Advertisement

Recent advances in lung cancer research: unravelling the future of treatment
- Review Article
- Published: 06 April 2024
Cite this article

- Luca Bertolaccini ORCID: orcid.org/0000-0002-1153-3334 1 ,
- Monica Casiraghi 1 , 2 ,
- Clarissa Uslenghi 1 ,
- Sebastiano Maiorca 1 &
- Lorenzo Spaggiari 1 , 2
813 Accesses
3 Citations
1 Altmetric
Explore all metrics
Lung cancer, a multifaceted disease, demands tailored therapeutic approaches due to its diverse subtypes and stages. This comprehensive review explores the intricate landscape of lung cancer research, delving into recent breakthroughs and their implications for diagnosis, therapy, and prevention. Genomic profiling and biomarker identification have ushered in the era of personalised medicine, enabling targeted therapies that minimise harm to healthy tissues while effectively combating cancer cells. The relationship between pulmonary tuberculosis and lung cancer is examined, shedding light on potential mechanisms linking these two conditions. Early detection methods, notably low-dose computed tomography scans, have significantly improved patient outcomes, emphasising the importance of timely interventions. There has been a growing interest in segmentectomy as a surgical intervention for early-stage lung cancer in recent years. Immunotherapy has emerged as a transformative approach, harnessing the body's immune system to recognise and eliminate cancer cells. Combining immunotherapy with traditional treatments, such as chemotherapy and targeted therapies, has shown enhanced efficacy, addressing the disease's heterogeneity and overcoming drug resistance. Precision medicine, guided by genomic profiling, has enabled the development of targeted therapies like tyrosine kinase inhibitors, offering personalised treatments tailored to individual patients. Challenges such as drug resistance and limited accessibility to advanced therapies persist, emphasising the need for collaborative efforts and innovative technologies like artificial intelligence. Despite challenges, ongoing interdisciplinary collaborations and technological advancements offer hope for a future where lung cancer is treatable and preventable, reducing the burden on patients and healthcare systems worldwide.
This is a preview of subscription content, log in via an institution to check access.
Access this article
Subscribe and save.
- Get 10 units per month
- Download Article/Chapter or eBook
- 1 Unit = 1 Article or 1 Chapter
- Cancel anytime
Price includes VAT (Russian Federation)
Instant access to the full article PDF.
Rent this article via DeepDyve
Institutional subscriptions
Similar content being viewed by others

The artificial intelligence and machine learning in lung cancer immunotherapy
Application of clinical bioinformatics in lung cancer-specific biomarkers.

Precision Medicine in Lung Cancer
Data availability.
Not applicable.
Cheng B, Xiong S, Li C, Liang H, Zhao Y, Li J et al (2020) An annual review of the remarkable advances in lung cancer clinical research in 2019. J Thorac Dis 12(3):1056–1069
Article PubMed PubMed Central Google Scholar
Ibodeng GO, Uche IN, Mokua R, Galo M, Odigwe B, Galeas JN, Dasgupta S (2023) A snapshot of lung cancer: where are we now?-a narrative review. Ann Transl Med 11(6):261
Article CAS PubMed PubMed Central Google Scholar
Bertolaccini L, Casiraghi M, Petrella F, Rampinelli C, Tessitore A, Spaggiari L (2022) A methodological quality evaluation of the published guidelines and recommendations about the lung cancer screening. Eur J Cancer Prev 31(1):19–25
Article PubMed Google Scholar
Duma N, Santana-Davila R, Molina JR (2019) Non-small cell lung cancer: epidemiology, screening, diagnosis, and treatment. Mayo Clin Proc 94(8):1623–1640
Article CAS PubMed Google Scholar
Hwang SY, Kim JY, Lee HS, Lee S, Kim D, Kim S et al (2022) Pulmonary tuberculosis and risk of lung cancer: a systematic review and meta-analysis. J Clin Med 11(3):765
Yaegashi LB, Baldavira CM, Prieto TG, Machado-Rugolo J, Velosa APP, da Silveira LKR et al (2021) In situ overexpression of matricellular mechanical proteins demands functional immune signature and mitigates non-small cell lung cancer progression. Front Immunol 12:714230
Bourgot I, Primac I, Louis T, Noel A, Maquoi E (2020) Reciprocal interplay between fibrillar collagens and collagen-binding integrins: implications in cancer progression and metastasis. Front Oncol 10:1488
Horne ZD, Jack R, Gray ZT, Siegfried JM, Wilson DO, Yousem SA et al (2011) Increased levels of tumor-infiltrating lymphocytes are associated with improved recurrence-free survival in stage 1A non-small-cell lung cancer. J Surg Res 171(1):1–5
Jamal-Hanjani M, Wilson GA, McGranahan N, Birkbak NJ, Watkins TBK, Veeriah S et al (2017) Tracking the evolution of non-small-cell lung cancer. N Engl J Med 376(22):2109–2121
Oliver AL (2022) Lung cancer: epidemiology and screening. Surg Clin North Am 102(3):335–344
Ribas A, Wolchok JD (2018) Cancer immunotherapy using checkpoint blockade. Science 359(6382):1350–1355
Sahin U, Derhovanessian E, Miller M, Kloke BP, Simon P, Lower M et al (2017) Personalized RNA mutanome vaccines mobilize poly-specific therapeutic immunity against cancer. Nature 547(7662):222–226
Maude SL, Frey N, Shaw PA, Aplenc R, Barrett DM, Bunin NJ et al (2014) Chimeric antigen receptor T cells for sustained remissions in leukemia. N Engl J Med 371(16):1507–1517
Franzi S, Mattioni G, Rijavec E, Croci GA, Tosi D (2022) Neoadjuvant chemo-immunotherapy for locally advanced non-small-cell lung cancer: a review of the literature. J Clin Med 11(9):2629
Szeto CH, Shalata W, Yakobson A, Agbarya A (2021) Neoadjuvant and adjuvant immunotherapy in early-stage non-small-cell lung cancer, past, present, and future. J Clin Med 10(23):5614
Chai Y, Wu X, Bai H, Duan J (2022) Combined immunotherapy with chemotherapy versus bevacizumab with chemotherapy in first-line treatment of driver-gene-negative non-squamous non-small cell lung cancer: an updated systematic review and network meta-analysis. J Clin Med 11(6):1655
Schiller JH, Harrington D, Belani CP, Langer C, Sandler A, Krook J et al (2002) Comparison of four chemotherapy regimens for advanced non-small-cell lung cancer. N Engl J Med 346(2):92–98
Gandhi L, Rodriguez-Abreu D, Gadgeel S, Esteban E, Felip E, De Angelis F et al (2018) Pembrolizumab plus chemotherapy in metastatic non-small-cell lung cancer. N Engl J Med 378(22):2078–2092
Tsuboi M, Herbst RS, John T, Kato T, Majem M, Grohe C et al (2023) Overall Survival with Osimertinib in Resected EGFR-mutated NSCLC. N Engl J Med 389(2):137–147
Dohopolski M, Iyengar P (2021) Oligometastatic non-small cell lung cancer: a narrative review of stereotactic ablative radiotherapy. Ann Palliat Med 10(5):5944–5953
Yuan Z, Wang Y, Zhang J, Zheng J, Li W (2019) A meta-analysis of clinical outcomes after radiofrequency ablation and microwave ablation for lung cancer and pulmonary metastases. J Am Coll Radiol 16(3):302–314
Chen Y, Luo H, Liu R, Tan M, Wang Q, Wu X et al (2023) Efficacy and safety of particle therapy for inoperable stage II–III non-small cell lung cancer: a systematic review and meta-analysis. Radiat Oncol 18(1):86
Harada H, Suefuji H, Mori K, Ishikawa H, Nakamura M, Tokumaru S et al (2023) Proton and carbon ion radiotherapy for operable early-stage lung cancer: 3-year results of a prospective nationwide registry. Int J Radiation Oncol Biol Phys 117(2):23
Article Google Scholar
de Koning HJ, van der Aalst CM, de Jong PA, Scholten ET, Nackaerts K, Heuvelmans MA et al (2020) reduced lung-cancer mortality with volume CT screening in a randomized trial. N Engl J Med 382(6):503–513
Huo B, Manos D, Xu Z, Matheson K, Chun S, Fris J et al (2023) Screening criteria evaluation for expansion in pulmonary neoplasias (SCREEN). Semin Thorac Cardiovasc Surg 35(4):769–780
Passiglia F, Cinquini M, Bertolaccini L, Del Re M, Facchinetti F, Ferrara R et al (2021) Benefits and harms of lung cancer screening by chest computed tomography: a systematic review and meta-analysis. J Clin Oncol 39(23):2574–2585
Qi SA, Wu Q, Chen Z, Zhang W, Zhou Y, Mao K et al (2021) High-resolution metabolomic biomarkers for lung cancer diagnosis and prognosis. Sci Rep 11(1):11805
Madama D, Martins R, Pires AS, Botelho MF, Alves MG, Abrantes AM, Cordeiro CR (2021) Metabolomic profiling in lung cancer: a systematic review. Metabolites 11(9):630
Planchard D, Kim TM, Mazieres J, Quoix E, Riely G, Barlesi F et al (2016) Dabrafenib in patients with BRAF(V600E)-positive advanced non-small-cell lung cancer: a single-arm, multicentre, open-label, phase 2 trial. Lancet Oncol 17(5):642–650
Araujo DC, Veloso AA, Borges KBG, Carvalho MDG (2022) Prognosing the risk of COVID-19 death through a machine learning-based routine blood panel: a retrospective study in Brazil. Int J Med Inform 165:104835
Chiu HY, Chao HS, Chen YM (2022) Application of artificial intelligence in lung cancer. Cancers (Basel) 14(6):1370
Christie JR, Lang P, Zelko LM, Palma DA, Abdelrazek M, Mattonen SA (2021) Artificial intelligence in lung cancer: bridging the gap between computational power and clinical decision-making. Can Assoc Radiol J 72(1):86–97
Goncalves S, Fong PC, Blokhina M (2022) Artificial intelligence for early diagnosis of lung cancer through incidental nodule detection in low- and middle-income countries-acceleration during the COVID-19 pandemic but here to stay. Am J Cancer Res 12(1):1–16
CAS PubMed PubMed Central Google Scholar
Goldsmith I, Chesterfield-Thomas G, Toghill H (2021) Pre-treatment optimization with pulmonary rehabilitation in lung cancer: making the inoperable patients operable. EClinicalMedicine 31:100663
Shields MD, Chen K, Dutcher G, Patel I, Pellini B (2022) Making the rounds: exploring the role of circulating tumor DNA (ctDNA) in non-small cell lung cancer. Int J Mol Sci 23(16):9006
Abbosh C, Frankell AM, Harrison T, Kisistok J, Garnett A, Johnson L et al (2023) Tracking early lung cancer metastatic dissemination in TRACERx using ctDNA. Nature 616(7957):553–562
Zaman FY, Subramaniam A, Afroz A, Samoon Z, Gough D, Arulananda S, Alamgeer M (2023) Circulating tumour DNA (ctDNA) as a predictor of clinical outcome in non-small cell lung cancer undergoing targeted therapies: a systematic review and meta-analysis. Cancers (Basel) 15(9):2425
Jaffee EM, Dang CV, Agus DB, Alexander BM, Anderson KC, Ashworth A et al (2017) Future cancer research priorities in the USA: a lancet oncology commission. Lancet Oncol 18(11):e653–e706
Saji H, Okada M, Tsuboi M, Nakajima R, Suzuki K, Aokage K et al (2022) Segmentectomy versus lobectomy in small-sized peripheral non-small-cell lung cancer (JCOG0802/WJOG4607L): a multicentre, open-label, phase 3, randomised, controlled, non-inferiority trial. Lancet 399(10335):1607–1617
Nakada T, Noda Y, Kato D, Shibasaki T, Mori S, Asano H et al (2019) Risk factors and cancer recurrence associated with postoperative complications after thoracoscopic lobectomy for clinical stage I non-small cell lung cancer. Thorac Cancer 10(10):1945–1952
Bedetti B, Bertolaccini L, Rocco R, Schmidt J, Solli P, Scarci M (2017) Segmentectomy versus lobectomy for stage I non-small cell lung cancer: a systematic review and meta-analysis. J Thorac Dis 9(6):1615–1623
Bertolaccini L, Prisciandaro E, Bardoni C, Cara A, Diotti C, Girelli L, Spaggiari L (2022) Minimally invasive anatomical segmentectomy versus lobectomy in stage IA non-small cell lung cancer: a systematic review and meta-analysis. Cancers (Basel) 14(24):6157
Wang P, Fu YH, Qi HF, He P, Wang HF, Li C, Liu XC (2023) Evaluation of the efficacy and safety of robot-assisted and video assisted thoracic surgery for early non-small cell lung cancer: a meta-analysis. Technol Health Care 32(2):511–523
Casiraghi M, Galetta D, Borri A, Tessitore A, Romano R, Diotti C et al (2019) Ten years’ experience in robotic-assisted thoracic surgery for early stage lung cancer. Thorac Cardiovasc Surg 67(7):564–572
Wang P, Wang S, Liu Z, Sui X, Wang X, Li X et al (2022) Segmentectomy and wedge resection for elderly patients with stage I non-small cell lung cancer: a systematic review and meta-analysis. J Clin Med 11(2):294
Bertolaccini L, Cara A, Chiari M, Diotti C, Glick N, Mohamed S et al (2023) Real-world survival outcomes of wedge resection versus lobectomy for cT1a/b cN0 cM0 non-small cell lung cancer: a single center retrospective analysis. Front Oncol 13:1226429
Bertolaccini L, Spaggiari L (2023) Is it time to cross the pillars of evidence in favor of segmentectomies in early-stage non-small cell lung cancer? Cancers (Basel) 15(7):1993
Zaraca F, Kirschbaum A, Pipitone MD, Bertolaccini L, Group PS (2023) Prospective randomized study on the efficacy of three-dimensional reconstructions of bronchovascular structures on preoperative chest CT scan in patients who are candidates for pulmonary segmentectomy surgery: the patches (prospective randomized study efficacy of three-dimensional reconstructions segmentecomy) study protocol. Trials 24(1):594
Komarnicki P, Musialkiewicz J, Stanska A, Maciejewski A, Gut P, Mastorakos G, Ruchala M (2022) Circulating neuroendocrine tumor biomarkers: past, present and future. J Clin Med 11(19):5542
Coudray N, Ocampo PS, Sakellaropoulos T, Narula N, Snuderl M, Fenyo D et al (2018) Classification and mutation prediction from non-small cell lung cancer histopathology images using deep learning. Nat Med 24(10):1559–1567
Biesinger M, Eicken N, Varga A, Weber M, Brndiar M, Erd G et al (2022) Lymph but not blood vessel invasion is independent prognostic in lung cancer patients treated by VATS-lobectomy and might represent a future upstaging factor for early stages. Cancers 14(8):1893
Asamura H, Nishimura KK, Giroux DJ, Chansky K, Hoering A, Rusch V, et al (2023) IASLC Lung Cancer Staging Project The New Database to Inform Revisions in the Ninth Edition of the TNM Classification of Lung Cancer. J Thorac Oncol 18(5): 564–575
Hardenberg MC, Patel B, Matthews C, Califano R, Garcia Campelo R, Grohe C et al (2022) The value of disease-free survival (DFS) and osimertinib in adjuvant non-small-cell lung cancer (NSCLC): an international Delphi consensus report. ESMO Open 7(5):100572
Wu YL, Tsuboi M, He J, John T, Grohe C, Majem M et al (2020) Osimertinib in resected EGFR-mutated non-small-cell lung cancer. N Engl J Med 383(18):1711–1723
Xu H, Baidoo AAH, Su S, Ye J, Chen C, Xie Y et al (2019) A comparison of EGFR mutation status in tissue and plasma cell-free DNA detected by ADx-ARMS in advanced lung adenocarcinoma patients. Transl Lung Cancer Res 8(2):135–143
Zou PC, Wang L, Liu B, Zhang HZ, Liu HC (2011) EGFR-targeted therapies combined with chemotherapy for treating advanced non-small-cell lung cancer: a meta-analysis. Diagnostics 9:38
Google Scholar
Solomon BJ, Bauer TM, Mok TSK, Liu G, Mazieres J, de Marinis F et al (2023) Efficacy and safety of first-line lorlatinib versus crizotinib in patients with advanced, ALK-positive non-small-cell lung cancer: updated analysis of data from the phase 3, randomised, open-label CROWN study. Lancet Respir Med 11(4):354–366
Hotta K, Hida T, Nokihara H, Morise M, Kim YH, Azuma K et al (2022) Final overall survival analysis from the phase III J-ALEX study of alectinib versus crizotinib in ALK inhibitor-naive Japanese patients with ALK-positive non-small-cell lung cancer. ESMO Open 7(4):100527
Shaw AT, Bauer TM, de Marinis F, Felip E, Goto Y, Liu G et al (2020) First-line lorlatinib or crizotinib in advanced ALK-positive lung cancer. N Engl J Med 383(21):2018–2029
Herbst RS, Baas P, Kim DW, Felip E, Perez-Gracia JL, Han JY et al (2016) Pembrolizumab versus docetaxel for previously treated, PD-L1-positive, advanced non-small-cell lung cancer (KEYNOTE-010): a randomised controlled trial. Lancet 387(10027):1540–1550
Isaacs J, Stinchcombe TE (2022) Neoadjuvant and adjuvant systemic therapy for early-stage non-small-cell lung cancer. Drugs 82(8):855–863
John AO, Ramnath N (2023) Neoadjuvant versus adjuvant systemic therapy for early-stage non-small cell lung cancer: the changing landscape due to immunotherapy. Oncologist 28(9):752–764
Wakelee H, Liberman M, Kato T, Tsuboi M, Lee SH, Gao S et al (2023) Perioperative pembrolizumab for early-stage non-small-cell lung cancer. N Engl J Med 389(6):491–503
Kogure Y, Hashimoto H, Oki M (2021) A randomized phase iii study of pembrolizumab versus pembrolizumab-carboplatin-pemetrexed for locally advanced or metastatic nonsquamous non-small-cell lung cancer with PD-L1 50% or more (LAPLACE-50): study protocol. Clin Lung Cancer 11:921–924
Download references
Acknowledgements
This work was partially supported by the Italian Ministry of Health with Ricerca Corrente and 5x1000 funds.
Ministero della Salute, 5 × 1000, Ricerca Corrente.
Author information
Authors and affiliations.
Department of Thoracic Surgery, IEO, European Institute of Oncology IRCCS, Via Ripamonti 435, 20141, Milan, Italy
Luca Bertolaccini, Monica Casiraghi, Clarissa Uslenghi, Sebastiano Maiorca & Lorenzo Spaggiari
Department of Oncology and Hemato-Oncology, University of Milan, Milan, Italy
Monica Casiraghi & Lorenzo Spaggiari
You can also search for this author in PubMed Google Scholar
Corresponding author
Correspondence to Luca Bertolaccini .
Additional information
Publisher's note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
Reprints and permissions
About this article
Bertolaccini, L., Casiraghi, M., Uslenghi, C. et al. Recent advances in lung cancer research: unravelling the future of treatment. Updates Surg (2024). https://doi.org/10.1007/s13304-024-01841-3
Download citation
Received : 06 March 2024
Accepted : 24 March 2024
Published : 06 April 2024
DOI : https://doi.org/10.1007/s13304-024-01841-3
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Lung cancer
- Comprehensive review
- Find a journal
- Publish with us
- Track your research
Thank you for visiting nature.com. You are using a browser version with limited support for CSS. To obtain the best experience, we recommend you use a more up to date browser (or turn off compatibility mode in Internet Explorer). In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript.
- View all journals
Lung cancer articles from across Nature Portfolio
Lung cancer arises in tissues of the lung, usually in the cells lining air passages. The two main types are small-cell lung cancer and non-small-cell lung cancer, according to the shape of cells under a microscope. The most common symptoms are coughing, shortness of breath and chest pains.
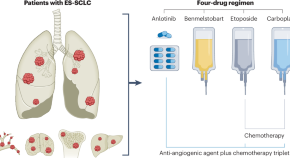
Navigating treatment combinations in small-cell lung cancer
The ETER701 trial demonstrates that a four-drug regimen, involving the addition of anti-angiogenesis therapy to immuno-chemotherapy, improves survival outcomes for extensive-stage small-cell lung cancer — but is more indeed better when it comes to treating this intractable disease?
Related Subjects
- Non-small-cell lung cancer
- Small-cell lung cancer
Latest Research and Reviews
Combining RAS(ON) G12C-selective inhibitor with SHP2 inhibition sensitises lung tumours to immune checkpoint blockade
GDP-bound form of KRASG12C inhibitors have been approved for the treatment of patients with advanced KRASG12C mutant non-small cell lung cancer (NSCLC), however resistance to these drugs is often observed. Here the authors report that combining a GTP-bound RAS G12C-selective inhibitor with SHP2 inhibition can sensitize lung tumours to immune checkpoint blockade.
- Panayiotis Anastasiou
- Christopher Moore
- Julian Downward
Comparison of methods for cancer stem cell detection in prognosis of early stages NSCLC
- Boutaîna Chandouri
- Thomas Naves
- Fabrice Lalloué
PRAD6A promotes lung adenocarcinoma cell proliferation and invasion through Serpina3
- Mingxin Liu
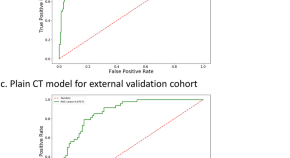
The study of plain CT combined with contrast-enhanced CT-based models in predicting malignancy of solitary solid pulmonary nodules
- Wenjia Zhang
- Xiaonan Cui
- Jinliang Niu
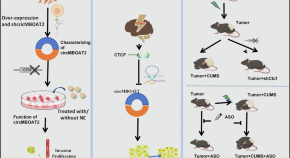
Chronic stress promotes non-small cell lung cancer (NSCLC) progression through circMBOAT2 upregulation mediated by CTCF
- Zhicong Chen
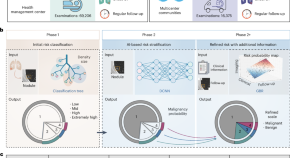
Data-driven risk stratification and precision management of pulmonary nodules detected on chest computed tomography
Trained on a cohort of 45,064 cases and validated on data acquired from mobile computed tomography scanners deployed in rural China, a lung cancer screening deep learning model is shown to outperform existing lung cancer risk scores.
- Chengdi Wang
News and Comment
The mariposa trials — implications for the treatment of egfr -mutant nsclc.
In the past 2 years, substantial improvements have been made in the management of advanced-stage EGFR -mutant non-small-cell lung cancer. Recent studies have suggested added benefit from the combination of third-generation tyrosine-kinase inhibitors with either chemotherapy or a bispecific antibody targeting EGFR and MET. Herein, we summarize these advances and their implications for clinical practice.
- Fatemeh Ardeshir-Larijani
- Suresh S. Ramalingam
Anlotinib plus benmelstobart and chemotherapy are effective in ES-SCLC
- Diana Romero
Osimertinib efficacious as maintenance therapy in patients with stage III NSCLC
- Peter Sidaway
Histological transformation to small-cell carcinoma
In this Journal Club, Oh and Kim discuss a study demonstrating the mechanisms underlying histological transformation of lung adenocarcinoma to neuroendocrine small-cell lung cancer.
- Tae Min Kim
Adjuvant alectinib improves outcomes in ALK -mutant NSCLC
Quick links.
- Explore articles by subject
- Guide to authors
- Editorial policies
Masks Strongly Recommended but Not Required in Maryland
Respiratory viruses continue to circulate in Maryland, so masking remains strongly recommended when you visit Johns Hopkins Medicine clinical locations in Maryland. To protect your loved one, please do not visit if you are sick or have a COVID-19 positive test result. Get more resources on masking and COVID-19 precautions .
- Vaccines
- Masking Guidelines
- Visitor Guidelines
News & Publications
Significant advances in lung cancer treatment bring hope.
Much is changing in the treatment of lung cancer and Susan Scott, M.D. , a fellowship-trained medical oncologist specializing in treating lung cancers at the Johns Hopkins Kimmel Cancer Center at Sibley Memorial Hospital, is very excited.
“More than 50% of patients with lung cancer are diagnosed at an early stage, before it's metastatic and has spread outside the chest,” says Dr. Scott. “These patients have been treated the same way for about 30 years, with little improvement. Just in the past year, we've had some exciting developments in how we help these patients.”
Historically, following diagnosis, patients with early-stage lung cancer had surgery sometimes followed by chemotherapy. A recent FDA-approved treatment that combines chemotherapy and immunotherapy, given before surgery, is bringing new hope.
Early Attack Before Surgery
This new treatment is given through an IV over the course of six to nine weeks. The patient receives a combination of two chemotherapies and one immunotherapy agent that work together to jumpstart the immune system to fight the cancer. When the patient is ready for surgery, their immune system is already primed to act as a surveillance mechanism to attack the cancer and prevent it from growing again. These targeted treatments before surgery provide an opportunity to start treating the cancer and shrinking the tumor while it is still in place.
“Even patients who are diagnosed before their disease spreads outside of the chest still have a high risk of the cancer coming back,” says Dr. Scott. “That is scary, and we want to minimize that risk as much as we can. The more can do at the beginning of treatment to essentially cure the cancer is where our research lies and where these exciting new changes come into play.”
Screening for Early Stage Lung Cancer
The identification of early stage lung cancer has increased significantly in the past five years because of low-dose CT screening for smokers. The U.S. Preventive Services Task Force recommends annual lung cancer screening with low-dose CT for people who:
- Have a 20 pack-year or more smoking history, and
- Smoke now or have quit within the past 15 years, and
- Are between 50 and 80 years old.
This screening often finds tumors before they spread outside the lung or cause any symptoms. Dr. Scott notes, “In the Washington, D.C., population we serve, about half of the patients we're seeing have never smoked.” For patients who don't smoke, lung cancer is sometimes found incidentally when they have a CT scan for another reason. Even though some patients present with symptoms, many of these patients will still have cancer that has not yet spread outside the chest and can benefit from this new therapy.
Hope for the Future
“Many lung cancer patients today are now managed like those who have a chronic disease,” says Dr. Scott. “The average life expectancy, even for patients with stage four disease, has tripled in the last several years. In 2021, there were more than 10 new drugs approved for lung cancer alone. That is astronomical for cancer. With clinical trials available at different stages of disease and different phases of treatment, and so many new approaches, we are providing more hope for our patients.”
The Sidney Kimmel Cancer Center at Sibley Memorial Hospital’s multidisciplinary team is comprised of thoracic surgeons, radiation oncologists, medical oncologists, interventional pulmonologists and pathologists who work together to develop a treatment plan tailored for each patient. For more information, visit hopkinscancerdc.org .
Ready to start planning your care? Call us at 800-525-2225 to make an appointment.
How Four Decades of Research Led to an Important Advance in Lung Cancer
Monday, May 24, 2021

