- Remote Access
- Save figures into PowerPoint
- Download tables as PDFs


9: Bacterial Meningitis
Jonathan C. Cho
- Download Chapter PDF
Disclaimer: These citations have been automatically generated based on the information we have and it may not be 100% accurate. Please consult the latest official manual style if you have any questions regarding the format accuracy.
Download citation file:
- Search Book
Jump to a Section
Patient presentation.
- Full Chapter
- Supplementary Content
Chief Complaint
“I have severe headaches and fevers.”
History of Present Illness
DJ is a 54-year-old Caucasian female who presents to the emergency department with worsening headache, neck pain, and back pain of 2 days duration. She also complains of low-grade fevers and chills that developed over the past 24 hours. Her son, who is present during her exam, states that she seems more lethargic and has difficulty maintaining her balance. In addition, she reports 3 to 4 episodes of nausea and vomiting.
Past Medical History
CHF, COPD, HTN, epilepsy, stroke, hypothyroidism, anxiety
Surgical History
Hysterectomy, cholecystectomy
Family History
Father had HTN and passed away from a stroke 4 years ago; mother has type II DM and epilepsy; brother has HTN
Social History
Divorced but lives with her two sons who are currently attending college. Smokes ½ ppd × 27 years and drinks alcohol occasionally.
Home Medications
Advair 250 mcg/50 mcg 1 puff BID
Albuterol metered-dose-inhaler 2 puffs q4h PRN shortness of breath
Alprazolam 0.5 mg PO daily
Aspirin 81 mg PO daily
Atorvastatin 20 mg PO daily
Carvedilol 6.25 mg PO BID
Citalopram 20 mg PO daily
Divalproex sodium 500 mg PO BID
Furosemide 20 mg PO daily
Levothyroxine 88 mcg PO daily
Levetiracetam 500 mg PO BID
Lisinopril 20 mg PO daily
Physical Examination
Vital signs.
Temp 101.2°F, P 72, RR 23 breaths per minute, BP 162/87 mm Hg, pO 2 91%, Ht 5′3″, Wt 56.4 kg
Lethargic, female with dizziness and in mild to moderate distress.
Normocephalic, atraumatic, PERRLA, EOMI, pale or dry mucous membranes and conjunctiva, poor dentition
Diminished breath sounds and crackles bilaterally.
Cardiovascular
NSR, no m/r/g
Soft, non-distended, non-tender, bowel sounds hyperactive
Genitourinary
Normal female genitalia, no complaints of dysuria or hematuria
Lethargic, oriented to place and person, (–) Brudzinski’s sign, (+) Kernig’s sign
Extremities
Get free access through your institution, pop-up div successfully displayed.
This div only appears when the trigger link is hovered over. Otherwise it is hidden from view.
Please Wait
Thank you for visiting nature.com. You are using a browser version with limited support for CSS. To obtain the best experience, we recommend you use a more up to date browser (or turn off compatibility mode in Internet Explorer). In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript.
- View all journals
- Explore content
- About the journal
- Publish with us
- Sign up for alerts
- Open access
- Published: 16 March 2021
A hospital-based study on etiology and prognosis of bacterial meningitis in adults
- Jun-Sang Sunwoo 1 ,
- Hye-Rim Shin 2 ,
- Han Sang Lee 3 ,
- Jangsup Moon 3 , 4 ,
- Soon-Tae Lee 3 ,
- Keun-Hwa Jung 3 ,
- Kyung-Il Park 5 ,
- Ki-Young Jung 3 ,
- Manho Kim 3 , 6 ,
- Sang Kun Lee 3 &
- Kon Chu 3
Scientific Reports volume 11 , Article number: 6028 ( 2021 ) Cite this article
5367 Accesses
17 Citations
Metrics details
- Central nervous system infections
Bacterial meningitis is a neurological emergency with high morbidity and mortality. We herein investigated clinical features, etiology, antimicrobial susceptibility profiles, and prognosis of bacterial meningitis in adults from a single tertiary center. We retrospectively reviewed medical records of patients with laboratory-confirmed bacterial meningitis from 2007 to 2016. Patients with recent neurosurgery, head trauma, or indwelling neurosurgical devices were classified as having healthcare-related meningitis. Causative microorganisms were identified by analyzing cerebrospinal fluid (CSF) and blood cultures, and antimicrobial susceptibility profiles were evaluated. We performed multiple logistic regression analysis to identify factors associated with unfavorable outcomes. We identified 161 cases (age, 55.9 ± 15.5 years; male, 50.9%), of which 43 had community-acquired and 118 had healthcare-related meningitis. CSF and blood culture positivity rates were 91.3% and 30.4%, respectively. In community-acquired meningitis patients, Klebsiella pneumoniae (25.6%) was the most common isolate, followed by Streptococcus pneumoniae (18.6%) and Listeria monocytogenes (11.6%). The susceptibility rates of K. pneumoniae to ceftriaxone, cefepime, and meropenem were 85.7%, 81.3%, and 100%, respectively. Among healthcare-related meningitis patients, the most common bacterial isolates were coagulase-negative staphylococci (28.0%), followed by Staphylococcus aureus (16.1%) and Enterobacter spp. (13.6%). Neurological complications occurred in 39.1% of the patients and the 3-month mortality rate was 14.8%. After adjusting for covariates, unfavorable outcome was significantly associated with old age (odds ratio [OR] 1.03, 95% confidence interval [CI] 1.00–1.06), neurological complications (OR 4.53, 95% CI 1.57–13.05), and initial Glasgow coma scale ≤ 8 (OR 19.71, 95% CI 4.35–89.40). Understanding bacterial pathogens and their antibiotic susceptibility may help optimize antimicrobial therapy in adult bacterial meningitis.
Similar content being viewed by others

