Jump to navigation


Experiment: Exploring Solubility - How Much Salt and Sugar Can Dissolve in Water?
Introduction:.
Solubility is a key concept in chemistry describing the maximum amount of a solute that can dissolve in a solvent at a given temperature. This experiment explores the solubility of common substances in water, illustrating the impact of molecular structure and temperature on solubility rates.
Materials You'll Need:
- Table salt (sodium chloride)
- Sugar (sucrose)
- Epsom salt (magnesium sulfate)
- Baking soda (sodium bicarbonate)
- Four small, identical containers or beakers
- A stirring rod or spoon
- Water at room temperature (approximately 20°C)
- Measuring spoons
- Graduated cylinder or measuring cup
- Scale for precise measurement
- Notebook and pen for recording observations
- Prepare the Containers: Use a permanent marker to label each container with the name of the substance you're testing: sodium chloride, sucrose, magnesium sulfate, and sodium bicarbonate.
- Measure Water: Use a graduated cylinder to measure 100 ml of water into each container.
- Test Solubility: Add the solutes to the water incrementally, stirring continuously until no more dissolves. Use a scale to weigh each teaspoon of solute before adding to the water. Record the weight of the solute added to reach the point of saturation.
Observations and Results:
The solubility of a substance in water is heavily dependent on the substance's chemical properties and the temperature of the water. In this experiment, conducted at room temperature, the following observations were made:
1. Sodium Chloride (Table Salt):
Observation: Incrementally added to water, sodium chloride dissolved readily until reaching its solubility limit, which is approximately 357 grams per liter at 20°C.
Result: At room temperature, a saturation point was observed at around 35.7 grams in 100 ml of water, which aligns with sodium chloride's known solubility.
2. Sucrose (Sugar):
Observation: Sucrose dissolved in water, appearing cloudy at first but clearing upon further dissolution.
Result: Sucrose's solubility at room temperature is about 200 grams in 100 ml of water, demonstrating a high solubility rate, contrary to what might be considered moderate.
3. Magnesium Sulfate (Epsom Salt):
Observation: Magnesium sulfate dissolved in water, showing high solubility initially but leaving some undissolved material after reaching saturation.
Result: The solubility of magnesium sulfate at 20°C is 26 grams per 100 ml, indicating a definitive solubility limit at room temperature.
4. Sodium Bicarbonate (Baking Soda):
Observation: Sodium bicarbonate showed solubility in water with an increasing amount until reaching a saturation point without visible residue.
Result: Sodium bicarbonate's solubility is lower than that of sodium chloride, at about 9 grams per 100 ml at room temperature. The observation made previously about 'no visible residue' pertains to an amount below its solubility threshold.
Comparison and Analysis:
The solubility experiment illustrated the differing solubility of the tested substances. Sodium chloride and sodium bicarbonate showed high and moderate solubility, respectively, while sucrose displayed a surprisingly high solubility rate, and magnesium sulfate demonstrated a high solubility up to a certain limit.
It's important to note that solubility is an equilibrium condition and is specific to the temperature at which it is measured. The results obtained offer a clear example of how solubility varies between substances and underlines the significance of accurate measurement and controlled conditions in solubility testing.
Conclusion:
This experiment underscores the variable solubility of different substances in water and the importance of understanding these differences for practical applications. It also highlights the importance of precise measurement and conditions when experimenting with solubility.
Safety Tips:
As with all chemistry experiments, safety is paramount. Ensure you wear protective gear and handle all materials with care. Proper disposal of chemicals is essential to maintaining safety and environmental standards.

- Sign in / Register
- Administration
- My Bookmarks
- My Contributions
- Activity Review
- Edit profile

The PhET website does not support your browser. We recommend using the latest version of Chrome, Firefox, Safari, or Edge.
