Last updated 2nd August 2024: Online ordering is currently unavailable due to technical issues. As we resolve the issues resulting from this, we are also experiencing some delays to publication. We are working hard to restore services as soon as possible and apologise for the inconvenience. For further updates please visit our website https://www.cambridge.org/news-and-insights/technical-incident
We use cookies to distinguish you from other users and to provide you with a better experience on our websites. Close this message to accept cookies or find out how to manage your cookie settings .

Login Alert

- > Case Studies in Communication Disorders
- > Man with stroke-induced Broca's aphasia

Book contents
- Case Studies in Communication Disorders
- Copyright page
- Section A Speech disorders
- Section B Language disorders
- Case study 10 Boy aged 7 years with developmental phonological disorder
- Case study 11 Portuguese-speaking girl aged 7 years with phonological disorder
- Case study 12 Boy aged 4;8 years with specific language impairment
- Case study 13 Boy with pragmatic language impairment
- Case study 14 Swedish-speaking girl with pragmatic language impairment
- Case study 15 Man aged 47 years with developmental dyslexia
- Case study 16 Boy aged 5;6 years with FG syndrome
- Case study 17 Boy with Floating-Harbor syndrome
- Case study 18 Woman aged 28 years with autism
- Case study 19 Girl with Sturge–Weber syndrome
- Case study 20 Man aged 47 years with temporal lobe epilepsy
- Case study 21 Girl aged 10 years with traumatic brain injury
- Case study 22 Girl aged 9;11 years with right cerebellar tumour
- Case study 23 Woman with post-irradiation speech and language disorder
- Case study 24 Woman aged 66 years with Wernicke's aphasia
- Case study 25 Woman aged 41 years with Broca's aphasia
- Case study 26 Man with stroke-induced Broca's aphasia
- Case study 27 Man aged 41 years with non-fluent aphasia
- Case study 28 Man aged 60 years with right hemisphere damage
- Case study 29 Man aged 24 years with closed head injury
- Case study 30 Woman aged 87 years with early-stage Alzheimer's disease
- Case study 31 Man aged 36 years with AIDS dementia complex
- Case study 32 Man aged 76 years with Parkinson's disease
- Case study 33 Man aged 37 years with Huntington's disease
- Section C Fluency disorders
- Section D Voice disorders
- Section E Hearing disorders
- Section F Psychiatric disorders
Case study 26 - Man with stroke-induced Broca's aphasia
from Section B - Language disorders
Published online by Cambridge University Press: 09 November 2016

Access options
Save book to kindle.
To save this book to your Kindle, first ensure [email protected] is added to your Approved Personal Document E-mail List under your Personal Document Settings on the Manage Your Content and Devices page of your Amazon account. Then enter the ‘name’ part of your Kindle email address below. Find out more about saving to your Kindle .
Note you can select to save to either the @free.kindle.com or @kindle.com variations. ‘@free.kindle.com’ emails are free but can only be saved to your device when it is connected to wi-fi. ‘@kindle.com’ emails can be delivered even when you are not connected to wi-fi, but note that service fees apply.
Find out more about the Kindle Personal Document Service .
- Man with stroke-induced Broca's aphasia
- Louise Cummings , Nottingham Trent University
- Book: Case Studies in Communication Disorders
- Online publication: 09 November 2016
- Chapter DOI: https://doi.org/10.1017/CBO9781316651100.029
Save book to Dropbox
To save content items to your account, please confirm that you agree to abide by our usage policies. If this is the first time you use this feature, you will be asked to authorise Cambridge Core to connect with your account. Find out more about saving content to Dropbox .
Save book to Google Drive
To save content items to your account, please confirm that you agree to abide by our usage policies. If this is the first time you use this feature, you will be asked to authorise Cambridge Core to connect with your account. Find out more about saving content to Google Drive .
Thank you for visiting nature.com. You are using a browser version with limited support for CSS. To obtain the best experience, we recommend you use a more up to date browser (or turn off compatibility mode in Internet Explorer). In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript.
- View all journals
- Explore content
- About the journal
- Publish with us
- Sign up for alerts
- Data Descriptor
- Open access
- Published: 02 August 2024
The stroke outcome optimization project: Acute ischemic strokes from a comprehensive stroke center
- John Absher 1 , 2 , 3 ,
- Sarah Goncher 1 ,
- Roger Newman-Norlund 4 ,
- Nicholas Perkins ORCID: orcid.org/0009-0002-6717-6716 1 , 2 , 3 ,
- Grigori Yourganov 5 ,
- Jan Vargas 1 , 2 , 3 na1 ,
- Sanjeev Sivakumar 1 , 3 na1 ,
- Naveen Parti 1 , 3 na1 ,
- Shannon Sternberg 3 na1 ,
- Alex Teghipco 4 ,
- Makayla Gibson ORCID: orcid.org/0000-0001-7309-8713 4 ,
- Sarah Wilson ORCID: orcid.org/0000-0002-5559-4569 6 ,
- Leonardo Bonilha 7 &
- Chris Rorden 4
Scientific Data volume 11 , Article number: 839 ( 2024 ) Cite this article
31 Accesses
Metrics details
- Computational neuroscience
- Outcomes research
- Predictive markers
Stroke is a leading cause of disability, and Magnetic Resonance Imaging (MRI) is routinely acquired for acute stroke management. Publicly sharing these datasets can aid in the development of machine learning algorithms, particularly for lesion identification, brain health quantification, and prognosis. These algorithms thrive on large amounts of information, but require diverse datasets to avoid overfitting to specific populations or acquisitions. While there are many large public MRI datasets, few of these include acute stroke. We describe clinical MRI using diffusion-weighted, fluid-attenuated and T1-weighted modalities for 1715 individuals admitted in the upstate of South Carolina, of whom 1461 have acute ischemic stroke. Demographic and impairment data are provided for 1106 of the stroke survivors from this cohort. Our validation demonstrates that machine learning can leverage the imaging data to predict stroke severity as measured by the NIH Stroke Scale/Score (NIHSS). We share not only the raw data, but also the scripts for replicating our findings. These tools can aid in education, and provide a benchmark for validating improved methods.
Similar content being viewed by others
Combining clinical and imaging data for predicting functional outcomes after acute ischemic stroke: an automated machine learning approach
Deep learning-based detection and segmentation of diffusion abnormalities in acute ischemic stroke
ISLES 2022: A multi-center magnetic resonance imaging stroke lesion segmentation dataset
Background & summary.
Stroke is a leading cause of long-term disability in the United States. Despite a decrease in stroke incidence per year of life among the elderly, this decline is counteracted by extended life expectancies and significant upswings in occurrences among younger adults. When paired with effective acute interventions that enhance survival, the overall result is a growing number of individuals living with stroke-related impairments 1 . Our overarching goal is to provide a large, public and diverse dataset that combines the medical imaging that is typical of acute stroke management along with demographic and impairment measures. These datasets can aid developers of tools to map brain injury, determine residual brain health, and create reliable diagnostic and prognostic measures.
Many “risk scores” have been developed to estimate the impact of acute ischemic stroke (AIS) and intracranial hemorrhage (ICH) in individual patients 2 , 3 , 4 , 5 and 94 individual de novo Clinically Predictive Models (CPMs) of stroke outcome were found in the Tufts PACE Clinical Prediction Model Registry (as of July 26, 2023) 6 . Stroke risk and stroke outcomes are influenced by many factors such as sex, racial/ethnic group, and socioeconomic class 6 , 7 . Validated CPMs for stroke are needed for predicting various outcomes, including overall functional recovery and the development of vascular dementia 6 , 8 .
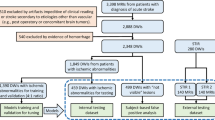
Prisma Health-Upstate has been collecting Get with the Guidelines (GWTG-stroke) data on all acute strokes seen at Greenville Memorial Hospital since 2009. GWTG data includes basic demographic information (age, race, sex), zip code, stroke etiology, vitals and blood work at admission and discharge, medical history and current medications, time to and type of thrombolytic therapy administered, complications, in-patient treatment and interventions, lifestyle interventions, stroke interventions, and neuroimaging data 9 . Because the GWTG data also includes two measures of post-stroke function, the NIH stroke scale (NIHSS) and modified Rankin Scale (mRS), we used these as the primary indicators of stroke severity for this dataset. However, we would note that each of these scales has its own limitations, and the best metrics of stroke impact should ideally be composed of data from multiple assessments, and include instructions for central adjudication, consistent rater training and correct application of novel statistical techniques 10 . More than 15,000 subjects are included in our local GWTG dataset. GWTG is used to promote the quality of hospital stroke care by tracking key processes and demographics known to relate to favorable outcomes, such as door-to-needle time or whether an AIS patient received thrombolytic 11 . Approximately 1100–1300 unique subjects are entered into our comprehensive stroke centers (CSC) GWTG database each year. GWTG data are important for understanding AIS outcomes, because they capture the systems of care that are influential in such outcomes. Collection and distribution of the data described in the SOOP repository is approved under protocol Pro00078716 of the Prisma Health Committee A (initial approval 10/29/2018, status = ongoing). Notably, the informed consent requirement for the current retrospective data analysis was waived by this Institutional Review Board. SOOP participants treated by study investigators may be recontacted and consented in person or remotely with the help of a legally authorized representative if needed, making future, prospective, longitudinal investigations a possibility. There are many distinct AIS outcomes. For example, weakness, numbness, visual loss, and cognitive dysfunction often result from stroke. Interactions among these factors may vary among subjects with an isolated, first-time AIS, compared to those individuals with recurrent or multifocal AIS. Also, stroke related cognitive impairments occur in up to a third of stroke patients 12 , 13 . Relatively few studies have attempted to predict specific outcomes such as motor function, aphasia, neglect, and depression 14 , 15 , 16 . Regardless of the measure of interest, a comprehensive approach to outcome prediction requires consideration of both known and unknown predictor variables and confounds–the quality of AIS care processes, patient characteristics, structural and functional consequences of acute and pre-existing brain damage, rehabilitation strategies, resilience factors, and other influences 17 , 18 , 19 , 20 , 21 . For example, age at stroke 22 , 23 and exercise are correlated with recovery, and factors like age, lesion volume, and residual brain health synergistically predict outcome. Large data repositories may capture information on a broad range of known and unknown outcome predictors and confounders to promote public health research 22 , 23 . Consequently, there is growing interest in data sharing consortia for AIS 24 , 25 . Such large data sharing collaboratives have been valuable adjuncts to understanding many diseases and disorders.
As an example, members of our team have experience applying machine learning to predict stroke sequelae and recovery trajectories in chronic and acute stroke. While these approaches have demonstrated potential, their effectiveness is often tempered by limitations such as small sample sizes, which can lead to overfitting or underfitting depending on the complexity of the algorithm used. Moreover, each algorithm carries inherent strengths and weaknesses that must be carefully considered to optimize model performance and ensure generalizability across diverse patient populations. The results to date demonstrate AIS outcome classification that is statistically significant, but insufficiently rigorous to change the standard of care for individuals.
While the majority of our group’s research has focused on diagnostic aspects of acute and chronic stroke, such as the relationship between lesion size, lesion location and chronic impairment, we acknowledge the critical importance of prognostic studies in predicting long-term outcomes on the basis of data available in the acute stage. Indeed, early prediction of likely recovery trajectories and chronic outcome is of paramount importance to stroke survivors, as well as their caregivers, as it can provide both a roadmap for future recovery as well as a set of expectations/limits in which to frame treatment outcomes. Accurate prediction of long-term outcomes likely requires the creation of comprehensive models that considers multiple factors, including lesion size and location and overall brain health along with various data such as demographic, medical, health and lifestyle factors. Additionally, it may be that the relative prognostic and diagnostic value of each of these factors differs in the acute and chronic stages of stroke recovery. Ultimately, gaining a better understanding of these interactions may be informative to clinicians and rehabilitation scientists striving to understand and manage stroke across time.Therefore, we combine GWTG quality data, clinical data from the electronic health record, and magnetic resonance imaging (MRI) morphometry data to examine a large population of AIS subjects from a large comprehensive stroke center (CSC). This paper introduces our efforts to develop a reproducible, sharable AIS CPM. An AIS data sharing consortium using these or similar methods could vastly improve outcome predictions in acute stroke 26 .
The term prognosis implies a type of clinical prediction model (CPM) that clinicians and families value immensely. AIS prognosis has both clinical and research significance. Stroke patients and their families are eager to know how they will recover, and how likely it is that long-term consequences like vascular dementia may develop. Likely, therapy teams rely on prognostic information to tailor their approach, deciding on the intensity and type of rehabilitation efforts. These can range from strategies aimed at restoring lost functions (rehabilitation) to those designed to help patients adapt to impairments through alternative techniques (compensatory strategies). Both forms are integral parts of the rehabilitation process, and the decision to emphasize one over the other, or how to effectively combine them, is heavily informed by an individual’s predicted recovery trajectory. Prognosis guides expectations and suggests the approaches we recommend clinically for every individual with AIS. Perfect prognostic information would enable research teams to detect meaningful effects of treatment interventions with smaller sample sizes, thus accelerating and economizing clinical trials. We focus in this report on our initial efforts to develop a CPM for prediction of stroke impairment using a large AIS population from a single CSC.
Publicly sharing clinical data can empower discoveries by scientists who do not have access to medical data. Further, datasets can also be aggregated to improve performance and overcome problems with local overfitting. Open datasets can also aid in education as well as providing shared benchmarks for validating and comparing competing solutions. Our SOOP dataset is similar to two other recent shared datasets. While we emphasize the differences, we note the potential for using these large, curated datasets synergistically. The ISLES 2022 dataset includes MR images and lesion maps from 400 stroke survivors. All individuals are from Europe, with consequences on training diversity 27 . The images are already completely brain extracted, which might limit methods that attempt to model image intensity homogeneity biases, as well as developing robust methods that can cope with diverse features such as wide diploic spaces and post bregmatic dips. The data also lacks demographic details beyond age, which limits the utility to developing automated lesion identification. Liu et al . 28 provide acute imaging and demographic data from 2888 individuals from the state of Maryland in the USA, capturing a more diverse population. This dataset also includes rich demographic and outcome measures. However, a limitation of this dataset is that the distribution the dataset is released as a restricted-use collection under a Data Use Agreement (DUA) which requires collaboration with a data review board and restrictions on data handling (e.g. data must be contained on an external drive where the computer is disconnected from the internet during all analyses). These restrictions limit the ability to use this dataset in many educational settings. In contrast to these existing works, our Stroke Outcome Optimization Project (SOOP) provides truly open imaging data from stroke survivors as well as similar data from individuals where stroke was excluded. Also, we provide both demographic measures as well as popular acute measures of stroke impairment and quality metrics.
While BIDS-capable pipelines exist for data from neurologically healthy adults, the presence of stroke can disrupt spatial normalization of imaging data 29 . Beyond providing the normalized acute stroke MRIs and associated clinical data, we also describe, validate and share a full processing pipeline that imports clinical data stored in the emerging BIDS-format for data sharing and generates impairment predictions. Adding data processed through this pipeline from additional stroke datasets may expand the range of impairments and outcomes that may be predicted.
Ethical statement
This retrospective evaluation of GWTG data, electronic health records extracts, and imaging data was approved by the local ethics boards. The dataset is considered exempt based on the retrospective nature of the study and the rigorous patient de-identification. Approval for re-contact and prospective examination of survivors from this cohort and their care partners has also been obtained. IRB approval for collection of the data contained in this repository was obtained from Prisma Health Committee A, Greenville SC (Pro00078716, 10-29-2018).
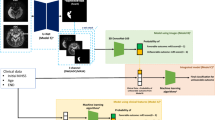
Our study sample included individuals captured within the GWTG database at Prisma Health-Upstate from the start of 2019 through the end of 2020, representing all identified acute stroke encounters over the entire two-year period. All participants included in this study were exempt from informed consent prior to participation, in accordance with approval received from the Institutional Review Board. Exclusion criteria were then applied. Individuals with subarachnoid, subdural, or intracerebral hemorrhage were excluded. Individuals lacking brain MRI were excluded. Individuals with stroke mimics, transient ischemic attacks, or other confounding structural or functional brain disorders (e.g., brain tumor, refractory epilepsy) were also excluded. The final sample included all eligible individuals with AIS, deemed unlikely to have significant major comorbidities to common clinical sequelae of stroke that could adversely impact outcome (n = 1415). Out of 1415 total participants, 305 had large-artery atherosclerosis, 343 had cardioembolism (e.g. atrial fibrillation/flutter, prosthetic heart valve, recent mI), 107 had small-vessel disease (e.g. subcortical, brain stem or lacunar infarct < 1.5 cm), 80 had a stroke of other determined etiology. In our study, 526 cases were reported as cryptogenic strokes, indicating that despite thorough diagnostic evaluations, no definitive cause could be identified. Additionally, 54 participants were classified as unable to determine (UTD), reflecting instances where insufficient documentation or inconclusive evidence prevented any stroke etiology classification. We also included 254 individuals where stroke was initially suspected (requiring the same imaging as for stroke) but later excluded as a probable diagnosis. Behavioral and demographic data were available and are provided for 1106 stroke survivors, with details including gender, age, race, body mass index, NIH stroke scale, mortal status, and acute Modified Rankin Scale. Speech and language pathology findings for the Western Aphasia Battery are provided for each of the subjects for whom this information is available. Of these, medical records listed 784 as white, 257 as black or African American, with 538 women and 568 men, age (after being limited to individuals aged 89 or less due to privacy concerns associated with distribution of age data above this value 30 ) ranges from 16 to 89 years with a median of 65, mean of 64.8 and a standard deviation of 14. NIHSS scores ranged from 0 to 30 with a median of 5 and a mean of 8 (standard deviation of 7.90).
Magnetic resonance imaging data
MRI scans for each person were completed within 30 days following their admission to the hospital. Most were obtained within 48 hours days of the acute stroke. For each individual we selected the T1-weighted, T2-weighted, Fluid Attenuated Inversion Recovery (FLAIR), and diffusion sequence that provided the best brain coverage and signal to noise ratio. The diffusion sequence included a TRACE image as well as an image with a contrast similar to an apparent diffusion coefficient (ADC). However, scan settings varied greatly between individuals, with sequence details stored in the text-based BIDS-format ‘sidecar’ provided with each Neuroimaging Informatics Technology Initiative (NIFTI) format image. In particular the T1w-modality varied tremendously, both in terms of coverage, resolution and contrast. We note that the clinically useful Gadolinium-enhanced T1w scans differ considerably from typical unenhanced sequences popular with basic science.
MRI images were converted from Digital Imaging and Communications in Medicine (DICOM) format to NIfTI format using dcm2niix 31 . We extended the ‘spm_deface’ script included with the latest version of Statistical Parametric Mapping software (SPM12) 32 to remove identifiable features from the face and neck. While some teams distribute images after complete brain extraction, we intentionally share images that include the scalp. Our rationale is that these regions can aid in modeling image inhomogeneity and our diverse dataset can help others develop brain extraction tools trained on a diverse dataset with features such as post-bregmatic depression and wide diploic spaces.
We also conducted stroke lesion mapping to identify and demarcate the extent of the injury. Specifically, three trained neuroscientists (RNN, MG, SW) manually traced lesion boundaries on each axial slice of participants’ T2w structural image. The percentage of patients with strokes including specific vascular regions, as defined by Faria’s digital arterial territory atlas 33 , can be found in Fig. 1 .