Epidemiology and outcomes of bacterial meningitis in the neonatal intensive care unit
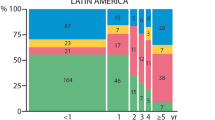
Outcome of childhood bacterial meningitis on three continents
Longer than 2 hours to antibiotics is associated with doubling of mortality in a multinational community-acquired bacterial meningitis cohort
Introduction.
Bacterial meningitis is a neurological emergency with high morbidity and mortality. Over 1.2 million cases of bacterial meningitis are estimated to occur annually worldwide 1 . Although adjunctive dexamethasone reduces the risk of unfavorable outcomes and death, neurological complications occur in approximately 30% of survivors 2 , 3 . Delayed antibiotic administration has been shown to significantly increase mortality and adverse outcomes at 3 months 4 . Therefore, early clinical suspicion and immediate antibiotic therapy are crucial in the initial management of bacterial meningitis.
Antibiotic treatments are determined empirically, based on the common causative pathogens of bacterial meningitis, age, host immune status, and predisposing conditions 5 . According to previous epidemiological studies, Streptococcus pneumoniae , Neisseria meningitidis , Haemophilus influenzae , and Listeria monocytogenes are the major bacterial pathogens responsible for community-acquired meningitis in adults 6 , 7 . On the other hand, the most common microorganisms associated with neurosurgical procedures and head trauma were coagulase-negative staphylococci (CoNS), Staphylococcus aureus , and gram-negative bacilli 8 . However, the epidemiology of bacterial meningitis has changed over the past 30 years. The introduction of conjugate vaccines significantly decreased the incidence of H. influenzae and S. pneumoniae meningitis and also shifted the age distribution of bacterial meningitis from children to older adults 9 , 10 . The increasing rate of antimicrobial resistance in S. pneumoniae is another important epidemiological trend that should be considered when selecting the appropriate antibiotic therapy 11 . However, there is little information on the etiology and antimicrobial susceptibility profiles of recent bacterial meningitis cases, especially in Korea. Therefore, we investigated the clinical, laboratory, and microbiological profiles of adult bacterial meningitis patients from a single tertiary center over a 10-year period.
Study subjects
We retrospectively reviewed the medical records of adult patients with laboratory-confirmed bacterial meningitis, who were treated in Seoul National University Hospital from 2007 to 2016. Bacterial meningitis was defined as follows according to the World Health Organization recommendation 12 . Suspected cases were defined as any person with clinical features of bacterial meningitis, such as fever, altered consciousness, and meningeal signs. Probable cases were defined as any suspected cases with cerebrospinal fluid (CSF) white blood cell (WBC) count > 100 cells/mm 3 , or CSF WBC count of 10–100 cells/mm 3 with either protein level > 100 mg/dL or glucose level < 40 mg/dL. Finally, laboratory-confirmed cases were defined as any suspected or probable cases in which bacterial pathogens were identified in CSF or blood cultures or bacterial antigen detection by CSF latex agglutination test. Only laboratory-confirmed bacterial meningitis cases were selected as study subjects and included in the analysis. Patients with tuberculous meningitis and meningoencephalitis due to non-bacterial pathogens were excluded from the study. When CSF and blood cultures showed discordant results, the isolate from the CSF culture was considered as the causative organism.
Bacterial meningitis cases were classified into community-acquired and healthcare-related meningitis, because they have a different spectrum of bacterial pathogens. Patients with recent neurosurgery (within 1 month of the onset of meningitis); head trauma; or indwelling neurosurgical devices, such as ventriculoperitoneal shunt, extraventricular drain, and lumbar drain, were classified as having healthcare-related meningitis. Patients without evidence of healthcare-related infection were classified as having community-acquired meningitis.
Clinical information
We analyzed demographic information, symptoms and signs at presentation, premorbid functional status, immunocompromised status, concurrent infection, indwelling neurosurgical devices, and recent neurosurgery or head trauma. Severe mental deterioration at admission was defined as an initial Glasgow Coma Scale (GCS) score ≤ 8. We evaluated the initial CSF profiles, including cell count with differential and protein and glucose levels. Clinical outcomes were measured using a modified Rankin Scale (mRS) score at discharge and 3 months after discharge. An unfavorable outcome was defined as an mRS score ≥ 4 at 3 months.
Antimicrobial susceptibility test
Antimicrobial susceptibility tests were performed and interpreted according to the Clinical and Laboratory Standards Institute. The results were reported as susceptible, intermediate, or resistant. A bacterial isolate was classified as non-susceptible to an antimicrobial agent when it tested as intermediate or resistant. Multi-drug resistance (MDR) was defined, according to the guidelines of the European Centre for Disease Prevention and Control 13 , as non-susceptibility to at least one agent in three or more antimicrobial categories. In particular, MDR of Streptococcus spp. was defined as non-susceptibility to penicillin and antimicrobials in two or more other non-β-lactam classes 14 .
Ethical statement
This study protocol was approved by the institutional review board (IRB) of Seoul National University Hospital (No. C-1705–016-851) and was performed in accordance with the principles of the Declaration of Helsinki. Because this was a retrospective medical chart review study, informed consent was not obtained from the participants and the IRB of Seoul National University Hospital granted a waiver of informed consent. All information gathered in this study was anonymized to preserve the participants’ privacy.
Statistical analysis
We performed a Student's t-test or a Pearson's chi-square test for between-group comparisons of continuous and categorical variables, respectively. Data that were not normally distributed are presented as median (interquartile range) and were analyzed using a Wilcoxon rank-sum test. We performed multivariate logistic regression analyses to identify factors related to clinical outcomes in bacterial meningitis patients. Dependent variables were unfavorable outcome and mortality at 3 months, analyzed separately. Patients with a premorbid disability, defined as a premorbid mRS score ≥ 3, were excluded from the analysis. Variables with P < 0.1 in univariate logistic analyses were included as independent variables. In addition, age, sex, and type of meningitis (healthcare-related vs community-acquired) were included as covariates. A two-tailed P -value < 0.05 was considered statistically significant and statistical analyses were performed using SPSS version 25 (IBM Corp. Armonk, NY, USA).
Clinical presentation
We identified 161 cases of which 43 had community-acquired meningitis and 118 had healthcare-related meningitis. Six patients, all of whom had a healthcare-related infection, experienced a second episode of bacterial meningitis. Of these, postoperative CSF leak occurred in one patient and intraventricular devices were implanted in the other five patients. Overall, the mean age was 55.9 ± 15.5 years and 50.9% of patients were male (Table 1 ). There was no significant seasonal variation ( P = 0.806). Regarding predisposing factors, immunocompromised conditions were present in 31 (19.3%) patients and a concurrent infection, such as pneumonia, catheter-related blood stream infection, or peritonitis, was found in 44 (27.3%) patients. Among the healthcare-related meningitis patients, 64.4% underwent recent neurosurgery and 62.7% had indwelling neurosurgical devices. At presentation, the classic triad of fever, neck stiffness, and altered mental status was found in 31.3% of patients. The initial GCS score was 12.3 ± 3.8 and severe mental deterioration was observed in 33 (20.5%) patients. Compared to healthcare-related patients, community-acquired meningitis patients were characterized by older age ( P < 0.001), lower initial GCS scores ( P = 0.032), and a higher rate of neck stiffness ( P = 0.049) and the classic symptom triad ( P = 0.003). Regarding predisposing conditions, patients with healthcare-related meningitis showed a higher prevalence of concurrent infections ( P = 0.027) and a lower prevalence of diabetes mellitus ( P = 0.034) than those with community-acquired meningitis.
Laboratory findings
Mean CSF opening pressure was 22.5 ± 10.6 cmH 2 O, and an elevated pressure ≥ 20 cmH 2 O was found in 54.3% of the patients (Table 2 ). Median CSF WBC count was 828.0/mm 3 (interquartile range [IQR], 256.3–2870.0), with 76.3% neutrophils and 14.0% lymphocytes. The median protein level was 201.8 mg/dL (IQR, 93.0–489.0) and CSF/blood glucose ratio was 0.28 (IQR, 0.07–0.47). Community-acquired meningitis patients showed higher CSF protein levels ( P = 0.005) and lower CSF glucose levels ( P = 0.002) than healthcare-related meningitis patients, although CSF WBC counts did not significantly differ between the two groups. In blood tests, thrombocytopenia, with a platelet count < 100, 000/mm 3 , was noted in 12.6% of the patients, whereas increased high-sensitivity C-reactive protein (hs-CRP) levels > 10 mg/dL were identified in 42.8% of the patients.
Causative microorganisms
Overall, CSF Gram stains and cultures were positive in 24.5% (34/139) and 91.3% (147/161) of patients, respectively. Blood cultures were positive in 30.4% (49/161) of patients and blood and CSF cultures were both positive in 21.7% (35/161) of patients. Bacterial antigen detection tests were only performed in 40 patients and six of these (15.0%) were positive, with five testing positive for S. pneumoniae and one testing positive for Streptococcus agalactiae .
The bacterial pathogens isolated from 161 bacterial meningitis cases are summarized in Table 3 . Mixed infections with two different species were found in four patients. In community-acquired meningitis patients, Klebsiella pneumoniae (25.6%) was the most common isolate, followed by S. pneumoniae (18.6%) and L. monocytogenes (11.6%). H. influenzae accounted for 4.7% of infections, but N. meningitidis was not detected. Among healthcare-related meningitis patients, the most common bacterial isolates were CoNS (28.0%), followed by S. aureus (16.1%) and Enterobacter spp. (13.6%). Streptococcus spp. were more common in community-acquired meningitis patients (34.9% vs. 4.2%, P < 0.001), whereas Staphylococcus spp. were more frequently isolated from healthcare-related meningitis patients (44.1% vs. 4.7%, P < 0.001). Furthermore, L. monocytogenes was only isolated from community-acquired meningitis patients, whereas CoNS and Enterobacter spp. were only isolated from healthcare-related meningitis patients.
Antimicrobial susceptibility profiles
The susceptibility rates of S. pneumoniae to penicillin G, cefotaxime, and vancomycin were 33.3%, 40.0%, and 100%, respectively (Supplementary Table S1 ). For streptococci other than S. pneumoniae , the susceptibility rates to penicillin and vancomycin were 62.5% and 100%, respectively. Among the S. aureus isolates, 85% were resistant to methicillin (oxacillin), but 100% were susceptible to vancomycin. S. epidermidis isolates showed similar profiles, with 90.5% resistant to methicillin (oxacillin), but 100% susceptible to vancomycin. The MDR rate of Staphylococcus spp. was 88.5%. Among gram-negative bacilli, the susceptibility rates of K. pneumoniae to ceftriaxone, cefepime, and meropenem were 85.7%, 81.3%, and 100%, respectively (Supplementary Table S2 ). Extended-spectrum β-lactamase (ESBL) producers were found to account for 18.8% (3/16) of the K. pneumoniae isolates, all of which were from healthcare-related meningitis patients. The MDR rates of K. pneumoniae , Pseudomonas spp., Acinetobacter spp., and Enterobacter spp. were 25.0%, 18.2%, 44.4%, and 56.3%, respectively. Overall, bacterial isolates from healthcare-related meningitis patients showed higher rates of MDR than those from community-acquired meningitis patients (69.1% vs. 25.0%, P < 0.001).
Complications and outcomes
The mean length of stay in the hospital was 76.7 ± 97.6 days, with 52.8% of patients treated in the intensive care unit. Mechanical ventilation was used in 36.0% of patients. Neurological complications occurred in 39.1% of patients and the most common complication was hydrocephalus (19.9%), followed by seizures (13.7%). Ischemic infarction and cerebral hemorrhage were more common in community-acquired patients than in healthcare-related patients ( P < 0.001 and 0.015, respectively; Table 4 ). Mortality rates at discharge and 3 months after discharge were 10.6% and 14.8%, respectively. Mortality rates and mRS scores at discharge and 3 months after discharge did not differ between community-acquired and healthcare-related meningitis patients, although 3-month mRS scores in healthcare-related meningitis patients tended to be higher than those in community-acquired meningitis patients ( P = 0.075).
We then assessed factors associated with unfavorable outcomes and mortality at 3 months in adult bacterial meningitis patients. Since 19 patients had no follow-up data at 3 months after discharge, we analyzed the outcome in 142 patients. In univariate analysis, an unfavorable outcome was associated with older age, neurological complications, concurrent infection, high hs-CRP levels, and an initial GCS score ≤ 8. However, neither positive Gram staining results, the MDR status of isolates, nor immunocompromised status were associated with an unfavorable outcome (Supplementary Table S3 ). After adjusting for covariates, an unfavorable outcome was significantly associated with older age (odds ratio [OR] 1.03, 95% confidence interval [CI] 1.00–1.06), neurological complications (OR 4.53, 95% CI 1.57–13.05), and an initial GCS score ≤ 8 (OR 19.71, 95% CI 4.35–89.40; Table 5 ). Multivariate analysis for mortality at 3 months showed similar results with higher mortality rates associated with neurological complications (OR 5.67, 95% CI 1.76–18.25) and an initial GCS score ≤ 8 (OR 5.31, 95% CI 1.47–19.11).
We additionally performed subgroup analysis on the unfavorable outcome for community-acquired and healthcare-related meningitis, respectively. In healthcare-related meningitis, older age (OR 1.07, 95% CI 1.02–1.11), neurological complications (OR 4.13, 95% CI 1.12–15.25), and an initial GCS score ≤ 8 (OR 39.93, 95% CI 2.61–610.86) were associated with the unfavorable outcome, which is similar to the results from the total subjects. By contrast, an initial GCS score ≤ 8 (OR 14.23, 95% CI 1.82–111.31) only remained significantly associated with the unfavorable outcome in community-acquired meningitis (Table S4 ).
In this study, we investigated clinical, laboratory, and microbiological profiles of adult bacterial meningitis. The composition of causative microorganisms was significantly different between community-acquired and healthcare-related meningitis. Streptococcus spp. accounted for 34.9% of community-acquired meningitis patients, whereas Staphylococcus spp. accounted for 44.1% of healthcare-related meningitis patients. At the species level, K. pneumoniae (25.6%) was the most common causative bacterium in community-acquired meningitis, followed by S. pneumoniae (18.6%) and L. monocytogenes (11.6%) . In healthcare-related meningitis patients, S. epidermidis (17.8%) was the most common causative bacterium, followed by S. aureus (16.1%). Mortality during hospitalization and 3 months after discharge were 10.6% and 14.8%, respectively. Older age, any neurological complications, and severe mental deterioration at admission were significantly associated with unfavorable outcomes, which is consistent with the results of previous studies 3 , 15 .
A notable finding in this study was that K. pneumoniae was the most common pathogen in community-acquired meningitis patients. This is consistent with data from Taiwan showing that K. pneumoniae was the leading causative pathogen, accounting for 44.9% of spontaneous bacterial meningitis patients 16 . Although a high incidence of K. pneumoniae meningitis, together with a low incidence of S. pneumoniae meningitis has been reported in Taiwan in the 1980s and 1990s, the exact cause has not been determined 17 . Diabetes mellitus has been reported as a risk factor for community-acquired meningitis and liver abscesses caused by K. pneumoniae 18 . In agreement with this, 54.5% (6/11) of community-acquired K. pneumoniae meningitis patients in our study had comorbid diabetes mellitus. However, this cannot explain the sudden change in the epidemiology of bacterial meningitis in Korea. An epidemiological study in Korean adults between 1998 and 2008 showed that K. pneumoniae was the third most common pathogen, but accounted for only 7.7% of community-acquired meningitis cases 19 . Selection bias due to a single-center study design may have contributed to our results. Nevertheless, our findings suggested that K. pneumoniae infection should be considered as a possible etiology of community-acquired bacterial meningitis, at least in the tertiary hospital setting. It also cannot be concluded whether the increase in K. pneumoniae meningitis is due to regional or racial influences in East Asian countries. Further epidemiological studies in other countries are needed to address this issue. All K. pneumoniae isolates from community-acquired meningitis patients were susceptible to cefotaxime and cefepime. ESBL-producing K. pneumoniae , all of which were isolated from healthcare-related meningitis patients, were resistant to ceftazidime and cefepime, but susceptible to imipenem and meropenem. These antimicrobial susceptibility profiles are consistent with previous studies 20 , suggesting that the third- and fourth-generation cephalosporins are appropriate for antibiotic therapy against community-acquired meningitis associated with K. pneumoniae , but carbapenems should be considered for the treatment of healthcare-related meningitis.
S. pneumoniae has traditionally been reported as the most common causative organism of community-acquired bacterial meningitis in adults 3 , 21 . However, S. pneumoniae only accounted for 18.6% of cases in our study, although it was the second most common species detected. The introduction of conjugate vaccines against S. pneumoniae is a possible explanation for the low frequency of pneumococcal meningitis. Pneumococcal vaccination significantly decreased the incidence of pneumococcal meningitis in children and adults, suggesting an indirect effect of vaccination through herd immunity 22 , 23 . The high rate of resistance of S. pneumoniae to penicillin and third-generation cephalosporins confirmed that antibiotics containing vancomycin are the appropriate empirical regimen for S. pneumoniae meningitis. The low frequency of H. influenzae meningitis in this study is also thought to be the result of H. influenzae type b vaccination 6 . In Korea, the incidence of H. influenzae meningitis in children has significantly decreased since 2001 24 , and in a previous study, H. influenzae was not isolated among 196 adult patients with community-acquired meningitis 19 .
L. monocytogenes was the third most common pathogen detected in our study population. It is well known that elderly individuals and immunocompromised patients are at a high risk of contracting L. monocytogenes meningitis 25 . In our study, the mean age of L. monocytogenes meningitis patients was 73.6 years (range, 66–83 years) and two (40%) patients were immunocompromised. The two (40%) immunocompromised patients died in hospital and the other two (40%) were severely disabled (mRS score, 5) at 3 months, thus confirming the high mortality and morbidity rates previously reported for L. monocytogenes meningitis 26 .
The frequency of healthcare-related meningitis (73.3%) was higher than that of community-acquired meningitis (26.7%) in our study. These findings are likely to be caused by selecting bacterial meningitis cases from the tertiary hospital. Patients with community-acquired meningitis, especially those with mild severity, might have been treated at a lower level hospital rather than being transferred to this hospital. It is also possible that the epidemiological trend of decreasing frequencies of pneumococcal and meningococcal meningitis contributed to a decrease in the overall number of community-acquired meningitis and a relative increase in the proportion of healthcare-related meningitis 10 . However, it cannot be concluded in this study, and a nationwide epidemiological investigation is required to address this issue. The most common microorganisms causing healthcare-related bacterial meningitis were CoNS, S. aureus , and gram-negative bacilli, which were consistent with the results of previous studies 17 , 21 . Staphylococcus spp. were mostly methicillin-resistant, but all were susceptible to vancomycin. Gram-negative bacilli showed moderate susceptibility to third- and fourth-generation cephalosporins, but high susceptibility to carbapenems. These antimicrobial susceptibility profiles support the use of vancomycin plus meropenem for the empirical treatment of healthcare-related meningitis 27 . Exceptionally, Acinetobacter spp. showed high resistance (44.4%) to meropenem. Therefore, if the Acinetobacter isolates are resistant to carbapenems, colistin or polymyxin B should be administered 28 .
There are several limitations of this study. Firstly, as a single-center study, our subjects may not represent the general population of adult bacterial meningitis patients in Korea. Recruitment from a tertiary hospital may have caused selection bias toward more severe cases. Moreover, although we collected data over a 10-year period, the sample size was relatively small, especially for community-acquired meningitis. Another limitation is the retrospective chart review design, which may have resulted in incomplete or inaccurate data collection. We first investigated the results of CSF examinations and cultures, and among probable cases and culture-positive cases, we reviewed the electrical medical records and selected those who met the definition of laboratory-confirmed bacterial meningitis. Nevertheless, we might have missed some cases, which is an inevitable limitation of the retrospective study. Furthermore, although we identified the clinical factors associated with unfavorable outcomes in bacterial meningitis patients, a causal relationship cannot be inferred from this study. Although we adjusted for the effect of the type of infection in the multivariate analysis, we cannot completely rule out the effect of neurosurgery itself on the unfavorable outcome.
In conclusion, we identified a spectrum of causative microorganisms for adult bacterial meningitis cases over a recent 10-year period. The increased proportion of K. pneumoniae infections and the decreased proportion of S. pneumoniae infections among community-acquired meningitis patients was particularly noteworthy. Our data on causative microorganisms and their antibiotic susceptibility profiles may help optimize determination of the appropriate empirical antimicrobial therapy for adult bacterial meningitis patients. However, further studies are required to confirm the changing epidemiology of causative pathogens and prognostic factors in adult bacterial meningitis.
Data availability
Deidentified data are available from the corresponding author upon reasonable request.
Liu, L. et al. Global, regional, and national causes of child mortality: an updated systematic analysis for 2010 with time trends since 2000. The Lancet 379 , 2151–2161. https://doi.org/10.1016/s0140-6736(12)60560-1 (2012).
Article Google Scholar
de Gans, J., van de Beek, D. & European Dexamethasone in Adulthood Bacterial Meningitis Study Investigators. Dexamethasone in adults with bacterial meningitis. N. Engl. J. Med. 347 , 1549–1556. https://doi.org/10.1056/NEJMoa021334 (2002).
Article CAS PubMed Google Scholar
van de Beek, D. et al. Clinical features and prognostic factors in adults with bacterial meningitis. N. Engl. J. Med. 351 , 1849–1859. https://doi.org/10.1056/NEJMoa040845 (2004).
Article PubMed Google Scholar
Auburtin, M. et al. Detrimental role of delayed antibiotic administration and penicillin-nonsusceptible strains in adult intensive care unit patients with pneumococcal meningitis: the PNEUMOREA prospective multicenter study. Crit. Care Med. 34 , 2758–2765. https://doi.org/10.1097/01.ccm.0000239434.26669.65 (2006).
Tunkel, A. R. et al. Practice guidelines for the management of bacterial meningitis. Clin. Infect. Dis. 39 , 1267–1284. https://doi.org/10.1086/425368 (2004).
Schuchat, A. et al. Bacterial meningitis in the United States in 1995 Active Surveillance Team. N. Engl. J. Med. 337 , 970–976. https://doi.org/10.1056/NEJM199710023371404 (1997).
Sigurdardottir, B., Bjornsson, O. M., Jonsdottir, K. E., Erlendsdottir, H. & Gudmundsson, S. Acute bacterial meningitis in adults A 20-year overview. Arch. Intern. Med. 157 , 425–430. https://doi.org/10.1001/archinte.1997.00440250077009 (1997).
Laxmi, S. & Tunkel, A. R. Healthcare-associated bacterial meningitis. Curr. Infect. Dis. Rep. 13 , 367–373. https://doi.org/10.1007/s11908-011-0190-z (2011).
Thigpen, M. C. et al. Bacterial meningitis in the United States, 1998–2007. N. Engl. J. Med. 364 , 2016–2025. https://doi.org/10.1056/NEJMoa1005384 (2011).
Castelblanco, R. L., Lee, M. & Hasbun, R. Epidemiology of bacterial meningitis in the USA from 1997 to 2010: a population-based observational study. Lancet. Infect. Dis 14 , 813–819. https://doi.org/10.1016/s1473-3099(14)70805-9 (2014).
Song, J. H. et al. Spread of drug-resistant Streptococcus pneumoniae in Asian countries: Asian Network for Surveillance of Resistant Pathogens (ANSORP) Study. Clin. Infect. Dis. 28 , 1206–1211. https://doi.org/10.1086/514783 (1999).
World Health Organization. Surveillance standards for vaccine-preventable diseases . 2nd edn, (World Health Organization, 2018)
Magiorakos, A. P. et al. Multidrug-resistant, extensively drug-resistant and pandrug-resistant bacteria: an international expert proposal for interim standard definitions for acquired resistance. Clin. Microbiol. Infect. 18 , 268–281. https://doi.org/10.1111/j.1469-0691.2011.03570.x (2012).
Richter, S. S. et al. Changing epidemiology of antimicrobial-resistant Streptococcus pneumoniae in the United States, 2004–2005. Clin. Infect. Dis. 48 , e23-33. https://doi.org/10.1086/595857 (2009).
Flores-Cordero, J. M. et al. Acute community-acquired bacterial meningitis in adults admitted to the intensive care unit: clinical manifestations, management and prognostic factors. Intensive Care Med. 29 , 1967–1973. https://doi.org/10.1007/s00134-003-1935-4 (2003).
Chang, W. N. et al. Changing epidemiology of adult bacterial meningitis in southern taiwan: a hospital-based study. Infection 36 , 15–22. https://doi.org/10.1007/s15010-007-7009-8 (2008).
Lu, C. H., Chang, W. N. & Chang, H. W. Adult bacterial meningitis in Southern Taiwan: epidemiologic trend and prognostic factors. J. Neurol. Sci. 182 , 36–44. https://doi.org/10.1016/s0022-510x(00)00445-7 (2000).
Ko, W.-C. et al. Community-acquired Klebsiella pneumoniae bacteremia: global differences in clinical patterns. Emerg. Infect. Dis. 8 , 160–166. https://doi.org/10.3201/eid0802.010025 (2002).
Article PubMed PubMed Central Google Scholar
Moon, S. Y. et al. Changing etiology of community-acquired bacterial meningitis in adults: a nationwide multicenter study in Korea. Eur. J. Clin. Microbiol. Infect. Dis. 29 , 793–800. https://doi.org/10.1007/s10096-010-0929-8 (2010).
Ku, Y.-H. et al. Klebsiella pneumoniae Isolates from Meningitis: Epidemiology Virulence and Antibiotic Resistance. Sci. Rep. 7 , 6634. https://doi.org/10.1038/s41598-017-06878-6 (2017).
Article ADS CAS PubMed PubMed Central Google Scholar
Durand, M. L. et al. Acute bacterial meningitis in adults. A review of 493 episodes. N. Engl. J. Med. 328 , 21–28. https://doi.org/10.1056/NEJM199301073280104 (1993).
Hsu, H. E. et al. Effect of pneumococcal conjugate vaccine on pneumococcal meningitis. N. Engl. J. Med. 360 , 244–256. https://doi.org/10.1056/NEJMoa0800836 (2009).
Article CAS PubMed PubMed Central Google Scholar
Tsai, C. J., Griffin, M. R., Nuorti, J. P. & Grijalva, C. G. Changing epidemiology of pneumococcal meningitis after the introduction of pneumococcal conjugate vaccine in the United States. Clin. Infect. Dis. 46 , 1664–1672. https://doi.org/10.1086/587897 (2008).
Cho, H. K. et al. The causative organisms of bacterial meningitis in Korean children in 1996–2005. J. Korean Med. Sci. 25 , 895–899. https://doi.org/10.3346/jkms.2010.25.6.895 (2010).
Brouwer, M. C., van de Beek, D., Heckenberg, S. G., Spanjaard, L. & de Gans, J. Community-acquired Listeria monocytogenes meningitis in adults. Clin. Infect. Dis. 43 , 1233–1238. https://doi.org/10.1086/508462 (2006).
Amaya-Villar, R. et al. Three-year multicenter surveillance of community-acquired Listeria monocytogenes meningitis in adults. BMC Infect. Dis. 10 , 324. https://doi.org/10.1186/1471-2334-10-324 (2010).
Tunkel, A. R. et al. Infectious diseases society of america’s clinical practice guidelines for healthcare-associated ventriculitis and meningitis. Clin. Infect. Dis. 64 , e34–e65. https://doi.org/10.1093/cid/ciw861 (2017).
Kim, B.-N. et al. Management of meningitis due to antibiotic-resistant Acinetobacter species. Lancet. Infect. Dis 9 , 245–255. https://doi.org/10.1016/S1473-3099(09)70055-6 (2009).
Download references
Acknowledgement
The abstract of this study was published under the title "Clinical and Microbiological Characteristics of Bacterial Meningitis in Adults" at the 145th Annual Meeting of the American Neurological Association ( https://doi.org/10.1002/ana.25865 ).
This work was supported by a research grant from Ildong Pharmaceutical, Co., Ltd, Seoul, South Korea (06-2019-1880).
Author information
Authors and affiliations.
Department of Neurosurgery, Seoul National University Hospital, Seoul, South Korea
Jun-Sang Sunwoo
Department of Neurology, Dankook University Hospital, Cheonan, South Korea
Hye-Rim Shin
Department of Neurology, Comprehensive Epilepsy Center, Biomedical Research Institute, Seoul National University Hospital, 101, Daehak-ro, Jongno-gu, Seoul, 03080, South Korea
Han Sang Lee, Jangsup Moon, Soon-Tae Lee, Keun-Hwa Jung, Ki-Young Jung, Manho Kim, Sang Kun Lee & Kon Chu
Rare Disease Center, Seoul National University Hospital, Seoul, South Korea
Jangsup Moon
Department of Neurology, Seoul National University Hospital Healthcare System Gangnam Center, Seoul, South Korea
Kyung-Il Park
Protein Metabolism and Neuroscience Dementia Medical Research Center, Seoul National University College of Medicine, Seoul, South Korea
You can also search for this author in PubMed Google Scholar
Contributions
J.S.S., H.R.S., H.S.L., and K.C. contributed to the conception and design of the study; all authors contributed to the acquisition, analysis, or interpretation of data; J.S.S. and K.C. contributed to drafting a significant portion of the manuscript.
Corresponding author
Correspondence to Kon Chu .
Ethics declarations
Competing interests.
The authors declare no competing interests.
Additional information
Publisher's note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions.
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ .
Reprints and permissions
About this article
Cite this article.
Sunwoo, JS., Shin, HR., Lee, H.S. et al. A hospital-based study on etiology and prognosis of bacterial meningitis in adults. Sci Rep 11 , 6028 (2021). https://doi.org/10.1038/s41598-021-85382-4
Download citation
Received : 17 November 2020
Accepted : 01 March 2021
Published : 16 March 2021
DOI : https://doi.org/10.1038/s41598-021-85382-4
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
This article is cited by
Bacterial meningitis in adults: a retrospective study among 148 patients in an 8-year period in a university hospital, finland.
- Sakke Niemelä
- Laura Lempinen
BMC Infectious Diseases (2023)
By submitting a comment you agree to abide by our Terms and Community Guidelines . If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.
Quick links
- Explore articles by subject
- Guide to authors
- Editorial policies
Sign up for the Nature Briefing newsletter — what matters in science, free to your inbox daily.
An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
- My Bibliography
- Collections
- Citation manager
Save citation to file
Email citation, add to collections.
- Create a new collection
- Add to an existing collection
Add to My Bibliography
Your saved search, create a file for external citation management software, your rss feed.
- Search in PubMed
- Search in NLM Catalog
- Add to Search
Viral Meningitis: A Pediatric Case Study
Affiliation.
- 1 University of Alabama at Birmingham School of Nursing.
- PMID: 33105178
- DOI: 10.1097/TME.0000000000000318
Meningitis is a significant viral, bacterial, or fungal infection of the meninges that cover and protect the brain and the spinal cord. Symptoms of meningitis may present rapidly or develop gradually over a period of days, manifesting with common prodromal flu-like symptoms of headache, photophobia, fever, nuchal rigidity, myalgias, and fatigue. Character and significance of symptoms vary by patient age. Symptoms of infection may improve spontaneously or worsen, becoming potentially lethal. Early recognition and treatment of meningitis are crucial to prevent morbidity and mortality. The case reviewed in this article focuses on viral meningitis in a pediatric patient that may be unrecognized or underreported because of indistinct symptoms. Epidemiology, pathophysiology, presentation, assessment techniques, diagnostics, clinical management, and health promotion relevant to viral meningitis are presented.
PubMed Disclaimer
Similar articles
- Fifteen-minute consultation: enterovirus meningitis and encephalitis-when can we stop the antibiotics? Drysdale SB, Kelly DF. Drysdale SB, et al. Arch Dis Child Educ Pract Ed. 2017 Apr;102(2):66-71. doi: 10.1136/archdischild-2016-310632. Epub 2016 Oct 27. Arch Dis Child Educ Pract Ed. 2017. PMID: 27789515 Review.
- [Prospective study of 59 cases of viral meningitis in children. Clinical and virologic diagnosis. Epidemiology and physiopathology]. Tardieu M, Dussaix E, Lebon P, Landrieu P. Tardieu M, et al. Arch Fr Pediatr. 1986 Jan;43(1):9-14. Arch Fr Pediatr. 1986. PMID: 3010893 French.
- Enteroviruses and meningitis. Wilfert CM, Lehrman SN, Katz SL. Wilfert CM, et al. Pediatr Infect Dis. 1983 Jul-Aug;2(4):333-41. doi: 10.1097/00006454-198307000-00019. Pediatr Infect Dis. 1983. PMID: 6310537 No abstract available.
- Management of central nervous system infections during an epidemic of enteroviral aseptic meningitis. Singer JI, Maur PR, Riley JP, Smith PB. Singer JI, et al. J Pediatr. 1980 Mar;96(3 Pt 2):559-63. doi: 10.1016/s0022-3476(80)80866-3. J Pediatr. 1980. PMID: 7359259
- Enterovirus infections: diagnosis and treatment. Sawyer MH. Sawyer MH. Semin Pediatr Infect Dis. 2002 Jan;13(1):40-7. doi: 10.1053/spid.2002.29756. Semin Pediatr Infect Dis. 2002. PMID: 12118843 Review.
- Predictive value of inflammatory factors on the efficacy of adjuvant Dexamethasone in the treatment of refractory purulent meningitis among pediatric patients. Zhong X, Niu Q, Yuan X. Zhong X, et al. J Med Biochem. 2024 Jun 15;43(4):406-412. doi: 10.5937/jomb0-37618. J Med Biochem. 2024. PMID: 39139150 Free PMC article.
- Acetaminophen. (2020). Retrieved from https://online.epocrates.com/drugs/306/acetaminophen
- Bartt R. (2012). Acute bacterial and viral meningitis. American Academy of Neurology, 18(6), 1255–1270. doi:10.1212/01.CON.0000423846.40147.4f
- Bennett J., Dolin R., Blaser M. (2019). Mandell, Douglas, and Bennett's principles and practice of infectious diseases (9th ed.). Philadelphia, PA: Elsevier.
- Ceftriaxone. (2020). Retrieved from https://online.epocrates.com/drugs/1623/ceftriaxone
- Centers for Disease Control and Prevention (CDC). (2018). Viral meningitis. Retrieved from https://www.cdc.gov/meningitis/viral.html
Publication types
- Search in MeSH
Related information
- PubChem Compound (MeSH Keyword)
LinkOut - more resources
Full text sources.
- Ingenta plc
- Ovid Technologies, Inc.
- Wolters Kluwer

- Citation Manager
NCBI Literature Resources
MeSH PMC Bookshelf Disclaimer
The PubMed wordmark and PubMed logo are registered trademarks of the U.S. Department of Health and Human Services (HHS). Unauthorized use of these marks is strictly prohibited.
Case-based learning: meningitis
Causes, diagnosis and initial management options for adults and children with meningitis.

