
Design an experiment to demonstrate hydrotropism.
To demonstrate hydrotropism, plant seeds in two identical containers with moist soil. Place one container in a uniformly moist environment and the other in a gradient (one side drier than the other). Observe root growth over several days. Roots in the gradient environment should grow towards the moist side, illustrating hydrotropism – the tendency of roots to grow towards moisture.
Let’s discuss in detail
The aim of this experiment is to demonstrate hydrotropism, the growth response of plants to moisture gradients. Begin by preparing two identical containers with moist, well-drained soil. Plant several seeds of the same species at equal depths in each container. Ensure that the seeds are known to exhibit hydrotropic responses, such as pea or bean seeds.
For one container (the experimental group), create a moisture gradient. This can be achieved by watering only one side of the container, keeping the other side relatively dry. The control group, the other container, should be uniformly watered to maintain consistent moisture throughout. This setup will allow you to compare root growth in a uniform moisture environment versus a gradient.
Download App for Class 10

Over the course of several days to a week, monitor and record the growth of the seedlings, focusing particularly on the direction of root growth. It’s important to keep all other conditions, such as light and temperature, consistent for both containers to ensure that any differences in growth direction are due to moisture levels alone.
In the container with the moisture gradient, observe the direction of the root growth. Hydrotropism should cause the roots to grow towards the moist side of the container, as roots naturally seek out water sources. In the control container, roots should grow downward more uniformly, following gravitropism (response to gravity) rather than hydrotropism.
Compare the root growth patterns in the two containers. The experimental group should exhibit a clear hydrotropic response, with roots growing towards the moisture. The control group’s roots should show a standard growth pattern, unaffected by a moisture gradient. This experiment demonstrates hydrotropism, highlighting how plants adapt their growth in response to environmental moisture conditions.
This simple experiment effectively illustrates the concept of hydrotropism, showing how roots navigate through soil to find water, an essential survival mechanism for plants in varying soil conditions.
Copyright 2024 by Tiwari Academy | A step towards Free Education


- Why Does Water Expand When It Freezes
- Gold Foil Experiment
- Faraday Cage
- Oil Drop Experiment
- Magnetic Monopole
- Why Do Fireflies Light Up
- Types of Blood Cells With Their Structure, and Functions
- The Main Parts of a Plant With Their Functions
- Parts of a Flower With Their Structure and Functions
- Parts of a Leaf With Their Structure and Functions
- Why Does Ice Float on Water
- Why Does Oil Float on Water
- How Do Clouds Form
- What Causes Lightning
- How are Diamonds Made
- Types of Meteorites
- Types of Volcanoes
- Types of Rocks
Hydrotropism
What is hydrotropism.
Hydrotropism is a type of tropic movement by which some plant parts, specifically root and stem , tend to move towards or away from water stimulus.
The term ‘hydrotropism’ is a combination of two words, ‘hydro’ and ‘tropism’. Here, ‘hydro ‘means ‘water’, and ‘tropism’ stands for ‘tropic movement’. Tropic movement is a directional movement displayed by a plant in response to any external stimuli, such as light, gravity, chemical, touch, temperature, and water. In this case, the external stimulus is water or moisture.
Example : The movement of plant roots towards water.
Based on whether the plant part moves towards or away from the water stimulus, hydrotropism can be of two types:
1. Positive hydrotropism: Here, the plant part tends to grow towards moisture or water stimulus. Example: Growth of plant roots towards relatively higher humidity or moisture content.
2. Negative hydrotropism: In this type, the plant part grows away from the water stimulus. Example: Growth of stem away from the moisture content.
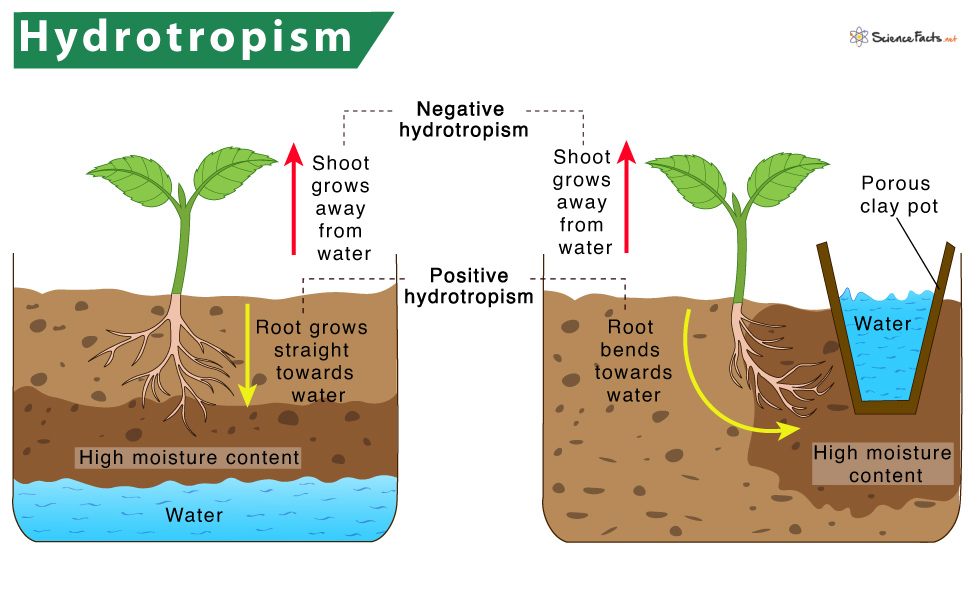
How does Hydrotropism Work in Plants
As discussed, the roots grow towards water as a response to hydrotropism. First, the root caps sense the moisture content and send a signal to the elongation zone of the root. Thus, the roots begin to grow towards that stimulus in search of more water. As water continuously moves in soil, the moisture gradient in the soil also keeps on changing. So, roots constantly change direction in response to the soil moisture content.
Why is Hydrotropism Important
- It modifies root growth towards areas with higher moisture content, thus helping in water uptake.
- It helps plants to obtain water efficiently even under drought conditions.
- Hydrotropism – Sciencedirect.com
- Hydrotropism: root growth responses to water – Cell.com
- Where’s the water? Hydrotropism in plants – Pnas.org
- Hydrotropism: how roots search for water – Academic.oup.com
Article was last reviewed on Thursday, February 9, 2023
Related articles
Leave a Reply Cancel reply
Your email address will not be published. Required fields are marked *
Save my name, email, and website in this browser for the next time I comment.
Popular Articles
Join our Newsletter
Fill your E-mail Address
Related Worksheets
- Privacy Policy
© 2024 ( Science Facts ). All rights reserved. Reproduction in whole or in part without permission is prohibited.
- Search Menu
- Sign in through your institution
- Advance articles
- Darwin Reviews
- Special Issues
- Expert View
- Flowering Newsletter Reviews
- Technical Innovations
- Editor's Choice
- Virtual Issues
- Community Resources
- Reasons to submit
- Author Guidelines
- Peer Reviewers
- Submission Site
- Open Access
- About Journal of Experimental Botany
- About the Society for Experimental Biology
- Editorial Board
- Advertising and Corporate Services
- Journals Career Network
- Permissions
- Self-Archiving Policy
- Dispatch Dates
- Journal metrics
- Journals on Oxford Academic
- Books on Oxford Academic

Article Contents
Introduction, plant hormones and hydrotropism, key hydrotropism genes, where and how do roots sense water, interaction between hydro- and gravitropism, can hydrotropism improve drought acclimation, conclusions and future directions, acknowledgements.
- < Previous
Hydrotropism: how roots search for water
- Article contents
- Figures & tables
- Supplementary Data
Daniela Dietrich, Hydrotropism: how roots search for water, Journal of Experimental Botany , Volume 69, Issue 11, 11 May 2018, Pages 2759–2771, https://doi.org/10.1093/jxb/ery034
- Permissions Icon Permissions
Fresh water is an increasingly scarce resource for agriculture. Plant roots mediate water uptake from the soil and have developed a number of adaptive traits such as hydrotropism to aid water foraging. Hydrotropism modifies root growth to respond to a water potential gradient in soil and grow towards areas with a higher moisture content. Abscisic acid (ABA) and a small number of genes, including those encoding ABA signal transducers, MIZ2/GNOM , and the hydrotropism-specific MIZ1 , are known to be necessary for the response in Arabidopsis thaliana , whereas the role of auxin in hydrotropism appears to vary depending on the plant species. This review will describe recent progress characterizing the hormonal regulation of hydrotropism. Recent advances in identifying the sites of hydrotropic perception and response, together with its interaction with gravitropism, will also be discussed. Finally, I will describe putative mechanisms for perception of the water potential gradient and a potential role for hydrotropism in acclimatizing plants to drought conditions.
Plants need to respond to a constantly changing environment and use tropisms to reposition organs for resource capture. Tropisms are directional growth movements that allow plants to respond to gravity, light, touch, water, salt, and oxygen ( Gilroy and Masson, 2008 ; Galvan-Ampudia et al. , 2013 ; Eysholdt-Derzsó and Sauter, 2017 ; Su et al. , 2017 ). In plant roots, gravity is described as the main driver determining the direction of root growth. Gravity is perceived in the columella cells of the root cap, where displacement of statoliths leads to a lateral gradient in the shootward auxin flux. More auxin flows through the lateral root cap and epidermis on the lower side of the root, leading to differential growth in the epidermis of the elongation zone, ultimately resulting in the root tip growing downwards ( Blancaflor et al. , 1998 ; Ottenschläger et al. , 2003 ; Swarup et al. , 2005 ; Friml, 2010 ; Rahman et al. , 2010 ). In comparison, relatively little is known about hydrotropism, the directional growth of plant roots towards a water source. Plant roots are able to perceive a water potential gradient in their surroundings and change the direction of the root tip through differential growth in the elongation zone.
Water is becoming increasingly scarce on our planet, and agriculture, which uses ~70% of all freshwater globally, claims the biggest share of this limited resource ( Davies and Bennett, 2015 ; World Water Assessment Programme, 2015 ). Making crop plants more resilient to drought stress has been highlighted as an important goal in a recent report on sustainable water use ( World Water Assessment Programme, 2015 ). Current strategies to improve water use efficiency through changes in root system architecture focus on increasing the steepness of roots to exploit water resources in lower soil horizons ( Henry et al. , 2011 ; Lynch, 2013 ; Uga et al. , 2013 ; Rogers and Benfey, 2015 ; Gao and Lynch, 2016 ). Hydrotropism allows roots to grow actively towards water sources which may be located in any direction. Understanding and modifying this response in plants should be considered as an additional strategy to pursue the goal of sustainable water use in agriculture.
Early descriptions of hydrotropism exist ( Bonnet, 1754 ; Knight, 1811 ) and, in the 19th century, Sachs, Molisch, Darwin, Wiesner, and others conducted experiments to determine which part of the root tip is necessary for the perception of the water signal ( Sachs, 1872 ; Darwin and Darwin, 1880 ; Wiesner, 1881 ; Molisch, 1883 ). Growing plant roots through a sieve with moist sawdust suspended at an angle or along the outside of a clay funnel, these experiments employed moisture gradients in air to observe the bending response of roots ( Fig. 1A , B ). Roots were covered in a mixture of olive oil and lamp black or cauterized with silver nitrate to determine the site of perception ( Darwin and Darwin, 1880 ). Although some of those studies came to the conclusion that the root tip is the site of perception for both gravity and water, others observed that the elongation zone is also able to perceive the water signal (for a review of early hydrotropism literature, see Hooker, 1915 ).

