Academia.edu no longer supports Internet Explorer.
To browse Academia.edu and the wider internet faster and more securely, please take a few seconds to upgrade your browser .
Enter the email address you signed up with and we'll email you a reset link.
- We're Hiring!
- Help Center

Comprehensive Organic Chemistry Experiments for the Laboratory Classroom

This expansive and practical textbook contains organic chemistry experiments for teaching in the laboratory at the undergraduate level covering a range of functional group transformations and key organic reactions.The editorial team have collected contributions from around the world and standardized them for publication. Each experiment will explore a modern chemistry scenario, such as: sustainable chemistry; application in the pharmaceutical industry; catalysis and material sciences, to name a few. All the experiments will be complemented with a set of questions to challenge the students and a section for the instructors, concerning the results obtained and advice on getting the best outcome from the experiment. A section covering practical aspects with tips and advice for the instructors, together with the results obtained in the laboratory by students, has been compiled for each experiment. Targeted at professors and lecturers in chemistry, this useful text will provide up to dat...

Related Papers
Journal of Chemical Education
David Pursell , Mai Tsoi
This text provides students with a comprehensive organic chemistry laboratory experience that emphasizes Green Chemistry principles. The Organic Chemistry I laboratory portion of the text uses classical experiments as vehicles for technique development and spectroscopic analysis. The Organic Chemistry II laboratory text section encompasses a multistep synthesis project specifically designed to leverage the techniques and instrumentation acquired by students during their Organic Chemistry I Laboratory experience.
Chiara Ghiron
KRYSTEL IRIS DE CASTRO
Innovative Publisher
Dr Akhil Nagar
The stratagem for literature searching in organic and inorganic synthesis increasingly becomes easier with the computer database. However, the dilemma has now become how to select one procedure instead of other. For neophyte, this is a difficult task, deciding the reaction conditions to be carried out to have the best chance of success. This practical procedure collection intended to serve lab-mate by sharing author’s own experience through most commonly used experimental procedures from established groups, reviewed journals and/or patents. Under the title of each experimental procedure, brief commentaries are often offered which summarize the authors’ personal experience. For the final products, detailed spectral data are not given because they simply take up too much space. We had a good time putting together these experimental procedures. We have even been using the manuscript ourselves quite often. Hopefully, you will find it as useful. We welcome your critique.
Journal of Engineering Research
Fernando León Cedeño
Nick Zyabko
Edward McIntee
Paul Morgan
AIP Conference Proceedings
Asep Kadarohman
Loading Preview
Sorry, preview is currently unavailable. You can download the paper by clicking the button above.
RELATED PAPERS
Rakesh Parashar
Journal of chemical …
Giannis Papaefstathiou
Richard Pagni
AGPHBooks (Academic Guru)
Dr. Rachana Mehta
Chemistry International
Ian Williams
kwanele sboniso
Jonathan Cannon
Medicinal Chemistry Research
Deretu Choma
Paul H. Mazzocchi
Elaisa Nacilla
Natalia Danilkina
Dwi Adhi Putra
LeRoy Kroll
sciepub.com SciEP
CHIMIA International Journal for Chemistry
CHEMISTRY HSSC – II
Fahad Zafar
Michael Edenborough
muduku ivan
Yadila Lopez
Nurul Athirah
Elijah St Germain
RELATED TOPICS
- We're Hiring!
- Help Center
- Find new research papers in:
- Health Sciences
- Earth Sciences
- Cognitive Science
- Mathematics
- Computer Science
- Academia ©2024
We’re fighting to restore access to 500,000+ books in court this week. Join us!
Internet Archive Audio

- This Just In
- Grateful Dead
- Old Time Radio
- 78 RPMs and Cylinder Recordings
- Audio Books & Poetry
- Computers, Technology and Science
- Music, Arts & Culture
- News & Public Affairs
- Spirituality & Religion
- Radio News Archive

- Flickr Commons
- Occupy Wall Street Flickr
- NASA Images
- Solar System Collection
- Ames Research Center

- All Software
- Old School Emulation
- MS-DOS Games
- Historical Software
- Classic PC Games
- Software Library
- Kodi Archive and Support File
- Vintage Software
- CD-ROM Software
- CD-ROM Software Library
- Software Sites
- Tucows Software Library
- Shareware CD-ROMs
- Software Capsules Compilation
- CD-ROM Images
- ZX Spectrum
- DOOM Level CD

