
- Why Does Water Expand When It Freezes
Gold Foil Experiment
- Faraday Cage
- Oil Drop Experiment
- Magnetic Monopole
- Why Do Fireflies Light Up
- Types of Blood Cells With Their Structure, and Functions
- The Main Parts of a Plant With Their Functions
- Parts of a Flower With Their Structure and Functions
- Parts of a Leaf With Their Structure and Functions
- Why Does Ice Float on Water
- Why Does Oil Float on Water
- How Do Clouds Form
- What Causes Lightning
- How are Diamonds Made
- Types of Meteorites
- Types of Volcanoes
- Types of Rocks

Who did the Gold Foil Experiment?
The gold foil experiment was a pathbreaking work conducted by scientists Hans Geiger and Ernest Marsden under the supervision of Nobel laureate physicist Ernest Rutherford that led to the discovery of the proper structure of an atom . Known as the Geiger-Marsden experiment, it was performed at the Physical Laboratories of the University of Manchester between 1908 and 1913.
The prevalent atomic theory at the time of the research was the plum pudding model that was developed by Lord Kelvin and further improved by J.J. Thomson. According to the theory, an atom was a positively charged sphere with the electrons embedded in it like plums in a Christmas pudding.
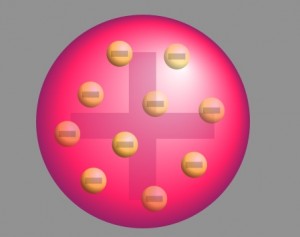
With neutrons and protons yet to be discovered, the theory was derived following the classical Newtonian Physics. However, in the absence of experimental proof, this approach lacked proper acceptance by the scientific community.
What is the Gold Foil Experiment?
Description.
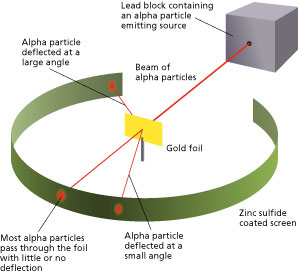
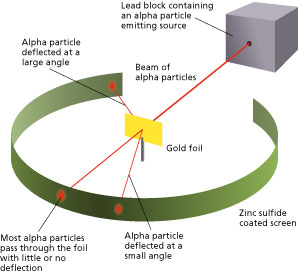
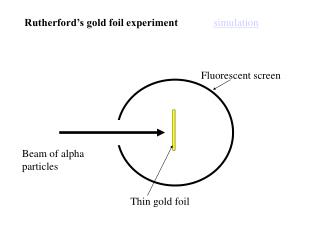
The method used by scientists included the following experimental steps and procedure. They bombarded a thin gold foil of thickness approximately 8.6 x 10 -6 cm with a beam of alpha particles in a vacuum. Alpha particles are positively charged particles with a mass of about four times that of a hydrogen atom and are found in radioactive natural substances. They used gold since it is highly malleable, producing sheets that can be only a few atoms thick, thereby ensuring smooth passage of the alpha particles. A circular screen coated with zinc sulfide surrounded the foil. Since the positively charged alpha particles possess mass and move very fast, it was hypothesized that they would penetrate the thin gold foil and land themselves on the screen, producing fluorescence in the part they struck.
Like the plum pudding model, since the positive charge of atoms was evenly distributed and too small as compared to that of the alpha particles, the deflection of the particulate matter was predicted to be less than a small fraction of a degree.
Observation
Though most of the alpha particles behaved as expected, there was a noticeable fraction of particles that got scattered by angles greater than 90 degrees. There were about 1 in every 2000 particles that got scattered by a full 180 degree, i.e., they retraced their path after hitting the gold foil.
Simulation of Rutherford’s Gold Foil Experiment Courtesy: University of Colorado Boulder
The unexpected outcome could have only one explanation – a highly concentrated positive charge at the center of an atom that caused an electrostatic repulsion of the particles strong enough to bounce them back to their source. The particles that got deflected by huge angles passed close to the said concentrated mass. Most of the particles moved undeviated as there was no obstruction to their path, proving that the majority of an atom is empty.
In addition to the above, Rutherford concluded that since the central core could deflect the dense alpha particles, it shows that almost the entire mass of the atom is concentrated there. Rutherford named it the “nucleus” after experimenting with various gases. He also used materials other than gold for the foil, though the gold foil version gained the most popularity.
He further went on to reject the plum pudding model and developed a new atomic structure called the planetary model. In this model, a vastly empty atom holds a tiny nucleus at the center surrounded by a cloud of electrons. As a result of his gold foil experiment, Rutherford’s atomic theory holds good even today.
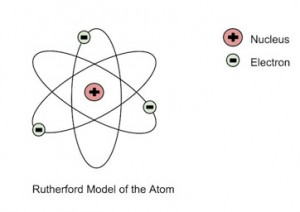
Rutherford’s Atomic Model
Rutherford’s Gold Foil Experiment Animation
- Rutherford demonstrated his experiment on bombarding thin gold foil with alpha particles contributed immensely to the atomic theory by proposing his nuclear atomic model.
- The nuclear model of the atom consists of a small and dense positively charged interior surrounded by a cloud of electrons.
- The significance and purpose of the gold foil experiment are still prevalent today. The discovery of the nucleus paved the way for further research, unraveling a list of unknown fundamental particles.
- Chemed.chem.purdue.edu
- Chem.libretexts.org
- Large.stanford.edu
- Radioa ctivity.eu.com
Article was last reviewed on Friday, February 3, 2023
Related articles
5 responses to “Gold Foil Experiment”
Super very much helpful to me,clear explanation about every act done by our Rutherford that is under different sub headings ,which is very much clear to ,to study .very much thanks to the science facts.com.thank u so much.
Good explanation,very helpful ,thank u ,so much
very clear and helpful, perfect for my science project!
Thank you for sharing the interactive program on the effects of the type of atom on the experiment! Looking forward to sharing this with my ninth graders!
Rutherford spearheaded with a team of scientist in his experiment of gold foil to capture the particles of the year 1911. It’s the beginning of explaining particles that float and are compacted . Rutherford discovered this atom through countless experiments which was the revolutionary discovery of the atomic nuclear . Rutherford name the atom as a positive charge and the the center is the nucleus.
Barack Hussein Obama
Mrs. Danize Obama
Leave a Reply Cancel reply
Your email address will not be published. Required fields are marked *
Save my name, email, and website in this browser for the next time I comment.
Popular Articles
Join our Newsletter
Fill your E-mail Address
Related Worksheets
- Privacy Policy
© 2024 ( Science Facts ). All rights reserved. Reproduction in whole or in part without permission is prohibited.
Sciencing_Icons_Science SCIENCE
Sciencing_icons_biology biology, sciencing_icons_cells cells, sciencing_icons_molecular molecular, sciencing_icons_microorganisms microorganisms, sciencing_icons_genetics genetics, sciencing_icons_human body human body, sciencing_icons_ecology ecology, sciencing_icons_chemistry chemistry, sciencing_icons_atomic & molecular structure atomic & molecular structure, sciencing_icons_bonds bonds, sciencing_icons_reactions reactions, sciencing_icons_stoichiometry stoichiometry, sciencing_icons_solutions solutions, sciencing_icons_acids & bases acids & bases, sciencing_icons_thermodynamics thermodynamics, sciencing_icons_organic chemistry organic chemistry, sciencing_icons_physics physics, sciencing_icons_fundamentals-physics fundamentals, sciencing_icons_electronics electronics, sciencing_icons_waves waves, sciencing_icons_energy energy, sciencing_icons_fluid fluid, sciencing_icons_astronomy astronomy, sciencing_icons_geology geology, sciencing_icons_fundamentals-geology fundamentals, sciencing_icons_minerals & rocks minerals & rocks, sciencing_icons_earth scructure earth structure, sciencing_icons_fossils fossils, sciencing_icons_natural disasters natural disasters, sciencing_icons_nature nature, sciencing_icons_ecosystems ecosystems, sciencing_icons_environment environment, sciencing_icons_insects insects, sciencing_icons_plants & mushrooms plants & mushrooms, sciencing_icons_animals animals, sciencing_icons_math math, sciencing_icons_arithmetic arithmetic, sciencing_icons_addition & subtraction addition & subtraction, sciencing_icons_multiplication & division multiplication & division, sciencing_icons_decimals decimals, sciencing_icons_fractions fractions, sciencing_icons_conversions conversions, sciencing_icons_algebra algebra, sciencing_icons_working with units working with units, sciencing_icons_equations & expressions equations & expressions, sciencing_icons_ratios & proportions ratios & proportions, sciencing_icons_inequalities inequalities, sciencing_icons_exponents & logarithms exponents & logarithms, sciencing_icons_factorization factorization, sciencing_icons_functions functions, sciencing_icons_linear equations linear equations, sciencing_icons_graphs graphs, sciencing_icons_quadratics quadratics, sciencing_icons_polynomials polynomials, sciencing_icons_geometry geometry, sciencing_icons_fundamentals-geometry fundamentals, sciencing_icons_cartesian cartesian, sciencing_icons_circles circles, sciencing_icons_solids solids, sciencing_icons_trigonometry trigonometry, sciencing_icons_probability-statistics probability & statistics, sciencing_icons_mean-median-mode mean/median/mode, sciencing_icons_independent-dependent variables independent/dependent variables, sciencing_icons_deviation deviation, sciencing_icons_correlation correlation, sciencing_icons_sampling sampling, sciencing_icons_distributions distributions, sciencing_icons_probability probability, sciencing_icons_calculus calculus, sciencing_icons_differentiation-integration differentiation/integration, sciencing_icons_application application, sciencing_icons_projects projects, sciencing_icons_news news.
- Share Tweet Email Print
- Home ⋅
- Science Fair Project Ideas for Kids, Middle & High School Students ⋅
About Rutherford's Gold Foil Experiment