Historic and modern assays for hydrotropism. (A) Cross-section of the assay described by Sachs and used by the Darwins. Mesh covers the bottom of a round metal frame. Filled with moist sawdust and suspended at an angle, roots can grow through the mesh and need to bend in order to maintain contact with moisture provided by the sawdust. Redrawn from Sachs (1872) . (B) Molisch’s hydrotropism assay. Roots grow through holes in the rim of a clay funnel connected to a water reservoir. Once roots reach the edge of the funnel, they have to bend in order to stay in contact with the moisture provided by the funnel surface. Redrawn from Molisch (1883) . (C) Moisture in air assay. Inside a box, seedlings are mounted on a water-soaked foam or agar bloc with the root tip pointing down, and suspended in air. The water potential gradient between the moisture-containing support and the surrounding air is further increased by a dish containing a concentrated salt solution. Roots need to bend around the edge of the support in order to stay in contact with their water supply. (D) Split-agar assay. Seedlings are placed in a square Petri dish on growth medium which is in direct contact with another growth medium containing an osmolyte. Diffusion of the osmolyte establishes a water potential gradient that is able to deflect root tip growth from following the gravity vector. (E, F) Rice root bending hydrotropically in the moisture in air assay (Nakajima et al ., Auxin transport and response requirements for root hydrotropism differ between plant species, Journal of Experimental Botany 2017, 68 , 3441–3456, by permission of the Society of Experimental Biology). (G, H) Arabidopsis thaliana roots bending hydrotropically in the split-agar assay. The white dashed line indicates the border between the two different growth media. The arrow labelled g indicates the gravity vector in all assays.
Loomis and Ewan (1936) conducted the first assessment of how plant roots grow in soil with different water availability. Utilizing the fact that soil of a certain moisture content does not lose water to adjacent, drier soil through capillary action ( Veihmeyer and Hendrickson, 1927 ), they created water gradients in soil to test the growth response of plant roots. Placing germinating seeds at the border between soil with either 4% or 11.8% moisture (the wilting coefficient of that soil was 7.1%), roots growing into the dry soil stopped their growth, whereas roots growing in the wet soil continued to grow and produced lateral roots ( Loomis and Ewan, 1936 ). This is not a directional growth response in the strictest sense, but slight modifications allowed hydrotropism to be observed. When the border between wet and dry soil was set at a 45° angle and seeds were placed in wet soil some distance away from the border between wet and dry soil, several plant species, including beans ( Phaseolus limensis and Phaseolus vulgaris ), buckwheat ( Fagopyrum esculentum ), and foxtail millet ( Setaria italica ), had roots that did not grow along the gravity vector into dry soil but were bending to follow the line between dry and wet soil ( Loomis and Ewan, 1936 ).
In recent years, there has been renewed interest in hydrotropism (reviewed in Monshausen and Gilroy, 2009 ; Cassab et al. , 2013 ; Moriwaki et al. , 2013 ; Shkolnik and Fromm, 2016 ), and hydrotropic responses have been shown for pea ( Pisum sativum ), cucumber ( Cucumis sativus ), wheat ( Triticum aestivum ), maize ( Zea mays ), rice ( Oryza sativa ), birdsfoot trefoil ( Lotus japonicus ), sitka spruce ( Picea sitchensis ), and Arabidopsis thaliana ( Table 1 ) ( Jaffe et al. , 1985 ; Takahashi and Scott, 1991 ; Coutts and Nicoll, 1993 ; Oyanagi et al. , 1995 ; Mizuno et al. , 2002 ; Takahashi et al. , 2002 ; Nakajima et al. , 2017 ). Although other methods to test hydrotropism exist ( Tsuda et al. , 2003 ; Eapen et al. , 2015 ), most hydrotropism assays are currently performed using two systems ( Fig. 1C–H ). For the moisture in air assay ( Fig. 1C , E , F ), seedlings are mounted on a support, usually foam or agar blocks, in such a way that just the very root tip is suspended in air. The support acts as a source of water, and the moisture gradient to the surrounding air is increased by placing the mounted seedlings in an enclosed environment with a concentrated salt solution ( Takahashi et al. , 2002 ; Morohashi et al. , 2017 ). Alternatively, a water potential gradient can be imposed in a split-agar-based system by adding an osmolyte, (e.g. sorbitol), to the growth medium and placing this in direct contact with the growth medium without additives ( Fig. 1D , G , H ). Seedlings are transferred to these plates and placed with their root tips a set distance away from the border between the two growth media ( Antoni et al. , 2016 ). In both cases, roots will experience a water potential gradient, with a wet (in contact with the foam/agar support or closer to the growth media without osmolyte) and dry (facing the air or closer to the growth media with osmolyte) side to the root. Roots showing a hydrotropic response will change the growth direction of the root tip, bending either around the supporting block or towards the medium with higher water potential. The resulting angle of deflection from vertical, gravitropic growth is then measured. These assays have been used to identify genes involved in hydrotropism and characterize cellular and molecular events of the response.
This review will provide an overview of the signalling pathways and genes involved in hydrotropism, species-specific differences in the response, putative mechanisms for perception of the water gradient, describe interaction between hydrotropism and gravitropism, possible contributions of hydrotropism to drought resilience, and concludes with a series of future directions for hydrotropism research.
Auxin plays a central role in several tropisms and might be involved in hydrotropism too. However, the requirement for auxin in the hydrotropic response varies depending on the plant species examined ( Table 1 ). In A. thaliana , the agravitropic auxin transport mutants aux1 and pin2 are not impaired in their hydrotropic response ( Takahashi et al. , 2002 ). Likewise, the auxin transport inhibitors 2,3,5-triiodobenzoic acid (TIBA), 1-naphthylphthalamic acid (NPA), and 3-chloro-4-hydroxyphenylacetic acid (CHPAA) are unable to block hydrotropism; in fact, treatment with TIBA or NPA leads to an earlier increase in root tip angle in hydrotropism assays, even though final angles remain the same ( Kaneyasu et al. , 2007 ; Shkolnik et al. , 2016 ). In addition, expression of the auxin reporters DII-Venus and DR5 remains unchanged throughout the hydrotropism response ( Ponce et al. , 2008 b ; Takahashi et al. , 2009 ; Shkolnik et al. , 2016 ). Even though hydrotropism in A. thaliana does not require auxin transport, a functioning response to auxin appears to be necessary for hydrotropism. Treatment with auxin response inhibitors led to contrasting results. Whereas p -chlorophenoxyisobutylacetic acid (PCIB) produced a decrease in hydrotropic response, addition of auxinole or α-(phenylethyl-2-oxo)-indole acetic acid (PEO-IAA) accelerated the response ( Kaneyasu et al. , 2007 ; Shkolnik et al. , 2016 ). These contrasting results could be due to different modes of action and specificities of inhibitors. Auxinole and PEO-IAA have been shown to bind to the auxin receptor TRANSPORT INHIBITOR RESPONSE 1 (TIR1), whereas the mode of action of PCIB is still unclear ( Oono et al. , 2003 ; Hayashi et al. , 2008 , 2012 ). PCIB is unable to reverse the effects of exogenous IAA application on root growth, which both auxinole and PEO-IAA are able to do ( Oono et al. , 2003 ; Hayashi et al. , 2008 , 2012 ). The more specific inhibitors auxinole and PEO-IAA indicate that auxin has a negative influence on hydrotropism in A. thaliana , but this awaits independent confirmation from experiments with auxin response mutants.
Plant species-specific differences in hydrotropism
| Plant species . | Gravitropism masks hydrotropism . | Root cap needed for hydrotropism . | Auxin transport inhibitor blocks hydrotropism . | Auxin response inhibitor blocks hydrotropism . | Auxin biosynthesis inhibitor blocks hydrotropism . | Hydrotropism genes . | References . |
|---|---|---|---|---|---|---|---|
| Pea | Yes | ND | Yes/no | Only at 100 µM PCIB | ND | ND | (1985); (2017) |
| Cucumber | Yes | No | Yes | Yes (PCIB) | ND | , | (2002); (2017) |
| No | No | No | Yes (PCIB), no (auxinole, PEO-IAA) | ND | , , , , , | (2002); (2007); (2007); (2009 ); (2010); (2013); (2016); (2017) | |
| Rice | No | No | Yes | Yes (PCIB) | Yes | ND | (2017) |
| No | ND | No | No (PCIB) | Yes | ND | (2017) |
| Plant species . | Gravitropism masks hydrotropism . | Root cap needed for hydrotropism . | Auxin transport inhibitor blocks hydrotropism . | Auxin response inhibitor blocks hydrotropism . | Auxin biosynthesis inhibitor blocks hydrotropism . | Hydrotropism genes . | References . |
|---|---|---|---|---|---|---|---|
| Pea | Yes | ND | Yes/no | Only at 100 µM PCIB | ND | ND | (1985); (2017) |
| Cucumber | Yes | No | Yes | Yes (PCIB) | ND | , | (2002); (2017) |
| No | No | No | Yes (PCIB), no (auxinole, PEO-IAA) | ND | , , , , , | (2002); (2007); (2007); (2009 ); (2010); (2013); (2016); (2017) | |
| Rice | No | No | Yes | Yes (PCIB) | Yes | ND | (2017) |
| No | ND | No | No (PCIB) | Yes | ND | (2017) |
ND, not determined
Auxin’s function in hydrotropism has been explored in four other plant species, cucumber, rice, birdsfoot trefoil, and pea. Gravitropism usually masks the hydrotropism response in both cucumber and pea, hence experiments with pea use the ageotropum mutant which is completely agravitropic, whereas experiments with cucumber seedlings are conducted either under microgravity or clinorotation, or after removal of the root tip ( Jaffe et al. , 1985 ; Morohashi et al. , 2017 ). In cucumber, the Aux/IAA gene CsIAA1 (sometimes also referred to as CsIAA12 ) is differentially expressed within 30 min of exposure to a gravity or water stimulus, with increased expression occurring on the concave side of the bending root ( Mizuno et al. , 2002 ). Increased expression on the concave side of hydrotropically bending roots has also been observed for other CsIAA genes ( Morohashi et al. , 2017 ). Treatment with the auxin transport inhibitors TIBA and 9-hydroxyfluorene-9-carboxylic acid (HFCA) strongly reduces the hydrotropic response in cucumber, while PCIB and brefeldin A (BFA) have a less strong inhibitory effect ( Morohashi et al. , 2017 ). CsPIN5 , which is localized in the epidermis and lateral root cap and like AtPIN2 may function in shootward transport of auxin from the root tip, is decreased on the convex side of gravitropically bending roots and on the dry side of roots exposed to a water potential gradient ( Morohashi et al. , 2017 ). Surprisingly, this differential CsPIN5 localization also takes places in hydrotropically stimulated roots that show no response because they are exposed to normal gravity ( Morohashi et al. , 2017 ). Auxin efflux transport inhibitors (HFCA, NPA, and TIBA) disrupt hydrotropism in the pea ageotropum mutant, whereas inhibitors of auxin influx (CHPAA and 1-naphthoxyacetic acid) do not seem to have a discernible effect on the response ( Nakajima et al. , 2017 ).
In rice, inhibitors of auxin transport (CHPAA and TIBA), response (PCIB), and biosynthesis (kynurenine) inhibit hydrotropism, and the effect of the latter can be rescued by exogenous application of IAA ( Nakajima et al. , 2017 ). Interestingly, the hydrotropic response of birdsfoot trefoil is only inhibited by kynurenine application, which again can be rescued by IAA application, whereas CHPAA, TIBA, and PCIB do not affect hydrotropism ( Nakajima et al. , 2017 ). It seems surprising that auxin biosynthesis, but not signalling, is necessary for hydrotropism in birdsfoot trefoil. Signal transduction through ABP1, which recently has been shown not to be involved in auxin signalling ( Enders et al. , 2015 ; Gao et al. , 2015 ), has been invoked to explain this discrepancy ( Nakajima et al. , 2017 ). An alternative explanation may be that PCIB is not specific enough to inhibit the response in L. japonicus , and that a more potent inhibitor (e.g. auxinole) could prove the necessity for auxin signalling. In summary, the involvement of auxin in hydrotropism varies widely in a plant species-specific manner. Plants usually have species-specific water requirements for successful completion of their life cycle, which might explain why gravitropism overrides hydrotropism in some species, whereas in others (e.g. rice) hydrotropism is independent of gravitropism, but still requires auxin. Understanding the role of auxin in hydrotropism will be important to understanding how gravi- and hydrotropic signals are integrated to determine the growth direction of the root tip.
Abscisic acid (ABA) is involved in many processes in plant development and physiological responses, but is perhaps best known for its function in the response to drought and osmotic stress ( Yamaguchi-Shinozaki and Shinozaki, 2006 ; Cutler et al. , 2010 ). The core components of the ABA signalling pathway consist of cytosolic receptors of the START-domain superfamily (PYR/PYL/RCAR), clade A, type 2C protein phosphatases (PP2C), and a subclass III Snf1-related kinases (SnRK2) ( Cutler et al. , 2010 ). ABA leads to the formation of a ternary receptor–hormone–phosphatase complex that relieves the inhibition of SnRK2 kinases by PP2C phosphatases, allowing the phosphorylation of downstream targets ( Fujii et al. , 2009 ; Ma et al. , 2009 ; Park et al. , 2009 ). In A. thaliana , the ABA biosynthesis mutant aba1-1 has a reduced hydrotropic response, but this defect is rescued by the exogenous application of ABA ( Takahashi et al. , 2002 ). ABA signal transduction mutants also have an altered hydrotropic response, with the gain-of-function PP2C mutant abi2-1 and a hextuple receptor mutant showing a reduced response, whereas it is increased in a loss-of-function quadruple pp2c mutant ( Takahashi et al. , 2002 ; Antoni et al. , 2013 ).
The most detailed exploration of the role of ABA signalling in hydrotropism has been conducted for the SnRK2 kinases. Three family members, SnRK2.2 , SnRK2.3 , and SnRK2.6 , are known to be involved in ABA signalling, and the snkr2.2 snrk2.3 double mutant has a strongly reduced hydrotropism ( Mustilli et al. , 2002 ; Fujii et al. , 2007 ; Dietrich et al. , 2017 ). Tissue-specific expression of SnRK2.2 in the double mutant background showed that expression in the cortex alone is able to rescue the response ( Dietrich et al. , 2017 ). Exogenous ABA at low concentrations promotes root elongation through increasing the length of root cells at maturity and, in the snkr2.2 snrk2.3 mutant, SnRK2.2 expression in the cortex was able to rescue this effect ( Dietrich et al. , 2017 ). A mathematical model examining the contribution of the cortex to root bending predicted that differential elongation in the cortex could be the driving force behind hydrotropic bending ( Dietrich et al. , 2017 ). This was further confirmed by blocking differential elongation in a tissue-specific manner, which only blocked hydrotropism if the cortex was affected ( Dietrich et al. , 2017 ). Together, this led to the proposal that ABA-mediated differential elongation in the cortex is the driving force behind the changes in growth direction observed in hydrotropism ( Dietrich et al. , 2017 ) ( Fig. 2 ). With auxin transport and response in the lateral root cap and epidermis driving gravitropism ( Swarup et al. , 2005 ), the distinct role of the cortex in hydrotropism indicates that there are tissue-specific and mechanistic differences between responses to gravity and water. The position of ABA in the signalling cascade for hydrotropism is currently unclear. The rescue of the hydrotropic defect of the aba1-1 mutant by application of exogenous ABA, which is non-directional, could be taken as an indication that hydrotropic signalling does not involve an ABA gradient across the root. On the other hand, hydrotropic signalling could involve changes in ABA sensitivity on the dry and wet side of the root. It is also still unknown if the water potential gradient across the root affects the radial transport of water and signalling molecules. It seems possible that a water potential gradient could lead to changes in the direction of water flow on the dry and wet side of the root, with water flowing towards the stele on the wet side and away from the stele on the dry side of the root. This differential water flow could affect the transport direction of signalling molecules, including ABA. These different hypotheses about the mechanism of ABA in the hydrotropic response still await experimental verification. In addition, the requirement for ABA in hydrotropism of plant species other than A. thaliana still needds to be examined.

