Information
- Author Services

Initiatives
You are accessing a machine-readable page. In order to be human-readable, please install an RSS reader.
All articles published by MDPI are made immediately available worldwide under an open access license. No special permission is required to reuse all or part of the article published by MDPI, including figures and tables. For articles published under an open access Creative Common CC BY license, any part of the article may be reused without permission provided that the original article is clearly cited. For more information, please refer to https://www.mdpi.com/openaccess .
Feature papers represent the most advanced research with significant potential for high impact in the field. A Feature Paper should be a substantial original Article that involves several techniques or approaches, provides an outlook for future research directions and describes possible research applications.
Feature papers are submitted upon individual invitation or recommendation by the scientific editors and must receive positive feedback from the reviewers.
Editor’s Choice articles are based on recommendations by the scientific editors of MDPI journals from around the world. Editors select a small number of articles recently published in the journal that they believe will be particularly interesting to readers, or important in the respective research area. The aim is to provide a snapshot of some of the most exciting work published in the various research areas of the journal.
Original Submission Date Received: .
- Active Journals
- Find a Journal
- Proceedings Series
- For Authors
- For Reviewers
- For Editors
- For Librarians
- For Publishers
- For Societies
- For Conference Organizers
- Open Access Policy
- Institutional Open Access Program
- Special Issues Guidelines
- Editorial Process
- Research and Publication Ethics
- Article Processing Charges
- Testimonials
- Preprints.org
- SciProfiles
- Encyclopedia

Article Menu

- Subscribe SciFeed
- Recommended Articles
- PubMed/Medline
- Google Scholar
- on Google Scholar
- Table of Contents
Find support for a specific problem in the support section of our website.
Please let us know what you think of our products and services.
Visit our dedicated information section to learn more about MDPI.
JSmol Viewer
Gait analysis in neurorehabilitation: from research to clinical practice.

Graphical Abstract
1. Introduction
2. search strategy, 3. neurodegenerative disorders, 3.1. parkinson’s disease, 3.2. multiple sclerosis, 3.3. cerebellar ataxia, 4. acquired brain injury, 4.1. stroke, 4.2. traumatic brain injury, 5. discussion, 5.1. clinical considerations about gait and postural dysfunctions, 5.2. clinical implications of nws, 5.3. clinical implications of ws, 5.4. future directions of gait analysis, 6. conclusions, author contributions, institutional review board statement, informed consent statement, data availability statement, conflicts of interest.
| | |
| “Parkinson’s disease” OR “multiple sclerosis” OR “cerebellar ataxia” OR “ataxia” OR “neurodegenerative disorders” OR “acquired brain injury” OR “stroke” OR “traumatic brain injury” | “wearable sensors” OR “gait platforms” OR “non-wearable sensors” OR “instrumented gait analysis” OR “objective gait evaluation” OR “inertial measurement units” OR “motion capture systems” OR “mobile application” OR “artificial intelligence” |
- Salchow-Hömmen, C.; Skrobot, M.; Jochner, M.C.E.; Schauer, T.; Kühn, A.A.; Wenger, N. Review—Emerging Portable Technologies for Gait Analysis in Neurological Disorders. Front. Hum. Neurosci. 2022 , 16 , 768575. [ Google Scholar ] [ CrossRef ]
- Li, S.; Francisco, G.E.; Zhou, P. Post-stroke Hemiplegic Gait: New Perspective and Insights. Front. Physiol. 2018 , 9 , 1021. [ Google Scholar ] [ CrossRef ] [ Green Version ]
- Loyd, B.J.; Dibble, L.E.; Weightman, M.M.; Pelo, R.; Hoppes, C.W.; Lester, M.; King, L.A.; Fino, P.C. Volitional Head Movement Deficits and Alterations in Gait Speed Following Mild Traumatic Brain Injury. J. Head Trauma Rehabil. 2022 , 38 , E223–E232. [ Google Scholar ] [ CrossRef ]
- di Biase, L.; Di Santo, A.; Caminiti, M.L.; De Liso, A.; Shah, S.A.; Ricci, L.; Di Lazzaro, V. Gait Analysis in Parkinson’s Disease: An Overview of the Most Accurate Markers for Diagnosis and Symptoms Monitoring. Sensors 2020 , 20 , 3529. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Coca-Tapia, M.; Cuesta-Gómez, A.; Molina-Rueda, F.; Carratalá-Tejada, M. Gait Pattern in People with Multiple Sclerosis: A Systematic Review. Diagnostics 2021 , 11 , 584. [ Google Scholar ] [ CrossRef ]
- Benedetti, M.G.; Piperno, R.; Simoncini, L.; Bonato, P.; Tonini, A.; Giannini, S. Gait abnormalities in minimally impaired multiple sclerosis patients. Mult. Scler. J. 1999 , 5 , 363–368. [ Google Scholar ] [ CrossRef ]
- Tunca, C.; Pehlivan, N.; Ak, N.; Arnrich, B.; Salur, G.; Ersoy, C. Inertial Sensor-Based Robust Gait Analysis in Non-Hospital Settings for Neurological Disorders. Sensors 2017 , 17 , 825. [ Google Scholar ] [ CrossRef ] [ Green Version ]
- Das, R.; Paul, S.; Mourya, G.K.; Kumar, N.; Hussain, M. Recent Trends and Practices toward Assessment and Rehabilitation of Neurodegenerative Disorders: Insights from Human Gait. Front. Neurosci. 2022 , 16 , 859298. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Hulleck, A.A.; Mohan, D.M.; Abdallah, N.; El Rich, M.; Khalaf, K. Present and future of gait assessment in clinical practice: Towards the application of novel trends and technologies. Front. Med. Technol. 2022 , 4 , 901331. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Bonanno, M.; De Luca, R.; Torregrossa, W.; Tonin, P.; Calabrò, R.S. Moving toward Appropriate Motor Assessment Tools in People Affected by Severe Acquired Brain Injury: A Scoping Review with Clinical Advices. Healthcare 2022 , 10 , 1115. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Bhidayasiri, R.; Martinez-Martin, P. Clinical Assessments in Parkinson’s Disease: Scales and Monitoring. Int. Rev. Neurobiol. 2017 , 132 , 129–182. [ Google Scholar ] [ CrossRef ]
- Wang, C.; Ruiz, A.; Mao-Draayer, Y. Assessment and Treatment Strategies for a Multiple Sclerosis Relapse. J. Immunol. Clin. Res. 2016 , 5 , 1032. [ Google Scholar ] [ PubMed ]
- Power, L.; Pathirana, P.; Horne, M.; Milne, S.; Marriott, A.; Szmulewicz, D.J. Instrumented Objective Clinical Examination of Cerebellar Ataxia: The Upper and Lower Limb—A Review. Cerebellum 2022 , 21 , 145–158. [ Google Scholar ] [ CrossRef ]
- Muro-de-la-Herran, A.; Garcia-Zapirain, B.; Mendez-Zorrilla, A. Gait analysis methods: An overview of wearable and non-wearable systems, highlighting clinical applications. Sensors 2014 , 14 , 3362–3394. [ Google Scholar ] [ CrossRef ] [ Green Version ]
- Schniepp, R.; Huppert, A.; Decker, J.; Schenkel, F.; Schlick, C.; Rasoul, A.; Dieterich, M.; Brandt, T.; Jahn, K.; Wuehr, M. Fall prediction in neurological gait disorders: Differential contributions from clinical assessment, gait analysis, and daily-life mobility monitoring. J. Neurol. 2021 , 268 , 3421–3434. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Pieruccini-Faria, F.; Black, S.E.; Masellis, M.; Smith, E.E.; Almeida, Q.J.; Li, K.Z.H.; Bherer, L.; Camicioli, R.; Montero-Odasso, M. Gait variability across neurodegenerative and cognitive disorders: Results from the Canadian Consortium of Neurodegeneration in Aging (CCNA) and the Gait and Brain Study. Alzheimer’s Dement. 2021 , 17 , 1317–1328. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Cicirelli, G.; Impedovo, D.; Dentamaro, V.; Marani, R.; Pirlo, G.; D’Orazio, T.R. Human Gait Analysis in Neurodegenerative Diseases: A Review. IEEE J. Biomed. Health Inform. 2022 , 26 , 229–242. [ Google Scholar ] [ CrossRef ]
- Marković, V.; Stanković, I.; Radovanović, S.; Petrović, I.; Lukić, M.J.; Mišković, N.D.; Svetel, M.; Kostić, V. Gait alterations in Parkinson’s disease at the stage of hemiparkinsonism—A longitudinal study. PLoS ONE 2022 , 17 , e0269886. [ Google Scholar ] [ CrossRef ]
- Pistacchi, M.; Gioulis, M.; Sanson, F.; De Giovannini, E.; Filippi, G.; Rossetto, F.; Zambito Marsala, S. Gait analysis and clinical correlations in early Parkinson’s disease. Funct. Neurol. 2017 , 32 , 28–34. [ Google Scholar ] [ CrossRef ]
- Del Din, S.; Godfrey, A.; Rochester, L. Validation of an Accelerometer to Quantify a Comprehensive Battery of Gait Characteristics in Healthy Older Adults and Parkinson’s Disease: Toward Clinical and at Home Use. IEEE J. Biomed. Health Inform. 2015 , 20 , 838–847. [ Google Scholar ] [ CrossRef ] [ Green Version ]
- Potter, M.V.; Cain, S.M.; Ojeda, L.V.; Gurchiek, R.D.; McGinnis, R.S.; Perkins, N.C. Evaluation of Error-State Kalman Filter Method for Estimating Human Lower-Limb Kinematics during Various Walking Gaits. Sensors 2022 , 22 , 8398. [ Google Scholar ] [ CrossRef ]
- Cameron, M.H.; Nilsagard, Y. Balance, gait, and falls in multiple sclerosis. Handb. Clin. Neurol. 2018 , 159 , 237–250. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Kempen, J.C.; Doorenbosch, C.A.; Knol, D.L.; de Groot, V.; Beckerman, H. Newly Identified Gait Patterns in Patients with Multiple Sclerosis May Be Related to Push-off Quality. Phys. Ther. 2016 , 96 , 1744–1752. [ Google Scholar ] [ CrossRef ] [ PubMed ] [ Green Version ]
- Severini, G.; Manca, M.; Ferraresi, G.; Caniatti, L.M.; Cosma, M.; Baldasso, F.; Straudi, S.; Morelli, M.; Basaglia, N. Evaluation of Clinical Gait Analysis parameters in patients affected by Multiple Sclerosis: Analysis of kinematics. Clin. Biomech. 2017 , 45 , 1–8. [ Google Scholar ] [ CrossRef ]
- Sehle, A.; Mündermann, A.; Starrost, K.; Sailer, S.; Becher, I.; Dettmers, C.; Vieten, M. Objective assessment of motor fatigue in multiple sclerosis using kinematic gait analysis: A pilot study. J. Neuroeng. Rehabil. 2011 , 8 , 59. [ Google Scholar ] [ CrossRef ] [ Green Version ]
- Hu, W.; Combden, O.; Jiang, X.; Buragadda, S.; Newell, C.J.; Williams, M.C.; Critch, A.L.; Ploughman, M. Machine learning corroborates subjective ratings of walking and balance difficulty in multiple sclerosis. Front. Artif. Intell. 2022 , 5 , 952312. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Kim, S.M.; Kim, D.H.; Yang, Y.; Ha, S.W.; Han, J.H. Gait Patterns in Parkinson’s Disease with or without Cognitive Impairment. Dement. Neurocogn. Disord. 2018 , 17 , 57–65. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- di Biase, L.; Raiano, L.; Caminiti, M.L.; Pecoraro, P.M.; Di Lazzaro, V. Parkinson’s Disease Wearable Gait Analysis: Kinematic and Dynamic Markers for Diagnosis. Sensors 2022 , 22 , 8773. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Rehman, R.Z.U.; Buckley, C.; Mico-Amigo, M.E.; Kirk, C.; Dunne-Willows, M.; Mazza, C.; Shi, J.Q.; Alcock, L.; Rochester, L.; Del Din, S. Accelerometry-Based Digital Gait Characteristics for Classification of Parkinson’s Disease: What Counts? IEEE Open J. Eng. Med. Biol. 2020 , 1 , 65–73. [ Google Scholar ] [ CrossRef ]
- Jakob, V.; Küderle, A.; Kluge, F.; Klucken, J.; Eskofier, B.M.; Winkler, J.; Winterholler, M.; Gassner, H. Validation of a Sensor-Based Gait Analysis System with a Gold-Standard Motion Capture System in Patients with Parkinson’s Disease. Sensors 2021 , 21 , 7680. [ Google Scholar ] [ CrossRef ]
- Alberto, S.; Cabral, S.; Proença, J.; Pona-Ferreira, F.; Leitão, M.; Bouça-Machado, R.; Kauppila, L.A.; Veloso, A.P.; Costa, R.M.; Ferreira, J.J.; et al. Validation of quantitative gait analysis systems for Parkinson’s disease for use in supervised and unsupervised environments. BMC Neurol. 2021 , 21 , 331. [ Google Scholar ] [ CrossRef ]
- Liu, R.; Wang, Z.; Qiu, S.; Zhao, H.Y.; Wang, C.; Shi, X.; Lin, F. A Wearable Gait Analysis and Recognition Method for Parkinson’s Disease Based on Error State Kalman Filter. IEEE J. Biomed. Health Inform. 2022 , 26 , 4165–4175. [ Google Scholar ] [ CrossRef ]
- Lizama, L.E.C.; Khan, F.; Lee, P.V.S.; Galea, M.P. The use of laboratory gait analysis for understanding gait deterioration in people with multiple sclerosis. Mult. Scler. J. 2016 , 22 , 1768–1776. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Buckley, E.; Mazzà, C.; McNeill, A. A systematic review of the gait characteristics associated with Cerebellar Ataxia. Gait Posture 2018 , 60 , 154–163. [ Google Scholar ] [ CrossRef ]
- Serrao, M.; Pierelli, F.; Ranavolo, A.; Draicchio, F.; Conte, C.; Don, R.; Di Fabio, R.; LeRose, M.; Padua, L.; Sandrini, G.; et al. Gait Pattern in Inherited Cerebellar Ataxias. Cerebellum 2011 , 11 , 194–211. [ Google Scholar ] [ CrossRef ]
- Matsushima, A.; Yoshida, K.; Genno, H.; Murata, A.; Matsuzawa, S.; Nakamura, K.; Nakamura, A.; Ikeda, S.-I. Clinical assessment of standing and gait in ataxic patients using a triaxial accelerometer. Cerebellum Ataxias 2015 , 2 , 9. [ Google Scholar ] [ CrossRef ] [ PubMed ] [ Green Version ]
- Zhou, H.; Nguyen, H.; Enriquez, A.; Morsy, L.; Curtis, M.; Piser, T.; Kenney, C.; Stephen, C.D.; Gupta, A.S.; Schmahmann, J.D.; et al. Assessment of gait and balance impairment in people with spinocerebellar ataxia using wearable sensors. Neurol. Sci. 2021 , 43 , 2589–2599. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Ilg, W.; Seemann, J.; Giese, M.; Traschütz, A.; Schöls, L.; Timmann, D.; Synofzik, M. Real-life gait assessment in degenerative cerebellar ataxia: Toward ecologically valid biomarkers. Neurology 2020 , 95 , e1199–e1210. [ Google Scholar ] [ CrossRef ]
- Ferrarello, F.; Bianchi, V.A.M.; Baccini, M.; Rubbieri, G.; Mossello, E.; Cavallini, M.C.; Marchionni, N.; Di Bari, M. Tools for Observational Gait Analysis in Patients With Stroke: A Systematic Review. Phys. Ther. 2013 , 93 , 1673–1685. [ Google Scholar ] [ CrossRef ] [ Green Version ]
- Gupta, A.; Taly, A.B. Post-stroke Gait Analysis in Rehabilitation Set-up: Observational or Instrumental! Neurol. India 2019 , 67 , 1041–1042. [ Google Scholar ] [ CrossRef ]
- Mohan, D.M.; Khandoker, A.H.; Wasti, S.A.; Alali, S.I.I.I.; Jelinek, H.F.; Khalaf, K. Assessment Methods of Post-stroke Gait: A Scoping Review of Technology-Driven Approaches to Gait Characterization and Analysis. Front. Neurol. 2021 , 12 , 650024. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Latorre, J.; Colomer, C.; Alcañiz, M.; Llorens, R. Gait analysis with the Kinect v2: Normative study with healthy individuals and comprehensive study of its sensitivity, validity, and reliability in individuals with stroke. J. Neuroeng. Rehabil. 2019 , 16 , 97. [ Google Scholar ] [ CrossRef ] [ PubMed ] [ Green Version ]
- Wang, Y.; Tang, R.; Wang, H.; Yu, X.; Li, Y.; Wang, C.; Wang, L.; Qie, S. The Validity and Reliability of a New Intelligent Three-Dimensional Gait Analysis System in Healthy Subjects and Patients with Post-Stroke. Sensors 2022 , 22 , 9425. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Balaban, B.; Tok, F. Gait Disturbances in Patients with Stroke. PM&R 2014 , 6 , 635–642. [ Google Scholar ] [ CrossRef ]
- Patterson, K.K.; Gage, W.H.; Brooks, D.; Black, S.E.; McIlroy, W.E. Evaluation of gait symmetry after stroke: A comparison of current methods and recommendations for standardization. Gait Posture 2010 , 31 , 241–246. [ Google Scholar ] [ CrossRef ]
- Laudanski, A.; Brouwer, B.; Li, Q. Measurement of Lower Limb Joint Kinematics using Inertial Sensors During Stair Ascent and Descent in Healthy Older Adults and Stroke Survivors. J. Health Eng. 2013 , 4 , 555–576. [ Google Scholar ] [ CrossRef ] [ Green Version ]
- Dever, A.; Powell, D.; Graham, L.; Mason, R.; Das, J.; Marshall, S.J.; Vitorio, R.; Godfrey, A.; Stuart, S. Gait Impairment in Traumatic Brain Injury: A Systematic Review. Sensors 2022 , 22 , 1480. [ Google Scholar ] [ CrossRef ]
- Reidy, J.; Mobbs, R.; Kim, J.; Brown, E.; Mobbs, R. Clinical gait characteristics in the early post-concussion phase: A systematic review. J. Clin. Neurosci. 2023 , 107 , 184–191. [ Google Scholar ] [ CrossRef ]
- Martini, D.N.; Parrington, L.; Stuart, S.; Fino, P.C.; King, L.A. Gait Performance in People with Symptomatic, Chronic Mild Traumatic Brain Injury. J. Neurotrauma 2021 , 38 , 218–224. [ Google Scholar ] [ CrossRef ]
- Belluscio, V.; Bergamini, E.; Tramontano, M.; Bustos, A.O.; Allevi, G.; Formisano, R.; Vannozzi, G.; Buzzi, M.G. Gait Quality Assessment in Survivors from Severe Traumatic Brain Injury: An Instrumented Approach Based on Inertial Sensors. Sensors 2019 , 19 , 5315. [ Google Scholar ] [ CrossRef ] [ Green Version ]
- Pitt, W.; Chen, S.-H.; Chou, L.-S. Using IMU-based kinematic markers to monitor dual-task gait balance control recovery in acutely concussed individuals. Clin. Biomech. 2020 , 80 , 105145. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Celik, Y.; Stuart, S.; Woo, W.; Godfrey, A. Gait analysis in neurological populations: Progression in the use of wearables. Med. Eng. Phys. 2020 , 87 , 9–29. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Stolze, H.; Klebe, S.; Baecker, C.; Zechlin, C.; Friege, L.; Pohle, S.; Deuschl, G. Prevalence of gait disorders in hospitalized neurological patients. Mov. Disord. 2004 , 20 , 89–94. [ Google Scholar ] [ CrossRef ]
- Ge, H.-L.; Chen, X.-Y.; Lin, Y.-X.; Ge, T.-J.; Yu, L.-H.; Lin, Z.-Y.; Wu, X.-Y.; Kang, D.-Z.; Ding, C.-Y. The prevalence of freezing of gait in Parkinson’s disease and in patients with different disease durations and severities. Chin. Neurosurg. J. 2020 , 6 , 17. [ Google Scholar ] [ CrossRef ]
- Tasseel-Ponche, S.; Delafontaine, A.; Godefroy, O.; Yelnik, A.P.; Doutrellot, P.-L.; Duchossoy, C.; Hyra, M.; Sader, T.; Diouf, M. Walking speed at the acute and subacute stroke stage: A descriptive meta-analysis. Front. Neurol. 2022 , 13 , 989622. [ Google Scholar ] [ CrossRef ]
- Moon, Y.; Sung, J.; An, R.; Hernandez, M.E.; Sosnoff, J.J. Gait variability in people with neurological disorders: A systematic review and meta-analysis. Hum. Mov. Sci. 2016 , 47 , 197–208. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Patel, P.; Enzastiga, D.; Casamento-Moran, A.; Christou, E.A.; Lodha, N. Increased temporal stride variability contributes to impaired gait coordination after stroke. Sci. Rep. 2022 , 12 , 12679. [ Google Scholar ] [ CrossRef ]
- Niechwiej-Szwedo, E.; Inness, E.; Howe, J.; Jaglal, S.; McIlroy, W.; Verrier, M. Changes in gait variability during different challenges to mobility in patients with traumatic brain injury. Gait Posture 2007 , 25 , 70–77. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Farrell, J.W., 3rd; Merkas, J.; Pilutti, L.A. The Effect of Exercise Training on Gait, Balance, and Physical Fitness Asymmetries in Persons with Chronic Neurological Conditions: A Systematic Review of Randomized Controlled Trials. Front. Physiol. 2020 , 11 , 585765. [ Google Scholar ] [ CrossRef ]
- Bishnoi, A.; Shankar, M.; Lee, R.; Hu, Y.; Hernandez, M.E. Effects of Therapeutic Intervention on Spatiotemporal Gait Parameters in Adults With Neurologic Disorder: Systematic Review and Meta-analysis. Arch. Phys. Med. Rehabil. 2023 , 104 , 451–474. [ Google Scholar ] [ CrossRef ]
- Keshner, E.A.; Lamontagne, A. The Untapped Potential of Virtual Reality in Rehabilitation of Balance and Gait in Neurological Disorders. Front. Virtual Real. 2021 , 2 , 641650. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Peppe, A.; Chiavalon, C.; Pasqualetti, P.; Crovato, D.; Caltagirone, C. Does gait analysis quantify motor rehabilitation efficacy in Parkinson’s disease patients? Gait Posture 2007 , 26 , 452–462. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Guner, S.; Inanici, F. Yoga therapy and ambulatory multiple sclerosis Assessment of gait analysis parameters, fatigue and balance. J. Bodyw. Mov. Ther. 2015 , 19 , 72–81. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Collins, J.-D.; Markham, A.; Service, K.; Reini, S.; Wolf, E.; Sessoms, P. A systematic literature review of the use and effectiveness of the Computer Assisted Rehabilitation Environment for research and rehabilitation as it relates to the wounded warrior. Work 2015 , 50 , 121–129. [ Google Scholar ] [ CrossRef ] [ PubMed ] [ Green Version ]
- Smith, A.J.; Lemaire, E.D. Temporal-spatial gait parameter models of very slow walking. Gait Posture 2018 , 61 , 125–129. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Imoto, D.; Hirano, S.; Mukaino, M.; Saitoh, E.; Otaka, Y. A novel gait analysis system for detecting abnormal hemiparetic gait patterns during robot-assisted gait training: A criterion validity study among healthy adults. Front. Neurorobot. 2022 , 16 , 1047376. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Khera, P.; Kumar, N. Role of machine learning in gait analysis: A review. J. Med. Eng. Technol. 2020 , 44 , 441–467. [ Google Scholar ] [ CrossRef ]
- Alfayeed, S.M.; Saini, B.S. Human Gait Analysis Using Machine Learning: A Review. In Proceedings of the 2021 International Conference on Computational Intelligence and Knowledge Economy (ICCIKE), Dubai, United Arab Emirates, 17–18 March 2021; pp. 550–554. [ Google Scholar ]
- Slemenšek, J.; Fister, I.; Geršak, J.; Bratina, B.; van Midden, V.M.; Pirtošek, Z.; Šafarič, R. Human Gait Activity Recognition Machine Learning Methods. Sensors 2023 , 23 , 745. [ Google Scholar ] [ CrossRef ]
- Fricke, C.; Alizadeh, J.; Zakhary, N.; Woost, T.B.; Bogdan, M.; Classen, J. Evaluation of Three Machine Learning Algorithms for the Automatic Classification of EMG Patterns in Gait Disorders. Front. Neurol. 2021 , 12 , 666458. [ Google Scholar ] [ CrossRef ]
- Liuzzi, P.; Carpinella, I.; Anastasi, D.; Gervasoni, E.; Lencioni, T.; Bertoni, R.; Carrozza, M.C.; Cattaneo, D.; Ferrarin, M.; Mannini, A. Machine learning based estimation of dynamic balance and gait adaptability in persons with neurological diseases using inertial sensors. Sci. Rep. 2023 , 13 , 8640. [ Google Scholar ] [ CrossRef ]
- Rastegari, E.; Azizian, S.; Ali, H. Machine learning and similarity network approaches to support automatic classification of Parkinson’s diseases using accelerometer-based gait analysis. In Proceedings of the 52nd Annual Hawaii International Conference on System Sciences, Maui, HI, USA, 8–11 January 2019; pp. 4231–4242. [ Google Scholar ]
- Harris, E.J.; Khoo, I.-H.; Demircan, E. A Survey of Human Gait-Based Artificial Intelligence Applications. Front. Robot. AI 2022 , 8 , 749274. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Luo, S.; Androwis, G.; Adamovich, S.; Su, H.; Nunez, E.; Zhou, X. Reinforcement Learning and Control of a Lower Extremity Exoskeleton for Squat Assistance. Front. Robot. AI 2021 , 8 , 702845. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Romijnders, R.; Warmerdam, E.; Hansen, C.; Schmidt, G.; Maetzler, W. A Deep Learning Approach for Gait Event Detection from a Single Shank-Worn IMU: Validation in Healthy and Neurological Cohorts. Sensors 2022 , 22 , 3859. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Kaur, R.; Motl, R.W.; Sowers, R.; Hernandez, M.E. A Vision-Based Framework for Predicting Multiple Sclerosis and Parkinson’s Disease Gait Dysfunctions—A Deep Learning Approach. IEEE J. Biomed. Health Inform. 2023 , 27 , 190–201. [ Google Scholar ] [ CrossRef ]
Click here to enlarge figure
| Reference n° | Gait Analysis System | Technology Description | Neurological Disorder | Clinical Implication | |
|---|---|---|---|---|---|
| Non- Wearable Sensors | Wearable Sensors | ||||
| [ ] | X | X | Three-dimensional gait analysis in laboratory, including optometric system, a dynamometric platform, and ad hoc software. | PD with 1.5–2 H&Y stage | Reduced gait speed and step length, showing bilateral extra rotation of knee, ankle, and foot. |
| [ ] | X | Triaxial accelerometer-based device placed on the fifth lumbar vertebrae and a double-sided tape. | PD with 1–3 H&Y stage | NA | |
| [ ] | X | Instrumented force-sensitive insole placed in patients’ shoes, with eight pressure-sensitive sensors. | PD with 2–3 H&Y stage | Stride-to-stride variability due to bradykinesia, loss of muscle synergies in the lower limb, and lack of rhythmicity. | |
| [ ] | X | X | Motion-capture based gait analysis compared to mobile sensor (inertial sensors) gait analysis, which were integrated in the mid-sole of the athletic shoes. | PD with 1–4 H&Y stage | Reduced gait speed, stride time, and length; increased duration stance phase time accompanied by a synchronic decreasing duration of swing phase time. |
| [ ] | X | X | Gait assessment through an optoelectronic (48 retroflected markers), inertial, and a smartphone-based capture system. | PD with <3 H&Y stage | NA |
| [ ] | X | Wearable device compared to Opti Track system, using an error state Kalman filter algorithm. | PD | NA | |
| [ ] | X | Stereophotogrammetric system (Vicon Motion Systems Ltd., Oxford, UK) and reflective markers to estimate joints’ angles. | MS with a score of ≤5–6 | MS patients showed reduced gait speed, which correlated with a decrease in cadence, step length, and swing time, and an increase in stance time. Additionally, authors found an increased pelvic tilt, which negatively correlates with the 6MWT. | |
| [ ] | X | X | Wireless AS200 system, comprising three line-scanning camera system and 11 active infrared markers attached on body’s patient, with a 2-mm accuracy. | MS with a mean score of 3.6 in EDSS | MS patients manifested changes in variability of movement gait patterns due to fatigue, altered motor coordination linked to additional activity of the antagonists, or insufficient strength produced by the agonists. |
| [ ] | X | Walkway sensor and machine learning (XGB) process to distinguish MS patients’ degree of severity based on their gait features. | MS with a mean score of 2.11 in EDSS | Step time and step width were considered as the most important variables to distinguish level of severity of MS subjects. | |
| [ ] | X | X | SMART-E stereophotogrammetric system (BTS, Milan, Italy) with eight infrared cameras (for acquiring kinematic data). Sensorized pathway with 2 piezoelectric force platforms (for acquiring kinetic data), 22 retro-reflective spherical markers for lower-body segments, and 15 markers for the upper body, placed on specific anatomic sites. | Spino-CA autosomal dominant (type 1 and 2) and Friedreich’s ataxia as recessive ataxia | Loss of lower limbs control during gait and of ability to stabilize a walking strategy over time. CA patients definitively lack a stable gait control behavior since the cerebellum functions of motor behavior and developing new motor patterns are altered. |
| [ ] | X | Triaxial accelerometer. | Spino-CA with a mean score of 3.9 for stance and gait in SARA | Gait velocity, cadence, step length, step regularity, and step repeatability are strongly correlated with disease duration. | |
| [ ] | X | Seven inertial sensors while performing two independent trials of gait and balance assessments. | CA | NA | |
| [ ] | X | Three Opal inertial sensors were attached on both feet and the posterior trunk at the level of L5 with elastic Velcro bands. | Spino-CA with a mean score of 3.6 for stance and gait in SARA | Minimal changes in gait spatial–temporal parameters can be considered as accurate markers for CA progression. | |
| Reference n° | Gait Analysis System | Technology Description | Neurological Disorder | Clinical Implication | |
|---|---|---|---|---|---|
| NWS | WS | ||||
| [ ] | X | A 10 m walkway with a pressure sensitive mat. Spatial–temporal parameters were registered using GaitRite mat, which contains a total of 13,824 sensors. | Post-stroke patients (both ischemic and hemorrhagic) | Most useful gait parameters are step length, swing time, and stance time. In addition, authors stated that asymmetry time values are not reliable parameters to assess gait in post-stroke patients. | |
| [ ] | X | Inertial Measurment Unit (IMU) system (Xsens Technology B.V., Enschede, The Netherlands, Hengelo) composed of seven inertial sensors. | Post-stroke patients | NA | |
| [ ] | X | Kinect v2, which included an 8-core Intel in addition to an ad hoc application designed to register the 3D position and orientation of the 25 human joints provided by the Kinect v2. | Post-stroke patients (both ischemic and hemorrhagic) | Results indicated that patients with a higher fall risk manifested lower gait velocity and cadence, a shorter stride and step length, and higher double support time. Additionally, the risk of falling was related to increased trunk and pelvic obliquity and tilt, and to decreased hip flexion–extension and ankle height variation. | |
| [ ] | X | Odonate 3D motion capture system in a mobile terminal and a workstation. This innovative a binocular depth camera combined with an artificial intelligence system to capture, analyze, and calculate gait parameters automatically. | Post-stroke patients | Alterations were found in spatial–temporal and kinematic parameters; thus, this new system can perform an objective gait assessment in five minutes, also in a home-based setting. | |
| [ ] | X | Five synchronized IMUs. | Severe TBI patients | Severe TBI patients present serious difficulties in maintaining balance during gait, especially movements of the head, which are the most impaired, probably related to vestibular dysfunctions due to traumatic events. Additionally, authors suggested to assess gait through dynamic balance skills during curved trajectories as in Figure-of-8 Walk Test. | |
| [ ] | X | Three IMUs were attached with elastic straps over both lateral ankles to detect gait phases and over the fifth lumbar vertebrae. | TBI | TBI patients manifest great imbalances in dynamic balance, especially in antero-medial weight shifting, when compared with healthy control subjects. | |
| The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
Share and Cite
Bonanno, M.; De Nunzio, A.M.; Quartarone, A.; Militi, A.; Petralito, F.; Calabrò, R.S. Gait Analysis in Neurorehabilitation: From Research to Clinical Practice. Bioengineering 2023 , 10 , 785. https://doi.org/10.3390/bioengineering10070785
Bonanno M, De Nunzio AM, Quartarone A, Militi A, Petralito F, Calabrò RS. Gait Analysis in Neurorehabilitation: From Research to Clinical Practice. Bioengineering . 2023; 10(7):785. https://doi.org/10.3390/bioengineering10070785
Bonanno, Mirjam, Alessandro Marco De Nunzio, Angelo Quartarone, Annalisa Militi, Francesco Petralito, and Rocco Salvatore Calabrò. 2023. "Gait Analysis in Neurorehabilitation: From Research to Clinical Practice" Bioengineering 10, no. 7: 785. https://doi.org/10.3390/bioengineering10070785
Article Metrics
Article access statistics, further information, mdpi initiatives, follow mdpi.

