
- History & Society
- Science & Tech
- Biographies
- Animals & Nature
- Geography & Travel
- Arts & Culture
- Games & Quizzes
- On This Day
- One Good Fact
- New Articles
- Lifestyles & Social Issues
- Philosophy & Religion
- Politics, Law & Government
- World History
- Health & Medicine
- Browse Biographies
- Birds, Reptiles & Other Vertebrates
- Bugs, Mollusks & Other Invertebrates
- Environment
- Fossils & Geologic Time
- Entertainment & Pop Culture
- Sports & Recreation
- Visual Arts
- Demystified
- Image Galleries
- Infographics
- Top Questions
- Britannica Kids
- Saving Earth
- Space Next 50
- Student Center

- Is mathematics a physical science?
- Why does physics work in SI units?


Franck-Hertz experiment
Our editors will review what you’ve submitted and determine whether to revise the article.
- Hyperphysics - Franck-Hertz Experiment
Franck-Hertz experiment , in physics, first experimental verification of the existence of discrete energy states in atoms, performed (1914) by the German-born physicists James Franck and Gustav Hertz .
Franck and Hertz directed low-energy electrons through a gas enclosed in an electron tube . As the energy of the electrons was slowly increased, a certain critical electron energy was reached at which the electron stream made a change from almost undisturbed passage through the gas to nearly complete stoppage. The gas atoms were able to absorb the energy of the electrons only when it reached a certain critical value, indicating that within the gas atoms themselves the atomic electrons make an abrupt transition to a discrete higher energy level . As long as the bombarding electrons have less than this discrete amount of energy, no transition is possible and no energy is absorbed from the stream of electrons. When they have this precise energy, they lose it all at once in collisions to atomic electrons, which store the energy by being promoted to a higher energy level.
Franck—Hertz Experiment
- First Online: 25 July 2009
Cite this chapter

- Friedel Weinert
627 Accesses
In 1913 Bohr took Rutherford's nucleus model of the hydrogen atom as the basis for his quantized atom model (► Bohr's atomic model; Rutherford atom). Although it was not the first, it was the first successful atom model. A year later, two Berlin experimenters, James Franck (1882–1964) and Gustav Hertz (1887–1975), unaware of Bohr's model and its implications, performed an experiment which later turned out to be one of its strongest corroborations. For the so-called Franck—Hertz experiment , they were awarded the Nobel Prize for Physics in 1925. In this experiment ► electrons are ejected from a cathode, C , into a tube filled with mercury gas (see Fig. 1). The energy of the electrons can be increased in a controllable manner by accelerating them towards the positively charged grid, G , through the potential difference V a . Electrons fly through the grid towards anode A . Between G and A , a small retarding voltage, V r , decelerates the electrons. They will only reach the anode A , if their energies V exceed V r , where they will be recorded by the ammeter A.
Collisions between the atoms and the electrons will occur. Only electrons with sufficient energy will cause the mercury atoms to make transitions to higher states of energy. The electrons will lose their energy to the atoms. When V a = 4.9 V, the curve drops very sharply.
This is a preview of subscription content, log in via an institution to check access.
Access this chapter
Subscribe and save.
- Get 10 units per month
- Download Article/Chapter or eBook
- 1 Unit = 1 Article or 1 Chapter
- Cancel anytime
- Available as PDF
- Read on any device
- Instant download
- Own it forever
- Compact, lightweight edition
- Dispatched in 3 to 5 business days
- Free shipping worldwide - see info
- Durable hardcover edition
Tax calculation will be finalised at checkout
Purchases are for personal use only
Institutional subscriptions
Similar content being viewed by others

The Franck-Hertz Experiments, 1911–1914 Experimentalists in Search of a Theory

Introduction

Paradigms and paradoxes: the ionization potential of atomic astatine (Z =85), polonium (Z = 84), and some other elements—what does this value tell us about the energetics of atomic and diatomic halogens
Primary literature.
J. Franck, G. Hertz: Über Zusammenstö e zwischen Elektronen und den Molekülen des Quecksilberdampfes und die Ionisierungsspannung desselben. Verhandlungen der Deutschen Physikalischen Gesellschaft 16 , 457–67 (1914). With supplementary texts and commentary by Armin Hermann in: Die Elektronenstoßversuche (Battenberg, München 1967, Dokumente der Naturwissenschaft 9)
Google Scholar
J. Franck, P. Jordan: Anregung von Quantenspüngen durch Stöße (Springer, Berlin 1926)