Clockwise, from upper left: Karen with her grandson, Giles, at Walt Disney World in July 2019, just before she started the trial for sotorasib; Karen at her son Zachary’s wedding in Georgia in October 2020; Karen with her sons Matthew (left) and Zachary Feiden in Washington, DC, in September 2020.
Update: On May 28, 2021, the US Food and Drug Administration granted accelerated approval to sotorasib (Lumakras TM ) for the treatment of advanced non-small cell lung cancer driven by the KRAS -G12C mutation in patients who have already received at least one other treatment. The approval was based on the clinical trials co-led by Memorial Sloan Kettering medical oncologist Bob Li.
Karen Milich got the surprise call at 7:30 on a Saturday night. It was Bob Li , her medical oncologist at Memorial Sloan Kettering, telling her that he had obtained a slot for her on a clinical trial of a brand-new experimental drug called sotorasib (AMG 510). “Dr. Li was so excited, and his excitement made me cry,” remembers Karen, who at that time had been living with advanced lung cancer for nearly a year and whose disease was continuing to spread despite other treatments.
She got up the next day, rented a car, and — together with her sister, brother-in-law, and nephew — drove 20 hours from her home in Florida to New York City. She arrived at Dr. Li’s office at 11:00 on Tuesday morning. About a week later, she started taking the drug.
That was August 2019. Since that time, Karen’s cancer has melted away. “I wake up every morning and take my AMG, just like other people take a daily aspirin,” says Karen, who previously had received chemotherapy, radiation, and immunotherapy. “I don’t feel any side effects from it at all.”
A Small Pill with a Big Story
Sotorasib looks like any other pill, but it represents a breakthrough in cancer science. In fact, the US Food and Drug Administration officially granted it a Breakthrough Therapy designation in December 2020. This means the drug has demonstrated substantial improvement over standard treatment and may be close to receiving approval for use beyond clinical trials.
A targeted therapy , sotorasib blocks a cancer-causing protein that results from a mutation in a gene called KRAS (pronounced “kay-rass”). KRAS , initially discovered in 1982 by scientists at the National Cancer Institute and multiple other academic centers, was one of the first cancer genes ever found. Mutations in KRAS and two related genes, HRAS and NRAS , are found in about 20% of all cancers.
Yet despite decades of research, scientists kept hitting roadblocks. That’s because the protein’s smooth, round shape lacked notches or grooves where drugs could attach. The mutant protein eventually was given a label by scientists: undruggable.
In the early 2000s, molecular testing for lung cancer started becoming commonplace. If doctors found certain mutations in patients’ tumors, they could prescribe drugs to go after those mutations. Finding a KRAS mutation in a tumor was like drawing the short straw: It meant that the promising targeted therapies that were being developed for other cancer genes would not work.
Looking for Better Treatments
When Karen was first diagnosed in the fall of 2018, receiving a drug to target her KRAS mutation was not an option. She initially was given chemotherapy and radiation at MSK Westchester .
After staying with her mother-in-law in New York for several months while receiving treatment, Karen was ready to return to her home in Florida. Dr. Li arranged for her see a doctor at the Miami Cancer Institute, where she received an immunotherapy drug. (The Miami Cancer Institute is a member of the MSK Cancer Alliance .)
Her cancer continued to grow. It spread to the peritoneum, which is the lining of the abdomen. At that point, she felt like she was running out of options. That’s when she learned she may be a candidate for the sotorasib trial.
Leading the Way in Lab Research
The trial came about thanks to years of hard work, much of it done at MSK. In a paper published in Science in 2016, MSK physician-scientists Piro Lito and Neal Rosen showed how it was possible to target KRAS in cancer cells. Their research built on early molecules that were originally developed by Kevan Shokat at University of California, San Francisco. These compounds inhibit the most common form of mutated KRAS in lung cancer called KRAS- G12C , which is found in about one in eight non-small cell lung cancers — including Karen’s.
Dr. Lito is a member of MSK’s Human Oncology and Pathogenesis Program and also is taking care of patients on the sotorasib trial. He’s played a key role in the development of these inhibitors, including a study published in Nature in early 2020 that showed on the molecular level why so many patients develop resistance to sotorasib and similar drugs as well as ways to overcome it.
“The clinical trials for KRAS inhibitors represent the efforts of many institutions,” Dr. Lito says. “But what really sets MSK apart in this area is the combination of preclinical and clinical development focused on understanding how these drugs work and the best way to administer them to patients.”
A Milestone for People with Lung Cancer
Today, scans show no sign of cancer in Karen’s lungs or anywhere else. In addition, liquid biopsies show no evidence of the cancer-causing mutation in her blood. Although many other patients in the trial eventually developed resistance to sotorasib, Karen has not. But if she does, MSK has additional trials under way that combine sotorasib with other drugs to overcome that resistance, based largely on Dr. Lito and Dr. Li’s research. MSK is also participating in trials of other drugs that target KRAS- G12C, currently led by medical oncologists Gregory Riely and Kathryn Arbour .
“Karen’s got a remarkable story, but she’s not the only one,” says Dr. Li, who is a member of MSK’s Early Drug Development Service , which focuses on early-stage clinical trials. “It’s a testament to what a milestone this is, to be able to target this protein that was previously considered to be really bad news.”
Karen, now 59, still lives in Florida, but since she started on the trial, she’s been staying in the New York City area. Because of her cancer diagnosis and the COVID-19 pandemic, she’s had to step back from her job as a restaurant manager. But that’s given her more time to spend with her 7-year-old grandson, Giles, who she calls “the apple of my eye and the love of my life.” This summer, they’ll be spending the entire month of July together, which has become a family tradition. “I’m thankful to God every day for Dr. Li, Sloan Kettering, and the trial,” Karen says. “They’re all incredible.”
She adds: “I’m also thankful for my whole family who supported me throughout treatment, especially my two sons.”
Dr. Li has served as an uncompensated advisor to Amgen, Genentech, Boehringer Ingelheim, Lilly, AstraZeneca, Daiichi Sankyo, and has received consulting fees from Guardant Health and Hengrui Therapeutics. He has received research grants to his institution from Amgen, Genentech, AstraZeneca, Daiichi Sankyo, Lilly, Illumina, GRAIL, Guardant Health, Hengrui Therapeutics, MORE Health, and Bolt Biotherapeutics. He has received academic travel support from Resolution Bioscience, MORE Health, and Jiangsu Hengrui Medicine. He is an inventor on two institutional patents at MSK (US62/685,057 and US62/514,661) and has intellectual property rights as a book author at Karger Publishers and Shanghai Jiao Tong University Press. Dr. Lito has served as a scientific advisor to Revolution Medicines and Black Diamond Therapeutics, and he has received grants to his institution from Amgen, Mirati, Revolution Medicines, Boehringer Ingelheim, and Vitrac Pharmaceuticals.
Related topics:

- Help & Support
- Lung Health & Diseases
- Lung Disease Lookup
- Lung Cancer
- Lung Cancer Basics
Lung Cancer Research
Why we need research.
Research provides hope and saves lives. This is especially true when it comes to lung cancer research. Lung cancer research can help develop better treatments, increasing the survival and quality of life for patients. Research can provide a better and longer future for those diagnosed with lung cancer as well and can also ultimately increase the number of survivors living with the disease.
The Lung Association supports lung cancer research so we can help prevent lung cancer cases, and failing that, prolong the lives of lung cancer patients. We have made some progress, but we plan to invest more, as lung cancer remains the leading cause of cancer deaths in the United States.
Our Lung Cancer Research Program
The American Lung Association is committed to funding lung cancer research. As part of our Awards and Grants Program , a large part of funds goes toward research on lung cancer prevention, treatment and quality of life. The primary goal of this lung cancer research program is simple: improve and save lives. The secondary goal is almost as important: To fund top-notch lung cancer researchers at important career crossroads to and gain long-term commitment to lung cancer research. Without the life-long dedication of lung cancer researchers and a large and active community of people trying to improve patients' lives, important and much-needed discoveries would be impossible.
What Research Is Being Done?
Thanks to the medical breakthroughs led by Lung Association researchers and their colleagues worldwide, our lung cancer researchers have made significant contributions to the field of lung cancer. For example, biomarker testing and targeted therapies have helped advance the area of personalized treatment (finding the unique genetic makeup of a person's tumor and developing and using drugs that are designed to be most effective for that patient).
Currently funded Lung Association researchers are:
- Finding out why some nonsmokers develop lung cancer
- Discovering new biomarkers as an early warning system to detect the spread of lung cancer
- Decoding the genetic mechanisms which cause lung cancer
- Understanding how the structure and regulation of chromosomes affect lung cancer
- Understanding sex-differences to customize lung cancer treatments
- Using next generation nanotechnology to target lung cancer
- Using a virus to treat lung cancer
- Overcoming obstacles for cellular immunotherapy against lung cancer
- Improving quality of life and access to healthcare for lung cancer patients after completing therapy
- Reversing drug resistance in lung cancers
- Identifying metabolic alterations in lung cancer-associated cachexia
- Testing methods to increase lung cancer screening among Quitline callers
Lung Cancer Researchers
Visit our Meet the Researchers section to view our lung cancer researchers and their studies.
Research Partnerships
Lung cancer interception dream team.
As a collaborative effort with Stand Up To Cancer and the LUNGevity Foundation, the Lung Cancer Interception Dream Team leverages a new approach to lung cancer prevention: cancer interception.
Learn more about the Dream Team .
How You Can Be a Part of Research
Lung cancer registry.
The Lung Cancer Registry is a database of medical information collected from thousands of lung cancer patients. Researchers study this health data to gain a better understanding of the disease, which can ultimately lead to better outcomes for patients. By participating in the Registry, you not only will help advance lung cancer research, but you will also be able to learn about new clinical trial opportunities that may help in your own treatment program.
Learn more about the Lung Cancer Registry and how to sign up.
Lung Cancer Clinical Trials
Read questions and answers about clinical trials and see our Lung Association listing of current trials .
Download our checklist to help you talk with your doctor about clinical trials.
You can also search the Lung Cancer Clinical Trials Matching Service , provided by a partnership between the American Lung Association and EmergingMed. Patients can search for clinical trials that match their specific diagnosis and treatment history.
Find a Clinical Trial
Learn more about clinical trial programs in your area by searching our list and be sure to discuss with your doctor whether a clinical trial is right for you.
Page last updated: June 7, 2024
Fund the future of lung health
This World Lung Day, help advance research that will define the treatments and cures of tomorrow.
Donate Today
Make a Donation
Your tax-deductible donation funds lung disease and lung cancer research, new treatments, lung health education, and more.
Become a Lung Health Insider
Join over 700,000 people who receive the latest news about lung health, including research, lung disease, air quality, quitting tobacco, inspiring stories and more!
Thank you! You will now receive email updates from the American Lung Association.
Select Your Location
Select your location to view local American Lung Association events and news near you.
Change Language
Lung helpline.
Talk to our lung health experts at the American Lung Association. Our service is free and we are here to help you.
Online Help
Our 24/7 cancer helpline provides information and answers for people dealing with cancer. We can connect you with trained cancer information specialists who will answer questions about a cancer diagnosis and provide guidance and a compassionate ear.
Chat live online
Select the Live Chat button at the bottom of the page
Call us at 1-800-227-2345
Available any time of day or night
Our highly trained specialists are available 24/7 via phone and on weekdays can assist through online chat. We connect patients, caregivers, and family members with essential services and resources at every step of their cancer journey. Ask us how you can get involved and support the fight against cancer. Some of the topics we can assist with include:
- Referrals to patient-related programs or resources
- Donations, website, or event-related assistance
- Tobacco-related topics
- Volunteer opportunities
- Cancer Information
For medical questions, we encourage you to review our information with your doctor.
Lung Cancer
- What Is Lung Cancer?
- Key Statistics for Lung Cancer
- What’s New in Lung Cancer Research?
- Lung Cancer Risk Factors
- What Causes Lung Cancer?
- Can Lung Cancer Be Prevented?
- Can Lung Cancer Be Found Early?
- Lung Nodules
- Signs and Symptoms of Lung Cancer
- Tests for Lung Cancer
- Non-Small Cell Lung Cancer Stages
- Small Cell Lung Cancer Stages
- Lung Cancer Survival Rates
- Questions to Ask About Lung Cancer
- Surgery for Non-Small Cell Lung Cancer
- Radiofrequency Ablation (RFA) for Non-Small Cell Lung Cancer
- Radiation Therapy for Non-Small Cell Lung Cancer
- Chemotherapy for Non-Small Cell Lung Cancer
- Targeted Drug Therapy for Non-Small Cell Lung Cancer
- Immunotherapy for Non-Small Cell Lung Cancer
- Palliative Procedures for Non-Small Cell Lung Cancer
Treatment Choices for Non-Small Cell Lung Cancer, by Stage
- Chemotherapy for Small Cell Lung Cancer
- Immunotherapy for Small Cell Lung Cancer
- Radiation Therapy for Small Cell Lung Cancer
- Surgery for Small Cell Lung Cancer
- Palliative Procedures for Small Cell Lung Cancer
- Treatment Choices for Small Cell Lung Cancer, by Stage
- Living as a Lung Cancer Survivor
- Second Cancers After Lung Cancer
- If You Have Non-small Cell Lung Cancer
- If You Have Small Cell Lung Cancer
- Lung Cancer Quiz
- Lung Cancer Videos
The treatment options for non-small cell lung cancer (NSCLC) are based mainly on the stage (extent) of the cancer, but other factors, such as a person’s overall health and lung function, as well as certain traits of the cancer itself, are also important.
Treating occult cancer
Treating stage 0 nsclc, treating stage i nsclc, treating stage ii nsclc, treating stage iiia nsclc, treating stage iiib nsclc, treating stage iva and ivb nsclc, nsclc that progresses or recurs after treatment.
If you smoke: one of the most important things you can do to be ready for treatment is to try to quit . Studies have shown that people who stop smoking after a diagnosis of lung cancer tend to have better outcomes than those who don’t.
For these cancers, malignant cells are seen on sputum cytology, but no obvious tumor can be found with bronchoscopy or imaging tests. They are usually early-stage cancers. Bronchoscopy and possibly other tests are usually repeated every few months to look for a tumor. If a tumor is found, treatment will depend on the stage.
Because stage 0 NSCLC is limited to the lining layer of the airways and has not invaded deeper into the lung tissue or other areas, it is usually curable by surgery alone. No chemotherapy or radiation therapy is needed.
If you are healthy enough for surgery , you can usually be treated by segmentectomy or wedge resection (removal of part of the lobe of the lung). Cancers in some locations (such as where the windpipe divides into the left and right main bronchi) may be treated with a sleeve resection, but in some cases, they may be hard to remove completely without removing a lobe (lobectomy) or even an entire lung (pneumonectomy).
For some stage 0 cancers, treatments such as photodynamic therapy (PDT), laser therapy, or brachytherapy (internal radiation) may be alternatives to surgery.
If you have stage I NSCLC, surgery may be the only treatment you need. Surgery will either take out the lobe of the lung that has the tumor (lobectomy) or take out a smaller piece of the lung (sleeve resection, segmentectomy, or wedge resection). At least some lymph nodes in the lung and in the space between the lungs will also be removed and checked for cancer.
Segmentectomy or wedge resection is generally an option only for very small stage I cancers and for patients with other health problems that make removing the entire lobe dangerous. Still, most surgeons believe it is better to do a lobectomy if the patient can tolerate it, as it offers the best chance for cure.
For people with stage I NSCLC that has a higher risk of coming back (based on size, location, or other factors), chemotherapy , immunotherapy , and possibly targeted therapy (ie. alectinib, osimertinib) after surgery may lower the risk that cancer will return. This is called adjuvant treatment.
After surgery, the removed tissue is checked to see if there are cancer cells at the edges of the surgery specimen (called positive margins ). This could mean that some cancer has been left behind, so a second surgery might be done to try to ensure that all the cancer has been removed (this might be followed by chemotherapy as well). Another option might be to use radiation therapy after surgery.
If you have serious health problems that prevent you from having surgery, you may get stereotactic body radiation therapy (SBRT) or another type of radiation therapy as your main treatment. Ablation may be another option if the tumor is small and you are not able to undergo surgery.
Neoadjuvant (pre-operative) chemotherapy with or without immunotherapy is usually offered to patients with stage II NSCLC. After neoadjuvant therapy, people who have stage II NSCLC and are healthy enough for surgery usually have the cancer removed by lobectomy or sleeve resection. Sometimes removing the whole lung (pneumonectomy) is needed.
Any lymph nodes likely to have cancer in them are also removed. The extent of lymph node involvement and whether or not cancer cells are found at the edges of the removed tissues are important factors when planning the next step of treatment.
After surgery, the removed tissue is checked to see if there are cancer cells at the edges of the surgery specimen. This might mean that some cancer has been left behind, so a second surgery might be done to try to remove any remaining cancer. This may be followed by additional treatment with either chemotherapy, targeted therapy (ie. alectinib, osimertinib), or immunotherapy (ie. atezolizumab, pembrolizumab, durvalumab).
The initial treatment for stage IIIA NSCLC may include some combination of radiation therapy , chemotherapy (chemo), immunotherapy, and/or surgery . For this reason, planning treatment for stage IIIA NSCLC often requires input from a medical oncologist, radiation oncologist, and a thoracic surgeon. Your treatment options depend on the size of the tumor, where it is in your lung, which lymph nodes it has spread to, your overall health, and how well you are tolerating treatment.
For stage IIIA lung cancers that is not able to be surgically removed, treatment usually starts with chemo, often combined with radiation therapy (called chemoradiation ). Surgery may be an option after this if the doctor thinks any remaining cancer can be removed and the patient is healthy enough.
For certain stage IIIA cancers that can be surgically removed, treatment usually startes with chemotherapy with or without immunotherapy, followed by surgery. Additional therapy after surgery (adjuvant therapy) might be needed depending on what is found during surgery. Options for adjuvant therapy include chemotherapy, targeted therapy (ie. alectinib, osimertinib) and/or immunotherapy.
If surgery, radiation, and chemoradiation are not likely to be good treatment options, treatment with an immunotherapy drug such as pembrolizumab (Keytruda) or cemiplimab (Libtayo) may be considered first.
Stage IIIB NSCLC has spread to lymph nodes that are near the other lung or in the neck, and may also have grown into important structures in the chest. These cancers can’t be removed completely by surgery .
As with other stages of lung cancer, treatment depends on the patient’s overall health. If you are in fairly good health you may be helped by chemotherapy (chemo) combined with radiation therapy (known as chemoradiation ). Some people can even be cured with this treatment. If the cancer stays under control after 2 or more treatments of chemoradiation, the immunotherapy drug durvalumab (Imfinzi) can be given for up to a year to help keep the cancer stable.
Patients who are not healthy enough for this combination are often treated with radiation therapy alone, or, less often, chemo alone. If surgery, radiation, and chemoradiation aren’t likely to be good treatment options, an immunotherapy drug such as pembrolizumab (Keytruda) or cemiplimab (Libtayo) may be considered as the first treatment.
These cancers can be hard to treat, so taking part in a clinical trial of newer treatments may be a good option for some people.
Stage IVA or IVB NSCLC has already spread when it is diagnosed. These cancers can be very hard to cure. Treatment options depend on where and how far the cancer has spread, whether the cancer cells have certain gene or protein changes, and your overall health.
If you are in otherwise good health, treatments such as surgery , chemotherapy (chemo), targeted therapy , immunotherapy , and radiation therapy may help you live longer and make you feel better by relieving symptoms, even though they aren’t likely to cure you.
Other treatments, such as photodynamic therapy (PDT) or laser therapy, may also be used to help relieve symptoms . In any case, if you are going to be treated for advanced NSCLC, be sure you understand the goals of treatment before you start.
NSCLC that has spread to only one other site (stage IVA)
Cancer that is limited in the lungs and has only spread to one other site (such as the brain) is not common, but it can sometimes be treated (and even potentially cured) with surgery and/or radiation therapy to treat the area of cancer spread, followed by treatment of the cancer in the lung. For example, a single tumor in the brain may be treated with surgery or stereotactic radiation, or surgery followed by radiation to the whole brain. Treatment for the lung tumor is then based on its T and N stages, and may include surgery, chemo, radiation, or some of these in combination.
NSCLC that has spread widely (stage IVB)
For cancers that have spread widely throughout the body, before any treatments start, your tumor will be tested for certain gene mutations (such as in the KRAS , EGFR , ALK , ROS1 , BRAF , RET , MET , or NTRK genes). If one of these genes is mutated in your cancer cells, your first treatment will likely be a targeted therapy drug.
Your tumor cells might also be tested for the PD-L1 protein . Tumors with higher levels of PD-L1 are more likely to respond to certain immunotherapy drugs (known as immune checkpoint inhibitors ), which might be an option either alone or along with chemo.
If the cancer has caused fluid buildup in the space around the lungs (a malignant pleural effusion), the fluid may be drained. If it keeps coming back, options include pleurodesis or placement of a catheter into the chest through the skin to let the fluid drain out. (Details of these are discussed in Palliative Procedures for Non-Small Cell Lung Cancer .)
As with other stages, treatment for stage IV lung cancer depends on a person’s overall health. For example, some people not in good health might get only 1 chemo drug instead of 2. For people who can’t have chemo, radiation therapy is usually the treatment of choice. Local treatments such as laser therapy, PDT, or stent placement may also be used to help relieve symptoms caused by lung tumors.
Because treatment is unlikely to cure these cancers, taking part in a clinical trial of newer treatments may be a good option.
You can also find more information about living with stage IV cancer in Advanced Cancer .
If cancer continues to grow during treatment (progresses) or comes back (recurs), further treatment will depend on the location and extent of the cancer, what treatments have been used, and on the person’s health and desire for more treatment. It’s important to understand the goal of any further treatment – if it is to try to cure the cancer, to slow its growth, or to help relieve symptoms. It is also important to understand the benefits and risks.
Smaller cancers that recur locally in the lungs can sometimes be treated again with surgery or radiation therapy (if it hasn’t been used before). Cancers that recur in the lymph nodes between the lungs are usually treated with chemo, possibly along with radiation if it hasn’t been used before. For cancers that return at distant sites, chemo, targeted therapies, and/or immunotherapy are often the treatments of choice.
For more on dealing with a recurrence, see Understanding Recurrence .
In some people, the cancer may never go away completely. These people may get regular treatments with chemo, radiation therapy, or other therapies to try to help keep the cancer in check. Learning to live with cancer that does not go away can be difficult and very stressful. It has its own type of uncertainty. Managing Cancer as a Chronic Illness talks more about this.

