- BUSINESS IDEAS
- HOW TO START
- Multi-generational Business Planning
- Services By Stunners
- CALCULATORS


How to Start a Clinical Research Organization

Clinical research organizations (CROs) play an important role in the design and execution of clinical studies, which are required to bring innovative medications and medical devices to market. A contract research organization (CRO) is a corporation that provides assistance and services to pharmaceutical, biotechnology, and medical device companies during the clinical trial process. The growing complexity of clinical trials has increased demand for CRO services in the US market, as corporations seek to outsource portions of the trial process to experienced specialists.
The purpose of this blog post is to provide a thorough guide on how to start a Clinical Research Organization, including step-by-step instructions and success suggestions in the clinical research sector. At the end of this essay, readers should have a firm grasp on the procedures required to launch their own CRO and position themselves for success in this expanding market.
Table of Contents
How to Start a Clinical Research Organization – Step by Step Guide
Step 1: overview of the market.
To establish a successful clinical research organization (CRO), it is necessary to first learn about the clinical research sector in the United States. The clinical research sector in the United States is a highly regulated and complicated market, with clinical research undertaken across a wide range of therapeutic fields. Clinical research of various forms is undertaken in the United States, including Phase I-IV clinical trials, observational studies, and registries. Clinical trials are the most common sort of clinical research, in which a novel medicine or medical technology is tested on human participants to establish its safety and efficacy.
Clinical research in the United States has a lengthy history, reaching back to the early twentieth century. With the establishment of the Food and Drug Administration (FDA) in 1938 and the implementation of the Clinical Laboratory Improvement Amendments (CLIA) in 1988, the business has become increasingly complicated and heavily regulated throughout time.
CROs have played a significant role in the clinical research business, supporting and assisting pharmaceutical, biotechnology, and medical device companies during the clinical trial process. Services such as study design, patient recruiting, data collecting and management, and regulatory support are included. Entrepreneurs wishing to create their own CRO can position themselves for success by studying the clinical research business in the United States and the function of CROs within it.
Step 2: Prepare Business Plan
A robust business plan is essential for launching a successful clinical research enterprise (CRO). A business plan serves as a road map for the company, ensuring that all parts of the firm are examined and planned for. An overview of the sector, target market analysis, marketing and sales strategies, financial projections, finance requirements, and operational details are all included in a well-written business plan. It also describes the company’s vision, mission, and values, as well as its unique selling proposition (USP), which differentiates it from other CROs in the market.

Startup Stunners has been providing high-quality business plan writing services for years, and we’re ready to assist you in developing a complete, effective strategy that will move your company ahead. Our team of professionals is committed to assisting you in achieving your company objectives and obtaining finance from banks, grants, or other sources. We’re here to help you succeed whether you’re a newbie, entrepreneur, or small company owner. Don’t put it off any longer; visit startupstunners.com/contact-us/ today and let us lead you to success!
Step 3: Establishing Clinical Trial Services
After you’ve gained a thorough understanding of the clinical research industry and the value of a business strategy, the next stage in launching a successful CRO is to establish clinical trial services. Clinical trial services are the foundation of a CRO because they are the core offering to pharmaceutical, biotechnology, and medical device businesses.
A CRO’s clinical trial services can vary depending on the company’s size and specialism. CROs commonly provide services such as study design, site selection, patient recruitment, data collection and management, and regulatory support. It is critical to have a skilled and experienced team on hand to provide these services, as they necessitate expertise and attention to detail to ensure that the clinical trial runs smoothly and produces accurate and reliable results.
A CRO must have a reputation for providing high-quality clinical trial services in order to form partnerships with pharmaceutical companies. Building a strong network of contacts within the industry, attending conferences and networking events, and showcasing previous successes to potential clients are all essential. A CRO can also distinguish itself by providing unique or specialized services that set it apart from competitors. A CRO, for example, may be an expert in a specific therapeutic area or provide cutting-edge technology solutions for data management and analysis.
Step 4: Develop Standard Operating Procedures (SOPs)
Developing Standard Operating Procedures (SOPs) is an essential step in launching a successful CRO. SOPs are written instructions that explain how to complete a task or activity in a consistent and uniform manner. They are significant because they ensure that all parts of the company are carried out in a methodical and structured manner, which is critical for ensuring the quality of clinical trial services supplied by the CRO.
A title page, purpose, scope, definitions, duties, procedures, and reference papers are all common components of SOPs. These parts contribute to the SOP being thorough and covering all aspects of the task or activity described. It is critical to include individuals who are skilled and knowledgeable in the area being documented while writing SOPs. This ensures that the SOP is correct and reflects the best practices and procedures for that particular task or activity.
Employee training on SOPs is critical to ensuring that they are followed consistently and accurately. Employees that have received SOP training understand the value of adhering to defined protocols and are better suited to do their duties. This training can take a variety of forms, including classroom instruction, hands-on instruction, and refresher courses. Furthermore, SOPs must be updated on a regular basis to reflect changes in rules, industry standards, or best practices.
SOPs ensure that all elements of the business are carried out in an uniform and organized manner, which is essential for ensuring the quality of clinical trial services supplied. A CRO can establish a strong basis for its operations and position itself for success in the clinical research sector by including experienced personnel in the formulation of SOPs, offering extensive training to staff, and constantly updating SOPs.
Step 5: Securing Funds
Obtaining money is a critical step in launching a CRO. CROs can obtain financing from a variety of sources, including:
- Equity financing: In exchange for finance, equity financing entails selling ownership shares in the Company to investors. This can be accomplished with the help of private equity firms, venture capitalists, or angel investors. Equity finance can provide large capital, but it also dilutes the company’s control.
- Debt financing: Debt financing is borrowing money from lenders such as banks or financial institutions and repaying it over time with interest. This can take the shape of a loan, credit line, or credit card. Debt financing can provide instant cash flow, but it also involves interest repayment, which can be a substantial burden for a fledgling business.
- Government funding: CROs can obtain government support in the form of grants, loans, or tax credits. These funding are often allocated to certain research fields or sectors and may impose stringent documentation and reporting requirements.
- Crowdfunding: Crowdfunding is the practice of soliciting small sums of money from a large number of individuals, generally through internet platforms. This can be an excellent alternative for CROs wishing to generate small sums of money or establish a network of supporters.
- Strategic alliances: Strategic alliances involve collaborating with other businesses or organizations to exchange resources, knowledge, and finance. This can be an excellent alternative for CROs wanting to secure finance by leveraging current ties and networks.
Step 6: Tips for Success in the clinical research industry
To compete in the clinical research sector, CROs must have a solid foundation, cultivate strong client relationships, and stay current on industry rules and best practices. Here are some pointers for achieving success in the clinical research industry:
- Concentrate on quality: CROs must build a reputation for offering top-notch services because quality is crucial in the clinical research sector. This includes making certain that all areas of the firm are standardized and organized, and that workers are well-trained and experienced.
- Establish good client relationships: Establishing strong client relationships is crucial for success in the clinical research sector. Listening to clients’ wants and concerns, being responsive and open, and following through on promises are all part of this. CROs may establish a solid reputation and position themselves for long-term success by fostering customer trust and loyalty.
- Maintain compliance with legislation: Because the clinical research sector is extensively regulated, CROs must maintain compliance with all relevant regulations and guidelines. This includes investing in regular personnel training and education as well as being up to date on changes in rules and best practices.
- Use technology to increase efficiency and quality: Technology is becoming increasingly crucial in the clinical research sector, and CROs that can use it to improve efficiency and quality will have a competitive advantage. Investing in technology solutions such as electronic data capture systems and clinical trial administration software is part of this.
- Concentrate on innovation: The clinical research market is continuously changing, and CROs who can innovate and adapt to changes will be well-positioned for success. This entails investing in R&D, being current on emerging trends and technology, and being ready to take prudent risks.
Step 7: Networking and partnership formation
Networking and forming alliances are critical for success in the clinical research sector. Here are some pointers for good networking and partnership formation:
- Attend industry conferences and events: Attending industry conferences and events is a terrific method to meet possible partners and collaborators. These gatherings allow attendees to network with industry experts, learn about new trends and technology, and form new business partnerships.
- Join industry groups and organizations: Another excellent strategy to network and form partnerships is to join industry associations and organizations. These associations and organizations give members access to industry experts and leaders, as well as educational and networking opportunities.
- Use social media: Social media sites such as LinkedIn and Twitter allow you to interact with industry people and develop new connections. CROs can establish new alliances and expand their brand by distributing thought leadership content and communicating with other industry professionals.
- Be aggressive in your approach: Creating alliances necessitates proactive outreach. CROs should identify suitable partners and approach them with a clear value proposition and a plan for how the collaboration may benefit both parties.
- Emphasize collaboration: Relationships thrive when they are collaborative and mutually beneficial. CROs should focus on developing partnerships based on shared values and goals that exploit both parties’ strengths.
- Follow up and keep in touch: Successful collaborations include continual contact and follow-up. CROs should make an attempt to maintain contact with partners and collaborators, as well as to cultivate these connections over time.
Step 8: Staying up-to-date with industry trends
Keeping current with industry developments is critical for clinical research success. Here are some of the reasons:
- Competitiveness: The clinical research sector is extremely competitive, and staying current on industry developments can provide CROs with a competitive advantage. CROs may provide better services and solutions to their clients by understanding the latest technology, methodologies, and approaches.
- Compliance: Because the clinical research sector is highly regulated, maintaining current on industry trends is crucial for assuring compliance. CROs may ensure that they are functioning in a compliant and ethical manner by remaining up to date on the current legislation and guidelines.
- Innovation: Because the clinical research market is always changing, being current on industry trends is critical for innovation. CROs can produce new services and solutions that match the changing needs of their clients by understanding the newest research and development trends.
- Reputation: Keeping up with market changes can help CROs establish themselves as industry leaders. CROs may demonstrate their expertise and establish themselves as trusted partners for their clients by releasing thought leadership content and engaging in industry events and forums.
- Efficiency: Keeping current on industry developments can also help CROs run more efficiently. CROs can enhance their bottom line and become more competitive by implementing new technology and practices that streamline their processes and cut costs.
Step 9: Technology
There are various technologies that can be used in clinical research, each with its own set of advantages. Here are a couple such examples:
- EDC Systems: EDC systems are software applications designed to gather, handle, and store clinical trial data. EDC systems can increase data quality and accuracy by streamlining data gathering and management procedures, reducing mistakes and inconsistencies, and streamlining data collection and management processes.
- Clinical Trial Management Systems (CTMS): CTMS are software tools developed to manage clinical trial operational features such as patient enrollment, study progress, and trial finances. CTMS can increase research efficiency, save expenses, and improve trial management overall.
- Electronic Patient-Reported Outcomes (ePRO) Systems: ePRO systems are software applications that enable patients to electronically complete study-related questionnaires and assessments, typically using a mobile device or PC. ePRO systems can increase patient compliance and engagement while decreasing data entry errors and improving data accuracy.
- Wearable Devices: Smartwatches and fitness trackers, for example, can be used to collect patient health data such as heart rate and activity levels. This information can be utilized to track patient health and the efficacy of treatments. Wearable technology can help boost patient involvement and compliance.
- Artificial Intelligence (AI): Artificial intelligence (AI) technologies such as machine learning and natural language processing can be used to examine enormous datasets and detect patterns and trends. AI can assist researchers in discovering new treatments and therapies, increasing patient recruitment and retention, and improving overall trial design and management.
To summarize, establishing a clinical research organization can be a difficult yet rewarding endeavor. It is critical to have a good business plan in place, to form relationships with pharmaceutical companies, to develop complete SOPs, to acquire funding, and to stay current on industry developments and innovations. CROs may provide high-quality clinical trial services and contribute to the advancement of medical research by following these steps and harnessing the latest technologies. With rising demand for CRO services in the US market, there has never been a better moment to establish a clinical research company.
Frequently Asked Questions
What is the definition of a clinical research organization (cro).
A clinical research organization (CRO) is a corporation that provides clinical trial support services to the pharmaceutical, biotechnology, and medical device sectors.
Why are contract research organizations (CROs) significant in clinical research?
Clinical research organizations (CROs) are significant in clinical research because they provide the infrastructure, resources, and knowledge needed to perform clinical trials rapidly and successfully. CROs also help to reduce the risks involved with clinical trials and assure regulatory compliance.
What forms of clinical research are carried out in the United States?
Clinical research in the United States includes drug trials, device trials, observational studies, and epidemiological research.
What exactly are normal operating procedures?
SOPs are written documents that outline the procedures, processes, and rules that a corporation uses to maintain consistency and quality in its operations.
How may CROs obtain funding?
CROs can obtain money in a variety of ways, including venture capital, private equity, grants, loans, and collaborations with pharmaceutical corporations.
How are clinical research technologies used?
Electronic data capture (EDC) systems, clinical trial management systems (CTMS), electronic patient-reported outcomes (ePRO) systems, wearable devices, and artificial intelligence are all extensively utilized in clinical research (AI).
What are some pointers for achieving success in the clinical research industry?
Building a professional and experienced staff, creating good partnerships with pharmaceutical companies, remaining up to speed with industry changes and technologies, and maintaining a focus on quality and compliance are some guidelines for success in the clinical research sector.
RELATED ARTICLES MORE FROM AUTHOR

How to Start a Dump Truck Business

How to Start a Pallet Business

How to Start a Powder Coating Business
Leave a reply cancel reply.
Save my name, email, and website in this browser for the next time I comment.

- Privacy Policy
- Terms of Service
© Copyright 2022 Startup Stunners. All rights reserved.
Got2bwireless.com
How to Start a Clinical Research Business: A Comprehensive Guide

