- Open access
- Published: 17 January 2024

Nutrient patterns and risk of diabetes mellitus type 2: a case-control study
- Morteza haramshahi 1 ,
- Thoraya Mohamed Elhassan A-Elgadir 2 ,
- Hamid Mahmood Abdullah Daabo 3 ,
- Yahya Altinkaynak 4 ,
- Ahmed Hjazi 5 ,
- Archana Saxena 6 ,
- Mazin A.A. Najm 7 ,
- Abbas F. Almulla 8 ,
- Ali Alsaalamy 9 &
- Mohammad Amin Kashani 10
BMC Endocrine Disorders volume 24 , Article number: 10 ( 2024 ) Cite this article
2559 Accesses
1 Altmetric
Metrics details
Backgrounds
Although the significance of diet in preventing or managing diabetes complications is highlighted in current literature, there is insufficient evidence regarding the correlation between nutrient patterns and these complications. The objective of this case-control study is to investigate this relationship by analyzing the dietary intake of nutrients in participants with and without type 2 diabetes (T2D).
A case-control study was conducted at the Tabriz Center of Metabolism and Endocrinology to investigate the relationship between nutrient patterns and type 2 diabetes (T2D). The study enrolled 225 newly diagnosed cases of T2D and 225 controls. The dietary intake of nutrients was assessed using a validated semi-quantitative food frequency questionnaire (FFQ). Principal component analysis using Varimax rotation was used to obtain nutrient patterns. Logistic regression analysis was performed to estimate the risk of T2D.
The participants’ mean (SD) age and BMI were 39.8 (8.8) years and 27.8 (3.6) kg/m2, respectively. The results identified three major nutrient patterns. The first nutrient pattern was characterized by high consumption of sucrose, animal protein, vitamin E, vitamin B1, vitamin B12, calcium, phosphorus, zinc, and potassium. The second nutrient pattern included fiber, plant protein, vitamin D, Riboflavin, Vitamin B5, copper, and Magnesium. The third nutrient pattern was characterized by fiber, plant protein, vitamin A, riboflavin, vitamin C, calcium, and potassium. Individuals in the highest tertile of nutrient pattern 3 (NP3) had a lower risk of T2D compared to those in the lowest tertile after adjusting for confounders. The odds ratio was 0.52 with a 95% confidence interval of 0.30–0.89 and a P_trend of 0.039.
This study found that conforming to a nutrient pattern consisting of plant protein, vitamin C, vitamin A, vitamin B2, potassium, and calcium is linked to a lower likelihood of developing T2D.The initial results suggest that following a nutrient pattern that includes these nutrients may reduce the risk of T2D. However, further research is required to confirm the relationship between nutrient patterns and T2D.
Peer Review reports
Type 2 diabetes is a significant concern for public health in developed nations. It leads to high rates of illness and death and places a significant financial burden on healthcare systems [ 1 , 2 ]. In the past few decades, there has been a sharp increase in the occurrence of diabetes, and is expected to continue increasing, with an estimated 693 million people living with the disease by 2045 [ 1 ]. Complications associated with type 2 diabetes can also contribute to premature death. A concerning aspect of the disease is that a significant proportion of cases (40%) go undetected [ 3 ], and there is also an increasing prevalence of prediabetes, which raises the risk of developing type 2 diabetes and other chronic diseases [ 1 ].
The connection between diet and type 2 diabetes has been extensively studied, including the examination of dietary patterns and individual foods or nutrient patterns [ 4 , 5 , 6 , 7 ]. Various sources have suggested that chronic diseases may be influenced by a combination of nutrients [ 8 ]. In the field of nutritional epidemiology, the examination of dietary patterns has emerged as a viable approach to investigate the correlation between diet and disease. This method involves using statistical techniques to combine multiple foods or nutrients into dietary or nutrient patterns, which are believed to provide a more detailed understanding of the connection between diet and disease. It has been suggested that the impact of individual nutrients or foods on chronic disease may be too subtle to detect, but their collective effect within a pattern may be more indicative [ 9 ].
There have been some recent studies examining the effect of nutrient patterns on chronic disease such as, non-alcoholic fatty liver, breast and gastric cancer, Polycystic Ovary Syndrome (PCOs) and metabolic syndrome [ 10 , 11 , 12 , 13 , 14 ]. For example, it was found that a nutrient pattern consisting mainly of protein, carbohydrates, and various sugars was linked to a higher risk of Metabolic Syndrome (MetS) in both men and women, whereas a pattern characterized by copper, selenium, and several vitamins was linked to greater odds of MetS [ 14 ]. A prospective study conducted among participants of the Tehran Lipid and Glucose Study indicates that a nutrient pattern rich in vitamin A, vitamin C, vitamin B6, potassium, and fructose is associated with a reduced risk of insulin-related disorders [ 15 ]. Although there have been limited investigations on the connection between nutrient patterns and the likelihood of developing diabetes, the present study seeks to explore this relationship by analyzing the adherence to different nutrient patterns and its effect on the risk of type 2 diabetes.
Study population
This study utilized a case-control design and involved participants between the ages of 18 and 60 who had been diagnosed with type 2 diabetes within the previous six months based on specific glucose level criteria (FBS levels of ≥ 126 mg/dl and 2 h-PG levels of ≥ 200 mg/dl [ 17 ]). Healthy individuals within the same age range were also included, with specific glucose level criteria (FBS levels of < 100 mg/dl and 2 h-PG levels of < 200 mg/dl [ 17 ]). The study excluded individuals with certain chronic diseases, Type 1 Diabetes, gestational diabetes, those following specific dietary patterns or taking certain medications, pregnant and breastfeeding women, those with a family history of diabetes or hypertension, and those who did not complete the food frequency questionnaire (more than 35 items) or whose reported energy intake was outside of a specific range (range of 800–4200 kcal [ 18 ]).
This study enrolled 450 adult participants, with 225 individuals in the case group and 225 in the control group. The case group was selected using a simple sampling method from patients diagnosed with diabetes at the Tabriz Center of Metabolism and Endocrinology as a referral center affiliated to tabriz University of Medical Sciences from January 2021 to March 2022, as well as through a two-stage cluster sampling method among patients referred to private endocrinologists to enhance the sample’s external validity. Participants in the control group were also selected through a two-stage cluster sampling method from individuals who had undergone blood glucose checkups at the Tabriz Center of Metabolism and Endocrinology, a referral center affiliated with Tabriz University of Medical Sciences, within the past six months. All participants provided informed consent at the beginning of the study. The study was financially supported by Tabriz University of Medical Sciences and is related to project NO. 1400/63,145.
Dietary assessment
To collect dietary intake information, personal interviews and a semi-quantitative food frequency questionnaire (FFQ) consisting of 168 food items were used [ 16 ]. The FFQ asked about the frequency of consumption for each item over the course of one year, with the year before diagnosis for the case group and the year before the interview for the control group. Participants were also asked about the frequency of consumption (per day, week, month, or year) for each type of food. to ensure consistency in measurements, a nutritionist provided instructions on converting the size of reported food items from household measures to grams using four scales. The quantity of food consumed by each individual was calculated based on their intake in grams and reported on a daily basis. The nutrient composition of all foods was derived by using modified nutritionist IV software.
Nutrient pattern assessment
We conducted factor analyses using a comprehensive set of 34 nutrients, encompassing various macronutrients, micronutrients, and other dietary components. These included sucrose, lactose, fructose, fiber, animal protein, plant protein, saturated fatty acids, monounsaturated fatty acids, polyunsaturated fatty acids, cholesterol, as well as an array of vitamins and minerals such as A, D, E, K, C, thiamine (B1), riboflavin (B2), niacin (B3), pantothenic acid (B5), pyridoxine (B6), folate (B9), B12, calcium, phosphorus, iron, zinc, copper, magnesium, manganese, chromium, selenium, sodium, potassium, and caffeine. The dietary intake of these 34 nutrients per 1,000 Kcal of energy intake was computed and utilized as input variables. Subsequently, nutrient patterns (NPs) were derived through principal component analysis (PCA) with varimax rotation, based on the correlation matrix. Factor scores for each participant were then calculated by aggregating the frequency of consumption and multiplying it by the factor loadings across all 34 nutrients. To assess the statistical correlation between variables and evaluate the adequacy of the sample size, we employed the Bartlett test of sphericity ( P < 0.001) and the Kaiser-Mayer-Olkin test (0.71), respectively.
Assessment of other variables
To obtain the participants’ anthropometric measurements, weight and height were measured using a seca scale, and the participants’ BMI was determined by dividing their weight in kilograms by the square of their height in meters. Waist circumference was measured using a metal anthropometric tape, and the participants’ hip circumference was measured using a metal anthropometric tape while standing [ 17 ]. Daily physical activity was measured using a physical activity questionnaire [ 18 ], and personal questioning was employed to gather information on population and socioeconomic characteristics, including marital status, academic degree, and smoking.
Statistical analysis
Statistical analysis was performed using the Statistical Package Software for Social Science, version 21. The normality of the data was assessed using Kolmogorov-Smirnov’s test and histogram chart. The characteristics and dietary intakes of the case and control groups were presented as mean ± SD or median and frequency (percentages). Independent sample t-tests and chi-square tests were used to compare continuous and categorical variables, respectively, between the case and control groups.
The participants’ mean (SD) age and BMI were 39.8 (8.8) years and 27.8 (3.6) kg/m2, respectively. The mean (SD) BMI in the case group was 30.5 ± 4.1, and in the control group, it was 25.2 ± 3.2 kg/m2. The mean (SD) physical activity in the case group was 1121 ± 611 MET/min/week, and in the control group, it was 1598 ± 940 MET/min/week. There were significant differences in BMI and physical activity between the two groups. The mean (SD) waist circumference in the case group was 109.32 ± 10.28 cm, and in the control group, it was 87.25 ± 9.35 cm. The mean (SD) hip circumference in the case group was 107.25 ± 8.61 cm, and in the control group, it was 91.44 ± 6.17 cm. The study identified three primary nutrient patterns (NPs) with eigenvalues greater than 2. Table 1 displays the factor loadings for nutrient patterns, which accounted for 56.11% of the total nutrient variation. The high intake of sucrose, animal protein, phosphorus, zinc, potassium, calcium, vitamin E, vitamin B1 and vitamin B12 were the distinguishing features of the first pattern. The second nutrient pattern was positively associated with copper, magnesium, fiber, vitamin D, B2, B5 and plant protein but had a negative correlation with lactose and saturated fatty acids. On the other hand, the high intake of fiber, vitamin A, B2, vitamin C, plant protein and potassium were the distinguishing features of the third pattern.
The following are the characteristics of T2D patients compared to the control group, as shown in Table 2 : Higher BMI, More likely to be smokers, Lower physical activity levels, higher FBS, HbA1C, Insulin ( p < 0.05). Other variables did not differ significantly between the two groups ( p > 0.05). Additionally, T2D patients had a greater intake of energy and vitamin B3 but consumed less plant protein, vitamin A, vitamin E, vitamin B2, and zinc ( p < 0.05).
Table 3 summarizes the partial correlation coefficient between NPs and food sources, with NP1 showing a strong positive correlation with low-fat dairy, NP2 with refined grains, and NP3 with fruits and vegetables.
Table 4 demonstrates the relationships between NPs and T2D. After adjusting for age and sex, there was no significant link between each nutrient pattern (NP) and T2D. However, when adjusting for other factors such as BMI, physical activity, smoking, and energy intake, individuals in the highest tertile of NP1 and NP2 did not show a significant association with T2D compared to those in the lowest tertile. On the other hand, those in the highest tertile of NP3 had a lower probability of developing T2D than those in the lowest tertile (OR: 0.52, 95%CI: 0.30–0.89, P_trend = 0.039).
In this study, three major NPs were identified. After adjusting for potential confounders, we observed a significant inverse association between the Third NP and the odds of T2D. The high intake of fiber, vitamin A, B2, vitamin C, plant protein and potassium were the distinguishing features of the third pattern.
Dietary patterns, such as healthy, Mediterranean, traditional, and Western dietary patterns, have recently received significant attention in studying the connection between diet and health. When looking at the relationship between nutrients and disease incidence, it is more challenging to evaluate when considering individual foods and the metabolism of all nutrients together [ 19 ]. It is therefore more effective to take a broader view and consider diet as a whole. Dietary and nutrient patterns can have a greater impact on health than specific nutrients or nutritional groups. There is supporting evidence that links high calorie or high glycemic index foods with an increased risk of T2D. The quality of one’s diet is also associated with the risk, progression, and side effects of T2D [ 20 ]. Establishing a desirable food pattern has become a priority in public health efforts to prevent T2D. By studying dietary and nutrient patterns, we can gain a comprehensive understanding of an individual’s overall diet beyond just the consumption of specific nutrients and food groups. Moreover, it is easier for people to understand health recommendations when presented as dietary patterns rather than focusing solely on individual nutrients [ 19 ].
A previous cross-sectional study investigated the relationship between NPs and fasting glucose and glycated hemoglobin levels among apparently healthy black South Africans. The study stratified 2,010 participants by gender and urban/rural status and identified three nutrient patterns per stratum. In rural women, a nutrient pattern driven by starch, dietary fiber, and B vitamins was significantly associated with lower fasting glucose and glycated hemoglobin levels. A nutrient pattern that included vitamin B1, zinc, and plant protein was linked to notable decreases in glycated hemoglobin and fasting glucose levels in rural men. These findings suggest that nutrient patterns that are plant-based are linked to lower levels of fasting glucose and glycated hemoglobin [ 21 ].
Iwasaki et al. found that specific nutrient patterns were associated with lower risks of MetS. One nutrient pattern high in potassium, fiber, and vitamins, while another pattern high in vitamin B2, saturated fatty acids and calcium [ 22 ]. A recent study found that a nutrient pattern characterized by high intake of calcium, potassium, fats, cholesterol, vitamins B2, B12, A, D, K and C was positively linked to MetS [ 23 ]. Salehi-Sahlabadi et al. found that adhering to a nutrient pattern rich in potassium, vitamin A, fructose, vitamin C and vitamin B6 was negatively associated with the likelihood of NAFLD [ 11 ]. A nutrient pattern high in potassium, vitamin A, vitamin B6, vitamin C and fructose was associated with a reduced risk of hyperinsulinemia, IR, and dyslipidemia among participants in Tehran, according to a prospective study [ 11 , 24 , 25 ].
Due to several variations among studies exploring NPs linked to chronic diseases, including differences in the number of nutrients, populations, study designs and outcomes there has been a considerable diversity in the identified NPs, with only a few NPs being replicated across studies. Our study is the first of its kind to explore the correlation between nutrient patterns and T2D in this context.
In our study, there was no association between NPs 1 and 2 and T2D. This lack of correlation may be attributed to the absence of harmful nutrients or food categories linked to diabetes in these NPs. NP3 in this study, unlike other NPs, is positively associated with beneficial food groups such as nuts, fruits, plant oil and vegetables, and negatively associated with unhealthy food groups like red-processed meat, snacks, high-fat dairy and refined grains. A recent systematic review and meta-analysis found that individuals who consumed higher amounts of fruits and vegetables had a lower risk of developing type 2 diabetes [ 26 ]. Moreover, the consumption of vegetables was found to have an inverse relationship with ALT, TC and LDL levels among adults, while fruit consumption was associated with a positive reduction in visceral fat [ 27 , 28 ]. Another study suggested that an increased intake of vegetables and fruits could potentially lower the risk of MetS [ 29 ]. According to a study, greater nut consumption was significantly linked to a reduced prevalence of T2D [ 30 ]. Consuming fruits and vegetables is a crucial component of a healthful dietary pattern that can lower the risk of type 2 diabetes [ 31 ]. On the other hand, Consuming a Western dietary pattern, which primarily consists of fast foods, high-fat dairy, refined grains, soft drinks and processed meat has been found to be correlated with an increased risk of type 2 diabetes [ 31 ].
Several mechanisms have been identified that explain the positive associations between the components of NP 3 and T2D or its risk factors. Vitamin intake has been shown to play a role in the development of T2D through various pathways. Consuming vitamin C has been found to have beneficial effects in reducing the risk of type 2 diabetes mellitus. These effects can be attributed to the following actions of vitamin C: vasodilator, cytoprotective, platelet anti-aggregator and anti-mutagenic. To achieve this, the body increases the production of several substances including prostaglandin E1, PGI2, endothelial nitric oxide, and lipoxin A4. Additionally, the body restores the Arachidonic Acid content to normal levels [ 32 ]. Vitamin A has a multifaceted role in cell regulation beyond its antioxidant function. It contributes to gene regulation, epithelial cell integrity, and resistance to infection. Research suggests that vitamin A also enhances antioxidant enzyme function in the body. Research has indicated a link between vitamin A deficiency and type 2 diabetes mellitus (T2DM), which suggests that vitamin A may have a role in the biology of T2DM [ 33 ]. Moreover, a meta-analysis has found that replacing animal protein with plant protein can lead to minor improvements in glycemic control for individuals with diabetes [ 34 ]. According to a recent meta-analysis, increasing the consumption of fruits, especially berries, yellow vegetables, cruciferous vegetables, green leafy vegetables is associated with a lower risk of developing type 2 diabetes. These results support the recommendation to incorporate more fruits and vegetables into the diet as a way to prevent various chronic diseases, including type 2 diabetes [ 35 ]. A study showed that maintaining adequate potassium intake could regulate insulin secretion and carbohydrate metabolism, leading to the prevention of obesity and metabolic syndrome (MetS) [ 36 ].
A number of research studies conducted in the Western societies have shown that Western dietary pattern including higher intake of red meat, processed meat, and refined grains is significantly associated with increased risk of T2D [ 37 , 38 ]. For example, in the 12-years cohort prospective study, van Dam et al. investigated dietary pattern of 42,504 American white men at the age range of 40–75 years old using the FFQ. After controlling the confounders, the risk of T2D increased 60% in people adherent to the western-like dietary pattern [ 38 ]. The rapid process of change in lifestyle, diets, and physical activity that have been occurred as a result of extended urbanization, improved economic status, change of work pattern toward jobs, and change in the processes of producing and distributing nutrients during the recent years in developing countries have led people to more consumption of fast food and processed foods [ 20 ].
Significant research has been conducted on the impact of nutrient type and sequence on glucose tolerance. Multiple studies have shown that manipulating the sequence of food intake can enhance glycemic control in individuals with type 2 diabetes in real-life situations. The glucose-lowering effect of preload-based nutritional strategies has been found to be more pronounced in type 2 diabetes patients compared to healthy individuals. Moreover, consuming carbohydrates last, as part of meal patterns, has been proven to improve glucose tolerance and reduce the risk of weight gain [ 39 ]. Recent findings on meal sequence further emphasize the potential of this dietary approach in preventing and managing type 2 diabetes [ 40 ].
Several studies have shown that food from a short supply chain has a significant impact on metabolic syndrome. The length of the food supply chain is important in determining the risk of metabolic syndrome in a population [ 41 ]. Research indicates that people who consume food from short supply chains have a lower prevalence of metabolic syndrome compared to those who consume food from long supply chains. Specifically, food from short supply chains is associated with lower levels of triglycerides and glucose, which leads to a reduced occurrence of metabolic syndrome [ 42 ]. Adhering to the Mediterranean diet with a short supply chain is also found to significantly reduce the prevalence of metabolic syndrome. Therefore, these studies provide evidence that food from short supply chains positively affects metabolic parameters and the occurrence of metabolic syndrome [ 41 ].
The study we conducted presented several advantages. It was the first case-control research to investigate the correlation between nutrient patterns and the likelihood of developing type 2 diabetes (T2D). While numerous studies have explored the relationship between dietary patterns and diabetes, there is a scarcity of research specifically focusing on nutrient patterns in individuals with type 2 diabetes. Furthermore, the collection of dietary intake data was carried out through face-to-face interviews conducted by trained dieticians to minimize measurement errors. However, this study also had some limitations. Case-control studies are susceptible to selection and recall biases. Additionally, the use of factor analysis to identify patterns, and the potential influence of research decisions on the number of factors and nutrient factor loadings in each pattern, should be considered. Lastly, despite the use of a validated semi-quantitative FFQ (food frequency questionnaire), there remains a possibility of measurement error due to dietary recall. The study’s findings and limitations contribute to the ongoing discourse on the role of nutrient patterns in the development of T2D and the importance of considering these factors in future research and preventive strategies.
Conclusions
The results of this study indicate that conforming to a nutrient pattern consisting of plant protein, vitamin C, vitamin A, vitamin B2, potassium, and calcium is linked to a lower likelihood of developing T2D. Our investigation did not reveal any significant correlation between other nutrient patterns and T2D risk. However, additional research is necessary to authenticate these initial findings and establish the correlation between nutrient patterns and T2D.
Data availability
Upon reasonable request, the corresponding author can provide the datasets that were produced and analyzed during the current study.
Ogurtsova K, Guariguata L, Barengo NC, Ruiz PL-D, Sacre JW, Karuranga S, et al. IDF Diabetes Atlas: global estimates of undiagnosed diabetes in adults for 2021. Diabetes Res Clin Pract. 2022;183:109118.
Article PubMed Google Scholar
Teo ZL, Tham Y-C, Yu M, Chee ML, Rim TH, Cheung N, et al. Global prevalence of diabetic retinopathy and projection of burden through 2045: systematic review and meta-analysis. Ophthalmology. 2021;128(11):1580–91.
Sugiyama T, Yanagisawa-Sugita A, Tanaka H, Ihana-Sugiyama N, Imai K, Ohsugi M, et al. Different incidences of diabetic retinopathy requiring treatment since diagnosis according to the course of diabetes diagnosis: a retrospective cohort study. Sci Rep. 2023;13(1):10527.
Article CAS PubMed PubMed Central Google Scholar
Hodge AM, English DR, O’Dea K, Giles GG. Dietary patterns and diabetes incidence in the Melbourne Collaborative Cohort Study. Am J Epidemiol. 2007;165(6):603–10.
Jannasch F, Kröger J, Schulze MB. Dietary patterns and type 2 diabetes: a systematic literature review and meta-analysis of prospective studies. J Nutr. 2017;147(6):1174–82.
Article CAS PubMed Google Scholar
Sami W, Ansari T, Butt NS, Ab Hamid MR. Effect of diet on type 2 diabetes mellitus: a review. Int J Health Sci. 2017;11(2):65.
Google Scholar
Miller V, Micha R, Choi E, Karageorgou D, Webb P, Mozaffarian D. Evaluation of the quality of evidence of the association of foods and nutrients with cardiovascular disease and diabetes: a systematic review. JAMA Netw open. 2022;5(2):e2146705–e.
Article PubMed PubMed Central Google Scholar
Salehi-Sahlabadi A, Teymoori F, Jabbari M, Momeni A, Mokari-Yamchi A, Sohouli M, et al. Dietary polyphenols and the odds of non-alcoholic fatty liver disease: a case-control study. Clin Nutr ESPEN. 2021;41:429–35.
Salehi-Abargouei A, Esmaillzadeh A, Azadbakht L, Keshteli AH, Feizi A, Feinle-Bisset C, et al. Nutrient patterns and their relation to general and abdominal obesity in Iranian adults: findings from the SEPAHAN study. Eur J Nutr. 2016;55:505–18.
Panjeshahin A, Salehi-Abargouei A, Ghadiri-Anari A, Rasouli A, Hosseinzadeh M. The Association between nutrient patterns and polycystic ovary syndrome: a case-control study. J Nutr Food Secur. 2022.
Salehi-Sahlabadi A, Teymoori F, Ahmadirad H, Mokhtari E, Azadi M, Seraj SS, et al. Nutrient patterns and non-alcoholic fatty liver disease in Iranian Adul: a case-control study. Front Nutr. 2022;9:977403.
Fereidani SS, Eini-Zinab H, Heidari Z, Jalali S, Sedaghat F, Rashidkhani B. Nutrient patterns and risk of breast cancer among Iranian women: a case-control study. Asian Pac J cancer Prevention: APJCP. 2018;19(9):2619.
CAS Google Scholar
Narmcheshm S, Sasanfar B, Hadji M, Zendehdel K, Toorang F, Azadbakht L. Patterns of nutrient intake in relation to gastric cancer: a case control study. Nutr Cancer. 2022;74(3):830–9.
Khayyatzadeh SS, Moohebati M, Mazidi M, Avan A, Tayefi M, Parizadeh SMR, et al. Nutrient patterns and their relationship to metabolic syndrome in Iranian adults. Eur J Clin Invest. 2016;46(10):840–52.
Teymoori F, Mokhtari E, Salehi P, Hosseini-Esfahani F, Mirmiran P, Azizi F. A nutrient pattern characterized by vitamin A, C, B6, potassium, and fructose is associated with reduced risk of insulin-related disorders: a prospective study among participants of Tehran lipid and glucose study. Diabetol Metab Syndr. 2021;13(1):12.
Esmaillzadeh A, Azadbakht L. Major dietary patterns in relation to general obesity and central adiposity among Iranian women. J Nutr. 2008;138(2):358–63.
Wang J, Thornton JC, Bari S, Williamson B, Gallagher D, Heymsfield SB, et al. Comparisons of waist circumferences measured at 4 sites. Am J Clin Nutr. 2003;77(2):379–84.
Committee IR. Guidelines for data processing and analysis of the International Physical Activity Questionnaire (IPAQ)-short and long forms. http://www.ipaq.ki.se/scoring.pdf ; 2005.
Mandalazi E, Drake I, Wirfält E, Orho-Melander M, Sonestedt E. A high diet quality based on dietary recommendations is not associated with lower incidence of type 2 diabetes in the Malmö Diet and Cancer Cohort. Int J Mol Sci. 2016;17(6):901.
Beigrezaei S, Ghiasvand R, Feizi A, Iraj B. Relationship between dietary patterns and incidence of type 2 diabetes. Int J Prev Med. 2019;10:122.
Chikowore T, Pisa PT, Van Zyl T, Feskens EJ, Wentzel-Viljoen E, Conradie KR. Nutrient patterns associated with fasting glucose and glycated haemoglobin levels in a black South African population. Nutrients. 2017;9(1):9.
Iwasaki Y, Arisawa K, Katsuura-Kamano S, Uemura H, Tsukamoto M, Kadomatsu Y, et al. Associations of nutrient patterns with the prevalence of metabolic syndrome: results from the baseline data of the Japan multi-institutional collaborative cohort study. Nutrients. 2019;11(5):990.
Sadeghi O, Sadeghi A, Mozaffari-Khosravi H, Shokri A. The association between nutrient patterns and metabolic syndrome among Iranian adults: cross-sectional analysis of Shahedieh cohort study. Public Health Nutr. 2021;24(11):3379–88.
Mottaghian M, Salehi P, Teymoori F, Mirmiran P, Hosseini-Esfahani F, Azizi F. Nutrient patterns and cardiometabolic risk factors among Iranian adults: Tehran lipid and glucose study. BMC Public Health. 2020;20:1–12.
Article Google Scholar
Teymoori F, Mokhtari E, Salehi P, Hosseini-Esfahani F, Mirmiran P, Azizi F. A nutrient pattern characterized by vitamin A, C, B6, potassium, and fructose is associated with reduced risk of insulin-related disorders: a prospective study among participants of Tehran lipid and glucose study. Diabetol Metab Syndr. 2021;13(1):1–13.
Halvorsen RE, Elvestad M, Molin M, Aune D. Fruit and vegetable consumption and the risk of type 2 diabetes: a systematic review and dose–response meta-analysis of prospective studies. BMJ Nutr Prev Health. 2021;4(2):519.
Mollahosseini M, Daneshzad E, Rahimi MH, Yekaninejad MS, Maghbooli Z, Mirzaei K. The association between fruit and vegetable intake and liver enzymes (aspartate and alanine transaminases) in Tehran, Iran. Ethiop J Health Sci. 2017;27(4):401–10.
Plaz Torres MC, Bodini G, Furnari M, Marabotto E, Zentilin P, Giannini EG. Nuts and non-alcoholic fatty liver disease: are nuts safe for patients with fatty liver disease? Nutrients. 2020;12(11):3363.
Lee M, Lim M, Kim J. Fruit and vegetable consumption and the metabolic syndrome: a systematic review and dose–response meta-analysis. Br J Nutr. 2019;122(7):723–33.
Muley A, Fernandez R, Ellwood L, Muley P, Shah M. Effect of tree nuts on glycemic outcomes in adults with type 2 diabetes mellitus: a systematic review. JBI Evid Synthesis. 2021;19(5):966–1002.
Beigrezaei S, Ghiasvand R, Feizi A, Iraj B. Relationship between dietary patterns and incidence of type 2 diabetes. Int J Prev Med. 2019;10.
Das UN. Vitamin C for type 2 diabetes mellitus and hypertension. Arch Med Res. 2019;50(2):11–4.
Iqbal S, Naseem I. Role of vitamin A in type 2 diabetes mellitus biology: effects of intervention therapy in a deficient state. Nutrition. 2015;31(7–8):901–7.
Viguiliouk E, Stewart SE, Jayalath VH, Ng AP, Mirrahimi A, De Souza RJ, et al. Effect of replacing animal protein with plant protein on glycemic control in diabetes: a systematic review and meta-analysis of randomized controlled trials. Nutrients. 2015;7(12):9804–24.
Wang PY, Fang JC, Gao ZH, Zhang C, Xie SY. Higher intake of fruits, vegetables or their fiber reduces the risk of type 2 diabetes: a meta-analysis. J Diabetes Invest. 2016;7(1):56–69.
Cai X, Li X, Fan W, Yu W, Wang S, Li Z, et al. Potassium and obesity/metabolic syndrome: a systematic review and meta-analysis of the epidemiological evidence. Nutrients. 2016;8(4):183.
van Dam RM, Rimm EB, Willett WC, Stampfer MJ, Hu FB. Dietary patterns and risk for type 2 diabetes mellitus in US men. Ann Intern Med. 2002;136(3):201–9.
Fung TT, Schulze M, Manson JE, Willett WC, Hu FB. Dietary patterns, meat intake, and the risk of type 2 diabetes in women. Arch Intern Med. 2004;164(20):2235–40.
Wheeler ML, Dunbar SA, Jaacks LM, Karmally W, Mayer-Davis EJ, Wylie-Rosett J, et al. Macronutrients, Food groups, and eating patterns in the management of diabetes: a systematic review of the literature, 2010. Diabetes Care. 2012;35(2):434–45.
Dwibedi C, Mellergård E, Gyllensten AC, Nilsson K, Axelsson AS, Bäckman M, et al. Effect of self-managed lifestyle treatment on glycemic control in patients with type 2 diabetes. Npj Digit Med. 2022;5(1):60.
Santulli G, Pascale V, Finelli R, Visco V, Giannotti R, Massari A, et al. We are what we eat: impact of food from short supply chain on metabolic syndrome. J Clin Med. 2019;8(12):2061.
De Rosa M, Giannotti R, Pascale A, Finelli R, Ilario M, Ciccarelli M, et al. P6280 food of short supply chain impacts metabolism and cardiovascular risk. A survey in Southern Italy. Eur Heart J. 2018;39(suppl1):ehy566. P6280.
Download references
Acknowledgements
The researchers express their gratitude towards all the individuals who volunteered to take part in the study.
This research received no external funding.
Author information
Authors and affiliations.
Faculty of medicine, Tabriz University of medical sciences, Tabriz, Iran
Morteza haramshahi
Department of clinical biochemistry, College of medicine, King Khalid University, Abha, Saudi Arabia
Thoraya Mohamed Elhassan A-Elgadir
Fharmacy Department, Duhok polytechnic, University Duhok, Kurdistan, Iraq
Hamid Mahmood Abdullah Daabo
Department of Medical Services and Techniques, Ardahan University, Ardahan, Turkey
Yahya Altinkaynak
Department of Medical Laboratory Sciences, College of Applied Medical Sciences, Prince Sattam bin Abdulaziz University, Jeddah, Saudi Arabia
Ahmed Hjazi
Department of Management, Uttaranchal Institute of Management, Uttaranchal University, Dehradun, Uttarakhand, India
Archana Saxena
Pharmaceutical Chemistry Department, College of Pharmacy, Al-Ayen University, Thi-Qar, Iraq
Mazin A.A. Najm
College of technical engineering, The Islamic University, Najaf, Iraq
Abbas F. Almulla
College of technical engineering, Imam Ja’afar Al-Sadiq University, Al‐Muthanna, 66002, Iraq
Ali Alsaalamy
Department of Medicinal Chemistry, Faculty of Pharmacy, Tehran Medical Sciences, Islamic Azad University, Tehran, Iran
Mohammad Amin Kashani
You can also search for this author in PubMed Google Scholar
Contributions
The study’s protocol was designed by M.K., M.H., and T.E., while H.A., Y.A., and A.H. carried out the research. A.S. analyzed the data and prepared the initial draft of the manuscript. M.N., A.FA., and A.A. interpreted the data and provided critical feedback on the manuscript. All authors reviewed and approved the final version of the manuscript.
Corresponding author
Correspondence to Mohammad Amin Kashani .
Ethics declarations
Ethics approval and consent to participate.
This study was performed in line with the principles of the Declaration of Helsinki. Informed consent was obtained from all participants or their legal guardians. Approval was granted by the Research Ethics Committee of Islamic Azad University of Medical Sciences (Approval number: IR.AUI.MEDICINE. REC.1401.147).
Consent for publication
Not applicable.
Competing interests
The authors declared no conflicts of interest.
Additional information
Publisher’s note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ . The Creative Commons Public Domain Dedication waiver ( http://creativecommons.org/publicdomain/zero/1.0/ ) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
Reprints and permissions
About this article
Cite this article.
haramshahi, M., A-Elgadir, T.M.E., Daabo, H.M.A. et al. Nutrient patterns and risk of diabetes mellitus type 2: a case-control study. BMC Endocr Disord 24 , 10 (2024). https://doi.org/10.1186/s12902-024-01540-5
Download citation
Received : 04 November 2023
Accepted : 09 January 2024
Published : 17 January 2024
DOI : https://doi.org/10.1186/s12902-024-01540-5
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Nutrient pattern
BMC Endocrine Disorders
ISSN: 1472-6823
- General enquiries: [email protected]
- Introduction
- Conclusions
- Article Information
BMI indicates body mass index (calculated as weight in kilograms divided by height in meters squared).
Data are least squares means derived from mixed linear models and adjusted for baseline hemoglobin A 1c and sex. Error bars indicate 95% CIs.
Error bars indicate 95% CIs. If the prespecified treatment target was reached at the medical consultation, the pharmacological treatment was halved. If unchanged values or an additional reduction was observed at the following medical consultation, the medical treatment was paused.
Trial Protocol
Statistical Analysis Plan
eTable 1. Per Protocol Analysis From Baseline to 12-Month Follow-up
eTable 2. Baseline-Observation-Carried-Forward and Multiple Imputation Analysis From Baseline to 12-Month Follow-up
eTable 3. Medical Consultation and Medical Adherence to Glucose-Lowering Medication
eTable 4. Medical Consultation and Medical Adherence to Lipid-Lowering Medication
eTable 5. Medical Consultation and Medical Adherence to Blood Pressure–Lowering Medication
eTable 6. Exercise and Daily Physical Activity for the Lifestyle Participants
eTable 7. Dietary Registration (3 Days) and Attendance for the Lifestyle Participants
eFigure 1. Reduction in Lipid-Lowering Medication
eFigure 2. Reduction in Blood Pressure–Lowering Medication
- Intensive Lifestyle Intervention for Type 2 Diabetes JAMA Comment & Response December 26, 2017 Dario Giugliano, MD, PhD; Maria Ida Maiorino, MD, PhD; Katherine Esposito, MD, PhD
- Intensive Lifestyle Intervention for Type 2 Diabetes—Reply JAMA Comment & Response December 26, 2017 Mathias Ried-Larsen, PhD; Mette Yun Johansen, MSc; Bente Klarlund Pedersen, DMSc
See More About
Select your interests.
Customize your JAMA Network experience by selecting one or more topics from the list below.
- Academic Medicine
- Acid Base, Electrolytes, Fluids
- Allergy and Clinical Immunology
- American Indian or Alaska Natives
- Anesthesiology
- Anticoagulation
- Art and Images in Psychiatry
- Artificial Intelligence
- Assisted Reproduction
- Bleeding and Transfusion
- Caring for the Critically Ill Patient
- Challenges in Clinical Electrocardiography
- Climate and Health
- Climate Change
- Clinical Challenge
- Clinical Decision Support
- Clinical Implications of Basic Neuroscience
- Clinical Pharmacy and Pharmacology
- Complementary and Alternative Medicine
- Consensus Statements
- Coronavirus (COVID-19)
- Critical Care Medicine
- Cultural Competency
- Dental Medicine
- Dermatology
- Diabetes and Endocrinology
- Diagnostic Test Interpretation
- Drug Development
- Electronic Health Records
- Emergency Medicine
- End of Life, Hospice, Palliative Care
- Environmental Health
- Equity, Diversity, and Inclusion
- Facial Plastic Surgery
- Gastroenterology and Hepatology
- Genetics and Genomics
- Genomics and Precision Health
- Global Health
- Guide to Statistics and Methods
- Hair Disorders
- Health Care Delivery Models
- Health Care Economics, Insurance, Payment
- Health Care Quality
- Health Care Reform
- Health Care Safety
- Health Care Workforce
- Health Disparities
- Health Inequities
- Health Policy
- Health Systems Science
- History of Medicine
- Hypertension
- Images in Neurology
- Implementation Science
- Infectious Diseases
- Innovations in Health Care Delivery
- JAMA Infographic
- Law and Medicine
- Leading Change
- Less is More
- LGBTQIA Medicine
- Lifestyle Behaviors
- Medical Coding
- Medical Devices and Equipment
- Medical Education
- Medical Education and Training
- Medical Journals and Publishing
- Mobile Health and Telemedicine
- Narrative Medicine
- Neuroscience and Psychiatry
- Notable Notes
- Nutrition, Obesity, Exercise
- Obstetrics and Gynecology
- Occupational Health
- Ophthalmology
- Orthopedics
- Otolaryngology
- Pain Medicine
- Palliative Care
- Pathology and Laboratory Medicine
- Patient Care
- Patient Information
- Performance Improvement
- Performance Measures
- Perioperative Care and Consultation
- Pharmacoeconomics
- Pharmacoepidemiology
- Pharmacogenetics
- Pharmacy and Clinical Pharmacology
- Physical Medicine and Rehabilitation
- Physical Therapy
- Physician Leadership
- Population Health
- Primary Care
- Professional Well-being
- Professionalism
- Psychiatry and Behavioral Health
- Public Health
- Pulmonary Medicine
- Regulatory Agencies
- Reproductive Health
- Research, Methods, Statistics
- Resuscitation
- Rheumatology
- Risk Management
- Scientific Discovery and the Future of Medicine
- Shared Decision Making and Communication
- Sleep Medicine
- Sports Medicine
- Stem Cell Transplantation
- Substance Use and Addiction Medicine
- Surgical Innovation
- Surgical Pearls
- Teachable Moment
- Technology and Finance
- The Art of JAMA
- The Arts and Medicine
- The Rational Clinical Examination
- Tobacco and e-Cigarettes
- Translational Medicine
- Trauma and Injury
- Treatment Adherence
- Ultrasonography
- Users' Guide to the Medical Literature
- Vaccination
- Venous Thromboembolism
- Veterans Health
- Women's Health
- Workflow and Process
- Wound Care, Infection, Healing
Others Also Liked
- Download PDF
- X Facebook More LinkedIn
Johansen MY , MacDonald CS , Hansen KB, et al. Effect of an Intensive Lifestyle Intervention on Glycemic Control in Patients With Type 2 Diabetes : A Randomized Clinical Trial . JAMA. 2017;318(7):637–646. doi:10.1001/jama.2017.10169
Manage citations:
© 2024
- Permissions
Effect of an Intensive Lifestyle Intervention on Glycemic Control in Patients With Type 2 Diabetes : A Randomized Clinical Trial
- 1 Centre of Inflammation and Metabolism, Rigshospitalet, University of Copenhagen, Copenhagen, Denmark
- 2 Centre for Physical Activity Research, Rigshospitalet, University of Copenhagen, Copenhagen, Denmark
- 3 CopenRehab, University of Copenhagen, Copenhagen, Denmark
- 4 Musculoskeletal Statistics Unit, Parker Institute, Copenhagen, Denmark
- 5 Frederiksberg Hospital, Copenhagen, Denmark
- 6 Department of Clinical Physiology and Nuclear Medicine, Herlev Hospital, Herlev, Denmark
- 7 Translational Research and Early Clinical Development, Cardiovascular and Metabolic Research, AstraZeneca, Mölndal, Sweden
- 8 Danish Diabetes Academy, Odense University Hospital, Odense, Denmark
- Comment & Response Intensive Lifestyle Intervention for Type 2 Diabetes Dario Giugliano, MD, PhD; Maria Ida Maiorino, MD, PhD; Katherine Esposito, MD, PhD JAMA
- Comment & Response Intensive Lifestyle Intervention for Type 2 Diabetes—Reply Mathias Ried-Larsen, PhD; Mette Yun Johansen, MSc; Bente Klarlund Pedersen, DMSc JAMA
Question Can an intensive lifestyle intervention achieve glycemic control comparable with standard care in patients with type 2 diabetes?
Findings In this randomized clinical trial of 98 adults with type 2 diabetes diagnosed for less than 10 years, and which was designed to assess equivalence, the lifestyle intervention vs standard care resulted in a mean change in hemoglobin A 1c level of −0.31% vs −0.04%, respectively. The 95% CI around the difference (−0.52% to −0.01%) exceeded the prespecified equivalence margin of ±0.4%.
Meaning An intensive lifestyle intervention did not meet the criterion for equivalence for glycemic control, but the direction of findings suggests potential benefit.
Importance It is unclear whether a lifestyle intervention can maintain glycemic control in patients with type 2 diabetes.
Objective To test whether an intensive lifestyle intervention results in equivalent glycemic control compared with standard care and, secondarily, leads to a reduction in glucose-lowering medication in participants with type 2 diabetes.
Design, Setting, and Participants Randomized, assessor-blinded, single-center study within Region Zealand and the Capital Region of Denmark (April 2015-August 2016). Ninety-eight adult participants with non–insulin-dependent type 2 diabetes who were diagnosed for less than 10 years were included. Participants were randomly assigned (2:1; stratified by sex) to the lifestyle group (n = 64) or the standard care group (n = 34).
Interventions All participants received standard care with individual counseling and standardized, blinded, target-driven medical therapy. Additionally, the lifestyle intervention included 5 to 6 weekly aerobic training sessions (duration 30-60 minutes), of which 2 to 3 sessions were combined with resistance training. The lifestyle participants received dietary plans aiming for a body mass index of 25 or less. Participants were followed up for 12 months.
Main Outcomes and Measures Primary outcome was change in hemoglobin A 1c (HbA 1c ) from baseline to 12-month follow-up, and equivalence was prespecified by a CI margin of ±0.4% based on the intention-to-treat population. Superiority analysis was performed on the secondary outcome reductions in glucose-lowering medication.
Results Among 98 randomized participants (mean age, 54.6 years [SD, 8.9]; women, 47 [48%]; mean baseline HbA 1c , 6.7%), 93 participants completed the trial. From baseline to 12-month follow-up, the mean HbA 1c level changed from 6.65% to 6.34% in the lifestyle group and from 6.74% to 6.66% in the standard care group (mean between-group difference in change of −0.26% [95% CI, −0.52% to −0.01%]), not meeting the criteria for equivalence ( P = .15). Reduction in glucose-lowering medications occurred in 47 participants (73.5%) in the lifestyle group and 9 participants (26.4%) in the standard care group (difference, 47.1 percentage points [95% CI, 28.6-65.3]). There were 32 adverse events (most commonly musculoskeletal pain or discomfort and mild hypoglycemia) in the lifestyle group and 5 in the standard care group.
Conclusions and Relevance Among adults with type 2 diabetes diagnosed for less than 10 years, a lifestyle intervention compared with standard care resulted in a change in glycemic control that did not reach the criterion for equivalence, but was in a direction consistent with benefit. Further research is needed to assess superiority, as well as generalizability and durability of findings.
Trial Registration clinicaltrials.gov Identifier: NCT02417012
First-line treatment of type 2 diabetes includes diet, physical activity, and weight loss prior to or in parallel with initiation of pharmacological therapy. 1 Whereas medication is effective in lowering hemoglobin A 1c (HbA 1c ) 2 in patients with type 2 diabetes, it is also associated with potential adverse drug interactions, 3 discomforts, 4 increased economic costs 5 and decreased quality of life. 6 Therefore, lifestyle interventions are needed that are able to maintain glycemic control to at least the same extent as medication.
In the Action for Health in Diabetes (Look AHEAD) study, reductions in HbA 1c and glucose-lowering medication were observed after 12 months of lifestyle intervention compared with diabetes support and education. 7 However, the clinical relevance of this and other lifestyle interventions is limited due to self-reported medication changes, use of drug-assisted weight loss and weight maintenance, and the subjective nature of unblinded, target-driven regulation of glucose-lowering medication. 8 - 10 To our knowledge, only 2 studies have implemented objective target-driven regulation of glucose-lowering medication when assessing the effect of lifestyle in patients with type 2 diabetes. 11 , 12 A randomized clinical trial showed that an intensive diet intervention maintained glycemic control in patients with type 2 diabetes, preventing an increased need for glucose-lowering medication. 11 The addition of walking provided no further improvements. 11 In contrast, improvement in glycemic control was reported when adding supervised exercise, but with no concurrent reduction in glucose-lowering medication. 12
The objective of this randomized clinical trial was to test the hypothesis that an intensive lifestyle intervention is equivalent compared with standard care in maintaining glycemic control in participants with type 2 diabetes diagnosed less than 10 years, and secondarily leads to a reduction in glucose-lowering medication.
This study was a single-center, assessor-blinded, randomized clinical trial that took place in Region Zealand and the Capital Region of Denmark from April 2015 to August 2016. The full protocol is included in Supplement 1 . This study was approved by the Scientific Ethical Committee at the Capital Region of Denmark. Guidelines from the Helsinki Declaration were followed and reporting in this article is aligned with CONSORT standards. All participants provided oral and written informed consent.
Participants were recruited via media and the Danish Diabetes Association and screened through telephone interview and medical examination. Inclusion criteria were type 2 diabetes diagnosed less than 10 years, body mass index (BMI; calculated as weight in kilograms divided by height in meters squared) of 25 to 40, and taking 2 or fewer glucose-lowering medications. Exclusion criteria were HbA 1c level greater than 9%, insulin-dependence, or presence of 1 or more of the following complications: diabetic retinopathy, macroalbuminuria (urine albumin-creatinine ratio ≥300 mg/g) or nephropathy (plasma creatinine ≥1.47 mg/dL [to convert to μmol/L, multiply by 88.4]). At least 6 weeks prior to baseline measurements, all participants had their glucose-lowering, lipid-lowering, and blood pressure–lowering medications titrated by the study endocrinologist to obtain prespecified treatment targets. 13 Response to the medical standardization did not constitute reason for exclusion. Medical standardization was performed to assess the effect of the lifestyle intervention without amplifying the result due to poorly regulated HbA 1c levels at baseline. The data were collected at Rigshospitalet, Copenhagen, Denmark.
Participants were randomized in permuted blocks of 3 and 6, stratified by sex, to either the lifestyle group or the standard care group in a 2:1 ratio. A computer-generated random number sequence was created by an independent statistician. The sequence was given to an external data manager with no involvement in the study procedures and concealed on a password-protected computer. After baseline measurements, participants were given consecutive numbers, which were forwarded to the external data manager, who subsequently returned the corresponding allocation to the study nurse. Blinding of the participants and the study nurse was not possible after group allocation. However, the study nurse solely delivered the standard care treatment and had no role in assessing the treatment actions, analyzing, or interpreting the data. All test personnel and adjudicators of outcomes were blinded.
All participants received standard care that included medical counseling, education in type 2 diabetes, and lifestyle advice by the study nurse at baseline and every 3 months for 12 months. The study endocrinologist, who regulated all glucose-lowering, lipid-lowering, and blood pressure–lowering medication, was blinded to group allocation and received all clinical variables from the study nurse. To minimize the risk of bias, prespecified treatment targets and algorithms 13 for glucose-lowering, lipid-lowering, and blood pressure–lowering medication were followed by the study endocrinologist to reach standardization across groups. The treatment target for glycemic control was 6.5% for HbA 1c level, and if this target was reached, the glucose-lowering medication dose was halved. If the HbA 1c level was unchanged or lower at the following medical consultation, the glucose-lowering medication was discontinued. If the participant experienced hypoglycemic events between medical consultations, they would contact the study nurse, and the blinded study endocrinologist would consider whether a reduction in glucose-lowering medication was necessary. If HbA 1c level exceeded 7.5%, the glucose-lowering medication was increased according to the prespecified algorithm. 13
The lifestyle participants additionally received an intensive lifestyle intervention, described in detail previously, 13 which consisted of 5 to 6 weekly aerobic sessions (duration 30-60 minutes), of which 2 to 3 sessions were combined with resistance training. For the first 4 months, all exercise sessions were supervised, and supervision was progressively reduced during the 12 months. All supervised training was performed in groups of 4 to 8 participants. Participants were given an individual dietary plan with a macronutrient distribution of 45% to 60% carbohydrate, 15% to 20% protein, and 20% to 35% fat (<7% saturated fat). During the first 4 months the total energy intake was restricted. Individual and group-based dietary counseling were offered by clinical dieticians and progressively reduced during the 12 months. Additionally, participants were encouraged to be physically active in their leisure time (≥10 000 steps per day). Steps and exercise sessions were objectively monitored with a smartwatch (Polar V800).
The primary outcome was change in HbA 1c level from baseline to 12-month follow-up. The secondary outcome was reduction in glucose-lowering medication from baseline to 12-month follow-up. Exploratory outcomes included changes from baseline to 12-month follow-up in total cholesterol, low-density lipoprotein (LDL) cholesterol, high-density lipoprotein (HDL) cholesterol, triglycerides, systolic and diastolic blood pressure, fasting insulin, fasting glucose, 2-hour glucose concentration following an oral glucose tolerance test, maximal oxygen uptake (V̇ o 2max ), weight, BMI, fat mass (total and abdominal), and lean body mass. We also explored the reduction in blood pressure lowering and lipid-lowering medication. Additionally, we analyzed the proportions of participants who discontinued their blinded, target-driven, glucose-lowering medical therapy between baseline to 12-month follow-up as well as the proportion of participants who increased their treatment according to the same prespecified algorithm between baseline to 12-month follow-up. In case of any adverse events the participants in the lifestyle group were encouraged to contact the intervention center and those in the standard care group were advised to contact the study nurse. At each medical consultation, the study nurse interviewed all participants about potential adverse events. All outcomes were presented to a blinded, adjudicated writing committee and group allocation was only revealed when consensus was achieved.
Measurements were performed in 1 laboratory and biochemical analyses were completed at the central laboratory (Rigshospitalet, Denmark) using standard procedures ( Supplement 1 ). Primary, secondary, and exploratory measurements were performed in 1 day, except the 2-hour oral glucose tolerance test, which was performed on a separate day 48 hours after discontinuation of glucose-lowering medication and exercise cessation.
The predefined margin of equivalences was ±0.4% for HbA 1c level in relation to between-group comparison and was decided by 2 clinical content experts (AAV and KBH). This margin was based on half of the effect that was considered a clinically relevant reduction in HbA 1c level leading to a reduction in the risk of microvascular complications in patients with newly diagnosed type 2 diabetes. 14 , 15 Moreover, the minimum detectable significant change in HbA 1c level and what is recommended as an acceptable noninferiority margin defined by the US Food and Drug Administration were considered. 16 , 17 This margin has been widely used in trials testing glucose-lowering medications in patients with type 2 diabetes. 18 - 21
In a two 1-sided test analysis for additive equivalence of 2-sample normal means with bounds ±0.4% [95% CI] for the mean difference and a significance level of .05, assuming a mean difference of 0 and a common SD of 0.9%, a total sample size of 120 participants assuming an allocation ratio of 2:1 would correspond to a power less than 50% (0.476). However, based on a superiority approach (in potential favor of standard care) it was decided (MR-L and RC) that a 95% CI excluding differences between groups of greater than 0.4 units would be interpreted as indicating the absence of a clinically meaningful difference. 22 According to the principle of sensitivity, a concept that refers to the sensitivity of the overall conclusions to various limitations of the data, assumptions, and analytic approaches to data analysis, 23 our estimates showed that including only 90 participants (60:30) would result in reasonable confidence limits. Thus, the sample size was based on feasibility within the local context enabling up to 120 participants to be enrolled. The sample size was truncated based on a formal stop rule defined as 24 months (August 2015) prior to the study end date of the preplanned 24-month follow-up (August 2017). 13
The full statistical analysis plan is available in Supplement 2 . The analysis of the primary outcome was performed according to the intention-to-treat principle. Imputations were not used to replace missing data in the primary analysis, but were included in a sensitivity analysis to assess missing data. According to Piaggio et al, 24 equivalence is declared if the entire 2-sided CI ([1-α] × 100%) is included within the equivalence margin. Accordingly, a 2-sided 95% CI for the difference in change in HbA 1c level from baseline to 12-month follow-up between groups was derived from a repeated-measures mixed linear model and equivalence was declared if the 95% CI of HbA 1c level change was completely within the prespecified equivalence range (−0.4% to +0.4%). 25 Equivalence was tested using two 1-sided tests. 26 The repeated-measures mixed linear models included participants as a random effect, with fixed factors for group (2 levels), time (4 levels for the continuous outcomes [ie, change in HbA 1c level from baseline]), and the corresponding interactions (adjusted for baseline values and sex). To assess the adequacy of the linear models, features were investigated via the predicted values and the residuals. Equivalence results are expressed with estimates of the group differences in the change from baseline and 95% CIs to represent precision of the estimates and P values for equivalence.
The analyses of the secondary outcome and the exploratory outcomes were based on a superiority assumption and presented as mean difference with 95% CI and P values for superiority. The secondary outcome (reduction in glucose-lowering medication) was reported as the between-group difference in the proportion of the participants (risk difference, percentage point), who reduced their need for glucose-lowering medication according to the prespecified algorithm from baseline to 12-month follow-up. 13 A reduction from baseline was scored as 0 (no reduction) or 1 (a reduction). Additionally, we explored the between-group difference in the proportion of participants, who completely discontinued their blinded, target-driven, glucose-lowering medical therapy from baseline to 12-month follow-up as well as the difference in the proportion of participants who increased their treatment from baseline to 12-month follow-up according to the same prespecified algorithm. The difference in proportion between the groups reducing, discontinuing, or increasing their medication at 12-month follow-up compared with baseline was tested using a χ 2 test. Difference in the median change of the medication score from baseline to 12-month follow-up was tested using a Wilcoxon rank-sum test.
Sensitivity analyses included the baseline-observation carried forward imputation technique, complete-case and multiple linear imputation analysis. In the multiple imputation procedure the missing values at 12-month follow-up were imputed including all covariates from the main model (eTable 2 in Supplement 3 ). β-Coefficients and standard errors were obtained from 30 imputed data sets and adjusted for the variability between imputations. 27 A per-protocol population was defined by adherence to medication and attendance at medical consultations in both groups and the completion of 70% or more of the prescribed exercise sessions for the lifestyle participants.
Statistical analyses were performed using STATA/IC (StataCorp), version 13.1, and the statistical significance level was set at α <.05 (2-tailed). A statistical analysis plan was described prior to analysis.
Between April 2015 and August 2015, a total of 878 participants were screened for inclusion, and, of these, 356 were excluded primarily due to having a diagnosis of type 2 diabetes for more than 10 years and insulin-dependence. Additionally, 382 participants withdrew primarily because of geographical distance. Of the 98 participants who were enrolled in the study, 64 participants were allocated to the lifestyle group and 34 participants to the standard care group ( Figure 1 ). At baseline, the participants had a mean age of 54.6 years (SD, 8.9) and mean HbA 1c level of 6.7% ( Table 1 ). At 12-month follow-up, no significant difference ( P = .22) in retention rates was observed between the groups (97% for the lifestyle group and 91% for the standard care group).
From baseline to 12-month follow-up, the mean HbA 1c level changed from 6.65% to 6.34% in the lifestyle group, and from 6.74% to 6.66% in the standard care group, with a mean between-group difference for change of −0.26% (95% CI, −0.52% to −0.01%). For the primary outcome, the difference in change for HbA 1c level from baseline to 12-month follow-up was not contained within the equivalence margin of ±0.4%, thus equivalence could not be declared in the intention-to-treat analysis ( P = .15) ( Table 2 ). In the per-protocol analysis the mean change in HbA 1c level decreased from 6.71% to 6.15% in the lifestyle group, and from 6.71% to 6.50% in the standard care group with a mean between-group difference of −0.36% [95% CI, −0.65% to −0.08%] ( P = .18). Thus, equivalence could not be declared (eTable 1 in Supplement 3 ). The analysis of the secondary outcome showed that the proportion of participants, who reduced the use of glucose-lowering medication from baseline to 12-month follow-up was higher in the lifestyle group (73%) compared with the standard care group (26%) (risk difference, 47.1% [95% CI, 28.6% to 65.3%]) ( Table 2 ), with a number needed to treat of 2.1 (95% CI, 1.6 to 3.5). The least-squares mean of HbA 1c level is shown in Figure 2 , and the mean reduction in glucose-lowering medication from baseline to 12-month follow-up is shown in Figure 3 .
Exploratory outcomes are presented in Table 2 . No group differences were observed in relation to reductions in lipid-lowering or blood pressure–lowering medication during the 12 months. Adherence to lipid-lowering and blood pressure–lowering medication are reported in eTables 4 and 5 in Supplement 3 , whereas the proportion of participants that reduced the use of lipid-lowering and blood pressure–lowering medication is illustrated in eFigures 1 and 2 in Supplement 3 . Post hoc analysis showed that more participants in the lifestyle group eliminated the use of glucose-lowering medication (56.3%) than the standard care group (14.7%) from baseline to 12-month follow-up (risk difference, 41.5% [95% CI, 24.5% to 58.6%]). A larger proportion of the standard care participants increased the use of glucose-lowering medication (44.1%) compared with lifestyle participants (10.9%) (risk difference, 33.2% [95% CI, 51.5% to 14.8%]). Thirty-two adverse events occurred in the lifestyle group ( Table 3 ). One participant in the lifestyle group experienced atrial fibrillation. Several sensitivity analyses confirmed the robustness of the primary analysis (eTable 2 in Supplement 3 ).
At 12-month follow-up, 71% of lifestyle participants and 83% of standard care participants adhered to the prescribed glucose-lowering medication (eTable 3 in Supplement 3 ). The lifestyle participants completed 82% of the prescribed exercise sessions, both aerobic and resistance training, during the 12 months (eTable 6 in Supplement 3 ) and attendance was 78% at the individual and dietary group sessions throughout year 1 (eTable 7 in Supplement 3 ).
The main finding was that an intensive lifestyle intervention was nonequivalent compared with standard care in relation to maintaining glycemic control, with the modest reduction in HbA 1c favoring the lifestyle group. Additionally, the lifestyle intervention led to a substantial and parallel reduction in glucose-lowering medication.
The finding that the lifestyle intervention resulted in a rejection of the equivalence hypothesis may appear unexpected as the utilized initial medical titration resulted in all participants being very close to the HbA 1c level treatment target at baseline measurement prior to the lifestyle intervention. Additionally, the treat-to-target approach intentionally induced a ceiling effect on HbA 1c level in both groups. Earlier studies have also addressed the effect of lifestyle on glycemic control and target-driven regulation of glucose-lowering medication. However, the results have been conflicting 11 , 12 and may to some extent be explained by reliance on advice-based exercise interventions 11 as opposed to supervision of exercise. 28 Furthermore, greater improvement in glycemic control is associated with higher levels of physical activity, 29 beyond the current physical activity recommendations for patients with type 2 diabetes. 30
In the Look AHEAD study, a baseline HbA 1c level of 7.2% was reduced by 0.6% in the lifestyle group after 1 year. 7 The corresponding numbers in the current study were 6.7% at baseline and −0.3% at year 1. The proportion of participants in the lifestyle group who reduced the use of glucose-lowering medication after 12 months was 73.5% in this study compared with 7.8% in the Look AHEAD study. 7 This may be due to several factors including different levels of supervised exercise and total exercise volume (duration, frequency, and intensity), which in this study far exceeded what was implemented in the Look AHEAD study. 31 The use of drug-assisted weight loss in Look AHEAD also differed markedly from this study and may limit the true effect of lifestyle intervention. Besides an extensive exercise intervention, the blinded, highly standardized, algorithm and target-driven approach to regulate glucose-lowering medication in both the lifestyle and standard care group was a major strength of this study compared with other studies. However, more adverse events were observed in the lifestyle group compared with standard care, which may be ascribed to higher susceptibility in this group in relation to, for example, mild hypoglycemia because of the combination of lifestyle and medical therapy.
This study has several limitations. First, only participants with type 2 diabetes diagnosed for less than 10 years were included. Prolonged diabetes duration, poor glycemic control, and insulin dependence 8 , 12 , 32 may reflect a more progressive disease state. As observed in the Look AHEAD study, better glycemic control and short diabetes duration at baseline were associated with a higher probability of meeting optimal care goals and remission of type 2 diabetes at 1-year follow-up. 8 , 33 Thus, the inclusion criteria in this study may limit generalizability. Second, the lifestyle intervention included several lifestyle elements, which challenges the interpretation of individual effects of each intervention component. Third, the self-reported dietary intake in this study is subject to biases and limitations. 34 Fourth, to be able to discriminate between the combined effect of medication and lifestyle in contrast to medication alone, a prespecified treatment algorithm using recommended first-line medical treatments 35 was employed, which led to a limited number of medications. Therefore, it is not possible to generalize the results to other combinations of glucose-lowering medications.
Among adults with type 2 diabetes diagnosed for less than 10 years, a lifestyle intervention compared with standard care resulted in a change in glycemic control that did not reach the criterion for equivalence, but was in a direction consistent with benefit. Further research is needed to assess superiority, as well as generalizability and durability of findings.
Corresponding Author: Mathias Ried-Larsen, PhD, Centre of Inflammation and Metabolism and Centre for Physical Activity Research, Rigshospitalet 7641, Blegdamsvej 9, DK-2100 Copenhagen, Denmark ( [email protected] ).
Accepted for Publication: July 10, 2017.
Author Contributions: Dr Ried-Larsen had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Concept and design: Johansen, MacDonald, K. Hansen, Karstoft, Christensen, Zacho, Vaag, B. Pedersen, Ried-Larsen.
Acquisition, analysis, or interpretation of data: Johansen, MacDonald, Karstoft, Christensen, Langberg, M. Pedersen, L. Hansen, Wedell-Neergaard, Nielsen, Iepsen, Vaag, Ried-Larsen.
Drafting of the manuscript: Johansen, MacDonald, Ried-Larsen.
Critical revision of the manuscript for important intellectual content: Johansen, K. Hansen, Karstoft, Christensen, M. Pedersen, L. Hansen, Zacho, Wedell-Neergaard, Nielsen, Iepsen, Langberg, Vaag, B. Pedersen, Ried-Larsen.
Statistical analysis: Christensen, Ried-Larsen.
Obtained funding: Langberg, Vaag, B. Pedersen, Ried-Larsen.
Administrative, technical, or material support: Johansen, M. Pedersen, L. Hansen, Wedell-Neergaard, Iepsen, Vaag, Ried-Larsen.
Supervision: Johansen, Karstoft, Christensen, Zacho, M. Pedersen, Langberg, Vaag, B. Pedersen, Ried-Larsen.
Conflict of Interest Disclosures: All authors have completed and submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Allan Vaag was appointed vice president for AstraZeneca’s Translational Research and Early Clinical Development during the completion of the study, but remained in the scientific steering committee of this study. Dr Christensen’s employer, the Parker Institute, Bispebjerg, and Frederiksberg Hospital, is supported by core grant OCAY-13-309 from the Oak Foundatian; he reports receiving personal fees from Abbott, AbbVie, Amgen, Axellus A/S, Bayer HealthCare Pharmaceuticals, Biogen Idec, Bristol-Myers Squibb, Cambridge Weight Plan, Celgene, Eli Lilly, Hospira, Ipsen, Janssen, Laboratories Expanscience, Merck Sharp & Dohme, Mundipharma, Norpharma, Novartis, Orkla Health, Pfizer, Roche, Rottapharm-Madaus, Sobi, Takeda, and Wyeth; personal fees from employment from Research Unit for Musculoskeletal Function and Physiotherapy, Institute of Sports Science and Clinical Biomechanics, and the University of Southern Denmark; grants pending and grant funding from Axellus A/S, AbbVie, Cambridge Weight Plan, Janssen, Merck Sharp & Dohme, Mundipharma, Novartis, and Roche; and being involved in many health care initiatives and research that could benefit from wide uptake of this publication including Cochrane, Outcome Measures in Rheumatology, International Dermatology Outcome Measures, RADS, and the Grading of Recommendations Assessment, Development and Evaluation Working Group. No other disclosures were reported.
Funding/Support: This project was funded by TrygFonden. The Centre for Physical Activity Research (CFAS) is supported by a grant from TrygFonden. Centre for Inflammation and Metabolism/CFAS is a member of the Danish Center for Strategic Research in Type 2 Diabetes (the Danish Council for Strategic Research, grants 09-067009 and 09-075724). The Contour Next glucose monitors were provided by Bayer A/S, Copenhagen, Denmark. This work was also supported by a grant from the Danish Diabetes Academy, which is supported by the Novo Nordisk Foundation (Dr Ried-Larsen).
Role of the Funder/Sponsor: The funders had no role in design and conduct of the study; collection, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
Reproducible Research Statement: Individual participant data that underlie the results reported in this article, after deidentification (text, tables, figures, and appendices), the study protocol, statistical analysis plan, and analytic code can be shared beginning 6 months after publication of 24-month follow-up article and ending 5 years following this article to researchers, who provide a methodologically sound proposal. Proposals should be directed to Mathias Ried-Larsen ( [email protected] ). To gain access, data requestors will need to sign a data access agreement.
Additional Contributions: We thank all participants for their effort, the supportive approach from the participants’ families, the Danish Diabetes Association for their assistance, and current and former staff at the Centre for Physical Activity Research, and the intervention assistants, physical trainers, and the clinical dietitians for their contribution to this study. They did not receive compensation for their contributions outside of their salaries. We also thank Rasmus Ø. Nielsen, PhD (Aarhus University), for helping with randomization and allocation procedures. He did not receive compensation for his contribution.
- Register for email alerts with links to free full-text articles
- Access PDFs of free articles
- Manage your interests
- Save searches and receive search alerts
Thank you for visiting nature.com. You are using a browser version with limited support for CSS. To obtain the best experience, we recommend you use a more up to date browser (or turn off compatibility mode in Internet Explorer). In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript.
- View all journals
- Explore content
- About the journal
- Publish with us
- Sign up for alerts
- Review Article
- Published: 20 July 2020
Diet and exercise in the prevention and treatment of type 2 diabetes mellitus
- Faidon Magkos ORCID: orcid.org/0000-0002-1312-7364 1 ,
- Mads F. Hjorth ORCID: orcid.org/0000-0001-9440-2737 1 &
- Arne Astrup ORCID: orcid.org/0000-0001-8968-8996 1
Nature Reviews Endocrinology volume 16 , pages 545–555 ( 2020 ) Cite this article
19k Accesses
234 Citations
120 Altmetric
Metrics details
- Type 2 diabetes
Evidence from observational studies and randomized trials suggests that prediabetes and type 2 diabetes mellitus (T2DM) can develop in genetically susceptible individuals in parallel with weight (that is, fat) gain. Accordingly, studies show that weight loss can produce remission of T2DM in a dose-dependent manner. A weight loss of ~15 kg, achieved by calorie restriction as part of an intensive management programme, can lead to remission of T2DM in ~80% of patients with obesity and T2DM. However, long-term weight loss maintenance is challenging. Obesity and T2DM are associated with diminished glucose uptake in the brain that impairs the satiating effect of dietary carbohydrate; therefore, carbohydrate restriction might help maintain weight loss and maximize metabolic benefits. Likewise, increases in physical activity and fitness are an important contributor to T2DM remission when combined with calorie restriction and weight loss. Preliminary studies suggest that a precision dietary management approach that uses pretreatment glycaemic status to stratify patients can help optimize dietary recommendations with respect to carbohydrate, fat and dietary fibre. This approach might lead to improved weight loss maintenance and glycaemic control. Future research should focus on better understanding the individual response to dietary treatment and translating these findings into clinical practice.
Studies show that weight loss can produce remission of type 2 diabetes mellitus (T2DM) in a dose-dependent manner.
In patients with T2DM and obesity, weight loss of ~15 kg, achieved by an intensive management programme involving calorie restriction, can lead to remission of T2DM in ~80% of individuals.
Long-term maintenance of weight loss and metabolic health in people who have undergone intensive lifestyle intervention is challenging.
Carbohydrate restriction might help maintain weight loss and maximize metabolic benefits.
When combined with calorie restriction and weight loss, increases in physical activity and fitness are an important contributor to T2DM remission.
Preliminary work suggests that pretreatment glycaemic status could be used to stratify patients in order to optimize dietary recommendations.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
24,99 € / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
195,33 € per year
only 16,28 € per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
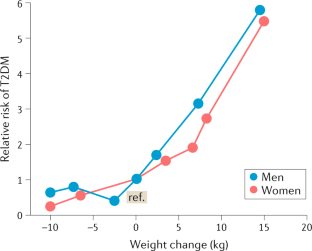
Similar content being viewed by others