The American Cancer Society medical and editorial content team
Our team is made up of doctors and oncology certified nurses with deep knowledge of cancer care as well as editors and translators with extensive experience in medical writing.
Araujo LH, Horn L, Merritt RE, Shilo K, Xu-Welliver M, Carbone DP. Ch. 69 - Cancer of the Lung: Non-small cell lung cancer and small cell lung cancer. In: Niederhuber JE, Armitage JO, Doroshow JH, Kastan MB, Tepper JE, eds. Abeloff’s Clinical Oncology . 6th ed. Philadelphia, Pa: Elsevier; 2020.
Chiang A, Detterbeck FC, Stewart T, Decker RH, Tanoue L. Chapter 48: Non-small cell lung cancer. In: DeVita VT, Lawrence TS, Rosenberg SA, eds. DeVita, Hellman, and Rosenberg’s Cancer: Principles and Practice of Oncology . 11th ed. Philadelphia, Pa: Lippincott Williams & Wilkins; 2019.
National Cancer Institute. Physician Data Query (PDQ). Health Professional Version. Non-Small Cell Lung Cancer Treatment. 2023. Accessed at https://www.cancer.gov/types/lung/hp/non-small-cell-lung-treatment-pdq on Jan 23, 2024.
National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines in Oncology: Non-Small Cell Lung Cancer. V.1.2024. Accessed at https://www.nccn.org/professionals/physician_gls/pdf/nscl.pdf on Jan 23, 2024.
Last Revised: May 2, 2024
American Cancer Society medical information is copyrighted material. For reprint requests, please see our Content Usage Policy .
American Cancer Society Emails
Sign up to stay up-to-date with news, valuable information, and ways to get involved with the American Cancer Society.
More in Lung Cancer
- About Lung Cancer
- Causes, Risk Factors, and Prevention
- Early Detection, Diagnosis, and Staging
- Treatment for Non-small Cell Lung Cancer
- Treatment for Small Cell Lung Cancer
- After Treatment
Help us end cancer as we know it, for everyone.

If this was helpful, donate to help fund patient support services, research, and cancer content updates.
- Patient Care & Health Information
- Diseases & Conditions
- Lung cancer
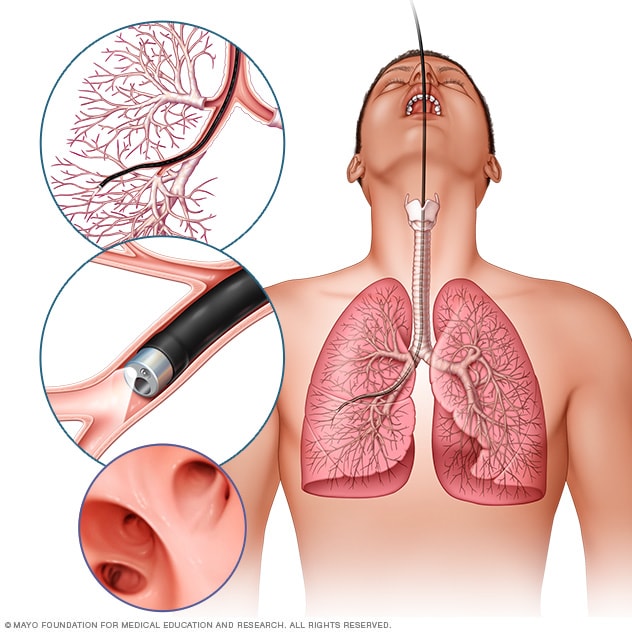
- Bronchoscopy
In flexible bronchoscopy, a healthcare professional inserts a thin, bendable tube through the mouth or nose into the lungs. A light and a small camera on the bronchoscope allow the health professional to look inside the lungs' airways.
Lung cancer diagnosis often starts with an imaging test to look at the lungs. If you have symptoms that worry you, a healthcare professional might start with an X-ray. If you smoke or used to smoke, you might have an imaging test to look for signs of lung cancer before you develop symptoms.
Testing healthy people for lung cancer
People with an increased risk of lung cancer may consider yearly lung cancer screening using low-dose CT scans. Lung cancer screening is generally offered to people 50 and older who smoked heavily for many years. Screening also is offered to people who have quit smoking in the past 15 years.
Discuss your lung cancer risk with your healthcare professional. Together you can decide whether lung cancer screening is right for you.
Tests to diagnose lung cancer
If your healthcare professional thinks you may have lung cancer, a number of tests can be used to look for cancerous cells and to rule out other conditions.
Tests may include:
- Imaging tests. Imaging tests make pictures of the body. They can show the location and size of the lung cancer. Tests might include X-ray, MRI , CT and positron emission tomography, which also is called a PET scan.
- Sputum cytology. Sputum is the mucus that is coughed up from the lungs. If you are coughing up sputum, it can be looked at under a microscope. The sputum can sometimes show lung cancer cells.
Biopsy. A biopsy is a procedure to remove a sample of tissue for testing in a lab.
Your healthcare team can perform a lung cancer biopsy several ways. One way is bronchoscopy. During bronchoscopy, a healthcare professional passes a lighted tube with a camera down your throat into your lungs to examine the area. Special tools can be passed through the tube to collect a sample of tissue.
Mediastinoscopy also is an option. During mediastinoscopy, an incision is made at the base of your neck. Surgical tools are then inserted behind your breastbone to take tissue samples from lymph nodes.
Another option is a needle biopsy. In a needle biopsy, your healthcare professional uses X-ray or CT images to guide a needle through the skin on your chest. The needle goes into the lung tissue to collect cells that could be cancerous.
A biopsy sample also may be taken from lymph nodes or other areas where cancer has spread.
Your cancer cells will be carefully tested in a lab to find out what type of lung cancer you have. The results can help determine the likely outcome of your cancer, called the prognosis, and guide your treatment.
Tests to determine the extent of the cancer
If you're diagnosed with lung cancer, you may have other tests to see if the cancer has spread. These tests help your healthcare team find out the extent of your cancer, also called the stage. Cancer staging tests often involve imaging tests. The tests might look for signs of cancer in your lymph nodes or in other parts of your body. Your healthcare team uses the cancer staging test results to help create your treatment plan.
Imaging tests may include MRI , CT , bone scans and PET scan. Not every test is right for every person. Talk with your healthcare professional about which procedures will work for you.
The stages of lung cancer range from 1 to 4. The lowest number means that the cancer is small and only in the lung. As the cancer grows larger or spreads outside of the lungs, the numbers get higher. A stage 4 lung cancer has spread to other areas of the body.
In small cell lung cancer, the stages may be called limited or extensive. In the limited stage, the cancer affects one lung and the area around it. In the extensive stage, the cancer has spread to the other lung or to other parts of the body.
- Care at Mayo Clinic
Our caring team of Mayo Clinic experts can help you with your lung cancer-related health concerns Start Here
More Information
Lung cancer care at Mayo Clinic
- Lung cancer screening
- Positron emission tomography scan
- Infographic: Lung Cancer
Treatment for lung cancer usually begins with surgery to remove the cancer. If the cancer is very large or has spread to other parts of the body, surgery may not be possible. Treatment might start with medicine and radiation instead. Your healthcare team considers many factors when creating a treatment plan. These factors may include your overall health, the type and stage of your cancer, and your preferences.
Some people with lung cancer choose not to have treatment. For instance, you may feel that the side effects of treatment will outweigh the potential benefits. When that's the case, your healthcare professional may suggest comfort care to treat only the symptoms the cancer is causing.
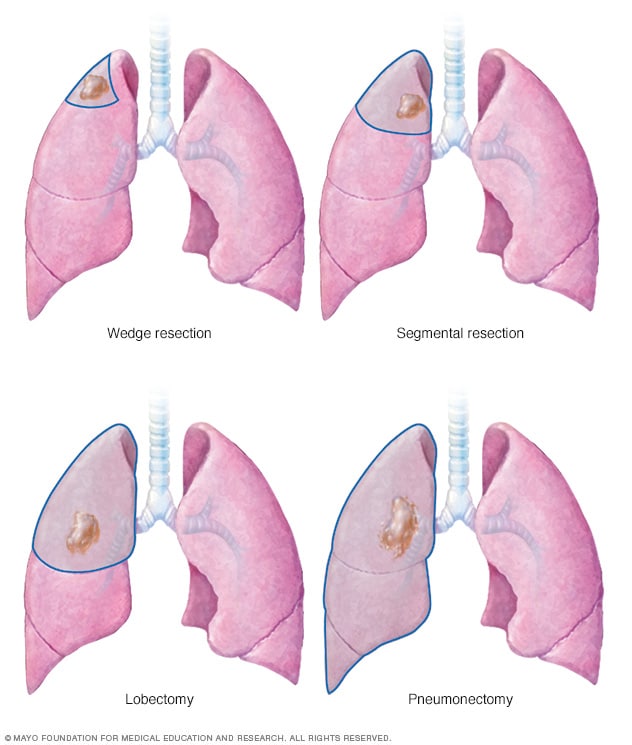
- Lung cancer surgery
Lung cancer surgery can involve removing a portion of the lung or the entire lung. An operation to remove the lung cancer and a small portion of healthy tissue is called a wedge resection. Removing a larger area of the lung is called segmental resection. Surgery to remove one lobe from a lung is called lobectomy. Removing an entire lung is called pneumonectomy.
During surgery, your surgeon works to remove the lung cancer and some healthy tissue around it. Procedures to remove lung cancer include:
- Wedge resection to remove a small section of lung that contains the cancer along with a margin of healthy tissue.
- Segmental resection to remove a larger portion of lung, but not an entire lobe.
- Lobectomy to remove the entire lobe of one lung.
- Pneumonectomy to remove an entire lung.
If you have surgery, your surgeon also may remove lymph nodes from your chest to test them for cancer.
Surgery may be an option if your cancer is only in the lungs. If you have a larger lung cancer, chemotherapy or radiation therapy may be used before surgery to shrink the cancer. Chemotherapy or radiation therapy also may be used after surgery if there's a risk that cancer cells were left behind or that your cancer may come back.
- Radiation therapy
Radiation therapy treats cancer with powerful energy beams. The energy can come from X-rays, protons or other sources. During radiation therapy, you lie on a table while a machine moves around you. The machine directs radiation to precise points on your body.
For lung cancer that has spread within the chest, radiation may be used before surgery or after surgery. It's often combined with chemotherapy treatments. If surgery isn't an option, combined chemotherapy and radiation therapy may be your first treatment.
For lung cancers that have spread to other areas of the body, radiation therapy may help relieve symptoms.
- Chemotherapy
Chemotherapy treats cancer with strong medicines. Many chemotherapy medicines exist. Most are given through a vein. Some come in pill form. A combination of medicines usually is given in a series of treatments over a period of weeks or months. Breaks in between are used to help you recover.
Chemotherapy is often used after surgery to kill any cancer cells that may remain. It can be used alone or combined with radiation therapy. Chemotherapy also may be used before surgery to shrink cancers and make them easier to remove.
In people with lung cancer that has spread, chemotherapy can be used to relieve pain and other symptoms.
Stereotactic body radiotherapy
Stereotactic body radiotherapy is an intense radiation treatment. This treatment aims beams of radiation from many angles at the cancer. Stereotactic body radiotherapy treatment is typically completed in one or a few treatments. Sometimes this treatment is called stereotactic radiosurgery.
Stereotactic body radiotherapy may be an option for people with small lung cancers who can't have surgery. It also may be used to treat lung cancer that spreads to other parts of the body, including the brain.
Targeted therapy
Targeted therapy for cancer is a treatment that uses medicines that attack specific chemicals in the cancer cells. By blocking these chemicals, targeted treatments can cause cancer cells to die. For lung cancer, targeted therapy may be used for people with cancer that spreads or comes back after treatment.
Some targeted therapies only work in people whose cancer cells have certain DNA changes. Your cancer cells may be tested in a lab to see if these medicines might help you.
Immunotherapy
Immunotherapy for cancer is a treatment with medicine that helps the body's immune system to kill cancer cells. The immune system fights off diseases by attacking germs and other cells that shouldn't be in the body. Cancer cells survive by hiding from the immune system. Immunotherapy helps the immune system cells find and kill the cancer cells.
For lung cancer, immunotherapy might be used after surgery to kill any cancer cells that remain. When surgery isn't an option, immunotherapy might help control the cancer.
Palliative care
Palliative care is a special type of healthcare that helps you feel better when you have a serious illness. If you have cancer, palliative care can help relieve pain and other symptoms. A healthcare team that may include doctors, nurses and other specially trained health professionals provides palliative care. The care team's goal is to improve quality of life for you and your family.
Palliative care specialists work with you, your family and your care team. They provide an extra layer of support while you have cancer treatment. You can have palliative care at the same time you're getting strong cancer treatments, such as surgery, chemotherapy or radiation therapy.
The use of palliative care with other proper treatments can help people with cancer feel better and live longer.
- Ablation therapy
- Brachytherapy
- Proton therapy
- Stop-smoking services
There is a problem with information submitted for this request. Review/update the information highlighted below and resubmit the form.
Get Mayo Clinic cancer expertise delivered to your inbox.
Subscribe for free and receive an in-depth guide to coping with cancer, plus helpful information on how to get a second opinion. You can unsubscribe at any time. Click here for an email preview.
Error Select a topic
Error Email field is required
Error Include a valid email address
To provide you with the most relevant and helpful information, and understand which information is beneficial, we may combine your email and website usage information with other information we have about you. If you are a Mayo Clinic patient, this could include protected health information. If we combine this information with your protected health information, we will treat all of that information as protected health information and will only use or disclose that information as set forth in our notice of privacy practices. You may opt-out of email communications at any time by clicking on the unsubscribe link in the e-mail.
Thank you for subscribing
Your in-depth coping with cancer guide will be in your inbox shortly. You will also receive emails from Mayo Clinic on the latest about cancer news, research, and care.
If you don’t receive our email within 5 minutes, check your SPAM folder, then contact us at [email protected] .
Sorry something went wrong with your subscription
Please, try again in a couple of minutes
Clinical trials
Explore Mayo Clinic studies testing new treatments, interventions and tests as a means to prevent, detect, treat or manage this condition.
Lifestyle and home remedies
Many people with lung cancer experience shortness of breath. Treatments such as supplemental oxygen and medicines are available to help you feel more comfortable. However, they aren't always enough.
To cope with shortness of breath, it may help to:
Try to relax
Feeling short of breath can be scary. But fear and anxiety only make it harder to breathe. When you begin to feel short of breath, choose an activity that helps you relax. Listen to music, imagine your favorite vacation spot, meditate or say a prayer.
Find a comfortable position
It may help to lean forward when you feel short of breath.
Focus on your breath
When you feel short of breath, focus your mind on your breathing. Instead of trying to fill your lungs with air, concentrate on moving the muscles that control your breathing. Try breathing through pursed lips and pacing your breaths with your activity.
Save your energy for what's important
If you're short of breath, you may become tired easily. Prioritize your tasks for the day so that you can save your energy for what needs to be done.
Tell your healthcare professional if you experience shortness of breath or if your symptoms worsen. There are many other treatments available to relieve shortness of breath.
Alternative medicine
Complementary and alternative lung cancer treatments can't cure your cancer. But complementary and alternative treatments can often be combined with your healthcare team's care to help relieve symptoms.
The American College of Chest Physicians suggests people with lung cancer may find comfort in:
Acupuncture
During an acupuncture session, a trained practitioner inserts small needles into precise points on your body. Acupuncture may relieve pain and ease cancer treatment side effects, such as nausea and vomiting.
Hypnosis is typically done by a therapist who leads you through relaxation exercises. The therapist may ask you to think pleasing and positive thoughts. Hypnosis may reduce anxiety, nausea and pain in people with cancer.
During massages, massage therapists use their hands to apply pressure to your skin and muscles. Massage can help relieve anxiety and pain in people with cancer. Some massage therapists are specially trained to work with people who have cancer.
Meditation is a time of quiet reflection in which you focus on something. It may be an idea, image or sound. Meditation may reduce stress and improve quality of life in people with cancer.
Yoga combines gentle stretching movements with deep breathing and meditation. Yoga may help people with cancer sleep better.
Coping and support
With time, you'll find what helps you cope with the uncertainty and distress of a cancer diagnosis. Until then, you may find that it helps to:
Learn enough about lung cancer to make decisions about your care
Ask your healthcare team about your cancer, including your test results, treatment options and, if you like, your prognosis. As you learn more about lung cancer, you may become more confident in making treatment decisions.
Keep friends and family close
Keeping your close relationships strong will help you deal with your lung cancer. Friends and family can provide the practical support you'll need, such as helping take care of your home if you're in the hospital. And they can serve as emotional support when you feel overwhelmed by having cancer.
Find someone to talk with
Find someone who is willing to listen to you talk about your hopes and fears. This may be a friend or family member. The concern and understanding of a counselor, medical social worker, clergy member or cancer support group also may be helpful.
Ask your healthcare team about support groups in your area. Other sources of information include the National Cancer Institute and the American Cancer Society.
Preparing for your appointment
Make an appointment with a doctor or other healthcare professional if you have any symptoms that worry you.
If your healthcare professional suspects that you have lung cancer, you'll likely be referred to a specialist. Specialists who treat lung cancer may include:
- Oncologists. Doctors who specialize in treating cancer.
- Pulmonologists. Doctors who diagnose and treat lung diseases.
- Radiation oncologists. Doctors who use radiation to treat cancer.
- Thoracic surgeons. Surgeons who operate on the lungs.
- Palliative care specialists. Doctors who treat signs and symptoms of cancer and cancer treatment.
Because appointments can be brief, it's a good idea to be prepared. Here's some information to help you get ready.
What you can do
- Be aware of any pre-appointment restrictions. At the time you make the appointment, be sure to ask if there's anything you need to do in advance, such as restrict your diet.
- Write down symptoms you're experiencing, including any that may not seem related to the reason for which you scheduled the appointment.
- Write down key personal information, including major stresses or recent life changes.
- Make a list of all medicines, vitamins or supplements you're taking and the doses. Or you may prefer to bring your medicine bottles to your appointment.
- Gather your medical records. If you've had a chest X-ray or a scan done by a different healthcare professional, try to get that file and bring it to your appointment.
- Consider taking a family member or friend along. Sometimes it can be difficult to remember all the information provided during an appointment. Someone who accompanies you may remember something that you missed or forgot.
- Write down questions to ask your healthcare team.
Questions to ask if you've been diagnosed with lung cancer
Your time with your healthcare team is limited, so preparing a list of questions can help you make the most of your time together. List your questions from most important to least important in case time runs out. For lung cancer, some basic questions to ask include:
- What type of lung cancer do I have?
- May I see the chest X-ray or CT scan that shows my cancer?
- What is causing my symptoms?
- What is the stage of my lung cancer?
- Will I need more tests?
- Should my lung cancer cells be tested for gene changes that may determine my treatment options?
- Has my cancer spread to other parts of my body?
- What are my treatment options?
- Will any of these treatment options cure my cancer?
- What are the potential side effects of each treatment?
- Is there one treatment that you think is best for me?
- Is there a benefit if I quit smoking now?
- What advice would you give a friend or family member in my situation?
- What if I don't want treatment?
- Are there ways to relieve the symptoms I'm experiencing?
- Can I enroll in a clinical trial?
- Should I see a specialist? What will that cost, and will my insurance cover it?
- Are there brochures or other material that I can take with me? What websites do you recommend?
Don't hesitate to ask other questions.
What to expect from your doctor
Be prepared to answer questions, such as:
- When did you first begin experiencing symptoms?
- Have your symptoms been ongoing or occasional?
- How severe are your symptoms?
- Do you wheeze when breathing?
- Do you have a cough that feels like you're clearing your throat?
- Have you ever been diagnosed with emphysema or chronic obstructive pulmonary disease?
- Do you take medicines for shortness of breath?
- What, if anything, seems to improve your symptoms?
- What, if anything, appears to worsen your symptoms?
- Non-small cell lung cancer. National Comprehensive Cancer Network. https://www.nccn.org/guidelines/guidelines-detail?category=1&id=1450. Accessed Dec. 4, 2023.
- Small cell lung cancer. National Comprehensive Cancer Network. https://www.nccn.org/guidelines/guidelines-detail?category=1&id=1462. Accessed Dec. 4, 2023.
- Niederhuber JE, et al., eds. Cancer of the lung: Non-small cell lung cancer and small cell lung cancer. In: Abeloff's Clinical Oncology. 6th ed. Elsevier; 2020. https://www.clinicalkey.com. Accessed Dec. 4, 2023.
- Non-small cell lung cancer treatment (PDQ) – Patient version. National Cancer Institute. https://www.cancer.gov/types/lung/patient/non-small-cell-lung-treatment-pdq. Accessed Dec. 4, 2023.
- Small cell lung cancer treatment (PDQ) – Patient version. National Cancer Institute. https://www.cancer.gov/types/lung/patient/small-cell-lung-treatment-pdq. Accessed Dec. 4, 2023.
- Lung cancer – non-small cell. Cancer.Net. https://www.cancer.net/cancer-types/lung-cancer/view-all. Accessed Dec. 4, 2023.
- Lung cancer – small cell. Cancer.Net. https://www.cancer.net/cancer-types/33776/view-all. Accessed Dec. 4, 2023.
- Detterbeck FC, et al. Executive Summary: Diagnosis and management of lung cancer, 3rd ed.: American College of Chest Physicians evidence-based clinical practice guidelines. Chest. 2013; doi:10.1378/chest.12-2377.
- Palliative care. National Comprehensive Cancer Network. https://www.nccn.org/guidelines/guidelines-detail?category=3&id=1454. Accessed Dec. 4, 2023.
- Lung cancer. World Health Organization. https://www.who.int/news-room/fact-sheets/detail/lung-cancer. Accessed Dec. 4, 2023.
- Cairns LM. Managing breathlessness in patients with lung cancer. Nursing Standard. 2012; doi:10.7748/ns2012.11.27.13.44.c9450.
- Warner KJ. Allscripts EPSi. Mayo Clinic. Jan. 13, 2020.
- Brown AY. Allscripts EPSi. Mayo Clinic. July 30, 2019.
- Searching for cancer centers. American College of Surgeons. https://www.facs.org/search/cancer-programs. Accessed Dec. 4, 2023.
- Temel JS, et al. Early palliative care for patients with metastatic non-small-cell lung cancer. New England Journal of Medicine. 2010; doi:10.1056/NEJMoa1000678.
- Dunning J, et al. Microlobectomy: A novel form of endoscopic lobectomy. Innovations. 2017; doi:10.1097/IMI.0000000000000394.
- Leventakos K, et al. Advances in the treatment of non-small cell lung cancer: Focus on nivolumab, pembrolizumab and atezolizumab. BioDrugs. 2016; doi:10.1007/s40259-016-0187-0.
- Dong H, et al. B7-H1, a third member of the B7 family, co-stimulates T-cell proliferation and interleukin-10 secretion. Nature Medicine. 1999;5:1365.
- Aberle DR, et al. Reduced lung-cancer mortality with low-dose computed tomographic screening. New England Journal of Medicine. 2011; doi:10.1056/NEJMoa1102873.
- Lung nodules: Can they be cancerous?
- Super Survivor Conquers Cancer
Associated Procedures
News from mayo clinic.
- Science Saturday: Study finds senescent immune cells promote lung tumor growth June 17, 2023, 11:00 a.m. CDT
- Era of hope for patients with lung cancer Nov. 16, 2022, 03:00 p.m. CDT
- Mayo Clinic Q&A podcast: Survivorship after surgery for lung cancer Nov. 15, 2022, 01:30 p.m. CDT
- Mayo Clinic Minute: Understanding lung cancer Nov. 02, 2022, 04:00 p.m. CDT
- Lung cancer diagnosis innovation leads to higher survival rates Nov. 02, 2022, 02:30 p.m. CDT
Products & Services
- A Book: Mayo Clinic Family Health Book
- Newsletter: Mayo Clinic Health Letter — Digital Edition
Mayo Clinic in Rochester, Minnesota, Mayo Clinic in Phoenix/Scottsdale, Arizona, and Mayo Clinic in Jacksonville, Florida, have been recognized among the top Pulmonology hospitals in the nation for 2024-2025 by U.S. News & World Report.
- Symptoms & causes
- Diagnosis & treatment
- Doctors & departments
Mayo Clinic does not endorse companies or products. Advertising revenue supports our not-for-profit mission.
- Opportunities
Mayo Clinic Press
Check out these best-sellers and special offers on books and newsletters from Mayo Clinic Press .
- Mayo Clinic on Incontinence - Mayo Clinic Press Mayo Clinic on Incontinence
- The Essential Diabetes Book - Mayo Clinic Press The Essential Diabetes Book
- Mayo Clinic on Hearing and Balance - Mayo Clinic Press Mayo Clinic on Hearing and Balance
- FREE Mayo Clinic Diet Assessment - Mayo Clinic Press FREE Mayo Clinic Diet Assessment
- Mayo Clinic Health Letter - FREE book - Mayo Clinic Press Mayo Clinic Health Letter - FREE book

