Sciencing_Icons_Science SCIENCE
Sciencing_icons_biology biology, sciencing_icons_cells cells, sciencing_icons_molecular molecular, sciencing_icons_microorganisms microorganisms, sciencing_icons_genetics genetics, sciencing_icons_human body human body, sciencing_icons_ecology ecology, sciencing_icons_chemistry chemistry, sciencing_icons_atomic & molecular structure atomic & molecular structure, sciencing_icons_bonds bonds, sciencing_icons_reactions reactions, sciencing_icons_stoichiometry stoichiometry, sciencing_icons_solutions solutions, sciencing_icons_acids & bases acids & bases, sciencing_icons_thermodynamics thermodynamics, sciencing_icons_organic chemistry organic chemistry, sciencing_icons_physics physics, sciencing_icons_fundamentals-physics fundamentals, sciencing_icons_electronics electronics, sciencing_icons_waves waves, sciencing_icons_energy energy, sciencing_icons_fluid fluid, sciencing_icons_astronomy astronomy, sciencing_icons_geology geology, sciencing_icons_fundamentals-geology fundamentals, sciencing_icons_minerals & rocks minerals & rocks, sciencing_icons_earth scructure earth structure, sciencing_icons_fossils fossils, sciencing_icons_natural disasters natural disasters, sciencing_icons_nature nature, sciencing_icons_ecosystems ecosystems, sciencing_icons_environment environment, sciencing_icons_insects insects, sciencing_icons_plants & mushrooms plants & mushrooms, sciencing_icons_animals animals, sciencing_icons_math math, sciencing_icons_arithmetic arithmetic, sciencing_icons_addition & subtraction addition & subtraction, sciencing_icons_multiplication & division multiplication & division, sciencing_icons_decimals decimals, sciencing_icons_fractions fractions, sciencing_icons_conversions conversions, sciencing_icons_algebra algebra, sciencing_icons_working with units working with units, sciencing_icons_equations & expressions equations & expressions, sciencing_icons_ratios & proportions ratios & proportions, sciencing_icons_inequalities inequalities, sciencing_icons_exponents & logarithms exponents & logarithms, sciencing_icons_factorization factorization, sciencing_icons_functions functions, sciencing_icons_linear equations linear equations, sciencing_icons_graphs graphs, sciencing_icons_quadratics quadratics, sciencing_icons_polynomials polynomials, sciencing_icons_geometry geometry, sciencing_icons_fundamentals-geometry fundamentals, sciencing_icons_cartesian cartesian, sciencing_icons_circles circles, sciencing_icons_solids solids, sciencing_icons_trigonometry trigonometry, sciencing_icons_probability-statistics probability & statistics, sciencing_icons_mean-median-mode mean/median/mode, sciencing_icons_independent-dependent variables independent/dependent variables, sciencing_icons_deviation deviation, sciencing_icons_correlation correlation, sciencing_icons_sampling sampling, sciencing_icons_distributions distributions, sciencing_icons_probability probability, sciencing_icons_calculus calculus, sciencing_icons_differentiation-integration differentiation/integration, sciencing_icons_application application, sciencing_icons_projects projects, sciencing_icons_news news.
- Share Tweet Email Print
- Home ⋅
- Science Fair Project Ideas for Kids, Middle & High School Students ⋅

About Rutherford's Gold Foil Experiment

Five Types of Atomic Models
Ernest Rutherford, originally from New Zealand, is credited as being the father of nuclear physics for his discoveries in atomic structure, even though Hantaro Nagaoka, a physicist from the Imperial University of Tokyo, first proposed the theory of the nucleus as it is known today. Rutherford's "gold foil experiment" led to the discovery that most of an atom's mass is located in a dense region now called the nucleus. Prior to the groundbreaking gold foil experiment, Rutherford was granted the Nobel Prize for other key contributions in the field of chemistry.
The popular theory of atomic structure at the time of Rutherford's experiment was the "plum pudding model." This model was developed in 1904 by J.J. Thompson, the scientist who discovered the electron. This theory held that the negatively charged electrons in an atom were floating in a sea of positive charge--the electrons being akin to plums in a bowl of pudding. Although Dr. Nagaoka had published his competing theory that electrons orbit a positive nucleus, akin to the way the planet Saturn is orbited by its rings, in 1904, the plum pudding model was the prevailing theory on the structure of the atom until it was disproved by Ernest Rutherford in 1911.
The gold foil experiment was conducted under the supervision of Rutherford at the University of Manchester in 1909 by scientist Hans Geiger (whose work eventually led to the development of the Geiger counter) and undergraduate student Ernest Marsden. Rutherford, chair of the Manchester physics department at the time of the experiment, is given primary credit for the experiment, as the theories that resulted are primarily his work. Rutherford's gold foil experiment is also sometimes referred to as the Geiger-Marsden experiment.
The gold foil experiment consisted of a series of tests in which a positively charged helium particle was shot at a very thin layer of gold foil. The expected result was that the positive particles would be moved just a few degrees from their path as they passed through the sea of positive charge proposed in the plum pudding model. The result, however, was that the positive particles were repelled off of the gold foil by nearly 180 degrees in a very small region of the atom, while most of the remaining particles were not deflected at all but rather passed right through the atom.
Significance
The data generated from the gold foil experiment demonstrated that the plum pudding model of the atom was incorrect. The way in which the positive particles bounced off the thin foil indicated that the majority of the mass of an atom was concentrated in one small region. Because the majority of the positive particles continued on their original path unmoved, Rutherford correctly deducted that most of the remainder of the atom was empty space. Rutherford termed his discovery "the central charge," a region later named the nucleus.
Rutherford's discovery of the nucleus and proposed atomic structure was later refined by physicist Niels Bohr in 1913. Bohr's model of the atom, also referred to as the Rutherford Bohr model, is the basic atomic model used today. Rutherford's description of the atom set the foundation for all future atomic models and the development of nuclear physics.
Related Articles
What are the 4 atomic models, what are the different kinds of models of atoms, what contributions did j.j. thomson make to the atom, james chadwick atomic theory, list of the atomic theories, who discovered iodine 131, who discovered the particle theory, cern plans to search for mysterious fifth force particle, who was the african american nuclear scientist who..., how to make a gold atom model, atomic structure of gold, what are an atom, electron, neutron and proton, the locations of protons, neutrons and electrons within..., how to know if an element has a positive or negative..., what are the properties of protons, six elements named after scientists.
Photo Credits
iSailorr/iStock/Getty Images
Find Your Next Great Science Fair Project! GO

