Problem Solving in Genetics: Content Hints Can Help
Affiliation.
- 1 Department of Molecular, Cellular, and Developmental Biology, University of Colorado-Boulder, Boulder, CO 80309.
- PMID: 31144570
- PMCID: PMC6755211
- DOI: 10.1187/cbe.18-06-0093
Problem solving is an integral part of doing science, yet it is challenging for students in many disciplines to learn. We explored student success in solving genetics problems in several genetics content areas using sets of three consecutive questions for each content area. To promote improvement, we provided students the choice to take a content-focused prompt, termed a "content hint," during either the second or third question within each content area. Overall, for students who answered the first question in a content area incorrectly, the content hints helped them solve additional content-matched problems. We also examined students' descriptions of their problem solving and found that students who improved following a hint typically used the hint content to accurately solve a problem. Students who did not improve upon receipt of the content hint demonstrated a variety of content-specific errors and omissions. Overall, ultimate success in the practice assignment (on the final question of each topic) predicted success on content-matched final exam questions, regardless of initial practice performance or initial genetics knowledge. Our findings suggest that some struggling students may have deficits in specific genetics content knowledge, which when addressed, allow the students to successfully solve challenging genetics problems.

Publication types
- Research Support, U.S. Gov't, Non-P.H.S.
- Educational Measurement
- Genetics / education*
- Least-Squares Analysis
- Probability
- Problem Solving*
- Recombination, Genetic / genetics
- Regression Analysis
Loading metrics
Open Access
Opinion Piece
Solving the missing heritability problem
* E-mail: [email protected]
Affiliation Big Data Institute, University of Oxford, Oxford, United Kingdom
- Alexander I. Young

Published: June 24, 2019
- https://doi.org/10.1371/journal.pgen.1008222
- Reader Comments
Citation: Young AI (2019) Solving the missing heritability problem. PLoS Genet 15(6): e1008222. https://doi.org/10.1371/journal.pgen.1008222
Editor: Jonathan Flint, University of California Los Angeles, UNITED STATES
Copyright: © 2019 Alexander I. Young. This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Funding: AY was supported by a grant from the Li Ka Shing foundation ( https://www.lksf.org/ ). The funder had no role in the preparation of the article.
Competing interests: The author declares that no competing interests exist.
The problem of missing heritability, that is to say the gap between heritability estimates from genotype data and heritability estimates from twin data, has been a source of debate for about a decade [ 1 ]. It might appear that the advent of whole genome sequence data on tens of thousands of people is poised to resolve the issue, but here I want to sound a note of caution: more sequence data does not mean methodological problems go away…
Heritability measures the overall importance of genetic inheritance in shaping differences between individuals and is defined as the fraction of trait variation in a population due to genetic inheritance [ 2 ]. The advent of twin studies[ 3 ] made it possible to estimate heritability by comparing the phenotypic similarity of identical (monozygotic) twins to non-identical (dizygotic) twins: since monozygotic twins are genetically identical, whereas non-identical twins are only half identical on average, greater similarity of identical over non-identical twins is evidence for a contribution of genetic variation to trait variation. However, the twin design makes several assumptions, most importantly that there is no greater environmental similarity of identical over non-identical twins. Whether twin studies have overestimated heritability for human traits, especially social and behavioural traits, remains controversial [ 4 ].
The dawn of the genome-wide association study (GWAS) era, around the year 2007, brought with it the question: can we identify specific genetic variations that explain the heritability estimated from twin studies? The small sample sizes of early GWAS meant they had power to identify only common genetic variants with relatively strong effects, and the amount of trait variation that these variants explained was typically only a small fraction of the heritability estimated by twin studies. For height, by 2010 around 40 variants had been identified that collectively explained around 5% of the variation in height, compared to a twin heritability of around 80% [ 5 ]. This gap became labelled 'the problem of missing heritability' and has stimulated heated debate ever since [ 1 ].
Many different explanations for the 'missing heritability' have been proposed [ 6 ]. I will focus on two: 1) that complex traits are highly polygenic and affected by many rare variants; 2) that twin studies have overestimated heritability. Note that both of these explanations could contribute to explaining the ‘missing heritability’. The idea behind 1) was that GWAS were not sufficiently powerful to detect the many genetic variants with weak effects on a trait like height, and the genotyping array technologies were not capturing the rare genetic variants that may explain a substantial fraction of the heritability [ 1 , 5 , 6 ]. The idea behind 2) was that twin studies were overestimating heritability, perhaps due to genetic interactions [ 7 ], gene-environment interactions[ 8 ], or violation of twin studies assumptions about the environment [ 4 ], so that less heritability was in fact missing.
The deepest solution to the missing heritability problem would involve identifying all of the causal genetic variants and measuring how much trait variation they explain. An intermediate step towards this solution is to show how much variation we could hope to explain from all measured genetic variation, even if we do not have the statistical power to identify all of the specific causal variants.
In 2010, a paper took a step towards this intermediate solution by showing that ~45% of the variation in height could be explained by the genetic variation captured on a particular genotyping array that measured ~250k common single nucleotide polymorphisms (SNPs) ( Table 1 ). This implied that the genetic variation captured on the genotyping array explained a lot more of the variation in height than the particular variants that had been identified to affect height at the time. Therefore, there were many common variants with relatively weak effects on height that had been missed by GWAS due to a lack of statistical power.
- PPT PowerPoint slide
- PNG larger image
- TIFF original image
https://doi.org/10.1371/journal.pgen.1008222.t001
The authors employed a methodology that later became termed GREML (Genomic Relatedness Restricted Maximum Likelihood) [ 9 , 10 ]. The GREML methodology estimates the variance explained by the SNPs by measuring how phenotypic similarity changes with SNP similarity. Typically, GREML restricts the analysis to distantly related individuals in order to avoid bias due to certain kinds of environmental effects shared between close relatives and genetic interactions [ 5 , 10 ]. The GREML methodology can only capture phenotypic variation explained by SNPs that are correlated with genotyped SNPs due to linkage disequilibrium (LD) [ 9 – 11 ]. Genotyping arrays mostly measure genetic variants that are common in the population, and most rare variants are in low LD with the common variants on a typical genotyping array [ 12 , 13 ], so the GREML methodology applied in 2010 was unable to capture most of the phenotypic variation explained by rare variants. In 2015, the GREML methodology was extended to include rarer genetic variations inferred by imputation [ 12 ], a statistical procedure that can infer genetic variants not measured on a genotyping array through reference to more complete genome sequence data. This increased the variance explained for height from 45% to 56% ( Table 1 ). The question then remained: was the ~80% number from twin studies too high, or do very rare variants that cannot be imputed accurately explain the gap?
One approach to answering this question is to extend the GREML methodology to high quality whole genome sequence (WGS) data[ 13 ], an extension that I’ll call GREML-WGS. WGS data directly measures all genetic variants. If GREML-WGS could show that the variance explained by all sequence variants was in line with twin heritability estimates, this would suggest that the full twin heritability is waiting to be unlocked by large samples with whole genome sequence data. Initial results suggest that a substantial fraction of height variation is explained by the effects of very rare variants that are not well imputed[ 13 ]. This result is plausible for traits under selection, which will tend to make alleles with large effects on traits rare in the population[ 14 ]. If the gap between heritability estimated from imputed SNPs and twin heritability is accounted for by the effects of rare variants that are not well imputed, this would be an important step towards solving the missing heritability problems and be informative of the genetic architecture of complex traits. I therefore outline a series of challenges that would need to overcome before we could be confident of such a result from GREML-WGS.
The first challenge is one of precision. The information used to estimate heritability from rare variants by GREML-WGS comes from the variation in sharing of rare variants among distantly related pairs of individuals [ 13 , 15 ]. However, distantly related individuals typically do not share any particular rare variant, so the variation in rare variant sharing is low. This means that large samples with high quality WGS data are required to obtain precise estimates, and such samples are not common yet. Based on the only existing application of GREML-WGS [ 13 ], a sample size of ~40,000 would produce estimates precise enough to be statistically distinguished from other heritability estimates ( Table 1 ). It is likely that this challenge will be overcome shortly, since samples of similar magnitude already exist [ 16 ].
However, when the challenge of achieving sufficient precision of GREML-WGS estimates is overcome, questions about methodological assumptions remain. The methodology assumes that effect sizes are normally distributed within each bin, where the variants have been divided into bins based upon their frequency and the strength of their correlations with other variants (LD). Since GREML makes inferences about the distribution of effect sizes, GREML heritability estimates can become biased when assumptions about the distribution of effect sizes are violated [ 17 ]. This could be more problematic for the rare variants used in GREML-WGS than for the common variants used in standard GREML, as one expects there to be a small fraction of rare variants with strong effects, implying a large deviation from the assumed normal distribution of effect sizes.
Population stratification presents another challenge for the GREML-WGS methodology. Population stratification occurs when two genetically distinct subpopulations have different mean trait values. This implies that any genetic variant that is differentiated between these subpopulations (which is usually due to chance, i.e. genetic drift) will be correlated with the trait even though it has no causal effect on the trait. Recently, two papers were published showing that stratification has affected genome-wide association studies of height, leading to spurious inferences about selection on height in Europe [ 18 – 20 ]. This work has shown that stratification can remain problematic even after attempting to correct for it using principal component analysis (PCA) [ 21 ], a technique that attempts to infer the major axes of genetic variation in a population (principal components), which are typically associated with geographic separation [ 22 ].
If mean trait values differ along the major principal components, adjusting for the major principal components can remove bias due to population stratification. However, it is hard to accurately infer all of the relevant axes of genetic variation that may be correlated with mean trait values in order to completely eliminate bias due to stratification [ 23 ]. The situation is even trickier for the rare variants used in GREML-WGS. Rare variants tend to have more complicated spatial distributions than common variants, making it even more difficult to infer the axes of genetic variation required to remove bias due to stratification [ 24 , 25 ].
The linear mixed model methodology underlying GREML methods can also be used to adjust for stratification in GWAS [ 30 – 32 ]. Linear mixed model GWAS methods model the effects of genome-wide SNPs jointly with the focal SNP, resulting in an estimate of the variance explained by the genome-wide SNPs and an estimate of the effect of the focal SNP. Linear mixed model GWAS can account for more complicated patterns of stratification than PCA by modelling the effects of all genome-wide SNPs, rather than considering stratification along the major principal components alone, leading to reduced bias in SNP effect estimates compared to PCA adjustment [ 32 ]. However, this implies that those stratification effects that linear mixed models pick up, but PCA misses, are likely to contribute to the heritability estimate from GREML, leading to an overestimation of heritability. Supporting this, I have found evidence that the heritability of height in Iceland was overestimated by a method that is very similar to GREML [ 27 ], and I suspect that this overestimation was due in part to population stratification that had not been properly controlled for by PCA.
The problem of population stratification is even trickier for the very rare variants used by GREML-WGS. The GREML-WGS methodology measures the contribution from rare variants in part by measuring the degree to which pairs of individuals who share rare variants tend to have more similar phenotypes than people who do not. However, if a pair of individuals share a very rare variant, then it is likely that they inherited this variant from a recent common ancestor, even if their genome-wide relatedness is low. Pairs of individuals who share a recent common ancestor are more likely to have similar environments than those who do not, implying that the GREML methodology could mistakenly infer contributions from rare genetic variants that are in fact environmental contributions. It is hard to see how this type of stratification could be corrected for by PCA because it is specific to particular pairs or clusters of individuals who share a recent common ancestor.
It will be difficult to assess the impact of population stratification on GREML-WGS without using some form of family data, where the randomisation of genetic material during meiosis can be used to disentangle genetic from environmental influences [ 34 – 37 ]. Family data can also be used to estimate heritability in a way that is robust to population stratification. Siblings vary in their relatedness due to random inheritance of the same or different copies of parental chromosomes. A method that I call Sib-Regression takes advantage of the random variation in relatedness between siblings in a family to estimate heritability with little bias from population stratification and environment [ 38 ]. However, Sib-Regression requires hundreds of thousands of genotyped siblings pairs to obtain precise estimates. Last year, I described a method, relatedness disequilibrium regression (RDR), that generalises Sib-Regression to all relative pair classes, gaining precision while retaining robustness to population stratification [ 27 ]. Ideally, one would obtain WGS data on a large sample of families and compare Sib-Regression, RDR, and GREML-WGS estimates.