Percentage of patients experiencing damage to 32 distinct vascular territories described by the digital arterial territory atlas created by Faria and colleagues 33 . The largest percentage of patients experienced MCA injury, but a significant number also experienced ACA and MLS/LLS injuries. The wide variety of lesion location, as well as the bilateral distribution (see Fig. 3 ) makes SOOP particularly useful to researchers and clinicians interested in recovery of functions that are primarily lateralized (i.e. language) or considered bilateral (i.e. motor).
While there is no universally accepted method for demarcation of acute stroke lesions, our process adheres to several established guidelines. All raters used MRIcroGL12 34 software to manually inspect and trace lesions on ADC diffusion weighted images (DWI) in which acute lesions appeared as hypointense. Three raters trained in the use of MRIcroGL12 in our lab, and experienced with the process of creating lesion masks performed the lesion demarcations (authors RN, MG, and SW). The first step in lesion demarcation was to scroll through the entire ADC image and locate area(s) that, with absolute certainty, contained acutely lesioned tissue. From there, the trained rater demarcated the region in all spatially adjacent (in the superior inferior direction) slices that appeared to contain contiguous lesioned tissue. Lesions data were then exported as binary NIFTI formatted files in subject native DWI space. In these files, a value of ‘1’ denotes lesioned voxels and ‘0’ denotes non-lesioned voxels. Notably, this newly created NIFTI file was aligned with and had the same dimensions as the DWI image on which it was drawn. Lesion masks were produced in native (subject specific) space, and the resulting lesion masks were also normalized to standard anatomical (MNI) space and associated neuroanatomical atlases. A similar process was used to identify participants that additionally showed evidence of chronic stroke lesions, which showed up as hypointense on the same ADC images (acute and chronic stroke lesion files are stored separately in the SOOP OpenNeuro database). Video recordings were made of all lesion demarcation, using Quicktime’s ‘New Screen Recording’ function, and are available upon reasonable request to the corresponding author.
Importantly, recent evidence suggests that not all lesioned tissue exhibits uniform damage characteristics. Specifically, work by Krishnamurthy and colleagues demonstrated that T2w/T1w MRI signal ratios can be used to identify pericavitational areas with varying degrees of tissue integrity, termed Tissue Integrity Gradation via T2w T1w Ratio (TIGR) 35 . This method reveals a gradient of damage within lesions, rather than a binary map like the one generated by our manual lesion demarcation approach, and this may provide for more sensitive lesion-symptom mapping. We acknowledge these developments and propose incorporating such advanced methodologies in future protocols to enhance the precision of lesion identification and characterization 36 .
Data Records
The anonymized images for the Stroke Outcome Optimization Project (SOOP) are available from OpenNeuro ( https://openneuro.org/datasets/ds004889 ). The imaging, demographic and behavioral measures are organized using the brain imaging data structure (BIDS) 35 and shared publicly on the OpenNeuro web site 37 . This curated structure provides human readable filenames with a clear file hierarchy for storing data. A benefit of this system is that it allows automated tools to process and aggregate datasets. Beyond the raw imaging data, the data is provided in text formats that allow inspection. Specifically, the demographic and impairment measures are stored in the tab-separated value text format spreadsheet ‘participants.tsv’, which includes a labeled header row to describe the variables, and each subsequent row provides the values for a single participant with the first column providing participant identification (e.g. ‘sub-11’). The text file ‘participants.json’ provides in-depth descriptors for each of these labels. The defaced imaging data is stored in a separate folder for each individual (e.g. ‘sub-11’). Each participant’s folder contains two subfolders: the ‘anat’ folder stores the anatomical scans (here the T1 and FLAIR modalities) and the ‘dwi’ folder stores the diffusion data (here the TRACE and ADC images). The MR images are stored in NIfTI format, with each including a text-format JSON file that provides sequence details. The root directory also contains a folder named ‘derivatives’ which includes the folder ‘lesion_masks’ containing one folder (e.g. ‘sub-11’) for each patient where a stroke was observed. These folders provide the lesion maps, drawn on the individual’s TRACE image. For individuals who had pre-existing injuries a total of three lesion maps are provided (‘-lesionChronic_mask’, ‘-lesionAcute_mask’, ‘-lesion_mask’) while those with only recent injury exclusively include the latter two images. The normalized FLAIR images for the SOOP participants are available from the Open Science Framework (OSF) 38 .
Technical Validation
Our focus on predicting NIH stroke scale, which is a popular but notably non-comprehensive measure of AIS severity 10 , may facilitate education, data sharing and collaboration. We emphasize that the provided dataset can be used by others to improve clinical tools, including automatic lesion mapping, spatial normalization, brain integrity measurements, and predicting outcomes. To demonstrate the richness of the data, we have provided simple scripts that illustrate current best practices to allow easy replication, education and a basic validation benchmark for comparing future tools and methods. Specifically, these scripts use the lesion maps drawn on the TRACE image to predict impairment on the NIH stroke scale.
Briefly, the first task is to warp the lesion masks drawn in the native space of each individual’s TRACE image to a common template image. Here we leverage the Clinical Toolbox for SPM 39 to first coregister the low-resolution TRACE image to the high resolution FLAIR image (this warps the lesion to FLAIR space) and subsequently conducts unified segmentation and normalization 40 to warp the individuals FLAIR image to a common template (so that the lesion maps from all individuals are in a standard space), as shown in Figs. 2 and 3 . We then calculate the proportion of injury for each region in a vascular atlas 33 , resulting in a tab separated value where each row provides information from a single subject and each column lists the proportion injury for each territory in the atlas. A script removes columns that are damaged in fewer than a specified proportion of the population. We chose 5% of the participants, following conventions to improve statistical power and spatial biases 41 . Therefore, Fig. 3 appears to omit anterior cerebral artery strokes, which occur at a frequency lower than 5% 42 , 43 . Note that these spreadsheets match the layout of the ‘participants.tsv’, allowing us to concatenate the lesion information, demographics and outcome measures for our subsequent analyses.

Example data for one individual (participant 342). For each individual we provide a scan with T1-weighting ( A ), a T2-weighted fluid-attenuated inversion recovery (FLAIR) ( B ), as well as two images from an echo-planar imaging diffusion sequence. With regards to the diffusion sequences shown, this study chose to use very short DWI sequences referred to Apparent Diffusion Coefficient (ADC) ( C ) and TRACE ( D ) scans, as opposed to longer (10–20 minute) DWI sequences used to calculate tractography. These shorter DWI scans allowed for detection of abnormal diffusion using a very short acquisition time, which is apt for clinical settings.
Finally, we provide a script (deep_learn.py) that computes a simple leave-one-out prediction of the NIH stroke scale based on the imaging measures as well as participant age (see Code Availability section). Users can simply download the entire SOOP project from our GitHub repository ( https://github.com/neurolabusc/StrokeOutcomeOptimizationProjectDemo ) and run the python file, deep_learn.py, to generate the graph in this manuscript (more detailed instructions for running deep_learn.py using Python are included on the GitHub page for this project). Note that the goal of machine learning is to use features synergistically to provide the best prediction. Participant age is known to predict initial stroke score 44 , though in our sample it does not prove a reliable predictor on its own. The analysis utilizes a TensorFlow-based implementation in Python, employing a sequential neural network architecture. The model consists of three layers: an initial dense layer with 64 nodes and a rectified linear unit (ReLU) activation function, followed by a second dense layer with 32 nodes and ReLU activation, and finally, an output layer with a single node. We also provide an identical analysis using support vector machines, which can sometimes be more robust for relatively small datasets. We wish to emphasize that our data is amenable to more sophisticated analyses, but our goal is to provide a simple solution using off-the-shelf solutions. Both models significantly predict stroke scale, with the Neural Network correlation r = 0.543, p-value < 0.00001, and the SVR r = 0.550, p-value < 0.00001. Results for both models are shown in Fig. 4 .

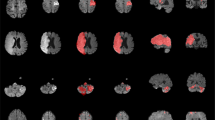
Top Panel: Bitmap image generated by our validation scripts for a single participant (#23). This image shows axial, sagittal and coronal slices, as well as a rendered image, in standard MNI space. The lesion, located in the right caudate nucleus, is depicted in red. White matter hyperintensities (periventricular) are visible on the anterior boundary of the left and right ventricles. Users can inspect this bitmap can as part of the quality assurance process. In particular, the unified segmentation and normalization method we use develops a virtuous cycle between the spatial warping and the tissue segmentation that drives the brain extraction. Therefore, an accurate volume rendering (right panel) is consistent with a successful spatial warping to standard space. Middle Panel: Despite the variable differences in quality and resolution of each individual FLAIR scan (renderings for four representative individuals shown on the left side), all are normalized into a standard space, as seen by the rendering of the mean normalized FLAIR scan from all individuals (right side). Bottom Panel: Lesion incidence map (N = 1461) for the SOOP dataset. Hotter colors show regions with higher injury incidence.