5X Challenge
Thanks to generous benefactors, your gift today can have 5X the impact to advance AI innovation at Mayo Clinic.
An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
The PMC website is updating on October 15, 2024. Learn More or Try it out now .
- Advanced Search
- Journal List
- HHS Author Manuscripts

Lung cancer: biology and treatment options
Hassan lemjabbar-alaoui.
a Department of Surgery, Thoracic Oncology Division, University of California, San Francisco 94143, USA.
Omer Hassan
Yi-wei yang, petra buchanan.
Lung cancer remains the leading cause of cancer mortality in men and women in the U.S. and worldwide. About 90% of lung cancer cases are caused by smoking and the use of tobacco products. However, other factors such as radon gas, asbestos, air pollution exposures, and chronic infections can contribute to lung carcinogenesis. In addition, multiple inherited and acquired mechanisms of susceptibility to lung cancer have been proposed. Lung cancer is divided into two broad histologic classes, which grow and spread differently: small-cell lung carcinomas (SCLC) and non-small cell lung carcinomas (NSCLC). Treatment options for lung cancer include surgery, radiation therapy, chemotherapy, and targeted therapy. Therapeutic-modalities recommendations depend on several factors, including the type and stage of cancer. Despite the improvements in diagnosis and therapy made during the past 25 years, the prognosis for patients with lung cancer is still unsatisfactory. The responses to current standard therapies are poor except for the most localized cancers. However, a better understanding of the biology pertinent to these challenging malignancies, might lead to the development of more efficacious and perhaps more specific drugs. The purpose of this review is to summarize the recent developments in lung cancer biology and its therapeutic strategies, and discuss the latest treatment advances including therapies currently under clinical investigation.
1. Introduction
Lung cancer, a highly invasive, rapidly metastasizing and prevalent cancer, is the top killer cancer in both men and women in the United States of America (USA). During 2014, an estimated 224,210 new cases and 159,260 deaths for lung cancer were predicted in the USA [ 1 ]. It causes more deaths per year than the next four leading causes of cancer (Colon/rectal, breast, pancreas, and prostate) death combined in the United States. Its incidence and mortality patterns are consistently associated with 20 or more years of smoking history. The individual susceptibility to tobacco-induced lung cancer may be dependent on competitive gene–enzyme interactions that affect activation or detoxification of procarcinogens and levels of DNA adduct formation as well as determined by the integrity of endogenous mechanisms for repairing lesions in DNA. Lung cancer is highly heterogeneous that can arise in many different sites in the bronchial tree, therefore presenting highly variable symptoms and signs depending on its anatomic location. 70% of patients diagnosed with lung cancer present with advanced stage disease (stage III or IV) ( Figure.1 ).