- Smithsonian Libraries
- FEDLINK (US)
- Lincoln Collection
- American Libraries
- Canadian Libraries
- Universal Library
- Project Gutenberg
- Children's Library
- Biodiversity Heritage Library
- Books by Language
- Additional Collections

- Prelinger Archives
- Democracy Now!
- Occupy Wall Street
- TV NSA Clip Library
- Animation & Cartoons
- Arts & Music
- Computers & Technology
- Cultural & Academic Films
- Ephemeral Films
- Sports Videos
- Videogame Videos
- Youth Media
Search the history of over 866 billion web pages on the Internet.
Mobile Apps
- Wayback Machine (iOS)
- Wayback Machine (Android)
Browser Extensions
Archive-it subscription.
- Explore the Collections
- Build Collections
Save Page Now
Capture a web page as it appears now for use as a trusted citation in the future.
Please enter a valid web address
- Donate Donate icon An illustration of a heart shape
Golden Book of Chemistry Experiments
Bookreader item preview, share or embed this item, flag this item for.
- Graphic Violence
- Explicit Sexual Content
- Hate Speech
- Misinformation/Disinformation
- Marketing/Phishing/Advertising
- Misleading/Inaccurate/Missing Metadata
plus-circle Add Review comment Reviews
26,020 Views
95 Favorites
DOWNLOAD OPTIONS
For users with print-disabilities
IN COLLECTIONS
Uploaded by hgoodall on April 10, 2011
SIMILAR ITEMS (based on metadata)
Resource Type: Virtual Labs
The Virtual Lab is an online simulation of a chemistry lab. It is designed to help students link chemical computations with authentic laboratory chemistry. The lab allows students to select from hundreds of standard reagents (aqueous) and manipulate them in a manner resembling a real lab. More information and offline downloads . Please scroll below to find our collection of pre-written problems, they have been organized by concept and ranked by difficulty.
Stoichiometry
The mole, molarity, and density, glucose dilution problem.
In this activity, students use the virtual lab to create a 0.025M glucose solution from a standard 1M glucose solution. First, they calculate the correct volumes of 1M glucose solution and water to mix together…
Acid Dilution Problem
In this activity, students use the virtual lab to create 500mL of 3M HCl solution from a concentrated stock solution of 11.6M HCl. They must first calculate the correct volumes of 11.6M HCl solution and water to…
Cola and Sucrose Concentration Problem
In this activity, students use the virtual lab to prepare a sucrose solution for a soda recipe. They next calculate the concentration of their solution in terms of molarity, percent mass and density. Finally, they…
Making Stock Solutions from Solids
In this activity, students use the virtual lab to create stock solutions starting from solid salts. Students must first calculate the correct amount of solid to make the solution. Next, they prepare the solution…
Identifying the Unknown Metal (Metals Density Problem)
In this activity, students use the virtual lab to identify an unknown metal by measuring its density and comparing their measurements to the densities of known metals.
Identifying an Unknown Liquid from its Density
In this activity students use the virtual lab to design an experiment to determine the identity of mislabeled bottles using the densities of the solutions inside.
Alcohol Density Problem
Determine the concentration of an alcohol solution from its density.
Reaction Stoichiometry and Limiting Reagents
Gravimetric determination of arsenic.
Set in the context of ground water contamination in Bangladesh, this stoichiometry and analytical chemistry activity examines the issues around identifying wells contaminated with arsenic. (Part of a larger online…
Determining Stoichiometric Coefficients
In this activity, students use the virtual lab to determine how 4 unknown substances react with each other including their stoichiometric coefficients.
Stoichiometry and Solution Preparation Problem
In this limiting reagents problem, students mix together solutions in different ratios in an attempt to produce a final solution that contains only 1 product.
Textbook Style Limiting Reagents Problems
Textbook-style practice limiting reagent exercises with that can be used as a way to "predict and check" your answers using the virtual lab.
Textbook Style Limiting Reagents Problem II
In this activity, students practice with experiments involving limiting reagents and the test their knowledge to determine the concentration of an unknown solution.
Predicting DNA Concentration
In this limiting reagents problem, students are given specific concentrations of DNA solutions and are asked to predict what products and reactants will remain after a specific volumes are mixed and reaction has…
