

Self Inflating Balloon: Baking Soda and Vinegar Balloon Experiment
This post may contain affiliate links.
Have you ever tried the baking soda and vinegar balloon experiment? This classic science experiment is really one of my favorites. It is an easy science experiment to do and it really is exciting to watch. It creates a self-inflating balloon that kids think is the coolest!
The reaction between the baking soda and vinegar cause the balloon to inflate all on its own! It is a show-stopper experiment for kids.
(See more of my STEM projects for kids.)

How to Do the Baking Soda and Vinegar Balloon Experiment:
Supplies you will need for this simple science experiment:.

Plastic empty water bottle or soda bottles, cleaned out Large Balloon White Vinegar (acetic acid) Baking Soda (or sodium bicarbonate) Small Funnel Spoon
TIP: Before starting the experiment, you will want to stretch out the balloon to make it more loose and easier to inflate.
Step 1- Pour 1-2 spoonfuls of baking soda into the opening of the balloon, using a funnel. You’ll need to shake it a bit to get it down into the base of the balloon.
Step 2- Use the funnel again and pour some vinegar into the empty plastic bottle until it is about an inch or two deep- maybe a few tablespoons of vinegar. Exact amounts do not matter. Add a few drops of food coloring to change it up if you want- totally unnecessary.

Step 3- Carefully stretch the opening of the balloon around the mouth of the bottle leaving it hanging down until you are ready for the reaction.
TIP: Don’t let any of the baking soda dump into the bottom of the bottle while attaching it.
Step 4- When you are ready to see the chemical reaction happen, lift up the balloon allowing the baking soda to fall down into the bottle.

This is when the fun starts! Baking soda and vinegar mix to create an awesome chemical reaction. The gas from combining the two will escape as bubbles of carbon dioxide gas that cause the balloon to inflate. It’s impressive. The more gas there is created, the larger the balloon will get.
Your kids, if they are anything like mine, will beg to do the experiment again, then 10 more times! This is really a perfect science project for kids to try on their own.

If you do repeat it, you will need fresh vinegar in the bottle. Once a reaction happens, it is not quite so strong the second time through. The balloon does not usually inflate again unless the vinegar is fresh.My kids were amazed and wanted to do it again and again and again. Stock up on baking soda and vinegar if you are planning this one! Luckily they are both quite inexpensive.
(It’s a good thing they are both so cheap!)
The Science Behind It: Why the Baking Soda and Vinegar Reaction Works?
When the baking soda and vinegar reaction happens, it is an acid-base reaction. Vinegar is the acid and baking soda is the base. This reaction between the two causes a gas called carbon dioxide to bubble and foam. This gas having nowhere else to go, expands the balloon making the self-inflating balloon happen.
Here is the chemical equation behind it: Baking soda + vinegar — yields carbon dioxide + water + sodium ion + acetate ion There is more to it than that, but that’s the basic explanation.
Try some variations to see if other reactions work:
Will baking powder work instead of baking soda?
Would lemon juice work instead of vinegar?
Could you do the same thing with an alka-seltzer tablet and soft drinks?
Use the scientific method to investigate different variations on this experiment to see how they work.
Want More Baking Soda and Vinegar Experiments?
We love the carbon dioxide reactions that these two substances create.
Check out these other ones we have done:
Easy Bottle Rocket Experiment
Bathtub Bottle Rocket
Film Canister Rocket Experiment
How to Make a Volcano experiment! (This one is fun because when the eruption occurs, the carbon dioxide bubbles pour over like lava!
Former school teacher turned homeschool mom of 4 kids. Loves creating awesome hands-on creative learning ideas to make learning engaging and memorable for all kids!
Similar Posts

Lunch Time Bingo & Lunch Packing Guide

Roll a Gingerbread Man- Preschool Christmas Game

4th Grade Homeschool Science- Real Science-4-Kids Review

How To Make a Lego Chess Set
Hundred Chart Puzzle with Printable

Fun Ways to Teach Division to Kids
What a great idea! I can’t believe I haven’t heard of this experiment. My girls are 12 and 11 and still love doing at home science projects. Although they use Time4Learning science curriculum it is always fun to do your own.
Thanks for the idea!
Awesome! I’m making a list of simple, fun experiments to do this summer, and I’m adding this one to it! We don’t seem to get to these types of experiments during the regular school year! Stopping by from HHH and new follower! Thanks for sharing your experiment!
My boys loved this experiment too. 🙂
- Pingback: Fizzing & Bubbling Science Experiments - Teach Beside Me
I do experiments with 4 year old grandson, he loves this one. We did the volcano as well and now he explains what happens to everyone he wants to show it too. Thank you for sharing, it is fun teaching when the things work as well as yours.
fantastic from a grammy
Nice , it is possible to send easy experiments for kids with the help of video
Leave a Reply Cancel reply
You must be logged in to post a comment.

Self Inflating Balloon Science Experiment: An Easy Science Project To Do With Kids
My son hates blowing up balloons [thanks to a balloon that blew up on his face once]. So this self inflating balloon science experiment is one he likes.
It’s very easy to do too. And if your child is looking for a science project to do this year , you might want to have a look at this experiment.
Topics : chemistry, air, carbon dioxide, balloon experiments , kitchen science
You will need:
- baking soda
- small glass bottle
The Self Inflating Balloon In The Gally Kids Headquarters
We did this experiment one Saturday afternoon when my son was just getting a little bit tired of all the educational experiments and activities we were doing (something I had planned for a series of videos on our Gally Kids Youtube channel ).
So I ended up doing a lot of the work while he watched the experiment (while playing with his Ultra Stealth Rider) and saying “Oh, it’s just like the erupting volcano!”
So yeah. If you have a child who has done an experiment with vinegar and baking soda ( or have one of the preschooler Chemistry science kits – which usually has this experiment included ) you’ll probably get exactly the same reaction.
But we are testing a different thing in this self inflating balloon experiment.
We’re showing the “explosion”. Instead, we’re showing them that a new gas is formed when these two things react with each other.
The baking soda went in first.
Well, to be honest, I did the vinegar first- which is a huuuge mistake! This meant that the funnel got wet and the baking soda wouldn’t get stuck to the funnel instead of flowing straight into the balloon.
So don’t do that.

After the baking soda, put the vinegar in the glass bottle.
Now a side note to the bottle. We used a small bottle here. And I suggest you do the same for a bigger and more spectacular balloon size.

Now comes the tricky part. This is when you put the balloon on the opening of the glass bottle. Young kids might need help with doing this properly.
Make sure that it’s tight and secure, all else, the pressure will cause the balloon to dislodge and all that air will escape.
Also when doing this, don’t let the baking soda fall into the vinegar yet.

And finally, it’s time to pour all that baking soda in.
As soon as they touch each other, a chemical reaction takes place. Bubbling starts and the balloon starts to inflate. I was surprised at how big the balloon inflated.

The Instructions: The Quick Version

- First, using the funnel, pour all the baking soda in the balloon.
- Then put the balloon aside and pour the vinegar into the small bottle using the same funnel.
- Next, carefully fit the balloon into the bottle opening. Make sure the baking soda doesn’t fall in while you’re doing this. And most importantly, make sure the balloon is tight and secure.
- Finally, pour all the baking soda in the vinegar
- Voila! your self-inflating balloon.
Self Inflating Balloon Experiment Video
Explanation.
When baking soda and vinegar mix, a chemical reaction takes place.
This chemical reaction produces carbon dioxide which you can “see” from all the bubbles.
Now all this extra air has nowhere to go except towards the balloon. And as the heavier carbon dioxide pushes the air, it inflates the balloon.
- The size of the inflated balloon will depend on many variables such as the size of the bottle and the vinegar and baking soda ratio.
- Make sure the balloon is tight and secure on the bottle opening or else it might slip off as soon as the reaction takes place releasing the carbon dioxide into the air.
Self-Inflating Balloon Science Experiment
The self-inflating balloon science experiment is a true science experiment that would be perfect for science fairs or a science lesson. What kids learn in this lesson is that different chemical reactions and gasses can be used to inflate balloons. The real question is, will any of the balloons be able to float?

