- Nutritional Medicine
- Food Science
- Nutrition and Dietetics
- Food Fortification

Food Fortification: The Advantages, Disadvantages and Lessons from Sight and Life Programs
- This person is not on ResearchGate, or hasn't claimed this research yet.

- Sight and Life

- Wageningen University & Research

- Sight and Life Foundation
Abstract and Figures

Discover the world's research
- 25+ million members
- 160+ million publication pages
- 2.3+ billion citations
- Rashmi Mathur

- Yongfeng Ding

- Kenneth H Brown
- Sonja Y Hess

- Monica Macaluso

- Parixit Prajapati
- Ragini Sharma
- Yongqiang Cheng

- Muhammad Tanveer Altaf

- K. T. Ravi Kiran
- Bhagya Vijayan
- Naveen Kumar
- Suhani Sinha
- Siddharth Tiwari
- Adam Drewnowski

- N.K. Bhullar

- Howarth E. Bouis

- Penjani Mkambula

- Jonathan Gorstein
- ANN NUTR METAB

- A. Bernardinho Munhaua

- Mark W. Rosegrant

- Rebecca A Heidkamp
- Ellen Piwoz
- Stuart Gillespie
- Zulfiqar A Bhutta

- Recruit researchers
- Join for free
- Login Email Tip: Most researchers use their institutional email address as their ResearchGate login Password Forgot password? Keep me logged in Log in or Continue with Google Welcome back! Please log in. Email · Hint Tip: Most researchers use their institutional email address as their ResearchGate login Password Forgot password? Keep me logged in Log in or Continue with Google No account? Sign up
An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
- My Bibliography
- Collections
- Citation manager
Save citation to file
Email citation, add to collections.
- Create a new collection
- Add to an existing collection
Add to My Bibliography
Your saved search, create a file for external citation management software, your rss feed.
- Search in PubMed
- Search in NLM Catalog
- Add to Search
Rice fortification: an emerging opportunity to contribute to the elimination of vitamin and mineral deficiency worldwide
Affiliation.
- 1 Centre for Health Innovation and Partnership, NSW Health Bldg 52B, Cumberland Hospital, 5, Fleet Street, North Parramatta, NSW 2151, Australia. [email protected]
- PMID: 23424896
- DOI: 10.1177/156482651203300410
Vitamin and mineral deficiencies are ranked among the top causes of poor health and disability in the world. These deficiencies damage developing brains, impair learning ability, increase susceptibility to infections, and reduce the work productivity of nations. Food fortification is a sustainable, cost-effective approach to reducing vitamin and mineral deficiency. As the staple food for an estimated 3 billion people, rice has the potential to fill an obvious gap in current fortification programs. In recent years, new technologies have produced fortified rice kernels that are efficacious in reducing vitamin and mineral deficiency. There are opportunities to fortify a significant share of rice that comes from large mills supplying centralized markets and national welfare programs in major rice-growing countries. The rice export markets, which handle 30 million MT of rice annually, also present a key fortification opportunity. The cost of fortifying rice is only 1.5% to 3% of the current retail price of rice. Countries that mandate rice fortification have the strongest evidence for achieving wide coverage and impact. The Rice Fortification Resource Group (RiFoRG), a global network of public and private partners that offers technical and advocacy support for rice fortification, has a vision of promoting rice fortification worldwide. It has a targeted approach, engaging multisector partners in key countries where the opportunities are greatest and there is receptivity to early adoption of large-scale rice fortification. The challenges are real, the imperative to address them is powerful, and the opportunities to deliver the promise of rice fortification are clear.
PubMed Disclaimer
Similar articles
- Rice fortification: its potential for improving micronutrient intake and steps required for implementation at scale. Piccoli NB, Grede N, de Pee S, Singhkumarwong A, Roks E, Moench-Pfanner R, Bloem MW. Piccoli NB, et al. Food Nutr Bull. 2012 Dec;33(4 Suppl):S360-72. doi: 10.1177/15648265120334S312. Food Nutr Bull. 2012. PMID: 23444717 Review.
- Fortifying food in the field to boost nutrition: case studies from Afghanistan, Angola, and Zambia. van den Briel T, Cheung E, Zewari J, Khan R. van den Briel T, et al. Food Nutr Bull. 2007 Sep;28(3):353-64. doi: 10.1177/156482650702800312. Food Nutr Bull. 2007. PMID: 17974369
- An overview of global rice production, supply, trade, and consumption. Muthayya S, Sugimoto JD, Montgomery S, Maberly GF. Muthayya S, et al. Ann N Y Acad Sci. 2014 Sep;1324:7-14. doi: 10.1111/nyas.12540. Epub 2014 Sep 15. Ann N Y Acad Sci. 2014. PMID: 25224455 Review.
- Review of the cost components of introducing industrially fortified rice. Roks E. Roks E. Ann N Y Acad Sci. 2014 Sep;1324:82-91. doi: 10.1111/nyas.12480. Epub 2014 Jun 24. Ann N Y Acad Sci. 2014. PMID: 24961588 Review.
- GAIN Premix Facility: an innovative approach for improving access to quality vitamin and mineral premix in fortification initiatives. Guinot P, Jallier V, Blasi A, Guyondet C, Van Ameringen M. Guinot P, et al. Food Nutr Bull. 2012 Dec;33(4 Suppl):S381-9. doi: 10.1177/15648265120334S314. Food Nutr Bull. 2012. PMID: 23444719
- Optimization of Vitamin B1, B2, and B6 Absorption in Nang Tay Dum Floating Rice Grains. Nguyen TTL, Pham TMN, Ho TB, Ly-Nguyen B. Nguyen TTL, et al. Foods. 2024 Aug 23;13(17):2650. doi: 10.3390/foods13172650. Foods. 2024. PMID: 39272416 Free PMC article.
- Starch Characteristics and Amylopectin Unit and Internal Chain Profiles of Indonesian Rice ( Oryza sativa ). Mogoginta JG, Murai T, Annor GA. Mogoginta JG, et al. Foods. 2024 Jul 31;13(15):2422. doi: 10.3390/foods13152422. Foods. 2024. PMID: 39123613 Free PMC article.
- Market assessment of fortified parboiled rice in Burkina Faso. Durand-Morat A, Wang YJ, Bassole IHN, Nkengla-Asi L, Yang W. Durand-Morat A, et al. PLoS One. 2024 Mar 13;19(3):e0297674. doi: 10.1371/journal.pone.0297674. eCollection 2024. PLoS One. 2024. PMID: 38478539 Free PMC article.
- Effect of Germination on Mineral Content Changes in Brown Rice (Oryza sativa L.). Li X, Ma C, Bian X, Fu Y, Zhang G, Liu X, Zhang N. Li X, et al. Biol Trace Elem Res. 2024 Mar 12. doi: 10.1007/s12011-024-04147-y. Online ahead of print. Biol Trace Elem Res. 2024. PMID: 38472512
- The Association Between Vitamin B1 Deficiency and Anemia Among Elderly Patients at a Rural Hospital in Japan: A Cross-Sectional Study. Fukunaga T, Ohta R, Sano C. Fukunaga T, et al. Cureus. 2023 Oct 17;15(10):e47173. doi: 10.7759/cureus.47173. eCollection 2023 Oct. Cureus. 2023. PMID: 38021762 Free PMC article.
Publication types
- Search in MeSH
Related information
- Cited in Books
LinkOut - more resources
Full text sources.
- MedlinePlus Health Information
Research Materials
- NCI CPTC Antibody Characterization Program
Miscellaneous
- NCI CPTAC Assay Portal

- Citation Manager
NCBI Literature Resources
MeSH PMC Bookshelf Disclaimer
The PubMed wordmark and PubMed logo are registered trademarks of the U.S. Department of Health and Human Services (HHS). Unauthorized use of these marks is strictly prohibited.
An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
The PMC website is updating on October 15, 2024. Learn More or Try it out now .
- Advanced Search
- Journal List
- Cochrane Database Syst Rev
Food fortification with multiple micronutrients: impact on health outcomes in general population
Vitamins and minerals are essential for growth and maintenance of a healthy body, and have a role in the functioning of almost every organ. Multiple interventions have been designed to improve micronutrient deficiency, and food fortification is one of them.
To assess the impact of food fortification with multiple micronutrients on health outcomes in the general population, including men, women and children.
Search methods
We searched electronic databases up to 29 August 2018, including the Cochrane Central Register of Controlled Trial (CENTRAL), the Cochrane Effective Practice and Organisation of Care (EPOC) Group Specialised Register and Cochrane Public Health Specialised Register; MEDLINE; Embase, and 20 other databases, including clinical trial registries. There were no date or language restrictions. We checked reference lists of included studies and relevant systematic reviews for additional papers to be considered for inclusion.
Selection criteria
We included randomised controlled trials (RCTs), cluster‐RCTs, quasi‐randomised trials, controlled before‐after (CBA) studies and interrupted time series (ITS) studies that assessed the impact of food fortification with multiple micronutrients (MMNs). Primary outcomes included anaemia, micronutrient deficiencies, anthropometric measures, morbidity, all‐cause mortality and cause‐specific mortality. Secondary outcomes included potential adverse outcomes, serum concentration of specific micronutrients, serum haemoglobin levels and neurodevelopmental and cognitive outcomes. We included food fortification studies from both high‐income and low‐ and middle‐income countries (LMICs).
Data collection and analysis
Two review authors independently screened, extracted and quality‐appraised the data from eligible studies. We carried out statistical analysis using Review Manager 5 software. We used random‐effects meta‐analysis for combining data, as the characteristics of study participants and interventions differed significantly. We set out the main findings of the review in 'Summary of findings' tables, using the GRADE approach.
Main results
We identified 127 studies as relevant through title/abstract screening, and included 43 studies (48 papers) with 19,585 participants (17,878 children) in the review. All the included studies except three compared MMN fortification with placebo/no intervention. Two studies compared MMN fortification versus iodised salt and one study compared MMN fortification versus calcium fortification alone. Thirty‐six studies targeted children; 20 studies were conducted in LMICs. Food vehicles used included staple foods, such as rice and flour; dairy products, including milk and yogurt; non‐dairy beverages; biscuits; spreads; and salt. Fourteen of the studies were fully commercially funded, 13 had partial‐commercial funding, 14 had non‐commercial funding and two studies did not specify the source of funding. We rated all the evidence as of low to very low quality due to study limitations, imprecision, high heterogeneity and small sample size.
When compared with placebo/no intervention, MMN fortification may reduce anaemia by 32% (risk ratio (RR) 0.68, 95% confidence interval (CI) 0.56 to 0.84; 11 studies, 3746 participants; low‐quality evidence), iron deficiency anaemia by 72% (RR 0.28, 95% CI 0.19 to 0.39; 6 studies, 2189 participants; low‐quality evidence), iron deficiency by 56% (RR 0.44, 95% CI 0.32 to 0.60; 11 studies, 3289 participants; low‐quality evidence); vitamin A deficiency by 58% (RR 0.42, 95% CI 0.28 to 0.62; 6 studies, 1482 participants; low‐quality evidence), vitamin B2 deficiency by 64% (RR 0.36, 95% CI 0.19 to 0.68; 1 study, 296 participants; low‐quality evidence), vitamin B6 deficiency by 91% (RR 0.09, 95% CI 0.02 to 0.38; 2 studies, 301 participants; low‐quality evidence), vitamin B12 deficiency by 58% (RR 0.42, 95% CI 0.25 to 0.71; 3 studies, 728 participants; low‐quality evidence), weight‐for‐age z‐scores (WAZ) (mean difference (MD) 0.1, 95% CI 0.02 to 0.17; 8 studies, 2889 participants; low‐quality evidence) and weight‐for‐height/length z‐score (WHZ/WLZ) (MD 0.1, 95% CI 0.02 to 0.18; 6 studies, 1758 participants; low‐quality evidence). We are uncertain about the effect of MMN fortification on zinc deficiency (RR 0.84, 95% CI 0.65 to 1.08; 5 studies, 1490 participants; low‐quality evidence) and height/length‐for‐age z‐score (HAZ/LAZ) (MD 0.09, 95% CI 0.01 to 0.18; 8 studies, 2889 participants; low‐quality evidence). Most of the studies in this comparison were conducted in children.
Subgroup analyses of funding sources (commercial versus non‐commercial) and duration of intervention did not demonstrate any difference in effects, although this was a relatively small number of studies and the possible association between commercial funding and increased effect estimates has been demonstrated in the wider health literature. We could not conduct subgroup analysis by food vehicle and funding; since there were too few studies in each subgroup to draw any meaningful conclusions.
When we compared MMNs versus iodised salt, we are uncertain about the effect of MMN fortification on anaemia (R 0.86, 95% CI 0.37 to 2.01; 1 study, 88 participants; very low‐quality evidence), iron deficiency anaemia (RR 0.40, 95% CI 0.09 to 1.83; 2 studies, 245 participants; very low‐quality evidence), iron deficiency (RR 0.98, 95% CI 0.82 to 1.17; 1 study, 88 participants; very low‐quality evidence) and vitamin A deficiency (RR 0.19, 95% CI 0.07 to 0.55; 2 studies, 363 participants; very low‐quality evidence). Both of the studies were conducted in children.
Only one study conducted in children compared MMN fortification versus calcium fortification. None of the primary outcomes were reported in the study.
None of the included studies reported on morbidity, adverse events, all‐cause or cause‐specific mortality.
Authors' conclusions
The evidence from this review suggests that MMN fortification when compared to placebo/no intervention may reduce anaemia, iron deficiency anaemia and micronutrient deficiencies (iron, vitamin A, vitamin B2 and vitamin B6). We are uncertain of the effect of MMN fortification on anthropometric measures (HAZ/LAZ, WAZ and WHZ/WLZ). There are no data to suggest possible adverse effects of MMN fortification, and we could not draw reliable conclusions from various subgroup analyses due to a limited number of studies in each subgroup. We remain cautious about the level of commercial funding in this field, and the possibility that this may be associated with higher effect estimates, although subgroup analysis in this review did not demonstrate any impact of commercial funding. These findings are subject to study limitations, imprecision, high heterogeneity and small sample sizes, and we rated most of the evidence low to very low quality. and hence no concrete conclusions could be drawn from the findings of this review.
Plain language summary
Impact of food fortification with multiple micronutrients on health
Review question Does multiple micronutrient fortification improve health?
Background Vitamins and minerals are important for growth and body functioning. Micronutrient deficiencies are common in many populations, and food fortification is one of the interventions to reduce the burden of micronutrient deficiencies and improve health in the general population. Food fortification involves adding micronutrients to processed foods. There have been studies with various single micronutrient fortification, dual micronutrient fortification and multiple micronutrient fortification, including zinc, iron, selenium, vitamin A, vitamin B complexes, vitamin C and vitamin E. We reviewed the evidence about the impact of food fortification with multiple micronutrients (MMNs) on health in the general population.
Study characteristics We included 43 studies (48 papers) in 19,585 participants (17,878 children) in this review. The evidence is current to August 2018. Most of the included studies assessed the impact of food fortification with MMN compared to placebo or to no intervention; two studies compared food fortification with MMN to iodised salt and one study compared food fortification with MMN to food fortification with calcium alone. Most of the studies (36 out of 43) targeted children. Twenty studies were conducted in developing countries. Food used for fortification included staple foods, such as rice and flour; dairy products, including milk and yogurt; non‐dairy beverages; biscuits; spreads; and salt. A high proportion of studies were funded by commercial sources (e.g. manufacturers of micronutrients), which can be associated with finding more beneficial effects than independently‐funded studies.
Key results Food fortification with MMN may reduce anaemia by 32%, iron deficiency anaemia by 72%, micronutrient deficiencies (including iron deficiency by 56%, vitamin A deficiency by 58%, vitamin B2 deficiency by 64%, vitamin B6 deficiency by 91% and vitamin B12 deficiency by 58%). MMN fortification may also improve child growth measured as weight for age and weight for height/length. We are uncertain of the effect of MMN fortification on zinc deficiency and child growth measured as height/length for age. The included studies did not report on any side effects associated with MMN fortification, including deaths and diseases. We are uncertain of the effect of food fortification with MMN compared to iodised salt for iron deficiency anaemia and vitamin A deficiency.
Quality of the evidence The quality of the evidence was low to very low, due to limitations in the study methods that could introduce a risk of bias, high heterogeneity (variation in the results from study to study), and small sample sizes.
Although the review suggests some positive effects of MMN fortification compared to no intervention, a number of other factors should also be considered. Firstly, there is no information on possible side effects of the MMN fortification. Secondly, we could not perform various subgroup analyses to identify whether MMN fortification is more effective in different population groups, food vehicles, dosage, duration of intervention and geographical region, due to limited number of studies in each subgroup. We performed a subgroup analysis to compare commercial and non‐commercially‐funded studies and did not find a significant difference between their results, although we remain cautious about these findings. Our results are uncertain, due to the low quality of the evidence.
Summary of findings
Description of the condition.
Vitamins and minerals are essential for growth and maintenance of a healthy body, and their deficiencies can lead to various diseases. Micronutrient deficiencies account for a substantial global burden of disease, with iron and vitamin A deficiency being among the 15 leading causes of global morbidity and mortality ( WHO 2002 ). Globally about 1.62 billion people are anaemic, with the highest prevalence among preschool children followed by pregnant women ( Benoist 2008 ). Iron, iodine, folate, vitamin A, and zinc deficiencies are the most widespread micronutrient deficiencies, and all of these are common contributors to poor growth, intellectual impairments, perinatal complications, and increased risk of morbidity and mortality ( Bailey 2015 ). In 2014, iron deficiency anaemia was one of the three most common causes of disability‐adjusted life years (DALYs) lost among adolescents along with other micronutrient deficiencies accounting for over 2500 DALYs per 100,000 adolescents ( Akseer 2017 ; WHO 2014 ). About 190 million preschool children and 19.1 million pregnant women are vitamin A‐deficient ( WHO 2009 ). Iodine and zinc deficiencies are estimated to affect 29% and 17% respectively of the world’s population ( Black 2013 ), with approximately 82% of pregnant women worldwide having inadequate zinc intake. Prevalence of suboptimal body stores of vitamins B6 and B12 have also been reported ( McLean 2008 ). Populations from developing countries are believed to be most affected, with multiple micronutrient (MMN) deficiencies frequently co‐existing among more than two billion people affected ( Bailey 2015 ; Best 2011 ; Dijkhuizen 2001 ; Ramakrishnan 2002 ; Stanger 2009 ).
Micronutrient deficiencies can result in impairments in mental and physical growth and development, and immune competence. They may also adversely affect reproductive outcomes ( Gibson 2002 ; Haimi 2014 ; Viteri 2002 ). MMN deficiencies are associated with increased incidence and severity of infectious illness and mortality from diarrhoea, measles, malaria and pneumonia ( Ibrahim 2017 ). In preschool children, zinc deficiency has been associated with an increased risk of diarrhoea, malaria and pneumonia, while vitamin A deficiency is associated with increased risk of mortality due to diarrhoea ( Black 2008 ; Black 2013 ). The prevalence of iron deficiency anaemia during pregnancy is a risk factor for maternal mortality ( Allen 2008 ).
Several strategies have been implemented to combat micronutrient deficiencies, including exclusive breastfeeding during the first six months of life, nutrition education, food rationing, control of parasitic infections and nutritional supplementation ( Bhutta 2008 ; Bhutta 2013 ). Food fortification is one of these strategies, in which a variety of micronutrient combinations can be added to foods, including zinc, iron, selenium, vitamin A, vitamin B complexes, vitamin C, vitamin D and vitamin E ( Allen 2006 ).
Description of the intervention
Food fortification adopts an integrated approach and provides support to reduce micronutrients malnutrition when other existing food supplies fail to do so ( Allen 2006 ). Food fortification is the process by which micronutrients are added to processed foods and has been applied at various levels and directed to different age groups ( De Lourdes Samaniego‐Vaesken 2012 ; Allen 2006 ). A range of micronutrient combinations has been used to fortify foods. There have been studies with various single micronutrient fortifications, dual micronutrient fortification and up to 20 micronutrient fortifications, including zinc, iron, selenium, vitamin A, vitamin B complexes, vitamin C and vitamin E. The advantage of fortification of food items consumed by the general population is that no or minimal behaviour change is required on the part of the population ( Serdula 2010a ; Serdula 2010b ). This provides an advantage in terms of coverage and efficiency. Food fortification could potentially also be cost‐effective, as it can be targeted at different age groups at a time ( Hurrell 1997 ; Lotfi 1996 ; Allen 2006 ). In contrast, supplementation depends upon a viable delivery mechanism and the availability and access to the intended individuals ( Harrison 2010 ). For fortification, however, issues related to safe and effective levels of micronutrients being used, the relevance of the micronutrients and appropriate food vehicle need to be considered ( Allen 2006 ; Allen 2008 ).
One of the issues concerning food fortification is that many of the these studies are funded by the manufacturers of fortified food products. The source of funding could be one of the biases in studies evaluating nutrition interventions if the researchers have a vested interest in the outcomes of the research. Industry‐funded research might skew the evidence towards solutions that favour industry interests by focusing on food components that can be manipulated and marketed by food companies ( Fabbri 2018 ). These concerns should therefore be explicitly evaluated when considering the evidence on food fortification.
How the intervention might work
Fortification could be mass fortification (that is, adding micronutrients to foods that are commonly consumed, such as flour, salt, sugar and cooking oil), or point‐of‐use fortification, which involves adding single‐dose packets of vitamins and minerals in powder form that can be sprinkled onto any ready‐to‐eat food consumed at home, school, nurseries, refugee camps or any other place where possible ( WHO 2014 ; Zlotkin 2005 ). For this review, we focus only on mass food fortification. Food fortification can combat micronutrient deficiencies at the population level at reasonable cost, making it a very efficient public health intervention.
Many trials have shown the positive impact of food fortification. Sazawal 2007 showed that milk fortified with multiple micronutrients reduced the odds for days with severe illnesses by 15% (95% confidence interval (CI) 5% to 24%), the incidence of diarrhoea by 18% (95% CI 7% to 27%) and the incidence of acute lower respiratory illness by 26% (95% CI 3% to 43%) in children. Another study by Osei 2010 reported improvement in vitamin A, vitamin B12, folate and total body iron status after fortification of school meals in a village in India. A review by Aaron 2015 reported a reduction in risk of anaemia (relative risk (RR) 0.58, 95% CI 0.29 to 0.88), iron deficiency (RR 0.34, 95%CI 0.21 to 0.55), and iron deficiency anaemia (RR 0.17, 95% CI 0.06 to 0.53) after use of fortified beverages in school‐aged children.
Concerns exist that food fortification may result in unacceptably high micronutrient levels among those consuming higher amounts of fortified foods. However, exceeding the upper intake level has not been shown to increase the risk of adverse effects, according to a review of data from national surveys conducted in European countries ( Hennessy 2013 ).
Why it is important to do this review
Several questions remain about the use of fortified foods. Many reviews to evaluate the use of fortified foods have suggested benefit, but usually focus on a particular food vehicle or a subset of the general population. A review by Eichler 2012 focused on MMN‐fortified dairy and cereal products delivered to pre‐school children in developing countries, and showed it to be effective in reducing anaemia. Das 2013 focused on the use of MMN‐fortified foods for women and children only. There is no evidence to suggest which combinations work best and whether certain combinations may work better with particular food vehicles. It is also unclear which population subgroups may derive the most benefit from these interventions and under what conditions.
This review serves as a comprehensive assessment of the effect of MMN fortification on the population as a whole, without being limited to certain age groups, regions or a particular food vehicle. Reviews of home or point‐of‐use fortification of food through micronutrient powders ( De‐Regil 2011 ; Salam 2013 ), and ready‐to‐use therapeutic food (RUTF) ( Schoonees 2019 ) already exist, and we have not focused on these areas.
To assess the impact of food fortification with MMNs on health outcomes in the general population, including men, women and children.
Criteria for considering studies for this review
Types of studies.
We have included:
- Randomised controlled trials (RCTs);
- Quasi‐randomised trials;
- Cluster‐RCTs (c‐RCTs);
- Controlled before‐after (CBA) studies;
- Interrupted time series (ITS) studies.
We intended to include quasi‐experimental study designs, CBA and ITS, along with RCTs, since we planned to assess the effectiveness of large‐scale programme evaluations that might not have been conducted in a randomised fashion. We applied no language or publication status restrictions.
Types of participants
We included studies that assess the effects of food fortification in the general population, including men, women and children. We also included studies that targeted fortification in specific populations (e.g. older people, pregnant women, women of reproductive age, and children at school through institutions such as schools or care facilities). We included studies from all countries, regardless of their level of income and development.
We excluded studies conducted among some special population groups, including critically‐ill people, anaemic people or people diagnosed with any specific diseases.
Types of interventions
Intervention: MMN fortification (three or more micronutrients) by any food vehicle, compared with a single micronutrient or no fortification.
We have not included studies evaluating point‐of‐use or home fortification of foods, therapeutic blended food or food supplementation.
Types of outcome measures
Primary outcomes.
- Anaemia (defined as haemoglobin (Hb) concentration < 11 g/dL)
- Iron‐deficiency anaemia (defined as Hb concentration < 11 g/dLwith serum ferritin < 15 µg/l)
- Deficiency of specific micronutrients (iron, zinc, vitamin A, B vitamins) (as defined by the World Health Organization (WHO) micronutrient deficiency cut‐offs)
- Anthropometric outcomes (e.g. incidence of stunting (defined as below minus two standard deviations from median height for age of reference population), wasting (defined as below minus two standard deviations from median weight for height of reference population) and underweight (defined as below minus two standard deviations from median weight for age of reference population)
- Morbidity (e.g. infectious diseases such as pneumonia, sepsis and diarrhoea)
- All‐cause mortality (defined as death due to any cause)
- Cause‐specific mortality (as defined by study authors) due to pneumonia, diarrhoea or malaria
Secondary outcomes
- Potential adverse outcomes (as defined by study authors)
- Serum haemoglobin levels (measured as g/dL)
- Serum concentration of specific micronutrients (folate, ferritin, vitamin A, B vitamins, zinc)
- Neuro‐developmental and cognitive outcomes
Search methods for identification of studies
Electronic searches.
We searched the following electronic databases for primary studies, without date or language restrictions. We conducted the final search on 29 August 2018.
- Cochrane Central Register of Controlled Trials (CENTRAL; 2018), in the Cochrane Library, including the Cochrane Effective Practice and Organisation of Care (EPOC) Group Specialised Register and Cochrane Public Health Specialised Register (searched 29 August 2018);
- MEDLINE and MEDLINE(R) In‐Process; and Other Non‐Indexed Citations Ovid (searched 29 August 2018);
- PubMed for the most recent six months to identify records that are 'Epub ahead of print'; (searched 29 August 2018);
- Embase Ovid (1974 to August 2018) (searched 29 August 2018);
- Cumulative Index to Nursing and Allied Health Literature (CINAHL) Plus EBSCOhost; 1937 to August 2018 (searched 29 August 2018);
- PsycINFO (www.apa.org/pubs/databases/index; searched 30 August 2018);
- Education Resources Information Center (ERIC) (eric.ed.gov/ searched 22 August 2018);
- Latin American and Caribbean Health Sciences Literature (LILACS); (lilacs.bvsalud.org/en; searched 29 August 2018);
- AGRIS (aims.fao.org/search/node searched 30 August 2018);
- Science Citation Index and Social Sciences Citation Index Web of Science (SCI and SSCI; 1970 to August 2018);
- Food Science and Technology Abstracts (www.ebsco.com/products/research‐databases/fsta‐food‐science‐and‐technology‐abstracts searched 31 August 2018);
- AgriCOLA (agricola.nal.usda.gov/; searched 28 August 2018);
- Global Index Medicus ‐ AFRO (indexmedicus.afro.who.int/ searched 31 August 2018);
- EMRO (www.emro.who.int/index.html searched 31 August 2018);
- Pan American Health library (PAHO)/WHO Institutional Repository for Information Sharing (iris.paho.org/xmlui; searched 30 August 2018);
- WHOLIS Global Index Medicus (WHO Library Database; search.bvsalud.org/ghl/?lang=en&submit=Search&where=REGIONAL; searched 29 August 2018);
- WPRO (https://www.who.int/library/databases/wpro/en/ searched 29 August 2018);
- Global Index Medicus (Index Medicus for the South‐East Asian Region; IMSEAR) (search.bvsalud.org/ghl/?lang=en&submit=Search&where=REGIONAL; searched 31 August 2018);
- 3ie Database of Impact studies (www.3ieimpact.org/evidence‐hub/impact‐evaluation‐repository; searched 29 August 2018);
- EPPI centre databases ‐ DoPHER (eppi.ioe.ac.uk/webdatabases4/Intro.aspx?ID=9 ) and TROPHI (eppi.ioe.ac.uk/webdatabases4/Intro.aspx?ID=12) searched 29 August 2018;
- OpenGrey (www.opengrey.eu/ searched 30 August 2018);
- Index to Conference Proceedings (mjl.clarivate.com/scope/scope_cpci‐s/ searched 30 August 2018);
- ClinicalTrials.gov (clinicaltrials.gov/), and WHO International Clinical Trials Registry Platform (ICTRP; www.who.int/ictrp/en/) (searched 30 August 2018).
We adapted the MEDLINE search strategy ( Appendix 1 ) for use in the other databases using the appropriate controlled vocabulary as applicable ( Appendix 2 ; Appendix 3 ; Appendix 4 ; Appendix 5 ; Appendix 6 ). We handsearched the journals and the proceedings of major relevant conferences relating to food and nutrition, including Micronutrient Forum, Nutrition and Food Sciences and Hidden Hunger. We also handsearched the journals in which the included studies appeared most frequently. We searched the top five journals (according to the number of included studies provided) for the previous 12 months.
Searching other resources
We checked the reference lists of included studies and relevant systematic reviews for additional papers to consider for inclusion.
Selection of studies
Two review authors (SBM and KM) independently assessed all the studies identified potentially for inclusion. We resolved any disagreement through discussion or, if required, consulted a third review author (RAS or JKD).
Data extraction and management
We designed a form for the extraction of data. Two of the review authors (from SBM, AM, ZL, KM and RK) extracted the data from each eligible study using the agreed form. We resolved discrepancies through discussion or, when required, consulted a third review author (JKD or RAS). We entered data into Review Manager 5 ( RevMan 2014 ), and checked them for accuracy by double data entry (SBM, ZL and RAS), having one review author entering data into a separate file and comparing the results. For the studies that reported outcomes at multiple time points, we extracted data for each time point and reported the outcomes at the last reported time period.
We used the PROGRESS checklist (place, race, occupation, gender, religion, education, socioeconomic status, social status) ( O’Neill 2014 ; Welch 2016 ) to record whether outcome data were reported by socio‐demographic characteristics known to be important from an equity perspective. We also recorded whether studies included specific strategies to address diversity or disadvantage. Where available, we extracted data on costs and process/implementation and source of funding of the primary studies. These details are presented in the Characteristics of included studies tables.
Assessment of risk of bias in included studies
Two review authors (from SBM, RAS, JKD and RK) independently assessed risks of bias (RoB) for each study using the criteria outlined in the Cochrane Handbook for Systematic Reviews of Interventions ( Higgins 2017 ), with the exception that we conducted 'Risk of bias' assessments at the level of each study, and not for individual outcomes. We used the Cochrane Effective Practice and Organisation of Care ( EPOC 2017 ) nine‐point criteria for non‐RCTs and CBA studies to determine the quality of all eligible studies. We did not find any eligible studies using an ITS study design. We resolved any disagreement by discussion or by involving a third assessor. We report the risks of bias for each study in the Characteristics of included studies table. We did not exclude studies on the grounds of their quality, but clearly report methodological quality when presenting the results of the studies.
Random sequence generation (checking for possible selection bias)
We have described for each included study the method used to generate the allocation sequence in sufficient detail to allow an assessment of whether it should produce comparable groups. We have assessed the methods as: • low risk of bias (any truly random process, e.g. random‐number table, computer random‐number generator); • high risk of bias (any non‐random process, e.g. odd or even date of birth, hospital or clinic record number); • unclear risk of bias.
Allocation concealment (checking for possible selection bias)
We have described for each included study the method used to conceal allocation to interventions prior to assignment and assessed whether intervention allocation could have been foreseen in advance of or during recruitment, or changed after assignment. We have assessed the methods as: • low risk of bias (e.g. telephone or central randomisation, consecutively‐numbered sealed opaque envelopes); • high risk of bias (open random allocation, unsealed or non‐opaque envelopes, alternation, date of birth); • unclear risk of bias.
Blinding of participants and personnel (checking for possible performance bias)
We have described for each included study the methods used, if any, to blind study participants and personnel from knowledge of which intervention a participant received. We have considered that studies are at low risk of bias if they were blinded, or if we judge that the lack of blinding would be unlikely to affect results. We have assessed blinding separately for different outcomes or classes of outcomes. We have assessed the methods as: • low, high or unclear risk of bias for participants; • low, high or unclear risk of bias for personnel.
Blinding of outcome assessment (checking for possible detection bias)
We have described for each included study the methods used, if any, to blind outcome assessors from knowledge of which intervention a participant received. We have assessed blinding separately for different outcomes or classes of outcomes. We have assessed methods used to blind outcome assessment as: • low, high or unclear risk of bias.
Incomplete outcome data (checking for possible attrition bias due to the amount, nature and handling of incomplete outcome data)
We have described for each included study, and for each outcome or class of outcomes, the completeness of data including attrition and exclusions from the analysis. We have stated whether attrition and exclusions were reported and the numbers included in the analysis at each stage (compared with the total randomised participants), reasons for attrition or exclusion where reported, and whether missing data were balanced across groups or were related to outcomes. We considered studies with more than 20% loss to follow‐up, or with an imbalanced loss to follow‐up in different groups, to have insufficient completeness of outcome data. We also looked at the amount and distribution across intervention groups and the reasons for outcomes being missing. Where sufficient information was reported, or was supplied by the trial authors, we have re‐included missing data in the analyses which we undertook. We assessed methods as: • low risk of bias (e.g. no or minimal missing outcome data, missing outcome data balanced across groups); • high risk of bias (e.g. numbers or reasons for missing data imbalance across groups, ’as treated’ analysis done with substantial departure of intervention received from that assigned at randomisation); • unclear risk of bias.
Selective reporting (checking for reporting bias)
We have described for each included study how we investigated the possibility of selective outcome reporting bias and what we found. We have assessed the methods as: • low risk of bias (where it is clear that all of the study’s prespecified outcomes and all expected outcomes of interest to the review have been reported); • high risk of bias (where not all the study’s prespecified outcomes have been reported, one or more reported primary outcomes were not prespecified, outcomes of interest are reported incompletely and so cannot be used, study fails to include results of a key outcome that would have been expected to have been reported); • unclear risk of bias.
We have described for each included study any important concerns we have about other possible sources of bias. We have assessed whether each study was free of other problems that could put it at risk of bias: • low risk of other bias; • high risk of other bias; • unclear whether there is a risk of other bias.
Additional criteria for cluster‐RCTs
We assessed and report five additional criteria for all the included c‐RCTs in the section of 'Other bias' in the RoB table. These are recruitment bias; baseline imbalance; loss of clusters; incorrect analysis; and comparability with individually randomised trials.
Recruitment bias
We have assessed whether the individuals were recruited to the trial after the clusters have been randomised. We have assessed the methods as:
- low, high or unclear risk of bias.
Baseline imbalance
We have assessed the reporting of the baseline comparability of clusters, or statistical adjustment for baseline characteristics. We have assessed the methods as:
Loss of clusters
we have assessed whether there was any loss of complete clusters or omission of complete clusters from the analysis. We have assessed the methods as:
Incorrect analysis
We have assessed whether appropriate analysis has been conducted for adjusting the clustering. We have assessed the methods as:
Comparability with individually randomised trial
We assessed the possible differences between the intervention effects in individually‐randomised and cluster‐randomised trails. We have assessed the methods as:
Overall risk of bias
We have made explicit judgments about whether studies are at high risk of bias, according to the criteria given in the Cochrane Handbook for Systematic Reviews of Interventions ( Higgins 2017 ). We assessed the likely magnitude and direction of the bias and whether we considered it likely to have had an impact on the findings. We judged the studies to be at overall high risk of bias if they were at high or unclear risk for any of the four criteria of allocation concealment, blinding of participants and personnel, blinding of outcome assessment or incomplete outcome data. We explored the impact of the level of bias by conducting sensitivity analyses for those studies with high or unclear risk of bias in any of the above four domains i.e. according to the method and adequacy of allocation concealment; blinding status of the participants/personnel and outcome assessor; or percentage lost to follow‐up or attrition of 20% or more, or with an imbalanced loss to follow‐up in different groups.
Measures of treatment effect
Dichotomous data.
For dichotomous data, we present results as a summary risk ratio (RR) with a 95% confidence interval (CI).
Continuous data
For continuous data, we have used the mean difference (MD) if outcomes were measured in the same way between trials. We would have used the standardised mean difference (SMD) to combine trials that measured the same outcome but with different methods. Where the studies reported change in continuous outcomes and did not report endline values, we combined these data using the MD.
Unit of analysis issues
Cluster‐randomised trials.
We have included cluster‐randomised trials in the analyses along with individually‐randomised trials. We used cluster‐adjusted estimates from c‐RCTs where available. If the studies had not adjusted for clustering, we attempted to adjust their standard errors using the methods described in the Cochrane Handbook for Systematic Reviews of Interventions ( Higgins 2019 ), using an estimate of the intra‐cluster correlation coefficient (ICC) derived from the trial. If the trial did not report the cluster‐adjusted estimated or the ICC, we imputed an ICC from a similar study included in the review, adjusting if the nature or size of the clusters was different (e.g. households compared to classrooms). We assessed any imputed ICCs using sensitivity analysis. We considered it reasonable to combine the results from both if there was little heterogeneity between the study designs and if we considered the interaction between the effect of intervention and the choice of randomisation unit to be unlikely. We have also acknowledged heterogeneity in the randomisation unit and performed a subgroup analysis to investigate the effects of the randomisation unit.
Studies with more than two treatment groups
When we identified studies with more than two intervention groups (multi‐arm studies), we combined groups to create a single pair‐wise comparison or used the methods set out in the Cochrane Handbook for Systematic Reviews of Interventions ( Higgins 2019 ) to avoid double‐counting study participants. For the subgroup analyses, when the control group was shared by two or more study arms, we divided the control group (events and total population) over the number of relevant subgroups to avoid double‐counting the participants.
Dealing with missing data
We have described missing data, including dropouts. Differential dropout rates can lead to biased estimates of the effect size, and bias may arise if the reasons for dropping out differ across groups. We have reported the reasons for dropout. If data were missing for some cases, or if the reasons for dropping out were not reported, we have contacted the authors. For included studies, we have noted levels of attrition. We have explored the impact of including studies with high levels of missing data in the overall assessment of treatment effect by using sensitivity analysis. For all outcomes, we have carried out analyses, as far as possible, on an intention‐to‐treat basis, i.e. we attempted to include all participants randomised to each group in the analyses, and all participants were analysed in the group to which they were allocated, regardless of whether or not they received the allocated intervention. The denominator for each outcome in each trial was the number randomised minus any participants whose outcomes were known to be missing.
Assessment of heterogeneity
We assessed the included studies for clinical, methodological, and statistical heterogeneity. We assessed clinical heterogeneity by comparing the distribution of important factors, such as the study participants, study setting, dose and duration of the intervention and co‐interventions. We evaluated methodological heterogeneity on the basis of factors such as the method of sequence generation, allocation concealment, blinding of outcome assessment, and losses to follow‐up. We have assessed statistical heterogeneity in each meta‐analysis using the T 2 , I 2 and Chi 2 statistics. We regard heterogeneity as substantial if I 2 was greater than 30% and either T 2 was greater than zero, or there was a low P value (< 0.10) in the Chi 2 test for heterogeneity.
Assessment of reporting biases
If there were 10 or more studies in the meta‐analysis we investigated reporting biases (such as publication bias) using funnel plots. We have assessed funnel plot asymmetry visually, and if asymmetry was visually apparent in any of the plots, we attempted to investigate it through sensitivity analysis (where possible) and compared the random‐effects with the fixed‐effect model. We considered non‐reporting bias as one of the possible explanations of the funnel plot asymmetry.
Data synthesis
We carried out the statistical analysis using the Review Manager 5 software. We categorised the studies into the following three comparisons:
- MMN fortification versus placebo or no intervention;
- MMN fortification versus iodised salt;
- MMN fortification versus calcium only fortification.
We used random‐effects meta‐analysis for combining data, as the characteristics of study participants and interventions differed significantly. We present the results as the average treatment effect with a 95% confidence interval, and the estimates of T 2 and I 2 . We used the Mantel‐Haenszel method for dichotomous data, the inverse variance for continuous data and generic Inverse variance for synthesis including data originating from c‐RCTs. We synthesised the findings from the RCTs and c‐RCTs together but did not pool non‐randomised studies, as we judged them to be too few in number. We have reported the findings from the non‐randomised studies separately.
GRADE and 'Summary of findings' tables
We have set out the findings of the primary outcomes in 'Summary of findings' tables, prepared using the GRADE approach ( Guyatt 2008 ) and using GRADE profiler software ( GRADEpro ). We have listed the outcomes for each comparison with estimates of relative effects along with the number of participants and studies contributing data for those outcomes. For each individual outcome, we assessed the quality of the evidence using the GRADE approach, which involves consideration of within‐study risk of bias (methodological quality), directness of evidence, heterogeneity, precision of effect estimates and risk of publication bias. We have rated the quality of the body of evidence for each key outcome as high, moderate, low or very low. We present these findings according to the standardised language adapted from Glenton 2010 .
Subgroup analysis and investigation of heterogeneity
We have conducted subgroup analyses on the basis of the following, where data permitted (where there were at least three studies in each subgroup):
- population (children, women of reproductive age, adults)
- baseline micronutrient status (malnourished, normal)
- various combination of MMNs (e.g. different numbers and types of micronutrients used)
- low‐to‐middle income countries versus high‐income countries
- duration of intervention (zero to six months, six to 12 months, more than 12 months)
- food vehicle used for fortification
- studies with and without commercial funding
We assessed differences between subgroups by interaction tests, and by inspection of the subgroups' CIs; non‐overlapping CIs indicate a statistically significant difference in treatment effect between the subgroups. Inferences for clinical relevance were based on these subgroup analyses, where possible. This ensured that the review's conclusions considered specific contextual factors in relation to food vehicle, target population and dose, among others.
Sensitivity analysis
We performed sensitivity analyses to examine the effect of removing studies at high overall risk of bias (those with high or unclear risk of bias according to the method and adequacy of allocation concealment, blinding status of the participants, or percentage lost to follow‐up, or attrition of 20% or more, or with an imbalanced loss to follow‐up in different groups).
Description of studies
See Characteristics of included studies ; Characteristics of excluded studies ; Additional tables.
Results of the search
We identified 5789 records, of which we screened 126 full texts and included 43 studies (from 48 papers) with 19,585 participants (17,878 children) in the review ( Figure 1 ).

