Your browser is not supported
Sorry but it looks as if your browser is out of date. To get the best experience using our site we recommend that you upgrade or switch browsers.
Find a solution
- Skip to main content
- Skip to navigation

- Back to parent navigation item
- Collections
- Sustainability in chemistry
- Simple rules
- Teacher well-being hub
- Women in chemistry
- Global science
- Escape room activities
- Decolonising chemistry teaching
- Teaching science skills
- Get the print issue
- RSC Education

- More navigation items
Source: Royal Society of Chemistry

Related video

Cosmetics, technical services chemist

Illustrate polymer properties with a self-siphoning solution

Demonstrate changes of state using volume differences
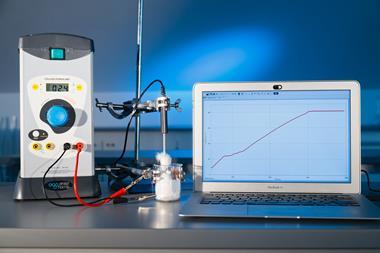
Demonstrating the heating curve of tert-butanol

Dynamite soap: The combustion of stoichiometric hydrogen–oxygen mixtures
Diffusion in action.

Ammonia meets hydrogen chloride in this classic diffusion demo
Watch the video and download the technician notes from the Education in Chemistry website: rsc.li/XXXXXXX
Download this
The technician notes as MS Word or pdf
Download all
Diffusion is one of the most common concepts taught to students – from early introductions to particles through transport in biology and kinetic theory in physics and chemistry. One of its most ubiquitous demonstrations is the reaction of ammonia with hydrogen chloride – but that doesn’t mean it’s not worth revisiting. This way, we can ensure we achieve the best and safest results, as well as the most authentic discussion of their meaning. Following a recent disappointment when rehearsing this demo, not only did I revisit the most recent CLEAPSS method – which rewarded me with some great tips for doing it even better – but I also gave some thought to the limitations of the conclusions that can be drawn from it.
Diffusion is one of the most common concepts taught to students – from early introductions to particles through transport in biology and kinetic theory in physics and chemistry. One of its most ubiquitous demonstrations is the reaction of ammonia with hydrogen chloride – but that doesn’t mean it’s not worth revisiting. This way, we can ensure the best and safest results are achieved, as well as the most authentic discussion of their meaning. Following a recent disappointment when rehearsing this demo, not only did I revisit the most recent CLEAPSS method ( LINK ) – which rewarded me with some great tips for doing it even better – but I also gave some thought to the limitations of the conclusions that can be drawn from it.
- 4 cm 3 concentrated hydrochloric acid (corrosive)
- 4 cm 3 concentrated 880 ammonia solution (corrosive (skin/eyes), irritating (respiratory), very toxic to aquatic life)
- 2 sample vials
- 2 100 cm 3 beakers
- 2 cotton buds
- Cotton wool
- 2 elastic bands
- Glass tube for gases to diffuse in, with bungs to fit each end (ideally approx 1 m long)
- Clamp and stand
- Large beaker of water for disposal
- Black paper
- Gloves (CLEAPSS members should consult GL349 )
- Splash-proof goggles
Preparation
Secure a glass tube horizontally in a clamp where the demonstration will take place. Check the bungs fit in the ends of the tube. Placing a piece of black paper behind the tube will improve the visibility of the white product.
Bore a small hole in each bung to snugly fit the stem of a cotton bud. Trim the bud to a length such that when inserted into the narrow end of the bung and placed in the sample vial, the tip of the bud touches the base of the vial. Once the stem of the bud has been secured in the bung, wrap extra wool around the end of the bud and hold this in place with an elastic band.
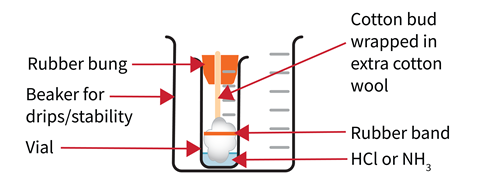
Set up your vial inside a beaker
Wear splash-proof goggles and work in a running fume cupboard. Place the vials into 100 cm 3 beakers to catch drips and reduce the risk of tipping. Add 4 cm 3 of concentrated hydrochloric acid to one sample vial and 4 cm 3 of concentrated ammonia to the other. When ready for the demonstration, take them to the bench and leave them at least one metre away from each other either end of the clamped tube.
In front of the class
Wearing gloves, dip the buds into their corresponding vials to absorb the liquids. Work with a colleague if possible to insert the two buds into the ends of the tube at the same time and place the beakers under the ends of the glass tube to catch any drips. After a couple of minutes, a white ring of ammonium chloride will form where the two gases meet. This will be closer to the source of the hydrogen chloride than to the source of the ammonia.
Safety and disposal
- Avoid skin contact with the chemicals – wear gloves and splash-proof goggles.
- Always use chemicals from a new or recently opened bottle. Open bottles of concentrated ammonia with caution in a working fume cupboard as pressure can build up, especially on warm days.
- Once the demonstration is complete, both buds and bungs can be placed in a waiting large beaker of water to dilute the remaining acid and base before washing down the sink with plenty of water.
Teaching goal
This demonstration is an excellent display of diffusion in action – especially when coupled with some of Bob Worley’s famous ‘ puddle experiments ’. The fact that the hydrogen chloride clearly travels a shorter distance than the ammonia is worth pointing out to students to illustrate the relationship between mass and kinetic energy of the relevant molecules. You can stretch the model still further to illustrate how Graham’s law is fraught with issues because the gases are not diffusing into each other directly, but rather through air.
Given the temperatures of the gases are the same, a common approach might be to suggest that their average kinetic energy and time taken for them to travel to the point of reaction must be the same. As such, the kinetic energy expression ( KE = ½mv 2 ) for the two gases reduces to the following, where d is the distance travelled by each gas:
d NH3 / d HCl = √36.5/17 ≈ 1.47
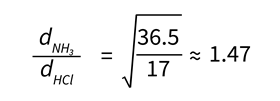
However, this method neglects the fact that the assumptions behind Graham’s law cannot apply. The different sizes of the molecules and their collision cross sections with other molecules come into play. In other words, the rate of diffusion depends not only on the molecules of HCl and NH 3 , but also on the properties of the (mainly) nitrogen and oxygen molecules into which they are diffusing. As such, the observed ratio may be slightly less than that predicted in the simplistic model above – the demo is perhaps best kept as a qualitative one at this level.
Technician notes - Ammonium and hydrogen chloride diffusion

More Declan Fleming

Demonstrations with dry ice

Non-burning paper: investigate the fire triangle and conditions for combustion

- Developing teaching practice
- Professional development
- Properties of matter
- Quantitative chemistry and stoichiometry
Latest videos

2024-08-27T06:00:00Z
By Declan Fleming

Research scientist, microplastics

2024-06-10T05:00:00Z
2024-04-22T05:38:00Z
Demonstrate concentration and density with a transition metal colloid cell
2024-02-19T10:06:00Z
Related articles

Diffusion of gases: ammonia and hydrogen chloride
In association with Nuffield Foundation Five out of five
A demonstration to show the diffusion of gases, using ammonia solution and hydrochloric acid. Includes kit list and safety instructions.

How to teach titration post-16
2024-07-08T05:32:00Z By Jo Haywood
Tips for teaching practical titration techniques and the underlying theory

How to teach polymers at post-16
2024-05-28T06:57:00Z By Martin Bluemel
Teaching strategies and resources to help learners master polymers and overcome misconceptions
3 readers' comments
Only registered users can comment on this article., more exhibition chemistry.

2024-08-27T06:00:00Z By Declan Fleming
Explore changes of state and neutralisation reactions with this trio of demonstrations using solid carbon dioxide

2024-06-10T05:00:00Z By Declan Fleming
Use this reworking of the classic non-burning £5 note demonstration to explore combustion with learners aged 11–16 years

2024-04-22T05:38:00Z By Declan Fleming
Demonstrate the tubeless siphon with poly(ethylene glycol) and highlight the polymer’s viscoelasticity to your 11–16 learners
- Contributors
- Print issue
- Email alerts
Site powered by Webvision Cloud
Skip to Content
Other ways to search:
- Events Calendar
G420: Graham’s Law of Diffusion – NH3 and HCl Diffusion
Introduction
A cotton swab is dipped into concentrated hydrochloric acid (producing hydrogen chloride gas) while a second on is dipped into concentrated aqueous ammonia (producing ammonia gas). Both cotton swabs are simultaneously inserted into opposite ends of a long glass Graham’s Law apparatus and placed on an overhead projector. A white ring of solid ammonium chloride forms where the two gases meet inside the tube.
NH3 + HCl → NH4Cl

To Conduct Demonstration
If necessary, protect non-glass surfaces of an overhead projector with clear plastic.
- Dip one Q-tip into concentrated hydrochloric acid (HCl) and a second cotton swab into concentrated aqueous ammonia (NH3).
- Simultaneously insert both cotton swabs into the ends of the glass apparatus securing the rubber septa.
- Place the glass apparatus on the overhead projector. Wait until a while ring of solid ammonium chloride forms where the hydrogen chloride and ammonia gases meet. The ammonia gas, having a lower molecular weight than the hydrogen chloride, will diffuse faster and travel a greater length of the tube. Consequently, the white ring of ammonium chloride will form much closer to hydrochloric acid end of the tube.
Reaction Time : 10 min
Wear safety goggles and latex gloves to protect the eyes and hands from the strong acid and base used in this demonstration. Keep the containers of concentrated hydrochloric acid and aqueous ammonia covered when not dipping the cotton. Work in a well ventilated area and avoid breathing the concentrated vapors of either reagent.
- This demo is more easily seen on an overhead projector than on a document camera with a dark background because of glare which is created by the camera on the dark background.
- Do not store concentrated acid and base in the box with the apparatus and the swabs. The chemicals interact coating everything in the box with a
L.R. Summerlin and J.L. Ealy Jr., Chemical Demonstrations: A Sourcebook for Teachers , 1985.
- G410: Gases – Boyle’s Law
- G430: Pressure and Temperature – The Collapsing Can
- G440: Evaporation and Expansion – The Drinking Bird
- G450: Effusion – Relative Effusion Rates of H2, He, and O2
- G460: Charle's Law
- G420: Prep Notes
- Mailing List
- Terms and Conditions
- © Copyright Notice
Graham’s law of diffusion — a quantitative demonstration
George B. Kauffman, California State University, Fresno Fresno CA

Kudos to Carlos Correa for his "Chemistry in Pictures Winner: Ammonia and hydrochloric acid" on the cover of the March 2014 issue. Chem 13 News readers should be interested in my related demonstration. 1 Not only does it involve active student participation, but it also provides quantitative proof of Graham's law that relates the ratio of the rates of diffusion of two gases.
High school chemistry teachers can use the familiar reaction between gaseous ammonia and hydrogen chloride in a quantitative demonstration of Graham’s law of diffusion (the rate of diffusion of a gas is inversely proportional to the square root of its molecular weight). Suspend a long, scrupulously dry tube horizontally as level as possible with a ring stand and clamp at each end. Saturate separate pieces of cotton with concentrated aqueous ammonia and hydrochloric acid and simultaneously insert them into the ends of the tube, which is then closed with rubber stoppers (Fig. 1). 2
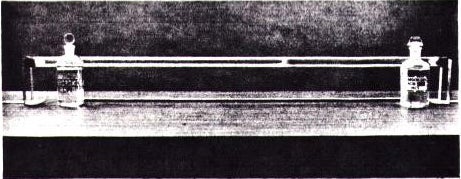
Fig. 1: Diffusion apparatus showing the deposit of ammonium chloride where the gases met.
Ask the class to predict whether the gases will meet at the center of the tube, closer to the NH 3 , or closer to the HCl (the correct answer). Within 10 minutes a white deposit of NH 4 Cl forms at the point where the gases meet:
NH 3 (g) + HCl(g) → NH 4 Cl(s)
Measure the distance of the deposit from each piece of cotton. According to Graham’s law, the ratio of the rates of diffusion is:
rNH 3 /rHCl = (mwHCl/mwNH 3 ) 1/2 = (36.46/17.03) 1/2 = 1.463
Considering the crudity of the apparatus, the absence of a diffusion plug, the presence of convection currents, and the fact that solutions of different concentrations rather than gases are used, the results obtained are usually remarkably close to the theoretical prediction. Tubes ranging in inner diameter from 1.7 cm to 2.4 cm give good results. The larger the diameter of the tube, the faster the deposit is formed, but the more spread out it is. The longer the tube, the better the quantitative results should be, but often this doesn’t happen because convection currents arise during the longer reaction time, causing mixing of the gases. A tube 130 cm long and 1.7 cm inner diameter gives satisfactory results. The deposit forms predominantly on the bottom of the tube because of gravity and the density of the gases.
The formation of NH 4 Cl from NH 3 and HCl can also be used in two related demonstrations.
If you take the stoppers from bottles of concentrated NH 3 and concentrated HCl and hold them within 5 cm of each other, the NH 4 Cl smoke forms on the HCl stopper because the NH 3 diffuses faster than the HCl does. 3
Years ago I did a demonstration to illustrate that students needn’t panic when they spill acids or bases on themselves but have time to go calmly to the sink. I used to invert an open bottle of concentrated NH 3 , the mouth of which is held tightly against the palm of one hand, and then repeat the process with a bottle of concentrated HCl on the other hand. When I brought my hands together, cupped them, and compressed them, smoke rings formed. I was careful not to allow the palms of my hands to touch because the heat of neutralization would have caused burns! (Fig. 2). I imagine this demonstration can be added to the many stories from the “good old days” and would no longer be acceptable for safety reasons.
References and notes
- George B. Kauffman, Ronald D. Ebner, Gaseous Diffusion: A Demonstration of Graham’s Law, Journal of College Science Teaching , September/October 1985 , 15(1), pages 78-79.
- Although it has long been known that “ammonium hydroxide” doesn’t exist, and although this fact was called to the attention of chemistry teachers more than six decades ago (John B. Davis, Ammonia and “Ammonium Hydroxide”, Journal of Chemical Education , October, 1953 , 30(10), page 511), bottles of aqueous ammonia are still almost invariably labeled “ammonium hydroxide, NH 4 OH”. You can use this fact to remind students not to believe everything that they read, even if it is embossed in glass.
- Herbert Franklin Davison, A Collection of Chemical Lecture Experiments, The Chemical Catalog Co., New York NY, 1926 , page 73.
More about September 2014

Department of Chemistry 200 University Ave. W Waterloo, Ontario, Canada N2L 3G1
gcsescience.com 19 gcsescience.com
Elements, Compounds and Mixtures
The Diffusion of Hydrogen Chloride and Ammonia Gas through Air to form Ammonium Chloride .
Cotton wool soaked in concentrated ammonia solution , NH 3 (aq) and concentrated hydrogen chloride solution (also called hydrochloric acid ) H Cl (aq) are placed at each end of a sealed tube . The cotton wool with ammonia solution gives off ammonia molecules ( NH 3 ). The cotton wool with hydrochloric acid gives off hydrogen chloride molecules ( HCl ).
HCl and NH 3 molecules diffuse through the air towards each other. When they meet , they react to form a white powder called ammonium chloride , NH 4 Cl .
Note that lighter ( smaller ) particles move more quickly than heavier ( larger ) ones at the same temperature .
gcsescience.com The Periodic Table Index Chemistry Quiz gcsescience.com
Home GCSE Chemistry GCSE Physics
Copyright © 2015 gcsescience.com. All Rights Reserved.

You are here
Search form.
- Request History
- Links to Applets
- Acceleration (A+0)
- Conservation of Energy (A+5)
- Frames of Reference (A+10)
- Friction (A+12)
- Forces (A+14)
- Gravitation (A+15)
- Linear Inertia (A+20)
- Rotational Inertia (A+25)
- Angular Momentum (A+30)
- Linear Momentum (A+35)
- Motion in One Dimension (A+37)
- Physical Measurements (A+45)
- Projectiles (A+50)
- Rotational Dynamics (A+55)
- Statics and Mechanical Equilibrium (A+60)
- Torque (A+65)
- Vectors (A+70)
- Mechanical Advantage (A+80)
- Chaotic Oscillations (B+5)
- Simple Harmonic Motion (B+10)
- Coupled Harmonic Oscillators (B+15)
- Forced Oscillations/Resonance (B+20)
- Travelling Waves (B+25)
- Superposition : Fourier Principles/Complex Waves (B+30)
- Interference (B+35)
- Sound Spectrum/Sources (B+45)
- Standing Waves/Resonance (B+50)
- Vibrational Modes (B+55)
- Speed of Sound (B+60)
- Doppler Shift (B+65)
- Shock Waves (B+67)
- Music and the Ear (B+70)
- Weather and the Atmosphere (C+0)
- Calorimetry (C+5)
- Carnot Cycle (C+10)
- Conduction (C+15)
- Convection (C+20)
- Engines and Pumps (C+22)
- Fluid Dynamics (C+25)
- Surface Tension and Capillary Action (C+27)
- Fluid Statics (C+30)
- Heat and Work (C+35)
- Heat Capacity (C+40)
- Heat of Fusion (C+45)
- Irreversibility and Fluctuations (C+50)
- Kinetic Theory and Gas Models (C+55)
- Liquification of a Gas (C+60)
- Molecular Models and Crystal Structure (C+62)
- Radiation (C+65)
- Temperature and Expansion (C+70)
- Thermometry (C+75)
- Triple Point (C+80)
- Mechanical Properties of Materials (C+90)
- Capacitance (D+0)
- Electromagnetic Oscillations (D+5)
- Electrostatics (D+10)
- Faraday's Law (D+15)
- Inductance (D+20)
- LCR Phase Relationships (D+25)
- Magnetic Fields (D+30)
- Magnetic Properties (D+35)
- Meters (D+40)
- Motors (D+45)
- Oscilloscopes (D+50)
- Resistance (D+55)
- Solid State and Semiconductors (D+60)
- Thermionic Emission (D+65)
- Thermoelectricity (D+70)
- Transformers (D+75)
- Voltaic Cells (D+80)
- Electrolysis (D+85)
- Color Perception (E+5)
- Diffraction (E+10)
- Gratings and Spectra (E+15)
- Holography (E+20)
- Interference (E+25)
- Lenses (E+30)
- Metallic Color (E+35)
- Mirrors (E+40)
- Polarization and Birefringence (E+45)
- Prisms (E+50)
- Propagation of Light (E+55)
- Refraction and Reflection (E+60)
- Spectral Energy (E+65)
- Cathode and Canal Rays (F+0)
- X-Rays (F+5)
- Electron Diffraction (F+10)
- Photoelectric Effect (F+15)
- Atomic Structure (F+20)
- Photons (F+18)
- Quantum Mechanical Barrier Penetration (F+30)
- Scattering (F+25)
- Elementary Particles (F+35)
- Cloud Chambers (F+45)
- Range of Alpha Particles (F+50)
- Franck-Hertz Experiment (F+55)
- Radioactivity (F+65)
- Accelerators (F+70)
- Fission and Fusion (F+80)
- Superconductivity (F+85)
- Zeeman Effect (F+90)
- Laser (F+95)
- Fuel Cells (F+100)
- Astronomy Slides (G+0)
- Astronomy Models (G+5)
- Optical Illusions (G+55)
- Audio Illusions (G+50)
- Perception (G+60)
- Request new password
Diffusion of Ammonia and HCl.
Primary tabs.
- View (active tab)
- What links here
(C)Properties of Heat and Matter - C+55: Kinetic Theory and Gas Models

UCB Taxonomy:
Popularity:.
- Log in to post comments
everything you need to study Chemistry from Year 8 to Year 13
1.3 diffusion.
Prior knowledge: You will need to have an understanding of how the states of matter differ, in terms of their particles.
Diffusion in liquids
When a drop of ink is placed into a beaker of water, the ink diffuses slowly throughout the whole volume of the water. It does this because both the water molecules and the ink particles are constantly moving, colliding with each other and randomly changing direction. It follows that the hotter the water is, the faster the particles will be moving around and the faster the ink will diffuse throughout the volume.