Dietary weight loss-induced improvements in metabolic function are enhanced by exercise in people with obesity and prediabetes
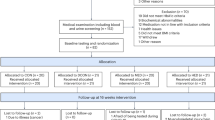
Effects of different doses of exercise and diet-induced weight loss on beta-cell function in type 2 diabetes (DOSE-EX): a randomized clinical trial

Effect of time restricted eating on body weight and fasting glucose in participants with obesity: results of a randomized, controlled, virtual clinical trial
International Diabetes Federation. IDF Diabetes Atlas 9th edn (International Diabetes Federation, 2019).
Zhu, Y. et al. Racial/ethnic disparities in the prevalence of diabetes and prediabetes by BMI: patient outcomes research to advance learning (PORTAL) multisite cohort of adults in the U.S. Diabetes Care 42 , 2211–2219 (2019).
Article PubMed PubMed Central Google Scholar
Magkos, F. Metabolically healthy obesity: what’s in a name? Am. J. Clin. Nutr. 110 , 533–539 (2019). A review of the dissociation between excess body weight and metabolic dysfunction .
Article PubMed Google Scholar
Willett, W. C., Dietz, W. H. & Colditz, G. A. Guidelines for healthy weight. N. Engl. J. Med. 341 , 427–434 (1999).
Article CAS PubMed Google Scholar
Prospective Studies Collaboration. Body-mass index and cause-specific mortality in 900,000 adults: collaborative analyses of 57 prospective studies. Lancet 373 , 1083–1096 (2009).
Article PubMed Central Google Scholar
Chan, J. M., Rimm, E. B., Colditz, G. A., Stampfer, M. J. & Willett, W. C. Obesity, fat distribution, and weight gain as risk factors for clinical diabetes in men. Diabetes Care 17 , 961–969 (1994).
Colditz, G. A., Willett, W. C., Rotnitzky, A. & Manson, J. E. Weight gain as a risk factor for clinical diabetes mellitus in women. Ann. Intern. Med. 122 , 481–486 (1995).
Hu, F. B. et al. Diet, lifestyle, and the risk of type 2 diabetes mellitus in women. N. Engl. J. Med. 345 , 790–797 (2001).
Kendall, D. M., Cuddihy, R. M. & Bergenstal, R. M. Clinical application of incretin-based therapy: therapeutic potential, patient selection and clinical use. Am. J. Med. 122 , S37–S50 (2009).
Mittendorfer, B., Magkos, F., Fabbrini, E., Mohammed, B. S. & Klein, S. Relationship between body fat mass and free fatty acid kinetics in men and women. Obesity 17 , 1872–1877 (2009).
Conte, C. et al. Multiorgan insulin sensitivity in lean and obese subjects. Diabetes Care 35 , 1316–1321 (2012).
Article CAS PubMed PubMed Central Google Scholar
Wilman, H. R. et al. Characterisation of liver fat in the UK Biobank cohort. PLoS One 12 , e0172921 (2017).
Article PubMed PubMed Central CAS Google Scholar
Pienkowska, J. et al. MRI assessment of ectopic fat accumulation in pancreas, liver and skeletal muscle in patients with obesity, overweight and normal BMI in correlation with the presence of central obesity and metabolic syndrome. Diabetes Metab. Syndr. Obes. 12 , 623–636 (2019).
Tabak, A. G. et al. Trajectories of glycaemia, insulin sensitivity, and insulin secretion before diagnosis of type 2 diabetes: an analysis from the Whitehall II study. Lancet 373 , 2215–2221 (2009). A prospective study of the temporal changes in metabolic function and glucose control along the natural history of T2DM .
Weir, G. C. & Bonner-Weir, S. Five stages of evolving beta-cell dysfunction during progression to diabetes. Diabetes 53 (Suppl. 3), 16–21 (2004).
Article Google Scholar
Astrup, A. & Finer, N. Redefining type 2 diabetes: ‘diabesity’ or ‘obesity dependent diabetes mellitus’? Obes. Rev. 1 , 57–59 (2000).
Leitner, D. R. et al. Obesity and type 2 diabetes: two diseases with a need for combined treatment strategies — EASO can lead the way. Obes. Facts 10 , 483–492 (2017).
Sjostrom, L. Review of the key results from the Swedish Obese Subjects (SOS) trial — a prospective controlled intervention study of bariatric surgery. J. Intern. Med. 273 , 219–234 (2013).
Jans, A. et al. Duration of type 2 diabetes and remission rates after bariatric surgery in Sweden 2007–2015: a registry-based cohort study. PLoS Med. 16 , e1002985 (2019).
Davies, M. J. et al. Efficacy of liraglutide for weight loss among patients with type 2 diabetes: the SCALE diabetes randomized clinical trial. JAMA 314 , 687–699 (2015).
Madsbad, S. & Holst, J. J. GLP-1 as a mediator in the remission of type 2 diabetes after gastric bypass and sleeve gastrectomy surgery. Diabetes 63 , 3172–3174 (2014).
MacDonald, P. E. et al. The multiple actions of GLP-1 on the process of glucose-stimulated insulin secretion. Diabetes 51 (Suppl. 3), 434–442 (2002).
Magkos, F. et al. Effects of moderate and subsequent progressive weight loss on metabolic function and adipose tissue biology in humans with obesity. Cell Metab. 23 , 591–601 (2016). A randomized controlled trial of the effects of progressive diet-induced weight loss on body composition and metabolic function .
Wing, R. R. et al. Long-term effects of modest weight loss in type II diabetic patients. Arch. Intern. Med. 147 , 1749–1753 (1987).
Henry, R. R., Wallace, P. & Olefsky, J. M. Effects of weight loss on mechanisms of hyperglycemia in obese non-insulin-dependent diabetes mellitus. Diabetes 35 , 990–998 (1986).
Markovic, T. P. et al. The determinants of glycemic responses to diet restriction and weight loss in obesity and NIDDM. Diabetes Care 21 , 687–694 (1998).
Henry, R. R., Scheaffer, L. & Olefsky, J. M. Glycemic effects of intensive caloric restriction and isocaloric refeeding in noninsulin-dependent diabetes mellitus. J. Clin. Endocrinol. Metab. 61 , 917–925 (1985).
Hughes, T. A., Gwynne, J. T., Switzer, B. R., Herbst, C. & White, G. Effects of caloric restriction and weight loss on glycemic control, insulin release and resistance, and atherosclerotic risk in obese patients with type II diabetes mellitus. Am. J. Med. 77 , 7–17 (1984).
Steven, S. & Taylor, R. Restoring normoglycaemia by use of a very low calorie diet in long- and short-duration type 2 diabetes. Diabet. Med. 32 , 1149–1155 (2015).
Lim, E. L. et al. Reversal of type 2 diabetes: normalisation of beta cell function in association with decreased pancreas and liver triacylglycerol. Diabetologia 54 , 2506–2514 (2011).
Taylor, R. et al. Remission of human type 2 diabetes requires decrease in liver and pancreas fat content but is dependent upon capacity for beta cell recovery. Cell Metab. 28 , 547–556.e3 (2018).
Al-Mrabeh, A. et al. Hepatic lipoprotein export and remission of human type 2 diabetes after weight loss. Cell Metab. 31 , 233–249 (2020). A prospective study evaluating the potential mechanisms of T2DM remission and relapse following lifestyle modification .
Taylor, R. Pathogenesis of type 2 diabetes: tracing the reverse route from cure to cause. Diabetologia 51 , 1781–1789 (2008).
Taylor, R. & Barnes, A. C. Can type 2 diabetes be reversed and how can this best be achieved? James Lind Alliance research priority number one. Diabet. Med. 36 , 308–315 (2019).
Brown, A. et al. Low-energy total diet replacement intervention in patients with type 2 diabetes mellitus and obesity treated with insulin: a randomized trial. BMJ Open Diabetes Res. Care 8 , e001012 (2020).
Gregg, E. W. et al. Association of an intensive lifestyle intervention with remission of type 2 diabetes. JAMA 308 , 2489–2496 (2012).
Annuzzi, G., Rivellese, A. A., Bozzetto, L. & Riccardi, G. The results of Look AHEAD do not row against the implementation of lifestyle changes in patients with type 2 diabetes. Nutr. Metab. Cardiovasc. Dis. 24 , 4–9 (2014).
Raynor, H. A. et al. Partial meal replacement plan and quality of the diet at 1 year: action for health in diabetes (Look AHEAD) trial. J. Acad. Nutr. Diet. 115 , 731–742 (2015).
Lean, M. E. et al. Primary care-led weight management for remission of type 2 diabetes (DiRECT): an open-label, cluster-randomised trial. Lancet 391 , 541–551 (2018).
Lean, M. E. J. et al. Durability of a primary care-led weight-management intervention for remission of type 2 diabetes: 2-year results of the DiRECT open-label, cluster-randomised trial. Lancet Diabetes Endocrinol. 7 , 344–355 (2019). A randomized controlled trial of diet-induced weight loss demonstrating that remission of T2DM depends on the amount of weight loss .
Heymsfield, S. B., Gonzalez, M. C., Shen, W., Redman, L. & Thomas, D. Weight loss composition is one-fourth fat-free mass: a critical review and critique of this widely cited rule. Obes. Rev. 15 , 310–321 (2014).
DeFronzo, R. A. et al. The effect of insulin on the disposal of intravenous glucose. Results from indirect calorimetry and hepatic and femoral venous catheterization. Diabetes 30 , 1000–1007 (1981).
Ferrannini, E. et al. The disposal of an oral glucose load in healthy subjects. A quantitative study. Diabetes 34 , 580–588 (1985).
American Diabetes Association. Standards of medical care in diabetes — 2020. Diabetes Care 43 , S1–S212 (2020).
Ajala, O., English, P. & Pinkney, J. Systematic review and meta-analysis of different dietary approaches to the management of type 2 diabetes. Am. J. Clin. Nutr. 97 , 505–516 (2013).
Hjorth, M. F., Zohar, Y., Hill, J. O. & Astrup, A. Personalized dietary management of overweight and obesity based on measures of insulin and glucose. Annu. Rev. Nutr. 38 , 245–272 (2018). A review of evidence supporting baseline glycaemia as a major predictor of weight loss success in response to dietary interventions .
Snorgaard, O., Poulsen, G. M., Andersen, H. K. & Astrup, A. Systematic review and meta-analysis of dietary carbohydrate restriction in patients with type 2 diabetes. BMJ Open. Diabetes Res. Care 5 , e000354 (2017).
Kirk, E. et al. Dietary fat and carbohydrates differentially alter insulin sensitivity during caloric restriction. Gastroenterology 136 , 1552–1560 (2009).
Wing, R. R. et al. Caloric restriction per se is a significant factor in improvements in glycemic control and insulin sensitivity during weight loss in obese NIDDM patients. Diabetes Care 17 , 30–36 (1994).
Look Ahead Research Group. Cardiovascular effects of intensive lifestyle intervention in type 2 diabetes. N. Engl. J. Med. 369 , 145–154 (2013).
Article CAS Google Scholar
Sjostrom, L. et al. Association of bariatric surgery with long-term remission of type 2 diabetes and with microvascular and macrovascular complications. JAMA 311 , 2297–2304 (2014).
Article PubMed CAS Google Scholar
Wing, R. R., Blair, E., Marcus, M., Epstein, L. H. & Harvey, J. Year-long weight loss treatment for obese patients with type II diabetes: does including an intermittent very-low-calorie diet improve outcome? Am. J. Med. 97 , 354–362 (1994).
Samkani, A. et al. A carbohydrate-reduced high-protein diet acutely decreases postprandial and diurnal glucose excursions in type 2 diabetes patients. Br. J. Nutr. 119 , 910–917 (2018).
Skytte, M. J. et al. A carbohydrate-reduced high-protein diet improves HbA1c and liver fat content in weight stable participants with type 2 diabetes: a randomised controlled trial. Diabetologia 62 , 2066–2078 (2019). A cross-over study showing that low-carbohydrate diets can improve metabolic risk factors in patients with T2DM without much weight loss .
Taylor, R., Al-Mrabeh, A. & Sattar, N. Understanding the mechanisms of reversal of type 2 diabetes. Lancet Diabetes Endocrinol. 7 , 726–736 (2019). A review of the mechanisms of T2DM remission .
Hellerstein, M. K. De novo lipogenesis in humans: metabolic and regulatory aspects. Eur. J. Clin. Nutr. 53 (Suppl. 1), 53–65 (1999).
van Wyk, H. J., Davis, R. E. & Davies, J. S. A critical review of low-carbohydrate diets in people with type 2 diabetes. Diabet. Med. 33 , 148–157 (2016).
Kodama, S. et al. Influence of fat and carbohydrate proportions on the metabolic profile in patients with type 2 diabetes: a meta-analysis. Diabetes Care 32 , 959–965 (2009).
Hamdy, O. et al. Fat versus carbohydrate-based energy-restricted diets for weight loss in patients with type 2 diabetes. Curr. Diab Rep. 18 , 128 (2018).
Forouhi, N. G., Misra, A., Mohan, V., Taylor, R. & Yancy, W. Dietary and nutritional approaches for prevention and management of type 2 diabetes. BMJ 361 , k2234 (2018).
Shan, Z., Guo, Y., Hu, F. B., Liu, L. & Qi, Q. Association of low-carbohydrate and low-fat diets with mortality among US adults. JAMA Intern. Med. 180 , 513–523 (2020).
Livesey, G. et al. Dietary glycemic index and load and the risk of type 2 diabetes: a systematic review and updated meta-analyses of prospective cohort studies. Nutrients 11 , 1280 (2019).
Article CAS PubMed Central Google Scholar
Livesey, G. et al. Dietary glycemic index and load and the risk of type 2 diabetes: assessment of causal relations. Nutrients 11 , 1436 (2019).
Hwang, J. J. et al. Blunted rise in brain glucose levels during hyperglycemia in adults with obesity and T2DM. JCI Insight 2 , e95913 (2017). A study showing that patients with obesity and T2DM have a blunted rise in brain blood glucose levels in response to carbohydrate ingestion, and this associates with their feelings of appetite and hunger .
Astrup, A. & Hjorth, M. F. Classification of obesity targeted personalized dietary weight loss management based on carbohydrate tolerance. Eur. J. Clin. Nutr. 72 , 1300–1304 (2018).
Frost, G. et al. The short-chain fatty acid acetate reduces appetite via a central homeostatic mechanism. Nat. Commun. 5 , 3611 (2014).
Trajkovski, M. & Wollheim, C. B. Physiology: microbial signals to the brain control weight. Nature 534 , 185–187 (2016).
Hjorth, M. F. et al. Pretreatment prevotella-to-bacteroides ratio and salivary amylase gene copy number as prognostic markers for dietary weight loss. Am. J. Clin. Nutr. 111 , 1079–1086 (2020).
Sanna, S. et al. Causal relationships among the gut microbiome, short-chain fatty acids and metabolic diseases. Nat. Genet. 51 , 600–605 (2019).
Yamada, Y. et al. A non-calorie-restricted low-carbohydrate diet is effective as an alternative therapy for patients with type 2 diabetes. Intern. Med. 53 , 13–19 (2014).
Tay, J. et al. A very low-carbohydrate, low-saturated fat diet for type 2 diabetes management: a randomized trial. Diabetes Care 37 , 2909–2918 (2014).
Balducci, S. et al. Physical exercise as therapy for type 2 diabetes mellitus. Diabetes Metab. Res. Rev. 30 (Suppl 1), 13–23 (2014).
Boule, N. G., Haddad, E., Kenny, G. P., Wells, G. A. & Sigal, R. J. Effects of exercise on glycemic control and body mass in type 2 diabetes mellitus: a meta-analysis of controlled clinical trials. JAMA 286 , 1218–1227 (2001).
Snowling, N. J. & Hopkins, W. G. Effects of different modes of exercise training on glucose control and risk factors for complications in type 2 diabetic patients: a meta-analysis. Diabetes Care 29 , 2518–2527 (2006).
Balducci, S. et al. Effect of an intensive exercise intervention strategy on modifiable cardiovascular risk factors in subjects with type 2 diabetes mellitus: a randomized controlled trial: the Italian Diabetes and Exercise Study (IDES). Arch. Intern. Med. 170 , 1794–1803 (2010).
Di Loreto, C. et al. Make your diabetic patients walk: long-term impact of different amounts of physical activity on type 2 diabetes. Diabetes Care 28 , 1295–1302 (2005).
Balducci, S. et al. Changes in physical fitness predict improvements in modifiable cardiovascular risk factors independently of body weight loss in subjects with type 2 diabetes participating in the Italian Diabetes and Exercise Study (IDES). Diabetes Care 35 , 1347–1354 (2012).
Balducci, S. et al. Effect of high- versus low-intensity supervised aerobic and resistance training on modifiable cardiovascular risk factors in type 2 diabetes: the Italian Diabetes and Exercise Study (IDES). PLoS One 7 , e49297 (2012).
Eriksson, K. F. & Lindgarde, F. Prevention of type 2 (non-insulin-dependent) diabetes mellitus by diet and physical exercise. The 6-year Malmo feasibility study. Diabetologia 34 , 891–898 (1991).
Saltin, B. et al. Physical training and glucose tolerance in middle-aged men with chemical diabetes. Diabetes 28 (Suppl. 1), 30–32 (1979).
Nagi, D. Diabetes in Practice 2nd edn (John Wiley & Sons, 2005).
Ades, P. A., Savage, P. D., Marney, A. M., Harvey, J. & Evans, K. A. Remission of recently diagnosed type 2 diabetes mellitus with weight loss and exercise. J. Cardiopulm. Rehabil. Prev. 35 , 193–197 (2015).
Ried-Larsen, M. et al. Type 2 diabetes remission 1 year after an intensive lifestyle intervention: a secondary analysis of a randomized clinical trial. Diabetes Obes. Metab. 21 , 2257–2266 (2019).
Johansen, M. Y. et al. Effect of an intensive lifestyle intervention on glycemic control in patients with type 2 diabetes: a randomized clinical trial. JAMA 318 , 637–646 (2017).
Vetter, M. L., Ritter, S., Wadden, T. A. & Sarwer, D. B. Comparison of bariatric surgical procedures for diabetes remission: efficacy and mechanisms. Diabetes Spectr. 25 , 200–210 (2012).
Bray, G. A., Krauss, R. M., Sacks, F. M. & Qi, L. Lessons learned from the POUNDS Lost Study: genetic, metabolic, and behavioral factors affecting changes in body weight, body composition, and cardiometabolic risk. Curr. Obes. Rep. 8 , 262–283 (2019).
Franz, M. J. & Evert, A. B. American Diabetes Association Guide to Nutrition Therapy for Diabetes 2 edn (American Diabetes Association, 2012).
Rowley, W. R., Bezold, C., Arikan, Y., Byrne, E. & Krohe, S. Diabetes 2030: insights from yesterday, today, and future trends. Popul. Health Manag. 20 , 6–12 (2017).
Gillies, C. L. et al. Pharmacological and lifestyle interventions to prevent or delay type 2 diabetes in people with impaired glucose tolerance: systematic review and meta-analysis. BMJ 334 , 299 (2007).
Knowler, W. C. et al. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N. Engl. J. Med. 346 , 393–403 (2002).
Lindstrom, J. et al. Sustained reduction in the incidence of type 2 diabetes by lifestyle intervention: follow-up of the Finnish Diabetes Prevention study. Lancet 368 , 1673–1679 (2006).
Pan, X. R. et al. Effects of diet and exercise in preventing NIDDM in people with impaired glucose tolerance. The Da Qing IGT and Diabetes study. Diabetes Care 20 , 537–544 (1997).
Li, G. et al. The long-term effect of lifestyle interventions to prevent diabetes in the China Da Qing Diabetes Prevention study: a 20-year follow-up study. Lancet 371 , 1783–1789 (2008).
Poulsen, S. K. et al. Health effect of the New Nordic Diet in adults with increased waist circumference: a 6-mo randomized controlled trial. Am. J. Clin. Nutr. 99 , 35–45 (2014).
Hjorth, M. F. et al. Pretreatment fasting plasma glucose and insulin modify dietary weight loss success: results from 3 randomized clinical trials. Am. J. Clin. Nutr. 106 , 499–505 (2017).
Ritz, C., Astrup, A., Larsen, T. M. & Hjorth, M. F. Weight loss at your fingertips: personalized nutrition with fasting glucose and insulin using a novel statistical approach. Eur. J. Clin. Nutr. 73 , 1529–1535 (2019). This article uses a novel statistical approach to model and estimate diet-induced weight loss according to baseline levels of glycaemia .
Due, A. et al. Comparison of 3 ad libitum diets for weight-loss maintenance, risk of cardiovascular disease, and diabetes: a 6-mo randomized, controlled trial. Am. J. Clin. Nutr. 88 , 1232–1241 (2008).
CAS PubMed Google Scholar
Hjorth, M. F., Due, A., Larsen, T. M. & Astrup, A. Pretreatment fasting plasma glucose modifies dietary weight loss maintenance success: results from a stratified RCT. Obesity 25 , 2045–2048 (2017).
Larsen, T. M. et al. Diets with high or low protein content and glycemic index for weight-loss maintenance. N. Engl. J. Med. 363 , 2102–2113 (2010).
Greenway, F. L. et al. A randomized, double-blind, placebo-controlled study of Gelesis100: a novel nonsystemic oral hydrogel for weight loss. Obesity 27 , 205–216 (2019).
Dansinger, M. L., Gleason, J. A., Griffith, J. L., Selker, H. P. & Schaefer, E. J. Comparison of the Atkins, Ornish, Weight Watchers, and Zone diets for weight loss and heart disease risk reduction: a randomized trial. JAMA 293 , 43–53 (2005).
Greenberg, I., Stampfer, M. J., Schwarzfuchs, D., Shai, I. & Group, D. Adherence and success in long-term weight loss diets: the dietary intervention randomized controlled trial (DIRECT). J. Am. Coll. Nutr. 28 , 159–168 (2009).
Sacks, F. M. et al. Comparison of weight-loss diets with different compositions of fat, protein, and carbohydrates. N. Engl. J. Med. 360 , 859–873 (2009). The largest and longest (to date) randomized study comparing the weight loss effectiveness of diets differing in macronutrient composition shows no differences among diets .
Download references
Author information
Authors and affiliations.
Department of Nutrition, Exercise and Sports, Faculty of Science, University of Copenhagen, Frederiksberg Campus, Copenhagen, Denmark
Faidon Magkos, Mads F. Hjorth & Arne Astrup
You can also search for this author in PubMed Google Scholar
Contributions
The authors contributed equally to all aspects of the article.
Corresponding author
Correspondence to Arne Astrup .
Ethics declarations
Competing interests.
M.F.H. and A.A. are co-inventors on a pending provisional patent application on the use of biomarkers for prediction of weight loss responses and co-founders/owners of the University of Copenhagen spin-out company Personalized Weight Management Research Consortium ApS (Gluco-diet.dk). A.A. is a consultant or advisory board member for Basic Research, USA, Beachbody, USA, BioCare Copenhagen, Denmark, Gelesis, USA, Groupe Éthique et Santé, France, McCain Foods Limited, USA, Nestlé Research Center, Switzerland, and Weight Watchers, USA. A.A. and M.F.H. are co-authors of a number of diet/cookery books, including personalized nutrition for weight loss, published in several languages. F.M. declares no competing interests.
Additional information
Peer review information.
Nature Reviews Endocrinology thanks P. Clifton, R. Taylor and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
An intermediate condition between normoglycaemia and type 2 diabetes mellitus, characterized by moderately elevated fasting or postprandial blood glucose or HbA 1c .
A relative ranking of foods according to their ability to increase blood glucose levels relative to a reference food (glucose or white bread) for the same amount of bioavailable carbohydrate.
An extension of the glycaemic index that takes into account the actual amount of available carbohydrate present in one serving of a food or in the whole diet.
Rights and permissions
Reprints and permissions
About this article
Cite this article.
Magkos, F., Hjorth, M.F. & Astrup, A. Diet and exercise in the prevention and treatment of type 2 diabetes mellitus. Nat Rev Endocrinol 16 , 545–555 (2020). https://doi.org/10.1038/s41574-020-0381-5
Download citation
Accepted : 12 June 2020
Published : 20 July 2020
Issue Date : October 2020
DOI : https://doi.org/10.1038/s41574-020-0381-5
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
This article is cited by
Effects of dietary interventions and intermittent fasting on hdl function in obese individuals with t2dm: a randomized controlled trial.
- Anja Pammer
- Anna Obermayer
- Gunther Marsche
Cardiovascular Diabetology (2024)
Comorbidities and use of health services in people with diabetes mellitus according to risk levels by adjusted morbidity groups
- Jaime Barrio-Cortes
- María Pilar Mateos-Carchenilla
- Montserrat Ruiz-López
BMC Endocrine Disorders (2024)
Hypothalamic POMC neuron-specific knockout of MC4R affects insulin sensitivity by regulating Kir2.1
- Haohao Zhang
Molecular Medicine (2024)
Knowledge, attitude, and practice toward weight management among diabetic patients in Qidong City, Jiangsu Province
- Xiaofeng Li
- Shengnan Cai
BMC Public Health (2024)
Metrnl: a promising biomarker and therapeutic target for cardiovascular and metabolic diseases
- Wen-sheng Dong
Cell Communication and Signaling (2024)
Quick links
- Explore articles by subject
- Guide to authors
- Editorial policies
Sign up for the Nature Briefing newsletter — what matters in science, free to your inbox daily.
An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
- My Bibliography
- Collections
- Citation manager
Save citation to file
Email citation, add to collections.
- Create a new collection
- Add to an existing collection
Add to My Bibliography
Your saved search, create a file for external citation management software, your rss feed.
- Search in PubMed
- Search in NLM Catalog
- Add to Search
Improving type 2 diabetes mellitus glycaemic control through lifestyle modification implementing diet intervention: a systematic review and meta-analysis
Affiliations.
- 1 Department of Preventive Medicine and Public Health, University of Granada, Avenida de la Investigación, 11, 18016, Granada, Spain. [email protected].
- 2 CIBER Epidemiología y Salud Pública (CIBERESP), Avenida Monforte de Lemos, 3-5, 28029, Madrid, Spain. [email protected].
- 3 Department of Obstetrics and Gynecology, McMaster University, 1280 Main Street West, Hamilton, ON, Canada.
- 4 High Institute of Pubic Health, Alexandria University, 165 El-Horreya Avenue - El-Ibrahimia, Alexandria, Egypt.
- 5 Department of Preventive Medicine and Public Health, University of Granada, Avenida de la Investigación, 11, 18016, Granada, Spain.
- 6 Fundación para la Investigación Biosanitaria de Andalucía Oriental (FIBAO), Avenida de Madrid, 15, 18018, Granada, Spain.
- 7 CIBER Epidemiología y Salud Pública (CIBERESP), Avenida Monforte de Lemos, 3-5, 28029, Madrid, Spain.
- 8 Department of Molecular Biology and Biochemical Engineering, University Pablo de Olavide, Carretera de Utrera, Km 1, 41013, Seville, Spain.
- 9 Instituto de Investigación Biosanitaria de Granada (ibs.Granada), Servicio Andaluz de Salud/Universidad de Granada, Avenida de Madrid, 15, 18018, Granada, Spain.
- PMID: 31781857
- DOI: 10.1007/s00394-019-02147-6
Purpose: Type 2 diabetes mellitus represents a significant health problem. Many studies have reported that intensive nutritional intervention by itself or in addition to medications is the best method to improve glycaemic control in type 2 diabetes mellitus. However, in clinical practice, dietary education is not implemented as an integral part in the management of type 2 diabetes mellitus. The purpose of this systematic review and meta-analysis is to analyse the scientific evidence concerning the role of nutritional intervention in the glycaemic control of type 2 diabetes mellitus.
Methods: We searched Pubmed, Scopus, Cochrane Library and Web of Science databases from inception till May 2019 for randomised controlled trials (RCTs) that include dietary interventions in the management of patients with type 2 diabetes mellitus.
Results: A total of 28 studies were included. Our results demonstrated that lifestyle interventions significantly lowered glycosylated haemoglobin (HbA 1c ) levels compared to the usual care for patients with type 2 diabetes mellitus, overall weighted mean difference, WMD = - 0.51 (- 0.67, - 0.35). Strategies combining individualized and group-based activities were the most effective, WMD = - 0.95 (- 1.24, - 0.66). Most of stratified analyses did not totally resolve heterogeneity, but improvement in HbA 1c levels has been consistently observed.
Conclusions: The available evidence from RCTs shows that lifestyle intervention is more effective than the standard care regarding the glycaemic control of type 2 diabetic patients, particularly when there is a weight loss. It is time to translate this evidence to the primary health care practice. The protocol of the present systematic review was registered in PROSPERO, registration number CRD42018090469.
Keywords: Diet; Glycaemic control; Lifestyle intervention; Meta-analysis; Systematic review; Type 2 diabetes mellitus.
PubMed Disclaimer
Similar articles
- Effectiveness of lifestyle-based weight loss interventions for adults with type 2 diabetes: a systematic review and meta-analysis. Terranova CO, Brakenridge CL, Lawler SP, Eakin EG, Reeves MM. Terranova CO, et al. Diabetes Obes Metab. 2015 Apr;17(4):371-8. doi: 10.1111/dom.12430. Epub 2015 Jan 14. Diabetes Obes Metab. 2015. PMID: 25523815 Review.
- Lifestyle advice, processes of care and glycaemic control amongst patients with type 2 diabetes in a South African primary care facility. Kalain A, Omole OB. Kalain A, et al. Afr J Prim Health Care Fam Med. 2020 Mar 24;12(1):e1-e6. doi: 10.4102/phcfm.v12i1.2163. Afr J Prim Health Care Fam Med. 2020. PMID: 32242428 Free PMC article.
- The effect of weight management interventions that include a diet component on weight-related outcomes in pregnant and postpartum women: a systematic review protocol. Spencer L, Rollo M, Hauck Y, MacDonald-Wicks L, Wood L, Hutchesson M, Giglia R, Smith R, Collins C. Spencer L, et al. JBI Database System Rev Implement Rep. 2015 Jan;13(1):88-98. doi: 10.11124/jbisrir-2015-1812. JBI Database System Rev Implement Rep. 2015. PMID: 26447010
- Effect of educational interventions on knowledge of the disease and glycaemic control in patients with type 2 diabetes mellitus: a systematic review and meta-analysis of randomised controlled trials. Shiferaw WS, Akalu TY, Desta M, Kassie AM, Petrucka PM, Aynalem YA. Shiferaw WS, et al. BMJ Open. 2021 Dec 9;11(12):e049806. doi: 10.1136/bmjopen-2021-049806. BMJ Open. 2021. PMID: 34887271 Free PMC article.
- Self-monitoring of blood glucose in type 2 diabetes: systematic review. Clar C, Barnard K, Cummins E, Royle P, Waugh N; Aberdeen Health Technology Assessment Group. Clar C, et al. Health Technol Assess. 2010 Mar;14(12):1-140. doi: 10.3310/hta14120. Health Technol Assess. 2010. PMID: 20226138 Review.
- Effect of patient-centered self-management intervention on glycemic control, self-efficacy, and self-care behaviors in South Asian adults with type 2 diabetes mellitus: A multicenter randomized controlled trial. Asmat K, Sivarajan Froelicher E, Dhamani KA, Gul R, Khan N. Asmat K, et al. J Diabetes. 2024 Sep;16(9):e13611. doi: 10.1111/1753-0407.13611. J Diabetes. 2024. PMID: 39264007 Free PMC article. Clinical Trial.
- Health Achieved Through Lifestyle Transformation (HALT): A Kaiser Permanente Diet and Lifestyle Intervention Program for Coronary Artery Disease and Type 2 Diabetes. Ergas IJ, Mauch LR, Rahbar JA, Kitazono R, Kushi LH, Misquitta R. Ergas IJ, et al. Am J Lifestyle Med. 2023 Mar 30;18(1):35-48. doi: 10.1177/15598276231164949. eCollection 2024 Jan-Feb. Am J Lifestyle Med. 2023. PMID: 39184268 Free PMC article.
- Examining the Impact of a Behavior Modification Program on Disease Prevention Behaviors among Individuals at Risk of Diabetes: A Quasi-Experimental Investigation. Phudphad T, Teravecharoenchai S, Khemtong P, Suksatan W. Phudphad T, et al. Eur J Investig Health Psychol Educ. 2024 Jul 6;14(7):1969-1980. doi: 10.3390/ejihpe14070131. Eur J Investig Health Psychol Educ. 2024. PMID: 39056646 Free PMC article.
- The incidence of diabetes mellitus and its determining factors in a Kurdish population: insights from a cohort study in western Iran. Najafi F, Moradinazar M, Khosravi Shadmani F, Pasdar Y, Darbandi M, Salimi Y, Ghasemi SR. Najafi F, et al. Sci Rep. 2024 Jul 9;14(1):15761. doi: 10.1038/s41598-024-66795-3. Sci Rep. 2024. PMID: 38977871 Free PMC article.
- Effects of Momordica charantia L. supplementation on glycemic control and lipid profile in type 2 diabetes mellitus patients: A systematic review and meta-analysis of randomized controlled trials. Zhang X, Zhao Y, Song Y, Miao M. Zhang X, et al. Heliyon. 2024 May 11;10(10):e31126. doi: 10.1016/j.heliyon.2024.e31126. eCollection 2024 May 30. Heliyon. 2024. PMID: 38784554 Free PMC article.
Publication types
- Search in MeSH
LinkOut - more resources
Full text sources.
- MedlinePlus Health Information
Miscellaneous
- NCI CPTAC Assay Portal
- Citation Manager
NCBI Literature Resources
MeSH PMC Bookshelf Disclaimer
The PubMed wordmark and PubMed logo are registered trademarks of the U.S. Department of Health and Human Services (HHS). Unauthorized use of these marks is strictly prohibited.
Log in using your username and password
- Search More Search for this keyword Advanced search
- Latest content
- BMJ NPH Collections
- BMJ Journals
You are here
- Online First
- Lifestyle medicine for type 2 diabetes: practice-based evidence for long-term efficacy of a multicomponent lifestyle intervention (Reverse Diabetes2 Now)
- Article Text
- Article info
- Citation Tools
- Rapid Responses
- Article metrics
other Versions
- You are currently viewing an earlier version of this article (January 17, 2023).
- View the most recent version of this article
- http://orcid.org/0000-0002-0863-4484 Gerda K Pot 1 , 2 ,
- Marieke CE Battjes-Fries 1 ,
- Olga N Patijn 1 ,
- Nynke van der Zijl 3 ,
- Hanno Pijl 4 and
- Peter Voshol 1
- 1 Louis Bolk Instituut, Department of Nutrition and Health , Bunnik , The Netherlands
- 2 King's College London Division of Diabetes and Nutritional Sciences , London , UK
- 3 General Practitioner , Amsterdam , The Netherlands
- 4 Leids Universitair Medisch Centrum , Leiden , Zuid-Holland , The Netherlands
- Correspondence to Dr Gerda K Pot, Louis Bolk Instituut, Bunnik 3981 AJ, Netherlands; g.pot{at}louisbolk.nl
Introduction A wealth of evidence supports short-term efficacy of lifestyle interventions in type 2 diabetes (T2D). However, little is known about long-term effects of lifestyle interventions in real-life settings.
Methods This observational, single-arm study evaluated long-term impact of ‘Voeding Leeft: Reverse-Diabetes2-Now’, a 6-month multicomponent lifestyle programme, on glycaemic control and glucose-lowering medication (GLmed) use, other T2D parameters and quality of life in 438 T2D participants at 6, 12, 18 and 24 months using paired sample t-tests, χ 2 and generalised linear models.
Results At 24 months, 234 participants provided information on GLmed and HbA1c (‘responders’). 67% of the responders used less GLmed, and 28% ceased all GLmed. Notably, 71% of insulin users at baseline (n=47 of 66 insulin users) were off insulin at 24 months. Mean HbA1c levels were similar at 24 months compared with baseline (55.6±12.8 vs. 56.3±10.5 mmol/mol, p=0.43), but more responders had HbA1c levels ≤53 mmol/mol at 24 months (53% vs 45% at baseline). Furthermore, triglyceride levels (−0.34±1.02 mmol/L, p=0.004), body weight (−7.0±6.8 kg, p<0.001), waist circumference (−7.9±8.2 cm, p<0.001), body mass index (−2.4±2.3 kg/m 2 , p<0.001) and total cholesterol/high-density lipoprotein (HDL) ratio (−0.22±1.24, p=0.044) were lower, while HDL (+0.17 ± 0.53 mmol/L, p<0.001) and low-density lipoprotein-cholesterol levels (+0.18 ± 1.06 mmol/L, p=0.040) were slightly higher. No differences were observed in fasting glucose or total cholesterol levels. Quality of life and self-reported health significantly improved.
Conclusion This study indicates robust, durable real-life benefits of this lifestyle group programme after up to 24 months of follow-up, particularly in terms of medication use, body weight and quality of life in T2D patients.
- nutritional treatment
- diabetes mellitus
This is an open access article distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited, appropriate credit is given, any changes made indicated, and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/ .
https://doi.org/10.1136/bmjnph-2020-000081
Statistics from Altmetric.com
Request permissions.
If you wish to reuse any or all of this article please use the link below which will take you to the Copyright Clearance Center’s RightsLink service. You will be able to get a quick price and instant permission to reuse the content in many different ways.
Introduction
Type 2 diabetes (T2D) is a prototypical non-communicable chronic disease (NCD), emanating from gene–behaviour interactions. 1 2 Unhealthy lifestyle factors, such as poor eating habits, physical inactivity, sleep deprivation and stress, contribute to the development of NCDs 3–5 and increase mortality risk. 6 7 Therefore, lifestyle modification should be a structural element of NCD treatment strategies. Although lifestyle intervention usually constitutes a component of the guidelines for clinical management of any NCD, current clinical practice primarily embraces drugs to ameliorate symptoms and prevent disease progression.
Several lifestyle intervention studies have shown promising effects in T2D patients. The DiRECT study 8 9 and the VirtaHealth trial 10 11 demonstrated a 46%–64% remission rate of T2D after 2 years. However, both DiRECT (very low-calorie meal replacement) and Virta (nutritional ketosis) interventions require radical changes of food consumption. Several other studies, evaluating more modest changes in one or two lifestyle components, yielded long-term (9 months to 4 years) benefits in T2D as well. 12–14 Most lifestyle interventions to date are primarily focused on one or two aspects of lifestyle involved in the aetiology of T2D. However, fully effective lifestyle advice encompasses nutrition, physical activity, sleep and stress management. 15 In addition, sustained behaviour change requires psychological support, for example cognitive behavioural support. 16 In 2015, the Foundation Voeding Leeft developed a multicomponent lifestyle intervention for T2D (‘Reverse-Diabetes2-Now’ (RD2N)) to support T2D patients in their efforts to change their lifestyle to remedy their disease. RD2N is a 6-month group programme using biometric feedback for personalised advice pertaining to the full range of lifestyle factors involved in the T2D pathogenesis. It focuses on improving skills rather than just knowledge of all relevant lifestyle components. In a pilot study, RD2N improved glucose control and reduced GLmed use in 72 T2D patients. 17 In this study, we report data of all participants who completed 24 months of follow-up.
Study population
T2D patients who started their RD2N programme between January and December 2017 were enrolled in this study. Patients were included using a stepped-wedge design, with ~20 patients per group per location starting each month (a ‘convenience sample’). Inclusion criteria for the RD2N programme were T2D diagnosis, age 18–75 years, body mass index (BMI) 25–41 kg/m 2 , ability to speak Dutch fluently and motivation to take part in a lifestyle intervention programme. Moreover, all participants used glucose-lowering medication (GLmed) at baseline. Exclusion criteria were use of an insulin pump, serious co-morbidities, for example, severe form of chronic obstructive pulmonary disease (Gold III or IV), bariatric surgery, eating disorders, heart failure (classes 2–4) or kidney failure (estimated Glomerular Filtration Rate/Modification of Diet in Renal Disease study equatione (eGFR/MDRD) <45 unit). Patients, as well as their physician, provided written informed consent.
Lifestyle intervention program
RD2N is a 6-month lifestyle intervention programme, extended by optional follow-up, to help T2D patients gain control over their disease by improving their health, nutrition and lifestyle skills 17 (see Box 1 for main elements of programme). Nutritional advice entailed increased intake of unprocessed/whole or minimally processed foods, being low in high glycaemic carbohydrates and fitting with a Mediterranean food pattern. 18 In addition, health and food literacy skills were increased by explaining the underlying pathophysiology of T2D in simple language and the effects of food on health, that is T2D is a disease with insulin resistance as most important feature and that the body cannot handle the intake of glucose very well. Moreover, information was provided to develop cooking skills, manage stress, tackle mental obstacles and implement physical activity routines. Participants were provided with 6-month intensive guidance by a multidisciplinary support team, including a dietitian, personal coach and nurse practitioner. To enhance effectiveness, partners/family of participants were also actively involved in the process, 19 and participants received instant feedback on their progress, for example measuring their own blood glucose levels before and after meals. 20 RD2N started with 2-day group training on location. Subsequently, groups were invited for a 1-day follow-up meeting after 1, 3 and 6 months. Meanwhile, all groups were encouraged to keep in regular contact with each other and the support team, using a protected online community platform. 19
Key success elements nutrition and lifestyle intervention programme
Intense training/contact coaches/knowledge transfer explaining underlying causes of disease.
Multidisciplinary (dietitian, personal coach and nurse practitioner).
Involvement social environment and create support group.
Nutritional advice: individual approach, not calorie restricting, fresh and unprocessed foods and three-meal approach.
Study design and setting
Researchers of the Louis Bolk Institute (the Netherlands) independently monitored the results of the RD2N programme, which was set-up and executed by personnel of Voeding Leeft. An observational, single-arm pretest and post-test design was used for this monitoring study. Recruitment of participants was executed by Voeding Leeft and was not part of this monitoring study. Data on primary outcome and most secondary outcome parameters at baseline were collected via the patients’ physician. As participants became more aware of the importance of the outcome biomarkers in the course of the programme, they were asked to self-report them during follow-up. Data on secondary study outcomes at baseline and all study outcomes during follow-up were collected via online questionnaires. One week prior to the initiation of the programme and 1 week before the final meeting at 6 months, as well as at 12, 18 and 24 months of follow-up, participants received an email with a link to these questionnaires. To promote completeness of the data at 24 months, the support team actively approached participants. At 6 and 24 months of follow-up, data on the primary outcome parameters were complemented with data from Voeding Leeft.
Primary outcome measures: GLmed and Hb1Ac values
Baseline GLmed use was assessed by asking participants to report on the dose and frequency of their GLmed. GLmed use was classified as (0) no medication, (1) only metformin, (2) metformin and/or sulfonylurea (SU) derivative or (3) metformin and/or SU derivative and/or insulin. GLmed was prescribed according to the Dutch general practitioners (GPs) guideline for T2D treatment. 21 HbA1c levels were measured as a usual component of routine physician follow-up. Participants self-reported their data to the investigators and/or to the data collection team at Voeding Leeft.
Secondary outcome measures
Secondary outcome measures included self-reported fasting blood glucose, height, weight, waist circumference and lipid profile (total cholesterol, high-density lipoprotein (HDL), low-density lipoprotein (LDL) and triglycerides). Participants were asked to report values as recently measured by their physician according to the Dutch GPs guideline standards 21 to the data collection team at Voeding Leeft at baseline and to the investigators during follow-up. Perceived health and quality of life were assessed with a 10-point Likert scale. The validated ‘Checklist Individual Strength’ questionnaire 22 was used to assess fatigue during the past 2 weeks on a 7-point scale (ranging from (not) to (very applicable)). Programme adherence was assessed with a 5-point Likert scale. Furthermore, at baseline, information was collected on date of birth, sex, education level and family structure at home.
Statistical analyses
Descriptive analyses were conducted to describe participants’ socio-demographic characteristics. Data were described as means±SD, if they were normally distributed, or n (percentage). Next, paired sample t-tests were conducted to evaluate the effects of the RD2N programme on change in each measured parameter (follow-up vs baseline) and χ 2 tests were used for categorical variables. Repeated measures analyses using generalised linear models were conducted to evaluate the effects of the RD2N programme on changes in each measured numerical parameter using data available at all time points. Furthermore, at 24 months analyses were stratified using three predefined subgroups: (1) HbA1c level ≤53 mmol/mol (‘low HbA1c starters’) or >53 mmol/mol (‘high HbA1c starters’), (2) use of GLmed at baseline (categorised into two main groups: category 1 (metformin only, termed ‘low GLmed’) vs categories 2 and 3 (ie, SU derivatives and/or insulin, termed ‘high GLmed’)) and (3) education level as proxy for socio-economic status (SES) as information on income was not available. These subgroups were predefined, as we were interested to know if any of them were more likely to respond to the intervention. People with bad metabolic control tend to respond better to (any) treatment for T2D and people with high SES are usually more amenable to lifestyle advice, and it would be useful for broad clinical implementation of the intervention to know if RD2N is particularly effective in patients using less or more medication. Results were interpreted as statistically significant when p<0.05 (two-sided) and SPSS (V.24.0) was used to conduct statistical analyses. Data were analysed using a per-protocol approach reporting data of those who provided data on the study outcome parameters at both baseline and 24 months (=‘responders’).
Of note, 438 participants, in 23 groups of 15–20 patients each were included in this study. Of these, 234 participants (53%) provided data on HbA1c and GLmed at baseline as well as 24 months, the ‘responders’. The baseline characteristics of the responders (n=234) were similar to those of all participants who started the programme (n=438) ( table 1 ). Due to missing or invalid answers, data on secondary outcomes at 24 months are presented for fewer participants, varying from 111 to 195 of the 438 participants (25%–45%) per outcome measure. The responders’ age ranged from 22 to 75 years, and was 61.3±8.5 years on average ( table 1 ). Just over half the responders were men (53%) and 54% had low or middle education.
- View inline
Demographic characteristics at baseline of all participants (n=438) and of those who provided data on HbA1c and GLmed use at both baseline and 24 months (‘responders’, n=234)
Primary outcome measures
All responders used GLmed at baseline, as GLmed use was an inclusion criterion of RD2N. GLmed use was less in 67% of responders after 24 months. Indeed, 28% of responders ceased all GLmed use, and 71% of responders using insulin at baseline was off insulin therapy at 24 months. In 2% of responders GLmed actually increased, and in 31% it remained stable as compared with baseline ( figure 1 ).
- Download figure
- Open in new tab
- Download powerpoint
Percentage of responders in various GLmed categories at baseline and at 24 months (n=234). SU, sulfonylurea.
Subgroup analyses showed that a similar fraction of responders with low (68%) versus high (66%) baseline HbA1c levels was able to lower their GLmed. However, the percentage of responders who were able to cease their GLmed use was higher in those with low baseline HbA1c levels (40%) compared with those with high baseline HbA1c levels (18%; online supplementary table 1 ). For different education-level subgroups, we found that the percentage of responders who were able to lower or cease their GLmed use at 24 months was highest for those in the low education group (79% and 30% for low vs 58% and 25% for middle education and 68% and 29% in high education; online supplementary table 1 ). We did not perform subgroup analyses for baseline GLmed here, as it goes without saying that the possibilities for stopping medication are greater in people who use more of it.
Supplemental material
Hba1c levels.
HbA1c levels initially declined, but gradually increased over time ( figure 2 ). HbA1c levels at 24 months were not different from baseline (−0.67±12.9 mmol/mol, p=0.430). At 24 months, 53% of responders had HbA1c levels ≤53 mmol/mol, compared with 45% at baseline.
Mean HbA1c levels (in mmol/mol) over time atbaseline, 6, 12, 18 and 24 months. The number of participants differs per timepoint, as these are the participants who provided data on HbA1c at both baseline and follow-up.
After stratification into predefined subgroups ( online supplementary table 2 ), we found that HbA1c levels were significantly lower at 24 months compared with baseline in responders who had high baseline HbA1c levels (−4.7±13.9 mmol/mol, p value <0.001). In contrast, HbA1c levels increased in those who had an HbA1c ≤53 mmol/mol at baseline (4.2±9.5 mmol/mol, p value <0.001). However, GLmed use declined substantially in the latter group, while HbA1c levels remained below the target value defined in treatment guidelines (ie, 53 mmol/mol). Subgroup analyses in low or high GLmed showed that only responders using just metformin at baseline had a significant decline in HbA1c levels (−3.6±9.3 mmol/mol, p=0.002 vs 0.5±13.9, p=0.62). We found no differences in effects on HbA1c between different education subgroups.
Glucose control and GLmed use combined
Forty-four per cent of responders reported the use of less medication and a lower HbA1c, 42% reported less medication or a lower HbA1c, leaving 14% reporting more medication or a higher HbA1c. Thus, the vast majority (86%) of responders reported at least one benefit of the intervention in terms of glucose control.
Other T2D biomarkers and health parameters
At 24 months, responders had significantly lower triglyceride levels (−0.34±1.02 mmol/L, p=0.004), total cholesterol/HDL ratio (−0.22±1.24 mmol/L, p=0.044), body weight (−7.01±6.8 kg, p<0.001), BMI (−2.36±2.28 kg/m 2 , p<0.001) and waist circumference (−7.9±8.2 cm, p<0.001), and higher HDL (0.17±0.53 mmol/L, p<0.001) and LDL levels (0.18±1.06 mmol/L, p=0.044) ( table 2 ). No statistical significant differences were found for fasting glucose and total cholesterol at 24 months compared with baseline. Also taking into account measurements at 6, 12 and 18 months we found similar results, except that significant changes in HDL, total cholesterol/HDL ratio and LDL at 24 months were not statistically significant, but it should be noted that numbers of responders included in the repeated measures analyses were lower.
Secondary outcomes: mean scores and changes of health parameters, self-perceived health, quality of life and fatigue at baseline and 24 months (of n=438)
Subgroup analyses showed similar findings for responders with low or high baseline HbA1c levels, except for triglycerides, which declined significantly only in responders with high baseline HbA1c (p<0.001; online supplementary table 3A ). Those with low baseline GLmed had similar results as those with high baseline GLmed ( online supplementary table 3B ). Interestingly, in regards to education level, we observed most prominent changes of HDL, total cholesterol/HDL ratio and triglycerides in those with low education level ( online supplementary table 3C ).
Subjective health parameters
Both self-perceived health and quality of life of responders were significantly higher at 24 months compared with baseline: self-perceived health increased by 0.4±1.5 (scale 1–10) (p<0.001) and quality of life increased by 0.3±1.3 (scale 1–10) (p=0.001). In addition, scores for fatigue were significantly lower at 24 months compared with baseline (−9.5±20.7 at a scale of 20–140, p<0.001). Results remained similar when performing repeated measures analyses also including information at 6, 12 and 18 months.
Subgroup analyses yielded similar results for those responders with low or high baseline HbA1c levels ( online supplementary table 3A ), except for quality of life, which only improved in those with high baseline HbA1c levels (p=0.012). Self-perceived health, quality of life and fatigue improved significantly only in responders in the high GLmed category at baseline ( online supplementary table 3B ). With regards to education level, self-reported health, quality of life and fatigue all improved significantly in those with low education level, whereas simply quality of life and fatigue improved in middle education level and only fatigue in those with a high education level ( online supplementary table 3C ).
Programme adherence and appreciation
At 24 months, 90% of responders reported to almost fully adhere to the nutrition guidelines for breakfast, 81% for lunch, 79% for dinner, 53% for snacks and 83% for drinks. Fifty-three per cent of responders indicated that they did not find it difficult to adhere to the programme guidelines, but 41% of the responders found it difficult to adhere to the programme guidelines at social events. Furthermore, 67% reported to be (very) motivated to continue adherence to the guidelines, and 92% of responders gave a score of ≥8 (scale 1–10) to recommend this programme to friends or family. There were no differences in recommending this programme to others in relation to education level (78% for low education, 80% for middle and 77% for high), although more responders with low education level indicated that eating according to the recommendations was (very) expensive (39% for low vs 34% for middle and 23% for high education level) and (very) difficult to adhere to the programme guidelines (31% for low vs 27% for middle and 18% for high education level).
The results of this study showed that a 6-month multidisciplinary group programme designed to promote health literacy and lifestyle skills improves clinical parameters as well as quality of life in a substantial percentage of T2D patients after up to 24 months of follow-up. Indeed, the vast majority of the responders reported to use less GLmed as compared with baseline. Moreover, 44% of responders reported to use less medication and a lower HbA1c, 42% reported the use of less medication or a lower HbA1c, leaving 18% to report a higher HbA1c or the use of more medication. In addition, their body weight and waist circumference declined, as well as their serum triglyceride levels and total cholesterol/HDL cholesterol ratio. Moreover, their quality of life, self-perceived health and fatigue were all significantly improved at 24 m.
In a recent pilot study evaluating the effects of RD2N treatment, 62% of responders reduced GLmed at 6 months. 17 The present study confirms that the treatment effectively sustains this benefit over 24 months, since two-thirds (67%) of all responders reduced their GLmed by that time. Indeed, 71% (47 of 66) of responders who used insulin at baseline were off insulin therapy at 24 months. These effects are of obvious clinical and economic benefit, even in the context of the non-significant reduction of HbA1c that was observed during the same follow-up period. Moreover, RD2N also seemed to bring about sustainable benefits in terms of other health parameters, including various components of the metabolic syndrome, as well as improvements in self-perceived health, quality of life and fatigue. It is difficult, if not impossible, to ascertain which elements of the programme are responsible for these durable effects. However, we suspect that the multidisciplinary approach, focusing on a broad spectrum of lifestyle skills rather than health literacy alone, as well as provision of biofeedback information on the effects of the intervention were of critical importance.
Subgroup analyses revealed that HbA1c levels increased slightly in responders who had baseline HbA1c levels ≤53 mmol/mol, while it declined in those with a baseline HbA1c >53 mmol/mol. However, GLmed use declined substantially in the former group (39% stopped GLmed entirely), while HbA1c levels remained below target values according to clinical treatment guidelines. This means that RD2N treatment significantly reduced medication use to control blood glucose in people with well-controlled HbA1c to begin with. Surprisingly, responders with low HbA1c and GLmed use at baseline did less well in terms of subjective health parameters, perhaps because they were in better shape to begin with.
About one-third of the responders in the present study had a lower education level. Interestingly, they were most successful in terms of reversal after 6 months, but the difference between education-level groups disappeared at 24 months. We consider this an important finding, as it shows that the programme is at least equally effective across groups with different education levels in the long term. This goes against conventional wisdom, which consistently suspects people with a lower education level to be less accessible and responsive to lifestyle intervention. However, the fact that participation in the programme was (obviously) voluntarily reflects intrinsic motivation to change lifestyle, which equally applies to people with different education levels.
Several other lifestyle interventions have also shown promising results in the treatment of T2D. The DiRECT study and Virta trial reported impressive success after 24 months of follow-up. 9 11 However, these interventions appear to be a considerable burden on the willpower of people, perhaps rendering them less suitable for a significant percentage of patients. Moreover, they primarily focus on nutrition as a target of lifestyle modification, while other lifestyle factors such as stress, sleep and physical activity are also well known to contribute to the pathogenesis of T2D. 23–26 These potential caveats do not mean that these interventions will turn out to be less useful in clinical practice. Indeed, it is increasingly recognised that the biological response to a lifestyle intervention (as well as the response to drugs) differs substantially between individuals. 27 28 In addition, sustained reversal of T2D requires lifelong adherence to lifestyle measures, therefore it is of critical importance for the success of any lifestyle intervention that it fits as closely as possible with the patients’ preferences. Some people undoubtedly prefer (intermittent) meal replacement to a more modest but structural modification of their daily diet. Others may like to focus on stress and physical activity rather than food. Thus, a broad variety of available effective interventions, offering distinct treatment options to patients, will benefit more people and it could well be possible that tackling a combination of lifestyle factors is key.
We report real-world data, which is a strength of our study, as it reflects the impact of RD2N in daily clinical practice and thus provides evidence for real-life robust results. However, data collection in everyday life is less well structured than in the context of a traditional clinical trial. The number of non-responders has been substantial in the present analyses (47%), which probably biased the results since it is reasonable to suppose that less successful participants were more reluctant to respond to information requests.
Moreover, our analyses did not compare RD2N with another intervention, placebo and/or standard medical care. The lack of a control comparison hampers interpretation of the observations in terms of causality. However, application of the Bradford Hill criteria 29 to our study suggests that a causal relationship between intervention and observed effects may be possible, as findings are biologically plausible and consistent over time. Also, we cannot be absolutely sure that RD2N is any better than regular medical care in the Netherlands. However, reduction of GLmed in the course of time is rare in clinical practice, so the substantial decline in GLmed in response to RD2N treatment supports the idea that it contributes to better diabetes care.
In conclusion, T2D is commonly considered a chronic progressive disease. Reduction of medication dose is rare in regular clinical care. The present report signifies the potential of RD2N treatment as multicomponent lifestyle intervention to improve T2D in a significant number of patients, particularly in terms of medication use, metabolic control, as well as quality of life. Using a multidisciplinary approach, focusing on a broad spectrum of lifestyle skills rather than health literacy alone, as well as provision of biofeedback information on the effects of the intervention seems a viable approach.
Acknowledgments
We are indebted to the participants of the ‘Reverse Diabetes2 Now’ programme. We thank the whole Reverse Diabetes2 Now team for executing the programme. Furthermore, we thank Lotte Schaffer for her help in data collection. We thank Nicole de Groot, Marianne de Visser, Connie Hoek, Dan Hoevenaars, Barbara Kerstens, Peter Klosse, Ronald Rier, Lotte Schaffer, Martijn van Beek, Albert van der Velde, Maaike de Vries and Renger Witkamp for their contributions to the programme. We thank Klaas Berkhof for his support to this project.
- English P ,
- Bassuk SS ,
- Bergmann MM ,
- Kröger J , et al
- Colpani V ,
- Jaspers L , et al
- Kvaavik E ,
- Ursin G , et al
- Leslie WS ,
- Barnes AC , et al
- Hallberg SJ ,
- McKenzie AL ,
- Williams PT , et al
- Athinarayanan SJ ,
- Hallberg SJ , et al
- Look AHEAD Research Group ,
- Conaway MR ,
- Crowther JQ , et al
- Di Onofrio V ,
- Di Dio M , et al
- Castelnuovo G ,
- Pietrabissa G ,
- Manzoni GM , et al
- Battjes-Fries MCE ,
- Patijn ON , et al
- Greaves CJ ,
- Sheppard KE ,
- Abraham C , et al
- Kim KM , et al
- De Grauw W ,
- Nijpels G , et al
- Vercoulen JH ,
- Alberts M ,
- Bleijenberg G , et al
- Chen J , et al
- Xie M , et al
- Hackett RA ,
- Zmora N , et al
- Caidahl K , et al
GKP and MCB-F contributed equally.
Contributors GKP analysed the data, drafted and revised the manuscript; MB-F designed the study, collected, collated and analysed the data and revised the manuscript; ONP collected data and revised the manuscript; NvdZ was responsible for the medical coordination of the intervention and revised the manuscript. HP is chief medical advisor of Voeding Leeft, and provided scientific input to design of the study and manuscript; PV co-designed the lifestyle intervention and the study, revised the manuscript and is responsible for the overall content of the manuscript (guarantor).
Funding This work was supported by Ekhaga (application number 2017-55) and by VGZ Health Insurance via the foundation Voeding Leeft.
Competing interests None declared.
Patient consent for publication Not required.
Ethics approval The Medical Ethical Reviewing Committee of Wageningen University (NL) reviewed the study protocol and is of the opinion that it does not fall within the remit of the Dutch ‘Medical Research Involving Human Subjects Act’ (17 January 2019).
Provenance and peer review Not commissioned; externally peer reviewed.
Data availability statement Data may be obtained from a third party and are not publicly available. Data may be obtained from a third party (Foundation Voeding Leeft) and are not publicly available.
Read the full text or download the PDF:
- - Google Chrome
Intended for healthcare professionals
- My email alerts
- BMA member login
- Username * Password * Forgot your log in details? Need to activate BMA Member Log In Log in via OpenAthens Log in via your institution

Search form
- Advanced search
- Search responses
- Search blogs
- News & Views
- Nutritional basis of...
Nutritional basis of type 2 diabetes remission
Read our food for thought 2020 collection.
- Related content
- Peer review
This article has a correction. Please see:
- Nutritional basis of type 2 diabetes remission - July 09, 2021
- Roy Taylor , professor 1 ,
- Ambady Ramachandran , professor 2 3 ,
- William S Yancy Jr , physician 4 ,
- Nita G Forouhi , professor 5
- 1 Magnetic Resonance Centre, Translational and Clinical Research Institute, Newcastle University, Newcastle upon Tyne, UK
- 2 India Diabetes Research Foundation, Chennai, India
- 3 Dr A Ramachandran’s Diabetes Hospitals, Chennai, India
- 4 Duke Lifestyle and Weight Management Center, Duke University Health System and Department of Medicine, Duke University Medical School, Durham, NC, USA
- 5 MRC Epidemiology Unit, University of Cambridge School of Clinical Medicine, Cambridge, UK
- Correspondence to R Taylor Roy.Taylor{at}ncl.ac.uk
Roy Taylor and colleagues explain how type 2 diabetes can be reversed by weight loss and avoidance of weight regain
Type 2 diabetes mellitus was once thought to be irreversible and progressive, but a series of clinical studies over the past 12 years have clarified the mechanisms that cause the disease. We now know that the processes that cause type 2 diabetes can be returned to normal functioning by restriction of food energy to achieve weight loss of around 15 kg. 1 Around half of people who are within the first 10 years of diagnosis and manage to follow food energy restriction can stop all diabetes medication and return to non-diabetic glucose control. 2 3 Remission is achieved when haemoglobin A 1c concentrations of 48 mmol/mol are recorded after weight loss and at least six months later without any anti-diabetic medications ( box 1 ). 4 Here we summarise the new understanding of type 2 diabetes and consider how different changes to food intake can achieve the necessary weight loss and maintenance required for remission of diabetes.
Definition of remission*
The consensus guideline from UK Primary Care Diabetes Society and Association of British Clinical Diabetologists lays out three criteria for remission of type 2 diabetes 4 :
Weight loss
Fasting plasma glucose <7 mmol/L or HbA 1c <48 mmol/mol (WHO diagnostic thresholds) on two occasions separated by at least six months
Attainment of these glycaemic parameters after complete cessation of all glucose lowering therapies
*Remission is sometimes used to describe meeting glycaemic targets even though hypoglycaemic drugs have not been stopped. Care must be taken in the interpretation of stated rates of remission.
What causes type 2 diabetes and remission?
In 2008 the twin cycle hypothesis postulated that there were vicious cycles of fat accumulation in the liver and pancreas that lead to the development of type 2 diabetes over at least a decade ( fig 1 ). 6 The hypothesis was developed from emerging knowledge on the relation between liver fat and control of the constant flow of glucose into the blood as well as observation that normal insulin secretion returned after substantial weight loss in people with type 2 diabetes. It predicted that major calorie restriction would lead to a rapid fall in liver fat, normalisation of liver insulin sensitivity, and decrease to normal levels of glucose production by the liver.