Unknown Concentration of DNA Solution Problem
In this advanced limiting reagent problem, students use the virtual lab to determine the concentration of a solution of DNA by reacting it with known amounts of a fluorescent dye which binds to the DNA.
Thermochemistry
Energy and enthalpy, camping problem i.
In this part of the MRE scenario, students measure the enthalpy of a reaction.
Camping Problem II
In this part of the MRE scenario, students determine change in the enthalpy of a reaction as the concentration of reactants are varied.
ATP Reaction (Thermochemistry and Bonding)
Determine the enthalpy of the ATP reaction.
Determining the Heat of Reaction in Aqueous Solution
In this activity, students perform an experiment to determine the heat of a reaction.
Coffee Problem
Use the virtual lab to determine how much milk to add to hot coffee to reach the desired temperature
Measuring the heat capacity of an engine coolant.
As an analytical chemist at a company developing new engine coolants your task is to determine the heat capacity of a newly developed product and then to determine if its heat capacity is greater of less than that…
Measuring the heat capacity of an engine coolant II (Advanced version)
Measure and compare the heat capacity of an unknown liquid with an unknown density.
Camping Problem III
In this part of the MRE scenario, students create solutions that when mixed, increase to a certain temperature.
Heats of Reaction - Hess' Law
This activity provides a demonstration of Hess' Law using three reactions: the solubility NaOH in water, the solubility NaOH in HCl and the reaction of a solution of HCl and a solution of NaOH.
Equilibrium
Lechatlier's principle, cobalt chloride and lechatlier’s principle.
In this activity, students safely explore the equilibrium reaction of the cobalt chloride reaction.
Equilibrium Calculations
Dna binding problem.
In this activity, students explore equilibrium constants in biochemical systems by measuring the binding constant of a DNA-Dye reaction.
Acid-Base Chemistry
Strong acids and bases, strong acid and base problems.
Textbook-style strong acid and base problems that can be checked using the Virtual Lab.
Determination of the pH Scale by the Method of Successive Dilutions
This activity was created as an accompaniment to an in-class demonstration of the method of successive dilutions using HCl, NaOH, a pH meter, and universal indicator solution. After the demonstration, students…
Weak Acids and Bases
Weak acid and base problems.
Textbook-style weak acid and base problems that can be checked using the Virtual Lab.
Determining the pKa and Concentration Ratio of a Protein in Solution
Use the virtual lab to determine the pKa of a protein then create a buffer solution with a specific concentration ratio of the protein in its protonated/ unprotonated form.
Unknown Acid and Base Problem
In this exercise, students graph the titration curve of an unknown acid and base to determine their pKa’s and concentrations.
Buffer Solutions
Creating a buffer solution.
An exercise to design a buffer solution with specific properties.
DNA - Dye Binding: Equilibrium and Buffer Solutions
Students examine equilibrium and buffer solutions in a biological setting.
Acid/Base Titrations
Standardization of naoh with a khp solution: acid base titration.
Use the Virtual Laboratory to standardize an unknown NaOH solution (approximately 0.2M) to four significant figures via titration with 25.00 mL of a KHP standard solution.
Solubility Product
Determining the solubility product.
Determine the solubility product constatnt (Ksp) for various solids.
Temperature and the Solubility of Salts
Examine the solubilities of salts based on temperature.
Determining the solubility of copper chloride at different temperatures
GIven the solubility of CuCl at 2 different temperatures, predict its solubility at a third temperature. Then test your prediction by creating the solution in the virtual lab
Oxidation/Reduction and Electrochemistry
Standard reduction potentials, exploring oxidation-reduction reactions.
Design an experiment to order Cu, Mg, Zn and Pb from strongest to weakest reducing agent.
Analytical Chemistry/Lab Techniques
Gravimetric analysis, unknown silver chloride.
Determine the concentration of Silver ion in a Silver Nitrate solution using gravimetric analysis
The ChemCollective site and its contents are licensed under a Creative Commons Attribution 3.0 NonCommercial-NoDerivs License.
Your browser is not supported
Sorry but it looks as if your browser is out of date. To get the best experience using our site we recommend that you upgrade or switch browsers.
Find a solution
- Skip to main content
- Skip to navigation

