- PRO Courses Guides New Tech Help Pro Expert Videos About wikiHow Pro Upgrade Sign In
- EDIT Edit this Article
- EXPLORE Tech Help Pro About Us Random Article Quizzes Request a New Article Community Dashboard This Or That Game Happiness Hub Popular Categories Arts and Entertainment Artwork Books Movies Computers and Electronics Computers Phone Skills Technology Hacks Health Men's Health Mental Health Women's Health Relationships Dating Love Relationship Issues Hobbies and Crafts Crafts Drawing Games Education & Communication Communication Skills Personal Development Studying Personal Care and Style Fashion Hair Care Personal Hygiene Youth Personal Care School Stuff Dating All Categories Arts and Entertainment Finance and Business Home and Garden Relationship Quizzes Cars & Other Vehicles Food and Entertaining Personal Care and Style Sports and Fitness Computers and Electronics Health Pets and Animals Travel Education & Communication Hobbies and Crafts Philosophy and Religion Work World Family Life Holidays and Traditions Relationships Youth
- Browse Articles
- Learn Something New
- Quizzes Hot
- Happiness Hub
- This Or That Game
- Train Your Brain
- Explore More
- Support wikiHow
- About wikiHow
- Log in / Sign up
- Education and Communications
- Medical Studies

How to Write a Medical Case Study Report
Last Updated: April 18, 2024 Approved
This article was medically reviewed by Mark Ziats, MD, PhD and by wikiHow staff writer, Jennifer Mueller, JD . Dr. Mark Ziats is an Internal Medicine Physician, Scientist, Entrepreneur, and the Medical Director of xBiotech. With over five years of experience, he specializes in biotechnology, genomics, and medical devices. He earned a Doctor of Medicine degree from Baylor College of Medicine, a Ph.D. in Genetics from the University of Cambridge, and a BS in Biochemistry and Chemistry from Clemson University. He also completed the INNoVATE Program in Biotechnology Entrepreneurship at The Johns Hopkins University - Carey Business School. Dr. Ziats is board certified by the American Board of Internal Medicine. There are 15 references cited in this article, which can be found at the bottom of the page. wikiHow marks an article as reader-approved once it receives enough positive feedback. In this case, 100% of readers who voted found the article helpful, earning it our reader-approved status. This article has been viewed 189,706 times.
You've encountered an interesting and unusual case on your rounds, and a colleague or supervising physician says, "Why don't you write up a case study report?" If you've never written one before, that might sound intimidating, but it's a great way to get started in medical writing. Case studies always follow a standard structure and format, so the writing is very formulaic once you get the hang of it. Read on for a step-by-step guide to writing your first case study report.
What is a case study report?

- Medical students or residents typically do the bulk of the writing of the report. If you're just starting your medical career, a case study report is a great way to get a publication under your belt. [2] X Research source

- If the patient is a minor or is incapable of giving informed consent, get consent from their parents or closest relative. [4] X Trustworthy Source PubMed Central Journal archive from the U.S. National Institutes of Health Go to source
- Your hospital likely has specific consent forms to use. Ask your supervising physician if you're not sure where to get one.
- Some journals also have their own consent form. Check your target journal's author or submission information to make sure. [5] X Research source
How is a case study report structured?

- Even though the introduction is the first part of a case study report, doctors typically write it last. You'll have a better idea of how to introduce your case study to readers after you've written it.
- Your abstract comes at the top, before the introduction, and provides a brief summary of the entire report. Unless your case study is published in an open-access journal, the abstract is the only part of the article many readers will see.

- Many journals offer templates and checklists you can use to make sure your case study includes everything necessary and is formatted properly—take advantage of these! Some journals, such as BMJ Case Reports , require all case studies submitted to use their templates.
Drafting Your Medical Case Study Report

- Patient description
- Chronological case history
- Physical exam results
- Results of any pathological tests, imaging, or other investigations
- Treatment plan
- Expected outcome of treatment
- Actual outcome of treatment

- Why the patient sought medical help (you can even use their own words)
- Important information that helped you settle on your diagnosis
- The results of your clinical examination, including diagnostic tests and their results, along with any helpful images
- A description of the treatment plan
- The outcome, including how and why treatment ended and how long the patient was under your care [11] X Trustworthy Source PubMed Central Journal archive from the U.S. National Institutes of Health Go to source

- You will need references to back up symptoms of the condition, common treatment, and the expected outcome of that common treatment.
- Use your research to paint a picture of the usual case of a patient with a similar condition—it'll help you show how unusual and different your patient's case is.
- Generally, aim for around 20 references—no fewer than 15, but no more than 25. [13] X Trustworthy Source PubMed Central Journal archive from the U.S. National Institutes of Health Go to source

- Close your discussion section with a summary of the lessons learned from the case and why it's significant to consider when treating similar cases in the future.
- Outline any open questions that remain. You might also provide suggestions for future research.

- In your conclusion, you might also give suggestions or recommendations to readers based on what you learned as a result of the case.
- Some journals don't want a separate conclusion section. If that's the case for one of your target journals, just move this paragraph to the end of your discussion section.
Polishing Your Report for Submission to Publishers

- Most titles are fewer than 10 words long and include the name of the disease or condition treated.
- You might also include the treatment used and whether the outcome was successful. When deciding what to include, think about the reason you wrote the case study in the first place and why you think it's important for other clinicians to read.

- Made a significant intellectual contribution to the case study report
- Was involved in the medical care of the patient reported
- Can explain and defend the data presented in the report
- Has approved the final manuscript before submission for publication

- Keep in mind that the abstract is not just going to be the first thing people read—it will often be the only thing people read. Make sure that if someone is going to walk away having only read the abstract, they'll still get the same message they would have if they read the whole thing.
- There are 2 basic types of abstract: narrative and structured. A narrative abstract is a single paragraph written in narrative prose. A structured abstract includes headings that correspond with the sections of the paper, then a brief summary of each section. Use the format preferred by your target journal.

- Look for keywords that are relevant to your field or sub-field and directly related to the content of your article, such as the name of the condition or specific treatments you used.
- Most journals allow 4-8 keywords but check the submission guidelines of your target journal to make sure.

- Blur out the patient's face as well as any tattoos, birthmarks, or unrelated scars that are visible in diagnostic images.

- It's common to thank the patient, but that's up to you. Even if you don't, include a statement indicating that you have the patient's written, informed consent to publish the information.
- Read the journal's submission guidelines for a definition of what that journal considers a conflict of interest. They're generally the same, but some might be stricter than others. [22] X Research source

- If you're not familiar with the citation style used by your target journal, check online for a guide. There might also be one available at your hospital or medical school library.
- Medical librarians can also help with citation style and references if you run into something tricky—don't just wing it! Correct citation style insures that readers can access the materials you cite.

- It's also a good idea to get a beta reader who isn't a medical professional. Their comments can help you figure out where you need to clarify your points.
- Read a lot of case studies published in your target journals—it will help you internalize the tone and style that journal is looking for.
Submitting Your Report to Publishers

- Look into the background and reputation of journals before you decide to submit to them. Only seek publication from reputable journals in which articles go through a peer-review process.
- Find out what publishing fees the journals charge. Keep in mind that open-access journals tend to charge higher publishing fees. [26] X Research source
- Read each journal's submission and editorial guidelines carefully. They'll tell you exactly how to format your case study, how long each section should be, and what citation style to use. [27] X Research source
- For electronic journals that only publish case reports, try BMJ Case Reports , Journal of Medical Case Reports , or Radiology Case Reports .

- If your manuscript isn't suitable for the journal you submitted to, the journal might offer to forward it to an associated journal where it would be a better fit.
- When your manuscript is provisionally accepted, the journal will send it to other doctors for evaluation under the peer-review process.
- Most medical journals don't accept simultaneous submissions, meaning you'll have to submit to your first choice, wait for their decision, then move to the next journal on the list if they don't bite.

- Along with your revised manuscript, include a letter with your response to each of the reviewer's comments. Where you made revisions, add page numbers to indicate where the revisions are that address that reviewer's comments.
- Sometimes, doctors involved in the peer review process will indicate that the journal should reject the manuscript. If that's the case, you'll get a letter explaining why your case study report won't be published and you're free to submit it elsewhere.

- Some journals require you to have your article professionally copy-edited at your own cost while others do this in-house. The editors will let you know what you're responsible for.

- With your acceptance letter, you'll get instructions on how to make payment and how much you owe. Take note of the deadline and make sure you pay it as soon as possible to avoid publication delays.
- Some journals will publish for free, with an "open-access option" that allows you to pay a fee only if you want open access to your article. [32] X Research source

- Through the publishing agreement, you assign your copyright in the article to the journal. This allows the journal to legally publish your work. That assignment can be exclusive or non-exclusive and may only last for a specific term. Read these details carefully!
- If you published an open-access article, you don't assign the copyright to the publisher. The publishing agreement merely gives the journal the right to publish the "Version of Record." [33] X Research source
How do I find a suitable case for a report?