Hydrotropism mechanism in Arabidopsis thaliana. A. thaliana roots exposed to a water potential gradient perceive reduced water availability through an as yet unknown mechanism in the elongation zone. Reactive oxygen species (ROS) are able to inhibit hydrotropism, but currently the stage at which the response is affected is unknown. Abscisic acid and MIZ2/GNOM are required for hydrotropism and could be involved in perception and differential growth. The role of auxin is currently unclear, but a lateral auxin gradient does not develop during hydrotropism in A. thaliana . Bending of the root tip is achieved by differential elongation of cortex cells; abscisic acid and expression of MIZ1 and SnRK2.2 in the cortex cell file are required for this. Hydrotropic bending of the root tip will trigger a gravitropic response through statolith relocalization, which provides feedback inhibition. Statoliths and differentially expanding cortex cells have been drawn for emphasis and are not to scale.
Forward genetic screens have only led to the isolation of a few hydrotropism-related genes in A. thaliana ; no hydrotropic response 1 ( nhr1 ) and altered hydrotropic response 1 ( ahr1 ) are semi-dominant mutants affected in hydrotropism ( Eapen et al. , 2003 ; Saucedo et al. , 2012 ). Homozygous nhr1 plants never reach the reproductive stage, and the genes affected in both mutants have not yet been cloned ( Eapen et al. , 2003 ; Saucedo et al. , 2012 ; Salazar-Blas et al. , 2017 ).
mizu-kussei 1 ( miz1 ), described by Kobayashi et al. (2007) , is caused by a recessive mutation in At2g41660. Apart from a complete absence of hydrotropism and slightly reduced root phototropism and waving, miz1 plants grow normally and in particular show a normal gravitropism response and root tip anatomy ( Kobayashi et al. , 2007 ). Overexpression of MIZ1 leads to increased root curvature in hydrotropism assays ( Miyazawa et al. , 2012 ). Unfortunately, MIZ1 is a protein of unknown function, containing only a conserved domain of uncharacterized function (DUF617 domain). Homologues containing a DUF617 domain have been found in rice and Physcomitrella patens but not in algae, suggesting that acquisition of MIZ1 function may have taken place during the evolution of land plants ( Kobayashi et al. , 2007 ). It is still unclear at which step of the hydrotropism response MIZ1 functions, but subcellular localization of MIZ1 showed that it is a soluble protein associated with the cytosolic side of the endoplasmatic reticulum (ER) membrane ( Yamazaki et al. , 2012 ). ABA and blue light are both able to up-regulate MIZ1 expression ( Moriwaki et al. , 2012 ). MIZ1 itself appears to influence auxin accumulation, as free IAA concentrations in miz1 and MIZ1 -overexpressing roots increase and decrease, respectively ( Moriwaki et al. , 2011 ). Whether this is directly linked to the role of MIZ1 in hydrotropism is unclear, and overexpression or loss of MIZ1 function do not affect PIN gene expression and localization ( Moriwaki et al. , 2011 ). A MIZ1–green fluoprescent protein (GFP) fusion under the control of its own promoter has shown that the protein is strongly expressed in cortex cells around the transition zone between the meristem and elongation zone, the lateral root cap and columella, and also, to a lesser extent, in the epidermis and stele, but MIZ1–GFP intensity and localization do not change during the hydrotropic response ( Yamazaki et al. , 2012 ; Moriwaki et al. , 2013 ). Recently, it was shown that expression of MIZ1 in the cortex alone is able to rescue the hydrotropism response of miz1 mutants, highlighting the important role of this tissue in hydrotropism ( Dietrich et al. , 2017 ) ( Fig. 2 ).
A second mutant isolated through forward screens, miz2 , is a weak GNOM allele (G951E) ( Miyazawa et al. , 2009 b ). GNOM is a GDP/GTP exchange factor for small G proteins of the ARF class (ARF-GEF) regulating intracellular vesicle trafficking, whose best characterized function is polar targeting of PIN proteins to the plasma membrane ( Geldner et al. , 2003 ). Importantly, miz2 does not affect auxin response or PIN localization ( Miyazawa et al. , 2009a , b ). The G951E mutation of miz2 is downstream of the Sec7 domain and affects an amino acid conserved in GNOM homologues in other plant species ( Miyazawa et al. , 2009 b ). Treatment with BFA, a known inhibitor of ARF-GEFs, phenocopies miz2 . In addition, the hydrotropic response of the BFA-resistant GN M696L allele cannot be blocked by BFA, whereas the weak gnom B/E allele is ahydrotropic ( Miyazawa et al. , 2009 b ). Supporting evidence of the importance of vesicle trafficking for hydrotropism comes from a phospholipase D mutant that is slightly impaired in hydrotropism ( Taniguchi et al. , 2010 ).
There appears to be no direct interaction between MIZ2 and MIZ1, as MIZ1–GFP is still correctly localized in the miz2 mutant ( Moriwaki et al. , 2011 ). Interestingly though, miz2 plants that overexpress MIZ1 show an ahydrotropic phenotype, demonstrating that MIZ2 is epistatic to MIZ1 ( Miyazawa et al. , 2012 ).
Which part of the root is able to sense a gradient in water availability is a question that has fascinated people since the early days of hydrotropism research. The Darwins describe experiments where covering the root tip with a mixture of olive oil and lamp black abolishes the hydrotropic response, concluding that the very root tip is necessary for the perception of gravity and water. This led them to coin the, since then much repeated, description of the root tip as the ‘brain of the root’ ( Darwin and Darwin, 1880 ). Darwin’s contemporaries already criticized those experiments, especially with regards to the effect of the applied mixture on root growth rates and the difficulties in applying the mixture in an even manner and to a precise region of the root ( Wiesner, 1881 ; Molisch, 1883 ). Similar problems affect more recent experiments. A role for the root cap in hydrotropism perception was reported for pea and maize, but root growth rates were not always recorded ( Takahashi and Scott, 1991 , 1993 ; Takahashi and Suge, 1991 ; Takano et al. , 1995 ; Hirasawa et al. , 1997 ). In addition, while surgical ablation experiments record the length of root tip removed, usually no relationship to anatomical markers along the root axis is given and it is therefore difficult to know whether just the columella or larger parts, including the meristem or perhaps even the elongation zone, were removed. Miyazawa et al. (2008) used heavy-ion microbeam irradiation and laser ablation to ablate either the columella or what is described as the elongation zone of A. thaliana roots, and reported conflicting results. While irradiation of the elongation zone led to a reduction in hydrotropic bending, the same treatment of the columella did not ( Miyazawa et al. , 2008 ). On the other hand, laser ablation of the columella did reduce the hydrotropic response ( Miyazawa et al. , 2008 ). However, root growth rates following both treatments were extremely slow, so that these results have to be considered with caution. More recently, laser ablation and microdissection were again used to determine the root tissue responsible for hydrotropism perception in A. thaliana . Root growth rates were reported for these experiments and were in the expected range. Whereas laser ablation of the columella inhibited the gravitropic response as reported by Blancaflor et al. (1998) , the hydrotropic response was not perturbed ( Dietrich et al. , 2017 ). Removal of the root cap and meristem by either laser ablation or microdissection also did not inhibit hydrotropism, demonstrating that the elongation zone of the root is able to perceive and respond to the hydrotropic signal ( Dietrich et al. , 2017 ) ( Fig. 2 ). While some contribution from the columella and root cap in hydrotropism perception cannot be totally excluded, these results place perception for hydro- and gravitropism in separate tissues. In addition, removal of the columella in rice and cucumber does not impair hydrotropism, demonstrating that in other plant species hydrotropism perception also does not depend on this tissue ( Morohashi et al. , 2017 ; Nakajima et al. , 2017 ; Fujii et al. , 2018 ).
How could roots be able to sense a water potential gradient in the elongation zone? The difference in water potential across the root is rather small, and was calculated to reach a maximum of <10 kPa across a 100 µm wide A. thaliana root during a standard split-agar hydrotropism assay, which is <3% of the maximum absolute water potential experienced at the root midline ( Dietrich et al. , 2017 ). Mechanosensitive ion channels could potentially be triggered by changes in cell volume if a root is exposed to a water potential gradient ( Hamilton et al. , 2015 ). Pea is the only plant species where turgor measurements have been performed during hydrotropism, but differences in turgor between the wet and dry side of the root were not observed ( Hirasawa et al. , 1997 ; Miyamoto et al. , 2002 ).
The miz2 (GN G951E ) phenotype strongly implies that membrane proteins play an important part in the hydrotropism response ( Miyazawa et al. , 2009 b ). Although GNOM is best known for its role in endosomal recycling of PIN proteins ( Geldner et al. , 2003 ), localization of PIN1 is unaffected in miz2 ( Miyazawa et al. , 2009 a ). Hydrotropism may rely on endosomal recycling of other proteins, trafficking from the ER to the Golgi or endocytosis, processes that also rely on GNOM ( Paez Valencia et al. , 2016 ).
It is highly likely that hydrotropism is intricately linked to water uptake and transport in the root. Radial water uptake from the soil towards the xylem vessels in the vasculature follows two paths, the apoplastic route along cell walls and the cell-to-cell path that is comprised of transcellular (across membranes) and symplastic (through plasmodesmata) transport ( Li et al. , 2014 ). Root hydraulic conductivity (Lp r ) is a measure for water transported through the root. Aquaporins are membrane channels that transport water and small neutral molecules, and one subfamily, the plasma membrane intrinsic proteins (PIPs), contributes significantly to Lp r ( Sutka et al. , 2011 ; Li et al. , 2014 ). How could aquaporins and changes in Lp r contribute to hydrotropic signalling? Lp r is reduced by abiotic stress in many plant species ( Aroca et al. , 2012 ). PIP activity is regulated at many levels—transcriptionally, translationally, through gating of the channel itself by phosphorylation, protons, or divalent cations, and by cellular trafficking ( Li et al. , 2014 )—and reduction of root hydraulic conductivity under abiotic stress could be achieved using any of these regulatory mechanisms. For salt stress, it was demonstrated that treatment with 100 mM sodium chloride reduces Lp r by ~60% within 1 h and decreases aquaporin transcript abundance ( Boursiac et al. , 2005 ). Down-regulation of aquaporin gene expression, however, takes longer than the decrease in Lpr, but other regulation mechanisms respond more rapidly to salt stress. At 45 min after the start of salt treatment, a substantial amount of a PIP2;1–GFP fusion protein had become internalized, and removal from the plasma membrane involved clathrin and membrane raft-associated pathways ( Boursiac et al. , 2008 ; Li et al. , 2011 ). Another pathway for removal of aquaporins from the plasma membrane involves tryptophan-rich sensory protein/translocator (TSPO), which is induced by abiotic stress and was shown to interact with PIP2;7, leading to internalization and autophagic degradation of the aquaporin ( Hachez et al. , 2014 ). In addition, PIP1;2 and PIP2;1 were recently shown to interact directly with receptor-like kinases (RLKs) in the plasma membrane and were regulated in their water transporting activity by this interaction ( Bellati et al. , 2016 ). Similar to the examples for salt and TSPO regulating PIPs at the plasma membrane, the low water potential during hydrotropism could affect the presence in the membrane of aquaporins through endosomal recycling, which would explain the requirement for MIZ2/GNOM ( Fig. 3 ). The interaction of aquaporins with RLKs could also be affected by low water potential, possibly leading to changes in cell elongation through signalling via the RLKs in addition to regulation of PIP activity by the RLKs ( Fig. 3 ). These hypothetical regulation mechanisms of aquaporin activity or membrane presence could lead to a change in hydraulic conductivity, with two possible outcomes. Cell or tissue growth could be affected by changes in Lp r , as was demonstrated for lateral root primordia emergence ( Péret et al. , 2012 ). Alternatively, hydraulic conductivity was shown to affect radial ABA transport along the apoplastic pathway through solvent drag ( Freundl et al. , 1998 ). This could lead to changes in ABA concentration on the dry and wet side of the root, driving differential cell elongation.