We can examine existing heritability estimates from RDR and Sib-Regression ( Table 1 ) to get a sense of what we might expect from precise GREML-WGS estimates. The RDR estimate of the heritability of height in Iceland is 55% (S.E. 4.4%). The Sib-Regression estimate is 68% (S.E. 9.6%), which gives an estimate of 68% (S.E. 7.9%) when combined with a previous estimate [ 30 ]. These estimates suggest that the heritability of height may be lower than estimated by twin studies. Furthermore, if one compares RDR and Sib-Regression estimates to twin estimates, the RDR and Sib-Regression estimates are consistently lower across traits ( Fig 1 ). This implies that even with WGS data there may still be some ‘missing heritability', in that there is still a gap between heritability estimated from robust genomic methods (RDR and Sib-Regression) and twin estimates.
The error bars give 95% confidence intervals for the estimates. The estimates are taken from Young et al. 2018 [ 27 ]. The RDR and Sib-Regression estimates are from Icelandic samples, and the Swedish twin estimates are taken from various publications utilising the Swedish twin registry [ 27 , 33 ].
https://doi.org/10.1371/journal.pgen.1008222.g001
While my methodological concerns about GREML-WGS might be answered through further analyses for a trait like height, my own work has shown that the GREML approach leads to substantial overestimation of heritability for traits like educational attainment [ 27 ]. This is due to the influence of indirect genetic effects (‘genetic nurture’) from relatives [ 35 ], which are the effects of genetic variants in relatives (mostly siblings and parents) on an individual through their environment. Family data is required to adjust for indirect genetic effects from relatives. Therefore, solving the problem of missing heritability for traits like educational attainment will require large samples of families with WGS data.
Further collection of family data may also contribute to solving a related puzzle about genetic prediction. By using the estimated effects of genome-wide SNPs, a model that predicts trait values from genotype data can be constructed, termed a polygenic score [ 38 ]. While the correlation between the polygenic score and educational attainment suggests that it can predict around 11–13% of the variation in educational attainment, within-family analyses suggest that at least half of this predictive ability comes from indirect genetic effects from relatives, population stratification, and assortative mating [ 35 , 39 ]. Similar results have been obtained for other cognitive and behavioural traits [ 40 , 41 ]. The within-family design removes the total influence of indirect genetic effects from relatives, assortative mating, and population stratification; however, the relative contribution of these different factors to polygenic prediction is not well characterised. Building a better understanding of assortative mating and the relationship between genetic and environmental influences on traits will form a key part of the deep solution to the missing heritability problem, which will also leverage whole genome sequence data to construct a more detailed understanding of genetic architecture and stronger polygenic predictors.
- View Article
- PubMed/NCBI
- Google Scholar
- 2. Falconer D. S. & Mackay T. F. C. Introduction to Quantitative Genetics . (Longman, 1996).
- 23. Chen, C. & Bloemendal, A. MIA: Christina Chen, PCA and stratification in GWAS; Alex Bloemendal, primer on random matrix theory. https://www.youtube.com/watch?v=B7ub92OLw1g (2019).
- 41. Mostafavi, H., Harpak, A., Conley, D. & Pritchard, J. K. Variable prediction accuracy of polygenic scores within an ancestry group.

Unlocking the Secrets of Genetic Variation – Strategies for Genetic Problem Solving
- Post author By admin-science
- Post date 20.12.2023
In the field of genetics, understanding the intricate puzzle of the genome is crucial. Geneticists delve into the realm of DNA, inheritance, genotypes, and mutations to unlock the secrets hidden within our genetic makeup. However, genetic problem solving is not a straightforward task. It requires a combination of analytical thinking, scientific expertise, and attention to detail.
One of the key aspects of genetics problem solving is identifying the problem itself. Whether it is a hereditary disease, a gene mutation, or a complex inheritance pattern, pinpointing the issue is vital for finding the right solution. Geneticists rely on various techniques, such as DNA sequencing and genetic testing, to unravel the mysteries encoded in our genes.
Once the problem is identified, the next step is to develop a systematic approach to solving it. This involves analyzing the available data, studying the inheritance patterns, and considering the potential impact of genetic variations. Strong analytical skills are essential in deciphering the complex puzzle of genetics and connecting the dots between genotype and phenotype.
Furthermore, effective genetics problem solving requires staying up-to-date with the latest research and advancements in the field. Geneticists must continuously expand their knowledge and keep pace with the ever-evolving understanding of the human genome. By integrating new discoveries into their problem-solving strategies, geneticists can approach challenges with fresh perspectives and innovative solutions.
In conclusion, genetics problem solving is a multifaceted task that demands a combination of scientific expertise, analytical thinking, and a constant pursuit of knowledge. By employing these approaches and techniques, geneticists can successfully tackle genetic challenges and contribute to our understanding of the complex world of genetics.
Understanding Genetics Challenges
The field of genetics focuses on the study of genes and their role in inheritable traits and characteristics. The genome, or complete set of genetic material, contains the instructions for building and maintaining an organism. Understanding genetics is crucial for solving problems related to inherited disorders, diseases, and other genetic challenges.
Genes, which are specific segments of DNA, determine an individual’s genotype, or genetic makeup. They play a significant role in shaping various traits, such as eye color, height, and susceptibility to certain diseases. However, genetic variations and mutations can sometimes lead to problems, making it necessary to understand and solve genetic challenges.
Genetic problem-solving involves analyzing and interpreting genetic data and information to identify the cause of a particular condition or trait. Scientists use various techniques, such as DNA sequencing and genetic testing, to study and analyze an individual’s genetic makeup. By identifying specific gene or chromosome abnormalities, researchers can gain insights into the underlying genetic challenge.
One common genetic challenge is understanding and predicting the inheritance patterns of genetic disorders. For example, certain diseases may be inherited in a dominant or recessive manner, meaning they can be passed on from one generation to the next. By studying family pedigrees and patterns of inheritance, scientists can make predictions about the likelihood of an individual inheriting a particular disorder.
Another genetic challenge is identifying and understanding the effects of mutations. Mutations are changes in the DNA sequence that can lead to altered gene function or protein production. Some mutations can cause genetic disorders, while others may have minimal or no impact on an individual’s health. Understanding the consequences of different mutations is essential for diagnosing and treating genetic disorders effectively.
In conclusion, genetics presents various challenges that require a systematic approach to solving problems related to inherited traits, disorders, and diseases. By understanding the intricacies of the genome, genes, inheritance patterns, and mutations, scientists can tackle genetic challenges effectively and make significant advancements in the field of genetics.
The Importance of Genetic Problem Solving
Genetic problem solving plays a crucial role in understanding the complexities of genetics, genes, and DNA. It involves identifying and addressing challenges related to inheritance, genotype, mutation, and the overall structure of the genome.
One of the main reasons why genetic problem solving is important is that it helps scientists and researchers gain a deeper understanding of various genetic disorders and diseases. By analyzing and solving genetic problems, they can identify the specific genes or mutations responsible for certain conditions. This knowledge is vital for developing targeted treatments and therapies.
Furthermore, genetic problem solving is essential for identifying patterns of inheritance. It allows scientists to determine how genetic traits are passed down from one generation to another. By understanding inheritance patterns, researchers can predict the likelihood of certain traits or disorders appearing in offspring, which can be crucial in genetic counseling.
Genetic problem solving also plays a significant role in genomic research. By analyzing genetic data and addressing challenges, scientists can uncover the underlying mechanisms of diseases and uncover potential targets for drug development. This information is invaluable in advancing personalized medicine and precision therapies.
In addition, genetic problem solving is vital for unraveling the intricate relationship between genes and environmental factors. It allows scientists to study the interplay between genetics and lifestyle, uncovering how our genes interact with external factors to influence our health and well-being.
In conclusion, genetic problem solving is a fundamental aspect of genetics research. It allows scientists and researchers to analyze complex genetic challenges and uncover valuable insights into the mechanisms of inheritance, diseases, and the interplay between genes and the environment. By tackling these challenges effectively, we can advance our understanding of genetics and pave the way for new discoveries and therapeutic interventions.
Genetic Testing and Diagnosis
Genetic testing and diagnosis play a crucial role in understanding and addressing genetic challenges. By analyzing an individual’s genotype, healthcare professionals can identify mutations and variations in genes that may be responsible for certain inherited conditions or diseases.
Identifying Mutations
Through genetic testing, scientists and doctors can identify specific mutations or changes in an individual’s genes. These mutations can have a significant impact on the inheritance pattern of certain traits or diseases. By pinpointing these mutations, healthcare professionals can provide more accurate diagnoses and personalized treatment plans.
Inheritance Patterns
Genetic testing also helps to determine the inheritance pattern of certain traits or diseases. This information is crucial for understanding the likelihood of an individual passing on specific genetic conditions to their children. There are several inheritance patterns, including autosomal dominant, autosomal recessive, and X-linked inheritance, each with their own implications for genetic counseling and family planning.
By understanding the inheritance patterns associated with certain conditions, healthcare professionals can provide individuals and families with valuable information about the risks and chances of passing on genetic disorders. This knowledge allows for informed decision-making and proactive measures to manage and prevent the occurrence of certain conditions.
Problem Solving in Genetics
Incorporating genetic testing and diagnosis into problem-solving in genetics is essential. By analyzing an individual’s genes and genome, scientists and doctors can unravel complex genetic challenges and develop effective strategies for managing and treating them.
The information obtained through genetic testing helps healthcare professionals identify the root causes of genetic disorders, allowing for targeted therapies and interventions. Additionally, genetic testing can inform healthcare professionals about an individual’s predisposition to certain conditions, enabling proactive measures to prevent or delay the onset of certain diseases.
Overall, genetic testing and diagnosis are vital components of problem-solving in genetics. By analyzing an individual’s genes and understanding their inheritance patterns, healthcare professionals can provide accurate diagnoses, personalized treatment plans, and valuable information for family planning and genetic counseling.
Genetics in Medicine and Healthcare
Genetics plays a crucial role in medicine and healthcare. Understanding the principles of genetics helps healthcare professionals diagnose and treat a variety of genetic disorders and diseases. By analyzing an individual’s DNA and genes, doctors can determine a person’s genetic makeup and identify potential health problems or genetic conditions they may be predisposed to.
The study of genetics allows for the understanding of how genes are inherited and how mutations can occur. Genes are segments of DNA that provide instructions for the development and functioning of our bodies. By studying genetics, researchers can better grasp the mechanisms behind certain diseases, allowing for targeted therapies and treatments.
Genetic testing has revolutionized the field of medicine and healthcare. By analyzing an individual’s DNA, doctors can identify specific genetic mutations or variations that may contribute to disease development. This information can then be used to provide personalized treatment options and interventions.
Problem solving in genetics is an essential skill in medicine and healthcare. Determining the genotype of an individual or understanding patterns of inheritance can help in diagnosing and treating genetic disorders. Being able to identify mutations or variations in genes allows for better management and prevention of diseases.
In conclusion, genetics plays a vital role in medicine and healthcare. The study of DNA, genes, and inheritance patterns allows for improved diagnosis, treatment, and prevention of genetic disorders and diseases. Problem solving in genetics is an essential skill for healthcare professionals, enabling them to provide personalized and effective care to patients.
Genetic Counseling and Support
Genetic counseling is an essential service for individuals and families facing genetic challenges. It involves providing information and support to help people understand the genetic basis of their condition and make informed decisions about their health and well-being.
Understanding the Genome
Genetic counselors play a crucial role in helping individuals comprehend the complexity of the human genome. They explain how genes are inherited, the impact of different genotypes on health, and the role of DNA in cellular functions. By breaking down complex concepts into understandable terms, genetic counselors empower individuals to navigate the genetic landscape and comprehend the potential genetic contributions to their condition.
Genetic Problem Solving
Genetic counselors guide individuals through the process of problem-solving genetic challenges. They help identify potential genetic mutations or abnormalities and explain the implications for the individual and their family members. By interpreting and analyzing genetic test results, genetic counselors provide clear explanations, answer questions, and outline available options for further evaluation or management.