- Back to parent navigation item
- Primary teacher
- Secondary/FE teacher
- Early career or student teacher
- Higher education
- Curriculum support
- Literacy in science teaching
- Periodic table
- Interactive periodic table
- Climate change and sustainability
- Resources shop
- Collections
- Remote teaching support
- Starters for ten
- Screen experiments
- Assessment for learning
Microscale chemistry
- Faces of chemistry
- Classic chemistry experiments
- Nuffield practical collection
- Anecdotes for chemistry teachers
- On this day in chemistry
- Global experiments
- PhET interactive simulations
- Chemistry vignettes
- Context and problem based learning
- Journal of the month
- Chemistry and art
- Art analysis
- Pigments and colours
- Ancient art: today's technology
- Psychology and art theory
- Art and archaeology
- Artists as chemists
- The physics of restoration and conservation
- Ancient Egyptian art
- Ancient Greek art
- Ancient Roman art
- Classic chemistry demonstrations
- In search of solutions
- In search of more solutions
- Creative problem-solving in chemistry
- Solar spark
- Chemistry for non-specialists
- Health and safety in higher education
- Analytical chemistry introductions
- Exhibition chemistry
- Introductory maths for higher education
- Commercial skills for chemists
- Kitchen chemistry
- Journals how to guides
- Chemistry in health
- Chemistry in sport
- Chemistry in your cupboard
- Chocolate chemistry
- Adnoddau addysgu cemeg Cymraeg
- The chemistry of fireworks
- Festive chemistry
- Education in Chemistry
- Teach Chemistry
- On-demand online
- Live online
- Selected PD articles
- PD for primary teachers
- PD for secondary teachers
- What we offer
- Chartered Science Teacher (CSciTeach)
- Teacher mentoring
- UK Chemistry Olympiad
- Who can enter?
- How does it work?
- Resources and past papers
- Top of the Bench
- Schools' Analyst
- Regional support
- Education coordinators
- RSC Yusuf Hamied Inspirational Science Programme
- RSC Education News
- Supporting teacher training
- Interest groups

- More navigation items
Tips, ideas and practical experiments to help you make the most of microscale chemistry in your classroom
On this page
- Getting started
Why microscale?
Microscale chemistry practicals.
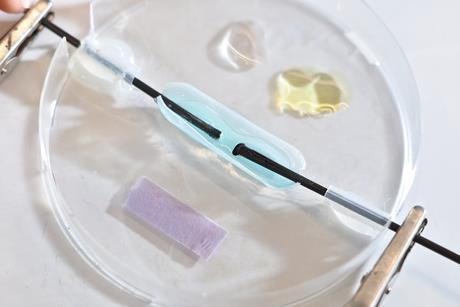
The fast guide to microscale practical work
By Maria Burke
Every chemistry teacher’s must-have guide to this no-fuss method for practical work
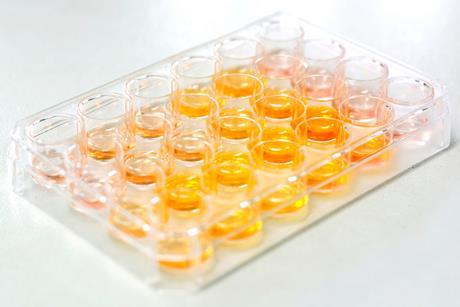
Apparatus and techniques for microscale chemistry
Find out what apparatus you need for common microscale experiments, learn about key techniques and discover how to prepare solutions of different elements.

Maximise learning with microscale
By Bob Worley
Find out why and how a small-scale approach to practical work reaps big rewards in your classroom
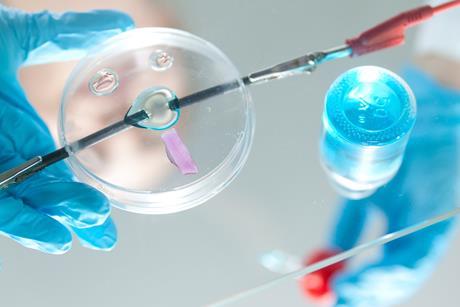
Making practical work more effective
By David Paterson
Microscale chemistry and well-ordered teaching sequences reduce cognitive overload

