there is no significant relationship among the parameters. Finally, using Varimax rotation with Kaiser Normalization PCA has been carried out on normalized data and so the covariance matrix coincides with the correlation matrix (Table 4 ). SPSS software was used to carry out both tests. The % variance and cumulative variance is also represented in Table 4 . This explains more than 76% of the total variance and shows only the first six components. However, more components might be needed as the first component by itself explains less than 25% of the variance. In the standardized ratings, six PCs explain more than 76% of the total variability so these PCs have been reasonably retained to reduce the dimension further.
The z-score analysis has been performed for 15 parameters in this study. Figure 5 shows the Box and whisker plot which displays the variability in z-score values of the data points. The maximum and minimum values in the box plot are represented via vertical black lines which in most cases lie beyond the boxes. The whiskers that are not considered outliers are extended to the most extreme data points and the outliers in the box plot are plotted individually using the ‘ + ’ symbol. To obtain a set of linearly transformed scores, Z-scores are estimated which can be simplified by plotting a straight-line graph between z-scores and corresponding scores. The parameters were subjected to PCA after the estimation of z-scores. Analysis of 15 parameters would still be a very tedious and expensive job so PCA has been performed which reduced the parameters using a statistical approach. PCs are independent axes where data is projected 22 , 36 . Table 3 shows the rotated factors loading, eigenvalues, individual variance, and cumulative variance of these PCs. The 6 PCs of these parameters account for 76.24% of the total variance and have individual eigenvalues > 1.
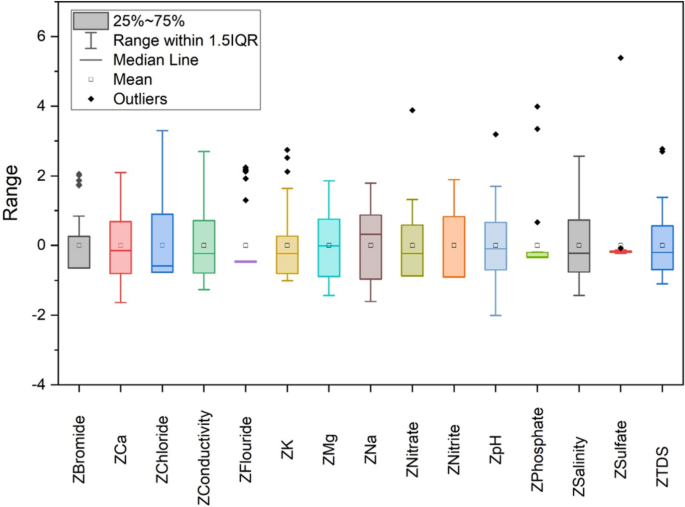
Box plot shows the z-score variability of data.
Figure 6 shows the relation between the first 2 and 3PCs that contributes maximum to the overall variance. The direction and length of the vectors in the 2D bi-plotare represented in Fig. 6 a which indicates the contribution of each variable to the two PCs in the bi-plot. For example, on the horizontal axis, the first PCs have positive coefficients for Chloride, Fluoride, Sulphate, Bromide, EC, TDS, Salinity, and Potassium while negative coefficients for nitrate, nitrite, phosphate, pH, calcium, magnesium, and sodium. That is why 8 vectors are directed into the right half of the plot and 7 are directed to the left half of the plot. Similarly, the PC2 has negative coefficients on the vertical axis for sulfate, phosphate, salinity, calcium, magnesium, and potassium whereas has positive coefficients for the remaining 9 variables. A point for the mean values of each parameter at all of the 31 locations was also included in this 2D biplot. Therefore, their relative locations can be determined from the plot as these points are scaled with respect to the maximum score value and maximum coefficient length. If the first 2 PCs do not explain enough of the variance in the data, then the 3-dimension plot proves to be quite useful. Figure 6 b shows the 3D representation of first 3 PCs.
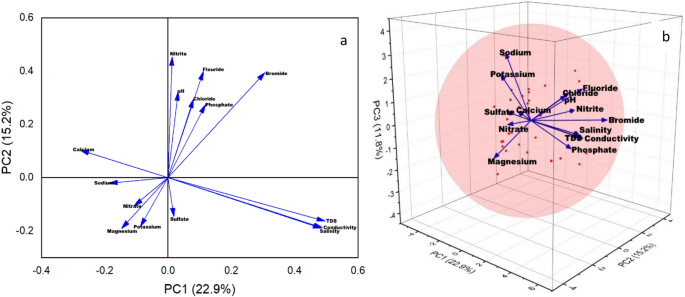
( a ) 2D biplot (2PCs) and ( b ) 3D plot (3PCs) of principal components.
Correlation analysis
Pearson linear correlation matrix was generated by using those parameters which were contributingamaximum of 6 PCs (> 0.35). Table 5 shows the most effective water quality parameters to define any co-variation. The obtained resultsindicateavery strong positive correlation between TDS and EC, and salinity. Salinity shows a strong positive correlation with TDS. pH shows weak and very weak positive correlation with all parameters except NO 2 ˉ. While Ca has a very weak positive correlation with NO 3 ˉ, Fˉ and pH; it has a very weak negative correlation with Clˉ, NO 2 ˉ, PO 4 3 ˉ and Br and has a moderate negative correlation with EC, TDS, and salinity. A similar very weak negative correlation is shown between K + and all the parameters except Clˉ, pH, and EC (shows very weak positive correlation). Na shows a very weak negative correlation with NO 3 ˉ, PO 4 3 ˉ, Brˉ, EC, TDS, and salinity; it has a very weak positive correlation with Fˉ, Clˉ, NO 2 ˉ and pH.Brˉ and moderate positive correlation with Fˉ and PO 4 3 ˉ and shows very weak negative correlation with NO 2 ˉ. Hence, correlation coefficients between pairs of water quality parameters concentrations indicate that Fluoride, Bromide, phosphate, and calcium values significantly correlate with pollutant parameters such as nutrient and trace elements. This suggested that these nutrients and trace elements have high values as they are significantly affecting TDS, salinity, and EC because ofthenatural geological activities in the study area.
Biological parameters for water quality assessment
A wide variety of pathogenic and non-pathogenic microorganisms are found in water bodies which may lead to unpleasant taste and odour in water. This may be served as an indicator and the main concern behind studying the microbiological quality of water. Bacteria, helminths, protozoa, and viruses are contaminants that are derived from feces, household waste, etc. Indicator organisms are generally used to analyze the microbiological quality of water and among them, coliform and E. coli are such indicators. In this work, the bacterial colony count of collected water samples from different locations of Tawang was found to be in the range of 7 CFU/ml to 20117 CFU/ml as mentioned in Table 7 . As per BIS Standards 29 the permissible limit of bacterial count in drinking water should be 0 CFU/100 ml.
Spatial distribution pattern
Figure 2 a–g represents the spatial distribution pattern of different water quality parameters in the location maps of sample collection area. Figure 2 a represents the spatial distribution pattern of the pH, which indicates that the central part along with NE-WS across the district shows the alkaline nature of surface water. On the other hand the NE-NW along with the central part represents the acidic nature of water. According to previous reports, fluoride is absorbed on a clay surface in acidic water,and desorbed in alkaline water. Hence, for fluoride dissolution, alkaline pH is more favorable 37 , 38 . The EC in the district is in the range of 136.1µS/cmto 1361µS/cm(Fig. 2 b). The TDS is also low in the central and NE-NW-SE-SW parts of the Tawang (Fig. 2 c). This shows that the EC and TDS have a significant positive correlation as evidence by the correlation matrix of the quality parameters (Table 5 ). The salinity map clearly and significantly indicates that it is within the permissible limit (600 mg/L) but unevenly distributed in the Tawang district (Fig. 2 d). A positive correlation is observed between the salinity, EC, and TDS of the surface water as mentioned in Table 5 . Most of the surface water in the spatial distribution graph showed alkaline nature which might be due to the presence of carbonatesand bicarbonates 39 .
The spatial distribution of Ca 2+ and Mg 2+ suggested varying concentrations within the permissible limit which reflects that the study area is characterized by soft surface water (Fig. 2 g and h). The study area is surrounded by rocks covered with snow, so the Ca 2+ and Mg 2+ ions present in the surface water might be due to the leaching of calcium and magnesium-bearing rocks. Ca 2+ shows a significant negative correlation with EC, TDS, and salinity. Na + is highest (within permissible limit) in the central part and some patches in the NW part of the district. It is also showing negative correlation with the TDS and salinity in the spatial distribution pattern and correlation matrix. The presence of K + is within thepermissible limit but it is covering the major portion of the district (Fig. 2 i) 40 . The spatial distribution pattern of sulphate (Fig. 2 q) and chloride (2 k) reveals that they are present within the permissible limit. The spatial distribution pattern of iron reveals its absence in the district (Fig. 2 j). Another important factor for the study area is fluoride (Fig. 2 m) which is mainly observed in the SE part of the district above the permissible limit, with a concentration of more than 10 mg/L which may lead to skeletal fluorosis 41 . Fluoride concentration in surface water depends on many factors such as temperature, pH, the solubility of fluorine bearing minerals, size, and type of geological formations, presence, and absence of complexing or precipitating ions and colloids, anion exchange capacity of water and the contact time during which the water remains in contact with the geological formations 12 , 40 , 42 .
Nitrate, nitrite, and phosphate in the surface water are mainly due to anthropogenic activities such as waste disposal, sanitary landfills, overapplication of fertilizers or improper manure management practices,etc. 43 . In this study, it was observed that the nitrate, nitrite, and phosphate are within the permissible limit which indicates absence of any such kind of activities (Fig. 2 n,o,p).
Water quality assessment using WQI
FOR A RAPID ASSESSMENT OF ENVIRONMENTAl impact,WQI can help us to decide overall water quality. WQI provides a value with a quick and understandable explanation of water quality. BIS 29 , US EPA 44 ,and WHO standard 19 parameter values were used for the calculation of WQI at different water sampling locations in Tawang, Arunachal Pradesh. The relative water quality parameters are presented in Table 6 .
In the present study, the water samples were collected during the winter season, and the calculated WQI results are mentioned in Table 7 . The average WQI value in Tawang is 82.49. The WQI results of maximum samples are in the range of 0–50 which are considered as good for drinking, while some samples are unsuitable for drinking showing value > 50. In terms of WQI, the Tawang water samples from most of the sites have good water quality and some have poor water quality. Therefore, WQI has been widely applied in the monitoring of water quality and plays a significant role in water resource management for suitable applications.
In this research, the WQI value calculated for sites 13, 14, 17, 18, and 25 was 24.58, 21.22, 23.96, 23.07 and 25.80 respectively. These results show that the water of these sites is suitable for drinking as it comes in the range of 0–25. Additionally, the WQI value calculated for site 1, 5, 6, 8, 9,11, 19, 20, 21A, 21B, 22, 26B, 29, and 31 was in the range of 26–50 which shows that the water from these sites is suitable for drinking after normal treatment. However, the WQI values in sites 2, 3, 4, 10, 16, 23, 24, 26A, 28, and 30 are more than 100. Because of the water flowfrom agricultural land and household wastewater into these sites,adecrease in water quality is observed leading to high WQI values.
Hydrogeochemical facies and rock water interaction
Based on their hydrogeochemical facies, Hill Piper trilinear diagram explains and classifies different types of water groups 45 . Figure 7 shows the uneven distribution of major ions which are plotted on the Hill-Piper trilinear diagram using Grapher Software (Grapher 16.3.410)( [email protected]). This diagram represents the major significant cations and anions responsible for the nature of surface water. It is comprised of two triangles at the base and one diamond shape at the top which categorized surface water into various six types (Ca 2+ - HCO 3 ˉ type, Na + - Clˉ type, Mixed Ca 2+ - Mg 2+ - Clˉtype, Mixed Ca 2+ -Na + - HCO 3 ˉ type, Na + - HCO 3 ˉ type and Ca 2+ - Clˉtype) 46 . A critical evaluation of the diagram reflects that majority of the samples (50%)fall under Ca 2+ - HCO 3 ˉtype and 10% of the samples showed Ca 2+ -Clˉ type. The study was conducted during winter season and from the results it is clear that weathering of rocks and precipitation are the major processes occurring in the surface water environment. Hydrochemistry of the investigated samples represents that the alkaline earth > alkali metals and weak acid > strong acidic anions. Major cations are present in order, No Dominant type ˃ Mg 2+ of the mean abundance while anions are present in mean abundance order of HCO 3 ˉ˃SO 4 2 ˉ. Hence, it can be concluded that the surface water in this study is polluted due to natural activities or rock-water interaction. Similar type of studies have been carried out to find the hydrogeochemistry and water quality ofRewalsar Lake of Lesser Himalayan,which showed that the alkaline earth surpass the alkaline metal and weak acid exceed to strong acid 5 , 47 .
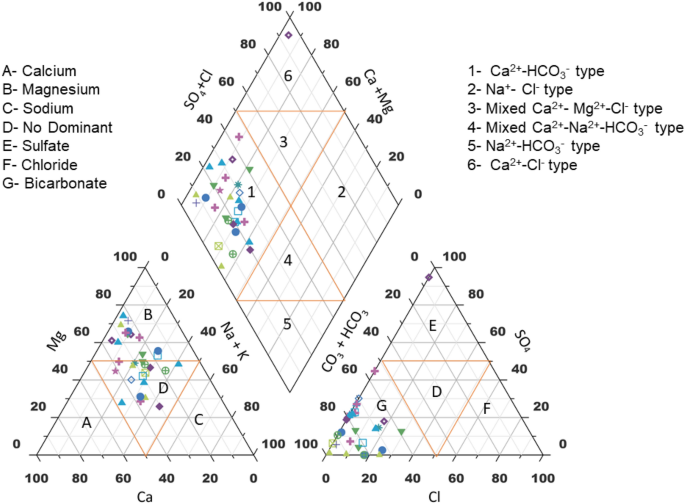
Hill Piper trilinear diagram.
The geochemical processes occurring in the surface water of the study area was further verified by Durov’s plot (Grapher16.3.410, [email protected]) 48 . This diagramconsistsof two ternary plotswhich are plotted against anions and cations of interest (data are normalized to 100%). The Durov plot reveals the relationship and properties of large sample groups while clustering the data points indicating the samples with similar chemical composition 49 . Figure 8 shows the Durov’s plot which evaluate the water type from geochemical process that affect the surface water. In this study, most of the surface water samples are plotted in field 5 which indicates that water exhibits simple dissolution or mixing (no dominant of cation or anion). Some of the samples fall in field 8 which indicates that the surface water has undergone reverse ion exchange with water minerals. Few of the surface water samples are plotted in field 6 which indicates the probable mixing or uncommon dissolution influences (SO 4 2- dominant or anion discriminant and Na dominant). In recent research on Parbati river overall water quality, hydrogeochemical characteristics and other chemical parameters were assessed. The results of Durov’s plot of this study are in line with the present research 50 .
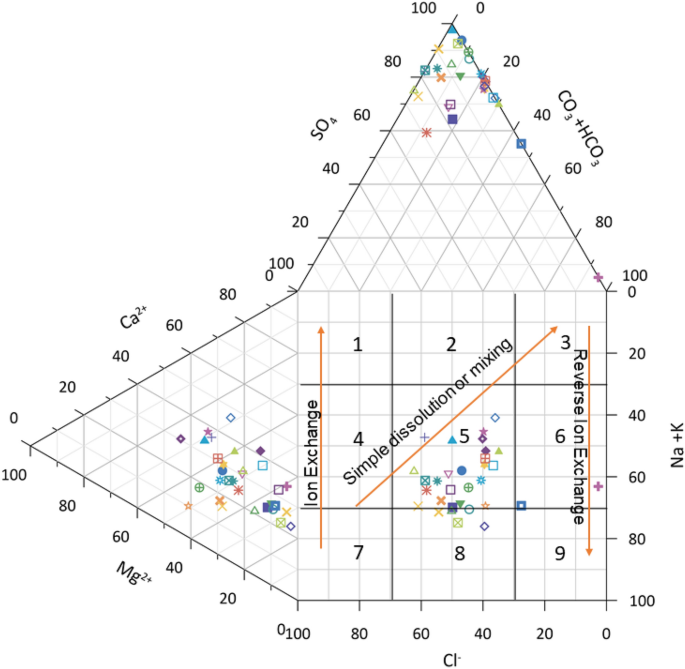
Durov plot illustrating hydrochemical processes involved in surface water/groundwater in different locations of Tawang area.
