All Research Articles
- Irish Research Council Enterprise Partnership Scheme
- Health Research Board
- Irish Research Council
- Science Foundation Ireland
- Health Research Board Ireland
- Irish Cancer Society
- Fulbright Commission (Fulbright-HRB Health Impact Award)
- Health Information and Quality Authority
- National Children's Research Ccentre
- Office of the Vice-President for Equality, Diversity and Inclusion, University of Galway
- Health Service Executive, National Doctors Training and Planning
- Health Research Board (HRB) Ireland
- This work is supported by the Health Research Board (HRB) of Ireland
- Health Research Board SPHeRE Programme (Structured Population and Health-services Research Education)
- Further Education Policy at the University of Galway, Ireland.
- University College Dublin

Are you a HRB-funded researcher?
Submission to HRB Open Research is open to all HRB grantholders or people working on a HRB-funded/co-funded grant on or since 1 January 2017. Sign up for information about developments, publishing and publications from HRB Open Research.
We'll keep you updated on any major new updates to HRB Open Research
The email address should be the one you originally registered with F1000.
You registered with F1000 via Google, so we cannot reset your password.
To sign in, please click here .
If you still need help with your Google account password, please click here .
You registered with F1000 via Facebook, so we cannot reset your password.
If you still need help with your Facebook account password, please click here .
If your email address is registered with us, we will email you instructions to reset your password.
If you think you should have received this email but it has not arrived, please check your spam filters and/or contact for further assistance.
Irish Research Council

Health Research Board expands the opportunity for the public, patients and carers to be involved in health research
- Share on Twitter
- Share on Facebook
Posted: 29 January, 2021
The Health Research Board (HRB) is announcing €5m* in funding to support research institutions develop a network to advance the involvement of the public, patients and carers in health and social care research, from generation of ideas to delivery of results.
The initiative, in collaboration with the Irish Research Council (IRC), will see the development of a national network of Public and Patient Involvement (PPI) centres across 17 higher education institutions** on an all-island basis.
The new Network grows and consolidates the work of its predecessor, PPI Ignite. The first of its kind in Ireland, this initiative saw the HRB and the IRC support five universities to catalyse change in Irish research culture by providing support for researchers to involve people in every stage of their research.
Commenting on the announcement, Dr Mairead O’Driscoll, CEO of the HRB said:
“Involving people in the work we do and the work we fund leads to improved research, improved outcomes and improved lives. People’s insights and life experience can inform that work in ways researchers operating in isolation can’t.”
This new PPI Ignite Network is the next step in the HRBs national leadership of involving people in research. Working with the Irish Research Council and all the partner institutions, we are helping to ensure that people and patients are involved at every stage of Irish health and social care research, right from the start, before pen is even put to paper for a research proposal.”
IRC Director Peter Brown also welcomed the award, saying:
“The exchange of knowledge and innovation is a key action in the IRC’s strategy, so I am delighted to further support this award announced today. This programme seeks to embed a culture of public and patient engagement in research across our higher education institutions and promotes richer, more meaningful research outcomes. Following recent joint initiatives such as the COVID-19 Rapid response call, the IRC are pleased to again be partnering with the HRB in support of research for societal benefit.”
NUI Galway is hosting the PPI Ignite Network Programme Office. Leading the team is Professor Sean Dineen, who said:
“We are excited to be given this opportunity to showcase what Ireland can deliver in terms of high quality, meaningful public and patient involvement in health and social care research. We look forward to working with our partner organisations to realise the potential of this investment.”
PPI is research undertaken ‘with’ or ‘by’ members of the public rather than ‘to’, ‘about’ or ‘for’ them, improving research quality and ensuring it’s relevant, useable, and in the best interests of the patient and general population. The new PPI Ignite Network will:
- Develop and deliver PPI education and training to undergraduate and postgraduate students, researchers, the public, patients, and community organisations, policymakers, and research funders
- Explore ways of making it easier to involve patients and the public in research, how to identify what is good PPI and how to measure the impact of PPI
- Ensure the voices of marginalized and disadvantaged groups are heard
- Establish an online PPI hub, annual PPI Festival and other events to share examples of good PPI, provide useful PPI resources and share knowledge and experience. The online hub will also connect patients and members of the public interested in being involved with researchers seeking PPI partners.
Wendy Costello is a patient advocate and PPI contributor, working with research groups in NUI Galway, University College Dublin and the National Children’s Research Centre. She says:
“As a patient advocate, I’m so excited to see the development of the PPI Ignite Network. This joined-up thinking will see a collaboration of the best minds in Ireland coming together to change the landscape of PPI here, and make it the envy of Europe. I can’t contain my excitement and want to roll my sleeves up and get stuck in! I’m particularly excited about the development of a PPI hub. This is something we’ve been pushing for. Matching researchers with the right people is crucial to research priorities and outcomes.”
The new network comprises the five original PPI Ignite universities, and two new universities working with 10 national partner organisations as follows:
** Lead sites:
- NUI Galway (hosting the PPI Ignite Network Programme Office)
- University of Limerick
- Trinity College Dublin
- University College Dublin
- Dublin City University
- University College Cork (new lead site)
- RCSI University of Medicine and Health Sciences (new lead site)
National partners:
- Queens University Belfast
- NUI Maynooth
- HSE Research & Development
- Health Research Charities Ireland
- Campus Engage
- HRB Trials Methodology Research Network
- International Collaboration for Participatory Research
A total of 54 local partners are also involved, including charities and community development groups, international and national academic collaborators, advocacy groups, Saolta and Ireland East Healthcare Groups, HSE Digital Transformation, Clinical Research Facilities, Dundalk IT and SFI Centres.
*The HRB will fund €2.3 million for the PPI Ignite Network. An additional €1 million and €1.6 million co-funding will come from the Irish Research Council (IRC) and the network’s lead sites respectively, leading to a total budget of exactly €4.9 million over five years.
More: Erasmus+ , Europe , Internationalisation , Mairéad McGuinness , National Botanic Gardens
Other Latest Posts
- Research on a new diagnostic tool for Alzheimer’s disease presented at the 30th Annual Alzheimer Day at Northwestern University, Chicago 16 May, 2024
- Metal Oxy-Sulfide and Oxide Nanomaterials: Photodegradation of Chemical Pollutants 9 May, 2024

16 May, 2024
Research on a new diagnostic tool for Alzheimer’s disease presented at the 30th Annual Alzheimer Day at Northwestern University, Chicago
Read Article

9 May, 2024
Metal Oxy-Sulfide and Oxide Nanomaterials: Photodegradation of Chemical Pollutants

An international partnership approach to developing dementia awareness strategies and research
Data protection notice.
Please read our updated Data Protection Notice .
Our use of cookies
We use necessary cookies to make our site work. We'd also like to set optional analytics cookies to help us improve it. We won't set these optional cookies unless you enable them. Using this tool will set a cookie on your device to remember your preferences.
For more detailed information about the cookies we use, see our Privacy Policy page
Necessary cookies
Necessary cookies enable core functionality such as security, network management, and accessibility. You may disable these by changing your browser settings, but this may affect how the website functions.
Analytics cookies Consent to Analytics cookies
We'd like to set Google Analytics cookies to help us to improve our website by collecting and reporting information on how you use it. The cookies collect information in a way that does not directly identify anyone.
Save and close

CLINICAL TRIAL NETWORKS
Clinical research networks.
HRB NCTO works with and supports a number of clinical trial networks. Clinical trial networks are groups of clinicians and scientists from across Ireland who have come together around a particular disease, or clinical interest. Investigators agree on research activities that are best conducted as a large group nationally rather than in isolation.
A number of clinical trials networks have been established and are listed below by research area. Some receive state funding while others are funded mainly through charitable funds. The Health Research Board have funded cancer research through Cancer Trials Ireland since 2002 and in recent years have funded the establishment of additional Clinical Trial Networks in, Critical Care, Paediatrics (in4Kids), Primary Care, Rare Disease, Infectious Disease, Diabetes and Dementia.
The clinical research network page is live and updated on an ongoing basis. If you are a lead of a clinical research network and would like it included on the HRB NCTO website, please contact [email protected].
HRB NCTO works with and supports a number of clinical research networks. Clinical research networks are groups of clinicians and scientists from across Ireland who have come together around a particular disease, or clinical interest. Investigators agree on research activities that are best conducted as a large group nationally rather than in isolation.
A number of clinical research networks have been established and are listed below by research area. Some receive state funding while others are funded mainly through charitable funds . The Health Research Board in recent years have funded the establishment of Clinical Trial Networks in, Critical Care, Paediatrics ( i n4Kids), Primary Care, Rare Disease, Infectious Disease, Diabetes and Dementia.
The clinical research network page is live and updated on an ongoing basis. If you are a lead of a clinical research network and would like it included on the HRB NCTO website, please contact [email protected].
RESEARCH AREAS
Cardiovascular & stroke, critical care, haematology, infectious disease, neurodegeneration, paediatrics, perioperative, primary care, rare disease, rare kidney disease, respiratory, rheumatology.

Cancer Trials Ireland
Cancer Trials Ireland sponsor and operate Cancer Clinical trials in Ireland and Europe. Its 50+ strong staff manage a portfolio of 100+ cancer studies. In the past 20 years almost 31,000 people have taken part in nearly 800 cancer clinical trials. In 2020, a survey of public attitudes revealed one in two people in Ireland would take part in a clinical trial.
Established in 1996, Cancer Trials Ireland (formally the All-Ireland Cooperative Oncology Research Group – ICORG) was established in 1996. It enables patients in Ireland to gain early access to novel cancer treatments and therapies. Cancer Trials Ireland operate cancer clinical trials across a number of disease areas – Breast, Gastrointestinal, Genitourinary, Gynaecology, Haematology/Lymphoma, Lung, etc. The organisation works closely with international collaborative groups such as ECOG, NSABP, ANZUP and has developed strong links with our pharmaceutical partners.
+353-1-6677211
BCNI: Blood Cancer Network Ireland
BCNI is a national clinical research network set up to benefit blood cancer patients in Ireland. BCNI offer clinical trials to blood cancer patients, providing the opportunity to test new, potentially life-saving treatments and drugs. BCNI has also established a biobank and blood cancer registry, which will further our knowledge and expertise in the field of blood cancer research and ultimately improve patient outcomes. BCNI has been funded by the Irish Cancer Society and Science Foundation Ireland since 2015.
BCNI is a collaborative network of clinicians, scientists, and population health experts with a shared interest in blood cancer research. Members of the national network are based at the NUI Galway/ University Hospital Galway, University College Cork/ Cork University Hospital, Trinity College Dublin/St James Hospital, Beaumont Hospital and the Mater Hospital, and the National Cancer Registry Ireland. The key investigators involved are Dr Eva Szegezdi, Dr Philip Murphy, Prof. Mary Cahill, Prof. Michael O’Dwyer, Prof. Paul Browne, Prof. Peter O’Gorman, Prof. Kerri Clough-Gorr and Dr John Quinn
The aim of BCNI is to provide blood cancer patients in Ireland with access to novel and innovative cancer treatments through the provision of early phase clinical trials. BCNI also collect information and samples from blood cancer patients in Ireland in order to improve our understanding and to uncover new ways to combat this disease.
+353-91-49-3811
HRB Stroke Clinical Trial Network Ireland
Stroke is the second leading cause of death in the world, the leading cause of new disability, and a major cause of dementia and health costs.
The HRB Stroke Clinical Trial Network Ireland is led by Professor Peter J Kelly, Mater University Hospital and University College Dublin. The Network will initially involve eight Irish hospitals, six leading universities, and all seven Hospital Groups, including colleagues from UCD, RCSI, Trinity College, UCC, NUI Galway, and University of Limerick. It will have strong links with international researchers in the UK, Europe, and North America. In addition to the HRB, other Network partners are the Irish Heart Foundation, who will fund new Stroke Research Nurses, and seven industry partners, who will fund education and training activities.
In the Network, Irish researchers in hospitals will:
- Join several new international trials of new treatments for emergency care, prevention, and recovery after stroke.
- Lead a new clinical trial aiming to prevent second strokes and heart attack after first stroke.
- Train new doctors, nurses, and therapists in how to perform safe high-quality clinical trials, and will work with patient groups and the private sector to bring new treatments to patients with stroke.
+353 (01) 7164576
HRB Irish Critical Care Clinical Trials Network

About the ICC-CTN
The Irish Critical Care Clinical Trials Network (ICC-CTN), set up in 2015 by Professor Alistair Nichol, has become a leading critical care research network in Ireland and globally. By supporting ICUs throughout Ireland and Europe, the ICC-CTN has helped to facilitate the roll-out of global trials in ICUs across the island of Ireland, giving Irish patients access to novel treatments. With a large network of collaborators around the world, the ICC-CTN has been able to secure both national and international funding, which has helped to grow the ICC-CTN team and widen the scope of the trials and research activities they conduct and support. This work has made a significant contribution to ICU research, informing evidence-based clinical practices and guidelines on a global scale.
Strategic Objectives
After receiving a renewal of funding from the HRB CTN-2021 award scheme, the network has begun the expansion of projects and activities including leadership in national and international PPIE groups, Training, Education, Methodology & more. Their main objectives are:
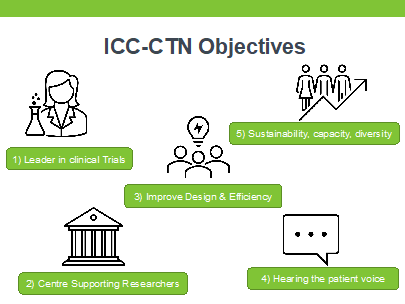
Research Priority Areas
The core thematic research areas include 1) Pandemic Preparedness 2) Out of hospital Cardiac Arrest, 3) Trauma, 4) Acute Respiratory Failure, 5) Acute Renal Failure. The ICC-CTN conducts and supports clinical trials and observational, non-interventional and pilot/feasibility studies in these core areas and more, including sepsis, sedation and delirium, nutrition, and physiotherapy.