Five Types of Atomic Models
Ernest Rutherford, originally from New Zealand, is credited as being the father of nuclear physics for his discoveries in atomic structure, even though Hantaro Nagaoka, a physicist from the Imperial University of Tokyo, first proposed the theory of the nucleus as it is known today. Rutherford's "gold foil experiment" led to the discovery that most of an atom's mass is located in a dense region now called the nucleus. Prior to the groundbreaking gold foil experiment, Rutherford was granted the Nobel Prize for other key contributions in the field of chemistry.
The popular theory of atomic structure at the time of Rutherford's experiment was the "plum pudding model." This model was developed in 1904 by J.J. Thompson, the scientist who discovered the electron. This theory held that the negatively charged electrons in an atom were floating in a sea of positive charge--the electrons being akin to plums in a bowl of pudding. Although Dr. Nagaoka had published his competing theory that electrons orbit a positive nucleus, akin to the way the planet Saturn is orbited by its rings, in 1904, the plum pudding model was the prevailing theory on the structure of the atom until it was disproved by Ernest Rutherford in 1911.
The gold foil experiment was conducted under the supervision of Rutherford at the University of Manchester in 1909 by scientist Hans Geiger (whose work eventually led to the development of the Geiger counter) and undergraduate student Ernest Marsden. Rutherford, chair of the Manchester physics department at the time of the experiment, is given primary credit for the experiment, as the theories that resulted are primarily his work. Rutherford's gold foil experiment is also sometimes referred to as the Geiger-Marsden experiment.
The gold foil experiment consisted of a series of tests in which a positively charged helium particle was shot at a very thin layer of gold foil. The expected result was that the positive particles would be moved just a few degrees from their path as they passed through the sea of positive charge proposed in the plum pudding model. The result, however, was that the positive particles were repelled off of the gold foil by nearly 180 degrees in a very small region of the atom, while most of the remaining particles were not deflected at all but rather passed right through the atom.
Significance
The data generated from the gold foil experiment demonstrated that the plum pudding model of the atom was incorrect. The way in which the positive particles bounced off the thin foil indicated that the majority of the mass of an atom was concentrated in one small region. Because the majority of the positive particles continued on their original path unmoved, Rutherford correctly deducted that most of the remainder of the atom was empty space. Rutherford termed his discovery "the central charge," a region later named the nucleus.
Rutherford's discovery of the nucleus and proposed atomic structure was later refined by physicist Niels Bohr in 1913. Bohr's model of the atom, also referred to as the Rutherford Bohr model, is the basic atomic model used today. Rutherford's description of the atom set the foundation for all future atomic models and the development of nuclear physics.
Related Articles
What are the 4 atomic models, what are the different kinds of models of atoms, what contributions did j.j. thomson make to the atom, james chadwick atomic theory, list of the atomic theories, who discovered iodine 131, who discovered the particle theory, cern plans to search for mysterious fifth force particle, who was the african american nuclear scientist who..., how to make a gold atom model, atomic structure of gold, what are an atom, electron, neutron and proton, the locations of protons, neutrons and electrons within..., how to know if an element has a positive or negative..., what are the properties of protons, six elements named after scientists.
Photo Credits
iSailorr/iStock/Getty Images
Find Your Next Great Science Fair Project! GO

Discovering the Nucleus: Rutherford’s Gold Foil Experiment

History of Chemistry: Rutherford Gold Foil Experiment
In this article, you will learn the history behind the Rutherford Gold Foil Experiment and the events that led to the discovery of the atomic nucleus. If you enjoy this article, check out our other history of chemistry articles linked below!
- Rutherford Atomic Model
- JJ Thompson cathode-ray tube
- Rutherfords Jar Experiment
- Molecular Geometry tutorial
- The structure of an atom
- Bohr Atomic Model
- Nuclear Reactions
Who was Ernest Rutherford?