We created an easy-to-modify script that attempts to predict NIH Stroke Scale (NIHSS) scores based on participant age and lesion load to each brain region described in the vascular territory brain atlas created by Faria and colleagues 33 . Our script deep_learn.py, which is contained in our open-source GitHub repository: https://github.com/neurolabusc/StrokeOutcomeOptimizationProjectDemo ), can be run in a Python environment or using Jupyter Notebooks, to predict NIHSS scores using two different algorithms: support vector regression (SVR - red) and neural network (NN - green). This GitHub page contains more detailed instructions on dependencies and how to run this script. Comparison of the performance of these algorithms shows that NN outperforms SVR for this classification task. Other researchers can easily modify this script to run it on subsets of our data (e.g. males vs. females, large vs. small lesions determined by a median split, etc) or compare the performance of other types of machine learning or AI models. *Each circle represents a unique participant. Lesion sizes were converted to z-scores and are represented by the size of each dot. Data points with predicted NIHSS Values > = 30 (N = 2) or < = 0 (N = 8) were excluded from the graph for visualization.
Usage Notes
The Stroke Outcome Optimization Project is publicly shared on OpenNeuro using the community developed BIDS structure to enable usage with any BIDS-compatible pipeline. We hope that this will encourage the development, validation and education for novel tools that are capable of handling multiple types of data that influence stroke outcome. The Technical Validation section describes a simple set of analyses using current best practices. The Matlab and Python scripts for reproducing these results are available from GitHub. By design, these scripts focus on simplicity for clarity and training. These scripts provide a basic validation benchmark so others can evaluate the performance of more sophisticated solutions.
Anonymizing and curating large datasets for public sharing requires substantial investment of resources. Our initial release focuses specifically on ischemic stroke. We recognize that our exclusion criteria impact the generalizability of machine learning predictions by omitting structural and clinical comorbidities, and hemorrhagic strokes, that are represented in our entire stroke cohort; by omitting infrequent stroke subtypes such as anterior cerebral artery strokes and hemorrhages, generalizability to an entire stroke population is limited. However, our future goal is to remove exclusion criteria systematically. We provide code and methodology that may be used for data collection across comprehensive stroke centers. Larger datasets will be required to model the impact of uncommon or rare influences on stroke outcome, and we plan to systematically incorporate such comorbidities into our evolving models. Educational and occupational background, race and ethnicity, tobacco, alcohol and drug use, treatment timing and success, and many other factors impact long term stroke outcome. The current work is our initial effort to develop a CPM for stroke using electronic health records (EHR) and MRI data that are routinely acquired during acute ischemic stroke management. We hope to stimulate machine learning methods that will enable a comprehensive accounting of many factors that may influence aphasia outcomes in particular, and eventually other stroke outcomes. We also plan on releasing additional behavioral data to researchers. These additional data will eventually include GWTG data including, comprehensive medical history and current medication information, medications, comorbidities, as well as estimated SES (based on zip-code) and possibly other MRI-derivatives (such as quantity and location of white matter hyperintensities, perivascular space and microbleeds) These details will be generalized to protect identities.
Code availability
We refined dcm2niix for converting the source DICOM MRI scans to BIDS format, with improvements incorporated in this open source software ( https://github.com/rordenlab/dcm2niix ). Our defacing method is available from GitHub ( https://github.com/neurolabusc/mydeface ). We provide minimal Matlab and Python scripts to organize, process, and analyze these data using machine learning. These scripts are all stored in a self-contained archive at GitHub ( https://github.com/neurolabusc/StrokeOutcomeOptimizationProjectDemo ), allowing others to replicate and extend the findings we describe in the Technical Validation section.
Writing Group Members. et al . Heart Disease and Stroke Statistics-2016 Update: A Report From the American Heart Association. Circulation 133 , e38–360 (2016).
Google Scholar
Weimar, C. et al . Prediction of recurrent stroke and vascular death in patients with transient ischemic attack or nondisabling stroke: a prospective comparison of validated prognostic scores. Stroke 41 , 487–493 (2010).
Article PubMed Google Scholar
O’Brien, E. C. et al . Quality of Care and Ischemic Stroke Risk After Hospitalization for Transient Ischemic Attack: Findings From Get With The Guidelines-Stroke. Circ. Cardiovasc. Qual. Outcomes 8 , S117–24 (2015).
Smith, E. E. et al . Risk score for in-hospital ischemic stroke mortality derived and validated within the Get With the Guidelines-Stroke Program. Circulation 122 , 1496–1504 (2010).
Menon, B. K. et al . Risk score for intracranial hemorrhage in patients with acute ischemic stroke treated with intravenous tissue-type plasminogen activator. Stroke 43 , 2293–2299 (2012).
Article CAS PubMed Google Scholar
Wessler, B. S. et al . Tufts PACE Clinical Predictive Model Registry: update 1990 through 2015. Diagn Progn Res 1 , 20 (2017).
Article PubMed PubMed Central Google Scholar
Benjamin, E. J. et al . Heart Disease and Stroke Statistics-2018 Update: A Report From the American Heart Association. Circulation 137 , e67–e492 (2018).
Wessler, B. S. et al . Clinical Prediction Models for Cardiovascular Disease: Tufts Predictive Analytics and Comparative Effectiveness Clinical Prediction Model Database. Circ. Cardiovasc. Qual. Outcomes 8 , 368–375 (2015).
Smaha, L. A. The American Heart Association get with the guidelines program. Am. Heart J. 148 , S46–S48 (2004).
Taylor-Rowan, M., Wilson, A., Dawson, J. & Quinn, T. J. Functional Assessment for Acute Stroke Trials: Properties, Analysis, and Application. Front. Neurol. 9 , 191 (2018).
Song, S. et al . Association of Get With The Guidelines-Stroke Program Participation and Clinical Outcomes for Medicare Beneficiaries With Ischemic Stroke. Stroke 47 , 1294–1302 (2016).
Mijajlović, M. D. et al . Post-stroke dementia - a comprehensive review. BMC Med. 15 , 11 (2017).
Pendlebury, S. T. et al . Methodological factors in determining rates of dementia in transient ischemic attack and stroke: (I) impact of baseline selection bias. Stroke 46 , 641–646 (2015).
Grefkes, C. & Fink, G. R. Connectivity-based approaches in stroke and recovery of function. Lancet Neurol. 13 , 206–216 (2014).
Rehme, A. K. et al . Individual prediction of chronic motor outcome in the acute post-stroke stage: Behavioral parameters versus functional imaging. Hum. Brain Mapp. 36 , 4553–4565 (2015).
Volz, L. J. et al . Time-dependent functional role of the contralesional motor cortex after stroke. Neuroimage Clin 16 , 165–174 (2017).
Article CAS PubMed PubMed Central Google Scholar
Yoshimoto, T. et al . Impact of Previous Stroke on Clinical Outcome in Elderly Patients With Nonvalvular Atrial Fibrillation: ANAFIE Registry. Stroke 53 , 2549–2558 (2022).
Gallanagh, S., Quinn, T. J., Alexander, J. & Walters, M. R. Physical activity in the prevention and treatment of stroke. ISRN Neurol. 2011 , 953818 (2011).
Liew, S.-L. et al . Association of Brain Age, Lesion Volume, and Functional Outcome in Patients With Stroke. Neurology 100 , e2103–e2113 (2023).
Garcia, D. A., Arredondo, R. & Morris, M. A review of rehabilitation strategies for stroke recovery. P roceedings of the (2012).
Yan, H.-Y. & Lin, H.-R. Resilience in Stroke Patients: A Concept Analysis. Healthcare (Basel) 10 , (2022).
Manolio, T. A. et al . New models for large prospective studies: is there a better way? Am. J. Epidemiol. 175 , 859–866 (2012).
Sudlow, C. et al . UK biobank: an open access resource for identifying the causes of a wide range of complex diseases of middle and old age. PLoS Med. 12 , e1001779 (2015).
Lees, K. R., Khatri, P. & STAIR IX Collaborators. Stroke Treatment Academic Industry Roundtable Recommendations for Individual Data Pooling Analyses in Stroke. Stroke 47 , 2154–2159 (2016).
Liebeskind, D. S. et al . Imaging in StrokeNet: Realizing the Potential of Big Data. Stroke 46 , 2000–2006 (2015).
Krakauer, J. W. & Marshall, R. S. The proportional recovery rule for stroke revisited. Annals of neurology 78 , 845–847 (2015).
Kopal, J., Uddin, L. Q. & Bzdok, D. The end game: respecting major sources of population diversity. Nat. Methods 20 , 1122–1128 (2023).
Liu, C.-F. et al . A large public dataset of annotated clinical MRIs and metadata of patients with acute stroke. Sci Data 10 , 548 (2023).
Article ADS PubMed PubMed Central Google Scholar
Brett, M., Leff, A. P., Rorden, C. & Ashburner, J. Spatial normalization of brain images with focal lesions using cost function masking. Neuroimage 14 , 486–500 (2001).
Office for Civil Rights (OCR). Guidance regarding methods for DE-identification of protected health information in accordance with the health insurance portability and accountability act (HIPAA) Privacy Rule. H HS.gov https://www.hhs.gov/hipaa/for-professionals/privacy/special-topics/de-identification/index.html (2012).
Li, X., Morgan, P. S., Ashburner, J., Smith, J. & Rorden, C. The first step for neuroimaging data analysis: DICOM to NIfTI conversion. J. Neurosci. Methods 264 , 47–56 (2016).
Friston, K. J., Ashburner, J. T., Nichols, T. E. & Penny, W. D. Statistical Parametric Mapping the Analysis of Funtional Brain Images . (Elsevier/Academic Press, Amsterdam, 2007).
Liu, C.-F. et al . Digital 3D Brain MRI Arterial Territories Atlas. Sci Data 10 , 74 (2023).
Rorden, C. & Brett, M. Stereotaxic display of brain lesions. Behav. Neurol. 12 , 191–200 (2000).
Gorgolewski, K. J. et al . The brain imaging data structure, a format for organizing and describing outputs of neuroimaging experiments. Sci Data 3 , 160044 (2016).
Krishnamurthy, L. C. et al . Not All Lesioned Tissue Is Equal: Identifying Pericavitational Areas in Chronic Stroke With Tissue Integrity Gradation via T2w T1w Ratio. Front. Neurosci. 15 , 665707 (2021).
Rorden, C., Absher, J. & Newman-Norlund, R. Stroke Outcome Optimization Project (SOOP). OpenNeuro https://doi.org/10.18112/openneuro.ds004889.v1.1.2 (2024).
Rorden, C., Absher, J. R., Gibson, M., Teghipco, A. & Newman-Norlund, R. Stroke Outcome Optimization Project (SOOP). OSF https://doi.org/10.17605/OSF.IO/YQKTJ (2024).
Rorden, C., Bonilha, L., Fridriksson, J., Bender, B. & Karnath, H.-O. Age-specific CT and MRI templates for spatial normalization. Neuroimage 61 , 957–965 (2012).
Ashburner, J. & Friston, K. J. Unified segmentation. Neuroimage 26 , 839–851 (2005).
Sperber, C. & Karnath, H. Impact of correction factors in human brain lesion‐behavior inference. Hum. Brain Mapp. 38 , 1692 (2017).
Arboix, A. et al . Infarction in the territory of the anterior cerebral artery: clinical study of 51 patients. BMC Neurol. 9 , 30 (2009).
Thirugnanachandran, T. et al . Anterior Cerebral Artery Stroke: Role of Collateral Systems on Infarct Topography. Stroke 52 , 2930–2938 (2021).
Simmons, C. A., Poupore, N. & Nathaniel, T. I. Age Stratification and Stroke Severity in the Telestroke Network. J. Clin. Med. Res . 12 , (2023).
Download references
Acknowledgements
Administrative support, space, and resources were provided by Clemson University and Prisma Health. Technical support was provided by Clemson University, Prisma Health, and the University of South Carolina. Philanthropic support was provided by Furman University, including stipends for several students: Elizabeth Nethercoat, Michael Garovich, Natalie Dunn, Molly Oroho, Cade Azzariti, Hailey Turk, and Davis Dear. Patrick Burton received stipend support through a Health Sciences Center Seed Grant. We also acknowledge Jenna Durham, Sarah Hierholzer, Leigh Ann Spell, Wes Wimpey, and Alex Ewing for their contributions. This work was supported by the National Institute of Health (P50DC014664, RF1MH133701). We would like to acknowledge the participants, students, faculty, and staff who have supported the Center for the Study of Aphasia Recovery.
Author information
These authors contributed equally: Jan Vargas, Sanjeev Sivakumar, Naveen Parti, Shannon Sternberg.
Authors and Affiliations
University of South Carolina School of Medicine, Greenville, SC, 29605, USA
John Absher, Sarah Goncher, Nicholas Perkins, Jan Vargas, Sanjeev Sivakumar & Naveen Parti
Clemson University School of Health Research, CUSHR, Clemson, SC, 29634, USA
John Absher, Nicholas Perkins & Jan Vargas
Departments of Medicine, Neurosurgery, and Radiology, Prisma Health, Greenville, SC, 29601, USA
John Absher, Nicholas Perkins, Jan Vargas, Sanjeev Sivakumar, Naveen Parti & Shannon Sternberg
Department of Psychology, University of South Carolina, Columbia, SC, 29203, USA
Roger Newman-Norlund, Alex Teghipco, Makayla Gibson & Chris Rorden
Partnership for an Advanced Computing Environment, Georgia Institute of Technology, Atlanta, GA, 30332, USA
Grigori Yourganov
Linguistics Program, University of South Carolina, Columbia, SC, 29203, USA
Sarah Wilson
Department of Neurology, University of South Carolina, Columbia, SC, 29208, USA
Leonardo Bonilha
You can also search for this author in PubMed Google Scholar
Contributions
J.A. designed the research study, supervised data acquisition, wrote the first draft of the manuscript, and edited the final versions for publication. C.R. developed the manuscript for the journal format and developed all scripts and validation testing. L.B. developed the machine learning scripts described in the manuscript. J.A., C.R., R.N., S.K. and A.T. performed the MRI processing and analysis. S.G. and J.A. evaluated each subject’s inclusion/exclusion criteria, and performed speech language pathology (SLP) ratings. Nicholas Perkins wrote the code responsible for EPIC and MRI data abstraction. C.R., J.V.M. and N.P. collaborated on MRI data collection, conversion to NIfTI format, de-identification, and coding. G.Y., J.A., S.K., A.T., R.N. and C.R. developed and maintained the neuroimaging software, and collaborated on data analysis. M.G. and S.W. assisted R.N. with manual lesion delineation. All authors contributed to editorial changes in the manuscript. All authors read and approved the final manuscript.
Corresponding author
Correspondence to John Absher .
Ethics declarations
Competing interests.
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/ .
Reprints and permissions
About this article
Cite this article.
Absher, J., Goncher, S., Newman-Norlund, R. et al. The stroke outcome optimization project: Acute ischemic strokes from a comprehensive stroke center. Sci Data 11 , 839 (2024). https://doi.org/10.1038/s41597-024-03667-5
Download citation
Received : 19 January 2024
Accepted : 22 July 2024
Published : 02 August 2024
DOI : https://doi.org/10.1038/s41597-024-03667-5
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
Quick links
- Explore articles by subject
- Guide to authors
- Editorial policies
Sign up for the Nature Briefing newsletter — what matters in science, free to your inbox daily.
Log in using your username and password
- Search More Search for this keyword Advanced search
- Latest content
- Global health
- BMJ Journals
You are here
- Volume 2014, Issue
- Broca aphasia
- Article Text
- Article info
- Citation Tools
- Rapid Responses
- Article metrics
- Takashi Watari 1 ,
- Taro Shimizu 1 ,
- Yasuharu Tokuda 2
- 1 Department of Internal Medicine , Tokyo Joto Hospital , Tokyo , Japan
- 2 Japan Community Healthcare Organization , Tokyo , Japan
- Correspondence to Dr Taro Shimizu, shimizutaro7{at}gmail.com
https://doi.org/10.1136/bcr-2014-208214
Statistics from Altmetric.com
Request permissions.
If you wish to reuse any or all of this article please use the link below which will take you to the Copyright Clearance Center’s RightsLink service. You will be able to get a quick price and instant permission to reuse the content in many different ways.
Description
An 80-year-old Japanese woman presented with sudden onset of speech disturbance and confusion. She was riding a bicycle when she suddenly felt unwell and subsequently noticed she could not find words to express her thoughts. A pedestrian found her sitting on the ground, at a loss for words and looking confused. She was brought to the emergency department for evaluation. On examination, she was alert, but looked very anxious, frustrated and confused. She was not oriented to time, place and person. She spoke hesitantly and non-fluently, she seemed not to be able to find words to respond (speaking and writing) to the physician's questions. She also showed impairment in repetition and comprehension to questions with complex syntax. The rest of the neurological examination was normal. Laboratory studies showed high cholesterol and elevated glycated haemoglobin of 8.2.
- Download figure
- Open in new tab
- Download powerpoint
Diffusion-weighted MRI showing ischaemic findings involving the Broca area.
Three-dimensional MR angiography showing a signal loss (arrows) at the distal point of the left middle cerebral artery.
Differential diagnosis of Broca aphasia
Ischaemic disease
Cerebral infarction
Transient ischaemic attack
Haemorrhage
Intracerebral haemorrhage
Traumatic injury
Subdural haematoma
Subarachnoid haemorrhage
Herpes encephalitis
West Nile encephalitis
Bacterial infection/abscess
Fungal abscess
Prion disease
Toxoplasmosis
Lyme disease
Degeneration
Alzheimer’s disease
Primary progressive aphasia
Amyotrophic lateral sclerosis
Demyelination
Multiple sclerosis
Acute disseminated encephalomyelitis
Primary brain tumour
Brain metastases
Sarcoidosis
Conversion disorder
Wernicke’s encephalopathy
Learning points
Broca aphasia should be suspected when a patient has difficulty in repetition and naming, and if dysfluency or inaccuracy of expression of speech and writing are detected.
The diagnosis is sometimes difficult because of the limited manifestation of symptoms.
- Daroff RB , et al
- Ochfeld E ,
- Newhart M ,
- Molitoris J , et al
Contributors TW wrote the manuscript. TS and YT revised the manuscript.
Competing interests None.
Patient consent Obtained.
Provenance and peer review Not commissioned; externally peer reviewed.
Read the full text or download the PDF:

Argye Elizabeth Hillis , MD
Vascular neurology.
- Johns Hopkins School of Medicine Faculty
4.9 of 5 stars
14 insurances accepted, professional titles.
- Director, Center of Excellence in Stroke Detection and Diagnosis, Sheikh Khalifa Stroke Institute
- Sheikh Khalifa Stroke Institute Professor of Acute Stroke Diagnoses and Management
- Director, Cerebrovascular Division of Neurology
- Executive Vice Chair, Department of Neurology
Primary Academic Title
Professor of Neurology
Dr. Argye Hillis is a professor of Neurology, with joint faculty appointments in Physical Medicine and Rehabilitation and in Cognitive Science. She is also the Sheikh Khalifa Stroke Institute Professor of Acute Stroke Diagnoses and Management. Prior to medical training and neurology residency, Dr. Hillis worked as a speech-language pathologist, and conducted clinical research focusing on understanding and treating aphasia and hemispatial neglect. She has brought these areas of experience to impact on her clinical research in neurology, which involves cognitive and neuroimaging studies of aphasia and hemispatial neglect due to acute stroke and focal dementias. She has published extensively on these topics in journals and textbooks. Dr. Hillis is Associate Editor of Stroke and has served as Associate editor of Brain, Annals of Neurology, Aphasiology, American Journal of Speech-Language Pathology, Neurocase, Cognitive Neuropsychology, and Language and Cognitive Processes and served as co-Editor and Chief of Behavioral Neurology. Dr. Hillis serves as the Executive Vice Chair of the Department of Neurology and the Director of the Cerebrovascular Division of Neurology at Johns Hopkins.
Centers and Institutes
Cerebrovascular Center
- #TomorrowsDiscoveries: Recovering After a Stroke - Argye Hillis, M.D.
- The Johns Hopkins Hospital Comprehensive Stroke Center

Recent News Articles and Media Coverage
Argye Hillis Among Stoke Researchers Awarded $11 Million NIH Grant Press Release (4/20/16)
Dealing with the emotional aspects of stroke rehab, Philadelphia Inquirer (04/13/2014)
Why You Get the Joke: Brain's Sarcasm Center Found, Live Science
Additional Academic Titles
Professor of Physical Medicine and Rehabilitation
Research Interests
Cognitive Deficits and Recovery after Right Hemisphere Stroke, Language Recovery After Stroke, Stroke Cognitive Outcome and Recovery (SCORE)
Lab Website
- The mission of the Stroke Cognitive Outcomes and Recovery (S.C.O.R.E.) Lab is to enhance knowledge of brain mechanisms that allow people recover language, empathy, and other cognitive and communicative functions after stroke, and to improve ways to facilitate recovery of these functions after stroke. We also seek to improve the understanding of neurobiology of primary progressive aphasia., and how to enhance communication in people with this group of clinical syndromes.
Research Summary
Following a stroke, an individual may experience speech, language, cognitive, or emotional problems. Dr. Hillis’ current research aims to improve the understanding of how language and other cognitive functions are represented and carried out in the brain, how they recover after injury, and how understanding these processes can contribute to evaluation and treatment of stroke and dementia. Specifically, current research studies include the following:
- Stroke Cognitive Outcome and Recovery (SCORE)
- Language Recovery After Stroke
- Cognitive Deficits and Recovery after Right Hemisphere Stroke
Dr. Hillis' current research combines longitudinal task-related and task-free functional imaging and structural imaging from the acute stage of stroke through the first year of recovery, with detailed cognitive and language assessments to improve our understanding how language and other cognitive functions recover after stroke. Her other avenue of research involves novel treatment studies and longitudinal imaging and language studies of Primary Progressive Aphasia. She has published extensively on these topics in journals and textbooks.
Google Scholar
http://scholar.google.com/scholar?q=Argye+E+Hillis&btnG=&hl=en&as_sdt=0%2C21
http://www.ncbi.nlm.nih.gov/pubmed/?term=hillis%2C+argye
Selected Publications
- SELECTED PUBLICATIONS (from >150 total) Hillis, A.E. (1989). Efficacy and generalization of treatment for aphasic naming errors. Archives of Physical Medicine and Rehabilitation, 70, 632-636. Caramazza, A. & Hillis, A.E. (1991). Lexical organization of nouns and verbs in the brain. Nature, 349,788-90. Hillis, A.E. & Caramazza, A. (1991). Category-specific naming and comprehension impairment: A double dissociation. Brain, 114, 2081-2094. Hillis, A.E. & Caramazza, A. (1995). Cognitive and neural mechanisms underlying visual and semantic processing. Journal of Cognitive Neuroscience, 7, 457-478. Hillis, A.E. & Caramazza, A. (1995). The representation of grammatical categories of words in the brain. Journal of Cognitive Neuroscience, 7, 396-407. Hillis, A.E., Boatman, D., Hart, J. & Gordon, B. (1999). Making sense out of jargon: a neurolinguistic and computational account of jargon aphasia. Neurology, 53, 1813-1824. Hillis, A.E., Wityk, R.J., Tuffiash, E., Beauchamp, N.J., Jacobs, M.A., Barker, P.B., Selnes, O.A. (2001). Hypoperfusion of Wernickes area predicts severity of semantic deficit in acute stroke. Annals of Neurology, 50, 561-566. Hillis, A.E., Wityk, R.J., Barker, P.B., Beauchamp, N.J., Gailloud, P., Murphy, K., Cooper, O., Metter, E.J. (2002). Subcortical aphasia and neglect in acute stroke: the role of cortical hypoperfusion, Brain,125, 1094-1104. Hillis, A.E., Tuffiash, E. & Caramazza, A. (2002). Modality specific deterioration in oral naming of verbs. Journal of Cognitive Neuroscience, 14, 1099-1108. Hillis, A.E., Wityk, R., Barker, P.B., Caramazza, A. (2003). Neural regions essential for writing verbs. Nature Neuroscience, 6, 19-20. Hillis, A.E., Oh, S., Ken, L. (2004). Deterioration of naming nouns versus verbs in primary progressive aphasia. Annals of Neurology, 55, 268-275. Hillis, A.E., Work, M., Breese, E.L., Barker, P.B., Jacobs, M.A. & Maurer, K. (2004). Re-examining the brain regions crucial for orchestrating speech articulation. Brain, 127, 1479-1487. Hillis, A.E., Newhart, M., Heidler, J., Barker, P.B., Herskovits, E., and Degaonkar, M. (2005). The roles of the visual word form area in reading. NeuroImage, 24, 548-559. Reineck, L., Agarwal, S. & Hillis, A.E. (2005). The diffusion-clinical mismatch predicts early language recovery in acute stroke. Neurology, 64, 828-833. Hillis, A.E., Newhart, M., Heidler, J., Barker, P.B., Degaonkar, M. (2005). Anatomy of spatial attention: insights from perfusion imaging and hemispatial neglect in acute stroke. Journal of Neuroscience, 25, 3161-7. Charles, R. & Hillis, A.E. (2005). Posterior Cortical Atrophy: clinical presentation and cognitive deficits compared to Alzheimers Disease. Behavioural Neurology, 16, 15-23. Hillis, A.E., Heidler-Gary, J., Newhart, M., Chang, S., Ken, L. & Bak, T. (2006). Naming and comprehension in primary progressive aphasia: the influence of grammatical word class. Aphasiology, 20, 246-256. Newhart, M., Ken, L., Kleinman, J.T., Heidler-Gary, J., & Hillis, A.E. (2007). Neural networks essential for naming and word comprehension. Cognitive and Behavioral Neurology, 20, 25-30. DeLeon, J., Gottesman, R.F., Kleinman, J.T., Newhart, M., Davis, C., Lee, A., Hillis, A.E. (2007) Neural regions essential for distinct cognitive processes underlying picture naming. Brain, 130, 1408-22. Heidler-Gary, J. & Hillis, A.E. (2007). Distinctions between the dementia in Amyotrophic Lateral Sclerosis with Frontotemporal Dementia and the dementia of Alzheimer''s Disease. Amyotrophic Lateral Sclerosis. Philipose, L.E., Gottesman, R.F., Newhart, M.; Kleinman, J.T.; Herskovits, E.H.; Pawlak, M.A., Marsh, E.B.; Davis, C.; Heidler-Gary, J.; Hillis, A.E. (2008). Neural regions essential for reading and spelling of words and pseudowords. Annals of Neurology. 481-492. Cloutman, L., Gottesman, R., Chaudhry, P., Davis, C., Kleinman, J.T., Pawlak, M., Herskovits, E.H., Kannan, V., Lee, A., Newhart, M., Heidler-Gary, J., Hillis, A.E. (2008)Where (in the brain) do semantic errors come from? Cortex. [Epub ahead of print] Medina, J., Kannan, V., Pawlak, M., Kleinman, J.T., Newhart, M., Davis, C., Heidler-Gary J.E., Herskovits, E.H., Hillis, A.E. (2008) Neural substrates of visuospatial processing in distinct reference frames: evidence from unilateral spatial neglect. Journal of Cognitive Neuroscience. [Epub ahead of print]. Cloutman, L., Gingis, L., Newhart, M., Davis, C., Heidler-Gary, J., Crinion, J., Hillis, A.E. (in press). A neural network critical for spelling. Annals of Neurology.
- Alpha Omega Alpha
- Phi Beta Kappa
- Research Fellow, National Stroke Association
- Fellow, American Heart Association
- Fellow, American Stroke Association
- Derek Denny-Brown Neurological Scholar Award, American Neurological Association
- Norman Geschwind Award in Behavioral Neurology, American Academy of Neurology
- Baltimore Top Docs 2015, Baltimore Magazine, 1/1/15
- Best Doctors in America, 1/1/14
Memberships
- Academy of Aphasia
- American Academy of Neurology, Section on Behavioral Neurology
- American Heart Association Stroke Council, Fellow;Abstract Reviewer for the International Stroke Conference (2003-2007); Scientific Session Chair, 2005, 2006
- American Neurological Association, Scientific Program Committee (2004-2006)
- Clinical Aphasiology Conference, Program committee 1985, 1992; Program Chair 2003; Conference Chair 2004; Steering Committee (2003-present)
- Faculty 1000 Medicine, Evaluation Board
- Society for Neuroscience
- World Federation of Neurology- Research Group on Aphasia and Cognitive Disorders, Chair, 2004-2008
Advances in Diagnosis, Treatment, and Prognosis of Primary Progressive Aphasia
- 601 North Caroline Street, Floor 5 , Baltimore , MD 21287
- phone: 410-955-9441
- fax: 410-955-6402
- 10753 Falls Road, Pavilion II STE 115 , Lutherville , MD 21093
- fax: 410-616-7231
Johns Hopkins University School of Medicine
Board certifications.
- First Health
- Geisinger Health Plan
- HealthSmart/Accel
- Johns Hopkins Health Plans
- Pennsylvania's Preferred Health Networks (PPHN)
- Point Comfort Underwriters
- Private Healthcare Systems (PHCS)
- UnitedHealthcare
- Veteran Affairs Community Care Network (Optum-VACCN)
The Patient Rating score is an average of all responses to physician related questions on the national CG-CAHPS Medical Practice patient experience survey through Press Ganey. Responses are measured on a scale of 1 to 5, with 5 being the best score. Comments are also gathered from our CG-CAHPS Medical Practice Survey through Press Ganey and displayed in their entirety. Patients are de-identified for confidentiality and patient privacy.
Knowledgeable, & Friendly
Dr, Hillis was fabulous!
She was very easy to talk to. Very friendly, nice & pleasant. Extremely knowledgeable In Neurology. I felt at ease In her care.
She is an expert in her field.
Very easy to talk to
Dr. Hillis was wonderful. Easy discussions and direct responses to questions.
Dr. Hillis is exceptional. I have had great physicians and she is still a cut above the rest.
Dr. Hillis was very thorough and a good listener. She was patient while waiting for my responses.
I am recovering from a serious illness and she was incredibly knowledgeable, caring, and concerned
Dr. Hillis is phenomenal!
Dr.Hillis is compassionate, and extremely knowledgeable and professional. She listens to our concerns and addresses them.
Dr. Hillis is a super star!!
Dr. Hillis seems very patient- and definitely knowledgeable. Was not able to offer me much in the way of a discharge plan but that is the nature of the illness. I will likely request a time soon when I can discuss his case in more detail when he is not present.
She was on top of my issue. Understood why we had the visit
We were so pleased with Dr Hillis. Dr Hillis went out of her way to make this experience the best possible. Spent time, explain things, answered questions. Dr Hillis realized that this appoint was very important to us and treated it that way.
Very committed "and goes the extra mile.
Dr. Hillis was caring, supportive, & very knowledgeable.
This was an issue with anomia, not a physical problem.
Dr Hillis is a pleasure to work with
Helped us understand the diagnosis and how we can manage it.
She is very concerned and friendly
Dr. Hillis is very thorough, knowledgeable, and has a genuine concern for her patients.
Dr. Hillis is professional and took the time to listen to my concerns and made recommendations.