During the last seven years, the ICC-CTN have been involved in the coordination of over 19 ICU clinical studies that have delivered clinical practice changing results to more than 1600 Irish patients and more than 800,000 patients globally. The network works with 18 Irish, and more than 30 European hospitals as part of a global collaboration that spans 63 countries and multiple partnerships.
Check out their Twitter and Website to find out more.

HRB Dementia Clinical Trials Network
HRB Dementia Clinical Trials Network (‘Dementia Trials Ireland’; DTI) is a multidisciplinary national dementia clinical research network led by Professor Iracema Leroi at St James’ Hospital, Dublin and Professor Seán Kennelly who is based at Tallaght University Hospital, Dublin. The DTI Steering Committee is supported by clinical and non-clinical dementia researchers from around Ireland including geriatricians, psychiatrists, neurologists, neuroimagers, PPI members, biostatisticians/trial methodologists and bioengineers. The objective of DTI is to significantly develop and expand the small dementia clinical trials’ portfolio to include studies of diverse types (proof of concept to implementation; non-pharmacological to pharmacological approaches) suitable for all stages and sub-types of dementia (i.e. from preclinical to advanced stage dementia). Our studies will apply different methods, including imaging, biomarkers and neuropsychological methods to assess healthy volunteers, carers and patients. The ultimate goal is to enable every person at risk of, or living with, dementia in Ireland, the opportunity to access clinical trials. This will entail upskilling the workforce to conduct trials, attracting more international trials (including industry-sponsored trials) and competing for funding for investigator-led trials within and beyond Ireland. A cornerstone of the work will be the inclusion of people with lived experience and their caregivers in the day-to-day development of DTI (patient and public involvement; PPI), as well as raising public and professional awareness of dementia and dementia trials throughout Ireland. The current study portfolio is a combination of investigator-led (i.e. www.sense-cog.eu ) and industry-sponsored studies. Current research focuses on Alzheimer’s, Lewy Body Disorders and other dementias, mild cognitive impairment, brain-injury and healthy volunteers.
DTI will work closely with and be aligned with a number of other HRB-supported clinical research entities including the HRB-National Clinical Trials Network (HRB-NCTO); HRB Trials Methodology Research Network (HRB-TMRN), the Clinical Research Facilities (Dublin, Cork and Galway), and the HRB’s national PPI network, IGNITE. Additional partners include universities and hospitals in Dublin (The Mater, St Vincent’s, St James’, Tallaght) Cork, Galway and Sligo.
ATLANTIC DiP (Diabetes In Pregnancy)
The ATLANTIC DiP research programme has focused on examining the outcomes of pregnancy for women with Type 1 and Type 2 Diabetes and the factors influencing these outcomes. The mission of ATLANTIC DIP is to improve the outcomes of pregnancy for women with Diabetes by promoting evidence based best practice before, during and after pregnancy.
HRB Diabetes Collaborative Clinical Trial-Network-Ireland (DCCTN)
Diabetes is one of the most common chronic diseases in Ireland and the number affected by diabetes is increasing at an alarming rate alongside the increase in obesity rates. Good diabetes control and treatment avoids diabetes complications, and quality of life is sustained. Patients with diabetes throughout the course of their disease receive treatment from multiple disciplines including pharmacotherapy, surgery, medical nutritional interventions, psychology, and physiotherapy. In addition, they are exposed to new and evolving technologies to measure glucose and deliver insulin. To further advance treatments and technologies for patients with diabetes a multi-disciplinary collaborative approach is required with representation from all the departments patients will encounter.
The HRB Diabetes Collaborative Clinical Trial Network is an all-island collaborative network, the aim is to bring together key stakeholders from different disciplines to design and work on a range of ambitious multi-centre diabetes clinical trials. These trials will have the aim of improving health and wellbeing for all patients with diabetes on the island of Ireland.
It is foremost in the minds of the Network members, that all Network activities have patient and public involvement and are complimentary to the priorities of the national HSE Diabetes clinical care programme. While it is early days for the Diabetes Collaborative CTN, the core working group is working hard to increase the collaboration between members, and access for patients to clinical trials.
You can find more information about the Diabetes Collaborative Clinical Trial Network at https://diabetestrialsctn.ie/ or emailing [email protected]
The Irish Network for VTE Research
The Irish Network for VTE Research (INViTE) is an Irish, patient-oriented research network aimed at developing and participating in excellent National and International venous thromboembolism (VTE)-related research. We are honoured to work with the patient group Thrombosis Ireland (http://thrombosisireland.ie) at every step along the way of study concept, design and development
INViTE was launched on 12th September 2018 by the Irish Minister for Business, Enterprise and Innovation Heather Humphries in Croke Park, Dublin and is a member of the prestigious INVENT-VTE (International Network of Venous Thromboembolism Clinical Networks; www.invent-vte.com).
Patient organization Thrombosis Ireland ( http://thrombosisireland.ie/ )
Clinician organizations:
VTE Ireland; Irish Association of Emergency Medicine
Translational research groups/Centres:
UCD Conway SPHERE Research Group ( http://www.ucd.ie/conway/research/researchgroups/ucdconwaysphere/ ); Irish Centre for Vascular Biology ( http://www.rcsi.ie/ICVB )
Hospital partners:
- University Hospital Limerick
- Rotunda Hospital Dublin
- National Maternity Hospital, Holles Street, Dublin
- St Vincent’s University Hospital Dublin
- Our Lady of Lourdes Hospital Drogheda
- St James’s Hospital Dublin;
- Beaumont Hospital Dublin
- Cork University Hospital
- Mater Misericordiae University Hospital Dublin
- University Hospital Galway
- Our Lady’s Hospital Navan
- Sligo University Hospital
- Midland Regional Hospital Tullamore
- Coombe Women and Infants Hospital, Dublin
- Belfast City Hospital (Due to join in 2021)
University partners:
University College Dublin, University of Limerick, Royal College of Surgeons in Ireland, University College Cork, Trinity College Dublin, (2021: Queens University, Belfast)
CRCs: University of Limerick CRU, RCSI, UCD, TCD CRCs
Inclusion Health Service, St James’s Hospital, Dublin
Department of Health Intelligence R&D, Irish Health Services Executive
Hospital Groups: Ireland East Hospital Group
INVENT-VTE (International Network of Venous Thromboembolism Clinical Networks; www.invent-vte.com )
Irish Hepatitis C Outcomes Research Network (ICORN)
Established in 2012, ICORN is a collaboration between clinical specialist groups, patient advocacy groups and healthcare service providers including the Irish Society of Gastroenterology (ISGE), the Infectious Diseases Society of Ireland (IDSI), and the National Centre for Pharmacoeconomics (NCPE). Clinicians from the seven hospitals with centres of excellence in gastroenterology, hepatology or infectious disease caring for patients with Hepatitis C (HCV) infection are involved, they are: Beaumont Hospital, Dublin; Cork University Hospital, Cork; Galway University Hospital, Galway; Mater Misericordiae University Hospital, Dublin; St. Luke’s Hospital, Kilkenny; St. James’s Hospital, Dublin and St. Vincent’s Hospital, Dublin.
The initial goal of this collaboration was to optimise the quality of care of patients with Hepatitis C (HCV) undergoing treatment with direct-acting antiviral therapy (DAAs). This prompted the design and implementation of treatment protocols for clinical sites and the establishment of the ICORN Treatment Registry, a prospective longitudinal treatment outcomes registry. The ICORN Treatment Registry facilitates clinical, basic science and translational medicine research for HCV infection. Other network research themes developed since 2012 include innovative research projects on models of care and screening for HCV infection.
ICORN affords the opportunity for collaborative, multi-centered, world-class research in patients with Hepatitis C in Ireland and is uniquely positioned as the leading platform for HCV clinical research in Ireland.
For further information, please contact: Dolores Barry, ICORN Programme Manager Tel: +353 1 896 4038 Email: [email protected]

ID-CTNI: Infectious Diseases Clinical Trials Network Ireland
ID-CTNI is led by Professor Paddy Mallon, St Vincent’s University Hospital and University College Dublin (UCD). The ID-CTNI network is hosted at the UCD Clinical Research Centre and supported by the UCD Centre for Experimental Pathogen Host Research (CEPHR).
The ID-CTNI network brings together a team with expertise in leading, directing and assisting Infectious Diseases clinical trials and investigations, both investigator and industry led. The multidisciplinary network comprises academic clinicians and researchers from:
- University College Dublin/St Vincent’s University Hospital & Mater Misericordiae University Hospital
- University College Cork/Cork University Hospital
- Trinity College Dublin/St James’s Hospital
- Royal College of Surgeons in Ireland/Beaumont Hospital
- University of Galway/Galway University Hospital
The objectives of the ID-CTNI include:
Implement a governance structure that improves Infectious Diseases clinical trial design, methods, and coordination in Ireland Integration of existing investigator-initiated clinical trials and the implementation of new multicentre clinical trials. Patient involvement will play a central role in the network and planning of clinical trials. The expertise within the ID-CTNI enables better integration of resources and training of new researchers in Infectious Diseases, this will better place ID-CTNI research internationally. Along with other clinical trial networks, Align and integrate with national strategic initiatives in Infectious Diseases to be better prepared for future pandemics
The unprecedented focus on Infectious Diseases arising from the COVID19 pandemic is providing opportunities for the ID-CTNI to develop international clinical trials with other clinical trials networks.
Current Multi-site Clinical Trials include:
EU-COVAT-2 BOOSTAVAC (COVID-19 Booster Vaccine Trial)
An International Multicentre, Phase 2, Randomised, Adaptive Protocol to determine need for, optimal timing of and immunogenicity of administering a 4th homologous mRNA vaccination dose against SARS-CoV-2 in the general population (18+ years) already vaccinated against SARS-CoV-2
COVIRL-002 – Tocilizumab for management of severe, non-critical COVID-19 infection.
An open-label, multi-centre, randomised trial comparing different doses of single-dose tocilizumab in adults with severe, non-critical, PCR-confirmed COVID-19 infection with evidence of progressive decline in respiratory function and evolving systemic inflammation on time to intubation, non-invasive ventilation and/or all-cause mortality.
SWIFT Study – Semaglutide’s Efficacy in Achieving Weight Loss for Those With HIV.
A randomised, controlled, parallel group, open-label definitive intervention trial evaluating the impact of a 28-week course of weekly, subcutaneous Semaglutide alongside dietary and exercise advice on weight loss, inflammation and immune function in obese people living with HIV on stable antiretroviral therapy compared to dietary and exercise advice alone.
A new Monkeypox Vaccine Trial study to be started soon.
For queries please contact: Dr Polina Smovolyk, Project Manager. Email: < [email protected] >
Motor Neurone Disease
Research Motor Neurone (RMN) was founded in 2007, for the purpose of promoting and facilitating research into the causes and treatments of motor neurone disease (MND), also known as ALS. RMN also strives to increase awareness of this incurable disease at both a national and international level. Ongoing research is needed to discover the cause, treatment and methods of improving quality of life for MND sufferers and their families.
Neurology Research Group, St. Vincent’s University Hospital
The Neurology Research Group in St. Vincent’s University Hospital (SVUH) was established over 15 years ago. It has expertise and experience running numerous clinical pharmaceutical trials, academic research studies – both clinical and basic science, and interventional studies with allied health professionals. Several national neurology registries and research databases are also co-ordinated from the SVUH site.
Their particular areas of excellence are neuroinflammatory disorders, in particular multiple sclerosis, movement disorders and cognitive neurology. They have a dedicated clinical facility and can run clinical trials in all other areas of neurology. Their principal Investigators are recognised as international experts in their field of research and are experienced national principle investigators for many clinical trials. In addition to four consultant neurologists, they have a dedicated research team consisting of neurology research registrars, clinical research nurse manager/ coordinator, 3 clinical research nurses and a research assistant. As well as dedicated clinical facilities they have office space for external monitors, laboratory facilities on site for sample processing and access to neuroimaging, neurophysiology, neuropsychology, neuropsychiatry and neuro-ophthalmology services to name but a few. They have on-going collaborations with a number of basic neuroscience and bioengineering groups in Ireland and internationally.
For further information please contact: Sinead Jordan, Neurology Research Nurse Manager Email: [email protected] Phone: (01) 2213592

Irish Network for Children’s Clinical Trials
In4kids is a HRB funded national paediatric clinical research network hosted by the INFANT Centre in University College Cork (UCC). The network’s vision is for better medicines, medical devices & interventions for babies, children & young people in Ireland. Children are not just small adults – they have distinct physiologic, developmental, and psychological characteristics. They deserve and must have the same access to high quality, evidence-based (from paediatric studies) health care as adults..
Through increased and enhanced collaboration amongst the paediatric research community in Ireland, In4kids will develop the national capacity for high-quality, ethical paediatric clinical research.
They welcome all of the Paediatric research community across Ireland to join them, by becoming members of the network. Benefits of this are access to the members only platform of bespoke supports and tools for conducting paediatric research, increased opportunities to collaborate on national clinical research projects and being kept informed on the recent advances in national and international paediatric research.
More information on In4kids membership is available here .
Follow them on Twitter and LinkedIn .
Subscribe to their newsletter .
Visit their website for further information.
For general enquiries email: [email protected]
HRB Mother & Baby Clinical Trials Network Ireland
The HRB Mother & Baby Clinical Trials Network Ireland (HRB M&B CTNI) is a new, exciting and unique partnership between the two most successful perinatal research entities currently operational in Ireland, INFANT and Perinatal Ireland.
This CTN represents a critical mass of obstetricians, neonatologists, midwives and allied professionals from seven of the largest maternity hospitals on the island of Ireland, which deliver over 55,000 babies per annum (almost three quarters of the total births on this island). Together, we have a strong track record in collaborative research and in the conduct of large-scale, international, multicentre, randomised controlled trials (RCTs) of diagnostics and interventions in pregnancy and neonates (e.g. PELICAN, IMPROvED, NEMO, TEST). In addition, we are acknowledged world leaders in longitudinal cohort studies such as ESPRiT, PORTO, GENESIS, SCOPE etc. and we have established and curate Ireland’s first birth cohort, BASELINE.
Perinatal disease accounts for nearly 10% of the global burden of disease. However, R&D investment in perinatal health remains small and nonstrategic; the number of registered pipeline drugs for perinatal conditions is only 1-5% of those for other major disease areas. One barrier to the development of better therapies and diagnostics for mothers and babies is the intrinsic complexity of conducting trials in these uniquely vulnerable populations and conducting long-term follow up. The Mother & Baby CTNI, built on a robust platform of existing collaborations and more than a decade of experience in the conduct of research in the perinatal population, will reverse this pattern.
The primary aim of the HRB M&B CTNI is to unite the combined experience of both the medical teams and resources in all centres. This combination provides a world class research infrastructure which is at the forefront of translation research regarding the transition of fundamental research into the clinical environment. HRB M&B CTNI has a balanced and extensive portfolio of both ‘home-grown’ and international clinical trials of novel interventions and diagnostics in pregnancy and neonates. The HRB M&B CTNI will facilitate greater national collaboration in the arena of perinatal trials and will ensure that Ireland maintains our place at the international forefront of this area of clinical research.
For all queries please contact: Dr Liz Tully, Network Manager. Email: [email protected] or Telephone: 01-4022540
Irish Centre for Fetal and Neonatal Translational Research (INFANT)