Ernest Rutherford is known as the father of nuclear physics. Born in Brightwater, New Zealand on August 30th, 1871, Rutherford was the fourth of twelve children. His father was a farmer and his mother a school teacher. From a very early age, Rutherford understood the importance of hard work and the power of education. In school, he excelled greatly and at the age of fifteen won an academic scholarship to study at Nelson Collegiate School. Then, at the age of 19, he won another academic scholarship to study at Canterbury College in Christchurch. A few years later he won another scholarship, the exhibition science scholarship, and he left New Zealand to study at Trinity College, Cambridge in England. While there, he conducted research at the Cavendish Laboratory under his advisor J.J. Thomson .

During his time at Cavendish Lab, Rutherford faced adversity from his peers. Because he was from New Zealand, he was often ostracized by fellow students. In the end, he used this as motivation to succeed. Which he did as he made a multitude of great discoveries through his research in gases and radioactivity. These included the discovery of different types of radiation, radiometric dating, and the nucleus of an atom.
The Rutherford Gold Foil Experiment
The experiment.
While working as a chair at the University of Manchester, Rutherford conducted the gold-foil experiment alongside Hans Geiger and Ernest Marsden. In this experiment, they shot alpha particles –which Rutherford had discovered years prior– directly at a piece of thin gold foil . As the alpha particles passed through, they would hit the phosphorescent screen encasing the foil. When the particles came into contact with the screen, there would be a flash.

Observations
Going into the experiment, Rutherford had formed preconceptions for the experiment based on J.J. Thomson’s plum pudding model . He predicted the alpha particles would shoot through the foil with ease. Some of the particles did manage to pass directly through the foil, but some veered from the path either bouncing back or deflecting. Rutherford found this to be an exciting observation and compared it to shooting a bullet at a piece of tissue and having it bounce back.
From this observation, two deductions were made. Firstly, he concluded most of the atom is composed of empty space. Secondly, he concluded there must be something small, dense, and positive inside the atom to repel the positively charged alpha particles. This became the nucleus, which in Latin means the seed inside of a fruit.
The Nuclear Model
The gold-foil experiment disproved J.J. Thomsons plum pudding model, which hypothesized the atom was positively charged spaced with electrons embedded inside. Therefore, giving way to the nuclear model. In this model, Rutherford theorized the atomic structure was similar to that of the solar system. Where the nucleus was in this middle and surrounded by empty space with orbiting electrons.
- Anatomy & Physiology
- Astrophysics
- Earth Science
- Environmental Science
- Organic Chemistry
- Precalculus
- Trigonometry
- English Grammar
- U.S. History
- World History
... and beyond
- Socratic Meta
- Featured Answers

- Rutherford's Gold Foil Experiment
Key Questions
Rutherford's experiment showed that the atom does not contain a uniform distribution of charge.
Explanation:
Thomson's plum pudding model viewed the atom as a massive blob of positive charge dotted with negative charges.
A plum pudding was a Christmas cake studded with raisins ("plums"). So think of the model as a spherical Christmas cake.
When Rutherford shot α particles through gold foil, he found that most of the particles went through. Some scattered in various directions, and a few were even deflected back towards the source.
He argued that the plum pudding model was incorrect. The symmetrical distribution of charge would allow all the α particles to pass through with no deflection.
Rutherford proposed that the atom is mostly empty space. The electrons revolve in circular orbits about a massive positive charge at the centre.
His model explained why most of the α particles passed straight through the foil. The small positive nucleus would deflect the few particles that came close.
The nuclear model replaced the plum pudding model. The atom now consisted of a positive nucleus with negative electrons in circular orbits around it .

- History & Society
- Science & Tech
- Biographies
- Animals & Nature
- Geography & Travel
- Arts & Culture
- Games & Quizzes
- On This Day
- One Good Fact
- New Articles
- Lifestyles & Social Issues
- Philosophy & Religion
- Politics, Law & Government
- World History
- Health & Medicine
- Browse Biographies
- Birds, Reptiles & Other Vertebrates
- Bugs, Mollusks & Other Invertebrates
- Environment
- Fossils & Geologic Time
- Entertainment & Pop Culture
- Sports & Recreation
- Visual Arts
- Demystified
- Image Galleries
- Infographics
- Top Questions
- Britannica Kids
- Saving Earth
- Space Next 50
- Student Center

What is the model of the atom proposed by Ernest Rutherford?
What is the rutherford gold-foil experiment, what were the results of rutherford's experiment, what did ernest rutherford's atomic model get right and wrong, what was the impact of ernest rutherford's theory.

Rutherford model
Our editors will review what you’ve submitted and determine whether to revise the article.
- UC Davis - The Rutherford Scattering Experiment
- Chemistry LibreTexts - Rutherford's Experiment- The Nuclear Model of the Atom

