
Personalised lessons and regular feedback to ensure you ace your exams! Book a free consultation today
100+ Video Tutorials, Flashcards and Weekly Seminars
Gain hands-on experience of how physics is used in different fields. Experience life as a uni student and boost your university application with our summer programme!
- Revision notes >
- A-level Chemistry Revision Notes >
- AQA A-Level Chemistry Revision Notes

Transition Metals - Iodine-Sodium Thiosulfate Titrations (A-Level Chemistry)
Carrying out a iodine-sodium thiosulfate titrations.
In order to find out the concentration of an oxidising agent , I odine-Sodium Thiosulfate titrations can be used.
Table of Contents
This involves adding an acidified solution of potassium iodid e (KI) to a solution of the oxidising agent under investigation. The iodide ions in solution will be oxidised to iodine :
I2 + 2e⁻ ⇌ 2I⁻
For example, if we were using potassium iodate (V) (KIO₃) as the oxidising agent, the reaction would be:
IO₃⁻ (aq) + 2I⁻ (aq) + 6H⁺ (aq) → 3I₂ (aq) + 3H₂O (l)
The higher the concentration of the oxidising agent, the more iodide ions will be oxidised to iodine.
In order to find out how many moles of iodine have been produced, the solution is titrated with a solution of sodium thiosulfate (Na₂S₂O₃) of known concentration . Iodine will react with the thiosulfate ions to form iodide ions once again, turning the solution from brown to colourless:
I₂ (aq) + 2S₂O₃²⁻ (aq) → 2I⁻ (aq) + 2S₄O₆²⁻ (aq)
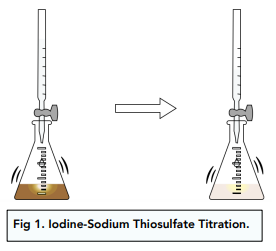
As the thiosulfate solution is added from the burette drop by drop, the iodine solution in the conical flask will gradually become a very pale yellow as the end point is approached. The end point of the titration can therefore be difficult to see.
If we add 2cm³ of starch solution , the reaction mixture will turn dark blue to indicate that iodine is still present. Now you can continue to add sodium thiosulfate drop by drop until the blue colour disappears completely, indicating that all the iodine has just reacted .
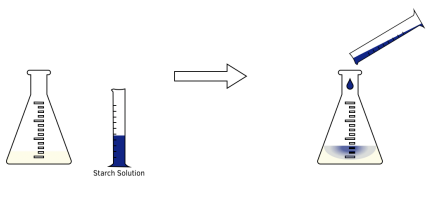
Calculating the Concentration of the Oxidising Agent
In order to find out the concentration of an oxidising agent , we have to carry out two simple stoichiometric calculations .
Worked example: A student adds 25.0 cm³ of potassium iodate (V) solution to an excess of acidified potassium iodide solution. He then titres the resulting solution with 0.120 mol dm-³ sodium thiosulfate solution. It takes 11.0 cm³ of sodium thiosulfate solution to reach the end point in the titration. Calculate the concentration of potassium iodate. (4 marks)
Step 1: Calculate the number of moles of sodium thiosulfate added in the titration.
Number of moles = concentration x volume Number of moles = [0.120 mol dm⁻³ x 11.0 cm³]/1000 = 1.32 x 10⁻³ mol
Step 2: Calculate the number of moles of iodine that have reacted in the titration.
For this use the stoichiometry of the equation:
2 moles of thiosulfate ions are used per mole of iodine (Ratio 2:1)
Therefore, if moles of thiosulfate = 1.32 x 10⁻³ mol Then moles of iodine = 1.32 x 10⁻³ mol / 2 = 6.60 x 10⁻⁴ mol
Step 3: Calculate the number of moles of oxidising agent.
3 moles of iodine are produced for every mole of iodate ions (Ratio 3:1)
Therefore, if moles of iodine = 6.60 x 10⁻⁴ mol Then moles of iodate = 6.60 x 10⁻⁴ mol / 3 = 2.20 x 10⁻⁴ mol
Step 4: Calculate the concentration of oxidising agent.
Number of moles = concentration x volume
Concentration = number of moles / volume Concentration= (2.20 x 10⁻⁴ mol / 25.0cm³) x 1000 = 0.00880 mol dm⁻³

