The best free cultural &
educational media on the web
- Online Courses
- Certificates
- Degrees & Mini-Degrees
- Audio Books

Read John Nash’s Super Short PhD Thesis with 26 Pages & 2 Citations: The Beauty of Inventing a Field
in Math | June 1st, 2015 1 Comment

Last week John Nash , the Nobel Prize-winning mathematician, and subject of the blockbuster film A Beautiful Mind , passed away at the age of 86. He died in a taxi cab accident in New Jersey.
Days later, Cliff Pickover highlighted a curious factoid: When Nash wrote his Ph.D. thesis in 1950, “Non Cooperative Games” at Princeton University, the dissertation (you can read it online here) was brief. It ran only 26 pages. And more particularly, it was light on citations. Nash’s diss cited two texts: One was written by John von Neumann & Oskar Morgenstern, whose book, Theory of Games and Economic Behavior (1944), essentially created game theory and revolutionized the field of economics; the other cited text, “Equilibrium Points in n‑Person Games,” was an article written by Nash himself. And it laid the foundation for his dissertation, another seminal work in the development of game theory, for which Nash won the Nobel Prize in Economic Sciences in 1994 .
The reward of inventing a new field, I guess, is having a slim bibliography.
Related Content:
A Brilliant Madness — 2002 Film on the Nobel Prize Winning Mathematician" href="http://www.openculture.com/2012/06/john_nash_ia_brilliant_madnessi.html" rel="bookmark nofollow">John Nash: A Brilliant Madness — 2002 Film on the Nobel Prize Winning Mathematician
The Shortest-Known Paper Published in a Serious Math Journal: Two Succinct Sentences
The World Record for the Shortest Math Article: 2 Words
Free Online Math Courses
by OC | Permalink | Comments (1) |
Related posts:
Comments (1), 1 comment so far.
This was shocking to know about the demise of John Nash. I had a chance to view the film “a beautiful mind” with a close friend, Steve Landfried in Wisconsin-Chicago where John Nash was a subject of this film. I am glad that Steve made this choice for me since I could see and feel all, that this magnificient scientist had gone through.This is still my favorite film because of its subject
Add a comment
Leave a reply.
Name (required)
Email (required)
XHTML: You can use these tags: <a href="" title=""> <abbr title=""> <acronym title=""> <b> <blockquote cite=""> <cite> <code> <del datetime=""> <em> <i> <q cite=""> <s> <strike> <strong>
Click here to cancel reply.
- 1,700 Free Online Courses
- 200 Online Certificate Programs
- 100+ Online Degree & Mini-Degree Programs
- 1,150 Free Movies
- 1,000 Free Audio Books
- 150+ Best Podcasts
- 800 Free eBooks
- 200 Free Textbooks
- 300 Free Language Lessons
- 150 Free Business Courses
- Free K-12 Education
- Get Our Daily Email
Free Courses
- Art & Art History
- Classics/Ancient World
- Computer Science
- Data Science
- Engineering
- Environment
- Political Science
- Writing & Journalism
- All 1500 Free Courses
- 1000+ MOOCs & Certificate Courses
Receive our Daily Email
Free updates, get our daily email.
Get the best cultural and educational resources on the web curated for you in a daily email. We never spam. Unsubscribe at any time.
FOLLOW ON SOCIAL MEDIA
Free Movies
- 1150 Free Movies Online
- Free Film Noir
- Silent Films
- Documentaries
- Martial Arts/Kung Fu
- Free Hitchcock Films
- Free Charlie Chaplin
- Free John Wayne Movies
- Free Tarkovsky Films
- Free Dziga Vertov
- Free Oscar Winners
- Free Language Lessons
- All Languages
Free eBooks
- 700 Free eBooks
- Free Philosophy eBooks
- The Harvard Classics
- Philip K. Dick Stories
- Neil Gaiman Stories
- David Foster Wallace Stories & Essays
- Hemingway Stories
- Great Gatsby & Other Fitzgerald Novels
- HP Lovecraft
- Edgar Allan Poe
- Free Alice Munro Stories
- Jennifer Egan Stories
- George Saunders Stories
- Hunter S. Thompson Essays
- Joan Didion Essays
- Gabriel Garcia Marquez Stories
- David Sedaris Stories
- Stephen King
- Golden Age Comics
- Free Books by UC Press
- Life Changing Books
Free Audio Books
- 700 Free Audio Books
- Free Audio Books: Fiction
- Free Audio Books: Poetry
- Free Audio Books: Non-Fiction
Free Textbooks
- Free Physics Textbooks
- Free Computer Science Textbooks
- Free Math Textbooks
K-12 Resources
- Free Video Lessons
- Web Resources by Subject
- Quality YouTube Channels
- Teacher Resources
- All Free Kids Resources
Free Art & Images
- All Art Images & Books
- The Rijksmuseum
- Smithsonian
- The Guggenheim
- The National Gallery
- The Whitney
- LA County Museum
- Stanford University
- British Library
- Google Art Project
- French Revolution
- Getty Images
- Guggenheim Art Books
- Met Art Books
- Getty Art Books
- New York Public Library Maps
- Museum of New Zealand
- Smarthistory
- Coloring Books
- All Bach Organ Works
- All of Bach
- 80,000 Classical Music Scores
- Free Classical Music
- Live Classical Music
- 9,000 Grateful Dead Concerts
- Alan Lomax Blues & Folk Archive
Writing Tips
- William Zinsser
- Kurt Vonnegut
- Toni Morrison
- Margaret Atwood
- David Ogilvy
- Billy Wilder
- All posts by date
Personal Finance
- Open Personal Finance
- Amazon Kindle
- Architecture
- Artificial Intelligence
- Beat & Tweets
- Comics/Cartoons
- Current Affairs
- English Language
- Entrepreneurship
- Food & Drink
- Graduation Speech
- How to Learn for Free
- Internet Archive
- Language Lessons
- Most Popular
- Neuroscience
- Photography
- Pretty Much Pop
- Productivity
- UC Berkeley
- Uncategorized
- Video - Arts & Culture
- Video - Politics/Society
- Video - Science
- Video Games
Great Lectures
- Michel Foucault
- Sun Ra at UC Berkeley
- Richard Feynman
- Joseph Campbell
- Jorge Luis Borges
- Leonard Bernstein
- Richard Dawkins
- Buckminster Fuller
- Walter Kaufmann on Existentialism
- Jacques Lacan
- Roland Barthes
- Nobel Lectures by Writers
- Bertrand Russell
- Oxford Philosophy Lectures
Receive our newsletter!
Open Culture scours the web for the best educational media. We find the free courses and audio books you need, the language lessons & educational videos you want, and plenty of enlightenment in between.
Great Recordings
- T.S. Eliot Reads Waste Land
- Sylvia Plath - Ariel
- Joyce Reads Ulysses
- Joyce - Finnegans Wake
- Patti Smith Reads Virginia Woolf
- Albert Einstein
- Charles Bukowski
- Bill Murray
- Fitzgerald Reads Shakespeare
- William Faulkner
- Flannery O'Connor
- Tolkien - The Hobbit
- Allen Ginsberg - Howl
- Dylan Thomas
- Anne Sexton
- John Cheever
- David Foster Wallace
Book Lists By
- Neil deGrasse Tyson
- Ernest Hemingway
- F. Scott Fitzgerald
- Allen Ginsberg
- Patti Smith
- Henry Miller
- Christopher Hitchens
- Joseph Brodsky
- Donald Barthelme
- David Bowie
- Samuel Beckett
- Art Garfunkel
- Marilyn Monroe
- Picks by Female Creatives
- Zadie Smith & Gary Shteyngart
- Lynda Barry
Favorite Movies
- Kurosawa's 100
- David Lynch
- Werner Herzog
- Woody Allen
- Wes Anderson
- Luis Buñuel
- Roger Ebert
- Susan Sontag
- Scorsese Foreign Films
- Philosophy Films
- February 2024
- January 2024
- December 2023
- November 2023
- October 2023
- September 2023
- August 2023
- February 2023
- January 2023
- December 2022
- November 2022
- October 2022
- September 2022
- August 2022
- February 2022
- January 2022
- December 2021
- November 2021
- October 2021
- September 2021
- August 2021
- February 2021
- January 2021
- December 2020
- November 2020
- October 2020
- September 2020
- August 2020
- February 2020
- January 2020
- December 2019
- November 2019
- October 2019
- September 2019
- August 2019
- February 2019
- January 2019
- December 2018
- November 2018
- October 2018
- September 2018
- August 2018
- February 2018
- January 2018
- December 2017
- November 2017
- October 2017
- September 2017
- August 2017
- February 2017
- January 2017
- December 2016
- November 2016
- October 2016
- September 2016
- August 2016
- February 2016
- January 2016
- December 2015
- November 2015
- October 2015
- September 2015
- August 2015
- February 2015
- January 2015
- December 2014
- November 2014
- October 2014
- September 2014
- August 2014
- February 2014
- January 2014
- December 2013
- November 2013
- October 2013
- September 2013
- August 2013
- February 2013
- January 2013
- December 2012
- November 2012
- October 2012
- September 2012
- August 2012
- February 2012
- January 2012
- December 2011
- November 2011
- October 2011
- September 2011
- August 2011
- February 2011
- January 2011
- December 2010
- November 2010
- October 2010
- September 2010
- August 2010
- February 2010
- January 2010
- December 2009
- November 2009
- October 2009
- September 2009
- August 2009
- February 2009
- January 2009
- December 2008
- November 2008
- October 2008
- September 2008
- August 2008
- February 2008
- January 2008
- December 2007
- November 2007
- October 2007
- September 2007
- August 2007
- February 2007
- January 2007
- December 2006
- November 2006
- October 2006
- September 2006
©2006-2024 Open Culture, LLC. All rights reserved.
- Advertise with Us
- Copyright Policy
- Privacy Policy
- Terms of Use
Politics and Elections
Incredible story of john nash and his short phd thesis.

This is a story about John Nash, the famed mathematician who inspired the movie 'A Beautiful Mind,' and his 27-page-long PhD thesis. He altered our understanding of the world in a mere 27 pages and shaped a life that, while cut short, was long in its reach and profound in its influence.
The year is 1950. John Nash, a young graduate student at Princeton, submits his PhD dissertation. It’s not a voluminous work stretching into hundreds of pages, as one might expect from groundbreaking research. Instead, it consists of a mere 27 pages. Titled “ Non-Cooperative Games ,” this unassuming document would go on to revolutionize the field of economics and game theory. As such it eventually earned Nash the Nobel Prize in Economic Sciences in 1994.
A minimalist approach where less is more
Nash’s thesis was unique not just in its length, but also in its reliance on citations. Only two sources were cited in the entire document. The first was the 1944 seminal work “ Theory of Games and Economic Behavior ” by John von Neumann and Oskar Morgenstern, the book that essentially laid the foundation for game theory.
The second was an article titled “ Equilibrium Points in n-Person Games ” — authored by none other than John Nash himself. This deliberate selection hinted at Nash’s confidence in his original ideas and his audacity to let them stand almost solely on their own merit.
In academic circles, the importance of a thesis is sometimes measured by its length and the number of citations it carries. Nash defied this unwritten rule in a spectacular manner. It was as if he was saying, “ Here are my ideas; they stand on their own .”
The essence of Nash’s brilliance
What made Nash’s dissertation extraordinary was its concept of equilibrium in “ non-cooperative ” games. A significant departure from traditional theories that primarily focused on “ cooperative ” games where participants can make binding agreements.
His work argued that in any game involving two or more players, each with their set of strategies, there exists an equilibrium where each player’s strategy is optimal given the strategies chosen by everyone else.
This would come to be known as the “ Nash Equilibrium. ”
Nash’s theories extended well beyond economics. They found applications in various domains, including biology, political science, and even philosophy. They provided a robust analytical framework to understand how individual decisions in complex systems could ultimately result in a stable, balanced outcome.
Nash’s impact was far-reaching and profound
The magnitude of the impact of Nash’s work can be best understood by the accolades it received. In 1994, nearly five decades after he wrote his thesis, Nash was awarded the Sveriges Riksbank Prize in Economic Sciences in Memory of Alfred Nobel – Nobel prize for Economics . This prestigious recognition signified the immense influence and lasting relevance of his work.