Sciencing_Icons_Science SCIENCE
Sciencing_icons_biology biology, sciencing_icons_cells cells, sciencing_icons_molecular molecular, sciencing_icons_microorganisms microorganisms, sciencing_icons_genetics genetics, sciencing_icons_human body human body, sciencing_icons_ecology ecology, sciencing_icons_chemistry chemistry, sciencing_icons_atomic & molecular structure atomic & molecular structure, sciencing_icons_bonds bonds, sciencing_icons_reactions reactions, sciencing_icons_stoichiometry stoichiometry, sciencing_icons_solutions solutions, sciencing_icons_acids & bases acids & bases, sciencing_icons_thermodynamics thermodynamics, sciencing_icons_organic chemistry organic chemistry, sciencing_icons_physics physics, sciencing_icons_fundamentals-physics fundamentals, sciencing_icons_electronics electronics, sciencing_icons_waves waves, sciencing_icons_energy energy, sciencing_icons_fluid fluid, sciencing_icons_astronomy astronomy, sciencing_icons_geology geology, sciencing_icons_fundamentals-geology fundamentals, sciencing_icons_minerals & rocks minerals & rocks, sciencing_icons_earth scructure earth structure, sciencing_icons_fossils fossils, sciencing_icons_natural disasters natural disasters, sciencing_icons_nature nature, sciencing_icons_ecosystems ecosystems, sciencing_icons_environment environment, sciencing_icons_insects insects, sciencing_icons_plants & mushrooms plants & mushrooms, sciencing_icons_animals animals, sciencing_icons_math math, sciencing_icons_arithmetic arithmetic, sciencing_icons_addition & subtraction addition & subtraction, sciencing_icons_multiplication & division multiplication & division, sciencing_icons_decimals decimals, sciencing_icons_fractions fractions, sciencing_icons_conversions conversions, sciencing_icons_algebra algebra, sciencing_icons_working with units working with units, sciencing_icons_equations & expressions equations & expressions, sciencing_icons_ratios & proportions ratios & proportions, sciencing_icons_inequalities inequalities, sciencing_icons_exponents & logarithms exponents & logarithms, sciencing_icons_factorization factorization, sciencing_icons_functions functions, sciencing_icons_linear equations linear equations, sciencing_icons_graphs graphs, sciencing_icons_quadratics quadratics, sciencing_icons_polynomials polynomials, sciencing_icons_geometry geometry, sciencing_icons_fundamentals-geometry fundamentals, sciencing_icons_cartesian cartesian, sciencing_icons_circles circles, sciencing_icons_solids solids, sciencing_icons_trigonometry trigonometry, sciencing_icons_probability-statistics probability & statistics, sciencing_icons_mean-median-mode mean/median/mode, sciencing_icons_independent-dependent variables independent/dependent variables, sciencing_icons_deviation deviation, sciencing_icons_correlation correlation, sciencing_icons_sampling sampling, sciencing_icons_distributions distributions, sciencing_icons_probability probability, sciencing_icons_calculus calculus, sciencing_icons_differentiation-integration differentiation/integration, sciencing_icons_application application, sciencing_icons_projects projects, sciencing_icons_news news.
- Share Tweet Email Print
- Home ⋅
- Science Fair Project Ideas for Kids, Middle & High School Students ⋅
Sugar Dissolves in Water Faster Than Salt Science Projects

Science Projects and Research With Salt, Sugar, Water and Ice Cubes
When a substance dissolves in another substance, it forms a solution. The substance being dissolved is called the solute, and the substance it is dissolving into is called the solvent. Sugar and salt both dissolve in solution relatively easily, but one dissolves quicker than the other. A simple experiment can determine which one dissolves faster.
Experiment Setup
To do this project you will need a supply of both salt and sugar as well as a way to measure out equal amounts of both substances. You will also need at least three solvents, with one of them being water. Suggested solvents include distilled vinegar and rubbing alcohol. Be sure to allow all three solvents to reach room temperature before you run the experiment. Label three cups with the names of the solvents and the word salt, then label the other three with the names of the solvents and the word sugar.
Collecting Data
Make a data table that includes all three solvents for both sugar and salt. The table should include a start time, stop time and elapsed time to record how long it took each solute to dissolve. For greater accuracy, run the test two or three times for each solute in each solvent and average the findings together. Perform the experiment by pouring equal amounts of your solvent in six cups. Add one teaspoon of salt to one of the cups and record how long it takes to dissolve. Repeat this for the other two solvents, then repeat again for sugar in all three solvents. Record all your data in your table.
