- Structure of Atom

Cathode Ray Experiment
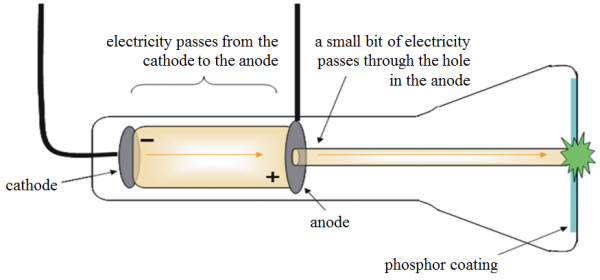
What is cathode ray tube.
A cathode-ray tube (CRT) is a vacuum tube in which an electron beam, deflected by applied electric or magnetic fields, produces a trace on a fluorescent screen.
The function of the cathode ray tube is to convert an electrical signal into a visual display. Cathode rays or streams of electron particles are quite easy to produce, electrons orbit every atom and move from atom to atom as an electric current.
Table of Contents
Cathode ray tube, recommended videos.
- J.J.Thomson Experiment
Apparatus Setup
Procedure of the experiment.
- Frequently Asked Questions – FAQs
In a cathode ray tube, electrons are accelerated from one end of the tube to the other using an electric field. When the electrons hit the far end of the tube they give up all the energy they carry due to their speed and this is changed to other forms such as heat. A small amount of energy is transformed into X-rays.
The cathode ray tube (CRT), invented in 1897 by the German physicist Karl Ferdinand Braun, is an evacuated glass envelope containing an electron gun a source of electrons and a fluorescent light, usually with internal or external means to accelerate and redirect the electrons. Light is produced when electrons hit a fluorescent tube.
The electron beam is deflected and modulated in a manner that allows an image to appear on the projector. The picture may reflect electrical wave forms (oscilloscope), photographs (television, computer monitor), echoes of radar-detected aircraft, and so on. The single electron beam can be processed to show movable images in natural colours.

J. J. Thomson Experiment – The Discovery of Electron
The Cathode ray experiment was a result of English physicists named J. J. Thomson experimenting with cathode ray tubes. During his experiment he discovered electrons and it is one of the most important discoveries in the history of physics. He was even awarded a Nobel Prize in physics for this discovery and his work on the conduction of electricity in gases.
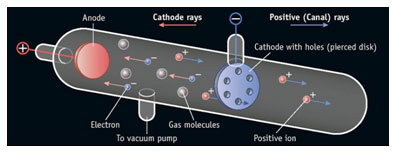
However, talking about the experiment, J. J. Thomson took a tube made of glass containing two pieces of metal as an electrode. The air inside the chamber was subjected to high voltage and electricity flowing through the air from the negative electrode to the positive electrode.
J. J. Thomson designed a glass tube that was partly evacuated, i.e. all the air had been drained out of the building. He then applied a high electric voltage at either end of the tube between two electrodes. He observed a particle stream (ray) coming out of the negatively charged electrode (cathode) to the positively charged electrode (anode). This ray is called a cathode ray and is called a cathode ray tube for the entire construction.
The experiment Cathode Ray Tube (CRT) conducted by J. J. Thomson, is one of the most well-known physical experiments that led to electron discovery . In addition, the experiment could describe characteristic properties, in essence, its affinity to positive charge, and its charge to mass ratio. This paper describes how J is simulated. J. Thomson experimented with Cathode Ray Tube.
The major contribution of this work is the new approach to modelling this experiment, using the equations of physical laws to describe the electrons’ motion with a great deal of accuracy and precision. The user can manipulate and record the movement of the electrons by assigning various values to the experimental parameters.

A Diagram of JJ.Thomson Cathode Ray Tube Experiment showing Electron Beam – A cathode-ray tube (CRT) is a large, sealed glass tube.
The apparatus of the experiment incorporated a tube made of glass containing two pieces of metals at the opposite ends which acted as an electrode. The two metal pieces were connected with an external voltage. The pressure of the gas inside the tube was lowered by evacuating the air.
- Apparatus is set up by providing a high voltage source and evacuating the air to maintain the low pressure inside the tube.
- High voltage is passed to the two metal pieces to ionize the air and make it a conductor of electricity.
- The electricity starts flowing as the circuit was complete.
- To identify the constituents of the ray produced by applying a high voltage to the tube, the dipole was set up as an add-on in the experiment.
- The positive pole and negative pole were kept on either side of the discharge ray.
- When the dipoles were applied, the ray was repelled by the negative pole and it was deflected towards the positive pole.
- This was further confirmed by placing the phosphorescent substance at the end of the discharge ray. It glows when hit by a discharge ray. By carefully observing the places where fluorescence was observed, it was noted that the deflections were on the positive side. So the constituents of the discharge tube were negatively charged.
After completing the experiment J.J. Thomson concluded that rays were and are basically negatively charged particles present or moving around in a set of a positive charge. This theory further helped physicists in understanding the structure of an atom . And the significant observation that he made was that the characteristics of cathode rays or electrons did not depend on the material of electrodes or the nature of the gas present in the cathode ray tube. All in all, from all this we learn that the electrons are in fact the basic constituent of all the atoms.
Most of the mass of the atom and all of its positive charge are contained in a small nucleus, called a nucleus. The particle which is positively charged is called a proton. The greater part of an atom’s volume is empty space.
The number of electrons that are dispersed outside the nucleus is the same as the number of positively charged protons in the nucleus. This explains the electrical neutrality of an atom as a whole.
Uses of Cathode Ray Tube
- Used as a most popular television (TV) display.
- X-rays are produced when fast-moving cathode rays are stopped suddenly.
- The screen of a cathode ray oscilloscope, and the monitor of a computer, are coated with fluorescent substances. When the cathode rays fall off the screen pictures are visible on the screen.
Frequently Asked Questions – FAQs
What are cathode ray tubes made of.
The cathode, or the emitter of electrons, is made of a caesium alloy. For many electronic vacuum tube systems, Cesium is used as a cathode, as it releases electrons readily when heated or hit by light.
Where can you find a cathode ray tube?
Cathode rays are streams of electrons observed in vacuum tubes (also called an electron beam or an e-beam). If an evacuated glass tube is fitted with two electrodes and a voltage is applied, it is observed that the glass opposite the negative electrode glows from the electrons emitted from the cathode.
How did JJ Thomson find the electron?
In the year 1897 J.J. Thomson invented the electron by playing with a tube that was Crookes, or cathode ray. He had shown that the cathode rays were charged negatively. Thomson realized that the accepted model of an atom did not account for the particles charged negatively or positively.
What are the properties of cathode rays?
They are formed in an evacuated tube via the negative electrode, or cathode, and move toward the anode. They journey straight and cast sharp shadows. They’ve got strength, and they can do the job. Electric and magnetic fields block them, and they have a negative charge.
What do you mean by cathode?
A device’s anode is the terminal on which current flows in from outside. A device’s cathode is the terminal from which current flows out. By present, we mean the traditional positive moment. Because electrons are charged negatively, positive current flowing in is the same as outflowing electrons.
Who discovered the cathode rays?
Studies of cathode-ray began in 1854 when the vacuum tube was improved by Heinrich Geissler, a glassblower and technical assistant to the German physicist Julius Plücker. In 1858, Plücker discovered cathode rays by sealing two electrodes inside the tube, evacuating the air and forcing it between the electrode’s electric current.
Which gas is used in the cathode ray experiment?
For better results in a cathode tube experiment, an evacuated (low pressure) tube is filled with hydrogen gas that is the lightest gas (maybe the lightest element) on ionization, giving the maximum charge value to the mass ratio (e / m ratio = 1.76 x 10 ^ 11 coulombs per kg).
What is the Colour of the cathode ray?
Cathode-ray tube (CRT), a vacuum tube which produces images when electron beams strike its phosphorescent surface. CRTs can be monochrome (using one electron gun) or coloured (using usually three electron guns to produce red, green, and blue images that render a multicoloured image when combined).
How cathode rays are formed?
Cathode rays come from the cathode because the cathode is charged negatively. So those rays strike and ionize the gas sample inside the container. The electrons that were ejected from gas ionization travel to the anode. These rays are electrons that are actually produced from the gas ionization inside the tube.
What are cathode rays made of?
Thomson showed that cathode rays were composed of a negatively charged particle, previously unknown, which was later named electron. To render an image on a screen, Cathode ray tubes (CRTs) use a focused beam of electrons deflected by electrical or magnetic fields.
For more information about cathode ray experiment, the discovery of electron or other sub-atomic particles, you can download BYJU’S – The learning app. You can also keep visiting the website or subscribe to our YouTube channel for more content.

Put your understanding of this concept to test by answering a few MCQs. Click ‘Start Quiz’ to begin!
Select the correct answer and click on the “Finish” button Check your score and answers at the end of the quiz
Visit BYJU’S for all Chemistry related queries and study materials
Your result is as below
Request OTP on Voice Call
Leave a Comment Cancel reply
Your Mobile number and Email id will not be published. Required fields are marked *
Post My Comment
Register with BYJU'S & Download Free PDFs
Register with byju's & watch live videos.