Subscribe to receive issue release notifications and newsletters from MDPI journals
An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
- My Bibliography
- Collections
- Citation manager
Save citation to file
Email citation, add to collections.
- Create a new collection
- Add to an existing collection
Add to My Bibliography
Your saved search, create a file for external citation management software, your rss feed.
- Search in PubMed
- Search in NLM Catalog
- Add to Search
Gait analysis: clinical facts
Affiliation.
- 1 Health Sciences Research Centre, University of Salford, Salford, UK - [email protected].
- PMID: 27618499
Gait analysis is a well-established tool for the quantitative assessment of gait disturbances providing functional diagnosis, assessment for treatment planning, and monitoring of disease progress. There is a large volume of literature on the research use of gait analysis, but evidence on its clinical routine use supports a favorable cost-benefit ratio in a limited number of conditions. Initially gait analysis was introduced to clinical practice to improve the management of children with cerebral palsy. However, there is good evidence to extend its use to patients with various upper motor neuron diseases, and to lower limb amputation. Thereby, the methodology for properly conducting and interpreting the exam is of paramount relevance. Appropriateness of gait analysis prescription and reliability of data obtained are required in the clinical environment. This paper provides an overview on guidelines for managing a clinical gait analysis service and on the principal clinical domains of its application: cerebral palsy, stroke, traumatic brain injury and lower limb amputation.
PubMed Disclaimer
Similar articles
- Differential effects of rhythmic auditory stimulation and neurodevelopmental treatment/Bobath on gait patterns in adults with cerebral palsy: a randomized controlled trial. Kim SJ, Kwak EE, Park ES, Cho SR. Kim SJ, et al. Clin Rehabil. 2012 Oct;26(10):904-14. doi: 10.1177/0269215511434648. Epub 2012 Feb 3. Clin Rehabil. 2012. PMID: 22308559 Clinical Trial.
- The Influence of a Constraint and Bimanual Training Program Using a Variety of Modalities, on Upper Extremity Functions and Gait Parameters Among Children with Hemiparetic Cerebral Palsy: A Case Series. Cohen-Holzer M, Sorek G, Schless S, Kerem J, Katz-Leurer M. Cohen-Holzer M, et al. Phys Occup Ther Pediatr. 2016;36(1):17-27. doi: 10.3109/01942638.2014.990549. Epub 2014 Dec 18. Phys Occup Ther Pediatr. 2016. PMID: 25521486
- Repeatability and validation of gait deviation index in children: typically developing and cerebral palsy. Massaad A, Assi A, Skalli W, Ghanem I. Massaad A, et al. Gait Posture. 2014;39(1):354-8. doi: 10.1016/j.gaitpost.2013.08.001. Epub 2013 Sep 11. Gait Posture. 2014. PMID: 24079975
- The effect of tuning ankle foot orthoses-footwear combination on the gait parameters of children with cerebral palsy. Eddison N, Chockalingam N. Eddison N, et al. Prosthet Orthot Int. 2013 Apr;37(2):95-107. doi: 10.1177/0309364612450706. Epub 2012 Jul 24. Prosthet Orthot Int. 2013. PMID: 22833518 Review.
- The locomotion laboratory as a clinical assessment system. Winter DA. Winter DA. Med Prog Technol. 1976;4(3):95-106. Med Prog Technol. 1976. PMID: 138792 Review.
- Machine learning-based gait adaptation dysfunction identification using CMill-based gait data. Yang H, Liao Z, Zou H, Li K, Zhou Y, Gao Z, Mao Y, Song C. Yang H, et al. Front Neurorobot. 2024 Jul 29;18:1421401. doi: 10.3389/fnbot.2024.1421401. eCollection 2024. Front Neurorobot. 2024. PMID: 39136036 Free PMC article.
- Development of an IMU based 2-segment foot model for an applicable medical gait analysis. Bauer L, Hamberger MA, Böcker W, Polzer H, Baumbach SF. Bauer L, et al. BMC Musculoskelet Disord. 2024 Jul 31;25(1):606. doi: 10.1186/s12891-024-07719-0. BMC Musculoskelet Disord. 2024. PMID: 39085824 Free PMC article.
- 3D motion analysis dataset of healthy young adult volunteers walking and running on overground and treadmill. Riglet L, Delphin C, Claquesin L, Orliac B, Ornetti P, Laroche D, Gueugnon M. Riglet L, et al. Sci Data. 2024 May 30;11(1):556. doi: 10.1038/s41597-024-03420-y. Sci Data. 2024. PMID: 38816523 Free PMC article.
- The applicability of markerless motion capture for clinical gait analysis in children with cerebral palsy. Wishaupt K, Schallig W, van Dorst MH, Buizer AI, van der Krogt MM. Wishaupt K, et al. Sci Rep. 2024 May 24;14(1):11910. doi: 10.1038/s41598-024-62119-7. Sci Rep. 2024. PMID: 38789587 Free PMC article.
- Can on-line gait training improve clinical practice? Study protocol for feasibility randomised controlled trial of an on-line educational intervention to improve clinician's gait-related decision-making in ambulant children and young people with cerebral palsy. Hebda-Boon A, Shortland AP, Birn-Jeffery A, Morrissey D. Hebda-Boon A, et al. Pilot Feasibility Stud. 2024 May 14;10(1):76. doi: 10.1186/s40814-024-01477-5. Pilot Feasibility Stud. 2024. PMID: 38745259 Free PMC article.
Publication types
- Search in MeSH
Related information
- Cited in Books
LinkOut - more resources
Full text sources.
- Minerva Medica
- MedlinePlus Consumer Health Information
- MedlinePlus Health Information
- Citation Manager
NCBI Literature Resources
MeSH PMC Bookshelf Disclaimer
The PubMed wordmark and PubMed logo are registered trademarks of the U.S. Department of Health and Human Services (HHS). Unauthorized use of these marks is strictly prohibited.
REVIEW article
Present and future of gait assessment in clinical practice: towards the application of novel trends and technologies.

- 1 Mechanical Engineering Department, Khalifa University, Abu Dhabi, United Arab Emirates
- 2 School of Mechanical and Aerospace Engineering, Monash University, Clayton Campus, Melbourne, Australia
- 3 Weill Cornell Medicine, New York City, NY, United States
- 4 Biomedical Engineering Department, Khalifa University, Abu Dhabi, United Arab Emirates
- 5 Health Engineering Innovation Center, Khalifa University, Abu Dhabi, United Arab Emirates
Background: Despite being available for more than three decades, quantitative gait analysis remains largely associated with research institutions and not well leveraged in clinical settings. This is mostly due to the high cost/cumbersome equipment and complex protocols and data management/analysis associated with traditional gait labs, as well as the diverse training/experience and preference of clinical teams. Observational gait and qualitative scales continue to be predominantly used in clinics despite evidence of less efficacy of quantifying gait.
Research objective: This study provides a scoping review of the status of clinical gait assessment, including shedding light on common gait pathologies, clinical parameters, indices, and scales. We also highlight novel state-of-the-art gait characterization and analysis approaches and the integration of commercially available wearable tools and technology and AI-driven computational platforms.
Methods: A comprehensive literature search was conducted within PubMed, Web of Science, Medline, and ScienceDirect for all articles published until December 2021 using a set of keywords, including normal and pathological gait, gait parameters, gait assessment, gait analysis, wearable systems, inertial measurement units, accelerometer, gyroscope, magnetometer, insole sensors, electromyography sensors. Original articles that met the selection criteria were included.
Results and significance: Clinical gait analysis remains highly observational and is hence subjective and largely influenced by the observer's background and experience. Quantitative Instrumented gait analysis (IGA) has the capability of providing clinicians with accurate and reliable gait data for diagnosis and monitoring but is limited in clinical applicability mainly due to logistics. Rapidly emerging smart wearable technology, multi-modality, and sensor fusion approaches, as well as AI-driven computational platforms are increasingly commanding greater attention in gait assessment. These tools promise a paradigm shift in the quantification of gait in the clinic and beyond. On the other hand, standardization of clinical protocols and ensuring their feasibility to map the complex features of human gait and represent them meaningfully remain critical challenges.
1. Introduction
Changes in the signature of gait, or the unique sequential walking pattern in humans, reveal key information about the status and progression of numerous underlying health challenges, from neurological and musculoskeletal conditions to cardiovascular and metabolic disease, and to ageing-associated ambulatory dysfunction and trauma. Accurate reliable identification of gait patterns and characteristics in clinical settings, as well as monitoring and evaluating them over time, enable effective tailored treatment, inform predictive outcome assessment, and an allow for an overall better practice of precision medicine.
In clinical gait assessment, both a person's “ability” to walk and “how” the individual walks are highly relevant. The walking ability of a person is typically based on two main aspects: how far can an individual walk and what is his/her tolerance level ( 1 ). For example, for post stroke gait assessment, 3-, 6-, or 10 min walk tests are used, in addition to Functional Ambulation Category (FAC), Short Physical Performance Battery (SPPB), and/or Motor Assessment Scale (MAS). Other clinical subjective assessment scales include the Unified Parkinson Disease Rating Scale (UPDRS) the Scale for the Rating and Assessment of Ataxia (SARA)], the Alzheimer's Disease Assessment Scale (ADAS), the Expanded Disability Status Scale (EDSS) the High-level MobilitARTIy Assessment Tool (HiMAT), and the Dynamic Gait Index ( 2 ). The quality of gait or “how” the person walks, on the other hand, highly depends on the quantification of gait patterns and accurate identification of specific gait characteristics. Despite evidence of the advantages of quantitative instrumented gait analysis (IGA) in clinical practice and recommendations by the National Institute for Health and Clinical Excellence (NICE) ( 3 ) identifying IGA is the preferrable choice for “gait-improving surgery”, it remains not well leveraged in clinical settings due to the high cost/cumbersome equipment and complex protocols/data analysis associated with traditional gait labs, as well as diverse training, experience and preference of clinical teams ( 3 – 5 ). Moreover, the use of IGA by allied health professionals, such as physiotherapists, occupational therapists and orthotists, and training also remain non standardized and limited ( 5 – 7 ).
Observational gait analysis continues to be popular among clinicians due to its inherent simplicity, availability, and low cost ( 8 ). On the other hand, the validity, reliability, specificity, and responsiveness ( 9 , 10 ) of these qualitative methods are controversial and increasingly being questioned ( 6 ). Furthermore, there is evidence to suggest that subjective clinical assessment scales may not be sensitive to disease severity and specific characteristics and may limit understanding of underlying disease mechanisms, hence adversely impacting optimal treatment ( 11 ). Examples of such scales include Multiple Sclerosis (MS), where subjective measures, such as the Expanded Disability Status Scale (EDSS), the Multiple Sclerosis Severity Scale (MSSS), Multiple Sclerosis Walking Scale (MSWS), and Multiple Sclerosis Functional Composite (MSFC), continue to be widely used in clinical practice. These scales have been criticized for lack of sensitivity ( 12 ), high interrater variability ( 13 ), as well as being prone to practice effects and variability ( 14 , 15 ). Similarly, clinical assessment of Parkinson's disease (PD) using the Unified Parkinson's Disease Rating Scale (UPDRS) is subjective and largely dependent on the expertise and experience of the clinicians, as well as the severity of the disease ( 16 ). In Stroke patients, assessment tests such as Functional Ambulation Category (FAC), Short Physical Performance Battery (SPPB), and/or Motor Assessment Scale (MAS) are typically employed, along with qualitative observational/visual gait analysis (using naked eye or video images). Nevertheless, the validity, reliability, specificity, and responsiveness of these qualitative methods are questioned ( 9 ), and although they may be useful for the rudimentary evaluation of some gait parameters, they are not adequate for analyzing the multifaceted aspects of gait variability and complexity ( 17 ).
Instrumented gait analysis (IGA), which can provide accurate and precise quantitative measurement of gait patterns and characteristics, has long been the gold standard for gait assessment in research practice ( 18 ). IGA generally refers to the use of instrumentation to capture and analyze a variety of human gait parameters (spatiotemporal, kinematic, and kinetic measures). Traditional IGA systems include motion capture systems, and force plates, instrumented walkways, and treadmills, while more recent systems comprise of miniaturized wearable sensing system, computational platforms and modalities ( 18 ). Literature on the clinical applicability and efficacy of IGA indicates that IGA-based quantitative assessment can improve the diagnosis, outcome prediction, and rehabilitation of various gait impairments as compared to conventional observational scales and techniques for gait dysfunction in a wide spectrum of diseases including MS, PD, Stroke, and Cerebral Palsy ( 9 – 13 ). A recent review on the clinical efficacy of IGA confirms that there is strong evidence that 3-D gait analysis, or 3DGA; has the potential to alter and reinforce treatment decisions; increases confidence in treatment planning and agreement among clinicians; can better identify diagnostic groups and expected treatment outcomes; and overall can improve patient outcomes if recommendations are followed ( 19 ).
Emerging at an unprecedented rate, wearable sensing systems and associated computational modalities are rapidly transforming the quality and accessibility of healthcare, spanning multiple applications from neurology and orthopedics to cardiovascular, metabolic, and mental health. Magnetic (e.g., magnetometers), inertial measurement (e.g., accelerometers and gyroscopes), and force sensors (e.g., insole foot pressure) nowadays offer unprecedented data capture opportunities that can overcome limitations of non-wearable devices due to their low-cost, less setup-time and complexity, lightweight, and portability, making them ideal for out-of-lab and continuous monitoring in the clinic and beyond ( 20 ). Magneto-inertial measurement units (MIMUs), in conjunction with force pressure sensors, have the capability of capturing spatiotemporal, kinematic, and kinetic gait data ( 2 ) rendering the concept of a mobile gait lab a reality. Such labs can inherently overcome the limitations of IGA traditional labs, providing less costly and cumbersome tools with potential for gait assessment in natural environments (clinics, homes, sports arenas, etc.), user friendly interfaces, and the opportunity to provide continuous real-time feedback to clinicians and patients, as well as tele rehabilitation capabilities. In addition, wearable systems allow for easy synchronization with other physiological measurement systems, including EMG, ECG, and EEG, towards the acquisition of invaluable multimodal continuous physiological data in various settings.
This scoping review aims to provide a summary of the current state of clinical gait assessment, including shedding light on gait pathologies and clinical indices and scales, as well as a roadmap for the development of future gait mobile labs- highlighting the clinical validity and reliability of the latest devices and data interpretation algorithms. The word novel in the title of this review reflects recent emergence/implementation of the technologies reviewed and/or recent commercialization. This includes wearable technologies, as well as AI-driven computational platforms. The remainder of the review is structured as follows: Section 2 describes the adopted methodology, including the approach, search strategy and selection criteria. Section 3 details clinical gait pathologies, relevant parameters, as well as current clinical gait assessment tools, scales, and indices, while Section 4 presents gait assessment technologies applicable to clinical settings, including state-of-the-art imaging techniques and wearable technologies, algorithms, and novel AI-driven computational platforms. Section 5 deliberates on the concept of a mobile gait lab for clinical applications. Section 6 highlights the limitations, while Section 7 presents the conclusive remarks and future work.
This review is aimed at summarizing various clinical gait pathologies and associated parameters, applicable gait analysis techniques and gait indices, and the latest trends in wearables systems and algorithms. To address this broader research objective, the authors adopted a scoping review approach rather than a systematic review approach. As reported in ( 21 ), scoping reviews are ideal for addressing a broader scope with a more expansive inclusion criterion.
2.1. Search criteria
A keyword search was performed in PubMed, Web of Science, Medline, and ScienceDirect databases, using a combination of search terms from the following groups: 1. (normal gait OR pathological gait OR gait parameters OR gait indices), 2. (gait assessment OR gait analysis), 3. (wearable systems OR wearable algorithms), 4. (inertial measurement units OR accelerometer OR gyroscope OR magnetometer OR insole sensors OR electromyography sensors). No limit for the year of publication was set, however, the search was last updated in December 2021. Only articles written in the English language were considered in this review. In addition, the reference list of the included articles was checked to identify additional relevant publications meeting the inclusion criteria. The literature search and data extraction were carried out independently by two authors (AAH, DMM) and any inconsistencies and disagreements discrepancies were resolved through following discussions with the other authors (NA, MER, KK).
This scoping review included original published works and review articles which met the following inclusion/exclusion criteria: (i) studies addressing various gait disorders and associated gait parameters, (ii) studies focusing on instrumented gait analysis techniques and gait indices, (iii) studies evaluating the use, validity, and reliability of wearable-based gait measurement devices/systems for measuring gait events, and evaluating and assessing gait dysfunction, (iv) studies concerning the applicability of sensor fusion techniques and algorithms applicable for wearable-based systems with application to gait analysis. The title and/or abstract of the studies were initially screened for suitability. The full-text articles meeting the inclusion criteria were obtained for data extraction and synthesis. A flowchart explaining the same is shown in Figure 1 .
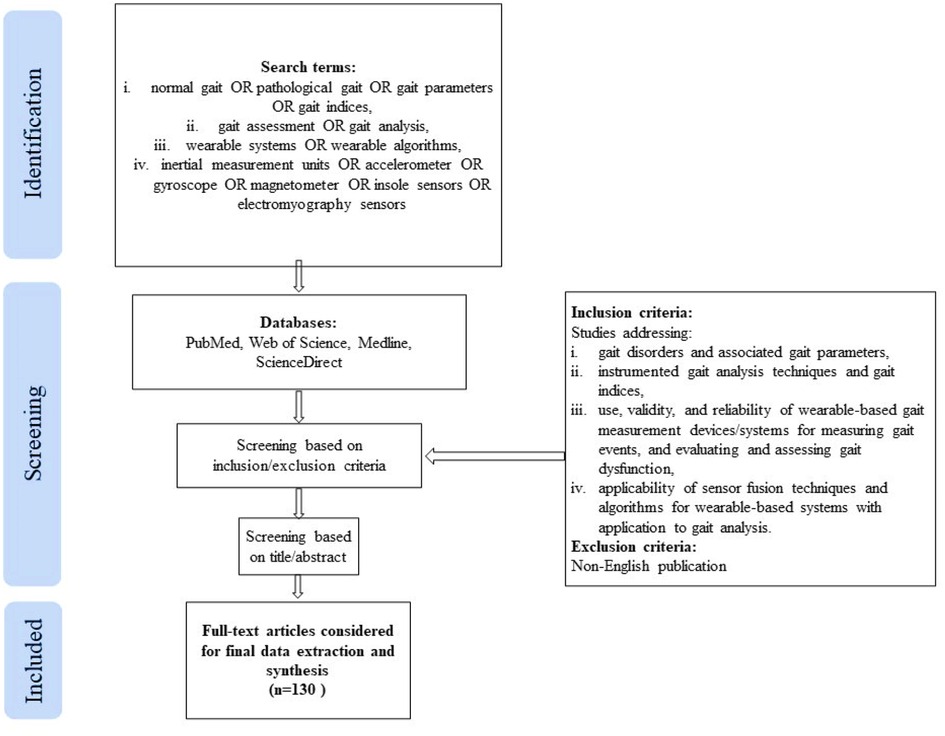
Figure 1 . Publication selection process.
3. Clinical gait pathologies and parameters
3.1. normal gait cycle and parameters.
Normal gait can be defined as a series of rhythmic, systematic, and coordinated movements of the limbs and trunk that results in the forward advancement of the body's center of mass ( 22 ). A result of intricate dynamic interactions between the central nervous system and feedback mechanisms ( 23 ), walking is characterized by individual gait cycles and functional phases ( Figure 2 ). A gait cycle consists of two main phases, stance, and swing, which are further divided into five and three functional phases, respectively. The stance phase corresponds to the duration between heel strike and toe-off of the same foot, constituting approximately 60% of the gait cycle. The swing phase begins with toe-off and ends with heel contact of the same foot and occupies 40% of the cycle. As each functional phase contributes to successfully accomplishing the goal of walking, healthy gait involves cyclic and complementary movements of the limbs under control. It is characterized by stance stability; toe clearance during the swing; pre-positioning at swing; sufficient step length; as well as mechanical and metabolic efficiency ( 24 ). Table 1 provides gait parameter ranges based on studies on healthy adults. Determining an appropriate normal range for many of the features is highly challenging as individuals exhibit a wide range of gait patterns across different age groups and gender ( 17 ).
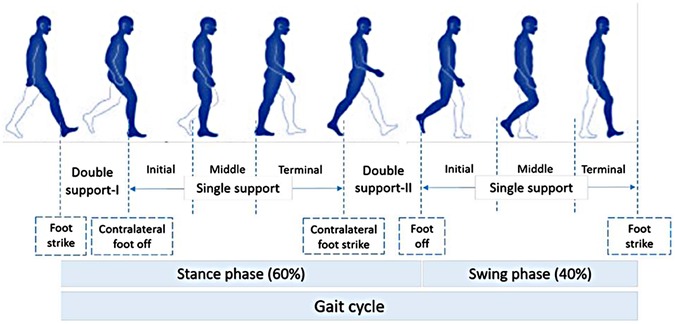
Figure 2 . Normal gait cycle (adapted from 1 ).