A research centre focused entirely on pregnancy, birth and early childhood.
INFANT is hosted by University College Cork (UCC), Ireland, and co-located in Cork University Hospital and Cork University Maternity Hospital. INFANT was established in 2013, building on over a decade of award-winning translational research with a mission to deliver pioneering translational research to improve health outcomes for mothers and babies.
INFANT’s research is underpinned by leadership in cross-disciplinary clinical and scientific research disciplines. From pregnancy to early childhood, INFANT conducts research in three core areas:
(1) Neuroscience and Development
(2) Foetal and Maternal Health and
(3) Nutrition and Growth.
INFANT has made many world-first scientific breakthroughs including: the development of an algorithm that detects seizures in new-born babies; the discovery and validation of biomarkers to detect brain injury in new-born babies, and the development of early predictive biomarkers for preeclampsia. Read more here.
Research at INFANT is led by a multidisciplinary team of investigators comprised of clinicians, scientists, and engineers with the support of the INFANT Operations and Clinical Research Teams.
Follow them on Twitter , Instagram , Facebook and LinkedIn .
Visit their website for more information.
Perinatal Ireland
Perinatal Ireland is a multi-centre research consortium which focuses on improving both women and children’s health all over Ireland. This improvement is achieved through the utilisation of advanced technologies including ultrasound which contributes to the enhanced detection and diagnosis levels in utero. Perinatal Ireland focuses primarily on areas of clinical improvement in women and children’s health such as intrauterine growth restriction (IUGR), discordant growth in twins, the prevention of stillbirth, prediction of preterm labour etc.
Perinatal Ireland has a unique position resulting from the access to large patient populations which facilitates innovative and ground-breaking clinical and translational research. Each member site of the consortium is equipped with dedicated ultrasound imaging equipment and software, with access to specialised expertise such as biostatistical support and a robust quality system. Perinatal Ireland has a strong track record in the conduct of national prospective observational studies such as the national twin study (ESPRIT) and the national growth restriction study (PORTO) whereby the knowledge gained from this research has proven beneficial to mother and babies across the island in the publication of national clinical care guidelines.
The consortium incorporates the main academic obstetric units across the island including The Rotunda Hospital (Dublin), The National Maternity Hospital (Holles St. Dublin), The Coombe Women and Infant’s University Hospital (Dublin), Cork University Maternity Hospital, University Maternity Hospital Limerick, University Hospital Galway, Royal Jubilee Maternity Hospital (Belfast).
Perioperative Clinical Trials Network Ireland
For all queries please contact: Prof. Donal J. Buggy , Network Manager & Professor of Anaesthesiology & Periop erative Medicine, UCD. Email: [email protected]

HRB Primary Care Clinical Trial Network Ireland
The HRB Primary Care Clinical Trials Network Ireland (CTNI) aims to support the creation of high-quality clinical evidence which improves patient outcomes in Irish primary care. To achieve this, they provide supports to researchers to develop and conduct clinical trials in primary care that address important and common problems. They then support the successful translation and implementation of this evidence into healthcare policy and practice.
The HRB Primary Care CTNI was established in 2015 as a collaborative partnership between NUI Galway, the Royal College of Surgeons in Ireland, Queen’s University Belfast, the Association of University Departments of General Practice in Ireland, and the Irish College of General Practitioners. Since establishment, it has supported more than 30 clinical studies, recruited almost 4,000 patients, leveraged funds of over €19,000,000, and published in leading international journal such as the New England Journal of Medicine, the Lancet, and the British Medical Journal.
In 2021, the HRB Primary Care CTNI was successful in securing funding for the next 5 years of operations, with a focus on achieving the following strategic objectives:
- To maximise the successful delivery of primary care trials in Ireland.
- To continue to build capacity for world-class clinical trials in Irish primary care, through the provision of financial supports to early career researchers, the promotion of primary care research education and dissemination, and the provision of seed funding to develop a roadmap for implementation of a national research IT infrastructure.
- To develop an agenda for Irish primary care clinical trials research, by leading a priority setting partnership to develop the ‘Top 10’ research priorities in chronic disease management, and a core outcome set (COS) for future trials in this area.
- To enhance patient and public involvement (PPI) in primary care research in Ireland, by continuing to grow the capacity of the Primary Care CTNI PPI group, including PPI in network oversight and portfolio development, and continuing our synergistic relationship with the national PPI network.
For more information, contact the Primary Care CTNI Network Manager, Dr Patrick Murphy, by emailing [email protected]
HRB Centre for Primary Care Research
The HRB Centre for Primary Care Research (HRB CPCR) has been funded as a national research centre since 2008. It is a collaborative research group, led by the Department of General Practice, RCSI with colleagues from General Practice in NUIG, TILDA and General Practice in TCD and the School of Pharmacy in Queen’s University Belfast. The emphasis on the second phase of funding is to develop and assess information and communication technology to improve the quality and safety of patient care. The following clinical areas are being covered: safe and effective prescribing; chronic disease management in diabetes; rational antibiotic use in the community and the value of a summary care record.
For all queries please email: [email protected]
Irish College of General Practitioners (ICGP)
The research unit of the ICGP aims to develop and support research and audits in general practice. The overall aim of the research unit is to contribute to the knowledge base of general practice and to support evidence-based practice. The ICGP Research Ethics Committee, granted formal recognition on 17th May 2005, reviews both CTIMP and non-CTIMP projects. The College provides research training and guidance to GPs and GP trainees through in a variety of formats and is a partner on the Primary Care Clinical Trials Network.
For all queries please email: [email protected]
HRB Rare Disease Clinical Trial Network:

The RD-CTN, a HRB research network, led by Prof Rachel Crowley and Prof Cormac McCarthy at University College Dublin, aims to enhance rare disease care and outcomes, with the following objectives:
- Act as a collaborative hub for trials in rare diseases
- Facilitate and support the conduct of trials in rare diseases
- Increase the opportunities for rare disease patients to access high-quality clinical trials.
This will result in increased in patient engagement; more trial opportunities for rare disease patients; increased opportunity for investigator-initiated trials; significant industry investment into Irish healthcare; enhanced knowledge of rare disease through patient focused research and parallel translation scientific studies.
Link to HRB announcement: https://www.hrb.ie/news/news-story/article/hrb-announces-e1-million-investment-for-rare-disease-clinical-trial-network-on-rare-disease-day-2022-1/
Website: rarediseaseresearch.ie
Current Clinical Trials:
TOPAZ is a randomised open-label clinical trial for people with osteogenesis imperfecta (OI). Osteogenesis Imperfecta (OI) is a rare genetic condition present from birth and is frequently called ‘brittle bone disease’. The study aims to investigate whether a treatment with a drug called teriparatide (TPTD) followed by treatment with another drug called Zoledronic acid (ZA) reduces the risk of broken bones occurring in people with OI.
Study design:
Participants will be randomised in a 1:1 ratio to receive treatment with teriparatide (TPTD) for 2 years followed by an infusion of ZA or to receive standard care for the duration of the study. All participants will be followed up for a total duration of between 2 and 5 years.
Inclusion criteria:
- Adult patient aged 18 years and over with a clinical diagnosis of osteogenesis imperfecta.
- Patients willing and able to consent and comply with the study protocol
Type of Study: Phase III/IV
Study Status: Ongoing
Principal Investigator: Prof Rachel Crowley
Contact: [email protected]
IMPALLA – 2 is a randomized, double-blind, placebo-controlled multicentre clinical trial investigating efficacy and safety of inhaled molgramostim (rhGM-CSF) in patients with Autoimmune Pulmonary Alveolar Proteinosis aPAP. Autoimmune PAP is caused by an immune system malfunction due to IgG antibodies that block the granulocyte-macrophage colony stimulating factor (GM-CSF) effect. GM-CSF is a protein that regulates clearance of surfactant (a mix of protein and fat) by alveolar macrophages. The surfactant accumulates in the air sacs of the lungs and eventually lead to an inability to breath. The aim of this trial is to confirm if Molgramostim Nebulizer Solution (inhaled molgramostim) improves function of the lungs in patients with autoimmune pulmonary alveolar proteinosis (aPAP).
The trial will include 2 periods; a double-blind treatment period consisting of up to 8 trial visits and an open-label follow-up period consisting of up to 5 trial visits.
Principal Investigator: Prof Cormac McCarthy
Contact: [email protected]
__________________________________________________________________________________________
For any other query, contact:
Suzanne McCormack
Rare Disease Clinical Trial Network Manager
Email: [email protected]
Tel: 086 8573927
VINE: The Vasculitis Irish Network
VINE is a collaboration between five Irish centres, Vasculitis Ireland Awareness and the National Vasculitis Patient Organisation. The network comprises of dedicated multi-disciplinary centres that provide a coordinated care path for patients with primary systemic small vessel vasculitis (PSV), from diagnosis to relapse and on to long term remission. It provides access to clinical trials for patients with PSV. The five centres maintain close ties and difficult cases are often discussed in a cross centre forum.
To provide a foundation for developing the service in Ireland and to support translational vasculitis research, the VINE network has established the Irish RKD Registry and Biobank which seeks to enrol 900 patients in Ireland with PSV, providing a rich longitudinal clinical database, which is linked to one of the most complete PSV biobanks in the world based in Trinity College Dublin. Further details are provided below.
The network is closely aligned with UKIVAS , the Vasculitis Rare Disease Group of the UK and Ireland and the European Vasculitis Society.
For further information please contact: Prof Mark Little, Trinity Health Kidney Centre, Tallaght Hospital Email: [email protected]
Rare Kidney Disease Registry and Biobank
The RKD registry and Biobank was established in 2012 and aims to address an unmet need in the study of rare kidney disease in Ireland including facilitation of clinical studies when sporadic cases are scattered throughout many hospitals in Ireland. As the principal investigator is prominent in the Systemic Vasculitis research arena, this condition is particularly strongly represented; some 350 cases have been recruited and sampled longitudinally, making this one of the most extensive vasculitis biobanks in the world.
The Registry is an integrated resource across AMNCH Hospital Tallaght, St James’s Hospital, Beaumont Hospital and Trinity College Dublin, with input from multiple other units across the country. It is funded by Science Foundation Ireland, Dublin Centre for Clinical Research, Trinity College MedDay and The Meath Foundation. Our goal in developing such a network is to link a robust patient registry (with capture of detailed longitudinal clinical data across multiple units) to collection of biological samples.
Respiratory and Asthma Research Network
The INCA™ Studies are a suite of clinical investigations. The focus of our research is in developing a novel technology which can be used in clinical practice as an objective assessment of patient adherence to inhaled therapy.
For further information please contact: Elaine Mac Hale, Programme manager: [email protected]
Arthritis Research Coalition
A recent report assessing rheumatology research excellence, measured by the number of citations per article published between 1996 and 2010, ranked Ireland 1st of the 35 richest countries. Major research outputs have included molecular studies on the pathogenesis of common rheumatic diseases, the discovery and validation of prognostic biomarkers, and clinical studies on novel therapies. These have relied on the recruitment of highly characterized patients populations and by the development of linked biorepositories of blood and synovial tissue.
Despite these successes the research activity has been reliant on a fragmented and poorly funded infrastructure. The development of national rheumatology research networks in many European countries including Britain, Sweden and The Netherlands highlights their value. The establishment of Arthritis Ireland chairs, and the development of a Dublin Centre of Excellence, will form the foundation of national rheumatology research strategy, however, the development of a research nurse network will seek to engage all clinicians, co-ordinate all research activities and ensure that Ireland remains on top.
The primary aim is recruit patients with common rheumatic diseases. and obtain biosamples that will underpin clinical research. A secondary aim is to increase national involvement in clinical trials of novel therapeutic agents.
The consortium lead is Prof Gerry Wilson. The leads by area are as follows: Rheumatoid Arthritis – Prof Doug Veale OA/Crystal arthritis – Prof Geraldine McCarthy Spondyloarthropathies – Dr Barry O’Shea Connective Tissue Diseases – Dr Grainne Murphy Paediatrics – Dr Orla Killeen Metabolic Bone- Dr John Carey
For further information: Email: [email protected]
National Surgical Research Support Centre (NSRSC)
The RCSI-NSRSC was set up to establish an all-island Irish Surgical Trial Network to enhance the collaboration and coordination of surgical research and trial activity in Ireland.
Objectives of the Network
Encourage and facilitate surgical trials and research to embed a culture of research in clinical practice.
Establish a trial portfolio to generate high quality evidence to inform policy and set professional standards & guidelines for Irish surgery to improve patient outcomes.
Provide training and education to surgical trainees to produce the next generation of academic leaders in surgery.
Showcase surgical trial activity to establish Ireland as an attractive place to conduct innovative surgical trials.
To provide every patient access to high quality surgical trials and innovative cutting-edge technology across the island of Ireland.
Supports offered
The NSRSC acts as a point of contact, local sponsor and trial coordinating centre for Investigators and international sponsors who would like to open trials here in Ireland. We offer access to a wide network of surgeons, surgical trainees, allied healthcare professionals and scientists participating in surgical research.
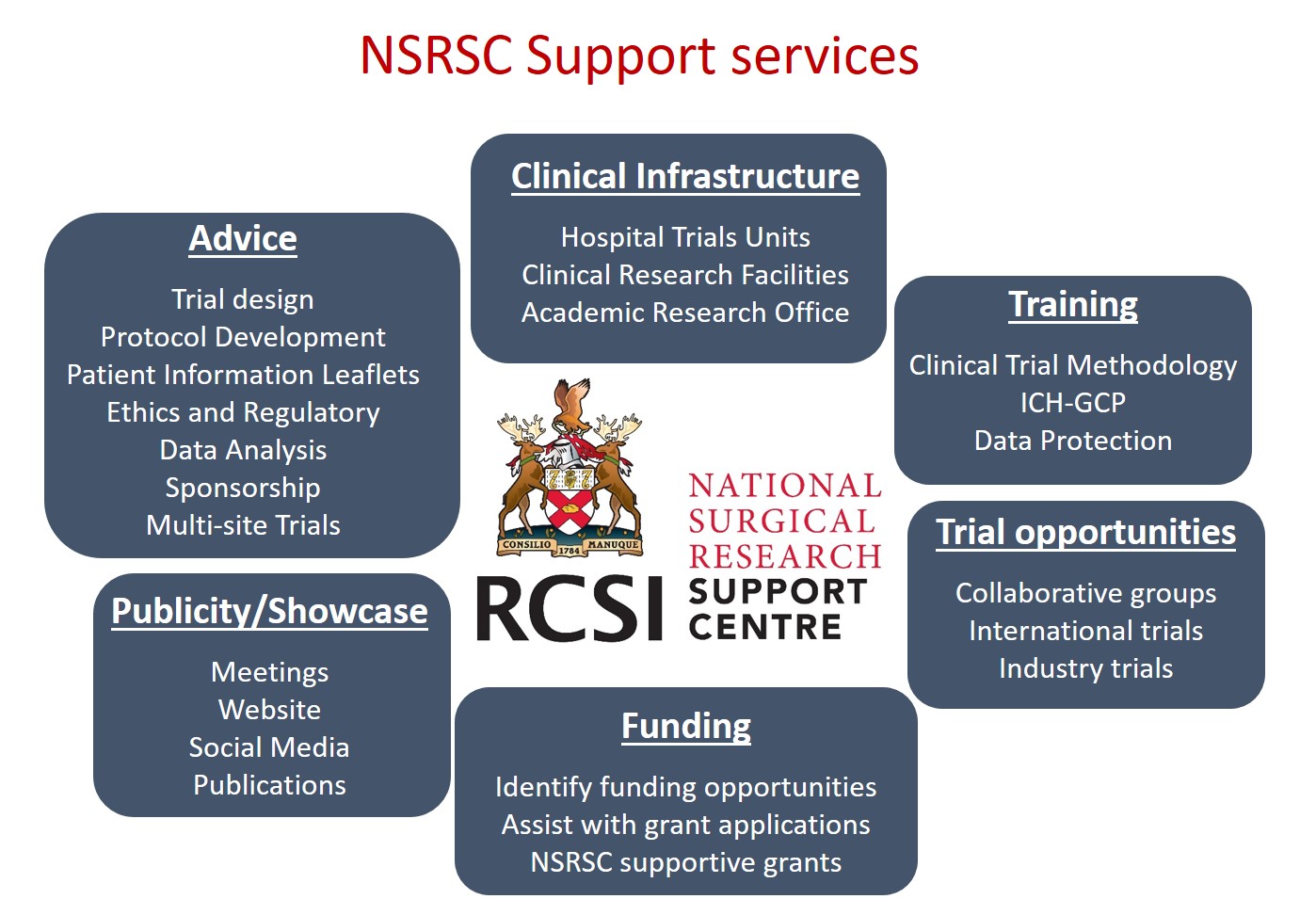
Types of surgical research supported
The centre supports trials across all surgical specialities and thematic areas.
Currently the NSRSC are supporting multi-centre Investigator-led Randomised Controlled trials across a range of areas including diabetic foot disease, appendicitis, bariatric surgery, wound healing devices, varicose vein surgery, cancer and chronic subdural haematoma.
One key area of focus is on infection prevention in patients undergoing a variety of surgical procedures.
The network also supports and coordinates international collaborative surgical trials, pilot/feasibility studies, observational and non-interventional research studies.
Check out the website, Twitter and LinkedIn or contact the NSRSC:
W eb : www.rcsi.com/NSRSC
Twitter : @RCSI_NSRSC
LinkedIn : www.linkedin.com/company/nsrsc
Email: [email protected]