Schematic illustration of the Non-Small Lung Cancer (NSCLC) staging. Of note, Squamous cell lung carcinomas arise in the epithelial cells of main and lobar Bronchi (not shown), whereas Adenocarcinomas originate in the peripheral lung tissue and arise in the epithelial cells of segmental bronchi. For stage IV NSCLC cancers, the incidence of distant metastasis to the extrathoracic organs is depicted. For each organ, the percentages represent the incidence of metastasis for squamous cell lung carcinomas and adenocarcinomas, respectively.
Squamous cell lung cancers (SQCLC) represent about 25%–30% of all lung cancers and tend to arise in the main bronchi and advance to the carina ( Table 1 ). Adenocarcinomas (AdenoCA) account for approximately 40% of all lung cancers and consist of tumors arising in peripheral bronchi. AdenoCAs advance by producing lobar atelectasis and pneumonitis. Bronchioloalveolar cancers (BAC), now reclassified into adenocarcinoma in situ (AIS) and minimally invasive adenocarcinoma (MIA), arise in alveoli and spread through the interalveolar connections. AIS and MIA describe patients with very good disease-free survival after complete resection (5-year rate nears 100%) [ 2 , 3 ]. Small cell lung cancers (SCLC) derived from the hormonal cells of the lung, are the most dedifferentiated cancers and tend to be central mediastinal tumors. SCLCs comprise 10%–15% of all lung cancers, and are extremely aggressive disseminating rapidly into submucosal lymphatic vessels and regional lymph nodes, and almost always present without a bronchial invasion. Large cell anaplastic carcinomas (LCAC), also termed NSCLC not otherwise specified (NOS), are more proximal in location and locally tend to invade the mediastinum and its structures early. NSCLC-NOS comprises about 10% of all NSCLC. and behaves similarly to small cell cancers with a rapid fatal spread. Pancoast cancer arises in superior sulcus and advances by local invasion into juxta-opposed structures. All lung cancer types can become multifocal in the lobe they arise in (T3), or spread into the lung of origin (T4), or spread to the contralateral lung (M1) ( Figure.1 ) [ 3 ]. The compression of mediastinal structures is associated invariably with advanced lymph node involvement, which can lead variously to esophageal compression and difficulty in swallowing, venous compression and congestion associated with collateral circulation, or tracheal compression. Signs of metastatic disease involving such remote sites as the liver, brain, or bone are seen before any knowledge of a primary lung lesion.
Types of Lung Cancer.
| Lung Cancer Type | % of All Lung Cancer | Anatomic Location |
|---|---|---|
| 25–30% | Arise in main bronchi and advance to the carina | |
| 40% | Arise in peripheral bronchi | |
| 10% | Tumors lack the classic glandular or squamous morphology | |
| 10–15% | Derive from the hormonal Cells Disseminate into submucosal lymphatic vessels and regional lymph nodes almost without a bronchial invasion |
2. International Staging System for Lung Cancer
Cancer staging is a critical step in the diagnosis process, and its objectives are multifarious including 1) Helping the clinician to recommend a treatment plan; 2) Giving some indication of prognosis; 3) Aiding in the evaluation of the results of treatment; 4) Facilitating the exchange of information between treatment centers; 5) Contributing to the continuing investigation of human cancer. The international TNM-based staging system describes the anatomical extent of the disease ( Table 3 ). The T category describes the size and extent of the primary tumor. The N category describes the extent of involvement of regional lymph nodes. The M category describes the presence or absence of distant metastatic spread. The addition of numbers to these categories describes the extent of the cancer. All possible combinations of the T, N, and M categories are then used to create TNM subsets ( Table 2 ). TNM subsets with similar prognoses are then combined into stage groupings. NSCLC stages range from one to four (I through IV). The lower the stage, the less the cancer has spread. SCLC is defined using two stages: Limited (confined to the hemithorax of origin, the mediastinum, or the supraclavicular lymph nodes) and extensive (spread beyond the supraclavicular areas) [ 4 ].
TNM stage grouping for NSCLC. TNM stand for the size and location of the T umor, the location of cancer in the lymph N odes and where the cancer has spread ( M etastases).
T = Primary tumor: T 1a (Tumor size ≤2 cm), T 1b (>2–3 cm): T 2a (>3–5 cm), T 2b (>5–7 cm); T 3 (>7 cm) and/or (Multiple tumor nodules in the same lobe); T 4 (Multiple tumor nodules (of any size) in the same lung but a different lobe).
N 0 = No regional lymph node metastasis; N 1 = Metastasis in ipsilateral peribronchial and/or ipsilateral hilar lymph nodes and intrapulmonary nodes, including involvement by direct extension; N 2 = Metastasis in ipsilateral mediastinal and/or subcarinal lymph node(s); N 3 = Metastasis in contralateral mediastinal, contralateral hilar, ipsilateral or contralateral scalene, or supraclavicular lymph node(s).
M = Distant metastasis: M 1a (Malignant pleural or pericardial effusions and/or Separate tumor nodules in the contralateral lung); M 2b (Distant metastasis in extrathoracic organs).
TNM-based staging system.
| Category | Description |
|---|---|
| Size and extent of the primary tumor | |
| Extent of involvement of regional lymph nodes | |
| Presence or absence of distant metastatic spread | |
| Extent of the cancer | |
| Pretreatment, Clinical stage | |
| Postsurgical stage | |
| Post-preoperative therapy stage | |
| Postsurgical stage with preoperative therapy |
The term stage, without further classification, relates to the pretreatment, clinical stage or cTNM [ 3 , 5 – 7 ]. cTNM is derived using the evidence available from clinical history and examination, blood tests, imaging, endoscopic examination, biopsy material, surgical examination, and any other test considered necessary prior to making a decision as to the appropriate treatment in any individual. If this decision leads to surgical treatment, then additional information becomes available at surgery and by pathological examination allowing a more accurate assessment of disease indicated by the pathological, postsurgical stage or pTNM. pTNM does not replace the cTNM, which should remain as a record in the patient’s notes. If the patient undergoes preoperative “induction” therapy, usually with either or both chemotherapy and radiotherapy, then a reassessment is made after this treatment, prior to a final decision on surgical treatment [ 3 , 5 – 7 ]. The evidence available from this process is used to create the ycTNM, and after surgical treatment in these circumstances, the postsurgical pathological extent of disease is described as ypTNM. At various points in the patient’s journey, events may allow or demand a reassessment of disease extent. An rTNM may be established if relapse occurs after a disease-free interval. An aTNM may be formulated if the disease is first discovered at an autopsy. In each case, previous assessments of TNM are retained in the patient records [ 3 , 5 – 7 ].
3. Lung cancer biology
Lung cancer cells have defects in the regulatory circuits that govern normal cell proliferation and homeostasis. The transformation from a normal to malignant lung cancer phenotype is thought to arise in a multistep fashion, through a series of genetic and epigenetic alterations, ultimately evolving into invasive cancer by clonal expansion [ 8 , 9 ]. Following the development of the primary cancer, continued accumulation of genetic and epigenetic abnormalities, acquired during clonal expansion, influences the processes of invasion, metastasis, and resistance to cancer therapy [ 8 , 9 ]. The identification and characterization of these molecular changes are of critical importance for improving disease prevention, early detection, and treatment. The knowledge of both a patient’s tumor characteristics and genetics will significantly advance the personalized prognosis and ideal treatment selection for each patient.
3.1. Genomic alterations
Recently, the Cancer Genome Atlas Research Network reported the molecular profiling of 230 lung adenocarcinomas. High somatic mutation rates were seen from the whole-exome sequencing (mean 8.87 mutations per megabase of DNA). Eighteen genes were identified to be significantly mutated both in the abovementioned 230 AdenoCA cases as well as in 182 AdenoCA tumors previously analyzed in similar fashion. Genomic alterations include point mutations (missense and nonsense mutations, frameshift and slicing site alterations), rearrangement (transversions and transitions) as well as somatic copy number alterations [ 10 ]. The alterations of kinase protein levels or activities have also been studied. Using RNA-seq analysis in 7000 tumors (20 solid cancer types), novel and recurrent kinase gene fusion events were identified. In lung adenocarcinomas (513 samples), fusion events were found in ROS1, RET, PRKCB, NTRK, MET and ALK genes and were found in PRKCB, PRKCA, PKN1, FGR, FGFR1, FGFR2 an FGFR3 genes in lung squamous cell carcinomas (492 samples) ( Table 5 ) [ 11 ]. These findings may have significant clinical impact and new therapeutic approaches could be developed targeting these alterations. Another large systematic genomic study reclassified 12 tumor types into 11 subtypes based on the sequencing data from 3527 tumor cases (DNA copy number, DNA methylation, mRNA expression, microRNA expression, protein expression and somatic point mutation). Somatic mutations such as KEAP1 and STK11 are preferentially mutated in LUAD-enriched tumors group, containing most of the lung adenocarcinoma cases, while CDKN2A, NOTCH1, MLL2 and NFE2L2 were found mutated preferentially in squamous-like tumors group encompassing most of the lung squamous cell carcinoma cases. Squamous-like tumors also showed frequent MYC amplification and loss of CDKN2A, RB1 and TP53. The reclassification generated new prognostic information that could be used to guide therapeutic decision [ 12 ].
Kinase fusion events identified in AdenoCA and SQCLC [ 12 ]
| | ||
| Gene name | Number of cases altered | Alteration |
| 5/513 | Known fusion | |
| 1/513 | New fusion | |
| 2/513 | Known kinase-novel indication | |
| 1/513 | New fusion | |
| 2/513 | Known fusion | |
| 8/513 | Known fusion | |
| | ||
| | ||
| 1/492 | Known fusion | |
| 1/492 | Known fusion | |
| 3/492 | Known fusion | |
| 1/492 | New fusion | |
| 1/492 | New fusion | |
| 2/492 | Known kinase-novel indication | |
| 1/492 | New fusion |
3.2. Molecular pathology of lung cancer
Several targetable genetic alterations have been identified in lung cancer [ 13 ] ( Table 6 ), including 1) Activating mutations in a number of proto-oncogenes including KRAS, EGFR, BRAF, PI3K, MEK and HER2. Noteworthy, EGFR (Epidermal growth factor receptor) plays a critical role in regulating normal cell proliferation, apoptosis, and other cellular functions. Approximately 10% of NSCLC patients in the US and 35% in East Asia have tumor associated EGFR mutations [ 14 – 16 ]. 2) Structural rearrangements in ALK, ROS1 and possibly RET. 3) Amplification of proto-oncogenes such as MET in adenocarcinomas, FGFR1 and DDR2 in squamous cell lung carcinomas. 4) Oncogenic gene overexpression by microRNAs (miRNAs). 5) Inactivation of Tumor Suppressor Genes (TSG), including TP53, RB1, CDKN2A, FHIT, RASSF1A, and PTEN. 6) Enhanced telomerase activity, which contributes to cellular immortality by maintaining telomere length through de novo synthesis of telomeres and elongation of existing telomeres (100% of SCLCs and 80% to 85% of NSCLCs). The hTERT gene is amplified in 57% of NSCLCs.
Oncogenes and tumor suppressor genes altered in NSCLC [ 14 ].
| AdenoCA (rare), SQCLC (20%, AKT3: 16%) | PI3K | |
| AdenoCA (3–13%) | RTK | |
| AdenoCA (6%), SQCLC (4%) | RAF | |
| AdenoCA (12%) | RB1/CDK | |
| SQCLC (3–8%) | RTK | |
| AdenoCA (40–50%), SQCLC (7%) | RTK | |
| AdenoCA (7–14%) | RTK | |
| SQCLC (2%) | RTK | |
| AdenoCA (1–3%), SQCLC (22%), SCLC (6%) | RTK | |
| SQCLC (3%) | RAS | |
| SCLC (95%) | RTK | |
| AdenoCA (30%), SQCLC (5%) | RAS | |
| AdenoCA (20%) | TP53 | |
| AdenoCA (25%) | RTK | |
| SCLC (10%) | Epigenetic regulation | |
| AdenoCA (31%), SQCLC (rare), SCLC (16%) | Transcriptional regulators | |
| AdenoCA (20%) | Developmental pathways | |
| AdenoCA (<1%), SQCLC (<1%) | RAS | |
| SQCLC (19%) | Oxidative stress response | |
| AdenoCA (rare), SQCLC (16%) | PI3K | |
| AdenoCA (1–2 %) | RTK | |
| AdenoCA (1.5%) | RTK | |
| SQCLC (21%) | Developmental pathways | |
| SQCLC (16%) | Developmental pathways | |
| AdenoCA (rare), SQCLC (8%) | PI3K | |
| AdenoCA (8%) | Epigenetic Regulation | |
| SQCLC (3%) | Developmental pathways | |
| AdenoCA (>20%), SQCLC (72%) | RB1/CK | |
| SCLC (9%) | Epigenetic Regulation | |
| SQCLC (7%) | Oxidative stress response | |
| SCLC (9%) | Epigenetic Regulation | |
| AdenoCA (11%), SQCLC (12%) | Oxidative stress response | |
| AdenoCA (15–30%), SQCLC (2%) | LKB1/AMPK | |
| SQCLC (19%) | Epigenetic Regulation | |
| AdenoCA (8–10%), SQCLC (11%) | RAS | |
| SQCLC (13%) | Developmental pathways | |
| SQCLC (4%) | RAS | |
| AdenoCA (rare), SQCLC (7%), SCLC (100%) | RB1/CDK | |
| AdenoCA (5%) | Epigenetic Regulation | |
| AdenoCA (10%) | Epigenetic Regulation | |
| AdenoCA (70%), SQCLC (80%), SCLC (70%) | TP53 | |
| SQCLC (6%) | PI3K |
Remarkably, scores of the aforementioned aberrations correlate with patient’s smoking history as well as with racial and gender differences, which suggest a possible role of the host’s genetic makeup as key determinants in lung carcinogenesis [ 8 , 9 ].
3.3. Clinical applications
Tremendous work has been conducted to translate the acquired information of these genetic anomalies into improvement of patient care in the clinic including early detection and treatment and prognosis prediction:
- Discovery of biomarkers for early detection of primary and recurrent disease: Currently, the diagnosis of lung cancer is primarily based on symptoms and lung cancer detection often occurs when curative intervention (i.e., surgery) is no longer possible. The five-year survival rate in early-stage, operable NSCLC is approximately 50%–70%, but drops to 2%–5% for patients whose cancers have spread distantly [ 17 ]. Numerous potential early lung cancer detection biomarkers, have been investigated. However, there are still no biomarkers for detection of lung cancer in clinical use due to the lack of either or both a robust sensitivity and specificity or a functional relevance of these biomarkers to lung carcinogenesis.
Platinum-based regimens are standard of care in advanced lung cancer. However, their clinical effectiveness is limited by cumulative haemato- and neuro-toxicities highlighting the need for alternative treatment strategies. ERCC1 functions as a key enzyme in nucleotide excision repair (NER). Low ERCC1 expression correlates with increased sensitivity to platinum-based therapy and high ERCC1 expression correlates with better overall prognosis in NSCLC [ 18 , 19 ]. Nearly 50% of NSCLC patients have low levels of ERCC1, and therefore could benefit from alternative therapies exploiting this tumor ERCC1 deficiency [ 19 ]. RRM1 is the regulatory subunit of ribonucleotide reductase essential for the deoxyribonucleotides (dNTP) synthesis.
RRM1 is the main target for the antimetabolite drug gemcitabine, which is an underpinning cancer therapy in the treatment of many malignancies including lung cancer. Gemcitabine directly binds to RRM1 and irreversibly inactivates ribonucleotide reductase [ 20 – 28 ]. High RRM1 levels are associated with tumor resistance and low RRM1 levels with tumor sensitivity to gemcitabine treatment [ 21 , 23 , 25 – 28 ].
Recent studies have suggested that low levels of the heparan sulfate 6-O-endosulfatase (SULF2) through methylation in NSCLC may be predictive of better survival and increase sensitivity to topoisomerase-1 inhibitors (TPI) [ 29 ]. SULF2 is overexpressed in many tumors including lung adenocarcinomas and lung squamous carcinomas to remove critical sulfation modifications from sulfated heparin sulfate proteoglycans (HSPGs) and thus release growth factors essential for tumor growth [ 30 – 32 ]. It was established that SULF2 methylation via induction of high expression of Ubiquitin-Like Modifier (ISG15) sensitizes lung cancer cells to TPIs via suppression of ubiquition and proteasomal degradation [ 29 ].
A number of new potentially targetable alterations were identified in NSCLC including FGFR1 amplification and DDR1 mutation found in squamous cell lung carcinomas. These alterations might be important prognostic and predictive factors for patient’s response to treatments with FGFR inhibitors or DDR1 inhibitors (e.g., Dasatinib) [ 33 , 34 ].
- Discovery of prognostic and predictive biomarkers: The prognostic and/or predictive value of an extensive panel of molecular markers has been tested in early and advanced stage lung cancer. In advanced NSCLC, positive EGFR mutation (10–15% of NSCLC) or ALK rearrangement (ALK-EML4 fusion) status (5–7% of NSCLC) was shown to be predictive for a significant clinical benefit from EGFR tyrosine kinase inhibitors (TKIs) [ 35 , 36 ], or ALK TKI (crizotinib) [ 37 – 40 ], respectively. ALK-EML4 fusion positivity in NSCLC seems to be a prognosticator of poor response to EGFR TKI therapy [ 41 – 44 ] as EGFR mutations and EML4-ALK fusions occur almost exclusively in adenocarcinomas. Nevertheless, EML4-ALK appears to be mutually exclusive to that of EGFR or KRAS mutations in NSCLC and is more common in never or former light smokers [ 45 ].
3.4. Tumor microenvironment
The tumor microenvironment and the complex interactions of its various cell types and their released signaling molecules are an emerging hallmark of cancer [ 46 ]. It consists of stromal cells, cancer-associated fibroblasts, stem cells and a comprehensive set of immune cells recruited into tumors. The tumor microenvironment is altered to suppress host immune responses, foster tumor growth, and help cancer cells evade immune surveillance [ 47 ]. The tumor-associated immune cells include tumor-associated macrophages (TAM), dendritic cell (DC) subsets, cytotoxic and regulatory T-cells (CTLs and Tregs), natural killer (NK) cells, and myeloid-derived suppressor cells (MDSC). The amounts of different immune cell subsets in the tumor microenvironment can vary considerably among patients and may be used as a predictor of treatment outcome and survival in certain cancers [ 48 – 50 ]. The altered tumor microenvironment is established by the cancer cells through the loss of MHC class I molecules, the loss of antigen variants, and the active secretion of several growth factors, such as vascular endothelial growth factor (VEGF) and granulocyte-macrophage colony-stimulating factor (GM-CSF) [ 51 ]. The immune cells present in the tumor microenvironment are functionally impaired, and the newly infiltrating immune cells become alternatively activated, resulting in a perturbed phenotype [ 51 ]..
Myeloid cells including myeloid-derived suppressor cells (MDSC), macrophages, and dendritic cells (DC), can act as regulatory cells in the tumor microenvironment. MDSCs exert their pro-tumor effects through the inhibition of T-cell proliferation and activation by increasing levels of NO synthase and arginase-1 [ 52 ] [ 51 , 53 , 54 ], releasing IL-10 and overproducing reactive oxygen species (ROS) [ 55 ]. In the peripheral blood of advanced stage NSCLC patients, increased levels of MSDC were detected compared to healthy controls and were associated with lower levels of CD8+ T-cells [ 56 ].
Tumor-associated macrophages (TAM) show a higher frequency of the pro-tumor M2-phenotype, which accumulate in the tumor stroma and correlate with poor patient outcome and decreased OS [ 57 ]. These alternatively activated macrophages show impaired ability to present antigen and appropriate co-stimulation to T-cells [ 51 ]. In contrast, macrophages of the M1 phenotype accumulate intratumorally and show antitumor functions through expression of HLA-DR, inducible nitric oxide synthase (iNOS) and TNF-α [ 58 ]. Furthermore, a high intratumoral density of CD68+ macrophages correlates with increased survival in NSCLC [ 59 ].
DCs are the most important antigen-presenting cells at the border of the innate and adaptive immune system. However, in the tumor microenvironment, DCs are often in an immature state that fail to prime T-cells efficiently due to low expression levels of co-stimulatory molecules such as CD80 and CD86 and a weak antigen presenting capacity [ 60 , 61 ]. Peripheral blood lymphocytes can also be used as a prognostic marker in NSCLC: 1) the total blood count of lymphocytes was shown to be associated with a lower hazard ratio for death [ 57 , 62 ]; 2) a neutrophil to lymphocyte ratio (NLR) >3.81 was identified in the same study as a predictor of survival in patients with Stage I NSCLC; 3)Treg cells were also detected at elevated levels in the peripheral blood of NSCLC patients compared to healthy controls [ 63 , 64 ].
In early-stage NSCLC, a tertiary lymphoid structure is detected in the tumor stroma that contains mature and follicular DC, CD4+ T-cells, and CD20+ B-cells. This tertiary lymphoid structure is defined as Tumor-induced Bronchus Associated Lymphoid Tissues (Ti-BALT) [ 48 , 65 ]. A small retrospective study demonstrated that the density of mature DC (DC-LAMP +) homing in the Ti-BALT correlates with disease-free survival, disease-specific survival, and OS in early-stage NSCLC patients [ 48 ].
Chronic inflammation can also play a significant role in the tumor environment through the release of reactive oxygen and nitrogen species, as well as TNF-α. Consequently, chronic inflammation can facilitate tumor growth via activation of NF-κB and the subsequent suppression of adaptive immune responses [ 48 ]. NSCLC tumors often show hypoxic areas, which leads to the release of pro-angiogenic factors such as VEGF, thereby increasing tumor angiogenesis [ 66 , 67 ].
3.5. Racial and ethnic diversity in lung cancer
There is considerable variation in cancer incidence as well as death rates among different racial and ethnic groups [ 68 ]. Although the cause of this racial and ethnic disparity in cancer risk and outcomes remains controversial [ 69 ], there is a growing consensus that the interaction of genetic and environmental factors including diet is at least partially responsible for the ethnic differences in cancer risk and outcome [ 70 ].
Regarding lung cancer, women have lower incidence and death rates than men. African-American men have the highest incidence and death rate in the United States, followed by White, American Indian or Alaskan Natives, Asian American or Pacific Islanders, and Hispanic/Latino men. In women, the highest rates are in white women, followed by American Indian or Alaskan Natives and African Americans, Asian American or Pacific Islanders, and Hispanic/Latino groups [ 68 ]. Moreover, clinical trials have shown that Asian ethnicity is an independent favorable prognostic factor for OS in NSCLC patients regardless of their smoking history. The frequency of the activated EGFR mutations is higher in East Asian patients as compared with Caucasian patients (30 vs. 7 %, respectively). Numerous studies showed that EGFR mutation-positive patients of Asian origin have better efficacy outcomes with first-line EGFR tyrosine kinase inhibitors (TKI), especially patients with adenocarcinoma histology and never smokers [ 71 ]. In contrast, prevalence of K-ras mutations is lower in Asian patients (<10 vs. 18 %) [ 71 ]. Deciphering these racial disparities requires the identification of risk factors for lung cancer in multiracial, multiethnic groups such as genetic polymorphisms and gene-environment interactions. Moreover, inclusion of minority groups in lung cancer screening and clinical trials may be advantageous in reducing these disparities.
Environmental factors/geography as well as socioeconomic status may also affect lung cancer susceptibility, treatment outcome, and survival rates [ 72 ]. Access to treatment and adherence to treatment regimen [ 73 ] appear to be an enabling factor for racial disparities in lung cancer. It has been shown that White and African-American patients with early-stage NSCLC who were eligible and received surgical resection had comparable survival rates. In contrast, African-Americans who did not undergo surgery (due to un-insurance, limited access to health care, fear to diagnosis or beliefs) had the lowest survival rate [ 73 ]
4. Treatment of Non-Small-Cell Lung Cancer
In this section, the standard and emerging treatments for early stage, advanced, and recurrent NSCLC, as well as brain metastasis will be discussed ( Table 7 ). The various drugs and corresponding targets mentioned in this section are summarized in ( Table 8 ).
Treatment options for NSCLC.
| Stage | Treatment options |
|---|---|
| Surgery | |
| Radiotherapy | |
| Surgery followed by chemotherapy (adjuvant) | |
| Surgery followed by chemotherapy | |
| Chemotherapy (neoadjuvant) followed by surgery | |
| Sequential or combined chemoradiation | |
| Radical surgery | |
| Chemotherapy or radiation | |
| Chemoradiation | |
| Chemotherapy (a combination of two cytotoxic drugs, platinum-based) | |
| Bevacizumab (VEGF antibody) + first line doublet combination chemotherapy | |
| Chemotherapy (single cytotoxic drug) | |
| EGFR targeted therapy (erlotinib and gefitinib) | |
| Docetaxel, pemetrexed, erlotinib and gefitinib | |
| External palliative radiation therapy, Cytotoxic chemotherapy, EGFR inhibitors (with or without EGFR mutations), EML4-ALK inhibitor (with EML-ALK translocations), Surgical resection (isolated cerebral metastases), Laser therapy or interstitial radiation therapy (endobronchial lesions), Stereotactic radiation surgery | |
| Whole Brain Radiotherapy (WBRT), Surgery, Stereotactic Radiosurgery (SRS) with and without WBRT, Systemic Therapy and Radiosensitization |
Drugs and corresponding targets.
| Drug | Target | Type |
|---|---|---|
| VEGF | Humanize VEGFR-trap | |
| TRAIL-R2 | Monoclonal antibody | |
| DR5/TRAIL-R2/TNFRSF10B | Monoclonal antibody | |
| PDGFR | Tyrosine kinase inhibitor | |
| DNA (SSB and DSB) | Alkylating agent | |
| VEGF | Monoclonal antibody | |
| DNA | Small molecule inhibitor | |
| PDGFR | Tyrosine kinase inhibitor | |
| VEGF | Small molecule inhibitor | |
| Histone deactylases (HDACs) | Small molecule inhibitor | |
| DNA | Small molecule inhibitor | |
| IGF-IR | Monoclonal antibody | |
| IGF-IR | Monoclonal antibody | |
| Tubulin | Small molecule inhibitor | |
| Histone deactylases (HDACs) | Small molecule inhibitor | |
| EGFR | Tyrosine kinase inhibitor | |
| Topoisomerase II | Small molecule inhibitor | |
| IGF-IR | Monoclonal antibody | |
| EGFR | Tyrosine kinase inhibitor | |
| DNA | Alkylating agent | |
| PARP-1 | Small molecule inhibitor | |
| CTLA-4 | Monoclonal antibody | |
| Topoisomerase I | Small molecule inhibitor | |
| PDGFR | Tyrosine kinase inhibitor | |
| DR4/TRAIL-R1 | Monoclonal antibody | |
| PD-L1 | Monoclonal antibody | |
| PDGFR | Tyrosine kinase inhibitor | |
| PD-L1 | Monoclonal antibody | |
| VEGFR-1/2/3, PDGFR-α/β, FGFR-1/2/3, Flt-3, Src family | Multiple target Tyrosine kinase inhibitor | |
| PD-1 | Monoclonal antibody | |
| PARP-1 | Small molecule inhibitor | |
| Tubulin | Small molecule inhibitor | |
| Histone deactylases (HDACs) | Small molecule inhibitor | |
| VEGFR-1/2/3, PDGFR-α/β, and FGFR-1 and 3 | Multiple target Tyrosine kinase inhibitor | |
| Thymidylate Synthase | Small molecule inhibitor | |
| PD-1 | Monoclonal antibody | |
| Histone deactylases (HDACs) | Small molecule inhibitor | |
| Histone deactylases (HDACs) | Small molecule inhibitor | |
| VEGFR-2/3, PDGFR-β, c-Kit, Raf, and Flt-3 | Multiple target Tyrosine kinase inhibitor | |
| VEGFR-1/2/3, PDGFR-α/β, c-Kit, Flt-3, and RET | Multiple target Tyrosine kinase inhibitor | |
| Immune-modulatory function | Glycoprotein, recombinant | |
| Topoisomerase I | Small molecule inhibitor | |
| CTLA-4 | Monoclonal antibody | |
| PARP-1 | Small molecule inhibitor | |
| Tubulin | Small molecule inhibitor | |
| Tubulin | Small molecule inhibitor | |
| Histone deactylases (HDACs) | Small molecule inhibitor | |
| Survivin | Small molecule inhibitor |
4.1. Treatment of early stage (stage I and Stage II) Non- Small-Cell Lung Cancer
The primary treatment for resectable and operable early stage disease (Stage I and II) is surgery [ 74 ] which provides the best option for long-term survival [ 75 ]. Five-year survival rates after surgical resection are 60%–80% for stage I NSCLC and 30%–50% for stage II NSCLC patients [ 76 ]. For patients refusing surgical resection or with unresectable tumors, primary radiotherapy can be used such as stereotactic body radiotherapy (SBRT) for high-risk patients or unresectable tumors [ 72 ]. However, post-surgery radiotherapy is not recommended for stage I and II patients [ 72 ]. To date, adjuvant platinum-based chemotherapy was shown to be beneficial for stage II NSCLC patients [ 77 ] and is the recommended treatment strategy for completely resected patients [ 72 ]. Conversely, a clear benefit has so far not been proven for adjuvant chemotherapy in stage I NSCLC patients [ 78 ].
4.2. Treatment of stage III Non- Small-Cell Lung Cancer
More than 70 % of NSCLC patients are diagnosed in advanced stages or metastatic disease [ 2 ] (stages III and IV). Stage III NSCLC is a heterogeneous disease, and varies from resectable tumors with microscopic metastases to lymph nodes to unresectable, bulky disease involving multiple nodal locations. The 5-year OS rate varies between 10% to 15% for stage IIIA-N2 disease and 2% to 5% for stage IIIA bulky disease with mediastinal involvement. In this heterogeneous population of stage III NSCLC patients, the treatment strategies, including radiotherapy, chemotherapy, and surgical resection are determined by the tumor location and whether it is resectable.
The standard treatment consists of surgery followed by chemotherapy for patients with resectable stage IIIA NSCLC. It has been shown that the adjuvant chemotherapy significantly prolonged OS rate in clinical studies [ 79 – 83 ] and that adjuvant radiation therapy can improve control of resected stage IIIA-N2 disease [ 84 ]. Meta-analyses of numerous clinical studies showed that neoadjuvant chemotherapy provides a modest 5% to 6% improvement in survival at five years [ 85 ].
For unresectable stage IIIA patients, standard treatment may include either a sequential or concurrent combination of chemotherapy and radiation therapy (chemoradiation), and external radiation therapy for patients who cannot be treated with combined therapy. Meta-analyses of multiple randomized clinical studies showed that platinum based chemoradiation therapy provides a significant 10% reduction in the risk of death when compared with radiation therapy alone [ 86 – 88 ]. Several clinical investigations showed that the radical surgery in Stage IIIA patients with bulky primary tumors may provide up to 50% increase in the 5-year survival rate as compared to patients with incomplete resection [ 89 – 91 ].
Stage IIIB NSCLC represents about 17.6 % of all lung cancers [ 92 ] with a 5-year survival rate of 3% to 7% [ 93 ]. The options and sequence of treatments for stage IIIB NSCLC are determined based on the site of tumor involvement and the patient’s performance status (PS) ( Table 4 ). Generally, patients with stage IIIB NSCLC do not benefit from surgery alone. The standard therapy for these patients consists of either a sequential combination of chemotherapy or external radiation therapy. As palliative treatment, Stage IIIB NSCLC may receive external radiation therapy alone to relieve pain and other symptoms to improve the quality of life.
Eastern Cooperative Oncology Group (ECOG) Performance Status . The ECOG scale and criteria are used to assess how a patient's disease is progressing, assess how the disease affects the daily living abilities of the patient, and determine appropriate treatment and prognosis.
| Grade | ECOG |
|---|---|
| Fully active, able to carry on all pre-disease performance without restriction | |
| Restricted in physically strenuous activity but ambulatory and able to carry out work of a light or sedentary nature, e.g., light house work, office work | |
| Ambulatory and capable of all self-care but unable to carry out any work activities. Up and about more than 50% of waking hours | |
| Capable of only limited self-care, confined to bed or chair more than 50% of waking hours | |
| Completely disabled. Cannot carry on any self-care. Totally confined to bed or chair | |
| Dead |
4.3. Treatment of Stage IV Non-Small Cell Lung Cancer
Stage IV NSCLC accounts for 40% of the newly diagnosed NSCLC patients. The choice of treatment for stage IV NSCLC patients depends on many factors including, comorbidity, PS, histology, and molecular genetic features of the cancer [ 94 ]. Standard treatment options for stage IV NSCLC disease may include palliative external radiation therapy, combination chemotherapy, combination chemotherapy and targeted therapy, and any Laser therapy or internal endoscopic radiation therapy as needed. Similar to radiation therapy, surgery could also be used in some cases to alleviate disease-related symptoms.
4.3.1. Chemotherapy
For NSCLC, Chemotherapy is usually well tolerated by patients with PS 0 and 1 but rarely effective in patients with a PS 3 and 4 where palliative care is preferred. Use of chemotherapy is controversial in PS 2 NSCLC patients, which represent nearly 40% of advanced stage NSCLC patients. Chemotherapy is recommended only for PS 2 patients who are reasonably fit, and awake for more than 50 % of the day.
i. First-line Chemotherapy
The median OS is 4.5 months when no chemotherapy is given to advanced metastatic NSCLC or after failure of all treatments. Use of chemotherapy improves the 1-year OS rate from 10%–20% up to 30%–50% [ 74 ]. A combination of two cytotoxic drugs is the recommended first-line therapy for Stage IV NSCLC patients with a PS of 0 or 1. Platinum (Cisplatin or carboplatin)-based combination therapies yield better response and OS rates than the non-platinum combination therapies. First line platinum-based chemotherapeutics may include doublets of cisplatin or carboplatin given in combination with taxanes (paclitaxel, docetaxel, or vinorelbine), antimetabolites (gemcitabine or pemetrexed), or vinca alkaloids (vinblastine) with comparable activity [ 95 ]. Use of single cytotoxic chemotherapy is preferred in stage IV patients with a PS of 2 due to their greater risk of toxicity and drug intolerance, comparing to patients with a PS 0 to 1.
ii. First-line combination chemotherapy with targeted therapy
Currently, the addition of bevacizumab, an antibody targeting VEGF, to first-line doublet combination chemotherapy, is supported for the treatment of stage IV NSCLC patients with exception (squamous carcinoma histology, brain metastasis, significant cardiovascular disease or a PS greater than 1) due to fatal bleeding concerns. The combination of bevacizumab with carboplatin and paclitaxel doublet appeared to be superior to the combination with cisplatin and gemcitabine.
4.3.2. EGFR tyrosine kinase inhibitors (first line)
The first of the approved targeted drugs for NSCLC patients are agents that specifically block the EGFR) such as tyrosine kinase inhibitor (TKI) Erlotinib (Tarceva) and gefitinib (Iressa). Mutations of EGFRs can lead to abnormal activation of this receptor triggering uncontrolled cell growth, which may account for several subsets of cancers including NSCLC.
Evidence from several randomized clinical trials demonstrated that use of single-agent gefitinib as a first-line therapy might be recommended for patients with activating EGFR mutations, particularly for patients who have contraindications to platinum therapy. Conversely, cytotoxic chemotherapy is preferred if EGFR mutation status is negative or unknown. Three large controlled and randomized trials showed that gefitinib or erlotinib are better than platinum combination chemotherapy as first-line treatment for stage IIIB or IV lung adenocarcinomas in nonsmokers or former light smokers in East Asia [ 35 , 36 , 96 , 97 ].. Data from these trials demonstrated that gefitinib or erlotinib improved PFS but not OS and have favorable toxicity profiles compared with combination chemotherapy of patients with chemotherapy-naïve and EGFR mutations adenocarcinoma. Similar benefits of erlotinib versus platinum-based chemotherapy as first-line were reported in one European large randomized clinical trial (PFS: 9.7 vs. 5.2 months, respectively) [ 98 ]. Neither erlotinib nor gefitinib is recommended for use in combination with cytotoxic chemotherapy as first-line therapy
4.3.3. Maintenance therapy following first-line chemotherapy
Maintenance therapy is the treatment continuation until disease progression of a cancer that has not advanced following the first-line therapy. The primary goal is to improve cancer-related symptoms, and, hopefully, improve survival time beyond that provided by the first-line therapy. It has lately gained great interest in the treatment of advanced NSCLC (stage IIIB and stage IV) [ 99 ]. To date, pemetrexed (Alimta), and erlotinib (Tarceva) are the two medications that have been approved by the FDA as maintenance therapy for advanced lung cancer. Evidence from two randomized controlled clinical trials showed a statistically significant improvement of PFS (4.1 to 4.3 months vs. 2.6 to 2.8 months), with the addition of pemetrexed as maintenance therapy following standard first-line platinum-based combination chemotherapy [ 100 – 103 ]. Remarkably, pemetrexed maintenance therapy appears to be effective only in patients with adenocarcinoma and large cell carcinoma as well as in patients with EGFR mutations in their tumors, but not in patients with squamous cell lung carcinoma.
Data from a large randomized controlled clinical trial showed improved survival (both PFS and OS) with erlotinib maintenance treatment following platinum-based chemotherapy in NSCLC patients without progressive disease (PFS: 12.3 weeks vs. 11.1 weeks) [ 104 ]. Similar to pemetrexed, erlotinib maintenance therapy improved outcome primarily in non-squamous cell lung carcinoma NSCLC patients. Patients with activating EGFR mutations in their tumors showed greatest PFS benefit comparing to patients with wild type EGFR who also experienced improvement in their median PFS. This trial also demonstrated that KRAS mutation status was a significant, negative prognostic factor for maintenance erlotinib-induced PFS [ 105 ]. Moreover, never-smoking women with better PS seems to derive the utmost survival advantage from maintenance erlotinib therapy.
4.3.4. Second- and third-line therapies in the treatment of advanced NSCLC
Docetaxel (Taxotere), pemetrexed, erlotinib, and gefitinib, are currently approved as second-line therapy for patients with advanced NSCLC who have failed first-line platinum-based therapy and have an acceptable PS.
Evidence from several randomized clinical trials and meta-analyses [ 106 – 108 ] showed that docetaxel in the second-line setting leads to better survival and quality of life (QoL) when compared to best supportive care [ 107 ] or to single agent ifosfamide or vinorelbine [ 109 ]. Pemetrexed yielded similar clinical response comparing to docetaxel (a median survival of about 8 months, one-year survival of 30%, and a response rate of 10%) [ 110 ] with better toxicity profile that may benefit older patients with a PS of 3 [ 111 ]. Pemetrexed also provided better outcome in lung adenocarcinoma patients, whereas docetaxel treatment was more effective in lung squamous cell carcinoma patients [ 112 ]. Erlotinib related response was more common in women with adenocarcinoma, never-smokers, or east-Asians, which is correlated with more frequent EGFR activating mutations [ 113 ]. To date, erlotinib is approved and may be recommended as second- or third-line therapy for patients with a PS of 0 to 3 who have not received prior erlotinib or gefitinib.
Because of the scarcity of data on cytotoxic chemotherapy as third-line therapy, there are no recommendations for or against using a cytotoxic chemotherapy in the third-line setting. However, Phase III clinical trials of docetaxel, erlotinib, gefitinib, and pemetrexed allowed patients to continue chemotherapy, as tolerated, until disease progression.
4.4. Standard Treatment Options for Recurrent NSCLC
Recurrent or relapsed NSCLC is a cancer that has progressed or returned following an initial treatment with surgery, radiation therapy, and/or chemotherapy. The cancer may return in the lung, brain, or other parts of the body. For NSCLC patients who have never been treated with chemotherapy, the treatment plan is similar to that of Stage IV NSCLC. For those patients who have already been treated with chemotherapy, standard treatment options may include: 1) External palliative radiation therapy, which achieves palliation of symptoms from a localized tumor mass [ 114 ], to relieve pain and other symptoms and improve the quality of life; 2) Cytotoxic chemotherapy [ 107 , 110 , 115 ]; 3) EGFR inhibitors (TKIs) in patients with or without EGFR mutations.; 4) EML4-ALK inhibitor (Crizotinib) in patients with EML-ALK translocations.[ 116 , 117 ]; 5) Surgical resection of isolated cerebral metastases (for selected patients who have a very small amount of cancer that has spread to the brain) [ 118 ]; 6) Laser therapy or interstitial radiation therapy using an endoscope (for endobronchial lesions) [ 119 ]. 7) Stereotactic radiation surgery (for selected patients who cannot have surgery) [ 120 , 121 ].
4.4.1. Cytotoxic Chemotherapy for Recurrent NSCLC
Evidence from clinical studies showed that use of cytotoxic chemotherapy and targeted therapy may achieve objective responses, albeit with small improvement in survival for patients with recurrent NSCLC [ 122 ]. In some trials, platinum based chemotherapy has also been shown to achieve palliation of symptoms, which occurred more often than the objective response in patients with good PS [ 123 , 124 ]. Treatment options for NSCLC patients whose cancer has recurred after platinum-based chemotherapy may include either new cytotoxic chemotherapy such as docetaxel [ 107 , 114 ] and pemetrexed [ 110 ], or a targeted therapy such as erlotinib [ 113 ], gefitinib [ 115 ], and crizotinib for cancers with EML4-ALK translocations [ 116 , 117 ]. Patients with squamous lung carcinomas benefit more from docetaxel, whereas those with non-squamous NSCLC appeared to benefit more from pemetrexed [ 125 ].
4.4.2. EGFR Inhibitors for Recurrent NSCLC
A large randomized phase III trial comparing gefitinib to placebo in recurrent NSCLC patients suggested that gefitinib might be a valid treatment for recurrent NSCLC patients with improved survival compared to placebo in never-smokers (median survival 8.9 mo vs. 6.1 mo), and Asian patients (median survival 9.5 mo vs. 5.5 mo) [ 126 ], In two large randomized, placebo controlled trials, erlotinib has also been shown to improve survival and quality of life in patients with recurrent NSCLC after first-line or second-line chemotherapy compared to placebo [ 113 , 127 ]. Moreover, erlotinib treatment also induced a greater improvement in patients’ symptoms, such as cough, pain, and difficulty in breathing, compared to placebo [ 113 ]. Conversely, erlotinib did not improve survival when compared to standard second-line chemotherapy with docetaxel or pemetrexed [ 128 ], in recurrent NSCLC patients after a first-line platinum combination therapy.
4.4.3. ALK/MET Inhibitors for Recurrent NSCLC
Translocations of EML4 and ALK occur on the short arm of chromosome 2, and the fusion of EML4 and ALK (normally a dormant gene) results in a constitutive activation of the ALK kinase. The EML4-ALK fusion oncogene has been identified in approximately 4% in the NSCLC population, and is generally found in individuals who do not typically respond to EGFR TKI therapy [ 40 , 45 ]. EGFR and EML4-ALK mutations appear to be mutually exclusive with exceptions. Tumors harboring the EML4-ALK fusion oncogene are sensitive to crizotinib, a selective, ATP-competitive ALK and MET/HGF dual TKI, which is FDA approved for the treatment of patients with locally advanced or metastatic ALK positive-NSCLC [ 117 ]. Crizotinib (XALKORI @ , Pfizer) is currently approved in Switzerland for treatment of patients with previously treated ALK-positive advanced NSCLC, and in the USA for treatment of patients with locally advanced or metastatic, ALK-positive NSCLC.
Crizotinib therapy has shown improvement in survival of patients with advanced, ALK-positive NSCLC compared to standard therapies for advanced NSCLC [ 117 ] [ 129 ]. Similar to the kinase inhibitors already used in clinic, such as imatinib and EGFR inhibitors, resistance to crizotinib frequently develops in patients’ tumors [ 130 ]. These tumors might either acquire additional ALK kinase domain mutations (i.e., L1196M, C1156Y mutations) that alter drug sensitivity [ 131 ], or other ALK alterations, including amplification, gain in copy number, and loss of ALK genomic rearrangement [ 132 ]. Furthermore, signaling through other kinases, such as EGFR, might compensate for ALK inhibition, thereby mediating resistance to ALK inhibitors [ 132 ]. Mutation in the KRAS gene was also shown to play a role in resistance to crizotinib and around 8 % of ALK-positive NSCLC patients were shown to harbor either a KRAS or EGFR mutation in addition [ 130 ].
4.5. Treatment of Second Primary Tumor
A second primary cancer is a separate cancer arising in a patient who had another cancer in the past. Second or higher order primary tumors account for about 6 to 10% of all cancer diagnoses, and are the fifth most commonly diagnosed cancer in Western countries. The risk of developing a second primary cancer may increase with the use of cancer therapies, such as chemotherapy and radiation therapy. However, it is crucially important to remember that this cancer therapy-related risk is minimal when compared to the benefits of treating the original primary cancer. Patients with lung cancer are at high risk of developing second primary lung cancers. However, it may be difficult to accurately determine whether the new tumor is a secondary primary cancer or a metastasis from the original cancer. Studies have shown that in the majority of lung cancer patients the new lesion is a second primary tumor. When the original primary tumor has been surgically removed, surgical resection of second primary tumors may achieve a 5-year survival rate of 60%, with a comparable expected operative morbidity and mortality to the primary surgery. Tumors 2 cm or smaller are associated with significant positive long-term prognostic factors for survival and freedom from recurrence following resection of a the second primary cancer [ 133 – 135 ]
4.6. Treatment of Brain Metastases
Brain metastases are a common problem in lung cancer patients and a significant cause of morbidity and mortality. Brain metastases are found in about 80% of SCLC and 30% NSCLC at two years from diagnosis [ 136 , 137 ]. Among the various histologies of NSCLC, the incidence of brain metastases in patients with adenocarcinoma and large cell carcinoma is greater than in patients with squamous cell carcinoma [ 138 , 139 ]. The median survival for untreated lung cancer patients with brain metastases is 4 to 7 weeks [ 140 – 142 ]. The treatment may be for relief of symptoms or therapeutic strategies. Treatment options for lung cancer patients with brain metastases may include Whole Brain Radiotherapy (WBRT), surgical resection, Stereotactic Radiosurgery (SRS), Systemic therapy and Radiosensitization, or a combination of these various treatment modalities.
a) Whole Brain Radiotherapy (WBRT)
WBRT is the standard of care for cerebral metastasis in lung cancer patients. Several randomized trails have assessed numerous WBRT dose and fractionation schedules but showed no significant difference in either survival times, or symptomatic response rates and duration. Nevertheless, the results of these trials have suggested better palliative effects from the more prolonged schedules and the choice of dose fractionation schedule should be based on patients’ prognosis [ 143 ]. Additionally, a systematic imaging study of dose response based on tumor size and histology, following WBRT (30 Gy in 10 fractions) [ 144 ], showed an improved response rate for smaller tumors without necrosis. The complete response rate was 37% for SCLC, 25% for squamous cell carcinoma, and 14% for non-breast adenocarcinoma.
Resection of a single brain metastasis combined with WBRT is a standard treatment option of brain metastases [ 134 , 145 ]. A prospective randomized study [ 134 ], demonstrated superiority of surgical removal of the brain tumor followed by radiotherapy over needle biopsy and radiotherapy, with lower recurrence rates at the site of the original metastasis (20% vs. 52%,), and a significantly longer time to recurrence of the original brain metastasis (median >59 weeks vs. 21, p < 0.0001). Moreover, the median survival with surgery and adjuvant WBRT was much longer than with WBRT alone (40 weeks vs. 15 weeks, p < 0.01). Patient’s functional independence (KPS score of ≥70) was also preserved much longer with combined surgery and WBRT than with radiation alone (median: 38 weeks vs. 8 weeks, p < 0.005).