Table 1 . Gait parameters for healthy individuals ( 1 ).
3.2. Gait parameters associated with pathology
Gait disorders are typically associated with deficits in the brain, spinal cord, peripheral nerves, muscles, joints, or bones. Some medical conditions leading to pathological gait include but not limited to muscular dystrophy, myelodysplasia, cerebral palsy, arthritis, osteoarthritis, head injury, lower limb amputation, multiple sclerosis, rheumatoid, spinal cord injury, parkinsonism, and stroke ( 25 ).
In neuromuscular conditions, the loss of central control affects the motion. In general, patients walk slower than healthy individuals and with compromised spatiotemporal, kinematic, and kinetic parameters. In older adults, a walking speed decline of 0.7% per year is observed, along with significant changes in cadence and step length. The aging population also exhibits lower knee extension at heel-strike and knee flexion during the swing phase ( 23 , 26 ). The following subsections describe some of the most common gait disorders and associated pathological parameters. The associated impacted parameters are summarized in Table 2 .

Table 2 . Effect of pathology on gait disorders.
3.2.1. Neurological gait disorders in elderly people
Gait ailments associated with aging lead to reduction in the quality of life and increased morbidity and mortality. Elderly patients exhibit complex gait disorders, and their dual task ability deteriorates due to a decline in their central resources ( 23 , 26 ).
Specific gait dysfunction noted in the elderly population are summarized as follows:
3.2.1.1. Hypokinetic-rigid gait disorders
Shuffling with a reduced step height and stride length characterizes hypokinetic gait disorder ( 27 ). Reduced arm swing with slow turning movements is also present in isolation. Festination, when patients use rapid small steps to maintain the feet beneath the forward moving trunk, is also observed. Ataxic elements include broad stance width and an increased variability in timing and amplitude of steps ( 27 ). Gait associated with underlying diseases, such as Parkinson's disease, cerebrovascular disease, and ventricular widening, is classified within hypokinetic-rigid gait disorders ( 27 , 28 ).
3.2.1.2. Cautious and careless gait
Defined as gait during which people move slowly with a wider base, and shorter stride, with minimal trunk movement, while the knees and elbows are bent. Whereas careless gait is when patients appear overly confident and walk insensitively fast. Careless gait is due to confusion and delirium associated with old age ( 27 ).
3.2.1.3. Dyskinetic gait or involuntary movements
Patients with post-anoxic encephalopathy exhibit bouncing gait and stance. This is also observed in patients with Parkinson's disease-causing excessive trunk movements contributing to falls. Several dystonic patients are reported to walk on their toes ( 27 , 28 ).
3.21.1.4. Psychogenic gait disorders
Gait dysfunction is common in elderly people due to adverse effects of drugs leading to extrapyramidal side-effects, sedation, orthostatic hypotension, behavioral abnormalities, or ataxia ( 27 , 28 ).
3.2.1.5. Fluctuating or episodic gait disorders
Elderly people often exhibit fluctuating or episodic gait disorder after exercise due to fatigue, and it might be an indication of underlying vascular or neurogenic limping. Freezing gait is part of hypokinetic-rigid syndrome ( 27 , 28 ).
3.2.2. Gait disorders in Parkinson's disease
PD is a neurological disorder which leads to cognition, where gait impairment deteriorates with disease progression, increasing reliance on cognition to control gait. Due to cognitive impairment with PD, the ability to compensate for gait disorders diminishes, leading to further gait impairment. PD is characterized by deficit in amplitude and gait speed, along with increased gait variability ( 29 ).
3.2.3. Gait in diabetic peripheral neuropathy
Neuropathy of motor, sensory, and autonomic components of the nervous system are one of the many complications of Type II Diabetes (T2D). An intact central and peripheral nervous system are essential to initiate and control healthy gait, along with sufficient muscle strength, bone, and joint movements in complete range for normal locomotion. Patients diagnosed with T2D take extra steps when walking in straight paths and during turns, along with an overall reduction in walking speed, step length, cadence, and fewer acceleration patterns as compared to age-matched healthy controls. Joint range of motion is also altered in T2D, where patients with diabetic peripheral neuropathy exhibit a reduced range of motion at the ankle joint in dorsi and plantar flexion and a reduced flexion and extension range of motion at the knee joint in both, as compared to non-diabetic people ( 24 ).
3.2.4. Post stroke gait
Hemiplegia after stroke contributes to significant reduction in gait performance. In stroke survivors, function of the cerebral cortex is usually impaired, whilst that of spinal cord is preserved. Dysfunction is typically demonstrated by a marked asymmetrical deficit. Decreased walking speed and cadence, in addition to longer gait cycle and double limb support as compared to healthy individuals. For hemiplegic stroke survivors, a reduced peak extension of the hip joint in late stance, varying peak lateral pelvis displacement, knee flexion and decreased plantarflexion of ankle at toe off are reported. The GRF (Ground Reaction Force) pattern is characterized as asymmetric, along with decreased amplitude of joint moments, at the lower limb joints on the paretic side ( 30 ).

3.2.5. Total hip arthroplasty (THA)
Large deficits in gait speed ( 31 ), stride length ( 32 , 33 ), sagittal hip range of motion ( 32 , 33 ), hip abduction moment-coronal plane ( 31 ), and negligible changes in transverse plane hip range of motion ( 31 ), deficiency in single limb support time ( 31 ), are reported in patients post THA as compared to healthy controls. Peak hip extension is typically reduced, whereas peak hip flexion remains similar as compared to controls. In addition, peak hip abduction moment is reduced along with peak hip external rotation moment ( 34 ).
3.3. Clinical gait assessment measures and indices
The use of observational gait analysis and subjective rating sales continues to be widespread in clinical settings, both as a diagnostic tool and as a prognostic measure, as previously mentioned. Although these techniques can be useful for the initial rudimentary evaluation of some gait parameters, the validity, reliability, specificity, and responsiveness of these qualitative methods are highly questionable. Researchers have therefore proposed various pathology-specific gait indices and summary measures ( 35 ) based on commercially available technologies with accepted levels of accuracy Table 3 . summarizes the current clinical gait summary measures, discrete and continuous gait indices, and non-linear approaches reported in literature, along with advantages and disadvantages.