While the 26 pages may have seemed trivial at the time, the depth and breadth of the ideas contained within were far from it. Many researchers and scholars have since built upon Nash’s principles, and his equilibrium concepts have become a standard teaching module in economics courses worldwide.
Paving the way for modern game theory
Before Nash, game theory was an interesting but limited field. His dissertation blew open the doors to various new interpretations and applications. For instance, Nash’s ideas are now routinely applied in everything from market economics to election strategies. You can find them in negotiations, and even evolutionary biology.
His theories are applied in fields as varied as artificial intelligence, where algorithms often use Nash equilibrium concepts for decision-making. They are visible to international politics, where countries apply non-cooperative game theories in diplomatic negotiations and strategy planning. His work even finds applications in evolutionary biology, as mentioned earlier, explaining survival strategies among different species.
A life fraught with challenges — genius, vulnerability and the enigma of recovery
John Nash’s mental health battles are commonly thought to have been a lifelong struggle. However, the truth, as explained by his biographer Sylvia Nasar, paints a more nuanced picture .
Nash experienced the onset of schizophrenia at age 30 and underwent treatment only up to age 40. After that point, he had what he described as “ aging out of it. ” Nash speculated that hormonal changes played a role in his recovery, a hypothesis that aligns with the principles of cognitive and behavioral therapies that help patients manage delusions rather than will them away.
Nash’s case was exceptional even among those with chronic schizophrenia. He was among the small fraction who experience spontaneous recoveries. Generally, only 8 to 10% of patience experience such recovery.
While his intellectual brilliance was undoubted, another underappreciated aspect of his life was the role his wife Alicia played in his recovery. As Nasar notes, Nash had a stable home and family to return to, providing a sanctuary of sorts that undoubtedly played a role in his well-being.
Hopes and recognition and the Nobel as a crowning achievement and a final cure
The Nobel Prize in 1994 wasn’t just a triumph of intellectual prowess; it served a dual role.
For Nash, the award was a recognition not just from the academic community but also a form of reintegration into a society, The same society that had perhaps distanced itself from him due to his mental illness.
According to Nasar, the Nobel “completed the cure” in the sense that Nash could re-enter a community that, despite years of estrangement, still held significance for him and to which he could contribute anew.
This external validation, while late in coming, offered a sense of closure and belonging. And that cannot be underestimated in understanding Nash’s multifaceted life journey.
The unshakable support of his family and the acknowledgment from a community that had once shunned him combined to provide a form of holistic recovery that is as awe-inspiring as it is rare. Nash’s story isn’t merely one of intellectual triumph but is equally a narrative about the resilience of the human spirit, underscored by the roles of love, belonging, and recognition.
A tale of two narratives — Hollywood vs. Reality
Nash’s life was depicted in the 2001 film “A Beautiful Mind,” inspired by Nash’s life. While the movie captured the essence of Nash’s genius and his battle with schizophrenia, it took several creative liberties.
One such discrepancy is the film’s portrayal of visual hallucinations, which Nash himself did not experience. Moreover, the movie chose to focus on the more dramatic aspects of his condition. At the same time it omitted significant elements of his personal life. For example, his divorce and eventual remarriage to Alicia.
What the film did accurately convey, however, was Nash’s resilience and the unwavering support he received from Alicia. It managed to humanize a man often perceived merely as a towering intellectual figure. It brought to light the everyday battles he fought against his own mind.
A final chapter
The story of John Nash and his wife Alicia took a heartbreaking turn on May 23, 2015. Just shortly after Nash had received the Abel Prize for his contributions to mathematics , the couple died in a car crash while returning home from the airport. It was a shocking and sudden end. Not just to a life marked by incredible highs and lows, but also to a love story that had withstood the test of time, illness, and even separation.
The news of their deaths reverberated across academic circles and beyond, offering a grim reminder of the unpredictability of life. At the time of his passing, Nash was still active in his work, never one to rest on his laurels. His death, therefore, served as a stark full stop to a career that had recently received another accolade, and a life journey that had been marked by extraordinary resilience.
The couple’s sudden departure was an unfortunate punctuation to an enduring narrative of love, perseverance, and intellectual curiosity. As much as Nash’s theories revolutionized economics and mathematics, his life story with Alicia provided a real-world testament to the power of human endurance and connection.
Though they left the world abruptly, the indelible marks both John and Alicia Nash made—on science, on perceptions of mental health, and on each other’s lives—endure as a testament to their shared journey. They may have departed, but their story continues to resonate, serving as a complex but ultimately inspiring legacy.
A lifetime of legacy in 27 pages and a life that stretched its bounds
In the grand arc of John Nash’s life, which was full and long, living to the age of 86, one can see echoes of the succinct but powerful document that made him famous.
His 27-page PhD thesis, “Non Cooperative Games,” was remarkable for distilling complex theories into an understandable, transformative format. Similarly, Nash’s long life was dense with accomplishments and challenges that transcended the years he lived.
While his life did stretch over eight decades, each chapter was marked by a density of experience. From the highs of academic achievements to the lows of mental health struggles, from the love and support of his family to the ultimate, tragic conclusion — each period of his life was as impactful as those concise 27 pages that first put him on the map.
The abrupt ending to Nash’s life was a cruel stroke of fate. However, in no way it diminishes the longevity or significance of his journey. If his thesis taught us that profound ideas don’t need a lot of space to make an impact, his life demonstrated that the impact of human existence isn’t solely measured by its length, but by its depth and the ripples it creates in the world around it.
John Nash did just that. Altered our understanding of the world in a mere 27 pages and shaped a life that, while cut short, was long in its reach and profound in its influence.

Don't stop reading
Inflation data visualization tutorial and a case study
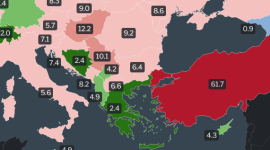
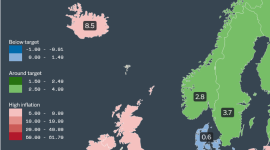
Inflation rates in Europe 2023 by country (Latest data and maps)

2024 Europe gas storage reserves – by country, updated daily

Top 200 most common last names in the USA: A full list and a racial distribution

List and data visualization of the top 20 countries polluting the oceans the most

List of Europe’s most dangerous and worst roads

Ethics and ethical data visualization: A complete guide
Seven principles in art and design that’ll improve your data visualizations
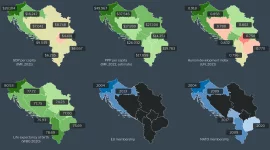
Comparison of Slovenia, Croatia, and Western Balkans in 2023 – economy and politics
Interactive map of Pangaea / Pangea with present-day borders and a globe
October in different languages of Europe, maps, and etymology
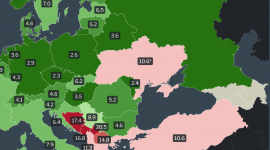
Unemployment rates in Europe, by country, latest data for 2022
Older articles, september in different languages of europe, maps, and etymology, road quality in europe: the best and worst roads in europe, here’s how heat waves are affecting your mental health and well-being, august in different languages of europe, maps, and etymology, the uk got its new national highest temperature record of 40.3 °c, how many healthy life years do europeans live.
MIT Libraries logo MIT Libraries

Year 91 – 1951: “Non-cooperative Games” by John Nash, in: Annals of Mathematics 54 (2)
Posted on April 7, 2011 in All years , Uncategorized

Over the past 60 years, game theory has been one of the most influential theories in the social sciences, pervasive in economics, political science, business administration, and military strategy – the disciplines most consulted by the powers-that-be for “real-world,” high-stakes decisions. But just as there would be no semiconductors or (God forbid) laser pointers if not for the abstruse mathematics of quantum theory, game theory can be traced back to theoretical work by academic mathematicians. In a set of papers in the 1950s, mathematician John Forbes Nash set forth breakthrough ideas that helped transform game theory from an ivory tower abstraction into an indispensable analytical tool used by strategists from Wall Street to the Pentagon.
The foundational game theory work of mathematician John von Neumann and economist Oskar Morgenstern, published in 1944, provided a framework for solutions to zero-sum games, where one player’s win was the other’s loss. Nash, in his dissertation research at Princeton (published in this and three other papers), extended game theory to n -person games in which more than one party can gain, a better reflection of practical situations. Nash demonstrated that “a finite non-cooperative game always has at least one equilibrium point” or stable solution. This result came to be called the “Nash equilibrium,” a situation where no one player can get a better payoff by changing strategies, so long as other players also keep their strategies. Using Nash’s framework, predictions can be made about the outcomes of strategic interactions.
Based on Nash’s advances, game theory developed into one of the pre-eminent tools of economics in the second half of the 20th century. In recognition of his breakthrough work, Nash was joint recipient of the Nobel Prize for Economics in 1994 for “pioneering analysis of equilibria in the theory of non-cooperative games.”
If visions of Russell Crowe have danced in your head while you’ve been reading this post, that’s probably because you remember that Crowe played John Forbes Nash in the 2001 film A Beautiful Mind (at least we hope that’s why). Part of the movie takes place at MIT, portraying Nash’s years as an instructor in mathematics at the Institute, where he worked from 1951 to 1959, until mental illness curtailed his mathematical career.
Find it in the library
.jpg?w=1)
Non-Cooperative Games, 1951
Brought to you by, more from fine printed books and manuscripts including americana.
The Masterpieces of John Forbes Nash Jr.
We’re sorry, something doesn't seem to be working properly.
Please try refreshing the page. If that doesn't work, please contact support so we can address the problem.
- First Online: 24 February 2019
Cite this chapter

- Camillo De Lellis 3 , 4
1741 Accesses
3 Citations
In this set of notes I follow Nash’s four groundbreaking works on real algebraic manifolds, on isometric embeddings of Riemannian manifolds and on the continuity of solutions to parabolic equations. My aim has been to stay as close as possible to Nash’s original arguments, but at the same time present them with a more modern language and notation. Occasionally I have also provided detailed proofs of the points that Nash leaves to the reader.
This is a preview of subscription content, log in via an institution to check access.
Access this chapter
- Available as PDF
- Read on any device
- Instant download
- Own it forever
- Available as EPUB and PDF
- Durable hardcover edition
- Dispatched in 3 to 5 business days
- Free shipping worldwide - see info
Tax calculation will be finalised at checkout
Purchases are for personal use only
Institutional subscriptions
Similar content being viewed by others

Analysis on Noncompact Manifolds and Index Theory: Fredholm Conditions and Pseudodifferential Operators