Rutherford Atomic Model
Definition of the rutherford model.
The Rutherford atomic model has 2 main parts: the nucleus, and the atom’s remaining space, occupied by electrons.
According to the model, the nucleus is a very small portion of the atom’s volume. It occupies a small space in the very center of the atom. Protons and neutrons make up the nucleus and define the atom’s chemical properties.
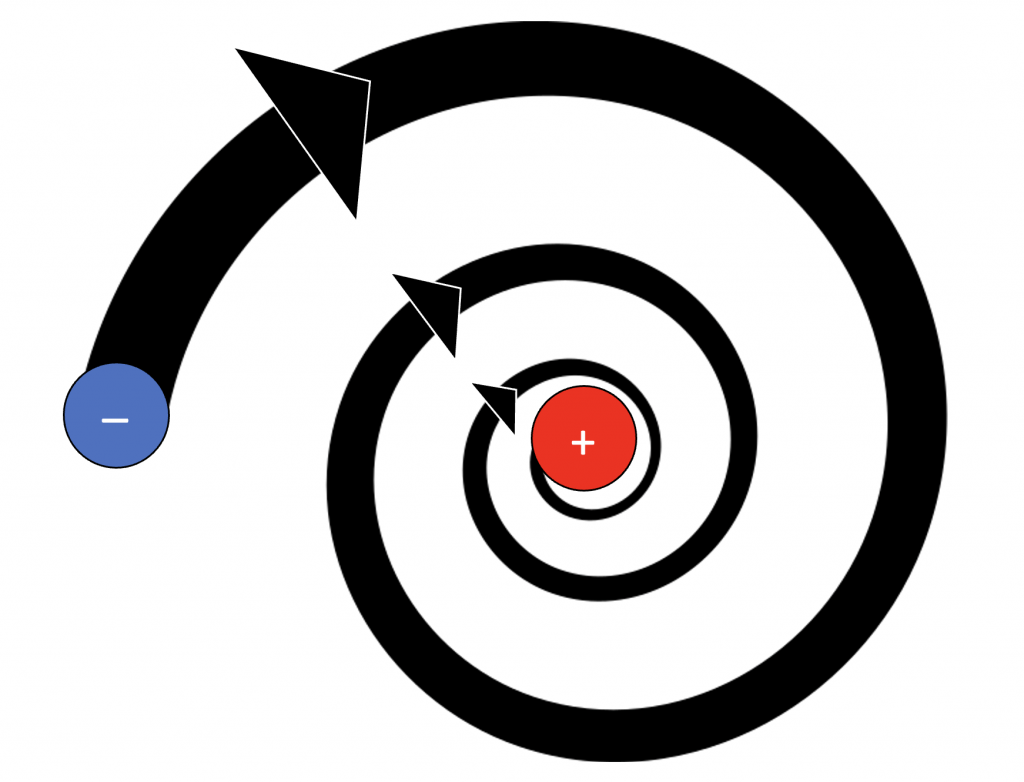
Rutherford also claimed in his model that electrons revolved around the nucleus in set orbits, like planets revolving around the Sun. This part of the theory was inaccurate, as explained in the last section.

Rutherford’s Gold Foil Experiment
The Rutherford gold foil experiment , also known as the scattering experiment, led to the creation of the model and explained the parts of the atom. In 1909, graduate student Ernest Marsden (under Ernest Rutherford’s supervision) fired alpha particles at a gold foil piece. Most of the particles passed directly through the foil, meaning that a majority of the space in each atom was unoccupied. However, a few particles were deflected, and some even backward. This must have been caused by tiny pockets of positive charge in the foil repelling the alpha particles back. Their discovery led to the creation of Rutherford’s model, in which the dense, positively-charged nucleus occupies a very small area in the center of each atom.
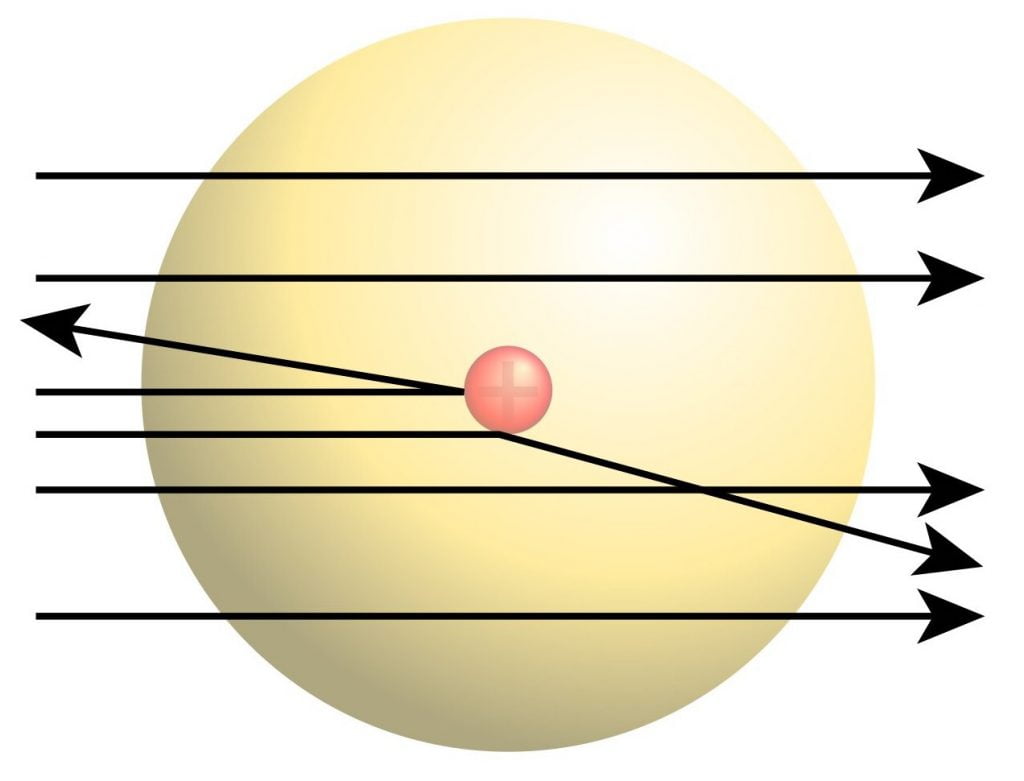
Shortcomings of the Rutherford Model
While common models today are based on the Rutherford atomic theory, it does not paint the complete picture:
- The model is missing parts and does not account for the location or distribution of electrons.
- Rutherford proposed that electrons orbit around the nucleus in set paths, but according to Maxwell’s theory , this is not possible because the atom would not be stable. Electromagnetic radiation from the electrons in orbit would cause the atom to collapse into the nucleus in 10 -8 seconds.
- Electrons increase and decrease energy levels randomly due to the acceleration and are not always in a standard circular orbit. They give off electromagnetic radiation due to the circular motion of orbiting; thus they must have some initial energy by the law of conservation of energy. The Rutherford atomic model does not account for the initial energy and subsequent energy level changes.
Further Reading
- Dalton’s Atomic Theory
- Bohr Atomic Model
- The Structure of an Atom
May, 1911: Rutherford and the Discovery of the Atomic Nucleus

