
- Longstreth GF, Thompson WG, Chey WD, et al. Functional bowel disorders. Gastroenterology. 2006;130:1480-1491. Abstract
- Patient Reported Outcomes Measurement Information System (PROMIS). National Institutes of Health. http://www.nihpromis.org/ Accessed August 12, 2011.
- 3Brandt LJ, Chey WD, Foxx-Orenstein AE, et al. An evidence-based systematic review on the management of irritable bowel syndrome. Am J Gastroenterol. 2009;104:S1-S34.
- Bijkerk CJ, de Wit NJ, Muris JWM, Whorwell PJ, Knottnerus JA, Hoes AW. Soluble or insoluble fibre in irritable bowel syndrome in primary care? Randomised placebo controlled trial. BMJ. 2009;339:B3154-B3160. Abstract
- Atkinson W, Sheldon TA, Shaath N, Whorwell PJ. Food elimination based on IgG antibodies in irritable bowel syndrome: a randomised controlled trial. Gut. 2004;53:1459-1464. Abstract
- Shepherd SJ, Parker FC, Muir JG, et al. Dietary triggers of abdominal symptoms in patients with irritable bowel syndrome: randomized placebo-controlled evidence. Clin Gastroenterol Hepatol. 2008;6:765-771. Abstract
- Shah ED, Basseri RJ, Chong K, et al. Abnormal breath testing in IBS: a meta-analysis. Dig Dis Sci. 2010;55:2441-2449. Abstract
- Frissora CL, Cash BD. Review article: the role of antibiotics vs. conventional pharmacotherapy in treating symptoms of irritable bowel syndrome. Aliment Pharmacol Ther. 2007;25:1271-1281. Abstract
- Pimental M, Lembo A, Chey W, et al. Rifaximin therapy for patients with irritable bowel syndrome without constipation. N Engl J Med. 2011;364:22-32. Abstract
- Camilleri M, Chey WY, Mayer EA, et al. A randomized controlled clinical trial of the serotonin type 3 receptor antagonist alosetron in women with diarrhea, predominant irritable bowel syndrome. Arch Intern Med. 2001;161:1733-1740. Abstract
- Nyhlin H, Bang C, Elsborg L, et al. A double-blind, placebo-controlled, randomized study to evaluate the efficacy, safety, and tolerability of tegaserod in patients with irritable bowel syndrome. Scand J Gastroenterol. 2004;39:119-126. Abstract
- Drossman DA, Chey WD, Johanson JF, et al. Clinical trial: lubiprostone in patients with constipation-associated irritable bowel syndrome-results of two randomized, placebo-controlled studies. Aliment Pharmacol Ther. 2009;29:329-341. Abstract
- Pimentel M, Park S, Mirocha J, et al. The effect of a nonabsorbed oral antibiotic (rifaximin) on the symptoms of irritable bowel syndrome. Ann Intern Med. 2006;145:557-563. Abstract
- DuPont HL, Jiang ZD. Influence of rifaximin treatment on the susceptibility of intestinal gram-negative flora and enterococci. Clin Microbiol Infect. 2004;10:1009-1011. Abstract
- Yang J, Lee HR, Low K, et al. Rifaximin versus other antibiotics in the primary treatment and retreatment of bacterial overgrowth in IBS. Dig Dis Sci. 2008;53:169-174. Abstract
- Pimentel M, Morales W, Chua K, et al. Effects of rifaximin treatment and retreatment in nonconstipated IBS subjects. Dig Dis Sci. 2011;56:2067-2072. Abstract

Faculty and Disclosures
As an organization accredited by the ACCME, Medscape, LLC, requires everyone who is in a position to control the content of an education activity to disclose all relevant financial relationships with any commercial interest. The ACCME defines "relevant financial relationships" as financial relationships in any amount, occurring within the past 12 months, including financial relationships of a spouse or life partner, that could create a conflict of interest.
Medscape, LLC, encourages Authors to identify investigational products or off-label uses of products regulated by the US Food and Drug Administration, at first mention and where appropriate in the content.
Brooks D. Cash, MD
Professor, Uniformed Services University of the Health Sciences; Chief of Medicine, National Naval Medical Center, Bethesda, Maryland
Disclosure: Brooks D. Cash, MD, has disclosed the following relevant financial relationships: Served as an advisor or consultant for: Salix Pharmaceuticals, Inc.; Takeda Pharmaceuticals North America, Inc.; Ironwood Pharmaceuticals, Inc.; Prometheus Laboratories Inc. Served as a speaker or a member of a speakers bureau for: Salix Pharmaceuticals, Inc.; Takeda Pharmaceuticals North America, Inc.; Prometheus Laboratories Inc. Received grants for clinical research from: Salix Pharmaceuticals, Inc. Dr. Cash does intend to discuss investigational drugs, mechanical devices, biologics, or diagnostics not approved by the FDA for use in the United States. Dr. Cash does intend to discuss off-label uses of drugs, mechanical devices, biologics, or diagnostics approved by the FDA for use in the United States.
Shari Weisenfeld, MD
Scientific Director, Medscape, LLC
Disclosure: Shari Weisenfeld, MD, has disclosed no relevant financial relationships.

A 32-Year-Old Woman With IBS: Clinical Outcomes and the Use of Antibiotics
Case presentation.
SN is a 32-year-old white woman who presents with symptoms of recurrent abdominal pain and loose stools. She states that she has experienced these symptoms since adolescence, with periods of improvement and worsening over the years. She notes that her symptoms were most pronounced when she was in college, and they improved during her pregnancy with her first child 4 years ago. Over the past year, her symptoms have been occurring more frequently and with greater severity. She also has been increasingly bothered by bloating and distention over the past 6 months. The bloating seems to worsen with food intake, while the distention progresses throughout the day. When questioned about abdominal pain, she describes it as 7 (on a scale of 10) at its worst and seemingly related to defecation, with acute worsening immediately prior to defecation and significant improvement after defecation. She has loose stools approximately one third of the time and often will have 2-3 bowel movements per day.
Other than her gastrointestinal symptoms, she considers herself healthy. She has no chronic illnesses or prior surgeries. She had an uncomplicated pregnancy and a vaginal delivery 4 years ago (gravida 1, para 1). She has no family history of organic gastrointestinal diseases such as inflammatory bowel disease, malignancy, or celiac disease.
SN is employed part time at an accounting firm. She jogs 2-3 miles 3-4 times a week, tries to eat 4-6 servings of fruits and vegetables daily, and takes a daily multivitamin, which she has taken for many years.
Her weight and other vital signs are within normal limits: height 5'6", weight 120 lb, blood pressure 108/64 mm Hg, pulse 60 beats per minute, and respiratory rate 12 breaths per minute.
On physical examination, she is a well-developed, well-nourished woman in no acute distress. Her physical examination is notable for mild tenderness to palpation in the left lower quadrant, but there is no rebound tenderness, guarding, or other peritoneal signs. The remainder of the physical examination is unremarkable.
IBS Presentations
The hallmark symptoms of irritable bowel syndrome (IBS) are abdominal pain or discomfort associated with at least two of the following characteristics: (1) pain or discomfort associated with a change in the form of the stool; (2) pain or discomfort associated with a change in the stool frequency; and/or (3) the pain or discomfort is relieved with defecation. The above characteristics are a liberal translation of the Rome III criteria for IBS. [1] The reality of clinical practice is that IBS can have a wide variety of clinical presentations. In addition to the "classic" symptom complex mentioned above, patients will often complain of other abdominal or defecatory symptoms such as bloating, gas, a prominent gastrocolic reflex, flatulence, distention, mucous in bowel movements, a sense of incomplete evacuation, and straining required for defecation.
IBS typically can be classified into 1 of 3 major categories, defined by the predominant stool pattern: IBS with diarrhea (IBS-D), IBS with constipation (IBS-C), and mixed IBS (IBS-M). Each subgroup accounts for about one third of IBS patient presentations, although the percentage of each subtype may vary depending upon geography and patient population. [1]
Treatments and Clinical Trials
Treatment for IBS is typically directed at improving individual symptoms. For IBS-C this usually involves laxative therapies, and for IBS-D this usually involves antidiarrheal medications. Antispasmodic medications have long been used to target the pain and discomfort that patients often experience with IBS; however, there is no compelling evidence from clinical trials performed in the United States to support their use. Other common pharmacologic therapies prescribed for IBS include antidepressants, probiotics, and antibiotics.
Just as therapy is often directed at specific symptoms, patient response in clinical practice is typically dependent on changes in those symptoms and can be highly variable. This is in contrast to the many clinical trials of therapies for IBS, where changes in composite or global symptom measures are used to assess therapeutic efficacy. It is important for clinicians to understand the differences between measures of efficacy in clinical trials and the individual responses seen in practice, while also recognizing the wide-ranging global effects of IBS on the lives of their patients so that they can move beyond simply asking about individual symptoms (Figure 1).
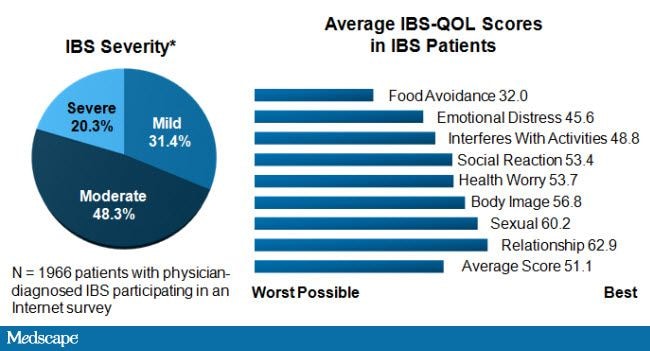
Figure 1. IBS profoundly affects QOL. Adapted from International Foundation for Functional Gastrointestinal Disorders; IBS patients: their illness experience and unmet need. Milwaukee, WI: International Foundation for Gastrointestinal Disorders; 2009.
IBS is a very subjective condition, without reliable biomarkers to help make the diagnosis or follow response to therapy, which has made developing clinical trial endpoints a difficult endeavor. Some trials evaluate the effects of therapy on individual IBS symptoms, while others use composite IBS scores or global IBS symptoms as their measure of efficacy. Still others use physiologic, rather than clinical, endpoints. Over the last decade, the US Food and Drug Administration (FDA) has favored a global endpoint for phase 3 clinical trials. It remains unclear if this is the optimal approach, especially because so much of the routine clinical practice surrounding IBS is directed at alleviating the individual symptoms patients experience.
In recent years, the National Institutes of Health has embarked on developing improved measures for patient-reported outcomes. The Patient Reported Outcome Measurement Information System (PROMIS®) aims to provide clinicians and researchers access to efficient, precise, valid, and responsive adult- and child-reported measures of health and well-being. [2] The stated strategic goals of this program are to (1) create and promulgate a set of qualitative and quantitative methodological standards for development and validation of instruments; (2) launch a sustainable entity that is able to promote the research, development, and dissemination activities for the network; (3) identify and prioritize a set of research and development opportunities that include clinical applications; and (4) disseminate information in order to forge strategic alliances to enhance the adoption of these standards in research, clinical practice, and policy. Currently, investigators from the University of California, Los Angeles, are working on a project under this program to develop and test a "gastrointestinal distress scale" that may have significant applicability for future IBS clinical trials.
Perhaps because IBS is such a heterogeneous disorder, there is no single therapeutic approach that has proven to be the de facto "first-line" treatment strategy. Generally, the aim of treatments for conditions that do not result in long-term health sequelae or impaired life expectancy, such as IBS, is to minimize the costs and possible adverse effects of therapies.
Multiple studies have considered the effects of nonpharmacologic therapies for IBS as well, including fiber supplementation or bulking agents, food-restriction diets, and psychological/cognitive behavior-based treatments. Among these 3 categories, psychological therapies have the best evidence supporting their use.
Bulking agents have traditionally been felt to be ineffective therapies for IBS. [3] However, Bijkerk and colleagues recently compared psyllium, bran, and a rice-flour placebo among patients with IBS and found that the percentage of patients responding, defined as adequate symptom relief, was significantly greater than placebo in the first (primary endpoint) and second months of therapy. Additionally, treatment with bran 10 g/day was also significantly better than placebo during the third month of treatment. [4]
Dietary restriction has been used by many patients with IBS in order to minimize gastrointestinal symptoms. Typically this includes limiting foods containing dairy sugars such as lactose, foods containing gluten, and most recently limiting fermentable oligosaccharides, disaccharides, monosaccharides, and polyols (FODMAPs). Examples of FODMAPs include fructose and lactose, sorbitol, fructans, and raffinose, which humans do not express suitable hydrolases for and thus are always poorly absorbed. There are a number of studies that support FODMAP-restricted diets. In one study, 150 outpatients with IBS were randomly assigned to 3 months of a diet excluding all foods for which they had elevated immunoglobulin G antibodies (enzyme-linked immunosorbent assay test) or a sham diet excluding the same number of foods but not those to which they had antibodies. [5] After 12 weeks, the true diet resulted in a 10% greater reduction in symptom score than the sham diet (mean difference of 39.95% [95% confidence interval (CI), 5-72]; P = .024) and a 26% greater reduction in fully compliant patients (difference 98.95% [95% CI, 52-144]; P < .001). Global rating also significantly improved in the true-diet group as a whole ( P = .048, number needed to treat [NNT] = 9) and even more in compliant patients ( P = .006, NNT = 2.5). All other outcomes showed trends favoring the true diet, and relaxing the diet led to a 24% greater deterioration in symptoms in those on the true diet (difference 52 [95% CI, 18-88]; P = .003).
In another double-blinded, randomized, controlled trial, 25 patients who had responded to dietary change consisting of a FODMAP-restricted diet were rechallenged with reintroduction of fructose, fructans, alone or in combination, or glucose for a maximum test period of 2 weeks. [6] Approximately 70% of patients receiving fructose, 77% receiving fructans, and 79% receiving a mixture were not adequately controlled compared with 14% receiving glucose ( P ≤ .02). FODMAP-restricted diets can be difficult to adhere to; however, in patients with IBS and fructose malabsorption, dietary restriction of fructose and/or fructans may improve symptoms and is a reasonable treatment option.
Another therapeutic strategy for IBS has emerged from investigations on the origins of IBS symptoms from the interaction of enteric bacteria with ingested carbohydrates. This theory has been popularized by multiple reports demonstrating that patients with IBS are more likely than non-IBS controls to have abnormal breath tests after ingestion of fermentable carbohydrates. [7] Furthermore, studies have demonstrated that normalization of breath tests with antibiotic therapy may be accompanied by an improvement in IBS symptoms. These observations have led to multiple trials using antibiotic therapy for IBS. [8] This area of research recently culminated with the publication of the TARGET 1 and 2 trials in the New England Journal of Medicine . [9] This report of the effects of antibiotic therapy for IBS by Pimentel and colleagues represents a major addition to the IBS therapy literature and details the results of 2 identical trials for nonconstipated IBS. Both of these large studies (n = 623 for TARGET 1; n = 637 for TARGET 2) were conducted in multiple sites throughout the United States and Canada and enrolled patients with mild to moderate symptoms of nonconstipation IBS according to the Rome II IBS diagnostic criteria. After a 1- to 2-week screening phase to confirm eligibility requirements, patients were randomly assigned via concealed allocation to receive either rifaximin or placebo, in a 1:1 ratio. After completing the 14-day treatment period, patients were evaluated for 10 additional weeks, in order to monitor the short-term durability of treatment effects and symptoms. Efficacy assessments were obtained daily by means of an interactive voice-response system over the course of the entire study and a clearly defined endpoint, adequate relief of global IBS symptoms for at least 2 of the 4 weeks during the primary evaluation period based on a binary response to a yes/no question. A key secondary endpoint, satisfactory relief of bloating, was assessed in a similar fashion as the primary endpoint over the same period of time.
In both studies, patients consistently fulfilled the criteria for relief of global IBS symptoms and bloating (Figure 2). A statistically significant proportion of patients randomly assigned to the rifaximin group, compared with those who received placebo, had adequate relief of global IBS symptoms (41% vs 32% pooled data, P < .001) and bloating (40% vs 30% pooled data, P < .001) for at least 2 of the first 4 weeks of the treatment assessment period. Moreover, these results were durable, with statistically significant differences favoring rifaximin for the relief of global symptoms and bloating through the10-week post-treatment observation period. Other important individual symptoms of IBS were assessed, including abdominal pain and stool consistency, and these endpoints were also more likely to improve with rifaximin compared with placebo. No clinically significant differences were observed in terms of treatment-emergent adverse events between patients in either treatment arm.
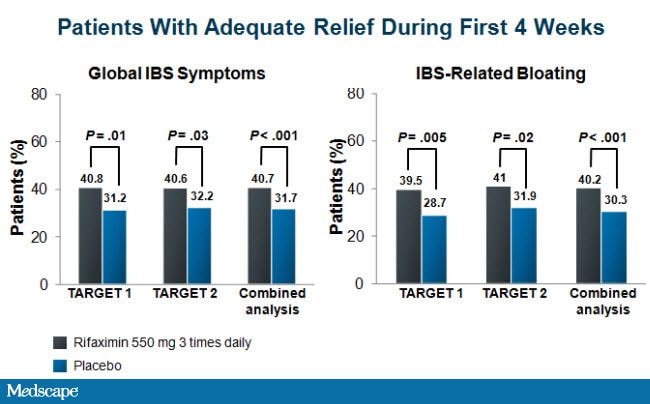
Figure 2. Efficacy of rifaximin in improving global IBS symptoms and IBS-related bloating. Adapted from Pimental M, et al. N Engl J Med . 2011;564:22-32. [9]
Clinical experience has demonstrated that rifaximin can significantly improve the gastrointestinal symptoms of some IBS patients. These results from the 2 large and well-designed clinical trials described offer convincing evidence that results that have been observed anecdotally for the last several years are in fact consistent and reproducible. The differences observed for the primary and secondary endpoints between rifaximin and placebo, while only 9%-10%, were similar to treatment differences observed in other phase 3 clinical trials of medications that have received FDA approval as therapies for IBS such as alosetron, tegaserod, and lubiprostone. [10-12]
The promising results of TARGET 1 and TARGET 2, however, do not completely resolve the issues surrounding the use of antibiotics for IBS. One of the most important questions remaining is the optimal means to identify probable responders. Some have suggested that breath test evidence of small intestinal bacterial overgrowth (SIBO) should be used as a treatment criterion, but previous studies of antibiotic therapy for IBS have not convincingly demonstrated a strong correlation between normalization of breath tests and clinical response. [13] As a practical matter, many clinicians have used positive breath tests to obtain third-party reimbursement for rifaximin, which is an expensive medication in the 1200- to 1650-mg doses used for IBS. Another question surrounding the use of antibiotics and probiotics for IBS is exactly how they are exerting an effect on the gastrointestinal tract of patients with IBS who respond to these therapies. Popular theories hold that these medications may decrease the density of fermenting bacteria in the small bowel, but there are other theories, such as anti-inflammatory effects that lead to alterations in enteric motility, secretion, and sensitivity, which have been put forth as possible explanations. Another concern surrounds the possibility of resistance, but there are abundant data in the literature demonstrating the safety of nonabsorbed antibiotics with respect to this issue. [14] Another issue, one that was raised by the FDA in their review of the clinical trial data during the approval deliberations for rifaximin, is the durability of response. While treatment differences between rifaximin and placebo persisted throughout the conclusion of the 12-week study period, a gradual diminution of the relief of global IBS symptoms and bloating was observed in both groups as the trial progressed. This observation mirrors community-based clinical experience. While there can be a dramatic response in some patients, symptom recurrence at a variable point after rifaximin appears to be common. Data on recurrence and retreatment effects with rifaximin, or any other antibiotic used for IBS, are crucial to obtain as there is very little guidance in the literature. What data that exist suggest that responders who experience recurrent IBS symptoms will respond to retreatment. [15,16]
Case Conclusion
Based on her long history of typical symptoms and lack of alarm features, SN is diagnosed with IBS. Her description allows for further categorization as IBS-D with prominent bloating. A complete blood count, thyroid studies, celiac sprue, and pregnancy testing are ordered. Based upon her age, lack of risk factors and alarm features, and intermittent loose stools, there was a discussion with the patient, and a decision was made not to perform colonoscopy at this time. SN is given educational material regarding IBS, including important Websites with IBS information like the International Foundation for Functional Gastrointestinal Disorders Website.
SN is anxious to avoid medications, and thus lifestyle modification with a FODMAP-restricted diet is prescribed. She returns 4 weeks later without symptom improvement, admitting that the diet was difficult to adhere to, particularly with other family members to consider. Her laboratory results are within normal limits. Rifaximin (550 mg 3 times a day for 14 days) is prescribed along with loperamide. SN is advised to begin with 2 mg of loperamide every morning and to increase by an additional 2 mg every week if her stool remains frequently soft and runny and to decrease to every other day should constipation occur.
SN returns after 4 weeks on this therapy and reports that her bloating and distention have almost completely resolved, her bowel habits are more predictable, and she has more solid stool. She reports occasional cramping and abdominal discomfort that signals the need to defecate, but this has been minimal and short lived. She does not want to pursue additional medical therapy at this time. She also has made some small changes to her diet like decreasing the amount and frequency of cruciferous vegetable and legumes she consumes, and she feels that this has helped her symptoms. She is advised about possible symptom recurrence over the next 6 months, especially bloating, and she has agreed to contact you for retreatment with rifaximin if she experiences a recurrence of symptoms.
Supported by an independent educational grant from Salix Pharmaceuticals.
The material presented here does not necessarily reflect the views of Medscape, LLC, or companies that support educational programming on www.medscape.org. These materials may discuss therapeutic products that have not been approved by the US Food and Drug Administration and off-label uses of approved products. A qualified healthcare professional should be consulted before using any therapeutic product discussed. Readers should verify all information and data before treating patients or employing any therapies described in this educational activity.
Medscape Education © 2011 Medscape, LLC

