[Updated] CBSE Class 12 Chemistry Lab Manual 2024-25 Session in PDF

CBSE Class 12 Chemistry Lab Manual is provided to the students to score well in the examinations. For a subject like Chemistry, it is important to remember the right reactions and what they result in is vital. Students must concentrate on Chemistry Practicals because it has been allocated 30 marks. They must try to get full marks in this section to increase their overall marks in the CBSE class 12 examination. Students should study the laws and theories before performing the experiments and are suggested to revise their practical notes before their examination. That’s why we are providing a Class 12 Chemistry Lab Manual for practice purposes to obtain a great score in the final examination.
Before we discuss the Class 12 Lab Manual, let us check the CBSE Class 12 Summary; Below, we have mentioned the complete CBSE Class 12 Summary. The student is advised to check out to complete the summary.
| 12th | |
| Chemistry | |
| CBSE | |
| Lab Manual | |

Class 12 Chemistry Lab Manual
Below we have mentioned the CBSE Chemistry Lab Manual for Class 12. Students have checked the complete Class 12 Chemistry Lab Manual in pdf for a great score in the final examination.
Example of Lab Manual
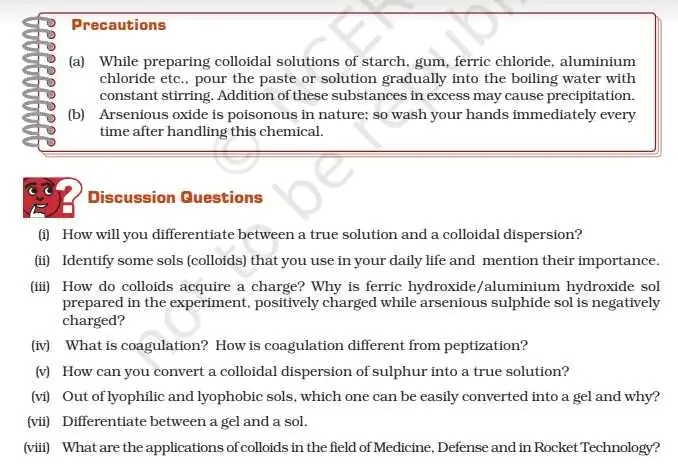
NOTE : The links given below for downloading Class 12 Chemistry Lab Manual in pdf format
| Unit 1 | Colloids | |
| Unit 2 | Chemical Kinetics | |
| Unit 3 | Thermochemistry | |
| Unit 4 | Electrochemistry | |
| Unit 5 | Chromatography | |
| Unit 6 | Titrimetric Analysis (Redox Reactions) | |
| Unit 7 | Systematic Qualitative Analysis | |
| Unit 8 | Tests for Functional Groups in Organic Compounds | |
| Unit 9 | Preparation of Inorganic Compounds | |
| Unit 10 | Preparation of Organic Compounds |
Class 12 Chemistry Lab Manual Projects
| 1 |
Class 12 Chemistry Syllabus
Check out the latest CBSE NCERT Class 12 Chemistry Syllabus. The syllabus is for the academic year 2024-25 sessions. First of all, check the CBSE Class 12 Chemistry Exam Pattern. Students should review the complete syllabus and exam pattern with the marking scheme.
Class 12 Chemistry Exam Pattern
In this section, we have mentioned the Class 12 Chemistry Exam Pattern. Students can check the Class 12 Chemistry Exam Pattern for the academic year 2024-25.
| Unit I | Solid State | 10 | 23 |
| Unit II | Solutions | 10 | “ |
| Unit III | Electrochemistry | 12 | “ |
| Unit IV | Chemical Kinetics | 10 | “ |
| Unit V | Surface Chemistry | 08 | “ |
| Unit VI | General Principles and Processes of Isolation of Elements | 08 | 19 |
| Unit VII | p -Block Elements | 12 | “ |
| Unit VIII | d -and f -Block Elements | 12 | “ |
| Unit IX | Coordination Compounds | 12 | “ |
| Unit X | Haloalkanes and Haloarenes | 10 | 28 |
| Unit XI | Alcohols, Phenols and Ethers | 10 | “ |
| Unit XII | Aldehydes, Ketones and Carboxylic Acids | 10 | “ |
| Unit XIII | Amines | 10 | “ |
| Unit XIV | Biomolecules | 12 | “ |
| Unit XV | Polymers | 08 | “ |
| Unit XVI | Chemistry in Everyday Life | 06 | “ |
| 70 |
NOTE:- To know more information about the Class 12 Chemistry Syllabus
Class 12 Chemistry Useful Resources
We have tried to bring CBSE Class 12 Chemistry NCERT Study Materials like Syllabus, Worksheet, Sample Paper, NCERT Solution, Important Books, Holiday Homework, Previous Year Question Paper, etc. You can visit all these important topics by clicking the links given.
| ChemistryNCERT Solution | |
You Should Also Checkout
- [Updated] CBSE Class 12 Sanskrit Core NCERT Book 2024-25 Session in PDF
- [Updated] Class 12 Physics Notes 2024-25: Syllabus, Hand Written, MCQ, Important Question in PDF!
- [Updated] CBSE Class 12 Summer Season Holiday Homework 2024-25 Session in PDF
- 2000+ Physics MCQs for NEET 2024: Chapterwise Important Question and Answer
About Editorial Staff
Meet Edufever Staff - Your Career Sherpas for the Path to Success! 🚀 We're a team of dedicated education enthusiasts, committed to providing authentic and reliable information to empower your career choices. From college selection to skill development, count on us to be your guiding Sherpas. Your queries and suggestions are always welcome - feel free to comment below. Let's embark on this transformative journey together! 💼💫
Leave a Comment Cancel reply
Notify me via e-mail if anyone answers my comment.
- CBSE Class 12
NCERT Class 12 Chemistry Lab Manual: Download Unit-Wise FREE PDFs
Cbse class 12 chemistry lab manual pdf: this article contains direct links to download the latest ncert chemistry lab manuals for class 12 chemistry. also, check the cbse class 12 chemistry practical syllabus and download the lab manuals accordingly..