Why I love microscale
Advocating a practical approach to practical work in the classroom

More from Education in Chemistry


Escape the classroom: and try microscale chemistry
By Adrian Allan
Escape room puzzles based on simple microscale reactions for students
Exhibition Chemistry demonstrations
Engage and inspire your students with these exciting demonstrations, including videos, practical guidance and teaching notes

The movement of ions: bringing electrolysis to life
Demonstrate the movement of positive and negative ions with a simpler, safer version of this classic demo

Dynamite soap: The combustion of stoichiometric hydrogen–oxygen mixtures
Add this quick demo to the end of a lesson on squeaky pops to show the dramatic impact of mixing chemicals in the correct proportions
Practical videos
Video resources featuring microscale experiments, designed to support flipped learning and live practical work
Displacement reactions – practical videos | 14–16 students
Video and resources investigating the displacement reactions between the halogens chlorine, bromine and iodine and their respective halides in microscale

Identifying ions – practical videos | 14–16 students
Video resource showing how to identify ions in various solutions. Flame tests, sodium hydroxide test (microscale) and tests for negative ions: carbonate, sulfate and halide ions

Reactivity of metals | practical videos | 14–16 years
Video with supporting resources featuring three experiments investigating the relative reactivity of metals, including metal displacement reactions in microscale

Electrochemical cells | practical videos | 16–18 students
Video and supporting resources to support electrochemistry practical work, including two microscale experiments, animation and cell diagrams
More microscale experiments and demonstrations

Precipitation reactions of lead nitrate
Compare the colours of various lead compounds to identify which would be good pigments in this microscale practical. Includes kit list and safety instructions.

Some reactions of sulfur dioxide
Observe the reactions of sulfur dioxide with potassium manganate (IV), iodide/iodate mixture and indicator solution. Includes kit list and safety instructions.

The determination of copper in brass
Try this microscale class practical to investigate how much copper there is in brass using nitric acid. Includes kit list and safety instructions.

Microscale reactions of hydrogen sulfide
Observe the reactions of hydrogen sulfide with lead nitrate, silver nitrate and potassium manganate(VII) in this microscale practical. Includes kit list and safety instructions.

Microscale reactions of ammonia
Try this practical to explore the reactions of ammonia with indicator solution, copper(II) sulfate solution and Nessler’s reagent. Includes kit list and safety instructions.

Measuring density
By measuring the relative mass of seawater and tap water, students will be able to discover the density of these liquids. Includes kit list and safety instructions.

The chemistry of thiosulfate ions
Sodium thiosulfate has several interesting reactions with a variety of chemicals. This experiment will let students explore and record these reactions. Includes kit list and safety instructions.
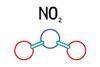
Some reactions of nitrogen dioxide
Using a range of chemicals and solutions, students can create an experiment that will explore some of the reactions of nitrogen dioxide. Includes kit list and safety instructions.

Testing acids and bases on a microscale
Test various substances with indicator solution and look for colour changes in this microscale class practical. Includes kit list and safety instructions.

Mass changes in chemical reactions
Perform two chemical reactions to see whether any mass changes occur in this microscale class practical. Includes kit list and safety instructions.

The oxidation of cyclohexanol by nitric acid
Perform a ring opening oxidation using nitric acid to produce the dicarboxylic acid, 1,6-hexanedioic acid (adipic acid) – and then use the solid crystals that form to determine a melting point. Includes kit list and safety instructions.

Exploring the chemistry of chromium, molybdenum and tungsten
Discover how transition elements differ in aspects of colour, precipitate formation, changes in oxidation state and equilibria. Includes kit list and safety instructions.

Brady’s test for aldehydes and ketones
Identify aldehydes and ketones using Brady’s reagent (2,4-dinitrophenylhydrazine) in this microscale experiment. Includes kit list and safety instructions.

The chemical properties of phenol
Observe and interpret some of the chemical reactions of hydroxybenzene (phenol), by adding five different substances to a Petri dish, and noting down findings. Includes kit list and safety instructions.

Preparing ethyne on a microscale
Generate ethyne gas with calcium carbide and test its properties in this microscale class practical. Includes kit list and safety instructions.

Observing chemical changes
Try this microscale practical to explore the chemical changes in displacement, redox and precipitation reactions. Includes kit list and safety instructions.