- Case report
- Open access
- Published: 03 August 2021
Pain and psyche in a patient with irritable bowel syndrome: chicken or egg? A time series case report
- Felicitas Engel 1 ,
- Tatjana Stadnitski 2 ,
- Esther Stroe-Kunold 1 ,
- Sabrina Berens 1 ,
- Rainer Schaefert 1 , 3 &
- Beate Wild ORCID: orcid.org/0000-0002-2279-8135 1
BMC Gastroenterology volume 21 , Article number: 309 ( 2021 ) Cite this article
2658 Accesses
3 Citations
1 Altmetric
Metrics details
Irritable bowel syndrome (IBS) appears to have a bidirectional interaction with both depressive and anxiety-related complaints. However, it remains unclear how exactly the psychological complaints, at the individual level, are related to somatic symptoms on a daily basis. This single case study investigates how somatic and psychological variables are temporally related in a patient with irritable bowel syndrome.
The patient was a woman in her mid-twenties with an IBS diagnosis. She reported frequent soft bowel movements (5–6 times per day), as well as flatulence and abdominal pain. She resembled a typical IBS patient; however, a marked feature of the patient was her high motivation for psychosomatic treatment as well as her willingness to try new strategies regarding the management of her symptoms. As an innovative approach this single case study used a longitudinal, observational, time series design. The patient answered questions regarding somatic and psychological variables daily over a period of twelve weeks with an online diary. The diary data was analysed using an autoregressive (VAR) modeling approach. Time series analyses showed that in most variables, strong same-day correlations between somatic (abdominal pain, daily impairment) and psychological time series (including coping strategies) were present. The day-lagged relationships indicated that higher values in abdominal pain on one day were predictive of higher values in the psychological variables on the following day (e.g. nervousness, tension, catastrophizing, hopelessness). The use of positive thinking as a coping strategy was helpful in reducing the pain on the following days.
In the presented case we found a high correlation between variables, with somatic symptoms temporally preceding psychological variables. In addition, for this patient, the use of positive thoughts as a coping strategy was helpful in reducing pain.
Peer Review reports
Irritable bowel syndrome (IBS) is characterized by recurrent abdominal pain that is associated with a change in frequency or form (appearance) of stool and can be related to defecation [ 1 ]. Currently, the symptom pattern is not sufficiently explained by peripheral organ pathology. IBS affects about 8% of the European population [ 2 ] and is most recently understood as a disorder of (microbiota-) gut-brain interaction [ 3 , 4 ] with a multifactorial origin that includes biological, psychological, and social factors [ 5 ]. Many patients who suffer from IBS also suffer from comorbid depressive or anxiety-related disorders [ 5 ]. Mood and anxiety disorders can precede or follow an IBS diagnosis due to the high discomfort caused by IBS [ 6 , 7 , 8 ]. By looking at specific psychological variables it was found that catastrophizing is directly associated with IBS symptom severity, while anxiety is indirectly related to IBS symptom severity [ 9 ].
While population-based studies suggest that IBS has a bidirectional interaction with both depressive and anxiety-related complaints, it remains unclear how exactly the psychological complaints, at the individual level, are related to somatic symptoms on a daily basis. Are increased psychological complaints (such as depression, tension, and nervousness) on one day preceded by IBS complaints on the previous day, or is it the other way around? A previous study showed that week-to-week stress and IBS symptoms were strongly cross-correlated in the same week, but were not temporally related across several weeks [ 10 ]. However, a day-by-day measure is needed to identify more fine-grained and direct relations. Furthermore, the focus of the study was on the mean values from a large patient sample, therefore potentially differing relationships in individual patients may not have been reflected in the aggregated data analysis.
Another interesting topic in patients suffering from IBS is the mutual relationship between coping strategies and IBS symptoms. A recent study reported that levels of coping resources were associated with gastrointestinal and extraintestinal symptom severity [ 11 ]. Also, catastrophizing and a lower self-perceived ability to reduce symptoms appeared to have a negative effect on health outcome in gastrointestinal disorders [ 12 ]. Interestingly, IBS patients have been reported to use passive coping strategies more frequently (such as escape-avoidance strategies instead of intended problem solving) compared to healthy controls [ 13 ]. Here too, the question arises to what extent coping strategies are related to IBS complaints and whether or not they are able to influence IBS complaints.
Overall, IBS symptoms and psychological distress are bi-directionally related, and coping strategies purportedly play an important role in the up- or down-regulation of IBS symptoms. However, individual mechanisms are not yet understood, and previous studies lack the longitudinal data on a day-by-day basis. Longitudinal data is necessary in order to obtain information about direct interactions, to better understand how temporal interactions between IBS symptoms and psychological complaints are related. As aggregated data can eliminate individual effects within the heterogeneous IBS patient sample, a single case study can provide important insights into specific mechanism to generate hypotheses for personalized clinical studies [ 14 ]. Conversely, inferences from singe case studies do not automatically apply to the patient population. However, results from single case studies can be used to generate hypotheses that can be examined in a sample of patients with similar characteristics.
This case study has, for the first time, applied a longitudinal time series design to a patient with IBS. Study objectives of this single-case analysis were: (1) to explore temporal relationships and interactions between the somatic and psychological complaints of the patient and (2) to investigate the impact of personal coping strategies on somatic symptoms.
Case presentation
Study design.
The study used a longitudinal, observational, single-case design. The study was approved by the medical ethics committee of the University Hospital Heidelberg. The patient was recruited in the frame of a pilot intervention study, conducted between July, 2014 and June, 2015 [ 15 ]. During her waiting period—and before the beginning of the therapy group—the patient answered questions daily regarding somatic and psychological complaints as well as coping strategies with the use of an online diary.
The diary data of the patient was collected following presentation in our outpatient specialty clinic for functional gastrointestinal disorders [ 16 ], and before group therapy. The data thereby showed the classic course of IBS without specific group intervention. The patient filled out the diaries from 10/2014 to 01/2015; over twelve weeks a total of 72 diary days were collected.
Measurements in the online diary
At the beginning of the study the patient received individual training in how to use the online diary; she was instructed to fill out the diary on a daily basis (between 4 pm and 12 am) via internet access. Validated questionnaires for IBS, as well as for psychological complaints and coping strategies, were used and adapted for the daily diary design. The most discriminating items of the questionnaires were derived in order to shorten the completion time of the diary (approximately 5–10 min). All items were rated by a visual analogue scale (VAS) with bipolar labels. The marked points were then converted by the computer program to a numeric scale ranging from 1 to 101. In addition, it was possible to enter a short free text in the diary.
For the measurement of somatic symptoms, we used the items “How severe is your abdominal (tummy) pain?” and “Please indicate how much your irritable bowel syndrome is affecting or interfering with your life today?”. Higher scores on these items reflected higher pain or higher somatic impairment. Psychological variables and coping strategies measured in the online diary are shown in Table 1 .
In January 2014, a German student (female in her mid-twenties), was referred to the outpatient specialty clinic of the University Hospital of Heidelberg for functional gastrointestinal disorders. She reported frequent soft bowel movements (5–6 times per day), as well as flatulence and abdominal pain. According to ROM-III [ 22 ] and the clinical assessment, an IBS (subtype IBS-diarrhea, IBS-D) was diagnosed. In addition, the patient was suffering from comorbid gluten, lactose, and sorbitol intolerance. No mental illness was present. Despite professional nutritional advice that included a gluten-, lactose- and sorbitol-reduced diet, gastrointestinal complaints persisted. In the course of the three-month follow-up appointments that included multimodal treatment [ 16 ] (04/2014, 07/2014, 11/2014), the patient correlated intestinal complaints and stress. She reported, for example, that the intestinal symptoms increased at the beginning of the semester and in the examination period. In the course of the diary study the patient did not describe any long-lasting stressor (such as an examination phase), but rather shorter week- or day-specific stressful events (such as Christmas holidays or looking for a part-time job) associated with an onset of IBS-symptoms on the same day. As an additional stressor, she described shame and the fear of a recurrence of the IBS complaints (particularly of soft bowel movements and flatulence), especially in social settings and situations where she could not easily reach a toilet. Relaxation techniques (yoga and gut-directed hypnosis using a CD) slightly improved her symptoms and the associated fear. Regarding the short stressful events, she described a good improvement of symptoms when using a strategy of calming down, with no further subsequent exacerbation. After the online diary study presented here, the patient received a group intervention [ 15 ] from which she has benefited.
In conclusion, according to IBS symptoms, symptom specific fears and avoidance behavior, the presented case of a young female patient resembled a typical IBS patient; however, a marked feature of the patient was her high motivation for psychosomatic treatment as well as her willingness to try new strategies regarding the management of her symptoms.
Statistical analysis
Initially, the following analyses were conducted for each time series: graphic examinations; calculations of descriptive statistics (range, median, mean, standard deviation), autocorrelation functions (ACF), and tests for stationarity with the Augmented Dickey–Fuller (ADF) procedure. Autocorrelation is the bivariate correlation of a time series with a lagged copy of itself. Therefore, instantaneous (lag = 0) autocorrelation is always equals one, significant autocorrelations on other lags imply predictability of the future time series values from the past values. Stability or instability as well as memory characteristics of time series can be inferred from their autocorrelation functions: non-zero autocorrelations at only a few lags are typical for stable short-memory processes, whereas significant autocorrelations on many lags indicate long memory or instability. Stationarity means that the statistical characteristics of a process under study do not change over time (e.g., exhibit no trends or distinct fluctuations of mean or variance). The Augmented Dickey-Fuller algorithms tests the null hypothesis “time series is stationary”.
In addition, cross-correlation functions (CCF), instantaneous correlations, and simultaneous regressions with psychological measures—both as dependent and somatic variables as predictors—were estimated. Cross-correlation measures similarity of two different time series as a function of the displacement of one relative to the other. Generally, instantaneous (lag = 0) correlations or simultaneous (lag = 0) regressions do not imply causation. For lagged correlations and regressions, however, it is different, since they explore the ability to predict the future values of a time series from prior values of another times series. The idea behind this is as follows: Since time does not run backwards, the cause cannot come after its effect. Therefore, events in the past can cause events to happen today, but future events cannot influence the present. The concept of Granger causality incorporates this idea: if lagged values of a time series X improve prediction of future values of a series Y, the former series Granger-causes the latter. For example, if lagged values of a somatic times series improve prediction of future values of a psychological one, the former series Granger-causes the latter. The vector autoregressive (VAR) methodology investigated the temporal dynamics between two or more time series by separating the time-lagged from the simultaneous relations. Therefore, temporal interdependencies between time series were analyzed using this approach. The VAR technique thereby allowed inferences about the temporal order of the effects by employing the temporal causality concept introduced by Granger. Furthermore, the VAR approach can handle time series that mutually influence each other and thus reveal feedback effects. In VAR modelling, interpretation of the regression coefficients is problematic because the lagged values of the dependent variables are used as predictors (i.e. dependent and independent variables are both endogenous, that is, determined and interrelated inside the organism or system), consequently, external influences can enter the autoregressive system exclusively through the residual term, which is also called “exogenous shock”. The behaviour of a VAR system can be modelled using impulse response analyses (IRA) and forecast error variance decompositions (FEVD). Impulse response functions (IRF) examine interdependencies within a VAR system by tracing the effect of an exogenous shock in one of the series on other variables. The FEVD estimates the amount of variance in each variable that can be explained by the other variables of the system during a specific period (h). For instance, in case of daily measurements, FEVD = 0.24 (h = 10) means that 24% of the forecast error variance in a dependent variable can be explained by exogenous shocks (random changes) of the predictors for a time horizon of 10 days.
The analyses were conducted using the R software. (Please consult Stadnitski & Wild (2019) and Stadnitski (2014, 2020) for descriptions, detailed explanations, and implementation of all analyses with the R software [ 23 , 24 , 25 ]).
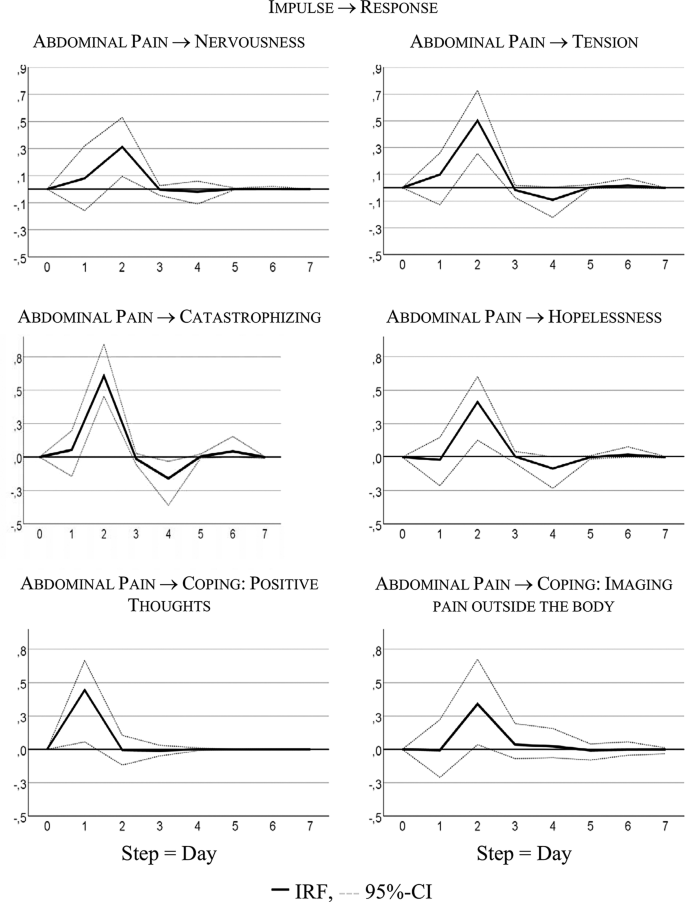
Figure 1 visualizes the patient’s development of somatic symptoms, abdominal pain (AP), and daily impairment (DI) over 72 successive days together with their autocorrelation and cross-correlation function. In both series there appeared strong discomfort with values distinctly higher than 20 on 7 days. Almost 90% of the measurements varied between 1 and 20 on the 100-point scale. The average (Mean AP = 11.10, DI = 14.35) and variability (Standard Deviation: AP = 15.90, DI = 18.55) were higher for DI than AP (see also Table 2 ). Both time series exhibited no trends. Figure 2 shows the time series of additional psychological variables and coping strategies.
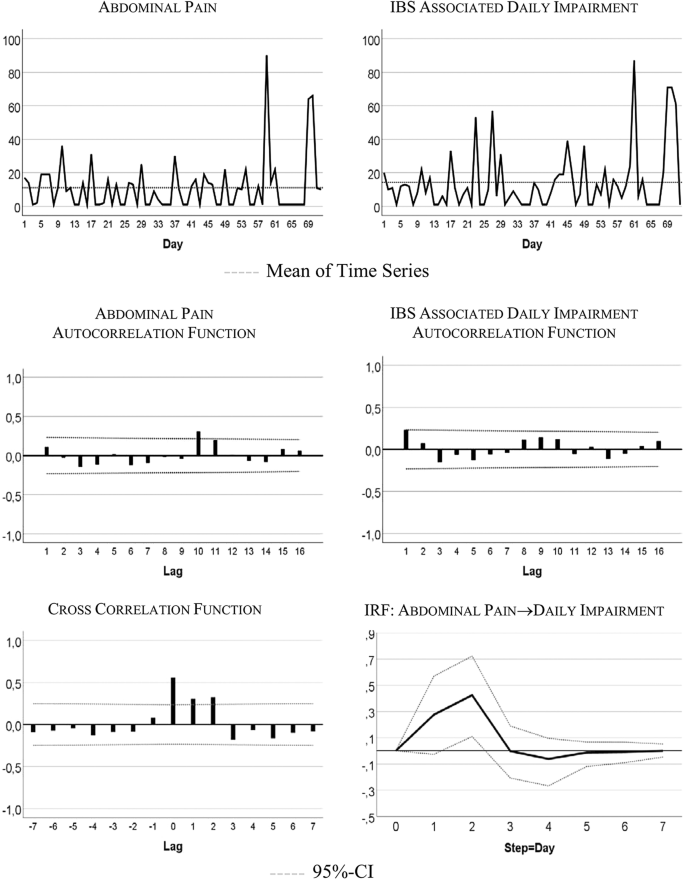
Somatic time series: abdominal pain (AP) and IBS-associated daily impairment (DI)
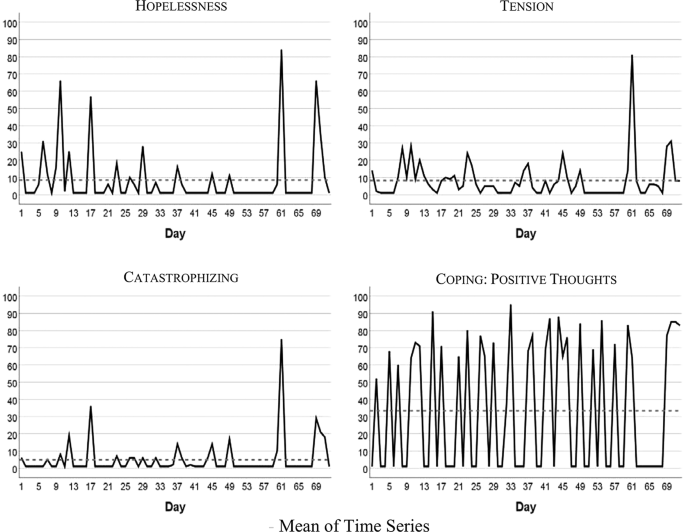
Time series of hopelessness, tension, catastrophizing, coping
The time series quantitatively reflect the free text descriptions of the patient. The highest scores in AP and DI were recorded between days 57 and 68 of the study period. In the free text passages of the diary the patient noted that she experienced the Christmas holidays (days 52–67) as a period of high stress and increased IBS pain. In addition, on days 59–61 she described the occurrence of menstrual cramps together with IBS-associated pain and impairment.
Table 2 summarizes characteristics of somatic and psychological and coping time series. In the majority of cases all of the series except “Coping with positive thoughts” (CPT) ranged between 1 and 20 on the 100-point scale, with high values observed about 10% of the time. CPT values alternated between very low and high with values equal on 1 out of 40 days, and values higher than 50 on 31 days. All series were stationary, i.e., exhibited no trends. Three series (tension, catastrophizing, and hopelessness) demonstrated no autocorrelations.
Table 3 shows instantaneous correlations between the somatic and psychological (including coping) time series. In most cases, strong and positive correlations were observed. Interestingly, the relationship between psychological and coping variables with DI was stronger than with AP. The amount of predicted variance (R 2 ) from linear regressions with psychological and coping measures as dependent variables and somatic variables as predictors varied between 12 and 94%. The non-significant correlation between depressiveness and abdominal pain could be due to the very limited range of the variable depressiveness over the course of the 72 days.
Table 4 summarizes the significant results of the VAR analyses for interdependencies between abdominal pain and psychological distress or coping strategies; only statistically significant findings from calculations for all possible combinations of variables are provided. Identified lagged or temporal relations showed mostly the same direction, indicating that previous values in the somatic variable (AP) were predictive of values in the psychological variables or coping strategies. The variance decomposition estimates show that somatic symptoms in the psychological (and coping) time series explain 12% to 41% of variability.
Figure 3 visualizes responses of psychological states and coping strategies to increases in AP; it shows that psychological and coping aspects reacted with higher symptoms to an increase in AP. For instance, increasing AP caused a strong delayed increase in catastrophizing: + 0.60 standard deviations, i.e., about 7 points on the 100-point scale.
Time lagged psychological variables
Table 4 shows that the bivariate system, including AP and CPT, is characterized by a bidirectional or feedback predictive causality. AP Granger-caused CPT with 24% of explained variance, CPT Granger-caused AP with 6% of explained variance. Both series also correlated instantaneously: r = 0.43, R² = 18%.
Figure 4 visualizes the feedback relationship. An increase in AP caused more CPT next day. Intensified CPT resulted in less pain on the subsequent day: i.e., a decrease of 0.25 standard deviations, 4-point on the 100-point scale.
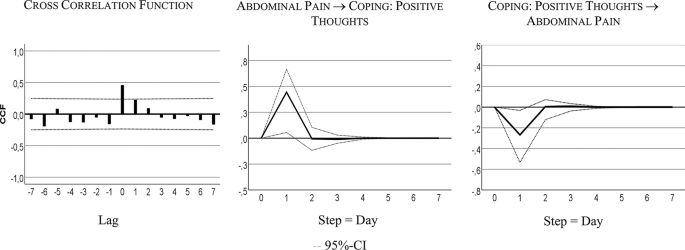
Cross-correlation and time lagged relationships: abdominal pain (AP) and coping with positive thoughts (CPT)
Discussion and conclusion
This is the first study to investigate the temporal relationships between somatic and psychological variables on a daily basis. We analyzed a female patient with IBS in her mid-twenties with symptoms of diarrhea, flatulence, and abdominal pain. She reported stress-related IBS symptoms as well as symptom related fears. In most variables, strong same-day correlations between somatic (especially daily impairment) and psychological (including coping) time series were observed. The day-lagged relationships indicated that higher values in abdominal pain on one day were predictive of higher values in psychological complaints (nervousness and tension) or of negative coping strategies (catastrophizing, hopelessness) on the following day. The use of positive thinking as a positive coping strategy was helpful in reducing the pain on the following days.
All variables remained stationary—that is, time series exhibited no trends over the measured time period (72 days). In the study period, the patient did not receive additional psychotherapeutic treatment, nor did she report long-lasting stressors. Therefore, we did not expect her symptoms to change over a longer period of time. The stability of IBS symptoms is supported by literature that usually describes IBS as a chronic disease. The diagnostic criteria for IBS also imply some symptom stability, because the symptoms must occur for a period of at least 3 months (with an onset at least 6 months prior the diagnosis) [ 22 ]. In addition, for IBS, population-based studies report a remission rate of about 55% only over a period of more than 10 years [ 26 ]. In addition to the general stationary trend of the variables, individual outliers with more severe symptoms were visible (e.g. the Christmas Holidays on days 52–67).
The patient stated that stressful or stress-free episodes would influence her symptoms; this was also reflected in the same-day analysis. In the free text of the diary the patient also described that in specific stressful situations she was ashamed of her symptoms and related consequences. The high same-day correlations between the somatic (AP, DI) and psychological time series (nervousness, tension, depressiveness, catastrophizing, hopelessness) reflect this interdependency—which the patient is aware of—between IBS symptoms and psychological state. Interestingly, this correlation was even higher for DI, meaning that functionality is especially important. The interaction between somatic and psychological distress is also described in previous studies. Midenfjord et al. (2019), for instance, showed in a cross-sectional study that IBS patients with psychological distress demonstrated more severe somatic symptoms and a lower quality of life [ 27 ]. Varni et al. (2017) found in a sample of pediatric patients with functional gastrointestinal disorders that somatic symptoms were differentially related to decreased health-related quality of life [ 28 ]. Another study reported a correlation between pain intensity and intensity of psychopathological symptoms (such as low spirits or anxiety) in IBS patients [ 29 ] while Dong et al. (2020) showed that IBS symptom severity predicted health-related quality of life influenced by stressful life events [ 30 ]. Interestingly, there is evidence that this association between current abdominal symptoms and psychological distress is not limited to functional gastrointestinal diseases but can also be seen in inflammatory bowel diseases [ 31 ]. The underlying physiological mechanism for the interaction between somatic and psychological distress could be explained by the concept of the (microbiome-) gut-brain axis. The (microbiome-)gut-brain axis refers to the complex network of connections between the microbiota, the enteric nervous system, and the central nervous system. [ 3 , 4 , 32 , 33 ]. Previous research has shown that the link between gastrointestinal symptoms and psychological distress could be based on a complex and bidirectional interaction between biological, psychological, and social factors [ 5 ]. For example, visceral hypersensitivity and an enhanced perceptual response to gastrointestinal sensations can trigger gastrointestinal specific anxiety [ 5 , 32 ]. On the other hand, psychosocial distress can lead, for instance, to an activation of the enteric and autonomic nervous system, which may trigger a change in smooth muscle activity or glandular secretion thus leading to IBS-symptoms. [ 32 ].
In addition to the daily correlation, it is also useful to look at day-to-day relationships in order to make time-delayed effects more visible and to answer the question whether or not psychological complaints precede IBS complaints, or vice versa. In literature, both perspectives are described for mental illnesses and IBS [ 6 , 7 , 8 ]. However, for this particular patient we found a strong time-delayed relationship between IBS symptoms, the following psychological complaints (nervousness, tension), and negative coping strategies (catastrophizing, hopelessness). This shows that having abdominal pain on one day was associated with more psychological stress the next day, not vice versa. This is in line with another study showing the temporal relationship that abdominal symptoms lead to increased stress and negative affect, while increased daily life stressors even lowered the IBS-symptoms [ 34 ]. This is interesting, as in literature frequently the opposite temporal direction or a feedback-loop is assumed [ 35 ]. Patel et al. (2016), for instance, investigated the relationship between sleep, mood and somatic symptoms in a sample of IBS patients and healthy controls over the course of 7 days [ 36 ]. In IBS patients, sleep disturbances were predictive for abdominal pain on the following day. Additional analyses showed that the sleep effects on abdominal pain in IBS patients could be mediated by depression and anxiety [ 36 ].
The question arises why our data show that the patient first develops gastrointestinal complaints and only afterwards psychological complaints. The patient herself had the impression that increased stress would lead to an increase in symptoms. For instance, during the short stressful event of applying for a new job the patient reported an onset of IBS complaints. She also reported that in this case the immediate application of a coping strategy (such as calming down) had helped her to reduce the symptoms. However, this sequence occurred over the course of only several hours—and would thus be reflected in the high same-day correlations of the time series (and not in the day-lagged correlations). On the other hand, shorter time intervals had been tested in Chan's study with an outcome similar to ours [ 34 ]. It is also possible that shorter daily stressors could also lead to a distraction from the IBS-symptoms, while longer stressors (like Christmas Holidays in the case of our study) may lead to an increase in symptoms.
Another interesting approach to feelings and symptoms of IBS is the concept of alexithymia. This concept states, among others, that feelings in IBS-patients may be misinterpreted as negative bodily sensations [ 37 ]. For our patient, this could mean that in stressful situations (such as job search or exam phases) she may initially perceive her feelings only physically and interpret them as a preliminary stage of a new outbreak of her IBS. The hyper-focus on the symptoms could initially intensify them. Shortly afterwards, the patient may get negative feelings from the IBS symptoms themselves.
The time-lagged correlation between IBS complaints and the following psychological complaints and negative coping strategies could be related to the patient’s social anxiety and the pressure to perform. In the free text of the diary the patient described that with the occurrence of abdominal complaints she would fear that soft bowel movements would follow, and that she would not be able to reach a toilet in a timely manner; she also felt ashamed when she had to leave certain events because of her IBS symptoms. Physiologically, this relationship between IBS complaints and following psychological distress could again be explained by the (microbiome-)gut-brain axis [ 5 , 32 ]. The occurrence of abdominal complaints (maybe as an expression of visceral hypersensitivity) can trigger gastrointestinal specific anxiety and the autonomic nervous systems as well as the hypothalamic pituitary axis are sending stress signals to the gut, resulting, among others, in a higher bowel motility and secretion leading to diarrhea and pain [ 32 ].
Interestingly, abdominal pain was not associated with a depressive feeling in general, but with negative processing (such as hopelessness and catastrophizing) as well as tense or anxious arousal (nervousness, tension). These negative feelings and coping strategies had no effect on the patient’s increased abdominal pain the next day; in contrast, the use of positive coping strategies was helpful.
The patient reported using positive coping strategies to reduce her symptoms; this was also seen in the data analysis. The intensified use of a specific coping strategy on one day (thinking of things the patient enjoyed doing) was followed by a decrease in pain on the subsequent day. Conversely, an increase in pain was followed by an increased use of this coping strategy. This corresponds to the clinical impression and the self-report of the patient: She considered relaxation techniques and new coping strategies such as distraction as beneficial for her condition. This result is supported by literature that considers psychotherapeutic treatment, including positive coping strategies, as a possible treatment of IBS [ 38 ].
In summary, the results of the time series analysis partly reflect the self-report of the patient as well as the clinical impression of the outpatient caretaker. However, our results expand upon these insights by showing temporal relationships between IBS symptoms and psychological variables over consecutive days—with psychological changes following changes in abdominal pain and related impairment. In addition, a mutual day-lagged relationship between IBS symptoms and coping could be detected.
This study has several implications: Overall, it shows that at the very least this patient is aware of her individual process of personal change, her fears, and her coping strategies––all of which to a large extent, could be confirmed by the time series analysis––an analysis that also provided additional information. This supports the hypothesis that individual characterizations are promising in terms of providing a better understanding of specific mechanisms, as well as an understanding of how temporal interactions between IBS symptoms and psychological symptoms are related. In clinical practice, practitioners should consider individual explanatory models of aggravating factors and coping strategies and stay open to psychosomatic as well as somatopsychic mechanisms. Previous psychological treatment recommendations for IBS patients concluded that a change in illness-specific cognitions as well as gastrointestinal anxiety as key mechanisms may have an effect on the outcomes of IBS symptom severity and quality of life [ 39 ]. In this case study, only positive thinking had a time-lagged effect on a decrease in abdominal pain, while catastrophizing and hopelessness were a result of having abdominal pain previously. Although it is not possible to generalize the results of an individual case, this supports the fact that treatments which more directly target abdominal symptoms (e.g., hypnotherapy) may have promising effects on IBS symptoms as well as associated psychological complaints. Therefore, a disorder-oriented integrative group intervention for IBS with gut-directed hypnotherapy seems promising [ 15 ].
From a methodological point of view, we have to point out that the here applied concept of Granger-causality does not equal causality. Causality according to Hill [ 40 ] can be assessed by using the following 9 criteria: strength, consistency, specificity, temporality, biological gradient, plausibility, coherence, experiment, analogy. The definition of Granger-causality, however, implies only that previous values of a time series X (e.g. somatic symptoms) improve prediction of future values of another series Y (e.g. nervousness of the patient). It does not imply the causality of X for Y.
Our study has several limitations. Firstly, we examined only one patient suffering from IBS; the generalizability of the results is therefore limited. We cannot simply transfer the results to other IBS patients but must carefully investigate further patient samples in regard to temporal relationships and interactions between somatic and psychological variables. Secondly, we were able to detect day-to-day changes only; shorter periods of time could not be captured. Nevertheless, previous studies mainly focused on longer time periods which is why this approach is still more advantageous in terms of capturing the direct relationships. Nevertheless, we were able to show a clear picture of a single IBS-patient. This is helpful as IBS is a complex illness with, in all likelihood, heterogeneous genesis and factors. A comprehensive case study could help identify subclasses of IBS to arrive at a better treatment and avoid dilution effects.
In conclusion we found in the presented case that somatic symptoms temporally precede psychological complaints. In addition, for this patient, the use of positive thoughts as a coping strategy was helpful in reducing pain. Further analyses should be conducted to verify if these relationships can be found in other patients who suffer from IBS symptoms.
Availability of data and materials
The datasets used and analysed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- Irritable bowel syndrome
Abdominal pain
IBS-associated daily impairment
Nervousness
Depressiveness
Pain-associated discomfort
Catastrophizing
Hopelessness
Coping with positive thoughts
Coping with imagining pain outside the body
Schmulson MJ, Drossman DA. What is new in Rome IV. J Neurogastroenterol Motil. 2017;23(2):151–63.
Article Google Scholar
Sperber AD, Dumitrascu D, Fukudo S, Gerson C, Ghoshal UC, Gwee KA, Hungin APS, Kang J-Y, Minhu C, Schmulson M. The global prevalence of IBS in adults remains elusive due to the heterogeneity of studies: a Rome Foundation working team literature review. Gut. 2017;66:1075-82.
Drossman DA, Hasler WL. Rome IV-functional GI disorders: disorders of gut-brain interaction. Gastroenterology. 2016;150(6):1257–61.
De Palma G, Collins SM, Bercik P, Verdu EF. The microbiota-gut-brain axis in gastrointestinal disorders: stressed bugs, stressed brain or both? J Physiol. 2014;592(Pt 14):2989–97.
Van Oudenhove L, Levy RL, Crowell MD, Drossman DA, Halpert AD, Keefer L, Lackner JM, Murphy TB, Naliboff BD. Biopsychosocial aspects of functional gastrointestinal disorders: how central and environmental processes contribute to the development and expression of functional gastrointestinal disorders. Gastroenterology. 2016;150(6):1355–67.
Jones MP, Tack J, Van Oudenhove L, Walker MM, Holtmann G, Koloski NA, Talley NJ. Mood and anxiety disorders precede development of functional gastrointestinal disorders in patients but not in the population. Clin Gastroenterol Hepatol. 2017; 15(7):1014–1020 e1014.
Ford AC, Forman D, Bailey AG, Axon AT, Moayyedi P. Irritable bowel syndrome: a 10-yr natural history of symptoms and factors that influence consultation behavior. Am J Gastroenterol. 2008;103(5):1229–39.
Koloski NA, Jones M, Talley NJ. Evidence that independent gut-to-brain and brain-to-gut pathways operate in the irritable bowel syndrome and functional dyspepsia: a 1-year population-based prospective study. Aliment Pharmacol Ther. 2016;44(6):592–600.
Article CAS Google Scholar
van Tilburg MA, Palsson OS, Whitehead WE. Which psychological factors exacerbate irritable bowel syndrome? Development of a comprehensive model. J Psychosom Res. 2013;74(6):486–92.
Blanchard EB, Lackner JM, Jaccard J, Rowell D, Carosella AM, Powell C, Sanders K, Krasner S, Kuhn E. The role of stress in symptom exacerbation among IBS patients. J Psychosom Res. 2008;64(2):119–28.
Wilpart K, Tornblom H, Svedlund J, Tack JF, Simren M, Van Oudenhove L. Coping skills are associated with gastrointestinal symptom severity and somatization in patients with irritable bowel syndrome. Clin Gastroenterol Hepatol. 2017;15(10):1565–71.
Drossman DA, Leserman J, Li Z, Keefe F, Hu YJ, Toomey TC. Effects of coping on health outcome among women with gastrointestinal disorders. Psychosom Med. 2000;62(3):309–17.
Jones MP, Wessinger S, Crowell MD. Coping strategies and interpersonal support in patients with irritable bowel syndrome and inflammatory bowel disease. Clin Gastroenterol Hepatol. 2006;4(4):474–81.
Gagnier JJ, Kienle G, Altman DG, Moher D, Sox H, Riley D, Group C. The CARE guidelines: consensus-based clinical case report guideline development. J Clin Epidemiol. 2014;67(1):46–51.
Berens S, Stroe-Kunold E, Kraus F, Tesarz J, Gauss A, Niesler B, Herzog W, Schaefert R. Pilot-RCT of an integrative group therapy for patients with refractory irritable bowel syndrome (ISRCTN02977330). J Psychosom Res. 2018;105:72–9.
Berens S, Kraus F, Gauss A, Tesarz J, Herzog W, Niesler B, Stroe-Kunold E, Schaefert R. A specialty clinic for functional gastrointestinal disorders in tertiary care: concept and patient population. Clin Gastroenterol Hepatol. 2017;15(7):1127–9.
Francis CY, Morris J, Whorwell PJ. The irritable bowel severity scoring system: a simple method of monitoring irritable bowel syndrome and its progress. Aliment Pharmacol Ther. 1997;11(2):395–402.
Derogatis LR, Melisaratos N. The Brief Symptom Inventory: an introductory report. Psychol Med. 1983;13(3):595–605.
Kroenke K, Spitzer RL, Williams JB. The PHQ-9: validity of a brief depression severity measure. J Gen Intern Med. 2001;16(9):606–13.
Kroenke K, Spitzer RL, Williams JB. The PHQ-15: validity of a new measure for evaluating the severity of somatic symptoms. Psychosom Med. 2002;64(2):258–66.
Robinson ME, Riley JL, Myers CD, Sadler IJ, Kvaal SA, Geisser ME, Keefe FJ. The Coping Strategies Questionnaire: a large sample, item level factor analysis. Clin J Pain. 1997;13(1):43–9.
Longstreth GF, Thompson WG, Chey WD, Houghton LA, Mearin F, Spiller RC. Functional bowel disorders. Gastroenterology. 2006;130(5):1480–91.
Stadnitski T. Multivariate time series analyses for psychological research: VAR, SVAR, VEC, SVEC models and cointegration as useful tools for understanding psychological processes. Hamburg: Dr. Kovač; 2014.
Stadnitski T, Wild B. How to deal with temporal relationships between biopsychosocial variables: a practical guide to time series analysis. Psychosom Med. 2019;81(3):289–304.
Stadnitski T. Time series analyses with psychometric data. PLoS ONE. 2020;15(4):e0231785.
Olafsdottir LB, Gudjonsson H, Jonsdottir HH, Bjornsson E, Thjodleifsson B. Natural history of functional gastrointestinal disorders: comparison of two longitudinal population-based studies. Dig Liver Dis. 2012;44(3):211–7.
Midenfjord I, Polster A, Sjovall H, Tornblom H, Simren M. Anxiety and depression in irritable bowel syndrome: Exploring the interaction with other symptoms and pathophysiology using multivariate analyses. Neurogastroenterol Motil. 2019;31(8):e13619.
Varni JW, Shulman RJ, Self MM, Nurko S, Saps M, Saeed SA, Patel AS, Dark CV, Bendo CB, Pohl JF, et al. Gastrointestinal symptoms predictors of health-related quality of life in pediatric patients with functional gastrointestinal disorders. Qual Life Res. 2017;26(4):1015–25.
Gorczyca R, Filip R, Walczak E. Psychological aspects of pain. Ann Agric Environ Med. 2013; Spec no. 1:23–27.
Dong Y, Baumeister D, Berens S, Eich W, Tesarz J. Stressful Life Events moderate the relationship between changes in symptom severity and health-related quality of life in patients with irritable bowel syndrome. J Clin Gastroenterol. 2020;54(5):445–51.
Berens S, Schaefert R, Baumeister D, Gauss A, Eich W, Tesarz J. Does symptom activity explain psychological differences in patients with irritable bowel syndrome and inflammatory bowel disease? Results from a multi-center cross-sectional study. J Psychosom Res. 2019;126:109836.
Tait C, Sayuk GS. The Brain-Gut-Microbiotal Axis: a framework for understanding functional GI illness and their therapeutic interventions. Eur J Intern Med. 2021;84:1–9.
Kennedy PJ, Cryan JF, Dinan TG, Clarke G. Irritable bowel syndrome: a microbiome-gut-brain axis disorder? World J Gastroenterol. 2014;20(39):14105–25.
Chan Y, So SH, Mak ADP, Siah KTH, Chan W, Wu JCY. The temporal relationship of daily life stress, emotions, and bowel symptoms in irritable bowel syndrome-Diarrhea subtype: a smartphone-based experience sampling study. Neurogastroenterol Motil. 2019;31(3):e13514.
Drossman DA. Functional gastrointestinal disorders: history, pathophysiology, clinical features, and Rome IV. Gastroenterology. 2016; 150(6):1262–1279. e1262.
Patel A, Hasak S, Cassell B, Ciorba MA, Vivio EE, Kumar M, Gyawali CP, Sayuk GS. Effects of disturbed sleep on gastrointestinal and somatic pain symptoms in irritable bowel syndrome. Aliment Pharmacol Ther. 2016;44(3):246–58.
Kano M, Endo Y, Fukudo S. Association between alexithymia and functional gastrointestinal disorders. Front Psychol. 2018;9:599.
Torkzadeh F, Danesh M, Mirbagher L, Daghaghzadeh H, Emami MH. Relations between coping skills, symptom severity, psychological symptoms, and quality of life in patients with irritable bowel syndrome. Int J Prev Med. 2019;10:72.
Windgassen S, Moss-Morris R, Chilcot J, Sibelli A, Goldsmith K, Chalder T. The journey between brain and gut: a systematic review of psychological mechanisms of treatment effect in irritable bowel syndrome. Br J Health Psychol. 2017;22(4):701–36.
Hill AB. The environment and disease: association or causation? Proc R Soc Med. 1965;58(5):295–300.
CAS PubMed PubMed Central Google Scholar
Download references
Acknowledgements
The authors thank the participating patient and the contributing research assistants.
Open Access funding enabled and organized by Projekt DEAL.
Author information
Authors and affiliations.
Department of General Internal Medicine and Psychosomatics, University of Heidelberg, Im Neuenheimer Feld 410, 69120, Heidelberg, Germany
Felicitas Engel, Esther Stroe-Kunold, Sabrina Berens, Rainer Schaefert & Beate Wild
Department of Quantitative Methods in Psychology, University of Ulm, Albert-Einstein-Allee 47, 89081, Ulm, Germany
Tatjana Stadnitski
Department of Psychosomatic Medicine, Division of Internal Medicine, University Hospital Basel, Hebelstrasse 2, 4031, Basel, Switzerland
Rainer Schaefert
You can also search for this author in PubMed Google Scholar
Contributions
BW, FE, ES and RS conceived and designed the study. FE, SB, ES and RS collected the data. TS statistically analyzed and all authors interpreted the data. FE, BW and TS drafted the manuscript. All authors critically revised the manuscript and provided important intellectual content. All authors read and approved the final manuscript.
Corresponding author
Correspondence to Beate Wild .
Ethics declarations
Ethics approval and consent to participate.
The study was approved by the medical ethics committee of the University Hospital Heidelberg.
Consent for publication
The patient gave written informed consent for analysis and publication of the data.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ . The Creative Commons Public Domain Dedication waiver ( http://creativecommons.org/publicdomain/zero/1.0/ ) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
Reprints and permissions
About this article
Cite this article.
Engel, F., Stadnitski, T., Stroe-Kunold, E. et al. Pain and psyche in a patient with irritable bowel syndrome: chicken or egg? A time series case report. BMC Gastroenterol 21 , 309 (2021). https://doi.org/10.1186/s12876-021-01879-2
Download citation
Received : 06 October 2020
Accepted : 08 July 2021
Published : 03 August 2021
DOI : https://doi.org/10.1186/s12876-021-01879-2
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Functional gastrointestinal disorders
- Time series analysis
- Single case study
BMC Gastroenterology
ISSN: 1471-230X
- General enquiries: [email protected]
Case study: Seeing success through a multimodal approach to IBS management
Mia*, a young student in her early 20s with a vegetarian diet high in FODMAPs, turned to dietitian Chloe Madigan for help with her irregular bowel. With 35 years of dietetic practice to draw from, Chloe was able to guide Mia towards a better quality of life thanks to a combination of gut-directed hypnotherapy and dietary therapy that supported her lifestyle.
Here’s Chloe’s approach.
Seeing the stress signals
“Mia is a college student who fits in part-time work as well, so she had a lot going on when she first came to see me.
“As soon as she walked into my consultation room, I thought of Nerva almost from the onset. She had a high anxiety level; you could just see it. She spoke very quickly and tried to tell me everything all at once, almost sounding panicked. I picked up on her stress right away.
“Her bowel pattern was irregular, and she was opening her bowel about two to three times a day, and she said it was often loose stool.
“We all know stress is a huge exacerbator of IBS. And I know it’s a sign of the times, but I’ve been seeing more and more patients like Mia who take on so many stressors in their life. Sometimes they don’t realize the impact this has on their gut health.
Mia, however, could already see that stress was exacerbating her bowel.
“She was also a vegan when she was a teenager, and then became more of a vegetarian. In addition to her IBS, what concerned me was she had low iron (but regular periods).”
DIY digestion
Like most patients, Mia had already tried a few things on her own like Metamucil, Movicol, and probiotics.
“She tried lots and lots and lots of different things before seeking professional support, but she just couldn’t get her bowel under control.
“Her diet was good and she was eating reasonably well. But as a vegetarian, she was relying heavily on high FODMAP vegetables, legumes, and whole grains.”
Before her first consultation with Chloe, she’d already met with GI and had a colonoscopy.
“She did this test as she was experiencing stomach cramps, excess wind, and bloating. So, it could have been either IBS or IBD. But the results from the colonoscopy were all fine.”
First steps to the full picture
With Mia, and all of her patients, Chloe always begins with a comprehensive assessment.
“I talk about their symptoms and ask how long it’s all been going on for. I like to determine what it’s not to confirm it is IBS. And then I look at their dietary intake.
“So often FODMAPs aren’t the problem – they’re just exacerbating the existing condition.
“But, having said that, I didn’t automatically put Mia on a low FODMAP diet – I don’t do this with any of my patients.
“I’ve been referring the low FODMAP diet since about 2008, so I’ve been dabbling in it for a long time! And obviously it’s evolved and been refined, but now I approach IBS very differently.
“In the past, I’d probably say that we all got a bit excited about the low FODMAP diet – finally we had a solution to offer people with IBS! And we were more willing to put people on the diet without too much consideration. Now, my approach is entirely different.
“What I find nine times out of 10 is if we improve people's dietary fiber intake, their fluid, and activity, we can often regulate the bowel, and then FODMAPs are better absorbed.
“However, I do refer Nerva and gut-directed hypnotherapy quite a bit. What I see happening all the time with patients like Mia is they all have this huge preoccupation with their gut and their symptoms and we need to quieten that down – this is when Nerva comes in for me.”
Merging the mind and gut
While Nerva and the low FODMAP diet are effective standalone options for IBS management, both Chloe and Mia believe teaming the two approaches together was what led to Mia’s life-changing improvements.
“I thought to myself at the time, ‘It’s probably not a bad time to trial the low FODMAP diet to start’. But I still talked to her about Nerva on that first visit too.
“The first roadblock was she told me she couldn’t afford it. But I reached out to Nerva Clinical Specialist, Eloise , who was able to offer Mia a compassionate access account, which was incredible. She was so, so grateful. For me, it was just great to see a patient who I knew would really benefit from gut-directed hypnotherapy be given the opportunity to access the program.
“She started the low FODMAP diet and Nerva at the same time, and she’s currently up to the reintroduction phase. She did have a few setbacks with the diet and was slightly less compliant. She started strictly, but it was the end of her university year, and heading into the Christmas holidays made it challenging to follow closely, so her improvements were more gradual.
“I also had her on partially hydrolyzed guar gum, which is a prebiotic to increase gut mobility and can reduce small bacterial overgrowth – it’s actually as effective as the antibiotics for this.
“I also recommended psyllium husk and Movicol to keep her bowel regulated if required. One of the problems for patients like Mia is if their bowel isn’t regulated, they’ll continue to get bloating. And when they’re hypersensitive like she is, it becomes even more complicated. So, I wanted to work on her physical symptoms, as well as her emotions and anxiety with Nerva.”
Moving from meditation to hypnotherapy
Mia says her meditation practice helped her embrace the Nerva program fully from the beginning.
“Before she came to me, she was already doing daily meditation and yoga. So, when I introduced her to gut-directed hypnotherapy, she was really excited by the idea as she could already see the benefits of meditation and yoga and liked the idea of expanding on this practice.
“After that first appointment, she went home and looked into Nerva for herself and said she found it to be a genuine, trustworthy resource, and the app felt honest and transparent to her.
“She called it ‘slow education’, meaning she liked how the in-app psychoeducation progressed and built on topics at a good pace.”
Mia was happy to share she experienced very few setbacks moving through the six-week program.
“She’s a very diligent, conscientious person, but also overly anxious and a perfectionist. So, she listened every day, no problem, and started on the maintenance plan right away.
“Something she did experience, however, is if she was having a significant flare-up, sitting down to actively listen and concentrate on a gut-directed hypnotherapy session made it worse. She couldn’t do yoga either and often got a sharp, growing pain on her left side.
“However, I have referred many patients to Nerva and most are textbook: they listen, they feel fantastic, and it helps them desensitize. Mia was an exception in this way.”
Score improvements before and after the intervention
Mia saw huge improvements in her scores at the end of Nerva.