The aim of BCNI is to provide blood cancer patients in Ireland with access to novel and innovative cancer treatments through the provision of early phase clinical trials. BCNI also collect information and samples from blood cancer patients in Ireland in order to improve our understanding and to uncover new ways to combat this disease..
Galway Diabetes Research Centre (GDRC)
GDRC is a joint venture between NUI Galway and Galway and Roscommon University Hospital Group, which bring together an interdisciplinary research consortium of active researchers and clinicians who have a track record in the field of diabetes. The centre is a hub for collaborative effort of researchers and clinicians, with a passion for this work, to provide a greater understanding of how diabetes develops and the underlying mechanisms, the development of new and better therapies for patients, and assessing health care interventions and delivery for patients.
The aim of the HRB Diabetes Collaborative Clinical Trial Network is to improve health and wellbeing for all patients with Diabetes through research and an active portfolio of ambitious multicentre trials on the island of Ireland. Regardless of geographical location or socioeconomic status, all patients will be facilitated to participate in relevant trials through a well-developed network of clinical sites supported by Clinical Research Facilities, Academic Institutions, and the National Clinical Care programme in Diabetes. This ambitious aim is supported by the individual network members who have considerable experience in investigator and industry led clinical trials. They can demonstrate individual success with national and international grant funding and the influence of their research on policy and guidelines. They have a broad international collaboration base with distinguished world experts in their fields. The network is building further on national investment in clinical research facilities (CRFs)/networks, bringing together academics, clinicians, health professionals, patients, industry, charities, and health care delivery networks. The network aims to collaborate fully with the existing CTNs. The network will have strong PPI input and will be overseen by the National HRB PPI hub, and trial designs will be robustly debated with the HRB TMRN and supported by core outcome sets (COSs) and Evidence Synthesis Ireland.
The network core working group will focus on development of (a) a research strategy (b) a governance structure (c) plan for interaction with industry (d) establishing a patient groups and public PPI group (d) sustainability plan with a focus on national and international grant applications, in addition to industry and SME fiscal and in-kind contributions. While they network members continue to put patients and patient improvement at the heart of diabetic clinical research.
Currently the network is in start-up phase, establishing sustainable governance structures, developing a consistent logo and brand identity, creating a website for network members, public and patient involvement and developing a research strategy. They can be contacted by emailing [email protected] .
Investigator Network for Inflammatory Bowel Disease Therapy in Ireland (INITIative)
INITIative is the first collaborative research network for Inflammatory Bowel Disease in Ireland. The network is open to clinical and scientific investigators with an interest in Crohn’s Disease and Ulcerative Colitis throughout the island of Ireland. Goals of the Network include:
- To foster collaboration and encourage multi-centre investigator initiated studies in Crohn’s Disease and Ulcerative Colitis in Ireland
- To attract and facilitate industry sponsored clinical trials in Crohn’s Disease and Ulcerative Colitis in Ireland in order to improve the access for patients in Ireland to investigational drugs and therapies
- To facilitate participation by Irish IBD investigators in European research consortia and networks
- To attract funding for and investment in research infrastructure to support research in Crohn’s Disease and Ulcerative Colitis in Ireland
- To foster all Ireland, European and Global collaboration in IBD research and improve the profile of IBD related research taking place in Ireland Current INITIAtive projects include several investigator initiated studies: GOAL-ARC and HARP-X. GOAL-ARC is a nationwide multi-centred investigator initiated randomized control trial to evaluate the use of personalized golimumab (GLM) dose adjustment in UC. The primary objective is to ascertain if dose adjustment of GLM, based on GLM drug levels and FCP levels, results in higher response and remission rates than standard dosing.
HARP-X is a pilot study that will determine the effect of tofacitinib maintenance therapy on endoscopic response in subjects with active, chronic, antibiotic dependent or refractory pouchitis.
INITIative continues to participate in numerous observational studies, including I-CARE, the first European prospective cohort study that will provide unique information on the long-term use of recommended therapy in IBD.
A key priority of the network is to expand research into non-pharmacologic interventions, including psychological and dietary interventions, nursing interventions, and methods to address patient quality of life. There are currently several studies in the pipeline looking at these important aspects of IBD. In addition, the network’s research priorities are targeted for understudied patient groups, including patients with stomas, proctitis and pouchitis that are generally excluded from industry sponsored studies.
For further information please contact:
Glen Doherty MB BCh PhD FRCPI FEBGH Senior Clinical Lecturer, University College Dublin and Consultant Gastroenterologist and Physician, Centre for Colorectal Disease, St. Vincent’s University Hospital
Annie Coe, BSN, MScResearch Nurse & INITIative Coordinator Centre for Colorectal Disease, St. Vincent’s University Hospital Email: [email protected]
For all queries please contact: Prof. Donal J. Buggy, Network Manager & Professor of Anaesthesiology & Periop erative Medicine, UCD. Email: [email protected]
- To enhance patient and public involvement (PPI) in primary care research in Ireland, by continuing to grow the capacity of the Primary Care CTNI PPI group, including PPI in network oversight and portfolio development, and continuing our synergistic relationship with the national PPI network. For more information, contact the Primary Care CTNI Network Manager, Dr Patrick Murphy, by emailing [email protected] or [email protected]
How we can help
News & Events
How can we help

Privacy Policy
Designed & Developed By Digigrow
Privacy Overview

Health Research, Inc. is an independent 501(c)(3) not for profit corporation governed by a Board of Directors which will consist of not less than five (5) and not more than thirty five (35) members, all of which are voting members. The NYS Commissioner of Health serves as the President of the HRI Board. Directors of the Corporation are elected by a majority vote of the Directors. The term for which a Director shall be elected shall be one (1) year.
The current Board consists of:
Andrew T. Ruby Board Secretary/Treasurer Director, Fiscal Management Group NYS Department of Health
Michael B. Sexton, Esq. Chief Operations Officer and General Counsel Roswell Park Comprehensive Cancer Center
Candace Johnson President and CEO Roswell Park Comprehensive Cancer Center
Tim Reynolds Director of Accounting and Auditing Marvin and Company, P.C.
Justin P. Runke, Partner Vice President and General Counsel Cayuga Health System
Mary Applegate Medical Director Howard Interventions
Michael Nazarko Retired New York State Department of Health
READ THIS AGREEMENT CAREFULLY BEFORE YOU CLICK THE “I AGREE TO THE LICENSE TERMS” BUTTON. BY CLICKING ON THE “I AGREE TO THE LICENSE TERMS” BUTTON, THE PERSON ACCEPTING THIS AGREEMENT ACKNOWLEDGES THAT (1) HE OR SHE IS AUTHORIZED TO ENTER INTO THIS AGREEMENT FOR AND ON BEHALF OF YOU, AND IS DOING SO, AND (2) HE OR SHE HAS READ, UNDERSTANDS AND AGREES THAT YOU SHALL BE BOUND BY THESE TERMS AND CONDITIONS AND ALL MODIFICATIONS AND ADDITIONS PROVIDED FOR. IF YOU DO NOT AGREE WITH THESE TERMS AND CONDITIONS, CLICK ON THE “RETURN” BUTTON AND INSTALLATION WILL TERMINATE. IF YOU ARE NOT AUTHORIZED TO ENTER INTO AND BIND YOUR INSTITUTION TO THIS AGREEMENT, CLICK ON THE “I AM NOT THE AUTHORIZED SIGNATORY” BUTTON.
I AGREE TO THE LICENSE TERMS (Click to agree and on following screen click Code->Download Zip to download 13MB File)
I DO NOT AGREE TO THE LICENSE TERMS (you must agree to the licensing terms to access the Sfold Download)
Health Research Board (HRB)
- Reference work entry
- First Online: 01 January 2018
- Cite this reference work entry

220 Accesses
Research & Development for Health, 73 Lower Baggot Street, Dublin, 2, Ireland Tel: (353) 1 234 5000 Fax: (353) 1 661 2335 Email: [email protected] Website: www.hrb.ie Contact: The Research Grants Manager
You have full access to this open access chapter, Download reference work entry PDF
The Health Research Board (HRB) comprises 16 members appointed by the Minister of Health, with eight of the members being nominated on the co-joint nomination of the universities and colleges. The main functions of the HRB are to promote or commission health research, to promote and conduct epidemiological research as may be appropriate at national level, to promote or commission health services research, to liaise and co-operate with other research bodies in Ireland and overseas in the promotion of relevant research and to undertake such other cognate functions as the Minister may from time to time determine.
All-Ireland Institute for Hospice and Palliative Care Fellowships
Subjects: Clinical, epidemiological, public health, statistics, health economics, social science, operational and management disciplines.
Purpose: To enable graduates with some appropriate relevant experience to pursue a career in health devices and research in Ireland.
Eligibility: Candidates must normally hold a primary degree in a discipline relevant to health services research, have acquired appropriate postgraduate experience in the field of health services and research, have support from an approved academic department or centre, have obtained the prior approval of a head of department for the research study being proposed and be Irish citizens or graduates from overseas with a permanent Irish resident status.
Level of Study: Postgraduate
Type: Fellowship
Value: Please consult the organization salary on a postdoctorate scale up to €7,500 per year for consumables
Length of Study: The maximum period of the award will be 3 years
Frequency: Annual
Study Establishment: Institutions approved by the Board, such as teaching hospitals, universities, research institutes and health boards in Ireland
Country of Study: Ireland
No. of awards offered: Varies
Application Procedure: Applicants must complete an online application form, available from the website.
Closing Date: September 30th
Funding: Government
No. of awards given last year: 4
No. of applicants last year: 20