JL / The Pharmaceutical Journal
Meningitis is the second leading infection-related cause of death in children in the world, second only to pneumonia [1] . It is responsible for more deaths than malaria, AIDS, measles and tetanus combined [1] . The disease is more prevalent in children under the age of four years and in teenagers. In England, there has been a decline in confirmed cases of invasive meningococcal disease over the past two decades, from 2,595 cases in 1999/2000 to 755 cases in 2017/2018, which is a small increase from the 748 cases reported in 2016 and 2017 [2] .
Pharmacists have a vital role in raising awareness of the signs and symptoms of meningitis, while also maximising the benefit of the national immunisation programme to reduce the incidence of the disease. This article will cover initial management options, with a focus on children and neonates.
Meningitis — inflammation of the membranes covering the brain and spinal cord (meninges) — can be caused by viruses, bacteria or fungi.
Meningococcal disease encompasses meningococcal septicaemia (25% of cases), meningococcal meningitis (15% of cases) or a combination of the two (60% of cases) [3] . Up to 95% of meningitis in the UK is aseptic, where there is no growth on cerebrospinal fluid (CSF) culture, usually with a viral aetiology (e.g. enteroviruses) [3] .
Bacterial meningitis is most commonly caused by Neisseria meningitidis (also known as meningococcus), although the main pathogens alter with age. As such, N. meningitidis , Streptococcus pneumoniae (also known as pneumococcus) and Haemophilus influenzae type b are the leading causes of meningitis in children older than three months; however, Streptococcus agalactiae , Escherichia coli , S. pneumoniae and Listeria monocytogenes are more common in children younger than three months [3] .
The bacteria that cause meningitis are very common — they are present in the nasopharynx in around one in ten people who may not ever show any symptoms of disease. The reasons why some people develop meningitis while others do not are not yet fully understood. However, it is thought that genetic variations in the genes for Factor H and Factor H-related proteins may have a role to play [4] . These proteins regulate a part of the body’s immune system called the complement system, which recognises and kills invading bacteria.
Risk factors
In general, young children are at the highest risk of bacterial meningitis and septicaemia, but other age groups, including older people, can also be vulnerable to specific types. One study found that meningococcal meningitis was less frequent in older patients, whereas pneumococcal, listerial and meningitis of unknown origin were more frequent [3], [5] . People with low immunity, for example, those with HIV or those having chemotherapy treatment for cancer, may also be at an increased risk.
Individual countries show seasonal patterns of bacterial meningitis. For instance, increased cases have been observed between the months of December and March in the United States, France and the UK [6] . There is also evidence that mass gatherings and exposure to cigarette and wood smoke can make people more susceptible to certain causes of meningitis and septicaemia, potentially from interference with mucosal immunity [7] .
Depending on the cause, cases of meningitis may be isolated. However, those who have been in close contact with someone with bacterial meningitis may be at increased risk of disease.
Pathophysiology
Infection occurs through transmission of contaminated respiratory droplets or saliva. Pili on the bacterial surface are thought to disrupt endothelial cell junctions in the blood–brain barrier, allowing the pathogens to penetrate it [8] . Once they have entered the subarachnoid space (the area of the brain between the arachnoid membrane and the pia mater), the pathogens replicate rapidly. This causes the production of several inflammatory mediators, including lymphocytes and inflammatory cytokines, as well as local immunoglobulin production by plasma cells. This enhances the influx of leukocytes into the CSF, which releases toxic substances that contribute to the production of cyctotoxic oedema and increased intracranial pressure. It is this process that can contribute to neurological damage and even death [9] , [10] .
Signs, symptoms and immediate management
Symptoms typically occur within 3–7 days after transmission [3] . Early, non-Âspecific symptoms of meningitis include:
- Irritability;
- Ill appearance;
- Refusing food/drink;
- Other aches and respiratory symptoms;
- Vomiting/nausea;
Healthcare professionals should be aware that classic signs of meningitis that include neck stiffness, bulging fontanelle and high-pitched cry are often absent in infants with bacterial meningitis [3] , [11] . Less common early symptoms include shivering, diarrhoea, abdominal pain and distention, coryza and other ear, nose and throat symptoms [3] .
General features of meningitis include a nonÂ-blanching rash that can appear anywhere on the body, altered mental state, shock, unconsciousness and toxic or moribund state. If a patient presents with these symptoms, the glass test (also known as the ‘Tumbler test’; see Figure 1) may be used to aid diagnosis, where the side of a clear glass should be firmly pressed against the rash; if it does not fade under pressure, the patient may have septicaemia and needs urgent medical attention (see Figure 2) [3] , [12] . However, it should be noted that the National Institute for Health and Care Excellence’s Clinical Knowledge Summary states that the glass test should not be used solely for diagnosing bacterial meningitis and meningococcal septicaemia [13] .
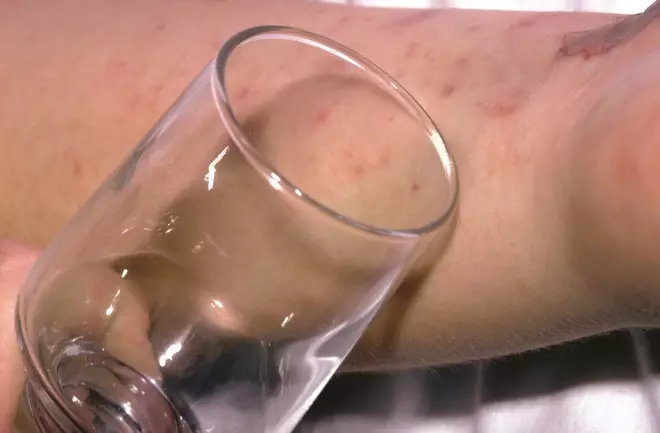
Figure 1: Glass or ‘tumbler’ test
Source: Alamy Stock Photo / Mediscan
A rash that does not fade under pressure is a sign of meningococcal septicaemeia. However, this test should not be used solely in diagnosis.
The classic rash associated with meningitis usually looks like small, red pin pricks that spreads quickly over the body and turns into red or purple blotches. However, a rash is not always present with meningitis and may be less visible in darker skin tones. It is, therefore, important to also check the soles of the feet, palms of the hands and eyelids in the patient with suspected meningitis [3] .
Furthermore, if the patient is a child or young person, it is important for healthcare professionals to consider other non-specific features of their presentation, such as the level of parental or carer concern (particularly compared with previous illness in the child or young person or their family), how quickly the illness is progressing, and clinical judgement of the overall severity of the illness [3] .

Figure 2: Immediate management of suspected meningitis in children and neonates
Source: National Institute for Health and Care Excellence [3]
CRP: C-reactive protein; CSF: cerebrospinal fluid; CT: computerised tomography; EDTA: ethylenedianinetetraacetic acid; FBC: full blood count; GCS: Glasgow coma scale; HSV: herpes simplex virus; ICP: intracranial pressure; IV: intravenous; LFT’s: liver function tests; LP: lumbar puncture; Mg: magnesium test; PCR: polymerase chain reaction; TB: tuberculosis; U+E’s: urea and electrolytes; WBC: white blood cell.
Prevention and vaccination
As meningitis can be caused by several different pathogens, there are several vaccinations available that can offer some protection against the disease (see Table) [10] .
| Meningitis B vaccine | This vaccine offers protection against meningococcal group B bacteria — a common cause of meningitis in young children in the UK | 8 weeks 16 weeks 1 year | N/A |
| 6-in-1 vaccine (DTaP/IPV/Hib/Hep B) | This vaccine offers protection against: | 8 weeks 12 weeks 16 weeks | N/A |
| Pneumococcal vaccine | This vaccine offers protection against serious infections caused by pneumococcal bacteria, including meningitis | 8 weeks 16 weeks 1 year | |
| Hib/Meningitis C vaccine | This vaccine offers protection against meningococcal group C bacteria that can cause meningitis | 1 year | as part of the combined meningitis ACWY vaccine |
| MMR vaccine | This vaccine offers protection against measles, mumps and rubella. Meningitis can sometimes occur as a complication of these infections | 1 year 3 years and 4 months | |
| ACWY: the meningococcal strains A, C, W and Y; DTaP: diphtheria, tetanus and pertussis vaccine; Hep B: heptatitis B; Hib: type b; IPV: inactivated poliovirus vaccine; MMR: measles, mumps and rubella Sources: NHS Choices | |||
Case studies
Several case studies show how assessment and treatment of meningitis varies by patient. All patients, events and incidents in these case studies are fictitious and should only be used as examples in the clinical setting.
Case study 1: a toddler with mild meningitis
Eva is a three-year-old girl who is on holiday with her grandparents. Eva is unusually tired and is complaining that her legs are aching. This morning, Eva’s grandparents noticed a very small purple rash on her leg, and so they have to come to the pharmacy for advice. Eva has no fever or any other symptoms, but her grandmother has a cold sore.
Assessment and diagnosis
The rash does not fade under pressure when a glass is pressed against it.
Petechiae and purpura are significantly more common with invasive meningococcal infection than with pneumococcal meningitis, which rarely presents with a rash [13] . However, although the glass test is widely promoted in patient information leaflets, the National Institute for Health and Care Excellence (NICE) has found no evidence on its use. The test is not promoted in the NICE guidelines. Consequently, the glass test should not be used as the only test for diagnosing the condition [12] . Public Health England is also informed that Eva may have meningitis, and 999 is called.
Treatment options
On arrival at hospital, Eva is showing signs of shock — she is tachycardic with increased drowsiness and cold peripheries. After having initial tests, she is treated for shock with a fluid bolus of 20mL/kg sodium chloride 0.9% over 10 minutes. A lumbar puncture is contraindicated in shock and, therefore, Eva is empirically started on intravenous (IV) ceftriaxone and steroids. She is also started on IV aciclovir, owing to her history of contact with the herpes simplex virus.
Advice and recommendations
Eva is treated with antibiotics for ten days and her grandparents are both prescribed rifampicin as chemoprophylaxis. Antibiotic prophylaxis should be given as soon as possible (ideally within 24 hours) after the diagnosis of the index case [12] .
Case study 2: a baby with meningitis
Katherine is a mother of two young children who comes into the pharmacy and asks for advice. She has a young baby, Jacob, who is six weeks old and Esmé who is four years old. Jacob has a blocked nose and fever. Katherine explains that Esmé had gastroenteritis with cold symptoms and fever last week, but no rash. Katherine is worried about Jacob and asks for advice.
Katherine brings her children into the consultation room for further assessment. Jacob has been more unsettled than usual and does not want to feed as much as normal. Upon examination, Jacob has a rash on his stomach and back, which his mother says was not present this morning. His rash looks like red blotches and does not fade with the glass test. Owing to his age, Jacob is too young to have received any vaccinations.
It is important to remain calm and inform Katherine that you think Jacob may have meningitis, as he has the characteristic rash, as well as other known symptoms. Jacob needs to be taken to hospital for emergency assessment and an ambulance is called.
On arrival at the hospital, Jacob has blood tests taken and a lumbar puncture. He is started on intravenous (IV) cefotaxime with amoxicillin (if he was three months or older, IV ceftriaxone would be administered) with full-volume maintenance fluids and enteral feeds as tolerated [3] . Corticosteroids must not be used in children aged younger than three months with suspected or confirmed bacterial meningitis.
Jacob has hourly observations initially for heart rate, blood pressure, respiratory rate, oxygen saturation, fluid balance and Glasgow Coma Scale (GCS). The GCS is a neurological scale used to describe the level of consciousness in a person following a traumatic brain injury — the lower the number, the more severe the brain injury. Public Health England is also informed that Jacob may have meningitis.
In children younger than three months, ceftriaxone may be used as an alternative to cefotaxime (with or without ampicillin or amoxicillin); however, it should not be used in premature babies or in babies with jaundice, hypoalbuminaemia or acidosis, as it may exacerbate hyperbilirubinaemia [3] .
The microbiology consultant calls the ward to confirm that Jacob has Group B streptococcal meningitis. As per the National Institute for Health and Care Excellence’s guidelines, Jacob will need treating with IV cefotaxime for at least 14 days [3] .
Before discharge, Katherine is given the contact details of several patient support organisations, including meningitis charities that can offer support and written information to signpost her to further help. Jacob has an audiology appointment booked in two weeks and will be seen by a paediatrician after this. At this appointment, the following morbidities will be considered:
- Hearing loss;
- Orthopaedic complications;
- Skin complications (including scarring from necrosis);
- Psychosocial problems;
- Neurological and developmental problems;
- Kidney failure.
Outcome of the advice
Jacob makes a full recovery from his meningitis with no lasting effects.
Case study 3: an adult with suspected meningitis
Jane is a paediatric haematology nurse who comes into the pharmacy asking to buy paracetamol. She says she has a terrible headache and upset stomach. She seems confused and disorientated; talking to her further highlights that something is not right.
Jane explains that she has not felt well since last night and has spent most of the day in bed, as she feels like she has no energy. However, some of what Jane also says does not make sense, and she is finding it hard to follow the conversation. She has no fever or rash.
Vomiting, severe headache and confusion are all symptoms of meningitis. Using a symptoms checker, such as the one by the Meningitis Research Foundation , to help with decision making.
Upon further questioning, it is clear that Jane must go to a hospital immediately and an ambulance is called. Jane presented with confusion and disorientation, which might indicate a stroke; however, bacterial meningitis can cause stroke.
When the paramedics arrive at the pharmacy, they find Jane has a Glasgow Coma Scale of 4/15. Once Jane arrives in hospital, they follow the stroke pathway, but she is now also febrile. Jane has a lumbar puncture and the results show she has bacterial meningitis. She also has a CT scan that shows an infarct on her right temporal lobe. Jane is treated in hospital with antibiotics and steroids, and eventually discharged to go home after three weeks.
Jane was working in the paediatric intensive care unit the week preceding the symptoms. She was looking after a child with Haemophilus influenzae type b (Hib). The patient was in a neutral pressure side room with a positive pressure lobby — this is an infection control measure to prevent the spread of microbial contaminants outside the patient’s side room. The lobby had been used to store an apheresis machine; however, the door between the side room and lobby had been left open, inadvertently leading to the exposure of Hib.
Although Jane has now fully recovered, she has to wear glasses owing to damage to her optical nerve. She also has tinnitus and occasionally suffers from severe headaches.
Recovering from meningitis/complications
Some of the most common complications associated with meningitis are [10] :
- Hearing loss, which may be partial or total — people who have had meningitis will usually have a hearing test after a few weeks to check for any problems;
- Recurrent seizures;
- Problems with memory and concentration;
- Problems with coordination, movement and balance;
- Learning difficulties and behavioural problems;
- Vision loss, which may be partial or total;
- Loss of limbs — amputation is sometimes necessary to stop the infection spreading through the body and remove damaged tissue;
- Bone and joint problems, such as arthritis;
- Kidney problems.
Overall, it is estimated that up to one in every ten cases of bacterial meningitis is fatal.
Useful resources
- Meningitis Research Foundation
- Meningitis Now
- National Institute for Health and Care Excellence clinical guideline [CG102]
[1] UNICEF, WHO, World Bank Group & United Nations. Levels and Trends in Child Mortality Report. 2017. Available at: https://www.unicef.org/publications/index_101071.html (accessed June 2019)
[2] Public Health England. Invasive meningococcal disease in England: annual laboratory confirmed reports for epidemiological year 2017 to 2018. 2018. Available at: https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/751821/hpr3818_IMD.pdf (accessed June 2019)
[3] National Institute for Health and Care Excellence. Meningitis (bacterial) and meningococcal septicaemia in under 16s: recognition, diagnosis and management. Clinical guideline [CG102]. 2015. Available at: https://www.nice.org.uk/guidance/cg102 (accessed June 2019)
[4] Davila S, Wright VJ, Khor CC et al . Genome-wide association study identifies variants in the CFH region associated with host susceptibility to meningococcal disease. Nat Genet 2010;42(9):772–776. doi: 10.1038/ng.640
[5] Domingo P, Pomar V, de Benito N & Coll P. The spectrum of acute bacterial meningitis in elderly patients. BMC Infect Dis 2013;13:108. doi: 10.1186/1471-2334-13-108
[6] Paireau J, Chen A, Broutin H et al . Seasonal dynamics of bacterial meningitis: a time-series analysis. Lancet Glob Health 2016;4(6):e370–e377. doi: 10.1016/S2214-109X(16)30064-X
[7] Cooper LV, Robson A, Trotter CL et al . Risk factors for acquisition of mening ococcal carriage in the African meningitis belt. Trop Med Int Health 2019;24(4):392–400. doi: 10.1111/tmi.13203
[8] Kolappan S, Coureuil M, Yu X et al . Structure of the Neisseria meningitidis type IV pilus. Nat Commun 2016;7:13015. doi: 10.1038/ncomms13015
[9] Tunkel AR & Scheld WM. Pathogenesis and pathophysiology of bacterial meningitis. Clin Microbiol Rev 1993;6(2):118–136. doi: 10.1128/CMR.6.2.118
[10] Sáez-Llorens X & McCracken GH Jr. Bacterial meningitis in children. Lancet 2003;361(9375):2139–2148. doi: 10.1016/S0140-6736(03)13693-8
[11] NHS Choices. Meningitis. 2019. Available at: https://www.nhs.uk/conditions/meningitis (accessed June 2019)
[12] Baines P, Reilly N & Gill A. Paediatric meningitis: clinical features and diagnosis. Clin Pharm 2009;1:307–310. URI: 10971150
[13] The National Institute for Health and Care Excellence. Clinical Knowledge Summaries: meningitis — bacterial meningitis and meningococcal disease. 2019. Available at: https://cks.nice.org.uk/meningitis-bacterial-meningitis-and-meningococcal-disease (accessed June 2019)
[14] NHS Choices. Pneumococcal vaccination. 2019. https://www.nhs.uk/conditions/vaccinations/pneumococcal-vaccination (accessed June 2019)
[15] Cooper LV, Robson A, Trotter C et al. Risk factors for acquisition of meningococcal carriage in the African meningitis belt. Trop Med Int Health 2019;24(4):392–400. doi: 10.1111/tmi.13203
You might also be interested in…
Government advises NHS service providers to hold PPE stocks in response to mpox risk
Test your knowledge of infectious diseases
Whooping cough resurgence as vaccination rates slump
- Subscribe to journal Subscribe
- Get new issue alerts Get alerts
Secondary Logo
Journal logo.
Colleague's E-mail is Invalid
Your message has been successfully sent to your colleague.
Save my selection
Viral Meningitis
A pediatric case study.
Editor(s): Jordan, Kathleen S. DNP, RN, FNP-BC, ENP-C, SANE-P, FAEN, FAANP, Column Editor
University of Alabama at Birmingham School of Nursing.
Corresponding Author: Sarah Freer, MSN, APRN, FNP-C, ENP-C, University of Alabama at Birmingham School of Nursing, 1720 2nd Ave S., Birmingham, AL 35294 ( [email protected] ).
Disclosure: The authors report no conflicts of interest.
Meningitis is a significant viral, bacterial, or fungal infection of the meninges that cover and protect the brain and the spinal cord. Symptoms of meningitis may present rapidly or develop gradually over a period of days, manifesting with common prodromal flu-like symptoms of headache, photophobia, fever, nuchal rigidity, myalgias, and fatigue. Character and significance of symptoms vary by patient age. Symptoms of infection may improve spontaneously or worsen, becoming potentially lethal. Early recognition and treatment of meningitis are crucial to prevent morbidity and mortality. The case reviewed in this article focuses on viral meningitis in a pediatric patient that may be unrecognized or underreported because of indistinct symptoms. Epidemiology, pathophysiology, presentation, assessment techniques, diagnostics, clinical management, and health promotion relevant to viral meningitis are presented.
Full Text Access for Subscribers:
Individual subscribers.