Additionally, genetic counselors provide emotional and psychological support throughout the genetic problem-solving process. They create a safe space for individuals to express their concerns, fears, and anxieties. Genetic counseling sessions can help individuals cope with the emotional impact of genetic information and make informed decisions regarding their health and future.
Genetic counseling is a multidisciplinary field that combines medical knowledge with counseling skills. It aims to empower individuals and families to understand and manage their genetic conditions effectively. Through genetic counseling and support, individuals can navigate the complex world of genetics, embrace proactive healthcare measures, and make informed decisions about their genetic health.
Advancements in Genetic Research
Genetic research has made significant progress in recent years, enabling scientists to explore and understand the complexities of the genetic code. These advancements have paved the way for new breakthroughs in problem-solving related to genes, genomes, and inheritance.
One of the key areas of advancement is the ability to study the structure and function of genes and the genome. With the advent of technologies like next-generation sequencing, scientists can now analyze millions of DNA sequences simultaneously, allowing for a comprehensive understanding of an individual’s genetic makeup.
This increased knowledge of the genome has opened up new avenues for solving genetic problems. Researchers can now identify specific gene mutations that may be responsible for certain diseases or conditions. By studying the genotype of individuals, scientists can better understand how genetic variations contribute to the development of certain traits or diseases.
Advancements in genetic research have also improved our understanding of inheritance patterns. Scientists can now trace the transmission of genetic traits through generations and identify patterns of inheritance. This knowledge can be invaluable in predicting the likelihood of a certain trait or disease being passed down in a family.
Moreover, advancements in genetic research have allowed for the development of targeted therapies and treatments. By understanding the genetic mutations underlying a disease, researchers can develop treatments that specifically target these mutations, offering more effective and personalized therapies.
In conclusion, advancements in genetic research have revolutionized our understanding of genes, genomes, and inheritance. These advancements have enabled scientists to tackle genetic challenges effectively, leading to improved diagnosis, treatment, and prevention of genetic diseases. As technology continues to advance, we can expect further breakthroughs in the field of genetics and the ability to solve even more complex genetic problems.
Genetic Disorders: Causes and Symptoms
When it comes to solving genetic problems, understanding the causes and symptoms of genetic disorders is crucial. Genetic disorders are the result of abnormalities in an individual’s genome, which is made up of their genes and DNA. These abnormalities can be caused by mutations or changes in the DNA sequence, leading to a variety of health conditions.
Causes of Genetic Disorders
Genetic disorders can be caused by a number of factors. One common cause is inherited genetic mutations, which are passed down from parent to child. These mutations can occur in various genes and can result in a wide range of disorders, including cystic fibrosis, sickle cell anemia, and Huntington’s disease.
Other genetic disorders are caused by spontaneous mutations, which occur randomly and are not inherited from a parent. These mutations can happen during DNA replication or due to environmental factors, such as exposure to radiation or certain chemicals.
Symptoms of Genetic Disorders
The symptoms of genetic disorders can vary widely, depending on the specific disorder and the individual affected. Some genetic disorders may result in physical abnormalities, such as birth defects or developmental delays. Others may cause chronic health conditions, such as diabetes or heart disease.
In some cases, genetic disorders may not present any symptoms at birth but can manifest later in life. For example, certain genetic mutations can increase the risk of developing certain types of cancer later in adulthood.
It is important to note that not all genetic disorders are severe or life-threatening. Some may cause only mild symptoms or have minimal impact on an individual’s overall health.
In conclusion, understanding the causes and symptoms of genetic disorders is crucial for effectively solving genetic problems. By identifying and diagnosing these disorders, healthcare professionals can develop targeted treatment plans and provide appropriate support to individuals and families affected by genetic conditions.
Treating Genetic Disorders
When it comes to genetics, treating genetic disorders requires a solid understanding of the underlying causes and mechanisms. Genes are the building blocks of DNA, and any mutation or anomaly in these genes can lead to a variety of disorders and conditions.
The first step in treating genetic disorders is to accurately diagnose the specific mutation or abnormality present in the patient’s genome. This may involve genetic tests and analysis of the individual’s DNA. Once the specific genotype is identified, medical professionals can develop targeted treatment plans.
Treatment options for genetic disorders can vary depending on the nature and severity of the condition. In some cases, medications may be prescribed to manage symptoms or slow down disease progression. For other disorders, surgical interventions or gene therapies may be necessary to correct or replace faulty genes.
Advances in genetic research have also led to the development of innovative treatments such as gene editing and gene therapy. These cutting-edge techniques aim to directly modify the patient’s genome, either by removing or replacing faulty genes. While these approaches are still being refined, they hold great promise for the future of treating genetic disorders.
It is important to note that treating genetic disorders is often a lifelong process, requiring ongoing monitoring and adjustment of treatment plans. Genetic counseling and support can also play a crucial role in helping individuals and families cope with the challenges posed by genetic conditions.
Overall, addressing genetic disorders requires a multidisciplinary approach, involving genetics experts, medical professionals, and support networks. By leveraging our understanding of genetics and utilizing the latest advancements in the field, we can work towards effectively treating and managing these complex conditions.
Gene Therapy: An Innovative Solution
Gene therapy is a cutting-edge approach to solving genetic challenges. By targeting specific genes and manipulating them, gene therapy aims to correct genetic defects and treat genetic disorders. This revolutionary technique holds great promise in the field of genetics, opening up new possibilities for solving complex genetic problems.
The Importance of Genes in Solving Genetic Challenges
Genes, which are segments of DNA, play a crucial role in determining our traits and characteristics. They carry the instructions for building proteins, which are the building blocks of our bodies. Understanding the function and structure of genes is essential for solving genetic challenges.
Genetics is the study of genes and how they are passed down through generations. It involves the study of inheritance, mutations, and the entire genome. With advancements in technology, scientists can now analyze an individual’s genotype, enabling them to identify potential genetic challenges and devise targeted solutions.
Gene Therapy: A Potential Solution
Gene therapy is an innovative approach that involves introducing functional genes or modifying existing ones to treat genetic disorders. It holds the potential to permanently cure genetic diseases by addressing the root cause at the genetic level. Through various delivery systems, such as viral vectors or gene editing tools like CRISPR-Cas9, gene therapy can target specific genes and correct genetic defects.
This revolutionary technique is still in its early stages but has shown promising results in treating various genetic disorders. By precisely manipulating genes, gene therapy can potentially provide long-term solutions to genetic challenges that were once considered incurable.
In conclusion, gene therapy represents a groundbreaking solution to the complex challenges posed by genetics. By understanding the role of genes in inheritance, mutations, and the overall genome, scientists can develop targeted strategies to tackle genetic disorders. With further advancements in technology and research, gene therapy holds tremendous potential in transforming the field of genetics and offering innovative solutions for genetic challenges.
Genomics and Personalized Medicine
In the field of genetics, genomics plays a crucial role in advancing personalized medicine. Genomics focuses on studying the entire set of genes, known as the genome, to understand the individual’s unique genetic makeup and how it influences their health and responses to different treatments. This approach is essential for solving complex genetic problems.
Personalized medicine utilizes the knowledge gained from genomics to tailor medical treatments and interventions to the specific genotype of each individual. By understanding the variations in genes and genetic mutations, healthcare providers can develop targeted therapies that are more effective and have fewer adverse effects.
Genomic data can help identify individuals who may be at risk of certain genetic diseases or conditions. By analyzing an individual’s genome, scientists can detect genetic variations that may increase the likelihood of developing specific disorders, allowing for early interventions and preventive measures.
Moreover, genomics enables the identification of disease-causing mutations and the understanding of how they impact an individual’s health. This knowledge is crucial for problem-solving in the field of genetics, as it allows researchers and clinicians to develop strategies to mitigate the effects of harmful mutations and potentially correct them.
Furthermore, genomics also plays a key role in the study of inheritance patterns and genetic disorders. By analyzing patterns of inheritance within families and understanding the underlying genetic mechanisms, scientists can better comprehend how genetic conditions are passed down from one generation to another. This understanding aids in the diagnosis and management of genetic disorders.
In conclusion, genomics is a powerful tool in the field of personalized medicine and genetic problem solving. By studying the genome and understanding the influence of genes, genomics provides valuable insights that can be applied to develop targeted therapies, identify individuals at risk of genetic diseases, and better understand inheritance patterns. These advancements contribute to more effective and personalized healthcare.
Genetic Technologies: Tools for Problem Solving
Genetic technologies have revolutionized the field of genetics and have provided scientists with powerful tools to tackle genetic challenges effectively. These technologies have enabled researchers to understand the intricate details of gene mutations and genotypes, ultimately leading to a better comprehension of how genetics affect various aspects of life.
One of the most significant technologies in the field of genetics is DNA sequencing. This technique allows scientists to decipher the complete sequence of an organism’s genome. By reading the sequence of nucleotides in DNA, researchers can identify specific genes and mutations that may be associated with certain traits or diseases. DNA sequencing has had a profound impact on our understanding of genetics and has opened up numerous avenues for further research and discovery.
Another important genetic technology is gene editing. This revolutionary tool allows scientists to precisely modify an organism’s DNA, resulting in targeted changes to the genome. Using techniques such as CRISPR-Cas9, researchers can add, delete, or alter specific genes, thus potentially correcting genetic mutations that cause inherited diseases. Gene editing holds immense promise for the treatment of genetic disorders and has sparked excitement within the scientific community as a potential solution to various genetic challenges.
The field of genetics also benefits from genetic testing technologies. These tests can provide valuable information about an individual’s genetic makeup and their risk of developing certain diseases. By analyzing an individual’s genome, genetic testing can identify mutations or variants that may indicate a predisposition to certain conditions. This information can then be used to inform medical decisions, such as preventive measures or personalized treatments.
Furthermore, advancements in genetics have contributed to a better understanding of inheritance patterns. Scientists can now accurately determine how traits are passed down from one generation to another, providing insights into the genetic basis of various characteristics. This knowledge helps in predicting the likelihood of certain traits or diseases being inherited, enabling individuals to make informed decisions regarding their health and reproduction.
In conclusion, genetic technologies have provided scientists with powerful tools to tackle genetic challenges effectively. From DNA sequencing and gene editing to genetic testing and understanding inheritance patterns, these tools have revolutionized the field of genetics and have the potential to shape the future of medicine and biology.
Genetic Engineering: From Theory to Practice
Genetic engineering is a field of science that involves the manipulation of an organism’s DNA to solve genetic challenges. It is a practical implementation of the concepts and theories developed in the study of genetics, with the goal of altering an organism’s genotype to achieve desired traits or outcomes.
The basis of genetic engineering lies in understanding the role of genes in determining an organism’s traits and inheritance. Genes are segments of DNA that contain the instructions for building and maintaining an organism’s body. By manipulating these genes, scientists can modify the genetic makeup of an organism, altering its characteristics and potentially solving genetic problems.
One of the key tools used in genetic engineering is the genome. The genome is the complete set of DNA in an organism, including all of its genes. By analyzing and understanding the genome, scientists can identify specific genes that are associated with certain traits or problems. This knowledge can then be used to develop strategies for modifying or correcting genetic issues.
Genetic engineering is not limited to any specific organism or problem. It can be applied to plants, animals, and even humans. For example, genetic engineering has been used to develop crops that are resistant to pests or diseases, improving agricultural yield and food security. It has also played a role in medical research, allowing scientists to study and treat genetic disorders.
In order to carry out genetic engineering, scientists use various techniques such as gene editing, gene therapy, and cloning. These techniques involve manipulating and altering DNA to achieve the desired changes in an organism’s genetic makeup. They require a deep understanding of genetics and the ability to solve complex genetic problems.
Overall, genetic engineering is a practical application of the theories and concepts developed in the study of genetics. It allows scientists to manipulate and modify an organism’s DNA to achieve desired outcomes. By understanding the role of genes in determining traits and inheritance, scientists can solve genetic challenges and pave the way for advancements in various fields, from agriculture to medicine.
Applications of Genetics in Agriculture
Genetics plays a crucial role in solving various challenges in agriculture. The knowledge of DNA, mutations, inheritance, and genomes allows scientists and farmers to improve crop yield, enhance plant quality, and develop disease-resistant varieties.