Hydrogeochemistry of water is altered and affected by different natural processes like evaporation, precipitation, rock weathering and combination of all these processes. These processes can be deciphered using Gibb’s diagram which is characterized by three chief zones: rock weathering dominance, precipitation, and evaporation. It is formed by plotting between the ratio of TDS vs Clˉ/(Clˉ + HCO 3 ˉ) and TDS vs Na + / (Na + + Ca 2+ ) for anions and cations respectively whereby all ions are expressed in meq/L.It is well known that the reactions occurinsurface water and essential minerals of the water play a vital role towards water quality. Additionally, it is also useful in knowing the primary mechanism of ion contribution in surface water 46 , 51 .
The Gibbs plot of surface water samples collected from Tawang area in winter season has been shown in Fig. 9 a and b. The samples collected from this area is prevalent with rock-water interaction and precipitation. Therefore, in plot 10a precipitation dominates over rock -water and evaporation while in plot 10b, rock- water interaction dominates over evaporation and precipitation. Hence, in this study the two primary factors that influenced the surface water chemistry are weathering of rock forming minerals and rainwater intrusion into the aquifer. The samples which lie in the evaporation zone are the indicative of the water influenced by sea water and in this study the samples have fallen into precipitation or rock- water dominant zone. While if any sample falls outside these three mentioned zones, then might be due to any anthropogenic activities.
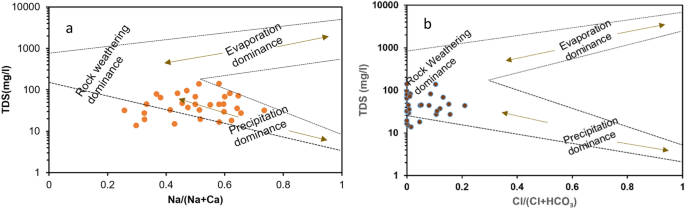
Gibbs diagrams ( a ) cations: TDS vs Na + /Na + + Ca 2+ ) ( b ) anions: TDS vs Cl/(Cl - + HCO 3 - )reveals water chemistry controlling mechanism.
Earlier studies on different water bodies in Himalayan regions also confirmed rock dominance as a main factor for controlling ionic composition 47 , 52 , 53 . The Himalayan water bodies are surrounded by rocks so the water percolation through the rocky lithology and longtime rock water interaction may result in high solute concentration. Furthermore, the less ionic composition in this study reflects the precipitation dominance which might be due to precipitation and melting of ice at higher altitude of Himalayan region 52 .
Figure 10 is the modified version of Durov plot and Piper Plot in which the two equilateral triangles are omitted. In case of Hill Piper plot, the type of water is determined on the data plot when the milliequivalent percentage of major anions and cations are plotted in each triangle irrespective of their triangular field. The central diamond field which provides the overall character of water is the extension of the triangular field. In contrast, the Chadha diagram is plotted between the difference in the alkaline earth (Ca 2+ + Mg 2+ ) and alkali metals (Na + + K + ) on X axis and the difference in weak acid anions (CO 3 2- + HCO 3 ˉ) and strong acid anions (Clˉ + SO 4 2 ˉ) on Y axis. Depending on the size of the scale chosen the plot can be square or rectangular field which has all the advantage of diamond- shaped field of Hill- Piper diagram. The rectangular field is divided into eight sub fields to represent the primary character of water. The eight sub fields are as follows: (1) Alkaline earths > alkali metals. (2) Alkali metals > alkaline earths. (3) Weak acidic anions > strong acidic anions. (4) Strong acidic anions > weak acidic anions. (5) Alkaline earths and weak acidic anions > alkaline metals and strong acidic anions respectively. This type of water has temporary hardness and in Chadha’s plot the position of the data points represents Ca 2+ –Mg 2+- HCO 3 ˉ-type, HCO 3 ˉ dominant Ca 2+ –Mg 2+ -type or Ca 2+ –Mg 2+ dominant HCO 3 ˉ -type waters. (6)Alkaline earths > alkali metals and strong acidic anions > weak acidic anions. This type of water has permanent hardness and during irrigation usage it does not deposit residual carbonate. The datapoints in the Chadha’s plot represents Ca 2+ –Mg 2+ -dominant Clˉ-type, Ca 2+ –Mg 2+ –Clˉ-type or Clˉ-dominant Ca 2+ –Mg 2+ -dominant Clˉ-type waters. (7) Alkali metals > alkaline earths and strong acidic anions > weak acidic anions. This type of water creates salinity problem and the data points in the Chadha’s plot represents Clˉ -dominant Na + -type, Na + -dominant Clˉ-type, Na + –Clˉ-type, or Na 2 SO 4 -type waters. (8) Alkali metals > alkaline earths and weak acidic anions > strong acidic anions. Residual sodium carbonate deposit and foaming problem occurs in such type of waters. The data points in the Chadha’s plot represents HCO 3 ˉ-dominant Na + -type, Na + –HCO 3 ˉ-type or Na + -dominant HCO 3 ˉ-type waters 54 , 55 . In present study, the points in the Chadha’s plots lies in different fields (1 and 2) and showing Clˉ -dominant Na + -type, Na + -dominant Clˉ-type, Na + –Clˉ-type, Na 2 SO 4 –Ca 2+ –Mg 2+ –HCO 3 ˉ-type, HCO 3 ˉ dominant Ca 2+ –Mg 2+ -type or Ca 2+ –Mg 2+ dominant HCO 3 ˉ-type waters. Most of the samples indicated nature of surface water in the Tawang area as hard (TH > 75) and influence permanent and temporary hardness 47 . The data points set in some location near field 5 and 7 suggested that alkaline earths and weak acidic anions exceedsalkali metals and strong acidic anions zone.
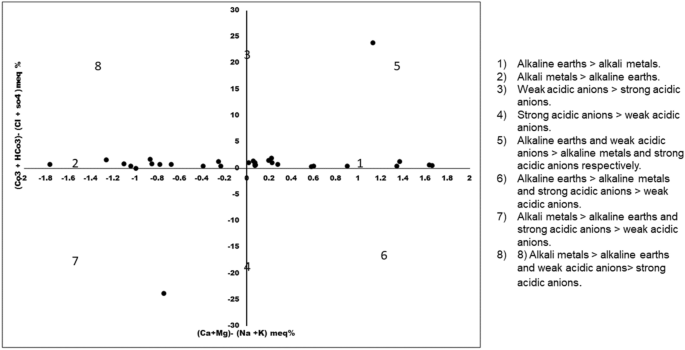
Chadha’s Plot for hydrogeochemical facies.
Figure 11 a–i shows the statistical analysis of certain pairs of parameters to elucidate the hydrologic processes. The concentration of Ca 2+ (R 2 = 0.0399), Na + (R 2 = 0.0159)and Mg 2+ (R 2 = 0.0081) in surface water have shown weak association with HCO 3 ˉ concentration. Similarly, HCO 3 ˉ + Clˉ have weak association with Na + + Mg 2+ + Ca 2+ ions in the surface water (R 2 = 0. 0476). However, Mg 2+ , Ca 2+ and Na + have also showed the weak association amongst them (Mg 2+ vs Ca 2+ , R 2 = 0. 0591 and Ca 2+ vs Na 2+ , R 2 = 0. 0005). Additionally, the weak association between the cations in the surface water with Clˉ was observed. Similar to this work surface water and groundwater of river Munda Basin was studied and the results are in line with the present study 56 .
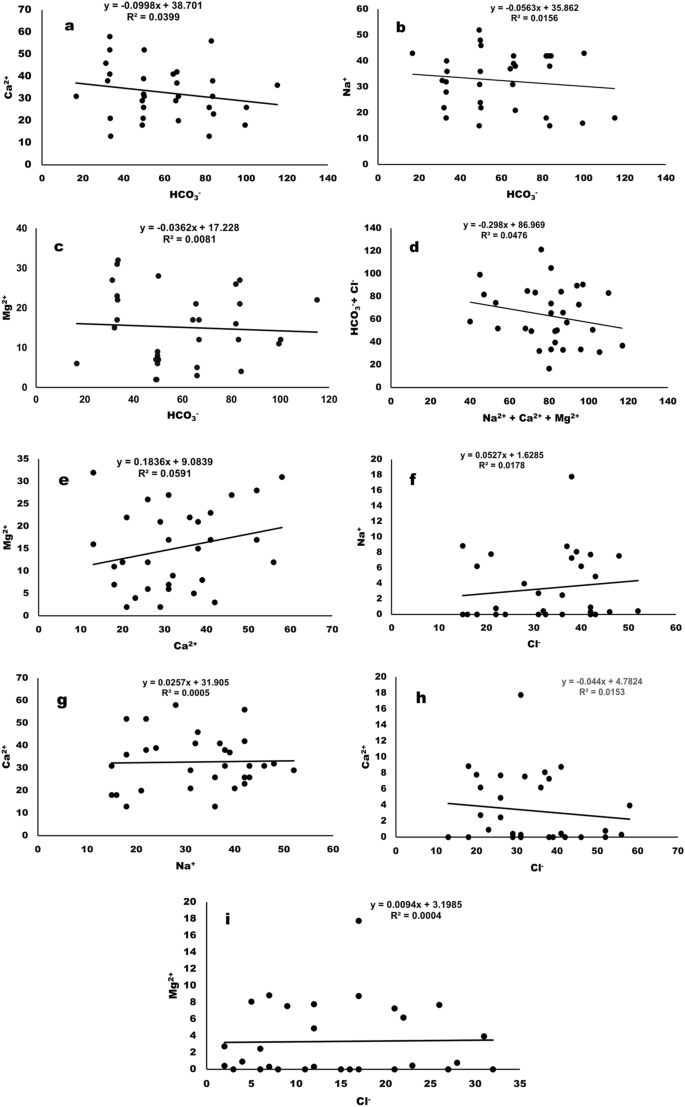
Hydrologic processes, statistical analyses of the correlations between ( a ) Ca 2+ vs HCO 3 - ( b ) Na 2+ vs. HCO 3 - ( c ) Mg 2+ vs. HCO 3 - ( d ) HCO 3 - + Cl - vs. Na + + Mg 2+ + Ca 2+ ( e ) Mg 2+ vs. Ca 2+ ( f ) Na 2+ vs. Cl - ( g ) Ca 2+ vs. Na 2+ ( h ) Ca 2+ vs. Cl - & ( i ) Ma 2+ vs. Cl - .
Schoeller’s index is also used to study ion exchange that occurs between the surface water and the host environment. To interpret the ion exchange behavior between the surfacewater and the host environment Eqs. ( 5 ) and ( 6 ) are used. This process is also known as chloro-alkaline indices and all the ions are expressed in meq/L. Depending on the Na + and K + exchange from water with Mg 2+ and Ca 2+ in rock/soil, or vice versa, CAI-I and CAI-II values may be positive or negative. If the Chloro-alkaline indices value is positive, it means Na + and K + exchange occurs in water with Mg 2+ and Ca 2+ while if it yields negative value this means ion exchange occurs between in Mg 2+ and Ca 2+ surface water and Na + and K + in rock/soil 57 , 58 , 59 . In the present study as shown in Fig. 12 , all the samples (100%) have generated negative values which revealed a presence of reverse ion exchange controlling surface water chemistry as well as rock-water interaction.
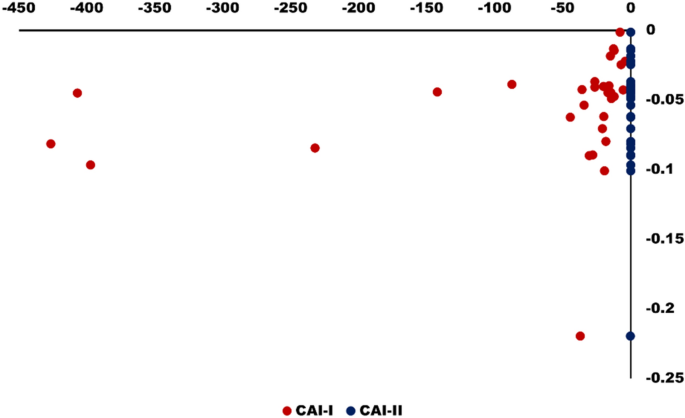
Scatter diagram shows the variation of chloro- alkaline indices of surface water samples in the study area.
Process controlling the surface water chemistry
The dissolution of different parent materials yield different ion combinations, so the geological formation occurring within the study area can be assigned to the process that influence the surface water in the Tawang area aquifer. Na + and K + are originating from silicate weathering while Ca 2+ and Mg 2+ are from carbonate weathering before evaporation. Additionally, SO 4 2- and Clˉ originate from evaporation while HCO 3 ˉ from carbonate silicates. Studies have been done by many researchers which prove that in water system calcite, dolomite, anhydrite and gypsum weathering and dissolution are very predominant processess.Anthropogenic activity to some extent also generates these parameters. The physiochemical characteristics are also affected by the dissolution of evaporites 47 , 60 .
The scatter plots were used to understand the source of major ions and ion exchange process affecting the surfacewater of Tawang area. These plots compared the different parameters in equivalent concentration (meq/L) 60 . Figure 13 a shows the scatter plot of (Ca 2+ + Mg 2+ ) versus (HCO 3 ˉ + SO 4 2 ˉ) which results in close to 1:1 equiline and highlighted that the water samples from different locations of Tawang has calcite, gypsum dissolution, anhydrite, and dolomite. Meanwhile, reverse ion exchange and carbonate weathering is demonstrated by the abundant amount of Ca 2+ + Mg 2+ while silicate weathering can be suggested by the presence of (HCO 3 ˉ + SO 4 2 ˉ) over (Ca 2+ + Mg 2+ ). This result revealed that all samples show dominance (HCO 3 ˉ + SO 4 2 ˉ) over (Ca 2+ + Mg 2+ ) indicating that silicate weathering is a reaction that affect the chemistry of the surface water samples in different location of Tawang. Furthermore, in two locationsthe reverse ion exchange is occurring due to excessive (Ca 2+ + Mg 2+ ) over (HCO 3 - + SO 4 2- ) 52 .
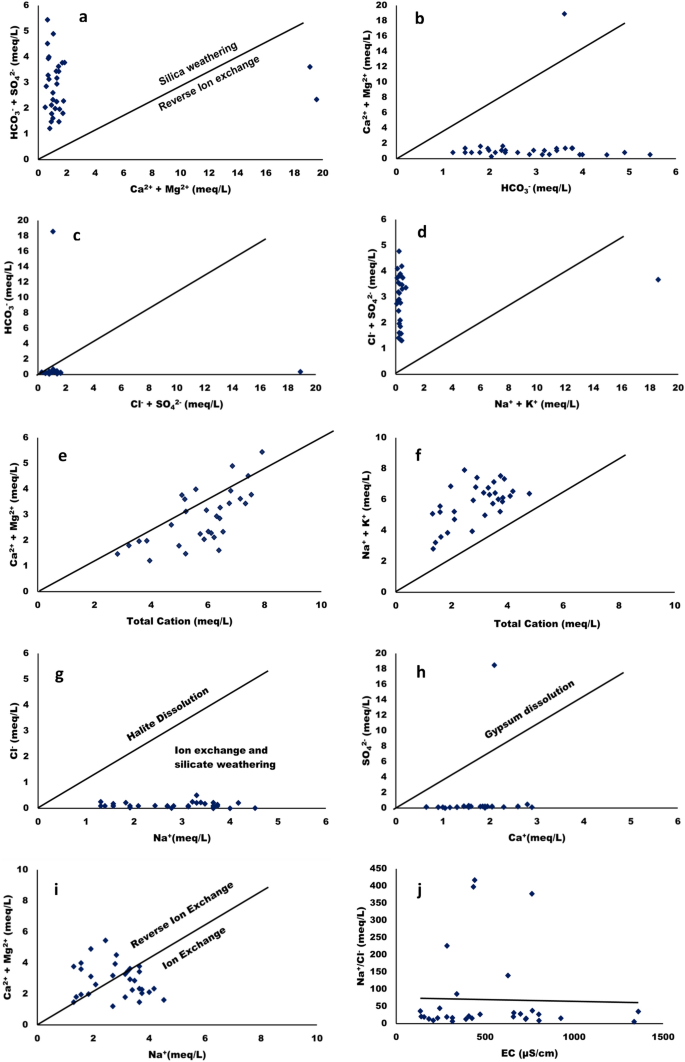
Scatter plots indicating sources of different parameters in surface water of Tawang area.