Table 3 . Clinical gait measures and indices ( 123 , 124 ).
4. Gait assessment technologies applicable to clinical settings
In the past couple of decades, remarkable technological advancement has been witnessed in the field of gait assessment and analysis, particularly in gait assessment technology. Instrumented walkways, both portable and non-portable, became a good alternative to complicated, bulky and non-portable traditional gait labs. These systems (for example the Walkway and StrideWay from Tekscan Inc., Boston, United States) are now widely used in research and to a limited extent in clinical practice. They typically include low-profile floor walkway systems equipped with grids of embedded sensors below the surface, which record foot-strike patterns as a function of time and space as an individual walks across the platform, and dedicated software which computes the various spatiotemporal gait measures Although these instrumented mats involve less setup time and are generally simple to operate as compared to traditional IGA labs, they are expensive, restrictive to specific operational environment to over-ground trials ( 36 , 37 ).
Marker-based optical motion capture (Mocap) is another rapidly emerging technology effective for obtaining 3D kinematic movement data. Passive Mocap systems [e.g., Vicon (Vicon Motion Systems Ltd, Oxford, United Kingdom) and ELITE optoelectronic system (BTS S. p .A., Milano, Italy)], include retro-reflective markers (that reflect the light emitted by high-resolution infrared cameras) attached to specific anatomic landmarks. The location of the marker is identified by decoding the camera images. Here, the markers must be calibrated for identification before the recording session commences. Active Mocap systems (e.g., Optotrak motion capture system; Northern Digital Inc., Waterloo, Canada), on the other hand, use light-emitting diode (LED) markers (reflect their own light powered by a battery), which are automatically identified ( 38 , 39 ). In the context of clinical relevance, although such systems yield extremely accurate reliable data, operational factors including infrastructure, non-portability, high cost, additional time required for initial set-up and calibration, operational complexity, and restrictions to indoor setup impose hurdles to their functional deployment in clinics and rehabilitation centers ( 84 ). Recently, more portable cost-effective alternatives, such as Microsoft Kinect (based on a depth sensor-based markerless motion capture solution) became the application of choice ( 40 ).
Optoelectronic systems (e.g., Optogait®, Microgate, Italy) have also been used to capture spatiotemporal gait parameters. These mainly consist of a transmitting and a receiving bar containing an infrared light. Interruptions of the communication between the emitter and receiver are detected by the system to calculate the various gait parameters ( 41 ).
An evolution in the measurement of gait kinetic parameters can also be witnessed in the last two decades. These parameters include ground reaction forces, and intersegmental joint reaction forces, moments, and powers. Instrumented walkways offer dynamic plantar pressure mapping but are expensive and do not provide joint kinetic data. Force plates are also used in various gait analysis studies ( 38 , 39 , 42 ). These are able to provide intersegmental joint reaction forces by using the ground reaction forces measured along with inverse dynamics models (Winters book) Chen et al. ( 93 ) developed a novel remote sensing technology called “Electrostatic Field Sensing (EFS)” for measuring human gait including stepping, walking, and running, and further extended the work to post-stroke gait. This technology is credited with several advantages, such as being non-contact, affordable, and allows long-time monitoring ( 43 ). Shoe insole systems represent another category of gait quantification tools and techniques. These systems are designed to allow for the recording of both dynamic plantar pressure and spatiotemporal data. F-scan (Tekscan Inc., Boston, United States) is an ultra-thin in-shoe pressure measurement system utilizing Force-Sensitive Resistive films (FSR) technology ( 44 ).
The characteristics of different measurement systems applicable to clinical settings are summarized in Table 4 , and the pros and cons of these systems are listed in Table 5 .
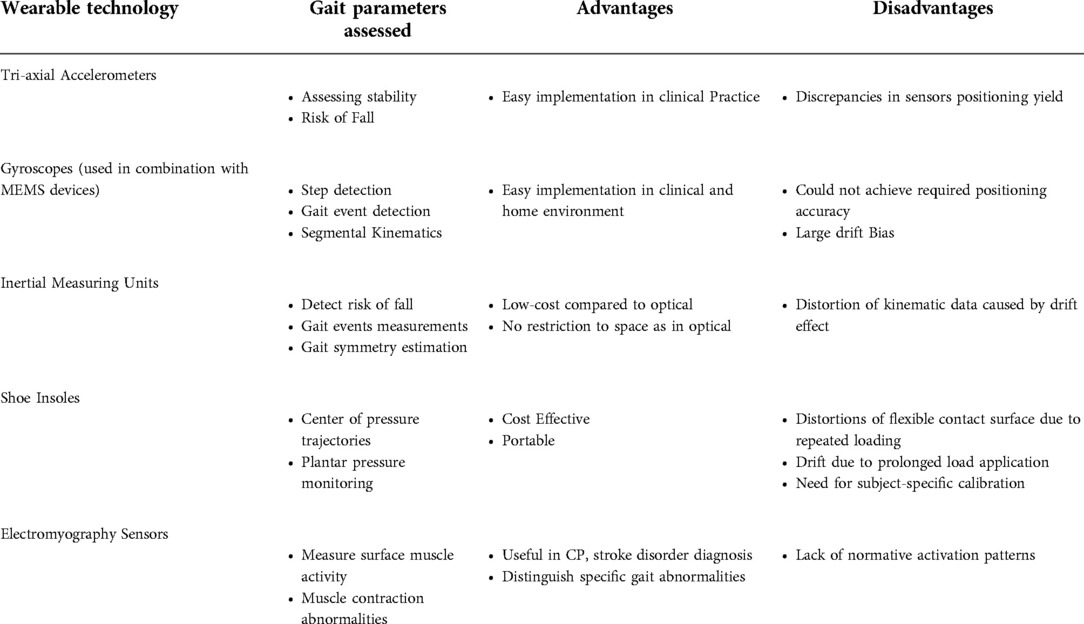
Table 4 . Portable wearable gait assessment tools.
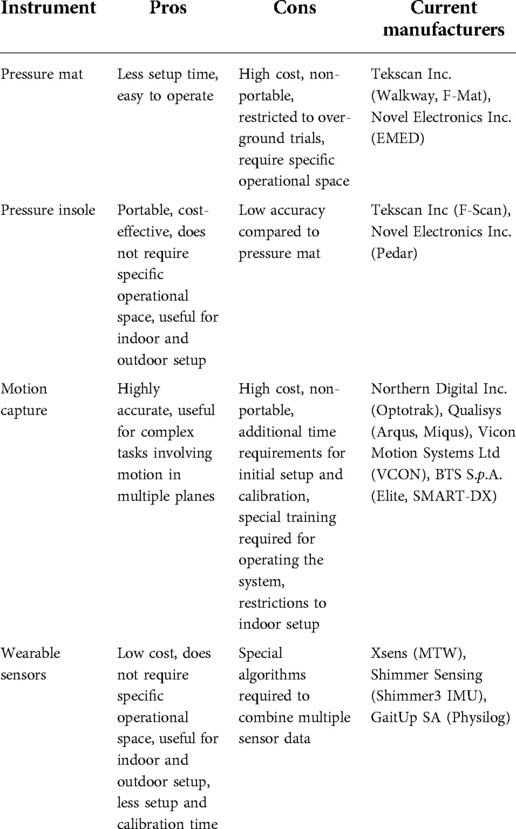
Table 5 . Pros and cons of different IGA systems.
Computational pipeline using computer vision techniques has been proposed as an ecological and precise method to quantify gait in children with neurodevelopmental disorders, along with the pose estimation software to obtain whole-body gait synchrony and balance ( 45 ). Speed, arm swing, postural control, and smoothness (or roughness) of movement features of gait for Parkinson's patients were extracted using videos processed by ordinal random forest classification model. Significant correlation between clinician labels and model estimates was reported, which provides gait impairment severity assessment in Parkinson's disease using single patient video, thereby reducing the need for sophisticated gait equipment ( 46 ). Computer vision-based gait assessment tools promise frequent gait monitoring using minimal resources ( 46 ). Deep learning to detect human subject in 2D images and then combining 3D sensing data to measure gait features has proven to be more robust than depth cameras in gait parameter acquisition ( 47 ).
4.1. Imaging techniques for gait assessment
As previously mentioned, marker-based optoelectronic systems are currently the most widely used systems in IGA among both research and clinical communities. On the other hand, one of the main sources of error inherent to these systems is the degree of movement of the skin, muscle, and other soft tissues, or the so- called soft tissue artifacts (STA), under the markers in relation to bony landmarks, hence violating the rigid body assumption underlying these methods ( 48 , 49 ). Moreover, STA varies by marker location in a unique and unpredictable manner, particularly during dynamic activities, which can make it unreliable for clinical applications ( 50 ).
Although not yet widespread in biomechanics, computer vision based markerless gait assessment methods offer a promising tool for gait assessment in research, as well clinical and sports biomechanics applications. By leveraging modern technologies, such as improved solvers, advanced image features and modern machine learning, markerless vision-based systems can reduce the required number of cameras, incorporating moving cameras, increasing the number of tracked individuals, and offering robust detection and fitting in diverse environments. On the other hand, issues such as accuracy and field-based feasibility remain to be addressed ( 51 ).
Three-dimensional imaging techniques have been successfully used to directly determine bone movements during walking as a gold reference standard to validate/improve current motion capture techniques ( 54 ). For example, researchers have resorted to quantifying STA by comparing with reference 3D kinematics of bone reconstructed from fluoroscopy-based tracking ( 53 ). Fluoroscopy has also emerged as a means for tracking position and orientation of underlying skeletal anatomy of the foot/ankle ( 54 ). Although single plane fluoroscopy yielded large errors when used to evaluate the accuracy of multi-segment foot models ( 49 ), dual fluoroscopy (DF) was found reliable and is considered as the current reference standard to compare joint angles ( 55 ). Combined with 2D/3D registration, video-fluoroscopy allows for accurate quantification of 3D joint motion free of STA ( 56 ). High-speed dual fluoroscopy (DF) has been reported to measure in-vivo bone motion of the foot and ankle with sub-millimeter and sub-degree errors ( 57 ). DF has also been used to evaluate multi-segment foot models and reported good agreement between DF and skin-marker data for the first metatarsal and sagittal plane measurements of the longitudinal arch ( 48 ).
Various researchers investigated the use of DF for clinical applications . In-vivo dual fluoroscopy was used to quantify the hip joint kinematics of patients with Femoroactabular impingement syndrome (FAIS) relative to asymptomatic, morphologically normal control participants during standing, level walking, incline walking and an unweighted functional activity. The kinematic position of the hip joint was obtained by registering projections of 3D computed Tomography models with DF images ( 58 ). Knee kinematic profiles were also obtained using 3D video-fluoroscopy and compared to actual and nominal flexion-extension, internal-external rotations, and antero-posterior translations profiles with optical mocap during stair climbing ( 59 ). Joint function for total talonavicular replacement after a complex articular fracture was evaluated using a full body gait analysis and 3D joint kinematics based on single-plane fluoroscopy ( 60 ). The 3D video fluoroscopic analysis was performed to assess joint motion of the replaced ankle ( 60 ). DF and CT imaging techniques were both employed to calculate in-vivo hip kinematics, along with model-based tracking, to compare the effect of different coordinate systems ( 61 ). Since marker-based systems are unable to accurately analyze talocrural or subtalar motion because the talus lacks palpable landmarks to place external markers ( 54 ), digitized video fluoroscopy was reportedly used to determine the sagittal plane motion of the medial longitudinal arch during dynamic gait ( 62 ). Characteristics of knee joint motion were also analyzed in 6DOF during treadmill walking using a dual fluoroscopy imaging system at different speeds ( 63 ).
DF uses anatomical landmarks visible on 3D CT reconstructions which substantially reduces errors due to STA ( 58 ). Computed tomography (CT) scans of participants are usually needed in DF to determine bone position from the DF images. Single plane fluoroscopy is restricted to 2D motion capture, while using a second FS allows for a full 3D analysis although a single gantry system has lower radiation than the biplane system with reported ionizing radiation levels of 10 µSv per trial ( 54 ). Stationary image intensifiers and static systems have a restricted field of view limiting their application to highly restricted movements ( 56 ). Moving fluoroscopes, consisting of a fluoroscopic unit mounted on a moving trolley which moves with the subject and is controlled by wire sensors to ensure that it remains in the field of view of the image intensifier ( 56 ), provide an enhanced field of view ideal for dynamic scenarios and moving joints.
Fluoroscopic systems designed for precise capture of bone movement and joint kinematics, unlike optical or inertial systems, are not yet commercially available, generally requiring in-house instrumentation and further performance evaluation. The evaluation would typically include determining the resolution of the hardware imaging chain, assessing how the hardware and software reduce or eliminate various distortions, and measuring static and dynamic accuracies and precisions based on precisely known motions and positions ( 64 ). Image quality is a major determinant of error in fluoroscopic applications ( 62 ). Pulse imaging of fluoroscopes, such as pulse width, limits image quality at a given frame rate. Increasing the pulse rate, which is function of pulse width, may add to radiation exposure, leading to an important tradeoff consideration between image quality and radiation exposure ( 63 ). Moving video-fluoroscopes reported lower gait velocity, step length, and cadence as compared to control conditions, indicating altered time distance parameters towards those of slow walking ( 56 ). So far, dynamic MRI used to define in- vivo talocrural and subtalar kinematics ( 65 ) does not allow data collection during normal gait.
Continued multidisciplinary collaborative efforts among biomechanists, imaging and computer vision experts, and clinicians are essential for fully leveraging these highly promising techniques in clinical applications.
4.2. Portable wearable systems for gait assessment
Wearable technology – the use of body-worn sensors to measure the characteristics of human locomotion, has recently emerged as an efficient, convenient, and most importantly, inexpensive option to quantitative gait analysis for both clinical and research-based applications ( Figure 3 ). In general, it uses individual sensor elements, such as accelerometers, gyroscopes, magneto resistive sensors, force/pressure sensors, goniometers, inclinometers, and electromyographic (EMG) sensors, or combined as an inertial measurement unit (IMU) ( 66 ). In comparison to conventional counterparts (e.g., walkway and camera based Mocap), wearable sensing enables continuous gait monitoring (> 2 h) outside the lab or clinic, allowing for replication of natural patterns of walking. Moreover, gait patterns over an ample distance could be measured as opposed to limited walking distance in a lab-based setting.
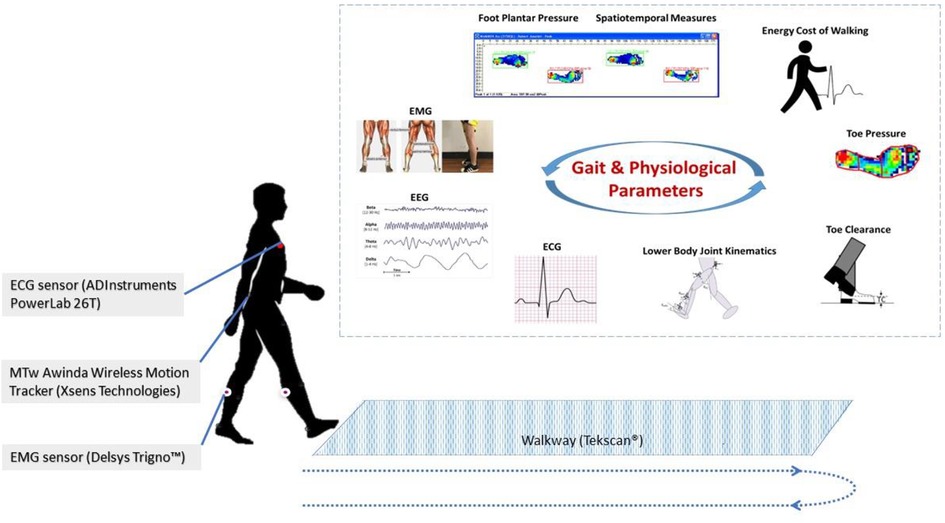
Figure 3 . Wearable gait lab
Accelerometers are often used in gait analysis for assessing stability and risk of fall. In a study which used a single tri-axial accelerometer mounted on the sacrum to analyze the risk of fall among 80 participants, accelerometry-based techniques were found to be able to detect subjects with increased risk of fall by employing appropriate machine learning techniques ( 66 ). In ( 67 ), a 3D accelerometer attached to the lower back was used for stability assessment of older adults. The applicability of a single accelerometer, worn on the back was further examined in ( 68 ), which highlights promising results for implementation in routine clinical practices. Considerable work has also been carried out to assess the consistency of gait characteristics obtained from accelerometers, where discrepancies in sensors positioning yield to critical errors ( 69 ). Furthermore, in ( 70 ), the authors have provided a comprehensive review on the use of accelerometry-based gait analysis techniques and their application to clinical settings.
Gyroscopes are also increasingly employed for gait studies. These devices measure angular velocity and are often combined with accelerometers and other micro-electromechanical systems (MEMS) devices to enhance performance through sensor fusion techniques. They have found applications in step detection, gait event detection, segmental kinematics, and more. For instance, a single gyroscope placed in the instep of the foot was successfully used to detect gait events, including heel strike, foot flat, heel off, and toe-off ( 71 ). Another study involved two gyroscopes, mounted on the lower left and right side of the waist to calculate walking steps and step length ( 72 ).
Magnetometers measure the magnetic field direction and intensity at a specific point. In combination with other inertial sensors (accelerometers and gyroscopes), they form a so-called inertial measurement unit (IMU), which can produce a drift-free estimation of gait parameters ( 73 ). Sophisticated commercialized IMUs (Physiolog 5 IMU, Gait Up, Switzerland, MTw Awinda, Xsens Technologies B.V., Netherlands), as well as in-house developed systems, were equally used for gait studies ( 74 ). In the context of human motion analysis, IMUs are employed for several possible goals, for example, to estimate the joint angles ( 74 ), to detect the risk of fall in an elderly population, long term monitoring of activities and symptoms ( 75 ), measurement of gait events, spatiotemporal parameters ( 76 , 77 , 78 ), ground reaction forces and moments ( 79 ), and estimation of gait symmetry ( 80 ). Mariani et al. (2010) used IMUs to measure foot kinematics in a study involving both young and elderly and reported the suitability of the system to clinical practice ( 81 ). Parisi et al. developed a low-cost system with a single IMU attached to the lower trunk to examine the gait characteristics of both hemiparetic and normal control subjects through measurement of spatiotemporal parameters, which showed excellent correlation with the parameters obtained from a standard reference system ( 78 ).
Insole systems for gait measurement and analysis represent a major category, which is cost-effective, portable, and applicable for both indoor and outdoor settings. Over the years, various technologies were developed ( 82 ), tested, and commercialized. These include capacitive sensors (Pedar system, Novel GmbH, Germany) ( 83 ), force-sensing resistors (FSR) (F-Scan, Tekscan Inc., United States) ( 84 ), and piezoresistive sensors (FlexiForce system, Tekscan, United States and ParoTec system, Paromed, Germany) ( 82 ). Researchers have adopted different approaches about the design, fabrication, and applications of insole systems. Both prefabricated and in-house fabricated insole systems have been tested for healthy and pathological gait ( 85 , 86 ). Some studies have also integrated inertial measurement units (IMU) with shoe insoles to enhance their capabilities. Despite the fact that these shoe-based systems have successfully been used for various gait analysis applications, they suffer from some drawbacks, such as (i) distortion of the flexible contact surface due to repeated loading, which leads to changes in the sensor response, (ii) drift in the output due to prolonged load application that causes heat inside the shoe, and (iii) need for subject-specific calibration that may alter accuracy ( 87 ). Mancinelli et al. (2012) presented ActiveGait – a novel sensorized shoe system for real-time monitoring of gait deviations associated with Cerebral Palsy in children. They reported that the severity of gait deviations can be estimated with an accuracy greater than 80% using the features derived from the center of pressure trajectories gathered from the shoe system ( 88 ). In ( 87 ), the authors designed a novel flexible foot insole system using an optoelectronic sensing technology for monitoring plantar pressure deviations in real-time. The system consists of an array of 64 sensing elements and onboard electronics for signal processing and transmission. Experimental validation was conducted on healthy subjects while walking at self-selected slow and normal speed. A commercial force plate (AMTI, Watertown, United States) was used as a reference system for benchmarking. Jagos et al. (2017), on the other hand, developed the eSHOE, which consists of four FSR sensors, a three-axis accelerometer, and a three-axis gyroscope, and reported good agreement with the gait parameters obtained from the GAITRite mat ( 89 ). Various other studies have also examined the applicability of shoe-based systems for gait analysis ( 85 , 90 – 92 ).
Another class of sensors that found major applications in gait studies is electromyography (EMG) sensors. Surface EMG is a non-invasive technique used to measure muscle activity. In ( 93 ), Lee et al. proposed a method using EMG signals to obtain biometrics from gait for personal identification methods. Another study adopted EMG techniques to understand the co-contraction patterns of thigh muscle during free walking using surface EMG ( 94 ). These research efforts emphasize the importance of wearable sensors in the study of human gait. The wearable systems discussed in this section are summarized in Table 6 .
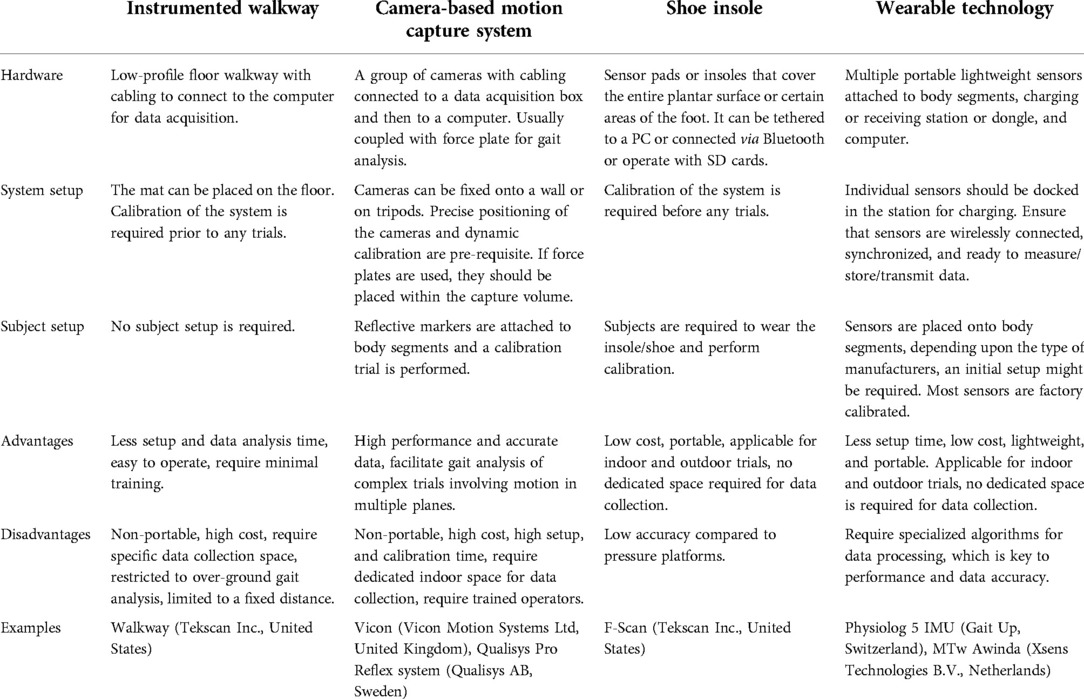
Table 6 . Instrumented gait analysis (IGA) systems and their features.
Although emerging new wearable technologies promise to enhance gait assessment and rehabilitation, there is limited research on the use of wearable technology to assess gait and mobility and its efficacy in clinical settings. According to a recently published review by Peters et. al. on the use of wearable technology to assess gait and mobility in stroke patients ( 95 ), most of the available studies are intervention studies conducted in laboratory settings that have used sensors to investigate change in cadence, step time variability, and gait speed. As wearable technologies continue to progress in affordability and accessibility, it is expected that such technologies would enable the gathering of movement-related data in “real-world” and various clinical settings. Importantly, these researchers indicated that so far only a limited number of studies examined reliability and validity of existing wearable devices, highlighting the need for more studies to examine psychometric and other properties when collecting gait and mobility information to determine which wearable technologies are most effective. Another recent review on the evaluation of the use of wearables in PD also indicates that novel technologies and wearables have the potential to enable early or differential diagnosis of PD, monitoring of motion state, prevention, or reduction of off-stage status, and assessing of movement complications. On the other hand, more research is required for the validation and the identification of more accurate markers of PD progression ( 96 ). Importantly, these authors warn that wearable devices may not be appropriate in cases of severe motor impairment, off-stage state, cognitive impairment, and for elderly patients and that further research is required for clinical validation.
4.3. Wearable-based gait computational algorithms
Besides sensor technology, sensor fusion algorithms play a critical role in predicting the accuracy/precision of these wearable-based systems. Most of the research has focused mainly on gait feature detection, daily physical activity monitoring, and gait data classification targeting disease diagnosis and user recognition. These algorithms are based on different data mining and AI technology, including machine learning, fuzzy computing, wavelet transforms, genetic algorithms, and data fusions. Alaqtash et al. ( 97 ) developed an intelligent fuzzy computational algorithm for characterizing gait in healthy, as well as impaired subjects. McCamley et al. established a method to calculate initial and final contact of gait using continuous wavelet transforms, employing waist-mounted inertial sensors ( 98 ). Another study cited the use of a single accelerometer mounted at the lower trunk and a corresponding algorithm to identify gait spatiotemporal parameters ( 68 ). A real-time gait event detection algorithm was proposed in ( 99 ) making use of adaptive decision rules. Further in ( 100 ), an original signal processing algorithm is developed to extract heel strike, toe strike, heel-off, and toe-off from an accelerometer positioned on the feet.
A novel gyroscope only (GO) algorithm was proposed in ( 101 ) to calculate knee angle through the integration of gyroscope-derived knee angular velocity. A zero-angle update algorithm was implemented to eliminate drift in the integral value. In addition, published work on noise-zero crossing (NZC) gait phase algorithm was also adapted. This method is applicable for continuous monitoring of gait data. Nukala et al. used support vector machines (SVM), KNN, binary decision trees (BDT), and backpropagation artificial neural network (BP-ANN) to classify the gait of patients from normal subjects, where features extracted from raw signals from gyroscopes and accelerometers were used as inputs. This study reported the highest overall classification accuracy of 100% with BP-ANN, 98% with SVM, 96% with KNN, and 94% with BDT ( 102 ).
Li et al. proposed DTW algorithm, sample entropy method, and empirical mode decomposition to calculate 3 main gait features of post-stroke subjects: symmetry, complexity character, and stepping stability. A k-nearest neighbor (KNN) classifier trained on the acquired features showed a promising result (area under the curve (AUC) of 0.94), which suggests the feasibility of such techniques to automatic gait analysis systems ( 43 ). Rastegari et al. employed a feature selection technique called maximum information gain minimum correlation (MIGMC) to extract gait data of subjects with Parkinson's Disease ( 103 ). The performance of several machine learning classifiers, including Support Vector Machines, Random Forest, AdaBoost, Bagging, and Naïve Bayes were also assessed to test the power of the feature set obtained.
The use of novel computational platforms, including Machine Learning, Support Vector Machine, and Neural Network approaches, are increasingly commanding greater attention in gait and rehabilitation research. Although their use in clinical settings are not yet well leveraged, these tools promise a paradigm shift in stroke gait quantification and rehabilitation, as they provide means for acquiring, storing and analyzing multifactorial complex gait data, while capturing its non-linear dynamic variability and offering the invaluable benefits of predictive analytics ( 1 ). A recent review article discussed the potential value of ML in gait analysis towards quantification and rehabilitation ( 104 ). The authors concluded that further evidence is required although preliminary data demonstrates that the control strategies for gait rehabilitation benefit from reinforcement learning and (deep) neural-networks due to their ability to capture participants' variability. This review paper demonstrated the success of ML techniques in detecting gait disorders, predicting rehabilitation length, and control of rehabilitation devices. Further work is needed for verification in clinical settings.
4.4. Data-driven gait rehabilitation in clinical settings
Quantitative gait assessment is invaluable towards disease-specific and patient specific rehabilitation/therapeutic interventions. Spatiotemporal, kinematic, and kinetic parameters obtained during instrumented gait assessment can help clinicians benchmark, devise strategies, and evaluate the effect of various rehabilitation interventions. Gait disorders not only affect these parameters, and patterns and time spent in the various gait phases, but can also highly impact gait symmetry, and regularity, depending on the disease and severity ( 105 ). Increasing evidence supports a data-driven physical rehabilitation approach to the treatment of functional gait disturbance ( 106 ). There are multiple examples in literature on the effective use of quantitative gait measures towards more effective data-driven rehabilitation. A recent review by Biase et al. ( 107 ) studied the most relevant technologies used to evaluate gait features and the associated algorithms that have shown promise to aid diagnosis and symptom monitoring towards rehabilitation in Parkinson's disease (PD) patients. They reported physical kinematic features of pitch, roll and yaw rotations of the foot during walking, based on which feature extraction and classification techniques, such as principal component analysis (PCA) and support vector machines (SVM) method were used to classify the PD patients. They also used gait features, including step duration, rise and fall gradients of the swing phase, as well as standard deviation of the minima as quantitative measures, for benchmarking and monitoring PD motor status during rehabilitation. Interestingly, this review sheds light on need to change the evaluated gait features as a function of disease progression. Another study was Pistacchi et al. ( 108 ) suggested spatiotemporal gait parameters, such as speed and step length, where reduced step length seems to be a specific feature of Parkinson's disease gait particularly in early disease stages. On the other hand, asymmetry, step shuffling, double-limb support and increased cadence are more common in mild to moderate stages, while advanced stages are more frequent freezing of gait (FOG) and motor blocks, reduced balance and postural control, motor fluctuations and dyskinesia ( 109 ). Researchers have also investigated the evaluation of ambulatory systems for gait analysis post hip replacement ( 110 ). They found gait characteristics such as stride length and velocity, as well as thigh and shank rotations different from healthy individuals and recommended their use to monitor post-surgical rehabilitation efficacy. Spatiotemporal gait parameters, such as step length, width and cadence have been used ( 111 ) to assess the effect of swing resistance and assistance rehabilitation on gait symmetry in hemiplegic patients. Investigators have also studied whether specific variables measured routinely at a rehabilitation center were predictors of gait performance of hemiparetic stroke patients ( 112 ). They found that motor control and balance were the best predictors of gait performance. A recent review article on assessment methods of post stroke gait suggests that multiple spatiotemporal, kinematic, and kinetic parameters can be useful in diagnosing post-stroke gait dysfunction and as quantitative measures to evaluate rehabilitation outcomes ( 1 ). Spatiotemporal characteristics of post-stroke gait include reduced step or stride length, increased step length on the hemiparetic side, wider base of support, greater toe-out angle, reduced walking speed and cadence. Stride time, stance period on both lower limb, and double support time are also increased, in addition to less time in stance and more time in swing phase for the paretic side, as well as asymmetries in spatial and temporal factors. Kinematic parameters associated with hemiplegic gait (reduced mean peak extension of the hip joint in late stance, alterations in the lateral displacement of the pelvis and flexion of the knee, and decreased plantarflexion of the ankle at toe-off, in addition to a significant decrease in peak hip and knee flexion during the swing phase, reduced knee extension prior to initial contact, as well as decreased ankle dorsiflexion during swing), and kinetic parameters (asymmetric patterns, as well as decreased amplitudes of the joint moments and joint powers at the hip, knee, and ankle joints on the paretic side) can be used as quantitative means to design and evaluate effective rehabilitation ( 113 – 115 ). IGA has also been successfully used to quantify and improve gait dysfunction associated with ageing and assess the risk of falling ( 116 ). Spatiotemporal gait parameters such as velocity, swing time, stride length, stride time- and double support time variability, as well as heel strike and toe off angles, and foot clearance, have been suggested as plausible indicative quantitative measures ( 116 ) to assess the risk of falling in elderly subjects. Inertial sensor-equipped shoes additionally provided heel strike and toe off angles, and foot clearance ( 116 ). The study ( 117 ) summarizes that multi-component exercise therapy which consisted of strength, ROM exercise, balance, flexibility and stretching exercises, circuit exercise training, and gait training was found to enhance gait function for individuals suffering with diabetic peripheral neuropathy compared to control groups using spatiotemporal gait parameters like velocity, cadence, step length, step time, double support time, stride length, stride time, ankle ROM. Gait assessment has potential to develop patient training paradigms for overcoming gait disorders ( 111 ).
5. Mobile gait lab for clinical applications and beyond
In recent decades, the healthcare field has witnessed a tremendous interest in the use of wearable sensing modalities and AI-driven data management/analysis techniques for patient diagnosis, monitoring, and rehabilitation. The portability, lightweight, ease of use, and high-power efficiency are some of the factors that promote applicability to a clinical platform.
There are few examples in literature demonstrating the potential success of using wearable-based systems for gait assessment in clinical settings. Prajapati et al. assessed the walking activity of inpatients with subacute stroke using commercial accelerometers attached above the ankle. They found that the walking bouts were shorter in duration and gait was more asymmetric ( 118 ). Studies have established test-retest reliability and accuracy of different sensor technologies; however, further validation trials are recommended prior to any clinical use. Hsu et al. assessed the test-retest reliability of an accelerometer-based system with infrared assist for measuring spatiotemporal parameters, including walking speed, step length, and cadence, as well as trunk control parameters, including gait symmetry, gait regularity, acceleration root mean square, and acceleration root mean square ratio of healthy subjects in hospital ( 119 ). This study showed excellent test-retest reliability of the parameters considered, and thus highlighting the reliability of an infrared assisted, trunk accelerometer-based device for clinical gait analysis. Another study investigated the concurrent validity and test-retest reliability of gait parameters (cadence, gait velocity, step time, step length, step time variability, and step time asymmetry) acquired from elderly subjects, using a tri-axial accelerometer attached to the center of body mass ( 120 ). In comparison to a reference GAITRite system, the acquired parameters showed good validity and reliability. Poitras et al. performed a systematic review of 42 studies assessing the reliability and validity of wearable sensors, specifically, IMUs, for quantifying the joint motion ( 121 ). Evidence suggests that IMU could be an alternative solution to an expensive motion capture system, as it shows good validity for lower-limb analysis involving fewer complex tasks. However, more work is needed to draw a better conclusion with regards to its reliability, as well as to standardize the protocol to get more accurate data in a clinical setting. Importantly, additional research efforts are needed to examine the responsiveness of wearables in free-living conditions in hospital settings.
6. Limitations
This review aimed to summarize available published work on the present and future of gait analysis in clinical settings. The focus was to highlight current systems, scales, and indices, as well as recent technology-driven gait characterization and analysis approaches and their applicability to clinical settings. Within this context, pathological gait associated with different disease, as well as ageing was briefly discussed. As such, this article may have not covered the complete spectrum of gait pathologies and associated parameters. A scoping (non-systematic) search methodology was selected to broaden the scope and integration of the three main aspects of focus (gait pathology, clinical assessment, recent tools, and technologies). In addition, we do not recommend any specific protocol over the other, as most of the papers incorporate different inclusion/exclusion criteria for subject selection, as well as different sampling sizes, which may render comparisons unrealistic.
7. Conclusive remarks and future work
This scoping review aimed to shed light on the status of gait assessment in clinical settings, as well as the state-of-the-art emerging tools and technologies and their potential clinical applicability. Clinical gait analysis continues to rely mainly on observational gait and quantitative scales and is hence subjective and suffers from variability and the lack of sensitivity influenced by the observer's background and experience. Based on the reviewed literature, quantitative IGA-based gait analysis, commonly used in research labs, has the capability of providing clinicians with accurate and reliable gait data for informed diagnosis and continuous monitoring. On the other hand, several factors, including high cost and infrastructure challenges; data variability, complexity, and multidimensionality; lack of sufficient knowledge and standardized training in clinical environments; and time constraints, continue to limit its wide-spread deployment. Rapidly emerging smart wearable technology and AI, including Machine Learning, Support Vector Machine, and Neural Network approaches, are increasingly playing a bigger role in gait assessment. Although their use in clinical settings is not yet well leveraged, these tools promise an unprecedented paradigm shift in the quantification of gait in the clinic and beyond, as they provide means for acquiring, storing, and analyzing multifactorial complex gait data, while capturing its non-linear dynamic variability and offering the invaluable benefits of predictive analytics.