“I think the bloating and wind were huge successes for Mia – moving from 70 to 21 for bloating and from 72 down to 21 is incredible for her. Though I’m sure these significant improvements were supported by her dietary changes as she wasn’t fermenting as much in her gut.
“But it was great to see her anxiety and depression PHQ-4 score halve! It was a really positive outcome.
"Both Mia and I feel like it was the combination of Nerva paired with the low FODMAP diet that really worked for her and made an impact – it was a balancing act for her.”
Reviewing the outcome
Chloe met with Mia recently to review her progress.
Mia said she’d had a few flare-ups and was experiencing stress that was mostly due to her university studies. “We’re going to do reintroductions in her diet once her bowel has stabilized a bit.
“She has noticed she can tolerate some FODMAPs, but she does experience a flare-up if she eats a lot. It was more challenging because she’s a vegetarian and reliant on particular foods like legumes, but I gave her options and meal plans.
“What I always emphasize to all of my IBS patients is they can not stay on the low FODMAP diet long term. It’s like injurying your leg and being in a cast for six weeks – you’ve got to learn to walk again.
“Reintroductions are important, especially for prebiotics, and it’s one of the reasons why I don’t generally put people on the low FODMAP diet unless I genuinely believe that a patient’s diet is high in FODMAPs, or I can see there’s a strong correlation between certain foods and their symptoms.
“Generally speaking, my first approach with gut health patients like Mia is to regulate their bowel, and around 90% of these patients will have issues with stress exacerbation, and that’s why Nerva is in the mix straight away.
“I’ve found that the combination of regulating the bowel and adding Nerva is what gives you the best results.”
Referring gut-directed hypnotherapy
Chloe is a strong advocate for using gut-directed hypnotherapy for IBS management for her clients.
“I’ve only worked for 3 days so far this week and I’ve prescribed GDH five times this week already! So, I do recommend it a lot, and this week I haven’t prescribed the low FODMAP diet once.
“For me, Nerva is this additional tool I use in conjunction with regulating someone’s bowel. Because once their bowel is out of whack and they’re getting all of this bloating, they become hyperfocused on it. And once they get more stressed, they get more bowel symptoms, and they’re on the roller coaster. A big part of my work with Mia was helping her become less fixated on her symptoms.
“I’m always careful suggesting Nerva and don’t want to offend anyone. How I approach a patient is I start by asking, ‘Do you feel that your bowel is worse when you’re under a lot of stress? Does it misbehave?’ And then this leads to, ‘Well, there’s a tool that can help with that and it involves a 15-minute commitment...’”
Chloe Madigan is an accredited practicing dietitian at Ochre Medical Centre in Wollongong, Australia.
*Name changed
Mindset Health only uses high-quality sources, including peer-reviewed research, to support our articles. We work with experts to ensure our content is helpful, accurate and trustworthy.
Book a consultation with Mindset Health to learn what we can offer you and your patients.
Expand your mindset. learn more about mindset health.
- Colonic Diseases
- Functional Colonic Diseases
- Digestive System Diseases
- Gastrointestinal Diseases
- Intestinal Diseases
- Internal Medicine
- Gastroenterology
- Irritable Bowel Syndrome
Irritable Bowel Syndrome: A Case Study
- ANIMA Indonesian Psychological Journal 31(4):180-191
- 31(4):180-191

- Universitas Surabaya
- This person is not on ResearchGate, or hasn't claimed this research yet.
Abstract and Figures

Discover the world's research
- 25+ million members
- 160+ million publication pages
- 2.3+ billion citations

- Shanti Eswaran
- Azar Kiamarsi

- Ki-Baik Hahm

- Tetsuo Arakawa
- Baojuan Han
- A P Manning
- W G Thompson

- S.H. Lovibond
- Peter F. Lovibond
- Recruit researchers
- Join for free
- Login Email Tip: Most researchers use their institutional email address as their ResearchGate login Password Forgot password? Keep me logged in Log in or Continue with Google Welcome back! Please log in. Email · Hint Tip: Most researchers use their institutional email address as their ResearchGate login Password Forgot password? Keep me logged in Log in or Continue with Google No account? Sign up

- Nutrition counsellor
- Food Allergy
- gastroenterology
- Heptologist In Delhi
- Liver transplant
Case Study On IBS
- Case Study On Irritable Bowel Syndrome (IBS)
What does Irritable bowel syndrome (IBS) mean?
Irritable bowel syndrome (IBS), also known as mucus colitis, nervous colon, and spastic colitis. IBS is a common functional disorder of the gastrointestinal tract. It is characterized by current episodes of abdominal pain, bloating, gas, and discomfort along with changes in the consistency of stool. This condition is heterogeneous, exhibiting variability in the symptoms reported within and between males and females.
What are the symptoms of IBS?
I ndividuals suffering from IBS will experience symptoms like belly discomfort, pain, diarrhea, constipation. However, these symptoms tend to change over some time. It can either be cured by treatment or may get severe over time, depending on the individual’s condition. Some common irritable bowel syndrome symptoms are listed below-
- Abdominal pains and cramps usually in the lower abdomen after taking meals
- Bloating of abdomen
- Increased gas
- Passing whitish mucus through the stool
- Constipation
- Harder or looser stool than usual
- Sudden urge to go to the washroom
- Food intolerance
- Weight loss and loss of appetite
Irritable bowel syndrome symptoms in females
Most of the symptoms of IBS in females are similar to males. Sometimes the symptoms tend to worsen during the time of menstruation. Women often experience worsening diarrhea just before menstrual periods. Bloating is a common symptom of IBS. Females with IBS are more likely to experience more bloating during their menstrual cycle than those women without IBS. However, menopausal women experience fewer symptoms than those women who are menstruating. Some females reported that their symptoms increased during pregnancy.
Irritable bowel syndrome symptoms in males
Researchers suggest due to hormonal differences, the male gut is less sensitive to signs of IBS than females. Males do experience problems with sexual intimacy just like females. They face difficulty in fulfilling their work and most likely suffer from depression. However, many think males simply avoid symptoms of IBS.
Irritable bowel syndrome symptoms in infants
If anyone or both the parents are suffering from this disorder, children are at higher risk. Both boys and girls are equally affected. They face symptoms like, belly pain that can persist for more than 3 months, mucus seen in the stool, loss of appetite, bloating, and gas.
Are you looking for a gastroenterologist? No worries we got you covered! Book an appointment with Dr. Nivedita Pandey, one of the best gastro doctors in Delhi. Can simply go for a gastroenterologist live chat session with her. She is there to solve all your tummy problems with compassionate care.
Irritable bowel syndrome (IBS) causes
Researchers are not aware of the exact cause of IBS. There are several factors like alteration in the gastrointestinal tract, food intolerance, hypersensitivity to pain. These factors are believed to be the causes of IBS. Let’s discuss some common causes of IBS-
- Brain-gut dysfunction- When there is a miscommunication between the nerves of the brain and gut, there are chances to develop IBS.
- Dysmotility- When individuals suffer from the abnormal movement of food in the gastrointestinal tract.
- Bacterial infection in the gastrointestinal tract.
- Overgrowth of small intestinal bacteria- An increase in number or change in the type of bacteria can cause IBS.
- Genetic influences- Researchers say that genes can make some people develop irritable bowel syndrome.
Mental disorders- Depression, stress, anxiety can be causes of IBS.
Irritable bowel syndrome symptoms treatment
When it comes to nonsurgical treatment, changing lifestyle and diet is the primary treatment for IBS. Some of the lifestyle changes are-
- Exercise regularly
- Avoid drinking alcohol and smoking
- Sleep properly
- Get involved in stress management and relaxation techniques
- Practice mindful training
- Avoid taking caffeine
- Walk regularly and practice yoga
- Go for psychotherapy and cognitive behavioral therapy
- Drink enough water and fluids
- Avoid taking foods that trigger your symptoms, look for foods with low-fat content, intake cooked vegetables other than cabbage, cauliflower, and broccoli which might cause bloating of the abdomen.
- For those who are lactose intolerant try to avoid dairy products that can trigger your symptoms.
- Enjoy eating chicken and fish
In case lifestyle and dietary changes do not provide relief to your symptoms, the individual should immediately seek urgent medical attention by consulting an online doctor chat or simply get in touch with the best gastroenterologist in Jammu. Most cases of IBP can be treated by nonsurgical measures. But if the symptoms get more severe, the patient might have to undergo surgery.
Irritable bowel syndrome risk factors
IBS can affect both females and males of all ages. However, it is more likely to affect individuals during their teens to adulthood. Researchers have studied that genes play a vital role in the development of IBS. Records show that more females are affected than males. The reason can be, males do not reach out for help to a specialist, and females experience hormonal changes during their menstrual cycle.
On the other hand, irritable bowel syndrome health risks can be mental illness, stress, depression, and traumatic events in their lives like sexual abuse. These conditions during irritable bowel syndrome can be cured by providing stress management and behavioral therapy to the patients to decrease the symptoms.
Only a small number of people have severe cases of irritable bowel syndrome. Severe symptoms can be treated with medication and therapies. Most patients can control their symptoms by changing their lifestyle and diet. These individuals come under the category of mild cases of irritable bowel syndrome. On the other hand, some individuals might have extremely serious symptoms like bloody diarrhea, shortness of breath, palpitation, swelling of the tummy. These individuals are considered the worst cases of irritable bowel syndrome. Later in their lives, they might be diagnosed with colon cancer.
Irritable bowel syndrome case study
A 43-year-old woman with a history of recurrent abdominal pain for 25 years, loose stool, fecal urgency. In the last 1-2 years, her symptoms have worsened and she experienced severe abdomen pain, fecal urgency, and inconsistency along with 6 to 8 times loose bowel movements per day. She is not exposed to alcohol. Her symptoms started deteriorating in recent years with the increase in stress in her personal and professional life. Due to her improper bowel movements, she had to quit her job which requires a lot of traveling and her body is not permitting to do that. There is no family history of irritable bowel syndrome or colon cancer. Physical examinations and laboratory test reports showed no signs of weight loss, no blood in stool, CPC and laboratory negative for celiac serologies, colonoscopy with biopsy was negative, and fecal protection was negative. Previous treatment of this patient included a low-FODMAP diet with mild improvement in symptoms. The patient wants to resolve physical urgency and inconsistency and at the same time hopes for a solution to her other symptoms.
Irritable bowel syndrome case definition
This particular case of a 43-year-old woman suffers from symptoms like abdominal pain, fecal urgency, inconsistency, and loose bowel movements. From the patient’s history, one of the renowned gastroenterologists in Delhi was able to conclude that it is a functional gastrointestinal disorder, called irritable bowel syndrome.
Irritable bowel syndrome case report
Diagnosis- The 43-year-old woman is suffering from irritable bowel syndrome. It is a digestive system disorder and is concerned with abnormal bowel movements, and abdomen pain.
Signs and symptoms- The signs and symptoms shown by this patient are recurrent abdominal pain, fecal inconsistency, loose stools for 6-8 times a day, fecal urgency and her symptoms have worsened in the last few years, with an increase in stress in her professional and personal life.
Treatment- Firstly, based on the patient’s history, the best gastroenterologist in Delhi and her team suggested going for therapeutic sessions like CBT (Cognitive-behavioral therapy). The next important thing they paid attention to is diet. The patient was asked to continue taking a low-FODMAP diet, but at the same time consulting a dietician is recommended, to make sure she is getting her daily nutrition requirements.
When it comes to pharmacological management, such patients with severe symptoms should consider taking therapies of rifaximin or eluxadoline. Lastly, the doctor made sure the patient is aware that her symptoms might be minimized with a better lifestyle and medication, but it’s not realistic to completely resolve the symptoms.
Follow up- A follow-up was recommended by the doctor. Approximately 5 to 6 weeks later, the patient visited the clinic again. Dr. Nivedita and her team ran some tests and diagnosed her. With the right treatment and therapies, her symptoms got minimized. Abdominal pain, fecal urgency, inconsistency have improved, also her anxiety and stress got better. The Patient was sent back home in a better state and her family was thankful to the doctor and her team.
To solve your gastroenterology problems, get in touch with Dr. Nivedita and fix your appointment online. You can find her among the top 10 gastroenterologists in Delhi. She is considered one of the best doctors in Patna for the stomach, the best liver specialist in Delhi NCR, and the best liver expert doctor in Jaipur. Also, her ability to treat patients online by gastroenterologist live chat is remarkable. So take this opportunity and get yourself and your loved ones treated.
Consult the best Hepatologist in India, Dr. Nivedita Pandey through online dr chat if any abnormality occurs. She is also well known for her nutritional counseling services online services of hepatologist or teleconsultation services. Fill the form below to book an appointment with us now. She is also famous for her care from afar service and as a food pipe specialist . You can also find her as the best liver specialist doctor in Patna , Bihar or hepatologist in Patna or the best doctor for hepatitis b in Patna or at the gastro and liver clinic Patna Bihar, Gooddeed Clinic and also as a gastroenterologist in Faridabad , the best gastro doctor in Delhi, NCR , a gastroenterologist In Uttarakhand , a liver specialist in Jhansi or Best female Gynecologist in Jhansi, best gastroenterologist in Jammu or best physician in Jammu city, take advantage of the online gastroenterology consultation to gastroenterologist live chat and receive the best treatment that your body deserves!
Is IBS considered a serious health condition?
The severity of irritable bowel syndrome varies from person to person. In some individuals, IBS leads to symptoms that are manageable and can be cured by changing their lifestyle. But for others, the symptoms might get severe and interfere with their quality of life. However, with proper treatment, the symptoms can be minimized.
Is IBS a common disorder?
IBS is the most common functional disorder of the gastrointestinal tract. It affects both men and women starting from the age of 2 to 60.
Privacy Preference Center
Privacy preferences.
An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
- My Bibliography
- Collections
- Citation manager
Save citation to file
Email citation, add to collections.
- Create a new collection
- Add to an existing collection
Add to My Bibliography
Your saved search, create a file for external citation management software, your rss feed.
- Search in PubMed
- Search in NLM Catalog
- Add to Search
An Evidence-based Approach to Therapy in IBS-D: A Case Study Compendium
Affiliation.
- 1 Lin Chang , MD David Geffen School of Medicine at UCLA Los Angeles, California.
- PMID: 22570639
- PMCID: PMC3338169
A burden on both patients and the healthcare system, irritable bowel syndrome (IBS) is a prevalent condition that can result in high medical costs, frequent visits to the doctor, missed work, and anxiety and depression in the patient. This chronic disorder causes abdominal pain or discomfort and is characterized by abnormal defecation that presents mainly as either constipation or diarrhea symptoms. IBS associated with diarrhea (IBS-D) accounts for approximately one third of all IBS patients. IBS-D treatment can be confusing and frustrating for both the patient and the physician, complicated by the fact that a specific therapeutic algorithm has not been developed. Treatment options are widely varied, consisting of both nonpharmacologic (dietary changes) and pharmacologic (loperamide and alosetron) interventions. Furthermore, mounting evidence suggests a possible role for small intestinal bacterial overgrowth in the pathogenesis of IBS-D; thus, both antibiotics (such as rifaximin) and probiotics are frequently used to treat patients. Although all of these interventions elicit some measure of symptom response in a proportion of treated patients, there is no standard of care for the treatment of IBS-D. Thus, physicians would benefit from knowledge of all of the strategies used to treat IBS-D, in order to treat patients appropriately.
PubMed Disclaimer
Global assessment of symptom relief…
Global assessment of symptom relief in irritable bowel syndrome patients. Data from Whorwell…
Positive lactulose breath test: odds…
Positive lactulose breath test: odds in irritable bowel syndrome versus controls. The arrow…
confidence interval
odds ratio.
Similar articles
- Short-course therapy for diarrhea-predominant irritable bowel syndrome: understanding the mechanism, impact on gut microbiota, and safety and tolerability of rifaximin. Chang C. Chang C. Clin Exp Gastroenterol. 2018 Sep 24;11:335-345. doi: 10.2147/CEG.S167031. eCollection 2018. Clin Exp Gastroenterol. 2018. PMID: 30288076 Free PMC article. Review.
- Evidence-based management of irritable bowel syndrome with diarrhea. Pimentel M. Pimentel M. Am J Manag Care. 2018 Jan;24(3 Suppl):S35-S46. Am J Manag Care. 2018. PMID: 29372991 Review.
- Current gut-directed therapies for irritable bowel syndrome. Chang HY, Kelly EC, Lembo AJ. Chang HY, et al. Curr Treat Options Gastroenterol. 2006 Jul;9(4):314-23. doi: 10.1007/s11938-006-0013-8. Curr Treat Options Gastroenterol. 2006. PMID: 16836950
- Diagnosis and treatment of diarrhea-predominant irritable bowel syndrome. Lacy BE. Lacy BE. Int J Gen Med. 2016 Feb 11;9:7-17. doi: 10.2147/IJGM.S93698. eCollection 2016. Int J Gen Med. 2016. PMID: 26929659 Free PMC article. Review.
- Management of irritable bowel syndrome with diarrhea: focus on eluxadoline. Jones J, Lembo A, Heidelbaugh J, Kuritzky L, Lacy B. Jones J, et al. Curr Med Res Opin. 2021 Apr;37(4):567-578. doi: 10.1080/03007995.2021.1888705. Epub 2021 Mar 3. Curr Med Res Opin. 2021. PMID: 33566707
- Intensive short-term dynamic psychotherapy for irritable bowel syndrome: a randomized controlled trial examining improvements in emotion regulation, defense mechanisms, quality of life, and IBS symptoms. Shafiei F, Dehghani M, Lavasani FF, Manouchehri M, Mokhtare M. Shafiei F, et al. Front Psychol. 2024 Mar 28;15:1293150. doi: 10.3389/fpsyg.2024.1293150. eCollection 2024. Front Psychol. 2024. PMID: 38605838 Free PMC article.
- Treating irritable bowel syndrome through an interdisciplinary approach. Nelkowska DD. Nelkowska DD. Ann Gastroenterol. 2020 Jan-Feb;33(1):1-8. doi: 10.20524/aog.2019.0441. Epub 2019 Nov 29. Ann Gastroenterol. 2020. PMID: 31892791 Free PMC article. Review.
- Irritable bowel syndrome: a disease still searching for pathogenesis, diagnosis and therapy. Bellini M, Gambaccini D, Stasi C, Urbano MT, Marchi S, Usai-Satta P. Bellini M, et al. World J Gastroenterol. 2014 Jul 21;20(27):8807-20. doi: 10.3748/wjg.v20.i27.8807. World J Gastroenterol. 2014. PMID: 25083055 Free PMC article. Review.
- Acupuncture-moxibustion in treating irritable bowel syndrome: how does it work? Ma XP, Hong J, An CP, Zhang D, Huang Y, Wu HG, Zhang CH, Meeuwsen S. Ma XP, et al. World J Gastroenterol. 2014 May 28;20(20):6044-54. doi: 10.3748/wjg.v20.i20.6044. World J Gastroenterol. 2014. PMID: 24876727 Free PMC article. Review.
- Lacy BE, Rosemore J, Robertson D, Corbin DA, Grau M, Crowell MD. Physicians' attitudes and practices in the evaluation and treatment of irritable bowel syndrome. Scand J Gastroenterol. 2006;41:892–902. - PubMed
- Saito YA, Locke GR, Talley NJ, Zinsmeister AR, Fett SL, Melton LJ., 3rd A comparison of the Rome and Manning criteria for case identification in epidemiological investigations of irritable bowel syndrome. Am J Gastroenterol. 2000;95:2816–2824. - PubMed
- Talley NJ, Zinsmeister AR, Van Dyke C, Melton LJ., 3rd Epidemiology of colonic symptoms and the irritable bowel syndrome. Gastroenterology. 1991;101:927–934. - PubMed
- Ford AC, Forman D, Bailey AG, Axon AT, Moayyedi P. Irritable bowel syndrome: a 10-yr natural history of symptoms and factors that influence consultation behavior. Am J Gastroenterol. 2008;103:1229–1239. quiz 1240. - PubMed
- Drossman DA, Li Z, Andruzzi E, et al. U.S. householder survey of functional gastrointestinal disorders. Prevalence, sociodemography, and health impact. Dig Dis Sci. 1993;38:1569–1580. - PubMed
Publication types
- Search in MeSH
Related information
- PubChem Compound
- PubChem Substance
LinkOut - more resources
Full text sources.
- Europe PubMed Central
- PubMed Central
- Citation Manager
NCBI Literature Resources
MeSH PMC Bookshelf Disclaimer
The PubMed wordmark and PubMed logo are registered trademarks of the U.S. Department of Health and Human Services (HHS). Unauthorized use of these marks is strictly prohibited.
JavaScript seems to be disabled in your browser. For the best experience on our site, be sure to turn on Javascript in your browser.
Free Shipping On U.S. Domestic Orders $75+