Meanwhile Fig. 13 b shows that the plot for (Ca 2+ + Mg 2+ ) vs HCO 3 ˉrepresents that the water samples have fallen below the 1:1 equiline which suggested that in all the locations silicate weathering impacts waterdominantly.If these points fall on or above the divider line, it indicates that HCO 3 ˉ in the surface water is controlled by alkaline earth metals as well as carbonate lithology 47 , 52 , 56 . Moreover, (SO 4 2 ˉ + Cl - ) vs HCO 3 ˉscattered plot is shown in Fig. 13 c in which SO 4 2- + Cl - has shown dominance over HCO 3 - at lower concentration range. Similarly, in Fig. 13 d a scattered plot was plotted between (SO 4 2 ˉ + Clˉ) vs (Na + + K + ) and it was found that the water sample points are above 1:1 line 55 .
The points plotted againstalkaline earth metals vs total cations in Fig. 13 e have fallen above and below the equiline but the maximum number of locations of water samples are below 1:1 line. This might be due to the presence of higher amount of Na + + K + with more dissolved solids. The high (Na + + K + )/total cation (50%) and low (Ca 2+ + Mg 2+ )/(Na + + K + ) (9.9%) means water chemical composition is greatly affected by silicate weathering along with less influence of carbonate dissolution. Additionally, if the soil is more influenced with alkalinity this could result in more (SO 4 2 ˉ + Clˉ) and from these findings it can be concluded that the soil source might be having Na 2+ SO 4 2 ˉ and K 2+ SO 4 2 ˉ. Figure 13 f shows the scattered plot of (Na + + K + ) vs total cation and the higher ratio of (Na + + K + ) present in the surface water due to silicate weathering or alkaline soil 47 , 56 . Furthermore, the Fig. 13 g shows the points between Clˉversus Na + to assess the impact of halite dissolution towards the surface water chemistry, whereby all the samples have fallen below the 1:1 equiline. Thus, it can be concluded that the source of Na + is silicate weathering which may be from Na-plagioclaseas mentioned in previous studies 61 .
Another primary process of salination that occurs in the surface water system is depicted using SO 4 2 ˉvs. Ca 2+ plot as shown in Fig. 13 h. In this plot the points are below 1:1 line which suggest that the gypsum dissolution is less in water samples. A positive relation of (Ca 2+ + Mg 2+ ) vs. Na + was observed in Fig. 13 i and it is used to assess the impact of ion exchange on surface water chemistry. In the present study the points are present on both sides of the 1:1 equiline indicative of both ion exchange and reverse ion exchange to occur in water. If the (Na + /Clˉ) vs EC (µS/cm) plot will yield a horizontal line,then it has been suggested that the evaporation process is occurring in the water system. If the Na + /Clˉ is nearly equal to 1, sodium will be a product of halite dissolution whereas Na + /Clˉ > 1 indicative of ions emitted from silicate minerals weathering. In the present work the Na + /Clˉmolar ratio is in the range of 5.2–417.2 as shown in Fig. 13 j which is greater than 1, indicative of silicate weathering as an indicative of Na + release to the surfacewater 61 .
Water suitability forirrigation purpose
Water quality must be monitored to maintain soil fertility and better crop output. The low quality water shows harmful impact on heavy clayed soil but can be used for irrigation of sandy and permeable soil through which chemical may pass deep down 47 . To know the suitability of water for irrigation certain parameters like Magnesium hazard (MH), Total hardness (TH), Permeability index (PI), Kelly Index (KI), Sodium adsorption rate (SAR), Sodium percentage (Na%) and Residual sodium carbonate (RSC) are used.
Szabolcs and Darab proposed a formula as mentioned in Table 8 for the calculation of Magnesium hazard (MH) in meq/L. According to this formula if MH < 50 meq/L then the water is suitable for irrigation while if MH > 50 meq/L then the water is unsuitable for irrigation 62 . In the present study as shown in Fig. 14a, 80% of water samples from different locations have MH scores within the safe limit of 50 meq/L indicating suitability for irrigation. While 20% of water samples have MH score > 50 meq/L which means these water samples have more Mg 2+ over Ca 2+ which adversely affect the soil quality leading to poor irrigation yield. In most of the surface water the state of equilibrium is maintained by the Ca 2+ and Mg 2+ ions,however if the in-equilibrium occurs between these two ions the soil will become alkaline due to high Mg 2+ concentration in water and subsequently leads to reduction in crop yield. Additionally, in highly saline and predominantly sodium dense water, Mg 2+ ions will negatively impact soil structure.
Due to precipitated Ca 2+ and Mg 2+ ions in water, the quality of permanent and temporary hardness can be deduced from action of soap on it. Calcium carbonate is the reason for temporary hardness in water which can be removed byheating,whilethepermanent hardness is due to the presence of both Ca 2+ and Mg 2+ ions andcan be removed by ion exchange. In the present study, the total hardness (TH) was calculated as per Toddmentioned in Table 8 and is expressed in mg/L. The total hardness in this study ranges from 73.75 ± 0.5 to 272.39 ± 0.08 mg/L as shown in Fig. 14 b whereby its optimal limit is 80–100 mg CaCO 3 /L.
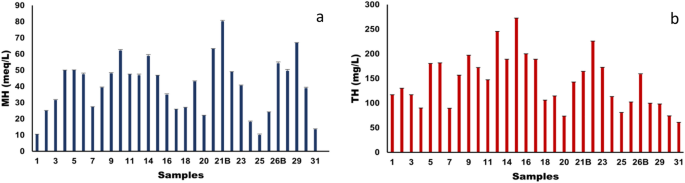
( a ) Magnesium hazard and ( b ) Total hardness of the water samples collected from different locations of Tawang.
The long-term use of Na + , Ca 2+ , Mg 2+ and HCO 3 ˉ rich water affects the soil permeability. To find out the suitability of water Doneen used the Permeability index (PI) and classified irrigation water in three classes which are Class-I, Class-II and Class-III. Figure 15 (Grapher16.3.410, [email protected]) shows the classification of irrigation water based on permeability index calculated as per the formula mentioned in Table 8 . According to this classification only Class-I and Class-II types of water are suitable for irrigation due to 75% or more maximum permeability score while Class-III is not suitable for irrigation due to 25% maximum permeability. In the present research work, 48% of the samples falls in Class-I and 45% of the water samples falls in Class-II, indicating that the water is good for irrigation purpose. However, 7% of water sample falls into Class-III, indicating that water is not suitable for irrigation.
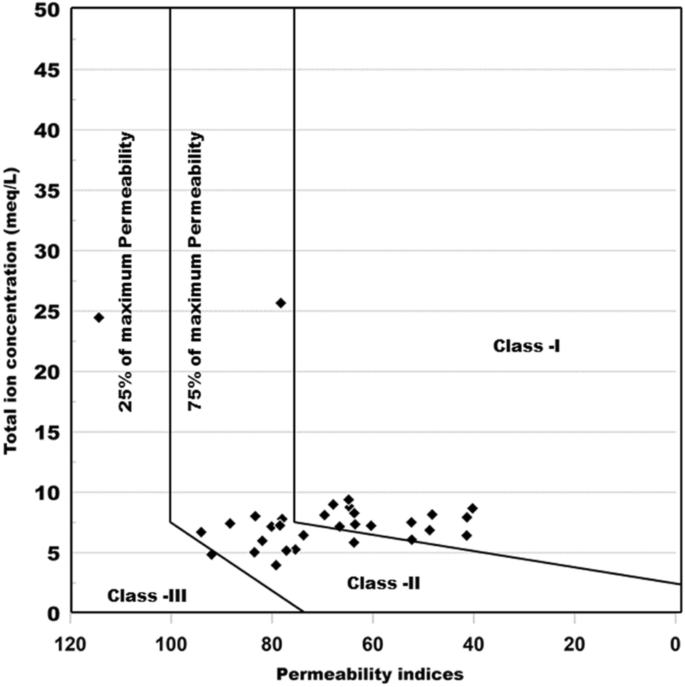
Irrigation water classification based on Permeability index.
Kelly index (KI)is also another useful method to classify water for irrigation. It can be calculated using the following formula as mentioned in Table 8 . According to this index, KI < 1.0 is indicative of good water for irrigation while KI > 1.0 is indicative of bad water and unsuitable for irrigation 47 , 52 . In the present study the 58% of water samples obtained from different location of Tawang has yielded KI values < 1.0 while 42% of them yielded KI value > 1.0. Hence, 58% of water samples from different location is suitable for irrigation.
Sodium adsorption rate (SAR) is a parameter to calculate the correlation between soluble divalent cations (Ca 2+ and Mg 2+ ) and Na + . If SAR value is higher, it means the Na + concentration is higher with respect to Ca 2+ and Mg 2+ . An alkaline soil is developed from high Na + concentration and reduced soil permeability. The SAR is determined by using formula mentioned in Table 8 and based on this the irrigation water is classified into four alkali categories: S1: low (0–10), S2: medium (10–18), S3: high (18–26) and S4: very high (> 26) 63 . In the present study as shown in Fig. 16 (Grapher 16.3.410, [email protected]), the SAR values in the study area ranges from 0.95 to 5.03 and as per Richard’s classification all the samples fall into the low sodium hazard and low to very high salinity hazard. These results support the findings of KI. Therefore, it can be concluded that the maximum water samples from different location of Tawangare suitable for irrigation purpose.
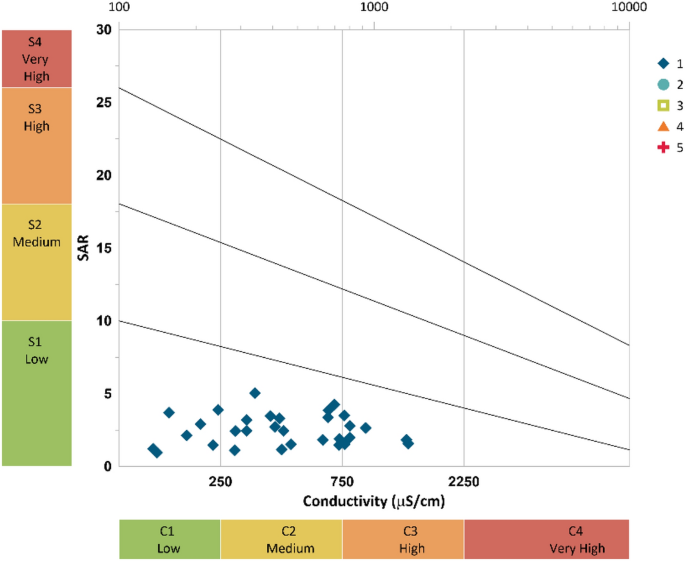
Classification of irrigation water using Wilcox diagram.
Sodium is deemed as an important cation in water irrigation due to its ability to reduce soil fertility, as high cation concentration shows negative effect on plant growth. The sodium percentage in this study was also calculated by using Wilcox formula as mentioned in Table 8 64 . According to BIS the maximum acceptable limit of Na% is 60% and above that limit it is harmful for growth of plants. Additionally, the higher concentration of sodium increases the hardness in soil leading to reduction in soil permeability 47 . Hence, based on the Table 8 , 20% of the samples lie in good category, 48% in permissible while 32% in doubtful in terms of their suitability for irrigation. Additionally, a graph has been plotted between Na% and EC as per Wilcox to determine the water suitability for irrigation and from Fig. 17 it was found that 94% of samples are excellent to good for agricultural usages. The remaining 6% of the samples lies in good to permissible category and can be used for irrigation purpose. From these results it can be concluded that for irrigation purpose water should have high concentration of Ca 2+ and Mg 2+ ions and lesser Na + .

Surface water rating using Sodium Percentage and Electrical Conductivity.
The residual sodium carbonates (RSC) are another method to check water irrigation property. RSC can be calculated using the difference in sum of carbonate and bicarbonate to the sum of calcium and magnesium in water as mentioned in Table 8 . The excess amount of carbonate and bicarbonate altered the soil physical property either by increasing its salinity or itself get precipitated leading to decrease in soil fertility. The high concentration of bicarbonate precipitate Ca 2+ and Mg 2+ in water and reduce their amount that ultimately leads tohigh concentration of CO 3 2- and HCO 3 ˉ in the solution expressed as HCO 3 ˉ hazard. RSC is also used to assess the relationship between alkaline earths with weak acids to assess the water quality for irrigation. If weak acid > alkaline earths, then soil permeability is damaged, as alkaline earths become precipitated in the soil. Additionally, excessive carbonate and bicarbonate in water also intrude alkaline earth over the permissible limit and finally affect the agricultural crop. The RSC value is categorized into safe for irrigation (< 1.25 epm), fair quality water (1.25–2.5 epm) and unsuitable for irrigation (> 2.5 epm) 47 , 52 , 56 .In this study, 99% of the surface water samples have been categorized as safe for irrigation while the remaining 1% unsuitable for irrigation. The negative RSC values indicates that the calcium and magnesium are partially precipitated.
In the present study, the water quality of 31 different locations of Tawangduring the winter season were evaluated from the viewpoint of its suitability for drinking and irrigation. For calculation of water quality, 15 parameters were selected represented by pH, TDS, anions, cations, EC, salinity, turbidity for PCA analysis. Different statistical toolssuch as PCA and coorelation analysis were used in this paper to derive the relationship between different parameters of water as mentioned below.
In the present study, the average WQI value is found to be 82.49. The WQI results of 61% samples are in the range of 0–50 which are considered as good for drinking, while 39% are unsuitable for drinking showing value > 50. In terms of WQI, the Tawang water samples from most of the sites have good water quality except some which show poor water quality.
Hill piper plot showed that the alkaline earth dominates alkali metal and weak acid exceeds strong acid. This plot also showed that the Ca 2+ –HCO 3 ˉ, Na + –HCO 3 ˉ and Na + –Clˉ type of water is dominant in this area.Gibbs plot also revealed that rock weathering is the main process which controls the surface water The dominance due to precipitation was also observed, which may be due to continuous outflow of surface water thus having short rock-water interaction. The silicate weathering is found to be dominant process rather than carbonate weathering due to rich silicate minerals lithology. For increasing concentration of alkali metals in the surface water of Tawang, reverse ion exchange process has played an active role. Both Na + and Ca 2+ are observed as dominant cations while HCO 3 ˉ and SO 4 2 ˉ as dominant anions. The values in the chloro-alkaline indices in this study were negative indicating the exchange of Ca 2+ and Mg 2+ ions by Na + and K + ions of rock material.
The scatter plot of Ca 2+ + Mg 2+ vs total cation and Na + + K + vs total cation has specified the silicate weathering as a dominant source of major ions. Furthermore, the scattered plot of (Ca 2+ + Mg 2+ ) vs (HCO 3 ˉ + SO 4 2 ˉ), (Ca 2+ + Mg 2+ ) vs HCO 3 ˉ and (SO 4 2 ˉ + Clˉ) vs HCO 3 ˉindicated that both silicate weathering and reverse ion exchange processes have played an important role in geochemical reactions.
The parameters like MH, KI, PI, SAR, Na% and RSC have shown that the water from different locations of Tawang is suitable for irrigation. Some water samples were found to be hard with permanent and temporary hardness. However 93% of the samples have shown PI score < 75, which indicates the suitability of the samples for irrigation.
Hence this study has thrown some light on the water quality of untouched surface water sources of theTawang area. The study has revealed the suitability of most of the water samples for human consumption as well as irrigation. From the detailed water quality analysis and geochemical characteristics it can be inferred that those surface water sources lying at an altitude of maximum 4465 m in the studied area have not been exposed to any kind of anthropogenic activities. Hence the obtained results are in coorelation with natural activities only, which make it even more valuabledatabase asset for future references.
Mukate, S. et al. Development of new integrated water quality index (IWQI) model to evaluate the drinking suitability of water. Ecol. Ind. 101 , 348–354 (2019).