Institutional Users
Not a subscriber.
You can read the full text of this article if you:
- + Favorites
- View in Gallery
Readers Of this Article Also Read
Handoff communication from the emergency department to primary care, initial evaluation and management of bacterial meningitis in an emergent....

HILLARY R. MOUNT, MD, AND SEAN D. BOYLE, DO
Am Fam Physician. 2017;96(5):314-322
Patient information : See related handout on meningitis , written by the authors of this article.
Author disclosure: No relevant financial affiliations.
The etiologies of meningitis range in severity from benign and self-limited to life-threatening with potentially severe morbidity. Bacterial meningitis is a medical emergency that requires prompt recognition and treatment. Mortality remains high despite the introduction of vaccinations for common pathogens that have reduced the incidence of meningitis worldwide. Aseptic meningitis is the most common form of meningitis with an annual incidence of 7.6 per 100,000 adults. Most cases of aseptic meningitis are viral and require supportive care. Viral meningitis is generally self-limited with a good prognosis. Examination maneuvers such as Kernig sign or Brudzinski sign may not be useful to differentiate bacterial from aseptic meningitis because of variable sensitivity and specificity. Because clinical findings are also unreliable, the diagnosis relies on the examination of cerebrospinal fluid obtained from lumbar puncture. Delayed initiation of antibiotics can worsen mortality. Treatment should be started promptly in cases where transfer, imaging, or lumbar puncture may slow a definitive diagnosis. Empiric antibiotics should be directed toward the most likely pathogens and should be adjusted by patient age and risk factors. Dexamethasone should be administered to children and adults with suspected bacterial meningitis before or at the time of initiation of antibiotics. Vaccination against the most common pathogens that cause bacterial meningitis is recommended. Chemoprophylaxis of close contacts is helpful in preventing additional infections.
Patients with meningitis present a particular challenge for physicians. Etiologies range in severity from benign and self-limited to life-threatening with potentially severe morbidity. To further complicate the diagnostic process, physical examination and testing are limited in sensitivity and specificity. Advanc`es in vaccination have reduced the incidence of bacterial meningitis; however, it remains a significant disease with high rates of morbidity and mortality, making its timely diagnosis and treatment an important concern. 1
WHAT IS NEW ON THIS TOPIC: BACTERIAL MENINGITIS
In 2015, the Advisory Committee on Immunization Practices gave meningococcal serogroup B vaccines a category B recommendation (individual clinical decision making) for healthy patients 16 to 23 years of age (preferred age 16 to 18 years).
| Diagnosis of meningitis is mainly based on clinical presentation and cerebrospinal fluid analysis. Other laboratory testing and clinical decision rules, such as the Bacterial Meningitis Score, may be useful adjuncts. | C | , – , – |
| Lumbar puncture may be performed without computed tomography of the brain if there are no risk factors for an occult intracranial abnormality. | C | , |
| Appropriate antimicrobials should be given promptly if bacterial meningitis is suspected, even if the evaluation is ongoing. Treatment should not be delayed if there is lag time in the evaluation. | B | , , , |
| Dexamethasone should be given before or at the time of antibiotic administration to patients older than six weeks who present with clinical features concerning for bacterial meningitis. | B | , – , , |
| Vaccination for , type B, and is recommended for patients in appropriate risk groups and significantly decreases the incidence of bacterial meningitis. | B | – |
Meningitis is an inflammatory process involving the meninges. The differential diagnosis is broad ( Table 1 ) . Aseptic meningitis is the most common form. The annual incidence is unknown because of underreporting, but European studies have shown 70 cases per 100,000 children younger than one year, 5.2 cases per 100,000 children one to 14 years of age, and 7.6 per 100,000 adults. 2 , 3 Aseptic is differentiated from bacterial meningitis if there is meningeal inflammation without signs of bacterial growth in cultures. These cases are often viral, and enterovirus is the most common pathogen in immunocompetent individuals. 2 , 4 The most common etiology in U.S. adults hospitalized for meningitis is enterovirus (50.9%), followed by unknown etiology (18.7%), bacterial (13.9%), herpes simplex virus (HSV; 8.3%), noninfectious (3.5%), fungal (2.7%), arboviruses (1.1%), and other viruses (0.8%). 5 Enterovirus and mosquito-borne viruses, such as St. Louis encephalitis and West Nile virus, often present in the summer and early fall. 4 , 6 HSV and varicella zoster virus can cause meningitis and encephalitis. 2
| Bacterial meningitis |
| Viral meningitis |
| Behçet syndrome |
| Benign recurrent lymphocytic meningitis (Mollaret meningitis) |
| Central nervous system abscess |
| Drug-induced meningitis (e.g., non-steroidal anti-inflammatory drugs, trimethoprim/sulfamethoxazole) |
| Ehrlichiosis |
| Fungal meningitis |
| Human immunodeficiency virus |
| Leptomeningeal carcinomatosis |
| Lyme disease (neuroborreliosis) |
| Neoplastic meningitis |
| Neurosarcoidosis |
| Neurosyphilis |
| Parasitic meningitis |
| Systemic lupus erythematosus |
| Tuberculous meningitis |
| Vasculitis |
Causative bacteria in community-acquired bacterial meningitis vary depending on age, vaccination status, and recent trauma or instrumentation 7 , 8 ( Table 2 9 ) . Vaccination has nearly eliminated the risk of Haemophilus influenzae and substantially reduced the rates of Neisseria meningitidis and Streptococcus pneumoniae as causes of meningitis in the developed world. 10 Between 1998 and 2007, the overall annual incidence of bacterial meningitis in the United States decreased from 1 to 0.69 per 100,000 persons. 1 This decrease has been most dramatic in children two months to 10 years of age, shifting the burden of disease to an older population. 1 Annual incidence is still highest in neonates at 40 per 100,000, and has remained largely unchanged. 1 Older patients are at highest risk of S. pneumoniae meningitis, whereas children and young adults have a higher risk of N. meningitidis meningitis. 1 , 11 Patients older than 60 years and patients who are immunocompromised are at higher risk of Listeria monocytogenes meningitis, although rates remain low. 11
| Infants younger than 1 month | (group B streptococcus), , , other gram-negative bacilli | Ampicillin plus cefotaxime (Claforan) |
| Alternative: ampicillin plus gentamicin | ||
| Children 1 to 23 months of age | , , , , | Vancomycin plus ceftriaxone |
| Alternative: meropenem (Merrem IV) plus vancomycin | ||
| Children and adults 2 to 50 years of age | , | Vancomycin plus ceftriaxone |
| Alternative: meropenem plus vancomycin | ||
| Adults older than 50 years or with altered cellular immunity or alcoholism | , , , aerobic gram-negative bacilli | Vancomycin plus ceftriaxone plus ampicillin |
| Alternative: meropenem plus vancomycin | ||
| Patients with basilar skull fracture or cochlear implant | , , group A beta-hemolytic streptococci | Vancomycin plus ceftriaxone |
| Alternative: meropenem plus vancomycin | ||
| Patients with penetrating trauma or post neurosurgery | , coagulase-negative staphylococci, aerobic gram-negative bacilli (including ) | Vancomycin plus cefepime |
| Alternative: meropenem plus vancomycin | ||
| Patients with cerebrospinal fluid shunt | Coagulase-negative staphylococci, , aerobic gram-negative bacilli (including ), | Vancomycin plus cefepime |
Presentation
Presentation can be similar for aseptic and bacterial meningitis, but patients with bacterial meningitis are generally more ill-appearing. Fever, headache, neck stiffness, and altered mental status are classic symptoms of meningitis, and a combination of two of these occurs in 95% of adults presenting with bacterial meningitis. 12 However, less than one-half of patients present with all of these symptoms. 12 , 13
Presentation varies with age. Older patients are less likely to have headache and neck stiffness, and more likely to have altered mental status and focal neurologic deficits 11 , 13 ( Table 3 11 – 13 ) . Presentation also varies in young children, with vague symptoms such as irritability, lethargy, or poor feeding. 14 Arboviruses such as West Nile virus typically cause encephalitis but can present without altered mental status or focal neurologic findings. 6 Similarly, HSV can cause a spectrum of disease from meningitis to life-threatening encephalitis. HSV meningitis can present with or without cutaneous lesions and should be considered as an etiology in persons presenting with altered mental status, focal neurologic deficits, or seizure. 15
| Headache | 87 to 92 | 60 to 77 |
| Neck stiffness | 83 to 86 | 31 to 78 |
| Nausea | 74 | 36 |
| Fever | 72 to 77 | 48 to 84 |
| Positive blood culture | 62 to 66 | 73 |
| Altered mental status | 60 to 69 | 84 |
| Focal neurologic deficit | 29 to 33 | 46 |
| Rash | 26 | 4 to 11 |
| Seizure | 5 | 5 |
| Papilledema | 3 | 4 |
The time from symptom onset to presentation for medical care tends to be shorter in bacterial meningitis, with 47% of patients presenting after less than 24 hours of symptoms. 16 Patients with viral meningitis have a median presentation of two days after symptom onset. 17
Examination findings that may indicate meningeal irritation include a positive Kernig sign, positive Brudzinski sign, neck stiffness, and jolt accentuation of headache (i.e., worsening of headache by horizontal rotation of the head two to three times per second). Physical examination findings have shown wide variability in their sensitivity and specificity, and are not reliable to rule out bacterial meningitis. 18 – 20 Examples of Kernig and Brudzinski tests are available at https://www.youtube.com/watch?v=Evx48zcKFDA and https://www.youtube.com/watch?v=rN-R7-hh5x4 .
Because of the poor performance of clinical signs to rule out meningitis, all patients who present with symptoms concerning for meningitis should undergo prompt lumbar puncture (LP) and evaluation of cerebrospinal fluid (CSF) for definitive diagnosis. Because of the risk of increased intracranial pressure with brain inflammation, the Infectious Diseases Society of America recommends performing computed tomography of the head before LP in specific high-risk patients to reduce the possibility of cerebral herniation during the procedure ( Table 4 ) . 7 , 21 , 22 However, recent retrospective data have shown that removing the restriction on LP in patients with altered mental status reduced mortality from 11.7% to 6.9%, suggesting it may be safe to proceed with LP in these patients. 22
| Altered mental status |
| Focal neurologic deficit |
| History of central nervous system disease |
| Hypertension with bradycardia |
| Immunosuppression |
| Papilledema |
| Respiratory abnormalities |
| Seizure (in the previous 30 minutes to one week) |
The CSF findings typical of aseptic meningitis are a relatively low and predominantly lymphocytic pleocytosis, normal glucose level, and a normal to slightly elevated protein level ( Table 5 9 ) . Bacterial meningitis classically has a very high and predominantly neutrophilic pleocytosis, low glucose level, and high protein level. This is not the case for all patients and can vary in older patients and those with partially treated bacterial meningitis, immunosuppression, or meningitis caused by L. monocytogenes . 11 It is important to use age-adjusted values for white blood cell counts when interpreting CSF results in neonates and young infants. 23 In up to 57% of children with aseptic meningitis, neutrophils predominate the CSF; therefore, cell type alone cannot be used to differentiate between aseptic and bacterial meningitis in children between 30 days and 18 years of age. 24
| × per L) | |||||
|---|---|---|---|---|---|
| Pyogenic (not ) | > 500 (0.50) | > 80 | Low | > 100 (1.00) | ~70% |
| > 100 (0.10) | ~50 | Normal | > 50 (0.50) | ~30% | |
| Partially treated pyogenic | > 100 | ~50 | Normal | > 70 (0.70) | ~60% |
| Aseptic, often viral | 10 to 1,000 (0.01 to 1.00) | Early: > 50 Late: < 20 | Normal | < 200 (2.00) | Not applicable |
| Tubercular | 50 to 500 (0.05 to 0.50) | < 30 | Low | > 100 | Rare |
| Fungal | 50 to 500 | < 30 | Low | Varies | Often high in cryptococcus |
CSF results can be variable, and decisions about treatment with antibiotics while awaiting culture results can be challenging. There are a number of clinical decision tools that have been developed for use in children to help differentiate between aseptic and bacterial meningitis in the setting of pleocytosis. The Bacterial Meningitis Score has a sensitivity of 99% to 100% and a specificity of 52% to 62%, and appears to be the most specific tool available currently, although it is not widely used. 25 – 27 The score can be calculated online at http://reference.medscape.com/calculator/bacterial-meningitis-score-child .
Serum procalcitonin, serum C-reactive protein, and CSF lactate levels can be useful in distinguishing between aseptic and bacterial meningitis. 28 – 33 C-reactive protein has a high negative predictive value but a much lower positive predictive value. 28 Procalcitonin is sensitive (96%) and specific (89% to 98%) for bacterial causes of meningitis. 29 , 30 CSF lactate also has a high sensitivity (93% to 97%) and specificity (92% to 96%). 31 – 33 CSF latex agglutination testing for common bacterial pathogens is rapid and, if positive, can be useful in patients with negative Gram stain if LP was performed after antibiotics were administered. This test cannot be used to rule out bacterial meningitis. 7
Because CSF enterovirus polymerase chain reaction testing is more rapid than bacterial cultures, a positive test result can prompt discontinuation of antibiotic treatment, thus reducing antibiotic exposure and cost in patients admitted for suspected meningitis. 34 Similarly, polymerase chain reaction testing can be used to detect West Nile virus when seasonally appropriate in areas of higher incidence. HSV and varicella zoster viral polymerase chain reaction testing should be used in the setting of meningoencephalitis.
INITIAL MANAGEMENT
Prompt recognition of a potential case of meningitis is essential so that empiric treatment may begin as soon as possible. The initial management strategy is outlined in Figure 1 . 7 , 9 Stabilization of the patient's cardiopulmonary status takes priority. Intravenous fluids may be beneficial within the first 48 hours, but further study is needed to determine the appropriate intravenous fluid management. 35 A meta-analysis of studies with variable quality in children showed that fluids may decrease spasticity, seizures, and chronic severe neurologic sequelae. 35 The next urgent requirement is initiating empiric antibiotics as soon as possible after blood cultures are drawn and the LP is performed. Antibiotics should not be delayed if there is any lag time in performing the LP (e.g., transfer to clinical site that can perform the test, need for head computed tomography before LP). 7 , 8 Droplet isolation precautions should be instituted for the first 24 hours of treatment. 23
ANTIMICROBIALS
Before CSF results are available, patients with suspected bacterial meningitis should be treated with antibiotics as quickly as possible. 8 , 22 , 36 , 37 Acyclovir should be added if there is concern for HSV meningitis or encephalitis. Door-to-antibiotic time lapse of more than six hours has an adjusted odds ratio for mortality of 8.4. 37 If CSF results are more consistent with aseptic meningitis, antibiotics can be discontinued, depending on the severity of the presentation and overall clinical picture. Selection of the appropriate empiric antibiotic regimen is primarily based on age ( Table 2 9 ) . Specific pathogens are more prevalent in certain age groups, but empiric coverage should cover most possible culprits. Viral meningitis (non-HSV) management is focused on supportive care.
Treatment of tuberculous, cryptococcal, or other fungal meningitides is beyond the scope of this article, but should be considered if risk factors are present (e.g., travel to endemic areas, immunocompromised state, human immunodeficiency virus infection). These patients, as well as those coinfected with human immunodeficiency virus, should be managed in consultation with an infectious disease subspecialist when available.
Length of treatment varies based on the pathogen identified ( Table 6 7 ) . Intravenous antibiotics should be used to complete the full treatment course, but outpatient management can be considered in persons who are clinically improving after at least six days of therapy with reliable outpatient arrangements (i.e., intravenous access, home health care, reliable follow-up, and a safe home environment). 7
| 7 | |
| 7 | |
| 10 to 14 | |
| 14 to 21 | |
| Aerobic gram-negative bacilli | 21 |
| ≥ 21 |
CORTICOSTEROIDS
Corticosteroids are traditionally used as adjunctive treatment in meningitis to reduce the inflammatory response. The evidence for corticosteroids is heterogeneous and limited to specific bacterial pathogens, 38 – 44 but the organism is not usually known at the time of the initial presentation. A 2015 Cochrane review found a nonsignificant reduction in overall mortality (relative risk [RR] = 0.90), as well as a significant reduction in severe hearing loss (RR = 0.51), any hearing loss (RR = 0.58), and short-term neurologic sequelae (RR = 0.64) with the use of dexamethasone in high-income countries. 41 The number needed to treat to decrease mortality in the S. pneumoniae subgroup was 18 and the number needed to treat to prevent hearing loss was 21. 38 , 41 There was a small increase in recurrent fever in patients given corticosteroids (number needed to harm = 16) with no worse outcome. 38 , 41
The best evidence supports the use of dexamethasone 10 to 20 minutes before or concomitantly with antibiotic administration in the following groups: infants and children with H. influenzae type B, adults with S. pneumoniae , or patients with Mycobacterium tuberculosis without concomitant human immunodeficiency virus infection. 7 , 8 , 42 , 45 Some evidence also shows a benefit with corticosteroids in children older than six weeks with pneumococcal meningitis. 45
Because the etiology is not known at presentation, dexamethasone should be given before or at the time of initial antibiotics while awaiting the final culture results in all patients older than six weeks with suspected bacterial meningitis. Dexamethasone can be discontinued after four days or earlier if the pathogen is not H. influenzae or S. pneumoniae , or if CSF findings are more consistent with aseptic meningitis. 46