Researchers are also paying increased attention to multisource and multi-modality sensor fusion approaches, which can further add value by integrating the output of multiple sensors to capture the complexity and variability of gait. Multimodality sensor fusion also allows for simultaneous monitoring of various physiological signals during locomotion, such as EMG, ECG, and EEG, where fusing these with various gait measures (spatiotemporal, kinematic, and kinetic) can shed light on underlying health conditions and disease etiology towards better informed outcome prediction and clinical decisions. As the volume of data from the variety of sensors, including electroencephalography, electro-oculography, electro-cardiography, and electromyography, motion capture and force sensors data, substantially increases, more AI-driven sophisticated data management and modeling are needed to quantify and interpret complex network AI/NN models. Models which include static and dynamic features, combined with sophisticated data reduction and individualized feature selection of the most relevant gait characteristics are needed to close the loop for this paradigm shift. Future work is warranted on a multidisciplinary level: to validate the clinical applicability and integration of the various sensing modalities, to ensure proper synchronization of the various systems for accurate continuous real-time monitoring, to develop and validate fast and reliable computational platforms, and to implement modular user-friendly interfaces easy to use in any environment.
In summary, instrumented gait analysis is a well-established tool for the quantitative assessment of gait dysfunction which could be effectively used for functional diagnosis, treatment/surgery/rehabilitation/planning, and progression monitoring for a wide spectrum of disease. The literature indicates that recent advancement in wearable technology and computationally advanced data analytics, including AI, can overcome the challenges of traditional gait labs, allowing for less costly, portable, and relatively simple gait testing protocols in clinical settings, as well as user-friendly data management, analysis, and interpretation computational platforms. On the other hand, the development of clinically driven standardized methodology and procedures is of paramount significance and remains largely unaddressed. These standardized practices should not only focus on quantitative gait diagnosis but should also incorporate sophisticated objective measures and 3-D dynamic gait profiles and markers for monitoring progress and outcome prediction and evaluation. Proper gait protocols should be devised and leveraged towards identifying gait characteristics that could be effectively used as early disease diagnostic markers. Importantly, training clinical teams at various levels, from doctors and surgeons to physiotherapists and other allied health professionals, on properly using these novel assessment and computational tools is equally important and warrants an equally rapid paradigm shift in training and practice in clinical settings towards patient-specific precise medicine.
Author contributions
AAH, MR and KK conceived the idea. AAH, DMM, KK and MR formulated the objective for this review. AAH designed the search strategy, conducted abstract screening and full text review, extracted the data, and drafted the manuscript. KK, NA, DMM, and AAH contributed to writing the manuscript. DMM and NA performed a part of the literature survey, including abstract screening, full text review, and data extraction. MR, and KK provided significant guidance on the content of the manuscript, overall supervision, and critical feedback. All authors contributed to the article and approved the submitted version.
This publication is based upon work supported by the HEIC at Khalifa University of Science and Technology.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Mohan DM, Khandoker AH, Wasti SA, Ismail Ibrahim Ismail Alali S, Jelinek HF, Khalaf K. Assessment methods of post-stroke gait: a scoping review of technology-driven approaches to gait characterization and analysis. Front Neurol . (2021) 12:650024. doi: 10.3389/fneur.2021.650024
PubMed Abstract | CrossRef Full Text | Google Scholar
2. Celik Y, Stuart S, Woo WL, Godfrey A. Gait analysis in neurological populations: progression in the use of wearables. Med Eng Phys . (2021) 87:9–29. doi: 10.1016/j.medengphy.2020.11.005
3. Rathinam C, Bateman A, Peirson J, Skinner J. Observational gait assessment tools in paediatrics–a systematic review. Gait Posture . (2014) 40:279–85. S0966-6362(14)00478-0 24798609
PubMed Abstract | Google Scholar
4. Eastlack ME, Arvidson J, Snyder-Mackler L, Danoff JV, McGarvey CL. Interrater reliability of videotaped observational gait-analysis assessments. Phys Ther . (1991) 71:465–72. doi: 10.1093/ptj/71.6.465
5. Hebda-Boon A, Zhang B, Amankwah A, Shortland AP, Morrissey D. Clinicians’ experiences of instrumented gait analysis in management of patients with cerebral palsy: a qualitative study. Phys Occup Ther Pediatr . (2022) 42(4):403–15. doi: 10.1080/01942638.2022.2037808
6. Toro B, Nester C, Farren P. A review of observational gait assessment in clinical practice. null . (2003) 19:137–49. doi: 10.1080/09593980307964
CrossRef Full Text | Google Scholar
7. Franki I, Desloovere K, De Cat J, Feys H, Molenaers G, Calders P, et al. The evidence-base for basic physical therapy techniques targeting lower limb function in children with cerebral palsy: a systematic review using the international classification of functioning, disability and health as a conceptual framework. J Rehabil Med . (2012) 44:385–95. doi: 10.2340/16501977-0983
8. Wallmann HW. Introduction to observational gait analysis. Home Health Care Manag Pract . (2009) 22:66–8. doi: 10.1177/1084822309343277
9. Ferrarello F, Bianchi VA, Baccini M, Rubbieri G, Mossello E, Cavallini MC, et al. Tools for observational gait analysis in patients with stroke: a systematic review. Phys Ther . (2013) 93:1673–85. doi: 10.2522/ptj.20120344
10. Turani N, Kemiksizoğlu A, Karataş M, Ozker R. Assessment of hemiplegic gait using the Wisconsin gait scale. Scand J Caring Sci . (2004) 18:103–8. doi: 10.1111/j.1471-6712.2004.00262.x
11. Dasgupta P, VanSwearingen J, Godfrey A, Redfern M, Montero-Odasso M, Sejdic E. Acceleration gait measures as proxies for motor skill of walking: a narrative review. IEEE Trans Neural Syst Rehabil Eng . (2021) 29:249–61. doi: 10.1109/TNSRE.2020.3044260
12. Noseworthy JH. Clinical scoring methods for multiple sclerosis. Ann Neurol . (1994) 36:S80–5. doi: 10.1002/ana.410360718
13. Noseworthy JH, Vandervoort MK, Wong CJ, Ebers GC. Interrater variability with the expanded disability Status scale (EDSS) and functional systems (FS) in a multiple sclerosis clinical trial. The Canadian cooperation MS study group. Neurol . (1990) 40:971–5. doi: 10.1212/wnl.40.6.971
14. Roxburgh RH, Seaman SR, Masterman T, Hensiek AE, Sawcer SJ, Vukusic S, et al. Multiple sclerosis severity score: using disability and disease duration to rate disease severity. Neurol . (2005) 64:1144–51. doi: 10.1212/01.WNL.0000156155.19270.F8
15. Vienne-Jumeau A, Quijoux F, Vidal PP, Ricard D. Value of gait analysis for measuring disease severity using inertial sensors in patients with multiple sclerosis: protocol for a systematic review and meta-analysis. Syst Rev . (2019) 8:15. doi: 10.1186/s13643-018-0918-z
16. Balaji E, Brindha D, Elumalai VK, Umesh K. Data-driven gait analysis for diagnosis and severity rating of Parkinson's Disease. Med Eng Phys . (2021) 91:54–64. S1350-4533(21)00028-X 34074466
17. Mohan DM, Khandoker AH, Wasti SA, Ismail Ibrahim Ismail Alali S, Jelinek HF, Khalaf K. Assessment methods of post-stroke gait: a scoping review of technology-driven approaches to gait characterization and Analysis. Front Neurol . (2021) 12:650024. doi: 10.3389/fneur.2021.650024
18. Cappozzo A. Gait analysis methodology. Hum Mov Sci . (1984) 3:27–50. doi: 10.1016/0167-9457(84)90004-6
19. Wren TAL, Tucker CA, Rethlefsen SA, Gorton GE 3rd, Õunpuu S. Clinical efficacy of instrumented gait analysis: systematic review 2020 update. Gait Posture . (2020) 80:274–9. S0966-6362(20)30181-8 32563727
20. Saboor A, Kask T, Kuusik A, Alam MM, Le Moullec Y, Niazi IK, et al. Latest research trends in gait analysis using wearable sensors and machine learning: a systematic review. IEEE Access . (2020) 8:167830–64. doi: 10.1109/ACCESS.2020.3022818
21. Munn Z, Peters MDJ, Stern C, Tufanaru C, McArthur A, Aromataris E. Systematic review or scoping review? Guidance for authors when choosing between a systematic or scoping review approach. BMC Med Res Methodol . (2018) 18:143. doi: 10.1186/s12874-018-0611-x
22. Winter DA. The biomechanics and motor control of humna gait: Normal, elderly and pathological . Waterloo: Ont.: Waterloo Biomechanics (1991).
23. Ali A, Sundaraj K, Ahmad B, Ahamed N, Islam A. Gait disorder rehabilitation using vision and non-vision based sensors: a systematic review. Bosnian Journal of Basic Medical Sciences . (2012) 12:193–202. doi: 10.17305/bjbms.2012.2484
24. Alam U, Riley DR, Jugdey RS, Azmi SS, Rajbhandari SS, D’août K, et al. Diabetic neuropathy and gait: a review. Diabetes Ther . (2017) 8:1253. doi: 10.1007/s13300-017-0295-y
25. Whittle MW. Clinical gait analysis: a review. Hum Mov Sci . (1996) 15:369–87. doi: 10.1016/0167-9457(96)00006-1
26. Fukuchi CA, Fukuchi RK, Duarte M. Effects of walking speed on gait biomechanics in healthy participants: a systematic review and meta-analysis. Syst Rev . (2019) 8:153. doi: 10.1186/s13643-019-1063-z
27. Snijders AH, van de Warrenburg BP, Giladi N, Bloem BR. Neurological gait disorders in elderly people: clinical approach and classification. Lancet Neurol . (2007) 6:63–74. S1474-4422(06)70678-0 [pii].17166803
28. Snijders AH, van de Warrenburg BP, Giladi N, Bloem BR. Neurological gait disorders in elderly people: clinical approach and classifi cation. Lancet Neurol . (2007) 6(1):63–74. doi: 10.1016/S1474-4422(06)70678-0
29. Intzandt B, Beck EN, Silveira CRA. The effects of exercise on cognition and gait in Parkinson's Disease: a scoping review. Neurosci Biobehav Rev . (2018) 95:136–69. doi: 10.1016/j.neubiorev.2018.09.018
30. Belda-Lois J, Mena-del Horno S, Bermejo-Bosch I, Moreno JC, Pons JL, Farina D, et al. Rehabilitation of gait after stroke: a review towards a top-down approach. J Neuroeng Rehabil . (2011) 8:66. doi: 10.1186/1743-0003-8-66
31. Bahl JS, Nelson MJ, Taylor M, Solomon LB, Arnold JB, Thewlis D. Biomechanical changes and recovery of gait function after total hip arthroplasty for osteoarthritis: a systematic review and meta-analysis. Osteoarthritis Cartilage . (2018) 26:847–63. S1063-4584(18)31013-6 [pii].29474993
32. Ewen AM, Stewart S, St Clair Gibson A, Kashyap SN, Caplan N. Post-operative gait analysis in total hip replacement patients—a review of current literature and meta-analysis. Gait Posture . (2012) 36:1–6. doi: 10.1016/j.gaitpost.2011.12.024
33. Kolk S, Minten MJ, van Bon GE, Rijnen WH, Geurts AC, Verdonschot N, et al. Gait and gait-related activities of daily living after total hip arthroplasty: a systematic review. Clin Biomech (Bristol, Avon) . (2014) 29:705–18. S0268-0033(14)00127-2 [pii].24951319
34. Kolk S, Minten MJM, van Bon GEA, Rijnen WH, Geurts ACH, Verdonschot N, et al. Gait and gait-related activities of daily living after total hip arthroplasty: a systematic review. Clin Biomech . (2014) 29:705–18. doi: 10.1016/j.clinbiomech.2014.05.008
35. Ladha C, Din S, Nazarpour K, Hickey A, Morris R, Catt M, et al. Toward a low-cost gait analysis system for clinical and free-living assessment. Annu Int Conf IEEE Eng Med Biol Soc . (2016) 2016:1874–7. doi: 10.1109/EMBC.2016.7591086. PMID: 28268692
36. Patterson KK, Gage WH, Brooks D, Black SE, McIlroy WE. Evaluation of gait symmetry after stroke: a comparison of current methods and recommendations for standardization. Gait Posture . (2010) 31:241–6. doi: 10.1016/j.gaitpost.2009.10.014
37. Patterson KK, Gage WH, Brooks D, Black SE, McIlroy WE. Changes in gait symmetry and velocity after stroke: a cross-sectional study from weeks to years after stroke. Neurorehabil Neural Repair . (2010) 24:783–90. doi: 10.1177/1545968310372091
38. Laudanski A, Brouwer B, Li Q. Measurement of lower limb joint kinematics using inertial sensors during stair ascent and descent in healthy older adults and stroke survivors. J Healthc Eng . (2013) 4:555–76. doi: 10.1260/2040-2295.4.4.555
39. Rastegarpanah A, Scone T, Saadat M, Rastegarpanah M, Taylor SJ, Sadeghein N. Targeting effect on gait parameters in healthy individuals and post-stroke hemiparetic individuals. J Rehabil Assist Technol Eng . (2018) 5:2055668318766710. doi: 10.1177/2055668318766710
40. Latorre J, Colomer C, Alcañiz M, Llorens R. Gait analysis with the kinect v2: normative study with healthy individuals and comprehensive study of its sensitivity, validity, and reliability in individuals with stroke. J Neuroeng Rehabil . (2019) 16:97. doi: 10.1186/s12984-019-0568-y
41. Iosa M, Bini F, Marinozzi F, Fusco A, Morone G, Koch G, et al. Stability and harmony of gait in patients with subacute stroke. J Med Biol Eng . (2016) 36:635–43. doi: 10.1007/s40846-016-0178-0 [pii].27853414
42. Sousa ASP, Silva A, Santos R, Sousa F, Tavares JMRS. Interlimb coordination during the stance phase of gait in subjects with stroke. Arch Phys Med Rehabil . (2013) 94:2515–22. S0003-9993(13)00537-6 [pii].23871877
43. Li M, Tian S, Sun L, Chen X. Gait analysis for post-stroke hemiparetic patient by multi-features fusion method. Sens (Basel) . (2019) 19(7):1737. doi: 10.3390/s19071737
44. Krishnan V, Khoo I, Marayong P, DeMars K, Cormack J. Gait training in chronic stroke using walk-even feedback device: a pilot study. Neurosci J . (2016) 2016:1–8. doi: 10.1155/2016/6808319
45. Ardalan A, Yamane N, Rao AK, Montes J, Goldman S. Analysis of gait synchrony and balance in neurodevelopmental disorders using computer vision techniques. Health Informatics J . (2021) 27:14604582211055650. doi: 10.1177/14604582211055650
46. Rupprechter S, Morinan G, Peng Y, Foltynie T, Sibley K, Weil RS, et al. A clinically interpretable computer-vision based method for quantifying gait in Parkinson's Disease. Sens (Basel) . (2021) 21:5437. doi: 10.3390/s21165437
47. Li Y, Zhang P, Zhang Y, Miyazaki K. 2019 41st annual international conference of the IEEE engineering in medicine and biology society (EMBC). Gait analysis using stereo camera in daily environment (2019). p. 1471–5
48. Kessler SE, Rainbow MJ, Lichtwark GA, Cresswell AG, D'Andrea SE, Konow N, et al. A direct comparison of biplanar videoradiography and optical motion capture for foot and ankle kinematics. Front Bioeng Biotechnol . (2019) 7:199. doi: 10.3389/fbioe.2019.00199
49. Tranberg R, Karlsson D. The relative skin movement of the foot: a 2-D roentgen photogrammetry study. Clin Biomech (Bristol, Avon) . (1998) 13:71–6. S0268003397000521 [pii].11415773
50. Nester C, Jones RK, Liu A, Howard D, Lundberg A, Arndt A, et al. Foot kinematics during walking measured using bone and surface mounted markers. J Biomech . (2007) 40:3412–23. doi: 10.1016/j.jbiomech.2007.05.019
51. Colyer SL, Evans M, Cosker DP, Salo AIT. A review of the evolution of vision-based motion analysis and the integration of advanced computer vision methods towards developing a markerless system. Sports Med Open . (2018) 4:24. doi: 10.1186/s40798-018-0139-y
52. Baker R. Gait analysis methods in rehabilitation. J Neuroeng Rehabil . (2006) 3:4. doi: 10.1186/1743-0003-3-4
53. Stagni R, Fantozzi S, Cappello A, Leardini A. Quantification of soft tissue artefact in motion analysis by combining 3D fluoroscopy and stereophotogrammetry: a study on two subjects. Clin Biomech (Bristol, Avon) . (2005) 20:320–9. doi: 10.1016/j.clinbiomech.2004.11.012
54. McHenry BD, Exten E, Long JT, Harris GF. Sagittal fluoroscopy for the assessment of hindfoot kinematics. J Biomech Eng . (2016) 138:4032445. doi: 10.1115/1.4032445
55. Roach KE, Foreman KB, MacWilliams BA, Karpos K, Nichols J, Anderson AE. The modified shriners hospitals for children Greenville (mSHCG) multi-segment foot model provides clinically acceptable measurements of ankle and midfoot angles: a dual fluoroscopy study. Gait Posture . (2021) 85:258–65. doi: 10.1016/j.gaitpost.2021.02.004
56. Hitz M, Schütz P, Angst M, Taylor WR, List R. Influence of the moving fluoroscope on gait patterns. PLoS One . (2018) 13:e0200608. doi: 10.1371/journal.pone.0200608
57. Roach KE, Wang B, Kapron AL, Fiorentino NM, Saltzman CL, Bo Foreman K, et al. In vivo kinematics of the tibiotalar and subtalar joints in asymptomatic subjects: a high-speed dual fluoroscopy study. J Biomech Eng . (2016) 138:910061. doi: 10.1115/1.4034263
58. Atkins PR, Fiorentino NM, Hartle JA, Aoki SK, Peters CL, Foreman KB, et al. In vivo pelvic and hip joint kinematics in patients with cam femoroacetabular impingement syndrome: a dual fluoroscopy study. J Orthop Res . (2020) 38:823–33. doi: 10.1002/jor.24509
59. Battaglia S, Belvedere C, Jaber SA, Affatato S, D'Angeli V, Leardini A. A new protocol from real joint motion data for wear simulation in total knee arthroplasty: stair climbing. Med Eng Phys . (2014) 36:1605–10. doi: 10.1016/j.medengphy.2014.08.010
60. Belvedere C, Cadossi M, Mazzotti A, Giannini S, Leardini A. Fluoroscopic and gait analyses for the functional performance of a custom-made total talonavicular replacement. J Foot Ankle Surg . (2017) 56:836–44. doi: 10.1053/j.jfas.2017.02.004
61. Uemura K, Atkins PR, Anderson AE. The effect of using different coordinate systems on in-vivo hip angles can be estimated from computed tomography images. J Biomech . (2019) 95:109318. doi: 10.1016/j.jbiomech.2019.109318
62. Wearing SC, Urry S, Perlman P, Smeathers J, Dubois P. Sagittal plane motion of the human arch during gait: a videofluoroscopic analysis. Foot Ankle Int . (1998) 19:738–42. doi: 10.1177/107110079801901105
63. Li G, Kozanek M, Hosseini A, Liu F, Van de Velde SK, Rubash HE. New fluoroscopic imaging technique for investigation of 6DOF knee kinematics during treadmill gait. J Orthop Surg Res . (2009) 4:6. doi: 10.1186/1749-799X-4-6
64. Iaquinto JM, Tsai R, Haynor DR, Fassbind MJ, Sangeorzan BJ, Ledoux WR. Marker-based validation of a biplane fluoroscopy system for quantifying foot kinematics. Med Eng Phys . (2014) 36:391–6. doi: 10.1016/j.medengphy.2013.08.013
65. Sheehan FT, Seisler AR, Siegel KL. In vivo talocrural and subtalar kinematics: a non-invasive 3D dynamic MRI study. Foot Ankle Int . (2007) 28:323–35. doi: 10.3113/FAI.2007.0323
66. Tao W, Liu T, Zheng R, Feng H. Gait analysis using wearable sensors. Sens (Basel, Switzerland) . (2012) 12:2255–83. doi: 10.3390/s120202255
67. Ihlen EAF, Weiss A, Beck Y, Helbostad JL, Hausdorff JM. A comparison study of local dynamic stability measures of daily life walking in older adult community-dwelling fallers and non-fallers. J Biomech . (2016) 49:1498–503. doi: 10.1016/j.jbiomech.2016.03.019
68. Bugané F, Benedetti MG, Casadio G, Attala S, Biagi F, Manca M, et al. Estimation of spatial-temporal gait parameters in level walking based on a single accelerometer: validation on Normal subjects by standard gait analysis. Comput Methods Programs Biomed . (2012) 108:129–37. doi: 10.1016/j.cmpb.2012.02.003
69. Rispens SM, Pijnappels M, van Schooten KS, Beek PJ, Daffertshofer A, van Dieën JH. Consistency of gait characteristics as determined from acceleration data collected at different trunk locations. Gait Posture . (2014) 40:187–92. doi: 10.1016/j.gaitpost.2014.03.182
70. Jarchi D, Pope J, Lee TKM, Tamjidi L, Mirzaei A, Sanei S. A review on accelerometry-based gait analysis and emerging clinical applications. IEEE Rev Biomed Eng . (2018) 11:177–94. doi: 10.1109/RBME.2018.2807182
71. Felix P, Figueiredo J, Santos C, Moreno J. ADAPTIVE REAL-TIME TOOL FOR HUMAN GAIT EVENT DETECTION USING A WEARABLE GYROSCOPE. (2017). 653 p.
72. Nasr A, Nadeem T. A Novel Technique for Gait Analysis Using Two Waist Mounted Gyroscopes. In: Anonymous - 2019 IEEE Global Communications Conference (GLOBECOM); (2019). p. 1-6.
73. Kun L, Inoue Y, Shibata K, Enguo C. Ambulatory estimation of knee-joint kinematics in anatomical coordinate system using accelerometers and magnetometers. IEEE Trans on Biomed Eng . (2011) 58:435–42. doi: 10.1109/TBME.2010.2089454
74. Rodríguez-Martín D, Pérez-López C, Samà A, Cabestany J, Català A. A wearable inertial measurement unit for long-term monitoring in the dependency care area. Sens . (2013) 13(10):14079–104. doi: 10.3390/s131014079
75. Seel T, Raisch J, Schauer T. IMU-Based Joint angle measurement for gait analysis. Sens . (2014) 14(4):6891–909. doi: 10.3390/s140406891
76. Teufl W, Lorenz M, Miezal M, Taetz B, Fröhlich M, Bleser G. Towards inertial sensor based Mobile gait analysis: event-detection and spatio-temporal parameters. Sens . (2019) 19(1):38. doi: 10.3390/s19010038
77. Hwang T-H, Reh J, Effenberg AO, Blume H. Real-Time gait analysis using a single head-worn inertial measurement unit. IEEE Trans on Cons Elec . (2018) 64:240–8. doi: 10.1109/TCE.2018.2843289
78. Parisi F, Ferrari G, Baricich A, D'Innocenzo M, Cisari C, Mauro A. Accurate gait analysis in post-stroke patients using a single inertial measurement unit. In: Anonymous - 2016 IEEE 13th International Conference on Wearable and Implantable Body Sensor Networks (BSN); (2016). p. 335-340).
79. Karatsidis A, Bellusci G, Schepers HM, De Zee M, Andersen MS, Veltink PH. Estimation of ground reaction forces and moments during gait using only inertial motion capture. Senss . (2017) 17(1):75. doi: 10.3390/s17010075
80. Sant’ Anna A, Wickström N, Eklund H, Zügner R, Tranberg R. Assessment of gait symmetry and gait normality using inertial sensors: in-lab and in-situ evaluation . Berlin, Heidelberg: Springer (2013).
81. Mariani B, Hoskovec C, Rochat S, Büla C, Penders J, Aminian K. 3D Gait assessment in young and elderly subjects using foot-worn inertial sensors. J Biomech . (2010) 43:2999–3006. doi: 10.1016/j.jbiomech.2010.07.003
82. Razak AHA, Zayegh A, Begg RK, Wahab Y. Foot plantar pressure measurement system: a review. Sens (Basel, Switzerland) . (2012) 12:9884–912. doi: 10.3390/s120709884
83. Novel® Pedar® System [Internet]. []. Available at: https://www.novel.de/products/pedar/
84. Tekscan® F-Scan® System.
85. Leitch KM, Birmingham TB, Jones IC, Giffin JR, Jenkyn TR. In-shoe plantar pressure measurements for patients with knee osteoarthritis: reliability and effects of lateral heel wedges. Gait Posture . (2011) 34:391–6. doi: 10.1016/j.gaitpost.2011.06.008
86. Malvade PS, Joshi AK, Madhe SP. In-sole Shoe Foot Pressure Monitoring for Gait Analysis. In: Anonymous - 2017 International Conference on Computing, Communication, Control and Automation (ICCUBEA); (2017). p. 1-4.
87. Crea S, Donati M, De Rossi S, Marco Maria Oddo CM, Vitiello N. A wireless flexible sensorized insole for gait analysis. Sens (Basel, Switzerland) . (2014) 14:1073–93. doi: 10.3390/s140101073
88. Mancinelli C, Patel S, Deming LC, Nimec D, Chu JJ, Beckwith J, et al. A novel sensorized shoe system to classify gait severity in children with cerebral palsy. Annu Int Conf IEEE Eng Med Biol Soc . (2012) 2012:5010–3. doi: 10.1109/EMBC.2012.6347118
89. Jagos H, Pils K, Haller M, Wassermann C, Chhatwal C, Rafolt D, et al. Mobile gait analysis via eSHOEs instrumented shoe insoles: a pilot study for validation against the gold standard GAITRite(®). J Med Eng Technol . (2017) 41:375–86. doi: 10.1080/03091902.2017.1320434
90. Arafsha F, Hanna C, Aboualmagd A, Fraser S, El Saddik A. Instrumented wireless SmartInsole system for Mobile gait analysis: a validation pilot study with tekscan strideway. J Sens and Actuator Netw . (2018) 7(3):36. doi: 10.3390/jsan7030036
91. Nagano H, Begg RK. Shoe-Insole technology for injury prevention in walking. Sens (Basel) . (2018) 18:1468. doi: 10.3390/s18051468
92. Lin F, Wang A, Zhuang Y, Tomita MR, Xu W. Smart insole: a wearable sensor device for unobtrusive gait monitoring in daily life. IEEE Trans on Indust Informat . (2016) 12:2281–91. doi: 10.1109/TII.2016.2585643
93. Lee M, Ryu J, Youn I. Biometric personal identification based on gait analysis using surface EMG signals. In: Anonymous - 2017 2nd IEEE International Conference on Computational Intelligence and Applications (ICCIA); (2017). p. 318-321).
94. Strazza A, Mengarelli A, Fioretti S, Burattini L, Agostini V, Knaflitz M, et al. Surface-EMG analysis for the quantification of thigh muscle dynamic co-contractions during Normal gait. Gait Posture . (2017) 51:228–33. doi: 10.1016/j.gaitpost.2016.11.003
95. Peters D, O’Brien E, Kamrud K, Roberts S, Rooney T, Thibodeau K, et al. Utilization of wearable technology to assess gait and mobility post-stroke: a systematic review. J Neuroeng Rehabil . (2021) 18(1):67. doi: 10.1186/s12984-021-00863-x
96. Lu R, Xu Y, Li X, Fan Y, Zeng W, Tan Y, et al. Evaluation of wearable sensor devices in Parkinson's Disease: a review of current Status and future prospects. Parkinson's Disease . (2020) 2020:4693019. doi: 10.1155/2020/4693019
97. Alaqtash M, Yu H, Brower R, Abdelgawad A, Sarkodie-Gyan T. Application of wearable sensors for human gait analysis using fuzzy computational algorithm. Eng Appl Artif Intell . (2011) 24:1018–25. doi: 10.1016/j.engappai.2011.04.010
98. McCamley J, Donati M, Grimpampi E, Mazzà C. An enhanced estimate of initial contact and final contact instants of time using lower trunk inertial sensor data. Gait Posture . (2012) 36:316–8. doi: 10.1016/j.gaitpost.2012.02.019
99. Felix P, Figueiredo J, Santos C, Moreno J. ADAPTIVE REAL-TIME TOOL FOR HUMAN GAIT EVENT DETECTION USING A WEARABLE GYROSCOPE. (2017). 653 p).
100. Boutaayamou M, Schwartz C, Stamatakis J, Denoël V, Maquet D, Forthomme B, et al. Development and validation of an accelerometer-based method for quantifying gait events. Med Eng Phys . (2015) 37:226–32. doi: 10.1016/j.medengphy.2015.01.001
101. Allseits E, Kim KJ, Bennett C, Gailey R, Gaunaurd I, Agrawal V. A novel method for estimating knee angle using two leg-mounted gyroscopes for continuous monitoring with Mobile health devices. Sens (Basel) . (2018) 18:2759. doi: 10.3390/s18092759
102. Nukala BT, Nakano T, Rodriguez A, Tsay J, Lopez J, Nguyen TQ, et al. Real-Time classification of patients with balance disorders vs. Normal subjects using a low-cost small wireless wearable gait sensor. Biosens . (2016) 6(4):58. doi: 10.3390/bios6040058
103. Rastegari E, Azizian S, Ali H. Machine Learning and Similarity Network Approaches to Support Automatic Classification of Parkinson's Diseases Using Accelerometer-based Gait Analysis. (2019).).
104. Khera P, Kumar N. Role of machine learning in gait analysis: a review. J Med Eng Technol . (2020) 44:441–67. doi: 10.1080/03091902.2020.1822940
105. Zhao H, Wang Z, Qiu S, Shen Y, Wang J. IMU-based gait analysis for rehabilitation assessment of patients with gait disorders . In: Anonymous (2017). 622–6.
106. Butz C, Iske C, Truba N, Trott K. Treatment of functional gait abnormality in a rehabilitation setting: emphasizing the physical interventions for treating the whole child. Innov Clin Neurosci . (2019) 16:18–21.31832259
107. di Biase L, Di Santo A, Caminiti ML, De Liso A, Shah SA, Ricci L, et al. Gait analysis in Parkinson's Disease: an overview of the most accurate markers for diagnosis and symptoms monitoring. Sens (Basel) . (2020) 20:3529. doi: 10.3390/s20123529
108. Pistacchi M, Gioulis M, Sanson F, De Giovannini E, Filippi G, Rossetto F, et al. Gait analysis and clinical correlations in early Parkinson's Disease. Funct Neurol . (2017) 32:28–34. doi: 10.11138/FNeur/2017.32.1.028
109. Mirelman A, Bonato P, Camicioli R, Ellis TD, Giladi N, Hamilton JL, et al. Gait impairments in Parkinson's Disease. Lancet Neurol . (2019) 18:697–708. doi: 10.1016/S1474-4422(19)30044-4
110. Aminian K, Trevisan C, Najafi B, Dejnabadi H, Frigo C, Pavan E, et al. Evaluation of an ambulatory system for gait analysis in hip osteoarthritis and after total hip replacement. Gait Posture . (2004) 20:102–7. doi: 10.1016/S0966-6362(03)00093-6
111. Yen SC, Schmit BD, Wu M. Using swing resistance and assistance to improve gait symmetry in individuals post-stroke. Hum Mov Sci . (2015) 42:212–24. doi: 10.1016/j.humov.2015.05.010
112. Bohannon RW. Gait performance of hemiparetic stroke patients: selected variables. Arch Phys Med Rehabil . (1987) 68:777–81.3675175
113. Nadeau S, Betschart M, Bethoux F. Gait analysis for poststroke rehabilitation: the relevance of biomechanical analysis and the impact of gait speed. Phys Med Rehabil Clin N Am . (2013) 24:265–76. doi: 10.1016/j.pmr.2012.11.007
114. Moseley A, Wales A, Herbert R, Schurr K, Moore S. Observation and analysis of hemiplegic gait: stance phase. Aust J Physiother . (1993) 39:259–67. doi: 10.1016/S0004-9514(14)60486-4
115. von Schroeder HP, Coutts RD, Lyden PD, Billings E Jr, Nickel VL. Gait parameters following stroke: a practical assessment. J Rehabil Res Dev . (1995) 32:25–31.7760264
116. Schülein S, Barth J, Rampp A, Rupprecht R, Eskofier BM, Winkler J, et al. Instrumented gait analysis: a measure of gait improvement by a wheeled walker in hospitalized geriatric patients. J Neuroeng Rehabil . (2017) 14:18. doi: 10.1186/s12984-017-0228-z
117. Melese H, Alamer A, Hailu Temesgen M, Kahsay G. Effectiveness of exercise therapy on gait function in diabetic peripheral neuropathy patients: a systematic review of randomized controlled trials. Diabetes Metab Syndr Obes . (2020) 13:2753–64. doi: 10.2147/DMSO.S261175
118. Prajapati SK, Gage WH, Brooks D, Black SE, McIlroy WE. A novel approach to ambulatory monitoring: investigation into the quantity and control of everyday walking in patients with subacute stroke. Neurorehabil Neural Repair . (2011) 25:6–14. doi: 10.1177/1545968310374189
119. Hsu C, Tsai Y, Yau C, Shie H, Wu C. Test-Retest reliability of an automated infrared-assisted trunk accelerometer-based gait analysis system. Sens . (2016) 16(8):1156. doi: 10.3390/s16081156
120. Byun S, Han JW, Kim TH, Kim KW. Test-Retest reliability and concurrent validity of a single tri-axial accelerometer-based gait analysis in older adults with Normal cognition. PLoS One . (2016) 11:e0158956. doi: 10.1371/journal.pone.0158956
121. Poitras I, Dupuis F, Bielmann M, Campeau-Lecours A, Mercier C, Bouyer LJ, et al. Validity and reliability of wearable sensors for joint angle estimation: a systematic review. Sens . (2019) 19(7):1555. doi: 10.3390/s19071555
122. Ro DH, Lee J, Lee J, Park JY, Han HS, Lee MC. Effects of knee osteoarthritis on hip and ankle gait mechanics. Adv Orthop . (2019) 2019:9757369. doi: 10.1155/2019/9757369
123. Viteckova S, Kutilek P, Svoboda Z, Krupicka R, Kauler J, Szabo Z. Gait symmetry measures: a review of current and prospective methods. Biomed Signal Process Control . (2018) 42:89–100. doi: 10.1016/j.bspc.2018.01.013
124. Cimolin V, Galli M. Summary measures for clinical gait analysis: a literature review. Gait Posture . (2014) 39:1005–10. doi: 10.1016/j.gaitpost.2014.02.001
Keywords: clinical gait assessment, gait technologies, gait measures, mobile gait lab, gait pathologies
Citation: Hulleck AA, Menoth Mohan D, Abdallah N, El Rich M and Khalaf K (2022) Present and future of gait assessment in clinical practice: Towards the application of novel trends and technologies. Front. Med. Technol. 4:901331. doi: 10.3389/fmedt.2022.901331
Received: 21 March 2022; Accepted: 17 November 2022; Published: 16 December 2022.
Reviewed by:
© 2022 Hulleck, Menoth Mohan, Abdallah, El Rich and Khalaf. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY) . The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
* Correspondence: Kinda Khalaf [email protected]
Specialty Section: This article was submitted to Diagnostic and Therapeutic Devices, a section of the journal Frontiers in Medical Technology
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
- Understanding Your Brain & Body
Gait Analysis Top Questions Answered
1. Why would my child need gait analysis?
The primary purpose of gait analysis is to provide a detailed understanding of a patient’s movement patterns so that more informed treatment decisions can be made. Gait analysis provides documentation of movement patterns such as joint angles and muscle activity during walking that cannot be determined through visual analysis alone. When this information is used in conjunction with a comprehensive clinical exam the best understanding of movement pathology is possible. Gait analysis is appropriate for a variety of complex conditions that involve impairments to the muscles, joints, nerves and bones that impact gait function.
2. What information will gait analysis tell my child's doctor?
Gait analysis will provide a detailed description of your child’s walking including joint angles and loads, muscle activity and details about speed and step lengths. Gait analysis findings can be used to better understand walking pathology therefore can be used to recommend interventions, including braces, medication, physical therapy, and surgery that are focused on improving walking. It is important to measure and understand what the doctor is proposing to treat to make the best treatment decision. Gait analysis can also be used to evaluate the effectiveness of treatments which is also a very important step.
3. I'm a teen or adult and I've never had gait analysis, is it still something I should consider?
Yes, teenagers and adults can have gait analysis. If you are concerned about changes in how you walk or if you’re not able to participate in usual activities, speak with your physician about whether gait analysis is a good option.
4. If my child is scheduled to have gait analysis, what are the pre-appointment considerations?
When scheduling your appointment, your doctor’s office will let you know what to wear to the appointment, how long the appointment will take (it’s usually several hours), and who will be involved in the testing. If you are wearing braces or use an ambulatory aid, it is important to bring these to the gait analysis appointment.
5. If my child has gait analysis, does that mean they will definitely get referred for surgery?
Having gait analysis done doesn’t mean that your child will get referred for surgery. The information from the analysis will help you, your child, and your care team determine the best treatments based upon your goals.
6. Who are all the healthcare providers involved in gait analysis and what are their roles?
The healthcare providers involved in gait analysis can include: orthopedic surgeon, physical therapist, kinesiologist, engineer, researchers, and technicians. This is a multidisciplinary and highly trained team of experts in both the collection and interpretation of gait analysis data.
7. Are children and their families happy with the outcomes of having gait analysis?
A gait analysis provides comprehensive data that improves the understanding of walking function and the possible causes of walking difficulties and in some cases after multiple gait analysis tests, can document an improvement or decline in walking function. It is important to understand prior to a gait analysis what is possible in terms of the information provided by gait analysis so that there are no disappointments following. Gait analysis allows for a better understanding of a walking problem. The outcomes in terms of treatment decisions related to gait analysis vary depending on the goals of treatment and the provider making the treatment decisions. Satisfaction with treatment outcomes depends on understanding all treatment options and setting realistic goals for the treatment and is not based up on the gait analysis procedure itself.
8. How long is a gait analysis appointment?
The length of gait analysis appointments can vary by the number of tests required, the level of disability and the ability of the individual to cooperate. The duration is generally between 2-3 hours. The best estimate of the time for a motion analysis for a given patient can be provided at the time of scheduling when the specifics of the motion analysis and the child are known.