- A rare disease, or unusual presentation of any disease
- An unusual combination of diseases or conditions
- A difficult or inconclusive diagnosis
- Unexpected developments or responses to treatment
- Personal impact
- Observations that shed new light on the patient's disease or condition

- There might be other members of your medical team that want to help with writing. If so, use one of these brainstorming sessions to divvy up writing responsibilities in a way that makes the most sense given your relative skills and experience.
- Senior doctors might also be able to name some journals that would potentially publish your case study. [36] X Research source
Expert Q&A
You Might Also Like

- ↑ https://www.elsevier.com/connect/authors-update/the-dos-and-donts-of-writing-and-publishing-case-reports
- ↑ https://www.bmj.com/content/350/bmj.h2693
- ↑ https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5686928/
- ↑ https://health.usf.edu/medicine/internalmedicine/im-impact/~/media/B3A3421F4C144FA090AE965C21791A3C.ashx
- ↑ https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2597880/
- ↑ https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6476221/
- ↑ https://www.springer.com/gp/authors-editors/authorandreviewertutorials/writing-a-journal-manuscript/title-abstract-and-keywords/10285522
- ↑ http://www.ncbi.nlm.nih.gov/pmc/articles/PMC2597880/
- ↑ https://thelancet.com/pb/assets/raw/Lancet/authors/tl-info-for-authors.pdf
- ↑ https://jmedicalcasereports.biomedcentral.com/articles/10.1186/s13256-017-1351-y
- ↑ https://guides.himmelfarb.gwu.edu/casereports
- ↑ https://casereports.bmj.com/pages/authors/
- ↑ https://jmedicalcasereports.biomedcentral.com/articles/10.1186/1752-1947-7-239
- ↑ https://research.chm.msu.edu/students-residents/writing-a-case-report
- ↑ https://authorservices.taylorandfrancis.com/publishing-your-research/moving-through-production/copyright-for-journal-authors/#
About This Article

Medical Disclaimer
The content of this article is not intended to be a substitute for professional medical advice, examination, diagnosis, or treatment. You should always contact your doctor or other qualified healthcare professional before starting, changing, or stopping any kind of health treatment.
Read More...
To start a medical case study report, first choose a title that clearly reflects the contents of the report. You’ll also need to list any participating authors and develop a list of keywords, as well as an abstract summarizing the report. Your report will need to include an introduction summarizing the context of the report, as well as a detailed presentation of the case. Don’t forget to include a thorough citation list and acknowledgements of anyone else who participated in the study. For more tips from our Medical co-author, including how to get your case study report published, keep reading! Did this summary help you? Yes No
- Send fan mail to authors
Reader Success Stories
Sep 5, 2020
Did this article help you?

Asfia Banu Pasha
Apr 10, 2017
Jun 20, 2021
Mar 1, 2017

Featured Articles

Trending Articles

Watch Articles

- Terms of Use
- Privacy Policy
- Do Not Sell or Share My Info
- Not Selling Info
wikiHow Tech Help Pro:
Level up your tech skills and stay ahead of the curve
Case Report: A Beginner’s Guide with Examples
A case report is a descriptive study that documents an unusual clinical phenomenon in a single patient. It describes in details the patient’s history, signs, symptoms, test results, diagnosis, prognosis and treatment. It also contains a short literature review, discusses the importance of the case and how it improves the existing knowledge on the subject.
A similar design involving a group of patients (with the similar problem) is referred to as case series.
Advantages of case reports
Case reports offer, in general a fast, easy and cheap way to report an unusual observation or a rare event in a clinical setting, as these have very small probability of being detected in an experimental study because of limitations on the number of patients that can be included.
These events deserve to be reported since they might provide insights on some exceptions to general rules and theories in the field.
Case reports are great to get first impressions that can generate new hypotheses (e.g. detecting a potential side effect of a drug) or challenge existing ones (e.g. shedding the light on the possibility of a different biological mechanism of a disease).
In many of these cases, additional investigation is needed such as designing large observational studies or randomized experiments or even going back and mining data from previous research looking for evidence for theses hypotheses.
Limitations of case reports
Observing a relationship between an exposure and a disease in a case report does not mean that it is causal in nature.
This is because of:
- The absence of a control group that provides a benchmark or a point of reference against which we compare our results. A control group is important to eliminate the role of external factors which can interfere with the relationship between exposure and disease
- Unmeasured Confounding caused by variables that influence both the exposure and the disease
A case report can have a powerful emotional effect (see examples of case reports below). This can lead to overrate the importance of the evidence provided by such case. In his book Against Empathy: The Case for Rational Compassion , Paul Bloom explains how a powerful story affects our emotions, can distort our judgement and even lead us to make bad moral choices.
When a case report describes a rare event it is important to remember that what we’re reading about is exceptional and most importantly resist generalizations especially because a case report is, by definition, a study where the sample is only 1 patient.
Selection bias is another issue as the cases in case reports are not chosen at random, therefore some members of the population may have a higher probability of being included in the study than others.
So, results from a case report cannot be representative of the entire population.
Because of these limitations, case reports have the lowest level of evidence compared to other study designs as represented in the evidence pyramid below:
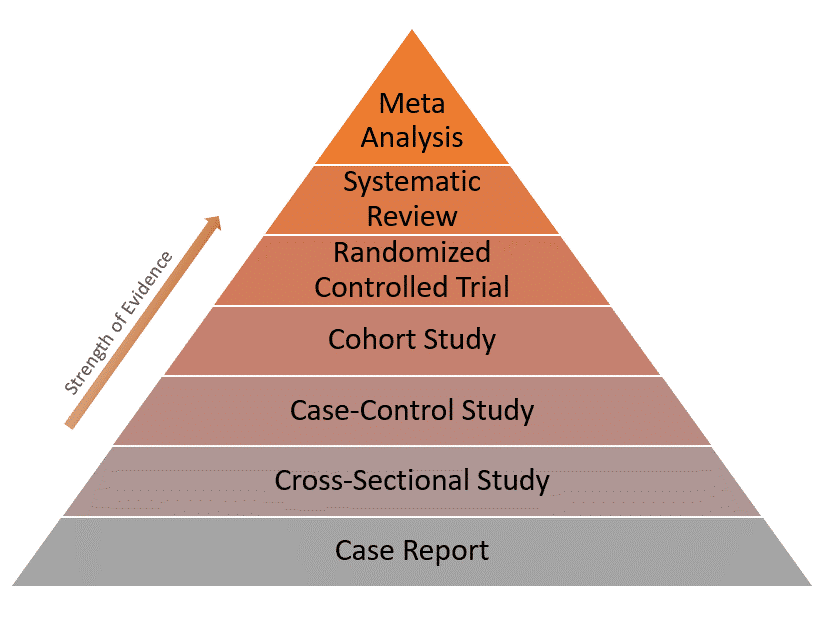
Real-world examples of case reports
Example 1: normal plasma cholesterol in an 88-year-old man who eats 25 eggs a day.
This is the case of an old man with Alzheimer’s disease who has been eating 20-30 eggs every day for almost 15 years. [ Source ]
The man had an LDL-cholesterol level of only 142 mg/dL (3.68 mmol/L) and no significant clinical atherosclerosis (deposition of cholesterol in arterial walls)!
His body adapted by reducing the intestinal absorption of cholesterol, lowering the rate of its synthesis and increasing the rate of its conversion into bile acid.
This is indeed an unusual case of biological adaptation to a major change in dietary intake.
Example 2: Recovery from the passage of an iron bar through the head
This is an interesting case of a construction foreman named Phineas Gage. [ Source ]
In 1848, due to an explosion at work, an iron bar passed through his head destroying a large portion of his brain’s frontal lobe. He survived the event and the injury only affected 1 thing: His personality!
After the accident, Gage became profane, rough and disrespectful to the extent that he was no longer tolerable to people around him. So he lost his job and his family.
His case inspired further research that focused on the relationship between specific parts of the brain and personality.
- Sayre JW, Toklu HZ, Ye F, Mazza J, Yale S. Case Reports, Case Series – From Clinical Practice to Evidence-Based Medicine in Graduate Medical Education . Cureus . 2017;9(8):e1546. Published 2017 Aug 7. doi:10.7759/cureus.1546.
- Nissen T, Wynn R. The clinical case report: a review of its merits and limitations . BMC Res Notes . 2014;7:264. Published 2014 Apr 23. doi:10.1186/1756-0500-7-264.
Further reading
- Case Report vs Cross-Sectional Study
- Cohort vs Cross-Sectional Study
- How to Identify Different Types of Cohort Studies?
- Matched Pairs Design
- Randomized Block Design