* This post may contain affiliate links for your convenience. Click here for my full disclosure.
What you’ll need for the self-inflating balloon experiment:
- Balloons (1 for each type of inflation material)
- Plastic squeeze bottles (1 for each type of inflation material)
- Baking soda
- Measuring cup
One thing to note before starting is that the yeast balloon takes some time to inflate. You’ll want to get that started before you dive into the other ones so you can compare them at the same time. We waited about 10 minutes after starting our yeast mixture to give it time to inflate before we did the other experiments.
What mixture will inflate a balloon the best?
Hypothesis:
The kids thought that the baking soda and vinegar would make the biggest balloon because they’ve made baking soda paint bombs before, and know the power of this reaction!

How to do the Self-Inflating Balloon Experiment:
To keep this scientific, add the same amount of inflation material into each bottle. We added about 3 tablespoons of hot water into one bottle, vinegar into another, and warm water into the third (for the yeast). We added 1 teaspoon of sugar along with half a yeast packet to the yeast bottle.

On top of each bottle, tape a balloon tightly around the spout so they can’t pop off.
Screw the lid on tightly to the yeast bottle and the hot water bottle.
Fill the cap with baking soda and quickly screw the lid onto the last bottle.

The baking soda and vinegar bottle will inflate the most and the fastest. In fact, ours nearly popped the lid off the bottle and made a huge mess, but we caught it in time. The yeast is a slow-inflating balloon, but it lasted the longest.

The hot water was barely enough air to help the balloon stand up straight.
What Kids Learn in the Self-Inflating Balloon Experiment
This experiment is a classic science experiment with a hypothesis, experiment, and results record. This experiment is simple, yet helps kids understand how the scientific process works. Additionally, who wouldn’t want to learn what the best replacement for helium in balloons is? Of course, some kids might be disappointed to learn that these balloons won’t actually float in the air, but that’s another lesson too! These balloons can’t float because none of the fillings are lighter than air!

Looking for more STEAM (Science, Technology, Engineering, Arts and Math) projects and inspiration?
Can’t get enough STEAM? We’ve got you covered. With the super affordable, super sweet STEAM Kids book! It has a Year’s Worth of Hands-on Science, Technology, Engineering, Art, & Math Activities for Kids. Plus a lot more…. And, you get a free STEAM Kids Valentine’s Day EBook with every purchase of the original STEAM Kids book.
FREE DOWNLOAD
Discover how to get siblings to get along even when all they do is annoy each other with the Sibling “Get Along” Poster Pack!
1 thought on “Self-Inflating Balloon Science Experiment”
This is much easier to do if you use 12-20 oz soda bottles and regular balloons instead of the water balloons pictured here.
Leave a Comment Cancel Reply
Your email address will not be published. Required fields are marked *
Save my name, email, and website in this browser for the next time I comment.

CONNECT WITH ME

Forgetful Momma
Secular Homeschooling
Self Inflating Balloon Experiment
How about some science fun with a balloon ? My seven-year-old was amazed by this. He had no idea what to expect when we started this self inflating balloon experiment.

This post contains affiliate links, see my disclosure policy for more information.
I used a Sharpie to draw a face on our balloon just to give it a little more fun, and to pretend we were growing a monster head with Halloween coming up soon.
Gauge loved this, I don’t know if you can hear him in the video or not, but he says “Did you know this would happen?!” Who knows inflating a balloon could be so much fun.
What You Need:
- baking soda
- water/pop bottle
Science Experiment E-Book
How to make a balloon self inflate
This is a science experiment simple enough for your kids to do themselves. My almost seven-year-old did it with very little help from me.
Start by measuring out half a cup of vinegar.

It is very helpful to have a funnel for the next part: pouring the vinegar into a bottle. A funnel means less will get spilled.

We dried off our funnel and used it to put half a tablespoon of baking soda into our balloon.

Remove the funnel from your balloon, and carefully fit it over the opening of your bottle without spilling any of the baking soda in the bottle. It will cause a reaction before you are ready.

Once you are ready and watching you can flip the balloon up to empty the baking soda into the bottle. The reaction does happen quite fast.
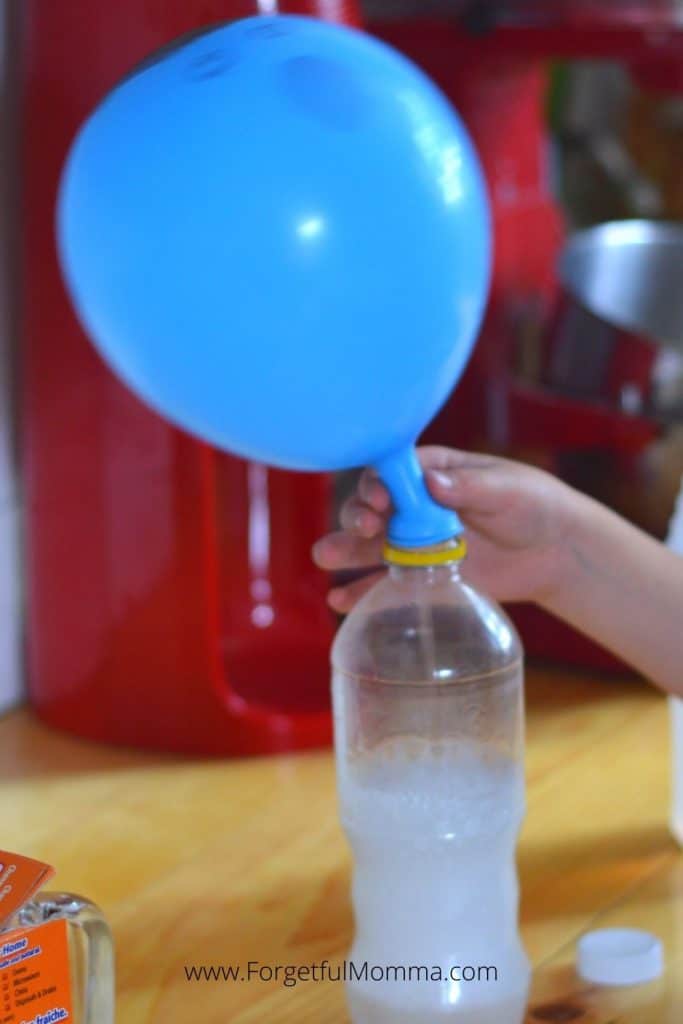
Now you just wait for the reaction. My favorite part of this is that this science experiment uses things you most likely have around your house already.
We have balloons from parties past “floating” around our house, which is probably why I have been finding activities to do with my kids using balloons . Time to use them up before another birthday, and party theme comes around.

What’s Happening:
The secret lies with vinegar and baking soda, two household staples that, when combined, create carbonic acid. Carbonic acid breaks down into water and carbon dioxide.
This experiment uses carbon dioxide to fill the balloon, giving the illusion that the balloon is self-inflating.

Self Inflating Balloon
Easy science experiment for kids using things you have in the kitchen.
- 1/2 cup of vinegar
- 1/2 tsp of baking soda
Instructions
- Pour 1/2 cup of vinegar into an old water or pop bottle.
- put 1/2 tbsp of baking soda into a balloon.
- Carefully, put the opening of the balloon over the water bottle.
- Empty the baking soda into the bottle, and into the vinegar and watch the reaction.
More Science for Kids
Heart Pumping Human Body Science Experiment
Homemade Lava Lamp for your Kids
Wizard’s Brew Fun is Brewing
- Skip to main content
- Skip to primary sidebar
- Amazon Favorites
- Search this website
A Dab of Glue Will Do
Little Learners, Big Ideas
PS PK K 1 2
Self-Inflating Balloon Science Experiment
I'd love it if you shared!
- Pinterest 1.2K

Science is all about testing, re-testing, searching, exploring, and playing. I’m almost convinced most scientists are kids who never quite grew up! Try this Self-Inflating Balloon Science Experiment with your kids today!

Children have a natural instinct to experiment and test, which is why science activities are so much fun with little ones! In the self-inflating balloon science experiment, children can test which chemical reaction produces the reaction that is best at inflating a balloon. What is even better about this experiment is that the science experiment is inexpensive and classroom-friendly. Little ones will have a blast testing all sorts of reactions beyond the three we tested in our example.
Getting the Self-Inflating Balloon Science Experiment Ready

Setting this experiment up is super easy, and it takes very little advanced prep. You will need:
- Balloons (1 for each reaction)
- Plastic bottles (1 for each reaction)
- Plastic bottles
All you have to do in advance is collect the materials and write the type of reaction on each balloon with a permanent marker.