CBSE Class 12 Chemistry Practical Syllabus 2024-25
PRACTICAL SYLLABUS ( 60 Periods)
Micro-chemical methods are available for several of the practical experiments. Wherever possible, such techniques should be used.
A. Surface Chemistry
- Lyophilic sol - starch, egg albumin and gum
- Lyophobic sol - aluminium hydroxide, ferric hydroxide, arsenous sulphide.
(b) Dialysis of sol-prepared in (a) above.
(c) Study of the role of emulsifying agents in stabilizing the emulsion of different oils.
B. Chemical Kinetics
(a) Effect of concentration and temperature on the rate of reaction between Sodium Thiosulphate and Hydrochloric acid.
(b) Study of reaction rates of any one of the following:
(i) Reaction of Iodide ion with Hydrogen Peroxide at room temperature using different concentration of Iodide ions.
(ii) Reaction between Potassium Iodate, (KIO3) and Sodium Sulphite: (Na2SO3) using starch solution as indicator (clock reaction).
C. Thermochemistry
Any one of the following experiments
i) Enthalpy of dissolution of Copper Sulphate or Potassium Nitrate.
ii) Enthalpy of neutralization of strong acid (HCI) and strong base (NaOH).
iii) Determination of enthaply change during interaction (Hydrogen bond formation) between Acetone and Chloroform.
D. Electrochemistry
Variation of cell potential in Zn/Zn2+|| Cu2+/Cu with change in concentration of electrolytes (CuSO4 or ZnSO4) at room temperature.
E. Chromatography
i) Separation of pigments from extracts of leaves and flowers by paper chromatography and determination of Rf values.
ii) Separation of constituents present in an inorganic mixture containing two cations only (constituents having large difference in Rf values to be provided).
F. Preparation of Inorganic Compounds
Preparation of double salt of Ferrous Ammonium Sulphate or Potash Alum. Preparation of Potassium Ferric Oxalate.
G. Preparation of Organic Compounds
Preparation of any one of the following compounds
i) Acetanilide ii) Di -benzal Acetone iii) p-Nitroacetanilide iv) Aniline yellow or 2 - Naphthol Anilinedye.
H. Tests for the functional groups present in organic compounds:
Unsaturation, alcoholic, phenolic, aldehydic, ketonic, carboxylic and amino (Primary) groups.
I Characteristic tests of carbohydrates, fats and proteins in pure samples and their detection in given foodstuffs.
J Determination of concentration/ molarity of KMnO4 solution by titrating it against a standard solution of:
i) Oxalic acid,
ii) Ferrous Ammonium Sulphate (Students will be required to prepare standard solutions by weighing themselves).
K Qualitative analysis
Determination of one cation and one anion in a given salt. Cation : Pb2+, Cu2+ As3+, Aℓ3+, Fe3+, Mn2+, Zn2+, Cu2+, Ni2+, Ca2+, Sr2+, Ba2+, Mg2+, NH4 + Anions: (CO3) 2- , S2- , (SO3) 2- , (NO2) - , (SO4) 2- , Cℓ - , Br- , I- , PO3- 4, (C2O4) 2- , CH3COO- ,NO3 - (Note: Insoluble salts excluded)
- Study of the presence of oxalate ions in guava fruit at different stages of ripening.
- Study of quantity of casein present in different samples of milk.
- Preparation of soybean milk and its comparison with the natural milk with respect to curd formation, effect of temperature, etc.
- Study of the effect of Potassium Bisulphate as food preservative under various conditions (temperature, concentration, time, etc.)
- Study of digestion of starch by salivary amylase and effect of pH and temperature on it.
- Comparative study of the rate of fermentation of following materials: wheat flour, gram flour, potato juice, carrot juice, etc.
- Extraction of essential oils present in Saunf (aniseed), Ajwain (carum), Illaichi (cardamom).
- Study of common food adulterants in fat, oil, butter, sugar, turmeric power, chilli powder and pepper.
NCERT Class 12 Chemistry Lab Manual PDF Unit-Wise
The CBSE Class 12 Science and other board students who follow NCERT can download their NCERT Class 12 Lab manuals from here.
| Lab Manual PDF |
| Lab Manual PDF |
| Lab Manual PDF |
| Lab Manual PDF |
| Lab Manual PDF |
| Lab Manual PDF |
| Lab Manual PDF |
| Lab Manual PDF |
| Lab Manual PDF |
| Lab Manual PDF |
| Lab Manual PDF |
Download these Chemistry Unit-wise practical manuals to get ready for the final board examination and prepare your practical files.
Important: CBSE Class 12 Science Mock Test Papers (All Subjects)
- CBSE Class 12 Biology Syllabus 2024-25
- CBSE Class 12 Syllabus (All Subject PDFs)
Get here latest School , CBSE and Govt Jobs notification and articles in English and Hindi for Sarkari Naukari , Sarkari Result and Exam Preparation . Download the Jagran Josh Sarkari Naukri App .
- India Post GDS Merit List 2024
- NDA Question Paper 2024
- RBI Grade B Admit Card 2024
- UP Police Constable Admit Card 2024
- SSC CGL Admit Card 2024
- UP Police Constable Question Paper 2024 PDF
- CDS Question Paper 2024
- RRB NTPC Recruitment 2024
- Teachers Day Speech
- Teachers Day Gift
- Free NCERT Books Download
- Education News
- NCERT Books
Latest Education News
Paris Paralympics 2024 India Medals list: किन भारतीयों ने जीते मेडल,पढ़ें सबके नाम
उत्तर प्रदेश के 8 रेलवे स्टेशनों को मिले नए नाम, यहां देखें नई लिस्ट
Jasdeep Singh Gill Story: कौन हैं जसदीप सिंह गिल? केमिकल इंजीनियर से धार्मिक गुरु बनने तक की कहानी
बिना पासपोर्ट और वीजा के विदेश की सैर, बस यह डॉक्यूमेंट रखें साथ
Brain Teaser: This High IQ Logic Puzzle Will Separate the Smart From the Smarter
Teacher’s Day 2024: डॉ. सर्वपल्ली राधाकृष्णन, जो राष्ट्रपति के रूप में दान कर देते थे आधे से अधिक वेतन
Picture Puzzle IQ Test: Find the mistake in the picture in 5 seconds!
Maharashtra Police SRPF Result 2022-23 OUT at mahapolice.gov.in, Download Here
Kerala SET Result 2024 OUT at lbsedp.lbscentre.in: Download KSET Marks Link
UP Scholarship 2024: Direct Link to Apply Online for Pre-Matric and Post-Matric Scheme, Check Eligibility, Other Details
Dr MGR Medical University Result 2024 OUT at tnmgrmu.ac.in; Direct Link to Download UG and PG Marksheet
Teachers Day 2024: How Dr. Sarvepalli Radhakrishnan's Birthday Became Teachers' Day in India? Details Here
MGSU Result 2024 OUT at mgsubikaner.ac.in, Direct Link to Download UG and PG Marksheet PDF
SSC CGL Admit Card 2024 Out at ssc.gov.in: Direct Link to Download Region Wise Tier 1 Hall Ticket PDF Here
Delhi Home Guard Admit Card 2024 OUT at dghgenrollment.in: Download Link Here
SSC CGL Reasoning Questions With Solution, Download PDF Here
Synonyms and Antonyms: 100+ Synonyms and Antonyms List with Examples and Meaning for SSC; Download PDF
SSC CGL Previous Year Question Paper PDF Download, Tier 1 & 2 PDFs with Solutions
Visual Skill Test: Find the odd woman face in the picture in 3 seconds!
KTET Result 2024 OUT at ktet.kerala.gov.in: Download Kerala TET Marks
Notes For Free
One Stop Repository
Titration (Oxalic Acid)
(a) To prepare 100ml of M/40 solution of oxalic acid.