The atom , as described by Ernest Rutherford , has a tiny, massive core called the nucleus . The nucleus has a positive charge. Electrons are particles with a negative charge. Electrons orbit the nucleus. The empty space between the nucleus and the electrons takes up most of the volume of the atom.
A piece of gold foil was hit with alpha particles , which have a positive charge. Most alpha particles went right through. This showed that the gold atoms were mostly empty space. Some particles had their paths bent at large angles. A few even bounced backward. The only way this would happen was if the atom had a small, heavy region of positive charge inside it.
The previous model of the atom, the Thomson atomic model , or the “plum pudding” model, in which negatively charged electrons were like the plums in the atom’s positively charged pudding, was disproved. The Rutherford atomic model relied on classical physics. The Bohr atomic model , relying on quantum mechanics, built upon the Rutherford model to explain the orbits of electrons.
The Rutherford atomic model was correct in that the atom is mostly empty space. Most of the mass is in the nucleus, and the nucleus is positively charged. Far from the nucleus are the negatively charged electrons. But the Rutherford atomic model used classical physics and not quantum mechanics. This meant that an electron circling the nucleus would give off electromagnetic radiation . The electron would lose energy and fall into the nucleus. In the Bohr model, which used quantum theory, the electrons exist only in specific orbits and can move between these orbits.
The gold-foil experiment showed that the atom consists of a small, massive, positively charged nucleus with the negatively charged electrons being at a great distance from the centre. Niels Bohr built upon Rutherford’s model to make his own. In Bohr’s model the orbits of the electrons were explained by quantum mechanics.
Rutherford model , description of the structure of atoms proposed (1911) by the New Zealand-born physicist Ernest Rutherford . The model described the atom as a tiny, dense, positively charged core called a nucleus, in which nearly all the mass is concentrated, around which the light, negative constituents , called electrons , circulate at some distance, much like planets revolving around the Sun .

The nucleus was postulated as small and dense to account for the scattering of alpha particles from thin gold foil, as observed in a series of experiments performed by undergraduate Ernest Marsden under the direction of Rutherford and German physicist Hans Geiger in 1909. A radioactive source emitting alpha particles (i.e., positively charged particles, identical to the helium atom nucleus and 7,000 times more massive than electrons) was enclosed within a protective lead shield. The radiation was focused into a narrow beam after passing through a slit in a lead screen. A thin section of gold foil was placed in front of the slit, and a screen coated with zinc sulfide to render it fluorescent served as a counter to detect alpha particles. As each alpha particle struck the fluorescent screen , it produced a burst of light called a scintillation, which was visible through a viewing microscope attached to the back of the screen. The screen itself was movable, allowing Rutherford and his associates to determine whether or not any alpha particles were being deflected by the gold foil.

Most alpha particles passed straight through the gold foil, which implied that atoms are mostly composed of open space. Some alpha particles were deflected slightly, suggesting interactions with other positively charged particles within the atom. Still other alpha particles were scattered at large angles, while a very few even bounced back toward the source. (Rutherford famously said later, “It was almost as incredible as if you fired a 15-inch shell at a piece of tissue paper and it came back and hit you.”) Only a positively charged and relatively heavy target particle, such as the proposed nucleus, could account for such strong repulsion. The negative electrons that balanced electrically the positive nuclear charge were regarded as traveling in circular orbits about the nucleus. The electrostatic force of attraction between electrons and nucleus was likened to the gravitational force of attraction between the revolving planets and the Sun. Most of this planetary atom was open space and offered no resistance to the passage of the alpha particles.
The Rutherford model supplanted the “plum-pudding” atomic model of English physicist Sir J.J. Thomson , in which the electrons were embedded in a positively charged atom like plums in a pudding. Based wholly on classical physics , the Rutherford model itself was superseded in a few years by the Bohr atomic model , which incorporated some early quantum theory . See also atomic model .
If you're seeing this message, it means we're having trouble loading external resources on our website.
If you're behind a web filter, please make sure that the domains *.kastatic.org and *.kasandbox.org are unblocked.
To log in and use all the features of Khan Academy, please enable JavaScript in your browser.
Chemistry archive
Course: chemistry archive > unit 1.
- The history of atomic chemistry
- Dalton's atomic theory
- Discovery of the electron and nucleus
Rutherford’s gold foil experiment

Want to join the conversation?
- Upvote Button navigates to signup page
- Downvote Button navigates to signup page
- Flag Button navigates to signup page

Video transcript
Stack Exchange Network
Stack Exchange network consists of 183 Q&A communities including Stack Overflow , the largest, most trusted online community for developers to learn, share their knowledge, and build their careers.
Q&A for work
Connect and share knowledge within a single location that is structured and easy to search.
Why Rutherford used only gold foil in his famous gold foil experiment?
why didn't Rutherford use an aluminium foil, or a silver foil. Why he used gold foil in his gold foil experiment?
- atomic-physics
- 1 $\begingroup$ That's really a question you need to ask from Geiger and Marsden: en.wikipedia.org/wiki/Geiger%E2%80%93Marsden_experiment . It might have something to do with the fact that gold can be hammered into extremely thin foils, which is not possible (as far as I know) with either aluminum or silver. That reason is also given in the Wikipedia article. $\endgroup$ – CuriousOne Commented Jun 5, 2016 at 10:01
4 Answers 4
He actually used also Aluminium, Silver, and Copper. He did so because he wanted to prove that the Rutherford cross section was proportional to $Z^2$.
In any case, he needed to use malleable material (metals) in order to achieve a micrometer-thin foil to prevent the entire $\alpha$ beam to be absorbed by the target.
- $\begingroup$ Hey this looks like a fantastic answer; can you give a citation for it? $\endgroup$ – Selene Routley Commented Jun 5, 2016 at 12:55
- $\begingroup$ Professor Longo said it during the Nuclear Physics course at Sapienza university. The material used are cited also on Wikipedia's article: en.wikipedia.org/wiki/Geiger –Marsden_experiment I had forgotten one element:he used tin, too. $\endgroup$ – Drebin J. Commented Jun 5, 2016 at 13:12
Is this true?
In a 1913 paper, The Laws of Deflexion of α Particles through Large Angles... Geiger and Marsden reused the above apparatus to measure how the scattering pattern varied with the square of the nuclear charge (i.e. if s ∝ Qn2). Geiger and Marsden didn't know what the positive charge of the nucleus of their metals were (they had only just discovered the nucleus existed at all), but they assumed it was proportional to the atomic weight, so they tested whether the scattering was proportional to the atomic weight squared. Geiger and Marsden covered the holes of the disc with foils of gold, tin, silver, copper, and aluminum. They measured each foil's stopping power by equating it to an equivalent thickness of air. They counted the number of scintillations per minute that each foil produced on the screen.
See Wikipedia
Yes, it is correct that Rutherford used other metallic atoms instead of gold. From using other metallic atoms, he drew the following conclusion that there shall be no change in his prior observations, if and only if the malleability of the metal is sufficive enough for the alpha particles to penetrate through, otherwise there shall be a lack of penetration of the alpha particles, thus different scattering of particles, which would ultimately for-go his previous experiment.
Geiger and Marsden first used Gold because it is a malleable metal and they could relatively easily produce foils of a thickness of around $1\; \mu$m which still is about 3500 atoms thick. Even so this was thin enough to observe an incoming alpha particle interacting with only one nucleus and not being absorbed by the foil.
Other malleable metals were then used to see what effect they had on the scattering of alpha particle. The parameter which they used to categorise a metal was its atomic weight as mentioned by @Mikhail in his question and they did find that the scattering was approximately proportional to the atomic weight squared.
It was Moseley who first systematically associated atomic number $Z$ with the number of positive charges in the nucleus.
Your Answer
Sign up or log in, post as a guest.
Required, but never shown
By clicking “Post Your Answer”, you agree to our terms of service and acknowledge you have read our privacy policy .
Not the answer you're looking for? Browse other questions tagged atomic-physics radiation scattering or ask your own question .
- Featured on Meta
- Announcing a change to the data-dump process
- We've made changes to our Terms of Service & Privacy Policy - July 2024
Hot Network Questions
- Commentaries relevant to doubled phrases in Aleinu
- The proof of conservation of momentum in Mechanics by Landau and Lifshitz
- Are there any examples of moving Manses?
- "between" two countries
- Is a Fizban's dragonborn's breath weapon magical?
- Why isn’t this formula used at all?
- Do I have legal grounds against a book by an ex which depicts private details of my life, including some false events, without permission?
- What is the color of the final disc(s)?
- RP2040 ADC calibration (schematic)
- How to Respond to Directive: "Move Our Entire Website to a CMS Service"
- Do some chemicals degrade at low temperatures?
- Accessing an overridden Greek letter
- How to make an operator form of Part[] to use with // (Postfix)
- How to reinstall a ceiling fan that fell out of ceiling?
- What happens if your flight is cancelled on the last day of your visa; does it vary by country/region?
- Is doping still a thing in pro cycling (2024)?
- Question on implementation of PBL (problem or project-based learning) in Linear Algebra course
- When non-resident US citizens vote, which state does their vote count for wrt the electoral college?
- DC motor pump always transports in same direction, regardless of polarity
- What exactly is code and how does it relate to law? Where does it fit into the hierarchy of law?
- How long should I boil a liquid mixture containing vanilla extract to vaporize the alcohol, when making ice cream?
- Can multi-threading improve performance of an IO-bound process?
- Does Wall of Ice still deal damage after being affected by a Wot4E Monk's Shape the Flowing River?
- Efficient way to remove nailed-down plywood flooring in attic without damaging it?