Study flow diagram showing results of the literature search.
Included studies
Types of studies We include 43 studies which meet the eligibility criteria. Most of the included studies (39) were RCTs, of which six studies were c‐RCTs ( DeGier 2016 ; Liu 1993 ; Perignon 2016 ; Rahman 2015 ; Vinodkumar 2009 ; Wang 2017 ). There were four CBA studies ( Abrams 2003 ; Adams 2017 ; Azlaf 2017 ; Mardones 2007 ).
Participants and Settings
Thirty‐six of the studies were conducted among children. Most of these (29) were conducted among pre‐school and school‐aged children ( Aaron 2011 ; Abrams 2003 ; Adams 2017 ; Ash 2003 ; Azlaf 2017 ; DeGier 2016 ; Economos 2014 ; Hieu 2012 ; Hyder 2007 ; Jinabhai 2001 ; Lopriore 2004 ; Nga 2009 ; Osendarp 2007 ; Perignon 2016 ; Petrova 2019 ; Pinkaew 2013 ; Pinkaew 2014 ; Powers 2016 ; Rahman 2015 ; Sazawal 2013 ; Solon 2003 ; Taljaard 2013 ; Thankachan 2012 ; Thankachan 2013 ; Van Stuijvenberg 1999 ; Vaz 2011 ; Vinodkumar 2009 ; Wang 2017 ; Zimmerman 2004 ). Four studies included infants aged from six months to 12 months ( Faber 2005 ; Gibson 2011 ; Liu 1993 ; Oelofse 2003 ), and three studies included children aged one to three years ( Nesamvuni 2005 ; Sazawal 2007 ; Villalpando 2006 ). Three studies targeted pregnant women ( Järvenpaa 2007 ; Mardones 2007 ; Tatala 2002 ), three studies targeted adults ( Tapola 2004 ; Tucker 2004 ; Van het Hof 1998 ), while one study targeted an elderly population aged over 70 years ( Chin A Paw 2000 ).
Twenty studies were conducted in lower‐middle income‐countries (LMICs) ( Aaron 2011 ; Adams 2017 ; Ash 2003 ; Azlaf 2017 ; DeGier 2016 ; Gibson 2011 ; Hieu 2012 ; Hyder 2007 ; Nga 2009 ; Perignon 2016 ; Rahman 2015 ; Sazawal 2007 ; Sazawal 2013 ; Solon 2003 ; Tatala 2002 ; Thankachan 2012 ; Thankachan 2013 ; Vaz 2011 ; Vinodkumar 2009 ; Zimmerman 2004 ), 13 in upper‐middle‐income countries (UMICs) ( Abrams 2003 ; Faber 2005 ; Jinabhai 2001 ; Liu 1993 ; Lopriore 2004 ; Nesamvuni 2005 ; Oelofse 2003 ; Pinkaew 2013 ; Pinkaew 2014 ; Taljaard 2013 ; Van Stuijvenberg 1999 ; Villalpando 2006 ; Wang 2017 ) and nine in high‐income countries (HICs) ( Chin A Paw 2000 ; Economos 2014 ; Järvenpaa 2007 ; Mardones 2007 ; Petrova 2019 ; Powers 2016 ; Tapola 2004 ; Tucker 2004 ; Van het Hof 1998 ). One trial was conducted in both LMICs and HICs ( Osendarp 2007 ).
We conducted a descriptive analysis of the PROGRESS‐Plus factors reported by included trials. We present an account of this analysis in Table 3 . Most trials did not directly report on these factors. The analysis suggests that equity‐related variables and analysis are commonly overlooked by trials, thus affecting the generation of evidence on how inequities are identified and how interventions can contribute to mitigate or reduce them. We present all PROGRESS‐Plus factors reported by the included trials in Table 4 .
| No. studies conducted in low‐middle‐income countries (LMICs): 20 No. studies conducted in high‐income countries (HICs): 16 One in both LMIC and HIC All with community‐dwelling individuals | |
| Most race and ethnicity categories were self‐reported. This category was scarcely reported in included studies (published data). In all, 34 studies did not report any data related to race or ethnicity. Three studies vaguely described ethnicity as: "Wagogo and Wakaguru people" ( ), "Farmers" ( ) and "mixed Berber and Arab descent" ( ) The lack of a representative sample by race/ethnicity impacts on the risk of bias in those studies that report this factor, and it is unknown in those that do not None of the studies reported on cultural or language variables | |
| This is one of the most under‐reported categories in the studies. None of the included studies reported on this factor, except for three studies indicating that some of their participants worked either as farmers, rice farmers or construction workers, skilled labourers, or garment factory workers ( ; ; ; ) | |
| This category was reported in almost every study, although some did not provide the specific distribution of the sample by sex when participants from both sexes were included. Most studies were conducted with children, but some also included adolescents, pregnant women and adults | |
| This is one of the most under‐reported categories in the studies. Only two studies mentioned that the participants were Muslims ( ; ) | |
| Two studies mentioned that the participants had primary level of education ( ; ); however, many studies were carried out in school settings | |
| This factor was mostly poorly reported or not reported at all in the published data of the included studies. 12 studies did report this factor with various degrees of detail: specified that the families were low‐income urban families; ; ; ; ; and specified that the participants had low socio‐economic status; mentioned that the participants were middle income; specified that the participants were Saharawi refugees; included participants from two different countries (One HIC: Australia, and other LMIC: Indonesia) and specified that the participants form Australia had higher socio‐economic status while those from Indonesia had middle to low socio‐economic status; mentioned that the participants belonged to poor per‐urban communities, while specified that the families had a monthly income of less than INR 2000 (USD 50) | |
| No study directly reported any measurement of social capital. Indirectly, some studies reported that participants were recruited through primary schools, non‐formal schools, housing complexes and home‐care organisations, thus indicating that participants had at least one social connection or network | |
| All studies reported on age, as this factor is essential for their analysis. Many also reported the participants’ Body Mass Index (BMI). Despite this being collected, the studies did not examined BMI from a social determinants of health perspective. Some studies reported other behavioural factors such as smoking, alcohol intake and physical activity. Studies including children also reported parent education, occupation, children's anthropometric status (stunted, wasted, underweight), infections, water source, cooking fuel and toilet use, although very infrequently | |
| Most studies recruited their participants through similar strategies: schools, mailings, printed ads and flyers distributed in university campuses, community centres, prenatal clinics or through advertisement on local radio and television. This strategy influences the composition of the samples and explains why many of them are not representative at population level. Most of the studies took place in LMICs and in children, hence the use of schools and community centres; however, this strategy may leave out literate individuals who are less connected to organisations and schools or with less access to newspapers and other written outlets |
HIC: high‐income countries; LMIC: low middle income countries
| LMIC (Nigeria) | ‐ | agrarian communities | Children (5 to 13 years) | ‐ | Primary | ‐ | Government‐operated primary schools | Household size, water source, cooking fuel, toilet type, maternal education and occupation, paternal education and occupation | Government‐operated primary schools | |
| UMIC (Botswana) | ‐ | ‐ | Children (5 to 11 years | ‐ | Primary | Lower‐income urban families | Public schools | Age BMI | Public schools | |
| LMIC (Bangladesh) | ‐ | ‐ | Children (6 to 11 years) | ‐ | Primary | Rural disadvantaged districts | Primary schools | ‐ | Primary schools | |
| LMIC (Morocco) | ‐ | Farming | Children (7 to 9 years) | ‐ | Primary | Rural low‐income communities | Primary school | Water, sanitation and hygiene indicators, parent education, family income and budget | Primary school | |
| LIC (Tanzania) | Wagogo and Wakaguru people | ‐ | Children 6 to 11 years | ‐ | ‐ | ‐ | 6 rural primary schools | Age BMI | Schools | |
| HIC (Netherlands) | ‐ | ‐ | Independently living, frail elderly men and women 70 years or older | ‐ | ‐ | ‐ | Housing complexes, home care organisations | ‐ | By mail from senior housing complexes, meals‐on‐wheels programmes, home‐care organisations, and general practitioners from the surroundings of Wageningen | |
| LMIC (Cambodia) | ‐ | ‐ | Children aged 9.71 ± 2.42 | ‐ | ‐ | ‐ | Primary schools in rural Kampong Speu province | ‐ | ‐ | |
| HIC ( USA) | ‐ | ‐ | Children 6 to 10 years | ‐ | ‐ | ‐ | ‐ | BMI | Children were recruited at Boston University Medical Center and Tufts Medical Center from the hospital paediatric clinics and through local print and online classified advertisements | |
| UMIC (South Africa) | ‐ | ‐ | Infants 6 to 12 months | ‐ | ‐ | Low SES | ‐ | ‐ | Infants were recruited through the community‐based health programme | |
| LMIC (Zambia) | ‐ | ‐ | Infants aged 6 months | ‐ | ‐ | Middle income | ‐ | Breastfeeding duration, Weight‐for‐age Z‐score, Length‐for‐age Z‐score, Weight‐for length Z‐score, BMI‐for‐age Z‐score, SES. Maternal education and HIV status | ‐ | |
| LMIC (Vietnam) | ‐ | ‐ | Children (6 to 9 years) | ‐ | ‐ | ‐ | ‐ | ‐ | School | |
| LMIC (Bangladesh) | ‐ | ‐ | Adolescent girls | ‐ | ‐ | Low SES | Non‐formal primary education (NFPE) 9 schools | SES, menstruation, BMI | ‐ | |
| HIC (Finland) | ‐ | ‐ | Pregnant women 19 to 40 years | ‐ | ‐ | ‐ | ‐ | BMI, BP, present diseases, current medication, alcohol consumption, smoking habits, physical activity, and use of vitamins and other nutrients | Health care units | |
| UMIC (South Africa) | ‐ | ‐ | Children aged 8 to 10 years | ‐ | ‐ | Rural community | ‐ | ‐ | Primary schools | |
| UMIC (Beijing, China) | ‐ | ‐ | Children aged 6 to 13 yrs | ‐ | ‐ | ‐ | Primary schools | Body weight and length, | ‐ | |
| UMIC (Algeria) | ‐ | ‐ | Children aged 3 to 6 yrs | ‐ | ‐ | Saharawi refugees | ‐ | ‐ | ‐ | |
| HIC (Chile) | Ethnically mixed families (Amerindian and Hispanic) | ‐ | Pregnant women | ‐ | ‐ | Urban health clinics | ‐ | ‐ | Antenatal clinics | |
| UMIC (South Africa) | ‐ | ‐ | Children aged 1 to 3 yrs | ‐ | ‐ | ‐ | ‐ | Demographic, socio‐economic and dietary data, height, weight, haemoglobin, hematocrit, serum retinol and retinol‐binding protein(RBP). Anthropometric, blood and serum | Children at the creches and the well‐baby clinic were screened and the first 60 undernourished children who had weight‐for‐age or height‐for‐age below the 5th percentile of the National Center for Health Statistics (NCHS) identified. The parents/guardians of these children were contacted and recruited to voluntarily participate in the study | |
| LMIC (Vietnam) | ‐ | Farming | Children aged 6 to 8 yrs | ‐ | ‐ | ‐ | ‐ | Sociodemographic characteristics of the children (age, sex, illness history, medical supplements), mothers (age, and education, family size, and household socioeconomic status) | Pupils were recruited from 2 schools that had been selected on the basis of a high prevalence of anaemia and parasite infestations among school children during an earlier survey | |
| UMIC (South Africa) | Black community, Kayamandi | Most of the inhabitants work in the industries in the city or as domestic workers in private homes | Children aged 6 to 12 months | ‐ | ‐ | Urban disadvantaged communities, low SES | ‐ | Baseline food intake | Local clinics | |
| HIC (Australia) and LMIC (Indonesia) | ‐ | ‐ | Children aged 6 to 10 years | ‐ | ‐ | South Australian government metropolitan schools of higher SES in Adelaide, and schools in the central district of Jakarta of middle to low SES | ‐ | BMI, MUAC, WAZ, HAZ, WHZ, highest education in household | In Australia, the intervention was home‐based, with the children being recruited through invitations distributed either through the schools or through an additional media drive. A general, unpersonalised invitation to the parents of children in the appropriate age range was distributed through the schools | |
| LMIC (Cambodia) | ‐ | Rice farming | Children aged 6 to 16 years | ‐ | ‐ | ‐ | Primary schools | Parasitic infection | All parents of children from the 20 schools were invited to attend a meeting at which the study procedures were explained. Written informed consent was obtained from the parents as was verbal assent from the participating children | |
| HIC (Spain) | ‐ | ‐ | Children aged 8 to 14 years | ‐ | ‐ | ‐ | ‐ | ‐ | School | |
| UMIC (Thailand) | ‐ | ‐ | Children aged 7 to 12 years Male : Female (10:10) | Muslim | ‐ | Low income | Schools in southern Thailand | ‐ | ‐ | |
| UMIC (Thailand) | ‐ | ‐ | Children aged 8 to 12 yrs Male : Female 12 : 13 | Muslim | ‐ | Low income | ‐ | Weight, height and BMI | ‐ | |
| HIC (UK) | ‐ | ‐ | Adolescent girls (aged 16 to 19 years) | ‐ | ‐ | ‐ | ‐ | ‐ | Schools and colleges | |
| LMIC (Bangladesh) | ‐ | ‐ | Children aged 6 to 15 yrs | ‐ | ‐ | ‐ | ‐ | BMI | ‐ | |
| LMIC (India) | ‐ | ‐ | Children aged 1 to 3 yrs | ‐ | ‐ | ‐ | ‐ | Father/mother literacy, father/mother occupation, SES, weight, height, wasted, stunted | ‐ | |
| LMIC (Bangladesh) | ‐ | ‐ | School‐attending children aged 6 to 9 years | ‐ | ‐ | ‐ | Primary schools | Mother age, mother education, father education, mother employment, father employment, father income | ‐ | |
| LMIC (Philippines) | ‐ | ‐ | School children from grades 1 to 6 | ‐ | ‐ | ‐ | ‐ | ‐ | School | |
| UMIC (South Africa) | ‐ | ‐ | School‐attending children aged 6 to 11 years | ‐ | ‐ | ‐ | ‐ | Stunted, wasted, underweight | ‐ | |
| HIC (Finland) | ‐ | ‐ | Healthy volunteers aged 26 to 65 yrs; 39 men and 29 women | ‐ | ‐ | ‐ | ‐ | BMI, BP | ‐ | |
| LIC (Tanzania) | ‐ | Semi‐arid, agricultural population | Pregnant women | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | |
| LMIC (India) | ‐ | Construction workers, skilled labourers, or garment factory workers | Children aged 6 to 12 years | ‐ | ‐ | Low SES | ‐ | Height, weight, BMI, stunting, SES, religion | ‐ | |
| LMIC (India) | ‐ | ‐ | School‐attending children aged 6 to 12 years | ‐ | ‐ | ‐ | ‐ | Height, weight, BMI, stunting, thinness, household head education, religion | ‐ | |
| HIC (USA) | ‐ | ‐ | Healthy volunteers aged 50 to 85 years | ‐ | ‐ | ‐ | ‐ | Education, ethnicity, smoker, alcohol intake, no. of medications used | Through advertisements in local newspapers, posters, radio, and mailing lists | |
| HIC (Netherlands) | ‐ | ‐ | Non‐smoking participants, healthy, aged 18 to 65 yrs | ‐ | ‐ | ‐ | ‐ | ‐ | Volunteers were recruited from employees of the laboratory and from inhabitants of Vlaardingen and the surrounding district | |
| UMIC (South Africa) | ‐ | ‐ | Children aged 6 to 11 years | ‐ | ‐ | Low SES | ‐ | Stunted, underweight, parasitic infection | ‐ | |
| LMIC (India) | ‐ | ‐ | Children aged 7 to 10.5 years | ‐ | ‐ | Middle socio‐economic groups | ‐ | ‐ | Schools | |
| UMIC (Mexico) | ‐ | ‐ | Infants aged 10 to 30 months | ‐ | ‐ | Poor peri‐urban communities | ‐ | Weight, length, SES indicators | Local health facility registry | |
| LMIC (India) | ‐ | ‐ | Children aged 5 to 18 years | ‐ | ‐ | The families of all the children had a monthly income of less than INR 2000 (USD 50) | Schools | ‐ | ‐ | |
| UMIC (China) | ‐ | ‐ | Children aged 12 to 14 years | ‐ | ‐ | ‐ | ‐ | Gender, weight | Schools | |
| LMIC (Morroco) | Mixed Berber and Arab descent | ‐ | Children aged 6 to 14 years | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | |
BMI: body mass index; BP: blood pressure; HIC: high‐income country; LIC: low‐income country; LMIC: low middle income country; MUAC: mid‐upper arm circumference; SES: socio‐economic status; UMIC: upper middle‐income country
Duration of Intervention
The duration of intervention varied from a minimum of eight weeks to a maximum of one year. The duration of intervention in 29 studies was six months or less ( Aaron 2011 ; Abrams 2003 ; Ash 2003 ; Chin A Paw 2000 ; DeGier 2016 ; Economos 2014 ; Faber 2005 ; Hieu 2012 ; Järvenpaa 2007 ; Jinabhai 2001 ; Liu 1993 ; Lopriore 2004 ; Nga 2009 ; Oelofse 2003 ; Perignon 2016 ; Petrova 2019 ; Pinkaew 2013 ; Pinkaew 2014 ; Powers 2016 ; Rahman 2015 ; Solon 2003 ; Tapola 2004 ; Tatala 2002 ; Thankachan 2012 ; Thankachan 2013 ; Tucker 2004 ; Vaz 2011 ; Villalpando 2006 ; Wang 2017 ), while in 14 studies the duration of intervention was between six months and one year ( Adams 2017 ; Azlaf 2017 ; Gibson 2011 ; Hyder 2007 ; Mardones 2007 ; Nesamvuni 2005 ; Osendarp 2007 ; Sazawal 2007 ; Sazawal 2013 ; Taljaard 2013 ; Van het Hof 1998 ; Van Stuijvenberg 1999 ; Vinodkumar 2009 ; Zimmerman 2004 ).
Food vehicles
Food vehicles used included staple food, such as rice and flour ( DeGier 2016 ; Faber 2005 ; Gibson 2011 ; Nesamvuni 2005 ; Oelofse 2003 ; Perignon 2016 ; Pinkaew 2013 ; Pinkaew 2014 ; Powers 2016 ; Rahman 2015 ; Thankachan 2012 ; Tucker 2004 ), dairy products, including milk and yogurt ( Azlaf 2017 ; Chin A Paw 2000 ; Mardones 2007 ; Petrova 2019 ; Sazawal 2007 ; Sazawal 2013 ; Van het Hof 1998 ; Villalpando 2006 ; Wang 2017 ), non‐dairy beverages ( Aaron 2011 ; Abrams 2003 ; Ash 2003 ; Economos 2014 ; Hyder 2007 ; Järvenpaa 2007 ; Osendarp 2007 ; Solon 2003 ; Taljaard 2013 ; Tapola 2004 ; Tatala 2002 ; Thankachan 2013 ; Vaz 2011 ), biscuits ( Adams 2017 ; Hieu 2012 ; Jinabhai 2001 ; Liu 1993 ; Nga 2009 ; Van Stuijvenberg 1999 ), spreads ( Lopriore 2004 ), and salt ( Vinodkumar 2009 ; Zimmerman 2004 ). Outcomes
Anaemia, micronutrient deficiencies, anthropometric measures and serum micronutrient levels were the most commonly reported outcomes. Eight studies reported neuro‐cognitive outcomes in children ( Faber 2005 ; Nga 2009 ; Osendarp 2007 ; Taljaard 2013 ; Thankachan 2012 ; Van Stuijvenberg 1999 ; DeGier 2016 ; Petrova 2019 ). None of the included studies reported on morbidity, adverse events, or all‐cause or cause‐specific mortality.
Fourteen of the included studies were fully commercially funded ( Ash 2003 ; Economos 2014 ; Järvenpaa 2007 ; Mardones 2007 ; Osendarp 2007 ; Petrova 2019 ; Powers 2016 ; Sazawal 2007 ; Taljaard 2013 ; Tapola 2004 ; Thankachan 2012 ; Thankachan 2013 ; Van Stuijvenberg 1999 ; Vaz 2011 ); 13 of the included studies had partial commercial funding ( Aaron 2011 ; Abrams 2003 ; Chin A Paw 2000 ; Faber 2005 ; Gibson 2011 ; Hyder 2007 ; Jinabhai 2001 ; Nesamvuni 2005 ; Pinkaew 2013 ; Pinkaew 2014 ; Solon 2003 ; Tatala 2002 ; Tucker 2004 ); 14 studies were non‐commercially funded ( Adams 2017 ; Azlaf 2017 ; Hieu 2012 ; Liu 1993 ; Rahman 2015 ; Villalpando 2006 ; DeGier 2016 ; Lopriore 2004 ; Nga 2009 ; Perignon 2016 ; Vinodkumar 2009 ; Zimmerman 2004 ; Sazawal 2013 ; Wang 2017 ), while two studies ( Van het Hof 1998 ; Oelofse 2003 ) did not specify the source of funding.
Excluded studies
We excluded 78 studies at full‐text screening. Common reasons for exclusion included point‐of‐use fortification, a pre‐post design without a control group, no outcomes of interest, and supplementation rather than fortification. See Characteristics of excluded studies for a full list of reasons for exclusion.
Risk of bias in included studies
See Figure 2 ; Figure 3 for the 'Risk of bias' summary and graph.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.

Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
Of the 39 included RCTs, we considered five to be at overall low risk of bias ( Perignon 2016 ; Petrova 2019 ; Rahman 2015 ; Sazawal 2007 ; Vaz 2011 ) , and the remaining 34 to be at overall high risk of bias, due to concerns around allocation concealment, blinding of participants, or incomplete outcome data.
We judged 18 studies to be at a low risk for random sequence generation ( DeGier 2016 ; Economos 2014 ; Faber 2005 ; Gibson 2011 ; Hieu 2012 ; Lopriore 2004 ; Nga 2009 ; Osendarp 2007 ; Perignon 2016 ; Petrova 2019 ; Rahman 2015 ; Sazawal 2007 ; Sazawal 2013 ; Tatala 2002 ; Thankachan 2012 ; Thankachan 2013 ; Vaz 2011 ; Wang 2017 ); we judged one study to be at high risk of bias for sequence generation ( Zimmerman 2004 ), while the rest were at unclear risk of bias.
We rated nine studies at a low risk for allocation concealment ( Chin A Paw 2000 ; DeGier 2016 ; Hieu 2012 ; Perignon 2016 ; Petrova 2019 ; Rahman 2015 ; Sazawal 2007 Sazawal 2013 ; Vaz 2011 ), one study at high risk of bias for allocation concealment ( Wang 2017 ), while the rest were at unclear risk of bias.
We judged 24 studies to be at low risk of bias for blinding of participants and personnel ( Aaron 2011 ; Ash 2003 ; DeGier 2016 ; Economos 2014 ; Gibson 2011 ; Hieu 2012 ; Hyder 2007 ; Lopriore 2004 ; Nga 2009 ; Osendarp 2007 ; Perignon 2016 ; Petrova 2019 ; Powers 2016 ; Rahman 2015 ; Sazawal 2007 ; Sazawal 2013 ; Solon 2003 ; Taljaard 2013 ; Thankachan 2012 ; Thankachan 2013 ; Van Stuijvenberg 1999 ; Vaz 2011 ; Villalpando 2006 ; Zimmerman 2004 ), four studies at high risk of bias for blinding of participants and personnel ( Chin A Paw 2000 ; Oelofse 2003 ; Van het Hof 1998 ; Wang 2017 ), while rest were at unclear risk of bias.
We rated 23 studies were at low risk of bias for blinding of outcome assessment ( Aaron 2011 ; Ash 2003 ; DeGier 2016 ; Economos 2014 ; Gibson 2011 ; Hieu 2012 ; Hyder 2007 ; Lopriore 2004 ; Nga 2009 ; Osendarp 2007 ; Perignon 2016 ; Petrova 2019 ; Powers 2016 ; Rahman 2015 ; Sazawal 2007 ; Sazawal 2013 ; Solon 2003 ; Thankachan 2012 ; Thankachan 2013 ; Van het Hof 1998 ; Vaz 2011 ; Villalpando 2006 ; Zimmerman 2004 ), four studies at high risk of bias for blinding of outcome assessment ( Chin A Paw 2000 ; Nesamvuni 2005 ; Oelofse 2003 ; Wang 2017 ), while the rest were at unclear risk of bias.
Incomplete outcome data
As the studies involved significant lifestyle changes and were carried out over a period of many weeks and months, dropouts were present, but these were either comparable in the different trial arms, or few and addressed and accounted for. We rated 26 studies at low risk of attrition bias ( Aaron 2011 ; Ash 2003 ; Faber 2005 ; Hyder 2007 ; Järvenpaa 2007 ; Nga 2009 ; Perignon 2016 ; Petrova 2019 ; Pinkaew 2013 ; Pinkaew 2014 ; Powers 2016 ; Rahman 2015 ; Sazawal 2007 ; Solon 2003 ; Taljaard 2013 ; Tapola 2004 ; Thankachan 2012 ; Thankachan 2013 ; Tucker 2004 ; Van het Hof 1998 ; Van Stuijvenberg 1999 ; Vaz 2011 ; Villalpando 2006 ; Vinodkumar 2009 ; Wang 2017 ; Zimmerman 2004 ), 12 studies at high risk of attrition bias ( Chin A Paw 2000 ; DeGier 2016 ; Economos 2014 ; Gibson 2011 ; Hieu 2012 ; Liu 1993 ; Lopriore 2004 ; Nesamvuni 2005 ; Oelofse 2003 ; Osendarp 2007 ; Sazawal 2013 ; Tatala 2002 ), while one study ( Jinabhai 2001 ) was rated at unclear risk of bias.
Selective reporting
Most of the studies did not report trial registration details, but in most cases the outcomes discussed in the paper were reported. We judged only one study ( DeGier 2016 ) to be at high risk for selective reporting, since it was powered to assess the micronutrient status but this outcome was not reported in the paper. There was a very minimal risk of reporting bias in the studies and generally we did not detect selective reporting. None of the included studies mentioned or reported on adverse effects.
Other potential sources of bias
We rated all studies at low risk for other potential bias.
For the six c‐RCTs ( DeGier 2016 ; Liu 1993 ; Perignon 2016 ; Rahman 2015 ; Vinodkumar 2009 ; Wang 2017 ), we have assessed and reported additional criteria. We found all six c‐RCTs to be at low risk for recruitment bias, baseline imbalance, loss of clusters, incorrect analysis, and for comparability with individually‐randomised trials.
Risk of bias for CBA
Four studies ( Abrams 2003 ; Adams 2017 ; Azlaf 2017 ; Mardones 2007 ) were assessed on additional criteria based on EPOC 2017 , since these were CBA studies.
We judged all four studies to be at high risk for random sequence generation, allocation concealment and knowledge of the allocated interventions adequately prevented during the study; we rated Mardones 2007 at high risk for incomplete outcome data, while all studies were at low risk for all other criteria, including baseline outcome measurements, baseline characteristics, incomplete outcome data, protection against contamination and selective outcome reporting. We found no other sources of bias.
Risk of bias for CBA studies are reported under the 'Other Bias' section of their respective 'Risk of bias' table.
Effects of interventions
See: Table 1 ; Table 2
for the main comparison
| General population Community and schools MMN fortification | |||||||
| Defined as haemoglobin (Hb) concentration < 11 g/dL Measured at after 6 months and 6 ‐ 12 months of intervention | 311 per 1000 | 211 per 1000 (174 to 382) | 3746 participants (11 studies) | ⊕⊕⊝⊝ low | ‐ | ||
| Iron deficiency Defined as serum ferritin < 15 µg/l Measured after 6 months and 6 ‐ 12 months of intervention | 253 per 1000 | 111 per 1000 (81 to 152) | 3289 participants (11 studies) | ⊕⊕⊝⊝ low | ‐ | ||
| Vitramin A deficiency Defined as serum retinol < 0.70 µmol/l Measured after 6 months of intervention | 222 per 1000 | 93 per 1000 (62 to 138) | 1482 participants (6 studies) | ⊕⊕⊝⊝ low | ‐ | ||
| Zinc deficiency Defined as serum zinc level < 0.66 mcg/mL Measured after 6 months and 6 ‐ 12 months of intervention | 490 per 1000 | 411 per 1000 (319 to 529) | 1490 participants (5 studies) | ⊕⊝⊝⊝ very low | ‐ | ||
| Weight‐for‐age z‐scores (WAZ) Measured as Z‐scores (standard deviation scores) Measured after 6 months and 6 ‐ 12 months of intervention | Mean WAZ score was −0.94 for the control group | Mean WAZ score 0.10 higher (0.02 to 0.17 higher) | 2889 participants (8 studies) | ⊕⊕⊝⊝ low | ‐ | ||
| Height‐for‐age z‐scores/length‐for‐age z‐scores (HAZ/LAZ) Measured as Z‐scores (standard deviation scores) Measured after 6 months and 6 ‐ 12 months of intervention | Mean HAZ/LAZ score was −1.18 for the control group | Mean HAZ/LAZ score 0.09 higher (0.01 to 0.18 higher) | 2889 participants (8 studies) | ⊕⊝⊝⊝ very low | ‐ | ||
| Weight‐for‐height z‐score/weight for length z‐score (WHZ/LHZ) Measured as Z‐scores (standard deviation scores) Measured after 6 months and 6‐12 months of intervention | Mean WHZ/LHZ score was −0.03 in the control group | Mean WHZ/WLZ score 0.10 higher (0.02 to 0.18 higher) | 1758 participants (6 studies) | ⊕⊕⊝⊝ low | ‐ | ||
| *The basis for the (e.g. the median control group risk across studies) is provided in footnotes. The (and its 95% confidence interval) is based on the assumed risk in the comparison group and the of the intervention (and its 95% CI). Confidence interval; Risk Ratio; Weight‐for‐age z‐score; Height‐for‐age z‐score/Length‐for‐age z‐score; : Weight‐for‐height z‐score/Length‐for‐height z‐score. | |||||||
| GRADE Working Group grades of evidence Further research is very unlikely to change our confidence in the estimate of effect. Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. We are very uncertain about the estimate. | |||||||
a Assumed risk is the mean of the post‐intervention values in the control groups in the included studies. b Downgraded by one level due to study limitations including lack of randomisation, blinding and attrition. c Downgraded by one level due to high heterogeneity (I 2 > 30%). d Downgraded by one level due to imprecision.
| General population Community MMN fortification Iodised salt | |||||||
| Anaemia was defined as a haemoglobin (Hb) concentration < 13 g/dL for boys and < 12 g/dL for girls Measured after 9 months of intervention | 698 per 1000 | 600 per 1000 (258 to 1403) | | 88 participants (1 study) | ⊕⊝⊝⊝ very low | ‐ | |
| Iron deficiency Defined as serum ferritin < 15 mg/L or serum transferrin concentration > 7.6 mg/L Measured after 9 months of intervention | 860 per 1000 | 843 per 1000 (705 to 1006) | | 88 participants (1 study) | ⊕⊝⊝⊝ very low | ‐ | |
| Vitamin A deficiency Defined as serum retinol less than 0.70 µmol/l or less than 20 ug/dL Measured after 9 and 10 months of intervention | 388 per 1000 | 74 per 1000 (27 to 213) | | 363 participants (2 studies) | ⊕⊝⊝⊝ very low | ‐ | |
| Zinc deficiency | ‐ | ‐ | ‐ | ‐ | ‐ | None of the included studies reported this outcome | |
| ‐ | ‐ | ‐ | ‐ | ‐ | None of the included studies reported these outcomes | ||
| *The basis for the (e.g. the median control group risk across studies) is provided in footnotes. The (and its 95% confidence interval) is based on the assumed risk in the comparison group and the of the intervention (and its 95% CI). Confidence interval; Risk Ratio | |||||||
| GRADE Working Group grades of evidence Further research is very unlikely to change our confidence in the estimate of effect. Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. We are very uncertain about the estimate. | |||||||
a Assumed risk taken from post‐intervention value in the iodised salt group of a single included study. b Downgraded by one level due to study limitations including lack of randomisation, blinding and attrition. c Downgraded by two levels due to serious imprecision, including small sample size. d Assumed risk is the mean of the post‐intervention values in the iodised salt groups in the included studies.
Comparison 1: Multiple micronutrient fortification versus placebo/no intervention
Forty studies compared MMN fortification with placebo or no intervention ( Aaron 2011 ; Abrams 2003 ; Adams 2017 ; Ash 2003 ; Azlaf 2017 ; Chin A Paw 2000 ; DeGier 2016 ; Faber 2005 ; Gibson 2011 ; Hieu 2012 ; Hyder 2007 ; Järvenpaa 2007 ; Jinabhai 2001 ; Liu 1993 ; Lopriore 2004 ; Mardones 2007 ; Nesamvuni 2005 ; Nga 2009 ; Oelofse 2003 ; Osendarp 2007 ; Perignon 2016 ; Petrova 2019 ; Pinkaew 2013 ; Pinkaew 2014 ; Powers 2016 ; Rahman 2015 ; Sazawal 2007 ; Sazawal 2013 ; Solon 2003 ; Taljaard 2013 ; Tapola 2004 ; Tatala 2002 ; Thankachan 2012 ; Thankachan 2013 ; Tucker 2004 ; Van het Hof 1998 ; Van Stuijvenberg 1999 ; Vaz 2011 ; Villalpando 2006 ; Wang 2017 ). Of these, Mardones 2007 did not measure any of the outcomes included in this review.
Primary Outcomes
Among the primary outcomes, included studies reported anaemia, micronutrient deficiencies (iron, zinc, vitamin A, B vitamins) and anthropometric outcomes (weight‐for‐age z‐score (WAZ), height/length‐for‐age z‐score (HAZ/LAZ) and weight‐for‐height/length z‐score (WHZ/WLZ)). None of the included studies reported morbidity, all‐cause mortality or cause‐specific mortality.
Anaemia: Pooled study results
MMN fortification may reduce anaemia by 32% when compared to placebo/no intervention (risk ratio (RR) 0.68, 95% confidence interval (CI) 0.56 to 0.84; I 2 = 61%; 11 studies, 3746 participants; low‐quality evidence; Analysis 1.1 ; Figure 4 ).

Forest plot of comparison: 1 MMN vs Placebo/No intervention, outcome: 1.1 Anaemia.

Comparison 1 MMN vs placebo/no intervention, Outcome 1 Anaemia.
Findings from Abrams 2003 and Adams 2017 were not pooled with the meta‐analysis since these were non‐randomised studies. Both Adams 2017 and Abrams 2003 reported reduced anaemia in the intervention group compared to the control group (RR 0.67, 95% CI 0.41 to 0.93 and OR 0.48, 95% CI 0.27 to 0.87, respectively).
Iron deficiency anaemia: Pooled study results
MMN fortification may reduce the prevalence of iron deficiency anaemia by 72% (RR 0.28, 95% CI 0.19 to 0.39; I 2 = 19%; 6 studies, 2189 participants; low‐quality evidence; Analysis 1.2 ; Figure 5 ).

Forest plot of comparison: 1 MMN vs Placebo, outcome: 1.2 Iron deficiency anaemia.

Comparison 1 MMN vs placebo/no intervention, Outcome 2 Iron deficiency anaemia.
Micronutrient deficiencies: Pooled study results
MMN fortification may reduce micronutrient deficiencies including iron deficiency by 56% (RR 0.44; 95% CI 0.32 to 0.60; I 2 = 54%; 11 studies, 3289 participants; low‐quality evidence; Analysis 1.3 ; Figure 6 ); vitamin A deficiency by 58% (RR 0.42, 95% CI 0.28 to 0.62; I 2 = 31%; 6 studies, 1482 participants; low‐quality evidence; Analysis 1.4 ; Figure 7 ); vitamin B2 deficiency by 64% (RR 0.36, 95% CI 0.19 to 0.68; 1 study, 296 participants; low‐quality evidence; Analysis 1.5 ); vitamin B6 deficiency by 91% (RR 0.09, 95% CI 0.02 to 0.38; I 2 = 0%; 2 studies, 301 participants; low‐quality evidence; Analysis 1.5 ) and vitamin B12 deficiency by 58% (RR 0.42, 95% CI 0.25 to 0.71; I 2 = 0%; 3 studies; n = 728; moderate‐quality evidence; Analysis 1.5 ). We are uncertain of the effect of MMN fortification on zinc deficiency (RR 0.84, 95% CI 0.65 to 1.08; I 2 = 74%: 5 studies, 1490 participants; very low‐quality evidence; Analysis 1.6 ; Figure 8 ).

Forest plot of comparison: 1 MMN vs placebo/no intervention, outcome: 1.3 Micronutrient deficiencies: Iron.

Forest plot of comparison: 1 MMN vs placebo/no intervention, outcome: 1.4 Micronutrient deficiencies: Vitamin A.

Forest plot of comparison: 1 MMN vs placebo/no intervention, outcome: 1.6 Micronutrient deficiencies: Zinc.

Comparison 1 MMN vs placebo/no intervention, Outcome 3 Micronutrient deficiencies: Iron.

Comparison 1 MMN vs placebo/no intervention, Outcome 4 Micronutrient deficiencies: Vitamin A.

Comparison 1 MMN vs placebo/no intervention, Outcome 5 Micronutrient deficiencies: B Vitamin.

Comparison 1 MMN vs placebo/no intervention, Outcome 6 Micronutrient deficiencies: Zinc.
Findings from Adams 2017 and Azlaf 2017 were not pooled with the meta‐analysis since these were not RCTs. Adams 2017 reported no effect of MMN fortification on zinc deficiency (RR 0.76, 95% CI 0.5 to 1.02), while Azlaf 2017 reported a significant effect of MMN fortification on vitamin A deficiency (prevalence of vitamin A deficiency of 4.3% in the fortified group compared to 25.2% in the control group (P < 0.001)) which are consistent with the findings of the meta‐analysis.
Anthropometric outcomes: Pooled study results weight for age
MMN fortification may improve WAZ (mean difference (MD) 0.10 z‐scores, 95% CI 0.02 to 0.17; I 2 = 26%; 8 studies, 2889 participants; low‐quality evidence; Analysis 1.7 ) and WHZ/WLZ (MD 0.10 z‐scores, 95% CI 0.02 to 0.18; I 2 = 5%; 6 studies, 1758 participants; low‐quality evidence; Analysis 1.8 ). We are uncertain about the effect of MMN fortification on HAZ/LAZ (MD 0.09, 95% CI 0.01 to 0.18; I 2 = 39%; 8 studies, 2889 participants; very low‐quality evidence; Analysis 1.9 ).

Comparison 1 MMN vs placebo/no intervention, Outcome 7 Anthropometric: WAZ.

Comparison 1 MMN vs placebo/no intervention, Outcome 8 Anthropometric: WHZ/WLZ.

Comparison 1 MMN vs placebo/no intervention, Outcome 9 Anthropometric: HAZ/LAZ.
Almost all the studies included in analyses of the primary outcomes were at high risk of bias. Sensitivity analysis removing all studies at overall high risk of bias left all primary analyses with only one or no included studies, indicating that risks of bias may have an important impact on the reported results.
Among the secondary outcomes, included trials reported on serum haemoglobin, serum micronutrient concentrations (folate, ferritin, vitamin A, B vitamins and zinc) and neuro‐cognitive outcomes. None of the included trials reported any potential adverse effects.
Serum haemoglobin: Pooled study results
MMN fortification may improve serum haemoglobin (MD 3.01 g/L, 95% CI 2.14 to 3.87; I 2 = 99%; 20 studies, 6985 participants; low‐quality evidence; Analysis 1.10 ).

Comparison 1 MMN vs placebo/no intervention, Outcome 10 Biochemical: Serum haemoglobin (g/L).
Serum micronutrient levels: Pooled study results
MMN fortification may improve serum ferritin (MD 8.27 μg/mL, 95% CI 3.26 to 13.27; I 2 = 88%; 7 studies, 2407 participants; low‐quality evidence; Analysis 1.11 ), vitamin B6 (MD 35.02 nmol/L, 95% CI 22.95 to 47.09; I 2 = 82%; 2 studies, 301 participants; low‐quality evidence; Analysis 1.12 ), vitamin B9 (folate) (MD 12.41 nmol/L, 95% CI 6.55 to 18.28; I 2 = 100%; 5 studies, 568 participants; low‐quality evidence; Analysis 1.12 ) and vitamin B12 (MD 61.90 pmol/L, 95% CI 53.56 to 70.23; I 2 = 100%; 6 studies, 893 participants; low‐quality evidence; Analysis 1.12 ). We are uncertain of the effect of MMN fortification on serum vitamin A (MD 0.04 μmol/L, 95% CI −0.01 to 0.09; I 2 = 68%; 13 studies, 2457 participants; low‐quality evidence; Analysis 1.13 ), on serum zinc (MD 0.25 ug/dL, 95% CI −0.05 to 0.55; I 2 = 76%; 15 studies, 4428 participants; low‐quality evidence; Analysis 1.14 ) or on vitamin B1 (MD 4.80 nmol/L, 95% CI −2.77 to 12.37; 1 study, 118 participants; low‐quality evidence; Analysis 1.12 ).

Comparison 1 MMN vs placebo/no intervention, Outcome 11 Biochemical: Serum ferritin (μg/mL).

Comparison 1 MMN vs placebo/no intervention, Outcome 12 Biochemical: B Vitamin.

Comparison 1 MMN vs placebo/no intervention, Outcome 13 Biochemical: Serum vitamin A (μmol/L).

Comparison 1 MMN vs placebo/no intervention, Outcome 14 Biochemical: Serum zinc (μg/dL).
Neuro‐cognitive outcome: single‐study results
Eight studies reported various neuro‐cognitive outcomes in children ( Faber 2005 ; Nga 2009 ; Osendarp 2007 ; Taljaard 2013 ; Thankachan 2012 ; Van Stuijvenberg 1999 ; DeGier 2016 ; Petrova 2019 ). We are uncertain of the effect of MMN fortification on motor development score (MD 1.10, 95% CI 0.17 to 2.03; 1 study, 266 participants; very low‐quality evidence; Analysis 1.15 ), Raven’s Colored Progressive Matrices test (RCPM) (MD 0.13, 95% CI −0.86 to 1.11; I 2 = 59%; 2 studies, 1124 participants; very low‐quality evidence; Analysis 1.15 ), general intelligence (MD −0.07, 95% CI −0.34 to 0.20; 1 study, 251 participants; very low‐quality evidence; Analysis 1.15 ), verbal learning and memory (MD 0.13, 95% CI −0.10 to 0.37; 1 study, 251 participants; very low‐quality evidence; Analysis 1.15 ), visual attention (MD 0.09, 95% CI −0.11 to 0.29; 1 study, 251 participants; very low‐quality evidence; Analysis 1.15 ) and coding (MD −0.53, 95% CI −1.26 to 0.21; 3 studies, 509 participants; I 2 = 0%; low‐quality evidence; Analysis 1.15 ).

Comparison 1 MMN vs placebo/no intervention, Outcome 15 Neuro‐cognitive outcomes.
Taljaard 2013 assessed cognition using the Kaufman Assessment Battery for Children version II (KABC) sub‐tests and the Hopkins Verbal Learning Test (HVLT), suggesting that fortification increased KABC Atlantis (intervention group mean score 5.9 compared to control group mean score 5) and HVLT Discrimination Index scores (intervention group mean score 14.7 compared to control group mean score 13.8); there was no effect on story completion, number recall, rover, triangles, word order, hand movements, recall and recognition. Van Stuijvenberg 1999 used cognitive tests designed to record speed of processing and capacity of working memory in tasks closely related to the intellectual skills required for schoolwork, suggesting improvement in the digit span forward task (short‐term memory) (P < 0.05) only, with no effect on any of the other cognitive functions (verbal fluency, digit copying, writing crosses, counting letters, cancelling letters, reading numbers, digit span backward task and counting backward).
Assessment of reporting bias
We were able to generate funnel plots for five outcomes, as they included 10 or more studies; these include anaemia, iron deficiency, serum haemoglobin, serum vitamin A and serum zinc ( Figure 9 ; Figure 10 ; Figure 11 ; Figure 12 ; Figure 13 ). The funnel plots for the outcomes of anaemia and serum haemoglobin were visually asymmetrical, suggesting that publication bias could be one of the possible sources of asymmetry. We compared the fixed‐effect model with the random‐effects model for these two anaemia outcomes. We found the estimates to be in a similar direction of effect with overlapping CIs, indicating that smaller studies were not systematically finding more positive results, and that there is no specific indication that reporting bias might be having an important impact on this outcome. For serum haemoglobin, the sensitivity analysis showed a notably smaller result using a fixed‐effect analysis, indicating that smaller studies on average reported more positive results than larger ones. Reporting bias may be one cause of this heterogeneity, although there may be other causes that we were not able to identify (see section on subgroup analysis below).

Funnel plot of comparison: 1 MMN vs Placebo/No intervention, outcome: 1.1 Anaemia.

Funnel plot of comparison: 1 MMN vs placebo/no intervention, outcome: 1.3 Micronutrient deficiencies: Iron.

Funnel plot of comparison: 1 MMN vs placebo/no intervention, outcome: 1.10 Biochemical: Serum haemoglobin (g/L).

Funnel plot of comparison: 1 MMN vs Placebo/No intervention, outcome: 1.13 Biochemical: Serum Vitamin A (umol/L).

Funnel plot of comparison: 1 MMN vs Placebo/No intervention, outcome: 1.14 Biochemical: Serum Zinc (ug/dL).
Subgroup analysis
We could only conduct subgroup analysis by duration of intervention (for the outcomes of WAZ and HAZ/LAZ) and by source of funding (for the outcomes of anaemia and iron deficiency). There were fewer than three studies in each subgroup for all other outcomes.
Subgroup analysis by the duration of intervention suggested no difference between the 'six months or less' intervention duration and 'more than six months to one year' intervention duration for WAZ ( Analysis 2.1 ) and HAZ/LAZ ( Analysis 2.2 ).

Comparison 2 MMN vs placebo/no intervention (Subgroup analysis by duration of intervention), Outcome 1 Anthropometric: WAZ.

Comparison 2 MMN vs placebo/no intervention (Subgroup analysis by duration of intervention), Outcome 2 Anthropometric: HAZ/LAZ.
Subgroup analysis by funding suggested no difference between non‐commercial, partial and full commercial funding for anaemia ( Analysis 3.1 ) and iron deficiency ( Analysis 3.2 ).

Comparison 3 MMN vs placebo/no intervention (Subgroup analysis by funding), Outcome 1 Anaemia.

Comparison 3 MMN vs placebo/no intervention (Subgroup analysis by funding), Outcome 2 Micronutrient Deficiencies: Iron.
Comparison 2: Multiple micronutrient fortification versus iodised salt
Two studies compared MMN fortification with iodised salt in children ( Vinodkumar 2009 ; Zimmerman 2004 ). We could not conduct any subgroup analysis for this comparison due to the limited number of studies.
Among the primary outcomes, included studies reported anaemia, iron deficiency anaemia and micronutrient deficiencies (iron and vitamin A). None of the included trials in this comparison reported anthropometric outcomes, morbidity, all‐cause mortality or cause‐specific mortality.
Anaemia: SIngle‐study result
We are uncertain of the effect of MMN fortification when compared to iodised salt for anaemia (RR 0.86, 95% CI 0.37 to 2.01; 1 study, 88 participants; very low‐quality evidence; Analysis 4.1 ).

Comparison 4 MMN vs iodised salt, Outcome 1 Anaemia.
We are uncertain of the effect of MMN fortification when compared to iodised salt for iron deficiency anaemia (RR 0.40, 95% CI 0.09 to 1.83; I 2 = 77%; 2 studies, 245 participants; very low‐quality evidence; Analysis 4.2 ).

Comparison 4 MMN vs iodised salt, Outcome 2 Iron deficiency anaemia.
Micronutrient deficiency: Single‐study result
We are uncertain of the effect of MMN fortification compared to iodised salt for iron deficiency (RR 0.98, 95% CI 0.82 to 1.17; 1 study, 88 participants; very low‐quality evidence; Analysis 4.3 ).

Comparison 4 MMN vs iodised salt, Outcome 3 Micronutrient deficiencies: Iron.
We are uncertain of the effect of MMN fortification compared to iodised salt on vitamin A deficiency when compared to iodised salt (RR 0.19, 95% CI 0.07 to 0.55; I 2 = 96%; 2 studies, 363 participants; very low‐quality evidence; Analysis 4.4 ).

Comparison 4 MMN vs iodised salt, Outcome 4 Micronutrient deficiencies: Vitamin A.
Among the secondary outcomes, included studies reported serum haemoglobin, serum micronutrient concentrations (ferritin, B vitamins, vitamin A and zinc). None of the included studies reported any potential adverse events or neuro‐cognitive outcomes.
Serum haemoglobin: pooled study results
MMN fortification when compared to iodised salt may improve serum haemoglobin (MD 10.20 g/L, 95% CI 3.06 to 17.35; I 2 = 96%; 2 trials, 559 participants; low‐quality evidence; Analysis 4.5 ).

Comparison 4 MMN vs iodised salt, Outcome 5 Biochemical: Serum haemoglobin (g/L).
Serum micronutrient levels: single‐study results
We are uncertain of the effect of MMN fortification compared to iodised salt on serum ferritin (MD 0.18, 95% CI −36.14 to 36.50; 1 study, 88 participants; very low‐quality evidence; Analysis 4.6 ), serum vitamin B9 (MD 5.04, 95% CI −0.92 to 11.00; 1 study, 95 participants; very low‐quality evidence; Analysis 4.7 ), serum vitamin B12 (MD 15,184, 95% CI 6336.35 to 24,031.65; 1 study, 95 participants; very low‐quality evidence; Analysis 4.7 ), serum vitamin A (MD 2.82, 95% CI −2.88 to 8.51; I 2 = 87%; 2 studies, 363 participants; very low‐quality evidence; Analysis 4.8 ) and serum zinc (MD 39.77, 95% CI −86.29 to 165.83; 1 study, 95 participants; very low‐quality evidence; Analysis 4.9 ).

Comparison 4 MMN vs iodised salt, Outcome 6 Biochemical: Serum ferritin (ug/L).

Comparison 4 MMN vs iodised salt, Outcome 7 Biochemical: B Vitamin.

Comparison 4 MMN vs iodised salt, Outcome 8 Biochemical: Serum vitamin A (umol/L).

Comparison 4 MMN vs iodised salt, Outcome 9 Biochemical: Serum zinc.
Comparison 3: Multiple micronutrient fortification versus calcium fortification alone
Only one trial ( Economos 2014 ) compared MMN fortification with calcium fortification in children.
None of the primary outcomes were reported in the trial.
Among the secondary outcomes, only serum micronutrient levels were reported.
Serum micronutrient levels: SIngle study results
We are uncertain of the effect of MMN fortification on serum vitamin E (MD 5.10, 95% CI 3.49 to 6.71; 1 study, 93 participants; very low‐quality evidence; Analysis 5.1 ) and serum vitamin D (MD 15.10, 95% CI 3.06 to 27.14; 1 study, 93 participants; very low‐quality evidence; Analysis 5.2 ), serum calcium (MD 0.00, 95% CI −0.17 to 0.17; 1 study, 88 participants; very low‐quality evidence; Analysis 5.3 ) and serum vitamin A (MD 0.10, 95% CI −0.03 to 0.23; 1 study, 88 participants; very low‐quality evidence; Analysis 5.4 ) compared to calcium fortification alone.

Comparison 5 MMN vs calcium fortification alone, Outcome 1 Biochemical: Serum vitamin E.

Comparison 5 MMN vs calcium fortification alone, Outcome 2 Biochemical: Serum vitamin D.

Comparison 5 MMN vs calcium fortification alone, Outcome 3 Biochemical: Serum calcium.