What Happens
In this experiment, sugar should dissolve faster in solvents than salt does. The reason for this is because the sugar molecules are bigger than the ions of dissolved salt. This allows for more water molecules to surround a single particle, pulling it into solution faster. Also, because a molecule of sugar is much larger than a sodium or chlorine atom, fewer molecules are found in a teaspoon of sugar than salt, leaving fewer molecules to be pulled into solution.
Changes in the Experiments
This experiment can be changed to include different variables. For example, the temperature of a solvent affects its ability to dissolve solutes. You could run the experiment again, using temperature as a variable for each solvent. Another variable you could test would be the solubility of different types of sugar or salt. Use the larger crystals of sea salt or the smaller crystals of powdered sugar to see if this affects solubility rates. Finally, another variable that could be added to the experiment is how much stirring the solution affects the ability of a solute to dissolve.
Related Articles
Science projects and research with salt, sugar, water..., how to measure solubility for a science project, ideas for a science fair project using kool-aid, experiments with salt melting ice, how to compute the freezing point of a mixture, why does salt melt ice faster than sugar, science projects with three variables for kids in fifth..., how to make a five percent solution with salt, difference between solubility & molarity, definitions of control, constant, independent and dependent..., science projects on what freezes faster: water or sugar..., science fair on how vitamin c & ibuprofen affect plant..., how to calculate melting & boiling points using molality, what is the definition of dissolve in chemistry, how does a sugar crystal grow, science projects using gummy worms, experiments with salt and sugar ice cubes, how to calculate solubilities, chemistry projects for diffusion in liquids.
- TeacherVision: Salt or Sugar: Which Dissolves Faster in Different Liquids?
- Ask a Scientist: Crystal Dissolving Rates
- JulianTrubin.com: Salt & Sugar Science Fair Projects
About the Author
Kevin Carr has been writing for a variety of outlets and companies since 1991. He has contributed to McGraw-Hill textbooks for middle school and high school, written for the Newspaper Network of Central Ohio and has been a featured film critic for online publications including 7M Pictures and Film School Rejects. Carr holds a Bachelor of Science in education.
Find Your Next Great Science Fair Project! GO

- Science Notes Posts
- Contact Science Notes
- Todd Helmenstine Biography
- Anne Helmenstine Biography
- Free Printable Periodic Tables (PDF and PNG)
- Periodic Table Wallpapers
- Interactive Periodic Table
- Periodic Table Posters
- Science Experiments for Kids
- How to Grow Crystals
- Chemistry Projects
- Fire and Flames Projects
- Holiday Science
- Chemistry Problems With Answers
- Physics Problems
- Unit Conversion Example Problems
- Chemistry Worksheets
- Biology Worksheets
- Periodic Table Worksheets
- Physical Science Worksheets
- Science Lab Worksheets
- My Amazon Books

How to Separate Salt and Sugar

If you spill sugar and salt together in your kitchen, it’s not worth the effort to separate them. But, you can separate salt and sugar mixtures as a science project to learn about chemical and physical properties and separation chemistry. Here are three ways to separate salt and sugar, plus one that seems like it should work, but really doesn’t.
Separate Salt and Sugar Using Solubility
Both salt and sugar dissolve in water. However, sugar (sucrose) is much more soluble in alcohol than salt (sodium chloride) is. For all practical purposes, salt is insoluble in alcohol. The solubility of salt is 14 g/kg in methanol (25 °C or 77 °F) and 0.65 g/kg in ethanol (25 °C or 77 °F). If you ever plan on eating the salt or sugar, use ethanol to separate the components of the mixture because methanol is toxic. If efficiency is your goal, use methanol because you’ll need less of it to dissolve the salt, leaving the sugar behind. Evaporate or boil off the alcohol to recover the salt.
Be aware this method doesn’t work nearly as well if you don’t use absolute alcohol. If you try to separate sugar and salt using 50% alcohol, it’s likely there will be enough water in the liquid to dissolve both components of the mixture!
Separate Salt and Sugar Using Density
The density of pure table salt (NaCl) is 2.17 g/cm 3 , while the density of pure table sugar (sucrose) is 1.587 g/cm 3 . So, to separate the pure solids, you could shake the mixture. The heavier salt will sink to the bottom of the container. While the material at the top of the container will be almost pure sugar and that at the bottom will be almost pure salt, it may be hard to tell where one compound ends and the other begins. You won’t be able to get 100% separation using only this method.