The Geiger - Marsden Experiment aka Rutherfords Gold Foil Experiment
Oct 25, 2014
240 likes | 867 Views
The Geiger - Marsden Experiment aka Rutherfords Gold Foil Experiment. The Geiger - Marsden Experiment aka Rutherfords Gold Foil Experiment. We get a planetary view of the atom Nucleus 1/10,000 atoms diameter 99.9% of atoms mass is in the nucleus BUT.....!!!. 1913 Niels Bohr.
Share Presentation
- stellar spectra
- exact wavelength
- spectra studies
- stellar spectral classification
- hydrogen balmer lines fading

Presentation Transcript
The Geiger - Marsden Experiment aka Rutherfords Gold Foil Experiment We get a planetary view of the atom Nucleus 1/10,000 atoms diameter 99.9% of atoms mass is in the nucleus BUT.....!!!
1913 Niels Bohr • An orbiting electron must be accelerating…..WHY??? • Changing direction • So, it must radiate energy….WHY? • Accelerating charges cause EM radiation • KE & Momentum should be lost due to E-M radiation. Electron should spiral inward to nucleus
Stellar Spectra Provide Info about Stars
62 52 42 n=32 n=6 n=1 (Ground State) n=3 (2nd excited state) n=2 (1st excited state) n=4 n=5 Larger Jump = More Energy = Bluer Wavelength
26 25 24 n=23 n=6 n=1 (Ground State) n=3 (2nd excited state) n=2 (1st excited state) n=4 n=5 Photons of all other energies (wavelengths) are ignored and pass on by unabsorbed.
Hydrogen Helium Oxygen Neon Iron
Stellar Spectra Provide Info about Stars • Nebula NGC 2363 • This nebula is a glowing gas cloud about 10,000,000 LY from Earth. • The hot stars in the Nebula emit high energy photons that are absorbed by the gas. • The heated gases produce an emission spectrum and the particular wavelength of the red light of the nebula is 656nm. The exact wavelength of Hydrogen.
The Suns Absorption Spectrum The Suns Absorption Spectrum from 420 – 430 nm. (TOP) The emission spectrum of Iron (Bottom)
Stellar Spectral Classification • In the late 1800’s astronomers were trying to organize and make sense of all the data they were collecting. • At the time, spectra studies were the most reliable, but there is a huge diversity of stellar spectra. • In 1870’s stars were classified into various letters based upon their spectral patterns. "Oh, Be A Fine Girl, Kiss Me!"
Stellar Spectral Classification Hydrogen Balmer Lines Very Weak due to extreme temps Hydrogen Balmer Lines Strongest Hydrogen Balmer Lines fading out and trace amounts of heavier elements starting to appear Stars containing heavier metals such as Calcium and Iron (Including the Sun, a G2 star) Stars containing Titanium Oxide
Stellar Spectral Classification • In short, the OBAFGKM system allows us to • Identify the surface temperature of the stars • Chemical composition of the stars
Key Properties of Nearby Stars So we can determine the distances and characteristics of Stars We need a better classification scheme Where 4πR2 = area of a sphere
- More by User

The experiment
The experiment. at the LHC. Stato dell’esperimento Attivita` di Pisa/Siena Composizione Gruppo Richieste 2014 Nicola Turini on behalf of the TOTEM PISA/SIENA Group.
618 views • 18 slides

Rutherford and The Gold Foil Experiment
Ernest Rutherford. Born on August 30, 1871 in Nelson, New Zealand. In 1889 he was awarded a University scholarship and he proceeded to the University of New Zealand, Wellington, where he entered Canterbury College.Later he graduatedM.A. in 1893 with a double first in Mathematics and Physical Sci
162 views • 7 slides
Contamination and D egradation of Perfluorinated S ulfonic A cid Membrane due to Swelling-Dehydration C ycles Shuang Ma Andersen 1 , Per Morgen 2 , Clause-Henning Solterbeck 3 and Eivind M. Skou 1 1 KBM, 2 IFK, University of Southern Denmark, Odense, Denmark
318 views • 1 slides
Experiment. Sol-gel on chip process. “Double layer non-hydrolytic reaction process” Reaction between deposited precursor layers. Zinc acetate. Zinc tin oxide. Annealing. Tin tert -butoxide. Substrate. Substrate. Non-hydrolytic reaction.
241 views • 8 slides