Clinical Research Training Fellowship in Nursing and Midwifery
Subjects: Nursing and midwifery.
Purpose: To provide experienced nurses and midwives with an opportunity to carry out research in clinical nursing or midwifery, leading to a postgraduate degree at the Master’s or doctoral level. These fellowships will provide nurses with the research experience necessary to develop their expertise as specialists in their chosen field of nursing or midwifery.
Eligibility: To be eligible for a fellowship a candidate must be registered as a nurse or midwife; have practised professional nursing or midwifery for at least 5 years; hold a post in nursing or midwifery practice or a post related to nursing or midwifery; have been employed in the Irish health services or an Irish academic Department of Nursing and/or Midwifery, within 2 years prior to the closing date for application to the Fellowship; confirm support approval from Head of Department in which the research study is being carried out; and provide evidence of academic supervision from a suitably qualified nurse or a midwife.
Level of Study: Graduate, Predoctorate
Value: Salary on postdoctoral scale and consumables of €7,500 per year
Length of Study: Up to 3 years
Study Establishment: Fellowships are tenable by nurses or midwives employed in a recognized health service or an Irish academic Department of Nursing and/or Midwifery and registered with an academic Department of Nursing and/or Midwifery or other relevant academic department
Application Procedure: Applicants can obtain an application form from our website.
Closing Date: December 14th
Clinician Scientist Award for Clinical Health Professionals
Subjects: Word class research clinical and translational research with a strong relevance to human health.
Purpose: To release outstanding medically or professionally qualified researchers in the health professions from some or all of their service commitment to conduct.
Eligibility: Medical consultants in the Irish health system or senior clinicians in health related disciplines who are qualified to hold a post in the Irish health service.
Level of Study: Research
Type: Award
Value: Research costs up to €500,000 for a maximum of 5 years plus supplemental funding up to €300,000 for the first 3 years (in certain cases) and pro-rata salary cost of the applicant
Length of Study: 5 years
Study Establishment: Any Irish teaching hospital or academic institution
No. of awards offered: 2–3
Application Procedure: Online application form available from website. Full applications from invited applicants only.
Closing Date: September 29th
No. of awards given last year: 1
No. of applicants last year: 5
HRB Postdoctoral Research Fellowships
Subjects: Researchers who hold a PhD and want to develop their career, as an advanced level in a health related discipline.
Purpose: Career development in health related disciplines.
Eligibility: Applicants must be postdoctorates with less than 5 years of postdoctoral experience.
Level of Study: Postdoctorate
Value: The fellowship will provide funding for salary (based on the Irish University Association salary scale for postdoctoral researchers) and salary-related costs for up to 3 years in addition to running costs, a training and development allowance, a dissemination allowance and a travel grant. Payment of the fellowship will be made through a host institution on the island of Ireland and a contract of employment should be issued by the host institution to the fellow
Study Establishment: A university, research hospital or institute
Closing Date: April
No. of awards given last year: 8
No. of applicants last year: 50
HRB Translational Research Awards
Purpose: The purpose of these awards were to enable researchers to establish and support teams working full time on extensive or long-term research programmes that have a clear link to patient care.
Eligibility: Open to candidates who hold a post in an established academic research centre, have an outstanding track record and have at least 5 years of research experience.
Value: €1,500,000
Study Establishment: Any Irish academic or research institution
Application Procedure: Online application form available from the HRB website.
Closing Date: March 26th
No. of applicants last year: 12
Additional Information: Check website for more details.
Research Leaders Awards
Eligibility: All applications must involve a partnership with at least one health-related partner organization involved in the delivery of health and social care and/or health and social care policy. Applications should be aligned with the strategic plans of the nominating organizations, and should reflect national priorities and strategies in health and social care.
Level of Study: Doctorate, Postdoctorate
Value: Each Research Leader Award consists of a contribution to salary support for the nominee up to the grade of Associate Professor, an attractive discretionary research support package of up to €600,000 over the period of the award, and a contribution to overhead expenses
Length of Study: A maximum of 5 years
Application Procedure: All applications must be made online using the HRB GEMS. (A link to GEMS is below.) To access the application form the nominating Higher Education Institution must provide the HRB with the contact details of their nominated Principal Investigator. The HRB will then invite the nominated candidate to initiate the application form.
Closing Date: March 31st
Additional Information: Please check at www.hrb.ie/research-strategy-funding/grants-and-fellowships/hrb-grants-and-fellowships/grant/133 for more information.
Research Training Fellowships for Healthcare Professionals 2016
Subjects: Biomedicine.
Purpose: To support medical and dental graduates with appropriate experience who are interested in gaining specialized clinical research training in a biomedical field in Ireland, leading to a PhD.
Eligibility: Candidates should be graduates in medicine or dentistry up to and including senior registrar or equivalent academic level. Applicants must be registered or have completed their higher specialized training.
Level of Study: Doctorate
Value: Part-time funding towards salary and related costs, student fees, running costs, dissemination and knowledge exchange costs, training costs and a travel grant to gain research experience abroad
Study Establishment: At an appropriate academic department in the Republic of Ireland or Northern Ireland
Application Procedure: Applicants must apply with the support of the head of an appropriate sponsoring laboratory in the Republic of Ireland. Candidates may apply to remain in their current laboratory, to return to one where they have worked before or to move to a new laboratory. Applicants have to fill in on online form available on the website.
Closing Date: November 5th
No. of applicants last year: 42
Additional Information: Proposals may be submitted for specialized research training or for training in a basic subject relevant to a particular clinical interest.
Editor information
Copyright information.
© 2018 Macmillan Publishers Ltd.
About this entry
Cite this entry.
(2018). Health Research Board (HRB). In: The Grants Register 2018. Palgrave Macmillan, London. https://doi.org/10.1007/978-1-349-94186-5_548
Download citation
DOI : https://doi.org/10.1007/978-1-349-94186-5_548
Published : 10 January 2018
Publisher Name : Palgrave Macmillan, London
Print ISBN : 978-1-137-59209-5
Online ISBN : 978-1-349-94186-5
eBook Packages : Education Reference Module Humanities and Social Sciences Reference Module Education
Share this entry
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Publish with us
Policies and ethics
- Find a journal
- Track your research

- Our approach
Scott Young, MD
Co-Executive Director and Senior Medical Director of CMI
Scott Young, MD, serves as associate executive director for Clinical Care and Innovation at The Permanente Federation. He also serves as the Executive Director and Senior Medical Director of Kaiser Permanente’s Care Management Institute. He leads a nationwide team that is integral to Kaiser Permanente’s commitment to improve the care and wellness of its 9.3 million members. His work includes commissioning the discovery, development and spread of programs and best practices focused on care delivery, education and member experience.
Dr. Young is former director for Health IT at the Agency for Healthcare Research and Quality. Prior to joining that agency, he served as a senior clinical advisor at the Centers for Medicare and Medicaid Services. Dr. Young’s policy experience also includes service as a Robert Wood Johnson health policy fellow in the office of U.S. Sen. Jeff Bingaman of New Mexico.
He is former executive vice president of the Utah HealthCare Institute, a not-for-profit organization providing clinical care, outreach programs, medical education, research, informatics and health policy services. Dr. Young is a founding member of Intermountain Health Care’s Utah Valley Family Practice Residency.
Dr. Young received his medical degree from the University of Oklahoma in 1987 and completed his training at the Fairfax Family Practice Residency. He is board certified in family medicine and a fellow of the American Academy of Family Physicians.
Joan Gelrud, RN, MSN, CPHQ, FACHE
Senior Vice President of National Health Plan and Hospital Quality and the Co-executive Director of CMI
Joan Gelrud leads work to advance Kaiser Permanente’s nation-leading excellence in quality, safety and experience. She oversees Care Experience, Risk and Patient Safety, the Design Consultancy, Quality Governance, Accreditation, Licensing and Regulation, and the Performance Institute. As the Co-Executive Director of the Care Management Institute, Joan, with her partner Scott Young, MD oversee the award-winning work of the interdisciplinary CMI team for Kaiser Permanente.
Joan joined Kaiser Permanente in September of 2015. In her position as Vice President of Quality, Regulatory and Risk Management for Mid-Atlantic States, Joan provided strategic leadership for Kaiser Permanente to compete as a quality leader in health care. She had responsibility for the quality and safety of care, treatment and services provided to members, including outcomes and accreditation, licensing, clinical risk management, peer review and credentialing. Joan was responsible for leveraging best practices across the region and served as the Chief Nurse Executive. Joan was also responsible for Infection Prevention, Employee Health, Regional Patient Care Services, Clinical Staff Education, Outreach to members and communities via mobile van services. Under the safety umbrella, Joan was responsible for patient and workforce safety across the region. She completed Kaiser Permanente’s Executive Leadership Program at Harvard University while in Mid-Atlantic States.
Prior to joining Kaiser Permanente, Joan was Vice President at MedStar St. Mary’s Hospital in Leonardtown, Maryland where responsibilities included Quality, Safety, Data and Analytics, Clinical Resource Management, Stroke Center, Organizational Learning and Research, Community Health Outreach, Human Resources, Imaging, Risk Management, Cardiology, Neurology, Respiratory Therapy, Rehabilitation Services, Patient Advocates, Pharmacy and Laboratory. Some of Joan’s operational accomplishments included opening practices to improve access to pediatric neurology, cardiology and pulmonary outpatient services, working with Children’s National Medical Center and MedStar Georgetown University Hospital. She expanded local interventional radiology and tele-neurology services with the MedStar Washington Hospital Center and MedStar Georgetown University Hospital. Her team achieved a Health Enterprise Zone designation in St. Mary’s County and a competitive state grant to improve access to healthcare, reduce readmissions and address social barriers to health.
Jim Bellows, PhD
Managing Director
Jim Bellows leads CMI’s research and development work, which includes supporting care delivery innovation projects, identifying specific population care practices that contribute to superior performance, and supporting and studying the spread of the most promising practices.
He is also responsible for CMI’s efforts in demonstrating the value of population care and consults with a variety of Program Office leaders and departments on areas related to program evaluation and performance measurement.
Dr. Bellows has been a lecturer at the University of California Berkeley School of Public Health, where he taught Health Care Quality – Measurement and Improvement from 2004 to 2007. He earned his PhD in health services research and health economics from the University of California Berkeley and joined CMI in July 2001 after several earlier careers. He received a master’s degree in public health from University of California Berkeley and a bachelor’s degree from the University of California Santa Cruz.
Maria A. Butler
Director of Business Operations
Maria Butler is the Director of Business Operations at Kaiser Permanente’s Care Management Institute. Maria joined CMI in 2006 and oversees the business function of the department; managing finance activities, human resources and administrative operations that support the department’s ability to achieve its business objectives. This management function works closely with the CMI Executive Directors, leadership, managers, and staff to ensure the smooth running of the organization by carrying out the department’s plan, anticipating risk, and solving problems creatively and effectively. Maria has a BA degree from San Francisco State University and over twenty years of experience in finance and administration having worked at APL Global Logistics, Crowley Maritime Corporation, Peet’s Coffee & Tea, and Saatchi & Saatchi Corporate Communications.
Tracy Cameron
Senior Director
Tracy Cameron leads the Care Management Institute’s work in Care Experience. In this role, she partners with regional and national leaders to improve access to care and provide our members with an excellent care experience across settings. She previously served as Director of Performance Assessment for The Permanente Federation and Director of Medical Economics in Kaiser Permanente’s Northern California region.
Prior to joining Kaiser Permanente in 1987, Tracy was a research scientist at Pacific Presbyterian Medical Center and the University of California at Berkeley, where she worked in public health research.
Tracy holds a bachelor’s degree in Sociology from Purdue University, as well as a master’s degree in Sociology and an MBA from the University of California, Berkeley.
Carol Cain, PhD
Senior Director, Clinical Information Services
Carol Cain, PhD, directs the Care Management Institute’s Clinical Information Services, including Kaiser Permanente’s Clinical Library, guideline and evidence services, and care delivery informatics. She co-leads the Permanente Federation’s Clinical Education portfolio, including national Continuing Medical Education activities and conferences, and quality improvement for Maintenance of Certification. She also coordinates the Care Management Institute’s partner relations with organizations outside Kaiser Permanente.
Carol has worked on care delivery improvement in several clinical pathways, including the transition from hospital to home, reducing hospital infections, managing chronic pain, and others, with the goal of expanding physician capacity in guiding clinical improvement projects, sharing pathways across Kaiser Permanente, and looking at ongoing reliability and quality in those pathways. She has also worked on design of the transition from hospital to home from a patient and caregiver perspective; spread of successful innovations; and care delivery implications of technology. She has a background in clinical informatics from Stanford and is currently teaching a biomedical informatics graduate course on designing IT innovations. She previously managed the health IT portfolio at AHRQ, the Agency for Healthcare Research and Quality.
Jeff Convissar, MD
Medical Director
Jeff Convissar, MD is the Medical Director at Kaiser Permanente’s Care Management Institute. He has been with Kaiser Permanente since 1992. Jeff was previously the Executive Director of National Risk Management. Prior to that, for 12 years he was an attending physician in the Emergency Department at the Kaiser Permanente Santa Clara Medical Center in Northern California. At Santa Clara, Jeff also served as the Assistant Chief of Quality. Jeff has a BA in Biology from Brown University, his MD is from the UCLA School of Medicine and ED residency training was completed at Harbor-UCLA Medical Center. Jeff is responsible for a variety of improvement initiatives at Kaiser Permanente.
Rivka Gordon, PA, MHS
Chief of Staff
Rivka Gordon is Chief of Staff to the Associate Executive Director of the Care Management Institute and is a member of the leadership team. Rivka is a Primary Care Physician Assistant with a Masters degree in Health Sciences from Duke University. In addition to her work at Kaiser Permanente, Rivka serves on Planned Parenthood Federation of America’s National Medical Committee and on the San Francisco Women’s Community Clinic and Tara Health Foundation boards of directors.
Craig W Robbins, MD, MPH
Medical Director, Clinical Information Services
Dr. Craig Robbins serves as Medical Director of the Center for Clinical Information Services in the KP Care Management Institute. He also serves as Medical Director for the KP Maintenance of Certification Portfolio at The Permanente Federation. He has been a family physician with the Colorado Permanente Medical Group since July 1998. In his CMI role, Dr. Robbins is the lead physician for the KP National Guidelines Program which has been an organizational member of the Guidelines International Network (G-I-N) since 2010. He is a current member of the G-I-N Board of Trustees, the Executive Advisory Board for the Kaiser Permanente Research Associates Evidence-based Practice Center (KPRA-EPC), and the Editorial Board for the National Guideline and Quality Metric Clearinghouses (NGC/NQMC). Dr. Robbins received his BS (1990) and MD (1993) from the University of Michigan and his MPH (1998) from the University of Pittsburgh. He completed his Family Medicine Residency (1993-6) at the University of Virginia and a Faculty Development Fellowship (1996-8) at the University of Pittsburgh Medical Center-St Margaret Hospital in Pittsburgh, PA.
Lisa Schilling, RN, MPH
Vice President
Lisa Schilling, RN, MPH, is national Vice President, Healthcare Performance Improvement and Director of Kaiser Permanente’s Center for Health System Performance. She leads the strategy to develop and implement a performance improvement system and planning to adopt its total health strategy in care delivery.
A leader with more than 20 years’ experience, Lisa serves on the editorial board of the Joint Commission Journal for Quality and Safety and has authored several publications on related topics.
Prior to Kaiser Permanente, she was the national director of critical care services at VHA Inc., focusing on improving delivery system performance.
Matthew C. Stiefel
Matt Stiefel directs Population Health in Kaiser Permanente’s Care Management Institute. He was a 2008-09 fellow with the Institute for Healthcare Improvement, and continues as a faculty member for the IHI Triple Aim. Matt joined Kaiser Permanente in 1981 as a medical economist, and later held management positions in Kaiser Permanente Northwest, directing planning, marketing, and medical economics. He joined the Care Management Institute in 1998. Prior to Kaiser Permanente, he served as a policy analyst on the Carter Administration Domestic Policy Staff and in the US Department of Health, Education and Welfare, and as a local health planner in the San Francisco bay area. He completed an MS in epidemiology from the Harvard School of Public Health in 2013, holds an MPA from the Wharton School, and a BA in psychology from Stanford.
Winston Wong, MD
Dr. Wong serves as Medical Director, Community Benefit, National Program Office, and is responsible for developing partnerships with communities and organizations in advancing population management and evidence based medicine, with a particular focus on health disparities and vulnerable populations. Previous to Kaiser Permanente, Dr. Wong served as a Captain in the U.S. Public Health Service and was awarded an Outstanding Service Medal. In addition to playing various advisory roles with CMMS, NIH and the National Academy of Medicine, and most recently to DHHS as an advisor on minority health, Wong currently serves on Boards representing public hospitals and school based health centers addressing issues of access and quality for diverse populations. He is also a Board member of the California Endowment, one of the nation’s largest health philanthropies. Bilingual in Cantonese and Toisan dialects, Dr. Wong continues a small practice in Family Medicine at Asian Health Services in Oakland, a federally qualified health center, where he previously served as Medical Director. Dr. Wong was featured as a “Face of Public Health” in the American Journal of Public Health. Dr. Wong received his undergraduate and Master’s degrees at UC Berkeley, his medical degree from UC San Francisco, and an Honorary Doctorate in Humane Letters from the A.T. Still School of Osteopathy.
- Terms & Conditions
- Privacy Practices
- Site policies
- Quick Facts
- Evaluation/Analytics
- Evidence Services
- Patient-Centered Improvement
- Breastfeeding Support
- Cardiovascular Health
- Spreading Effective Practices
- Publications
- See us on facebook
- See us on twitter
- See us on youtube
- See us on linkedin
- See us on instagram
Trust, human-centered AI and collaboration the focus of inaugural RAISE Health symposium
Artificial intelligence experts discuss how to integrate trustworthy AI into health care, why multi-disciplinary collaboration is crucial and the potential for generative AI in research.
May 17, 2024 - By Hanae Armitage