Assessment Of Afasia in Stroke Patients: Case Study
- Dwi Febryanto Universitas Diponegoro
- Retnaningsih Universitas Diponegoro
- Fitria Handayani Universitas Diponegoro
Introduction: Aphasia is understood as difficulty in understanding or producing language caused by disorders involving the brain hemispheres. Early assessment of aphasia is very important to prevent the emergence of telegraphic speech styles, improve welfare, independence, social participation, quality of life, reduce length of stay and care costs, but there is little literature on this subject, especially in stroke patients. Purpose : This study is to provide an overview of the assessment of aphasia in stroke patients. Methods: The design of a case study involving 6 participants and data collection was carried out by conducting an assessment using the Language Aphasia Screening Test (LAST) instrument which was monitored for 3 days, including monitoring errors for naming images, monitoring mismatches repeating words and sentences, monitoring spontaneous pronunciation, monitoring image comprehension, monitoring comprehension of verbal instructions. Results: A total of 6 ischemic stroke patients were found wrong in repeating words and sentences. Conclusion: In aphasic stroke patients all language modalities are impaired, ranging from spontaneous speech, repetition, naming, language comprehension, reading and writing
Al-Khawaja, I., Wade, D. T., & Collin, C. F. (1996). Bedside screening for aphasia: a comparison of two methods. Journal of Neurology, 243(2), 201-204. Article | Crossref
Berthier, M. L. (2005). Poststroke aphasia. Drugs & aging, 22(2), 163-182. Article | Crossref
Chang, T., Gajasinghe, S., & Arambepola, C. (2015). Prevalence of stroke and its risk factors in urban Sri Lanka: population-based study. Stroke, 46(10), 2965-2968. Article | Crossref
Corallo, F., Bonanno, L., Buono, V. L., De Salvo, S., Rifici, C., Pollicino, P., ... & Bramanti, A. (2017). Augmentative and alternative communication effects on quality of life in patients with locked-in syndrome and their caregivers. Journal of Stroke and Cerebrovascular Diseases, 26(9), 1929-1933. Article | Crossref
Dronkers, N., & Baldo, J. V. (2010). Language: Aphasia. In Encyclopedia of Neuroscience (pp. 343-348). Elsevier Ltd. Article | Crossref
El Hachioui, H., Visch-Brink, E. G., de Lau, L. M., van de Sandt-Koenderman, M. W., Nouwens, F., Koudstaal, P. J., & Dippel, D. W. (2017). Screening tests for aphasia in patients with stroke: a systematic review. Journal of neurology, 264(2), 211-220. Article | Crossref
Enderby, P. M., Wood, V. A., Wade, D. T., & Hewer, R. L. (1986). The Frenchay Aphasia Screening Test: a short, simple test for aphasia appropriate for non-specialists. International rehabilitation medicine, 8(4), 166-170. Article | Crossref
Erdodi, L., & Roth, R. (2017). Low scores on BDAE Complex Ideational Material are associated with invalid performance in adults without aphasia. Applied Neuropsychology: Adult, 24(3), 264-274. Article | Crossref
Flamand-Roze, C., Falissard, B., Roze, E., Maintigneux, L., Beziz, J., Chacon, A., ... & Denier, C. (2011). Validation of a new language screening tool for patients with acute stroke: the Language Screening Test (LAST). Stroke, 42(5), 1224-1229. Article | Crossref
Flowers, H. L., Flamand-Roze, C., Denier, C., Roze, E., Silver, F. L., Rochon, E., ... & Langdon, C. (2015). English adaptation, international harmonisation, and normative validation of the Language Screening Test (LAST). Aphasiology, 29(2), 214-236. Article | Crossref
Indonesia, P. P. N. (2016). Standar Intervensi Keperawatan Indonesia. Jakarta: PPNI. Book
Kemenkes, R. I. (2014). Situasi kesehatan jantung. Pusat Data Dan Informasi Kementerian Kesehatan RI. Article
Kertesz, A. (2007). Is there a need for standardized aphasia tests? Why, how, what and when to test aphasics. Journal Aphasiology. 313-317. Article | Crossref
Koenig-Bruhin, M., Vanbellingen, T., Schumacher, R., Pflugshaupt, T., Annoni, J. M., Müri, R. M., ... & Nyffeler, T. (2016). Screening for language disorders in stroke: German validation of the language screening test (LAST). Cerebrovascular diseases extra, 6(1), 27-31. Article | Crossref
Lumbantobing, S. M. (2000). Neurologi klinik pemeriksaan fisik dan mental. Jakarta: Balai Penerbit FK UL. Book
Marshall, R. C., & Campbell, S. (2013). Treatment of Aphasia In The Acute Care Setting : Getting Off on the Right Foot. Lexington: University of Kentucky. Article
Pinzon, R., & Asanti, L. (2010). Awas stroke! pengertian, gejala, tindakan, perawatan dan pencegahan. Penerbit Andi. Book
Poslawsky, I. E., Schuurmans, M. J., Lindeman, E., & Hafsteinsdóttir, T. B. (2010). A systematic review of nursing rehabilitation of stroke patients with aphasia. Journal of clinical nursing, 19(1‐2), 17-32. Article | Crossref
Prins, R., & Maas, W. (2002). Afasia Deskripsi Pemeriksaan Penanganan. Jakarta: Fakultas Kedokteran Universitas Indonesia. Article
Shehata, G. A., El Mistikawi, T., Al Sayed, K. R., & Hassan, H. S. (2015). The effect of aphasia upon personality traits, depression and anxiety among stroke patients. Journal of affective disorders, 172, 312-314. Article | Crossref
Silbernagl, S., & Lang, F. (2007). Teks & atlas berwarna patofisiologi. Jakarta: EGC. Book
Suwardianto, H. (2018). Level Of Perception Emergency Skills In Youth Red Cross. Journal Of Nursing Practice, 2(1), 17-24. Article | Crossref
Suwardianto, H., & Rimawati, R. (2018). Explicit Instruction Model (EIM): Daily Training Emergencies Preparedness (DTEP) Toward Skills of Participants the Youth Red Cross. In The 2nd Joint International Conferences (Vol. 2, No. 2, pp. 403-410). Article
Suwardianto, H., Richard, S. D., Prasetyo, A., & Utami, R. S. (2017). PHYSICAL FUNCTION–TARDIVE DYSKINESIA (PFTD) ON CRITICAL PATIENTS IN INTENSIVE CARE UNIT. Jurnal Ners, 12(2), 196-204. Article | Crossref
Venketasubramanian, N., Tan, L. C., Sahadevan, S., Chin, J. J., Krishnamoorthy, E. S., Hong, C. Y., & Saw, S. M. (2005). Prevalence of stroke among Chinese, Malay, and Indian Singaporeans: a community-based tri-racial cross-sectional survey. Stroke, 36(3), 551-556. Article | Crossref
Wang, W., Jiang, B., Sun, H., Ru, X., Sun, D., Wang, L., ... & Chen, Z. (2017). Prevalence, incidence, and mortality of stroke in China: results from a nationwide population-based survey of 480 687 adults. Circulation, 135(8), 759-771. Article | Crossref
Weiner, H., & Levitt, L. P. (2000). Buku saku neurologi. Jakarta. EGC. Book
Yusiana, M. A & Suwardianto, H. (2014). The Effectiveness Of Deep Breathing and Slow Stroke Back Massage to Decrease The Blood Pressure On A Patient With Hypertension. Indonesian Nursing Journal of Education and Clinic (INJEC). 1(1). 31-39. Article | Crossref

How to Cite
- Endnote/Zotero/Mendeley (RIS)

|
|
|
|
| and Scope |
| Team |


Current Issue
Published by:
Nursing Study Program
Institut Ilmu Kesehatan STRADA Indonesia
Manila Street Number 37, Sumberece, Kediri, East Java, Indonesia 64133
Website: https://thejnp.org/index.php/jnp
Email: [email protected]
Whatsapp: +62 857 4895 9055
Journal Of Nursing Practice is licensed under a Creative Commons Attribution-ShareAlike 4.0 International License .

Case Study - Moderate Broca’s Aphasia
Moderate broca's aphasia (brain injury).

Time since brain injury: 3 years
- Relatively good comprehension in context
- Sentence-level reading
- Good awareness of deficits and breakdowns
- Difficulty finding specific words in conversation
- Limited ability to write single words
- Speech was mostly single nouns
She completed a 8-week program with tDCS
And my aphasia coach software homework daily..

- Learned a wider variety of strategies to help during breakdowns
- Began speaking in grammatical sentences of 4+ words independently
- Learned how to use her phone as a strategy
- Self-correcting errors in verb tense and grammatical structure
- Improved ability to accurately write longer words and sentences
- What's Happening Here?
- Departments & Units
- Academic Programs
- How To Apply
- Financial Wellness
- CHS Global Initiatives
- Health Education Building
- Active Research Funding
- Undergraduate Research
- Centers and Institutes
- Samaritan's Touch
- CSD Academic Clinic
- Voice and Swallow Clinic
- Runner’s Clinic
- UK Pediatric Therapies
- Dean's Messages
- Programming
- Diversity Healthcare Program
- DEI Program Grant
- Online Order Forms
- Business/HR Forms
- Submit Feedback to Staff Council
- Helpdesk Request
- IT Tutorials
- New Publication Request
- Branding Resources
- Graphic Design Request
- Faculty Handbook
- Faculty Council
- Order CHS Apparel
- Compliance and Standards
- Education Abroad
- Student Leadership and Involvement
- Graduate/Professional Student Resources
- Other Resources
- Office of the Dean
- Office of Academic Affairs & Undergraduate Education
- Office of Advancement
- Office of Assessment
- Creative Services
- Office of Faculty Advancement and Clinical Engagement
- Office of Finance and Administration
- Office of Student Affairs
- Office of Technology Services
- Research & Scholarship Support Program
- External Grant Proposal Review Program
- Faculty Toolkit for Undergraduate Research
- Preceptor Resources
- Photo Galleries
- Gateway Magazine
- The Way Podcast
- Hall of Fame Inductees
- Alumni Info Update
- Class Notes
- Opportunities to Give
- Ways to Give
- Advancement Council
- Emeritus Faculty
- Organizational Chart
Aphasia Classes Solve Escape Room for Final Project

By Ryan Clark CHS Communications Director
“This is the first class where I’ve ever done something like this.”
That was how Abby Short described Thursday’s CSD 677: Aphasia and Related Disorders class, where the graduate students were tasked with solving an Escape Room as their final project.
“I think what’s so cool about it is it’s so hands-on,” said Short, a first-year graduate student in the Communication Sciences and Disorders department . “It’s teaching us those practical, clinical skills, giving us a true learning experience in how to be a clinician in the future.”

“After reading about Educational Escape Rooms in nursing, as well as athletic training, and speaking with colleagues in (Medical Laboratory Science), I knew this innovative, team-based project aligned with the objectives of my final class project,” Page said. “I strive to develop engaging and exciting projects that raise awareness of aphasia and challenge students' creative critical thinking skills.”
Every four minutes someone acquires aphasia, which is often caused by a stroke, the National Aphasia Association reports. In 2022 in Kentucky, diseases of the cerebrovascular system (stroke) were the fifth leading cause of death, and one-third of strokes result in aphasia. Out of 60 diseases and 15 health conditions, aphasia has the largest negative impact on health-related quality of life.
“As future speech-language pathologists, students in this class will likely serve individuals with aphasia,” Page said. “They require a clear understanding of aphasia’s impact on quality of life to provide person-centered care.”
The importance — and the fun, were not lost on the students.
“We’re all having a really good time, working hard,” Short said. “I’m used to sitting down, and it has to be quiet to take an exam. To have a group learning experience like this, to play a huge role in the completion of the class, is awesome.”
Page said she hopes students can get a better understanding of aphasia.
“The aim is for students to better understand aphasia by applying course content to solve puzzles related to a person with aphasia,” she said. “Each group of four students will receive a different case study and solve puzzles related to the individual's medical history, assessment, goals, and appropriate treatments. By integrating educational content into a game format, I hope to make learning about aphasia more dynamic and enjoyable.”
“In real life, we may not always have a textbook with us,” Short said. “I feel like (Dr. Page) has prepared us well. We’re all having a good time.”
Students and individuals with aphasia will also educate the community in an effort to spread aphasia awareness. They will present:
- Tuesday, Aug. 6 at 10 a.m. at Southland Christian Church and at 12:30 p.m. at the High Street YMCA.
- Wednesday, Aug. 7 at 2 p.m. at Central Library in Fayette County
See our PHOTO GALLERY of the class here!