Comparison 5 MMN vs calcium fortification alone, Outcome 4 Biochemical: Serum vitamin A (umol/L).
Summary of main results
This review summarises findings from 43 studies (48 papers) with 19,585 participants (17,878 children). Most of the included studies compared MMN fortification with placebo/no intervention; two studies compared MMN fortification with iodised salt and one study compared it with calcium fortification alone. Most of the included studies targeted children, so the overall evidence generated from this review applies to children. We rated most of the evidence as of low to very low quality, due to study limitations, imprecision, high heterogeneity and small sample size. When compared to placebo/no intervention, MMN fortification may reduce anaemia, iron deficiency anaemia and micronutrient deficiencies (including iron, vitamin A, vitamin B2 and vitamin B6 deficiency). We are uncertain of the effect of MMN fortification on WAZ, WHZ/WLZ, HAZ/LAZ and other micronutrient deficiencies (including zinc and vitamin B12). Among the secondary outcomes, MMN fortification may improve serum haemoglobin, serum folate, serum ferritin, serum vitamin A and serum vitamin B12; but we are uncertain of the effect on serum zinc, serum vitamin B1 and serum vitamin B6 and any of the neuro‐cognitive outcomes. None of the included trials reported morbidity, all‐cause mortality, cause‐specific mortality or adverse events.
Two studies in children compared MMN fortification with iodised salt. We are uncertain of the effect of MMN fortification on anaemia, iron deficiency anaemia, vitamin A deficiency, serum ferritin, vitamin B9, vitamin B12, vitamin A and zinc; MMN fortification compared to iodised salt may improve haemoglobin. One trial compared MMN fortification with calcium fortification alone in children, showing inconclusive results on serum vitamin E and serum vitamin D, serum calcium and serum vitamin A in the MMN fortification group compared to calcium fortification alone.
Most of the included studies did not directly report on PROGRESS‐Plus factors or the equity‐related variables, and none of the studies reported on adverse events.
Overall completeness and applicability of evidence
This review summarises findings from 43 studies conducted between 1998 and 2018. Most of the studies were conducted in low‐ and middle‐income countries, apart from nine studies from high‐income countries, including Australia, Finland, Spain, Netherlands and the USA. Most of the studies compared MMN fortification with placebo, reporting all the primary outcomes except for morbidities, all‐cause mortality and cause‐specific mortality. None of the included trials reported on any adverse events of food fortification with MMN.
Most of the included studies targeted children, and hence the conclusions apply predominantly to children. Twenty‐nine studies were conducted among pre‐school and school‐aged children; four studies included infants aged between six and 12 months; four studies included children aged one to three years; three studies targeted pregnant women; three studies targeted adults, while one study targeted an elderly population aged over 70 years. Trials had interventions of variable duration, ranging from eight weeks to a maximum of one year. Trials used different food vehicles and numbers and concentrations of micronutrients, and the frequency of intake was also not uniform. There is limited information on the baseline nutritional status of the trial participants.
We were unable to conduct planned subgroup analysis by the various study designs, population groups, baseline micronutrient status, various combination of MMNs, low‐to‐middle income countries versus high‐income countries and the food vehicle used for fortification, due to the limited number of trials with various outcomes. Subgroup analyses comparing different durations of intervention did not identify any significant differences in outcomes. Future updates of this review could add data to various subgroups, if available at that time, which could lead to more meaningful conclusions.
Most of the included studies were fully or partially commercially funded, with a few trials being non‐commercially funded. Subgroup analyses comparing commercial versus non‐commercial funding did not find a significant difference in outcomes, although this was not a large number of studies, and the level of commercial funding remains a concern, because of the possibility of conflict of interest, and the possible association between commercial funding and more positive findings ( Fabbri 2018 ). Independent trials and evaluations are needed to truly assess the impact of food fortification with MMN.
None of the included trials reported adverse events, which limits the completeness and applicability of the existing evidence. A descriptive analysis of the PROGRESS‐Plus factors reported by included studies suggests that most studies lack direct reporting on these factors. Equity‐related variables and analyses were commonly missing from the included studies, thus affecting the availability of evidence on how inequities are identified and how food fortification with MMN can contribute to mitigate or reduce them.
Quality of the evidence
We judged most of the outcomes to be of low to very low quality. Outcomes were mainly downgraded due to study limitations, high heterogeneity and imprecision. Study limitations included a lack of blinding of outcome assessment, incomplete outcome data and inconsistency among studies reporting the outcome. There was high heterogeneity for most of the reported outcomes. None of the included studies reported morbidity, all‐cause or cause‐specific mortality. Information on random sequence generation and allocation concealment was unclear in half of the included studies. In more than half of the included studies the methods used to conceal allocation were not described. Blinding of participants and personnel was also not clearly reported in many studies, while some of the studies were judged to be at a high risk of bias for blinding of outcome assessors. We also rated studies at high risk of bias for incomplete outcome data. This represented a major limitation, as most of the studies were fully or partially commercially funded. Lack of information on dietary intake and baseline nutritional status was another limitation of the review.
Potential biases in the review process
We were aware of the possibility of introducing bias at every stage of the reviewing process. We developed a comprehensive search strategy for a list of pre‐identified databases to capture the eligible studies. We tried to minimise bias in a number of ways; two review authors assessed eligibility for inclusion, carried out data extraction and assessed risks of bias. Nevertheless, the process of assessing risk of bias, for example, is not an exact science and includes many personal judgements. While we tried to be as inclusive as possible in our search strategies, the literature identified was predominantly written in English and published in North American and European journals. Although we tried to assess reporting bias, we largely relied on information available in the published trial reports, meaning that reporting bias was not usually apparent.
Agreements and disagreements with other studies or reviews
Our findings agree with another systematic review ( Das 2013 ), which concluded that food fortification with MMN reduced anaemia and improved serum haemoglobin, ferritin and retinol. Another review ( Best 2011 ) evaluating the impact of MMN fortification on micronutrient status, growth, health, and cognitive development of school children also suggested that MMN fortification improved micronutrient status and reduced anaemia prevalence, with some studies reporting positive effects on morbidity, growth, and cognitive outcomes, but the overall effects on these outcomes were equivocal. This review did not conduct any meta‐analyses. A more recent review evaluating the impact of MMN‐fortified non‐dairy beverage interventions in school‐aged children in LMICs, suggested improved serum haemoglobin and ferritin and reduced anaemia and iron deficiency anaemia ( Aaron 2015 ).
Reviews on home fortification with MMN suggests that it is effective in reducing anaemia and iron deficiency in children aged six months to 23 months ( De‐Regil 2011 ). There was very limited evidence on home fortification for pregnant women, suggesting that micronutrient powders for point‐of‐use fortification of foods did not have any clear effect on maternal anaemia and haemoglobin at or near term, compared with multiple micronutrient supplements ( Suchdev 2014 ). Another review of micronutrient powders in women and children also suggests that they are effective in improving anaemia and haemoglobin among children reporting lack of impact on growth; evidence of increased diarrhoea requires careful consideration before recommending the intervention for large‐scale implementation ( Salam 2013 ).
Implications for practice
The evidence from this review suggests that MMN fortification when compared to placebo may improve anaemia, iron deficiency anaemia, micronutrient deficiencies (including iron, vitamin A, vitamin B2 and vitamin B6 deficiency), serum haemoglobin, serum folate, serum ferritin, serum vitamin A, serum vitamin B12 and some motor and cognitive outcomes. However, there are a number of other factors that should also be considered. Firstly, the quality of the evidence was low to very low. Secondly, there are no reported data to assess possible side effects of the MMN fortification. Thirdly, we could not draw reliable conclusions from various subgroup analyses on population groups, food vehicles, dosage and region, due to a limited number of studies in each subgroup and measuring varying outcomes. Lastly, we remain cautious about the level of commercial funding among the included studies, although a direct effect of commercial funding was not demonstrated in this review.
Implications for research
The findings of our review provide a number of implications for future research. Future research should focus on generating high‐quality evidence with longer follow‐ups, and assessing the impact in various population groups. The evidence can be consolidated with the use of larger sample sizes and better study designs. It would also be important for study authors to report allocation, randomisation and blinding procedures in detail. There is a need for non‐commercially funded studies and independent evaluations. There are limited data on how fortification affects population groups with variable baseline health status and underlying micronutrient deficiencies and levels of malnutrition. Research should also focus on evaluating the direct health outcomes, including morbidities, mortality and adverse events, especially in LMIC settings. It is also important to report on equity variables for future studies, to assess whether food fortification has any impact on equity.
| 19 February 2020 | Amended | Typo corrected in Plain language summary (removal of the word 'Title in heading) |
Acknowledgements
We thank members of Cochrane Public Health for their extensive editorial support during the preparation and finalisation of this review.
We would also like to acknowledge Ms. Sultana Jabeen for assisting us with the PROGRESS‐PLUS criteria.
Appendix 1. MEDLINE search strategy
(food or crops or crop or flour or salt or salts or fish or soy foods or sauce* or cereals or carbohydrates or sugar* or Oryza sativa or rice* or milk or bread or oil or oils or beverages or yogurt or margarine or cheese or maize* or condiments or triticum or wheat* or spice or spices or curry powder* or fats or fat or dairy).mp. OR exp Zea mays/
2. Micronutrients
(iron or ferr* or iodine or vitamin a or beta carotene or folic or folate* or micronutrients).mp.
3. Fortification
(enrich* or forti* or enhance* or refine*).mp.
1 AND 2 AND 3
Appendix 2. Embase search strategy
1. (food or 'food supply' or crop or bread or flour or salt or 'fish products' or 'soy food*' or sauce or sugar or wheat or triticum or rice or cereal* or grain* or cheese or dairy or cake* or biscuit* or juice* or yogurt or chocolate or margarine or milk or oil or butter or cream or yogurt or beverages or spice or 'curry powder*' or 'dietary fats' or fat or fats or condiment*)
2. Iron or ferr* or Iodine or Vitamin A or beta Carotene or Folic Acid or folate* or Micronutrients
3. enrich* or forti* or enhance* or refine*
Appendix 3. CINAHL
1. (food or “food supply” or crop or bread or flour or salt or “fish products” or “soy foods” or sauce or sugaror wheat or triticum or rice or cereal* or grain* or cheese or dairy or cake* or biscuit* or juice* or yogurt or chocolate or margarine or milk or maize or oil or butter or cream or yogurt or beverages or Spices or "curry powder*" or fat or fats or condiment*)
3. (enrich* or forti* or enhance* or refine*)
Appendix 4. Cochrane
(Food or "Food Supply" or "Agricultural Crops" or crop or Flour or Salts or "Fish Products" or "Soy Foods" or sauce* or Cereals or "Dietary Carbohydrates" or sugar* or "Oryza sativa" or rice or Milk or Bread or Oils or Beverages or Yogurt or Margarine or Cheese or "Zea mays" or maize* or Condiments or Triticum or wheat* or Spices or "curry powder" or "Dietary Fats" or fat or fats or "Dairy Products") AND (Iron or ferr* or Iodine or "Vitamin A" or "beta Carotene" or "Folic Acid" or folate or Micronutrients) AND (enrich or fortified or enhance or refine)
Appendix 5. WHOLIS
words or phrase “food or food supply or crop or bread or flour or salt or fish product or soy food or sauce or sugar or wheat or triticum or rice or cereal or grain or cheese or dairy or cake or biscuit or juice or yogurt or chocolate or margarine or milk or oil or butter or cream or yogurt or beverages or spices or curry powder or dietary fat or fats or fat” AND words or phrase "fortified or fortification or enriched or enhanced or refined” AND Iron or ferr* or Iodine or Vitamin A or beta Carotene or Folic Acid or folate* or Micronutrients
Appendix 6. Others
Fortification or fortified or enriched (Subject)
Fortified (All fields) OR Enrich (All fields) OR fortification (All fields)
African Index Medicus:
fortification or fortified or enriched (titles and keywords)
fortification or fortified or enriched (with atleast one word)
fortified or fortification or enriched
allintitle: fortified OR fortification OR enriched
fortified OR fortification OR enriched
3ie Database of Impact studies
fortification OR enriched OR fortified (Health and Nutrition and Population subcategory
fortification OR enriched OR fortified (Free text terms)
fortification OR fortified AND food discipline:(05T ‐ Health services, health administration, community care services) discipline:(06H ‐ Food technology, food microbiology)
Clinical trials.gov:
"food fortification" OR fortified (restricted to interventional studies only)
http://apps.who.int/trialsearch/AdvSearch.aspx
"food fortification" OR fortified or enriched (titles)
Food Science and Technology Abstracts
fortification OR enriched OR fortified
AgriCOLA: https://agricola.nal.usda.gov/
Edited (no change to conclusions)
Data and analyses
Comparison 1.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 11 | 3746 | Risk Ratio (Random, 95% CI) | 0.68 [0.56, 0.84] | |
| 6 | 2189 | Risk Ratio (M‐H, Random, 95% CI) | 0.28 [0.19, 0.39] | |
| 11 | 3289 | Risk Ratio (Random, 95% CI) | 0.44 [0.32, 0.60] | |
| 6 | 1482 | Risk Ratio (Random, 95% CI) | 0.42 [0.28, 0.62] | |
| 4 | Risk Ratio (Random, 95% CI) | Subtotals only | ||
| 5.1 Vitamin B2 | 1 | 296 | Risk Ratio (Random, 95% CI) | 0.36 [0.19, 0.68] |
| 5.2 Vitamin B6 | 2 | 301 | Risk Ratio (Random, 95% CI) | 0.09 [0.02, 0.38] |
| 5.3 Vitamin B12 | 3 | 728 | Risk Ratio (Random, 95% CI) | 0.42 [0.25, 0.71] |
| 5 | 1490 | Risk Ratio (M‐H, Random, 95% CI) | 0.84 [0.65, 1.08] | |
| 8 | 2889 | Mean Difference (IV, Random, 95% CI) | 0.10 [0.02, 0.17] | |
| 6 | 1758 | Mean Difference (IV, Random, 95% CI) | 0.10 [0.02, 0.18] | |
| 8 | 2889 | Mean Difference (IV, Random, 95% CI) | 0.09 [0.01, 0.18] | |
| 20 | 6985 | Mean Difference (Random, 95% CI) | 3.01 [2.14, 3.87] | |
| 7 | 2407 | Mean Difference (Random, 95% CI) | 8.27 [3.26, 13.27] | |
| 7 | Mean Difference (Random, 95% CI) | Subtotals only | ||
| 12.1 Vitamin B1 (nmol/L) | 1 | 118 | Mean Difference (Random, 95% CI) | 4.8 [‐2.77, 12.37] |
| 12.2 Vitamin B6 (nmol/L) | 2 | 301 | Mean Difference (Random, 95% CI) | 35.02 [22.95, 47.09] |
| 12.3 Vitamin B9 (nmol/L) | 5 | 568 | Mean Difference (Random, 95% CI) | 12.41 [6.55, 18.28] |
| 12.4 Vitamin B12 (pmol/L) | 6 | 893 | Mean Difference (Random, 95% CI) | 61.90 [53.56, 70.23] |
| 13 | 2457 | Mean Difference (Random, 95% CI) | 0.04 [‐0.01, 0.09] | |
| 15 | 4428 | Mean Difference (IV, Random, 95% CI) | 0.25 [‐0.05, 0.55] | |
| 6 | Mean Difference (Random, 95% CI) | Subtotals only | ||
| 15.1 Motor development score | 1 | 266 | Mean Difference (Random, 95% CI) | 1.1 [0.17, 2.03] |
| 15.2 Raven's coloured matrices | 2 | 1124 | Mean Difference (Random, 95% CI) | 0.13 [‐0.86, 1.11] |
| 15.3 General intelligence | 1 | 251 | Mean Difference (Random, 95% CI) | ‐0.07 [‐0.34, 0.20] |
| 15.4 Verbal learning and memory | 1 | 251 | Mean Difference (Random, 95% CI) | 0.13 [‐0.10, 0.37] |
| 15.5 Visual attention | 1 | 251 | Mean Difference (Random, 95% CI) | 0.09 [‐0.11, 0.29] |
| 15.6 Coding | 3 | 509 | Mean Difference (Random, 95% CI) | ‐0.53 [‐1.26, 0.21] |
Comparison 2
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 8 | 2889 | Mean Difference (IV, Random, 95% CI) | 0.10 [0.02, 0.17] | |
| 1.1 ≤ six months | 5 | 1598 | Mean Difference (IV, Random, 95% CI) | 0.06 [‐0.02, 0.14] |
| 1.2 > six months to one year | 3 | 1291 | Mean Difference (IV, Random, 95% CI) | 0.13 [‐0.00, 0.25] |
| 8 | 2889 | Mean Difference (IV, Random, 95% CI) | 0.09 [0.01, 0.18] | |
| 2.1 ≤ six months | 5 | 1598 | Mean Difference (IV, Random, 95% CI) | 0.05 [‐0.04, 0.14] |
| 2.2 > six months to one year | 3 | 1291 | Mean Difference (IV, Random, 95% CI) | 0.14 [0.01, 0.27] |
Comparison 3
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 11 | Risk Ratio (Random, 95% CI) | 0.68 [0.56, 0.84] | ||
| 1.1 Non‐commercial funding | 4 | Risk Ratio (Random, 95% CI) | 0.43 [0.17, 1.10] | |
| 1.2 Partial commercial funding | 3 | Risk Ratio (Random, 95% CI) | 0.66 [0.54, 0.81] | |
| 1.3 Full commercial funding | 4 | Risk Ratio (Random, 95% CI) | 0.77 [0.65, 0.92] | |
| 11 | Risk Ratio (Random, 95% CI) | 0.43 [0.32, 0.59] | ||
| 2.1 Non‐commercial funding | 4 | Risk Ratio (Random, 95% CI) | 0.45 [0.18, 1.08] | |
| 2.2 Partial commercial funding | 3 | Risk Ratio (Random, 95% CI) | 0.23 [0.10, 0.56] | |
| 2.3 Full commercial funding | 4 | Risk Ratio (Random, 95% CI) | 0.45 [0.32, 0.64] |
Comparison 4
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 | Risk Ratio (Random, 95% CI) | Totals not selected | ||
| 2 | 245 | Risk Ratio (Random, 95% CI) | 0.40 [0.09, 1.83] | |
| 1 | Risk Ratio (Random, 95% CI) | Totals not selected | ||
| 2 | 363 | Risk Ratio (Random, 95% CI) | 0.19 [0.07, 0.55] | |
| 2 | 559 | Mean Difference (Random, 95% CI) | 10.20 [3.06, 17.35] | |
| 1 | 88 | Mean Difference (Random, 95% CI) | 0.18 [‐36.14, 36.50] | |
| 1 | Mean Difference (Random, 95% CI) | Totals not selected | ||
| 7.1 Vitamin B9 | 1 | Mean Difference (Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 7.2 Vitamin B12 | 1 | Mean Difference (Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 2 | 363 | Std. Mean Difference (Random, 95% CI) | 2.82 [‐2.88, 8.51] | |
| 1 | Mean Difference (Random, 95% CI) | Subtotals only |
Comparison 5
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | ||
| 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | ||
| 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | ||
| 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected |
Characteristics of studies
Characteristics of included studies [ordered by study id].
| Methods | Randomised controlled trial | |
| Participants | Participants in the study were 566 apparently healthy male and female children attending 2 government‐operated primary schools located in Akanga and Akaleku, Nasarawa State, Nigeria, who met the following criteria: 5 – 13 year old and haemoglobin ≥ 70 g/L | |
| Interventions | Intervention (n = 288): Children received a single daily serving of a multi‐micronutrient beverage or a placebo beverage 5 days/week for 6 months. The beverages were isoenergetic and were composed of a proprietary blend of precooked maize and soy protein isolate, providing 689 kJ energy, 4.1 g fat, and 5.2 g protein/serving. Control (n = 278): Placebo Food vehicle: Beverage Dose: Retinol palmitate, 1 mg retinol equivalents, D‐biotin, 47 mg, ascorbic acid, 60 mg, cholecalciferol, 1.2 mg, dl‐a‐tocopherol acetate, 6.5 mg, folic acid, 200 mg, niacinamide, 18 mg, calcium D‐pantothenate, 2.3 mg, pyridoxine HCl, 2 mg, riboflavin, 1.6 mg, thiamine HCl, 1 mg, calcium carbonate and calcium lactate, 84 mg, copper sulfate 5‐hydrate, 1.2 mg, potassium iodate, 150 mg, ferrous bisglycinate chelate and ferrous sulfate, 14 mg, magnesium oxide, 49 mg, manganese glycinate chelate, 4.5 mg, molybdenum amino acid chelate, 29 mg, monosodium phosphate anhydrous, 187 mg, potassium chloride, 276 mg, selenium amino acid complex, 24.8 mg, vanadium nicotinate glycinate chelate, 25 mg, zinc glycinate chelate and zinc oxide, 15 mg, bioflavonoids, 87.5 mg Duration: 6 months Additional Interventions: Per school policy, all children were given a single 200 mg dose of albendazole 1 week prior to the baseline blood draw and 1 month prior to the final blood draw | |
| Outcomes | Haemoglobin, serum ferritin, serum retinol, serum zinc | |
| Notes | Supported by funding from Global Alliance for Improved Nutrition (Geneva, Switzerland). The multi‐micronutrient beverage was developed by International Nutrition and Sport S.A. (Pty) Limited Study duration: January to August 2007 | |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Rosters were obtained from both schools and, using a stratified sampling scheme, children were randomly assigned to groups at the individual level proportionate to the number of male and female students in each school and class level." Comment: Insufficient information to permit judgement |
| Allocation concealment (selection bias) | Unclear risk | Quote: "Rosters were obtained from both schools and, using a stratified sampling scheme, children were randomly assigned to groups at the individual level proportionate to the number of male and female students in each school and class level." Comment: Insufficient information to permit judgement |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "Products were masked for taste, colour, aroma, texture, and packaging and were labelled with unique product codes, which were maintained by the manufacturer until after the data were analysed." Comment: Adequately done |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "Products were masked for taste, colour, aroma, texture, and packaging and were labelled with unique product codes, which were maintained by the manufacturer until after the data were analysed." Comment: Adequately done |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Intervention group: 18/288 Control: 14/278 Overall 6% attrition rate Comment: Low attrition rate unlikely to affect results |
| Selective reporting (reporting bias) | Low risk | Comment: Trial registration or published protocol not identified, but the outcomes specified in the methodology section have been reported in the results |
| Other bias | Low risk | No additional bias identified |
| Methods | Controlled before‐after study | |
| Participants | Conducted in Botswana. 311 Children were considered eligible for the study if they were 5 – 11 years old, weighed > 15 kg, had a haemoglobin concentration > 60 g/L and had no known chronic illnesses such as HIV or recent acute illnesses | |
| Interventions | Intervention (n = 164): Children were to receive an average of 240 mL daily of the fortified beverage under direct observation. The treatment group received a fruit‐flavoured beverage containing 419 kJ/240 mL with a proprietary blend of micronutrients. 7 servings were given a week for a total of 1680 ml/week. Because it was not possible to administer drinks on weekends, we gave 2 drinks to each participant on Monday and Friday Control (n = 147): Placebo Food vehicle: Fruit‐flavoured beverage Dose: B‐carotene, 2400 ug, riboflavin 0.4 mg, niacin 2.7 mg, pyridoxine HCl 0.5 mcg, folic acid 140 ug, cyanocobalamin 1 ug, ascorbic acid 60 mg, dl‐a ‐tocopherol acetate 7.5 mg, tricalcium phosphate 120 mg, ferrous bisglycinate chelate 7 mg, potassium iodide 60 g, zinc gluconate 3.75 mg Duration: 8 weeks | |
| Outcomes | Weight, mid‐upper arm circumference, haemoglobin, retinol, ferritin, vitamin B12, folate and riboflavin status | |
| Notes | This project was financed in part with federal funds from the USDA/ARS under Cooperative Agreement number 58–6250‐6–001 and by The Minute Maid Company, Houston, TX Study duration: Study dates not reported. | |
| Random sequence generation (selection bias) | High risk | Quote: "All students in one school were provided the experimental beverage, whereas the control beverage was given at the other school. By assigning the subjects to either the fortified beverage or control, using their school for assignment, we hoped to be able to avoid compromising the study by an error in administration, while still obtaining meaningful results." Comment: High risk |
| Allocation concealment (selection bias) | High risk | Quote: "All students in one school were provided the experimental beverage, whereas the control beverage was given at the other school. By assigning the subjects to either the fortified beverage or control, using their school for assignment, we hoped to be able to avoid compromising the study by an error in administration, while still obtaining meaningful results." Comment: High risk |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote: "On the basis of input from local medical and educational officials, it was determined that there was a substantial risk of confusing the beverages by attempting to provide more than one drink at each school, and that maintaining blinding would then be nearly impossible." Comment: High risk |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Quote: "On the basis of input from local medical and educational officials, it was determined that there was a substantial risk of confusing the beverages by attempting to provide more than one drink at each school, and that maintaining blinding would then be nearly impossible." Comment: High risk |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Intervention group: 19/164 Control group: 29/147 Overall 15% attrition rate Comment: Low attrition rate |
| Selective reporting (reporting bias) | Low risk | Comment: Published protocol not identified, but the outcomes specified in the methodology section have been reported in the results |
| Other bias | Unclear risk | Baseline outcome measurements: Quote: "The baseline anthropometric and biochemical studies supported the equivalence of the two groups" Comment: Low risk Baseline characteristics: Quote: ""There were no significant differences in baseline characteristics between subjects who completed the study and those who did not." Comment: Low risk Protection against contamination: Quote: "On the basis of input from local medical and educational officials, it was determined that there was a substantial risk of confusing the beverages by attempting to provide more than one drink at each school, and that maintaining blinding would then be nearly impossible. Two schools were required to provide an adequate number of subjects for the trial." Comment: Low risk |
| Methods | Controlled before‐after study conducted among all primary school‐going children aged 6 ‐ 11 years in 10 disadvantaged sub‐districts in Bangladesh from September 2011 to November 2012 | |
| Participants | 368 primary school children at baseline and 351 children at endline | |
| Interventions | Intervention (n = 191): Daily administration of a packet of fortified biscuit to all primary school‐going children aged 6 to 11 years. Biscuit ingredients were: wheat flour (69% by weight); sugar (12%); vegetable fat (hydrogenated‐75% and liquid‐25% ‐ 13%); soya flour (6%); iodised salt (0.5%); leavening agent (1.0%) and micronutrient premix (1.5 kg premix in 998.5 kg biscuit dough). The fortified biscuit was prepared to provide 300 kcal per single 75 gm packet (approximately 15% of daily calorie requirements), and a range of micronutrients contributing to about 75% of the daily requirements of vitamin A, folate, iron, iodine, zinc and magnesium Energy: 450 kcal Moisture (maximum): 4.5% Protein: 10 ‐ 15 g Fat: 15 g Calcium: 212.5 ‐ 287.5 mg, magnesium: 127.5 ‐ 172.5 mg, vitamin A (retinol): 212.5 ‐ 287.5 mcg, vitamin D: 1.615 ‐ 2.185 mcg, vitamin E: 4.25 ‐ 5.75 mg, Vitamin B1: 0.425 ‐ 0.575 mg, vitamin B2: 0.595 ‐ 0.805 mg, vitamin B3 (niacin): 5.1 ‐ 6.9 mg, vitamin B5 (pantothenic acid): 2.55 ‐ 3.45 mg, vitamin B6: 0.85 ‐ 1.15 mg, vitamin B12: 0.425 ‐ 0.575 mcg, folic acid: 680 ‐ 920 mcg, vitamin C: 17.0 ‐ 23.0 mg, iron: 9.35 ‐ 12.65 mg, iodine: 63.75 ‐ 86.25 mcg zinc: 7.00 ‐ 8.00 mg Control area (n = 177): did not receive any intervention Duration: 12 months | |
| Outcomes | Haemoglobin levels, micronutrient (ferritin, folic acid, vitamin B12, retinol, zinc, iodine, vitamin D) levels, anaemia | |
| Notes | This study was supported by the European Union (EU), through a sub‐contract from the James P. Grant School of Public Health, BRAC University, Dhaka Bangladesh Study duration: September 2011 to November 2012 | |
| Random sequence generation (selection bias) | High risk | Quote: "The quantitative component assessed the impact of micronutrient fortification on 351 children aged 6 ‐ 11 years using a cohort pre‐post research design with a control group." Comment: High risk |
| Allocation concealment (selection bias) | High risk | Quote: "The quantitative component assessed the impact of micronutrient fortification on 351 children aged 6 ‐ 11 years using a cohort pre‐post research design with a control group." Comment: High risk |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Comment: The control group did not receive any intervention. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Comment: The control group did not receive any intervention. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Intervention group: 2/191 Control group: 15/177 Overall 4.6% attrition rate Comment: Low risk for attrition bias |
| Selective reporting (reporting bias) | Low risk | Comment: Published protocol not identified, but the outcomes specified in the methodology section have been reported in the results |
| Other bias | Unclear risk | Baseline outcome measurements: Quote: "at baseline, the characteristics of primary school students in intervention and control groups were largely similar." Comment: Low risk Baseline characteristics: Quote: "at baseline, the characteristics of primary school students in intervention and control groups were largely similar." Comment: Low risk Protection against contamination: Quote:"Similar measurements were made on a control group of primary school children living in adjacent sub‐districts where the program had not been implemented." Comment: Low risk |
| Methods | Randomised controlled trial | |
| Participants | Conducted in Tanzania. Participants included 830 rural children (aged 6 – 11 years) attending primary schools | |
| Interventions | Intervention (n = 382): Fortified beverage. One serving of the beverage was provided at school during the morning recess Control (n = 392): Unfortified beverage provided 90 kcal in each 25 g individual‐serving sachet Dose: iron 5.4 mg, Vit A 1750 IU, iodine 45 ug, zinc 5.25 mg, ascorbic acid 72 mg, riboflavin 0.6 mg, folic acid 0.14 mg, vit B 12.3 ug, B6 0.7 mg, E 10.5 mg in 25 g sachet Food vehicle: The content of 1 sachet was mixed with 250 mL previously boiled water to make a pleasant‐tasting, orange‐flavoured beverage Duration: 6 months | |
| Outcomes | Serum haemoglobin, ferritin, protoporphyrin, retinol, height, weight , BMI | |
| Notes | The dietary supplement used was developed and produced by food technologists at Procter & Gamble and was made available in the form of a multiple‐micronutrient beverage powder. The beverage was developed to be nutritionally adequate and pleasant‐ tasting without problems of nutrient instability, off colour, or off flavour Study duration: November–December 1995 to July–August 1996 | |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Each child in each stratum was then randomly allocated to receive either the fortified or unfortified beverage in a double‐blind manner." Comment: Insufficient information to permit judgement |
| Allocation concealment (selection bias) | Unclear risk | Quote: "Each child in each stratum was then randomly allocated to receive either the fortified or unfortified beverage in a double‐blind manner." Comment: Insufficient information to permit judgement |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "The fortified beverage and the unfortified (placebo) beverage were identical in terms of taste and appearance", "The research team, schoolteachers, and schoolchildren were blinded as to whether the sachets were fortified or unfortified, i.e., the meaning of the label colours was not revealed" Comment: Adequately done |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote:"The research team, schoolteachers, and schoolchildren were blinded as to whether the sachets were fortified or unfortified, i.e., the meaning of the label colours was not revealed" Comment: Adequately done |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Total loss to follow‐up 7% (56/830), Comment: Low attrition rate |
| Selective reporting (reporting bias) | Low risk | Comment: Trial registration or published protocol not identified, but the outcomes specified in the methodology section have been reported in the Results |
| Other bias | Low risk | No additional bias identified |
| Methods | A double‐blind CBA study conducted among school‐aged children aged 7 ‐ 9 years living in a rural and mountainous area of Morocco between February and October 2012 | |
| Participants | 194 school children aged 7 ‐ 9 years were recruited from 3 primary schools | |
| Interventions | Children were divided into 2 groups to receive 200 ml of either: fortified milk (fortified milk group: FMG) or non‐fortified milk (non‐fortified milk group: NFMG) The intervention (n = 79) was delivered to children by the headmaster or by the teachers with consumption of milk during the morning under close supervision Energy (Kcal): 154.8 Fat (%): 5.8 Protein (g): 5.8 Lipids (g): 6 Carbohydrates (g): 19.44 Calcium (mg): 240, iron (mg): 4.2, iodine (g): 45, vitamin A (g): 240, vitamin D3 (g): 3, Control group (n = 115): NFMG Duration: 9 months | |
| Outcomes | Serum vitamin A levels and vitamin A deficiency | |
| Notes | Milk was provided by the Foundation for Child Nutrition. Study duration: February to October 2012 | |
| Random sequence generation (selection bias) | High risk | Quote: "This study is a longitudinal interventional, double‐blind (participants and assessors), and controlled one." Comment: High risk |
| Allocation concealment (selection bias) | High risk | Quote: "This study is a longitudinal interventional, double‐blind (participants and assessors), and controlled one." Comment: High risk |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "The fortified and unfortified milk were similar in macro‐nutrient composition, taste, aroma, texture and packaging but not in micronutrients content" Comment: Low risk |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "The fortified and unfortified milk were similar in macro‐nutrient composition, taste, aroma, texture and packaging but not in micronutrients content" Comment: Low risk |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Interventiong roup: 10/79 Control group: 4/115 Over 7% attrition rate Comment: Low risk of attrition |
| Selective reporting (reporting bias) | Low risk | Comment: The study was retrospectively registered in the Pan African Clinical Trial. Registry with the identification number PACTR201410000896410. Outcomes specified in the methodology have been reported in the results section. |
| Other bias | Unclear risk | Baseline outcome measurements: Comment: Table 2 reports similar baseline outcome measurements between the two groups hence judged to be at low risk of bias for baseline outcome measurements Baseline characteristics: Quote: "The two groups of children were well‐balanced with respect to age and gender.." Comment: Low risk Protection against contamination: Comment: The intervention and control milk were provided in different school hence judged to be at low risk of contamination. |
| Methods | Randomised controlled trial | |
| Participants | Conducted among 224 independently‐living, frail elderly men and women in the Netherlands The inclusion criteria included: age 70 or older, a need for care services (e.g. home care, meals‐on‐wheels), not participating regularly in physical activities of moderate to high intensity (weekly more than 30 minutes of brisk walking, cycling, gymnastics), BMI (based on self‐reported height and weight) ≤ 25 kg/m or involuntary weight loss, non‐institutionalised, not taking multivitamin supplements for the last month, no terminal disease or rapidly deteriorating health status, and the ability to comprehend the procedures of the study | |
| Interventions | Participants were divided into the following 4 groups: For this review, we have only included data from groups 2 and 4 from the above Intervention: Participants were asked to eat 1 fruit product (100 g portions juice and compote) and 1 dairy product (100 g portions vanilla custard and fruit yogurt, 75 g portions vanilla fruit soft curd cheese) daily for 17 weeks. They were allowed to eat the products either in addition to their daily diet or as a replacement Control: Placebo Food vehicle: A number of fruit and dairy products were enriched with several vitamins and minerals for which elderly people’s intake or status is frequently low Dose: vitamin D (7.5 mg), E (8.9 mg), B1 (1 mg), B2 (1.4 mg), B6 (1.1 mg), folic acid (0.25 mg), B12 (2.5 mg), and C (70 mg), calcium (225 mg), magnesium (75 mg), zinc (4.75 mg), iron (4.25 mg), and iodine (0.24 mg) Duration: 17 weeks | |
| Outcomes | Serum pyridoxine, serum ascorbic acid, serum folate, serum zInc, Serum Iron, mean fitness score, mean performance score | |
| Notes | The Dutch Health Research Council, The Hague, The Netherlands; and Wiebe Visser of the Dutch Dairy Foundation on Nutrition and Health, Maarssen, Roche Nederland B.V., Friesland Coberco Dairy Foods B.V., Campina Melkunie–Mona Division, Bekina Lebensmittel GmbH, subsidiary of Royal Numico NV. Study duration: Enrollment done between January through July 1997 and the intervention continued for 17 weeks. | |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Eligible subjects .. were randomly assigned to: supervised group exercise ...; enriched food products ...; both ..; or a control group .... Group assignment took place before baseline measurements with sealed envelopes." Comment: Insufficient information to permit judgement |
| Allocation concealment (selection bias) | Low risk | Quote: "Group assignment took place before baseline measurements with sealed envelopes." Comment: Adequately done |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote: "The nutritional intervention was intended to be double blinded." Comment: Probably not done |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Quote: "The nutritional intervention was intended to be double blinded." Comment: Probably not done |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Total loss to follow‐up of 26% (56/217) Comment: High attrition rate |
| Selective reporting (reporting bias) | Low risk | Comment: Trial registration or published protocol not identified, but the outcomes specified in the methodology section have been reported in the Results |
| Other bias | Low risk | No other potential sources of bias identified |
| Methods | Cluster‐randomised controlled trial | |
| Participants | Conducted in Cambodia. The clusters were 16 primary schools in rural Kampong Speu province, of which 4 were randomly selected for each study group (n = 1977 children). Schools were eligible if they participated in the World Food Program school meal programme and all children were served breakfast daily | |