By studying the genotype of plants, scientists can identify and manipulate specific genes responsible for desirable traits. This enables the development of crops with improved drought tolerance, disease resistance, and nutritional content. For example, genetic engineering has facilitated the creation of insect-resistant crops, such as Bt corn, which reduces the need for chemical pesticides.
Genetic techniques also enable the preservation and improvement of livestock breeds. By selectively breeding animals with desired traits, farmers can enhance characteristics such as milk production, meat quality, and disease resistance. Additionally, genetics helps in identifying and eliminating harmful genetic disorders in livestock populations, ensuring healthier and more productive animals.
Furthermore, genetics aids in crop breeding programs by accelerating the process of developing new varieties with desired traits. Through techniques like marker-assisted selection, scientists can identify plants with specific genes of interest and breed them to create improved varieties. This approach saves time and resources by eliminating the need for lengthy traditional breeding methods.
The application of genetics in agriculture also extends to the field of precision farming. By analyzing the genetic makeup of soil and crops, farmers can determine the optimal amount of fertilizers, pesticides, and water required for maximum efficiency. This allows for targeted and sustainable farming practices, reducing chemical usage and minimizing environmental impact.
In conclusion, the application of genetics in agriculture has revolutionized the industry by providing solutions to various challenges. By understanding and manipulating genes, scientists and farmers can improve crop yield, enhance livestock breeds, develop disease-resistant varieties, and optimize farming practices. These advancements contribute to sustainable and efficient agriculture, benefiting both farmers and consumers.
Genetic Diversity and Conservation
Genetic diversity is a fundamental concept in genetics and plays a crucial role in solving genetic challenges. It refers to the variety of genes and alleles within a population or species. Genetic diversity is important for several reasons:
1. Adaptability
Genetic diversity allows a population to be adaptable to changes in their environment. Different individuals may carry different sets of genes, which provide variations in traits that can be advantageous under certain conditions. This diversity increases the chances of survival and reproduction for a population.
2. Conservation of Species
Understanding genetic diversity is essential for conservation efforts. By studying the genetic makeup of individuals within a population, conservationists can identify the level of genetic variation. Low genetic diversity increases the risk of extinction, as it reduces the population’s ability to adapt to changing environments and increases the vulnerability to diseases.
Conservation strategies are aimed at preserving genetic diversity by protecting habitats, preventing habitat fragmentation, and implementing breeding programs that promote outbreeding (breeding individuals with different genetic backgrounds).
3. Mutation Detection
Genetic diversity also plays a vital role in solving genetic problems by providing the genetic variation needed for mutation detection. Mutations are changes in the DNA sequence of genes and are a common source of genetic disorders. By comparing the DNA sequences of individuals with and without a particular disorder, scientists can identify the specific mutations responsible for the condition.
4. Genotype-Phenotype Mapping
Another aspect of genetic diversity is its contribution to solving the problem of mapping genotype to phenotype. Genes determine the traits and characteristics of an individual, and genetic diversity allows for the identification of different genetic variants associated with specific phenotypic traits. By analyzing the genetic diversity within a population, scientists can identify the genetic factors responsible for phenotypic variations.
In conclusion, genetic diversity is a fundamental aspect of genetics problem solving. It enables adaptability, aids in conservation efforts, facilitates mutation detection, and assists in mapping genotypes to phenotypes. Understanding and preserving genetic diversity is crucial for the long-term survival and well-being of populations and species.
Implications of Genetic Discoveries
The discoveries made in the field of genetics have profound implications for various aspects of our lives. Understanding the genotype and genome of individuals has revolutionized the way we approach and solve genetic problems.
Advancements in Problem Solving
By studying the DNA and genetic makeup of individuals, scientists can now identify potential genetic disorders and diseases much earlier. This allows for timely intervention and treatment, ultimately improving the quality of life for affected individuals.
Furthermore, the knowledge of genetic mutations and their implications has opened up new opportunities for solving inherited genetic problems. Genetic counseling can now provide accurate information on the likelihood of passing on genetic disorders, helping individuals make informed decisions about family planning.
Impact on Inheritance and Family Planning
Understanding the genetics involved in inheritance has significant implications for family planning. The ability to identify and screen for genetic mutations allows couples to assess the risks of passing on genetic disorders to their children.
This knowledge empowers individuals and couples to make informed choices about having biological children, potentially reducing the prevalence of genetic disorders in future generations.
Moreover, the advancements in genetics have made it possible to detect genetic abnormalities in embryos during the process of in vitro fertilization. This enables parents to make decisions about selective embryo implantation, reducing the chances of passing on inherited genetic disorders.
In conclusion, the discoveries in genetics have had far-reaching implications for solving genetic problems, understanding inheritance patterns, and making informed decisions about family planning. The understanding of the genotype and genome has opened up new possibilities for improving the lives of individuals affected by genetic disorders and shaping the future of genetic health.
Genetic Privacy and Ethics
As we delve deeper into the field of genetic problem solving, it is important to consider the implications of genetics on our privacy and ethical boundaries. With the advancement of technology, our ability to decode and manipulate the building blocks of life, such as DNA and genes, has become increasingly powerful.
One of the concerns surrounding genetic privacy relates to the potential misuse or misinterpretation of genetic information. This can occur when someone’s genetic data is used without their consent for purposes that may not be in their best interest. For example, insurance companies could discriminate against individuals based on their genetic predisposition to certain health conditions.
Another aspect to consider is the ethical implications of genetic modification and gene editing. With the ability to manipulate the genome comes the responsibility to use this technology in an ethically responsible manner. While genetic modification has the potential to address certain genetic disorders and improve human health, it also raises questions about the boundaries of what is considered acceptable in terms of altering natural inheritance.
Protecting genetic privacy is crucial in order to build trust and confidence in genetics research and its applications. It is important for individuals to have control over their genetic information and to be able to make informed decisions about how it is used. This involves ensuring that individuals have the right to give or withhold consent for the use of their genetic data, and that there are strict regulations and protocols in place to prevent misuse of this information.
In conclusion, as we continue to make progress in solving genetic problems and understanding the complexities of our DNA and genes, we must also carefully consider the ethical implications and privacy concerns that arise. Balancing the need for genetic advancements with protecting the privacy and rights of individuals is crucial for building a responsible and ethical future in genetics research and application.
Educating the Public about Genetics
Understanding the complexities of genetics is crucial for individuals to make informed decisions about their health and well-being. The human genome contains all of the genetic information that determines our unique traits, including our susceptibility to certain diseases. By educating the public about genetics, we can empower individuals to better understand their own inheritance and the potential problems they may face.
One of the key concepts in genetics is the inheritance of traits, which is passed down from parents to their offspring. This process is controlled by our genotype, which is the combination of genes we inherit from our parents. Each gene is a segment of DNA that provides instructions for building and maintaining our bodies.
By explaining the mechanisms of inheritance and the role of genes in our health, we can help the public understand the importance of genetic testing. Genetic testing can help identify potential health risks and guide individuals in making decisions about their lifestyle and medical care. By knowing our genetic makeup, we can take proactive steps to prevent or manage certain genetic conditions.
Genetic counseling is another important aspect of educating the public about genetics. Genetic counselors are trained professionals who can help individuals understand their genetic information and provide guidance on reproductive options and family planning. Through genetic counseling, individuals and families can make well-informed decisions about their genetic health.
Moreover, by raising awareness about genetics, we can promote research and development in the field. Scientific breakthroughs in genetics have the potential to lead to new treatments and therapies for genetic diseases. By supporting genetic research, we can contribute to solving complex genetic problems and improving the health outcomes of individuals and populations.
In conclusion, education plays a vital role in empowering the public to understand and tackle genetic challenges effectively. By providing knowledge about the genome, genetics, inheritance, and the importance of genetic testing and counseling, we can enable individuals to make informed decisions for their health. Through increased awareness and support for genetic research, we can drive progress in solving genetic problems and improve the lives of individuals affected by genetic conditions.
Collaboration for Genetic Problem Solving
Genetic problem solving can be a complex and challenging task. It requires an understanding of the inheritance patterns, the structure of the genome, and the different types of mutations that can occur in the DNA. To effectively tackle genetic challenges, collaboration plays a crucial role.
In a collaborative environment, scientists and researchers with diverse expertise can come together to share their knowledge and skills. This collaboration allows for a more holistic approach to problem solving, as different perspectives can lead to innovative solutions.
When it comes to genetics, collaboration can help in several ways. For example, by pooling resources and sharing data, researchers can collectively analyze large datasets and discover patterns that may not be apparent to individual researchers. This can lead to breakthroughs in understanding genotype-phenotype correlations or identifying disease-causing mutations.
Collaboration also allows for the exchange of ideas and methodologies. Different labs may have different techniques or technologies at their disposal, and by working together, researchers can combine their strengths and leverage each other’s expertise. This can lead to more efficient problem solving and accelerate the pace of genetic research.
Furthermore, collaboration fosters an environment of open communication and learning. By working with others, researchers can expand their knowledge and skills through the transfer of information and experiences. This type of collaborative learning can be invaluable in the rapidly evolving field of genetics, where new discoveries and techniques are constantly emerging.
In conclusion, collaboration is essential in genetic problem solving. By bringing together experts from different disciplines and allowing for the sharing of knowledge and resources, collaboration enhances problem-solving capabilities and accelerates genetic research. In the face of the complex challenges posed by genetics, collaboration is key to unlocking the mysteries of inheritance, the genome, and the mechanisms underlying genetic diseases.
Advocacy for Genetic Research
Advocacy for genetic research plays a crucial role in understanding the complex interplay between mutation and disease. By studying the genome and DNA, scientists can identify potential problems and work towards finding solutions. This field of study, known as genetics, aims to unravel the mysteries of inheritance and how genes influence our health and well-being.
Through advocacy efforts, researchers can secure the resources necessary to fund genetic studies and experiments. This financial support allows scientists to investigate the underlying causes of genetic disorders, such as Huntington’s disease or cystic fibrosis, which can ultimately lead to breakthroughs in treatment and prevention.
Advocacy for genetic research also promotes awareness and understanding among the general public. By highlighting the importance of genetics and its role in health, advocates can dispel misconceptions and reduce the stigma often associated with genetic conditions. This increased awareness fosters a more supportive environment for individuals and families affected by genetic disorders.
Furthermore, advocacy efforts can help shape public policies and regulations related to genetic research. By engaging with lawmakers and policymakers, advocates can influence decisions that affect the research and development of genetic therapies. This collaboration ensures that genetic research remains a priority and receives the necessary support from both public and private sectors.
In conclusion, advocacy for genetic research is vital for innovation and progress in solving genetic problems. By advocating for increased funding, raising public awareness, and influencing policy decisions, advocates play a crucial role in advancing our understanding of genetics and improving the lives of those affected by genetic diseases.
The Future of Genetic Problem Solving
Advancements in genetic research and technology have paved the way for innovative approaches to solving genetic problems. With the understanding of genotypes, DNA, mutations, and inheritance patterns, scientists are now equipped with powerful tools to address various genetic challenges.
Genetic problem solving is a complex process that involves analyzing and interpreting genetic data to identify the underlying causes of diseases, disorders, or traits. It requires a deep understanding of genetics and the ability to navigate through vast amounts of genomic information.
In the future, as our understanding of genetics continues to expand, we can expect more precise and efficient methods of solving genetic problems. New technologies such as CRISPR-Cas9 offer the potential for targeted gene editing, allowing scientists to correct genetic mutations and potentially cure genetic disorders.
Genomic medicine and personalized treatments
One exciting development in the future of genetic problem solving is the emergence of genomic medicine. By analyzing an individual’s DNA, scientists can tailor treatments and therapies to their specific genetic makeup. This personalized approach has the potential to revolutionize medicine, leading to more effective treatments with fewer side effects.
The ability to predict an individual’s risk of developing certain diseases based on their genetic profile opens up new possibilities for preventative medicine. With this knowledge, individuals can make informed lifestyle choices and undergo regular screenings to detect potential health issues early on, leading to better overall outcomes.