Doing the Self-Inflating Balloon Science Experiment
Heat some water on the stove or in a microwave . If you don’t have access to something to heat water in the classroom, you can always switch out the hot water for yeast and sugar.

Fill one bottle with 5 tablespoons of hot water . Add the “water” balloon to the top.
Fill another bottle with 5 tablespoons of hot water. Drop in an alka-seltzer tablet and cover with the “alka-seltzer” balloon.

Add 5 tablespoons of vinegar to the third bottle. Drop in two tablespoons of baking soda and quickly add the “baking soda” balloon.
Stand back and watch.

In our experiment, the water balloon didn’t inflate much at all. The hot air was not pressurized enough to inflate the balloon. The alka-seltzer tablet filled the balloon a little, but the baking soda and vinegar balloon had the best reaction. We thought the balloon was going to pop off the bottle!
The Science Behind the Self-Inflating Balloon Experiment

Alka-seltzer tablets and baking soda and vinegar both produce the same CO2 reaction. When an acid is mixed with a base, the two mixtures react through an endothermic reaction (a reaction that makes the mixture feel colder), creating CO2 gas which bubbles up through the liquid and out into the air, which inflates the balloon. The heat rising from the hot water was not strong enough to inflate our balloon. However, hotter water or perhaps more water in the bottle might have done the trick.
More Science Activities and Ideas
Walking Water Science for Kids
Science Notebook
Plants Unit
Want science planned for you ALL YEAR LONG?!
Do you want science planned for the ENTIRE CALENDAR YEAR !? This Endless Science Mega Bundle will save you so much time and keep your students engaged and excited about learning . This amazing resource contains 53 science topics including life science , physical science , earth science , and animal studies .
You May Also Enjoy These Posts

Reader Interactions
April 24, 2022 at 2:24 pm
Real clean internet site, thanks for this post.
[…] Learn more: A Dab of Glue Will Do […]
[…] Blowing up balloons with chemistry! […]
[…] Self-Inflating Balloon Science Experiment – (adabofgluewilldo.com) […]
[…] Self-Inflating Balloon […]
[…] Image Source/ Tutorial: A Dab of Glue Will Do […]
Leave a Comment Cancel reply
Your email address will not be published. Required fields are marked *
This site uses Akismet to reduce spam. Learn how your comment data is processed .

The Magic of Self-Inflating Balloons: Fun Science Demo!

At The Kids Point , we believe that learning science can be just as fun as it is educational. Self-inflating balloons are a fantastic way to demonstrate the wonders of chemistry and physics in a way that’s engaging and magical. This demonstration involves a simple chemical reaction that inflates a balloon without the need for a pump. Here’s everything you need to know to create this exciting science demo, perfect for kids and families looking for a hands-on learning experience.
Understanding the Science Behind Self-Inflating Balloons
Self-inflating balloons are a product of a chemical reaction between baking soda and vinegar. When these two substances interact, they produce carbon dioxide gas, which inflates the balloon. To understand this process better, let’s break down the science involved:
- Chemical Reaction : The core of the self-inflating balloon is a simple acid-base reaction. Baking soda (sodium bicarbonate) is a base, and vinegar (acetic acid) is an acid. When mixed, they react to form carbonic acid, which quickly decomposes into carbon dioxide gas and water. The chemical equation for this reaction is: NaHCO3+CH3COOH→CO2+H2O+CH3COONa\text{NaHCO}_3 + \text{CH}_3\text{COOH} \rightarrow \text{CO}_2 + \text{H}_2\text{O} + \text{CH}_3\text{COONa}NaHCO3+CH3COOH→CO2+H2O+CH3COONa
- Gas Production : The carbon dioxide gas produced in this reaction is what causes the balloon to inflate. Carbon dioxide is a gas at room temperature and occupies more space than the solid or liquid reactants, so it pushes against the walls of the balloon, causing it to expand.
- Pressure and Volume : According to Boyle’s Law, if the volume of a gas increases (as the balloon expands), the pressure inside the balloon also increases until it reaches equilibrium with the external pressure. This is why the balloon continues to inflate until it reaches its maximum capacity.
Materials Needed
To demonstrate the magic of self-inflating balloons, you will need the following materials:
- Baking Soda : A common household item and the base in the reaction.
- Vinegar : An acid that reacts with baking soda.
- A Small Balloon : The self-inflating balloon in the demonstration.
- A Small Plastic Bottle : To contain the reaction.
- A Funnel : To help with adding baking soda to the balloon.
- A Measuring Spoon : For accurate measurement of baking soda.
- A Measuring Cup : For vinegar.
How to Make a Self-Inflating Balloon: Step-by-Step Instructions
- Prepare the Balloon : Using a funnel, carefully pour a few tablespoons of baking soda into the balloon. Make sure not to overfill the balloon, as it needs space to expand.
- Prepare the Vinegar Solution : Measure about 1/4 cup of vinegar and pour it into the plastic bottle. You can adjust the quantity depending on how much reaction you want to observe.
- Attach the Balloon : Stretch the mouth of the balloon over the opening of the plastic bottle, but make sure not to let the baking soda fall into the vinegar just yet.
- Initiate the Reaction : Once the balloon is securely attached to the bottle, lift the balloon so that the baking soda falls into the vinegar. Immediately, you will start to see bubbles forming as the reaction begins.
- Watch the Magic : As the reaction progresses, the balloon will start to inflate with carbon dioxide gas. This process may take a few seconds, so be patient and watch the balloon expand.
- Optional – Add More Baking Soda and Vinegar : If you want to extend the demonstration, you can repeat the process with more baking soda and vinegar.
Exploring the Science Behind the Fun
To deepen your understanding and make the demonstration more educational, consider explaining the following concepts:
- Reaction Rate : The rate at which the balloon inflates can be affected by the amount of baking soda and vinegar used. More of either reactant will speed up the reaction and produce more carbon dioxide gas.
- Gas Laws : Introduce basic concepts of gas laws, such as Boyle’s Law and Charles’s Law, to explain how gases behave under different conditions.
- Pressure and Volume : Discuss how the pressure inside the balloon increases as the volume of gas increases and how this relates to the balloon’s inflation.
- Safety Tips : Emphasize the importance of handling vinegar and baking soda safely, and ensure that the demonstration is conducted in a well-ventilated area.
Making It Fun: Variations and Extensions
To keep the fun going and explore more scientific concepts, try these variations and extensions of the self-inflating balloon demo:
- Color Changing Reaction : Add a few drops of food coloring to the vinegar before starting the reaction. This adds a visual element to the demonstration and makes it more engaging for kids.
- Different Types of Balloons : Experiment with balloons of different sizes and shapes to see how they affect the inflation process.
- Measurement and Comparison : Measure how much baking soda and vinegar are needed to inflate the balloon to different sizes. This can lead to discussions about measurement, volume, and chemical reactions.
- Temperature Effects : Conduct the experiment at different temperatures to see how temperature affects the reaction rate and the amount of gas produced.
- Alternative Gases : Explore other types of chemical reactions that produce gases, such as using yeast to produce carbon dioxide for inflating balloons.
Educational Value
The self-inflating balloon experiment is not only a fun and magical activity but also an excellent educational tool. It helps students understand chemical reactions, gas laws, and the principles of pressure and volume. The hands-on nature of the experiment makes complex scientific concepts more accessible and engaging, fostering a love for science and curiosity about the world.
By incorporating these concepts into your demonstration, you can make the self-inflating balloon experiment a memorable learning experience. Whether you’re a teacher, a parent, or just a science enthusiast, this simple yet captivating experiment offers endless opportunities for exploration and discovery.
The magic of self-inflating balloons at The Kids Point showcases the incredible science behind everyday chemical reactions. This fun and easy-to-perform demonstration provides a tangible way to explore key scientific concepts, making learning an exciting adventure. Gather your materials, invite friends and family, and let the magic of self-inflating balloons bring a touch of scientific wonder to your day!
Kids Science
Give a comment Cancel reply
Save my name, email, and website in this browser for the next time I comment.
Cool Science Experiments Headquarters
Making Science Fun, Easy to Teach and Exciting to Learn!
Science Experiments
Balloon Blow-up Science Experiment
Can you blow up a balloon without using your mouth? In this simple science experiment, we’re going to show you how to do it with only a few everyday items you probably already have in your home. It makes a great experiment for young children because the set-up is simple and it only takes a few minutes to get to the exciting finale.
In addition to a video demonstration and detailed printable instructions, we also have the scientific explanation of how this simple chemical reaction works making it perfect for older scientists too.