Diffusion in gases
To demonstrate diffusion in gases, a long glass tube is set up with cotton wool soaked with hydrochloric acid at one end, and cotton wool soaked with ammonia at the other end.

The hydrogen chloride and the ammonia gases diffuse along the tube from either end, because the particles are constantly, randomly moving. Where they meet, they react forming a white ‘smoke ring’ of ammonium chloride.
ammonia + hydrogen chloride → ammonium chloride
The smoke ring is not formed in the middle of the tube, but nearer to the end with the hydrochloric acid. This tells us that the ammonia molecules travel further than the hydrogen chloride molecules in the same amount of time, in other words they diffuse more quickly. This is because they are lighter: ammonia has a mass of 17 units while hydrogen chloride has a mass of 36.5 units.
Share this:

- Copy shortlink
- Report this content
- Manage subscriptions
An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
- My Bibliography
- Collections
- Citation manager
Save citation to file
Email citation, add to collections.
- Create a new collection
- Add to an existing collection

Add to My Bibliography
Your saved search, create a file for external citation management software, your rss feed.
- Search in PubMed
- Search in NLM Catalog
- Add to Search
The counterdiffusion of HCl and NH 3 : An experimental and modeling analysis of topochemistry, diffusion, reaction, and phase transitions
Affiliations.
- 1 Department of Chemistry, Colorado State University, Fort Collins, Colorado 80523, USA.
- 2 Department of Mathematics, Colorado State University, Fort Collins, Colorado 80523, USA.
- 3 Department of Mathematics, Louisiana State University, Baton Rouge, Louisiana 70803, USA.
- 4 Department of Chemical and Biological Engineering, Colorado State University, Fort Collins, Colorado 80523, USA.
- PMID: 31005123
- DOI: 10.1063/1.5083927
Vapor-phase ammonia, NH 3(g) , and hydrochloric acid, HCl (g) , undergo a series of complex reactions, including nucleation and growth, to form solid ammonium chloride, NH 4 Cl (s) . The counterdiffusional experiment, whereby HCl (g) and NH 3(g) diffuse from opposite ends of a tube and react to form spatiotemporally complex patterns, has a rich history of study. In this paper, we combine experimental data, molecular simulations, and analysis and simulations of a partial differential equation model to address the questions of where the first unobserved vapor product NH 4 Cl (g) and visually observable precipitate NH 4 Cl (s) form and how these positions depend on experimental parameters. These analyses yield a consistent picture which involves a moving reaction front as well as previously unobserved heterogeneous nucleation, wall nucleation, and homogeneous nucleation. The experiments combined with modeling allow for an estimate of the heterogeneous and homogeneous nucleation thresholds for the vapor-to-solid phase transition. The results, synthesized with the literature on this vapor-to-particle reaction, inform a discussion of the details of the reaction mechanism, including the role of water, which concludes the paper.
PubMed Disclaimer
Similar articles
- Ammonia vapour in the mouth as a diagnostic marker for Helicobacter pylori infection: preliminary 'proof of principle' pharmacological investigations. Dun CDR, Blac M, Cowell DC, Penaul C, Ratcliffe NM, Spence R, Teare C. Dun CDR, et al. Br J Biomed Sci. 2001;58(2):66-75. Br J Biomed Sci. 2001. PMID: 11440209
- Nucleation probability in binary heterogeneous nucleation of water-n-propanol vapor mixtures on insoluble and soluble nanoparticles. Wagner PE, Kaller D, Vrtala A, Lauri A, Kulmala M, Laaksonen A. Wagner PE, et al. Phys Rev E Stat Nonlin Soft Matter Phys. 2003 Feb;67(2 Pt 1):021605. doi: 10.1103/PhysRevE.67.021605. Epub 2003 Feb 24. Phys Rev E Stat Nonlin Soft Matter Phys. 2003. PMID: 12636690
- Smog chamber study of the role of NH 3 in new particle formation from photo-oxidation of aromatic hydrocarbons. Li K, Chen L, White SJ, Yu H, Wu X, Gao X, Azzi M, Cen K. Li K, et al. Sci Total Environ. 2018 Apr 1;619-620:927-937. doi: 10.1016/j.scitotenv.2017.11.180. Epub 2017 Nov 29. Sci Total Environ. 2018. PMID: 29734638
- Recent developments in the kinetic theory of nucleation. Ruckenstein E, Djikaev YS. Ruckenstein E, et al. Adv Colloid Interface Sci. 2005 Dec 30;118(1-3):51-72. doi: 10.1016/j.cis.2005.06.001. Epub 2005 Aug 30. Adv Colloid Interface Sci. 2005. PMID: 16137628 Review.
- Vapor-liquid nucleation: the solid touch. Yarom M, Marmur A. Yarom M, et al. Adv Colloid Interface Sci. 2015 Aug;222:743-54. doi: 10.1016/j.cis.2014.07.011. Epub 2014 Aug 6. Adv Colloid Interface Sci. 2015. PMID: 25172583 Review.
Related information
Linkout - more resources, full text sources.
- Silverchair Information Systems

- Citation Manager
NCBI Literature Resources
MeSH PMC Bookshelf Disclaimer
The PubMed wordmark and PubMed logo are registered trademarks of the U.S. Department of Health and Human Services (HHS). Unauthorized use of these marks is strictly prohibited.
Thank you for visiting nature.com. You are using a browser version with limited support for CSS. To obtain the best experience, we recommend you use a more up to date browser (or turn off compatibility mode in Internet Explorer). In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript.
- View all journals
- Explore content
- About the journal
- Publish with us
- Sign up for alerts
- Review Article
- Open access
- Published: 30 August 2024
Hygroscopic behavior and aerosol chemistry of atmospheric particles containing organic acids and inorganic salts
- Fang Tan 1 ,
- Hongbin Zhang 1 ,
- Kaihui Xia ORCID: orcid.org/0009-0006-4826-5085 2 ,
- Bo Jing ORCID: orcid.org/0000-0001-7741-5384 3 ,
- Xiaohong Li 1 ,
- Shengrui Tong ORCID: orcid.org/0000-0003-2432-5830 4 &
- Maofa Ge ORCID: orcid.org/0000-0002-1771-9359 4 , 5
npj Climate and Atmospheric Science volume 7 , Article number: 203 ( 2024 ) Cite this article
1 Altmetric
Metrics details
- Atmospheric chemistry
- Environmental impact
Aerosol hygroscopic behavior plays a central role in determining climate effects and environmental influence of atmospheric particulates. Water-soluble organic acids (WSOAs) constitute a significant fraction of organic aerosols. These organic acids have a complex impact on aerosol hygroscopicity due to their physical and chemical interactions with atmospheric inorganic salts. The mixing of WSOAs with inorganic salts exerts a multiple influence on the hygroscopic growth and phase behaviors of aerosol particles, largely depending on the composition ratio, acid properties, particle size and interactions between particle components. The WSOAs play a critical role in determining water uptake characteristics of aerosol particles, especially in the low and moderate RH ranges. The previous studies reveal the occurrence of aerosol chemistry related to chloride/nitrate/ammonium depletions in aerosol droplets containing WSOAs and inorganic salts. The potential influence of WSOAs on the atmospheric recycling of HCl/HNO 3 /NH 3 due to the chloride/nitrate/ammonium depletion may contribute to the atmospheric budget of reactive gases. A fundamental understanding for the hygroscopic behavior and aerosol chemistry of inorganic–WSOA systems is essential for the accurate parameterization of aerosol behaviors in atmospheric models. However, there is still lack of a comprehensive understanding of the hygroscopicity and related aerosol chemistry of internally mixed inorganic–WSOA systems. The present review comprehensively summarizes the impacts of WSOAs on hygroscopicity and phase changes of atmospherically relevant inorganic salts in aerosol particles especially under subsaturated conditions, and overviews the recent advances on aerosol chemistry related to the hygroscopic process for the internally mixed inorganic–WSOA aerosols.
Similar content being viewed by others

Global variability in atmospheric new particle formation mechanisms
Global organic and inorganic aerosol hygroscopicity and its effect on radiative forcing
Characteristics and optical properties of atmospheric aerosols based on long-term AERONET investigations in an urban environment of Pakistan
Introduction.
Atmospheric aerosols exert a considerable influence on the Earth’s climate by directly absorbing or scattering solar radiation and by indirectly modifying cloud properties through acting as cloud condensation nuclei (CCN) or ice nuclei (IN) 1 , 2 , 3 . Aerosol particles can absorb and release water when they undergo relative humidity (RH) cycles, which is generally termed as hygroscopic property. Aerosol hygroscopicity plays a central role in determining climate effects and environmental influence of atmospheric particulates. Water uptake can govern the liquid water content, composition, size and phase state (liquid, semisolid, or solid) of aerosol particles. The liquid water in the particle phase could serve as a medium for aqueous-phase and multiphase chemistry, facilitating the formation of sulfate, nitrate and secondary organic aerosols (SOAs) through the partitioning of reactive species between the gas phase and the particle phase and subsequent aqueous-phase or multiphase processing 4 , 5 , 6 , 7 , 8 , 9 , 10 .
The hygroscopic process could lead to phase changes of aerosol particles in addition to changes in the liquid water content. The phase behavior of aerosols controls the ability of atmospheric particles to impact air quality and climate, due to the fact that the types of particle phase states have a critical influence on aerosol properties and processes including reactivity, hygroscopic and optical properties 11 . Aerosol particles under atmospherically relevant conditions may undergo phase transitions including the deliquescence transition, the efflorescence transition and the liquid-liquid phase separation (LLPS) when exposed to varying RH. The deliquescence transition refers to the phase transformation of the solid phase into the aqueous phase when a solid particle is exposed to increasing RH (i.e., hydration process). The crystalline substances such as (NH 4 ) 2 SO 4 and NaCl exhibit no apparent water uptake before they undergo prompt deliquescence transitions at a definite RH, indicated as deliquescence relative humidity (DRH) or deliquescence point. Amorphous substances such as nitrates and organics could absorb water continuously and undergo gradual deliquescence far below the deliquescence point of their crystalline counterparts 12 , 13 . When exposed to the decreasing RH (i.e., dehydration process), the aqueous particle may undergo a distinct efflorescence transition with the phase transformation of the aqueous phase into the crystalline solid phase at a definite RH, indicated as efflorescence relative humidity (ERH) or crystallization relative humidity (CRH). In contrast to the thermodynamics-determined deliquescence process, the efflorescence process is kinetically controlled due to the presence of the activation barrier for the formation of a crystal nucleus. The aqueous particles would become supersaturated with solute rather than undergo efflorescence or crystallization at the DRH. As a result, aerosol efflorescence transitions generally occur at a much lower RH than that at which deliquescence transitions occur, and thus a hysteresis exists between the two phase-transitions for the same aerosol particle. Apparently, considering the RH history instead of just the ambient RH is crucial for determining the phase state (liquid, solid) of aerosol particles. In addition to deliquescence and efflorescence transitions, aerosol particles containing organics may undergo LLPS during the RH cycles, resulting in a two–phase (or three liquid phases 14 ) with a core–shell or partially engulfed morphology 15 , 16 , 17 , 18 .
Atmospheric particles have complex hygroscopic behaviors. The current findings reveal that ambient aerosols may exhibit sudden or gradual deliquescence transitions, which are strongly correlated with aerosol inorganic composition such as nitrate and ammoniated sulfate 19 , 20 , 21 , 22 . It was found that deliquescence may occur for aerosols with a relatively large size but vanish for smaller aerosols in ambient air 23 . Field measurements also observed that atmospheric particles can undergo crystallization followed by gradual deliquescence with RH fluctuating down and up, suggesting the importance of RH history on aerosol phase transitions 22 . Ambient organic aerosols generally show gradual water uptake without obvious phase transitions 24 and exist in amorphous or liquid states 25 , 26 , 27 , 28 . The hygroscopicity and phase behavior of atmospheric particles is substantially dependent on the particle properties including the chemical composition, particle-size and mixing state, and on ambient conditions such as RH and temperature 29 , 30 , 31 . Aerosol particles are generally composed of multicomponent inorganic–organic mixtures. The inorganic fractions are dominated by sulfate and nitrate salts for continental aerosols and NaCl for sea spray aerosols. Organic material accounts for 20% to 90% in fine particulate mass, for which a considerable fraction of the organic aerosol is identified as water-soluble organic acids (WSOAs) 11 , 32 . The WSOAs contain a series of organic acids such as formic and acetic acid, low molecular weight dicarboxylic acids, and some multifunctional organic acids. Due to the influence of volatility on the partitioning of WSOAs to the particle phase, the low molecular weight dicarboxylic acids and multifunctional organic acids with relatively low vapor pressure can exist stably in aerosol particles and have a substantial impact on the hygroscopic behavior of atmospheric particles 33 , 34 . Field measurements show that oxalic acid is the most prevalent dicarboxylic acid, typically followed by malonic and succinic acids 32 . The hygroscopic behavior of WSOAs in aerosol particles could deviate substantially from the bulk counterparts. For example, some WSOA particles exhibit gradual and considerable water uptake at much lower RH than their deliquescence point measured by bulk methods 33 , 34 , 35 , 36 . It is found that the single or mixed WSOA aerosol particles tend to exist as liquid or amorphous semi-solid, resulting in the humidity-induced glass transition and continuous water uptake during the hygroscopic process 12 , 13 , 37 . This is consistent with the field and laboratory observations for ambient organic aerosols, which can adopt a highly viscous liquid, semi-solid, or glass state under atmospherically relevant conditions 25 , 28 , 38 . WSOAs such as dicarboxylic acids could exhibit surface activity with enhanced surface concentrations in aqueous aerosols and cloud droplets, resulting in considerable redistribution of surface-active organic acid molecules from the interior bulk of a droplet to the surface phase, termed bulk–surface partitioning 39 , 40 . This redistribution phenomenon plays a large role in physicochemical properties including the CCN activity of aerosol particles 39 , 40 , 41 . In addition, organic acid dissociation regulated by the droplet surface may also influence the cloud response to organic aerosols 42 .
The mixing of WSOAs with atmospherically relevant inorganic salts exerts a complex influence on the hygroscopic growth and phase behavior of internally mixed systems, largely depending on the composition ratio, acid properties, particle size and interactions between particle components 30 , 43 , 44 , 45 , 46 , 47 , 48 , 49 , 50 . The presence of WSOAs may promote or inhibit the crystallization of inorganic salts, enhance or reduce water uptake by aerosol particles, and even shape the particle structure such as phase-separated and gel structures 30 , 43 , 47 , 48 , 51 , 52 , 53 . The unique phase behaviors including LLPS and surface partitioning of WSOA molecules in aqueous particles containing WSOAs and inorganic salts can even enhance cloud droplet activation, which are not considered in classic Köhler theory 54 , 55 , 56 . Atmospherically relevant inorganic salts are found to enhance the enrichment of organic acid molecules on the aqueous aerosol surface, thus regulating the CCN activity of atmospheric particles 39 , 41 . In addition, the aerosol chemistry related to the formation of organic salts has been observed for mixtures of WSOAs and inorganic salts in aqueous aerosols undergoing the dehydration process 49 , 57 , 58 , leading to the modified particle composition and variation in hygroscopicity 45 , 47 , 59 . Aerosol thermodynamic models developed for internally mixed systems composed of inorganics and dicarboxylic acids have not incorporated the phase behavior and aerosol chemistry, which leads to the deficiency in modeled water uptake by inorganic–WSOA aerosol systems 36 , 47 , 48 , 60 , 61 . A fundamental understanding for the hygroscopic behavior and aerosol chemistry of inorganic–WSOA systems is essential for the accurate parameterization of aerosol hygroscopicity in atmospheric models. However, there is still a lack of a comprehensive picture on a molecular scale for the hygroscopic behavior and related aerosol chemistry of inorganic–WSOA systems. The present review comprehensively summarizes the impacts of WSOAs on hygroscopicity and phase changes of atmospherically relevant inorganic salts in aerosol particles especially under subsaturated conditions, and overviews the recent advances on aerosol chemistry related to the hygroscopic process for inorganic–WSOA aerosol systems. This review is structured as follows: introducing measurement techniques and modeling methods for aerosol hygroscopicity; summarizing hygroscopicity and phase behaviors of WSOAs and their internally mixed systems with inorganic salts; overviewing recent advances on aerosol chemistry between WSOAs and inorganic salts; assessing open and unresolved questions that remain, and the implications of a better understanding of water uptake behaviors of atmospheric particles containing WSOAs and inorganic salts.
Methodologies in characterizing aerosol hygroscopicity
Measurement techniques.
In the past decades, a large number of experimental approaches have been developed to characterize the hygroscopic behavior and water content of aerosol particles generated in laboratories and collected from ambient conditions. These approaches are complementary in their capabilities, inferring water content, morphology, phase state and compositions of aerosol particles spanning various size ranges. The measurement approaches for aerosol hygroscopicity under subsaturated conditions have been fully summarized in previous publications 31 , 62 . Instruments for CCN measurements can be referred to the ref. 63 . Briefly, we only describe some of these developments that are generally applied in the hygroscopicity measurement. Spectroscopic techniques such as Raman spectroscopy are powerful tools to identify the aerosol composition, water content and particle phase state by component characteristic peaks 46 , 64 , 65 , which have been widely applied in studies on the hygroscopicity, phase transition and chemical process of aerosol particles. The hygroscopicity tandem differential mobility analyzer (HTDMA) is one of the common techniques used for measurements on submicron aerosol hygroscopicity 36 , 43 , 66 . The HTDMA system can detect size distributions of the suspended aerosol particles under various RH conditions and acquire the particle size information for estimation of hygroscopic growth factors (GFs), which indicate the water uptake ability of aerosol particles and are defined as a ratio of the particle mobility diameter at a specific RH to the mobility diameter under dry conditions such as RH < 5%. For supermicron particles, electrodynamic balances (EDBs) have been developed to explore the hygroscopicity of single particles with a particle radius size ranging from 5 to 50 μm 31 , 67 . The EDB measurements can characterize the water content in aerosol particles with varying components by providing the mass growth factor, which is calculated as a ratio of the mass of a particle at a certain RH relative to the mass of the particle under dry (RH < 5%) conditions. HTDMA measures the water uptake for a distribution of particle sizes with low dispersity whereas the EDB is developed for single particle measurements. The humidograms derived from HTDMA and EDB measurements can provide the information on the water contents and phase behaviors of aerosol particles during RH cycles, which can be indicated by GFs and the growth curve shapes, respectively. For example, the abrupt jump or drop in the growth curve typically suggests the occurrence of phase transitions in aerosol particles whereas the smooth curve often reflects gradual water uptake or release without obvious deliquescence and efflorescence transitions. The hygroscopic nephelometer system has been developed for field measurements of ambient aerosol hygroscopicity 22 , 68 , 69 , 70 and phase behaviors 19 , 22 , 71 , 72 . This technique could monitor the dynamic hygroscopic behavior of atmospheric aerosols by measuring the scattering enhancement factor f(RH). The overall hygroscopicity parameter and aerosol liquid water contents can be derived from the measured f(RH) values 68 . The humidified nephelometer system has an advantage in providing insights into the hygroscopicity of aerosol particles with a broad size range 68 , 69 , 70 .
Modeling methods
Köhler theory describes hygroscopic growth and cloud droplet activation of aerosol particles, relating RH, composition and diameter of a spherical droplet, expressed in the following form 73 , 74 :
Here, a w is the water activity in the particle phase, σ s/a is the surface tension of a droplet at the solution/air interface, M w is the molar mass of water, R is the ideal gas constant, T is the temperature, ρ w is the density of water, D p is the wet particle diameter. The Köhler equation combines the Raoult effect and the Kelvin effect, which are represented by the water activity term and the exponential term in Eq. ( 1 ), respectively. The hygroscopic growth of aerosol particles below 95% RH is typically determined by the Raoult effect while the Kelvin effect, influenced by the surface tension, has a large impact on the cloud droplet activation 75 .
According to κ-Köhler theory proposed by Petters and Kreidenweis 76 , a single hygroscopicity parameter κ is applied for describing the hygroscopic abilities of aerosol particles. It can be derived based on hygroscopic growth measurements under subsaturated conditions (κ GF ) or CCN activity measurements under supersaturated conditions (κ CCN ) 76 , 77 :
where GF(RH) and D(RH) is the growth factor and the particle diameter at a specific RH, respectively, and D 0 is the diameter of the dry particle. For highly soluble organics, the κ -values are typically consistent between κ GF and κ CCN in contrast to the observed discrepancies for slightly soluble organics 76 , 78 .
where D d is the dry particle diameter and s c is the critical supersaturation ratio.
According to the method proposed by Kreidenweis et al. 79 , the continuous water uptake behavior of aerosol particles can be well described with a polynomial fit equation:
The coefficients a , b and c are determined by the fit to GF vs. a w measurement data.
The Zdanovskii–Stokes–Robinson (ZSR) approach is widely applied to estimate water uptake by multicomponent particles, assuming that the total water uptake is equal to the simple addition of water uptake by each component in the particles at a given RH 43 , 47 , 48 . Although this method is based on the simple assumption that no interactions exist between particle components and thus each component absorbs water independently, it is a powerful tool to predict water uptake of internally mixed systems containing inorganic salts and organic species especially at high RH in the atmosphere 30 , 43 . The deviations of ZSR–predictions from the hygroscopic growth of internally mixed particles typically imply the influence of phase states, morphology effects, or chemical interactions between particle components on the water uptake 36 , 47 , 80 . The hygroscopic growth factor (GF mix ) of the mixed particles is expressed by the ZSR relation 81 , 82 , as follows:
where GF k is the hygroscopic growth factor of pure component k , and ε k is the volume fraction of component k in the dry mixture. The volume fraction ( ε k ) of component k is calculated as:
where w k is the mass fraction of species k and ρ k is the corresponding density. For multicomponent aerosol particles, the overall value of κ can be estimated by the ZSR rule 76 :
where ε i and κ i are the volume fraction of component i in the dry mixture and the single hygroscopicity parameter of component i , respectively.
The atmospheric community has developed thermodynamics models to describe the hygroscopic growth and phase transition of aerosol systems present in the atmosphere. The most common models include the Extended Aerosol Inorganics Model (E-AIM) and Aerosol Inorganic–Organic Mixtures Functional groups the Activity Coefficients (AIOMFAC). The E-AIM can treat the solution thermodynamics including predictions of the water activity, phase state, and equilibrium partitioning of the atmospheric inorganic aerosol systems 83 , and was later extended to cover organic components by combining with the Universal Quasi-Chemical Functional Group Activity Coefficient (UNIFAC) model 84 , 85 , 86 . In contrast to E-AIM, the AIOMFAC model covers a larger amount of the organic functional groups and inorganic ions typically present in atmospheric aerosols. AIOMFAC describes the non-ideal mixing in the aqueous phase of multicomponent aerosols composed of organic and inorganic components and explicitly accounts for interactions such as salt–effects between organic functional groups and inorganic ions by combining a Pitzer-like electrolyte solution model with a UNIFAC-based group contribution approach 87 , 88 . Thus, the AIOMFAC could not only estimate the water uptake of complex mixtures but also the phase behavior like LLPS in aerosol particles 88 .
Hygroscopic behavior of aerosol particles containing water-soluble organic acids and inorganic salts
Water-soluble organic acid.
The WSOAs exhibit distinct hygroscopic behaviors, largely depending upon the solubility and phase state. A detailed description of hygroscopic behaviors of typical organic acids including oxalic acid (OA), malonic acid (MA), succinic acid (SA), adipic acid (AA), citric acid (CA) and phthalic acid (PA) is summarized below. The chemical properties of these organic acids are presented in Table 1 . The hygroscopicity of these acids has been measured by multiple measurement methods. Theory predictions and bulk measurements show that the DRH of OA, SA and AA is greater than 97% RH, which can be expected due to their low solubility 33 , 34 , 35 . As shown in Fig. 1 , no hygroscopic growth and no deliquescence transitions of OA, SA and AA occur in the RH range between 5% and 90%, as observed in various measurement techniques including the EDB and HTDMA 33 , 34 , 36 , 89 , 90 , 91 . Also, it has been found that the crystalline anhydrous OA particles could be transformed into the crystalline OA dihydrate during hydration between 10% and 30% RH 64 , 90 . However, some HTDMA measurements indicate substantial and continuous water uptake by OA particles above 45% RH upon hydration 12 , 34 , 92 , due to the influence of the particle phase state. Mikhailov et al. 12 demonstrated that initial OA particles generated in their HTDMA measurements should remain in an amorphous solid state, which could undergo a humidity-induced glass transition and thus result in gradual water uptake starting from low RH. When OA particles exist in a crystalline solid phase, no obvious water uptake was observed prior to the deliquescence point ( > 97%) in the HTDMA measurement 36 . The hygroscopic growth of OA aerosol particles at low RH reflects that hygroscopic behaviors of amorphous organic substances are not solely determined by their solubility and deliquescence points.