Type 2 diabetes develops as long term intake of excess food energy leads to accumulation of liver fat, driven by a vicious cycle of hepatic insulin resistance and hyperinsulinaemia. The raised liver fat level causes increased hepatic export of very low density lipoprotein (VLDL) triglycerides. If the subcutaneous fat depot cannot accommodate this, ectopic fat will build up, including in the pancreas. In people with susceptible β cells, the acute insulin response to food becomes diminished and de novo lipogenesis from glucose is enhanced. β Cell function can be restored if liver fat is reduced through weight loss. Figure is modified from Al-Mrabeh et al with permission 5
- Download figure
- Open in new tab
- Download powerpoint
Testing the hypothesis required a sure-fire way of achieving around 15 kg weight loss, and one of the most striking findings of the 2011 Counterpoint study was the acceptability of a low calorie liquid diet for a short planned period. 1 People with type 2 diabetes in the study achieved an average of over 15 kg weight loss in eight weeks during normal living. 1 Participants’ initially high levels of liver and pancreas fat fell to normal ranges, with decreased hepatic glucose output and improved β cell function. The study included only people who had had diabetes diagnosed within four years, but a subsequent study found that remission was much less likely after 10 years of diabetes. 2
These studies set the scene for Direct (Diabetes Remission Clinical Trial), a randomised controlled trial in primary care of a low calorie diet with structured follow-up compared with conventional management according to best practice guidelines. This study confirmed widespread acceptability, with almost 30% of those invited accepting to participate and an average weight loss of 14.5 kg. 7 Primary care nurses or dietitians worked with patients in the intervention group, and 36% (53/149) achieved remission for two years. 8
Direct also showed that people in remission could return to normal maximal insulin secretion rates if they maintained their weight after initial rapid weight loss. 9 This complete return to normal functional β cell mass is remarkable. Previously, both clinical and histological studies on the pancreas found that β cell capacity declined to around 50% by the time of diagnosis, and death or apoptosis of the β cells had been assumed. But we now know that excess fat exposure causes β cells to de-differentiate, losing ability to secrete insulin 10 —most likely through downregulation of the genes controlling insulin production. The return to normal for a large group of people who used to have type 2 diabetes shows the potential for β cell recovery. Some individuals remain in remission for many years provided weight is not regained. 11
Type 2 diabetes is characterised by accumulation of more fat in the liver and pancreas than an individual can tolerate. Different people have different fat thresholds, and this explains why only around half of people diagnosed with type 2 diabetes are obese and some have a healthy body mass index. 12 13 The excess fat within liver cells causes insulin resistance, and this entirely resolves if liver fat falls to low-normal levels. 1 2 14 Once this happens insulin can act normally again, restraining the outpouring of glucose from the liver into the blood and rapidly normalising fasting blood glucose concentrations.
Because the liver supplies triglyceride to the rest of the body, the sudden fall in liver fat causes the high rate of triglyceride supply to fall to normal. 14 As a result, fat levels inside the pancreas gradually decrease, along with all ectopic fat depots. Gradually, normal insulin response to eating is restored. 1 2 14 15
Any sustained decrease in calorie intake is able to remove the excess intra-organ fat. For example, the enforced sudden decrease in food intake after bariatric surgery brings about remission by the same underlying mechanisms as voluntary dieting. 15 16 Bariatric surgery necessitates nil by mouth for a period followed by much reduced food intake and achieves around 64% remission of diabetes at two years. 17
In the UK Prospective Diabetes Study, normalisation of fasting glucose levels was reported in 15% of participants following an initial dietary weight loss phase. 18 The Look-Ahead randomised trial compared intensive physical activity advice plus dietary restriction with conventional management of type 2 diabetes. 11 Although diabetes remission was not an outcome measure, the modest weight loss achieved led to remission in 11.5% of participants in the intensive lifestyle intervention group. Merely providing the information on the degree of weight loss required for remission can allow motivated people to achieve this for themselves using their preferred method. 19
Key components of dietary advice
Low carbohydrate versus low calorie diets.
Much noise and confusion surround the “best” macronutrient composition in dietary advice for weight loss. Low fat diets used to be favoured. This was because fat contains a higher density of calories (9 kcal/g) than carbohydrate and protein (4 kcal/g), coupled with concerns about the cardiovascular risks of higher fat diets. On the other hand, interest is increasing in low or very low carbohydrate diets for weight loss because carbohydrate is the primary contributor to post-prandial glycaemia. Table 1 summarises the main evidence comparing low calorie and low carbohydrate diets.
The controversy about low carbohydrate or low calorie approaches to remission of type 2 diabetes: Areas of agreement and disagreement
- View inline
Randomised trials in general populations (not specific for diabetes) show that both low calorie and low carbohydrate diets can be effective for weight loss as long as participants can adhere to the diet. 33 Studies show slightly greater weight loss up to one year with low carbohydrate diets than low fat diets, with a modest difference of around 1 kg body weight, 34 35 but the scanty randomised trial evidence at two years shows no difference between diets. 20 36
A non-randomised study with intensive follow-up reported that a very low carbohydrate approach in people with type 2 diabetes can achieve and sustain weight loss of 12 kg at two years. 28 Moreover, a two year follow-up of a cohort (not selected for diabetes) in a single British general practice reported a decrease in median weight of 8.3 kg at two years on a low carbohydrate diet (50-130 g/day). 30 For glycaemic control, a non-randomised study of a diet with less than 30 g/day of carbohydrate reported 58% of participants achieving HbA 1c <48 mmol/mol, but metformin was not discontinued. 37 However, non-randomised or uncontrolled studies report data on completers and are therefore not directly comparable with randomised trials, which analyse by intention to treat and outcomes. Randomised trials are generally considered to provide the highest quality of evidence when they are feasible.
To our knowledge only one randomised trial has reported diabetes remission rates after a low carbohydrate diet intervention in patients with poorly controlled glycaemia. However, as metformin was continued the participants did not meet the current definition of remission ( box 1 ). Participants in the intervention group lost 3.7 kg more than those in the comparator group and 11% (12/109) achieved HbA 1c <48 mmol/mol compared with 0/117 in the comparator group. 31
Heterogeneity in definitions also makes interpretation difficult. What constitutes a low carbohydrate diet varies widely across studies from <45% of total energy 22 to ketogenic levels of intake of under 50 g/day (<10% of energy). 28 Box 2 gives a standardised definition.
What is a low carbohydrate diet?
The term “low carbohydrate” is used in various ways. Recommendations for consistency of approach have been made, the most widely used being that of Feinman and colleagues 38 :
Very low carbohydrate: 20 to 50 g/day (≤10% of energy, based on 2000 kcal/day)
Low carbohydrate: >50 to <130 (>10% to <26%)
Moderate carbohydrate: 130 to 230 (26% to 45%)
High carbohydrate >230 (>45%)
Dietary adherence is always problematic, with substantial differences in prescribed and attained macronutrient intakes. The best diet for longer term success will be one which is easiest for an individual to adhere to in the long term.
Dietary restriction through eating strategies
Portion control is an established strategy for weight loss, as is the concept of fasting: short term dietary self-restraint was traditionally associated with religious practices. Intermittent fasting has become popular more recently. Daily or alternate day fasting aims for roughly 25% lower intake of food energy: the 5:2 diet reduces intake to 500 to 700 calories a day for two days each week, while time restricted feeding limits eating to within a 6 to 8 hour window each day (for instance, omit breakfast and eat only between 12 pm and 6 pm). Systematic reviews show that each of these eating strategies can be effective, with reports of weight loss of up to 13% of baseline weight, 39 and that different intermitting fasting approaches achieve similar weight losses as the traditional continuous energy restriction approach. 40 41 However, the existing randomised trials are of short duration with small sample size and heterogeneity across studies, and further research is warranted to test whether these approaches can be effective for the remission of type 2 diabetes. 39 42 Beyond weight loss, intermittent fasting may have longer term effects on health and longevity. 43 Challenges such as hunger and cravings on fasting days could be too great for some despite evidence that these diminish over time. 43
Dietary quality
Food is eaten within overall sociocultural contexts, and focusing solely on the quantity or type of macronutrients may be over simplistic. Different food sources affect physiological pathways differently, including appetite, satiety, hunger, and diet induced thermogenesis. Reducing all carbohydrates indiscriminately may take away the benefits from the consumption of fibre and wholegrain. Decades of research have clarified the importance of distinguishing between saturated, unsaturated, and trans fats for cardiometabolic disease. 44 45 46 Furthermore, even considering saturated fats as a group is not sufficiently discriminatory to understand health effects because individual saturated fatty acids differ in their association with type 2 diabetes. 47
The importance of food sources rather than macronutrient type is highlighted by the associations of meat and dairy, which are both typically high in saturated fat and protein, with cardiometabolic risk. Some types of dairy such as fermented dairy (yoghurt or cheese) are associated inversely with type 2 diabetes and cardiovascular disease, whereas red and processed meat are positively associated. 45 48 49
Advice on foods consumed within an overall dietary pattern may facilitate better longer term adherence. Evidence supports the benefits of Mediterranean-type diets for several health outcomes, although this dietary pattern is not singularly superior or easier to adhere to. Other effective dietary patterns include DASH (dietary approaches to stop hypertension), the healthy eating index, Nordic diet, and vegetarian or other meal plans, but more research is needed. 50 Consensus is also emerging that avoidance of ultraprocessed foods and increased consumption of fresh, whole foods has health benefits, including for weight and glycaemic control. Food based dietary guidelines that move beyond a focus on macronutrients and consider overall dietary and social contexts would communicate our current knowledge on nutrition and its effect on type 2 diabetes more comprehensively.
Remission in ethnically diverse and global populations
Most participants in studies on remission of type 2 diabetes carried out in western countries have been white, and background nutritional patterns of other ethnicities have to be considered. 7 51 The Look-Ahead study included around 38% ethnic minority participants (mainly Hispanic and African American). Although not a primary aim of the study, remission of type 2 diabetes was observed in proportion to weight loss (11.5% (248/2157) at year 1 and 7.3% (150/2056) at year 4, with weight loss of 8.6% and 4.7%, respectively); no association of ethnicity with remission was observed. 11 A large community based analysis from the Kaiser Permanente Northern California Registry showed a higher likelihood of remission in African Americans than in the white population, with overall seven year remission of 4.6% among people with type 2 diabetes for less than two years. 52 A similar retrospective survey of people aged over 65 years observed higher rates of non-surgical remission after eight years in Asian and Hispanic people than in white and African American groups. 53
South Asians achieve remission after a low calorie liquid diet similarly to white Europeans. 54 A two year prospective study of a low calorie diet and advice to walk daily in a young South Asian population with recent onset type 2 diabetes found 75% remission at three months and 69% at two years. HbA 1c was <39 mmol/mol in 53% of participants at three months and in 47% at two years; 22% had HbA 1c 39-47 mmol/mol at both time points. 55 Similar observations were made in a Thai population: 79% had achieved remission at 12 weeks (with an average weight loss of 10 kg) and 30% had maintained remission at 12 months. 56 A trial in a Middle Eastern population observed remission in 61% of those allocated to total diet replacement and lifestyle intervention. 25
A recent study in Barbados on a predominantly African Caribbean population observed rates of weight loss induced remission similar to those documented in Direct. 32 This was achieved over eight weeks by using a hypocaloric liquid diet (760 kcal) with withdrawal of diabetes medication on day 1 of the diet. Nine of the 11 (82%) participants who lost at least 10 kg achieved non-diabetic fasting blood glucose levels compared with six of 14 (43%) who lost <10 kg. Remission of prediabetes by weight loss and physical activity has also been shown in Indian populations, with significant improvements in insulin resistance and β cell function. 57 58
Evidence on ways to improve long term remission
The US national registry has documented the feasibility of people maintaining substantial weight loss over 10 years and has provided important insights into nutritional and other factors. 59 Weight regain was fastest for participants in the early years of follow-up, with decreasing rates over each of the first five years followed by stable maintenance over the subsequent five years, suggesting that maintenance requires less effort over time. Many personal factors influence what we eat and therefore how well weight loss is maintained, including age, sex, genetics, ethnicity, body fat status, level of physical activity, and family and social culture. But there are also profound wider influences on food intake. These include food availability, accessibility, cost, advertising, ready availability of fast food takeaways and home delivery options, and price promotions for processed energy dense foods.
Psychological study of participants in weight loss studies of remission has shown support from family and friends has a critical role in both achieving weight loss and avoiding regain. 23 60 Eating is a social activity, with individuals tending to eat similarly to their family and friendship groups. The psychological term “behaviour contagion” is descriptive, and it is notable that spouses or partners often report weight loss. Given that type 2 diabetes runs in families, all, including children, are likely to benefit.
Continued support from healthcare professionals, irrespective of composition of food advised, is one strategy to avoid weight regain and sustain diabetes remission. In Direct a “rescue plan” of partial or total meal replacement was offered to participants who regained 2 kg or 4 kg, respectively. 8 More research is needed, but observational evidence indicates that maintaining weight loss over 10 years requires sustained dietary change, regular physical activity, and frequent self-weighing. 59 61
Population strategies, including education, dietary guidelines, and empowerment to make healthy food choices, such as clear food labelling, are necessary but not yet universally available. Evidence supports the case for other population “nudge” interventions, including taxation, restriction of fast food outlets near schools, and reducing the size and appeal of food portions, packages, and tableware to influence the quantities of food and beverages consumed. 62 Another potentially clinically and economically effective strategy is food prescription to promote healthier eating. Pilot data from the US on people with uncontrolled type 2 diabetes and food insecurity shows substantial reductions in HbA 1c in those who received fresh food on prescription. 63
Future directions
Type 2 diabetes can be reversed by substantial weight loss in the early years after diagnosis, and the pathophysiological basis of this is now clear. Long term maintenance of weight loss brings about lasting remission, but this is more difficult to achieve than weight loss. Strategies to optimise the avoidance of weight regain in the long term need to be developed and rigorously tested in all populations. Population strategies are also required to enable healthier food choices and prevent the current excessive weight gain during childhood and adult life. Long term surveillance of people with type 2 diabetes in remission is needed to determine whether it also decreases the rates of vascular events and weight related cancers.
Key messages
Type 2 diabetes develops when personal tolerance for fat levels in the liver and pancreas are exceeded
Weight loss sufficient to reverse this will permit return to non-diabetic blood glucose in the early years after diagnosis
Remission is durable provided weight regain is avoided
Avoidance of weight regain can be achieved by various strategies and individuals must find the dietary strategies most suited to them alongside increased physical activity
To enable healthful dietary intakes in populations, policy interventions such as taxation on calorie dense foods and restrictions on portion size are needed.
Competing interests: RT and NGF are unpaid members of the Joint SACN/NHS-England/Diabetes-UK Working Group to review the evidence on lower carbohydrate diets compared with current government advice for adults with type 2 diabetes. RT has received fees for educational lectures from Lilly and Janssen and is author of Life Without Diabetes (Short Books). WY is a member of the medical review board for dietdoctor.com and has contributed to guidelines for American Diabetes Association and Guideline Central regarding nutrition and health. The views expressed are the authors’ own. RT has research funding from Diabetes UK (17/0005645 and 13/0004691). NGF was supported by Medical Research Council Epidemiology Unit (MC_UU_00006/3) and NIHR Biomedical Research Centre Cambridge: Nutrition, Diet, and Lifestyle Research Theme (IS-BRC-1215-20014) and NGF is a NIHR Senior Investigator. The views expressed are those of the authors and not necessarily those of the NIHR, the Department of Health and Social Care, or any other organisations they are associated with.
Provenance and peer review: Commissioned; externally peer reviewed.
This article is one of a series commissioned by The BMJ. Open access fees for the series were funded by Swiss Re, which had no input into the commissioning or peer review of the articles. The BMJ thanks the series advisers, Nita Forouhi, Dariush Mozaffarian, and Anna Lartey for valuable advice and guiding selection of topics in the series.
This is an Open Access article distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/ .
- Hollingsworth KG ,
- Aribisala BS ,
- Mathers JC ,
- Al-Mrabeh A ,
- Zhyzhneuskaya S ,
- Hambling C ,
- Zhyzhneuskaya SV ,
- Leslie WS ,
- Barnes AC ,
- Kim-Muller JY ,
- Wagenknecht LE ,
- Look AHEAD Research Group
- Walker JJ ,
- Scottish Diabetes Research Network Epidemiology Group
- Lingvay I ,
- Livingston E
- Panunzi S ,
- Carlsson L ,
- De Gaetano A ,
- Sainsbury E ,
- Kizirian NV ,
- Partridge SR ,
- Colagiuri S ,
- Korsmo-Haugen HK ,
- Brurberg KG ,
- Murray SW ,
- Rehackova L ,
- Araújo-Soares V ,
- Adamson AJ ,
- Sniehotta FF
- Kanazawa A ,
- Zaghloul H ,
- Chagoury O ,
- Astbury NM ,
- Aveyard P ,
- Nickless A ,
- Athinarayanan SJ ,
- Hallberg SJ ,
- Saslow LR ,
- Summers C ,
- Aikens JE ,
- Khalid AA ,
- Yancy WS Jr . ,
- Crowley MJ ,
- Johnston BC ,
- Kanters S ,
- Bandayrel K ,
- de Melo IS ,
- de Oliveira SL ,
- da Rocha Ataide T
- Tobias DK ,
- Manson JE ,
- Ludwig DS ,
- Willett W ,
- van Zuuren EJ ,
- Fedorowicz Z ,
- Kuijpers T ,
- McKenzie AL ,
- Williams PT ,
- Feinman RD ,
- Pogozelski WK ,
- O’Driscoll T ,
- Clarke RE ,
- Coulter SN ,
- Rynders CA ,
- Thomas EA ,
- Catenacci VA ,
- Melanson EL
- Headland M ,
- Clifton PM ,
- de Cabo R ,
- Mozaffarian D
- Forouhi NG ,
- Krauss RM ,
- Koulman A ,
- Schwingshackl L ,
- Hoffmann G ,
- Lampousi AM ,
- Appleby PN ,
- Bradbury KE ,
- Schulze MB ,
- Martínez-González MA ,
- Lichtenstein AH ,
- Dambha-Miller H ,
- Strelitz J ,
- Karter AJ ,
- Parker MM ,
- Moffet HH ,
- Tangelloju S ,
- Little BB ,
- Esterhay RJ ,
- Choudhari PK ,
- Mahajan RR ,
- Sarathi V ,
- Chaithanya HB ,
- Dwarakanath CS
- Umphonsathien M ,
- Prutanopajai P ,
- Aiam-O-Ran J ,
- Snehalatha C ,
- Nanditha A ,
- Araujo-Soares V ,
- Thomas JG ,
- Hollands GJ ,
- Shemilt I ,
- Marteau TM ,
- Passaretti M ,
- Coolbaugh S
Advertisement
A whole-food, plant-based intensive lifestyle intervention improves glycaemic control and reduces medications in individuals with type 2 diabetes: a randomised controlled trial
- Open access
- Published: 21 September 2024
Cite this article
You have full access to this open access article

- Cody J. Hanick 1 ,
- Courtney M. Peterson 1 ,
- Brenda C. Davis 2 ,
- Joan Sabaté 3 &
- John H. Kelly Jr. 4 , 5
608 Accesses
9 Altmetric
Explore all metrics
Aims/hypothesis
We conducted the largest and longest clinical trial comparing a whole-food, plant-based intervention with standard medical care (SMC) in individuals with type 2 diabetes.
We randomised (parallel-arm; computerised 1:1 randomisation ratio) 169 adults aged 18–75 years with type 2 diabetes in the Marshall Islands to an intensive whole-food, plant-based intervention with moderate exercise (PB+Ex) or SMC for 24 weeks. The PB+Ex intervention included 12 weeks of meals, exercise sessions and group classes. Primary outcomes were glycaemic control (HbA 1c , glucose, insulin and HOMA-IR) and glucose-lowering medication use. Secondary outcomes included lipids, blood pressure, heart rate and C-reactive protein. Only lab analysts were blinded.
Compared with SMC ( n =90 randomised; n =70 analysed), the PB+Ex ( n =79 randomised; n =66 analysed) intervention decreased HbA 1c by an additional 14 mmol/mol (1.3%) at week 12 (−22 vs −7 mmol/mol [−2.0% vs −0.7%]; p <0.0001) and 8 mmol/mol (0.7%) at week 24 (−16 vs −8 mmol/mol [−1.4% vs −0.7%]; p =0.01). Concomitantly, 63% of medicated PB+Ex participants reduced their glucose-lowering medications (vs 24%; p =0.006), and 23% of PB+Ex participants with a baseline HbA 1c <75 mmol/mol (<9%) achieved remission. Additionally, the PB+Ex intervention reduced weight (−2.7 kg; p <0.0001), C-reactive protein (−11 nmol/l; p =0.005) and cardiovascular medication use compared with SMC. At intermediate timepoints, it improved glucose, insulin, HOMA-IR, cholesterol, triglycerides and heart rate, but not at week 24.
Conclusions/interpretation
A whole-food, plant-based lifestyle intervention was more effective for improving glycaemic control than SMC. It also reduced the need for diabetes and cardiovascular medications and induced diabetes remission in some participants. Therefore, it is an effective, evidence-based lifestyle option for individuals with type 2 diabetes.
Trial registration
ClinicalTrials.gov NCT03862963
This research was funded by the Department of the Army (W81XWH-05-1-0547). CJH received support through a National Institutes of Health Predoctoral T32 Obesity Fellowship (T32 HL105349).
Graphical Abstract
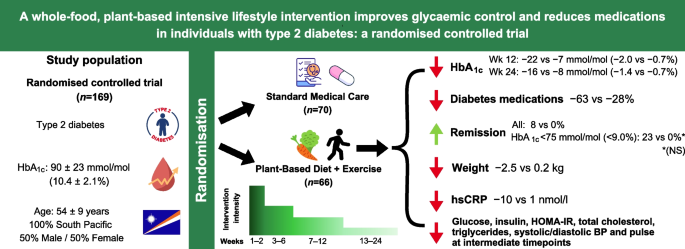
Similar content being viewed by others

The BROAD study: A randomised controlled trial using a whole food plant-based diet in the community for obesity, ischaemic heart disease or diabetes

An Update on the Effects of Plant-Based Diets on Cardiometabolic Factors in Adults with Type 2 Diabetes Mellitus
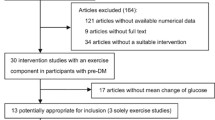
Comparison of control fasting plasma glucose of exercise-only versus exercise-diet among a pre-diabetic population: a meta-analysis
Avoid common mistakes on your manuscript.

Introduction
Diabetes is a pressing global health issue, affecting 10.5% of adults worldwide [ 1 ]. Although the causes of type 2 diabetes are complex, diet is a leading risk factor. Data from the Global Burden of Disease study suggest that a poor diet (low in vegetables, fruit, whole grains and fibre, and high in sugar-sweetened beverages, processed meat and red meat) is the second leading risk factor for diabetes, behind only a high BMI [ 2 ].
Increasingly, data suggest that a healthy diet can not only prevent type 2 diabetes but may also treat and reverse it. The Diabetes Remission Clinical Trial (DiRECT) found that a diet very low in energy (very-low-calorie diet [VLCD]) decreased HbA 1c by 10 mmol/mol (0.9%), and 46% of participants with early-stage type 2 diabetes went into remission vs only 4% receiving standard care [ 3 ]. However, VLCDs fail to improve HbA 1c in up to 40% of individuals, they are difficult to adhere to and only a minority of individuals are willing to try them [ 3 , 4 , 5 ]. Moreover, VLCDs pose several health risks, including headaches, dizziness, muscle cramps and bone loss [ 3 ].
Therefore, improving diet quality may be a better approach for treating type 2 diabetes. Of these approaches, low-carbohydrate diets, particularly ketogenic diets, are the most well-studied. Clinical trials suggest ketogenic diets lower HbA 1c [ 6 ], reduce diabetes medications [ 6 ] and induce diabetes remission in some individuals [ 7 ]. However, low-carbohydrate diets are associated with increased mortality risk [ 8 ] and can actually impair glucose tolerance [ 9 , 10 , 11 ] and increase cholesterol and inflammation [ 11 , 12 ].
An alternative, potentially more promising, approach is whole-food, plant-based (WFPB) diets, which are predominated by whole foods such as vegetables, fruits, legumes, whole grains, nuts and seeds. WFPB diets include not only vegetarian and vegan diets but also other plant-predominant diets that incorporate meat and seafood. Clinical trials in adults with type 2 diabetes have found that WFPB diets lower HbA 1c [ 13 , 14 , 15 ], reduce the need for diabetes medications [ 16 , 17 , 18 , 19 , 20 ] and allow some individuals to wean off all medications [ 16 , 18 , 19 ]. For example, one small study found that 55% of individuals with insulin-dependent type 2 diabetes no longer needed exogenous insulin after only 16 days on a WFPB diet [ 19 ]. In addition, WFPB diets can reduce body weight without deliberate energy restriction [ 21 , 22 ], have fewer side effects than VLCDs and ketogenic diets and may also have better adherence and acceptability [ 23 , 24 ].
Although promising, most studies examining WFPB diets and diabetes are small and short-term. Thus, we conducted the largest and longest randomised controlled trial to compare a WFPB intervention vs standard medical care (SMC) in participants with type 2 diabetes. We conducted the clinical trial in the Republic of the Marshall Islands (RMI), which has the seventh-highest diabetes prevalence globally [ 25 ]. The country’s high prevalence has been partially attributed to its increased reliance on imported foods, including white rice, refined flour, sugar-sweetened beverages and canned meats [ 26 , 27 , 28 , 29 , 30 , 31 ], which provides an excellent milieu to test whether improving diet quality can treat type 2 diabetes. To mirror other intensive lifestyle interventions, we paired a WFPB diet with moderate exercise and hypothesised that the combination would be more effective than SMC for improving glycaemic control and cardiovascular health and would reduce the need for glucose-lowering medications.
Study design
We conducted a 24 week parallel-arm, randomised controlled trial comparing a whole-food, plant-based intervention with moderate exercise (PB+Ex) with SMC in adults with type 2 diabetes. The study was approved by the institutional review board at Loma Linda University (protocol no. 59105) and an ad hoc institutional review board assembled by the RMI Minister of Health.
Study population
We enrolled adults with type 2 diabetes aged 18–75 years. Participants either had an HbA 1c ≥64 mmol/mol (≥8.0%) or were diagnosed with type 2 diabetes and taking glucose-lowering medication. Exclusion criteria included heart disease, changes in glucose-lowering medications in the past 3 months and physical or medical conditions that hinder participation. Enrolment was generally representative of Marshallese adults with type 2 diabetes (see the electronic supplementary material [ ESM ]). Participants provided written informed consent before participating, and demographic variables including biological sex were collected by self-report. Participants were enrolled in five cohorts and randomised in a 1:1 ratio generated by Microsoft Excel. Participants were randomised a few days before baseline data collection to give them time to make accommodations to attend weekly classes. Further details on recruitment, randomisation, the lifestyle intervention and the methods are provided in the protocol manuscript [ 32 ].
Standard care
The control group was treated using glucose-lowering pharmacotherapy, according to SMC in the RMI. They were instructed to maintain their current diet and physical activity levels.
PB+Ex intervention
The PB+Ex intervention is described in detail in the protocol manuscript [ 32 ]. In brief, the PB+Ex group was instructed to eat a WFPB diet permitting minimal animal products and to exercise 30–60 min/day for 24 weeks. During weeks 1–12, the PB+Ex group received prepared meals, attended group exercise sessions and received group instruction on eating healthfully, cooking, exercising and managing stress (see ESM Table 1 for a list of class topics). The intervention was culturally tailored and developed in partnership with the Marshallese government and local diabetes clinics and included Marshallese staff and popular foods (see the ESM for a detailed description of the cultural adaptation and positionality statements). The intensity of support progressively decreased, with participants attending 15–21 h/week of group classes in weeks 1–2, 8–10 h/week in weeks 3–6 and 4–5 h/week in weeks 7–12. During weeks 13–24 (the follow-up phase), participants were instructed to follow the intervention on their own.
The prescribed diet was high in fibre (35 g/ 4184 kJ), low in fat (20–25% of energy; saturated fat <7% of energy), moderate in protein (10–15% of energy) and low in sodium (<2400 mg/day). During weeks 1–2 (the intensive phase), PB+Ex participants received 12 prepared meals/week and were instructed to consume no animal products and minimal ground grains and refined carbohydrates. Thereafter, participants received 2 meals/week during weeks 3–6 and 1 meal/week during weeks 7–12. During weeks 3–12, participants could consume small amounts of animal foods, oils, fat-rich foods and processed foods, following a four-tiered food classification system [ 33 ]. Specifically, they were instructed to consume 75–100% of energy from whole, unprocessed plant foods (tier 1), such as vegetables, legumes, whole grains and fruit. The remainder of their diet could include ≤25% lightly processed foods (tier 2), ≤10% moderately processed foods and moderate-fat animal products (tier 3) and ≤5% heavily processed foods and high-fat animal products (tier 4).
The PB+Ex group was instructed to do moderate-intensity aerobic and resistance exercise 60 min/day during weeks 1–2 and 30–60 min/day during weeks 3–24. During weeks 1–2, participants attended 1 h group exercise classes 4 days/week. Thereafter, they attended group exercise classes twice a week during weeks 3–6 and once a week during weeks 7–12. Participants were also counselled to walk 10–20 min before breakfast and after lunch and dinner.
Cohort differences
To increase intervention intensity, participants in cohorts 3–5 of the PB+Ex group repeated weeks 1 and 2 during weeks 4 and 6. This included a repeat of educational sessions, meals provided and exercise classes.
Study outcomes
Outcomes were assessed at weeks 0, 2, 6, 12 and 24. The primary outcome was glycaemic control, measured by HbA 1c , fasting glucose, fasting insulin, HOMA-IR and diabetes medication use. Secondary endpoints were cardiovascular risk factors, including body weight, waist circumference, lipids, systolic blood pressure (SBP), diastolic blood pressure (DBP), resting heart rate, high-sensitivity C-reactive protein (hsCRP) and cardiovascular medication use. Only lab analysts performing the serum assays were blinded.
Serum chemistry
HbA 1c , glucose, insulin, total cholesterol, HDL-cholesterol, triglycerides and hsCRP were analysed blinded at the Clinical Laboratory Improvement Amendments-approved laboratory in the Ministry of Health’s Hospital, while LDL-cholesterol was calculated using the Friedewald equation. Triglyceride values >4.52 mmol/l (>400 mg/dl) were Winsorised to minimise the effect of outliers on the analyses. LDL-cholesterol values were treated as missing whenever triglyceride values exceeded 4.52 mmol/l. hsCRP values ≥95 mmol/l (≥10 mg/l) were considered indicative of acute infection and treated as missing.
Medication use
Primary care physicians and/or the Diabetes Wellness Clinic’s clinicians adjusted participants’ medications based on glucometer and/or serum glucose values. PB+Ex participants on insulin were monitored daily with glucometers and instructed to reduce insulin doses when glucose fell to <3.9 mmol/l (<70 mg/dl) or hypoglycaemic symptoms manifested. For SMC participants on insulin, their physicians were responsible for adjusting their medication doses. Diabetes medication use was quantified using the medication effect score (MES) [ 25 ], which estimates the HbA 1c reduction expected from all glucose-lowering pharmacotherapy [ 34 ]. Diabetes remission was defined as achieving HbA 1c <48 mmol/mol (<6.5%) after not using glucose-lowering medications for at least 3 months.
Statistical power
The required sample size was estimated using the variances observed in HbA 1c and glucose in cohorts 1–2. A sample size of n =120 was needed to have 80% power to detect a 1.1 mmol/l (20 mg/dl) difference in glucose and an 11 mmol/mol (1.0%) difference in HbA 1c , given α=0.05.
Statistical analyses
Analyses were performed with two-sided tests and α=0.05, primarily using SAS (version 9.4; SAS Institute; Cary, NC, USA). Baseline data were compared using independent samples t tests or the Mann–Whitney U test if neither raw nor transformed values were normal. The main analysis was intention-to-treat. Continuous data were analysed using linear mixed models, adjusting for baseline values, sex and/or cohort whenever statistically merited. Categorical data were analysed using Fisher’s exact test. Missing medication doses were singly imputed whenever a missing dose was flanked by two timepoints with identical doses and were otherwise treated as missing. When HbA 1c values were missing, remission status was singly imputed by assuming HbA 1c values changed by no more than 16 mmol/mol (1.5%) between weeks 0 and 2 and by no more than 33 mmol/mol (3.0%) between each subsequent pair of timepoints. Insulin and HOMA-IR were analysed only in participants not on insulin, while MES was analysed only in those taking glucose-lowering medication. The proportion of participants who decreased their medication doses was calculated in the subgroup of participants on the medication(s) at baseline. Lastly, to calculate diabetes remission and decreases in medication doses, we included everyone with sufficient data at either week 12, week 24 or both timepoints, in order to increase the sample size and improve accuracy in the estimated proportions.
Participants
As shown in ESM Fig. 1 , we screened 530 people. Of these, 361 did not meet the eligibility criteria, primarily due to HbA 1c being out of range and secondarily due to cardiovascular pathologies, such as angina. We randomised 169 participants (SMC: n =90; PB+Ex: n =79), but 31 withdrew before baseline data were collected. Seventy-two participants received the SMC intervention, while 66 received the PB+Ex intervention. Twenty-eight participants (20%) were lost to follow-up at week 24, and retention was similar between groups ( p =1.00). Two completers were excluded from the analyses after we later discovered that they no longer had diabetes at baseline. There were no adverse events related to the protocol. Participant characteristics are summarised in Table 1 . All participants were of Pacific Islander descent, and 50% were female. Participants had a mean age (±SD) of 54 ± 9 years, a BMI of 29.8 ± 4.9 kg/m 2 , an HbA 1c of 90 ± 23 mmol/mol (10.4 ± 2.1%) and a fasting glucose of 12.9 ± 4.2 mmol/l, indicating high rates of uncontrolled type 2 diabetes. Sixty-one per cent of participants used ≥1 glucose-lowering agent, and metformin and sulfonylureas were each used by 40% of participants. Only 9% of participants were on insulin. Thirty-two per cent of participants were taking ≥1 cardiovascular medication. All participant characteristics were similar between groups at baseline, except SBP, which was 8 mmHg higher in the PB+Ex group ( p =0.04). Thus, blood pressure analyses were adjusted for baseline values.
Glycaemic outcomes
ESM Table 2 summarises the results for all cardiometabolic outcomes. Glycaemic outcomes are illustrated in Fig. 1 . Compared with SMC, the PB+Ex intervention reduced HbA 1c by an additional 13 mmol/mol (1.2%) at week 6 (−20 vs −7 mmol/mol [−1.8% vs −0.6%]; 95% CI −19, −7 mmol/mol [−1.7%, −0.6%]; p <0.0001), 14 mmol/mol (1.3%) at week 12 (−22 vs −7 mmol/mol [−2.0% vs −0.7%]; 95% CI −20, −8 mmol/mol [−1.8%, −0.8%]; p <0.0001) and 8 mmol/mol (0.7%) at week 24 (−16 vs −8 mmol/mol [−1.4% vs −0.7%]; 95% CI −14, −1 mmol/mol [−1.3%, −0.1%]; p =0.01) (Fig. 1 a). Since many participants decreased their glucose-lowering medication doses, we used the MES to calculate the true reduction in HbA 1c if medication doses had not been adjusted (i.e. HbA 1c +MES; Fig. 1 b). Had medication doses not been changed, the PB+Ex intervention would have reduced HbA 1c by an additional 13 mmol/mol (1.2%) at week 6 (−21 vs −8 mmol/mol [−1.9% vs −0.8%]; 95% CI −20, −6 mmol/mol [−1.8%, −0.5%]; p =0.0004), 19 mmol/mol (1.7%) at week 12 (−26 vs −7 mmol/mol [−2.4% vs −0.6%]; 95% CI −26, −12 mmol/mol [−2.4%, −1.1%]; p <0.0001) and 11 mmol/mol (1.0%) at week 24 (−18 vs −7 mmol/mol [−1.6% vs −0.6%]; 95% CI −18, −3 mmol/mol [−1.7%, −0.3%]; p =0.005). The PB+Ex intervention also reduced glucose by an additional 3.2 mmol/l at week 2 (95% CI −4.4, −2.0 mmol/l; p <0.0001), 3.0 mmol/l at week 6 (95% CI −4.2, −1.8 mmol/l; p <0.0001) and 2.1 mmol/l at week 12 (95% CI −3.3, −0.9 mmol/l; p =0.0007) relative to SMC, but not at week 24 ( p =0.24) (Fig. 1 c). Among participants not taking insulin ( n =124), the PB+Ex intervention reduced fasting insulin at week 6 by 17.4 pmol/l (−12.6 vs −4.8 pmol/l; 95% CI −32.4, −2.5 pmol/l; p =0.02) but did not affect insulin at any other timepoint ( p ≥0.17; Fig. 1 d). The PB+Ex intervention also reduced HOMA-IR at week 2 (−1.74; 95% CI −3.37, −0.11; p =0.04), week 6 (−2.98; 95% CI −4.61, −1.35; p =0.0004) and week 12 (−1.74; 95% CI −3.25, −0.23; p =0.02), but not at week 24 ( p =0.53) (ESM Fig. 2 b).

Glycaemic control. The PB+Ex intervention was more effective than SMC at improving ( a ) HbA 1c and ( b ) the MES plus HbA 1c (HbA 1c +MES), which measures the true effect on HbA 1c if glucose-lowering medication doses had not been changed. The PB+Ex intervention also improved ( c ) fasting glucose at all timepoints except week 24 and ( d ) fasting insulin at week 2 only. Data shown are least-squares means ± SEMs. * p <0.05
Diabetes remission and medication use
Diabetes remission rates and medication use are shown in Fig. 2 . Eight per cent ( n =5) of PB+Ex participants achieved diabetes remission vs 0% in the SMC group ( n =127; p =0.02; Fig. 2 a). All PB+Ex participants who achieved remission had a baseline HbA 1c <75 mmol/mol (<9.0%). In a post hoc analysis, the PB+Ex intervention induced remission in 23% of participants with a baseline HbA 1c <75 mmol/mol (<9.0%), although this was not statistically different from the SMC group (vs 0%; n =36; p =0.13; Fig. 2 b). In addition, 63% of PB+Ex participants reduced their baseline dose of glucose-lowering medications vs only 24% of SMC participants ( n =56; p =0.006). This was mirrored by statistically significant decreases in MES at all timepoints, including at week 12 (−6 mmol/mol [−0.5%]; 95% CI −8, −3 mmol/mol; p =0.0002) and week 24 (−9 mmol/mol [−0.7%]; 95% CI −12, −5 mmol/mol; p <0.0001) ( n =91; ESM Fig. 2 a). Lastly, 67% of PB+Ex participants reduced their dose of one or more cardiovascular medications vs only 15% of the SMC group ( n =19; p =0.046).
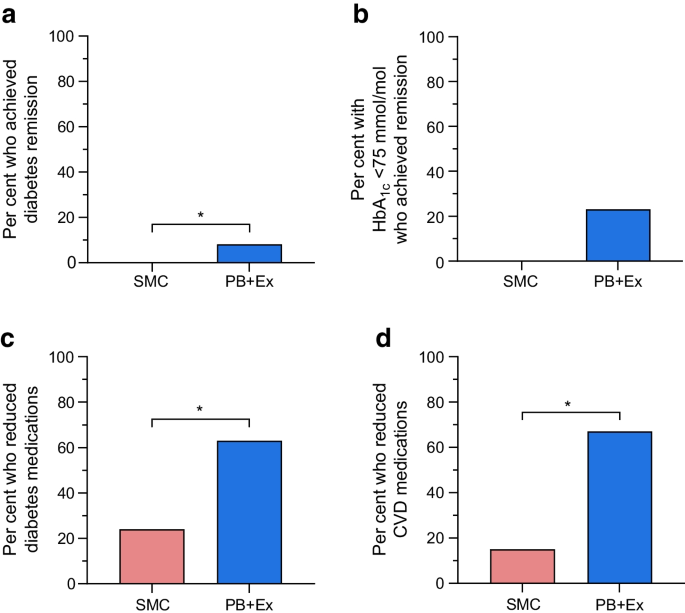
Diabetes remission and medication use. ( a ) PB+Ex was more effective than SMC at inducing diabetes remission. ( b ) About one-quarter of PB+Ex participants with a baseline HbA 1c <75 mmol/mol (<9%) achieved remission. The PB+Ex group also significantly reduced their doses of ( c ) diabetes medications and ( d ) cardiovascular medications. Data shown are proportions (%). * p <0.05
Body weight and cardiovascular disease risk factors
Figure 3 shows the effects on body weight and cardiovascular risk factors. Relative to SMC, the PB+Ex intervention modestly reduced body weight at all timepoints, including week 2 (−1.4 kg; 95% CI −2.2, −0.6 kg; p =0.001), week 6 (−2.6 kg; 95% CI −3.4, −1.7 kg; p <0.0001), week 12 (−2.5 kg; 95% CI −3.4, −1.6 kg; p <0.0001; Fig. 3 a) and week 24 (−2.7 kg; 95% CI −3.6, −1.8 kg; p <0.0001). Similarly, waist circumference was significantly reduced in the PB+Ex group at weeks 6 (−1.8 cm; 95% CI −3.6, 0.0 cm; p =0.04), 12 (−1.9 cm; 95% CI −3.7, −0.1 cm; p =0.04) and 24 (−3.8 cm; 95% CI −5.8, −1.8 cm; p =0.0002), but not at week 2 ( p =0.34; ESM Fig. 2 c). The PB+Ex intervention also reduced total cholesterol by an additional 0.47 mmol/l at week 2 (95% CI −0.76, −0.19 mmol/l; p =0.001) and 0.38 mmol/l at week 6 (95% CI −0.67, −0.08 mmol/l; p =0.01), but not at week 12 or week 24 ( p ≥0.34; Fig. 3 b). This was driven by large decreases in triglycerides at week 2 (−0.47 mmol/l; 95% CI −0.72, −0.23 mmol/l; p =0.0002), week 6 (−0.28 mmol/l; 95% CI −0.53, −0.04 mmol/l; p =0.02) and week 12 (−0.38 mmol/l; 95% CI −0.63, −0.13 mmol/l; p =0.003), but not at week 24 ( p =0.09; Fig. 3 c). There were no differences in LDL-cholesterol ( p ≥0.13; Fig. 3 d) or HDL-cholesterol ( p ≥0.09; ESM Fig. 1 d) at any timepoint. In addition, the PB+Ex intervention reduced SBP by 8 mmHg at both week 2 (95% CI −14, −1 mmHg; p =0.02) and week 6 (95% CI −14, −1 mmHg; p =0.03), but not at week 12 or week 24 ( p ≥0.13; Fig. 3 e). Similarly, the PB+Ex intervention reduced DBP by 5 mmHg at week 2 (95% CI −9, −2 mmHg; p =0.003) and 4 mmHg at week 6 (95% CI −8, 0 mmHg; p =0.03), but not at week 12 or week 24 ( p ≥0.08; Fig. 3 f). Relative to SMC, the PB+Ex intervention also reduced heart rate by 4 beats/min at week 6 (95% CI −8, −1 beats/min; p =0.02) and 5 beats/min at week 12 (95% CI −8, −1 beats/min; p =0.02), but not at week 2 or week 24 ( p ≥0.10; Fig. 3 g). Lastly, the PB+Ex intervention more effectively lowered hsCRP at all timepoints, including week 2 (−14 nmol/l; 95% CI −21, −6 nmol/l; p =0.0003), week 6 (−14 nmol/l; 95% CI −22, −7 nmol/l; p =0.0003), week 12 (−9 nmol/l; 95% CI −16, −1 nmol/l; p =0.02) and week 24 (−11 nmol/l; 95% CI −19, −4 nmol/l; p =0.005; Fig. 3 h).
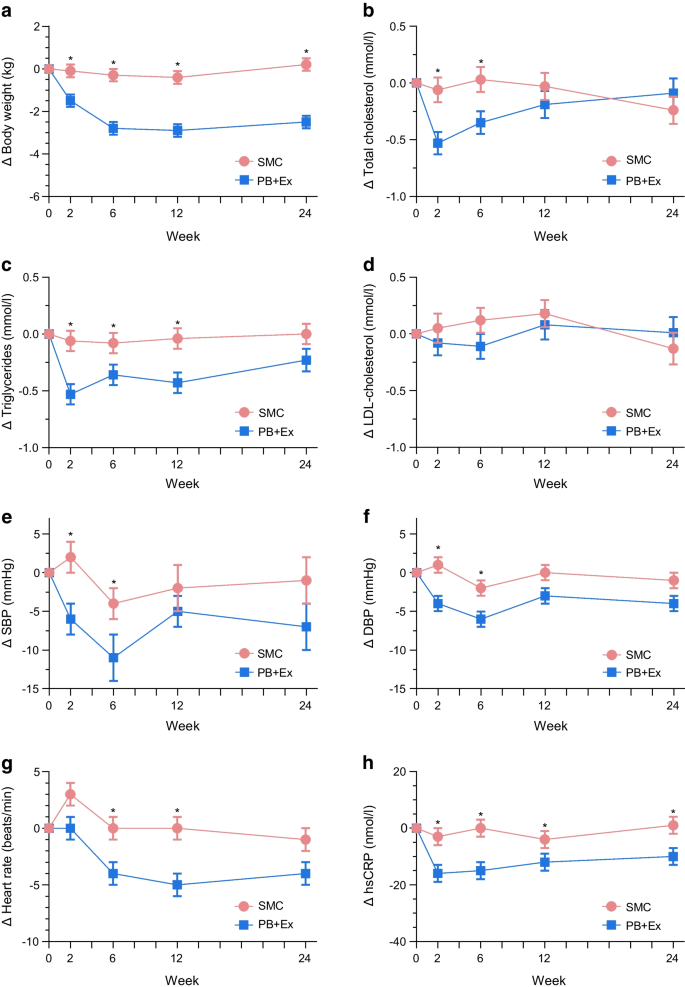
Cardiovascular disease risk factors. The PB+Ex intervention was more effective than SMC at lowering ( a ) body weight and ( h ) hsCRP. The PB+Ex intervention improved ( b ) total cholesterol, ( c ) triglycerides, ( e ) SBP, ( f ) DBP and ( g ) heart rate at intermediate timepoints but not at week 24. There were no differences in ( d ) LDL-cholesterol. Data shown are least-squares means ± SEMs. * p <0.05
We conducted the largest and longest randomised controlled trial to compare a WFPB intervention vs SMC in individuals with type 2 diabetes. We implemented a lifestyle intervention involving a WFPB diet including limited animal products and moderate exercise, which progressively decreased in intensity. The PB+Ex intervention was superior to SMC for improving HbA 1c , hsCRP, weight and waist circumference. The PB+Ex intervention also reduced the need for diabetes and cardiovascular medications and induced type 2 diabetes remission in some participants. At interim timepoints, the PB+Ex intervention improved nearly every cardiometabolic endpoint, although these differences attenuated as the intervention intensity decreased.
The PB+Ex intervention was far more effective at improving glycaemic control than SMC centred on medication management: it decreased HbA 1c by an additional 14 mmol/mol (1.3%) at week 12 and 8 mmol/mol (0.7%) at week 24. Importantly, the ‘true’ effect on HbA 1c levels was even larger than this because the SMC group increased their dose of glucose-lowering medications by the equivalent of 360 mg/day of metformin at week 24, whereas the PB+Ex group reduced their dose by 820 mg/day (a between-group difference of 1180 mg/day) [ 34 ]. After adjusting for medication changes, the PB+Ex intervention lowered HbA 1c by an additional 19 mmol/mol (1.7%) at week 12 and 11 mmol/mol (1.0%) at week 24 relative to SMC. Such a large improvement in HbA 1c levels could dramatically reduce the risks of comorbidities, particularly myocardial infarction and microvascular complications, and profoundly improve clinical management of type 2 diabetes. Interestingly, much of the glycaemic improvement occurred in the first 2 weeks of the study before any substantial weight loss occurred. The PB+Ex intervention decreased fasting glucose by a dramatic 4.0 mmol/l relative to baseline within only 2 weeks. This suggests that the improvements were due to changes in diet quality and/or physical activity rather than weight loss. For comparison, the glycaemic improvements we observed were much larger than the 7–11 mmol/mol (0.6–1.1%) reduction in HbA 1c reported in other clinical trials on plant-based diets [ 17 , 35 , 36 ] or the 3–4 mmol/mol (0.3–0.4%) reported in meta-analyses [ 13 , 37 ]. Plus, most studies on plant-based interventions report no improvements in fasting glucose or insulin [ 17 , 35 , 36 , 38 , 39 ]. The larger effects we observed may be due to the very high amounts of whole foods, the addition of moderate exercise, the provision of prepared meals and intensive instruction, participants having higher baseline HbA 1c values than in the USA [ 27 ] and/or participants having a lower quality diet at baseline [ 28 , 29 , 30 , 31 , 40 , 41 ]. A complex interplay of socioeconomic, geopolitical, and cultural factors involving limited arable land, displacement, a remote location, unemployment, and poverty have decreased access to nutritious foods [ 29 , 42 ].
The PB+Ex intervention also reduced the need for glucose-lowering and cardiovascular medications in roughly two-thirds of PB+Ex participants. Our intervention induced a greater reduction in medication use than in any other clinical trial on plant-based diets [ 15 , 17 , 35 , 36 , 38 , 39 , 43 ]. The PB+Ex intervention also induced type 2 diabetes remission in 8% of all participants and 23% of those with a baseline HbA 1c <75 mmol/mol (<9.0%). The participants who went into remission lost modest or no weight (~0–6 kg), and one even gained weight. For comparison, in the DiRECT trial, where participants’ mean baseline HbA 1c was 61 mmol/mol (7.7%), only 7% of participants who lost 0–5 kg went into remission [ 3 ]. This important finding suggests that type 2 diabetes remission is possible through improving diet quality and/or increasing physical activity, even if individuals do not lose weight.
We also investigated the effects of a PB+Ex intervention on cardiovascular risk factors. The PB+Ex intervention reduced total cholesterol and triglycerides at intermediate timepoints but did not affect LDL-cholesterol. It is unclear why we found a dramatic reduction in triglycerides but not LDL-cholesterol, as this conflicts with a meta-analysis reporting that plant-based diets lower total and LDL-cholesterol but do not affect triglycerides [ 44 ]. The PB+Ex intervention also decreased SBP by 8 mmHg at both week 2 and week 6 and DBP by 5 and 4 mmHg at weeks 2 and 6, respectively. However, the effects vanished as the intervention intensity waned. The effects we observed at intermediate time points were larger than those reported in a meta-analysis of plant-based diets, which reported 3 and 2 mmHg improvements in SBP and DBP, respectively [ 45 ]. The PB+Ex intervention also decreased heart rate by 4 and 5 beats/min at weeks 6 and 12, respectively, compared with the SMC, although the effect lost significance by week 24 ( p =0.10). These intermediate improvements in triglycerides, blood pressure and heart rate may partially explain why plant-based diets are associated with lower cardiovascular disease incidence and mortality risk [ 46 ]. Finally, the PB+Ex intervention reduced hsCRP at all timepoints, indicating the intervention decreased inflammation. Notably, biological sex was not a statistically significant covariate for any cardiometabolic outcome, suggesting no differences between males and females.
The strengths of this study include the innovative diet approach, large sample size, long duration, high male representation (50%), high-priority population, the cultural adaptation and using a lifestyle intervention that progressively decreased in intensity. The latter factor provided us with a unique opportunity to test different ‘doses’ of the PB+Ex intervention. Interestingly, during the most intensive phase, participants experienced robust improvements in nearly every cardiometabolic endpoint, although the effects for fasting glucose, insulin, triglycerides, heart rate and blood pressure waned as intervention intensity decreased. This suggests that a WFPB diet with moderate exercise can improve most cardiometabolic risk factors, but the effects depend on the intervention intensity and/or level of adherence. Limitations of this study include that no adherence data or diet records were collected; participants were randomised 3–5 days prior to baseline testing; some SMC participants adopted elements of the PB+Ex lifestyle intervention (which likely diluted estimates of the true treatment effects); no Marshallese citizens assisted with study design, data interpretation or manuscript writing; and there were minor differences in the intervention intensity in cohorts 1–2 vs 3–5 (although there were no statistically significant differences between cohorts in virtually all outcomes).
In conclusion, a WFPB lifestyle intervention with moderate exercise was more effective than SMC at improving glycaemic control, body weight, waist circumference and inflammation. It also lessened the need for glucose-lowering and cardiovascular medications and induced type 2 diabetes remission in some participants. Overall, our findings support the ‘food as medicine’ concept and suggest that WFPB interventions with moderate exercise may dramatically reduce the risk of comorbidities. A WFPB diet with moderate exercise can be offered as a highly effective, evidence-based lifestyle intervention for individuals with type 2 diabetes.
Abbreviations
Diastolic blood pressure
Diabetes Remission Clinical Trial
High-sensitivity C-reactive protein
Medication effect score
Whole-food, plant-based intervention with moderate exercise
Republic of the Marshall Islands
Systolic blood pressure
Standard medical care
Very-low-calorie diet
Whole-food, plant-based
Khan MAB, Hashim MJ, King JK, Govender RD, Mustafa H, Al Kaabi J (2020) Epidemiology of type 2 diabetes - global burden of disease and forecasted trends. J Epidemiol Glob Health 10(1):107–111. https://doi.org/10.2991/jegh.k.191028.001
Article PubMed PubMed Central Google Scholar
Ong KL, Stafford LK, McLaughlin SA et al (2023) Global, regional, and national burden of diabetes from 1990 to 2021, with projections of prevalence to 2050: a systematic analysis for the Global Burden of Disease Study 2021. Lancet 402(10397):203–234. https://doi.org/10.1016/s0140-6736(23)01301-6
Article Google Scholar
Lean ME, Leslie WS, Barnes AC et al (2018) Primary care-led weight management for remission of type 2 diabetes (DiRECT): an open-label, cluster-randomised trial. Lancet 391(10120):541–551. https://doi.org/10.1016/s0140-6736(17)33102-1
Article PubMed Google Scholar
Steven S, Hollingsworth KG, Al-Mrabeh A et al (2016) Very low-calorie diet and 6 months of weight stability in type 2 diabetes: pathophysiological changes in responders and nonresponders. Diabetes Care 39(5):808–815. https://doi.org/10.2337/dc15-1942
Article CAS PubMed Google Scholar
Paisey RB, Frost J, Harvey P et al (2002) Five year results of a prospective very low calorie diet or conventional weight loss programme in type 2 diabetes. J Hum Nutr Diet 15(2):121–127. https://doi.org/10.1046/j.1365-277x.2002.00342.x
Rafiullah M, Musambil M, David SK (2022) Effect of a very low-carbohydrate ketogenic diet vs recommended diets in patients with type 2 diabetes: a meta-analysis. Nutr Rev 80(3):488–502. https://doi.org/10.1093/nutrit/nuab040
Goldenberg JZ, Day A, Brinkworth GD et al (2021) Efficacy and safety of low and very low carbohydrate diets for type 2 diabetes remission: systematic review and meta-analysis of published and unpublished randomized trial data. BMJ 372:m4743. https://doi.org/10.1136/bmj.m4743
Noto H, Goto A, Tsujimoto T, Noda M (2013) Low-carbohydrate diets and all-cause mortality: a systematic review and meta-analysis of observational studies. PLoS One 8(1):e55030. https://doi.org/10.1371/journal.pone.0055030
Article CAS PubMed PubMed Central Google Scholar
Rosenbaum M, Hall KD, Guo J et al (2019) Glucose and lipid homeostasis and inflammation in humans following an isocaloric ketogenic diet. Obesity (Silver Spring) 27(6):971–981. https://doi.org/10.1002/oby.22468
Hall KD, Guo J, Courville AB et al (2021) Effect of a plant-based, low-fat diet versus an animal-based, ketogenic diet on ad libitum energy intake. Nat Med 27(2):344–353. https://doi.org/10.1038/s41591-020-01209-1
Webster CC, van Boom KM, Armino N et al (2020) Reduced glucose tolerance and skeletal muscle GLUT4 and IRS1 content in cyclists habituated to a long-term low-carbohydrate, high-fat diet. Int J Sport Nutr Exerc Metab 30(3):210–217. https://doi.org/10.1123/ijsnem.2019-0359
Chawla S, Tessarolo Silva F, Amaral Medeiros S, Mekary RA, Radenkovic D (2020) The effect of low-fat and low-carbohydrate diets on weight loss and lipid levels: a systematic review and meta-analysis. Nutrients 12(12):3774. https://doi.org/10.3390/nu12123774
Toumpanakis A, Turnbull T, Alba-Barba I (2018) Effectiveness of plant-based diets in promoting well-being in the management of type 2 diabetes: a systematic review. BMJ Open Diabetes Res Care 6(1):e000534. https://doi.org/10.1136/bmjdrc-2018-000534
de Carvalho GB, Dias-Vasconcelos NL, Santos RKF, Brandão-Lima PN, da Silva DG, Pires LV (2020) Effect of different dietary patterns on glycemic control in individuals with type 2 diabetes mellitus: a systematic review. Crit Rev Food Sci Nutr 60(12):1999–2010. https://doi.org/10.1080/10408398.2019.1624498
Jenkins DJ, Jones PJ, Abdullah MM et al (2022) Low-carbohydrate vegan diets in diabetes for weight loss and sustainability: a randomized controlled trial. Am J Clin Nutr 116(5):1240–1250. https://doi.org/10.1093/ajcn/nqac203
Dunaief DM, Fuhrman J, Dunaief JL, Ying G (2012) Glycemic and cardiovascular parameters improved in type 2 diabetes with the high nutrient density (HND) diet. Open J Prev Med 2(3):364–371. https://doi.org/10.4236/ojpm.2012.23053
Article CAS Google Scholar
Kahleova H, Matoulek M, Malinska H et al (2011) Vegetarian diet improves insulin resistance and oxidative stress markers more than conventional diet in subjects with Type 2 diabetes. Diabet Med 28(5):549–559. https://doi.org/10.1111/j.1464-5491.2010.03209.x
Karlsen M, Panigraphi G, Kelly J (2021) 503-P: intensive lifestyle interventions for treatment towards remission of type 2 diabetes: a case series. Diabetes 70(Suppl 1):503-P. https://doi.org/10.2337/db21-503-P
Anderson JW, Ward K (1979) High-carbohydrate, high-fiber diets for insulin-treated men with diabetes mellitus. Am J Clin Nutr 32(11):2312–2321. https://doi.org/10.1093/ajcn/32.11.2312
Campbell TM, Campbell EK, Attia J et al (2023) The acute effects of a DASH diet and whole food, plant-based diet on insulin requirements and related cardiometabolic markers in individuals with insulin-treated type 2 diabetes. Diabetes Res Clin Pract 202:110814. https://doi.org/10.1016/j.diabres.2023.110814
Austin G, Ferguson JJA, Garg ML (2021) Effects of plant-based diets on weight status in type 2 diabetes: a systematic review and meta-analysis of randomised controlled trials. Nutrients 13(11):4099. https://doi.org/10.3390/nu13114099
Tran E, Dale HF, Jensen C, Lied GA (2020) Effects of plant-based diets on weight status: a systematic review. Diabetes Metab Syndr Obes 13:3433–3448. https://doi.org/10.2147/dmso.S272802
Melina V, Craig W, Levin S (2016) Position of the academy of nutrition and dietetics: vegetarian diets. J Acad Nutr Diet 116(12):1970–1980. https://doi.org/10.1016/j.jand.2016.09.025
Moore WJ, McGrievy ME, Turner-McGrievy GM (2015) Dietary adherence and acceptability of five different diets, including vegan and vegetarian diets, for weight loss: the New DIETs study. Eat Behav 19:33–38. https://doi.org/10.1016/j.eatbeh.2015.06.011
Sun H, Saeedi P, Karuranga S et al (2022) IDF Diabetes Atlas: Global, regional and country-level diabetes prevalence estimates for 2021 and projections for 2045. Diabetes Res Clin Pract 183:109119. https://doi.org/10.1016/j.diabres.2021.109119
Ahlgren I, Yamada S, Wong A (2014) Rising oceans, climate change, food aid, and human rights in the Marshall Islands. Health Hum Rights 16(1):69–80
PubMed Google Scholar
Hankosky ER, Schapiro D, Gunn KB, Lubelczyk EB, Mitroi J, Nelson DR (2023) Gaps remain for achieving HbA1c targets for people with type 1 or type 2 diabetes using insulin: results from NHANES 2009–2020. Diabetes Ther 14(6):967–975. https://doi.org/10.1007/s13300-023-01399-0
Kodish SR, Matean M, Grey K et al (2022) Conceptualizing multi-level determinants of infant and young child nutrition in the Republic of Marshall Islands-a socio-ecological perspective. PLOS Glob Public Health 2(12):e0001343. https://doi.org/10.1371/journal.pgph.0001343
McElfish PA, Smith L, Sparks K et al (2019) A community-based participatory approach to promote healthy eating among Marshallese. Hawaii J Health Soc Welf 78(11):332–337
PubMed PubMed Central Google Scholar
Gittelsohn J, Dyckman W, Tan ML et al (2006) Development and implementation of a food store-based intervention to improve diet in the Republic of the Marshall Islands. Health Promot Pract 7(4):396–405. https://doi.org/10.1177/1524839905278620
Dela Cruz R, Novotny R, Wilkens LR et al (2023) Diet quality of young children in the US-Affiliated Pacific’s Children’s Healthy Living (CHL) program. J Acad Nutr Diet 123(12):1781–1792. https://doi.org/10.1016/j.jand.2023.08.003
Davis BC, Jamshed H, Peterson CM et al (2019) An intensive lifestyle intervention to treat type 2 diabetes in the republic of the Marshall islands: protocol for a randomized controlled trial. Front Nutr 6:79. https://doi.org/10.3389/fnut.2019.00079
Davis B, Barnard T (2003) Defeating diabetes: a no-nonsense approach to type 2 diabetes and the diabesity epidemic. Healthy Living Publications, Summertown, TN, USA
Google Scholar
Alexopoulos AS, Yancy WS, Edelman D et al (2021) Clinical associations of an updated medication effect score for measuring diabetes treatment intensity. Chronic Illn 17(4):451–462. https://doi.org/10.1177/1742395319884096
Lee YM, Kim SA, Lee IK et al (2016) Effect of a brown rice based vegan diet and conventional diabetic diet on glycemic control of patients with type 2 diabetes: a 12-week randomized clinical trial. PLoS One 11(6):e0155918. https://doi.org/10.1371/journal.pone.0155918
Barnard ND, Cohen J, Jenkins DJ et al (2009) A low-fat vegan diet and a conventional diabetes diet in the treatment of type 2 diabetes: a randomized, controlled, 74-wk clinical trial. Am J Clin Nutr 89(5):1588s–1596s. https://doi.org/10.3945/ajcn.2009.26736H
Viguiliouk E, Kendall CW, Kahleová H et al (2019) Effect of vegetarian dietary patterns on cardiometabolic risk factors in diabetes: a systematic review and meta-analysis of randomized controlled trials. Clin Nutr 38(3):1133–1145. https://doi.org/10.1016/j.clnu.2018.05.032
Barnard ND, Levin SM, Gloede L, Flores R (2018) Turning the waiting room into a classroom: weekly classes using a vegan or a portion-controlled eating plan improve diabetes control in a randomized translational study. J Acad Nutr Diet 118(6):1072–1079. https://doi.org/10.1016/j.jand.2017.11.017
Bunner AE, Wells CL, Gonzales J, Agarwal U, Bayat E, Barnard ND (2015) A dietary intervention for chronic diabetic neuropathy pain: a randomized controlled pilot study. Nutr Diabetes 5(5):e158. https://doi.org/10.1038/nutd.2015.8
Gittelsohn J, Haberle H, Vastine AE, Dyckman W, Palafox NA (2003) Macro- and microlevel processes affect food choice and nutritional status in the republic of the marshall islands. J Nutr 133(1):310s–313s. https://doi.org/10.1093/jn/133.1.310S
Hingle M, Short E, Aflague T et al (2023) Food security is associated with higher diet quality among children of the US-Affiliated Pacific Region. J Nutr 153(3):848–856. https://doi.org/10.1016/j.tjnut.2023.01.015
Yamada S, Palafox N (2001) On the biopsychosocial model: the example of political economic causes of diabetes in the Marshall Islands. Fam Med 33(9):702–704
CAS PubMed Google Scholar
Nicholson AS, Sklar M, Barnard ND, Gore S, Sullivan R, Browning S (1999) Toward improved management of NIDDM: a randomized, controlled, pilot intervention using a lowfat, vegetarian diet. Prev Med 29(2):87–91. https://doi.org/10.1006/pmed.1999.0529
Koch CA, Kjeldsen EW, Frikke-Schmidt R (2023) Vegetarian or vegan diets and blood lipids: a meta-analysis of randomized trials. Eur Heart J 44(28):2609–2622. https://doi.org/10.1093/eurheartj/ehad211
Lee KW, Loh HC, Ching SM, Devaraj NK, Hoo FK (2020) Effects of vegetarian diets on blood pressure lowering: a systematic review with meta-analysis and trial sequential analysis. Nutrients 12(6):1604. https://doi.org/10.3390/nu12061604
Quek J, Lim G, Lim WH et al (2021) The association of plant-based diet with cardiovascular disease and mortality: a meta-analysis and systematic review of prospect cohort studies. Front Cardiovasc Med 8:756810. https://doi.org/10.3389/fcvm.2021.756810
Download references
Author information
Authors and affiliations.
Department of Nutrition Sciences, University of Alabama at Birmingham, Birmingham, AL, USA
Cody J. Hanick & Courtney M. Peterson
Brenda Davis Nutrition Consultation Services, Kelowna, BC, Canada
Brenda C. Davis
School of Public Health, Center for Nutrition, Lifestyle, and Disease Prevention, Loma Linda University, Loma Linda, CA, USA
Joan Sabaté
Department of Preventive Medicine, School of Medicine, Loma Linda University, Loma Linda, CA, USA
John H. Kelly Jr.
Lifestyle Health Education Inc., Rocky Mount, VA, USA
You can also search for this author in PubMed Google Scholar
Corresponding author
Correspondence to John H. Kelly Jr. .
Ethics declarations
Acknowledgements.
The authors are extremely grateful to the government of the Republic of the Marshall Islands, especially the Ministry of Health for providing Marshallese staff and medical professionals, sample processing and the building to set up the Diabetes Wellness Center; the RMI Diabetes Wellness Clinic for providing aid in recruitment and medical care for SMC participants; and the Canvasback Missions staff, who were invaluable in working with the Ministry of Health and conducting this trial. The authors also thank R. D. Harris (former physician at The Meridian Senior Retirement Center) and J. D. Spence (President and co-founder of Canvasback Missions, Inc.) for their help in conducting the trial and coordinating logistics. An abstract and a research poster were presented virtually at the American Diabetes Association’s 82nd Scientific Sessions on 3 June 2022.
Data availability
The datasets generated during and/or analysed in the current study are available from the corresponding author upon reasonable request.
Open access funding provided by SCELC, Statewide California Electronic Library Consortium. This research was funded by the Department of the Army (W81XWH-05-1-0547). The sponsor had no role in the design and conduct of the trial or in the analysis, interpretation, and publication of data. CJH received support through a National Institutes of Health Predoctoral T32 Obesity Fellowship (T32 HL105349). The content is solely the responsibility of the authors and does not necessarily reflect the views of the National Institutes of Health.
Authors’ relationships and activities
BCD is the author of the books Defeating Diabetes and The Kick Diabetes Cookbook: An Action Plan and Recipes for Defeating Diabetes . The authors declare that there are no other relationships or activities that might bias, or be perceived to bias, their work.
Contribution statement
JHK designed the study, with input from JS. BCD and JHK conducted the study, including designing the menus, administering the intervention and acquiring data. CJH and CMP performed the statistical analyses and drafted the manuscript. All authors interpreted study data and revised and approved the final version of the manuscript. JHK is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Additional information
Publisher's note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
ESM (PDF 229 KB)
Rights and permissions.
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ .
Reprints and permissions
About this article
Hanick, C.J., Peterson, C.M., Davis, B.C. et al. A whole-food, plant-based intensive lifestyle intervention improves glycaemic control and reduces medications in individuals with type 2 diabetes: a randomised controlled trial. Diabetologia (2024). https://doi.org/10.1007/s00125-024-06272-8
Download citation
Received : 06 March 2024
Accepted : 19 July 2024
Published : 21 September 2024
DOI : https://doi.org/10.1007/s00125-024-06272-8
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Cardiovascular disease
- Diabetes remission
- Dietary intervention
- Glycaemic control
- Lifestyle intervention
- Plant-based diet
- Randomised controlled trial
- Type 2 diabetes
- Find a journal
- Publish with us
- Track your research
REVIEW article
The role of nutrition in the prevention and intervention of type 2 diabetes.