What is the Bochner Technique and Where is it Applied

Fred Gehring’s Mathematics
In a short autobiographical note, cf. [ 80 , Ch. 2], Nash states that he made his important discovery while completing his PhD at Princeton. In his own words “…I was fortunate enough, besides developing the idea which led to “NonCooperative Games”, also to make a nice discovery relating manifolds and real algebraic varieties. So, I was prepared actually for the possibility that the game theory work would not be regarded as acceptable as a thesis in the mathematics department and then that I could realize the objective of a Ph.D. thesis with the other results.”
Here we are using the nontrivial fact that in a connected real analytic manifold any pair of points can be joined by a real analytic arc. One simple argument goes as follows: use first Whitney’s theorem to assume, without loss of generality, that Σ is a real analytic submanifold of \(\mathbb R^N\) . Fix two points p and q and use the existence of a real analytic projection in a neighborhood of Σ to reduce our claim to the existence of a real analytic arc connecting any two points inside a connected open subset of the Euclidean space. Finally use the Weierstrass polynomial approximation theorem to show the latter claim.
The projection of an algebraic subvariety is not always an algebraic subvariety: here as well we are taking advantage of the genericity of the projection.
Many thanks to Riccardo Ghiloni for suggesting this argument, which follows closely the proof of [ 55 , Lem. 3.2].
Observe that in this context the closure in the Euclidean topology coincides with the Zariski closure.
Closed manifolds can be C 1 isometrically immersed in lower dimension: already at the time of Nash’s paper this could be shown in \(\mathbb R^{2n-1}\) (for n > 1!) using Whitney’s immersion theorem. Nowadays we can use Cohen’s solution of the immersion conjecture to lower the dimension to n − a ( n ), where a ( n ) is the number of 1’s in the binary expansion of n , cf. [ 17 ].
This is what Nash calls “a stage”, cf. [ 73 , p. 391].
Although the term is nowadays rather common, it was not introduced by Nash, neither in [ 73 ] nor in the subsequent paper [ 75 ].
In his paper Nash claims indeed a much larger K ( n ), cf. [ 73 , bottom of p. 386].
The argument of Nash is slightly different, since it covers the space of positive definite matrices with appropriate simplices.
Nash cites Steenrod’s classical book, [ 94 ].
Nash writes Also they could be obtained by orthogonal propagation , cf. [ 73 , top of p. 387].
The term free was not coined by Nash, but introduced later in the literature by Gromov.
It must be observed that Nash employs this fact without explicitly stating it and he does not prove it neither he gives a reference. He uses it twice, once in the proof of Theorem 29 and once in the proof of Proposition 35 , and although in the first case one could appeal to a more elementary argument, I could not see an easier way in the second.
Indeed Nash does not give any argument and just refers to a similar reasoning that he uses in Proposition 35 below.
Nash suggests an alternative argument which avoids the discussion of the dimensions of \(\mathcal {C} (p, L)\) and \(\mathcal {B}\) . One can apply his result on real algebraic varieties to find an embedding v which realizes v ( Σ ) as a real algebraic submanifold, cf. Theorem 1 . Then any set of coefficients \(A^r_{ij}\) which is algebraically independent over the minimal field \(\mathbb F\) of definition of v ( Σ ) (see Proposition 12 ) belongs to the complement of \(\mathcal {B}\) . Since \(\mathbb F\) is finitely generated over the rationals (see Proposition 12 ), it has countable cardinality and the conclusion follows easily.
In Nash’s paper the operator is called S θ , where θ corresponds to ε −1 . Since it is nowadays rather unusual to parametrize a family of convolutions as Nash does, I have switched to a more modern convention.
Nash does not take advantage of this simple remark and introduces instead a rather unusual notation to keep track of all the estimates for the intermediate norms in the bounds corresponding to ( 59 ), ( 60 ) and ( 61 ).
In fact, De Giorgi’s statement is stronger, since in his theorem ∥ v ∥ ∞ in ( 125 ) is replaced by the L 2 norm of v (note that the power of r should be suitably adjusted: the reader can easily guess the correct exponent using the invariance of the statement under the transformation u r ( x ) = u ( rx )).
Indeed, it was known that the first partial derivatives of the minimizer satisfy a uniformly elliptic partial differential equation with measurable coefficients. De Giorgi’s stronger version of Theorem 50 would then directly imply the desired Hölder estimate. Nash’s version was also sufficient, because a theorem of Stampacchia guaranteed the local boundedness of the first partial derivatives, cf. [ 93 ].
Nash does not provide any argument nor reference, he only briefly mentions that Theorem 48 follows from Theorem 51 using a regularization scheme and the maximum principle. Note that a derivation of the latter under the weak regularity assumptions of Theorem 48 is, however, not entirely trivial: in Sect. 5.8 we give an alternative argument based on a suitable energy estimate.
In order to simplify the notation we omit the domain of integration when it is the entire space.
The first two equations are the first two equations from [ 77 , p. 487, (1)] whereas the third should correspond to [ 77 , p. 488, (1c)]. The latter is derived by Nash from the third equation in [ 77 , p. 487, (1)], which in turn corresponds to the classical conservation law for the entropy, see, for instance, [ 61 , (49.5)]. The third equation of [ 77 , p. 487, (1)] contains two typos, which disappear in [ 77 , p. 488, (1c)]. The latter however contains another error: Nash has η and ζ in place of \(\frac {\eta }{\rho T S_T}\) and \(\frac {\zeta }{\rho T S_T}\) , but it is easy to see that this would not be consistent with the way he describes its derivation.
Nash’s error has no real consequence for the rest of the note, since he treats the coefficients in front of \(\mathcal {S} (v)_{ij} \mathcal {S} (v)_{ij}\) and (div v ) 2 as arbitrary real analytic functions of ρ and T and the same holds for \(\frac {\eta }{\rho T S_T}\) and \(\frac {\zeta }{\rho T S_T}\) under the assumption S T ≠ 0. The latter inequality is needed in any case even to treat Nash’s “wrong” equation for T .
Indeed Nash does not mention the positivity of S T , although this is certainly required by his argument when he reduces the existence of solutions of ( 240 ) to the existence of a solutions of a suitable parabolic system, cf. [ 77 , (6) and (7)]: the equation in T is parabolic if and only if \(\frac {\varkappa }{\rho T S_T}\) is positive.
I also have the impression that his argument does not really need the positivity of S and p , although these are quite natural assumptions from the thermodynamical point of view.
In the modern literature it is customary to take an equivalent definition of X through formal power series; we refer to [ 58 ] for the latter and for several important subtleties related to variants of the Nash arc space.
In fact, Nash claims the proposition with any algebraic subset W of V in place of V s but, although the proposition does hold for W = V s , it turns out to be false for a general algebraic subset W ; cf. [ 21 , Ex. 3.7] for a simple explicit counterexample.
S. Akbulut and H. King. On approximating submanifolds by algebraic sets and a solution to the Nash conjecture. Invent. Math. , 107(1):87–98, 1992.
MathSciNet MATH Google Scholar
A. G. Akritas. Sylvester’s forgotten form of the resultant. Fibonacci Quart. , 31(4):325–332, 1993.
A. D. Alexandrov. Intrinsic geometry of convex surfaces . OGIZ, Moscow-Leningrad, 1948.
Google Scholar
G. E. Andrews, R. Askey, and R. Roy. Special functions. Cambridge: Cambridge University Press, 1999.
MATH Google Scholar
D. G. Aronson. Bounds for the fundamental solution of a parabolic equation. Bull. Amer. Math. Soc. , 73:890–896, 1967.
D. G. Aronson. Non-negative solutions of linear parabolic equations. Ann. Scuola Norm. Sup. Pisa (3) , 22:607–694, 1968.
R. F. Bass. Diffusions and elliptic operators . Springer-Verlag, New York, 1998.
R. F. Bass. On Aronson’s upper bounds for heat kernels. Bull. London Math. Soc. , 34(4):415–419, 2002.
J. Bochnak, M. Coste, and M.-F. Roy. Real Algebraic Geometry . Springer-Verlag, Berlin, 1998.
J. F. Borisov. The parallel translation on a smooth surface. IV. Vestnik Leningrad. Univ. , 14(13):83–92, 1959.
J. F. Borisov. C 1, α -isometric immersions of Riemannian spaces. Doklady , 163:869–871, 1965.
T. Buckmaster, C. De Lellis, P. Isett, and L. Székelyhidi, Jr. Anomalous dissipation for 1∕5-Hölder Euler flows. Ann. of Math. (2) , 182(1):127–172, 2015.
T. Buckmaster, C. De Lellis, L. Székelyhidi, Jr., and V. Vicol. Onsager’s conjecture for admissible weak solutions. Comm. Pure Appl. Math. , to appear.
C. Burstin. Ein Beitrag zum Problem der Einbettung der Riemannschen Räume in euklidischen Räumen. Rec. Math. Moscou , 38(3–4):74–85, 1931.
E. Cartan. Sur la possibilité de plonger un espace Riemannien donné dans un espace Euclidien. Ann. Soc. Polon. Math. , 6:1–7, 1928.
H. Cartan. Variétés analytiques réelles et variétés analytiques complexes. Bull. Soc. Math. France , 85:77–99, 1957.
R. L. Cohen. The immersion conjecture for differentiable manifolds. Ann. of Math. (2) , 122(2):237–328, 1985.
S. Cohn-Vossen. Zwei Sätze über die Starrheit der Eiflächen. Nachrichten Göttingen , 1927:125–137, 1927.
S. Conti, C. De Lellis, and L. Székelyhidi, Jr. h -principle and rigidity for C 1, α isometric embeddings. In Nonlinear partial differential equations , volume 7 of Abel Symp. , pages 83–116. Springer, Heidelberg, 2012.
T. de Fernex. Three-dimensional counter-examples to the Nash problem. Compos. Math. , 149(9):1519–1534, 2013.
T. de Fernex. The space of arcs of an algebraic variety. In Algebraic geometry: Salt Lake City 2015 , Proc. Sympos. Pure Math., Vol. 97.1, pp. 169–197, 2015.
E. De Giorgi. Sulla differenziabilità e l’analiticità delle estremali degli integrali multipli regolari. Mem. Accad. Sci. Torino. Cl. Sci. Fis. Mat. Nat. (3) , 3:25–43, 1957.
E. De Giorgi. Un esempio di estremali discontinue per un problema variazionale di tipo ellittico. Boll. Un. Mat. Ital. (4) , 1:135–137, 1968.
E. De Giorgi. Selected papers . Springer-Verlag, Berlin, 2006. Edited by L. Ambrosio, G. Dal Maso, M. Forti, M. Miranda and S. Spagnolo.
C. De Lellis, D. Inauen, and L. Székelyhidi, Jr. A Nash–Kuiper theorem for \(C^{1,\frac {1}{5}-\delta }\) immersions of surfaces in 3 dimensions. Rev. Mat. Iberoam. , 34(3):1119–1152, 2018.
C. De Lellis, H. King, J. Milnor, Nachbar J., L. Székelyhidi, Jr., C. Villani, and J. Weinstein. John Forbes Nash, Jr. 1928–2015. Notices of the AMS , 63(5):492–506, 2017.
C. De Lellis and L. Székelyhidi, Jr. Dissipative continuous Euler flows. Invent. Math. , 193(2):377–407, 2013.
M. Demazure, H. Pinkham, and B. Teissier, editors. Séminaire sur les Singularités des Surfaces , volume 777 of Lecture Notes in Mathematics . Springer, Berlin, 1980. Held at the Centre de Mathématiques de l’École Polytechnique, Palaiseau, 1976–1977.
Y. Eliashberg and N. Mishachev. Introduction to the h-principle . American Mathematical Society, Providence, RI, 2002.
L. C. Evans. Partial differential equations . American Mathematical Society, Providence (R.I.), 1998.
L. C. Evans and R. F. Gariepy. Measure theory and fine properties of functions . CRC Press, Boca Raton, FL, 1992.
E. B. Fabes and D. W. Stroock. A new proof of Moser’s parabolic Harnack inequality using the old ideas of Nash. Arch. Rational Mech. Anal. , 96(4):327–338, 1986.
E. Feireisl. Dynamics of viscous compressible fluids . Oxford University Press, Oxford, 2004.
J. Fernández de Bobadilla and M. P. Pereira. The Nash problem for surfaces. Ann. of Math. (2) , 176(3):2003–2029, 2012.
A. Friedman. Partial differential equations of parabolic type . Prentice-Hall, 1964.
P. D. González Pérez and B. Teissier. Toric geometry and the Semple-Nash modification. Rev. R. Acad. Cienc. Exactas Fís. Nat. Ser. A Math. RACSAM , 108(1):1–48, 2014.
M. J. Greenberg. Rational points in Henselian discrete valuation rings. Inst. Hautes Études Sci. Publ. Math. , (31):59–64, 1966.
M. L. Gromov. Isometric imbeddings and immersions. Dokl. Akad. Nauk SSSR , 192:1206–1209, 1970.
M. L. Gromov. Partial differential relations . Springer-Verlag, Berlin, 1986.
M. L. Gromov and V. A. Rohlin. Imbeddings and immersions in Riemannian geometry. Uspehi Mat. Nauk , 25(5 (155)):3–62, 1970.
M. Günther. Zum Einbettungssatz von J. Nash. Math. Nachr. , 144:165–187, 1989.
M. Günther. Isometric embeddings of Riemannian manifolds. In Proceedings of the International Congress of Mathematicians, Vol. I, II (Kyoto, 1990) , pages 1137–1143. Math. Soc. Japan, Tokyo, 1991.
R. S. Hamilton. The inverse function theorem of Nash and Moser. Bull. Amer. Math. Soc. (N.S.) , 7(1):65–222, 1982.
W. Hao, S. Leonardi, and J. Nečas. An example of irregular solution to a nonlinear Euler-Lagrange elliptic system with real analytic coefficients. Ann. Scuola Norm. Sup. Pisa Cl. Sci. (4) , 23(1):57–67, 1996.
R. Hartshorne. Algebraic geometry . Springer-Verlag, New York-Heidelberg, 1977.
A. Hatcher. Algebraic Topology . Cambridge University Press, Cambridge, 2002.
G. Herglotz. Über die Starrheit der Eiflächen. Abh. Math. Semin. Hansische Univ. , 15:127–129, 1943.
H. Hironaka. Resolution of singularities of an algebraic variety over a field of characteristic zero. I, II. Ann. of Math. (2) 79 (1964), 109–203; ibid. (2) , 79:205–326, 1964.
M. W. Hirsch. Differential Topology . Springer-Verlag, New York-Heidelberg, 1976.
E. Hopf. Zum analytischen Charakter der Lösungen regulärer zweidimensionaler Variationsprobleme. Math. Z. , 30:404–413, 1929.
P. Isett. A Proof of Onsager’s Conjecture. Ann. Math. (2) , 188:871–963, 2018.
S. Ishii and J. Kollár. The Nash problem on arc families of singularities. Duke Math. J. , 120(3):601–620, 2003.
H. Jacobowitz. Implicit function theorems and isometric embeddings. Ann. of Math. (2) , 95:191–225, 1972.
M. Janet. Sur la possibilité de plonger un espace Riemannien donné à n dimensions dans un espace Euclidien à \(\frac {n(n+1)}{2}\) dimensions. C. R. Acad. Sci., Paris , 183:942–943, 1926.
Z. Jelonek. On the extension of real regular embedding. Bull. Lond. Math. Soc. , 40(5):801–806, 2008.
J. M. Johnson and J. Kollár. Arc spaces of cA -type singularities. J. Singul. , 7:238–252, 2013.
A. Källén. Isometric embedding of a smooth compact manifold with a metric of low regularity. Ark. Mat. , 16(1):29–50, 1978.
J. Kollár and A. Némethi. Holomorphic arcs on singularities. Invent. Math. , 200(1):97–147, 2015.
S. G. Krantz and H. R. Parks. A Primer of Real Analytic Functions . Birkhäuser Verlag, Basel, 1992.
N. H. Kuiper. On C 1 -isometric imbeddings. I, II. Nederl. Akad. Wetensch. Proc. Ser. A. 58 = Indag. Math. , 17:545–556, 683–689, 1955.
L. D. Landau and E. M. Lifshitz. Course of theoretical physics. Vol. 6, Fluid dynamics . Pergamon Press, Oxford, second edition, 1987.
H. Lewy. On the existence of a closed convex surface realizing a given Riemannian metric. Proc. Natl. Acad. Sci. USA , 24:104–106, 1938.
P.-L. Lions. Mathematical topics in fluid mechanics. Vol. 2 . The Clarendon Press, Oxford University Press, New York, 1998. Compressible models, Oxford Science Publications.
J. W. Milnor and J. D. Stasheff. Characteristic classes . Princeton University Press, Princeton, N. J., 1974.
C. Mooney and O. Savin. Some Singular Minimizers in Low Dimensions in the Calculus of Variations. Arch. Ration. Mech. Anal. , 221(1):1–22, 2016.
C. B. Morrey, Jr. On the solutions of quasi-linear elliptic partial differential equations. Trans. Am. Math. Soc. , 43:126–166, 1938.
C. B. Morrey, Jr. Second-order elliptic systems of differential equations. In Contributions to the theory of partial differential equations , Annals of Mathematics Studies, no. 33, pages 101–159. Princeton University Press, Princeton, N. J., 1954.
J. Moser. A new technique for the construction of solutions of non-linear differential equations. Proc. Nat. Acad. Sci. USA , 47:1824–1831, 1961.
J. Moser. On Harnack’s theorem for elliptic differential equations. Comm. Pure Appl. Math. , 14:577–591, 1961.
J. Moser. A rapidly convergent iteration method and non-linear differential equations. II. Ann. Scuola Norm. Sup. Pisa (3) , 20:499–535, 1966.
J. Moser. A rapidly convergent iteration method and non-linear partial differential equations. I. Ann. Scuola Norm. Sup. Pisa (3) , 20:265–315, 1966.
J. Nash. Real algebraic manifolds. Ann. of Math. (2) , 56:405–421, 1952.
J. Nash. C 1 isometric imbeddings. Ann. of Math. (2) , 60:383–396, 1954.
J. Nash. A path space and the Stiefel-Whitney classes. Proc. Nat. Acad. Sci. U.S.A. , 41:320–321, 1955.
J. Nash. The imbedding problem for Riemannian manifolds. Ann. of Math. (2) , 63:20–63, 1956.
J. Nash. Continuity of solutions of parabolic and elliptic equations. Amer. J. Math. , 80:931–954, 1958.
J. Nash. Le problème de Cauchy pour les équations différentielles d’un fluide général. Bull. Soc. Math. France , 90:487–497, 1962.
J. Nash. Analyticity of the solutions of implicit function problems with analytic data. Ann. of Math. (2) , 84:345–355, 1966.
J. Nash. Arc structure of singularities. Duke Math. J. , 81(1):31–38 (1996), 1995.
J. Nash. The essential John Nash . Princeton University Press, Princeton, NJ, 2002. Edited by H. W. Kuhn and S. Nasar.
L. Nirenberg. The determination of a closed convex surface having given line element . ProQuest LLC, Ann Arbor, MI, 1949. Thesis (Ph.D.)–New York University.
L. Nirenberg. The Weyl and Minkowski problems in differential geometry in the large. Comm. Pure Appl. Math. , 6:337–394, 1953.
A. Nobile. Some properties of the Nash blowing-up. Pacific J. Math. , 60(1):297–305, 1975.
C. Plénat and M. Spivakovsky. The Nash problem and its solution: a survey. J. Singul. , 13:229–244, 2015.
A. V. Pogorelov. Izgibanie vypuklyh poverhnosteı̆ . Gosudarstv. Izdat. Tehn.-Teor. Lit., Moscow-Leningrad, 1951.
A. V. Pogorelov. Extrinsic geometry of convex surfaces . American Mathematical Society, Providence, R.I., 1973.
W. Rudin. Principles of mathematical analysis . McGraw-Hill Book Co., New York-Auckland-Düsseldorf, third edition, 1976.
L. Schläfli. Nota alla memoria del sig. Beltrami, “Sugli spazii di curvatura costante”. Annali di Mat. (2) , 5:178–193, 1871.
J. Schwartz. On Nash’s implicit functional theorem. Comm. Pure Appl. Math. , 13:509–530, 1960.
J. G. Semple. Some investigations in the geometry of curve and surface elements. Proc. London Math. Soc. (3) , 4:24–49, 1954.
M. Shiota. Nash manifolds , volume 1269 of Lecture Notes in Mathematics . Springer-Verlag, Berlin, 1987.
M. Spivakovsky. Sandwiched singularities and desingularization of surfaces by normalized Nash transformations. Ann. of Math. (2) , 131(3):411–491, 1990.
G. Stampacchia. Sistemi di equazioni di tipo ellittico a derivate parziali del primo ordine e proprietà delle estremali degli integrali multipli. Ricerche Mat. , 1:200–226, 1952.
N. Steenrod. The Topology of Fibre Bundles . Princeton Mathematical Series, vol. 14. Princeton University Press, Princeton, N. J., 1951.
V. Šverák and X. Yan. A singular minimizer of a smooth strongly convex functional in three dimensions. Calc. Var. Partial Differential Equations , 10(3):213–221, 2000.
V. Šverák and X. Yan. Non-Lipschitz minimizers of smooth uniformly convex functionals. Proc. Natl. Acad. Sci. USA , 99(24):15269–15276, 2002.
L. Székelyhidi, Jr. The h -principle and turbulence. ICM 2014 Proceedings Volume , 2014.
R. Thom. Espaces fibrés en sphères et carrés de Steenrod. Ann. Sci. Ecole Norm. Sup. (3) , 69:109–182, 1952.
A. Tognoli. Su una congettura di Nash. Ann. Scuola Norm. Sup. Pisa (3) , 27:167–185, 1973.
L. D. Tráng and B. Teissier. On the mathematical work of Professor Heisuke Hironaka. Publ. Res. Inst. Math. Sci. , 44(2):165–177, 2008.
A. H. Wallace. Algebraic approximation of manifolds. Proc. London Math. Soc. (3) , 7:196–210, 1957.
A. Weil. Foundations of Algebraic Geometry . American Mathematical Society, New York, 1946.
H. Weyl. Über die Bestimmung einer geschlossenen konvexen Fläche durch ihr Linienelement. Zürich. Naturf. Ges. 61, 40–72, 1916.
J. H. C. Whitehead. On C 1 -complexes. Ann. of Math. (2) , 41:809–824, 1940.
H. Whitney. Differentiable manifolds. Ann. of Math. (2) , 37(3):645–680, 1936.
H. Whitney. The self-intersections of a smooth n -manifold in 2 n -space. Ann. of Math. (2) , 45:220–246, 1944.
S.-T. Yau. Open problems in geometry. In Differential geometry: partial differential equations on manifolds (Los Angeles, CA, 1990) , volume 54 of Proc. Sympos. Pure Math. , pages 1–28. Amer. Math. Soc., Providence, RI, 1993.
Download references
Acknowledgements
I am very grateful to Helge and Ragni for entrusting to me this portion of the Nash volume, a wonderful occasion to deepen my understanding of the mathematics of a true genius, who has had a tremendous influence in my own work.
Most of the manuscript has been written while I was visiting the CMSA at Harvard and I wish to thank Shing-Tung Yau and the staff at CMSA for giving me the opportunity to carry on my work in such a stimulating environment.
Several friends and colleagues have offered me kind and invaluable help with various portions of this note. In particular I wish to thank Davide Vittone for giving me several precious suggestions with the Sects. 3 and 4 and reading very carefully all the manuscript; Gabriele Di Cerbo, Riccardo Ghiloni and János Kollár for clarifying several important points concerning Sect. 2 and pointing out a few embarassing mistakes; Tommaso de Fernex and János Kollar for kindly reviewing a first rather approximate version of Sect. 6.4 ; Eduard Feireisl for his suggestions on Sect. 6.3 ; Cedric Villani for allowing me to steal a couple of paragraphs from his beautiful review of [ 76 ] in the Nash memorial article [ 26 ]; Francois Costantino for helping me with a delicate topological issue; Jonas Hirsch and Govind Menon for proofreading several portions of the manuscript; Helge Holden for going through all the manuscript with extreme care.
This work has been supported by the grant agreement 154903 of the Swiss National Foundation.
Author information
Authors and affiliations.
School of Mathematics, Institute for Advanced Study, Princeton University, Princeton, NJ, USA
Camillo De Lellis
Universität Zürich, Zürich, Switzerland
You can also search for this author in PubMed Google Scholar
Corresponding author
Correspondence to Camillo De Lellis .