JUMP TO SECTION: Instructions | Video Tutorial | How it Works
Supplies Needed
- Small Soda Bottle
- Baking Soda
Balloon Blow-up Science Lab Kit – Only $5

Use our easy Balloon Blow-up Science Lab Kit to grab your students’ attention without the stress of planning!
It’s everything you need to make science easy for teachers and fun for students — using inexpensive materials you probably already have in your storage closet!
Balloon Blow Up Science Experiment Instructions
Step 1 – Start with some questions: How do you blow up a balloon? What if I told you that you couldn’t blow air into it, do you think you could still inflate (blow-up) the balloon? Then observe the supplies for the experiments. Do you think they can be use to blow up the balloon? If so how? Write down your hypothesis (prediction).

Step 2 – Using a funnel, pour about a third of a cup of vinegar into the bottle. We used Apple Cider Vinegar, but any type of vinegar will work.

Step 3 – Then insert another funnel into the mouth of the balloon. We recommend using two different funnels. One funnel for filling the bottle with vinegar and one for the balloon. However, you can do the experiment with only one funnel. Just make sure you completely wash and dry the funnel after you add the vinegar and before you put it into the balloon. This is very important.

Step 4 – Place two teaspoons of baking soda into the funnel so it falls into the balloon. When the balloon is filled with the baking soda, carefully remove it from the funnel.

Step 5 – Next, secure the mouth of the balloon over the mouth of the bottle. Take your time doing this and don’t let any of the baking soda fall out of the balloon and into the bottom of the bottle. Take a moment to make some observations. What will happen if we lift up the balloon? Write down your hypothesis (prediction) and then test to see if you were right!

Step 6 – While holding the bottle, lift the end of the balloon and allow the baking soda to drop into the bottle.

Step 7 – What happens to the balloon? Was your hypothesis correct? Wondering what caused the balloon to inflate? Find out the answer in the how does this experiment work section below.
Video Tutorial
How Does the Science Experiment Work?
When baking soda (a base) and vinegar (an acid) are mixed together they create a chemical reaction that results in the formation of carbon dioxide gas. Gases do not have a specific shape or volume, rather they expand rapidly filling their container. Gases expand rapidly because their particles move at high speeds in all directions. As the carbon dioxide gas fills the bottle, it has nowhere else to go so it begins to fill the balloon. As the carbon dioxide gas fills the balloon, the balloon inflates. The more gas that is created, the larger the balloon will inflate.
The baking soda and vinegar chemical reaction will continue to inflate the balloon as long as there is still baking soda and vinegar to react. Once the reaction between baking soda and vinegar has stopped, the balloon will slowly begin to deflate.
An acid is a substance that tastes bitter, reacts with metals and carbonates, and turns blue litmus paper red. A base is a substance that tastes bitter, feels slippery, and turns red litmus paper blue.
Other Ideas to Try
Does changing the amount of baking soda and vinegar change the size of the balloon when it inflates? What would happen if you used another acid like lemon juice instead of the vinegar? Would it react the same with the baking soda?
I hope you enjoyed the experiment. Here are some printable instructions:

Instructions
- Using a funnel, pour about a third of a cup of vinegar into the bottle. Tip: I used Apple Cider Vinegar, but any kind of vinegar will work.
- Then insert another funnel into the mouth of the balloon. Tip: It is best to have two funnels, one for filling the bottle with vinegar and one for the balloon. If you only have one funnel, it is important that you completely wash and dry the funnel after you add the vinegar and before you put it into the balloon.
- Place two teaspoons of baking soda into the funnel so it falls into the balloon. Then remove the balloon from the funnel.
- Next, secure the the mouth of the balloon over the top of the bottle. Tip: Don’t let any of the baking soda drop into the bottle…yet!
- While holding the bottle, lift the end of the balloon allowing the baking soda to drop into the bottle.
- Watch in amazement as the balloon magically inflates!

Reader Interactions
November 2, 2017 at 11:00 am
Yeah but don’t just eyeball the measurements of things because if you use to much baking soda it will make the baloon spring a leak and all sorts of stuff will fly out and make a big mess.
I speak form experience
Seriously, don’t do this
April 21, 2018 at 10:26 am
I did this experiment and it is perfect!
You need to hold properly the bottle when you mix the baking soda into vinegar.
May 22, 2019 at 8:57 am
We’re doing science experiments at school and this one is brilliant! I loved it a lot.
June 22, 2020 at 11:15 am
I love this experiment! My balloon grew 6 inches!
June 19, 2023 at 11:17 pm
I tried and it worked well – Exited to do such experiment
Leave a Reply Cancel reply
Your email address will not be published. Required fields are marked *
Save my name, email, and website in this browser for the next time I comment.

- Privacy Policy
- Disclosure Policy
Copyright © 2024 · Cool Science Experiments HQ
Choose an Account to Log In

Notifications
Science project, how to inflate a balloon using baking soda and vinegar.

What is the project about?
Students learn about gas and chemical reactions by discovering how to inflate a balloon using baking soda and vinegar.
What are the goals?
The goal of the project is to demonstrate the power of gas procuded when of baking soda and vinegar are mixed. The goal is for the balloon to be blown up by the gas created.
Materials and Equipment / Ingredients
What materials are required.
- Balloon (1 per student)
- Small bottle (cleaned glass beverage bottle will work well) (1 per student)
- Small funnel (1 per student)
- Baking soda (2 tablespoons per student)
- Vinegar (4 ounces per student)
Where can the materials be found?
Introduction, research questions.
- What do you think will happen when baking soda and vinegar come in contact (what will be produced)?
- What do you think will happen to the balloon attached?
- Why does the balloon stop blowing up (why does the reaction stop)?

Terms, Concepts and Questions to Start Background Research
For the parent/student, what terms and concepts are required to better understand the project.
The terms carbon dioxide, chemical reaction, reactants, and endothermic should be reviewed.
Experimental Procedure
- Using the funnel, add the baking soda to each balloon (two people may be needed for this; one person to hold the balloon open and the other person to put the baking soda inside of the balloon).
- Pour the vinegar into the bottle.
- Carefully fit the balloon over the bottle opening (be careful not to drop the baking soda into the vinegar yet).
- Once the balloon is fitted snugly on the nozzle, hold up the balloon and allow the baking soda to fall into the vinegar.
- Observe the chemical reaction and effect on the balloon.
- Record observations.
Bibliography / References to related books / Links to related sites on the web
Related learning resources, add to collection, create new collection, new collection, new collection>, sign up to start collecting.
Bookmark this to easily find it later. Then send your curated collection to your children, or put together your own custom lesson plan.
Get Your ALL ACCESS Shop Pass here →

Baking Soda and Vinegar Balloon Experiment
Combine a fizzing baking soda and vinegar reaction with balloon play with this easy-to-set-up balloon science experiment for kids . Find out how to blow up a balloon with baking soda and vinegar. Grab a few simple ingredients from the kitchen, and you have fantastic chemistry for kids.

BAKING SODA AND VINEGAR BALLOON EXPERIMENT
Don’t have vinegar for this experiment? Try a citric acid like lemon juice, and check out our citric acid and baking soda experiment here.
- Baking Soda
- Empty Water Bottles
- Measuring Spoons
- Funnel {optional but helpful)

BLOW-UP BALLOON EXPERIMENT SETUP:
Step 1. Blow up the balloon a bit to stretch it out some, and use the funnel and teaspoon to add baking soda to the balloon. We started with two teaspoons and added a teaspoon for each balloon.
Step 2. Fill the container with vinegar halfway.
Step 3. When your balloons are all made up, attach them to the containers making sure you have a good seal!
Step 4. Next, lift up the balloon to dump the baking soda into the container of vinegar. Watch your balloon blow up!
To get the most gas out of it, we swirled around the container to get it all going!