Diffusion of gases on a microscale
Try this class practical to explore the diffusion of gases and how relative molecular mass affects rate of diffusion. Includes kit list and safety instructions.

Redox chemistry with dichromate ions
Observe the colour changes that occur with the reduction of dichromate ions by hydrogen peroxide. Includes kit list and safety instructions.

Oxidation states of iron
Compare the two main oxidation states of iron and consider explanations for differences in this microscale practical. Includes kit list and safety instructions.

Microscale reactions of metals with acids
Try this class practical to explore reactivity series with various metals as they react with acids on a microscale. Includes kit list and safety instructions.

Unsaturation test with potassium manganate(VII)
Use a solution of potassium manganate to test for unsaturation in organic compounds in this microscale practical. Includes kit list and safety instructions.

Properties of group 2 elements
Microscale experiment where various anion solutions are added to drops of group 2 element cations. Includes kit list and safety instructions.

Testing for unsaturation with bromine on a microscale
Try this class experiment to prepare elemental bromine and use it to test for unsaturation in organic compounds. Includes kit list and safety instructions.

Oxygen and methylene blue
Reacting hydrogen peroxide, and potassium manganate together will produce detectable oxygen so by using methylene blue solution, and a gas generating apparatus students can test for the presence of oxygen in this practical. Includes kit list and safety instruction.

Synthesis of aspirin on a microscale
Use this class practical to produce aspirin in a microscale esterification reaction using phosphoric acid as a catalyst. Includes kit list and safety instructions.

Energy changes in neutralisation
Study energy changes in two chemical reactions using thermometer strips to measure temperature in this experiment. Includes kit list and safety instructions.

Formation of TCP (2,4,6-trichlorohydroxybenzene)
Delve into preparing TCP by reacting hydroxybenzene (phenol) with chlorine gas, and create this distinctive smelling compound.

Investigating redox reactions on a microscale
Carry out two redox reactions and observe and interpret the results in this microscale class practical. Includes kit list and safety instructions.

The microscale synthesis of indigo dye
Carry out a microscale organic synthesis, the result of which will leave students with indigo dye. Includes kit list and safety instructions.

Finding out how much salt there is in seawater
Use the microscale titration apparatus to titrate silver nitrate solution against sea water

The treatment of oil spills
Tackle the real-life environment problem of oil spills in your classroom, by creating and then treating a micro version of an oil event. Includes kit list and safety instructions.

Some reactions of carbon dioxide
Create carbon dioxide from marble chips and acid, then test for its reaction with barium hydroxide by observing the carbonate precipitate. Includes kit list and safety instructions.

The microscale synthesis of azo dyes
Synthesise an azo dye, and use it to change the colour of cotton, with this class experiment. Includes kit list and safety instructions.

Sulfate and carbonate solubility of Groups 1 and 2
Try this microscale practical to explore the properties of elements in Groups 1 and 2 as they form various precipitates. Includes kit list and safety instructions.

Measuring an equilibrium constant on a microscale
Use your microscale titration apparatus to determine the equilibrium constant for the reaction between silver(I) and iron(II) ions

Exploring the properties of the carvones
Test the smell of each enantiomer of carvone and detect the differences

Measuring the amount of vitamin C in fruit drinks
Explore ascorbic acid in fruit drinks through titration in this experiment, with specimen results and calculations, stock solutions, and detailed notes included.

Displacement reactions of metals on a microscale
Examine the reactions between various metals and metal salt solutions in this microscale class practical. Includes kit list and safety instructions.

Electrolysis using a microscale Hoffman apparatus
Investigate the electrolysis of sodium sulfate solution using a microscale Hoffman apparatus in this class practical. Includes kit list and safety instructions.

The chemistry of silver
Discover the properties of silver compounds with redox reactions, complex formation and colour/state changes. Includes kit list and safety instructions.

Transition elements and complex compounds microscale experiment | 16–18 years
Try this microscale practical investigating the transition elements, complex formation and change in oxidation state. Includes kit list and safety instructions

Analysis of aspirin tablets on a microscale
Try this microscale class practical to analyse aspirin tablets and find out how much salicylic acid is present. Includes kit list and safety instructions.
The temperature changes induced by evaporation
Explore the rate of evaporation for a trio of liquids, using just a temperature strip, and our worksheet. Includes kit list and safety instructions.