Editor information
Editors and affiliations.
Department of Mathematical Sciences, NTNU Norwegian University of Science and Technology, Trondheim, Norway
Helge Holden
Department of Mathematics, University of Oslo, Oslo, Norway
Ragni Piene
Rights and permissions
Reprints and permissions
Copyright information
© 2019 Springer Nature Switzerland AG
About this chapter
De Lellis, C. (2019). The Masterpieces of John Forbes Nash Jr.. In: Holden, H., Piene, R. (eds) The Abel Prize 2013-2017. The Abel Prize. Springer, Cham. https://doi.org/10.1007/978-3-319-99028-6_19
Download citation
DOI : https://doi.org/10.1007/978-3-319-99028-6_19
Published : 24 February 2019
Publisher Name : Springer, Cham
Print ISBN : 978-3-319-99027-9
Online ISBN : 978-3-319-99028-6
eBook Packages : Mathematics and Statistics Mathematics and Statistics (R0)
Share this chapter
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Publish with us
Policies and ethics
- Find a journal
- Track your research
Princeton University Library

SPECIAL COLLECTIONS
Special Collections Schedule - Summer 2024
The Special Collections reading rooms in Firestone and Mudd Libraries will be closed on the following upcoming holidays: Monday, May 27 (Memorial Day), Wednesday, June 19 (Juneteenth), Thursday, July 4 (Independence Day), and Monday, September 2 (Labor Day). We will be closing at 12:00pm on Friday, June 14 and will be closed all day on Friday, June 28. We will also begin our Summer Hours, 9am-4:15pm, on Monday, June 3. During this time we stop paging at 3:45pm.
You are here
- Collections
- Princeton University Archives
University Archives

John Nash's Dissertation
Non-cooperative Games, May 1950, is available in PDF format Non-Cooperative_Games_Nash.pdf . The dissertation is provided for research use only.
[Note: Chapter 6 of The Essential John Nash, edited by Harold W. Kuhn and Sylvia Nasar (Princeton, New Jersey: Princeton University Press, 2001) contains a facsimile of Nash's 1950 Ph.D. dissertation on non-cooperative games.]
In the movie A Beautiful Mind there is a scene in which faculty members present their pens to Nash. What is the origin of the pen ceremony? When did it start?
The scene in the movie, A Beautiful Mind, in which mathematics professors ritualistically present pens to Nash was completely fabricated in Hollywood. No such custom exists. What it symbolizes is that Nash was accepted and recognized in the mathematics community for his accomplishments. While some movies are based on books, the film A Beautiful Mind states that it was inspired by the life of John Nash. There are many discrepancies between the book and the film.
May I see Nash’s graduate school records?
John F. Nash, Jr's records have been digitzed and are available to view here: Graduate Alumni Records
May I see Nash’s faculty file personnel records?
Personnel files transferred to the archives after 2003 : Files are closed until 100 years after the person's year of birth or 5 years after the person's year of death, whichever is longer. Therefore, John F. Nash's personell files are closed until, June 13, 2028.
May I have a copy of Nash’s 1994 Nobel Prize acceptance speech?
At the Nobel Prize Award ceremony, His Majesty the King of Sweden hands each Laureate a diploma, a medal, and a document confirming the Prize amount. The Laureates do not give acceptance speeches . The scene in the movie A Beautiful Mind in which Nash thanks his wife Alicia for her continued support during his illness is fictional.
Laureates are each invited to give an hour-long lecture; however, the Nobel committee did not ask Nash to do so, due to concerns over his mental health.
Additional Resources
Princetoniana Committee Oral History Project Records , Interview with Harold Kuhn, Part 1, pp. 31-40. In this part of the interview, Prof. Kuhn discusses his behind the scenes work for John Nash’s Nobel Prize.
Historical Subject Files Collection, 1746-2005 : A Beautiful Mind.
Article: John Nash automobile accident May 23, 2015, in Monroe Township, New Jersey.

John F Nash PhD

Project Details
In this page you can find Nash’s PhD thesis:
- Original document
- Transcribed into tex/pdf *
*: Thanks to Rebeca Duarte Miguel for this, and to Jeek Midford for spotting some spelling mistakes.
Others form category

List of Experimental Economic Labs of the world
Game Theory Online Tools

Game Theory Society
Experiments on SciOn

Game Theory Nobelists

Software for Experiments

Online courses about Game Theory

GT in the media

Top 10 Game Theorists on Google Scholar
ETH Controversies Workshops

Modellbildung: Game theory and strategic interaction

Game Theory Movie

In the Ron Howard movie “A Beautiful Mind,” Russell Crowe plays John Nash, a major figure in game theory. Although it was a good movie, it totally misconstrues Nash’s doctoral dissertation and how it fit into the pre-existing literature. In this episode, Bob sets the record straight.
Mentioned in the Episode and Other Links of Interest:
- John Nash’s doctoral dissertation .
- Bob’s link for Liberty Classroom . (If you click this link and then register, the site will know you came from me.)
- Sylvia Nasar’s book that inspired the Ron Howard movie about Nash. #CommissionsEarned (As an Amazon Associate I earn from qualifying purchases.)
- A good explanation of the Allais Paradox (which shows limits of expected utility theory).
- Bob Murphy Show ep. 96 , which starts with critique of use of game theory.
- Help support the Bob Murphy Show.
The audio production for this episode was provided by Podsworth Media .
About the author, Robert
Christian and economist, Chief Economist at infineo, and Senior Fellow with the Mises Institute.
Thank you for this podcast. I recall watching that movie nearly 20 years ago, and thinking they had got it backwards too. I love equilibrium theory in general, and the Nash equilibrium in particular. Though I do a lot with optimization (mostly MIPs….in a variety of industries), I’ve not had a lot of opportunity to formulate and solve equilibrium models. Great stuff, nonetheless. Thanks again!
Leave a Comment Cancel Reply
Save my name, email, and website in this browser for the next time I comment.
This is the best letter of recommendation ever
by Dylan Matthews

John Nash — the Princeton game theorist who shared the 1994 Nobel Prize in economics and was portrayed by Russell Crowe in A Beautiful Mind — died in a car crash on May 23, along with his wife, Alicia. In light of his passing, Princeton has digitized his academic file and released it to the public; you can see the full PDF here .
Probably the best document it contains is a recommendation letter by Richard Duffin, Nash's undergraduate advisor at the Carnegie Institute of Technology (now Carnegie Mellon), to Solomon Lefschetz, a math professor at Princeton, where Nash was applying to grad school:

The letter was successful. Nash went on to get his PhD at Princeton. His dissertation, "Non-cooperative games," introduced a concept that would become known as the "Nash equilibrium," a crucial concept in game theory. Khan Academy explains the idea, and how it relates to the famous prisoner's dilemma game, here:
Nash's dissertation has been cited about 7,189 times , according to Google Scholar, though that excludes many uses of the term "Nash equilibrium" that don't cite the actual paper. "If Nash got a dollar for every time someone wrote or said 'Nash equilibrium,'" Princeton economist Avinash Dixit once wrote, "he would be a rich man."
Nash submitted his thesis in May 1950, when he was 21 years old. It was a mere 27 pages long, which includes an acknowledgements section and a very short bibliography. The entire dissertation cites only two sources: game theory's founders John von Neumann and Oskar Morgenstern, and John Nash.