a oxalic acid data from Mikhailov et al. 12 and Jing et al. 36 . b malonic acid, succinic acid and phthalic acid data from Jing et al. 36 ; adipic acid data from Han et al. 97 ; citric acid data from Peng et al. 33 .
In the EDB and HTDMA measurements, MA particles generally exhibit continuous water uptake without obvious phase transitions below its DRH of ~70% RH 33 , 34 , 36 . Such hygroscopic behavior indicates MA particles likely remain in a metastable state and retain some water even under dry conditions 33 . The similar water uptake behavior is also observed for PA and multifunctional WSOAs with a relatively high solubility such as citric acid, malic acid and tartaric acid 33 , 44 . The apparently gradual water uptake at low RH reveals that water uptake of some WSOAs is not solubility limited but strongly influenced by the phase state of the aerosols. Despite their solubilities, the WSOA aerosols with a large number of miscible components may remain fully liquid (or amorphous solid) even in the absence of water 37 . Jing et al. 36 found that the hygroscopic species such as levoglucosan and malonic acid exert a strong influence on water uptake and phase behaviors of OA, even suppressing OA crystallization completely in the mixed particles. The metastable state of organic acid particles at low RH can account for the continuous water uptake and the enhancement in CCN activity beyond their solubility limitation 93 . The field measurements and laboratory studies also suggest that secondary organic aerosols (SOAs) could exist as highly viscous semi-solids or amorphous glassy solids under ambient conditions 25 , 28 .
The hygroscopicity (κ GF or κ CCN ) of organics is found to be related with the water solubility, molecular weight, functional group and O:C ratio 94 , 95 , 96 , 97 . For the highly soluble species (solubility > 7 × 10 – 1 g mL -1 ), their hygroscopicity is largely regulated by the molecular weight while a negative correlation is found between hygroscopicity and molecular volume 97 . For slightly soluble organics, larger solubility corresponds to higher hygroscopicity. In addition, the hygroscopicity of organics generally increases with the O:C ratio. The higher hygroscopicity is observed for organic acids with more functional groups when the acids have the same carbon number 97 . The addition of phenyl radical to organic acid molecular structures may reduce the hygroscopicity of dicarboxylic acids 98 . It is found that UNIFAC model typically well describes the hygroscopic growth of simple and unbranched dicarboxylic acids, while the increasing molecular complexity through the addition of alkyl branches may enhance the disparity between UNIFAC predictions and measurements 99 . Additionally, the hygroscopicity of multicomponent organic acid aerosols can be highly non-additive, as indicated by the deviation of the simple additive rule (ZSR) from the measured growth of internally mixed organic acid systems 36 , 100 . This phenomenon suggests that the specific non-ideal interactions between WSOAs in mixed particles could also regulate the aerosol water-uptake behaviors. The CCN activity of aerosols is typically determined by the chemical composition, phase behavior, morphology and size of the particle 55 , 101 , 102 , 103 . For WSOAs, the solubility and phase behavior play a key role in the cloud droplet activations 41 , 93 , 98 , 104 , 105 . The highly soluble acids (e.g., malonic and glutaric acid) exhibit high CCN activity, well described by the Köhler theory assuming the full dissolution of the dry particle 106 , 107 . The slightly soluble acids (e.g., adipic acid) typically have the low capability of CCN activations 76 , 104 , 108 . It was found that κ CCN increased with solubility for slightly soluble acids, while it decreased with molecular volume for highly soluble acids 98 . For some organic acids with the low solubility (e.g., phthalic acid), they may still show enhancement in CCN activity and activate almost as if they were completely dissolved in spite of the bulk solubility limit 93 . This can be attributed to the phase behavior, i.e., the existence of the particles in a metastable or amorphous state at low RH 93 , 109 .
Ammonium sulfate aerosols internally mixed with water-soluble organic acids
WSOAs have a dramatic impact on the hygroscopic growth and phase behavior of ammonium sulfate (AS) in aerosol particles. The effects of representative WSOAs on the hygroscopic behavior of AS are summarized below.
Oxalic acid
The presence of OA exerts a significant influence on the hygroscopicity of AS aerosols 36 , 43 , 64 , 92 , 110 , 111 . It was found in a HTDMA study that OA in submicron particles composed of AS and OA (1.5:1, mass ratio) can prevent the crystallization of AS, thus resulting in continuous water uptake without a clear deliquescence transition during the hydration process 43 , seen in Fig. 2 . As shown in Fig. 2 , some other HTDMA studies indicate that the submicron internally mixed AS–OA particles with an equal mass ratio do not exhibit any hygroscopic growth until reaching the deliquescence point at ~77% RH slightly lower than pure AS 36 , 111 , consistent with the bulk measurements for eutonic mixtures 89 , 112 . The phase state of OA at low RH may explain the discrepancy in hygroscopic growth of AS–OA particles. The existence of amorphous or liquid-like OA at low RH may hinder the nucleation of AS 43 , while the formation of crystalline OA dihydrate upon dehydration does not prevent the crystallization of AS 36 . Jing et al. 36 also revealed that the full deliquescence of OA above the DRH of mixed AS–OA particles leads to higher water uptake relative to the ZSR modeled results without taking the OA dissolution into account, as indicated by the agreement between measured growth factors and predictions from the E-AIM assuming the complete dissolution of the solid acid in the aqueous phase. From a thermodynamic equilibrium view, the mixed particles composed of AS and OA with an equal mass ratio at high RH should still contain a certain amount of solid OA due to the low solubility. The formation of metastable solid may lead to the complete OA dissolution and thus contributes to the water uptake of submicron particles at high RH 36 , 111 .

1.5:1 case from measurements of Prenni et al. 43 . 1:1 case from measurements of Jing et al. 36 . Predicted growth curves from the ZSR and E-AIM assuming oxalic acid dihydrate are indicated by orange and blue lines, respectively. In the model calculations for 1:1 (mass ratio) mixed particles, the E-AIM estimates are based on the assumption of the complete dissolution of OA after deliquescence while the GF = 1 for OA is used for ZSR predictions 36 .
More complicated hygroscopic behaviors occur for the micron-sized AS–OA mixtures. Wang et al. 64 investigated the hygroscopic behaviors of micron-sized particles containing AS and OA with different organic–to–inorganic molar ratios (OIRs) using confocal Raman spectroscopy. They observed enhanced CRH of mixed AS–OA droplets with increasing OA content and a slight shift in the DRH of AS in mixed AS–OA particles with an OIR of 1:1 to a lower RH at ~77%. Their measurements showed that water uptake by the AS–OA mixed particles at high RH during the deliquescence process was dramatically lower than that during the efflorescence process and decreased with increasing OA content. This phenomenon differs from hygroscopic characteristics of typically mixed systems containing inorganic salts and water-soluble organic compounds, of which deliquescence and efflorescence growth curves are generally converged above the deliquescence point 30 , 45 , 61 , 80 , 113 , 114 , 115 , 116 . Raman and infrared spectra revealed the formation of ammonium hydrogen oxalate (NH 4 HC 2 O 4 ) and ammonium hydrogen sulfate (NH 4 HSO 4 ) derived from interactions between OA and AS in aerosols during the efflorescence process 64 , 110 , which should be responsible for the suppressed deliquescence growth of mixed particles 64 . The mixed AS–OA particles with an OIR of 3:1 even exhibit no obvious water uptake below 90% RH due to the considerable formation of solid NH 4 HC 2 O 4 with a high DRH.
As shown in Fig. 3 , Raman spectra and micrographs suggest the presence of solid NH 4 HC 2 O 4 on the particle surface and variations in components between the shell and the core of the particle upon dehydration. In contrast, the HTDMA studies for AS–OA mixed particles do not suggest the specific interactions between OA and AS related to the water uptake under high RH conditions 36 , 43 , 111 . It is worth noting that aerosols in HTDMA studies underwent rapid drying and the total residence time for transformation of droplets into dry particles in the drying section of the HTDMA was typically tens of seconds 36 , 43 , much shorter than the entire efflorescence time of 10–12 h in the Raman spectroscopy measurements 64 . In the HTDMA experiments, the faster drying rate and smaller particles with a submicron size result in the higher supersaturations of an aqueous phase than in the Raman spectra study, likely leading to the reduced dissociation of OA and the less HC 2 O 4 - formed in the droplets and thus inhibiting the formation of NH 4 HC 2 O 4 . Wang et al. 64 observed one-step efflorescence of rapidly dried particles composed of AS and OA (1:1, molar ratio) occurring at 47 ± 2.5% RH, compared to the two-step efflorescence of slowly dried particles occurring at 75% and 44.3% RH, and identified no characteristic peaks of NH 4 HC 2 O 4 and NH 4 HSO 4 in the spectra of the rapidly dried particles. In contrast to the HTDMA studies, Wei et al. 117 observed that the formation of NH 4 HSO 4 in 100 nm AS–OA (1:1, mass ratio) particles deposited on the substrate for FTIR measurements could promote the water uptake of mixed particles starting from low RH ~ 50% prior to the deliquescence when the particles underwent a deposition time of ∼ 12 h after being dried by a diffusion dryer. Overall, the current studies indicate that the phase variability of OA along with the chemical reactions with AS plays a critical role in determining the hygroscopicity of AS particles internally mixed with OA. The drying process for aerosol droplets has an impact on the reactions of OA with AS in the aerosols under kinetic control. It can be concluded that the various drying or efflorescence processes may affect the potential interplay between aerosol components and hygroscopic growth of multicomponent particles.