Latest Research Trends in Gait Analysis Using Wearable Sensors and Machine Learning: A Systematic Review
- September 2020
- IEEE Access 8

- Tallinn University of Technology

Abstract and Figures

Discover the world's research
- 25+ million members
- 160+ million publication pages
- 2.3+ billion citations
- Jingzhe Sun
- Songah Jeong
- Zihao Zheng

- SENSORS-BASEL
- Yong-Gyun Kim
- Sungjoon Kim

- Seong-Ho Jang

- Eman N. Marzban
- Abobakr Khalil Al-Shamiri

- Lorenzo Scalise
- Ming-Chang Lee
- Jia-Chun Lin
- Sokratis Katsikas

- Xiaohui Jia

- Abdullah Alshahrani
- Abrar Almjally

- Ke-Ling Hsu
- Yi-Yuan Chen
- Peter P. K. Chan
- Ben M.F. Lam

- Wen-Shuai Hu

- Elisabeth Kraus

- Recruit researchers
- Join for free
- Login Email Tip: Most researchers use their institutional email address as their ResearchGate login Password Forgot password? Keep me logged in Log in or Continue with Google Welcome back! Please log in. Email · Hint Tip: Most researchers use their institutional email address as their ResearchGate login Password Forgot password? Keep me logged in Log in or Continue with Google No account? Sign up
An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
Preview improvements coming to the PMC website in October 2024. Learn More or Try it out now .
- Advanced Search
- Journal List
- J Neuroengineering Rehabil