The ethical implications
As with any advancement in science, genetic problem solving raises important ethical considerations. Issues surrounding privacy, discrimination, and consent must be carefully addressed to ensure that the benefits of genetic research are accessible to all individuals, while minimizing potential harm.
Conclusion: The future of genetic problem solving holds great promise. With advancements in technology and our growing understanding of genetics, we have the potential to solve complex genetic challenges and improve human health. However, it is crucial that these advancements are accompanied by responsible ethical considerations to ensure that the benefits reach everyone and are used for the greater good.
Addressing Genetic Challenges on a Global Scale
Genetics plays a crucial role in understanding and solving various health challenges faced by populations worldwide. The study of genetics enables researchers and healthcare professionals to identify and address genetic mutations that can lead to diseases and disorders.
One of the primary components of genetics is DNA, the molecule that contains the hereditary information passed down from parents to offspring. By studying the structure and function of DNA, scientists can better understand how mutations occur and how they can impact an individual’s health.
Inheritance patterns also play a significant role in genetic challenges. The way genes are passed down from one generation to the next can influence the likelihood of inheriting certain diseases or traits. Understanding these inheritance patterns is essential for identifying individuals who may be at a higher risk and implementing preventive measures accordingly.
Advancements in genome sequencing technologies have revolutionized the field of genetics and have made it possible to analyze an individual’s entire genetic makeup, known as their genome. By studying an individual’s genome, researchers can identify specific genes or variations that may contribute to genetic challenges, such as susceptibility to certain diseases or adverse drug reactions.
Addressing genetic challenges on a global scale requires a multidisciplinary approach. Scientists, healthcare professionals, policymakers, and researchers from around the world must collaborate to develop innovative solutions and interventions. This collaboration can involve sharing genetic data, establishing genetic counseling services, and implementing policies that support genetic research and education.
The complexity of genetic challenges necessitates the development and implementation of effective solving strategies. This includes conducting extensive research to identify the underlying causes of genetic mutations, developing targeted therapies and treatments, and providing genetic counseling and testing services to affected individuals and families.
Education and awareness are also crucial in addressing genetic challenges globally. By increasing public knowledge about genetics and the importance of early detection and prevention, individuals can make informed decisions about their health and seek appropriate medical interventions when necessary.
In conclusion, addressing genetic challenges on a global scale requires a comprehensive and collaborative approach. Through advancements in genetics, researchers and healthcare professionals can better understand the causes and effects of genetic mutations and develop effective strategies to tackle these challenges. By fostering international cooperation and promoting education and awareness, we can work towards improving the health and well-being of populations worldwide.
Empowering Individuals Through Genetic Knowledge
Understanding the intricacies of the genome and DNA has allowed us to gain insight into the underlying principles of genetics. Knowing our genotype and understanding how inheritance works can empower individuals to make informed decisions and take control of their genetic health.
By studying genes and their role in inheritance, scientists have been able to identify the risk factors for various genetic disorders. This knowledge helps individuals and their families in making important decisions about their health and potential treatment options.
Genetic mutations are a common occurrence in the human population, and understanding how they can impact our health is crucial. Armed with this information, individuals can take proactive steps to manage their genetic health and seek appropriate medical interventions when necessary.
Problem solving in genetics involves identifying and understanding the underlying genetic causes of a particular issue. This knowledge can help individuals better address and manage genetic challenges they may face in their lives.
By encouraging the dissemination of genetic knowledge and promoting genetic literacy, we can empower individuals to take charge of their genetic health. This includes educating people about the importance of genetic testing, counseling, and the potential implications of genetic information.
In conclusion, genetic knowledge is a powerful tool that can empower individuals to make informed decisions about their health. Understanding the intricacies of our genes and genetic inheritance can help individuals better navigate genetic challenges and take proactive steps towards their genetic well-being.
Overcoming Stigma Associated with Genetic Conditions
Dealing with genetic conditions can be challenging enough without the added burden of societal stigma. Genetic conditions are linked to variations or mutations in an individual’s genes, DNA, or genome, which can cause a range of health and developmental issues. These conditions are not a result of personal choice, but rather the luck of the genetic lottery.
However, due to a lack of understanding and misconceptions, individuals with genetic conditions often face judgment, discrimination, and social exclusion. This stigma can cause significant emotional and psychological distress, as well as hinder access to healthcare and support services.
Overcoming stigma associated with genetic conditions requires a multifaceted approach that involves education, awareness, and advocacy. Education plays a crucial role in dispelling myths and misconceptions about genetics and genetic conditions. By providing accurate information and promoting genetic literacy, we can help society understand that these conditions are not something to be ashamed of.
Additionally, raising awareness about the prevalence and impact of genetic conditions can help break down the walls of stigma. Sharing stories of individuals and families living with genetic conditions can humanize the experience and foster empathy. Genetic conditions are not isolated incidents but affect a significant portion of the population, making it essential to recognize and address the challenges they face.
Advocacy is another vital tool in the fight against stigma. Advocacy efforts can involve supporting legislation and policies that protect the rights of individuals with genetic conditions, promoting inclusion in all aspects of society, and challenging discriminatory practices. By speaking up and advocating for change, we can ensure that individuals with genetic conditions are treated with dignity and respect.
It is essential to emphasize that genetic conditions do not define a person’s worth or potential. Everyone deserves equal opportunities and support regardless of their genotype. We must recognize that genetic variations are a normal part of human diversity and should not be a source of stigma or discrimination.
Overcoming the stigma associated with genetic conditions is not something that can be achieved overnight. It requires ongoing effort, understanding, and compassion from society as a whole. Together, we can create a more inclusive and accepting world for individuals with genetic conditions, where they can thrive and reach their full potential.
Genetics and Psychological Health
The relationship between genetics and psychological health has been a subject of extensive research. Our DNA, housed within the genome, encodes the information that defines our unique characteristics and traits, including our physical and psychological attributes.
Mutations in our DNA can occur during the replication process, leading to changes in our genetic code. These mutations can have an impact on our psychological health and can contribute to the development of mental disorders.
The inheritance of certain genes can also play a role in our psychological health. While many psychological traits are influenced by a combination of genes and environmental factors, certain conditions, such as schizophrenia and bipolar disorder, have a strong genetic component.
Understanding the genotype, or the combination of genes an individual possesses, can help identify potential risks or vulnerabilities to certain psychological disorders. By studying the specific genes associated with different conditions, researchers can gain insight into the biological mechanisms underlying these disorders.
Genetic research has revealed that there is no one-size-fits-all approach to mental health. The interactions between genes and the environment are complex, and different genetic variations can result in different outcomes for individuals facing similar environmental challenges.
By studying the intersection of genetics and psychological health, researchers are working towards developing personalized approaches to mental health treatment. Genetic testing and analysis can help identify individuals who may be more susceptible to certain disorders, allowing for early intervention and targeted treatments.
- Understanding the genetic basis of psychological health
- Identifying genetic mutations and their impact on mental well-being
- Exploring the inheritance patterns of mental disorders
- Examining the role of genotype in psychological vulnerability
- Personalized approaches to mental health based on genetic testing
In conclusion, genetics plays a significant role in psychological health. By understanding the genetic factors that contribute to mental disorders, researchers and healthcare professionals can work towards personalized treatments and interventions, improving the well-being of individuals affected by these conditions.
Creating Accessible Solutions for Genetic Challenges
When it comes to genetic challenges, the complex nature of DNA and mutations can make problem-solving a daunting task. However, with the advancement in genetic research, scientists are finding innovative ways to tackle these challenges.
One of the key aspects of solving genetic problems is understanding the genotype of an individual. Genotype refers to the genetic makeup of an organism, which is determined by the combination of genes inherited from both parents. By studying the inheritance patterns and analyzing the genome, scientists can identify the genetic basis of various disorders and conditions.
Accessible solutions for genetic challenges involve utilizing the knowledge of genes and their functions. Genes are segments of DNA that contain instructions for the production of proteins, which play crucial roles in the body. By understanding the functions of specific genes, scientists can identify the underlying causes of genetic disorders and develop targeted solutions.
Moreover, advancements in technology have enabled researchers to analyze and interpret vast amounts of genetic data. With the help of high-throughput sequencing technologies, scientists can examine the entire genome of an individual, allowing for a comprehensive understanding of genetic variations that may be associated with certain conditions.
To effectively tackle genetic challenges, collaborations and interdisciplinary approaches are essential. The complexity of genetics requires the expertise of geneticists, molecular biologists, bioinformaticians, and other specialists. By combining their knowledge and skills, these professionals can collectively work towards finding solutions for genetic challenges.
In conclusion, solving genetic challenges requires a deep understanding of DNA, mutations, genotypes, inheritance patterns, genomes, and genes. Through the application of innovative technologies and interdisciplinary collaborations, scientists are making significant progress in creating accessible solutions for genetic disorders and conditions.

Key Takeaways: Effective Genetic Problem Solving
- Understanding mutations and their impact on the genome is crucial for effective genetic problem solving.
- Genetic problems often involve analyzing the DNA sequence, identifying genes, and predicting inheritance patterns.
- Analyzing the genotype of individuals and understanding how it relates to phenotypic traits is essential.
- Applying problem-solving skills to genetic scenarios involves careful observation, critical thinking, and logical reasoning.
- Using Punnett squares and pedigrees can help visualize and solve inheritance patterns.
- Problem-solving in genetics often requires understanding complex concepts such as gene linkage, gene expression, and genetic crosses.
- Keeping up with advances in technology, such as CRISPR, can provide new avenues for solving genetic challenges.
- Collaboration and communication with experts and peers can enhance problem-solving abilities in genetics.
- Developing a comprehensive understanding of genetic principles, such as Mendelian inheritance and genetic variation, is foundational to solving genetic problems.
- Practicing problem-solving through solving genetic case studies can improve analytical skills and application of genetic knowledge.
What are some common genetic challenges people face?
Some common genetic challenges people face include inherited diseases, genetic disorders, and the risk of passing on certain genetic conditions to their children.
How can genetic challenges be effectively tackled?
Genetic challenges can be effectively tackled through a combination of genetic testing, counseling, and medical interventions. By identifying the genetic issue, individuals and healthcare professionals can develop appropriate strategies and treatment plans.
What is genetic testing and how does it help in problem solving?
Genetic testing is a process that analyzes an individual’s DNA to identify possible genetic variations or mutations. It helps in problem solving by providing important information about the genetic cause of a condition or disease, enabling targeted treatment and management plans.
Are there any ethical considerations in tackling genetic challenges?
Yes, there are ethical considerations in tackling genetic challenges. Issues such as genetic discrimination, consent, privacy, and the potential misuse of genetic information must be carefully addressed to ensure ethical and responsible practices in genetic problem solving.
What are some future advancements in genetic problem solving?
Some future advancements in genetic problem solving include advancements in gene editing technologies like CRISPR, personalized medicine based on an individual’s genetic profile, and improved understanding of complex genetic interactions that affect human health and traits.
Related posts:
- Free Genetic Problem Worksheet – Practice Genetics with Our Interactive Exercises
- Effective Strategies for Solving Complex Genetic Problems and Achieving Desired Outcomes
- Discover the Answer Key to Genetics Problems and Unlock the Secrets of Heredity
- Test your genetics knowledge with these challenging practice problems!
- The Complete Answers and Solutions for Genetics Problems Worksheet
- Genetic Algorithm – A Powerful Tool for Problem Solving
- Unveiling hidden truths – The power of genetic forensics in crime solving and justice
- The Astonishing World of the Genetic Detective
- Using Forensic Genetic Genealogy to Solve Cold Cases – A Breakthrough in Crime Investigation
- The Knapsack Problem Genetic Algorithm – Solving the Challenge of Optimizing Resource Allocation
Genetic Algorithms and Applications
- Living reference work entry
- First Online: 18 November 2023
- Cite this living reference work entry

- Jonathan Thompson 3
147 Accesses
Genetic algorithms are extremely popular methods for solving optimization problems. They are a population-based method that combine solutions to produce offspring using operators including crossover and mutation. This chapter introduces the general concept of genetic algorithms before describing their main features including the creation of the initial population, the choice of parents, the crossover and mutation operators, and the means for updating the population. The importance of the parameters is discussed , and various interesting adaptations for genetic algorithms are discussed including hybridization, parallelization, and means of maintaining population diversity. Applications are described for the graph coloring problem, nurse scheduling problem, and the job shop scheduling problem, and it is shown that genetic algorithms are still a relevant and current solution method for a wide range of problems.