Do you dream of starting your own clinical research business, but don’t know where to begin? Fear not, my friend! Starting any business can be a daunting task, but with the right information and guidance, it can also be a rewarding and satisfying journey. In this article, we will explore the steps you need to take to start your own clinical research business.
First things first, you need to have a solid plan. This means conducting research, understanding your target market, and creating a business plan that outlines your goals and objectives. Once you have a clear plan in place, the next step is to acquire the necessary knowledge and expertise in the clinical research field. This may involve continuing education, networking, and gaining experience through internships or working for other companies within the industry.
Finally, it’s time to take action and start building your business. This involves setting up your physical space, creating a website and marketing materials, and developing relationships with industry contacts and potential clients. Remember, starting any business requires hard work, dedication, and a willingness to adapt to changing circumstances. With a solid plan, the right skills, and the determination to succeed, you too can start your own successful clinical research business. Conducting a Market Analysis
Before starting a clinical research business, it is crucial to conduct a thorough market analysis. This involves researching the market and competitors to identify opportunities and potential challenges that may affect the success of your business.
- Research the target market: You need to identify the demographics, behaviors, and needs of the target market. Understanding the needs of your prospective patients and service providers will enable you to customize your services to meet those needs, thus increasing your chances of success.
- Determine the current trends and demands: Identifying the current trends and demands in the market will enable you to develop services that meet those needs.
- Identify potential competitors: Research your competitors to determine their strengths, weaknesses, and pricing strategies. This information will help you position your services competitively.
To conduct a more in-depth analysis, it’s recommended to create SWOT analysis. This technique will help you identify the strengths, weaknesses, opportunities, and threats that affect your business.
Your potential business partners and investors will also want to see the results of your market analysis, so invest time and resources into this aspect of your business planning.
Developing a Business Plan
Developing a business plan is essential for starting a clinical research business. The business plan outlines the company’s goals, strategies, and financial projections, and helps investors and stakeholders understand the nature and potential of the business.
A well-crafted business plan should include the following:
- Executive summary – a concise overview of the business and its objectives.
- Company description – a detailed description of the business, including its history, mission, and services.
- Market analysis – a study of the target market, competitors, and industry trends.
- Services and products – a breakdown of the products and services offered by the business.
- Marketing and sales strategies – a plan for promoting and selling the business’s products and services.
- Management structure – an overview of the management and organizational structure of the business.
- Financial projections – a detailed financial analysis of the business, including revenue, expenses, and profit projections.
- Appendix – supporting documents and information, such as resumes, contracts, and market research data.
In addition to these elements, the business plan should also consider potential risks and contingencies, such as changes in regulations or unexpected market shifts. It should also include a timeline for achieving goals and milestones, as well as a plan for adapting and revising the plan as necessary.
Developing a business plan is a crucial step in starting a successful clinical research business. A comprehensive and well-researched plan can help attract investors and stakeholders, guide decision making, and ensure the long-term success of the business.
| Pros | Cons |
|---|---|
| Provides a roadmap for the business | Requires significant time and effort |
| Helps attract investors and stakeholders | May require consulting with experts for market research and financial analysis |
| Guides decision-making and adaptation | May not account for unexpected market shifts or changes in regulations |
Overall, a well-developed business plan is an essential tool for starting and maintaining a thriving clinical research business.
Legal Considerations
Starting a clinical research business involves complying with legal requirements. Understanding the legal considerations is crucial to avoid legal liabilities and ensure the safety of participants in your clinical trials. Below are some of the important legal considerations:
- Good Clinical Practice (GCP) regulations: GCP regulations are a set of international ethical and scientific quality standards for designing, conducting, recording, and reporting clinical research involving human subjects. Compliance with GCP regulations ensures that the rights, safety, and well-being of clinical trial participants are protected. It is important to ensure that your clinical research business complies with GCP regulations.
- Obtaining regulatory approvals: Before starting any clinical trial, regulatory approvals must be obtained from the relevant authorities in your country. Regulatory approvals include approval from the ethics committee or institutional review board (IRB) and approval from the regulatory agency responsible for the oversight of clinical trials (such as the FDA in the United States). Failure to obtain regulatory approvals can result in legal liabilities, fines, and even criminal charges.
- Intellectual property protection: Protecting intellectual property (IP) is crucial in any business, including clinical research. The IP in clinical research includes patents, trademarks, copyrights, and trade secrets. Protecting your IP ensures that your business maintains its competitive edge and gains recognition in the market. You can protect your IP by obtaining patents and trademarks, registering copyrights, and maintaining confidentiality agreements and nondisclosure agreements (NDAs) with employees and stakeholders.
It is important to consult with legal experts to ensure compliance with all legal requirements when starting a clinical research business. Additionally, you should keep yourself updated with the changing legal landscape relating to clinical research, by subscribing to relevant publications and attending conferences and seminars.
Below is a table showing some of the legal documents that are required for conducting clinical trials:
| Legal Document | Purpose |
|---|---|
| Protocol | Describes the objectives, design, methodology, statistical considerations, and organization of the clinical trial. |
| Informed consent form (ICF) | Explains the purpose, risks, and benefits of the clinical trial to the participant and gives the participant the opportunity to decide whether or not to participate in the trial. |
| Institutional review board (IRB) approval letter | Indicates that the IRB has reviewed and approved the clinical trial protocol and ICF. |
| Investigator’s brochure | Provides background information about the investigational product, including its safety and efficacy data. |
| Case report form (CRF) | Used to collect data during the clinical trial. It includes information about the participant, the investigational product, and the efficacy and safety outcomes. |
Understanding the legal considerations when starting a clinical research business is essential for the success of your business. By complying with the legal requirements, you can ensure the safety of your participants, protect your IP, and avoid legal liabilities.
Regulatory Compliance
When starting a clinical research business, regulatory compliance is crucial. All clinical research must adhere to regulations and guidelines set by governing bodies such as the Food and Drug Administration (FDA), the International Conference on Harmonisation (ICH), and the Institutional Review Board (IRB). Failure to comply with these regulations can lead to legal action, loss of credibility, and harm to study participants.
- Develop a thorough understanding of all regulatory requirements and guidelines before beginning any clinical research.
- Ensure that all study documents and procedures adhere to these regulations and guidelines.
- Implement quality assurance processes to ensure ongoing compliance throughout the study.
It is important to note that regulatory requirements and guidelines can vary depending on the type of research being conducted, such as studies involving drugs or medical devices. Consulting with regulatory experts and seeking advice from governing bodies can help ensure compliance and reduce the risk of non-compliance.
IRB Approval
The Institutional Review Board (IRB) plays an integral role in the clinical research process. All studies involving human subjects must be reviewed and approved by an IRB to ensure that the study is ethical and that the rights and welfare of participants are protected.
When starting a clinical research business, it is important to have a thorough understanding of the IRB approval process. This includes:
- Preparing and submitting a comprehensive study protocol to the IRB for review
- Addressing any concerns or questions raised by the IRB during the review process
- Following all IRB requirements and guidelines during the study
Data Management
Data management is a critical component of clinical research. Proper management and documentation of study data ensures accuracy, security, and compliance with regulatory requirements. This includes:
- Establishing data management processes and protocols before beginning the study
- Using secure and reliable data management systems that meet regulatory requirements
- Ensuring all study data is properly documented and stored, including informed consent forms and case report forms
| Data Management Best Practices: |
|---|
| Develop comprehensive data management plans that outline data collection, storage, and analysis procedures |
| Implement quality control processes to ensure data accuracy, completeness, and consistency |
| Conduct regular data audits to identify and address any issues or anomalies |
By implementing best practices for data management, clinical research businesses can ensure regulatory compliance and produce high-quality data for analysis and reporting.
Staffing and Recruitment
One of the most crucial aspects of starting a clinical research business is staffing and recruitment. Human resources are the backbone of any organization, and it is imperative to have the right team in place. Here are some essential steps to follow when staffing and recruiting for your clinical research business:
- Define the job description – Before starting the recruitment process, create a clear job description that outlines the responsibilities, duties, and requirements of the position. Make sure the job description is specific and detailed to attract the right candidates.
- Create a competitive compensation package – Talented individuals are in high demand, and it is essential to offer a competitive compensation package that includes health benefits, retirement plans, bonuses, and other incentives.
- Utilize online job postings – Use online job boards such as LinkedIn, Indeed, and Glassdoor to find potential candidates. These platforms have a vast pool of job seekers and allow you to filter candidates based on education, experience, and location.
Another effective way to recruit talent is through referrals. Ask your industry peers, colleagues, and friends if they know anyone who fits the job description. Offering a referral bonus can encourage individuals to refer qualified candidates to your organization.
Once you have received applications, the next step is to conduct a thorough screening process. This may include reviewing resumes and cover letters, conducting phone interviews, and scheduling in-person interviews. During the interview process, assess the candidate’s fit for the role and company culture, their problem-solving abilities, and communication skills.
Finally, after selecting the right candidate, it is essential to provide adequate training to ensure they are equipped to handle the job and have a clear understanding of the company’s goals and objectives.
| Step | Actions |
|---|---|
| 1 | Create a detailed job description |
| 2 | Offer a competitive compensation package |
| 3 | Utilize online job postings and referrals |
| 4 | Conduct phone and in-person interviews |
| 5 | Provide adequate training for new hires |
By following these steps and putting the right recruitment and staffing strategies in place, your clinical research business can attract and retain top talent, leading to a successful and thriving business.
Identifying Funding Opportunities
Identifying funding opportunities is one of the most important steps to establish a successful clinical research business. Funding opportunities help researchers support their projects and contribute to expanding scientific knowledge. Here are some techniques to help in identifying funding opportunities:
- Search for federal funding options – The federal government provides funding for a wide range of research projects. The National Institutes of Health (NIH) is the largest provider of biomedical research funding in the United States. Grants.gov is another online platform where you can find a listing of federal grants available for different categories.
- Explore private foundations – Private foundations also provide funding for clinical research projects. Examples include the Bill and Melinda Gates Foundation, the Susan G. Komen Foundation, and the American Cancer Society. These foundations usually have specific areas of interest and eligibility criteria for applicants.
- Network with industry partners – Networking with industry partners is another valuable strategy for identifying funding opportunities. Industry partners often invest in research projects that align with their business strategies. Collaboration with industry partners can also offer researchers access to valuable resources and expertise.
Once funding opportunities have been identified, it is crucial to follow the guidelines carefully and ensure that the application meets the eligibility criteria. Additionally, researchers should ensure that they have available resources and equipment to carry out their project if funding is awarded.
Establishing Partnerships and Collaborations
Starting a clinical research business requires a substantial amount of investment, both in terms of finances and time. Establishing partnerships and collaborations with other businesses can be advantageous for startups to share responsibilities and resources. Here are some ways to establish partnerships and collaborations:
- Identify Potential Partners: Before establishing partnerships, it is essential to identify potential partners, such as pharmaceutical companies, contract research organizations, and academic institutions, among others.
- Assess Their Capabilities: Evaluating the capabilities of potential partners is vital to avoid any conflicts that may arise during the partnership. Consider their expertise, experience, financial standing, and reputation.
- Outline Terms and Conditions: After identifying and evaluating partners, draw up an agreement that outlines the terms and conditions of the partnership. Such agreements should include the scope of work, responsibilities, financial contributions, and timelines.
Formal partnerships and collaborations can take different forms, such as joint ventures, strategic alliances, and licensing agreements. These collaborations can foster research and development, knowledge sharing, and the pooling of financial resources to achieve common goals. For example, an academic institution may partner with a clinical research organization to conduct a clinical trial on a new drug or therapy, and share the results with the pharmaceutical industry.
Table 1 below shows some benefits of establishing partnerships and collaborations:
| Benefits of Partnerships and Collaborations |
|---|
| Shared expertise, knowledge, and resources |
| Lower costs and reduced risks |
| Access to new markets and customers |
| Faster time-to-market and increased innovation |
| Increased credibility and visibility |
Establishing partnerships and collaborations is an effective way to grow a clinical research business. It enables startups to share risks, costs, and resources, and leverage the expertise and knowledge of other businesses. However, partnerships and collaborations require effective communication, clear agreements, and mutual trust to achieve the goals of all parties involved.
Developing Standard Operating Procedures (SOPs)
Standard Operating Procedures (SOPs) are comprehensive documents that outline how a specific task or process should be executed. SOPs are critical for clinical research as they ensure consistency, accuracy, and compliance with regulatory standards. Here are 8 steps to developing effective SOPs for your clinical research business:
- Identify the task: Determine which tasks require an SOP and prioritize them.
- Involve the experts: Invite personnel with the necessary expertise to draft the SOP.
- Gather references: Collect relevant regulations, guidelines, and research papers to support the SOP.
- Define the scope: Outline the objective, purpose, and intended audience of the SOP.
- Develop the format: Decide on the structure, style, and format of the SOP, ensuring it is user-friendly and easy to update.
- Write the content: Draft the SOP in a clear and concise language, specifying the steps, responsibilities, timelines, and possible risks involved.
- Validate the SOP: Review and revise the SOP with all stakeholders to ensure it reflects their feedback and is compliant with regulations.
- Implement and train: Communicate the SOP to everyone involved in the task and provide the necessary training to ensure they understand and follow it.
Following these steps will result in the development of effective SOPs that are essential for the smooth and compliant execution of clinical research tasks.
Moreover, SOPs should be periodically reviewed, updated, and revised to ensure they remain relevant and compliant with changing regulations and guidelines. A documented process should also be in place for revisions and updates to maintain compliance throughout the clinical research process.
| BENEFITS OF EFFECTIVE SOPs |
|---|
| Minimizes errors and inconsistencies |
| Ensures compliance with regulations and standards |
| Provides direction, guidance, and consistency |
| Increases productivity and efficiency |
| Enhances quality control and data integrity |
Implementing effective SOPs is critical to the success and reputation of your clinical research business. It demonstrates your commitment to quality, ethics, and regulatory compliance, and sets a foundation for the growth and sustainability of your business.
Implementing Quality Assurance and Quality Control Measures
Implementing quality assurance and quality control measures is an essential step in starting a clinical research business. Quality assurance (QA) involves ensuring that all processes and procedures are conducted in a systematic, standardized, and consistent manner. Quality control (QC) involves monitoring and verifying that the processes and procedures are implemented correctly and producing accurate and reliable outcomes.
- Developing Standard Operating Procedures (SOPs) – SOPs are a set of written instructions that outline the steps for carrying out a specific task. Developing SOPs for all clinical research activities ensures that all staff is aware of the processes and procedures that need to be followed, and they are implemented consistently.
- Training Staff – Staff needs to be appropriately trained to understand and implement the SOPs. Training should be provided to both new and existing employees to ensure that they are up-to-date with the latest procedures and policies.
- Assigning Roles and Responsibilities – A clear delineation of roles and responsibilities ensures that all staff knows their responsibilities and the accountability for specific tasks.
Another critical aspect is monitoring and verifying that the processes and procedures are correct and producing accurate outcomes. This process is known as Quality Control (QC). To implement QC effectively, you need to establish a Quality control unit (QCU), responsible for assessing quality control measures. The QCU follows the following steps to ensure the integrity and accuracy of research data:
- Implementing Data Cleaning Procedures – Data cleaning is the process of identifying and correcting errors in collected data. A well-designed data cleaning protocol ensures the accuracy and integrity of the data for analysis.
- Performing Site Visits – Site visits ensure that data is being collected and recorded accurately and consistently. They also help to monitor study compliance and detect problems early.
- Adverse Event Reporting and Monitoring – Adverse events are unexpected or unfavorable outcomes that may occur during a clinical trial or research study. A system must be in place to report and monitor adverse events to minimize the risk of harm to patients.
Overall, implementing Quality Assurance and Quality Control measures is essential to ensure that clinical research activities are conducted appropriately, accurately, and ethically. A poorly designed and executed study not only compromises patient safety but also impacts the reliability of the data and the credibility of the research.
| Benefits of Implementing Quality Assurance and Quality Control Measures |
|---|
| Ensures compliance with regulatory requirements |
| Minimizes risks to patients and staff |
| Maximizes the quality and reliability of research data |
| Provides a systematic approach to conducting research activities |
Therefore, a well-conducted study ensures that data collected is accurate and useful, leading to reliable results and conclusions. Implementing QA/QC measures are the key to achieving these objectives.
Identifying Potential Clients and Developing Marketing Strategies
In the clinical research business, identifying potential clients is a crucial first step in developing effective marketing strategies. Here are ten essential steps to identify potential clients:
- 1. Identify your target market: Knowing your target market helps you focus your marketing efforts on specific clients who require your services.
- 2. Conduct market research: Research your potential clients to find out their challenges, needs, pain points, and how your services can solve their problems.
- 3. Know your competitors: Identify your competitors and what distinguishes your services, and how you can offer value-added services to your clients.
- 4. Build a brand: Develop a brand that distinguishes you from your competitors and aligns with the needs of your target market.
- 5. Attend conferences and events: Attend conferences and events relevant to your target market to network and identify potential clients.
- 6. Leverage social media: Use social media platforms to publish relevant content, engage with your audience, and drive traffic to your website.
- 7. Build a website: A well-designed website that highlights your services and positions you as a thought leader in the industry helps you attract potential clients.
- 8. Use email marketing: Build an email list of potential clients and use email campaigns to inform them about your services and promotions.
- 9. Develop partnerships: Build strategic partnerships with other businesses in the industry to increase your reach and identify potential clients.
- 10. Offer exceptional customer service: Delivering exceptional customer service helps you retain clients, get referrals, and build a positive reputation in the industry.
Once you have identified potential clients, the next step is to develop marketing strategies that resonate with them. These strategies should communicate how your services solve their challenges, and how your company distinguishes itself from competitors. Here are a few proven marketing strategies:
- Create a blog: Publish educational and informative content that establishes you as a thought leader and gives you credibility with potential clients.
- Offer case studies: Case studies showcase your experience in solving real problems for clients and validate your services to potential clients.
- Run paid ads: Paid ads on social media platforms and search engines help you increase your reach and attract potential clients.
Remember, developing effective marketing strategies requires a deep understanding of your potential clients and how you can help them solve their problems. Stay committed to understanding your target audience, and your efforts will pay off in the form of new clients and revenue growth.
| Subsection: | Identifying Potential Clients and Developing Marketing Strategies |
|---|---|
| Content: | 10 essential steps to identify potential clients and several proven marketing strategies to attract potential clients. |
Identifying potential clients and developing marketing strategies can be a complex process that requires constant refinement. However, by staying committed to understanding your target audience and delivering exceptional services, you can establish a reputation as a reliable provider of clinical research services and drive growth for your business.
Frequently Asked Questions – Starting a Clinical Research Business
1. what qualifications do i need to start a clinical research business.
To get started in this field, you will typically need at least a bachelor’s degree in a related field such as biology, chemistry, or healthcare administration. Having additional certifications or previous experience in clinical research can also be very helpful.
2. What kind of funding is required to start a clinical research business?
Starting a clinical research business can be quite expensive, given the need for specialized equipment and software. Some common funding options include angel investors, venture capitalists, and government grants.
3. How do I go about finding potential clients for my clinical research business?
Potential clients typically include pharmaceutical companies, medical device manufacturers, and academic research institutions. You can start by networking with professionals in these fields or attending industry conferences and events.
4. What are some common challenges I may face when starting my own clinical research business?
Some common challenges include the need for a lot of funding upfront, navigating complex regulations and guidelines, and finding and retaining top talent. It’s important to plan for these challenges and develop strategies to overcome them.
5. How can I ensure that my clinical research business is operating ethically and within all regulatory guidelines?
It’s important to stay up-to-date on all relevant regulations and guidelines, and to work with experienced consultants or advisors who can help ensure that your business is operating ethically and within the law.
6. What are some key factors that will determine the success of my clinical research business?
Key factors include your ability to attract and retain high-quality talent, develop strong relationships with clients, stay on top of the latest advances and trends in the field, and successfully manage your finances.
7. How long does it typically take for a clinical research business to become profitable?
This can vary widely depending on a variety of factors, including the services you offer, the size of your client base, and your overall financial management. In general, it can take several years to become profitable in this field.
Closing Thoughts
We hope this guide has been helpful in getting you started on the path to starting your own clinical research business! Remember to stay committed, plan carefully, and seek out the support and resources you need along the way. Thanks for reading, and we hope to see you back here soon!
How to Start a Laboratory Testing Business: A Comprehensive Guide How to Start a Medical Laboratory Business: A Step-by-Step Guide How to Start a Home Dialysis Business: A Step-by-Step Guide How to Start a Chiropractic Business: A Comprehensive Guide How to Start a Drug Testing Business: Tips and Strategies How to Start a Lab Testing Business: A Step-by-Step Guide
Writing Business Plans for a Life Science Startup or Clinical Program
Topic Relevance by Timeline
The business plan is an important tool for raising capital, finding strategic partners, recruiting, and providing an internal guide on how to drive a company’s growth.
The plan should include an executive overview, introduction to the management team, market and competitive analyses, value proposition, operating plan, financial projections, and potential risks.
The plan should be concise, well written, and dynamic. Details behind key assumptions should be included.
Common business plan pitfalls include focusing only on the product without framing it in the context of the consumers/patients, the market dynamics, and the ecosystem in which it will be launched, as well as giving financials that are too aggressive and precise given the stage the company is in.
New founders should consider engaging experts to help test assumptions as they develop the key parts of the business plan, including the financial projections.
Many of the same concepts for writing a business plan for a startup apply to creating a business plan for a new clinical program or expanding operations within a health system.
Introduction
Building a life science startup is a long and complex endeavor, and the skills required are very different from the knowledge and training that academic scientists undergo. The process of developing a business plan ensures the team has tested their vision/strategy, and the plan can be used as a roadmap to guide their operations. It also serves as an important communication document when seeking investment in the business. Topics familiar to experienced grant writers—such as the significance, innovation, and approach topics from National Institutes of Health (NIH) grant applications—are relevant and necessary for framing the business plan. The plan also helps to keep the management team accountable, but sage entrepreneurs will recognize that they will often have to revise the original plan over time, based on clinical trial results, regulatory milestones, and market changes. There are several kinds of business plans, generally described as either one-page plans (business model canvas or lean launchpad) and traditional, full-length plans. While one-page business plans are a useful and simple tool for rapid, early iteration, the startup team will ultimately have to write a more detailed plan to secure funding (“ Business Model Canvas ”'; Osterwalder and Pigneur ). In this chapter, we focus on the later and will outline the key elements of a full-length biomedical business plan, highlight the pitfalls to avoid, and provide potential resources for new founders to get their plans started.
It is important to note that developing a sound business plan is also highly relevant for supporting smaller-scale clinical programs, operational investments, and intrapreneurship efforts (see the chapter on " Intrapreneurship: Strategic Approaches for Managing Disruptive Innovation in Your Clinical and Research Projects "). Please see the section at the end of the chapter that outlines the key differences for these types of business plans.
Key Elements of a Business Plan
A) executive s ummary.
The executive summary is a one to four page overview of the existing problem/need in the market and how the new product or service has a unique value proposition that addresses that need (Markowitz) . It is the reader’s first impression of the business, and investors often read only the summary, so it is important for it to be compelling ( Figure 1 ). Consider it a concise but more formal “elevator pitch” that highlights what the company is and why the product or service will win in the market (Cohen) . The remainder of the executive summary should include the short- and long-term goals, key points in the strategic plan, the business model, summary of financial projections, and information about employees and location (Valentin) .
Elements in an Executive Summary.
B) Management team and advisors
This section introduces the management team, their backgrounds, and how their expertise aligns with their particular roles in the business. In addition to the management team, there should be a Scientific Advisory Board (SAB), which helps to guide technology decisions, and a Board of Directors (BoD) which helps to guide all key decisions for the company. SAB and BoD members should be chosen based on their ability to provide industry knowledge and key industry connections that can help the company grow. Having industry experts involved in the company gives assurance—especially to investors—that the technology and operations have been vetted and are supported by those who know the market well.
C) Market Overview
This section should describe the overall landscape of the market, including the size of the market, key segments, historical and expected market growth, and key drivers or trends that may impact the problem the startup is trying to solve.
Market size estimates for life science businesses often include the incidence and prevalence of the disease/condition related to the problem, the estimated medical cost burden—in the U.S. or globally—associated with the condition, and the estimated cost for the consumer/patient with the current available solutions (see the chapter “ Conducting Insightful Market Research ”). It may be useful to describe any relevant preclinical and clinical data that support why this problem exists and highlight the market need. While a large market size is usually viewed positively, it is important that the information provided focus on the market size relevant to the startup’s specific solution. For example, a business plan for an intervention to treat prescription opioid addiction should include details about the global market for opioid drugs, the most recent trends and the expected growth rate of prescribed opioid use and abuse, the morbidity and mortality associated with opioid abuse, and the estimated annual cost for the treatment of opioid abuse and addiction, including the subsequent medical and mental health costs. To demonstrate market size in this example, estimates would include the number of emergency department visits, hospitalizations, intensive care unit admissions, and procedures for overdose or opioid abuse–related complications, as well as the current number, length, and cost of drug rehabilitation admissions.
In terms of the current market, it is useful to describe how the market is divided into customer/patient segments, which may be by geographic region, demographics, psychographic, or customer type (in healthcare, this might be pediatric vs. adult, or inpatient hospital vs. outpatient setting), among others, who are using a certain type of product/service. In the above example of the opioid market, there is segmentation in application (pain relief, anesthesia, cough suppression, diarrhea suppression, or treating addiction) and by geography (North America, Europe, Asia-Pacific, South America, and the Middle East/Africa, which can be further broken down by key countries), which can then be mapped to the types of opioid products with different mechanisms of action (for example, short acting vs. long acting opioids). If the market has clear customer/market segments, there are likely different drivers of demand in each of them, which should be well understood since the startup will want to provide product/service offerings that meet the needs of those segments. Describing the market structure, how this market is segmented, and projected growth rate of target segments will make it easier to determine which segments are the most valuable and to describe how they will be targeted.
As the market data are outlined, it is important to tie back to the product/service offering and how it is uniquely positioned to fulfill the unmet need(s) within the particular target markets (see the chapter “ Identifying Unmet Needs: Problems that Need Solutions ”). Use market research, economic trends, and even patient and provider behaviors, if appropriate, to determine what sector(s) of the market the product fits best. Understand the needs of patients, providers, and hospitals and why the startup’s strategy will meet these needs, in order to better prepare the marketing plan for the product. Moreover, it is important to highlight the attributes of the market that support the offering, such as a large addressable market size, rapid growth in the market segment(s) of interest, and/or the level of competition in these segments. These are dimensions that are critical to stakeholders when they evaluate the business plan.
D) Competitive Landscape
The problem the startup is seeking to solve is one that multiple incumbents are likely already addressing today, either directly or indirectly. This section should provide an overview of the current offerings in the market, where they fall short, and how the new offering fills a current gap in the market. In the above sections, the problem should have been outlined and framed in a way that there would be critical dimensions that matter to stakeholders (patients, physicians, providers, payers). It would be helpful to describe how these competitive offerings compare across these dimensions, which sets up the next section on value proposition.
Understanding the competitors’ product and services, market share, current and past strategies, strengths and weaknesses, the threats they pose to the startup, and the opportunities they make available are integral to a thorough and useful analysis of the competition. This is not just an exercise to learn about other businesses; it will also help identify the strengths and weakness of the startup’s business strategy (see the chapter “ Startup Company Formation and Management ”). Consider developing a basic profile of each of the current direct competitors in the market with these characteristics and include it in the Appendix.
Typical sources of information are company websites and marketing materials (Hisrich et al.) . Academic libraries can also provide a wealth of information through their subscriptions. Other helpful strategies include browsing media outlets for press releases and public relations information, social media, and former customers’ testimonials on how they perceive the competition.
E) Value Proposition
Building off the market need and competitive landscape analysis, the value proposition of the startup’s solution should be articulated in this next section. Against the dimensions that matter to stakeholders, this section of the plan should describe how the new solution will outperform the competition. The value proposition statement is a key way to succinctly demonstrate the measurable benefit that the patient or provider would get from the new product or service, and why patients or health care providers would choose it over existing solutions. Money savings, time, and convenience add to the value of a product. It is important to explain this in a way that can be understood by both scientific and nonscientific audiences. Describe the product/service without revealing too much proprietary information since the business plan may be distributed beyond the intended recipients.
The research results that led to the development of the new product should be shared. This may include pilot data, preclinical/animal model studies, and/or clinical trials, depending on what stage of testing the product has undergone (see the chapter “ Pre-Clinical Animal Models ”). Provide preliminary data and reference specific publications that support the product. In addition, any results of prototype testing should be included.
In the life sciences realm, even if the product solution meets the needs of a patient, ensuring that it fits into the medical ecosystem is imperative. Understanding the infrastructure of a hospital, including the physicians, the administrators, the insurance payers, and whether or not the new offering will improve a patient’s quality of life or improve outcomes such that payers will reimburse the startup for its technology is critical to success. It is also important to articulate (if applicable) whether or not the offering can be dropped into existing treatment algorithms/processes or if changes will need to be made to how work is done to adopt the solution. If a lot of re-training or adjustments around the rest of the ecosystem are required, the value proposition will be more challenging since a lot of changes will need to be made to adopt the solution.
As an update to this chapter, I recommend “The Triple Win Framework” to write a successful value proposition. Here is a quick intro course to using the framework. —
|
|---|
F) Operating Plan
With the market and competitive landscape outlined, and the company’s value proposition defined, this section should describe how the company will execute to capitalize on the opportunity. The operating plan should begin with a thorough explanation of the business model—how the company will work successfully with clients, suppliers, manufacturers, and partners to generate profit. Include here the organizational structure of the company. Next, describe all critical technical, regulatory, and strategic milestones. Finally, outline any functional details about daily execution (Friend and Zehle) .
There are numerous business models, and any one industry may have several examples of successful companies using different approaches. For example, is the company going to adopt an integrator model, where they will build out everything needed to launch the offering, or will they adopt an orchestrator model, where they will partner with people for certain core competencies (e.g., manufacturers) to bring the solution to market? The operating plan should describe the selected model and explain why it is preferable to alternatives. Referencing the leading players highlighted in the competitive landscape section and contrasting against their business models may also be helpful.
Healthcare businesses must deal with reimbursement, fee schedules, billing systems, managed care contracts, and licensing, along with operational issues. The operating plan must address how these challenges will be handled and how the company will get paid, either through insurance reimbursement, by employers, on a fee-for-service basis, or directly by consumer payments. The long sales cycle in most health care businesses is particularly challenging for startups and requires keen long-term planning.
Reimbursement by third-party payers to hospitals and physicians is one of the determinants in whether or not a product will ultimately make it to market, whether it will be used by healthcare providers and patients, and how accessible the product will be. For most technology in healthcare, the payers account for most of the purchasing. Understanding the payers’ reimbursement process, their reimbursement terms, their method for determining the amount to be paid to the provider, and their policy on out-of-pocket cost sharing with the patients is integral as the reimbursement method will impact return on investment for the business (see the chapter “ Reimbursement Strategies and CPT Codes for Device Development ”).
After establishing the business model, it is important to provide an overview of the significant milestones the company foresees. Include any remaining technical development goals, any regulatory approvals the company will face, and other strategic imperatives, such as licenses to related technology, critical partnerships, or protecting intellectual property. Biotechnology and biomedical devices may also need to go through extensive regulatory and legal processes before approval. These processes are outlined elsewhere in this textbook (see the chapter “ FDA Device Regulation: 510(k), PMA ” and, “ FDA Drug Regulation: Investigational New Drug Applications ”).
Each of these milestones should include a description of the task, due date, budget, and responsible person. Due dates and budgets should be ranged since it is difficult to have 100% clarity; milestones function as the management team’s commitment to investors, and the company’s ability to complete these goals will be assessed.
Young businesses should also provide details about the market entry strategy to penetrate the targeted market effectively and to reach revenue and profit expectations. To develop this strategy, engaging with and understanding the ecosystem early on can help improve the design of the new offering and ensure that the solution can be reimbursed. Ideally, during this process one should meet with representatives across the ecosystem to understand what they care about and what the startup will need to deliver in order for them to embrace the new offering.
The technical side of the operating plan should include tactical steps and a timeline for implementing the plan and making the business operational. As a reference point, mention what has been done thus far. Explain how the business will operate, describing the current production process but also the planned process once the company is at scale. Include high-level details about labor, materials, technology, facilities, equipment, manufacturing processes, distribution plan, supply chain, and quality-control measures.
G) Financial Projections
In this section, the team must articulate the financials of the company and show that they have a solid understanding of their expenses, future revenue, and the projected timeline for achieving revenue goals. There will be many assumptions that go into these estimates, so it is important to provide ranges and to explain the assumptions behind the projections. Potential investors will review them to gauge the robustness of an entrepreneur’s understanding of the challenges that lie ahead.
A startup’s financial statements should detail the anticipated financial performance over time (for example: expenses, assets, liabilities, and working capital). Since the financial performance of the startup is dependent on future events (e.g., regulatory approvals or clinical trial partnerships), the financial projections will likely need to be in the format of a ‘pro forma’ budget, which projects future revenues and expenses based on a set of assumptions. Projections of financial statements should go far enough into the future to help readers see where the business can go when it matures or reaches an exit point. Outlined below are the three major parts of the financial plan ( Kolchinsky ; Friend and Zehle ).
The income statement shows the revenue, expenses, and profit for the business over a specific time period. If there are multiple sources of revenue, these may be itemized for future comparisons over time. Early on in the business development, this may be generated monthly and eventually quarterly or annually. An income statement showing earnings before interest, taxes, depreciation, and amortization (EBITDA) acts as a frequent proxy for a cash flow statement.
A statement of cash flow projections shows what the company expects to bring in and how much it will be spending each month. This includes tracking the cash revenues and cash disbursements for the month, and reconciling these two against the opening balance from the previous month. It is important to demonstrate that the startup can adhere to a budget and not overspend consistently. Thus, conservative estimates are preferred; this will increase the level of expenses, but the company should be able to justify why those expenses are needed. Additionally, the statement should show how much working capital the startup needs to pay the bills early on, and how long it will take to have a positive cash flow (bringing in more money than the company is spending). By estimating conservatively, the team can ensure that the company will have enough financial support (i.e., runway) to achieve the milestones without falling short of cash and going bankrupt.
The balance sheet highlights any major working capital requirements and includes assets, liabilities, and equity. Rather than showing trends, the balance sheet reflects these as of a set date.
The additional components of the financial plan detail how the company expects to make money selling the product. These include:
the cost of the product, what factors go into the unit cost, and the plans for bringing these costs down when the company is at scale;
cost estimates for equipment, facilities, inventory, and day-to-day operations, including salaries;
the price the company expects to receive for the product and why it is achievable, especially in the context of reimbursement.
Due to the long process of taking a product to market in healthcare businesses, there should be a section in the financial plan about the capital required for the various regulatory milestones. As outlined in the operating plan, all product development, technical, and regulatory milestones would come with an estimated budget and timeframe for completion. Included in this is the cost of the Food and Drug Administration’s drug review process—a major consideration in the financial projections for life science startups. From preclinical research to the Investigational New Drug application, to clinical trials, to the New Drug Application submission and review, this process is expensive and takes many years to complete. How far along a product is in this process will factor greatly into how much money will be necessary to complete Phase 1, 2, and/or 3 trials, as well as the subsequent regulatory requirements.
Clinical trials can incur substantial costs beyond distributing the study drug/device and the associated study procedures. Site costs, fees for storage, technology solutions, and safety monitoring, core lab fees, and study staff salaries—for scientists, physicians, project managers, data managers, research coordinators, biostatisticians, and site management, including regulatory visits and investigator meetings—all must be budgeted into the cost of each phase, and the timeline to complete the data collection should be considered. In addition, up to 30% of any grant funding may need to go toward administrative overhead to carry out the study, if implemented at an academic medical center. In some cases, academic entrepreneurs can establish sponsored research agreements that allow components of the preclinical or clinical research to occur at their university (see chapters on “Post Alliance Agreements and Sponsored Research Agreements” and “ Understanding Conflict of Interest for Academic Entrepreneurs ”). As mentioned earlier, many of these projections may be pro forma because they will rely on the achievement of other milestones prior to implementation.
H) Risks/Anticipated Problems
All business plans should include a section on anticipated risks/problems and potential alternative strategies. This can demonstrate to investors that the entrepreneur has thought through potential challenges and has plans to either prevent them from occurring, or backup plans to mitigate the consequences. Generally speaking, a balanced approach is helpful here—not hiding or obfuscating major challenges, especially those that have befallen other companies, but at the same time not overwhelming the reader with negativity. At the very least, this can be a thought exercise for the startup and may identify issues that had not previously been considered. While a business plan is not a legal document or binding contract, intentional distortion of facts can come back to haunt a company.
Business Plans for Clinical or Operational Programs
There will be many situations where an idea is not yet at the stage of becoming a company, but requires investment to drive growth or sustain operations. Examples include developing a new clinical program or service (e.g., a novel surveillance protocol postsurgical intervention that will result in diagnostic testing revenue) or expanding operations (e.g., building a new facility to treat patients who have an eating disorder). These situations also call for business plans in order to garner support and investment—in this case, the investment would be coming internally from the health system or institution rather than outside investors.
The key components of these business plans are executive summary, background, proposal description, market and competitive analyses, operating plan, metrics for success, financial projections, and potential risks. Many of the same concepts from earlier apply, but the key differences include: 1) the business plan should specify the dollar amount of the resources needed to make the plan operational; and 2) the proposal description, operating plan, financial projections, and risks should take into account the impact of the plan on the existing operations of the system.
As mentioned earlier, most health systems will have internal strategy and finance teams, which are helpful resources for developing business plans and should be consulted early on to help with the financial projections as well as the anticipated system impacts of the plan. The remainder of this section outlines each part of the business plan and focuses on the key differences from a startup business plan.
Executive summary: In addition to the points covered in the startup executive summary, the executive summary for a new program/service/facility should include the “ask”—what resources (e.g., capital, new full-time employees, or other operating expenses) are being requested to support the new program. The executive summary should also state the expected financial return on the investment from the perspective of the institution (usually in terms of annual steady-state contribution margin or total incremental contribution margin over a period of time, usually seven years).
Background: This section should describe the current state of operations. If the authors are proposing a new clinical program, for example, they should describe the patient population being addressed, how they are currently being served, and the current volumes. It should also describe the limitations of the current state, the unmet need, and what factors necessitate a new solution.
Proposal description: The authors should describe what new program/service/facility is being proposed, and how it addresses the current challenges. This section should also describe how the proposal will lead to growth—will the program reach a new patient population not previously served? Have a greater geographic reach? Result in greater utilization of other services at the institution? This is also an appropriate place to describe other benefits of the plan, including improved quality, safety, patient experience, efficient resource utilization, etc. It is important for this section to align with the institution’s priorities.
Market and competitive analyses: This section should look very much the same as described above in the startup business plan.
Operating plan: This section should build on the proposal description and go into more technical detail. Details should include how the program will be staffed, what type of services will be delivered, where they will be delivered, hours of operation, pricing and reimbursement, and what the impact will be on downstream services and the rest of the institution. Thus, it requires a detailed understanding of how the institution currently operates, so the new proposal can realistically be integrated without excessive disruptions to operant workflow.
Metrics for success: The authors should list two–three measurable metrics that will show the success of the program. These can include volume, financial, patient/staff satisfaction, or other related metrics. Ideally, there should be targets for each metric (e.g., “increase volume by 10% above baseline by year 3”). For plans where measurable impact will not be realized until several years out, milestone-based targets are also acceptable as near-term goals (e.g., “achieve regulatory approval by X date”). It is preferable that these metrics be ones that the institution already monitors, thus enhancing alignment with current priorities and facilitating the ability to add this new project. In some cases, though, it may be necessary to develop new metrics, which should be done in consultation with the institution’s leadership.
Financial projections: This section should include all components outlined above, with the exception of a balance sheet, since the plan is only describing a subset of operations within a larger system. For the pro forma income statement, it is useful to show multiple views: a base case view (if applicable), which shows current operations of the program/facility; an incremental view, which just shows the incremental revenue and expenses associated with the proposed plan; and a strategic view, which is the addition of the base case and incremental. It is also highly recommended to have a separate section outlining the assumptions used to develop the financial projections (e.g., data source, patient population, growth projections, operational start date and ramp-up speed). As these business plans are usually in the context of a large academic institution, it is helpful for planning purposes for the financial statements to specify the organizational entity to which the projected revenues and expenses will accrue (e.g., Hospital vs. Practice Plan vs. Research).
Risks and mitigation: This section should look very much the same as described above in the startup business plan. In addition, the plan should consider potential risks/negative impacts to other parts of the institution. For example, if the plan proposes to build a new facility in a suburban location, will it potentially cannibalize volume from the main location, and if so, what is the plan to mitigate revenue loss?
The same tips on business plan writing apply here. Since clinical and operational programs need to function within the environment of an existing system, socializing the plan early and often is especially critical for gaining support, both for funding the plan and for implementing it. In some cases, this may involve obtaining regulatory approval from internal institutional entities, such as the institutional review board (IRB), legal counsel, or information services (especially if information protected under the Health Insurance Portability and Accountability Act (HIPAA) is involved). Factoring time for these approvals is crucial.
External Resources
In academic entrepreneurship, there are a host of extra challenges that must be considered, including who owns the intellectual property, technology transfer agreements, regulatory procedures, and conflict of interest, among others (Kolchinsky) . At the same time, being part of an academic institution provides many resources not available to non-academic entrepreneurs, including business planning, legal, and funding support. Networking and taking advantage of other schools within the academic system who have experts in these fields will improve the entrepreneur’s knowledge, especially when entrepreneurship is not one’s first career or when this is one’s first experience with developing a product. This can be particularly valuable when estimating costs related to preclinical and clinical research. Many academic centers have clinical research offices that have pre-populated budget templates, which can help to ensure that all aspects of the clinical trial are adequately budgeted. Most academic systems also have corporate Strategy and Finance teams that can help with business analyses as well, such as market/competitive dynamics and projecting future demand (whether that be in terms of patient volume, demand for the product, etc.). In addition, university libraries may provide online access to publications and market research related to the product and the industry. For specific tasks, such as preparing an exit strategy that involves an initial public offering (IPO), extensive external legal and financial consultations are generally required.
Tips on Business Plan Writing
Remember that a business plan is dynamic, changing with the growth of the company and as the company pivots due to unexpected challenges or market trends. The plan should be comprehensive, and key points, data, and strategies should be consistent throughout. Be mindful of including important details without going into excessive minutiae. Lastly, this is a professional document, and it can be the first and lasting impression of the company on potential investors, so appearance matters. To this end, recruit trusted editorial support to make sure the plan is consistent from beginning to end and to check for correct spelling, grammar, and formatting.
Writing a business plan is a process. It requires brainstorming, researching, prewriting, drafting, revising, and editing. The business plan is not only an internal company tool for thinking about the future, it is also about presenting the company’s idea, solution, marketing strategy, financial projections, and long-term vision to prospective investors. It is an introspective exercise that each executive team should utilize to help identify misconceptions before they become costly, to organize and streamline team members’ efforts, to focus on the big picture rather than getting caught up in short-term actions, and to establish performance standards for the company (Valentin) . It is important that all key stakeholders participate in the development and writing of the business plan. As the money at risk increases, the benefits of having a well-developed business plan increase as well.
U.S. Small Business Administration .
BPlans , produced by Palo Alto Software, is a free resource for new entrepreneurs with tools, webinars, and templates for writing a business plan.
Business Plan for Scientists .
Business Plans Handbook : Craft, D. Business Plans Handbook, vol. 42, pp. vii–viii. Gale, 2018. Gale Virtual Reference Library .
The contents of this chapter represent the opinions of the chapter authors and editors. The contents should not be construed as legal advice. The contents do not necessarily represent the official views of any affiliated organizations, partner organizations, or sponsors. For programs or organizations mentioned in this chapter, the authors encourage the reader to directly contact the relevant organization for additional information.
Great article, thank you!
Thanks, that's exactly what I was looking for! I read an article about tech company business plan , everything is quite detailed. But I needed a narrower specialty. Your post is just right!
Author Linda Miller and her colleague Brendon Ardietta recently conducted a CHOP workshop on articulating your idea as a “triple win” in healthcare: value for patients, clinicians/researchers, and financers (e.g. CHOP, grant funders, VCs). See video and handouts