Writing a Case Report
This page is intended for medical students, residents or others who do not have much experience with case reports, but are planning on writing one.
What is a case report? A medical case report, also known as a case study, is a detailed description of a clinical encounter with a patient. The most important aspect of a case report, i.e. the reason you would go to the trouble of writing one, is that the case is sufficiently unique, rare or interesting such that other medical professionals will learn something from it.
Case reports are commonly of the following categories :
- Rare diseases
- Unusual presentation of disease
- Unexpected events
- Unusual combination of diseases or conditions
- Difficult or inconclusive diagnosis
- Treatment or management challenges
- Personal impact
- Observations that shed new light on a disease or condition
- Anatomical variations
It is important that you recognize what is unique or interesting about your case, and this must be described clearly in the case report.
Case reports generally take the format of :
1. Background
2. Case presentation
3. Observations and investigation
4. Diagnosis
5. Treatment
7. Discussion
Does a case report require IRB approval?
Case reports typically discuss a single patient. If this is true for your case report, then it most likely does not require IRB approval because it not considered research. If you have more than one patient, your study could qualify as a Case Series, which would require IRB review. If you have questions, you chould check your local IRB's guidelines on reviewing case reports.
Are there other rules for writing a case report?
First, you will be collecting protected health information, thus HIPAA applies to case reports. Spectrum Health has created a very helpful guidance document for case reports, which you can see here: Case Report Guidance - Spectrum Health
While this guidance document was created by Spectrum Health, the rules and regulations outlined could apply to any case report. This includes answering questions like: Do I need written HIPAA authorization to publish a case report? When do I need IRB review of a case report? What qualifies as a patient identifier?
How do I get started?
1. We STRONGLY encourage you to consult the CARE Guidelines, which provide guidance on writing case reports - https://www.care-statement.org/
Specifically, the checklist - https://www.care-statement.org/checklist - which explains exactly the information you should collect and include in your case report.
2. Identify a case. If you are a medical student, you may not yet have the clinical expertise to determine if a specific case is worth writing up. If so, you must seek the help of a clinician. It is common for students to ask attendings or residents if they have any interesting cases that can be used for a case report.
3. Select a journal or two to which you think you will submit the case report. Journals often have specific requirements for publishing case reports, which could include a requirement for informed consent, a letter or statement from the IRB and other things. Journals may also charge publication fees (see Is it free to publish? below)
4. Obtain informed consent from the patient (see " Do I have to obtain informed consent from the patient? " below). Journals may have their own informed consent form that they would like you to use, so please look for this when selecting a journal.
Once you've identified the case, selected an appropriate journal(s), and considered informed consent, you can collect the required information to write the case report.
How do I write a case report?
Once you identify a case and have learned what information to include in the case report, try to find a previously published case report. Finding published case reports in a similar field will provide examples to guide you through the process of writing a case report.
One journal you can consult is BMJ Case Reports . MSU has a multi-year open access publishing agreement with the publisher BM J , and an institutional fellowship with BMJ Case Reports which allows MSU faculty, staff and students to publish in this journal for free. See this page for a link to the journal and more information on publishing- https://lib.msu.edu/collections/scholcomm/support
There are numerous other journals where you can find published case reports to help guide you in your writing.
Do I have to obtain informed consent from the patient?
The CARE guidelines recommend obtaining informed consent from patients for all case reports. Our recommendation is to obtain informed consent from the patient. Although not technically required, especially if the case report does not include any identifying information, some journals require informed consent for all case reports before publishing. The CARE guidelines recommend obtaining informed consent AND the patient's perspective on the treatment/outcome (if possible). Please consider this as well.
If required, it is recommended you obtain informed consent before the case report is written.
An example of a case report consent form can be found on the BMJ Case Reports website, which you can access via the MSU library page - https://casereports.bmj.com/ . Go to "Instructions for Authors" and then "Patient Consent" to find the consent form they use. You can create a similar form to obtain consent from your patient. If you have identified a journal already, please consult their requirements and determine if they have a specific consent form they would like you to use.
Seek feedback
Once you have written a draft of the case report, you should seek feedback on your writing, from experts in the field if possible, or from those who have written case reports before.
Selecting a journal
Aside from BMJ Case Reports mentioned above, there are many, many journals out there who publish medical case reports. Ask your mentor if they have a journal they would like to use. If you need to select on your own, here are some strategies:
1. Do a PubMed search. https://pubmed.ncbi.nlm.nih.gov/
a. Do a search for a topic, disease or other feature of your case report
b. When the results appear, on the left side of the page is a limiter for "article type". Case reports are an article type to which you can limit your search results. If you don't see that option on the left, click "additional filters".
c. Review the case reports that come up and see what journals they are published in.
2. Use JANE - https://jane.biosemantics.org/
3. Check with specialty societies. Many specialty societies are affiliated with one or more journal, which can be reviewed for ones that match your needs
4. Search through individual publisher journal lists. Elsevier publishes many different medical research journals, and they have a journal finder, much like JANE ( https://journalfinder.elsevier.com/ ). This is exclusive to Elsevier journals. There are many other publishers of medical journals for review, including Springer, Dove Press, BMJ, BMC, Wiley, Sage, Nature and many others.
Is it free to publish ?
Be aware that it may not be free to publish your case report. Many journals charge publication fees. Of note, many open access journals charge author fees of thousands of dollars. Other journals have smaller page charges (i.e. $60 per page), and still others will publish for free, with an "open access option". It is best practice to check the journal's Info for Authors section or Author Center to determine what the cost is to publish. MSU-CHM does NOT have funds to support publication costs, so this is an important step if you do not want to pay out of pocket for publishing
*A more thorough discussion on finding a journal, publication costs, predatory journals and other publication-related issues can be found here: https://research.chm.msu.edu/students-residents/finding-a-journal
Gagnier JJ, Kienle G, Altman DG, Moher D, Sox H, Riley D. 2013. The CARE guidelines: Consensus-based clinical case reporting guideline development. Glob Adv Health Med . 2:38-43. doi: 10.7453/gahmj.2013.008
Riley DS, Barber MS, Kienle GS, AronsonJK, von Schoen-Angerer T, Tugwell P, Kiene H, Helfand M, Altman DG, Sox H, Werthmann PG, Moher D, Rison RA, Shamseer L, Koch CA, Sun GH, Hanaway P, Sudak NL, Kaszkin-Bettag M, Carpenter JE, Gagnier JJ. 2017. CARE guidelines for case reports: explanation and elaboration document. J Clin Epidemiol . 89:218-234. doi: 10.1016/j.jclinepi.2017.04.026
Guidelines to writing a clinical case report. 2017. Heart Views . 18:104-105. doi: 10.4103/1995-705X.217857
Ortega-Loubon C, Culquichicon C, Correa R. The importance of writing and publishing case reports during medical education. 2017. Cureus. 9:e1964. doi: 10.7759/cureus.1964
Writing and publishing a useful and interesting case report. 2019. BMJ Case Reports. Link to document
Camm CF. Writing an excellent case report: EHJ Case Reports , Case of the Year 2019. 2020. European Heart Jounrnal. 41:1230-1231. https://doi.org/10.1093/eurheartj/ehaa176
*content developed by Mark Trottier, PhD
- Search Menu
Sign in through your institution
- Advance articles
- AHFS First Release
- AJHP Voices
- AJHP Residents Edition
- Supplements
- Top Twenty-Five Articles
- ASHP National Surveys of Pharmacy Practice in Hospital Settings
- Medication Safety
- Pharmacy Technicians
- Specialty Pharmacy
- Emergency Preparedness and Clinician Well-being
- Author Guidelines
- Submission Site
- Open Access
- Information for Reviewers
- Self-Archiving Policy
- Author Instructions for Residents Edition
- Advertising and Corporate Services
- Advertising
- Reprints and ePrints
- Sponsored Supplements
- Editorial Board
- Permissions
- Journals on Oxford Academic
- Books on Oxford Academic
- < Previous
How to write a patient case report
- Article contents
- Figures & tables
- Supplementary Data
Henry Cohen, How to write a patient case report, American Journal of Health-System Pharmacy , Volume 63, Issue 19, 1 October 2006, Pages 1888–1892, https://doi.org/10.2146/ajhp060182
- Permissions Icon Permissions
Purpose. Guidelines for writing patient case reports, with a focus on medication-related reports, are provided.
Summary. The format of a patient case report encompasses the following five sections: an abstract, an introduction and objective that contain a literature review, a description of the case report, a discussion that includes a detailed explanation of the literature review, a summary of the case, and a conclusion. The abstract of a patient case report should succinctly include the four sections of the main text of the report. The introduction section should provide the subject, purpose, and merit of the case report. It must explain why the case report is novel or merits review, and it should include a comprehensive literature review that corroborates the author’s claims. The case presentation section should describe the case in chronological order and in enough detail for the reader to establish his or her own conclusions about the case’s validity. The discussion section is the most important section of the case report. It ought to evaluate the patient case for accuracy, validity, and uniqueness; compare and contrast the case report with the published literature; derive new knowledge; summarize the essential features of the report; and draw recommendations. The conclusion section should be brief and provide a conclusion with evidence-based recommendations and applicability to practice.
Conclusion. Patient case reports are valuable resources of new and unusual information that may lead to vital research.
American Society of Health-System Pharmacists members
Personal account.
- Sign in with email/username & password
- Get email alerts
- Save searches
- Purchase content
- Activate your purchase/trial code
- Add your ORCID iD
Institutional access
Sign in with a library card.
- Sign in with username/password
- Recommend to your librarian
- Institutional account management
- Get help with access
Access to content on Oxford Academic is often provided through institutional subscriptions and purchases. If you are a member of an institution with an active account, you may be able to access content in one of the following ways:
IP based access
Typically, access is provided across an institutional network to a range of IP addresses. This authentication occurs automatically, and it is not possible to sign out of an IP authenticated account.
Choose this option to get remote access when outside your institution. Shibboleth/Open Athens technology is used to provide single sign-on between your institution’s website and Oxford Academic.
- Click Sign in through your institution.
- Select your institution from the list provided, which will take you to your institution's website to sign in.
- When on the institution site, please use the credentials provided by your institution. Do not use an Oxford Academic personal account.
- Following successful sign in, you will be returned to Oxford Academic.
If your institution is not listed or you cannot sign in to your institution’s website, please contact your librarian or administrator.
Enter your library card number to sign in. If you cannot sign in, please contact your librarian.
Society Members
Society member access to a journal is achieved in one of the following ways:
Sign in through society site
Many societies offer single sign-on between the society website and Oxford Academic. If you see ‘Sign in through society site’ in the sign in pane within a journal:
- Click Sign in through society site.
- When on the society site, please use the credentials provided by that society. Do not use an Oxford Academic personal account.
If you do not have a society account or have forgotten your username or password, please contact your society.
Sign in using a personal account
Some societies use Oxford Academic personal accounts to provide access to their members. See below.
A personal account can be used to get email alerts, save searches, purchase content, and activate subscriptions.
Some societies use Oxford Academic personal accounts to provide access to their members.
Viewing your signed in accounts
Click the account icon in the top right to:
- View your signed in personal account and access account management features.
- View the institutional accounts that are providing access.
Signed in but can't access content
Oxford Academic is home to a wide variety of products. The institutional subscription may not cover the content that you are trying to access. If you believe you should have access to that content, please contact your librarian.
For librarians and administrators, your personal account also provides access to institutional account management. Here you will find options to view and activate subscriptions, manage institutional settings and access options, access usage statistics, and more.
Short-term Access
To purchase short-term access, please sign in to your personal account above.
Don't already have a personal account? Register
| Month: | Total Views: |
|---|---|
| January 2019 | 253 |
| February 2019 | 915 |
| March 2019 | 810 |
| April 2019 | 784 |
| May 2019 | 759 |
| June 2019 | 735 |
| July 2019 | 687 |
| August 2019 | 511 |
| September 2019 | 377 |
| October 2019 | 265 |
| November 2019 | 237 |
| December 2019 | 97 |
| January 2020 | 205 |
| February 2020 | 151 |
| March 2020 | 143 |
| April 2020 | 103 |
| May 2020 | 115 |
| June 2020 | 133 |
| July 2020 | 129 |
| August 2020 | 154 |
| September 2020 | 133 |
| October 2020 | 147 |
| November 2020 | 85 |
| December 2020 | 84 |
| January 2021 | 104 |
| February 2021 | 105 |
| March 2021 | 108 |
| April 2021 | 153 |
| May 2021 | 109 |
| June 2021 | 87 |
| July 2021 | 124 |
| August 2021 | 113 |
| September 2021 | 126 |
| October 2021 | 126 |
| November 2021 | 101 |
| December 2021 | 137 |
| January 2022 | 105 |
| February 2022 | 105 |
| March 2022 | 149 |
| April 2022 | 186 |
| May 2022 | 116 |
| June 2022 | 155 |
| July 2022 | 115 |
| August 2022 | 111 |
| September 2022 | 176 |
| October 2022 | 123 |
| November 2022 | 126 |
| December 2022 | 101 |
| January 2023 | 151 |
| February 2023 | 127 |
| March 2023 | 168 |
| April 2023 | 147 |
| May 2023 | 103 |
| June 2023 | 50 |
| July 2023 | 76 |
| August 2023 | 117 |
| September 2023 | 94 |
| October 2023 | 138 |
| November 2023 | 115 |
| December 2023 | 76 |
| January 2024 | 103 |
| February 2024 | 108 |
| March 2024 | 108 |
| April 2024 | 85 |
| May 2024 | 63 |
| June 2024 | 58 |
| July 2024 | 94 |
| August 2024 | 74 |
| September 2024 | 59 |