Iodine-Sodium Thiosulfate Titration with Copper (II) ions
An alloy is the combination of metals with other metals or elements.
An iodine-sodium thiosulfate titration can be used to calculate the percentage composition of copper metal in an alloy such as brass. The titration goes as follows:
1. Prepare a a solution of the alloy . A known mass of the alloy is first dissolved in concentrated nitric acid and the mixture made up to 250cm³ by adding deionised water. 25cm³ of the mixture is pipetted into a separate conical flask. Sodium carbonate solution is then slowly added until a white precipitate forms, indicating that any leftover acid has been neutralised. The precipitate can be removed by adding a bit of ethanoic acid .
2. Add an excess of potassium iodide solution . The iodide ions will reduce copper(II) ions in solution to copper (I) ions , forming a wash-off white precipitate of copper (I) iodide .
2Cu²⁺ (aq) + 4I⁻ (aq) → 2CuI (s) + I₂ (aq)
3. Titrate the resulting mixture with sodium thiosulfate solution. Fill a burette with sodium thiosulfate solution of known concentration and add it to the alloy mixture drop by drop until all of the iodine has reacted.
4. Calculate the percentage of copper in the alloy. The number of moles of copper can be calculated from the stoichiometric ratio of Cu²⁺ to I₂ derived from the reaction equation. This can then be used to calculate the mass of copper contained in the alloy sample used and hence its percentage composition.
Transition metals are elements in the periodic table that have partially filled d orbitals in their valence electron shells. They have unique physical and chemical properties that make them useful in various industries and applications.
An Iodine-Sodium Thiosulfate Titration is a laboratory experiment used to determine the amount of iodine present in a sample. Sodium Thiosulfate is used as the titrant, and iodine reacts with it to produce a yellow color. The reaction is monitored until the color disappears, which indicates the end point of the titration. The volume of Sodium Thiosulfate used is then used to calculate the amount of iodine in the sample.
The steps involved in an Iodine-Sodium Thiosulfate Titration are: 1. Preparation of the iodine solution: A known volume of iodine is dissolved in a solvent to make the solution to be titrated. 2. Preparation of the sodium thiosulfate solution: Sodium Thiosulfate is dissolved in water to make a solution that will be used as the titrant. 3. Titration of the iodine solution: A few drops of starch are added to the iodine solution. The sodium thiosulfate solution is then slowly added to the iodine solution while stirring. The reaction produces a yellow color, which disappears when the end point is reached. 4. Calculation of the amount of iodine: The volume of sodium thiosulfate used at the end point is recorded and used to calculate the amount of iodine in the sample.
Starch is used in an Iodine-Sodium Thiosulfate Titration as an indicator to indicate the end point of the reaction. When starch is added to the iodine solution, it reacts with iodine to form a blue-black complex. The appearance of the blue-black color indicates the end point of the titration.
The accuracy of an Iodine-Sodium Thiosulfate Titration can be determined by repeating the experiment several times and calculating the average value. The deviation of the values obtained from the average can be used to determine the accuracy of the experiment. Additionally, the use of a standardized sodium thiosulfate solution can also improve the accuracy of the experiment.
Iodine-Sodium Thiosulfate Titrations are commonly used in analytical chemistry to determine the amount of iodine in a sample. The method is widely used in various industries, such as water treatment, agriculture, and food science, to monitor the levels of iodine in water, soil, and food samples.
Still got a question? Leave a comment
Leave a comment, cancel reply.
Save my name, email, and website in this browser for the next time I comment.
AQA 3.1.1 Atomic structure
Ionisation energies (a-level chemistry), atomic structure – electron arrangement (a-level chemistry), atomic structure – electrons in atoms (a-level chemistry), atomic structure – mass spectrometry (a-level chemistry), atomic structure – element isotopes (a-level chemistry), atomic structure – atomic and mass number (a-level chemistry), atomic structure – subatomic particles (a-level chemistry), aqa 3.1.10 equilibrium constant kp for homogeneous systems, equilibrium constant for homogenous systems – le chatelier’s principle in gas equilibria (a-level chemistry), equilibrium constant for homogenous systems – gas equilibria and kp (a-level chemistry), equilibrium constant for homogeneous system – changing kp (a-level chemistry), equilibrium constant for homogenous systems – gas partial pressures (a-level chemistry), aqa 3.1.11 electrode potentials and electrochemical cells, acids and bases – drawing ph curves (a-level chemistry), acids and bases – acid-base indicators (a-level chemistry), acids and bases – dilutions and ph (a-level chemistry), electrode potentials and electrochemical cells – commercial applications of fuel cells (a-level chemistry), electrode potentials and electrochemical cells – electrochemical cells reactions (a-level chemistry), electrode potentials and electrochemical cells – representing electrochemical cells (a-level chemistry), electrode potentials and electrochemical cells – electrode potentials (a-level chemistry), electrode potentials and electrochemical cells – half cells and full cells (a-level chemistry), aqa 3.1.12 acids and bases, acids and bases – titrations (a-level chemistry), acids and bases – buffer action (a-level chemistry), acids and bases – ph of strong bases (a-level chemistry), acids and bases – ionic product of water (a-level chemistry), acids and bases – more ka calculations (a-level chemistry), acids and bases – the acid dissociation constant, ka (a-level chemistry), acids and bases – the ph scale and strong acids (a-level chemistry), acids and bases – neutralisation reactions (a-level chemistry), acids and bases – acid and base strength (a-level chemistry), acids and bases – the brønsted-lowry acid-base theory (a-level chemistry), aqa 3.1.2 amount of substance, amount of substance – percentage atom economy (a-level chemistry), amount of substance – calculating percentage yields (a-level chemistry), amount of substance – stoichiometric calculations (a-level chemistry), amount of substance – balancing chemical equations (a-level chemistry), amount of substance – empirical and molecular formulae (a-level chemistry), amount of substance – further mole calculations (a-level chemistry), amount of substance- the mole and the avogadro constant (a-level chemistry), amount of substance – measuring relative masses (a-level chemistry), amount of substance – the ideal gas equation (a-level chemistry), aqa 3.1.3 bonding, periodicity – classification (a-level chemistry), bonding – hydrogen bonding in water (a-level chemistry), bonding – forces between molecules (a-level chemistry), bonding – bond polarity (a-level chemistry), bonding – molecular shapes (a-level chemistry), bonding – predicting structures (a-level chemistry), bonding – carbon allotropes (a-level chemistry), bonding – properties of metallic bonding (a-level chemistry), bonding – properties of covalent structures (a-level chemistry), bonding – covalent bonds (a-level chemistry), aqa 3.1.4 energetics, aqa 3.1.5 kinetics, kinetics – the maxwell–boltzmann distribution and catalysts (a-level chemistry), kinetics – the collision theory and reaction rates (a-level chemistry), aqa 3.1.6 chemical equilibria, calculations with equilibrium constants (a-level chemistry), chemical equilibria applied to industry (a-level chemistry), chemical equilibria and le chatelier’s principle (a-level chemistry), aqa 3.1.7 oxidation, reduction and redox, oxidation, reduction and redox equations – balancing redox equations (a-level chemistry), oxidation, reduction and redox equations – redox processes (a-level chemistry), oxidation, reduction and redox equations – oxidation states (a-level chemistry), aqa 3.1.8 thermodynamics, thermodynamic – calculations involving free energy (a-level chemistry), thermodynamic – gibbs free energy (a-level chemistry), thermodynamic – entropy change predictions (a-level chemistry), thermodynamic – total entropy changes (a-level chemistry), thermodynamic – introduction to entropy (a-level chemistry), thermodynamic – calculating enthalpy changes of solution (a-level chemistry), thermodynamic – enthalpy of solution (a-level chemistry), thermodynamic – enthalpy of hydration (a-level chemistry), thermodynamic – calculations involving born-haber cycles (a-level chemistry), thermodynamic – construction of born-haber cycles (a-level chemistry), aqa 3.1.9 rate equations, rate equations – reaction determining steps (a-level chemistry), rate equations – reaction half lives (a-level chemistry), rate equations – uses of clock reactions (a-level chemistry), rate equations – determining orders of reactions graphically (a-level chemistry), rate equations – determining order of reaction experimentally (a-level chemistry), rate equations – temperature changes and the rate constant (a-level chemistry), rate equations – the rate constant (a-level chemistry), rate equations – introduction to orders of reactions (a-level chemistry), rate equations – the rate equation (a-level chemistry), rate equations – measuring rate of reaction (a-level chemistry), aqa 3.2.1 periodicity, periodicity – trends along period 3 (a-level chemistry), aqa 3.2.2 group 2, the alkaline earth metals, uses of group 2 elements and their compounds (a-level chemistry), reactions of group 2 elements (a-level chemistry), group 2, the alkaline earth metals (a-level chemistry), aqa 3.2.3 group 7(17), the halogens, the halogens -halide ions and their reactions (a-level chemistry), the halogens – disproportionation reactions in halogens (a-level chemistry), the halogens – reactions with halogens (a-level chemistry), the halogens – group 7, the halogens (a-level chemistry), aqa 3.2.4 properties of period 3 elements, properties of period 3 elements – properties of period 3 compounds (a-level chemistry), properties of period 3 elements – reactivity of period 3 elements (a-level chemistry), aqa 3.2.5 transition metals, transition metals – autocatalysis of transition metals (a-level chemistry), transition metals – transition metals as homogeneous catalysts (a-level chemistry), transition metals – transition metals as heterogeneous catalysts (a-level chemistry), transition metals – examples of redox reactions in transition metals (a-level chemistry), transition metals – iodine-sodium thiosulfate titrations (a-level chemistry), transition metals – carrying titrations with potassium permanganate (a-level chemistry), transition metals – redox titrations (a-level chemistry), transition metals – redox potentials (a-level chemistry), transition metals – redox reactions revisited (a-level chemistry), transition metals – ligand substitution reactions (a-level chemistry), aqa 3.2.6 reactions of ions in aqueous solution, reactions of ions in aqueous solutions – metal ions in solution (a-level chemistry), aqa 3.3.1 introduction to organic chemistry, introduction to organic chemistry – structural isomers (a-level chemistry), introduction to organic chemistry – e/z isomerism (a-level chemistry), introduction to organic chemistry – reaction mechanisms in organic chemistry (a-level chemistry), introduction to organic chemistry – general formulae (a-level chemistry), introduction to organic chemistry – introduction to functional groups (a-level chemistry), introduction to organic chemistry – naming and representing organic compounds (a-level chemistry), aqa 3.3.10 aromatic chemistry, aromatic chemistry – friedel-crafts acylation and alkylation (a-level chemistry), aromatic chemistry – halogenation reactions in benzene (a-level chemistry), aromatic chemistry – electrophilic substitution reactions in benzene (a-level chemistry), aromatic chemistry – improved benzene model (a-level chemistry), aromatic chemistry – introduction to benzene (a-level chemistry), aqa 3.3.11 amines, amines – nitriles (a-level chemistry), amines – properties and reactivity of amines (a-level chemistry), amines – amine synthesis (a-level chemistry), amines – introduction to amines (a-level chemistry), aqa 3.3.12 polymers, polymer disposal (a-level chemistry), polymer biodegradability (a-level chemistry), condensation polymers (a-level chemistry), polyamide formation (a-level chemistry), aqa 3.3.13 amino acids, amino acids, proteins and dna – dna replication (a-level chemistry), amino acids, proteins and dna – dna (a-level chemistry), amino acids, proteins and dna – enzyme action (a-level chemistry), amino acids, proteins and dna – structure of proteins (a-level chemistry), amino acids, proteins and dna – structure of amino acids (a-level chemistry), aqa 3.3.14 organic synthesis, organic synthesis – considerations in organic synthesis (a-level chemistry), organic synthesis – organic synthesis: aromatic compounds (a-level chemistry), organic synthesis – organic synthesis: aliphatic compounds (a-level chemistry), aqa 3.3.15 nmr, analytical techniques – high resolution ¹h nmr (a-level chemistry), analytical techniques – types of nmr: hydrogen (a-level chemistry), analytical techniques – types of nmr: carbon 13 (a-level chemistry), analytical techniques – nmr samples and standards (a-level chemistry), analytical techniques – nuclear magnetic resonance spectroscopy (a-level chemistry), aqa 3.3.16 chromatography, analytical techniques – different types of chromatography (a-level chemistry), analytical techniques – chromatography (a-level chemistry), aqa 3.3.2 alkanes, alkanes – obtaining alkanes (a-level chemistry), alkanes – alkanes: properties and reactivity (a-level chemistry), aqa 3.3.3 halogenoalkanes, halogenoalkanes – environmental impact of halogenalkanes (a-level chemistry), halogenoalkanes – reactivity of halogenoalkanes (a-level chemistry), halogenoalkanes – introduction to halogenoalkanes (a-level chemistry), aqa 3.3.4 alkenes, alkenes – addition polymerisation in alkenes (a-level chemistry), alkenes – alkene structure and reactivity (a-level chemistry), aqa 3.3.5 alcohols, alcohols – industrial production of alcohols (a-level chemistry), alcohols – alcohol reactivity (a-level chemistry), alcohols – alcohol oxidation (a-level chemistry), alcohols – introduction to alcohols (a-level chemistry), aqa 3.3.6 organic analysis, organic analysis – infrared (ir) spectroscopy (a-level chemistry), organic analysis – identification of functional groups (a-level chemistry), aqa 3.3.7 optical isomerism, optical isomerism (a-level chemistry), aqa 3.3.8 aldehydes and ketones, aldehydes and ketones – reactions to increase carbon chain length (a-level chemistry), aldehydes and ketones – testing for carbonyl compounds (a-level chemistry), aldehydes and ketones – reactivity of carbonyl compunds (a-level chemistry), aldehydes and ketones – carbonyl compounds (a-level chemistry), aqa 3.3.9 carboxylic acids, carboxylic acids and derivatives – structure of amides (a-level chemistry), carboxylic acids and derivatives – acyl groups (a-level chemistry), carboxylic acids and derivatives – properties and reactivity of esters (a-level chemistry), carboxylic acids and derivatives – properties and reactivity of carboxylic acids (a-level chemistry), 21: organic synthesis, 29: intro to organic chemistry, aromatic chemistry – benzene nomenclature (a-level chemistry), cie 1: atomic structure, bonding – ion formation (a-level chemistry), cie 10: group 2, cie 11: group 17, cie 13: intro to as organic chemistry, cie 14: hydrocarbons, cie 15: halogen compounds, cie 16: hydroxy compounds, cie 17: carbonyl compounds, cie 18: carboxylic acids and derivatives, cie 19: nitrogen compounds, cie 2: atoms, molecules and stoichiometry, cie 20: polymerisation, cie 22: analytical techniques, cie 23: chemical energetics, cie 24: electrochemistry, cie 25: equilibria, cie 27: group 2 elements, cie 28: chemistry of transition elements, transition metals – colour in transition metal ions (a-level chemistry), transition metals – optical isomerism in complex ions (a-level chemistry), transition metals – cis-trans isomerism in complex ions (a-level chemistry), transition metals – complex ion shape (a-level chemistry), transition metals – ligands (a-level chemistry), transition metals – introduction to complex ions (a-level chemistry), cie 3: chemical bonding, bonding – properties of ionic bonding (a-level chemistry), cie 30: hydrocarbons, aromatic chemistry – reactivity of substituted benzene (a-level chemistry), cie 31: halogen compounds, cie 32: hydroxy compounds, cie 33: carboxylic acids and derivatives, cie 34: nitrogen compounds, cie 35: polymerisation, cie 36: organic synthesis, cie 37: analytic techniques, analytical techniques – deuterium use in ¹h nmr (a-level chemistry), cie 4: states of matter, cie 6: electrochemistry, cie 7: equilibria, cie 8: reaction kinetics, cie 9: the periodic table, cie: 26: reaction kinetics, catalysts, edexcel topic 1: atomic structure and the periodic table, edexcel topic 10: equlibrium 1, edexcel topic 11: equilibrium 2, edexcel topic 12: acid-base equilibria, edexcel topic 13: energetics 2, edexcel topic 14: redox 2, edexcel topic 15: transition metals, edexcel topic 16: kinetics 2, edexcel topic 17: organic chemistry 2, edexcel topic 18: organic chemistry 3, organic synthesis – practical purification techniques (a-level chemistry), organic synthesis – practical preparation techniques (a-level chemistry), edexcel topic 19: modern analytical techniques 2, edexcel topic 2a & b: bonding and structure, edexcel topic 3: redox 1, edexcel topic 4: inorganic chemistry & the periodic table, the halogens – testing for ions (a-level chemistry), edexcel topic 5: formulae, equations and amounts of substance, edexcel topic 6: organic chemistry 1, edexcel topic 7: modern analytical techniques 1, edexcel topic 8, edexcel topic 9: kinetics 1, ocr 2.1.1 atomic structure and isotopes, ocr 2.1.2 compounds, formulae and equations, ocr 2.1.3 amount of substance, ocr 2.1.4 acids, ocr 2.1.5 redox, ocr 2.2.1 electron structure, ocr 2.2.2 bonding and structure, ocr 3.1.1 periodicity, ocr 3.1.2 group 2, ocr 3.1.3 the halogens, ocr 3.1.4 qualitative analysis, ocr 3.2.2 reaction rates, ocr 3.2.3 chemical equilibrium, ocr 4.1.1 basic concepts of organic chemistry, ocr 4.1.2 alkanes, ocr 4.1.3 alkenes, ocr 4.2.1 alcohols, ocr 4.2.2 haloalkanes, ocr 4.2.3 organic synthesis, ocr 4.2.4 analytical techniques, ocr 5.1.1 rates, equilibrium and ph, ocr 5.1.2 how fast, ocr 5.1.3 acids, bases and buffers, ocr 5.2.1 lattice enthalpy, thermodynamic – enthalpy key terms (a-level chemistry), thermodynamic – lattice enthalpies (a-level chemistry), ocr 5.2.2 enthalpy and entropy, ocr 5.2.3 redox and electrode potentials, ocr 5.3.1 transition elements, precipitation reactions of metal ions in solution (a-level chemistry), ocr 5.3.2 qualitative analysis, ocr 6.1.1 aromatic compounds, ocr 6.1.2 carbonyl compounds, ocr 6.1.3 carboxylic acids and esters, ocr 6.2.1 amines, ocr 6.2.2 amino acids, amides and chirality, ocr 6.2.3 polyesters and polyamides, ocr 6.2.4 carbon–carbon bond formation, ocr 6.2.5 organic synthesis, ocr 6.3.1 chromatography and qualitative analysis, ocr 6.3.2 spectroscopy, related links.
- A-level Chemistry Past Papers
Boost your A-Level Chemistry Performance
Get a 9 in A-Level Chemistry with our Trusted 1-1 Tutors. Enquire now.
100+ Video Tutorials, Flashcards and Weekly Seminars. 100% Money Back Guarantee
Gain hands-on experience of how physics is used in different fields. Boost your university application with our summer programme!
Learn live with other students and gain expert tips and advice to boost your score.