REPEAT TESTING
Repeat LP is generally not needed but should be considered to evaluate CSF parameters in persons who are not clinically improving after 48 hours of appropriate treatment. Repeating the LP can identify resistant pathogens, confirm the diagnosis if initial results were negative, and determine the length of treatment for neonates with a gram-negative bacterial pathogen until CSF sterilization is documented. 7 , 47
Prognosis varies by age and etiology of meningitis. In a large analysis of patients from 1998 to 2007, the overall mortality rate in those with bacterial meningitis was 14.8%. 1 Worse outcomes occurred in those with low Glasgow Coma Scale scores, systemic compromise (e.g., low CSF white blood cell count, tachycardia, positive blood cultures, abnormal neurologic examination, fever), alcoholism, and pneumococcal infection. 11 – 13 , 16 Mortality is generally higher in pneumococcal meningitis (30%) than other types, especially penicillin-resistant strains. 12 , 48 , 49 Viral meningitis outside the neonatal period has lower mortality and complication rates, but large studies or reviews are lacking. One large cohort study found a 4.5% mortality rate and a 30.9% rate of complications, such as developmental delay, seizure disorder, or hearing loss, for childhood encephalitis and meningitis combined. 50 Tuberculous meningitis also has a higher mortality rate (19.3%) with a higher risk of neurologic disease in survivors (53.9%). 51 A recent prospective cohort study also found that males had a higher risk of unfavorable outcomes (odds ratio = 1.34) and death (odds ratio = 1.47). 52
Complications from bacterial meningitis also vary by age ( Table 7 1 , 11 , 12 , 46 , 53 – 56 ) . Neurologic sequelae such as hearing loss occur in approximately 6% to 31% of children and can resolve within 48 hours, but may be permanent in 2% to 7% of children. 53 – 56 An audiology assessment should be considered in children before discharge. 8 Follow-up should assess for hearing loss (including referral for cochlear implants, if present), psychosocial problems, neurologic disease, or developmental delay. 57 Testing for complement deficiency should be considered if there is more than one episode of meningitis, one episode plus another serious infection, meningococcal disease other than serogroup B, or meningitis with a strong family history of the disease. 57
| No sequelae | 83.6 |
| Cognitive impairment or low IQ | 45 |
| Academic limitations | 29.9 |
| Reversible hearing loss | 6.7 to 31 |
| Spasticity or paresis | 3.5 |
| Deafness | 2.4 to 7 |
| Seizure disorder | 1.8 to 4.2 |
| Mortality | 0.3 to 3.8 |
| Focal neurologic deficits | 37 to 50 |
| Cardiorespiratory failure | 29 to 38 |
| Seizures | 15 to 24 |
| Mortality | 14.8 to 21 |
| Hearing loss | 14 to 69 |
| Hemiparesis | 4 to 6 |
VACCINATION
Vaccines that have decreased the incidence of meningitis include H. influenzae type B, S. pneumoniae , and N. meningitidis . 58 – 60 Administration of one of the meningococcal vaccines that covers serogroups A, C, W, and Y (MPSV4 [Menomune], Hib-MenCY [Menhibrix], MenACWY-D [Menactra], or MenACWY-CRM [Menveo]) is recommended for patients 11 to 12 years of age, with a booster at 16 years of age. However, the initial dose should be given earlier in the setting of a high-risk condition, such as functional asplenia or complement deficiencies, travel to endemic areas, or a community outbreak. 60 There are also two available vaccines for meningococcal type B strains (MenB-4C [Bexsero] and MenB-FHbp [Trumenba]) to be used in patients with complement disease or functional asplenia, or in healthy individuals at risk during a serogroup B outbreak as determined by the Centers for Disease Control and Prevention. 60
The Advisory Committee on Immunization Practices recently added a category B recommendation (individual clinical decision making) for consideration of vaccination with serogroup B vaccines in healthy patients 16 to 23 years of age (preferred age of 16 to 18 years). 60 , 61 The serogroup B vaccines are not interchangeable, so care should be taken to ensure completion of the series with the same brand that was used for the initial dose.
CHEMOPROPHYLAXIS
Treatment with chemoprophylactic antibiotics should be given to close contacts 7 , 62 , 63 ( Table 8 9 , 14 , 64 – 68 ) . Appropriate antibiotics should be given to identified contacts within 24 hours of the patient's diagnosis and should not be given if contact occurred more than 14 days before the patient's onset of symptoms. 63 Options for chemoprophylaxis are rifampin, ceftriaxone, and ciprofloxacin, although rifampin has been associated with resistant isolates. 62 , 63
| (postexposure prophylaxis) | Living in a household with one or more unvaccinated or incompletely vaccinated children younger than 48 months | Rifampin | 20 mg per kg per day, up to 600 mg per day, for four days | — |
| (postexposure prophylaxis) | Close contact (for more than eight hours) with someone with infection | Ceftriaxone | Single intramuscular dose of 250 mg (125 mg if younger than 15 years) | — |
| Contact with oral secretions of someone with infection | ||||
| Ciprofloxacin | Adults: single dose of 500 mg | Rare resistant isolates | ||
| Rifampin | Adults: 600 mg every 12 hours for two days | Not fully effective and rare resistant isolates | ||
| Children one month or older: 10 mg per kg every 12 hours for two days | ||||
| Children younger than one month: 5 mg per kg every 12 hours for two days | ||||
| (group B streptococcus; women in the intrapartum period) | Previous birth to an infant with invasive infection | Penicillin G | Initial dose of 5 million units intravenously, then 2.5 to 3 million units every four hours during the intrapartum period | — |
| Colonization at 35 to 37 weeks' gestation | ||||
| If allergic to penicillin: | ||||
| Bacteriuria during pregnancy | Cefazolin | 2 g followed by 1 g every eight hours | — | |
| High risk because of fever, amniotic fluid rupture for more than 18 hours, or delivery before 37 weeks' gestation | ||||
| Clindamycin | 900 mg every eight hours | Clindamycin susceptibility must be confirmed by antimicrobial susceptibility test | ||
| Vancomycin | 15 to 20 mg per kg every 12 hours | — |
This article updates a previous article on this topic by Bamberger . 9
Data Sources: The terms meningitis, bacterial meningitis, and Neisseria meningitidis were searched in PubMed, Essential Evidence Plus, and the Cochrane database. In addition, the Infectious Diseases Society of America, the National Institute for Health and Care Excellence, and the American Academy of Pediatrics guidelines were reviewed. Search dates: October 1, 2016, and March 13, 2017.
The authors thank Thomas Lamarre, MD, for his input and expertise.
Thigpen MC, Whitney CG, Messonnier NE, et al.; Emerging Infections Program Network. Bacterial meningitis in the United States, 1998–2007. N Engl J Med. 2011;364(21):2016-2025.
Kupila L, Vuorinen T, Vainionpää R, Hukkanen V, Marttila RJ, Kotilainen P. Etiology of aseptic meningitis and encephalitis in an adult population. Neurology. 2006;66(1):75-80.
Martin NG, Iro MA, Sadarangani M, Goldacre R, Pollard AJ, Goldacre MJ. Hospital admissions for viral meningitis in children in England over five decades: a population-based observational study. Lancet Infect Dis. 2016;16(11):1279-1287.
Lee BE, Davies HD. Aseptic meningitis. Curr Opin Infect Dis. 2007;20(3):272-277.
Hasbun R, Rosenthal N, Balada-Llasat JM, et al. Epidemiology of meningitis and encephalitis in the United States from 2011–2014 [published online ahead of print April 17, 2017]. Clin Infect Dis . https://academic.oup.com/cid/article-abstract/doi/10.1093/cid/cix319/3737650/Epidemiology-of-Meningitis-and-Encephalitis-in-the?redirectedFrom=fulltext [login required]. Accessed April 30, 2017.
Petersen LR, Brault AC, Nasci RS. West Nile virus: review of the literature. JAMA. 2013;310(3):308-315.
Tunkel AR, Hartman BJ, Kaplan SL, et al. Practice guidelines for the management of bacterial meningitis. Clin Infect Dis. 2004;39(9):1267-1284.
National Institute for Health and Care Excellence. Meningitis (bacterial) and meningococcal septicaemia in children and young people. Quality standard (QS19); June 2012. https://www.nice.org.uk/guidance/qs19 . Accessed October 1, 2016.
Bamberger DM. Diagnosis, initial management, and prevention of meningitis. Am Fam Physician. 2010;82(12):1491-1498.
McIntyre PB, O'Brien KL, Greenwood B, van de Beek D. Effect of vaccines on bacterial meningitis worldwide. Lancet. 2012;380(9854):1703-1711.
Weisfelt M, van de Beek D, Spanjaard L, Reitsma JB, de Gans J. Community-acquired bacterial meningitis in older people. J Am Geriatr Soc. 2006;54(10):1500-1507.
van de Beek D, de Gans J, Spanjaard L, Weisfelt M, Reitsma JB, Vermeulen M. Clinical features and prognostic factors in adults with bacterial meningitis [published correction appears in N Engl J Med . 2005;352(9): 950]. N Engl J Med. 2004;351(18):1849-1859.
Wang AY, Machicado JD, Khoury NT, Wootton SH, Salazar L, Hasbun R. Community-acquired meningitis in older adults: clinical features, etiology, and prognostic factors. J Am Geriatr Soc. 2014;62(11):2064-2070.
Sáez-Llorens X, McCracken GH. Bacterial meningitis in children. Lancet. 2003;361(9375):2139-2148.
Bijlsma MW, Brouwer MC, Kasanmoentalib ES, et al. Community-acquired bacterial meningitis in adults in the Netherlands, 2006–14:a prospective cohort study. Lancet Infect Dis. 2016;16(3):339-347.
Ihekwaba UK, Kudesia G, McKendrick MW. Clinical features of viral meningitis in adults: significant differences in cerebrospinal fluid findings among herpes simplex virus, varicella zoster virus, and enterovirus infections. Clin Infect Dis. 2008;47(6):783-789.
Steiner I, Benninger F. Update on herpes virus infections of the nervous system. Curr Neurol Neurosci Rep. 2013;13(12):414.
Waghdhare S, Kalantri A, Joshi R, Kalantri S. Accuracy of physical signs for detecting meningitis: a hospital-based diagnostic accuracy study. Clin Neurol Neurosurg. 2010;112(9):752-757.
Curtis S, Stobart K, Vandermeer B, Simel DL, Klassen T. Clinical features suggestive of meningitis in children: a systematic review of prospective data. Pediatrics. 2010;126(5):952-960.
Nakao JH, Jafri FN, Shah K, Newman DH. Jolt accentuation of headache and other clinical signs: poor predictors of meningitis in adults. Am J Emerg Med. 2014;32(1):24-28.
Joffe AR. Lumbar puncture and brain herniation in acute bacterial meningitis: a review. J Intensive Care Med. 2007;22(4):194-207.
Glimåker M, Johansson B, Grindborg Ö, Bottai M, Lindquist L, Sjölin J. Adult bacterial meningitis: earlier treatment and improved outcome following guideline revision promoting prompt lumbar puncture. Clin Infect Dis. 2015;60(8):1162-1169.
Siegel JD, Rhinehart E, Jackson M, Chiarello L; Healthcare Infection Control Practices Advisory Committee. 2007 guideline for isolation precautions: preventing transmission of infectious agents in healthcare settings. https://www.cdc.gov/hicpac/pdf/isolation/Isolation2007.pdf . Accessed October 1, 2016.
Negrini B, Kelleher KJ, Wald ER. Cerebrospinal fluid findings in aseptic versus bacterial meningitis. Pediatrics. 2000;105(2):316-319.
Nigrovic LE, Kuppermann N, Macias CG, et al.; Pediatric Emergency Medicine Collaborative Research Committee of the American Academy of Pediatrics. Clinical prediction rule for identifying children with cerebrospinal fluid pleocytosis at very low risk of bacterial meningitis. JAMA. 2007;297(1):52-60.
Nigrovic LE, Malley R, Kuppermann N. Meta-analysis of bacterial meningitis score validation studies. Arch Dis Child. 2012;97(9):799-805.
Dubos F, Korczowski B, Aygun DA, et al. Distinguishing between bacterial and aseptic meningitis in children: European comparison of two clinical decision rules [published correction appears in Arch Dis Child . 2011;96(4):407]. Arch Dis Child. 2010;95(12):963-967.
Gerdes LU, Jørgensen PE, Nexø E, Wang P. C-reactive protein and bacterial meningitis: a meta-analysis. Scand J Clin Lab Invest. 1998;58(5):383-393.
Henry BM, Roy J, Ramakrishnan PK, Vikse J, Tomaszewski KA, Walocha JA. Procalcitonin as a serum biomarker for differentiation of bacterial meningitis from viral meningitis in children: evidence from a meta-analysis. Clin Pediatr (Phila). 2016;55(8):749-764.
Vikse J, Henry BM, Roy J, Ramakrishnan PK, Tomaszewski KA, Walocha JA. The role of serum procalcitonin in the diagnosis of bacterial meningitis in adults: a systematic review and meta-analysis. Int J Infect Dis. 2015;38:68-76.
Huy NT, Thao NT, Diep DT, Kikuchi M, Zamora J, Hirayama K. Cerebrospinal fluid lactate concentration to distinguish bacterial from aseptic meningitis: a systemic review and meta-analysis. Crit Care. 2010;14(6):R240.
Sakushima K, Hayashino Y, Kawaguchi T, Jackson JL, Fukuhara S. Diagnostic accuracy of cerebrospinal fluid lactate for differentiating bacterial meningitis from aseptic meningitis: a meta-analysis. J Infect. 2011;62(4):255-262.
Viallon A, Desseigne N, Marjollet O, et al. Meningitis in adult patients with a negative direct cerebrospinal fluid examination: value of cytochemical markers for differential diagnosis. Crit Care. 2011;15(3):R136.
Archimbaud C, Chambon M, Bailly JL, et al. Impact of rapid enterovirus molecular diagnosis on the management of infants, children, and adults with aseptic meningitis. J Med Virol. 2009;81(1):42-48.
Maconochie IK, Bhaumik S. Fluid therapy for acute bacterial meningitis. Cochrane Database Syst Rev. 2016(11):CD004786.
Sudarsanam TD, Rupali P, Tharyan P, Abraham OC, Thomas K. Pre-admission antibiotics for suspected cases of meningococcal disease. Cochrane Database Syst Rev. 2013(8):CD005437.
Proulx N, Fréchette D, Toye B, Chan J, Kravcik S. Delays in the administration of antibiotics are associated with mortality from adult acute bacterial meningitis. QJM. 2005;98(4):291-298.
van de Beek D, Farrar JJ, de Gans J, et al. Adjunctive dexamethasone in bacterial meningitis: a meta-analysis of individual patient data. Lancet Neurol. 2010;9(3):254-263.
Mongelluzzo J, Mohamad Z, Ten Have TR, Shah SS. Corticosteroids and mortality in children with bacterial meningitis. JAMA. 2008;299(17):2048-2055.
Thwaites GE, Nguyen DB, Nguyen HD, et al. Dexamethasone for the treatment of tuberculous meningitis in adolescents and adults. N Engl J Med. 2004;351(17):1741-1751.
Brouwer MC, McIntyre P, Prasad K, van de Beek D. Corticosteroids for acute bacterial meningitis. Cochrane Database Syst Rev. 2015(9):CD004405.
Prasad K, Singh MB, Ryan H. Corticosteroids for managing tuberculous meningitis. Cochrane Database Syst Rev. 2016(4):CD002244.
Beardsley J, Wolbers M, Kibengo FM, et al.; CryptoDex Investigators. Adjunctive dexamethasone in HIV-associated cryptococcal meningitis. N Engl J Med. 2016;374(6):542-554.
Fritz D, Brouwer MC, van de Beek D. Dexamethasone and long-term survival in bacterial meningitis. Neurology. 2012;79(22):2177-2179.
Kimberlin DW, Long SS, Brady MT, Jackson MA, eds. Red Book: 2015 Report of the Committee on Infectious Diseases . 30th ed. Elk Grove Village, Ill.: American Academy of Pediatrics; 2015:370,631.
van de Beek D, de Gans J, Tunkel AR, Wijdicks EF. Community-acquired bacterial meningitis in adults. N Engl J Med. 2006;354(1):44-53.
Costerus JM, Brouwer MC, van der Ende A, van de Beek D. Repeat lumbar puncture in adults with bçacterial meningitis. Clin Microbiol Infect. 2016;22(5):428-433.
Schuchat A, Robinson K, Wenger JD, et al. Bacterial meningitis in the United States in 1995. Active Surveillance Team. N Engl J Med. 1997;337(14):970-976.
Edmond K, Clark A, Korczak VS, Sanderson C, Griffiths UK, Rudan I. Global and regional risk of disabling sequelae from bacterial meningitis: a systematic review and meta-analysis. Lancet Infect Dis. 2010;10(5):317-328.
Rantakallio P, Leskinen M, von Wendt L. Incidence and prognosis of central nervous system infections in a birth cohort of 12,000 children. Scand J Infect Dis. 1986;18(4):287-294.
Chiang SS, Khan FA, Milstein MB, et al. Treatment outcomes of childhood tuberculous meningitis: a systematic review and meta-analysis. Lancet Infect Dis. 2014;14(10):947-957.
Dias SP, Brouwer MC, Bijlsma MW, van der Ende A, van de Beek D. Sex-based differences in adults with community-acquired bacterial meningitis: a prospective cohort study. Clin Microbiol Infect. 2017;23(2):121.e9-121.e15.
Richardson MP, Reid A, Tarlow MJ, Rudd PT. Hearing loss during bacterial meningitis [published correction appears in Arch Dis Child . 1997;76(4):386]. Arch Dis Child. 1997;76(2):134-138.
Baraff LJ, Lee SI, Schriger DL. Outcomes of bacterial meningitis in children: a meta-analysis. Pediatr Infect Dis J. 1993;12(5):389-394.
Chandran A, Herbert H, Misurski D, Santosham M. Long-term sequelae of childhood bacterial meningitis: an underappreciated problem. Pediatr Infect Dis J. 2011;30(1):3-6.
Worsøe L, Cayé-Thomasen P, Brandt CT, Thomsen J, østergaard C. Factors associated with the occurrence of hearing loss after pneumococcal meningitis. Clin Infect Dis. 2010;51(8):917-924.
National Institute for Health and Care Excellence. Meningitis (bacterial) and meningococcal septicaemia in under 16s: recognition, diagnosis and management. Clinical guideline (CG102); updated February 2015. https://www.nice.org.uk/guidance/cg102 . Accessed October 3, 2016.
Patel M, Lee CK. Polysaccharide vaccines for preventing serogroup A meningococcal meningitis. Cochrane Database Syst Rev. 2005(1):CD001093.
Swingler G, Fransman D, Hussey G. Conjugate vaccines for preventing Haemophilus influenzae type B infections. Cochrane Database Syst Rev. 2007(2):CD001729.
MacNeil JR, Rubin L, McNamara L, Briere EC, Clark TA, Cohn AC Meningitis and Vaccine Preventable Diseases Branch, Division of Bacterial Diseases, National Center for Immunization and Respiratory Diseases, CDC. Use of MenACWY-CRM vaccine in children aged 2 through 23 months at increased risk for meningococcal disease: recommendations of the Advisory Committee on Immunization Practices, 2013. MMWR Morb Mortal Wkly Rep. 2014;63(24):527-530.
MacNeil JR, Rubin L, Folaranmi T, Ortega-Sanchez IR, Patel M, Martin SW. Use of serogroup B meningococcal vaccines in adolescents and young adults: recommendations of the Advisory Committee on Immunization Practices, 2015. MMWR Morb Mortal Wkly Rep. 2015;64(41):1171-1176.
Zalmanovici Trestioreanu A, Fraser A, Gafter-Gvili A, Paul M, Leibovici L. Antibiotics for preventing meningococcal infections. Cochrane Database Syst Rev. 2013(10):CD004785.
Cohn AC, MacNeil JR, Clark TA, et al. Prevention and control of meningococcal disease: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep. 2013;62(RR-2):1-28.
Chaudhuri A, Martinez-Martin P, Kennedy PG, et al. EFNS guideline on the management of community-acquired bacterial meningitis: report of an EFNS Task Force on acute bacterial meningitis in older children and adults [published correction appears in Eur J Neurol . 2008;15(8):880]. Eur J Neurol. 2008;15(7):649-659.
Verani JR, McGee L, Schrag SJ Division of Bacterial Diseases, National Center for Immunization and Respiratory Diseases, Centers for Disease Control and Prevention. Prevention of perinatal group B streptococcal disease—revised guidelines from CDC, 2010. MMWR Recomm Rep. 2010;59(RR-10):1-36.
Telisinghe L, Waite TD, Gobin M, et al. Chemoprophylaxis and vaccination in preventing subsequent cases of meningococcal disease in household contacts of a case of meningococcal disease: a systematic review. Epidemiol Infect. 2015;143(11):2259-2268.
Gilbert DN, Moellering RC Jr., Eliopoulos GM, Chambers HF, Saag MS, eds. The Sanford Guide to Antimicrobial Therapy . 39th ed. Sperryville, Va.: Antimicrobial Therapy; 2009:9.
Bradley JS, Nelson JD, Barnett ED, et al. Nelson's Pediatric Antimicrobial Therapy . 23rd ed. Elk Grove Village, Ill.: American Academy of Pediatrics; 2017:226.
Continue Reading