This is a preview of subscription content, log in via an institution to check access.
Access this chapter
Institutional subscriptions
Aickelin U (2002) An indirect genetic algorithm for set covering problems. J Oper Res Soc 53(10):1118–1126
Article MATH Google Scholar
Aickelin U, Dowsland K (2004) An indirect genetic algorithm for a nurse-scheduling problem. Comput Oper Res 31(5):71–778
Bettemir O, Sonmez R (2015) Hybrid genetic algorithm with simulated annealing for resource-constrained project scheduling. J Manag Eng 31(5):04014082
Article Google Scholar
Bindu M, Sabu M (2020) A hybrid feature selection approach using artificial bee colony and genetic algorithm. In: Advanced computing and communication technologies for high performance applications. IEEE, Piscataway, pp 211–216
Google Scholar
Burke E, Newall J, Weare R (1996) A memetic algorithm for university exam timetabling. In: First international conference on the practice and theory of automated timetabling, pp 241–250
Cattaruzza D, Absi N, Feillet D, Vidal T (2014) A memetic algorithm for the multi trip vehicle routing problem. Eur J Oper Res 236(1):833–848
Article MathSciNet MATH Google Scholar
Chang P, Huang W, Ting C (2010) Dynamic diversity control in genetic algorithm for mining unsearched solution space in TSP problems. Expert Syst Appl 37(3):1863–1878
Cowling P, Kendall G, Soubeiga E (2000) Hyperheuristic approach to scheduling a sales summit. In: Proceedings of the third international conference of practice and theory of automated timetabling, vol 2079, pp 176–190
Cowling P, Kendall G, Han L (2002) An investigation of a hyperheuristic genetic algorithm applied to a trainer scheduling problem. In: Proceedings of the 2002 congress on evolutionary computation, IEEE, vol 2, pp 1185–1190
Davis L (1991) Handbook of genetic algorithms. Van Nostrand Reinhold, New York
Davoodi M, Golsefidi M, Mesgari M (2019) A hybrid optimisation method for vehicle routing problem using artificial bee colony and genetic algorithm. Int Arch Photogram Remote Sens Spatial Inf Sci 42:293–297
Dawkins R (1976) The selfish gene. Oxford University Press, New York
De Jong K (1975) An analysis of the behaviour of a class of genetic adaptive systems. Doctoral thesis, University of Michigan
Della Croce F, Tadei R, Volta G (1995) A genetic algorithm for the job shop problem. Comput Oper Res 22(1):15–24
Douiri S, Elbernoussi S (2015) Solving the graph coloring problem via hybrid genetic algorithms. J King Saud Univ – Eng Sci 27(1):114–118
Duan H, Yu X (2007) Hybrid ant colony optimisation using memetic algorithm for travelling salesman problem. In: IEEE international symposium on approximate dynamic programming and reinforcement learning, pp 92–95
Eiben A, Hinterding R, Michalewicz Z (1999) Parameter control in evolutionary algorithms. IEEE Trans Evol Comput 3(2):124–141
Ferrucci F, Salza P, Sarro F (2018) Using Hadoop mapreduce for parallel genetic algorithms: a comparison of the global, grid and Island models. Evol Comput 26(4):535–567
Galinier P, Hao K (1999) Hybrid evolutionary algorithms for graph coloring. J Comput Optim 3:379–397
Glass C, Prugel-Bennett A (2003) Genetic algorithm for graph coloring: exploration of Galinier and Hao’s algorithm. J Comput Optim 7:229–236
Goldberg D (1989) Genetic algorithms in search: optimization and machine learning. Addison-Wesley, Reading
MATH Google Scholar
Goncalves J, Mendes J, Resende M (2005) A hybrid genetic algorithm for the job shop scheduling problem. Eur J Oper Res 167(1):77–95
Grefenstette J (1981) Parallel adaptive algorithms for function optimisation. Technical report CS-81-19, Vanderbilt University, Nashville
Grobler J, Engelbrecht A, Kendall G, Yadavalli V (2015) Heuristic space diversity control for improved meta-hyper-heuristic performance. Inf Sci 300:49–62
Han L, Kendall G (2003) Guided operators for a hyper-heuristic genetic algorithm. In: Australasian joint conference on artificial intelligence, pp 807–820
Harik G, Lobo F (1999) A parameter-less genetic algorithm. GECCO 99:258–267
Holland J (1975) Adaptation and artificial systems. University of Michigan Press, Ann Arbor
Jat S, Yang S (2011) A hybrid genetic algorithm and Tabu search approach for post enrolment course timetabling. J Sched 14:617–637
Article MathSciNet Google Scholar
Kim S, Ko Y, Uhmn S, Kim J (2014) A strategy to improve performance of genetic algorithm for nurse scheduling problem. Int J Softw Eng Appl 8(1):53–62
Kundu S, Mahato M, Mahanty B, Acharyya S (2008) Comparative performance of simulated annealing and genetic algorithm in solving nurse scheduling problem. In: Proceedings of the international multi conference of engineers and computer scientists, vol 1, pp 961–1000
Li X, Gao L (2016) An effective hybrid genetic algorithm and Tabu search for flexible job shop scheduling problem. Int J Prod Econ 174:93–110
Li T, Yin Y, Yang B, Hou J, Zhou K (2022) A self-learning bee colony and genetic algorithm hybrid for cloud manufacturing services. Computing 104:1977–2003
Lozano M, Herrera F, Cano J (2008) Replacement strategies to preserve useful diversity in steady-state Genetic Algorithms. Inf Sci 178:4421–4433
Lu Z, Hao J (2010) A memetic algorithm for graph coloring. Eur J Oper Res 203(1):241–250
Maenhout B, Vanhoucke M (2008) Comparison and hybridisation of crossover operators for the nurse scheduling problem. Ann Oper Res 159:333–353
Michalewicz Z (1995) A survey of constraint handling techniques in evolutionary computation methods. In: Proceedings of the fourth annual conference on evolutionary programming, San Diego, pp 135–155
Michalewicz Z, Fogel D (2013) How to solve it: modern heuristics. Springer, Berlin
Moz M, Pato M (2007) A genetic algorithm approach to a nurse rerostering problem. Comput Oper Res 34:667–691
Nabeel R (2010) Hybrid genetic algorithms with great deluge for course timetabling. Int J Comput Sci Netw Soc 10:283–288
Park B, Choi H, Kim H (2003) A hybrid genetic algorithm for the job shop scheduling problems. Comput Ind Eng 45(4):597–613
Pezzella F, Morganti G, Ciaschetti G (2008) A genetic algorithm for the flexible job-shop scheduling problem. Comput Oper Res 35(10):3202–3212
Rothlauf F (2011) Design of Modern heuristics: principles and applications. Springer, Berlin
Book MATH Google Scholar
Salhi S (2017) Heuristic search: the emerging science of problem solving. Palgrave Macmillan, Cham
Book Google Scholar
Thangiah SD (2019) A hybrid genetic algorithm, simulated annealing and Tabu search heuristic for vehicle routing problems with time windows. In: Practical handbook of genetic algorithms. CRC Press, Boca Raton, pp 347–384
Chapter Google Scholar
Thomas J, Chaudhari N (2014) Design of efficient packing system using genetic algorithm based on hyper-heuristic approach. Adv Eng Softw 73:45–52
Whitley D (1989) The GENITOR algorithm and selection pressure: why rank-based allocation of reproductive trials is best. In: Proceedings of the third international conference on genetic algorithms, pp 116–121
Zhu K, Liu Z (2004) Population diversity in permutation-based genetic algorithm. In: European conference on machine learning, pp 537–547
Zukhri Z, Paputungan I (2013) A hybrid optimisation algorithm based on genetic algorithm and ant colony optimisation. Int J Artif Intell Appl 4(5):63–75
Download references
Author information
Authors and affiliations.
Cardiff University, Cardiff, UK
Jonathan Thompson
You can also search for this author in PubMed Google Scholar
Corresponding author
Correspondence to Jonathan Thompson .
Editor information
Editors and affiliations.
Institute of Artificial Intelligence, MIT World Peace University, Pune, Pune, Maharashtra, India
Anand J. Kulkarni
Faculty of Engineering and IT, University of Technology Sydney, Ultimo, NSW, Australia
Amir H. Gandomi
Section Editor information
Institute of Artificial Intelligence, Dr Vishwanath Karad MIT World Peace University, Pune, India
Rights and permissions
Reprints and permissions
Copyright information
© 2023 Springer Nature Singapore Pte Ltd.
About this entry
Cite this entry.
Thompson, J. (2023). Genetic Algorithms and Applications. In: Kulkarni, A.J., Gandomi, A.H. (eds) Handbook of Formal Optimization. Springer, Singapore. https://doi.org/10.1007/978-981-19-8851-6_30-1
Download citation
DOI : https://doi.org/10.1007/978-981-19-8851-6_30-1
Received : 31 July 2023
Accepted : 10 August 2023
Published : 18 November 2023
Publisher Name : Springer, Singapore
Print ISBN : 978-981-19-8851-6
Online ISBN : 978-981-19-8851-6
eBook Packages : Springer Reference Intelligent Technologies and Robotics Reference Module Computer Science and Engineering
- Publish with us
Policies and ethics
- Find a journal
- Track your research
- Analysis of Algorithms
- Backtracking
- Dynamic Programming
- Divide and Conquer
- Geometric Algorithms
- Mathematical Algorithms
- Pattern Searching
- Bitwise Algorithms
- Branch & Bound
- Randomized Algorithms
Genetic Algorithms
- Greedy Algorithms
- Math Algorithms
- Algorithms Tutorial
- A* Search Algorithm
- Algorithms Design Techniques
- Greedy Best first search algorithm
- Crossover in Genetic Algorithm
- Simple Genetic Algorithm (SGA)
- Genetic Drift
- Search Algorithms in AI
- ML - Convergence of Genetic Algorithms
- Steady State Genetic Algorithm (SSGA)
- Sequential Covering Algorithm
- Encoding Methods in Genetic Algorithm
- Learn-One-Rule Algorithm
- Algorithms | Misc | Question 14
- Algorithms | Searching | Question 6
- Flajolet Martin Algorithm
Genetic Algorithms(GAs) are adaptive heuristic search algorithms that belong to the larger part of evolutionary algorithms. Genetic algorithms are based on the ideas of natural selection and genetics. These are intelligent exploitation of random searches provided with historical data to direct the search into the region of better performance in solution space. They are commonly used to generate high-quality solutions for optimization problems and search problems.
Genetic algorithms simulate the process of natural selection which means those species that can adapt to changes in their environment can survive and reproduce and go to the next generation. In simple words, they simulate “survival of the fittest” among individuals of consecutive generations to solve a problem. Each generation consists of a population of individuals and each individual represents a point in search space and possible solution. Each individual is represented as a string of character/integer/float/bits. This string is analogous to the Chromosome.
Foundation of Genetic Algorithms
Genetic algorithms are based on an analogy with the genetic structure and behavior of chromosomes of the population. Following is the foundation of GAs based on this analogy –
- Individuals in the population compete for resources and mate
- Those individuals who are successful (fittest) then mate to create more offspring than others
- Genes from the “fittest” parent propagate throughout the generation, that is sometimes parents create offspring which is better than either parent.
- Thus each successive generation is more suited for their environment.
Search space
The population of individuals are maintained within search space. Each individual represents a solution in search space for given problem. Each individual is coded as a finite length vector (analogous to chromosome) of components. These variable components are analogous to Genes. Thus a chromosome (individual) is composed of several genes (variable components).

Fitness Score
A Fitness Score is given to each individual which shows the ability of an individual to “compete” . The individual having optimal fitness score (or near optimal) are sought.
The GAs maintains the population of n individuals (chromosome/solutions) along with their fitness scores.The individuals having better fitness scores are given more chance to reproduce than others. The individuals with better fitness scores are selected who mate and produce better offspring by combining chromosomes of parents. The population size is static so the room has to be created for new arrivals. So, some individuals die and get replaced by new arrivals eventually creating new generation when all the mating opportunity of the old population is exhausted. It is hoped that over successive generations better solutions will arrive while least fit die.