In 1909, Ernest Rutherford’s student reported some unexpected results from an experiment Rutherford had assigned him. Rutherford called this news the most incredible event of his life.
In the now well-known experiment, alpha particles were observed to scatter backwards from a gold foil. Rutherford’s explanation, which he published in May 1911, was that the scattering was caused by a hard, dense core at the center of the atom–the nucleus.
Ernest Rutherford was born in New Zealand, in 1871, one of 12 children. Growing up, he often helped out on the family farm, but he was a good student, and received a scholarship to attend the University of New Zealand. After college he won a scholarship in 1894 to become a research student at Cambridge. Upon receiving the news of this scholarship, Rutherford is reported to have said, “That’s the last potato I’ll ever dig.”
At Cambridge, the young Rutherford worked in the Cavendish lab with J.J. Thomson, discoverer of the electron. Rutherford’s talent was quickly recognized, and in 1898 he took a professorship at McGill University in Montreal. There, he identified alpha and beta radiation as two separate types of radiation, and studied some of their properties, though he didn’t know that alphas were helium nuclei. In 1901 Rutherford and chemist Frederick Soddy found that one radioactive element can decay into another. The discovery earned Rutherford the 1908 Nobel Prize in Chemistry, which irritated him somewhat because he considered himself a physicist, not a chemist. (Rutherford is widely quoted as having said, “All science is either physics or stamp collecting”)
In 1907 Rutherford returned to England, to the University of Manchester. In 1909, he and his colleague Hans Geiger were looking for a research project for a student, Ernest Marsden. Rutherford had already been studying the scattering of alpha particles off a gold target, carefully measuring the small forward angles through which most of the particles scattered. Rutherford, who didn’t want to neglect any angle of an experiment, no matter how unpromising, suggested Marsden look to see if any alpha particles actually scattered backwards.
Marsden was not expected to find anything, but nonetheless he dutifully and carefully carried out the experiment. He later wrote that he felt it was a sort of test of his experimental skills. The experiment involved firing alpha particles from a radioactive source at a thin gold foil. Any scattered particles would hit a screen coated with zinc sulfide, which scintillates when hit with charged particles. Marsden was to sit in the darkened room, wait for his eyes to adjust to the darkness, and then patiently stare at the screen, expecting to see nothing at all.
Instead, Marsden saw lots of tiny, fleeting flashes of yellowish light, on average more than one blip per second.
He could hardly believe what he saw. He tested and retested every aspect of the experiment, but when he couldn’t find anything wrong, he reported the results to Rutherford.
Rutherford too was astonished. As he was fond of saying, “It was as if you fired a 15-inch shell at a piece of tissue paper and it came back and hit you."
About one in every few thousand of the alpha particles fired at the gold target had scattered at an angle greater than 90 degrees. This didn’t fit with the prevailing model of the atom, the so-called plum pudding model developed by J.J. Thomson. In this model electrons were believed to be stuck throughout a blob of positively charged matter, like raisins in a pudding. But this sort of arrangement would only cause small angle scattering, nothing like what Marsden had observed.
After thinking about the problem for over a year, Rutherford came up with an answer. The only explanation, Rutherford suggested in 1911, was that the alpha particles were being scattered by a large amount of positive charge concentrated in a very small space at the center of the gold atom. The electrons in the atom must be orbiting around this central core, like planets around the sun, Rutherford proposed.
Rutherford carried out a fairly simple calculation to find the size of the nucleus, and found it to be only about 1/100,000 the size of the atom. The atom was mostly empty space.
In March 1911, Rutherford announced his surprising finding at a meeting of the Manchester Literary and Philosophical Society, and in May 1911, he published a paper on the results in the Philosophical Magazine .
Later Rutherford and Marsden tried the experiment with other elements as the target, and measured their nuclei as well.
The solar system model was not immediately accepted. One obvious problem was that according to Maxwell’s equations, electrons traveling in a circular orbit should radiate energy, and therefore slow down and fall into the nucleus. A solar system atom wouldn’t last long.
Fortunately, Niels Bohr was soon able to save the solar system model by applying new ideas from quantum mechanics. He showed that the atom could stay intact if electrons were only allowed to occupy certain discrete orbitals.
Though Rutherford still didn’t know what was in this nucleus he had discovered (protons and neutrons would be identified later), his insight in 1911, which overturned the prevailing plum pudding model of the atom, had opened the way for modern nuclear physics.
Ernie Tretkoff
Join your society.
If you embrace scientific discovery, truth and integrity, partnership, inclusion, and lifelong curiosity, this is your professional home.
Rutherford Experiment and Atomic Collisions
Claimed by: Lia McSweeney (Fall 2023)
- 1.1 A Mathematical Model
- 1.2 A Computational Model
- 2.2 Middling
- 2.3 Difficult
- 3 Connectedness
- 5.1 Further Reading
- 5.2 External Links
- 6 References
The Main Idea
Rutherford's Gold Foil Experiment helped detect that there was a large positively charged mass in the center of an atom: the nucleus. The experiment was done through the use of atomic collisions. Under the instruction of Rutherford, Hans Geiger and Ernest Marsden pointed a beam of alpha particles at a thin foil of metal and measured the scattering pattern by using a fluorescent screen. The scientists noted that some alpha particles bounced in random directions. This was not originally hypothesized due to the idea that, at most the alpha particle should experience only a 90° scattering angle. This helped lead to the discovery of the nucleus and a highly compact positively charged center.
Rutherford studied the particles that uranium and its derivatives emitted and how these particles affected certain materials. Rutherford created a method to record the position of each alpha particle by circling the bombarded object with a ZnS coated sheet. This sheet would emit a flash of light when hit by an alpha particle, allowing Rutherford to accurately measure the deflection of each alpha particle. This gave Rutherford a counting mechanism for theses particles he wanted to study. Rutherford then began to study the angles that negatively charged particles deflected when they collided with a thin metal foil. This was the beginning of his most famous study: the gold foil experiment. Knowing the relative mass of these negatively charged particles and their quick speed, he hypothesized that they would pierce the metal foil but then collide with the atoms dispersed inside the foil resulting in the small deflections. These deflections were extremely small, usually by a degree. In 1911, Ernest Rutherford took this experiment further and worked with his assistants, Hans Geiger and Ernest Marsden, to carry out an experiment that tested the plum pudding model. They shot alpha (helium 2+) particles at gold foil in order to measure the deflection of the particles as they come off of the other side. They decided to see if these deflections could occur at larger angles greater than 90 degrees. Through countless trials, they found an extremely small portion of these deflections to occur at angles larger than 90 degrees. Rutherford wondered how these large deflections occurred and concluded that there existed an extremely small and positively charged area in the atom that resulted in these huge deflections. He eventually named this area the nucleus. What happened during these deflections was that most particles would become slightly deflected by small angles due to the positive atoms. However, some would collide directly with nucleus resulting in the deflections that were greater than 90 degrees. These occurred rarely because the nucleus was such a small size so the probability of these atoms hitting the nucleus was very low. This experiment helped indicate that the atom is made predominantly of empty space with a small nucleus with protons and electrons placed extremely far away from the nucleus in their own cloud. Rutherford devised the name “proton” to describe the positive particles in the nucleus. He thought that a neutral particle existed in the nucleus too, but its existence wasn’t confirmed until 1932 when James Chadwick proved it.
A Mathematical Model
Rutherford modeled the effect the alpha particle has on the electrons of the gold atom. He did this by calculating the potential electric energy between the particle and the atom using the formula below. Rutherford came up with several equations to numerically describe these deflections. Based on the equations below, the number of particles scattered at a certain angle is directly proportional to the thickness of the metal foil and the square of the nucleus’ charge but inversely proportional to the particle’s velocity raised to the fourth power.
[math]\displaystyle{ {U_{elec}} = {\frac{1}{4πε_0}}{\frac{q_{α}q_{Au}}{r}} }[/math]
r = center to center distance between particle and atom
[math]\displaystyle{ {\frac{1}{4πε_0}} = {9*10^9}{\frac {N*m^2}{C^2}} }[/math]
[math]\displaystyle{ {q_α} }[/math] = charge of alpha particle
[math]\displaystyle{ {q_{Au}} }[/math] = charge of gold nucleus
In this instance the charge of the alpha particle is equal to 2e and the charge of the gold particle is equal to 79e.
Another important part of atomic collisions is that they are inelastic collisions. This is shown by the conservation of both momentum and kinetic energy. Take the alpha particle and gold particle for example.
[math]\displaystyle{ {\vec{p_{α,i}}} = {\vec{p_{α,f}}}+ {\vec{p_{Au,f}}} }[/math]
[math]\displaystyle{ {\vec{K_{α,i}}} = {\vec{K_{α,f}}}+ {\vec{K_{Au,f}}} }[/math]
Where [math]\displaystyle{ {\vec{p}} }[/math] is momentum and [math]\displaystyle{ {\vec{K}} }[/math] is kinetic energy.
A Computational Model
Much like the mathematical model, the collision can be modeled computationally using the same formulas. Here is a video of a VPython mode of a continuous stream of alpha particles with exaggerated interaction for easy viewing:
Example Problems
The scattering of alpha particles from nuclei is mathematically modeled from the Coulomb force and treated as an orbit. For a ZnS detector at a specific angle with respect to the incident beam, the number of particles per unit area striking the detector is given by the Rutherford formula: [math]\displaystyle{ N(θ) = {\frac{N_inLZ^2k^2e^4}{4r^2KE^2sin^4(θ/2)}} }[/math] where [math]\displaystyle{ N_i = \text {number of incident alpha particles} }[/math] [math]\displaystyle{ n = \text {atoms per unit volume in target} }[/math] [math]\displaystyle{ L = \text {thickness of target} }[/math] [math]\displaystyle{ Z = \text {atomic number of target} }[/math] [math]\displaystyle{ e = \text {electron charge} }[/math] [math]\displaystyle{ k = \text {Coulomb's constant} }[/math] [math]\displaystyle{ r = \text {target to detector distance} }[/math] [math]\displaystyle{ KE = \text {kinetic energy of alpha} }[/math] [math]\displaystyle{ θ = \text {scattering angle} }[/math]
Find the number of particles per unit area striking the detector given the following values: [math]\displaystyle{ N_i = 5 }[/math] alpha particles [math]\displaystyle{ n = 8.4866 * 10^{22} \text {atoms in 1} cm^3 }[/math] [math]\displaystyle{ L = 1 cm }[/math] [math]\displaystyle{ Z = 26 }[/math] [math]\displaystyle{ e = -1 }[/math] [math]\displaystyle{ k = 8.988 * 10^9 }[/math] [math]\displaystyle{ r = 10 cm }[/math] [math]\displaystyle{ KE = (1/2)*m*v^2 }[/math] where [math]\displaystyle{ v_a = 1.53 * 10^7 m/s \text {and mass of the alpha particle is} 6.64424*10^27 kg }[/math] [math]\displaystyle{ θ = 0.18 degrees }[/math]
Plug each number into the equation (make sure units cancel).
[math]\displaystyle{ N(0.18) = {\frac{5*8.4866*10^{22}*1*26^2*{(8.988*10^9)}^2*-1^4}{4*10^2*{((1/2)(6.64424*10^27){(1.53*10^-7)}^2)}^2*sin^4(0.18/2)}} }[/math]
= 1.57341 * 10^27 particles striking the surface per cm
Rutherford found that the fraction of particles scattered at an angle [math]\displaystyle{ θ }[/math] or greater can be modeled by the equation [math]\displaystyle{ F_{θ} ≈ e^{(−θ/θ^2_m)} }[/math] . At what angle would Rutherford have found a fraction of [math]\displaystyle{ 10^{45} }[/math] particles to be at that angle or greater than? ( [math]\displaystyle{ θ_m ≈ 1 }[/math] for a gold leaf foil)
Using the formula [math]\displaystyle{ F_{θ} ≈ e^{(−θ/θ^2_m)} }[/math] , we can rearrange to solve for θ by taking the log of both sides:
[math]\displaystyle{ log(F_{θ}) = −θ/θ^2_m }[/math]
Then, we can multiple by [math]\displaystyle{ -θ^2_m }[/math] to find:
[math]\displaystyle{ θ = −(θ^2_m)log(F_{θ}) }[/math]
Plugging in the given information, [math]\displaystyle{ θ = −(1^2)log(10^{45}) = 45 }[/math] . Therefore, a fraction of [math]\displaystyle{ 10^{45} }[/math] particles are scattered at about an angle of 45 degrees or greater.
A proton and an electron are a distance [math]\displaystyle{ {7.2*10^{-9}m} }[/math] apart. What is the electric potential energy of the system consisting of the proton and the electron?
[math]\displaystyle{ {U_{elec}} = {\frac{1}{4πε_0}}{\frac{q_{+}q_{-}}{r}} }[/math]
[math]\displaystyle{ {U_{elec}} = {9*10^{9}}{\frac {N*m^2}{C^2}}*{\frac{1.6*10^{-19}*(-1.6*10^{-19})}{7.2*10^{-9}}} = {-3.2*10^{-28}}{J} }[/math]
Connectedness
This topic is related to the study of chemical engineering. Without the discovery of the nucleus, any progress in this field would be limited based on the interaction of atomic particles. This would also hinder the medical field for very similar reason. Much of the understanding of sciences has its roots in the understanding of the atom and its functions. This experiment and the idea of atomic collisions helped to widen the atomic grasp. One of the best industrial examples of atomic collisions is the Large Hadron Collider.