| Interventions | Intervention: Children received 1 of 3 types of fortified rice or placebo (unfortified white rice) 6 days a week for 6 months: UltraRice_original (n = 479), UltraRice_improved (n = 500), NutriRice (n = 506) Control: Placebo (n = 492) Food vehicle: 3 types of fortified rice Dose: UltraRice original: Iron 10.67 mg, zinc 3 mg, vitamin B1 1.1 mg, folate 0.2 mg; UltraRice improved: retinol 0.64 mg, iron 7.55 mg, zinc 2.0 mg, vitamin B1 1.4 mg, vitamin B3 12 mg, folate 0.3 mg, vitamin B12 0.004; NutriRice: retinol 0.29 mg, iron 7.46 mg, zinc 3.7 mg, vitamin B1 0.7 mg, vitamin B3 8 mg, vitamin B6 0.92 mg, folate 0.1 mg, vitamin B12 0.001 mg Duration: 6 months Additional interventions: After baseline data collection, all children received a single dose of 500 mg mebendazole | |
| Outcomes | Hookworm infection risk; cognitive outcomes | |
| Notes | The research described received funding from United States Department of Agriculture/FAS through a grant (FFE‐442‐2012/038‐ 00, 10.608) to PATH and internal funding from WFP (through the WFP/DSM partnership) and IRD Study duration: November 2012 to June 2013. | |
| Random sequence generation (selection bias) | Low risk | Quote: "Three different randomizations, combining different schools to one intervention, were separately generated based on a list number of children per school by iteration to fit the predefined criteria of group size (within 10% of the mean)." Comment: Adequately done |
| Allocation concealment (selection bias) | Low risk | Quote: "A researcher not involved in the field work (MAD) blindly picked one of the three randomizations, and allocated each group of schools to an intervention arm." Comment: Adequately done |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "The entire research team and all participants and caregivers were blinded to the allocation. The code was only known to one person with WFP, responsible to allocate the correct type of rice to the right school." Comment: Adequately done |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "The entire research team and all participants and caregivers were blinded to the allocation. The code was only known to one person with WFP, responsible to allocate the correct type of rice to the right school." Comment: Adequately done |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Placebo group: 184/492 UltraRice_original: 123/479 UltraRice_improved: 188/500, NutriRice: 281/506 Loss to follow‐up ranges from 26% to 44% per group Comment: High attrition rate |
| Selective reporting (reporting bias) | High risk | The trial is registered on ClinicalTrials.gov NCT01706419. The study was powered for its primary outcomes (micronutrient status), which are not reported here |
| Other bias | Low risk | Comment: No additional biases identified |
| Methods | Randomised controlled trial | |
| Participants | 176 healthy children (aged 6 ‐ 10 years) were recruited to participate in a 12‐week double‐ blind, randomised controlled trial at Boston University Medical Center and Tufts Medical Center in Boston, MA. In January through June 2005 and 2006, children were recruited from the hospital paediatric clinics and through local print and online classified advertisements Exclusion criteria used to screen potential participants included a history of rickets, diabetes, intestinal malabsorption (i.e. cystic fibrosis, fat malabsorption syndrome, or Crohn’s disease) or severe medical illness, including renal failure; allergies to orange juice; any medical conditions precluding daily consumption of orange juice; currently taking, or having taken < 1 month before start of study, a prescription vitamin D supplement | |
| Interventions | All 3 intervention groups consumed 2 x 240‐mL (16 oz) glasses of juice a day. Total daily intake of micronutrients by study group was as follows: Orange juice preparations were isocaloric and provided 110 kcal/240 mL for a total contribution of 220 kcal/day. Orange juice was home‐delivered every 2 weeks, and log sheets of deliveries were maintained. Study participants were instructed to drink 2 x 240‐mL glasses of orange juice a day using a re‐useable cup holding 8 oz (240 mL) to measure juice Duration: 12 weeks The interventions arms eligible for the review were CaDEA (2) and Ca alone (3). | |
| Outcomes | Calcium, phosphorous, albumin, alkaline phosphatase, 25‐hydroxyvitamin D, parathyroid hormone, retinol, a‐tocopherol | |
| Notes | Sponsored by The Beverage Institute for Health & Wellness, The Coca Cola Company, Atlanta, GA Study duration: January through June during 2005 and 2006. | |
| Random sequence generation (selection bias) | Low risk | Quote: "Participants were randomised by a computer‐generated code into one of the three beverage intervention groups." Comment: Adequately done |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "Beverages were blind‐packaged in colour and number coded containers by the manufacturer (Minute Maid)." Comment: Adequately done |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "Beverages were blind‐packaged in colour and number coded containers by the manufacturer (Minute Maid)." Comment: Adequately done |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Total loss to follow‐up 25% Comment: High attrition rate |
| Selective reporting (reporting bias) | Low risk | Comment: Trial registration or published protocol not identified, but the outcomes specified in the methodology section have been reported in the Results |
| Other bias | Low risk | No additional bias identified |
| Methods | Randomised controlled trial | |
| Participants | The study area was in The Valley of a Thousand Hills in KwaZulu‐Natal province, South Africa. All eligible infants (n = 361) who were aged 6 – 12 months at baseline were asked to participate | |
| Interventions | Intervention (n = 180):The finely milled maize meal was fortified to supply 3 mg carotene, 11 mg iron (ferrous fumarate), and 3 mg zinc (zinc sulfate) per 40 g dry product, Ascorbic acid (sodium ascorbate) was added (56 mg/40 g dry product) to enhance iron absorption. The maize meal was further fortified with certain nutrients that are limited in the diet of South African children, so that it supplied 110 g copper, 10 g selenium, 0.4 mg riboflavin, 0.15 mg vitamin B6, 0.25 g vitamin B12, and 2.5 mg vitamin E per 40 g dry product Control (n = 181): Same porridge with no added nutrients Dose: The mothers helped to identify a suitable portion size, which was set at 20 g dry product, mixed with 125 mL milk or water. The dry product was packed in individual 25 g colour‐coded sachets; the additional 5 g/sachet allowed for spillage and the mother’s tasting. An intake of 2 sachets a day was recommended, consumed as either 1 or 2 meals Food vehicle: Porridge Duration: 6 months | |
| Outcomes | Motor development, anthropometrics (weight, length, LAZ, WAZ, WLZ), serum haemoglobin, ferritin, retinol and zinc, CRP | |
| Notes | Supported by the Thrasher Research Fund and the Community‐based Health Programme of The Valley Trust. Tiger Food Brands Limited donated the fortified‐porridge product Study duration: First phase: February to August 2002 Second phase: September 2002 to March 2003. | |
| Random sequence generation (selection bias) | Low risk | Quote: "The allocation schedule was generated in blocks of 8 by the drawing of a sticker from a container that contained 4 yellow and 4 green stickers. Infants were randomly assigned in the order that they completed the baseline survey." Comment: Adequately done |
| Allocation concealment (selection bias) | Unclear risk | "Color‐coding was used to distinguish between the 2 treatment groups. The project leader was aware of which porridge each of the groups was receiving, because the fortified porridge had a slight yellow colour due to the carotene used as fortificant." Comment: Cannot ascertain if this affected outcome |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Quote: "The mothers and community health workers were not aware of which porridge was fortified. All baseline and postintervention measurements were done in a blinded manner." "The project leader was aware of which porridge each of the groups was receiving, because the fortified porridge had a slight yellow colour due to the ‐carotene used as fortificant." Comment: Participant blinding conducted adequately, but cannot ascertain if personnel blinding was adequate |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Quote: "All baseline and postintervention measurements were done in a blinded manner." "The project leader was aware of which porridge each of the groups was receiving, because the fortified porridge had a slight yellow colour due to the carotene used as fortificant." Comment: Cannot ascertain if personnel blinding was adequate |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Intervention group: 36/180 Control group: 36/181 Overall 19.12% loss to follow‐up Comment: Low attrition rate |
| Selective reporting (reporting bias) | Low risk | Comment: Trial registration or published protocol not identified, but the outcomes specified in the methodology section have been reported in the Results |
| Other bias | Low risk | No additional bias identified |
| Methods | Randomised controlled trial | |
| Participants | This 12‐month RCT was conducted on 6‐month‐old Zambian infants (n = 743) living in Chilenje, a middle‐income area of Lusaka, Zambia. The study was conducted from October 2005 to July 2009. Eligible infants aged 6 months ± 2 weeks whose parents or guardians gave written informed consent were randomly assigned to receive either a richly micronutrient‐fortified porridge or a basal porridge | |
| Interventions | Intervention (n = 373): Caregivers received individual instructions by the project nutritionist about how to prepare and cook the porridge according to the package directions and how to feed the Chilenje porridge in place of other porridges. They were each supplied with a spoon (5 mL) to measure the amount of porridge flour (i.e. 7 level spoonfuls of dry flour, ˜ 30 g dry flour), and a plastic feeding cup graduated in millilitres to measure the volume of water required (i.e. 250 mL). The slurry was cooked (5 – 10 minutes) and transferred into the graduated plastic feeding cup, which was then used to feed the child. The volume of porridge fed to the child was noted by the caregiver at the end of each feeding (in millilitres). Porridges prepared according to the package directions had an energy density of 0.76 kcal/g (1 kcal = 4.18 kJ), 16% energy from protein, and an analysed phytate content of 5.8 g/kg (dry weight) Control (n = 370): Placebo Food vehicle: Porridge Dose: Vitamin A, retinol equivalents 6.5 ug/kg, vitamin C, 2 g/kg, cholecalciferol, 0.1 mg/kg, thiamine (mononitrate), 9 mg/kg, riboflavin, 11.2 mg/kg, niacin (niacinamide), 140 mg/kg, pyridoxine (HCl), 8.6 mg/kg, folate, 2.21 mg/kg, vitamin B12, 9.75 mg/kg, pantothenic acid, 40.3 mg/kg, iron (ferrous fumarate), 250 mg/kg, zinc (oxide), 200 mg/kg, copper (gluconate), 3.2 mg/kg, manganese (sulphate monohydrate), 12 mg/kg, selenium (sodium selenite) 0.2 mg/kg, Calcium 6.8 g/kg, Phosphorous 5.3 g/kg, Magnesium (oxide) 943 mg/kg. Duration: 1 year Additional interventions: All infants in the RCT were supplemented with vitamin A capsules at their 6‐, 12‐, and 18‐month clinic visits, according to the standard protocol of care, through the government national vitamin A supplementation programme | |
| Outcomes | Anaemia, iron deficiency, iron deficiency anaemia, zinc deficiency | |
| Notes | Supported by the Bill and Melinda Gates Foundation. Micronutrients were provided by DSM Nutritional Products, Isando, South Africa Study duration: October 2005 to July 2009 | |
| Random sequence generation (selection bias) | Low risk | Quote: " Eligible infants aged 6 months ± 2 wk whose parents or guardians gave written informed consent were randomly assigned to receive either a richly micronutrient‐fortified porridge or a basal porridge using a block randomisation scheme, with a block length of 20." Comment: Adequately done |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "The investigators, clinic staff, outcomes assessors, and participants were unaware of the intervention assignment and knowledge of treatment groups became known only after the database was finalized. An exit questionnaire indicated that the blinding was effective." Comment: Adequately done |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "The investigators, clinic staff, outcomes assessors, and participants were unaware of the intervention assignment and knowledge of treatment groups became known only after the database was finalized. An exit questionnaire indicated that the blinding was effective." Comment: Adequately done |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Intervention: 83/373 Control: 84/370 Loss to follow‐up of 23.3% and 23.7% in control and intervention groups respectively Comment: High attrition rate |
| Selective reporting (reporting bias) | Low risk | This trial was registered at the UK National Institute for Health Research, Current Controlled Trials, ISRCTN Register (www.controlled‐trials.com/mrct/trial/835053/ISRCTN37460449) as ISRCTN37460449 |
| Other bias | Low risk | No additional bias identified. |
| Methods | Randomised controlled trial | |
| Participants | School children (n = 403) aged 6 – 9 years in grade 1 ‐ 3 of 5 primary schools were recruited. The schools were located in 3 communes of 2 districts (Bac Tra My and Tien Phuoc) of Quang Nam province, 900 km south of Hanoi, Vietnam, where micronutrient deficiencies were known to exist. The schools were selected based on their proximity to the general hospital of Tam Ki so that blood samples could be processed within 4 hours of collection | |
| Interventions | Intervention: The treatment groups were as follows: Daily fortified biscuit group and placebo tablet once a week (FB); Daily non‐fortified biscuits and placebo tablet once a week (control group, C); Fe tablet once a week and daily non‐fortified biscuits (weekly Fe pharmaceutical supplementation group, SUP). Biscuits were distributed for 6 months during the break time (09.00 – 09.30 hours), 5 days a week excluding school holidays, weekends and public holidays Control: Placebo and Iron supplement Food vehicle: Biscuit Dose: A daily ration of 5 biscuits (approximately 30 g) covered 50% of the RNI of a 9‐year‐old child for vitamin A (all‐transretinol), Fe (iron fumarate), Zn (zinc sulphate) and iodine, 40 % of the requirements of Cu, vitamin C, thiamin, riboflavin, vitamins B6, B12, E and niacin, 35% of the requirements of Mg, 20% of the requirements of Ca, vitamin D and folate and 7% of the requirements of Mn, Se, K, chloride, Na, fluoride, pantothenic acid, vitamin K and biotin Duration: 6 months Additional interventions: All the children were de‐wormed by the health services of the Quang Nam province with mebendazole (500 mg) a few days after the start of the study The intervention arms eligible for this review were FB and C | |
| Outcomes | Serum haemoglobin, transferrin receptor, ferritin, retinol, zinc, body iron | |
| Notes | Supported by Decentralized French co‐operation, Sight and Life and IRD Study duration: November 2005 to May 2006 | |
| Random sequence generation (selection bias) | Low risk | Quote: "From an alphabetical name list of the children attending the five selected schools, children were randomly assigned into three treatment groups using a computer‐generated random list and all children from all schools were allocated to the three groups." Comment: Adequately done |
| Allocation concealment (selection bias) | Low risk | Quote: "All field staff and researchers as well as teachers were blinded for the group allocation that was kept in a sealed envelope at the NIN until the end of data analysis." Comment: Adequately done |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "All field staff and researchers as well as teachers were blinded for the group allocation that was kept in a sealed envelope at the NIN until the end of data analysis." Comment: Adequately done |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "All field staff and researchers as well as teachers were blinded for the group allocation that was kept in a sealed envelope at the NIN until the end of data analysis." Comment: Adequately done |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Overall loss to follow‐up: 106/403 Total loss to follow‐up 26.3%. Comment: High attrition rate |
| Selective reporting (reporting bias) | Low risk | Comment: Trial registration or published protocol not identified, but the outcomes specified in the methodology section have been reported in the Results |
| Other bias | Low risk | No additional bias identified |
| Methods | Randomised controlled trial | |
| Participants | Conducted in 54 non‐formal primary education (NFPE) schools operated by the Bangladesh Rural Advancement Committee (BRAC, one of the largest national non‐governmental organisations in the world) in Sherpur district, 300 km northeast of Dhaka city. Participants were adolescent girls (n = 1125). Adolescent boys attending the schools (around 30% of the students) were included in the randomisation process and were provided the same beverages to avoid sharing. However, they were not included in any aspect of the data collection or analysis | |
| Interventions | Intervention (n = 559): Fortified orange‐flavoured powdered beverage. The contents of 2 sachets, which contained 90 g powder, were dissolved in 1000 mL of tube‐well water. Each student received 200 mL of the reconstituted fortified or non‐fortified beverage daily Control (n = 566): equal quantity of a non‐fortified orange‐flavoured powdered beverage (identical to the fortified beverage in terms of weight, colour, flavour, and appearance) as a control Dose: micronutrient‐fortified powder in 1 serving (200 mL): Iron, mg 7.0, vitamin A, IU (RE) 1296 (389), iodine, mg 75, zinc, mg 7.5, vitamin C, mg 120, riboflavin, mg 0.91, folic acid, mg 120, vitamin B12, mg 1.0, vitamin B6, mg 1.0, vitamin E, mg 10, niacin, mg 5.0 Food Vehicle: Powdered orange‐flavoured beverage Duration: 6 days a week for 12 months | |
| Outcomes | Haemoglobin and serum levels of ferritin, retinol, zinc, and CRP. Anthropometric measurements including height, weight, and MUAC | |
| Notes | Supported by the Micronutrient Initiative, Ottawa, Canada. Supplement was provided by Procter & Gamble Study duration: Not specified. | |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Randomization was done by listing all selected children, assigning them with random numbers, and dividing the odd numbers from the even numbers to form the 2 groups." Comment: Insufficient information to permit judgement |
| Allocation concealment (selection bias) | Unclear risk | Comment: Insufficient information to permit judgement |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote:"The sachets containing the fortified and non‐fortified beverage differed only in the sachet’s colour (blue or yellow). Researchers, school teachers, shastho shebikas (BRAC community health workers), and students did not know whether the blue or yellow Coloured sachets contained the fortified beverage." "The decoding was done only by the manufacturer after the study was completed and the data analysed." Comment: Adequately done |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote:"The sachets containing the fortified and non‐fortified beverage differed only in the sachet’s colour (blue or yellow). Researchers, schoolteachers, shastho shebikas (BRAC community health workers), and students did not know whether the blue or yellow Coloured sachets contained the fortified beverage." "The decoding was done only by the manufacturer after the study was completed and the data analysed." Comment: Adequately done |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Intervention group: 77/559 Control group: 59/566 12.1% total loss to follow‐up Comment: Low attrition rate unlikely to affect results |
| Selective reporting (reporting bias) | Low risk | Comment: Trial registration or published protocol not identified, but the outcomes specified in the methodology section have been reported in the Results |
| Other bias | Low risk | No additional bias identified |
| Methods | A community‐based double‐blind, randomised, placebo‐controlled trial in 11 rural South African primary schools in 1995 | |
| Participants | The study randomly allocated 579 children aged between 8 and 10 years into 6 study groups | |
| Interventions | The intervention groups were managed as follows: Group 1: de‐worming, biscuits fortified with a combination of micronutrients (vitamin A and iron) and other nutrients (defined below); Group 2: de‐worming, biscuits fortified with vitamin A; Group 3: de‐worming, non‐fortified biscuits (no micronutrients); Group 4: not de‐wormed, biscuits fortified with vitamin A, iron and other nutrients; Group 5: not de‐wormed, biscuits fortified with vitamin A; Group 6: not de‐wormed, non‐fortified biscuits. The 2 biscuits given daily to groups 1 and 4 contained vitamin B (25% RDA, 0.25 mg), vitamin A (50% RD, 350 J.tg), iron in the form of FeEDTA (50% RDA, 5 mg), calcium (25% RDA, 200 mg) and zinc (25% RDA, 2.5 mg) The 2 biscuits given daily to groups 2 and 5 supplied 100% (700 J.tg) RDA vitamin A Duration: 4 months For this review, we have included data from Group 4 and Group 6 only | |
| Outcomes | Micronutrient status (serum retinol, haemoglobin, hematocrit, serum ferritin, serum iron and percentage transferrin saturation); helminthic infections (prevalence and intensity); nutritional status (weight, height and knee‐heel length were measured, and serum albumin levels were assessed) and scholastic and cognitive tests | |
| Notes | This study was supported by a research grant from the Human Sciences Research Council, the British Council supported the UK academic Iink, the fortified biscuits were supplied by SASKO, the albendazole tablets by Smith Kline Beecham and the praziquantel tablets by Bayer Study duration: 4 months in 1995 | |
| Random sequence generation (selection bias) | Unclear risk | Quote: "The subjects were randomly allocated into six study groups." Comment: Insufficient information |
| Allocation concealment (selection bias) | Unclear risk | Comment: Insufficient information |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Quote: "A community‐based, double‐blind, randomised, placebo‐controlled trial" Comment: Insufficient information |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Quote: "A community‐based, double‐blind, randomised, placebo‐controlled trial" Comment: Insufficient information |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Comment: The CONSORT flow diagram was not provided |
| Selective reporting (reporting bias) | Low risk | No information on trial registration provided. Outcomes specified in the methods section have been reported in the Results |
| Other bias | Low risk | Comment: No additional bias identified |
| Methods | Randomised controlled trial | |
| Participants | Conducted in Finland. 72 pregnant women from the city of Oulu were recruited for the study | |
| Interventions | Intervention (n = 40): During the 8‐week intervention period, the women followed their habitual diet, except that 1000 mL of their daily liquids were replaced by fortified or normal mineral water Control (n = 32): Placebo Food vehicle: Fortified mineral water Dose: Potassium (mg) 141, magnesium (mg) 53, calcium (mg) 800, sodium (mg) 6, vitamin B6 (mg) 1.5, vitamin B12 (mg) 2.1, folic acid (mg) 470, vitamin D (mg) 5.0 Duration: 8 weeks | |
| Outcomes | Serum folate, vitamin B12, erythrocyte folate, plasma homocysteine | |
| Notes | Supported by Olvi PLC, Iisalmi, Finland Study duration: Not specified. | |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Quote: "A randomised, placebo‐controlled, double‐blind parallel study design was used." Comment: Insufficient information on how blinding was performed |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Quote: "A randomised, placebo‐controlled, double‐blind parallel study design was used." Comment: Insufficient information on how blinding was performed |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Overall loss to follow‐up: 8/74 8.4% loss to follow‐up Comment: Low attrition rate |
| Selective reporting (reporting bias) | Low risk | Trial registration or published protocol not identified, but the outcomes specified in the methodology section have been reported in the Results |
| Other bias | Low risk | No additional bias identified |
| Methods | Cluster‐randomised controlled trial | |
| Participants | Conducted in China from February 1990 to June 1990 on healthy full‐term infants (n = 226) born without complication and with birth weights > 2.5 kg aged 6 ‐ 13 months at the outset | |
| Interventions | Intervention (n = 77): Fortified rusks were either eaten dry or were taken in liquid form. after dispersion in water Control (n = 87): Unfortified rusk Dose: wheat flour, sugar and vegetable oil. MMN per rusk (17g): calcium 300 mg, iron 5 mg, zinc 3 mg, vitamin A 224 ug, vitamin D 4 ug, thiamine 0.15 ug, riboflavin 0.2 mg, niacin 2.5 mg, cyanocobalamin 0.3 ug, folic acid 25 ug Food vehicle: Rusk Duration: 3 months | |
| Outcomes | Anthropometric measurements (body weight, length), a clinical examination, blood samples (free erythrocyte porphyrin, plasma ferritin, erythrocyte glutathione reductase activation coeff, vitamin E and retinol), and diet histories (24‐hour recall) | |
| Notes | Supported in part by a grant from the United Kingdom Department of Trade and Industry Study duration: February 1990 to June 1990 | |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information provided to permit judgement |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information provided to permit judgement |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Insufficient information provided to permit judgement |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient information provided to permit judgement |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Total loss to follow‐up was 24.9% Comment: High attrition rate may affect outcomes |
| Selective reporting (reporting bias) | Low risk | No information on trial registration provided. Outcomes specified in the methods section have been reported in the Results |
| Other bias | Low risk | No additional bias identified (i) recruitment bias: Low risk (ii) baseline imbalance: Low risk (iii) loss of clusters: Low risk (iv) incorrect analysis: Low risk (v) comparability with individually randomised trials: Low risk |
| Methods | Randomised controlled trial | |
| Participants | Conducted between May 1998 and January 1999 at Saharawi refugee camps near the town of Tindouf in southwest Algeria, on children (n = 374) aged 3 – 6 years with height‐for‐age z scores (HAZ) ≤ −2.0 with use of World Health Organization and National Center for Health Statistics (WHO/NCHS) reference median were eligible | |
| Interventions | Children were assigned to 1 of 5 groups: Dose: per 100 g; Calcium (mg) 1000, potassium (mg) 1134, phosphorus (mg) 635, magnesium (mg) 156, iron (mg) 42, zinc (mg) 41, copper (mg) 2, vitamin A (ug) 2000, vitamin D (ug) 50, vitamin E (mg) 20, vitamin C (mg) 125, vitamin B1 (mg) 4, vitamin B2 (mg) 4, vitamin B6 (mg) 4, vitamin B12 (ug) 4, folate (ug) 500, pantothenic acid (mg) 25, niacin (mg) 50 Food vehicle: Spread Duration: 6 months Additional: metronidazole or mebendazole treatment For this review, we have merged data from FS and FSM groups as the intervention group, and have merged data from US and USM groups as the control group | |
| Outcomes | Growth: knee‐heel length, weight, height, WAZ, WHZ, HAZ, stunting, underweight and wasting, haemoglobin levels, anaemia, morbidity, faecal macroscopy and egg counts | |
| Notes | Supported by the Italian nongovernmental organization Comitato Internazionale per lo Sviluppo dei Popoli (CISP) as part of a grant from the European Commission Humanitarian Office (ECHO) Study duration: May 1998 and January 1999 | |
| Random sequence generation (selection bias) | Low risk | Quote: "With use of a simple computer‐generated randomisation method." Comment: Adequately done |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information provided to permit judgement |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "Neither the field assistants nor the investigator was aware of group assignment. The codes were revealed only after all subjects had completed the trial." "The supplements were colour coded at production, and the key revealing the code was kept by the manufacturer in France." Comment: Adequately done |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "Neither the field assistants nor the investigator was aware of group assignment. The codes were revealed only after all subjects had completed the trial." Comment: Adequately done |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Total loss to follow‐up 32.1% Comment: High attrition rate may affect outcome |
| Selective reporting (reporting bias) | Low risk | Comment: No information on trial registration provided. Outcomes specified in the methods section have been reported in the Results |
| Other bias | Low risk | No additional bias identified |
| Methods | A non‐blinded, controlled before‐after study conducted among pregnant women at 19 antenatal clinics and delivered at 2 maternity hospitals in Santiago, Chile between May 2002 and February 2003 | |
| Participants | 970 pregnant women | |
| Interventions | Pregnant women were assigned to receive regular powdered milk (n = 477) or a milk product fortified with multiple micronutrients and omega‐3 fatty acids (n = 493). Women in the experimental group received 2 kg per month of powdered milk (Mamans or product M, produced by Parmalat SpA, Parma, Italy), fortified with multiple micronutrients: Energy (kcal): 521.0 Protein (g): 25.0 Fats (g): 21.0 Milk fat: 10.5 Vegetable fat: 10.5 Polyunsaturated fatty acids: 5.3 Omega‐3 fatty acids: 0.9 Omega‐6 fatty acids" 4.4 Carbohydrates (g): 58 Lactose: 3 Vitamins A (mg): 1200, thiamine (B1) (mg): 1.0, riboflavin (B2) (mg): 1.0, pyridoxine (B6) (mg): 2.0, B12 (mg): 1.5, C (mg): 110, D3 (mg): 15, E (mg): 45, niacin (PP) (mg): 10, biotin (mg): 45, folic acid (mg): 600, Ca (mg): 960, P (mg): 720, Mg (mg): 90, Zn (mg): 12, Fe (mg): 27, Bioavailable Fe (mg): 4.5, Se (mg): 15 | |
| Outcomes | Maternal anthropometry, birthweight, duration of gestation, infant length, infant head circumference, preterm birth, low birthweight | |
| Notes | Supported by Parmalat SpA, Italy, the company that provided the Maman product. Study duration: May 2002 and February 2003 | |
| Random sequence generation (selection bias) | High risk | Quote: "A total of 1173 women were considered eligible, and they were recruited and randomised." Comment: No details of randomisation provided. |
| Allocation concealment (selection bias) | High risk | Quote: "A total of 1173 women were considered eligible, and they were recruited and randomised." Comment: No details of allocation provided. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote: "Non‐blinded, randomised controlled study" Comment: Not done |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Quote: "Non‐blinded, randomised controlled study" Comment: Not done |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Comment: Fortified milk group: 224/589 Regular powdered milk group: 219/552 Overall 41.4% attrition rate |
| Selective reporting (reporting bias) | Low risk | Comment: No information on trial registration provided. Outcomes specified in the methods section have been reported in the results |
| Other bias | Unclear risk | Baseline outcome measurements: Quote: "Selected baseline biological and social variables were similar between the control and the experimental groups with the exception of gestational age at recruitment, which was slightly higher in group M (intervention group)." Comment: Low risk Baseline characteristics: Quote: "Selected baseline biological and social variables were similar between the control and the experimental groups with the exception of gestational age at recruitment, which was slightly higher in group M (intervention group)." Comment: Low risk Protection against contamination: Low risk |
| Methods | Randomised controlled trial | |
| Participants | Using the creche and clinic as entry points into the community in Oukasie, Brits, in the North West Province of South Africa, all 1 – 3‐year‐old children (n = 60) at the creches and the well‐baby clinic were screened and the first 60 undernourished children who had weight‐for‐age or height‐for‐age below the 5th percentile of the National Center for Health Statistics (NCHS) reference identified | |
| Interventions | Intervention: undernourished 1 – 3‐year‐old children and their households were randomly allocated to either an experimental (n = 30): or control group (n = 30). The households (families) in the experimental group received a vitamin‐fortified maize meal and those in the control group unfortified maize meal. Between 25 and 50 kg (depending on usual monthly consumption) of maize meal flour was provided to the families per month to replace all maize meal consumed by these households Control: Unfortified maize meal Dose: 1700 IU vitamin A, 0.61 mg thiamine, 0.62 mg riboflavin and 0.56mg pyridoxine Food vehicle: Maize meal porridge Duration: 12 months | |
| Outcomes | Weight, height, haemoglobin, hematocrit, serum retinol, serum RBP | |
| Notes | The study was funded by grants from the National Research Foundation, Potchefstroom University for Christian Higher Education, Hoffman La Roche (Switzerland), Roche Vitamin and Fine Chemicals and a gift of maize from Maizecor. Study duration: Not specified. | |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Quote: "The study design was a randomised, parallel, single‐blind intervention (families were blinded)" Comment: No blinding of personnel |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Quote: "The study design was a randomised, parallel, single‐blind intervention (families were blinded)." Comment: Not done |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Intervention group: 9/30 Control group: 7/30 Comment: High attrition rate |
| Selective reporting (reporting bias) | Low risk | Comment: Trial registration not specified. Outcomes specified in the methods section have been reported in the Results |
| Other bias | Low risk | No additional bias identified |
| Methods | Randomised controlled trial | |
| Participants | Conducted in Vietnam. Pupils were recruited from 2 schools that had been selected on the basis of a high prevalence of anaemia and parasite infestations among school children during an earlier survey. In total, 642 children aged 6 – 8 years in 20 classes were available, of which 510 children were randomly selected | |
| Interventions | Intervention: The 4 intervention groups were: Food vehicle: Fortified biscuit Dose: Iron (ferrous fumarate), 6 mg, zinc (zinc sulfate), 5.6 mg, iodine (potassium iodide), 35 ug, vitamin A (retinyl acetate), 300 ug RE, thiamine (thiamine mononitrate), 1 mg, riboflavin, 0.9 mg, vitamin B6, 1.1 mg, niacin (niacinamid), 10.5 mg NE, vitamin B12, 1.5 ug, folic acid, 120 ug, vitamin C, 28.4 mg, calcium (CaHPO4), 150 mg, cholecalciferol, 74 ug, magnesium, 40 mg, selenium (sodium salt), 6.8 ug, potassium (citrate), 378 mg, phosphorus, 70 mg, pantothenic acid, 3 mg, vitamin E, 2.8 ug, vitamin K, 10 ug, biotin (D‐biotin), 18 ug Duration: 6 months Additional interventions: A single dose of intestinal anthelminthic treatment as orange‐flavoured chewable tablets containing 400 mg albendazole (Vidoca) or identical placebo tablet was given. De‐worming with albendazole was given to all children at the end of the study after the final stool sample collection. The intervention arms eligible for this review were MMF and the placebo groups | |
| Outcomes | Haemoglobin, serum ferritin, transferrin receptor, retinol. zinc, body iron | |
| Notes | Supported by the Neys‐van Hoogstraten Foundation, The Netherlands, and Ellison Medical Foundation Study duration: January to June 2007 | |
| Random sequence generation (selection bias) | Low risk | Quote: "510 pupils were allocated to 1 of the 4 intervention groups based on a computer‐generated list, matched on age (12‐mo age groups) and sex, and using a block size of 8 by one of the researchers not involved in the field work." Comment: Adequately done |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "The codes of fortified and non‐fortified biscuits, Alb, and placebo were kept by the manufacturers and by a member of the institute staff not directly involved in the study until the data analysis was finished." Comment: Adequately done |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "The code of fortified and non‐fortified biscuits, Alb, and placebo were kept by the manufacturers and by a member of the institute staff not directly involved in the study until the data analysis was finished." Comment: Adequately done |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 8.6% total loss to follow‐up Comment: Low attrition rate |
| Selective reporting (reporting bias) | Low risk | Trial registration not specified. Outcomes specified in the methods section have been reported in the Results |
| Other bias | Low risk | No additional bias identified |
| Methods | A community‐based randomised trial conducted among 6‐ to 12‐month‐old infants from a black urban disadvantaged community in the Western Cape, South Africa between March 1999 and June 2000 | |
| Participants | 60 children aged approximately 6 months were randomly selected from all mothers visiting the local clinic with their infants | |
| Interventions | The experimental group (n = 30) received a micronutrient‐fortified complementary food throughout the 6‐month period, while the control group (n = 30) did not receive any complementary food, but continued their normal diet Energy (kJ): 1304 Protein (g): 12 Fat (g): 6 Carbohydrate (g): 54.8 Vitamin A (iu): 1200, vitamin C (mg): 40, vitamin B1 (mg): 0.64, vitamin B2 (mg): 0.24, niacin (mg): 3.2, calcium (mg): 368, iron (mg): 8, vitamin D (iu): 160, vitamin E (iu): 4, biotin (mg): 20, folic acid (mg): 17.6, pantothenic acid (mg): 0.6, vitamin B12 (mg): 0.6, vitamin B6 (mg): 0.24, phosphorous (mg): 232, iodine (mg): 26, zinc (mg): 5.6, potassium (mg): 632, sodium (mg): 272, chloride (mg): 440 | |
| Outcomes | Serum retinol, iron, haemoglobin, zinc, weight, length, weight for age Z‐score, height for age Z‐score, weight for height Z‐score | |
| Notes | No sample size calculations done a priori. Funding was not specified Study duration: March 1999 to June 2000 | |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Each infant was randomly allocated to either an experimental or a control group." Comment: Insufficient information |
| Allocation concealment (selection bias) | Unclear risk | Quote: "Each infant was randomly allocated to either an experimental or a control group." Comment: Insufficient information |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Probably not done |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Probably not done |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Intervention group: 14/30 Control group: 16/30 High attrition rates |
| Selective reporting (reporting bias) | Low risk | Comment: Trial registration not specified. Outcomes specified in the methods section have been reported in the Results |
| Other bias | Low risk | Comment: No additional bias identified |
| Methods | Randomised controlled trial | |
| Participants | Conducted in Australia and Indonesia. The trials were conducted from August 2003 to April 2005 in children (n = 396) aged 6 – 10 years from South Australian government metropolitan schools of higher socio‐economic status in Adelaide and from schools in the central district of Jakarta of middle to low socio‐economic status (n = 384) | |
| Interventions | Intervention: The studies in Australia and Indonesia both used a 2‐x‐2 factorial design in which the children were individually randomly allocated to 1 of 4 intervention groups: A fruit‐flavoured drink (soy 0.6%) was used as the vehicle for all treatments, which were added as powders Food vehicle: Fruit‐flavoured drink Dose: Iron as NaFeEDTA 10 mg, zinc as zinc sulfate 5 mg, vitamin A as retinol acetate 400 ug, folate 150 ug, vitamin B6 1 mg, vitamin B12 1.5 ug, vitamin C 45 mg Duration: 1 year The intervention arms eligible for this review were Arms 1 and 4 | |