Email alerts
Citing articles via.
- Recommend to Your Librarian
Affiliations
- Online ISSN 1535-2900
- Print ISSN 1079-2082
- Copyright © 2024 American Society of Health-System Pharmacists
- About Oxford Academic
- Publish journals with us
- University press partners
- What we publish
- New features
- Open access
- Rights and permissions
- Accessibility
- Media enquiries
- Oxford University Press
- Oxford Languages
- University of Oxford
Oxford University Press is a department of the University of Oxford. It furthers the University's objective of excellence in research, scholarship, and education by publishing worldwide
- Copyright © 2024 Oxford University Press
- Cookie settings
- Cookie policy
- Privacy policy
- Legal notice
This Feature Is Available To Subscribers Only
Sign In or Create an Account
This PDF is available to Subscribers Only
For full access to this pdf, sign in to an existing account, or purchase an annual subscription.

The Carl T. Hayden Veterans Affairs (VA) Medical Center in Phoenix, Arizona, demonstrates that grid-interactive efficient building (GEB) strategies and technologies can be realistically deployed across buildings with substantial energy and cost savings.
The project implemented both energy conservation measures (ECMs) and grid-interactive technologies and controls strategies, making it a leading example of a smart, sustainable, and efficient commercial building.
Ultimately, these ECM retrofits helped the medical center achieve over 25% energy savings and an ENERGY STAR ® rating of 99, making it one of the most energy-efficient medical centers in the United States.
This case study highlights the challenges and the lessons learned throughout the project development and implementation, in addition to some of the best practices for project management, operations, load shifting and peak management, and heating, ventilation, and air-conditioning (HVAC) system management.
Download the case study.
Quick Links
- - Google Chrome
Intended for healthcare professionals
- My email alerts
- BMA member login
- Username * Password * Forgot your log in details? Need to activate BMA Member Log In Log in via OpenAthens Log in via your institution

Search form
- Advanced search
- Search responses
- Search blogs
- How to present patient...
How to present patient cases
- Related content
- Peer review
- Mary Ni Lochlainn , foundation year 2 doctor 1 ,
- Ibrahim Balogun , healthcare of older people/stroke medicine consultant 1
- 1 East Kent Foundation Trust, UK
A guide on how to structure a case presentation
This article contains...
-History of presenting problem
-Medical and surgical history
-Drugs, including allergies to drugs
-Family history
-Social history
-Review of systems
-Findings on examination, including vital signs and observations
-Differential diagnosis/impression
-Investigations
-Management
Presenting patient cases is a key part of everyday clinical practice. A well delivered presentation has the potential to facilitate patient care and improve efficiency on ward rounds, as well as a means of teaching and assessing clinical competence. 1
The purpose of a case presentation is to communicate your diagnostic reasoning to the listener, so that he or she has a clear picture of the patient’s condition and further management can be planned accordingly. 2 To give a high quality presentation you need to take a thorough history. Consultants make decisions about patient care based on information presented to them by junior members of the team, so the importance of accurately presenting your patient cannot be overemphasised.
As a medical student, you are likely to be asked to present in numerous settings. A formal case presentation may take place at a teaching session or even at a conference or scientific meeting. These presentations are usually thorough and have an accompanying PowerPoint presentation or poster. More often, case presentations take place on the wards or over the phone and tend to be brief, using only memory or short, handwritten notes as an aid.
Everyone has their own presenting style, and the context of the presentation will determine how much detail you need to put in. You should anticipate what information your senior colleagues will need to know about the patient’s history and the care he or she has received since admission, to enable them to make further management decisions. In this article, I use a fictitious case to …
Log in using your username and password
BMA Member Log In
If you have a subscription to The BMJ, log in:
- Need to activate
- Log in via institution
- Log in via OpenAthens
Log in through your institution
Subscribe from £184 *.
Subscribe and get access to all BMJ articles, and much more.
* For online subscription
Access this article for 1 day for: £50 / $60/ €56 ( excludes VAT )
You can download a PDF version for your personal record.
Buy this article
Call/Text/Whatsapp:
+1 (888-687-4420)
24/7/365 Available
- College Essay
- Argumentative Essay
- Expository Essay
- Narrative Essay
- Descriptive Essay
- Scholarship Essay
- Admission Essay
- Reflective Essay
- Nursing Essay
- Economics Essay
Assignments
- Term Papers
- Research Papers
- Case Studies
- Dissertation
- Presentation
- Editing Help
- Cheap Essay Writing
- How to Order
Writing A Case Study
Case Study Format
Simple Case Study Format for Students to Follow

People also read
A Complete Case Study Writing Guide With Examples
Understand the Types of Case Study Here
Brilliant Case Study Examples and Templates For Your Help
Having trouble making your case studies stand out? Finding it hard to organise your story? You're not alone!
Many students struggle with case study writing !
Imagine spending a lot of time on your case studies, but they don't grab your reader's interest. But don't worry!
In this guide, we will go step by step through case study formatting, along with practical tips to make your research stand out from the rest! By following our step-by-step approach, you can understand how to write a case study assignment well.
So, let’s get started!