Around the early 1900s, very little was known about atoms besides the ground breaking experiments conducted by J.J. Thompson in 1897. Thompson discovered what we call the electron. He hypothesized that electrons were negatively charged particles. It was also speculated that there must be a positive charge to balance out the negative charge from the electron. This "Plum Pudding Model" was invented by Thompson. This model assumed that matter consists of atoms which are overall positively charged, but with some type of negative electron charge throughout it. The electrons function as the "plum" which was evenly distributed through a positively charged "pudding".
With the knowledge of the plum pudding model of the atom, Ernst Rutherford and a small group of scientists set out to discover the properties behind alpha particles. The experiment, now known as the Gold Foil Experiment, was used to test this in 1911. It involved launching alpha particles at a small piece of gold foil. It was hypothesized that the alpha particle would be deflected at times, but at an angle because it was assumed that the alpha particle was more dense than the gold foil atom. They registered deflected particles through light emissions that would occur when the alpha particle hit the light source. Much to their surprise, some of the alpha particles they launched bounced straight back. This demonstrated that the gold particle was more massive than expected. It led to the discovery that the atom contained a positively charged nucleus. This was a major break through in the study of the atom in that it showed what the atom's composition was and how it act around other atoms.
Collisions is a related helpful page to get a foundation in collisions. Elastic Collisions and Inelastic Collisions are also useful, and Scattering: Collisions in 2D and 3D takes a broader look at the principles involved in the Rutherford Experiment.
Further Reading
Another page on Rutherford's Experiment.
External Links
MIT video of an experiment confirming Rutherford's model.
Chabay, R.W., & Sherwood, B.A. (2015). Collisions. In Fiorillo, J. Editor & Rentrop, A. Editor (Eds.), Matter and Interactions (383-410). John Wiley & Sons, Inc.
“Ernest Rutherford.” New Page 2, chemed.chem.purdue.edu/genchem/history/gold.html.
"History of Rutherford Experiment". HyperPhysics. Web. 03 Dec. 2015. Retrieved from: < http://hyperphysics.phy-astr.gsu.edu/hbase/hframe.html >.
“Rutherford Scattering.” MIT OpenCourseWare, MIT Department of Physics , ocw.mit.edu/courses/physics/8-13-14-experimental-physics-i-ii-junior-lab-fall-2016-spring-2017/experiments/rutherford-scattering/MIT8_13-14F16-S17exp15.pdf.
Navigation menu
- Structure of Atom
- Rutherford Atomic Model And Its Limitations
Rutherford Atomic Model and Limitations
Define rutherford atomic model.
Rutherford Atomic Model – The plum pudding model given by J. J. Thomson failed to explain certain experimental results associated with the atomic structure of elements. Ernest Rutherford, a British scientist conducted an experiment and based on the observations of this experiment, he explained the atomic structure of elements and proposed Rutherford’s Atomic Model.
Table of Contents
- Rutherfords Alpha Scattering Experiment
Observations of Rutherford’s Alpha Scattering Experiment
Rutherford atomic model, limitations of rutherford atomic model, recommended videos, frequently asked questions – faqs.