More in this stream

40 years ago today, one man saved us from world-ending nuclear war

How gun ownership became a powerful political identity

Why scientists are cloning black-footed ferrets
Most popular, openai insiders are demanding a “right to warn” the public, the backlash against children’s youtuber ms rachel, explained, take a mental break with the newest vox crossword, an artist snuck an anti-semitic message into marvel’s newest x-men comic book, the secret to modern friendship, according to real friends, today, explained.
Understand the world with a daily explainer plus the most compelling stories of the day.
More in Science

The surprisingly subtle recipe making heat waves worse

This changed everything

10 big things we think will happen in the next 10 years

The last 10 years, explained

The internet peaked with “the dress,” and then it unraveled

The science of near-death experiences

Is Hunter Biden being prosecuted because of politics?

Kate Middleton’s cancer diagnosis, explained

Why are we so obsessed with morning routines?

Our identity crisis on immigration

Why lying on the internet keeps working
An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
Preview improvements coming to the PMC website in October 2024. Learn More or Try it out now .
- Advanced Search
- Journal List
- Drug Des Devel Ther
Non-Alcoholic Steatohepatitis (NASH) – A Review of a Crowded Clinical Landscape, Driven by a Complex Disease
Julia m fraile.
1 Scottish Biologics Facility, University of Aberdeen, Aberdeen, AB25 2ZP, UK
2 Elasmogen Ltd, Aberdeen, AB25 2ZP, UK
Soumya Palliyil
Caroline barelle, andrew j porter, marina kovaleva.
Non-alcoholic steatohepatitis (NASH) is a progressive form of non-alcoholic fatty liver disease (NAFLD), characterized by chronic inflammation and accumulation of fat in liver tissue. Affecting estimated 35 million people globally, NASH is the most common chronic liver condition in Western populations, and with patient numbers growing rapidly, the market for NASH therapy is projected to rise to $27.2 B in 2029. Despite this clinical need and attractive commercial opportunity, there are no Food and Drug Administration (FDA)-approved therapies specifically for this disease. Many have tried and unfortunately failed to find a drug, or drug combination, capable of unravelling the complexities of this metabolic condition. At the time of writing this review, only Zydus Cadila’s new drug application for Saroglitazar had been approved (2020) for NASH therapy in India. However, it is hoped that this dearth of therapy options will improve as several drug candidates progress through late-stage clinical development. Obeticholic acid (Intercept Pharmaceuticals), Cenicriviroc (Allergan), Aramchol (Galmed Pharmaceuticals), Resmetirom (Madrigal Pharmaceuticals), Dapagliflozin and Semaglutide (Novo Nordisk) are in advanced Phase 3 clinical trials, while Belapectin (Galectin Therapeutics), MSDC-0602K (Cirius Therapeutics), Lanifibranor (Inventiva), Efruxifermin (Akero) and Tesamorelin (Theratechnologies) are expected to start Phase 3 trials soon. Here, we have performed an exhaustive review of the current therapeutic landscape for this disease and compared, in some detail, the fortunes of different drug classes (biologics vs small molecules) and target molecules. Given the complex pathophysiology of NASH, the use of drug combination, different mechanisms of actions and the targeting of each stage of the disease will likely be required. Hence, the development of a single therapy for NASH seems challenging and unlikely, despite the plethora of later stage trials due to report. We therefore predict that clinical, patient and company interest in pipeline and next-generation therapies will remain high for some time to come.
Introduction
Non-alcoholic steatohepatitis (NASH) is a severe form of non-alcoholic fatty liver disease (NAFLD), characterized by the presence of liver inflammation and hepatocyte injury (ballooning) due to fat accumulation. 1 Although it develops typically in the absence of excessive alcohol consumption, NAFLD is related to an unhealthy diet and a lack of physical activity. Affecting 35 million people globally, NAFLD is the most common chronic liver condition in Western populations and, with patient numbers growing rapidly, the market is expected to rise towards $27.2 B in 2029. 2 There are four different clinical phases described for NAFLD. Phase 1 is characterized by simple steatosis and is considered harmless. Some patients progress to Phase 2 developing inflammation and ballooning (NASH). Phase 3 is defined by the presence of NASH with persistent inflammation resulting in liver fibrosis (scarring), which is considered the strongest predictor of liver-related events in NASH patients. Over time, this 3rd stage can lead to a more serious condition, such as liver cirrhosis (Phase 4) or even cancer, where a liver transplant is the only therapy option. In addition to liver-specific pathology, a diagnosis of NASH is also associated with increased cardiometabolic risk and represents the leading cause of death for these patients. 3 Last year, a group of experts started a new debate on the best terminology for NAFLD and proposed Metabolic Associated Fatty Liver Disease (MAFLD) as a more appropriate term to reflect the heterogeneity of this disease. 4 Although the incorporation of this new term leads to a change in the diagnostic criteria, it does not affect the prevalence of the condition in the population. 5 Since no decision regarding which term should be used had been made by the time of writing this revision, we decided to maintain NAFLD in this article.
During the early stages of this disease, patients often show few or non-specific symptoms, such as tiredness, fatigue or abdominal pain, and therefore NASH is often not diagnosed until later stages of disease progression, using invasive techniques such as liver biopsy. 1 A late on-set diagnosis has given NASH the dubious nom de plume of the “Silent Killer”. Due to the lack of a cost-effective and minimally invasive diagnostic test, the prevalence of this disease can only be estimated. In a meta-analysis of several studies, the worldwide prevalence of NAFLD was 25.24%. Among this population, biopsy-confirmed NASH was reported in 59.1% patients. 6 Even with earlier diagnosis or improved diagnostic options, NASH patients are currently unable to benefit from a portfolio of treatment options that would typically be available to patients suffering from other major disease indications, such as rheumatoid arthritis or some types of cancer. Reduction in liver fat by bariatric surgery has been shown to reverse NASH and fibrosis in severely obese patients. 7 However, at the time of article submission, there were no Food and Drug Administration (FDA) or European Medicines Agency (EMA) approved NASH-specific drugs, and life-style modifications focused on a healthy diet and exercise were the primary recommendations for patients. Hopefully, this situation is about to change, as several drug candidates are in late-stage clinical trials. 8
The histological evidence for a NASH diagnosis is determined by the NAFLD activity score (NAS), which is a composite of steatosis, inflammation and hepatocyte ballooning and represents a measure of disease activity. Once a diagnosis has been made, the rate of disease progression is represented by the NASH Clinical Research Network (CRN) fibrosis score: 0 (none), 1 (mild or moderate), 2 (perisinusoidal fibrosis with portal/periportal fibrosis), 3 (bridging fibrosis) and 4 (cirrhosis). 9 Using this fibrosis severity score, the FDA and EMA have encouraged sponsors to focus their drug development activities on those stages of the disease with greatest need, defined as non-cirrhotic NASH with liver fibrosis (fibrosis score greater than 1 but less than 4). Regulators have also highlighted the need to identify less-invasive biomarkers to supplant liver biopsy as the gold standard for diagnosis and assessment of the disease, enabling much broader testing and earlier diagnosis, as well as accelerated drug development outcomes. Furthermore, for approval of Phase 3 clinical studies, sponsors have been recommended to evaluate NASH and fibrosis independently and to consider the following two primary endpoints: (i) improvement of liver fibrosis greater than or equal to stage 1 (NASH CRN fibrosis score) without worsening of NASH and/or (ii) resolution of NASH (defined by the absence of isolated fatty liver disease or simple steatosis) with no worsening of liver fibrosis. 10
In this review, we have summarized the complex therapeutic landscape of NAFLD/NASH and compared the different drug classes and pathway targets, focusing on those compounds that are in late-stage clinical development and by definition are better placed to succeed in the “race” for the first FDA-approved and specific therapeutic option against NASH.
NASH (Mono) Therapies in Late-Stage Development
Saroglitazar Magnesium (Lipaglyn) is a first-in-class therapy already approved in India for the treatment of type 2 diabetes (T2D) and dyslipidemia that acts as a dual peroxisome proliferator-activator receptor (PPAR) alpha and gamma agonist, lowering high blood triglycerides and blood sugar. The PPAR family is composed of three members, PPARα, PPARγ and PPARδ, which are ubiquitously expressed in different organs and play an essential role in lipid and glucose metabolism, and inflammation. 11 Zydus Cadila Ltd’s new drug application (NDA) for Saroglitazar was accepted in 2020 for NASH treatment in India after achieving positive results in a Phase 3 clinical trial, EVIDENCES II, in Indian NASH patients ( Figure 1 ). After 52 weeks of treatment, the trial successfully met primary and secondary endpoints, demonstrating histological improvement of NASH by liver biopsy. In another Phase 2 clinical trial, EVIDENCES I, Saroglitazar showed improvement measured as a reduction in liver enzymes and lipid values in NAFLD patients. Outside India, a Phase 2 EVIDENCES IV trial in the United States (ClinicalTrials.gov {"type":"clinical-trial","attrs":{"text":"NCT03061721","term_id":"NCT03061721"}} NCT03061721 ) completed in October 2020 and met its primary endpoint of reduced ALT in NAFLD and NASH patients after 16 weeks of treatment with Saroglitazar. 12

Phase 3 clinical therapies for NASH treatment aligned to different stages of the disease. NASH progression is represented by the NASH CRN fibrosis score: NAFLD (none), F1 (mild or moderate), F2 (perisinusoidal fibrosis with portal/periportal fibrosis), F3 (bridging fibrosis) and F4 (cirrhosis). Company, drug name and targets are indicated for each clinical therapy.
Obeticholic acid (Ocaliva) is a semisynthetic analog of the bile acid, chenodeoxycholic acid, and acts as a Farnesoid X receptor (FXR) agonist ( Figure 2 ). FXR is a member of the nuclear receptor superfamily that regulates a variety of genes involved in bile acid synthesis and transport, and in glucose and lipid metabolism. 13 Already approved for the treatment of primary biliary cholangitis (PBC), Obeticholic acid is being developed by Intercept Pharmaceuticals Inc and was considered to be “leading the field” until the FDA rejected the company’s NDA for NASH treatment in June 2020. Intercept’s NDA application was based on an interim analysis from their 18-month Phase 3 REGENERATE (ClinicalTrials.gov {"type":"clinical-trial","attrs":{"text":"NCT02548351","term_id":"NCT02548351"}} NCT02548351 ) clinical trial of Obeticholic acid with nearly 2500 NASH patients with fibrosis enrolled ( Figure 1 ). 14 The study demonstrated an improvement of liver fibrosis without worsening of NASH as one of the two endpoints that the FDA requires for securing approval. However, the regulatory agency has asked the company to provide longer term data from the Phase 3 REGENERATE clinical study with specific reference to efficacy and safety and declined to support an accelerated approval for this medication. The FDA also reported several active liver toxicity signals associated with Obeticholic acid treatment, not already mentioned on its current drug label. In addition, an increase in low-density lipoprotein cholesterol and total cholesterol has been observed after treatment with Obeticholic acid in the Phase 2 FLINT clinical trial (ClinicalTrials.gov {"type":"clinical-trial","attrs":{"text":"NCT01265498","term_id":"NCT01265498"}} NCT01265498 ). 15 Undeterred by this initial set-back, Intercept Pharmaceuticals are also sponsoring another Phase 3 REVERSE clinical trial (ClinicalTrials.gov {"type":"clinical-trial","attrs":{"text":"NCT03439254","term_id":"NCT03439254"}} NCT03439254 ) to evaluate the efficacy and safety of Obeticholic acid in patients with compensated cirrhosis due to NASH.