Potential mechanisms for perception and response to low water potential. Low water potential could affect the membrane presence or activity of plasma membrane intrinsic proteins (PIPs). This could affect cell elongation through several independent pathways: PIPs were shown to interact directly with receptor-like kinases (RLKs) in the plasma membrane. This interaction was shown to regulate PIP activity, but could potentially also affect signalling from the RLK to change cell elongation. Changes in aquaporin activity or presence due to low water potential will also lead to a change in hydraulic conductivity, with two possible outcomes. Hydraulic conductivity could affect cell elongation directly (as demonstrated for lateral root primordia), but can also affect radial ABA transport in the root. Changes in local ABA concentration could be the driver of differential cell elongation, leading ultimately to root bending. Perception would not necessarily require sensing of a water potential gradient at opposing sides of the root, but could work through a water potential set point, below which PIP membrane presence or activity changes, initiating the signal cascade leading to cell elongation. MIZ2/GNOM is required to facilitate cycling of PIPs (and RLKs) to and from the plasma membrane in this model. Aquaporin regulation in a single layer or all tissue layers of the root may be necessary for this mechanism.
These hypothetical perception mechanisms linked to aquaporins would not necessarily require sensing of the water potential gradient at opposing sides of the root, but could utilize a water potential set point, below which aquaporin membrane presence or activity changes, setting in motion the signalling cascade leading to cell elongation. However, at the moment, the identity of the hydrotropic signal perceived by the root is still unclear.
Several tropisms can adjust the growth direction of the root tip, and interaction and competition between the responses to different environmental cues will determine the final growth direction. To understand hydrotropism, its interaction with gravitropism is central. It has been argued that gravitropism determines the ‘default’ growth direction of the root, which is then adjusted by tropic responses to other environmental cues ( Blancaflor and Masson, 2003 ; Rosquete and Kleine-Vehn, 2013 ; Krieger et al. , 2016 ). In the interaction between hydro- and gravitropism, a clear distinction has to be drawn between plant species that depend on auxin and its transport for their hydrotropic response and those where hydrotropism is independent of development of a lateral auxin gradient. In those plant species which require auxin transport, the gravitropic response can be assumed to counteract hydrotropism, unless the water potential gradient aligns with the gravity vector. This would explain why hydrotropism in pea and cucumber can only be observed if gravitropism has been removed. Still, there are plants (e.g. rice), that rely on auxin transport for both tropisms but react to a water gradient in the presence of gravity. How can such differences be explained? It is still unclear whether plant species requiring auxin for hydrotropism develop a lateral auxin gradient during the response. If they do, species-specific differences in the interaction between gravi- and hydrotropism could be due to differences in the establishment of these auxin gradients.
Another factor influencing the interaction between the two tropisms could be timing and sensitivity of each response. Presentation time, defined as the minimum exposure time needed to elicit a response, has been determined for the gravitropism response of various plant roots ( Kiss and Sack, 1989 ; Kiss et al. , 1996 ; Hou et al. , 2003 ). Usually, root curvature in response to a 90° stimulus is plotted against stimulation time and the presentation time determined by regression analysis ( Kiss et al. , 1996 ). For hydrotropism, the presentation time has so far only been determined for ageotropum peas following the method described for gravitropism ( Stinemetz et al. , 1996 ). Equally, data on the strength of the water potential gradient necessary for triggering hydrotropism are scarce ( Takano et al. , 1995 ). Natural variation has been reported to exist for gravitropic presentation times ( Tanimoto et al. , 2008 ; Moulia and Fournier, 2009 ), and a more detailed examination of presentation times and response strength for both hydro- and gravitropism should help to understand species-specific differences in the interaction between those tropisms.
In A. thaliana , hydrotropism is independent of the development of a lateral auxin gradient ( Shkolnik et al. , 2016 ). Plants treated with auxin transport and response inhibitors ( Shkolnik et al. , 2016 ) and the pgm1 mutant which lacks statoliths ( Takahashi et al. , 2003 ) show a faster hydrotropic response. Together with the observation that statolith degradation occurs in roots exposed to a water potential gradient in A. thaliana and Raphanus sativus ( Takahashi et al. , 2003 ; Ponce et al. , 2008 a ), this has been taken as evidence to support the hypothesis that gravitropic responsiveness needs to be reduced so that hydrotropism can take place. In contrast, exposure of roots to 150 mM sodium chloride leads to agravitropic growth and degradation of statoliths, but several salt overly sensitive mutants, which display the same agravitropic growth on medium with salt, retain their statoliths ( Sun et al. , 2008 ). This indicates that statolith degradation on exposure to environmental stress may be a mere correlation and not causative for the response. In addition, the agravitropic pin2 and aux1 mutants do not have an accelerated hydrotropic response ( Takahashi et al. , 2002 ). Detailed analysis of the kinetics of gravitropism shows that the rate of gravitropic root bending in A. thaliana depends on the stimulation angle, with smaller stimulation angles resulting in reduced bending rates ( Mullen et al. , 2000 ). In addition, a threshold angle of 15° from the vertical has to be reached before 50% of a population of seedlings respond gravitropically ( Mullen et al. , 2000 ). Therefore, a water potential gradient can lead to a substantial change in root angle before a gravity response is triggered. Furthermore, this gravitropic response will be slow to begin with, as the stimulation angle is small.
Recently, a study investigated the interaction between hydro- and gravitropism and the role of reactive oxygen species (ROS) ( Krieger et al. , 2016 ). A very interesting observation of this study was that hydro- and gravitropism lead to bending of the root tip in different regions, with gravitropic bending initiating relatively close to the root tip in the distal elongation zone whereas hydrotropic bending takes place in a more shootward region of the elongation zone (central elongation zone) ( Krieger et al. , 2016 ), which provides further confirmation that gravitropism and hydrotropism employ different tissues in their bending mechanisms.
Auxin-induced ROS production is necessary for gravitropism ( Joo et al. , 2001 ), and using the fluorescent dye dihydrorhodamine-123, Krieger et al. (2016) demonstrated that 2 h after gravistimulation a transient ROS increase was visible on the concave side of the distal elongation zone of the bending root. Using the moisture in air assay, a ROS increase was observed on the concave side of the central elongation zone of hydrotropically bending roots ( Krieger et al. , 2016 ). However, when calcium chloride was replaced with distilled water in the assay (i.e. under conditions that do not induce hydrotropic bending in roots), a similar ROS increase in the same location was observed ( Krieger et al. , 2016 ). ROS distribution, however, was unchanged when hydrotropism was induced in roots using the split-agar assay, and the authors attribute the spurious ROS accumulation in the moisture in air assay to the mechanical tension the roots were under ( Krieger et al. , 2016 ). Treatment with ROS scavengers and NADPH oxidase inhibitors showed that ROS production in fact inhibited hydrotropism ( Krieger et al. , 2016 ). Ascorbate peroxidase ( apx1-2 ) and respiratory burst oxidase homologue ( rbohC ) mutants showed decreased and increased hydrotropic curvature, respectively, further confirming the inhibition of hydrotropism by ROS ( Krieger et al. , 2016 ). How ROS inhibit hydrotropism is currently unknown. Interestingly though, the same study also showed that after 4 h of hydrostimulation, a 90° gravitropic stimulus was unable to elicit an increase in ROS or a lateral auxin gradient ( Krieger et al. , 2016 ). Clearly more work is still necessary to understand exactly how hydro- and gravitropism interact, but this is an exciting first glimpse showing that hydrotropism is able to influence the gravitropic response.
Drought stress is a major limiting factor in crop production, and complex plant responses exist to escape, avoid, or tolerate limited water availability ( Wery, 2005 ; Gaur et al. , 2008 ). Drought can lead to an increase in the root to shoot ratio of plants, usually due to shoot growth being more strongly affected by drought ( Blum, 2005 ), and maintaining yield under drought conditions can be linked to a well-developed root system, particularly in those regions of the soil still containing water ( Comas et al. , 2013 ). Irrigation is used to prevent drought stress in crops, and agriculture uses 70% of globally available freshwater, mostly for this purpose ( Du et al. , 2015 ; World Water Assessment Programme, 2015 ). Climate change, however, will make water availability more unpredictable, with increased likelihoods for extreme weather events and changes in rainfall patterns ( IPCC, 2014 ). A variety of strategies are pursued to make agricultural water use more sustainable and ‘produce more crop per drop’ ( Morison et al. , 2008 ; Du et al. , 2015 ). How could hydrotropism, which allows roots to forage for water in soil, contribute to this? Conservation tillage, which minimizes the amount of soil disturbance, increases soil water availability through improved physical soil properties, increased organic matter, and reduced evaporation due to crop residue left on the surface ( Triplett and Dick, 2008 ), and is now widely adopted in many rain-fed agriculture systems ( Brunel et al. , 2013 ; Peiretti and Dumanski, 2014 ). Currently little is known about water distribution in soils under conservation tillage, but water may be more heterogeneously distributed than under conventional tillage, which would make crops with an increased hydrotropism response more efficient. For agricultural systems using irrigation, deficit and partial root zone drying (PRD) irrigation systems have been demonstrated to increase the water use efficiency in a number of crops ( Kang and Zhang, 2004 ). In these systems, less water than is needed to cover evapotranspiration demand is supplied, sometimes only to part of the root system (PRD). As a result, plants produce less shoot biomass and decrease stomatal conductance, whilst still producing similar or slightly reduced yields compared with fully irrigated crops. Under PRD, it is thought that the drying part of the root system produces a signal that regulates stomatal conductance, whereas the irrigated part supplies the shoot with sufficient water to produce the crop ( Kang and Zhang, 2004 ; Sobeih et al. , 2004 ). The applied effects of PRD on plant growth have been extensively studied, and are reviewed elsewhere ( Kang and Zhang, 2004 ). For root growth, it was shown that PRD leads to an increase in tomato root dry weight, particularly in those parts of the root system that were rewatered after a previous drying period ( Mingo et al. , 2004 ), and an increase in the root surface area of maize ( Zhenchang et al. , 2016 ).
That hydrotropism can contribute to directional root growth in soil has been demonstrated for A. thaliana grown in soil microcosms with a lateral water gradient ( Iwata et al. , 2013 ). Plants showed increased root growth in the area with higher water content ( Iwata et al. , 2013 ). This behaviour was dependent on a functioning hydrotropism response, as plants overexpressing MIZ1 had an increased tendency to grow roots in soil with high water content, whereas miz1 plants grew roots in a random fashion, unrelated to water distribution in the soil ( Iwata et al. , 2013 ). In maize, a recent study tried to link hydrotropic responsiveness to yield under PRD irrigation and drought ( Eapen et al. , 2017 ). The hydrotropic response of part of a collection of maize hybrid lines from the Drought Tolerance Maize for Africa project was analysed at 4 d after germination, and representative lines with strong and weak hydrotropic responses were tested in field trials ( Eapen et al. , 2017 ). Although one line with a strong hydrotropic response showed increased yield under PRD irrigation and drought stress, the results were more ambiguous for other lines ( Eapen et al. , 2017 ). Interestingly, however, there seemed to be a stronger correlation between root weight and grain yield in the lines with a strong hydrotropic response compared with those lines which only weakly responded to the stimulus ( Eapen et al. , 2017 ). Root biomass and root system architecture traits might have been confounding factors in this study, and highlight the need for rigorous experimental design when assessing the contribution of hydrotropism to crop performance. These new developments are an indication that crops with an improved hydrotropic response could be beneficial in agricultural systems using conservation tillage or deficit/PRD irrigation systems, contributing to improved water use efficiency.
Hydrotropism research has taken a leap forward in the last few years with a number of discoveries describing the site of perception, bending mechanism, and interaction with gravitropism. Hydrotropism has now been shown to exist in an increasing number of plant species and, interestingly, species-specific mechanistic differences in the response exist. New techniques will allow us to understand this tropism and how it contributes to water uptake and drought responses in plants.
Although progress has been made in understanding hydrotropism, many more questions still remain open. Most importantly, it is still unclear what the water signal is and how it is perceived. With the recent discovery that the columella may not be necessary for hydrotropism and that the signal can be perceived by the elongation zone of A. thaliana ( Dietrich et al. , 2017 ), the pool of potential candidates for hydrotropism perception has widened and changed again. Development of sensors for calcium, ABA, pH, ROS, and other signalling molecules has improved dramatically over recent years ( Nagai et al. , 2004 ; Jones et al. , 2014 ; Waadt et al. , 2014 ; Krieger et al. , 2016 ), but these sensors may still not be sensitive enough to detect changes during hydrotropism. It might be necessary to determine the signal indirectly, and a better understanding of hydrotropism response kinetics may help in this respect. The presentation time for the hydrotropic signal has been determined so far only for pea, and water potential gradients used in assays are usually chosen on the basis of returning the maximum response without affecting root growth ( Stinemetz et al. , 1996 ; Takahashi et al. , 2002 ). A systematic evaluation of presentation times and the strength of the water potential gradient needed to trigger the response may inform the search for the elusive water signal. New developments in microfluidic devices now allow precise delivery of stimuli at high spatial and temporal resolution, and will be instrumental in determining these parameters ( Meier et al. , 2010 ; Stanley et al. , 2018 ). Once the signal for hydrotropism has been found, it should be easier to connect genes known to be involved in hydrotropism (e.g. MIZ1 , MIZ2/GNOM , and ABA genes) to the signal transduction pathway of the hydrotropic response. Alternatively, a reverse approach could be used, starting from these known components to search for interaction partners that are specific to hydrotropism.
In A. thaliana , the cortex tissue has been shown to play an important role in hydrotropism, with evidence that differential elongation in this tissue drives the bending response ( Dietrich et al. , 2017 ). Other plant species in which hydrotropism has been observed have a cortex consisting of multiple cell layers, and it will be interesting to see whether hydrotropic bending in those species uses the same mechanistic principle to drive the bending response.
The interaction of hydrotropism with other tropisms, gravitropism in particular, is another area of great interest. For those plant species that require auxin transport for hydrotropism, it will be important to determine whether a lateral auxin gradient develops during the response and how such a gradient is affected by gravitropism. Mathematical modelling has provided new insights into gravitropism ( Swarup et al. , 2005 ; Band et al. , 2012 ) and has been used to investigate the bending response in hydrotropism ( Dietrich et al. , 2017 ). Development of new models that combine hydro- and gravitropic responses will be an important part of understanding how these tropisms interact and direct root tip growth angles.
Until now, all hydrotropism experiments have been performed on primary roots of plants. Wiesner and Molisch already observed that lateral roots grow more easily in the direction of water than primary roots ( Wiesner, 1881 ; Molisch, 1883 ). Hydrotropism research needs to extend its scope and investigate the response of lateral roots. Lateral roots, which have a different gravitropic set point angle and are therefore less responsive to gravity than primary roots ( Roychoudhry et al. , 2013 , 2017 ), are in theory more responsive to water potential gradients. Lateral roots make up the majority of any plant root system and, although hydrotropism assays for lateral roots will be technically more difficult, these should give us a better appreciation of whether hydrotropism is able to increase water uptake.
Ultimately, hydrotropic responses will have to be assessed in soil. Methods now exist that allow the visualization of roots and water in soil and to compute water fluxes into the root ( Daly et al. , 2015 , 2018 ). Development of a hydrotropic assay in soil will be a necessity to understand the true contribution of this tropism to water uptake and drought acclimation in plants.
The author would like to thank Malcolm J. Bennett and Darren M. Wells for discussion of the manuscript. This work was supported by the Leverhulme Trust [grant no. RPG-2016-409].
Antoni R , Dietrich D , Bennett MJ , Rodriguez PL . 2016 . Hydrotropism: analysis of the root response to a moisture gradient . Methods in Molecular Biology 1398 , 3 – 9 .
Google Scholar
Antoni R , Gonzalez-Guzman M , Rodriguez L et al. 2013 . PYRABACTIN RESISTANCE1-LIKE8 plays an important role for the regulation of abscisic acid signaling in root . Plant Physiology 161 , 931 – 941 .
Aroca R , Porcel R , Ruiz-Lozano JM . 2012 . Regulation of root water uptake under abiotic stress conditions . Journal of Experimental Botany 63 , 43 – 57 .
Band LR , Wells DM , Larrieu A et al. 2012 . Root gravitropism is regulated by a transient lateral auxin gradient controlled by a tipping-point mechanism . Proceedings of the National Academy of Sciences, USA 109 , 4668 – 4673 .
Bellati J , Champeyroux C , Hem S , Rofidal V , Krouk G , Maurel C , Santoni V . 2016 . Novel aquaporin regulatory mechanisms revealed by interactomics . Molecular and Cellular Proteomics 15 , 3473 – 3487 .
Blancaflor EB , Fasano JM , Gilroy S . 1998 . Mapping the functional roles of cap cells in the response of Arabidopsis primary roots to gravity . Plant Physiology 116 , 213 – 222 .
Blancaflor EB , Masson PH . 2003 . Plant gravitropism. Unraveling the ups and downs of a complex process . Plant Physiology 133 , 1677 – 1690 .
Blum A . 2005 . Drought resistance, water-use efficiency, and yield potential—are they compatible, dissonant, or mutually exclusive ? Australian Journal of Agricultural Research 56 , 1159 – 1168 .
Bonnet C . 1754 . Recherches sur l’usage des feuilles dans les plantes et sur quelques autres sujets relatifs a l’histoire de la vegetation . Goettingen, Leiden : Elie Luzac .
Google Preview
Boursiac Y , Boudet J , Postaire O , Luu DT , Tournaire-Roux C , Maurel C . 2008 . Stimulus-induced downregulation of root water transport involves reactive oxygen species-activated cell signalling and plasma membrane intrinsic protein internalization . The Plant Journal 56 , 207 – 218 .
Boursiac Y , Chen S , Luu DT , Sorieul M , van den Dries N , Maurel C . 2005 . Early effects of salinity on water transport in Arabidopsis roots. Molecular and cellular features of aquaporin expression . Plant Physiology 139 , 790 – 805 .
Brunel N , Seguel O , Acevedo E . 2013 . Conservation tillage and water availability for wheat in the dryland of central Chile . Journal of Soil Science and Plant Nutrition 13 , 622 – 637 .
Cassab GI , Eapen D , Campos ME . 2013 . Root hydrotropism: an update . American Journal of Botany 100 , 14 – 24 .
Comas LH , Becker SR , Cruz VM , Byrne PF , Dierig DA . 2013 . Root traits contributing to plant productivity under drought . Frontiers in Plant Science 4 , 442 .
Coutts MP , Nicoll BC . 1993 . Orientation of the lateral roots of trees: II. Hydrotropic and gravitropic responses of lateral roots of Sitka spruce grown in air at different humidities . New Phytologist 124 , 277 – 281 .
Cutler SR , Rodriguez PL , Finkelstein RR , Abrams SR . 2010 . Abscisic acid: emergence of a core signaling network . Annual Review of Plant Biology 61 , 651 – 679 .
Daly KR , Mooney SJ , Bennett MJ , Crout NM , Roose T , Tracy SR . 2015 . Assessing the influence of the rhizosphere on soil hydraulic properties using X-ray computed tomography and numerical modelling . Journal of Experimental Botany 66 , 2305 – 2314 .
Daly KR , Tracy SR , Crout NMJ , Mairhofer S , Pridmore TP , Mooney SJ , Roose T . 2018 . Quantification of root water uptake in soil using X-ray computed tomography and image-based modelling . Plant, Cell and Environment 41 , 121 – 133 .
Darwin C , Darwin F . 1880 . The power of movement in plants . London : John Murray .
Davies WJ , Bennett MJ . 2015 . Achieving more crop per drop . Nature Plants 1 , 15118 .
Dietrich D , Pang L , Kobayashi A et al. 2017 . Root hydrotropism is controlled via a cortex-specific growth mechanism . Nature Plants 3 , 17057 .
Du T , Kang S , Zhang J , Davies WJ . 2015 . Deficit irrigation and sustainable water-resource strategies in agriculture for China’s food security . Journal of Experimental Botany 66 , 2253 – 2269 .
Eapen D , Barroso ML , Campos ME , Ponce G , Corkidi G , Dubrovsky JG , Cassab GI . 2003 . A no hydrotropic response root mutant that responds positively to gravitropism in Arabidopsis . Plant Physiology 131 , 536 – 546 .
Eapen D , Martínez JJ , Cassab GI . 2015 . Assays for root hydrotropism and response to water stress . In: Blancaflor EB , ed. Plant gravitropism: methods and protocols . New York : Springer New York , 133 – 142 .
Eapen D , Martínez-Guadarrama J , Hernández-Bruno O , Flores L , Nieto-Sotelo J , Cassab GI . 2017 . Synergy between root hydrotropic response and root biomass in maize ( Zea mays L.) enhances drought avoidance . Plant Science 265 , 87 – 99 .
Enders TA , Oh S , Yang Z , Montgomery BL , Strader LC . 2015 . Genome sequencing of Arabidopsis abp1-5 reveals second-site mutations that may affect phenotypes . The Plant Cell 27 , 1820 – 1826 .
Eysholdt-Derzsó E , Sauter M . 2017 . Root bending is antagonistically affected by hypoxia and ERF-mediated transcription via auxin signaling . Plant Physiology 175 , 412 – 423 .
Freundl E , Steudle E , Hartung W . 1998 . Water uptake by roots of maize and sunflower affects the radial transport of abscisic acid and its concentration in the xylem . Planta 207 , 8 – 19 .
Friml J . 2010 . Subcellular trafficking of PIN auxin efflux carriers in auxin transport . European Journal of Cell Biology 89 , 231 – 235 .
Fujii H , Chinnusamy V , Rodrigues A , Rubio S , Antoni R , Park SY , Cutler SR , Sheen J , Rodriguez PL , Zhu JK . 2009 . In vitro reconstitution of an abscisic acid signalling pathway . Nature 462 , 660 – 664 .
Fujii N , Miyabayashi S , Sugita T et al. 2018 . Root-tip-mediated inhibition of hydrotropism is accompanied with the suppression of asymmetric expression of auxin-inducible genes in response to moisture gradients in cucumber roots . PLoS One 13 , e0189827 .
Fujii H , Verslues PE , Zhu JK . 2007 . Identification of two protein kinases required for abscisic acid regulation of seed germination, root growth, and gene expression in Arabidopsis . The Plant Cell 19 , 485 – 494 .
Galvan-Ampudia CS , Julkowska MM , Darwish E , Gandullo J , Korver RA , Brunoud G , Haring MA , Munnik T , Vernoux T , Testerink C . 2013 . Halotropism is a response of plant roots to avoid a saline environment . Current Biology 23 , 2044 – 2050 .