a - d The upper panel shows the micrographs for efflorescence behaviors of internally mixed particles. The lower panel indicates the spatial distribution of chemicals within mixed oxalic acid–ammonium sulfate (OIR = 3:1) particles at 74.4% RH. Raman spectra show the formation of NH 4 HC 2 O 4 on the surface shell in contrast to the chemicals at the core dominated by oxalic acid and ammonium sulfate in addition to water. Reprinted with permission from Wang et al. 64 .
Malonic acid
The presence of malonic acid could extend the RH range within which AS remains in the aqueous phase of aerosols. The previous studies performed with bulk solutions, supermicron particles and submicron particles at temperatures ranging from 293 to 303 K indicate that CRH and DRH of AS–MA systems generally decrease with an increase in organic mass fraction less than ~50% 30 , 43 , 51 , 89 , 112 , 118 . Braban and Abbatt 118 found that the crystallization of both the MA and AS components was mutually suppressed by each other in aerosols. The submicron 1:1 (by masses) AS–MA particles measured using the HTDMA show gradual water uptake without a deliquescence transition upon hydration, suggesting the inhibition of AS crystallization during the drying process 43 , 119 . Choi and Chan 30 also observed apparent water uptake by supermicron 1:1 (by moles) AS–MA particles prior to the full AS deliquescence in the scanning EDB measurements. The model methods, including ZSR, E-AIM and AIOMFAC, assuming complete dissolution of MA in the mixture starting from low RH, could well reproduce the HTDMA-measured water uptake by submicron AS–MA particles during the deliquescence process 43 , 111 . However, obvious overpredictions of model methods relative to the EDB measurements occur in the medium RH range between 40% and 70% for the supermicron AS–MA particles upon hydration 30 , 67 , 120 , likely resulting from the incomplete dissolution of MA and thus less contribution to water content in the supermicron particles 61 . Raman spectra also identified solid inclusions of MA in supermicron AS–MA particles 67 , suggesting the heterogeneous crystallization of a fraction of MA induced by AS in contrast to the suppressed crystallization of MA in submicron particles 118 . This difference is likely due to the size dependence of the crystallization of mixed AS–MA particles 121 . Classical nucleation theory indicates that CRH decreases with the reduction in the particle volume, and thus less possibility of crystallization nucleation of MA can be expected for submicron particles than for supermicron particles 51 , 121 . The comparisons between the experimental results and the model predictions highlight the role of the solid phases present in the water uptake by mixed particles in the medium RH range, as well as the particle-size effects on the aerosol hygroscopic growth and phase behavior.
Succinic acid
The effect of succinic acid on the crystallization of AS remains uncertain. Both enhanced and decreased CRH of equimolar mixed AS–SA particles, relative to the pure AS, were observed in the EDB measurements 30 , 67 . Laskina et al. 46 reported the agreement between CRHs of equal mass mixed particles and pure AS with a micron size and found lower CRH of the mixed particles with a submicron size compared to pure AS. The possible reason is that efflorescence, as a kinetic-controlled process, is influenced by multiple factors such as particle size, temperature, and trace impurities, which obscure the role of SA in the crystallization of AS. As for the deliquescence process, previous studies show that succinic acid has no measurable effect on the deliquescence point (around 80% RH) of AS, as indicated by bulk measurements for the eutonic AS–SA mixtures 89 , 112 and by the EDB and micro-Raman spectroscopy measurements for the micron-sized particles 30 , 46 . In contrast, the HTDMA measurements indicated that DRH of submicron (100 nm) mixed AS–SA particles with an equal mass ratio shifted to a lower RH at 73%–76% RH compared to ~80% RH for AS 43 , 46 , 119 . Veghte et al. 52 found that mixed AS–SA particles with a diameter < 170 nm adopted a homogeneous structure while a phase separated morphology occurred for larger particles. The discrepancies in the DRHs of submicron and micron AS–SA particles can be attributed to the particle mixing state, i.e., homogeneous or heterogeneous structure 46 .
In addition, the phase state of SA also plays an important role in the water uptake of mixed particles. The HTDMA measurements show that the hygroscopic growth of submicron particles composed of AS and SA with an equal mass ratio upon hydration could be well described by the ZSR approach assuming that SA remained solid and thus did not participate in the water uptake 43 . Whereas, the EDB measurements from Choi and Chan 30 revealed that water uptake of equimolar mixed AS–SA particles with a micron size at the deliquescence point agreed well with ZSR predictions assuming that SA was completely dissolved in the aqueous phase. Clegg and Seinfeld 61 proposed that for a system at thermodynamic equilibrium the equimolar mixed AS–SA particles at the deliquescence point should consist of aqueous (NH 4 ) 2 SO 4 , and a small amount of aqueous succinic acid in equilibrium with the solid acid, consistent with the Raman observations of the existence of solid SA features even at 90% RH 67 . They found that predicted water uptake at the DRH from the extended ZSR model based on the thermodynamic equilibrium assumption was markedly lower than the EDB results measured by Choi and Chan 30 . The deviations suggest the complete dissolution of SA at the DRH of mixed particles, likely due to the existence of a metastable state of SA 61 .In spite of its high DRH near 99% RH, SA could considerably contribute to the water uptake of mixed particles of AS and SA at high RH by the complete dissolution of a metastable solid phase relative to the stable crystallized phase. Also, the existence of a metastable state of SA may even induce the water uptake of mixed particles around equimolar composition in an intermediate RH range (30–80%), substantially lower than the DRH of the mixed system near 80% 122 . Infrared spectra reveal that the ion-molecule interactions between the organic and inorganic components likely destabilize the crystal structure relative to the pure solid SA and allow the partial and complete dissolution of SA prior to and above the deliquescence point 122 .
Adipic acid
Adipic acid has a multiple impact on the hygroscopic behavior of AS, largely depending on the organic content. Bulk measurements reveal that the less soluble adipic acid has no measurable effect on the DRH of AS in eutonic mixtures 89 . Prenni et al. 43 found that the hygroscopic growth of internally mixed AS–AA particles with the organic mass percentage less than 50% could be well described by the ZSR approach assuming that AA did not participate in the water uptake. Their HTDMA measurements indicate that the hygroscopic features of mixed particles resemble those of pure AS. However, the situation becomes complicated when the organic content is larger than 50% by mass percentage, seen in Fig. 4 . There exist substantial discrepancies between the ZSR prediction and hygroscopic growth measured using the HTDMA and EDB at RH higher than the DRH of AS for mixed particles with a high organic content, and shifts in DRH to lower RH 80 . For example, higher and lower water uptake relative to ZSR predictions in both hydration and dehydration branches was observed for the 1:3 and 1:4 (AS: AA, mass ratio) AS–AA mixtures, respectively 80 . As shown in Fig. 4 (the lower panel), the EDB measurements even verified a pre-deliquescence water uptake starting at 45% RH lower than the deliquescence point (80% RH) of AS, which was not detected by the HTDMA. The complex hygroscopic behavior of AA-dominated particles is likely due to the morphological effects. It was found that AS–AA mixed particles with a major organic fraction contained a conglomerate of nanocrystals with cracks, pores, and veins 80 . For the AA-dominated particles, adipic acid could always stay in a solid state below its CRH ( ≥ 93% 123 ,), encapsulating some fractions of AS residing in organic veins and pores. Due to the Kelvin effect, enhanced water uptake can be expected in such pores and veins compared to that of a flat surface or the convex particle surface. When the AA fraction increases to a certain extent, more enclosed AS unexposed to the humid air would lead to the suppressed hygroscopic growth relative to the model estimates. Therefore, morphological effects should be responsible for the enhanced or reduced water uptake of AA-dominated particles 44 , 80 , 124 .

The 2:1.1 case resembles the ZSR – predicted curve, while substantial deviations exist between measurements and ZSR predictions in the 1:3.3 case. The data from Sjogren et al. 80 is also presented in the 1:3.3 case. Reprinted with permission from Zardini et al. 44 .
Yeung et al. 123 observed that AA solids did not promote the crystallization of AS, as indicated by the similar crystallization RH of mixed particles to that of pure AS. Also, their measurements from the micro-Raman spectroscopy and EDB showed that early water uptake before complete deliquescence was more pronounced in mixed particles with a lower mass fraction of AA (AA less than 30%), which differed from the HTDMA results. They attributed the prior water uptake by AS-dominated particles to the formation of a minor amount of ammonium adipate from the reaction between AS and AA. A recent study using the High-humidity-HTDMA and model methods (ideal solution and UNIFAC) revealed that the microscopic mechanisms related to the Raoult effect could influence hygroscopic growth of internally mixed AS–AA particles in the high RH range between 80% and 99.5% 125 . It was found that two dynamic processes, including surface partitioning of AA and non-ideal properties of aqueous droplets, led to the lower hygroscopicity by affecting water activity. Whereas, the surface partitioning of AA inducing the surface tension reduction enhances the hygroscopicity of mixed AS–AA particles under high RH (near 100%) 126 and supersaturation conditions 54 . The possible reason is that the Kelvin effect, related to the surface tension reduction resulting from the droplet surface partition of AA, prevails over the Raoult effect under supersaturation conditions, and thus promotes the water uptake and cloud droplet activation 54 , 126 . Overall, the previous findings on internally mixed AS–AA particles highlight the importance of multiple factors, including physical morphology effects and chemical processes such as the surfactant effect, on the hygroscopic behaviors of aerosol particles in the low, medium, and high RH range.
Citric acid
Citric acid could significantly alter the hygroscopic growth of AS. The internally mixed particles with an equimolar ratio lose and absorb water continuously over the whole RH range 30 , 44 . The smooth hygroscopic growth without an apparent phase transition indicates that the mixed particles likely exist in a liquid state even at low RH. The presence of CA dramatically inhibited the crystallization of AS 30 . In addition, the AS–CA mixture exhibits much lower water uptake compared with the ZSR predictions, suggesting specific interactions between the components 44 . A recent study also confirmed that the addition of AS to CA leads to a reduction in the water uptake at a fixed low RH, accompanied with an increase in the viscosity over several orders of magnitude 50 . The observed increase in viscosity with increasing inorganic content in AS–CA mixtures also suggests the existence of strong ion–molecule interactions. The calculated results from the Density Functional Theory (DFT) method indicate the presence of complexation between sulfate ions and carboxylic groups of citric acid under low RH conditions, which could lead to the formation of strong H-bonding between hydrogen citrate and hydrogen sulfate 127 . Therefore, the current results for mixed AS–CA particles underline the potential role of specific ion–molecule interactions on aerosol hygroscopicity.
CCN properties of AS–WSOA particles
Previous studies reveal that trace levels of AS significantly decrease the activation diameter of slightly soluble organic aerosols such as adipic and succinic acid, and thus enhance cloud droplet activation 104 , 128 . For the less surface-active WSOAs (e.g., oxalic acid), the hygroscopicity (κ CCN ) of their mixture with AS typically agrees well with the predictions based on the ZSR rule 98 , 129 and the corresponding CCN activity can be well described by the Köhler theory without accounting for surface partitioning 129 , 130 . For more surface-active organic acids (e.g., adipic and octanedioic acid), it is found that the organic fractions in mixtures with AS have a strong impact on the hygroscopicity (κ CCN ) 98 . For example, the hygroscopicity (κ CCN ) of mixed particles with an organic mass fraction less than 60% is comparable to that derived from the ZSR method 98 , 129 , while much higher hygroscopicity than the ZSR-predictions occurs for mixed particles with an organic mass fraction larger than 75%, likely due to surface tension reduction induced by the surface-active acids 98 . In addition, the current findings also underscore the importance of surface activity of WSOAs in cloud droplet activations 39 , 41 , 54 . It is observed that AS aerosols coated with a series of dicarboxylic acids (e.g., malonic and succinic acid), exhibit much larger CCN activation diameters than predictions from the κ-Köhler theory assuming the dissolution of organics within the bulk droplet rather than surface partitioning, for which the discrepancies are diminished when accounting for the surface tension effects of organics within the Köhler theory 54 .
Microphysical mechanisms influencing hygroscopicity of AS-WSOA aerosol particles
WSOAs play a critical role in determining the water uptake characteristics of AS, especially in the low and moderate RH range. WSOAs could contribute to water uptake by aerosols containing AS at low RH. Field observations also indicate the substantial impact of water-soluble organic matter on the hygroscopicity of fine particles under low RH conditions 131 . Comparisons between the experimental measurements and the model predictions highlight the role of phase behaviors, morphology effects and chemical interactions between AS and WSOA on the hygroscopic growth of aerosol particles. The partial or complete dissolution of AS in the liquid organic components can account for the enhanced water uptake at moderate RH compared to model predictions 36 , 132 , 133 . Current findings indicate that the particle morphology (i.e. homogeneous or phase-separated) could regulate not only the CCN activity but also the water uptake by aerosol particles under subsaturated conditions 103 , 134 , 135 , 136 . For the same internally mixed AS − WSOA system, a phase-separated particle structure (partially engulfed morphology) may lead to the larger CCN activation diameters relative to the homogeneous morphology 103 . For aerosols under subsaturated conditions, the core–shell morphology of particles with solid or semisolid organic coatings (organic acid or secondary organic matter) can substantially reduce the hygroscopic growth of aerosols at high RH relative to that of the same compositional particles with the homogeneous morphology 136 , 137 . It should be noted that thin organic coatings (monomolecular films) may not affect water condensation on aerosol droplets while they considerably reduce the water evaporation rate 138 .
One prevalent phase behavior relevant to aerosol hygroscopicity is LLPS, which is driven by the non-ideal mixing of water with aerosol components 17 , 18 , 55 , 102 . LLPS refers to the phase-separated structure with a core–shell or partially engulfed morphology, for which the well mixed liquid phase of the particle is generally separated into a salt-rich phase and an organic-rich phase 139 (or a low-polarity organic-rich phase and a higher-polarity organic-rich phase for the organic aerosol particle free of inorganic salt 140 , 141 , or three liquid phases containing two organic-rich phases and an aqueous inorganic-rich phase 14 ). Song et al. 16 observed the occurrence of LLPS in internally mixed dicarboxylic acids–AS particles during the dehydration and hydration process. The occurrence of LLPS depends on the average O:C (oxygen–to–carbon elemental ratio), organic to inorganic mass ratio and particle size of aerosols as well as ambient conditions including temperature and RH 18 , 139 , 142 , among which the average O:C ratio and particle size are the key factors affecting the core−shell mixing structure of atmospheric fine particles with diameters in submicronmeters 139 , 143 . LLPS always occurs for AS particles containing WSOAs with O: C < 0.56, and never occurs for mixture with O: C > 0.80 142 . As for the regime 0.56 < O: C < 0.80, the specific types and compositions of organic functional groups determine the occurrence of LLPS. However, Ott et al. 144 found that the addition of non-phase-separating organic acids such as SA, CA and MA with a certain amount could prevent the occurrence of LLPS in aerosol particles containing AS and WSOAs that are expected to undergo the LLPS. The occurrence of LLPS accompanied with the formation of the liquid organic film (organic-rich phase) on the particle surface could promote aerosol droplet growth in supersaturated regimes and lower the surface tension of the aerosol droplet, resulting in enhanced CCN activity 55 , 56 , 102 . This is because the Kelvin effect determined by the surface tension becomes comparable to or even prevails over the Raoult effect in controlling hygroscopicity under high RH and supersaturated conditions, which can explain discrepancies between subsaturated hygroscopicity and CCN activity 55 , 126 . As shown in Fig. 5 , Malek et al. 56 developed the ZSR model accounting for LLPS with O: C ratios, which can describe the enhanced hygroscopicity of internally mixed AS–organic acid systems. For WSOAs that does not undergo LLPS, the accumulation of organic acid molecules on the droplet surface and enhanced surface partitioning could occur in their mixture with AS under high RH and supersaturated conditions 145 , which also leads to the larger growth diameters of droplets than predictions from classical Köhler theory 54 . The established thermodynamic models considering surface tension reductions due to equilibrium bulk–surface partitioning 54 and LLPS 55 explain the discrepancies in CCN activity of organic aerosols.

a Experimental contour plot of κ-values extrapolated from experimental κ-values. The vertices of the triangles represent 100% pure compounds (weight percent compositions): bottom left vertex = 100% AS, bottom right vertex = 100% 2MGA, and top vertex = 100% sucrose. The color gradient indicates the range of κ-hygroscopicity values for the ternary system. The region for the assumed occurrence of LLPS is outlined in the dotted green lines. b The ternary system’s κ-experimental versus κ-predicted values obtained from the ZSR rule (light blue triangles), the ZSR/O:C hygroscopicity model (green squares), and the ZSR/O:C-LLPS hygroscopicity model (brown circles). The black dotted line is the referenced line of 1:1 ratio; the gray region shows a 95% prediction interval across the 1:1 line. Reprinted with permission from Malek et al. 56 .
Bulk–surface partitioning
According to Köhler theory, the cloud droplet activations of aerosol particles are determined by Raoult effect (water activity) and Kelvin effect (surface curvature related to surface tensions), in addition to particle size. The traditional viewpoint assumes that the surface tension of an aerosol droplet can simply be treated as that of pure water in climate models for CCN predictions because the droplet is highly diluted near activation, which has been challenged for aerosol particles containing surface-active organics 54 , 55 , 146 , 147 , 148 , 149 . Previous laboratory and field measurements demonstrate that surface-active organics could considerably reduce the surface tension of aerosols or finite-sized droplets relative to the value for water 40 , 147 , 149 , 150 , 151 . Notably, the surface tension reduction is found to be particle-size-dependent and does not match the value for macroscopic bulk solutions 147 , 148 . In contrast to the macroscopic solutions, the high surface-to-volume ratio of aerosol droplets could enhance partitioning of surface-active organic molecules from the interior aqueous phase to the droplet surface, which decreases the bulk concentration and reduces surface tension of the aerosol droplet 40 , 41 . The surface partitioning phenomenon has been observed for aerosol droplets containing WSOAs. For example, the accumulation of succinic acid molecules in the surface region can even lead to a tenfold surface concentration as compared with the saturated bulk solutions 145 . The presence of inorganic salts such as AS could further drive the surface enrichment of organic acid molecules in aerosol droplets 40 , 145 . Such surface partitioning, depleting interior-phase concentration, can increase water activity (i.e., Raoult effect and thus decrease the droplet growth) while concomitantly lowering the surface tension (i.e., Kelvin effect and thus enhance the droplet growth) 54 , 55 . As a result, the surface partitioning may result in counteracting effects on CCN activity. However, the previous findings reveal that surface tension reduction may play a more important role than the Raoult effect related to surface partitioning in cloud droplet activity of organic-dominated aerosols 54 , 55 or ultrafine particles in clean environments 146 . Previous experimental and modeling studies indicate that the Köhler equation without treatment of bulk–surface partitioning of organics including WSOAs may fail to capture the cloud droplet activation behavior of organic aerosols 39 , 41 , 54 , 152 . Several thermodynamic models have been developed to treat bulk–surface partitioning of surface-active organics, which are incorporated within Köhler theory to improve the estimation of cloud droplet activations. These models include the Gibbs surface approach, the molecular monolayer surface model, the AIOMFAC-based LLPS approach with possible partial surface coverage, and the compressed film surface model 39 , 153 . The distinct surface activity and phase behavior like LLPS of aerosol particles containing inorganic salts and organics such as WSOAs may not only substantially modify cloud droplet activations but also influence atmospheric chemistry related to the aerosols 40 , 154 .
Nitrate aerosols internally mixed with water-soluble organic acids
Nitrate is one of the major inorganic components in atmospheric particles and becomes dominant in fine particles in some urban areas. The water uptake and phase behavior of nitrate can dramatically influence the particle’s physicochemical properties and multiphase chemistry processes. The nitrate salts such as NH 4 NO 3 , NaNO 3 and Ca(NO 3 ) 2 tend to stay in the liquid-like or amorphous state even under dry conditions and absorb water continuously from low RH without apparent phase transitions in submicron particles 48 , 155 , 156 . However, the phase state of NH 4 NO 3 in internally mixed particles could be substantially affected by slightly soluble WSOAs 48 , 157 . The presence of OA or SA could facilitate the crystallization of NH 4 NO 3 upon dehydration and result in the occurrence of deliquescence transitions for mixed particles with 50% acid by mass relative to the pure NH 4 NO 3 particles 48 . In contrast, the crystallization of NH 4 NO 3 was not observed for NH 4 NO 3 -dominated particles containing 25% OA or SA by mass 48 , 157 , 158 . The crystallization of NH 4 NO 3 depends on the fractions of slightly soluble WSOAs in the mixed NH 4 NO 3 –WSOA particles. This phenomenon can be explained by the fact that the solid organic acid lowers the nucleation barrier to NH 4 NO 3 efflorescence, but it can be completely dissolved in NH 4 NO 3 –dominated particles 157 .
In the case of NaNO 3 –containing particles (seen in Fig. 6 ), the continuous water uptake occurs for NaNO 3 particles with 50% OA or 50% SA by mass, suggesting the absence of NaNO 3 crystallization in the mixed systems during the hygroscopic process 48 . The contradicting observations for NH 4 NO 3 –containing particles can be explained by the higher hygroscopicity of NaNO 3 than NH 4 NO 3 , which does not favor the existence of solid acids promoting the NaNO 3 crystallization. The water uptake by NaNO 3 particles with 50% OA by mass agrees well with the ZSR predictions assuming the complete dissolution of OA 48 , suggesting that the liquid-like NaNO 3 likely inhibits the crystallization of OA. However, Ma et al. 59 observed that NaNO 3 –OA (2:1, inorganic-organic molar ratio) particles exhibited no hygroscopic growth upon hydration, dramatically deviating from the ZSR estimates. They found that the formation of sodium oxalate with low hygroscopicity due to nitrate depletion can account for the water uptake discrepancies. It is found that malonic acid does not alter the phase state of NH 4 NO 3 and NaNO 3 , as indicated by the continuous water uptake by mixed particles and the agreement between measured and ZSR-modeled growth, assuming that NH 4 NO 3 or NaNO 3 always exists in the liquid state 48 . In contrast, the submicron particles containing Ca(NO 3 ) 2 and MA exhibit an obvious decrease in particle size and particle shrinkage (seen in Fig. 7 ) in low humidity range between 10 and 40% RH, and thus the whole hygroscopic behavior substantially deviates from the ZSR prediction 48 . A similar hygroscopic behavior was observed for internally mixed Ca(NO 3 ) 2 –citric acid particles 158 , NH 4 NO 3 –protein particles 155 and SOA particles derived from methylglyoxal–methylamine aqueous reactions upon hydration below 50% RH 159 . The Ca(NO 3 ) 2 –MA (1:1, by mass) particle has a compact spherical structure at 30% RH relative to the gel-like structure under dry conditions 48 . This unexpected behavior of particle shrinkage upon hydration typically results from the humidity-induced microstructural rearrangement from porous, gel-like structures into more compact spheres for (semi-)solid amorphous particles 12 . The hygroscopic behavior of Ca(NO 3 ) 2 –MA aerosols suggests a strong influence of interactions between particle components on the microscopic structure and water uptake of particles. Richards et al. 53 demonstrate that cooperative ion–molecule interactions between carboxylic groups of low-molecular-weight di/polycarboxylic acids and divalent inorganic ions such as Ca 2+ enable the solidification of particles and humidity-dependent gel–phase transition in organic-inorganic aerosols, which are responsible for the obvious deviations from the ZSR-modeled hygroscopic behavior.

a NaNO 3 –oxalic acid (OA), b NaNO 3 –malonic acid (MA), c NaNO 3 –succinic acid (SA), and d NaNO 3 –phthalic acid (PA). The green line indicates the hygroscopic growth predicted from the ZSR method. For the NaNO 3 –OA mixture, the ZSR–predicted curve based on the GFs for OA from Mikhailov et al. 12 is presented by the dashed line. The shaded area shows the potential water-uptake contributed by the oxalic acid component. Reproduced with permission from Jing et al. 48 .