Let's get acquainted ? What is your name?
Nice to meet you, {{name}} what is your preferred e-mail address, nice to meet you, {{name}} what is your preferred phone number, what is your preferred phone number, just to check, what are you interested in, when should we call you.
It would be great to have a 15m chat to discuss a personalised plan and answer any questions
What time works best for you? (UK Time)
Pick a time-slot that works best for you ?
How many hours of 1-1 tutoring are you looking for?
My whatsapp number is..., for our safeguarding policy, please confirm....
Please provide the mobile number of a guardian/parent
Which online course are you interested in?
What is your query, you can apply for a bursary by clicking this link, sure, what is your query, thank you for your response. we will aim to get back to you within 12-24 hours., lock in a 2 hour 1-1 tutoring lesson now.
If you're ready and keen to get started click the button below to book your first 2 hour 1-1 tutoring lesson with us. Connect with a tutor from a university of your choice in minutes. (Use FAST5 to get 5% Off!)
Redox Titration -Thiosulfate & Iodine ( Edexcel International A Level Chemistry )
Revision note.

Core Practical 13b: Thiosulfate & Iodine Titration
Iodine-thiosulfate titrations.
- A redox reaction occurs between iodine and thiosulfate ions:
2S 2 O 3 2– (aq) + I 2 (aq) → 2I – (aq) + S 4 O 6 2– (aq)
- The light brown/yellow colour of the iodine turns paler as it is converted to colourless iodide ions
- When the solution is a straw colour, starch is added to clarify the end point
- The solution turns blue/black until all the iodine reacts, at which point the colour disappears.
- This titration can be used to determine the concentration of an oxidising agent , which oxidises iodide ions to iodine molecules
- The amount of iodine is determined from titration against a known quantity of sodium thiosulfate solution
Worked example
Analysis of household bleach
Chlorate(I) ions, ClO - , are the active ingredient in many household bleaches.
10.0 cm 3 of bleach was made up to 250.0 cm 3 . 25.0 cm 3 of this solution had 10.0 cm 3 of 1.0 mol dm -3 potassium iodide and then acidified with 1.0 mol dm -3 hydrochloric acid.
ClO - (aq) + 2I - (aq) + 2H + (aq) → Cl - (aq) + I 2 (aq) + H 2 O (l)
This was titrated with 0.05 mol dm -3 sodium thiosulfate solution giving an average titre of 25.20 cm 3 .
2S 2 O 3 2- (aq) + I 2 (aq) → 2I - (aq) + S 4 O 6 2- (aq)
What is the concentration of chlorate(I) ions in the bleach?
Answer:
- Therefore, 1 : 2 ratio of ClO - (aq) : S 2 O 3 2- (aq)
- Number of moles of ClO - (aq) in 250.0 cm 3 = 6.30 x 10 -4 x 10 = 6.30 x 10 -3 moles
- 10 cm 3 bleach = 6.30 x 10 -3 moles of ClO - ions
- 1.0 dm 3 bleach = 0.630 moles of ClO - ions
- Therefore, the concentration of ClO - ions in the bleach is 0.630 mol dm -3
General sequence for redox titration calculations
- Write down the half equations for the oxidant and reductant
- Deduce the overall equation
- Calculate the number of moles of manganate(VII) or dichromate(VI) used
- Calculate the ratio of moles of oxidant to moles of reductant from the overall redox equation
- Calculate the number of moles in the sample solution of the reductant
- Calculate the number of moles in the original solution of reductant
- Determine either the concentration of the original solution or the percentage of reductant in a known quantity of sample
You've read 0 of your 10 free revision notes
Unlock more, it's free, join the 100,000 + students that ❤️ save my exams.
the (exam) results speak for themselves:
Did this page help you?
- Advanced Inorganic & Organic Chemistry Core Practicals
- Formulae & Equations
- Amount of Substance
- Atomic Structure
- Electrons & Ions
- The Periodic Table
- Ionic & Metallic Bonding & Structure
- Covalent Bonding & Structure
- Introductory Organic Chemistry
Author: Richard
Richard has taught Chemistry for over 15 years as well as working as a science tutor, examiner, content creator and author. He wasn’t the greatest at exams and only discovered how to revise in his final year at university. That knowledge made him want to help students learn how to revise, challenge them to think about what they actually know and hopefully succeed; so here he is, happily, at SME.
Preparation and Standardization of Sodium thiosulphate
Sodium thiosulphate solution is standardized against potassium dichromate in presence of hydrochloric acid and potassium iodide. Potassium dichromate oxidizes the iodide ion in acidic medium to equivalent amount of iodine. The iodine formed in the reaction oxidizes sodium thiosulphate giving sodium tetrathionate ion and the end point is detected by starch solution.
Hence, based on the above theory our aim is to prepare and standardize sodium thiosulphate using potassium dichromate and potassium iodide in presence of hydrochloric acid and starch solution as indicator. 1
REQUIREMENTS
Apparatus: , chemicals: , preparation of sodium thiosulphate solution.
Dissolve 25 g of sodium thiosulphate in CO 2 free water and make the volume upto 1000 ml. Keep the solution aside and filter to remove any cloudieness, if appears.
Preparation of starch solution
Standardization of sodium thiosulphate.
Dissolve 0.125 g of accurately weighed potassium dichromate in 25 ml of water present in a 250 ml erlenmeyer flask. Add 10 ml of hydrochloric acid and 2 g of potassium iodide, stopper, shake and keep in dark for 15 min. Add 100 ml of water to the above mixture and titrate with sodium thiosulphate using starch as the indicator. Near end point the color will be changed from dark blue to bottle green. Each ml of 0.1 M sodium thiosulphate is equivalent to 0.04904 g of K 2 Cr 2 O 7 .
Tabulation of standardization
Normality of sodium thiosulphate = (Weight of Pot.dichromate)/(Volume of sod.thiosulfate consumed)× 0.04904
From the above experiment it was evident that sodium thiosulpahte can be effectively standardized by using potassium dichromate and iodide with hydrochloric acid and starch as the visual indicator. After performing the calculations, strength of the prepared sodium thiosuplhate solution was found to be……..N.
🔴 Labmonk Scholarships. Click here
Leave a Comment Cancel reply
We Labmonk, some scientific researchers unite to design a platform for getting sources of different lab protocols and discuss various research related issues.
Quick links
Choose your topic.
Academia.edu no longer supports Internet Explorer.
To browse Academia.edu and the wider internet faster and more securely, please take a few seconds to upgrade your browser .
Enter the email address you signed up with and we'll email you a reset link.
- We're Hiring!
- Help Center