College of Health Sciences | 900 South Limestone | University of Kentucky | Phone - (859) 323-1100 |
- © University of Kentucky
- An Equal Opportunity University
- Accreditation
An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
Preview improvements coming to the PMC website in October 2024. Learn More or Try it out now .
- Advanced Search
- Journal List
- Front Neurol
- PMC11236691
Perspective on the clinical management of post-stroke aphasia and dysphagia using repetitive transcranial magnetic stimulation (rTMS)
Anastasios m. georgiou.
1 The Cyprus Rehabilitating Aphasia and Dysphagia (C-RAD) Lab, Department of Rehabilitation Sciences, Cyprus University of Technology, Limassol, Cyprus
2 The Brain and Neurorehabilitation Lab, Department of Rehabilitation Sciences, Cyprus University of Technology, Limassol, Cyprus
1 Introduction
Stroke, a prevalent cause of disability worldwide, presents individuals with a range of formidable challenges; notably aphasia, reported at rates of 7%−77% across high- and middle-income countries ( 1 ), and dysphagia, with incidence rates reaching up to 80% ( 2 ). Despite their distinct manifestations, these conditions are pivotal for survival, human communication, and overall quality of life (QoL) post-stroke. Within the domain of medical speech pathology, aphasia and dysphagia have historically been key areas of therapeutic intervention, primarily through behavioral therapies. However, the advent of technological advancements has fueled interest in exploring noninvasive brain stimulation techniques (NIBST), such as repetitive transcranial magnetic stimulation (rTMS), for their potential in leveraging neuroplasticity to address these post-stroke deficits.
2 Repetitive TMS applications in stroke-induced aphasia and dysphagia
Since the 1990s, rTMS has attracted attention as a promising therapy for treating problems associated with various neurological disorders, notably including aphasia and dysphagia post-stroke. This interest is driven by the safety and non-invasive nature of rTMS, with ongoing research uncovering new treatment options. Several factors, as analyzed below, are intricately related to the clinical effects of rTMS in the treatment of stroke induced aphasia and dysphagia.
2.1 The role of brain laterality
For over a century after Broca's and Wernicke's first reports, language has been hypothesized to be lateralized to the inferior frontal and superior temporal areas of the left hemisphere in adults. Building on this understanding, research applying rTMS as a treatment for aphasia following stroke commonly adopts one of two strategies that are principally informed by models of post-stroke brain reorganization. The first strategy involves down-regulating neuronal activity in right hemisphere regions, typically by targeting contralateral areas homologous to the lesion, using inhibitory stimulation protocols that apply low-frequency rTMS [e.g., ( 3 – 5 )]. The second aims to increase activity in perilesional areas of the left hemisphere using excitatory forms of stimulation that employ high-frequency rTMS [e.g., ( 6 , 7 )]. Some scientists have used a combined approach involving both suppressing activity in the right hemisphere and up-regulating the left hemisphere utilizing bilateral rTMS [e.g., ( 8 , 9 )]. In any case, it is well established that language processes rely on bilaterally distributed brain networks and therefore the role of the right hemisphere is neither disregarded nor overlooked. For instance, while the lesion size in the left hemisphere is the most significant predictor of stroke induced aphasia recovery after 6 months, the volume of the long segment of the right arcuate fasciculus is a good predictor of longitudinal recovery as well ( 10 ). Hence, suppression of neuronal activity in the right hemisphere targets the hyperactive right pars triangularis (pTr), which works maladaptively for recovery, in order to facilitate modulation of the right pars opercularis (pOp) that in turn promotes language recovery via secondary pathways [see ( 11 ) for details].
While the concept of brain laterality is widely accepted within the aphasia research community, the cerebral control of swallowing presents a more debated topic among dysphagia researchers. Cumulative research findings suggest that both reflexive and volitional swallowing are regulated by various cortical and subcortical regions (i.e., primary motor cortex, primary somatosensory cortex, insula, cingulate cortex, supplementary motor area, premotor cortex, auditory cortex, inferior frontal gyrus, parietooccipital and prefrontal cortex, operculum, putamen, thalamus, global pallidus, internal capsule, cerebellum, corpus callosum, basal ganglia, caudate, pons, midbrain and inferior parietal lobule) and damage to these areas can lead to dysphagia. These areas are interconnected in distinct groups within and between both hemispheres - for a comprehensive review on the topic, refer to Cheng et al. ( 12 ) and the references within. Even though several human studies indicate hemispheric dominance in swallowing, either favoring the left [e.g., ( 13 – 15 )] or the right [e.g., ( 16 , 17 )], the degree of laterality varies among individuals with activation patterns and laterality shifting between preparation and execution stages ( 12 ). Additionally, it remains unclear if dysphagia symptoms are more likely to arise from lesions in the left or right hemisphere. Some studies find no link between the lesioned hemisphere and dysphagia severity, while others suggest more severe dysphagia with either right of left hemispheric lesions ( 12 ). Given these complexities and refraining from necessarily viewing the right hemisphere as maladaptive for swallowing improvement post-stroke, as posited by the interhemispheric inhibition model ( 18 ), both excitatory and inhibitory rTMS paradigms have been applied unilaterally to either the left or right hemispheres, or bilateral stimulation has been utilized [e.g., ( 19 – 27 )]. It is important to note though that several studies have not reported favorable outcomes with rTMS for post-stroke swallowing rehabilitation ( 28 – 31 ). So, maybe it is insufficient to solely take into consideration the various nodes of swallowing and lesion location. Of particular interest are the key areas influencing long-term recovery from post-stroke dysphagia. Evidence suggests that in ischemic stroke, the risk of aspiration is more likely to persist beyond the first week when both the frontal operculum and insular cortex are affected ( 32 ). Furthermore, damage to the insular cortex may impair ipsilesional cortical reorganization and slow recovery, with lesions affecting more than 50% of that node linked to impaired oral intake after 4 weeks and those affecting < 25% of it associated with recovery ( 33 ). Given this, rTMS can be strategically used to target the insular cortex, potentially enhancing cortical reorganization, and accelerating the recovery of swallowing function in stroke patients.
Recently, researchers have begun to investigate the potential of the cerebellum to facilitate recovery for post-stroke aphasia and dysphagia using rTMS. Specifically, low-frequency rTMS has been explored for post-stroke aphasia ( 34 ), while three studies ( 35 – 37 ) have applied high-frequency rTMS to the cerebellum for dysphagia, yielding favorable outcomes. These findings suggest that rTMS targeting the cerebellum holds promise for promoting recovery in both conditions. With regards to aphasia, it is assumed that neuromodulation of the right cerebellum regulates functional connectivity between the right cerebellum and the cerebral areas involved in language processing thereby facilitating language gains. With regards to dysphagia, the physiologic mechanism of action involves the cerebellum indirectly modulating neuronal activity not only in the brainstem but also in other brain areas involved in swallowing, such as the insular cortex and cerebrum.
Nonetheless, there is currently no consensus in relation to the optimal stimulation site (i.e., affected, unaffected, or both hemispheres, or cerebellum) and precise stimulation parameters for dysphagia and aphasia post-stroke. This lack of agreement highlights the urgent need for additional research to find the most effective rTMS approaches for treating these conditions. Additionally, standardization of stimulation protocols and rigorous investigation into individual patient characteristics may lead to the development of personalized treatment strategies.
2.2 Importance of baseline clinical, neuroradiologic and instrumental assessment
Baseline clinical and neuroradiologic assessments for aphasia are essential components for the successful application of rTMS in post-stroke rehabilitation. Comprehensive baseline assessments enable the (i) determination of the nature and extent of aphasia, (ii) recognition of potential risks and (iii) possible forecasting of recovery trajectories. Such assessments also guide more precise and effective intervention strategies tailored to individual patient needs. Precise information on the syndrome (e.g., global vs. motor vs. sensory aphasia) and its severity is crucial because these factors are associated with different prognoses. Recovery from aphasia is a dynamic process, and it is often observed that one type of aphasia evolves into another ( 38 , 39 ). For instance, global aphasia typically evolves into Broca's aphasia ( 40 ), indicating that rTMS protocols may preferably focus initially on broader language functions before targeting speech production. Similarly, patients with vascular etiology presenting with Broca's aphasia, which often improves to anomic aphasia ( 40 ), may benefit from rTMS protocols designed to progressively enhance fluency and word retrieval. For patients with vascular etiology presenting with Wernicke's aphasia, who show potential for comprehension improvement and evolution to conduction or transcortical sensory aphasia ( 40 ), rTMS may focus on enhancing auditory comprehension, semantic processing, and naming. Understanding these prognostic nuances would allow clinicians to tailor rTMS protocols to the specific type of aphasia and its likely progression, optimizing rehabilitation outcomes for post-stroke patients. Such considerations are currently lacking in the existing literature.
Baseline clinical, neuroradiologic, and instrumental assessment for post-stroke dysphagia is also very important when considering the application of rTMS for rehabilitation purposes. Such assessments serve as the foundation for understanding the underlying physiological and neurological mechanisms contributing to stroke induced dysphagia. Clinical assessment provides valuable information on the patient's swallowing function, including the severity of dysphagia and associated symptoms. Neuroradiologic examinations help with the identification of structural abnormalities or lesions in the brain that may be contributing to swallowing difficulties, offering insights into the neuroanatomical basis of dysphagia. Instrumental assessments, such as videofluoroscopy (VFS) or fiberoptic endoscopic examination of swallowing (FEES), allow for real-time visualization of the swallowing process, enabling clinicians to identify specific impairments and tailor interventions accordingly. By conducting comprehensive assessments across these domains, clinicians can gain a holistic understanding of the patient's dysphagia profile, thereby informing the development of targeted rTMS treatment protocols aimed at addressing the underlying neural mechanisms and improving swallowing function.
2.3 Measuring and control in rTMS studies: aphasia vs. dysphagia post-stroke
In studying dysphagia and aphasia, researchers encounter unique challenges in measuring and controlling variables. Dysphagia research benefits from a more straightforward measurement process due to the universal nature of swallowing as a physiological function. This universality enables consistent assessment and interpretation of treatment outcomes across patients from different cultural backgrounds. On the other hand, aphasia studies face complexities in terms of measuring variables due to the diverse linguistic and cultural contexts in which they are conducted. In aphasia research standardizing assessment tools and outcome measures across various linguistic contexts is very important as it allows for meaningful comparisons across studies and ensures the validity and reliability of research findings.
Moreover, in comparison to dysphagia trials, controlling factors in aphasia studies, such as the implementation of behavioral interventions, introduces additional challenges. Behavioral therapies for aphasia involve a diverse range of approaches, with varying regimens applied across different studies. With regards to speech and language therapy (SALT) that is used as an adjuvant therapy in rTMS trials, significant inconsistencies in SALT types and intensities are observed across studies and such inconsistencies can impact the interpretation of results and the ability to make meaningful comparisons across studies. For instance, one relevant study focused on language comprehension and expression with a 30-min program ( 41 ), another trial implemented a 30- min SALT program focusing on naming ( 42 ) and another research utilized a 45-min SALT regimen targeting the reactivation of word retrieval ( 3 ). Other studies have implemented a 45-min SALT regimen tailored to address patient-specific language difficulties [e.g., ( 43 – 45 )]. The lack of SALT standardization across studies hampers the assessment of rTMS efficacy, making it difficult to discern the specific outcomes of rTMS from those of SALT. As a result, the true extent of improvement in language abilities ascribed to rTMS remains uncertain. On the other hand, dysphagia studies experience fewer differences in controlling factors, as interventions often focus on physiological aspects of swallowing that are less influenced by cultural and/or linguistic factors. Overall, while dysphagia research benefits from a more streamlined tracking process and simpler control factors, aphasia studies entail greater complexity due to linguistic and cultural diversity, especially when considering bilingual or multilingual stroke patients.
The possibility that rTMS may prime the brain to receive behavioral aphasia and dysphagia therapy raises interesting questions about the possible synergistic effects of combining these interventions. Repetitive TMS has demonstrated neuromodulatory effects on cortical excitability and neural plasticity, which, theoretically, can enhance the brain's responsiveness to subsequent interventions. Nevertheless, the extent to which rTMS primes the brain for behavioral treatment, particularly in the context of post-stroke aphasia and dysphagia, remains an active area of research. With regards to post-stroke aphasia, recent studies indicate that when rTMS is used as a standalone treatment, it holds promise in facilitating language and/or cognitive gains [e.g., ( 5 , 46 – 48 )]. With regards to stroke-induced dysphagia recent (MAs) analyses have indicated mixed outcomes [e.g., ( 49 – 52 )]. Therefore, the question arises: is behavioral therapy indispensable, or can it be substituted by rTMS treatments? Well, while rTMS has the capacity to modulate neuronal activity directly and indirectly, potentially accelerating neuroplasticity and recovery, behavioral therapy provides essential benefits that rTMS alone cannot fully induce, such as personalized strategies for daily functioning and psychosocial support. Thus, an integrated approach that combines both behavioral therapy and rTMS might offer the most comprehensive and effective treatment for stroke induced aphasia and dysphagia. Additional research is required to comprehensively understand the potential of rTMS as a standalone therapy and to determine the best protocols for its use in conjunction with behavioral treatments.
2.4 Assessing the merits of meta-analyses
A notable shortcoming of both systematic review (SRs) and meta-analyses (MAs) is that their conclusions can vary significantly with the inclusion or exclusion of certain studies. Also, the credibility of this type of research relies on the quality of the studies SRs and MAs include, as biased studies can exacerbate overall bias and therefore lead to misleading conclusions ( 53 ). In the field of rTMS for post-stroke aphasia and dysphagia, there is currently a noticeable trend among researchers to generate multiple MAs, surpassing the number of available primary studies. While MAs are valuable tools for synthesizing existing evidence and offering insights into intervention effectiveness, the disproportionate increase in MAs, in comparison to primary studies, raises concerns regarding the reliability and robustness of their findings. This surge in the number of MAs leads to redundancy and a decrease in the overall quality of research in these areas and therefore highlights the importance of maintaining a balance between conducting MAs and primary studies.
Umbrella reviews (URs) enhance the objectivity of SRs by offering a comprehensive overview and quality control. They also streamline research and foster collaboration, thus reducing redundancy and improving research quality amidst the proliferation of MAs. A recent UR on the effectiveness of rTMS for dysphagia in stroke patients ( 54 ) revealed significant overlap among studies included in various MAs, which is unsurprising given the disproportionately larger number of MAs compared to primary studies on the topic. Repeatedly including the same studies in MAs consolidates evidence and strengthens statistical power, yet it may also reinforce specific result patterns, emphasize potentially misleading outcomes, and result in an overly precise yet inaccurate estimation of intervention effectiveness. This issue becomes particularly worrisome when SRs receive low ratings in methodological quality, as it casts doubt on the reliability of conclusions derived from the MAs contained within these SRs. This was the case for two umbrella reviews, one for rTMS post-stroke aphasia ( 55 ) the other one for rTMS post-stroke dysphagia ( 54 ). Both URs found that published SRs, with or without MAs, of randomized controlled trials (RCTs) on the topics exhibit subpar methodological quality and therefore the evidence concerning the effectiveness of rTMS in promoting language and swallowing improvements post-stroke is inconclusive.
To address challenges associated with SRs and MAs, researchers should prioritize methodological rigor in their execution. Specifically, efforts to enhance the quality of SRs should align with established guidelines, alongside fostering collaboration among researchers to avoid duplication of efforts and ensure that the synthesis of evidence is comprehensive and reliable.
2.5 The importance of dissociating clinical from statistical significance
The distinction between clinical and statistical significance is very important in rTMS research for post-stroke aphasia and dysphagia. While statistical significance is very important for researchers, clinical significance is more important for individuals with aphasia or dysphagia post-stroke and their caregivers. Despite achieving statistical significance in treatment outcomes, the failure to accommodate the needs and fulfill the expectations of participants and their caregivers underscores the importance of measuring clinical significance. Quality of life encompasses a wide range of aspects, including physical health, mental health, emotional wellbeing, and the ability to engage in meaningful activities. For patients recovering from stroke, improvements in these areas can be just as important as neurological recovery. Therefore, incorporating QoL measures tailored to post-stroke aphasia and dysphagia would allow for a comprehensive understanding of treatment effectiveness and its implications on patients' lives. Despite the significance of QoL measures, they are seldom utilized in rTMS studies addressing these conditions. To advance research in those fields, it is imperative to integrate ecological outcome measures that reflect the real-world impact of interventions, facilitating a more holistic evaluation of treatment effectiveness and patient outcomes.
3 Discussion
Despite ongoing and increasing research on rTMS in stroke-induced aphasia and dysphagia, as of 2020, Lefaucheur et al. ( 56 ) concluded in their extensive review of rTMS studies up to 2018 that the evidence supporting rTMS effects on post-stroke aphasia remains insufficient for drawing definitive conclusions and making recommendations. Similarly, with regards to dysphagia post-stroke, Lefaucheur et al. ( 56 ) assert that due to the variability in results and protocols, it remains uncertain whether rTMS provides therapeutic benefits for patients experiencing persistent dysphagia in the post-acute or chronic stages of stroke. But, given that post-stroke dysphagia often exhibits rapid recovery, it is advised to administer rTMS in the early stages of the disease to maximize therapeutic benefits ( 56 ).
The use of rTMS as a treatment option for post-stroke aphasia and dysphagia shows potential but also poses challenges. While rTMS is a non-invasive approach with the potential to induce neuroplastic changes, making it a possibly effective intervention for both conditions, the current state of research reveals uncertainty. The heterogeneity in study methodologies, stimulation protocols, and outcome measures and the lack of consensus regarding optimal stimulation sites and ecological outcome measures make it difficult to synthesize evidence, develop standardized treatment guidelines and enhance the clinical relevance of findings. Also, the variability in tracking variables and controlling factors, particularly in post-stroke aphasia trials, highlights the importance of standardization and international collaboration to enable meaningful comparisons across diverse linguistic and cultural contexts. To improve the precision and applicability of future research, studies must clearly define target groups and include robust risk stratification based on key prognostic markers for aphasia and dysphagia, such as lesion size and location, overall health status, and baseline functional abilities. For instance, the predictive swallowing score (PRESS) model ( 57 ) uses easily measured predictors suitable for various clinical settings that, according to the researchers, can be further refined by adding instrumental biomarkers and advanced neuroimaging, improving decision-making, and promoting personalized medicine for patients that have suffered a stroke. Addressing all the aforementioned gaps and challenges, this non-invasive neuromodulation approach may become an effective add-on or standalone treatment for improving functional outcomes and QoL for individuals affected by stroke induced aphasia and/or dysphagia.
Author contributions
AG: Writing – review & editing, Methodology, Conceptualization.
Funding Statement
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
Conflict of interest
The author declares that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
- My Bibliography
- Collections
- Citation manager
Save citation to file
Email citation, add to collections.
- Create a new collection
- Add to an existing collection
Add to My Bibliography
Your saved search, create a file for external citation management software, your rss feed.
- Search in PubMed
- Search in NLM Catalog
- Add to Search
The resolving stroke and aphasia. A case study with computerized tomography
- PMID: 426669
- DOI: 10.1001/archneur.1979.00500400087016
A 39-year-old man suffered an intracerebral hemorrhage in the region of the left internal capsule deep to Wernicke's area. The location of the lesion was confirmed by computerized tomography (CT) performed two days postictally. Two weeks after admission, the Boston Diagnostic Aphasia Examination (BDAE) diclosed Wernicke's aphasia. We hypothesize that the hematoma exerted pressure on Wernicke's cortical area, thus causing the resulting Wernicke's aphasia at that time. A CT scan three months later showed absorption of the hematoma, with a residual low-density lesion deep to Wernicke's area, in the region of the arcuate fasciculus. At that time, BDAE testing disclosed a mild conduction aphasia. Serial CT scanning combined with discriminating clinical evaluation of aphasia provides a valuable opportunity for study of the processes underlying stroke resolution and aphasia.
PubMed Disclaimer
Similar articles
- Etiology of stroke in patients with Wernicke's aphasia. Knepper LE, Biller J, Tranel D, Adams HP Jr, Marsh EE 3rd. Knepper LE, et al. Stroke. 1989 Dec;20(12):1730-2. doi: 10.1161/01.str.20.12.1730. Stroke. 1989. PMID: 2595736
- Neuroanatomical correlates of the post-stroke aphasias studied with cerebral blood flow SPECT scanning. Jodzio K, Gasecki D, Drumm DA, Lass P, Nyka W. Jodzio K, et al. Med Sci Monit. 2003 Mar;9(3):MT32-41. Med Sci Monit. 2003. PMID: 12640350
- Lesion localization in aphasia with cranial computed tomography and the Boston Diagnostic Aphasia Exam. Naeser MA, Hayward RW. Naeser MA, et al. Neurology. 1978 Jun;28(6):545-51. doi: 10.1212/wnl.28.6.545. Neurology. 1978. PMID: 565884
- Global aphasia due to thalamic hemorrhage: a case report and review of the literature. Kumar R, Masih AK, Pardo J. Kumar R, et al. Arch Phys Med Rehabil. 1996 Dec;77(12):1312-5. doi: 10.1016/s0003-9993(96)90199-9. Arch Phys Med Rehabil. 1996. PMID: 8976318 Review.
- Post-stroke language disorders. Sinanović O, Mrkonjić Z, Zukić S, Vidović M, Imamović K. Sinanović O, et al. Acta Clin Croat. 2011 Mar;50(1):79-94. Acta Clin Croat. 2011. PMID: 22034787 Review.
Publication types
- Search in MeSH
Related information
Linkout - more resources, full text sources.
- Silverchair Information Systems
- Citation Manager
NCBI Literature Resources
MeSH PMC Bookshelf Disclaimer
The PubMed wordmark and PubMed logo are registered trademarks of the U.S. Department of Health and Human Services (HHS). Unauthorized use of these marks is strictly prohibited.
- Introduction
- Conclusions
- Article Information
eTable 1. BWHS Data Collection on Racism
eTable 2. Multivariable Associations of Perceived Interpersonal Racism With Incident Definite Stroke Confirmed by Medical Records
eTable 3. Associations of Perceived Interpersonal Racism With Incident Stroke by Subgroups
eFigure 1. Kaplan-Meier Survival Curve for the Association Between Perceived Racism in Everyday Life With Incident Stroke
eFigure 2. Kaplan-Meier Survival Curve for the Association Between Perceived Racism in Job, Housing and by the Police With Incident Stroke
Data Sharing Statement
See More About
Sign up for emails based on your interests, select your interests.
Customize your JAMA Network experience by selecting one or more topics from the list below.
- Academic Medicine
- Acid Base, Electrolytes, Fluids
- Allergy and Clinical Immunology
- American Indian or Alaska Natives
- Anesthesiology
- Anticoagulation
- Art and Images in Psychiatry
- Artificial Intelligence
- Assisted Reproduction
- Bleeding and Transfusion
- Caring for the Critically Ill Patient
- Challenges in Clinical Electrocardiography
- Climate and Health
- Climate Change
- Clinical Challenge
- Clinical Decision Support
- Clinical Implications of Basic Neuroscience
- Clinical Pharmacy and Pharmacology
- Complementary and Alternative Medicine
- Consensus Statements
- Coronavirus (COVID-19)
- Critical Care Medicine
- Cultural Competency
- Dental Medicine
- Dermatology