(b)Using this calculate the molarity and strength of the given KMnO 4 solution.
APPARATUS AND CHEMICALS REQUIRED –
Oxalic acid, weighing bottle, weight box, volumetric flask, funnel, distilled water, chemical balance, beakers, conical flask, funnel, burette, pipette, clamp stand, tile, dilute H 2 SO 4 , KMnO 4 solution.
(a) THEORY –
Oxalic acid is a dicarboxylic acid having molar mass 126gmol -1 . It is a primary standard and has the molecular formula COOH-COOH.2H 2 O. Its equivalent mass is 126/2 = 63 as its n factor is 2 as per the following reaction:
COOH-COOH → 2CO 2 + 2H + + 2e – .
- Weigh a clean dry bottle using a chemical balance.
- Add 3.15g more weights to the pan containing the weights for the weighing bottle.
- Add oxalic acid in small amounts to the weighing bottle, so that the pans are balanced.
- Remove the weighing bottle from the pan.
- Using a funnel, transfer the oxalic acid to the volumetric flask.
- Add a few drops of distilled water to dissolve the oxalic acid.
- Make up the volume to the required level using distilled water .
- The standard solution is prepared.
(b) T HEORY –
- The reaction between KMnO 4 and oxalic acid is a redox reaction and the titration is therefore called a redox titration.
- Oxalic acid is the reducing agent and KMnO 4 is the oxidizing agent.
- KMnO 4 acts as an oxidizing agent in all the mediums; i.e. acidic, basic and neutral medium.
- KMnO 4 acts as the strongest oxidizing agent in the acidic medium and therefore dil. H 2 SO 4 is added to the conical flask before starting the titration.
- The titration between oxalic acid and KMnO 4 is a slow reaction, therefore heat the oxalic acid solution to about 60 0 C to increase the rate of the reaction.
IONIC EQUATIONS INVOLVED:
Reduction Half: MnO 4 – + 8H + + 5e – → Mn 2+ + 4H 2 O] X 2
Oxidation Half: C 2 O 4 2- → 2CO 2 + 2e – ] X 5
Overall Equation: 2MnO 4 – + 16H + + 5C 2 O 4 2- → 2Mn 2+ + 10CO 2 + H 2 O
INDICATOR – KMnO 4 acts as a self indicator.
END POINT – Colourless to light pink (KMnO 4 in the burette)
PROCEDURE –
1. Fill the burette with KMnO 4 solution.
2. Pipette out 10ml. of oxalic acid solution into the conical flask.
3. Add half a test tube of dil. H 2 SO 4 and heat the solution to about 60 0 C to increase the rate of the reaction.
4. Keep a glazed tile under the burette and place the conical flask on it.
5. Note down the initial reading of the burette.
6. Run down the KMnO 4 solution into the conical flask dropwise with shaking.
7. Stop the titration when a permanent pink colour is obtained in the solution.
8. This is the endpoint. Note down the final burette reading.
9. Repeat the experiment until three concordant values are obtained.
OBSERVATION TABLE: (TO BE PUT UP ON THE BLANK SIDE USING A PENCIL)
Volume of Oxalic Acid solution taken = 10mL
| I nitial | Final | Used (mL) | |
| 1 | 16 | 26.5 | 10.5 |
| 2 | 26.5 | 36.9 | 10.4 |
| 3 | 36.9 | 47.4 | 10.5 |
Concordant Value = 10.5mL
CALCULATIONS: (TO BE PUT UP ON THE BLANK SIDE USING A PENCIL)
Calculation of amount of oxalic acid to be weighed to prepare 100ml M/20 solution:
Molecular Mass of Oxalic Acid = 126g/mole
1000 cm 3 of 1M oxalic acid require 126g oxalic acid.
1000 cm 3 of M/40 oxalic acid require =126/40g = 3.15g
Using formula:
N 1 M 1 V 1 = N 2 M 2 V 2
Where N 1 = 5 (for KMnO 4 ), V 1 = 10.5 , M 1 = ?
N 2 = 2 (for oxalic acid), V 2 = 10ml, M 2 = 1/40
M 1 = [2*(1/40)*10]/[5*10.5] = 1/105M = 0.0095M
S trength = M X Molar Mass = 158 *( 1/105) = 1.504g/L
RESULT- (ON RULED SIDE ) –
The Molarity of KMnO 4 = 0.0095M
And the strength of KMnO 4 = 1.504g/L
You can also get Class XII Practicals on Biology , Physics , Chemistry , and Physical Education .
36 Replies to “Titration (Oxalic Acid)”
Thanks for guidance
Helpful , to a large extent
Thanks for guidance and good theory
Thanq for giving information regarding practicals
Calculation is 100% right….. Bcz in our school sir has told from other formula….. M1V1/N1 =M2V2/N2….plz tell which one is right….
In ur formula N1 and N2 are stoicio.. Coeff of kmno4 and (cooh)2 in balanced formula respectively
formula for the calculation of normaility—- no.of gram equivalent = normality x volume no. of gram equivalent = molarity x volume x n-factor correct formula is- M1 X V1 X N1 = M2 X V2 X N2
Gorgeous one guys 🤗
Gorgeous one guy 🤗
Really it is very nice 🐞🐞🐞
Thanks a lot for this free notes…
Thanks for help
is this observations are right…??
How to prepare M/10 kmno4
Oxalic acid ka weight 0.315 g hoga n ki 3.15g
much helpful to me really thank you …..
I think the weight of oxalic acid should be 0.315g not 3.15g because we have to make 100ml solution not 1000ml solution.
Really helpful 👍, thanku
Very helpful👍
Mazza aaya👌
Very Helpful. Thanks!!
Thank you from the bottom of my heart.😘😘😘😘😘
Thankyou so much for this pratical
Leave a Reply Cancel reply
Your email address will not be published. Required fields are marked *
Experience Teachmint X - AI driven Interactive Flat Panels and Smart Boards
More from Veena Nayak
Recommended content, learn from anywhere on any device.
Top Leader by G2
Top Performer by SourceForge
Top Leader by SoftwareSuggest
Ranked Amongst Top 25 Companies by LinkedIn
ISO27001 Certified
Most Preferred Workplace
We use cookies to enhance site navigation and analyse usage, read our Privacy Policy for more.
NCERT Solutions for Class 6, 7, 8, 9, 10, 11 and 12
NCERT Solutions for Class 12 Chemistry (Newly Updated For 2023-2024)
NCERT Solutions for Class 12 Chemistry : The NCERT solutions provided here will enhance the concepts of the students, as well as suggest alternative methods to solve particular problems to the teachers. The target is to direct individuals towards problem solving strategies, rather than solving problems in one prescribed format. The links below provide the detailed solutions for all the Class 12 Chemistry problems.
Chemistry is much more than the language of Science. We have made sure that our solutions reflect the same. We aim to aid the students with answering the questions correctly, using logical approach and methodology. The NCERT Solutions provide ample material to enable students to form a good base with the fundamentals of the subject.
Class 12 Chemistry NCERT Solutions (2023-24)
The solutions have been especially designed to help the students write concise answers in the board examinations, as well as prepare well for objective questions that the students face in JEE and NEET.
- Chapter 1 The Solid State
- Chapter 2 Solutions
- Chapter 3 Electro chemistry
- Chapter 4 Chemical Kinetics
- Chapter 5 Surface Chemistry
- Chapter 6 General Principles and Processes of Isolation of Elements
- Chapter 7 The p Block Elements
- Chapter 8 The d and f Block Elements