Therapeutic landscape for NAFLD/NASH with targeted pathways. Current clinical therapies for NASH are grouped into different target classes and clinical development stages. A cross indicates that the drug has been discontinued for NASH therapy from the company’s pipeline.
Although Obeticholic acid had been heralded as the most advanced drug for the treatment of NASH, the FDA response to their NDA, together with the potential for liver toxicity problems, was encouraging enough for Intercept’s competitors to continue playing their part in this drug race. Indeed, Intercept Pharmaceuticals themselves have developed another FXR agonist for NASH treatment, INT-787, which, whilst still in preclinical studies, is believed to be more selective than Obeticholic acid. The company plans to start clinical trials with INT-787 in 2021.
Resmetirom, or MGL-3196, is a first-in-class oral thyroid hormone receptor-beta (THRβ) agonist developed by Madrigal Pharmaceuticals Inc ( Figure 2 ). THRβ is the main receptor for thyroid hormones in the liver and plays an essential role in lipid metabolism. 16 Phase 2 clinical data showed that Resmetirom treatment resulted in a significant reduction in hepatic fat in patients with NASH, 17 and supported its advancement into Phase 3 clinical development ( Figure 1 ). Madrigal Pharmaceuticals is currently sponsoring two Phase 3 studies: MAESTRO-NASH (ClinicalTrials.gov {"type":"clinical-trial","attrs":{"text":"NCT03900429","term_id":"NCT03900429"}} NCT03900429 ) and MAESTRO-NAFLD-1 (ClinicalTrials.gov {"type":"clinical-trial","attrs":{"text":"NCT04197479","term_id":"NCT04197479"}} NCT04197479 ), comprising patients at different stages of the disease. MAESTRO-NASH began in 2019 and is expected to enroll up to 2000 NASH patients with fibrosis (CRN stage 2 or 3). This trial is divided into two different arms. Initially, 900 patients will be enrolled with the primary endpoint of NASH resolution after 52 weeks of treatment, with at least a 2-point reduction in NAS and no worsening of fibrosis. Additional 1100 patients will be added to the study after the first year of treatment to evaluate the reduction in liver-related adverse events or progression to cirrhosis for up to further 54 months. Early data demonstrated liver fat reduction after three months of treatment with Resmetirom and was also shown to be a predictor for NASH resolution and fibrosis reduction in subsequent liver biopsy. Madrigal’s second Phase 3 study, MAESTRO-NAFLD-1, was initiated at the end of 2019 and is expected to enroll 700 patients with early stages of the disease (NAFLD or presumed NASH). Unlike MAESTRO-NASH trial, MAESTRO-NAFLD-1 is a non-biopsy study and its primary endpoint is to evaluate the effect of Resmetirom on the incidence of adverse effects, compared to placebo. The company expects to release data from this study by the end of 2021. Separate from its benefits in liver-related conditions, Madrigal Pharmaceuticals hopes to also highlight Resmetirom’s potential to decrease the elevated cardiovascular risk seen in NASH patients, through a reduction in heart attacks and strokes both during the trials and patient follow-up.
Cenicriviroc (CVC), is a novel, orally administered, potent, small molecule agonist that acts to block chemokine 2 and 5 receptors (CCR2/CCR5), both with well-known roles in liver inflammation and fibrosis ( Figure 2 ). 18 CVC has been developed by Allergan Inc (an AbbVie Inc company) and has received a Fast Track designation by the FDA for the treatment of NASH. Although CVC treatment failed to meet its primary endpoint in the Phase 2b CENTAUR study (ClinicalTrials.gov {"type":"clinical-trial","attrs":{"text":"NCT02217475","term_id":"NCT02217475"}} NCT02217475 ) defined as histological improvement in NAS without worsening of fibrosis, 19 it did improve levels of measurable liver fibrosis without worsening NASH. This data provided enough confidence for the company to continue to evaluate the efficacy and safety of this drug in a Phase 3 AURORA trial ( Figure 1 ) (ClinicalTrials.gov {"type":"clinical-trial","attrs":{"text":"NCT03028740","term_id":"NCT03028740"}} NCT03028740 ), enrolling approximately 2000 NASH patients with fibrosis (CRN stage 2 or 3). 20 Like others in this space, this study was thought to be performed in two arms: (i) lasting approximately one year to examine the improvement in liver fibrosis by at least 1 stage with no worsening of NASH in patients and (ii) lasting further 7 years to analyze long-term clinical outcomes by following histopathologic progression to cirrhosis, other liver-related outcomes and all-cause mortality in patients with stage 3 fibrosis. However, at the beginning of 2021 the AURORA phase 3 clinical trial terminated due to lack of efficacy on the results of the first part of the study. The company has not indicated future plans for CVC yet, but the termination of the AURORA study sows a seed of doubt about its efficacy as a monotherapy. Allergan is also conducting a 48-week Phase 2b TANDEM study (ClinicalTrials.gov {"type":"clinical-trial","attrs":{"text":"NCT03517540","term_id":"NCT03517540"}} NCT03517540 ) in collaboration with Novartis AG to evaluate the safety and efficacy of a combination of Tropifexor, a FXR agonist, and CVC in 200 patients with biopsy-proven NASH with fibrosis (CRN stage 2 or 3) ( Figure 2 ). 21
Aramchol is a first-in-class, once-daily, oral stearoyl-coenzyme A desaturase-1 (SCD1) modulator developed by Galmed Pharmaceuticals Ltd and granted FDA Fast Track designation status for the treatment of NASH. SCD1 is considered a mediator of liver steatosis and fibrosis because of its role in fatty acid biosynthesis. 22 , 23 In 2018, the company published the results of their Phase 2b ARREST clinical trial (ClinicalTrials.gov {"type":"clinical-trial","attrs":{"text":"NCT02279524","term_id":"NCT02279524"}} NCT02279524 ) that documented Aramchol treatment in 247 NASH patients who were all clinically overweight or obese and had prediabetes or T2D. This one-year study demonstrated liver fat reduction, biochemical improvement and both NASH and fibrosis resolution, with favorable safety and tolerability profiles. Buoyed by these results, Galmed Pharmaceuticals started, one year later, their 52-week Phase 3/4 ARMOR study (ClinicalTrials.gov {"type":"clinical-trial","attrs":{"text":"NCT04104321","term_id":"NCT04104321"}} NCT04104321 ) with NASH patients with advanced fibrosis (CRN stage 2 or 3) to evaluate the efficacy of Aramchol on NASH resolution, fibrosis improvement and a series of clinical outcomes related to NASH progression ( Figure 1 ). Primary completion of this study is estimated in 2022.
Galectin Therapeutics Inc, Cirius Therapeutics Inc, Inventiva Pharma SA, Akero Therapeutics Inc and Theratechnologies Inc, are also moving their NASH therapeutic candidates into later stage clinical development, encouraged by patient outcomes, safety profiles and the hitting of key endpoints in early clinical studies.
Galectin Therapeutics has started recruiting NASH patients with compensated cirrhosis for a Phase 2b/3 NAVIGATE clinical study (ClinicalTrials.gov {"type":"clinical-trial","attrs":{"text":"NCT04365868","term_id":"NCT04365868"}} NCT04365868 ) to begin evaluating the safety and efficacy of Belapectin (GR-MD-02), a complex carbohydrate that targets Galectin-3, a β‐galactoside‐binding lectin that plays an important role in inflammatory responses, 24 for the prevention of esophageal varices. The company already reported in their Phase 2b NASH-CX clinical trial (ClinicalTrials.gov {"type":"clinical-trial","attrs":{"text":"NCT02462967","term_id":"NCT02462967"}} NCT02462967 ) that Belapectin treatment, when compared to the placebo arm, safely reduced the hepatic venous pressure gradient in NASH cirrhosis patients without esophageal varices. 25 Galectin Therapeutics’ next NAVIGATE study will consist of an 18-month Phase 2b extension study followed by an interim analysis to consider any adaptive treatment modifications before a final evaluation in an 18-month Phase 3 trial. Endpoints will assess the proportion of patients who develop new esophageal varices after 18 months of Belapectin treatment compared to placebo (primary) and the effect of this treatment on the incidence of long-term clinically significant cirrhosis-related events (key secondary endpoint).
Cirius Therapeutics is another company planning to enter Phase 3 development for NASH treatment with its drug MSDC-0602K. This second-generation thiazolidinedione is designed to modulate the mitochondrial pyruvate carrier (MPC), a protein complex that regulates the entry of pyruvate into the mitochondria, with minimum activation of PPARγ ( Figure 2 ). 26 The company demonstrated in their 12-month Phase 2b EMMINENCE clinical trial (ClinicalTrials.gov {"type":"clinical-trial","attrs":{"text":"NCT02784444","term_id":"NCT02784444"}} NCT02784444 ), involving over 400 subjects with biopsy proven NASH (CRN stage 2 or 3), that treatment with MSDC-0602K significantly improved glycemic control and liver enzyme levels compared to the placebo arm. Furthermore, MSDC-0602K treatment was well tolerated and resulted in a dose-dependent improvement in liver histopathology, which, although not being statistically significant, supported the continuation to Phase 3 MMONARCh-1 clinical development in NAFLD/NASH patients with T2D (ClinicalTrials.gov {"type":"clinical-trial","attrs":{"text":"NCT03970031","term_id":"NCT03970031"}} NCT03970031 ).
Lanifibranor is an oral small molecule that activates all three PPAR isoforms (PPARα, PPARδ and PPARγ), inducing anti-fibrotic, anti-inflammatory and other beneficial metabolic changes in the body, and delivers these outcomes by decreasing triglyceride levels and increasing high-density lipoprotein cholesterol levels and insulin sensitization ( Figure 2 ). Developed by Inventiva, it is the only pan-PPAR agonist in clinical development for NASH. The company completed a 24-week Phase 2b NATIVE clinical trial (ClinicalTrials.gov {"type":"clinical-trial","attrs":{"text":"NCT03008070","term_id":"NCT03008070"}} NCT03008070 ) and demonstrated that the higher dose used in this study (1200 mg per day) reduced by at least two points the steatosis activity fibrosis (SAF) score (SAF is a measure that combines the degree of hepatocellular inflammation and cellular ballooning) with no worsening of fibrosis (primary endpoint). The study also met secondary endpoints with the same dose, which included resolution of NASH with no worsening of fibrosis and improvement of fibrosis without worsening of NASH. Inventiva was pleased to be able to highlight that Lanifibranor is the only candidate drug to achieve statistically significant results for both of the primary endpoints specified by the FDA and the EMA, a key deliverable for those companies and drugs seeking accelerated approval during Phase 3 development. The FDA responded to this strong 2b data set by indicating that a single pivotal Phase 3 study could be sufficient to support the filing of an NDA in the United States. This feedback from regulators, together with Breakthrough Therapy designation in NASH, encouraged the initiation of a Phase 3 NATIVE3 pivotal clinical trial with Lanifibranor, which is expected to recruit its first patients sometime in 2021.
Efruxifermin (EFX), formerly AKR-001, is an analogue fusion protein of FGF21, which is a member of the fibroblast growth factor family that regulates multiple metabolic pathways and cellular processes, 27 and was “designed” by Akero to increase insulin sensitivity, improve lipoproteins, reduce liver fat and inflammation and reverse levels of fibrosis ( Figure 2 ). The decision to continue EFX development in 2020 was taken after positive histological results demonstrated a reduction in liver fat and improvement in liver fibrosis, obtained in a 16-week Phase 2a BALANCED study in NASH patients. 28 Akero now intends to evaluate the efficacy of EFX in NASH patients in a Phase 2b/3 adaptive clinical trial. This two-part study will be composed of a 24-week Phase 2b HARMONY trial that has already started and is designed to select an appropriate patient dose, which will then be used to inform and further evaluate their drug in a more expansive Phase 3 study.
Tesamorelin is an FDA-approved medication for the treatment of HIV-associated lipodystrophy developed by Theratechnologies. Tesamorelin is a synthetic form of growth-hormone-releasing hormone (GHRH), which participates in a wide range of physiological pathways, including the stimulation of the growth hormone secretion from the pituitary gland. 29 Data presented by the company from a 12-month clinical trial evaluating the effect of Tesamorelin on liver fat and steatohepatitis in HIV-infected NAFLD patients (ClinicalTrials.gov {"type":"clinical-trial","attrs":{"text":"NCT02196831","term_id":"NCT02196831"}} NCT02196831 ) demonstrated that participants treated with this compound had a greater reduction in hepatic fat fraction compared to those receiving placebo ( Figure 2 ). 30 In December 2020, the FDA approved the continuation of Tesamorelin to Phase 3 development for NASH treatment and recommended a meeting with the company to discuss the details of the proposed trial design. The study is expected to start in Q3 2021 and will enroll patients with liver-biopsy confirmed NASH (CRN stage 2 or 3). After 18 months of treatment with Tesamorelin or placebo, a second liver biopsy will be performed and NASH resolution with no worsening of fibrosis or fibrosis improvement ≥1 stage with no worsening of NASH will be compared between both groups (primary endpoints). Theratechnologies is planning its strategy to get approval from the EMA to initiate a Phase 3 clinical trial of Tesamorelin for NASH treatment also in Europe.
Anti-Diabetic Drugs for NASH Treatment
NAFLD is closely associated with T2D, 31 but despite this established clinical link there is currently no go-to pharmacotherapy for NASH patients with T2D. Pioglitazone (Actos), a PPARγ agonist developed by Takeda Pharmaceutical Company Limited, is the only recommended medication for patients with both pathologies. However, the FDA and European Association for the Study of the Liver (EASL) have voiced several safety concerns that have prevented any enthusiastic support for its wide clinical use. Pioglitazone in combination with vitamin E improved liver histology in patients with NASH and T2D (ClinicalTrials.gov {"type":"clinical-trial","attrs":{"text":"NCT01002547","term_id":"NCT01002547"}} NCT01002547 ). 32 In this regard, a Phase 3 PIVENS study (ClinicalTrials.gov {"type":"clinical-trial","attrs":{"text":"NCT00063622","term_id":"NCT00063622"}} NCT00063622 ) demonstrated that treatment with vitamin E showed better results than placebo in improving liver histology in NASH patients without diabetes. 33 Although the EASL guideline states that vitamin E may be used in non-cirrhotic and non-diabetic NASH patients, they advise that further studies are required for firm recommendations to be made concerning extending vitamin E usefulness.
Currently, several new diabetic agents are in clinical trials for NASH treatment, including sodium-glucose co-transporter-1/2 (SGLT1/2) inhibitors and glucagon-like peptide 1 receptor (GLP-1R) agonists ( Figure 2 ). 34 Ahead of these newer agents, Dapagliflozin, Liraglutide, Dulaglutide and Semaglutide are examples of already approved anti-diabetic therapies that are now under late-stage consideration for NASH. Of those molecules that appear to be progressing well, Dapagliflozin, developed by Bristol-Myers Squibb and AstraZeneca Ltd, is currently in Phase 3 and is an inhibitor of SGLT2, which in turn impedes glucose reabsorption in the proximal tubule leading to glucosuria and plasma glucose reduction ( Figure 1 ). The Dapagliflozin DEAN study (ClinicalTrials.gov {"type":"clinical-trial","attrs":{"text":"NCT03723252","term_id":"NCT03723252"}} NCT03723252 ) is still recruiting and will evaluate its safety and efficacy on NASH histological improvement in approximately 100 participants.
Semaglutide, developed by Novo Nordisk A/S, is approved for the treatment of T2D, functions as an agonist of GLP-1R and plays an essential role in insulin secretion ( Figure 2 ). 34 A 72-week Phase 2 clinical study was performed using Semaglutide to evaluate the efficacy and safety of three different doses of subcutaneous administration versus placebo in 320 subjects with biopsy-confirmed NASH and liver fibrosis stages 1, 2 or 3 (ClinicalTrials.gov {"type":"clinical-trial","attrs":{"text":"NCT02970942","term_id":"NCT02970942"}} NCT02970942 ). For this study: (i) NASH resolution without worsening of fibrosis and (ii) improvement of at least one fibrosis stage with no worsening of NASH were defined as primary and secondary endpoints. Only patients with stage 2 or 3 fibrosis levels were assessed in this cohort (230 individuals). A fuller range of analyses were performed on all 320 patients and indicated that the higher dose of Semaglutide (0.4 mg per day) resulted in a significantly higher percentage of patients with NASH showing resolution of the condition with no worsening of fibrosis when compared to placebo. It is also worth noting, however, that no significant differences were observed between the different doses used and the percentage of patients with an improvement in fibrosis staging, including the placebo control. 35 The company has recently started recruiting patients with non-cirrhotic NASH in a Phase 3 clinical trial (ClinicalTrials.gov {"type":"clinical-trial","attrs":{"text":"NCT04822181","term_id":"NCT04822181"}} NCT04822181 ) that is expected to last for approximately 5 years ( Figure 1 ). In addition, Novo Nordisk and Gilead Sciences Inc have recently completed an extensive Phase 2a clinical trial (ClinicalTrials.gov {"type":"clinical-trial","attrs":{"text":"NCT03987074","term_id":"NCT03987074"}} NCT03987074 ) that evaluates the safety, tolerability, and efficacy of Semaglutide alone or in combination with Cilofexor (FXR agonist) and/or Firsocostat (ACC inhibitor), in more than 100 NASH patients with a fibrosis score of 2 or 3 ( Figure 2 ). All combinations were well tolerated, and a statistically significant improvement in hepatic steatosis and liver injury was observed in the combination arms compared to Semaglutide alone (at 24 weeks in post-hoc analysis). Novo Nordisk and Gilead have planned a further Phase 2b study beginning in the second half of 2021 to analyze the effect of Semaglutide alone and in combination with Cilofexor and/or Firsocostat on liver fibrosis improvement and NASH resolution in 440 NASH patients with compensated cirrhosis.
Discontinued Therapeutics for NASH
Over the last few years, several pharmaceutical companies have failed to find a drug for the treatment of NASH typically because of a lack of efficacy, toxicity or elements of both. Two higher-profile medications that were discontinued after failing Phase 3 clinical trials were Selonsertib (Gilead) and Elafibranor (Genfit SA). Selonsertib is an oral small molecule inhibitor of the apoptosis signal-regulating kinase 1 (ASK1) and eventually failed in two different Phase 3 trials, STELLAR-3 (ClinicalTrials.gov {"type":"clinical-trial","attrs":{"text":"NCT03053050","term_id":"NCT03053050"}} NCT03053050 ) and STELLAR-4 (ClinicalTrials.gov {"type":"clinical-trial","attrs":{"text":"NCT03053063","term_id":"NCT03053063"}} NCT03053063 ). Both studies enrolled over 1600 patients in total and were designed to evaluate the safety and efficacy of Selonsertib when used to treat NASH patients with stage 3 fibrosis (STELLAR-3) or compensated cirrhosis, stage 4 fibrosis (STELLAR-4). Although Selonsertib was generally well tolerated with no safety concerns, none of the studies achieved their primary endpoint of an improvement in fibrosis without worsening of NASH (after 48 weeks of treatment). 36 Gilead also tested Selonsertib as part of a combination therapy with Firsocostat and Cilofexor in a Phase 2b ATLAS clinical trial (ClinicalTrials.gov {"type":"clinical-trial","attrs":{"text":"NCT03449446","term_id":"NCT03449446"}} NCT03449446 ) with NASH patients with advanced fibrosis (CRN stage 3 or 4). Again, all treatments, either alone or in dual combination, were well tolerated but none met their primary endpoint of reducing fibrosis without worsening of NASH. However, improvements in multiple secondary endpoints involving measurements of fibrosis and liver function were observed in patients treated with both Firsocostat and Cilofexor, when compared with the placebo group. 37