Discovering the electron: JJ Thomson and the Cathode Ray Tube

Concept Introduction: JJ Thomson and the Discovery of the Electron
The discovery of the electron was an important step for physics, chemistry, and all fields of science. JJ Thomson made the discovery using the cathode ray tube. Learn all about the discovery, the importance of the discovery, and JJ Thomson in this tutorial article.
Further Reading on the Electron
Electron Orbital and Electron Shapes Writing Electron Configurations Electron Shells What are valence electrons? Electron Affinity Aufbau Principle
Who was JJ Thomson?
JJ Thomson was an English physicist who is credited with discovery of the electron in 1897. Thompson was born in December 1856 in Manchester, England and was educated at the University of Manchester and then the University of Cambridge, graduating with a degree in mathematics. Thompson made the switch to physics a few years later and began studying the properties of cathode rays. In addition to this work, Thomson also performed the first-ever mass spectrometr y experiments, discovered the first isotope and made important contributions both to the understanding of positively charged particles and electrical conductivity in gases.
Thomson did most of this work while leading the famed Cavendish Laboratory at the University of Cambridge. Although he received the Nobel Prize in physics and not chemistry, Thomson’s contributions to the field of chemistry are numerous. For instance, the discovery of the electron was vital to the development of chemistry today, and it was the first subatomic particle to be discovered. The proton and the neutron would soon follow as the full structure of the atom was discovered.
What is a cathode ray tube and why was it important?
Prior to the discovery of the electron, several scientists suggested that atoms consisted of smaller pieces. Yet until Thomson, no one had determined what these might be. Cathode rays played a critical role in unlocking this mystery. Thomson determined that charged particles much lighter than atoms , particles that we now call electrons made up cathode rays. Cathode rays form when electrons emit from one electrode and travel to another. The transfer occurs due to the application of a voltage in vacuum. Thomson also determined the mass to charge ratio of the electron using a cathode ray tube, another significant discovery.

How did Thomson make these discoveries?
Thomson was able to deflect the cathode ray towards a positively charged plate deduce that the particles in the beam were negatively charged. Then Thomson measured how much various strengths of magnetic fields bent the particles. Using this information Thomson determined the mass to charge ratio of an electron. These were the two critical pieces of information that lead to the discovery of the electron. Thomson was now able to determine that the particles in question were much smaller than atoms, but still highly charged. He finally proved atoms consisted of smaller components, something scientists puzzled over for a long time. Thomson called the particle “corpuscles” , not an electron. George Francis Fitzgerald suggested the name electron.
Why was the discovery of the electron important?
The discovery of the electron was the first step in a long journey towards a better understanding of the atom and chemical bonding. Although Thomson didn’t know it, the electron would turn out to be one of the most important particles in chemistry. We now know the electron forms the basis of all chemical bonds. In turn chemical bonds are essential to the reactions taking place around us every day. Thomson’s work provided the foundation for the work done by many other important scientists such as Einstein, Schrodinger, and Feynman.
Interesting Facts about JJ Thomson
Not only did Thomson receive the Nobel Prize in physics in 1906 , but his son Sir George Paget Thomson won the prize in 1937. A year earlier, in 1936, Thomson wrote an autobiography called “Recollections and Reflections”. He died in 1940, buried near Isaac Newton and Charles Darwin. JJ stands for “Joseph John”. Strangely, another author with the name JJ Thomson wrote a book with the same name in 1975. Thomson had many famous students, including Ernest Rutherford.
Discovery of the Electron: Further Reading
Protons, Neutrons & Electrons Discovering the nucleus with gold foil Millikan oil drop experiment Phase Diagrams
J.J. Thomson’s Cathode Ray Tube Experiment
In 1897, J.J. Thomson conducted a groundbreaking experiment using a cathode ray tube that revolutionized our understanding of atomic structure and subatomic particles . His experiment, conducted at Cambridge’s Cavendish Laboratory, involved manipulating cathode rays with electric and magnetic fields.
This experiment provided evidence that cathode rays were composed of tiny particles, rather than waves in the now-rejected ether. It laid the foundation for our understanding of atomic structure and paved the way for advancements in particle physics . The significance of Thomson’s cathode ray tube experiment continues to resonate in the field of science.
Key Takeaways:
The significance of j.j. thomson’s cathode ray tube experiment.
This experiment led Thomson to propose his “plum pudding” model of the atom, which suggested that atoms consisted of a positively charged “pudding” with negatively charged electrons embedded within it. Thomson’s experiment and subsequent theories about the nature of cathode rays and electrons paved the way for further advancements in particle physics and the development of the modern atomic model.
“Thomson’s cathode ray tube experiment revolutionized our understanding of atomic structure and subatomic particles. His discovery of the charge-to-mass ratio of electrons laid the foundation for future advancements in the field of particle physics.” – Dr. Emily Johnson, Physics Professor
The Influence on Future Physicists
His contributions to the field of particle physics revolutionized our understanding of the microscopic world and set the stage for groundbreaking discoveries in the years to come. Without Thomson’s cathode ray tube experiment, our knowledge of atomic structure and subatomic particles would be vastly different, and the field of particle physics may not have progressed to the extent it has.
Contributions to Modern Physics
Applications and legacy of j.j. thomson’s cathode ray tube experiment.
The cathode ray tube experiment conducted by J.J. Thomson not only revolutionized our understanding of atomic structure and subatomic particles but also had a significant impact beyond the realm of scientific research.
Recognizing the significance of his contributions, J.J. Thomson was awarded the Nobel Prize in Physics in 1906. This prestigious accolade serves as a testament to the profound impact his experiments had on the field of particle physics and the advancement of scientific knowledge.
What was J.J. Thomson’s Cathode Ray Tube Experiment?
When and where did the experiment take place, how did thomson manipulate the cathode rays.
Thomson was able to manipulate the cathode rays using electric and magnetic fields.
What did Thomson discover through his experiments?
What evidence did thomson’s experiments provide about cathode rays, what was the significance of thomson’s discovery of electrons, what was thomson’s proposed model of the atom.
Thomson proposed the “plum pudding” model of the atom, which suggested that atoms consisted of a positively charged “pudding” with negatively charged electrons embedded within it.
What applications did cathode ray tubes have beyond scientific research?
What was j.j. thomson’s legacy in the field of particle physics, similar posts, which is the electron configuration for zinc, what is 1s2 2s2 2p6 3s2 3p6 4s1, understanding the electron configuration of cu+, electron configuration for fe2+ ion, dalton’s contribution to the periodic table, what is the electron configuration for ru.
All Subjects
Atomic Physics
Study guides for every class, that actually explain what's on your next test, thomson's cathode ray experiment, from class:.
Thomson's Cathode Ray Experiment was a groundbreaking study conducted by J.J. Thomson in 1897 that led to the discovery of the electron as a fundamental particle. In this experiment, Thomson used a cathode ray tube to observe the behavior of cathode rays, which are streams of electrons emitted from a negatively charged electrode. His findings challenged existing atomic models and introduced the concept of subatomic particles, significantly influencing the development of atomic theory.
congrats on reading the definition of Thomson's Cathode Ray Experiment . now let's actually learn it.
5 Must Know Facts For Your Next Test
- Thomson's experiment demonstrated that cathode rays were deflected by electric and magnetic fields, indicating that they carried a negative charge.
- The results of the experiment allowed Thomson to estimate the charge-to-mass ratio of the electron, providing essential insights into its properties.
- His work paved the way for the rejection of Dalton's atomic model, which viewed atoms as indivisible particles.
- Thomson received the Nobel Prize in Physics in 1906 for his investigations of the conduction of electricity through gases, including his cathode ray studies.
- The discovery of the electron marked a significant shift in atomic theory, leading to further research into atomic structure and quantum mechanics.
Review Questions
- Thomson's Cathode Ray Experiment challenged existing atomic theories by providing evidence that atoms were not indivisible, as previously thought. Before his work, Dalton's model posited that atoms were the smallest units of matter. However, Thomson's findings revealed that atoms contained smaller negatively charged particles—electrons. This discovery prompted scientists to reconsider the structure of the atom and laid the groundwork for future models.
- Thomson's findings significantly influenced subsequent developments in atomic theory by introducing the concept of subatomic particles and prompting the development of new models to explain atomic structure. The Plum Pudding Model was proposed by Thomson himself to illustrate how electrons are embedded within a positively charged 'soup.' This paved the way for later models, such as Rutherford's nuclear model and Bohr's planetary model, which built on Thomson's foundational work and refined our understanding of atomic structure.
- Thomson's Cathode Ray Experiment had a profound impact on modern physics by fundamentally changing our understanding of atomic structure. The identification of electrons as subatomic particles allowed scientists to explore complex interactions within atoms, leading to advancements in fields like quantum mechanics and chemistry. The experiment set a precedent for experimental approaches in physics, showcasing how empirical evidence could reshape theoretical frameworks. Today, Thomson's work is recognized as a cornerstone in the evolution of atomic theory, influencing ongoing research into particle physics and the nature of matter.
Related terms
Cathode Ray Tube : A vacuum tube containing one or more electron guns that produces images when an electron beam strikes a phosphorescent surface.
Electron : A negatively charged subatomic particle that orbits the nucleus of an atom and plays a key role in chemical bonding and electricity.
Plum Pudding Model : An early model of atomic structure proposed by J.J. Thomson, suggesting that atoms are composed of a uniform positively charged 'soup' with negatively charged electrons embedded within it.
" Thomson's Cathode Ray Experiment " also found in:
© 2024 fiveable inc. all rights reserved., ap® and sat® are trademarks registered by the college board, which is not affiliated with, and does not endorse this website..

- Foundations
- Write Paper
Search form
- Experiments
- Anthropology
- Self-Esteem
- Social Anxiety

Cathode Ray Experiment
The electric experiment by j.j. thomson.
J. J. Thomson was one of the great scientists of the 19th century; his inspired and innovative cathode ray experiment greatly contributed to our understanding of the modern world.
This article is a part of the guide:
- Ben Franklin Kite
- Physics Experiments
- Brownian Movement
Browse Full Outline
- 1 Physics Experiments
- 2 Ben Franklin Kite
- 3 Brownian Movement
- 4 Cathode Ray Experiment

Like most scientists of that era, he inspired generations of later physicists, from Einstein to Hawking .
His better-known research proved the existence of negatively charged particles, later called electrons, and earned him a deserved Nobel Prize for physics. This research led to further experiments by Bohr and Rutherford, leading to an understanding of the structure of the atom.