- Chapter 9 Coordination Compounds
- Chapter 10 Haloalkanes and Haloarenes
- Chapter 11 Alcohols Phenols and Ethers
- Chapter 12 Aldehydes Ketones and Carboxylic Acids
- Chapter 13 Amines
- Chapter 14 Biomolecules
- Chapter 15 Polymers
- Chapter 16 Chemistry in Everyday Life
HC Verma Concepts of Physics
Class 12 Chemistry Chapter 1 The Solid State
This chapter deals with the solid state. It is comprised of 11 subtopics. This chapter deals with crystalline and amorphous solids, as well as imperfections in solids. It explains the unit cell in detail, and then builds up to explain the solid state. The solutions are made in accordance with the NCERT text. It justifies the concepts explained in the textbook, and helps the students reinforce the fundamentals.
Class 12 Chemistry Chapter 2 Solutions
The second chapter deals with types of solutions, and their properties. The concepts dealt with are Raoult’s law, concentration of solutions, vapor pressure of liquid solutions, abnormal molar masses, and colligative properties. The back and in-chapter exercises are made to reinforce the concepts, and the solutions aid the students in the same. This chapter promises a chunk of 5 marks in the Board exam.
Class 12 Chemistry Chapter 3 Electrochemistry
This chapter deals with the electrochemical cell. Students also learn about the galvanic cell and the electrolytic cell. The chapter explains the standard potential of the cell, Gibbs energy of cell reaction, and the relation with the equilibrium constant. The students will learn the Kohlrausch Law and its applications.
Class 12 Chemistry Chapter 4 Chemical Kinetics
This chapter deals with the kinetics, or the rate of a reaction. The topics covered include the factors affecting the rate of a reaction, the integrated rate equation, Pseudo First Order reactions, and the collision theory of chemical reactions. This chapter is responsible from engineering and medical sciences point of view too.
Class 12 Chemistry Chapter 5 Surface Chemistry
The fifth chapter deals with emulsions, adsorption, adsorption isotherms, factors controlling adsorption of gases and liquids, and catalysts and their role in industry. The chapter further explores colloids and their applications.
Class 12 Chemistry 6 General Principles and Processes of Isolation of Elements
This chapter deal with the inorganic chemistry portion of the syllabus. The chapter explores the occurence of metals, the concentration of ores, cleaning and extraction, of crude metal from ores, thermodynamic and electrochemical principles of metallurgy, and the refining process
Class 12 Chemistry Chapter 7 p Block Elements
The chapter deals with the Group 15 elements, Oxides of Nitrogen, Allotropes of Phosphorus, Group 16 elements, simple oxides, allotropes of sulfur, chlorine, and other elements.
Class 12 Chemistry Chapter 8 d and f Block Elements
The chapter deals with the properties of transition elements, colored ions and complex compounds, variation in sizes of elements, ionization enthalpies, magnetic properties, and oxidation states. The chapter deals with d block elements and their properties and variations.
Class 12 Chemistry Chapter 9 Coordination Compounds
The compounds dealt with here, are relatively and the concepts are novel, and therefore the students are required to pay special attention to the chapter. The chapter explains Werner’s theory of Coordination compounds, Bonding in Coordination compounds, Nomenclature, Isomerism and others. The chapter can be well understood through the exercises and solutions, that help to reinforce the fundamentals.
Class 12 Chemistry Chapter 10 Haloalkanes and Haloarenes
Students learn the concepts of IUPAC nomenclature of Haloalkanes and Haloarenes. They will also learn the applications of organometallic reactions, preparation of haloalkanes and haloarenes, and stereochemistry. The students also learn to design the reaction mechanism.
Class 12 Chemistry Chapter 11 Alcohols, Phenols and Ethers
This chapter also begins with the IUPAC nomenclature of alcohols, phenols and ethers. They also deal with the preparation of alcohols, phenols, and ethers. They further explore the properties of the same.
Class 12 Chemistry Chapter 12 Aldehydes, Ketones and Carboxylic Acids
The next ones in line are the aldehydes, ketones and carboxylic acids. The IUPAC names, to start with, the chapter explores their preparation methods, the key reactions, characteristic tests, and other properties.
Class 12 Chemistry Chapter 13 Amines
The ten subtopics include the preparation methods, the physical properties, the chemical reactions, the methods to prepare diazonium salts, the importance of diazonium salts in synthesis of Aromatic Compounds.
Class 12 Chemistry Chapter 14 Biomolecules
In this chapter, students learn about biomolecules like glucose, fructose, starch, glycogen, etc. They get to learn about the structure, the classes, and the properties of carbohydrates and proteins. They also learn about the reactions of some biomolecules.
Class 12 Chemistry Chapter 15 Polymers
This chapter deals with the science of polymers, the various types of polymers, whether addition or condensation, linear or cross linked, their reactions, and their properties. This chapter also deals with some well known polymers and their applications.
Class 12 Chemistry Chapter 16 Chemistry in Everyday Life
This chapter deals with the importance of chemistry in everyday life, mainly drug action, drug classification and uses, and the target interaction, along with chemicals in food and cleansing agents.
NCERT textbooks are prescribed by CBSE as the best books for preparation of the school as well as board examinations. Designed in a fairly simple language, we have made sure that the solutions cover all the fundamental concepts. The solutions are hundred percent error free, and easily comprehensible.
Free Resources
NCERT Solutions
Quick Resources
- NCERT solutions for class 12 Chemistry
- Active page
Chemistry, in general, is the most important subject of class 12 th science class. The NCERT Solutions for Class 12 Chemistry is prepared by experts to assists students in their learning. The team at Physics Wallah comprises of experts having years of experience. This NCERT solutions for class 12 th chemistry can assist your overall progress in the upcoming board examination. The team at Physics Wallah has maintained the top class standard over the years.
Since our team is already acquainted with the type of question papers in the final examination. The NCERT Solutions for class 12 chemistry is a precious text for students. The versatility of our study material is not only restrained to board exams. Students can also refer this study material for various other competitive exams as well.