The blue solid and orange dashed lines represent the hygroscopic growth predicted from the ZSR method and GF = 1, respectively. The shaded area indicates no water uptake by the mixed aerosols under low RH conditions. Reproduced with permission from Jing et al. 48 .
The current findings reveal the significant influence of WSOAs on the hygroscopic behavior of nitrates in atmospheric aerosols. The nitrate salt–WSOA aerosols exhibit varying phase behaviors and hygroscopic growth depending upon the type of components in the particles. While pure nitrate particles generally show continuous water uptake and remain in a liquid-like state even under dry conditions, the deliquescence transitions still likely occur for internally mixed particles containing organic acids with a high deliquescence point such as oxalic acid and succinic acid 48 , 158 . However, the organic acids do not efficiently initiate the crystallization of nitrate in submicron particles when the mass fraction of organic acids is less than 50% in internally mixed particles. Sodium nitrate relative to ammonium nitrate more likely exists in the liquid-like state in submicron particles even when particles contain a considerable fraction of WSOAs with a high deliquescence point. Due to the absolute abundance of nitrate relative to WSOAs in atmospheric particulates, the presence of WSOAs does not likely alter the amorphous or liquid-like phase state of nitrates 48 , 160 . The relative amount of nitrates has been increasingly growing in atmospheric particulates of some urban areas. The enhanced nitrate content extends the RH range in which the aerosol particles contain liquid water and remain in the liquid state 161 . For example, in the East Asian megacities, urban particles with a ∼ 50% nitrate mass fraction can stay in a non-solid state even at RH < 20% 162 .
Sodium chloride aerosols internally mixed with water-soluble organic acids
Sea salt aerosols, generated at the ocean surface and injected into the atmosphere, constitute important fractions of atmospheric particles in the troposphere. NaCl represents the major component of sea salt aerosols. It has been found that NaCl is generally mixed with WSOAs in ambient particles. The hygroscopicity of NaCl is substantially influenced by the presence of WSOAs in the internally mixed systems. The hygroscopic behaviors of the NaCl particles internally mixed with WSOAs are strongly influenced by the type and composition fraction of organic acids 30 , 47 , 163 , as seen in Fig. 8 . The presence of WSOAs could even react with NaCl in aerosol particles, resulting in the formation of organic salts and release of HCl (i. e., chloride depletion, seen in Fig. 9a ) that further alters the particle composition and hygroscopicity 47 , 57 , 163 . While chloride depletion may occur in the NaCl–WSOA systems, the changes in particle composition due to the formation of organic salts exert little influence on the water uptake of mixtures except for the oxalic–acid–containing particles 30 , 45 , 47 , 115 . For example, the hygroscopic growth of both submicron and supermicron NaCl particles containing malonic acid or glutaric acid can be well described in the whole RH range by the E-AIM that assumes nominal (or unchanged) compositions in particles and thus does not take the chloride depletion into consideration 45 , 47 , 115 . The possible reason for the agreement between measurements and predictions is that the organic salts such as malonate and glutarate have similar hygroscopicity to the corresponding organic acids 164 . Choi and Chan 30 also reported that the water content of NaCl particles internally mixed (1:1, mole ratio) with one organic acid (malonic acid, succinic acid, glutaric acid and citric acid) after complete deliquescence above 70% RH agreed well with the ZSR predictions assuming no interactions between aerosol components. Also, κ CCN values for aerosol particles composed of NaCl and dicarboxylic acids (e.g., malonic acid or succinic acid) are in agreement with ZSR predictions 98 .

a NaCl–oxalic acid (OA), b NaCl–malonic acid (MA), c NaCl–succinic acid (SA), and d NaCl–OA–MA. ZSR–predicted curves based on solid or liquid OA (data from Mikhailov et al. 12 ) assumption are indicated by orange solid and dashed lines, respectively. The blue solid lines represent predicted curves from E-AIM. Hygroscopic growth factors of NaCl–OA particles with an equal mass ratio are also shown in hollow squares for comparison in the panel ( d ). Reproduced with permission from Peng et al. 47 . Copyright 2016 American Chemical Society.

a Mean elemental ratios of chloride to sodium (Cl/Na) as a function of particle size for NaCl, glutaric acid (GA)–NaCl, and malonic acid (MA)–NaCl particles with varying molar ratios. Dash lines represent the nominal value of Cl/Na = 1 in pure NaCl particles. Error bars correspond to a standard deviation of 1. Reprinted with permission from Ghorai et al. 45 . Copyright 2014 American Chemical Society. b Mean elemental ratios of nitrogen to sodium (N/Na) as a function of particle size (in diameter) for MA–NaNO 3 particles with varying molar ratios. Black solid diamonds represent the reference data for pure NaNO 3 particles. Dash lines represent the nominal value of N/Na = 1 in pure NaNO 3 particles. Reprinted with permission from Wang and Laskin 58 . Copyright 2014 John Wiley & Sons, Inc.
The WSOAs, except for oxalic acid, exert a similar influence on the hygroscopicity of NaCl as compared to AS. For soluble organic acids such as malonic acid and citric acid, they promote obvious water uptake by the mixed particles at low and moderate RH ranges and lower the DRH of NaCl from 75% RH to ~70% RH or even lead to the smooth growth without clear deliquescence transitions when organic acid content increases from the low to high fractions 30 , 47 . For organic acids with limited solubility (e.g., succinic acid), the apparent deliquescence transitions occur for NaCl–succinic acid particles with varying mixing ratios, and water uptake of mixed particles is consistent with the ZSR simulations for organic acid content less than 50% by mass percentage 47 . In the case of succinic–acid–dominated particles, it is found that ZSR method underpredicts the hygroscopic growth of NaCl–SA (1:3, inorganic-organic mass ratio) particles above the DRH, likely due to the partial dissolution or the morphology effects of SA that enhance the water uptake of mixed systems 47 . However, the acid displacement reaction between oxalic acid and NaCl has a substantial influence on the hygroscopicity of mixed particles. Previous studies indicate that the presence of OA markedly reduces the water uptake of NaCl–OA particles above 75% RH relative to the ZSR predictions 47 , 91 , due to the formation of oxalate salts with a high deliquescence point 164 . The lower hygroscopicity κ and weaker CCN activity derived from CCN measurements for NaCl–OA particles is also observed when it was compared with ZSR estimates 129 . As a result, the acid displacement reaction of NaCl with OA likely has a critical impact on the hygroscopicity of NaCl particles containing OA under both subsaturated and supersaturated conditions.
As NaCl is generally internally mixed with multiple organic acids in ambient particles, some studies largely using the HTDMA have also investigated the influence of binary and multiple component organic acids on the hygroscopic behavior of NaCl. Peng et al. 47 observed that the addition of MA into NaCl–OA systems promoted the hygroscopic growth of mixed particles starting from low RH and the ternary mixtures exhibited continuous water uptake relative to the NaCl–OA particles (seen in Fig. 8 d), indicating that the initial particles may stay in a liquid-like state even under dry conditions. In addition, the presence of MA reconciles the discrepancies in water uptake between experimental results and model predictions without taking chloride depletion into account. Thus, the coexisting MA substantially diminishes the influence of chloride depletion along with changes in particle composition on the hygroscopic growth of ternary NaCl–OA–MA systems. Moore and Raymond 165 found that the measured hygroscopic growth of mixtures containing NaCl and ten dicarboxylic acids above the deliquescence point is consistent with the ZSR estimates for the organic acid mass fractions not larger than 50%, while the overestimates from the ZSR occur for the organic-acid-dominated mixtures. Wang et al. 166 also reported the agreements between measured water uptake of mixtures containing NaCl and organic components (levoglucosan, succinic acid, phthalic acid, and humic acid) and the ZSR estimates for the organic acid mass fractions not larger than 50% as compared to the underestimates from ZSR for the organics–dominated mixtures.
Due to the influence of chloride depletion, the NaCl–WSOA mixture aerosols generally exist as either a ternary NaCl–WSOA–organic sodium salt system or a binary NaCl–organic sodium salt system or a WSOA–organic sodium salt system depending upon the initial acid content, rather than a binary NaCl–WSOA system during the hygroscopic process 45 , 163 . The chemical compositional evolution of the NaCl–WSOA aerosols during the water evaporation-condensation process may affect the mixing states and phase states of particles, which further modifies the hygroscopic behaviors of NaCl–WSOA aerosols under subsaturated conditions 163 . However, under supersaturated conditions, Kelvin effects related to surface tension reduction exert a great influence on the CCN activity of NaCl–WSOA particles. For example, the κ CCN value of NaCl–AA mixed particles with an organic volume fraction of 60% can be up to 0.734, even higher than that of NaCl–MA (0.639) 98 , which shows an opposite trend with respect to hygroscopic growth of a single acid under subsaturated conditions. This discrepancy could be ascribed to the surface tension reduction induced by the surface-active species like AA.
Ammonium sulfate aerosols internally mixed with water-soluble carboxylic salts
In the atmosphere, WSOA can be transformed into corresponding organic salts through neutralization with NH 3 or displacement of hydrogen ions by metal ions in the particle phase. Field measurements observe a significant fraction of water-soluble organic salts in atmospheric particulates. The formation of organic salts plays a critical role in regulating the volatility, water content, and phase state of organic-acid-containing particles. Wu et al. 164 investigated the effects of atmospherically relevant water-soluble carboxylic salts on the hygroscopic behaviors of (NH 4 ) 2 SO 4 using a HTDMA. They observed the lower DRH and comparable water uptake after the deliquescence for the mixtures of (NH 4 ) 2 SO 4 and organic salts relative to that of pure (NH 4 ) 2 SO 4 particles, implying that the contribution to water uptake by organic salts in these particles was close to that of (NH 4 ) 2 SO 4 . Schroeder and Beyer 167 revealed that the onset DRH of organic salt − (NH 4 ) 2 SO 4 mixtures was always lower than that of each pure component, regardless of the fraction of organic salts in the mixture. Wang et al. 49 found that aqueous droplets containing (CH 2 ) n (COONa) 2 ( n = 1, 2) and (NH 4 ) 2 SO 4 may undergo ammonium depletion with the release of gaseous NH 3 during the efflorescence process, likely resulting from the synergistic effect between NH 4 + dissociation and carboxylic anion hydrolysis. The ammonium depletion was accompanied with the formation of corresponding organic acids and monosodium dicarboxylates in mixed particles with a high and low (NH 4 ) 2 SO 4 content, respectively. The changes in the particle composition may reduce water uptake by internally mixed organic salt–(NH 4 ) 2 SO 4 systems upon hydration as compared to that upon dehydration 49 , revealing that potential interactions between water-soluble organic salts and (NH 4 ) 2 SO 4 in aqueous aerosols affect the repartitioning of NH 3 between the condensed and gas phases and thus alter hygroscopic properties of aerosol particles.
Aerosol chemistry of atmospheric particles containing water-soluble organic acids and inorganic salts
Field observations have indicated the substantial chloride and nitrate depletion in sea spray aerosols and continental aerosols 168 , 169 . The WSOAs have been found to contribute to the chloride depletion and nitrate depletion through displacement reactions with chloride salts (e.g., NaCl) and nitrate salts (e.g., NaNO 3 ) in aerosol particles (seen in Fig. 9 ), resulting in the repartitioning of HCl and HNO 3 into the gas phase 57 , 58 , 170 , 171 , 172 , 173 . The aerosol chemistry related to these depletions are presented as Eqs. ( 9 )–( 12 ):
where, HA and A - represent the WSOA and the corresponding organic acid anion, respectively. The equilibrium of reactions (9)–(12) shifting to the right can lead to the continuous degassing of HCl and HNO 3 . The resulting loss of chloride and nitrate anions in the particle phase is termed as chloride depletion and nitrate depletion, respectively.
Previous studies demonstrate that the extent of depletions in internally mixed HA–NaCl and HA–nitrate particles is determined by the release of HCl(g) and HNO 3 (g) departing from the particle phase 57 , 58 . Although these reactions are not favored in bulk solutions, aerosol droplets with a relatively high surface-to-volume ratio yet facilitate unexpected multiphase chemistry 174 , 175 , 176 , especially during the dehydration or efflorescence process 163 , 177 . The depletion reactions can occur at high RH (e.g., 80%) and they are not confined to the dehydration process 163 , 174 . From the viewpoint of thermodynamics, the differences between acidity and volatility of HA and those of HCl/HNO 3 control the equilibrium conditions of these multiphase reactions in aqueous droplets. One study based on the multiphase buffer theory indicates that the effective equilibrium constants (K*) for the depletion reactions in aerosol droplets are considerably higher than the classical reaction equilibrium constants (K) for bulk solutions, as indicated by the apparent enhancement factors (K*K -1 ) of 10 7 − 10 10 and 10 2 − 10 5 for chloride depletion and nitrate depletion, respectively 177 . Thermodynamic analysis also suggests that the lower aerosol water content would drive the depletion reactions to a larger extent 177 , consistent with the experimental observations of substantial depletion during the dehydration process 163 , 178 . The laboratory studies reveal that the larger WSOA content relative to NaCl/NaNO 3 content in aerosol particles as well as the higher acidity of organic acids results in greater chloride/nitrate depletion 45 , 58 . This can be explained by the fact that these depletion reactions are driven by the liberation and formation of HCl/HNO 3 , which largely depends on the available H + ions dissociated from the organic acid in the aqueous aerosols. The nitrate salt type also exerts an influence on nitrate depletion with the depletion extent following the order: Mg(NO 3 ) 2 > Ca(NO 3 ) 2 > NaNO 3 , which is due to the ion-molecular interactions such as complexation between Ca 2+ or Mg 2+ and carboxylic acids 179 . From the viewpoint of kinetics, the rate constants for the depletion reactions are regulated by the generation of HCl or HNO 3 and subsequent release from the air-water interface into the gas phase 174 . The kinetics analysis shows that the depletion reactions proceed rapidly in aerosol droplets containing a dicarboxylic acid and NaCl or NaNO 3 with a radius less than 10 μm, for which the characteristic reaction time is always within the scope of lifetime of atmospheric particles 177 , as seen in Fig. 10 . The experimental results also reveal that the first-order rate constants for nitrate and chloride depletions at 80% RH range from 10 −3 to 10 −4 s −1 , indicating the occurrence of appreciable depletions within the lifetime of aerosol droplets 174 . Additionally, the higher reaction rate occurs for the chloride depletion than for the nitrate depletion in aerosol droplets 174 . However, the experimental measurements show that these depletion reactions have much lower rates than the theoretical predictions based on the diffusion-limited mass-transfer equation 174 , 177 , 180 . The discrepancies between experiments and calculations likely result from the differences in partial pressures of HCl/HNO 3 and droplet viscosities.
Panels (left), (middle) and (right) show the temporal evolution of reaction extents, ϵ ( t ), for the chloride, nitrate, and ammonium depletion, respectively, in aerosol microdroplets with a radius of 1 μm at 90% RH. The inorganics and organics in aerosols are mixed in a stoichiometric ratio. The style of the black curves indicates the type of dicarboxylic acids (H 2 A) or the corresponding disodium salts (Na 2 A) involved in the depletion reactions–oxalic acid (OA) or oxalate (dotted curves), malonic acid (MA) or malonate (dashed-dotted curves), succinic acid (SA) or succinate (dashed curves) and glutaric acid (GA) or glutarate (solid curve). Reprinted with permission from Chen et al. 177 . Copyright 2022 American Chemical Society.
The depletion reactions between WSOAs and inorganic salts (e.g., NaCl and NaNO 3 ) provide a recycling mechanism of HCl/HNO 3 and potentially contribute to the atmospheric HCl/HNO 3 budget 58 . Meanwhile, the multiphase chemistry related to organic acids and inorganic salts leads to the formation of carboxylate salts and thus alters the particle composition. For example, Li et al. 163 found that the mixed NaCl–dicarboxylic acid (DCA) aerosols undergoing the hygroscopic process remained as a ternary NaCl–DCA–DCA sodium salt system or a binary NaCl–DCA sodium salt or a DCA–DCA sodium salt system, depending on the initial NaCl to DCA mixing ratio. The DCA sodium salt is identified as monosodium dicarboxylate in most of the internally mixed systems except for NaCl–oxalic acid particles with a NaCl molar fraction of 75% in which both disodium oxalate and monosodium oxalate were formed 163 , 178 . The current studies indicate that the produced oxalate salts with a high DRH ( > 90%) may substantially reduce the water uptake by the mixed particle of oxalic acid with NaCl or NaNO 3 during the hydration process and the reduction in water content becomes larger for the mixture with a higher oxalic acid fraction 47 , 59 . In contrast, the internally mixed system containing other WSOAs (e.g., malonic acid or glutaric acid) and NaCl does not exhibit obvious differences in hygroscopicity from the model predictions assuming the unchanged composition in the particle phase 45 , 47 , 91 , 115 . Thus, the influence of chloride/nitrate depletion on aerosol hygroscopicity is regulated by the type and content of organic acids in aerosol particles.
The current studies also reveal the influence of particle properties such as phase state and size on the chloride/nitrate depletion. The phase state of the particle is found to play a role in determining the depletion extent 47 , 58 , 172 . The NaCl–OA mixed particles could stay in a liquid-like state during the dehydration process due to the addition of malonic acid, resulting in the inhibition of chloride depletion during the hygroscopic process 47 . Wang and Laskin 58 also reported that the investigated WSOA–nitrate particles in a metastable state after dehydration still retained some water at low RH and led to the lower release of HNO 3 into the gas phase from the fresh particles than that from the particles stored for several weeks, which can be attributed to the low diffusion of HNO 3 in the highly viscous matrix 13 . The phase transformation of mixed NaNO 3 –OA particles from amorphous solid to semisolid and liquid during the deliquescence process was observed to be accompanied by an enhancement in the nitrate depletion extent and HNO 3 release rate, likely due to the generation of more available NO 3 − ion 172 . For the size effects, the model simulation indicates the reduction in chloride depletion extent with the droplet radius in the range between 1 μm and 10 μm for NaCl–DCA mixed systems, with the calculated results based on the Maxwell steady-state diffusive mass transfer equation 181 . Nevertheless, the laboratory studies reveal that no significant size-dependence of chloride/nitrate depletion was observed for the WSOA–NaCl (or NaNO 3 ) mixed particles with a size of 0.1–10 μm 45 , 57 , 58 , as seen in Fig. 9 . Only slight size-dependence was observed for Ca(NO 3 ) 2 particles internally mixed with glutaric or citric acid 58 .
WSOAs can be transformed into water-soluble carboxylate salts through neutralization with NH 3 or displacement by metal ions in the particle phase. One study has observed the ammonium loss (a low ammonium−sulfate molar ratio < 2) and degassing of NH 3 (i.e., ammonium depletion) in aerosol droplets containing (NH 4 ) 2 SO 4 with (CH 2 ) 2 (COONa) 2 or CH 2 (COONa) 2 during the efflorescence process 49 . This ammonium depletion process is likely triggered by the synergistic effect between NH 4 + dissociation and dicarboxylic anion hydrolysis, and driven by NH 3 partitioning into the gas phase (seen in Eqs. ( 13 )–( 14 )) 49 .
where Na 2 A and NaHA represent the disodium dicarboxylate and the monosodium dicarboxylate, respectively. The Na 2 C 2 O 4 –(NH 4 ) 2 SO 4 mixtures exhibit no apparent ammonium depletion, which can be explained by the weaker hydrolysis of oxalate anions 49 . The ammonium depletion could continuously acidify the aerosol during the reaction process and result in the formation of organic acids and monosodium dicarboxylate salts in mixed particles with a high and low (NH 4 ) 2 SO 4 content, respectively 49 , 182 . The model simulation suggests that the ammonium depletion process generally has a much shorter reaction timescale than that of chloride/nitrate depletion, and that the higher threshold aerosol water content (AWC) for the occurrence of ammonium depletion in Na 2 A–(NH 4 ) 2 SO 4 droplets than the typical AWC of continental aerosols may drive ammonium depletion in urban aerosols 177 , as seen in Fig. 10 . Field observations have confirmed a low ammonium–sulfate ratio (< 2) and high acidity for the sulfate aerosol particulates in the eastern United States despite excess NH 3 concentrations, inconsistent with the predictions from thermodynamic models 183 , 184 . The low ammonium−sulfate ratio (< 2) exhibits a strong correlation with the nonvolatile cations (e.g., Na + ) 185 , 186 . The aerosol chemistry between water-soluble organic salts and (NH 4 ) 2 SO 4 in aqueous aerosols provides a feasible mechanism for the ammonium loss and the repartition of NH 3 between the condensed and gas phases.
The aerosol chemistry related to chloride/nitrate/ammonium depletion in aerosol particles reveals the potential influence of WSOAs on the atmospheric recycle of HCl/HNO 3 /NH 3 , which may contribute to the atmospheric budget of reactive gases. Meanwhile, the changes in particle composition likely modify the physicochemical properties (e.g., hygroscopicity and pH) of aerosols and relevant chemical processes. For example, some experimental measurements indicate that chloride/nitrate depletion could induce an increase in pH of organic acid microdroplets internally mixed with NaCl or NaNO 3 174 , 180 .
Concluding assessment and outlook
Hygroscopicity and phase state play a central role in determining the climate effects and environmental influence of atmospheric particles. The current studies reveal the complicated phase behaviors of WSOAs and the substantial influence of WSOAs on the hygroscopicity of atmospherically inorganic salts. As shown in Fig. 11 , the interactions between WSOAs and inorganic salts exert a significant influence on the water uptake and phase behavior of atmospheric particles. The aerosol thermodynamic model without accounting for non-ideal interactions, phase behavior and microphysical particle-structure would result in deviations in modelled water uptake by organic-inorganic aerosols, especially in the low and intermediate RH range. This humidity region is typically where a single simplified approach, such as ZSR, fails to capture the hygroscopic growth of multicomponent aerosols with the variability in particle phase states 132 . The application of the simplified approach for modelling hygroscopic growth in atmospheric climate models may lead to uncertainties in estimates of aerosol radiative properties. To further advance the understanding of aerosol chemistry and hygroscopicity, future research directions are outlined below:
Further studies are needed to better constrain the key parameters regulating the phase state and morphology of organic-inorganic aerosols as well as to explore the impacts of diverse phase behaviors on the regional air quality and global climate. The divalent metal ion–organic acid molecule interactions in aerosol particles are found to create a supramolecular network and thus mould the particle structure, resulting in the formation of a gel structure and unexpectedly low water uptake for aerosol particles 48 , 53 . However, it is still not clear how these supramolecular effects influence the physicochemical properties and related environmental impacts of ambient aerosol particles on a regional and global scale. This highlights the need to better understand the fundamental microphysics determining the particle phase and morphology.
Previous studies on submicron particles have largely focused on the deliquescence growth, as compared to the limited information on the dehydration or efflorescence process. Some studies uncover the importance of RH history in shaping the phase behavior of aerosol particles in addition to RH and chemical composition 22 , 67 . For example, the aerosol particle may stay liquid at a certain RH during the efflorescence process while remaining solid at the same RH during the deliquescence process. Specially, as the submicron particles frequently show differences in water uptake and phase behaviors compared to supermicron particles, it merits additional investigation into the efflorescence behaviors of internally mixed WSOA–inorganic salt particles with a submicron size. Some limited studies have indicated the temporal evolution of the hygroscopicity and phase transitions such as LLPS for aerosol containing AS and WSOAs 64 , 187 , underlining the importance of nucleation kinetics. Further efforts are needed to clarify the kinetic mechanisms that influence aerosol hygroscopicity and phase transitions.
The presence of surface-active organic acids is observed to enhance cloud droplet activation through surface partitioning. However, there are still some unresolved questions on the role of organic acids in the cloud droplet activation. The aerosol droplets containing surface-active organic acids and inorganic salts under high RH or supersaturated conditions may undergo LLPS and bulk–surface partitioning, with bulk depletion accompanied with surface tension reduction. The phase behavior of bulk–surface partitioning with bulk depletion for organic acid molecules could increase water activity and thus decrease the aerosol hygroscopicity due to the Raoult effect. However, the concomitant surface tension reduction related to the Kelvin effect can promote the aerosol growth and cloud droplet activation. The counteracting effects remain to be generally established in atmospheric models for a better assessment of CCN activity of ambient aerosols 188 .
Dicarboxylic acids could contribute to the particle nucleation by bonding with sulfuric acid molecules 189 , 190 . The interactions between water and organic acid molecules have also been found to participate in and favor the new particle formation (NPF) 191 , 192 , 193 . However, the microphysics related to the hygroscopicity of nanocluster aerosol (sub-4 nm) remain unknown due to challenges in experimental and theoretical methods. The insufficient characterization for the hygroscopic behavior of nanocluster aerosols limits the understanding of the new particle formation and thus future investigations are recommended to this interesting topic.
The aerosol chemistry relevant to chloride/nitrate/ammonium depletion has implications for atmospheric impacts of aerosol particles. The depletion reactions modify particle composition and hygroscopic properties. Previous laboratory studies on these depletion processes have focused on simple binary organic-inorganic component particles. A recent study reveals that sea salt aerosol containing a sea salt surrogate (98.5% sodium chloride, 0.9% sulfate, calcium and magnesium salts and 0.5% water in mass) and malonic acid (1:3, salt-acid mass ratio) has much lower water uptake than the ZSR estimates at high RH, inconsistent with the results for the binary NaCl–malonic acid mixture 47 , 194 . More efforts should be made for the understanding of depletion influence on the hygroscopic behaviors of aerosol particles with complex components.
One study on SOA mixed with NaCl or NaNO 3 indicates that higher particle viscosity at low temperatures and RH can hinder these depletion reactions 195 . The ambient aerosol may undergo complex phase transitions such as LLPS that could impact particle reactivity and gas-particle partitioning of HCl/HNO 3 /NH 3 . The phase state effects on depletion reactions remain unclear for atmospheric particles with diverse phase behaviors. The mass transfer limitation in semi-solid or highly viscous liquid aerosols may inhibit and delay the release of gas molecule HCl/HNO 3 /NH 3 from the particle phase 13 , 196 , 197 . The influence of controlling factors on the depletion process merits further investigation. In addition, the release and recycling of HCl/HNO 3 /NH 3 from aerosol particles have implications for atmospheric chemistry. Further experimental and modeling studies are warranted for assessment of the impacts of these aerosol chemistries between WSOAs and inorganic salts on the regional and global atmospheric environment.