What is a Cathode Ray Tube?
Even without consciously realizing it, most of us are already aware of what a cathode ray tube is.
Look at any glowing neon sign or any ‘old-fashioned’ television set, and you are looking at the modern descendants of the cathode ray tube.
Physicists in the 19th century found out that if they constructed a glass tube with wires inserted in both ends, and pumped out as much of the air as they could, an electric charge passed across the tube from the wires would create a fluorescent glow. This cathode ray also became known as an ‘electron gun’.
Later and improved cathode ray experiments found that certain types of glass produced a fluorescent glow at the positive end of the tube. William Crookes discovered that a tube coated in a fluorescing material at the positive end, would produce a focused ‘dot’ when rays from the electron gun hit it.
With more experimentation, researchers found that the ‘cathode rays’ emitted from the cathode could not move around solid objects and so traveled in straight lines, a property of waves. However, other researchers, notably Crookes, argued that the focused nature of the beam meant that they had to be particles.
Physicists knew that the ray carried a negative charge but were not sure whether the charge could be separated from the ray. They debated whether the rays were waves or particles, as they seemed to exhibit some of the properties of both. In response, J. J. Thomson constructed some elegant experiments to find a definitive and comprehensive answer about the nature of cathode rays.

Thomson’s First Cathode Ray Experiment
Thomson had an inkling that the ‘rays’ emitted from the electron gun were inseparable from the latent charge, and decided to try and prove this by using a magnetic field.
His first experiment was to build a cathode ray tube with a metal cylinder on the end. This cylinder had two slits in it, leading to electrometers, which could measure small electric charges.
He found that by applying a magnetic field across the tube, there was no activity recorded by the electrometers and so the charge had been bent away by the magnet. This proved that the negative charge and the ray were inseparable and intertwined.
Thomson's Cathode Ray Second Experiment
Like all great scientists, he did not stop there, and developed the second stage of the experiment, to prove that the rays carried a negative charge. To prove this hypothesis, he attempted to deflect them with an electric field.
Earlier experiments had failed to back this up, but Thomson thought that the vacuum in the tube was not good enough, and found ways to improve greatly the quality.
For this, he constructed a slightly different cathode ray tube, with a fluorescent coating at one end and a near perfect vacuum. Halfway down the tube were two electric plates, producing a positive anode and a negative cathode, which he hoped would deflect the rays.
As he expected, the rays were deflected by the electric charge, proving beyond doubt that the rays were made up of charged particles carrying a negative charge. This result was a major discovery in itself, but Thomson resolved to understand more about the nature of these particles.
Thomson's Third Experiment
The third experiment was a brilliant piece of scientific deduction and shows how a series of experiments can gradually uncover truths.
Many great scientific discoveries involve performing a series of interconnected experiments, gradually accumulating data and proving a hypothesis .
He decided to try to work out the nature of the particles. They were too small to have their mass or charge calculated directly, but he attempted to deduce this from how much the particles were bent by electrical currents, of varying strengths.
Thomson found out that the charge to mass ratio was so large that the particles either carried a huge charge, or were a thousand times smaller than a hydrogen ion. He decided upon the latter and came up with the idea that the cathode rays were made of particles that emanated from within the atoms themselves, a very bold and innovative idea.
Later Developments
Thomson came up with the initial idea for the structure of the atom, postulating that it consisted of these negatively charged particles swimming in a sea of positive charge. His pupil, Rutherford, developed the idea and came up with the theory that the atom consisted of a positively charged nucleus surrounded by orbiting tiny negative particles, which he called electrons.
Quantum physics has shown things to be a little more complex than this but all quantum physicists owe their legacy to Thomson. Although atoms were known about, as apparently indivisible elementary particles, he was the first to postulate that they had a complicated internal structure.
Thomson's greatest gift to physics was not his experiments, but the next generation of great scientists who studied under him, including Rutherford, Oppenheimer and Aston. These great minds were inspired by him, marking him out as one of the grandfathers of modern physics.
- Psychology 101
- Flags and Countries
- Capitals and Countries
Martyn Shuttleworth (Sep 22, 2008). Cathode Ray Experiment. Retrieved Oct 28, 2024 from Explorable.com: https://explorable.com/cathode-ray-experiment
You Are Allowed To Copy The Text
The text in this article is licensed under the Creative Commons-License Attribution 4.0 International (CC BY 4.0) .
This means you're free to copy, share and adapt any parts (or all) of the text in the article, as long as you give appropriate credit and provide a link/reference to this page.
That is it. You don't need our permission to copy the article; just include a link/reference back to this page. You can use it freely (with some kind of link), and we're also okay with people reprinting in publications like books, blogs, newsletters, course-material, papers, wikipedia and presentations (with clear attribution).
Want to stay up to date? Follow us!
Save this course for later.
Don't have time for it all now? No problem, save it as a course and come back to it later.
Footer bottom
- Privacy Policy

- Subscribe to our RSS Feed
- Like us on Facebook
- Follow us on Twitter
J. J. Thomson
The Discovery of the Electron (J. J. Thomson)
In 1897, J. J. Thomson found that the cathode rays can be deflected by an electric field, as shown below. By balancing the effect of a magnetic field on a cathode-ray beam with an electric field, Thomson was able to show that cathode "rays" are actually composed of particles. This experiment also provided an estimate of the ratio of the charge to the mass of these particles.
In the SI system, charge is measured in units of coulombs. By definition, one coulomb is the charge carried by a current of one ampere that flows for one second: 1 C = 1 amp-s. When Thomson's data are converted to SI units, the charge-to-mass ratio of the particles in the cathode-ray beam is about 10 8 coulomb per gram.
Thomson found the same charge-to-mass ratio regardless of the metal used to make the cathode and the anode. He also found the same charge-to-mass ratio regardless of the gas used to fill the tube. He therefore concluded that the particles given off by the cathode in this experiment are a universal component of matter. Although Thomson called these particles corpuscles , the name electron, which had been proposed by George Stoney several years earlier for the fundamental unit of negative electricity, was soon accepted.
The Raisin Pudding Model of the Atom (J. J. Thomson)
Thomson recognized one of the consequences of the discovery of the electron. Because matter is electrically neutral, there must be a positively charged particle that balances the negative charge on the electrons in an atom. Furthermore, if electrons are very much lighter than atoms, these positively charged particles must carry the mass of the atom. Thomson therefore suggested that atoms are spheres of positive charge in which light, negatively charged electrons are embedded, much as raisins might be embedded in the surface of a pudding. At the time Thomson proposed this model, evidence for the existence of positively charged particles was available from cathode-ray tube experiments.
- Anatomy & Physiology
- Astrophysics
- Earth Science
- Environmental Science
- Organic Chemistry
- Precalculus
- Trigonometry
- English Grammar
- U.S. History
- World History
... and beyond
- Socratic Meta
- Featured Answers

- Cathode Ray Tube Experiment
Key Questions
Thomson's experiments with cathode ray tubes helped him to discover the electron.
This ushered in a model of atomic structure referred to as the plum pudding model. I like to think of it like a sphere shaped chocolate chip cookie since plum pudding is not super popular in the US.
The cookie dough (they didn't know what it was yet) is positively charged and the chocolate chips (electrons) are negatively charged and scattered randomly throughout the cookie (atom). The positive and negative charges cancel producing a neutral atom.