Standardization of Sodium Thiosulphate (Na2S2O3) solution with a Potassium Dichromate (K2Cr2O7) solution.

Related Papers
Jeremiah Fernandex
Theory Aqueous iodine solutions normally contain potassium iodide (KI), which acts to keep the iodine in solution. This is due to the fact that an equilibrium is set up as follows: I 2 + I-I 3-I 3-is much more soluble than I 2 and it is as I 3-the iodine is kept in solution. In this experiment, a standard (0.06 M) solution of iodine is generated in the conical flask by reacting a standard (0.02 M) solution of potassium iodate, for each titration, with excess potassium iodide. Iodine is liberated from iodate and iodide according to the equation: IO 3-+ 5I-+ 6H + → 3I 2 + 3H 2 O The iodine solution, which is a golden-brown colour, can be titrated against sodium thiosulfate solution. The sodium thiosulfate solution is placed in the burette and, as it is added to the conical flask, it reacts with the iodine and the colour of the solution fades. When it reaches a pale yellow colour, a few drops of a freshly prepared starch solution are added. The solution becomes blue-black, and the titration is continued until it goes colourless. The titration reaction may be represented by the equation: I 2 + 2S 2 O 3 2-→ 2I-+ S 4 O 6 2-(Note that in this experiment a standard solution of iodine is used to standardise a sodium thiosulfate solution. But you also need to know that a standard solution of sodium thiosulfate can be used to standardise an iodine solution.)
Mohr Method
Nazira Mukhanbetova
Objective: Determination of chloride in solid and liquid samples by the Mohr Method Learning Outcome: • Students understand the terms volumetric analysis, morarity, molality normality and redox titration. • Students acquire the skill to prepare standard solutions of silver nitrate and sodium chloride. • Students understand the apparatus used for a titration. • Students acquire the skill to perform the precicpitation-titration in the real lab after understanding the different steps. Titration is a process by which the concentration of an unknown substance in solution is determined by adding measured amounts of a standard solution that reacts with the unknown. Then the concentration of the unknown can be calculated using the stoichiometry of the reaction and the number of moles of standard solution needed to reach the so called end point. Precipitation titrations are based upon reactions that yield ionic compounds of limited solubility. Classification of methods precipitation titration (on titrant): 1.
Mohd Zulhelmy Ahmad
dimas prasetyo
Novem Ylayron
Nurul Syafiqah
Sintia Lestari
Ajay Sharma
Determination of thiosulphate using photochemical exchange reaction of sodium nitroprusside has been investigated. It is an inexpensive, faster and convenient quantitative method. Sodium nitroprusside is a photolabile complex which undergoes photochemical ligand exchange reactions rapidly. Some recent efforts have been made to utilise such reactions for the estimation of some nitrogen containing anions and electron rich organic molecules. The progress of the reaction is observed spectrophotometrically. The effects of different parameters like pH, change of concentration of sodium nitroprusside, concentration of ligands, light intensity etc. on percentage error was investigated. The efforts were made to minimise the percentage error and some optimum conditions were obtained. Such reaction can be used for the determination of thiosulphate in the range of millimoles to micromoles; hence it is important to know whether such estimations can be done successfully and that too with the desi...
James Baker
Yana Mariana
Loading Preview
Sorry, preview is currently unavailable. You can download the paper by clicking the button above.
RELATED PAPERS
Luis Bayro Garibay
Béla Noszál
Journal of Chemical Education
Jennifer Garcia
Farhang Awlqadr
Dr Mohammad Harun-Ur- Rashid
Rese Javellana
Atharva Kulkarni
Darrell Nordstrom
Nirmala Bardiya
Uma Chatterjee
Zunair Ali Syed
David Randall
Analytical Chemistry
Darryl Siemer
Malcolm Clark
Ali Albakaa
WigglyTop xxx
Maddy Parsons
DARREN WONG ZHENG HAO
Engrid Strike
Claudius D'Silva
nik muhammad izzat

RELATED TOPICS
- We're Hiring!
- Help Center
- Find new research papers in:
- Health Sciences
- Earth Sciences
- Cognitive Science
- Mathematics
- Computer Science
- Academia ©2024
Master Chemistry
Iodometric Titration: Principle, Example, Advantages
Written By Adeel Abbas
Table of Contents
Iodometric titration is a method of quantitative analysis that involves the indirect determination of the concentration of an oxidizing agent in a sample solution.