In patients with multiple brain metastases, surgery is typically limited to the resection of the dominant, symptomatic lesion. Various studies have shown that surgery combined with adjuvant WBRT or stereotactic radiosurgery (SRS) has similar survival outcome in patients with multiple lesions compared with patients with single brain metastasis or a single lesion [ 146 – 148 ]. About 50% of patients treated with resection and postoperative radiation therapy develop recurrence in the brain [ 118 ]. Few patients with recurrent brain metastasis and good PS, but without progressive metastases outside of the brain, may be treated with surgery or stereotactic radiation surgery [ 118 , 120 ]. However, most patients with recurrent brain metastasis may be treated with additional radiation therapy, albeit with a limited palliative benefit [ 149 ].
c) Stereotactic Radiosurgery (SRS) with and without WBRT
Stereotactic radiosurgery (SRS) is a form of non-invasive radiation therapy that focuses high-power energy on a precisely defined small target (e.g. the center of the tumor). The suggested mechanisms of SRS-induced tumor killing are radiation-induced DNA damage, endothelial cell apoptosis, microvascular dysfunction, and induction of a T-cell response against the tumor [ 150 – 152 ]. Because of the generally small size and well-defined margin of brain metastases at presentation [ 153 , 154 ], SRS may be an effective alternative to surgery for up to four small brain metastases (up to 4 cm in size) [ 154 ].
Several studies showed that SRS might achieve better prognosis and prolonged survival in lung cancer patients with good PS, no systemic disease, and longer survival time from the diagnosis of primary disease [ 155 – 160 ]. The addition of SRS to WBRT could be beneficial for patients who are not eligible for surgery due to tumor location in the brain or other medical contradictions.. A large randomized controlled Phase III trial study, showed that the local recurrence at one year decreased significantly with the combination of WBRT and SRS (18 vs. 29%, p = 0.01) [ 161 ]. A planned sub-analysis, in patients with a single brain metastasis revealed an improved median survival (6.5 vs. 4.9 months; p = 0.039) and improved quality of life with WBRT and SRS.[ 161 – 163 ]. The addition of adjuvant WBRT to SRS yielded a significant increase in the average time to deterioration in patients with one to four brain metastases (16.5 months vs. 7.6 months, p = 0.05) although no survival advantage was observed [ 164 ].
d) Systemic Therapy and Radiosensitization
In 30–70% of patients with a single brain metastasis, lung cancer is the primary disease [ 165 ]. Generally, most chemotherapeutic agents are unable to cross the blood brain barrier reach the CNS. However, the endothelium leakiness of the tumor vessels, which may disrupt the blood-brain barrier, is well documented in human cancer, particularly in case of macroscopic metastases or relapsed disease. In keeping, several small phase II studies demonstrated that chemotherapy alone yields response rates of brain metastases of 43%–100% and 0%–38% for metastases from SCLC and NSCLC, respectively [ 165 ]. However, combining chemotherapies (thalidomide, teniposide, topotecan, paclitaxel, and cisplatin) to WBRT did not demonstrate survival benefit although some showed enhanced response rates [ 166 – 175 ].
Radiosensitizing agents, such as motexafin gadolinium (Xcytrin) and efaproxyn (efaproxiral or RSR-13), may increase oxygen levels in the tumor and therefore enhance its sensitivity to radiation therapy. However, initial trials showed that the addition of radiosensitizers to WBRT may improve response rate and time to progression (TTP), but not survival. Overall, the evidence to date does not support the clinical use of chemotherapy or radiosensitizers in conjunction with WBRT in the treatment of brain metastases.
4.7. Role of Angiogenesis Inhibitors in NSCLC
Angiogenic pathways provide an important target in NSCLC treatment since they foster tumor growth through the development of new blood vessels. The complex process of angiogenesis is regulated by pro-angiogenic factors such as vascular endothelial growth factor (VEGF), fibroblast growth factor (FGF), platelet-derived growth factor (PDGF), as well as angiopoietins [ 176 ]. Currently, only the monoclonal antibody bevacizumab, targeting circulating VEGF, is approved for first-line treatment of advanced NSCLC in combination with platinum-base chemotherapy [ 176 ]. Several anti-angiogenic agents are under clinical investigation, including sorafenib and sunitinib.
A randomized phase III trial (ECOG 4599) assessing the efficacy of bevacizumab in combination with first-line platinum-based chemotherapy (carboplatin/paclitaxel) showed significantly improved response rates (35 % vs. 15 %, P<0.001), PFS (6.2 vs. 4.5 months, P<0,001), and overall survival (OS) (12.3 vs. 10.3 months, P=0.003) in the antibody group, compared to chemotherapy alone [ 177 ]. Further analysis revealed that baseline tumor cavitation is the most significant risk factor for the fatal side effect after bevacizumab therapy [ 178 ]. The trial also suggested that patients with adenocarcinoma histology might benefit more from the treatment with bevacizumab [ 179 ].
However, resistance to treatment with anti-VEGF agents is a challenge and occurs in all patients eventually. This resistance might, at least partially, be caused by up-regulation of compensatory angiogenic pathways, e.g. through PDGF or FGF signaling [ 176 ]. Multi-targeted anti-angiogenesis therapies therefore represent an interesting treatment strategy for NSCLC patients. In keeping, the multiple tyrosine kinase inhibitor (TKI) sorafenib which targets VEGFR-2/3, PDGFR-β, c-Kit, Raf, and Flt-3, produced promising response rates in several phase I and II studies [ 176 ]. Although sorafenib showed activity as single agent in NSCLC patients, it did not show an added improvement when combined with the carboplatin, paclitaxel and EGFR TKI (erlotinib) [ 176 ]. Interestingly, Sorafenib treatment resulted in an increased disease control rates in NSCLC patients with K-Ras mutations [ 176 ]. Several phase II/III studies for sorafenib in NSCLC are still ongoing [ 176 ].
Another multiple TKI, sunitinib, targeting VEGFR-1/2/3, PDGFR-α/β, c-Kit, Flt-3, and RET, have also been evaluated in NSCLC patients [ 176 ]. Similar to sorafenib, sunitinib demonstrated singe-agent activity in pretreated NSCLC patients but did not show promising results when combined with paclitaxel/carboplatin alone, with bevacizumab or with erlotinib in two phase II trials [ 180 , 181 ]. Moreover, several other agents inhibiting VEGFR in combination with PDGFR are currently in clinical development, including cediranib, axitinib, motesanib, and linifinib [ 176 ].
Conversely, the angiogenesis inhibitors have not proven to increase the efficacy of standard platinum-based chemotherapy in advanced NSCLC possibly due to lower doses required to reduce toxicities [ 176 ]. In addition to dual-targeted therapies, several agents with activity against three angiogenic pathways (VEGFR, PDGFR, and FGFR) are currently under clinical investigation. An example of the aforementioned inhibitors is the small molecule inhibitor nintedanib which targets VEGFR-1/2/3, PDGFR-α/β, FGFR-1/2/3, as well as Flt-3 and Src family members [ 182 ]. Nintedanib achieved disease stabilization in 46 % of the patients, with a median PFS of 6.9 weeks and median OS of 21.9 weeks, in stage IIIB/IV advanced NSCLC patients [ 183 ]. Pazopanib is another interesting multi TKI, targeting VEGFR-1/2/3, PDGFR-α/β, and FGFR-1 and 3 [ 184 ]. In a phase II study treating naïve stage I/II resectable NSCLC patients, pazopanib showed significant activity as single agent. 86 % of the pazopanib treated patients had a reduction in tumor volume, with two patients achieving a reduction of over 50 %. Pazopanib is currently under investigation in advanced NSCLC [ 176 ].
4.8. Other Molecular Targeted Agents, under Clinical Evaluation for NSCLC Treatment
A) talactoferrin.
The glycoprotein lactoferrin was first described as an iron-binding protein in breast milk and shows immune-modulatory functions [ 185 , 186 ]. The human recombinant lactoferrin, talactoferrin, is given orally, and is able to recruit immature dendritic cells (DC) into the gut-associated lymphoid tissue, where cross-presentation of tumor antigens and subsequent DC maturation can occur [ 186 ]. Preclinical data also showed an increase in splenic NK cell activity and inhibition of NSCLC tumor growth with talactoferrin [ 187 ]. A double blind, placebo controlled phase II study using talactoferrin as monotherapy showed improved OS (median 6.1 vs, 3.7 months after a follow-up of 15.2 months). When combining with chemotherapy (carboplatin and paclitaxel) in treating advanced stage IIIB/IV NSCLC patients, talactoferrin also showed a promising trend for disease-control rates in the intention-to-treat and evaluable population [ 188 ].
b) Insulin-like Growth Factor Inhibitors
The insulin-like growth factor system (IGF system) comprises two receptors: Insulin-like growth factor 1 receptor (IGF-IR) and IGF-IIR with their respective ligands: Insulin-like growth factors 1 and 2 (IGF-1 and IGF-2) and six high-affinity IGF binding proteins (IGFBP) that function as carrier proteins for these ligands. IGF 1 and 2 are involved in the regulation of the development and growth of somatic tissues, as well as carbohydrate metabolism [ 189 , 190 ]. The IGF signaling pathway promotes cell growth by stimulating cell proliferation and differentiation. Additionally, IGF-IR, but not IGF-IIR, signaling inhibits apoptosis [ 191 ]. These ligands bind to the extracellular domain of the IGF receptor 1 (IGF-1R), which is expressed on many normal human cells [ 192 , 193 ], and overexpressed in many cancers, including lung cancer. Furthermore, increased IGF-1 levels, and decreased IGFBP-3/4 level correlate with a higher risk of lung cancer [ 190 , 194 ].
A number of inhibitors for the IGF signaling pathway have been developed, including monoclonal antibodies and small-molecule TKIs, which target the intracellular domain of the receptor [ 190 ]. Figitumumab, a monoclonal antibody against IGF-1R, showed significantly increased overall response rates compared to chemotherapy alone (54 % vs. 42 %, P<0.0001) in a phase II trial in previously untreated locally advanced or metastatic NSCLC patients, Squamous tumors showed a response rate of 78 % and PFS (12-week) of 89 % [ 195 ]. However, two phase III trials that used Figitumumab in combination with chemotherapy in non-adenocarcinoma NSCLC were closed due to severe lethal adverse events and unmet primary endpoint [ 190 , 196 , 197 ]. Two other monoclonal antibodies against IGF ligands, cixutumumab and dalotuzumab, as well as small molecule TKIs are currently under clinical investigation [ 190 ].
c) Histone Deacetylase Inhibitors
Histones are nuclear structural enzymes and, as part of the chromatin, are involved in nucleosomal DNA organization and gene regulation. Conformational changes in DNA structure are regulated by histone acetylation and deacetylation, a mechanism that is often affected in tumor cells [ 198 ]. Histone deactylases (HDACs) are involved in chromatin condensation and repression of gene expression and are frequently overexpressed in many cancers [ 190 ]. Contrary to genetic mutations, the epigenetic modifications induced by HDACs are reversible, and therefore, HDACs are an attractive target for cancer therapy [ 198 ]. Various HDAC inhibitors have been developed and shown to modulate the acetylation status of several important cellular proteins involved in tumor cell growth and proliferation, including p53, HSP90, STAT3, subunits of NFκ-B and α-tubulin [ 190 , 199 , 200 ]. Moreover, the HDAC inhibitors can also modify the cell cycle and lower the apoptotic threshold [ 190 , 199 , 200 ]. Many HDAC inhibitors showed anticancer activity in cell culture and animal models of carcinogenesis. Two of these HDAC inhibitors, suberoylanilide hydroxamic acid (SAHA, Vorinostat) and Romidepsin (Depsipeptide, FK228), have already been FDA approved for the treatment of cutaneous T-cell lymphoma (CTCL). The addition of vorinistat to chemotherapy was able to improve the response rate compared to the placebo addition to chemotherapy (34 vs. 12.5 %, P=0.48) in treating advanced stage IIIB/IV NSCLC patients [ 201 , 202 ]. However, a phase III trial of vorinostat in combination with carboplatin and paclitaxel was stopped due to increased adverse effects, and a lack of efficacy in the vorinostat group [ 203 ]. A number of other HDAC inhibitors including entinostat, pivanex, cI-994, panobinostat, and romidepsin, are currently under clinical investigation for treatment of NSCLC [ 204 ].
d) Pro-Apopototic Agents
Apoptosis has long been known as a hallmark of cancer, and cancer cells exploit both upregulation of antiapoptotic as well as downregulation of pro-apoptotic mechanisms [ 46 , 205 ]. Novel pro-apoptotic drugs are currently being investigated for the treatment of NSCLC. To date, both Mapatumumab, a high-affinity monoclonal antibody against the death receptor DR4/TRAIL-R1, and a pro-apoptotic agent apomab did not show clinical benefit as monotherapy or in combining with chemotherapies (carboplatin and paclitaxel) in clinical trials [ 206 , 207 ] [ 208 ] [ 190 , 209 ]. A number of other new pro-apoptotic agents, such as Conatumumab (targeting DR1) and YM155 (targeting survivin), are currently under clinical investigation for the treatment of NSCLC and have shown synergistic effects in combination with chemotherapy [ 190 , 210 , 211 ].
4.9. Immunotherapy
A) immune checkpoint inhibitors.
Tumors ascribe certain immune-checkpoint pathways as a chief mechanism of immune resistance, particularly against T cells that are specific for tumor antigens. Several of these immune checkpoints are initiated by ligand–receptor interactions, and thus are amenable to inhibition by antibodies or modulated by recombinant forms of ligands or receptors [ 186 ]. Two monoclonal antibodies, ipilimumab and tremelimumab, have been used successfully in NSCLC against the cytotoxic T-lymphocyte-associated antigen (CTLA-4), an inhibitory T-cell co-receptor found on activated T-cells and regulatory T-cell subsets [ 186 , 212 ]. A multicenter double-blind phase II trial showed that the combination of ipilimumab and chemotherapy (carboplatin or paclitaxel) significantly improved the immune-related PFS in advanced stage IIB/IV NSCLC patients with squamous cell carcinoma (without prior chemotherapy) [ 186 , 213 ]. Tremelimumab has also been tested in a randomized, phase II trial as maintenance after first-line chemotherapy, compared to best supportive care. However, the results of this trial showed no improvement in PFS [ 214 ].
2) PD-1 and PD-L1
The immune-checkpoint receptor, programmed death-1 (PD-1), is a promising target, for stimulation of antitumor immune responses by the patient's own immune system. Unlike CTLA4, the main role of PD-1 is to control the activity of T cells in peripheral tissues at the time of an inflammatory response to infection and to limit autoimmunity [ 215 – 222 ]. This translates into a major immune resistance mechanism within the tumor microenvironment [ 223 – 225 ]. Another interesting immunotherapeutic option is the direct targeting of PD-1 ligands (PD-L1), B7-H1/PD-L1 and B7-DC/PD-L2. It has been shown that B7-H1/PD-L1 is selectively upregulated in many human cancers including lung cancer [ 223 , 226 ]. An encouraging phase I study showed clinical activity of PD-L1 blocking agents in NSCLC [ 223 , 226 ].
A dose-escalation study testing a monoclonal antibody against PD-1 (MDX-1106) in the treatment of refractory-metastatic solid tumors (melanoma, renal cell cancer, colon cancer, NSCLC), showed objective responses in five of 49 NSCLC patients [ 222 ]. This result highlights the potential activity of anti-PD-1 against a non-immunogenic tumor [ 226 ]. Another study assessed the safety and antitumor activity of the anti-PD-1 monoclonal antibody BMS-936558 or nivolumab in patients with advanced tumors (NSCLC, melanoma, prostate, renal cell, and colon cancer) [ 227 ]. Durable, objective responses (partial and complete) were observed in 18 % of NSCLC patients. Significantly, the objective responses to anti-PD-1 therapy and clinical benefit correlated with PD-L1 expression by tumor cells (P=0.025 and 0.005, respectively). Although the expression of PD-L1 by infiltrating immune cells did not significantly correlate with objective response (P=0.14), marked correlation with clinical benefit was reported (P=0.038). The toxic side effects were milder with PD-1 inhibition compared to CTLA-4 inhibition, thus underlining the importance of targeting immune checkpoint-pathways with better benefit-to-toxicity ratios [ 186 , 226 ].
Several clinical trials are currently investigating immune checkpoint inhibitors, such as anti-CTLA-4 (nivolumab) and anti-PD-1 (ipilimumab), as monotherapy or in combination with chemotherapy in NSCLC [ 228 ]. Recently, results from two phase III trials (CheckMate-017 and 057) showed an OS benefit with Nivolumab compared to docetaxel in both nonsquamous and squamous NSCLC. In the phase III CheckMate-017 trial [ 229 ], there was a 41% OS improvement with nivolumab compared to docetaxel in the squamous setting. In the phase III CheckMate-057 trial [ 230 ], the OS benefit with nivolumab was 27% in patients with nonsquamous NSCLC. Based on data from CheckMate-017 trial, Nivolumab is now approved by the FDA in squamous NSCLC.
Recent publications evaluated expression of PD-1 and PD-L1 in NSCLC. Patients with KRAS mutations were shown to express higher levels of PD-1 compared to patients with wild type KRAS, whereas increased levels of PD-L1 were detected in patients with EGFR mutations or ALK translocations [ 231 ]. Interestingly, the clinical profile of PD-1 expressing patients included male smokers with adenocarcinoma more frequently, whereas PD-L1 was more frequently expressed in female non-smokers with adenocarcinoma. Both PD-1 and PD-L1 were recently shown to be upregulated through activation of EGFR, thereby leading to immune evasion [ 232 , 233 ]. Furthermore, patients with EGFR mutations and increased expression of PD-L1 showed a higher response rate to treatment with the EGFR-TKIs gefitinib or erlotinib, compared to PD-L1 negative patients, which resulted in longer TTP (11.7 vs. 5.7 months, P<0.0001) and OS (21.9 vs. 12.5 months, P=0.09) [ 231 ]. Taken together, these recent studies suggest that combination of EGFR TKIs with PD-1 inhibitors might be beneficial in treatment of NSCLC [ 231 , 232 ].
Another study analyzed the mechanism of combining immunotherapy with immune checkpoint inhibition in a B16 tumor mouse model. A TLR agonist enhanced GM-vaccine (TEGVAX) was shown to induce anti-tumor immune responses in vivo , which was associated with IFN-y dependent upregulation of PD-L1 in the tumor microenvironment. Combined treatment with TEGVAX and PD-1 inhibition led to regression of established tumors, whereas PD-1 inhibition alone did not induce anti-tumor immune responses [ 234 ]. Other antibodies against PD-1, such as pembrolizumab (MK-3475), MPDL3280A, and MEDI4736 are currently being investigated and have shown promising results in Phase 1 clinical trials [ 235 ]. A recent study used whole-exome sequencing to investigate the genomic determinants of response in two independent cohorts of NSCLC treated with this therapy [ 236 ]. This study revealed that a higher nonsynonymous mutation burden in tumors was associated with improved ORR, durable clinical benefit, and PFS. Pembrolizumab clinical efficacy also correlated with the molecular smoking signature, higher neoantigen burden, and DNA repair and replication pathway mutations (e.g., mutations in POLD1, POLE, MSH2, Rad51, Rad17, DNA-PK) [ 236 ]. Remarkably, pembrolizumab–induced neoantigen-specific T cell reactivity was also observed in the peripheral blood, thus, suggesting possible blood-based assays to monitor response during anti–PD-1 therapy.
b) Vaccine Therapy for NSCLC
Vaccination against pathogens is one of the most important developments in modern medicine and saves millions of lives each year. For advanced NSCLC patients, median OS is about one year, and only 3.5 % survive five years after diagnosis, despite the addition of new therapies to standard chemotherapy [ 186 ]. Therefore, vaccinations for solid tumors, either preventive (for tumors related to infections such as human papilloma virus-associated cervical cancer [ 237 ]) or therapeutic (breaking tolerance and achieving long lasting response in tumors such as ipilimumab (anti-CTLA-4) in advanced melanomas [ 226 , 238 ]), have long been seen as the ultimate treatment option for cancer patients.
As many other cancers, NSCLC belongs to the non-immunogenic tumors and therefore the identification of tumor specific immunogenic antigens for vaccine therapy presents a major challenge. The most promising results of vaccines for NSCLC patients have been observed in the adjuvant setting and in locally advanced NSCLC [ 190 ]. A Summary of the various vaccine therapy evaluated in NSCLC is shown in Table 9 .
Types of vaccine therapy for NSCLC.
| BEC2/BCG | glycosphingolipid GD3 vaccine | Combines a monoclonal antibody that mimics the glycosphingolipid GD3 with the adjuvant bacillus Calmette-Guerin |
|---|---|---|
| Allogeneic vaccine | Four irradiated lung cancer cell lines and an antisense plasmid against TGF-beta | |
| EGF vaccine | Cyclophosphamide and EGF | |
| Mesothelin vaccine | Genetically-engineered Listeria monocytogenes | |
| p53 vaccine | Produced from patients’ autologous peripheral blood mononuclear cells (PBMCs) | |
| MUC-1 vaccine | Synthetic peptide derived from the mucin 1 | |
| Melanoma-associated antigen A3 vaccine | Tumor specific antigen Mage-A3 | |
| PRAME vaccine | Recombinant PRAME protein combined with the AS15 Adjuvant System | |
| MVA-MUC1-IL2 vaccine | Modified vaccinia ankara encoding human MUC-1 antigen and interleukin-2 |
Mucin-1 (MUC1) is a glycoprotein present on normal epithelial tissue and in various cancers, including NSCLC [ 66 , 239 ]. A mutated MUC1protein overexpressed in cancer cells shows aberrant glycosylation pattern that is antigenically different from wild-type protein expressed on normal epithelial cells [ 66 ]. L-BLP25 is a synthetic vaccine against the core peptide of MUC1 combining the peptide with cyclophosphamide as an adjuvant [ 186 , 240 ]. Recently updated data from a phase IIB randomized study treating stage IIIB/IV NSCLC patients with L-BLP25 showed significant improvement in the vaccine group comparing to the supportive group (3-year survival rates: 31 vs. 17 % [ 241 ]).
The efficacy of TG4010, a recombinant vaccinia virus that combines the human MUC1 and interleukin-2 coding sequences [ 66 ], in combination with cisplatin and vinorelbine or as monotherapy has been investigated in a randomized phase II study for advanced NSCLC patients [ 242 , 243 ]. A subgroup with a detectable CD8+ T-cell response was able to generate an immune response against MUC1 and had longer median survival [ 243 ]. Data from another phase II study comparing the vaccine plus chemotherapy with chemotherapy alone in advanced NSCLC patients with confirmed MUC1 expression showed that higher numbers of activated NK cells might suppress DC and effector T-cells and result in decreased median OS rates as well as increased adverse effects. [ 244 ] [ 243 ].
CimaVax EGF is a new vaccine that is being developed for NSCLC treatment. This vaccine is made up of a low dose cyclophosphamide and EGF. It works as an immunoadjuvant to reduce the inhibition of Tsuppressor cells and to stimulate the production of anti-EGF antibodies that may inhibit EGF binding EGFR on cancer cells, and consequently decrease cancer cells growth [ 66 , 243 , 245 , 246 ].
A phase I study showed that the production of anti-EGF antibodies and serum EGF levels after use of the EGF-based vaccine, correlate with increased survival rates in NSCLC patients [ 247 ]. A randomized phase II trial comparing the CimaVax vaccine to best supportive care in stage IIIB/IV patients [ 248 ] showed minimal toxicity and good antigen responses in 51.4% of the patients although the median OS did not improve [ 66 ]. Longer median survival rates were seen in good antigen responders than in poor antigen responders (11.7 vs. 3.6 months) [ 243 ]. In addition, longer median OS rates were seen in patients with EGF levels below 168 pg/ml (13 vs. 5.6 months) and under 60 years (11.57 vs. 5.33 months, P=0.0124) [ 243 ]. The vaccine has been approved for clinical trial development in the US stage IIIB/IV patients [ 243 ].
C. Melanoma-associated antigen (MAGE)
Melanoma-associated antigen A3 vaccine (MAGE-A3) uses a tumor specific antigen, which is expressed in 35 % of NSCLC, most frequently in squamous cell carcinomas [ 186 ]. It ranges from 16 % in stage IA to 48 % in stage IIIB and may be associated with poor prognosis [ 66 , 249 , 250 ]. The MAGE-A3 vaccine, initially developed for metastatic melanoma patients, showed a positive sign of activity after 28 months in a phase II adjuvant therapy study in early stage NSCLC [ 251 ]. Prior to treatment, the NSCLC tumors were analyzed by gene expression profiling to identify a gene signature that correlates with the clinical activity of the vaccine [ 252 ]. The identified signature of genes related to the immune system overlapped with the gene set detected in the melanoma trial. Reduction in the relative recurrence risk after treatment with the MAGE-A3 vaccine showed a 2-fold increase for tumors containing the gene signature (57%), compared to the non-selected population (25%). This result underlines the value of the stratification of patient subpopulations and suggests that gene profiling for a certain tumor microenvironment or immune cells might have a predictive value in cancer immunotherapy [ 66 , 251 – 253 ].
D. TGF beta
The allogeneic vaccine belagenpumatucel-L combines four irradiated lung cancer cell lines (two adenocarcinoma, 1 squamous cell and one large cell carcinoma) with an antisense plasmid against TGF-b (transforming growth factor beta) [ 186 ], a poor prognostic factor in NSCLC. TGF-b2 suppresses dendritic cells, NK cells, and activated cytotoxic T lymphocytes and thereby may help tumors to escape immunosurveillance [ 186 ]. The use of four tumor cell lines in the belagenpumatucel-L vaccine increases the number of tumor antigens and the suppression of TGF-b expression through the antisense plasmid removes a major source of immune suppression at the injection site [ 186 ]. Preclinical data showed that the inhibition of TGF-beta2 could help to break the tolerance and to increase the immunogenicity of tumor vaccines [ 66 , 254 , 255 ]. Patients who received a high dose of the vaccine showed a significantly improved OS compared to low dose group in a randomized, dose-variable phase II trial with 75 NSCLC patients (stages II-IV) [ 255 ] [ 186 ].
Another important tumor antigen for vaccine therapy is PRAME (preferentially expressed antigen of melanoma), which has recently been shown to contribute to carcinogenesis in NSCLC [ 256 ]. As the name suggests, it was first detected in a melanoma patient [ 67 ], and is expressed in a variety of tumors [ 66 ]. PRAME seems to function via the suppression of the retinoic acid receptor (RAR), a signaling pathway which regulates cell death and cell cycle [ 66 , 257 ].. Overexpression of PRAME might be used by tumor cells to escape suppressive RAR signaling, thereby fostering tumor-progression [ 66 ]. Clinical data suggests that poor clinical outcome of some patient subpopulations correlates with PRAME expression in neuroblastoma [ 258 ] and breast cancer [ 259 – 261 ].
A new PRAME vaccine combining the purified recombinant PRAME protein with an adjuvant (liposomal preparation with the AS15 adjuvant system) has been developed. A currently ongoing phase II study (PEARL study) is evaluating the efficacy of the PRAME vaccine in resected NSCLC. This study enrolled patients radically resected for NSCLC, stages IA (T1b), IB, II or IIIA, and whose tumors exhibit PRAME expression. The primary endpoint of the study is disease-free survival (DFS) [ 257 , 262 ].
5. Small-cell lung cancer
Small cell lung cancer (SCLC) accounts for approximately 15% of all lung carcinomas [ 4 , 263 ]. The highest risk-factor for development of SCLC is smoking, and the decrease in percentage of smokers and amount of cigarettes smoked per person in the US might explain the recent decrease in SCLC incidence rates [ 263 ]. 30% of SCLC patients are diagnosed with limited-stage disease (LS-SCLC), where the cancer is confined to the hemithorax, the mediastinum, or the supraclavicular lymph nodes. 70% of SCLC patients are diagnosed with extensive-stage disease (ES-SCLC), with tumors spreading beyond the supraclavicular areas [ 4 , 263 ]. Although SCLC is initially more responsive to chemotherapy and radiation therapy than all other lung cancer types, it is very difficult to cure, due to its aggressive growth and its wide dissemination at the time of diagnosis.
As for other lung cancers, the treatment options for SCLC patients are determined by histology, stage, and general health and comorbidities of the patient. For patients with LS-SCLC, standard treatment options include platinum based chemotherapy and radiation therapy, combination chemotherapy alone, surgery followed by chemotherapy or chemoradiation therapy, and prophylactic cranial irradiation [ 264 ]. For patients with ES-SCLC patients, current treatment recommendations include combination chemotherapy, radiation therapy and prophylactic cranial irradiation [ 264 ]. For recurrent SCLC, the treatment options are chemotherapy and palliative therapy. Although chemotherapy and radiotherapy can lead to strong initial responses in SCLC, disease recurrence is common. Notwithstanding the improvements in diagnosis and therapy made during the past two decades, the current prognosis for patients with SCLC remains substandard. Untreated SCLC is the most aggressive of all type of lung cancers, with a median survival from diagnosis of only 2 to 4 months. The 2 years DFS, following treatment of SCLC patients, remains a dismal 10% [ 265 ]. Moreover, the OS at 5 years of all population of SCLC patients is merely 5% to 10% [ 4 , 265 – 267 ], with a better prognosis for patients with LS-SCLC (5-year survivals of 14%) than for patients with ES-SCLC [ 4 , 266 , 268 , 269 ]. In patients who showed complete response to chemoradiation, prophylactic cranial radiation may avert brain metastases recurrence, and thus, increase patients’ survival [ 270 , 271 ].
Although surgery or chemotherapy alone can improve survival in patients with LS-SCLC, a greater improvement in long-term survival has been shown with combination therapy [ 269 , 272 ]. Particularly combining chemotherapy with thoracic radiation therapy (TRT) increases OS by 5% compared to chemotherapy alone [ 4 , 273 – 276 ]. Although median survival of 6 to 12 months may be achieved in patients with ES-SCLC with the currently available therapy, long-term DFS is rare in these patients [ 4 , 275 , 276 ].
Targeted therapies in SCLC
A) vegf inhibitors.
Inhibition of circulating VEGF with bevacizumab has been studied in ES-SCLC. Chemotherapy naïve patients treated with cisplatin, irinotecan, and bevacizumab, showed an ORR of 75 %, a median OS of 11.6 months, and a median PFS of 7.0 months [ 277 ]. A study investigating cisplatin, etoposide, and bevacizumab in previously untreated ES-SCLC patients showed that a higher baseline level of vascular cell adhesion molecule (VCAM) was associated with a higher risk of progression or death, compared to lower levels of VCAM, but no other biomarkers could be correlated with treatment outcome [ 278 ].
Other VEGF inhibitors, including the multi-kinase inhibitors sorafenib, sunitinib, and cediranib, are currently under clinical investigations in SCLC. [ 279 ] [ 264 ]. Aflibercept (AVE0005) is a fully humanized protein that contains immunoglobulin domains from the two VEGF receptors VEGFR1/2, fused to the constant region of IgG1. These soluble receptors work as a VEGFR-trap and inhibit binding of VEGF to its common receptors. Aflibercept is currently investigated in combination with topotecan in previously treated ES-SCLC [ 264 ].
b) EGFR inhibitors
Mutation of the EGFR is less frequent in SCLC compared to NSCLC, and only around 4% of patients were shown to harbor the mutation [ 280 ]. In a phase II study, patients were stratified according to chemosensitive or chemo-refractory relapsed SCLC and treated with gefitinib. However, the abovementioned study could not demonstrate a gefitinib benefit for SCLC patients [ 281 ].
c) Bendamustine
This cytotoxic agent causes DNA breaks through its alkylating activity. Compared with other alkylating agents, bendamustine causes more extensive and durable DNA single- and double-strand breaks [ 264 ]. Combining bendamustine with carboplatin in treating ES-SCLC [ 282 ], the ORR was 72.7 %, with a median TTP of 5.2 months and median survival time of 8.3 months.. As a single agent in second- and third-line setting in patients with relapsed/refractory SCLC, Bendamustine is well tolerated and effective agent [ 283 ].
d) Immunotherapy
To date, only few studies have evaluated immunotherapy in the treatment of SCLC [ 243 ]. The BEC2/BCG vaccine combines a monoclonal antibody that mimics the glycosphingolipid GD3, which is selectively expressed in SCLC, with the adjuvant bacillus Calmette-Guerin [ 243 ]. The BEC2/BCG vaccine has been demonstrated to develop antibodies against GD3 in Melanoma patients. In an early clinical study, although only 30% of the SCLC patients (5/15, ES/LS-SCLC=8/7) developed measureable anti-GD3 antibodies, the median OS was 20.5 months (relapse-free survival was longer in patients who developed measurable anti-GD3 antibodies) [ 243 , 284 , 285 ]..
Another novel vaccine (INGN-225) targeting p53 protein was developed, and is currently under investigation for treatment of SCLC [ 243 ]. The INGN-225 vaccine is produced from patients’ autologous peripheral blood mononuclear cells (PBMCs). The autologous PBMCs are cultured in the presence of IL-4 and granulocyte-macrophage colony-stimulating factor (GM-CSF) prior to incubation with a viral construct containing wild-type p53 (adenovirus Ad.p53). In a preclinical study, dendritic cells transfected with Ad.p53 were able to induce cytotoxic T lymphocytes following vaccination [ 243 ]. In a recent phase I/II study.14 of the 54 enrolled ES-SCLC patients demonstrated a positive immune response and showed increased median survival (12.6 vs. 8.2 months, P=0.131) [ 243 , 286 ]. Among those responsive patients, 78.6% responded to second-line chemotherapy treatment.
6. Palliative Care for Patients with Lung Cancer
A high percentage of lung cancer patients suffers from substantial symptom burden, including fatigue, loss of appetite and weight loss, as well as dyspnea, hemoptysis, and chest pain [ 287 ]. Better quality of life, lower depressive symptoms, higher median survival and fewer needs on aggressive end-of-life care were observed in patients receiving early palliative care combined with standard oncologic care compared to standard oncologic alone for metastatic NSCLC.[ 287 ]. Megestrol acetate (MA), an appetite stimulant, is one of the supporting treatments that have shown success in treating cancer-related anorexia (CRA) and improve quality of life. Combining MA with olanzapine (OLN) for the treatment of CRA in 80 patients with either advanced gastrointestinal cancer or advanced lung cancer patients (stages III and IV) resulted in a significant improvement of mean symptom scores as measured by the MD Anderson Symptom Inventory (MDASI) [ 287 ] suggesting that combination MA and OLN may be an effective palliative therapy for patients with CRA [ 288 ]..
7. Mesothelioma
Malignant Mesothelioma (MM) is an extremely aggressive tumor affecting about 3,000 new patients in the United States annually [ 289 ]. The incidence of the disease in the US is expected to rise steadily and peak with about 85,000 new MM cases over the course of the next 20 years [ 290 , 291 ]. MM arises from the surface serosal cells of the pleura and, less frequently, from the peritoneum. Exposure to asbestos is a wellestablished cause, with occupational exposure being documented in 70–80% of MM patients [ 292 – 294 ]. MM is sub-typed into three forms according to the histological morphology: epithelial, sarcomatoid, and biphasic. Diffuse MM comprises about 75% of mesotheliomas diagnosed [ 295 ].
Treatment of MM with surgery, chemotherapy, or radiation therapy is rarely curative. Clinical trials of single modality treatment with extrapleural pneumonectomy or pleurectomy, chemotherapy or radiation therapy, have not shown significant improvement in survival compared with supportive treatment. Median survival has ranged from 10 to17 months [ 296 ]. Sugarbaker et al. reported a 15% 5-year survival with multimodal therapy and a 25% 5-year survival in patients who underwent complete surgical resection [ 297 ]. Recent trials of new-generation platinum- and pemetrexed based regimens have reported encouraging results. In particular, a phase II trial of pemetrexed plus cisplatin for MM reported a median survival of 12 months compared with nine months after treatment with cisplatin alone [ 298 ]. Despite these promising results, long-term survival with currently available treatment is rare. Therefore, novel meaningful therapies for MM are urgently needed. Recent advances have shown that tumors carrying activating mutations in some cellular proto-oncogenes are particularly sensitive to targeted therapies directed against the mutant proteins [ 14 , 16 , 299 – 307 ].
Role of immunotherapy in malignant mesothelioma
A number of immunotherapy strategies have been tested in mesothelioma patients, with varying degrees of success.
a) Mesothelin
Mesothelin is an immunogenic glycoprotein specifically overexpressed in malignant mesothelioma, NSCLC, ovarian, and pancreatic cancers [ 186 ]. CRS-207, is a genetically-engineered, double-deleted bactria Listeria monocytogenes strain expressing the human mesothelin [ 186 ]. Phagocytic cells, such as macrophages and dendritic cells, take up CRS-207, and mesothelin is subsequently expressed and processed through the MHC I presentation pathway. This process is predicted to activate T cells in order to attack mesothelin-positive mesothelioma cells. In preclinical studies, CRS-207 was shown to elicit anti-mesothelin cell-mediated immunity [ 186 ]. In a phase I dose-escalation study treating patients with advanced mesothelin expression and treatmentrefractory cancers, with CRS-207, mesothelin-specific T-cell responses were in one of five patients with mesothelioma, with 15 months or more survival after the first dose [ 186 ]. Patients who received sequential CRS-207 treatment with prior immunotherapy or subsequent local radiation therapy benefited the most. CRS-207 is currently investigated in combination with first-line chemotherapy in mesothelioma patients.
b) WT1 analogue peptide vaccine
The transcription factor Wilms’ tumor suppressor gene 1 (WT1) is frequently overexpressed in mesothelioma and other solid and hematopoietic tumors [ 186 ]. Recently, a multivalent WT1 peptide analog vaccine was developed [ 186 ]. A CD-4+ T-cell proliferation to WT1-specific peptides was seen in six of nine patients, and a CD8+ T-cell response was detected in six of six HLA-A0201 patients in an early study investigating the WT1 peptide analog vaccine in MM and NSCLC patients. Stimulated T-cells also showed cytotoxicity against WT1 positive cells [ 308 ]. A subsequent randomized phase II study is currently investigating the adjuvant WT1 analog peptide vaccine in MM patients who have completed combined modality therapy [ 309 ].
c) Dendritic cell vaccine
Dendritic cells (DCs) are the most potent antigen-producing cells, capable of sensitizing T cells to both new and recall antigens. DC-based cancer immunotherapy is aimed at using these cells to prime specific antitumor immunity through the generation of effector cells that attack and kill tumors. Dendritic cells can be matured using a standard cytokine cocktail and pulsed with autologous tumor cell lysates, which presents a source of tumor antigens for immunotherapy [ 186 ].
A phase I study has shown that the DC vaccine was well tolerated in newly diagnosed MM patients, who experienced a partial response or stable disease after previous combination chemotherapy [ 310 ]. A significant humoral response to keyhole limpet hemocyanin, a marker for immune response, was detected in serum samples from all patients. Nine patients had successful lymphocyte activation according to increased levels of granzyme B expressing CD3 and CD8 T-cells. However, no correlation between the clinical responses and the humoral or cellular immune responses was observed [ 186 ].
Another small Phase I/II study evaluated the feasibility, safety, immunogenicity, and clinical efficacy of consolidation treatment with autologous DC transfected with mRNA encoding the malignant mesothelioma-associated WT1 antigen [ 311 ]. Ten patients with unresectable MM and non-progressive disease after platinum/pemetrexed-based chemotherapy underwent leukapheresis: Isolating CD14+ monocytes to produce mature DC, followed by electroporation of WT1 mRNA and biweekly intradermal vaccinations [ 311 ].. DC vaccination was well tolerated; no systemic toxicity was recorded. The 6-, 12- and 18-month survival rates were 100%, 90%, and 75%, respectively. In vivo evidence of vaccine-elicited immunity to the DC vaccine administered was obtained in nine of the ten enrolled patients. The OS data suggest that adjuvant DC-based immunotherapy may provide a clinical benefit for patients with MM [ 311 ].
8. The future of thoracic malignancies
Despite the intensive research and development of several new targeted agents and immunotherapies, survival rates for lung cancer and mesothelioma patients remain dismal. More studies are still needed to identify the underlying genetic alterations and predispositions affecting clinical outcome. Early detection and treatment of these cancers may help dramatically in the improvement of patients’ survival. Over 60% of lung cancer patients are in fact diagnosed at late stages of the disease, where current treatment modalities are unlikely to be effective. The 5-year OS rate is less than 10 % in patients with advanced disease, and greater than 70 % in patients with stage 1 disease. Reliable biomarkers are greatly needed to predict sensitivity to each therapeutic modality in thoracic malignancies that could support optimal selection of treatment on individual patient basis as well as for early detection of lung cancer that could improve its prognosis.
Acknowledgments
This work was supported in part by The Doctors Cancer Foundation grant A118560, the American Cancer Society grant IRG-97-150-13, and The NIH/NCI U01 grant NIH Grants 5U01CA168878.
Abbreviations
| SCLC | Small-Cell Lung Carcinomas |
| NSCLC | Non-Small Cell Lung Carcinomas |
| MM | Malignant Mesothelioma |
| AdenoCA | Adenocarcinomas |
| SQCLC | Squamous Cell Lung Cancers |
| LCAC | Large Cell Anaplastic Carcinomas |
| OS | Overall Survival |
| PFS | Progression-Free Survival |
| TTP | Time to Progression |
| ORR | Objective Response Rate |
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Competing interests :
An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
- My Bibliography
- Collections
- Citation manager
Save citation to file
Email citation, add to collections.
- Create a new collection
- Add to an existing collection
Add to My Bibliography
Your saved search, create a file for external citation management software, your rss feed.
- Search in PubMed
- Search in NLM Catalog
- Add to Search
The American Cancer Society National Lung Cancer Roundtable strategic plan: Current challenges and future directions for shared decision making for lung cancer screening
Affiliations.
- 1 The University of Texas MD Anderson Cancer Center, Houston, Texas, USA.
- 2 Department of Medical Oncology, Thomas Jefferson University, Philadelphia, Pennsylvania, USA.
- 3 University of Michigan, Ann Arbor, Michigan, USA.
- 4 Veterans Affairs Ann Arbor Center for Clinical Management Research, University of Michigan Medical School, Institute for Health Policy Innovation, Ann Arbor, Michigan, USA.
- 5 University of Iowa Carver College of Medicine, Iowa City, Iowa, USA.
- 6 University of Iowa Holden Comprehensive Cancer Center, Iowa City, Iowa, USA.
- 7 Division of Cancer Epidemiology and Genetics, National Cancer Institute, Bethesda, Maryland, USA.
- 8 Respiratory Institute, Cleveland Clinic, Cleveland, Ohio, USA.
- 9 Informed Consulting LLC, Marblehead, Massachusetts, USA.
- 10 Division of General Medicine and Clinical Epidemiology, University of North Carolina at Chapel Hill, Chapel Hill, North Carolina, USA.
- 11 Lineberger Comprehensive Cancer Center, University of North Carolina at Chapel Hill, Chapel Hill, North Carolina, USA.
- 12 Ralph H. Johnson Veterans Affairs Medical Center, Charleston, South Carolina, USA.
- 13 Medical University of South Carolina, Charleston, South Carolina, USA.
- 14 Center for Early Cancer Detection Science, American Cancer Society, Atlanta, Georgia, USA.
- 15 Center for Healthcare Organization & Implementation Research, VA Boston Healthcare System, Boston, Massachusetts, USA.
- 16 The Pulmonary Center, Boston University School of Medicine, Boston, Massachusetts, USA.
- 17 National Center for Lung Cancer Screening, Veterans Health Administration, Washington, District of Columbia, USA.
- PMID: 39302231
- DOI: 10.1002/cncr.35382
Shared decision making (SDM) between health care professionals and patients is essential to help patients make well informed choices about lung cancer screening (LCS). Patients who participate in SDM have greater LCS knowledge, reduced decisional conflict, and improved adherence to annual screening compared with patients who do not participate in SDM. SDM tools are acceptable to patients and clinicians. The importance of SDM in LCS is emphasized in recommendations from professional organizations and highlighted as a priority in the 2022 President's Cancer Panel Report. The updated 2022 national coverage determination from the Centers for Medicare & Medicaid Services reaffirms the value of SDM in offering LCS to eligible beneficiaries. The Shared Decision-Making Task Group of the American Cancer Society National Lung Cancer Roundtable undertook a group consensus process to identify priorities for research and implementation related to SDM for LCS and then evaluated current knowledge in these areas. Priority areas included: (1) developing feasible, adaptable SDM training programs for health care professionals; (2) understanding the impact of alternative health system LCS models on SDM practice and outcomes; (3) developing and evaluating new patient decision aids for use with diverse populations and in varied settings; (4) offering conceptual clarity about what constitutes a high-quality decision and developing appropriate quality measures; and (5) studying the use of prediction-augmented screening to support SDM in practice. Gaps in current research in all areas were observed. The authors conclude with a research and implementation agenda to advance the quality and implementation of SDM for persons who might benefit from LCS.
Keywords: cancer care delivery; cancer screening; decision aids; decision making; lung neoplasms; shared.
© 2024 American Cancer Society. This article has been contributed to by U.S. Government employees and their work is in the public domain in the USA.
PubMed Disclaimer
- Aberle DR, Adams AM, et al. Reduced lung‐cancer mortality with low‐dose computed tomographic screening.N Engl J Med. 2011;365(5):395‐409. doi:10.1056/NEJMoa1102873
- Moyer VA, U.S. Preventive Services Task Force. Screening for lung cancer: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med. 2014;160(5):330‐338. doi:10.7326/M13‐2771
- Wender R, Fontham ET, Barrera E Jr, et al. American Cancer Society lung cancer screening guidelines. CA Cancer J Clin. 2013;63(2):107‐117. doi:10.3322/caac.21172
- Wood DE. National Comprehensive Cancer Network (NCCN) Clinical Practice Guidelines for Lung Cancer Screening. Thorac Surg Clin. 2015;25(2):185‐197. doi:10.1016/j.thorsurg.2014.12.003
- Bach PB, Mirkin JN, Oliver TK, et al. Benefits and harms of CT screening for lung cancer: a systematic review. JAMA. 2012;307(22):2418‐2429. doi:10.1001/jama.2012.5521
Publication types
- Search in MeSH
Related information
Grants and funding.
- American Cancer Society National Lung Cancer Roundtable
LinkOut - more resources
Full text sources.