JJ Thompson’s Discovery of Electron: Cathode Ray Tube Experiment Explained
JJ Thomson discovered the electron in 1897 and there are tons of videos about it. However, most videos miss what JJ Thomson himself said was the motivating factor: a debate about how cathode rays move. Want to know not only how but why electrons were discovered?
Table of Contents
The start of jj thomson, how thomson discovered electrons: trials and errors, thomson’s conclusion.
A short history of Thomson: Joseph John Thomson, JJ on papers, to friends, and even to his own son [1] , was born in Lancashire, England to a middle class bookseller. When he was 14 years old, Thomson planned to get an apprenticeship to a locomotive engineer but it had a long waiting list, so, he applied to and was accepted at that very young age to Owen’s college.
Thompson later recalled that, “the authorities at Owens College thought my admission was such a scandal – I expect they feared that students would soon be coming in perambulators – that they passed regulations raising the minimum age for admission, so that such a catastrophe should not happen again.
[2] ” While in school, his father died, and his family didn’t have enough money for the apprenticeship. Instead, he relied on scholarships at universities – ironically leading him to much greater fame in academia. In 1884, at the tender age of 28, Thomson applied to be the head of the Cavendish Research Institute.
He mostly applied as a lark and was as surprised as anyone to actually get the position! “I felt like a fisherman who…had casually cast a line in an unlikely spot and hooked a fish much too heavy for him to land. [3] ” Suddenly, he had incredible resources, stability and ability to research whatever he wished.
He ended up having an unerring ability to pinpoint interesting phenomena for himself and for others. In fact, a full eight of his research assistants and his son eventually earned Nobel Prizes, but, of course, like Thomson’s own Nobel Prize, that was in the future.
Why did J. J. Thomson discover the electron in 1897? Well, according to Thomson: “the discovery of the electron began with an attempt to explain the discrepancy between the behavior of cathode rays under magnetic and electric forces [4] .” What did he mean by that?
Well, a cathode ray, or a ray in a vacuum tube that emanates from the negative electrode, can be easily moved with a magnet. This gave a charismatic English chemist named William Crookes the crazy idea that the cathode ray was made of charged particles in 1879!
However, 5 years later, a young German scientist named Heinrich Hertz found that he could not get the beam to move with parallel plates, or with an electric field. Hertz decided that Crookes was wrong, if the cathode ray was made of charged particles then it should be attracted to a positive plate and repulsed from a negative plate.
Ergo, it couldn’t be particles, and Hertz decided it was probably some new kind of electromagnetic wave, like a new kind of ultraviolet light. Further, in 1892, Hertz accidentally discovered that cathode rays could tunnel through thin pieces of metal, which seemed like further proof that Crookes was so very wrong.
Then, in December of 1895, a French physicist named Jean Perrin used a magnet to direct a cathode ray into and out of an electroscope (called a Faraday cylinder) and measured its charge. Perrin wrote, “the Faraday cylinder became negatively charged when the cathode rays entered it, and only when they entered it; the cathode rays are thus charged with negative electricity .
[5] ” This is why JJ Thomson was so confused, he felt that Perrin had, “conclusive evidence that the rays carried a charge of negative electricity” except that, “Hertz found that when they were exposed to an electric force they were not deflected at all.” What was going on?
In 1896, Thomson wondered if there might have been something wrong with Hertz’s experiment with the two plates. Thomson knew that the cathode ray tubes that they had only work if there is a little air in the tube and the amount of air needed depended on the shape of the terminals.
Thomson wondered if the air affected the results. Through trial and error, Thomson found he could get a “stronger” beam by shooting it through a positive anode with a hole in it. With this system he could evacuate the tube to a much higher degree and, if the vacuum was good enough, the cathode ray was moved by electrically charged plates, “just as negatively electrified particles would be.
[6] ” (If you are wondering why the air affected it, the air became ionized in the high electric field and became conductive. The conductive air then acted like a Faraday cage shielding the beam from the electric field.)
As stated before, Heinrich Hertz also found that cathode rays could travel through thin solids. How could a particle do that? Thomson thought that maybe particles could go through a solid if they were moving really, really fast. But how to determine how fast a ray was moving?
Thomson made an electromagnetic gauntlet. First, Thomson put a magnet near the ray to deflect the ray one-way and plates with electric charge to deflect the ray the other way. He then added or reduced the charge on the plates so that the forces were balanced and the ray went in a straight line.
He knew that the force from the magnet depended on the charge of the particle, its speed and the magnetic field (given the letter B). He also knew that the electric force from the plates only depended on the charge of the particle and the Electric field. Since these forces were balanced, Thomson could determine the speed of the particles from the ratio of the two fields.
Thomson found speeds as big as 60,000 miles per second or almost one third of the speed of light. Thomson recalled, “In all cases when the cathode rays are produced their velocity is much greater than the velocity of any other moving body with which we are acquainted. [7] ”
Thomson then did something even more ingenious; he removed the magnetic field. Now, he had a beam of particles moving at a known speed with a single force on them. They would fall, as Thomson said, “like a bullet projected horizontally with a velocity v and falling under gravity [8] ”.
Note that these “bullets” are falling because of the force between their charge and the charges on the electric plates as gravity is too small on such light objects to be influential. By measuring the distance the bullets went he could determine the time they were in the tube and by the distance they “fell” Thomson could determine their acceleration.
Using F=ma Thomson determine the ratio of the charge on the particle to the mass (or e/m). He found some very interesting results. First, no matter what variables he changed in the experiment, the value of e/m was constant. “We may… use any kind of substance we please for the electrodes and fill the tube with gas of any kind and yet the value of e/m will remain the same.
[9] ” This was a revolutionary result. Thomson concluded that everything contained these tiny little things that he called corpuscles (and we call electrons). He also deduced that the “corpuscles” in one item are exactly the same as the “corpuscles” in another. So, for example, an oxygen molecule contains the same kind of electrons as a piece of gold! Atoms are the building blocks of matter but inside the atoms (called subatomic) are these tiny electrons that are the same for everything .
The other result he found was that the value of e/m was gigantic, 1,700 times bigger than the value for a charged Hydrogen atom, the object with the largest value of e/m before this experiment. So, either the “corpuscle” had a ridiculously large charge or it was, well, ridiculously small.
A student of Thomson’s named C. T. R. Wilson had experimented with slowly falling water droplets that found that the charge on the corpuscles were, to the accuracy of the experiment, the same as the charge on a charged Hydrogen atom! Thomson concluded that his corpuscles were just very, very, tiny, about 1,700 times smaller then the Hydrogen atom [1] . These experiments lead Thomson to come to some interesting conclusions:
- Electrons are in everything and are well over a thousand times smaller then even the smallest atom.
- Benjamin Franklin thought positive objects had too much “electrical fire” and negative had too little. Really, positive objects have too few electrons and negative have too many. Oops.
- Although since Franklin, people thought current flowed from the positive side to the negative, really, the electrons are flowing the other way. When a person talks about “current” that flows from positive to negative they are talking about something that is not real! True “electric current” flows from negative to positive and is the real way the electrons move. [although by the time that people believed J.J. Thomson, it was too late to change our electronics, so people just decided to stick with “current” going the wrong way!]
- Since electrons are tiny and in everything but most things have a neutral charge, and because solid objects are solid, the electrons must be swimming in a sea or soup of positive charges. Like raisons in a raison cookie.
The first three are still considered correct over one hundred years later. The forth theory, the “plum pudding model” named after a truly English “desert” with raisins in sweet bread that the English torture people with during Christmas, was proposed by Thomson in 1904.
In 1908, a former student of Thomson’snamed Ernest Rutherford was experimenting with radiation, and inadvertently demolished the “plum pudding model” in the process. However, before I can get into Rutherford’s gold foil experiment, I first want to talk about what was going on in France concurrent to Thomson’s experiments.
This is a story of how a new mother working mostly in a converted shed discovered and named the radium that Rutherford was experimenting with. That woman’s name was Marie Sklodowska Curie, and that story is next time on the Lightning Tamers.
[1] the current number is 1,836 but Thomson got pretty close
[1] p 14 “Flash of the Cathode Rays: A History of JJ Thomson’s Electron” Dahl
[2] Thompson, J.J. Recollections and Reflections p. 2 Referred to in Davis & Falconer JJ. Thompson and the Discovery of the Electron 2002 p. 3
[3] Thomson, Joseph John Recollections and Reflections p. 98 quoted in Davis, E.A & Falconer, Isabel JJ Thomson and the Discovery of the Electron 2002 p. 35
[4] Thomson, JJ Recollections and Reflections p. 332-3
[5] “New Experiments on the Kathode Rays” Jean Perrin, December 30, 1985 translation appeared in Nature, Volume 53, p 298-9, January 30, 1896
[6] Nobel Prize speech?
Related Posts
How the inquisition led to the vacuum pump.
How in the world would the inquisition lead to the invention of the vacuum pump? …

The True Father of Electricity: Stephen Gray
On May 1, 1729 a retired clothing dyer noticed a single feather moving in a…
How Static Electricity Works
How did gold lead to the first rules of electricity? And, why is it ½…
Georg Matthias Bose: Crazy Experiments of the Enlightenment
Am I really going to light a fire with my bare finger? Of course, I…
Leave a Comment Cancel Reply
Your email address will not be published. Required fields are marked *
Save my name, email, and website in this browser for the next time I comment.
J.J. Thomson

by: Ann Johnson
- 1.1 Biography
- 2 Electron Discovery
- 3 Cathode Ray Experiments
- 4 Isotopes and Mass Spectrometry
- 5.1 Further reading
- 5.2 External links
- 6 References
The Main Idea
J. J. Thomson was a Nobel Prize winning English physicist who used cathode rays to discover electrons. He also developed the mass spectrometer.
J. J. Thomson was born on December 18th, 1856 in England. His father wished he would become an engineer, however he could not find an apprenticeship. He attended Trinity College at Cambridge, and eventually headed the Cavendish Laboratory. Thomson married one of his students, Rose Paget, in 1892. They had two children, Joan and George Thomson. George eventually became a physicist and earned a Nobel Prize of his own. J. J. Thomson published over 200 papers and 13 books. He died on August 30th, 1940 in Cambridge and is buried in Westminster Abbey.
Electron Discovery
J. J. Thomson discovered the electron in 1897 while performing experiments on electric discharge in a high-vacuum cathode ray tube. He interpreted the deflection of the rays by electrically charged plates and magnets as "evidence of bodies much smaller than atoms." He later suggested that the atom is best represented as a sphere of positive matter, through which electrons are positioned by electrostatic forces.
Cathode Ray Experiments
A cathode ray tube is a glass tube with wiring inserted on both ends, and as much air as possible pumped out of it. Cathode rays were discovered to travel in straight lines, just like waves do. Physicists knew that the ray had an electric charge, and they were trying to figure out if that electric charge could be separated from the ray.
Thomson had the hypothesis that the ray and charge were inseparable, and designed experiments using a magnetic field to prove this was true. He first built a cathode ray tube with a metal cylinder at the end. The cylinder had slits in it that were attached to electrometers, that could measure electric charges. When he applied a magnetic field across the tube, no activity was recorded by the electrometers. This meant the charge had been bent away by the magnet. This proved his theory that the charge and the ray were inseparable.
Isotopes and Mass Spectrometry
After discovering the electron, Thomson started studying positive rays. Positive rays behaved very differently from cathode rays, and he found that each ray followed its own parabolic path based on its detection on the photographic plate. He reasoned that no two particles would follow the same path unless they possessed the same mass-to-charge ratio. He correctly suggested that the positively charged particles were formed by the loss of an electron (isotopes). This created the field of mass spectrometry, which is still used very heavily today.
Properties of matter, including mass and charge, are related to Thomson's work with electrons and the mass spectrometer.
Further reading
Thomson, J. J. (June 1906). "On the Number of Corpuscles in an Atom". Philosophical Magazine 11: 769–781. doi:10.1080/14786440609463496. Archived from the original on 19 December 2007. Retrieved 4 October 2008. Leadership and creativity : a history of the Cavendish Laboratory, 1871 - 1919
External links
http://www.cambridgenetwork.co.uk/news/cambridge-physicist-is-streets-ahead/
http://thomson.iqm.unicamp.br/thomson.phphttp://www.chemheritage.org/discover/online-resources/chemistry-in-history/themes/atomic-and-nuclear-structure/thomson.aspx http://www.biography.com/people/jj-thomson-40039 http://study.com/academy/lesson/jj-thomsons-cathode-ray-tube-crt-definition-experiment-diagram.htmlhttps://explorable.com/cathode-ray-experiment
[[Category:Notable Scientists]
Navigation menu
Subatomic science: JJ Thomson's discovery of the electron
Read about how JJ Thomson announced his discovery of the electron at the Royal Institution in this blog by our Head of Heritage and Collections.
JJ Thomson, while familiar to scientists, is not necessarily a name most people would recognise; however, anyone who has undertaken any science at school will have heard of an electron.
It is Thomson we have to thank for discovering this fundamental breakthrough in science and announcing his discovery to the world during a lecture here at the Royal Institution in 1897.
What is an electron?
The technical definition is:
"An electron is a stable subatomic particle with a negative electrical charge. Unlike protons and neutrons, electrons are not constructed from even smaller components."
As a non-scientist this definition is something I have heard before but must confess is not something that means a great deal to me. It is an explanation in its basic form but doesn’t convey really what an electron is or what the impact of its discovery made
John Dalton's atomic theory
Prior to 1897, scientists had hypothesised about the makeup of the universe at the atomic and subatomic level but had not been able to prove any theories. The atom had been known about for many years.
In 1808, chemist John Dalton developed an argument that led to a realisation: that perhaps all matter, the things or objects that make up the universe are made of tiny, little bits.
These are fundamental and indivisible bits and named after the ancient Greek words ‘a’ meaning not and ‘tomos’ meaning cut therefore ‘atomos’ or uncuttable. Atoms.
JJ Thomson's cathode ray tube experiments
Thomson, a highly respected theoretical physics professor at Cambridge University, undertook a series of experiments designed to study the nature of electric discharge in a high-vacuum cathode-ray tube – he was attempting to solve a long-standing controversy regarding the nature of cathode rays, which occur when an electric current is driven through a vessel from which most of the air or other gas has been pumped out.
This was something that many scientists were investigating at the time. It was Thomson that made the breakthrough however, concluding through his experimentation that particles making up the rays were 1,000 times lighter than the lightest atom, proving that something smaller than atoms existed.
Thomson likened the composition of atoms to plum pudding, with negatively-charged ‘corpuscles’ dotted throughout a positively charged field.