Article CAS Google Scholar
Wu, Z. et al. Assessing river water quality using water quality index in Lake Taihu Basin, China. Sci. Total Environ. 612 , 914–922 (2018).
Article ADS CAS PubMed Google Scholar
Uddin, M. G., Nash, S. & Olbert, A. I. A review of water quality index models and their use for assessing surface water quality. Ecol. Ind. 122 , 107218 (2021).
Noori, R. et al. A critical review on the application of the National sanitation foundation water quality index. Environ. Pollut. 244 , 575–587 (2019).
Article CAS PubMed Google Scholar
Kumar, R. et al. Hydro-geochemical characteristics of glacial meltwater from Naradu Glacier catchment, Western Himalaya. Environmental Earth Sciences 78 (24), 1–12 (2019).
Duraisamy, S. et al. Hydrogeochemical characterization and evaluation of groundwater quality in Kangayam taluk, Tirupur district, Tamil Nadu, India, using GIS techniques. Environ. Geochem. Health 41 (2), 851–873 (2019).
Adimalla, N. & Taloor, A. K. Hydrogeochemical investigation of groundwater quality in the hard rock terrain of South India using Geographic Information System (GIS) and groundwater quality index (GWQI) techniques. Groundw. Sustain. Dev. 10 , 100288 (2020).
Article Google Scholar
Şener, Ş, Şener, E. & Davraz, A. Evaluation of water quality using water quality index (WQI) method and GIS in Aksu River (SW-Turkey). Sci. Total Environ. 584–585 , 131–144 (2017).
Article ADS PubMed CAS Google Scholar
Tripathi, M. & Singal, S. K. Use of Principal Component Analysis for parameter selection for development of a novel Water Quality Index: A case study of river Ganga India. Ecol. Ind. 96 , 430–436 (2019).
Tian, Y. et al. Using a water quality index to assess the water quality of the upper and middle streams of the Luanhe River, northern China. Sci. Total Environ. 667 , 142–151 (2019).
Ewaid, S. H. et al. Development and evaluation of a water quality index for the Iraqi Rivers. Hydrology 7 (3), 67 (2020).
Ram, A. et al. Groundwater quality assessment using water quality index (WQI) under GIS framework. Appl. Water Sci. 11 (2), 46 (2021).
Article ADS CAS Google Scholar
Abba, S. I. et al. Emerging evolutionary algorithm integrated with kernel principal component analysis for modeling the performance of a water treatment plant. J. Water Process Eng. 33 , 101081 (2020).
Taloor, A. K. et al. Spring water quality and discharge assessment in the Basantar watershed of Jammu Himalaya using geographic information system (GIS) and water quality Index (WQI). Groundw. Sustain. Dev. 10 , 100364 (2020).
Kawo, N. S. & Karuppannan, S. Groundwater quality assessment using water quality index and GIS technique in Modjo River Basin, central Ethiopia. J. Afr. Earth Sc. 147 , 300–311 (2018).
Zanotti, C. et al. Groundwater and surface water quality characterization through positive matrix factorization combined with GIS approach. Water Res. 159 , 122–134 (2019).
Rice, E.W., Baird, R.B. & Eaton A.D. In Standard Methods for the Examination of Water and Wastewater , 23rd edn (American Public Health Association, American Water Works Association, Water Environment Federation, 2017)
Standard, I., Drinking water-specification. 1st Revision, IS, 1991. 10500 .
Cotruvo, J. A. 2017 WHO guidelines for drinking water quality: First addendum to the fourth edition. J. Am. Water Works Assoc. 109 (7), 44–51 (2017).
Federation, W.E. and A. Association, Standard methods for the examination of water and wastewater. American Public Health Association (APHA): Washington, DC, USA, 2005. 21 .
Ewaid, S. H., Abed, S. A. & Kadhum, S. A. Predicting the Tigris River water quality within Baghdad, Iraq by using water quality index and regression analysis. Environ. Technol. Innov. 11 , 390–398 (2018).
Mahapatra, S. S. et al. Prediction of water quality using principal component analysis. Water Qual. Expo. Health 4 (2), 93–104 (2012).
Tokatli, C. Drinking water quality assessment of Ergene River Basin (Turkey) by water quality index: Essential and toxic elements. Sains Malay. 48 (10), 2071–2081 (2019).
Călmuc, V.A., et al ., Various methods for calculating the water quality index. Analele Universității” Dunărea de Jos” din Galați. Fascicula II, Matematică, fizică, mecanică teoretică/Annals of the” Dunarea de Jos” University of Galati. Fascicle II, Mathematics, Physics, Theoretical Mechanics , 2018. 41 (2): p. 171–178.
Anyanwu, E.D. and C.S. Emeka, Application of water quality index in the drinking water quality assessment of a southeastern Nigeria river. Food Environ. Saf. J., 18 (4), 308–314. (2020).
Ustaoğlu, F., Tepe, Y. & Taş, B. Assessment of stream quality and health risk in a subtropical Turkey river system: A combined approach using statistical analysis and water quality index. Ecol. Ind. 113 , 105815 (2020).
Menberu, Z., Mogesse, B. & Reddythota, D. Evaluation of water quality and eutrophication status of Hawassa Lake based on different water quality indices. Appl. Water Sci. 11 (3), 1–10 (2021).
Khatri, N. et al. Analysis and assessment of ground water quality in Satlasana Taluka, Mehsana district, Gujarat, India through application of water quality indices. Groundw. Sustain. Dev. 10 , 100321 (2020).
BIS, I.S.D.W.S., Bureau of Indian Standards. New Delhi, 2012: p. 2–3.
Devesa, R. & Dietrich, A. Guidance for optimizing drinking water taste by adjusting mineralization as measured by total dissolved solids (TDS). Desalination 439 , 147–154 (2018).
Wu, T. & Brant, J. A. Magnetic field effects on pH and electrical conductivity: Implications for water and wastewater treatment. Environ. Eng. Sci. 37 (11), 717–727 (2020).
Kumar, R. et al. Distribution of trace metal in Shaune Garang catchment: evidence from particles and nanoparticles. Mater. Today: Proc. 15 , 586–594 (2019).
CAS Google Scholar
Dubey, R., et al. , Survey of natural water sources of Tawang region and studies of their physico-chemical and bacterial contamination of water. 2020.
Balázs, B. et al. Extracting water-related features using reflectance data and principal component analysis of Landsat images. Hydrol. Sci. J. 63 (2), 269–284 (2018).
Shrestha, N. Factor analysis as a tool for survey analysis. Am. J. Appl. Math. Stat. 9 (1), 4–11 (2021).
Jang, D. & Choi, G. Estimation of non-revenue water ratio for sustainable management using artificial neural network and Z-score in Incheon, Republic of Korea. Sustainability 9 (11), 1933 (2017).
Mukherjee, I. & Singh, U. K. Groundwater fluoride contamination, probable release, and containment mechanisms: A review on Indian context. Environ. Geochem. Health 40 (6), 2259–2301 (2018).
Zango, M. S. et al. Hydrogeochemical controls and human health risk assessment of groundwater fluoride and boron in the semi-arid North East region of Ghana. J. Geochem. Explor. 207 , 106363 (2019).
Kumari, M. & Rai, S. Hydrogeochemical evaluation of groundwater quality for drinking and irrigation purposes using water quality index in semi arid region of India. J. Geol. Soc. India 95 (2), 159–168 (2020).
Ram, A. et al. Assessment of groundwater quality using water quality index (WQI) in Kulpahar watershed, District Mahoba, Uttar Pradesh, India. J. Indian Assoc. Environ. Manag. (JIAEM) 40 (3), 24–38 (2020).
Google Scholar
Mridha, D. et al. Fluoride exposure and its potential health risk assessment in drinking water and staple food in the population from fluoride endemic regions of Bihar, India. Groundw. Sustain. Dev. 13 , 100558 (2021).
Narsimha, A. & Sudarshan, V. Contamination of fluoride in groundwater and its effect on human health: A case study in hard rock aquifers of Siddipet, Telangana State India. Appl. Water Sci. 7 (5), 2501–2512 (2017).
Nieder, R., Benbi, D. K. & Reichl, F. X. Reactive water-soluble forms of nitrogen and phosphorus and their impacts on environment and human health. In Soil components and human health 223–255 (Springer, 2018).
Chapter Google Scholar
Agency, U.E.P., Drinking water standards and health advisories . 2018, US Environmental Protection Agency Washington, DC.
Ram, A. et al. Groundwater quality assessment using water quality index (WQI) under GIS framework. Appl. Water Sci. 11 (2), 1–20 (2021).
Tanvir Rahman, M. A. T. M. et al. Groundwater characterization and selection of suitable water type for irrigation in the western region of Bangladesh. Appl. Water Sci. 7 (1), 233–243 (2017).
Gaury, P. K., Meena, N. K. & Mahajan, A. Hydrochemistry and water quality of Rewalsar Lake of Lesser Himalaya, Himachal Pradesh, India. Environ. Monitor. Assess. 190 (2), 1–22 (2018).
Ravikumar, P., Somashekar, R. & Prakash, K. A comparative study on usage of Durov and Piper diagrams to interpret hydrochemical processes in groundwater from SRLIS river basin, Karnataka, India. Elixir Earth Sci. 2015 (80), 31073–31077 (2015).
Lloyd, J.W. and Heathcote, J., Natural inorganic hydrochemistry in relation to ground water. 1985.
Sharma, G. et al. Application of multivariate statistical analysis and water quality index for quality characterization of Parbati River, Northwestern Himalaya, India. Discover. Water 1 (1), 5 (2021).
Gibbs, R. Mechanisms controlling world water chemistry. Sci. J. 170 , 1088–1090 (1970).
ADS CAS Google Scholar
Kumar, P., Mahajan, A. K. & Kumar, A. Groundwater geochemical facie: Implications of rock-water interaction at the Chamba city (HP), northwest Himalaya, India. Environ. Sci. Pollut. Res. Int. 27 (9), 9012–9026 (2020).
Kumar, R. et al. Hydro-geochemical analysis of meltwater draining from Bilare Banga glacier, Western Himalaya. Acta Geophys. 67 (2), 651–660 (2019).
Article ADS Google Scholar
Chadha, D. A proposed new diagram for geochemical classification of natural waters and interpretation of chemical data. Hydrogeol. J. 7 (5), 431–439 (1999).
Jain, C., Sharma, S. & Singh, S. Physico-chemical characteristics and hydrogeological mechanisms in groundwater with special reference to arsenic contamination in Barpeta District, Assam (India). Environ. Monit. Assess. 190 (7), 1–16 (2018).
Shamsuddin, M. K. N. et al. Geochemical characteristic and water quality index of groundwater and surface water at Lower River Muda Basin, Malaysia. Arab. J. Geosci. 12 (9), 1–27 (2019).
Schoeller, H., Geochemistry of groundwater. In ‘Groundwater Studies–an International Guide for Research and Practice’.(Eds RH Brown, AA Konoplyantsev, J. Ineson, and VS Kovalevsky.) pp. 1–18 . 1977, UNESCO: Paris, France.
Barik, R. & Pattanayak, S. K. Assessment of groundwater quality for irrigation of green spaces in the Rourkela city of Odisha, India. Groundw. Sustain. Dev. 8 , 428–438 (2019).
Kant, N., Singh, P. K. & Kumar, B. Hydrogeochemical characterization and groundwater quality of Jamshedpur urban agglomeration in Precambrian terrain, Eastern India. J. Geol. Soc. India 92 (1), 67–75 (2018).
Kumar, P. & Kumar, P. Removal of cadmium (Cd-II) from aqueous solution using gas industry-based adsorbent. SN Appl. Sci. 1 (4), 1–8 (2019).
Li, X. et al. Hydro-geochemistry of the river water in the Jiulongjiang River basin, Southeast China: Implications of anthropogenic inputs and chemical weathering. Int. J. Environ. Res. Public Health 16 (3), 440 (2019).
Article CAS PubMed Central Google Scholar
Szabolcs, I. & Darab, K. Radio-active technique for examining the improving effect of CaCO 3 on alkali (Szik) soils. Acta Agron. Hung. 13 , 93–101 (1964).
McGeorge, W.T. Diagnosis and Improvement of Saline and Alkaline Soils. Soil Science Society of America Journal , 18 , 348–348. https://doi.org/10.2136/sssaj1954.03615995001800030032x (1954).
Wilcox, L., Classification and use of irrigation waters . 1955: US Department of Agriculture.
Download references
Acknowledgements
The authors want to acknowledge the Government of Arunachal Pradesh and Indian Army for their support during sample collection.
No funding is provided for this research work.
Author information
Authors and affiliations.
Defence Research Laboratory, Strategic Product Development Division, DRDO, Tezpur, Assam, India
Nisha Gaur, Arpan Sarkar, Dhiraj Dutta, B. J. Gogoi, Rama Dubey & Sanjai Kumar Dwivedi
You can also search for this author in PubMed Google Scholar
Contributions
Dr. N.G.–Microbiological Testing of the sample. Mr. A.S.–Water sample collection and Field work. Mr. D.D.–Physico chemical testing of the sample and statistical analysis of the data. Dr. B.J.G.–Geo-location collection and GIS based data compilation. Dr. R.D.– Writing of the Manuscript. Dr. S.K.D.–Overall Guidance and final editing of the manuscript.
Corresponding author
Correspondence to Rama Dubey .
Ethics declarations
Competing interests.
The authors declare no competing interests.
Additional information
Publisher's note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ .
Reprints and permissions
About this article
Cite this article.
Gaur, N., Sarkar, A., Dutta, D. et al. Evaluation of water quality index and geochemical characteristics of surfacewater from Tawang India. Sci Rep 12 , 11698 (2022). https://doi.org/10.1038/s41598-022-14760-3
Download citation
Received : 02 August 2021
Accepted : 13 June 2022
Published : 09 July 2022
DOI : https://doi.org/10.1038/s41598-022-14760-3
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
This article is cited by
Using isometric log-ratio in compositional data analysis for developing a groundwater pollution index.
- Kyoung-Ho Kim
- Seong-Taek Yun
Scientific Reports (2024)
Hydrogeochemical factors influencing the dynamics of groundwater characteristics in eco-sensitive areas of the Southern Western Ghats, India
- Pankaj Bakshe
- Mini Chandran
- Ravin M. Jugade
Water quality assessment of hydrochemical parameters and its spatial–temporal distribution: a case study of water resources in the Kebir Rhumel Basin, Algeria
- Fatma Elhadj Lakouas
- Lotfi Mouni
Euro-Mediterranean Journal for Environmental Integration (2024)
Changes in the characteristics of water quality parameters under the influence of dam construction
- Raoof Mostafazadeh
- Ali Nasiri Khiavi
Environment, Development and Sustainability (2024)
Hydrogeochemical processes controlling surface water quality for irrigation in a Mediterranean wetland ecosystem, Northeast Algeria
- Faouzi Zahi
- Abdelmalek Drouiche
- Mohamed Djidel
Environmental Monitoring and Assessment (2024)
Quick links
- Explore articles by subject
- Guide to authors
- Editorial policies
Sign up for the Nature Briefing: Microbiology newsletter — what matters in microbiology research, free to your inbox weekly.
Advertisement
Groundwater quality assessment using water quality index (WQI) under GIS framework
- Original Article
- Open access
- Published: 12 February 2021
- Volume 11 , article number 46 , ( 2021 )
Cite this article
You have full access to this open access article

- Arjun Ram 1 ,
- S. K. Tiwari 2 ,
- H. K. Pandey 3 ,
- Abhishek Kumar Chaurasia 1 ,
- Supriya Singh 4 &
- Y. V. Singh 5
66k Accesses
165 Citations
Explore all metrics
Groundwater is an important source for drinking water supply in hard rock terrain of Bundelkhand massif particularly in District Mahoba, Uttar Pradesh, India. An attempt has been made in this work to understand the suitability of groundwater for human consumption. The parameters like pH, electrical conductivity, total dissolved solids, alkalinity , total hardness, calcium, magnesium, sodium, potassium, bicarbonate, sulfate, chloride, fluoride, nitrate, copper, manganese, silver, zinc, iron and nickel were analysed to estimate the groundwater quality. The water quality index (WQI) has been applied to categorize the water quality viz: excellent, good, poor, etc. which is quite useful to infer the quality of water to the people and policy makers in the concerned area. The WQI in the study area ranges from 4.75 to 115.93. The overall WQI in the study area indicates that the groundwater is safe and potable except few localized pockets in Charkhari and Jaitpur Blocks. The Hill-Piper Trilinear diagram reveals that the groundwater of the study area falls under Na + -Cl − , mixed Ca 2+ -Mg 2+ -Cl − and Ca 2+ - \({\text{HCO}}_{3}^{ - }\) types. The granite-gneiss contains orthoclase feldspar and biotite minerals which after weathering yields bicarbonate and chloride rich groundwater. The correlation matrix has been created and analysed to observe their significant impetus on the assessment of groundwater quality. The current study suggests that the groundwater of the area under deteriorated water quality needs treatment before consumption and also to be protected from the perils of geogenic/anthropogenic contamination.