Separate Salt and Sugar Using Crystal Shape
If you have infinite time and patience, you can separate sugar and salt in a mixture with a magnifying glass and pair of tweezers. Salt crystals are cubic, while sugar crystals are monoclinic hexagons.
What About Using Melting Point?
Sugar is a covalent compound, while salt is an ionic compound. So, you might predict you can separate sugar and salt using melting point . The melting point of salt is very high (800.7 °C or 1473.3 °F). The problem is sugar decomposes at 186 °C (367 °F) rather than melts. If you try to separate the components of the mixture using heat, all you’ll get is burned sugar (carbon) and salt. Save this method for separating salt and sand (although there are better options).
- Burgess, J (1978). Metal Ions in Solution . New York: Ellis Horwood. ISBN 978-0-85312-027-8.
- Rumble, John (ed.) (2019). CRC Handbook of Chemistry and Physics (100th ed.). CRC Press. ISBN:978-1138367296.
- Westphal, Gisbert et al. (2002) “Sodium Chloride” in Ullmann’s Encyclopedia of Industrial Chemistry . Wiley-VCH, Weinheim. doi: 10.1002/14356007.a24_317.pub4
- Wilson, Ian D.; Adlard, Edward R.; Cooke, Michael; et al., eds. (2000). Encyclopedia of Separation Science . San Diego: Academic Press. ISBN 978-0-12-226770-3.
Related Posts
Salt or Sugar: Which Dissolves Faster in Different Liquids?
Solutions are nothing more than mixtures of different compounds or elements. You encounter solutions every day without even realizing it.
Even the air you breathe-which contains water-is a solution of a liquid and a gas. If you drank a soda today, you actually drank of solution of a gas dissolved in flavored water. If you're wearing a bracelet made of sterling silver, you're wearing a solution of two metals.
In this experiment, you'll be working with a liquid solution, which is one of three types of solutions. The other types are gaseous solutions and solid solutions.
So What Seems to Be the Problem?
Mixing a liquid in a gas makes another type of solution, called a gaseous solution. An example of this type of solution is humidity. Humidity is water (a liquid) dissolved in air (a gas).
In a solid solution , such as sterling silver, copper that has been heated at high temperatures is mixed with silver that also has been heated until it melts. The copper is the solute , which is the substance that will dissolve into the solvent . The silver is the solvent.
The type of solution is determined by the state of matter of the solvent. If the substance doing the dissolving is a liquid, the solution is called a liquid solution. If the solvent is a gas, the solution is called a gaseous solution. And you guessed right: A solid solvent will form a solid solution.
There are a few factors that generally increase the amount of solute that can be dissolved. If you want to dissolve more sugar in the same amount of water, for instance, you could heat the water. You also could grind the sugar into smaller particles to increase its surface area, or you could stir the mixture.
In the years that you've been using salt and sugar on your foods, you've probably noticed that each piece of salt-which actually is a crystal-is a little smaller than each piece of sugar, which also is a crystal.
The problem you'll be attempting to solve in this experiment is whether sugar or salt dissolves faster when mixed into various liquids. Does the size of the pieces affect how quickly they mix with the liquid?
When you dissolve sugar or salt in a liquid-say, water-what happens is that the sugar molecules move to fit themselves between the molecules of water within a glass or beaker. The illustration below shows how the different molecules are arranged in the container.
In your experiment, you'll see how salt and sugar molecules move within different liquids and dissolve at different rates.
The title of this section, "Salt or Sugar: Which Dissolves Faster in Different Liquids?" could serve as your project title, if you want. You also could consider one of the following titles for your project:
- The Great Salt vs. Sugar Dissolving Contest
- Using Salt and Sugar to Explore How Substances Dissolve
Whatever name you choose is fine. Let's take a minute now to consider why this project is a valuable use of your time.
What's the Point?
And when the excessive solute has been dissolved by heating the solution, it's said to be supersaturated .
The point of this experiment, in addition to learning whether salt or sugar dissolves faster in various liquids, is to learn how molecules interact in a solution.