| Outcomes | Haemoglobin, serum ferritin, vitamin B12, zinc, transferrin receptor, body iron, general intelligence, verbal learning and memory, visual attention | |
| Notes | Supported by Unilever Netherlands BV Study duration: August 2003 to April 2005 | |
| Random sequence generation (selection bias) | Low risk | Quote: "In Australia, the children were randomly assigned to intervention groups on entry in the study. In Indonesia, the children were stratified by school before being randomly assigned. Random assignment was done by means of a computer‐generated list in both countries." Comment: Adequately done |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "The supplement powders were indistinguishable in colour and taste and were color‐coded. The codes remained unknown to both investigators and participants until the study was completed, all data had been entered, and initial analyses had been performed." Comment: Adequately done |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "The supplement powders were indistinguishable in colour and taste and were color‐coded. The codes remained unknown to both investigators and participants until the study was completed, all data had been entered, and initial analyses had been performed." Comment: Adequately done |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Australia 37%, Indonesia 7.1% loss to follow‐up Comment: High attrition rate for the study in Australia may have affected outcomes |
| Selective reporting (reporting bias) | Low risk | The trial is registered with the Netherlands Trials Registry as Trial N324 (NTR362). No evidence of selective reporting |
| Other bias | Low risk | No additional bias identified |
| Methods | Cluster‐Randomised controlled trial | |
| Participants | The study was conducted between November 2012 and July 2013 in 20 primary schools (n = 2440 children) from 5 districts of Kampong Speu province in Cambodia. Children attending the selected schools were eligible to be part of the study if they were 6 – 16 years of age, written informed consent was obtained from parent/caregiver, and the child did not have a mental or severe physical handicap. Children with severe anaemia (defined as haemoglobin concentration < 70 g/L) were excluded | |
| Interventions | Intervention: The four intervention groups were: Breakfast was distributed 6 days a week for 6 months Food vehicle: Fortified rice Dose: URO, URN, NutriRice Iron (mg) 10.67, 7.55, 7.46, zinc (mg) 3.04, 2.02, 3.68, vitamin B1 (mg) 1.06, 1.43, 0.69, folic acid (mg) 0.17, 0.28, 0.14, vitamin A (IU) 0, 2140, 960, vitamin B3 (mg) 0, 12.57, 7.98, vitamin B12 (µg) 0, 3.8, 1.26, vitamin B6 (mg) 0, 0, 0.92 Duration: 6 months Additional interventions: Children were de‐wormed using mebendazole just after the baseline and endline, according to the standard procedures of the Ministry of Health, Cambodia The intervention arms eligible for this review were Arms 3 and 4. | |
| Outcomes | Haemoglobin, serum ferritin, transferrin receptor, body iron | |
| Notes | Supported by USDA/FAS, WFP‐DSM consortium, and IRD Study duration: November 2012 to July 2013 | |
| Random sequence generation (selection bias) | Low risk | Quote: "The 16 selected schools were randomly allocated to one of the four intervention groups using a computer generated list with predefined criteria of group size." Comment: Adequately done |
| Allocation concealment (selection bias) | Low risk | Quote: "Rice was packaged in bags containing a letter (A‐H) according to allocation.." Comment: Adequately done |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "Randomization was done by one of the researchers (M.A.D.) not involved in the field work and the codes were not known by any researchers or field staff during implementation, thus assuring the study was double‐blind." Comment: Adequately done |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "Randomization was done by one of the researchers (M.A.D.) not involved in the field work and the codes were not known by any researchers or field staff during implementation, thus assuring the study was double‐blind." Comment: Adequately done |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 7.9% total loss to follow‐up. Comment: Low attrition rate |
| Selective reporting (reporting bias) | Low risk | Comment: The trial was registered at ClinicalTrials.gov (Identifier: NCT01706419). No evidence of selective reporting |
| Other bias | Low risk | No additional bias identified. (i) recruitment bias: Low risk (ii) baseline imbalance: Low risk (iii) loss of clusters: Low risk (iv) incorrect analysis: Low risk (v) comparability with individually randomised trials: Low risk |
| Methods | A randomised, controlled, double‐blind trial conducted among children at 3 schools in Granada, Spain | |
| Participants | 119 children aged 8 – 14 years | |
| Interventions | Children were randomly allocated to a fortified milk group or a regular full‐milk control group for a duration of 5 months. Children in the Fortified group (n = 60) consumed 0.6 L/day of a fortified milk beverage containing vitamins (A, B complex, C, D and E), minerals (calcium, phosphorus, zinc), fish oils (with high levels of DHA and EPA), oleic acid, and carbohydrates (sugar and honey) (Puleva Ma®) Energy (Kcal/kJ): 69/288 Proteins (g): 3.0 Carbohydrates (g): 7.4 Total fat (g): 3.0 Saturated fatty acids (g): 1.2 Monounsaturated fatty acids (g); 1.5 Polyunsaturated fatty acids (g): 0.3 Omega‐3 (mg): 35, docosahexaenoic acid (DHA) (mg): 20, eicosapentaenoic acid (EPA) (mg): 10, vitamin A (retinol) (mg): 120, vitamin B1 (mg): 0.21, vitamin B2 (mg): 0.24, vitamin B3 (mg): 2.7, pantothenic acid (mg): 0.9, vitamin B6 (mg): 0.3, biotin (mg): 22.5, folic acid (mg): 30.0, vitamin B12 (mg): 0.15, vitamin C (mg): 9.0, vitamin D (mg): 0.75, vitamin E (mg): 1.5, calcium (mg): 140, zinc (mg): 2.25 Children in the Control group (n = 59) consumed 0.6 L/day of regular full milk | |
| Outcomes | Biochemical indicators (HDL, LDL, TG, DHA, ferritin, iron, calcium, vitamin D, vitamin E); Anthropometric measures (BMI, waist circumference); Cognitive tests (digital span, letter number sequencing, coding, symbol/animal search) | |
| Notes | The study was funded by Lactalis Puleva SL. One of the authors is currently employed and one of the authors was employed by Biosearch Life, which is part of Lactalis Study duration: January to June (year not specified). | |
| Random sequence generation (selection bias) | Low risk | Quote: "Randomization was done with the program SIGESMU." Comment: Adequately done |
| Allocation concealment (selection bias) | Low risk | Quote: "The two beverages were labelled Product A and Product B" Comment: Adequately done |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "all persons involved in the execution of the study were blind to their true content. The beverages were supplied in vacuum‐sealed tetrabrik containers with blank surfaces, without any trademarks or identification." Comment: Adequately done |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "all persons involved in the execution of the study were blind to their true content. The beverages were supplied in vacuum‐sealed tetrabrik containers with blank surfaces, without any trademarks or identification." Comment: Adequately done |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Fortified milk group: 52/60 Regular milk group: 51/59 |
| Selective reporting (reporting bias) | Low risk | Comment: Trial registration not specified. Outcomes specified in the methods section have been reported in the Results |
| Other bias | Low risk | No additional bias identified |
| Methods | Randomised controlled trial | |
| Participants | The study was conducted in Satun province, on the west coast of southern Thailand, where most of the population is Muslim. The study was performed in 8 primary schools (n = 203) in the Muang district, which included mainly children from low‐income families. The schools had 4‐ to 12‐year‐old children (kindergarten to grade 6) who were provided with a school lunch programme (5 days a week), which was partly subsidised by the government | |
| Interventions | Intervention (n = 101): The fortified rice was mixed with the natural rice and cooked by local cooks at a central kitchen in Satun town, which had been specifically set up for the study. The cooked rice was weighed into individual portions of 140 g into a color‐coded container that was labelled with the child's name. The weight was regularly controlled by research assistants. The rice was transported to the 8 schools by the research assistants and the 140 g of cooked rice (triple‐fortified rice or unfortified rice) was given to each child. The rice was consumed with foods such as soup or curry, which was provided by the school lunch programme. The rice meal was fed 5 days a week Control: Placebo (n = 102) Food vehicle: Fortified rice Dose: 10 mg iron, 9 mg zinc, and 1050 mg vitamin A/g extruded rice Duration: 6 months Additional interventions: After completion of the study, all children who remained deficient in any of the micronutrients in either group received supervised treatment of the respective micronutrient(s) according to local policies | |
| Outcomes | Serum zinc, retinol, haemoglobin, ferritin | |
| Notes | The study was supported by Medicor Foundation (Triesen, Liechtenstein) and The Royal Thai Government Scholarship. Dr. Paul Lohmann GmbH (Emmerthal, Germany) provided iron and zinc compounds and DSM Nutritional Products Ltd. (Basel, Switzerland) provided the vitamin A compound Study duration: July 2009 to March 2010 | |
| Random sequence generation (selection bias) | Unclear risk | Comment: Insufficient information to permit judgement |
| Allocation concealment (selection bias) | Unclear risk | Comment: Insufficient information to permit judgement |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Comment: Insufficient information to permit judgement |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Comment: Insufficient information to permit judgement |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Intervention group: 7/101 Control group: 14/102 In total, 21 children (˜ 10%) were lost for final analysis and 182 completed the study according to the protocol Comment: Low attrition rate |
| Selective reporting (reporting bias) | Low risk | Comments: This trial was registered at www.clinicaltrials.gov as NCT01061307. No evidence of selective reporting |
| Other bias | Low risk | Comment: No additional bias identified |
| Methods | Randomised controlled trial | |
| Participants | The study was performed in a peri‐urban area of the Muang district, Satun province, on the west coast of southern Thailand. Most of the population was Muslim and the participants were primarily from low‐income families. One primary school in the Muang district, with children aged 4 – 12 years, was selected for the study (n = 50). The school provided a free lunch meal (5 days a week) that was partly subsidised by the government | |
| Interventions | Intervention(n = 25): 1 group was given the triple‐fortified rice containing Fe, Zn, and vitamin A (fortified group) Control: Unfortified rice (n = 25) Food vehicle: Triple‐fortified rice Dose: 10 mg Fe, 9 mg Zn, and 1.05 mg vitamin A/g extruded rice Duration: 2 months | |
| Outcomes | Serum retinol, vitamin A deficiency | |
| Notes | Supported by Medicor Foundation (Triesen, Liechtenstein), the International Atomic Energy Agency (Vienna, Austria), and the Royal Thai Government Scholarship Study duration: August to November 2010 | |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Quote: "The VA efficacy study was a double‐blind, randomised, controlled trial." Comment: Insufficient information to permit judgement |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient information to permit judgement |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: "Of the 50 children who started the intervention, 45 children completed it." Intervention group: 2/25 Control group: 3/25 Comment: Low attrition rate |
| Selective reporting (reporting bias) | Low risk | This trial was registered at clinicaltrials.gov as NCT01199445. No evidence of selective reporting |
| Other bias | Low risk | No additional bias identified |
| Methods | A randomised, double‐blind, placebo‐controlled intervention trial was conducted in girls recruited at ages 16 – 19 years, from schools and colleges in Sheffield, UK between July 2012 and May 2013 | |
| Participants | 71 adolescent girls aged 16 – 19 years were selected from schools, colleges and Universities within the Sheffield area | |
| Interventions | Intervention (n = 34): Girls were randomised to receive 50 g fortified with 150 ml semi‐skimmed milk daily for 12 weeks, as a breakfast or as a supper Energy (kcal): 257 Fat (g): 3.1 Carbohydrate (g): 47.8 Sugars (g): 15.9 Vitamin D (μg): 4.15, vitamin C (mg): 51.5, vitamin B1 (mg): 1.21, vitamin B2 (mg): 1.71, niacin (mg): 17.8, vitamin B6 (mg): 1.74, folic acid (μg): 176, vitamin B12 (μg): 1.45, iron (mg): 6.5, calcium (mg): 215 Control (n = 37): Unfortified cereal | |
| Outcomes | Plasma ferritin, haemoglobin, micronutrient intake | |
| Notes | The Kelloggs Company of Great Britain provided the cereal for the study and financial support for the research. The salary of one of the authors was provided by Kelloggs Company of Great Britain Study duration: July 2012 and May 2013 | |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Volunteers were randomised in blocks of twelve to receive a daily intake of either fortified or unfortified cereal." Comment: Insufficient information |
| Allocation concealment (selection bias) | Unclear risk | Not specified. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "Unfortified and fortified cereal was provided and the identity of each was blinded to the researchers and participants." Comment: Adequately done |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "Unfortified and fortified cereal was provided and the identity of each was blinded to the researchers and participants." Comment: Adequately done. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Retained in study: 73/78 CONSORT flow diagram does not specify the loss to follow‐up according to the study group assignment |
| Selective reporting (reporting bias) | Low risk | Registered with Current Controlled Trials (Registration: ISRCTN55141306) and prespecified outcomes reported |
| Other bias | Low risk | The study enrolled voluntary participants and an Amazon voucher for GBP 30 was offered on completion of the study, although it was not considered a source of bias |
| Methods | Cluster‐randomised controlled trial | |
| Participants | The study sites included 7 out of the total 16 unions (approximately 65 villages) (n = 352 children) of Mirsarai sub‐district in the south‐eastern part of Bangladesh. Assuming that 7 – 9 eligible children (aged 6 – 15 years) would be available from each bari and using a statistics book generated random‐number table, a total of 44 baris were randomly selected from the total listed baris for distribution of the flour. Among the 44 selected baris, 22 baris were randomly assigned to the intervention group and 22 baris to the control group (control) | |
| Interventions | Intervention (n = 203): Throughout the trial period, the project staff distributed the flour once every week. In order to prevent participants sharing of chapattis with other members of a bari, the same amount of flour was also allocated to other members of that bari during this period. Children received chapattis made from 100 g of fortified or unfortified wheat flour daily for 6 months Control (n = 149): Unfortified flour Food vehicle: Chappattis made from fortified wheat flour Dose: Vitamin A 212 ug, iron 6.6 mg, thiamine 0.64 mg, riboflavin 0.40 mg, folic acid 0.15 mg, zinc oxide 3.3 mg, niacin as niacinamide 5.3 mg Duration: 6 months | |
| Outcomes | Serum retinol, ferritin, transferrin receptor, haemoglobin | |
| Notes | Funded by a grant from the MOST project (Contract No. HRN‐AA‐00–98‐00047‐00) and by support to the Mirsarai field area by USAID Cooperation Agreement number 388‐A‐00–97‐00032‐00 Study duration: Not specified. | |
| Random sequence generation (selection bias) | Low risk | Quote: "Using a statistics book generated random number table, a total of 44 baris were randomly selected from the total listed baris for distribution of the flour. Among the 44 selected baris, 22 baris were randomly assigned to the intervention group and 22 baris to the control group (control)." Comment: Adequately done |
| Allocation concealment (selection bias) | Low risk | Quote: "A person not involved with the study assigned the baris to six different codes of flour (A, B, C, D, E and F) for distribution of the flour bags." Comment Adequately done |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "It was only after completion of the analysis, the groups were unblinded." Comment: Adequately done |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "It was only after completion of the analysis, the groups were unblinded." Comment: Adequately done |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Intervention group: 12/203 Control group: 6/149 5% total loss to follow‐up Comment: Low attrition rate |
| Selective reporting (reporting bias) | Low risk | Comment: Trial registration not specified. Outcomes specified in the methods section have been reported in the Results |
| Other bias | Low risk | No additional bias identified (i) recruitment bias: Low risk (ii) baseline imbalance: Low risk (iii) loss of clusters: Low risk (iv) incorrect analysis: Low risk (v) comparability with individually randomised trials: Low risk |
| Methods | Randomised controlled trial | |
| Participants | The trial was carried out from April 2002 to April 2004, in Sangam Vihar, a peri‐urban community located on the outskirts of New Delhi, India. All permanent resident families in the area with children aged 1 – 3 years (n = 633) were invited to participate in the study, and their consent sought. Children who were exclusively or predominantly breast‐fed or allergic to milk were excluded. Children with severe malnutrition needing rehabilitation or chronic/severe illness requiring hospitalisation or special treatment were to be excluded | |
| Interventions | Intervention (n = 316): Fonterra Brands (Singapore) Pte. Ltd. provided 32 g single‐serve sachets of fortified milk powder and control for the study. At enrolment, the procedure for preparing milk was clearly explained and demonstrated to mothers. Each week, the milk assistants delivered 21 sachets at home and advised the mother to feed the child 3 sachets a day Control (n = 317): Unfortified milk Food vehicle: Fortified powdered milk Dose: Fortified milk (3 servings a day) was designed to deliver additional amounts of zinc (7.8 mg), iron (9.6 mg), selenium (4.2 ug), copper (0.27 mg), vitamin A (156 ug), vitamin C (40.2 mg), vitamin E (7.5 mg) Duration: 1 year Additional interventions: At enrolment, all children who had severe anaemia (haemoglobin < 70 g/L) were given therapeutic doses of iron for 3 months in addition to their assigned intervention | |
| Outcomes | Haemoglobin, hematocrit, protoporphyrin, ferritin, transferrin receptor, zinc, weight velocity, height velocity, WHZ, WAZ, HAZ | |
| Notes | Supported from the grants of Fonterra Brands (Singapore) Pte. Ltd Study duration: April 2002 to April 2004 | |
| Random sequence generation (selection bias) | Low risk | Quote: "Letter codes A through D were used to identify four groups (across two separate trials). In‐house computer software generated a random sequence of group codes with permuted block length of 16." Comment: Adequately done |
| Allocation concealment (selection bias) | Low risk | Quote: "Group codes from 1 to 6 were used to identify the fortified and non‐fortified yoghurt" Comment: Adequately done |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "The supplementation sachets were identical in colour, size (weight 32 g), taste and packaging and were labelled with a letter code. The investigators and the study team were blinded to the identity of the letter codes. Fonterra Brands Pte. Ltd. provided code identification to investigators after finishing of the trial, at the time of analysis." Comment: Adequately done |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "The supplementation sachets were identical in colour, size (weight 32 g), taste and packaging and were labelled with a letter code. The investigators and the study team were blinded to the identity of the letter codes. Fonterra Brands Pte. Ltd. provided code identification to investigators after finishing of the trial, at the time of analysis." Comment: Adequately done |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Intervention group: 27/316 Control group: 36/317 Comment: Low attrition rate |
| Selective reporting (reporting bias) | Low risk | This trial was registered at ClinicalTrials.gov: NCT00980733 and there was no evidence of selective reporting |
| Other bias | Low risk | Comments: No additional bias identified |
| Methods | Randomised controlled trial | |
| Participants | The study was conducted between June 2008 and March 2010 in primary schools of Gabtali town of Bogra district in the Rajshahi Division (n = 1010), Northern Bangladesh. The selected schools were in close proximity to a yoghurt factory. The inclusion criteria for enrolment into the study were children aged 6 to 9 years attending selected schools, who were likely to remain in the same school, and parents providing consent. Children with severe malnutrition needing nutritional rehabilitation or chronic/severe illness requiring hospitalisation or special treatment were excluded and referred for treatment | |
| Interventions | Intervention (n = 501): Children allocated to the yoghurt groups received 1 cup of the yoghurt (60 g) daily during the lunch break of the school for 1 year. The feeding session was strictly monitored and supervised by the field workers and class teachers and the compliance to the intervention was recorded in compliance record forms. A separate list was prepared for children who were absent from school and their respective yoghurt cups were delivered at home in the afternoon Control (n = 509): Unfortified yogurt Food vehicle: Fortified yogurt Dose: Calcium 85 (mg), phosphorus 67 (mg), iron 3.3 (mg), zinc 3.0 (mg), iodine 40 (μg), vitamin A 140 (μg) Duration: 12 months | |
| Outcomes | Haemoglobin, serum ferritin, transferrin receptor, zinc, iodine, RBP, body iron stores, weight velocity, height velocity, WAZ, HAZ, BMIz | |
| Notes | Global Alliance for Improved Nutrition funded the study Study duration: June 2008 and March 2010 | |
| Random sequence generation (selection bias) | Low risk | Quote: "Using in‐house computer software, a random sequence of group codes with a permuted block length of 6 was generated to randomly allocate the individual child to one of the two yoghurt groups." Comment: Adequately done |
| Allocation concealment (selection bias) | Low risk | Quote: "Group codes from 1 to 6 were used to identify the fortified and non‐fortified yoghurt." Comment: Adequately done |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "The codes of the groups were not known to the investigators, field team, teachers, children or anyone involved in the study during the field implementation. Cups were prepared and labelled with group codes a day in advance at a factory in Bogra. The yoghurt for the two intervention groups was identical in packaging, appearance, taste and smell." Comment: Adequately done |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "The codes of the groups were not known to the investigators, field team, teachers, children or anyone involved in the study during the field implementation. Cups were prepared and labelled with group codes a day in advance at a factory in Bogra. The yoghurt for the two intervention groups was identical in packaging, appearance, taste and smell." Comment: Adequately done |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Intervention group: 227/501 Control group: 216/509 Comment: High attrition rate |
| Selective reporting (reporting bias) | Low risk | Comment: This trial was registered at ClinicalTrial.gov: NCT00980733 and all prespecified outcomes have been reported in the Results |
| Other bias | Low risk | Comment: No additional bias identified |
| Methods | An individually‐randomised, double‐blind, placebo‐controlled field efficacy trial among schoolchildren in the municipality of Balete, located in the province of Batangas in the Philippines | |
| Participants | 831 children in grades 1 – 6 were enrolled from 4 elementary schools | |
| Interventions | Participants were randomised into 1 of the 4 following groups and received beverage for 16 weeks: Group 1 received fortified beverage with anthelmintic therapy (n = 203); Group 2 received fortified beverage with placebo anthelmintic therapy (n = 209); Group 3 received non‐fortified beverage with anthelmintic therapy (n = 213); Group 4 received non‐fortified beverage with placebo anthelmintic therapy (n = 206). The fortified beverage contained a single serving (25 g sachets) with iron (4.8 mg), vitamin A (700 IU), iodine (48 μg), zinc (3.75 mg), vitamin C (75 mg), riboflavin (0.46 mg), folic acid (0.06 mg), vitamin B12 (0.5 μg), vitamin B6 (0.5 mg), vitamin E (2.5 mg), and niacin (2.5 mg) For this review, we have only included data from Group 2 and Group 4 | |
| Outcomes | Weight, height, weight for age Z‐score, height for age Z‐score, weight for height Z‐score, haemoglobin, anaemia, urinary iodine, physical fitness and cognitive performance | |
| Notes | The trial was funded by the Nutrition Center of the Philippines and Procter & Gamble Co Study duration: October 1998 to March 1999 | |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Study participants were assigned, through randomizations at the individual level, to one of four different treatment groups." Comment: Insufficient information |
| Allocation concealment (selection bias) | Unclear risk | Not specified. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "Placebo beverage and placebo anthelmintic pills were indistinguishable from their counterparts in appearance, smell, and taste." Comment: Adequatelyt done |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "Placebo beverage and placebo anthelmintic pills were indistinguishable from their counterparts in appearance, smell, and taste." Comment: Adequately done |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Overall loss to follow‐up 43/851 |
| Selective reporting (reporting bias) | Low risk | Trial registration not specified. Outcomes specified in the methods section have been reported in the Results |
| Other bias | Low risk | No additional bias identified. |
| Methods | Randomised controlled trial | |
| Participants | Conducted in primary school children (n = 414) between the ages of 6 and 11 years in a peri‐urban settlement in the North West province in South Africa. The study was conducted in 3 preselected primary schools chosen by the Department of Education. Learners at all 3 schools were provided a single daily meal, sponsored by the National School Nutrition Programme. The inclusion criteria were as follows: (1) no health condition that would make cognitive testing impractical (e.g. dyslexia and hearing difficulties); (2) 6 – 10 years old by January 2010; (3) no use of medication or supplements that could affect nutritional status | |
| Interventions | Intervention: The four different formulations of the beverages were as follows: Food vehicle: Fortified beverage Dose: Vitamin A (ug RE) 400, vitamin E (mg) 7·5, vitamin C (mg) 60, vitamin B2 (mg) 0·4, nicotinamide (mg) 2·7, vitamin B6 (mg) 0·5, folic acid (ug) 140, vitamin B12 (ug) 1·0, calcium (mg) 120, iron (mg) 7·0, zinc (mg) 3·75, iodine (ug) 60 Duration: 8 months Additional interventions: Children were de‐wormed at the baseline with 200 mg (100 mg twice daily) of mebendazole for 3 consecutive days For this review, we have only included data from MNNS and CNS groups. | |
| Outcomes | Haemoglobin, serum ferritin, protoporphyrin, transferrin receptor, zinc, retinol | |
| Notes | Supported by a research grant from Coca Cola South Africa (Pty) Study duration: March 2010 to November 2010 | |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "The colourant Yellow Sunset E110 was used to give a similar colour to the micronutrient‐containing beverages that had b‐carotene. All the beverage formulations were, therefore, identical in colour and taste. The participants, investigators and school assistants were blinded to treatment assignments." Comment: Adequately done |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Comment: Insufficient information to permit judgement |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 3.9% total loss to follow‐up Comment: Low attrition rate |
| Selective reporting (reporting bias) | Low risk | Comment: Trial registration not specified. Outcomes specified in the methods section have been reported in the Results |
| Other bias | Low risk | Comment: No additional bias identified |
| Methods | Randomised controlled trial | |
| Participants | 66 participants were recruited to take part in the study from eastern Finland. They fulfilled the following inclusion criteria: age 26 – 65 years, normal liver, kidney and thyroid function, and no history of unstable coronary artery disease (i.e. myocardial infarction, coronary artery bypass graft (CABG), or percutaneous transluminal coronary angioplasty (PTCA) within the previous 6 months), transient Ischaemic attack, kidney stones or malignant diseases. People with alcohol abuse (above 45 g of ethanol a day) or those that had used vitamin supplements (B or D vitamins) within 2 months prior to the study were excluded | |
| Interventions | Intervention (n = 31): During the intervention period, the participants followed their habitual diet except that 750 ml of liquids were replaced with the mineral water. They were not allowed to use calcium supplements and foods fortified with calcium and/or any B group vitamin during the study Control (n = 29): Placebo Food vehicle: Fortified mineral water Dose: Potassium (mg) 141, magnesium (mg) 53, calcium (mg) 563, sodium (mg) 6, vitamin B6 (mg) 1, vitamin B12 (ug) 7.5, folic acid (ug) 563, vitamin D (ug) 0.6 Duration: 8 weeks | |
| Outcomes | Serum folate, erythrocyte folate, serum vitamin B12, calcium, alkaline phosphatase, plasma homocysteine | |
| Notes | Supported by Olvi PLC, Iisalmi, Finland Study duration: Not specified. | |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Insufficient information to permit judgement |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient information to permit judgement |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: "Of the 62 subjects who were randomised, 60 (39 men and 21 women) completed the study. Two subjects discontinued the study: one dropped out due to adverse effects (abdominal discomfort, diarrhoea, nausea and vomiting) of the test mineral water and one subject moved away." Comment: Low attrition rate |
| Selective reporting (reporting bias) | Low risk | Trial registration not specified. Outcomes specified in the methods section have been reported in the Results |
| Other bias | Low risk | Quote: "Men and women were randomised separately. In addition, the randomisation was carried out separately for couples and single persons, in order to ensure the same mineral water for both spouses." Comment: Low risk of bias as randomisation was performed adequately on all groups |
| Methods | Randomised controlled trial | |
| Participants | Conducted in pregnant women 12 and 34 weeks pregnant in Tanzania from August 1999 to October 1999 (n = 439). | |
| Interventions | Intervention (n = 129): The fortified beverage mix was packaged in 25 g packets. Each woman was asked to consume the beverage thus produced twice a day with meals Control (n = 130): non‐fortified beverage mix of identical appearance, colour and taste. Was packaged in similar, but different coloured 25 g packets and served as the placebo Dose: Iron 10.8 mg, vitamin A 1050 RE, iodine 90 ug, zinc 10.5 mg, vitamin C 144 mg, riboflavin 1.2 mg, folic acid 280 ug, vit B12 6 ug, B6 1.4 mg, niacin 10 mg, vit E 21 mg. Food vehicle: orange‐flavoured micronutrient‐fortified powdered beverage mix containing 11 micronutrients Duration: 8 weeks Additional Interventions: Just before the women left the antenatal clinic, staff provided the mothers with a 2‐week supply of an iron/folic acid supplement that contained 60 mg of elemental iron and 500 g of folic acid to be taken on a daily basis. Women who were found to have parasitic infections were treated with a single dose of albendazole (400 mg) | |
| Outcomes | Height, weight, mid‐upper arm circumference (MUAC) and skinfold thickness, Hemoglobin, TSH, retinol, CRP, ferritin | |
| Notes | The micronutrient supplement was developed and produced by Procter & Gamble Company. Supported by a Micronutrient Initiative grant, Procter & Gamble Company, UNICEF Tanzania, Tanzania Food and Nutrition Centre and Cornell University. Study date: August 1999 to October 1999 | |
| Random sequence generation (selection bias) | Low risk | Quote: "At each of the six study centres, a block randomisation (10 subjects in each block) was used to assign women into either the micronutrient‐fortified (experimental) group or the non‐fortified (control) group." Comment: Adequately done |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Quote: "This study was a randomised, placebo‐controlled double‐blind effectiveness trial." Comment: Insufficient information to permit judgement |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Quote: "This study was a randomised, placebo‐controlled double‐blind effectiveness trial." Comment: Insufficient information to permit judgement |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Quote: "Of those enrolled (439), 121 women were lost to follow‐up and 59 mothers delivered before their 8‐wk postintervention visit (Fig. 1). Because delivery affects haemoglobin and other variables that were measured, the main statistical analyses were restricted to the 259 (59% of enrolled women; 127 experimental and 132 placebo) women who were still pregnant and were still in the study after 8 wk of supplementation, regardless of their gestational age at entry into the study." Comment: Attrition rate may have affected outcomes |
| Selective reporting (reporting bias) | Low risk | Trial registration not specified. Outcomes specified in the methods section have been reported in the Results |
| Other bias | Low risk | No additional bias identified |
| Methods | Randomised controlled trial | |
| Participants | The study was carried out in a convenience sample of children attending 4 primary schools in the Bangalore Urban District of Karnataka State in South India. These schools were selected because they cater to the educational needs of children from low socio‐economic neighbourhoods and are situated within the Bangalore urban region. 258 consenting children aged 6 – 12 years were included. Weight and height were measured and venous blood was collected to identify children with haemoglobin (Hb) concentrations < 115 g/L for 6 – 11 years and < 120 g/L for 12‐year‐olds. Those who did not intend to use micronutrient supplements other than those administered at school during the study, or who did not intend to migrate or withdraw from school during the study period were eligible for inclusion in the study. Participants with Hb < 90 g/L or who had any chronic illness requiring long‐term use of medication, physical handicaps, or severe malnutrition (weight‐for‐age Z‐score or height‐for‐age Z‐score < −3) were excluded | |
| Interventions | Intervention: The 3 types of rice: 1. High iron: 12.5 mg Fe/100 g (n = 86) 2. Low iron: 6.5 mg Fe/ 100 g (n = 86) 3. Control, ˜ 100 g raw rice/meal (n = 86) Food Vehicle: Two types of fortified rice; high and low iron Dose: All mg/100g, vitamin A 0.5, thiamine 0.38, niacin 5, vitamin B6 0.38, vitamin B12 0.00075, folate 0.075, iron 12.5 high iron group; 6.25 low iron group, zinc 3 Duration: 6 months Additional Interventions: All study children were de‐wormed under the supervision of the research staff with 400 mg albendazole (Low‐Cost Pharmaceuticals) before the study and near the study midpoint | |
| Outcomes | Haemoglobin, serum ferritin, transferrin receptor, protoporphyrin, retinol, zinc, thiamine, vitamin B12, plasma homocysteine | |
| Notes | Supported by DSM, Mumbai, India Study duration: July 2009 to March 2010 | |
| Random sequence generation (selection bias) | Low risk | Quote: "A block randomisation with a computer‐generated list in blocks of 20 was used to assign children to one of the 3 intervention groups." Comment: Adequately done |
| Allocation concealment (selection bias) | Unclear risk | Quote: "Each group was randomly assigned a distinct colour code, which remained unknown to both the study staff and the children until the completion of the study." Comment: Insufficient information to permit judgement |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "Each group was randomly assigned a distinct colour code, which remained unknown to both the study staff and the children until the completion of the study." Comment: Adequately done |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "All data were entered and initial analyses were performed prior to unmasking of the study." Comment: Adequately done |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 10% total loss to follow‐up Comment: Low attrition rate |
| Selective reporting (reporting bias) | Low risk | Comment: This trial was registered at the Clinical Trials Registry of India as CTRI/20/09/091/000941 and there was no evidence of selective reporting |
| Other bias | Low risk | Comment: No additional bias identified |
| Methods | Randomised controlled trial | |
| Participants | This study was carried out in children attending the St Joseph primary school in Kolar and Franciscan School, Bangalore, South India. 246 children aged 6 – 12 years were included. Weight and height were measured and 8 ml of venous blood was collected for determination of Hb and serum ferritin (SF). Children with SF levels < 20 ug/l and who were not intending to use micronutrient supplements during the study, to migrate or withdraw from school during the study period, were eligible for inclusion in the study. Children with anaemia (Hb < 8 g/dl), chronic illness, physical handicaps or severe malnutrition (weight‐for‐age (WAZ) or height‐for‐age (HAZ) Z‐score <‐3) were excluded | |
| Interventions | Intervention (n = 122): The MMN‐fortified beverage provided between 20% and 70% of the recommended daily allowance of micronutrients (vitamin A, B2, B12, C, folic acid, iron and zinc) for children between 6 and 12 years. The beverage contained 6 mg iron/serving as ferrous gluconate along with 27 mg of vitamin C in an orange‐flavoured base. The drinks were provided 6 days/week for a period of 8 weeks Control (n = 124): Unfortified beverage Food vehicle: Fortified beverage Dose: Vitamin A 243 (ug), vitamin C 27 (mg), vitamin B2 0.63 (mg), vitamin B12 1.27 (ug), folic acid 35 (ug), iron 5.9 (mg), zinc 1.2 (mg) Duration: 8 weeks | |
| Outcomes | Haemoglobin, serum ferritin, transferrin receptor, protoporphyrin, vitamin A, B12, C, zinc, folate, body iron stores | |
| Notes | Supported by Coca‐Cola, India Study duration: January 2010 to March 2010 | |
| Random sequence generation (selection bias) | Low risk | Quote: "A block randomisation with a computer‐generated list in blocks of 20 each was used to assign children to one of the two intervention groups." Comment: Adequately done |