NCERT Solutions for Class 12 Chemistry Chapters (Updated 2022-23)
- Chapter 1 The Solid State
- Chapter 2 Solutions
- Chapter 3 Electrochemistry
- Chapter 4 Chemical Kinetics
- Chapter 5 Surface Chemistry
- Chapter 6 General Principles and Processes of Isolation of Elements
- Chapter 7 The p-Block Elements
- Chapter 8 The d & f Block Elements
- Chapter 9 Coordination Compounds
- Chapter 10 Haloalkanes and Haloarenes
- Chapter 11 Alcohols, Phenols, and Ethers
- Chapter 12 Aldehydes, Ketones, and Carboxylic Acids
- Chapter 13 Amines
- Chapter 14 Biomolecules
- Chapter 15 Polymers
- Chapter 16 Chemistry in Everyday Life
NCERT Solutions for Class 12 Chemistry Free PDF Download

Chapter- 1 The Solid State
This chapter explains the general characteristics of the solid state, the classification of solids, the crystal lattice, unit cell imperfections, and solids. The matter has three states – solid, liquid, and gas. Solids are then divided into – amorphous and crystalline. Amorphous has no form, and Crystalline has a definite shape. NCERT Solutions is a perfect guide to get a firm grasp on these concepts. For further reinforcement of concepts, students can also access NCERT sample solutions available on Physics Wallah.
Also Check- Important questions for class 12 chemistry Chapter 1 Solid state
Chapter- 2 Solutions
A solution is a mixture of the two or more components. This chapter helps the students to understand the types of solutions, the concentration of solutions, solubility of gases and solid in a liquid, ideal and non ideal solutions, the vapour pressure of liquid solutions, Raoult’s Law. Various problems based on finding the molarity, mass percentage and Henry’s Law constant, mole fraction, are also present here. These concept are not only important for the Class 12 exams but also are of higher importance in competitive exams like JEE Mains, JEE Advanced etc.
Also Check- Important questions for class 12 chemistry Chapter 2 Liquid Solution
Chapter- 3 Electrochemistry
An electrochemistry is a branch of chemistry that deals with the relationship between electrical and chemical energy produced in redox reactions and their conversion. The concepts covered in this chapter are –Redox reactions; conductance in electrolytic solutions, Kohlrausch’s Law, molar and specific conductivity variations of conductivity with concentration, electrolysis and laws of electrolysis (elementary idea), Galvanic cells and dry cell – electrolytic cells; lead accumulator, standard electrode potential, EMF of a cell, Nernst equation and their application to chemical cells, fuel cells; corrosion.
Also Check- Important questions for class 12 chemistry Chapter 3 Electrochemistry
Chapter- 4 Chemical Kinetics
This chapter will provide a good understanding of the rate of a chemical reaction, the Arrhenius equation, the rate dependence of a reaction, and the collision theory of a chemical reaction. The chemical kinetics is the branch of chemistry that deals with the rate of a chemical reaction, the factors that affect it, and the mechanism of the reaction. We have three types according to the rate of reaction: instantaneous, slow, and moderately slow reactions. The topics which are covered in this chapter are- Rate of a reaction (average and instantaneous), temperature, factors affecting rates of reaction, concentration catalyst; order and molecularity of a reaction; rate law and specific rate constant, integrated rate equations, and half-life (only for zero and first order reactions); concept of collision theory (elementary idea, no mathematical treatment).
Also Check- Important questions for class 12 chemistry Chapter 4 Chemical Kinetics
Chapter- 5 Surface Chemistry
Surface chemistry deals with important properties such as catalysis, adsorption, and colloids, which include gel and emulsion. After studying this chapter, students will understand the interface phenomenon and its importance, adsorption and its classification, the mechanism of adsorption, and the factors that control adsorption. Further solving questions from the textbook and previous year's questions will strengthen the preparation of CBSE students for the exams.
Also Check- Important questions for class 12 chemistry Chapter 5 Surface chemistry
Chapter- 6 General Principles and Processes of Isolation of Elements
Metallurgy is a scientific and technological process of isolating metal from its ores. Students will also discussed about the fundamental principles and developments in this field, besides explaining the processes and reactions of metal extraction. The Aluminum is the most abundant metal found on the earth’s crust which is 8.3% by weight. So cleaning the ore, i.e., removal of particles like clay, sand, etc., is known as the concentration or dressing of the ore.
Also Check- Important questions for class 12 chemistry Chapter 6 General principle and process of isolation of elements
Chapter- 7 The p-Block Elements
The history of p-block elements has a history that takes us back to the 19th century. A groups of elements 13, 14, 15, 16, 17, and 18 are known as p-block elements. They exist in 3 physical states – metal, non-metal, and metalloids. For better clarification of these concepts, students can refer to NCERT Solutions available at Physics Wallah. A clear explanation of each concept will help students score well in the final exam.
Also Check- Important questions for class 12 chemistry Chapter 7 P-Block elements
Chapter- 8 The d- and f-Block Elements
The elements lying between the s-block and p-block elements are known as d-block or transition elements. The inner transition series is called f-block elements. This chapter introduces concepts such as General introduction, electronic configuration, occurrence and characteristics of transition metals, properties of the first-row transition metals in general trends– metallic character, ionization enthalpy, oxidation states, ionic radii, colour, catalytic property, magnetic properties, interstitial compounds, alloy formation. Preparation and properties of K2, Cr 2 O 7 , and KMnO 4 .
Lanthanoids: electronic configuration, oxidation states, chemical reactivity, and lanthanoid contraction. Actinoids: Electronic configuration, oxidation states.
Also Check- Important questions for class 12 chemistry Chapter 8 D and F block
Chapter- 9 Coordination Compounds
The coordination of compounds is a rigid portion of modern inorganic chemistry. In this chapter, students will be able to learn about Werner’s Theory of Coordination Compounds, Introduction, ligands, coordination number, color, magnetic properties and shapes, IUPAC nomenclature of mononuclear coordination compounds, bonding; isomerism, the importance of coordination compounds (in qualitative analysis, extraction of metals and biological systems). They will also discuss the bonding in meta carbonyls which are important for the board exams. These concepts are important for CBSE and competitive exams, so more importance should be given when scoring marks.
Also Check- Important questions for class 12 chemistry Chapter 9 Co-ordination compounds
Chapter- 10 Haloalkanes and Haloarenes
A halogen derivative of hydrocarbons is known as haloalkanes. They are classified based on the number of hydrogen atoms in them. An aromatic compound in which the halogens are attached directly to the carbon atom of the aromatic ring is known as haloarenes. In this chapter, students will also discuss the preparation methods, chemical and physical properties, and uses of organohalogen compounds. The reactions that is involved in the preparation of haloalkanes and haloarenes are clearly explained in this chapter to help students achieve good exam results. These chapters cover Haloalkanes: Nomenclature, nature of C-X bond, physical and chemical properties, and mechanism of substitution reactions.
Haloarenes: Substitution reactions, nature of C-X bond. Uses and environmental effects of – dichloromethane, trichloromethane, tetrachloromethane, iodoform, freons, DDT.
Also Check- Important questions for class 12 chemistry Chapter 10 Haloalkanes
Chapter- 11 Alcohols, Phenols and Ethers
The classification of alcohols and phenols is based on the number of -OH groups present. Compounds that have one -OH group are called monohydric alcohols and phenols. Compounds with two, three, or more -OH groups are called dihydric, trihydric, or polyhydric alcohols and phenols. Students will learn about the reactions related to the process of producing alcohols from phenols, alcohols, and ethers. It will also help students to become familiar with the physical properties of alcohols, phenols, and ethers. Topics covered in these chapters are-Alcohols:
- Nomenclature, physical and chemical properties (of primary alcohols only), methods of preparation.
- Secondary and tertiary alcohols, identification of primary.
- The mechanism of dehydration uses some important compounds – methanol and ethanol.
Phenols: Nomenclature, methods of preparation, physical and chemical properties, acidic nature of phenol, electrophilic substitution reactions, uses of phenols. Ethers: Nomenclature, methods of preparation, physical and chemical properties, uses.
Also Check- Important questions for class 12 chemistry Chapter 11 Phenol and ethers
Chapter- 12 Aldehydes, Ketones and Carboxylic Acids
Aldehydes, ketones, and carboxylic acids are most important in organic chemistry. Aldehydes and ketones can be obtained by hydration of alkynes, ozonolysis of alkenes and oxidation of alcohols. Carboxylic acids can be obtained by oxidation of aldehydes or primary alcohols. This chapter is very important and fetches more marks in the board exam. For this, students must learn all the concepts and revise them regularly to get good scores. Topics covered in these chapters are- Aldehydes and Ketones: Nomenclature, methods of preparation, nature of carbonyl group, mechanism of nucleophilic addition and physical and chemical properties, reactivity of alpha hydrogen in aldehydes; uses. Carboxylic Acids: Nomenclature, acidic nature, methods of preparation, physical and chemical properties; uses.
Also Check- Important questions for class 12 chemistry Chapter 12 Aldehyde and ketones
Chapter- 13 Amines