G Johnstone Stoney coins the term 'electron'
Thomson explained within his lecture all of his experiments and the results, never mentioning the word electron but instead sticking to corpuscles to explain these tiny particles in the same terms as biological cells (corpuscles are a minute body or cell in an organism).
Such would they have remained if not for the term 'electron' coined by G Johnstone Stoney who in 1891 denoted the unit of charge found in experiments that passed electrical current through chemicals.
It was then in 1897 after Thomson’s publication of his research that Irish physicist George Francis Fitzgerald suggested that the term be applied to Thomson's research instead of corpuscles to better describe these newly discovered subatomic particles.
JJ Thomson and the Royal Institution
Thomson had a long-standing relationship with the Royal Institution during his long academic career in Cambridge, lecturing many times on the development of physics through Discourses and educational lectures to all ages.
Thomson was a great friend of Sir William Henry Bragg and Sir William Lawrence Bragg, who jointly won the Nobel Prize in 1915 for the development of x-ray crystallography, and who were both former Director’s of the Royal Institution.
JJ Thomson's Nobel Prize
Thomson received the Nobel Prize for his work in Physics in 1906 and was knighted in 1908. The studies of nuclear organisation that continue even to this day and the further identification of elementary particles have all followed the accomplishments of Thomson and his discovery in 1897.
More about the history of the Ri

Art, culture and society History of science
Letters to gwendoline – wwi bragg family correspondence.
One story of Gallipoli told through letters home in memory of Anzac Day
History of science
The birth of electric motion.
As we celebrate the bicentenary of Faraday's invention of the electric motor in 1821, our Head of Heritage and Collections

Who discovered the greenhouse effect?
John Tyndall set the foundation for our modern understanding of the greenhouse effect, climate change, meteorology, and weather

- Scientific Biographies
Joseph John “J. J.” Thomson
In 1897 Thomson discovered the electron and then went on to propose a model for the structure of the atom. His work also led to the invention of the mass spectrograph.

The British physicist Joseph John “J. J.” Thomson (1856–1940) performed a series of experiments in 1897 designed to study the nature of electric discharge in a high-vacuum cathode-ray tube, an area being investigated by many scientists at the time.
Thomson interpreted the deflection of the rays by electrically charged plates and magnets as evidence of “bodies much smaller than atoms” (electrons) that he calculated as having a very large value for the charge-to-mass ratio. Later he estimated the value of the charge itself.
Structure of the Atom and Mass Spectrography
In 1904 Thomson suggested a model of the atom as a sphere of positive matter in which electrons are positioned by electrostatic forces. His efforts to estimate the number of electrons in an atom from measurements of the scattering of light, X, beta, and gamma rays initiated the research trajectory along which his student Ernest Rutherford moved.
Thomson’s last important experimental program focused on determining the nature of positively charged particles. Here his techniques led to the development of the mass spectrograph. His assistant, Francis Aston, developed Thomson’s instrument further and with the improved version was able to discover isotopes—atoms of the same element with different atomic weights—in a large number of nonradioactive elements.
Early Life and Education
Ironically, Thomson—great scientist and physics mentor—became a physicist by default. His father intended him to be an engineer, which in those days required an apprenticeship, but his family could not raise the necessary fee. Instead young Thomson attended Owens College, Manchester, which had an excellent science faculty. He was then recommended to Trinity College, Cambridge, where he became a mathematical physicist.

In 1884 he was named to the prestigious Cavendish Professorship of Experimental Physics at Cambridge, although he had personally done very little experimental work. Even though he was clumsy with his hands, he had a genius for designing apparatus and diagnosing its problems. He was a good lecturer, encouraged his students, and devoted considerable attention to the wider problems of science teaching at university and secondary levels.
Ties to the Chemical Community
Of all the physicists associated with determining the structure of the atom, Thomson remained most closely aligned to the chemical community. His nonmathematical atomic theory—unlike early quantum theory—could also be used to account for chemical bonding and molecular structure (see Gilbert Newton Lewis and Irving Langmuir ). In 1913 Thomson published an influential monograph urging chemists to use the mass spectrograph in their analyses.
A Nobel Prize
Thomson received various honors, including the Nobel Prize in Physics in 1906 and a knighthood in 1908. He also had the great pleasure of seeing several of his close associates receive their own Nobel Prizes, including Rutherford in chemistry (1908) and Aston in chemistry (1922).
Featured image: Science History Institute
Browse more biographies

Søren Sørensen

Carl von Linde

Robert Burns Woodward
Copy the above HTML to republish this content. We have formatted the material to follow our guidelines, which include our credit requirements. Please review our full list of guidelines for more information. By republishing this content, you agree to our republication requirements.

- Table of Contents
- Random Entry
- Chronological
- Editorial Information
- About the SEP
- Editorial Board
- How to Cite the SEP
- Special Characters
- Advanced Tools
- Support the SEP
- PDFs for SEP Friends
- Make a Donation
- SEPIA for Libraries
- Back to Entry
- Entry Contents
- Entry Bibliography
- Academic Tools
- Friends PDF Preview
- Author and Citation Info
- Back to Top
Supplement to Experiment in Physics
Appendix 7: evidence for a new entity: j.j. thomson and the electron.
In discussing the existence of electrons Ian Hacking has written, “So far as I’m concerned, if you can spray them then they are real” (Hacking 1983, p. 23). He went on to elaborate this view. “We are completely convinced of the reality of electrons when we set out to build—and often enough succeed in building—new kinds of device that use various well-understood causal properties of electrons to interfere in other more hypothetical parts of nature” (p. 265).
Hacking worried that the simple manipulation of the first quotation, the changing of the charge on an oil drop or on a superconducting niobium sphere, which involves only the charge of the electron, was insufficient grounds for belief in electrons. His second illustration, which he believed more convincing because it involved several properties of the electron, was that of Peggy II, a source of polarized electrons built at the Stanford Linear Accelerator Center in the late 1970s. Peggy II provided polarized electrons for an experiment that scattered electrons off deuterium to investigate the weak neutral current. Although I agree with Hacking that manipulability can often provide us with grounds for belief in a theoretical entity, [ 1 ] his illustration comes far too late. Physicists were manipulating the electron in Hacking’s sense in the early twentieth century. [ 2 ] They believed in the existence of electrons well before Peggy II, and I will argue that they had good reasons for that belief.
The position I adopt is one that might reasonably be called “conjectural” realism. It is conjectural because, despite having good reasons for belief in the existence of an entity or in the truth of a scientific law, we might be wrong. At one time scientists had good reason to believe in phlogiston and caloric, substances we now have good reason to believe don’t exist. My position includes both Sellars’ view that “to have good reason for holding a theory is ipso facto to have good reason for holding that the entities postulated by the theory exist” (Sellars 1962, p. 97), and the “entity realism” proposed by Cartwright (1983) and by Hacking (1983). Both Hacking, as noted above, and Cartwright emphasize the manipulability of an entity as a criterion for belief in its existence. Cartwright also stresses causal reasoning as part of her belief in entities. In her discussion of the operation of a cloud chamber she states, “…if there are no electrons in the cloud chamber, I do not know why the tracks are there” (Cartwright, 1983, p.99). In other words, if such entities don’t exist then we have no plausible causal story to tell. Both Hacking and Cartwright grant existence to entities such as electrons, but do not grant “real” status to either laws or theories, which may postulate or apply to such entities.
In contrast to both Cartwright and Hacking, I suggest that we can also have good reasons for belief in the laws and theories governing the behavior of the entities, and that several of their illustrations implicitly involve such laws. [ 3 ] I have argued elsewhere for belief in the reality of scientific laws (Franklin 1996). In this section I shall concentrate on the reality and existence of entities, in particular, the electron. I agree with both Hacking and Cartwright that we can go beyond Sellars and have good reasons for belief in entities even without laws. Hacking and Cartwright emphasize experimenting with entities. I will argue that experimenting on entities and measuring their properties can also provide grounds for belief in their existence.
In this section I will discuss the grounds for belief in the existence of the electron by examining J.J. Thomson’s experiments on cathode rays. His 1897 experiment on cathode rays is generally regarded as the “discovery” of the electron.
The purpose of J.J. Thomson’s experiments was clearly stated in the introduction to his 1897 paper.
The experiments discussed in this paper were undertaken in the hope of gaining some information as to the nature of Cathode Rays. The most diverse opinions are held as to these rays; according to the almost unanimous opinion of German physicists they are due to some process in the aether to which—inasmuch as in a uniform magnetic field their course is circular and not rectilinear—no phenomenon hitherto observed is analogous: another view of these rays is that, so far from being wholly aetherial, they are in fact wholly material, and that they mark the paths of particles of matter charged with negative electricity (Thomson 1897, p. 293).
Thomson’s first order of business was to show that the cathode rays carried negative charge. This had presumably been shown previously by Perrin. Perrin placed two coaxial metal cylinders, insulated from one another, in front of a plane cathode. The cylinders each had a small hole through which the cathode rays could pass onto the inner cylinder. The outer cylinder was grounded. When cathode rays passed into the inner cylinder an electroscope attached to it showed the presence of a negative electrical charge. When the cathode rays were magnetically deflected so that they did not pass through the holes, no charge was detected. “Now the supporters of the aetherial theory do not deny that electrified particles are shot off from the cathode; they deny, however, that these charged particles have any more to do with the cathode rays than a rifle-ball has with the flash when a rifle is fired” (Thomson 1897, p. 294).
Thomson repeated the experiment, but in a form that was not open to that objection. The apparatus is shown in Figure 14. The two coaxial cylinders with holes are shown. The outer cylinder was grounded and the inner one attached to an electrometer to detect any charge. The cathode rays from A pass into the bulb, but would not enter the holes in the cylinders unless deflected by a magnetic field.