- The Eighth Affiliated Hospital, Sun Yat-sen University, Shenzhen, China
Type 2 diabetes (T2D) is a rapidly growing epidemic, which leads to increased mortality rates and health care costs. Nutrients (namely, carbohydrates, fat, protein, mineral substances, and vitamin), sensing, and management are central to metabolic homeostasis, therefore presenting a leading factor contributing to T2D. Understanding the comprehensive effects and the underlying mechanisms of nutrition in regulating glucose metabolism and the interactions of diet with genetics, epigenetics, and gut microbiota is helpful for developing new strategies to prevent and treat T2D. In this review, we discuss different mechanistic pathways contributing to T2D and then summarize the current researches concerning associations between different nutrients intake and glucose homeostasis. We also explore the possible relationship between nutrients and genetic background, epigenetics, and metagenomics in terms of the susceptibility and treatment of T2D. For the specificity of individual, precision nutrition depends on the person’s genotype, and microbiota is vital to the prevention and intervention of T2D.
Introduction
Diabetes mellitus, previously considered as a disease of minor significance to health, is now becoming one of the main threats to human health both in developed and developing countries ( Zimmet et al., 2001 ). There has been an explosive increase in the number of people diagnosed with diabetes in recent decades worldwide ( King et al., 1998 ). According to the ninth edition of the IDF Diabetes Atlas in 2019, 488 million adults aged 20–99 years live with diabetes in the world, and the number will reach 578 million by 2030 and 700 million by 2045. It is estimated that 4.2 million adults aged 20–79 years will die of diabetes, which accounts for 11.3% of all deaths. And this is equivalent to eight deaths every minute.
Diabetes is defined as a metabolic disease characterized by persistent hyperglycemia caused by multiple factors including genetics, nutrition, environment, and physical activity. There are two main forms of diabetes, type 1 diabetes and type 2 diabetes (T2D) ( Alberti and Zimmet, 1998 ). T2D accounts for more than 90% of all diabetes cases ( Zimmet et al., 2001 ), and the diabetes epidemic particularly relates to T2D. Insulin resistance and/or abnormal insulin secretion are the main characters of T2D. Apart from the heightened genetic susceptibility of ethnic groups, environmental and behavioral factors are also very important in the development of T2D. Globalization results in altered dietary and lifestyle habits ( Malik et al., 2013 ), such as taking more high-fat or high-carbohydrate foods and sedentary lifestyles with low energy expenditure ( Zimmet et al., 2001 ). Diets induce multiple metabolic processes and modify the metabolism homeostasis of the organism ( Manore et al., 2017 ). Therefore, unhealthy dietary habits such as Western diet have been one of the most important drivers of glucose metabolism disorder that leads to diabetes finally ( Rico-Campa et al., 2019 ).
The increase in the prevalence of T2D is associated with a concomitant rise in the incidence of metabolic disorders. Long-term high glucose levels will trigger chronic metabolic syndrome and include obesity ( Schwartz and Porte, 2005 ), cardiovascular disease, retinopathy, nephropathy, dyslipidemia, and hypertension ( Moller, 2001 ). T2D now represents a risk of coronary heart disease, and nearly 80% of diabetic mortality is diabetes-induced cardiovascular disease ( Haffner et al., 1998 ). The life qualities of patients with diabetes decrease largely for the serious diabetes complications.
Diet alone or with hypoglycemic agents is the way to control blood glucose levels in the treatment of T2D ( Zimmet et al., 2001 ; Ley et al., 2014 ). Different diets with varied nutrient composition result in changes of metabolites and gut microbiome that are responsible for the glucose metabolism of the whole body ( Qin et al., 2012 ; Guasch-Ferre et al., 2016 ). For example, different amino acid content diets can lead to alterations of plasma branched-chain amino acid (BCAA) concentrations, which are linked to the risk of T2D ( Garcia-Perez et al., 2017 ). Fiber- and protein-enriched diet changed the abundance of Akkermansia muciniphila , decreasing fasting glucose levels of participants ( Dao et al., 2016 ). However, the interactions between dietary and glucose metabolism need further study to understand the importance of its actions for glucose management. It is important to identify and make suitable dietary solutions that can diminish the prevalence of diabetes and its related complications ( San-Cristobal et al., 2015 ). These include different kinds of food and also healthy dietary habits.
Genome-wide association studies (GWASs) have revealed many genetic variants related to the susceptibility of complex diseases, and moreover, the interactions between genetic information and nutrition are attracting more attention recently, namely, nutrigenetics. Because of the genetic variability between individuals, the responses to dietary are different. Also, the specific diet and nutrition modify gene expression, epigenetic features, and gut microbiome to personalize the response to interventions. This prompts us to explore more possibilities to understand the pathophysiological mechanisms and precision nutrition solutions to prevent and manage T2D more efficiently.
Herein, first, we introduce the major metabolic pathway related to T2D, namely, insulin signaling pathway, and the compounding factors as well. Then, the roles of macronutrient, micronutrient, and other chemicals in maintaining metabolic homeostasis of the body and their effects on T2D are reviewed in detail. In addition, some nutritional recommendations for T2D are summarized. From the perspective of precision nutrition, we review the diet interactions with genetic background, epigenetics, and gut microbiota contributing to the risk of T2D. Also, responses to dietary interventions mainly aiming at weight loss and management of insulin resistance are screened for their interaction with genetic, epigenetic features, and gut microbiota.
Regulation of Glucose Metabolism
Circulating blood glucose is derived from diet via intestinal absorption, and the process of glucose production is called gluconeogenesis and glycogen breakdown ( Rines et al., 2016 ). Current therapeutic approaches to treat T2D rely on the molecular signaling pathways and targets that impair glucose homeostasis. Insulin signaling pathway dysregulation or insulin resistance is the main reason for T2D. Insulin is an endocrine peptide hormone secreted by the pancreas, and it binds to membrane-bound receptors in target cells of liver, adipose tissue, and skeletal muscle to trigger metabolic responses to numerous stimuli ( Petersen and Shulman, 2018 ). Insulin exerts its low glucose function by binding to the insulin receptor (INSR), and then activated INSR recruits phosphotyrosine-binding scaffold proteins such as the INSR substrate (IRS) family. IRS proteins have NH 2 -terminal pleckstrin homology (PH) and PTB domains that target them to activate INSR. Then, the tyrosine phosphorylated IRS proteins recruit PI3K heterodimers that contain a regulatory p85 subunit and a catalytic p110 subunit. PI3K catalyzes the production of phosphatidylinositol-3,4,5-tris-phosphate (PIP 3 ) from PIP 2 and PIP 3 and then recruits proteins with PH domains to the plasma membrane, such as pyruvate dehydrogenase kinase 1, which directly phosphorylates AKT. The activated AKT phosphorylates many downstream substrates in various signaling pathways, making it a key node in insulin signaling ( Petersen and Shulman, 2018 ). The activated insulin signaling decreases glucose production, increases glycogen synthesis, and also increases glucose uptake into peripheral tissues such as skeletal muscle and adipose tissue ( Figure 1 ).
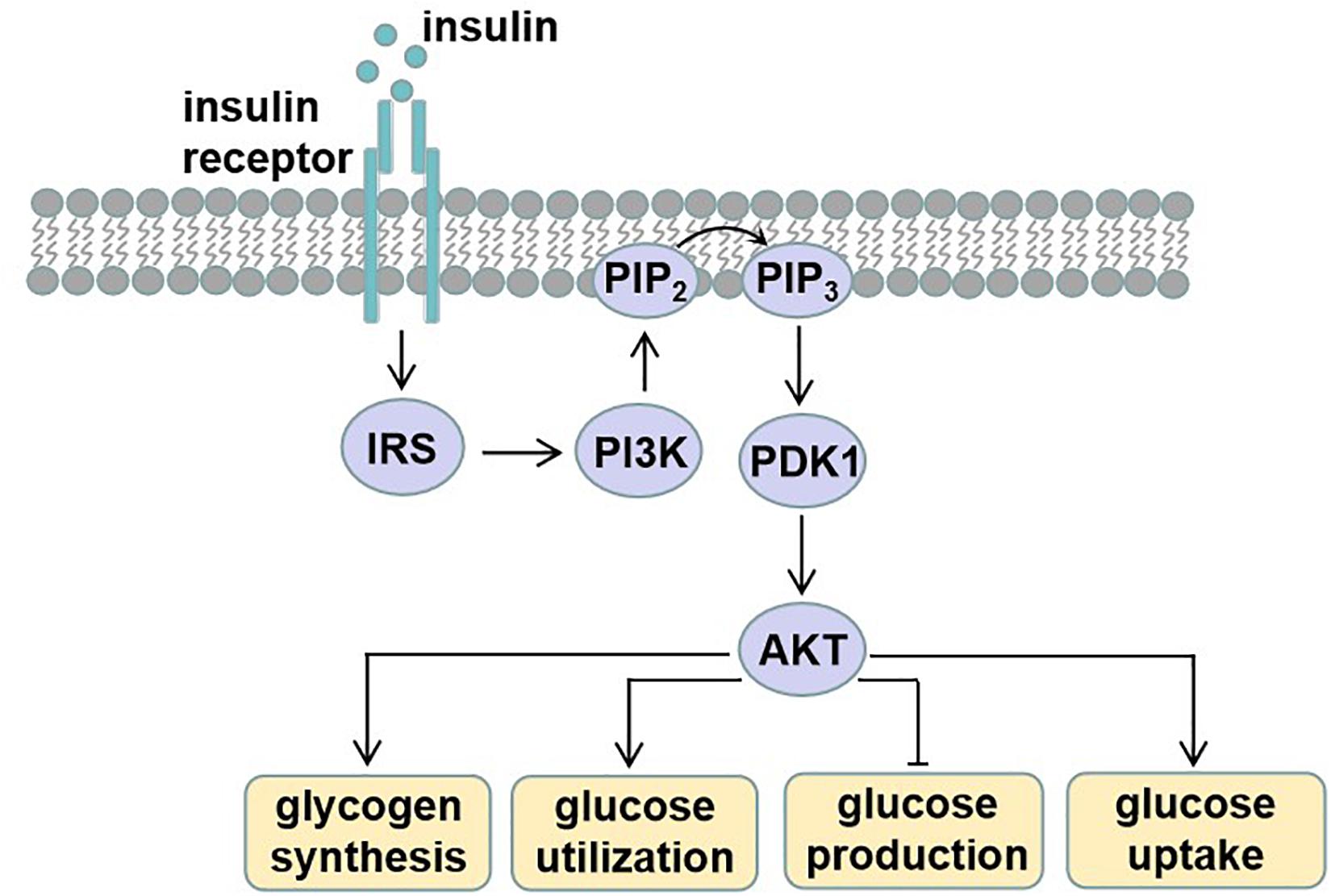
Figure 1. Insulin signaling. Insulin binds and activates insulin receptor (INSR), causing phosphorylation of insulin receptor substrate (IRS). Tyrosine phosphorylated IRS proteins recruit phosphatidylinositide-3 (PI3K), which catalyzes the production of phosphatidylinositol-3,4,5-tris-phosphate (PIP 3 ) from PIP 2 . PIP 3 then recruits proteins with PH domains such as pyruvate dehydrogenase kinase 1 (PDK1), which phosphorylates activating protein kinase B (AKT). These effector proteins mediate the effects of insulin on glucose production, utilization, and uptake, as well as glycogen synthesis.
The dysfunction of insulin signaling will cause insulin resistance, which is a complex metabolic disorder that is closely linked to many pathways including lipid metabolism, energy expenditure, and inflammation ( Figure 2 ). Hepatic lipid accumulation is known to cause insulin resistance ( Samuel and Shulman, 2012 ). Diacylglycerol species activate protein kinase C (PKC), which results in impaired insulin signaling ( Perry et al., 2014 ). An excess of lipid accumulation in liver is often accompanied by hepatic inflammation. Kupffer cells and macrophages will decrease insulin sensitivity by secreting proinflammatory molecules, which activate serine/threonine kinases such as c-Jun N-terminal kinase (JNK) and IκB kinase that in turn impair insulin signaling ( Lackey and Olefsky, 2016 ). Moreover, lipid accumulation triggers the unfolded protein response (UPR) pathway, which impairs insulin signaling ( Ozcan et al., 2004 ). UPR may also alter hepatokine secretion and consequently contribute to the development of insulin resistance ( Koska et al., 2008 ). Energy expenditure disorder leads to obesity and insulin resistance, because non-esterified fatty acids impair β-cell functions, reduce PI3K signaling, and enhance gluconeogenic enzyme expressions ( Kahn et al., 2006 ). What is more, increased release of tumor necrosis factor α (TNF-α), interleukin 6 (IL6), and monocyte chemotactic protein 1 are all found to be responsible for the development of insulin resistance ( Kahn et al., 2006 ). In addition, hepatokines, proteins produced from liver and secreted into the circulation, also play important roles in regulating insulin signaling ( Meex and Watt, 2017 ). Retinol-binding protein 4 (RBP4), α2-macroglobulin (A2M), fetuin A (FETUA), fetuin B (FETUB), hepassocin (FGL1), leukocyte cell–derived chemotaxin 2 (LECT2), and selenoprotein P (SELENOP) are negative regulators of insulin sensitivity, and they will cause insulin resistance, whereas fibroblast growth factor 21 (FGF21), sex hormone–binding globulin (SHBG), adropin, and angiopoietin-like protein 4 (ANGPTL4) are positive regulators ( Lai et al., 2008 ; Meex and Watt, 2017 ).
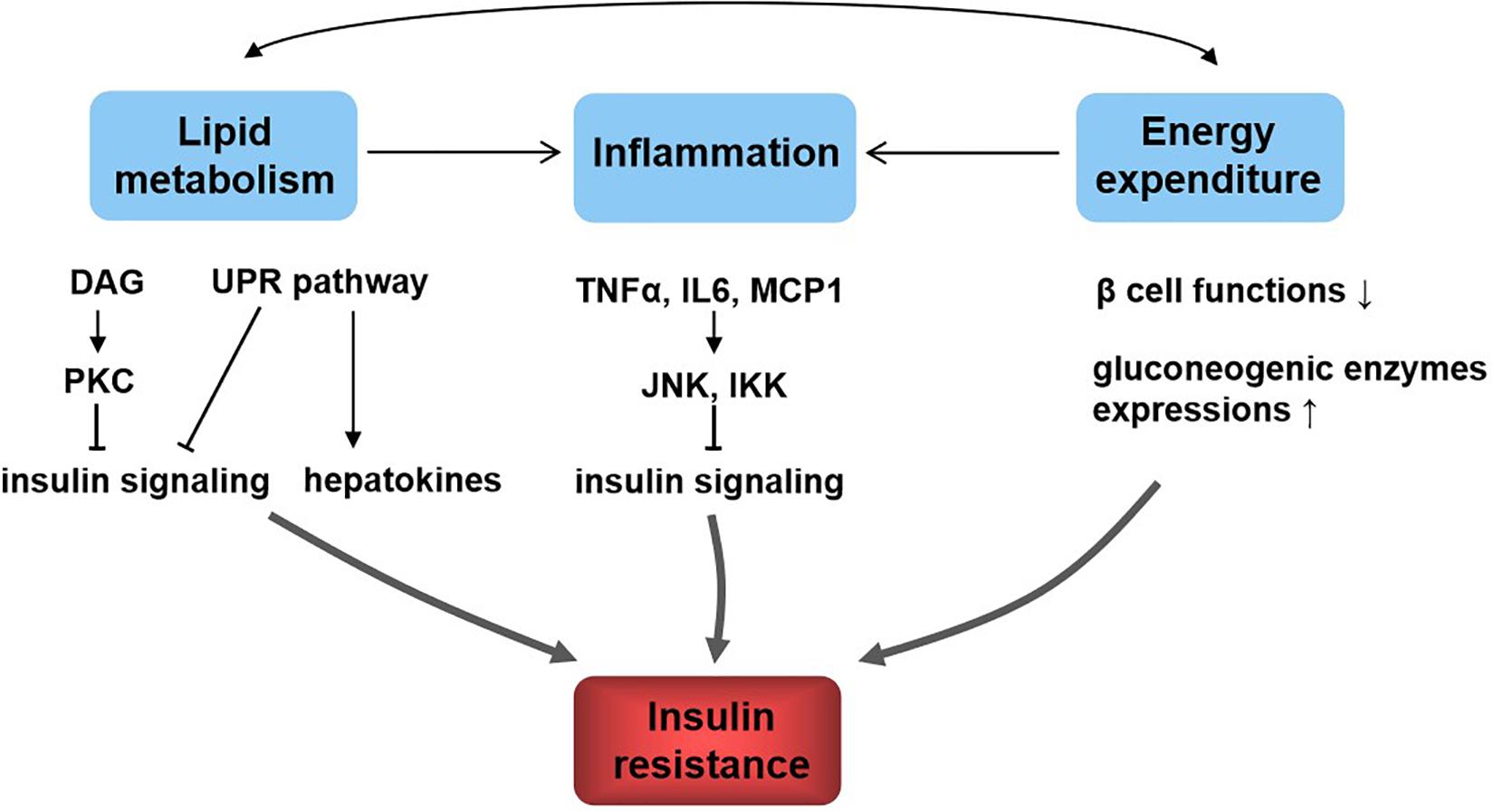
Figure 2. Relationship between lipid metabolism, energy metabolism, inflammation, and insulin resistance. Lipid metabolism and energy metabolism disorder lead to inflammation and affect each other. These all contribute to insulin resistance. The underlying mechanisms include diacylglycerol (DAG), activate protein kinase C (PKC) and lipid accumulation, trigger the unfolded protein response (UPR) pathway, and result in insulin signaling inhibition; UPR affects hepatokine secretion to induce insulin resistance; inflammatory molecules such as tumor necrosis factor α (TNF-α), interleukin 6 (IL6), and monocyte chemotactic protein 1 (MCP1) activate c-Jun N-terminal kinase (JNK) and IκB kinase (IKK), which in turn impair insulin signaling; energy homeostasis disorder impairs β-cell functions, reduces PI3K signaling, and enhances gluconeogenic enzyme expressions, resulting in insulin resistance.
Macronutrient and T2D
Carbohydrate.
It needs a precise control of glucose metabolism to maintain metabolic homeostasis of the body. Hormonal regulation and the related enzyme transcription induced by different metabolites in response to glucose availability are mainly responsible for the control. Insulin induces INSR autophosphorylation and then recruits and phosphorylates IR substrates 1 and 2 (IRS1/2). This results in phosphatidylinositide-3, 4, 5-P3 (PIP3) production, and activating protein kinase B (PKB/AKT) ( Saltiel and Kahn, 2001 ). Thus, it promotes glucose uptake by different tissues, including liver, adipose tissue, and skeletal muscle; inhibits hepatic glucose output; increases glycogen synthesis; and decreases glycogen decompose ( Zhang et al., 2009 ). Insulin induces anabolic responses such as ribosome biogenesis and protein synthesis, which are dependent on nutritional state. The mTOR/S6K1 signaling pathway is also activated by insulin, which plays a vital role in the regulation of glucose homeostasis ( Um et al., 2006 ). Glucose released by diet stimulates the production of PI3P, recruiting proteins to endosomal membranes and finally activating mTOR/S6K1 signaling pathway ( Um et al., 2006 ).
Glucose homeostasis involves different pathways that are carried out in part by the transcriptional control of related genes. Carbohydrate induces the expressions of these enzymes, including pyruvate kinase, glucokinase, ATP citrate lyase, and acetyl CoA carboxylase ( Haro et al., 2019 ). And these genes are regulated by the carbohydrate-responsive element-binding protein (ChREBP) ( Figure 3 ), which is a helix–loop–helix leucine zipper transcription factor ( Lee and Cha, 2018 ). It plays a very important role in sugar-induced lipogenesis and glucose homeostasis by regulating carbohydrate digestion and transport ( Yamashita et al., 2001 ). In response to glucose, ChREBP forms a heterodimer and activates the target genes transcriptions, which contain carbohydrate response element motifs. Except for its glucose sensor role, ChREBP is also essential for fructose induced lipogenesis in liver and intestine possibly via the ChREBP-FGF21 signaling axis ( Fisher et al., 2017 ).
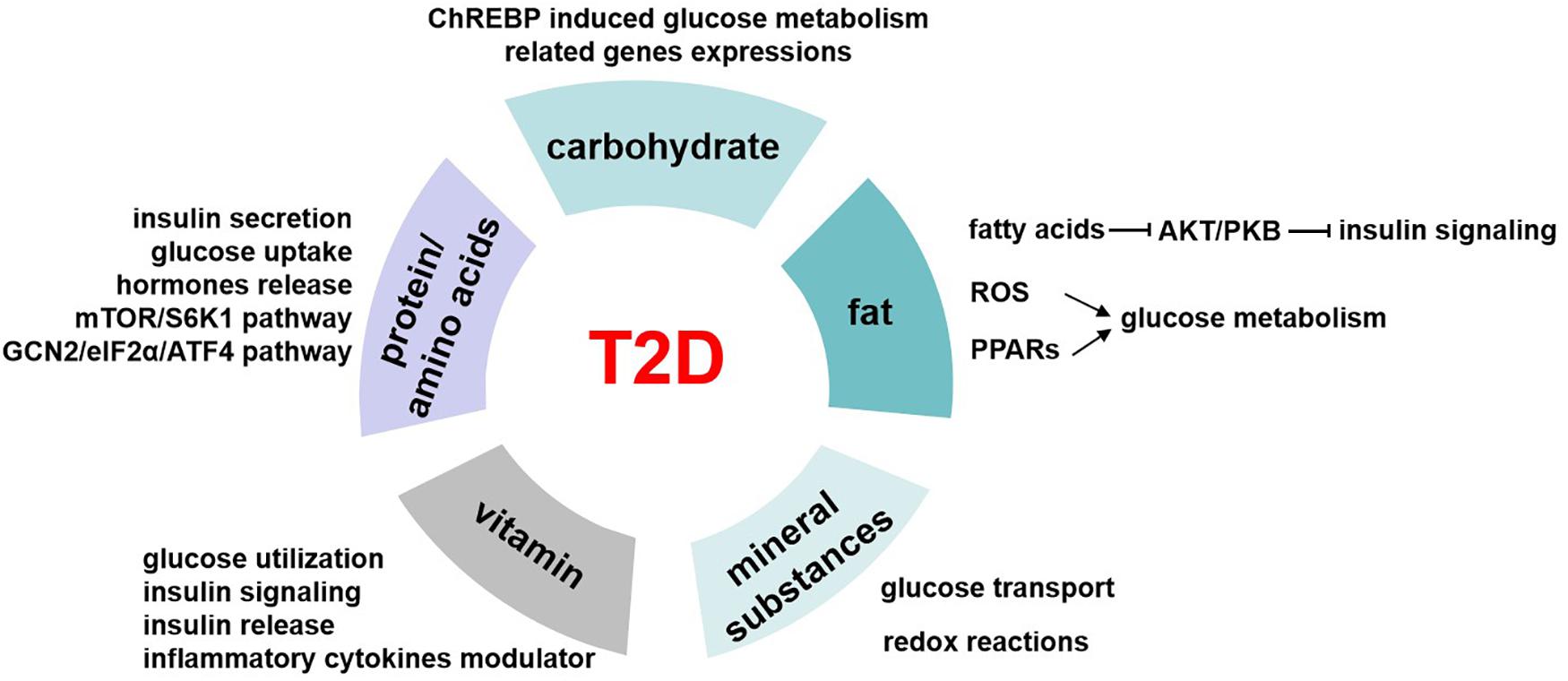
Figure 3. The role of nutrients in T2D. Carbohydrates regulate glucose homeostasis through carbohydrate-responsive element-binding protein (ChREBP) induced glucose metabolism–related genes expressions. Fatty acids inhibit AKT/PKB activation and therefore impair insulin signaling pathway. Besides, fat induces reactive oxygen species (ROS) generation in mitochondrial and activates peroxisome proliferator-activated receptors (PPARs), and all these mediate the regulation of fat on glucose metabolism. The possible underlying pathways or mechanisms of protein/amino acids affecting glucose levels include insulin secretion, glucose uptake, hormone release, mTOR/S6K1 signaling pathway, and GCN2/eIF2α/ATF4 transduction pathway. Mineral substances are activating cofactors and coenzymes for metabolism control, oxidative stress, and genetic transcription. This makes them play roles in glucose transport and redox reactions, which finally affect glucose homeostasis. Vitamin has a role in regulating glucose utilization, insulin signaling, and insulin release from β cells to maintain blood glucose levels, and it is also the modulator of inflammatory cytokines related to glucose metabolism.
Carbohydrate foods that promote sustained but low glucose levels may have benefits to metabolic control of diabetes and its complications. Diets with slow-release carbohydrates lower the glucose and insulin responses throughout the day and improve the capacity for fibrinolysis, which may be a potential therapy to T2D ( Russell et al., 2016 ). When syrup is included in a diabetic diet, it is good to consider sucrose rather than fructose ( Wheeler and Pi-Sunyer, 2008 ). In a short-term trial of T2D patients, scientists showed that isocaloric fructose replacement of other carbohydrates such as sucrose and starch improved glycemic control and had no effects on insulin signaling ( Cooper et al., 2012 ). However, it should take more account to the point that high sucrose or fructose diet is not recommended to diabetic individuals and others who have impaired glucose metabolism. Besides, certain diet components may affect the regulation role of foods on glucose levels. For example, diet with fiber, certain proteins, or lipids to mix may influence the rates of carbohydrate digestion and absorption, which may be beneficial to T2D patients ( Russell et al., 2016 ). Polyols have been used as sugar replacers for about 80 years. Clinical trial showed that polyols had a role in lowering serum glucose levels in T2D patients ( Mohsenpour et al., 2019 ), which may provide a new strategy to manage T2D. Dietary fibers, which are mainly found in cereals, fruits, vegetables, or legumes, showed close associations with T2D. Increased fiber intake, especially soluble fiber, played a beneficial role in improving glycemic control in patients with T2D ( Chandalia et al., 2000 ).
Our body obtains kinds of lipid metabolites from diet intake directly or generated intracellularly by liver and adipose tissue in different pathways. Lipidomics help us better understand the circulating lipid species. Among these, some are considered as biomarkers related to insulin resistance, such as stearic acid and deoxysphingolipids, and saturation and chain length of fatty acids ( Meikle and Summers, 2017 ). High-fat diet–induced insulin resistance and T2D have been largely known since 20 years ago. High-fat diet increases lipid accumulation in cells and leads to obesity. Excess of fat increases proinflammatory cytokines and other hormones or factors involved in insulin resistance ( Kahn et al., 2006 ). Free fatty acids inhibit Akt/PKB activation, thus impairing the insulin signaling pathway ( Bruce and Febbraio, 2007 ). Besides, the reactive oxygen species generation in mitochondrial is increased, which also affects the glucose homeostasis ( Bruce and Febbraio, 2007 ). Peroxisome proliferator-activated receptors (PPARs) function as lipid sensors that can be activated by both dietary fatty acids and their derivatives. PPARs regulate the expression of genes involved in a variety of processes including glucose and lipid metabolism, immune response, and cell growth ( Evans et al., 2004 ; Figure 3 ). PPARα is vital in regulating fatty acid oxidation and therefore has indirect effects on improving glucose metabolism ( Evans et al., 2004 ). Besides, PPARα activates tribbles pseudokinase 3 (TRB3), a direct target, to inhibit AKT activation and impairs insulin sensitivity ( Du et al., 2003 ). PPARγ is an effector of adipogenesis via C/EBP and is responsible for the glucose regulation.
Scientists investigated the impact of fatty acid intake on blood glucose and insulin in the diet of adults with T2D and found that replacement of saturated fats with monounsaturated fatty acids (MUFAs) or polyunsaturated fatty acids may improve their glucose or insulin tolerance ( Russell et al., 2016 ). And in vitro experiments have also confirmed that MUFAs or oleate rather than palmitate prevents insulin resistance ( Gao et al., 2009 ). Postprandial hyperlipidemia is common in T2D patients, and it was shown that omega-3 fatty acids could reduce postprandial lipids but may not correct them completely ( Tomlinson et al., 2020 ). However, the role of trans -fats in regulating glucose control is still controversial. Meta-analysis showed cholesterol-rich diet had a positive relationship with T2D risk ( Tajima et al., 2014 ). Besides, supplementation of plant sterols or stanols lowered serum cholesterol levels ( Derosa et al., 2018 ) that may be indirectly beneficial to glucose metabolism.
Dietary proteins are vital to life for its important role in acquiring essential amino acids to maintain protein synthesis and degradation and supporting cellular processes such as cell growth and development ( Tremblay et al., 2007 ). In recent years, more and more studies have shown that proteins had different effects on glucose homeostasis by affecting insulin action and secretion except for body weight and feeding behavior. In normal or diabetic humans, dietary proteins stimulate insulin secretion so as to reduce glycemia ( Spiller et al., 1987 ). High-protein diets seem to have beneficial effects on weight loss and glucose metabolism, significantly increase insulin sensitivity, and decrease inflammation in the short term ( Russell et al., 2016 ). But long-term high-protein intake seems to result in insulin resistance in the whole body, by increasing mTOR/S6K1 signaling pathway and stimulating gluconeogenesis and high glucagon turnover ( Linn et al., 2000 ). Studies showed that a 6-month high-protein diet (1.87 ± 0.26 g protein/kg body weight per day) in healthy individuals increased fasting glucose levels, impaired hepatic glucose output suppression by insulin, and enhanced gluconeogenesis ( Linn et al., 2000 ). On the other hand, low-protein diets (5%–10% protein calories) suggested improved insulin sensitivity that is beneficial to T2D, and this may be realized through the general control non-derepressible 2 (GCN2)/transcription factor 4 (ATF4)/FGF21 signaling pathway ( Haro et al., 2019 ; Figure 3 ).
Soy protein is one kind of protein that is good for its hypolipidemic and hypocholesterolemic benefits in humans ( Anderson et al., 1995 ). Studies showed that soy protein intake can positively affect glucose metabolism in addition to its effect on decreasing serum lipids. In comparison with casein, soy protein reduced fasting glucose and insulin levels in animals and prevented insulin resistance induced by a high-sucrose diet. Moreover, in humans, it was also revealed that soy protein decreased glucose levels compared to casein ( Hubbard et al., 1989 ). And this function might be explained by the differential hormonal response. Besides, soy protein can also stimulate INSR mRNA expression and thereby increase insulin signaling in fat and liver finally improve insulin sensitivity in these tissues ( Iritani et al., 1997 ).
Fish protein is another protein and widely known protein for years, as Alaska and Greenland populations have a low incidence of T2D for taking large amounts of fish. In lean fish, protein is the most abundant nutrient; consumption of fish protein showed improved cholesterol transport via high-density lipoprotein and reduced triglycerides via very low-density lipoprotein ( Chen et al., 2020 ). Meanwhile, compared to casein-fed animals, cod protein–fed rats were protected against insulin resistance induced by sucrose or in saturated fat ( Lavigne et al., 2000 ) by stimulating glucose uptake by skeletal muscle ( Lavigne et al., 2001 ). Cod protein activated PI3K/AKT signaling pathway and selectively improved GLUT4 translocation to the T tubules, improving glucose transport in response to insulin ( Tremblay et al., 2003 ). Moreover, human studies also showed cod protein exerted beneficial effects to T2D. Cod protein induced a lower insulin-to-glucose ratio compared with milk protein ( von Post-Skagegard et al., 2006 ) and increased postmeal plasma insulin concentrations compared with beef protein ( Tremblay et al., 2007 ).
Protein breakdown or synthesis leads to the change of amino acids levels. There are eight amino acids that cannot be produced inside the body but must come from food. Amino acids are considered as gene expression regulators such as CHOP, which is important to glucose metabolism ( Tremblay and Marette, 2001 ). Amino acids activate the mTOR/S6K1 pathway, and the activation of mTOR inhibits PI3K that results in insulin resistance ( Kimball and Jefferson, 2006 ). BCAAs are kind of important amino acids in regulating homeostasis. BCAAs regulate the release of hormones, including leptin (LEP), GLP-1, and ghrelin, which affects glucose control ( Potier et al., 2009 ). Besides, BCAAs regulate glucose metabolism partly through activating the mTORC1/PKC signaling pathway ( Vary and Lynch, 2007 ).
A healthy and balanced diet should meet all the requirements in amino acids and proteins from varied sources in appropriate proportions. The canonical pathway to respond to amino acid deficiency is amino acid response ( Chou et al., 2012 ). When the essential amino acids decrease, it would cause the deacetylation of the corresponding tRNAs. Uncharged tRNAs bind and activate the GCN2 kinase, and then the activated GCN2 phosphorylates the eukaryotic initiation factor 2α (eIF2α), and induces ATF4 activation ( Hao et al., 2005 ). Numerous studies have shown that increasing dietary levels of BCAAs had a positive effect on T2D ( Lynch and Adams, 2014 ), whereas others suggested that deficiency of BCAAs was beneficial for improving insulin sensitivity and glucose tolerance. Leucine deprivation or methionine deficiency all showed improved insulin sensitivity, energy expenditure, and thermogenesis via GCN2/eIF2α/ATF4/FGF21 transduction pathway ( Haro et al., 2019 ).
Micronutrient and T2D
Mineral substances.
As micronutrient, mineral substances are required at very low concentrations for the normal growth but play important roles in maintaining metabolism homeostasis ( Shenkin, 2006 ). Some of the mineral substances are activating cofactors and coenzymes for metabolism control, oxidative stress, and genetic transcription. The deficiency of mineral substances was shown to have relationship with T2D ( Figure 3 ).
Selenium is a vital component of enzymes for redox reactions such as glutathione peroxidase and thioredoxin reductase in human body, and importantly, the dose range to toxicity is very narrow ( Sun et al., 2013 ). The main dietary sources of selenium are cereals, black tea, milk, mushrooms, soybeans, bamboo shoots, nuts, and broccoli ( Rayman et al., 2008 ). Appropriate concentration of selenium intake can act as an insulin minetic to attenuate diabetes, with the role of decreasing glucose and insulin tolerance, thus preventing hepatic insulin resistance ( Zhou et al., 2013 ). However, high selenium concentration will result in gluconeogenesis, and fasting blood glucose (FBG) levels increased and therefore have a risk to diabetes ( Ogawa-Wong et al., 2016 ).
Vanadium is common in nature but appears at very low concentrations in humans. It occurs with proteins such as transferrin, albumin, and hemoglobin that are vital to the physiological processes ( Pessoa and Tomaz, 2010 ). In vitro and in vivo researches suggested that vanadium had insulin-mimetic properties and may be a potential therapeutic agent to T2D ( Domingo and Gomez, 2016 ). Oral administration of 1 mg/kg per day of vanadyl sulfate for 4 weeks significantly decreased glucose levels in diabetes patients. The possible mechanism underlying this might be through increasing GLUT translocation to plasma membrane and then resulting in glucose transport increase ( Cohen et al., 1995 ).
Chromium plays an important role in glucose metabolism by enhancing the binding of insulin to INSR ( Cefalu and Hu, 2004 ). Clinical trials suggested 4 months’ supplementation of chromium significantly decreased postprandial and also fasting glucose levels. Mechanisms underlying this beneficial function of chromium may partly be explained by the increase of GLUT2 expression and the activation of PI3K/AKT pathway in skeletal muscle ( Panchal et al., 2017 ).
Zinc is an important component of enzymes that play vital roles in regulating insulin sensitivity and glucose homeostasis. Researches showed that, in patients with T2D, the concentrations of zinc in plasma and tissues are lower ( Russell et al., 2016 ). Zinc supplementation improved insulin sensitivity and glucose tolerance in diabetic mice models ( Chen et al., 2000 ) and was found to have similar functions in humans ( Russell et al., 2016 ).
High sodium intake leads to a higher risk of hypertension and cardiovascular diseases in patients with diabetes mellitus. Sodium intake increases natriuresis via PPARδ/SGLT2 pathway and subsequently regulates glucose metabolism of type 2 diabetic patients ( Zhao et al., 2016 ). In contrast, another substance, magnesium, was suggested to decrease the risk of cardiovascular diseases in T2D patients. Magnesium deficiency was associated with diabetes risk, whereas magnesium supplementation could attenuate insulin resistance and improve glycemic control in T2D patients ( Wa et al., 2018 ).
In recent years, vitamin has received increased attention because of its roles in regulating the development of T2D by modulating insulin resistance and pancreatic β-cell functions ( Figure 3 ). Among these, vitamins D and E are the two most popular types. Vitamin D was used to be a regulator of bone metabolism but was found to have various clinical functions. It is a key hormone involved in calcium and phosphorous balance with several derivatives ( Muscogiuri et al., 2017 ). Vitamin D receptor (VDR) is found in the pancreatic β cells and insulin response tissues such as skeletal muscle and adipose tissue ( Fan et al., 2016 ). Studies showed that vitamin D affected glucose utilization in VDR-dependent manner in muscle and adipose tissue and activated PPARδ, which is a transcription factor involved in fatty acid metabolism ( Grammatiki et al., 2017 ). Besides, vitamins modulated insulin action and insulin sensitivity by directly stimulating INSR gene expressions ( Maestro et al., 2000 ) or altered calcium flux to influence insulin release of β cells ( Muscogiuri et al., 2017 ). Moreover, vitamin is a negative modulator of inflammatory cytokine such as TNF-α and IL6, which are closely related to insulin resistance ( Garbossa and Folli, 2017 ).
Insulin resistance is the main diagnosis in most T2D patients, and vitamin D deficiency was found to result in insulin resistance and metabolic syndrome such as hypogonadotrophic, renal diseases and cardiovascular complications ( Garbossa and Folli, 2017 ). And some beneficial effects of vitamin D supplementation have been reported. In several clinical trials, vitamin D administration decreased serum fasting glucose levels and improved Homeostatic Model Assessment of Insulin Resistance index in T2D patients ( Talaei et al., 2013 ; Grammatiki et al., 2017 ). Vitamin E is a fat-soluble vitamin, which is well known for its antioxidant capacity. Besides, it also functions on cell cycle, cell signaling, lipid metabolism, and inflammation ( Gray et al., 2011 ). Several years ago, vitamin E has been reported to have a role in regulation of insulin sensitivity ( Galmes et al., 2018 ). Vitamin E supplementation significantly decreased plasma glucose and hemoglobin A 1 c (HbA 1 c ) levels ( Paolisso et al., 1993 ). The underlying mechanisms may include several pathways. For its antioxidant capacity, vitamin E alters IRS1 phosphorylation, thus affecting insulin signaling ( Gray et al., 2011 ). Besides, vitamin E was shown to directly regulate gene expression such as PPARγ, which plays important roles in insulin sensitivity ( Landrier et al., 2009 ).