Each new generation has on average more “better genes” than the individual (solution) of previous generations. Thus each new generations have better “partial solutions” than previous generations. Once the offspring produced having no significant difference from offspring produced by previous populations, the population is converged. The algorithm is said to be converged to a set of solutions for the problem.
Operators of Genetic Algorithms
Once the initial generation is created, the algorithm evolves the generation using following operators – 1) Selection Operator: The idea is to give preference to the individuals with good fitness scores and allow them to pass their genes to successive generations. 2) Crossover Operator: This represents mating between individuals. Two individuals are selected using selection operator and crossover sites are chosen randomly. Then the genes at these crossover sites are exchanged thus creating a completely new individual (offspring). For example –
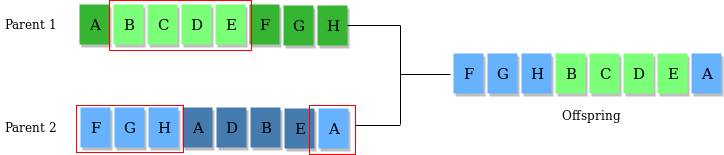
3) Mutation Operator: The key idea is to insert random genes in offspring to maintain the diversity in the population to avoid premature convergence. For example –
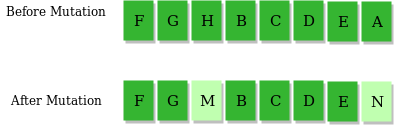
The whole algorithm can be summarized as –
Example problem and solution using Genetic Algorithms
Given a target string, the goal is to produce target string starting from a random string of the same length. In the following implementation, following analogies are made –
- Characters A-Z, a-z, 0-9, and other special symbols are considered as genes
- A string generated by these characters is considered as chromosome/solution/Individual
Fitness score is the number of characters which differ from characters in target string at a particular index. So individual having lower fitness value is given more preference.
Note: Every-time algorithm start with random strings, so output may differ
As we can see from the output, our algorithm sometimes stuck at a local optimum solution, this can be further improved by updating fitness score calculation algorithm or by tweaking mutation and crossover operators.
Why use Genetic Algorithms
- They are Robust
- Provide optimisation over large space state.
- Unlike traditional AI, they do not break on slight change in input or presence of noise
Application of Genetic Algorithms
Genetic algorithms have many applications, some of them are –
- Recurrent Neural Network
- Mutation testing
- Code breaking
- Filtering and signal processing
- Learning fuzzy rule base etc
Please Login to comment...
Similar reads, improve your coding skills with practice.
What kind of Experience do you want to share?
Scientists find a likely cause of many unexplained cases of intellectual disability: A genetic disorder
A newly identified neurodevelopmental disorder may explain tens of thousands of cases of intellectual disability whose cause was previously unknown, according to a new study.
The research, published Friday in the journal Nature Medicine, investigates the effects of mutations in the gene RNU4-2, which is found in all animals, plants and fungi.
The gene plays an important role in gene splicing — the process of cutting out portions of genetic material and stitching others together. Ernest Turro, the new study’s senior author and an associate professor of genetics and genomic science at the Icahn School of Medicine at Mount Sinai, said that in theory, mutations in the RNU4-2 gene could disrupt that splicing process, ultimately leading to abnormal brain development and intellectual disability.
This type of disability is characterized by significant limitations to a person’s ability to learn, reason, problem-solve, communicate or socialize, and it is often indicated by a low IQ. People with the disorder might also have seizures, motor delays, small heads, short stature or low muscle mass, according to the research.
The researchers hope that genetic tests for intellectual disabilities in children can quickly be updated to screen for the mutations.
“A considerable number of families will finally be able to have a genetic diagnosis,” Turro said.
Dr. Hakon Hakonarson, director of the Center for Applied Genomics at Children’s Hospital of Philadelphia, who was not involved in the study, said that because most cases of intellectual disability don’t have a known cause, the findings could “explain a good number of cases that are currently unexplained.”
The study estimates that up to 1 in 20,000 young people might have the condition. Researchers don’t know about the life expectancy associated with the disorder, so they have not estimated its prevalence among older adults, but Turro said some people with the genetic mutation have lived into adulthood.
The estimate suggests that the condition is slightly less common than Rett syndrome, a genetic disorder that causes babies to rapidly lose coordination, speech and mobility and affects about 1 in 10,000 female infants.
But Dr. Jeffrey Gruen, a professor of pediatrics and genetics at Yale School of Medicine who was not part of the research, said mutations in the RNU4-2 gene may turn out to be less common than the study suggests. He also questioned whether everyone with the mutations would have obvious learning or developmental issues.
“There are probably tens of thousands of people around the world that carry this, but does it cause intellectual disability in those tens of thousands? I don’t know,” he said. Gruen added, however, that the discovery is significant.
Hakonarson said the mutations probably cause at least some symptoms.
“The likelihood that this is disease-causing with these variants — which are not seen, by the way, in healthy people — is almost 100%,” he said.
The findings are based on data from the National Genomic Research Library, which contains information about the genomes — the entirety of a person’s genetic code — of people in the U.K. The study looked at the genomes of more than 77,000 participants.
Historically, studies of neurodevelopmental disorders have only looked at a small portion of the genome — specifically, so-called coding genes that are involved in the production of proteins. Of the 1,427 genes linked to intellectual disability, all but nine are coding genes.
Instead, Turro and his research team looked at noncoding genes — which don’t produce proteins — in about 5,500 people with intellectual disabilities. Mutations in the RNU4-2 gene were strongly associated with that group, compared with around 46,000 people who did not have intellectual disabilities.
“There’s no question this paper is going to provoke a lot of studies now,” Hakonarson said. “People are going to go hunting for additional genes, because there’s a lot of noncoding RNA genes.”
The mutations in the RNU4-2 gene seem to occur at random, so they most likely can’t be passed from parent to child. For that reason, getting a diagnosis could be a comfort to parents who want to have more children, Turro said.
The researchers said it will be quite some time before they figure out whether the disorder can be treated with drugs or gene therapy.
“These are an extremely tough group of disorders to tackle therapeutically,” Andrew Mumford, a co-author of the study and research director of the South West England NHS Genomic Medicine Service, said on a call with reporters.
But even without an available treatment, he added, families often benefit from having a diagnosis.
“It helps them come to terms with the impact,” he said. “Being able to tell someone, ‘Yes, we have found the cause of development disorder in your child’ is incredibly powerful.”
Gruen said the discovery could also help connect families whose children have the same genetic condition so they can share stories and offer support.
“You could get some idea of what the future holds for them,” Gruen said. “Is this something that could be remediated? Can we expect there to be language? Can we expect there to be motor issues? That’s also very, very important to know.”
Aria Bendix is the breaking health reporter for NBC News Digital.

- Manage Email Subscriptions
- How to Post to DZone
- Article Submission Guidelines
- Manage My Drafts
Cloud Native : How are orgs leveraging a cloud-centric state of development that allow their applications to remain resilient and scalable?
Join our Cloud Native Virtual Roundtable where, alongside great panelists, we'll dive into the essential pillars of cloud-native technology.
Launch your software development career: Dive head first into the SDLC and learn how to build high-quality software and teams.
AI Automation Essentials . Check out this Refcard for all things AI automation, including model training, data security, and more.
- AI Explained: The Critical Phases of Training and Inference
- Optimizing Model Training: Strategies and Challenges in Artificial Intelligence
- Search for Rail Defects (Part 3)
- Architecting High-Performance Supercomputers for Tomorrow's Challenges
- Deciding When and When Not To Use Infrastructure as Code
- A Guide to Regression Analysis Forecasting in Python
- HTTP API: Key Skills for Smooth Integration and Operation, Part 2
- Parsing Structured Environment Variables in Rust
- Data Engineering
Enhancing Vehicle Routing Problems With Deep Reinforcement Learning and Metaheuristics
Combining machine learning methods and traditional optimization techniques for logistics and fleet management execution optimizations..
Join the DZone community and get the full member experience.
The Vehicle Routing Problem (VRP) is a fundamental challenge in logistics and supply chain management, involving the optimization of routes for a fleet of vehicles to deliver goods to a set of customers. The problem's complexity increases with the number of vehicles, delivery points, and constraints such as delivery windows, vehicle capacities, and traffic conditions. Recent advancements in deep reinforcement learning ( DRL ) and metaheuristics offer promising solutions to enhance VRP efficiency and scalability.
Understanding the Vehicle Routing Problem
The VRP can be seen as an extension of the Traveling Salesman Problem ( TSP ), where multiple vehicles must visit a set of locations and return to a central depot. The goal is to minimize the total travel distance or time while satisfying constraints such as vehicle capacity and delivery windows. The combinatorial nature of VRP makes it computationally challenging, especially as the problem size grows.
Metaheuristics for Solving VRP
Metaheuristics are high-level problem-independent algorithmic frameworks that guide other heuristics to explore the solution space effectively. They are particularly useful for solving complex optimization problems like VRP. Some popular metaheuristics include:
- Genetic Algorithms (GA) : Inspired by natural selection, GA uses operations like selection, crossover, and mutation to evolve a population of solutions.
- Simulated Annealing (SA) : Mimicking the annealing process in metallurgy, SA explores the solution space by probabilistically accepting worse solutions to escape local optima.
- Ant Colony Optimization (ACO) : Based on the foraging behavior of ants, ACO uses pheromone trails to guide the search for optimal routes.
- Tabu Search (TS) : Employing a memory structure, TS avoids revisiting recently explored solutions, helping to escape local optima.
While metaheuristics are effective, their performance can be further enhanced by integrating them with machine learning techniques.
Deep Reinforcement Learning for VRP
Deep Reinforcement Learning (DRL) combines reinforcement learning (RL) with deep neural networks, enabling agents to learn optimal policies for decision-making in complex environments. In the context of VRP, DRL can be used to train agents to make routing decisions that optimize delivery efficiency.
Enhanced State Representation
DRL can enhance VRP by providing richer state representations. A DRL agent can process various features such as vehicle positions, remaining capacities, and customer demands, capturing the complex interactions between these elements. For example, a neural network can encode the state of each vehicle and customer into high-dimensional embeddings, allowing the agent to make more informed routing decisions.
Dynamic Decision-Making
Traditional metaheuristics often rely on static rules for exploring the solution space. In contrast, DRL enables dynamic decision-making by continuously updating policies based on real-time feedback. This adaptability is crucial for VRP, where traffic conditions and customer demands can change dynamically.
Exploration and Exploitation Balance
DRL algorithms, such as Q-learning and Policy Gradient methods, balance exploration (trying new routes), and exploitation (optimizing known routes). This balance helps in discovering better solutions while avoiding suboptimal local minima.
Integrating DRL With Metaheuristics
Combining DRL with metaheuristics can significantly improve VRP solutions. DRL can provide initial solutions and adaptive policies, while metaheuristics can refine these solutions through their robust search capabilities. Here’s how this integration works:
- Initialization with DRL : Use a trained DRL agent to generate an initial set of feasible routes. The agent considers factors like current traffic conditions, vehicle capacities, and delivery deadlines.
- Refinement with Metaheuristics : Apply metaheuristic techniques to refine the DRL-generated routes. For example, use Genetic Algorithms to evolve the initial solutions by iteratively applying crossover and mutation.
- Adaptive optimization : Use the feedback from the metaheuristic optimization process to further train the DRL agent. This iterative approach helps the agent learn from the refinements and improve its initial solution generation.
Practical Example: Capacitated VRP With Time Windows
Let's consider a practical example of solving a Capacitated VRP with Time Windows (CVRPTW) using DRL and metaheuristics.
1. Problem Formulation
- A set of customers with specific demand and time windows.
- A fleet of vehicles with limited capacity.
- A depot as the starting and ending point.
- Minimize total travel distance while ensuring all deliveries are made within the specified time windows and vehicle capacity constraints.
2. DRL for Initial Solution
DRL can be used to generate a high-quality initial solution by training a policy network. The network outputs the probability of selecting the next customer to visit, considering factors like remaining capacity, current location, and time windows.