Case Study: Irritable Bowel
- ← Back to Articles

Samantha came to see me after struggling with irritable bowel syndrome for nine months. Samantha’s main symptoms were frequent loose stools, severe abdominal bloating and flatulence. She was extremely distressed by these symptoms, due to their intensity and duration.
The abdominal bloating and discomfort became progressively worse as the day went on; so much so that by the evening Samantha said she looked 6 months pregnant. She said to me “I can’t leave the house at night because none of my clothes fit. It’s a good thing I live alone because the amount of gas I pass each night after dinner would drive everyone away”.
Samantha had seen her local GP about these symptoms and was referred to a gastroenterologist to rule out serious pathology. Nothing abnormal was detected and Samantha was diagnosed with IBS. The gastroenterologist suggested Samantha may want to try a low FODMAP diet and left it at that.
Samantha had done a quick internet search on low FODMAP diets but felt quite overwhelmed, so she wanted some personalised help.
After asking Samantha to complete a dietary questionnaire I discovered that her diet is based on wheat and milk. Nearly every meal and snack contains both of those foods. In my experience, gluten and dairy products are the biggest offenders in producing IBS symptoms. I asked Samantha to completely avoid all dairy products and gluten for one month and return at that time for another consultation. People with weak digestion usually have trouble digesting certain types of fiber in vegetables – known as FODMAPS. I asked Samantha to avoid onions, cabbage, broccoli and cauliflower for the moment, because those foods are particularly high in FODMAPS and they do have a bad reputation for causing gas. Once her digestion improves, she will probably be able to digest FODMAPS again.
I gave Samantha BactoClear capsules , in order to reduce the levels of pathogenic organisms in her gut that caused bloating and gas. I also gave her a glutamine supplement , to restore the integrity of her intestinal lining, give her symptom relief and reduce inflammation in her gut. When she finished the BactoClear capsules, Samantha was to take the probiotic Floratone, to restore levels of good bacteria in her bowel. I made sure she was not consuming any carbonated beverages, chewing gum or artificially sweetened foods.
I asked Samantha to take digestive enzymes with every meal. Supplementing with enzymes is a very effective way of alleviating symptoms of gas and bloating. The enzymes would help Samantha to digest her food more effectively. This means undigested food wouldn’t sit around in her intestines and provide food for unfriendly bacteria, which create gases.
Samantha returned one month later with a significant reduction in symptoms. Her stool was much better formed and she was only having two bowel movements a day. Flatulence and bloating were greatly reduced. She noticed some bloating and flatulence whenever she consumed almonds, and if she ate too much gluten free bread. Several of my patients have reported a sensitivity to almonds, therefore I asked Samantha to eat other types of nuts in small quantities and watch for a reaction. I told Samantha that the majority of gluten free bread is made of high glycemic index grains and therefore high in sugar. Ideally it would be an occasional food, and she is better off basing her diet on naturally gluten free foods such as vegetables, seafood, poultry, eggs, meat, and fruit, along with small amounts of nuts and seeds, and good fats. She found this eating plan quite acceptable and was thrilled with the symptom relief it offered her.
The above symptoms have not been evaluated by the FDA and are not intended to diagnose, treat or cure any disease.

Know someone who might benefit from this article? Share it!
Join the Conversation
Shop Our Products by Health Interest
Hormone & thyroid health, liver & gallbladder health, digestive health, bone & joint health, women's health, immune health, healthy aging, general health.
By submitting your email address you agree to joining our mailing list.
Most Recent Articles
- Lemon And Thyme Roasted Green Beans
- Men With Gout Have Increased Prostate Cancer Risk
- Perimenopause May Trigger Anxiety Or Depression
- Improve Your Kidneys
- Raspberry Overnight Oats
Most Popular Articles
- 5 Ways To Reverse A Fatty Liver
- Why Is Livatone Plus The World's Leading Liver Support Supplement?
- Things you must know if you don’t have a gallbladder
- What to Do If You Don't Have a Gallbladder?
- Your Skin Reflects Your Liver
1-888-75-LIVER
Monday to Friday, 9:00 am to 5:00 pm MST
Satisfaction Guaranteed
If it’s faulty or wrongly described, we’ll replace it.
Episode Intro
Podcast transcript, insiders guide to gut health – tony’s transformation.
Welcome to episode 32 of the Inside Knowledge. I’m Anna Mapson.
My IBS Case Study
I’d like to introduce you to Tony.
Tony, I worked with this year, and his big problem when we started working together was severe pain every single night that was coming from a bile acid build up during the night. He had bile acid reflux, so that’s where the bile that we produce to help us emulsify fats was backing up into his stomach and causing him a lot of pain.
The reason it was dripping out is he didn’t have a gallbladder. He’d had it removed about 30 years prior. And it was really affecting his sleep , really bad pain he was getting, and he also had issues around constipation . So he was passing one bowel movement every day, but he was really struggling.
He also felt that he had some problems with gastric dumping. So this is where your stomach is emptying too quickly into the small intestine.
Restrictive diet for IBS
All of these problems together had led him to have a really, really restrictive diet. So not only was he waking up several times throughout the night, He was also on such a small selection of foods, so his energy levels were really low , he’d lost a lot of weight.
He was extremely low in mood and just felt like things had hit a real crisis point. He was only eating eight different foods, and actually one of those foods was and fruit juice. So it was a really reduced amount of different things and eating those on repeat every day.
Losing hope
But with a sense of hopelessness, there was a sense that he was not going to survive this health condition.
He really felt like things had got so bad that he was just wasting away, and he didn’t know where to turn to. Obviously, he’d been to his doctors lots of times. He’d really been back and forth, to his different healthcare providers, and they had run lots of different tests, they had given him some medication to help excrete the bile out of the body, and that was helping to an extent, but not really.
How Tony chose me as his IBS nutritionist
The reason that Tony said he wanted to work with me is that he knew from our first discovery call where we went through his issues that I had a good understanding of his health conditions, Of diet and food and what things can do. And by that I just understood what the medication was I knew a lot about the different medical terms that he was using. I was able to challenge him a little bit on different diet things that he may have heard online. Or read about that actually, I thought weren’t helpful.
So he knew that we were gonna have a good working relationship and that we would be able to be on the same page about things. So I think that’s what really helped him is that discovery call, just feeling like actually I can learn from you and I want to work with you.
And I have to say he is one of the most committed people I’ve ever worked with in terms of taking everything on and really going for it.
How Tony reintroduced foods to his IBS diet
So as an example of that, one of the first things I wanted to do is try and get him eating. a variety of different foods. I wanted to increase the amount of fibre that he was getting to help increase bowel movements. And ease that sense of constipation that he was getting. I also really wanted him to eat more protein because I was really concerned that he was not able to sustain his muscle mass and would eventually start to lose even more weight if he couldn’t increase his amount of calories.
I started off by suggesting to him that he would introduce green beans. Little thin green beans, and at such a gentle way we would start off with one bean on one day. The next day we would increase it to two beans, and the next day to three beans.
Starting with green beans
So that was my first suggestion, and I said you don’t need to do anything, just the first step is to go out, buy the beans, and we will chat in a few days and see how it’s gone.
One of the examples of how I think he was so committed is by the time I’d spoken to him, he had already bought the beans and he’d already done day one and day two. He was just on it. He was so keen to make some changes.
And even though these were tiny changes, he was really prepared to give it a go. Despite the anxiety and the worry that this would not work for him, that there would be a further problem by following my advice.
Finding the right nutritionist for your gut issues
This is a really crucial part of working with someone. So if you have a call with me or someone else, another nutritionist. And you don’t really get the sense that they know what they’re talking about. That they have got examples of having done this before. And that you just don’t believe what they’re suggesting is going to be helpful.
It’s not going to work. You have to buy in to the process and you have to buy in to the thought that this person is the right one for you. And it might not be the right one. Like, you can shop around and find different nutritional therapists all over the world. People who are doing a similar job to me but in a different style.
You’ve got to find the right person for you.
Increasing protein to help gain weight – a case study
The next thing that I really wanted him to focus on was increasing his protein and this was where it didn’t go so well. I asked him to start taking some whey powder because when people are sensitive to FODMAPs, that’s the carbohydrate element of lots of foods, lactose can be something that people react to.
It can make you bloated, it can give you gas, it can cause diarrhoea, all kinds of things that people don’t always get on well with in terms of drinking cow’s milk. However, I have had some really good results with people taking whey protein., That’s the protein from dairy and adding it to their food, or adding it to a smoothie or something. The problem was, is that he wasn’t having anything that he could mix it with, so it was really difficult.
Early reintroduction of milk – a bad choice!
So what he decided to do was buy some milk and mix the whey protein with milk.
This was terrible, and he started getting a lot of gas, and even within one day, he just decided this was not going to work.
So, what we did instead was move back towards focusing on other types of protein.
He kept the whey protein because it keeps for a long time, won’t go off, and we started thinking about how he could eat more eggs. Eggs were another protein he was prepared to try. So starting off with just one, then having maybe two scrambled eggs, and then maybe moving on to an omelette.
Adding more vegetables
The next vegetable that he increased was butternut squash. So once he’d got to three green beans, I said keep it at that level for a few days. We’re going to start to aim for 80 grams, which is one portion of vegetables. So I wanted him to aim for 80 grams. But once he’d got to say about 40 grams, we started to reintroduce another vegetable, which in this case was butternut squash.
And again, starting with really small amounts, little cubes of mashed up soft boiled vegetables. Really easy to digest and just helped him to get a bit more confidence.
That was just in the first sort of two weeks, we just introduced those things. So green beans, the whey, which went terribly, and then butternut squash and eggs.
In this way, he’s starting to increase his fiber, starting to increase some protein containing foods. Now alongside all of this, there was education really around Digestion and how long things take to move through you, so I asked him to do a stool transit time to check how long food was taking for him to eat, uh, and then how long he would see it in the toilet.
This was really important because he was feeling that some foods were giving him an immediate sense of constipation or bad bowel movement.
Learning about gut transit time in IBS
And I talked a lot about how long food takes to move through us. If you want some details on that, go and listen to episode one about normal digestion . But, effectively, you’re looking for like 24 hours, 36 hours for food to start coming out.
That’s normal. So something you literally just eat is not going to cause you constipation or a hard stool the next time that you go to the toilet.
It’s kind of uncoupling that sense of what you’re eating, giving you an immediate problem. We also had a lot of conversations about what is constipated, what does that actually mean.
Sense of incomplete evacuation
If you’re going to the toilet every day and the stools are relatively soft, and you’re having sufficient amount of stool come out of you, that’s not constipated. And yet, some people feel that they haven’t got rid of everything, and there’s this sense of incomplete evacuation. That is really common. Now it doesn’t necessarily mean that you’re constipated and that you need laxatives or that you need to change your diet.
Sometimes more food is the answer to constipation
It could just be needing to eat more food because sometimes we need more bulk to move along. A lot of things are getting absorbed through the gut and so sometimes you need to add more fibre in, in order to get a more bulky stool. And that is actually what happened in this case. So the more food he ate by the time he got through a month. Or a month and a half that actually he was getting a really good stool. It was happening because he was eating more fibre, more bulk that could be passed through the gut.
Tony also introduced potatoes. These were good foods for him because we could cook them in a variety of ways and led him to eat a bit more of an interesting diet. Also kiwi fruit because I stressed about how it was a good way, low FODMAP way, to increase stools and move away from constipation. But he also introduced a whole range of proteins including beef, salmon, chicken and even nuts as well.
Normalising the diet – adding flavour
And some days he ended up going two to three times a day. This was in about a month into our journey of working together. I also really wanted him to focus on flavour giving food. So things like green herbs, like parsley, coriander, mint. These are really great additions to boost the flavour profile of your meals and make them a bit more desirable.
When you’ve had a history of eating the same food again and again, it just gets so boring. And so naturally you don’t. Really feel much of an appetite. So in order to stimulate your appetite to be able to eat more It’s good to increase flavours things like ginger, citrus fruits These can all be really helpful in small doses to begin with till you feel confident with the flavour And then you can just go wild with those things like green leafy herbs. Because they’re actually really good for you as well and can contain things like iron and magnesium.
Slow digestion from eating protein
Going back to the protein, Tony really had a sense that when he ate too much protein, his stomach just shut down.
He felt like things did not move through his gut for a really long time. He felt too full eating a small amount of protein. So when we’ve introduced these things like beef and the salmon, at first he was just eating a really small amount once a day. Obviously, I knew that this is not enough protein to keep him going.
However, he was prepared to keep going with it and just stay at, for example, three days, eating one amount of beef for three days in a row, just at lunchtime. After that period, once he felt like that was being okay and tolerated, he was able to eat. Protein twice a day, for example, having eggs in the morning, having the chicken at lunch and then even moving on to having three amounts of protein throughout the day.
Gradually increasing the food volume
As his confidence grew, we also introduced snacks in between meals, and these were additional ways to eat foods that he just quite fancied eating. That he actually just ate for enjoyment, but also to try to up the nutrient content. And up the calorie content because he really didn’t want to lose any more weight.
So this feeling that protein was bad for him that was closing down his system and shutting things down was actually really key to address because without that level of protein I didn’t feel like he was going to get enough energy and enough strength to start and start feeling better overall. We need a lot of fats, proteins, carbs.
Like we need the balance of all of these things to have a good, healthy response and be able to create neurotransmitters that help us feel happier. And just feeling like we’ve got energy to want to go out for a walk. We need nutrients for all of these things. Basically feeling like you’re having a good life rather than just existing.
Case study learning – Eating more can lead to a better appetite
And so trying to get all of those different. Balance of nutrients in was really important in order to make it feel like life isn’t just about this condition or the problems. Actually, he felt like the more he ate, his appetite came back.
So, he ended up saying, I’ve got a huge appetite this week. About two months into working together, and he had not felt hungry for so long.
One of the best points for me in this was that he said he once had a normal bowel movement. It was the first he’d had in four years.
And he was really happy and we had a little mini celebration about that. Because this is a real turning point. And it felt… to him like a normal bowel movement. I really attribute this to eating more food and eating a bigger variety of foods. Because it was enabling him to have a better bowel movement. But also enabling him to feel more energy, feel a bit more positive.
Lastly – bringing back the FODMAPs
One of the final areas he introduced was the really high FODMAP foods, including onions, garlic, and also wheat.
I talked a lot about the prebiotic qualities of these foods, like they are really good for your gut bacteria. How grains as well, like whole grains, including wheat, rice, these can be very beneficial foods for supporting your gut bacteria.
Starting supplements for IBS
With this client, I didn’t use many supplements. One of the things I suggested that he would do would be start taking some magnesium powder. And this was specifically at the beginning when I had really understood that he was feeling constipated. He also had a high degree of anxiety. So we used magnesium citrate powder.
The reason I like using a powder with people is because you can titrate it up and down. You can increase the dose, and you can make very small doses if you prefer. So powder is really good in that sense, particularly when people are nervous about taking different supplements.
Butyrate – the gut bacteria’s creation
I also suggested that he start taking butyrate, which is a short chain fatty acid produced by your gut bacteria.
But if you don’t have many good bacteria in the gut, then sometimes taking it can be a way to accelerate progress. Changing the environment of the gut by actually putting this short chain fatty acid in. There’s three types of short chain fatty acids that our good gut bacteria make. Butyrate is one of them.
Benefits of short chain fatty acids
The other ones are acetate and propionate. These are anti Inflammatory. They are fuel for the colon cells and they can actually move around the body and have an anti inflammatory effect. But specifically in the gut, they really help to change the environment of the gut. So sometimes taking a butyrate supplement may be helpful.
It’s not important for everybody and the best way to get that is through food and fibre. But at the beginning when he wasn’t really eating much fibre, I thought that this might help accelerate his changes. And towards the end of working together, we talked about introducing some probiotics as well. He was keen to do that, but I wouldn’t have thought it was essential.
It was just another like additional future proofing to try and really keep, keep things working as they were.
Client’s feedback on this case
One of the really nice things that we ended on was him saying this is better than I ever thought it could be for myself. Like, I can really see myself getting better now and that sense of hope was really moving for me.
I have to say, I really enjoyed working with this person because he was so engaged in the suggestions that I gave him.
Toning the throat muscles
Another thing that was slightly different that I hadn’t done before was encouraged him to use an iQuoro device. This is like a little plastic device you put in your mouth and bite down on it. It tries to help you tone the muscles in your throat and your neck and can be quite helpful for people with various issues around swallowing. Sometimes like if dysphagia where you can’t swallow very well or you have silent reflux. It’s just about helping coordinate the muscles of your throat, your esophagus and, and the tongue as well, and strengthening those up.
The thing about this device is that it only really works if you continue to do it. It’s a bit like weight training. It’s not like a once and done and you’ve done it. You have to keep going and keep those muscles working. But if it allows you to eat better and get the sense that your diet is improving, I think it’s worth doing some of those things sometimes in order to sustain the improvements.
And that’s the other thing about when I say people are really motivated. When you are in such a bad place and you can see some signs of improvement, of course you’re going to be more motivated. There are other people who I’ve worked with who, it takes a long while for them to start to see changes and they don’t feel as motivated, and I really get that.
If you’re not seeing progress, it feels just all like an uphill struggle, whereas in this case… It was pretty quick, I have to say, and rapidly saw some improvements in the way that he was feeling. So, I hope there’s been something from that that you can take that’s useful for your situation, your digestion.
Join the Gut Reset
If you’re interested in working with me, I work with people specifically over three months in my gut reset. Check out the show notes for links to my website where you can find out a bit more about how I work. I work with you wherever you are in the world, as long as the time differences work and we can align our schedules to talk during my working hours, then I’m happy to work with you wherever you are.
And I also have a whole range of education. That is given as part of the Gut Reset in videos and things that are pre recorded that you can do in your own time. So you can learn about gut health, about diet and healthy nutrition, as well as getting the regular coaching calls. Because I speak to people each week and I think for me that’s a big part of why I see this transformation or why I see this Process being effective is that I really get to know people and what will work.
And I think that’s where people make the biggest improvements is because if things aren’t working it’s only another few days until we speak again and we can really problem solve and work out what would be better for you. What could you start, stop, change to actually get the improvements that you need.
Right, I will leave it there for next week.
Ep.61 – Forcing your body – The IBS struggle
Forcing your body with IBSWelcome to episode 61 of the Inside Knowledge Podcast for people with IBS. I'm Anna Mapson. I've picked this topic about forcing your body to do things that you don't want it to do because it's something I've seen more and more. Or maybe I've...
Ep.60 – Is the FODMAP diet worth the hassle?
Is the FODMAP diet worth it? Welcome to episode 60 of the Inside Knowledge podcast. I'm Anna Mapson. This week I've chosen to speak about the low FODMAP diet because it has coincided with me launching the ultimate low FODMAP diet guide, which will help you understand...
Ep.59 – How to get better sleep when you have IBS
Sleep and IBSHello, welcome to episode 59 of the Inside Knowledge. I'm Anna Mapson. The reason for picking sleep as a topic for a podcast is that a lot of my clients really struggle with sleep. It can be down to things like waking up because your symptoms are waking...
Ep.58 – Case study – Vegan slow transit constipation to normal
Sian's IBS story - vegan diet with constipationWelcome to episode 58 of the Inside Knowledge. I'm Anna Mappson. Choosing case studies to share with you is always really good. It gives me a chance to reflect a little bit on What's gone well and actually how my practice...
Ep.57 – Should we be eating 30 plants a week
Should we eat 30 plants a weekWelcome to episode 57 of the Inside Knowledge podcast for people with IBS. I'm Anna Mapson. You've probably heard me talk a lot on this podcast already about the importance of diet variety to help feed our gut bacteria and cover essential...
Ep.56 – IBS advice to ignore by an IBS nutritionist
Breathing techniques for IBSWelcome to episode 56 of the Inside Knowledge podcast. I'm Anna Mapson. My podcast has turned one this week. I've been running weekly episodes for a year now, and I wanted to take this time just to reflect a little bit on things that I have...
Ep.55 – The truth about stomach acid
The truth about stomach acidWelcome to episode 55 of the Inside Knowledge podcast. I'm Anna Mapson. Today I want to talk about stomach acid and particularly about low stomach acid and not having enough. I think I will do a separate episode on high stomach acid or...
Ep.54 – Hidden IBS trigger ingredients
Breathing techniques for IBSWelcome to episode 54 of the Inside Knowledge podcast for people with IBS. I'm Anna Mapson. This episode is going to highlight a couple of things that you might be seeing in your food ingredient listings that could be contributing to your...
Ep.53 – How your childhood eating could be affecting your IBS
Breathing techniques for IBSwelcome to episode 53 of the Inside Knowledge podcast for people with IBS, I'm Anna Mapson. In my work as an IBS nutritionist, I work with people all over the world who have all different kinds of relationships to food I find it so...
Ep.52 – The possible cause of your IBS-Diarrhoea
Breathing techniques for IBSWelcome to episode 52 of the Inside Knowledge podcast. I'm Anna Mappson. The reason I selected bile acid diarrhoea as a topic for this week's podcast is that I see it quite often linked with SIBO, that is small intestine bacteria...
Improve IBS without changing your diet?
Download my top 5 non-food changes for better digestion.
Super simple tips for relief from painful and embarrassing IBS symptoms
ImmunoNutrition
Every meal is an opportunity to heal, case study examples.
Case study: IBS
The following case study aims to show you how I work with a client as a Nutritional Therapist. Irritable Bowel Syndrome (IBS) is one of the most common conditions that clients come to see me for. Many aim to see relief from an array of symptoms such as constipation, reflux, diarrhoea, wind, headaches, bloating and food intolerances alongside a host of additional symptoms that they did not know could be related, including joint aches and pains, weight loss or gain and fatigue. In this case study I will show you how a client’s symptoms may present and how functional diagnostic testing and Nutritional Therapy can help.
James was a 50-year-old gentleman when he first came to see me. He had been suffering from a range of IBS symptoms for over 25 years which began after a particularly stressful period in his life. His symptoms included constipation, diarrhoea, flatulence and abdominal pain alongside extreme fatigue and insomnia. More recently, he had begun to experience joint pain which, alongside his fatigue, was preventing him from leading the active life he previously had. He had been to see his Doctor who had recommended a colonoscopy, endoscopy and some blood tests. However, all the results came back within the ‘normal’ range. Despite this evaluation, his symptoms persisted.
James ate quite a healthy and well-balanced diet. Because of this, he was even more confused as to why his symptoms were so bad. He had already identified that certain foods were problematic for him, and had tried removing these, however although initially his symptoms had lessened, the relief was not long lasting.