Other Chemicals and T2D
Besides macronutrients and micronutrients, others such as phytochemicals and bioactives that are widely distributed in diets or chemicals (such as alcohol) also have potential effects on T2D. Phytochemicals or bioactives exist in fruits, flowers, wood, seeds, bark, and stems, and some of them are found in traditional Chinese medicine ( Zhao et al., 2019 ). They have been reported for their beneficial and therapeutic roles on diabetes in various studies. Phytochemical compounds such as lignans or flavonoids protect against oxidative stress and help diabetic wound healing ( Bacanli et al., 2019 ). Bioactives, such as curcumin, capsaicin, berberine, celastrol, or artemisinin, were shown to improve insulin sensitivity to combat diabetes ( Zhao et al., 2019 ). Despite the promising benefits, the molecular activity and toxicity of these numerous phytochemicals and bioactives need to be explored in further studies.
Alcohol is closely related to diseases such as fatty liver, cardiovascular diseases, and also T2D. Recently, a dose-response meta-analysis suggested that light and moderate alcohol intake may reduce the risk of T2D, whereas heavy alcohol intake showed inconclusive association ( Knott et al., 2015 ).
Nutritional Recommendations for T2D
Prospective studies and clinical trials suggest different nutritional recommendations for the prevention and management of T2D. And they all highlight the importance of dietary habits and lifestyles. For example, calorie restriction and exercise are helpful to reduce the risk of T2D. From the perspective of nutrients, the quality is more important than the quantity. To better improve glucose control in T2D patients, diets rich in fruits, vegetables, legumes, and whole grains are recommended. Low-carbohydrate, low-GI (glycemic index), and high-protein diet patterns will protect us from hyperglycemia incidence. Moreover, moderate consumption of nuts and alcohol is also beneficial ( Ley et al., 2014 ). Different populations or individuals have different foods, dietary habits, and disease susceptibility as well, so nutritional strategies should vary according to their cultures and genetic background.
Diet With Genetics, Epigenetics, and Metagenomics Involved in the Risk of T2D
Genetic backgrounds and environments (e.g., high-fat and high-energy dietary habits, and a sedentary lifestyle) are major factors that contribute to high susceptibility of T2D. The impressive progress of next-generation sequencing (NGS) technology has enabled genome sequencing to be obtained in a cheap and reliable large-scale manner, which provides a comprehensive description of genetic variants including single-nucleotide polymorphisms (SNPs), copy number variations, and other structural variants. Various technologies combined with NGS are developed to explore an increasingly diverse range of biological problems extensively for transcriptome, epigenome, and microbiome. Genetic variants account for only 5–10% for the observed heritability of T2D ( Schwenk et al., 2013 ). Recent advances in precision nutrition have recognized that an individual’s diet may increase the disease risk of T2D by interacting with specific gene variants, affecting the expression of genes, modifying the epigenetic features, or altering microbial composition involved in critical metabolic pathways.
Genetic Variants and Diet Interactions
Genetic variants are the most widely studied features in the field of precision nutrition, and the GWASs have generated extensive knowledge about the genetic background of T2D ( Table 1 ; Hindy et al., 2012 ; Ortega-Azorin et al., 2012 ; Ericson et al., 2013 ; Hwang et al., 2013 ; Ouhaibi-Djellouli et al., 2014 ; InterAct Consortium, 2016 ; Park et al., 2017 ; Schuler et al., 2017 ). For example, The α-ketoglutarate–dependent dioxygenase (FTO) and melanocortin-4 receptor (MC4R) genes were confirmed to be obesity-associated loci, which promotes researchers to study the association of these variants with T2D. GWASs showed these two genes were not significantly associated with diabetes, and conversely, SNP–diet interactions were found to play an important role in the risk of T2D ( Ortega-Azorin et al., 2012 ). FTO rs9939609 and MC4R rs17782313 polymorphisms conferred a higher risk of T2D in subjects with low adherence to the Mediterranean diet. Transcription factor 7–like 2 protein (TCF7L2) was reported to play an important role in the pathogenesis of T2D, and the rs7903146 polymorphism was associated with a high risk of T2D in an Algerian population ( Ouhaibi-Djellouli et al., 2014 ). In addition, the risk was increased in the subjects with both rs7903146 SNP and high dessert and milk intakes, which had higher fasting plasma glucose concentration.
Table 1. SNPs–diet interactions increase the risk of type 2 diabetes.
In order to better understand the cumulative effect of known T2D-related genetic variants, genetic risk score (GRS) has been developed. For example, 22 T2D-related SNPs identified by GWAS were chosen, where 15 SNPs affect β-cell function, and 7 SNPs affect insulin response, and the number of risk alleles present for each SNP was summed as a GRS for each individual ( Layton et al., 2018 ). The GRSs were found to be significantly related to the risk of T2D in African Americans. There were also several studies using GRS to examine the effect of SNPs on diet interactions and disease risk. In the Malmö Diet and Cancer cohort (1991–1996) in Sweden ( Ericson et al., 2018 ), GRS and dietary risk score (DRS) were found to be associated with risk of T2D independently, and the individuals with both high GRS and DRS have the highest risk of T2D. However, no interaction was observed between GRS and dietary intakes in terms of disease risk. Likewise, the same observation was reported in the EPIC-InterAct case-cohort study restricted to Mediterranean diet ( Langenberg et al., 2014 ). However, in United States men, a Western dietary pattern, characterized by a high intake of processed meat, red meat, refined cereals, butter, eggs and high-fat dairy products, showed a significant interaction with the GRS based on 10 T2D-associated SNPs, to increase the risk of T2D ( Qi et al., 2009 ).
The Effect of Dietary Intakes on Gene Expression and Epigenetic Modification
Besides interacting with genetic background, diet styles have been shown to change transcriptions related to T2D and increase the disease risk ( Table 2 ). There are two types of transcriptome studies focusing on the gene expression change response to long-term dietary interventions or differentially expressed transcripts comparing the conditions from different habitual dietary exposures. Dietary intervention studies were usually carried out in rat or mice, and the effects on the metabolism were tested in both maternal and offspring. Low-protein diets in rat model down-regulated the expression of NR1H3 and then increased the expression of hepatic gluconeogenic genes (including G6PC and HSD11B1) and consequently resulted in glucose intolerance in adult offspring ( Vo et al., 2013 ). Vitamin D deficiency in pregnant rat induced the down-regulation of nuclear factor κB inhibitor α (Iκbα) and resulted in insulin resistance in the offspring, which was associated with persistently increased inflammation ( Zhang et al., 2014 ). Chromium was reported to regulate blood glucose first in 1959 ( Mertz and Schwarz, 1959 ), a recent study using a mouse model found that chromium deficiency increased T2D susceptibility by downregulating insulin signaling genes to result in glucose intolerance ( Zhang et al., 2017 ).
Table 2. Dietary intakes changing gene expression and epigenetics increase the risk of type 2 diabetes.
Epigenetics, including DNA methylation, histone modification, non-coding RNAs, chromatin structure, and so on, can regulate gene expression without changing the DNA coding sequence. Epigenetics are inheritable and reversible processes and involved in every aspect of life, for example, cell differentiation, embryogenesis, and development. In recent years, researches have recovered that epigenetic changes play an important role in various diseases including cancers, mental disorders, immune disease, diabetes, and cardiovascular diseases. In several population studies ( Chambers et al., 2015 ; Dayeh et al., 2016 ; Wahl et al., 2017 ), DNA methylation markers were reported to be significantly associated with T2D incidence, and the DNA methylation risk score was able to predict the risk of T2D. The environment and lifestyle can directly interact with the genome to modify the epigenetics, and their influence can even be passed to the next generation. In the previous study, low-protein diet decreased the acetylation of histone H3 surrounding NR1H3 promoter to silence its expression and increased the risk of T2D in the offspring ( Vo et al., 2013 ). Iκbα expression was found to be repressed potentially by Iκbα methylation when vitamin D was deficient ( Zhang et al., 2014 ). DNA methylation profiling of the maternal liver tissue with chromium restriction diet revealed hypermethylated genes mainly involved in insulin signaling pathway; these genes were downregulated and consequently promoted T2D ( Zhang et al., 2017 ). Likewise, magnesium and calcium deficiency increased the risk of T2D by inducing DNA methylation aberrations in genes related to glucocorticoid metabolism ( Takaya et al., 2011 , 2013 ).
The Effect of Dietary Intakes on Gut Microbiota
Gut microbiome is related to the pathogenesis of most chronic diseases, for example, controlling body weight and regulating insulin resistance. Among the environmental factors contributing to T2D, diet plays an important role through changing the gut microbiome. With the technology advances recently, 16S rRNA gene amplicon sequencing, shotgun metagenomic, and metatranscriptomic sequencing have been well established and widely used for comprehensive mapping of gut microbes. In a recent review ( Gurung et al., 2020 ), the authors summarized 42 observational studies about bacterial microbiome and T2D and reported that five genera (including Bifidobacterium , Bacteroides , Faecalibacterium , Akkermansia , and Roseburia ) were negatively associated with T2D, whereas the genera of Ruminococcus , Fusobacterium , and Blautia were positively associated with T2D. It is known that gut microbiota influences the nutrition absorption, and correspondingly, nutrition modulates the composition of gut microbiota. Several literatures have studied how food intakes change the gut microbiome and then promote T2D. From 59 T2D patients, high-carbohydrate, high-fat, and high-protein diets were found to increase counts of Clostridium clusters IV and XI and decreased counts of Bifidobacterium species, order Lactobacillales , and Clostridium cluster IV in gut; therefore, fecal short-chain fatty acid (SCFA) production was decreased subsequently, leading to metabolic disorders, which increased the blood insulin levels and insulin resistance ( Yamaguchi et al., 2016 ).
These scientific advances allow us to predict individual risk by taking into account the genetic, epigenetic information, and dietary habits, thus enabling personalized prevention of the disease by formulating dietary recommendations.
Diet With Genetics, Epigenetics, and Metagenomics Involved in the Intervention of T2D
Dietary intervention is an important way to control blood glucose levels in the treatment of T2D. There have been more recognitions that nutrition adjustment for T2D, which mainly aim at adjusting the metabolic disorders (i.e., insulin resistance), has different responses, given the individuals’ genetic features. Dietary interventions can also change the expression and epigenetic feature of genes involved in the important metabolic pathway, whereas the expression profiles and epigenetic markers can be used to predict personalized response. Moreover, the gut microbiota compositions can be modulated directly by nutrition during dietary interventions.
SNPs–Diet Interactions Showing Differential Responses to Dietary Intervention
FTO rs1558902 polymorphisms with high-fat diet were reported to improve the insulin sensitivity differently rather than low-fat diet from a randomized weight-loss dietary interventional trial ( Zheng et al., 2015 ). Similarly, food interventions aimed at restricting caloric intake or modifying energy derived from fat, protein, or carbohydrates were screened with several SNPs, showing that different SNPs–diet interactions resulted in varied response in terms of weight loss, fasting insulin, and HOMA-IR ( Grau et al., 2010 ; Qi et al., 2011 , 2012 , 2015 ; Xu et al., 2013 ; Huang T. et al., 2015 ). In a POUNDS LOST trial ( Huang et al., 2016 ), a 2-year low-protein weight-loss diet for individuals with low diabetes GRS was found to significantly improve β-cell function and insulin resistance, whereas a high-protein diet might be more beneficial for patients with high GRS.
Epigenetic Modification After Dietary Intervention
Epigenetics play an important role in the metabolic disorders contributing to T2D, whereas lifestyle interventions aiming at diet and physical activity can reversely change the epigenetics and metabolic pathways. The current nutritional recommendations for diabetes management mostly aim to achieve modest weight loss and maintenance. There are not much direct studies about dietary intervention for T2D patients; however, several weight loss programs studied the interactions between DNA methylation and diet intervention. In a trial using 27 obese women with an 8-week low-calorie diet to study the interindividual difference ( Cordero et al., 2011 ), good responders with a successful weight loss showed lower methylation of LEP and TNF-α promoter in adipose tissue and improved the lipid profile and fat mass percentage after the dietary intervention. This observation indicates the potential to predict the efficiency of weight loss by dietary intervention using DNA methylation of LEP and TNF-α promoter. Similarly, differential methylation of five regions located in or near AQP9, DUSP22, HIPK3, TNNT1, and TNNI3 genes was discovered between high and low responders to a weight loss intervention ( Moleres et al., 2013 ). Interestingly, a pilot study reported that DNA methylation patterns of RYR1, TUBA3C, and BDNF in peripheral blood mononuclear cell were changed after weight loss intervention, and the DNA methylation pattern in the successful weight loss maintainers for up to 3 years after intervention was similar to normal-weight individuals rather than obese participants ( Huang Y.T. et al., 2015 ). Therefore, DNA methylation markers might be used to predict body weight maintenance after weight loss.
Modulation of Gut Microbiota After Dietary Intervention
Unhealthy food intakes, e.g., Western diet, might change gut microbiota to increase the risk of T2D; conversely, the gut microbiome can be used as a target for the treatment of T2D. Several studies have shown that dietary intervention can modulate gut microbiota composition to treat T2D ( Table 3 ). Deficiency in SCFA production has been associated with T2D ( Puddu et al., 2014 ). A randomized clinical study using fecal shotgun metagenomic sequencing uncovered that high-fiber diet increased the abundance of SCFA-producing microbiota in TD2 patients to alleviate their phenotype ( Zhao L. et al., 2018 ). Similarly, fiber-rich macrobiotic Ma-Pi 2 diet or a recommended control diet for T2D treatment ( Candela et al., 2016 ) was found to have a positive impact on modulating gut microbe dysbiosis, especially recovering the SCFA-producing microbiome such as Faecalibacterium , Roseburia , Lachnospira , Bacteroides , and Akkermansia . Moreover, the Ma-Pi 2 diet showed the potential to reverse proinflammatory dysbiosis in T2D by counteracting the increase in the proinflammatory groups, such as Collinsella and Streptococcus . Low-calorie formula diet was proven to have a favorable impact on gut microbiome in a standardized three-phase weight loss program for T2D patients ( Taheri et al., 2019 ). The result showed that all of the participants lost their weight and accompanied by a significant improvement of glucose metabolism indicated by a reduction of HbA 1 c , fasting glucose, and insulin. Meanwhile, both the phylogenetic diversity and β diversity markedly shifted during the end of the low-calorie formula diet. Based on the epidemiological studies, increased circulating BCAAs are associated with insulin resistance and T2D. A randomized crossover trial performed on T2D patients identified that decreased intake of BCAAs was negatively relevant to postprandial insulin secretion ( Karusheva et al., 2019 ). Meanwhile, the analysis of fecal microbiome showed enrichment in Bacteroidetes but decrease of Firmicutes .
Table 3. Dietary interventions modulate gut microbiota.
There were also many researches using animal models to investigate dietary intervention for T2D. A report demonstrated that pumpkin polysaccharide had the ability to ease the phenotype of T2D in rat model induced by high-fat diet and streptozotocin and selectively enriched the butyric acid–producing microbiota in rat gut, which is the potential mechanism ( Liu et al., 2018 ). In a non-obese type 2 diabetic animal model, the pancreatectomized rats were provided diets supplemented with aronia, red ginseng, shiitake mushroom, and nattokinase (AGM) ( Yang et al., 2018 ). After 12 weeks’ feed, the experimental group showed enhanced insulin secretion and reduced insulin resistance, as well as improved the gut microbiome dysbiosis. According to traditional Chinese medicine, oil tea containing green tea and ginger has potential to treat various ailments ( Lin et al., 2018 ). Lin et al. (2018) orally gavaged the db/db mice with oil tea for 8 weeks and tested FBG, oral glucose tolerance test, and lipid levels. The result showed that oil tea can effectively suppress the blood glucose elevation, and meanwhile the gut microbiota was markedly enriched with Lachnospiraceae. It was also reported that the high-fat diet–fed mice showed reduced insulin resistance after oral gavage red pitaya β-cyanins for 14 weeks ( Song et al., 2016 ). 16S rRNA sequencing analysis found the structure of gut microbiota was modulated especially with the decreased ratio of Firmicutes and Bacteroidetes and increased relative abundance of Akkermansia . In another streptozotocin-induced type 2 diabetic mice model, Lessonia nigrescens ethanolic extract was shown to decrease FBG levels ( Zhao C. et al., 2018 ). The gene and protein of PI3K in liver were upregulated, whereas JNK was significantly downregulated. Meanwhile, the gut microbiota was enriched with beneficial bacteria, Barnesiella , and had less abundances of Clostridium and Alistipes .
In summary, previous studies have shown that the specific dietary (e.g., high-fiber, low-fat, or low-calorie formula diet) can regulate insulin secretion and resistance through modulating the gut microbiota. Therefore, modification of the gut bacteria composition by dietary intervention might be a feasible method to alleviate the symptom of T2D. However, concrete conclusion remains to be obtained by future well-designed and long-term studies. Especially, more effort is expected to study the role of individual food compounds or nutrients in regulating the metabolism to prevent and treat T2D.
Type 2 diabetes is a metabolic disease characterized by insulin resistance and/or abnormal insulin secretion that is caused by multiple factors including genetics, nutrition, and physical activity. Insulin signaling dysregulation in glucose metabolism is the major mechanism contributing to T2D, and the factors involved in this pathway can be targets for prevention and intervention of T2D. Growing evidence suggest the important role of nutrition in developing T2D, and the mechanisms behind are explored, respectively, in terms of five main nutrients, namely, proteins, carbohydrates, fats, vitamins, and minerals. Previous suggestions for management of T2D are usually made based on average population; however, with the advance of precision medicine, precision nutrition has attracted increasing attention in T2D. Genetic predisposition combined with diet specifically influences the risk of developing T2D for individuals, and food intakes change gene expression, epigenetic features, and gut microbiota to characterize individuals’ response to prevention and treatment by adjusting dietary patterns. However, precision nutrition is still in its infancy, and the studies performed are not comprehensive and sometimes have contradicted conclusions, possibly due to the limited sample size, varied population, and unstandardized study design. In conclusion, nutrition plays a big role in the prevention and intervention of T2D, and precision nutrition holds promise for future therapeutic strategies.
Author Contributions
YG and QL conceived the idea and wrote the manuscript with input from ZH, DS, and QG. YG prepared the figures. QL and ZH prepared the tables. All authors edited and approved the final manuscript.
This review was supported by the National Natural Science Foundation of China (No. 81700750), Guangdong Natural Science Foundation (No. 2019A1515111174), Shenzhen Outbound post-doctoral research funding (CZBSHKYJJ002), the Fundamental Research Funds for the Central Universities, Sun Yat-sen University (19ykpy02), the Basic Research Start-up Project of The Eighth Affiliated Hospital of Sun Yat-sen University (GCCRCYJ022), and The Eighth Affiliated Hospital of Sun Yat-sen University Outstanding Youth Reserve Talent Science Fund (FBJQ2019004).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Alberti, K. G., and Zimmet, P. Z. (1998). Definition, diagnosis and classification of diabetes mellitus and its complications. Part 1: diagnosis and classification of diabetes mellitus provisional report of a WHO consultation. Diabet Med. 15, 539–553. doi: 10.1002/(sici)1096-9136(199807)15:7<539::aid-dia668>3.0.co;2-s
CrossRef Full Text | Google Scholar
Anderson, J. W., Johnstone, B. M., and Cook-Newell, M. E. (1995). Meta-analysis of the effects of soy protein intake on serum lipids. N. Engl. J. Med. 333, 276–282. doi: 10.1056/NEJM199508033330502
PubMed Abstract | CrossRef Full Text | Google Scholar
Bacanli, M., Dilsiz, S. A., Basaran, N., and Basaran, A. A. (2019). Effects of phytochemicals against diabetes. Adv. Food Nutr. Res. 89, 209–238. doi: 10.1016/bs.afnr.2019.02.006
Bruce, C. R., and Febbraio, M. A. (2007). It’s what you do with the fat that matters! Nat. Med. 13, 1137–1138. doi: 10.1038/nm1007-1137
Candela, M., Biagi, E., Soverini, M., Consolandi, C., Quercia, S., Severgnini, M., et al. (2016). Modulation of gut microbiota dysbioses in type 2 diabetic patients by macrobiotic Ma-Pi 2 diet. Br. J. Nutr. 116, 80–93. doi: 10.1017/S0007114516001045
Cefalu, W. T., and Hu, F. B. (2004). Role of chromium in human health and in diabetes. Diabetes Care 27, 2741–2751. doi: 10.2337/diacare.27.11.2741
Chambers, J. C., Loh, M., Lehne, B., Drong, A., Kriebel, J., Motta, V., et al. (2015). Epigenome-wide association of DNA methylation markers in peripheral blood from Indian Asians and Europeans with incident type 2 diabetes: a nested case-control study. Lancet Diabetes Endocrinol. 3, 526–534. doi: 10.1016/S2213-8587(15)00127-8
Chandalia, M., Garg, A., Lutjohann, D., von Bergmann, K., Grundy, S. M., and Brinkley, L. J. (2000). Beneficial effects of high dietary fiber intake in patients with type 2 diabetes mellitus. N. Engl. J. Med. 342, 1392–1398. doi: 10.1056/NEJM200005113421903
Chen, M. D., Song, Y. M., and Lin, P. Y. (2000). Zinc effects on hyperglycemia and hypoleptinemia in streptozotocin-induced diabetic mice. Horm. Metab. Res. 32, 107–109. doi: 10.1055/s-2007-978600
Chen, Z., Franco, O. H., Lamballais, S., Ikram, M. A., Schoufour, J. D., Muka, T., et al. (2020). Associations of specific dietary protein with longitudinal insulin resistance, prediabetes and type 2 diabetes: the rotterdam study. Clin. Nutr. 39, 242–249. doi: 10.1016/j.clnu.2019.01.021
Chou, C. J., Affolter, M., and Kussmann, M. (2012). A nutrigenomics view of protein intake: macronutrient, bioactive peptides, and protein turnover. Prog. Mol. Biol. Transl. Sci. 108, 51–74. doi: 10.1016/B978-0-12-398397-8.00003-4
Cohen, N., Halberstam, M., Shlimovich, P., Chang, C. J., Shamoon, H., and Rossetti, L. (1995). Oral vanadyl sulfate improves hepatic and peripheral insulin sensitivity in patients with non-insulin-dependent diabetes mellitus. J. Clin. Invest. 95, 2501–2509. doi: 10.1172/JCI117951
Cooper, A. J., Forouhi, N. G., Ye, Z., Buijsse, B., Arriola, L., Balkau, B., et al. (2012). Fruit and vegetable intake and type 2 diabetes: ePIC-InterAct prospective study and meta-analysis. Eur. J. Clin. Nutr. 66, 1082–1092. doi: 10.1038/ejcn.2012.85
Cordero, P., Campion, J., Milagro, F. I., Goyenechea, E., Steemburgo, T., Javierre, B. M., et al. (2011). Leptin and TNF-alpha promoter methylation levels measured by MSP could predict the response to a low-calorie diet. J. Physiol. Biochem. 67, 463–470. doi: 10.1007/s13105-011-0084-4
Dao, M. C., Everard, A., Aron-Wisnewsky, J., Sokolovska, N., Prifti, E., Verger, E. O., et al. (2016). Akkermansia muciniphila and improved metabolic health during a dietary intervention in obesity: relationship with gut microbiome richness and ecology. Gut 65, 426–436. doi: 10.1136/gutjnl-2014-308778
Dayeh, T., Tuomi, T., Almgren, P., Perfilyev, A., Jansson, P. A., de Mello, V. D., et al. (2016). DNA methylation of loci within ABCG1 and PHOSPHO1 in blood DNA is associated with future type 2 diabetes risk. Epigenetics 11, 482–488. doi: 10.1080/15592294.2016.1178418
Derosa, G., Catena, G., Raddino, R., Gaudio, G., Maggi, A., D’Angelo, A., et al. (2018). Effects on oral fat load of a nutraceutical combination of fermented red rice, sterol esters and stanols, curcumin, and olive polyphenols: a randomized, placebo controlled trial. Phytomedicine 42, 75–82. doi: 10.1016/j.phymed.2018.01.014
Domingo, J. L., and Gomez, M. (2016). Vanadium compounds for the treatment of human diabetes mellitus: a scientific curiosity? A review of thirty years of research. Food Chem. Toxicol. 95, 137–141. doi: 10.1016/j.fct.2016.07.005
Du, K., Herzig, S., Kulkarni, R. N., and Montminy, M. (2003). TRB3: a tribbles homolog that inhibits Akt/PKB activation by insulin in liver. Science 300, 1574–1577. doi: 10.1126/science.1079817
Ericson, U., Hindy, G., Drake, I., Schulz, C. A., Brunkwall, L., Hellstrand, S., et al. (2018). Dietary and genetic risk scores and incidence of type 2 diabetes. Genes Nutr. 13:13. doi: 10.1186/s12263-018-0599-1
Ericson, U., Rukh, G., Stojkovic, I., Sonestedt, E., Gullberg, B., Wirfält, E., et al. (2013). Sex-specific interactions between the IRS1 polymorphism and intakes of carbohydrates and fat on incident type 2 diabetes. Am. J. Clin. Nutr. 97, 208–216. doi: 10.3945/ajcn.112.046474
Evans, R. M., Barish, G. D., and Wang, Y. X. (2004). PPARs and the complex journey to obesity. Nat. Med. 10, 355–361. doi: 10.1038/nm1025
Fan, Y., Futawaka, K., Koyama, R., Fukuda, Y., Hayashi, M., Imamoto, M., et al. (2016). Vitamin D3/VDR resists diet-induced obesity by modulating UCP3 expression in muscles. J. Biomed. Sci. 23:56. doi: 10.1186/s12929-016-0271-2
Fisher, F. M., Kim, M., Doridot, L., Cunniff, J. C., Parker, T. S., Levine, D. M., et al. (2017). A critical role for ChREBP-mediated FGF21 secretion in hepatic fructose metabolism. Mol. Metab. 6, 14–21. doi: 10.1016/j.molmet.2016.11.008
Galmes, S., Serra, F., and Palou, A. (2018). Vitamin E metabolic effects and genetic variants: a challenge for precision nutrition in obesity and associated disturbances. Nutrients 10:1919. doi: 10.3390/nu10121919
Gao, D., Griffiths, H. R., and Bailey, C. J. (2009). Oleate protects against palmitate-induced insulin resistance in L6 myotubes. Br. J. Nutr. 102, 1557–1563. doi: 10.1017/S0007114509990948
Garbossa, S. G., and Folli, F. (2017). Vitamin D, sub-inflammation and insulin resistance. A window on a potential role for the interaction between bone and glucose metabolism. Rev. Endocr. Metab. Disord 18, 243–258. doi: 10.1007/s11154-017-9423-2
Garcia-Perez, I., Posma, J. M., Gibson, R., Chambers, E. S., Hansen, T. H., Vestergaard, H., et al. (2017). Objective assessment of dietary patterns by use of metabolic phenotyping: a randomised, controlled, crossover trial. Lancet Diabetes Endocrinol. 5, 184–195. doi: 10.1016/S2213-8587(16)30419-3
Grammatiki, M., Rapti, E., Karras, S., Ajjan, R. A., and Kotsa, K. (2017). Vitamin D and diabetes mellitus: causal or casual association? Rev. Endocr. Metab. Disord. 18, 227–241. doi: 10.1007/s11154-016-9403-y
Grau, K., Cauchi, S., Holst, C., Astrup, A., Martinez, J. A., Saris, W. H., et al. (2010). TCF7L2 rs7903146-macronutrient interaction in obese individuals’ responses to a 10-wk randomized hypoenergetic diet. Am. J. Clin. Nutr. 91, 472–479. doi: 10.3945/ajcn.2009.27947
Gray, B., Swick, J., and Ronnenberg, A. G. (2011). Vitamin E and adiponectin: proposed mechanism for vitamin E-induced improvement in insulin sensitivity. Nutr. Rev. 69, 155–161. doi: 10.1111/j.1753-4887.2011.00377.x
Guasch-Ferre, M., Hruby, A., Toledo, E., Clish, C. B., Martinez-Gonzalez, M. A., Salas-Salvado, J., et al. (2016). Metabolomics in prediabetes and diabetes: a systematic review and meta-analysis. Diabetes Care 39, 833–846. doi: 10.2337/dc15-2251
Gurung, M., Li, Z., You, H., Rodrigues, R., Jump, D. B., Morgun, A., et al. (2020). Role of gut microbiota in type 2 diabetes pathophysiology. EBioMedicine 51:102590. doi: 10.1016/j.ebiom.2019.11.051
Haffner, S. M., Lehto, S., Ronnemaa, T., Pyorala, K., and Laakso, M. (1998). Mortality from coronary heart disease in subjects with type 2 diabetes and in nondiabetic subjects with and without prior myocardial infarction. N. Engl. J. Med. 339, 229–234. doi: 10.1056/NEJM199807233390404
Hao, S., Sharp, J. W., Ross-Inta, C. M., McDaniel, B. J., Anthony, T. G., Wek, R. C., et al. (2005). Uncharged tRNA and sensing of amino acid deficiency in mammalian piriform cortex. Science 307, 1776–1778. doi: 10.1126/science.1104882
Haro, D., Marrero, P. F., and Relat, J. (2019). Nutritional Regulation of Gene Expression: carbohydrate-, Fat- and Amino Acid-Dependent Modulation of Transcriptional Activity. Int. J. Mol. Sci. 20:1386. doi: 10.3390/ijms20061386
Hindy, G., Sonestedt, E., Ericson, U., Jing, X. J., Zhou, Y., Hansson, O., et al. (2012). Role of TCF7L2 risk variant and dietary fibre intake on incident type 2 diabetes. Diabetologia 55, 2646–2654. doi: 10.1007/s00125-012-2634-x
Huang, T., Huang, J., Qi, Q., Li, Y., Bray, G. A., Rood, J., et al. (2015). PCSK7 genotype modifies effect of a weight-loss diet on 2-year changes of insulin resistance: the POUNDS LOST trial. Diabetes Care 38, 439–444. doi: 10.2337/dc14-0473
Huang, T., Ley, S. H., Zheng, Y., Wang, T., Bray, G. A., Sacks, F. M., et al. (2016). Genetic susceptibility to diabetes and long-term improvement of insulin resistance and beta cell function during weight loss: the Preventing Overweight Using Novel Dietary Strategies (POUNDS LOST) trial. Am. J. Clin. Nutr. 104, 198–204. doi: 10.3945/ajcn.115.121186
Huang, Y. T., Maccani, J. Z. J., Hawley, N. L., Wing, R. R., Kelsey, K. T., and McCaffery, J. M. (2015). Epigenetic patterns in successful weight loss maintainers: a pilot study. Int. J. Obes. 39, 865–868. doi: 10.1038/ijo.2014.213
Hubbard, R., Kosch, C. L., Sanchez, A., Sabate, J., Berk, L., and Shavlik, G. (1989). Effect of dietary protein on serum insulin and glucagon levels in hyper- and normocholesterolemic men. Atherosclerosis 76, 55–61. doi: 10.1016/0021-9150(89)90193-7
Hwang, J. Y., Park, J. E., Choi, Y. J., Huh, K. B., Chang, N., and Kim, W. Y. (2013). Carbohydrate intake interacts with SNP276G>T polymorphism in the adiponectin gene to affect fasting blood glucose, HbA1C, and HDL cholesterol in Korean patients with type 2 diabetes. J. Am. Coll. Nutr. 32, 143–150. doi: 10.1080/07315724.2013.791795
InterAct Consortium (2016). Investigation of gene-diet interactions in the incretin system and risk of type 2 diabetes: the EPIC-InterAct study. Diabetologia 59, 2613–2621. doi: 10.1007/s00125-016-4090-5
Iritani, N., Sugimoto, T., Fukuda, H., Komiya, M., and Ikeda, H. (1997). Dietary soybean protein increases insulin receptor gene expression in Wistar fatty rats when dietary polyunsaturated fatty acid level is low. J. Nutr. 127, 1077–1083. doi: 10.1093/jn/127.6.1077
Kahn, S. E., Hull, R. L., and Utzschneider, K. M. (2006). Mechanisms linking obesity to insulin resistance and type 2 diabetes. Nature 444, 840–846. doi: 10.1038/nature05482
Karusheva, Y., Koessler, T., Strassburger, K., Markgraf, D., Mastrototaro, L., Jelenik, T., et al. (2019). Short-term dietary reduction of branched-chain amino acids reduces meal-induced insulin secretion and modifies microbiome composition in type 2 diabetes: a randomized controlled crossover trial. Am. J. Clin. Nutr. 110, 1098–1107. doi: 10.1093/ajcn/nqz191
Kimball, S. R., and Jefferson, L. S. (2006). New functions for amino acids: effects on gene transcription and translation. Am. J. Clin. Nutr. 83, 500S–507S. doi: 10.1093/ajcn/83.2.500S
King, H., Aubert, R. E., and Herman, W. H. (1998). Global burden of diabetes, 1995-2025: prevalence, numerical estimates, and projections. Diabetes Care 21, 1414–1431. doi: 10.2337/diacare.21.9.1414
Knott, C., Bell, S., and Britton, A. (2015). Alcohol consumption and the risk of type 2 diabetes a systematic review and dose-response meta-analysis of more than 1.9 million individuals from 38 observational studies. Diabetes Care 38, 1804–1812. doi: 10.2337/dc15-0710
Koska, J., Stefan, N., Permana, P. A., Weyer, C., Sonoda, M., Bogardus, C., et al. (2008). Increased fat accumulation in liver may link insulin resistance with subcutaneous abdominal adipocyte enlargement, visceral adiposity, and hypoadiponectinemia in obese individuals. Am. J. Clin. Nutr. 87, 295–302. doi: 10.1093/ajcn/87.2.295
Lackey, D. E., and Olefsky, J. M. (2016). Regulation of metabolism by the innate immune system. Nat. Rev. Endocrinol. 12, 15–28. doi: 10.1038/nrendo.2015.189
Lai, K. K., Kolippakkam, D., and Beretta, L. (2008). Comprehensive and quantitative proteome profiling of the mouse liver and plasma. Hepatology 47, 1043–1051. doi: 10.1002/hep.22123
Landrier, J. F., Gouranton, E., El Yazidi, C., Malezet, C., Balaguer, P., Borel, P., et al. (2009). Adiponectin expression is induced by vitamin E via a peroxisome proliferator-activated receptor gamma-dependent mechanism. Endocrinology 150, 5318–5325. doi: 10.1210/en.2009-0506
Langenberg, C., Sharp, S. J., Franks, P. W., Scott, R. A., Deloukas, P., Forouhi, N. G., et al. (2014). Gene-lifestyle interaction and type 2 diabetes: the EPIC interact case-cohort study. PLoS Med. 11:e1001647. doi: 10.1371/journal.pmed.1001647
Lavigne, C., Marette, A., and Jacques, H. (2000). Cod and soy proteins compared with casein improve glucose tolerance and insulin sensitivity in rats. Am. J. Physiol. Endocrinol. Metab. 278, E491–E500. doi: 10.1152/ajpendo.2000.278.3.E491
Lavigne, C., Tremblay, F., Asselin, G., Jacques, H., and Marette, A. (2001). Prevention of skeletal muscle insulin resistance by dietary cod protein in high fat-fed rats. Am. J. Physiol. Endocrinol. Metab. 281, E62–E71. doi: 10.1152/ajpendo.2001.281.1.E62
Layton, J., Li, X., Shen, C., de Groot, M., Lange, L., Correa, A., et al. (2018). Type 2 diabetes genetic risk scores are associated with increased type 2 diabetes risk among african americans by cardiometabolic status. Clin. Med. Insights Endocrinol. Diabetes 11:1179551417748942. doi: 10.1177/1179551417748942
Lee, H. J., and Cha, J. Y. (2018). Recent insights into the role of ChREBP in intestinal fructose absorption and metabolism. BMB Rep. 51, 429–436. doi: 10.5483/bmbrep.2018.51.9.197
Ley, S. H., Hamdy, O., Mohan, V., and Hu, F. B. (2014). Prevention and management of type 2 diabetes: dietary components and nutritional strategies. Lancet 383, 1999–2007. doi: 10.1016/S0140-6736(14)60613-9
Lin, R., He, X., Chen, H., He, Q., Yao, Z., Li, Y., et al. (2018). Oil tea improves glucose and lipid levels and alters gut microbiota in type 2 diabetic mice. Nutr. Res. 57, 67–77. doi: 10.1016/j.nutres.2018.05.004
Linn, T., Santosa, B., Gronemeyer, D., Aygen, S., Scholz, N., Busch, M., et al. (2000). Effect of long-term dietary protein intake on glucose metabolism in humans. Diabetologia 43, 1257–1265. doi: 10.1007/s001250051521
Liu, G., Liang, L., Yu, G., and Li, Q. (2018). Pumpkin polysaccharide modifies the gut microbiota during alleviation of type 2 diabetes in rats. Int. J. Biol. Macromol. 115, 711–717. doi: 10.1016/j.ijbiomac.2018.04.127
Lynch, C. J., and Adams, S. H. (2014). Branched-chain amino acids in metabolic signalling and insulin resistance. Nat. Rev. Endocrinol. 10, 723–736. doi: 10.1038/nrendo.2014.171
Maestro, B., Campion, J., Davila, N., and Calle, C. (2000). Stimulation by 1,25-dihydroxyvitamin D3 of insulin receptor expression and insulin responsiveness for glucose transport in U-937 human promonocytic cells. Endocr. J. 47, 383–391. doi: 10.1507/endocrj.47.383
Malik, V. S., Willett, W. C., and Hu, F. B. (2013). Global obesity: trends, risk factors and policy implications. Nat. Rev. Endocrinol. 9, 13–27. doi: 10.1038/nrendo.2012.199
Manore, M. M., Larson-Meyer, D. E., Lindsay, A. R., Hongu, N., and Houtkooper, L. (2017). Dynamic energy balance: an integrated framework for discussing diet and physical activity in obesity prevention-is it more than eating less and exercising more? Nutrients 9:905. doi: 10.3390/nu9080905
Meex, R. C. R., and Watt, M. J. (2017). Hepatokines linking nonalcoholic fatty liver disease and insulin resistance. Nat. Rev. Endocrinol. 13, 509–520. doi: 10.1038/nrendo.2017.56
Meikle, P. J., and Summers, S. A. (2017). Sphingolipids and phospholipids in insulin resistance and related metabolic disorders. Nat. Rev. Endocrinol. 13, 79–91. doi: 10.1038/nrendo.2016.169
Mertz, W., and Schwarz, K. (1959). Relation of glucose tolerance factor to impaired intravenous glucose tolerance of rats on stock diets. Am. J. Physiol. 196, 614–618. doi: 10.1152/ajplegacy.1959.196.3.614
Mohsenpour, M. A., Kaseb, F., Nazemian, R., Mozaffari-Khosravi, H., Fallahzadeh, H., and Salehi-Abargouei, A. (2019). The effect of a new mixture of sugar and sugar-alcohols compared to sucrose and glucose on blood glucose increase and the possible adverse reactions: a phase I double-blind, three-way randomized cross-over clinical trial. Endocrinol. Diabetes Nutr. 66, 647–653. doi: 10.1016/j.endinu.2018.12.008
Moleres, A., Campion, J., Milagro, F. I., Marcos, A., Campoy, C., Garagorri, J. M., et al. (2013). Differential DNA methylation patterns between high and low responders to a weight loss intervention in overweight or obese adolescents: the EVASYON study. FASEB J. 27, 2504–2512. doi: 10.1096/fj.12-215566
Moller, D. E. (2001). New drug targets for type 2 diabetes and the metabolic syndrome. Nature 414, 821–827. doi: 10.1038/414821a
Muscogiuri, G., Altieri, B., Annweiler, C., Balercia, G., Pal, H. B., Boucher, B. J., et al. (2017). Vitamin D and chronic diseases: the current state of the art. Arch. Toxicol. 91, 97–107. doi: 10.1007/s00204-016-1804-x
Ogawa-Wong, A. N., Berry, M. J., and Seale, L. A. (2016). Selenium and metabolic disorders: an emphasis on type 2 diabetes risk. Nutrients 8:80. doi: 10.3390/nu8020080
Ortega-Azorin, C., Sorli, J. V., Asensio, E. M., Coltell, O., Martinez-Gonzalez, M. A., Salas-Salvado, J., et al. (2012). Associations of the FTO rs9939609 and the MC4R rs17782313 polymorphisms with type 2 diabetes are modulated by diet, being higher when adherence to the Mediterranean diet pattern is low. Cardiovasc Diabetol 11:137. doi: 10.1186/1475-2840-11-137
Ouhaibi-Djellouli, H., Mediene-Benchekor, S., Lardjam-Hetraf, S. A., Hamani-Medjaoui, I., Meroufel, D. N., Boulenouar, H., et al. (2014). The TCF7L2 rs7903146 polymorphism, dietary intakes and type 2 diabetes risk in an Algerian population. BMC Genet. 15:134. doi: 10.1186/s12863-014-0134-3
Ozcan, U., Cao, Q., Yilmaz, E., Lee, A. H., Iwakoshi, N. N., Ozdelen, E., et al. (2004). Endoplasmic reticulum stress links obesity, insulin action, and type 2 diabetes. Science 306, 457–461. doi: 10.1126/science.1103160
Panchal, S. K., Wanyonyi, S., and Brown, L. (2017). Selenium, vanadium, and chromium as micronutrients to improve metabolic syndrome. Curr. Hypertens. Rep. 19:10. doi: 10.1007/s11906-017-0701-x
Paolisso, G., D’Amore, A., Giugliano, D., Ceriello, A., Varricchio, M., and D’Onofrio, F. (1993). Pharmacologic doses of vitamin E improve insulin action in healthy subjects and non-insulin-dependent diabetic patients. Am. J. Clin. Nutr. 57, 650–656. doi: 10.1093/ajcn/57.5.650
Park, S., Kim, B. C., and Kang, S. (2017). Interaction effect of PGC-1α rs10517030 variants and energy intake in the risk of type 2 diabetes in middle-aged adults. Eur. J. Clin. Nutr. 71, 1442–1448. doi: 10.1038/ejcn.2017.68
Perry, R. J., Samuel, V. T., Petersen, K. F., and Shulman, G. I. (2014). The role of hepatic lipids in hepatic insulin resistance and type 2 diabetes. Nature 510, 84–91. doi: 10.1038/nature13478
Pessoa, J. C., and Tomaz, I. (2010). Transport of therapeutic vanadium and ruthenium complexes by blood plasma components. Curr. Med. Chem. 17, 3701–3738. doi: 10.2174/092986710793213742
Petersen, M. C., and Shulman, G. I. (2018). Mechanisms of insulin action and insulin resistance. Physiol. Rev. 98, 2133–2223. doi: 10.1152/physrev.00063.2017
Potier, M., Darcel, N., and Tome, D. (2009). Protein, amino acids and the control of food intake. Curr. Opin. Clin. Nutr. Metab. Care 12, 54–58. doi: 10.1097/MCO.0b013e32831b9e01
Puddu, A., Sanguineti, R., Montecucco, F., and Viviani, G. L. (2014). Evidence for the gut microbiota short-chain fatty acids as key pathophysiological molecules improving diabetes. Mediators Inflamm. 2014:162021. doi: 10.1155/2014/162021
Qi, L., Cornelis, M. C., Zhang, C., van Dam, R. M., and Hu, F. B. (2009). Genetic predisposition, Western dietary pattern, and the risk of type 2 diabetes in men. Am. J. Clin. Nutr. 89, 1453–1458. doi: 10.3945/ajcn.2008.27249
Qi, Q., Bray, G. A., Hu, F. B., Sacks, F. M., and Qi, L. (2012). Weight-loss diets modify glucose-dependent insulinotropic polypeptide receptor rs2287019 genotype effects on changes in body weight, fasting glucose, and insulin resistance: the preventing overweight using novel dietary strategies trial. Am. J. Clin. Nutr. 95, 506–513. doi: 10.3945/ajcn.111.025270
Qi, Q., Bray, G. A., Smith, S. R., Hu, F. B., Sacks, F. M., and Qi, L. (2011). Insulin receptor substrate 1 gene variation modifies insulin resistance response to weight-loss diets in a 2-year randomized trial: the Preventing Overweight Using Novel Dietary Strategies (POUNDS LOST) trial. Circulation 124, 563–571. doi: 10.1161/CIRCULATIONAHA.111.025767
Qi, Q., Zheng, Y., Huang, T., Rood, J., Bray, G. A., Sacks, F. M., et al. (2015). Vitamin D metabolism-related genetic variants, dietary protein intake and improvement of insulin resistance in a 2 year weight-loss trial: pOUNDS Lost. Diabetologia 58, 2791–2799. doi: 10.1007/s00125-015-3750-1
Qin, J., Li, Y., Cai, Z., Li, S., Zhu, J., Zhang, F., et al. (2012). A metagenome-wide association study of gut microbiota in type 2 diabetes. Nature 490, 55–60. doi: 10.1038/nature11450
Rayman, M. P., Infante, H. G., and Sargent, M. (2008). Food-chain selenium and human health: spotlight on speciation. Br. J. Nutr. 100, 238–253. doi: 10.1017/S0007114508922522
Rico-Campa, A., Martinez-Gonzalez, M. A., Alvarez-Alvarez, I., Mendonca, R. D., de la Fuente-Arrillaga, C., Gomez-Donoso, C., et al. (2019). Association between consumption of ultra-processed foods and all cause mortality: sUN prospective cohort study. BMJ 365:l1949. doi: 10.1136/bmj.l1949
Rines, A. K., Sharabi, K., Tavares, C. D., and Puigserver, P. (2016). Targeting hepatic glucose metabolism in the treatment of type 2 diabetes. Nat. Rev. Drug Discov. 15, 786–804. doi: 10.1038/nrd.2016.151
Russell, W. R., Baka, A., Bjorck, I., Delzenne, N., Gao, D., Griffiths, H. R., et al. (2016). Impact of diet composition on blood glucose regulation. Crit. Rev. Food Sci. Nutr. 56, 541–590. doi: 10.1080/10408398.2013.792772
Saltiel, A. R., and Kahn, C. R. (2001). Insulin signalling and the regulation of glucose and lipid metabolism. Nature 414, 799–806. doi: 10.1038/414799a
Samuel, V. T., and Shulman, G. I. (2012). Mechanisms for insulin resistance: common threads and missing links. Cell 148, 852–871. doi: 10.1016/j.cell.2012.02.017
San-Cristobal, R., Navas-Carretero, S., Celis-Morales, C., Brennan, L., Walsh, M., Lovegrove, J. A., et al. (2015). Analysis of dietary pattern impact on weight status for personalised nutrition through on-line advice: the food4Me spanish cohort. Nutrients 7, 9523–9537. doi: 10.3390/nu7115482
Schuler, R., Osterhoff, M. A., Frahnow, T., Mohlig, M., Spranger, J., Stefanovski, D., et al. (2017). Dietary fat intake modulates effects of a frequent ace gene variant on glucose tolerance with association to type 2 diabetes. Sci. Rep. 7:9234. doi: 10.1038/s41598-017-08300-7
Schwartz, M. W., and Porte, D. Jr. (2005). Diabetes, obesity, and the brain. Science 307, 375–379. doi: 10.1126/science.1104344
Schwenk, R. W., Vogel, H., and Schurmann, A. (2013). Genetic and epigenetic control of metabolic health. Mol. Metab. 2, 337–347. doi: 10.1016/j.molmet.2013.09.002
Shenkin, A. (2006). Micronutrients in health and disease. Postgrad. Med. J. 82, 559–567. doi: 10.1136/pgmj.2006.047670
Song, H., Chu, Q., Yan, F., Yang, Y., Han, W., and Zheng, X. (2016). Red pitaya betacyanins protects from diet-induced obesity, liver steatosis and insulin resistance in association with modulation of gut microbiota in mice. J. Gastroenterol. Hepatol. 31, 1462–1469. doi: 10.1111/jgh.13278
Spiller, G. A., Jensen, C. D., Pattison, T. S., Chuck, C. S., Whittam, J. H., and Scala, J. (1987). Effect of protein dose on serum glucose and insulin response to sugars. Am. J. Clin. Nutr. 46, 474–480. doi: 10.1093/ajcn/46.3.474
Sun, M., Liu, G., and Wu, Q. (2013). Speciation of organic and inorganic selenium in selenium-enriched rice by graphite furnace atomic absorption spectrometry after cloud point extraction. Food Chem. 141, 66–71. doi: 10.1016/j.foodchem.2013.03.002
Taheri, S., Frost, F., Storck, L. J., Kacprowski, T., Gärtner, S., Rühlemann, M., et al. (2019). A structured weight loss program increases gut microbiota phylogenetic diversity and reduces levels of Collinsella in obese type 2 diabetics: a pilot study. PLoS One 14:e0219489. doi: 10.1371/journal.pone.0219489
Tajima, R., Kodama, S., Hirata, M., Horikawa, C., Fujihara, K., Yachi, Y., et al. (2014). High cholesterol intake is associated with elevated risk of type 2 diabetes mellitus - a meta-analysis. Clin. Nutr. 33, 946–950. doi: 10.1016/j.clnu.2014.03.001
Takaya, J., Iharada, A., Okihana, H., and Kaneko, K. (2011). Magnesium deficiency in pregnant rats alters methylation of specific cytosines in the hepatic hydroxysteroid dehydrogenase-2 promoter of the offspring. Epigenetics 6, 573–578. doi: 10.4161/epi.6.5.15220
Takaya, J., Iharada, A., Okihana, H., and Kaneko, K. (2013). A calcium-deficient diet in pregnant, nursing rats induces hypomethylation of specific cytosines in the 11beta-hydroxysteroid dehydrogenase-1 promoter in pup liver. Nutr. Res. 33, 961–970. doi: 10.1016/j.nutres.2013.07.015
Talaei, A., Mohamadi, M., and Adgi, Z. (2013). The effect of vitamin D on insulin resistance in patients with type 2 diabetes. Diabetol. Metab. Syndr. 5:8. doi: 10.1186/1758-5996-5-8
Tomlinson, B., Chan, P., and Lam, C. W. K. (2020). Postprandial hyperlipidemia as a risk factor in patients with type 2 diabetes. Expert Rev Endocrinol Metab 15, 147–157. doi: 10.1080/17446651.2020.1750949
Tremblay, F., Lavigne, C., Jacques, H., and Marette, A. (2003). Dietary cod protein restores insulin-induced activation of phosphatidylinositol 3-kinase/Akt and GLUT4 translocation to the T-tubules in skeletal muscle of high-fat-fed obese rats. Diabetes 52, 29–37. doi: 10.2337/diabetes.52.1.29
Tremblay, F., Lavigne, C., Jacques, H., and Marette, A. (2007). Role of dietary proteins and amino acids in the pathogenesis of insulin resistance. Annu. Rev. Nutr. 27, 293–310. doi: 10.1146/annurev.nutr.25.050304.092545
Tremblay, F., and Marette, A. (2001). Amino acid and insulin signaling via the mTOR/p70 S6 kinase pathway. A negative feedback mechanism leading to insulin resistance in skeletal muscle cells. J. Biol. Chem. 276, 38052–38060. doi: 10.1074/jbc.M106703200
Um, S. H., D’Alessio, D., and Thomas, G. (2006). Nutrient overload, insulin resistance, and ribosomal protein S6 kinase 1. S6K1. Cell Metab. 3, 393–402. doi: 10.1016/j.cmet.2006.05.003
Vary, T. C., and Lynch, C. J. (2007). Nutrient signaling components controlling protein synthesis in striated muscle. J. Nutr. 137, 1835–1843. doi: 10.1093/jn/137.8.1835
Vo, T. X., Revesz, A., Sohi, G., Ma, N., and Hardy, D. B. (2013). Maternal protein restriction leads to enhanced hepatic gluconeogenic gene expression in adult male rat offspring due to impaired expression of the liver X receptor. J. Endocrinol. 218, 85–97. doi: 10.1530/JOE-13-0055
von Post-Skagegard, M., Vessby, B., and Karlstrom, B. (2006). Glucose and insulin responses in healthy women after intake of composite meals containing cod-, milk-, and soy protein. Eur. J. Clin. Nutr. 60, 949–954. doi: 10.1038/sj.ejcn.1602404
Wa, E. L. I, Naser, A., Taleb, M. H., and Abutair, A. S. (2018). The effects of oral magnesium supplementation on glycemic response among type 2 diabetes patients. Nutrients 11;44. doi: 10.3390/nu11010044
Wahl, S., Drong, A., Lehne, B., Loh, M., Scott, W. R., Kunze, S., et al. (2017). Epigenome-wide association study of body mass index, and the adverse outcomes of adiposity. Nature 541, 81–86. doi: 10.1038/nature20784
Wheeler, M. L., and Pi-Sunyer, F. X. (2008). Carbohydrate issues: type and amount. J. Am. Diet. Assoc. 108(4 Suppl. 1), S34–S39. doi: 10.1016/j.jada.2008.01.024
Xu, M., Qi, Q., Liang, J., Bray, G. A., Hu, F. B., Sacks, F. M., et al. (2013). Genetic determinant for amino acid metabolites and changes in body weight and insulin resistance in response to weight-loss diets: the preventing overweight using novel dietary strategies (POUNDS LOST) trial. Circulation 127, 1283–1289. doi: 10.1161/CIRCULATIONAHA.112.000586
Yamaguchi, Y., Adachi, K., Sugiyama, T., Shimozato, A., Ebi, M., Ogasawara, N., et al. (2016). Association of Intestinal Microbiota with Metabolic Markers and Dietary Habits in Patients with Type 2 Diabetes. Digestion 94, 66–72. doi: 10.1159/000447690
Yamashita, H., Takenoshita, M., Sakurai, M., Bruick, R. K., Henzel, W. J., Shillinglaw, W., et al. (2001). A glucose-responsive transcription factor that regulates carbohydrate metabolism in the liver. Proc. Natl. Acad. Sci. U.S.A. 98, 9116–9121. doi: 10.1073/pnas.161284298
Yang, H., Kim, M., Kwon, D., Kim, D., Zhang, T., Ha, C., et al. (2018). Combination of aronia, red ginseng, shiitake mushroom and nattokinase potentiated insulin secretion and reduced insulin resistance with improving gut microbiome dysbiosis in insulin deficient type 2 diabetic rats. Nutrients 10:948. doi: 10.3390/nu10070948
Zhang, B. B., Zhou, G., and Li, C. (2009). AMPK: an emerging drug target for diabetes and the metabolic syndrome. Cell Metab. 9, 407–416. doi: 10.1016/j.cmet.2009.03.012
Zhang, H., Chu, X., Huang, Y., Li, G., Wang, Y., Li, Y., et al. (2014). Maternal vitamin D deficiency during pregnancy results in insulin resistance in rat offspring, which is associated with inflammation and Ikappabalpha methylation. Diabetologia 57, 2165–2172. doi: 10.1007/s00125-014-3316-7
Zhang, Q., Sun, X., Xiao, X., Zheng, J., Li, M., Yu, M., et al. (2017). Dietary chromium restriction of pregnant mice changes the methylation status of hepatic genes involved with insulin signaling in adult male offspring. PLoS One 12:e0169889. doi: 10.1371/journal.pone.0169889
Zhao, C., Yang, C., Chen, M., Lv, X., Liu, B., Yi, L., et al. (2018). Regulatory efficacy of brown seaweed lessonia nigrescens extract on the gene expression profile and intestinal microflora in type 2 diabetic mice. Mol. Nutr. Food Res. 62:1700730. doi: 10.1002/mnfr.201700730
Zhao, C., Yang, C., Wai, S. T. C., Zhang, Y., Paoli, P., Wu, Y., et al. (2019). Regulation of glucose metabolism by bioactive phytochemicals for the management of type 2 diabetes mellitus. Crit. Rev. Food Sci. Nutr. 59, 830–847. doi: 10.1080/10408398.2018.1501658
Zhao, L., Zhang, F., Ding, X., Wu, G., Lam, Y. Y., Wang, X., et al. (2018). Gut bacteria selectively promoted by dietary fibers alleviate type 2 diabetes. Science 359, 1151–1156. doi: 10.1126/science.aao5774
Zhao, Y., Gao, P., Sun, F., Li, Q., Chen, J., Yu, H., et al. (2016). sodium intake regulates glucose homeostasis through the PPARdelta/adiponectin-mediated SGLT2 pathway. Cell Metab. 23, 699–711. doi: 10.1016/j.cmet.2016.02.019
Zheng, Y., Huang, T., Zhang, X., Rood, J., Bray, G. A., Sacks, F. M., et al. (2015). dietary fat modifies the effects of FTO genotype on changes in insulin sensitivity. J. Nutr. 145, 977–982. doi: 10.3945/jn.115.210005
Zhou, J., Huang, K., and Lei, X. G. (2013). Selenium and diabetes–evidence from animal studies. Free Radic. Biol. Med. 65, 1548–1556. doi: 10.1016/j.freeradbiomed.2013.07.012
Zimmet, P., Alberti, K. G., and Shaw, J. (2001). Global and societal implications of the diabetes epidemic. Nature 414, 782–787. doi: 10.1038/414782a
Keywords : nutrition, diet, genetics, epigenetics, gut microbiota, type 2 diabetes
Citation: Guo Y, Huang Z, Sang D, Gao Q and Li Q (2020) The Role of Nutrition in the Prevention and Intervention of Type 2 Diabetes. Front. Bioeng. Biotechnol. 8:575442. doi: 10.3389/fbioe.2020.575442
Received: 23 June 2020; Accepted: 17 August 2020; Published: 15 September 2020.
Reviewed by:
Copyright © 2020 Guo, Huang, Sang, Gao and Li. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY) . The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yajie Guo, [email protected] ; Qingjiao Li, [email protected]
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Click through the PLOS taxonomy to find articles in your field.
For more information about PLOS Subject Areas, click here .
Loading metrics
Open Access
Peer-reviewed
Research Article
Effects of an instructional WhatsApp group on self-care and HbA1c among female patients with Type 2 diabetes mellitus
Roles Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Project administration, Resources, Software, Validation, Visualization, Writing – review & editing
Affiliations Faculty of Nursing, Medical/Surgical Department, King Abdulaziz University, Jeddah, Saudi Arabia, Medical Department Rabigh General Hospital, Rabigh, Saudi Arabia
Roles Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Project administration, Resources, Software, Supervision, Validation, Visualization, Writing – review & editing
Affiliations Faculty of Nursing, Medical/Surgical Department, King Abdulaziz University, Jeddah, Saudi Arabia, Faculty of Nursing, Ain Shams University, Cairo, Egypt
Roles Conceptualization, Formal analysis, Investigation, Methodology, Project administration, Resources, Software, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing
* E-mail: [email protected]
Affiliation Faculty of Nursing, Medical/Surgical Department, King Abdulaziz University, Jeddah, Saudi Arabia
- Riham Saud Alhazmy,
- Asmaa Hamdi Khalil,
- Hayfa Almutary