- Content Index

How to start a clinical research site
31st Jul 2018

Establishing a new research site can be exciting but challenging – to be successful there needs to be a lot of preparation and hard work
Starting a clinical research site is an exciting opportunity for the ambitious research professional looking to progress from employee to site ownership, or the medical entrepreneur looking to expand a portfolio with a lucrative investment. Clinical research provides financial and altruistic appeal: a successful site will conduct a diversity of studies with commensurate compensation. The data generated (from trials conducted) could possibly contribute to a regulatory agency submission for new drug approval. At the very least, a successful site will impact the drug development process.
Beyond the altruism is the enormity of work involved in establishing an investigative site. From funding to infrastructure, staffing to strategic connections, it takes business and clinical acumen to start and a salesperson’s mentality to sustain a successful study site.
Appropriate funding is integral to starting and continuing the site momentum until new trials can sustain the effort. The investigational site founders must know the amount needed to start the site, and how long the funding will cover all costs until xxx deadline and the need to re-fund or re-evaluate the effort.
Some prospective site owners self-fund-with personal savings or business loans, while others align with investors or physician groups looking to expand into clinical research. Gualberto Perez MD, founder and president at Research GCP, and a successful research site owner, has participated in several successful financial strategies for site start up. Perez states that “it all depends on the size of your start up. When I first started many years ago (25 approximately) we commenced in a big way with a full-blown phase I unit— multiple private investors; we later borrowed from a bank”.
Ten years later Perez opened his late-phase site GCP Research with his own funding. He grew it organically in a financially graded fashion, expanding to other services and embarking into outpatient phase I work. His success is due to his expertise, and the incredible work ethic needed to start an investigational site.
The right location is dependent on the site model and overall objective. An ambitious early phase site may need a larger facility to accommodate specific serial testing (blood, respiratory, vitals) and visit duration (overnight and or extended patient stays), while a medical office/clinic would accommodate the needs of an investigational site conducting simpler late-phase trials. The basic location requirements are examination room/bed, locked area for refrigerated or ambient study drug storage, secure storage for supplies/records, staff and monitor work areas, a room for specific activities (if needed) such as blood draws/electrocardiogram/pulmonary function testing and a lab area for blood/urine processing, (centrifuges) storage/shipment (freezers). There are additional lab certificate/waiver requirements dependent on the type and extent of lab testing to be completed at the site.
Establishing infrastructure with standardised clinical research process (informed consent, safety reporting, deviation reporting, study drug administration/destruction, staff on boarding and training, etc.) and staff job descriptions will promote consistency and transparency with job performance. Centralised filing of staff licensure and training certificates (for such things as good clinical practice, equipment, and computer systems/study devices) to correspond with the delegation of authority log demonstrates due diligence and appropriate task delegation.
Patient recruitment is integral to successful study conduct and sponsor consideration for repeat business. For a dedicated research site, affiliation with hospitals, clinics and/or physician practices is critical to identify and enroll patients, as well as recruitment staff to develop tools/tactics to advertise the studies. Alternatively, some investigators house the research department within their physical medical practice and may have the opportunity to review their practice database for potential patients.
Hiring staff
The ideal functional team includes the experienced physician investigator, study nurse/coordinator/data manager (to divide clinical and administrative, scheduling and data management duties), experienced finance/legal/business personnel to handle contracts, budgets, and business strategy to optimise site growth, and key individuals with the network/relationships to attract investigational studies.
Role is also driven by site size and business model. A study coordinator at a smaller physician practice will likely wear many hats and complete study duties as well as contracts, regulatory and recruitment. A larger research site will have separate personnel for clinical, data management, legal and administrative responsibilities, as well as dedicated business development staff to cultivate relationships.
The smart administrator or investigator will develop connections with local imaging, ophthalmology, spirometry, e.g. medical offices that perform ancillary testing, so that if a study presents with specific safety testing requirements for study drug, the ancillary facilities are already vetted and capabilities confirmed. This strengthens a site’s credibility and helps diversity their therapeutic portfolio.
Dan Sfera, CEO of DSCS CRO and “The Clinical Trials Guru” podcast host, as well as a successful investigative site owner, feels that adaptability and salesmanship are two critical traits for successful site owners. Sfera says that the effort should not be underestimated when starting an investigational site.
“If there are any important lessons that I’ve learned to own a clinical research site, it is the fact that there are almost no rules or guidelines or standards for how to structure your business. When starting out, it could be very challenging and overwhelming for somebody coming from a structured environment, such as a CRA or a study coordinator, to go out on his/her own and become a site owner. At the end of the day, the most important concept that you need to understand and master is the art of salesmanship. You must understand that from the very minute you start your site as a business owner, you are a sales person first. It does not matter what your background is. It does not matter what you’ve done in the past. You have to master sales.”
Sfera also gives two pivotal examples of adaptability as a site owner; “the ability to adapt. I talk about this all the time. Your first principal investigator will probably leave you at some point. The studies that you specialise in at some point will go through a downcycle. The patients that used to get referred to you will at some point stop. The same thing goes for your employee’s retention.”
Whatever the motivation, or business model pursued, an individual with the tenacity, experience and work ethic has the potential to build a successful research site that will benefit society and the employees vested to make it successful.
News alerts
Latest content.

Contact details
PharmaTimes Media Ltd. Mansard House Church Road Little Bookham Leatherhead Surrey KT23 3JG
E: [email protected] E: [email protected] T: +44 (0)20 7240 6999 F: +44 (0)20 7240 4479
A Comprehensive Guide to Clinical Research Organizations (CROs)

Clinical Research Organizations (CROs) play a crucial role in the pharmaceutical, biotechnology, and medical device industries. They provide support to companies in the form of research services outsourced on a contract basis. In this comprehensive guide, we will explore what CROs are, who their clients are, the stages of the research process they are typically involved in, and delve into the exciting career opportunities within the field of clinical research.
Table of Contents
Introduction to Clinical Research Organizations (CROs) The Role of CROs in the Research Process Services Offered by CROs Clients and Partners of CROs Careers in Clinical Research Clinical Research Associate (CRA) Roles and Responsibilities Educational and Professional Requirements for Clinical Research Careers Advancement Opportunities in Clinical Research Tips for Success in Clinical Research Careers Resources and Professional Organizations Conclusion
Introduction to Clinical Research Organizations (CROs)
Clinical Research Organizations (CROs) are companies that provide support to the pharmaceutical, biotechnology, and medical device industries in the form of research services outsourced on a contract basis. They offer a wide range of services , including biopharmaceutical development, clinical development, clinical trials management, and pharmacovigilance. CROs aim to simplify the entry into drug markets and streamline the drug development process by providing specialized expertise and resources.
The Role of CROs in the Research Process
CROs play a crucial role in the research process, from the early stages of drug discovery and development to the final stages of clinical trials and commercialization. They work closely with their clients to design and execute clinical trials, ensuring adherence to regulatory requirements and ethical standards. CROs also provide support in data management, statistical analysis, and the preparation of regulatory submissions.
Services Offered by CROs
CROs offer a wide range of services to their clients, including but not limited to:
Biopharmaceutical development : CROs assist in the development of new drugs, from preclinical studies to early-phase clinical trials. Clinical development: CROs design and manage clinical trials, ensuring compliance with regulatory requirements and ethical standards.
Clinical trials management: CROs oversee all aspects of clinical trials, including site selection, patient recruitment, data collection, and safety monitoring.
Pharmacovigilance : CROs monitor the safety of drugs and medical devices during clinical trials and after they are on the market.
Real-world evidence and outcomes research : CROs collect and analyze data from real-world sources, such as electronic health records, to generate evidence on the safety and effectiveness of drugs and medical devices. CROs conduct studies to evaluate the effectiveness and safety of drugs and medical devices in real-world settings.
Clients and Partners of CROs
CROs work with a diverse range of clients, including pharmaceutical companies, biotechnology firms, medical device manufacturers, research institutions, and government organizations . They collaborate closely with their clients to ensure that research studies are conducted efficiently, safely, and in compliance with regulatory requirements. CROs also partner with academic institutions and foundations to support their research initiatives.
Careers in Clinical Research
Clinical research offers exciting career opportunities for individuals interested in the scientific, regulatory, and operational aspects of drug development.
Careers in clinical research span a wide range of roles, including Clinical Research Associate (CRA), Clinical Project Manager, Data Manager, Biostatistician, and Medical Writer, among others. These roles require a combination of scientific knowledge, attention to detail, critical thinking, and strong communication skills.
Clinical Research Associate (CRA) Roles and Responsibilities
Clinical Research Associates (CRAs) play a crucial role in the execution and monitoring of clinical trials. Their responsibilities include site selection and initiation, monitoring study progress, ensuring compliance with protocols and regulatory requirements, and maintaining accurate and complete documentation. CRAs work closely with investigators, study coordinators, and other stakeholders to ensure that trials are conducted safely and efficiently.
Educational and Professional Requirements for Clinical Research Careers
Careers in clinical research typically require a strong educational background in life sciences or a related field. Many positions, such as CRAs, require a bachelor's or master's degree in a scientific discipline. Professional certifications, such as the Certified Clinical Research Associate (CCRA) certification, can enhance career prospects and demonstrate expertise in the field.
Advancement Opportunities in Clinical Research
Clinical research offers ample opportunities for career advancement and professional growth. Experienced professionals can progress to more senior roles, such as Clinical Project Manager or Clinical Operations Director, where they oversee the planning and execution of multiple clinical trials. Continuing education, networking, and staying updated with industry trends are essential for career advancement in clinical research.
Tips for success in seeking a Career in Clinical Research
To succeed in clinical research careers, professionals should continuously develop their scientific knowledge, stay updated with regulatory requirements, and enhance their communication and project management skills. Networking, building relationships with key stakeholders, and seeking mentorship can also contribute to career advancement in the field.
Resources and Professional Organizations for Clinical Research Professionals
Several resources and professional organizations cater to the needs of clinical research professionals. These include industry publications, online forums, conferences, and professional associations. Organizations such as the Association of Clinical Research Professionals (ACRP) and the Society of Clinical Research Associates (SoCRA) provide educational resources, networking opportunities, and professional certifications for clinical research professionals.
Clinical Research Organizations (CROs) play a vital role in the pharmaceutical, biotechnology, and medical device industries by providing research services on a contract basis. They offer a wide range of services to support the development and execution of clinical trials, ensuring compliance with regulatory requirements and ethical standards.
Careers in clinical research offer exciting opportunities for individuals interested in the scientific, regulatory, and operational aspects of drug development. By partnering with CROs and pursuing careers in clinical research, professionals can contribute to the advancement of medical science and the development of innovative therapies.
If you're interested in exploring careers opportunities in clinical research, view our current vacancies at ICON today.
Sign up for post alerts
Icon & you the potential of together..
Careers that improve the lives of patients, our clients and each other. Are you ready to make a difference?
Related jobs at ICON
UK, Reading
Full Service - Development & Commercialisation Solutions
Johannesburg
Remote Working
Hybrid: Office/Remote
Business Area
ICON Full Service & Corporate Support
Job Categories
Clinical Programming
Description
At ICON, it’s our people that set us apart. Our diverse teams enable us to become a better partner to our customers and help us to fulfil our mission to advance and improve patients’ lives. Our ‘Own I
Expiry date

Clinical Trial Management
ICON Strategic Solutions
ICON plc is a world-leading healthcare intelligence and clinical research organisation. From molecule to medicine, we advance clinical research providing outsourced services to pharmaceutical, biotech
2024-113639

India, Chennai
Drug Safety
We are currently seeking a Pharmacovigilance Associate to join our diverse and dynamic team. Pharmacovigilance Associate What You Will Be Doing: Review and process safety events (pre-marketing, post

Netherlands
Clinical Monitoring
As a Clinical Research Associate, based in the Netherlands you will be joining the world’s largest & most comprehensive clinical research organisation, powered by healthcare intelligence.
2024-113053

Related stories
.png)
Teaser label
Content type
Publish date
Building a Career in Clinical Research Are you considering a career that blends purpose, innovation, and growth? We sat down with Andrew Purchase, Site Head at ICON Swansea, to learn about his jou
Discover Andrew’s inspiring career journey at ICON since joining as a graduate in 2006.

From Navy Corpsman to ICON Associate Project Manager: Elizabeth's Journey ICON is proud to spotlight Elizabeth, a recent addition to our team as an Associate Project Manager through the Veteran Le
Discover Elizabeth's inspiring journey from a Navy Corpsman to an Associate Project Manager at ICON Plc.
.png)
In today's competitive job market, staying ahead of the curve is crucial. As artificial intelligence (AI) continues to reshape various industries, it's also transforming the way we search for and
Learn how to use AI for resume building, job matching, and interview prep in our comprehensive guide.
Recently viewed jobs
A better career. A better world. A better you.
Browse popular job categories below or search all jobs above