First consultation
During his first consultation, James and I discussed all of his symptoms in great detail. I spoke to James about the need to get additional blood works from his Doctor and also spoke about a functional stool test. A stool test is a test which is able to provide a comprehensive picture of your gastrointestinal tract- the results of which can therefore ensure that any intervention is based upon your specific needs. If you are struggling to break down and absorb the nutrients in your food, then problems can arise elsewhere in your body, even if you eat a healthy diet.
The results of James’ stool test highlighted several issues. It demonstrated that he had an overgrowth of some bacteria that would generally not be present in a healthy gut. He also had an overgrowth of yeast and a parasite. In addition to this, several markers in the stool test were raised, indicating that his immune system had been triggered. The triggering of this immune response indicated an immune response to both the overgrowth of these bacteria, the parasite and specific trigger foods.
We discussed his results and I explained how his symptoms (such as constipation and bloating) were associated with the presence of these bacteria, yeast, the parasite and that the food intolerances that he was experiencing can develop as a consequence of a reduced integrity of the intestinal lining- or ‘leaky gut’ as it is commonly known.

Protocol introduced:
Due to the presence of a parasite and an overgrowth of both bacteria and yeast in James’ gastrointestinal tract, the approach we used was based upon the 5 R protocol. Although the protocol appears to be linear, some of these stages can be introduced concurrently depending upon your symptoms:
- Remove Food allergens were identified and removed and the bacterial and yeast parasites were removed using a combination of herbs and other supplements.
- Replace At the same time as removing triggers of inflammation, I supported James’ digestion using specific digestive enzymes (which the stool test had highlighted as being low). James’ diet was tweaked to ensure that it was nutrient dense and could nourish his health.
- Reinoculated Once the parasites, bacteria and yeast infection had been cleared to as great an extent as possible, probiotics were introduced carefully to help reinoculated his gut with beneficial bacteria.
- Repair Specific nutrients that help repair the intestine lining were then added whilst ensuring to keep inflammatory foods out of his system.
- Rebalance We ensured to focus on other factors that were possible triggers of James’ inflammation, including stress and reduced sleep- both of which can affect the health of your intestine.
The results:
Within 8 weeks of following the nutritional therapy programme, the long-lasting abdominal pain that James had been experiencing, had more or less gone. James was having a regular ‘normal’ bowel movement daily and felt more in control of his health and symptoms. After 4 months on the plan, he reported that not only had his gut health improved dramatically, so too had his sleep, skin health and mental wellbeing. He also had more energy and was starting to return to exercise.

You may have had your symptoms for years; you may be struggling to cope – however there is hope. Understanding and addressing the cause of your concerns is central to ensuring that your health will improve.
Case study: Chronic Fatigue
The following case study aims to show you how I work with a client as a Nutritional Therapist. Many clients who come to see me have a diagnosis of chronic fatigue or are suffering with persistent, ongoing fatigue. Many clients also experience a host of additional varied symptoms, including (amongst many others) joint pain, reduced mobility, light and sound sensitivity, restless leg syndrome, digestive health concerns (such as diarrhoea, constipation, flatulence, food intolerances), insomnia, heart palpitations, impaired cognitive function and changes in their weight. Women also often experience menstrual pain, fatigue, irritability, breast tenderness and headaches. Many clients are also taking a range of prescription medications for a range of reasons, for example to try and support their appetite, reduce their pain or improve their sleep. In this case study I will show you how a client’s symptoms may present and how functional diagnostic testing and Nutritional Therapy can help.
Anna was a 28-year-old lady when she first came to see me. She was diagnosed with CFS over 5 years ago and despite introducing a range of techniques that were recommended by her GP (such as pacing and shifting, resting, regularly snacking) her fatigue persisted. Anna also reported that she had been experiencing ongoing pain in her joints for which she was taking prescribed medication and over the counter medications alongside introducing distraction techniques and heat therapy. Anna found the ongoing pain to be particularly problematic and disabling and sought resolution to this pain as a primary goal. Anna was also experiencing chronic stress due to her illness, repeated throat infections (which were regularly treated using antibiotics), insomnia, light and sound sensitivity and cognitive difficulties which, alongside her fatigue and pain, impaired her capacity to work. She had been to see her Doctor and a Consultant, had had a range of blood tests (all the results came back within the ‘normal’ range). Despite this evaluation, her symptoms persisted.
Anna tried her best to eat as well as she could, however because of her fatigue and pain, she struggled to shop and cook and relied heavily upon family members (with whom she lived), to help her. Much of the food she ate was convenience food that was premade. She also frequently relied upon sweet foods and foods high in carbohydrates alongside caffeinated beverages to fuel her depleted energy reserves throughout the day.

During her first consultation, Anna and I discussed all of her symptoms in great detail. I requested a copy of her most recent blood tests from her GP and spoke to her about the need to get additional blood works from her Doctor in order to rule out any functional cause of her symptoms. In addition, we discussed the various functional tests that were most appropriate for her needs and symptoms and consequently both a stool and Organic Acid test were ordered.
The results of Anna’s OAT test highlighted mitochondrial dysfunction and a deficiency of specific nutrients. It also demonstrated reduced absorption and a lowered intake of vital nutrients. Her stool test demonstrated an overgrowth of some bacteria that would generally not be present in a healthy gut alongside raised inflammatory markers. In addition, her GP blood test results demonstrated the need for additional thyroid testing and supplementation.
At her second consultation, we discussed her results and I explained how her leaky gut and mitochondrial dysfunction were linked and possible causes of these based upon her background medical history, diet, blood test results and lifestyle. A plan was then introduced to address these problems.

The presence of both bacteria and yeast in Anna’ gastrointestinal tract alongside nutrient deficiencies, were affecting her energy production cycle (Krebs cycle). A 5 R protocol was introduced whereby we:
- Removed We identified and removed food allergens (including IgE reactions that were identified in the hospital setting) whilst also introducing specific supplements and dietary protocols to challenge the overgrowth of yeast and bacteria.
- Replaced At the same time as removing triggers of inflammation, I supported Anna’ digestion with specific nutrients that were highlighted as depleted. Anna’ diet was also tweaked to ensure that it was nutrient dense and could nourish her health.
- Reinoculated Once the bacteria and yeast infection had been cleared to as great an extent as possible, and proinflammatory foods had been reduced, various probiotics were slowly introduced carefully to help reinoculated her gut with beneficial bacteria.
- Repair Specific nutrients that help repair the intestine lining were then added whilst ensuring to keep inflammatory foods out of her system.
- Rebalance We ensured to focus on other factors that were possible triggers of Anna’ inflammation. Other health care professionals that were able to support additional triggers were introduced.

Within 8 weeks of following the nutritional therapy programme, the long-lasting abdominal and muscular-skeletal pains that Anna had been experiencing, had more or less gone. Anna was having a regular ‘normal’ bowel movement daily and felt more in control of her diet and lifestyle. Her energy levels started to improve, so too had her digestion, sleep, skin health and mental wellbeing. She was able to concentrate much better and no longer experienced heart palpitations. Anna continues to improve on a daily basis and greatly values having more independence and a greater control and understanding of the underlying causes of her diagnosis. Anna is no longer reliant upon medication to control her symptoms and pain.

Share this:
Blog at WordPress.com.

- Copy shortlink
- Report this content
- Manage subscriptions

Case study : Irritable Bowel Syndrome (IBS), Male, 41 years old
Case study 3 : Irritable Bowel Syndrome (IBS), Male, 41 years old
Fair health state, IBS in history 15 years, followed by a low back injury at age of 15, injury of cervical spine later in life. Not taking any medication, in the past was taking anti-inflammatory and immodium. Diet is not great, drinking cider, wine, eating spicy foods. History of IBS: started off discomfort in the lower abdomen in response to stress further developed to an agonising pain and instant desire to go to toilet. Very disturbing for life. Observation: Periodically stressed, sleep well. Abdomen is distended. Palpation: lower abdomen and sacral area are tender to palpation.
- 1st treatment – Assessment, shows that the Active region is cervical. Treated with Flex Array – significant relaxation + 6 points
- 2nd treatment – pain in the lower back focused on Lumbar zone
- 3rd & 4th treatments – treatment of the lower abdomen and Flex array on the Lumbar-Sacral zone;
- 4th & 5th treatments – exacerbation of pain in the lower abdomen, focused on the Sigmoid projection,;
Treatment outcome – mid course – pain is a lot less and even poor diet would not stimulate it.
- 6th and 7th treatments – focus on the Lumbar back region with Flex array and working on the upper abdomen (complaint zone);
- 8th treatment –Assessment – Active zone shifted to mid Thoracic, focused on it.
- 9th and 10th treatments – spinal roots L4, L5 with Flex array focused on the dermatomes.
- 11th and 12th treatments – assessment + 6 points, readings shifted again to Low back. Treated with the Flex array.
Treatment result: Pain is completely absent, even when stressed or poor diet.
Recommended to purchase a home use device to maintain the result and get further improvement.
A resent progress was checked three years from his first treatment.
During the first year he used his personal device occasionally, mostly as a preventative treatment. Now the patient reported that there were no symptoms of pain or discomfort, even in stressful situations.
An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
The PMC website is updating on October 15, 2024. Learn More or Try it out now .
- Advanced Search
- Journal List
- Gastroenterol Hepatol (N Y)
- v.15(1); 2019 Jan
Irritable Bowel Syndrome and Dietary Interventions
Irritable bowel syndrome (IBS) is the most prevalent functional gastrointestinal disorder, affecting approximately 14% of the global population. Symptoms of IBS are some of the most common reasons that primary care providers refer patients to gastroenterologists. IBS has a significant economic impact on the health care system and greatly reduces patients’ quality of life. The precise cause of IBS remains unknown, but likely involves a variety of factors, such as infection, inflammation, medication, and stress, in a genetically predisposed individual. Physicians can diagnose patients with IBS by obtaining a careful history and physical examination, performing limited testing, and applying the Rome IV criteria. Treating IBS symptoms can be challenging, as no medication cures the disorder. Thus, treatment focuses on improving symptoms and quality of life. Many patients report that symptoms develop from, or are exacerbated by, food. A number of physiologic and biochemical processes can occur with food ingestion that may produce heightened symptoms of IBS. Therefore, dietary interventions to improve IBS symptoms appear to be a reasonable treatment approach. This article discusses the evidence supporting dietary interventions for the treatment of IBS.
Irritable bowel syndrome (IBS) is the most commonly encountered functional gastrointestinal disorder, with a worldwide prevalence of approximately 14%. 1 IBS is a chronic disorder for many patients and is associated with markedly elevated health care costs and a reduction in patients’ quality of life. 2 , 3 The disorder can be diagnosed using the Rome IV criteria in combination with a careful history, physical examination, and limited diagnostic tests. 2 - 4
Although the exact pathophysiology of IBS remains unknown and differs in extent and magnitude from patient to patient, alterations in the gut microbiome, 5 , 6 disturbances in gastrointestinal motility, changes in the enteric nervous system, coexisting psychological distress, and visceral hypersensitivity all likely play a role. 2 - 4 These different pathophysiologic processes lead to variations in symptom expression, making IBS a heterogeneous disorder. Targeted pharmacotherapy for IBS has been largely unfruitful due to a lack of clarity regarding local gastrointestinal nervous system and central modulation mechanisms involved in visceral hyperalgesia, as well as the multiple neurotransmitters involved in this hypersensitive state. 7 - 9 Not surprisingly, treating IBS symptoms can very often be challenging, and no validated treatment algorithm exists.
A variety of pharmacologic therapies are available to treat IBS symptoms; however, many patients prefer to avoid medications and desire alternative approaches. 10 Dietary modifications to treat IBS symptoms have received significant attention lately, in part due to the recognition that many IBS patients report that foods appear to induce or exacerbate their symptoms. 11 , 12 Some patients believe they are able to identify the specific offending items; however, several studies show that when patients are rechallenged with the foods they perceive as triggers, they do not report the same symptoms. 1 , 2 , 13 , 14 Although certain foods have been traditionally recognized as triggers for diarrhea, abdominal pain, gas, and bloating, no formal research existed to prove or disprove their cause-effect relationship or their therapeutic benefits until the 1940s, when reports of malabsorption of different carbohydrates, as well as their relationship with gastrointestinal symptoms, were first published. 15 , 16
The research methodology of studies for placebo-controlled dietary interventions requires a more sophisticated design than a drug or nutrient trial, during which a similar capsule or tablet without the active ingredient can be delivered to the control group. A properly controlled diet study can be performed by developing sham diets that are comparable in feasibility and complexity both for teaching and/or instructing (to minimize investigator bias) and for following (to minimize patient bias) when compared to the studied diet. 17 Placebo and nocebo effects cannot be underplayed in these trials, as the clinical effects from a diet change can be influenced by several factors, including patients’ expectations, previous responses to particular diets, taste preferences, and personal and cultural beliefs regarding the impact of food in health. 17 Therefore, a rigorous design is needed for the development of the control group in dietary advice trials. 18
A number of different diets are now promoted to treat IBS symptoms, and these include regimens that exclude carbohydrates, fermentable foods, gluten, and substances that might create food-related antibodies. 13 , 19 Despite significant interest in this area from patients and providers, carefully controlled prospective studies evaluating the safety and efficacy of these diets remain limited. This article reviews the different diets available to treat IBS symptoms using the most recent data from the literature, focusing primarily on diets low in fermentable oligosaccharides, disaccharides, monosaccharides, and polyols (FODMAPs) and excluding gluten, as these 2 diets have been the most carefully studied and are commonly employed by patients.
The Low–Fermentable Oligosaccharide, Disaccharide, Monosaccharide, and Polyol Diet
The low-FODMAP diet was developed at Monash University in Australia by Dr Peter Gibson and Dr Susan Shepherd, and is now commonly used to treat IBS symptoms, based on both biologic plausibility and evidence from prospective trials showing improvement in symptoms in approximately 75% of patients. 3 , 13 , 15 The low-FODMAP diet has progressively gained ground in mainstream media over the last 12 years, with growing notations in websites, blogs, tweets, and vlogs. Food companies are now even incorporating the term into their labels. As of September 2018, a search for the term FODMAP identified over 11,000 videos on the YouTube platform and 180,000 posts on Instagram. Most Twitter posts mentioning the hashtag #low-FODMAP originate from Australia, the United States, the United Kingdom, and Canada.
Fermentable Oligosaccharides, Disaccharides, Monosaccharides, and Polyols and Irritable Bowel Syndrome Symptoms
FODMAPs are short-chain carbohydrates that are characterized by limited (or minimal) small intestine absorption, intense bacterial fermentation to short-chain fatty acids (SCFAs), and high osmotic activity. 5 , 20 Ong and colleagues demonstrated that dietary FODMAPs induced hydrogen and methane production in the intestines of patients with IBS. 21 The changes in pH levels, and the probable changes in gut flora, 4 - 6 , 22 may alter colonic epithelial function and may also cause local inflammation, thereby contributing to changes in colonic function. 5 , 20 When superimposed on baseline symptoms of abdominal pain, these changes can significantly heighten gastrointestinal symptoms in patients with IBS, especially those of excessive gas, bloating, and loose stools. 23 , 24
Other research has proposed alternative mechanisms by which the ingestion of FODMAPs could cause symptoms; however, there are scant data to support the hypotheses. For example, elevated SCFAs could stimulate mucosal release of 5-hydroxytryptamine (serotonin) and the production of histamine, causing a localized neuro-inflammatory response involving mast cell activation. These factors could contribute to a detrimental change in intestinal secretion, sensitivity, and motility, causing or worsening IBS symptoms. 25
The potential role of a diet high in FODMAPs in the development of IBS symptoms can be seen in the Figure. 26 , 27
Phases of the Low–Fermentable Oligosaccharide, Disaccharide, Monosaccharide, and Polyol Diet
The low-FODMAP diet intervention for IBS patients consists of 3 distinct phases: the restriction or elimination phase, the reintroduction or rechallenge phase, and the maintenance or personalized phase. 28 , 29 During the initial phase, patients eliminate FODMAPs from their diets. Importantly, the low-FODMAP diet is meant to last only 4 to 6 weeks, and it is essentially a method to determine whether symptoms are related to specific foods. It is not designed for long-term use. During the second phase, after noting symptom improvement or resolution, foods containing FODMAPs are reintroduced gradually, with the goal of identifying tolerance to individual ingredients and specific symptom triggers among fermentable carbohydrates. This phase lasts several weeks, if not longer, as foods are slowly reintroduced. After reviewing and interpreting results from the food rechallenge phase, the goals of the third phase are to continue the intake of foods that were well-tolerated and to restrict foods that produced symptoms (ie, trigger foods). As the tolerance to different FODMAPs can change over time, patients can attempt to reintroduce their trigger foods a few months after symptom control if they so desire. 30

Proposed mechanisms of FODMAP ingestion and symptoms in IBS.
Study Results
The results from all studies thus far, including observational case-control studies and randomized, controlled trials, generally support the use of a low-FODMAP diet for patients with IBS, as 50% to 80% of patients report some benefits compared to using a regular or habitual diet ( Table 1 ). 3 , 13 , 26 , 31 , 32 Two studies were conducted comparing the low-FODMAP diet to commonly recommended IBS diets (National Institute for Health and Care Excellence [NICE] or modified NICE guidelines). 33 , 34 NICE, modified NICE, and low-FODMAP diets were reported to be effective 33 , 34 ; however, one study showed significantly better results in the low-FODMAP diet group, particularly with regard to pain and bloating. 34 A randomized study compared the low-FODMAP diet to a moderate-FODMAP Australian diet, finding better outcomes for IBS patients who followed the low-FODMAP diet. 35
Studies Comparing the Low-FODMAP Diet to Other Dietary Interventions for IBS Patients
| Study | Comparison | Summary and/or Comments |
|---|---|---|
| Staudacher et al | Low-FODMAP diet vs NICE guidelines diet | =.038). |
| Halmos et al | Low-FODMAP diet vs modified-FODMAP diet | The low-FODMAP diet improved overall symptom scores as well as flatulence, abdominal pain, and bloating. |
| Bohn et al | Low-FODMAP diet vs traditional IBS diet | Similar results for both diets; no significant statistical difference was found |
| Eswaran et al | Low-FODMAP diet vs modified NICE guidelines diet | |
| Hustoft et al | Low-FODMAP diet vs high-FODMAP diet | Three weeks of a low-FODMAP diet improved IBS symptoms, decreased serum levels of proinflammatory IL-6 and IL-8, and decreased levels of fecal bacteria and total SCFAs. |
| McIntosh et al | Low-FODMAP diet vs high-FODMAP diet | |
| Staudacher et al | species | |
| Zahedi et al | Low-FODMAP diet vs standard dietary advice for diarrhea-predominant IBS | Although both groups had improvement in symptoms, the low-FODMAP diet group had better results for symptom scores, abdominal pain, bloating, stool frequency, and consistency. |
FODMAP, fermentable oligosaccharide, disaccharide, monosaccharide, and polyol; IBS, irritable bowel syndrome; IL, interleukin; NICE, National Institute for Health and Care Excellence; SCFA, short-chain fatty acid.
The low-FODMAP diet has also been compared to nondietary interventions, including hypnotherapy and yoga. Gut-directed hypnotherapy has been shown to be comparably effective to a low-FODMAP diet approach; however, the combination of both interventions did not add any significant therapeutic benefits. 36 , 37 Hatha yoga also appears to be beneficial to IBS patients and had a positive impact on patients’ symptoms, with similar results to a low-FODMAP diet. 38 , 39
Advantages of a Low–Fermentable Oligosaccharide, Disaccharide, Monosaccharide, and Polyol Diet
The main advantage of the low-FODMAP diet is in the positive results of several studies performed throughout the world, as a medication or intervention rarely elicits positive symptom control in over half of treated patients. The interest and benefits appear to extend beyond Western societies and English-speaking countries, with recent publications analyzing local diets and exploring the applicability of a low-FODMAP diet in South, East, and Southeast Asia. 40 , 41 A well-conducted study from Colombia of 50 adult IBS patients showed improvement in both symptoms and quality of life. 42 The results observed thus far in different trials show a consistent pattern and rate of response in patients with IBS treated with a low-FODMAP diet. However, a comprehensive meta-analysis by Dionne and colleagues analyzed 7 different low-FODMAP studies in IBS patients and concluded that the overall quality of the data was very low (using Grading of Recommendations Assessment, Development, and Evaluation criteria). 32
Data on the long-term use of a low-FODMAP diet are limited. In a retrospective study by Maagaard and colleagues, some clinical benefit was observed in 57% to 74% of patients at 14 to 16 months follow-up. 43
Challenges of a Low–Fermentable Oligosaccharide, Disaccharide, Monosaccharide, and Polyol Diet
Well-known disadvantages of the low-FODMAP diet are its complexity (difficult to teach, difficult to follow, and labor-intensive), expense, and potential nutritional deficiencies. 26 , 44 The first dietary management counseling appointment is estimated to last approximately 1 hour, 44 which is extremely difficult for a busy primary care provider to perform in the current medical climate. This time commitment leads to some reluctance from physicians in recommending this diet to patients. Incomplete education may then lead to partial or complete noncompliance in clinical practice. In addition, patients need to devote time to planning and shopping for a low-FODMAP diet, which can also reduce compliance.
Any elimination diet generates concern about potential nutritional deficiencies, inappropriate calorie intake resulting in weight loss, and deleterious consequences in body composition. 25 Farida and colleagues studied the micronutrient intake during the first phase of the low-FODMAP diet, and found a higher daily intake of vita-min B6, but a significantly lower daily intake of calcium, retinol, riboflavin, thiamin, and transfatty acids. 45
A pilot study analyzed 26 patients with IBS before and after an 8 week–long low-FODMAP diet with regard to nutritional status and body composition, which were evaluated using bioelectrical impedance vector analysis, anthropometric data, and laboratorial serum studies. 46 Although statistically significant changes were observed in serum albumin and lipids after the introduction of a low-FODMAP diet, the differences were very small and the laboratory results remained within normal range. Overall, this study did not show detrimental effects on body composition or nutritional status in a small group of IBS patients treated with a low-FODMAP diet. 46
Teaching a Low–Fermentable Oligosaccharide, Disaccharide, Monosaccharide, and Polyol Diet to Patients
In all of the studies published to date, including both prospective and retrospective studies, registered dietitians provided diet education to patients. 47 , 48 Unfortunately, not all patients have access to dietitians, and most insurance companies will not pay for a nutrition consult to discuss the implementation of a low-FODMAP diet. Therefore, alternative methods of teaching IBS patients how to follow a low-FODMAP diet are required. Nurse-led dietary counseling has been attempted in 2 different studies, with neither producing conclusive or promising results. 49 , 50 Another study focused on a dietitian-led, group education intervention, comparing it to one-onone education, with promising results: the group-led program was clinically effective and reasonable with regard to costs. 51 Kinrade and colleagues also found that 82% of patients had symptom improvement after receiving low-FODMAP education via group sessions. 52 However, the challenge with group-led discussions for the low-FODMAP diet remains the lack of insurance coverage.
Given the paucity of proven similarly efficacious teaching methods, current guidelines still recommend that low-FODMAP dietary guidance can only be given by a health care professional with expertise in dietary management. 53 , 54 No studies have compared dietitian-led interventions to other methods of dietary management, nor have any studies evaluated an educational program delivered by a dietitian in a one-on-one session vs a group session.
The Gluten-Free Diet
The elimination of gluten from the diet of IBS patients has demonstrated efficacy beyond patients with celiac disease. 13 , 55 - 57 A number of studies published within the last 6 years have investigated the role of gluten in patients with IBS. Biesiekierski and colleagues 55 enrolled 34 patients meeting Rome III criteria for IBS into a double-blind, placebo-controlled, rechallenge dietary study. Patients had noted previous improvement in IBS symptoms with a gluten-free diet, and were randomized to receive a high-gluten diet (16 g/day) or a gluten-free diet during a 6-week period. For the high-gluten group, carbohydrate-depleted wheat gluten was added to the same gluten-free base mix used for the gluten-free group. The majority of patients exposed to gluten (68%) reported uncontrolled symptoms compared with patients exposed to placebo (40%; P <.001). No differences between rates of celiac serology, fecal lactoferrin, or C-reactive protein levels, or measures of intestinal permeability were found between the groups.
Vazquez-Roque and colleagues 57 performed a 4-week, prospective, randomized, controlled trial evaluating 45 patients with diarrhea-predominant IBS who did not have celiac disease, and found a reduction in patient-reported stool frequency ( P =.04) with a gluten-free diet, with the most pronounced effect in those patients who were HLA-DQ2– or HLA-DQ8–positive ( P =.019). Biesiekierski and colleagues followed up their 2011 study 55 with a double-blind, crossover study of 37 IBS patients of all subtypes without celiac disease who had previously reported improvement with a gluten-free diet for at least 6 weeks before study enrollment. 56 Patients were prescribed a low-FODMAP diet, and, following a 2-week run-in, were randomized to a high-gluten diet (16 g/day), a low-gluten diet (2 g/day), or placebo (no gluten). After 1 week, patients were randomized to the second arm, and then the third arm. The 2-week low-FODMAP run-in delivered an improvement in gastrointestinal symptoms ( P <.001), whereas during the 1-week diet study period, symptoms worsened in all patients ( P <.001) irrespective of their diet. These findings highlight the likely role that factors other than gluten play in patients using a gluten-free diet for IBS symptoms.
Elli and colleagues 58 and Zanwar and colleagues 59 conducted double-blind, placebo-controlled, gluten rechallenge trials in patients with IBS and negative celiac testing for 3 and 4 weeks, respectively. The first study 58 implemented a 7-day crossover using gluten capsules, with 18 of 53 patients (34%) developing worse symptoms with gluten exposure. However, a substantial number of patients (14/48; 29.2%) also noted symptoms in the placebo challenge. The second study 59 showed an increase in gastrointestinal symptoms with a wheat bread challenge compared to gluten-free bread (55.7% vs 33.3%; P <.05). It has been suggested that the presence of additional components in both the gluten capsules and the wheat bread could be responsible for at least part of the effect. 60
Challenges of a Gluten-Free Diet
The main limitations of the current literature on gluten-free diets for IBS lie in small study sample sizes and concern for contamination of the vehicle of gluten exposure. To this point, a large meta-analysis reviewing 1726 studies evaluating the efficacy of a gluten-free diet on the management of IBS recently found insufficient evidence to recommend this diet for IBS symptoms, as findings were not statistically significant. 32
Isolation of gluten from the diet without also removing other potential symptom-driving substances is both difficult to study and nebulous for IBS patients. It is possible that many IBS patients improve on a gluten-free diet, as it also reduces fructan intake, a significant component of modern wheat products. 61 Skodje and colleagues performed a double-blind, placebo-controlled, crossover challenge to discover the effect of gluten (without fructan) and the effect of fructan (without gluten) in patients with self-reported gluten sensitivity. 61 Their results weaken the role of gluten and strengthen the symptom-inducing effect of fructans in patients with self-reported sensitivity to rye, wheat, and barley. 61
Additional Diets for the Management of Irritable Bowel Syndrome
Given that 70% to 89% of patients with IBS report exacerbation of symptoms with specific foods, 12 , 26 , 62 it is not surprising that patients would attempt to reduce or eliminate symptom-producing foods from their diets. One of the limitations of exclusion diets is that, thus far, clinicians have been unable to identify (and, therefore, unable to develop validated diagnostic testing for) the specific mechanisms by which individual foods cause gastrointestinal symptoms. If such tests existed, a more efficient approach would be possible: clinicians would be able to immediately recommend the elimination of individual foods rather than going through the process of elimination/restriction followed by reintroduction/personalization. There are insufficient data to recommend panel allergy testing with immunoglobulin (Ig) G for patients who meet the criteria for IBS, although this testing is a common request from patients in clinical practice. Furthermore, panel blood tests can cost up to $1000. 63 Based on current guidelines, food-specific serum IgG4 indicates only repeated exposure to food components, and does not represent allergy, intolerance, or hypersensitivity. 64 , 65
Prior to the growing popularity of the low-FODMAP diet, a traditional IBS diet had been routinely recommended in clinical practice in the United Kingdom, and dietary guidelines were published and updated by NICE. 28 Contrary to the low-FODMAP diet, the traditional IBS diet focuses on the number of meals, and when, how, and how much to eat, rather than the content of the diet itself. 33 General recommendations for the traditional IBS diet are summarized in Table 2 . Although the NICE or modified NICE recommendations have not been compared to placebo, there are 3 trials comparing them to the low-FODMAP diet, all showing similar positive results for the studied diets. 33 , 34 , 53
General Recommendations for the Traditional IBS Diet 28 , 80
| Encouraged Habits and Behaviors | Limited Intake | Other Considerations |
|---|---|---|
| Eating regular meals and limiting the volume/amount of food per meal | The intake of fat, spicy foods, and sorbitol (an artificial sweetener found in diabetic/ slimming/sugar-free drinks and sweets) should be minimized in patients with diarrhea-predominant IBS or mixed IBS. | If increasing the fiber intake is advised, the option should be for soluble instead of insoluble fiber, as insoluble fiber is discouraged for patients with IBS. |
| Eating slowly and chewing thoroughly | Daily fruit intake should be limited to 3 portions. | Although adding probiotics is safe in patients with IBS, they do not seem to provide benefit regarding symptom control and, therefore, are not recommended. |
| No long gaps between meals or skipping meals | The intake of coffee, tea, alcohol, and carbonated beverages should be reduced. |
IBS, irritable bowel syndrome.
A study by Lenhart and colleagues found that the majority of gastroenterologists practicing in the United States recommend dietary changes to more than 75% of their IBS patients, but very few (21%) refer them to a dietitian. 66 The diets most commonly recommended by gastroenterologists for IBS patients are low-FODMAP, lactose-free, high-fiber, gluten-free, and low-fat diets.
Besides the previously discussed low-FODMAP and gluten-free diets, over half of IBS patients decide to self-manage and follow different dietary interventions to improve their symptoms prior to seeking advice from a gastrointestinal physician. 66 The Paleolithic diet, very low–carbohydrate diet (or ketogenic diet), and IgG-based avoidance diet are commonly recommended in clinical practice and on blogs and different social media channels. However, there is a lack of substantial evidence for the majority of these specialized diets, as summarized in Table 3 .
Evidence Behind Other Diets Used to Treat IBS 13
| Type of Diet | Description of Diet | Supporting Evidence |
|---|---|---|
| Lactose-free diet | No lactose-containing products, except if products are treated with lactase (ie, lactose-free cow’s milk, lactose-free yogurt) | Only helpful for patients with lactose intolerance; not efficacious for patients with IBS without lactose intolerance , |
| Low-fructose/fructan diet | Avoid foods high in fructose and fructans. | |
| Paleolithic diet | Only foods available during the Paleolithic era: seafood, lean meat, fruits, vegetables, nuts, and seeds; no processed food, dairy, added salt, added sugar, grains, legumes, or alcohol | No studies available |
| Specific carbohydrate diet | Reduce ingestion of disaccharides and polysaccharides. | Trend toward improvement in symptoms, but not statistically significant; inferior results compared to the low-FODMAP diet |
| IgG-based avoidance diet | Exclude foods to which patients have increased serum IgG antibodies. | |
| Very low–carbohydrate diet or ketogenic diet | ||
| Fiber supplementation | Psyllium supplement | |
| Low-fat diet | Less than 27 g of fat per day, considering a diet of 2000 kcal/day | |
| Low-fiber diet | Less than 10-15 g of fiber/day | No evidence available; common practice is to recommend decreasing fiber for patients with diarrhea-predominant IBS to increase transit time |
| Low-histamine diet | and atopic dermatitis, and one study for pediatric patients with chronic digestive complaints. No evidence to support this diet for IBS currently |
FODMAP, fermentable oligosaccharide, disaccharide, monosaccharide, and polyol; IBS, irritable bowel syndrome; Ig, immunoglobulin; RCT, randomized, controlled trial.
Patients as Consumers
The notion from Hippocrates of food as medicine seems to be regaining popularity, especially among millennials, who are interested in a holistic approach both for disease prevention and for treatment, and prefer nonpharmaco-logic interventions, if available. 31 Driven by consumers’ desire for foods that optimize health and/or prevent chronic illnesses, the market for functional foods has been one of the fastest-growing existing food sectors over the last decade. 31 , 67 The general trend of consumer commitment to gluten-free diets has sparked a dramatic growth in this market, increasing 136% from 2013 to 2015, and, thus, creating an $11.6 billion annual industry as of 2015. 68
As previously mentioned, the complexity of the low-FODMAP diet and its different phases is a limiting factor for its use and compliance. In an effort to assist patients, Dr Gibson’s group at Monash University created a certification program to assist consumers to easily identify low-FODMAP foods. 30 The online application is available for smartphones, and is designed to help patients choose the dishes and ingredients that are appropriate for the different phases of their diet. 30
Considerations Regarding Dietary Interventions in Irritable Bowel Syndrome Patients
The concern about orthorexia, or orthorexia nervosa, is another important factor when recommending an elimination or restrictive diet. The term orthorexia was first introduced in the literature in 1998 to describe an obsession with healthy eating, 69 , 70 and, although not formally recognized as a disease by the most recent Diagnostic and Statistical Manual of Mental Disorders , the number of publications and social media references to the term is on the rise. 71 Signs and symptoms described in the literature include compulsive checking of nutritional labels; avoidance of a high number of food groups; inability to eat anything not deemed healthy, clean, or pure; high levels of stress in relation to eating; and unhealthy time investment in planning or worrying about future meals. 72 , 73
There are no studies currently linking IBS and orthorexia, nor orthorexia and a low-FODMAP diet or a gluten-free diet. However, orthorexia may innocently start as a simple desire to improve one’s eating habits or health, 74 such as with a recommended IBS diet, and then slowly evolve into toxic and anxiety-generating behaviors.
Another eating disorder that could be of concern when treating IBS patients with dietary modifications is avoidant/restrictive food intake disorder (ARFID). 75 This is characterized by an avoidant and/or restrictive eating behavior that negatively impacts the intake of macro- or micronutrients, potentially causing calorie and/or protein malnutrition. ARFID can be distinguished from anorexia nervosa by the lack of worry about one’s weight. 75 For this reason, when following IBS patients undergoing dietary changes, clinicians should be vigilant to the development of extreme and unnecessary dietary restrictions and avoidant behaviors that are not objectively beneficial to the patients and could cause nutritional deficiencies in the long term.
The role of diets for the treatment of IBS symptoms is complex and remains poorly defined. Investigations into the relationship between diet and IBS symptoms have been limited by small sample sizes, placebo effects, and the lack of specificity of symptoms. More complicated diets are difficult for patients, and patient recall of diet is often poor. Cheap, effective, point-of-care testing for food intolerances are lacking, and cross-contamination of specific dietary IBS triggers is likely prevalent. Initial management of IBS with dietary adjustment involves either single-food elimination for common culprits such as lactose and fructose, or potentially a larger elimination diet (eg, low-FODMAP) with targeted reintroduction after 4 weeks, under the guidance of a registered dietitian. Expensive and unproven commercial food-specific allergy testing should be avoided. Future larger studies likely requiring multicenter designs are needed to further define the efficacy of specific dietary options for IBS patients.
- Current Issue
- Supplements