Figure 14. Thomson’s apparatus for demonstrating that cathode rays have negative charge. The slits in the cylinders are shown. From Thomson (1897).
When the cathode rays (whose path was traced by the phosphorescence on the glass) did not fall on the slit, the electrical charge sent to the electrometer when the induction coil producing the rays was set in action was small and irregular; when, however, the rays were bent by a magnet so as to fall on the slit there was a large charge of negative electricity sent to the electrometer…. If the rays were so much bent by the magnet that they overshot the slits in the cylinder, the charge passing into the cylinder fell again to a very small fraction of its value when the aim was true. Thus this experiment shows that however we twist and deflect the cathode rays by magnetic forces, the negative electrification follows the same path as the rays, and that this negative electrification is indissolubly connected with the cathode rays (Thomson 1897, p. 294–295, emphasis added).
This experiment also demonstrated that cathode rays were deflected by a magnetic field in exactly the way one would expect if they were negatively charged material particles. [ 4 ]

Figure 15. Thomson’s apparatus for demonstrating that cathode rays are deflected by an electric field. It was also used to measure \(\bfrac{m}{e}\). From Thomson (1897).
There was, however, a problem for the view that cathode rays were negatively charged particles. Several experiments, in particular those of Hertz, had failed to observe the deflection of cathode rays by an electrostatic field. Thomson proceeded to answer this objection. His apparatus is shown in Figure 15. Cathode rays from C pass through a slit in the anode A, and through another slit at B. They then passed between plates D and E and produced a narrow well-defined phosphorescent patch at the end of the tube, which also had a scale attached to measure any deflection. When Hertz had performed the experiment he had found no deflection when a potential difference was applied across D and E. He concluded that the electrostatic properties of the cathode ray are either nil or very feeble. Thomson admitted that when he first performed the experiment he also saw no effect. “on repeating this experiment [that of Hertz] I at first got the same result [no deflection], but subsequent experiments showed that the absence of deflexion is due to the conductivity conferred on the rarefied gas by the cathode rays”. [ 5 ] On measuring this conductivity it was found that it diminished very rapidly as the exhaustion increased; it seemed that on trying Hertz’s experiment at very high exhaustion there might be a chance of detecting the deflexion of the cathode rays by an electrostatic force (Thomson 1897, p. 296). Thomson did perform the experiment at lower pressure [higher exhaustion] and observed the deflection. [ 6 ]
Thomson concluded:
As the cathode rays carry a charge of negative electricity, are deflected by an electrostatic force as if they were negatively electrified, and are acted on by a magnetic force in just the way in which this force would act on a negatively electrified body moving along the path of these rays, I can see no escape from the conclusion that they are charges of negative electricity carried by particles of matter. (Thomson 1897, p. 302) [ 7 ]
Having established that cathode rays were negatively charged material particles, Thomson went on to discuss what the particles were. “What are these particles? are they atoms, or molecules, or matter in a still finer state of subdivision” (p. 302). To investigate this question Thomson made measurements on the charge to mass ratio of cathode rays. Thomson’s method used both the electrostatic and magnetic deflection of the cathode rays. [ 8 ] The apparatus is shown in Figure 15. It also included a magnetic field that could be created perpendicular to both the electric field and the trajectory of the cathode rays.
Let us consider a beam of particles of mass \(m\) charge \(e\), and velocity \(v\). Suppose the beam passes through an electric field F in the region between plates D and E, which has a length \(L\). The time for a particle to pass through this region \(t = \bfrac{L}{v}\). The electric force on the particle is \(Fe\) and its acceleration \(a = \bfrac{Fe}{m}\). The deflection d at the end of the region is given by
Now consider a situation in which the beam of cathode rays simultaneously pass through both \(F\) and a magnetic field \(B\) in the same region. Thomson adjusted \(B\) so that the beam was undeflected. thus the magnetic force was equal to the electrostatic force.
This determined the velocity of the beam. Thus,
Each of the quantities in the above expression was measured so the \(\bfrac{e}{m}\) or \(\bfrac{m}{e}\) could be determined.
Using this method Thomson found a value of \(\bfrac{m}{e}\) of \((1.29\pm 0.17) \times 10^{-7}\). This value was independent of both the gas in the tube and of the metal used in the cathode, suggesting that the particles were constituents of the atoms of all substances. It was also far smaller, by a factor of 1000, than the smallest value previously obtained, \(10^{-4}\), that of the hydrogen ion in electrolysis.
Thomson remarked that this might be due to the smallness of \(m\) or to the largeness of \(e\). He argued that \(m\) was small citing Lenard’s work on the range of cathode rays in air. The range, which is related to the mean free path for collisions, and which depends on the size of the object, was 0.5 cm. The mean free path for molecules in air was approximately \(10^{-5}\) cm. If the cathode ray traveled so much farther than a molecule before colliding with an air molecule, Thomson argued that it must be much smaller than a molecule. [ 9 ]
Thomson had shown that cathode rays behave as one would expect negatively charged material particles to behave. They deposited negative charge on an electrometer, and were deflected by both electric and magnetic fields in the appropriate direction for a negative charge. In addition the value for the mass to charge ratio was far smaller than the smallest value previously obtained, that of the hydrogen ion. If the charge were the same as that on the hydrogen ion, the mass would be far less. In addition, the cathode rays traveled farther in air than did molecules, also implying that they were smaller than an atom or molecule. Thomson concluded that these negatively charged particles were constituents of atoms. In other words, Thomson’s experiments had given us good reasons to believe in the existence of electrons.
Return to Experiment in Physics
Copyright © 2023 by Allan Franklin < allan . franklin @ colorado . edu > Slobodan Perovic < sperovic @ f . bg . ac . rs >
- Accessibility
Support SEP
Mirror sites.
View this site from another server:
- Info about mirror sites
The Stanford Encyclopedia of Philosophy is copyright © 2023 by The Metaphysics Research Lab , Department of Philosophy, Stanford University
Library of Congress Catalog Data: ISSN 1095-5054
KentChemistry HOME
Dalton's Model of the Atom / J.J. Thomson / Millikan's Oil Drop Experiment / Rutherford / Niels Bohr / DeBroglie / Heisenberg / Planck / Schrödinger / Chadwick
Plum Pudding
Discovery of the Electron: Cathode Rays
- First Online: 05 August 2020
Cite this chapter

- Bruce D. Popp 2
401 Accesses
Poincaré’s work on dynamics of the electron provides a classical theory of subatomic charged particles to accompany the experimental work done over the decade following Jean Perrin’s work in 1895 . This chapter is the first of two parts that look at the discovery of the electron, the experimental work that established that electrical charge is discrete and that electrons have a mass that is small compared to hydrogen.
J. J. Thomson measured the charge-to-mass ratio of cathode rays, establishing that they were particles (not radiation) and providing a distinctive property with which to identify the same particle in other contexts, including ionized gases and the photoelectric effect. This is an interpretation of what we mean when we say that he discovered the electron.
This is a preview of subscription content, log in via an institution to check access.
Access this chapter
Subscribe and save.
- Get 10 units per month
- Download Article/Chapter or eBook
- 1 Unit = 1 Article or 1 Chapter
- Cancel anytime
- Available as PDF
- Read on any device
- Instant download
- Own it forever
- Available as EPUB and PDF
- Compact, lightweight edition
- Dispatched in 3 to 5 business days
- Free shipping worldwide - see info
- Durable hardcover edition
Tax calculation will be finalised at checkout
Purchases are for personal use only
Institutional subscriptions
Similar content being viewed by others