Similar content being viewed by others


Evaluation of Groundwater Quality for Drinking Purposes Using the WQI and EWQI in Semi-Arid Regions in India
Water quality index for assessment of drinking groundwater purpose case study: area surrounding Ismailia Canal, Egypt
Groundwater quality evaluation using Water Quality Index (WQI) under GIS framework for Mandya City, Karnataka
Avoid common mistakes on your manuscript.
Introduction
In India, there has been a tremendous increase in the demand for groundwater due to rapid growth of population, accelerated pace of industrialization and urbanization (Yisa and Jimoh 2010 ). The availability and quality of groundwater are badly affected at an alarming rate due to anthropogenic activities viz. overexploitation and improper waste disposal (industrial, domestic and agricultural) to groundwater reservoirs (Panda and Sinha 1991; Kavitha et al. 2019a , 2019b ). Consequently, human health is seriously threatened by the prevailing agricultural practices particularly in relation to excessive application of fertilizers; unsanitary conditions and disposal of sewage into groundwater (Panigrahi et al. 2012 ). The groundwater quality also varies with depth of water, seasonal changes, leached dissolved salts and sub-surface environment (Gebrehiwot et al. 2011 ). According to the World Health Organization (WHO 2017 ), about 80% of all the diseases in human beings are water-borne. Once the groundwater is contaminated, it is difficult to ensure its restoration and proper quality by preventing the pollutants from the source. It, therefore, becomes imperative to monitor the quality of groundwater regularly, and to device ways and means to protect it from contamination. The quality of groundwater is deciphered using various physical, chemical and biological characteristics of water (Diersing and Nancy 2009 ; Panneerselvam et al. 2020a ). It is a measure of health and hygiene of groundwater concerning the need and purpose of human consumption (Johnson et al. 1997 ; Panneerselvam et al. 2020b ).
In recent years, the assessment and monitoring of groundwater quality on a regular basis is being carried out using Geographic Information System (GIS) technique added with the IDW interpolation method and has proved itself as a powerful tool for evaluating and analysing spatial information of water resources (Aravindan et al. 2010 ; Shankar et al. 2010 , 2011a , b ; Venkateswaran et al. 2012 ; Selvam et al. 2013b; Magesh and Elango 2019 ; Balamurugan et al. 2020b ; Soujanya Kamble et al. 2020 ). It is an economically feasible and time-efficient technique for transforming huge data sets to generate various spatial distribution maps and projections revealing trends, associations and sources of contaminants/pollutants. In this work, GIS technique has been used for spatial evaluation of various groundwater quality parameters.
In this study, the physicochemical properties of forty-three groundwater samples collected from wells and hand pumps were determined and compared with international standards of WHO for drinking and domestic uses based on Water Quality Index (WQI). The WQI was first developed by Horton ( 1965 ) based on weighted arithmetical calculation. A number of researchers (Brown et al. 1972 ; GEMS UNEP 2007; Kavitha and Elangovan 2010 ; Alobaidy et al. 2010 ; Shankar and Kawo 2019 ; Bawoke and Anteneh 2020 developed various WQI models based on weighing and rating of different water quality parameters which is derived by the weighted arithmetic method. The WQI is a dimensionless number with values ranking between 0 and 100. The WQI is a unique digital rating expression that expresses overall water quality status viz. excellent, good, poor, etc. at a certain space and time based on various water quality parameters. Thus, the WQI is being used as an important tool to compare the quality of groundwater and their management (Jagadeeswari and Ramesh 2012 ) in a particular region; and is helpful for selecting appropriate economically feasible treatment process to cope up with the concerned quality issues. It depicts the composite impact of different water quality parameters and communicates water quality information to the public and legislative policy-makers to shape strong policy and implement the water quality programs (Kalavathy et al. 2011 ) by the government.
Mineral intractions strongly influence groundwater hydrochemistry in aquifers and disintegration of minerals from various source rocks (Cerar and Urbanc 2013 ; Modibo Sidibé et al. 2019 ). Hydrochemistry of the analysed samples indicates that the mean abundance of major cations is present in order of Na ++ > Ca 2+ > Mg 2+ > K + while major anions in order of \({\text{HCO}}_{3}^{ - }\) > \({\text{NO}}_{3}^{ - }\) > Cl − > \({\text{SO}}_{4}^{2 - }\) > F − . The study shows that the sodium is dominant alkali while calcium and magnesium are the dominant alkaline earth metal leached in the aquafer due to rock water interaction affecting the quality of groundwater. Sodium in aquafer is derived from the weathering of halite and silicate minerals such as feldspar (Khan et al. 2014 ; Mostafa et al. 2017 ). The critical evaluation of Hill-Piper Trilinear diagram reflects Na + -Cl − , mixed Ca 2+ -Mg 2+ -Cl − , Ca 2+ - \({\text{HCO}}_{3}^{ - }\) , mixed Ca 2+ -Na + - \({\text{HCO}}_{3}^{ - }\) , Na + - \({\text{HCO}}_{3}^{ - }\) and Ca 2+ -Cl − type hydro-chemical facies in decreasing order of dominance. The Hydro-chemical characterization of groundwater reveals that the nature of aquifer is controlled by type of water, source and level of contamination (Aghazadeh et al. 2017 ; Brhane 2018 ). Hence, in order to keep the health of any aquaculture system, particularly an aquifer system at an optimal level, certain water quality indicators or parameters must be regularly monitored and controlled. Therefore, the objective of the study is to calculate the WQI of groundwater in order to assess its suitability for human consumption using the GIS interpolation technique and statistical approach in the study area.
Mahoba district is the south-western district of Uttar Pradesh which is adjacent to the state of Madhya Pradesh in south and Hamirpur (UP) in the north. The study area falls under the survey of India (SOI) toposheets no. 54O and 63C lies between latitude N25°01′30″ to N25°39′40″ and longitude E79°15′00″ to E80°10′30″ and covers an area of approximately 2933.59 km 2 . River Dhasan separates the district Mahoba from Jhansi in the west. A certainpart of Jhansi and Banda district has been merged in newly constructed Mahoba district in 1995 (bifurcated from Hamirpur). Mahoba district consists of three tehsils Kulpahar, Charkhari, Mahoba and four blocks Panwari, Jaitpur, Charkhari, Kabrai (Fig. 1 a). Kabrai is the biggest block fromaerial coverage as well as population point of view. Jaitpur is the smallest block from aerial coverage and Charkhari from population point of view. The study area experiences a typical subtropical climate punctuated by long and intense summer, with distinct seasons. The area receives an average annual precipitation of 864 mm mainly from the south-west monsoon. The temperature of the coldest month (January) is 8.3°C while the temperature of the hottest month (May) shoots upto 47.5°C. The entire area under investigation is characterised by highly jointed/fractured Bundelkhand granite (Archean age) with thin soil cover. Physiographically , the area is characterised by Bundelkhand massif terrain and is marked by the occurrence of solitary or clustered hillocks and intervening low relief with undulating plains. Two major physiographic units are: (1) Southern part having high relief with hillocks- This is south of 20°25′ N latitude & maximum altitude is 340 mamsl, reserved forest. Granitoids and intervening pegmatitic veins and numbers of quartz veins are observed. (2) Northern part relatively low relief with lower hillocks- In between 25°25′N and 25°39′N latitude and maximum altitude is 310 mamsl. The area in and around Panwari is mainly covered with thick alluvium, and hard rock is encountered only below 35 mbgl, coverage with seasonal forest. Pedi plain, pediment inselberg and buried pediplains are present.
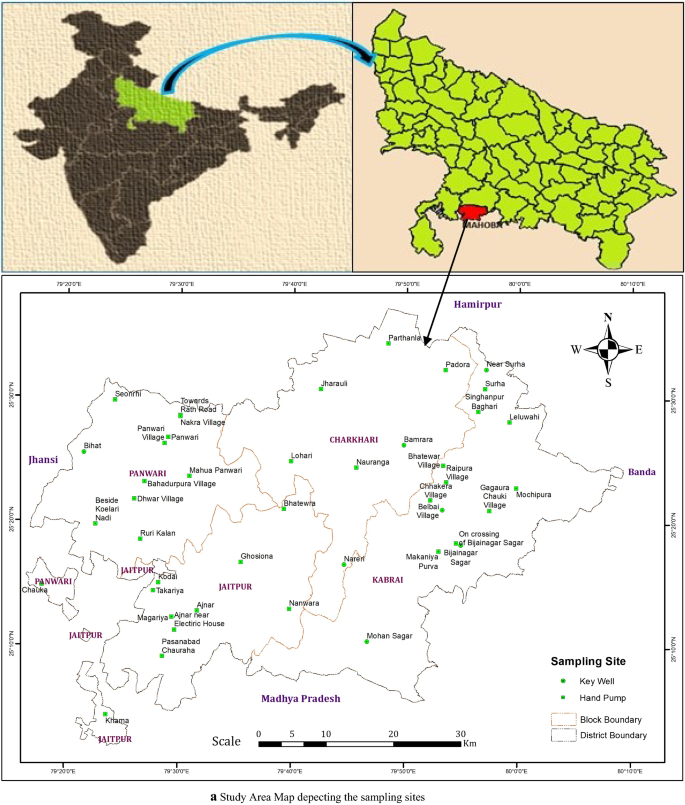
a Study area map depicting the sampling sites. b Geological map of study area
Geological and hydrogeological set-up
The granite, particularly leucogranite, older and younger alluvium consisting of clay, silt, sand and gravel mainly comprises the study area. The geological set-up of the study area indicates that the most dominant lithology is leucogranite covering mainly central and eastern part while recent alluvium covers the northern part (Fig. 1 b). At places, few patches of pink granite have also been recorded which appears enclosed in leucogranite or adjacent to its outcrop.The occurrence of groundwater is highly uncertain and unpredictable in this hilly and rugged terrain as it does not allow percolation and storages underground. The presence of porosity depends on the intensity of weathering and rock fracture which is responsible for groundwater occurrence, its quantity and flow mostly in permeable zones of weathered rock formations and under secondary porosity in the deep fractured zone. Groundwater recharge in the study area is triggered by the depth of overburden 7 m (Jaitpur-Kulpahar area) to 35 m (parts of Mahoba Tahsil and Charkhari block) as well as the intensity of weathering.
Materials and methods
The groundwater samples were collected during pre-monsoon (June 2016) period from the study area according to standard procedures of the American Public Health Association (APHA, 2017). The sampling locations were marked with the help of global positioning system (GPS) as shown in the Fig. 1 a. Samples were collected from the location through hand pump (depth: approx. 40 m) and dug wells (depth: 8–30 m ) as shown in Fig. 2 a–t. The collecting bottles (High-Density Polythene, HDPE) of one-litre capacity each were sterilized under the aseptic condition to avoid unpredictable contamination and subsequent changes in the characteristics of groundwater. Water samples were filtered using Whatman 42 filter paper (pore size 2.5 μm) prior to collection in the bottle. The sample was kept in the ice-box (portable) and brought to NABL accredited (ISO 17,025: 2017) laboratory of Central Ground Water Board (CGWB), Lucknow and Department of Soil Science & Agricultural Chemistry, Banaras Hindu University, Varanasi, UP, India. The samples were stored in a chemical laboratory at temperature 4–5 °C. The samples for metallic parameters were added 2 ml elemental grade nitric acid to obtain the pH 2–3 after acidification. The samples were pre-filtered in the laboratory to carry out the analysis. In the present study, a total of 20 groundwater quality parameters of forty-three samples were analysed as per test standard methods (APHA 2017) in the laboratory except for unstable parameters viz. hydrogen ion concentration (pH), electrical conductivity (EC) and total dissolved solids (TDS) which are determined by portable device (pH-meter, EC-meter and TDS-meter) in situ. Alkalinity (AK), Total hardness (TH), calcium (Ca 2+ ), magnesium (Mg 2+ ), bicarbonate ( \({\text{HCO}}_{3}^{ - }\) ) and chloride (Cl − ) were analysed using volumetric titrations; sodium (Na + ) and potassium (K + ) were analysed using systronics flame photometer model 129; nitrate ( \({\text{NO}}_{3}^{ - }\) ), fluoride (F − ), sulfate ( \({\text{SO}}_{4}^{2 - }\) ), were analysed using shimadzu 1800 spectrophotometer. Prior to analysis of the heavy metals viz. copper (Cu), manganese (Mn), silver (Ag), zinc (Zn), iron (Fe) and nickel (Ni); the groundwater samples were acidified with 1:1 nitric acid and concentrated ten times. The samples were subjected to analysis using Shimadzu 6701 Atomic Absorption Spectrophotometer (AAS) on flame mode with hollow cathode lamps of metal under analysis. The concentration of metal is displayed on the monitor. The standards of the metallic parameters were prepared from National Institute of Standards and Technology (NIST) certified (Certified Reference Materials) CRM as per NABL guidelines of 17,025:2017.
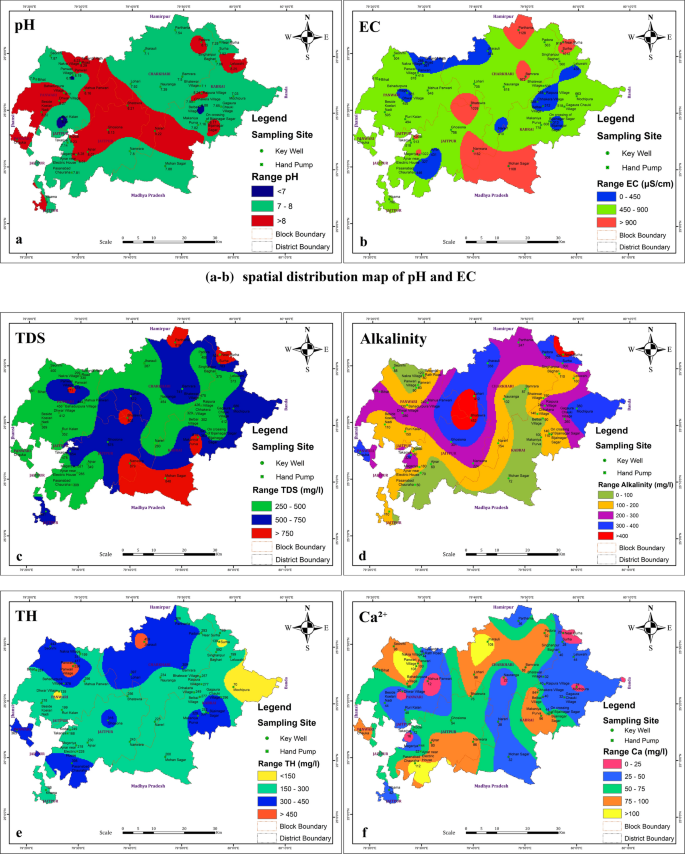
a – b Spatial distribution map of pH and EC. c – h Spatial distribution map of TDS, AK, TH, Ca 2+ , Mg 2+ and Na + . i – n : spatial distribution map of K + , \({\text{HCO}}_{3}^{ - }\) , \({\text{SO}}_{4}^{2 - }\) , Cl − , F − and \({\text{NO}}_{3}^{ - }\) . o – t Spatial distribution map of Cu, Mn, Ag, Zn, Fe and Ni
The quality assurance and quality control (QA/QC) procedure of the data has been considered during the study. Approximately half of the volume (500 ml) of samples were specially separated and checked in the laboratory to ensure QA/QC mechanisms. The accuracy of the chemical analysis has been validated by charge balance errors and samples < 5% error were considered.