The orange dashed box shows the aerosol chemistry relevant to the depletion processes enhanced by the dehydration process. The blue dashed box indicates the diversity in phase transitions during the aerosol water-uptake process.
Data availability
All the data are reproduced and adapted from the literature listed in the References.
Carslaw, K. S. et al. Large contribution of natural aerosols to uncertainty in indirect forcing. Nature 503 , 67–71 (2013).
Article CAS Google Scholar
Bellouin, N. et al. Bounding global aerosol radiative forcing of climate change. Rev. Geophys . 58 https://doi.org/10.1029/2019RG000660 (2020).
Burrows, S. M. et al. Ice-nucleating particles that impact clouds and climate: Observational and modeling research needs. Rev. Geophys . 60 https://doi.org/10.1029/2021RG000745 (2022).
Tan, F. et al. Heterogeneous reactions of NO 2 with CaCO 3 –(NH 4 ) 2 SO 4 mixtures at different relative humidities. Atmos. Chem. Phys. 16 , 8081–8093 (2016).
Faust, J. A., Wong, J. P. S., Lee, A. K. Y. & Abbatt, J. P. D. Role of aerosol liquid water in secondary organic aerosol formation from volatile organic compounds. Environ. Sci. Technol. 51 , 1405–1413 (2017).
Li, K. et al. Enhanced light scattering of secondary organic aerosols by multiphase reactions. Environ. Sci. Technol. 51 , 1285–1292 (2017).
Xia, K. et al. Heterogeneous reaction of HCOOH on NaCl particles at different relative humidities. J. Phys. Chem. A 122 , 7218–7226 (2018).
Wang, J. et al. Fast sulfate formation from oxidation of SO 2 by NO 2 and HONO observed in Beijing haze. Nat. Commun. 11 , 2844 (2020).
Wang, W. et al. Sulfate formation is dominated by manganese-catalyzed oxidation of SO 2 on aerosol surfaces during haze events. Nat. Commun. 12 , 1993 (2021).
Lv, S. et al. Nitrate-enhanced gas-to-particle-phase partitioning of water-soluble organic compounds in Chinese urban atmosphere: Implications for secondary organic aerosol formation. Environ. Sci. Technol. Lett. 10 , 14–20 (2023).
Kanakidou, M. et al. Organic aerosol and global climate modelling: a review. Atmos. Chem. Phys. 5 , 1053–1123 (2005).
Mikhailov, E., Vlasenko, S., Martin, S. T. & Koop, T. & Pöschl, U. Amorphous and crystalline aerosol particles interacting with water vapor: conceptual framework and experimental evidence for restructuring, phase transitions and kinetic limitations. Atmos. Chem. Phys. 9 , 9491–9522 (2009).
Koop, T., Bookhold, J., Shiraiwa, M. & Pöschl, U. Glass transition and phase state of organic compounds: dependency on molecular properties and implications for secondary organic aerosols in the atmosphere. Phys. Chem. Chem. Phys. 13 , 19238–19255 (2011).
Huang, Y. et al. Coexistence of three liquid phases in individual atmospheric aerosol particles. Proc. Natl. Acad. Sci. USA 118 https://doi.org/10.1073/pnas.2102512118 (2021).
Reid, J. P. et al. The morphology of aerosol particles consisting of hydrophobic and hydrophilic phases: hydrocarbons, alcohols and fatty acids as the hydrophobic component. Phys. Chem. Chem. Phys. 13 , 15559–15572 (2011).
Song, M., Marcolli, C., Krieger, U. K., Zuend, A. & Peter, T. Liquid-liquid phase separation and morphology of internally mixed dicarboxylic acids/ammonium sulfate/water particles. Atmos. Chem. Phys. 12 , 2691–2712 (2012).
You, Y. et al. Images reveal that atmospheric particles can undergo liquid–liquid phase separations. Proc. Natl. Acad. Sci. USA 109 , 13188–13193 (2012).
You, Y., Renbaum-Wolff, L. & Bertram, A. K. Liquid–liquid phase separation in particles containing organics mixed with ammonium sulfate, ammonium bisulfate, ammonium nitrate or sodium chloride. Atmos. Chem. Phys. 13 , 11723–11734 (2013).
Article Google Scholar
Kuang, Y. et al. Deliquescent phenomena of ambient aerosols on the North China Plain. Geophys. Res. Lett. 43 , 8744–8750 (2016).
Sun, J. et al. Key role of nitrate in phase transitions of urban particles: Implications of important reactive surfaces for secondary aerosol formation. J. Geophys. Res. -Atmos. 123 , 1234–1243 (2018).
Meng, X. et al. Particle phase state and aerosol liquid water greatly impact secondary aerosol formation: insights into phase transition and its role in haze events. Atmos. Chem. Phys. 24 , 2399–2414 (2024).
Qiao, H. et al. Unlocking the mystery of aerosol phase transitions governed by relative humidity history through an advanced outdoor nephelometer system. Geophys. Res. Lett . 51 https://doi.org/10.1029/2023GL107179 (2024).
Jose, C. et al. Complex hygroscopic behavior of ambient aerosol particles revealed by a piezoelectric technique. ACS Earth Space Chem. 8 , 983–991 (2024).
Pajunoja, A. et al. Adsorptive uptake of water by semisolid secondary organic aerosols. Geophys. Res. Lett. 42 , 3063–3068 (2015).
Virtanen, A. et al. An amorphous solid state of biogenic secondary organic aerosol particles. Nature 467 , 824–827 (2010).
Pajunoja, A. et al. Phase state of ambient aerosol linked with water uptake and chemical aging in the southeastern US. Atmos. Chem. Phys. 16 , 11163–11176 (2016).
Bateman, A. P. et al. Sub-micrometre particulate matter is primarily in liquid form over Amazon rainforest. Nat. Geosci. 9 , 34–37 (2016).
Reid, J. P. et al. The viscosity of atmospherically relevant organic particles. Nat. Commun. 9 , 956 (2018).
Martin, S. T. Phase transitions of aqueous atmospheric particles. Chem. Rev. 100 , 3403–3454 (2000).
Choi, M. Y. & Chan, C. K. The effects of organic species on the hygroscopic behaviors of inorganic aerosols. Environ. Sci. Technol. 36 , 2422–2428 (2002).
Krieger, U. K., Marcolli, C. & Reid, J. P. Exploring the complexity of aerosol particle properties and processes using single particle techniques. Chem. Soc. Rev. 41 , 6631–6662 (2012).
Kawamura, K. & Bikkina, S. A review of dicarboxylic acids and related compounds in atmospheric aerosols: Molecular distributions, sources and transformation. Atmos. Res. 170 , 140–160 (2016).
Peng, C., Chan, M. N. & Chan, C. K. The hygroscopic properties of dicarboxylic and multifunctional acids: Measurements and UNIFAC predictions. Environ. Sci. Technol. 35 , 4495–4501 (2001).
Prenni, A. J. et al. The effects of low molecular weight dicarboxylic acids on cloud formation. J. Phys. Chem. A 105 , 11240–11248 (2001).
Zamora, I. R., Tabazadeh, A., Golden, D. M. & Jacobson, M. Z. Hygroscopic growth of common organic aerosol solutes, including humic substances, as derived from water activity measurements. J. Geophys. Res.-Atmos . 116 https://doi.org/10.1029/2011jd016067 (2011).
Jing, B. et al. Hygroscopic behavior of multicomponent organic aerosols and their internal mixtures with ammonium sulfate. Atmos. Chem. Phys. 16 , 4101–4118 (2016).
Marcolli, C., Luo, B. & Peter, T. Mixing of the organic aerosol fractions: Liquids as the thermodynamically stable phases. J. Phys. Chem. A 108 , 2216–2224 (2004).
Zobrist, B., Marcolli, C., Pedernera, D. A. & Koop, T. Do atmospheric aerosols form glasses? Atmos. Chem. Phys. 8 , 5221–5244 (2008).
Vepsäläinen, S., Calderón, S. M., Malila, J. & Prisle, N. L. Comparison of six approaches to predicting droplet activation of surface active aerosol – Part 1: moderately surface active organics. Atmos. Chem. Phys. 22 , 2669–2687 (2022).
Prisle, N. L. Surfaces of atmospheric droplet models probed with synchrotron XPS on a liquid microjet. Acc. Chem. Res. 57 , 177–187 (2024).
Schmedding, R. & Zuend, A. A thermodynamic framework for bulk–surface partitioning in finite-volume mixed organic–inorganic aerosol particles and cloud droplets. Atmos. Chem. Phys. 23 , 7741–7765 (2023).
Sengupta, G., Zheng, M. & Prisle, N. L. Impact of acidity and surface-modulated acid dissociation on cloud response to organic aerosol. Atmos. Chem. Phys. 24 , 1467–1487 (2024).
Prenni, A. J., DeMott, P. J. & Kreidenweis, S. M. Water uptake of internally mixed particles containing ammonium sulfate and dicarboxylic acids. Atmos. Environ. 37 , 4243–4251 (2003).
Zardini, A. A. et al. A combined particle trap/HTDMA hygroscopicity study of mixed inorganic/organic aerosol particles. Atmos. Chem. Phys. 8 , 5589–5601 (2008).
Ghorai, S., Wang, B., Tivanski, A. & Laskin, A. Hygroscopic properties of internally mixed particles composed of NaCl and water-soluble organic acids. Environ. Sci. Technol. 48 , 2234–2241 (2014).
Laskina, O. et al. Size matters in the water uptake and hygroscopic growth of atmospherically relevant multicomponent aerosol particles. J. Phys. Chem. A 119 , 4489–4497 (2015).
Peng, C., Jing, B., Guo, Y.-C., Zhang, Y.-H. & Ge, M.-F. Hygroscopic behavior of multicomponent aerosols involving NaCl and dicarboxylic acids. J. Phys. Chem. A 120 , 1029–1038 (2016).
Jing, B. et al. Hygroscopic behavior of atmospheric aerosols containing nitrate salts and water-soluble organic acids. Atmos. Chem. Phys. 18 , 5115–5127 (2018).
Wang, N. et al. Hygroscopicity and compositional evolution of atmospheric aerosols containing water-soluble carboxylic acid salts and ammonium sulfate: Influence of ammonium depletion. Environ. Sci. Technol. 53 , 6225–6234 (2019).
Sheldon, C. S. et al. Exploring the hygroscopicity, water diffusivity, and viscosity of organic–inorganic aerosols – a case study on internally-mixed citric acid and ammonium sulfate particles. Environ. Sci. -Atmos. 3 , 24–34 (2023).
Parsons, M. T., Knopf, D. A. & Bertram, A. K. Deliquescence and crystallization of ammonium sulfate particles internally mixed with water-soluble organic compounds. J. Phys. Chem. A 108 , 11600–11608 (2004).
Veghte, D. P., Altaf, M. B. & Freedman, M. A. Size dependence of the structure of organic aerosol. J. Am. Chem. Soc. 135 , 16046–16049 (2013).
Richards, D. S. et al. Ion-molecule interactions enable unexpected phase transitions in organic-inorganic aerosol. Sci. Adv . 6 https://doi.org/10.1126/sciadv.abb5643 (2020).
Ruehl, C. R., Davies, J. F. & Wilson, K. R. An interfacial mechanism for cloud droplet formation on organic aerosols. Science 351 , 1447–1450 (2016).
Ovadnevaite, J. et al. Surface tension prevails over solute effect in organic-influenced cloud droplet activation. Nature 546 , 637–641 (2017).
Malek, K. et al. Liquid–liquid phase separation can drive aerosol droplet growth in supersaturated regimes. ACS Environ. Au 3 , 348–360 (2023).
Laskin, A. et al. Tropospheric chemistry of internally mixed sea salt and organic particles: Surprising reactivity of NaCl with weak organic acids. J. Geophys. Res.-Atmos . 117 https://doi.org/10.1029/2012jd017743 (2012).
Wang, B. & Laskin, A. Reactions between water-soluble organic acids and nitrates in atmospheric aerosols: Recycling of nitric acid and formation of organic salts. J. Geophys. Res. -Atmos. 119 , 3335–3351 (2014).
Ma, Q. et al. A comprehensive study about the hygroscopic behavior of mixtures of oxalic acid and nitrate salts: Implication for the occurrence of atmospheric metal oxalate complex. ACS Earth Space Chem. 3 , 1216–1225 (2019).
Clegg, S. L. & Seinfeld, J. H. Thermodynamic models of aqueous solutions containing inorganic electrolytes and dicarboxylic acids at 298.15 K. 1. The acids as nondissociating components. J. Phys. Chem. A 110 , 5692–5717 (2006).
Clegg, S. L. & Seinfeld, J. H. Thermodynamic models of aqueous solutions containing inorganic electrolytes and dicarboxylic acids at 298.15 K. 2. Systems including dissociation equilibria. J. Phys. Chem. A 110 , 5718–5734 (2006).
Tang, M. et al. A review of experimental techniques for aerosol hygroscopicity studies. Atmos. Chem. Phys. 19 , 12631–12686 (2019).
Kreidenweis, S. & Asa-Awuku, A. Treatise on geochemistry Ch. 5.13, 331-361 (Elsevier Ltd., 2014).
Wang, X. et al. Hygroscopic behavior and chemical composition evolution of internally mixed aerosols composed of oxalic acid and ammonium sulfate. Atmos. Chem. Phys. 17 , 12797–12812 (2017).
Liang, Z., Chu, Y., Gen, M. & Chan, C. K. Single-particle Raman spectroscopy for studying physical and chemical processes of atmospheric particles. Atmos. Chem. Phys. 22 , 3017–3044 (2022).
Svenningsson, B. et al. Hygroscopic growth and critical supersaturations for mixed aerosol particles of inorganic and organic compounds of atmospheric relevance. Atmos. Chem. Phys. 6 , 1937–1952 (2006).
Ling, T. Y. & Chan, C. K. Partial crystallization and deliquescence of particles containing ammonium sulfate and dicarboxylic acids. J. Geophys. Res.-Atmos . 113 https://doi.org/10.1029/2008jd009779 (2008).
Kuang, Y. et al. A novel method for deriving the aerosol hygroscopicity parameter based only on measurements from a humidified nephelometer system. Atmos. Chem. Phys. 17 , 6651–6662 (2017).
Kuang, Y. et al. Distinct diurnal variation in organic aerosol hygroscopicity and its relationship with oxygenated organic aerosol. Atmos. Chem. Phys. 20 , 865–880 (2020).
Kuang, Y. et al. Contrasting effects of secondary organic aerosol formations on organic aerosol hygroscopicity. Atmos. Chem. Phys. 21 , 10375–10391 (2021).
Fierz-Schmidhauser, R. et al. Measurement of relative humidity dependent light scattering of aerosols. Atmos. Meas. Tech. 3 , 39–50 (2010).
Titos, G. et al. Effect of hygroscopic growth on the aerosol light-scattering coefficient: A review of measurements, techniques and error sources. Atmos. Environ. 141 , 494–507 (2016).
Köhler, H. The nucleus in and the growth of hygroscopic droplets. Trans. Faraday Soc. 32 , 1152–1161 (1936).
Seinfeld, J. H. & Pandis, S. N. Atmospheric chemistry and physics: from air pollution to climate change . 712-714 (John Wiley & Sons, 2016).
Wex, H., Stratmann, F., Topping, D. & McFiggans, G. The Kelvin versus the Raoult term in the Köhler equation. J. Atmos. Sci. 65 , 4004–4016 (2008).
Petters, M. D. & Kreidenweis, S. M. A single parameter representation of hygroscopic growth and cloud condensation nucleus activity. Atmos. Chem. Phys. 7 , 1961–1971 (2007).
Carrico, C. M. et al. Aerosol hygroscopicity and cloud droplet activation of extracts of filters from biomass burning experiments. J. Geophys. Res.-Atmos . 113 https://doi.org/10.1029/2007JD009274 (2008).
Chan, M. N., Kreidenweis, S. M. & Chan, C. K. Measurements of the hygroscopic and deliquescence properties of organic compounds of different solubilities in water and their relationship with cloud condensation nuclei activities. Environ. Sci. Technol. 42 , 3602–3608 (2008).
Kreidenweis, S. M. et al. Water activity and activation diameters from hygroscopicity data - Part I: Theory and application to inorganic salts. Atmos. Chem. Phys. 5 , 1357–1370 (2005).
Sjogren, S. et al. Hygroscopic growth and water uptake kinetics of two-phase aerosol particles consisting of ammonium sulfate, adipic and humic acid mixtures. J. Aerosol Sci. 38 , 157–171 (2007).
Stokes, R. H. & Robinson, R. A. Interactions in aqueous nonelectrolyte solutions. I. Solute-solvent equilibria. J. Phys. Chem. 70 , 2126–2131 (1966).
Malm, W. C. & Kreidenweis, S. M. The effects of models of aerosol hygroscopicity on the apportionment of extinction. Atmos. Environ. 31 , 1965–1976 (1997).
Wexler, A. S. & Clegg, S. L. Atmospheric aerosol models for systems including the ions H + , NH 4 + , Na + , SO 4 2− , NO 3 − , Cl − , Br − , and H 2 O. J. Geophys. Res.-Atmos . 107 https://doi.org/10.1029/2001JD000451 (2002).
Fredenslund, A., Jones, R. L. & Prausnitz, J. M. Group-contribution estimation of activity coefficients in nonideal liquid mixtures. AIChE J. 21 , 1086–1099 (1975).
Hansen, H. K., Rasmussen, P., Fredenslund, A., Schiller, M. & Gmehling, J. Vapor-liquid equilibria by UNIFAC group contribution. 5. Revision and extension. Ind. Eng. Chem. Res. 30 , 2352–2355 (1991).
Wittig, R., Lohmann, J. & Gmehling, J. Vapor−liquid equilibria by UNIFAC group contribution. 6. Revision and extension. Ind. Eng. Chem. Res. 42 , 183–188 (2003).
Zuend, A., Marcolli, C., Peter, T. & Seinfeld, J. H. Computation of liquid-liquid equilibria and phase stabilities: implications for RH-dependent gas/particle partitioning of organic-inorganic aerosols. Atmos. Chem. Phys. 10 , 7795–7820 (2010).
Zuend, A. et al. New and extended parameterization of the thermodynamic model AIOMFAC: calculation of activity coefficients for organic-inorganic mixtures containing carboxyl, hydroxyl, carbonyl, ether, ester, alkenyl, alkyl, and aromatic functional groups. Atmos. Chem. Phys. 11 , 9155–9206 (2011).
Brooks, S. D., Wise, M. E., Cushing, M. & Tolbert, M. A. Deliquescence behavior of organic/ammonium sulfate aerosol. Geophys. Res. Lett . 29 https://doi.org/10.1029/2002gl014733 (2002).
Braban, C. F., Carroll, M. F., Styler, S. A. & Abbatt, J. P. D. Phase transitions of malonic and oxalic acid aerosols. J. Phys. Chem. A 107 , 6594–6602 (2003).
Ma, Q., Ma, J., Liu, C., Lai, C. & He, H. Laboratory study on the hygroscopic behavior of external and internal C 2 –C 4 dicarboxylic acid–NaCl mixtures. Environ. Sci. Technol. 47 , 10381–10388 (2013).
CAS Google Scholar
Zhang, C. et al. Hygroscopic growth of aerosol particles consisted of oxalic acid and its internal mixture with ammonium sulfate for the relative humidity ranging from 80% to 99.5%. Atmos. Environ. 252 , 118318 (2021).
Huff Hartz, K. E. et al. Cloud condensation nuclei activation of limited solubility organic aerosol. Atmos. Environ. 40 , 605–617 (2006).
Massoli, P. et al. Relationship between aerosol oxidation level and hygroscopic properties of laboratory generated secondary organic aerosol (SOA) particles. Geophys. Res. Lett . 37 https://doi.org/10.1029/2010GL045258 (2010).
Suda, S. R. et al. Influence of functional groups on organic aerosol cloud condensation nucleus activity. Environ. Sci. Technol. 48 , 10182–10190 (2014).
Wang, J. et al. Cloud droplet activation of secondary organic aerosol is mainly controlled by molecular weight, not water solubility. Atmos. Chem. Phys. 19 , 941–954 (2019).
Han, S. et al. Hygroscopicity of organic compounds as a function of organic functionality, water solubility, molecular weight, and oxidation level. Atmos. Chem. Phys. 22 , 3985–4004 (2022).
Xiong, C. et al. Reconsideration of surface tension and phase state effects on cloud condensation nuclei activity based on the atomic force microscopy measurement. Atmos. Chem. Phys. 22 , 16123–16135 (2022).
Marsh, A. et al. Influence of organic compound functionality on aerosol hygroscopicity: dicarboxylic acids, alkyl-substituents, sugars and amino acids. Atmos. Chem. Phys. 17 , 5583–5599 (2017).
Marsh, A., Rovelli, G., Miles, R. E. H. & Reid, J. P. Complexity of measuring and representing the hygroscopicity of mixed component aerosol. J. Phys. Chem. A 123 , 1648–1660 (2019).
Farmer, D. K., Cappa, C. D. & Kreidenweis, S. M. Atmospheric processes and their controlling influence on cloud condensation nuclei activity. Chem. Rev. 115 , 4199–4217 (2015).
Liu, P. et al. Resolving the mechanisms of hygroscopic growth and cloud condensation nuclei activity for organic particulate matter. Nat. Commun. 9 , 4076 (2018).
Altaf, M. B., Dutcher, D. D., Raymond, T. M. & Freedman, M. A. Effect of particle morphology on cloud condensation nuclei activity. ACS Earth Space Chem. 2 , 634–639 (2018).
Bilde, M. & Svenningsson, B. CCN activation of slightly soluble organics: the importance of small amounts of inorganic salt and particle phase. Tellus B 56 , 128–134 (2004).
Kreidenweis, S. M., Petters, M. D. & DeMott, P. J. Deliquescence-controlled activation of organic aerosols. Geophys. Res. Lett . 33 https://doi.org/10.1029/2005GL024863 (2006).
Pradeep Kumar, P., Broekhuizen, K. & Abbatt, J. P. D. Organic acids as cloud condensation nuclei: Laboratory studies of highly soluble and insoluble species. Atmos. Chem. Phys. 3 , 509–520 (2003).
Sun, J. & Ariya, P. A. Atmospheric organic and bio-aerosols as cloud condensation nuclei (CCN): A review. Atmos. Environ. 40 , 795–820 (2006).
Hori, M., Ohta, S., Murao, N. & Yamagata, S. Activation capability of water soluble organic substances as CCN. J. Aerosol Sci. 34 , 419–448 (2003).