Gastroenterology & Hepatology
April 2023 - volume 19, issue 4, supplement 2, understanding the current approaches in the management of ibs-c: a case study.
Patient Case
A 36-year-old woman presents with persistent abdominal pain and constipation ( Table 1 ) . Upon inquiry, she states that she has experienced abdominal and bowel-related symptoms since she was in college. Her abdominal symptoms include intermittent cramps that typically occur in the left lower quadrant, nearly constant bloating that worsens during menstrual periods, and frequent episodes of constipation. She reports that her hard, small stools are associated with a feeling of incomplete emptying. She typically moves her bowels every other day. She denies seeing blood in her stool, fever, or unexplained weight loss, and she is not awakened at night by her symptoms. She exercises most days and has a normal body mass index (BMI). Her previous medical history includes an appendectomy at the age of 16 years. She reports no prior pregnancies (G0P0). Her family history is notable for breast and lung cancer, but no colorectal or gastric cancer.
The patient has tried to self-manage her symptoms through diet. Specifically, she has separately tried eliminating both gluten and dairy from her diet, with no improvement. Approximately 3 years ago, she tried the low-FODMAP (fermentable oligosaccharides, disaccharides, monosaccharides, and polyols) diet at the recommendation of a friend. She reports that the low-FODMAP diet decreased her bloating initially, but over several months the bloating returned. The low-FODMAP diet also worsened her constipation, and she therefore subsequently stopped the diet. Upon inquiry, the patient admits that she limits many social events because of her symptoms and has been sexually inactive for a year. She reports that her symptoms, particularly the pain and bloating, are so debilitating that she calls in sick and skips work about 1 day per month.
She recently started a new job as a clerk at a law firm and expresses concern that she has not accrued enough personal leave to be able to take time off from work. Because she is otherwise healthy, she has not undergone regular physical examinations, and she has no history with this doctor’s office. She is now presenting to a primary care physician (PCP) who was recommended to her, and she is inquiring about whether she should see a gastroenterologist.
On physical examination, the patient exhibits mild abdominal distension, and mild tympany is heard during abdominal percussion. Mild tenderness is noted in the left lower quadrant. A complete blood cell (CBC) count, thyroid-stimulating hormone (TSH) test, and compre hensive metabolic panel (CMP) are ordered, which show no abnormalities. The PCP recommends that the patient continue her low-fiber diet, additionally recommends an over-the-counter polyethylene glycol (PEG) laxative, and refers her to a local gastroenterologist.
The patient immediately makes an appointment but must wait approximately 3 months for her first visit. At the appointment, the gastroenterologist takes a thorough history and performs a rectal examination. This reveals normal relaxation of the pelvic floor, anal sphincter, and puborectalis muscle, and proper contraction of the abdominal wall muscles is noted when the patient is asked to simulate defecation. When hearing that the addition of the PEG laxative has exacerbated the patient’s bloating and that she therefore has discontinued it, the gastroenterologist instead prescribes 8 µg of lubiprostone twice a day and recommends a follow-up appointment in 2 months. The gastroenterologist also recommends that the patient begin a diary of her daily symptoms and diet.
After 2 months, the patient again presents to the gastroenterologist. She reports that she has tolerated the lubiprostone well but that relief of her symptoms has been limited. Review of her symptom diary does not reveal any clear food triggers. The gastroenterologist recommends that she switch to a medication of a different class, and she begins treatment with 50 mg of tenapanor twice a day.
At a follow-up appointment 2 months later, the patient reports less bloating and abdominal pain. She states that the frequency of her bowel movements has increased, and she is now having bowel movements most days. She reports no noticeable side effects from the medication.
Overview of IBS
In 2016, the fourth iteration of the Rome Diagnostic Criteria for Irritable Bowel Syndrome (IBS; Rome IV criteria) was released (Table 2). 1 Developed by expert consensus, the Rome IV criteria incorporated key changes designed to improve their clinical utility and to reflect an increased understanding of IBS pathophysiology. In the Rome IV criteria, IBS is defined as a disorder of brain-gut interactions in which recurrent abdominal pain on average at least 1 day per week is associated with 2 or more of the following: related to defecation; associated with a change in the frequency of stool; and associated with a change in the form (appearance) of stool. Notably, these criteria must have been met for the previous 3 months with an onset of symptoms at least 6 months before the diagnosis.
Abnormal bowel movements are classified with the Bristol Stool Form Scale (BSFS), which ranges from type 1 to type 7. 2 BSFS types 1 and 2 are associated with constipation, whereas types 6 and 7 are associated with diarrhea. A proper identification of the patient’s predominant stool type on days with abnormal stools is important for a correct diagnosis and identification of the subtype of IBS.
The 4 distinct IBS subtypes recognized are the following: IBS with constipation (IBS-C), IBS with diarrhea (IBS-D), IBS with mixed or alternating bowel habits (IBS-M), and IBS without a significant pattern of abnormal stool (IBS-U). 3 Once the pattern of stool type is determined with the BSFS, the Rome IV criteria can be applied to make an appropriate determination of a patient’s subtype. For example, IBS-C is diagnosed when more than 25% of a patient’s bowel movements are BSFS type 1 or 2 and fewer than 25% are type 6 or 7. In contrast, IBS-D is diagnosed when more than 25% of a patient’s bowel movements are BSFS type 6 or 7 and fewer than 25% are BSFS type 1 or 2.
With respect to IBS-C in particular, abdominal pain and constipation are considered to be the hallmark symptoms. A diagnosis of IBS-C is based on a medical history and a physical examination that include an evaluation of gastrointestinal symptoms, especially to identify alarm signs (eg, new symptoms in a patient older than 50 years, unintended weight loss, hematochezia, symptoms that awaken the patient at night, fever, acute or rapidly progressing symptoms, and a family history of colorectal cancer or inflammatory bowel disease). 4
A multifactorial pathophysiology has been proposed for IBS-C, with a wide range of potential mechanisms (Table 3). 5,6 Changes in gut motility are thought to lead to decreased colonic contractions and water imbalances, which result in hard stools and infrequent defecation. 7,8 Intestinal permeability may be altered when widening of the tight junctions between the intestinal epithelial cells results in an inflammatory response close to nerve fibers throughout the gut epithelium. 9,10 Patients with IBS-C may also exhibit visceral hypersensitivity—that is, enhanced sensitization of afferent nerve pathways within the gut. 9,11 And finally, changes in gut microbiota and other triggers of gut inflammation and immune activation have been proposed as potential pathophysiologic mechanisms. 9,10
IBS-C puts a significant burden on patients, as was exemplified by the IBS in America survey of 1667 individuals who met the Rome III criteria for IBS-C. 12 The objective of the survey was to explore the attitudes of patients with IBS-C and better understand their experiences living with IBS-C. More than half of the survey participants reported that their symptoms were very or extremely bothersome. When they were asked, “What would you be willing to give up for 1 month of IBS-C symptom relief?” their responses included the Internet (21%), their cell phone (25%), sex (42%), caffeine (58%), and alcohol (62%). In this same survey, individuals with IBS-C were more likely to report feelings of self-consciousness, avoidance of sex, difficulty concentrating, and feeling unable to reach their full potential.
Studies have also demonstrated the negative effect of IBS-C on measures of health-related quality of life (QoL). When a group of patients who had IBS-C was compared with a matched group of patients who did not have IBS-C, the individuals with IBS-C reported significantly poorer health-related QoL (Figure 1). 13 The physical component summary and mental component summary scores were lower, and overall work and activity impairment was greater, in the patients with IBS-C than in the matched comparison group. Other studies have also reported high levels of absenteeism and presenteeism among individuals with IBS. 14-16
These studies reflect the significant unmet need of patients with IBS-C, a large proportion of whom reportedly do not respond adequately to treatment. The results of an online questionnaire of more than 1300 people with IBS-C, reported in 2018, found that despite treatment with a prescription IBS-C medication, 77% continued to experience residual abdominal and stool-related symptoms. 17 Abdominal bloating/distension was the most frequent of the symptoms. The heterogeneous nature of IBS-C, and the large proportion of patients who continue to suffer despite treatment, point to the need for innovative agents with novel mechanisms of action.
Evidence for the Pharmacologic Management of IBS-C
In the United States, 2 organizations have provided evidence-based guidelines for the management of IBS: the American College of Gastroenterology (ACG) and the American Gastroenterological Association (AGA). The ACG guidelines, which include topics relevant to the diagnosis and management of both IBS-C and IBS-D, suggest “a positive diagnostic strategy as compared to a diagnostic strategy of exclusion be used to improve time to initiating appropriate therapy.” 3 This strategy involves a careful history and physical examination and the use of a standard definition to make a diagnosis, with a limited number of diagnostic tests. The AGA guidelines focus specifically on the pharmacologic management of IBS-C, and a separate guideline focuses on the pharmacologic management of IBS-D. 18,19
The management of IBS-C encompasses a wide range of interventions, including behavioral modifications, nonpharmacologic approaches, and pharmacologic agents. Behavioral modifications include regular exercise, adequate hydration, and sufficient sleep. 4,20,21 Dietary recommendations include avoiding foods known to trigger gastrointestinal symptoms (eg, sodas, fatty or fried foods, spicy foods, and foods containing artificial sugars). Diets should include regular servings of fruits and vegetables. Ensuring that an adequate amount of fiber is included in the diet is also important; when possible, commercially available fibers such as psyllium, which is primarily a soluble fiber, are recommended. Of note, insoluble fibers such as bran fiber may worsen symptoms and are generally not recommended for the management of IBS-C. 22,23 Changing toileting behavior so that the patient is in more of a squatting position (eg, raising the knees above the hips) and limiting time on the toilet to 10 to 15 minutes are recommended. 4,23 The AGA guideline recommends over-the-counter osmotic (eg, PEG) laxatives and fiber (eg, psyllium) as first-line treatments for IBS-C. For patients whose predominant symptom is pain, neuromodulators such as low-dose tricyclic antidepressants (TCAs) should be considered. Selective serotonin reuptake inhibitors (SSRIs) are generally not recommended, given the limited number of studies showing efficacy. 19
During the past several years, the US Food and Drug Administration (FDA) has approved several agents for the treatment of IBS-C. These relieve both abdominal pain and constipation. Currently, 5 medications are approved by the FDA for the treatment of IBS-C (Table 4). 24
Sodium/Hydrogen Exchange Transporter Inhibitor
Tenapanor is an inhibitor of the sodium/hydrogen exchange transporter isoform 3 (NHE3), which is expressed on the apical surface of the small intestine and colon and is primarily responsible for the absorption of dietary sodium. 25-27 NHE3 inhibition acts via 3 mechanisms. First, tenapanor decreases the absorption of dietary sodium, so that luminal water content is retained, intestinal transit time is accelerated, and stool is softened. Second, it has been shown in animal models that tenapanor decreases intestinal permeability by narrowing the tight junctions between intestinal epithelial cells. 9,10 Third, it has also been shown in animal models that tenapanor reduces visceral hypersensitivity, a common finding in patients with IBS-C. 9,28 It is important to note that the relevance to humans of the effects seen in animal models is not known. Importantly, tenapanor is locally acting, with minimal systemic absorption.
The efficacy and safety of tenapanor for the treatment of IBS-C were established in 2 placebo-controlled, randomized phase 3 trials, T3MPO-1 and T3MPO-2. 29,30 Patients with IBS-C were randomized to receive tenapanor (50 mg twice daily) or placebo for 12 weeks, followed by a 4-week randomized withdrawal period in T3MPO-1 (606 adults) and 26 weeks in T3MPO-2 (593 adults). Enrollment was restricted to patients with IBS-C who met the Rome III criteria (which were current at the time of study design) and who, at baseline, reported an average weekly stool frequency of 5 or fewer spontaneous bowel movements (SBMs) and 3 or fewer complete spontaneous bowel movements (CSBMs). Other eligibility criteria included a self-reported average weekly stool consistency of BSFS types 1 through 3, an average weekly abdominal pain score of 3 or higher (on a scale of 0 to 10, with 0 indicating no pain and 10 the worst imaginable pain), no liquid stools, and no mushy stools for more than 1 SBM. The primary endpoint was an overall response for 6 or more of the first 12 weeks of treatment; an overall response was defined as a decrease of 30% or more in average weekly worst abdominal pain score and an increase of at least one CSBM from baseline, both in the same week.
In T3MPO-1, a significantly higher percentage of the patients treated with tenapanor than of those who received placebo met the primary endpoint (27.0% vs 18.7%; P =0.020). 29 The percentages of patients with an abdominal pain response and with a CSBM response were also higher in the tenapanor arm than in the placebo arm (44.0% vs 33.1%; P =0.008 and 33.9% vs 29.4%; P =0.270, respectively). The patients treated with tenapanor experienced significantly greater improvements in abdominal symptoms (including abdominal discomfort, bloating, cramping, and fullness) and global IBS treatment measures (including stool consistency and IBS severity) in comparison with the patients treated with placebo.
The results of T3MPO-2 were similar, including those for the primary endpoint of overall response in 6 or more of the first 12 weeks of treatment (36.5% with tenapanor vs 23.7% with placebo; P <0.001) (Figure 2). 30 Considered separately, the abdominal pain responses were 49.8% vs 38.3% ( P =0.004), and the CSBM responses were 47.4% vs 33.3% ( P <0.001). Reductions in abdominal pain were reported with tenapanor as early as 1 week after the start of treatment, and the tenapanor-treated patients experienced a 54% decrease in abdominal pain from baseline to week 26. Reports of severe abdominal pain showed a 78% reduction from baseline (55%) to week 26 (12%).
In T3MPO-2, a durable response required patients to meet the response criteria for at least 3 of the final 4 weeks of the first 12 weeks of the treatment period. Durable response rates were significantly higher with tenapanor than with placebo; the durable abdominal pain responses were 34.8% vs 26.7% ( P =0.028), the durable CSBM responses were 21.2% vs 5.7% ( P <0.001), and the durable combined responses were 18.1% vs 5.0% ( P <0.001). Tenapanor was associated with significant improvements in the mean change from baseline in the average weekly number of CSBMs over time, as well as in the average weekly abdominal pain score over time. On average, over the 26-week treatment period, the patients treated with tenapanor had 3.3 CSBMs per week, a frequency that falls within the healthy range for adults. Tenapanor also reduced abdominal symptoms (including bloating, fullness, discomfort, and cramping) as early as 1 week after the start of treatment. Tenapanor was associated with a 41% improvement in the QoL score from baseline to week 26 and with a 3-fold increase in the number of patients reporting the highest QoL scores at the end of treatment.
Diarrhea was more frequently reported with tenapanor than with placebo (14.6% vs 1.7% in T3MPO-1 and 16.0% vs 3.7% in T3MPO-2). 29,30 The onset of diarrhea was usually within 1 week of the start of treatment and was typically transient and mild to moderate in severity. Other adverse events more frequently reported with tenapanor than with placebo included nausea, abdominal distension, and flatulence.
Guanylate Cyclase-C Agonists
The FDA has approved 2 guanylate cyclase-C (GC-C) agonists, linaclotide and plecanatide, for the treatment of IBS-C. Linaclotide and plecanatide are both peptides that act as selective agonists at the GC-C receptor on the luminal surface of intestinal enterocytes. 31,32 The endogenous ligands for the GC-C receptor promote intestinal secretion in response to a meal, and binding of these peptides results in increased levels of cyclic guanosine monophosphate (cGMP), a second messenger that plays a critical role in the regulation and secretion of intestinal fluid into the intestinal lumen.
Each of the 2 GC-C agonists has been demonstrated to have efficacy in randomized, controlled phase 3 trials. For example, linaclotide showed better efficacy than placebo (33.7% vs 13.9%; P <0.0001) in a phase 3 trial in which the primary endpoint was a reduction of 30% or more in worst abdominal pain plus an increase of at least one CSBM weekly, both for 6 or more of 12 treatment weeks. 33 Plecanatide also showed efficacy in comparison with placebo for the same primary endpoint in 2 phase 3 trials (study 1: 30.2% vs 17.8%, P <0.001; study 2: 21.5% vs 14.2%, P =0.009). 34 Across these phase 3 trials, diarrhea was the most frequently reported adverse event with the 2 peptides (linaclotide and plecanatide).
Chloride Channel Type II Agonist
The prostaglandin E1 derivative lubiprostone activates the intestinal chloride channel type 2 on the apical surface of small intestinal enterocytes, resulting in chloride efflux into the luminal cavity. 35 This process triggers fluid secretion into the luminal cavity, which softens stool and accelerates intestinal transit. In 2 phase 3 trials, the percentage of overall responders was significantly higher in the patients treated with lubiprostone (8 µg twice daily) than in those who received placebo (17.9% vs 10.1%; P =0.001). 36 In these trials, an overall responder was defined as a monthly responder for 2 or more of 3 treatment months; a monthly responder was defined as a patient who experienced at least moderate relief for 4 of 4 weeks or significant relief for 2 of 4 weeks. Nausea (7%) was the most frequently reported adverse event with lubiprostone.
Serotonin (5-HT 4 ) Receptor Agonist
The serotonin (5-HT 4 ) receptor agonist tegaserod is FDA-approved with an indication for the treatment of IBS-C in women younger than 65 years. However, the manufacturer withdrew tegaserod from the market in 2022; the withdrawal was reportedly based on a business decision and did not reflect the efficacy or safety of this agent. 37
IBS-C is a common disorder with negative effects on health-related QoL. Currently, 5 agents are approved by the FDA for the treatment of IBS-C, one of which has been withdrawn from the market. Unfortunately, no head-to-head trials have been performed. However, a systematic review and network meta-analysis conducted in 2018 to examine the relative safety and efficacy of FDA-approved agents for the treatment of IBS-C confirmed that each of them was significantly more effective than placebo for decreasing global symptoms. 38 Patients with an inadequate response to fiber and osmotic laxatives (eg, PEG) may benefit from one of the FDA-approved agents that have been shown to relieve both abdominal pain and constipation.
Disclosures
Dr Lembo has performed consulting for Aeon, Ardelyx, Cara Care, Gemelli, Gimoti, Ironwood Pharmaceuticals, Neurogastrx, OrphoMed, Takeda, and Vibrant Pharma; and has stock in Allurion, Bristol Myers Squibb, Johnson & Johnson.
1. Lacy BE, Patel NK. Rome criteria and a diagnostic approach to irritable bowel syndrome. J Clin Med . 2017;6(11):99.
2. Lewis SJ, Heaton KW. Stool form scale as a useful guide to intestinal transit time. Scand J Gastroenterol . 1997;32(9):920-924.
3. Lacy BE, Pimentel M, Brenner DM, et al. ACG Clinical Guideline: management of irritable bowel syndrome. Am J Gastroenterol . 2021;116(1):17-44.
4. Mearin F, Lacy BE, Chang L, et al. Bowel disorders. Gastroenterology . 2016:S0016-5085(16)00222-5.
5. Saha L. Irritable bowel syndrome: pathogenesis, diagnosis, treatment, and evidence-based medicine. World J Gastroenterol . 2014;20(22):6759-6773.
6. Spiller R, Major G. IBS and IBD – separate entities or on a spectrum? Nat Rev Gastroenterol Hepatol . 2016;13(10):613-621.
7. Camilleri M. Peripheral mechanisms in irritable bowel syndrome. N Engl J Med . 2012;367(17):1626-1635.
8. Camilleri M. Management of the irritable bowel syndrome. Gastroenterology . 2001;120(3):652-668.
9. Barbara G, Barbaro MR, Fuschi D, et al. Inflammatory and microbiota-related regulation of the intestinal epithelial barrier. Front Nutr . 2021;8:718356.
10. Camilleri M, Lasch K, Zhou W. Irritable bowel syndrome: methods, mechanisms, and pathophysiology. The confluence of increased permeability, inflammation, and pain in irritable bowel syndrome. Am J Physiol Gastrointest Liver Physiol . 2012;303(7):G775-G785.
11. Farzaei MH, Bahramsoltani R, Abdollahi M, Rahimi R. The role of visceral hypersensitivity in irritable bowel syndrome: pharmacological targets and novel treatments. J Neurogastroenterol Motil . 2016 30;22(4):558-574.
12. Ballou S, McMahon C, Lee HN, et al. Effects of irritable bowel syndrome on daily activities vary among subtypes based on results from the IBS in America survey. Clin Gastroenterol Hepatol . 2019;17(12):2471-2478.e3.
13. DiBonaventura M, Sun SX, Bolge SC, Wagner JS, Mody R. Health-related quality of life, work productivity and health care resource use associated with constipation predominant irritable bowel syndrome. Curr Med Res Opin . 2011;27(11):2213-2222.
14. Drossman DA, Li Z, Andruzzi E, et al. U.S. householder survey of functional gastrointestinal disorders. Prevalence, sociodemography, and health impact. Dig Dis Sci . 1993;38(9):1569-1580.
15. Frändemark Å, Törnblom H, Jakobsson S, Simrén M. Work productivity and activity impairment in irritable bowel syndrome (IBS): a multifaceted problem. Am J Gastroenterol . 2018;113(10):1540-1549.
16. Paré P, Gray J, Lam S, et al. Health-related quality of life, work productivity, and health care resource utilization of subjects with irritable bowel syndrome: baseline results from LOGIC (Longitudinal Outcomes Study of Gastrointestinal Symptoms in Canada), a naturalistic study. Clin Ther . 2006;28(10):1726-1735.
17. Quigley EMM, Horn J, Kissous-Hunt M, Crozier RA, Harris LA. Better Understanding and Recognition of the Disconnects, Experiences, and Needs of Patients with Irritable Bowel Syndrome with Constipation (BURDEN IBS-C) Study: Results of an Online Questionnaire. Adv Ther . 2018;35(7):967-980.
18. Chang L, Sultan S, Lembo A, Verne GN, Smalley W, Heidelbaugh JJ. AGA Clinical Practice Guideline on the Pharmacological Management of Irritable Bowel Syndrome With Constipation. Gastroenterology . 2022;163(1):118-136.
19. Lembo A, Sultan S, Chang L, Heidelbaugh JJ, Smalley W, Verne GN. AGA Clinical Practice Guideline on the Pharmacological Management of Irritable Bowel Syndrome With Diarrhea. Gastroenterology . 2022;163(1):137-151.
20. Patel A, Hasak S, Cassell B, et al. Effects of disturbed sleep on gastrointestinal and somatic pain symptoms in irritable bowel syndrome. Aliment Pharmacol Ther . 2016;44(3):246-258.
21. Anti M, Pignataro G, Armuzzi A, et al. Water supplementation enhances the effect of high-fiber diet on stool frequency and laxative consumption in adult patients with functional constipation. Hepatogastroenterology . 1998;45(21):727-732.
22. Ford AC, Moayyedi P, Chey WD, et al; ACG Task Force on Management of Irritable Bowel Syndrome. American College of Gastroenterology monograph on management of irritable bowel syndrome. Am J Gastroenterol . 2018;113(suppl 2):1-18.
23. Chey WD, Kurlander J, Eswaran S. Irritable bowel syndrome: a clinical review. JAMA . 2015;313(9):949-958.
24. Drugs@FDA: FDA-Approved Drugs. Accessed April 4, 2023.
25. Eutamene H, Charmot D, Navre M, et al. Visceral antinociceptive effects of RDX5791, a first-in-class minimally systemic NHE3 inhibitor on stress-induced colorectal hypersensitivity to distension in rats. Gastroenterology . 2011;140:S-57-S-58.
26. Spencer AG, Labonte ED, Rosenbaum DP, et al. Intestinal inhibition of the Na+/H+ exchanger 3 prevents cardiorenal damage in rats and inhibits Na+ uptake in humans. Sci Transl Med . 2014;6(227):227ra36.
27. Sinagra E, Rossi F, Raimondo D, et al. Tenapanor for the treatment of irritable bowel syndrome with constipation. Expert Rev Clin Pharmacol . 2020;13(5):473-479.
28. Li Q, King A, Liu L, et al. Tenapanor reduces IBS pain through inhibition of TRPV1-dependent neuronal hyperexcitability in vivo. Poster presented at: the World Congress of Gastroenterology at The American College of Gastroenterology Annual Scientific Meeting; October 13–18, 2017; Orlando, FL. P2027.
29. Chey WD, Lembo AJ, Rosenbaum DP. Efficacy of tenapanor in treating patients with irritable bowel syndrome with constipation: a 12-week, placebo-controlled phase 3 trial (T3MPO-1). Am J Gastroenterol . 2020;115(2):281-293.
30. Chey WD, Lembo AJ, Yang Y, Rosenbaum DP. Efficacy of tenapanor in treating patients with irritable bowel syndrome with constipation: a 26-week, placebo-controlled phase 3 trial (T3MPO-2). Am J Gastroenterol . 2021;116(6):1294-1303.
31. Thomas RH, Allmond K. Linaclotide (Linzess) for irritable bowel syndrome with constipation and for chronic idiopathic constipation. P&T . 2013;38(3):154-160.
32. Kamuda JA, Mazzola N. Plecanatide (Trulance) for chronic idiopathic constipation and irritable bowel syndrome with constipation. P&T . 2018;43(4):207-232.
33. Chey WD, Lembo AJ, Lavins BJ, et al. Linaclotide for irritable bowel syndrome with constipation: a 26-week, randomized, double-blind, placebo-controlled trial to evaluate efficacy and safety. Am J Gastroenterol . 2012;107(11):1702-1712.
34. Brenner DM, Fogel R, Dorn SD, et al. Efficacy, safety, and tolerability of plecanatide in patients with irritable bowel syndrome with constipation: results of two phase 3 randomized clinical trials. Am J Gastroenterol . 2018;113(5):735-745.
35. Lacy BE, Levy LC. Lubiprostone: a novel treatment for chronic constipation. Clin Interv Aging . 2008;3(2):357-364.
36. Drossman DA, Chey WD, Johanson JF, et al. Clinical trial: lubiprostone in patients with constipation-associated irritable bowel syndrome—results of two randomized, placebo-controlled studies. Aliment Pharmacol Ther . 2009;29(3):329-341.
37. Alfasigma USA, Inc. Press release: ZELNORM® (tegaserod) Notice of Withdrawal from Market. Accessed March 29, 2023. http://www.myzelnorm.com/assets/pdfs/Press%20Release%20on%20Notice%20of%20Withdrawal.pdf.
38. Black CJ, Burr NE, Quigley EMM, Moayyedi P, Houghton LA, Ford AC. Efficacy of secretagogues in patients with irritable bowel syndrome with constipation: systematic review and network meta-analysis. Gastroenterology . 2018;155(6):1753-1763.
Information
- Author Services
Initiatives
You are accessing a machine-readable page. In order to be human-readable, please install an RSS reader.
All articles published by MDPI are made immediately available worldwide under an open access license. No special permission is required to reuse all or part of the article published by MDPI, including figures and tables. For articles published under an open access Creative Common CC BY license, any part of the article may be reused without permission provided that the original article is clearly cited. For more information, please refer to https://www.mdpi.com/openaccess .
Feature papers represent the most advanced research with significant potential for high impact in the field. A Feature Paper should be a substantial original Article that involves several techniques or approaches, provides an outlook for future research directions and describes possible research applications.
Feature papers are submitted upon individual invitation or recommendation by the scientific editors and must receive positive feedback from the reviewers.
Editor’s Choice articles are based on recommendations by the scientific editors of MDPI journals from around the world. Editors select a small number of articles recently published in the journal that they believe will be particularly interesting to readers, or important in the respective research area. The aim is to provide a snapshot of some of the most exciting work published in the various research areas of the journal.
Original Submission Date Received: .
- Active Journals
- Find a Journal
- Proceedings Series
- For Authors
- For Reviewers
- For Editors
- For Librarians
- For Publishers
- For Societies
- For Conference Organizers
- Open Access Policy
- Institutional Open Access Program
- Special Issues Guidelines
- Editorial Process
- Research and Publication Ethics
- Article Processing Charges
- Testimonials
- Preprints.org
- SciProfiles
- Encyclopedia