Top 17 Clinical Research Organizations (CRO) in 2023
In clinical research and treatment development, clinical research organizations (CROs) are frequently a sponsor’s most important partner and ally.
Depending on the nature of the clinical trial, and your existing capabilities as a sponsor to run the trial, the CRO company of your choice will typically be responsible for facilitating most of the micro and macro processes that go into designing and running a successful clinical trial.
When contracting a CRO to help you with your trial, you are transferring over a large portion of responsibility into the hands of your clinical research partner. The CRO of your choice will have the responsibility to control a variety of factors and processes of a clinical trial, and depending on their expertise, team structures, service offerings, internal resources and many other capabilities.
Your ability to find and contract a top CRO company that is the right fit for your unique trial will be a determinant of whether or not you will be able to operate a high-quality clinical trial that meets your expected timelines, budget and delivers a top-notch patient experience.
At ClaraHealth (a patient-centric recruitment acceleration platform) , we have put together an extensive list of the top CRO companies in the US and around the world.
This is not a cro rankings list, but rather a compiled list of some of the top clinical research organizations around the world. We have highlighted their strengths and core service offerings to make it easier for you to find the right fit clinical research partner.
In addition, we’ve put together a list of 9 fundamental questions to ask the prospective clinical research organization , which will help you to save time and ensure a right fit in picking the CRO.
Formerly known as Quintiles and IMS Health, IQVIA is one of the largest CROs in the world, with a large range of service offerings to help advance clinical research.
The company was founded in North Carolina in 1982, and has since grown to over 88,000 employees in more than 100 countries.
Some clinical trial solutions offered by IQVIA include:
- Assistance with protocol design
- Design of phase 1 clinical trials
- Assessment and improvement of phase 2 and 3 clinical trials
- Site identification & selection
- Patient recruitment
- Access to global laboratories via their wholly owned subsidiary Q2 Solutions
Parexel is a global clinical research organization that was founded in 1982, and specializes in conducting clinical studies on behalf of its pharmaceutical partners in order to accelerate and ensure the drug approval process of up-and-coming potential treatments. It currently operates in more than 50 countries, and is run by more than 18,000 employees around the world.
The company has a wide range of service offerings, covering nearly every type of clinical trial service to assist sponsors in running successful clinical studies.
Some clinical trial solutions offered by Parexel include:
- Clinical trial design and development for early phase, phase 2 & 3, and late phase clinical trials
- Clinical data management
- Decentralized clinical trials
- Clinical supply chain management
- Medical writing
- Regulatory affairs consulting
- Pharmacovigilance
3. PRA Health Sciences
PRA Health Sciences is one of the largest contract research organizations in the world. Founded in 1976 under the name “Anti-Inflammatory Drug Study Group”, the company was renamed to PRA in 1982. PRA Health Sciences employees more than 17,000 people, and provides coverage to more than 90 countries.
In 2021, PRA Health Sciences was acquired by the Ireland-headquartered global CRO leader ICON, which is also reviewed in this list.
Some clinical trial solutions offered by PRA Health Sciences include:
- Decentralized Clinical Trials Platform
- Protocol Consultation & Study Design
- Onsite Support services
- Customized Solutions for Biotech (such as asset valuation, regulatory strategy, engagement and support, drug development strategy and funding solutions)
- Clinical Diagnostics
- Site Commercial Solutions
- PRA’s Laboratories for Drug Development
Headquartered in Ireland, ICON was founded in 1990 in Dublin by co-founders John Climax and Ronan Lambre. The company has since grown to be one of the largest CROs in the world. As of September 2020, the company employs more than 15,000 people in 94 locations and across 40 countries.
ICON offers clinical research services which include consulting, clinical development and commercialization across a wide range of therapeutic areas.
In 2021, ICON acquired PRA Health Sciences, which is another CRO and global leader in clinical research services.
Some clinical trial solutions offered by ICON:
- Commercial Positioning
- Early Phase
- Functional Services Provision
- Laboratories
- Language Services
- Medical Imaging
- Real World Intelligence
- Site & Patient Solutions
- COVID-19 Clinical Operations
5. Syneos Health
Formerly known as InVentiv Health Incorporated and INC Research, Syneos Health is a publicly listed and global contract research organization. The company is based in Morrisville, North Carolina, and specializes in assisting companies with late-stage clinical trials. Syneos Health currently employs more than 25,000 people, and has offices across 91 locations.
In early 2018, INC Research was acquired inVentiv Health, and the merged company was named Syneos Health.
Some clinical trial solutions offered by Syneos Health include:
- Decentralized Clinical Trials Solutions
- Bioanalytical Solutions
- Phase II-III/Phase IIIb-IIIV
- Medical Device Diagnostics
- Clinical Data Management
- Clinical Project Management
- Clinical Monitoring
- Drug Safety & Pharmacovigilance
- Site and Patient Access
6. Labcorp Drug Development (Formerly Covance)
Formerly known as Covance and renamed to Labcorp Drug Development in early 2021, this CRO is one of the largest contract research organizations in the world. The company claims to provide the world’s largest central laboratory network, and has been rated as one of the best places to work for LGBTQ+ equality by the Human Rights Campaign organization in 2018 to 2021. Currently, Labcorp employs over 70,000 people and is able to support clinical research efforts in almost 100 countries around the world.
Some clinical trial solutions offered by Labcorp Drug Development include:
- Preclinical Services
- Clinical Trials
- Clinical Trial Laboratory Services
- Post-Marketing Solutions
- Medical Devices
- Data & Technology
Also known as Pharmaceutical Product Development, PPD is a large global contract research organization headquartered in Wilmington, North Carolina. Started as a one-person consulting firm in 1985, PPD has grown to over 27,000 employees worldwide, and provides a wide range of clinical research services to pharmaceutical and biotech companies.
Some clinical trial solutions offered by PPD include:
- Clinical Development
- Early Development
- Peri- and Post-Approval
- PPD Biotech
- PPD Laboratories
- Product Development and Consulting
- Site and Patient Centric Solutions
8. Fisher Clinical Services
Part of Thermo Fisher Scientific, Fisher Clinical Services is a global clinical research organization with headquarters in Center Valley, Philadelphia.
The company has been in the business of clinical supply chain management for over 20 years, and is focused exclusively on working with the packaging and distribution requirements of clinical trials across the globe.
Some clinical trial solutions offered by Fisher Clinical Services include:
- Biologistics Management
- Cell & Gene Therapy
- Clinical Ancillary Management
- Clinical Label Services
- Clinical Trial Packaging & Storage
- Clinical Supply Optimization Services
- Cold Chain Management & Expertise
- Direct-to-Patient
- Distribution & Logistics
- Strategic Comparator Sourcing
- Public Health Research
Established in 1997 under the name Kiecana Clinical Research, KCR is a full-service contract research organization that provides a variety of services for clinical monitoring, safety & pharmacovigilance, clinical project management, quality assurance and regulatory affairs.
KCR operates globally, and has offices in North America, Western Europe, Central Europe and Eartern Europe. The company currently employs more than 700 staff.
Some clinical trial solutions offered by KCR include:
- Trial Execution
10. Medpace
Founded in 1992 and based in Cincinnati, Ohio, Medpace is a midsize clinical contract research organization. The company has operations in over 45 countries, and employs over 2,800 people. Medpace provides support services for Phase I-IV clinical trials for pharmaceutical and biotechnology companies, which include central laboratory services and regulatory services.
Some clinical trial solutions offered by Medpace include:
- Biostatistics and Data Sciences
- Clinical Trial Management
- Drug Safety and Pharmacovigilance
- Medical Writing
- Quality Assurance
- Regulatory Affairs
- Risk-Based Monitoring
- Medpace Laboratories
11. Clintec
Now in business for over 22 years, Clintec is a medium-sized global contract research organization for pharmaceutical, biotech and medical device industries, with large expertise in oncology and rare diseases.
The company provides the flexibility and agility of a smaller-sized CRO, while also having a wide global coverage that large CRO companies are known for. Clintec is based in more than 50 countries, and was acquired by the leading global CRO IQVIA in late 2018.
Some clinical trial solutions offered by Clintec include:
- Project Management
- Data Management
- Biostatistics
- Global Feasibilities
- Patient Recruitment & Retention
12. Worldwide Clinical Trials
Bringing over 30 years of experience to the clinical research market, Worldwide Clinical Trials is a leading medium-sized global contract research organization. Founded by physicians with a dedication and commitment to advancing medical research, Worldwide Clinical Trials was the first customer-centric CRO.
Currently the company has coverage in more than 60 countries, and has extensive experience in a wide range of therapeutic areas, including central nervous system, metabolic, cardiovascular, oncology, rare diseases and general medicine.
Some clinical trial solutions offered by Worldwide Clinical Trials include:
- Bioanalytical Lab
- Early Phase Development
- Clinical Phase IIB-II Clinical Trials
- Phase IIIB-IV Clinical Trials
- Trial Management Technologies
Named #1 CRO in the world for operational excellence at the 2021 CRO Leadership Awards, CTI Clinical Trial And Consulting Services is a medium-sized global contract research organization that has been serving pharmaceutical companies since 1999.
Based in Covington, Kentucky, CTI has offices around the world in more than 60 countries, with coverage in North America, Europe, Latin America, Middle-East, Africa, and Asia-Pacific regions.
Some clinical trial solutions offered by CTI include:
- Feasibility
- Regulatory Affairs Study Start-Up
- Medical Monitoring
- Safety & Pharmacovigilance
- Clinical Services
14. Wuxi AppTec
Founded in 2000 as WuXi PharmaTech in the city of Wuxi, China, Wuxi AppTec has grown from a single laboratory into a leading global contract research organization with more than 28,000 employees, including 23,000 scientists and more than 30 research & development and manufacturing sites around the world.
With offices in Asia, U.S, Europe and the Middle East, the company is able to provide coverage to more than 30 countries around the world.
Some clinical trial solutions offered by Wuxi AppTec include:
- Small Molecule Drug R&D and Manufacturing
- Cell Therapy and Gene Therapy
- Drug R&D and Medical Device Testing
- Clinical Services (Phase I-IV)
15. Advanced Clinical
Founded in 1994 and based out of Deerfield, Illinois, Advanced Clinical is a midsize and full-service CRO that helps sponsors with running clinical trials. The company employs more than 700 staff, and offers a wide variety of services across many therapeutic areas. Advanced Clinical has global representation in over 50 countries around the world.
Some clinical trial solutions offered by Advanced Clinical include:
- eTMF & Document Management
- Global Medical Services
- Quality & Validation
16. Pharm-Olam
Pharm-Olam is a leading midsize CRO with global headquarters located in Houston, Texas and its European headquarters in Bracknell, United Kingdom. The company employs more than 800 staff, and has 25 offices around the world, with a global coverage in more than 60 countries.
The company has therapeutic expertise in 5 areas, including Rare & Orphan Disease, Infectious Disease & Vaccine, Oncology-Hematology, Allergy and Autoimmune.
Some clinical trial solutions offered by Pharm-Olam include:
- Study Feasibility
- Site Activation
- Patient Recruitment
- Medical Affairs
- Compliance & Training
- Clinical Monitoring & Operations
17. Clinipace
Founded in 2003 and based out of Morrisville, North Carolina, Clinipace is a global midsize full-service CRO with a focus on solution customization for clinical trials. The company has a large global coverage in more than 50 countries, and has offices in North America, South America, Europe and Asia-Pacific regions.
Clinipace’s therapeutic focus areas include Oncology, Nephrology and Urology, Rare Disease, Gastroenterology and Women’s Health. The company also has complete therapeutic expertise in Infectious Disease & Vaccines, Cardiology, CNS, Immunology, and Respiratory.
Some clinical trial solutions offered by Clinipace include:
- Clinical Analytics
- Clinical Technology and Ecosystem
- Functional Service Partnership (FSP)
- Regulatory & Strategic Product Development
9 Fundamental Questions To Ask A Top CRO Company Before Signing The Contract
1. which services does the cro provide.
CROs offload a lot of operational tasks from trial sponsors, which can touch any component of clinical trial operations. From formulating an overall study strategy and implementing technologies to support the operational processes of the trial, to picking and identifying sites, and supporting patients during the trial, the range of clinical services offered by a CRO tends to be vast and inclusive of all the typical services and support you will require for running a successful clinical trial.
However, not all CROs are the same in their service offerings, or are able to offer the same depth of capability within a seemingly same clinical trial support process. For this reason it is important to understand exactly which kind of clinical services and support you are looking to receive from the prospective CRO when running your clinical trial.
While services such as clinical monitoring and clinical trial management are offered by the majority of CROs, the specific needs of each trial are unique, and for this reason it is important to first identify what will be the unique services your trial requires. Completing this internal analysis first will help you to understand the extent to which a potential CRO partner will be able to provide all of these services.
Some CROs specialize in specific clinical trial functions which the company may label as a “core services”, in which case this is a sign the company will have more expertise, experience, and will be set up in a way to maximize their capabilities in providing support for these services compared to other services that the CRO offers.
For example, a CRO may include patient recruitment as part of its “core services”, which implies that they are highly skilled in and have the necessary infrastructure to design and implement a high-quality patient recruitment strategy.
Clara Health CRO Support Services: At Clara Health our specialty services include technology-augmented digital and patient advocacy recruitment, as well as patient support via our signature patient recruitment platform, which we use to upgrade clinical trials and deliver results sponsors look for in their recruitment and retention campaigns.
At Clara, we work alongside CROs to supplement and support clinical trials with modern and personalized capabilities that CROs do not typically have the bandwidth, corporate structure or infrastructure to support.
If you would like to learn more about exactly how our platform can upgrade your unique trial, feel free to book a Free 30 Minute Consultation Session Here with one of our in-house experts.
2. What Related Experience Does The CRO Have?
It is helpful to ask the prospective CRO company if they have any relevant experience in running clinical trials that would be an asset in designing and running your study. Previous experience in a related therapeutic area or in running a trial with a similar design allows CROs to have a deeper understanding into potential opportunities and challenges, increasing the likelihood of your clinical study being successful.
For example, if a sponsor is planning to run a trial in oncology, for the purpose of site identification and selection it would be valuable to partner with a CRO vendor that has expertise in this area, as they likely already have a good understanding of which sites will lead to optimal results.
However, it is also important to consider all factors when selecting a CRO vendor and not to rely on therapeutic experience as the sole qualifier for whether or not a potential CRO is a fit for your trial. While previous experience is beneficial, some sponsors close themselves off from working with vendors that have not worked in their therapeutic area, which significantly limits options when choosing a CRO partner that is truly a good fit for their clinical study.
This can impact the end result of your clinical study, as sponsors that are not successful in choosing a CRO vendor that is the right overall fit may face difficulties if the needs of their clinical study aren’t being properly met.
Clara Health: We have worked to provide support for clinical trials across a wide range of therapeutic areas and trial designs. Our specialty is filling in the gaps that CROs traditionally did not have to think about, which include digital patient recruitment, patient advocacy recruitment, and technology-augmented patient support.
Additionally, we are constantly building our proprietary data and running tests in a variety of therapeutic areas. These research efforts allow us to have a detailed understanding of the expected level of difficulty when recruiting particular patient populations, as well as allow us to predict with accuracy which segments of the targeted population will be likely to qualify in a particular study.
3. What Are The Communication Workflows & Expectations For Performing And Delivering Contracted Services?
It is important that you clarify what the expectations for communication will be between your prospective CRO vendor and your internal teams, as you will most likely be working with the CRO of your choice for the entire duration of your clinical trial.
There are a vast variety of factors and success determinants for a clinical trial, which are continuously undergoing change as the study unfolds. For this reason, it is recommended that you work with a CRO that is proactive in their communication, so that you are kept up to date with information about important changes as your clinical trial progresses.
A vendor that is proactive rather than reactive in their communication and approach to dealing with arising issues is one of the most important qualities in CRO. Challenging situations will naturally arise, and the promptness with which they are taken care of will significantly impact your clinical trial’s degree of success. Therefore, seeking a vendor that is able to match the standard of communication that you as a sponsor would like to experience throughout the duration of your partnership is one of the most critical steps in determining which CRO is the right fit for your clinical trial.
We’ve included a few additional questions pertaining to the communication structure and reporting expectations that you can ask a prospective CRO vendor to determine the degree of fit in this particular category:
Communication Expectations:
- If we were to move forward with you, which of your team members will be our main point of contact?
- How available will you be outside of the scheduled meetings to address any of our concerns or additional requests?
- What will be the frequency at which update meetings will be conducted, and who will be present at those meetings?
- Which clinical study processes will be reported on, and what will be the workflow for how we will receive this information?
- What will be the cadence at which we will receive progress reports?
- Would we be able to access metrics electronically via an interactive dashboard, or will you send us formal reports?
Clara Health: At Clara Health, we directly interact and actively work with several key stakeholders involved in running a clinical trial, which includes sponsors, CROs, sites, and patients. This unique position allows us to have a centralized perspective which helps us to see all the moving parts of a clinical trial at the same time, which helps to identify issues and relay this vital information and insight back to the sponsor (or other appropriate stakeholders) in the shortest time possible.
The ability to access this perspective allows us to gather the most accurate, complete, and up-to-date information about how the clinical trial is unfolding, and quickly becomes very valuable to sponsors for their clinical trial.
As an example, we may receive feedback from patients about having an unsatisfactory experience with a particular study site. We are able to aggregate and analyze this information, and relay our findings back to the sponsor and the study site to improve the experience for other patients.
4. What Is The CRO’s Client Satisfaction Record?
It is a good practice to request information or metrics from the prospective CRO vendor that can point to the degree of satisfaction of their past clients. Prior to signing the contract, vendors will naturally do their best to uplift their image and future value to you during their sales conversations with you and your team. It can be tricky to get an objective understanding of what the partnership experience will actually entail, especially when there are multiple vendors fighting for your commitment.
We recommend that you ask the prospective vendor to provide success metrics regarding areas of clinical trial operations that are going to be important for your trial.
For example, you may be interested in learning about the vendor’s relationship to finances, in which case it will be useful to ask them about situations in which they went over the planned budget, and investigate into the reasons behind that. Alternatively you may be concerned about potential delays in timelines, in which case it would be helpful to learn about metrics regarding the CRO’s ability to meet timeline expectations.
You may also request to talk to the prospective CRO’s past clients, which will help you to gain insight into what the relationship was like and give you the opportunity to examine if the way in which the particular CRO manages its relationships and performs its services meets the expectations that you would have for your potential relationship and for your clinical trial.
Clara Health: At Clara Health, our relationships with our partners and with our patients are most important to us. In the unique position where we fit in the clinical trial process, we have the opportunity to directly co-create the clinical trial patient experience with a variety of stakeholders, including sponsors, sites, CROs, and patients.
Our company’s values and culture have been directed and developed to be such that the client and patient experience is at the top of priority for all of our internal teams, and we work to provide the best quality of care to all stakeholders.
We have many testimonials from every type of partner we’ve worked with which we can happily share with you.
5. How Do You Adapt When Encountering Challenges With Running A Clinical Trial?
It is inevitable that challenges and unforeseen changes will arise throughout the operational clinical trial process, and for this reason it is important to work with a CRO vendor that can provide you with evidence of their flexibility and ability to adapt to sudden changes.
The ideal CRO partner is one that is highly consultative throughout the entire process, and has an ability and the initiative to deal with challenges at their seed stage, prior to them turning into major obstacles for the success of your trial.
CROs naturally have a large reach, and there are a lot of different clinical trial mechanisms and processes that are under their control. They are able to monitor and respond to what is going on in every key link in the chain of the clinical trial operation.
It is reasonable to expect this level of oversight from a CRO, and additional questions that can help you gain insight into this include:
- What are some examples where the CRO was effective at monitoring the health of clinical trials they’ve helped operate in the past?
- How quickly does the CRO respond to challenges or opportunities for improving the clinical trial experience?
- How well does the CRO gather & process information from study sites, study teams, patients & the sponsor, and what are their typical data analysis workflows?
It is also recommended to speak to the prospective CROs past clients to help you gain insight into how well they respond and adapt to the naturally arising challenges in clinical trials.
Clara Health: While CROs do have a large reach within the clinical trial, no CRO has complete visibility into every clinical process. They are not typically set up to support full visibility, which can manifest as a potential threat to your clinical trial as it unfolds. This is especially true for parts of the clinical trial processes that CROs naturally do not specialize and often subcontract, such as clinical trial recruitment.
At Clara, we are in a unique position in relation to other key partners involved in operating the clinical trial. We are in direct and frequent contact with patients, CROs, study sites, study teams, and the sponsor, and have a very deep understanding of the patient pipeline. This allows us the unique ability to go very deep into specific parts of the recruitment chain and investigate what is working and what is not working.
In addition, Clara functions as a resource for all partners in the clinical trial. For example, we work directly with site teams to ensure that they have access to a 3rd party that they can relay their needs to and receive fast support in case there is anything they require that can improve the patient recruitment process.
6. Which Parts Of Operating The Clinical Trial Will You Be Outsourcing?
Since there are so many processes and mechanisms that go into operating a clinical trial, CROs will always outsource some parts of running and managing the study. While you can expect that the prospective CRO will subcontract some of the work, it is important to find out which exact parts the clinical study will be outsourced.
There are certain basic and key clinical processes (such as site selection) that CROs almost always help with, and if you find that these parts of your trial are going to be subcontracted to another company, it is recommended to find out why the CROs operations are set up this way and how this would impact the service you will receive.
Ultimately what matters to you as a partner and client is that the quality of service and care that you will receive will be up to standard, and meet what was promised and what you are expecting. While this trust is important after you have signed the contract, it is recommended that prior to entering into such a significant commitment that you have evidence and the conviction that the CRO of your choice is truly the right fit and will deliver the quality of service that was being discussed.
Since it is impossible to predict exactly what the quality of this relationship and services performed will actually be like in practice, it is recommended that you understand the details of what will be done for your trial and how. Investigating how the CRO outsources and subcontracts services for a clinical trial will help you to gain necessary insight that you would need to make the correct vendor selection decision.
Clara Health: At Clara, we maximize the effectiveness of the digital component across the entire digital & recruitment spectrum, which is added on top of the existing capabilities of the CROs and other vendors involved in operating your clinical trial. In addition, we offer services that augment the CROs efforts, which has the potential to significantly improve the patient experience, operations flows, recruitment and retention performance, which is so important in ensuring the success of a clinical trial.
For example, if a CRO wants to have a great site relationship, we are able to come in as a third party on behalf of the sponsor and CRO and act as a resource and additional support for sites.
In another example, If a sponsor wants to have great relationships with the patient community, Clara is able to come in on behalf of the sponsor and develop these relationships while being perceived more neutrally by the patient community.
7. Do You Have Experience Running International Trials?
If you are planning on operating an international clinical trial, it is recommended to work with a CRO that has extensive experience in this area. While many CROs will offer near-global coverage, the level of experience with specific geographic locations can significantly vary from one vendor to another.
It is important to work with a CRO that has experience running clinical trials in the specific countries and regions you are planning to conduct your research in. Being compliant with the local rules and regulations for clinical testing is a very complex process that requires existing understanding and familiarity in order to ensure logistical smoothness and to mitigate legal risks. In operating a clinical trial, there are a multitude of clinical services and processes, which can greatly vary across the many regions in which you can conduct clinical testing.
A CRO that is lacking experience in operating international trials or operating in particular regions where you plan on conducting research may not be able to meet your desired quality and agility expectations, and therefore may not be the right fit for your international clinical trial.
Clara Health: In the past, we have provided international patient recruitment and digitally-augmented trial support services for clinical trials in the EU, Canada, UK, Australia and South America.
Clara Health is fully compliant to operate international studies everywhere in the world, with the exception of Russia and China.
8. What Is Your Relationship With Patients?
Patient-centric approach to designing and operating a clinical trial is becoming more and more crucial in the clinical research space. The ability of a sponsor and their CRO partner to understand the needs and characteristics of their target patient community is a significant determinant of whether or not the study will be a success.
A sponsor that has close and authentic relationships with the patient community tends to have a deeper understanding of how to create the best clinical trial experience that will attract patients and keep their interest throughout the clinical trial.
In addition, strong relationships with patients allow sponsors and CROs to forecast recruitment and patient retention pipeline with much higher accuracy. This ability is critical for ensuring the success of the trial and mitigating the risk of low enrollment. After an understanding of the patient population is acquired, sponsors gain the necessary insight to design a clinical trial that is not only favorable to their research results, but is also practical and will result in the enrollment numbers they are looking for.
While many CROs have already recognized the importance of patient-centricity and evolved the ways in which they design and operate clinical trials, other CROs have not yet made such a pivot in their values. It is important to understand the degree of importance the prospective CRO places on creating a favorable patient experience, and what kind of infrastructure the company has to support it.
At Clara, we recommend choosing a CRO partner that is adapting to the patient-centric model which is becoming more and more important for running a successful clinical trial.
Clara Health: Since early stages of our development, we’ve had a dedicated patient advocacy team that has been integral in shaping our company’s vision and operations. We have built our entire platform and recruitment infrastructure around creating the best experience for patients. Our teams, corporate values, service offerings and company infrastructure all work in the service of the patient.
In addition, over the many years of being in business we have heavily invested in building authentic patient community relationships that span across a variety of therapeutic areas. This has given us a unique ability to receive feedback directly from patients that is genuine and authentic around marketing materials, strategy for patient recruitment, and other services that we build for specific trials.
This ability to build partnerships with the patient community in an authentic way gives us a very unique ability to engage with the patient community on behalf of a pharmaceutical company, allowing our sponsor & CRO partners the opportunity to start conversations with patients through our in-house patient advocacy team.
If you would like to learn how Clara can help you to build a strong & authentic relationship with your target patient community, get in touch with us and we’d be happy to share our capabilities and previous results with you as they relate to your current or upcoming clinical trial.
9. How Is The CRO Going To Utilize Patient Input For Developing The Trial?
In the initial stages of clinical trial design, sponsors often determine the ideal patient profiles that would help them to drive the most favorable research outcomes for their study. While it is important for the success of your trial to determine who your ideal patients are, very often these projections do not match up with what is viable in practice.
At Clara, we often encounter study protocols that are not set up realistically for successful recruitment to be possible.
Common mistakes that are made when determining trial eligibility criteria and trial design include:
- Overestimating the interest in the clinical trial from the target patient population
- A lack of patient focus in the trial design
- A lack of convenience for patients in their participation
- Complicated and/or inefficient study experience flows
- Crafting the eligibility criteria around the patient population that is most likely to lead to favorable study outcomes, without conducting sufficient research to more accurately estimate the recruitment and retention difficulty of the group for a particular study
It is natural for there to be a “push & pull” between the research ideal and the real world practicality. It is important to determine the correct balance between these two sides for your trial, as going too far in either direction will decrease the chance of your clinical study’s success.
The nature of the industry as it is right now is such that there is excess research idealization and not enough emphasis on patient centricity. This distorted orientation has resulted in many clinical trials being unsuccessful, negatively impacting sponsors, patients and the entire clinical trials industry.
The ideal CRO partner should help you make sure that your protocol design sets your study up for success. The CRO should be able to help you determine the proper balance between the research ideal and the real world practicality, and back up their findings with sufficient research and patient data that can project your trial being a success.
Clara Health: When formulating a recruitment and retention plan for our clients, we begin with conducting thorough research into the target trial patient population. This allows us to get a clear understanding of which recruitment channels will yield the best results and what kind of marketing materials will resonate with the prospective study participants.
To ensure accuracy and real-world applicability of our research, we consult and collaborate with our internal patient advocacy and patient support teams, as well as with our clients and patients representing the target trial patient profiles. We then tie our findings back with any existing proprietary data that we have in connection with the therapeutic area or the prospective target patient group.
Our unique position within the clinical recruitment chain gives us the presence and deep-rooted access needed to effectively tap into any of the three patient traffic sources: digital recruitment, offline recruitment, or patient advocacy recruitment.
Once a recruitment campaign has gone live, we constantly monitor, analyze and optimize our performance to make sure that the processes we have in place are as efficient as possible and drive the greatest results. In addition, we have the capability to layer in any traditional advertising (such as billboard ads) if requested by the study sponsor.