- Published: September 18, 2024
- https://doi.org/10.1371/journal.pone.0305845
- Reader Comments
Aims and objectives
To assess the effect of an instructional WhatsApp group on self-care and HbA1c levels among female patients with type 2 diabetes mellitus (T2DM).
T2DM is a chronic disease that requires effective self-care. WhatsApp is a free application that can be effectively used for patient education.
This study used a quasi-experimental design.
A convenience sample of 62 female participants was recruited from the medical outpatient clinic of a tertiary hospital. The Diabetes Self-Care Scale was used to assess the self-care profiles of the participants pre- and post-intervention. HbA1c samples were also collected at baseline and three months after receiving instructions from the WhatsApp group. Sociodemographic and clinical data were collected during the pre-intervention stage.
The mean HbA1c level decreased from 8.61 ± 1.70 to 7.92 ± 1.60 after implementing the WhatsApp group instructions; the values showed a significant difference (t-value = 5.107 and P -value < 0.001). The post-test mean score of total self-care was higher than the pre-test mean score (t-value = 12.359, P -value <0.001), indicating a highly significant difference.
Conclusions
The study demonstrated that the instructional WhatsApp group is an effective method for improving self-care and HbA1c levels in patients with T2DM. This study suggests the use of WhatsApp group instructions as a teaching method in the healthcare system for the education and follow-up of patients with T2DM.
Relevance to clinical practice
The findings support the need to initiate effective and dynamic interventional follow-ups through WhatsApp groups for patients with T2DM to improve their self-care and HbA1c levels and ultimately reduce the burden on hospitals and governments.
Citation: Alhazmy RS, Khalil AH, Almutary H (2024) Effects of an instructional WhatsApp group on self-care and HbA1c among female patients with Type 2 diabetes mellitus. PLoS ONE 19(9): e0305845. https://doi.org/10.1371/journal.pone.0305845
Editor: Nimesh Lageju, BP Koirala Institute of Health Sciences, NEPAL
Received: February 19, 2024; Accepted: June 5, 2024; Published: September 18, 2024
Copyright: © 2024 Alhazmy et al. This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Data Availability: All relevant data are within the paper and its Supporting Information files.
Funding: The author(s) received no specific funding for this work.
Competing interests: The authors have declared that no competing interests exist.
Introduction
Diabetes mellitus (DM) is an alarming global health issue that can lead to serious complications if uncontrolled or not managed appropriately. It is one of the fastest growing global health emergencies of the 21st century [ 1 ]. In 2019, DM reached pandemic proportions with a worldwide prevalence of 9% (463 million adults) [ 2 ]. More than half a billion people worldwide have developed DM, and approximately 1 in 10 adults have the disease [ 1 ]. The number of cases has increased over the past two years [ 1 ]. In addition, DM is one of the most common diseases that cause mortality and morbidity in Saudi Arabia. According to a review of national data, DM affects 8.5% of the total adult population of Saudi Arabia [ 3 ].
DM complications are associated with frequent and prolonged hospitalizations, which increase the burden on individuals and the healthcare system [ 4 ]. According to the American Diabetes Association (2018), the total estimated cost of a diabetes diagnosis in the United States in 2017 was $327 billion; this value includes medical costs and reduced patient productivity [ 5 ]. The chronic nature of this disease requires self-care and self-management to prevent possible complications.
The digital health revolution provides helpful tools that support healthy practices among people with chronic noncommunicable diseases (NCDs), such as diabetes [ 6 ]. This includes using mobile health applications in patients’ education. A growing number of studies demonstrate the effectiveness of using mobile apps for lifestyle changes and self-management in people with chronic NCDs such as diabetes, hypertension, and cardiac diseases [ 7 , 8 ]. According to a cross-sectional study with 1119 participants, the majority of respondents believed that using mobile health to prevent NCDs would be beneficial (62%), and that it would enable patients to manage their lifestyle modifications (59%) [ 9 ]. In addition, mobile apps were found to be effective tools for those in rural areas [ 8 ] and across all age groups [ 9 ]. However, choosing the appropriate mobile apps, such as WhatsApp, to enhance health still needs more investigation.
Type 2 diabetes mellitus (T2DM) is a metabolic disorder that occurs as a result of insulin resistance and impaired insulin production by islet β cells in the pancreas; this condition leads to elevated blood glucose levels, resulting in increased glycated hemoglobin (HbA1c) levels [ 10 , 11 ]. In 1990, T2DM was the 18th leading cause of mortality and the 9th cause of morbidity. In 2020, it ranked as the 9th cause of worldwide mortality and the 7th cause of morbidity [ 12 ]. According to the World Health Organization (2020), DM is the 7th leading cause of death among women in Saudi Arabia [ 13 ]. Various factors contribute to the increase in the total number of patients with DM, and they include an aging population and a rising obesity rate [ 14 , 15 ]. In addition, the rate of obesity in women is higher than that in men [ 16 ].
HbA1c is a diagnostic tool and objective measure that healthcare providers and researchers use to assess the clinical outcomes of patients with diabetes. It represents the average blood glucose levels of individuals over the previous 2–3 months based on the presumed half-life of red blood cells [ 17 ]. According to the Saudi Diabetes Clinical Practice Guidelines, the normal HbA1c level is 4%–5.6% [ 18 ]. The American Diabetes Association (2021) recommended that A1c should be less than 7.0% in adults with diabetes [ 19 ], and A1c 8% indicates poor diabetes control [ 17 ].
DM is a complex, long-term illness that requires regular medical assistance and multifaceted risk-reduction methods that are beyond glucose management. Given the unavailability of a definitive cure, secondary prevention is the best approach. Appropriate patient education regarding self-care can delay or prevent the onset of acute and chronic complications [ 20 ]. Self-care involves many aspects, such as diet, physical activities, medication adherence, blood glucose monitoring, problem solving, and coping skills [ 21 ]. The critical element for controlling diabetes is patients’ self-care management.
Several recent studies suggested the use of diabetes self-management education (DSME) to control the disease [ 22 – 24 ]. In these studies, the HbA1c levels of patients with T2DM who participated in DSME decreased by 0.71%–1.57% relative to those of patients on standard therapy [ 22 – 24 ]. With the development of technology, mobile applications have been broadly used to communicate and deliver information in a simple and easy manner. WhatsApp is a free messenger application that can be used across multiple platforms such as Android and iPhone devices [ 25 ]. Instructional WhatsApp groups can create a competitive environment to decrease the level of glycosylated hemoglobin by providing instructions by educators. Additionally, group members can encourage one another to achieve their primary goals [ 26 ]. The recent literature suggests that WhatsApp is an effective medical learning tool [ 27 ]. In Saudi Arabia, 71% of the total population uses WhatsApp, and people spend an average of three hours and two minutes on social media [ 25 ]. In addition, a study conducted in Saudi Arabia showed that Saudi women tend to learn through WhatsApp [ 28 ]. The theoretical framework for this study is based on the trans-theoretical model (TTM) of stages of change established by James Prochaska and Carlo DiClemente (the 1980s) [ 29 ]. The model has been used to help people develop healthy behaviors, including weight loss, exercise, and quitting unhealthy behaviors. In addition, it presents a health-promotion strategy that considers behavioral change as a series of steps [ 30 ]. At present, few studies have focused on the effects of instructional WhatsApp groups on self-care and HbA1c levels in female patients with T2DM, especially those in Saudi Arabia. Hence, the current study aimed to assess the effect of instructional WhatsApp groups on self-care and HbA1c levels among female patients with T2DM. Therefore, the findings of this study may help identify new strategies for managing T2DM.
Research objectives
The aim was achieved through the following objectives:
- Assessing the level of self-care and HbA1c among type 2 diabetic female patients.
- Providing diabetes self-care-related instruction through WhatsApp group.
- Measuring the effect of instructional WhatsApp group on self-care and level of HbA1c among type 2 diabetic patients.
Research hypothesis
• Type 2 diabetes female patients’ self-care will improve post implementing WhatsApp group instruction.
• HbA1c will decrease among female patients with type 2 diabetes post implementing WhatsApp group instruction.
Materials and methods
A quasi-experimental design (pre- and post-test) was used in this study.
Participants
The inclusion criteria were female adults with T2DM who could read and write Arabic and use WhatsApp on their cellphones. The exclusion criteria were patients using a different application, pregnant women with gestational diabetes, post-operative patients, patients with hearing or visual disabilities, and those with other comorbidities that would prevent them from participating in the study (e.g., mental illness and cerebrovascular accident).
Data were gathered from the medical outpatient clinic of the Rabigh General Hospital in the Western region of Saudi Arabia. The sample size was 89, which was calculated using the Raosoft program based on a report of the population size at this hospital in 2020 (114 female patients with T2DM), a level of confidence of 95%, a margin of error of 0.05, and a probability value of 0.5. A total of 70 patients who met the inclusion and exclusion criteria were recruited to participate in the study; 8 of them withdrew from the study during the intervention phase (3 left the WhatsApp group, and 5 did not complete the post-test). Therefore, the final sample size was 62 patients who completed three months of the WhatsApp intervention and the post-test ( Fig 1 ).
- PPT PowerPoint slide
- PNG larger image
- TIFF original image
https://doi.org/10.1371/journal.pone.0305845.g001
Data collection
The data collection process was divided into three phases: pre-test, intervention, and post-test.
Pre-test phase.
After obtaining ethical approval, the researcher met with the head nurse of the clinic to facilitate data collection. Initially, the medical records of female patients with diabetes were reviewed to identify those who met the inclusion criteria. Patient names, file numbers, and phone numbers were recorded to facilitate the communication with potential participants (files without phone numbers were excluded).
Female patients with T2DM were contacted to check if they could read and write Arabic and if they had a smartphone with the WhatsApp application. A total of 76 females who met the inclusion criteria were invited to participate in the study; 6 of them refused to participate. Those who agreed to participate in the study signed an informed consent form and were then provided with pre-test questionnaires to gather baseline data about their self-care. Blood samples were collected for HbA1c analysis.
Intervention phase.
The duration of the intervention phase was three months. Initially, the WhatsApp group was created and moderated by one researcher who is a registered nurse and diabetes educator and two other researchers who are associate professors in medical–surgical nursing. Instructions regarding diabetes self-care were provided through the WhatsApp group in the form of pictures, videos, and daily messages. The WhatsApp group was open daily from Sundays to Thursdays, from 6:00 p.m. to 8:00 p.m., for discussions, questions, or any clarifications or concerns. All instructions were sent to the group daily, and questions were answered by the moderator accordingly. Private conversations were not permitted. Depending on the amount of information presented during the first month, each topic was presented for 1–3 days. Patients were motivated to follow the instructions in the WhatsApp groups for the next 2 months.
Post-test phase.
After the completion of the intervention phase, an appointment schedule was sent for the post-test and HbA1c analysis and was sent to the patients. The patients were divided according to their code numbers, with 15–19 patients scheduled for the post-test and HbA1c analysis per day. The patients were invited again the day before the appointment, and a private message through WhatsApp was sent on the day of the appointment as a reminder. After completing the same questionnaires, a blood sample was withdrawn for HbA1c analysis. The results were then compared with the previous ones.
Data were collected using two structured, validated questionnaires. The first one was aimed at assessing the patients’ demographic and clinical data. It included demographic data such as age, marital status, level of education, and working status. It also covered clinical data such as duration of disease, methods used to treat diabetes, family history related to diabetes, comorbidities, and education about self-care for diabetes. The second questionnaire used was the Diabetes Self-Care Scale. This scale was adapted from Lee and Fisher (2005) and modified in the current study to measure self-care practices related to diabetes [ 31 ]. The modified scale includes 28 statements that are related to self-care activities and are grouped into seven domains. These domains are dietary control (five statements); exercise (three statements); blood glucose monitoring (two statements); medication adherence (three statements); follow-up (three statements); foot care (five statements); and other self-care practices related to hygiene, diabetes identification, and avoidance of complications (seven statements). The responses to these statements were rated on a 6-point Likert scale, with the choices ranging from 1 “strongly disagree” to 6 (“strongly agree”). The scores for each domain and the total scale were calculated, and the mean scores were calculated and categorized as good, moderate, or poor. The intervals between the three categories were calculated by subtracting the lowest value from the highest value for every domain and the total and then dividing the results by 3.
Ethical consideration
Ethical approval was obtained from the Ethics Committee of the Faculty of Nursing, King Abdulaziz University (Ref No. 2M. 79) and from the National Board Review of the Ministry of Health at Jeddah Research Center (IRB No. H-02-J-002) to collect data from the hospital in the Rabigh, Makkah region where the study was conducted.
Written informed consent was obtained from all participants who agreed to participate in the study after explaining the research objective during the interviews. A summary of the search, purpose, duration, advantages, and disadvantages of the intervention was provided in Arabic. The ethical aspects of the study were based on research ethics and principles. The patients were informed that their participation was voluntary and that they had the right to continue or withdraw. Confidentiality and anonymity were protected by providing a code number for each participant at the data collection stage. In addition, all data gathered during the study were kept confidential, and only the researchers had access to personal information.
Statistical analysis
Data analysis was performed using the Statistical Package for Social Science (version 23.0). Descriptive analyses using frequency, percentage, mean, and standard deviation (SD) were performed to determine the distribution of the study participants’ sociodemographic variables and clinical data. Normal distribution was evaluated using the kurtosis and skewness test and Jarque–Bera test. A paired sample t-test was used to compare the self-care domains and HbA1c before and after implementing the WhatsApp group instructions. The significance of the results was categorized using P-values: P ≤ 0.05 was considered statistically significant; P ≤ 0.01, P ≤ 0.001 was considered highly statistically significant, and P > 0.05 was considered non-significant. Cohen’s d was used to measure the effect size, with d ≤ 0.2, 0.2 < d < 0.8, and d > 0.8 indicating small, moderate, and large effect sizes, respectively.
Demographic and clinical characteristics
Table 1 presents the participants’ demographic characteristics. The mean age of the study participants was 47.6 ± 9.74. Most study participants were married (66.1%) while a few (8.1%) were single. Regarding education level, 58.1% of the study participants had less than a secondary level of education while 17.7% had a bachelor’s degree. In terms of occupation, 77.4% of the study participants were not employed.
https://doi.org/10.1371/journal.pone.0305845.t001
The clinical characteristics of the patients are presented in Table 2 . Approximately one-quarter of the sample (21%) had T2DM for 15 years or more, and only a small percentage (8%) had T2DM for less than a year. In terms of treatment, 58.1% of the participants used oral antidiabetics while 4.8% used diet and exercise. Furthermore, 64.5% of the patients had a family history of diabetes. More than half of them (54.8%) indicated that they had previously received diabetes self-care education.
https://doi.org/10.1371/journal.pone.0305845.t002
Diabetes self-care among study participants before and after the implementation of the WhatsApp group instructions
As shown in Table 3 , the paired samples t-test was used to compare the mean scores of the self-care domains among the study participants before and after implementing the WhatsApp group instructions at a significance level of α = 0.05. Moreover, the effect size was calculated using Cohen’s d, with the values d ≤ 0.2, 0.2 < d < 0.8, and d ≥ 0.8 indicating small, moderate, and large effect sizes, respectively.
https://doi.org/10.1371/journal.pone.0305845.t003
With regard to dietary control, Table 3 shows that the post-test mean score is higher than the pre-test mean score with a calculated t value = 5.176 and P-value < 0.001, indicating a highly statistically significant difference between them. Measuring the effect size of the implementation of the WhatsApp group instructions on the level of dietary control revealed a Cohen’s d = 0.657 (> 0.2 and < 0.8), indicating that the effect of the implementation was moderate.
Regarding the exercise domain, the Table 3 shows that the post-test mean score was higher than the pre-test mean score. The calculated paired t-value = 10.079 and P-value < 0.001 denoted the highly statistically significant difference between the scores. Specifically, the level of exercise among the study participants increased and improved because of the implementation of the WhatsApp group instructions, with Cohen’s d = 1.280 > 0.8, which indicated a large effect size.
With regard to blood glucose monitoring, the same table shows that the post-test mean score was higher than the pre-test mean score. The calculated paired t-value was 10.479 while the P—value < 0.001, indicating the highly statistically significant difference between the scores. Measuring the effect size of the implementation of the WhatsApp group instructions on the level of blood glucose monitoring revealed a Cohen’s d = 1.331 > 0.8, indicating a large effect.
Regarding medication adherence as a self-care domain, Table 3 shows that the post-test mean score was higher than the pre-test mean score. The paired t-value = 4.237 and P-value < 0.001 indicated a highly statistically significant difference. Measuring the effect size of the implementation of the WhatsApp group instructions on the level of medication adherence based on Cohen’s d revealed a value of d = 0.538 > 0.2, indicating a moderate effect.
In relation to the participants’ follow-up before and after implementing the WhatsApp group instructions, the same table shows that the post-test mean score increased more than the pre-test mean score, with the tabulated t-value being 6.478 and P-value < 0.001, which indicated the highly statistically significant difference between them. Measuring the effect size of the implementation of the WhatsApp group instructions on the level of follow-up using Cohen’s revealed a value of d = 0.823 > 0.8, which indicated a large effect.
Regarding foot care after implementing the WhatsApp group instructions, Table 3 shows that the post-test mean score was higher than the pre-test mean score. The paired t-value = 6.725 and P-value < 0.001 indicated the highly statistically significant difference between them. The effect size of implementing the WhatsApp group instructions was large, with Cohen’s d = 0.854 > 0.8.
For the other self-care practices related to hygiene, diabetes identification, and avoidance of complications, Table 3 shows that the post-test mean score was higher than the pre-test mean score. The paired t-value was 10.921 while the P-value < 0.001, indicating a highly statistically significant difference. The effect size of implementing the WhatsApp group instructions was large, with Cohen’s d = 1.387 > 0.8.
In relation to total self-care, the post-test mean score was higher than the pre-test mean score. The t-value was 12.359 while P-value < 0.001, indicating a highly statistically significant difference between them. The effect size of implementing the WhatsApp group instructions on the level of total self-care was large, with Cohen’s d = 1.570 > 0.8.
HbA1c among study participants before and after the implementation of the WhatsApp group instructions
Table 4 illustrates the difference between the mean score of the HbA1c levels among the study participants before and after the implementation of the WhatsApp group instructions. The mean HbA1c level among the study participants decreased from 8.61 ± 1.70 to 7.92 ± 1.60 after implementing the WhatsApp group instructions. The t-value = 5.107 and P-value < 0.001, with an absolute reduction of 0.69, indicated the highly statistically significant difference between them. The effect size of implementing the WhatsApp group instructions on the level of HbA1c was moderate given Cohen’s d = 0.649 > 0.2.
https://doi.org/10.1371/journal.pone.0305845.t004
The current study demonstrated the effectiveness of using the instructional WhatsApp group on self-care and HbA1c among female patients with T2DM. Subjective and objective data were used to assess the changes in clinical outcomes. The results showed that the instructional WhatsApp group improved all domains of self-care and HbA1c levels.
Regarding dietary control, the mean score of all dietary control items increased, indicating an improvement in self-care under this domain. This finding is in line with those of previous studies [ 22 , 32 ]. Changing one’s lifestyle, particularly eating habits, necessitates regular reminders and increased motivation. In this regard, WhatsApp group instructions appear to be an effective method.
The study showed that the total mean scores in the exercise domain improved significantly after the implementation of the WhatsApp group instructions. This finding is consistent with the study of ElGerges (2020), who used traditional education and followed the patients for three months; their study revealed a significant improvement in the exercise domain [ 22 ]. A similar finding was reported in another study conducted in Saudi Arabia that measured the effect of a WhatsApp-based intervention on promoting physical activity among female college students in Abha [ 33 ]. This study found that social network-based interventions (WhatsApp) contribute to improvements in physical activity. However, some studies revealed that the exercise domain did not improve significantly after participation in the studies [ 32 , 34 , 35 ]. These findings could be due to the continuous support and encouragement through the group in which participants are asked to download a step-counting application and share photos of their walking areas after receiving the related knowledge.
For blood glucose monitoring, the total mean scores improved significantly after the implementation of the WhatsApp group instructions. These findings are congruent with those of ElGerges (2020) and Zheng et al. (2019), who reported a positive relationship between DSME and blood glucose monitoring and a significant improvement in the post-test mean scores in relation to blood glucose monitoring [ 22 , 36 ]. Meanwhile, Dinar et al. (2019) and Hailu et al. (2019) found no relationship between DSME and blood glucose monitoring [ 32 , 34 ]. The discrepancy in some of the findings across studies may be related to several factors, including the strategies used to remind participants. The findings of the current study may be attributed to the fact that the patients were constantly reminded of the need to monitor their blood glucose, document it, and analyze the readings. In addition, notebooks were distributed to the participants during the pre-test visit to record their blood glucose levels. These practices motivated the participants to follow instructions and change their lifestyle.
Medication adherence in patients with chronic diseases remains challenging. The clinical outcomes of patients with DM are usually related to medication adherence. In this study, an instructional WhatsApp group was used to assess its effect on patient adherence to medications. The results showed that the total mean score of the medication adherence domain improved significantly after the implementation of the WhatsApp group instructions. This finding is consistent with those of ElGerges (2020) and Zheng et al. (2019), who found positive patient outcomes regarding medication adherence after implementing diabetes self-management education [ 22 , 36 ]. Furthermore, a study conducted in the Kingdom of Saudi Arabia (KSA) showed that compliance rates for individuals with diabetes range from 60% to 80% for insulin and from 65% to 85% for oral antidiabetic drugs [ 37 ]. However, Sartori et al. (2020), who used the WhatsApp application to assess the impact of education on medication adherence, reported that the findings were clinically significant but not statistically significant [ 38 ]. In addition, previous studies revealed that medication adherence for T2DM did not improve significantly with the WhatsApp application [ 34 , 35 ]. Regardless of these differences in the findings of previous studies, the recent literature has reported high medication compliance among patients with diabetes after using WhatsApp group instructions [ 37 ]. Increasing knowledge, awareness, and correction of concepts related to medicines through WhatsApp groups may convince patients and contribute to great adherence to medicines.
This study also found that the intervention had a positive effect on patient follow-up. The total mean score in this domain improved significantly after the implementation of the WhatsApp group instructions; this result is similar to the findings of a previous study [ 34 ]. The use of WhatsApp instructions contributed to increased compliance with follow-ups through online clinical and face-to-face visits. Consultations with physicians when experiencing extremely high or extremely low blood glucose levels also increased. Not following-up is usually due to the fear of censure from healthcare providers and concerns about laboratory results. Such issues are often attributable to noncompliance with medical regimens. In the current study, the patients showed high compliance with their regimens. The motivation for patients to visit the clinic may be their enthusiasm for knowing their laboratory results after committing to medication, exercise, and nutrition.
The current study also demonstrated significant improvements in foot care following the implementation of the WhatsApp group instructions. Several studies have reported similar findings [ 22 , 32 , 34 , 36 ]. Patients with DM seem to be interested in this aspect. In addition, KSA is a country of Islam and Muslims who pray five times a day. Hence, the feet should be inspected five times as well through ablution ( wudu ). During the study intervention, the patients were encouraged to practice foot care by giving them simple instructions to follow and reminding them continuously. In addition, the complications associated with diabetic feet were explained to them.
For other self-care practices related to hygiene, diabetes identification, and avoidance of complications, significant improvements were noted in the mean scores following the implementation of the WhatsApp group instructions. In the WhatsApp group, the patients were encouraged to wear diabetes identification, maintain their self-cleaning regimen to prevent infections, and search the Internet or ask a healthcare provider when they have a new issue. In doing so, they may increase their awareness regarding these points.
Overall, self-care improved significantly following the implementation of the WhatsApp group instruction. This result is consistent with studies that lasted for three months [ 22 , 24 , 36 ]. By contrast, Waller et al. (2021) revealed that the total mean self-care score of the patients with T2DM did not improve significantly [ 35 ]. The positive findings regarding self-care in our study may be attributed to the fact that the instructions given through WhatsApp were carefully designed to suit different age groups according to their educational and social levels. The instructions were also validated by specialists in the field (endocrine consultant, medical consultant, and diabetic educator). Each part of the self-care program was provided separately, and feedback was obtained daily to ensure adherence to the recommended instructions. In the group, the patients were encouraged to share their experiences with one another through a group chat where they also shared new food recipes with pictures. Thus, they were motivated to learn and follow the instructions to achieve their goals.
The current study assessed the effect of the instructional WhatsApp group on HbA1c and found a significant improvement in the level of HbA1c with an absolute reduction of 0.69 after implementing the WhatsApp instructions. Previous studies also found a positive impact of using WhatsApp groups on HbA1c levels [ 22 , 23 , 26 , 39 – 41 ]. A few studies also demonstrated an improvement in HbA1c [ 35 ]. Often, commitments in the overall domains (i.e., diet control, exercise, blood glucose monitoring, medication adherence, foot care, and follow-up in self-care) would reflect objective results such as HbA1c results. In addition, sharing blood glucose level readings during daily follow-up could contribute to improving HbA1c levels.
The strength of this study lies in using a convenient teaching method through a WhatsApp group for giving instructions to female patients with type 2 DM and assessing its effect on their self-care and HbA1c level, where there is scanty research on this field. However, this study had some limitations. There was no follow-up measurement for self-care or HbA1c after six months or one year. Thus, longitudinal studies are recommended to assess the continued benefit of the intervention. Also, using a small sample size from one clinical site could restrict the generalizability of the study to Saudi Arabia. In addition, the quasi-experimental designs may be associated with the Hawthorne affect [ 42 ]. To reduce the possibility of this bias, we use an objective measure (the HbA1c test) to evaluate the effectiveness of the applied intervention.
T2DM is one of the most remarkable diseases of the 21st century, threatening patients’ physical and psychological well-being. The instructional WhatsApp group effectively improved patient self-care and HbA1c levels. We recommend the adoption of WhatsApp group instructions as a teaching method in the healthcare system for the continuous education and follow-up of patients with diabetes.
Nurses in administration, bedside, and clinics play an important role in providing care to females with T2DM. Secondary prevention is required to avoid or prevent complications and involves the development of standardized guidelines to improve self-care practices and HbA1c levels among patients in healthcare centers and hospitals. These guidelines include the following: providing T2DM patients with a recording book before discharge; assessing self-care levels during hospital discharge and during the follow-up period in the clinics; and initiating effective, dynamic interventional follow-up through WhatsApp groups for female patients with T2DM to improve their self-care and HbA1c levels.
Supporting information
S1 dataset..
https://doi.org/10.1371/journal.pone.0305845.s001
- View Article
- PubMed/NCBI
- Google Scholar
An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
The PMC website is updating on October 15, 2024. Learn More or Try it out now .
- Advanced Search
- Journal List
- Int J Nurs Sci
- v.6(1); 2019 Jan 10
Interventions for self-management of type 2 diabetes: An integrative review
Roger carpenter.
a West Virginia University School of Nursing, Morgantown, WV, 26506, USA
Toni DiChiacchio
b Faculty Practice & Community Engagement, West Virginia University, Morgantown, WV, 26506, USA
Kendra Barker
Associated data.
Type 2 diabetes mellitus has been identified as one of the most challenging chronic illnesses to manage. Since the management of diabetes is mainly accomplished by patients and families, self-management has become the mainstay of diabetes care. However, a significant proportion of patients fail to engage in adequate self-management. A priority research question is how do interventions affect the self-management behaviors of persons with Type 2 diabetes?
Purpose/Objectives
The purpose of this integrative review is to provide a summary and critique of interventions that support diabetes self-management in the patient with Type II diabetes mellitus.
An integrative review design, with a comprehensive methodological approach of reviews, allowing inclusion of experimental and non-experimental studies.
A comprehensive search was conducted via Ebscohost using databases of Academic Search Complete, CINAHL, Health Source: Nursing/Academic Edition, MEDLINE, PsycArtiCLES, and PsycInfo. The final number of papers used for this review were: motivational interviewing (6), peer support/coaching (10), problem solving therapy (3), technology-based interventions (30), lifestyle modification programs (7), patient education (11), mindfulness (3), and cognitive behavioral therapy (5).
Studies were examined from seventeen countries including a broad range of cultures and ethnicities. While interventions have shown mixed results in all interventional categories, many studies do support small to modest improvements in physiologic, behavioral, and psychological outcome measures. Considerable heterogeneity of interventions exists. The most commonly reported physiologic measure was HbA1c level. Outcome measures were collected mostly at 6 and 12 months. Duration of most research was limited to one year.
Conclusions
Research exploring the impact of interventions for self-management has made major contributions to the care of persons with type 2 diabetes, from offering suggestions for improving care, to stimulating new questions for research. However, implications for clinical practice remain inconclusive, and limitations in existing research suggest caution in interpreting results of studies.
1. Introduction
Type 2 diabetes mellitus has been identified as one of the most challenging chronic illnesses to manage [ 1 ]. The demands of diabetes and the integration of complex self-management regimens into daily life have been shown to produce high levels of emotional distress, and to leave people feeling overwhelmed, frustrated, and discouraged [ 2 , 3 ]. These demands also lead to reduced well-being, anxiety, and depression [ 4 , 5 ].
Since the management of diabetes is mainly accomplished by patients and families, self-management has become the mainstay of diabetes care. Self-management is the process of actively engaging in self-care activities with the goals of improving one's behaviors and well-being. Self-management includes meal planning, planned physical activity, blood glucose monitoring, taking diabetes medicines, and of managing episodes of illness and of low and high blood glucose. Self-management treatment plans are individually developed in consultation with a variety of health care professionals such as doctors, nurses, dietitians, and pharmacists [ 6 ].
Maintaining tight glycemic control through self-management can significantly reduce complications associated with diabetes [ 7 , 8 ]. However, self-management of diabetes and tight glycemic control are complex, and can be further complicated by issues related to adherence to treatment plans. Most research on diabetes has found that a significant proportion of patients fail to engage in adequate self-management [ [9] , [10] , [11] ]. Suboptimal adherence to self-management is well documented as negatively influencing outcomes in people with diabetes [ [12] , [13] , [14] ].
From the State of the Science on Nursing Best Practices for Diabetes Self-Management [ 15 ], research priorities include exploring the concept of diabetes self-management. Priority research questions include asking what affects self-management in persons with diabetes (literacy, communication skills, psychosocial factors, demographics), and how do interventions affect the self-management behaviors of persons with diabetes? Therefore, the purpose of this integrative review is to provide a summary and critique of interventions that support diabetes self-management in the patient with Type II diabetes mellitus.
2.1. Search method
A comprehensive search was conducted via Ebscohost using the following databases: Academic Search Complete, CINAHL, Health Source: Nursing/Academic Edition, MEDLINE, PsycArtiCLES, and PsycInfo. Search terms included diabetes mellitus, type 2 in the abstract, self care and self-management as a subject term, and “randomized controlled trial” in any field. Limits were set to include only peer-reviewed quantitative studies of adults written in the English language, and between January 2007–January 2018. In addition, the Cochrane Library was searched for a review on self-management.
The initial search yielded 98 articles that were abstracted for topics of self-management intervention. Fifty seven sources were excluded due to not meeting inclusion criteria or being duplicate. Of the remaining sources included for review, the major topics included: motivational interviewing (3), peer support/coaching (6), problem solving therapy (2), technology-based interventions (15), lifestyle modification programs (3), patient education (10), and a grouping of studies organized under psychoeducational interventions (3) that included topics of cognitive behavioral therapy and mindfulness.
Reference lists of retrieved sources were then searched. In addition, a final search of each of these topics was done using keywords and/or topics: diabetes mellitus, type 2, self care, self-management, and the name of the specific intervention topic. Review of reference lists of all included sources extended the date range from 2004 to 2018. At the completion of all searches and reviews, the final number of papers used for this review were: motivational interviewing (6), peer support/coaching (10), problem solving therapy (3), technology-based interventions (30), lifestyle modification programs (7), patient education (11), mindfulness (3), and cognitive behavioral therapy (5).
2.2. Inclusion criteria and quality appraisal
Three doctorally prepared nurses knowledgeable in the area of diabetes independently screened all retrieved sources for inclusion criteria and quality. After independent screening, the three nurses met to discuss inclusion criteria and quality appraisal, and to come to consensus.
To be included in the final review, each article was screened for the following inclusion criteria: Included only adults with type 2 diabetes, identified an intervention, provided quantitative empirical (Meta-analysis, systematic review, RCT, quasi-experimental, cohort study, or descriptive) evaluative support, and included an outcome variable of self-management, operationalized as: physiological indicator (i.e., blood glucose level, HbA1c, blood pressure, weight, cholesterol), psychosocial indicator (i.e., depression, emotional adjustment, stage of change, stress, or support), self-management outcomes (i.e., diet, exercise, medication, SBGM pattern), and knowledge.
To evaluate the quality of the papers included in this review, papers were assigned a grade according to the American Diabetes Association (ADA) evidence grading system for clinical practice recommendations [ 16 ]. Studies needed to qualify as a Grade A, B, or C to be evaluated. We used the grading system to evaluate the quality of the evidence and selected only those studies of higher quality for inclusion in this review as these are studies that are well supported for changes in practice. Grade C studies were included because although it is a lower level of evidence, some of these research studies provided additional helpful insight and information about specific interventional categories. The quality of evidence for this review can be summarized as follows: for motivational interviewing, four grade A, and two grade C studies; for peer support/coaching, eight grade A, and two grade C studies; for problem solving therapy, three grade A studies; for technology-based interventions, 22 grade A, four grade B, and four grade C studies; for lifestyle modification programs, seven grade A studies; for patient education, ten grade A, and one grade C studies; , for mindfulness, one grade A, and two grade C studies; and for cognitive behavioral therapy, four grade A, and one grade C studies.
3.1. Intervention categories
The purpose of this integrative review is to provide a summary and critique of interventions that support diabetes self-management in the patient with type 2 diabetes mellitus. The results will be presented as follows: 1) Overview of the intervention, 2) research of the intervention in chronic disease populations, including type 1 diabetes, and 3) empirical evidence of the intervention specific to type 2 diabetes.
3.1.1. Motivational interviewing
Motivational interviewing (MI) is a patient-centered approach to facilitating behavior change by helping patients explore and resolve their ambivalence about changing behavior. Developed by Miller and Rollnick [ 17 ], the goal of MI is to explore the patient's ambivalence to behavior change in a way that the patient is more likely to change behavior in the desired direction. MI is based on the following principles: motivation to change is a state, not an individual trait, that may fluctuate over time and between situations, and can be influenced to change in a particular direction; it is the patient's task to resolve this ambivalence to change, rather than the practitioner's; and the practitioner's role is to recognize this ambivalence and be directive in helping the patient to explore and resolve this ambivalence [ 17 ].
MI has been used in health care consultation in the treatment of a variety of health problems, including alcoholism, substance abuse, smoking cessation, eating disorders, and psychiatric treatment adherence [ 18 ]. Systematic reviews examining the impact of MI on a broad range of chronic diseases, including diabetes (both type 1 and type 2), asthma, substance and alcohol abuse, addiction, and psychiatric disorders, have produced mixed results. MI has been evaluated to be effective in helping patients change behaviors related to disease self-management, even in brief encounters [ 19 ]. On the other hand, another systematic review of eight RCTs using MI to improve health behaviors in persons with both type 1 and type 2 diabetes concluded that the evidence does not support the use of MI to improve self-management behaviors [ 20 ].
For this integrative review, one systematic review, four randomized controlled trials (RCTs), and one descriptive study were reviewed (see Table 1 ). Locations of research included four studies in the United States and one in Taiwan. In a systematic review aimed at exploring the gaps in what is known about MI and its impact on behavior change and clinical outcomes, 14 RCTs were reviewed. Results suggest that MI has some impact on diet behavior changes and weight loss [ 21 ]. Four RCTs included one MI session, and two included four and five MI sessions. Studies showed improvements in self-management behaviors related to diet, weight loss, and HbA1c [ [22] , [23] , [24] ], self-efficacy and quality of life [ 23 , 25 ]. One study showed weak support for MI due to improvement seen in both the intervention and control groups when MI was added to standard DSME [ 22 ]. Improvements in depression and smoking cessation were not reported [ 23 , 26 ].
Table 1
Motivational interviewing intervention studies.
| Study & Location | Design | Sample | Outcome Measures | Intervention (I) and Control (C) Groups | Results |
|---|---|---|---|---|---|
| Smith-West et al. (2007) [ ] US | RCT | = 217 Female: 100% Mean age: 53 African American: 38% | Weight, BMI, HbA1c, collected at baseline, 6, 12, and 18 months. | Participants enrolled in a group-based weight control program, receiving 42 sessions (weekly for 6 months, then biweekly for 6 months, then monthly for 6 months for total of 18 months). (I): ( = 109) Five MI sessions offered (the first session before the first group meeting) at baseline, 3, 6, 9, and 12 months. Sessions lasted 45 min. Led by psychologists; (LTA = 6); Intervention delivered by psychologist; Intervention fidelity addressed. (C): ( = 108) [Attention Control group] Educational sessions (not MI sessions), the same number and length as the intervention group's MI sessions, that focused on topics of womens health; (LTA = 9). | MI group: significantly more weight loss at 6 and 18 months; significant HbAlc reduction at 6 months (0.8%), but not at 18 months. |
| Chen et al. (2012) [ ] Taiwan | RCT | = 250 Female: 50.2% Age range: 26-87 Chinese-speaking | HbA1c, self-management, self-efficacy, QOL, depression, anxiety, & stress, collected at baseline and 3 months post-intervention. | (I): ( = 104) Usual care plus a 45–60 min MI approach done 2 weeks after collection of baseline data; (LTA = 21); Intervention delivered by a nurse; Intervention fidelity not addressed. (C): ( = 110) Usual care; (LTA = 15). | Significant improvements in self-management, self-efficacy, QOL, and HbA1c (8.97 ± 2.17 decreased to 8.16 ± 1.73). Depression Anxiety Scale showed no significant change. |
| Welch et al. (2011) [ ] US | RCT | = 234 Female: 59% Mean age: 55.7 Caucasian: 84% | HbA1c, distress, self-care behaviors (SMBG, diet, exercise, & medication adherence), depression, satisfaction, & self-efficacy, collected at baseline and 6 months. | 4 groups: (1) DSME + MI with webtool; (2) DSME + MI without webtool; (3) DSME with webtool; (4) DSME without webtool. Intervention groups = 118 (LTA = 28). Intervention delivered by Certified Diabetes Educator; Intervention fidelity addressed. Control groups = 116 (LTA = 22). | Significant change in HbA1c over study period in total sample (reduction of 0.58%). Mediators of distress and self-care significantly associated with change in HbA1c for both groups. |
| Calhoun et al. (2010) [ ] US | Descriptive | = 20 Female: 53.8% Mean age: 54.0 American Indian | Glucose, HbA1c, & self-reported psychological (distress, locus of control, QOL, depression, stages of change), exercise, & diet, collected at baseline and 3 months post-intervention. | (I): ( = 20) A program with a baseline assessment, two MI sessions within 3 weeks of baseline (each session lasting 30 min), then 3 months post-intervention assessment. (LTA = 0). Intervention delivered by “trained interventionist”; Intervention fidelity not addressed. | Significant improvements in depressive symptoms, fatalism, treatment satisfaction (QOL tool), social/vocational worry (QOL tool). No significant change in HbA1c. |
| Hokanson et al. (2006) [ ] US | RCT | = 114 Female: 43% Age range: 21-80 White: 88% | Prevalence of smoking, self-efficacy, HbA1c, weight loss, lipids, & BP collected at baseline, 3 and 6 months follow-up. | (I): ( = 57) Face-to-face MI session (20–30 min) done at initial visit (done 3 months after baseline assessment and enrollment into study), and an additional 3–6 telephone counseling sessions (first call 1 week after MI session). Nicotine replacement therapy offered free to intervention group; (LTA = not described); Intervention delivered by “trained research staff”; Intervention fidelity not addressed. (C): ( = 57) Usual care; (LTA = not described). | No significant differences between groups at 6 months in smoking cessation. No significant differences in psychosocial variables. HbA1c improved in both groups (from >7% to <7% at 6 months). |
| Ekong & Kavookjian, (2016) [ ] | Systematic review | = 14 RCTs(US, UK, Taiwan, Thailand, Denmark, Netherlands) | Health behaviors for diabetes and any targeted clinical outcome. | Studies varied in length and frequency of MI sessions. Outcome variables included self-management behaviors of diet, physical activity, alcohol reduction and smoking cessation, HbA1c, BP, BMI, weight reduction, and cholesterol levels. | MI had some impact on diet behaviors and weight loss, and may show promise for dietary behaviors. |
3.1.2. Coaching/peer health coaching/peer support
Coaching, peer health coaching, and peer support use health care providers or volunteers, collectively referred to as coaches or peer supporters, to provide self-management support for persons who may be considered peers or who have the same health condition [ 27 , 28 ]. These coaches and peers can include patients, community health workers, lay educators, family members, and health care professionals. Peer health coaching is based on the idea that a patient will connect to others who have similar experiences [ 29 ]. Regardless as to the type of coaching or peer support, the goal is to engage and motivate patients in self-management.
Coaching and peer support interventions have been well documented in diabetes education. In the context of diabetes support, coaches and peers can have multiple roles, including educator, advocate, cultural translator, mentor, case manager, and group facilitator [ 27 ]. Peer coaching and support is most commonly delivered by a trained peer, and focuses on self-management interventions that are time limited and based on a scripted standardized curricula [ 30 ]. In terms of effectiveness, peer health coaching and support have been successful in improving self-management and in lowering HbA1c [ 31 ]. Because of these favorable results, peer health coaching and support has received increased interest as a model for more long-term diabetes self-management support interventions.
For this integrative review, ten randomized controlled trials (RCT) were reviewed (see Table 2 ). Locations of research included seven studies in the United States, and one in the Netherlands, Thailand, and Australia. Six studies compared peer-led interventions, two compared health professional-led interventions, one compared a CHW intervention, and one used a family-oriented approach to self-management, with all intervention groups being compared to usual care. The duration of the interventions ranged from 4 weeks to 18 months. Studies showed improvements in self-efficacy and knowledge of self-management [ [32] , [33] , [34] , [35] , [36] ]. Results for reduction in HbA1c were mixed. Four studies described reductions in HbA1c levels in peer-led intervention groups and CHWs [ 1 , 28 , 31 , 37 ]; three studies showed no significant reduction in HbA1c levels [ 32 , 33 , 38 ].
Table 2
Peer health coaching/peer.
| Study & Location | Design | Sample | Outcome Measures | Intervention (I) and Control (C) Groups | Results |
|---|---|---|---|---|---|
| Nishita et al. (2012) [ ] US | RCT | = 190 Female: 62.6% Mean age: 48.5 Hawaiian or Asian: 71% | Height, weight, and HbA1c, & self-reported self-efficacy, & QOL, collected at baseline, 6 and 12 months. | (I): ( = 128) Over 12 months, individualized, self-directed support from life coach and a pharmacist. Appointments made by individual participants (LTA = 45); Intervention delivered by pharmacist and “trained” life coach (bachelor's degree in social sciences); Intervention fidelity addressed. (C): ( = 62) No treatment; (LTA = 10). | No significant difference between groups on HbA1c or BMI. Self-efficacy and QOL improved in those subjects who had 10 or more sessions. |
| Ruggiero et al. (2014) [ ] US | RCT | = 270 Female: 68.8% Mean age: 53.2 African American: 52.6%; Hispanic/Latino: 47.4% | A1c, BMI, & self-reported self-care, depressive symptoms, & confidence, collected at baseline, 6 and 12 months. | (I): ( = 136) Medical Assistant coaching intervention delivered by trained MA's over a 12-month period with in-person contacts at regular clinic visits (30 min sessions), and monthly follow-up phone calls in between visits. The focus was on providing information and skills to make informed self-care choices and changes; (LTA = 43); Intervention delivered by medical assistants; Intervention fidelity addressed. (C): ( = 134) Usual care; (LTA = 51). | All groups reported improvements in self-care across time, but no intervention effect was found. No differences were found in HbA1c between groups or across time. |
| Wichit et al. (2017) [ ] Thailand | RCT | = 140 Female: 72.8% Mean age: 58.4 | Self-management activities, QOL, self-efficacy, and HbA1c, collected at baseline, 5 weeks and 13 weeks. | (I): ( = 70) Family intervention consisting of three 2-h group session delivered at baseline, 5 weeks and 9 weeks. Groups of 8–12 dyads (patient and family member); (LTA = 3); Intervention delivered by nurse; Intervention fidelity not addressed. (C): ( = 70) Usual care; (LTA = 3). | Improvements seen in self-efficacy, self-management, and QOL in the intervention group. No between group differences in HbA1c. |
| Wu et al. (2010) [ ] Australia | RCT | = 30 Female: 28.6% Mean age range: 62.7–71.5 | Self-reported self-efficacy, self-management behavior, & knowledge, collected at baseline & 4-week follow up. | (I): ( = 15) Usual care plus peer support (Peer CDSMP). The program is 3 face to face sessions with research nurse (week 1), and follow up weeks 2–4 by peers who used weekly one telephone call and two text messages after each phone call; Intervention delivered by nurses and “trained” peers; Intervention fidelity not addressed. (C): ( = 13) Usual care. | Significant differences in knowledge were found for the intervention group, but no differences between the two groups over time for self-efficacy and self-management. |
| Van der Wulp et al. (2012) [ ] Netherlands | RCT | = 133 Female: 45.4% Mean age: 54 | Self-reported self-efficacy, coping, diet, physical activity, well-being, depressive symptoms, & distress, collected at baseline, 3 and 6 months. | (I): ( = 68) Three monthly home visits by a peer (expert patient) with a follow up phone call or email within two weeks after each visit. Visit 1 explored areas of lifestyle change. Visit 2 had participants assign importance and feasibility to proposed lifestyle changes, and set goals related to those changes. Visit 3 evaluated goals; (LTA = 9); Intervention delivered by “trained” expert patient peers; Intervention fidelity addressed. (C): ( = 65) Usual care; (LTA = 5). | The peer-lead coaching intervention improved self-efficacy in patients experiencing low self-efficacy. No significant differences were found in remaining outcome variables. |
| Carrasquillo et al. (2017) [ ] US | RCT | = 300 Female: 55% Mean age: 55.2 Latino | BP, lipids, HbA1c, BMI, & self-reported diet, physical activity, and medication adherence, collected at baseline and 12 months. | (I): ( = 150) CHW intervention for 12 months that included 4 home visits and 12 phone calls, and additional monthly CHW led educational groups; (LTA = 39). Intervention delivered by a “trained” CHW; Intervention fidelity addressed. (C): ( = 150) Enhanced usual care that included additional mailed educational materials; (LTA = 46). | The intervention group had lower HbA1c (reduction of 0.51), compared to control. No difference in any other outcome variables. |
| Moskowitz et al. (2013) [ ] US | RCT | = 299 Female: 51.4–53% Mean age: 54.1–56.3 | A1c, and self-reported depression, social support, literacy, & self-management, collected at baseline and at 6 months. | (I): ( = 148) Coaching intervention with peer coaches interacting with patients in person – telephone contact 2 times/month, and an in-person contact 2 or more times over 6 months. Intervention delivered by “trained” peers; Intervention fidelity not addressed. (C): ( = 151) Usual care. Study attrition not addressed | Peer health coaching was more effective in lowering HbA1c for patients with low medication adherence and self-management than for patients with higher levels of adherence and self-management. |
| Sinclair et al. (2013) [ ] US | RCT | = 82 Female: 63% Mean age: 53-55 Hawaii | A1c, height, weight, BP, & lipids, collected at baseline and 6 months. | (I): ( = 48) Diabetes self-management program (Partners in Care), led by peer educators. Focus on knowledge and skills related to blood glucose monitoring, adherence to medications, healthy eating, physical activity, and stress reduction; (LTA = 14); Intervention delivered by “trained” peer educators; Intervention fidelity addressed. (C): ( = 34) Wait listed for intervention; (LTA = 3). | Significant reduction in HbA1c (reduction of 1.6) and distress in intervention group at 6 months. |
| Thom et al. (2013) [ ] US | RCT | = 299 Female: >50% Mean age: 56.1 African American: 30.7–37.5% | A1c, lipids, height, weight, BMI, & BP, collected at baseline and 6 months. | (I): ( = 148) Coaching intervention with peer coaches interacting with patients in person - telephone contact 2 times/month, and an in-person contact 2 or more times over 6 months; (LTA = 8); Intervention delivered by “trained” peer coaches; Intervention fidelity not addressed. (C): ( = 151) Usual care; (LTA = 16). | At 6 months, significant differences in HbA1c levels, with reduction of 1.07% in intervention group, and only 0.3% in the usual care group. |
| Tang et al. (2015) [ ] US | RCT | = 106 Female: 67% Mean age: 56.3 African American | HbA1c, lipids, BP, BMI, waist circumference, & self-reported distress, & social support, collected at baseline, 3, 9, and 15 months. | (I): ( = 54) 3 months DSME plus 12 months peer support; (LTA = 20); Intervention delivered by nurses and peer leaders; Intervention fidelity not addressed. (C): ( = 52) 3 months DSME; (LTA = 20). | No significant changes in HbA1c between groups. |
3.1.3. Problem solving therapy/problem solving
Problem solving therapy (PST) is an intervention approach for behavior change that entails a series of cognitive operations used to figure out what to do when the way to reach a goal is not apparent [ 39 ]. The goal of PST is to facilitate behavior change, aiming to facilitate positive emotional reactions and reduce negative emotional reactions [ 40 ]. PST involves teaching the patient a step-by-step process to solving life problems, generally broken down into two major parts: applying a problem solving orientation to life, and using problem solving skills [ 41 ]. PST is based on teaching the following skills: (1) identifying a problem, (2) defining the problem, (3) understanding the problem, (4) setting goals related to the problem, (5) identifying alternative solutions, (6) evaluating and choosing best alternatives, (7) implementing alternatives, and (8) evaluating the effort at problem solving [ 42 ].
PST has a long history in clinical and counseling psychology to address multiple mental health disorders, family and relational distress, stress management and coping skills, and substance abuse [ 39 ]. PST has been a frequently used component of interventions within diabetes education and care, usually one component of a larger diabetes self-management intervention. PST has been recognized as an important process, intervention, and skill in diabetes self-management [ 43 ].
For this integrative review, three studies were reviewed: a RCT, a systematic review, and a meta-analysis (see Table 3 ). Locations of research included the United States, with systematic reviews including studies from English and Chinese electronic databases. The RCT compared an intensive program including eight PST session to a condensed program including just one PST session. Results showed a significant difference in HbA1c (0.71%) in the intensive PST group [ 44 ]. The systematic review assessed 56 papers exploring the association of PST to diabetes self-management and control. Six studies used PST as an intervention for adults. Results of the review suggest that evidence for the effectiveness of PST on HbA1c is weak [ 45 ]. The meta-analysis assessed 16 RCTs of interactive self-management interventions, with seven being specific to PST. The studies specific to PST showed a mean difference of −0.39% when comparing intervention to control groups, demonstrating a significant reduction in HbA1c [ 46 ].
Table 3
Problem-solving therapy.
| Study & Location | Design | Sample | Outcome Measures | Intervention (I) and Control (C) Groups | Results |
|---|---|---|---|---|---|
| Hill-Briggs et al. (2011) [ ] US | RCT | = 56 Female: 58.9% Mean age: 61.3 African American | A1c, lipids, BP, literacy, & self-reported depression, knowledge, health problems, barriers, self-management, & satisfaction, collected at baseline, 1-week post-intervention, & 3 months. | (I - Intensive group): ( = 29) 1 session (diabetes and CVD education session + 8 PST session - delivered bi-weekly, 8–10 participants/group); (LTA = 3); Intervention delivered by “trained interventionist”; Intervention fidelity addressed. (I - Condensed group): ( = 27) CVD education session + one PST session; (LTA = 1). | Intensive group had significant improvement in SBP, DBP, LDL, and cholesterol, improved HbA1c (reduction of 0.71%), problem solving skills, self-management behavior of diet, and knowledge. |
| Hill-Briggs et al. (2007) [ ] | Systematic review | = 52 Qualitative, quantitative, cross-sectional prospective, RCTs, and quasi-experimental designs Type 1 and type 2 diabetes | Problem solving, self-management behaviors, physiological, psychosocial, and process outcomes. | Six studies of adults (out of 52 studies) used problem solving as an intervention. | Ineffective problem solving was associated with poor glycemic control; more studies are needed to make conclusions about the impact of problem solving on self-management; evidence for problem solving effectiveness on HbA1c is inconsistent and weak. |
| Cheng et al. (2017) [ ] | Meta-analysis | = 16 RCTs Adults type 2 diabetes English and Chinese | A1c | Seven studies of adults (out of 16 studies) used problem solving as an intervention. | Problem solving studies showed a mean difference in HbA1c of −0.39% (95% CI: −.73% to −.05%; p = .03). |
3.1.4. Technology-based interventions
Technology based interventions involve the use of equipment, devices, or tools to augment care through improved communication and increased ability to process information. Often referred to as telehealth, these various modalities include telephone, teleconferencing by video, computer, and internet/web-based technology [ 47 ]. Technology based interventions incorporate various technological modalities to monitor outcomes, provide self-management education, and deliver self-management strategies.
In general, technology based interventions have been used to provide support for patients with multiple health conditions including heart disease, chronic lung disease, and diabetes [ 48 ]. These telehealth interventions have been developed in response to access to care issues in various rural and regional communities [ 47 ]. The telephone is a customary technology that is commonly available for communication with patients [ 49 ]. More advanced telephone management includes mobile phone-based applications, referred to commonly as apps, which allow smart-phone applications and texting in addition to basic telephone components. Videoconferencing requires even more complex technology, such as webcams and software to communicate by video. Computer-assisted modules (CAM) typically include computer hardware and software that provide programs for education and/or support. CAMs can be further stratified to include web-based interventions. A final category includes mixed modalities of these various components. Systematic reviews of technology interventions for mixed populations of type 1 and type 2 diabetes have shown limited to no impact on hemoglobin HbA1c [ 47 , 50 , 51 ]. For this integrative review, 30 studies were reviewed (see Table 4 ). Research on telehealth yielded articles on telephone/mobile phone (16), computer-assisted modules (2), web-based interventions (7) and mixed modalities (5). Locations of research included eleven studies in the United States, and thirteen studies done in eight different countries.
Table 4
Technology based interventions.
| Study & Location | Design | Sample | Outcome Measures | Intervention (I) and Control (C) Groups | Results |
|---|---|---|---|---|---|
| Wu et al. (2010) [ ] | Systematic review & meta-analysis | = 7 RCTs, ≥16 years old Type 2 diabetes White: 81% | A1c | Telephone follow up interventions directed at improving self-management in comparison with a control group in which the telephone was the only difference in the intervention being provided. (I): = 1020. (C): = 744. | Standardized effect of the telephone follow up showed a mean weighted difference in HbA1c of −0.44% in favor of the intervention. |
| Graziano et al. (2009) [ ] US | RCT | = 120 Female: 45% Mean age: 62 White: 77% | A1c, medication changes, SMBG, & self-reported perceived severity, perceived susceptibility, perceived benefits, barriers, & attitudes, collect at baseline and 90 days. | (I): ( = 62) Usual care plus a daily automated prerecorded voice message relaying a short (less than 1 min) message focused on self-care behaviors to influence attitudes and beliefs, and reduce barriers for self-care behaviors; (LTA = 1); Intervention delivered by “investigator”; Intervention fidelity not addressed. . (C): ( = 58) Usual care; (LTA = 4). | No significant change in HbA1c or secondary outcomes between groups, except for SMBG. The telephone group had significant increase in frequency of SMBG. |
| Williams et al.(2012) [ ] Australia | RCT | = 120 Female: 37% Mean age: 57.4 Australian born: 70%. | A1c, & self-reported health-related QOL, collected at baseline and 6 months. | (I): ( = 60) Telephone Linked Care (TLC) with automated interactive telephone response, where users had to call in weekly. Calls lasted 5–20 min, and system gave feedback and encouragement based on participant responses; (LTA = 9); Intervention delivered by “coordinator”; Intervention fidelity not addressed. (C): ( = 60) Usual care; (LTA = 5). | The intervention group had a significant reduction in HbA1c (0.8%) compared to the control group (0.2%), and in mental health related QOL. |
| Lim et al.(2011) [ ] Korea | RCT | = 154 Females: 55.8% Mean age: 67.5 Korean | A1c, weight, BMI, glucose levels, lipids, & SMBG, collected at baseline, 3 and 6 months. | Three groups: (I-1): ( = 51) SMBG group; (LTA = 4). (I-2): ( = 51) U-healthcare group that received a glucometer that transmitted SMBG readings to the Clinical Decision Support Server, with subsequent participant feedback message on their mobile phone; (LTA = 2). Intervention delivered by diabetologists, nurses, dieticians, and exercise trainers; Intervention fidelity not addressed. (C): ( = 52) Usual care; (LTA = 4). | U-healthcare group had significant improvement in HbA1c and SMBG, but did not meet study goal of less than 7% for HbA1c. No other significant findings. |
| Walker et al. (2011) [ ] US | RCT | = 526 Female: 67.1% Mean age: 55.5 Black: 62%; Hispanic: 23%; 77% foreign born | A1c, medication adherence (pill counts), & self-reported self-management behaviors, collected at baseline and 12 months. | (I): ( = 262) Telephone intervention involving 10 calls at 4–6 week intervals from a health educator over a 12-month period. Focus was on medication and life style changes (no face-to-face interaction); (LTA = 34); Intervention delivered by “health educators” supervised by nurses; Intervention fidelity addressed. (C): ( = 264) Print materials only (no face-to-face interaction). Outcome variables of HbA1c, medication adherence (pill counts), and self-reported self-management behaviors collect at baseline and 12 months; (LTA = 48). | Telephone group had greater reduction in HbA1c (0.23% ± 1.1%) over 1 year, and improved medication adherence among those not taking insulin. No significant changes in self-management behaviors were related to HbA1c changes. |
| Trief et al. (2016) [ ] US | RCT | = 280 Female: 38.4% Mean age: 56.8 30% self-described minority | A1c, BMI, BP, distress, self-efficacy, depressive symptoms, & satisfaction collected at baseline, 4, 8, and 12 months. | Three arms: (IC): ( = 94) Individual call group, with 2 phone sessions, plus 10 additional calls (50–55 min) addressing self-management; (LTA = 1); Intervention delivered by dieticians; Intervention fidelity addressed. (CC): ( = 104) Collaborative couple call group, with 2 phone sessions, plus 10 additional calls (50–55 min) addressing self-management; (LTA = 7). (DE): ( = 82) Diabetes education with 2 phone sessions and no additional contact; (LTA = 4). | Significant reduction in HbA1c in all groups with no difference between groups. The Collaborative Couples intervention resulted in lasting improvements in HbA1c, obesity, and psychosocial variables. |
| Goode et al. (2015) [ ] Australia | RCT | = 302 Female: 72% Mean age: 57.8 Caucasian: 43.7% | Weight, PA, HbA1c, & diet collected at baseline, 6, 18, and 24 months. | (I): ( = 135) 18-month intervention with 27 phone calls, weekly for first 4 weeks, then every 2 weeks for 5 months, then monthly for the remaining 12 months. Counseling to increase PA, diet, and weight loss provided. Given pedometer and digital scales; (LTA = 33); Intervention delivered by counselors with bachelor's-level training in nutrition and dietetics; Intervention fidelity addressed. (C): ( = 144) Usual care plus educational brochures; (LTA = 13). | Increased dose of intervention was associated in greater weight loss. |
| Sacco et al. (2009) [ ] US | RCT | = 62 Female: 58% Mean age: 52 Caucasian: 77% African American: 14.5% Hispanic: 8.1% | A1c, BMI, and self-report of symptoms, depression, knowledge, self-efficacy, awareness of goals, and adherence to diet, SMBG, foot care, & medications, collected at baseline and 6 months. | (I): ( = 31) Telephone coaching call weekly for 3 months, then bi-weekly for additional 3 months. Telephone sessions averaged 17.4 min. Telephone sessions were guided by a Weekly Coaching Checklist addressing self-care, and reviewed weekly blood glucose readings; (LTA = 10); Interventions delivered by undergraduate psychology students; Intervention fidelity addressed. (C): ( = 31) Usual care; (LTA = 4). | Significant treatment effects on adherence, diabetes-related medical symptoms, and depression Symptoms. No significant effects on BMI or HbA1c. |
| Anderson et al. (2010) [ ] US | RCT | = 295 Female: 58% Age: > 18 White: 26–27% Other: 62–65% with majority being African American or Hispanic | Weight, BMI, HbA1c, lipids, & BP, and self-reported overall health, depressive symptoms, diet and physical activity, collected at baseline, 6 and 12 months. | (I): ( = 146) One-year of telephonic disease management with phone calls including a brief clinical assessment, self-management discussion. Patients were called weekly, bi-weekly or monthly depending on a risk-stratification, or if the patient requested a change in call frequency; (LTA = 52); Intervention delivered by nurses; Intervention fidelity not addressed. (C): ( = 149) Usual care; (LTA = 32). | No significant difference in HbA1c or other secondary outcome measures after 12 months. |
| Frosch et al. (2011) [ ] US | RCT | = 201 Female: 50% Mean age: 55 Latino: 55%; African American:16%; White: 20% | A1c, lipids, BP, BMI, & prescribed medications, and self-reported knowledge of self-management behaviors, collected at baseline, 1 and 6 months. | (I): ( = 100). A 24-min video behavior support intervention with a workbook and 5 sessions of telephone coaching by a trained diabetes nurse. The telephone sessions varied in length from 15 to 60 min with a cap of 150 min total. Time intervals between calls determined collaboratively; (LTA = 17); Intervention delivered by nurses; Intervention fidelity not addressed. (C): ( = 101) Usual care; (LTA = 14). | No significant overall reduction in HbA1c between groups. Secondary outcome measures were nonsignificant. |
| Nesari et al. (2010) [ ] Iran | RCT | = 61 Female: 71.7% Mean age: 51 Iranian | A1c, and self-reported disease characteristics, diet, exercise, medications, foot care, and SMBG, collected at baseline and after 12 weeks. | (I): ( = 30) Telephone follow up 12 weeks, twice weekly for the first month and then weekly for second and third months. Each session averaged 20 min and each person received 16 phone calls. Calls included self-management education, and medication adjustments coordinated by the nurse and consulting endocrinologist; (LTA = 0); Intervention delivered by nursing student; Intervention fidelity not addressed. (C): ( = 30) Usual care; (LTA = 1). | No significant HbA1c change between groups; Significant changes in adherence for diet, exercise, foot care, medication taking and SMBG. |
| Wayne et al. (2015) [ ] Canada | RCT | = 131 Female: 72% Mean age: 53.2 Black: 45%; Caucasian: 27% | A1c, weight, BMI, & waist circumference collected at baseline, 3 and 6 months. | (I): ( = 67) 6-month intervention using a health coach and smart phone, with 24/7 access to coach; (LTA = 19); Intervention delivered by behavior-change counseling specialist; Intervention fidelity not addressed. (C): ( = 64) Using health coach, but no smart phone; (LTA = 15). | No difference between groups in HbA1c reduction. Both groups reduced HbA1c (−0.84 intervention; −0.81 control). |
| Cui et al. (2106) [ ] | Systematic review | = 13 Adults with type 2 diabetes from 7 countries: Finland, Norway, US, Korea, Spain, Canada, Netherlands | A1c Baseline and at study completion | Thirteen RCTs compared mHealth smart phone applications to control groups receiving usual care only. Studies included a primary outcome variable of HbA1c, and measured change in HbA1c. | Significant reduction in HbA1c by 0.40% (p < .01) mean difference, when compared to control group. |
| Wu et al.(2018) [ ]. | Systematic review & meta-analysis | = 17 Adults with type 2 diabetes | A1c Baseline and at study completion | Seventeen RCTs of smartphone technology that used apps or internet access via the smartphone or personal digital assistants, compared to a control group receiving usual care only. Outcome variable of HbA1c, and measured change in HbA1c. | Meta-analysis showed a pooled HbA1c reduction of −0.51% when comparing smartphone technology to usual care. |
| Aikens et al.(2015) [ ] US | Descriptive comparative study | = 301 Male: 92.8% Mean age 66.7 Caucasian: 92.8% from Veterans Affairs clinics | Self-reported self-management behaviors, physical & mental functioning, depressive symptoms, & distress, collected at baseline, 3 and 6 months. | Two intervention groups: a 3 month group ( = 108), and a 6 month group ( = 193). The intervention was an Interactive voice response (IVR) mobile health service with questions via a tree-structured algorithm and verbal reinforcement for self-management. Calls were 5–10 min, and performed weekly for 3 or 6 months. A pattern of abnormal blood glucose or BP triggered a clinician notification for follow up. Attrition for total sample 23%, more likely in the 6-month group; Intervention delivered by research team; Intervention fidelity addressed. | Significant improvements in all health outcomes (except psychological functioning), and in self-management behaviors of medications, SMBG, and foot care. Duration of study had no significant effects on IVR outcomes. |
| Hou et al. (2016) [ ] | Systematic review | = 14 RCTs Adults with type 1 or type 2 diabetes | A1c (baseline and follow up, and not self-reported) | Ten RCTs (out of 14) were of type 2 diabetes, and using a total 9 different apps for type 2 diabetes. Apps were designed to improve self-management by providing personalize feedback on self-monitoring of blood glucose, diet, and physical activity | All studies of type 2 diabetes reported a mean reduction in HbA1c of 0.49% compared to controls. |
| Pal et al. (2014) [ ] | Systematic review | = 16 RCTs(UK) Adults with type 2 diabetes | A1c, BP, lipids, weight, death, health-related QOL, changes in cognition, behaviors, social support, emotional outcomes, adverse effect, complications, & economic data. | Interventions included those that were computer-based and interactive with users to generate tailored content aimed at improving self-management. | Computer-based interventions had a small effect on HbA1c, with a pooled effect of −0.2%, with the sub-group of mobile phone-based interventions having a larger effect (−0.50%) on HbA1c. No evidence of benefit for other biological, cognitive, behavioral or emotional outcomes. |
| Jaipakdee et al. (2015) [ ] Thailand | RCT | = 403 Females: 76.7% Mean age: 61.3 | A1c, glucose, weight, BMI, BP, waist circumference, and self-reported depressive symptoms, self-management behaviors, & QOL, collected at baseline, 3 and 6 months. | (I): ( = 203) DSMS over 6 months with computer-assisted instruction (CAI) that included educational sessions by computer plus a monthly 3 h educational session; (LTA = 9); Intervention delivered by nurses; Intervention fidelity addressed. (C): ( = 200) Usual care; (LTA = 16). | Significant improvements in HbA1c (reduction of 0.34), fasting blood glucose, health behaviors, and QOL in intervention group. |
| Pacaud et al. (2012) [ ] Canada | RCT | = 79 Female: 52.9% Mean age: 54.2 | A1c, diabetes knowledge, self-efficacy, self-care behaviors, satisfaction, QOL, collected at baseline, 3, 6, 9, & 12 months. | Two intervention conditions: (I-1): ( = 18) Web static group; (I-2): ( = 29) Web interactive group. (C): ( = 21) Standard face-to-face care. All groups received 60–90 min assessment with trained clinician and research assistant. Follow up during study was done by same clinicians for each group.(LTA: of the 79 enrolled, LTA 25% web static group, 16% face-to-face group, 2.6% web interactive group. . | Significant findings when comparing website use, such that higher website use was associated with higher knowledge, self-efficacy, and lower HbA1c. |
| Hansel et al. (2017) [ ] France | RCT | = 120 Female: 66.7% Mean age: 57 | Weight, waist circumference, BMI, lipids, HbA1c, aerobic fitness, & self-reported diet, physical activity, & satisfaction collected at baseline and 4 months. | (I): ( = 60) Web-based support tool designed to improve lifestyle habits, including diet and PA. Participants progress through modules as they answer questions. Human contact is limited to technical support. Program runs on a personal computer; (LTA = 11); Intervention delivered by study team; Intervention fidelity not addressed. (C): ( = 60) Usual care; (LTA = 5). | Significant improvements in HbA1c, weight and waist circumference in intervention group at 4 months. |
| Avdal et al. (2011) [ ] RCT Turkey | = 122 Female: 50.8% Mean age: 51.5 | A1c & rate of attendance at health check visits were collected at baseline and 6 months. | (I): ( = 61) Web site intervention that provided information, education, and feedback; (LTA = 9); Intervention delivered by nurses; Intervention fidelity not addressed. . (C): ( = 61) Usual care; (LTA = 8). | The intervention group had a mean reduction (0.13) in HbA1c, and increased health check visits. No significant changes seen in the control group. | |
| Glasko et al. (2012) [ ] US | RCT | = 463 Female: 50% Mean age: 58 White: 72%, African American: 15%, Latino 21% | A1c, BMI, lipids, BP, health literacy, and self-reported diet, physical activity, medication adherence, self-efficacy, problem solving, supportive sources, health status, distress, collected at baseline, 4 and 12 months. | 3 arm trial using CASM, an internet-based computer assisted self-management intervention. (Group 1): ( = 169) CASM (LTA = 49). (Group 2): ( = 162) CASM+, with added human support; (LTA = 38). (Group 3): ( = 132) Enhanced usual care group that included a computer-based health risk appraisal feedback and recommended preventive care behaviors but did not include the key intervention procedures; (LTA = 18). Intervention delivered by research team; Intervention fidelity not addressed. | Internet based programs significantly improved health care behaviors compared to usual care. All conditions improved moderately on biological and psychosocial outcomes, but between group differences not significant. |
| Lorig et al. (2010) [ ] US | RCT | = 761 Female: 76% Mean age: 54.3 White: 76% | A1c, and self-reported health status, health care utilization, patient activation, self-efficacy, distress, & physical activity, collected at baseline, 6, and 18 months. | 3 arm trial: (Group 1): ( = 259) Internet-based Diabetes Self-Management Program (IDSMP) that included a 6-week asynchronous training program with 6 weekly sessions and a reference book; (LTA = 50). (Group 2): ( = 232) IDSMP plus e-mail reinforcement; (LTA = 46). Intervention delivered by “trained” peer facilitators; Intervention fidelity not addressed. (C): ( = 270) Usual care; (LTA = 32). | Significant improvements in HbA1c, patient activation, and self-efficacy at 6 months, and self-efficacy and patient activation at 18 month, for the intervention groups. No changes in other health or behavioral indicators. |
| Heinrich et al. (2012) [ ] Netherlands | RCT | = 99 Female: Mean age: | Diabetes self-management knowledge, and use of website intervention, collected at baseline and two weeks. | (I): ( = 43) Web-based Diabetes Interactive Education Programme (DIEP) that provides an overview of type 2 diabetes in seven chapters; (LTA = 7); Interventions delivered by research team; intervention fidelity not addressed. (C): ( = 56) Usual care; (LTA = 2). | Significant improvement in knowledge scores in the experimental group at post-test. The total time spent on the website averaged 58 min, and was not correlated to increased knowledge. |
| Tang et al. (2013) [ ] US | Cohort study | = 415 Female: 40% Mean age: 54 White: 59% Asian: 21% Hispanic: 10% | A1c, BP, lipids, cardiovascular risk, and self-reported knowledge, distress, depression & treatment satisfaction, collected at 6 and 12 months. | (I): ( = 202): An online, disease management support system that included wirelessly uploaded home glucometer readings with graphical feedback, comprehensive patient-specific diabetes summary status report, nutrition and exercise logs, insulin record, online messaging with the health team; (LTA = 9); Intervention delivered by nurses and dieticians; Intervention fidelity not addressed. (C): ( = 213) Usual care; (LTA = 24). | Compared to usual care, the intervention group had significant HbA1c reduction at 6 months (reduction of 1.32), but no significant differences between groups on HbA1c at 12 months. |
| Jackson et al. (2006) [ ] | Systematic review | = 26 RCTs & observational studies Type 1 & type 2 diabetes | A1c, weight, BP, micro-albumin, lipids, creatinine, depression, hematocrit, & health care utilization, self-care behaviors, satisfaction, & cost. | 14 out of 26 studies were RCTs. Studies used various technologies including internet (3 RCTs), telephone (4 RCTs), and computer-assisted integration of clinical information (7 RCTs). | Six out of 14 RCTs showed significant declines in HbA1c (>1%) when compared with controls. Overall increases in patient satisfaction with the interventions, personal health care, perceived support, QOL, and knowledge. |
| Fisher et al. (2013) [ ] US | Cohort study | = 392 Female: 53.8% Mean age: 56 White: 40% Asian: 19% African American: 16% Hispanic: 11% | A1c, & self-reported diabetes distress, & self-reported physical activity, diet, & medication adherence, collected at baseline, 4 and 12 months. | 3 Intervention groups: All groups received live phone calls at weeks 2, 4, 7, 12, 24, 28, 34 & 48 to check progress. (Group 1): ( = 150) Computer-Assisted Self-Management (CASM) is a 40 min web-based diabetes program with interactive self-management feedback, and a booster program at month 5; (LTA = 29). (Group 2): ( = 146) CASM plus PST (CAPS) included a 60-min in-person intervention which introduced PST in addition to the CASM and a live booster session at month 5; (LTA = 29). (Group 3): ( = 96) Leap Ahead (LEAP) is a minimal intervention with a 20-min computer-delivered health risk appraisal along with diabetes information regarding healthy living, and a repeat risk appraisal at month 5; (LTA = 15). Intervention delivered by nonprofessional college graduate interventionists; Intervention fidelity not addressed. | No significant time or group main effects were found for HbA1c. Significant reductions in distress across all three groups without significant between group differences. |
| Noh et al. (2010) [ ] Korea | Cohort study | = 44 Female: 22.5% Mean age: 42 Koreans | A1c, fasting and post-prandial blood glucose levels, collected at baseline and 6 months. | (I): ( = 24), eMOD intervention is a web-based system providing diabetes education that participants can log into when convenient by either cell phone or computer; (LTA = 4); Intervention delivered by research team; Intervention fidelity not addressed. (C): ( = 20) Received education books with content similar to eMOD website; (LTA = 0). | A1c reduction (1.53%) and post-prandial blood glucose decreased significantly over time in the eMOD group, with significant relationship between change in HbA1c and frequency of access to eMOD. |
| Greenwood et al. (2015) [ ] US | RCT | = 90 Female: 23% Mean age: 58 Caucasian 64% | A1c, diabetes knowledge, self-management activities, & self-efficacy collected at baseline and 6 months. | (I): ( = 45) A telehealth remote monitoring system using a tablet connected to a modem and a glucometer that has a touch screen to answer daily health questions. Data is sent to a certified diabetes educator. 84 daily sessions delivered; (LTA = 4); Intervention delivered by certified diabetes educators; Intervention fidelity not addressed. (C): ( = 45) Usual care; (LTA = 5). | Both groups lowered HbA1c with a significant difference (−.41%) at 6 months, with greater reduction in the intervention group. |
| Wild et al. (2016) [ ] UK | RCT | = 321 Female: 33.3% Mean age: 61 Ethnicity/race not reported | A1c, BP, weight, lipids, self-reported self-management, & QOL collected at baseline and 9 months. | (I): ( = 160) 9-month telehealth intervention using remote monitoring equipment for weight, BP, and blood glucose, with the information being delivered via modem to nurses. Advice then given to participant based on data at weekly intervals, and as needed; (LTA = 14); Intervention delivered by research team; Intervention fidelity not addressed. (C): ( = 161) Usual care; (LTA = 22). | Intervention group showed reduction in HbA1c (0.51%), and blood pressure. No differences between groups in weight, self-management behaviors, or QOL. |
For telephone interventions, a systematic review with meta-analysis of seven RCTs examining the impact of telephone follow up interventions on glucose control found little impact on glycemic control, with a mean weighted difference in HbA1c of −0.44% in favor of the intervention [ 35 ]. Eleven RCTs studied the impact of telephone interventions on glycemic control, symptoms, and self-management behaviors. Two RCTs that explored the impact of automated response systems showed no improvement in HbA1c [ 49 , 52 ]. Nine RCTs examined live telephonic interactive interventions that involved a consultation, counseling, or coaching interaction(s), demonstrating mixed results on self-management behaviors. Five studies demonstrated improvements in HbA1c [ [53] , [54] , [55] , [56] ], weight loss [ 56 ], and symptoms [ 57 ]. However, four studies found no significant change impact on HbA1c level [ [58] , [59] , [60] , [61] ]. In a descriptive study by Aikens et al. [ 62 ], improvements in self-management behaviors were noted (medication adherence self-monitoring blood glucose, foot care) of varying significance.
More specifically, mobile phone technology and access to these devices is increasing the use of this technology in self-management of diabetes. Two systematic reviews of mobile phone applications designed to improve glycemic control by supporting type 2 diabetes self-management report an overall mean reduction in HbA1c of 0.40% and 0.49% when compared to controls [ 63 , 64 ]. One systematic review with meta-analysis also demonstrated a reduction in HbA1c of 0.51% when comparing smart phone to standard care [ 65 ].
Two studies examined CAMs and the impact on physiologic and psychosocial outcomes. A systematic review of 16 RCTs examined the impact of computer-based diabetes intervention, showing only a small benefit on reduction of HbA1c level, with no other evidence of benefit noted on cardiovascular risk factors, QOL and health status [ 66 ]. A RCT assessed the effectiveness of a computer-assisted diabetes self-management intervention, finding no significant HbA1c improvement and only small improvements in fasting plasma glucose and body weight [ 67 ].
Seven studies examined web-based interventions and the impact on physiologic and psychosocial outcomes, including six RCTs and a cohort study. Of the six RCTs, one showed significant improvement in HbA1c, weight, and waist circumference at four months [ 68 ] three showed improved HbA1c at six months [ [69] , [70] , [71] ], and two showed improvement in knowledge scores, healthcare behaviors, and HbA1c [ 72 , 73 ]. The cohort study showed a significant HbA1c reduction at six months but not at twelve month [ 74 ].
The mixed modalities studies were all CAMs & telephone and included one systematic review, two cohort studies, and two RCTs. The systematic review included six studies that found significant declines in HbA1c and an overall increase in satisfaction, personal health care, knowledge and quality of life [ 75 ]. Cohort studies showed mixed results with significant changes to HbA1c; however one found significant reductions in distress [ 76 , 77 ]. In the RCTs, intervention groups using telehealth with provider feedback showed significant decreases in HbA1c [ 78 ] and blood pressure, but no differences in weight, self-management adherence behaviors, or QOL [ 79 ].
3.1.5. Lifestyle modification programs
Lifestyle modification program (LMP) is a general description given to an intervention designed to promote health through lifestyle and behavior change. LMPs can include a wide range of topics, including diet, exercise, medications, and stress; can occur in a wide range of settings, including healthcare organizations, workplaces, and the community; and can be delivered through a variety of mediums ranging from face-to-face, to telephonic, to online technologies. LMP's have a long history in diabetes care, and typically combine interventions targeting diet, exercise, medication management, and behavior modification. Individualizing LMPs has been identified as a key to their success.
Seven RCTs were reviewed with various LMP interventions (see Table 5 ). Locations of research included three in the United States, and one in the United Kingdom, Canada, and the Netherlands. Programs ranged from twelve months to two years in length. Of these seven, only one study had a short-term statistically significant impact on HbA1c but this did not persist over the duration of the study [ 80 ]. Of the multiple LMP outcome variables, the most positive impacts were noted in diet (6/7 studies) [ 10 , [80] , [81] , [82] , [83] , [84] ]; physical activity (4/7 studies) [ 10 , 81 , 83 , 84 ]; self-efficacy (2/6 studies) [ 80 , 85 ]; and stress (2/6 studies) [ 82 , 83 ].
Table 5
Lifestyle modification programs.
| Study & Location | Design | Sample | Outcome Measures | Intervention (I) and Control (C) Groups | Results |
|---|---|---|---|---|---|
| Rosal et al. (2011) [ ] US | RCT | = 252 Female: 76.5% Age: > 18 Latino | Fasting glucose, HbA1c, BP, weight, BMI, waist circumference, medication intensity, physical activity, BGSM, diet, knowledge, & self-efficacy, collected at baseline, 4 and 12 months post-intervention. | (I): ( = 124) A 1-year long program with 12 weekly sessions with follow up phase of 8 monthly sessions. Focus of program: DM knowledge, attitudes, self-management, cultural tailoring; (LTA = 19); Intervention delivered by a nutritionist or health educator and “trained” and lay individuals; Intervention fidelity not addressed. (C): ( = 128) Usual care; (LTA = 16). | Significant difference in HbA1c at 4 months (reduction 0.88), but not at 12 months. Significant changes at 12 months for diabetes knowledge, self-efficacy, BGSM, and diet self-management. |
| Clark et al. (2004) [ ] UK | RCT | = 166 Female: 42% Mean age: 59.5 United Kingdom | Self-management activities, diet behaviors, physical activity, weight, BMI, waist circumference, lipids, HbA1c, stages of change, barriers, & self-efficacy, collected at baseline, 12, 24, and 52 weeks. | (I): ( = 50) Tailored LMP with meetings with interventionist at baseline, and weeks 12, 24, and 52, for goal setting and MI techniques for behavior change. Follow up phone calls by interventionist at weeks 1, 3, and 7; (LTA = 2); Intervention delivered by an “interventionist”; Intervention fidelity not addressed. (C): ( = 50) Usual care; (LTA = 4). | Fat intake reduced and physical activity increased in intervention group. No other significant differences between groups. |
| Thoolen et al. (2009) [ ] Netherlands | RCT | = 227 Female: 45% Mean age: 62 | BMI, & self-reported intentions, self-efficacy, proactive coping, self-care behaviors, physical activity, diet, & medications, collected at baseline, 3 and 12 months. | (I): ( = 89) Proactive coping intervention lead by RN, two individual and 4 group sessions (each session 2 h), over 12 weeks. Taught a 5-step proactive coping plan, involving goal setting and planning processes; (LTA = 11); Intervention delivered by nurses; Intervention fidelity not addressed. (C): ( = 108) Usual care; (LTA = 4). | Diet and physical activity behavior improved, resulting in significant weight loss at 12 months; proactive coping was a better predictor of long-term self-management than intentions or self-efficacy. |
| Toobert et al. (2007) [ ] US | RCT | = 289 Female: 100% Mean age: 61 Post-menopausal women | Self-reported lifestyle behaviors (diet, physical activity, smoking, stress management), social support, problem solving, self-efficacy, depression, QOL, & cost analysis, collected at baseline, 6, 12, and 24 months. | (I): ( = 163) Mediterranean Lifestyle Program (MLP), a 2 and a half days retreat, followed by 4-h weekly meetings for the first 6 months addressing diet, PA, stress management, and support groups. After 6 months, participants randomized to either (a) faded schedule of weekly meeting led by lay leader, or (b) 4 meetings over 18 months led by project staff to complete a personalized computer assisted program; Intervention delivered by a dietician, exercise physiologist, stress-management instructor, and professional and lay support group leaders; Intervention fidelity not addressed. (C): ( = 116) Usual care. LTA = 15% of total randomized sample. | Significant improvements at all time points for diet, stress management, & problem solving ability. Improvements noted in physical activity, social resources, and self-efficacy. |
| Toobert et al. (2011) [ ] US | RCT | = 280 Female: 100% Mean age: 55.6–58.7 Latina | Problem solving (coded by interviewers), and self-reported self-efficacy, social support, diet, stress management, & physical activity, collected at baseline, 6 and 12 months. | (I): ( = 142) Usual care plus Viva Bien program - a 12-month lifestyle modification program addressing diet, stress management techniques, exercise, smoking cessation, problem solving. Involves a 2 and a half days retreat followed by weekly 4-h meetings for 6 months, then twice monthly for 6 months. Intervention delivered by “study staff”; Intervention fidelity not addressed. (C): ( = 138) Usual care. LTA: 23.2% intervention group; 21.7% control group. | Significant improvements in behavior change (diet, practice of stress management, exercise, and engagement in social support), and HbA1c; however, these changes were not maintained at 12 months. Improvements in psychosocial outcomes (problem solving, self-efficacy, and perceived support). |
| McGowan (2015) [ ] Canada | RCT | = 361 Male: 54–64% Mean age range: 63.8–64.6 | HbA1c, lipids, weight, BMI, BP, waist circumference, self-reported self-efficacy, attitudes, behaviors, health status, & QOL, collected at baseline, 6 and 12 months. | Three groups: (I-1): ( = 130) DSMP program - lead by pairs of trained lay leaders, groups of 10–16 meet once a week for 2.5 h over a 6 wee time period; (LTA = 44) (I-2): ( = 109) CDSMP (same as DSMP, but not specific to diabetes); (LTA = 46). Both intervention groups led by ““trained program leaders”; Intervention fidelity not addressed. (C): ( = 122) Usual care; (LTA = 33). | Significant improvements in 5 of 30 outcome measures: fatigue, cognitive symptom management, self-efficacy, communication with physician, and diabetes empowerment. Marginal differences in HbA1c between both groups. Both programs effective in bringing about positive changes, but little difference between the programs. |
| Markle-Reid et al. (2018) [ ] Canada | RCT | = 159 Female: 55.9% Age: 30% aged 65 to 69, 40% aged 70 to 74, and 30% aged 75 and older. | HRQOL, mental health, & self-efficacy, collected at baseline and 6 months after intervention | (I): ( = 80) Participated in a community-based lifestyle modification program focused on self-efficacy, self-management, holistic care, and individual and caregiver engagement. The program, delivered by trained nurses, dietitians, program coordinator, and peer volunteers, involved 3 in-home visits, monthly group sessions, monthly case conferences, and on-going nurse-led care coordination. (LTA = 5). Fidelity addressed. (C): ( = 79) Usual care. (LTA = 13). | Intervention group showed improved quality of life and self-management and reduced depressive symptoms. |
3.1.6. Education
Identified as a critical element in the care for all people with diabetes, diabetes self-management education (DSME) has been a long-standing intervention in the care of persons with diabetes [ 86 ]. DSME has evolved over time to include behavioral and affective strategies [ 87 ], and biopsychosocial treatment models addressing both medical and psychosocial needs of persons with diabetes [ 88 ]. Educational interventions can be administered by peers or professionals, to individual or groups, in short term or extended sessions, and by different modalities. Current thought on optimal diabetes self-management is that DSME needs to be followed by diabetes self-management support (DSMS) [ 89 ]. DSMS involves several essential components that must be maintained long-term to prevent diabetes-related complications: adherence to diet, physical activity, treatments, and monitoring checks [ 90 ].
The National Standards for Diabetes Self-Management Education and Support are reviewed and revised approximately every five years by a Task Force jointly convened by the American Association of Diabetes Educators (AADE) and American Diabetes Association (ADA) [ 86 ]. While there are many models of DSME, the standards do not endorse any one approach, but rather, specifies what constitutes effective self-management strategies [ 86 ]. Many studies have explored the impact of DSME on self-management with outcomes measures covering a range of physiological, behavioral, and psychosocial variables. Research suggests that DSME is associated with changes in diabetes knowledge, clinical outcomes, self-efficacy, and quality of life [ 91 ].
For this review, eleven sources were reviewed: one systematic review, two meta-analysis, and eight RCTs. Locations of research included the United States, Sweden, Australia, Saudi Arabia, Japan, and Norway. Looking specifically at HbA1c as the outcome, a systematic review of 118 DSME interventions found that DSME resulted in a significant decrease in HbA1c [ 91 ]. Two meta-analyses analyzing RCTs specific to persons with type 2 diabetes show that the benefits of DSME are modest [ 92 ] and that the positive effects tend to gradually decline over time [ 93 ]. Eight RCTs conducted in six countries were reviewed with various educational interventions (see Table 6 ). Sample sizes ranged from 75 to 670 participants with the intervention groups ranging from 36 to 335 individuals in the RCTs. Statistically significant improvements in select biophysical, psychosocial, and self-management measures, including knowledge [ 94 , 95 ], distress and quality of life [ 96 , 97 ], and physiologic outcomes [ 98 , 100 ]. One study found no differences in biophysical or self-management behaviors [ 101 ].
Table 6
Educational interventions.
| Study & Location | Design | Sample | Outcome Measures | Intervention (I) and Control (C) Groups | Results |
|---|---|---|---|---|---|
| Chrvala, Sherr, & Lipman (2016) [ ] | Systematic review | = 118 RCTs >18 years, type 1 and type 2 diabetes. | Must have HbA1c as outcome variable. | Out of 118 RCTs, most reported on a single discrete DSME intervention with follow up HbA1c level at 3 months or greater. Several RCTs compared 2 or 3 methods of DSME to a control condition. | 61.9% of studies reported significant change in HbA1c, with an average reduction of 0.57. Education hours <10 were associated with a greater proportion of interventions with significant reductions in HbA1c. |
| Klein et al. (2013) [ ] | Meta-analysis | = 52 RCT Type 2 diabetes, age > 18. | HbA1c values at baseline and post-intervention. | Of the 52 RCTs, 17 had 13 weeks or less for length of intervention, 17 had 14–16 weeks of intervention, and 19 had 27 or more weeks of intervention. | DSME resulted in significant reductions in HbA1c compared to control conditions. However, most participants did not achieve recommended HbA1c level. |
| Adolfsson et al. (2007) [ ] Sweden | RCT | = 101 Female: 41% Mean age: 63 Sweden | HbA1c, BMI, and self-reported confidence in diabetes knowledge, self-efficacy, & satisfaction, collected at baseline and 1-year follow up. | (I): ( = 42) A group of 5–8 participants had 4-5 empowerment group education sessions, and a follow up session within 7 months; (LTA = 8); Intervention delivered by “trained” doctors and nurses; Intervention fidelity addressed. (C): ( = 46) Usual care; (LTA = 5). | Higher confidence in diabetic knowledge only statistically significant difference in intervention group. No significant change in HbA1c. |
| Campbell et al. (2013) [ ] Australia | RCT | = 670 Female: 46.3% Mean age: 55.7 Australia | Self-reported self-efficacy, and self-management behaviors, collected at baseline, 4 weeks, and 6 months. | (I): ( = 335) Received Fact Sheets and DVD comprising patient narratives of type II diabetes management during a 3-week intervention; (LTA = 49); Intervention delivery personnel and intervention fidelity not addressed. (C): ( = 335) Received diabetic Fact Sheets only; (LTA = 23). | Mean difference in self-efficacy was 7.2 better in intervention group. Change in self-care behaviors during previous 7 days significantly greater in intervention group. |
| Beverly et al. (2013) [ ] US | RCT | = 135 Female: 51% Mean age: 59 Caucasian: 75% | HbA1c, weight, BMI, waist circumference, BP, pedometer readings, fitness assessment, blood glucose, and self-reported self-care, symptoms, coping, distress, QOL, confidence, and health literacy, collected at baseline, 3, 6, and 12 months. | (I): ( = 68) Four 1-h group education sessions each with a different topic (diabetes overview, healthy eating, BGL monitoring, natural course of diabetes); (LTA = 10); Intervention delivered by RNs and dieticians; Intervention fidelity not addressed. (C): ( = 67) Two classes 2 h in length focused on BP & cholesterol; (LTA = 4). | Intervention group had modest improvement in HbA1c at 3 months (reduction of 0.4%), with no maintenance of improvement at 6 and 12 months. Control group had no improvement of HbA1c at any time. Both groups improved frequency of self-care, QOL, distress and frustration over time. |
| Sugiyama et al. (2015) [ ] US | RCT | = 516 Female: 70% Mean age: 63 Latino: 61%; African American: 39% | HbA1c, and self-reported mental and physical health-related QOL, & social support, collected at baseline and 6 months. | All given 2 h training on SMBG. (I): ( = 258) Six weekly small group self-care sessions based on empowerment model. Sessions were for 2 h, with 8–10 persons per group; (LTA = 55); Intervention delivered by trained “health educators”; Intervention fidelity addressed. (C): ( = 258) Six lectures on geriatric topics unrelated to diabetes; (LTA = 62). | Education increased health-related QOL, and significant reduction in HbAlc (0.4%) compared to control. |
| Mohamed et al. (2013) [ ] Saudi Arabia | RCT | = 430 Female: majority Mean age: 53.5 Saudi Arabia | HbA1c, fasting glucose, lipids, BMI, BP, albumin/creatinine ratio, and self-reported knowledge, attitudes, & practice, collected at baseline, 6 and 12 months. | (I): ( = 215) CSSEP (culturally sensitive structured education program), consisting of 4 educational sessions following the ADA standards of care clinical and behavioral goals, 3–4 h each, in groups 10–20 patients; (LTA = 106); Intervention delivered by “educators”; Intervention fidelity not addressed. (C): ( = 215) Usual care; (LTA = 34). | Significant improvements in intervention group in HbA1c reduction (0.55%), fasting blood sugar, BMI, albumin/creatinine ratio, knowledge, attitude & practice. |
| Moriyama et al. (2009) [ ] Japan | RCT | = 75 Female: 54% Mean age: 65.8 Japan | Weight, abdominal circumference, BP, fasting blood glucose, HbA1c, lipids, and self-reported QOL, stage of change, goal attainment, & self-check, collected at baseline and 3, 6, 9, and 12 months. | (I): ( = 50) Monthly face-to-face individual sessions, 30 min each, after clinical exam. Required patient setting behavioral goals on exercise and diet and contact every 2 weeks to check if practicing goal setting behaviors over the next 12 months; (LTA = 8); Intervention delivered by “educator”; Intervention fidelity addressed. (C): ( = 25) Usual care; (LTA = 2). | Intervention group had significant improvements in weight, HbA1c reduction (0.55%), self-efficacy, dieting and exercise stages, QOL, diastolic BP, total cholesterol and complication prevention behaviors. |
| Sperl-Hillen et al. (2011) [ ] US | RCT | = 623 Female: 49.4% Mean age: 61.8 Caucasian: 65.2%; Hispanic: 22.1% | HbA1c, weight, waist circumference, BP, and self-reported depression, general health, support, attitudes, caring ability, distress, understanding, empowerment, diet, & physical activity, collected at baseline, 1, and 4 months. | 3 groups: (I-1): ( = 243) Group education using Conversation Maps in four 2-h sessions with groups scheduled at 1 week intervals, 8–10 people per group; (LTA = 29). (I-2): ( = 246) Individual education; 3 sessions, 1 h each, one month intervals; (LTA = 37). Intervention delivered by “trained” nurses and dieticians; Intervention fidelity addressed. (C): ( = 134) Usual care (LTA = 13). | HbA1c reduction in all groups (0.27, 0.51, 0.24) but significantly more with individual education, compared to group education or usual care. Individual education improved physical health, but not mental health scores. |
| Rygg et al. (2012) [ ] Norway | RCT | = 146 Female: 45% Mean age: 66 White Norwegians: 100% | BP, BMI, HbA1c, lipids, creatinine, and self-reported patient activation, QOL, distress, global health, diabetes knowledge, & self-management skills, collected at 6 and 12 months. | (I): ( = 73) DSME group of 8–10 patients, 15 h of education over 3 sessions, one week between each session; (LTA = 9); Intervention delivered by “trained” nurses; Intervention fidelity not addressed. (C): ( = 73) Usual care; (LTA = 4). | No difference in primary outcomes between groups at 12 months. Diabetes knowledge and some self-management skills improved significantly in the intervention group. |
| Ferguson et al. (2015) [ ] | Systematic review and meta-analysis | = 13, with 11 included in meta-analysis. Hispanic or Latino majority. | A1c Baseline and at follow up. Follow up periods ranged from 6 months to 5 years. | Studies included a DSME intervention in combination with primary care. Seven RCTs included culturally tailored DSME; 9 reported the level of involvement of the primary care provider. Five of 13 studies reported statistically significant changes in HbA1c in the intervention group; Six found no significant changes in HbA1c between groups. | The pooled effect across studies was and HbA1c reduction of −0.25 (95% CI, −0.42 to −0.07, P = .01), indicating a greater improvement in glycemic control for the intervention group at 6 months–12 months. |
3.1.7. Mindfulness
Mindfulness is a type of meditation practice that has been described as being attentive to the present moment in an open and non-judgmental way [ 102 ]. Described as both a trait that can vary between persons, and a skill that can be learned, the concept of mindfulness has measureable aspects including: non-reacting, observing, acting with awareness, describing, and non-judging [ 103 ]. Mindfulness as an intervention engages and strengthens an individual's internal resources for optimization of health through self-awareness and taking responsibility for one's life choices [ 104 ]. Mindfulness interventions emphasize different practices, depending on the philosophy of mediation practice used, and can incorporate components of stress reduction therapy, cognitive behavior therapy, and spiritual components. However, while mindfulness interventions take on a variety of forms, most follow a systematic procedure for developing self-awareness, and have clear learning objectives based on theory and science [ 105 ].
Mindfulness interventions have be used in chronic disease care to address symptom management and the emotional distress caused by disease and its management. Research suggests that mindfulness has a negative association with both anxiety and depression symptoms in a sample of 666 persons with type 1 and 2 diabetes [ 106 ], and was negatively correlated with depression and positively correlated with health-related quality of life in a sample of 75 adults with type 2 diabetes [ 107 ]. A mindfulness-based cognitive therapy intervention has been shown to reduce emotional distress and increase quality of life in persons with type 1 and 2 diabetes [ 108 ]. In a systematic review of 45 studies using meditation interventions for chronic disease, Chan and Larson [ 105 ] conclude that meditation improved symptoms of anxiety, depression, and chronic disease; but conclude that the lack of consistency across diseases and types of meditation interventions warrants further research.
For this integrative review, mindfulness was studied as an intervention in three studies. Locations of research included the United States and Germany. The frequency and length of mindfulness interventions included a one-day workshop, to two 90-min session two months apart, and weekly meetings for 8 weeks. In a RCT of 81 persons from the community with type 2 diabetes, providing education and teaching mindfulness and acceptance of diabetes, compared to providing education alone resulted in improvements in HbA1c at three months post-intervention [ 109 ]. However, two other RCTs did not find improvements in physical measures of diabetes. An 8-week mindfulness-based intervention compared to a control group demonstrated lower levels of self-reported depression and improved health status at a one-year follow up, but no differences in albuminuria [ 110 ]. In a cohort study, a mindfulness-based eating intervention was compared to an educational intervention over a six-month period, resulting in no significant differences between groups for change in weight or diet intake [ 111 ]. See Table 7 .
Table 7
Mindfulness.
| Study & Location | Design | Sample | Outcome Measures | Intervention (I) and Control (C) Groups | Results |
|---|---|---|---|---|---|
| Gregg et al. (2007) [ ] US | RCT | = 81 Female: 46.9% Mean age: 50.9 Hispanic: 28.4% | A1c, and self-reported self-management (diet, exercise, and blood glucose monitoring), knowledge, treatment satisfaction, & acceptance, collected at baseline and 3 months. | (I): ( = 43) The ACT condition, involving a one-day workshop with education, acceptance, and mindfulness training; (LTA = 10); Intervention delivered by “author of manual”; Intervention fidelity not addressed. (C): ( = 38) Education alone; (LTA = 3). | ACT condition more likely to use the coping strategies, to report better diabetes self-care, and to have HbA1c values in the target range. ACT had no significant effect on HbA1c. |
| Hartmann et al. (2012) [ ] Germany | RCT | = 110 Males: 78.1% Mean age: 59 European | Albuminuria, and self-reported psychiatric comorbidity, levels of Depression, & stress, collected at baseline & 12 months. | (I): ( = 53) Mindfulness based stress reduction (MBSR) intervention, groups of 6–10 participants, meeting weekly for 8 weeks, with a booster session after 6 months; (LTA = 10); Intervention delivered by psychologist and resident physician; Intervention fidelity not addressed. (C): ( = 57) Usual care; (LTA = 6). | The MBSR group showed significant reduction in psychosocial distress, but not on albuminuria. o significant reduction in HbA1c. |
| Miller et al. (2014) [ ] US | Cohort | = 68 Female: 63.5% Mean age: 54 Caucasian: 76.5% | Weight, & self-reported diet, knowledge, outcome, expectancy, self-efficacy, anxiety, depression, & mindfulness, collected at baseline, post-intervention, then again 1 month and 3 months after the second data collection. | Group 1: ( = 32) Mindful based eating awareness training (MB-EAT), 2 CDs to guide mindfulness meditation, encouraged to meditate 6 days/week and to practice mini-meditations at other times, basic information on self-management; (LTA = 5). Group 2: ( = 36) Smart Choice (SC) intervention which is behavioral DSME and in-depth nutrient information. All groups had 90 min 1 and 3 month follow up session reviewing key principals of interventions; (LTA = 11);. For both groups, intervention delivered by dietician and social worker; Intervention fidelity addressed. | Both groups with significant improvements in depressive symptoms, expectations, self-efficacy, and cognitive control regarding eating behaviors. |
3.1.8. Cognitive behavioral therapy
Cognitive behavioral therapy (CBT) is a form of psychotherapy focused on problem-solving through improving negative thinking and behavior [ 112 ]. In CBT, the therapist focuses on the impact that dysfunctional thoughts have on current behavior and future functioning. CBT is aimed at evaluating, challenging, and modifying a patient's dysfunctional beliefs (cognitive restructuring) [ 113 ]. CBT is used as an intervention for multiple disorders including but not limited to, anxiety, depression, panic disorder, phobias, obsessive compulsive disorder, post-traumatic stress, schizophrenia, anger, eating disorders, somatic disorders, and chronic pain syndromes [ 114 ].
In relation to the study of diabetes, CBT has been used as an intervention to treat depression due to its association with glycemic control and self-management. The incidence of major depression as a comorbid condition in both type 1 and type 2 diabetes is well documented, estimated to affect 15–20% of persons with diabetes [ 115 ]. Furthermore, depression, even at low levels, has been associated with suboptimal adherence, worse diabetes control, and risk of complications [ 116 ]. In an early study done by Lustman et al. [ 117 ], the use of CBT with supportive diabetes education demonstrated effectiveness in treatment for major depression and potential improvement in glycemic control in persons with type 2 diabetes. Following this work, other studies have explored the use of CBT for treatment of depression and the impact on glycemic control. CBT has been explored in studies of both type 1 and type 2 diabetes mellitus, demonstrating positive effects of CBT on depressive symptoms, but with mixed findings on the impact on glycemic control. In a study of 94 outpatients with diabetes and depressive symptoms, improvements in depressive symptoms and HbA1c, and in self-reported depressive symptoms, anxiety, well-being, and diabetes-related distress were found [ 118 ]. Additional studies of both type 1 and type 2 diabetes using CBT interventions show improvements in depression, but are inconclusive regarding the impact on improving self-management and physical health outcomes [ 119 , 120 ].
For this integrative review, five studies examined the use of CBT for depression and the relationship to type 2 diabetes self-management: Three RCTs, one systematic review, and one meta-analyses (see Table 8 ). Two of the RCTs were done in the United States, and one in Germany. One study included five weekly 90-min CBT sessions, and two studies included eight to twelve one-hour weekly CBT sessions. All RCTs compared the intervention group to usual care that included diabetes self-management education. The RCTs show improvements in depression and distress [ 121 , 123 ], but only one study showed improvements in glycemic monitoring and control [ 123 ]. In a systematic review and meta-analysis of RCTs of psychological interventions to improve glycemic control in persons with type 2 diabetes, 23 out of 25 RCTs examined CBT as the intervention. Results suggest that there are improvements in long-term glycemic control and psychological distress, but not in weight control and blood glucose level [ 124 ]. In a meta-analyses of 45 RCTs assessing efficacy of psychological interventions for self-management of type 2 diabetes in adults from mainland China, 33 studies focused on CBT as the intervention. Analysis suggest that CBT was more effective than the control condition in reducing HbA1c, depression, and anxiety [ 125 ].
Table 8
Cognitive behavioral therapy.
| Study & Location | Design | Sample | Outcome Measures | Intervention (I) and Control (C) Groups | Results |
|---|---|---|---|---|---|
| Hermanns et al.(2015) [ ] Germany | RCT | = 214 Female: 56.5% Mean age: 43.3 German-speaking | Self-reported depressive symptoms, distress, self-care activities, well-being, QOL, diabetes acceptance, & treatment satisfaction, collected at baseline, immediately after intervention, then 6 and 12 months. | (I): ( = 106) DIAMOS program, delivered by psychologist using CBT, comprised of five 90-min lessons; (LTA = 13); Intervention delivered by “certified” psychologist; Intervention fidelity not addressed. (C): ( = 108) Usual care, consisting of a group-based diabetes education program; (LTA = 20). | 12-month follow up showed significant reduction in depressive symptoms, and diabetes related distress in the intervention group. |
| Penckofer et al. (2012) [ ] US | RCT | = 74 Females Mean age 54.8 White 63%; Black 29%; Hispanic: 8% | Fasting glucose, HbA1c, & self-reported depression, anxiety, anger, health related QOL, & knowledge, collected at baseline, 3 and 6 months. | (I): ( = 38) One hour CBT intervention done in group sessions, delivered by a nurse, weekly for 8 weeks; (LTA = 12); Interventions delivered by nurses; Intervention fidelity addressed. (C): ( = 36) Usual care; (LTA = 2). | CBT significantly reduced depression, anxiety, and anger symptoms compared to usual care, but there were no significant differences between groups on HbA1c levels. |
| Safren et al. (2014) [ ] US | RCT | = 87 Female: 50% Mean age: 55-58 Majority Caucasian | A1c, medication adherence, SMBG, distress & self-reported depression, collected at baseline, 4, 8, and 12 months. | (I): ( = 45) CBT for adherence & depression + ETAU (enhanced treatment as usual). Received 9–12 CBT sessions over 4 months; (LTA = 5); Intervention delivered by a “therapist”; Intervention fidelity not addressed. (C): ( = 42) ETAU with series of diabetes support and adherence interventions (included one meeting with nurse educator, two with dietician, one with adherence counselor); (LTA = 4). | Intervention group at 4 months: statistically significant improvement in medication adherence, SMBG, reduction in HbA1c (0.63%), & improvement in depression score. At 8 & 12 months medication adherence, HbA1c and SMBG adherence maintained in CBT group. |
| Ismail et al. (2004) [ ] | Systematic review | = 25 RCTs Psychological interventions for Type 2 diabetes control | A1c, blood glucose, weight, BMI, & psychological distress. | 23 studies of adults (out of 25 studies) used CBT as an intervention in relation to diabetes control in type 2 diabetes. | In persons receiving psychological therapies, there are improvements in long term glycemic control (mean HbA1c reduction of 0.32%), and psychological distress, but not in weight or blood glucose level. |
| Chapman et al. (2015) [ ] | Meta-analysis | = 45 RCTs(US and China) Psychological interventions for type 2 diabetes control | HbA1c, blood glucose, anxiety, depression, & QOL. | 33 studies of adults (out of 45 studies) used CBT as an intervention in relation to diabetes control in type 2 diabetes. | CBT was more effective than the control condition in reducing HbA1c (SMD = −0.97), depression, and anxiety. |
4. Synthesis
4.1. impact of interventions.
This integrative review examined 70 studies (8 systematic reviews, 3 meta-analyses, 53 RCTs, 4 cohort, and 2 descriptive), summarizing eight categories of interventions targeting physiologic, behavioral, and psychological outcomes in patients with type 2 diabetes. Studies were examined from seventeen countries including a broad range of cultures and ethnicities within the research, including Caucasian American, African American, Native American, Hispanic/Latino, European, Canadian, Australian, Middle-Eastern, and Asian populations.
While interventions have shown mixed results in all interventions categories, many studies do support small to modest improvements in physiologic, behavioral, and psychological outcome measures. Interventions have shown small to modest improvements for HbA1c. Often the significant HbA1c change was only within the intervention group, but not significant when compared between groups. Levels of improvement ranged from 0.13% to 1.6% reductions, with the highest reductions seen in peer support/coaching and technology-based interventions. Small to modest improvements were also seen in physiologic outcomes of weight loss, behavioral outcomes of self-reported diet and physical activity, and psychological outcomes of self-reported improvement in self-efficacy and reduction in distress.
4.2. Attributes of interventions
In addition to a wide variety of interventions being tested for self-management of type 2 diabetes, considerable heterogeneity of interventions exist within similar types of interventions. Areas of heterogeneity included length, duration, and number of sessions, content, method of delivery (i.e., in-person and technology-based, individual or group-based), and facilitation (i.e., self-directed, health care professional, peer). For example, motivational interviewing interventions ranged in length from one 60-min session to five 45-min sessions over one year, could be either individual or group based sessions, including face-to-face and self-directed internet based sessions. Considerable variation was found in all intervention categories in this review. This heterogeneity makes it difficult to aggregate findings on specific interventions.
A wide range of professionals and non-professionals were used for intervention delivery. Out of 59 studies, 18 (30%) had nurses facilitating the interventions, with most being education or technology interventions. Twenty-three studies used non-specified personnel to deliver the intervention, including health educators, trained personnel, and peer and/or lay persons. Most of these studies included a peer or coaching intervention, or a lifestyle modification program. Ten studies indicated that a research team delivered the intervention, mostly of which were technology-based interventions. Other types of professionals delivering interventions included certified diabetes educators, psychologist/counselors, pharmacists, dieticians and nutritionists, exercise physiologists and trainers, and social workers, medical assistants, physicians, and students. While only 30% of the studies had nurses as interventionists, they are well positioned to contribute to all intervention types. As it was noted with the exception of mindfulness, nurses were the only professionals used as interventionists across all types of interventions in this review. However, it is also to be noted that components of mindfulness have been embedded within some larger multi-modality and education interventions that have been led by nurses.
In addition to heterogeneity, many intervention approaches are multi-modal, and include components of different categories of intervention in one intervention program. For example, a life style management program may include components of education, motivational interviewing, and technology. Technology interventions, while focusing on the use of the specific technology, may include education, problem solving, and peer coaching. And while the use of multi-modal approach may be beneficial to helping to improve self-management, this overlap makes it challenging to separate out impact of specific interventions. And lastly, fidelity of interventions is another area of consideration. Out of 59 studies, only 21 (35.5%) addressed procedures for intervention fidelity.
4.3. Outcome measures and attrition
The studies in this review examined the impact of a self-management intervention on the major outcomes of physiologic measures of disease control, self-management behaviors, and psychological outcomes. The most commonly reported physiologic measure of disease control was HbA1c level. Other commonly used physiologic measures included weight, BMI, waist circumference, and blood lipid levels. The most commonly reported behavioral outcomes were for diet and physical activity. Other behavioral outcomes included SMBG and medication adherence. In addition, behavioral outcomes were mainly self-report. The most commonly reported psychological outcomes were self-efficacy and distress, and as in behavioral outcomes, these outcomes were also mainly self-reported.
Outcome measures were collected mostly at 6 months (19 studies) and 12 months (22 studies) follow up. Twelve studies collected outcome data at three to four months, two at 18 months, and two studies at 24 months. Overall, duration of most research was limited to one year.
In terms of attrition rates, the majority of studies (64.4%) had less than 20% attrition at final data collection time. Approximately 25% had 1.2–10% attrition, and 39% had 10.0–20% attrition. Three studies had attrition rates between 32.6 and 37.7%, with study duration lasting between 12 and 15 months. The majority of studies report attrition as a number or percentage, with limited information about participant characteristics and attrition. Five studies did not report attrition.
5. Discussion
5.1. impact of interventions.
The results of the integrative review support prior reports from the literature on diabetes self-management. A vast amount of literature exists describing intervention research for diabetes self-management. Interventions in general have demonstrated short-term improvements in glycemic control [ 126 , 127 ], and in promoting knowledge, self-efficacy, and in distress reduction [ 46 ]. However, results of intervention effectiveness are inconsistent [ 45 ], with many studies producing mixed results in relation to physiological, behavioral, and psychosocial outcomes.
The levels of improvement of HbA1c in this integrative review ranged from 0.13% to 1.6%. To elaborate on those findings, most studies that showed improvements in HbA1c had reductions of approximately 0.50%. Of the four studies that had showed HbA1c reductions of greater that 1.00%, three of them collected outcome data at 6 months, and two had sample sizes less than 65 subject. These finding bring consideration to the question of statistical versus clinical significance. It has been suggested that 0.5% HbA1c is a clinically significant change [ 128 ]. This reference to this reduction in HbA1c is drawn from the earlier work of the Diabetes Control and Complications Trial Research Group [ 129 ], and the UK Prospective Diabetes Study [ 7 ]. A difference in HbA1c of only approximately 2% between intensive and standard treatment groups demonstrated significant differences in outcome risks [ 129 ], and even lower differences in HbA1c (7.0% intensive vs 7.9% conventional treatment) demonstrated significant reduction of microvascular complications in persons with type 2 diabetes [ 7 ].
5.2. Intervention heterogeneity and fidelity assessment
The results of this integrative review demonstrated that in addition to a wide variety of interventions being tested for self-management of type 2 diabetes, there is considerable heterogeneity of interventions that exists within similar types of interventions. This result is also reported in systematic reviews on interventions for self-management of type 2 diabetes [ 23 , 124 , 125 ] describing considerable variability in studies with respect to methods of intervention delivery, duration, and intensity, and in measurement of outcome variables and follow-up interval [ 91 ]. In addition, many intervention approaches are multi-modal and include components of different categories of intervention in one intervention program. This overlap makes it challenging to separate out impact of specific interventions, and makes it challenging to aggregate findings and draw solid conclusions on the impact on outcomes of physiologic, behavioral, and psychological outcomes [ 35 , 91 ].
Fidelity of interventions is another area of consideration. In this integrative review, out of 59 studies, only 21 (35.5%) addressed procedures for intervention fidelity. Intervention fidelity has been identified as a limitation in diabetes self-management research, with issues concerning inconsistency in intervention delivery, quality in training to assure fidelity, and lack of fidelity assessment [21, 44, [ 125 ]. A systematic review specific to intervention fidelity in diabetes self-management interventions reported that intervention fidelity of interventions remains under-investigated [ 130 ], with most fidelity assessment done through direct observation, and with intervention dose being assessed by self-reported measures [ 130 ].
5.3. Outcome measures
The most commonly reported physiologic measure of disease control was HbA1c level. This is consistent with the diabetes literature on treatment and research [ 7 , 131 ], with HbA1c being considered the gold standard for glycemic control. HbA1c reflects average glycemia over approximately 3 months and has strong predictive value for diabetes complications [ 132 ], and provides the most objective and reliable information about glucose control of patients with type 2 diabetes. Most studies in this review reported HbA1c value changes between groups from points in time, as opposed to identifying target HbA1c reduction value. While a specific number or percentage considered to be the target value for reduction has not been identified or consistently used in reference for HbA1c reduction, the common approach has been consistency in lowering HbA1c. Consistent with the literature, studies in this review referenced the American Diabetes Association [ 132 ] goal for HbA1c for most adults to be 7%, and presented HbA1c results in terms of reductions towards that goal.
The most commonly reported self-management behavioral outcomes were for diet and physical activity. Diet and physical activity are two of the four major cornerstones of care for self-management of diabetes [ 133 ]. Poor diet and physical inactivity are major contributors to disabilities that result from diabetes. The importance of proper nutrition and physical activity in reducing rates of disease and death from chronic diseases has been well-established [ 8 , 134 ]. The balancing of diet and physical activity are well-established keys to managing diabetes [ 132 ], and in many cases, the most challenging of the self-management behaviors to manage due to being complicated and difficult to integrate into daily life [ 135 ]. In addition, they can be challenging to measure, with most measures in research studies being self-report. Self-report measures may present certain limitations in capturing aspects of dietary and physical activity behavior, with over-reporting being a known problem [ 68 , 136 ].
The most commonly reported psychosocial outcomes were self-efficacy and distress. Self-efficacy and distress have received considerable attention in the chronic disease and diabetes literature. Self-efficacy has been defined as the judgment of capabilities to organize and execute courses of action required to attain desired types of performance and expected outcomes [ 137 ]. Diabetes distress has been described as unique emotional issues directly related to the burdens and worries of living with a chronic disease [ 11 ]. Both self-efficacy and distress have been associated with diabetes self-management and HbA1c levels [ 138 , 139 ]. In general, a broad range of interventions have favorable impact on both self-efficacy and distress, however, sustaining impact on glycemic control and self-management behaviors remains a challenge. Successful treatment and management of emotional needs of patients is needed so that people can be successful with diabetes self-management [ 122 ]. And as in the measurement of diet and physical activity, measures of self-efficacy and distress are self-reported, thus the risk of over-reporting on these variables exists.
Outcome measures were collected mostly at 6 months (19 studies) and 12 months (22 studies) follow up. For studying the impact of interventions on physiologic, behavioral, and psychological outcomes, this timeline presents limitations. Research suggests that results of interventions begin to diminish over twelve months [ 46 ], and that longer follow up periods extending beyond twelve months are needed [ 75 ]. However, the challenges of longitudinal studies are well documented. Challenges such as incomplete and interrupted follow-up with study participants, attrition with loss to follow-up over time, and the generally increased time and financial demands associated with longitudinal research are implicit in study designs [ 140 ].
5.4. Limitations of this review
Because this was an integrative review we chose to include systematic reviews, meta-analyses, RCTs, and descriptive work. This was done in order to not miss nuances found within individual studies that can sometimes occur with larger review studies. However, because some of the RCTs may have also been in larger review studies, there may be some duplication of findings and enhanced or diminished intervention impact. Because of the exhaustive nature of the literature on this topic, it is challenging to stay informed of the entirety of the body of work in this area. Thus, not every piece of evidence, nor every aspect of intervention success/failure maybe completely accounted. And lastly, because of the multi-modal aspects of interventions, it was difficult to initially categorize the broad array of interventions. In each selected category, there may be other interventions. Thus the true impact of a singular category (i.e.: coaching, technology-based, etc.) is difficult to separate out and report outcomes.
5.5. Recommendations for future research
Based on the synthesis of findings from this review, the following recommendations for future research are offered. To address the concerns of multi-modal interventions, research that includes a theoretical basis/model of investigation would be beneficial to explicitly describe and provide rationale for the foundation of the intervention. Complex interventions need to be developed based on theoretical frameworks, which is important because a simple explanation or model applied to a complex intervention risks overstating the causal contribution of the intervention [ 141 ]. All elements of a complex intervention need to be identified and described, giving the intervention its theoretical and pragmatic basis that is thought to account for the effectiveness of the intervention [ 142 , 143 ]. Using a theoretical framework provides a guide to appropriately implement, and analyze the intervention [ 144 ].
Research studies need to include full protocols/descriptions of the interventions to provide researchers with the details for comparison and reproduction of the intervention. Many intervention descriptions are too brief or ambiguous, making it difficult to identify specific actions taken, and in turn, making replication challenging. Often words such as self-management, education program, or healthy lifestyle are used with little clarity into what exactly constitutes the intervention. Mixed modality approaches make it difficult to sort out contributions of components of intervention, or to examine the association of components with each other and the impact on outcomes. For example, tech-based interventions may be enhanced by adding coaching components.
Long-term studies and analysis are needed to assist in evaluating the ways in which study variables impact self-management behavior. Longer follow up may provide participants more opportunity to implement strategies targeting behavior change. Prolonged follow up is needed to monitor maintenance of skills gained, many of which may improve over time (i.e. problem solving skills, CBT). In addition, research incorporating more objective measures of self-management is needed. Much of the self-management behaviors are self-report. More objective measures, in addition to HbA1c, for self-management are needed. Objective measures of physical activity and diet are needed.
While efforts have been made to expand the diversity of research participants, many groups continue to be under-represented in diabetes research. More strategies for recruiting representative numbers of ethnic minorities and underserved populations, and research seeking to determine whether interventions are equally effective in these groups is needed. There is a need for new strategies to control the growing diabetes epidemic in the underserved and marginalized population, to better understand diabetes self-management patterns and correlates, and to identify and overcome barriers to self-care in an effort to identify effective culturally tailored self-management interventions [ 33 ].
And lastly, care delivery models that incorporate what is known about effective interventions in the management of diabetes is an area of nursing research wide open for investigation. Specifically, the role of the registered nurse in the management of diabetes care. An interesting point to consider about the issues with intervention heterogeneity, fidelity, and duration focuses on the role of the nurse in the primary care setting. All of the interventions included in this review fall within the scope of practice of general practice nurses in the primary care setting. The RN can be uniquely positioned as part of an inter-professional team to take on expanded primary care functions in managing the complex care of patients with diabetes, leading complex care management teams, and comprehensive care coordination between the primary care home and providers of care services [ 145 ].
6. Conclusion
Diabetes is a global health problem, as evidenced by the findings of this integrative review. The vast amount of research exploring the impact of interventions for self-management has made major contributions to the care of persons with type 2 diabetes, from offering suggestions for improving care, to stimulating new questions for research. However, implications for clinical practice remain inconclusive [ 126 ], and there remain limitations in the existing body of research, suggesting caution in interpreting results of studies. Moving research forward with attention to intervention development, study design features, and exploring innovative care delivery models offers potential to move this body of research forward to achieving impactful and sustainable physiologic, behavioral, and psychosocial outcomes, and improve the health of those with type 2 diabetes.
Funding or conflicts of interest
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Peer review under responsibility of Chinese Nursing Association.
Appendix A Supplementary data to this article can be found online at https://doi.org/10.1016/j.ijnss.2018.12.002 .
Appendix A. Supplementary data
The following is the Supplementary data to this article:
Log in using your username and password
- Search More Search for this keyword Advanced search
- Latest content
- For authors
- Browse by collection
- BMJ Journals
You are here
- Volume 14, Issue 9
- Comparative effectiveness trial of metformin versus insulin for the treatment of gestational diabetes in the USA: clinical trial protocol for the multicentre DECIDE study
- Article Text
- Article info
- Citation Tools
- Rapid Responses
- Article metrics
- http://orcid.org/0000-0002-8043-556X Kartik K Venkatesh 1 ,
- Cora MacPherson 2 ,
- Rebecca G Clifton 3 ,
- Camille E Powe 4 ,
- Anna Bartholomew 5 ,
- Donna Gregory 1 ,
- Anne Trinh 6 ,
- Ann Scheck McAlearney 6 ,
- Lauren G Fiechtner 7 ,
- Patrick Catalano 8 ,
- Donna Rice 9 ,
- Sharon Cross 6 ,
- Huban Kutay 1 ,
- Steven Gabbe 10 ,
- William A Grobman 6 ,
- Maged M Costantine 6 ,
- Ashley N Battarbee 11 ,
- Kim Boggess 12 ,
- Vivek Katukuri 13 ,
- Kacey Eichelberger 14 ,
- Tania Esakoff 15 ,
- Maisa N Feghali 16 ,
- Lori Harper 17 ,
- Anjali Kaimal 18 ,
- Martha Kole-White 19 ,
- Hector Mendez-Figueroa 20 ,
- Malgorzata Mlynarczyk 21 ,
- Anthony Sciscione 22 ,
- Lydia Shook 4 ,
- Nasim C Sobhani 23 ,
- David M Stamilio 24 ,
- Erika Werner 25 ,
- Samantha Wiegand 26 ,
- Chloe A Zera 27 ,
- Noelia M Zork 28 ,
- George Saade 21 ,
- Mark B Landon 1
- 1 Department of Obstetrics and Gynecology , The Ohio State University , Columbus , Ohio , USA
- 2 Department of Epidemiology , George Washington University School of Public Health and Health Services , Washington , District of Columbia , USA
- 3 George Washington University School of Public Health and Health Services , Washington , District of Columbia , USA
- 4 Massachusetts General Hospital , Boston , Massachusetts , USA
- 5 College of Medicine , The Ohio State University , Columbus , Ohio , USA
- 6 The Ohio State University , Columbus , Ohio , USA
- 7 Mass General Hospital for Children , Boston , Massachusetts , USA
- 8 Department of Obstetrics and Gynecology , Tufts University , Medford , Oregon , USA
- 9 DiabetesSisters , Raleigh , North Carolina , USA
- 10 Ohio State University College of Medicine , Columbus , Ohio , USA
- 11 The University of Alabama , Birmingham , Alabama , USA
- 12 The University of North Carolina , Chapel Hill , North Carolina , USA
- 13 University of New Mexico School of Medicine , Albuquerque , New Mexico , USA
- 14 Prisma Health , Greenville , South Carolina , USA
- 15 Cedars-Sinai Medical Center , Los Angeles , California , USA
- 16 University of Pittsburgh , Pittsburgh , Pennsylvania , USA
- 17 The University of Texas , Austin , Texas , USA
- 18 University of South Florida , Tampa , Florida , USA
- 19 Brown University , Providence , Rhode Island , USA
- 20 The University of Texas Health Science Center , Houston , Texas , USA
- 21 EVMS , Norfolk , Virginia , USA
- 22 Christiana Care Health Services Inc , Wilmington , Delaware , USA
- 23 UCSF , San Francisco , California , USA
- 24 Wake Forest University School of Medicine , Winston-Salem , North Carolina , USA
- 25 Tufts Medical Center , Boston , Massachusetts , USA
- 26 Premier Health Miami Valley Hospital , Dayton , Ohio , USA
- 27 Department of Obstetrics and Gynecology , BIDMC , Boston , Massachusetts , USA
- 28 Columbia University Irving Medical Center , New York , New York , USA
- Correspondence to Dr Kartik K Venkatesh; kartik.venkatesh{at}osumc.edu
Introduction Gestational diabetes mellitus (GDM) is one of the most common medical complications of pregnancy. Glycaemic control decreases the risk of adverse pregnancy outcomes for the affected pregnant individual and the infant exposed in utero. One in four individuals with GDM will require pharmacotherapy to achieve glycaemic control. Injectable insulin has been the mainstay of pharmacotherapy. Oral metformin is an alternative option increasingly used in clinical practice. Both insulin and metformin reduce the risk of adverse pregnancy outcomes, but comparative effectiveness data from a well-characterised, adequately powered study of a diverse US population remain lacking. Because metformin crosses the placenta, long-term safety data, in particular, the risk of childhood obesity, from exposed children are also needed. In addition, the patient-reported experiences of individuals with GDM requiring pharmacotherapy remain to be characterised, including barriers to and facilitators of metformin versus insulin use.
Methods and analysis In a two-arm open-label, pragmatic comparative effectiveness randomised controlled trial, we will determine if metformin is not inferior to insulin in reducing adverse pregnancy outcomes, is comparably safe for exposed individuals and children, and if patient-reported factors, including facilitators of and barriers to use, differ between metformin and insulin. We plan to recruit 1572 pregnant individuals with GDM who need pharmacotherapy at 20 US sites using consistent diagnostic and treatment criteria for oral metformin versus injectable insulin and follow them and their children through delivery to 2 years post partum. More information is available at www.decidestudy.org .
Ethics and dissemination The Institutional Review Board at The Ohio State University approved this study (IRB: 2024H0193; date: 7 December 2024). We plan to submit manuscripts describing the results of each study aim, including the pregnancy outcomes, the 2-year follow-up outcomes, and mixed-methods assessment of patient experiences for publication in peer-reviewed journals and presentations at international scientific meetings.
Trial registration number NCT06445946 .
- Diabetes in pregnancy
- Clinical Trial
- Maternal medicine
This is an open access article distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited, appropriate credit is given, any changes made indicated, and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/ .
https://doi.org/10.1136/bmjopen-2024-091176
Statistics from Altmetric.com
Request permissions.
If you wish to reuse any or all of this article please use the link below which will take you to the Copyright Clearance Center’s RightsLink service. You will be able to get a quick price and instant permission to reuse the content in many different ways.
STRENGTHS AND LIMITATIONS OF THIS STUDY
DECIDE ( www.decidestudy.org ) is a patient-centred and pragmatic comparative-effectiveness randomised control trial that will compare oral metformin versus injectable insulin for the prevention of adverse pregnancy outcomes and the safety of postpartum outcomes among pregnant individuals with gestational diabetes mellitus who require pharmacotherapy and for their exposed children.
Strengths of the DECIDE trial include a non-inferiority clinical trial design, assessment of postpartum outcomes to confirm safety, integration of patient-reported outcomes and inclusion of a racially, ethnically and geographically diverse population.
Limitations of the DECIDE trial include no follow-up beyond 2 years post partum and assessment of participant and infant anthropometry and adiposity by physical exam instead of imaging.
Challenges of this trial will include recruitment across 20 US sites and postpartum retention.
Introduction
Gestational diabetes mellitus (GDM) is one of the most common medical complications of pregnancy and affects nearly 400 000 or ~1 in 10 pregnant individuals in the USA every year. 1–3 The incidence of GDM has more than doubled in the past decade in an environment of rising prevalence of advanced reproductive age and obesity. 4 5 Moreover, GDM has risen inequitably among racially and ethnically minoritised and lower-income individuals. 2 6 More than one in four infants born to individuals with GDM will experience an adverse neonatal outcome, such as large-for-gestational age (LGA) birth weight, hypoglycaemia or hyperbilirubinaemia. 7–9 After delivery, individuals with prior GDM are at >10 fold increased risk of diabetes, and infants exposed to GDM are at 2-fold increased risk of obesity. 10 11
The goal of GDM treatment is to achieve optimal glycaemic control and prevent adverse pregnancy outcomes. 9 12 The initial therapeutic approach is dietary modification and regular exercise, 12 13 but >1 in 4 individuals will not achieve glucose control with these interventions. 13–15 When pharmacological treatment is needed, guidelines from the American Diabetes Association (ADA) and American College of Obstetricians and Gynecologists (ACOG) recommend insulin as the first-line medication, 14 16 while the Society for Maternal-Fetal Medicine states both insulin and metformin are reasonable medication options. 17
In the past, insulin has been the first-line option because it provides glycaemic control, improves pregnancy outcomes and does not cross the placenta. 7 18 19 An alternative to insulin is metformin, which also provides glycaemic control and improves pregnancy outcomes ( table 1 ). 18–20
- View inline
Advantages versus disadvantages of metformin versus insulin
Patients and providers may prefer metformin to insulin because it is convenient to take as an oral pill, well-tolerated, cheaper and practical when medication is needed for a brief time period. Additionally, metformin does not cause hypoglycaemia and reduces gestational weight gain. Metformin use is increasing in clinical practice—while insulin remains the most common medication for GDM, one in three individuals with GDM in the USA were prescribed metformin by late 2018. 21 22 Yet metformin has limitations, including that more than one in four individuals will ultimately need supplemental insulin to achieve glucose control, and there is known placental transfer. Historically, another oral agent, glyburide, had been used, but guidelines have since advised against its use following trials that showed it did not appear to be efficacious. 18 23
Follow-up data on metformin from individuals with prior GDM and their exposed children are limited. 24 Extant data suggest that children exposed to metformin had similar body fat composition but slightly higher body mass index (BMI) compared with those exposed to insulin 25 26 ; but recent population-based data show no difference in BMI. 27 However, these studies were limited due to inadequate randomised controlled trial (RCT) follow-up and observed heterogeneity in the effect across different study sites. Also, whether participant metabolic health postpartum varies based on prior metformin versus insulin exposure in pregnancy requires further study. 28
Deciding between metformin and insulin can be challenging for patients and providers given variation in treatment guidelines, provider recommendations and lack of conclusive comparative efficacy and safety data. 29 Understanding whether patients take medications as directed, how satisfied they are with their medication decision, and how their medication decision impacts their pregnancy experience may help to explain observed heterogeneity of treatment effects (HTE). 30 Patient perspectives on barriers to and facilitators of metformin versus insulin use may identify opportunities to improve outcomes. 31
DECIDE: A Comparative Effectiveness Trial of Oral Metformin vs Injectable Insulin for the Treatment of Gestational Diabetes is a randomised, patient-centred, open-label and pragmatic comparative effectiveness trial in pregnancy with postpartum follow-up. This protocol is written in accordance with the Standard Protocol Items: Recommendations for Interventional Trials 2013 statement. 32
Aims and hypotheses
Primary aims.
Aim 1: To evaluate whether pregnant individuals randomised to metformin are not inferior to pregnant individuals randomised to insulin for the composite adverse neonatal outcome (LGA birth weight, hypoglycaemia, hyperbilirubinemia or death).
Aim 2: To evaluate whether mean BMI at 2 years of age is higher in the offspring of pregnant individuals randomised to metformin.
Aim 3: To understand facilitators and barriers associated with metformin versus insulin use and HTE to facilitate evidence-based pharmacotherapy.
Primary hypotheses
Aim 1: We hypothesise that metformin is not inferior or worse than insulin by an absolute margin or difference of more than 8% in the composite adverse neonatal outcome.
Aim 2: We hypothesise that metformin does not result in increased child BMI at 2 years (not inferior by an absolute margin of 0.31 kg/m 2 ) compared with insulin.
Aim 3: We hypothesise that patient-reported factors associated with metformin compared with insulin use will be different, which is important to identify to enable clinical implementation of study findings.
Secondary aims
We will compare outcomes at delivery between pregnant individuals randomised to metformin versus insulin (hypertensive disorder of pregnancy, gestational weight gain, mode of delivery and obstetric anal sphincter injuries) and their infants (preterm birth, mechanical ventilation, neonatal intensive care unit (NICU) admission, oxygen support, respiratory distress syndrome and small-for-gestational-age at birth); as well as the frequency of treatment supplementation with insulin among pregnant individuals randomised to metformin.
We will compare outcomes at 2 years post partum between individuals randomised to metformin versus insulin (obesity, anthropometry, adiposity, diabetes, cholesterol and hypertension) and their children (obesity, anthropometry and adiposity).
We will compare patient-reported outcomes (PROs) at randomisation (mental and physical health; Diabetes Knowledge Questionnaire (DKQ), Diabetes Distress Scale (DDS) and Diabetes Management Self-Efficacy Scale (DMSES); lifestyle; health behaviours and diet), and at 6 weeks and 2 years post partum for the individual (pregnancy and childbirth experiences; treatment adherence and satisfaction; Maternal-Infant Bonding Scale (MIBS); lactation practices; lifestyle; health behaviours and diet) and child (lifestyle; health behaviours and diet).
Methods and analysis
DECIDE is a randomised, controlled, open-label, patient-centred and pragmatic multicentre comparative effectiveness trial that is designed to determine whether metformin is not inferior to insulin in reducing adverse pregnancy outcomes and is comparably safe for exposed pregnant individuals and children and to identify patient-reported factors associated with metformin versus insulin that facilitate and enable implementation of study findings ( online supplemental file 1 ).
Supplemental material
The DECIDE Study Consortium includes 20 clinical sites under a clinical coordinating centre (CCC) and an independent data coordinating centre (DCC). The consortium is governed by a steering committee and guided by a patient advisory board and stakeholder engagement group. Data management, coordination and analysis will be completed by the DCC, led by the study statisticians (CM and RGC). Participant data will be collected, stored and maintained in OpenClinica, a browser-independent electronic data capture system. Enrolled individuals will be randomised in a 1:1 ratio of metformin to insulin within the web-based data management system according to a computer-generated permuted block design with variable block sizes. Randomisation will be stratified by study site.
Individuals will be recruited across 20 US clinical sites with diabetes and prenatal care programmes ( figure 1 ). These sites have been selected with the goal of achieving racial and ethnic, urban and rural, and geographical diversity at both academic and community-based medical centres. Individuals who continue to receive routine prenatal care in their local community, and then receive high-risk prenatal and diabetes care from the clinical site will also be eligible for study participation. After delivery, individuals and their infants will be followed up with data ascertainment at 6 weeks and 2 years post partum.
- Download figure
- Open in new tab
- Download powerpoint
Geographic distribution of DECIDE sites across the USA. CCC, clinical coordinating centre; DCC, data coordinating centre.
Inclusion criteria
Inclusion criteria are age >18 years, singleton pregnancy, gestational age between 20 0/7 and 31 6/7 weeks, GDM diagnosis between 20 0/7 and 31 6/7 weeks, requiring medication for glycaemic control, and willingness and ability to attend 2-year follow-up visit ( table 2 ). The decision to initiate medication will be consistent with current US recommendations, defined as ≥30% elevation of either fasting or 1-hour or 2-hour postprandial glucose values in the prior week.
Inclusion and exclusion criteria
Exclusion criteria
We will exclude individuals who have known underlying chronic kidney disease; a fetus with a chromosomal, genetic or major structural malformation; contraindication to metformin or insulin; pregestational diabetes (either type 1 or 2); early-onset GDM <20 weeks; prior haemoglobin A1c >6.5%; concurrent enrollment in a trial with a primary aim that influences the primary study outcome; planned delivery at an outside clinical site where access to medical records cannot be obtained for outcome data abstraction; language barrier (appropriate translation resources unavailable at the site); participation in this trial in a previous pregnancy and fasting hyperglycaemia defined as >115 mg/dL for ≥50% glucose values in the past week ( table 2 ). We include fasting hyperglycaemia as an exclusion criterion as prior data suggest that individuals with this finding are likely to require insulin to achieve glycaemic control. 20
Recruitment
The start date for recruitment is 1 August 2024 and is anticipated to end by 1 May 2026, with final data collection at the 2-year follow-up ending on 1 May 2028. All individuals who present for prenatal care at sites in the DECIDE Study Consortium will be screened for eligibility. Individuals who meet study criteria will be approached for participation by study staff, which will include a study pamphlet with a weblink and QR code ( www.decidestudy.org ) and a 3 min video about GDM medication management ( https://youtu.be/CGmYCmF4vDo ). After eligibility is confirmed, individuals will be asked to participate after study information is given. Individuals who agree will complete the written informed consent process (see online supplemental file 2 for sample consent document). Reasons for ineligibility and rates of declining to participate will be collected. The patient advisory board will review recruitment and retention materials to create participant-friendly information and to assist with provider trainings. 33
Pregnancy (aim 1)
Baseline visit.
A research team member or healthcare provider will ask patients if they are interested in joining the study either in person or virtually. Those who are interested will be given an orientation to the study by a research team member ( figure 2 ). Written informed consent will be obtained in English or Spanish. Enrolled individuals will be randomised in a 1:1 ratio to metformin or insulin. Study staff will inform the provider about randomisation arm via telephone, email and the electronic medical record. Because this is an open label, non-blinded pragmatic trial comparing two treatments that are standard of care, individuals allocated to either arm will obtain their medication from their preferred pharmacy with a prescription from their provider, which will account for brand of insulin on formularies of their insurance plans.
Flow diagram of DECIDE study events.
Using defined data fields, we will record participant demographics, medical history and obstetric characteristics. Participants will complete standardised surveys at randomisation to assess lifestyle, health behaviours, diet, mental and physical health during pregnancy, DKQ, DDS and DMSES.
Follow-up visits
Consistent with a pragmatic trial, all dosing changes will be performed by the participant’s provider. The frequency of participant clinical encounters will be about every 2 weeks, which is standard clinical practice for GDM management, 34 with virtual or in-person clinic visits at the discretion of the provider. Study staff will visit with participants monthly (preferably in person and otherwise virtually), ask them about adherence to assigned treatment, review side effects related to their medication including nausea and symptoms of hypoglycaemia and assess medication adherence. Additionally, study staff will review and abstract the following information from the participant’s medical record: capillary blood glucose log values or continuous glucose monitoring logs for the past 1 week period, current metformin and insulin dosing, type(s) of insulin, and gestational weight gain. Finally, study staff will assess for adverse events (AEs) and serious AEs (SAEs) at each study visit.
The assigned treatment (insulin vs metformin) will be discontinued at delivery. The provider will have the responsibility for intrapartum and postpartum management. Data on intrapartum and postpartum glycaemic management will be abstracted by the study team. 35 Additionally, participant and infant data will be collected from the EHR until hospital discharge. We will collect comprehensive antepartum and intrapartum data, such as labour and delivery details; glycaemic control and neonatal outcomes. To address concerns about placental transfer of medication and fetal safety, cord blood and placental samples will be collected when possible for further analyses.
Postpartum follow-up through 2 years (aim 2)
~6 weeks post partum.
At ~6–8 weeks post partum, participants will complete standardised surveys with the study team in person, by a virtual platform, or by mail, per participant and site preference. This visit will include standardised measures to assess treatment adherence and satisfaction, lactation, lifestyle, health behaviours, diet, pregnancy and childbirth experience, and MIBS. Additional participant and child postpartum data through the ~6 weeks postpartum visit will be collected from the EHR. Testing for diabetes at the postpartum visit is standard of care. We will collect these results and emphasise best practices to increase uptake of diabetes screening. 36 Research staff will actively maintain contact with participants every 6 months after delivery by telephone, email or post.
2-years post partum
At or after 2 years post partum, participants will be invited to return for an in-person assessment and physical exam of both the participant and child. Participants will complete standardised surveys to assess lifestyle, health behaviours and diet in the mother and child. Physical exam of the child will include growth (weight and height), anthropometry (arm and abdominal circumference) and adiposity (skinfolds) measurements. For the participant, blood pressure, height, weight, anthropometry (waist and hip circumferences) and adiposity (skinfolds) will be obtained. The assessor at the visit will be masked to study arm assignment. A calibrated scale with stadiometer will be used for weight and height, a tape measure for anthropometry and callipers for skinfold measurements. As has been done in prior GDM cohorts (co-I PC), all sites will undergo central training and assessment in these techniques to promote standardisation of measurements and adherence to the study protocol. 37 38 Ongoing training and strict monitoring of the study team measurement techniques will be performed and regular assessment of interobserver variation will be conducted via training videos every 6 months. For the postpartum individual, blood will be obtained using standard venipuncture techniques, including for haemoglobin A1c, cholesterol panel and 2-hour 75 g oral glucose tolerance test using standardised collection procedures. 39
Mixed-methods assessment (aim 3)
In years 2–4 of the study, 150 individuals across all sites will be invited to complete a 30–45 min interview approximately 6 weeks after delivery. Individuals will be purposefully recruited from each study site and across each year of the study to ensure diversity with respect to race and ethnicity, age, insurance status and study arm. Participants will be given the option to be interviewed by phone or via video (eg, zoom).
A semistructured interview guide has been developed and refined based on feedback from patients and experts on qualitative research methods ( online supplemental file 2 ). These interviews will be performed centrally at OSU under the supervision of co-I ASM. The guide will include open-ended questions in the following domains: DECIDE trial participation, GDM and pregnancy, medications to treat GDM, experiences taking medication to treat GDM, and GDM and postpartum health. 31 During the interviews, participants will be asked to describe their experiences using metformin and/or insulin with question probes to address specific aspects of their experiences, including barriers to and facilitators of metformin and insulin use, and the factors that might improve adherence and pregnancy experience. The draft guide will be pilot tested and finalised prior to use in the study. All interviews will be audio recorded and transcribed verbatim to allow rigorous qualitative analysis.
Patient and public involvement
Patient and stakeholder interactions have helped develop the research question, comparators, study participants characteristics and relevant outcomes. 31 The DECIDE investigator team will engage key opinion leaders, patients with a history of GDM or living with diabetes and stakeholders, including public and private-sector insurers, advocacy organisations and professional societies, to elicit feedback through the Patient Advisory Board (co-I SC) and Stakeholder Engagement Board (co-I AT), both of which were established for this study and DiabetesSisters (co-I DR), a national patient advocacy organisation dedicated to women living with diabetes. The team will draw on participatory learning approaches, such as adaptive management, rapid assessments, data-driven decision-making and human-centred design to codevelop recommendations to inform project improvement.
The outcomes in this study include both clinical outcomes and PROs that cumulatively measure obstetric and perinatal morbidity and mortality that impact quality of life, well-being and pregnancy experience. Clinical outcomes relevant to contemporary practice are based on prior GDM pharmacotherapy trials, 20 23 40 meta-analyses comparing treatment strategies for GDM to prevent adverse outcomes, 18 19 26 engagement with stakeholder organisations and providers, 41 and core outcome sets for GDM that used a Delphi methodology. 42 43 PROs 44 are based on validated and standardised instruments that address birth experiences, living with diabetes, and treatment experiences and adherence; systematic reviews of prior qualitative studies of patient experiences with GDM; and testimonials from affected individuals and their families. 29 30 45–47
Primary clinical outcomes
The primary pregnancy outcome (aim 1) is a neonatal composite adverse outcome of LGA birth weight, hypoglycaemia, hyperbilirubinaemia and/or death ( table 3 ). 48 This measure is based on neonatal outcomes causally related to glycaemic control and consistent with that used in recent trials 23 and meta-analyses. 19
Primary and secondary outcomes
In the follow-up at 2 years, the primary child outcome is BMI as a continuous measure, which is consistent with prior GDM RCT follow-up studies and meta-analyses. 18 19 40 An updated sex-adjusted US reference will be used for standardisation of height and weight for age. 49 Anthropometry will be measured with standardised protocols that have been successfully implemented in prior GDM cohorts at birth (co-I PC). 37 38 50
Secondary clinical outcomes
Secondary pregnancy outcomes ( table 3 ) include neonatal outcomes (preterm birth, small-for-gestational-age, NICU admission, mechanical ventilation by duration, oxygen support by type and duration, and respiratory distress syndrome by clinical features and oxygen or respiratory support for any time during the first 72 hours after birth and participant outcomes (hypertensive disorder of pregnancy, mode of delivery, total gestational weight gain and obstetric anal sphincter injuries).
In the follow-up at 2 years, secondary child outcomes include obesity and measures of adiposity and anthropometry. Secondary participant outcomes at 2 years will include type 2 diabetes, obesity, pre-diabetes, hypertension, metabolic profile, and measures of anthropometry and adiposity.
Patient-reported measures
At randomisation, baseline assessments will include mental and physical health (PROMIS Global Short Form 51 52 ; DKQ, 53 DDS, 54 DMSES 55 and social determinants of health (Accountable Health Communities Health-Related Social Needs Screening Tool 56 and Williams Everyday Discrimination Scale 57 ( table 3 ).
At ~6 weeks postpartum follow-up visit, PROs will include treatment adherence and satisfaction Treatment Satisfaction Questionnaire for Medication 58 and Acceptability of Treatment 20 ; infant feeding practices (CDC Infant Feeding Practices, selected questions) 59 ; pregnancy and childbirth experience (Birth Satisfaction Scale-Revised Indicator) 60 ; MIBS 61 ; International Physical Activity Questionnaire (IPAQ), short-form 62 ; Mini-EAT (Eating Assessment Tool) 63 and the Brief Infant Sleep Questionnaire-Revised Short Form (selected questions). 64
At 2 years, PROs of the postpartum individual will include IPAQ, long-form 62 ; Mini-EAT 63 ; social determinants of health 56 ; mental and physical health 51 and of the child will include CDC Child Health and Diet Survey (selected questions) 65 ; Movement Behaviour Questionnaire (selected questions) 66 ; Brief Infant Sleep Questionnaire-Revised Short Form (selected questions) 64 and the Child Eating Behaviour Questionnaire. 67
Pharmacotherapy management
Study guidelines for metformin and insulin management including initiation, dosing, titration and monitoring have been developed based on current clinical guidelines. 68 Pharmacotherapy will be initiated when ≥30% of fasting and/or postprandial glucose values are elevated in the past week. Given this is a pragmatic trial, clinical practice may vary slightly across sites based on local standard-of-care and individualised provider–patient decision-making.
Treatment initiation and titration
Metformin (either extended or immediate release) will be started at 500 mg two times per day and titrated to a maximum daily dose of 2500 mg. Participants randomised to metformin will be given uniform advice by study personnel on how to minimise gastrointestinal distress, such as taking study tablets prior to meals and using antiemetics.
Providers will be encouraged to use trimester-specific and weight-based insulin dosing criteria for both basal and prandial insulins for up to a total of 4 daily injections. Consistent with clinical practice, some participants may be managed with a single dose of intermediate-acting or long-acting insulin at night to treat isolated fasting hyperglycaemia, while others may require additional treatment of postprandial hyperglycaemia with shorter-acting insulin. The sites’ insulin formularies may include rapid (Novolog, Humalog and Aspart), intermediate (Humulin N, Novolin N and NPH) and long-acting insulins (detemir, Lantus and degludec) with comparable efficacy in pregnancy. Participant insurance coverage will be considered when selecting insulin type.
We will standardise the incorporation of best practices regarding metformin and insulin titration per ADA and ACOG guidelines. Providers at each site will be instructed on the study protocol and trained on study procedures, including glycaemic monitoring ~1–2 weeks and uptitration. Glucose assessment by those participants electing self-monitored blood glucose monitoring will be performed at fasting and three times postprandial; those who elect continuous glucose monitoring will be asked to similarly document their fasting and postprandial values. Adherence to these goals will be monitored by research staff monthly from participant interviews and medical record review. Concerns regarding protocol adherence will be discussed with site PIs. Weekly participant glucose logs and total metformin and insulin doses and type of insulin will be recorded and considered in data analysis.
Treatment supplementation
Participants receiving metformin will have insulin supplemented (ie, addition of insulin to base regimen of metformin) only if they have not achieved euglycaemia for at least 30% of glucose values after approaching the maximum daily dose of metformin (>2000 mg or the maximum tolerated dose). Participants will be asked to continue taking metformin after treatment supplementation with insulin, which is generally the current clinical practice. In rare circumstances (0%–2%) in which severe gastrointestinal distress or intolerable side effects are present with metformin, participants may be prescribed insulin before reaching the maximum daily dose of metformin (2500 mg) or switched to insulin entirely. 69 Reasons that patients and providers decide on treatment supplementation will be collected.
Data safety and monitoring
An independent data safety monitoring board (DSMB) has been created to provide oversight of trial accrual and of privacy and safety of study participants. DSMB members have appropriate expertise (obstetrics and gynaecology, maternal–fetal medicine, endocrinology, neonatology, bioethics and biostatistics). The DSMB will meet to review the protocol prior to study initiation and then yearly to review study progress. The DCC will provide reports to the DSMB that include recruitment, protocol adherence and safety outcomes.
Detailed information about AEs and SAEs will be collected and evaluated throughout the trial. If a patient develops an SAE, the primary clinician in collaboration with the site PI will ascertain the safety of continuing the intervention. All unanticipated and possibly study-related AEs and SAEs will be reported to the IRB per regulatory reporting guidelines. Metformin may be temporarily stopped in the setting of acute kidney injury or intravenous contrast administration. Metformin has been reported to be very rarely associated with lactic acidosis (<10 cases per 100 000 patient-years), although the validity of this association has been challenged. 70 We will include lactic acidosis on metformin as a safety stopping rule.
Statistical analysis plan
Sample size and power.
Published data suggest that upwards of 30% of individuals with GDM have an associated adverse neonatal outcome. Using data from recent meta-analyses that compared the two treatment regimens, 18 19 71 and the most recent RCT (although comparing glyburide to insulin) that assessed the same primary composite outcome as in our study, 23 we estimate the frequency of the primary composite perinatal outcome to be 28% with insulin. To be conservative, we have used an estimate of 25%.
We have chosen a non-inferiority trial design because metformin’s advantages in terms of cost and ease (eg, oral, no refrigeration needed, less costly) suggest that metformin may be the preferred first-line treatment for GDM if it were found to be non-inferior to insulin in terms of efficacy and safety. 41 A non-inferiority margin of 8% was selected for the primary outcome based on a survey and interviews we conducted in January to June 2021 with each of our 20 site PIs, all of whom are maternal-fetal medicine specialists, as well as interviews with 144 patients. This conservative margin is also consistent with recent non-inferiority RCTs for GDM. 23 Additionally, we estimate that 20% of individuals who are randomised to metformin will require supplemental insulin, 21 which is lower than prior trials because we will exclude those with fasting hyperglycaemia (>115 mg/dL for >50% in the prior week) who are at the highest risk of failing metformin. 20
Based on the above assumptions, we plan to enrol 1572 individuals to determine if metformin is non-inferior to insulin for the composite primary outcome, with 90% power, one-sided significance level of 0.025, a loss to follow-up at delivery of 2% and 20% supplementation with insulin in addition to metformin.
For the 2-year follow-up, if outcomes are obtained on 1415 participants (ie, a loss to follow-up rate of 10%), there will be 90% power to rule out an effect size of at least 0.172 SD. This translates to a 0.31 unit difference in BMI or a 0.29 kg mean difference in child weight. 25 There will be 80% power to rule out an effect size of at least 0.149 SD, or a 0.27 unit difference in BMI or a 0.25 kg mean difference.
Analyses for pregnancy outcomes (aim 1)
We will use descriptive statistics to characterise participants to determine comparability of treatment groups at baseline. As an intention-to-treat analysis, the comparison is between individuals randomised to start metformin regardless of whether they later required supplemental insulin or stopped metformin due to side effects and switched to insulin versus individuals randomised to start on insulin. Analyses of the primary outcome will consist of summarising the proportions of trial participants with the primary endpoint for each group and calculating the corresponding between-group risk difference (insulin minus metformin) with 95% CIs.
Data analyses will adhere to the CONSORT (Consolidated Standards of Reporting Trials) guidelines and follow the intention-to-treat principle in which patients are analysed in the group to which they were randomised, regardless of whether they received the assigned intervention or altered their assigned medication prior to delivery. Metformin will be determined as non-inferior if the lower 95% confidence limit for the risk difference is −8 percentage points or greater (ie, closer to 0). If treatment groups differ on a pretreatment factor known to be a risk factor for the outcome, the analysis will adjust for these differences and an adjusted risk difference will be reported. If metformin is determined to be non-inferior to insulin, a superiority test will be conducted without adjusting the type I error, with metformin considered superior if the lower 95% confidence limit for the risk difference is more than 0.
Interim analyses
Since the sample size estimate is based on the assumption that the primary endpoint rate will be 25% in the insulin group, it is important to evaluate this proportion in the study after 20% of the participants (N=315) have delivered. In addition, the proportion of patients in the metformin group who require supplemental insulin will be reported. Once 50% of the participants have delivered (N=786), a formal interim analysis will be performed to determine whether metformin is inferior to insulin, with an upper boundary for the stopping rule for harm based on a one-sided type I error of 0.025 and the Lan-DeMets generalisation of the O’Brien-Fleming boundary. If the upper confidence bound for the risk difference is less than 0, the DSMB will evaluate this in the context of the other safety outcomes. We also plan to calculate conditional power given the observed data and conditional on future data showing no difference between treatment strategies. If the conditional power is high (>90%) that the neonatal composite rate will be more that 8% higher in the metformin arm, the DSMB will consider termination for futility, although any decision to terminate the study would not be reached solely on statistical grounds but on a number of clinical and statistical considerations.
Analyses for postpartum follow-up through 2 years (aim 2)
Child BMI is the primary outcome at 2 years of age. Analyses will consist of summarising the mean BMI standardised for age and sex for each group and calculating the corresponding between-group mean difference with 95% CIs using generalised linear models. Metformin will be determined as non-inferior to insulin if the lower 95% confidence limit for the mean difference is 0.31 units or greater (ie, closer to 0). Additional analyses as detailed above for the primary neonatal composite in the RCT will be performed, including for measures of child adiposity and anthropometry. Fetal sex will be evaluated for predefined interaction analyses with treatment group, and anthropometry will be standardised by sex-specific standards. 48
Mixed-methods analyses (aim 3)
We will use the constant comparative method and a grounded theory approach to analyse interview data. 72 This iterative approach to analysis will include reading interview transcripts and discussing findings among investigators as the study progresses. Our approach will enable exploration of emergent themes and ensure saturation in data collection. Analysis will prioritise the elucidation of key concepts from individuals’ interview statements (extraction), conceptual development based on constant comparative analysis, and classification of data through code development. 72 73 The coding team (co-I ASM) will create a preliminary coding dictionary based on the interview guide, defining broad categories of findings to enable coding of responses to interview questions. Frequent discussions among coding team members will allow the characterisation of emergent codes and ensure agreement about identified themes and subthemes. ATLAS.ti software will be used to support the analysis process.
Subgroup analyses
Treatment effectiveness for subgroups may differ due to barriers related to social determinants of health (eg, race/ethnicity), bioavailability of medication, physiologic insulin resistance (eg, BMI) or factors related to GDM and its severity (eg, maternal age, gestational age at medication initiation) ( online supplemental file 3 ). We will employ existing rigorous checklist for addressing the design, analysis and context of subgroup analyses. 74 These risk factors were selected based on differences in the frequency of GDM and adverse pregnancy and postpartum outcomes, and hence, at least a theoretical possibility as to why HTE may exist. We will formally assess for effect modification (interaction effect). Should we note significant heterogeneity of treatment effect across these prespecified groups (p<0.05), we will then systematically examine two-way effect modification. Should there be evidence of HTE, the proposed exploratory subgroup analyses will employ a non-inferiority approach consistent with the overall trial design and analysis plan.
Missing data and sensitivity analyses
We will investigate the robustness of the observed differences between the two groups with respect to any missing data. First, an inverse probability weighting (IPW) analysis will be conducted with each case weighted by the inverse probability of being a complete case. Under a missing-at-random mechanism, the IPW approach would result in an unbiased estimate of the difference between groups assuming a correctly specified model for the missing data. Second, a tipping-point analysis will describe the additional number of events in the insulin group versus the metformin group among the participants with missing data that would change the conclusion related to non-inferiority. In addition, a sensitivity analysis will be performed among participants in the metformin group who did not require supplemental insulin versus participants randomised to insulin only.
Participants will be asked to provide contact information (eg, phone, email and address) for themselves and two relatives who would know how to contact them. Research staff will actively maintain contact with participants throughout their pregnancies and by telephone, email or post, every 6 months after delivery. Participants will be asked to verify or update information at each contact. We will also maintain contact with participants and their families through flyers, cards and electronic communications in order to provide study updates.
Compensation
Participant reimbursement will be provided for completing assessments at multiple time points: randomisation (US$100), 6 weeks post partum (in person, virtual and/or telephone) (US$50) and 2-year follow-up visits for the participant and child (in person) (US$125). Participants selected for qualitative interviews will receive additional compensation (US$100).
Ethics and dissemination
The OSU Institutional Review Board (IRB), which will serve as the single IRB of record for all sites, has approved this protocol. All protocol amendments will be communicated for approval to the OSU IRB. Before a site may start the trial, it must be certified, which involves certification of research staff and an IRB reliance agreement with the single IRB.
We will submit study results for publication in peer-reviewed journals. The DCC and CCC will maintain access to the final trial dataset, and a limited deidentified dataset will be released via the online portal of the primary funder. A key component of our dissemination plan will be increasing patient and provider awareness about the comparative effectiveness results. Our partnership with DiabetesSisters and the Stakeholder Engagement Group will be leveraged for dissemination of results, including appropriate forums (eg, meetings, newsletters, social media communities, online videos). We will share accessible evidence-based factsheets and provide our primary publications for free download, including to study participants.
In this two-arm, open-label, pragmatic, comparative effectiveness RCT, we will examine whether metformin is not inferior to insulin in reducing adverse pregnancy outcomes and is comparably safe for exposed mothers and children, and whether patient-reported factors including facilitators and barriers of medication use differ between metformin versus insulin use. The DECIDE trial will randomise 1572 pregnant individuals with GDM who need pharmacotherapy at 20 US sites—with uniform diagnostic and treatment criteria—to oral metformin versus injectable insulin and follow them and their children through delivery and then to 2 years post partum.
The proposed comparative effectiveness study is designed to inform one of the most frequent medication decisions in pregnancy. The clinical equipoise that currently exists in use of these medications for GDM underscores that a trial with pregnancy and postpartum follow-up in a diverse, representative and contemporary US population is necessary and will fill a key knowledge gap affecting everyday practice, patient experience and clinical outcomes. 41 These themes, listed in bold below, have been identified as critical by stakeholders including patients, providers, researchers and professional societies.
Fill a critical evidence gap with regard to the optimal pharmacotherapy for individuals with GDM to prevent adverse pregnancy outcomes
Among the major limitations of the RCTs to date are (1) using varying GDM diagnostic criteria, (2) unclear criteria or guidelines for supplemental insulin, (3) lack of sufficient power for important outcomes, (4) insufficient long-term assessment of outcomes in exposed children, (5) unreported patterns of hyperglycaemia potentially influencing treatment effectiveness and (6) results from populations that do not reflect a contemporary US population. DECIDE will address each of these limitations with uniform diagnostic and treatment criteria and inclusion of 20 academic and community centres representative of major US geographical regions with diverse population characteristics.
Identify the long-term outcomes of metformin versus insulin on pregnant person and child health
Experts have cautioned that a GDM treatment trial without a plan for robust postnatal follow-up will not meaningfully fill the evidence gap and allow best practices to be determined. 24 71 DECIDE embeds a seamless, preplanned and rigorous follow-up of all randomised mother–child dyads.
Characterise patient experiences of individuals with GDM requiring pharmacotherapy
An in-depth understanding of patient and other key stakeholder perspectives on barriers to and facilitators of metformin versus insulin use is necessary to identify opportunities to improve outcomes. DECIDE includes PROs and outcomes that focus on the same constructs to bolster patient and stakeholder confidence. 75 DECIDE also assesses patient experiences, such as medication side effects, whether patients take medicines as directed, how satisfied they are with their medication choice, and how their medication choice impacts their pregnancy and postpartum experience, which may explain observed HTEs.
Active patient and stakeholder engagement
The proposed study is designed with the goal of informing healthcare decisions, both by filling an important evidence gap and by ensuring that the evidence provided is aligned with and informed by patients and other healthcare partners. While conducting the study, we will engage with the patient advisory board and stakeholder engagement group, which includes patients, patient advocates, clinicians, researchers, purchasers, payors, industry, health systems and policy-makers. We will discuss the study protocol and startup in a cooperative learning environment, and these stakeholders will be invited to participate in data analysis to add their perspectives to promote authenticity.
Limitations and strengths
Limitations.
First, while randomisation to pharmacotherapy minimises selection bias, lack of patient and provider blinding to treatment can introduce bias. Second, because this is a pragmatic RCT, variations in insulin formulary and differences in medication titration may result in heterogeneity in outcomes. To minimise the impact of variation of treatment effects across study sites, we have instituted uniform criteria for treatment initiation, defined as ≥30% elevated glucose values in the prior week. Also, the DECIDE manual of operations will contain guidelines for insulin and metformin management and standardised glycaemic targets for medication titration. We will stratify randomisation by site, and we will consider adjustment for site in analyses via both stratification and interaction effects. Finally, we include follow-up through 2 years postpartum, although longer follow-up may be necessary to assess the long-term impact of pharmacotherapy on outcomes.
We have powered our study to a conservative non-inferiority margin, which is consistent with recent non-inferiority RCTs for GDM 23 and allows for substitution of supplemental insulin for those on metformin. Second, we examine postpartum safety following exposure to metformin versus insulin on child and maternal/paternal health. Third, we integrate rigorous assessment of patient preferences and values through PROs, standardised measures and qualitative interviews as part of the RCT and follow-up. Finally, DECIDE includes a racially, ethnically and geographically diverse patient population with broad inclusion criteria reflective of obstetric practice to maximise relevance, impact and generalisability.
Ethics statements
Patient consent for publication.
Not applicable.
- Freaney PM , et al
- Venkatesh K ,
- Powe C , et al
- Gregory EC ,
- Harrington K ,
- Cameron NA , et al
- Venkatesh KK ,
- Buchanan TA ,
- HAPO Study Cooperative Research Group
- Catalano PM ,
- McIntyre HD ,
- Cruickshank JK , et al
- Landon MB ,
- Varner MW , et al
- Scholtens DM ,
- Kuang A , et al
- International Weight Management in Pregnancy (i-WIP) Collaborative Group
- Thom E , et al
- American College of Obstetricians and Gynecologists
- ↵ International Physical Activity Questionnaire (IPAQ) , Available : https://sites.google.com/site/theipaq/references [Accessed 12 Jul 2021 ].
- Brown FM , et al
- Society for Maternal-Fetal Medicine
- Balsells M ,
- García-Patterson A ,
- Solà I , et al
- Butalia S ,
- Gutierrez L ,
- Lodha A , et al
- Gao W , et al
- Castillo-Camelo W , et al
- Harrison RK , et al
- Barbour LA ,
- Scifres C ,
- Valent AM , et al
- Tarry-Adkins JL ,
- Engel SM , et al
- Donovan LE ,
- Zinman B , et al
- Glasziou P , et al
- Van Ryswyk E ,
- Middleton P ,
- Shute E , et al
- Trinh A , et al
- Tetzlaff JM ,
- Altman DG , et al
- Benizri N ,
- Burns K , et al
- Niznik CM ,
- Szmuilowicz ED , et al
- Cate JJM , Society for Maternal-Fetal Medicine (SMFM)
- Thomas AJ ,
- Avallone DA , et al
- Josefson JL ,
- Nodzenski M ,
- Talbot O , et al
- Potter JM ,
- Hickman PE ,
- Oakman C , et al
- Obolonkin V , et al
- Barbour LA , et al
- Bogdanet D ,
- Griffin TP , et al
- Nielsen KK ,
- O’Reilly S ,
- Wu N , et al
- Van Houten HK , et al
- Damm P , et al
- Wang Y , et al
- Churruca K ,
- Ellis LA , et al
- Kleinman KP ,
- Belfort MB , et al
- Prevention CfDCa
- Centers for Disease Control and Prevention
- Creedy DK , et al
- International Consortium for Health Outcomes Measurement (ICHOM)
- Garcia AA ,
- Villagomez ET ,
- Brown SA , et al
- Polonsky WH ,
- Earles J , et al
- Sangruangake M ,
- Jirapornkul C ,
- Centers for medicaid and Medicare Services
- Williams DR ,
- Jackson JS , et al
- Atkinson MJ ,
- Hass SL , et al
- Martin CR ,
- Hollins Martin C ,
- Kumar R , et al
- Marshall AL ,
- Sjöström M , et al
- Lara-Breitinger KM ,
- Medina Inojosa JR ,
- Li Z , et al
- Pediatric Sleep Council
- Terranova CO ,
- Brookes DSK , et al
- Carnell S ,
- Bakris G , et al. , American Diabetes Association Professional Practice Committee
- Ozanne SE ,
- DeFronzo R ,
- Fleming GA ,
- Chen K , et al
- Simmonds M ,
- Bryant M , et al
- Walter SD , et al
- Rivera SC ,
- Aiyegbusi OL , et al
Contributors KKV, CM, RGC, GS and ML designed the study. KKV, CM, RGC, AB, DG and ML wrote the methods manuscript. CP, ASM, LF, PC, AT and DR provided oversight for study design and implementation. CM and RGC provided statistical support and oversight. Under the clinical oversight of KKV, ML, MC, ANB, KB, KE, TE, MNF, LH, AK, MK-W, HM-F, MM, AS, NS, DS, SW, and CAZ assisted with the clinical trial development and execution. All authors revised the manuscript for relevant scientific content and approved the final version of the manuscript.
Funding This work was supported through a Patient-Centered Outcomes Research Institute (PCORI) BPS-2022C3-30268.
Disclaimer All statements in this report, including its findings and conclusions, are solely those of the authors and do not necessarily represent the views of the Patient-Centered Outcomes Research Institute (PCORI), its Board of Governors or Methodology Committee.
Map disclaimer The depiction of boundaries on this map does not imply the expression of any opinion whatsoever on the part of BMJ (or any member of its group) concerning the legal status of any country, territory, jurisdiction or area or of its authorities. This map is provided without any warranty of any kind, either express or implied.
Competing interests None declared.
Patient and public involvement Patients and/or the public were involved in the design, or conduct, or reporting, or dissemination plans of this research. Refer to the Methods section for further details.
Provenance and peer review Not commissioned; peer reviewed for ethical and funding approval prior to submission.
Supplemental material This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Read the full text or download the PDF:

- Previous Article
- Next Article
Introduction
Research design and methods, conclusions, article information, diabetes, prediabetes, and brain aging: the role of healthy lifestyle.
- Split-Screen
- Article contents
- Figures & tables
- Supplementary Data
- Peer Review
- Open the PDF for in another window
- Cite Icon Cite
- Get Permissions
Abigail Dove , Jiao Wang , Huijie Huang , Michelle M. Dunk , Sakura Sakakibara , Marc Guitart-Masip , Goran Papenberg , Weili Xu; Diabetes, Prediabetes, and Brain Aging: The Role of Healthy Lifestyle. Diabetes Care 20 September 2024; 47 (10): 1794–1802. https://doi.org/10.2337/dc24-0860
Download citation file:
- Ris (Zotero)
- Reference Manager
Diabetes is a well-known risk factor for dementia. We investigated the association between (pre)diabetes and older brain age and whether this can be attenuated by modifiable lifestyle behaviors.
The study included 31,229 dementia-free adults from the UK Biobank between the ages of 40 and 70 years. Glycemic status (normoglycemia, prediabetes, or diabetes) was ascertained based on medical history, medication use, and HbA 1c measured at baseline. Information on cardiometabolic risk factors (obesity, hypertension, low HDL, and high triglycerides) and lifestyle behaviors (smoking, drinking, and physical activity) was also collected at baseline. Participants underwent up to two brain MRI scans over 11 years of follow-up. Brain age was estimated using a machine learning model based on 1,079 brain MRI phenotypes and used to calculate brain age gap (BAG; i.e., brain age minus chronological age).
At baseline, 13,518 participants (43.3%) had prediabetes and 1,149 (3.7%) had diabetes. Prediabetes (β = 0.22 [95% CI 0.10, 0.34]) and diabetes (2.01 [1.70, 2.32]) were both associated with significantly higher BAG, and diabetes was further associated with significant increase in BAG over time (0.27 [0.01, 0.53]). The association between (pre)diabetes and higher BAG was more pronounced in men and in people with two or more cardiometabolic risk factors. In joint exposure analysis, having a healthy lifestyle (i.e., no smoking, no heavy drinking, and high physical activity) significantly attenuated the diabetes-BAG association.
Diabetes and even prediabetes are associated with accelerated brain aging, especially among men and people with poor cardiometabolic health. However, a healthy lifestyle may counteract this.
Graphical Abstract