Rutherford’s Alpha Scattering Experiment
Rutherford conducted an experiment by bombarding a thin sheet of gold with α-particles and then studied the trajectory of these particles after their interaction with the gold foil.

Rutherford, in his experiment, directed high energy streams of α-particles from a radioactive source at a thin sheet (100 nm thickness) of gold. In order to study the deflection caused to the α-particles, he placed a fluorescent zinc sulphide screen around the thin gold foil. Rutherford made certain observations that contradicted Thomson’s atomic model .
The observations made by Rutherford led him to conclude that:
- A major fraction of the α-particles bombarded towards the gold sheet passed through the sheet without any deflection, and hence most of the space in an atom is empty .
- Some of the α-particles were deflected by the gold sheet by very small angles, and hence the positive charge in an atom is not uniformly distributed . The positive charge in an atom is concentrated in a very small volume .
- Very few of the α-particles were deflected back, that is only a few α-particles had nearly 180 o angle of deflection. So the volume occupied by the positively charged particles in an atom is very small as compared to the total volume of an atom .
Based on the above observations and conclusions, Rutherford proposed the atomic structure of elements. According to the Rutherford atomic model:
- The positive charge and most of the mass of an atom is concentrated in an extremely small volume. He called this region of the atom as a nucleus.
- Rutherford’s model proposed that the negatively charged electrons surround the nucleus of an atom. He also claimed that the electrons surrounding the nucleus revolve around it with very high speed in circular paths. He named these circular paths as orbits.
- Electrons being negatively charged and nucleus being a densely concentrated mass of positively charged particles are held together by a strong electrostatic force of attraction.
Although the Rutherford atomic model was based on experimental observations, it failed to explain certain things.
- Rutherford proposed that the electrons revolve around the nucleus in fixed paths called orbits. According to Maxwell, accelerated charged particles emit electromagnetic radiations and hence an electron revolving around the nucleus should emit electromagnetic radiation. This radiation would carry energy from the motion of the electron which would come at the cost of shrinking of orbits. Ultimately the electrons would collapse in the nucleus. Calculations have shown that as per the Rutherford model, an electron would collapse into the nucleus in less than 10 -8 seconds. So the Rutherford model was not in accordance with Maxwell’s theory and could not explain the stability of an atom .
- One of the drawbacks of the Rutherford model was also that he did not say anything about the arrangement of electrons in an atom which made his theory incomplete.
- Although the early atomic models were inaccurate and failed to explain certain experimental results, they formed the base for future developments in the world of quantum mechanics .
Register with BYJU’S to learn more topics of chemistry such as Hybridization, Atomic Structure models and more.
| Lactose | |
The Gold Foil Experiment