Properties of stereoisomers
By soaking cotton wool in two limonene enantiomers, and adding a stereoisomer, students can explore the differences between each chemical and discuss how they each might react in different conditions. Includes kit list and safety instructions.

Using a microscale conductivity meter
Explore electrical conductivity with this practical that allows students to test different materials for how well a current will pass through them. Includes kit list and safety instructions.

Microscale extraction of copper
In association with Nuffield Foundation
Try this practical to reduce copper(II) oxide to copper using hydrogen, revealing their positions in the reactivity series. Includes kit list and safety instructions.

Practical potions microscale | 11–14 years
Observe chemical changes in this microscale experiment with a spooky twist.

Microscale technicians in trouble! investigation
Some solutions have been mixed up – help the technicians work out which is which

About this collection
A number of the practicals and demonstrations featured on this page are based on experiments previously published in Microscale chemistry: experiments in miniature . Royal Society of Chemistry members receive a 35% discount at the RSC bookshop.
- Contributors
- Email alerts
Site powered by Webvision Cloud
Practical analytical chemistry lab, manual lab
- December 2018
- In book: Practical analytical chemistry lab, manual lab (pp.1-28)

- Mustansiriyah University
Abstract and Figures

Discover the world's research
- 25+ million members
- 160+ million publication pages
- 2.3+ billion citations
- Recruit researchers
- Join for free
- Login Email Tip: Most researchers use their institutional email address as their ResearchGate login Password Forgot password? Keep me logged in Log in or Continue with Google Welcome back! Please log in. Email · Hint Tip: Most researchers use their institutional email address as their ResearchGate login Password Forgot password? Keep me logged in Log in or Continue with Google No account? Sign up
Experience Teachmint X - AI driven Interactive Flat Panels and Smart Boards

More from AARTI PATEL
Recommended content, learn from anywhere on any device.
Top Leader by G2
Top Performer by SourceForge
Top Leader by SoftwareSuggest
Ranked Amongst Top 25 Companies by LinkedIn
ISO27001 Certified
Most Preferred Workplace
We use cookies to enhance site navigation and analyse usage, read our Privacy Policy for more.