Ammonia derivatives are amines that are obtained by replacing hydrogen. From this chapter, students will be able to understand the nomenclature, structure, and properties of amines. Amines are important organic compounds that contain nitrogen. Numerous examples of amine basicity determinations, reactions, and amine synthesis are briefly explained in this chapter. Students will gain a good grasp of these concepts by answering the end-of-chapter questions. These chapters cover Amines: Nomenclature, classification, structure, methods of Preparation, and physical and chemical properties that identify primary, secondary and tertiary amines. Cyanides and Isocyanides will be mentioned at relevant places in context. Diazonium salts: chemical reactions, Preparation, and importance in synthetic organic chemistry.
Also Check- Important questions for class 12 chemistry Chapter 13 Amines
Chapter- 14 Biomolecules
Organic compounds present as basic components in various cells of a living organism are called biomolecules. These include carbohydrates, proteins, vitamins, enzymes, and nucleic acids. The interactions of biomolecules form the molecular logic of life processes. Basic molecules such as vitamins and mineral salts play an important role in the functioning of organisms. Topics covered in these chapters are- Carbohydrates: Classification (aldoses and ketoses), monosaccharides (glucose and fructose), oligosaccharides (lactose, sucrose, maltose), polysaccharides (cellulose, starch, glycogen); importance. Proteins: Elementary idea of α – amino acids, peptide bond, polypeptides, proteins, primary structure, secondary structure, tertiary structure and quaternary structure (qualitative concept only), denaturation of proteins; enzymes. Vitamins: Classification and functions. Nucleic Acids: DNA and RNA.
Also Check- Important questions for class 12 chemistry Chapter 14 Biomolecules
Chapter- 15 Polymers
This chapter includes concepts like – monomer, polymer, and polymerization. Its classification is based on source, structure, and polymerization. The types of polymerization are – addition polymerization and condensation polymerization. Important concepts of this chapter are clearly explained in the NCERT textbook. Students who aspire to score well in the exams are advised to study this chapter thoroughly and clearly understand these concepts.
Also Check- Important questions for class 12 chemistry Chapter 15 Polymers
Chapter- 16 Chemistry in Everyday Life
An area of human life is affected by chemistry. The principles of chemistry have benefited people in many ways. The topics discussed in this chapter are – drugs and their classification, drug target interactions, the therapeutic action of different standards of drugs, and chemicals in food and detergents. Chemistry is a tough subject and its requires a lot of practice to remember chemical reactions and formulas. So, NCERT Solutions at Physics Wallah will give you a strong overview of important topics.
Also Check- Important questions for class 12 chemistry Chapter 16 Chemistry in everyday life
CBSE Marking Scheme 2022-23
Class 12 is a major stage in a student's life. To help them grasp all the concepts effectively, the CBSE board has divided the entire course into sections with marks per the exam pattern. All the chapters are divided based on weightage and the importance of marks from the CBSE examination point of view. This aims to provide quality education to class 12 students and help them achieve their future goals. It also provides a solid foundation of basic concepts that are important for exams.
| 1 | Solutions | 7 |
| 2 | Electrochemistry | 9 |
| 3 | Chemical Kinetics | 7 |
| 4 | d-and f-Block Elements | 7 |
| 5 | Coordination Compounds | 7 |
| 6 | Haloalkanes and Haloarenes | 6 |
| 7 | Alcohols, Phenols and Ethers | 6 |
| 8 | Aldehydes, Ketones and Carboxylic Acids | 8 |
| 9 | Amines | 6 |
| 10 | Biomolecules | 7 |
Why NCERT Solutions for Class 12 Chemistry are important?
Chemistry consist of various typical theories as well numerical part. Many a time’s students get stuck in problems and complex theories. This hinders the smooth learning curve of students. In order to prevent any type of time wastage, the team has come up with NCERT solutions for class 12 chemistry study material.
Since chemistry has a wide syllabus to cover, so it becomes a bit difficult to comprehend at the time of revision. Students can easily revise the study material right before their examinations. The NCERT solutions for class 12 chemistry has been divided into chapter wise section. Our team has made extensive efforts to research the most possible questions from year question papers. Students can find defiantly find various important marked questions in the study material. Since syllabus of class 12th chemistry is very wide & comprises of various unnecessary things. The team at Physics Wallah has completely removed unnecessary things and made learning easy.
Step-by-step guidance is provided to critical numerical. Students can also find various short tricks to solve critical concepts and solutions. The experts in our team drafting NCERT solutions for class 12 chemistry are well versed in the topics. Students can find various intrinsic details in the NCERT solutions for class 12 chemistry prepared by our team. Chemistry is the subject with wide entices, we have inculcated short solving techniques in our syllabus.
How to Study NCERT Solutions for Class 12 chemistry effectively?
Whether it’s your board examinations or national level competitive examination, NCERT solutions for class 12 chemistry is a one-stop solution for all your needs. Students can also find various dedicated sections in study material for information on critical problems. The very ideology we follow at Physics Wallah is to help students with hassle-free learning. This NCERT solutions for class 12 chemistry can help students clear all their conceptual doubts.
Why Physics Wallah is best for NCERT Solutions for Class 12 chemistry?
Students looking for expert guidance can study through NCERT solutions for class 12 chemistry provided by Physics Wallah. We have been helping needy students by providing the most coveted study material for no dime. Students can access our NCERT solutions for class 12 chemistry for completely free. All they need to do is to sign up with us. Avail the precious study material created by experts of their field right at your home at no cost.
Approach to use NCERT solution for Class 12 chemistry for physical chemistry
Physical chemistry required conceptual clarity over the subject. One must know how to apply the concept in numerical. To become expert in numerical one must start with solid state chapter this is the chapter which didn’t required help for any other chapter and interesting too.In NCERT this chapter is explained with awesome figures which will help you to understand the structure of solid and what Bravis wants to explain. Try to solve the question of text book and after that take the help of NCERT solution for class 12 chemistry.
Don’t jump to solutions directly. Liquid solution required revision of stoichiometry chapter of class 11. Befor liquid solutions chapter you must know the term molarity, normality and other concentration term. Read the NCERT text book for the theory understand the concept of Raoult law and colligative properties solve the question given in the text book of NCERT. Take reference of NCERT solution for class 12 chemistry.
Electrochemistry and chemical kinetics topics are very important and carries high weightage in class 12 board exam. All questions given in NCERT text book must be solved completely. You can take help of Physics Wallah chemistry formula page to learn all formula required for these chapter.
Approach to use NCERT Solution for Class 12 chemistry for organic chemistry
At the beginning of Organic chemistry for class 12 one must revise few topics of class 11 organic chemistry such as.
- Chemical bonding
- General organic chemistry
- Hydrocarbon
It is very important to have clarity in the above topics before starting class 12 alkyl halide chapter. After revision start with alkyl halide. Read the theory of SN 1 and SN 2 reaction mechanism try to find the mechanism of each reaction and how the reaction proceed and for mechanism detail you can take reference of Physics Wallah organic section. Read the chapter make your own notes and write all reaction and solve the text book questions at last take the help of NCERT solution for class 12 chemistry.
Frequently Asked Question (FAQs)
Q1. How to score good marks in CBSE Board class 12 chemistry with the help of the NCERT textbook?
Ans. To score good marks in class 12 chemistry you must be strong in all three parts of chemistry physical, inorganic, and organic chemistry. You need a different approach in all three parts of chemistry for example to do well in physical chemistry focus on theory and solve numerical given in the NCERT textbook.
Inorganic chemistry is highly dependent on your notes so prepare a good note form the NCERT textbook and note down all bullet points in it. Understand all reaction mechanisms given in the NCERT textbook.
Q2. What is the right approach to reading the NCERT class 12 chemistry Textbook for the Board exam?
Ans. For board exam, the NCRT textbook is highly recommended and must be read line by line. The best approach to score good marks in class 12 chemistry is to make good notes from chapter-1 to chapter-16. Solve all questions given in the NCERT exercise.
Q3. What are the right strategies to deal with inorganic chemistry for the board exam?
Ans. Inorganic chemistry must be done through the NCERT textbook. It is sufficient and consists of all the important points which are required to score good marks in class 12 chemistry.
Q4. Is NCERT class 12 chemistry text is enough for the entrance exam?
Ans . Yes now a day all most all entrance exam is conducted by NTA that includes JEE and NEET and the recommended book of NTA is NCERT. Check out the previous year's paper you can easily do an analysis that almost 75 % of questions are asked directly from the NCERT textbook.
Q5. What are the most important chapters of class 12 chemistry for the board exam?
Ans. The most important chapters of class 12 chemistry are electrochemistry, liquid solution, coordination compounds, and carbonyl compounds.
Related Chapters
- NCERT solutions for class 12 Maths
- NCERT Solutions for Class 12 Biology