The gold Foil experiment
The gold Foil experiment . ( Rutherford’s experiment ). introductio n.
1.28k views • 12 slides

Ernest Rutherford & the Gold Foil Experiment
Ernest Rutherford & the Gold Foil Experiment. By Jake Easton & James Lampmann. Birth. He was born on August 30, 1871 in Nelson, New Zealand His parents were James and Martha Rutherford He was one of 12 kids. Education. In 1887, he won a scholarship to Nelson College
699 views • 15 slides

Experiment. Objectives Test beam damage potential in different materials. 108 plates. 30 cm. 6 cm. 6 cm. Four intensities [SPS-beam type @ 450 GeV ]: A=1.3x10 12 , B=2.6x10 12 , C=5.3x10 12 , D=7.9x10 12. Verena Kain (2005). A B D C. Experiment.
246 views • 6 slides
Experiment. ZTO TFT, sol-gel on chip process. 1. Effect of tin precursor (300°C, wet annealing). 1) Tin isopropoxide. 2) Tin(IV) chloride. 3) Tin(IV) tert -butoxide. Mobility 0.014. Mobility 0.007. Result. 2. Effect of catalyst (acetic acid) 300°C, dry annealing. Acetic acid.
188 views • 7 slides

Experiment. Here is an experiment that demonstrates Ferson’s point (see Ferson, Sarkissian and Simin, Journal of Financial Markets 2 (1), 49-68, February 1999)
220 views • 9 slides

Moisture Measurement in Meat Products by Temperature Controlled Microwave Drying to a Constant Weight S. P. Hailey 1* , C.R. Moser 1 , B.J. Haire 1 , J.T. Keeton 2 1 CEM Corporation, Matthews, USA; 2 Texas A&M University, USA [email protected]. Experiment.
486 views • 28 slides

Rare Kaon Decays. @. ISTRA+. EXPERIMENT. Viacheslav Duk INR RAS QUARKS 2006. INR/IHEP Protvino-Moscow, Russia. Talk outline. 1. Kaon physics 2. ISTRA+ experimental setup 3. Recent results on kaon decays @ ISTRA+ 4. Study of K → µ - ν γ @ ISTRA+ 5. Conclusions.
463 views • 24 slides
Rutherford’s gold foil experiment
Rutherford’s gold foil experiment. simulation. Fluorescent screen. Beam of alpha particles. Thin gold foil. Rutherford’s Model of the Atom. 1. The positive charge is concentrated in the nucleus. This is a very small part of the atom.
589 views • 11 slides

1. Get out homework. 2. Get your gummy bear and finish the worksheet. Yes, I know you don’t have much. 3. Design your own experiment! I will give you directions. HW: Test on Sept. 16/17. Experiment. Gummy Bears or Sponge capsules Clear IV/DV Create and Experimental Design Diagram
337 views • 2 slides

Rutherford’s Gold Foil Experiment
Rutherford’s Gold Foil Experiment. T.T. Rutherford’s gold-foil experiment. Before this experiment, we assumed that:. The atom is a solid sphere. It contains negative electrons. This was called the Plum Pudding Model. Positive material. Negative electrons.
245 views • 7 slides

The experiment. at LHC. Stefano Lami INFN Pisa on behalf of the TOTEM Collaboration. jet. b. b. jet. TOTEM Physics Overview. Total cross-section. Ultimately ~1% precision. Elastic Scattering. Over a wide t range. Forward physics.
425 views • 32 slides

~ The experiment
~ The experiment. Colossians 3:12-14. ~ The experiment. Colossians 3:12-14.
348 views • 27 slides

Ernest Rutherford and the Gold Foil Experiment
Ernest Rutherford and the Gold Foil Experiment. -Jordan Heffler and Nate Rose. Ernest Rutherford. Born August 30, 1871 Born in Bridgewater, New Zealand Died October 19, 1937 Worked with Hans Geiger and Ernest Marsden on Gold Foil Experiment
384 views • 12 slides
Experiment. Imposing treatments on the subjects in order to compare the results. The objects on which the treatment is imposed on are called experimental units (human subjects ).
336 views • 25 slides

Experiment. Obtain a tube of water and a straw. Exhale deeply through the straw into the water. Make observations. Why might your observations be different than the people near you?. pH Indicator Bromothymol Blue. acidic. basic. pH scale pH = -log [H+] a. [H+]=1 • 10 -1.
239 views • 11 slides
Experiment. Subjecting the sample to a controlled treatment. The objects on which the treatment is imposed on are called subjects. The response variable measures an outcome or result of a study.
355 views • 18 slides

The Experiment
The Experiment. Chapter 7. Experiments. Simple social experiments allow us to test targeted hypotheses about a specific social process Experiments gives us clear evidence about a particular causal relationship
352 views • 33 slides

The experiment. at the LHC. Prime Misure Attivita ` di Pisa/Siena Composizione Gruppo Richieste 2012 Stefano Lami on behalf of the TOTEM PISA/SIENA Group. RP. T1. T2. T1: 3.1 < | h | < 4.7 T2: 5.3 < | h | < 6.5. 147. 220.
231 views • 22 slides
Physical Review D
Covering particles, fields, gravitation, and cosmology.
- Collections
- Editorial Team
Bounds on new Majoron models from the Heidelberg-Moscow experiment
M. günther, j. hellmig, g. heusser, m. hirsch, h. v. klapdor-kleingrothaus, b. maier, h. päs, f. petry, y. ramachers, h. strecker, m. völlinger, a. balysh, s. t. belyaev, a. demehin, a. gurov, i. kondratenko, d. kotel'nikov, v. i. lebedev, and a. müller, phys. rev. d 54 , 3641 – published 1 september 1996.
- Citing Articles (20)
In recent years several new Majoron models were invented to avoid the shortcomings of the ordinary models while leading to observable decay rates in double β experiments. We give the first experimental half-life bounds on double β decays with new Majoron emission and derive bounds on the effective neutrino-Majoron couplings from the data of the Ge 76 Heidelberg-Moscow experiment. While stringent half-life limits for all decay modes and the coupling constants of the ordinary models were obtained, small matrix elements and phase space integrals result in much weaker limits on the effective coupling constants of the new Majoron models.
- Received 13 November 1995
DOI: https://doi.org/10.1103/PhysRevD.54.3641
©1996 American Physical Society
Authors & Affiliations
- Max-Planck-Institut für Kernphysik, P.O. Box 10 39 80, D-69029 Heidelberg, Germany
- Russian Science Center Kurchatov Institute, 123 182 Moscow, Russia
- Istituto Nazionale di Fisica Nucleare, I-67010 Assergi, Italy
- * Spokesmen of the collaboration.
References (Subscription Required)
Vol. 54, Iss. 5 — 1 September 1996
Access Options
- Buy Article »
- Log in with individual APS Journal Account »
- Log in with a username/password provided by your institution »
- Get access through a U.S. public or high school library »