Structure of Atom Class 11 Chemistry

Drawbacks of Rutherford Atomic Model

What was the speciality of Rutherford’s atomic model?
Rutherford was the first to determine the presence of a nucleus in an atom. He bombarded α-particles on a gold sheet, which made him encounter the presence of positively charged specie inside the atom.
What is Rutherford’s atomic model?
Rutherford proposed the atomic structure of elements. He explained that a positively charged particle is present inside the atom, and most of the mass of an atom is concentrated over there. He also stated that negatively charged particles rotate around the nucleus, and there is an electrostatic force of attraction between them.
What are the limitations of Rutherford’s atomic model?
Rutherford failed to explain the arrangement of electrons in an atom. Like Maxwell, he was unable to explain the stability of the atom.
What kind of experiment did Rutherford’s perform?
Rutherford performed an alpha scattering experiment. He bombarded α-particles on a gold sheet and then studied the trajectory of these α-particles.
What was the primary observation of Rutherford’s atomic model?
Rutherford observed that a microscopic positively charged particle is present inside the atom, and most of the mass of an atom is concentrated over there.

Put your understanding of this concept to test by answering a few MCQs. Click ‘Start Quiz’ to begin!
Select the correct answer and click on the “Finish” button Check your score and answers at the end of the quiz
Visit BYJU’S for all Chemistry related queries and study materials
Your result is as below
Request OTP on Voice Call
| CHEMISTRY Related Links | |

Leave a Comment Cancel reply
Your Mobile number and Email id will not be published. Required fields are marked *
Post My Comment
I am very much happy with the answer i got from this site, because you provide me with clearest and more understandable answer more than I expect. i really recommended the site, thanks.
What can I say, you people you’re the best! coz I tried to search for the failures of Rutherford any where and couldn’t but with you I found them. Thanks 🙏 I therefore recommend others to use this platform in order to get what they are searching for. I love guys
this clearer than I xpect. thanks alot
I am very happy with the answer that I obtained, however Ernest Rutherford’s Atomic Model never had any neutrons in the nucleus. James Chadwick discovered the neutron later in 1932. However, the limitations and observations of his theory on this web page seem to be correct.
It is very useful to me thaks
It’s a brilliant website with clear concept. Answers provided are really up to mark with best quality .
I ‘m contented with the explanation given. It seems much clearer with the interpretations following the observations.
I’m highly thankful to this website which made me clear about Rutherford method I was confused about this and worried also butt I’m now damn confident that it will surely help me and I’ll get good grades ❤️
Loads of love❤️
Register with BYJU'S & Download Free PDFs
Register with byju's & watch live videos.
- Alpha-Particle Scattering and Rutherford’s Nuclear Model of Atom
In 1911, Rutherford, along with his assistants, H. Geiger and E. Marsden, performed the Alpha Particle scattering experiment , which led to the birth of the ‘nuclear model of an atom ’ – a major step towards how we see the atom today.
Suggested Videos
J.j thomson’s plum-pudding model.
In 1897-98, the first model of an atom was proposed by J.J. Thomson. Famously known as the Plum-pudding model or the watermelon model, he proposed that an atom is made up of a positively charged ball with electrons embedded in it. Further, the negative and positive charges were equal in number , making the atom electrically neutral.
Figure 1 shows what Thomson’s plum-pudding model of an atom looked like. Ernest Rutherford, a former research student working with J.J. Thomson, proposed an experiment of scattering of alpha particles by atoms to understand the structure of an atom.
Rutherford, along with his assistants – H. Geiger and E. Marsden – started performing experiments to study the structure of an atom. In 1911, they performed the Alpha particle scattering experiment, which led to the birth of the ‘nuclear model of an atom’ – a major step towards how we see the atom today.

Figure 1. Source: Wikipedia
Browse more Topics under Atoms
- Atomic Spectra
- Bohr Model of the Hydrogen Atom
The Alpha Particle Scattering Experiment
They took a thin gold foil having a thickness of 2.1×10 -7 m and placed it in the centre of a rotatable detector made of zinc sulfide and a microscope. Then, they directed a beam of 5.5MeV alpha particles emitted from a radioactive source at the foil. Lead bricks collimated these alpha particles as they passed through them.
After hitting the foil, the scattering of these alpha particles could be studied by the brief flashes on the screen. Rutherford and his team expected to learn more about the structure of the atom from the results of this experiment.
Source: Wikipedia
Observations
Here is what they found:
- Most of the alpha particles passed through the foil without suffering any collisions
- Around 0.14% of the incident alpha particles scattered by more than 1 o
- Around 1 in 8000 alpha particles deflected by more than 90 o
These observations led to many arguments and conclusions which laid down the structure of the nuclear model on an atom.
Conclusions and arguments
The results of this experiment were not in sync with the plum-pudding model of the atom as suggested by Thomson. Rutherford concluded that since alpha particles are positively charged, for them to be deflected back, they needed a large repelling force. He further argued that for this to happen, the positive charge of the atom needs to be concentrated in the centre, unlike scattered in the earlier accepted model.
Hence, when the incident alpha particle came very close to the positive mass in the centre of the atom, it would repel leading to a deflection. On the other hand, if it passes through at a fair distance from this mass, then there would be no deflection and it would simply pass through.
He then suggested the ‘nuclear model of an atom’ wherein the entire positive charge and most of the mass of the atom is concentrated in the nucleus. Also, the electrons are moving in orbits around the nucleus akin to the planets and the sun. Further, Rutherford also concluded from his experiments that the size of the nucleus is between 10 -15 and 10 -14 m.
According to Kinetic theory, the size of an atom is around 10 -10 m or around 10,000 to 100,000 times the size of the nucleus proposed by Rutherford. Hence, the distance of the electrons from the nucleus should be around 10,000 to 100,000 times the size of the nucleus.
This eventually implies that most of the atom is empty space and explains why most alpha particles went right through the foil. And, these particles are deflected or scattered through a large angle on coming close to the nucleus. Also, the electrons having negligible mass, do not affect the trajectory of these incident alpha particles.
Alpha Particle Trajectory
The trajectory traced by an alpha particle depends on the impact parameter of the collision. The impact parameter is simply the perpendicular distance of each alpha particle from the centre of the nucleus. Since in a beam all alpha particles have the same kinetic energy, the scattering of these particles depends solely on the impact parameter.
Hence, the particles with a small impact parameter or the particles closer to the nucleus, experience large angle of scattering. On the other hand, those with a large impact parameter suffer no deflection or scattering at all. Finally, those particles having ~zero impact parameter or a head-on collision with the nucleus rebound back.
Coming to the experiment, Rutherford and his team observed that a really small fraction of the incident alpha particles was rebounding back. Hence, only a small number of particles were colliding head-on with the nucleus. This, subsequently, led them to believe that the mass of the atom is concentrated in a very small volume.
Electron Orbits
In a nutshell, Rutherford’s nuclear model of the atom describes it as:
- A small and positively charged nucleus at the centre
- Surrounded by revolving electrons in their dynamically stable orbits
The centripetal force that keeps the electrons in their orbits is an outcome of:
- The positively charged nucleus and
- The negatively charged revolving electrons.
Solved Example for You
Question: Rutherford, Geiger and Marsden, directed a beam of alpha particles on a foil of which metal
Solution: Gold
Customize your course in 30 seconds
Which class are you in.