Optional Art: Go ahead and use a sharpie to draw emojis, shapes, or fun pictures on your balloons before filling them with baking soda.
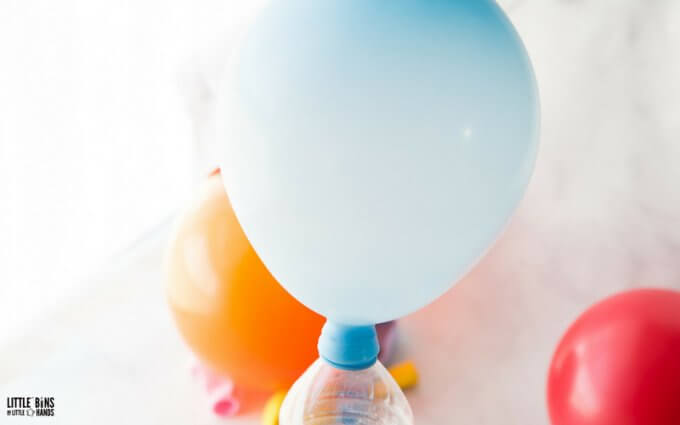
BALLOON EXPERIMENT TIPS
My son suggested we try different amounts of baking soda in our experiment to see what would happen. Also, will the balloon size grow bigger if more vinegar is in the bottle?
Always encourage your kids to ask questions and wonder what will happen if…
This is also a great way to encourage inquiry, observation, and critical thinking skills. You can read more about teaching the scientific method to kids here.
Make predictions! Ask questions! Share observations!

Be cautious with the amount of baking soda you add, as the reaction will get bigger each time. Safety goggles are always great for young scientists!
You could see the difference in the baking soda we put in the balloons! The red balloon with the least baking soda inflated the least. The blue balloon with the most inflated the most.
What else can you do with baking soda? Check out these unique baking soda experiments!
HOW DOES THE BALLOON EXPERIMENT WORK?
The science behind this baking soda and vinegar balloon science experiment is a chemical reaction between an acid and base . The base is the baking soda and the acid is vinegar. When the two ingredients mix, the balloon baking soda experiment gets its lift!
That lift is gas, carbon dioxide, or CO2. As the gas tries to leave the plastic container, it goes up into the balloon because of the tight seal you have created. Check out states of matter experiments !
The gas has nowhere to go and is pushing against the balloon it blows it up. Similar to how we exhale carbon dioxide when we blow up balloons ourselves.
We love exploring simple chemistry you can do at home or in the classroom. Science that isn’t too crazy but is still lots of fun for kids! You can check out more cool chemistry experiments .
Read more about the science behind baking soda and vinegar experiments .
WHAT IS THE SCIENTIFIC METHOD FOR KIDS?
The scientific method is a process or method of research. A problem is identified, information about the problem is gathered, a hypothesis or question is formulated from the information, and the hypothesis is tested with an experiment to prove or disprove its validity. Sounds heavy…
What in the world does that mean?!? The scientific method should be used as a guide to help lead the process. It’s not set in stone.
You don’t need to try and solve the world’s biggest science questions! The scientific method is all about studying and learning things right around you.
As kids develop practices that involve creating, gathering data evaluating, analyzing, and communicating, they can apply these critical thinking skills to any situation.
Learn more about the scientific method and how to use it.
Even though the scientific method feels like it is just for big kids…
This method can be used with kids of all ages! Have a casual conversation with younger kiddos or do a more formal notebook entry with older kiddos!
Click here to get your FREE Science Challenge Calendar

MORE SCIENCE EXPERIMENTS WITH BALLOONS
Have leftover balloons? Why not try one of these fun and easy balloon science experiments below!
- Explore physics with a balloon rocket
- Try this screaming balloon experiment
- Make a balloon-powered car
- Try a pop rocks and soda balloon experiment
- Learn about static electricity with a balloon and cornstarch experiment
- Bend water with a balloon.

Helpful Science Resources To Get You Started
Here are a few resources that will help you introduce science more effectively to your kiddos or students and feel confident yourself when presenting materials. You’ll find helpful free printables throughout.
- Best Science Practices (as it relates to the scientific method)
- Science Vocabulary
- 8 Science Books for Kids
- All About Scientists
- Free Science Worksheets
- Science Supplies List
- Science Tools for Kids
- Scientific Method for Kids
- Easy Science Fair Projects
- Citizen Science Guide
- Join us in the Club
Printable Science Projects For Kids
If you’re looking to grab all of our printable science projects in one convenient place plus exclusive worksheets and bonuses like a STEAM Project pack, our Science Project Pack is what you need! Over 300+ Pages!
- 90+ classic science activities with journal pages, supply lists, set up and process, and science information. NEW! Activity-specific observation pages!
- Best science practices posters and our original science method process folders for extra alternatives!
- Be a Collector activities pack introduces kids to the world of making collections through the eyes of a scientist. What will they collect first?
- Know the Words Science vocabulary pack includes flashcards, crosswords, and word searches that illuminate keywords in the experiments!
- My science journal writing prompts explore what it means to be a scientist!!
- Bonus STEAM Project Pack: Art meets science with doable projects!
- Bonus Quick Grab Packs for Biology, Earth Science, Chemistry, and Physics

42 Comments
Need more info on experiments. Thanks, Miranda
What information would you like?
thanks a lot very funny experiment
Your welcome!
(I was thinking that the pint bottle was going to blow up I got really scared first time I saw a science magic) but I can make smoke come out of my mouth it is very simple
I’m doing a Science Fair Project on this, but I don’t know and how to do the table and graphs, like the data and stuff. Can you help me?
And it’s due May 18, 2016 🙁
this is cool thanks you verry much
Your welcome! Try drawing on the balloons too!
Does the size of the container or size of balloon have any affect on how the balloon will blow up?
Yes, it will because of the space the gas has to fill once the baking soda and vinegar are combined. Great experiment to try different sizes using the same amounts of both vinegar and baking soda.
my team did the balloon inflating thing and it was fun
Is it safe for kids to do this experiment in school
I would think it would be as it is just baking soda and vinegar. You would need to use your best judgement of course. We have never had a balloon explode.
hi this is STEM project . can anyone explain how to connect – T technology E Engineering M mathematics through this experiment . thanks in advance
I will look into my information. Remember a STEM project does not need to contain each of the 4 pillars of STEM but at least two. I can tell you we used math {measuring} and science {chemical reaction}.
Definitely is cool
i love yo stuff
- Pingback: Tutors Only: Week 16 Science Experiment – ccricelake
If we wanted to use this for a science fair project what would the Question asking be?
How much baking soda/vinegar is needed to inflate balloon completely. Or, which acid is better vinegar or lemon juice? Do different shape balloons fill better?
We just did this experiment, but we only used one balloon. My kids are 2.5, 4 and 7 so we have a range of ability levels, but I wanted to add my kids’ favorite part! We took the balloon off the bottle and tied it shut, careful not to lose the gas. And then I blew a balloon up the same size, I asked them which one they thought would hit the ground first as I held them even in the air. Try it out!!
That’s awesome! We will def have to try that. What a great idea!
Where did you find your containers to hold the baking soda and vinegar?
- Pingback: Fun With Balloons
- Pingback: Grow Sugar Crystals for Edible Rock Candy Chemistry Experiment
- Pingback: Summer Science Camp for Young Scientists : 5 Days of Fun!
- Pingback: Exploding Science Experiments for Kids | My Home Based Life
- Pingback: Discrepant Event | Colleen Boyds teacher e-portfolio
- Pingback: 100+ STEM Projects for Kids (With Free Cheat Sheets)
- Pingback: Insanely Rad Pre K Science Projects for Your Curious Little Einsteins - MyVyllage
- Pingback: 30 Incredible Chemistry Experiments - 123 Homeschool 4 Me
- Pingback: Rainbow Science Experiments and STEM Ideas (St. Patricks Day)
- Pingback: 50 Simple Science Experiments with Supplies You Already Have
- Pingback: Science Experiments for Preschoolers - Round Rock Teravista
- Pingback: Science Experiments for Preschoolers - Sienna Plantation
- Pingback: M&M Candy Experiment For Kids | Little Bins for Little Hands
- Pingback: The BEST Very Simple Science Experiments for Kids to Try Anywhere
- Pingback: Kitchen Science Experiments and Activities for Kids
- Pingback: 60 Very Simple Science Experiments Your Kids Will Love
- Pingback: 25 Must Try Science Experiments For Kids | Little Bins for Little Hands
- Pingback: Bump Club And Beyond
Comments are closed.

Subscribe to receive a free 5-Day STEM Challenge Guide
~ projects to try now ~.