Secondary Literature
A. P. French, E. F. Taylor: An Introduction to Quantum Physics (Stanley Thornes Publishers, Cheltenham 1998, 30–1, Chapman & Hall 1979)
K. Krane: Modern Physics (Wiley, New York 1983, 169–70)
P. A. Tipler: Modern Physics (Worth Publishers, New York 1978, 155–7)
H. Geiger, K. Scheel eds.: Quanten (Springer, Berlin 1926, Handbuch der Physik 23)
J. Lemmerich: Max Born, James Franck: Physiker in ihrer Zeit (Reichert, Wiesbaden 1982)
J. Lemmerich: Aufrecht im Sturm (Spektrum, Berlin 2005) (Biography of J. Franck)
J. Kuczera: Gustav Hertz (Teubner, Leipzig 1985)
Book Google Scholar
Download references
You can also search for this author in PubMed Google Scholar
Editor information
Editors and affiliations.
Department of Physics, The City College of New York, 138th St. & Convent Ave., New York, NY, 10031, USA
Daniel Greenberger
Section for the History of Science & Technology, University of Stuttgart, Keplerstr. 17, Stuttgart, D-70174, Germany
Klaus Hentschel
Department of Social Sciences and Humanities, University of Bradford, Bradford, BD7 1DP, UK
Friedel Weinert
Rights and permissions
Reprints and permissions
Copyright information
© 2009 Springer-Verlag Berlin Heidelberg
About this chapter