Recent Concepts
- chapter 14-Biomolecules
- chapter 15-Polymers
- chapter 16-Chemistry In Everyday Life

Talk to Our counsellor


- Andhra Pradesh
- Chhattisgarh
- West Bengal
- Madhya Pradesh
- Maharashtra
- Jammu & Kashmir
- NCERT Books 2022-23
- NCERT Solutions
- NCERT Notes
- NCERT Exemplar Books
- NCERT Exemplar Solution
- States UT Book
- School Kits & Lab Manual
- NCERT Books 2021-22
- NCERT Books 2020-21
- NCERT Book 2019-2020
- NCERT Book 2015-2016
- RD Sharma Solution
- TS Grewal Solution
- TR Jain Solution
- Selina Solution
- Frank Solution
- Lakhmir Singh and Manjit Kaur Solution
- I.E.Irodov solutions
- ICSE - Goyal Brothers Park
- ICSE - Dorothy M. Noronhe
- Micheal Vaz Solution
- S.S. Krotov Solution
- Evergreen Science
- KC Sinha Solution
- ICSE - ISC Jayanti Sengupta, Oxford
- ICSE Focus on History
- ICSE GeoGraphy Voyage
- ICSE Hindi Solution
- ICSE Treasure Trove Solution
- Thomas & Finney Solution
- SL Loney Solution
- SB Mathur Solution
- P Bahadur Solution
- Narendra Awasthi Solution
- MS Chauhan Solution
- LA Sena Solution
- Integral Calculus Amit Agarwal Solution
- IA Maron Solution
- Hall & Knight Solution
- Errorless Solution
- Pradeep's KL Gogia Solution
- OP Tandon Solutions
- Sample Papers
- Previous Year Question Paper
- Important Question
- Value Based Questions
- CBSE Syllabus
- CBSE MCQs PDF
- Assertion & Reason
- New Revision Notes
- Revision Notes
- Question Bank
- Marks Wise Question
- Toppers Answer Sheets
- Exam Paper Aalysis
- Concept Map
- CBSE Text Book
- Additional Practice Questions
- Vocational Book
- CBSE - Concept
- KVS NCERT CBSE Worksheets
- Formula Class Wise
- Formula Chapter Wise
- Toppers Notes
- Most Repeated Question
- Diagram Based Question
- Study Planner
- JEE Previous Year Paper
- JEE Mock Test
- JEE Crash Course
- JEE Sample Papers
- Important Info
- SRM-JEEE Previous Year Paper
- SRM-JEEE Mock Test
- VITEEE Previous Year Paper
- VITEEE Mock Test
- BITSAT Previous Year Paper
- BITSAT Mock Test
- Manipal Previous Year Paper
- Manipal Engineering Mock Test
- AP EAMCET Previous Year Paper
- AP EAMCET Mock Test
- COMEDK Previous Year Paper
- COMEDK Mock Test
- GUJCET Previous Year Paper
- GUJCET Mock Test
- KCET Previous Year Paper
- KCET Mock Test
- KEAM Previous Year Paper
- KEAM Mock Test
- MHT CET Previous Year Paper
- MHT CET Mock Test
- TS EAMCET Previous Year Paper
- TS EAMCET Mock Test
- WBJEE Previous Year Paper
- WBJEE Mock Test
- AMU Previous Year Paper
- AMU Mock Test
- CUSAT Previous Year Paper
- CUSAT Mock Test
- AEEE Previous Year Paper
- AEEE Mock Test
- UPSEE Previous Year Paper
- UPSEE Mock Test
- CGPET Previous Year Paper
- BCECE Previous Year Paper
- JCECE Previous Year Paper
- Crash Course
- Previous Year Paper
- NCERT Based Short Notes
- NCERT Based Tests
- NEET Sample Paper
- Previous Year Papers
- Quantitative Aptitude
- Numerical Aptitude Data Interpretation
- General Knowledge
- Mathematics
- Agriculture
- Accountancy
- Business Studies
- Political science
- Enviromental Studies
- Mass Media Communication
- Teaching Aptitude
- Verbal Ability & Reading Comprehension
- Logical Reasoning & Data Interpretation
- CAT Mock Test
- CAT Important Question
- CAT Vocabulary
- CAT English Grammar
- MBA General Knowledge
- CAT Mind Map
- CAT Study Planner
- CMAT Mock Test
- SRCC GBO Mock Test
- SRCC GBO PYQs
- XAT Mock Test
- SNAP Mock Test
- IIFT Mock Test
- MAT Mock Test
- CUET PG Mock Test
- CUET PG PYQs
- MAH CET Mock Test
- MAH CET PYQs
- NAVODAYA VIDYALAYA
- SAINIK SCHOOL (AISSEE)
- Mechanical Engineering
- Electrical Engineering
- Electronics & Communication Engineering
- Civil Engineering
- Computer Science Engineering
- CBSE Board News
- Scholarship Olympiad
- School Admissions
- Entrance Exams
- All Board Updates
- Miscellaneous
- State Wise Books
- Engineering Exam
Maharashtra Class 12th Chemistry 2023 : Practical Book & Solution; Download PDF

SHARING IS CARING If our Website helped you a little, then kindly spread our voice using Social Networks. Spread our word to your readers, friends, teachers, students & all those close ones who deserve to know what you know now.
Maharashtra Class 12th Chemistry 2023 : Practical Book & Solution; Download PDF
In this article, we help students with Chemistry Practical Handbook for Class 12th Maharashtra board. You can find chapter-wise links for Chemistry practical handbook solutions.
The best part is that every material on this site is free of cost. This practical book is great to help students with their self-studies. Practical answers are given in detail with proper and understandable solutions.
Many students face difficulty in finding the proper practical Chemistry Handbook. Class 12th is an important year and it is very important to score well in this class. Hope the provided pdf helps you to lower your pressure.
| Experiment No.1 | |
| Experiment No.3 | |
| Experiment No.4 | |
| Experiment No.7 | |
| . Experiment No.8 | |
| Experiment No.9 | |
| Experiment No.10 | |
| Experiment No.11 | |
| Experiment No.12 | |
| Experiment No.18 | |
| Experiment No.19 | |
| Experiment No. 20 | |
| Experiment No. 21 | |
| Experiment No. 22 | |
| Experiment No. 23 | |
| Activity No.1 | |
| Activity No.2 | |
| Activity No.3 | |
Maharashtra Board Class 12 Study Material

- NCERT Solutions for Class 12 Maths
- NCERT Solutions for Class 10 Maths
- CBSE Syllabus 2023-24
- Social Media Channels
- Login Customize Your Notification Preferences
- JEE Advanced: Maharashtra Board Releases Category-wise Top 20 Percentile cut-off for IIT Admission 23 May, 2024, 1:47 pm
- Maharashtra HSC 12th Result 2024 Link Active : MSBSHSE 12th Results Declared; Direct Link Here 21 May, 2024, 1:01 pm
- Maharashtra HSC 12th Result 2024 Out: Check Toppers Name, Marks, Pass Percentage, Merit List, District & Result Full Statistics 21 May, 2024, 12:01 pm
- Maharashtra HSC 12th Result 2024: MSBSHSE Board will Declare 12th Results Today at 1 PM 21 May, 2024, 10:43 am
- Maharashtra Board HSC 12th Result 2024 Date, Time Confirmed: MSBSHSE to Release HSC 12th Result Tomorrow 21st May at 1 PM, Official Notice Here 20 May, 2024, 1:40 pm
- Maharashtra Board HSC 12th History Exam 2024 : Most Important Question Answers 15 March, 2024, 5:56 pm
- Maharashtra Board HSC 12th Exam 2024 : Geography Most Important Question Answers 14 March, 2024, 4:06 pm
- Maharashtra Board HSC 12th Exam 2024 : Economics Most Important Question Answers 8 March, 2024, 6:26 pm
- Maharashtra Board HSC 12th Exam 2024 : Biology Most Important Question Answers 5 March, 2024, 5:01 pm

JEE Advanced: Maharashtra Board Releases Category-wise Top 20 Percentile cut-off for IIT Admission

- Second click on the toggle icon

Provide prime members with unlimited access to all study materials in PDF format.
Allow prime members to attempt MCQ tests multiple times to enhance their learning and understanding.
Provide prime users with access to exclusive PDF study materials that are not available to regular users.