Authorization Required
Other options.
- Buy Article »
- Find an Institution with the Article »
Download & Share
Sign up to receive regular email alerts from Physical Review D
- Forgot your username/password?
- Create an account
Article Lookup
Paste a citation or doi, enter a citation.

- Introduction
- About Kate Sharpley
- Subscribing
- Publications
- Organizations
Copyright information
The story of the Moscow gold: How the Spanish war was lost
Francisco Olaya 'El Oro de Negrin'
(Ediciones Madre Tierra, Mostoles 1990)
This book is the product of almost thirty years investigation, involving examination of thousands of books and pamphlets, around a million documents, and the combing of 32 archives in Spain and beyond. Olaya's work is an attempt to come up with a satisfactory explanation of the denouement of the Spanish civil war. He is highly critical of the leadership of the PSOE (Socialist Workers Party of Spain, now in power) during the civil war.
In great and documented detail Olaya examines the whole topic of what has hitherto been known as the 'Moscow gold' and which he re-christens 'Negrin's gold', gold to the tune of 5,500 million pesetas (1937). About half of this sum wound up in the Soviet Union. A small portion went to France. The remainder passed to the republican government's purchasing commissions, set up by Indalecio Prieto of the PSOE to obtain war material.
In 1954 Jose Peirats was commissioned by the CNT -in-exile to write his monumental five volume 'La CNT en la revolucion espanola'. In the course of his researches he was accorded access to documentation belonging to the CNT and in London in the keeping of Polgare. When Polgare died in 1957, access to the documents was offered to Olaya by CNT colleagues aware of his researches. Among the documents, he discovered copies of 52 letters written to Negrin by his special agent, identified only as 'C'.
One of the reports from 'C' is an account of an exchange between Salvador do Madariaga (the philosopher and original wartime Ambassador to Britain) and the British Foreign Minister, in which British preoccupation with helping the Francoist side was evident. Olaya, using textual clues, attempted to Identify 'C'.
At first Olaya suspected one Calvino, who had figured in all the Purchasing Commissions ('C' had complained of corruptions by these commissions). Calvino however denied this and further investigations led Olaya to conclude 'C' had been PSOE luminary Celestino Alvarez. This was confirmed to him by two agents who had been operating on behalf of the CNT - FAI in Paris at the time. Further inquiries led Olaya to records from Turkish customs and cargo checks (by a French secret agent) of shipping that passed through the Dardanelles en route to Spain with foodstuffs and war materials. Olaya lists this information in an appendix to his book.
A record of the accidental discovery (at the bottom of a crate of goods being returned to the USSR as defective or unusable) of gold led Olaya to query the conventional account of the shipment of Spain's gold reserves to the USSR 'for safe keeping'. Other seemingly unrelated evidence led to the conclusion that, aside from the usual shipments, gold was removed from Spain via the diplomatic pouch to Prague and, also, unrecorded, aboard other vessels.
Olaya holds that the war was lost by the republic due to corruption in the Purchasing Commissions plus the failure of Negrin and Prieto (when so informed by 'C') to take remedial action.
Olaya argues that half of the gold reserves were was sent to the USSR and half to France, partly for their use of the Purchasing Commissions and partly to open accounts in the name of specific individuals… an account in Negrin's name held 390 million francs, one in the name of Julio Lopez Masegase held 198 millions. Olaya's book details all these. He says that so far no account has been taken of the assets seized from Franco's supporters, reckoned at almost three times the value of the gold held in Spain's treasury.
Franco was later able to recover a part of what had been described as Negrin's personal treasure. Negrin's ability to realise the value of his gold in France makes nonsense of the claim that gold had to be removed to the USSR for safekeeping. Olaya states: 'I wanted to check out everything sold by C who was Negrin's informant on activities taking place abroad but I wanted confirmation from other sources. To my surprise, I was to amass a wealth of documents that confirmed and expanded upon the whole business'.
In 1988 Olaya's book in manuscript was a finalist for the 'Espejo de Espana' prize awarded by Planeta publishers. However, Planeta refused to publish it, as did all of the other major publishers in Spain. As a result, the book has been issued by Ediciones Madre Tierra (Mother Earth) of Mostoles. Its author claims: 'The book is based on documents and we are not championing any interests or making partisan propaganda, merely telling the whole truth'.
The book's appearance has coincided with a PSOE desire to sell itself to the electorate under the slogan of '100 years of Integrity with the Socialist Party'. Olaya himself has explained 'it may appear that the book has emerged at an opportune time, but no, that is mere coincidence'. ~ Paul S.
In KSL: Bulletin of the Kate Sharpley Library No. 1 [1991]
- Negrin Lopez, Juan (1887-1956)
- Olaya, Francisco
- Partido Socialista Obrero Espanol PSOE
- Russia / Russian Empire / Soviet Union
- Spanish Revolution and Spanish Civil War SCW
Similar items
- KSL: Bulletin of the Kate Sharpley Library No. 1 [1991] .
- Enric (Henri) Melich Gutiérrez (1925-2021) .
- Imanol, - . Further Data on Riojan Involvement in Guerrilla Groups in France and Spain .
- Revision 6 : Post from the Camps [1 August 1939] .
- Araquistain, Luis . A Socialist Exposes the Counter-Revolutionary Role of the Communist Party of Spain [Leaflet, 1939] .