How to Start a Clinical Research Organization
By: Author Tony Martins Ajaero
Home » Business ideas » Healthcare and Medical » Clinical Research
Do you want to start a clinical research organization? If YES, here is a 20-step guide on how to start a clinical research organization with no money.
Please note that if you intend to start this type of business, it is advisable to first look at the existing laws and zonal regulations in the country or state you reside in to know what is expected of you.
The bottom line is that you need to pay a visit to the regulatory bodies in your country to get all the information you need before you can legally open your clinical research organization in your city.
Suggested for You
- Clinical Research Company Business Plan [Sample Template]
You can start your clinical research business from a small town in the United States. If you are good at what you do when it comes to coming up with breakthrough clinical research works and workable solutions to challenges for players in the healthcare cum medical industry, it won’t be too long before your brand becomes a nationally recognized brand.
Steps on How to Start a Clinical Research Organization Business
Understand the industry.
The Contract Research industry that clinical research business is a subset of, is perhaps one of the fastest growing and largest industries in the world because the wealth of any nation depends on the health of the nation. There is hardly any country where the healthcare and medical industry is not handled with all seriousness.
As a matter of fact, the healthcare industry cum Contract Research industry is known to gulp over 10 percent of gross domestic product (GDP) of most developed countries.
The Contract Research industry includes companies that provide research services on a contract basis to the pharmaceutical and biotechnology industries.
Contract research organizations (CRO) provide biopharmaceutical development, preclinical research, clinical research, and clinical trial management. Many CROs specifically provide clinical study and clinical trial support for drugs or medical devices.
A recent report published by IBISWorld shows that the Contract Research Industry is indeed experiencing rapid growth. The report shows that over the past five years, demand for industry services has risen as clients increasingly use their research and development (R&D) budgets to outsource to CROs.
As a result, industry revenue is expected to grow at an annualized rate of 7.1 percent to $17.1 billion in the five years to 2016, which includes a 6.3 percent projected growth in 2016 alone.
The industry experienced a period of aggressive growth over the past five years, benefiting from an aging US population and an expansion in private health-related research and development (R&D) expenditures.
As a result, industry revenue is expected to grow in the five years to 2016. Over the next five years, the industry will continue to benefit from increased R&D spending and an aging US population in the increasing need of disease treatment.
The industry will also benefit from rising demand from biopharmaceutical companies. IBISWorld forecasts that industry revenue will increase over the five years to 2022. Statistics have it that in the United States of America, the industry is worth $17bn, with an estimated growth rate of 7.1 percent.
There are about 3,585 registered and licensed contract research organizations cum clinical research businesses in the United States and they are responsible for employing about 5,513,669 people.
LabCorp and Quintiles are the leaders in this industry and they can boast of having the lion share of the available market in the United States.
If you are considering starting your own clinical research company in the United States, then you should try and work around the industry barriers.
The truth is that the barriers to entry in the contract research industry are high due to the significant regulatory requirements and the experience and strength of incumbents.
Some of the factors that encourage entrepreneurs to start their own clinical research business could be that the business can easily get support from the government at all levels and the business is indeed a profitable venture despite the legislation governing the industry.
Conduct Market Research and Feasibility Studies
- Demographics and Psychographics
The demographic and psychographic composition of those who require the services of clinical research organizations cut across all players in the health and medical care industry such as dental clinics, pharmaceutical companies, biotechnology companies, biomedical companies, Ministry of Health, medical devices development companies, and all medical clinics and hospitals.
In essence, the demographic composition for clinical research organizations is all encompassing. So, if you are thinking of starting your own clinical research business, then you should look beyond marketing your research services in your city, but also across the United States of America and even in other parts of the world.
Decide on a Niche
There is no niche area of specialization in the clinical research business since clinical research business is a niche area in the Contract Research Organizations industry. Although some clinical research organizations may decide to specialize in any or all of the following;
- Performing clinical research for pharmaceutical, biomedical and biotechnology companies
- Performing clinical research for medical device manufacturers
- Data management and regulation adherence services
- Development, advancement, and formulation of devices and medications

The Level of Competition in the Industry
The competition that exists in the clinical research organization goes beyond competitions in your city; it is both national and international.
This is because, major clinical research organizations can be contracted from any part of the United States or abroad especially in Israel. So, it will be right to say the competition in the clinical research business is highly competitive.
The truth is that no matter the level of competition in an industry, if you have done your due diligence and you brand and promote your services or business properly, you will always make headway in the industry.
Just ensure you come up with breakthrough clinical research works and workable solutions for players in the healthcare cum medical research industry and you know how to reach out to your target market.
But over and above, there are several clinical research organizations scattered all around the United States. So, if you choose to start your own business in the United States, you will definitely meet stiffer competitions amongst clinical research organizations in the United States.
Know Your Major Competitors in the Industry
Here are some of the most popular clinical research organizations in the United States of America and the world at large;
- Advanced BioScience Laboratories
- AlphaGenesis Incorporated (AGI)
- Covance (LabCorp)
- Syneos Health
- PRA Health Sciences
- Bio Analytical Research Corporation (BARC)
- Blue Sky BioServices
- Charles River Laboratories
- Clinical Device Group (CDG)
- Clinical Research Consulting, Inc.
- Davinci Biomedical Research Products, Inc.
- Edinger Medical Group and Research Center
- eResearchTechnology, Inc (ERT)
- Fast-Track Drugs and Biologics
- Fountain Medical Development (FMD)
- Global Drug Development Experts (GDDE)
- STATKING Clinical Services
- Symphony Pharma Life Sciences.
Economic Analysis
If you are looking towards successfully launching a business and maximizing profits, then you need to ensure that you get your economic analysis right and try as much as possible to adopt best practices in the industry you choose to build a business in.
Clinical research business is no longer a green business, as a matter of fact, you will come across several brand names when you source for clinical research organizations in your state.
So, if you are mapping out your economic analysis, you should carry out thorough market survey and costing of the required research materials, branding and other costs needed to successfully run the business.
Over and above, if you are considering starting a clinical research business, then your concern should not be limited to the cost of setting up the business and churning out a quality clinical research works, but also on branding and on how to build a robust business network.
The truth is that if you are able to build a robust business network, you are sure going to maximize profits in the business.
Decide Whether to Buy a Franchise or Start from Scratch
Unfortunately, you can hardly find a franchise of a clinical research organization to purchase meaning that if you want to own a clinical research business, you must be ready to start from the scratch. The truth is that it will pay you to start your business from the scratch.
Starting from the scratch will afford you the opportunity to conduct thorough market surveys and feasibility studies before choosing a location to launch the business.
Please note that most of the big and successful clinical research organizations around started from the scratch and they were able to build a solid business brand. It takes dedication, hard work, and determination to achieve business success.
Know the Possible Threats and Challenges You Will Face
If you decide to start your own clinical research organization today, one of the major challenges you are likely going to face is the presence of well–established clinical research organizations whose research works and solutions are used by major players all across the United States.
The only way to avoid this challenge is to create your own market perhaps by working with smaller health and medical organizations.
Other threats that you are likely going to face is economic downturn and unfavorable government policies (slowdown in releasing funding for Research and Development organization). As a matter of fact, the growth of a few dominant operators has led to an increasing level of concentration.
Choose the Most Suitable Legal Entity (LLC, C Corp, S Corp)
When considering starting a clinical research organization, the legal entity you choose will go a long way to determine how big the business can grow.
Generally, you have the options of choosing a general partnership, limited liability company, or a sole proprietorship for your business.
Ordinarily, sole proprietorship should have been the ideal business structure for a small-scale clinical research company especially if you are just starting out with a moderate start-up capital.
But if your intention is to grow the business and conduct clinical research for clients all across the United States of America, then choosing a sole proprietor is not an option for you. Limited Liability Company, LLC, or even general partnership will cut it for you.
Setting up an LLC protects you from personal liability. If anything goes wrong in the business, it is only the money that you invested into the limited liability company that will be at risk. It is not so for sole proprietorships and general partnerships.
Limited liability companies are simpler and more flexible to operate and you don’t need a board of directors, shareholder meetings and other managerial formalities.
These are some of the factors you should consider before choosing a legal entity for your clinical research organization; limitation of personal liability, ease of transferability, admission of new owners and investors’ expectation, and of course taxes.
If you take your time to study the various legal entities to use for your clinical research organization, you will agree that limited liability company; an LLC is most suitable.
You can start this type of business as a limited liability company (LLC) and in the future convert it to a ‘C’ corporation or an ‘S’ corporation especially when you have plans of going public.
Upgrading to a ‘C’ corporation or ‘S’ corporation will give you the opportunity to grow your clinical research organization so as to compete with major players in the industry; you will be able to generate capital from venture capital firms, you will enjoy separate tax structure, and you can easily transfer ownership of the company.
Choose a Catchy Business Name from the ideas Below
Normally, when it comes to choosing a name for your business, you should be creative because whatever name you choose for your business will go a long way to create a perception of what the business represents.
Typically, it is the norm for people to follow the trend in the industry they intend to operate from when naming their business. If you are considering starting your own clinical research business, here are some catchy names that you can choose from;
- Research Pro® Clinical Research Organization, Inc.
- Beth Martinez® Clinical Research Organization, LLC
- Lazarus Morphy and Co® Clinical Research Organization, Inc.
- International Clinical Research Corporation, Inc.
- Michelle Monk® Clinical Research Organization USA LLC.
- Shannon Stev® Clinical Research Organization, Inc.
- Larry Chris® Clinical Research Organization, Inc.
- Clinical Research and More® Inc.
- Lance Institute® Clinical Research Organization USA LLC
- South Gate® Clinical Research Organization, Inc.
Discuss With an Agent to Know the Best Insurance Policies for You
In the United States and in most countries of the world, you can’t operate a business without having some of the basic insurance policies that are required by the industry you want to operate from. Thus, it is imperative to create a budget for insurance and perhaps consult an insurance broker to guide you in choosing the best and most appropriate insurance policies for your business.
Here are some of the basic insurance policies that you should consider purchasing if you want to start your own clinical research organization in the United States of America;
- General insurance
- Health insurance
- Liability insurance
- Risk Insurance
- Workers compensation
- Building/Property insurance
- Overhead expense disability insurance
- Business owner’s policy group insurance
- Payment protection insurance
You can contact the following leading insurance companies in the United States of America to purchase the needed insurance policies for your clinical research business;
- Allstate Insurance Group
- Liberty Mutual
- Progressive Insurance Group (PGR)
- Health Care Service Corporation (HCSC)
- New York Life Insurance Company
- Lincoln National Life Insurance Company
- MassMutual (Massachusetts Mutual Life Insurance Company)
- Northwestern Mutual Life Insurance Company
Protect your Intellectual Property With Trademark, Copyrights, Patents
If you are considering starting your own clinical research organization, you need to file for intellectual property protection/trademark. This is because the nature of the business makes it impossible for you to successfully run the business without having any cause to challenge anybody in court for illegally making use of your company’s intellectual properties.
So, in essence, if you want to leverage on these business opportunities within the industry and also to protect your unique design, company’s logo, and other documents or software that are unique to you or even operation concepts, then you can go ahead to file for intellectual property protection. If you want to register your trademark, you are expected to begin the process by filing an application with the USPTO.
Get the Necessary Professional Certification
Aside from your ability to come up with clinical researches, professional certification is one of the main reasons why you will be able to stand as a clinical researcher. You are strongly encouraged to pursue professional certifications; it will go a long way to show your commitment to the business.
Certification validates your competency and shows that you are highly skilled, committed to your career, and up-to-date in the market. These are some of the certifications you can work towards achieving if you want to run your own clinical research organization;
- ACRP Certified Professional.
- ACRP Medical Device Professional Subspecialty
- ACRP Project Manager Subspecialty
Please note in order to become a certified clinical research associate, you should complete 3,000 hours performing essential job duties or 1,500 hours of equivalent work experience requirements through ACRP certifications or approved clinical research degree programs accredited by the Council for Higher Education. Submit a resume documenting and demonstrating job performance.
Get the Necessary Legal Documents You Need to Operate
It is a fact that you cannot successfully run any business in the United States without the proper documentation. If you do, it won’t be too long before the long hands of the law catch up with you. These are some of the basic legal documents that you are expected to have in place if you want to legally run your own business in the United States of America;
- Business and liability insurance
- Federal Tax Payer’s ID
- State Permit
- Certificate of Incorporation
- Business License
- Business Plan
- Employment Agreement (offer letters)
- Operating Agreement for LLCs
- Insurance Policy
- Online Terms of Use (if you have a website)
- Online Privacy Policy Document (basically for online payment portal)
- Company Bylaws
- Memorandum of Understanding (MoU)
Raise the Needed Startup Capital
Starting a standard clinical research organization is capital intensive even if you choose to launch the business on a small scale and you only have a handful of full–time employees on your payroll.
Leasing of a standard laboratory facility that can accommodate your research equipment and staff members and equipping the facility are part of what will consume a large chunk of your startup capital, in essence, if you choose to start the business on a small scale, you will still have to source for fund to finance the business.
No doubt when it comes to financing a business, one of the first things you should consider is to write a good business plan.
If you have a good and workable business plan document in place, you may not have to labor yourself before convincing your bank, investors and your friends to invest in your business. Here are some of the options you can explore when sourcing for start-up capital for your clinical research business;
- Raise money from personal savings and sale of personal stocks and properties
- Raise money from investors and business partners
- Sell shares to interested investors
- Apply for a loan from your bank or banks
- Pitch your business idea and apply for business grants and seed funding from government, donor organizations, and angel investors
- Source for soft loans from your family members and your friends.
Choose a Suitable Location for your Business
When it comes to choosing a location for your clinical research business, the rule of thumb is that you should be guided by the demand for such services and easy access to labor and research materials. Of course, if you are able to secure a central location for your clinical research organization, it will enable you cut the cost of transporting access to medical facilities.
It cannot be overemphasized that the location you choose to open your clinical research business is key to the success of the business, hence entrepreneurs are willing to rent or lease a facility in a visible location; a location where the demography consist of robust players in the health and medical industry.
If you make the mistake of renting or leasing a facility for your clinical research organization in a not too visible or hidden location simply because it is cheap, then you must be prepared to spend more in promoting the business and perhaps giving direction to potential clients.
It is important to note that a business facility in good location does not come cheap hence you should be able to allocate enough fund for leasing / renting in your budget. If you are new to the dynamics of choosing a location for your business, then you should feel free to talk to a business consultant or a realtor.
Most importantly, before choosing a location for your clinical research organization, ensure that you first conduct a thorough feasibility study and market survey. The possibility of you coming across a similar business that just closed shop in the location you want to open yours can’t be ruled out.
Having said that, these are some of the cities in the United States of America where you can locate your clinical research organization;
- Brattleboro, Vermont
- Tucson, Arizona
- Silver Spring, Maryland
- Rowland Heights, California
- Portland, Oregon
- Richmond, Virginia
- Green Bay, Wisconsin
- Plano, Texas
Hire Employees for your Technical and Manpower Needs
If you are considering setting up a clinical research organization, it means that you should be prepared to set up a standard research center. If you don’t have enough cash, you can start from a small laboratory.
As regard leasing or outright purchase of a research facility or laboratory facility, the choice is dependent on your financial standings, but the truth is that to be on the safe side, it is advisable to start off with a short–term rent/lease while test running the business in the location. If things work out as planned, then you go on a long-term lease or outright purchase of the property but if not, then move on and source for another ideal location.
When it comes to hiring employees for a standard clinical research business, you should make plans to hire a competent Chief Researcher / Chief Executive Officer (you can occupy this role), Clinical Research Fellows, Clinical Research Associates, Office Administrator, Accountant, Marketing Executives and Client Service Executive. On the average, you will need a minimum of 5 to 15 key staff members to run a small-scale but standard clinical research organization.
The Service Delivery Process of the Business
This is how clinical research organizations work; as an independent organization that steps into the development process, once a pharma company has identified a promising new molecule, a clinical research organization will organize and conduct clinical trials to test the new molecule in humans.
Clinical research organizations engage in performing clinical research for pharmaceutical, biomedical and biotechnology companies, and performing clinical research for medical device manufacturers. Clinical research organizations will also carry out data management and regulation adherence services and of course, development, advancement, and formulation of devices and medications.
Write a Marketing Plan Packed With ideas & Strategies
Running a business requires that you should be proactive when it comes to marketing your goods or services. If you choose to launch a clinical research organization, then you must employ strategies that will help you attract customers, or else you will likely struggle with the business because there are well–known brands that determine the market direction for clinical research industry.
Your marketing strategy will center on breakthrough research works, quality and pricing, and above all excellent customer service. The truth is that if you are able to put the above stated in place, you won’t struggle to retain your old customers and at the same time win over new customers.
So, when you are drafting your marketing plans , make sure that you create a compelling company profile. Aside from your qualifications and experience, it is important to clearly state what you have been able to achieve in time past. This will help boost your chances when marketing your clinical research services. Here are some of the platforms you can utilize to market your clinical research organization;
- Introduce your clinical research organizations by sending introductory letters alongside your brochure to pharmaceutical companies, biotechnology companies, biomedical companies, ministry of health, hospitals and medical clinics and medical devices development companies, and other key stakeholders in the United States of America and Canada.
- Advertise on the internet on blogs and forums, and also on social media like Twitter, Facebook, LinkedIn to get your message across
- Create a basic website for your business so as to give your business an online presence
- Directly market your clinical research organization
- Join local clinical research associations for industry trends and tips
- Advertise our business in international and national newspapers, local TV and radio stations
- List your business on yellow pages ads (local directories)
- Encourage the use of word of mouth marketing (referrals)
Work Out a Reasonable Pricing for your Services
One key factor that will help you offer your clinical research work at rock bottom prices is to purchase your research materials and supplies directly from sources as against going through third parties. The truth is that if you source your research materials directly from sources you will get them at a premium price.
You can also try as much as possible to work with independent contractors and marketers, it will help you save cost of paying sales and marketing executives. So also, if you are able to secure a business partnership as it relates to getting referrals, then you will be able to get the right pricing and of course maximize profits from your business.
Develop Iron-clad Competitive Strategies to Help You Win
The clinical research business is not so much a competitive industry because of the few players in the industry, but you must come up with a unique and highly creative strategy to be able to outsmart your competitors in the industry. Part of what you need to do in order to stay competitive in the industry is to ensure that your clinical research works are well designed to fit with the trends and safety must also be your guiding principle.
In other to stay competitive in this industry, you must ensure that your clients are always comfortable when making use of your clinical research works and always ensure that you pay attention to details when carrying out your job. The truth is that if there are fluctuations in the quality of your research works, customers can choose to shift allegiance and settle for other options available.
Brainstorm Possible Ways to Retain Clients & Customers
When it comes to business, no matter the industry that you choose to pitch your tent in, one of the easiest ways to increase customer retention and perhaps attract new customers is to produce results and satisfy your customers always. If your customers are satisfied with your products and service delivery, they can hardly source for alternative service providers.
If your services and customer service fluctuate , you are likely going to struggle to get your customers coming back. Ensure that you offer your customers incentives if you want to retain them and of course continue to generate repeated sales from them and also attract new customers.
Part of what you need to do to achieve this is to track progress, results, or outputs with the aim of improving on them quickly as the case demands. When it comes to managing your customers and building a loyal clientele base , you should purchase customized CRM software.
With a customized CRM system, you can easily stay in touch with your clients (you can carry out quick surveys, you can introduce new products and prices to them without any hitch, you can felicitate with them on their birthdays and other anniversaries, you can keep track of their progress, you can send bulk SMS and customized emails and above all, you can easily receive compliant and feedback from them).
Develop Strategies to Boost Brand Awareness and Create a Corporate Identity
If you are in business and you are not conscious about boosting your brand awareness and communicating your corporate identity, then you should be ready to take on whatever the society portrays your business to be. One of the secrets of larger corporations is that they are willing to spend fortunes to boost their brand awareness and to continue to communicate their corporate identity the way they want people to perceive them to be.
No matter the industry you belong to, the truth is that the market is dynamic and it requires consistent brand awareness and brand boosting cum promotion to continue to appeal to your target market. Here are the platforms you can leverage on to boost your brand and create a corporate identity for your clinical research organizations – business;
- Place adverts on both print (newspapers and health magazines) and electronic media platforms
- Sponsor relevant international and community-based events
- Leverage on the internet and social media platforms like; Instagram, Facebook , Twitter, YouTube, Google + et al to promote your clinical research organization
- Install your billboards in strategic locations all around your city or state
- List your clinical research organization – business in local directories / yellow pages
- Advertise your clinical research organization – business in your official website and employ strategies that will help you pull traffic to the site
- Position your Flexi Banners at strategic positions in the location where your clinical research organization – business is located.
- Ensure that all your staff members wear your branded shirts and all your vehicles are branded with your company logo.
- WHY HALLORAN
OUR SERVICES
We take a personalized and integrated approach to help our clients position their pipelines and companies for success offering a full range of development and commercialization services.
We are Halloran, a life science consulting firm, partnering to enrich your product development and business growth. We propel your organization further; positively impacting the health and wellbeing of patients around the world.

The Ever-Changing Business Model of Clinical Trials
Where Are the Clinical Trials?
A decentralized clinical trial contains any virtual component that enables a trial to still operate, but just different from the standard brick-and-mortar site trial. A lot has been accomplished over the past two years since our industry experienced a major operational disruption due to COVID-19. The pandemic catalyzed the adoption of decentralized clinical trial components, especially with the necessary and increased adoption of remote and in-home care.
A single new model has not reached its moment where it’s now the standard, and in fact, quite the opposite. The business model is ever-changing due to the vast array of trial execution options. COVID-19, though not without significant operational disruption, has incited more patient and sponsor optionality than ever before. There is a full spectrum of models ranging from fully decentralized, fully centralized, to hybrid trials that bring optionality both to site and patients.
These decentralized component options that enable fully decentralized and hybrid trials will not go away, but rather; trial design options will increase. With the global pandemic still ongoing, Clinical Ink’s consensus is that many of the digital interventions will become permanent. For example, bring your own devices (BYOD) will increase, remote hybrid trials will increase, clinical operations will grow even more complex as a result, and much more.
So, what do all these options mean for clinical trial design? The team at Clinical Ink recently shared their insight to a group of clinical operations professionals at Halloran’s Clinical Operations Retreat for Executives (CORE) in Sonoma, California. Fresh from the event, here are a few lessons to share that illuminate the options and limitations of decentralized trials and ways to reach success.
Clinical Trial Optionality Is Here to Stay
The clinical trial options – fully decentralized, fully centralized, and hybrid – allow for a broad spectrum of decentralized and hybrid-trial designs. And that fully virtual model is slowly and gradually migrating from smaller early-phase and post-approval studies toward larger pivotal trials. But in the near term, many sponsors, investigators, and research-service providers expect virtual trials to remain limited to a narrow set of use cases, like a well-characterized drug with few adverse events.
While most clinical trials are not likely to be entirely virtual, they will use one or more decentralized components based on suitability for their endpoints, patient populations, and treatments. Through their observations, hybrid models will be increasing with the increase of decentralized components to support a trial’s operations.
Before the pandemic, an Industry Standard Research survey in December of 2019 found that 38 percent of pharma and contract-research organizations expected virtual trials to be a major component of their portfolios, and 48 percent expected to run a trial with most activities conducted in participants’ homes. When asked the same questions a year later, the responses were 100 percent and 89 percent, respectively.
Bring Your Own Device Studies – Key Risks and Lessons Learned
Clinical Ink recently worked with a company that launched a Phase III study on a sleep disorder enrolling seventy-five patients at fifty-six sites throughout the US and Europe. In that study, the patients needed 1-3 daily care clinical outcome diary entries for a three-month period. 90% of the patients in the sleep disorder trial brought their own device (BYOD), while 10% of the devices were provisioned to the patients. Some of those that brought their own device used older models and had poor battery life – a key problem that’s often overlooked. Looking at those diary entries, 92% of the patients were compliant with their data entries. As a result, BYOD increased compliance and patient engagement but did not solve for those inevitable BYOD glitches that will arise. Understanding the risks, like poor battery life, will help a sponsor plan ahead and mitigate data risk.
Clinical Ink also conducted a study that compared a BYOD to a provisioned device to the overall data that was captured. There was a high correlation between the different hardware, and the positive results were a direct outcome from having all the screens show the exact same data. In summary, if patients bring their own devices, the sponsor will need a plan in place on evaluating and monitoring those devices to ensure accurate and timely data. Perhaps the solution is a virtual patient concierge service to help with these issues. And, as a result, data retrieved from a BYOD or a provisioned device will likely lead to the same overall data as long as the exact same data is captured from device to device.
Zooming out for a moment, Clinical Ink launched a recent survey and found that 94% of people are willing to use their own phone for a study and the same amount of people know how to download a digital application on their phone. But to provide an effective and efficient experience for the patient bringing their own device, as a sponsor, you’ll need to keep it super simple – do not make the tool complex as patients need to be familiar with the technology in order to get the data and results the sponsor needs for the trial. Remember, whether patients are using a BYOD, a provisioned device, or both modes, ensure the screens capture the exact same data.
Up until recently, the industry has been unaware of the successful drug applications that use BYOD data, causing some concern that regulators will not accept the data. This year though, the industry’s largest BYOD study – for the Pfizer COVID-19 vaccine development – collected primary safety data using a reactogenicity diary completed by patients. This is a promising indicator for future research, and it underlines beliefs that data collected through BYOD can be just as robust and reliable as the data collected using provisioned devices.
The Vast Options Ahead of Us
Looking at the theme addressed in this article – execution optionality – it all comes down to flexible options for sites and patients. Moving away from the standard brick-and-mortar trial to a world with many more options that benefit both the site and the patient sounds ideal. But there are headaches when it comes to shifting to a new operational mode. We have so many more options to select. But as we’ve heard before, no two trials are the same. Every trial is different, meaning, not all decentralized components that work for one trial will work for another. And there will be limitations such as the ones discussed in this article. Knowing those limitations – and the perspectives of your patients – early in the planning phase is essential to leaning into new clinical trial models.
About Clinical Ink
Clinical Ink is a global technology company offering data certainty from source to submission through their Lunexis eSource clinical technology and configurable direct data capture, eCOA, ePRO+, and eConsent modules. Their suite of solutions for capturing and integrating electronic data from sites, clinicians, patients, and caregivers naturally enhances clinical trial workflows.
About Halloran and CORE
Halloran is a full-service boutique life science consulting firm with offerings across strategy and program leadership, regulatory, clinical, and quality practices. Halloran partners with the most cutting-edge life sciences companies to enrich their product development and business growth through their expansive industry knowledge. From early development to commercialization, their flexible and integrated services are tailored to providing the know-how and a team of cross-functional subject matter experts to propel organizations further. Because of their commitment to their clients and their patients, clients choose Halloran time and time again.
The Clinical Operations Retreat for Executives (CORE) was launched in 2004 –an invitation-only meeting that brings together an exclusive group of senior leaders in clinical development to discuss and debate the most pressing issues around the business of product development and building enduring companies in this space. This conference is a one-of-a-kind peer roundtable where executives learn from each other and share best practices. CORE is hosted by Halloran Consulting Group and founded by Laurie Halloran.
Halloran Consulting Group
Related articles.