The inverse distance weighted (IDW) interpolation technique used in this study is now-adays an effective tool for spatial interpolation of groundwater quality parameters leading to the generation of spatial distribution maps (Magesh et al. 2013 ; Kawo and Shankar 2018 ; Balamurugan et al. 2020b ; Sarfo and Shankar 2020 ). The weights were assigned to various parameters at each location based on distance and were calculated, taking into consideration the closest specified locations. The distribution of each groundwater quality parameter has been demarcated in different zones on spatial distribution map viz. acceptable/desirable and permissible limits according to BIS (2012, 2015) and WHO ( 2017 ) for drinking purpose. The statistical analysis and correlation matrix of the analysed groundwater quality parameters have been laid down as shown in Tables 1 and 2 , respectively.
The water quality index (WQI)
The WQI has been determined using the drinking water quality standard recommended by the World Health Organization (WHO 2017 ). The Water Quality Index has been calculated using the weighted arithmetic method, which was originally proposed by Horton ( 1965 ) and developed by Brown et al. ( 1972 ). The weighted arithmetic water quality index (WQI) is represented in the following way:
where n = number of variables or parameters, W i = unit weight for the i th parameter, Q i = quality rating (sub-index) of the i th water quality parameter.
The unit weight ( W i ) of the various water quality parameters are inversely proportional to the recommended standards for the corresponding parameters.
where, W i = unit weight for the i th parameter, S n = standard value for i th parameters, K = proportional constant,
The value of K has been considered ‘1′ here and is calculated using the mentioned equation below:
According to Brown et al. ( 1972 ), the value of quality rating or sub-index ( Q i ) is calculated using the equation as given below:
where V o = observed value of i th parameter at a given sampling site, V i = ideal value of i th parameter in pure water, S n = standard permissible value of i th parameter.
All the ideal values (V i ) are taken as zero for drinking water except pH and dissolved oxygen (Tripathy and Sahu 2005 ). In case of pH, the ideal value is 7.0 (for natural/pure water) while the permissible value is 8.5 (for polluted water). Similarly, for dissolved oxygen, the ideal value is 14.6 mg/L while the standard permissible value for drinking water is 5 mg/L. Therefore, the quality rating for pH and Dissolved Oxygen are calculated from the equations respectively as shown below:
where, V pH = observed value of pH, V do = observed value of dissolved oxygen.
If, Q i = 0 implies complete absence of contaminants while 0 < Q i < 100 implies that, the contaminants are within the prescribed standard. When Q i > 100 implies that, the contaminants are above the standards.
The classification of water quality, based on its water quality index (WQI) after Brown et al. ( 1972 ); Chatterjee and Raziuddin ( 2002 ) and Shankar and Kawo ( 2019 ) have been considered here in this study for further reference which is mentioned in Table 3 .
Result and discussion
Groundwater quality parameters.
In this study based on the selected parameters as discussed above the groundwater quality maps have been prepared with the help of ArcGIS software 10.1 as shown in Fig. 2 a–t. In the following lines, the various parameters considered in the study are being discussed: The Bureau of Indian Standard (BIS 2012, 2015) and World Health Organization (WHO 2017 ) of drinking water standards have been considered as a reference in this study.
Hydrogen ion concentration (pH)
It is an important indicator for assessing the quality and pollution of any aquifer system as it is closely related to other chemical constituents of water. The presence of hydrogen ion concentration is measured in terms of pH range. Water, in its pure form shows a neutral pH which indicates hydrogen ion concentration. In the present study, the range of pH varies between 6.81 (minimum) to 8.32 (maximum) which is within the acceptable limit (6.5–8.5, avg: 7.81) indicating the alkaline nature of groundwater (ideal range of pH for human consumption: 6.5–8.5).
Electrical conductivity (EC)
In fact, it is a measure of the ability of any substance or solution to conduct electrical current through the water. EC is directly proportional to the dissolved material in a water sample. The desirable limit of EC for drinking purpose is 750 µS/cm. In this study, the electrical conductivity varies between 286 and 1162 µS/cm. High EC at some sites suggests the mixing of sewage in groundwater as these sites are near dense urbanization.
Total dissolved solids (TDS)
The weight of residue expresses it after a water sample is evaporated to dry state. It includes calcium, magnesium, sodium, potassium, carbonate, bicarbonate, chloride and sulfate. In the present study, it ranges between 280 to 879 mg/l (< 500 mg/l TDS for potable water as per BIS.). The agricultural practices, residential runoff, leaching of soil causing contamination and point source water pollution discharge from industrial or sewage treatment plants are the primary sources for TDS (Boyd 2000 ).
Alkalinity (AK)
It is a measure of the carbonate, bicarbonate and hydroxide ions present in water. The desirable limit of alkalinity in potable water is 200 mg/l, above which the taste of water becomes unpleasant. In the study area, the alkalinity ranges between 50 to 452 mg/l, which is within the permissible limit (600 mg/l).
Total hardness (TH)
It is the amount of dissolved calcium and magnesium in the water. Water moving through soil and rock dissolves naturally occurring minerals and carries them into the groundwater as it is a great solvent for calcium and magnesium. In this study, hardness ranges between 70 to 592 mg/l, which is within the permissible limits (600 mg/l). The high concentration of TH in groundwater may cause heart disease and kidney stone in human beings.
Calcium (Ca 2+ )
It enters into the aquifer system from the leaching of calcium bearing minerals. In the study area, the calcium concentration ranges from 12 to 112 mg/l and is within the permissible limit (200 mg/l). The lesser concentration of Ca 2+ in the groundwater satisfies the chemical weathering and dissolution of fluorite, consequently resulting in an increase of fluoride concentration.
Magnesium (Mg 2+ )
It is an important parameter responsible for the hardness of the water. In the study area, the concentration ranges between 2.4 to 120 mg/l and is present in little excess of the permissible limit (100 mg/l).
Sodium (Na + )
It is a highly reactive alkali metal. It is present in most of the groundwater. Many rocks and soils contain sodium compounds, which easily dissolves to liberate sodium in groundwater. In the study area, it ranges from 48.71 to 244.4 mg/l. The high concentration of Na + indicates weathering of rock-forming minerals i.e., silicate minerals (alkali feldspars) and/or dissolution of soil salts present therein due to evaporation (Stallard and Edmond 1983 ). In the aquifers, the high Na + concentration in groundwater may be related to the mechanism of cation exchange (Kangjoo Kim and Seong-Taekyun 2005).
Potassium (K + )
It is present in many minerals and most of the rocks. Many of these rocks are relatively soluble and releases potassium, the concentration of which increases with time in groundwater. In this study, it varies between 0.87 to 2.7 mg/l.
Bicarbonate ( \({\text{HCO}}_{3}^{ - }\) )
It is produced by the reaction of carbon dioxide with water on carbonate rocks viz. limestone and dolomite. The carbon-dioxide present in the soil reacts with the rock-forming minerals is responsible for the presence of bicarbonate, producing an alkaline environment in the groundwater. In the study area it varies between 36.61 to 536.95 mg/l and is within the permissible limit of 600 mg/l.
Sulfate ( \({\text{SO}}_{4}^{2 - }\) )
It is dissolved and leached from rocks containing gypsum, iron sulfides, and other sulfur bearing compounds. In the present study, it ranges between the 2.23 to 75.17 mg/l, which is well within the acceptable limit of 200 mg/l.
Chloride (Cl − )
In the present study the Cl − ranges between 70.92 to 276.59 mg/l which exceed the permissible limit (250 mg/l). The higher value of chlorine in groundwater makes it hazardous to human health (Pius et al. 2012 ; Sadat-Noori et al. 2014 ).
Fluoride (F − )
In groundwater fluoride is geogenic in nature. It is the lightest halogen, and one of the most reactive elements (Kaminsky et al. 1990 ). It usually occurs either in trace amounts or as a major ion with high concentration (Gaciri and Davies 1993 ; Apambire et al. 1997 ; Fantong et al. 2010 ). The groundwater contains fluorides released from various fluoride-bearing minerals mainly as a result of groundwater-host rock interaction. The study area comprising granite, granitic gneiss etc. is commonly found to contain fluorite (CaF 2 ) as an accessory mineral (Ozsvath 2006 ; Saxena and Ahmed 2003 ) which plays a significant role in controlling the geochemistry of fluoride (Deshmukh et al. 1995 ). In addition to fluorite it is also abundant in other rock-forming minerals like apatite, micas, amphiboles, and clay minerals (Karro and Uppin 2013 ; Narsimha and Sudarshan 2013 ; Naseem et al. 2010 ; Jha et al. 2010 ; Rafique et al. 2009 ; Carrillo-Rivera et al. 2002 ). In the present study, the fluoride concentration ranges from 0.11 to 3.91 mg/l. The concentration of fluoride exceeds the permissible limit (1.5 mg/l) in about 25% of the groundwater samples.
Nitrate ( \({\text{NO}}_{3}^{ - }\) )
Nitrate is naturally occurring ions and is a significant component in the nitrogen cycle. However, nitrate ion in groundwater is undesirable as it causes Methaemoglobinaemia in infants less than 6 months of age (Egereonu and Nwachukwu 2005 ). In general, its higher concentration causes health hazards if present beyond the permissible limit, 45 mg/l (Kumar et al. 2012 , 2014 ). In the study area, its concentration ranges from 86.95 to 210.4 mg/l. It is in excess of the permissible limits throughout the study area. The higher values of nitrate in potable water increases the chances of gastric ulcer/cancer, and other health hazards to infants and pregnant women (Rao 2006 ) also birth malformations and hypertension (Majumdar and Gupta 2000 ). The area under study is granite-gneiss terrain where the atmospheric nitrogen is fixed and added to the soil as ammonia through lightning storms, bacteria present in soil and root of plants. Further, animal wastes, plants and animals remain also undergo ammonification in the soil producing ammonia which undergoes nitrification/ammonia oxidation by Nitrosomonas and Nitrobacter bacteria to form nitrate (Rivett et al. 2008 ; Galloway et al. 2004). Granitic rocks contain nitrogen concentrations up to 250 mg Nkg −1 with ammonium partitioned into the orthoclase feldspar to a greater extent than muscovite or biotite (Boyd et al. 1993 ). Geologic nitrogen (nitrogen contained in bedrock) contribute to the ecosystem with nitrogen saturation (more nitrogen available than required by biota) leading to leaching of nitrogen and consequently elevating nitrate concentrations in groundwater (Dahlgren 1994 ; Holloway et al. 1998 ). Nitrogen released through weathering has a greater impact on soil and water quality. Also, denitrification is significant in modifying the level to which nitrogen released through weathering of bedrock influencing the supply of nitrate in groundwater (McCray et al. 2005 ).
Copper (Cu)
It is a naturally occurring metal in rock, soil, plants, animals, and groundwater in very less concentration. The concentration of Cu may get enriched into the groundwater through quarrying and mining activities, farming practices, manufacturing operations and municipal or industrial waste released. Cu gets into drinking water either by contaminating of well water or corrosion of copper pipes in case of water is acidic. In this study, it ranges between 0 and 0.0078 mg/l, which is within the permissible limit (0.05 mg/l).
Manganese (Mn)
It occurs naturally in groundwater, especially in an anaerobic environment. The concentrations of Mn in groundwater is dependent upon rainfall chemistry, aquifer lithology, geochemical environment, groundwater flow paths and residence time, etc. which may vary significantly in space and time. It may be released by the leaching of the overlying soils and minerals in underlying rocks as well as from the minerals of the aquifer itself in groundwater. In the present study, manganese ranges between 0.005 and 0.221 mg/l, which is within the permissible limit (0.3 mg/l).
It naturally occurs usually in the form of insoluble and immobile oxides, sulfides and some salts. It is rarely present in groundwater, surface water and drinking water at concentrations above 5 µg/litre (WHO 2017 ). In the present study, the silver ranges between 0.000 and 0.021 mg/l, which is within the permissible limit (0.1 mg/l).
Though it occurs in significant quantities in rocks, groundwater seldom contains zinc above 0.1 mg/l. In the present study, the groundwater shows the negligible concentration of Zn (0.0136 mg/l) which is well within the acceptable limit (5 mg/l).
The most common sources of iron in groundwater is weathering of iron-bearing minerals and rocks. The iron occurs naturally in the reduced Fe 2+ state in the aquifer, but its dissolution increases its concentration in groundwater. Iron in this state is soluble and generally does not create any health hazard. If Fe 2+ state is oxidised to Fe 3+ state in contact with atmospheric oxygen or by the action of iron-related bacteria which forms insoluble hydroxides in groundwater. So, the concentration of iron in groundwater is often higher than those measured in surface water. In the present study, the iron ranges between 0.0994 and 0.4018 mg/l, which is within of the permissible limit 1.0 mg/l (BIS 2015).
Nickel (Ni)
The primary source of nickel in groundwater is from the dissolution of nickel ore bearing rocks. The source of nickel in drinking water is leaching from metals in contact such as water supply pipes and fittings. Ni usually occurs in the divalent state, but oxidation states of + 1, + 3, or + 4 may also exist in nature. In the study area, it ranges between 0 and 0.0408 mg/l, and it crosses the permissible limit (0.02 mg/l).
Statistical analysis, correlation matrix and relative weightage
The relative weightage, general statistical analysis and correlation matrix of groundwater quality parameters are tabulated in Tables 4 , 1 and 2 , respectively. The correlation matrix of various 20 groundwater quality parameters, including 6 heavy metals was created and has been analysed using MS Excel 2016 Table 2 . Out of these, eight parameters viz. TDS, EC, Na + , Alkalinity, TH, Ca 2+ , Mg 2+ , \({\text{HCO}}_{3}^{ - }\) are significantly correlated, reflecting more than 0.50 correlation value. Further, TDS vs EC, Na + vs Alkalinity, TH as CaCO 3 − vs Ca 2+ and Mg 2+ , \({\text{HCO}}_{3}^{ - }\) vs Alkalinity and Na + indicates most relevant correlation having a significant impetus on the overall assessment of the quality of groundwater than any other major radicals and physical parameters. However, the majority of quality parameters are positively correlated with each other. A critical analysis of the correlation matrix for the heavy metals indicates that Cu is positively correlated with EC, TDS, Na + , K + , Cl − and \({\text{NO}}_{3}^{ - }\) . Similarly, Mn is positively correlated with pH, EC, TDS and Cu. While, Ag is positively correlated with pH, Ca 2+ , Mg 2+ , K + , TH, Cl − , \({\text{NO}}_{3}^{ - }\) and Mn. Further, Fe is positively correlated with TDS, Mg 2+ , Na + , TH, \({\text{HCO}}_{3}^{ - }\) , \({\text{SO}}_{4}^{2 - }\) , \({\text{NO}}_{3}^{ - }\) , Cu and Ag. Similarly, Ni is positively correlated with pH, EC, TDS, Ca 2+ , K + , \({\text{NO}}_{3}^{ - }\) and Mn.
The higher concentration of Ni, Fe and Mn may trigger the presence of other heavy metals viz. Pb, Cd and Cr which are very sensitive and significant heavy metal and needs to be observed carefully in future for groundwater quality in the study area. The presence of Fe, \({\text{SO}}_{4}^{2 - }\) and \({\text{NO}}_{3}^{ - }\) may trigger the presence of Cd (Chaurasia et al. 2018 ).