Gao Y , Lynch JP . 2016 . Reduced crown root number improves water acquisition under water deficit stress in maize ( Zea mays L.) . Journal of Experimental Botany 67 , 4545 – 4557 .
Gao Y , Zhang Y , Zhang D , Dai X , Estelle M , Zhao Y . 2015 . Auxin binding protein 1 (ABP1) is not required for either auxin signaling or Arabidopsis development . Proceedings of the National Academy of Sciences, USA 112 , 2275 – 2280 .
Gaur PM , Krishnamurthy L , Kashiwagi J . 2008 . Improving drought-avoidance root traits in chickpea ( Cicer arietinum L.)—current status of research at ICRISAT . Plant Production Science 11 , 3 – 11 .
Geldner N , Anders N , Wolters H , Keicher J , Kornberger W , Muller P , Delbarre A , Ueda T , Nakano A , Jürgens G . 2003 . The Arabidopsis GNOM ARF-GEF mediates endosomal recycling, auxin transport, and auxin-dependent plant growth . Cell 112 , 219 – 230 .
Gilroy S , Masson PH , eds . 2008 . Plant tropisms . Oxford : Blackwell Publishing Ltd .
Hachez C , Veljanovski V , Reinhardt H , Guillaumot D , Vanhee C , Chaumont F , Batoko H . 2014 . The Arabidopsis abiotic stress-induced TSPO-related protein reduces cell-surface expression of the aquaporin PIP2;7 through protein–protein interactions and autophagic degradation . The Plant Cell 26 , 4974 – 4990 .
Hamilton ES , Schlegel AM , Haswell ES . 2015 . United in diversity: mechanosensitive ion channels in plants . Annual Review of Plant Biology 66 , 113 – 137 .
Hayashi K , Neve J , Hirose M , Kuboki A , Shimada Y , Kepinski S , Nozaki H . 2012 . Rational design of an auxin antagonist of the SCF(TIR1) auxin receptor complex . ACS Chemical Biology 7 , 590 – 598 .
Hayashi K-I , Tan X , Zheng N , Hatate T , Kimura Y , Kepinski S , Nozaki H . 2008 . Small-molecule agonists and antagonists of F-box protein–substrate interactions in auxin perception and signaling . Proceedings of the National Academy of Sciences, USA 105 , 5632 – 5637 .
Henry A , Gowda VRP , Torres RO , McNally KL , Serraj R . 2011 . Variation in root system architecture and drought response in rice ( Oryza sativa ): phenotyping of the OryzaSNP panel in rainfed lowland fields . Field Crops Research 120 , 205 – 214 .
Hirasawa T , Takahashi H , Suge H , Ishihara K . 1997 . Water potential, turgor and cell wall properties in elongating tissues of the hydrotropically bending roots of pea ( Pisum sativum L) . Plant, Cell and Environment 20 , 381 – 386 .
Hooker HD . 1915 . Hydrotropism in roots of Lupinus albus . Annals of Botany 29 , 265 – 283 .
Hou G , Mohamalawari DR , Blancaflor EB . 2003 . Enhanced gravitropism of roots with a disrupted cap actin cytoskeleton . Plant Physiology 131 , 1360 – 1373 .
IPCC . 2014 . Climate change 2014: synthesis report. Contribution of working groups I, II and II to the fifth assessment report of the intergovernmental panel on climate change . Core Writing Team , Pachauri RK , Meyer LA , eds. Geneva, Switzerland : IPCC .
Iwata S , Miyazawa Y , Fujii N , Takahashi H . 2013 . MIZ1-regulated hydrotropism functions in the growth and survival of Arabidopsis thaliana under natural conditions . Annals of Botany 112 , 103 – 114 .
Jaffe MJ , Takahashi H , Biro RL . 1985 . A pea mutant for the study of hydrotropism in roots . Science 230 , 445 – 447 .
Jones AM , Danielson JA , Manojkumar SN , Lanquar V , Grossmann G , Frommer WB . 2014 . Abscisic acid dynamics in roots detected with genetically encoded FRET sensors . eLife 3 , e01741 .
Joo JH , Bae YS , Lee JS . 2001 . Role of auxin-induced reactive oxygen species in root gravitropism . Plant Physiology 126 , 1055 – 1060 .
Kaneyasu T , Kobayashi A , Nakayama M , Fujii N , Takahashi H , Miyazawa Y . 2007 . Auxin response, but not its polar transport, plays a role in hydrotropism of Arabidopsis roots . Journal of Experimental Botany 58 , 1143 – 1150 .
Kang S , Zhang J . 2004 . Controlled alternate partial root-zone irrigation: its physiological consequences and impact on water use efficiency . Journal of Experimental Botany 55 , 2437 – 2446 .
Kiss JZ , Sack FD . 1989 . Reduced gravitropic sensitivity in roots of a starch-deficient mutant of Nicotiana sylvestris . Planta 180 , 123 – 130 .
Kiss JZ , Wright JB , Caspar T . 1996 . Gravitropism in roots of intermediate-starch mutants of Arabidopsis . Physiologia Plantarum 97 , 237 – 244 .
Knight T . 1811 . On the causes which influence the direction of the growth of roots . Philosophical Transactions of the Royal Society of London 101 , 209 – 219 .
Kobayashi A , Takahashi A , Kakimoto Y , Miyazawa Y , Fujii N , Higashitani A , Takahashi H . 2007 . A gene essential for hydrotropism in roots . Proceedings of the National Academy of Sciences, USA 104 , 4724 – 4729 .
Krieger G , Shkolnik D , Miller G , Fromm H . 2016 . Reactive oxygen species tune root tropic responses . Plant Physiology 172 , 1209 – 1220 .
Li G , Santoni V , Maurel C . 2014 . Plant aquaporins: roles in plant physiology . Biochimica et Biophysica Acta 1840 , 1574 – 1582 .
Li X , Wang X , Yang Y , Li R , He Q , Fang X , Luu DT , Maurel C , Lin J . 2011 . Single-molecule analysis of PIP2;1 dynamics and partitioning reveals multiple modes of Arabidopsis plasma membrane aquaporin regulation . The Plant Cell 23 , 3780 – 3797 .
Loomis WE , Ewan LM . 1936 . Hydrotropic responses of roots in soil . Botanical Gazette 97 , 728 – 743 .
Lynch JP . 2013 . Steep, cheap and deep: an ideotype to optimize water and N acquisition by maize root systems . Annals of Botany 112 , 347 – 357 .
Ma Y , Szostkiewicz I , Korte A , Moes D , Yang Y , Christmann A , Grill E . 2009 . Regulators of PP2C phosphatase activity function as abscisic acid sensors . Science 324 , 1064 – 1068 .
Meier M , Lucchetta EM , Ismagilov RF . 2010 . Chemical stimulation of the Arabidopsis thaliana root using multi-laminar flow on a microfluidic chip . Lab on a Chip 10 , 2147 – 2153 .
Mingo DM , Theobald JC , Bacon MA , Davies WJ , Dodd IC . 2004 . Biomass allocation in tomato ( Lycopersicon esculentum ) plants grown under partial rootzone drying: enhancement of root growth . Functional Plant Biology 31 , 971 – 978 .
Miyamoto N , Ookawa T , Takahashi H , Hirasawa T . 2002 . Water uptake and hydraulic properties of elongating cells in hydrotropically bending roots of Pisum sativum L . Plant and Cell Physiology 43 , 393 – 401 .
Miyazawa Y , Ito Y , Moriwaki T , Kobayashi A , Fujii N , Takahashi H . 2009 a . A molecular mechanism unique to hydrotropism in roots . Plant Science 177 , 297 – 301 .
Miyazawa Y , Moriwaki T , Uchida M , Kobayashi A , Fujii N , Takahashi H . 2012 . Overexpression of MIZU-KUSSEI1 enhances the root hydrotropic response by retaining cell viability under hydrostimulated conditions in Arabidopsis thaliana . Plant and Cell Physiology 53 , 1926 – 1933 .
Miyazawa Y , Sakashita T , Funayama T et al. 2008 . Effects of locally targeted heavy-ion and laser microbeam on root hydrotropism in Arabidopsis thaliana . Journal of Radiation Research 49 , 373 – 379 .
Miyazawa Y , Takahashi A , Kobayashi A , Kaneyasu T , Fujii N , Takahashi H . 2009 b . GNOM-mediated vesicular trafficking plays an essential role in hydrotropism of Arabidopsis roots . Plant Physiology 149 , 835 – 840 .
Mizuno H , Kobayashi A , Fujii N , Yamashita M , Takahashi H . 2002 . Hydrotropic response and expression pattern of auxin-inducible gene, CS-IAA1, in the primary roots of clinorotated cucumber seedlings . Plant and Cell Physiology 43 , 793 – 801 .
Molisch H . 1883 . Untersuchungen ueber den Hydrotropismus. Sitzungsberichte k. k . Akademie Wien 88 , 897 – 943 .
Monshausen GB , Gilroy S . 2009 . The exploring root–root growth responses to local environmental conditions . Current Opinion in Plant Biology 12 , 766 – 772 .
Morison JI , Baker NR , Mullineaux PM , Davies WJ . 2008 . Improving water use in crop production . Philosophical Transactions of the Royal Society B: Biological Sciences 363 , 639 – 658 .
Moriwaki T , Miyazawa Y , Fujii N , Takahashi H . 2012 . Light and abscisic acid signalling are integrated by MIZ1 gene expression and regulate hydrotropic response in roots of Arabidopsis thaliana . Plant, Cell and Environment 35 , 1359 – 1368 .
Moriwaki T , Miyazawa Y , Kobayashi A , Takahashi H . 2013 . Molecular mechanisms of hydrotropism in seedling roots of Arabidopsis thaliana (Brassicaceae) . American Journal of Botany 100 , 25 – 34 .
Moriwaki T , Miyazawa Y , Kobayashi A , Uchida M , Watanabe C , Fujii N , Takahashi H . 2011 . Hormonal regulation of lateral root development in Arabidopsis modulated by MIZ1 and requirement of GNOM activity for MIZ1 function . Plant Physiology 157 , 1209 – 1220 .
Morohashi K , Okamoto M , Yamazaki C et al. 2017 . Gravitropism interferes with hydrotropism via counteracting auxin dynamics in cucumber roots: clinorotation and spaceflight experiments . New Phytologist 215 , 1476 – 1489 .
Moulia B , Fournier M . 2009 . The power and control of gravitropic movements in plants: a biomechanical and systems biology view . Journal of Experimental Botany 60 , 461 – 486 .
Mullen JL , Wolverton C , Ishikawa H , Evans ML . 2000 . Kinetics of constant gravitropic stimulus responses in Arabidopsis roots using a feedback system . Plant Physiology 123 , 665 – 670 .
Mustilli AC , Merlot S , Vavasseur A , Fenzi F , Giraudat J . 2002 . Arabidopsis OST1 protein kinase mediates the regulation of stomatal aperture by abscisic acid and acts upstream of reactive oxygen species production . The Plant Cell 14 , 3089 – 3099 .
Nagai T , Yamada S , Tominaga T , Ichikawa M , Miyawaki A . 2004 . Expanded dynamic range of fluorescent indicators for Ca 2 + by circularly permuted yellow fluorescent proteins . Proceedings of the National Academy of Sciences, USA 101 , 10554 – 10559 .
Nakajima Y , Nara Y , Kobayashi A , Sugita T , Miyazawa Y , Fujii N , Takahashi H . 2017 . Auxin transport and response requirements for root hydrotropism differ between plant species . Journal of Experimental Botany 68 , 3441 – 3456 .
Oono Y , Ooura C , Rahman A , Aspuria ET , Hayashi K , Tanaka A , Uchimiya H . 2003 . p-Chlorophenoxyisobutyric acid impairs auxin response in Arabidopsis root . Plant Physiology 133 , 1135 – 1147 .
Ottenschläger I , Wolff P , Wolverton C , Bhalerao RP , Sandberg G , Ishikawa H , Evans M , Palme K . 2003 . Gravity-regulated differential auxin transport from columella to lateral root cap cells . Proceedings of the National Academy of Sciences, USA 100 , 2987 – 2991 .
Oyanagi A , Takahashi H , Suge H . 1995 . Interactions between hydrotropism and gravitropism in the primary seminal roots of Triticum aestivum L . Annals of Botany 75 , 229 – 235 .
Paez Valencia J , Goodman K , Otegui MS . 2016 . Endocytosis and endosomal trafficking in plants . Annual Review of Plant Biology 67 , 309 – 335 .
Park SY , Fung P , Nishimura N et al. 2009 . Abscisic acid inhibits type 2C protein phosphatases via the PYR/PYL family of START proteins . Science 324 , 1068 – 1071 .
Peiretti R , Dumanski J . 2014 . The transformation of agriculture in Argentina through soil conservation . International Soil and Water Conservation Research 2 , 14 – 20 .
Péret B , Li G , Zhao J et al. 2012 . Auxin regulates aquaporin function to facilitate lateral root emergence . Nature Cell Biology 14 , 991 – 998 .
Ponce G , Rasgado FA , Cassab GI . 2008 a . Roles of amyloplasts and water deficit in root tropisms . Plant, Cell and Environment 31 , 205 – 217 .
Ponce G , Rasgado F , Cassab GI . 2008 b . How amyloplasts, water deficit and root tropisms interact ? Plant Signaling and Behavior 3 , 460 – 462 .
Rahman A , Takahashi M , Shibasaki K , Wu S , Inaba T , Tsurumi S , Baskin TI . 2010 . Gravitropism of Arabidopsis thaliana roots requires the polarization of PIN2 toward the root tip in meristematic cortical cells . The Plant Cell 22 , 1762 – 1776 .
Rogers ED , Benfey PN . 2015 . Regulation of plant root system architecture: implications for crop advancement . Current Opinion in Biotechnology 32 , 93 – 98 .
Rosquete MR , Kleine-Vehn J . 2013 . Halotropism: turning down the salty date . Current Biology 23 , R927 – R929 .
Roychoudhry S , Del Bianco M , Kieffer M , Kepinski S . 2013 . Auxin controls gravitropic setpoint angle in higher plant lateral branches . Current Biology 23 , 1497 – 1504 .
Roychoudhry S , Kieffer M , Del Bianco M , Liao CY , Weijers D , Kepinski S . 2017 . The developmental and environmental regulation of gravitropic setpoint angle in Arabidopsis and bean . Scientific Reports 7 , 42664 .
Sachs J . 1872 . Ablenkung der Wurzel von ihrer normalen Wachsthumsrichtung durch feuchte Koerper . In: Sachs J , ed. Arbeiten des Botanischen Institus in Wuerzburg . Leipzig : Wilhelm Engelmann , 209 – 222 .
Salazar-Blas A , Noriega-Calixto L , Campos ME , Eapen D , Cruz-Vázquez T , Castillo-Olamendi L , Sepulveda-Jiménez G , Porta H , Dubrovsky JG , Cassab GI . 2017 . Robust root growth in altered hydrotropic response1 ( ahr1 ) mutant of Arabidopsis is maintained by high rate of cell production at low water potential gradient . Journal of Plant Physiology 208 , 102 – 114 .
Saucedo M , Ponce G , Campos ME , Eapen D , García E , Luján R , Sánchez Y , Cassab GI . 2012 . An altered hydrotropic response ( ahr1 ) mutant of Arabidopsis recovers root hydrotropism with cytokinin . Journal of Experimental Botany 63 , 3587 – 3601 .
Shkolnik D , Fromm H . 2016 . The Cholodny–Went theory does not explain hydrotropism . Plant Science 252 , 400 – 403 .
Shkolnik D , Krieger G , Nuriel R , Fromm H . 2016 . Hydrotropism: root bending does not require auxin redistribution . Molecular Plant 9 , 757 – 759 .
Sobeih WY , Dodd IC , Bacon MA , Grierson D , Davies WJ . 2004 . Long-distance signals regulating stomatal conductance and leaf growth in tomato ( Lycopersicon esculentum ) plants subjected to partial root-zone drying . Journal of Experimental Botany 55 , 2353 – 2363 .
Stanley CE , Shrivastava J , Brugman R , Heinzelmann E , van Swaay D , Grossmann G . 2018 . Dual-flow-RootChip reveals local adaptations of roots towards environmental asymmetry at the physiological and genetic levels . New Phytologist 217 , 1357 – 1369 .
Stinemetz C , Takahashi H , Suge H . 1996 . Characterization of hydrotropism: the timing of perception and signal movement from the root cap in the agravitropic pea mutant ageotropum . Plant and Cell Physiology 37 , 800 – 805 .
Su SH , Gibbs NM , Jancewicz AL , Masson PH . 2017 . Molecular mechanisms of root gravitropism . Current Biology 27 , R964 – R972 .
Sun F , Zhang W , Hu H , Li B , Wang Y , Zhao Y , Li K , Liu M , Li X . 2008 . Salt modulates gravity signaling pathway to regulate growth direction of primary roots in Arabidopsis . Plant Physiology 146 , 178 – 188 .
Sutka M , Li G , Boudet J , Boursiac Y , Doumas P , Maurel C . 2011 . Natural variation of root hydraulics in Arabidopsis grown in normal and salt-stressed conditions . Plant Physiology 155 , 1264 – 1276 .
Swarup R , Kramer EM , Perry P , Knox K , Leyser HM , Haseloff J , Beemster GT , Bhalerao R , Bennett MJ . 2005 . Root gravitropism requires lateral root cap and epidermal cells for transport and response to a mobile auxin signal . Nature Cell Biology 7 , 1057 – 1065 .
Takahashi N , Goto N , Okada K , Takahashi H . 2002 . Hydrotropism in abscisic acid, wavy, and gravitropic mutants of Arabidopsis thaliana . Planta 216 , 203 – 211 .
Takahashi H , Miyazawa Y , Fujii N . 2009 . Hormonal interactions during root tropic growth: hydrotropism versus gravitropism . Plant Molecular Biology 69 , 489 – 502 .
Takahashi H , Scott TK . 1991 . Hydrotropism and its interaction with gravitropism in maize roots . Plant Physiology 96 , 558 – 564 .
Takahashi H , Scott TK . 1993 . Intensity of hydrostimulation for the induction of root hydrotropism and its sensing by the root cap . Plant, Cell and Environment 16 , 99 – 103 .
Takahashi H , Suge H . 1991 . Root hydrotropism of an agravitropic pea mutant, ageotropum . Physiologia Plantarum 82 , 24 – 31 .
Takahashi N , Yamazaki Y , Kobayashi A , Higashitani A , Takahashi H . 2003 . Hydrotropism interacts with gravitropism by degrading amyloplasts in seedling roots of Arabidopsis and radish . Plant Physiology 132 , 805 – 810 .
Takano M , Takahashi H , Hirasawa T , Suge H . 1995 . Hydrotropism in roots: sensing of a gradient in water potential by the root cap . Planta 197 , 410 – 413 .
Taniguchi YY , Taniguchi M , Tsuge T , Oka A , Aoyama T . 2010 . Involvement of Arabidopsis thaliana phospholipase Dzeta2 in root hydrotropism through the suppression of root gravitropism . Planta 231 , 491 – 497 .
Tanimoto M , Tremblay R , Colasanti J . 2008 . Altered gravitropic response, amyloplast sedimentation and circumnutation in the Arabidopsis shoot gravitropism 5 mutant are associated with reduced starch levels . Plant Molecular Biology 67 , 57 – 69 .
Triplett GB , Dick WA . 2008 . No-tillage crop production: a revolution in agriculture ! Agronomy Journal 100 , 153 – 165 .
Tsuda S , Miyamoto N , Takahashi H , Ishihara K , Hirasawa T . 2003 . Roots of Pisum sativum L. exhibit hydrotropism in response to a water potential gradient in vermiculite . Annals of Botany 92 , 767 – 770 .
Uga Y , Sugimoto K , Ogawa S et al. 2013 . Control of root system architecture by DEEPER ROOTING 1 increases rice yield under drought conditions . Nature Genetics 45 , 1097 – 1102 .
Veihmeyer FJ , Hendrickson AH . 1927 . Soil-moisture conditions in relation to plant growth . Plant Physiology 2 , 71 – 82 .
Waadt R , Hitomi K , Nishimura N , Hitomi C , Adams SR , Getzoff ED , Schroeder JI . 2014 . FRET-based reporters for the direct visualization of abscisic acid concentration changes and distribution in Arabidopsis . eLife 3 , e01739 .
Wery J . 2005 . Differential effects of soil water deficit on the basic plant functions and their significance to analyse crop responses to water deficit in indeterminate plants . Australian Journal of Agricultural Research 56 , 1201 – 1209 .
Wiesner J . 1881 . Das Bewegungsvermoegen der Pflanzen . Wien : Alfred Hoelder .
World Water Assessment Programme (WWAP) . 2015 . The United Nations World Water Development Report 2015: water for a sustainable world . Paris : UNESCO .
Yamaguchi-Shinozaki K , Shinozaki K . 2006 . Transcriptional regulatory networks in cellular responses and tolerance to dehydration and cold stresses . Annual Review of Plant Biology 57 , 781 – 803 .
Yamazaki T , Miyazawa Y , Kobayashi A , Moriwaki T , Fujii N , Takahashi H . 2012 . MIZ1, an essential protein for root hydrotropism, is associated with the cytoplasmic face of the endoplasmic reticulum membrane in Arabidopsis root cells . FEBS Letters 586 , 398 – 402 .
Zhenchang W , Xiaofei Y , Liang F , Jianbin Z . 2016 . Partial rootzone drying irrigation increase root surface area, root hydraulic conductivity and water use efficiency in maize . International Journal of Environmental Monitoring and Analysis 4 , 146 – 153 .
| Month: | Total Views: |
|---|---|
| February 2018 | 59 |
| March 2018 | 207 |
| April 2018 | 89 |
| May 2018 | 334 |
| June 2018 | 197 |
| July 2018 | 57 |
| August 2018 | 50 |
| September 2018 | 49 |
| October 2018 | 71 |
| November 2018 | 61 |
| December 2018 | 42 |
| January 2019 | 42 |
| February 2019 | 40 |
| March 2019 | 47 |
| April 2019 | 67 |
| May 2019 | 186 |
| June 2019 | 164 |
| July 2019 | 173 |
| August 2019 | 176 |
| September 2019 | 314 |
| October 2019 | 499 |
| November 2019 | 563 |
| December 2019 | 373 |
| January 2020 | 424 |
| February 2020 | 587 |
| March 2020 | 505 |
| April 2020 | 846 |
| May 2020 | 482 |
| June 2020 | 506 |
| July 2020 | 333 |
| August 2020 | 377 |
| September 2020 | 483 |
| October 2020 | 631 |
| November 2020 | 907 |
| December 2020 | 622 |
| January 2021 | 772 |
| February 2021 | 736 |
| March 2021 | 1,042 |
| April 2021 | 879 |
| May 2021 | 877 |
| June 2021 | 519 |
| July 2021 | 677 |
| August 2021 | 549 |
| September 2021 | 762 |
| October 2021 | 776 |
| November 2021 | 866 |
| December 2021 | 648 |
| January 2022 | 713 |
| February 2022 | 785 |
| March 2022 | 986 |
| April 2022 | 882 |
| May 2022 | 923 |
| June 2022 | 467 |
| July 2022 | 588 |
| August 2022 | 804 |
| September 2022 | 1,154 |
| October 2022 | 959 |
| November 2022 | 934 |
| December 2022 | 572 |
| January 2023 | 694 |
| February 2023 | 793 |
| March 2023 | 602 |
| April 2023 | 518 |
| May 2023 | 550 |
| June 2023 | 378 |
| July 2023 | 423 |
| August 2023 | 479 |
| September 2023 | 815 |
| October 2023 | 660 |
| November 2023 | 560 |
| December 2023 | 562 |
| January 2024 | 1,063 |
| February 2024 | 935 |
| March 2024 | 878 |
| April 2024 | 611 |
| May 2024 | 688 |
| June 2024 | 424 |
| July 2024 | 328 |
| August 2024 | 286 |
Email alerts
Citing articles via.
- Recommend to your Library
Affiliations
- Online ISSN 1460-2431
- Print ISSN 0022-0957
- Copyright © 2024 Society for Experimental Biology
- About Oxford Academic
- Publish journals with us
- University press partners
- What we publish
- New features
- Open access
- Institutional account management
- Rights and permissions
- Get help with access
- Accessibility
- Advertising
- Media enquiries
- Oxford University Press
- Oxford Languages
- University of Oxford
Oxford University Press is a department of the University of Oxford. It furthers the University's objective of excellence in research, scholarship, and education by publishing worldwide
- Copyright © 2024 Oxford University Press
- Cookie settings
- Cookie policy
- Privacy policy
- Legal notice
This Feature Is Available To Subscribers Only
Sign In or Create an Account
This PDF is available to Subscribers Only
For full access to this pdf, sign in to an existing account, or purchase an annual subscription.
Tropism Twist
Grade level.
Students investigate how light affects plant growth by observing changes in a plant’s growth and movement as light availability is altered through an experiment. Grades 3-5
Estimated Time
50 minutes plus observations for 10 days
Materials Needed
For the teacher:
- Build an example of the phototropism box according to directions
- Utility knife
For each group:
- Shoebox and/or cardboard milk cartons (have students bring these from home)
- Thick cardboard sections
- Clear plastic cup (6 oz.)
- Potting soil or peat pots
- Two bean seeds
For each student:
- Tropism Twist activity sheet
phototropism: a plant’s bending and growing towards a light source
Background Agricultural Connections
Although plants don’t have the ability to move from their rooted position, they do have the ability to respond to stimuli such as temperature, animals, moisture, gravity, and light. Tropisms are plant growth movements toward or away from a specific stimulus in nature. They help plants achieve optimal growth. Tropism comes from the Greek word, “to turn.”
Phototropism , photo meaning light, is the growth of a plant toward light. For plants, this light source is the sun, but artificial alternatives can also stimulate phototropism. This ability is very useful for plants, enabling them to position their leaves and flowers to efficiently receive the light energy they need for photosynthesis.
Plants have special receptors made of chemical pigments known as phytochromes. When phytochromes absorb visible wavelengths of light they emit a chemical signal that produces a hormone known as auxin. Auxins cause the cells on the shaded side of a plant to elongate more than cells on the sunny side. The growth of cells on the light-receiving side of the plant is inhibited. As a result, plants bend and twist towards the light.
In this lesson, we will focus on phototropism. However, there are a couple of other types of tropisms displayed by plants that are also important. Gravitropism causes stems of plants to grow up and roots to grow down. Hydrotropism causes plant roots to grow towards water.
- Show students a handful of sunflower seeds. Use a picture if actual sunflower seeds aren't available.
- Sunflowers produce seeds which can be eaten, pressed for oil, or used in birdseed.
- Sunflower seeds are produced mostly in North and South Dakota, Minnesota, Kansas, Colorado, Nebraska, Texas and California. 1
- The type of sunflowers which are grown for seeds can grow to be 10 feet tall.
- The head of a mature sunflower is usually about 15" in diameter.
- Sunflowers are typically planted in the spring and harvested in the fall.
- Summarize the lifecycle of the sunflower using pictures to illustrate.
- Define the word, phototropism. Break the word down into smaller pieces, explaining that “photo” means “light” and “tropism” means “to turn.” Draw on prior knowledge and remind students that plants receive their energy from the sun. Some plants move so that the surface of their leaves receive the most sun rays.
- Tell students that in this lesson, they will design an experiment so they can observe phototropism in action.
Explore and Explain
- Distribute the Tropism Twist activity sheet to each student. Ask students to write a hypothesis for the testable question, “Does light affect the direction that a seedling will grow?” in the appropriate place on their worksheet.
- Divide students into lab groups consisting of 3-4 students. Distribute shoebox, scissors, duct tape, and cardboard. Instruct students to write their names on the bottom of the shoebox.