Fei-Fei Li and Lloyd Minor give opening remarks at Stanford Medicine's first RAISE Health Symposium on May 14. Steve Fisch
Most people captured by artificial intelligence have all had something of an “aha” moment that opens their minds to a world of opportunities. During the inaugural RAISE Health symposium on May 14, Lloyd Minor , MD, dean of the Stanford School of Medicine and vice president for medical affairs at Stanford University, shared his.
Asked to summarize a discovery he’d made related to the inner ear, a curious Minor turned to generative AI. “I asked, ‘What is superior canal dehiscence syndrome?’” Minor told a group of nearly 4,000 symposium attendees. In seconds, a few paragraphs appeared.
“They were good — really good,” he said. “The information was brought together into a concise and, by and large, accurate and well-prioritized description of the disorder. It was quite remarkable.”
Minor’s excitement was shared by many at the half-day event, which was born of the RAISE Health initiative, a project launched by Stanford Medicine and the Stanford Institute for Human-Centered Artificial Intelligence (HAI) to guide the responsible use of AI in biomedical research, education and patient care. Speakers explored what it means to bring AI into the folds of medicine in a way that’s not just helpful for physicians and scientists, but transparent, fair and equitable for patients.
“We believe this is a technology to augment and enhance humanity,” said Fei-Fei Li , a professor of computer science at the Stanford School of Engineering who leads RAISE Health with Minor and is the co-director of HAI. From generating new molecular sequences that could give rise to new antibiotics, to mapping biodiversity, to uncovering hidden bits of basic biology, AI is accelerating scientific discovery, she said. But it’s not all beneficial. “All of these applications can have unintended consequences, and we need computer scientists to work with multiple stakeholders — from doctors and ethicists…to security experts and more — to develop and deploy [AI] responsibly,” she said. “Initiatives like RAISE Health show that we’re committed to this.”
The alignment of Stanford Medicine’s three entities — the School of Medicine, Stanford Health Care and Stanford Medicine Children’s Health — and its connection to the rest of Stanford University puts it in a unique position as experts grapple with AI development, governance and integration in health and medicine, Minor said.
“We’re ideally suited to be a pioneer in advancing and deploying AI in responsible ways, covering the gamut from fundamental biological discovery, enhancing drug development, making clinical trial processes more efficient, all the way through the actual delivery of health care and the way we run our health care delivery system,” he said.
What ethical integration looks like
Some speakers underscored a simple concept: Focus on the user — in this case, the patient or the physician — and all else will follow. “It’s putting patients at the center of everything that we do,” said Lisa Lehmann, MD, PhD, director of bioethics at Brigham and Women’s Hospital. “We need to be thinking about their needs and priorities.”

From left: Moderator Mohana Ravindranath of STAT News; Jessica Mega; Peter Lee of Microsoft Research; and Sylvia Plevritis, professor of biomedical data science, discuss the role of AI in medical research. Steve Fisch
Speakers on one panel — which included Lehmann, Stanford Medicine bioethicist Mildred Cho , PhD, and Michael Howell, MD, chief clinical officer at Google — pointed to the complex nature of a hospital system, highlighting the need to understand the purpose of any intervention before implementing it and to ensure that all systems developed are inclusive, with input from the populations it’s meant to help.
One key to that is transparency — being explicit about where the data used to train the algorithm came from, what the algorithm was originally intended for and whether future patient data will continue to help the algorithm learn, among other factors.
“Trying to predict ethical problems before they become consequential [means] finding a perfect sweet spot of knowing enough about the technology that you can make some ascertainment of it, but getting to it before [an issue] spreads further,” said Danton Char , MD, associate professor of pediatric anesthesiology, perioperative and pain medicine. One of the key steps, he said, is to identify all the stakeholders who could be impacted by a technology and take note of how they would want those questions answered for themselves.
Jesse Ehrenfeld, MD, president of the American Medical Association, discussed four drivers of adoption for any digital health tool, including those powered by AI. Does it work? Does it work in my institution? Who pays for it? Who is liable?
Michael Pfeffer , MD, chief information officer for Stanford Health Care, highlighted a recent example in which many of those questions were tested with care providers at Stanford Hospital. Clinicians were offered assistance from a large language model that drafts initial notes to patient inbox messages. While the drafts weren’t perfect, the clinicians who helped develop the technology reported that the model lightened their workload.
“There are three big things that we’ve been focusing on: safety, efficacy and inclusion. We’re physicians. We take this oath to ‘do no harm,’” said Nina Vasan , MD, clinical assistant professor of psychiatry and behavioral sciences, who joined a panel with Char and Pfeffer. “That needs to be the first way that we’re assessing any of these tools.”
Nigam Shah , MBBS, PhD, professor of medicine and of biomedical data sciences, kicked off a discussion with a jarring statistic, although he gave the audience fair warning. “I speak in bullet points and numbers, and sometimes they tend to be very direct,” he said.
To Shah, the success of AI hinges on our ability to scale it. “Doing the science right for one model takes about 10 years, and if every one of the 123 fellowship and residency programs wanted to test and deploy one model at that level of rigor, with our current ways of organizing work and [testing] it at every one of our sites to make sure it works properly, it would be $138 billion,” Shah said. “We can’t afford it. So, we have to find a way to scale, and we have to scale doing good science. The skills for rigor reside in one place, and the skills for scale reside in another, and hence, we’re going to need these kinds of partnerships.”

Associate dean Euan Ashley and Mildred Cho (at front table) attend the RAISE Health Symposium. Steve Fisch
The way to get there, according to a number of speakers at the symposium, is public-private partnership, such as that being modeled through the recent White House Executive Order on the Safe, Secure, and Trustworthy Development and Use of Artificial Intelligence and the Coalition for Health AI , or CHAI.
“The public-private partnerships [with] the most potential are [between] academia, the private sector and the public sector,” said Laura Adams, a senior advisor at the National Academy of Medicine. The government can bring public credibility, academic medical centers can bring legitimacy, and the technical expertise and compute time can come from the private sector, she noted. “All of us are better than any one of us, and we’re recognizing…that we don’t have a prayer of reaching the potential of [AI] unless we understand how to interact with each other.”
Innovating in AI, filling gaps
AI is also making an impact in research, whether scientists are using it to probe the dogma of biology, predict new synthetic molecular sequences and structures to underpin emerging therapeutics, or even to help them summarize or write scientific papers, several speakers said.
“There’s an opportunity to see the unknown,” said Jessica Mega , MD, a cardiologist at Stanford Medicine and co-founder of Alphabet’s Verily. Mega pointed to hyperspectral imaging, which captures features of an image that are invisible to the human eye. The idea is to use AI to detect patterns, for example, in pathology slides, unseen by humans that are indicative of disease. “I encourage people to push for the unknown. I think everyone here knows someone who is suffering from a health condition that needs something beyond what we can offer today,” Mega said.
There was also a consensus among panelists that AI systems will provide new means of identifying and combating biased decision making, whether it’s made by humans or AI, and opportunities to figure out where that bias is coming from.
“Health is more than health care,” was a statement echoed by multiple panelists. The speakers stressed that researchers often overlook social determinants of health — such as socioeconomic status, ZIP codes, education level, and race and ethnicity — when they are collecting inclusive data and enrolling participants for studies. “AI is only going to be as good as the data that the models are trained on,” said Michelle Williams , ScD, a professor of epidemiology at Harvard University and a visiting professor of epidemiology and population health at Stanford Medicine. “If we are looking for improving health [and] decreasing disparities, we’re going to have to make sure that we are collecting high-quality data on human behaviors, as well as the social and physical environment.”
Natalie Pageler , MD, clinical professor of pediatrics and of medicine, shared that cancer data aggregates often exclude data from pregnant people, creating inherent biases in models and exacerbating an existing gap in health care.
As with any emerging technology, there are ways that AI can make things better and ways it can make things worse, said David Magnus , PhD, professor of pediatrics and of medicine. The risk, Magnus said, is that AI systems learn about inequitable health outcomes driven by social determinants of health and reinforce them through their outputs. “AI is a mirror that reflects the society that we’re in,” he said. “I’m hopeful that every time we get an opportunity to shine a light on a problem — hold up that mirror to ourselves — it will be a spur for things to get better.”
If you weren’t able to attend the RAISE Health symposium, recordings of the sessions can be found here .