Paper Due? Why Suffer? That's our Job!
- 1. How to Format a Case Study
- 2. Case Study Format Template
- 3. Case Study Format Examples
How to Format a Case Study
When it comes to crafting a compelling case study, understanding how to write case study format is key to presenting your research effectively.
If you are wondering how to make case study format, here are the elements to include in your case study paper format.
Create an interesting title for your work. Keep it simple and short.
Here you need to briefly elaborate on the accomplishment. What you have done and how you got there.
Write about the entire story in one paragraph followed by 2-3 bullet points to display the case study contents.
An introduction about what the case study is all about.
Describe the challenges of the customer prior to using your product or service. Explain the long-term goals or objectives that the customer set out to achieve.
In this 2-3 paragraph section describe how your product or service specifically benefited and helped achieve the goals. You can also use percentages to show your contributions.
In the relevant section of your case study, add 1-2 quotes and visuals to support the story you are telling. You can also use icons to summarise information and highlight areas of your research.
Figure out what a study means and look at where else we can learn more are really important for making academic work have a bigger impact.
Call to action is optional but adding one can encourage your readers to take some action after learning your work.
Case Study Formatting Guidelines
Effective case study formatting is essential to convey your insights clearly and engage your audience. Follow these guidelines to ensure your case study is well-organised and impactful:
- Opt for easily readable fonts like Arial, Calibri, or Times New Roman.
- Maintain a consistent font size, typically 12 points for the body text.
- Set line spacing to double-spaced for the entire document.
- Use bullet points for concise and scannable information presentation.
- Employ numbered lists for sequences of steps or chronological order of events.
- Bold or italicize key phrases to draw attention to critical points; use underline sparingly.
- Choose left, center, or justified alignment based on your overall design.
- Make your headings clear and organized so readers know what's important.
If you need further assistance, check our case study format for students pdf here:
How To Write A Case Study Pdf
Case Study Format Template
Case studies can be used for different purposes. In social sciences, it can help you understand the problems of other people.
In businesses, it can help you earn the trust of potential customers. But do you even know what are the different types of case study and how to write one?
Refer to this case study format pdf before you start writing your own document. This student case study format sample contains all the information you might need when gathering information for your case study.
Case Study Format Examples
Case study examples are the best way to learn the basic techniques for writing a great case study on your own.
Explore these short case study sample pdfs to gain insights into presenting your research cohesively:
For your help, we have also compiled real-life case study examples along with a format that you can refer to while writing your own.
APA Case Study Format
If you are asked to write a case study in APA format, keep in mind there are some specific requirements that you need to adhere to.
Here is a case study APA format example for you to learn how to format a case study.
Business Case Study Format
Business case studies can help businesses sell products or services to prospects. Here is a perfect example for you to learn how to write an impressive business case study.
Case Study Format For MBA Students
Case Study Format Nursing
Writing a great nursing case study can be tough. That’s why we have provided a case study format for nursing students to use as a guide in creating their work.
Refer to this family case study format example if you are writing a nursing case study for the first time.
Nursing Case Study Format
Harvard Business School Case Study Format
Looking for HBS style business case study? Here is one for you to read and take hints and ideas to prepare this type of case study like a professional.
Tough Essay Due? Hire Tough Writers!
Medical Case Study Format
Writing medical case studies is helpful in medical practices as it gives a lot of information about different diseases. Look at this example and learn how to write a detailed medical case study.
Case Study Format Psychology
To study how the human mind works, you need a clear and organised method. Follow this easy psychology case study format to explore the details of psychological research:
Case Study Format Psychology
To sum it up, getting good at writing case studies means combining a clear structure, good storytelling, and smart presentation. If you follow the tips I've shared in this blog, you're on your way to crafting engaging stories that grab people's attention.
If your case study is causing problems, consider getting professional help. Our essay service is designed to help you secure top grades by meeting the criteria set by professors.
Our skilled writers are here to assist with any type of assignment you may have. Explore our case study writing service to relieve your stress and excel academically.
Write Essay Within 60 Seconds!

Dr. Barbara is a highly experienced writer and author who holds a Ph.D. degree in public health from an Ivy League school. She has worked in the medical field for many years, conducting extensive research on various health topics. Her writing has been featured in several top-tier publications.