Weinert, F. (2009). Franck—Hertz Experiment. In: Greenberger, D., Hentschel, K., Weinert, F. (eds) Compendium of Quantum Physics. Springer, Berlin, Heidelberg. https://doi.org/10.1007/978-3-540-70626-7_74
Download citation
DOI : https://doi.org/10.1007/978-3-540-70626-7_74
Published : 25 July 2009
Publisher Name : Springer, Berlin, Heidelberg
Print ISBN : 978-3-540-70622-9
Online ISBN : 978-3-540-70626-7
eBook Packages : Physics and Astronomy Physics and Astronomy (R0)
Share this chapter
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Publish with us
Policies and ethics
- Find a journal
- Track your research
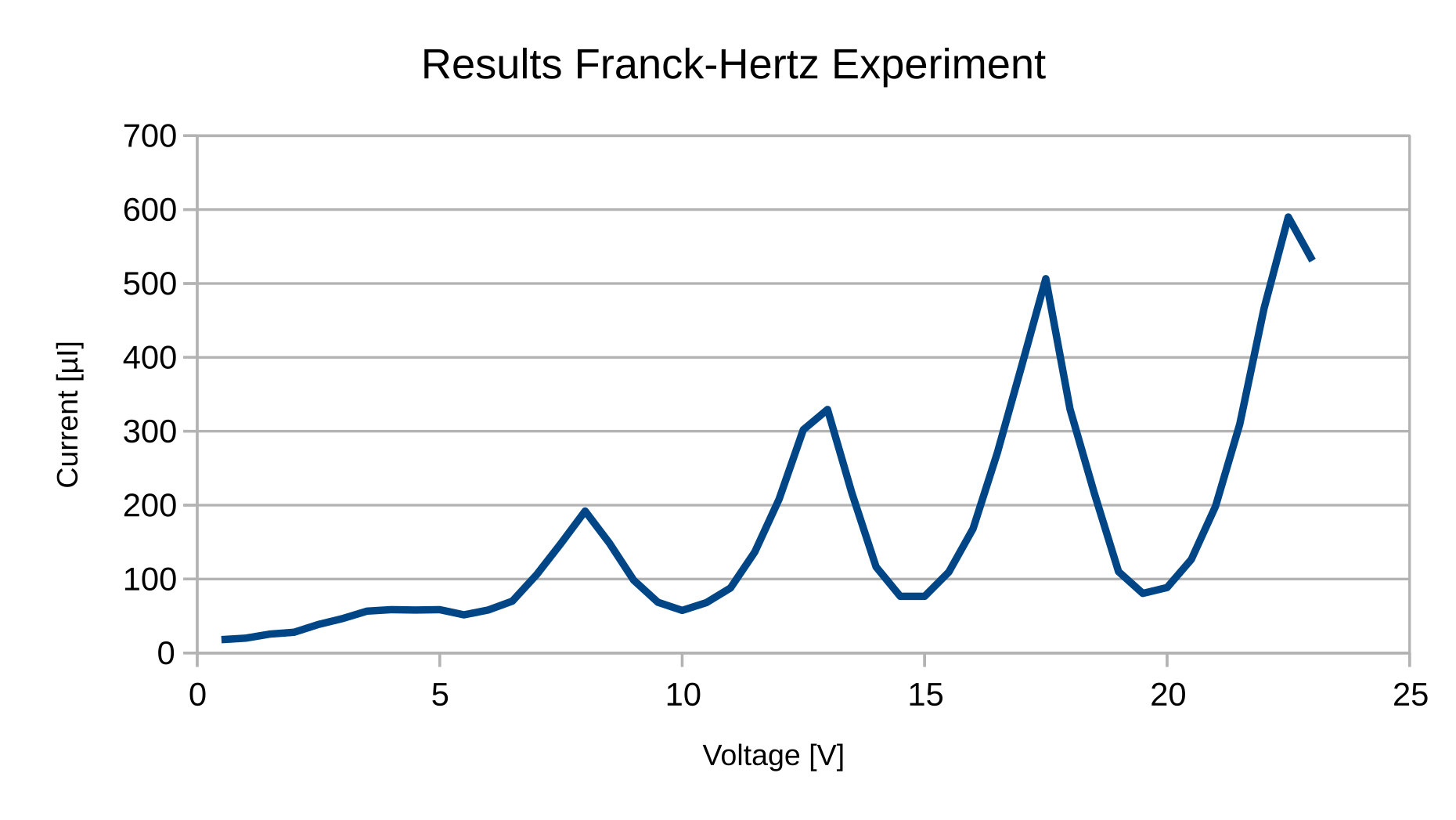
Franck-Hertz Experiment
- To demonstrate the concept of quantization of energy levels according to Bohr's model
- To record the Franck-Hertz Curve for mercury
- To measure the discontinuous energy emission of free electrons for inelastic collision
- To interpret the measurement results as representing discrete energy absorption by mercury atoms
- Frank-Hertz tube (Hg)
- Electric oven to heat up the Hg
- Frank-Hertz supply unit
- Temperature sensor
- Two-channel oscilloscope or PC
- Adjust $U_1= 0.5, 0.7, 1, 1.5\,\mathrm{V}$ and $U_3 = 10\,\mathrm{V}$.
- Record the current versus $U_2$ (0 to 80 V).
- Record the voltage corresponding to each peak