About Stanford Medicine
Stanford Medicine is an integrated academic health system comprising the Stanford School of Medicine and adult and pediatric health care delivery systems. Together, they harness the full potential of biomedicine through collaborative research, education and clinical care for patients. For more information, please visit med.stanford.edu .
Hope amid crisis
Psychiatry’s new frontiers

- U.S. Department of Health & Human Services

- Virtual Tour
- Staff Directory
- En Español
You are here
News releases.
News Release
Friday, May 24, 2024
Scientists map networks regulating gene function in the human brain
NIH-funded research details the brain’s cellular and molecular regulatory elements and their impact on brain function.
A consortium of researchers has produced the largest and most advanced multidimensional maps of gene regulation networks in the brains of people with and without mental disorders. These maps detail the many regulatory elements that coordinate the brain’s biological pathways and cellular functions. The research, supported the National Institutes of Health (NIH), used postmortem brain tissue from over 2,500 donors to map gene regulation networks across different stages of brain development and multiple brain-related disorders.
“These groundbreaking findings advance our understanding of where, how, and when genetic risk contributes to mental disorders such as schizophrenia, post-traumatic stress disorder, and depression,” said Joshua A. Gordon, M.D., Ph.D., director of NIH’s National Institute of Mental Health (NIMH). “Moreover, the critical resources, shared freely, will help researchers pinpoint genetic variants that are likely to play a causal role in mental illnesses and identify potential molecular targets for new therapeutics.”
The research is published across 15 papers in Science, Science Advances, and Scientific Reports. The papers report findings along several key themes:
- Population-level analyses that link genetic variants, regulatory elements, and different molecular forms of expressed genes to regulatory networks at the cellular level, in both the developing brain and adult brain
- Single-cell-level maps of the prefrontal cortex from individuals diagnosed with mental disorders and neurodevelopmental disorders
- Experimental analyses validating the function of regulatory elements and genetic variants associated with quantitative trait loci (segments of DNA that are linked with observable traits)
The analyses expand on previous findings, exploring multiple cortical and subcortical regions of the human brain. These brain areas play key roles in a range of essential processes, including decision-making, memory, learning, emotion, reward processing, and motor control.
Approximately 2% of the human genome is composed of genes that code for proteins. The remaining 98% includes DNA segments that help regulate the activity of those genes. To better understand how brain structure and function contribute to mental disorders, researchers in the NIMH-funded PsychENCODE Consortium are using standardized methods and data analysis approaches to build a comprehensive picture of these regulatory elements in the human brain.
In addition to these discoveries, the papers also highlight new methods and tools to help researchers analyze and explore the wealth of data produced by this effort. These resources include a web-based platform offering interactive visualization data from diverse brain cell types in individuals with and without mental disorders, known as PsychSCREEN. Together, these methods and tools provide a comprehensive, integrated data resource for the broader research community.
The papers focus on the second phase of findings from the PsychENCODE Consortium. This effort aims to advance our understanding of how gene regulation impacts brain function and dysfunction.
“These PsychENCODE Consortium findings shed new light on how gene risk maps onto brain function across developmental stages, brain regions, and disorders,” said Jonathan Pevsner, Ph.D., chief of the NIMH Genomics Research Branch. “The work lays a strong foundation for ongoing efforts to characterize regulatory pathways across disorders, elucidate the role of epigenetic mechanisms, and increase the ancestral diversity represented in studies.”
The PsychENCODE papers published in Science and Science Advances are presented as a collection on the Science website.
About the National Institute of Mental Health (NIMH): The mission of the NIMH is to transform the understanding and treatment of mental illnesses through basic and clinical research, paving the way for prevention, recovery and cure. For more information, visit the NIMH website.
About the National Institutes of Health (NIH): NIH, the nation's medical research agency, includes 27 Institutes and Centers and is a component of the U.S. Department of Health and Human Services. NIH is the primary federal agency conducting and supporting basic, clinical, and translational medical research, and is investigating the causes, treatments, and cures for both common and rare diseases. For more information about NIH and its programs, visit www.nih.gov .
NIH…Turning Discovery Into Health ®
Connect with Us
- More Social Media from NIH
ExploreDegrees Archive, 2011-12
Explore courses, alphabetical index, bulletin archive.
This archived information is dated to the 2011-12 academic year only and may no longer be current.
For currently applicable policies and information, see the current Stanford Bulletin .
Health Research and Policy
Emeriti: (Professors) Dan Bloch, John Farquhar, Victor R. Fuchs
Chair: Phil Lavori
Co-Chair: Robert Tibshirani
Professors: Laurence Baker, Bradley Efron, Trevor Hastie, Victor W. Henderson, Mark Hlatky, John Ioannidis, Iain M. Johnstone, Abby C. King, Philip W. Lavori, Ying Lu, Yvonne Maldonado, Richard A. Olshen, Julie Parsonnet, Robert Tibshirani, Alice S. Whittemore, Dee W. West, Wing Wong
Associate Professor: M. Kate Bundorf, Lorene M. Nelson, Chiara Sabatti
Assistant Professors: Marc Coram, Allison Kurian, Mei-Chiung Shih, Weiva Sieh, Lu Tian
Assistant Professors (Clinical): Rita Popat, Kristin Sainani
Courtesy Professors: Mary Goldstein, Paul Heidenreich, Daniel Kessler, Alex Macario, Douglas Owens, Paul Wise
Courtesy Associate Professors: Jay Bhattacharya, David R. Rogosa
Courtesy Assistant Professors : Grant Miller
Senior Lecturer: Irene Corso
Lecturers: Raymond Balise, Scarlett Gomez, Laurel Habel, De Kun Li, David Lilienfeld, Cynthia O'Malley, Caroline Tanner, Stephen Van Den Eeden
Consulting Professors: Gary Friedman, Elizabeth Holly, Marion Lee, George Lundberg, Peggy Reynolds
Consulting Associate Professors: Paul Barnett, Sally Glaser, Pamela Horn-Ross, Esther John, Ciaran Phibbs
Consulting Assistant Professors: Ellen Chang, Christina Clarke-Dur, Theresa Keegan, Bang Nguyen, Ingrid Oakley-Girvan, Rudy Rull, Todd Wagner
Mail Code: 94305-5405
Phone: (650) 723-5456
Web Site: http://hrp.stanford.edu
Courses offered by the Department of Health Research and Policy are listed under the subject code HRP on the Stanford Bulletin's ExploreCourses web site .
The Department of Health Research and Policy has three principal areas of scholarly interest:
- Biostatistics deals with scientific methodology in the medical sciences, emphasizing the use of statistical techniques.
- Epidemiology is the study of the distribution and determinants of illness and impairment in human populations. Epidemiology training provides analytic tools for clinical and translational research, including studies of disease etiology, prevention, and therapy.
- Health Services Research is concerned with many aspects of health policy analysis in the public and private sectors
Graduate Programs in Health Research Policy
The Program in Epidemiology and the Program in Health Services Research are housed in the Department of Health Research and Policy. These programs offer M.S. degrees in Epidemiology and in Health Services Research. Students with an interest in pursuing advanced degrees with an emphasis on biostatistics can do so through programs offered by the Department of Statistics. Division of Biostatistics faculty participate in these programs.
For additional information, address inquiries to the Educational Coordinator, Department of Health Research and Policy, Stanford University School of Medicine, HRP Redwood Building, Room T-152F, Stanford, California 94305-5405.
© Stanford University - Office of the Registrar . Archive of the Stanford Bulletin 2011-12. Terms of Use | Copyright Complaints
- Quick Finder
- Accessibility

You are here:
- Publications
<- Back to: publication list
Published: 24 August 2021
Health Research Board Annual Report 2020
Contents include: Chairperson and Chief Executive's report; a timeline of the HRB's key initiatives in response to the COVID-19 pandemic in 2020: a snapshot of other important HRB activities during 2020; key deliverables in line with the HRB strategy 2016-2020; and Appendices.
Part 2 contains the corporate governance and financial statements which have been approved by the Office of the Comptroller and Auditor General. This is also available for download (below).
Publication (PDF, 3 MB)
- An Bord Taighde Slainte Tuarascáil Bhliantúil 2020 Cuid 1 (PDF, 5 MB)
- Health Research Board Annual Report 2020 Part 2 (PDF, 465 KB)

Re-use includes copying, issuing copies to the public, publishing, broadcasting and translating into other languages. It also covers non-commercial research and study.
Full information on the regulations is available from the Public Sector Information (PSI) Portal at the following web address https://data.gov.ie/psi . The Public Sector Information Portal will always have the most up to date PSI Licence.

USF takes early steps toward $200 million research facility

The plan was approved at a meeting of the Board of Trustees Finance Committee on Wednesday. University officials emphasize this plan is "very very preliminary."
It’s big, it’s tall and it has a hefty price tag.
The University of South Florida is taking preliminary steps toward building a new, seven-story research facility on its Tampa campus. It’s estimated to cost over $200 million.
The proposed Translational Research Institute would focus on internal research rather than research by outside biotech companies. It will house about 100 National Institutes of Health-funded investigators and about 1,000 other researchers.
“It's the future state of research for the university,” said Carole Post, USF’s vice president of facilities at a meeting of the Board of Trustees Finance Committee on Wednesday. “We know how much emphasis there has been on that.”
USF president Rhea Law said the building is one key to the goal of enhancing the university’s research enterprise.
"We have a very bold and aggressive plan to enhance the numbers of our research,” Law said. “The one way we can do that is with the proper facilities to support it. So this is a part of that plan."
USF became part of the Association of American Universities last year, which should mean additional federal funds for research projects like this.
The building is now at the top of USF's state funding priorities list . A planned oceanographic building on the USF St. Petersburg campus has been moved down to number two. A STEM nursing facility now sits third on the priority list. The proposal has it being built on the USF Sarasota-Manatee campus.
The new proposal was approved by the committee, but USF officials emphasize this plan is "very very preliminary."
If approved, the building would be constructed on a currently empty lot on the northwest side of the Tampa campus, at the corner of USF Holly and USF Magnolia.

- News & Media
- Chemical Biology
- Computational Biology
- Ecosystem Science
- Cancer Biology
- Exposure Science & Pathogen Biology
- Metabolic Inflammatory Diseases
- Advanced Metabolomics
- Mass Spectrometry-Based Measurement Technologies
- Spatial and Single-Cell Proteomics
- Structural Biology
- Biofuels & Bioproducts
- Human Microbiome
- Soil Microbiome
- Synthetic Biology
- Computational Chemistry
- Chemical Separations
- Chemical Physics
- Atmospheric Aerosols
- Human-Earth System Interactions
- Modeling Earth Systems
- Coastal Science
- Plant Science
- Subsurface Science
- Terrestrial Aquatics
- Materials in Extreme Environments
- Precision Materials by Design
- Science of Interfaces
- Friction Stir Welding & Processing
- Dark Matter
- Flavor Physics
- Fusion Energy Science
- Neutrino Physics
- Quantum Information Sciences
- Emergency Response
- AGM Program
- Tools and Capabilities
- Grid Architecture
- Grid Cybersecurity
- Grid Energy Storage
- Earth System Modeling
- Energy System Modeling
- Transmission
- Distribution
- Appliance and Equipment Standards
- Building Energy Codes
- Advanced Building Controls
- Advanced Lighting
- Building-Grid Integration
- Building and Grid Modeling
- Commercial Buildings
- Federal Performance Optimization
- Resilience and Security
- Grid Resilience and Decarbonization
- Building America Solution Center
- Energy Efficient Technology Integration
- Home Energy Score
- Electrochemical Energy Storage
- Flexible Loads and Generation
- Grid Integration, Controls, and Architecture
- Regulation, Policy, and Valuation
- Science Supporting Energy Storage
- Chemical Energy Storage
- Waste Processing
- Radiation Measurement
- Environmental Remediation
- Subsurface Energy Systems
- Carbon Capture
- Carbon Storage
- Carbon Utilization
- Advanced Hydrocarbon Conversion
- Fuel Cycle Research
- Advanced Reactors
- Reactor Operations
- Reactor Licensing
- Solar Energy
- Wind Resource Characterization
- Wildlife and Wind
- Community Values and Ocean Co-Use
- Wind Systems Integration
- Wind Data Management
- Distributed Wind
- Energy Equity & Health
- Environmental Monitoring for Marine Energy
- Marine Biofouling and Corrosion
- Marine Energy Resource Characterization
- Testing for Marine Energy
- The Blue Economy
- Environmental Performance of Hydropower
- Hydropower Cybersecurity and Digitalization
- Hydropower and the Electric Grid
- Materials Science for Hydropower
- Pumped Storage Hydropower
- Water + Hydropower Planning
- Grid Integration of Renewable Energy
- Geothermal Energy
- Algal Biofuels
- Aviation Biofuels
- Waste-to-Energy and Products
- Hydrogen & Fuel Cells
- Emission Control
- Energy-Efficient Mobility Systems
- Lightweight Materials
- Vehicle Electrification
- Vehicle Grid Integration
- Contraband Detection
- Pathogen Science & Detection
- Explosives Detection
- Threat-Agnostic Biodefense
- Discovery and Insight
- Proactive Defense
- Trusted Systems
- Nuclear Material Science
- Radiological & Nuclear Detection
- Nuclear Forensics
- Ultra-Sensitive Nuclear Measurements
- Nuclear Explosion Monitoring
- Global Nuclear & Radiological Security
- Disaster Recovery
- Global Collaborations
- Legislative and Regulatory Analysis
- Technical Training
- Additive Manufacturing
- Deployed Technologies
- Rapid Prototyping
- Systems Engineering
- 5G Security
- RF Signal Detection & Exploitation
- Climate Security
- Internet of Things
- Maritime Security
- Artificial Intelligence
- Graph and Data Analytics
- Software Engineering
- Computational Mathematics & Statistics
- High-Performance Computing
- Visual Analytics
- Lab Objectives
- Publications & Reports
- Featured Research
- Diversity, Equity, Inclusion & Accessibility
- Lab Leadership
- Lab Fellows
- Staff Accomplishments
- Undergraduate Students
- Graduate Students
- Post-graduate Students
- University Faculty
- University Partnerships
- K-12 Educators and Students
- STEM Workforce Development
- STEM Outreach
- Meet the Team
- Internships
- Regional Impact
- Philanthropy
- Volunteering
- Available Technologies
- Industry Partnerships
- Licensing & Technology Transfer
- Entrepreneurial Leave
- Atmospheric Radiation Measurement User Facility
- Electricity Infrastructure Operations Center
- Energy Sciences Center
- Environmental Molecular Sciences Laboratory
- Grid Storage Launchpad
- Institute for Integrated Catalysis
- Interdiction Technology and Integration Laboratory
- PNNL Portland Research Center
- PNNL Seattle Research Center
- PNNL-Sequim (Marine and Coastal Research)
- Radiochemical Processing Laboratory
- Shallow Underground Laboratory
Waters to Serve on Scientific Data Editorial Board
Publisher will draw on her expertise in public health and infectious disease