Paper Due? Why Suffer? That’s our Job!
Keep reading
Redirect Notice
Inclusion of women and minorities as participants in research involving human subjects.
Learn about the policy for the Inclusion of Women and Minorities in NIH-funded research and how to comply with this policy in applications and progress reports.
NIH is mandated by the Public Health Service Act sec. 492B, 42 U.S.C. sec. 289a-2 to ensure the inclusion of women and members of racial and ethnic minority groups in all NIH-funded clinical research in a manner that is appropriate to the scientific question under study. The primary goal of this law is to ensure that research findings can be generalizable to the entire population. Additionally, the statute requires clinical trials to be designed to analyze whether study outcomes differ for women and members of racial and ethnic minority groups.
Implementation
Applications & proposals.
All NIH-funded studies that meet the NIH definition for clinical research must address plans for the inclusion of women and minorities within the application or proposal. Using the PHS Human Subjects and Clinical Trial Information Form, applications and proposals should describe the composition of the proposed study population in terms of sex or gender, racial, and ethnic groups, and provide a rationale for the proposed section. Any exclusions based on sex or gender, race, or ethnicity must include a rationale and justification based on a scientific or ethical basis. Investigators should also plan for appropriate outreach programs and activities to recruit and retain the proposed study population consistent with the purposes of the research project. Refer to the PHS Human Subjects and Clinical Trial Information Form Instructions for complete guidance on what to address in your application.
Peer Review
Scientific Review Groups will assess each application/proposal as being "acceptable" or "unacceptable" with regard to the inclusion of racial and ethnic minorities and women in the research project. For additional information on review considerations, refer to the Guidelines for the Review of Inclusion in Clinical Research . For information regarding the coding used to rate inclusion during peer review, see the list of NIH Peer Review Inclusion Codes .
Progress Reports
NIH recipients/offerors must collect and annually report information on sex or gender race, and ethnicity in progress reports. Refer to this Decision Tree for help determining reporting expectations for different types of studies.
Special Considerations for NIH-defined Phase III Clinical Trials
Applications & Proposals: If the proposed research includes an NIH-defined Phase III Clinical Trial , evidence must be reviewed to show whether or not clinically important differences in the intervention effect by sex or gender, race, and/or ethnicity are to be expected. The application or proposal must address plans for the valid analysis of group differences on the basis of sex or gender, race, and ethnicity unless there is clear evidence that such differences are unlikely to be seen.
Progress Reports: For projects involving NIH-defined Phase III Clinical Trials, annual Research Performance Progress Reports (RPPRs) should include a statement indicating the status of analyses of the primary outcome by sex or gender, race, and ethnicity. The results of these analyses should be included in the “Project Outcomes” section of the RPPR. See the Sample Project Outcomes page for an example.
Registering & Reporting in ClinicalTrials.gov: NIH-defined Phase III Clinical Trials that also meet the definition of an applicable clinical trial must report the results of the valid analysis of group differences in ClinicalTrials.gov. The valid analyses should be done for each primary outcome measure by sex or gender, and race and/or ethnicity. Upon study registration in ClinicalTrials.gov, outcome measures should be pre-specified by sex or gender, and race and/or ethnicity to prepare for reporting results in this stratified manner. Refer to the Guidance for Valid Analysis Reporting and NOT-OD-18-014 for additional information.
Policy Notices and Procedures
| Amendment: NIH Policy and Guidelines on the Inclusion of Women and Minorities as Subjects in Clinical Research | Amendment to the on the inclusion of women and minorities as subjects in clinical research. Includes requirement that recipients conducting applicable NIH-defined Phase III clinical trials ensure results of valid analyses by sex or gender, race, and/or ethnicity are submitted to ClinicalTrials.gov. | November 28, 2017 |
| NIH Policy and Guidelines on the Inclusion of Women and Minorities as Subjects in Clinical Research – Amended | Updated NIH policy on the inclusion of women and minorities as subjects in clinical research, which supersedes the and . | October 9, 2001 |
| NIH Policy and Guidelines on the Inclusion of Women and Minorities as Subjects in Clinical Research | Consolidated and concise summary of the on the inclusion of women and minorities in clinical research. | October 9, 2001 |
| NIH Policy on Reporting Race and Ethnicity Data: Subjects in Clinical Research | Additional guidance and instruction for using the revised minimum standards for maintaining, collecting, and presenting data on race and ethnicity. | August 8, 2001 |
| Infographic that walks through the elements of the existing dataset or resource definition to help users understand whether how it applies to their research. | August 2, 2024 | |
| This one-page resource highlights allowable costs for NIH grants that can be utilized to enhance inclusion through recruitment and retention activities. Allowable costs listed in the NIH Grants Policy Statement are provided with examples of inclusion-related activities. | August 10, 2023 | |
| May 19, 2022 | ||
| In Part 1 of this NIH All About Grants podcast miniseries, NIH’s Inclusion Policy Officer Dawn Corbett tells us how to consider inclusion plans when putting together an application. | April 20, 2022 | |
| NIH’s Inclusion Policy Officer Dawn Corbett covers inclusion plans during peer review and post-award in Part 2 of this NIH All About Grants podcast miniseries. | April 20, 2022 | |
| : Recruitment and Retention | Document listing resources on recruitment and retention of women, racial and ethnic minorities, and individuals across the lifespan. Resources include toolkits, articles, and more. | May 9, 2022 |
| Analyses by Sex or Gender, Race and Ethnicity for NIH-defined Phase III Clinical Trials | Guidance for understanding the definition of valid analysis and links to key resources for investigators and recipeients | March 8, 2022 |
| : Including Diverse Populations in NIH-funded Clinical Research | Video presentation by the NIH Inclusion Policy Officer for the NIH Grants Conference PreCon event, Human Subjects Research: Policies, Clinical Trials, & Inclusion, in December 2022. The presentation explains NIH inclusion policies and requirements for applicants and recipients. | January 27, 2023 |
| Announcing the availability of data on sex or gender, race, and ethnicity by NIH Research, Condition, and Disease Classification (RCDC) category. | April 11, 2022 | |
| Inclusion statistics by NIH RCDC category | Report on the representation of participants in human subjects studies from fiscal years 2018-2021 for FY2018 projects associated with the listed Research, Condition, and Disease Categorization (RCDC) categories. | April 11, 2022 |
| Reporting the Results of Valid Analyses | The "All About Grants" podcast featuring an interview with the Inclusion Policy Officer about valid analysis reporting for the Inclusion of Women and Minorities policy. | August 6, 2018 |
| HSS overview and training information | As of June 9, 2018, the Human Subjects System (HSS) replaced the Inclusion Management System (IMS). Similar to IMS, HSS is used by NIH staff, grant applicants, and recipients to manage human subjects information, including inclusion information. | May 25, 2018 |
| Valid Analysis Reporting in ClinicalTrials.gov for Applicable NIH-Defined Phase III Clinical Trials | This guidance document describes the required ClinicalTrials.gov reporting of valid analysis results for applicable NIH-defined Phase III clinical trials. The guidance includes examples and recommendations for creating the NIH-required outcomes during registration and entering results for reporting. | May 21, 2018 |
| Continuing to Strengthen Inclusion Reporting on NIH-funded Phase III Trials | Blog post by NIH's Deputy Director of Extramural Research, Dr. Mike Lauer describing valid analysis and the reporting requirements for applicable NIH-Defined Phase III clinical trials. | January 8, 2018 |
| Applying the Inclusion of Women and Minorities Policy | A tool for understanding how to monitor inclusion based on sex or gender, race and ethnicity in research. | January 3, 2018 |
| Inclusion of Women and Minorities in Clinical Research | Reports published by the Department of Health and Human Services. The data tables included in these reports provide documentation of the monitoring of inclusion with some degree of analysis. | September, 2017 |
Upcoming Events
DHSR One pager of resources for external users
- Human Subjects Research
- NIH Office of Research on Women's Health (ORWH)
- National Institute on Minority Health and Health Disparities (NIMHD)
- Diversity and Inclusion in Clinical Trials (NIMHD)
- For NIH Staff
Have additional questions? Contact your program officer or the Inclusion policy team: [email protected]
An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
The PMC website is updating on October 15, 2024. Learn More or Try it out now .
- Advanced Search
- Journal List
- J Med Radiat Sci
- v.60(3); 2013 Sep
Tips for writing a case report for the novice author
A case report is a description of important scientific observations that are missed or undetectable in clinical trials. This includes a rare or unusual clinical condition, a previously unreported or unrecognized disease, unusual side effects to therapy or response to treatment, and unique use of imaging modalities or diagnostic tests to assist diagnosis of a disease. Generally, a case report should be short and focussed, with its main components being the abstract, introduction, case description, and discussion. This article discusses the essential components of a case report, with the aim of providing guidelines and tips to novice authors to improve their writing skills.
Introduction
For many doctors and other healthcare professionals, writing a case report represents the first effort at getting articles published in medical journals and it is considered a useful exercise in learning how to write scientifically due to similarity of the basic methodology. 1 Case reports aim to convey a clinical message. 2 , 3 Despite different types of case reports, they all aim to enhance the reader's knowledge on the clinical manifestations, the diagnostic approach (with a focus on imaging modalities for case reports published in medical imaging/radiology journals), or the therapeutic alternatives of a disease. 2 – 4 Thus, a case report worthy of reading should contain both useful practical messages and educational purpose. 2 – 5
Although case reports are regarded by some as the lowest (some even do not list the case reports at all) in the hierarchy of evidence in the medical literature, publishing case reports allow for anecdotal sharing of individual experiences, providing essential sources of information for the optimum care of patients. In the hierarchy of evidence-based medicine, randomized controlled trials are placed at the top, superseded by systematic reviews and meta-analyses, followed by prospective experimental trials, then observational studies, case–control studies, and case series at the bottom. 1 , 6 – 8 Most authors are now aware of the impact factor of journals to which they submit their studies. Case reports are infrequently cited, and therefore, publishing case reports is likely to decrease the journal's impact factor. 9 This has led many editors to remove case report sections from their journals. 10
On the other hand, it has been pointed out by others that case reports that are carefully prepared and interpreted with appropriate caution play a valuable role in both the advancement of medical knowledge and the pursuit of education. 11 – 16 Vandenbroucke 17 listed five roles of potential contribution to defend the publication of case reports:
- Recognition and description of a new disease
- Recognition of rare manifestations of a known disease
- Elucidation of the mechanisms of a disease
- Detection of adverse or beneficial side effects of drugs (and other treatments)
- Medical education and audit
Two main roles are recognized for case reports published in medical imaging and radiology journals: as sources of new knowledge and as important means for education and learning. The case report as a source of new knowledge refers to visualization of a new manifestation or finding, or clearer demonstration of a known feature of a disease, using a new imaging technology or an imaging method. 18 , 19 Figure 1 is an example showing 3D virtual endoscopy and the unique intraluminal views of the coronary lumen provided by this new visualization tool. 18 The case report as a means for teaching and learning can be manifested as publication of characteristic and instructive cases for educational features. An example is that British Journal of Radiology (BJR) used to publish six to seven case reports in its monthly issue; however, it has changed the format to publishing “Case of the Month” since May 2012. Educational value instead of extreme rarity is the main virtue of a case report worthy of publication. 2 , 3