- PRO Courses Guides New Tech Help Pro Expert Videos About wikiHow Pro Upgrade Sign In
- EDIT Edit this Article
- EXPLORE Tech Help Pro About Us Random Article Quizzes Request a New Article Community Dashboard This Or That Game Happiness Hub Popular Categories Arts and Entertainment Artwork Books Movies Computers and Electronics Computers Phone Skills Technology Hacks Health Men's Health Mental Health Women's Health Relationships Dating Love Relationship Issues Hobbies and Crafts Crafts Drawing Games Education & Communication Communication Skills Personal Development Studying Personal Care and Style Fashion Hair Care Personal Hygiene Youth Personal Care School Stuff Dating All Categories Arts and Entertainment Finance and Business Home and Garden Relationship Quizzes Cars & Other Vehicles Food and Entertaining Personal Care and Style Sports and Fitness Computers and Electronics Health Pets and Animals Travel Education & Communication Hobbies and Crafts Philosophy and Religion Work World Family Life Holidays and Traditions Relationships Youth
- Browse Articles
- Learn Something New
- Quizzes Hot
- Happiness Hub
- This Or That Game
- Train Your Brain
- Explore More
- Support wikiHow
- About wikiHow
- Log in / Sign up
- Education and Communications
- Science for Kids
How to Blow up a Balloon With Baking Soda and Vinegar
Last Updated: February 2, 2024 Fact Checked
wikiHow is a “wiki,” similar to Wikipedia, which means that many of our articles are co-written by multiple authors. To create this article, 50 people, some anonymous, worked to edit and improve it over time. This article has been fact-checked, ensuring the accuracy of any cited facts and confirming the authority of its sources. This article has been viewed 415,859 times. Learn more...
Learn how to inflate a balloon in this fun science experiment using these common kitchen ingredients! Balloons inflated this way are filled with carbon dioxide produced by the two ingredients reacting. They do not contain helium, so they will not float.
Inflating the Balloon

- You can try this with any kind of vinegar, but the inflation might take longer or require more vinegar to work. Other types of vinegar tend to be more expensive as well.
- Vinegar can damage metal containers, potentially adding an unpleasant taste to food and drink stored in that container. If you have no plastic bottles, use a high-quality stainless steel bottle to minimize the chance of this happening. Weakening the vinegar with an equal amount of water might also help, and won't prevent the balloon from inflating. [2] X Research source

- If you don't have a funnel, you can place a plastic straw into a pile of baking soda, put your finger over the top hole of the straw, then poke the straw into the balloon and lift your finger. Tap the straw to get the baking soda to fall out, and repeat until the balloon is at least 1/3 of the way full. [4] X Research source

- Shake the bottle gently to mix the two ingredients if there's not much fizzing.

- Don't go overboard. The bottle should never be more than about 1/3 full of vinegar.
Grasping how the Process Works

- Baking soda is another word for the molecule sodium bicarbonate .
- White vinegar is a mixture of acetic acid and water. Only the acetic acid reacts with the baking soda.

- Although the definition of acid and base can get complicated, you can compare the differences between the original substances and the "neutralized" result to see there are obvious changes. For instance, vinegar has a strong smell and can be used to dissolve grime and dirt. After being mixed with baking soda, it smells much less strongly and is no more effective at cleaning than water is.

- NaHCO 3 + HC 2 H 3 O 2 (aq) → NaC 2 H 3 O 2 (aq) + H 2 O(l) + CO 2 (g)
- The letters in parentheses show the state the chemicals are in during and after the reaction: (g)as, (l)iquid, or (aq)ueous. "Aqueous" means the chemical is dissolved in water.
Community Q&A
- This method can also be used in homemade cardboard or plastic rockets and you can make them go a long way if ingredients are out right. The reason it blows up is because the reaction creates gas, and the pressure builds up. Thanks Helpful 0 Not Helpful 0
- You can use lime juice instead of vinegar. Thanks Helpful 0 Not Helpful 0

- If the balloon is fully inflated and the liquid is still fizzing, the balloon might be about to explode. Decide whether you have time to pull off the balloon, or whether you should just cover your face before it gets spattered! Thanks Helpful 69 Not Helpful 25
Things You'll Need
- Baking Soda
- Bottle with narrow neck
- Funnel (optional)
You Might Also Like

- ↑ https://www.cityofsacramento.org/-/media/Corporate/Files/ParksandRec/4thR/4r-SAH2-BakingSodaVinegarBalloonExp.pdf?la=en
- ↑ https://www.exploratorium.edu/science_explorer/balloon_blowup.html
- ↑ http://www.education.com/science-fair/article/balloon-gas-chemical-reaction/
- ↑ https://www.education.com/science-fair/article/balloon-gas-chemical-reaction/
- ↑ https://www.pbs.org/parents/crafts-and-experiments/inflate-a-balloon-with-baking-soda-and-vinegar
- ↑ https://www.cmosc.org/balloon-blow-up-science-experiment/
About This Article
To blow up a balloon with baking soda and vinegar, pour 1–2 inches of white vinegar into a plastic bottle. Next, hold a balloon loosely by the neck, fit a funnel or plastic straw into it, and pour 2 tablespoons of baking soda through it into the balloon. Then, stretch the neck of the balloon over the top of the bottle before lifting the balloon up over the bottle. The baking soda will fall out of the balloon, through the neck of the bottle, and into the vinegar. The 2 ingredients will fizz and react to create carbon dioxide, which will then inflate your balloon! If you want to learn more about the chemical reaction that occurs, keep reading! Did this summary help you? Yes No
- Send fan mail to authors
Reader Success Stories
Akime Yashito
Jan 10, 2017
Did this article help you?
Jan 24, 2018
Coco Wright
Nov 29, 2016
Monserrath Garza
Oct 27, 2017
Emmanuel Nikoi
Nov 17, 2016

Featured Articles

Trending Articles

Watch Articles

- Terms of Use
- Privacy Policy
- Do Not Sell or Share My Info
- Not Selling Info
Don’t miss out! Sign up for
wikiHow’s newsletter

Mama's Must-Haves
Your cart is currently empty!

Self inflating balloon experiment
Have you ever tried to inflate a balloon without using your mouth? In this experiment, you can! Create a self-inflating balloon by mixing vinegar and baking soda in an empty pop bottle to create carbon dioxide gas. If you secure a balloon on top of this bottle, it will capture the gas and blow the balloon up!
Learn how to re-create this simple science experiment at home by reading the post below.

What is the self-inflating balloon experiment?
This is an excellent activity for young scientists – Especially those who love learning outside! Learn why we think science for kids is so important in this post.
Primary Connections explains that when you mix vinegar (an acid) and baking soda (a base) , you create a carbon dioxide gas . Now, as we saw in our volcano experiment with the same chemical reaction, this gas will cause a bubbling eruption that flows out of the container.
But, when you affix a balloon on top of the bottle, the gas has nowhere to go… Except for the balloon! Usually, we are using the air from our lungs to fill a balloon when we blow it up. This air is also a gas. But in this experiment, we are trapping carbon dioxide inside of the balloon to create a self-inflating balloon!
Download a scientific method worksheet before starting
This is totally optional, but you may want to head over to my TpT store and download the Scientific Method worksheet for free. This way, you can emphasize key learning moments while introducing your preschooler to the scientific method. By practicing this, they will become confident in their ability to hypothesize, test, and evaluate experiments!
Plus, if you write down the step-by-step instructions and tuck this away in a folder for later, they can re-create the same activity another time.

Making a self-inflating balloon: What you’ll need
All of the materials required for this experiment are common household staples! Since you’ve got to act quickly to capture the gas, this experiment is best suited for older children (or requires adult assistance).
Here are the materials you will need:
- An empty 2L pop or water bottle
- 2 tbsp Baking soda
- 1 and a half cups vinegar
- Either two funnels or a funnel and a spoon
*This amount will blow up the balloon a little bit – I ended up adding more vinegar to see how much I could inflate my balloon!