More in AFP
More in pubmed.
Copyright © 2017 by the American Academy of Family Physicians.
This content is owned by the AAFP. A person viewing it online may make one printout of the material and may use that printout only for his or her personal, non-commercial reference. This material may not otherwise be downloaded, copied, printed, stored, transmitted or reproduced in any medium, whether now known or later invented, except as authorized in writing by the AAFP. See permissions for copyright questions and/or permission requests.
Copyright © 2024 American Academy of Family Physicians. All Rights Reserved.
- Campus Directory
- Current Students
- Faculty & Staff

Meningitis - Page 1
A 14-year old male complained to his parents of feeling quite ill with a headache, dizziness, nausea, and feeling very weak. After a rise in his body temperature, an increase in the severity of his headache, and the development of a rash, his mother took him into their medical clinic where he was examined by a family nurse practitioner (FNP). Upon examination, the FNP noted the patient also complained of a stiffness in his neck and nausea. The patient had a temperature of 103.5 and an increased heart rate. She noted a rash had developed on parts of the patient's extremities and wrists. Concerned with meningitis, she consulted the clinic's family practice physician and asked her to examine the patient.
Upon his examination, a complete blood count (CBC), blood for culture and lumbar puncture (LP) were performed since there was a strong clinical suspicion of bacterial meningitis. The LP examination included tests for the presence of bacteria (Gram stain), cell count and differential, glucose and protein. A culture of the fluid is also a standard test and detects the type of bacteria, if any, that may be present. Radiology was called to perform a CAT scan on the patient.
- 1. Which health professional would perform the lumbar puncture?
- 2. Which health professional would examine the CSF fluid?
View Meningitis: Signs, Symptoms, Risk Factors video and answer the following questions:
- 3. What is meningitis?
- 4. List 2 types of meningitis. Which of these is more severe?
- 5. How is meningitis transmitted?
- 6. Who is most at risk for contracting meningitis?
- 7. What are common symptoms of meningitis?
- 8. What are possible short and long-term complications of meningitis?
View a student's meningitis story video (this one is worth watching)
Case Report
- Open access
- Published: 28 August 2024
Aseptic meningitis with recurrent headache episodes, vomiting, and central fever as first manifestation of isolated neurosarcoidosis: a case report
- Athina-Maria Aloizou 1 ,
- Theresa Anne Gabriel 1 ,
- Carsten Lukas 2 ,
- Ralf Gold 1 &
- Jeremias Motte 1
BMC Neurology volume 24 , Article number: 299 ( 2024 ) Cite this article
Metrics details
Neurosarcoidosis is a rare entity, usually within the context of systematic sarcoidosis. Isolated neurosarcoidosis and especially a manifestation with pachymeningitis is a notable rarity.
A 26-year-old patient presented to the emergency department with acute onset, recurrent episodes of occipital headaches spreading over the whole cranium and vomiting without food consumption, for three days. The clinical examination did not reveal any neurological deficits. The laboratory exams showed no pathological findings. A CT examination with angiography did not detect any acute intracranial or vessel pathology. A lumbar puncture was performed to rule out subarachnoid hemorrhage. The results showed a lymphocytic pleocytosis of 400/µL, elevated protein levels of 1077 mg/dL and reduced glucose levels (CSF: 55 mg/dL, Serum: 118 mg/dL). Extensive infectiological examinations did not reveal any signs of infection, including Borrelia spp. and M. tuberculosis. No positive auto-antibodies or vasculitis-related auto-antibodies were detected. The CSF analysis showed negative oligoclonal bands but an isolated increase in β2-microglobulin, neopterin, and IL-2R levels. The MRI examination revealed a dural gadolinium-enhancement, pronounced in the basal cerebral structures and the upper segment of the cervical spine, consistent with neurosarcoidosis. Corticosteroid treatment rapidly led to a significant improvement of the symptoms. No systemic manifestations of sarcoidosis were found.
Conclusions
This case report aims to highlight aseptic meningitis with atypical, acute onset headache attacks as a possible manifestation of isolated neurosarcoidosis. Neurosarcoidosis is a clinical entity that requires prompt treatment to avoid permanent neurological deficits.
Peer Review reports
Introduction/background
Sarcoidosis is a multisystemic granulomatous disease that commonly affects the lungs, skin, and eyes, with an estimated incidence of 10–20/100,000 [ 1 ]. Neurosarcoidosis occurs in 5–20% of patients with systemic sarcoidosis. However, the available information on neurosarcoidosis is limited, as the available single-center studies carry a significant degree of variability [ 2 ]. The cranial nerves are most commonly affected in neurosarcoidosis, though it can also manifest as polyneuropathy, hydrocephalus, seizures, meningitis, and myelitis. An isolated neurosarcoidosis, without systemic manifestation, represents a notable rarity [ 3 , 4 , 5 ]. Diagnosing isolated neurosarcoidosis can be challenging, due to atypical manifestations, numerous mimics, and the inherent difficulty of acquiring tissue samples for biopsy. Diagnostic criteria were recently made available and require a rigorous exclusion of other possible causes [ 4 ]. In this case report, we describe a young patient with acute-onset, recurrent headaches, vomiting, and central fever, without meningism signs. The patient’s CSF and MRI findings were compatible with aseptic meningitis and neurosarcoidosis. The purpose of this report is to raise awareness on this atypical clinical manifestation of isolated neurosarcoidosis.
Case presentation
A 26-year-old male patient with no prior medical history, including migraine or other headache disorders, presented to our emergency department with recurrent headache attacks for the past three days. The first attack had an acute onset with no identifiable triggering factors, and an intensity of 7–9/10. The headache was initially located in the occipital region and then spread throughout the entirety of the cranium. Phono- or photophobia were not reported. The patient then presented recurrent headache episodes of this character, in the morning and in the evening, while medication such as ibuprofen could only slightly improve the pain. Additionally, the patient reported daily vomiting episodes, occurring even without food consumption. No other symptoms, namely fever, diarrhea, or neurological deficits, were reported. Nicotin, alcohol, and drug consumption were denied. The patient reported working as a security guard in a refugee camp.
Physical examination showed no focal neurological deficits or meningism. Vital parameters were normal, and the initial laboratory examination revealed no pathological findings. A cerebral CT scan (computed tomography) with angiography was carried out, to exclude a cerebral hemorrhage, vascular pathology, and sinus venous thrombosis; no pathological findings were found. A lumbar puncture to exclude a subarachnoid hemorrhage with greater certainty was then performed. The CSF (cerebrospinal fluid) pressure was measured at 15mmHg. The initial CSF findings revealed a lymphocytic pleocytosis (400/µL) with elevated protein (1077 mg/dL) and reduced glucose levels (CSF: 55 mg/dL, Serum: 118 mg/dL), without erythrocytes or siderophages. Empirical treatment with acyclovir, ampicillin, and ceftriaxone was initiated. The PCR (polymerase chain reaction) examination of the CSF did not detect any common CNS (central nervous system) infectious agents, while additional specific examinations, including single-PCR for Herpes Simplex 1/2 and Varicella Zoster Viruses, Borrelia ASI, next generation sequencing, 16 S-rDNA (bacterial universal) PCR, and panfungal PCR, were also negative. The CSF culture was sterile, and a native MRI of the brain showed no pathological findings. The antimicrobial treatment did not result in any clinical improvement. Instead, the patient started experiencing a fever of up to 39 °C. Repeated blood and urine cultures were sterile, while a lung X-ray and abdomen CT were unremarkable. Due to the patient’s professional background, a Quantiferon test was performed, along with tests for West Nile Virus, Dengue Fever and Yellow Fever antibodies, all of which were negative. Syphilis, HIV, and Hepatitis B/C examinations were also negative. Two Doppler ultrasound examinations, also during an acute headache attack, did not detect any vasospasm. The lumbar puncture was repeated three days later, revealing comparable findings with the first puncture. The cytological examination did not reveal any malignant cells. Furthermore, the oligoclonal bands and an array of auto-antibodies regarding systemic autoimmune disorders, vasculitis, and autoimmune encephalitis were negative (antinuclear-ANA and extranuclear-ENA antigen antibodies, anti-MOG, -AQP4, -Hu, -Ri, -ANNA-3, -Tr/DNER, -Ma1, -Ma2/Ta, -GAD65, -Ampiphysin, -NMDA, -AMPA, -GABA-b-Receptor, -LGI1, -CASPR2, -IgLON5, -DPPX, -Myelin, -CARPVIII, -Glycin, -mGluR1, -mGluR5, -GABA-a-Receptor, -Rho GTPase activating protein 26, -Recoverin, -GluRD2, -Flotillin 1/2, -ZIC4, -ITPR1, -Homer3, -Neurochondrin, -Neurexin-3-alpha, -ERC1, Sez6l2, -AP3B2, -Contactin1, -Neurofascin 155/186, -ATP1A3, -KCNA2, -Dopamin Receptor 2 antibodies). A second MRI examination of the brain and cervical spine with gadolinium enhancement revealed dural gadolinium enhancement, pronounced in the basal cerebral structures and the upper segment of the cervical spine, with no evidence of other intracranial or intramedullary pathology. Notably, we observed an isolated increase of β2-microglobulin, neopterin, and IL-2R levels in CSF, with normal serum and CSF ACE (angiotensin converting enzyme) levels. Intravenous corticosteroid treatment with methylprednisolone was initiated due to suspicion of neurosarcoidosis, resulting in immediate symptom improvement reported by the patient. An affection of the peripheral nervous system and the lungs could be excluded by the means of electroneurography and bronchoalveolar lavage. The patient was discharged with oral prednisolone (1 mg/kg). The patient remained asymptomatic after six weeks but suffered from various corticosteroid adverse effects, namely weight gain, acne, peripheral edema, and facial swelling, so a corticosteroid tapering plan and infliximab were initiated. The decision to initiate infliximab was based on the patient’s young age and possible need for long-term therapy, keeping in mind potential long-term side-effects of other immunosuppressive medications, and his wish to father children in the near future. The patient tolerated infliximab well. A new MRI after approximately 8 weeks revealed an improvement of the dural gadolinium enhancement (Fig. 1 ). In the last follow-up, 8 months after diagnosis, the patient had suffered no relapses, and only complained of light, non-persistent headaches, while exhibiting no focal neurological signs. The oral corticosteroid therapy could be minimized, the infliximab treatment was carried on.

T1-weighted MRI sequences with Gadolinium enhancement. The left image demonstrates the follow-up MRI after approximately 8 weeks, with improvement of the meningeal enhancement (red arrows), pronounced at the basal brain structures and the spinal cord
In this case, we discuss an atypical manifestation of isolated neurosarcoidosis, presenting with recurrent acute onset headaches, vomiting, and central fever, which were associated with an aseptic meningitis responsive to corticosteroids. Neurosarcoidosis usually presents in patients with systemic sarcoidosis, in approximately 5% of cases, making isolated neurosarcoidosis a rarity [ 6 ], while meningitis also appears in only approximately 16% of neurosarcoidosis cases [ 2 ]. Pachymeningitis is also rarer, with fever only seldom mentioned [ 7 ]. As such, the rarity of this patient lies in the atypical clinical manifestation, including recurrent acute headaches, central fever, and the absence of cranial nerve involvement, in the context of pachymeningitis in isolated neurosarcoidosis.
According to the widely used criteria by Zajicek et al. (1999) [ 8 ], the patient was diagnosed with “possible neurosarcoidosis”. In these criteria, a definite diagnosis can be set only on the basis of a biopsy, while a probable neurosarcoidosis is considered when evidence of systemic sarcoidosis is present, either through positive histology, including Kveim test, and/or at least two indirect indicators from Gallium scan, chest imaging and serum ACE. A rigorous exclusion of other possible causes and evidence of CNS inflammation is also required. However, it has been frequently intonated that even the pathological identification of granulomas is not 100% definite for the diagnosis of the disease [ 4 ]. Sensitive and specific biomarkers are lacking; ACE is the most commonly known test for sarcoidosis, with epitheloid and giant cells of the granulomas producing an abundant amount of the enzyme, respective of the granulomas present, and therefore often applied in disease monitoring [ 9 ]. However, its sensitivity and specificity in both serum and CSF are questionable, since several granulomatous diseases can lead to its increase [ 10 ], and the numbers of patients with elevated ACE have ranged from 30 to 80% among different studies [ 9 ]. As a 2016 meta-analysis reported, CSF ACE was increased in less than half of neurosarcoidosis cases, and serum ACE in about a third [ 2 ], with our patient also demonstrating normal values. Other aseptic meningitis cases with neurosarcoidosis also demonstrated normal ACE values as well [ 11 ], especially in serum [ 12 , 13 ]. IL-2R could represent another useful marker, since the accumulation of activated T-cells and the subsequent stimulated expression of IL-2R is a long-known pathological characteristic of sarcoidosis [ 14 ]. Although not specific for sarcoidosis, it has shown potential as a diagnostic and prognostic marker in this disease [ 10 ]. Similarly, β2-microglobulin, as a marker of lymphocytic activation, has also been reported as elevated in sarcoidosis patients, with often normal values of ACE [ 15 ], while elevated neopterin, a product of monocyte activation, has also been noted in some sarcoidosis cases, albeit lacking specificity [ 16 ]. Neopterin is also individually expressed in the CNS, with no correlations between serum and CSF levels; for neuroinflammatory disorders, higher CSF levels are noted in both infections and autoimmune disorders, albeit more pronounced of infections [ 17 ]. This is consistent with the diagnosis of isolated neurosarcoidosis in our patient, where neopterin was only elevated in CSF.
Building on previous efforts to define criteria and commenting on the many faces of neurosarcoidosis with absence of definite radiological and laboratory markers, Stein et al. (2018) proposed an updated version of the criteria [ 4 ]. These also included peripheral nerve pathology and a more “vague” description of typical inflammatory findings, considering how some older tests are now obsolete in modern practice, while also requiring pathological confirmation of systemic sarcoidosis for “probable”, and a nervous system biopsy positive for sarcoidosis for a “definite” diagnosis. Applying these criteria, our patient still received a “possible neurosarcoidosis” diagnosis.
Neurosarcoidosis requires prompt treatment in order to avoid residual neurological deficits. In several case-reports of neurosarcoidosis with atypical manifestation, corticosteroid/immunosuppressive treatment led to full remission, with patients free of symptoms in the follow-up [ 18 ], though other patients only achieved partial remission [ 11 ] or developed chronic issues such as hypopituitarism, cognitive impairment, and paraplegia [ 5 , 12 , 19 ], highlighting the need of high clinical suspicion and early treatment initiation; notably, the 2016 meta-analysis reported a complete remission in only 27% of included neurosarcoidosis cases [ 2 ]. There is no universal consensus and no available randomized trials regarding long-term immunosuppression in neurosarcoidosis, which still remains an individualized decision based on clinical severity and patient profiles, with the goal of sparing corticosteroids and avoiding permanent disability. Azathioprine and methotrexate are “traditional” immunosuppressive agents commonly used, though their clinical effect can take months to appear, and anti-TNF (tumor necrosis factor) monoclonal antibodies are increasingly being introduced early in the immunosuppressive treatment of neurosarcoidosis [ 20 ]. Infliximab in particular has shown very good rates of clinical remission in regards to CNS involvement, also in refractory cases [ 21 ], and has in fact been the most commonly administered treatment in neurosarcoidosis, as revealed in a recent meta-analysis [ 22 ]. Due to the young age of our patient and his desire to have children in the near future, the gravidity of his pachymeningitis, and the side-effects of oral corticosteroids occurring early, a long-term and fast acting immunosuppression was deemed crucial, thus leading us in choosing infliximab.
Though a notable rarity, isolated neurosarcoidosis should also be considered in cases of aseptic meningitis non responsive to antibiotic and antiviral treatment; ACE should not be considered as an absolute marker of sarcoidosis, with other markers such as IL-2R, β2-microglobulin, and neopterin in CSF being helpful in these cases.
Data availability
All data generated or analysed during this study are included in this published article.
Abbreviations
angiotensin converting enzyme
cerebrospinal fluid
computed tomography
interleucin 2 receptor, MRI: magnetic resonance tomography imaging
polymerase chain reaction
Iannuzzi MC, Rybicki BA, Teirstein AS, Sarcoidosis. N Engl J Med. 2007;357(21):2153–65.
Article CAS PubMed Google Scholar
Fritz D, van de Beek D, Brouwer MC. Clinical features, treatment and outcome in neurosarcoidosis: systematic review and meta-analysis. BMC Neurol. 2016;16(1):220.
Article PubMed PubMed Central Google Scholar
Park BJ, Ray E, Bathla G, Bruch LA, Streit JA, Cho TA, et al. Single Center experience with isolated spinal cord neurosarcoidosis. World Neurosurg. 2021;156:e398–407.
Article PubMed Google Scholar
Stern BJ, Royal W 3rd, Gelfand JM, Clifford DB, Tavee J, Pawate S, et al. Definition and Consensus Diagnostic Criteria for Neurosarcoidosis: from the Neurosarcoidosis Consortium Consensus Group. JAMA Neurol. 2018;75(12):1546–53.
Galnares-Olalde JA, Berebichez-Fridman R, Gomez-Garza G, Mercado M, Moreno-Sanchez F, Alegria-Loyola MA. Not everything is as it seems: neurosarcoidosis presenting as leptomeningitis. Clin Case Rep. 2018;6(4):596–602.
Crawford F, Alvi SA, Brahimaj B, Byrne R, Kocak M, Wiet RM. Neurosarcoidosis presenting as isolated bilateral Cerebellopontine Angle Tumors: Case Report and Review of the literature. Ear Nose Throat J. 2019;98(8):NP120–4.
Shen J, Lackey E, Shah S, Neurosarcoidosis. Diagnostic challenges and mimics a review. Curr Allergy Asthma Rep. 2023;23(7):399–410.
Zajicek JP, Scolding NJ, Foster O, Rovaris M, Evanson J, Moseley IF, et al. Central nervous system sarcoidosis–diagnosis and management. QJM. 1999;92(2):103–17.
Zheng SY, Du X, Dong JZ. Re-evaluating serum angiotensin-converting enzyme in sarcoidosis. Front Immunol. 2023;14:950095.
Article CAS PubMed PubMed Central Google Scholar
Chopra A, Kalkanis A, Judson MA. Biomarkers in sarcoidosis. Expert Rev Clin Immunol. 2016;12(11):1191–208.
Pandey A, Stoker T, Adamczyk LA, Stacpoole S. Aseptic meningitis and hydrocephalus secondary to neurosarcoidosis. BMJ Case Rep. 2021;14(8).
Held EP, Iglesia EA, Johnson AS, Fang JY, Wilson MH, Abel TW, et al. Neurosarcoidosis presenting as aseptic meningitis in an immunosuppressed renal transplant recipient. Transplantation. 2016;100(10):e96–100.
Abata J, Bazer D, Koroneos N, Syritsyna O. Isolated neurosarcoidosis presenting as Chronic Progressive Pachymeningitis. Case Rep Neurol Med. 2023;2023:2140740.
PubMed PubMed Central Google Scholar
Hunninghake GW, Crystal RG. Pulmonary sarcoidosis: a disorder mediated by excess helper T-lymphocyte activity at sites of disease activity. N Engl J Med. 1981;305(8):429–34.
Selroos O, Klockars M. Relation between clinical stage of sarcoidosis and serum values of angiotensin converting enzyme and beta2-microglobulin. Sarcoidosis. 1987;4(1):13–7.
CAS PubMed Google Scholar
Kraaijvanger R, Janssen Bonas M, Vorselaars ADM, Veltkamp M. Biomarkers in the diagnosis and prognosis of sarcoidosis: current use and future prospects. Front Immunol. 2020;11:1443.
Molero-Luis M, Casas-Alba D, Orellana G, Ormazabal A, Sierra C, Oliva C, et al. Cerebrospinal fluid neopterin as a biomarker of neuroinflammatory diseases. Sci Rep. 2020;10(1):18291.
Vahabi Z, Sikaroodi H, Abdi S, Hashemi A. Chronic meningitis as the first presentation of sarcoidosis: an uncommon finding. Iran J Neurol. 2011;10(1–2):29–31.
Szabo B, Crisan D, Tompa I, Szabo I. Isolated neurosarcoidosis. Rom J Morphol Embryol. 2011;52(3 Suppl):1139–42.
PubMed Google Scholar
Bradshaw MJ, Pawate S, Koth LL, Cho TA, Gelfand JM. Neurosarcoidosis: pathophysiology, diagnosis, and treatment. Neurol Neuroimmunol Neuroinflamm. 2021;8(6).
Sakkat A, Cox G, Khalidi N, Larche M, Beattie K, Renzoni EA, et al. Infliximab therapy in refractory sarcoidosis: a multicenter real-world analysis. Respir Res. 2022;23(1):54.
Chaiyanarm S, Satiraphan P, Apiraksattaykul N, Jitprapaikulsan J, Owattanapanich W, Rungjirajittranon T et al. Infliximab in neurosarcoidosis: a systematic review and meta-analysis. Ann Clin Transl Neurol. 2023.
Download references
Acknowledgements
No further acknowledgements.
No funding was received for the current study,
Open Access funding enabled and organized by Projekt DEAL.
Author information
Authors and affiliations.
Neurology Department, St. Josef Hospital Bochum, Ruhr University Bochum, Gudrunstr. 56, 44791, Bochum, Germany
Athina-Maria Aloizou, Theresa Anne Gabriel, Ralf Gold & Jeremias Motte
Institute of Neuroradiology, St. Josef-Hospital Bochum, Ruhr University Bochum, Bochum, Germany
Carsten Lukas
You can also search for this author in PubMed Google Scholar
Contributions
Conception: AA, JM. First draft preparation: AA. Interpretation of findings: TG, RG, JM. Manuscript reviewing: TG, CL, RG.
Corresponding author
Correspondence to Athina-Maria Aloizou .
Ethics declarations
Ethics approval and consent to participate.
No Ethics approval was needed, the patient granted his written informed consent.
Consent for publication
Written informed consent for publication has been obtained from the participants in this study.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ . The Creative Commons Public Domain Dedication waiver ( http://creativecommons.org/publicdomain/zero/1.0/ ) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
Reprints and permissions
About this article
Cite this article.
Aloizou, AM., Gabriel, T.A., Lukas, C. et al. Aseptic meningitis with recurrent headache episodes, vomiting, and central fever as first manifestation of isolated neurosarcoidosis: a case report. BMC Neurol 24 , 299 (2024). https://doi.org/10.1186/s12883-024-03794-x
Download citation
Received : 03 February 2024
Accepted : 07 August 2024
Published : 28 August 2024
DOI : https://doi.org/10.1186/s12883-024-03794-x
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Aseptic meningitis
- Neurosarcoidosis
- Sarcoidosis
- Neuroimmunology
BMC Neurology
ISSN: 1471-2377
- General enquiries: [email protected]
- Alzheimer's disease & dementia
- Arthritis & Rheumatism
- Attention deficit disorders
- Autism spectrum disorders
- Biomedical technology
- Diseases, Conditions, Syndromes
- Endocrinology & Metabolism
- Gastroenterology
- Gerontology & Geriatrics
- Health informatics
- Inflammatory disorders
- Medical economics
- Medical research
- Medications
- Neuroscience
- Obstetrics & gynaecology
- Oncology & Cancer
- Ophthalmology
- Overweight & Obesity
- Parkinson's & Movement disorders
- Psychology & Psychiatry
- Radiology & Imaging
- Sleep disorders
- Sports medicine & Kinesiology
- Vaccination
- Breast cancer
- Cardiovascular disease
- Chronic obstructive pulmonary disease
- Colon cancer
- Coronary artery disease
- Heart attack
- Heart disease
- High blood pressure
- Kidney disease
- Lung cancer
- Multiple sclerosis
- Myocardial infarction
- Ovarian cancer
- Post traumatic stress disorder
- Rheumatoid arthritis
- Schizophrenia
- Skin cancer
- Type 2 diabetes
- Full List »
share this!
September 4, 2024
This article has been reviewed according to Science X's editorial process and policies . Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
reputable news agency
What new college students should know about bacterial meningitis
by Mayo Clinic News Network
As students head off to college, close quarters in new spaces could put them at risk for contagious illnesses, including bacterial meningitis. Dr. Tina Ardon, a Mayo Clinic family medicine physician, explains the common ways meningitis spreads and the best way to prevent an infection.
Meningitis is a condition that inflames the membranes around the brain and spinal cord. While there are several forms of meningitis, an infection caused by bacteria is considered the most severe form, and may lead to seizures, vision loss or death if not treated quickly.
"Meningitis often presents with symptoms such as fever, a stiff neck, headache, maybe even mental status changes, and sometimes even a very distinct rash on the skin," says Dr. Ardon.
Bacterial meningitis, also known as meningitis B, can spread by sneezing or kissing, and sharing straws or drinks, among other ways. Dr. Ardon says college students are a group at higher risk for contracting meningitis due to their living conditions .
"They're in a dormitory, in residence halls, spending a lot of time together in close quarters," says Dr. Ardon.
Preventing meningitis starts with two rounds of booster shots: one around 11 or 12 years old, and another at 16.
"At college age, we have the opportunity to potentially boost the dose if it's been some time and also offer another vaccine that protects against meningitis B," says Dr. Ardon.
2024 Tribune Content Agency, LLC.
Explore further
Feedback to editors