IMAGES
VIDEO
COMMENTS
General Practical Chemistry EXPERIMENTS REOPRTS 101 Chem & 104 Chem Text Book: Practical General Chemistry By Dr. Ahmad Al-Owais & Dr. Abdulaziz Al-Wassil. 2 LIST OF EXPERIMENTS Week Date Experiment Book Page 1 Determination of a Liquid Density 131 - 133 2 Preparation of a Standard Solution of Sodium Carbonate 63 - 86
The recent development of syllabi and textual material is an attempt to implement this basic idea. The present laboratory manual will be complementary to the textbook of Chemistry for Class XI. It is in continuation to the NCERT's efforts to improve upon comprehension of concepts and practical skills among students.
Expertly communicate the excitement of chemistry with these time-tested classroom practicals. These resources have been compiled from the book Classic chemistry experiments: a collection of 100 chemistry experiments developed with the support of teachers throughout the UK.. If you'd like to buy a copy of the book, visit our online bookshop.If you're a Royal Society of Chemistry member, don't ...
Experiment 1: Measurement and Density 10 1 Measurements and Density INTRODUCTION EVALUATION OF EXPERIMENTAL DATA One of the most generally accepted axioms in chemistry is that, despite all of the advances in theory during that past fifty years, chemistry is still an experimental science. The
doing any experiment or whenever any experiment is being done in the laboratory around you (eye protection must meet ANSI Z87.1 impact standards). Repeated failure to wear approved eye protection will result in dismissal from the experiment being conducted. 2. If you should get an irritating substance in your eye, move quickly to the eye washer
The list of required practicals for chemistry is shown in the table below with suggested practicals. Topic 1.2. Obtaining and using experimental data for deriving empirical formulas from reactions involving mass changes. Determine the formula of MgO Determine the formula of hydrated copper sulfate. Topic 1.3.
Core practical 2. Make up a volumetric solution and carry out a simple acid-base titration Measuring mass accurately: In many experiments the best method for measuring mass is 1. Measure mass on 2 or 3d.p. balance of a weighing bottle with the required quantity of solid in it 2. Empty mass into reaction vessel/flask 3. Reweigh the now empty ...
This expansive and practical textbook contains organic chemistry experiments for teaching in the laboratory at the undergraduate level covering a range of functional group transformations and key organic reactions.The editorial team have collected contributions from around the world and standardized them for publication.
Conduct a microscale experiment to show learners how to perform this procedure. Tried and tested resources for teaching chemistry practicals and delivering engaging demonstrations. Includes screen experiments to reinforce or prepare students for practicals as well mapped practicals which fulfill the required activities in your exam specification.
Department of Chemistry. Laboratory Manual. 5.301 Chemistry Laboratory Techniques. Prepared by Katherine J. Franz and Kevin M. Shea with the assistance of Professors Rick L. Danheiser and Timothy M. Swager Revised by J. Haseltine, Kevin M. Shea, Sarah A. Tabacco, Kimberly L. Berkowski, Anne M. Rachupka and John J. Dolhun. IAP 2013.
Making nylon: the 'nylon rope trick'. The 'nylon rope trick' is a classic of chemistry classrooms, by mixing decanedioyl dichloride and in cyclohexane you can create a solution that will form nylon strings when floated on an aqueous solution of 1,6-diaminohexane. Kit list and safety instructions included.
Very well designed book with a lot of educational information and experiments. Very lucid approach to performing experiments in an amateur chemistry lab. Simple projects such as building your own laboratory weighing balance from a tin can, how to test conductivity of solutions, making your own pH indicator dyes, how to make rayon, are described.
The Mole Concept. A mole (mol) is a unit of amount. 1 mol of something is equal to 6.022x1023 of that thing. For example, 1 mol of H2 molecules is equal to 6.022x1023 H2 molecules. 1 mol of goats is 6.022x1023 goats. There are two important formulas for determining the number of moles present of a compound.
The Virtual Lab is an online simulation of a chemistry lab. It is designed to help students link chemical computations with authentic laboratory chemistry. The lab allows students to select from hundreds of standard reagents (aqueous) and manipulate them in a manner resembling a real lab. More information and offline downloads. Please scroll below to find our collection of pre-written problems ...
Chemistry is a practical science and an appropriate correlation between teaching theory and practical leads to a better understanding. The detailed study of theory, behind an experiment in practical enables the students to perform the experiment properly. So I have dealt with the theory of experiments in details.
1. Name of the experiment and date of performing the experiment. 2. Aim of the experiment: Please be specific while writing this. For example, "To determine the rate of reaction of A and B using method of D". 3. Main Concepts/Principles used in the design of the experiment: Again, you have to be specific to the experiment.
ORGANIC CHEMISTRY 121 EXPERIMENT 1 SYNTHESIS OF ASPIRIN FROM SALICYLIC ACID Aspirin is one of the oldest and most common drugs in use today. It is both an analgesic (pain killer) and antipyretic (reduces fever). One method of preparation is to react salicylic acid (1 ) with acetic anhydride (2) and a trace amount of acid (equation 1). OH COOH O ...
chemistry practical experiments - Free download as PDF File (.pdf), Text File (.txt) or read online for free. This document appears to be notes from a chemistry experiment involving the qualitative analysis and identification of salts. The experiment involved heating salts with different reagents and observing any color changes. Observations such as the color of solutions and precipitates ...
A number of the practicals and demonstrations featured on this page are based on experiments previously published in Microscale chemistry: experiments in miniature. Royal Society of Chemistry members receive a 35% discount at the RSC bookshop. Tips, ideas and practical experiments to bring microscale chemistry into your classroom, using only ...
Analytical chemistry is the study of the separation, identification, and quantification of the chemical components (elements or compounds) of natural and artificial materials (sample), and the ...
Notes of B.Sc 1st Yr Prctcl Maths, Chemistry Practical B.Sc1stYr Chem Pract.pdf - Study Material. Experience Teachmint X - AI driven Interactive Flat Panels and Smart Boards ... Experiment 5 (Scheme 1): b-sc. Chem101Thchem102Th. 1 Likes. 102 Views. Copied to clipboard Dr Dharvinder Kumar. Nov 14, 2021. Study Material.