Until recently, Genfit was considered the most advanced competitor to Intercept Pharmaceuticals Obeticholic acid, with its own oral treatment, Elafibranor, which acts simultaneously on nuclear receptors shown to play critical roles in the development of NASH, PPARα and PPARδ. Unlike other small molecules targeting PPARs, Elafibranor did not demonstrate the adverse effects commonly associated with PPARγ activation, with good safety and tolerability profiles in patients. In Genfit’s large international Phase 2b trial, Elafibranor delivered NASH resolution without worsening of fibrosis and improved the metabolism of triglycerides and lipids in patients with advanced disease. 38 This success encouraged the company to design a 72-week Phase 3 RESOLVE-IT clinical trial (ClinicalTrials.gov {"type":"clinical-trial","attrs":{"text":"NCT02704403","term_id":"NCT02704403"}} NCT02704403 ) to evaluate the efficacy and safety of Elafibranor, compared to placebo, in more than 1000 patients with NASH and fibrosis. Unfortunately, Genfit was disappointed to report in May 2020 that the study did not meet its key primary endpoints. Although both safety and tolerability profiles of Elafibranor were good, only 19.2% of the patients who received this drug achieved NASH resolution without worsening of fibrosis, compared to 14.7% of patients in the placebo arm. For fibrosis, the results were even starker, with 24.5% of Elafibranor-treated patients showing fibrosis improvement of at least one stage compared to an almost comparable 22.4% of patients in the placebo arm. No statistically significant differences between Elafibranor and placebo groups were found in these or any other metabolic parameters. Although Genfit will continue to develop Elafibranor for the treatment of PBC, the company has decided to terminate its Phase 3 RESOLVE-IT trial. Genfit has not abandoned liver disease completely as they are still committed to testing the anti-fibrotic properties of the repurposed Nitazoxanide, originally considered an anti-parasitic drug, and are now in a Phase 2 study with patients with NASH-induced stage 2 or 3 staging of fibrosis ( Figure 2 ). In addition, Genfit is seeking regulatory approval for a new diagnosis and disease management technology, NIS4™, which claims to be a non-invasive test capable of identifying patients at highest risk of liver disease, based on the quantification of specific biomarkers present in their blood when considered in combination with a series of, patient specific, metabolic risk factors.
Antibodies in Development for NASH
Most treatments for NASH that have made it all the way to clinical trials are based on the use of small molecules, typically inhibitors or agonists. In other disease areas such as inflammation or oncology, monoclonal antibodies (mAbs) have slowly begun to dominate as the “go-to” class for drug therapy with drugs such as Humira ® selling in excess of $100 B during its patent protected lifespan. However, for the treatment of metabolic diseases such as NASH, the jury is still out on the ability or potency of mAbs to have the same impact on disease progression. To date, only a few mAb therapies have made it all the way to clinical studies ( Table 1 ). The efficacy of Simtuzumab, a monoclonal antibody developed by Gilead and directed against lysyl oxidase-like-2 (LOXL2), an amine oxidase that promotes liver fibrosis by driving covalent crosslinking of collagen fibers, 39 , 40 was evaluated in two Phase 2b clinical trials involving patients with advanced NASH. Unfortunately, both studies were stopped after 96 weeks due to a lack of efficacy. 41 Tiziana Life Sciences Plc terminated their Phase 2 NASH study, designed to evaluate the efficacy of Foralumab. This orally administered mAb recognized CD3, a member of the immunoglobulin superfamily, which acts as a mediator for signal transduction and is expressed by a high-percentage of circulating peripheral T cells. 42 Another orally delivered mAb that failed to meet its clinical end points for NASH was IMM-124E. Developed by Immuron Ltd, this polyclonal antibody mixture was specific to lipopolysaccharide (LPS) and other pathogenic bacterial components in the human gastrointestinal tract. Data from its Phase 2 clinical trial (ClinicalTrials.gov {"type":"clinical-trial","attrs":{"text":"NCT02316717","term_id":"NCT02316717"}} NCT02316717 ) showed that treatment with IMM-124E did not produce any evidence of clinical benefit and was not able to reduce the fat content of the liver in NASH patients, but it did decrease serum LPS levels, as well as two biomarkers associated with liver function (AST, ALT). The end came for IMM-124E when negative results were also reported in a second Phase 2 clinical study involving pediatric NAFLD patients (ClinicalTrials.gov {"type":"clinical-trial","attrs":{"text":"NCT03042767","term_id":"NCT03042767"}} NCT03042767 ). This study also failed to meet its primary endpoint, showing no substantial changes in ALT levels when compared to the placebo control arm.
Antibodies in Clinical Development for the Treatment of NASH
The flexibility and potency of mAb drugs continue to be improved with format refinements often originally made as treatments for oncology, eventually being adopted by drug-discovery teams tackling other disease classes. One such refinement or improvement is the ability of a single mAb (or mAb-related drug format) to recognize and bind two different drug targets. This bi-specific capability can aid therapeutic potency, specificity and reduce off-target side effects. BFKB8488A (Genentech Inc) is a full-length, humanized bispecific antiFGFR1c/KLB agonist antibody that selectively activates FGFR1 in a KLB-dependent manner and mimics the actions of FGF21. 43 Genentech has already demonstrated in a Phase 1b clinical trial (ClinicalTrials.gov {"type":"clinical-trial","attrs":{"text":"NCT03060538","term_id":"NCT03060538"}} NCT03060538 ) that the subcutaneous injection of BFKB8488A was safe and adequately tolerated, with nausea as the most significant adverse effect. Early indicators of efficacy were also reported with improved markers of cardiometabolic and liver health observed in patients with both T2D and NAFLD, with a dose- and exposure-dependent reduction of liver fat in the NAFLD cohorts in particular. 43 Encouraged by these results, Genentech has recently initiated a Phase 2b BANFF clinical trial (ClinicalTrials.gov {"type":"clinical-trial","attrs":{"text":"NCT04171765","term_id":"NCT04171765"}} NCT04171765 ) to evaluate the efficacy, safety and pharmacokinetics of BFKB8488A in NASH patients with a fibrosis score between 2 and 3 ( Figure 2 and Table 1 ).
NGM313 is a monoclonal antibody, originally developed by NGM Biopharmaceuticals Inc, that selectively activates FGFR1c/KLB by binding to a unique epitope of KLB and acts as an insulin sensitizer. NGMBio’s Phase 1b clinical study demonstrated that a single subcutaneous dose of this antibody significantly reduced liver fat content and improved multiple metabolic parameters in obese, insulin-resistant subjects with NAFLD. Merck & Co Inc in-licensed NMG313 in 2019 (renamed MK-3655) and has recently started recruiting pre-cirrhotic NASH patients, with or without diabetes, into a Phase 2b clinical study to analyze MK-3655 effects on NASH resolution without worsening of fibrosis, after 52 weeks of treatment ( Figure 2 and Table 1 ). Hopefully, these “next-generation” mAbs from Genentech and Merck with boosted specificity and potency will succeed where others have failed, and we may also see the first-approved antibodies for the treatment of metabolic diseases such as NASH.
Chemomab is developing CM-101, a blocking mAb that targets CCL24, which is a chemokine that modulates inflammatory and fibrotic processes through its receptor CCR3. 44 CM-101 was tested in a 12-week Phase 1b clinical trial to evaluate its safety, tolerability, pharmacokinetics and pharmacodynamics in NAFLD patients with a follow-up study planned in a similar cohort of NASH patients. Results communicated by the company in January 2021 demonstrated that treatment with CM-101 decreased serum biomarkers of fibrosis and inflammation with no issues with safety or tolerability. Chemomab has recently started enrolling patients in a Phase 2a SPLASH clinical trial that will evaluate the effects of CM-101 in 40 NASH patients with fibrosis stage 2 or 3 ( Figure 2 and Table 1 ). Data from this study are expected in H1 2022.
Finally, Nimacimab, also known as RYI-018 or JNJ-2463, is another antibody at a much earlier clinical stage of development ( Figure 2 and Table 1 ). Nimacimab (Bird Rock Bio Inc) is an antagonist antibody with potentially anti-fibrotic, anti-inflammatory and a beneficial metabolic mechanism of action. Nimacimab targets the cannabinoid receptor 1 (CB1), a G-protein coupled receptor, which has been reported to play a role in hepatic inflammation and fibrosis. 45 Bird Rock Bio successfully completed a Phase 1b clinical trial that evaluated the safety, tolerability and pharmacokinetics of their antibody in NAFLD patients and their intention to conduct a Phase 2b clinical study has been confirmed.
At a much earlier point in their development (late stage pre-clinical) than any of the studies described above, companies such as Elasmogen Ltd are using the power and flexibility of their soloMER drug discovery platforms to produce smaller antibody-like biologics for the treatment of later stage liver disease, and fibrosis in particular. Whilst the details of this program remain confidential, the use of bi- and tri-specific formats has been disclosed, including the use of binding domains that employ receptor-mediated internalization to enter cells, facilitating the delivery of small molecules conjugated to their biologic targeting domains. This strategy delivers the promise of increasing potency still further through the combination of biologic and small molecule drugs as a single targeted therapy.
Multiple Points of Intervention – Which Drug-Target Class is “Winning”
Possible winners – going it alone.
NAFLD is a complicated, heterogeneous disease, with multiple possible points of therapeutic intervention. In general, therapeutic candidates currently in the clinic are focused on modulating metabolic pathways, reducing inflammation and/or fibrosis. 46 PPAR agonists represent a targeted therapy that reduces steatosis and inflammation. Several drugs that modulate the activity of these nuclear receptors have been investigated as treatments for NASH in patients ( Figure 2 ). Two of the most advanced, Elafibranor (Genfit) and Seladelpar (CymaBay Therapeutics Inc), did not meet their primary endpoints, were discontinued and together throw into question the whole approach of using PPAR agonists as a NASH treatment class. However, Inventiva has recently caused a re-evaluation of the field by reporting positive results (June 2020) from their Phase 2 NATIVE trial with Lanifibranor, a pan-PPAR agonist.
FXR ligands are a second class of molecules also capable of reducing the levels of both inflammation and fibrosis in patient trials. Several FXR-activating medications are currently under clinical development, each with slightly different structures and pharmacodynamic effects ( Figure 2 ). The most promising, with a clinically demonstrated positive effect on hepatic metabolism, is Obeticholic acid. Unfortunately, Intercept Pharmaceuticals, the drug’s developers, received a less than positive response from the FDA in June 2020 with potential liver toxicity flagged as a possible barrier to approval and a request for further clinical data issued. FGF analogs, closely related to FXR agonists, are also being evaluated as a potential NASH therapy. So far, treatment with several FGF19 and FGF21 agonists (both small molecules and bi-specific mAbs) has shown improvement in several metabolic parameters and a reduction in liver fat ( Figure 2 ). Thus, Efruxifermin (Akero), Pegbelfermin (Ambrx Inc/Bristol Myers Squibb), BFKB8488A (Genentech), Aldafermin (NGM Biopharmaceuticals) and MK-3655 (Merck & Co) are currently being studied in Phase 2b development ( Figure 2 ). It is noteworthy that Aldafermin has recently failed a Phase 2b ALPINE 2/3 study for NASH patients with stage 2 or 3 of liver fibrosis, but NGM Biopharmaceuticals are still evaluating this drug in a Phase 2b ALPINE 4 trial in NASH patients with severe fibrosis and compensated fibrosis. Within this target class, there is a secondary race happening between mAbs and small molecule inhibitors. In other disease areas, mAbs have been shown to deliver improvements in specificity (and potency), safety profile, and dosing frequency (4-week serum half-life), and whilst clinical development times can be shorter than small molecules, mAbs are also large, complex and more costly to manufacture at scale, especially where newer mAb formats prove to be the candidate molecule of choice.
Antagonistic drugs, such as VK2809 (Viking Therapeutics Inc) and Resmetirom (Madrigal Pharmaceuticals), targeting liver THRβ are gaining clinical traction. Now in Phase 2b and Phase 3, respectively, both have reported positive results, especially the reduction of lipids. Currently, in a class on its own, Aramchol (Galmed) has a unique mechanism of action, modulating the activity of SCD1. Although this is a target that other companies have yet to fall behind, the data so far suggest that this strategy is capable of reducing key metabolic markers of NASH and even fibrosis staging levels.
Whilst much of this review has focused on the treatment of earlier stage disease, the lack of reliable diagnostic tests (currently biopsy remains the “gold-standard”) means that patients continue to present with late-stage disease. Therefore, anti-fibrotic therapies targeting stellate cell activation or extracellular matrix production will still be required for some time to come. Allergan will need to indicate future plans for CVC after the termination of the Phase 3 AURORA trial, Galectin Therapeutics is currently evaluating Belapectin in a Phase 2b clinical study and new entrants, such as Elasmogen Ltd with their soloMER platform, are also developing anti-fibrotic/anti-stellate therapies.
Co-Therapies – Working Together to Win the Race
Although most NASH clinical trials are based on a single agent (mono) therapy, the complex pathophysiology of this disease and the multiple often redundant “escape” pathways to therapy, strongly suggest that the development of a single drug capable of effectively treating most patients is becoming increasingly challenging and unlikely. 47 Hence, the combination of therapies with different but complementary mechanisms of actions is now being considered by more and more drug developers as the best route to enhance efficiency, slow disease progression or even reversing NASH ( Figure 2 ). Several drugs are currently being tested in combination with FXR agonists. The combinations of Semaglutide/Cilofexor/Firsocostat (Novo Nordisk and Gilead), Tropifexor/CVC (Allergan and Novartis) and Selonsertib/Cilofexor/Firsocostat (Gilead, now discontinued) have all been discussed in more detail above. In other drug combination studies, Novartis is recruiting 380 NASH patients with stage 2 or 3 CRN fibrosis to test Licogliflozin, an SGLT1/2 inhibitor, and Tropifexor alone or in combination, in a Phase 2 ELIVATE clinical study (ClinicalTrials.gov {"type":"clinical-trial","attrs":{"text":"NCT04065841","term_id":"NCT04065841"}} NCT04065841 ), which is expected to complete in 2023. Metacrine Inc is evaluating another FXR agonist, MET409, in a Phase 2a combination trial (ClinicalTrials.gov {"type":"clinical-trial","attrs":{"text":"NCT04702490","term_id":"NCT04702490"}} NCT04702490 ) with Empagliflozin (Jardiance), a SGLT2 inhibitor, in patients with T2D and NASH. Results are expected in the fourth quarter of 2021. Pfizer Inc has considered combination therapies as well and assessed the effect of PF-05221304 (an ACC inhibitor) and PF-06865571 (a DGAT2 inhibitor) on whole-liver fat in subjects with NAFLD compared to placebo in a short, 6-week Phase 2a clinical trial (ClinicalTrials.gov {"type":"clinical-trial","attrs":{"text":"NCT03776175","term_id":"NCT03776175"}} NCT03776175 ). Both drugs alone and in combination significantly reduced liver fat when compared to placebo, but unfortunately, there was no evidence of additivity (or synergy) with no significant differences between each monotherapy, when compared with the combination arm. At the end of 2020, Pfizer communicated that PF-05221304 was no longer in its pipeline and it was discontinued as a monotherapy. Furthermore, Pfizer has recently included a Phase 1 clinical trial in their pipeline to evaluate PF-06865571 in combination with PF-06882961 (Danuglipron), a GLP-1R agonist, for the treatment of NASH.
Clinical trials involving monotherapies are still much higher in number than combination studies and are typically further in their clinical drug journey than most combination studies. However, we would predict that our growing understanding of the complexity of liver disease will almost certainly drive an increase in business development activity and the signing of more agreements between pharmaceutical companies eager to combine their, hopefully, complementary therapeutic agents.
Liver disease represents one of the greatest areas of unmet medical need in the developed world, with the lack of a reliable and user-friendly diagnostic and the absence of tailored drug therapies combining to drive a relentless increase in the number of patients globally. Clinical intervention is woefully inadequate and is still often limited to changes in life-style advice, a situation that has altered little in over 10 years. However, what has changed in the last decade is the development of numerous therapies in later stage clinical trials that could offer real hope, and soon, to patients struggling with metabolic liver diseases, such as NASH and NAFLD. The complex and heterogeneous nature of these diseases has generated a portfolio of drug classes (from small molecule inhibitors to antibodies), each being evaluated as the first approved therapy specific to NASH. Whilst one of the pharmaceutical companies described in this review may indeed be a winner in the race for the first universally accepted medication for NASH as a monotherapy, it is likely that this will simply be the end of the beginning rather than the beginning of the end for NASH, NAFLD and liver disease therapeutic options. The plethora of drug classes currently being evaluated and the possible benefits of combining agents with different and complementary mechanisms of action will keep this area of drug discovery active and productive for another 10 years at least.
Caroline Barelle, Andrew J Porter, and Marina Kovaleva are employees of Elasmogen Ltd. Soumya Palliyil is an employee of University of Aberdeen. Julia M Fraile reports being a Knowledge Transfer Partnership-Associate (Innovate UK) between the University of Aberdeen and Elasmogen Ltd, during the conduct of the study. Andrew J Porter reports personal fees from Elasmogen Ltd, during the conduct of the study. The authors report no other potential conflicts of interest in this work.