Introduction and Historical Background

The Interaction of Radiation with Matter

Introduction to Part II
Another aspect of Jean Perrin’s paper is worth noting. He wrote that he looked for positive charges corresponding to the negatively charged cathode rays and writes, “I think I found them in the same region where the cathode rays form.” (Perrin, 1895 ) These could be the channel rays (kanalstrahlen) discussed by Poincaré in (Poincaré, La dynamique de l’électron, 1908) section III and translated on page 107 of this book.
https://data.bnf.fr/fr/12744499/henri_pellat/
When I, in an undergraduate physics laboratory many years ago, measured the mass-to-charge ratio of an electron, glasswork and vacuum pumps were not involved; I was shown an instrument on a laboratory table, told “do this,” “measure that,” and given a handout with more information.
For information on who the Maxwellians were and what they did, see (Hunt, 1991 ). Notably, Oliver Heaviside, whose contribution to mathematical notation is discussed in Chap. 6 , is among them.
To provide a relevant example, but without suggesting that it is more or less deserving of critique, consider the first chapter provided by A. B. Pippard from the University of Cambridge for the book Electron: a Centenary Volume (Springford, 1997 ). The adjective continental is used three times in the first 10 pages.
An English adaptation of this paper was published within two months in The Electrician (Kaufmann , The Development of the Electron Idea, 1901b ). The adaptation does not include this list of references.
A commercial gas mixture used for lighting.
Falconer, I. (1987). Corpuscles, Electrons and Cathode Rays: J. J. Thomson and the ‘Discovery of the Electron’. British Journal for the History of Science , 241–276.
Google Scholar
Halley, E. (1705). Synopsis of the Astronomy of Comets. London.
Hunt, B. J. (1991). The Maxwellians. Ithaca, NY: Cornell University Press.
Kaufmann, W. (1897). Die magnetische Ablenkbarkeit der Kathodenstrahlen und ihre Abhängigkeit vom Entladungspotential. Annalen der Physik, ser. 3, 61 , 544–552.
Article ADS Google Scholar
Kaufmann, W. (1901a). Die Entwicklung des Electronenbegriffs. Physikalische Zeitschrift, 3 (1), 9–15.
Kaufmann, W. (1901b, November 8). The Development of the Electron Idea. The Electrician, 48 , 95–97.
Larmor, J. (1893). A Dynamical Theory of the Electric and Luminiferous Medium. Proceedings of the Royal Society of London, 54 , 438–461.
National Institute of Standards and Technology. (n.d.-a). CODATA Value: electron charge to mass quotient . Retrieved November 12, 2019, from https://physics.nist.gov/cgi-bin/cuu/Value?esme
National Institute of Standards and Technology. (n.d.-b). CODATA Value: proton-electron mass ratio . Retrieved July 6, 2019, from https://physics.nist.gov/cgi-bin/cuu/Value?mpsme
Nobel Media AB 2019. (n.d.). The Nobel Prize in Physics 1906. Retrieved March 16, 2019, from NobelPrize.org : https://www.nobelprize.org/prizes/physics/1906/summary/
Perrin, J. (1895, December). Nouvelles propriétés des rayons cathodiques. Comptes Rendus des Séances de l’Académie des Sciences, 121 , 1130–34.
Poincaré, H. (1906). Sur la dynamique de l’électron. Rendiconti del circolo matematico di Palermo, 21 , 129–176.
Article MATH Google Scholar
Poincaré, H. (1908). La dynamique de l’électron. Revue générale des sciences pures et appliquées, 19 , 386–402.
MATH Google Scholar
Smith, G. E. (2001). J. J. Thomson and the Electron. 1897–99. In J. Z. Buchwald, & J. Warwick, Histories of the Electron: The Birth of Microphysics (pp. 21–76). Cambridge, MA: Dibner Institute for the History of Science and Technology.
Springford, M. (Ed.). (1997). Electron: a Centenary Volume. Cambridge: Cambridge University Press.
Stoney, G. J. (1891). On the Cause of Double Lines and of Equidistant Satellites in the Spectra of Gases. Scientific Transactions of the Royal Dublin Society, IV , 563–680.
Stoney, G. J. (1894). Of the “Electron” or Atom of Electricity. Philosophical Magazine and Journal of Science, Fifth Series, 38 , 418–420.
Article Google Scholar
Thomson, J. J. (1897a, October). Cathode Rays. Philosophical Magazine and Journal of Science, Fifth Series, 44 , 293–316.
Thomson, J. J. (1897b, May 21). Cathode Rays. The Electrician, 39 , 104–9.
Thomson, J. J. (1897c, February). On the cathode rays. Proceedings of the Cambridge Philosophical Society, 9 , 243–4.
Thomson, J. J. (1898). On the Charge of Electricity Carried by the Ions Produced by Röntgen Rays. Philosophical Magazine and Journal of Science, Fifth Series, 46 , 528–45.
Thomson, J. J. (1899). On the Masses of Ions and Gases at Low Pressures. Philosophical Magazine, Fifth Series, 48 , 547–567.
Wiechert, E. (1897a). Über das Wesen der Elektricität. Schriften der Physikalisch-Ökonomischen Gesellschaft zu Königsberg., 38 , 3–20.
Wiechert, E. (1897b). Ueber das Wesen der Elektricität. Naturwissenschaftliche Rundschau, 12 , 237–239.
Download references
Author information
Authors and affiliations.
Norwood, MA, USA
Bruce D. Popp
You can also search for this author in PubMed Google Scholar
Rights and permissions
Reprints and permissions
Copyright information
© 2020 Springer Nature Switzerland AG
About this chapter
Popp, B.D. (2020). Discovery of the Electron: Cathode Rays. In: Henri Poincaré: Electrons to Special Relativity. Springer, Cham. https://doi.org/10.1007/978-3-030-48039-4_7
Download citation
DOI : https://doi.org/10.1007/978-3-030-48039-4_7
Published : 05 August 2020
Publisher Name : Springer, Cham
Print ISBN : 978-3-030-48038-7
Online ISBN : 978-3-030-48039-4
eBook Packages : Physics and Astronomy Physics and Astronomy (R0)
Share this chapter
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Publish with us
Policies and ethics
- Find a journal
- Track your research
Talk to our experts
1800-120-456-456
- Cathode Ray Experiment