- Citation Manager
NCBI Literature Resources
MeSH PMC Bookshelf Disclaimer
The PubMed wordmark and PubMed logo are registered trademarks of the U.S. Department of Health and Human Services (HHS). Unauthorized use of these marks is strictly prohibited.
Lung Cancer—Patient Version
Lung cancer includes two main types: non-small cell lung cancer and small cell lung cancer. Smoking causes most lung cancers, but nonsmokers can also develop lung cancer. Explore the links on this page to learn more about lung cancer treatment, prevention, screening, statistics, research, clinical trials, and more.
PDQ Treatment Information for Patients
- Non-Small Cell Lung Cancer Treatment
- Small Cell Lung Cancer Treatment
- Pleuropulmonary Blastoma
- Childhood Tracheobronchial Tumors
More information
- Late Effects of Treatment for Childhood Cancer (PDQ®)
- Drugs Approved for Lung Cancer
- Clinical Trials to Treat Non-Small Cell Lung Cancer
- Clinical Trials to Treat Small Cell Lung Cancer
Causes & Prevention
Pdq prevention information for patients.
- Lung Cancer Prevention
- Cancer-Causing Substances in the Environment
- Smokefree.gov
- Clinical Trials to Prevent Non-Small Cell Lung Cancer
- Clinical Trials to Prevent Small Cell Lung Cancer
PDQ Screening Information for Patients
- Lung Cancer Screening
- National Lung Screening Trial Patient and Physician Guide pdf file (39 KB)
- Clinical Trials to Screen for Non-Small Cell Lung Cancer
- Clinical Trials to Screen for Small Cell Lung Cancer