- Diabetes and Endocrinology
- Diagnostic Test Interpretation
- Drug Development
- Electronic Health Records
- Emergency Medicine
- End of Life, Hospice, Palliative Care
- Environmental Health
- Equity, Diversity, and Inclusion
- Facial Plastic Surgery
- Gastroenterology and Hepatology
- Genetics and Genomics
- Genomics and Precision Health
- Global Health
- Guide to Statistics and Methods
- Hair Disorders
- Health Care Delivery Models
- Health Care Economics, Insurance, Payment
- Health Care Quality
- Health Care Reform
- Health Care Safety
- Health Care Workforce
- Health Disparities
- Health Inequities
- Health Policy
- Health Systems Science
- History of Medicine
- Hypertension
- Images in Neurology
- Implementation Science
- Infectious Diseases
- Innovations in Health Care Delivery
- JAMA Infographic
- Law and Medicine
- Leading Change
- Less is More
- LGBTQIA Medicine
- Lifestyle Behaviors
- Medical Coding
- Medical Devices and Equipment
- Medical Education
- Medical Education and Training
- Medical Journals and Publishing
- Mobile Health and Telemedicine
- Narrative Medicine
- Neuroscience and Psychiatry
- Notable Notes
- Nutrition, Obesity, Exercise
- Obstetrics and Gynecology
- Occupational Health
- Ophthalmology
- Orthopedics
- Otolaryngology
- Pain Medicine
- Palliative Care
- Pathology and Laboratory Medicine
- Patient Care
- Patient Information
- Performance Improvement
- Performance Measures
- Perioperative Care and Consultation
- Pharmacoeconomics
- Pharmacoepidemiology
- Pharmacogenetics
- Pharmacy and Clinical Pharmacology
- Physical Medicine and Rehabilitation
- Physical Therapy
- Physician Leadership
- Population Health
- Primary Care
- Professional Well-being
- Professionalism
- Psychiatry and Behavioral Health
- Public Health
- Pulmonary Medicine
- Regulatory Agencies
- Reproductive Health
- Research, Methods, Statistics
- Resuscitation
- Rheumatology
- Risk Management
- Scientific Discovery and the Future of Medicine
- Shared Decision Making and Communication
- Sleep Medicine
- Sports Medicine
- Stem Cell Transplantation
- Substance Use and Addiction Medicine
- Surgical Innovation
- Surgical Pearls
- Teachable Moment
- Technology and Finance
- The Art of JAMA
- The Arts and Medicine
- The Rational Clinical Examination
- Tobacco and e-Cigarettes
- Translational Medicine
- Trauma and Injury
- Treatment Adherence
- Ultrasonography
- Users' Guide to the Medical Literature
- Vaccination
- Venous Thromboembolism
- Veterans Health
- Women's Health
- Workflow and Process
- Wound Care, Infection, Healing
Get the latest research based on your areas of interest.
Others also liked.
- Download PDF
- X Facebook More LinkedIn
Sheehy S , Aparicio HJ , Palmer JR, et al. Perceived Interpersonal Racism and Incident Stroke Among US Black Women. JAMA Netw Open. 2023;6(11):e2343203. doi:10.1001/jamanetworkopen.2023.43203
Manage citations:
© 2024
- Permissions
Perceived Interpersonal Racism and Incident Stroke Among US Black Women
- 1 Slone Epidemiology Center, Boston University, Boston, Massachusetts
- 2 Boston University Chobanian & Avedisian School of Medicine, Boston, Massachusetts
- 3 Boston Medical Center, Boston, Massachusetts
- 4 Boston University Center for Antiracist Research, Boston, Massachusetts
- 5 Boston University School of Public Health, Boston, Massachusetts
- 6 Beth Israel Deaconess Medical Center, Boston, Massachusetts
Question Is perceived interpersonal racism associated with stroke risk?
Findings In this cohort study of 48 375 US Black women, those who reported experiencing interpersonal racism in situations involving employment, housing, and the police had an estimated 38% increased risk of stroke compared with women who reported no such experiences.
Meaning These findings suggest that the high burden of racism experienced by Black US women may contribute to racial disparities in stroke incidence.
Importance Black individuals in the US experience stroke and stroke-related mortality at younger ages and more frequently than other racial groups. Studies examining the prospective association of interpersonal racism with stroke are lacking.
Objective To examine the association of perceived interpersonal racism with incident stroke among US Black women.
Design, Setting, and Participants The Black Women’s Health Study, a prospective cohort study of 59 000 Black women from across the US, assessed the longitudinal association between perceived interpersonal racism and stroke incidence. Stroke-free participants were followed up from 1997 until onset of stroke, death, loss to follow-up, or the end of the study period (December 31, 2019). Cox models were used to estimate hazard ratios (HRs) and 95% CIs, adjusting for major confounders, including education, neighborhood socioeconomic environment, and cardiometabolic factors. Data analysis was performed from March 2021 until December 2022.
Exposure On a questionnaire completed in 1997, participants reported experiences of racism in everyday life and when dealing with situations that involved employment, housing, and interactions with police.
Main Outcomes and Measures Strokes were identified through self-report on biennial questionnaires, medical records adjudication, and linkage with the National Death Index.
Results In 1997, 48 375 Black women (mean [SD] age, 41 [10] years) provided information on perceived interpersonal racism and were free of cardiovascular disease and cancer. During the 22 years of follow-up, 1664 incident stroke cases were identified; among them, 550 were definite cases confirmed by neurologist review and/or National Death Index linkage. Multivariable HRs for reported experiences of racism in all 3 domains of employment, housing, and interactions with police vs no such experiences were 1.38 (95% CI, 1.14-1.67), a 38% increase, for all incident cases and 1.37 (95% CI, 1.00-1.88) for definite cases. For comparisons of women in the highest quartile of everyday interpersonal racism score vs women in the lowest quartile, multivariable HRs were 1.14 (95% CI, 0.97-1.35) for analyses that included all incident stroke and 1.09 (95% CI, 0.83-1.45) for analyses that included definite cases only.
Conclusions and Relevance In this study, Black women who reported experiences of interpersonal racism in situations involving employment, housing, and interactions with police appeared to have an increased risk of stroke, even after accounting for demographic and vascular risk factors, suggesting that the high burden of racism experienced by Black US women may contribute to racial disparities in stroke incidence.
Black US individuals are especially vulnerable to stroke, with a 2- to 3-fold higher stroke incidence 1 - 4 and 1.2-fold higher stroke mortality than White US individuals. 5 Black women, in particular, experience stroke and stroke-related mortality at higher rates and earlier onset than women in any other racial group. 6 , 7
Racism in the US (both interpersonal racism and structural racism) disproportionally affects Black individuals. Racism is a complex construct and exists in multiple forms and at multiple levels. 8 - 11 Direct evidence linking racism with incident stroke is quite limited. The Reasons for Geographic and Regional Differences in Stroke study 12 provided important evidence that risk of stroke increased with increasing numbers of adverse social determinants of health, but racism was not directly assessed. The Multi-Ethnic Study of Atherosclerosis 13 reported no association of race-based discrimination with incident cardiovascular disease (CVD), but the association specifically with stroke was not reported. Some studies 14 - 16 have reported subclinical CVD associated with discrimination, although the results were inconsistent. The Black Women’s Health Study (BWHS), begun in 1995, asked a series of validated questions on experiences of racism in 1997, allowing for a prospective examination of the association of perceived interpersonal racism with incident stroke among Black women for 22 years of follow-up.
The Boston University Medical Campus institutional review board approved this cohort study. Study participants provided written informed consent. This report follows the Strengthening the Reporting of Observational Studies in Epidemiology ( STROBE ) reporting guidelines for observational studies.
The BWHS 17 is an ongoing, prospective cohort study that initially enrolled 59 000 participants through Essence Magazine and Black professional organizations (94% of the BWHS participants came from the Essence Magazine subscription lists). The BWHS is the largest contemporary cohort of Black women in the US, and most Black women were from 17 states across the mainland US, including California, Colorado, Georgia, Illinois, Indiana, Kansas, Louisiana, Maryland, Massachusetts, Michigan, Minnesota, New Jersey, New York, Oklahoma, South Carolina, Virginia, and Wisconsin, and Washington, District of Columbia. BWHS participants live in neighborhoods with a wide range of neighborhood socioeconomic status (SES). Black women aged 21 to 69 years (median age at baseline, 38 years) enrolled in 1995 by completing health questionnaires. In the initial questionnaire, respondents provided data on demographic characteristics, socioeconomic factors, medical conditions, and lifestyle factors. All but 5% of BWHS participants were born in the US. 17 Every 2 years, participants update health information on follow-up mailed and web health questionnaires. Follow-up through 2019 is complete for 86.2% of person-years.
Stroke cases were ascertained through (1) biennial BWHS questionnaires, which asked participants to report whether a physician had given them a diagnosis of stroke and the year of the diagnosis; (2) stroke medical records, which were reviewed and by a committee of neurologists (H.J.A., V.-A.L., and J.G.S.); and (3) the National Death Index (NDI). 18 On the 1995 BWHS questionnaire, participants were asked, “Has a doctor ever told you that you have any of the following conditions” (yes or no), and among the list was stroke. If yes, participants were asked to mark the age at which stroke was first diagnosed (<30, 30-39, 40-49, or ≥50 years). On the 1997 BWHS questionnaire and onward, participants were specifically asked, “If a doctor has told you that you had a stroke, please indicate when it was first diagnosed.” Self-reported stroke by the participant was followed by collection of detailed diagnostic information from medical records, including from inpatient hospitalizations and outpatient clinic visits. Among 1976 BWHS participants who reported a physician-diagnosed stroke, we were able to obtain releases and relevant portions of the medical record for 618. All medical records, including neuroimaging where available, were independently reviewed by an adjudication committee of vascular neurologists (H.J.A., V.-A.L., and J.G.S.), using the World Health Organization definition of stroke 19 : a new focal (or at times global) neurological impairment of sudden onset and of presumed vascular origin. 19 These included ischemic stroke, intracerebral hemorrhage, and subarachnoid hemorrhage. In addition, retinal and spinal cord infarction were included in this definition. Events not meeting this definition but with clinical evidence consistent with stroke, including (1) stroke that had been aborted by thrombolysis or mechanical thrombectomy and (2) symptoms lasting less than 24 hours with visible infarction on neuroimaging (ie, transient ischemic attack with demonstration of an ischemic lesion on magnetic resonance diffusion-weighted imaging), were also classified as stroke events. A reviewer with uncertainty about the status of the event forwarded it to a second reviewer. A joint discussion then took place between 2 to 3 reviewers to achieve a consensus (H.J.A., V.-A.L., or J.G.S.). Uncertainty was resolved through committee meetings for adjudication.
Appropriate medical records were obtained for 618 of 1976 participants who reported stroke. Among them, 450 (73%) were confirmed through adjudication and 168 were disconfirmed. Interrater reliability between the independent reviewers for adjudication of a confirmed stroke event was excellent (κ = 92%). Fatal stroke was ascertained through linkage with the NDI ( International Statistical Classification of Diseases and Related Health Problems, Tenth Revision [ ICD-10 ] codes I60-I69).
For the present analysis, the primary end point includes stroke confirmed by medical records review or NDI linkage, or self-reported stroke cases for which medical records could not be obtained. Stroke confirmed by medical records review or NDI were considered as definite cases. The secondary end point was restricted to definite cases only.
BWHS questions on racism and/or discrimination were adapted from an instrument developed by Williams et al, 20 designed to measure perceived interpersonal racism. We assessed perceived interpersonal racism experienced by the individual in everyday life and perceived interpersonal racism when dealing with situations that involved employers, housing, and police (eTable 1 in Supplement 1 ). 21 - 23 The present analysis is based on 1997 responses to the perceived interpersonal racism questions, 20 , 24 which represent perceived racism at midlife for most of the participants.
Five questions addressed interpersonal racism in everyday activities, such as, “People act as if they think you are dishonest,” with 5 responses ranging from never (scored as 0) to almost every day (scored as 4). The score for interpersonal racism in everyday life was the mean of responses to all 5 questions (possible scores, 0-4) and was categorized into quartiles. Three other questions asked whether the respondent had ever experienced discrimination owing to her race in employment, housing, and the police. 21 - 25 The score for perceived racism in institutional settings was the sum of positive responses to the 3 questions about employment, housing, and police (possible scores, 0, 1, 2, and 3). The BWHS has previously reported associations of perceived interpersonal racism with increased risks of type 2 diabetes 26 and obesity, 22 decreased subjective cognitive function, 23 and other outcomes. 21 , 24 , 27 - 34
Covariates were chosen a priori on the basis of factors known to be associated with increased risk of stroke. We included age (continuous), body mass index (calculated as weight in kilograms divided by height in meters squared; ≤24.9, 25-29.9, 30-34.9, and ≥35), education level (≤12, 13-15, 16, and ≥17 years), neighborhood socioeconomic status (SES) in quintiles, 35 vigorous physical activity (<1, 1-4 hours, or ≥5 hours per week), cigarette smoking (never, current <15 cigarettes per day, current ≥15 cigarettes per day, quit <10 years ago, and quit ≥10 years ago), history of diabetes (yes or no), history of hypertension (yes or no), history of coronary heart disease (yes or no), history of hyperlipidemia (yes or no), history of depression (yes or no), insurance status (have health insurance, yes or no), and indicators for health care utilization (had visited a doctor or nurse practitioner for a general physical and/or had your own regular physician or nurse practitioner, yes or no). All covariate values were taken from the 1997 questionnaire, the point at which the exposure, perceived interpersonal racism, was measured.
Hypertension, reported by participants on biennial questionnaires, was defined as physician-diagnosed hypertension together with use of an antihypertensive medication or diuretic, or use of an antihypertensive alone. In a prior validation study, 36 138 of 139 self-reports of hypertension (99%) were confirmed by medical records. Women who reported a diagnosis of diabetes occurring at age 30 years or older were considered to have type 2 diabetes. In a validation study, 37 217 of 229 women (94%) who reported diabetes were confirmed by their physicians to have type 2 diabetes.
Multivariable adjusted Cox proportional hazard models were used to estimate hazard ratios (HRs) and 95% CIs. For each study participant, follow-up was from 1997 until the onset of incident stroke, death, loss to follow-up, or the end of study follow-up (December 31, 2019), whichever came first. On the basis of known risk factors for stroke, we decided a priori to examine potential effect modification by baseline covariates, including age, residence in the so-called Stroke Belt (ie, Alabama, Arkansas, Georgia, Louisiana, Mississippi, North Carolina, South Carolina, and Tennessee), 38 educational level, and neighborhood SES. We used the Wald test to assess the significance of interaction. We also derived Kaplan-Meier survival curves for each of racism categories in the primary analysis. All analyses were performed using SAS statistical software version 9.4 (SAS Institute). All analyses involved 2-sided tests with a significance level of P < .05. Data analysis was performed from March 2021 until December 2022.
In 1997, 48 375 women (mean [SD] age, 41 [10] years) provided information on perceived interpersonal racism and were free of CVD (843 participants) and cancer (1615 participants). Compared with women in the lowest quartile of perceived interpersonal racism score, those in the highest quartile were similar with respect to neighborhood SES, body mass index, smoking, and physical activity ( Table 1 ). They were more likely to have a higher educational level. A total of 27 155 women (59%) perceived racism in employment, 16 109 (35%) perceived racism in housing, and 11 046 (24%) perceived racism in interactions with police. In the present study, 5159 participants (11%) reported yes to all 3 questions on perceived racism in employment, housing, and interactions with police, and 13 951 women (30%) reported no to all 3 questions. In this study, the correlation coefficient between everyday racism and housing, employment, and police scores was 0.36. Women who reported racism in employment, housing, and the police were more likely to live in high SES neighborhoods, less likely to live in the South, and more likely to have a higher educational level than those who reported no such experience ( Table 1 ).
During follow-up from 1997 through 2019, a total of 1664 incident strokes were identified. As shown in Table 2 and eFigures 1 and 2 in Supplement 1 , the age-adjusted HR for stroke was 1.28 (95% CI, 1.09-1.50; P for trend < .001) for women in the highest quartile of perceived interpersonal racism in everyday life vs those in the lowest quartile. The multivariable HR was 1.14 (95% CI, 0.97-1.35; P for trend = .03). When comparing women who experienced racism in employment, housing, and the police with those with no such experience, the age-adjusted HR for stroke was 1.42 (95% CI, 1.18-1.70; P for trend < .001). The multivariable HR was 1.38 (95% CI, 1.14-1.67; P for trend < .001), or a 38% increase, for women who answered yes to all 3 domains (employment, housing, and police) vs women who answered no to all 3 domains.
We repeated the analyses restricted to 550 definite cases that were confirmed through neurologist adjudication and/or the NDI ( Table 3 ). For perceived interpersonal racism in everyday life, there was no association with risk of stroke (multivariable adjusted HR, 1.09; 95% CI, 0.83-1.45; P for trend = .66). For women who reported interpersonal racism in all 3 of the domains of employment, housing, and police compared with none of the domains, the multivariable adjusted HR was 1.37 (95% CI, 1.00-1.88; P for trend = .05) ( Table 3 ). In a sensitivity analysis that restricted definite strokes to only those with medical records adjudicated by study neurologists, the comparable HR was 1.61 (95% CI, 1.09-2.37) (eTable 2 in Supplement 1 ).
Across strata of various subgroups, perceived interpersonal racism in employment, housing, and the police was consistently associated with elevated stroke risk (eTable 3 in Supplement 1 ). For perceived interpersonal racism in everyday life, the HR was most increased among participants in the lowest quartile of neighborhood SES ( P for interaction = .001) (eTable 3 in Supplement 1 ).
In this large, prospective cohort study of 48 375 Black women, we found that participants who reported experiencing interpersonal racism in 3 institutional settings had an estimated 38% increased risk of stroke compared with women who reported no such experiences. The elevated stroke risk was present in analyses of all incident strokes, as well as in analyses restricting to definite strokes. For perceived interpersonal racism in everyday life, stroke risk was elevated in an analysis that included all stroke cases, but there was no evidence of an increased risk of stroke based on analyses of the smaller number of definite cases.
Disproportionate numbers of Black US individuals face multiple adverse experiences over the life course, including racism and poverty; these are recognized as social determinants of health. 39 , 40 Racism may act as a psychosocial stressor and thereby elevate systemic inflammation, impair endothelial function, and dysregulate the hypothalamic-pituitary-adrenal axis. 41 - 43 Previous studies have linked perceived interpersonal racism with worse mental health outcomes, 41 higher risk of hypertension, 24 , 44 - 48 increased systolic blood pressure, 48 unhealthy behavior and lifestyles, 49 , 50 higher allostatic load, 51 - 53 higher inflammatory markers, 54 hormone dysregulation, 55 , 56 and shorter telomere length. 27 , 57 Although several large prospective cohort studies have investigated stroke risk factors among Black US individuals, direct evidence about perceived racism and incident stroke is very limited. The Jackson Heart Study 14 reported higher risk of subclinical CVD associated with discrimination, but did not examine stroke end points. The Multi-Ethnic Study of Atherosclerosis 13 reported no association of racial discrimination with risk of all CVD, but associations with stroke were not reported. Our study provides direct evidence on perceived racial discrimination at the interpersonal level in relation to subsequent occurrence of incident stroke.
Strengths of our study include the prospective cohort design with a large sample of Black women, wide geographic distribution, long duration of follow-up from 1997 until 2019, large number of incident strokes, and detailed information on potential confounding factors. Our study also has some limitations. First, the concept of racism is complex, and racism exists in multiple forms and at multiple levels. Structural racism 58 (ie, the ways societies reinforce racial differences in access to housing, education, employment, health care, and criminal justice) may also affect stroke risk and is not captured by questions about interpersonal racism. Second, our primary stroke end point included self-reported stroke. BWHS participants responded to the question, “Has a doctor ever told you that you have stroke?” Medical records were not available for all stroke events, either because the participant declined to sign a medical records release or because the appropriate hospital was not identified or did not have the records. A team of vascular neurologists performed a careful review of records that were obtained. The confirmation rate of 73% is similar to that reported in other studies of stroke. 59 Prior studies have shown that the question, “Has a doctor ever told you that you have stroke?” has good sensitivity and specificity for identifying patients in the community with a diagnosis of stroke. 59 , 60
Third, perceived racism captures an individual’s perceptions of an experience of racism and relies on a participant’s self-report. Perceived racism is inevitably subject to measurement error. An individual who experiences racism may overreport or underreport these sensitive events or change their interpretations and perceptions of these experiences over time. Because self-reported information on stroke was collected prospectively by biennial questionnaire, it is highly unlikely that stroke events were reported differentially with respect to the perceived interpersonal racism level reported in 1997. However, it is possible that BWHS participants who survived a stroke with severe deficits may have been less likely to respond.
Fourth, despite our efforts to control for confounders and risk factors for stroke, our study is observational and may still have been subject to unmeasured and residual confounding. For example, in our study, we were unable to adjust for history of atrial fibrillation, a risk factor for stroke. However, given the extensive list we included for major confounder adjustment, it is highly unlikely that our study will have such a strong unmeasured confounder.
Fifth, our study consisted of Black women with high educational levels compared with the general population of Black women. Participants in the BWHS needed to have sufficient literacy to complete health questionnaires every 2 years. BWHS participants represent the educational levels of Black US women, with underrepresentation of women with the lowest level of education (ie, <12 years). Future studies among Black women with lower levels of education are needed.
In this investigation of perceived interpersonal racism in relation to incident stroke, experiences of interpersonal racism in everyday life were associated with higher risk of incident stroke, although there was no association for the smaller group of definite strokes. However, in both analyses, individuals who reported experiencing interpersonal racism in situations concerning housing, employment, and the police appeared to have an increased risk, estimated to be 38%, compared with women who reported no such experiences. It is possible that the high burden of racism experienced by Black US individuals may contribute to racial disparities in stroke incidence.
Accepted for Publication: October 4, 2023.
Published: November 10, 2023. doi:10.1001/jamanetworkopen.2023.43203
Open Access: This is an open access article distributed under the terms of the CC-BY License . © 2023 Sheehy S et al. JAMA Network Open .
Corresponding Author: Shanshan Sheehy, ScD, Slone Epidemiology Center, Boston University, 72 E Concord St, L-7, Boston, MA 02118 ( [email protected] ).
Author Contributions: Dr Sheehy had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Concept and design: Sheehy, Cozier, Rosenberg.
Acquisition, analysis, or interpretation of data: All authors.
Drafting of the manuscript: Sheehy.
Critical review of the manuscript for important intellectual content: All authors.
Statistical analysis: Sheehy.
Obtained funding: Sheehy, Palmer.
Administrative, technical, or material support: Sheehy, Cozier.
Supervision: Sheehy, Aparicio, Palmer, Rosenberg.
Conflict of Interest Disclosures: Dr Lioutas reported receiving personal fees from Qmetis and grants from the National Institutes of Health outside the submitted work. No other disclosures were reported.
Funding/Support: This study was funded by the National Institutes of Health (grants R01CA058420, UM1CA164974, U01CA164974, L30 NS093634, and R01MD015085). Dr Aparicio is supported by an American Academy of Neurology Career Development Award and by Boston University’s Aram V. Chobanian Assistant Professorship.
Role of the Funder/Sponsor: The funders had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
Data Sharing Statement: See Supplement 2 .
Additional Contributions: We thank the staff and participants in the Black Women’s Health Study.
- Register for email alerts with links to free full-text articles
- Access PDFs of free articles
- Manage your interests
- Save searches and receive search alerts