What are Cathode Rays?
Cathode rays are a beam of negatively charged electrons traveling from the negative end of an electrode to the positive end within a vacuum, across a potential difference between the electrodes.
How Do the Cathode Rays Work?
The cathode is a negative electrode, Anode is the positive electrode. Since electrons are repelled by the negative electrode, the cathode is the source of cathode rays inside a vacuum environment. When a potential difference is applied, the electrons jump to an excited state and travel at high speeds to jump back-and-forth inside the vacuum glass chamber and when some cathode rays certain molecules of the cathode screen, they emit light energy. A wire is connected from anode to cathode to complete the electrical circuit.
Construction of a Cathode Ray Tube
Its Basic Components are: -
Electron Gun Assembly: - It is the source of the electron beams. The electron gun has a heater, cathode, pre-accelerating anode, focusing anode and accelerating anode.
Deflecting Plates: - They produce a uniform electrostatic field only in one direction, and accelerate particles in only one direction.
Screen: - The inner layer of the screen is coated with phosphorus, and produces fluorescence when cathode rays hit the screen by a process of phosphorus excitation.
Aquadag: - It is an aqueous solution of graphite used to collect the secondary emitted electrons which are required to keep the cathode ray in electrical equilibrium.
What is the Cathode Ray Tube Experiment?
In 1897, great physician J.J. Thompson, conducted his first cathode ray tube experiment to prove that rays emitted from an electron gun are inseparable from the latent charge. He built his cathode ray tube with a metal cylinder on the other end. The metal had two small diversions(slits), leading to an electrometer that could measure a small electric charge. From the first experiment, he discovered that the electrometers stopped measuring electric charge. From this, he deduced that the electric charge and the cathode rays must be combined and are the same entity.
Then he conducted a Second experiment, to prove the charge carried by the cathode rays was negative or positive. Now, he put a negatively charged metal plate on one side of the cathode rays to go past the anode, and a positively charged metal plate on the other side. Instead of an electrometer at one end of the Cathode Ray Tube, he used a fluorescent coated tube that would glow where the cathode ray hit it. When the charged metal plates were introduced he found that the cathode rays bent away from the negative plate and towards the positive plate. This proved that the cathode rays were negatively charged.
Then he performed the third experiment, to know the nature of the particles and reduce the mass of the particles as they had too small of a mass to be calculated directly. For the experiment, he used the cathode ray tube and with a high applied potential difference between the two electrodes, with the negatively charged cathode producing the cathode rays. He had already deduced that the particles were negatively charged. Firstly, he applied an electric field in the path between anode and cathode and measured the deflections from the straight path. Now he applied a magnetic field across the cathode ray tube by using an external magnetic field. The cathode ray is deflected by the magnetic field. Now he changed the direction of the external magnetic field and found that the beam of electrons is deflected in the opposite direction. From this experiment, he concluded that the electrostatic deflection is the same as the electromagnetic deflection for the cathode rays and he was able to calculate the charge to mass ratio of the electron.
After these three experiments, he deduced that inside the atom there consist of a subatomic particle, originally named ‘corpuscle’, then changed to ‘electron’ which is 1800 times lighter than the mass of hydrogen atom (Lightest atom).
Formula Used
The derivation of the formula used to calculate the charge to the mass ratio:
For Electric Field the force on a particle is
Force(F)=Charge(Q)*Electric field(E) ---<1>
For Magnetic Field the force on a particle moving with velocity is:
F=q*velocity(v)*Magnetic Field(B) ---<2>
From 1 and 2 we get,
V=E/B ----<3>
From the definition of Force,
Acceleration(a)= Force(f)/mass(m) ----<4>
Combining 1 and 4
a=q*E/m ----<5>
From Newton’s law Of motion, vertical displacement is:
Y= (1/2)*a*t*t ----<6>
From 5 and 6
q/m=(2*y*v*v)/x*x*E
Cathode Ray Tubes (CRT)
The cathode ray tube (CRT) is a vacuum tube, in which electrons are discharged from the cathode and accelerated through a voltage, and thereby gains acceleration of some 600 km/s for every volt. These accelerated electrons collide into the gas inside the tube, thereby allowing it to glow. This enables us to see the path of the beam. Helmholtz coil, a device for producing a region of nearly uniform magnetic field, is also used to apply a quantifiable magnetic field by passing a current through them.
A magnetic field will cause a force to act on the electrons which are perpendicular to both the magnetic field and their direction of travel. Thus, a circular path will be followed by a charged particle in a magnetic field. The faster the speed of a charged particle in a magnetic field, the larger the circle traced out in a magnetic field. Contrarily, the larger the magnetic field needed for a given radius of curvature of the beam. The paths of the electrons are distorted by the magnet in CRT Tv when they are brought near the screen. The picture on the screen appears when the electrons accurately hit phosphors on the back of the screen. Because of this, different colors of light are emitted on the screen when the electrons are impacted. Hence, the electrons are forced to settle in the wrong place, thereby causing the distortion of the image and the psychedelic colors.
Postulates of J.J. Thomson’s Atomic Model
After the Cathode ray tube experiment, Thomson gave one of the first atomic models including the newly discovered particle.
His model stated: -
An atom resembles a sphere of positive charge with a negative charge present inside the sphere.
The positive charge and the negative charge were equal in magnitude and thus the atom had no charge as a whole and is electrically neutral.
His model resembles a plum pudding or watermelon. It assumed that positive and negative charge inside an atom is randomly spread across the whole sphere like the red part of the watermelon (positive charge) and the black seeds (negative charge).
Practical Uses of Cathode Ray Tube Experiment
In ancient times, the cathode ray tubes were used in the beam where the electron was considered with no inertia but have higher frequencies and can be made visible for a short time.
Many scientists were trying to get the secrets of cathode rays, while others were in search of the practical uses or applications of cathode ray tube experiments. And the first search was ended and released in 1897 which was introduced as Karl Ferdinand Braun’s oscilloscope. It was used for producing luminescence on a chemical affected screen in which cathode rays were allowed to pass through the narrow aperture by focusing into the beams that looked like a dot. This dot was passed for scanning across the screen which was represented visually by the electrical pulse generator.
Then during the first two to three decades of the twentieth century, inventors continued to search the uses of cathode ray tube technology. Then inspired by Braun's oscilloscope, A. A. Campbell advised that a cathode ray tube would be used for projecting video images on the screen. But, this technology of the time did not get matched with the vision of Campbell-Swinton. It was only until 1922, when Philo T. Farnsworth developed a magnet to get focused on the stream of electrons on the screen, for producing the image. Thus, the first kind of it, Farnsworth, was quickly backed up by Zworykin’s kinescope, known as the ancestor of modern TV sets.
Nowadays, most image viewer devices are made with the help of cathode ray tube technology including the guns of electrons which are used in huge areas of science as well as medical applications. One such use for cathode-ray tube research is the microscope invented by Ernst Ruska in 1928. The microscope based on electrons uses the stream of electrons to magnify the image as the electrons have a small wavelength which is used for magnifying the objects which are very small to get resolved by visible light. Just like Plucker and Crookes work, Ernst Ruska used a strong field of magnetic lines for getting it focused on the stream of electrons into an image.
Solved Example:
Question: The charge of an electron e=1.602∗10−19 and its is mass m=9.11∗10−31. The stopping potential of an electron traveling in a cathode ray tube is V=5V. Find the velocity of an electron traveling (where charge of an electron e=1.602∗10−19 and mass m=9.11810−31).
Answer: Here we need to find the velocity of traveling electrons using the given stopping potential.
We know that eV=12mv2, the charge(e) and mass(m) of the electron is also given as,
e=1.602∗10−19 and m=9.11∗10−31
By substituting the values of e, m, V.(1.602∗10−19)(5)
=12(9.11∗10−31)(v2)v2
=(1.602∗10−19)(5)(2)9.11∗10−31v
=1.33∗106m/s
FAQs on Cathode Ray Experiment
1. What is the procedure of the Cathode Ray Experiment?
The apparatus of Cathode Ray Experiment is arranged in such a way that the terminals have high voltage with the internal pressure, which is reduced by removing the air inside the CRT. Because of the high voltage in the terminal, the partial air inside it is ionized and hence gas becomes the conductor. The electric current propagates as a closed-loop circuit. In order to recognize and measure the ray produced, a dipole is set up. The cathode rays will begin deflecting and repel from the dipole and move towards the anode because of the dipole. The phosphorescent substance is arranged in such a way that the rays strike the substance. And hence, it causes small sparks of light, which detects the stream of rays.
2. What are Cathode ray tubes?
Cathode ray tubes (CRT) are a presentation screen that produces pictures as a video signal. Cathode ray tubes (CRT) is a type of vacuum tube that displays pictures when electron beams from an electron gun hit a luminous surface. The CRT produces electron beams, accelerates them at high speed, and thereby deflecting them to take pictures on a phosphor screen. Electronic presentation gadgets being the most established and least expensive electronic presentation innovation, were initially made with CRTs. CRTs work at any aspect ratio, at any resolution, and geometry without the need to resize the picture. CRTs work on the principle of an optical and electromagnetic phenomenon, called cathodoluminescence.
3. What are the applications of Cathode ray tubes?
The following are the applications of Cathode ray tubes.
The main components of a cathode ray tube (CRT) includes A Vacuum tube holding an electron cannon and a screen lined with phosphors.
The technology of Cathode ray tubes is used by Televisions and computer monitors. Three electron cannons correlate to corresponding types of phosphors in color CRTs, one for each main color viz red, green, and blue.
Ancient computer terminals and black and white televisions are examples of monochromatic CRTs.
cathode ray tube (CRT) is also used in oscilloscopes, which are machines that display and analyze the waveform of electronic signals.
A cathode ray amusement device was the very first video game to be produced, which were used in old military radar screens.
4. What are the basic principles of the CRT?
There are three basic principles of the CRT as the following:
Electrons are released into a vacuum tube from very hot metal plates.
The released electrons are accelerated and their direction of movement is controlled by using either a magnetic field from a coil that is carrying an electric current or by a voltage between metal plates.
A high-velocity beam of electrons hits some materials such as zinc sulfide. The spot is created on the fluorescent screen, and it causes material, called a phosphor, to glow, giving a spot of light as wide as the beam.
5. How to understand the concept of the Cathode Ray Experiment easily?
Students can understand the concept of the Cathode Ray Experiment easily with the help of a detailed explanation of the Cathode Ray Experiment provided on Vedantu. Vedantu has provided here a thorough explanation of the Cathode Ray Experiment along with Cathode Rays, How Do the Cathode Rays Work, Construction of a Cathode Ray Tube, Postulates of J.J. Thomson’s Atomic Model, and Practical Uses of Cathode Ray Tube Experiment along with examples. Students can learn the concepts of all the important topics of Science subject on Vedantu.

NCERT Study Material

COMMENTS
Learn how J.J. Thomson discovered electrons by using a cathode ray tube, a vacuum tube that produces a beam of electrons. Find out the apparatus setup, the procedure of the experiment, the conclusion and the uses of cathode ray tubes.
Learn how JJ Thomson used a cathode ray tube to discover the electron in 1897 and measure its mass to charge ratio. Find out the importance of this discovery for physics and chemistry and some interesting facts about Thomson.
Learn how Thomson used a cathode ray tube to discover electrons and revolutionize our understanding of atomic structure. Explore the significance, applications and legacy of his experiment and its impact on modern physics.
Learn how J.J. Thomson used cathode ray tubes to discover the electron, a negatively charged subatomic particle. Find out the definition, diagram and history of cathode ray tubes and their ...
Thomson's Cathode Ray Experiment was a groundbreaking study conducted by J.J. Thomson in 1897 that led to the discovery of the electron as a fundamental particle. In this experiment, Thomson used a cathode ray tube to observe the behavior of cathode rays, which are streams of electrons emitted from a negatively charged electrode. His findings challenged existing atomic models and introduced ...
Learn how Thomson used a cathode ray tube to prove that cathode rays were negatively charged particles, later called electrons. Explore the history and significance of his experiments and their impact on the development of modern physics.
J. J. Thomson used a cathode ray tube to show that cathode rays are composed of particles, which he called electrons. He also estimated the charge-to-mass ratio of electrons and proposed the raisin pudding model of the atom.
Learn how J.J. Thomson discovered the electron and the plum pudding model of the atom using a cathode ray tube. See key questions, explanations, and examples related to Thomson's experiment and its impact on atomic theory.
Learn how JJ Thomson discovered the electron in 1897 by using a cathode ray tube to measure the charge and speed of the particles. Find out how he resolved the debate about how cathode rays move and why it changed scientific thought about atoms.
The outcome of the Cathode Ray Tube Experiment, conducted by J.J. Thomson, was the discovery of the electron. Thomson demonstrated that cathode rays were composed of negatively charged particles, which he called "corpuscles" (later renamed electrons). This experiment provided evidence for the existence of subatomic particles and led to the development of the modern atomic model.
Learn about J.J. Thomson, the Nobel Prize winning English physicist who discovered the electron and developed the mass spectrometer. Find out how he used cathode rays, electric and magnetic fields, and positive rays to explore the structure of atoms.
Learn how Thomson used a cathode ray tube to prove the existence of subatomic particles in 1897 and how he announced his discovery at the Royal Institution. Find out the origin of the term electron and Thomson's Nobel Prize and legacy.
The British physicist Joseph John "J. J." Thomson (1856-1940) performed a series of experiments in 1897 designed to study the nature of electric discharge in a high-vacuum cathode-ray tube, an area being investigated by many scientists at the time.
In this section I will discuss the grounds for belief in the existence of the electron by examining J.J. Thomson's experiments on cathode rays. His 1897 experiment on cathode rays is generally regarded as the "discovery" of the electron. The purpose of J.J. Thomson's experiments was clearly stated in the introduction to his 1897 paper.
This was found to be constant regardless of the gas used in the tube and the metal of the cathode and was approximately 1000 times less than the value calculated for hydrogen ions in the electrolysis of liquids. The electron is discovered, J J Thomson publishes his discovery of a subatomic particle common to all matter.
J. J. Thomson measured the charge-to-mass ratio and velocity of cathode rays and concluded that they are made up of negatively charged particles of very small mass. He contrasted his electrified-particle theory with the ether-vortex theory and cited Jean Perrin's experiment as evidence.
Thompson was able to measure the ratio of their mass to their speed. Cathode "rays" had been known for some time before Thomson. They were first observed as experiments in gas discharge tubes started to exploit better and better vacuums (the early experiments observed the varying forms of discharge in low pressure gases; cathode rays only become significant when there is very little gas left ...
Learn about the cathode ray experiment conducted by J J Thomson in 1897 to discover the electron and its properties. Find out the construction, formula and uses of cathode ray tubes.
In this MIT lecture, at 7:22, the professor says that when J.J. Thomson added a positively charged plate on one side of the cathode ray and a negatively charged plate on the other side, he observed a large deflection towards the positive plate, and a small deflection towards the negative plate (see image below).. This observation implied that hydrogen gas consists of positively charged ...