Photo by Andrea Starr | Pacific Northwest National Laboratory
Katrina Waters , a Laboratory Fellow and Chief Scientist at Pacific Northwest National Laboratory, will serve as an editorial board member for Scientific Data , a Nature journal that’s dedicated to the sharing and reuse of research data.
In a letter to Waters, the publication’s chief editor says Scientific Data has “grown steadily as the uptake for research data sharing increases.” As a result, their editorial board is expanding to include more subject matter experts who can help research communities engage in data sharing. Waters is a long-time advocate of data sharing, particularly as it relates to her field of expertise: the intersection of environmental exposures and infectious diseases in humans.
Waters is a corresponding author on two publications in this journal that exemplify the principles and advantages of data sharing. In a recent publication , she and a team of collaborators shared a large compendium of data: more than 24,000 raw genomics, proteomics, metabolomics, and lipidomics datasets collected over six years for a National Institute of Allergy and Infectious Diseases project focused on studying host response to viral infections . In 2023, Waters and her team published a data resource that links environmental chemical exposure at contaminated sites to biological phenotypes, supporting the joint Oregon State University and PNNL Superfund Research Program funded by the National Institute of Environmental Health Sciences .
In addition to her research programs, Waters leads an internal research program at PNNL called the Predictive Phenomics Initiative , which is focused on developing capabilities to measure and manipulate phenotypes of interest for future applications related to the bioeconomy, human health, and environmental sustainability. She holds joint faculty appointments with Oregon State University and the University of Washington.
Published: May 29, 2024
Research topics
Lab-level communications priority topics.
- HEOR Resources
Find the latest ISPOR news and announcements on the Society’s news site.
- Top 10 HEOR Trends
- The Future of HE+OR
- About Real-World Evidence
- Clinical Outcomes
- Digital Health, Devices, and Diagnostics
- Economic Evaluation
- Epidemiology
- Healthcare Delivery
- Health Policy
- Health Technology Assessment
- Methodology
- Patient-Centered Research
- Real-World Data
- Specialized Treatment Areas
- CHEERS Related Videos
- Health Technology Assessment Central
- ISPOR Presentations Database
- Media Center
- HEOR News Desk
- Company Listings
- Sponsored Webinars
- Assessing the Evidence
- US Healthcare System Overview-Background
- US Healthcare System Overview-Decision Makers and Influencers
- US Healthcare System Overview-Pharmaceuticals
- US Healthcare System Overview-Medical Devices and In Vitro Diagnostics
- US Healthcare System Overview-Documentation Requirements
- US Healthcare System Overview-Acknowledgements
- US Healthcare System Overview-References
- Pharmacoeconomic Guidelines Around the World
- COVID-19 Resources
Stay Connected
Already have an ISPOR Profile?
Click here to manage your subscriptions.
Subscribe to the HEOR News Desk
Dr eberechukwu onukwugha assumes role of president.
Lawrenceville, NJ, USA —May 28, 2024— ISPOR—The Professional Society for Health Economics and Outcomes Research announced today the results of its recent elections. The Society’s membership selected its new president-elect and 4 new board members. The 2024-2025 board will assume office on July 1 and includes the following members.
Eberechukwu Onukwugha, PhD—President Dr Onukwugha will assume the role of 2024-2025 president after serving as ISPOR’s president-elect over the past year. She is a professor in the Department of Practice, Sciences, and Health Outcomes Research and the executive director of Pharmaceutical Research Computing at the University of Maryland School of Pharmacy in Baltimore, Maryland, USA.
Brian O’Rourke, PharmD—Immediate Past President Dr O’Rourke moves into the role of immediate past president having served as president for the 2023-2024 term. He is currently president of Brian O’Rourke Health Care Consulting, Inc in Ottawa, Ontario, Canada and previously served as the president and chief executive officer of the Canadian Agency for Drugs and Technologies in Health (CADTH) from 2009-2020.
Uwe Siebert, MD, MPH, MSc, ScD—President-Elect Dr Siebert was recently elected as president-elect and will move into the role of president during the 2025-2026 term. He is professor of Public Health, Medical Decision Making, and Health Technology Assessment (HTA), and chair of the Department of Public Health, Health Services Research, and HTA at UMIT TIROL - University for Health Sciences and Technology in Austria. He is also adjunct professor of Epidemiology and Health Policy and Management at the Harvard Chan School of Public Health and affiliated with the Institute for Technology Assessment at the Massachusetts General Hospital, Harvard Medical School, Boston, MA, USA.
Elisabeth Fenwick, PhD—Director Dr Fenwick was recently elected as a new director and is chief scientific officer in OPEN Health’s HEOR & Market Access practice, based in Oxford, England, UK.
Ramiro E. Gilardino MD, MHS, MSc—Director Dr Gilardino was recently elected as a new director and acts as the global access and health technology assessment policy leader at MSD Innovation & Development (known as Merck in the United States and Canada).
Daniel Ollendorf, PhD—Director Dr Ollendorf was recently elected as a new director and is the chief scientific officer and director of health technology assessment methods and engagement at the Institute for Clinical and Economic Review (ICER).
Katja Rudell, PhD—Director Dr Rudell was recently elected as a new director and is senior director and global team lead of the Clinical Outcomes Assessment Science team at Parexel.
Returning board members include:
Dalia Dawoud, PhD—Director Dr Dawoud is a returning board director who is an associate director (research) at the National Institute for Health and Care Excellence (NICE) in London, England, UK.
Lucinda Orsini, DPM, MPH—Director Dr Orsini a returning director who is vice president for value and outcomes research at COMPASS Pathways in Skillman, New Jersey, USA.
Amy K. O’Sullivan, PhD—Director Dr O’Sullivan is a returning director who is senior vice president and chief scientific officer at Ontada in Boston, Massachusetts, USA.
Sean D. Sullivan, BScPharm, MSc, PhD—Treasurer Dr Sullivan is returning as the Society’s treasurer and is professor of pharmacy and public health at the University of Washington School of Pharmacy in Seattle, Washington, USA and visiting professor at the London School of Economics and Political Science in London, England, UK.
Rob Abbott—CEO and Executive Director Mr Abbott is chief executive officer and executive director at ISPOR—The Professional Society for Health Economics and Outcomes Research in Lawrenceville, New Jersey, USA.
ABOUT ISPOR ISPOR—The Professional Society for Health Economics and Outcomes Research (HEOR), is an international, multistakeholder, nonprofit dedicated to advancing HEOR excellence to improve decision making for health globally. The Society is the leading source for scientific conferences, peer-reviewed and MEDLINE ® -indexed publications, good practices guidance, education, collaboration, and tools/resources in the field. Website | LinkedIn | Twitter (@ispororg) | YouTube | Facebook | Instagram
Related Stories
When and how to conduct health technology assessments for biosimilars.
May 13, 2024
ISPOR Announces Patient-Centered Research Summit 2024
Apr 23, 2024
ISPOR 2024 Plenaries and Speakers Announced
Apr 22, 2024
Your browser is out-of-date
ISPOR recommends that you update your browser for more security, speed and the best experience on ispor.org. Update my browser now

COMMENTS
New HRB report reveals how available and accessible alcohol is in Ireland. The latest alcohol overview from the Health Research Board reveals that despite a decline in pubs, Ireland still ranks 3rd highest in the world for the number of pubs per head, and three-in-four people live within walking distance of a premises licensed to sell alcohol.
The Health Research Board (HRB) is participating in the THCS Joint Transnational Call 2024 to support Irish researchers to engage in transnational collaborative research in the field of health and care systems under the call entitled "Innovate to Prevent: Personalised Prevention in Health and Care Services". ...
The Health Research Board (HRB) is a government agency responsible for funding, co-ordination, and oversight of medical research in Ireland. History [ edit ] In 1986, the Government of Ireland amalgamated the Medical Research Council of Ireland and the Medico-Social Research Board to establish the HRB under the Health (Corporate Bodies) Act ...
Michelle Hanlon, Brian McGuire, Claire MacGilchrist, Rosie Dunne, Ellen Kirwan, Deirdre Ní Neachtain, Ketan Dhatariya, Virginie Blanchette, Hannah Durand, Anda Dragomir, Caroline McIntosh. Peer Reviewer Raquel Marques. Funder. This work is supported by the Health Research Board (HRB) of Ireland. PUBLISHED 18 Apr 2024.
The Health Research Board is the lead agency in Ireland supporting and funding health research. It also manages a number of health information systems. #ResearchEvidenceAction ...
The Health Research Board (HRB) is a state agency that supports research and provides evidence to prevent illness, improve health and transform patient care. Help us improve our site Leave feedback. Do not include any personal details in the box below. The information you submit will be analysed to improve the site and will not be responded to ...
The Health Research Board (HRB) comprises 16 members appointed by the Minister of Health, with eight of the members being nominated on the co-joint nomination of the universities and colleges. The main functions of the HRB are to promote or commission health research, to promote and conduct epidemiological research as may be appropriate at ...
With the support of the Health Research Board, host institution University College Cork, Enterprise Ireland and the seven University-based Clinical Research Facilities/Centres (CRFs/Cs) in the Republic of Ireland the Health Research Board National Clinical Trials Office (HRB NCTO), will deliver on the aims of the National Clinical Trials Coordination Programme 2021 over the 3-year term of its ...
Videos connected to HRB activities
The Health Research Board (HRB) is announcing €5m* in funding to support research institutions develop a network to advance the involvement of the public, patients and carers in health and social care research, from generation of ideas to delivery of results.
The Health Research Board in recent years have funded the establishment of Clinical Trial Networks in, Critical Care, Paediatrics (in4Kids), Primary Care, Rare Disease, Infectious Disease, Diabetes and Dementia. The clinical research network page is live and updated on an ongoing basis. If you are a lead of a clinical research network and would ...
Purpose: The APRO aims to support a strategic programme of applied research in health and social care that will have an impact on the health and social care of individuals, population health and the health system in Ireland and beyond.. Eligibility: 1.The researchers in each team should come from a variety of disciplinary backgrounds. The inclusion of researchers from relevant disciplines not ...
Commonwealth Health Research Board. Life-to-Date, CHRB has made 306 grant awards totaling $26.6 million in grant funding. When institutional matching funds added, total cumulative grant funds total $38.4 million. CHRB grant recipients have leveraged approximately $78 million in additional private and federal grant funds to further their ...
Health Research, Inc. is an independent 501(c)(3) not for profit corporation governed by a Board of Directors which will consist of not less than five (5) and not more than thirty five (35) members, all of which are voting members. The NYS Commissioner of Health serves as the President of the HRI Board. Directors of the Corporation are elected ...
Federal workers in the health arena may provide direct patient care, regulate how others provide care, set payment rates and policies, conduct medical or health systems research, regulate products ...
Commonwealth Health Research Board P.O. Box 1971 [Mailing] 101 N. 14th Street, 2nd Floor [Delivery] Richmond, Virginia 23218-1971 www.chrb.org
The County of Santa Clara Public Health Department is conducting a door-to-door survey in Gilroy, San Martin, and Morgan Hill from April 24 to 26, 2024. The goal of the survey is to better understand the community's thoughts and knowledge on climate change, extreme weather events, and emergency preparedness.
State-of-the-art medical school, translational research facility to be completed by 2026. Construction will begin this spring on the first of three buildings in the New Jersey Health + Life Science Exchange (HELIX), a public-private development planned in downtown New Brunswick, following approval today by the Rutgers University Board of Trustees.
The Health Research Board (HRB) comprises 16 members appointed by the Minister of Health, with eight of the members being nominated on the co-joint nomination of the universities and colleges. The main functions of the HRB are to promote or commission health research, to promote and conduct epidemiological research as may be appropriate at ...
National Board of Echocardiography ; American Society of Echocardiography Examination 2000 ; Medical Societies. 1992-present: Member, American College of Physicians ... Stanford University, Department of Health Research and Policy, School of Medicine, Stanford, CA. 1995 - 1997: M.S. in Health Services Research;
He earned his PhD in health services research and health economics from the University of California Berkeley and joined CMI in July 2001 after several earlier careers. ... since 2010. He is a current member of the G-I-N Board of Trustees, the Executive Advisory Board for the Kaiser Permanente Research Associates Evidence-based Practice Center ...
Stanford Medicine is an integrated academic health system comprising the Stanford School of Medicine and adult and pediatric health care delivery systems. Together, they harness the full potential of biomedicine through collaborative research, education and clinical care for patients. For more information, please visit med.stanford.edu.
About the National Institutes of Health (NIH): NIH, the nation's medical research agency, includes 27 Institutes and Centers and is a component of the U.S. Department of Health and Human Services. NIH is the primary federal agency conducting and supporting basic, clinical, and translational medical research, and is investigating the causes ...
The Board of Trustees Executive Officers The President Committees and Panels Appointed by the President The Provost Schools of the University ... address inquiries to the Educational Coordinator, Department of Health Research and Policy, Stanford University School of Medicine, HRP Redwood Building, Room T-152F, Stanford, California 94305-5405.
Health Research Board Publisher Health Research Board Place of publication Dublin ISSN number 0791220X Rights. Re-use includes copying, issuing copies to the public, publishing, broadcasting and translating into other languages. It also covers non-commercial research and study.
It will house about 100 National Institutes of Health-funded investigators and about 1,000 other researchers. "It's the future state of research for the university," said Carole Post, USF's vice president of facilities at a meeting of the Board of Trustees Finance Committee on Wednesday. "We know how much emphasis there has been on that."
Publisher will draw on her expertise in public health and infectious disease. Katrina Waters, a Laboratory Fellow and Chief Scientist at Pacific Northwest National Laboratory, will serve as an editorial board member for Scientific Data, a Nature journal that's dedicated to the sharing and reuse of research data. In a letter to Waters, the ...
Lawrenceville, NJ, USA —May 28, 2024— ISPOR—The Professional Society for Health Economics and Outcomes Research announced today the results of its recent elections. The Society's membership selected its new president-elect and 4 new board members. The 2024-2025 board will assume office on July 1 and includes the following members.