- Place the lid on the front of the box. Hold the box up to the light. Look through your two-inch hole and make certain that this hole is the only source for light to get into the box. Carefully duct tape over any other cracks or crevices that may be letting light in. Do not tape the box shut.
- Using paper to create a pattern, cut two pieces the height of the inside of the shoebox and half the width. Trace the pattern on stiff cardboard and cut them out. Tape them into the box as shown.
- After tropism boxes are complete, instruct students to use the designated planting station to plant two bean seeds for their group experiment. The planting station should be supplied with newspaper, 6-ounce plastic cups, potting soil, bean seeds, water spray bottles, craft sticks, masking tape, and markers for labeling.
- Place planted seeds in a lighted area and wait for the seeds to germinate. When the seedlings are approximately two inches tall, place the watered seedlings into the shoebox as shown.
- Close the box, tape it, and place it by a sunny window so the square hole on the top can be exposed to the light.
- After five days, carefully shine a flashlight through the square hole to observe the plant growth. It is best not to disturb plants during this testing period. It can alter the final outcome.
- In another 3-5 days, check to see if the plant has grown enough to reach the top of the box. Remove the shoebox lid once the plant has reached the top of the shoebox. Have students record their observations and answer the questions on their Tropism Twist worksheet.
- Have students plant the bean seeds, then build the tropism boxes on another day while you are waiting for seeds to germinate.
- Using different kinds of seeds, test to see if different kinds of seedlings display phototropism more than others. Do some seedlings bend and twist the moment they germinate? Do other seedlings show no sign of phototropism? Compare and contrast growth rate and angle of growth rate between seedlings.
Plant sunflower plants in large pots or outside. Once the sunflower plants begin to flower have students observe the flowers throughout the day. Explain to students that sunflower plants display heliotropism. Heliotropism is a plant behavior where the flower of the plant will follow the sun throughout the course of the day. Plants do this to maximize the light they receive during daylight hours.
Have students plant bean seeds as described in the lesson. Place nylon netting over the cup and tie it closed so the cup’s contents cannot be displaced. Tell students that they are going to study a different kind of tropism called gravitropism, or (geotropism). Gravitropism is a plant’s movement in response to gravity. It causes roots to grow down and the shoots to grow up towards the sky. By using a clear cup, students will be able to observe the growth pattern of both the roots and shoots.
Plant bean seeds as described in the lesson. Create cone shaped covers made from different colors of cellophane. Research wavelengths and how colors are absorbed at different wavelengths. Test to see if color affects plant growth.
After conducting these activities, review and summarize the following key concepts:
- Sunflowers are an agricultural crop grown by farmers. Their seeds are produced for food, oil, or animal feed.
- Because plants receive their energy from the sun through photosynthesis, some plants "move" in order to face the sun and receive the most energy.
- http://www.sunflowernsa.com/
Acknowledgements
This lesson was originally developed in 1993 through a partnership between the California Department of Food and Agriculture, California Farm Bureau Federation, Fertilizer Inspection Advisory Board, Fertilizer Research and Education Program and the California Foundation for Agriculture in the Classroom. It was updated in 2013 with funding from the California Foundation for Agriculture in the Classroom and a grant from the California Department of Food and Agriculture’s Fertilizer Research and Education Program.
Original Author: Pamela Emery Executive Director: Judy Culbertson Illustrator: Erik Davison Layout and Design: Nina Danner
Recommended Companion Resources
- Bottle Biology
- Sunflower House
- Troubled Waters
Mandi Bottoms, Shaney Emerson, and Robin Satnick
Organization
California Foundation for Agriculture in the Classroom
How can we help?
Send us a message with your question or comment.
Popular Cities
Take Class 10 Tuition from the Best Tutors
Book a Free Demo
- CBSE Class 10 Syllabus
- NCERT Class 10 Solutions
10. Design an experiment to demonstrate hydrotropism.
Asked by Sumitha 22/12/2021 Last Modified 15 Apr
Learn Control and co-ordination in animals and plants
Please enter your answer
To demonstrate hydrotropism, one could set up an experiment using a potted plant placed horizontally with its roots positioned at one end of a transparent container filled with moist soil. By observing the direction of root growth over time, one can demonstrate how the roots grow towards the moist soil, indicating the plant's response to the water gradient. Alternatively, one could use a gel medium with a water gradient to more precisely control the water distribution and observe root growth towards the water source.
Now ask question in any of the 1000+ Categories, and get Answers from Tutors and Trainers on UrbanPro.com
Related Lessons
Recommended Articles
Meet Raghunandan.G.H, a B. Tech Tutor from...
Raghunandan is a passionate teacher with a decade of teaching experience. Being a skilled trainer with extensive knowledge, he provides high-quality BTech, Class 10 and Class 12 tuition classes. His methods of teaching with real-time examples makes difficult topics simple to understand. He explains every concept in-detail...
Read full article >
Meet Swati, a Hindi Tutor from Bangalore
Swati is a renowned Hindi tutor with 7 years of experience in teaching. She conducts classes for various students ranging from class 6- class 12 and also BA students. Having pursued her education at Madras University where she did her Masters in Hindi, Swati knows her way around students. She believes that each student...
Meet Mohammad Wazid, a skilled trainer for...
Mohammad Wazid is a certified professional tutor for class 11 students. He has 6 years of teaching experience which he couples with an energetic attitude and a vision of making any subject easy for the students. Over the years he has developed skills with a capability of understanding the requirements of the students. This...
Quest Academy - Institute of the month
Quest Academy is a professional Bangalore based NEET and JEE (Main + Advanced) training institute. The academy was incorporated in 2015 to cater to the needs of students, who aim to crack competitive exams by connecting with the best brains around. The institute helps students enhance their skills and capabilities through...
Looking for Class 10 Tuition ?
Learn from the Best Tutors on UrbanPro
Are you a Tutor or Training Institute?
| Male Female Please select your gender. | |
| Please enter Password Sorry, this phone number is not verified, Please login with your email Id. |
By signing up, you agree to our Terms of Use and Privacy Policy .
Already a member?
Looking for Class 10 Tuition Classes?
The best tutors for Class 10 Tuition Classes are on UrbanPro
- Select the best Tutor
- Book & Attend a Free Demo
- Pay and start Learning