As you saw in the preceding illustration, the water molecules take up most of the room in the container. But there is still some available space in which the sugar or salt molecules can fit. Through your experiment, you'll learn how fast the sugar molecules fit into those spaces, as compared to the salt particles.
Knowing this will help you better understand the process that occurs as a substance dissolves.
The control in your experiment will be water. The other liquids in which you dissolve salt and sugar will be the variables.
Remember when you conduct your experiment that it's very important that the liquids you use are all the same temperature. You already learned that sugar dissolves faster in a warm liquid than in a cool one, so you know it wouldn't be an accurate experiment if some of the liquids you use are warm and some are cold. The temperature of the liquid would become a variable.
Therefore, all the liquids you use-including water-should be at room temperature. If you normally keep them in the fridge, be sure to allow them to sit out on the counter overnight until they are all the same temperature.
To give you a little more flexibility when you conduct the experiment, you may choose the liquids in which you'll dissolve sugar and salt. There's no point in having to go out and buy additional liquids if you've already got what you need.
Just make sure you choose liquids that are different from each other in taste, color, odor, and purpose. You'll also need to select those that allow you to observe the salt and sugar as it dissolves. If you use milk or orange juice, for example, you won't be able to watch the salt and sugar dissolve. Some suggestions for liquids to consider are:
- White vinegar
- Glass cleaner (such as Windex)
- Tea or iced tea (each at room temperature)
- Apple juice
- Rubbing alcohol
All of these are commonly found around the house, perhaps saving you a trip to the store.
What Do You Think Will Happen?
Now that you know how solutions are formed and some of the factors that will affect the speed at which the sugar and salt you'll be using will dissolve, you should be able to make a good guess as to which one will dissolve faster.
While you won't know until after your experiment if properties of the different liquids you choose will affect the rate at which the salt and sugar dissolve, you do know that salt crystals are generally smaller than sugar crystals. And you know that the temperature of the liquids will not be a factor in your experiment.
Just try to use your past experiences, the information you've read earlier in this section, and your common sense to come up with a sound hypothesis.
Remember that your hypothesis must be stated as an objective sentence, not a question. So go ahead and -make your guess as to whether the salt or sugar will dissolve faster, and let's get started with the experiment.
Materials You'll Need for This Project
Some liquids suggested for use in this experiment are white vinegar, club soda, ginger ale, glass cleaner, rubbing alcohol, apple juice, lemonade, and tea. If you want to substitute another liquid for one or more of the ones suggested, that's fine. Just be sure that all liquids are clear and at room temperature.
The amounts of materials listed below are enough for you to conduct the experiment three times with each liquid. You'll need:
- 12 clear, plastic cups (10 ounce [300 ml])
- One permanent marker
- One (1 teaspoon) (5.0 ml) measuring spoon
- One ( 1 2 teaspoon) (2.5 ml) measuring spoon
- One (1 cup) (240 ml) measuring cup
- 8 teaspoons (40 ml) salt, divided in 16 ( 1 2 teaspoon) portions
- 8 teaspoons (40 ml) sugar, divided in 16 ( 1 2 teaspoon) portions
- 48 ounces (1,440 ml) water at room temperature
- 24 ounces (720 ml) each of five different, clear liquids, all at room temperature
- One clock or watch with a second hand
- One clear plastic cup containing eight fluid ounces (240 ml) water at room temperature
Remember to make sure that all liquids are at room temperature.
Conducting Your Experiment
When you've gathered all your materials, you'll be ready to begin your experiment. Just follow these steps:
- Using the permanent marker, write "salt" on six of the plastic cups, and "sugar" on the other six.
- Place 1 / 2 teaspoon (2.5 ml) of salt into each of the six cups labeled "salt."
- Place 1 / 2 teaspoon (2.5 ml) of sugar into each of the six cups labeled "sugar."
- Add 8 ounces (240 ml) water to one cup containing salt, and one cup containing sugar. Immediately record the time at which the water was added on a data chart similar to the one shown in the next section, "Keeping Track of Your Experiment."
- Observe the solutes (salt and sugar) dissolving in the solvent (water). Record on the data chart the time at which it appears to you that each solute has completely dissolved. These times will probably not be the same.