Guest Column | June 14, 2018
Cro selection 101 — how to get started.
By Joelle Herman , president, NeoTrials, LLC

This three-part article series will look at how best to procure, manage, and implement best practices in this complicated market.
As companies continue to look for smarter ways to develop drugs, biologics, and devices, outsourcing clinical trials to contract research organizations (CROs) and niche providers continues to grow. A recent report from Statistica projects that global CRO revenues will reach $52.3 billion by 2021 just in locations outside of the Asia-Pacific (APAC) region. While that is impressive, APAC, which is one of the fastest growing markets in the world, is projected to grow at a 20 percent compound annual growth rate (CAGR), compared to 11.4 percent “rest of world” revenue. 1 This growth is substantial and plays into the critical nature of how product developers procure and utilize CROs. This is a complex process whether you are a large biopharma or a small one poised to initiate clinical trials. The complexity of a successful procurement can be managed with good early planning and a strategic approach.
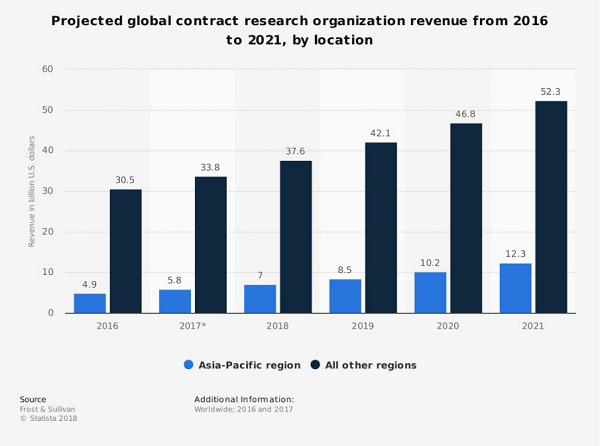
Champion/Selection Committee
Before you can go out and get bids, the needs of the project/program must be defined by an internal champion or selection committee. A person or team closest to the science or the person responsible for leading the program team or, in a small company, the person managing the clinical trial(s) often are best positioned to lead this effort. A champion will coordinate the entire process across multiple functions (project management, medical, finance, clinical operations, etc.) for the sole purpose of planning the best outsourcing strategy. The champion will ensure a process workflow, define roles/responsibilities, identify challenges (i.e., to eliminate silos or divisions because of competing priorities or lack of vested interest), and ensure goals are defined and agreed to by the selection committee.
A committee that is composed of multidisciplinary leaders often can determine the CRO that is the best fit for the organization. Not all CROs are aligned with your organizational culture, practices, or expertise, so it is a best practice to define these parameters. The selection committee must also have a vested and committed interest in this process. Depending on the size of the organization, the committee may want to define and agree on a communication strategy. This ensures the workflow is moving ahead as per plan and that all members are accountable for their roles and tasks. Additionally, good planning establishes how to maximize core assets and utilize internal resources, supplemented with external resources. A champion and selection committee will define exactly what tasks the CRO will perform and which procedures/processes will govern various tasks. For example, the sponsoring company will establish how the investigator site contracts will be negotiated and who will legally be bound by the agreement(s). CROs bidding on the work need to know exactly what tasks they will perform in order to provide their best approach. With that, the champion/committee will create a specifications document to communicate standard processes, tasks, and tools it is looking to procure. By further defining the scope of the services being procured, whether full service or partial service, companies create a strategic sourcing approach for the clinical trial.
Procurement/Strategic Sourcing
In order to establish and assess the best fit, the committee may want to utilize its vendor network, current CROs, and/or conduct a competition through public announcement of the procurement. A competition is not a new concept, but how you conduct the competition is key, especially if the project is partnered with another commercial entity or federal agency (i.e., federally funded). Also, as the committee considers long-term planning or portfolio management, it seems logical to maximize efficiencies with relationship management. How can teams reduce cost and time in this repetitive process? Commercial procurement models generally take one of three forms. The preferred provider model, which creates a more collaborative relationship with a CRO, ensures repeat business and allows for streamlined buying power. The performance-based model or managed services model drives accountability through incentives/penalties using milestones/target achievements. Another model is used when sponsors want to harness innovation to select the best value-added solution. Some CROs have new technology or innovative approaches aligned with the company’s goals to augment internal resources. Making these requirements clear to CROs that range from generalists to specialists with partial- to full-service clinical research functions allows the CRO procurement filter process to be maximized for best fit. What does this mean for the selection committee? What level of detail and criteria are needed?
The request for proposal (RFP) documentation is a vital piece in communicating the sponsor/client’s needs. The RFP will be addressed in Part 2 of this series, but before you can create an RFP, the committee must define criteria for selection. Matching chemistry like perception or perceived value is important on the surface, but the real measures of potential performance are the following criteria.

Risk management practices are now expected to be incorporated into the selection and oversight of CROs and other vendors. Find out more in SAM Sather's webinar:
CRO Oversight Post ICH GCP E6 (R2) Addendum
Selection Criteria For CRO Selection
- Specific therapeutic area and indication experience
- Team member experience in the indication
- Approach to current challenges (demonstrated understanding of enrollment, feasibility, access to KOLs, and investigative sites with the right population and experience)
- Responsiveness and ability to be limber with internal and external processes/systems (don’t forget to conduct a qualification audit as a contingency to an agreement)
- Quality/consistency of performance/financial stability/inspection history 2
- Value (determine the best service for best price)
- Transparency (confirm the scope of service delineates the client will have access to systems)
- Collaboration and communication planning (ask if they have escalation strategies)
- How do they plan (ask for examples of schedules, what plans will be part of the service, i.e., project plans, risk management plan, subcontractor management plan, monitoring plan, statistical analysis plan, data and pharmacovigilance plans)
According to Comprehend, 3 a clinical intelligence software provider, the three most common overlooked selection criteria are transparency, collaborative partnership, and real-time responsiveness. These intangible factors should be part of any strategic selection process. Sponsors struggle to quantify these factors, but to win the business, CROs can proactively demonstrate these intangibles with solid evidence. This isn’t one-sided. CROs can only provide their best services when all parties model transparent and collaborative relationships.
Future articles in this series will cover: developing a CRO selection workflow and checklist, addressing documentation and regulation concerns, and applying best practices to overcome the major challenges in outsourcing.
References:
- https://www.statista.com/statistics/817599/revenue-forecast-for-pharma-cros-by-location/
- https://www.pharmoutsourcing.com/Featured-Articles/172751-What-to-Look-for-in-Selecting-a-CRO-CMO-and-How-to-Ensure-the-Right-Choice-A-Quality-Assurance-Perspective/
- https://www.comprehend.com/overlooked-criteria-in-cro-selection/
About the Author:

Like what you are reading?
Sign up for our free newsletter, newsletter signup.

- All Services
- Research Sites
- Sponsors & CROs
- Independent
- Testimonials

- Work With Us
Adding to cart....

$49.97 / 2,499 points
Clinical research site business plan.
Elevate your healthcare venture with the “Clinical Research Company Business Plan” by Erik Lombere, MBA. This meticulously crafted document serves as a blueprint for launching and operating a successful clinical research site. Backed by industry insights, it offers strategic planning, financial projections, and step-by-step guidance to navigate this complex field. If you’re passionate about contributing to medical advancements and building a lucrative business, this plan is your roadmap to success. Secure your copy today!
Maximize Impact
Efficiency up.
Boost workflow speed by 40%, reducing project turnaround time significantly.
Cost Savings
Slash operational costs by up to 30%, optimizing budget allocation effectively.
Support 48H
Receive responsive support within 48 hours, ensuring smooth project progression.
Product Reviews
Share your valuable feedback with us through a review and earn points towards redeeming a complimentary item of your choice.
No reviews yet.

Let Us Know How We Did
Write a review and get 3 points towards a free templete when signed in!
Create Your Account and Start Earning Rewards Now
Embark on a rewarding journey with our exclusive points system! Sign up today and immediately earn 250 points, opening the door to a plethora of benefits. Use these points to download our Clinical Research CV Template for free, or save them to unlock discounts on other valuable products and services. Our point system is designed to grow with you – earn more points by leaving reviews, purchasing products, and engaging with our services. Every action not only enhances your professional toolkit but also brings exciting rewards. Create your account now and begin accumulating points towards your next career-enhancing resource!
Reimagining the Operating Model for a Clinical Research Organization

A well-established clinical research organization (CRO) specializing in the design and delivery of agile clinical trials engaged Clarkston for strategy services to help reimagine what their ideal future operating model should look like.
Given the company’s long-range plan (LRP), the CFO and the executive team wanted to define the major strategic considerations and triggers that could require fundamental changes to their operating model in the mid- to long-term, specifically those derived from key revenue milestone changes, global expansion, M&A activity, the addition of new enabling technology platforms (e.g., ERP), and those focused around cost-containment activities (e.g., onshore/offshore and/or insource/outsource efforts).
Download the Full Case Study Here
Clarkston’s team began with an in-depth assessment of the current state of the finance organization, followed by several conversations with former CFOs to gather perspectives from a variety of executives across different industry sub-segments. Clarkston then defined key finance activities (ranging from highly repeatable and transactional-intensive to highly strategic) to help our client understand core competency areas vs. those primed for delegation.
Through this engagement, the CFO and the executive team were able to clearly define and understand current organizational challenges and, more importantly, what and how to prepare for the future.
Download the full CRO Operating Model case study here, learn more about our Strategy Consulting Services or contact us below.
Contact Us to Learn More
Related Insights

Driving the Future of Clinical Research: Key Takeaways from MAGI 2024
Our clinical operations experts Erica Parks Murray and Laurie Stone recently attended MAGI 2024: The Clinical Research Conference in New ...

How to Prepare for ICH E6(R3): The Future of Clinical Trials
The International Council for Harmonisation (ICH) Good Clinical Practice (GCP) guidelines received a major facelift in a global effort to ...
Top 3 Reasons Why You Need to Incorporate DEI into Clinical Trials Today
Within the dynamic landscape of clinical trials, the incorporation of Diversity, Equity, and Inclusion (DE+I) into patient pools is frequently ...
Subscribe to All Posts
- Life Sciences
- Consumer Products
You may unsubscribe from these communications at any time.
- Email This field is for validation purposes and should be left unchanged.
Top 10 largest clinical research organizations
We take a look at the 10 biggest contract research organizations in the pharma sector in 2022.

The drug discovery process is complex. The clinical stage is particularly resource-intensive, something that has led to the rise in demand for contract research organizations (CROs) that can support drug manufacturers at each stage of the process, from discovery to approval. The core activities a CRO can provide include (but are not limited to) clinical trial management, data research and project management.
Despite an initial downturn at the start of the pandemic, Covid-19 created a spike in the number of clinical trials taking place due to the need for vaccines and drugs to tackle the virus. In the coming years, we expect the rise of technologies that enable decentralized clinical trials to help expand the market share of CROs.
Currently the global CRO services market is projected to grow from US$73.38 bn to $163.48 bn by 2029. Here Pharma IQ takes a look at the 10 biggest CROs in pharma today.
Founded in 1982, IQVIA is an American multinational company formed through the merger of Quintiles, a leading provider of product development and integrated healthcare services, and IMS Health, a global information and technology services company. The latter has enabled company to have a strong focus on digital solutions and analytics. In 2017, Quintiles IMS rebranded to IQVIA.
Laboratory Corporation of America Holdings, also known as Labcorp, is an American company that operates one of the largest clinical laboratory networks in the world. In an average week Labcorp processes tests on more than 3 million patient specimens. In 2020 the company earned revenue in excess of $14 bn.
Syneos Health
Founded in 1999, Syneos Health was created following the merger of two biopharmaceutical companies: INC Research and inVentive health. Today it has offices in more than 110 countries and offers services as a CRO as well as consultancy.
Founded in 1985 as a one-person consultancy firm, Pharmaceutical Product Development (PPD) is a global contract research organization that provides drug development, lab and lifecycle management services. In 2020 the company made US$4.7bn and the following year it became part of Thermo Fisher Scientific.
Headquartered in Dublin, Icon provides strategic management and support for clinical development from the compound selection stage through to clinical trials. Its services include clinical trials management, biometric activities, investigator recruitment and outcomes research.
Parexel was founded in 1982 and acquired by private equity firm Pamplona Capital in 2017 in a deal worth $5 bn. In 2021 it was bought by EQT Private Equity and Goldman Sachs Asset Management. The company conducts clinical trials and operates in more than 50 countries. It makes around $3 bn in revenue annually.
Charles River Laboratories
Founded in 1947, today this company specializes in cell and gene therapies as well as lab services for the pharmaceutical, medical device and biotech industries. As of 2021 it operates more than 90 facilities in 20 countries and has an annual revenue of $3.54 bn.
Award-winning MedPace has offices on six continents, with headquarters in Ohio where it has a clinical research campus and a number of clinical and bioanalytical laboratories. It provides clinical trial services for Phase I-IV studies in the biotech, pharma and medical device industries. Its revenue has been growing steadily year- on-year and is currently almost $1 bn.
CTI Clinical Trial and Consulting Services
This organization was founded in 1999 to provide clinical trial services and bring new drugs to market. It operates in more than 60 countries and since its inception has contributed to the approval of more than 150 new drugs and medical devices around the world.
WuXi AppTec
Founded in 2000 in Shanghai, WuXi AppTec is the newest company in our top 10. It provides services across the entire development cycle including small molecule R&D and manufacturing, biologics R&D and manufacturing, cell and gene therapy. It currently operates in 18 locations across China, Iceland and the US.
Quick links
- How to improve the success rate of clinical trials
- Webinar: The technology enabling decentralized clinical trials
- The challenges of handling and delivering highly potent oncology drugs
Get exclusive access to member-only articles, reports, videos, interviews, webinars and other premium content from industry experts and thought leaders by signing up to Pharma IQ here .
Upcoming Events
Ai for pharma & healthcare.
24 - 26 September, 2024 Amsterdam Marriott Hotel

SmartLab Exchange Europe
February 25 - 26, 2025 Novotel Amsterdam City, Netherlands

Pharma Contract Manufacturing
26 - 27 March, 2025 Berlin

SmartLab Exchange USA 2025
08 - 09 April, 2025 Le Méridien, Fort Lauderdale, Florida, USA

Clinical Trial Supply Forum
14-16 May Brussels, Belgium

Subscribe to our Free Newsletter
Insights from the world’s foremost thought leaders delivered to your inbox.
Latest Webinars
Pharma iq's power list 2022: in conversation with pharma's top leaders.
2022-10-18 02:00 PM - 03:00 PM BST

A post-pandemic 3D view of the patient and supply journey
2022-06-01 04:30 PM - 05:30 PM CET

Discover how targeted radiotherapy induced toxicity can be identified with imaging
2022-04-28 01:00 PM - 02:00 PM EST

RECOMMENDED

FIND CONTENT BY TYPE
- Infographics
Pharma IQ COMMUNITY
- Advertise With Us
- User Agreement
- Cookie Policy
- Become a Member Today
- Pharma IQ App
ADVERTISE WITH US
Reach Pharmaceuticals & Biotechnology professionals through cost-effective marketing opportunities to deliver your message, position yourself as a thought leader, and introduce new products, techniques and strategies to the market.
JOIN THE Pharma IQ COMMUNITY
Join Pharma & Biotech today and interact with a vibrant network of professionals, keeping up to date with the industry by accessing our wealth of articles, videos, live conferences and more.

Pharma IQ, a division of IQPC
Careers With IQPC | Contact Us | About Us | Cookie Policy
Become a Member today!
PLEASE ENTER YOUR EMAIL TO JOIN FOR FREE
Already an IQPC Community Member? Sign in Here or Forgot Password Sign up now and get FREE access to our extensive library of reports, infographics, whitepapers, webinars and online events from the world’s foremost thought leaders.
We respect your privacy, by clicking 'Subscribe' you will receive our e-newsletter, including information on Podcasts, Webinars, event discounts, online learning opportunities and agree to our User Agreement. You have the right to object. For further information on how we process and monitor your personal data click here . You can unsubscribe at any time.
Clinical Researcher
How the CRO Shift to Digital is Transforming Clinical Research
Clinical Researcher May 18, 2021

Clinical Researcher—May 2021 (Volume 35, Issue 4)
TRIALS & TECHNOLOGY
Clinical transformation accelerated during COVID-19 to speed trial execution. As companies raced to find treatments and vaccines, life sciences researchers came together to deliver innovation faster than ever. Despite these leaps forward, the industry recognizes the pace is not sustainable due to the regulatory changes and cross-company collaboration it took to get there. To drive long-lasting change in trials, more work remains to modernize clinical systems, and contract research organizations (CROs) are leading the way.
CROs are taking significant action to speed study execution by investing in new digital strategies and technologies that bring together study processes in trials. In fact, 90% of CROs say they have initiatives in place to unify clinical operations, according to a recent study .{1} These efforts are driving more streamlined and connected ways of conducting research.
By increasing efficiency in major clinical areas and improving collaboration across the trial ecosystem, CROs are advancing the industry toward patient-centric digital trials. The ongoing modernization elevates the industry to a new level of connectivity that will benefit life sciences and patients for years to come.
Modernizing Across the Clinical Landscape for Faster Execution
CROs are implementing digital approaches across the clinical spectrum to speed trials. New solutions are making it easier to modernize specific areas, and there’s been an acceleration in the adoption of purpose-built applications.
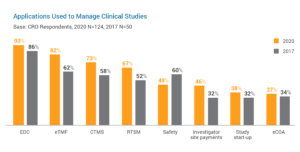
Study start-up is an area with significant potential to speed trial cycle times and improve overall efficiency in studies. This explains why 38% of CROs are using purpose-built study start-up applications, signaling a shift from manual methods like spreadsheets and e-mail to advanced solutions. The key drivers accelerating change among CROs are improving start-up times (73%) and reducing manual processes (52%). These advancements in study start-up will have a positive downstream impact on trials as more sponsors outsource early trial activities like site feasibility and selection to CROs.
In data management , one of the earliest areas to implement process automation, a growing number of leading CROs are looking to innovative clinical data management applications to run higher quality studies for sponsors.{2} This new breed of solution enables study builds in six weeks or less and delivers the agility to satisfy even the most complex data requirements.
CROs are also mitigating delays and issues in studies much quicker than ever before. With complete study oversight, clinical trial management systems (CTMS) empower CROs to identify problem areas and make instant decisions. This form of proactive trial management increases efficiency for CROs and research sites alike. The shift from spreadsheets and homegrown systems to digital clinical trial management has been key in standardizing study processes for faster execution.
Trial master file (TMF) management is undergoing vast transformation as more CROs move to eTMF applications with advanced digital and collaboration capabilities. Modern eTMF is driving positive improvements for CROs in TMF accuracy (70%) and completeness (63%) over other methods like paper and local file systems. eTMF is a vital tool for improved information sharing and better collaboration in trials.
Automating Information Exchange for Improved Collaboration
An overreliance on paper and manual processes in clinical trials make it difficult to share information with key stakeholders. The growing complexity and increasing volumes of data in studies are making it even harder. For CROs, streamlining information sharing is a top priority to reduce manual processes (78%), speed study execution (61%), and improve collaboration with sponsors and sites (57%).
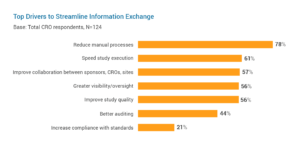
CROs are breaking down the paper barriers by adopting solutions that automate information flow. These new technologies connect investigator site systems with CRO clinical operations to streamline processes like safety letter distribution and study closeout transfers. By transforming information exchange from manual and paper-based to digital and automated, CROs are improving collaboration in studies.
Investments in decentralized approaches are also improving information sharing across trial stakeholders. For example, informed consent has been traditionally managed manually on paper. Solutions like eConsent digitize the process; eConsent makes it easier for patients to understand and provide informed consent and for CROs and sites to share information. More CROs are enabling sites to use eConsent for a simpler, paperless consenting process.
CROs are Digitizing Processes and Connecting Systems for Faster Trials
Sponsors outsourcing clinical trials expect to gain access to innovative technologies and approaches. To offer differentiated services and deliver efficiencies to sponsors, more CROs are digitizing processes and connecting systems. During the pandemic, this proved crucial to ensure continuity in trials. Many companies experienced delays because study monitors could not verify data at research sites. The CROs that unified study documents and data, and had the digital capabilities to enable remote monitoring, kept trials moving forward.
Looking ahead, these same organizations embracing digital will speed trials and lower overall costs, delivering transformational change for the industry. We’re only at the tip of the iceberg, but the advancements will be swift. Thank you, CROs, for moving the industry toward future trials that are digital, connected, and fast.
- Veeva Systems. 2020. Veeva Unified Clinical Operations Survey: Annual CRO Report
- Veeva Systems. 2020. 6 of the Top 7 Global CROs Partner with Veeva to Run Faster Clinical Trials for Sponsors

Jim Reilly is Vice President for Vault R&D with Veeva Systems. For the last 15 years, he has held a variety of senior positions in life sciences technology, leading software delivery and sales in clinical operations, regulatory, clinical data standards, and content management. He is responsible for customer engagement, market adoption, and strategic alliances across the entire Veeva Vault R&D product portfolio.
Sorry, we couldn't find any jobs that match your criteria.

The Industry Shift Toward Decentralized Clinical Trials: Impacts on Quality Management, Participant Outcomes, and Data Management

Insights into the Clinical Research Associate Career Pathway

The Future of Multi-Omics in Cancer Clinical Trials

Sign in to your site’s geographic location:
Business continuity plan for clinical trial sites: why electronic source matters during covid-19.
The impact of COVID-19 on clinical trials has likely become obvious for your site during this phase of the pandemic. Sites are struggling with screening holds, limited staff, an influx of sponsor requests while staff is not on-site, the challenge of retaining enrolled subjects, and more. It is now more important than ever to implement a business continuity plan for reopening your clinical research site. Be prepared for potential new waves of COVID-19 that could result in individual staff members being quarantined or the site having to take precautionary measures. This means that on short notice the site must be in a position to limit patient-staff interactions, and ensure that the PI and Sponsor representatives have remote access to patient data.
Before joining Clinical Research IO , I was a manager at a women’s health site. I oversaw the selection and implementation of the CRIO eSource – eRegulatory – CTMS system at my site. I knew this was the right decision because it allowed us to control quality and improve efficiency. But when the pandemic hit and we had to modify our procedures, I really knew that we had made the right decision. Unlike many sites I knew, we were able to adapt very quickly to the new circumstances, maintaining our productivity while enabling remote monitoring for our sponsors.
With CRIO, the system fully integrated all of our modules into one log-in: Scheduling, eSource , eRegulatory , Finance, and Recruitment. We could collect data electronically during the visit and then review and query that data remotely and in real-time. We could give read-only access as needed, and restrict access to different modules to different users. We could give our monitors instant access only to the studies they were assigned to and view and respond to their queries remotely. These capabilities became life-savers when we had to institute work-from-home and prohibit monitors from visiting our site.
Even though parts of our country are opening up again, most epidemiologists are forecasting future waves of the pandemic, which could necessitate many of the same measures sites have recently undertaken – work-from-home, virtual visits, restricted monitor travel, etc. Having CRIO implemented at your site will allow you to adapt instantly to these changes.
Here are the things we were able to do with CRIO during the pandemic:
1. We easily minimized the number of research site staff who had to be onsite:
- Coordinators took separate shifts that did not overlap. When one person was in the office seeing patients, other personnel could be at home, doing EDC entry, answering queries, processing regulatory documents or documenting phone call visits to patients.
- Our investigators could stay home and enter progress notes, conduct phone visits and record the source in real-time, and sign off on labs and visit notes.
- Our finance person could track receivables in real-time, as the data was being completed, and send invoices in a timely manner so as to maintain cash flow. We could see in an instant which invoices and visits were overdue on payment, and take appropriate follow up.
If you’d like to learn more about how CRIO-enabled clinical trial sites are allowing their staff to work remotely, schedule a demo with our team to learn more about how CRIO’s CTMS.
2. CRCs, Lab staff, and Investigators had access to the subject chart at the same time.
PIs could write a progress note immediately after seeing a patient while the CRC finished the visit. Lab techs were able to document blood draw times while the coordinator uploaded relevant visit files.
3. We were able to give our CRAs remote monitoring access at any time.
Our CRAs could view our data and leave their queries behind, and we could respond to and close out those queries from home. By the way, because of the built-in edit checks, we had a lot fewer queries to address, and saved a lot of time not having to scan and email source in.
4. We were able to adapt our eSource on the fly.
This way we could complete certain procedures on-site and certain procedures, and/or visits remotely. We were able to implement virtual visits and at-home deliveries of IP by creating appropriate procedures, adding them to our library, then adding them as needed to the appropriate studies.
5. We used CRIO’s automated text reminder feature to add COVID screening questions…
And a reminder to patients to wear a mask to their visit!
6. We could build and add a COVID-19 screening questionnaire per CDC guidelines (temperature check, etc.) to the beginning of each study visit.
Using that screening questionnaire, we could then generate an invoiceable charge to the sponsor, if that’s what our clinical trial agreement allowed.
No one could have predicted that we would be dealing with something as disruptive and devastating as COVID-19. Having CRIO really allowed us to adapt our processes while maintaining data quality and PI oversight. Based on this experience, I became such a strong advocate of the CRIO system that I joined their team – my job is to help other sites implement the system and gain the efficiencies I experienced firsthand.
If you’d like to learn more, you can talk directly to our team here .