COMMENTS
The Franck-Hertz experiment was the first electrical measurement to clearly show the quantum nature of atoms, and thus "transformed our understanding of the world". ... Franck and Hertz's original paper reported anode currents up to about 15 V, as illustrated in the figure above. Additional maxima and minima occur when current is measured to ...
data set. You will need to read through the paper to learn the details; a copy of the paper is linked on the course website. You will also run the same experiment for a Franck-Hertz tube containing neon. eon has a much higher energy for the first excited state, so the peaks in the I(V accel) curve are more widely spaced.
Franck-Hertz experiment to be finally interpreted rigor-ously after almost 100 years. The details are reported in this colloquium. At the outset we emphasise that the classic Franck-Hertz apparatus involves the passage of low current, low density electrons through a gas, and may therefore be categorised as a "swarm" experiment [1,13-16 ...
In 1911, James Franck and Gustav Hertz began a collaboration to investigate the nature of collisions of slow electrons with gas molecules that led to a series of carefully planned and executed experiments, culminating in their discovery of inelastic collisions of electrons with mercury vapor atoms in 1914. This paper tells the story of their collaboration and the eventual reinterpretation of ...
The 1914 experiment of James Franck and Gustav Hertz provided a graphic demonstration of quantization properties of atoms, thereby laying the foundations of modern atomic physics. This article revisits the experiment on the occasion of its Centenary, compares traditional and modern interpretations, and focuses in particular on the link between microscopic processes, which are governed by the ...
Franck-Hertz experiment, in physics, first experimental verification of the existence of discrete energy states in atoms, performed (1914) by the German-born physicists James Franck and Gustav Hertz.. Franck and Hertz directed low-energy electrons through a gas enclosed in an electron tube.As the energy of the electrons was slowly increased, a certain critical electron energy was reached at ...
The Franck-Hertz Experiment. The Franck-Hertz experiment, first undertaken shortly after Bohr's theory of the atom was pre-sented, provided one of the early indications that atoms had discrete energy levels. In this ex-periment, electrons are accelerated and pass through mercury vapor, where they lose energy by inelastic scattering in ...
Franck-Hertz Experiment Marnik Metting van Rijn February 8, 2020 Abstract The Franck-Hertz experiment was conducted on a mercury tube and the data analysed using ... The paper presenting the experiment mostly known as the Franck-Hertz experiment was published on April 24, 1914. James Franck and Gustav Hertz were awarded the Nobel Prize in Physics
In this experiment the Franck-Hertz tube, which contains a drop of mercury, is placed in a heated oven. The mercury drops evaporates until the vapor is saturated, i.e. the rate of evaporation equals the rate of condensation. By changing the temperature T of the oven, you will be able to change the vapor pressure and hence the density.
The Franck-Hertz Experiment 1 Introduction This is a classic experiment which provided strong evidence against classical mechanics and in the favor of the Bohr model of quantized energy states. In 1914, James Franck and Gustav Hertz bom-barded mercury atoms with a beam of electrons and showed that the electrons lost discrete amounts
2.1 The Franck-Hertz Experiment The Franck-Hertz experiment, preformed in 1914, is an experiment for con rming the Bohr Model of that atom. It was found that when electrons in a potential eld were passed through mercury vapour they experienced an energy loss in disinct steps, and that that the mercury gave an emission line at = 254nm.
Franck and Hertz's original experiment included a window through which the wavelength of the emitted photons could be measured, which they found to be 2536 A [2]. By measuring the current of electrons exiting the tube of gas as a function of their energy, Franck and Hertz observed dips in the measured current corresponding to
Experiment versus Theory. In 1925 the Nobel Prize in Physics was awarded jointly to James Franck (1882-1964) and Gustav L. Hertz (1887-1975) "for their discovery of the laws governing the impact of an electron upon an atom" 1.They conducted an ingenious set of experiments in 1913 and 1914 in which they measured the ionization potential of different gases 2; studied collisions between ...
Figure 1: Schematic diagram of the Franck-Hertz tube. On average, these accelerating electrons will travel a distance, L, (referred to as the mean free path) before colliding with one of the background mercury atoms. As a result, they will pick up some average increase in kinetic energy before making a collision (either elastic or inelastic).
The Franck-Hertz Experiment Introduction ... angles relative to the original direction. This means that in the first approximation, we may treat elastic collisions as if they did not occur (although the elastically scattered electrons do ... One example is a paper by Rapior, Sengstock, and Baev (RSB), published in 2006 [3]. Among the effects ...
1 Department of Physics, Işık University, Istanbul. April 2, 2015. Abstract. The Franck-Hertz experiment demonstrates the theory of. quantiza tion of ene rgy. Purpo se of this exp eriment is to ...
Nowadays, there is a multitude of experiments that go under the Franck-Hertz suit. Even commercial devices exist, projected, built, and marketed for the sole purpose of performing the Franck-Hertz experiment. In this paper we present two cardinal experiments of those, developed by Goucher and Einsporn, respectively.
Franck—Hertz Experiment. In 1913 Bohr took Rutherford's nucleus model of the hydrogen atom as the basis for his quantized atom model ( Bohr's atomic model; Rutherford atom). Although it was not the first, it was the first successful atom model. A year later, two Berlin experimenters, James Franck (1882-1964) and Gustav Hertz (1887-1975 ...
Theory. James Franck and Gustav Hertz conducted an experiment in 1914, which demonstrated the existence of excited states in mercury atoms. It confirms the prediction of quantum theory that electrons occupy only discrete, quantized energy states. This experiment supports Bohr's model of atoms. For this great invention, they have been awarded ...
The Franck-Hertz experiment with neon gas is modelled as an idealised steady-state Townsend experiment and analysed theoretically using (a) multi-term solution of Boltzmann equation and (b) Monte ...
Franck--Hertz experiment in mercury vapor to generate antibunched and photon-number squeezed ... Archived (PDF) from the original. on 4 January 2022. Retrieved 4 January 2022. 20. ^ Teich, ...
An Alternative Interpretation of the Franck-Hertz Experiment. ... An original substantiation of the physical essence of the phenomenon under consideration is proposed, based on the relativistic ...