How to make a self-inflating vinegar balloon
This classic science experiment is super easy. But, be sure to act quickly because as soon as the baking soda falls into the vinegar, the chemical reactions will start!
Here are the step-by-step instructions
- Remove the lid from your bottle
- Place a funnel into the top of the bottle for the liquid
- Pour the vinegar into the bottle first , then remove the funnel
- Either place a second funnel in or use a spoon to pour the baking soda in
- Quickly and carefully stretch the balloon over the top of the bottle
- Watch carefully! The chemical reaction between the two substances will make the balloon inflate
That’s it! The kids will have so much fun watching the self-inflating balloon science… But be careful to take the balloon off before it bursts! Otherwise, you’ll have a huge mess to clean.
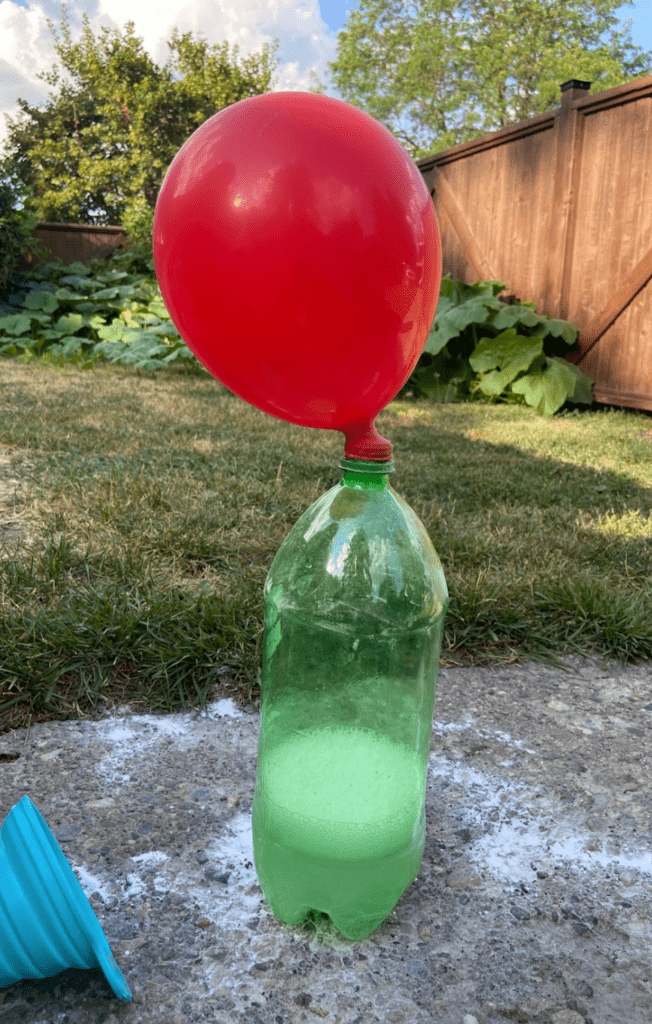
That’s all there is to this self-inflating balloon experiment! Pretty simple, hey? With just balloons, baking soda, vinegar, and an empty bottle, you can create a self-blowing balloon in just a few minutes. This happens because the acid (vinegar) and the base (baking soda) create a chemical reaction inside the water bottle. Since the balloon prevents the gas from escaping, the carbon dioxide flows into the balloon, making it appear to magically inflate all by itself.
Have you tried this self-inflating balloon science experiment (sometimes also called a ‘vinegar balloon experiment’)? We would love to hear how it went!
Here are some other science experiments you may enjoy
If you’re into science experiments, you and your kids will love these:
- Create a tissue paper butterfly that flaps its wings thanks to static electricity
- Explore how the sun moves while creating beautiful shadow art
More science posts
Searching for more science experiments and science-related activities to do with your little ones? Check out this category to see all of our posts about science for kids!
Leave a Reply
Your email address will not be published. Required fields are marked *

Self-Inflating Balloon Experiment
- March 25, 2020
- No Comments
If the children in your life are anything like mine, they LOVE to experiment! It is so fun to watch their facial expressions, as they experience the chemical reactions that take place, such as in this self-inflating balloon experiment. It’s a mixture of pure joy and wonder!
In this self-inflating balloon experiment, children use an acid-base reaction to inflate a balloon. However, there are countless variations that can be done, which I list below.
In addition, this experiment can be performed by children of all ages. Even my 2½ year old had a blast inflating her balloon several times! Simply increase or decrease the difficulty or comparisons being made in this experiment, depending on the age of the child.
Supplies Needed
Chances are, you have all you need to perform this experiment right in your own home! The equipment and materials lists are super simple.
The equipment recommended for this experiment includes:
- Small Measuring cup
- 8 to 12 oz. Bottle

The only two materials needed for this experiment are:
- Baking Soda
- Distilled White Vinegar

The chemicals in this experiment are non-toxic and inexpensive. Therefore, this is a great project for scientists of all ages to perform repeatedly!
The Experiment
To begin the experiment, first add about ¼ cup of vinegar to an 8 to 12 ounce bottle with a narrow opening. Refrain from adding too much vinegar, as this will cause the reaction to fizz up into the balloon.

Next, pour 1 tablespoons of baking soda into a balloon. It helps to use a funnel to perform this step. We also found it helpful to use a toothpick to stir the baking soda into the funnel and break up any clumps that may have occurred.

Once the baking soda is in the balloon, affix the balloon to the mouth of the bottle, being careful not to get any baking soda into the bottle, just yet.
Finally, lift the balloon up into the air, emptying the baking soda into the bottle. Watch the balloon inflate, as the baking soda mixes with the vinegar!
Tip: Have your little scientist perform this experiment on a tray or in a shallow baking dish of some sort, just in case the bottle tips over and the mixture spills.
The Science
The balloon inflates as a result of a chemical reaction between baking soda (sodium bicarbonate, a base) and vinegar (acetic acid), which produces an unstable carbonic acid. The carbonic acid immediately separates into water and carbon dioxide (CO2). As pressure builds up in the bottle, the CO2 bubbles out of the bottle and into the balloon, causing it to expand. The more CO2 that is created, the larger the balloon will expand!
Another interesting thing to mention is that the acid-base reaction is endothermic, which means the chemical reaction makes the mixture feel colder.

Self-Inflating Balloon Experiment Variations
Children will have a blast testing all sorts of variations to this experiment. Try these variations to see which chemical reaction is best at inflating a balloon! To make the self-inflating balloon experiment even more educational, record your hypotheses and analyze the effects.
Consider measuring the size of the balloon before and after it has inflated. Draw a 1 cm long black line on the balloon with a marker before adding the baking soda. After performing the experiment, record the time it took for the line to expand to 5 cm in length.
2. Change the Acid
Predict what will happen if you change the amount of vinegar. (i.e. 2 tablespoons, ½ cup, etc.) Does this effect how long it takes the line to get to 5 cm in length?
Does it change the reaction if you use lemon juice , soda , or citric acid dissolved in water as an acid, instead of distilled white vinegar?
Predict what will happen if you use hot water instead of distilled white vinegar. (Be sure to have an adult handle the hot water for little ones!)
3. Change the Base
Predict what will happen if you change the amount of baking soda. (i.e. 1 teaspoon, 2 tablespoons, etc.) Does this effect how long it takes the line to get to 5 cm in length?
Does it change the reaction if you use laundry detergent , an Alka-Seltzer tablet or a Mentos tablet as a base, instead of baking soda?
4. Temperature
Consider taking the temperature of the liquid in the bottle before and after the reaction. What has happened?
Predict what will happen if you change the temperature of the vinegar or other acid. (i.e. refrigerated vs. room temperature, etc.) Does this effect how long it takes the line to get to 5 cm in length?
5. Other Ways?
In what other way(s) can you change the amount of CO2 emanating from the chemical reaction to make the balloon inflate even more? Consider changing the chemicals, the size of the bottle, and the size of the balloon.
Tip: While performing this self-inflating balloon experiment, remember to keep all other variables the same.
Acid-base experiments are fun to experiment with at any time of the year. Fortunately, baking soda and vinegar are so inexpensive, children can experiment again and again and again! So, stock up on these supplies. You never know when you will need them for easy entertaining and loads of learning! Good luck!