| Allocation concealment (selection bias) | Unclear risk | Quote: "Each group was randomly assigned a distinct colour code, which remained blinded to both the study staff and children until the completion of the study." Comment: Insufficient information to permit judgement |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "Each group was randomly assigned a distinct colour code, which remained blinded to both the study staff and children until the completion of the study. All data were entered and initial analyses were performed before unmasking the study." Comment: Adequately done |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "Each group was randomly assigned a distinct colour code, which remained blinded to both the study staff and children until the completion of the study. All data were entered and initial analyses were performed before unmasking the study." Comment: Adequately done |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Intervention group: 2/122 Control group: 1/124 0.01% total loss to follow‐up Comment: Low attrition rate |
| Selective reporting (reporting bias) | Low risk | Trial registration not specified. Outcomes specified in the methods section have been reported in the Results |
| Other bias | Low risk | No additional bias identified |
| Methods | Randomised controlled trial | |
| Participants | Conducted in the USA. Volunteers were recruited through advertisements in local newspapers, posters, radio, and mailing lists. 215 adults (93 men and 122 women) 50 to 85 years old, completed the protocol, of whom 196 were non‐Hispanic white, 12 were African‐American, 4 were Asian‐American, and 3 were of another ethnicity | |
| Interventions | Intervention (n = 93): The enrolled participants were randomly assigned to consume breakfast cereal fortified with the RDAs of folic acid, vitamin B‐6, and vitamin B‐12 (400 ug, 2 mg, and 6 ug, respectively) per 1‐cup (0.24 L) serving or an identical cereal without the addition of these vitamins Control (n = 96): Unfortified cereal Food vehicle: Fortified cereal Dose: Analysis of the ready‐to‐eat cereal after fortification showed that the actual content was 440 ug folic acid, 1.8 mg vitamin B‐6, and 4.8 ug vitamin B‐12 per serving. Both cereals contained 100 kcal, 24 g carbohydrate, 1 g dietary fibre, 0.35 mg thiamine, 0.34 mg riboflavin, and 4.0 mg niacin per serving Duration: 12 weeks | |
| Outcomes | Serum folate, vitamin B12, B6, plasma homocysteine | |
| Notes | Supported by a grant from the Kellogg Company and by the US Department of Agriculture Agricultural Research Service (contract 53‐3K06‐01) Study duration: Not specified | |
| Random sequence generation (selection bias) | Unclear risk | Quote: "The study statistician (GED) randomly assigned the subjects to 1 of the 2 groups." Comment: Insufficient information to permit judgement |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Quote: "All staff members that interacted with the subjects were blind to the group assignments." Comment: Blinding of participants is not mentioned |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient information to permit judgement |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 10% total loss to follow‐up Comment: Low attrition rate |
| Selective reporting (reporting bias) | Low risk | Trial registration not specified. Outcomes specified in the methods section have been reported in the Results |
| Other bias | Low risk | No additional bias identified |
| Methods | Randomised controlled trial | |
| Participants | Conducted in the Netherlands. Non‐smoking adults, healthy as assessed by a medical investigation, between 18 and 65 years old, were eligible for participation in the study. The volunteers (n = 31) did not use vitamin C, E, carotenoid, selenium or zinc supplements. They were not using a medically prescribed diet or slimming regimen and had been weight‐stable for at least 1 month prior to the start of the study. Women were not pregnant or lactating. Volunteers were recruited from employees of Unilever Research Laboratorium and from inhabitants of Vlaardingen and the surrounding district | |
| Interventions | Intervention (n = 15): 15 g/d of an antioxidant fortified margarine Control (n = 16): 15 g/d of an ordinary unfortified margarine Food vehicle: Fortified margarine Dose: vitamin C 121 mg, vitamin E 31 mg, a‐carotene 2.7 mg, b‐carotene 5.3 mg Duration: 9 months | |
| Outcomes | Serum vitamin E, a‐carotene, b‐carotene, vitamin C, albumin, uric acid, total antioxidant activity | |
| Notes | Funding not specified Study duration: Not specified | |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote: "Due to the difference in colour of the margarines, the study was only blind to the analysts analysing the blood samples." Comment: Not done |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "Due to the difference in colour of the margarines, the study was only blind to the analysts analysing the blood samples." Comment: Adequately done |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: "Fifteen men and sixteen women completed the study. The volunteer who withdrew from participation in the study did so for medical reasons not related to the experimental treatment." Comment: Low attrition rate |
| Selective reporting (reporting bias) | Low risk | Trial registration not specified. Outcomes specified in the methods section have been reported in the Results |
| Other bias | Low risk | No additional bias identified |
| Methods | Randomised controlled trial | |
| Participants | The study population consisted of children aged 6 – 11 years in grades 1 – 5 of the Ndunakazi Primary School, a school in a rural mountainous area ˜ 60 km northwest of Durban, KwaZuluNatal, South Africa, and serving a community characterised by low socio‐economic status (n = 228) | |
| Interventions | Intervention (n = 115): The biscuits and cold drinks were distributed daily during the school week during the first 2 hours of the school day. No intervention took place during school holidays, weekends, or public holidays; the supplement was provided for a total of 215 days, or 43 weeks Control (n = 113): Placebo Food vehicle: Fortified biscuit Dose: Iron 5.9 (mg), b‐carotene 2.0 (mg), iodine 95.4 (ug) Duration: 12 months Additional Interventions: To enhance the absorption of iron, a vitamin C–fortified cold drink was given to the intervention group; the control group received an unfortified cold drink (placebo) All the children were dewormed (400 mg albendazole) at 4‐monthly intervals during the 12‐month randomised controlled trial, and on a further three occasions during the subsequent 18‐month follow‐up period. | |
| Outcomes | Serum retinol, ferritin, iron, transferrin saturation, haemoglobin, hematocrit, urinary iodine, white blood cell count | |
| Notes | Supported by a grant from SASKO Pty Ltd, who also donated the fortified products and placebo; the anthelmintic tablets were donated by SmithKline Beecham Pharmaceuticals Pty, Ltd Study duration: May 1995 to June 1996 | |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "The other group received an unfortified biscuit similar to the fortified biscuit in macronutrient composition, taste, and appearance. To avoid the exchange of biscuits and cold drinks between classmates, the intervention and control groups were seated on opposite sides of the classroom." Comment: Adequately done |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient information to permit judgement |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 5.3% total loss to follow‐up Comment: Low attrition rate |
| Selective reporting (reporting bias) | Low risk | Trial registration not specified. Outcomes specified in the methods section have been reported in the Results |
| Other bias | Low risk | No additional bias identified |
| Methods | A double‐blind, placebo‐controlled, randomised trial in children aged between 7 and 10½ years from 3 schools in Bangalore, India | |
| Participants | 300 clinically healthy school‐age children aged 7 to 10½ years old | |
| Interventions | Children were allocated to 1 of 3 study arms: Duration: 120 days. For this review, we included data for groups F and U | |
| Outcomes | Micronutrient status included thiamine, riboflavin, folate, niacin, iron, pyridoxal phosphate, and vitamins B12 and C | |
| Notes | This trial was sponsored by GlaxoSmithKline Consumer Healthcare Ltd, Gurgaon, India Study duration: | |
| Random sequence generation (selection bias) | Low risk | Quote: "The block randomisation technique was employed to generate 20 blocks (10 each of girls and boys to ensure equal gender distribution) each of size 15. The participants in each block were individually randomised to 1 of the 3 treatment groups based on a computer‐generated randomisation sequence." Comment: Adequately done |
| Allocation concealment (selection bias) | Low risk | Quote: "The computer‐generated sequence of randomisation with study arm allocation was restricted to a single person (T.T.)" Comment: Adequately done |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "The sponsor retained the codes for the product (F and U groups) and a copy was kept with a faculty member not involved with the study at the site in the event of an emergency. These codes were broken once all biochemical assessments (except thiamine and niacin) were completed and after database lock." Comment: Adeqautely done |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "The sponsor retained the codes for the product (F and U groups) and a copy was kept with a faculty member not involved with the study at the site in the event of an emergency. These codes were broken once all biochemical assessments (except thiamine and niacin) were completed and after database lock." Comment: Adeqautely done |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: Overall loss to follow‐up: 13/287 Low risk of attrition bias |
| Selective reporting (reporting bias) | Low risk | Comment: This trial was registered at clinicaltrials.gov as NCT00876018 and the prespecified outcomes have been reported |
| Other bias | Low risk | No additional bias identified |
| Methods | Randomised controlled trial | |
| Participants | This randomised clinical trial was carried out in a poor peri‐urban community of 5000 inhabitants in the outskirts of Puebla, a city located 120 km east of Mexico City. Healthy children (n = 115), 10 – 30 months of age at the beginning of the study, were selected from a registry of children younger than 5 years of age living in the community | |
| Interventions | Intervention: Children were randomly assigned to drink 400 mL/d (200 mL in the morning, 200 mL in the evening) of cow’s whole milk (distributed as milk powder) either fortified (FM) with 5.28 mg/400 mL of iron as ferrous gluconate and other micronutrients (n = 58) or non‐fortified milk (NFM) (iron concentration: 0.2 mg/400 mL) (n = 57). Food vehicle: Fortified milk Dose: unit/kg dry powder Iron (ferrous gluconate), 109.8 mg, zinc (zinc oxide), 109.8 mg, retinol palmitate, 449 ug, vitamin C (sodium ascorbate), 998 mg, folic acid, 669 ug Duration: 6 months | |
| Outcomes | Haemoglobin, serum ferritin, transferrin receptor, zinc | |
| Notes | Supported in part by The Ministry of Social Development of Mexico and Instituto Nacional de Salud Publica Study duration: Not specified | |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Children were randomly assigned to drink 400 mL/d (200 mL in the morning, 200 mL in the evening) of cow’s whole milk .. " Comment: Insufficient information to permit judgement |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "The packages of FM and NFM were undistinguishable, except for a color‐coded band in the upper corner of the sachet. The colour code was unknown to researchers, field workers, and users and was disclosed after data analysis." Comment: Adequately done |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "The packages of FM and NFM were undistinguishable, except for a color‐coded band in the upper corner of the sachet. The colour code was unknown to researchers, field workers, and users and was disclosed after data analysis." Comment: Adequately done |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Intervention group: 10/68 Control group: 5/62 11.5% total loss to follow‐up Comment: Low attrition rate |
| Selective reporting (reporting bias) | Low risk | Trial registration details not specified. All the prespecified outcomes in the methods section have been reported in the Results section |
| Other bias | Low risk | No additional bias identified |
| Methods | Cluster‐randomised controlled trial | |
| Participants | 3 residential schools were randomly selected as the experimental schools and 3 other residential schools as the controls in the city of Chennai, Tamilnadu, South India (n = 402) | |
| Interventions | Intervention (n = 213): Multiple micronutrient‐fortified salt Control (n = 189): Iodised salt Food vehicle: Fortified salt Dose: Vitamin A 3000 IU, vitamin B1 1 mg, vitamin B2 1 mg, vitamin B6 1 mg, niacin 5 mg, iron 1000 ppm, iodine 40 ppm, folic acid 100 mcg, vitamin B12 4 mcg, zinc 10 mg Duration: 9 months Additional interventions: Both the experimental and the control children were given a tablet of albendazole (400 mg) at baseline, at 4 months, and post‐intervention after 9 months | |
| Outcomes | Haemoglobin, serum ferritin, transferrin receptor, CRP, vitamin A, B12, folate, zinc, body iron stores, prevalence of angular stomatitis | |
| Notes | Supported by Task Force Sight and Life Study duration: July 2005 to April 2006 | |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Three residential schools were randomly selected as the experimental schools and three other residential schools as the controls.." Comment: Insufficient information to permit judgement |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Insufficient information to permit judgement |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient information to permit judgement |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: 11.7% total loss to follow‐up Low attrition rate |
| Selective reporting (reporting bias) | Low risk | Comment: Trial registration details not specified. All the prespecified outcomes in the methods section have been reported in the Results section |
| Other bias | Low risk | No additional bias identified |
| Methods | A cluster‐randomised controlled trial among healthy Chinese middle‐school students, aged 12 to 14 years, between June 2015 and January 2016 | |
| Participants | 360 students were enrolled from Xi’an Middle School | |
| Interventions | Participating children were allocated to either an intervention group (n = 177) or a control group (n = 183). Intervention group students were given 250 mL micronutrient‐fortified milk (Future Star, Mengniu Dairy Company Limited, Hohhot, China) per day for 6 months; students of the control group were provided with pure milk with approximately the same caloric value of the fortified milk (Milk Deluxe, China Mengniu Dairy Company Limited, Hohhot, China) Energy KJ: 332 Protein g: 3.1 Fat g: 3.6 Carbohydrate g: 8.6 Sodium mg: 58 Vitamin A g RE: 78, vitamin D g: 1.5, vitamin E mg ‐TE: 2.0, vitamin B2 mg: 0.09, pantothenic acid mg: 0.2, phosphorus mg: 70, calcium mg: 100, Zinc mg: 0.34 | |
| Outcomes | Micronutrient deficiencies (iron, vitamin D, vitamin B2, vitamin B12, selenium); academic performance; motivation and learning strategy scores | |
| Notes | This work was financially supported by a grant (Grant No. 81101333) from the National Natural Science Foundation of China and a grant (Grant No. 13‐168‐201608) from China Medical Board Study duration: June 2015 and January 2016 | |
| Random sequence generation (selection bias) | Low risk | Quote: "Participating children were allocated to either an intervention group (n = 177) or a control group (n = 183) with random number table by the research staff." Comment: Adequately done |
| Allocation concealment (selection bias) | High risk | Not done |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote: "Children, study investigators and the data analyst were not blinded to the treatment allocation" Comment: Not done |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Quote: "Children, study investigators and the data analyst were not blinded to the treatment allocation" Comment: Not done |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Intervention group: 40/177 Control group: 24/183 |
| Selective reporting (reporting bias) | Low risk | Trial registration details not specified. All the pre‐specified outcomes in the methods section have been reported in the Results section |
| Other bias | Low risk | Comment: No additional biases identified. (i) recruitment bias: Low risk (ii) baseline imbalance: Low risk (iii) loss of clusters: Low risk (iv) incorrect analysis: Low risk (v) comparability with individually randomised trials: Low risk |
| Methods | Randomised controlled trial | |
| Participants | Conducted in Morocco. The participants were children 6 – 14 years old from 2 neighbouring primary schools | |
| Interventions | Intervention: Group 1 (IS group) was given IS, i.e. salt fortified with 25 ug I/g salt (n = 83) Group 2 (TFS group) was given TFS, i.e. salt triple‐fortified with 25 ug iodine, 60 ug vitamin A, and 2 mg iron/g salt (n = 74) Food vehicle: Triple‐fortified salt Dose: 25 ug iodine, 60 ug vitamin A, and 2 mg iron/g salt. Duration: 10 months | |
| Outcomes | Haemoglobin, serum transferrin, ferritin, zinc, protoporphyrin, body iron, retinol, RBP | |
| Notes | Supported by the Thrasher Research Fund (Salt Lake City), the Foundation for Micronutrients in Medicine (Rapperswil, Switzerland), and the Swiss Federal Institute of Technology (Zurich, Switzerland) Study duration: Not specified | |
| Random sequence generation (selection bias) | High risk | Quote: "Because each participating family shared a monthly salt portion, children were randomly divided by household into 2 groups." Comment: Not adequately done |
| Allocation concealment (selection bias) | Unclear risk | Comment: Insufficient information to permit judgement |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "Both investigators and households were blind to group assignment" Comment: Adequately done |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "Both investigators and households were blind to group assignment" Comment: Adequately done |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: "Of the 159 children who began the study, 157 completed it; 2 children in the TFS group moved away." Comment: Low attrition rate |
| Selective reporting (reporting bias) | Low risk | Comment: Trial registration details not specified. All the pre‐specified outcomes in the methods section have been reported in the results section |
| Other bias | Low risk | No additional bias identified |
BMI: body mass index; CRP: C‐reactive protein; DHA: docosahexaenoic acid; HAZ: height‐for‐age Z‐score; HDL: high‐density lipoprotein; LAZ: length‐for‐age Z‐score; LD: low‐density lipoprotein; MUAC: middle upper arm circumference; RBP: retinol binding protein; RDA: recommended daily allowance; RNI: recommended nutritional intake; TG: triglycrides; WAZ: wight‐for‐age Z‐score; WHZ: weight‐for‐height Z‐score; WLZ: weight‐for‐length Z‐score
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| The study does not have an appropriate control group | |
| This study involves comparison of use of alternative natural foods with standard foods rather than the use of fortified food vehicles | |
| This study only used 2 micronutrients (Iron and vitamin A) | |
| This study included children who were anaemic at baseline | |
| This study included participants who were anaemic at baseline | |
| This study reported multiple cross‐sectional surveys | |
| This study was a pre‐post study without a control group | |
| This study compares diets instead of singular food vehicles with little reference to the exact included foods in those diets | |
| This study did not mention the specific micronutrients | |
| This study assessed in‐home fortification using micronutrient powders | |
| This study evaluates supplementation | |
| This study provided fortified blended foods | |
| This study included participants with osteoarthritis | |
| This study focused on specific population group (HIV exposed children) | |
| This study assessed in‐home fortification using micronutrient powders | |
| This study was a pre‐post study without a control group | |
| This study compares various fortified blended foods | |
| This study was a pre‐post study without a control group | |
| This study was a pre‐post study without a control group | |
| This study was a pre‐post design without a control group | |
| This is a cross‐sectional study | |
| This study focused on blended foods | |
| This study assess ready‐to‐use therapeutic food | |
| This study was a pre‐post study without a control group | |
| This study assessed fortified blended foods | |
| This study did not have an appropriate control group | |
| This study did not have an appropriate control group | |
| This study included non‐isocaloric supplement | |
| This study did not have an appropriate control group | |
| This study assessed point‐of‐use fortification | |
| This study did not have an appropriate control group | |
| This study assessed point‐of‐use fortification | |
| This study focused on ready‐to‐use therapeutic food and spreads | |
| This study focused on ready‐to‐use therapeutic food | |
| This study had a pre‐post design without a control group | |
| This study did not have an appropriate control group | |
| This study compares milk MMN fortifier with MMN supplement | |
| This study assessed ready‐to‐use therapeutic food | |
| This study assessed iron and selenium fortification only | |
| This is a pre‐post study without a control group | |
| This study supplemented a drink; did not assess fortification | |
| This study did not have an appropriate control group | |
| This study did not have an appropriate control group | |
| This study did not have an appropriate control group | |
| This study focused on a specific population group (critically ill people) | |
| This study did not have an appropriate control group | |
| This study was a before‐after study without a control group | |
| This is study assessed point‐of‐use home fortification | |
| This study did not have an appropriate control group | |
| This is study assessed point‐of‐use home fortification | |
| This study provided multiple micronutrient supplement, not fortification | |
| This study included participants who were already anaemic | |
| This is study assessed point‐of‐use home fortification | |
| This study assessed ready‐to‐use therapeutic food | |
| This study assessed ready‐to‐use therapeutic food. | |
| This study assessed ready‐to‐use therapeutic food | |
| This study assessed bioavailability in cell cultures | |
| This study assessed supplementation; not fortification | |
| This study reported nutrient intakes only | |
| This study is a cross‐sectional survey | |
| This study did not have an appropriate control group | |
| This study assessed supplementation; not fortification | |
| This is a pre‐post study without a control group | |
| This study is a cross‐sectional survey | |
| This study involves calcium supplementation | |
| This is a pre‐post study without a control group | |
| This is study assessed point‐of‐use home fortification | |
| This is a pre‐post study without a control group | |
| This study did not have an appropriate control group | |
| This is an observational study | |
| This is study assessed point‐of‐use home fortification | |
| The study focused on folic acid fortification alone | |
| This study assessed lipid‐based nutrient supplement | |
| This is a pre‐post study without a control group | |
| This is a pre‐post study without a control group | |
| This study assessed only 2 micronutrients for fortification (iron and vitamin A) | |
| This study assessed supplementation rather than fortification | |
| This study assessed supplementation rather than fortification |
Differences between protocol and review
- Background has been updated to reflect current information.
- We have modified the search strategy from the protocol, in consultation with the Information Scientist.
- We have now specified that we excluded studies conducted among special population groups including critically‐ill people, anaemic people or people diagnosed with any specific diseases. We also excluded studies on therapeutic blended food and food supplementation.
- We have removed the text pertaining to contacting the trial authors for more data, since this was not needed and hence not done.
- We have removed subgroup analysis by study design (RCTs/non‐RCTs, CBA/ITS).
- We have specified that we have only conducted subgroup analysis where there were at least three studies in each subgroup.
- We have modified the methods used to assess reporting bias to indicate that we used visual assessment rather than statistical tests, and that we explored any asymmetric funnel plots using sensitivity analysis to compare the fixed‐effect and random‐effects meta‐analyses.
- One of our prespecified primary outcomes was anthropometric measures and we had intended to include stunting, wasting and underweight. However, since none of the included studies reported these outcomes, we could not report them. We reported WAZ, HAZ/LAZ and WHZ as anthropometric outcomes, since they were reported in the included studies.
- We could not conduct all of the planned subgroup analyses under every comparison due to a limited number of included studies reporting on the relevant comparisons.
Contributions of authors
All authors contributed to the development of the review. Rohail Kumar (RK), Anoosh Moin (AM), Kashif Mukhtar (KM) and Salman Bin Mahmood (SBM) developed and ran the search strategy and obtained copies of the studies; Jai K Das (JKD), SBM and Rehana A Salam (RAS) selected which studies to include; RK, AM, KM, SBM and Zohra Lassi (ZL) extracted data from studies and entered data into Review Manager 5; SBM, JKD, ZL and RAS carried out and interpreted the analysis. RAS and JKD evaluated the studies according to the PROGRESS‐PLUS criteria. JKD, RAS, SBM, ZL and Zulfiqar A Bhutta (ZAB) drafted the final review, with input from all the authors.
Sources of support
Internal sources.
- Aga Khan University, Pakistan.
External sources
- No sources of support supplied
Declarations of interest
JKD: no competing interests.
RAS: no competing interests.
SBM: no competing interests.
AM: no competing interests.
RK: no competing interests.
KM: no competing interests.
ZL: Participated in a Nestlé Nutrition Institute workshop on Health and Nutrition in Adolescents and Young Women: preparing for the next generation for the related publication Nestlé Nutrition Institute Series Volume 80 (2015)
ZAB: Participated in a Nestlé Nutrition Institute workshop on Health and Nutrition in Adolescents and Young Women: preparing for the next generation and co‐edited the related publication Nestlé Nutrition Institute Series Volume 80 (2015). ZAB declares previous travel support from the Nestlé Nutrition Institute for attendance at a meeting on fortification strategies at the University of Winterthur, Winterthur, Switzerland, in October 2011. ZAB received an institutional grant from GAIN on fortification program evidence review in 2014. The paper is currently in press.
References to studies included in this review
Aaron 2011 {published data only}.
- Aaron GJ, Kariger P, Aliyu R, Flach M, Iya D, Obadiah M, et al. A multi‐micronutrient beverage enhances the vitamin A and zinc status of Nigerian primary schoolchildren . Journal of Nutrition 2011; 141 ( 8 ):1565‐72. [ PubMed ] [ Google Scholar ]
Abrams 2003 {published data only}
- Abrams SA, Mushi A, Hilmers DC, Griffin IJ, Davila P, Allen L. A multinutrient‐fortified beverage enhances the nutritional status of children in Botswana . Journal of Nutrition 2003; 133 ( 6 ):1834‐40. [ PubMed ] [ Google Scholar ]
Adams 2017 {published data only}
- Adams AM, Ahmed R, Latif AM, Rasheed S, Das SK, Hasib E, et al. Impact of fortified biscuits on micronutrient deficiencies among primary school children in Bangladesh . PloS One 2017; 12 ( 4 ):e0174673. [ PMC free article ] [ PubMed ] [ Google Scholar ]
Ash 2003 {published data only}
- Ash DM, Tatala SR, Frongillo EA Jr, Ndossi GD, Latham MC. Randomized efficacy trial of a micronutrient‐fortified beverage in primary school children in Tanzania . American Journal of Clinical Nutrition 2003; 77 ( 4 ):891‐8. [ PubMed ] [ Google Scholar ]
Azlaf 2017 {published data only}
- Azlaf M, Hamdouchi A, Benjeddou K, Zahrou FZ, Menchawy I, Kari K, et al. School fortified milk improves vitamin A status of rural children in Morocco: A longitudinal interventional and controlled study 1 . Mediterranean Journal of Nutrition and Metabolism 2017; 10 ( 1 ):13‐27. [ Google Scholar ]
Chin A Paw 2000 {published data only}
- Chin A Paw MJ, Jong N, Pallast EG, Kloek GC, Schouten EG, Kok FJ. Immunity in frail elderly: a randomized controlled trial of exercise and enriched foods . Medicine and Science in Sports and Exercise 2000; 32 ( 12 ):2005‐11. [ PubMed ] [ Google Scholar ]
DeGier 2016 {published data only}
- Gier B, Campos Ponce M, Perignon M, Fiorentino M, Khov K, Chamnan C, et al. Micronutrient‐fortified rice can increase hookworm infection risk: a cluster randomized trial . PLoS One 2016; 11 ( 1 ):e0145351. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Fiorentino M, Perignon M, Kuong K, Groot R, Parker M, Burja K, et al. Effect of multi‐micronutrient‐fortified rice on cognitive performance depends on premix composition and cognitive function tested: results of an effectiveness study in Cambodian schoolchildren . Public Health Nutrition 2018; 21 ( 4 ):816‐27. [ PMC free article ] [ PubMed ] [ Google Scholar ]
Economos 2014 {published data only}
- Economos CD, Moore CE, Hyatt RR, Kuder J, Chen T, Meydani SN, et al. Multinutrient‐fortified juices improve vitamin D and vitamin E status in children: a randomized controlled trial . Journal of the Academy of Nutrition and Dietetics 2014; 114 ( 5 ):709‐17. [ PubMed ] [ Google Scholar ]
Faber 2005 {published data only}
- Faber M, Kvalsvig JD, Lombard CJ, Benadé AJ. Effect of a fortified maize‐meal porridge on anemia, micronutrient status, and motor development of infants . American Journal of Clinical Nutrition 2005; 82 ( 5 ):1032‐9. [ PubMed ] [ Google Scholar ]
Gibson 2011 {published data only}
- Gibson RS, Kafwembe E, Mwanza S, Gosset L, Bailey KB, Mullen A, et al. A micronutrient‐fortified food enhances iron and selenium status of Zambian infants but has limited efficacy on zinc . Journal of Nutrition 2011; 141 ( 5 ):935‐43. [ PubMed ] [ Google Scholar ]
Hieu 2012 {published data only}
- Hieu NT, Sandalinas F, Sesmaisons A, Laillou A, Tam NP, Khan NC, et al. Multi‐micronutrient‐fortified biscuits decreased the prevalence of anaemia and improved iron status, whereas weekly iron supplementation only improved iron status in Vietnamese school children . British Journal of Nutrition 2012; 108 ( 8 ):1419‐27. [ PubMed ] [ Google Scholar ]
Hyder 2007 {published data only}
- Hyder SM, Haseen F, Khan M, Schaetzel T, Jalal CS, Rahman M, et al. A multiple‐micronutrient‐fortified beverage affects hemoglobin, iron, and vitamin A status and growth in adolescent girls in rural Bangladesh . Journal of Nutrition 2007; 137 ( 9 ):2147‐53. [ PubMed ] [ Google Scholar ]
Järvenpaa 2007 {published data only}
- Järvenpää J, Schwab U, Lappalainen T, Päkkilä M, Niskanen L, Punnonen K, et al. Fortified mineral water improves folate status and decreases plasma homocysteine concentration in pregnant women . Journal of Perinatal Medicine 2007; 35 ( 2 ):108‐14. [ PubMed ] [ Google Scholar ]
Jinabhai 2001 {published data only}
- Jinabhai CC, Taylor M, Coutsoudis A, Coovadia HM, Tomkins AM, Sullivan KR. A randomized controlled trial of the effect of antihelminthic treatment and micronutrient fortification on health status and school performance of rural primary school children . Annals of Tropical Paediatrics 2001; 21 ( 4 ):319‐33. [ PubMed ] [ Google Scholar ]
Liu 1993 {published data only}
- Liu DS, Bates CJ, Yin TA, Wang XB, Lu CQ. Nutritional efficacy of a fortified weaning rusk in a rural area near Beijing . American Journal of Clinical Nutrition 1993; 57 ( 4 ):506‐11. [ PubMed ] [ Google Scholar ]
Lopriore 2004 {published data only}
- Lopriore C, Guidoum Y, Briend A, Branca F. Spread fortified with vitamins and minerals induces catch‐up growth and eradicates severe anemia in stunted refugee children aged 3‐6 y . American Journal of Clinical Nutrition 2004; 80 ( 4 ):973‐81. [ PubMed ] [ Google Scholar ]
Mardones 2007 {published data only}
- Mardones F, Urrutia MT, Villarroel L, Rioseco A, Castillo O, Rozowski J, et al. Effects of a dairy product fortified with multiple micronutrients and omega‐3 fatty acids on birth weight and gestation duration in pregnant Chilean women . Public Health Nutrition 2008; 11 ( 1 ):30‐40. [ PubMed ] [ Google Scholar ]
Nesamvuni 2005 {published data only}
- Nesamvuni AE, Vorster HH, Margetts BM, Kruger A. Fortification of maize meal improved the nutritional status of 1‐3‐year‐old African children . Public Health Nutrition 2005; 8 ( 5 ):461‐7. [ PubMed ] [ Google Scholar ]
Nga 2009 {published data only}
- Nga TT, Winichagoon P, Dijkhuizen MA, Khan NC, Wasantwisut E, Furr H, et al. Multi‐micronutrient‐fortified biscuits decreased prevalence of anemia and improved micronutrient status and effectiveness of deworming in rural Vietnamese school children . Journal of Nutrition 2009; 139 ( 5 ):1013‐21. [ PubMed ] [ Google Scholar ]
- Nga TT, Winichagoon P, Dijkhuizen MA, Khan NC, Wasantwisut E, Wieringa FT. Decreased parasite load and improved cognitive outcomes caused by deworming and consumption of multi‐micronutrient fortified biscuits in rural Vietnamese schoolchildren . American Journal of Tropical Medicine and Hygiene 2011; 85 ( 2 ):330‐40. [ PMC free article ] [ PubMed ] [ Google Scholar ]
Oelofse 2003 {published data only}
- Oelofse A, Raaij JM, Benade AJ, Dhansay MA, Tolboom JJ, Hautvast JG. The effect of a micronutrient‐fortified complementary food on micronutrient status, growth and development of 6‐to 12‐month‐old disadvantaged urban South African infants . International Journal of Food Sciences and Nutrition 2003; 54 ( 5 ):399‐407. [ PubMed ] [ Google Scholar ]
Osendarp 2007 {published data only}
- Osendarp SJ, Baghurst KI, Bryan J, Calvaresi E, Hughes D, Hussaini M, et al. NEMO Study Group. Effect of a 12‐mo micronutrient intervention on learning and memory in well‐nourished and marginally nourished school‐aged children: 2 parallel, randomized, placebo‐controlled studies in Australia and Indonesia . American Journal of Clinical Nutrition 2007; 86 ( 4 ):1082‐93. [ PubMed ] [ Google Scholar ]
Perignon 2016 {published data only}
- Perignon M, Fiorentino M, Kuong K, Dijkhuizen MA, Burja K, Parker M, et al. Impact of multi‐icronutrient fortified rice on hemoglobin, iron and vitamin A status of Cambodian schoolchildren: a double‐blind cluster‐randomized controlled trial . Nutrients 2016; 8 ( 1 ):29. [DOI: 10.3390/nu8010029] [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
Petrova 2019 {published data only}
- Petrova D, Litrán MA, García‐Mármol E, Rodríguez‐Rodríguez M, Cueto‐Martín B, López‐Huertas E, et al. Еffects of fortified milk on cognitive abilities in school‐aged children: results from a randomised‐controlled trial . European Journal of Nutrition 2019; 58 ( 5 ):1863‐72. [ PubMed ] [ Google Scholar ]
Pinkaew 2013 {published data only}
- Pinkaew S, Winichagoon P, Hurrell RF, Wegmuller R. Extruded rice grains fortified with zinc, iron, and vitamin A increase zinc status of Thai school children when incorporated into a school lunch program . Journal of Nutrition 2013; 143 ( 3 ):362‐8. [DOI: 10.3945/jn.112.166058] [ PubMed ] [ CrossRef ] [ Google Scholar ]
Pinkaew 2014 {published data only}
- Pinkaew S, Wegmuller R, Wasantwisut E, Winichagoon P, Hurrell RF, Tanumihardjo SA. Triple‐fortified rice containing vitamin A reduced marginal vitamin A deficiency and increased vitamin A liver stores in school‐aged Thai children . Journal of Nutrition 2014; 144 ( 4 ):519‐24. [ PubMed ] [ Google Scholar ]
Powers 2016 {published data only}
- Powers HJ, Stephens M, Russell J, Hill MH. Fortified breakfast cereal consumed daily for 12 wk leads to a significant improvement in micronutrient intake and micronutrient status in adolescent girls: a randomised controlled trial . Nutrition Journal 2015; 15 ( 1 ):69. [ PMC free article ] [ PubMed ] [ Google Scholar ]
Rahman 2015 {published data only}
- Rahman AS, Ahmed T, Ahmed F, Alam MS, Wahed MA, Sack DA. Double‐blind cluster randomised controlled trial of wheat flour chapatti fortified with micronutrients on the status of vitamin A and iron in school‐aged children in rural Bangladesh . Maternal & Child Nutrition 2015; 11 Suppl 4 :120‐31. [ PMC free article ] [ PubMed ] [ Google Scholar ]
Sazawal 2007 {published data only}
- Sazawal S, Dhingra U, Dhingra P, Hiremath G, Kumar J, Sarkar A, et al. Effects of fortified milk on morbidity in young children in north India: community based, randomised, double masked placebo controlled trial . BMJ 2007; 334 ( 7585 ):140. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Sazawal S, Dhingra U, Dhingra P, Hiremath G, Sarkar A, Dutta A, et al. Micronutrient fortified milk improves iron status, anemia and growth among children 1‐4 years: a double masked, randomized, controlled trial . PLoS One 2010; 5 ( 8 ):e12167. [ PMC free article ] [ PubMed ] [ Google Scholar ]
Sazawal 2013 {published data only}
- Sazawal S, Habib A, Dhingra U, Dutta A, Dhingra P, Sarkar A, et al. Impact of micronutrient fortification of yoghurt on micronutrient status markers and growth ‐ a randomized double blind controlled trial among school children in Bangladesh . BMC Public Health 2013; 13 :514. [ PMC free article ] [ PubMed ] [ Google Scholar ]
Solon 2003 {published data only}
- Solon FS, Sarol JN Jr, Bernardo AB, Solon JA, Mehansho H, Sanchez‐Fermin LE, et al. Effect of a multiple‐micronutrient‐fortified fruit powder beverage on the nutrition status, physical fitness, and cognitive performance of schoolchildren in the Philippines . Food and Nutrition Bulletin 2003; 4 ( 2 ):129‐40. [ PubMed ] [ Google Scholar ]
Taljaard 2013 {published data only}
- Taljaard C, Covic NM, Graan AE, Kruger HS, Smuts CM, Baumgartner J, et al. Effects of a multi‐micronutrient‐fortified beverage, with and without sugar, on growth and cognition in South African schoolchildren: a randomised, double‐blind, controlled intervention . British Journal of Nutrition 2013; 110 ( 12 ):2271‐84. [ PubMed ] [ Google Scholar ]
Tapola 2004 {published data only}
- Tapola NS, Karvonen HM, Niskanen LK, Sarkkinen ES. Mineral water fortified with folic acid, vitamins B6, B12, D and calcium improves folate status and decreases plasma homocysteine concentration in men and women . European Journal of Clinical Nutrition 2004; 58 ( 2 ):376‐85. [ PubMed ] [ Google Scholar ]
Tatala 2002 {published data only}
- Makola D, Ash DM, Tatala SR, Latham MC, Ndossi G, Mehansho H. A micronutrient‐fortified beverage prevents iron deficiency, reduces anemia and improves the hemoglobin concentration of pregnant Tanzanian women . Journal of Nutrition 2003; 133 ( 5 ):1339‐46. [ PubMed ] [ Google Scholar ]
- Tatala SR, Ash D, Makola D, Latham M, Ndosi G, Grohn Y. Effect of micronutrient fortified beverage on nutritional anaemia during pregnancy . East African Medical Journal 2002; 79 ( 11 ):598‐603. [ PubMed ] [ Google Scholar ]
Thankachan 2012 {published data only}
- Thankachan P, Rah JH, Thomas T, Selvam S, Amalrajan V, Srinivasan K, et al. Multiple micronutrient‐fortified rice affects physical performance and plasma vitamin B‐12 and homocysteine concentrations of Indian school children . Journal of Nutrition 2012; 142 ( 5 ):846‐52. [ PubMed ] [ Google Scholar ]
Thankachan 2013 {published data only}
- Thankachan P, Selvam S, Surendran D, Chellan S, Pauline M, Abrams SA, et al. Efficacy of a multi micronutrient‐fortified drink in improving iron and micronutrient status among schoolchildren with low iron stores in India: a randomised, double‐masked placebo‐controlled trial . European Journal of Clinical Nutrition 2013; 67 ( 1 ):36‐41. [ PubMed ] [ Google Scholar ]
Tucker 2004 {published data only}
- Tucker KL, Olson B, Bakun P, Dallal GE, Selhub J, Rosenberg IH. Breakfast cereal fortified with folic acid, vitamin B‐6, and vitamin B‐12 increases vitamin concentrations and reduces homocysteine concentrations: a randomized trial . American Journal of Clinical Nutrition 2004; 79 ( 5 ):805‐11. [ PubMed ] [ Google Scholar ]
Van het Hof 1998 {published data only}
- het Hof KH, Tijburg LB, Boer HS, Wiseman SA, Weststrate JA. Antioxidant fortified margarine increases the antioxidant status . European Journal of Clinical Nutrition 1998; 52 ( 4 ):292‐9. [ PubMed ] [ Google Scholar ]