What will be the result of Rutherford's gold foil experiment, if Thomson's model of the atom was true?
Rutherford gold foil experiment: rutherford bombarded a thin sheet of gold foil with alpha particles and studied the path of the scattered particles. he gave his atomic model on this basis: an atom is essentially empty space (since most of the rays traveled straight through), something very solid (nucleus) resides inside every atom, causing some of the rays to "bounce back," and the nucleus is positively charged, causing some of the rays to be deflected at strange angles (like repels like, as in a magnet). result of rutherford's gold foil experiment, if thomson's model of the atom was true: in rutherford's model, the particles were deflected at large angles but if thomson's model was true, then the alpha particles would deflect at smaller angles. also, the speed of the particles would be decreased due to the repulsion of the diffused positive charge..

The gold foil used in Rutherford's experiment was _____ atoms thick.
What will happen if alpha particles strike electron in gold foil experiment performed by rutherford?
If the Thomson model is considered to be correct what could be the observation of the Rutherford alpha ray scattering experiment?


IMAGES
VIDEO
COMMENTS
Size of the Nucleus. It was possible to obtain the size of the nucleus through Rutherford's experiment. We can calculate the size of the nucleus, by obtaining the point of closest approach of an alpha particle. By shooting alpha particles of kinetic energy 5.5 MeV, the point of closest approach was estimated to be about 4×10 -14 m.
Gold-foil experiment: The gold foil experiment was designed by Rutherford. In his experiment, the α particles were made to come down on a thin gold foil.; Alpha particles α are made up of two protons and two neutrons tightly bound together which is identical to Helium-4.; Many of the α particles passed linearly through the gold foil.; Some of the particles deviated at small angles.
Learn how and why J. J. Thompson's plum pudding model of an atom was disproved by Rutherford and team through an experiment. Also, know about the different ...
The gold foil experiment was a pathbreaking work conducted by scientists Hans Geiger and Ernest Marsden under the supervision of Nobel laureate physicist Ernest Rutherford that led to the discovery of the proper structure of an atom. Known as the Geiger-Marsden experiment, it was performed at the Physical Laboratories of the University of ...
Prior to the groundbreaking gold foil experiment, Rutherford was granted the Nobel Prize for other key contributions in the field of chemistry. History. The popular theory of atomic structure at the time of Rutherford's experiment was the "plum pudding model." This model was developed in 1904 by J.J. Thompson, the scientist who discovered the ...
The Nuclear Model. The gold-foil experiment disproved J.J. Thomsons plum pudding model, which hypothesized the atom was positively charged spaced with electrons embedded inside. Therefore, giving way to the nuclear model. In this model, Rutherford theorized the atomic structure was similar to that of the solar system.
Rutherford's diffraction experiment tests diffraction via a thin foil made of gold metal. Opposite the gold foil is a screen that emits a flash of light when struck by a particle. The passing of many of the particles through suggested the condensed nucleus version of the atom model.
Rutherford conducted an experiment by bombarding a thin sheet of gold with α-particles and then studied the trajectory of these particles after their interaction with the gold foil. Rutherford, in his experiment, directed high energy streams of α-particles from a radioactive source at a thin sheet (100 nm thickness) of gold.
The nucleus was postulated as small and dense to account for the scattering of alpha particles from thin gold foil, as observed in a series of experiments performed by undergraduate Ernest Marsden under the direction of Rutherford and German physicist Hans Geiger in 1909. A radioactive source emitting alpha particles (i.e., positively charged particles, identical to the helium atom nucleus and ...
Well, that is quite an interesting question. You see, the detector the speaker speaks about here is actually a film of Zinc Sulphide positioned around the gold foil, with a small space to let the alpha particles, as mentioned by the speaker. Now, the Zinc Sulphide screen has fluorescent properties, i.e., when the scattered alpha particles hit ...
Now, the mass of the electron was known to be very small at the time of Rutherford's experiment. Even if ALL the electrons in the gold atom would gatter in a small nucleus, it would not be enough : by Newton's third law, an equal force would be exerted on the alpha particle and on the negative charge, therefore the latter would have 80 times ...
Yes, it is correct that Rutherford used other metallic atoms instead of gold. From using other metallic atoms, he drew the following conclusion that there shall be no change in his prior observations, if and only if the malleability of the metal is sufficive enough for the alpha particles to penetrate through, otherwise there shall be a lack of penetration of the alpha particles, thus ...
Given below are two statements:Statement I: Rutherford's gold foil experiment cannot explain the line spectrum of hydrogen atom.Statement II: Bohr's model of hydrogen atom contradicts Heisenberg's uncertainty principle.In the light of the above statement, choose the most appropriate answer from the options given below:
This was the wellknown gold foil experiment, in which it was observed that one particle in about 8,000 bounced off a thin foil of gold rather than passing through it. This surprised everyone, and as Rutherford stated, "It was about as credible as if you had fired a 15-inch shell at a piece of tissue paper and it came back and hit you ...
Rutherford in 1911, carried out an experiment called 'Gold foil experiment' and could conclude the nature of an atom and the position of the protons present in the atom. He used gold foil because gold has high malleability and can be hammered into thin sheets.
Rutherford's gold foil experiment. Rutherford's gold foil experiment. simulation. Fluorescent screen. Beam of alpha particles. Thin gold foil. Rutherford's Model of the Atom. 1. The positive charge is concentrated in the nucleus. This is a very small part of the atom. 582 views • 11 slides
In recent years several new Majoron models were invented to avoid the shortcomings of the ordinary models while leading to observable decay rates in double $\\ensuremath{\\beta}$ experiments. We give the first experimental half-life bounds on double $\\ensuremath{\\beta}$ decays with new Majoron emission and derive bounds on the effective neutrino-Majoron couplings from the data of the $^{76 ...
Other seemingly unrelated evidence led to the conclusion that, aside from the usual shipments, gold was removed from Spain via the diplomatic pouch to Prague and, also, unrecorded, aboard other vessels. Olaya holds that the war was lost by the republic due to corruption in the Purchasing Commissions plus the failure of Negrin and Prieto (when ...
In Rutherford's experiment, a thin gold foil was bombarded with alpha particles. According to Thomson's "plum-pudding" where atoms are to be made up of electrons embedded in a loose positive charge cloud, Alpha particles should have passed through the foil with little or no deflection because the diffused, massless positive charge cloud will ...
Result of Rutherford's gold foil experiment, if Thomson's model of the atom was true: In Rutherford's model, the particles were deflected at large angles but if Thomson's model was true, then the alpha particles would deflect at smaller angles. Also, the speed of the particles would be decreased due to the repulsion of the diffused positive charge.