New study shows cells get involved in unhealthy relationships after acute kidney injury in mice
32 minutes ago

Air pollution linked to higher risk of infertility in men
11 hours ago

Insulin and metformin combo aids diabetic foot ulcer healing, new study finds

Researchers advocate for tissue-engineering approach for arthritis relief
12 hours ago

Breaking the link between obesity and atrial fibrillation with a new cellular target

Researchers reveal key LAG3 mechanisms that could enhance cancer immunotherapy
13 hours ago

Banning friendships can backfire: Moms who 'meddle' make bad behavior worse

Heavy metal cadmium may be tied to memory issues for some

New study shows that 'super spikes' can increase track running speed by 2%

Keratin gene study pinpoints mutations associated with 'spindle hair'
14 hours ago
Related Stories
Sep 1, 2022

Nigeria first to use 'revolutionary' meningitis jab: WHO
Apr 13, 2024

US officials warn of increase in bacterial illnesses that can lead to meningitis and possibly death
Mar 28, 2024

CDC warns Muslim pilgrims to Saudi Arabia of meningitis outbreak
May 22, 2024
What you should know about meningitis
Oct 15, 2019

Meningitis kills 20 Nigerian school students
Feb 29, 2024
Recommended for you
Tired muscles can indeed be a pain in the neck, spine movement study shows
15 hours ago

Immune pathways can prevent lung healing after viral infection, study shows
16 hours ago

Investigational mpox mRNA vaccine more effectively reduces disease severity in primates compared to available vaccines
18 hours ago

First narrow-spectrum antibiotic successfully eliminates Fusobacterium nucleatum, a gum disease pathogen

T-cell responses influence patient outcomes in SARS-CoV-2 pneumonia, study finds
Let us know if there is a problem with our content.
Use this form if you have come across a typo, inaccuracy or would like to send an edit request for the content on this page. For general inquiries, please use our contact form . For general feedback, use the public comments section below (please adhere to guidelines ).
Please select the most appropriate category to facilitate processing of your request
Thank you for taking time to provide your feedback to the editors.
Your feedback is important to us. However, we do not guarantee individual replies due to the high volume of messages.
E-mail the story
Your email address is used only to let the recipient know who sent the email. Neither your address nor the recipient's address will be used for any other purpose. The information you enter will appear in your e-mail message and is not retained by Medical Xpress in any form.
Newsletter sign up
Get weekly and/or daily updates delivered to your inbox. You can unsubscribe at any time and we'll never share your details to third parties.
More information Privacy policy
Donate and enjoy an ad-free experience
We keep our content available to everyone. Consider supporting Science X's mission by getting a premium account.
E-mail newsletter
An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
Preview improvements coming to the PMC website in October 2024. Learn More or Try it out now .
- Advanced Search
- Journal List
- Lippincott Open Access

Acute bacterial meningitis
Emma c. wall.
a Francis Crick Institute
b NIHR Mucosal Pathogens Research Unit, Department of Infection, Division of Infection and Immunity, University College London, London, UK
Jia Mun Chan
Robert s. heyderman, purpose of review.
Community-acquired bacterial meningitis is a continually changing disease. This review summarises both dynamic epidemiology and emerging data on pathogenesis. Updated clinical guidelines are discussed, new agents undergoing clinical trials intended to reduce secondary brain damage are presented.
Recent findings
Conjugate vaccines are effective against serotype/serogroup-specific meningitis but vaccine escape variants are rising in prevalence. Meningitis occurs when bacteria evade mucosal and circulating immune responses and invade the brain: directly, or across the blood–brain barrier. Tissue damage is caused when host genetic susceptibility is exploited by bacterial virulence. The classical clinical triad of fever, neck stiffness and headache has poor diagnostic sensitivity, all guidelines reflect the necessity for a low index of suspicion and early Lumbar puncture. Unnecessary cranial imaging causes diagnostic delays. cerebrospinal fluid (CSF) culture and PCR are diagnostic, direct next-generation sequencing of CSF may revolutionise diagnostics. Administration of early antibiotics is essential to improve survival. Dexamethasone partially mitigates central nervous system inflammation in high-income settings. New agents in clinical trials include C5 inhibitors and daptomycin, data are expected in 2025.
Clinicians must remain vigilant for bacterial meningitis. Constantly changing epidemiology and emerging pathogenesis data are increasing the understanding of meningitis. Prospects for better treatments are forthcoming.
INTRODUCTION
Acute bacterial meningitis (ABM) is a disease with rapid onset, outbreak and epidemic potential, and high rates of mortality and morbidity [ 1 , 2 ]. Considerable advances have been made in the last 30 years towards epidemic management and disease control through vaccination, and understanding the contributions of both host and pathogen to clinical outcomes. In this review, we will summarise the rapidly changing epidemiology of ABM in the context of new vaccines. We will show how new unbiased genomics technologies are revealing specific host–pathogen interactions that cause inflammation and brain damage. Additionally, we will summarise which new adjunctive treatments are in development and describe how the current Severe Acute Respiratory Syndrome CoronaVirus2 (SARS-CoV2) pandemic may impact on the WHO's efforts to defeat meningitis by 2030.

no caption available
EPIDEMIOLOGY AND IMPACT OF VACCINATION
Community-acquired bacterial meningitis is predominately caused by three pathogens, Streptococcus pneumoniae , Neisseria meningitidis and Haemophilus influenzae type B. Additionally, Streptococcus suis in Southeast Asia, Listeria monocytogenes, Group B Streptococci, and Gram-negative bacteria such as Escherichia coli and Klebsiella pneumoniae , cause meningitis in specific groups, including neonates, pregnant women, transplant recipients and older adults [ 3 ]. Worldwide, the number of reported cases of bacterial meningitis to global surveillance sites rose between 2006 and 2016, with incidence strongly related to poverty (SDI) [ 3 ]. However, the geographical incidence varies significantly. In well-resourced settings, ABM incidence has fallen to below 0.5–1.5/100 000 population [ 4 , 5 , 6 ▪▪ ]. Contrastingly, in countries in the African Sahel region, where epidemic meningitis due to N. meningitidis and S. pneumoniae persists, incidence reaches 1000/100 000 cases [ 3 , 7 – 9 ]. Beyond the meningitis belt, the incidence in Africa approaches 2.5–25/100 000 per population [ 10 , 11 ].
Bacterial meningitis is globally associated with cooler, drier seasons [ 9 ]. It is likely that climate change will impact on meningitis incidence but modelling data are lacking [ 11 ]. Social distancing measures introduced to mitigate the spread of SARS-CoV2 during the CoronaVirus Infectious Diseases 2019 pandemic are also predicted to lead to a 20–30% decrease in meningitis incidence [ 12 ▪ , 13 ].
Global meningitis epidemiology is highly dynamic; changes in the last 25 years amongst adults and children have been influenced by the widespread use of conjugate vaccines [ 14 – 16 ], the HIV-1 epidemic [ 17 – 19 ], the roll-out of antiretroviral and antibacterial treatment including prevention of mother-to-child transmission [ 20 , 21 ], and significant progress on development and poverty reduction strategies (SDG), including improved maternal and neonatal care [ 22 ].
Vaccination remains the most important pillar of the WHO-led roadmap towards defeating meningitis by 2030 [ 23 ]. A summary of all available vaccines against the three common pathogens is given in Table Table1 1 .
Currently available vaccinations against meningitis-pathogens
| Vaccine formulation | Vaccine name | Serotypes covered | Protein conjugate | Commercially available vaccine |
| Polysaccharide | PPV-23 | 1, 2, 3, 4, 5, 6B, 7F, 8, 9V, 9N, 10A, 11A, 12F, 14, 15B, 17F, 18C, 19F, 19A, 20, 22F, 23F, 33F | NA | Pneumovax |
| Conjugate | PCV-7 | 4, 6B, 9V, 14, 18C, 19F, 23F | CRM197 | Prevenar |
| Conjugate | PCV-10 | 1, 4, 5, 6B, 7F, 9V, 14, 18C, 19A, 19F | Protein D, diphtheria toxoid, tetanus toxoid | Synflorex |
| Conjugate | PCV-10 | 1, 5, 6A, 6B, 7F, 9V, 14, 19A, 19F | CRM197 | Pneumosil |
| Conjugate | PCV-13 | 1, 3, 4, 5, 6A, 6B, 7F, 9V, 14, 18C, 19A, 19F | CRM197 | Prevenar 13 |
| Conjugate | MenACWY | ACWY | CRM197, diphtheria toxoid | Menactra, MenveoSerum Institute of India (in development) |
| Polysaccharide | MPSV4 | ACWY | NA | Menimmune |
| Conjugate | MenC | C | CRM197 or tetanus toxoid | Menitorix, NeisVac-C, Menjugate, Meningitec |
| Conjugate | Hib_MenCY-TT | CY, Hib | Tetanus toxoid | MenHibrix |
| Conjugate | Men A | A | Tetanus toxoid | MenAfriVac |
| Protein | Men B bivalent vaccine | B | Not used | Trumemba |
| Protein | 4CMenB | B | Not used | Bexsero |
| Conjugate | Monovalent | Type b | CRM197 | Menitorix, Pediacel |
NA, Not available; PCV, Pneumococcal Conjugate Vaccine; PPV, Pneumococcal Polysaccharide Vaccine.
Streptococcus pneumoniae
S. pneumoniae is the commonest cause of ABM world-wide. Reports of reduction in paediatric invasive pneumococcal disease (IPD), following pneumococcal conjugate vaccine introduction in higher-income countries, were rapidly followed by evidence of herd immunity in the wider adult population, particularly the elderly [ 24 – 26 ]. Incidence of S. pneumoniae meningitis is estimated to have fallen by 48% in children [ 14 , 16 , 27 ]. However, parallel reports have emerged of IPD, including meningitis, caused by nonvaccine serotypes [ 14 , 28 – 30 ]. To mitigate against serotype replacement and better prevent meningitis, new approaches to pneumococcal vaccine design are under development, including whole capsule and protein vaccines [ 31 – 34 , 35 ▪ ].
Neisseria meningitidis
Conjugate meningococcal vaccines are highly effective in preventing meningitis caused by individual serogroups. Serogroup C Incidence has declined dramatically following the introduction of Men-C vaccine in children in many high-income countries [ 36 – 38 ]. Epidemic meningitis caused by serogroup A in the Sahel region of Africa has been dramatically reduced by low-cost MenAfriVac serogroup A conjugate vaccine by 92% [ 39 , 40 ]. However, virulent clones of other serogroups have subsequently emerged (C, W, X) and epidemics of meningococcal meningitis continue to occur in the Sahel [ 41 , 42 ].
As serogroup C disease declined, serogroup B emerged as the leading cause of meningococcal meningitis in high SDI countries [ 15 ]. In 2015, the UK government introduced protein-based serogroup B vaccine 4CMenB (Bexsero) to all children under 2 years. UK cases of invasive serogroup B in children have declined 75% with an estimated overall vaccine efficacy of 54% [ 43 ]. However, disease due to other serogroups including W and Y remains problematic. MenC conjugate vaccine has now been replaced with quadrivalent MenACWY vaccine for all teenagers and young adults in the UK [ 38 ].
Haemophilus influenzae
Hib vaccination in 1989 led to dramatic reductions in paediatric meningitis between 75 and 95% [ 44 , 45 ]. Subsequently, Hib meningitis has virtually been eliminated globally in countries with effective Expanded Programme of Immunisations (EPI), but persists where vaccination coverage is poor including India, Nigeria, Pakistan and the Democratic Republic of Congo [ 16 , 44 , 46 , 47 ]. Hib conjugate vaccines are estimated to have reduced Hib meningitis by 49% globally 2000–2016 [ 3 ], and paediatric deaths by 90% over the same time period [ 16 ]. However, it is concerning that non-type b stains such as Hia are emerging [ 42 ].
Group B Streptococcus
Streptococcus agalactiae (Group B Streptococcus , GBS) primarily causes meningitis in neonates but also causes sepsis in older adults with co-morbidities and young adults who have consumed contaminated fish [ 48 ]. Serotypes Ia, Ib, II, III and V account for 98% of human carriage serotypes isolated globally [ 49 ]. Clonal complex 17 (CC17) strains have been shown to be hypervirulent, accounting for more than 80% of the disease [ 50 , 51 ]. GBS disease-causing lineages have distinct niche adaptation and virulence characteristics [ 52 , 53 ]. The most promising strategy to eliminate neonatal meningitis caused by GBS is vaccination in pregnancy, trials are ongoing [ 54 – 57 ].
PATHOGENESIS
The pathogenesis of most ABM follows a sequential pattern: nasopharyngeal colonization, bloodstream invasion across the mucosa, circulation of bacteria to the central nervous system (CNS), and subsequent CNS entry [ 58 ▪ , 59 ]. In ABM caused by L. monocytogenes , GBS and S. suis , bacteraemia has a gastrointestinal or genitourinary tract source [ 52 , 60 , 61 ]. Occasionally, ABM is acquired through direct CNS invasion through the cribriform plate [ 62 , 63 ]. In the majority of immunocompetent individuals, colonisation of the nasopharynx by S. pneumoniae and N. meningitidis is cleared by mucosal immunity, despite epithelial invasion [ 58 ▪ ]. Co-infection with S. pneumoniae and respiratory viruses such as influenza causes a heightened inflammatory state associated with both pneumococcal and meningococcal invasion [ 64 – 66 ], indeed preceding influenza is associated with seasonal ABM [ 11 , 67 ▪ ].
Bacteraemia usually precedes translocation across the blood–brain barrier (BBB) and/or blood–cerebrospinal fluid barrier into the CNS. Under basal conditions, the CNS environment is under continuous immunological surveillance [ 68 ]. This is achieved through the complexity of the BBB, where pericytes, astrocytes, microglia and specialised endothelial cells work in synergy to both resist pathogen invasion and kill bacteria on entry [ 68 ] (Fig. (Fig.1). 1 ). Bacteria breach the BBB by interacting with laminin receptors and exploiting endocytic pathways, for example via Platelet Activating Factor Receptor signalling [ 69 – 72 ] (Fig. (Fig.1). 1 ). However, mechanisms by which ABM-causing bacteria subvert CNS barriers to cause meningitis are not fully described.