Rastak, N. et al. Microphysical explanation of the RH‐dependent water affinity of biogenic organic aerosol and its importance for climate. Geophys. Res. Lett. 44 , 5167–5177 (2017).
Miñambres, L., Méndez, E., Sánchez, M. N., Castaño, F. & Basterretxea, F. J. Water uptake of internally mixed ammonium sulfate and dicarboxylic acid particles probed by infrared spectroscopy. Atmos. Environ. 70 , 108–116 (2013).
Bouzidi, H., Zuend, A., Ondráček, J., Schwarz, J. & Ždímal, V. Hygroscopic behavior of inorganic–organic aerosol systems including ammonium sulfate, dicarboxylic acids, and oligomer. Atmos. Environ. 229 , 117481 (2020).
Wise, M. E., Surratt, J. D., Curtis, D. B., Shilling, J. E. & Tolbert, M. A. Hygroscopic growth of ammonium sulfate/dicarboxylic acids. J. Geophys. Res. -Atmos. 108 , 4638 (2003).
Chan, M. N. & Chan, C. K. Hygroscopic properties of two model humic-like substances and their mixtures with inorganics of atmospheric importance. Environ. Sci. Technol. 37 , 5109–5115 (2003).
Gysel, M. et al. Hygroscopic properties of water-soluble matter and humic-like organics in atmospheric fine aerosol. Atmos. Chem. Phys. 4 , 35–50 (2004).
Pope, F. D., Dennis-Smither, B. J., Griffiths, P. T., Clegg, S. L. & Cox, R. A. Studies of single aerosol particles containing malonic acid, glutaric acid, and their mixtures with sodium chloride. I. Hygroscopic growth. J. Phys. Chem. A 114 , 5335–5341 (2010).
Estillore, A. D. et al. Water uptake and hygroscopic growth of organosulfate aerosol. Environ. Sci. Technol. 50 , 4259–4268 (2016).
Wei, X. et al. Technical note: Real-time diagnosis of the hygroscopic growth micro-dynamics of nanoparticles with Fourier transform infrared spectroscopy. Atmos. Chem. Phys. 22 , 3097–3109 (2022).
Braban, C. F. & Abbatt, J. P. D. A study of the phase transition behavior of internally mixed ammonium sulfate - malonic acid aerosols. Atmos. Chem. Phys. 4 , 1451–1459 (2004).
Hämeri, K., Charlson, R. & Hansson, H.-C. Hygroscopic properties of mixed ammonium sulfate and carboxylic acids particles. AIChE J. 48 , 1309–1316 (2002).
Yeung, M. C. & Chan, C. K. Water content and phase transitions in particles of inorganic and organic species and their mixtures using micro-Raman spectroscopy. Aerosol Sci. Tech. 44 , 269–280 (2010).
Parsons, M. T., Riffell, J. L. & Bertram, A. K. Crystallization of aqueous inorganic−malonic acid particles: Nucleation rates, dependence on size, and dependence on the ammonium-to-sulfate ratio. J. Phys. Chem. A 110 , 8108–8115 (2006).
Miñambres, L., Sánchez, M. N., Castaño, F. & Basterretxea, F. J. Hygroscopic properties of internally mixed particles of ammonium sulfate and succinic acid studied by infrared spectroscopy. J. Phys. Chem. A 114 , 6124–6130 (2010).
Yeung, M. C., Lee, A. K. Y. & Chan, C. K. Phase transition and hygroscopic properties of internally mixed ammonium sulfate and adipic acid (AS-AA) particles by optical microscopic imaging and Raman spectroscopy. Aerosol Sci. Tech. 43 , 387–399 (2009).
Zelenay, V. et al. Direct observation of water uptake and release in individual submicrometer sized ammonium sulfate and ammonium sulfate/adipic acid particles using X-ray microspectroscopy. J. Aerosol Sci. 42 , 38–51 (2011).
Zhang, C. et al. Surfactant effect on the hygroscopicity of aerosol particles at relative humidity ranging from 80% to 99.5%: Internally mixed adipic acid-ammonium sulfate particles. Atmos. Environ. 266 , 118725 (2021).
Ruehl, C. R. & Wilson, K. R. Surface organic monolayers control the hygroscopic growth of submicrometer particles at high relative humidity. J. Phys. Chem. A 118 , 3952–3966 (2014).
Shi, X.-M. et al. Hygroscopicity of internally mixed particles composed of (NH 4 ) 2 SO 4 and citric acid under pulsed RH change. Chemosphere 188 , 532–540 (2017).
Broekhuizen, K., Kumar, P. P. & Abbatt, J. P. D. Partially soluble organics as cloud condensation nuclei: Role of trace soluble and surface active species. Geophys. Res. Lett . 31 https://doi.org/10.1029/2003GL018203 (2004).
Frosch, M., Prisle, N. L., Bilde, M., Varga, Z. & Kiss, G. Joint effect of organic acids and inorganic salts on cloud droplet activation. Atmos. Chem. Phys. 11 , 3895–3911 (2011).
Abbatt, J., Broekhuizen, K. & Kumar, P. P. Cloud condensation nucleus activity of internally mixed ammonium sulfate/organic acid aerosol particles. Atmos. Environ. 39 , 4767–4778 (2005).
Tao, J. et al. Significant impact of water-soluble organic matter on hygroscopicity of fine particles under low relative humidity condition. Sci. Total Environ. 907 , 167980 (2024).
Hodas, N., Zuend, A., Mui, W., Flagan, R. C. & Seinfeld, J. H. Influence of particle-phase state on the hygroscopic behavior of mixed organic–inorganic aerosols. Atmos. Chem. Phys. 15 , 5027–5045 (2015).
Liu, Q. et al. Hygroscopicity of internally mixed multi-component aerosol particles of atmospheric relevance. Atmos. Environ. 125 , 69–77 (2016).
Chan, M. N., Lee, A. K. Y. & Chan, C. K. Responses of ammonium sulfate particles coated with glutaric acid to cyclic changes in relative humidity: Hygroscopicity and Raman characterization. Environ. Sci. Technol. 40 , 6983–6989 (2006).
Wang, W. et al. Effect of mixing structure on the water uptake of mixtures of ammonium sulfate and phthalic acid particles. Atmos. Chem. Phys. 21 , 2179–2190 (2021).
Li, W. et al. Organic coating reduces hygroscopic growth of phase-separated aerosol particles. Environ. Sci. Technol. 55 , 16339–16346 (2021).
Maskey, S. et al. Effect of mixing structure on the hygroscopic behavior of ultrafine ammonium sulfate particles mixed with succinic acid and levoglucosan. Particuology 13 , 27–34 (2014).
Davies, J. F., Miles, R. E. H., Haddrell, A. E. & Reid, J. P. Influence of organic films on the evaporation and condensation of water in aerosol. Proc. Natl. Acad. Sci. USA 110 , 8807–8812 (2013).
Freedman, M. A. Liquid–liquid phase separation in supermicrometer and submicrometer aerosol particles. Acc. Chem. Res. 53 , 1102–1110 (2020).
Song, M., Liu, P., Martin, S. T. & Bertram, A. K. Liquid–liquid phase separation in particles containing secondary organic material free of inorganic salts. Atmos. Chem. Phys. 17 , 11261–11271 (2017).
Mahrt, F. et al. Phase behavior of internal mixtures of hydrocarbon-like primary organic aerosol and secondary aerosol based on their differences in oxygen-to-carbon ratios. Environ. Sci. Technol. 56 , 3960–3973 (2022).
Song, M., Marcolli, C., Krieger, U. K., Zuend, A. & Peter, T. Liquid‐liquid phase separation in aerosol particles: Dependence on O:C, organic functionalities, and compositional complexity. Geophys. Res. Lett . 39 https://doi.org/10.1029/2012gl052807 (2012).
Li, W. et al. Microscopic evidence for phase separation of organic species and inorganic salts in fine ambient aerosol particles. Environ. Sci. Technol. 55 , 2234–2242 (2021).
Ott, E.-J. E., Tackman, E. C. & Freedman, M. A. Effects of sucrose on phase transitions of organic/inorganic aerosols. ACS Earth Space Chem. 4 , 591–601 (2020).
Werner, J. et al. Surface partitioning in organic–inorganic mixtures contributes to the size-dependence of the phase-state of atmospheric nanoparticles. Environ. Sci. Technol. 50 , 7434–7442 (2016).
Lowe, S. J. et al. Key drivers of cloud response to surface-active organics. Nat. Commun . 10 https://doi.org/10.1038/s41467-019-12982-0 (2019).
Bzdek, B. R., Reid, J. P., Malila, J. & Prisle, N. L. The surface tension of surfactant-containing, finite volume droplets. Proc. Natl. Acad. Sci. USA 117 , 8335–8343 (2020).
Bain, A., Ghosh, K., Prisle, N. L. & Bzdek, B. R. Surface-area-to-volume ratio determines surface tensions in microscopic, surfactant-containing droplets. ACS Cent. Sci. 9 , 2076–2083 (2023).
Fan, T. et al. Evidence of surface-tension lowering of atmospheric aerosols by organics from field observations in an urban atmosphere: Relation to particle size and chemical composition. Environ. Sci. Technol. 58 , 11363–11375 (2024).
Ruehl, C. R. et al. Strong evidence of surface tension reduction in microscopic aqueous droplets. Geophys. Res. Lett . 39 https://doi.org/10.1029/2012GL053706 (2012).
Gen, M., Hibara, A., Phung, P. N., Miyazaki, Y. & Mochida, M. In situ surface tension measurement of deliquesced aerosol particles. J. Phys. Chem. A 127 , 6100–6108 (2023).
Prisle, N. L., Raatikainen, T., Laaksonen, A. & Bilde, M. Surfactants in cloud droplet activation: mixed organic-inorganic particles. Atmos. Chem. Phys. 10 , 5663–5683 (2010).
Vepsäläinen, S., Calderón, S. M. & Prisle, N. L. Comparison of six approaches to predicting droplet activation of surface active aerosol – Part 2: Strong surfactants. Atmos. Chem. Phys. 23 , 15149–15164 (2023).
Su, H., Cheng, Y. & Pöschl, U. New multiphase chemical processes influencing atmospheric aerosols, air quality, and climate in the anthropocene. Acc. Chem. Res. 53 , 2034–2043 (2020).
Mikhailov, E., Vlasenko, S. & Niessner, R. & Pöschl, U. Interaction of aerosol particles composed of protein and salts with water vapor: hygroscopic growth and microstructural rearrangement. Atmos. Chem. Phys. 4 , 323–350 (2004).
Gibson, E. R., Hudson, P. K. & Grassian, V. H. Physicochemical properties of nitrate aerosols: Implications for the atmosphere. J. Phys. Chem. A 110 , 11785–11799 (2006).
Lightstone, J. M., Onasch, T. B., Imre, D., Oatis, S. & Deliquescence efflorescence, and water activity in ammonium nitrate and mixed ammonium nitrate/succinic acid microparticles. J. Phys. Chem. A 104 , 9337–9346 (2000).
Wang, Z. et al. Importance of water-soluble organic acid on the hygroscopicity of nitrate. Atmos. Environ. 190 , 65–73 (2018).
Hawkins, L. N. et al. Formation of semisolid, oligomerized aqueous SOA: Lab simulations of cloud processing. Environ. Sci. Technol. 48 , 2273–2280 (2014).
Sun, J. et al. Role of WSOCs and pH on ammonium nitrate aerosol efflorescence: Insights into secondary aerosol formation. Environ. Sci. Technol. 57 , 20074–20084 (2023).
Qiu, Y. et al. Predicting atmospheric particle phase state using an explainable machine learning approach based on particle rebound measurements. Environ. Sci. Technol. 57 , 15055–15064 (2023).
Liu, Y. C. et al. Enhanced nitrate fraction: Enabling urban aerosol particles to remain in a liquid state at reduced relative humidity. Geophys. Res. Lett . 50 https://doi.org/10.1029/2023gl105505 (2023).
Li, X., Wu, L., Lee, J.-S. & Ro, C.-U. Hygroscopic behavior and chemical reactivity of aerosols generated from mixture solutions of low molecular weight dicarboxylic acids and NaCl. Phys. Chem. Chem. Phys. 23 , 11052–11064 (2021).
Wu, Z. J., Nowak, A., Poulain, L., Herrmann, H. & Wiedensohler, A. Hygroscopic behavior of atmospherically relevant water-soluble carboxylic salts and their influence on the water uptake of ammonium sulfate. Atmos. Chem. Phys. 11 , 12617–12626 (2011).
Moore, R. H. & Raymond, T. M. HTDMA analysis of multicomponent dicarboxylic acid aerosols with comparison to UNIFAC and ZSR. J. Geophys. Res.-Atmos . 113 https://doi.org/10.1029/2007jd008660 (2008).
Wang, Y. et al. Water uptake of multicomponent organic mixtures and their influence on hygroscopicity of inorganic salts. J. Environ. Sci. 45 , 156–163 (2016).
Schroeder, J. R. & Beyer, K. D. Deliquescence relative humidities of organic and inorganic salts important in the atmosphere. J. Phys. Chem. A 120 , 9948–9957 (2016).
Kerminen, V.-M., Teinilä, K., Hillamo, R. & Pakkanen, T. Substitution of chloride in sea-salt particles by inorganic and organic anions. J. Aerosol Sci. 29 , 929–942 (1998).
Bauer, S. E. et al. Nitrate aerosols today and in 2030: a global simulation including aerosols and tropospheric ozone. Atmos. Chem. Phys. 7 , 5043–5059 (2007).
Braun, R. A. et al. Impact of wildfire emissions on chloride and bromide depletion in marine aerosol particles. Environ. Sci. Technol. 51 , 9013–9021 (2017).
Jing, B. et al. Hygroscopic properties of potassium chloride and its internal mixtures with organic compounds relevant to biomass burning aerosol particles. Sci. Rep . 7 https://doi.org/10.1038/srep43572 (2017).
Ma, S., Li, Q. & Zhang, Y. A comprehensive study on hygroscopic behaviour and nitrate depletion of NaNO 3 and dicarboxylic acid mixtures: implications for nitrate depletion in tropospheric aerosols. Atmos. Chem. Phys. 22 , 10955–10970 (2022).
Edwards, E. L. et al. Sea salt reactivity over the northwest Atlantic: an in-depth look using the airborne ACTIVATE dataset. Atmos. Chem. Phys. 24 , 3349–3378 (2024).
Angle, K. J. & Grassian, V. H. Direct quantification of changes in pH within single levitated microdroplets and the kinetics of nitrate and chloride depletion. Chem. Sci. 14 , 6259–6268 (2023).
Wilson, K. R. & Prophet, A. M. Chemical kinetics in microdroplets. Annu. Rev. Phys. Chem . https://doi.org/10.1146/annurev-physchem-052623-120718 (2024).
Li, L.-F. et al. Rethinking urban haze formation: Atmospheric sulfite conversion rate scales with aerosol surface area, not volume. One Earth https://doi.org/10.1016/j.oneear.2024.05.007 (2024).
Chen, Z., Liu, P., Su, H. & Zhang, Y.-H. Displacement of strong acids or bases by weak acids or bases in aerosols: Thermodynamics and kinetics. Environ. Sci. Technol. 56 , 12937–12944 (2022).
Li, X., Gupta, D., Lee, J., Park, G. & Ro, C.-U. Real-time investigation of chemical compositions and hygroscopic properties of aerosols generated from NaCl and malonic acid mixture solutions using in situ Raman microspectrometry. Environ. Sci. Technol. 51 , 263–270 (2017).
Saha, S. & Mathi, P. Exploring the hygroscopicity and chemical composition evolution in organic-inorganic aerosols: A study on internally mixed malonic acid-metal (Na + , Ca 2+ , Mg 2+ ) nitrates. Chemosphere 336 , 139260 (2023).
Jing, X., Chen, Z., Huang, Q., Liu, P. & Zhang, Y.-H. Spatiotemporally resolved pH measurement in aerosol microdroplets undergoing chloride depletion: An application of in situ Raman microspectrometry. Anal. Chem. 94 , 15132–15138 (2022).
Chen, Z., Liu, P., Liu, Y. & Zhang, Y.-H. Strong acids or bases displaced by weak acids or bases in aerosols: Reactions driven by the continuous partitioning of volatile products into the gas phase. Acc. Chem. Res. 54 , 3667–3678 (2021).
Li, L.-F., Chen, Z., Liu, P. & Zhang, Y.-H. Direct measurement of pH evolution in aerosol microdroplets undergoing ammonium depletion: A surface-enhanced raman spectroscopy approach. Environ. Sci. Technol. 56 , 6274–6281 (2022).
Silvern, R. F. et al. Inconsistency of ammonium–sulfate aerosol ratios with thermodynamic models in the eastern US: a possible role of organic aerosol. Atmos. Chem. Phys. 17 , 5107–5118 (2017).
Nah, T. et al. Characterization of aerosol composition, aerosol acidity, and organic acid partitioning at an agriculturally intensive rural southeastern US site. Atmos. Chem. Phys. 18 , 11471–11491 (2018).
Guo, H., Nenes, A. & Weber, R. J. The underappreciated role of nonvolatile cations in aerosol ammonium-sulfate molar ratios. Atmos. Chem. Phys. 18 , 17307–17323 (2018).
Zheng, G., Su, H. & Cheng, Y. Revisiting the key driving processes of the decadal trend of aerosol acidity in the U.S. ACS Environ. Au 2 , 346–353 (2022).
Huang, Q. et al. Experimental phase diagram and its temporal evolution for submicron 2-methylglutaric acid and ammonium sulfate aerosol particles. Phys. Chem. Chem. Phys. 26 , 2887–2894 (2024).
Prisle, N. L. A predictive thermodynamic framework of cloud droplet activation for chemically unresolved aerosol mixtures, including surface tension, non-ideality, and bulk–surface partitioning. Atmos. Chem. Phys. 21 , 16387–16411 (2021).
Lin, Y. et al. Interaction between succinic acid and sulfuric acid–base clusters. Atmos. Chem. Phys. 19 , 8003–8019 (2019).
Fang, X. et al. Observational evidence for the involvement of dicarboxylic acids in particle nucleation. Environ. Sci. Technol. Lett. 7 , 388–394 (2020).
Zhang, R. et al. Atmospheric new particle formation enhanced by organic acids. Science 304 , 1487–1490 (2004).
Arquero, K. D., Gerber, R. B. & Finlayson-Pitts, B. J. The role of oxalic acid in new particle formation from methanesulfonic acid, methylamine, and water. Environ. Sci. Technol. 51 , 2124–2130 (2017).
Li, X. & Bourg, I. C. Phase state, surface tension, water activity, and accommodation coefficient of water–organic clusters near the critical size for atmospheric new particle formation. Environ. Sci. Technol. 57 , 13092–13103 (2023).
Liu, H. et al. Relative humidity dependence of growth factor and real refractive index for sea salt/malonic acid internally mixed aerosols. J. Geophys. Res.-Atmos . 128 https://doi.org/10.1029/2022jd037579 (2023).
Wang, B. et al. Reactivity of liquid and semisolid secondary organic carbon with chloride and nitrate in atmospheric aerosols. J. Phys. Chem. A 119 , 4498–4508 (2014).
Shiraiwa, M., Ammann, M., Koop, T. & Pöschl, U. Gas uptake and chemical aging of semisolid organic aerosol particles. Proc. Natl. Acad. Sci. USA 108 , 11003–11008 (2011).
Shiraiwa, M., Zuend, A., Bertram, A. K. & Seinfeld, J. H. Gas–particle partitioning of atmospheric aerosols: interplay of physical state, non-ideal mixing and morphology. Phys. Chem. Chem. Phys. 15 , 11441–11453 (2013).
Tang, I. N. & Munkelwitz, H. R. Composition and temperature dependence of the deliquescence properties of hygroscopic aerosols. Atmos. Environ., Part A 27 , 467–473 (1993).
Apelblat, A., Dov, M., Wisniak, J. & Zabicky, J. Osmotic and activity coefficients of HO 2 CCH 2 C(OH)(CO 2 H)CH 2 CO 2 H (citric acid) in concentrated aqueous solutions at temperatures from 298.15 K to 318.15. K. J. Chem. Thermodyn. 27 , 347–353 (1995).
Download references
Acknowledgements
We gratefully appreciate financial support from the National Natural Science Foundation of China (Nos. 42130606 and 22321004).
Author information
Authors and affiliations.
School of Chemical Engineering, Guizhou Minzu University, Guiyang, 550025, People’s Republic of China
Fang Tan, Hongbin Zhang & Xiaohong Li
Department of Environmental Science and Engineering, University of Science and Technology of China, Hefei, 230026, People’s Republic of China
Jinan Academy of Agricultural Sciences, Jinan, 250100, People’s Republic of China
State Key Laboratory for Structural Chemistry of Unstable and Stable Species, Institute of Chemistry, Chinese Academy of Sciences, Beijing, 100190, People’s Republic of China
Shengrui Tong & Maofa Ge
University of Chinese Academy of Sciences, Beijing, 100049, People’s Republic of China
You can also search for this author in PubMed Google Scholar
Contributions
M.G., B.J. and K.X. conceived and coordinated this paper. B.J. and S.T. contributed to the discussions and reviewed the manuscript. K.X., H.Z. and X.L. participated in editing the first and final versions of the paper. F.T. and B.J. prepared the manuscript and addressed the referees’ comments with the contribution from all the other co-authors.
Corresponding authors
Correspondence to Kaihui Xia or Maofa Ge .
Ethics declarations
Competing interests.
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ .
Reprints and permissions
About this article
Cite this article.
Tan, F., Zhang, H., Xia, K. et al. Hygroscopic behavior and aerosol chemistry of atmospheric particles containing organic acids and inorganic salts. npj Clim Atmos Sci 7 , 203 (2024). https://doi.org/10.1038/s41612-024-00752-9
Download citation
Received : 27 April 2024
Accepted : 21 August 2024
Published : 30 August 2024
DOI : https://doi.org/10.1038/s41612-024-00752-9
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
Quick links
- Explore articles by subject
- Guide to authors
- Editorial policies
Sign up for the Nature Briefing: Anthropocene newsletter — what matters in anthropocene research, free to your inbox weekly.