21 CFR Part 11 Regulation Compliance Update
Compliance Update: The 21 CFR Part 11 Regulation is a cornerstone of conducting clinical trials in today’s world. The release of the regulation 1997 established guidelines for the use of electronic records and electronic signatures in FDA-regulated industries and had a significant impact on the pharmaceutical and medical device industries. The FDA began working on...

The Current State of Clinical Trials in Ukraine—A Conversation with Dr. Roman Fishchuk
Meet Dr. Roman Fishchuk, an esteemed otorhinolaryngologist (ENT) whose journey in the healthcare field has been marked by a commitment to providing medical care and innovative treatment options through clinical trials in his native Ukraine. Having graduated from Ivano-Frankivsk National Medical University, Dr. Fishchuk specialized in otolaryngology and completed a Master’s degree at the University...

Transitioning to CRIO? How to Get Your Data Ready for Migration
You have just signed on to use CRIO and you are excited to get started. Even if you are moving primarily from paper, you may have data that you want to migrate into CRIO rather than having to re-enter it. For many of our new clients, they have a wealth of information available in spreadsheets...
Get articles delivered to your inbox, every week
An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
The PMC website is updating on October 15, 2024. Learn More or Try it out now .
- Advanced Search
- Journal List
- v.15(2); 2023 Feb
- PMC10023071

Clinical Trials and Clinical Research: A Comprehensive Review
Venkataramana kandi.
1 Clinical Microbiology, Prathima Institute of Medical Sciences, Karimnagar, IND
Sabitha Vadakedath
2 Biochemistry, Prathima Institute of Medical Sciences, Karimnagar, IND
Clinical research is an alternative terminology used to describe medical research. Clinical research involves people, and it is generally carried out to evaluate the efficacy of a therapeutic drug, a medical/surgical procedure, or a device as a part of treatment and patient management. Moreover, any research that evaluates the aspects of a disease like the symptoms, risk factors, and pathophysiology, among others may be termed clinical research. However, clinical trials are those studies that assess the potential of a therapeutic drug/device in the management, control, and prevention of disease. In view of the increasing incidences of both communicable and non-communicable diseases, and especially after the effects that Coronavirus Disease-19 (COVID-19) had on public health worldwide, the emphasis on clinical research assumes extremely essential. The knowledge of clinical research will facilitate the discovery of drugs, devices, and vaccines, thereby improving preparedness during public health emergencies. Therefore, in this review, we comprehensively describe the critical elements of clinical research that include clinical trial phases, types, and designs of clinical trials, operations of trial, audit, and management, and ethical concerns.
Introduction and background
A clinical trial is a systematic process that is intended to find out the safety and efficacy of a drug/device in treating/preventing/diagnosing a disease or a medical condition [ 1 , 2 ]. Clinical trial includes various phases that include phase 0 (micro-dosing studies), phase 1, phase 2, phase 3, and phase 4 [ 3 ]. Phase 0 and phase 2 are called exploratory trial phases, phase 1 is termed the non-therapeutic phase, phase 3 is known as the therapeutic confirmatory phase, and phase 4 is called the post-approval or the post-marketing surveillance phase. Phase 0, also called the micro-dosing phase, was previously done in animals but now it is carried out in human volunteers to understand the dose tolerability (pharmacokinetics) before being administered as a part of the phase 1 trial among healthy individuals. The details of the clinical trial phases are shown in Table Table1 1 .
This table has been created by the authors.
MTD: maximum tolerated dose; SAD: single ascending dose; MAD: multiple ascending doses; NDA: new drug application; FDA: food and drug administration
| Clinical trial phase | Type of the study | Nature of study |
| Phase 0 | Exploratory | Examines too low (1/100 ) concentrations (micro-dosing) of the drug for less time. Study the pharmacokinetics and determine the dose for phase I studies. Previously done in animals but now it is carried out in humans. |
| Phase I, Phase Ia, Phase Ib | Non-therapeutic trial | Around <50 healthy subjects are recruited. Establishes a safe dose range, and the MTD. Examines the pharmacokinetic and pharmacodynamic effects. Usually single-center studies. Phase Ia: SAD, and MTD. Duration of one week to several months depending on the trial and includes 6-8 groups of 3-6 participants. Phase Ib: MAD and the dose is gradually narrowed down. Three groups of 8 individuals each. |
| Phase II, Phase IIa, Phase IIb | Exploratory trial | Recruiting around 5-100 patients of either sex. Examines the effective dosage and the therapeutic effects on patients. It decides the therapeutic regimen and drug-drug interactions. Usually, multicentre studies. Phase IIa: Decides the drug dosage, includes 20-30 patients, and takes up to weeks/months. Phase IIb: Studies dose-response relationship, drug-drug interactions, and comparison with a placebo. |
| Phase III | Therapeutic confirmatory trial | More than 300 patients (up to 3000) of either sex are recruited in this study and are multicentric trials. Pre-marketing phase examines the efficacy and the safety of the drug. Comparison of the test drug with the placebo/standard drug. Adverse drug reactions/adverse events are noted. Initiate the process of NDA with appropriate regulatory agencies like the FDA. |
| Phase IV | Post-approval study | After approval/post-licensure and post-marketing studies/surveillance studies. Following up on the patients for an exceptionally long time for potential adverse reactions and drug-drug interactions. |
Clinical research design has two major types that include non-interventional/observational and interventional/experimental studies. The non-interventional studies may have a comparator group (analytical studies like case-control and cohort studies), or without it (descriptive study). The experimental studies may be either randomized or non-randomized. Clinical trial designs are of several types that include parallel design, crossover design, factorial design, randomized withdrawal approach, adaptive design, superiority design, and non-inferiority design. The advantages and disadvantages of clinical trial designs are depicted in Table Table2 2 .
| Trial design type | Type of the study | Nature of study | Advantages/disadvantages |
| Parallel | Randomized | This is the most frequent design wherein each arm of the study group is allocated a particular treatment (placebo (an inert substance)/therapeutic drug) | The placebo arm does not receive the trial drug, so may not get the benefit of it |
| Crossover | Randomized | The patient in this trial gets each drug and the patients serve as a control themselves | Avoids participant bias in treatment and requires a small sample size. This design is not suitable for research on acute diseases. |
| Factorial | Non-randomized | Two or more interventions on the participants and the study can provide information on the interactions between the drugs | The study design is complex |
| Randomized withdrawal approach | Randomized | This study evaluates the time/duration of the drug therapy | The study uses a placebo to understand the efficacy of a drug in treating the disease |
| Matched pairs | Post-approval study | Recruit patients with the same characteristics | Less variability |
There are different types of clinical trials that include those which are conducted for treatment, prevention, early detection/screening, and diagnosis. These studies address the activities of an investigational drug on a disease and its outcomes [ 4 ]. They assess whether the drug is able to prevent the disease/condition, the ability of a device to detect/screen the disease, and the efficacy of a medical test to diagnose the disease/condition. The pictorial representation of a disease diagnosis, treatment, and prevention is depicted in Figure Figure1 1 .

This figure has been created by the authors.
The clinical trial designs could be improvised to make sure that the study's validity is maintained/retained. The adaptive designs facilitate researchers to improvise during the clinical trial without interfering with the integrity and validity of the results. Moreover, it allows flexibility during the conduction of trials and the collection of data. Despite these advantages, adaptive designs have not been universally accepted among clinical researchers. This could be attributed to the low familiarity of such designs in the research community. The adaptive designs have been applied during various phases of clinical trials and for different clinical conditions [ 5 , 6 ]. The adaptive designs applied during different phases are depicted in Figure Figure2 2 .

The Bayesian adaptive trial design has gained popularity, especially during the Coronavirus Disease-19 (COVID-19) pandemic. Such designs could operate under a single master protocol. It operates as a platform trial wherein multiple treatments can be tested on different patient groups suffering from disease [ 7 ].
In this review, we comprehensively discuss the essential elements of clinical research that include the principles of clinical research, planning clinical trials, practical aspects of clinical trial operations, essentials of clinical trial applications, monitoring, and audit, clinical trial data analysis, regulatory audits, and project management, clinical trial operations at the investigation site, the essentials of clinical trial experiments involving epidemiological, and genetic studies, and ethical considerations in clinical research/trials.
A clinical trial involves the study of the effect of an investigational drug/any other intervention in a defined population/participant. The clinical research includes a treatment group and a placebo wherein each group is evaluated for the efficacy of the intervention (improved/not improved) [ 8 ].
Clinical trials are broadly classified into controlled and uncontrolled trials. The uncontrolled trials are potentially biased, and the results of such research are not considered as equally as the controlled studies. Randomized controlled trials (RCTs) are considered the most effective clinical trials wherein the bias is minimized, and the results are considered reliable. There are different types of randomizations and each one has clearly defined functions as elaborated in Table Table3 3 .
| Randomization type | Functions |
| Simple randomization | The participants are assigned to a case or a control group based on flipping coin results/computer assignment |
| Block randomization | Equal and small groups of both cases and controls |
| Stratified randomization | Randomization based on the age of the participant and other covariates |
| Co-variate adaptive randomization/minimization | Sequential assignment of a new participant into a group based on the covariates |
| Randomization by body halves or paired organs (Split body trials) | One intervention is administered to one-half of the body and the comparator intervention is assigned to another half of the body |
| Clustered randomization | Intervention is administered to clusters/groups by randomization to prevent contamination and either active or comparator intervention is administered for each group |
| Allocation by randomized consent (Zelen trials) | Patients are allocated to one of the two trial arms |
Principles of clinical trial/research
Clinical trials or clinical research are conducted to improve the understanding of the unknown, test a hypothesis, and perform public health-related research [ 2 , 3 ]. This is majorly carried out by collecting the data and analyzing it to derive conclusions. There are various types of clinical trials that are majorly grouped as analytical, observational, and experimental research. Clinical research can also be classified into non-directed data capture, directed data capture, and drug trials. Clinical research could be prospective or retrospective. It may also be a case-control study or a cohort study. Clinical trials may be initiated to find treatment, prevent, observe, and diagnose a disease or a medical condition.
Among the various types of clinical research, observational research using a cross-sectional study design is the most frequently performed clinical research. This type of research is undertaken to analyze the presence or absence of a disease/condition, potential risk factors, and prevalence and incidence rates in a defined population. Clinical trials may be therapeutic or non-therapeutic type depending on the type of intervention. The therapeutic type of clinical trial uses a drug that may be beneficial to the patient. Whereas in a non-therapeutic clinical trial, the participant does not benefit from the drug. The non-therapeutic trials provide additional knowledge of the drug for future improvements. Different terminologies of clinical trials are delineated in Table Table4 4 .
| Type of clinical trial | Definition |
| Randomized trial | Study participants are randomly assigned to a group |
| Open-label | Both study subjects and the researchers are aware of the drug being tested |
| Blinded (single-blind) | In single-blind studies, the subject has no idea about the group (test/control) in which they are placed |
| Double-blind (double-blind) | In the double-blind study, the subjects as well as the investigator have no idea about the test/control group |
| Placebo | A substance that appears like a drug but has no active moiety |
| Add-on | An additional drug apart from the clinical trial drug given to a group of study participants |
| Single center | A study being carried out at a particular place/location/center |
| Multi-center | A study is being carried out at multiple places/locations/centers |
In view of the increased cost of the drug discovery process, developing, and low-income countries depend on the production of generic drugs. The generic drugs are similar in composition to the patented/branded drug. Once the patent period is expired generic drugs can be manufactured which have a similar quality, strength, and safety as the patented drug [ 9 ]. The regulatory requirements and the drug production process are almost the same for the branded and the generic drug according to the Food and Drug Administration (FDA), United States of America (USA).
The bioequivalence (BE) studies review the absorption, distribution, metabolism, and excretion (ADME) of the generic drug. These studies compare the concentration of the drug at the desired location in the human body, called the peak concentration of the drug (Cmax). The extent of absorption of the drug is measured using the area under the receiver operating characteristic curve (AUC), wherein the generic drug is supposed to demonstrate similar ADME activities as the branded drug. The BE studies may be undertaken in vitro (fasting, non-fasting, sprinkled fasting) or in vivo studies (clinical, bioanalytical, and statistical) [ 9 ].
Planning clinical trial/research
The clinical trial process involves protocol development, designing a case record/report form (CRF), and functioning of institutional review boards (IRBs). It also includes data management and the monitoring of clinical trial site activities. The CRF is the most significant document in a clinical study. It contains the information collected by the investigator about each subject participating in a clinical study/trial. According to the International Council for Harmonisation (ICH), the CRF can be printed, optical, or an electronic document that is used to record the safety and efficacy of the pharmaceutical drug/product in the test subjects. This information is intended for the sponsor who initiates the clinical study [ 10 ].
The CRF is designed as per the protocol and later it is thoroughly reviewed for its correctness (appropriate and structured questions) and finalized. The CRF then proceeds toward the print taking the language of the participating subjects into consideration. Once the CRF is printed, it is distributed to the investigation sites where it is filled with the details of the participating subjects by the investigator/nurse/subject/guardian of the subject/technician/consultant/monitors/pharmacist/pharmacokinetics/contract house staff. The filled CRFs are checked for their completeness and transported to the sponsor [ 11 ].
Effective planning and implementation of a clinical study/trial will influence its success. The clinical study majorly includes the collection and distribution of the trial data, which is done by the clinical data management section. The project manager is crucial to effectively plan, organize, and use the best processes to control and monitor the clinical study [ 10 , 11 ].
The clinical study is conducted by a sponsor or a clinical research organization (CRO). A perfect protocol, time limits, and regulatory requirements assume significance while planning a clinical trial. What, when, how, and who are clearly planned before the initiation of a study trial. Regular review of the project using the bar and Gantt charts, and maintaining the timelines assume increased significance for success with the product (study report, statistical report, database) [ 10 , 11 ].
The steps critical to planning a clinical trial include the idea, review of the available literature, identifying a problem, formulating the hypothesis, writing a synopsis, identifying the investigators, writing a protocol, finding a source of funding, designing a patient consent form, forming ethics boards, identifying an organization, preparing manuals for procedures, quality assurance, investigator training and initiation of the trial by recruiting the participants [ 10 ].
The two most important points to consider before the initiation of the clinical trial include whether there is a need for a clinical trial, if there is a need, then one must make sure that the study design and methodology are strong for the results to be reliable to the people [ 11 ].
For clinical research to envisage high-quality results, the study design, implementation of the study, quality assurance in data collection, and alleviation of bias and confounding factors must be robust [ 12 ]. Another important aspect of conducting a clinical trial is improved management of various elements of clinical research that include human and financial resources. The role of a trial manager to make a successful clinical trial was previously reported. The trial manager could play a key role in planning, coordinating, and successfully executing the trial. Some qualities of a trial manager include better communication and motivation, leadership, and strategic, tactical, and operational skills [ 13 ].
Practical aspects of a clinical trial operations
There are different types of clinical research. Research in the development of a novel drug could be initiated by nationally funded research, industry-sponsored research, and clinical research initiated by individuals/investigators. According to the documents 21 code of federal regulations (CFR) 312.3 and ICH E-6 Good Clinical Practice (GCP) 1.54, an investigator is an individual who initiates and conducts clinical research [ 14 ]. The investigator plan, design, conduct, monitor, manage data, compile reports, and supervise research-related regulatory and ethical issues. To manage a successful clinical trial project, it is essential for an investigator to give the letter of intent, write a proposal, set a timeline, develop a protocol and related documents like the case record forms, define the budget, and identify the funding sources.
Other major steps of clinical research include the approval of IRBs, conduction and supervision of the research, data review, and analysis. Successful clinical research includes various essential elements like a letter of intent which is the evidence that supports the interest of the researcher to conduct drug research, timeline, funding source, supplier, and participant characters.
Quality assurance, according to the ICH and GCP guidelines, is necessary to be implemented during clinical research to generate quality and accurate data. Each element of the clinical research must have been carried out according to the standard operating procedure (SOP), which is written/determined before the initiation of the study and during the preparation of the protocol [ 15 ].
The audit team (quality assurance group) is instrumental in determining the authenticity of the clinical research. The audit, according to the ICH and GCP, is an independent and external team that examines the process (recording the CRF, analysis of data, and interpretation of data) of clinical research. The quality assurance personnel are adequately trained, become trainers if needed, should be good communicators, and must handle any kind of situation. The audits can be at the investigator sites evaluating the CRF data, the protocol, and the personnel involved in clinical research (source data verification, monitors) [ 16 ].
Clinical trial operations are governed by legal and regulatory requirements, based on GCPs, and the application of science, technology, and interpersonal skills [ 17 ]. Clinical trial operations are complex, time and resource-specific that requires extensive planning and coordination, especially for the research which is conducted at multiple trial centers [ 18 ].
Recruiting the clinical trial participants/subjects is the most significant aspect of clinical trial operations. Previous research had noted that most clinical trials do not meet the participant numbers as decided in the protocol. Therefore, it is important to identify the potential barriers to patient recruitment [ 19 ].
Most clinical trials demand huge costs, increased timelines, and resources. Randomized clinical trial studies from Switzerland were analyzed for their costs which revealed approximately 72000 USD for a clinical trial to be completed. This study emphasized the need for increased transparency with respect to the costs associated with the clinical trial and improved collaboration between collaborators and stakeholders [ 20 ].
Clinical trial applications, monitoring, and audit
Among the most significant aspects of a clinical trial is the audit. An audit is a systematic process of evaluating the clinical trial operations at the site. The audit ensures that the clinical trial process is conducted according to the protocol, and predefined quality system procedures, following GCP guidelines, and according to the requirements of regulatory authorities [ 21 ].
The auditors are supposed to be independent and work without the involvement of the sponsors, CROs, or personnel at the trial site. The auditors ensure that the trial is conducted by designated professionally qualified, adequately trained personnel, with predefined responsibilities. The auditors also ensure the validity of the investigational drug, and the composition, and functioning of institutional review/ethics committees. The availability and correctness of the documents like the investigational broacher, informed consent forms, CRFs, approval letters of the regulatory authorities, and accreditation of the trial labs/sites [ 21 ].
The data management systems, the data collection software, data backup, recovery, and contingency plans, alternative data recording methods, security of the data, personnel training in data entry, and the statistical methods used to analyze the results of the trial are other important responsibilities of the auditor [ 21 , 22 ].
According to the ICH-GCP Sec 1.29 guidelines the inspection may be described as an act by the regulatory authorities to conduct an official review of the clinical trial-related documents, personnel (sponsor, investigator), and the trial site [ 21 , 22 ]. The summary report of the observations of the inspectors is performed using various forms as listed in Table Table5 5 .
FDA: Food and Drug Administration; IND: investigational new drug; NDA: new drug application; IRB: institutional review board; CFR: code of federal regulations
| Regulatory (FDA) form number | Components of the form |
| 483 | List of objectionable conditions/processes prepared by the FDA investigator and submitted to the auditee at the end of the inspection |
| 482 | The auditors submit their identity proofs and notice of inspections to the clinical investigators and later document their observations |
| 1571 | This document details the fact that the clinical trial is not initiated before 30 days of submitting the IND to the FDA for approval. The form confirms that the IRB complies with 21 CFR Part 56. The form details the agreement to follow regulatory requirements and names all the individuals who monitor the conduct and progress of the study and evaluate the safety of the clinical trial |
| 1572 | This form details the fact that the study is conducted after ethics approval ensures that the study is carried out according to protocol, informed consent, and IRB approval |
Because protecting data integrity, the rights, safety, and well-being of the study participants are more significant while conducting a clinical trial, regular monitoring and audit of the process appear crucial. Also, the quality of the clinical trial greatly depends on the approach of the trial personnel which includes the sponsors and investigators [ 21 ].
The responsibility of monitoring lies in different hands, and it depends on the clinical trial site. When the trial is initiated by a pharmaceutical industry, the responsibility of trial monitoring depends on the company or the sponsor, and when the trial is conducted by an academic organization, the responsibility lies with the principal investigator [ 21 ].
An audit is a process conducted by an independent body to ensure the quality of the study. Basically, an audit is a quality assurance process that determines if a study is carried out by following the SPOs, in compliance with the GCPs recommended by regulatory bodies like the ICH, FDA, and other local bodies [ 21 ].
An audit is performed to review all the available documents related to the IRB approval, investigational drug, and the documents related to the patient care/case record forms. Other documents that are audited include the protocol (date, sign, treatment, compliance), informed consent form, treatment response/outcome, toxic response/adverse event recording, and the accuracy of data entry [ 22 ].
Clinical trial data analysis, regulatory audits, and project management
The essential elements of clinical trial management systems (CDMS) include the management of the study, the site, staff, subject, contracts, data, and document management, patient diary integration, medical coding, monitoring, adverse event reporting, supplier management, lab data, external interfaces, and randomization. The CDMS involves setting a defined start and finishing time, defining study objectives, setting enrolment and termination criteria, commenting, and managing the study design [ 23 ].
Among the various key application areas of clinical trial systems, the data analysis assumes increased significance. The clinical trial data collected at the site in the form of case record form is stored in the CDMS ensuring the errors with respect to the double data entry are minimized.
Clinical trial data management uses medical coding, which uses terminologies with respect to the medications and adverse events/serious adverse events that need to be entered into the CDMS. The project undertaken to conduct the clinical trial must be predetermined with timelines and milestones. Timelines are usually set for the preparation of protocol, designing the CRF, planning the project, identifying the first subject, and timelines for recording the patient’s data for the first visit.
The timelines also are set for the last subject to be recruited in the study, the CRF of the last subject, and the locked period after the last subject entry. The planning of the project also includes the modes of collection of the data, the methods of the transport of the CRFs, patient diaries, and records of severe adverse events, to the central data management sites (fax, scan, courier, etc.) [ 24 ].
The preparation of SOPs and the type and timing of the quality control (QC) procedures are also included in the project planning before the start of a clinical study. Review (budget, resources, quality of process, assessment), measure (turnaround times, training issues), and control (CRF collection and delivery, incentives, revising the process) are the three important aspects of the implementation of a clinical research project.
In view of the increasing complexity related to the conduct of clinical trials, it is important to perform a clinical quality assurance (CQA) audit. The CQA audit process consists of a detailed plan for conducting audits, points of improvement, generating meaningful audit results, verifying SOP, and regulatory compliance, and promoting improvement in clinical trial research [ 25 ]. All the components of a CQA audit are delineated in Table Table6 6 .
CRF: case report form; CSR: clinical study report; IC: informed consent; PV: pharmacovigilance; SAE: serious adverse event
| Product-specific audits program | Pharmacovigilance audits program |
| Protocol, CRF, IC, CSR | |
| Supplier | Safety data management |
| Clinical database | |
| Investigator site | Communications and regulatory reporting |
| Clinical site visit | |
| Study management | Signal detection and evaluation |
| SAE reporting | |
| Supplier audits program | Risk management and PV planning |
| Supplier qualification | |
| Sponsor data audit during the trial | Computerized system |
| Preferred vendor list after the trials | |
| Process/System audits program | Suppliers |
| Clinical safety reporting | |
| Data management | Regulatory inspection management program |
| Clinical supply | |
| Study monitoring | Assist with the audit response |
| Computerized system | Pre-inspection audit |
Clinical trial operations at the investigator's site
The selection of an investigation site is important before starting a clinical trial. It is essential that the individuals recruited for the study meet the inclusion criteria of the trial, and the investigator's and patient's willingness to accept the protocol design and the timelines set by the regulatory authorities including the IRBs.
Before conducting clinical research, it is important for an investigator to agree to the terms and conditions of the agreement and maintain the confidentiality of the protocol. Evaluation of the protocol for the feasibility of its practices with respect to the resources, infrastructure, qualified and trained personnel available, availability of the study subjects, and benefit to the institution and the investigator is done by the sponsor during the site selection visit.
The standards of a clinical research trial are ensured by the Council for International Organizations of Medical Sciences (CIOMS), National Bioethics Advisory Commission (NBAC), United Nations Programme on Human Immunodeficiency Virus/Acquired Immunodeficiency Syndrome (HIV/AIDS) (UNAIDS), and World Medical Association (WMA) [ 26 ].
Recommendations for conducting clinical research based on the WMA support the slogan that says, “The health of my patient will be my first consideration.” According to the International Code of Medical Ethics (ICME), no human should be physically or mentally harmed during the clinical trial, and the study should be conducted in the best interest of the person [ 26 ].
Basic principles recommended by the Helsinki declaration include the conduction of clinical research only after the prior proof of the safety of the drug in animal and lab experiments. The clinical trials must be performed by scientifically, and medically qualified and well-trained personnel. Also, it is important to analyze the benefit of research over harm to the participants before initiating the drug trials.
The doctors may prescribe a drug to alleviate the suffering of the patient, save the patient from death, and gain additional knowledge of the drug only after obtaining informed consent. Under the equipoise principle, the investigators must be able to justify the treatment provided as a part of the clinical trial, wherein the patient in the placebo arm may be harmed due to the unavailability of the therapeutic/trial drug.
Clinical trial operations greatly depend on the environmental conditions and geographical attributes of the trial site. It may influence the costs and targets defined by the project before the initiation. It was noted that one-fourth of the clinical trial project proposals/applications submit critical data on the investigational drug from outside the country. Also, it was noted that almost 35% of delays in clinical trials owing to patient recruitment with one-third of studies enrolling only 5% of the participants [ 27 ].
It was suggested that clinical trial feasibility assessment in a defined geographical region may be undertaken for improved chances of success. Points to be considered under the feasibility assessment program include if the disease under the study is related to the population of the geographical region, appropriateness of the study design, patient, and comparator group, visit intervals, potential regulatory and ethical challenges, and commitments of the study partners, CROs in respective countries (multi-centric studies) [ 27 ].
Feasibility assessments may be undertaken at the program level (ethics, regulatory, and medical preparedness), study level (clinical, regulatory, technical, and operational aspects), and at the investigation site (investigational drug, competency of personnel, participant recruitment, and retention, quality systems, and infrastructural aspects) [ 27 ].
Clinical trials: true experiments
In accordance with the revised schedule "Y" of the Drugs and Cosmetics Act (DCA) (2005), a drug trial may be defined as a systematic study of a novel drug component. The clinical trials aim to evaluate the pharmacodynamic, and pharmacokinetic properties including ADME, efficacy, and safety of new drugs.
According to the drug and cosmetic rules (DCR), 1945, a new chemical entity (NCE) may be defined as a novel drug approved for a disease/condition, in a specified route, and at a particular dosage. It also may be a new drug combination, of previously approved drugs.
A clinical trial may be performed in three types; one that is done to find the efficacy of an NCE, a comparison study of two drugs against a medical condition, and the clinical research of approved drugs on a disease/condition. Also, studies of the bioavailability and BE studies of the generic drugs, and the drugs already approved in other countries are done to establish the efficacy of new drugs [ 28 ].
Apart from the discovery of a novel drug, clinical trials are also conducted to approve novel medical devices for public use. A medical device is defined as any instrument, apparatus, appliance, software, and any other material used for diagnostic/therapeutic purposes. The medical devices may be divided into three classes wherein class I uses general controls; class II uses general and special controls, and class III uses general, special controls, and premarket approvals [ 28 ].
The premarket approval applications ensure the safety and effectiveness, and confirmation of the activities from bench to animal to human clinical studies. The FDA approval for investigational device exemption (IDE) for a device not approved for a new indication/disease/condition. There are two types of IDE studies that include the feasibility study (basic safety and potential effectiveness) and the pivotal study (trial endpoints, randomization, monitoring, and statistical analysis plan) [ 28 ].
As evidenced by the available literature, there are two types of research that include observational and experimental research. Experimental research is alternatively known as the true type of research wherein the research is conducted by the intervention of a new drug/device/method (educational research). Most true experiments use randomized control trials that remove bias and neutralize the confounding variables that may interfere with the results of research [ 28 ].
The variables that may interfere with the study results are independent variables also called prediction variables (the intervention), dependent variables (the outcome), and extraneous variables (other confounding factors that could influence the outside). True experiments have three basic elements that include manipulation (that influence independent variables), control (over extraneous influencers), and randomization (unbiased grouping) [ 29 ].
Experiments can also be grouped as true, quasi-experimental, and non-experimental studies depending on the presence of specific characteristic features. True experiments have all three elements of study design (manipulation, control, randomization), and prospective, and have great scientific validity. Quasi-experiments generally have two elements of design (manipulation and control), are prospective, and have moderate scientific validity. The non-experimental studies lack manipulation, control, and randomization, are generally retrospective, and have low scientific validity [ 29 ].
Clinical trials: epidemiological and human genetics study
Epidemiological studies are intended to control health issues by understanding the distribution, determinants, incidence, prevalence, and impact on health among a defined population. Such studies are attempted to perceive the status of infectious diseases as well as non-communicable diseases [ 30 ].
Experimental studies are of two types that include observational (cross-sectional studies (surveys), case-control studies, and cohort studies) and experimental studies (randomized control studies) [ 3 , 31 ]. Such research may pose challenges related to ethics in relation to the social and cultural milieu.
Biomedical research related to human genetics and transplantation research poses an increased threat to ethical concerns, especially after the success of the human genome project (HGP) in the year 2000. The benefits of human genetic studies are innumerable that include the identification of genetic diseases, in vitro fertilization, and regeneration therapy. Research related to human genetics poses ethical, legal, and social issues (ELSI) that need to be appropriately addressed. Most importantly, these genetic research studies use advanced technologies which should be equally available to both economically well-placed and financially deprived people [ 32 ].
Gene therapy and genetic manipulations may potentially precipitate conflict of interest among the family members. The research on genetics may be of various types that include pedigree studies (identifying abnormal gene carriers), genetic screening (for diseases that may be heritable by the children), gene therapeutics (gene replacement therapy, gene construct administration), HGP (sequencing the whole human genome/deoxyribonucleic acid (DNA) fingerprinting), and DNA, cell-line banking/repository [ 33 ]. The biobanks are established to collect and store human tissue samples like umbilical tissue, cord blood, and others [ 34 ].
Epidemiological studies on genetics are attempts to understand the prevalence of diseases that may be transmitted among families. The classical epidemiological studies may include single case observations (one individual), case series (< 10 individuals), ecological studies (population/large group of people), cross-sectional studies (defined number of individuals), case-control studies (defined number of individuals), cohort (defined number of individuals), and interventional studies (defined number of individuals) [ 35 ].
Genetic studies are of different types that include familial aggregation (case-parent, case-parent-grandparent), heritability (study of twins), segregation (pedigree study), linkage study (case-control), association, linkage, disequilibrium, cohort case-only studies (related case-control, unrelated case-control, exposure, non-exposure group, case group), cross-sectional studies, association cohort (related case-control, familial cohort), and experimental retrospective cohort (clinical trial, exposure, and non-exposure group) [ 35 ].
Ethics and concerns in clinical trial/research
Because clinical research involves animals and human participants, adhering to ethics and ethical practices assumes increased significance [ 36 ]. In view of the unethical research conducted on war soldiers after the Second World War, the Nuremberg code was introduced in 1947, which promulgated rules for permissible medical experiments on humans. The Nuremberg code suggests that informed consent is mandatory for all the participants in a clinical trial, and the study subjects must be made aware of the nature, duration, and purpose of the study, and potential health hazards (foreseen and unforeseen). The study subjects should have the liberty to withdraw at any time during the trial and to choose a physician upon medical emergency. The other essential principles of clinical research involving human subjects as suggested by the Nuremberg code included benefit to the society, justification of study as noted by the results of the drug experiments on animals, avoiding even minimal suffering to the study participants, and making sure that the participants don’t have life risk, humanity first, improved medical facilities for participants, and suitably qualified investigators [ 37 ].
During the 18th world medical assembly meeting in the year 1964, in Helsinki, Finland, ethical principles for doctors practicing research were proposed. Declaration of Helsinki, as it is known made sure that the interests and concerns of the human participants will always prevail over the interests of the society. Later in 1974, the National Research Act was proposed which made sure that the research proposals are thoroughly screened by the Institutional ethics/Review Board. In 1979, the April 18th Belmont report was proposed by the national commission for the protection of human rights during biomedical and behavioral research. The Belmont report proposed three core principles during research involving human participants that include respect for persons, beneficence, and justice. The ICH laid down GCP guidelines [ 38 ]. These guidelines are universally followed throughout the world during the conduction of clinical research involving human participants.
ICH was first founded in 1991, in Brussels, under the umbrella of the USA, Japan, and European countries. The ICH conference is conducted once every two years with the participation from the member countries, observers from the regulatory agencies, like the World Health Organization (WHO), European Free Trade Association (EFTA), and the Canadian Health Protection Branch, and other interested stakeholders from the academia and the industry. The expert working groups of the ICH ensure the quality, efficacy, and safety of the medicinal product (drug/device). Despite the availability of the Nuremberg code, the Belmont Report, and the ICH-GCP guidelines, in the year 1982, International Ethical Guidelines for Biomedical Research Involving Human Subjects was proposed by the CIOMS in association with WHO [ 39 ]. The CIOMS protects the rights of the vulnerable population, and ensures ethical practices during clinical research, especially in underdeveloped countries [ 40 ]. In India, the ethical principles for biomedical research involving human subjects were introduced by the Indian Council of Medical Research (ICMR) in the year 2000 and were later amended in the year 2006 [ 41 ]. Clinical trial approvals can only be done by the IRB approved by the Drug Controller General of India (DGCI) as proposed in the year 2013 [ 42 ].
Current perspectives and future implications
A recent study attempted to evaluate the efficacy of adaptive clinical trials in predicting the success of a clinical trial drug that entered phase 3 and minimizing the time and cost of drug development. This study highlighted the drawbacks of such clinical trial designs that include the possibility of type 1 (false positive) and type 2 (false negative) errors [ 43 ].
The usefulness of animal studies during the preclinical phases of a clinical trial was evaluated in a previous study which concluded that animal studies may not completely guarantee the safety of the investigational drug. This is noted by the fact that many drugs which passed toxicity tests in animals produced adverse reactions in humans [ 44 ].
The significance of BE studies to compare branded and generic drugs was reported previously. The pharmacokinetic BE studies of Amoxycillin comparing branded and generic drugs were carried out among a group of healthy participants. The study results have demonstrated that the generic drug had lower Cmax as compared to the branded drug [ 45 ].
To establish the BE of the generic drugs, randomized crossover trials are carried out to assess the Cmax and the AUC. The ratio of each pharmacokinetic characteristic must match the ratio of AUC and/or Cmax, 1:1=1 for a generic drug to be considered as a bioequivalent to a branded drug [ 46 ].
Although the generic drug development is comparatively more beneficial than the branded drugs, synthesis of extended-release formulations of the generic drug appears to be complex. Since the extended-release formulations remain for longer periods in the stomach, they may be influenced by gastric acidity and interact with the food. A recent study suggested the use of bio-relevant dissolution tests to increase the successful production of generic extended-release drug formulations [ 47 ].
Although RCTs are considered the best designs, which rule out bias and the data/results obtained from such clinical research are the most reliable, RCTs may be plagued by miscalculation of the treatment outcomes/bias, problems of cointerventions, and contaminations [ 48 ].
The perception of healthcare providers regarding branded drugs and their view about the generic equivalents was recently analyzed and reported. It was noted that such a perception may be attributed to the flexible regulatory requirements for the approval of a generic drug as compared to a branded drug. Also, could be because a switch from a branded drug to a generic drug in patients may precipitate adverse events as evidenced by previous reports [ 49 ].
Because the vulnerable population like drug/alcohol addicts, mentally challenged people, children, geriatric age people, military persons, ethnic minorities, people suffering from incurable diseases, students, employees, and pregnant women cannot make decisions with respect to participating in a clinical trial, ethical concerns, and legal issues may prop up, that may be appropriately addressed before drug trials which include such groups [ 50 ].
Conclusions
Clinical research and clinical trials are important from the public health perspective. Clinical research facilitates scientists, public health administrations, and people to increase their understanding and improve preparedness with reference to the diseases prevalent in different geographical regions of the world. Moreover, clinical research helps in mitigating health-related problems as evidenced by the current Severe Acute Respiratory Syndrome Coronavirus-2 (SARS-CoV-2) pandemic and other emerging and re-emerging microbial infections. Clinical trials are crucial to the development of drugs, devices, and vaccines. Therefore, scientists are required to be up to date with the process and procedures of clinical research and trials as discussed comprehensively in this review.
The content published in Cureus is the result of clinical experience and/or research by independent individuals or organizations. Cureus is not responsible for the scientific accuracy or reliability of data or conclusions published herein. All content published within Cureus is intended only for educational, research and reference purposes. Additionally, articles published within Cureus should not be deemed a suitable substitute for the advice of a qualified health care professional. Do not disregard or avoid professional medical advice due to content published within Cureus.
The authors have declared that no competing interests exist.