Multiplanar reformatted image showing the left coronary artery with coronary stent implanted (arrows) at the ostium of left main stem (A). Virtual endoscopy views of the proximal segment of left coronary artery (B), left anterior descending (C), and left circumflex (D). The internal wall of these coronary branches looks smooth on virtual endoscopy images with no sign of intraluminal irregularity. (Reprint with permission from Reference. 18 )
Writing a case report can be educational for the author as well as for potential readers. 13 Whether in the context of reporting something potentially new or presenting an instructive example of something well known, the author's first and most important task is to search and read extensively on the topic. 20 This article aims to provide guidance on the novice author for writing case reports. Although it is recognized that these guidelines and tips for writing case reports are insufficient for making a successful author, they do help inexperienced authors to exercise and develop basic skills needed in medical writing.
The structure of the case report
Case reports are shorter than most other types of articles. Case reports should encompass the following five sections: an abstract, an introduction with a literature review, a description of the case report, a discussion that includes a detailed explanation of the literature review, and a brief summary of the case and a conclusion. 21 , 22 Tables, figures, graphs, and illustrations comprise the supplementary parts and will enhance the case report's flow and clarity. Unlike original articles, case reports do not follow the usual IMRAD (introduction, methods, results, and discussion) format of manuscript organization. As the format for case reports varies greatly among different journals, it is important for authors to read carefully and follow the target journal's instructions to authors.
The title is the first component of a case report that will be read by readers. Therefore, it should be concise, informative, and relevant to the subject. The ideal title should attract the reader's attention and state the focus on a particular issue, without being too cumbersome or artificial. 23 Redundant words such as “case reports” or “review of the literature” should be omitted, and ostentatious words such as “unique case” or “first report of” should be avoided. 1 , 5 Table 1 lists the titles of case reports that were published in BJR ( British Journal of Radiology ) and JMIRO ( Journal of Medical Imaging and Radiation Oncology ) between 2012 and 2013.
A list of case reports published in BJR and JMIRO between 2012 and 2013
| (BJR) | (JMIRO) |
|---|---|
| Severe back pain and lower extremities weakness in a young male | Case of bilateral non-traumatic subperiosteal orbital haematomas |
| A painful forefoot mass | Spinal arachnoiditis as a consequence of aneurysm-related subarachnoid haemorrhage |
| An 85-year-old male with abdominal pain and previous gastric surgery | IVC filter limb penetration of the caval wall during retroperitoneal surgery/lymph node dissection |
| A right atrial mass – but where is it coming from? | Haemobilia – a rare presentation of intrabiliary hydatid disease |
| An unusual case of duodenal beaking | Pulmonary arteriovenous malformation: a rare anterior mediastinal mass |
| Cystic renal mass in a patient with previous Wilm's tumour | Neuroimaging findings in acute ethylene glycol poisoning |
| Can you diagnose this condition on plain radiography? | Inducible myocardial ischaemia diagnosed using computed tomography dipyridamole stress myocardial perfusion technique |
| Unsuspected cystic left upper quadrant mass | Partial anomalous pulmonary venous return in patients with pulmonary hypertension |
| An uncommon cause of abdominal pain following blunt abdominal trauma | Uncommon pulmonary metastasis presenting as pulmonary infarction with tumour emboli in two cases |
| “Primum non nocere” – first, do no harm | Musculoskeletal CPD revision: cases from the New Zealand bone and soft tissue tumour registry |
| An unusual incidental finding |
IVC, inferior vena cava; CPD, continuing professional development.
The abstract
Like other types of articles, it is necessary to include a short summary that gives an overall idea about the content of the case report. The abstract is usually quite brief and generally shorter than that for other types of articles, and it typically has a word limit of 100 words or less. The abstract should be unstructured, pose the clinical question or diagnostic problem, and provide essential information which allows for easier retrieval from electronic database and helps researchers determine their levels of interest in the case report. 5
The introduction
The introduction should be concise and immediately attract the attention and interest of the reader. The introduction should provide background information on why the case is worth reading and publishing, and provides an explanation of the focus of the case report, for example: “We present/report a case of ….” Merit of the case report needs to be explained in light of the previous literature, thus, a focussed comprehensive literature review is required to corroborate the author's claim in this section. The author should bear in mind that a more detailed literature review belongs to the discussion, although critical evaluation of the literature is still required. 5 For some journals, such as BJR (case of the month), there is no Introduction section and the body of the case reports starts immediately with a description of the case.
The case description/summary
The case description or summary is the focus of the case report. The case is best presented in chronological order and in enough detail for the reader to establish his or her own conclusions about the case's validity. 5 , 21 The current medical condition and medical history, including relevant family history, should be clearly described in chronological order, typically comprising clinical history, physical examination findings, investigative results, including imaging and laboratory results, differential diagnosis, management, follow-up, and final diagnosis. 1 , 24 The following paragraph is an example of describing the patient's history:
A 34-year-old female was admitted to the outpatient department due to an increasing lump on the right thigh, which she stated as having been present for 5 years. A painful feeling sometimes occurred in the right upper leg. There was no complaint of lower limb weakness, no history of trauma and the patient was otherwise in good health. On physical examination, a deep seated round mass was detected and located on the right thigh with a size of 25 × 25 × 15 cm, showing hard consistency and non-mobile features ( Fig. 2 A). 25 Open in a separate window Figure 2 (A) Photograph showing a huge lump in the anterior part of the right thigh. (B) Radiographs revealed a bulged soft tissue mass in anterior compartment of right lower thigh showing predominantly radiolucent density with multiple chondroid matrix of calcification. Bone structure is still intact. (Reprint with permission from Reference. 25 )
All important negative findings should also be provided. The author's own interpretation or inferences should be avoided in the body of a case report. Tables/figures should be used to reveal chronological findings or to compare observations using different methods. The following paragraph is another example on the detailed description of using different methods both imaging and diagnostic:
Radiographs showed a bulge soft tissue mass in the right lower thigh having predominantly radiolucent density with multiple chondroid matrix of calcification ( Fig. 2 B), but the bone cortex is still intact. An MRI was obtained to further define the extent and nature of the lesion, confirming heterogeneous soft tissue mass in the anterior compartment of the muscle of the right lower thigh which mostly consisted of fat tissue, thick septation and some nodular non-adipose components. T2-weighted images through the tumour demonstrated high signal intensity comparable with the signal intensity of fat. Fat-suppressed T2-weighted images through the distal part of the tumour showed suppression of the signal through the central fatty components and lobular high signal intensity component at the peripheral rim. 25
In particular, figures need a brief but clear description. In the case of surgery and pathology specimens, the author is advised to provide a comprehensive summary of the surgical procedure and detailed pathologist's report. 5 , 25 The following paragraph is an excerpt from the case report published in the Australasian Medical Journal (AMJ):
The patient was admitted to the surgical ward with preparation for open surgery. The abdomen was opened through the site of the previous incision, and an abscess was observed and drained. A hole was detected in the peritoneal fascia. The anterior duodenum was oedematous and thickened with coverage of fibrin. A small perforated duodenal ulcer was seen. Graham patch procedure was performed to repair the perforated duodenal ulcer with two drains put in place and then the abdomen was closed. The patient was managed with intravenous fluids, as well as analgesics and antibiotics. 26
It is worth noting that patient confidentiality must be preserved. Patient demographics such as age and gender, and occasionally, race and occupation are referred to in the first sentence. In order to reduce the possibility of identifying the patient, the patient's initials, date of birth, and other identifiers such as hospital number must not be used.
The discussion
The discussion is the most important section of the case report. The discussion serves to summarize and interpret the key findings of the case report, to contrast the case report with what is already known in the literature and justify its uniqueness, to derive new knowledge and applicability to practice, and to draw clinically useful conclusions. 2 , 21 In comparing the new case with prior knowledge, the author should briefly summarize the published literature and show in what aspect the present case differs from those previously published, and thus deserves to be read and published. The discussion section of a case report is not designed to provide a comprehensive literature review and citation of all references; therefore, all the references cited should be critically evaluated.
Any limitations of the case should be stated and the significance of each limitation described. The value that the case adds to the current literature should be highlighted, so should the lessons that may be learnt from the case presented, especially if new recommendations for patient diagnosis (with use of an imaging modality) or management, could be put forward. 2 , 5 , 21 The following paragraph is an excerpt from a case report with regard to the concluding statement in the discussion:
This case report highlights the importance of using CT in making accurate diagnosis in patients with abdominal pain due to suspected GI tract perforation. In particular, appropriate selection of CT scanning protocol, such as with oral contrast administration is necessary to ensure timely diagnosis and improve patient management. 26
In the last paragraph, the author should provide the main conclusion of the case report based on the evidence reviewed in the discussion section. A concise statement of the lesson to be learnt from the case could be stated with justifiable evidence-based recommendations. This section should be concise and not exceed one paragraph. 14 , 21
The references
The references listed at the end of the case report should be carefully chosen by virtue of their relevance. References should provide additional information for readers interested in more detail than can be found in the case report, and they should support any specific points highlighted. 14 Some journals restrict the number of references to no more than 15 for a case report.
A case report will not have as much potential impact on the clinical practice of healthcare as randomized controlled trials or other research articles. However, case reports provide valuable sources of new and unusual information for clinicians to share their anecdotal experiences with individual cases, make others aware of unusual presentations or complications, and deliver the educational and teaching message. Well-written and appropriately structured case reports with meticulous attention to the very minute details will contribute to the medical literature and can still enrich our knowledge in today's evidence-based medical world. Table 2 provides the suggested checklist for reporting case reports. Guidelines and tips for writing case reports are not enough for becoming a successful author; however, they are considered helpful for inexperienced or novice authors to exercise and improve their skills needed in medical writing.
Checklist for writing case reports (based on advice in existing literature). 27
| Title |
| Should be brief and informative. |
| Abstract |
| Should facilitate retrieval with electronic searching. |
| Has a word limit of 100 words or less. |
| Introduction |
| Should be concise and attract the reader's attention. |
| Describe the uniqueness of the case and how the case contributes to the existing literature. |
| Is the message new and relevant to the medical imaging specialists? |
| Case report |
| Clearly describe the current medical condition and medical history in chronological order. |
| Provide details of the clinical presentation and examinations, including those from imaging and laboratory studies. |
| Describe the treatments, follow-up, and final diagnosis adequately. |
| Discussion |
| Summarize the essential features and compare the case report with the literature. |
| Explain the rationale for reporting the case. |
| State the lessons/experiences that may be learnt from the case report, and how things can be managed differently in a similar situation/case. |
| References |
| Should be relevant to the topic. |
| Limited to less than 15. |
| Figures and tables |
| Limited to one table, and two to three figures. |
| Illustrations should be effective. |
Conflict of Interest
None declared.