Type 2 diabetes (hereafter, diabetes) is a well-established risk factor for cognitive impairment and has been associated with approximately double the risk of dementia ( 1–3 ). In brain MRI studies, diabetes has been related to global brain atrophy, increased burden of small-vessel disease, and microstructural lesions before the onset of cognitive symptoms ( 4 ). While prediabetes has been related to more modest levels of many of the cerebrovascular and neurodegenerative abnormalities associated with overt diabetes in some MRI studies ( 5 , 6 ), the association of prediabetes with cognitive decline and dementia remains controversial, with previous studies reporting conflicting results ( 7–10 ).
Recently, modeling methods have been introduced to estimate brain age based on MRI features such as volume loss, cortical thinning, white matter degradation, loss of gyrification, and ventricle enlargement ( 11 ). Brain age gap (BAG) reflects the difference between brain age and chronological age. Having an older-appearing brain for one’s chronological age—that is, a high BAG—can indicate deviation from the normal aging process and has been linked to mortality and increased risk of cognitive decline and dementia ( 11 ). Early detection of accelerated brain aging could support timely identification and intervention for people who are most at risk for developing dementia.
A growing body of cross-sectional studies has linked diabetes to brain age that is between 0.85 and 4.6 years older than chronological age ( 12–18 ), but longitudinal evidence on the association between diabetes and changes in brain age is lacking, and the relationship between prediabetes and brain age has not been explored. Given the heterogeneity of the diabetes population, another important consideration is how clinically relevant factors, such as sex, comorbidities, and lifestyle behaviors, might influence the association between (pre)diabetes and brain age. A variety of lifestyle behaviors, including physical activity and smoking/alcohol avoidance, have been related to decelerated brain aging ( 12 , 16 , 19 , 20 ), but whether a healthy lifestyle can counteract the detrimental influence of (pre)diabetes is unknown.
To address these questions, we comprehensively investigated the relationship between hyperglycemia and brain aging, leveraging detailed neuroimaging data from the UK Biobank covering six different MRI modalities in >30,000 middle-aged and older adults. Specifically, we aimed to 1 ) examine the cross-sectional and longitudinal relationship between (pre)diabetes and BAG; 2 ) explore the role of sex and cardiometabolic risk factors in these associations; and 3 ) investigate whether a healthy lifestyle, characterized by high physical activity and abstention from smoking and heavy drinking, can attenuate the influence of (pre)diabetes on BAG.
Study Design and Population
The UK Biobank is an ongoing longitudinal study including >500,000 adults between the ages of 40 and 70 from across the United Kingdom ( 21 ). Between 2006 and 2010, participants took part in a baseline examination at 1 of 22 assessment centers across the country consisting of physical and medical assessments and a series of questionnaires about sociodemographic information and lifestyle behaviors. Approximately 9 years later, between 2014 and 2020, >40,000 participants additionally underwent a brain MRI scan. Beginning in 2019, participants were invited to return for a follow-up brain MRI scan.
Selection of the study population is illustrated in Supplementary Fig. 1 . The analysis was restricted to 34,296 participants who underwent brain MRI scans and had complete information on all available imaging-derived phenotypes (IDPs). We then excluded 630 participants with chronic neurological disorders (including dementia) at the time of the MRI scan (see Supplementary Table 1 for details), 15 with type 1 diabetes, and 2,422 with missing information on baseline HbA 1c , leaving a sample of 31,229, including 2,414 who underwent two MRI scans.
All data collection procedures have been approved by the UK National Research Ethics Service (Ref 11/NW/0382) and the use of the data for the present analyses were additionally approved by the Regional Ethical Review Board in Stockholm, Sweden (Ref 2024-00520-01). All participants provided informed consent at baseline.
Assessment of Prediabetes and Diabetes
Baseline diabetes and prediabetes were defined according to the American Diabetes Association standard diagnostic criteria ( 22 ). Participants were classified as having diabetes if they had any one of the following: medical record of diabetes, use of glucose-lowering medications, self-reported history of diabetes, or HbA 1c ≥6.5% (see Supplementary Table 2 for field codes). Among diabetes-free participants, prediabetes was defined as HbA 1c 5.7% to 6.4%, and normoglycemia was defined as HbA 1c <5.7%. Diabetes was further categorized according to level of glycemic control: <7.0% (well-controlled), ≥7.0 to <8.0% (moderately controlled), or ≥8.0% (poorly controlled) ( 23 ).
Acquisition of Brain IDPs
Brain MRI scans were conducted using a Siemens Skyra 3T scanner. Detailed descriptions of the UK Biobank brain MRI image acquisition and processing protocols have been previously published ( 24 , 25 ) and are summarized in Supplementary Table 3 .
A total of 1,079 IDPs were extracted across six MRI modalities: 165 from T1-weighted MRI, 1 from T2-fluid attenuated inversion recovery (FLAIR), 14 from T2*, 675 from diffusion MRI, 210 from resting-state functional MRI (fMRI), and 14 from task fMRI. Briefly, T1-weighted imaging provides information on the volume and thickness of different brain regions, T2-FLAIR imaging detects white matter hyperintensities (reflecting vascular brain damage), T2* detects brain microbleeds, diffusion MRI assesses white matter microstructural integrity, resting-state fMRI measures brain activity at rest for assessment of intrinsic functional connectivity of neural networks, and task fMRI does so when the participant is performing a task or experiencing a sensory stimulus (in this case, a face/shapes matching task) ( 24 ). A full list of all 1,079 IDPs is provided in Supplementary Material .
Machine Learning-Based Estimation of Brain Age and BAG
The procedure for brain age estimation has been described in previous studies ( 26 , 27 ). A detailed description is available in the Supplementary Material , and the workflow is illustrated in Supplementary Fig. 2 .
Briefly, from the entire sample of participants with complete brain MRI data ( N = 34,296), we first identified 4,355 healthy individuals between the ages of 40 and 70 with no ICD-10 diagnoses and who were free from self-reported long-term illness, disability, or frailty (Field ID: 2188) and self-reported fair or poor health status (Field ID: 2178) ( Supplementary Table 4 ). These participants were randomly allocated in a 4:1 ratio to a training set ( n = 3,484) and a validation set ( n = 871). Next, all 1,079 IDPs were Z standardized and nine machine learning models were trained for modeling brain age in the training set. These included least absolute shrinkage and selection operator regression (LASSO), eXtreme gradient boosting, and support vector regression, which were combined with three possible feature selection strategies (no feature selection, FeatureWiz, or recursive feature elimination with cross validation). Bayesian optimization was performed to optimize the hyperparameters of all nine models through 100 epochs ( Supplementary Tables 5 and 6 ). Once optimized, all nine models were applied to the validation set so that their performance could be compared. Ultimately, the LASSO model without feature selection achieved the lowest mean absolute error ( Supplementary Table 7 ) and was therefore chosen to predict brain age for the entire sample. Of the 1,079 IDPs, 285 contributed significantly to the brain age estimate and are listed in Supplementary Table 8 .
Next, because brain age tends to be overpredicted in younger individuals and underpredicted in older individuals, we corrected brain age estimates for age bias as follows ( 28 , 29 ): brain age corrected = [brain age original – β/α] , where coefficients α and β are the slope and intercept of brain age training set = α × chronological age training set + β ( Supplementary Fig. 3 ).
Finally, BAG, which represents the difference between an individual’s brain age and their chronological age, was calculated as BAG = brain age – age time of MRI . Positive values for BAG indicate a brain that is older (i.e., less healthy) and negative values for BAG indicate a brain that is younger (i.e., more healthy) than expected based on the individual’s chronological age.
Assessment of Covariates
Sociodemographic factors.
Education (college/university vs. not) was dichotomized based on the highest level of formal education attained. Socioeconomic status (SES) was assessed using the Townsend deprivation index, a measure of neighborhood-level socioeconomic deprivation based on the prevalence of unemployment, household overcrowding, car nonownership, and home nonownership in a given postcode of residence.
Cardiometabolic Risk Factors
Cardiometabolic risk factor burden was operationalized in terms of the components of the metabolic syndrome (MetS) ( 30 ). BMI was calculated using height and weight measurements from the baseline examination and classified as underweight (<20 kg/m 2 ), normal weight (≥20 to <25 kg/m 2 ), overweight (≥25 to <30 kg/m 2 ), or obese (≥30 kg/m 2 ). Hypertension was defined based on self-report, blood pressure measurement (systolic ≥140 mmHg, diastolic ≥90 mmHg), or antihypertensive medication use. HDL cholesterol and triglycerides were measured from blood samples collected at baseline. A score reflecting cardiometabolic risk factor burden (ranging from 0 to 4) was generated according to the total number of MetS components present, including obesity, hypertension, low HDL (<40 mg/dL [1.03 mmol/L] for men and <50 mg/dL [1.29 mmol/L] for women), and high triglycerides (≥150 mg/dL [1.7 mmol/L]). (Notably, the fifth MetS component, hyperglycemia, was not included because it was already considered as the exposure in all analyses.)
Lifestyle Behaviors
Information was collected on three readily modifiable lifestyle behaviors: smoking, alcohol drinking, and physical activity. Smoking status was categorized as nonsmoker, former smoker, or current smoker according to self-report. Intake of various alcoholic beverages was self-reported and converted into U.K. alcohol units (1 unit = 8 g ethanol) ( 31 ). Alcohol consumption was categorized as nondrinker, light/moderate drinking (≤14 units/week), or heavy drinking (>14 units/week) according to current U.K. guidelines on alcohol consumption for both men and women ( 32 ). Physical activity was measured using the International Physical Activity Questionnaire. Participants were classified as inactive (<600 MET-min/week), moderate (600 to <3,000 MET-min/week), or active (≥3,000 MET-min/week); 600 MET-min/week is equivalent to the World Health Organization recommendation of 150 min of moderate-intensity or 75 min of vigorous physical activity per week ( 33 ). An optimal lifestyle was defined as never smoking, no or light/moderate alcohol consumption, and high physical activity.
Alzheimer Disease-Related Polygenic Risk Score
Alzheimer disease (AD)-related polygenic risk score (PRS AD ) was obtained from the UK Biobank’s Standard PRS Set ( 34 ). Briefly, PRS AD represents the Z-standardized sum of each participant’s number of AD-related alleles (including the well-known APOE ε4 polymorphism) weighted by the strength of each allele’s association with AD ( 34 ).
Statistical Analysis
Baseline characteristics of the study participants by glycemic status were assessed using χ 2 tests for categorical variables and one-way ANOVA for continuous variables.
Linear regression models were used to estimate β-coefficients and 95% CIs for the association between glycemic status at baseline and BAG at the time of brain MRI. Least-squares means of BAG in the normoglycemia, prediabetes, and diabetes groups were additionally estimated from the margins of the linear regression models. Similar analyses were conducted using HbA 1c as a continuous variable. Restricted cubic splines with three knots at fixed percentiles of the HbA 1c distribution (10th, 50th, and 90th) were used to model the possible nonlinear association between HbA 1c and BAG. Among participants who underwent two brain MRI scans, linear mixed-effects models were used to estimate β-coefficients and 95% CIs for the association between glycemic status and changes in BAG between the first and second scans. The fixed effect included baseline glycemic status, follow-up time (in years), and their interaction. The random effect included random intercept and slope, allowing individual differences in BAG to be reflected at baseline and across follow-up.
Next, stratified linear regression models were used to explore the role of sex (women vs. men) and cardiometabolic health (0–1 vs. ≥2 risk factors) in the association between glycemic status and BAG. Finally, we performed joint exposure analysis by incorporating a six-category indicator variable that combined glycemic status (normoglycemia, prediabetes, or diabetes) and lifestyle (optimal or nonoptimal) into the linear regression model. Interactions between glycemic status and sex, cardiometabolic risk factor level, and lifestyle were assessed by incorporating the cross-product term into the models.
All models were first basic adjusted for sociodemographic factors (i.e., age, sex, education, and SES), followed by further adjustment for number of cardiometabolic risk factors, lifestyle behaviors (i.e., smoking, alcohol consumption, and physical activity), and PRS AD . Missing values for covariates were imputed using fully conditional specification, with estimates pooled from five iterations.
In sensitivity analysis, we repeated the main analyses 1 ) using BAG calculated based on brain age estimates from other candidate machine learning models; 2 ) using nonimputed data; 3 ) after adding an additional covariate for brain MRI assessment center; 4 ) after excluding participants with possible prodromal/undiagnosed dementia (i.e., incident dementia during follow-up; n = 42) or possible cognitive impairment (i.e., baseline cognitive test scores <25th percentile; n = 7,806) to minimize the possibility of reverse causality; and 5 ) using diabetes status defined at the time of brain MRI scan to address the possibility of changes in glycemic status since baseline. All analyses were performed using Stata SE 16.0 software (StataCorp, College Station, TX). P values <0.05 were considered statistically significant.
Data and Resource Availability
Requests for access to the UK Biobank data can be made here: https://www.ukbiobank.ac.uk/enable-your-research/apply-for-access .
Baseline Characteristics
Baseline characteristics of the 31,229 study participants (mean age 54.8 ± 7.5; 53.0% female) are summarized in Table 1 . At baseline, 13,518 participants (43.3%) had prediabetes and 1,149 (3.7%) had diabetes. Compared with participants with normoglycemia, those with (pre)diabetes were more likely to be older, male, have a lower education level and SES, be physically inactive, and have cardiometabolic risk factors. The study sample was comparatively younger and had greater educational attainment, higher SES, and a more favorable cardiometabolic risk profile compared with the UK Biobank population as a whole ( Supplementary Table 9 ).
Baseline characteristics of the 31,229 study participants by glycemic status
| . | . | By glycemic status . | . | ||
|---|---|---|---|---|---|
| Characteristics . | Full sample ( = 31,229) . | Normoglycemia ( = 16,562) . | Prediabetes ( = 13,518) . | Diabetes ( = 1,149) . | value . |
| Age, years | |||||
| At baseline | 54.8 ± 7.5 | 53.1 ± 7.5 | 56.7 ± 7.1 | 57.5 ± 7.0 | <0.001 |
| At time of brain MRI | 63.7 ± 7.6 | 62.0 ± 7.6 | 65.6 ± 7.2 | 66.4 ± 7.2 | <0.001 |
| Female sex | 16,556 (53.0) | 9,015 (54.4) | 7,105 (52.6) | 436 (38.0) | <0.001 |
| College/university-educated | 14,503 (46.6) | 8,006 (48.5) | 6,038 (44.8) | 459 (40.2) | <0.001 |
| Townsend deprivation index | −1.9 ± 2.7 | −1.9 ± 2.7 | −2.0 ± 2.7 | −1.5 ± 2.9 | <0.001 |
| BMI, kg/m | 26.5 ± 4.2 | 25.9 ± 3.8 | 26.9 ± 4.3 | 30.2 ± 5.5 | <0.001 |
| Underweight (<20) | 799 (2.6) | 490 (3.0) | 302 (2.2) | 7 (0.6) | <0.001 |
| Normal (20–25) | 11,651 (37.3) | 6,870 (41.5) | 4,613 (34.2) | 168 (14.6) | |
| Overweight (25–30) | 13,382 (42.9) | 7,020 (42.4) | 5,910 (43.8) | 452 (39.4) | |
| Obese (≥30) | 5,367 (17.2) | 2,163 (13.1) | 2,683 (19.9) | 521 (45.4) | |
| Hypertension | 6,594 (21.1) | 2,832 (17.1) | 3,197 (23.7) | 565 (49.2) | <0.001 |
| HDL, mg/dL | 57.2 ± 14.6 | 58.1 ± 14.5 | 57.0 ± 14.5 | 48.6 ± 13.2 | <0.001 |
| Triglycerides, mg/dL | 144.5 ± 84.0 | 135.0 ± 79.3 | 152.5 ± 85.3 | 186.4 ± 108.8 | <0.001 |
| HbA , % | 5.7 ± 0.5 | 5.4 ± 0.2 | 5.9 ± 0.2 | 7.1 ± 1.0 | <0.001 |
| Smoking | <0.001 | ||||
| Nonsmoker | 19,036 (61.1) | 10,446 (63.2) | 8,007 (59.4) | 583 (50.8) | |
| Former smoker | 10,258 (32.9) | 5,241 (31.7) | 4,537 (33.6) | 480 (41.8) | |
| Current smoker | 1,873 (6.0) | 847 (5.1) | 941 (7.0) | 85 (7.4) | |
| Alcohol consumption | <0.001 | ||||
| Nondrinker | 1,970 (7.2) | 886 (6.0) | 983 (8.4) | 101 (10.4) | |
| Low/moderate drinking | 11,785 (42.9) | 6,237 (42.4) | 5,134 (43.7) | 414 (42.4) | |
| Heavy drinking | 13,705 (49.9) | 7,599 (51.6) | 5,645 (48.0) | 461 (47.2) | |
| Physical activity | <0.001 | ||||
| Low | 4,880 (18.1) | 2,575 (17.8) | 2,049 (17.7) | 256 (26.0) | |
| Moderate | 11,320 (41.9) | 6,022 (41.7) | 4,896 (42.3) | 402 (40.8) | |
| High | 10,801 (40.0) | 5,835 (40.4) | 4,638 (40.0) | 328 (33.3) | |
| ε4 carrier | 7,322 (27.5) | 3,992 (28.1) | 3,105 (27.1) | 225 (23.8) | 0.008 |
| PRS | 0.04 ± 0.99 | 0.05 ± 0.99 | 0.04 ± 0.98 | −0.04 ± 0.96 | 0.026 |
| . | . | By glycemic status . | . | ||
|---|---|---|---|---|---|
| Characteristics . | Full sample ( = 31,229) . | Normoglycemia ( = 16,562) . | Prediabetes ( = 13,518) . | Diabetes ( = 1,149) . | value . |
| Age, years | |||||
| At baseline | 54.8 ± 7.5 | 53.1 ± 7.5 | 56.7 ± 7.1 | 57.5 ± 7.0 | <0.001 |
| At time of brain MRI | 63.7 ± 7.6 | 62.0 ± 7.6 | 65.6 ± 7.2 | 66.4 ± 7.2 | <0.001 |
| Female sex | 16,556 (53.0) | 9,015 (54.4) | 7,105 (52.6) | 436 (38.0) | <0.001 |
| College/university-educated | 14,503 (46.6) | 8,006 (48.5) | 6,038 (44.8) | 459 (40.2) | <0.001 |
| Townsend deprivation index | −1.9 ± 2.7 | −1.9 ± 2.7 | −2.0 ± 2.7 | −1.5 ± 2.9 | <0.001 |
| BMI, kg/m | 26.5 ± 4.2 | 25.9 ± 3.8 | 26.9 ± 4.3 | 30.2 ± 5.5 | <0.001 |
| Underweight (<20) | 799 (2.6) | 490 (3.0) | 302 (2.2) | 7 (0.6) | <0.001 |
| Normal (20–25) | 11,651 (37.3) | 6,870 (41.5) | 4,613 (34.2) | 168 (14.6) | |
| Overweight (25–30) | 13,382 (42.9) | 7,020 (42.4) | 5,910 (43.8) | 452 (39.4) | |
| Obese (≥30) | 5,367 (17.2) | 2,163 (13.1) | 2,683 (19.9) | 521 (45.4) | |
| Hypertension | 6,594 (21.1) | 2,832 (17.1) | 3,197 (23.7) | 565 (49.2) | <0.001 |
| HDL, mg/dL | 57.2 ± 14.6 | 58.1 ± 14.5 | 57.0 ± 14.5 | 48.6 ± 13.2 | <0.001 |
| Triglycerides, mg/dL | 144.5 ± 84.0 | 135.0 ± 79.3 | 152.5 ± 85.3 | 186.4 ± 108.8 | <0.001 |
| HbA , % | 5.7 ± 0.5 | 5.4 ± 0.2 | 5.9 ± 0.2 | 7.1 ± 1.0 | <0.001 |
| Smoking | <0.001 | ||||
| Nonsmoker | 19,036 (61.1) | 10,446 (63.2) | 8,007 (59.4) | 583 (50.8) | |
| Former smoker | 10,258 (32.9) | 5,241 (31.7) | 4,537 (33.6) | 480 (41.8) | |
| Current smoker | 1,873 (6.0) | 847 (5.1) | 941 (7.0) | 85 (7.4) | |
| Alcohol consumption | <0.001 | ||||
| Nondrinker | 1,970 (7.2) | 886 (6.0) | 983 (8.4) | 101 (10.4) | |
| Low/moderate drinking | 11,785 (42.9) | 6,237 (42.4) | 5,134 (43.7) | 414 (42.4) | |
| Heavy drinking | 13,705 (49.9) | 7,599 (51.6) | 5,645 (48.0) | 461 (47.2) | |
| Physical activity | <0.001 | ||||
| Low | 4,880 (18.1) | 2,575 (17.8) | 2,049 (17.7) | 256 (26.0) | |
| Moderate | 11,320 (41.9) | 6,022 (41.7) | 4,896 (42.3) | 402 (40.8) | |
| High | 10,801 (40.0) | 5,835 (40.4) | 4,638 (40.0) | 328 (33.3) | |
| ε4 carrier | 7,322 (27.5) | 3,992 (28.1) | 3,105 (27.1) | 225 (23.8) | 0.008 |
| PRS | 0.04 ± 0.99 | 0.05 ± 0.99 | 0.04 ± 0.98 | −0.04 ± 0.96 | 0.026 |
Data are presented as means ± SD or n (%). Missing data: 92 for education level; 28 for Townsend deprivation index; 30 for BMI; 15 for hypertension; 4,030 for HDL; 1,406 for triglycerides; 62 for smoking status; 3,769 for alcohol consumption; 4,228 for physical activity level; 4,638 for APOE ε4 status; and 242 for PRS AD .
Indicates significant ( P value <0.05) pairwise comparison (reference group = normoglycemia).
Prediabetes, Diabetes, and BAG
Compared with normoglycemia, prediabetes (β = 0.22 [95% CI 0.10, 0.34]) and diabetes (β = 2.01 [1.70, 2.32]) were associated with significantly higher BAG ( Table 2 ). Specifically, brain age was on average 0.50 years older than chronological age among people with prediabetes and 2.29 years older than chronological age among people with diabetes ( Fig. 1A ). BAG rose as high as 4.18 years among people with poorly controlled diabetes (HbA 1c ≥8.0%). Consistent with this, HbA 1c as a continuous variable was associated with significantly higher BAG (β = 0.77 [0.65, 0.90]), and the restricted cubic spline analysis showed a strong increase in BAG with higher levels of HbA 1c ( Fig. 1B ).
Cross-sectional and longitudinal associations between glycemic status and BAG: results from linear regression and linear mixed-effects models
| . | . | BAG . | |||
|---|---|---|---|---|---|
| . | . | Basic adjusted . | Multiadjusted . | ||
| Glycemic status . | Participants ( ) . | β (95% CI) . | value . | β (95% CI) . | value . |
| Cross-sectional | |||||
| Normoglycemia | 16,562 | Reference | Reference | ||
| Prediabetes | 13,518 | 0.32 (0.20, 0.44) | <0.001 | 0.22 (0.10, 0.34) | <0.001 |
| Diabetes | 1,149 | 2.40 (2.10, 2.71) | <0.001 | 2.01 (1.70, 2.32) | <0.001 |
| HbA <7.0% | 671 | 1.81 (1.42, 2.21) | <0.001 | 1.43 (1.04, 1.83) | <0.001 |
| HbA ≥7.0% to <8.0% | 303 | 2.62 (2.04, 3.20) | <0.001 | 2.19 (1.61, 2.77) | <0.001 |
| HbA ≥8.0% | 175 | 4.29 (3.53, 5.05) | <0.001 | 3.90 (3.15, 4.66) | <0.001 |
| HbA (continuous) | 0.95 (0.82, 1.08) | <0.001 | 0.77 (0.65, 0.90) | <0.001 | |
| Longitudinal | |||||
| Normoglycemia × time | 1,354 | Reference | Reference | ||
| Prediabetes × time | 982 | −0.03 (−0.12, 0.07) | 0.597 | −0.03 (−0.12, 0.07) | 0.596 |
| Diabetes × time | 78 | 0.27 (0.01, 0.53) | 0.045 | 0.27 (0.01, 0.53) | 0.045 |
| HbA (continuous) × time | 0.13 (0.03, 0.23) | 0.012 | 0.13 (0.03, 0.23) | 0.012 | |
| . | . | BAG . | |||
|---|---|---|---|---|---|
| . | . | Basic adjusted . | Multiadjusted . | ||
| Glycemic status . | Participants ( ) . | β (95% CI) . | value . | β (95% CI) . | value . |
| Cross-sectional | |||||
| Normoglycemia | 16,562 | Reference | Reference | ||
| Prediabetes | 13,518 | 0.32 (0.20, 0.44) | <0.001 | 0.22 (0.10, 0.34) | <0.001 |
| Diabetes | 1,149 | 2.40 (2.10, 2.71) | <0.001 | 2.01 (1.70, 2.32) | <0.001 |
| HbA <7.0% | 671 | 1.81 (1.42, 2.21) | <0.001 | 1.43 (1.04, 1.83) | <0.001 |
| HbA ≥7.0% to <8.0% | 303 | 2.62 (2.04, 3.20) | <0.001 | 2.19 (1.61, 2.77) | <0.001 |
| HbA ≥8.0% | 175 | 4.29 (3.53, 5.05) | <0.001 | 3.90 (3.15, 4.66) | <0.001 |
| HbA (continuous) | 0.95 (0.82, 1.08) | <0.001 | 0.77 (0.65, 0.90) | <0.001 | |
| Longitudinal | |||||
| Normoglycemia × time | 1,354 | Reference | Reference | ||
| Prediabetes × time | 982 | −0.03 (−0.12, 0.07) | 0.597 | −0.03 (−0.12, 0.07) | 0.596 |
| Diabetes × time | 78 | 0.27 (0.01, 0.53) | 0.045 | 0.27 (0.01, 0.53) | 0.045 |
| HbA (continuous) × time | 0.13 (0.03, 0.23) | 0.012 | 0.13 (0.03, 0.23) | 0.012 | |
Basic-adjusted models included age, sex, education, and socioeconomic status. Multiadjusted models additionally included cardiometabolic risk factor burden, smoking status, alcohol drinking, physical activity, and PRS AD .

Relationship between glycemic status and BAG. A : Least-squares means and SDs of BAG in participants with normoglycemia, prediabetes, and diabetes. B : The relationship between HbA 1c (as a continuous variable) and BAG is modeled using restricted cubic splines. The red line and red shaded area represent the least-squares means and 95% CIs of BAG as a function of baseline HbA 1c . Gray bars represent the distribution of HbA 1c in the study population. C : The relationship between glycemic status and changes in BAG is modeled using linear mixed-effects models. All models were adjusted for age, sex, education, SES, cardiometabolic risk factor burden, smoking status, alcohol drinking, physical activity, and PRS AD .
In an exploratory longitudinal analysis among the 2,414 participants (7.7%) who underwent two brain MRI scans, diabetes was associated with a 0.27-year annual increase in BAG ( Table 2 and Fig. 1C ). No significant relationship was detected between prediabetes and changes in BAG, although HbA 1c as a continuous variable was associated with a significant increase in BAG (β = 0.13 [95% CI 0.03, 0.23]).
Sex- and Cardiometabolic Burden–Stratified Analyses
In stratified analyses ( Fig. 2A and Supplementary Tables 10 and 11 ), the association between diabetes and higher BAG was more pronounced in men compared with women (β = 2.32 [95% CI 1.90, 2.74] vs. 1.51 [1.04, 1.99]) and people with a higher burden of cardiometabolic risk factors (0–1 risk factors: 1.91 [1.45, 2.36]; ≥2 risk factors: 2.20 [1.74, 2.66]). The same was true for prediabetes. Specifically, brain age was on average 0.75 years older than chronological age among men with prediabetes, compared with only 0.27 years older for women. Moreover, BAG rose to 2.63 years for men with diabetes compared with 1.76 years for women. Similarly, among individuals with two or more cardiometabolic risk factors, prediabetes and diabetes were associated with an average BAG of 1.32 and 3.08 years compared with 0.24 and 1.96 years, respectively, among their counterparts with a lower cardiometabolic risk factor burden.
Role of sex, cardiometabolic risk factor burden, and healthy lifestyle in the association between glycemic status and BAG. A : Least-squares means and SDs of BAG among participants with normoglycemia, prediabetes, and diabetes, stratified by sex and cardiometabolic burden. Significant interactions were detected between glycemic status and sex ( P < 0.001) and between glycemic status and cardiometabolic burden ( P < 0.001). Models were adjusted for age, education, SES, cardiometabolic risk factor burden, smoking status, alcohol drinking, physical activity, and PRS AD as well as sex or cardiometabolic risk factor burden, depending on the stratification factor. B : β-Coefficients for the joint effect on glycemic status and lifestyle on BAG. A significant interaction was detected between glycemic status and healthy lifestyle ( P = 0.04). Models were adjusted for age, sex, education, SES, cardiometabolic risk factor burden, and PRS AD . Note: The reference group was changed to (pre)diabetes and optimal lifestyle when assessing whether lifestyle significantly modified the (pre)diabetes-BAG association.
Significant interactions were detected between glycemic status and both sex and cardiometabolic burden with respect to BAG ( P < 0.001 for all).
Role of a Healthy Lifestyle
In joint exposure analysis, an optimal healthy lifestyle (i.e., nonsmoking, no or light/moderate drinking, and high physical activity) significantly attenuated the association between diabetes and BAG ( Fig. 2B and Supplementary Table 12 ). Brain age was on average only 0.78 years older than chronological age among people with diabetes and an optimal lifestyle compared with 2.46 years older with a nonoptimal lifestyle. Therefore, healthy lifestyle was related to a 1.68-year reduction in BAG. More modest reductions in BAG were seen between individuals with normoglycemia and prediabetes and an optimal vs. nonoptimal lifestyle, respectively, although the difference for individuals with prediabetes was not statistically significant. A significant interaction was detected between glycemic status and lifestyle ( P = 0.04).
Sensitivity Analyses
Sensitivity analyses are described in detail in the Supplementary Material . Overall, similar results were obtained when we repeated the analyses using BAG calculated based on brain age estimates from other candidate machine learning models ( Supplementary Table 13 ), using nonimputed data ( Supplementary Table 14 ), after additionally adjusting for brain MRI assessment center ( Supplementary Table 15 ), after excluding 42 participants with possible prodromal/undiagnosed dementia ( Supplementary Table 16 ), and after excluding 7,806 participants with possible cognitive impairment ( Supplementary Table 16 ). Moreover, 558 people with normoglycemia or prediabetes transitioned to diabetes during the ∼9-year period between baseline and the first MRI scan ( Supplementary Fig. 4 ), but results remained consistent using diabetes status defined at the time of this scan ( Supplementary Table 17 ).
In this large-scale neuroimaging study, diabetes and even prediabetes were related to significantly older brain age in relation to chronological age, and diabetes was further associated with significant widening of the gap between brain and chronological age over time. These associations were more pronounced in men and people with poorer cardiometabolic health but may be counteracted with a healthy lifestyle characterized by physical activity and abstention from smoking and heavy drinking.
Diabetes was associated with a BAG of 2.29 years in the current study, consistent with previous reports in which diabetes has been related to a BAG between 0.85 and 4.6 years ( 12–16 ). Drawing on the >2,000 participants in our study who underwent two brain MRI scans, we further determined that diabetes was associated with a 0.27-year annual increase in BAG over time, a compelling signal that diabetes is related not only to older brain age but also to an accelerated pace of brain aging. In line with this, a small study ( n = 25) exploring the longitudinal relationship between diabetes and brain aging reported that BAG widened by an estimated 0.2 years annually among people with diabetes ( 12 ).
Notably, whereas most previous studies estimated brain age used only T1-weighed imaging ( 12–15 , 17 , 18 ), ours leveraged information across six brain MRI modalities (T1-weighted imaging plus T2-FLAIR, T2*, diffusion MRI, resting-state fMRI, and task fMRI). A recent study also conducted using UK Biobank data concluded that whereas T1-weighted imaging is the MRI modality with the highest independent accuracy for brain age estimation, the best performance is achieved when multiple MRI modalities are combined ( 16 ).
Owing to our use of multimodal brain MRI data to estimate brain age, combined with the large sample size, we were able to detect a modest but highly statistically significant association between prediabetes ( P < 0.001) and higher BAG. In light of conflicting findings on the relationship between prediabetes and cognitive impairment and dementia ( 7–10 ), our results provide compelling evidence that prediabetes may accelerate brain aging during the very earliest stages of dementia development. Given the substantial and growing prevalence of prediabetes—estimated at ∼9% of the global population ( 35 )—even a modest effect of prediabetes on brain health could make a substantial difference at the population level. Encouragingly, prediabetes is a reversible state, and population-based studies have demonstrated that it is more common for people with prediabetes to regress to normoglycemia than progress to overt diabetes ( 36 , 37 ). Potential benefits for brain health could be yet another motivation to tighten glycemic control during this critical window.
Considering the heterogeneity of the diabetes population, we additionally investigated the role of a variety of other biological factors in the relationship between (pre)diabetes and brain age. In stratified analyses, the association between diabetes and higher BAG was more pronounced in men compared with women (2.63 vs. 1.76 years) and people with two or more as opposed to zero or one cardiometabolic risk factors (3.08 vs. 1.96 years). The prediabetes-BAG association was also stronger in men (0.75 vs. 0.27 years) and people with a higher cardiometabolic risk factor burden (1.32 vs. 0.24 years). Two previous studies have also reported a stronger relationship between brain age and diabetes among men ( 13 , 15 ), and the stronger diabetes-BAG association in in the context of a poorer cardiometabolic health is generally consistent with what has been observed for the diabetes-dementia association ( 2 , 3 ). These results highlight the complex interplay between hyperglycemia, sex, and cardiometabolic factors on brain health and underscore the importance of identifying populations that may benefit most from preventative interventions.
Although lifestyle behaviors such as a healthy diet, smoking/alcohol avoidance, physical activity, and social engagement have been associated with younger brain age ( 12 , 16 , 19 , 20 ), a relevant and so-far unexplored question is whether a healthy lifestyle can counteract the damaging influence of existing risk factors, such as diabetes, on brain aging. In our study, a lifestyle characterized by high physical activity and avoidance of smoking and heavy drinking significantly attenuated the association between diabetes and higher BAG. These results provide the encouraging suggestion that adoption of these healthy lifestyle behaviors could improve brain health among people with diabetes, although interventional studies are warranted to verify this hypothesis. Our findings are consistent with previous studies highlighting the mitigating role of lifestyle behaviors in the association between diabetes and dementia ( 38 , 39 ) and emphasize the significance of a healthy lifestyle for not only cardiometabolic health but also the brain.
There are several potential biological pathways through which (pre)diabetes may impact brain health. Hyperglycemia, the defining pathophysiological feature of diabetes, can promote endothelial dysfunction, oxidative stress, systemic inflammation, and the accumulation of advanced glycation end products ( 1 ). Together these contribute to disruption of blood-brain barrier permeability (exposing the brain to potentially toxic substances, leading to abnormal neuronal activity), demyelination and loss of axons (leading to brain atrophy and disruptions in neurotransmitter signaling), and alterations in Ca 2+ signaling (leading to excitotoxicity and disruptions in gene expression) ( 1 ). Additionally, the micro- and macrovascular complications of diabetes can contribute to brain atherosclerosis and cerebrovascular pathologies that may lower the threshold for neurodegeneration ( 1 ). Finally, the insulin resistance that characterizes diabetes has been linked to AD-related processes, including amyloid-β generation, τ-hyperphosphorylation, and impaired amyloid-β clearance ( 1 ). A healthy lifestyle may enhance cardiovascular and metabolic health, thereby minimizing the impact of hyperglycemia, insulin resistance, and vascular damage.
Strengths of this study include the large sample size and the use of multimodal brain MRI data to estimate brain age. However, some limitations should be acknowledged. First, healthy volunteer bias in the UK Biobank could limit the generalizability of our findings and may have contributed to an underestimation of the observed associations. Selection bias may be stronger in our sample because it was restricted to participants who underwent a brain MRI scan, a comparatively younger and more cardiometabolically healthy subgroup ( Supplementary Table 9 ).
Second, diet could not be considered in the healthy lifestyle construct due to a high proportion of missing data (35%); additional analyses integrating diet into the optimal lifestyle measure are presented in Supplementary Table 18 .
Third, there is the possibility of reverse causality insofar as having an older brain may contribute to the development of (pre)diabetes by making it more difficult to manage medical conditions and adhere to a healthy lifestyle. However, results remained consistent in sensitivity analyses excluding participants with possible cognitive impairment or prodromal dementia ( Supplementary Table 16 ), suggesting that reverse causality is unlikely to have a major impact on our findings.
Additionally, misclassification of baseline glycemic status may have occurred because HbA 1c is less sensitive than alternative measures such as fasting plasma glucose or the oral glucose tolerance test ( 40 ). Moreover, because HbA 1c was measured only at baseline, we could not assess changes in glycemic control or progression/reversion of prediabetes in relation to BAG.
Finally, longitudinal data were available for only 2,414 participants (7.7%). Repeat collection of brain MRI scans is still ongoing, presenting an opportunity for future studies to explore the longitudinal relationship between (pre)diabetes and brain aging in greater detail.
In conclusion, the current study provides evidence that hyperglycemia—including diabetes and even prediabetes—may contribute to accelerated brain aging. These associations were more pronounced in men and people with poorer cardiometabolic health but were attenuated with a healthy lifestyle characterized by physical activity and abstention from smoking and heavy drinking. Our findings highlight diabetes and prediabetes as ideal targets for lifestyle-based interventions to promote brain health.
This article contains supplementary material online at https://doi.org/10.2337/figshare.26417971 .
G.P. and W.X. are co-last authors.
Acknowledgments. The authors would like to express their gratitude to the UK Biobank study participants and the staff involved in the UK Biobank data collection and management.
Funding. A.D. received funding from Alzheimerfonden (AF-993470) and Demensfonden. W.X. received grants from the Swedish Research Council (No. 2021-01647), the Swedish Council for Health, Working Life and Welfare (No. 2021-01826), Alzheimerfonden, and the Karolinska Institutet Board of Research. G.P. received funding from the Riksbankens Jubileumsfond (No. P20-0779) and Swedish Research Council (No. 2019-02804). M.G.M. received funding from the Marianne and Markus Wallenberg Foundation (No. 2020.0013) and Swedish Research Council (No. 2021-02046).
Duality of Interest. No potential conflicts of interest relevant to this article were reported.
Author Contributions. A.D. conducted the statistical analyses, performed the literature search, and drafted the first version of the manuscript. A.D. and W.X. contributed to the conception and design of the study. J.W. and H.H. created the brain age variable. J.W., H.H., M.M.D., S.S., M.G.-M., G.P., and W.X. interpreted the data and provided critical revisions to the manuscript. All authors made a significant contribution to finalizing the manuscript and approved the final version for publication. A.D. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Handling Editors. The journal editors responsible for overseeing the review of the manuscript were Elizabeth Selvin and Alka M. Kanaya.
Email alerts
- Online ISSN 1935-5548
- Print ISSN 0149-5992
- Diabetes Care
- Clinical Diabetes
- Diabetes Spectrum
- Standards of Medical Care in Diabetes
- Scientific Sessions Abstracts
- BMJ Open Diabetes Research & Care
- ShopDiabetes.org
- ADA Professional Books
Clinical Compendia
- Clinical Compendia Home
- Latest News
- DiabetesPro SmartBrief
- Special Collections
- DiabetesPro®
- Diabetes Food Hub™
- Insulin Affordability
- Know Diabetes By Heart™
- About the ADA
- Journal Policies
- For Reviewers
- Advertising in ADA Journals
- Reprints and Permission for Reuse
- Copyright Notice/Public Access Policy
- ADA Professional Membership
- ADA Member Directory
- Diabetes.org
- X (Twitter)
- Cookie Policy
- Accessibility
- Terms & Conditions
- Get Adobe Acrobat Reader
- © Copyright American Diabetes Association
This Feature Is Available To Subscribers Only
Sign In or Create an Account

IMAGES
VIDEO
COMMENTS
It is a crucial component in the care of people with type 1 diabetes, and it becomes increasingly important in the care of patients with type 2 diabetes who have a constellation of comorbidities, all of which must be managed for successful disease outcomes.
Abstract. Unhealthy behaviours, including diet and physical activity, coupled with genetic predisposition, drive type 2 diabetes (T2D) occurrence and severity; the present review aims to summarise the most recent nutritional approaches in T2D, outlining unmet needs. Guidelines consistently suggest reducing energy intake to counteract the ...
Type 2 diabetes (T2D) is a rapidly growing epidemic, which leads to increased mortality rates and health care costs. Nutrients (namely, carbohydrates, fat, protein, mineral substances, and vitamin), sensing, and management are central to metabolic homeostasis, ...
Introduction Prevalence of type 2 diabetes (T2D) is increasing rapidly and lifestyle interventions to reverse diabetes are seen as a possible solution to stop this trend. New practice-based evidence is needed to gain more insight in the actual, and above all scientific, basis for these claims. Methods This observational study with a pretest post-test design aimed to pilot a 6-month ...
The nutrition Consensus Report (1) and four featured papers (2 - 5) in the special section on nutrition in this issue of Diabetes Care focus on nutrition therapy and medical nutrition therapy (MNT) in the management and prevention of diabetes.
Backgrounds Although the significance of diet in preventing or managing diabetes complications is highlighted in current literature, there is insufficient evidence regarding the correlation between nutrient patterns and these complications. The objective of this case-control study is to investigate this relationship by analyzing the dietary intake of nutrients in participants with and without ...
This randomized clinical trial compared the effects of an intensive lifestyle intervention vs standard care on glycemic control and medication reduction among participants with non-insulin-dependent type 2 diabetes.
Background: Although the body of evidence indicates clear benefits of dietary modifications for prevention of type-2 diabetes mellitus (T2DM), it may be difficult for healthcare providers to recommend which diet interventions or dietary factors are appropriate ...
Abstract Objective: The objective of this Expert Consensus Statement is to assist clinicians in achieving remission of type 2 diabetes (T2D) in adults using diet as a primary intervention. Evidence-informed statements agreed upon by a multi-disciplinary panel of expert healthcare professionals were used.
Nutrition therapy provided by dietitians was associated with better clinical parameters of type 2 diabetes, including clinically significant improved glycaemic control, across diverse multiethnic patient groups from all six inhabited continents. This conclusion should be reflected in clinical guidel …
This Review highlights the evidence from clinical trials that diet-induced weight loss interventions can be used to treat type 2 diabetes mellitus. The composition of the diet and the importance ...
Abstract Purpose: Type 2 diabetes mellitus represents a significant health problem. Many studies have reported that intensive nutritional intervention by itself or in addition to medications is the best method to improve glycaemic control in type 2 diabetes mellitus.
Introduction A wealth of evidence supports short-term efficacy of lifestyle interventions in type 2 diabetes (T2D). However, little is known about long-term effects of lifestyle interventions in real-life settings. Methods This observational, single-arm study evaluated long-term impact of 'Voeding Leeft: Reverse-Diabetes2-Now', a 6-month multicomponent lifestyle programme, on glycaemic ...
Common ground on dietary approaches for the prevention, management, and potential remission of type 2 diabetes can be found, argue Nita G Forouhi and colleagues Dietary factors are of paramount importance in the management and prevention of type 2 diabetes. Despite progress in formulating evidence based dietary guidance, controversy and confusion remain. In this article, we examine the ...
Questions What type of lifestyle modification should be prescribed for L.H.? What was the outcome after 2 months of treatment? Can risk factors for diabetes be reversed in a short period of time with intense exercise and diet?
To investigate the effects of combined diet-and-exercise interventions in patients with type 2 diabetes mellitus (T2DM). ... Further studies are necessary to compare the effectiveness of other diet-and-exercise combinations than those analyzed in this review and to clarify the role of other interacting factors, e.g. patients' medication. ...
Comprehensive lifestyle interventions are effective strategies to prevent type 2 diabetes among at-risk populations in LMICs. The heterogeneity identified in our results should be considered when using these interventions to address the onset of type 2 diabetes.
Roy Taylor and colleagues explain how type 2 diabetes can be reversed by weight loss and avoidance of weight regain Type 2 diabetes mellitus was once thought to be irreversible and progressive, but a series of clinical studies over the past 12 years have clarified the mechanisms that cause the disease. We now know that the processes that cause type 2 diabetes can be returned to normal ...
Aims/hypothesis We conducted the largest and longest clinical trial comparing a whole-food, plant-based intervention with standard medical care (SMC) in individuals with type 2 diabetes. Methods We randomised (parallel-arm; computerised 1:1 randomisation ratio) 169 adults aged 18-75 years with type 2 diabetes in the Marshall Islands to an intensive whole-food, plant-based intervention with ...
Abstract Type 2 diabetes mellitus (T2DM) accounts for >90% of the cases of diabetes in adults. Resistance to insulin action is the major cause that leads to chronic hyperglycemia in diabetic patients. T2DM is the consequence of activation of multiple pathways and factors involved in insulin resistance and β-cell dysfunction.
The comparative effectiveness of glucose-lowering medications for use with metformin to maintain target glycated hemoglobin levels in persons with type 2 diabetes is uncertain. In this trial involv...
Biotechnol., 15 September 2020. The Role of Nutrition in the Prevention and Intervention of Type 2 Diabetes. Type 2 diabetes (T2D) is a rapidly growing epidemic, which leads to increased mortality rates and health care costs. Nutrients (namely, carbohydrates, fat, protein, mineral substances, and vitamin), sensing, and management are central to ...
Studies such as PLATEDIAN (Telemedicine on Metabolic Control in Type 1 Diabetes Mellitus Andalusian Patients), provide positive results and conclude that a telemedicine intervention has a similar impact to the traditional office-based intervention in terms of glycemic control, acute complications, and perceived QOL (Ruiz de Adana et al., 2020).
Study with Quizlet and memorize flashcards containing terms like Mandana is given a prescription for metformin, a biguanide medication prescribed for management of type 2 diabetes. What best describes how metformin works to control blood glucose? a. It delays carbohydrate digestion. b. It increases urinary glucose excretion. c. It delays stomach emptying. d. It improves insulin secretion. e ...
Aims and objectives To assess the effect of an instructional WhatsApp group on self-care and HbA1c levels among female patients with type 2 diabetes mellitus (T2DM). Background T2DM is a chronic disease that requires effective self-care. WhatsApp is a free application that can be effectively used for patient education. Design This study used a quasi-experimental design. Methods A convenience ...
Background Type 2 diabetes mellitus has been identified as one of the most challenging chronic illnesses to manage. Since the management of diabetes is mainly accomplished by patients and families, self-management has become the mainstay of diabetes care. However, a significant proportion of patients fail to engage in adequate self-management.
Introduction Gestational diabetes mellitus (GDM) is one of the most common medical complications of pregnancy. Glycaemic control decreases the risk of adverse pregnancy outcomes for the affected pregnant individual and the infant exposed in utero. One in four individuals with GDM will require pharmacotherapy to achieve glycaemic control. Injectable insulin has been the mainstay of ...
Type 2 diabetes (hereafter, diabetes) is a well-established risk factor for cognitive impairment and has been associated with approximately double the risk of dementia ().In brain MRI studies, diabetes has been related to global brain atrophy, increased burden of small-vessel disease, and microstructural lesions before the onset of cognitive symptoms ().