Article Menu

- Subscribe SciFeed
- Recommended Articles
- Google Scholar
- on Google Scholar
- Table of Contents
Find support for a specific problem in the support section of our website.
Please let us know what you think of our products and services.
Visit our dedicated information section to learn more about MDPI.
JSmol Viewer
Irritable bowel syndrome: current landscape of diagnostic guidelines and therapeutic strategies.

1. Introduction
2. ibs diagnostic approach: american college of gastroenterology (acg) and british society of gastroenterology (bsg) comparison, 2.1. positive diagnostic strategy, 2.2. crp, fecal calprotectin, and fecal lactoferrin, 2.3. celiac disease serology, 2.4. colonoscopy, 2.5. anorectal physiology testing, 3. ibs management, 3.1. dietary modification, 3.1.1. low-fodmap diet, 3.1.2. soluble fibers, 3.2. psychotherapy.
Click here to enlarge figure
3.3. IBS Abdominal Pain and Global Symptom Management
3.3.1. antispasmodics, 3.3.2. peppermint oil, 3.3.3. probiotics, 3.4. ibs-d pharmacologic management, 3.4.1. loperamide, 3.4.2. bile acid sequestrants, 3.4.3. rifaximin, 3.4.4. eluxadoline, 3.4.5. tricyclic antidepressants, 3.4.6. alosetron, 3.5. ibs-c pharmacologic management, 3.5.1. laxatives, 3.5.2. lubiprostone, 3.5.3. linaclotide, 3.5.4. plecanatide, 3.5.5. tenapanor, 3.5.6. tegaserod, 4. recent advancements in ibs pharmacology, 4.1. olorinab, 4.2. dextofisopam, 4.3. mrx1234, 5. conclusions, author contributions, institutional review board statement, informed consent statement, data availability statement, conflicts of interest.
- Black, C.J.; Ford, A.C. Global burden of irritable bowel syndrome: Trends, predictions and risk factors. Nat. Rev. Gastroenterol. Hepatol. 2020 , 17 , 473–486. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Patel, N.; Shackelford, K. Irritable Bowel Syndrome. In StatPearls ; StatPearls Publishing: Treasure Island, FL, USA, 2023. [ Google Scholar ]
- Rome Foundation. Rome IV Diagnostic Criteria for FGIDs . Available online: https://theromefoundation.org/rome-iv/rome-iv-criteria/ (accessed on 20 June 2024).
- Drossman, D.A.; Camilleri, M.; Mayer, E.A.; Whitehead, W.E. AGA technical review on irritable bowel syndrome. Gastroenterology 2002 , 123 , 2108–2131. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Karantanos, T.; Markoutsaki, T.; Gazouli, M.; Anagnou, N.P.; Karamanolis, D.G. Current insights in to the pathophysiology of Irritable Bowel Syndrome. Gut Pathog. 2010 , 2 , 3. [ Google Scholar ] [ CrossRef ]
- Tillisch, K.; Mayer, E.A.; Labus, J.S. Quantitative meta-analysis identifies brain regions activated during rectal distension in irritable bowel syndrome. Gastroenterology 2011 , 140 , 91–100. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Oswiecimska, J.; Szymlak, A.; Roczniak, W.; Girczys-Poledniok, K.; Kwiecien, J. New insights into the pathogenesis and treatment of irritable bowel syndrome. Adv. Med. Sci. 2017 , 62 , 17–30. [ Google Scholar ] [ CrossRef ]
- Saha, L. Irritable bowel syndrome: Pathogenesis, diagnosis, treatment, and evidence-based medicine. World J. Gastroenterol. 2014 , 20 , 6759–6773. [ Google Scholar ] [ CrossRef ]
- Tian, C.M.; Zhang, Y.; Yang, M.F.; Xu, H.M.; Zhu, M.Z.; Yao, J.; Wang, L.S.; Liang, Y.J.; Li, D.F. Stem Cell Therapy in Inflammatory Bowel Disease: A Review of Achievements and Challenges. J. Inflamm. Res. 2023 , 16 , 2089–2119. [ Google Scholar ] [ CrossRef ]
- Panes, J.; Garcia-Olmo, D.; Van Assche, G.; Colombel, J.F.; Reinisch, W.; Baumgart, D.C.; Dignass, A.; Nachury, M.; Ferrante, M.; Kazemi-Shirazi, L.; et al. Expanded allogeneic adipose-derived mesenchymal stem cells (Cx601) for complex perianal fistulas in Crohn’s disease: A phase 3 randomised, double-blind controlled trial. Lancet 2016 , 388 , 1281–1290. [ Google Scholar ] [ CrossRef ]
- Dave, M.; Dev, A.; Somoza, R.A.; Zhao, N.; Viswanath, S.; Mina, P.R.; Chirra, P.; Obmann, V.C.; Mahabeleshwar, G.H.; Menghini, P.; et al. MSCs mediate long-term efficacy in a Crohn’s disease model by sustained anti-inflammatory macrophage programming via efferocytosis. NPJ Regen. Med. 2024 , 9 , 6. [ Google Scholar ] [ CrossRef ]
- El-Salhy, M. Possible role of intestinal stem cells in the pathophysiology of irritable bowel syndrome. World J. Gastroenterol. 2020 , 26 , 1427–1438. [ Google Scholar ] [ CrossRef ]
- Wilkins, T.; Pepitone, C.; Alex, B.; Schade, R.R. Diagnosis and management of IBS in adults. Am. Fam. Physician 2012 , 86 , 419–426. [ Google Scholar ] [ PubMed ]
- Werlang, M.E.; Palmer, W.C.; Lacy, B.E. Irritable Bowel Syndrome and Dietary Interventions. Gastroenterol. Hepatol. 2019 , 15 , 16–26. [ Google Scholar ]
- Berry, S.K.; Chey, W.D. Integrated Care for Irritable Bowel Syndrome: The Future Is Now. Gastroenterol. Clin. N. Am. 2021 , 50 , 713–720. [ Google Scholar ] [ CrossRef ]
- Lacy, B.E.; Pimentel, M.; Brenner, D.M.; Chey, W.D.; Keefer, L.A.; Long, M.D.; Moshiree, B. ACG Clinical Guideline: Management of Irritable Bowel Syndrome. Am. J. Gastroenterol. 2021 , 116 , 17–44. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Vasant, D.H.; Paine, P.A.; Black, C.J.; Houghton, L.A.; Everitt, H.A.; Corsetti, M.; Agrawal, A.; Aziz, I.; Farmer, A.D.; Eugenicos, M.P.; et al. British Society of Gastroenterology guidelines on the management of irritable bowel syndrome. Gut 2021 , 70 , 1214–1240. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Begtrup, L.M.; Engsbro, A.L.; Kjeldsen, J.; Larsen, P.V.; Schaffalitzky de Muckadell, O.; Bytzer, P.; Jarbol, D.E. A positive diagnostic strategy is noninferior to a strategy of exclusion for patients with irritable bowel syndrome. Clin. Gastroenterol. Hepatol. 2013 , 11 , 956–962.e951. [ Google Scholar ] [ CrossRef ]
- Sayuk, G.S.; Wolf, R.; Chang, L. Comparison of Symptoms, Healthcare Utilization, and Treatment in Diagnosed and Undiagnosed Individuals With Diarrhea-Predominant Irritable Bowel Syndrome. Am. J. Gastroenterol. 2017 , 112 , 892–899. [ Google Scholar ] [ CrossRef ]
- Menees, S.B.; Powell, C.; Kurlander, J.; Goel, A.; Chey, W.D. A meta-analysis of the utility of C-reactive protein, erythrocyte sedimentation rate, fecal calprotectin, and fecal lactoferrin to exclude inflammatory bowel disease in adults with IBS. Am. J. Gastroenterol. 2015 , 110 , 444–454. [ Google Scholar ] [ CrossRef ]
- van Rheenen, P.F.; Van de Vijver, E.; Fidler, V. Faecal calprotectin for screening of patients with suspected inflammatory bowel disease: Diagnostic meta-analysis. BMJ 2010 , 341 , c3369. [ Google Scholar ] [ CrossRef ]
- Zhou, X.L.; Xu, W.; Tang, X.X.; Luo, L.S.; Tu, J.F.; Zhang, C.J.; Xu, X.; Wu, Q.D.; Pan, W.S. Fecal lactoferrin in discriminating inflammatory bowel disease from irritable bowel syndrome: A diagnostic meta-analysis. BMC Gastroenterol. 2014 , 14 , 121. [ Google Scholar ] [ CrossRef ]
- Irvine, A.J.; Chey, W.D.; Ford, A.C. Screening for Celiac Disease in Irritable Bowel Syndrome: An Updated Systematic Review and Meta-analysis. Am. J. Gastroenterol. 2017 , 112 , 65–76. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Cash, B.D.; Rubenstein, J.H.; Young, P.E.; Gentry, A.; Nojkov, B.; Lee, D.; Andrews, A.H.; Dobhan, R.; Chey, W.D. The prevalence of celiac disease among patients with nonconstipated irritable bowel syndrome is similar to controls. Gastroenterology 2011 , 141 , 1187–1193. [ Google Scholar ] [ CrossRef ]
- Chey, W.D.; Hashash, J.G.; Manning, L.; Chang, L. AGA Clinical Practice Update on the Role of Diet in Irritable Bowel Syndrome: Expert Review. Gastroenterology 2022 , 162 , 1737–1745.e5. [ Google Scholar ] [ CrossRef ]
- Spiegel, B.M.; Gralnek, I.M.; Bolus, R.; Chang, L.; Dulai, G.S.; Naliboff, B.; Mayer, E.A. Is a negative colonoscopy associated with reassurance or improved health-related quality of life in irritable bowel syndrome? Gastrointest. Endosc. 2005 , 62 , 892–899. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Black, T.P.; Manolakis, C.S.; Di Palma, J.A. “Red flag” evaluation yield in irritable bowel syndrome. J. Gastrointest. Liver Dis. 2012 , 21 , 153–156. [ Google Scholar ] [ CrossRef ]
- Mulak, A.; Paradowski, L. Anorectal function and dyssynergic defecation in different subgroups of patients with irritable bowel syndrome. Int. J. Color. Dis. 2010 , 25 , 1011–1016. [ Google Scholar ] [ CrossRef ]
- Suttor, V.P.; Prott, G.M.; Hansen, R.D.; Kellow, J.E.; Malcolm, A. Evidence for pelvic floor dyssynergia in patients with irritable bowel syndrome. Dis. Colon Rectum 2010 , 53 , 156–160. [ Google Scholar ] [ CrossRef ]
- Baker, J.; Eswaran, S.; Saad, R.; Menees, S.; Shifferd, J.; Erickson, K.; Barthelemy, A.; Chey, W.D. Abdominal Symptoms Are Common and Benefit from Biofeedback Therapy in Patients with Dyssynergic Defecation. Clin. Transl. Gastroenterol. 2015 , 6 , e105. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Patcharatrakul, T.; Gonlachanvit, S. Outcome of biofeedback therapy in dyssynergic defecation patients with and without irritable bowel syndrome. J. Clin. Gastroenterol. 2011 , 45 , 593–598. [ Google Scholar ] [ CrossRef ]
- Cozma-Petrut, A.; Loghin, F.; Miere, D.; Dumitrascu, D.L. Diet in irritable bowel syndrome: What to recommend, not what to forbid to patients! World J. Gastroenterol. 2017 , 23 , 3771–3783. [ Google Scholar ] [ CrossRef ]
- Gibson, P.R.; Shepherd, S.J. Personal view: Food for thought--western lifestyle and susceptibility to Crohn’s disease. The FODMAP hypothesis. Aliment. Pharmacol. Ther. 2005 , 21 , 1399–1409. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Magge, S.; Lembo, A. Low-FODMAP Diet for Treatment of Irritable Bowel Syndrome. Gastroenterol. Hepatol. 2012 , 8 , 739–745. [ Google Scholar ]
- Black, C.J.; Staudacher, H.M.; Ford, A.C. Efficacy of a low FODMAP diet in irritable bowel syndrome: Systematic review and network meta-analysis. Gut 2022 , 71 , 1117–1126. [ Google Scholar ] [ CrossRef ]
- Bellini, M.; Tonarelli, S.; Nagy, A.G.; Pancetti, A.; Costa, F.; Ricchiuti, A.; de Bortoli, N.; Mosca, M.; Marchi, S.; Rossi, A. Low FODMAP Diet: Evidence, Doubts, and Hopes. Nutrients 2020 , 12 , 148. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Staudacher, H.M. Nutritional, microbiological and psychosocial implications of the low FODMAP diet. J. Gastroenterol. Hepatol. 2017 , 32 (Suppl. 1), 16–19. [ Google Scholar ] [ CrossRef ]
- Staudacher, H.M.; Lomer, M.C.; Anderson, J.L.; Barrett, J.S.; Muir, J.G.; Irving, P.M.; Whelan, K. Fermentable carbohydrate restriction reduces luminal bifidobacteria and gastrointestinal symptoms in patients with irritable bowel syndrome. J. Nutr. 2012 , 142 , 1510–1518. [ Google Scholar ] [ CrossRef ]
- El-Salhy, M.; Ystad, S.O.; Mazzawi, T.; Gundersen, D. Dietary fiber in irritable bowel syndrome (Review). Int. J. Mol. Med. 2017 , 40 , 607–613. [ Google Scholar ] [ CrossRef ]
- Bijkerk, C.J.; Muris, J.W.; Knottnerus, J.A.; Hoes, A.W.; de Wit, N.J. Systematic review: The role of different types of fibre in the treatment of irritable bowel syndrome. Aliment. Pharmacol. Ther. 2004 , 19 , 245–251. [ Google Scholar ] [ CrossRef ]
- Moayyedi, P.; Quigley, E.M.; Lacy, B.E.; Lembo, A.J.; Saito, Y.A.; Schiller, L.R.; Soffer, E.E.; Spiegel, B.M.; Ford, A.C. The effect of fiber supplementation on irritable bowel syndrome: A systematic review and meta-analysis. Am. J. Gastroenterol. 2014 , 109 , 1367–1374. [ Google Scholar ] [ CrossRef ]
- Van Oudenhove, L.; Crowell, M.D.; Drossman, D.A.; Halpert, A.D.; Keefer, L.; Lackner, J.M.; Murphy, T.B.; Naliboff, B.D.; Levy, R.L. Biopsychosocial Aspects of Functional Gastrointestinal Disorders. Gastroenterology 2016 , 150 , 1355–1367. [ Google Scholar ] [ CrossRef ]
- Lackner, J.M.; Jaccard, J.; Radziwon, C.D.; Firth, R.S.; Gudleski, G.D.; Hamilton, F.; Katz, L.A.; Keefer, L.; Krasner, S.S.; Ma, C.X.; et al. Durability and Decay of Treatment Benefit of Cognitive Behavioral Therapy for Irritable Bowel Syndrome: 12-Month Follow-Up. Am. J. Gastroenterol. 2019 , 114 , 330–338. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Everitt, H.A.; Landau, S.; O’Reilly, G.; Sibelli, A.; Hughes, S.; Windgassen, S.; Holland, R.; Little, P.; McCrone, P.; Bishop, F.L.; et al. Cognitive behavioural therapy for irritable bowel syndrome: 24-month follow-up of participants in the ACTIB randomised trial. Lancet Gastroenterol. Hepatol. 2019 , 4 , 863–872. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Jacobs, J.P.; Gupta, A.; Bhatt, R.R.; Brawer, J.; Gao, K.; Tillisch, K.; Lagishetty, V.; Firth, R.; Gudleski, G.D.; Ellingson, B.M.; et al. Cognitive behavioral therapy for irritable bowel syndrome induces bidirectional alterations in the brain-gut-microbiome axis associated with gastrointestinal symptom improvement. Microbiome 2021 , 9 , 236. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Whorwell, P.J.; Prior, A.; Faragher, E.B. Controlled trial of hypnotherapy in the treatment of severe refractory irritable-bowel syndrome. Lancet 1984 , 2 , 1232–1234. [ Google Scholar ] [ CrossRef ]
- Flik, C.E.; Laan, W.; Zuithoff, N.P.A.; van Rood, Y.R.; Smout, A.; Weusten, B.; Whorwell, P.J.; de Wit, N.J. Efficacy of individual and group hypnotherapy in irritable bowel syndrome (IMAGINE): A multicentre randomised controlled trial. Lancet Gastroenterol. Hepatol. 2019 , 4 , 20–31. [ Google Scholar ] [ CrossRef ]
- Lovdahl, J.; Tornblom, H.; Ringstrom, G.; Palsson, O.S.; Simren, M. Randomised clinical trial: Individual versus group hypnotherapy for irritable bowel syndrome. Aliment. Pharmacol. Ther. 2022 , 55 , 1501–1511. [ Google Scholar ] [ CrossRef ]
- American Gastroenterological Association. Clinical Decision Support Tool: IBS Treatment ; American Gastroenterological Association: Bethesda, MD, USA, 2022; Volume 163. [ Google Scholar ]
- Chang, L.; Sultan, S.; Lembo, A.; Verne, G.N.; Smalley, W.; Heidelbaugh, J.J. AGA Clinical Practice Guideline on the Pharmacological Management of Irritable Bowel Syndrome with Constipation. Gastroenterology 2022 , 163 , 118–136. [ Google Scholar ] [ CrossRef ]
- Lembo, A.; Sultan, S.; Chang, L.; Heidelbaugh, J.J.; Smalley, W.; Verne, G.N. AGA Clinical Practice Guideline on the Pharmacological Management of Irritable Bowel Syndrome with Diarrhea. Gastroenterology 2022 , 163 , 137–151. [ Google Scholar ] [ CrossRef ]
- Su, G.L.; Ko, C.W.; Bercik, P.; Falck-Ytter, Y.; Sultan, S.; Weizman, A.V.; Morgan, R.L. AGA Clinical Practice Guidelines on the Role of Probiotics in the Management of Gastrointestinal Disorders. Gastroenterology 2020 , 159 , 697–705. [ Google Scholar ] [ CrossRef ]
- Ruepert, L.; Quartero, A.O.; de Wit, N.J.; van der Heijden, G.J.; Rubin, G.; Muris, J.W. Bulking agents, antispasmodics and antidepressants for the treatment of irritable bowel syndrome. Cochrane Database Syst. Rev. 2011 , 2011 , CD003460. [ Google Scholar ] [ CrossRef ]
- Martinez-Vazquez, M.A.; Vazquez-Elizondo, G.; Gonzalez-Gonzalez, J.A.; Gutierrez-Udave, R.; Maldonado-Garza, H.J.; Bosques-Padilla, F.J. Effect of antispasmodic agents, alone or in combination, in the treatment of Irritable Bowel Syndrome: Systematic review and meta-analysis. Rev. Gastroenterol. Mex. 2012 , 77 , 82–90. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Page, J.G.; Dirnberger, G.M. Treatment of the irritable bowel syndrome with Bentyl (dicyclomine hydrochloride). J. Clin. Gastroenterol. 1981 , 3 , 153–156. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Ritchie, J.A.; Truelove, S.C. Treatment of irritable bowel syndrome with lorazepam, hyoscine butylbromide, and ispaghula husk. Br. Med. J. 1979 , 1 , 376–378. [ Google Scholar ] [ CrossRef ]
- Khanna, R.; MacDonald, J.K.; Levesque, B.G. Peppermint oil for the treatment of irritable bowel syndrome: A systematic review and meta-analysis. J. Clin. Gastroenterol. 2014 , 48 , 505–512. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Weerts, Z.; Masclee, A.A.M.; Witteman, B.J.M.; Clemens, C.H.M.; Winkens, B.; Brouwers, J.; Frijlink, H.W.; Muris, J.W.M.; De Wit, N.J.; Essers, B.A.B.; et al. Efficacy and Safety of Peppermint Oil in a Randomized, Double-Blind Trial of Patients With Irritable Bowel Syndrome. Gastroenterology 2020 , 158 , 123–136. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Black, C.J.; Moayyedi, P.; Quigley, E.M.M.; Ford, A.C. Peppermint Oil in Irritable Bowel Syndrome. Gastroenterology 2020 , 159 , 395–396. [ Google Scholar ] [ CrossRef ]
- Pimentel, M.; Lembo, A. Microbiome and Its Role in Irritable Bowel Syndrome. Dig. Dis. Sci. 2020 , 65 , 829–839. [ Google Scholar ] [ CrossRef ]
- Liu, H.N.; Wu, H.; Chen, Y.Z.; Chen, Y.J.; Shen, X.Z.; Liu, T.T. Altered molecular signature of intestinal microbiota in irritable bowel syndrome patients compared with healthy controls: A systematic review and meta-analysis. Dig. Liver Dis. 2017 , 49 , 331–337. [ Google Scholar ] [ CrossRef ]
- Crouzet, L.; Gaultier, E.; Del’Homme, C.; Cartier, C.; Delmas, E.; Dapoigny, M.; Fioramonti, J.; Bernalier-Donadille, A. The hypersensitivity to colonic distension of IBS patients can be transferred to rats through their fecal microbiota. Neurogastroenterol. Motil. 2013 , 25 , e272–e282. [ Google Scholar ] [ CrossRef ]
- Didari, T.; Mozaffari, S.; Nikfar, S.; Abdollahi, M. Effectiveness of probiotics in irritable bowel syndrome: Updated systematic review with meta-analysis. World J. Gastroenterol. 2015 , 21 , 3072–3084. [ Google Scholar ] [ CrossRef ]
- Ford, A.C.; Harris, L.A.; Lacy, B.E.; Quigley, E.M.M.; Moayyedi, P. Systematic review with meta-analysis: The efficacy of prebiotics, probiotics, synbiotics and antibiotics in irritable bowel syndrome. Aliment. Pharmacol. Ther. 2018 , 48 , 1044–1060. [ Google Scholar ] [ CrossRef ]
- Liu, R.; Staller, K. Update on Eluxadoline for the Treatment of Irritable Bowel Syndrome with Diarrhea: Patient Selection and Perspectives. Drug Des. Devel Ther. 2020 , 14 , 1391–1400. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Cann, P.A.; Read, N.W.; Holdsworth, C.D.; Barends, D. Role of loperamide and placebo in management of irritable bowel syndrome (IBS). Dig. Dis. Sci. 1984 , 29 , 239–247. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Ford, A.C.; Moayyedi, P.; Chey, W.D.; Harris, L.A.; Lacy, B.E.; Saito, Y.A.; Quigley, E.M.M. American College of Gastroenterology Monograph on Management of Irritable Bowel Syndrome. Am. J. Gastroenterol. 2018 , 113 , 1–18. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Lacy, B.E. Diagnosis and treatment of diarrhea-predominant irritable bowel syndrome. Int. J. Gen. Med. 2016 , 9 , 7–17. [ Google Scholar ] [ CrossRef ]
- Slattery, S.A.; Niaz, O.; Aziz, Q.; Ford, A.C.; Farmer, A.D. Systematic review with meta-analysis: The prevalence of bile acid malabsorption in the irritable bowel syndrome with diarrhoea. Aliment. Pharmacol. Ther. 2015 , 42 , 3–11. [ Google Scholar ] [ CrossRef ]
- Wong, B.S.; Camilleri, M.; Carlson, P.; McKinzie, S.; Busciglio, I.; Bondar, O.; Dyer, R.B.; Lamsam, J.; Zinsmeister, A.R. Increased bile acid biosynthesis is associated with irritable bowel syndrome with diarrhea. Clin. Gastroenterol. Hepatol. 2012 , 10 , 1009–1015.e3. [ Google Scholar ] [ CrossRef ]
- Camilleri, M. Advances in understanding of bile acid diarrhea. Expert Rev. Gastroenterol. Hepatol. 2014 , 8 , 49–61. [ Google Scholar ] [ CrossRef ]
- Odunsi-Shiyanbade, S.T.; Camilleri, M.; McKinzie, S.; Burton, D.; Carlson, P.; Busciglio, I.A.; Lamsam, J.; Singh, R.; Zinsmeister, A.R. Effects of chenodeoxycholate and a bile acid sequestrant, colesevelam, on intestinal transit and bowel function. Clin. Gastroenterol. Hepatol. 2010 , 8 , 159–165. [ Google Scholar ] [ CrossRef ]
- Camilleri, M.; Acosta, A.; Busciglio, I.; Boldingh, A.; Dyer, R.B.; Zinsmeister, A.R.; Lueke, A.; Gray, A.; Donato, L.J. Effect of colesevelam on faecal bile acids and bowel functions in diarrhoea-predominant irritable bowel syndrome. Aliment. Pharmacol. Ther. 2015 , 41 , 438–448. [ Google Scholar ] [ CrossRef ]
- Fodor, A.A.; Pimentel, M.; Chey, W.D.; Lembo, A.; Golden, P.L.; Israel, R.J.; Carroll, I.M. Rifaximin is associated with modest, transient decreases in multiple taxa in the gut microbiota of patients with diarrhoea-predominant irritable bowel syndrome. Gut Microbes 2019 , 10 , 22–33. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Pimentel, M.; Lembo, A.; Chey, W.D.; Zakko, S.; Ringel, Y.; Yu, J.; Mareya, S.M.; Shaw, A.L.; Bortey, E.; Forbes, W.P.; et al. Rifaximin therapy for patients with irritable bowel syndrome without constipation. N. Engl. J. Med. 2011 , 364 , 22–32. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Lembo, A.; Pimentel, M.; Rao, S.S.; Schoenfeld, P.; Cash, B.; Weinstock, L.B.; Paterson, C.; Bortey, E.; Forbes, W.P. Repeat Treatment With Rifaximin Is Safe and Effective in Patients With Diarrhea-Predominant Irritable Bowel Syndrome. Gastroenterology 2016 , 151 , 1113–1121. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Dove, L.S.; Lembo, A.; Randall, C.W.; Fogel, R.; Andrae, D.; Davenport, J.M.; McIntyre, G.; Almenoff, J.S.; Covington, P.S. Eluxadoline benefits patients with irritable bowel syndrome with diarrhea in a phase 2 study. Gastroenterology 2013 , 145 , 329–338.e321. [ Google Scholar ] [ CrossRef ]