- CCGRT, Mumbai
- CCGRT-Hyderabad
- CCGRT-Kolkata
- Dadra & Nagar Haveli
- Hubli - Dharwad
- ICSI PILIBHIT STUDY CENTRE
- Kanchipuram
- Khed (Ratnagiri)
- Lakshadweep
- Muzaffarnagar
- Palayamkottai
- Perumbavoor
- Ramanathapuram
- Shri Ganga Nagar
- Tiruchirapalli
- Tirunelveli
- Vaniyambadi,Vellore District
- Vizianagram
- Bhubaneswar
- Chhatrapati Sambhajinagar
- Gandhinagar (Gift City)
- Navi Mumbai
- Thiruvananthapuram
- Visakhapatnam
- ICSI Overseas Centre, Australia lnc. ICSI Overseas Centre, Australia -->
- ICSI Overseas Centre, Canada ICSI Overseas Centre, Canada -->
- ICSI Middle East (DIFC) NPIO, Dubai ICSI Middle East (DIFC) NPIO, Dubai -->
- ICSI Overseas Centre, Singapore ICSI Overseas Centre, Singapore -->
- ICSI Overseas Centre, UK --> ICSI Overseas Centre, UK
- ICSI Overseas Centre, USA ICSI Overseas Centre, USA -->
- ICSI International ADR Centre
- Aa Increase
- Aa Decrease

Sample Case Studies
- Case Digest Series - 8
- Case Digest Series - 7
- Case Digest Series - 6
- Case Digest Series - 5
- Case Digest Series - 4
- Case Digest Series - 3
- Case Digest Series - 2
- Case Digest Series - 1

IMAGES
VIDEO
COMMENTS
A case report is a detailed report of the symptoms, signs, diagnosis, treatment, and follow-up of an individual patient. Case reports usually describe an unusual or novel occurrence and as such, remain one of the cornerstones of medical progress and provide many new ideas in medicine. Some reports contain an extensive review of the relevant ...
It is best to simply tell the story and let the outcome speak for itself. With these points in mind, let's begin the process of writing the case study: Title page: Title: The title page will contain the full title of the article. Remember that many people may find our article by searching on the internet.
First steps. Begin by sitting down with your medical team to discuss the interesting aspects of the case and the learning points to highlight. Ideally, a registrar or middle grade will mentor you and give you guidance. Another junior doctor or medical student may also be keen to be involved. Allocate jobs to split the workload, set a deadline ...
The aim of this study was to get junior doctors to evaluate an online presentation as part of the process of developing a beginner's guide to writing a clinical case report. Materials and methods. In response to our previous studies an online presentation concerning how to write a clinical case report was provided for junior doctors.
Title of case. You do not need to include "a case report" in the title - you may be cryptic if you wish. Summary. This will be freely available online. Up to 150 words summarising the case presentation and outcome. We need a good flavour of the case - emphasise the learning points. Background.
Writing a case report is an excellent way of documenting these findings for the wider medical community—sharing new knowledge that will lead to better and safer patient care. For many medical students and junior doctors, a case report may be their first attempt at medical writing. A published case report will look impressive on your ...
Below is the general format adopted for most case reports. Summarise your case report in a sentence. Mention how rare this condition is, and why your case report is important eg as a differential to consider in a patient presenting with X, Y and Z. Narrate your case in a way that is easy and enjoyable to follow.
State the medical condition or situation. Demonstrate that the case is related to medical condition or situation. Provide a very brief synopsis of case: make the problem in (2) clearly related and include a brief outcome statement. State the significance of this case for readers or within the literature (optional).
Case studies always follow a standard structure and format, so the writing is very formulaic once you get the hang of it. Read on for a step-by-step guide to writing your first case study report. Steps. Section 1 of 6: ... To start a medical case study report, first choose a title that clearly reflects the contents of the report. ...
retrospective study in Zambia, TB was more commonly diagnosed among children with Kwashiorkor (47%) compared to Marasmus-Kwashiorkor (24%) and marasmus (29%) [7]. The majority had pulmonary TB, while TB meningitis, lymphadenitis (as our case) and disseminated TB were the most common forms of extra-pulmonary TB.
A clinical case report or case study is a means of disseminating new knowledge gained from clinical practice. Clinical case reports are the first-line evidence in medical literature as they present original observations. This article provides detailed guidance on how to identify, write, and publish a case report.
A case report is a descriptive study that documents an unusual clinical phenomenon in a single patient. It describes in details the patient's history, signs, symptoms, test results, diagnosis, prognosis and treatment. It also contains a short literature review, discusses the importance of the case and how it improves the existing knowledge on the subject.
The oldest example of a preserved clinical case in medical literature is a text from an ancient Egyptian papyrus dating from the 16 th to the 17 th dynasty, ... FORMAT FOR WRITING A CASE REPORTS. ... Cohen L, editors . London: Routledge; 2011. Case studies, in Research Methos in Education; p. 289. [Google Scholar] 10.
A medical case report, also known as a case study, is a detailed description of a clinical encounter with a patient. The most important aspect of a case report, i.e. the reason you would go to the trouble of writing one, is that the case is sufficiently unique, rare or interesting such that other medical professionals will learn something from it.
Abstract. Purpose. Guidelines for writing patient case reports, with a focus on medication-related reports, are provided. Summary. The format of a patient case report encompasses the following five sections: an abstract, an introduction and objective that contain a literature review, a description of the case report, a discussion that includes a detailed explanation of the literature review, a ...
A Medical Case Study Template is a systematic tool used primarily by healthcare professionals and students to document, analyze, and present individual patient cases. It serves as a structured guide, ensuring that all critical aspects of the case are captured and discussed in a standardized format. This includes patient information, clinical ...
A 44-year-old woman presented with cough, dyspnea, and chest pain. On examination, she had tachycardia and hypotension. Evaluation revealed SARS-CoV-2 RNA in a nasopharyngeal swab, as well as eleva...
Composing a Case Study A case study is a document used in many disciplines that examines unique or intriguing cases in order to gain some insight or understanding about the topic. Clinical case studies often look at individual cases or study different types of clinical practice. A case study may involve qualitative or quantitative research methods.
Case reports are a time-honored, important, integral, and accepted part of the medical literature. Both the Journal of Medical Case Reports and the Case Report section of BioMed Central Research Notes are committed to case report publication, and each have different criteria.Journal of Medical Case Reports was the world's first international, PubMed-listed medical journal devoted to ...
This case study highlights the challenges and the lessons learned throughout the project development and implementation, in addition to some of the best practices for project management, operations, load shifting and peak management, and heating, ventilation, and air-conditioning (HVAC) system management.
/ Sample Case Studies Sample Case Studies / case snippets case snippets; Case Snippets. Jurisprudence Interpretation & General Law; Company Law; Capital Market & Securities Law; Competition Law; Labour Law; Insolvency & Bankruptcy Law; Banking & Insurance Laws; Intellectual Property Law; Direct Tax; Goods and Services Tax;
Presenting patient cases is a key part of everyday clinical practice. A well delivered presentation has the potential to facilitate patient care and improve efficiency on ward rounds, as well as a means of teaching and assessing clinical competence. 1 The purpose of a case presentation is to communicate your diagnostic reasoning to the listener, so that he or she has a clear picture of the ...
Case study examples are the best way to learn the basic techniques for writing a great case study on your own. Explore these short case study sample pdfs to gain insights into presenting your research cohesively: For your help, we have also compiled real-life case study examples along with a format that you can refer to while writing your own.
Structure Your Report. Once you have selected the journal of submission, carefully reread the author instructions to structure your submission. The JACC: Case Reports authors instructions suggest a specific structure for a clinical case: history of presentation, physical examination, past medical history, differential diagnosis, investigations, management (medical/interventions), discussion ...
Purpose. NIH is mandated by the Public Health Service Act sec. 492B, 42 U.S.C. sec. 289a-2 to ensure the inclusion of women and members of racial and ethnic minority groups in all NIH-funded clinical research in a manner that is appropriate to the scientific question under study. The primary goal of this law is to ensure that research findings can be generalizable to the entire population.
Introduction. For many doctors and other healthcare professionals, writing a case report represents the first effort at getting articles published in medical journals and it is considered a useful exercise in learning how to write scientifically due to similarity of the basic methodology.1 Case reports aim to convey a clinical message.2,3 Despite different types of case reports, they all aim ...
Sample Case Studies. Case Digest Series - 8; Case Digest Series - 7; Case Digest Series - 6; Case Digest Series - 5; Case Digest Series - 4; Case Digest Series - 3; Case Digest Series - 2; Case Digest Series - 1