- Small measuring cup
- 8 – 12 oz. Bottle
- ¼ Cup White Vinegar
- 1 Tbsp Baking Soda
Instructions
- Pour white vinegar into a bottle.
- Use a funnel to pour baking soda into a balloon.
- Attach the balloon to the mouth of the bottle. Be careful not to get any baking soda into the bottle until you are ready to proceed with the experiment.
- Hold the balloon up and let the baking soda pour down into the bottle. Watch the balloon expand, as gasses fill the balloon!
Have you made a balloon self-inflate with your kids or grandkids? Please share below.
Related Articles:
- Glitter Volcanoes
- 10 Fun Halloween Candy Activities
- DIY Slingshots
Leave a Comment Cancel Reply
You must be logged in to post a comment.
Error: Contact form not found.
OPERATION: Self-Inflating Balloon aka “Thermodynamics”

by Tim Werle
August 22, 2011, in in door projects , operations, no comments.
INTRODUCTION:
The First Law of Thermodynamics , in its simplest form, states matter and energy are neither created nor destroyed by natural means . Prior to Einstein, the two conservation laws (energy and mass) were independent. With the recognition that mass was a form of energy (E=MC 2 ), the First Law of Thermodynamics includes mass as a form of energy. Believing the Universe and everything it contains came from nothing, by natural processes, violates this fundamental law of science.
- 100 ml white vinegar (about 6.5 Tablespoons)
- 10 g baking soda (about 2 teaspoons)
- 500ml plastic water bottle (everyday small water bottle)
- plastic spoon
- scale or balance
- paper towels
INSTRUCTIONS:
- Add 100ml of vinegar to the 500 mL plastic water bottle.
- Stretch your balloon out for about a minute so that it will inflate easily.
- Using the white plastic spoon, add 3 level teaspoons of baking soda to your balloon. (Use the funnel to avoid spilling)
- While keeping all the baking soda in the balloon, carefully place the mouth of the balloon over the opening of the plastic water bottle to make a tight seal. The balloon will hang to the side of the bottle. ***Do not allow any of the baking soda to enter the bottle.***
- Use your scale to find the mass of the “closed system” (bottle, vinegar, balloon, and baking soda) and record in the Data Table.
- While the balloon is still attached to the bottle, lift the balloon so the baking soda falls into the bottle and combines with the vinegar. Swirl gently.
- Record & draw observations.
- Use your scale to find the mass of the “closed system” once the chemical reaction has completed. ***Be sure to keep balloon attached***
- Record the info into the data table.
OBSERVATIONS:
What evidence did you observe to indicate a chemical reaction took place?
- What happened chemically in this experiment is represented in the chemical equation below:
- NaHCO 3 + CH 3 COOH is now NaOOCCH 3 + H 2 0 + CO 2
- (Baking Soda) (Vinegar) is now (Sodium Acetate) (Water) (Carbon Dioxide)
You can observe from the chemical equation above that no new elements were created.
- Knowing this, explain where the gas came from?
LIFE APPLICATION:
This simple experiment can give us confidence that God’s Word is not in opposition with science but the contrary; science continues to confirm God’s Word is true. The First Law of Thermodynamics tells us the Universe could not have come from nothing by natural processes.
- Explain why you can boldly accept a literal interpretation of Genesis 1:1 “In the beginning, God created the heavens and the earth”?
Click here to get the free Life Application and Observation Extended Study Workbook
Recommended posts.

OPERATION: Time Capsule
December 19, 2011

OPERATION: White As Snow
December 11, 2011

OPERATION: Memory Stone
November 28, 2011
Leave a Reply Cancel reply
Your email address will not be published. Required fields are marked *
Notify me of follow-up comments by email.
Notify me of new posts by email.
SUBSCRIBE NEWSLETTER

IMAGES
VIDEO
COMMENTS
Learn how to make a balloon inflate by itself using baking soda and vinegar in this easy science experiment. Find out the science behind the reaction, try variations and see more fun ideas with baking soda and vinegar.
In this simple science experiments video, we go over how to make a self-inflating balloon using just a bottle, bicarbonate of soda, vinegar and (of course) a...
Learn how to make a balloon inflate by itself using vinegar and baking soda. This easy science project shows kids the chemical reaction that produces carbon dioxide and pushes the air into the balloon.
Learn how to make a balloon inflate by breaking a pouch of citric acid and baking soda inside it. Explore the chemistry behind the reaction, the products formed, and the FAQs about self-inflating balloons.
Experimental Procedure: Put the water bottle where it will stand upright securely, or have a partner hold it. Fill it halfway with vinegar. Give the balloon a good stretching, like you would if you were about to blow it up. Use the funnel to put the baking soda inside the balloon. Gently shake the balloon until all the baking soda goes to the ...
Learn how to make balloons inflate with different chemical reactions and gasses, such as yeast, baking soda, vinegar, and hot water. Compare the sizes and durations of the balloons and find out which one is the best replacement for helium.
On-Site. Pour 50 mL 10% citric acid solution in labeled cup. Place 2 tsp. baking soda in a separate, labeled cup. Arrange the cups, latex balloon, spoon/spatula, funnel (if desired), flask/bottle, and 2-3 self-inflating balloons within easy reach. Set candle, lighter, and beaker in a safe location, at least 3 m away from participants.
Learn how to inflate a balloon using a chemical reaction. Want to feel more like a scientist? Check out our lab reports and more experiments for students by ...
Self Inflating Balloon Experiment. This post contains affiliate links, see my disclosure policy for more information. ... Instructions. Pour 1/2 cup of vinegar into an old water or pop bottle. put 1/2 tbsp of baking soda into a balloon. Carefully, put the opening of the balloon over the water bottle. ...
Learn how to inflate a balloon with alka-seltzer, hot water, or baking soda and vinegar. This fun and easy activity shows how chemical reactions produce CO2 gas that fills the balloon.
How to Make a Self-Inflating Balloon: Step-by-Step Instructions. Prepare the Balloon: Using a funnel, carefully pour a few tablespoons of baking soda into the balloon. Make sure not to overfill the balloon, as it needs space to expand. ... The self-inflating balloon experiment is not only a fun and magical activity but also an excellent ...
Learn how to inflate a balloon without blowing into it using baking soda and vinegar. This simple chemical reaction creates carbon dioxide gas that fills the balloon.
make a balloon self-inflate - science experiment. What you need: White vinegar. Step 1: Measure out half a cup of white vinegar. Step 2: Pour half a cup of vinegar inside the water bottle. Make sure you don't spill any or you'll be in for a mess! Step 3: Either get a funnel or improvise one out of paper like me.
Stretch the neck of a balloon over the narrow end of the powder funnel. Pour 1 level scoop of one powder into the funnel and balloon. Make sure all of the powder goes into the rounded portion of the balloon. Carefully remove the balloon from the funnel. Place one small rubber band over the neck of the balloon.
How to make a self inflating balloon. Clean a 1 liter bottle and let dry. Using a funnel, add 1 teaspoon of baking soda to the bottle. Place the small end of the funnel into the opening of the balloon. Hold carefully and pour the vinegar into the balloon. Carefully stretch out the open end of the balloon and place over the mouth of the bottle ...
Experimental Procedure. Using the funnel, add the baking soda to each balloon (two people may be needed for this; one person to hold the balloon open and the other person to put the baking soda inside of the balloon). Pour the vinegar into the bottle. Carefully fit the balloon over the bottle opening (be careful not to drop the baking soda into ...
We started with two teaspoons and added a teaspoon for each balloon. Step 2. Fill the container with vinegar halfway. Step 3. When your balloons are all made up, attach them to the containers making sure you have a good seal! Step 4. Next, lift up the balloon to dump the baking soda into the container of vinegar. Watch your balloon blow up!
X Research source. 3. Stretch the neck of the balloon over the top of the bottle. Be careful not to spill the baking soda while you do this. Hold the balloon's neck with both hands and stretch it over the top of the plastic bottle containing vinegar. Have a friend keep the bottle steady if the table or bottle is wobbly.
Here are the step-by-step instructions. Remove the lid from your bottle. Place a funnel into the top of the bottle for the liquid. Pour the vinegar into the bottle first, then remove the funnel. Either place a second funnel in or use a spoon to pour the baking soda in. Quickly and carefully stretch the balloon over the top of the bottle.
Instructions. Pour white vinegar into a bottle. Use a funnel to pour baking soda into a balloon. Attach the balloon to the mouth of the bottle. Be careful not to get any baking soda into the bottle until you are ready to proceed with the experiment. Hold the balloon up and let the baking soda pour down into the bottle.
INSTRUCTIONS: Add 100ml of vinegar to the 500 mL plastic water bottle. Stretch your balloon out for about a minute so that it will inflate easily. Using the white plastic spoon, add 3 level teaspoons of baking soda to your balloon. (Use the funnel to avoid spilling)
Hands-on Chemistry Activities Inflate a Balloon, Tabletop, Page 5 . Facilitate the activity . Invite participation 1. Introduce the activity by showing both a flat and an inflated self-inflating balloon. This is a self-inflating balloon before being inflated and after. What is interesting about
Learn about the science behind the self-inflating balloon experiment