Pseudo Code: DRL-Based Initial Solution
3. enhancing with metaheuristics.
Once an initial solution is obtained, metaheuristics can refine it. For example, Simulated Annealing (SA) can be applied to explore the solution space and escape local optima.
Pseudo Code: Simulated Annealing Refinement
Future prospects and applications.
Integrating DRL with metaheuristics for VRP is a promising research area with potential applications in various industries. Future research can explore:
- Multi-objective optimization: Balancing multiple objectives such as cost, time, and environmental impact.
- Scalability: Enhancing the scalability of combined DRL-metaheuristic approaches for larger and more complex VRP instances.
- Real-time adaptation: Developing DRL agents that can adapt to real-time changes in traffic, weather, and customer demands.
Enhancing Vehicle Routing Problems with Deep Reinforcement Learning and Metaheuristics offers a powerful approach to tackle the complexities of modern logistics. By leveraging the strengths of both techniques, this hybrid method provides scalable, adaptive, and efficient solutions, paving the way for innovative applications in supply chain management and beyond. As research progresses, the integration of DRL and metaheuristics holds great promise for optimizing a wide range of combinatorial optimization problems.
Opinions expressed by DZone contributors are their own.
Partner Resources
- About DZone
- Send feedback
- Community research
- Advertise with DZone
CONTRIBUTE ON DZONE
- Become a Contributor
- Core Program
- Visit the Writers' Zone
- Terms of Service
- Privacy Policy
- 3343 Perimeter Hill Drive
- Nashville, TN 37211
- [email protected]
Let's be friends:
- Share full article
For more audio journalism and storytelling, download New York Times Audio , a new iOS app available for news subscribers.

- June 7, 2024 • 30:00 Real Teenagers, Fake Nudes: The Rise of Deepfakes in American Schools
- June 6, 2024 • 23:38 The Fight Over the Next Pandemic
- June 5, 2024 • 30:42 Biden’s Push to End the War in Gaza
- June 4, 2024 • 29:17 A Conversation With President Zelensky
- June 3, 2024 • 32:07 How Trump’s Conviction Could Reshape the Election
- May 31, 2024 • 31:29 Guilty
- May 30, 2024 • 25:21 The Government Takes On Ticketmaster
- May 29, 2024 • 29:46 The Closing Arguments in the Trump Trial
- May 28, 2024 • 25:56 The Alitos and Their Flags
- May 24, 2024 • 25:18 Whales Have an Alphabet
- May 23, 2024 • 34:24 I.C.C. Prosecutor Requests Warrants for Israeli and Hamas Leaders
- May 22, 2024 • 23:20 Biden’s Open War on Hidden Fees
How Trump’s Conviction Could Reshape the Election
The guilty verdict in his manhattan criminal trial is set to become a key piece in the 2024 campaign..
Hosted by Michael Barbaro
Featuring Maggie Haberman and Reid J. Epstein
Produced by Rachel Quester , Asthaa Chaturvedi , Mooj Zadie and Diana Nguyen
Edited by Devon Taylor
Original music by Marion Lozano , Elisheba Ittoop and Pat McCusker
Engineered by Chris Wood
Listen and follow The Daily Apple Podcasts | Spotify | Amazon Music | YouTube
Last week, Donald J. Trump became the first U.S. former president to be convicted of a crime when a jury found that he had falsified business records to conceal a sex scandal.
Nate Cohn, who is the chief political analyst at The Times, Maggie Haberman, a senior political correspondent, and Reid J. Epstein, who also covers politics, discuss how the conviction might shape the remaining months of the presidential race.
On today’s episode

Nate Cohn , who is the chief political analyst for The New York Times.

Maggie Haberman , a senior political correspondent for The New York Times.

Reid J. Epstein , who covers politics for The New York Times.

Background reading
The political fallout is far from certain, but the verdict will test America’s traditions and legal institutions .
Watch a video analysis of whether this newfound moment sticks politically.
Democrats are pushing President Biden to make Mr. Trump’s felonies a top 2024 issue .
There are a lot of ways to listen to The Daily. Here’s how.
We aim to make transcripts available the next workday after an episode’s publication. You can find them at the top of the page.
The Daily is made by Rachel Quester, Lynsea Garrison, Clare Toeniskoetter, Paige Cowett, Michael Simon Johnson, Brad Fisher, Chris Wood, Jessica Cheung, Stella Tan, Alexandra Leigh Young, Lisa Chow, Eric Krupke, Marc Georges, Luke Vander Ploeg, M.J. Davis Lin, Dan Powell, Sydney Harper, Mike Benoist, Liz O. Baylen, Asthaa Chaturvedi, Rachelle Bonja, Diana Nguyen, Marion Lozano, Corey Schreppel, Rob Szypko, Elisheba Ittoop, Mooj Zadie, Patricia Willens, Rowan Niemisto, Jody Becker, Rikki Novetsky, John Ketchum, Nina Feldman, Will Reid, Carlos Prieto, Ben Calhoun, Susan Lee, Lexie Diao, Mary Wilson, Alex Stern, Sophia Lanman, Shannon Lin, Diane Wong, Devon Taylor, Alyssa Moxley, Summer Thomad, Olivia Natt, Daniel Ramirez and Brendan Klinkenberg.
Our theme music is by Jim Brunberg and Ben Landsverk of Wonderly. Special thanks to Sam Dolnick, Paula Szuchman, Lisa Tobin, Larissa Anderson, Julia Simon, Sofia Milan, Mahima Chablani, Elizabeth Davis-Moorer, Jeffrey Miranda, Renan Borelli, Maddy Masiello, Isabella Anderson, Nina Lassam and Nick Pitman.
Maggie Haberman is a senior political correspondent reporting on the 2024 presidential campaign, down ballot races across the country and the investigations into former President Donald J. Trump. More about Maggie Haberman
Reid J. Epstein covers campaigns and elections from Washington. Before joining The Times in 2019, he worked at The Wall Street Journal, Politico, Newsday and The Milwaukee Journal Sentinel. More about Reid J. Epstein
Advertisement

IMAGES
VIDEO
COMMENTS
Solutions to Genetics Problems This chapter is much more than a solution set for the genetics problems. Here you will find details concerning the assumptions made, the approaches taken, the predictions that are reasonable, and strategies that you can use to solve any genetics problem. The value of this chapter depends on you.
The Punnett square is a valuable tool, but it's not ideal for every genetics problem. For instance, suppose you were asked to calculate the frequency of the recessive class not for an Aa x Aa cross, not for an AaBb x AaBb cross, but for an AaBbCcDdEe x AaBbCcDdEe cross. If you wanted to solve that question using a Punnett square, you could do it - but you'd need to complete a Punnett square ...
Help with basic genetics problems, including the use of the Punnett square and rules of probability to solve monohybrid, dihybrid and even - wait for it - YE...
Problem solving is an integral part of doing science, yet it is challenging for students in many disciplines to learn. We explored student success in solving genetics problems in several genetics content areas using sets of three consecutive questions for each content area. To promote improvement, we provided students the choice to take a content-focused prompt, termed a "content hint ...
Problem solving is a critical skill in many disciplines but is often a challenge for students to learn. To examine the processes both students and experts undertake to solve constructed-response problems in genetics, we collected the written step-by-step procedures individuals used to solve problems in four different content areas.
BSC 2012. BSC 2011. MENDELIAN GENETICS PROBLEMS. The following problems are provided to develop your skill and test your understanding of solving problems in the patterns of inheritance. They will be most helpful if you solve them on your own. However, you should seek help if you find you cannot answer a problem.
to solve problems in the major areas of genetics. Each chapter begins with Study Hints, in which we suggest ways of organizing your approach to a topic or take you step-by-step through a problem-solving exercise. Some areas, such as linkage and mapping, are given special attention, since they are
Genetics Learning, Problem Solving in Genetics, Biology Teacher Education. Genetics, the central point of developments in the . field of biology, is a particularly difficult subject for teachers and students; since it involves relations between the events of different levels of biologi- cal organization. ...
Problem solving is an integral part of doing science, yet it is challenging for students in many disciplines to learn. We explored student success in solving genetics problems in several genetics content areas using sets of three consecutive questions for each content area. To promote improvement, w …
Genetics problems are solved entirely by logic! How to Start. Begin by writing out all of the information you have about the gene(s) and alleles in question. If you aren't given symbols to use, follow these rules: 1. All alleles of the same gene must be given some version of the same symbol 2. If you have complete dominance, assign a capital ...
Therefore, solving the problem of missing heritability for traits like educational attainment will require large samples of families with WGS data. Further collection of family data may also contribute to solving a related puzzle about genetic prediction. By using the estimated effects of genome-wide SNPs, ...
Explain. 6. You are a scientist performing the first analysis of the genetic basis for the inheritance of flower color in. a certain species of wildflower. You begin your investigation by observing that there are four different flower colors in the local wild population: white, red, blue and purple.
However, genetic problem solving is not a straightforward task. It requires a combination of analytical thinking, scientific expertise, and attention to detail. One of the key aspects of genetics problem solving is identifying the problem itself. Whether it is a hereditary disease, a gene mutation, or a complex inheritance pattern, pinpointing ...
Abstract. Genetic algorithms are extremely popular methods for solving optimization problems. They are a population-based method that combine solutions to produce offspring using operators including crossover and mutation. This chapter introduces the general concept of genetic algorithms before describing their main features including the ...
Exercise #2 — Solving Genetics Problems Report Sheets In this activity, the class will be divided into groups. Each group will be assigned a set of problems to solve. It may help to solve the problems using the following guidelines: 1. Assign letters (alleles) to the various characteristics. 2. Determine the phenotype and genotype of each ...
Problems Involving One Gene. 1. In cats, long hair is recessive to short hair. A true-breeding (homozygous) short-haired male is mated to. a long-haired female. What will their kittens look like? 2. Two cats are mated. One of the parent cats is long-haired (recessive allele).
In this article, I am going to explain how genetic algorithm (GA) works by solving a very simple optimization problem. The idea of this note is to understand the concept of the algorithm by solving an optimization problem step by step. Let us estimate the optimal values of a and b using GA which satisfy below expression.
This type of relationship between alleles, with a heterozygote phenotype intermediate between the two homozygote phenotypes, is called incomplete dominance. We can still use Mendel's model to predict the results of crosses for alleles that show incomplete dominance. For example, self-fertilization of a pink plant would produce a genotype ratio ...
GA is a population-based metaheuristic developed by John Holland in the 1970s. GA uses techniques inspired from nature, more specifically evolution, to find an optimal or near-optimal solution towards a problem. It applies evolution concepts such as reproduction and survival of the fittest to solve a problem.
Mendelian genetics questions. Google Classroom. Suppose a white-furred rabbit breeds with a black-furred rabbit and all of their offspring have a phenotype of gray fur. What does the gene for fur color in rabbits appear to be an example of? Choose 1 answer:
Genetic algorithms are often applied as an approach to solve global optimization problems. As a general rule of thumb genetic algorithms might be useful in problem domains that have a complex fitness landscape as mixing, i.e., mutation in combination with crossover, is designed to move the population away from local optima that a traditional ...
AuPrerequisites: Genetic Algorithm, Travelling Salesman ProblemIn this article, a genetic algorithm is proposed to solve the travelling salesman problem. Genetic algorithms are heuristic search algorithms inspired by the process that supports the evolution of life. The algorithm is designed to replicate the natural selection process to carry genera
Scientists find a likely cause of many unexplained cases of intellectual disability: A genetic disorder. Many neurodevelopmental issues have no known cause. But new research points to mutations in ...
solving genetics problems and determining whether the use of certain processes increases the likelihood of success. The general process of solving a problem has been described as building a mental model in which prior knowledge can be used to represent ways of thinking through a problem state (Johnson-Laird, 2010). Processes used in problem solv-
They are particularly useful for solving complex optimization problems like VRP. Some popular metaheuristics include: ... Genetic Algorithms (GA): Inspired by natural selection, GA uses operations ...
Last week, Donald J. Trump became the first U.S. former president to be convicted of a crime when a jury found that he had falsified business records to conceal a sex scandal. Nate Cohn, who is ...