Take Class 10 Tuition with the Best Tutors
The best Tutors for Class 10 Tuition Classes are on UrbanPro

This website uses cookies
We use cookies to improve user experience. Choose what cookies you allow us to use. You can read more about our Cookie Policy in our Privacy Policy
- About UrbanPro.com
- Terms of Use
- Privacy Policy

UrbanPro.com is India's largest network of most trusted tutors and institutes. Over 55 lakh students rely on UrbanPro.com, to fulfill their learning requirements across 1,000+ categories. Using UrbanPro.com, parents, and students can compare multiple Tutors and Institutes and choose the one that best suits their requirements. More than 7.5 lakh verified Tutors and Institutes are helping millions of students every day and growing their tutoring business on UrbanPro.com. Whether you are looking for a tutor to learn mathematics, a German language trainer to brush up your German language skills or an institute to upgrade your IT skills, we have got the best selection of Tutors and Training Institutes for you. Read more
- JEE Main Exam
- JEE Advanced Exam
- BITSAT Exam
- View All Engineering Exams
- Colleges Accepting B.Tech Applications
- Top Engineering Colleges in India
- Engineering Colleges in India
- Engineering Colleges in Tamil Nadu
- Engineering Colleges Accepting JEE Main
- Top IITs in India
- Top NITs in India
- Top IIITs in India
- JEE Main College Predictor
- JEE Main Rank Predictor
- MHT CET College Predictor
- AP EAMCET College Predictor
- GATE College Predictor
- KCET College Predictor
- JEE Advanced College Predictor
- View All College Predictors
- JEE Advanced Cutoff
- JEE Main Cutoff
- GATE Registration 2025
- JEE Main Syllabus 2025
- Download E-Books and Sample Papers
- Compare Colleges
- B.Tech College Applications
- JEE Main Question Papers
Quick links
- Mechanical Engineering
- Civil Engineering
- Aeronautical Engineering
- Information Technology
- Electronic Engineering
Quick Links
- Information Technology Courses
- Programming Courses
- Web Development Courses
- Data Analytics Courses
- Big Data Analytics Courses
- IT Colleges in Tamil Nadu
- IT Colleges in Uttar Pradesh
- Colleges Accepting Admissions
- MCA Colleges in India
- BCA Colleges in India
- Sample Papers
- Free Ebooks
- QnA - Get answers to your doubts
- Careers360 Youtube Channel
- Top Pharmacy Colleges in India
- Pharmacy Colleges in Pune
- Pharmacy Colleges in Mumbai
- Colleges Accepting GPAT Score
- Pharmacy Colleges in Lucknow
- List of Pharmacy Colleges in Nagpur
- GPAT Result
- GPAT 2024 Admit Card
- GPAT Question Papers
- Free Sample Papers
- RUHS Pharmacy Admission Test
- NCHMCT JEE 2024
- Mah BHMCT CET
- Top Hotel Management Colleges in Delhi
- Top Hotel Management Colleges in Hyderabad
- Top Hotel Management Colleges in Mumbai
- Top Hotel Management Colleges in Tamil Nadu
- Top Hotel Management Colleges in Maharashtra
- B.Sc Hotel Management
- Hotel Management
- Diploma in Hotel Management and Catering Technology
- List of Popular Branches
Diploma Colleges
- Top Diploma Colleges in Maharashtra
Other Exams
- SSC CHSL 2024
- UP PCS 2024
- UGC NET 2024
- RRB NTPC 2024
- IBPS PO 2024
- IBPS Clerk 2024
- IBPS SO 2024
- UPSC IAS 2024
- SSC CGL 2024
- IBPS RRB 2024
- Previous Year Sample Papers
- Free Competition E-books
- Sarkari Result
- QnA- Get your doubts answered
- UPSC Previous Year Sample Papers
- CTET Previous Year Sample Papers
- SBI Clerk Previous Year Sample Papers
- NDA Previous Year Sample Papers
Upcoming Events
- NDA Application Form 2024
- UPSC IAS Application Form 2024
- CDS Application Form 2024
- CTET Admit card 2024
- HP TET Result 2023
- SSC GD Constable Admit Card 2024
- UPTET Notification 2024
- SBI Clerk Result 2024
- CBSE Class 10th
- CBSE Class 12th
- UP Board 10th
- UP Board 12th
- Bihar Board 10th
- Bihar Board 12th
Top Schools
- Top Schools in India
- Top Schools in Delhi
- Top Schools in Mumbai
- Top Schools in Chennai
- Top Schools in Hyderabad
- Top Schools in Kolkata
- Top Schools in Pune
- Top Schools in Bangalore
Products & Resources
- JEE Main Knockout April
- NCERT Notes
- NCERT Syllabus
- NCERT Books
- RD Sharma Solutions
- Navodaya Vidyalaya Admission 2024-25
- NCERT Solutions
- NCERT Solutions for Class 12
- NCERT Solutions for Class 11
- NCERT solutions for Class 10
- NCERT solutions for Class 9
- NCERT solutions for Class 8
- NCERT Solutions for Class 7
Top Countries
- Study in USA
- Study in UK
- Study in Canada
- Study in Australia
- Study in Ireland
- Study in Germany
- Study in China
- Study in Europe
Student Visas
- Student Visa Canada
- Student Visa UK
- Student Visa USA
- Student Visa Australia
- Student Visa Germany
- Student Visa New Zealand
- Student Visa Ireland
- Top University in USA
- Top University in Canada
- Top University in Ireland
- Top Universities in UK
- Top Universities in Australia
- Best MBA Colleges in Abroad
- Business Management Studies Colleges
- CUET PG 2024
- IGNOU B.Ed Admission 2024
- DU Admission 2024
- UP B.Ed JEE 2024
- LPU NEST 2024
- IIT JAM 2024
- IGNOU Online Admission 2024
- Universities in India
- Top Universities in India 2024
- Top Colleges in India
- Top Universities in Uttar Pradesh 2024
- Top Universities in Bihar
- Top Universities in Madhya Pradesh 2024
- Top Universities in Tamil Nadu 2024
- Central Universities in India
- CUET DU Cut off 2024
- IGNOU Date Sheet 2024
- CUET DU CSAS Portal 2024
- CUET Response Sheet 2024
- CUET Result 2024
- CUET Participating Universities 2024
- CUET Previous Year Question Paper
- CUET Syllabus 2024 for Science Students
- E-Books and Sample Papers
- CUET College Predictor 2024
- CUET Exam Date 2024
- CUET Cut Off 2024
- NIRF Ranking 2024
- IGNOU Exam Form 2024
- CUET PG Counselling 2024
- CUET Answer Key 2024
- MAH MBA CET Exam
- View All Management Exams
Colleges & Courses
- MBA College Admissions
- MBA Colleges in India
- Top IIMs Colleges in India
- Top Online MBA Colleges in India
- MBA Colleges Accepting XAT Score
- BBA Colleges in India
- XAT College Predictor 2025
- SNAP College Predictor
- NMAT College Predictor
- MAT College Predictor 2024
- CMAT College Predictor 2024
- CAT Percentile Predictor 2024
- CAT 2024 College Predictor
- Executive MBA
- Part Time MBA
- Distance MBA
- XAT Registration
- Top MBA Entrance Exams 2024
- AP ICET Counselling 2024
- GD Topics for MBA
- CAT Exam Date 2024
- Download Helpful Ebooks
- IIM Fees Structure

Online Courses
- JEE Main One Month Course
- NEET One Month Course
- IBSAT Free Mock Tests
- IIT JEE Foundation Course
- Knockout BITSAT 2024
- Career Guidance Tool
Engineering Preparation
- Knockout JEE Main 2024
- Test Series JEE Main 2024
- JEE Main 2024 Rank Booster
Medical Preparation
- Knockout NEET 2024
- Test Series NEET 2024
- Rank Booster NEET 2024
Top Streams
- IT & Software Certification Courses
- Engineering and Architecture Certification Courses
- Programming And Development Certification Courses
- Business and Management Certification Courses
- Marketing Certification Courses
- Health and Fitness Certification Courses
- Design Certification Courses
Specializations
- Digital Marketing Certification Courses
- Cyber Security Certification Courses
- Artificial Intelligence Certification Courses
- Business Analytics Certification Courses
- Data Science Certification Courses
- Cloud Computing Certification Courses
- Machine Learning Certification Courses
- View All Certification Courses
- UG Degree Courses
- PG Degree Courses
- Short Term Courses
- Free Courses
- Online Degrees and Diplomas
- Compare Courses
Top Providers
- Coursera Courses
- Udemy Courses
- Edx Courses
- Swayam Courses
- upGrad Courses
- Simplilearn Courses
- Great Learning Courses
- AIIMS Nursing
- Top Medical Colleges in India
- Top Medical Colleges in India accepting NEET Score
- Medical Colleges accepting NEET
- List of Medical Colleges in India
- List of AIIMS Colleges In India
- Medical Colleges in Maharashtra
- Medical Colleges in India Accepting NEET PG
- NEET College Predictor
- NEET PG College Predictor
- NEET MDS College Predictor
- NEET Rank Predictor
- DNB PDCET College Predictor
- NEET Result 2024
- NEET Asnwer Key 2024
- NEET Cut off
- NEET Online Preparation
- Download Helpful E-books
- MS (Master of Surgery)
- Compare Law Collages
- CLAT Syllabus 2025
- CLAT Previous Year Question Paper
- Corporate Law
- Top Law Colleges in India
- Law College Accepting CLAT Score
- List of Law Colleges in India
- Top Law Colleges in Delhi
- Top NLUs Colleges in India
- Top Law Colleges in Chandigarh
- Top Law Collages in Lucknow
Predictors & E-Books
- CLAT College Predictor
- MHCET Law ( 5 Year L.L.B) College Predictor
- AILET College Predictor
- NID DAT Exam
- Pearl Academy Exam
Predictors & Articles
- NIFT College Predictor
- UCEED College Predictor
- NID DAT College Predictor
- NID DAT Syllabus 2025
- NID DAT 2025
- Design Colleges in India
- Top NIFT Colleges in India
- Fashion Design Colleges in India
- Top Interior Design Colleges in India
- Top Graphic Designing Colleges in India
- Fashion Design Colleges in Delhi
- Fashion Design Colleges in Mumbai
- Top Interior Design Colleges in Bangalore
- NIFT Result 2024
- NIFT Fees Structure
- NIFT Syllabus 2025
- Free Design E-books
- List of Branches
- Careers360 Youtube channel
- Fashion Designing
- Interior Design
- Textile Design
- Communication Design
- Accessory Designing
- Jewellery Design
- IPU CET BJMC 2024
- JMI Mass Communication Entrance Exam 2024
- IIMC Entrance Exam 2024
- Media & Journalism colleges in Delhi
- Media & Journalism colleges in Bangalore
- Media & Journalism colleges in Mumbai
- List of Media & Journalism Colleges in India
- Mass Communication
- Event Management
Top Courses & Careers
- Bachelor of Commerce (B.Com)
- Master of Commerce (M.Com)
- Company Secretary
- Cost Accountant
- Charted Accountant
- Credit Manager
- Financial Advisor
- Top Commerce Colleges in India
- Top Government Commerce Colleges in India
- Top Private Commerce Colleges in India
- Top M.Com Colleges in Mumbai
- Top B.Com Colleges in India
- CA Intermediate
- CA Foundation
- CS Executive
- CS Professional
- Difference between CA and CS
- Difference between CA and CMA
- CA Full form
- CMA Full form
- CS Full form
- CA Salary In India
Get Answers to all your Questions

- Design an experiment to demonstrate hydrotropism.
- #CBSE 10 Class
- #Science Textbook for Class X
- #Control and Coordination
Q.5. Design an experiment to demonstrate hydrotropism.
Answers (2).

The movement of the plant in the direction of the stimulus is called tropism.
AIM: To demonstrate hydrotropism in plants.
PROCEDURE :
i. Plant a seedling in a vessel containing soil.
ii. Adjacent to the seedling put a porous pot containing water.
iii. Leave the set up for a few days.

OBSERVATION :
iv. On examining the roots of seedlings it is observed that the roots bend towards the source of water and do not grow straight.
It confirms that plant shows hydrotropism as the roots bend towards the porous pot of water. Hydrotropism is a plant growth response in which the direction of growth is determined by a stimulus of the gradient in water concentration.
Pankaj Sanodiya
1 Take a porous pot and fill it with water.
2 keep a few freshly germinated pea seedling in a dried sand.
3 As the water is not available in sand the root growing will bend towards porous pot filled with water.
4 you will observe a hydrotropic curvature of the root as it grows towards water
5 This bending of root show the movement as a response towards water.
Manpreet Singh
Similar questions.
- Three coins are tossed simultaneously 200 times with the following frequencies of different outcomes:If the three coins are simultaneously tossed again, compute the probability of 2 heads coming up
- Use Euclid' s division algorithm to find the HCF of : 135 and 225
- Use Euclid' s division algorithm to find the HCF of :196 and 38220
Trending Articles/News

CBSE Class 10 compartment result 2024 soon; passing marks, websites to check
CBSE Class 10 compartment result 2024 expected soon at cbseresults.nic.in; grading system, passing marks
CBSE Class 10 compartment result 2024 declared; 48.83% pass
Latest Question
- A sum of money under compound interest doubles itself in 4 years. In how many years will it become 16 times itself? Option: 1 1
- A certain loan amounts, under compound interest, compounded annually earns an interest of Rs.1980 in the second year and Rs.2178 in the third year. How much interest did it earn in the first year?<
Ask your Query
Create Your Account
- I am already a member
Welcome Back :)
To keep connected with us please login with your personal information by phone
Dont't have an account? Register Now
Register to post Answer
- Class 6 Maths
- Class 6 Science
- Class 6 Social Science
- Class 6 English
- Class 7 Maths
- Class 7 Science
- Class 7 Social Science
- Class 7 English
- Class 8 Maths
- Class 8 Science
- Class 8 Social Science
- Class 8 English
- Class 9 Maths
- Class 9 Science
- Class 9 Social Science
- Class 9 English
- Class 10 Maths
- Class 10 Science
- Class 10 Social Science
- Class 10 English
- Class 11 Maths
- Class 11 Computer Science (Python)
- Class 11 English
- Class 12 Maths
- Class 12 English
- Class 12 Economics
- Class 12 Accountancy
- Class 12 Physics
- Class 12 Chemistry
- Class 12 Biology
- Class 12 Computer Science (Python)
- Class 12 Physical Education
- GST and Accounting Course
- Excel Course
- Tally Course
- Finance and CMA Data Course
- Payroll Course
Interesting
- Learn English
- Learn Excel
- Learn Tally
- Learn GST (Goods and Services Tax)
- Learn Accounting and Finance
- GST Tax Invoice Format
- Accounts Tax Practical
- Tally Ledger List
- GSTR 2A - JSON to Excel
Are you in school ? Do you love Teachoo?
We would love to talk to you! Please fill this form so that we can contact you
Questions from inside the book
- NCERT Questions
- Teachoo Questions
Question 5 Page 122 - Chapter 7 Class 10 - Control and Coordination
Last updated at April 16, 2024 by Teachoo
Design an experiment to demonstrate hydrotropism.
Plant movements that take place along a particular direction are called Tropic movements. When the stimulus that causes the movement is water , it is called hydrotropism .
Plants grow towards regions of water if there is a source of water nearby.
The following experiment demonstrates hydrotropism:
- Take two beakers and label them A and B
- In A, add moist soil and sow a seed
- In B, fill half with moist soil and half with dry soil. At the side of the moist soil place a water source also. Sow a seed.
- Let the plants grow.