Model of BBB environment during bacterial meningitis. ABM pathogen (depicted here as blue diplococci) in the bloodstream cross the capillary endothelium using both transcellular and paracellular routes. Bacteria may also be carried across the BBB by infiltrating phagocytes (Trojan Horse strategy). Recognition of the pathogen via sensing of PAMPs leads to the activation of resident immune cells such as microglia, macrophages, astrocytes and pericytes and production of DAMPs. These cells produce a coordinated inflammatory response to contain bacteria and recruit more neutrophils to the CSF compartment. This host response, while important for killing bacteria, activates a fibrinolytic and coagulation cascade. When advanced, these processes lead to sustained tissue damage, BBB breakdown and leakage, causing death or lifelong neurological sequalae in survivors. ABM, acute bacterial meningitis; BBB, blood–brain barrier.
In the 10–30% of ABM cases without concurrent bacteraemia [ 73 ], bacteria may interact with gangliosides, adhere to the olfactory bulb, invade the olfactory epithelium and directly translocate to the brain [ 63 , 74 – 77 ]. Pneumococcal strains causing nonhematogenous meningitis tend to be less frequently studied using bacteraemia-based animal models [ 75 – 77 ].
Inflammation and exacerbation of tissue damage in acute bacterial meningitis
Bacteria replicate rapidly in the relatively immune-privileged CNS compartment [ 78 ], releasing Pathogen Associated Molecular Pattern (PAMP)s that bind to toll-like receptors including 2,3,4 and 9, triggering the release of Damage-Accoiated Molecular Pattern Signallings (DAMPs) via Nuclear Factor Kappa-light-chain-enhancer of activated B cells (NF-κB) activation [ 79 – 82 ]. The subsequent release of extracellular cytokines and chemokines including Chemokine family Ligand 8 and cerebrospinal fluid (CSF)-3 drives a rapid influx of neutrophils to the CSF compartment [ 83 ▪▪ , 84 ].
Bacterial PAMPs and virulence proteins exert direct damage on the delicate structures of the CNS. Pneumococcal virulence factors, including capsule and pneumolysin, reduce microglia motility and chemotaxis [ 85 ▪ ]. Pneumolysin, a cytolysin and Toll Like Receptor 4 agonist is implicated in directly toxic effects on host cells, particularly within the BBB and hippocampus [ 86 , 87 ]. Others stimulate CERB binding protein (CBP) and receptor for advanced glycation end products (RAGE), increasing Tumour Necrosis Factor alpha (TNF-α) levels and promoting BBB disruption [ 88 , 89 ▪ ].
Host-detection of bacteria within the CNS triggers a highly inflammatory, and predominately ineffective host response, associated with further tissue damage. Sustained inflammation exacerbates tissue damage, leading to death or irreversible neurological damage [ 73 , 90 , 91 ]. Neutrophil infiltration is important for bacterial elimination [ 92 ]. However, neutrophils can directly damage the CNS [ 93 ]. Neutrophil extracellular traps (NETs) unexpectedly impaired CNS pneumococcal clearance and increased inflammatory damage in an experimental model [ 83 ▪▪ ]. Damaging DAMPs released both from neutrophil degranulation and NF-κB signalling include myeloperoxidase, matrix-metalloproteinases, TNF-α and prostaglandins [ 94 ▪ , 95 , 96 , 97 ▪ ]. Neutrophil-mediated inflammation is strongly associated with dysfunctional coagulation and fibrinolytic cascade in the CNS, including an excess of the anaphylatoxin complement C5 [ 98 ].
Clinical improvement with dexamethasone adjunctive therapy in both Hib and pneumococcal meningitis demonstrates the importance of host-mediated inflammation in ABM [ 99 , 100 ]. Dexamethasone may reduce NF-κB signalling and cytokine release [ 101 ].
Leveraging new technology to interrogate acute bacterial meningitis pathogenesis
Bacterial genome-wide association studies (GWAS) have revealed loci that are implicated in invasiveness, tissue tropism and the ability to cause CNS disease [ 102 ▪▪ , 103 ▪▪ , 104 , 105 ]. Single Nucleotide Polymorphisms (SNPs) in the raf operon determine pneumococcal tropism for ear/brain or lungs in an intranasal challenge model [ 106 ▪ , 107 ]. Additionally, SNPs in raf modulated neutrophil recruitment, leading to strain-dependent clearance [ 106 ▪ ].
Gene expression in S. pneumoniae is niche dependent, highlighting the importance of bacterial metabolism in pathogenesis [ 108 , 109 ▪▪ ]. In a quantitative proteomics study of ABM, the abundance of pneumococcal protein Elongation Factor Tu in CSF associated with severity in human disease [ 97 ▪ ]. In a murine model, proteins AliB and competence peptides were implicated in pathogenesis [ 110 ]. Joint human–pathogen GWAS studies of meningitis patients suggest that genetic differences in the host response exert greater effects on susceptibility and disease severity than bacterial genotype. This GWAS identified variants in the CCDC3 gene associated with disease severity [ 102 ▪▪ ]. CCDC3 is a multifunction gene involved in the metabolism and suppression of NF-κB–TNFα activation in endothelial cells [ 111 ].
NEW DIRECTIONS IN DIAGNOSTICS AND CLINICAL MANAGEMENT
Early recognition and initiation of appropriate antimicrobials are essential to minimise death and complications from ABM. The differential diagnosis in patients presenting with headache, fever, neck stiffness or altered mental state is broad: the classical meningitis triad has limited diagnostic sensitivity [ 112 ]. A high index of clinical suspicion is thus required to diagnose ABM [ 113 ]. Lumbar puncture is essential, and should be undertaken promptly before CSF is rendered sterile by broad-spectrum antibiotics [ 114 ].
Many patients with ABM present with an altered level of consciousness, leading clinicians to frequently request cranial imaging prior to diagnostic lumbar puncture. Early Lumbar puncture (LP) is strongly associated with higher diagnostic yield from the CSF; delays in LP for cranial imaging lead to substantial reductions in yield from either CSF bacterial culture or PCR [ 114 ]. Delays to diagnosis are linked to worse clinical outcomes [ 114 – 116 ]. Cranial imaging (either CT or MRI) in patients with clear clinical signs and symptoms of meningitis without focal neurology is thus not recommended in the majority of patients with suspected ABM [ 117 , 118 ]. CT has poor inter-reporting reliability to predict the risk of cerebral herniation in ABM [ 119 ]. The American, British and European infection societies meningitis guidelines all recommend immediate LP in cases of suspected ABM without delay for CT/MRI in immunocompetent adults with suspected ABM who have a stable GCS of ≥12/15 without seizures [ 119 – 123 ]. Important contraindications to LP include shock, respiratory compromise, or coagulopathy.
The diagnosis of ABM is dependent on the analysis of CSF. The leukocyte count remains the strongest predictive value of ABM. Diagnostic models including clinical, CSF and blood data show little additional benefit beyond clinical judgement [ 111 ]. Antibiotic administration prior to LP commonly renders the CSF sterile, thus clinicians are increasingly dependent on diagnostic PCR. Recent data suggest that while small multiplex panels targeting Hib, meningococci and pneumococci are highly sensitive and specific [ 123 ], larger panels that include viral, nosocomial and rarer community-acquired pathogens have varying sensitivity and specificity and are not currently recommended [ 124 ]. More recently, direct next-generation sequencing (NGS) and metagenomics of CSF have been proposed to detect pathogens in cases with a high index of clinical suspicion of ABM but negative PCR tests [ 125 ▪▪ ]. While this approach is promising, constraints around cost, bioinformatics expertise and clinically relevant turnaround times have limited clinical use of NGS to date [ 124 ].
All guidelines recommend patients with suspected ABM should receive parenteral antibiotics within 1 h. However, only 46% of patients in a clinical research study were reported to meet this target, limited by delays in the emergency department [ 126 , 127 ]. Antibiotic choice should be determined by patient risk group, patient allergies, and local guidelines informed by epidemiology, including antimicrobial resistance. Penicillin resistance in S. pneumoniae is 15–20% in some settings, but remains <5% in N. meningitidis [ 128 , 129 ]. However, quinolone resistance in N. meningitidis reaches 70% in Southeast Asia [ 15 , 130 ]. Diagnostic uncertainty in culture-negative meningitis often leads to prolonged dual antibiotic and antiviral therapies, which may be associated with nosocomial complications [ 114 , 131 ▪ ].
Adjunctive therapies
Adjunctive treatments are designed to reduce secondary inflammation in ABM and decrease the morbidity associated with CNS tissue damage. Inflammation is associated with secondary complications of ABM, including death, deafness, stroke, epilepsy and learning difficulties [ 91 , 131 ▪ , 132 – 134 ]. Delayed cerebral thrombosis is a rare complication of ABM that can occur up to 2 weeks post-admission [ 135 , 136 ].
In hospitals in high-income settings, patients presenting with suspected pneumococcal meningitis should receive adjunctive dexamethasone to reduce mortality [ 90 , 137 ]. In low-income settings, dexamethasone is only indicated in cases of suspected S. suis meningitis in Southeast Asia to reduce deafness [ 137 , 138 ]. In other settings, particularly in Low and Middle Income Countries in Africa, dexamethasone is ineffective and should not be given [ 139 ].
Other previously tested adjuncts, including hypothermia and glycerol, have been shown to be potentially harmful and should not be administered [ 140 , 141 ].
Emerging therapeutic targets
Empirical antibiotic treatment in most centres for suspected ABM is the third-generation cephalosporin, ceftriaxone [ 92 ]. However, bacterial lysis by ceftriaxone releases DAMPs that may prolong damaging inflammation even as bacteria killed [ 88 ]. Research in animal models has strongly suggested bacteriostatic antibiotics are associated with less CNS inflammation and improve outcomes [ 142 ]. In clinical practice, there are little data to suggest different clinical outcomes occur between bacteriostatic vs. bactericidal antibiotics [ 143 ]. As such, there are continued efforts to develop alternatives that reduce sequalae in survivors. A phase 2 clinical trial evaluating the adjunctive use of a nonlytic antibiotic, daptomycin, for pneumococcal meningitis is currently underway (ClinicalTrials.gov identifier NCT03480191). Adjunctive administration of daptomycin may dampen the inflammatory effects of ceftriaxone through currently unknown mechanisms [ 144 ].
The damaging coagulation and fibrinolytic cascade in CSF are triggered partly by excess complement C5 [ 98 ]. Inhibition of C5 improved outcomes in a murine model, clinical trials of C5 antagonists are currently underway [ 145 ].
Newer therapeutic agents with intriguing survival data in animal models are not yet in clinical trials. These include DNAse-1, targeted at disrupting ineffective NETosis, the possible neuroprotective effects of metformin, and matrix-metalloproteinase inhibitors targeted on preventing enzymatic tissue breakdown [ 83 ▪▪ , 146 – 148 ]. Proposed adjunctive antipneumococcal therapy includes targeting pneumolysin and P4, a pneumococcal peptide that may inhibit replication [ 149 , 150 ].
CONCLUSIONS
Community-acquired bacterial meningitis presents ongoing formidable epidemiological and clinical challenges. The ability of meningitis-causing pathogens to evolve in the ecological niche of the nasopharynx during carriage, and escape serotype-specific vaccines has led to new strategies to eliminate disease carriage through serotype-independent vaccination. The outcome of CNS host-pathogen interactions determines clinical sequelae, influenced by host genetic susceptibility.
CSF analysis is essential to make a diagnosis of ABM, leukocyte count remains the most effective predictor of ABM over newer models. Nonindicated cranial imaging introduces significant diagnostic delays. Multiplex PCR panels have increasing utility in ABM diagnostics, however NGS remains a research tool.
Patients with ABM continue to experience significant complications, including death, stroke and deafness. Adjunctive dexamethasone improves survival in high-income countries only, the results of clinical trials of more targeted approaches are awaited. Effective and affordable, pan-serogroup vaccination remains a crucial goal if we are to eliminate this devastating disease.
Acknowledgements
Financial support and sponsorship.
E.W. is supported by a postdoctoral clinical research fellowship from the Francis Crick Institute, and the National Institute for Health Research University College London Hospitals Department of Health's NIHR Biomedical Research Centre. E.C. and R.H. are funded by the National Institute for Health Research (NIHR) (project reference 16/136/46) using UK aid from the UK Government to support global health research. The views expressed in this publication are those of the author(s) and not necessarily those of the NIHR or the UK government.
E.C and R.H are funded by the Medical Research Council grant number MR/T016329/1 and the National Institute for Health Research (NIHR) (project reference 16/136/46) using UK aid from the UK Government to support global health research. The views expressed in this publication are those of the author(s) and not necessarily those of the NIHR or the UK government.
Conflicts of interest
There are no conflicts of interest.
REFERENCES AND RECOMMENDED READING
Papers of particular interest, published within the annual period of review, have been highlighted as:
- ▪ of special interest
- ▪▪ of outstanding interest

IMAGES
VIDEO
COMMENTS
Acute Bacterial Meningitis Case Study Bacterial meningitis is a life-threatening infection of the linings or meninges of the brain and spinal cord. Survivors may experience hearing loss or deafness, brain damage, seizures, and/or the retention of fluid on the brain. Symptoms may be mistaken for the flu. Find out what happens to a 14-year-old when bacteria invade his central nervous system.
We present the case of sudden death in a 2-year-old child. The patient died approximately 30 min after hospital admission before any routine diagnostic procedures were undertaken. Presence of whole-body rash and fulminant course of the disease raised suspicion of meningococcal septicemia. An autopsy was performed seven days after death when the ...
This case study was based on a popular press news article about Krystle Beauchamp Gridley's experience with meningitis while in college (). Students in an introductory neuroscience course read the popular press news article as well as an empirical ...
A 19-year-old man was admitted to the pediatric ICU because of shock, multiple organ failure, and rash. Twenty hours before admission, abdominal pain and nausea developed after he ate leftovers fro...
Differential diagnosis This case represented a diagnostic dilemma because of the discordance between clinical and initial investigation findings. The clinical features were strongly suggestive of meningitis, most likely of viral or bacterial aetiology, but the initial CSF examination was normal.
Read chapter 9 of Infectious Diseases: A Case Study Approach online now, exclusively on AccessPharmacy. AccessPharmacy is a subscription-based resource from McGraw Hill that features trusted pharmacy content from the best minds in the field.
Introduction Bacterial meningitis is a neurological emergency with high morbidity and mortality. Over 1.2 million cases of bacterial meningitis are estimated to occur annually worldwide 1 ...
The case reviewed in this article focuses on viral meningitis in a pediatric patient that may be unrecognized or underreported because of indistinct symptoms. Epidemiology, pathophysiology, presentation, assessment techniques, diagnostics, clinical management, and health promotion relevant to viral meningitis are presented.
Case-based learning: meningitis Causes, diagnosis and initial management options for adults and children with meningitis.
Join our expert to discuss the intricacies of a complicated adult meningitis case and review pertinent guidelines, literature, and contemporary clinical evidence. Learners will be guided to challenge their knowledge and review symptoms, clinical presentation, and treatment principles for meningitis through a robust, hands-on case study.
The case reviewed in this article focuses on viral meningitis in a pediatric patient that may be unrecognized or underreported because of indistinct symptoms. Epidemiology, pathophysiology, presentation, assessment techniques, diagnostics, clinical management, and health promotion relevant to viral meningitis are presented.
The etiologies of meningitis range in severity from benign and self-limited to life-threatening with potentially severe morbidity. Bacterial meningitis is a medical emergency that requires prompt ...
1 INTRODUCTION. Meningitis is a serious health condition that caused 236,000 deaths and 2.51 million incident cases globally in 2019. 1 While there is a decrease in age-standardized mortality rate from 7.5 per 100,000 population in 1990 to 3.3 in 2019, bacterial meningitis remains a significant concern with 112,000 deaths and 1.28 million incident cases reported in children younger than 5 ...
This review summarizes recent changes in the treatment of adults with community-acquired bacterial meningitis. It explains the initial assessment and management, the use of adjunctive corticosteroi...
The following fictional case is intended as a learning tool within the Pathology Competencies for Medical Education (PCME), a set of national standards for teaching pathology. These are divided into three basic competencies: Disease Mechanisms and Processes, ...
Learn how to diagnose and manage meningitis in adults from the Internal Medicine Journal, a leading publication from the Royal Australasian College of Physicians.
Meningitis - Case Summary. 1. "Meningitis is an infection of the fluid of a person's spinal cord and the fluid that surrounds the brain. People sometimes refer to it as spinal meningitis. Meningitis is usually caused by a viral or bacterial infection. Knowing whether meningitis is caused by a virus or bacterium is important because the severity ...
A Dutch nationwide prospective cohort study, involving 2220 patients with bacterial meningitis, demonstrated cerebral venous thrombosis in 1% of the patients. 29 S. pneumoniae (65%) and S. pyogenes (8%) were the most common causative pathogens. 29 The most common thrombosed sinus was the transverse sinus (69%), followed by the sigmoid sinus 46% ...
A case of meningococcal meningitis in an adult male in March 2000 in Hong Kong is reported. The main features were fever for one day with headache, vomiting, dizziness, diffuse maculo-papular rash, and one purpuric rash on the dorsum of right hand and stable vital signs. Incidentally, there were three other unreported cases of meningococcal ...
The causes and features of chronic meningeal inflammation or infection differ from those of acute meningitis. The evaluation includes a search for systemic disorders such as sarcoid, as well as inf...
Meningitis - Page 1. A 14-year old male complained to his parents of feeling quite ill with a headache, dizziness, nausea, and feeling very weak. After a rise in his body temperature, an increase in the severity of his headache, and the development of a rash, his mother took him into their medical clinic where he was examined by a family nurse ...
One study also reported on the presence of one of a number of signs/symptoms across categories (Joffe 1983). No studies reported on the distribution or duration of rash, limb or body pain, or cardiac symptoms, as signs/symptoms of bacterial meningitis in babies and children.
This is a case of streptococcus pneumoniae meningitis in an immunocompromised patient. This case discusses the presentation, management, and complications of a 57-year-old female with past medical history significant for Hodgkin's lymphoma in remission and rheumatoid arthritis on immunosuppressive therapy who presented to the emergency ...
This case report aims to highlight aseptic meningitis with atypical, acute onset headache attacks as a possible manifestation of isolated neurosarcoidosis. ... can lead to its increase , and the numbers of patients with elevated ACE have ranged from 30 to 80% among different studies . As a 2016 meta-analysis reported, ...
Bacterial meningitis, also known as meningitis B, can spread by sneezing or kissing, and sharing straws or drinks, among other ways. Dr. Ardon says college students are a group at higher risk for ...
Meningitis occurs when bacteria evade mucosal and circulating immune responses and invade the brain: directly, or across the blood-brain barrier. Tissue damage is caused when host genetic susceptibility is exploited by bacterial virulence.
Cortical laminar necrosis (CLN) is a rare neurological complication that refers to ischemic injury of selective neuronal cortical layers. This condition often gets triggered by hypoxia, hypoglycemia, status epilepticus, immunosuppressive therapy, and rarely infection. This case report highlights the clinical presentation, diagnostic challenges, management, and outcomes of a patient who ...
-- For Pediatric Indication, in Parallel with Ongoing VAX-24 Study, Company Plans to Initiate VAX-31 Infant Phase 2 Study in First Quarter of 2025 Following IND Application Submission and Clearance -- ... which is associated with high case-fatality rates, antibiotic resistance and meningitis. To address this need, VAX-31 was designed to ...