All Study Guide at one Place!
Tuesday, june 21, 2016, to detect one acid and one basic radical salt experiment : bacl2 chemistry class 12 cbse full practical.

Author & Editor
I love blogging!
14 comments:
Thank you so much. It helped lot during last time
thank you..........
Thanks...... Its help me a lot.....
Great help. Thanks
Thanks a lot.....
Thanks a lot
Thank you so much ��
Thank you so much 😊
Thanks 👍 🙏 it help me alot
Post a Comment
Popular Posts

Blog Archive
- ► December (3)
- ► July (19)
- To detect the presence of protein in a given food ...
- Titration 2 - Class 12 CBSE Practical
- Titration - 1 : Class 12 CBSE Chemistry Practical
- To Prove the presence of Starch in Potatoes : Clas...
- Functional Group 2 : Alcohol And Carboxylic Acid |...
- Functional Group 2 : Aldehyde and Ketone | Class ...
- Functional Group 1 : Alcohol and Phenol | Class 12...
- To Detect One Acid And One Basic Radical Salt Expe...
- To Detect one Acid and one Basic Radical Salt Expe...
- To detect one Acid and one Basic Radical from salt...
- ► May (4)
AD (728x90)
Blogger news, contributors.
- Shantanu Sharma
Special Deals on Books
Copyrights @ All Study Guide at one Place! - Blogger Templates By Templateism | Templatelib
- (91) 9407565896
- [email protected]
- Templateism

IMAGES
VIDEO
COMMENTS
To Prepare a pure sample of the complex potassiumtrioxalatoferrate (III) | Experiment No 13 | class 12th Chemistry | Maharashtra Board View & Download PDF (Google drive) : PDF Download Link ...
CBSE Chemistry Practical Class 12 covers the list of practicals, experiments and activities to be performed for the exam. Also, get the Chemistry Lab Manual Class 12 which help you in performing the experiments and suggest you the project work.
Experiment No.14 | To prepare dibenzalacetone | 12th Chemistry Practical Shamsu SN
CBSE Class 12 Chemistry Lab Manual Introduction to Basic Laboratory Equipment Viva Questions with Answers. Surface Chemistry Exp-2.1 : To prepare colloidal solution (sol) of starch. Exp-2.2 : To prepare a colloidal solution of gum. Exp-2.3 : To prepare colloidal solution (or sol) of egg albumin. Exp-2.4 : To prepare ferric hydroxide, [Fe(OH)3] sol. Exp-2.5 : To prepare aluminium hydroxide, […]
Solution Hub 361 subscribers 12 3.2K views 11 months ago Class 12 Chemistry Practical Book Chemistry Practical Book Maharashtra Board Class 12 ...more
Welcome to Master Notes, your academic companion for classes 9 to 12. Today, we embark on a journey of "Class 12 Chemistry Practical Files," demystifying the art and science behind these crucial components of your chemistry curriculum.
Check out the latest CBSE NCERT Class 12 Chemistry Syllabus. The syllabus is for the academic year 2024-25 sessions. First of all, check the CBSE Class 12 Chemistry Exam Pattern. Students should review the complete syllabus and exam pattern with the marking scheme.
Note:- Chemical equations of Experiment 3 to 14 are to be written on blank pages. Observation table of experiment 13 to 16 are to be drawn on blank pages. is also included in the practical syllabus. For pro work, contact the teacher for the top Project report should be hand written. Start each experiment form a new page.
Here are the latest NCERT Class 12 practical manuals provided for Physics, Chemistry, Biology, and Mathematics (PCMB). These lab manuals comprise all the experiments that are meant to be conducted ...
Check & download free PDF for NCERT lab manual for CBSE Class 12 Chemistry and prepare for CBSE 12th Chemistry practicals & board exam 2020-21.
NCERT Class 12 Chemistry Lab Manual PDF: The practical manuals are curriculum-based textbooks comprised of unit-wise experiments with their aim, theory, materials, observation, and expected results.
Spread the loveAIM - (a) To prepare 250ml of M/20 solution of Mohr's salt. (b) Using this calculate the molarity and strength of the given KMnO4 solution. APPARATUS AND CHEMICALS REQUIRED- Mohr's salt, weighing bottle, weight box, volumetric flask, funnel, distilled water, chemical balance, dilute H2SO4, beakers, conical flask, funnel, burette, pipette, clamp stand, tile, KMnO4 ...
Chemistry Lab Manual NCERT Solutions Class 12 Chemistry Sample Papers. In volumetric analysis, the quantities of the constituents present in the given unknown solution are determined by measuring the volumes of the solutions taking part in the given chemical reaction. The main process of this analysis is called titration which means the ...
Spread the loveAIM: - (a) To prepare 100ml of M/40 solution of oxalic acid. (b)Using this calculate the molarity and strength of the given KMnO4 solution. APPARATUS AND CHEMICALS REQUIRED- Oxalic acid, weighing bottle, weight box, volumetric flask, funnel, distilled water, chemical balance, beakers, conical flask, funnel, burette, pipette, clamp stand, tile, dilute H2SO4, KMnO4 solution ...
The Experiment Lab Manual Class 12 is a guide for students aiming to perform various laboratory experiments to learn about Class 12 Chemistry Experiment. It is study material prepared by NCERT subject matter experts for the assistance of Class 12 students to prepare well and perform well in the Chemistry Laboratory.
Notes of Class 12 Chemistry, Chemistry Lab manual (12th).pdf - Study Material
NCERT solutions for class 12 Chemistry solved by LearnCBSE.in expert teachers from latest edition books and as per NCERT (CBSE) guidelines. The Chemistry NCERT Solutions Class 12 provides extensive step-by-step solutions to diffcult problmes and equations which prepare students to crack difficulty levels in easiest way.
Chemistry, in general, is the most important subject of class 12th science class. The NCERT Solutions for Class 12 Chemistry is prepared by experts to assists students in their learning.
CBSE Chemistry Practicals and Experiments - List of chemistry practicals and experiments with detailed instructions, safety advice and background information. Chemistry Practical Class 12, 11, 10 and 9 covers the list of practicals, experiments and activities to be performed for the exam.
CBSE Class 12 Lab Manual for Chapter Experiment No 13 The laboratory is important for making the study complete, especially for a subject like Science and Maths. CBSE has included the practicals in secondary class intending to make students familiarised with the basic tools and techniques used in the labs. With the help of this, they can successfully perform the experiments listed in the CBSE ...
Maharashtra Class 12th Chemistry 2023 : Practical Book & Solution; Download PDF In this article, we help students with Chemistry Practical Handbook for Class 12th Maharashtra board. You can find chapter-wise links for Chemistry practical handbook solutions.
Please subscribe our YouTube Channel for further Experiments. This video includes Experiment No 6,7,8,9,10. Experiment 1 to 5 Video Link : • Chemistry Class 12th Practical Experi...
You can see the Practical No.17 of Class 12 CBSE Chemistry Course :To Detect one Acid and one Basic Radical Salt Experiment : BaCl2 . I have included all the contents ; AIM , Theory , Appratus , Chemicals Required , Reactions , Procedure , Result and Precautions .