Spatial distribution pattern
The spatial distribution pattern of the contour maps of the groundwater quality parameters have been generated as represented in Fig. 2 a–t. The spatial distribution pattern of the pH indicates that the central part along NW–SE across the district with some scattered small patches throughout indicating the presence of alkaline groundwater (Fig. 2 a). In acidic water, fluoride is adsorbed on a clay surface, while in alkaline water, fluoride is desorbed from solid phases; therefore, alkaline pH is more favourable for fluoride dissolution, (Keshavarzi et al. 2010 ; Rafique et al. 2009 ; Saxena and Ahmed 2003 ; Rao 2009 ; Ravindra and Garg 2007 ; Vikas et al. 2009 ). The southern portion of the district in Kabrai Block is having high TDS (> 750 mg/l) in groundwater (Fig. 2 c) due to poor fluxing and highly weathered rock formations. Similarly, EC is mainly highest (> 900 mg/l) in the southern part with small scattered patches in central and NE part of the district (Fig. 2 b). This is in consonance with the higher TDS (significant positive correlation with EC) as evidenced by the correlation matrix of the quality parameters (Table 2 ). The alkalinity map clearly and significantly indicates that it is highest in the central part surrounded by gradually decreasing alkalinity outwards (Fig. 2 d). The bicarbonates trigger the alkalinity in groundwater (Adams et al. 2001 ). The quality of groundwater in a major portion of the study area is alkaline in nature, indicating that the dissolved carbonates are predominantly in the form of bicarbonates. A positive correlation is observed between the alkalinity of groundwater and fluoride (Table 2 ), consequently releasing fluoride in the groundwater. The spatial distribution map of Ca 2+ suggests varying concentration within permissible limit throughout the study area (Fig. 2 f) due to the presence of alkali feldspar in granite. Similarly, Mg 2+ is also distributed unevenly but falls within permissible limit with an exception in NE part of the district (Fig. 2 g). The spatial distribution pattern of TH reflects that the study area is characterized by moderately hard groundwater.
Figure 2 e The Ca 2+ and Mg 2+ ions present in the groundwater are possibly derived from leaching of calcium and magnesium bearing rock-formations in the study area. The fluoride in groundwater shows a negative correlation with Ca 2+ , indicating the high value of fluoride in groundwater in association with low Ca 2+ content. The correlation matrix clearly marks a significant positive correlation among Na + , alkalinity and TDS, which is being reflected from their respective spatial distribution maps (Fig. 2 c, d and h). Na + is highest in the central part (with small patches in the eastern part and insignificantly in the western part) which is in conformity with the alkalinity and TDS spatial distribution patterns. Although, the presence of K + is insignificant and its lower concentration within the permissible limit is covering a major portion of the district due to poor weathering of orthoclase. Its distribution pattern indicates conformity more or less with the TDS and Na + (Fig. 2 c, h, and i). \({\text{HCO}}_{3}^{ - }\) is an important quality parameter showing significant positive correlation (> 0.50) with alkalinity and Na + (Table 2 ) which is also reflected in the spatial distribution pattern of these parameters (Fig. 2 d, h, j). Although sulphate ( \({\text{SO}}_{4}^{2 - }\) ) is an important quality parameter. It is present within the permissible limit in the study.
area (Fig. 2 k). Chloride is slightly in excess in a larger patch, particularly in SE-part of the study area which may cause a health hazard. It is revealed from the spatial distribution map of chloride (Fig. 2 l). This is due to poor fluxing and presence of halite mineral. Fluoride (F − ) is an important quality parameter, especially with respect to the study area where it is present noticeably in scattered patches throughout the district. It is observed that mainly in NE part, the central part and SE part of the district the concentration of fluoride is in excess (2.82 mg/l to 3.91 mg/l) of permissible limit 1.5 mg/l (Fig. 2 m). The higher concentration (> 3.0 mg/l) of fluoride may lead to skeletal fluorosis (Raju et al 2009 ). Several factors viz. temperature, pH, presence or absence of complexing or precipitating ions and colloids, the solubility of fluorine bearing minerals (biotite and apatite), anion exchange capacity of the aquifer (OH − with F − ), size and type of geological formations traversed by groundwater and the contact time during which water remains in contact with the formation are responsible for fluoride concentration in groundwater (Apambire et al. 1997 ). The lithology of fractured rock reveals that it contains more fluoride bearing minerals than massive rocks (Pandey et al. 2016 ). Nitrate (NO 3 − ) in groundwater is mainly anthropogenic in nature which could be due to leaching from waste disposal, sanitary landfills, over-application of inorganic nitrate fertilizer or improper manure management practice (Chapman 1996 ). In this study, it is observed that nitrate is in excess of the permissible limits with varying degree of concentration throughout the district, causing health hazard (Fig. 2 n). The area under study is granite-gneiss terrain where the atmospheric nitrogen is fixed and added to the soil as ammonia through lightning storms, bacteria present in soil and plants roots. Further, animal wastes, plants and animals remain also undergo ammonification in the soil producing ammonia which undergoes nitrification. The high values of nitrate in groundwater samples in the area may be due to unlined septic tanks and unplanned sewerage system that contaminates to the phreatic aquifer (Hei et al. 2020 ). Proper monitoring and concerned regulated effort are consistently required to get the assessment of nitrate impact on human health.
As far as heavy metals concentration in groundwater is concerned, Cu does not mark its noticeable presence (Fig. 2 o). Another, naturally occurring quality parameter is Mn which shows its presence within the permissible limit (Fig. 2 p). Silver and Zinc do not show any remarkable presence in the study area (Fig. 2 q and r). The study reveals a higher concentration of iron in groundwater in the Eastern part of the district due to secondary porosity and where ferrous (Fe 2+ ) ion usually occurs below the water table. The Fe 2+ after converting into Ferric (Fe 3+ ) state, becomes harmful and precipitated. This condition can be avoided naturally by raising the water table through groundwater recharging the affected area (Fig. 2 s). Nickel shows its remarkable presence in smaller patches in different areas (Fig. 2 t) due to the presence of heavy minerals like rutile and apatite.
Water quality index
The water quality index (WQI) map has been prepared using ArcGIS 10.1 on the basis of the selectively chosen quality parameters to decipher the various quality classes viz. excellent, good, poor, very poor and unsuitable at each hydro-station for drinking purpose (Tables 3 and 5 ; Fig. 3 ). The WQI Map of the study area indicates that major portion is having excellent (0–25) quality of groundwater while very poor (75–100) to unsuitable (> 100) quality is prevailing in small pockets in SW part (Fig. 3 ). The map clearly indicates that the quality of groundwater in Panwari Block belongs to excellent to good categories as for as potability for human consumption is concerned.
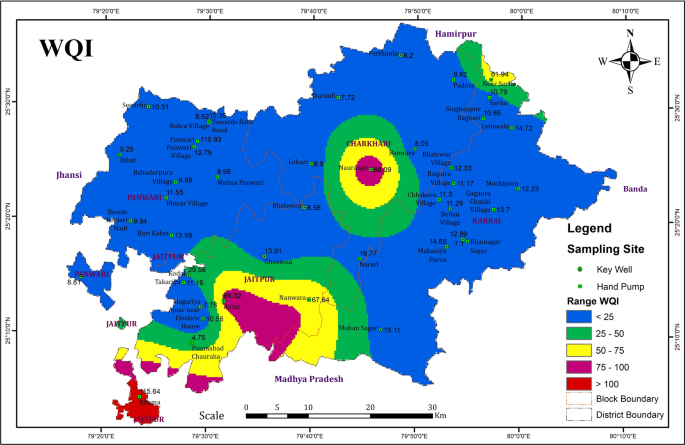
Water quality index map of the study area, District Mahoba
There is gradual variation in groundwater quality from very poor to excellent at the central part and outwards in the Charkhari Block. There is no noticeable change in the quality of groundwater except in the SW part of the Kabari Block. In the Jaitpur block, there is a significant.
variation in the quality class and the SW part (Nanwara, Ajnar and Khama) is characterized by poor, very poor and unsuitable categories (Fig. 3 ). Remaining part of the block falls under good to excellent groundwater quality. Overall, the quality of groundwater belongs to the excellent category in a major portion of the study area and is suitable for drinking as well as domestic uses.
Hydro-chemical facies
The major ions analysed are unevenly distributed and have been plotted on a Hill-Piper Trilinear diagram (Fig. 4 ). This diagram is comprised of two triangles at the base and one diamond shape at the top to represent the major significant cations and anions responsible for the nature of groundwater (Balamurugan et al. 2020a ). The piper diagram is used to categorize groundwater into various six types such as Ca 2+ - \({\text{HCO}}_{3}^{ - }\) type, Na + -Cl − type, mixed Ca 2+ -Mg 2+ -Cl − type, Ca 2+ -Na + - \({\text{HCO}}_{3}^{ - }\) type, Na + - \({\text{HCO}}_{3}^{ - }\) type and Ca 2+ -Cl − type. A critical evaluation of the diagram reflects that 32.56% of the samples fall under Na + -Cl − type, 30.23% of the samples under mixed Ca 2+ -Mg 2+ -Cl − type, 16.28% of the samples under Ca 2+ - \({\text{HCO}}_{3}^{ - }\) type, 13.95% of the samples under mixed Ca 2+ -Na + - \({\text{HCO}}_{3}^{ - }\) type, 4.65% of the samples under Na + - \({\text{HCO}}_{3}^{ - }\) type and 2.33% of the samples under Ca 2+ -Cl − type. Further, the observation reveals that the samples are distributed mainly into Na + -Cl − type, mixed Ca 2+ -Mg 2+ -Cl − type and Ca 2+ - \({\text{HCO}}_{3}^{ - }\) type reflecting higher concentration of sodium and calcium bearing salt/mineral. Hydrochemistry of the analysed samples indicate that the major cations are present in order Na + > Ca 2+ > Mg 2+ > K + of mean abundance while anions are present in the mean abundance order of \({\text{HCO}}_{3}^{ - }\) > \({\text{NO}}_{3}^{ - }\) > Cl − > \({\text{SO}}_{4}^{2 - }\) > F − (Table 1 ). This reveals that sodium, chloride and bicarbonate dominate the ionic concentration in the groundwater due to action of weathering of minerals like halite and dolomite as well as ion exchange process.
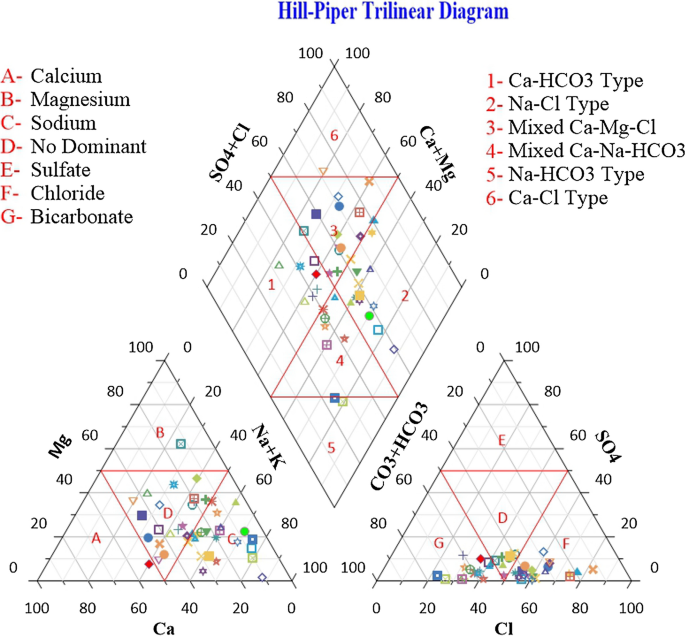
Types of groundwater
The outcome of the present research in the hard rock area of the Bundelkhand region of India reveals that the groundwater has been deteriorated due to both geogenic and anthropogenic activities.
The study area is comprised mainly of granite and alkali granite, specifically in extreme southern which is responsible for leaching of fluoride in groundwater.
The thickness of overburden (loose soil and weathered rock) in the northern part of the study area is negligible. Therefore, there is a poor fluxing of groundwater which in turn triggers the concentration of TDS, fluoride and bicarbonate in groundwater.
Anthropogenic activities like unlined septic tanks and unplanned sewerage system have triggered the nitrate concentration in groundwater, particularly in the central and northern part of the study area. The rest of the area is safe and has potable groundwater. In addition, the area under study is granite-gneiss terrain where the atmospheric nitrogen is fixed and added to the soil as ammonia through natural lightning, bacteria present in soil and plants roots. Further, ammonification of animal wastes, plants and animal remains produces ammonia which undergoes nitrification.
Hydro-chemical facies reveal that the nature of groundwater is Na + -Cl − , mixed Ca 2+ -Mg 2+ -Cl − and Ca 2+ - \({\text{HCO}}_{3}^{ - }\) type in the study area.
The high value of WQI has been found, which is due to the higher values of chloride, fluoride, nitrate, manganese, iron, and nickel in the groundwater, which warrants immediate attention.
On the basis of WOI, it is concluded that the groundwater is safe and potable in the study area except for localized pockets in Jaitpur and Charkhari Blocks.
Adams S, Titus R, Pietersen K, Tredoux G, Harris C (2001) Hydrochemical characteristics of aquifers near Sutherland in the Western Karoo, South Africa. J Hydrol 241:91–103
Article Google Scholar
Aghazadeh N, Chitsazan M, Golestan Y (2017) Hydrochemistry and quality assessment of groundwater in the Ardabil area. Iran Appl Water Sci 7:3599–3616
Alobaidy AM, Abid HS, Maulood BK (2010) Application of water quality index for assessment of Dokan lake ecosystem, Kurdistan region, Iraqi. J Water Res Prot 2:792–798
Apambire WB, Boyle DR, Michel FA (1997) Geochemistry, genesis, and health implications of fluoriferous groundwater in the upper regions of Ghana. Environ Geol 33:13–22
APHA, AWWA, WEF (2017) Standard Methods for the Examination of Water and Wastewater. 23th edition., APHA, Washington, DC. v.6.
Aravindan S, Shankar K, Ganesh BP, Rajan KD (2010) Hydrogeochemical mapping of in the hard rock area of Gadilam River basin, using GIS technique. Tamil Nadu Indian J Appl Geochem 12(2):209–216
Google Scholar
Balamurugan P, Kumar PS, Shankar K (2020a) Dataset on the suitability of groundwater for drinking and irrigation purposes in the Sarabanga River region, Tamil Nadu, India. Data in Brief 29:105255
Balamurugan P, Kumar PS, Shankar K, Nagavinothini R, Vijayasurya K (2020b) Non-carcinogenic risk assessment of groundwater in Southern Part of Salem District in Tamilnadu, India. J Chil Chem Soc 65:4697–4707
Bawoke GT, Anteneh ZL (2020) Spatial assessment and appraisal of groundwater suitability for drinking consumption in Andasa watershed using water quality index (WQI) and GIS techniques: Blue Nile Basin. Northwestern Ethiopia Cogent Eng 7(1):1748950
BIS (Bureau of Indian Standard) (2012) Indian standard drinking water specification, second revision, pp.1–16.
BIS (Bureau of Indian Standard) (2015) Indian standard drinking water specification. second revision, pp.2–6.
Boyd CE (2000) Water Quality an Introduction. Kluwer Acadamic Publi-shers, Boston, USA, p 330
Boyd SR, Hall A, Pillinger CT (1993) The measurement of d15N in crustal rocks by static vacuum mass spectrometry: Application to the origin of the ammonium in the Cornubian batholith, southwest England. Geochim Cosmochim Acta 57:1339–1347
Brhane GK (2018) Characterization of hydro chemistry and groundwater quality evaluation for drinking purpose in Adigrat area, Tigray, northern Ethiopia. Water Sci 32:213–229
Brown RM, Mccleiland NJ, Deiniger RA, O’Connor MF (1972) Water quality index-crossing the physical barrier. Res Jerusalem 6:787–797
Carrillo-Rivera JJ, Cardona A, Edmunds WM (2002) Use of abstraction regime and knowledge of hydrogeological conditions to control high-fluoride concentration in abstracted groundwater: San Luis Potosı basin, Mexico. J Hydrol 261:24–47
Cerar S, Urbanc J (2013) Carbonate chemistry and isotope characteristics of groundwater of Ljubljansko polje and Ljubljansko Barje aquifers in Slovenia. Sci World J 2013:1–11
Chapman D (1996) Water Quality Assessment-A guide to use of biota, sediments and water environmental monitoring 2nd Edition EPFN Spon. London. p.626.