Gait analysis methods in rehabilitation
Richard baker.
1 Hugh Williamson Gait Analysis Service, Royal Children's Hospital, Parkville, Victoria, Australia
2 Gait CCRE, Murdoch Children's Research Institute, Parkville, Victoria, Australia
3 Department of Mechanical and Manufacturing Engineering, University of Melbourne, Parkville, Australia
4 Musculoskeletal Research Centre, La Trobe University, Bundoora, Victoria, Australia
This is an Open Access article distributed under the terms of the Creative Commons Attribution License ( http://creativecommons.org/licenses/by/2.0 ), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Introduction
Brand's four reasons for clinical tests and his analysis of the characteristics of valid biomechanical tests for use in orthopaedics are taken as a basis for determining what methodologies are required for gait analysis in a clinical rehabilitation context.
Measurement methods in clinical gait analysis
The state of the art of optical systems capable of measuring the positions of retro-reflective markers placed on the skin is sufficiently advanced that they are probably no longer a significant source of error in clinical gait analysis. Determining the anthropometry of the subject and compensating for soft tissue movement in relation to the under-lying bones are now the principal problems. Techniques for using functional tests to determine joint centres and axes of rotation are starting to be used successfully. Probably the last great challenge for optical systems is in using computational techniques to compensate for soft tissue measurements. In the long term future it is possible that direct imaging of bones and joints in three dimensions (using MRI or fluoroscopy) may replace marker based systems.
Methods for interpreting gait analysis data
There is still not an accepted general theory of why we walk the way we do. In the absence of this, many explanations of walking address the mechanisms by which specific movements are achieved by particular muscles. A whole new methodology is developing to determine the functions of individual muscles. This needs further development and validation. A particular requirement is for subject specific models incorporating 3-dimensional imaging data of the musculo-skeletal anatomy with kinematic and kinetic data.
Methods for understanding the effects of intervention
Clinical gait analysis is extremely limited if it does not allow clinicians to choose between alternative possible interventions or to predict outcomes. This can be achieved either by rigorously planned clinical trials or using theoretical models. The evidence base is generally poor partly because of the limited number of prospective clinical trials that have been completed and more such studies are essential. Very recent work has started to show the potential of using models of the mechanisms by which people with pathology walk in order to simulate different potential interventions. The development of these models offers considerable promise for new clinical applications of gait analysis.
For the purposes of this paper gait analysis will be assumed to refer to the instrumented measurement of the movement patterns that make up walking and the associated interpretation of these. The core of most contemporary gait analysis is the measurement of joint kinematics and kinetics. Other measurements regularly made are electromyography (EMG), oxygen consumption and foot pressures. A systematic physical examination of the patient is usually conducted as part of a gait analysis.
Rehabilitation is a clinical discipline and this paper will thus concentrate on clinical gait analysis. Richard Brand [ 1 , 2 ] proposed four reasons for performing any clinical test (see Table Table1). 1 ). The third of these might actually be taken as a definition of the word clinical i.e. a clinical test is one conducted in order to select from among different management options for a patient (including the possibility of not intervening).
Reasons performing clinical tests as stated by Brand [1, 2])
| 1. to distinguish Diagnosis between disease entities (diagnosis). |
| 2. to determine severity of disease or in jury (i.e. assessment or evaluation) |
| 3. to select among treatment options |
| 4. to predict prognosis |
Much contemporary gait analysis is done for the purpose of clinical research . This differs from clinical testing in that the reason is not to make clinical decisions for the individual patient, but to learn about a condition affecting a group of patients or the effect of an intervention. It is important to remember that the criteria for valid clinical research may not be the same as those for valid clinical testing. For example if a measurement made on a patient cannot be relied upon because of random errors then that measurement will not be useful for clinical purposes. By increasing the number of patients in a sample however, even measurements with quite large random errors can result in meaningful conclusions in clinical research. This paper will focus on gait analysis for clinical use. It will also focus on methodology rather than areas of clinical application.
Brand's [ 1 , 2 ] other three possible reasons for performing any clinical test are to distinguish between disease entities (diagnosis), to determine the severity, extent or nature of a disease or injury (assessment), and to predict outcomes of intervention (or the absence of intervention). The monitoring of the progress of a patient's condition either following intervention or in its absence might be regarded as an additional reason. This modification of Brand's approach is summarised in Table Table2 2 .
Reasons performing clinical gait analysis (modified from Brand [1, 2])
| Clinical gait analysis is performed to allow the selection from amongst treatment options (including the possibility of not intervening). This is based on one or more of: |
| 1. between disease entities. |
| 2. of the severity, extent or nature of a disease or injury. |
| 3. progress in the presence or absence of intervention. |
| 4. of the outcome of intervention (or the absence of intervention). |
Brand went on to propose a number of criteria for assessing the usefulness of biomechanical measurements in general which, with some modification, can be used as criteria for the usefulness of all clinical gait analysis. These are listed in Table Table3. 3 . The first requirement of any clinical measurement is that it should characterise the patient, that is if the patient attends on two separate occasions, between which his or her condition might be considered as stable, the measurements taken should be similar. This requires that the measurement technique itself is repeatable but also that the quantity being measured is stable and independent of factors such as mood, motivation or pain. Measurements can be repeatable and stable without necessarily being accurate (representative of a specific physical quantity). Such tests can be clinically useful but will be much easier to interpret if they are also accurate. In an era of evidence based clinical practice it is essential that any measurement techniques are appropriately validated which must include assessments of both their repeatability and accuracy.
Criteria for biomechanical measures (extracted from text of Brand [1])
| Reproducible |
| Stable (independent of mood, motivation and pain) |
| Accurate |
| Appropriately validated |
| Capable of distinguishing between normal and abnormal |
| Must not alter the function it is measuring |
| Reported in form analogous to accepted clinical concepts |
| Cost-effective |
| Not observable by the skilled clinician |
In order to perform a diagnostic function it is necessary for measurements to be able to distinguish normal from abnormal patterns of movement and also between the characteristics of one disease entity and another. There are two aspects to this. The first is having measurement systems capable of working to adequate precision. The second is a knowledge of what characterises normal walking or a particular disease entity.
The requirement for patient assessment pre-supposes that a diagnosis does not give sufficient information to determine the most appropriate management for a patient and that measuring the precise characteristics of a patient's condition are essential for this. Measurements thus have to be sufficiently precise to reveal clinically important differences between patients with the same diagnosis. For monitoring purposes measurements need to be sufficiently precise to be able to determine whether a patient's condition is stable, improving or deteriorating.
Brand suggested that the measurement technique should not affect the function it is measuring. The walking performed in a gait analysis laboratory however, with the patient concentrating on what they are doing in an idealised environment, is not necessarily representative of their normal walking. At the very least this must be taken into account when interpreting results.
Gait analysis should reveal information that is useful to the clinician and this will generally require that results are reported in terms analogous to accepted clinical concepts. It must be cost-effective, that is the benefit of performing the test must be worth the cost. This balance need not necessarily be determined in purely financial terms but the financial cost of gait analysis is a significant factor. Finally there is no point doing any clinical test if the results could be obtained sufficiently well by simply observing the patient
The information obtained by assessing the patient is that used for selecting management options. This process does not, therefore, make further demands on the measurement systems but does require an understanding of how the patient's condition is likely to be affected by an intervention (or none) to a level sufficient to determine which options are preferable. Prediction of outcomes takes this one stage further to being able to determine not only which management option is best but also how the patient will be after that intervention.
This sequential analysis of the four potential purposes of clinical tests reveals a progression from just requiring reliable and precise measurements to the additional requirement of having an understanding of how such information is incorporated into clinical practice. The state of the art is that the measurement component of gait analysis can reasonably be described as an objective process whereas the interpretation component is predominantly subjective.
Making the interpretive component more objective can be achieved in two ways. The first is to develop a general theory of how people walk whether they have recognised pathology or not. As long ago as 1982 Cappozzo lamented, "The approaches to clinical gait analysis and evaluation are not supported by general theories" [ 3 ] and despite over 20 years of intense activity this is still a reasonable summary of the state of the art. The second approach, which must operate in the absence of the former, is to conduct clinical research to ascertain the outcome of particular interventions on groups of patients characterised by certain measurements. Most of the knowledge base used in the interpretive component of gait analysis comes from such studies. It is because there are relatively few studies available to base such interpretations on that the subjective element of interpretation is necessary in contemporary clinical gait analysis.
Modern clinical gait analysis traces its origins back to the early 1980s with the opening of the laboratory developed by the United Technologies Corporation at Newington, Connecticut and those provided with equipment by Oxford Dynamics (later to become Oxford Metrics) in Boston, Glasgow and Dundee. Retro-reflective markers were placed on the skin in relation to bony landmarks. These were illuminated stroboscopically and detected by modified video cameras. If two or more cameras detect a marker and the position and orientation of these cameras are known then it is possible to detect the three-dimensional position of that marker [ 4 ].
Whilst the basic principles remain the same as the earliest systems, the speed, accuracy and reliability has advanced beyond all recognition. It is not uncommon now to find clinical systems using 8, 10 or more cameras functioning at over 100 Hz and capable of detecting reliably the presence of many tens of markers of between 9 and 25 mm diameter. Calibration of the systems (the determination of the position, orientation and optical and electronic characteristics of the cameras) can generally be accomplished in less than a minute. Marker positions from clinical trials can be reconstructed and markers labelled automatically in real time (although this feature is often not essential for clinical studies). The determination of the accuracy of such systems is now generally limited by the accuracy of any alternative means to determine marker position and can be taken to be of the order of 1 mm. This is probably an order of magnitude smaller than other sources of error in determining joint kinematics and kinetics. This particular measurement technology has thus reached a mature state of development that, whilst advances will almost certainly continue, already probably delivers all that is required by conventional gait analysis [ 5 ].
The same cannot be said of the computer models used to derive joint kinematics and kinetics from the marker position data supplied by the measurement hardware. Almost all commercially available clinical systems use some variant of the Conventional Gait Model [ 6 ] which has been referred to as the Newington, Gage, Davis [ 7 ], Helen Hayes, Kadaba [ 8 , 9 ] or Vicon Clinical Manager (VCM) model. This was developed using the minimum number of markers possible to determine 3-dimensional kinematics and kinetics [ 10 , 11 ] of the lower limb at a time when measurement systems were only capable of detecting a handful of markers. It assumes three degree of freedom joints for the hip and knee and a two degree of freedom joint at the ankle. The model is hierarchical requiring the proximal segments to have been detected in order that distal segments can be defined and incorporates regression equations to determine the position of the hip joint centre with respect to pelvic markers. Kinetics are determined using an inverse dynamics approach which generally requires considerable filtering to give any useful signals. An alternative system the Cleveland Clinic Model based around a cluster of markers on a rigid base attached to each segment is the only other widely used model. Unfortunately documentation of this model in the scientific literature is very poor.
The problem of limited repeatability
The primary problem of current measurement technology is that of reliability in routine clinical use. Several studies have now been reported in which a single subject has been analysed in a number of different laboratories [ 12 - 14 ]. These have shown a degree of variability between sites that would appear to be sufficient to undermine clinical applications. In retrospect, the original studies of the reliability are flawed. There was no such study of the Davis implementation of the model and the statistics used by Kadaba et al [ 8 , 9 ] to report reliability of their implementation probably acted to mask deficiencies. In particular, use of relative measures of reliability such as the coefficient of multiple correlation (CMC) makes interpretation of findings difficult. Almost all reliability studies have been done on subjects without pathology where marker placement is reasonably straightforward. Reliability for clinical populations is rarely reported in the literature and is almost certainly inferior.
At least one recent study has shown that it is possible to get levels of reliability sufficient to justify the continued clinical use of gait analysis within a single centre [ 15 ]. Too few centres however are providing evidence to establish that this is the rule rather than the exception.
Source of error: Model calibration
There are two principal sources of error. The first is the difficulty determining the anthropometry of the individual subject (known as model calibration ). This has two aspects, placing markers accurately with respect to specific anatomical landmarks and determining the location of the joint centres (and other anatomical features) in relation to these markers. Failure to place markers accurately is probably the single greatest contributor to measurement variability in contemporary clinical gait analysis. This is partly a matter of appropriate staff training and quality assurance but at least as important, and more fundamental, is the problem that many of the landmarks used to guide marker placement are not themselves particularly well defined in patients with certain conditions [ 16 ]. Even when bony landmarks are sharply defined an increasing number of patients have a considerable thickness of subcutaneous fat that makes palpation difficult.
The Conventional Gait Model uses regression equations to determine the position of the hip joint centre in relation to the pelvis. Both Bell's [ 17 - 19 ] and Davis' [ 7 ] equations are commonly used and there is now good evidence that neither is satisfactory in healthy adults [ 20 ]. There have still been no published studies of whether either is valid for healthy children. Children with orthopaedic conditions including cerebral palsy may often have dysplasia of the hip or deformity of the pelvis, and it is exceedingly unlikely that any form of regression equation could be used in these patients to determine hip joint position.
Methods for moving away from anatomical landmarks and regressions equations for determining joint centres have been around for nearly a decade, the process being known as anatomical calibration [ 21 ]. They rely on calibration movements to be performed before capturing walking data and some form of fitting of the measured marker positions to an underlying model of how the body moves. The simplest example is probably the determination of the hip joint centre. It is assumed that the hip joint moves as a ball and socket joint about centre of rotation fixed in the pelvis. Any marker on the femur would thus be expected to describe a path on the surface of a sphere centred on the hip joint centre when the hip joint is moving. A least squares fit of the measured data to such a sphere allows the location of that joint centre to be determined [ 20 , 22 ]. Similar approaches are applicable to determine that axis of the knee joint which for this purpose has often been assumed to be a simple hinge joint.
Various approaches to fitting data to an underlying model have been attempted and many seem to give reasonable results [ 20 , 22 - 28 ]. Such techniques have not so far been widely accepted into clinical practice probably because there is a perception that such calibration trials are too difficult for patients to execute. At least one clinical lab however has now committed itself to implementing such techniques into routine practice and has reported failure to perform test adequately in only one of over 700 patients tested so far.
Sources of error: Soft tissue artefact
The second source of error is the degree of movement of the skin, muscle and other soft tissues in relation to the bones that occurs during walking. This is perhaps most marked in relation to the rotational profile of the hip. Lamoreux [ 29 ], as far back as 1991, reported that with optimal placement of thigh wands only 65% of transverse plane hip joint rotation was detected and that with poor placement this could be as little as 35%.
The problem of skin and other soft tissue movement is more problematic than that of model calibration. Lu and O'Connor where the first to propose fitting a model of how the body is expected to move to marker co-ordinate data [ 30 ] using an optimisation approach. This model uses a least squares fit, similar to some of the techniques described above for model calibration, and thus makes no assumptions about the nature of the soft tissue movement. Other similar models have now been made commercially available [ 26 ]. More recent studies have started to try to map out the movement of markers with respect to the underlying bones [ 31 , 32 ]. If such movement can be characterised as a function of joint angle then, in principle, this knowledge could be built into a model to allow such movements to be compensated for. Such mapping is only likely to be useful if it can be shown that soft tissue movement is consistent across a range of subjects and activities. It is not clear at present whether these conditions are satisfied. A particular problem in regard to mapping soft tissue movement is that of defining what the "true" movement of the bones is. In the absence of any gold standard a variety of assumptions are being used most of which have serious limitations.
The development of a gold standard method for determining joint movement will probably require a move away from skin-mounted markers (or other sensors). Once such technology is available however it is quite possible that this will supersede the presently available systems. The cost of any such new systems however is likely to prohibit ready clinical availability in the foreseeable future.
There has been some work done on markerless optical methods. By placing a number of video cameras around a subject and tracing the silhouette of the walking subject on each it is possible to generate a 3-dimentional silhouette of that subject. This has already been achieved but the next step of using such a silhouette to determine the co-ordinate systems associated with the moving body segments has not yet been satisfactorily achieved.
It is possible that the problem of skin movement can only be satisfactorily addressed by making direct measurements of bone position. It is now possible to take 3-dimensional images of bones (and muscles) using MRI but only within a very restricted capture volume [ 33 - 35 ]. The image processing problem of automatically determining a bone embedded axis system from such images has yet to be solved satisfactorily. Similarly both uniplanar and biplanar cine fluoroscopy [ 36 - 40 ] has been used to detect the 3-dimensional movement of the internal knee prostheses during a variety of movements. This is possible because a knowledge of the exact size and shape of the prosthetic components and their opacity to x-rays greatly simplifies the image processing problem. Using similar techniques to determine the movement of joints has also been reported [ 41 - 43 ]
Methods for interpreting clinical gait analysis data
The second element of clinical gait analysis is the interpretation of data. Conventions for describing 3-dimensional joint kinematics and kinetics are well formulated. Many laboratories are augmenting conventional kinematics and kinetics with muscle length and, less commonly, moment arm graphs. Normal patterns of movement as represented by these data are now generally fairly well understood by clinical specialists although there is actually very little normative data published in the peer-reviewed literature. Similarly, many abnormal patterns of movement are quite widely recognised by clinicians but there few published attempts at formal classification of these [ 44 - 46 ]. Many clinicians have learnt to associate particular abnormal patterns in particular patient groups with particular impairments of body structure and function. Intervention based on such an understanding often leads to a normalisation of gait patterns at subsequent assessments (e.g. [ 47 - 55 ]). It is on this basis that clinical gait analysis operates at present.
Despite the widespread acceptance of many of these conventions there are still problems. Baker [ 56 ] demonstrated that the Euler sequence used to calculate pelvic angles gives rise to data that can be mis-leading to clinicians and proposed an alternative to correct this which is yet to be adopted widely within clinical analysis. Methods for interpreting angles in three dimensions, either in terms of Euler/Cardan rotations or the Grood and Suntay convention [ 57 , 58 ] are not well understood either by clinicians or many bioengineers. A recent attempt to standardise the reporting of joint angles [ 59 ] proposed a different convention to that of the Conventional Gait Model and the continuing debate as to which is preferable illustrates this confusion [ 60 , 61 ]. Joint moments are generally reported with reference to orthogonal axis systems fixed in the distal (Conventional Gait Model) or proximal segments (or occasionally the laboratory axis system). These differ significantly depending on the axis system chosen [ 6 , 62 ] yet there has been no debate about which if any is preferable. Reporting moments about orthogonal axis systems and joint rotations about non-orthogonal ones leads to difficulties in relating the moments to the changes in joint angles to which they are related. The use of muscle moment arms will be discussed further below but it is interesting that there is no straightforward definition of the meaning of the term moment arm in three dimensions [ 63 ] and it is often not clear how such data should be interpreted.
Perhaps the most important limitation of our present understanding of human walking, however, is that it is primarily descriptive. We know what happens rather than why it happens. Many in the clinical gait analysis community regard kinematics as descriptive but contend that kinetics explain movement patterns. This is almost certainly misguided. Kinetics are simply another set of measurements and can thus only be descriptive.
There have been various attempts at establishing a theory of walking but none is particularly convincing. Saunders, Inman and Eberhart's determinants of normal walking [ 64 ] are perhaps the best known of these. Recent publications however have questioned how the detail of these reflects experimental data [ 65 - 70 ]. Gage [ 71 , 72 ] based his pre-requisites of gait on earlier work by Perry [ 73 ] but these are best regarded as pointers to where particular patients are deficient rather than explanations of how they are achieving walking with or without pathology.
Perhaps the closest we have come so far to understanding why we walk the way we do has come from the work of Pandy and Anderson [ 74 , 75 ]. They have shown that it is possible to construct a mathematical simulation of muscle function during normal walking based on the assumption that the total consumption of energy per unit distance walked is minimised. The authors, however, commented that the model seems more dependent on the boundary conditions imposed than on the nature of the optimisation function. Further, because of the complex nature of the optimisation process driving the model it is still difficult to explain how the precise characteristics of any particular feature of the walking pattern affect the overall calculation of energy expenditure. So far such a model has only been constructed for normal walking.
Conceptually, modifying such models to incorporate a specific abnormality of the musculo-skeletal anatomy such as a leg length discrepancy or contracture of a particular muscle is reasonably straightforward. It is much less certain whether such techniques can be applied at all to patients with neuromuscular pathology who are most frequently seen by clinical gait analysis services. Optimisation techniques assume that movements are controlled in such a way that a specific control function is minimised. In many neuromuscular conditions (Cerebral Palsy, Parkinson's disease, adult hemiplegia) the problem is one of a loss of central control and this would appear to invalidate any techniques modelling human movement as an optimised process.
If such models are developed it will be interesting to see whether they give any insights into the clinical management of patients. Further it will be interesting to see whether their use leads to an understanding of why we walk the way we do which can be formulated as theories that are applicable without the use of such complex models.
Whilst the answer to this question still seems as far away as ever, significant advances have been made over recent years in understanding the mechanisms by which we walk particularly in the way that muscles act. For many years it was assumed that a muscle's anatomical position determines how it acts. It was assumed for example that the action of the hamstrings, passing behind the knee, was always to flex the knee. It is only comparatively recently that biomechanists have come to appreciate that any individual muscle has an effect on all the segments of the body and that in some circumstances this may result in a muscle having an action different to its anatomical function [ 76 - 81 ]. It is now fairly well accepted, for example, that the hamstrings functions as a knee extensor during early stance in normal walking because its effect in extending the hip has a secondary tendency to extend the knee which is greater than its direct effect as an anatomical knee flexor [ 82 ].
Such work depends on knowing the joint kinematics and kinetics and inertial properties of the body segments. These can be used to estimate the forces in individual muscles [ 81 - 83 ]. This is an indeterminate problem so is dependent on an optimisation approach (and the validity of this in neuromuscular pathology is questioned in the same way as that of the simulations described above). Once the muscle forces are known forward modelling can be used to determine the effect that a given muscle is having on any segment (or joint) of the body. Until very recently the first part of this problem, the estimation of muscle forces had not been achieved which limited the application of the second part, the forward modelling to data obtained from the simulations described above [ 74 , 75 ]. Recently methods have been develop to estimate the muscle forces required to generate measured joint kinematics and ground reaction forces and have been used both to understand the function of individual muscles during pathological gait and predict the effect of interventions [ 84 , 85 ]. These have been based on scaled models of the adult musculo-skeletal anatomy.
There is also considerable debate at present about the validity of these techniques (the simulations, the estimations of muscle forces and the forward modelling). Whilst the general principles are sound the techniques are known to be extremely sensitive to certain aspects of their implementation (and may be sensitive to many more). For example the forward modelling in particular is sensitive to how the interaction between the foot and the floor is modelled with there being no clear consensus as to the most appropriate method for this [ 74 , 77 , 81 ].
Methods for understanding the effect of intervention
Understanding how to interpret clinical gait analysis data is not itself sufficient to allow selection from amongst treatment options (Table (Table1). 1 ). For this it is also necessary to know what effect the available interventions are likely to have on someone's walking pattern. If we had a general theory of walking then it might be possible to develop a theoretical basis for considering the effect of any intervention. For patients whose walking could be modelled using a simulation based on specific musculo-skeletal abnormalities it might be possible to use similar simulations to model what might happen if partial correction of those abnormalities were attempted (obviously full correction would restore normal walking!). The author is unaware of any published work at this level at present.
There are then two methods for understanding the effects of intervention in these patients; clinical research to establish what the actual effect of a given intervention is or using knowledge of the mechanisms of walking to predict the effect of modifying the characteristics of the musculo-skeletal anatomy.
By far the most common approach to date has been the use of clinical research – the comparison of gait patterns before and after a particular intervention [ 47 - 55 , 86 - 88 ]. Even so there have been comparatively few studies that have given conclusive findings. Many studies which claim to have done so have quite serious methodological flaws. This is particularly true of research into orthopaedic surgery for children with CP where researchers have used retrospective audits of clinical practice to try and answer specific questions. Many of these studies attempt to make inferences about individual procedures which have only ever been performed as part of a multi-level surgical package [ 47 , 49 - 51 , 54 , 55 ]. It is impossible to tell from these studies which effects are due to the particular procedure being considered and which are due to the overall package. Several studies have attempted to separate out those effects by dividing patients into those who have and those who have not had a particular procedure as part of the overall package of surgery and use methods to compare groups similar to those that would be used for a randomised clinical trial [ 47 , 54 ]. The validity of this approach is questionable, however, because generally the two groups of patients were not similar to start with. Those that had the procedure had it because it was considered that the patient needed it and vice versa. Comparison of the two groups to give insight into the effect of the procedure is thus invalid.
An alternative to the use of clinical trials is to use knowledge of the mechanisms of walking as a basis for modelling the effect of changing that mechanism. Reports of such studies are now starting to emerge. For example Arnold et al. [ 84 ] have reported a subject specific model of a cerebral palsy patient with a stiff knee gait and used it to predict the effect of three different potential interventions. These indicated a preferable intervention and the post-intervention gait data showed at least qualitative agreement with the theoretical predictions.
Competing interests
The author has received research funding from Oxford Metrics Plc (Oxford, UK)
- Brand RA. Can Biomechanics contribute to clinical orthopaedic assessments. Iowa Orthopaedic Journal. 1987; 9 :61–64. [ Google Scholar ]
- Brand RA, Crowninshield RD. Comment on criteria for patient evaluation tools. Journal of Biomechanics. 1981; 14 :655. doi: 10.1016/0021-9290(81)90093-2. [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Cappozzo A. Considerations on clinical gait evaluation. Journal of Biomechanics. 1983; 16 :302. doi: 10.1016/0021-9290(83)90202-6. [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Cappozzo A, Della Croce U, Leardini A, Chiari L. Human movement analysis using stereophotogrammetry. Part 1: theoretical background. Gait and Posture. 2005; 21 :186–196. [ PubMed ] [ Google Scholar ]
- Chiari L, Della Croce U, Leardini A, Cappozzo A. Human movement analysis using stereophotogrammetry. Part 2: instrumental errors. Gait and Posture. 2005; 21 :197–211. doi: 10.1016/j.gaitpost.2004.04.004. [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Baker R, Rodda J. All you ever wanted to know about the conventional gait model but were afraid to ask. Melbourne, Women and Children's Health; 2003. [ Google Scholar ]
- Davis RB, Ounpuu S, Tyburski D, Gage JR. A gait analysis data collection and reduction technique. Human Movement Science. 1991; 10 :575–587. doi: 10.1016/0167-9457(91)90046-Z. [ CrossRef ] [ Google Scholar ]
- Kadaba MP, Ramakrishnan HK, Wootten ME. Measurement of lower extremity kinematics during level walking. Journal of Orthopaedic Research. 1990; 8 :383–391. doi: 10.1002/jor.1100080310. [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Kadaba MP, Ramakrishnan HK, Wootten ME, Gainey J, Gorton G, Cochran GVB. Repeatability of kinematic, kinetic, and electromyographic data in normal adult gait. Journal of Orthopaedic Research. 1989; 7 :849–860. doi: 10.1002/jor.1100070611. [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Ounpuu S, Gage JR, Davis RB. Three-dimensional lower extremity joint kinetics in normal pediatric gait. Journal of Pediatric Orthopaedics. 1991; 11 :341–349. [ PubMed ] [ Google Scholar ]
- Ounpuu O, Davis RB, Deluca PA. Joint kinetics: Methods, interpretation and treatment decision-making in children with cerebral palsy and myelomeningocele. Gait and Posture. 1996; 4 :62–78. doi: 10.1016/0966-6362(95)01044-0. [ CrossRef ] [ Google Scholar ]
- Noonan KJ, Halliday S, Browne R, O'Brien S, Kayes K, J F. Inter-observer variability of gait analysis in patients with cerebral palsy. Journal of Pediatric Orthopaedics. 2003; 23 :279–287. doi: 10.1097/00004694-200305000-00001. [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Gorton G, Hebert D, Goode B. Assessment of the kinematic variability between 12 Shriners motion analysis laboratories. Gait and Posture. 2001; 13 :247. [ PubMed ] [ Google Scholar ]
- Gorton G, Hebert D, Goode B. Assessment of kinematic variability between 12 Shriners motion analysis laboratories part 2: Short term follow up. Gait and Posture. 2002; 16 (suppl 1) :S65–66. [ Google Scholar ]
- Schwartz MH, Trost JP, Wervey RA. Measurement and management of errors in quantitative gait data. Gait and Posture. 2004; 20 :196–203. doi: 10.1016/j.gaitpost.2003.09.011. [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Della Croce U, Leardini A, Chiari L, Cappozzo A. Human movement analysis using stereophotogrammetry. Part 4: assessment of anatomical landmark misplacement and its effects on joint kinematics. Gait and Posture. 2005; 21 :226–237. doi: 10.1016/j.gaitpost.2004.05.003. [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Bell AL, Brand RA, Pedersen DR. Prediction of hip joint center location from external landmark: ; Atlanta, Georgia. 1988. p. 212. [ Google Scholar ]
- Bell AL. A comparison of the accuracy of several hip centre location prediction methods. Journal of Biomechanics. 1990; 23 :617–621. doi: 10.1016/0021-9290(90)90054-7. [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Bell AL, Brand RA, Pedersen DR. Prediction of hip joint centre location from external landmarks. Human Movement Science. 1989; 8 :3–16. doi: 10.1016/0167-9457(89)90020-1. [ CrossRef ] [ Google Scholar ]
- Leardini A, Cappozzo A, Catani F, Toksvig-Larsen S, Petitto A, Sforza V, Cassanelli G, Giannini S. Validation of a functional method for the estimation of hip joint centre location. Journal of Biomechanics. 1999; 32 :99–103. doi: 10.1016/S0021-9290(98)00148-1. [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Cappozzo A, Catani F, Della Croce U, Leardini A. Position and orientation in space of bones during movement: anatomoical frame definition and determination. Clinical Biomechanics. 1995; 10 :171–178. doi: 10.1016/0268-0033(95)91394-T. [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Piazza SJ, Okita N, Cavanagh PR. Accuracy of the functional method of hip joint center location: effects of limited motion and varied implementation. Journal of Biomechanics. 2001; 34 :967–973. doi: 10.1016/S0021-9290(01)00052-5. [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Hicks JL, Richards JG. Clinical applicability of using spherical fitting to find hip joint centers. Gait and Posture. 2005; 22 :138–145. doi: 10.1016/j.gaitpost.2004.08.004. [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Piazza SJ, Erdemir A, Okita N, Cavanagh PR. Assessment of the functional method of hip joint center location subject to reduced range of hip motion. Journal of Biomechanics. 2004; 37 :349–356. doi: 10.1016/S0021-9290(03)00288-4. [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Camomilla V, Cereatti A, Vannozzi G, Cappozzo A. An optimised protocol for hip joint centre determination using the functional method. Journal of Biomechanics. 2005. pp. In press, available on–line. [ PubMed ]
- Charlton IW, Tate P, Smyth P, Roren L. Repeatability of an optimised lower body model. Gait and Posture. 2004; 20 :213–221. doi: 10.1016/j.gaitpost.2003.09.004. [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Schwartz MH, Rozumalski A. A new method for estimating joint parameters from motion data. Journal of Biomechanics. 2005; 38 :107–116. [ PubMed ] [ Google Scholar ]
- Reinbolt JA, Schutte JF, Fregly BJ, Koh BI, Haftka RT, George AD, Mitchell KH. Determination of patient-specific multi-joint kinematic models through two-level optimization. Journal of Biomechanics. 2005; 38 :621–626. doi: 10.1016/j.jbiomech.2004.03.031. [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Lamoreux LW. Errors in thigh axial rotation measurements using skin mounted markers. 1991. pp. 372–373.
- Lu TW, O'Connor JJ. Bone position estimation from skin marker co-ordinates using global optimisatoin with joint constraints. Journal of Biomechanics. 1999; 32 :129–134. doi: 10.1016/S0021-9290(98)00158-4. [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Alexander EJ, Andriacchi TP. Correcting for deformation in skin-based marker systems. Journal of Biomechanics. 2001; 34 :355–361. doi: 10.1016/S0021-9290(00)00192-5. [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Leardini A, Chiari L, Della Croce U, Cappozzo A. Human movement analysis using stereophotogrammetry. Part 3. Soft tissue artifact assessment and compensation. Gait and Posture. 2005; 21 :212–225. doi: 10.1016/j.gaitpost.2004.05.002. [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Asakawa DS, Pappas GP, Blemker SS, Drace JE, Delp SL. Cine phase-contrast magnetic resonance imaging as a tool for quantification of skeletal muscle motion. Seminars on Musculoskelet Radiology. 2003; 7 :287–295. doi: 10.1055/s-2004-815676. [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Rebmann AJ, Sheehan FT. Precise 3D skeletal kinematics using fast phase contrast magnetic resonance imaging. Journal of Magnetic Resonance Imaging. 2003; 17 :206–213. doi: 10.1002/jmri.10253. [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Barrance PJ, Williams GN, Novotny JE, Buchanan TS. A method for measurement of joint kinematics in vivo by registration of 3-D geometric models with cine phase contrast magnetic resonance imaging data. Journal of Biomechanical Engineering. 2005; 127 :829–837. doi: 10.1115/1.1992524. [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Banks S, Bellemans J, Nozaki H, Whiteside LA, Harman M, Hodge WA. Knee motions during maximum flexion in fixed and mobile-bearing arthroplasties. Clinical Orthopaedics and Related Research. 2003:131–138. doi: 10.1097/01.blo.0000063121.39522.19. [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Banks SA, Fregly BJ, Boniforti F, Reinschmidt C, Romagnoli S. Comparing in vivo kinematics of unicondylar and bi-unicondylar knee replacements. Knee Surg Sports Traumatol Arthrosc. 2005; 13 :551–556. doi: 10.1007/s00167-004-0565-x. [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Banks SA, Hodge WA. Implant design affects knee arthroplasty kinematics during stair-stepping. Clinical Orthopaedics and Related Research. 2004:187–193. doi: 10.1097/01.blo.0000138956.04316.ac. [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Banks SA, Hodge WA. 2003 Hap Paul Award Paper of the International Society for Technology in Arthroplasty. Design and activity dependence of kinematics in fixed and mobile-bearing knee arthroplasties. Journal of Arthroplasty. 2004; 19 :809–816. doi: 10.1016/j.arth.2004.04.011. [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Stagni R, Fantozzi S, Cappello A, Leardini A. Quantification of soft tissue artefact in motion analysis by combining 3D fluoroscopy and stereophotogrammetry: a study on two subjects. Clinical Biomechanics. 2005; 20 :320–329. doi: 10.1016/j.clinbiomech.2004.11.012. [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Fregly BJ, Rahman HA, Banks SA. Theoretical accuracy of model-based shape matching for measuring natural knee kinematics with single-plane fluoroscopy. Journal of Biomechanical Engineering. 2005; 127 :692–699. doi: 10.1115/1.1933949. [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Li G, DeFrate LE, Park SE, Gill TJ, Rubash HE. In vivo articular cartilage contact kinematics of the knee: an investigation using dual-orthogonal fluoroscopy and magnetic resonance image-based computer models. American Journal of Sports Medicine. 2005; 33 :102–107. doi: 10.1177/0363546504265577. [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Li G, Wuerz TH, DeFrate LE. Feasibility of using orthogonal fluoroscopic images to measure in vivo joint kinematics. Journal of Biomechanical Engineering. 2004; 126 :314–318. doi: 10.1115/1.1691448. [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Rodda JM, Graham HK, Carson L, Galea MP, Wolfe R. Sagittal gait patterns in spastic diplegia. Journal of Bone and Joint Surgery. 2004; 86 :251–258. doi: 10.1302/0301-620X.86B2.13878. [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Winters TF, Gage JR, Hicks R. Gait patterns in spastic hemiplegia in children and young adults. Journal of Bone and Joint Surgery. 1987; 69a :437–441. [ PubMed ] [ Google Scholar ]
- Hullin MG, Robb JE, Loudon IR. Gait patterns in children with hemiplegic spastic cerebral palsy. Journal of Pediatric Orthopaedics. 1996; 5 :547–251. [ PubMed ] [ Google Scholar ]
- Novacheck TF, Trost JP, Schwartz MH. Intramuscular psoas lengthening improves dynamic hip function in children with cerebral palsy. Journal of Pediatric Orthopaedics. 2002; 22 :158–164. doi: 10.1097/00004694-200203000-00004. [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Rose SA, DeLuca PA, Davis RBIII, Ounpuu S, Gage JR. Kinematic and kinetic evaluation of the ankle after lengthening of the gastrocnemuis fascia in children with cerebral palsy. Journal of Pediatric Orthopaedics. 1993; 13 :727–732. [ PubMed ] [ Google Scholar ]
- Ounpuu S, DeLuca P, Davis R, Romness M. Long-term effects of femoral derotation osteotomies: an evaluation using three-dimensional gait analysis. Journal of Pediatric Orthopaedics. 2002; 22 :139–145. doi: 10.1097/00004694-200203000-00001. [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Ounpuu S, Muik E, Davis RB, Gage JR, DeLuca PA. Rectus femoris surgery in children with cerebral palsy. Part II: A comparison between the effect of transfer and release of the distal rectus femoris on knee motion. Journal of Pediatric Orthopaedics. 1993; 13 :331–335. [ PubMed ] [ Google Scholar ]
- Ounpuu S, Muik E, Davis RB, Gage JR, DeLuca PA. Rectus femoris surgery in children with cerebral palsy. Part I: The effect of rectus femoris transfer location on knee motion. Journal of Pediatric Orthopaedics. 1993; 13 :325–330. [ PubMed ] [ Google Scholar ]
- Pirpiris M, Trivett A, Baker R, Rodda J, Nattrass GR, Graham HK. Femoral derotation osteotomy in spastic diplegia. Proximal or distal? Journal of Bone and Joint Surgery. 2003; 85 :265–272. doi: 10.1302/0301-620X.85B2.13342. [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Pirpiris M. Department of Paediatrics. , University of Melbourne; 2002. Single event multi-level surgery in spastic diplegia: comprehensive outcome analysis. [ Google Scholar ]
- DeLuca P, Ounpuu O, Davis RB, Walsh J. Effect of hamstrings and psoas lengthening on pelvic tilt in patients with spastic diplegic cerebral palsy. Journal of Pediatric Orthopaedics. 1998; 18 :712–718. doi: 10.1097/00004694-199811000-00004. [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Gage J, Perry J, Hicks R, Koop S, Wernt J. Rectus femoris transfer to improve knee function of children with cerebral palsy. Developmental Medicine and Child Neurology. 1987; 29 :159–166. [ PubMed ] [ Google Scholar ]
- Baker R. Pelvic angles: a mathematically rigorous definition which is consistent with a conventional clinical understanding of the terms. Gait and Posture. 2001; 13 :1–6. doi: 10.1016/S0966-6362(00)00083-7. [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Grood ES, Suntay WJ. A joint coordinate system for the clinical description of three-dimensional motions: Application to the knee. Transactions of the ASME, Journal of Biomechanical Engineering. 1983; 105 :136–143. [ PubMed ] [ Google Scholar ]
- Chao EYS. Justification of triaxial goniometer for the measurement of joint rotation. Journal of Biomechanics. 1980; 13 :989–1006. doi: 10.1016/0021-9290(80)90044-5. [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Wu G, van der Helm FC, Veeger HE, Makhsous M, Van Roy P, Anglin C, Nagels J, Karduna AR, McQuade K, Wang X, Werner FW, Buchholz B. ISB recommendation on definitions of joint coordinate systems of various joints for the reporting of human joint motion--Part II: shoulder, elbow, wrist and hand. Journal of Biomechanics. 2005; 38 :981–992. doi: 10.1016/j.jbiomech.2004.05.042. [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Baker R. ISB recommendation on definition of joint coordinate systems for the reporting of human joint motion-part I: ankle, hip and spine. J Biomech. 2003; 36 :300–2; author reply 303-4. doi: 10.1016/S0021-9290(02)00336-6. [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Schache A, Baker R, Vaughan C. Differences in lower limb transverse plane joint moments during gait when expressed in two alternative reference frames. Journal of Biomechanics. 2006; In press [ PubMed ] [ Google Scholar ]
- Pandy MG. Moment arm of a muscle force. Exercise and Sports Science Reviews. 1999; 27 :79–118. [ PubMed ] [ Google Scholar ]
- Saunders JBDM, Inman VT, Eberhart HD. The major determinants in normal and pathological gait. Journal of Bone and Joint Surgery. 1953; 35A :543–728. [ PubMed ] [ Google Scholar ]
- Ortega J, Farley C. Minimising vertical excursion of centre of mass movement does not reduce metabolic cost in walking: ; Toledo, OH. 2003. [ Google Scholar ]
- Gard SA, Childress DS. The effect of pelvic list on the vertical displacement of the trunk during normal walking. Gait and Posture. 1997; 5 :233–238. doi: 10.1016/S0966-6362(96)01089-2. [ CrossRef ] [ Google Scholar ]
- Gard SA, Childress DS. The influence of stance-phase knee flexion on the vertical displacement of the trunk during normal walking. Archives of Physical Medicine and Rehabilitation. 1999; 80 :26–32. doi: 10.1016/S0003-9993(99)90303-9. [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Gard SA, Childress DS. What determins the vertical displacement of the body during normal walking? Journal of Prosthetics and Orthotics. 2001; 13 :64–67. doi: 10.1097/00008526-200109000-00009. [ CrossRef ] [ Google Scholar ]
- Kerrigan DC, Riley PO, Lelas J, Della Croce U. Quantification of pelvic rotation as a determinant of gait. Archives of Physical Medicine and Rehabilitation. 2001; 82 :217–220. doi: 10.1053/apmr.2001.18063. [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Kerrigan DC, Della Croce U, Marciello M, Riley PO. A refined view of the determinants of gait: significance of heel rise. Archives of Physical Medicine and Rehabilitation. 2000; 81 :1077–1080. doi: 10.1053/apmr.2000.6306. [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Gage JR. Gait Analysis in Cerebral Palsy. Oxford, Mac Keith Press; 1991. [ Google Scholar ]
- Gage JR. The treatment of gait problems in cerebral palsy. London, Mac Keith Press; 2004. [ Google Scholar ]
- Perry J. Normal and pathological gait. In: Bunch WH, editor. Atlas of orthotics. St Louis, CV Mosby; 1985. pp. 76–111. [ Google Scholar ]
- Anderson FC, Pandy MG. Dynamic optimization of human walking. Journal of Biomechanical Engineering. 2001; 123 :381–390. doi: 10.1115/1.1392310. [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Anderson FC, Ziegler JM, Pandy MG, Whalen RT. Application of high-performance computing to numerical simulation of human movement. Journal of Biomechanical Engineering. 1995; 117 :155–157. [ PubMed ] [ Google Scholar ]
- Zajac FE, Neptune RR, Kautz SA. Biomechanics and muscle contraction of human walking: Part I: Introduction to concepts, power transfer, dynamics and simulations. Gait and Posture. 2002; 16 :215–232. doi: 10.1016/S0966-6362(02)00068-1. [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Zajac FE, Neptune RR, Kautz SA. Biomechanics and muscle co-ordination of human walking: Part II: Lessons from dynamical simulations and clinical implications. Gait and Posture. 2003; 17 :1–17. doi: 10.1016/S0966-6362(02)00069-3. [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Neptune RR, Kautz SA, Zajac FE. Contributions of the individual ankle plantar flexors to support, forward progression and swing initiation during walking. Journal of Biomechanics. 2001; 34 :1387–1398. doi: 10.1016/S0021-9290(01)00105-1. [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Kepple T, Siegel K, Stanhope S. Relative contributions of the lower extremity joint moments to forward progression and support during gait. Gait and Posture. 1997; 6 :1–8. doi: 10.1016/S0966-6362(96)01094-6. [ CrossRef ] [ Google Scholar ]
- Anderson FC, Pandy MG. Static and dynamic optimization solutions for gait are practically equivalent. Journal of Biomechanics. 2001; 34 :153–161. doi: 10.1016/S0021-9290(00)00155-X. [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Anderson FC, Pandy MG. Individual muscle contributions to support in normal walking. Gait and Posture. 2003; 17 :159–169. doi: 10.1016/S0966-6362(02)00073-5. [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Arnold AS, Anderson FC, Pandy MG, Delp SL. Muscular contributions to hip and knee extension during the single limb stance phase of normal gait: a framework for investigating the causes of crouch gait. Journal of Biomechanics. 2005; 38 :2181–2189. doi: 10.1016/j.jbiomech.2004.09.036. [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Anderson FC, Goldberg SR, Pandy MG, Delp SL. Contributions of muscle forces and toe-off kinematics to peak knee flexion during the swing phase of normal gait: an induced position analysis. Journal of Biomechanics. 2004; 37 :731–737. doi: 10.1016/j.jbiomech.2003.09.018. [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Arnold AS, Anderson FC, Liu M, Goldstein S, Thelen D, Ounpuu S, Delp SL. Biomechanical efficacy of treatments for stiff-knee gait: a simulation-based case study: ; Portland, Oregon, USA. 2005. [ Google Scholar ]
- Liu M, Arnold AS, Goldberg SR, Anderson FC, Thelen , Ounpuu S, Delp SL. Quadriceps force in stance limits knee flexion in swing: insight from a subject specific simulation of stiff-knee gait: ; Portland, Oregon, USA. 2005. [ Google Scholar ]
- Baker RJ, Jasinski M, Maciag-Tymecka I, Michalowska-Mrozek J, Bonikwski M, Carr LJ, MacLean J, Lin JP, Lynch B, Theologis T, Wendorff J, Eunson P, Cosgrove A. Botulinum toxin treatment of spasticity in diplegic cerebral palsy: a randomized, double-blind, placebo-controlled, dose-ranging study. Developmental Medicine and Child Neurology. 2002; 44 :666–675. doi: 10.1017/S0012162201002730. [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Eames NWA, Baker R, Hill N, Graham HK, Taylor T, Cosgrove A. The effect of botulinum toxin A on gastrocnemius length: magnitude and duration of response. Developmental Medicine and Child Neurology. 1999; 41 :226–232. doi: 10.1017/S0012162299000493. [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Saraph V, Zwick E, Zwick G, Steinwender C, Steinwender G, Linhart W. Multilevel surgery in spastic diplegia: evaluation by physical examination and gait analysis in 25 children. Journal of Pediatric Orthopaedics. 2002; 22 :150–157. doi: 10.1097/00004694-200203000-00003. [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Saraph V, Zwick E, Auner C, Schneider F, Steinwender G, Linhart W. Gait improvement surgery in diplegic children: How long do improvements last? Journal of Pediatric Orthopaedics. 2005; 25 :263–267. doi: 10.1097/01.bpo.0000151053.16615.86. [ PubMed ] [ CrossRef ] [ Google Scholar ]