- Shell Model
- Frank Hertz Experiment
- Effects of Radiation
- Ionizing Radiation
- Quantum Mechanics
One response to “Atomic Spectra”
i really have learnt alot, but its is difficult for me to register because my country(Nigeria) is not on the listed countries. pls kindly include if you can
Leave a Reply Cancel reply
Your email address will not be published. Required fields are marked *
Download the App

Rutherford Scattering ( AQA GCSE Physics )
Revision note.

Physics Project Lead
Rutherford Scattering
Alpha scattering.
- Physicist, Ernest Rutherford was instructing two of his students, Hans Geiger and Ernest Marsden to carry out the experiment
- They were directing a beam of alpha particles (He 2+ ions) at a thin gold foil
- They expected the alpha particles to travel through the gold foil, and maybe change direction a small amount
- Most of the alpha particles passed straight through the foil
- Some of the alpha particles changed direction but continued through the foil
- A few of the alpha particles bounced back off the gold foil
- The bouncing back could not be explained by the Plum Pudding model, so a new model had to be created

When alpha particles are fired at thin gold foil, most of them go straight through, some are deflected and a very small number bounce straight back
The Nuclear Model
- Ernest Rutherford made different conclusions from the findings of the experiment
- The table below describes the findings and conclusions of A, B and C from the image above:
Alpha Scattering Findings and Conclusions Table

- Rutherford proposed the nuclear model of the atom
- Nearly all of the mass of the atom is concentrated in the centre of the atom (in the nucleus)
- The nucleus is positively charged
- Negatively charged electrons orbit the nucleus at a distance
- The nuclear model could explain experimental observations better than the Plum Pudding model

The Nuclear model replaced the Plum Pudding model as it could better explain the observations of Rutherford’s Scattering Experiment
You've read 0 of your 10 free revision notes
Get unlimited access.
to absolutely everything:
- Downloadable PDFs
- Unlimited Revision Notes
- Topic Questions
- Past Papers
- Model Answers
- Videos (Maths and Science)
Join the 100,000 + Students that ❤️ Save My Exams
the (exam) results speak for themselves:
Did this page help you?
Author: Ashika
Ashika graduated with a first-class Physics degree from Manchester University and, having worked as a software engineer, focused on Physics education, creating engaging content to help students across all levels. Now an experienced GCSE and A Level Physics and Maths tutor, Ashika helps to grow and improve our Physics resources.
Rutherford's Experiment to Understand β-Rays
Nikolas martelaro february 20, 2017, submitted as coursework for ph241 , stanford university, winter 2017, introduction.
| Experimental Setup to determine existence of β-rays. (Source: N. Martelaro) |
Scientific experiments are the foundation for building our understanding of the physical world. While experiments may seem complex to the non-scientist, in reality, many influential scientific discoveries have been made with beautifully simple experiments. One such example is Earnest Rutherford's experiment on the nature of uranium radiation and its ability to pass through various materials. [1] Using the fact that the radiation from Uranium ionizes a gas, thus creating charges particles, Rutherford was able to measure the current produced by a sample of uranium placed between two charged metal plates. By placing metal foils on top of the uranium, Rutherford showed that part of the ionizing radiation was stopped while another part appeared to pass through the foils. This helped to confirm the work of Becquerel, showing that there were two components of the ionizing radiation. [2] These two rays were given the names α-rays and β-rays. While Becquerel's works suggested the existence of the two types of rays, there was still little understanding of their nature. Specifically, through how much and what types of material did these rays pass through? Rutherford aimed to explore this using his simple and beautiful experiment and was able to show the differences between α and β radiation absorption. This experiment would later lead to him advising Geiger and Mardsen's famous gold foil experiment, whereupon α particles moving through gold foil were shown to sometimes deflect, suggesting the positive nuclear core model of the atom that we know today. [3] This report gives an overview of the design and results of Rutherford's experiment, which is described in detail in his 1899 paper. [1] It should be noted that Rutherford details a number of experiments in his paper, only the experiment that confirmed the existence of α and β rays will be discussed here.
Known Theory of Uranium Radiation
At the time of Rutherford's experiment, uranium was know to emit an ionizing radiation similar to x-rays. When subjected to a gas, this radiation would create positively and negatively charged particles. This allows the gas to be a temporary conductor of electricity and would allow an electric potential and current to be measured. From Becquerel's work, it was known that the radiation would penetrate solid material, but it was not known through how much material. It was also known that the rays emitted from uranium had varying powers.
From this theory, Rutherford hypothesized that the rays emitting from uranium would be complex, composed of different types of rays. He proposed that testing how well the rays penetrated metal foils may help to show what the characteristics of the rays were.
Experiment Setup
Rutherford's experimental setup was quite simple. A diagram of it is shown in Fig. 1. A sample of uranium is placed between two plates A and B. Plate A is charged by a battery to 50 V, while plate B is connected to the sensing element of an electrometer. This creates an electrical potential between the plates. Due to the ionizing radiation coming from the uranium, the gas in between the plates will become electrically charged. Positive ions will move away from plate A while negative ion will move toward it. This will induce a small current between the plates. As this current flows in the gas, it will create a charge on the sensing element of the electrometer.
The electrometer works by having four separated quadrants. The quadrants are hollow inside, much like a bicycle tire without a tube. The diagonally opposing quadrants are connected together electrically. One set of quadrants is connected to earth ground while the other acts as the sensing quadrant and is connected to Plate B. A metal vane (or needle) is placed inside this hollow area of the quadrants. The vane hangs from a thread, allowing it to spin inside of the quadrants. The vane is then charged. When a charge is induced on Plate B and the sensing quadrant of the electrometer, it begins to spin the vane due to the opposing electrical forces, similar to how magnets with the north poles facing each other will repel each other. The degree that the vane turns is associated with the voltage, while the rate that the vane turns is associated with the current.
To explore the nature of the radiation, Rutherford covered the uranium with a thin sheet of metal foil. He then measured the "rate of leak" given by the electrometer vane when in constant motion (indicating a specific amount of current, and thus an amount of charge induced by the ionizing radiation). By placing successive sheets of foil, Rutherford was able to see how much the rate of leak diminished, indicating how much of the radiation was blocked.