You will notice that;
- In beaker A , the root grows straight as there is moist soil equally around
- In beaker B , the roots grow towards the side of the moist soil.
This experiment shows that plant roots have the tendency to grow towards a water sourc e.
This is hydrotropism .
HYDROTROPISM

Maninder Singh
CA Maninder Singh is a Chartered Accountant for the past 14 years and a teacher from the past 18 years. He teaches Science, Economics, Accounting and English at Teachoo
Hi, it looks like you're using AdBlock :(
Please login to view more pages. it's free :), solve all your doubts with teachoo black.
Study Material
.avif)
Design an experiment to demonstrate hydrotropism.
Hydrotropism is the process of the directional growth of plant roots towards a water source. Moist soil on one side and dry land on the other are used for the germination of seeds. The seedling first moves downward due to positive gravity. Later, it starts turning towards the wet ground.
Experiments that can prove hydrotropism
- Take 1 and 2 beakers.
- Fill the beaker 1 with moist soil to sow the seeds.
- In one part of beaker 2, add dry soil, and in another section, add moist soil. Sow the seeds.
- Next, place the tiny water beaker.
- To ensure the plants flourish, let them rest for a while.
- The plants will grow normally due to the moist soil's presence, and their roots will be straight.
- The pant in beaker 2 will grow towards the tiny water beaker placed next to it.
- Plants exhibit hydrotropism when they move towards the porous source.
- Hydrotropism is the directional growth of plant roots toward or away from water.

Class 10 NCERT Books Pdf
Class 10 Important Questions Pdf
Class 9 NCERT Books Pdf
CBSE Class 10 Sample Papers
Class 9 NCERT Solutions Pdf
Class 9 ncert exemplar.
To Download PDF
Please verify your Whatsapp number first, so you can download this pdf immediately
Please type a valid 10 digit whatsapp number

OTP sent, check your whatsapp
Your OTP is incorrect, Please enter valid OTP
Buy Latest Books
Teacher's Corner

- Mathematics (Standard)
- Mathematics (Basic)
- English L&L
- English Communicative
- Social Science
- Information Technology
- English Core
- Mathematics
- Accountancy
- Business Studies
- Political Science
- Computer Application
- Science (Hindi )
- Maths (Hindi)
- Social Science (Hindi )
- Applied Maths
- Physical Education
- History & Civics
- Literature in English
- English Language
- 10 Year Solved Papers
- Class 10 Science
- Class 10 Maths
- Class 12 Physics
- Class 12 Chemistry
- Class 12 Maths
- Class 12 Biology
- Class 12 PCB Combo
- Class 12 PCM Combo
- Math Standard
- Computer Applications
- Class 10 English
- Class 12 English
Design an experiment to demonstrate hydrotropism.
Chemical coordination in plants.
Growth movement of the roots of the plant towards water is called hydrotropism.
Below experiment demonstrates hydrotropism:
- Take two beakers 1 and 2.
- In beaker 1 add moist soil and sow the seeds.
- In beaker 2 add dry soil in one part and moist soil in another part and sow the seeds. Also, place a small porous pot containing water in the dry part adjacent to the seedling.
- Leave the set up for few days so that the plant can grow.
Observation
- In beaker 1, the plant grows normally and the roots are straight.
- In beaker 2, the roots bend towards the source of water and do not grow straight.
This experiment confirms that roots of a plant show positive hydrotropism as they bend towards the source of water (porous pot).
Answered By
Related Questions
Give an example of a plant hormone that promotes growth., what are plant hormones, how is the movement of leaves of the sensitive plant different from the movement of a shoot towards light, how do auxins promote the growth of a tendril around a support.
Confirm Password *
Username or email *
Forgot Password
Lost your password? Please enter your email address. You will receive a link and will create a new password via email.
You must login to ask question.
Tiwari Academy Discussion
Design an experiment to demonstrate hydrotropism.
NCERT Solution for Class 10 Science Chapter 7 Control and Coordination NCERT Books for Session 2022-2023 CBSE Board and UP Board Intext Questions Page No-122 Questions No-5
Take two small beakers and label them as A and B. Fill beaker A with water. Now make a cylindrical-shaped roll from a filter paper and keep it as a bridge between beaker A and beaker B, as shown in the figure. Attach few germinating seeds in the middle of the filter paper bridge. Now, cover the entire set-up with a transparent plastic container so that the moisture is retained. Observation: The roots of the germinating seeds will grow towards beaker A.This experiment demonstrates the phenomenon of hydrotropism
For more answers visit to website: https://www.tiwariacademy.com/ncert-solutions/class-10/science/chapter-7/
- Share on Facebook
- Share on Twitter
- Share on LinkedIn
- Share on WhatsApp
Take two glass troughs A and B and fill each one of them two-thirds with soil. In trough A plant a tiny seedling. In trough B plant a similar seedling and place a clay pot inside the soil. Water the soil in trough A daily and uniformly. Do not water the soil in trough B but put some water in the clay pot. Leave both the troughs for a few days. Now, dig up the seedlings carefully from both the troughs without damaging their roots. We will find that the root of seedling in trough A is straight. On the other hand, the root of seedling in trough B is found to be bent to the right side i.e., towards the clay pot containing water. In trough A, the root of the seedling gets water from both sides. But in trough B, the roots get water oozing out from the clay pot which is kept on the right side. Therefore, the root of seedling in trough B grows and bends towards the source of water to the right side. This experiment shows that the root of a plant grows towards water. In other words, the root of a plant is hydrotropism.
Hydro’ means water. Hydrotropism means growth towards water.
Materials Required: Seed, A big container, Porous water pot, water and Sand.
Procedure: • The tray should be big enough to accommodate the porous pot. • Fill the tray with sand and insert some seeds in it. • Make a pit in the sand and insert the porous pot in it. • Fill the porous pot with water. • Leave the set up for about a week.
Observation: After a week when seeds are taken out, it is observed that roots grow in the direction of the porous pot. This shows hydrotropic movement in roots.
To demonstrate hydrotropism in plants. Procedure : i. Plant a seedling in a vessel containing soil. ii. Adjacent to the seedling put a porous pot containing water. iii. Leave the set up for few days. Observation : iv. On examining the roots it is observed that the roots bend towards the source of water and do not grow straight. result : It confirms that plant shows hydrotropism as the roots bend towards the porous pot of water. As hydrotropism is a plant growth response in which the direction of growth is determined by a stimulus of gradient in water concentration.
Following things are required for this experiment: Seeds of bean, a deep tray, sand, a porous flower pot
Procedure: (i) The tray should be big enough to accommodate the porous pot. (ii) Fill the tray with sand and insert some seeds in it. (iii) Make a pit in the sand and insert the porous pot in it. (iv) Fill the porous pot with water. (v) Leave the set up for about a week.
Hydrotropism is the growth or movement of a plant in response to water. To design an experiment to demonstrate hydrotropism, you can set up a simple investigation using plant seedlings. Here’s a step-by-step guide:
Materials: 1. Plant Seeds (e.g., mung beans or radish seeds) 2. Petri dishes or small pots 3. Potting soil 4. Watering can or spray bottle 5. Transparent plastic barrier or divider (to create a water gradient) 6. Light source 7. Ruler or measuring tape 8. Marker or labels
Procedure: 1. Seed Germination:
» Plant the seeds in separate pots or sections of a Petri dish filled with potting soil. Ensure uniform conditions for germination, including temperature, light, and moisture.
2. Setup of Water Gradient:
» Create a water gradient by placing a transparent plastic barrier or divider in the middle of the experimental setup. This will allow you to water only one side of the plants, creating a gradient of water availability.
3. Labeling:
» Label each section or pot with the type of seed and mark one side as the “Watered” side and the other side as the “Dry” side.
4. Watering:
» Water only one side of the setup, ensuring that the “Watered” side receives ample water, while the “Dry” side remains relatively dry. Be careful not to overwater or underwater; maintain consistent conditions except for water availability.
5. Light Exposure:
» Place the entire setup under a light source to ensure uniform light conditions for the seedlings.
6. Observation and Measurement:
» Regularly observe and measure the growth of the seedlings. Focus on the direction of root growth. Use a ruler or measuring tape to measure the length of the roots.
7. Recording Data:
» Record your observations in a notebook. Note any differences in root growth between the “Watered” side and the “Dry” side.
8. Analysis:
» Analyze the data to determine if there is a significant difference in root growth direction between the watered and dry sides. Look for evidence of hydrotropic responses in the roots. Expected Results: If hydrotropism is occurring, you should observe a directional growth of the roots toward the watered side of the setup. The roots will likely exhibit a curvature, demonstrating the plant’s ability to sense and respond to water availability.
This experiment allows you to investigate how plants adjust their root growth in response to water gradients, providing evidence of hydrotropism.
Leave an answer Cancel reply
Save my name, email, and website in this browser for the next time I comment.
What is hydrotropism? Describe an activity to demonstrate hydrotropism.
In plant biology, hydrotropism is a plant’s growth response toward water sources. hydrotropism, which is triggered by plant hormones, can be a positive or negative response, whereby the plant will either turn away from water concentrations, protecting itself from oversaturation or move towards them, protecting itself in times of drought. activity to demonstrate hydrotropism: take a plant (pea seedling) in a nude jar filled with sand. now place a porous pet filled with water in the wide jar. roots of the plant will green towards water and bond towards the water source showing hydrotropism..
Question 5 Design an experiment to demonstrate hydrotropism.
What is hydrotropism in plants?

Question 5 Design an experiment to demonstrate hydrotropism.
Experiment to demonstrate hydrotropism 1. Take a clay pot, fill it partially with water and place it in the soil. 2. Now put some germinating seeds in the soil. 3. After one or two days you will observe that the roots of all the germinating seeds will grow towards the clay pot. 4. This experiment proves that roots of germinating seed exhibit positive hydrotropism.

Give an experiment to demonstrate diffusion

Talk to our experts
1800-120-456-456
Design an experiment to demonstrate hydrotropism.
- Question Answer
- Design an experiment to demons...

Repeaters Course for NEET 2022 - 23

IMAGES
COMMENTS
3. The process of root growth or migration towards a water source is known as hydrotropism. Experiment to prove hydrotropism: Procedure. 1 and 2 beakers are taken. Beaker 1 is filled with moist soil, which is used to sow the seeds. In one part of beaker 2, dry soil is added, while in another section, moist soil is added, and the seeds are sown
Procedure. Take two beakers 1 and 2. In beaker 1 add moist soil and sow the seeds. In beaker 2 add dry soil in one part and moist soil in another part and sow the seeds. Also, place a small beaker of water just adjacent to it. Keep it for some time so that the plants can grow. Result. It was found that in beaker 1 due to the presence of moist ...
Design an experiment to demonstrate hydrotropism📲PW App Link - https://bit.ly/PW_APP🌐PW Website - https://bit.ly/PW_APP📌 PHYSICS WALLAH OTHER CHANNELS :🌐...
Presented by www.shikshaabhiyan.com This video is a part of the series for CBSE Class 10, Biology demo videos for the chapter "Control & Coordination." In th...
The aim of this experiment is to demonstrate hydrotropism, the growth response of plants to moisture gradients. Begin by preparing two identical containers with moist, well-drained soil. Plant several seeds of the same species at equal depths in each container. Ensure that the seeds are known to exhibit hydrotropic responses, such as pea or ...
Hydrotropism is a type of tropic movement by which some plant parts, specifically root and stem, tend to move towards or away from water stimulus. The term 'hydrotropism' is a combination of two words, 'hydro' and 'tropism'. Here, 'hydro 'means 'water', and 'tropism' stands for 'tropic movement'. Tropic movement is a ...
Until now, all hydrotropism experiments have been performed on primary roots of plants. Wiesner and Molisch already observed that lateral roots grow more easily in the direction of water than primary roots (Wiesner, 1881; Molisch, 1883). Hydrotropism research needs to extend its scope and investigate the response of lateral roots.
Design an experiment to demonstrate hydrotropism.Class: 10Subject: BIOLOGYChapter: CONTROL AND COORDINATIONBoard:CBSEYou can ask any doubt from class 6-12, ...
Show students a completed tropism testing box and guide them through the steps of creating their own boxes. Use the diagrams to guide students through the construction process. Carefully draw and cut out a two-inch square from the middle section of one end of the box. Students may need help from the teacher and the teacher's utility knife.
1 Answer. To demonstrate hydrotropism, one could set up an experiment using a potted plant placed horizontally with its roots positioned at one end of a transparent container filled with moist soil. By observing the direction of root growth over time, one can demonstrate how the roots grow towards the moist soil,...
1 Take a porous pot and fill it with water. 2 keep a few freshly germinated pea seedling in a dried sand. 3 As the water is not available in sand the root growing will bend towards porous pot filled with water. 4 you will observe a hydrotropic curvature of the root as it grows towards water. 5 This bending of root show the movement as a ...
Design an experiment to demonstrate hydrotropism. Answer Plant movements that take place along a particular direction are called Tropic movements. When the stimulus that causes the movement is water , it is called hydrotropism . Plants grow towards regions of water if there is a source of water nearby.
Design an experiment to demonstrate hydrotropism. Answer: Hydrotropism is the process of the directional growth of plant roots towards a water source. Moist soil on one side and dry land on the other are used for the germination of seeds. The seedling first moves downward due to positive gravity. Later, it starts turning towards the wet ground.
Hydrotropism (hydro- "water"; tropism "involuntary orientation by an organism, that involves turning or curving as a positive or negative response to a stimulus") [1] is a plant's growth response in which the direction of growth is determined by a stimulus or gradient in water concentration. A common example is a plant root growing in humid air bending toward a higher relative humidity level.
Hydrotropism is demonstrated by the plant's roots bending in the direction of the porous water-holding vessel. A water concentration gradient stimulus controls the direction of development in hydrotropism, a particular sort of plant growth response. Summary: Design an experiment to Demonstrate Hydrotropism.
Growth movement of the roots of the plant towards water is called hydrotropism. Below experiment demonstrates hydrotropism: Procedure. Take two beakers 1 and 2. In beaker 1 add moist soil and sow the seeds. In beaker 2 add dry soil in one part and moist soil in another part and sow the seeds.
NCERT InText Questions (Page-105) Q.5 - Control and Coordination | Class 10 | NCERT Solution Series | SCIENCE (BIOLOGY)In this video, we will discuss Questio...
Hydrotropism is the growth or movement of a plant in response to water. To design an experiment to demonstrate hydrotropism, you can set up a simple investigation using plant seedlings. Here's a step-by-step guide: Materials: 1. Plant Seeds (e.g., mung beans or radish seeds) 2. Petri dishes or small pots.
Describe an activity to demonstrate hydrotropism. In plant biology, hydrotropism is a plant's growth response toward water sources. Hydrotropism, which is triggered by plant hormones, can be a positive or negative response, whereby the plant will either turn away from water concentrations, protecting itself from oversaturation or move towards ...
Design an experiment to demonstrate hydrotropism. Open in App. Solution. Experiment to demonstrate hydrotropism 1. Take a clay pot, fill it partially with water and place it in the soil. 2. Now put some germinating seeds in the soil. 3. After one or two days you will observe that the roots of all the germinating seeds will grow towards the clay ...
Design an experiment to demonstrate hydrotropism.Solution:To demonstrate hydrotropism in plants.Procedure :i. Plant a seedling in a vessel containing soil.ii...
The process of hydrotropism can be observed with the help of a simple experiment. The experiment will help to understand the whole process of plant movement that occurs in presence of any water source. For the experiment, we will need to have two beakers, soil, plant seeds and water. First of all, we have to take moist soil in beaker 1 and in ...
Q.5 Design an experiment to demonstrate hydrotropism.#controlandcoordination #ncertsolutions