This redox titration relies on the reaction between the oxidizing agent and iodide ions to produce iodine, which is then titrated using a standardized sodium thiosulfate solution.
The endpoint of the titration is indicated by the disappearance of the deep blue color of the starch-iodine complex, offering high precision in determining analyte concentration.
In our previous discussions, we have covered the broader concept of titration , which encompasses various types such as acid-base, redox, complexometric, and precipitation titrations. Each type of titration serves a specific purpose in quantitative analysis, allowing us to determine the concentration of analytes in a wide range of samples.
Moreover, we have explored the uses of titration in diverse fields, ranging from pharmaceuticals to environmental monitoring. This powerful analytical tool enables scientists to measure the concentration of substances with accuracy and reliability, providing valuable insights into chemical processes and ensuring quality control in various industries.
In the titration, the choice of indicators plays a crucial role in determining the endpoint of the titration reaction. We have delved into the fascinating world of indicators, both natural and synthetic, which serve as vital tools in visualizing the completion of titration reactions.
Natural indicators , such as litmus, turmeric, and red cabbage extract, harness the inherent color-changing properties of organic compounds. On the other hand, synthetic indicators , such as phenolphthalein and methyl orange, are carefully synthesized compounds specifically designed for titrimetric applications.
Now, let us delve into the intricacies of iodometric titration and discover its principles, procedure, advantages, and practical applications.
Principle of Iodometric Titration
Iodometric titration is a method used to measure the amount of an oxidizing agent in a solution. It works by mixing the oxidizing agent with iodide ions, which causes iodine to be released. To know when the reaction is complete, we use starch solution as an indicator. The starch forms a blue color when it reacts with iodine. We can tell that the reaction is finished when the blue color fades away.
Procedure of Iodometric Titration
To perform an iodometric titration, we need to follow a step-by-step process carefully. First, we dissolve an oxidizing substance in a suitable liquid. Then, we add sulfuric acid, hydrochloric acid, or acetic acid to make the solution acidic. This acid helps the next part of the reaction. Next, we introduce chlorine, which causes the release of iodide ions.
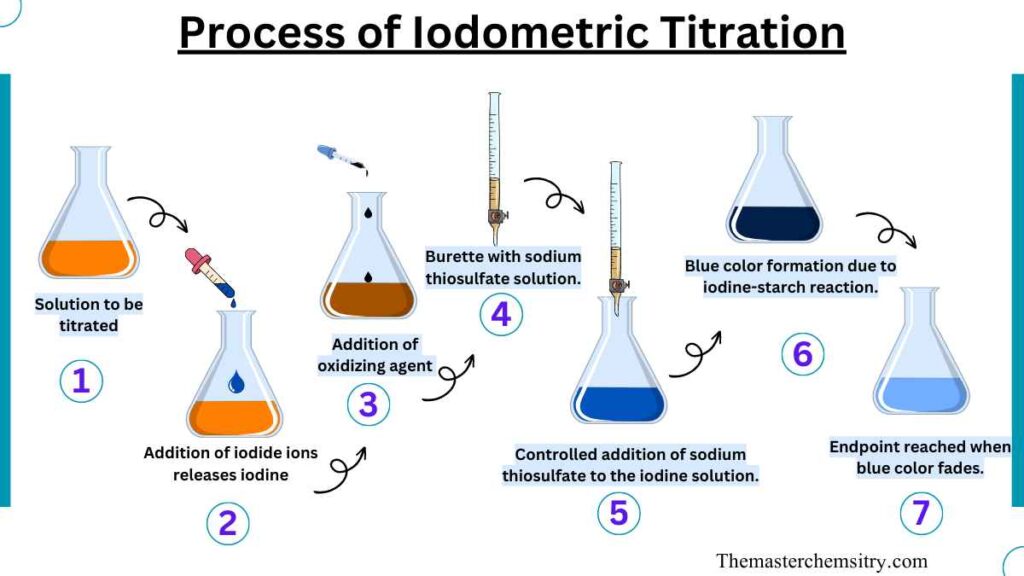
The freed iodide ions are then titrated using a standardized solution of sodium thiosulfate. As the sodium thiosulfate reacts with the iodine, the solution changes color from yellow to a lighter and more diluted shade. Finally, we add a starch indicator to the solution, and the color shifts dramatically from a deep blue to a pale yellow. This change in color tells us that the titration is complete.
Advantages of Iodometric Titration
Iodometric titration offers several noteworthy advantages, solidifying its position as a valuable analytical technique. Firstly, it allows for the precise determination of both reducing and oxidizing agents, expanding its analytical versatility. The visible color change associated with the formation of the starch-iodine complex provides a convenient visual indicator for identifying the endpoint of the titration.
Additionally, iodometric titration requires only small quantities of chemicals or substances, making it a cost-effective and efficient analytical method. The presence of iodine in starch imparts a vivid blue color change, facilitating the easy detection of the completion of the reaction. Moreover, iodometric titration offers the opportunity to visually observe reactivity at equilibrium points, fostering a deeper understanding of redox chemistry and its applications.
Applications of Iodometric Titration
Let us explore some practical applications of iodometric titration, demonstrating its relevance in analytical chemistry. One notable example involves the standardization of sodium thiosulfate (Na 2 S 2 O 3 ) using potassium dichromate (K 2 Cr 2 O 7 ). By accurately determining the concentration of the sodium thiosulfate solution, we can precisely quantify the concentration of the potassium dichromate, a powerful oxidizing agent.
Another application of iodometric titration involves the estimation of Cu(II) (copper(II) oxide) using a sodium thiosulfate solution. This method enables the determination of copper(II) oxide concentration in a given sample, contributing to industries reliant on copper-based materials.
Furthermore, iodometric titration finds application in the estimation of vitamin C, a potent reducing agent, through the iodometric method. This allows for the accurate quantification of vitamin C concentration in various biological samples, shedding light on its role in human health and nutrition.
The realm of iodometric titration unfolds as a captivating avenue in analytical chemistry, empowering scientists to unravel the mysteries of oxidizing agents’ concentrations. With a comprehensive understanding of its principles, procedure, advantages, and practical applications, we equip ourselves with the tools to navigate the intricate world of redox analysis.
Your browser is not supported
Sorry but it looks as if your browser is out of date. To get the best experience using our site we recommend that you upgrade or switch browsers.
Find a solution
- Skip to main content
- Skip to navigation

- Back to parent navigation item
- Primary teacher
- Secondary/FE teacher
- Early career or student teacher
- Higher education
- Curriculum support
- Literacy in science teaching
- Periodic table
- Interactive periodic table
- Climate change and sustainability
- Resources shop
- Collections
- Remote teaching support
- Starters for ten
- Screen experiments
- Assessment for learning
- Microscale chemistry
- Faces of chemistry
- Classic chemistry experiments
- Nuffield practical collection
- Anecdotes for chemistry teachers
- On this day in chemistry
- Global experiments
- PhET interactive simulations
- Chemistry vignettes
- Context and problem based learning
- Journal of the month
- Chemistry and art
- Art analysis
- Pigments and colours
- Ancient art: today's technology
- Psychology and art theory
- Art and archaeology
- Artists as chemists
- The physics of restoration and conservation
- Ancient Egyptian art
- Ancient Greek art
- Ancient Roman art
- Classic chemistry demonstrations
- In search of solutions
- In search of more solutions
- Creative problem-solving in chemistry
- Solar spark
- Chemistry for non-specialists
- Health and safety in higher education
- Analytical chemistry introductions
- Exhibition chemistry
- Introductory maths for higher education
- Commercial skills for chemists
- Kitchen chemistry
- Journals how to guides
- Chemistry in health
- Chemistry in sport
- Chemistry in your cupboard
- Chocolate chemistry
- Adnoddau addysgu cemeg Cymraeg
- The chemistry of fireworks
- Festive chemistry
- Education in Chemistry
- Teach Chemistry
- On-demand online
- Live online
- Selected PD articles
- PD for primary teachers
- PD for secondary teachers
- What we offer
- Chartered Science Teacher (CSciTeach)
- Teacher mentoring
- UK Chemistry Olympiad
- Who can enter?
- How does it work?
- Resources and past papers
- Top of the Bench
- Schools' Analyst
- Regional support
- Education coordinators
- RSC Yusuf Hamied Inspirational Science Programme
- RSC Education News
- Supporting teacher training
- Interest groups