Chatterjee C, Raziuddin M (2002) Determination of Water Quality Index (WQI) of a degraded river in Asansol industrial area (West Bengal). Nat Environ Pollut Technol 1:181–189
Chaurasia AK, Pandey HK, Tiwari SK, Prakash R, Pandey P, Ram A (2018) Groundwater quality assessment using water quality index (WQI) in parts of Varanasi district, Uttar Pradesh, India. J Geol Soc India 92:76–82
Dahlgren RA (1994) Soil acidification and nitrogen saturation from weathering of ammonium-bearing rock. Nature 368:838–841
Deshmukh AN, Shah KC, Sriram A (1995) Coal Ash: a source of fluoride pollution, a case study of Koradi thermal power station, District Nagpur. Maharashtra Gondwana Geol Mag 9:21–29
Diersing N, Nancy F (2009) Water quality: Frequently asked questions. Florida Brooks National Marine Sanctuary, Key West, FL
Egereonu UU, Nwachukwu UL (2005) Evaluation of the surface and groundwater resources of Efuru River Catchment, Mbano, South Eastern. Nigeria J Assoc Adv Model Simulat Tech Enterpr 66:53–71
Fantong WY, Satake H, Ayonghe SN, Suh EC, Adelana SM, Fantong EBS, Zhang J (2010) Geochemical provenance and spatial distribution of fluoride in groundwater of Mayo Tsanaga River Basin, Far North Region, Cameroon: implications for incidence of fluorosis and optimal consumption dose. Environ Geochem Health 32:147–163
Gaciri SJ, Davies TC (1993) The occurrence and geochemistry of fluoride in some natural waters of Kenya. J Hydrol 143:395–412
Galloway JN, Dentener FJ, Capone DG, Boyer EW, Howarth RW, Seitzinger SP, Asner GP, Cleveland CC, Green PA, Holland EA, Karl DM (2004) Nitrogen cycles: past, present, and future. Biogeochemistry 70:153–226
Gebrehiwot AB, Tadesse N, Jigar E (2011) Application of water quality index to assess suitablity of groundwater quality for drinking purposes in Hantebet watershed, Tigray, Northern Ethiopia. ISABB J Food Agric Sci 1:22–30
GEMS U (2007) Global drinking water quality index development and sensitivity analysis report. United Nations Environment Programme Global Environment Monitoring System/Water Programme. p.58.
Hei L, Bouchaou L, Tadoumant S, Reichert B (2020) Index-based groundwater vulnerability and water quality assessment in the arid region of Tata city (Morocco). Groundw Sustain Dev:100344
Holloway JM, Dahlgren RA, Hansen B, Casey WH (1998) Contribution of bedrock nitrogen to high nitrate concentrations in stream water. Nature 395:785–788
Horton RK (1965) An index number system for rating water quality. J Water Pollut Control Federation 373:303–306
Jagadeeswari PB, Ramesh K (2012) Deciphering fresh and saline groundwater interface in south Chennai coastal aquifer, Tamil Nadu, India. Int J Res Chem Environ 2:123–132
Jha SK, Nayak AK, Sharma YK (2010) Potential fluoride contamination in the drinking water of Marks Nagar, Unnao district, UP, India. Environ Geochem Health 32:217–226
Johnson DL, Ambrose SH, Bassett TJ, Bowen ML, Crummey DE, Isaacson JS, Winter-Nelson AE (1997) Meanings of environmental terms. J Environ Qual 26:581–589
Kalavathy S, Sharma TR, Suresh KP (2011) Water quality index of river Cauvery in Tiruchirappalli district, Tamilnadu. Arch Environ Sci 5:55–61
Kaminsky LS, Mahoney MC, Leach J, Melius J, Jo Miller M (1990) Fluoride: benefits and risks of exposure. Crit Rev Oral Biol Med 1:261–281
Kangjoo K, Yun ST (2005) Buffering of sodium concentration by cation exchange in the groundwater system of a sandy aquifer. Geochem J 39:273–284
Karro E, Uppin M (2013) The occurrence and hydrochemistry of fluoride and boron in carbonate aquifer system, central and western Estonia. Environ Monit Assess 185:3735–3748
Kavitha MT, Divahar R, Meenambal T, Shankar K, VijaySingh R, Haile TD, Gadafa C (2019a) Dataset on the assessment of water quality of surface water in Kalingarayan Canal for heavy metal pollution, Tamil Nadu. Data in Brief 22:878–884
Kavitha MT, Shankar K, Divahar R, Meenambal T, Saravanan R (2019b) Impact of industrial wastewater disposal on surface water bodies in Kalingarayan canal, Erode district, Tamil Nadu, India. Arch Agric Environ Sci 4(4):379–387
Kavitha R, Elangovan K (2010) Ground water quality characteristics at Erode district, Tamilnadu India. Int J Environ Sci 1:163–175
Kawo NS, Shankar K (2018) Groundwater quality assessment using water quality index and GIS technique in Modjo River Basin, Central Ethiopia. J Afr Earth Sci 147:300–311
Keshavarzi B, Moore F, Esmaeili A, Rastmanesh F (2010) The source of fluoride toxicity in Muteh area, Isfahan. Iran Environ Earth Sci 61:777–786
Khan D, Hagras MA, Iqbal N (2014) Groundwater quality evaluation in Thal Doab of Indus Basin of Pakistan. Int J Modern Eng Res 4:36–47
Kumar SK, Chandrasekar N, Seralathan P, Godson PS, Magesh NS (2012) Hydrogeochemical study of shallow carbonate aquifers, Rameswaram Island, India. Environ Monit Assess 184:4127–4138
Kumar SK, Logeshkumaran A, Magesh NS, Godson PS, Chandrasekar N (2014) Hydro-geochemistry and application of water quality index (WQI) for groundwater quality assessment, Anna Nagar, part of Chennai City, Tamil Nadu, India. Appl Water Sci 5:335–343
Magesh NS, Elango L (2019) Spatio-temporal variations of fluoride in the groundwater of Dindigul District, Tamil Nadu, India: a comparative assessment using two interpolation techniques. GIS Geostat Techn Groundwater Sci:283–296.
Magesh NS, Krishnakumar S, Chandrasekar N, Soundranayagam JP (2013) Groundwater quality assessment using WQI and GIS techniques, Dindigul district, Tamil Nadu, India. Arab J Geosci 6:4179–4189
Majumdar D, Gupta N (2000) Nitrate pollution of groundwater and associated human health disorders. Indian J Environ Health 42:28–39
McCray JE, Kirkland SL, Siegrist RL, Thyne GD (2005) Model parameters for simulating fate and transport of on-site wastewater nutrients. Ground Water 43:628–639
Modibo Sidibé A, Lin X, Koné S (2019) Assessing groundwater mineralization process, quality, and isotopic recharge origin in the Sahel Region in Africa. Water 11:789
Mostafa MG, Uddin SMH, Haque ABMH (2017) Assessment of hydrogeochemistry and groundwater quality of Rajshahi City in Bangladesh. Appl Water Sci. 7:4663–4671
Narsimha A, Sudarshan V (2013) Hydrogeochemistry of groundwater in Basara area, Adilabad District, Andhra Pradesh, India. J Appl Geochem 15:224–237
Naseem S, Rafique T, Bashir E, Bhanger MI, Laghari A, Usmani TH (2010) Lithological influences on occurrence of high-fluoride groundwater in Nagar Parkar area, Thar Desert, Pakistan. Chemosphere 78:1313–1321
Ozsvath DL (2006) Fluoride concentrations in a crystalline bedrock aquifer Marathon County, Wisconsin. Environ Geol 50:132–138
Panda RB, Sahu BK, Garnaik BK, Sinha BK, Nayak A (1991) Investigation of water quality of Brahmani River. Indian J Environ Health 33:45–50
Pandey HK, Duggal SK, Jamatia A (2016) Fluoride contamination of groundwater and it’s hydrogeological evolution in District Sonbhadra (UP) India. Proc Natl Acad Sci, India, Sect A 86:81–93
Panigrahi T, Das KK, Dey BS, Panda RB (2012) Assessment of Water Quality of river Sono, Balasore. Int J Environ Sci 3:49–56
Panneerselvam B, Paramasivam SK, Karuppannan S et al (2020a) A GIS-based evaluation of hydrochemical characterisation of groundwater in hard rock region, South Tamil Nadu. India Arab J Geosci 13(17):1–22
Panneerselvam, B., Karuppannan, S., & Muniraj, K. (2020b). Evaluation of drinking and irrigation suitability of groundwater with special emphasizing the health risk posed by nitrate contamination using nitrate pollution index (NPI) and human health risk assessment (HHRA). Human and Ecological Risk Assessment: An Int J, pp 1–25.
Pius A, Jerome C, Sharma N (2012) Evaluation of groundwater quality in and around Peenya industrial area of Bangalore, South India using GIS techniques. Environ Monit Assess 184:4067–4077
Rafique T, Naseem S, Usmani TH, Bashir E, Khan FA, Bhanger MI (2009) Geochemical factors controlling the occurrence of high fluoride groundwater in the Nagar Parkar area, Sindh, Pakistan. J Hazard Mater 171:424–430
Raju NJ, Dey S, Das K (2009) Fluoride contamination in groundwater of Sonbhadra district, Uttar Pradesh, India. Curr Sci 96:979–985
Rao NS (2006) Nitrate pollution and its distribution in the groundwater of Srikakulam district, Andhra Pradesh, India. Environ Geol 51:631–645
Rao NS (2009) Fluoride in groundwater, Varaha River Basin, Visakhapatnam District, Andhra Pradesh, India. Environ Monit Assess 152:47–60
Ravindra K, Garg VK (2007) Hydro-chemical survey of groundwater of Hisar city and assessment of defluoridation methods used in India. Environ Monit Assess 132:33–43
Rivett MO, Russ SR, Morgan P, Smith JW, Bemment N, CD, (2008) Nitrate attenuation in groundwater: a review of biogeochemical controlling processes. Water Res 42:4215–4232
Sadat-Noori SM, Ebrahimi K, Liaghat AM (2014) Groundwater quality assessment using the Water Quality Index and GIS in Saveh-Nobaran aquifer. Iran Environ Earth Sci 71:3827–3843
Sarfo AK, Shankar K (2020) Application of geospatial technologies in the COVID-19 fight of Ghana. Transact Indian Nat Acad Eng 5:193–204
Saxena V, Ahmed S (2003) Inferring the chemical parameters for the dissolution of fluoride in groundwater. Environ Geol 43:731–736
Selvam S, Manimaran G, Sivasubramanian P (2013) Hydrochemical characteristics and GIS-based assessment of groundwater quality in the coastal aquifers of Tuticorin Corporation, Tamilnadu, India. Appl Water Sci 3:145–159
Shankar K, Aravindan S, Rajendran S (2010) GIS based groundwater quality mapping in Paravanar River Sub-Basin, Tamil Nadu, India. Int J Geomat Geosci 1:282–296
Shankar K, Aravindan S, Rajendran S (2011) Hydrogeochemistry of the Paravanar river sub-basin, Cuddalore District, Tamilnadu, India. E-J Chem 8:835–845
Shankar K, Aravindan S, Rajendran S (2011) Spatial distribution of groundwater quality in Paravanar river sub basin, Cuddalore district, Tamil Nadu. Int J Geomat Geosci 1:914–931
Shankar K, Kawo NS (2019) Groundwater quality assessment using geospatial techniques and WQI in North East of Adama Town, Oromia Region. Ethiopia Hydrospatial Anal 3(1):22–36
Soujanya Kamble B, Saxena PR, Kurakalva RM, Shankar K (2020) Evaluation of seasonal and temporal variations of groundwater quality around Jawaharnagar municipal solid waste dumpsite of Hyderabad city. India SN Appl Sci 2:498
Stallard RF, Edmond JM (1983) Geochemistry of the Amazon: 2. The influence of geology and weathering environment on the dissolved load. J Geophys Res: Oceans 88:9671–9688
Tripathy JK, Sahu KC (2005) Seasonal hydrochemistry of groundwater in the Barrier Spit system of the Chilika Lagoon, India. J Environ Hydrol 13:1–9
Venkateswaran S, Karuppannan S, Shankar K (2012) Groundwater quality in Pambar sub-basin, Tamil Nadu, India using GIS. Int J Recent Sci Res 3:782–787
Vikas C, Kushwaha RK, Pandit MK (2009) Hydrochemical status of groundwater in district Ajmer (NW India) with reference to fluoride distribution. J Geol Soc India 73:773–784
WHO (2017) Guideline for drinking water quality, 4th edn. World Health Organization, Geneva
Yisa J, Jimoh T (2010) Analytical studies on water quality index of river Landzu. Am J Appl Sci. 7:453–458
Download references
Acknowledgements
Our thanks go to Central Groundwater Board (CGWB), Lucknow, NR, Region and Department of Soil Science & Agricultural Chemistry, Banaras Hindu University, Varanasi for their valuable support during the chemical analysis of groundwater samples.
There is no sponsored/finical assistance is involved in the present research work.
Author information
Authors and affiliations.
Department of Geology, Institute of Science, CAS, Banaras Hindu University, Varanasi, 221005, India
Arjun Ram & Abhishek Kumar Chaurasia
UGC-HRDC, Banaras Hindu University, Varanasi, 221005, India
S. K. Tiwari
Department of Civil Engineering, Motilal Nehru National Institute of Technology Allahabad, Prayagraj, 211004, UP, India
H. K. Pandey
Central Groundwater Board, Lucknow, 226021, UP, India
Supriya Singh
Department of Soil Science & Agricultural Chemistry, Banaras Hindu University, Varanasi, 221005, India
Y. V. Singh
You can also search for this author in PubMed Google Scholar
Corresponding author
Correspondence to H. K. Pandey .
Ethics declarations
Conflict of interest.
This is certified that there is no conflict of interest either academic or commercial.
Ethical standards
The research work is original in nature and does not contain the others findings accept referred work. Therefore, the entire manuscript follows the Ethical Standards.
Additional information
Publisher's note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ .
Reprints and permissions
About this article
Ram, A., Tiwari, S.K., Pandey, H.K. et al. Groundwater quality assessment using water quality index (WQI) under GIS framework. Appl Water Sci 11 , 46 (2021). https://doi.org/10.1007/s13201-021-01376-7
Download citation
Received : 27 May 2020
Accepted : 25 January 2021
Published : 12 February 2021
DOI : https://doi.org/10.1007/s13201-021-01376-7
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Groundwater
- Hydrochemistry
- Piper diagram
- Bundelkhand massif
- Find a journal
- Publish with us
- Track your research

IMAGES
VIDEO
COMMENTS
Water quality index (WQI) is one of the most used tools to describe water quality. It is based on physical, chemical, and biological factors that are combined into a single value that ranges from 0 to 100 and involves 4 processes: (1) parameter selection, (2) transformation of the raw data into common scale, (3) providing weights and (4 ...
Article. Open access. Published: 05 March 2024. Groundwater quality assessment using water quality index and principal component analysis in the Achnera block, Agra district, Uttar Pradesh,...
The water quality index (WQI) model is a popular tool for evaluating surface water quality. It uses aggregation techniques that allow conversion of extensive water quality data into a single value or index.
Evaluation of water quality index and geochemical characteristics of surfacewater from Tawang India. Nisha Gaur, Arpan Sarkar, Dhiraj Dutta, B. J. Gogoi, Rama Dubey & Sanjai Kumar...
The main aim of the current research is to determine the acceptability of drinking water sources using the Water Quality Index (WQI) values. The Bureau of Indian Standard (BIS 2012) was used to evaluate the WQI and evaluate the quality of water for the water sources.
This study employs cutting-edge AI algorithms to forecast the Water Quality Index (WQI) and classify water quality. Machine Learning models have been developed to predict WQI.
This study was performed to assess water quality in the Narmada River, the third-longest river in India. Water samples were collected from 6 major sampling stations with 17 sampling points. Nine water quality parameters were analyzed to calculate the water quality index (WQI), followed by multivariate statistical evaluation.
Volume 43, Part 6, 2021, Pages 3447-3451. Assessment of water quality Index and study of the impact of pollution on the rivers of Kerala. Author links open overlay panel. Jyothi S.N. a. , Gevargis Muramthookil Thomas a. , Rohith Raj R.V. b. , Akhil Masetti b. , Akhil Tammana b. , Manaswini Motheram b. , Nikhil Chowdary Gutlapalli b. Show more.
quality, as widely adopted in water quality research (Rah- mati et al. 2019 ; Singha et al. 2021 ; Manzar et al. 2022 ; Kumar et al. 2023 ; Sajib et al. 2023 ).
The water quality index (WQI) map has been prepared using ArcGIS 10.1 on the basis of the selectively chosen quality parameters to decipher the various quality classes viz. excellent, good, poor, very poor and unsuitable at each hydro-station for drinking purpose (Tables 3 and 5; Fig. 3).