IMAGES
VIDEO
COMMENTS
A 49-year-old woman was brought to the ED 2 hours after the onset of hemiplegia and aphasia during a transatlantic flight. Examination revealed evidence of acute ischemic stroke. Additional diagnos...
Go to Case Rubric. Kanika is a 38-year-old right-handed woman who was working as a social media coordinator for an international organization when she had a stroke in early June. Prior to the stroke, Kanika lived alone and frequently saw her sister and nieces, who lived in a nearby state. Her apartment is on the second floor, and there is no ...
Efficacy of behavioral speech and language therapy for aphasia. The literature on aphasia therapy is marred by mostly single-case design and small group studies (e.g., references [13-15]).Perhaps because of this historical focus on underpowered studies, the efficacy of aphasia therapy was debated for decades and, until relatively recently, remained highly controversial [16-18].
Some causes of Broca aphasia include traumatic brain injury, tumors, brain infections, and degenerative illnesses. However, the most common cause of infarct is a thrombus or emboli in the middle cerebral artery or the internal carotid artery. We present a case of Broca aphasia that was initially interpreted as confusion.
Cheng W, Zhang Y, He L. MRI features of stroke-like episodes in mitochondrial encephalomyopathy with lactic acidosis and stroke-like episodes. Front Neurol 2022;13:843386-843386. Crossref
Pharmacological agents already approved for the treatment of other neurological and psychiatric disorders have been studied in patients with post-stroke aphasia. Several small case studies, open-label trials, and small randomized clinical trials show that pharmacotherapy provides benefits in stroke patients with aphasia; however, benefits are ...
Case study 25 Woman aged 41 years with Broca's aphasia; Case study 26 Man with stroke-induced Broca's aphasia; Case study 27 Man aged 41 years with non-fluent aphasia; Case study 28 Man aged 60 years with right hemisphere damage; Case study 29 Man aged 24 years with closed head injury; Case study 30 Woman aged 87 years with early-stage ...
Shehata et al.'s cross-sectional study of 30 post-stroke patients with aphasia and 31 without aphasia found that aphasia was linked to a higher degree of psychosis (p = 0.001)—psychosis was more linked to aphasic stroke survivors than non-aphasic stroke survivors (Shehata et al., 2015). However, methodological limitations and relatively ...
Background and Purpose— Recently, a combined repetitive transcranial magnetic stimulation (rTMS) and activation positron emission tomography (PET) study showed essential language function of the right inferior frontal gyrus (IFG) in some right-handed acute poststroke aphasics. We reexamined these patients in the chronic phase to test whether the right IFG remained essential for language ...
CASE 2 A 62 year old woman with a history of hypertension and hyperlipidemia presented to a primary stroke center with sudden onset of weakness of the right side. On examination, she had a global aphasia, left gaze preference, right homonymous hemianopsia (field cut), right facial droop, dysarthria, and right hemiplegia (NIH Stroke Scale = 22).
Stroke is a leading cause of disability, and Magnetic Resonance Imaging (MRI) is routinely acquired for acute stroke management. Publicly sharing these datasets can aid in the development of ...
Conduction Aphasia. Age 64. Time since stroke: 3 months. 1 week program. [email protected]. 727.823.2529. Read stories about our graduates to see what we can accomplish together. These are actually typical results. Each case below has been selected to provide a variety of aphasia types, client ages, etc.
We aimed to characterize difficulties in famous face naming in three poststroke aphasic patients with a lesion limited to the left mid-posterior temporal language regions, sparing the anterior temporal lobe. The patients did not present semantic deficits specific to known people. Nonetheless, they s …
Broca aphasia is characterised by severe impairment in expressing speech and writing. 1 Comprehension is sometimes affected. Broca aphasia stems from neurological damage to the Broca area. The differential diagnosis is broad, encompassing vascular, infectious, inflammatory or degenerative conditions ( box 1 ).
METHODS. To address these questions, a prospective cross‐sectional case-control study was developed. The case group consisted of patients with post‐stroke aphasia included in the DULCINEA trial (NCT04289493).Full details of the design of this clinical trial are available elsewhere [].Briefly, the primary inclusion criteria were as follows: (i) non‐fluent aphasia due to ischaemic stroke ...
Case Study - Broca's Aphasia. Age: 33. Time Since Stroke: 6 months. Georgia had a stroke at a young age, losing her independent lifestyle as a teacher and gifted artist. She had to move back home with her parents and couldn't drive, but she had very good friends who maintained close contact with her. She had a great sense of humor.
Her other avenue of research involves novel treatment studies and longitudinal imaging and language studies of Primary Progressive Aphasia. She has published extensively on these topics in journals and textbooks. ... Cooper, O., Metter, E.J. (2002). Subcortical aphasia and neglect in acute stroke: the role of cortical hypoperfusion, Brain,125 ...
This study found that the overall prevalence of PSD was 46.6%. Prevalence is most influenced by the diagnosis method. Hypertension, history of stroke, atrial fibrillation, patient age, and stroke severity were risk factors significantly associated with PSD. The prevalence of aphasia, dysarthria, res …
Early assessment of aphasia is very important to prevent the emergence of telegraphic speech styles, improve welfare, independence, social participation, quality of life, reduce length of stay and care costs, but there is little literature on this subject, especially in stroke patients. Purpose: This study is to provide an overview of the ...
Began speaking in grammatical sentences of 4+ words independently. Learned how to use her phone as a strategy. Self-correcting errors in verb tense and grammatical structure. Improved ability to accurately write longer words and sentences. Case Study Moderate Broca's Aphasia (brain injury) Age: 27 Time since brain injury: 3 years.
Every four minutes someone acquires aphasia, which is often caused by a stroke, the National Aphasia Association reports. In 2022 in Kentucky, diseases of the cerebrovascular system (stroke) were the fifth leading cause of death, and one-third of strokes result in aphasia. ... "Each group of four students will receive a different case study ...
This was the case for two umbrella reviews, one for rTMS post-stroke aphasia the other one for rTMS post-stroke dysphagia . Both URs found that published SRs, with or without MAs, of randomized controlled trials (RCTs) on the topics exhibit subpar methodological quality and therefore the evidence concerning the effectiveness of rTMS in ...
A 39-year-old man suffered an intracerebral hemorrhage in the region of the left internal capsule deep to Wernicke's area. The location of the lesion was confirmed by computerized tomography (CT) performed two days postictally. Two weeks after admission, the Boston Diagnostic Aphasia Examination (BD …
The study, "Listening to classical music influences brain connectivity in post-stroke aphasia: a pilot study," was authored by Maryane Chea, Amina Ben Salah, Monica N. Toba, Ryan Zeineldin ...
The Multi-Ethnic Study of Atherosclerosis 13 reported no association of race-based discrimination with incident cardiovascular disease (CVD), but the association specifically with stroke was not reported. Some studies 14-16 have reported subclinical CVD associated with discrimination, although the results were inconsistent. The Black Women's ...