- More navigation items
The chemistry of thiosulfate ions
This experiment will allow students to find out some interesting chemical reactions of sodium thiosulphate, record, observe, and understand this compound
Students will induce reactions between sodium thiosulfate and other chemicals. This practical takes place in three parts, with each part showing learners a new side of this complex substance.
This experiment should take 20 minutes.
Equipment
- Eye protection
- Student worksheet
- Clear plastic sheet (eg OHP sheet)
Solutions should be contained in plastic pipettes. See the accompanying guidance on apparatus and techniques for microscale chemistry , which includes instructions for preparing a variety of solutions.
- Sodium thiosulphate, 0.1 mol dm –3
- Silver nitrate, 0.1 mol dm –3
- Sodium chloride, 0.1 mol dm –3
- Potassium bromide, 0.2 mol dm –3
- Potassium iodide, 0.2 mol dm –3
- Iron(III) nitrate, 0.1 mol dm –3
- Copper(II) sulfate, 0.2 mol dm –3
- Iodine solution, 0.05 mol dm –3 in 0.2 mol dm –3 KI
Health, safety and technical notes
- Read our standard health and safety guidance .
- Wear eye protection for part B and splash resistant goggles to BS EN166 3 for part C.
- Silver nitrate, AgNO 3 (aq), 0.1 mol dm –3 is an eye irritant (see CLEAPSS Hazcard HC087 ). Keep separate from organic waste containers.
- Copper(II) sulfate 0.2 mol dm –3 causes eye damage and is toxic to aquatic life (see CLEAPSS Hazcard HC027c ).
- Iron(III) nitrate, Fe(NO 3 ) 3 .9H 2 O(aq), 0.1 mol dm –3 (see CLEAPSS Hazcard HC055c ), potassium bromide, KBr(aq), 0.2 mol dm –3 , and potassium iodide, KI(aq), 0.2 mol dm –3 (see CLEAPSS Hazcard HC047b ), are low hazard. As is Iodine solution 0.05 mol dm –3 , but this is also toxic to aquatic life (see CLEAPSS Hazard HC054 ).
- Sodium thiosulphate, 0.1 mol dm –3 is low hazard (see CLEAPSS RB087 for preparation and Hazcard HC9 5a ).
Part A — The reaction between thiosulfate ions and iodine solution:
- Cover the worksheets with a clear plastic sheet.
- Put one drop of iodine solution in the box provided on the worksheet.
- Add two drops of thiosulfate solution.
- Observe, comment and write an equation for the reaction.
What type of reaction are you observing?
Part B — The reaction between thiosulfate and silver halide:
- To form the silver halides, first put one drop of silver nitrate solution into each of the empty boxes provided on the worksheet, then add one drop of potassium bromide solution and potassium iodide solutions into the appropriate boxes. Observe and comment.
- Add three drops of sodium thiosulfate solution to each box and stir with the end of a pipette.
- Observe and comment.
What explanations can you give for your observations?
Part C — The reaction between thiosulfate ions and transition metal ions:
- Put two drops of iron(III) solution in the first box provided on the worksheet.
- Put two drops of iron(III) solution and one drop of copper(II) solution in the second box provided.
- Put two drops of copper(II) solution in the third box provided.
- Add one drop of thiosulfate solution to each box and observe carefully, especially the second box.
Observations
The brown colour of iodine is discharged as it is reduced by thiosulfate ions:
I 2 (aq) + S 2 O 3 2– (aq) → 2I – (aq) + S 4 O 6 2– (aq)
The addition of halide ions to the silver nitrate solution produces precipitates of the silver halides – pale yellow (silver bromide) and deeper yellow (silver iodide). Silver bromide dissolves readily in sodium thiosulfate solution, whereas silver iodide is less soluble. This could be used as a test to distinguish a bromide from an iodide.
| Ag+(aq) | + | X-(aq) | → | AgX(s) |
| Silver | Halide | Silver | ||
| Ion | Ion | Halide |
The dissolution of silver bromide in thiosulfate solution is used in the fixing stage in photographic developing. Here, thiosulfate is used to dissolve unreacted silver bromide through the formation of soluble complexes such as Ag(S 2 O 3 ) 2 3– (aq).
The reaction of iron(III) with thiosulfate produces a deep violet complex anion, Fe(S 2 O 3 ) 2 – . This decomposes slowly with the fading of the violet colour:
Fe(S 2 O 3 ) 2 — (aq) + Fe 3+ (aq) → 2Fe 2+ (aq) + S 4 O 6 2– (aq)
The presence of copper(II) ions catalyses the decomposition reaction, and the violet colour fades more rapidly.
Thiosulfate reduces Cu(II) to Cu(I) and complexes the Cu(I):
2S 2 O 3 2– + 2Cu 2+ (aq) → 2Cu + (aq) + S 4 O 6 2– (aq)
2Cu + (aq) + 2S 2 O 3 2– → Cu 2 (S 2 O 3 ) 2 2– (aq)
The characteristic blue colour of copper(II) fades, leaving a colourless solution containing the complex ion Cu 2 (S 2 O 3 ) 2 2– (aq).
The chemistry of thiosulfate ions – teacher notes
The chemistry of thiosulfate ions – student sheet.
S. W. Breuer, Microscale practical organic chemistry . Lancaster: Lancaster University, 1991.
Additional information
This resource is part of our Microscale chemistry collection, which brings together smaller-scale experiments to engage your students and explore key chemical ideas. The resources originally appeared in the book Microscale chemistry: experiments in miniature , published by the Royal Society of Chemistry in 1998.
© Royal Society of Chemistry
Health and safety checked, 2018
- 16-18 years
- Practical experiments
- Redox chemistry
- Reactions and synthesis
Specification
- (c) redox reaction between Cu²⁺ and I⁻ and the determination of the liberated iodine with S₂O₃²⁻
- (o) reaction between aqueous Ag⁺ and halide ions followed by dilute aqueous NH₃
Related articles

4 ways to teach redox in terms of electrons
2024-07-03T05:06:00Z By Kristy Turner
Use these teacher-tested approaches to help learners gain a deeper understanding of redox reactions

Help learners master equilibrium and reversible reactions
2024-06-24T06:59:00Z By Emma Owens
Use this poster, fact sheet and storyboard activity to ensure your 14–16 students understand dynamic equilibrium

Non-burning paper: investigate the fire triangle and conditions for combustion
2024-06-10T05:00:00Z By Declan Fleming
Use this reworking of the classic non-burning £5 note demonstration to explore combustion with learners aged 11–16 years
1 Reader's comment
Only registered users can comment on this article., more experiments.