IMAGES
VIDEO
COMMENTS
Instrumented gait analysis (IGA), which can provide accurate and precise quantitative measurement of gait patterns and characteristics, has long been the gold standard for gait assessment in research practice . IGA generally refers to the use of instrumentation to capture and analyze a variety of human gait parameters (spatiotemporal, kinematic ...
solid line 1 - right stride length. dashed line 4 - left stride width. dashed line 2 - right stride width. In the left gait cycle, dotted line 6 and dashed line 3 enclose a polygon. If the ...
Gait analysis is not a new area of research. Many approaches were used traditionally to analyze gait. After intensive research, it is concluded that gait analysis approaches have been categorized into four major parts: Semi-Subjective Analysis, Objective Analysis, Machine learning techniques, and gait analysis using pose estimation.
Gait analysis, accomplished by either simple observation or three-. dimensional analysis with measurement of joint angles (kinematics), joint forces. (kinetics), muscular activity, foot pressure ...
When brain damage occurs, gait and balance are often impaired. Evaluation of the gait cycle, therefore, has a pivotal role during the rehabilitation path of subjects who suffer from neurological disorders. Gait analysis can be performed through laboratory systems, non-wearable sensors (NWS), and/or wearable sensors (WS). Using these tools, physiotherapists and neurologists have more objective ...
Research Question: This study aimed to review and summarize research efforts applicable to quantification and analyses of post-stroke gait with focus on recent technology-driven gait characterization and analysis approaches, including the integration of smart low cost wearables and Artificial Intelligence (AI), as well as feasibility and ...
Discussion. In the decade since our last review, the volume of studies pertaining to human gait analysis has increased almost 10-fold. This illustrates the growing use of 3DGA as both a clinical and research tool. Previously, over 100 studies were identified that dealt with technical efficacy (type 1).
Research in the field of gait analysis has also seen a rapid rise. A search on Google Scholar using the key phrase "gait analysis" revealed 28 results from the year 1973, of which only eight were relevant to human gait analysis, and of those, five were theses. A similar search for the year 2020 returned around 10,200 results.
The results of multiple linear regression analyses exploring the effects of age and sex on spatiotemporal gait parameters, adjusted for BMI and test center are shown in Table 4.Increasing age was associated with significant lower performance for mean values and CoV for all gait parameters, except for the mean value of stride width (P = 0.861) and CoV of double support time (P = 0.186).
Background: This paper updates our 2011 systematic review on the clinical efficacy of three-dimensional instrumented gait analysis (3DGA). Research question: What is the current evidence base pertaining to the clinical efficacy of 3DGA? Methods: We identified English language articles published from September 2009 to October 2019 reporting primary research that used typical motion analysis ...
In this narrative review, we aimed to summarize the most used gait analysis systems in neurological patients, shedding some light on their clinical value and implications for neurorehabilitation practice. Keywords: gait analysis, neurorehabilitation, neurological disorders, wearable sensors, non-wearable sensors. 1.
Gait analysis is a well-established tool for the quantitative assessment of gait disturbances providing functional diagnosis, assessment for treatment planning, and monitoring of disease progress. There is a large volume of literature on the research use of gait analysis, but evidence on its clinical routine use supports a favorable cost ...
The latest research on gait analysis comparing the advantages and disadvantages of the different systems leads us to conclude that, although objective quantification of the different parameters is rigorously carried out, these studies do not cover the need to extend the measurement capacity of WS systems in order to provide gait information ...
The aim of this research is to review various approaches for Gait Analysis and specifically clinical gait analysis.This paper includes the discussion on the background details of gait, related ...
Instrumented gait analysis (IGA), which can provide accurate and precise quantitative measurement of gait patterns and characteristics, has long been the gold standard for gait assessment in research practice . IGA generally refers to the use of instrumentation to capture and analyze a variety of human gait parameters (spatiotemporal, kinematic ...
Clinical gait analysis (CGA) is a systematic approach to comprehensively evaluate gait patterns, quantify impairments, plan targeted interventions, and evaluate the impact of interventions. However, international standards for CGA are currently lacking, resulting in various national initiatives.
Background. Although we are not conscious of its complexity, gait is a complex activity for human beings. It requires high motor control, and its pathologies have a harmful effect on personal autonomy and daily life activities [].Gait analysis with motion capture (MoCap) technology in the usual clinical practice is called 'clinical gait analysis' []; it is considered an important ...
8. How long is a gait analysis appointment? The length of gait analysis appointments can vary by the number of tests required, the level of disability and the ability of the individual to cooperate. The duration is generally between 2-3 hours. The best estimate of the time for a motion analysis for a given patient can be provided at the time of ...
Gait analysis. Gait analysis is a way to assess the dynamic posture and coordination during movement. This analysis is a means to evaluate, record, and make any necessary corrections for a smooth gait. During this analysis the therapist needs to note the minor shifts in movement such as rotations and tilts or knee movement and foot placement.
Gait is the locomotion attained through the movement of limbs and gait analysis examines the patterns (normal/abnormal) depending on the gait cycle.
These approaches have been successful in interpreting the biomechanical meaning of PCs in several clinical research questions of interest (e.g., differences in walking gait patterns between subjects with and ... Furthermore, in biomechanical gait analysis it is widely known that variability of the data is very high which involves within-trial ...
Introduction. For the purposes of this paper gait analysis will be assumed to refer to the instrumented measurement of the movement patterns that make up walking and the associated interpretation of these.The core of most contemporary gait analysis is the measurement of joint kinematics and kinetics. Other measurements regularly made are electromyography (EMG), oxygen consumption and foot ...
Gait analysis is also used extensively in research and to aid development of improved clinical practice. An assessment of a patient's gait may require the compilation of data from multiple sources, demanding several hours for processing and interpretation, or may make use of one or two tools for rapid, real-time feedback to patients during a ...