- Lembo, A.J.; Lacy, B.E.; Zuckerman, M.J.; Schey, R.; Dove, L.S.; Andrae, D.A.; Davenport, J.M.; McIntyre, G.; Lopez, R.; Turner, L.; et al. Eluxadoline for Irritable Bowel Syndrome with Diarrhea. N. Engl. J. Med. 2016 , 374 , 242–253. [ Google Scholar ] [ CrossRef ]
- Lacy, B.E.; Chey, W.D.; Cash, B.D.; Lembo, A.J.; Dove, L.S.; Covington, P.S. Eluxadoline Efficacy in IBS-D Patients Who Report Prior Loperamide Use. Am. J. Gastroenterol. 2017 , 112 , 924–932. [ Google Scholar ] [ CrossRef ]
- Brenner, D.M.; Sayuk, G.S.; Gutman, C.R.; Jo, E.; Elmes, S.J.R.; Liu, L.W.C.; Cash, B.D. Efficacy and Safety of Eluxadoline in Patients With Irritable Bowel Syndrome With Diarrhea Who Report Inadequate Symptom Control With Loperamide: RELIEF Phase 4 Study. Am. J. Gastroenterol. 2019 , 114 , 1502–1511. [ Google Scholar ] [ CrossRef ]
- Cangemi, D.J.; Lacy, B.E. Management of irritable bowel syndrome with diarrhea: A review of nonpharmacological and pharmacological interventions. Ther. Adv. Gastroenterol. 2019 , 12 , 1756284819878950. [ Google Scholar ] [ CrossRef ]
- US Food and Drug Administration. FDA Drug Safety Communication: FDA Warns about Increased Risk of Serious Pancreatitis with Irritable Bowel Drug Viberzi (Eluxadoline) in Patients without a Gallbladder ; Center for Drug Evaluation and Research: Beltsville, MD, USA, 2017. [ Google Scholar ]
- Xie, C.; Tang, Y.; Wang, Y.; Yu, T.; Wang, Y.; Jiang, L.; Lin, L. Efficacy and Safety of Antidepressants for the Treatment of Irritable Bowel Syndrome: A Meta-Analysis. PLoS ONE 2015 , 10 , e0127815. [ Google Scholar ] [ CrossRef ]
- Vahedi, H.; Merat, S.; Momtahen, S.; Kazzazi, A.S.; Ghaffari, N.; Olfati, G.; Malekzadeh, R. Clinical trial: The effect of amitriptyline in patients with diarrhoea-predominant irritable bowel syndrome. Aliment. Pharmacol. Ther. 2008 , 27 , 678–684. [ Google Scholar ] [ CrossRef ]
- Lacy, B.E.; Nicandro, J.P.; Chuang, E.; Earnest, D.L. Alosetron use in clinical practice: Significant improvement in irritable bowel syndrome symptoms evaluated using the US Food and Drug Administration composite endpoint. Ther. Adv. Gastroenterol. 2018 , 11 , 1756284818771674. [ Google Scholar ] [ CrossRef ]
- Tong, K.; Nicandro, J.P.; Shringarpure, R.; Chuang, E.; Chang, L. A 9-year evaluation of temporal trends in alosetron postmarketing safety under the risk management program. Ther. Adv. Gastroenterol. 2013 , 6 , 344–357. [ Google Scholar ] [ CrossRef ]
- Camilleri, M.; Chey, W.Y.; Mayer, E.A.; Northcutt, A.R.; Heath, A.; Dukes, G.E.; McSorley, D.; Mangel, A.M. A randomized controlled clinical trial of the serotonin type 3 receptor antagonist alosetron in women with diarrhea-predominant irritable bowel syndrome. Arch. Intern. Med. 2001 , 161 , 1733–1740. [ Google Scholar ] [ CrossRef ]
- Bharucha, A.E.; Wald, A. Chronic Constipation. Mayo Clin. Proc. 2019 , 94 , 2340–2357. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Chapman, R.W.; Stanghellini, V.; Geraint, M.; Halphen, M. Randomized clinical trial: Macrogol/PEG 3350 plus electrolytes for treatment of patients with constipation associated with irritable bowel syndrome. Am J Gastroenterol 2013 , 108 , 1508–1515. [ Google Scholar ] [ CrossRef ]
- Talley, N.J. Pharmacologic therapy for the irritable bowel syndrome. Am. J. Gastroenterol. 2003 , 98 , 750–758. [ Google Scholar ] [ CrossRef ]
- Wilson, N.; Schey, R. Lubiprostone in constipation: Clinical evidence and place in therapy. Ther. Adv. Chronic Dis. 2015 , 6 , 40–50. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Bonetto, S.; Fagoonee, S.; Battaglia, E.; Grassini, M.; Saracco, G.M.; Pellicano, R. Recent advances in the treatment of irritable bowel syndrome. Pol. Arch. Intern. Med. 2021 , 131 , 709–715. [ Google Scholar ] [ CrossRef ]
- Johanson, J.F.; Morton, D.; Geenen, J.; Ueno, R. Multicenter, 4-week, double-blind, randomized, placebo-controlled trial of lubiprostone, a locally-acting type-2 chloride channel activator, in patients with chronic constipation. Am. J. Gastroenterol. 2008 , 103 , 170–177. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Atluri, D.K.; Chandar, A.K.; Bharucha, A.E.; Falck-Ytter, Y. Effect of linaclotide in irritable bowel syndrome with constipation (IBS-C): A systematic review and meta-analysis. Neurogastroenterol. Motil. 2014 , 26 , 499–509. [ Google Scholar ] [ CrossRef ]
- Rao, S.S.; Quigley, E.M.; Shiff, S.J.; Lavins, B.J.; Kurtz, C.B.; MacDougall, J.E.; Currie, M.G.; Johnston, J.M. Effect of linaclotide on severe abdominal symptoms in patients with irritable bowel syndrome with constipation. Clin. Gastroenterol. Hepatol. 2014 , 12 , 616–623. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Johnston, J.M.; Kurtz, C.B.; Macdougall, J.E.; Lavins, B.J.; Currie, M.G.; Fitch, D.A.; O’Dea, C.; Baird, M.; Lembo, A.J. Linaclotide improves abdominal pain and bowel habits in a phase IIb study of patients with irritable bowel syndrome with constipation. Gastroenterology 2010 , 139 , 1877–1886.e2. [ Google Scholar ] [ CrossRef ]
- Brenner, D.M.; Fogel, R.; Dorn, S.D.; Krause, R.; Eng, P.; Kirshoff, R.; Nguyen, A.; Crozier, R.A.; Magnus, L.; Griffin, P.H. Efficacy, safety, and tolerability of plecanatide in patients with irritable bowel syndrome with constipation: Results of two phase 3 randomized clinical trials. Am. J. Gastroenterol. 2018 , 113 , 735–745. [ Google Scholar ] [ CrossRef ]
- Herekar, A.; Shimoga, D.; Jehangir, A.; Shahsavari, D.; Yan, Y.; Karunaratne, T.B.; Sharma, A. Tenapanor in the Treatment of Irritable Bowel Syndrome with Constipation: Discovery, Efficacy, and Role in Management. Clin. Exp. Gastroenterol. 2023 , 16 , 79–85. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Chey, W.D.; Lembo, A.J.; Yang, Y.; Rosenbaum, D.P. Efficacy of Tenapanor in Treating Patients With Irritable Bowel Syndrome With Constipation: A 26-Week, Placebo-Controlled Phase 3 Trial (T3MPO-2). Am. J. Gastroenterol. 2021 , 116 , 1294–1303. [ Google Scholar ] [ CrossRef ]
- Wechsler, E.V.; Shah, E.D. Diarrhea-Predominant and Constipation-Predominant Irritable Bowel Syndrome: Current Prescription Drug Treatment Options. Drugs 2021 , 81 , 1953–1968. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Sayuk, G.S.; Tack, J. Tegaserod: What’s Old Is New Again. Clin. Gastroenterol. Hepatol. 2022 , 20 , 2175–2184.e19. [ Google Scholar ] [ CrossRef ]
- Tack, J.; Camilleri, M.; Chang, L.; Chey, W.D.; Galligan, J.J.; Lacy, B.E.; Muller-Lissner, S.; Quigley, E.M.; Schuurkes, J.; De Maeyer, J.H.; et al. Systematic review: Cardiovascular safety profile of 5-HT(4) agonists developed for gastrointestinal disorders. Aliment. Pharmacol. Ther. 2012 , 35 , 745–767. [ Google Scholar ] [ CrossRef ]
- Wright, K.; Rooney, N.; Feeney, M.; Tate, J.; Robertson, D.; Welham, M.; Ward, S. Differential expression of cannabinoid receptors in the human colon: Cannabinoids promote epithelial wound healing. Gastroenterology 2005 , 129 , 437–453. [ Google Scholar ] [ CrossRef ]
- Dothel, G.; Chang, L.; Shih, W.; Barbaro, M.R.; Cremon, C.; Stanghellini, V.; De Ponti, F.; Mayer, E.A.; Barbara, G.; Sternini, C. micro-opioid receptor, beta-endorphin, and cannabinoid receptor-2 are increased in the colonic mucosa of irritable bowel syndrome patients. Neurogastroenterol. Motil. 2019 , 31 , e13688. [ Google Scholar ] [ CrossRef ]
- Kikuchi, A.; Ohashi, K.; Sugie, Y.; Sugimoto, H.; Omura, H. Pharmacological evaluation of a novel cannabinoid 2 (CB2) ligand, PF-03550096, in vitro and in vivo by using a rat model of visceral hypersensitivity. J. Pharmacol. Sci. 2008 , 106 , 219–224. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Chang, L.; Cash, B.D.; Lembo, A.; Kunkel, D.C.; English, B.A.; Lindstrom, B.; Gu, G.; Skare, S.; Gilder, K.; Turner, S.; et al. Efficacy and safety of olorinab, a full agonist of the cannabinoid receptor 2, for the treatment of abdominal pain in patients with irritable bowel syndrome: Results from a phase 2b randomized placebo-controlled trial (CAPTIVATE). Neurogastroenterol. Motil. 2023 , 35 , e14539. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Leventer, S.M.; Raudibaugh, K.; Frissora, C.L.; Kassem, N.; Keogh, J.C.; Phillips, J.; Mangel, A.W. Clinical trial: Dextofisopam in the treatment of patients with diarrhoea-predominant or alternating irritable bowel syndrome. Aliment. Pharmacol. Ther. 2008 , 27 , 197–206. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Quigley, E.M.M.; Markinson, L.; Stevenson, A.; Treasure, F.P.; Lacy, B.E. Randomised clinical trial: Efficacy and safety of the live biotherapeutic product MRx1234 in patients with irritable bowel syndrome. Aliment. Pharmacol. Ther. 2023 , 57 , 81–93. [ Google Scholar ] [ CrossRef ]
| Soluble Fiber | Mechanism of Action | Origin | Common Brands |
|---|---|---|---|
| Psyllium | Stool bulking agent that draws water into the gut lumen | Plantago Ovata psyllium husk | Metamucil |
| Methylcellulose | Stool bulking agent that draws water into the gut lumen | Processed plant cellulose | Citrucel |
| Polycarbophil | Stool bulking agent that draws water into the gut lumen | Synthetic polymer of polyacrylic acid cross-linked with divinyl glycol | FiberCon |
| Drug | IBS Subtype | Mechanism of Action | FDA Approval | AGA Recommendation [ , , ] | ACG Recommendation [ ] | BSG Recommendation [ ] |
|---|---|---|---|---|---|---|
| Antispasmodics | Global IBS symptoms and abdominal pain | Relax smooth muscle to decrease visceral hypersensitivity | Not specifically for IBS treatment | |||
| Peppermint Oil | Global IBS symptoms and abdominal pain | L-methanol relaxes smooth muscle via calcium channel inhibition | Listed as generally safe | N/A | ||
| Probiotics | Global IBS symptoms and abdominal pain | Live microorganism strains that impact the gut microbiome | No | |||
| Loperamide | IBS-D | μ-opioid receptor agonist | Yes | |||
| Bile Acid Sequestrants | IBS-D | Reduction of bile acids in the gut to decrease bile acid malabsorption | Yes | N/A | N/A | |
| Rifaximin | IBS-D | Non-systemic, oral antibiotic altering gut microbiome | Yes | |||
| Eluxadoline | IBS-D | μ- and κ- opioid receptor agonist, δ-opioid receptor antagonist | Yes | |||
| Tricyclic Antidepressants | IBS-D | Serotonin transporter (SERT) and norepinephrine transporter (NET) inhibition | Yes | |||
| Alosetron | IBS-D | Selective 5-HT antagonist | Yes | |||
| Lubiprostone | IBS-C | Chloride channel activator | Yes | |||
| Linaclotide | IBS-C | Guanylate cyclase-C agonist | Yes | |||
| Plecanatide | IBS-C | Guanylate cyclase-C agonist | Yes | |||
| Tenapanor | IBS-C | NHE3 inhibitor | Yes | N/A | ||
| Tegaserod | IBS-C | 5-HT agonist | Yes (Women <65-years-old) | |||
| Olorinab | IBS-D/IBS-C | Cannabinoid receptor-2 agonist | Currently in phase II trials | N/A | N/A | N/A |
| Dextofisopam | IBS-D/IBS-M | Modulation of autonomic function | Currently in phase II trials | N/A | N/A | N/A |
| MRx1234 | IBS-M | Live biotherapeutic that competes with sulfate-reducing bacteria in the gut | Currently in phase II trials | N/A | N/A | N/A |
| Laxatives | Mechanism of Action | Origin | Common Brands |
|---|---|---|---|
| Polyethylene glycol | Osmotic load draws water into the gastrointestinal lumen. | Derived from petroleum | MiraLAX, GoLytely, Glycolax |
| Bisacodyl | Stimulates enteric neurons to promote peristalsis. | Synthetic compound | Dulcolax, Ducodyl |
| Senna | Stimulates peristalsis and increases water in the gastrointestinal lumen. | Derived from dried leaflets or fruits of Cassia senna (C. acutifolia) | Senokot, Senna Lax, Ex-Lax, Senexon |
| Docusate sodium | Lowers the surface tension between feces and water, allowing lipids to enter and soften stool. | Synthetic compound | Colace, Dulcolax Stool Softener |
| Lactulose | Osmotic load draws water into the gastrointestinal lumen. Primarily used for decreasing intestinal ammonia in hyperammonemia from hepatic encephalopathy. | Derived from lactose | Unavailable over-the-counter |
| The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
Share and Cite
Chuy, D.S.; Wi, R.S.; Tadros, M. Irritable Bowel Syndrome: Current Landscape of Diagnostic Guidelines and Therapeutic Strategies. Gastroenterol. Insights 2024 , 15 , 786-809. https://doi.org/10.3390/gastroent15030056
Chuy DS, Wi RS, Tadros M. Irritable Bowel Syndrome: Current Landscape of Diagnostic Guidelines and Therapeutic Strategies. Gastroenterology Insights . 2024; 15(3):786-809. https://doi.org/10.3390/gastroent15030056
Chuy, Dareen S., Ryan S. Wi, and Micheal Tadros. 2024. "Irritable Bowel Syndrome: Current Landscape of Diagnostic Guidelines and Therapeutic Strategies" Gastroenterology Insights 15, no. 3: 786-809. https://doi.org/10.3390/gastroent15030056
Article Metrics
Article access statistics, further information, mdpi initiatives, follow mdpi.

Subscribe to receive issue release notifications and newsletters from MDPI journals

IMAGES
VIDEO
COMMENTS
Other common pharmacologic therapies prescribed for IBS include antidepressants, probiotics, and antibiotics. Just as therapy is often directed at specific symptoms, patient response in clinical practice is typically dependent on changes in those symptoms and can be highly variable. This is in contrast to the many clinical trials of therapies ...
Introduction. Irritable bowel syndrome (IBS) is the most prevalent gastrointestinal (GI) diagnosis with a world-wide prevalence of 14% 1.It is a functional GI disorder characterized by bloating, diarrhea, constipation, abdominal pain, and presents with a high prevalence of psychological comorbidity of depression and anxiety. 2 The pathophysiology of IBS is marked by complex interactions ...
Irritable bowel syndrome (IBS) is a highly prevalent disorder that reduces patients' quality of life and imposes a significant economic burden to the healthcare system. The prevalence of IBS in the United States is estimated to be 9 -22%, 1 - 3 and the yearly incidence is approximately 1.5%. 4 IBS is one of the most common medical disorders ...
BM = bowel movement; IBS-C = irritable bowel syndrome with constipation; IBS-D = irritable bowel syndrome with diarrhea; IBS -M = mixed irritable bowel syndrome. Note: Frequency represents the frequency with which each symptom or impact was included by concept elicitation participants in their list of the 5 most important IBS symptoms to treat.
Background Irritable bowel syndrome (IBS) appears to have a bidirectional interaction with both depressive and anxiety-related complaints. However, it remains unclear how exactly the psychological complaints, at the individual level, are related to somatic symptoms on a daily basis. This single case study investigates how somatic and psychological variables are temporally related in a patient ...
Mia*, a young student in her early 20s with a vegetarian diet high in FODMAPs, turned to dietitian Chloe Madigan for help with her irregular bowel. With 35 years of dietetic practice to draw from, Chloe was able to guide Mia towards a better quality of life thanks to a combination of gut-directed hypnotherapy and dietary therapy that supported her lifestyle.
The IBS in America survey showed that three-quarters of per - sons with IBS symptoms tried an average of 3.6 nonprescrip-tion products before seeking medical care. 8,9 Abdominal pain was the most common reason people sought medical care. CASE STUDY 1 SC is a 25-year-old woman with symptoms of constipation that
Publication types. Case Reports. A 26-year-old Caucasian woman presented with a two-year history of depression concomitant with irritable bowel syndrome (IBS-C; constipation subtype, gas/bloating). Past evaluation resulted in a clinical diagnosis of IBS-C in August of 2015. Between August and November of 2015, the patient developed ….
Irritable Bowel Syndrome (IBS) is a functional disorder marked by some gastrointestinal symptoms frequently associated with extradigestive symptoms with unknown organic causes. In this study the ...
Irritable bowel syndrome (IBS), also known as mucus colitis, nervous colon, and spastic colitis. IBS is a common functional disorder of the gastrointestinal tract. It is characterized by current episodes of abdominal pain, bloating, gas, and discomfort along with changes in the consistency of stool. This condition is heterogeneous, exhibiting ...
A burden on both patients and the healthcare system, irritable bowel syndrome (IBS) is a prevalent condition that can result in high medical costs, frequent visits to the doctor, missed work, and anxiety and depression in the patient. ... An Evidence-based Approach to Therapy in IBS-D: A Case Study Compendium Gastroenterol Hepatol (N Y). 2010 ...
Case Study: Irritable Bowel. Samantha came to see me after struggling with irritable bowel syndrome for nine months. Samantha's main symptoms were frequent loose stools, severe abdominal bloating and flatulence. She was extremely distressed by these symptoms, due to their intensity and duration. The abdominal bloating and discomfort became ...
The included studies garnered a range of experiences and needs of adults living with IBS. From the thematic synthesis, four themes were identified: (1) physical, psychological and social consequences; (2) impact of IBS on working adults; (3) dealing with IBS; and (4) sources of support and support needs of the adults.
Overview of IBS. In 2016, the fourth iteration of the Rome Diagnostic Criteria for Irritable Bowel Syndrome (IBS; Rome IV criteria) was released (Table 2). 1 Developed by expert consensus, the Rome IV criteria incorporated key changes designed to improve their clinical utility and to reflect an increased understanding of IBS pathophysiology.In the Rome IV criteria, IBS is defined as a disorder ...
Practical Evaluation and Management of Irritable Bowel Syndrome with Diarrhea: A Case Study Approach. e Bowel Syndrome with Diarrhea: A Case Study ApproachBrian E. Lacy, MD, PhDL. ING OBJECTIVES Identify patients who are appropriately di-agnosed based on history and symptoms Describe the roles of t. ome-IV criteria, colonoscopy,
How Tony's Transformation in the Gut Reset was achieved through IBS diet and lifestyle changes. Case Study on IBS improvements. 07812010412 [email protected]. 0 Items. Work With Me. 1:1 Gut Reset - 3 month programme ... How Tony reintroduced foods to his IBS diet. So as an example of that, one of the first things I wanted to do is ...
Case Study Examples. The following case study aims to show you how I work with a client as a Nutritional Therapist. Irritable Bowel Syndrome (IBS) is one of the most common conditions that clients come to see me for. Many aim to see relief from an array of symptoms such as constipation, reflux, diarrhoea, wind, headaches, bloating and food ...
This case study has, for the first time, applied a longitudinal time series design to a patient with IBS. Study objectives of this single-case analysis were: (1) to explore temporal relationships and interactions between the somatic and psychological complaints of the patient and (2) to investigate the impact of personal coping strategies on ...
irritable bowel syndrome (IBS). The disruption occurring in the GBA determines the changes in intestinal motility and secretion, causes visceral hypersensitivity and leads to cellular alterations of the entero-endocrine and immune systems. Microbiota may interplay with multiple of these different pathophysiological IBS targets.5 Case study
Case study 3 : Irritable Bowel Syndrome (IBS), Male, 41 years old. Fair health state, IBS in history 15 years, followed by a low back injury at age of 15, injury of cervical spine later in life. Not taking any medication, in the past was taking anti-inflammatory and immodium. Diet is not great, drinking cider, wine, eating spicy foods.
Study Results. The results from all studies thus far, including observational case-control studies and randomized, controlled trials, generally support the use of a low-FODMAP diet for patients with IBS, as 50% to 80% of patients report some benefits compared to using a regular or habitual diet (Table 1). 3, 13, 26, 31, 32 Two studies were conducted comparing the low-FODMAP diet to commonly ...
Understanding the Current Approaches in the Management of IBS-C: A Case Study. Download PDF. Patient Case. ... For example, IBS-C is diagnosed when more than 25% of a patient's bowel movements are BSFS type 1 or 2 and fewer than 25% are type 6 or 7. In contrast, IBS-D is diagnosed when more than 25% of a patient's bowel movements are BSFS ...
Irritable bowel syndrome (IBS) is a disorder of the gut-brain axis with pronounced adverse effects on physical health, psychological health, and overall quality of life. Diagnostic strategies can vary, highlighting a need to synthesize best-practice guidelines. Particularly, the American College of Gastroenterology and the British Society of Gastroenterology both support a positive ...