IMAGES
VIDEO
COMMENTS
Last week John Nash, the Nobel Prize-winning mathematician, and subject of the blockbuster film A Beautiful Mind, passed away at the age of 86. He died in a taxi cab accident in New Jersey. Days later, Cliff Pickover highlighted a curious factoid: When Nash wrote his Ph.D. thesis in 1950, "Non Cooperative Games" at Princeton University, ...
Joun NASH. (Received October 11, 1950) Introduction. Von Neumann and Morgenstern have developed a very fruitful theory of two-person zero-sum games in their book Theory of Games and Economic Be- havior. This book also contains a theory of n-person games of a type which we would call cooperative. This theory is based on an analysis of the ...
John Forbes Nash, Jr. (June 13, 1928 - May 23, 2015), known and published as John Nash, was an American mathematician who made fundamental contributions to game theory, real algebraic geometry, ... Nash earned a PhD in 1950 with a 28-page dissertation on non-cooperative games. ...
The essence of Nash's brilliance. What made Nash's dissertation extraordinary was its concept of equilibrium in "non-cooperative" games.A significant departure from traditional theories that primarily focused on "cooperative" games where participants can make binding agreements. His work argued that in any game involving two or more players, each with their set of strategies, there ...
NON-COOPERATIVE GAMES. John F. Nash. Published in Classics in Game Theory 1 September 1951. Mathematics. Classics in Game Theory. we would call cooperative. This theory is based on an analysis of the interrelationships of the various coalitions which can be formed by the players of the game. Our theory, in contradistinction, is based on the ...
Nash, in his dissertation research at Princeton (published in this and three other papers), extended game theory to n-person games in which more than one party can gain, a better reflection of practical situations. Nash demonstrated that "a finite non-cooperative game always has at least one equilibrium point" or stable solution. This ...
First edition, offprint issue of Nash's doctoral thesis, formulating the theory of non-cooperative games and describing the Nash equilibrium, for which he was awarded the 1994 Nobel Prize in Economic Sciences. In his biographical essay for the Nobel Prize, Nash humbly noted, "As a graduate student I studied mathematics fairly broadly and I was ...
At Princeton, Nash added to his prodigious achievements, finishing his dissertation - the work on non-cooperative games and equilibrium that would bring him the Nobel Prize - in his second year. (Nash also invented the board game Hex, a game independently created by Danish mathematician Piet Hein.)
cf. [44], Nash was prepared for the possibility that the game theory work would not be regarded as acceptable as a thesis at the Princeton mathematics department.
Abstract. John F. Nash, Jr., submitted his Ph.D. Dissertation entitled Non-cooperative games to Princeton University in 1950. Read it 58 years later, and you will find the germs of various later developments in game theory. Some of these are presented below, followed by a discussion concerning dynamic aspects of equilibrium.
http://www.jstor.org Non-Cooperative Games Author(s): John Nash Source: The Annals of Mathematics, Second Series, Vol. 54, No. 2, (Sep., 1951), pp. 286-295
10.1 Introduction. John Forbes Nash Jr was born in 1928 in Bluefield, West Virginia, USA. His father was a World War I veteran and electrical engineer who had come to Bluefield from Texas to work for a power company, while his mother Virginia was a native of Bluefield and was an English and Latin teacher.
These notes leave aside Nash's celebrated PhD thesis on game theory and focus on the remaining four fundamental papers that have started an equal number of revolutions in their respective topics, namely the 1952 note on real algebraic varieties, the 1954 paper on C 1 isometric embeddings, the 1956 subsequent work on smooth isometric ...
After his famous PhD thesis in game theory (and a few companion notes on the topic) Nash. directed his attention to geometry and specifically to the classical problem of embedding. smooth ...
John Nash's Dissertation. Non-cooperative Games, May 1950, is available in PDF format Non-Cooperative_Games_Nash.pdf.The dissertation is provided for research use only. [Note: Chapter 6 of The Essential John Nash, edited by Harold W. Kuhn and Sylvia Nasar (Princeton, New Jersey: Princeton University Press, 2001) contains a facsimile of Nash's 1950 Ph.D. dissertation on non-cooperative games.]
Project Details. In this page you can find Nash's PhD thesis: Original document. Transcribed into tex/pdf *. *: Thanks to Rebeca Duarte Miguel for this, and to Jeek Midford for spotting some spelling mistakes.
John F. Nash, Jr., submitted his Ph.D. dissertation entitled Non-Cooperative Games to Princeton University in 1950. Read it 58 years later, and you will find the germs of various later developments in game theory. Some of these are presented below, followed by a discussion concerning dynamic aspects of equilibrium.
In the Ron Howard movie "A Beautiful Mind," Russell Crowe plays John Nash, a major figure in game theory. Although it was a good movie, it totally misconstrues Nash's doctoral dissertation and how it fit into the pre-existing literature. In this episode, Bob sets the record straight. Mentioned in the Episode and Other Links of…
Nash's dissertation has been cited about 7,189 times, according to Google Scholar, though that excludes many uses of the term "Nash equilibrium" that don't cite the actual paper.
Nash Flows Over Time Dissertation, Berlin 2020 Reviewers: Prof. Dr. Martin Skutella, Prof. Dr. José Correa and Prof. Dr. Neil Olver Technische Universität Berlin Combinatorial Optimization & Graph Algorithms Institute of Mathematics Straße des 17. Juni 136 10623 Berlin. Acknowledgments
Dwayne A. Nash This dissertation examines the origins and social impact of New York's stop-and-frisk law, which authorizes police to stop, question and frisk people without a warrant or probable cause to believe crime was committed. Several observers associate it with a recent history of
Table of Contents List of Tables vii List of Figures ix Abstract xii Chapter 1: Introduction 1 1.1 Path Planning ...
What was John Nash's thesis? Nash earned a PhD in 1950 with a 28-page dissertation on non-cooperative games. The thesis, written under the supervision of doctoral advisor Albert W. Tucker, contained the definition and properties of the Nash equilibrium, a crucial concept in non-cooperative games.
Introduction. Non-alcoholic steatohepatitis (NASH) is a severe form of non-alcoholic fatty liver disease (NAFLD), characterized by the presence of liver inflammation and hepatocyte injury (ballooning) due to fat accumulation.1 Although it develops typically in the absence of excessive alcohol consumption, NAFLD is related to an unhealthy diet and a lack of physical activity.
Thesis. Dec 2021; Gbenga Ayodele Owolabi; ... David H Nash; Margaret Stack; The scale of modern blades means that tip speeds in excess of 100ms -1 are now common in utility scale turbines ...
A Dissertation by LANDON DANIEL NASH Submitted to the Office of Graduate and Professional Studies of Texas A&M University in partial fulfillment of the requirements for the degree of DOCTOR OF PHILOSOPHY Chair of Committee, Duncan J. Maitland Committee Members, Elizabeth Cosgriff-Hernandez Balakrishna Haridas David Staack