- Calculate the elapsed time during which the dissolving occurred. Take the time at which the water was added to the cups and the dissolving started, and subtract it from the time the dissolving ended. This gives you the total minutes it took for the salt and sugar to completely dissolve in the liquid.
- Repeat steps 4 through 6, using each different liquid instead of the water.
- Wash, rinse, and thoroughly dry each of the 12 cups.
- Repeat steps 2 through 8 two more times, for a total of three trials for each of the six liquids.
- Calculate an average dissolving time for the salt and the sugar in each of the six liquids.
Remember that to find the average time it took for the salt and sugar to dissolve in each liquid, you add the three times recorded for each one, then divide them by three. The number you get when you divide the times is the average time.
Keeping Track of Your Experiment
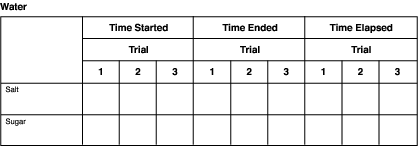
Charts such as the following one can be used to record information for each solvent. Simply change the names of the solvents in the heading.
Be sure to record the times as you go along. Don't depend on your memory to write them down later. You're going to have a lot of numbers by the time you finish your experiment.
Putting It All Together
What did you notice about the rates at which the salt and the sugar dissolved? Did you prove your hypothesis to be correct? Or incorrect? Could you detect any type of pattern as you added the salt and sugar to the various liquids? Was it obvious that the salt dissolved better and faster in some liquids compared to the sugar? Can you think of any reasons for which that might have occurred?
Do you think that the chemical natures of the solute and the solvent affected the dissolving rates? Use the information you gathered when you researched your topic to help you answer these questions.
The more you know about your project, the better able you'll be to analyze your data correctly and come up with a sound conclusion.
Further Investigation
As mentioned earlier, the factors affecting the solubility of solid solutes are:
- Increasing or decreasing the temperature of the solvent
- Increasing the surface area of the solute
If you wanted to take this project a step or two further, you could design an experiment that would test one-or perhaps all-of these variables.
You could easily compare the rate at which sugar cubes dissolve in liquid with the dissolving rate of granulated sugar.
Or you could use the same solute-say, sugar-and test whether stirring the solution caused it to dissolve faster. Heating and cooling the solvent as you add the same solute also would be a possibility for further experimentation.
If you're curious and willing to experiment, you probably can think of many variations for this project. And, because the experiment calls for only common, inexpensive materials, you should be able to experiment to your heart's content.
Easy and fun hands-on chemistry experiment
Students learn about molecules and solutions with this hands-on science activity, performing a series of experiments to determine whether sugar or salt dissolves faster when mixed into various liquids.
Looking for project-based learning?
Check out our collection of hands-on projects that combine math, ELA, and science concepts with 21st Century and social-emotional skills!
Featured Middle School Resources

Related Resources


IMAGES
VIDEO
COMMENTS
Solubility is a key concept in chemistry describing the maximum amount of a solute that can dissolve in a solvent at a given temperature. This experiment explores the solubility of common substances in water, illustrating the impact of molecular structure and temperature on solubility …
What happens when sugar and salt are added to water? Pour in sugar, shake in salt, and evaporate water to see the effects on concentration and conductivity. Zoom in to see how different sugar and salt compounds dissolve.
In this experiment, sugar should dissolve faster in solvents than salt does. The reason for this is because the sugar molecules are bigger than the ions of dissolved salt. This allows for more water molecules to surround a …
Both salt and sugar dissolve in water. However, sugar (sucrose) is much more soluble in alcohol than salt (sodium chloride) is. For all practical purposes, salt is insoluble in alcohol. The solubility of salt is 14 g/kg in …
When you dissolve sugar or salt in a liquid-say, water-what happens is that the sugar molecules move to fit themselves between the molecules of water within a glass or beaker. The illustration below shows how the different molecules are …
Several common liquids, such as water, rubbing alcohol, and club soda, will have solids such as salts, sand, and baking soda added to them to determine which solids dissolve in which liquids at room temperature.
Spoons. Microwave. Stovetop. Pots to heat water. Timer. Design and perform an experiment that explores at least two of the three factors that affect solubility to determine if salt or sugar …