COMMENTS
A demonstration to show the diffusion of gases, using ammonia solution and hydrochloric acid. Includes kit list and safety instructions.
High School Chemistry A classic demonstration experiment regularly carried out in High School Chemistry classes. Equipment: Glass tube and rubber bungs Concentrated hydrochloric acid Concentrated ...
This demonstration is an excellent display of diffusion in action - especially when coupled with some of Bob Worley's famous ' puddle experiments '. The fact that the hydrogen chloride clearly travels a shorter distance than the ammonia is worth pointing out to students to illustrate the relationship between mass and kinetic energy of ...
G420: Graham's Law of Diffusion - NH3 and HCl Diffusion Introduction A cotton swab is dipped into concentrated hydrochloric acid (producing hydrogen chloride gas) while a second on is dipped into concentrated aqueous ammonia (producing ammonia gas).
Diffusion of NH3 and HCl North Carolina School of Science and Mathematics 117K subscribers Subscribed 524 162K views 12 years ago Part of NCSSM CORE collection: This video shows the relative rates ...
Diffusion of gases: "white ring" experiment. NH3 and HCl diffuse towards each other forming NH4Cl - YouTube
This motion causes gases to travel across space (diffuse) and completely mix with each other. It is this diffusion that eventually causes one to notice smells such as perfume, fish, ammonia, etc. In this experiment, the rates of diffusion of two gases, ammonia (NH3) and hydrogen chloride (HCl), will be investigated.
Soak a ball of cotton wool in aqueous ammonia and insert a few centimetres into the glass tube. Rinse the tweezers in water, and dispose of excess ammonia. Close the end with the rubber bung. At the same time, insert a cotton wool ball soaked in hydrochloric acid at the other end. Rinse the tweezers again in water to avoid corrosion.
Demonstrating diffusion with ammonia and hydrogen chloride A classic demonstration to show the movement of molecules in gases.
Experimental values for the relative rates of diffusion of NH3 and HCl through the air in the tube can then be obtained by measuring the distance traveled by each gas and dividing by the time required for the appearance of the ammonium chloride precipitate.
Kudos to Carlos Correa for his "Chemistry in Pictures Winner: Ammonia and hydrochloric acid" on the cover of the March 2014 issue. Chem 13 News readers should be interested in my related demonstration. Not only does it involve active student participation, but it also provides quantitative proof of Graham's law that relates the ratio of the rates of diffusion of two gases.
The relative rates of diffusion of ammonia to hydrogen chloride can be observed in a simple experiment. Cotton balls are soaked with solutions of ammonia and hydrogen chloride (hydrochloric acid) and attached to two different rubber stoppers.
Elements, Compounds and Mixtures The Diffusion of Hydrogen Chloride and Ammonia Gas through Air to form Ammonium Chloride. Cotton wool soaked in concentrated ammonia solution, NH 3(aq) and concentrated hydrogen chloride solution (also called hydrochloric acid) H Cl (aq) are placed at each end of a sealed tube.
More: www.melscience.com Chemistry experiment: diffusion of hydrogen chloride (HCl) and ammonia (NH3) gases through air to form ammonium chloride smoke (NH4Cl).
Rate of diffusion for HCl = Distance traveled by HCl Time required for ring formation Calculate the ratio between the rate of diffusion of NH3(g) and the rate of diffusion of HCl(g).
Diffusion of Ammonia and HCl. Diffusion of Ammonia and HCl. Cotton swabs dipped into HCl and NH40H respectively are inserted into the ends of the tube. The diffusion velocity of the gases is inversely proportional to the square root of the molecular mass; this results in approximately 3:2 diffusion rate ratio.
Diffusion in gases To demonstrate diffusion in gases, a long glass tube is set up with cotton wool soaked with hydrochloric acid at one end, and cotton wool soaked with ammonia at the other end. The hydrogen chloride and the ammonia gases diffuse along the tube from either end, because the particles are constantly, randomly moving.
Here is an experiment to show the different rates of diffusion of two different gases, ammonia and hydrogen chloride. In the experiment the ammonia molecules move further than the hydrogen ...
The diffusion rates (velocities) of HCl and NH3 gases will be compared. Hydrogen chloride fumes will come from hydrochloric acid and ammonia fumes will come from aqueous ammonia.
Abstract Vapor-phase ammonia, NH 3 (g), and hydrochloric acid, HCl (g), undergo a series of complex reactions, including nucleation and growth, to form solid ammonium chloride, NH 4 Cl (s). The counterdiffusional experiment, whereby HCl (g) and NH 3 (g) diffuse from opposite ends of a tube and react to form spatiotemporally complex patterns, has a rich history of study. In this paper, we ...
This video attempts to show the rate of diffusion of gases along with (hopefully) a suitable and easy to understand explanation of the experiment.Please do n...
The potential influence of WSOAs on the atmospheric recycling of HCl/HNO3/NH3 due to the chloride/nitrate/ammonium depletion may contribute to the atmospheric budget of reactive gases.