Coping with Cancer
The information in this section is meant to help you cope with the many issues and concerns that occur when you have cancer.
For the best browsing experience please enable JavaScript. Instructions for Microsoft Edge and Internet Explorer , other browsers

- About cancer
- Get involved
- Our research
- Funding for researchers
- Cancer types
- Cancer in general
- Causes of cancer
- Coping with cancer
- Health professionals
- Do your own fundraising
- By cancer type
- By cancer subject
- Our funding schemes
- Applying for funding
- Managing your research grant
- How we deliver our research
- Find a shop
- Shop online
- Our eBay shop
- Our organisation
- Current jobs
- Cancer news

Metastatic lung cancer
Metastatic lung cancer means that the cancer has spread from where it started in the lung to other parts of the body. It is also called advanced lung cancer.
What is metastatic lung cancer?
Metastatic lung cancer is when the cancer has spread from the lung and gone to another part of the body.
Symptoms of metastatic lung cancer
Symptoms of metastatic lung cancer can vary depending on where the cancer has spread to.
Treatment for lung cancer
Your treatment depends on several factors. These include what type of lung cancer you have, how big it is and whether it has spread (the stage). It also depends on your general health.
Living with lung cancer
There is support available during and after treatment to help you cope. This includes support from your clinical nurse specialist, cancer charities, community services, and family and friends.
It’s a worrying time for many people and we want to be there for you whenever - and wherever - you need us. Cancer Chat is our fully moderated forum where you can talk to others affected by cancer, share experiences, and get support. Cancer Chat is free to join and available 24 hours a day.
Visit the Cancer Chat forum
About Cancer generously supported by Dangoor Education since 2010.

Find a clinical trial
Search our clinical trials database for all cancer trials and studies recruiting in the UK
Cancer Chat forum
Talk to other people affected by cancer
Nurse helpline 0808 800 4040
Questions about cancer? Call freephone 9 to 5 Monday to Friday or email us
We use cookies to understand how you use our site and to improve your experience. This includes personalizing content and advertising. To learn more, click here . By continuing to use our site, you accept our use of cookies, revised Privacy Policy and Terms of Service .

New to Zacks? Get started here.
Member Sign In
Don't Know Your Password?

- Zacks #1 Rank
- Zacks Industry Rank
- Zacks Sector Rank
- Equity Research
- Mutual Funds
- Mutual Fund Screener
- ETF Screener
- Earnings Calendar
- Earnings Releases
- Earnings ESP
- Earnings ESP Filter
- Stock Screener
- Premium Screens
- Basic Screens
- Thematic Screens
- Research Wizard
- Personal Finance
- Money Management
- Retirement Planning
- Tax Information
- My Portfolio
- Create Portfolio
- Style Scores
- Testimonials
- Zacks.com Tutorial
Services Overview
- Zacks Ultimate
- Zacks Investor Collection
- Zacks Premium
Investor Services
- ETF Investor
- Home Run Investor
- Income Investor
- Stocks Under $10
- Value Investor
- Top 10 Stocks
Other Services
- Method for Trading
- Zacks Confidential
Trading Services
- Black Box Trader
- Counterstrike
- Headline Trader
- Insider Trader
- Large-Cap Trader
- Options Trader
- Short Sell List
- Surprise Trader
- Alternative Energy

You are being directed to ZacksTrade, a division of LBMZ Securities and licensed broker-dealer. ZacksTrade and Zacks.com are separate companies. The web link between the two companies is not a solicitation or offer to invest in a particular security or type of security. ZacksTrade does not endorse or adopt any particular investment strategy, any analyst opinion/rating/report or any approach to evaluating individual securities.
If you wish to go to ZacksTrade, click OK . If you do not, click Cancel.
Image: Bigstock
Study: Merck and Daiichi's Lung Cancer Treatment Shows Promise
Merck ( MRK Quick Quote MRK - Free Report ) announced that a phase study evaluating its Daiichi Sankyo-partnered HER3-directed DXd antibody drug conjugate (ADC), patritumab deruxtecan, for treating EGFR-mutated non-small cell lung cancer (NSCLC), met its primary endpoint of progression-free survival (PFS).
The HERTHENA-Lung02 evaluated the efficacy and safety of patritumab deruxtecan versus pemetrexed and platinum chemotherapy for treating locally advanced or metastatic EGFR-mutated NSCLC in patients who had received prior EGFR tyrosine kinase inhibitor treatment. In the study, patritumab deruxtecan demonstrated a statistically significant improvement in PFS — the study’s primary endpoint — versus platinum plus pemetrexed induction chemotherapy.
As regards overall survival, a key secondary endpoint of the study, the data were immature at the time of the analysis. The study will continue to further assess overall survival.
Patients with metastatic EGFR-mutated NSCLC who are initially treated with an EGFR TKI sometimes experience disease progression. This creates a need for therapies like patritumab deruxtecan as treatment options for this type of lung cancer in the second-line setting are limited.
Merck plans to discuss the data with regulatory authorities to decide the next steps.
Merck’s stock has risen 8.5% so far this year compared with an increase of 25.9% for the industry .

More on MRK’s Patritumab Deruxtecan
A biologics license application (BLA) seeking accelerated approval for patritumab deruxtecan for previously-treated EGFR-mutated NSCLC is already under review in the United States supported by data from the HERTHENA-Lung01 pivotal phase II study.
In June, the FDA issued a complete response letter to the BLA based on observations made after the inspection of a third-party manufacturing facility. The FDA has not requested any additional efficacy/safety studies, nor has it identified any issues related to the safety and efficacy of the candidate. Merck is working closely with the FDA and the third-party manufacturer to resolve the issue.
Merck’s Deal With Daiichi Sankyo
Merck acquired global co-development and co-commercialization rights to patritumab deruxtecan/MK-1022 and two other ADCs, raludotatug deruxtecan/MK-5909 and ifinatamab deruxtecan/MK-2400 from Japan’s Daiichi Sankyo in October last year for a total potential consideration of up to $22 billion. While raludotatug deruxtecan is being developed in phase II/III study for ovarian cancer, ifinatamab deruxtecan is being studied for small-cell lung cancer in phase III and colorectal, bladder, endometrial and head and neck cancers in phase II.
Daiichi Sankyo has retained exclusive rights for the development of the candidates in Japan. In August this year, Merck expanded its deal with Daiichi to co-develop and co-commercialize MK-6070, an investigational T-cell engager targeting delta-like ligand 3, which it obtained from its acquisition of Harpoon Therapeutics.
Other Companies Making ADC Products
ADCs are being considered a disruptive innovation in the pharmaceutical industry as these will enable better treatment of cancer by harnessing the targeting power of antibodies to deliver cytotoxic molecule drugs to tumors.
Daiichi Sankyo has six ADCs in clinical development across multiple types of cancer, being developed utilizing its DXd ADC technology. It markets Enhertu, a HER2-directed ADC for HER2-mutated breast, lung and gastric cancers, in partnership with AstraZeneca ( AZN Quick Quote AZN - Free Report ) . Daiichi Sankyo and AstraZeneca have also developed datopotamab deruxtecan (Dato-DXd), a TROP2-directed ADC. Dato-DXd is under FDA review for advanced nonsquamous NSCLC as well as previously treated metastatic HR-positive, HER2-negative breast cancer. The sixth ADC candidate is DS-3939, a TA-MUC1-directed ADC, which Daiichi Sankyo is developing on its own.
Pfizer ( PFE Quick Quote PFE - Free Report ) also has a strong portfolio of ADC drugs, which were added with last year’s acquisition of Seagen. The December 2023 acquisition of Seagen added four ADCs — Adcetris, Padcev, Tukysa and Tivdak — to Pfizer’s portfolio. Adcetris, Padcev, Tukysa and Tivdak contributed $279 million, $394 million, $121 million and $33 million, respectively, to Pfizer’s oncology revenues in the second quarter. Pfizer is particularly witnessing strong demand for Padcev.
MRK’s Rank and Stock to Consider
Merck has a Zacks Rank #4 (Sell) currently.
Merck & Co., Inc. Stock Price and Consensus

Merck & Co., Inc. price-consensus-chart | Merck & Co., Inc. Quote
A top-ranked large drugmaker is Eli Lilly ( LLY Quick Quote LLY - Free Report ) , carrying a Zacks Rank #1 (Strong Buy). You can see the complete list of today’s Zacks #1 Rank stocks here .
Earnings estimates for 2024 have risen from $13.71 to $16.49 per share over the past 60 days. For 2025, earnings estimates have risen from $19.42 to $23.97 per share over the same timeframe. LLY’s stock is up 55.5% year to date.
Lilly beat estimates in each of the last four quarters, delivering a four-quarter average earnings surprise of 69.07%.
See More Zacks Research for These Tickers
Normally $25 each - click below to receive one report free:.
AstraZeneca PLC (AZN) - free report >>
Pfizer Inc. (PFE) - free report >>
Merck & Co., Inc. (MRK) - free report >>
Eli Lilly and Company (LLY) - free report >>
Published in
This file is used for Yahoo remarketing pixel add
Due to inactivity, you will be signed out in approximately:
23/102 DOAC thromboprophylaxis during treatment for lung cancer
This opportunity is now closed.
- Opportunity status: Closed
- Type: Programme
- Opening date: 27 July 2023
- Closing date: 24 January 2024 at 1:00 pm
- Reference ID: 33882
The Health Technology Assessment (HTA) Programme is accepting Stage 1 applications to their commissioned workstream for this primary research topic.
In order to apply you will need to carefully review the:
- Commissioning brief
- Stage 1 application supporting information
- Stage 1 guidance notes
Applications received by the advertised closing date will be considered at a first-stage funding committee meeting, and successful applicants will then be invited to submit a Stage 2 application. Applicants will have 8 weeks to complete and submit their Stage 2 application form, which will then be considered at the following HTA funding committee meeting. For more information, please read the commissioning brief.
All primary research projects are expected to establish a programme appointed Study Steering Committee and it is important that you read the Research Governance Guidance before completing your application. Costs incurred by this committee should be included in the budget as appropriate.
You can review information about this funding opportunity in our application system
Supporting Information
- HTA tips for applicants
- Word version of the Stage 1 application form
Please note that the Word version of the Stage 1 application form is to be used as a guide and to assist with completion of the online application form only, for example to see how many characters are accepted in each section and how the printed complete form is laid out. Please do not try to use this as an application form, you must apply using the online form available through the links available when calls are open. You should also refer to the application form guidance notes.
Contact Details
- For help with your application contact [email protected]
- For more information about the funding Programme, visit the HTA Page
- Got a research idea and not sure how to turn it into a funding application? The free NIHR Research Support Service (RSS) supports researchers in England to apply for funding, and to develop and deliver clinical and applied health, social care and public health research post award. Find out how the RSS can help you .
Studies within a trial or a review
- This funding opportunity is eligible for researchers to embed a study within a trial or a review . These studies should evaluate alternative ways of undertaking a trial or a review process.
Share this page

IMAGES
VIDEO
COMMENTS
NCI funds and oversees both early- and late-phase clinical trials to develop new treatments and improve patient care. Trials are available for both non-small cell lung cancer treatment and small cell lung cancer treatment. Lung Cancer Research Results. The following are some of our latest news articles on lung cancer research:
The Lung Cancer Master Protocol, or Lung-MAP, is a precision medicine research study for people with advanced non-small cell lung cancer that has continued to grow after treatment. Patients are assigned to different study drug combinations based on the results of genomic profiling of their tumors.
Lung cancer is one of the most frequently diagnosed cancers and the leading cause of cancer-related deaths worldwide with an estimated 2 million new cases and 1·76 million deaths per year. Substantial improvements in our understanding of disease biology, application of predictive biomarkers, and refinements in treatment have led to remarkable progress in the past two decades and transformed ...
Jianxing He and Wenhua Liang's research group from Guangzhou Institute of Respiratory Health launched a clinical study (CCTC-1901) in relation to sintilimab treatment in early-stage multiple primary lung cancer (MPLC) with ground-glass density . This is a single-center, single-arm, prospective Simon's two-stage design phase II clinical ...
The review paper accounts for the novel and current immunotherapy and targeted therapy available for lung cancer in clinical trials and in the research phases in depth. Special attention is being paid to mark out single or multiple genes that are required for malignancy and survival while developing targeted therapies for lung cancer treatment ...
Introduction. Lung cancer is one of the most prevalent and deadly diseases globally, necessitating continuous research and exploration of innovative treatment strategies. In recent years, lung cancer research has witnessed remarkable advancements, revolutionising the approaches to diagnosis, therapy, and prevention [1, 2]. This manuscript ...
Abstract. Despite a global pandemic that continued to inflict chaos and confusion on the world, resulting in fewer cancer screenings and delayed surgeries, remarkable lung cancer treatment advancements were made in 2021. From immunotherapy in the adjuvant setting to the approval of the first-in-class, highly selective inhibitor of KRAS G12C ...
Dr. Li presented results from a new clinical trial, CodeBreaK101, suggesting that sotorasib shows great potential as a first-line treatment for certain lung cancer patients when it is combined with the chemotherapy drugs carboplatin and pemetrexed.. Most people responded to the drug combination — meaning their tumor shrank or disappeared — and the treatment appeared to be safe.
In this Review, the authors provide an overview of the epidemiology, diagnosis, prognosis and treatment of patients with lung cancers harbouring these rare alterations. The importance of expedited ...
Lung cancer articles from across Nature Portfolio. Lung cancer arises in tissues of the lung, usually in the cells lining air passages. The two main types are small-cell lung cancer and non-small ...
Research into causes, prevention and treatment of lung cancer is ongoing in many medical centers. Read about the latest in lung cancer research. ... but smoking is still responsible for most lung cancer deaths. Research is continuing on: Ways to help people quit smoking and stay tobacco-free through counseling, ...
The more can do at the beginning of treatment to essentially cure the cancer is where our research lies and where these exciting new changes come into play." Screening for Early Stage Lung Cancer. The identification of early stage lung cancer has increased significantly in the past five years because of low-dose CT screening for smokers.
Pragmatica-Lung is a phase 3 clinical trial for people with non-small cell lung cancer that has spread beyond the lungs (stage 4 cancer). Researchers seek to confirm if the combination of pembrolizumab and ramucirumab helps people with lung cancer live longer than if they were on standard chemotherapy. This trial is part of a broader effort by NCI and FDA to modernize clinical trials.
Lung cancer persists as the main cause of cancer death in people globally, driven by the ongoing tobacco epidemic and the emergence of risk factors such as ionising radiation exposure and ambient pollution. Nearly 2 million people die of lung cancer each year, a far higher death rate than other common cancer types. However, developments over the past 10 years have dramatically improved the ...
Yet despite decades of research, scientists kept hitting roadblocks. That's because the protein's smooth, round shape lacked notches or grooves where drugs could attach. The mutant protein eventually was given a label by scientists: undruggable. In the early 2000s, molecular testing for lung cancer started becoming commonplace.
Up until 2000, lung cancer was a highly lethal disease. Treatment options were extremely limited, with only platinum-based chemotherapy agents available, resulting in a 1-year survival rate of 33% in patients with advanced disease. The approval of the first breakthrough EGFR-targeted therapy in 2003 and the identification of specific somatic driver mutations on the EGFR gene in 2004, which ...
Help us end cancer as we know it, for everyone. Donate with Confidence. Cancer information, answers, and hope. Available every minute of every day. Get an overview of the types of lung cancer, recent key statistics, and learn more about lung cancer research.
The American Lung Association is committed to funding lung cancer research. As part of our Awards and Grants Program, a large part of funds goes toward research on lung cancer prevention, treatment and quality of life. The primary goal of this lung cancer research program is simple: improve and save lives.
1 Introduction. Although lung cancer incidence has declined in the United States in recent years [], a disease including non-small cell lung cancer (NSCLC) and small cell lung cancer (SCLC), remains the number 1 cause of cancer-related death [].Over the past 20 years, significant improvements have been made in NSCLC treatment with the advent of targeted therapies [3-6] for genetic ...
Lung Cancer Research. As one of the world's largest cancer research centers, MD Anderson is leading the investigation into new methods of lung cancer diagnosis and treatment. Each patient benefits from the most advanced research, delivered as rapidly as possible. Our current lung cancer research efforts include:
Treating stage IIIA NSCLC. The initial treatment for stage IIIA NSCLC may include some combination of radiation therapy, chemotherapy (chemo), immunotherapy, and/or surgery. For this reason, planning treatment for stage IIIA NSCLC often requires input from a medical oncologist, radiation oncologist, and a thoracic surgeon.
Using Advanced AI to Improve Predictions of Treatment Side Effects in Lung Cancer: This research uses cutting-edge artificial intelligence (AI) techniques, including large language models like ChatGPT-4, to better predict potential side effects in lung cancer patients undergoing proton therapy.
Treatment for lung cancer usually begins with surgery to remove the cancer. If the cancer is very large or has spread to other parts of the body, surgery may not be possible. Treatment might start with medicine and radiation instead. Your healthcare team considers many factors when creating a treatment plan.
In addition, multiple inherited and acquired mechanisms of susceptibility to lung cancer have been proposed. Lung cancer is divided into two broad histologic classes, which grow and spread differently: small-cell lung carcinomas (SCLC) and non-small cell lung carcinomas (NSCLC). Treatment options for lung cancer include surgery, radiation ...
Affiliations 1 The University of Texas MD Anderson Cancer Center, Houston, Texas, USA.; 2 Department of Medical Oncology, Thomas Jefferson University, Philadelphia, Pennsylvania, USA.; 3 University of Michigan, Ann Arbor, Michigan, USA.; 4 Veterans Affairs Ann Arbor Center for Clinical Management Research, University of Michigan Medical School, Institute for Health Policy Innovation, Ann Arbor ...
Lung cancer includes two main types: non-small cell lung cancer and small cell lung cancer. Smoking causes most lung cancers, but nonsmokers can also develop lung cancer. Explore the links on this page to learn more about lung cancer treatment, prevention, screening, statistics, research, clinical trials, and more.
The results of the randomized phase 2 clinical trial, which included187 individuals who experienced cachexia with lung, pancreatic, or colorectal cancer, were reported in the New England Journal ...
Treatment for lung cancer. Your treatment depends on several factors. These include what type of lung cancer you have, how big it is and whether it has spread (the stage). ... Cancer Research UK is a registered charity in England and Wales (1089464), Scotland (SC041666), the Isle of Man (1103) and Jersey (247). A company limited by guarantee ...
This creates a need for therapies like patritumab deruxtecan as treatment options for this type of lung cancer in the second-line setting are limited. Merck plans to discuss the data with ...
23/102 DOAC thromboprophylaxis during treatment for lung cancer. Back to all funding opportunities. Download Print document. ... The free NIHR Research Support Service (RSS) supports researchers in England to apply for funding, and to develop and deliver clinical and applied health, social care and public health research post award. ...