IMAGES
VIDEO
COMMENTS
Rutherford gold-foil experiment In 1909 Rutherford disproved Sir J.J. Thomson's model of the atom as a uniformly distributed substance. Because only very few of the alpha particles in his beam were scattered by large angles after striking the gold foil while most passed completely through, Rutherford knew that the gold atom's mass must be ...
Rutherford overturned Thomson's model in 1911 with his famous gold-foil experiment, in which he demonstrated that the atom has a tiny, massive nucleus. Five years earlier Rutherford had noticed that alpha particles beamed through a hole onto a photographic plate would make a sharp-edged picture, while alpha particles beamed through a sheet of mica only 20 micrometres (or about 0.002 cm ...
The Rutherford model was devised by Ernest Rutherford to describe an atom. Rutherford directed the Geiger-Marsden experiment in 1909, which suggested, upon Rutherford's 1911 analysis, that J. J. Thomson 's plum pudding model of the atom was incorrect. Rutherford's new model [ 1] for the atom, based on the experimental results, contained new ...
A replica of an apparatus used by Geiger and Marsden to measure alpha particle scattering in a 1913 experiment. The Rutherford scattering experiments were a landmark series of experiments by which scientists learned that every atom has a nucleus where all of its positive charge and most of its mass is concentrated. They deduced this after measuring how an alpha particle beam is scattered when ...
Rutherford's gold foil experiment is also sometimes referred to as the Geiger-Marsden experiment. Features. The gold foil experiment consisted of a series of tests in which a positively charged helium particle was shot at a very thin layer of gold foil. The expected result was that the positive particles would be moved just a few degrees from ...
Rutherford's Gold Foil Experiment. The Rutherford gold foil experiment, also known as the scattering experiment, led to the creation of the model and explained the parts of the atom.In 1909, graduate student Ernest Marsden (under Ernest Rutherford's supervision) fired alpha particles at a gold foil piece. Most of the particles passed directly through the foil, meaning that a majority of ...
In the now well-known experiment, alpha particles were observed to scatter backwards from a gold foil. Rutherford's explanation, which he published in May 1911, was that the scattering was caused by a hard, dense core at the center of the atom-the nucleus. Ernest Rutherford was born in New Zealand, in 1871, one of 12 children.
Rutherford's Gold Foil Experiment helped detect that there was a large positively charged mass in the center of an atom: the nucleus. The experiment was done through the use of atomic collisions. Under the instruction of Rutherford, Hans Geiger and Ernest Marsden pointed a beam of alpha particles at a thin foil of metal and measured the ...
Observations of Rutherford's Alpha Scattering Experiment. The observations made by Rutherford led him to conclude that: A major fraction of the α-particles bombarded towards the gold sheet passed through the sheet without any deflection, and hence most of the space in an atom is empty.; Some of the α-particles were deflected by the gold sheet by very small angles, and hence the positive ...
Ernest Rutherford's famous gold foil experiment involves the scattering of alpha particles as they pass through a thin gold foil.It led to a better understan...
A British Physicist "Ernest Rutherford" proposed a model of the atomic structure known as Rutherford's Model of Atoms. He conducted an experiment where he bombarded α-particles in a thin sheet of gold. In this experiment, he studied the trajectory of the α-particles after interaction with the thin sheet of gold.
What did Rutherford do in his famous experiment? Rutherford's diffraction experiment tests diffraction via a thin foil made of gold metal. Opposite the gold foil is a screen that emits a flash of light when struck by a particle. The passing of many of the particles through suggested the condensed nucleus version of the atom model.
The Rutherford Experiment helps us to understand the structure of atoms. The experiment showed the presence of a positively charged kernel.
6. According to Rutherford, most of the atom's mass is concentrated in the electrons. True | False. 7. Ernest Rutherford won the Noble Prize for his model of the atom. True | False. 8. An atom ...
To see all my Chemistry videos, check outhttp://socratic.org/chemistryIn 1911, Ernest Rutherford and his colleagues discovered the nucleus of the atom using ...
Rutherford, along with his assistants - H. Geiger and E. Marsden - started performing experiments to study the structure of an atom. In 1911, they performed the Alpha particle scattering experiment, which led to the birth of the 'nuclear model of an atom' - a major step towards how we see the atom today. Figure 1. Source: Wikipedia.
Alpha Scattering. In 1909 a group of scientists were investigating the Plum Pudding model. Physicist, Ernest Rutherford was instructing two of his students, Hans Geiger and Ernest Marsden to carry out the experiment. They were directing a beam of alpha particles (He 2+ ions) at a thin gold foil. They expected the alpha particles to travel ...
Rutherford aimed to explore this using his simple and beautiful experiment and was able to show the differences between α and β radiation absorption. This experiment would later lead to him advising Geiger and Mardsen's famous gold foil experiment, whereupon α particles moving through gold foil were shown to sometimes deflect, suggesting the ...
Rutherford Scattering - PhET Interactive Simulations
The Rutherford atomic model was correct in that the atom is mostly empty space. Most of the mass is in the nucleus, and the nucleus is positively charged. Far from the nucleus are the negatively charged electrons. But the Rutherford atomic model used classical physics and not quantum mechanics. This meant that an electron circling the nucleus ...
These experiments led Rutherford to describe the atom as containing mostly empty space, with a very small, dense, positively charged nucleus at the center, which contained most of the mass of the atom, with the electrons orbiting the nucleus. Rutherford's gold foil experiments (and other metal foil experiments) involved firing positively ...