‘Gold’ coins on a microscale | 14–16 years
By Dorothy Warren and Sandrine Bouchelkia
Practical experiment where learners produce ‘gold’ coins by electroplating a copper coin with zinc, includes follow-up worksheet

Practical potions microscale | 11–14 years
By Kirsty Patterson
Observe chemical changes in this microscale experiment with a spooky twist.

Antibacterial properties of the halogens | 14–18 years
By Kristy Turner
Use this practical to investigate how solutions of the halogens inhibit the growth of bacteria and which is most effective
- Contributors
- Email alerts
Site powered by Webvision Cloud

IMAGES
VIDEO
COMMENTS
Answer. Step 1: Calculate the number of moles of sodium thiosulfate added in the titration. Number of moles = concentration x volume. Number of moles = [0.120 mol dm⁻³ x 11.0 cm³]/1000 = 1.32 x 10⁻³ mol. Step 2: Calculate the number of moles of iodine that have reacted in the titration. For this use the stoichiometry of the equation:
Chemistry 120: Experiment 3 Preparation of Standard Sodium Thiosulfate Solution and Determination of Hypochlorite in a Commercial Bleach Product Iodine can be used as an oxidizing agent in many oxidation-reduction titrations and iodide can be used as a reducing agent in other oxidation-reduction titrations: I2 + 2 e - = 2 I- (1)
The titration screen experiment is a helpful resource to support students to work through the calculations. Another redox titration involves titrating sodium thiosulfate into an unknown quantity of iodine using starch as an indicator.
How can the reaction of iodine and thiosulfate ions be used as a titration?00:00 - Introduction00:06 - Reaction between Iodine and Sodium Thiosulfate00:46 - ...
METTLER TOLEDO Page 1 of 4 Titration ApplicationM009 METTLER TOLEDO Application M009 . Titer Determination of Na. 2. S. 2. O. 3. 0.1 mol/L . Method for titer determination of sodium thiosulfate by redox titration using potassium iodate as the primary standard. Preparation and Procedures . CAUTION ‐ Use safety goggles, a lab coat and wear gloves.
Pipette 10-ml aliquot of prepared standard potassium dichromate solution into 100 ml flask. Add. 10 ml of 10 % KI solution and 10 ml of 1 M H2SO4 solution. Cover the flask with a stopper and put ...
The titration reaction may be represented by the equation: I2 + 2S 2O3 2- → 2I-+ S 4O6 2- Concentration of sodium thiosulfate solution (Note that in this experiment a standard solution of iodine is used to standardise a sodium thiosulfate solution. But you also need to know that a standard solution of sodium thiosulfate can be used to ...
This application report describes the general procedure for the titer determination of Sodium thiosulfate solutions. The titer is a dimensionless number about 1 for correcting the indicated concentration. In the software of the titration devices and application reports from SI Analytics®, the term "Titer" describes the exact
11. Continue to titrate with sodium thiosulfate solution, adding the solution drop wise from the burette as the end point is reached. 12. Record your titre from your first titration in the results table. 13. Perform further titrations until concordant results are obtained. Rough titration Accurate titration 1 Accurate titration 2 Burette ...
Determining the concentration of sodium thiosulfate solution. ... (II) sulfate titration. Mandatory experiment 4.6 - Determination of the amount of iron in an iron tablet. Mandatory experiment 4.7 - An iodine/thisulfate titration. Related articles. Ideas 4 ways to teach redox in terms of electrons. 2024-07-03T05:06:00Z By Kristy Turner.
Iodine-Thiosulfate Titrations. A redox reaction occurs between iodine and thiosulfate ions: 2S2O32- (aq) + I2 (aq) → 2I-(aq) + S4O62- (aq) The light brown/yellow colour of the iodine turns paler as it is converted to colourless iodide ions. When the solution is a straw colour, starch is added to clarify the end point.
2. action of Sodium Thiosulfate and Hydrochloric Acid continuedDiscussionSodium thiosulfate react. ion 1).Na2S2O3(aq) + 2HCl(aq) → S(s) + SO2(g) + 2NaCl(aq) Equation 1The kinetics of the reaction can be analyzed by graphing the. oncentration of Na2S2O3 as a function of both reaction time and 1/time. A plot of concentration versus time gives a ...
f. Sodium Thiosulfate working solution (0.018 M: reagent grade): Bring 100 mL of the sodium thiosulfate stock solution to 1000 mL with distilled water in a 1 liter volumetric flask. This solution is stored in a refrigerator and used for titrations. If a ready-made 0.1 N solution was used for the stock, a working solution of 0.01 N will be fine. g.
1. The indicator (starch) in the iodometric titration is not added in the early stage of the experiment as in acid-base titrations. Starch is only added after titration has begun, i.e. when the colour of the reaction mixture has changed from brown to a light yellow colour. Starch is a colloid that can absorb iodine and form a complex. When this
Standardization of sodium thiosulphate. Dissolve 0.125 g of accurately weighed potassium dichromate in 25 ml of water present in a 250 ml erlenmeyer flask. Add 10 ml of hydrochloric acid and 2 g of potassium iodide, stopper, shake and keep in dark for 15 min. Add 100 ml of water to the above mixture and titrate with sodium thiosulphate using ...
Fill the buret to just above the 0 mL mark with sodium thiosulfate solution. Allow a few milliliters to pour through the buret tip so that any trapped air can be flushed through. Read and record the initial buret level to the nearest 0.05 mL. Perform a 10-fold quantitative dilution of bleach.
The titration reaction may be represented by the equation: I 2 + 2S 2 O 3 2-→ 2I-+ S 4 O 6 2-(Note that in this experiment a standard solution of iodine is used to standardise a sodium thiosulfate solution. But you also need to know that a standard solution of sodium thiosulfate can be used to standardise an iodine solution.)
In this experiment, iodometric titration using a standardized 0 M sodium thiosulfate solution is utilized to determine the percent of copper in an unknown brass sample. To do so, a specified unknown brass sample is digested to liberate copper into its soluble cupric ion (Cu2+) form. Then, excess iodide is added in the form of potassium iodide ...
Applications of Iodometric Titration. Let us explore some practical applications of iodometric titration, demonstrating its relevance in analytical chemistry. One notable example involves the standardization of sodium thiosulfate (Na 2 S 2 O 3) using potassium dichromate (K 2 Cr 2 O 7). By accurately determining the concentration of the sodium ...
This experiment will allow students to find out some interesting chemical reactions of sodium thiosulphate, record, observe, and understand this compound. Students will induce reactions between sodium thiosulfate and other chemicals. This practical takes place in three parts, with each part showing learners a new side of this complex substance.