A Career with Clinical Research Organizations in Business Development (BD)
Business development team plays an essential role in all types of industries. In pharmaceutical and clinical research industries business development teams perform many important functions suchas understanding financial aspects of running clinical studies, setting objectives and goals for the successful completion of the projects, identify and approach new clients for new business opportunities and so on.
The Clinical Business Development team specialises in bidding for and obtaining new projects from new clientsacross various therapeutic areas of interest. The team supports in employing business tactics by building and applying strategic business plans to assist sales development and key account management techniques to make sure effective conductof studies. The Business Development team may be engaged in the recruiting and developing of new sites in order to achieveorganizational growth targets. The Business Development Executive (BDE) is responsibleto achieve business development objectives set bythe organization on a monthly and yearly basis. The BDE mayalso be supporting the Site Managers / Lead Coordinators in accomplishing their patient enrollment goals via the oversight of study marketing plans and educational materials.
Education Qualification & Training Requirements for Clinical Business Development (BD) Executive Position
Bachelor / Masters Degree in life science or health care related area; MBA will be advantage for people who want to begin their career in business development.
Experience / Training Required for Clinical Business Development Executive Position
Multinational CRO’s look for people who have 2-3 years relevant experience in business development or clinical research, but there are many CRO’s who hire freshers who have health care related degree and a PG Diploma in Clinical Research and Business Development .
Job Responsibilities of Clinical Business Development Team
- Recognize and prepare clinical trial research plans with CRO’s, pharmaceutical and biotech organizations.
- Explore various pharmaceutical organization’s pipelines to find out new and up coming clinical study possibilities with a focus on obtaining new therapeutic areas, new contacts, new pharmaceutical/biotech organizations and new CROs in the industry.
- Make calls to targeted companies, seek out new contacts, finish all surveys, make follow-up calls and evaluate web sites to make sure that all possible studies are being analyzed.
- Accountable for supporting with external advertising and provide support in enrollment of subjects for ongoing clinical research at study sites.
- Recognition of new possibilities for pipeline molecules / products
- Research of all new products/ideas coming to market and evaluate business viability
- Accomplish or exceed organizational goals that have been put in placeby the organization.
- Comply with all organization procedures, instructions and directives for the achievement of organization objectives and generate sales. Maintain an automated monitoring of all ongoing leads, sales and works in process.
- Be aware to market research reports, competitive strategies/studies and convey this information to management.
- Analyse new business models, methodologies, and markets that would maximize revenue share of the organization.
- Attend yearly industry conferences to enhance Clinical Research Benefits and to obtain further data on research that can help new business opportunities.
- Support with determining and recruiting new doctors to work as researchers at trial sites.
Annual Pay Scale for Clinical Business Development (BD) Executive
| Country | Salary | Currency |
| U.S. | 90K | USD |
| UK | 75K | GBP |
| Australia | 60K | AD |
| Singapore | 100K | SGD |
| India | 4– 6Lakh | INR |
Related posts:

Profile cancel
Email Not published

Clinical Operations Specialist
About the role.
Key Responsibilities:
- Evaluation of investigators fees (country level budget/Grant Plan) estimates per country.
- Negotiation of investigators fees and country related study costs; and supporting Clinical Project Manager (CPM) ensuring accurate planning, tracking and reporting of study budget.
- Set-up and coordination of third-party vendors (i.e. central lab, investigators’ meeting organization) and monitoring partner, ensuring all information, documentation and material in place for study start.
- Effective and smooth workflow between study participants (i.e. third-party vendors and monitoring partner).
- Follow-up with vendors and monitoring partners on day to day operations (recruitment reports, delivery of study kits...)
- The set-up and maintenance of studies in Clinical Trial Management Systems (CTMS), ensuring all key documents are present and filed as appropriate in Trial Master File (TMF)
- Ensuring availability of study material for monitoring partner/sites
Essential Requirements:
- Life Science degree or equivalent
- 3+ years’ operational experience of clinical study execution in a pharmaceutical company or contract research organization
- Strong technical and organizational skills, details oriented, thorough knowledge of Good Clinical practice and presentation and tact skills
- Consistent track record to establish effective working relationship in a matrix and multicultural environment and willingness to act accountably in project/study management
- Strong customer focused mentality and proficient English (oral and written)
You’ll receive: Monthly pension contribution matching your individual contribution up to 3% of your gross monthly base salary; Risk Life Insurance (full cost covered by Novartis); 5-week holiday per year; (1 week above the Labour Law requirement) ; 4 paid sick days within one calendar year in case of absence due to sickness without a medical sickness report; Cafeteria employee benefit program – choice of benefits from Benefit Plus Cafeteria in the amount of 12,500 CZK per year; Meal vouchers in amount of 105 CZK for each working day (full tax covered by company); MultiSport Card, Employee Share Purchase Plan. Find out more about Novartis Business Services: https://www.novartis.cz/
Commitment To Diversity And Inclusion :
Novartis is committed to building an outstanding, inclusive work environment and diverse teams representative of the patients and communities we serve.
Why Novartis: Helping people with disease and their families takes more than innovative science. It takes a community of smart, passionate people like you. Collaborating, supporting and inspiring each other. Combining to achieve breakthroughs that change patients’ lives. Ready to create a brighter future together? https://www.novartis.com/about/strategy/people-and-culture
Join our Novartis Network: Not the right Novartis role for you? Sign up to our talent community to stay connected and learn about suitable career opportunities as soon as they come up: https://talentnetwork.novartis.com/network
Benefits and Rewards: Read our handbook to learn about all the ways we’ll help you thrive personally and professionally: https://www.novartis.com/careers/benefits-rewards
Accessibility and accommodation
Novartis is committed to working with and providing reasonable accommodation to all individuals. If, because of a medical condition or disability, you need a reasonable accommodation for any part of the recruitment process, or in order to receive more detailed information about the essential functions of a position, please send an e-mail to [email protected] and let us know the nature of your request and your contact information. Please include the job requisition number in your message.
Novartis is committed to building an outstanding, inclusive work environment and diverse teams' representative of the patients and communities we serve.


IMAGES
VIDEO
COMMENTS
A Sample Clinical Research Business Plan Template. 1. Industry Overview. The Contract Research Organizations industry that clinical research business is a subset of, is perhaps one of the fastest growing and largest industries in the world. Obviously, there is hardly any country where the healthcare and medical industry is not handled with all ...
Table of Contents. How to Start a Clinical Research Organization - Step by Step Guide. Step 1: Overview of the Market. Step 2: Prepare Business Plan. Step 3: Establishing Clinical Trial Services. Step 4: Develop Standard Operating Procedures (SOPs) Step 5: Securing Funds. Step 6: Tips for Success in the clinical research industry.
2. Conduct market research: Research your potential clients to find out their challenges, needs, pain points, and how your services can solve their problems. 3. Know your competitors: Identify your competitors and what distinguishes your services, and how you can offer value-added services to your clients. 4.
It is important to note that developing a sound business plan is also highly relevant for supporting smaller-scale clinical programs, operational investments, and intrapreneurship efforts (see the chapter on "Intrapreneurship: Strategic Approaches for Managing Disruptive Innovation in Your Clinical and Research Projects"). Please see the ...
Clinical research provides financial and altruistic appeal: a successful site will conduct a diversity of studies with commensurate compensation. The data generated (from trials conducted) could possibly contribute to a regulatory agency submission for new drug approval. At the very least, a successful site will impact the drug development process.
Clinical Research Organizations (CROs) play a crucial role in the pharmaceutical, biotechnology, and medical device industries. They provide support to companies in the form of research services outsourced on a contract basis. In this comprehensive guide, we will explore what CROs are, who their clients are, the stages of the research process ...
9. KCR. Established in 1997 under the name Kiecana Clinical Research, KCR is a full-service contract research organization that provides a variety of services for clinical monitoring, safety & pharmacovigilance, clinical project management, quality assurance and regulatory affairs.
Here are some of the options you can explore when sourcing for start-up capital for your clinical research business; Raise money from personal savings and sale of personal stocks and properties. Raise money from investors and business partners. Sell shares to interested investors.
Our small clinical research business was facing challenges and needed a business plan. This organization conducts clinical research trials, was incorporated in 2008, and is located in Central V alley, CA. The organization st arted with two share holders (whom were also employees), and has since expanded to three share holders and four employees.
The business model is ever-changing due to the vast array of trial execution options. COVID-19, though not without significant operational disruption, has incited more patient and sponsor optionality than ever before. There is a full spectrum of models ranging from fully decentralized, fully centralized, to hybrid trials that bring optionality ...
Selection Criteria For CRO Selection. According to Comprehend, 3 a clinical intelligence software provider, the three most common overlooked selection criteria are transparency, collaborative partnership, and real-time responsiveness. These intangible factors should be part of any strategic selection process.
The Start-up Time and Readiness Tracking (START) study by Tufts Center for Study of Drug Development reported that nearly 11% of sites selected are never activated primarily due to budget and contract issues.1 CenterWatch's 2019 Financial and Operating Benchmarks survey noted that contract and budget negotiations remain the biggest SSU ...
Elevate your healthcare venture with the "Clinical Research Company Business Plan" by Erik Lombere, MBA. This meticulously crafted document serves as a blueprint for launching and operating a successful clinical research site. Backed by industry insights, it offers strategic planning, financial projections, and step-by-step guidance to navigate this complex field. If you're passionate about ...
A well-established clinical research organization (CRO) specializing in the design and delivery of agile clinical trials engaged Clarkston for strategy services to help reimagine what their ideal future operating model should look like. Given the company's long-range plan (LRP), the CFO and the executive team wanted to define the major ...
Laboratory Corporation of America Holdings, also known as Labcorp, is an American company that operates one of the largest clinical laboratory networks in the world. In an average week Labcorp processes tests on more than 3 million patient specimens. In 2020 the company earned revenue in excess of $14 bn. Syneos Health.
Costs of clinical trials have been rising in the United States (DeVol et al., 2011). The workshop's third session, "Aligning Cultural and Financial Incentives," explored opportunities for developing a new business model for clinical trials based on the seamless integration of technological advances and research activities to realize increased efficiencies and reduced costs. Individual ...
Obtain Workday Number. All studies with any type of funding will have a Workday number (formerly called Lawson number). Confirm Your Study is Built in EPIC. Once you have your approved IRB number, Workday number, and have the study activated in OnCore, the OnCore system automatically sends the study info to be built in EPIC.
After selecting a single conceptual framework or a combination of a few frameworks, a clinical study can be completed in two fundamental steps: study design and study report. Three study designs should be planned in sequence and iterated until satisfaction: the theoretical design, data collection design, and statistical analysis design [7].
Clinical Researcher—May 2021 (Volume 35, Issue 4) TRIALS & TECHNOLOGY Jim Reilly Clinical transformation accelerated during COVID-19 to speed trial execution. As companies raced to find treatments and vaccines, life sciences researchers came together to deliver innovation faster than ever. Despite these leaps forward, the industry recognizes the pace is not sustainable due to the regulatory ...
Building a Research Program. research program, consider site infrastructure including:The financial and philosophic dedication of one's institution: confirm that there is an understanding across the institution of the challenges. and commitment that accompany building a research program. Building a budget is a helpful tool to understand.
The impact of COVID-19 on clinical trials has likely become obvious for your site during this phase of the pandemic. Sites are struggling with screening holds, limited staff, an influx of sponsor requests while staff is not on-site, the challenge of retaining enrolled subjects, and more. It is now more important than ever to implement a business...
The clinical study is conducted by a sponsor or a clinical research organization (CRO). A perfect protocol, time limits, and regulatory requirements assume significance while planning a clinical trial. ... 1.54, an investigator is an individual who initiates and conducts clinical research . The investigator plan, design, conduct, monitor ...
Business development team plays an essential role in all types of industries. In pharmaceutical and clinical research industries business development teams perform many important functions such as understanding financial aspects of running clinical studies, setting objectives and goals for the successful completion of the projects, identify and approach new clients for new business ...
Externally, clinical trial sponsors and clinical research organizations will routinely conduct such audits, since they are intended to improve the functioning at an individual site and also to ensure that if the US Food and Drug Administration (FDA) ever audits the site, the records are appropriately maintained.
Key Responsibilities: Evaluation of investigators fees (country level budget/Grant Plan) estimates per country.Negotiation of investigators fees and country related study costs; and supporting Clinical Project Manager (CPM) ensuring accurate planning, tracking and reporting of study budget.Set-up and coordination of third-party vendors (i.e. central lab, investigators' meeting organization ...