Van Stuijvenberg 1999 {published data only}
- Stuijvenberg ME, Kvalsvig JD, Faber M, Kruger M, Kenoyer DG, Benadé AJ. Effect of iron‐, iodine‐, and beta‐carotene‐fortified biscuits on the micronutrient status of primary school children: a randomized controlled trial . American Journal of Clinical Nutrition 1999; 69 ( 3 ):497‐503. [ PubMed ] [ Google Scholar ]
Vaz 2011 {published data only}
- Vaz M, Pauline M, Unni US, Parikh P, Thomas T, Bharathi AV, et al. Micronutrient supplementation improves physical performance measures in Asian Indian school‐age children . Journal of Nutrition 2011; 141 ( 11 ):2017‐23. [ PubMed ] [ Google Scholar ]
Villalpando 2006 {published data only}
- Villalpando S, Shamah T, Rivera JA, Lara Y, Monterrubio E. Fortifying milk with ferrous gluconate and zinc oxide in a public nutrition program reduced the prevalence of anemia in toddlers . Journal of Nutrition 2006; 136 ( 10 ):2633‐37. [ PubMed ] [ Google Scholar ]
Vinodkumar 2009 {published data only}
- Vinodkumar M, Erhardt JG, Rajagopalan S. Impact of a multiple‐micronutrient fortified salt on the nutritional status and memory of schoolchildren . International Journal for Vitamin and Nutrition Research 2009; 79 ( 5‐6 ):348‐61. [ PubMed ] [ Google Scholar ]
Wang 2017 {published data only}
- Wang X, Hui Z, Dai X, Terry PD, Zhang Y, Ma M, et al. Micronutrient‐fortified milk and academic performance among Chinese middle school students: a cluster‐randomized controlled trial . Nutrients 2017; 9 ( 3 ):226. [ Google Scholar ]
Zimmerman 2004 {published data only}
- Zimmermann MB, Wegmueller R, Zeder C, Chaouki N, Biebinger R, Hurrell RF, et al. Triple fortification of salt with microcapsules of iodine, iron, and vitamin A . American Journal of Clinical Nutrition 2004; 80 ( 5 ):1283‐90. [ PubMed ] [ Google Scholar ]
References to studies excluded from this review
Aburto 2010 {published data only}.
- Aburto NJ, Ramirez‐Zea M, Neufeld LM, Flores‐Ayala R. The effect of nutritional supplementation on physical activity and exploratory behavior of Mexican infants aged 8‐12 months . European Journal of Clinical Nutrition 2010; 64 ( 6 ):644‐51. [ PubMed ] [ Google Scholar ]
Agte 2006 {published data only}
- Agte V, Jahagirdar M, Chiplonkar S. GLV supplements increased plasma beta‐carotene, vitamin C, zinc and hemoglobin in young healthy adults . European Journal of Nutrition 2006; 45 ( 1 ):29‐36. [ PubMed ] [ Google Scholar ]
Anand 2007 {published data only}
- Anand K, Lakshmy R, Janakarajan VN, Ritvik A, Misra P, Pandey RM, et al. Effect of consumption of micronutrient fortified candies on the iron and vitamin A status of children aged 3‐6 years in rural Haryana . Indian Pediatrics 2007; 44 ( 11 ):823. [ PubMed ] [ Google Scholar ]
Angeles Agdeppa 2011 {published data only}
- Angeles‐Agdeppa I, Magsadia CR, Capanzana MV. Fortified juice drink improved iron and zinc status of schoolchildren . Asia Pacific Journal of Clinical Nutrition 2011; 20 ( 4 ):535‐43. [ PubMed ] [ Google Scholar ]
Angeles‐Agdeppa 2017 {published data only}
- Angeles‐Agdeppa I, Magsadia CR, Aaron GJ, Lloyd BB, Hilmers DC, Bhutta ZA. A Micronutrient Fortified Beverage Given at Different Dosing Frequencies Had Limited Impact on Anemia and Micronutrient Status in Filipino Schoolchildren . Nutrients 2017; 9 ( 9 ):1002. [ PMC free article ] [ PubMed ] [ Google Scholar ]
Barkley 2015 {published data only}
- Barkley JS, Wheeler KS, Pachón H. Anaemia prevalence may be reduced among countries that fortify flour . British Journal of Nutrition 2015; 114 ( 2 ):265‐73. [ PubMed ] [ Google Scholar ]
Baró 2003 {published data only}
- Baró L, Fonollá J, Peña JL, Martínez‐Férez A, Lucena A, Jiménez J, et al. n‐3 Fatty acids plus oleic acid and vitamin supplemented milk consumption reduces total and LDL cholesterol, homocysteine and levels of endothelial adhesion molecules in healthy humans . Clinical Nutrition 2003; 22 ( 2 ):175‐82. [ PubMed ] [ Google Scholar ]
Berasategi 2011 {published data only}
- Berasategi I, Cuervo M, Las Heras AR, Santiago S, Martínez JA, Astiasarán I, et al. The inclusion of functional foods enriched in fibre, calcium, iodine, fat‐soluble vitamins and n‐3 fatty acids in a conventional diet improves the nutrient profile according to the Spanish reference intake . Public Health Nutrition 2011; 14 ( 3 ):451‐8. [ PubMed ] [ Google Scholar ]
Bishop 1996 {published data only}
- Bishop WB, Laubscher I, Labadarios D, Rehder P, Louw ME, Fellingham SA. Effect of vitamin‐enriched bread on the vitamin status of an isolated rural community‐‐a controlled clinical trial . South African Medical Journal 1996; 86 ( 4 ):458‐62. [ PubMed ] [ Google Scholar ]
Chen 2011 {published data only}
- Chen K, Zhang X, Li TY, Chen L, Wei XP, Qu P, et al. Effect of vitamin A, vitamin A plus iron and multiple micronutrient‐fortified seasoning powder on infectious morbidity of preschool children . Nutrition 2011; 27 ( 4 ):428‐34. [ PubMed ] [ Google Scholar ]
Cheung 2016 {published data only}
- Cheung YB, Xu Y, Mangani C, Fan YM, Dewey KG, Salminen SJ, et al. Gut microbiota in Malawian infants in a nutritional supplementation trial . Tropical Medicine & International Health 2016; 21 ( 2 ):283‐90. [ PubMed ] [ Google Scholar ]
Christian 2015 {published data only}
- Christian P, Shaikh S, Shamim AA, Mehra S, Wu L, Mitra M, et al. Effect of fortified complementary food supplementation on child growth in rural Bangladesh: a cluster‐randomised trial . International Journal of Epidemiology 2015; 44 ( 6 ):1862‐76. [ PMC free article ] [ PubMed ] [ Google Scholar ]
Colker 2002 {published data only}
- Colker CM, Swain M, Lynch L, Gingerich DA. Effects of a milk‐based bioactive micronutrient beverage on pain symptoms and activity of adults with osteoarthritis: a double‐blind, placebo‐controlled clinical evaluation . Nutrition 2002; 18 ( 5 ):388‐92. [ PubMed ] [ Google Scholar ]
Filteau 2010 {published data only}
- Filteau S, Kasonka L, Gibson R, Gompels UA, Jaffar S, Kafwembe E, et al. Micronutrient fortification to improve growth and health of maternally HIV‐unexposed and exposed Zambian infants: a randomised controlled trial . PloS One 2010; 5 ( 6 ):e11165. [ PMC free article ] [ PubMed ] [ Google Scholar ]
Gathwala 2007 {published data only}
- Gathwala G, Chawla M, Gehlaut VS. Fortified human milk in the small for gestational age neonat . Indian Journal of Pediatrics 2007; 74 ( 9 ):815‐8. [ PubMed ] [ Google Scholar ]
Gershoff 1977 {published data only}
- Gershoff SN, McGandy RB, Suttapreyasri D, Promkutkao C, Nondasuta A, Pisolyabutra U, et al. Nutrition studied in Thailand. II. Effects of fortification of rice with lysine, threonine, thiamin, riboflavin, vitamin A, and iron on preschool children . American Journal of Clinical Nutrition 1977; 30 ( 7 ):1185‐95. [ PubMed ] [ Google Scholar ]
Glosz 2018 {published data only}
- Glosz C, Schaffner A, Reaves S, Manary M, Papathakis P. Effect of nutritional interventions on micronutrient status in pregnant Malawian women with moderate malnutrition: A randomised, controlled trial . Nutrients 2018; 10 ( 7 ):879. [ PMC free article ] [ PubMed ] [ Google Scholar ]
Goyle 2010 {published data only}
- Goyle A, Prakash S. Effect of supplementation of micronutrient fortified biscuits on haemoglobin and serum iron levels of adolescent girls from Jaipur city, India . Nutrition and Food Science 2010; 40 ( 5 ):477‐84. [ Google Scholar ]
Grieger 2009 {published data only}
- Grieger JA, Nowson CA. Use of calcium, folate, and vitamin D₃‐fortified milk for 6 months improves nutritional status but not bone mass or turnover, in a group of Australian aged care residents . Journal of Nutrition for the Elderly 2009; 28 ( 3 ):236‐54. [ PubMed ] [ Google Scholar ]
Hoffman 2007 {published data only}
- Hoffman JR, Kang J, Ratamess NA, Jennings PF, Mangine GT, Faigenbaum AD. Effect of nutritionally enriched coffee consumption on aerobic and anaerobic exercise performance . Journal of Strength and Conditioning Research 2007; 21 ( 2 ):456‐9. [ PubMed ] [ Google Scholar ]
Hund 2013 {published data only}
- Hund L, Northrop‐Clewes CA, Nazario R, Suleymanova D, Mirzoyan L, Irisova M, et al. A novel approach to evaluating the iron and folate status of women of reproductive age in Uzbekistan after 3 years of flour fortification with micronutrients . PLoS One 2013; 8 ( 11 ):e79726. [DOI: 10.1371/journal.pone.0079726] [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
Huybregts 2009 {published data only}
- Huybregts L, Roberfroid D, Lanou H, Menten J, Meda N, Camp J, et al. Prenatal food supplementation fortified with multiple micronutrients increases birth length: a randomized controlled trial in rural Burkina Faso . American Journal of Clinical Nutrition 2009; 90 ( 6 ):1593‐600. [ PubMed ] [ Google Scholar ]
Iannotti 2016 {published data only}
- Iannotti L, Dulience SJ, Joseph S, Cooley C, Tufte T, Cox K, et al. Fortified snack reduced anemia in rural school‐aged children of Haiti: a cluster‐randomized, controlled trial . PloS One 2016; 11 ( 12 ):e0168121. [ PMC free article ] [ PubMed ] [ Google Scholar ]
Jaatinen 2014 {published data only}
- Jaatinen N, Korpela R, Poussa T, Turpeinen A, Mustonen S, Merilahti J, et al. Effects of daily intake of yoghurt enriched with bioactive components on chronic stress responses: a double‐blinded randomized controlled trial . International Journal of Food Sciences and Nutrition 2014; 65 ( 4 ):507‐14. [ PubMed ] [ Google Scholar ]
Janmohamed 2016 {published data only}
- Janmohamed A, Karakochuk CD, Boungnasiri S, Chapman GE, Janssen PA, Brant R, et al. Prenatal supplementation with Corn Soya Blend Plus reduces the risk of maternal anemia in late gestation and lowers the rate of preterm birth but does not significantly improve maternal weight gain and birth anthropometric measurements in rural Cambodian women: a randomized trial . American Journal of Clinical Nutrition 2016; 103 ( 2 ):559‐66. [ PubMed ] [ Google Scholar ]
Kanellakis 2012 {published data only}
- Kanellakis S, Moschonis G, Tenta R, Schaafsma A, Heuvel EG, Papaioannou N, et al. Changes in parameters of bone metabolism in postmenopausal women following a 12‐month intervention period using dairy products enriched with calcium, vitamin D, and phylloquinone (vitamin K(1)) or menaquinone‐7 (vitamin K (2)): the Postmenopausal Health Study II . Calcified Tissue International 2012; 90 ( 4 ):251‐62. [ PubMed ] [ Google Scholar ]
Krebs 2012 {published data only}
- Krebs NF, Mazariegos M, Chomba E, Sami N, Pasha O, Tshefu A, et al. Randomized controlled trial of meat compared with multi‐micronutrient‐fortified cereal in infants and toddlers with high stunting rates in diverse settings . American Journal of Clinical Nutrition 2012; 96 ( 4 ):840‐7. [ PMC free article ] [ PubMed ] [ Google Scholar ]
Kruger 2010 {published data only}
- Kruger MC, Schollum LM, Kuhn‐Sherlock B, Hestiantoro A, Wijanto P, Li‐Yu J, et al. The effect of a fortified milk drink on vitamin D status and bone turnover in post‐menopausal women from South East Asia . Bone 2010; 46 ( 3 ):759‐67. [ PubMed ] [ Google Scholar ]
Kumar 2007 {published data only}
- Kumar MV, Rajagopalan S. Multiple micronutrient fortification of salt and its effect on cognition in Chennai school children . Asia Pacific Journal of Clinical Nutrition 2007; 16 ( 3 ):505‐11. [ PubMed ] [ Google Scholar ]
Kumar 2008 {published data only}
- Kumar MV, Rajagopalan S. Trial using multiple micronutrient food supplement and its effect on cognition . Indian Journal of Pediatrics 2008; 75 ( 7 ):671‐8. [ PubMed ] [ Google Scholar ]
Kumar 2014 {published data only}
- Kumar MV, Nirmalan PK, Erhardt JG, Rahmathullah L, Rajagopalan S. An efficacy study on alleviating micronutrient deficiencies through a multiple micronutrient fortified salt in children in South India . Asia Pacific Journal of Clinical Nutrition 2014; 23 ( 3 ):413‐22. [ PubMed ] [ Google Scholar ]
Kuriyan 2016 {published data only}
- Kuriyan R, Thankachan P, Selvam S, Pauline M, Srinivasan K, Kamath‐Jha S, et al. The effects of regular consumption of a multiple micronutrient fortified milk beverage on the micronutrient status of school children and on their mental and physical performance . Clinical Nutrition 2016; 35 ( 1 ):190‐8. [ PubMed ] [ Google Scholar ]
Kuusipalo 2006 {published data only}
- Kuusipalo H, Maleta K, Briend A, Manary M, Ashorn P. Growth and change in blood haemoglobin concentration among underweight Malawian infants receiving fortified spreads for 12 weeks: a preliminary trial . Journal of Pediatric Gastroenterology and Nutrition 2006; 43 ( 4 ):525‐32. [ PubMed ] [ Google Scholar ]
Lartey 1999 {published data only}
- Lartey A, Manu A, Brown KH, Peerson JM, Dewey KG. A randomized, community‐based trial of the effects of improved, centrally processed complementary foods on growth and micronutrient status of Ghanaian infants from 6 to 12 mo of age . American Journal of Clinical Nutrition 1999; 70 ( 3 ):391‐404. [ PubMed ] [ Google Scholar ]
Layrisse 1996 {published data only}
- Layrisse M, Chaves JF, Bosch V, Tropper E, Bastardo B, Gonzalez E. Early response to the effect of iron fortification in the Venezuelan population . American Journal of Clinical Nutrition 1996; 64 ( 6 ):903‐7. [ PubMed ] [ Google Scholar ]
Lönnerdal 1994 {published data only}
- Lönnerdal B, Hernell O. Iron, zinc, copper and selenium status of breast‐fed infants and infants fed trace element fortified milk‐based infant formula . Acta Paediatrica 1994; 83 ( 4 ):367‐73. [ PubMed ] [ Google Scholar ]
Loui 2004 {published data only}
- Loui A, Raab A, Wagner M, Weigel H, Grüters‐Kieslich A, Brätter P, et al. Nutrition of very low birth weight infants fed human milk with or without supplemental trace elements: a randomized controlled trial . Journal of Pediatric Gastroenterology and Nutrition 2004; 39 ( 4 ):346‐53. [ PubMed ] [ Google Scholar ]
Lucas 1996 {published data only}
- Lucas A, Fewtrell MS, Morley R, Lucas PJ, Baker BA, Lister G, et al. Randomized outcome trial of human milk fortification and developmental outcome in preterm infants. . American Journal of Clinical Nutrition 1996; 64 ( 2 ):142‐51. [ PubMed ] [ Google Scholar ]
Lutter 2007 {published data only}
- Lutter CK, Rodríguez A, Fuenmayor G, Avila L, Sempertegui F, Escobar J. Growth and micronutrient status in children receiving a fortified complementary food . Journal of Nutrition 2008; 138 ( 2 ):379‐88. [ PubMed ] [ Google Scholar ]
Malpeli 2013 {published data only}
- Malpeli A, Ferrari MG, Varea A, Falivene M, Etchegoyen G, Vojkovic M, et al. Short‐term evaluation of the impact of a fortified food aid program on the micronutrient nutritional status of Argentinian pregnant women . Biological Trace Element Research 2013; 155 ( 2 ):176‐83. [ PubMed ] [ Google Scholar ]
Manders 2009 {published data only}
- Manders M, Groot LC, Hoefnagels WH, Dhonukshe‐Rutten RA, Wouters‐Wesseling W, Mulders AJ, et al. The effect of a nutrient dense drink on mental and physical function in institutionalized elderly people . Journal of Nutrition, Health & Aging 2009; 13 ( 9 ):760‐7. [ PubMed ] [ Google Scholar ]
Manno 2011 {published data only}
- Manno D, Siame J, Larke N, Baisley K, Kasonka L, Filteau S. Effect of multiple micronutrient‐fortified food on mild morbidity and clinical symptoms in Zambian infants: results from a randomised controlled trial . European Journal of Clinical Nutrition 2011; 65 ( 10 ):1163. [ PubMed ] [ Google Scholar ]
Matilsky 2009 {published data only}
- Matilsky DK, Maleta K, Castleman T, Manary MJ. Supplementary feeding with fortified spreads results in higher recovery rates than with a corn/soy blend in moderately wasted children . The Journal of Nutrition 2009; 139 ( 4 ):773‐8. [ PMC free article ] [ PubMed ] [ Google Scholar ]
McNulty 1996 {published data only}
- McNulty H, Eaton‐Evans J, Cran G, Woulahan G, Boreham C, Savage JM, et al. Nutrient intakes and impact of fortified breakfast cereals in schoolchildren . Archives of Disease in Childhood 1996; 75 ( 6 ):474‐81. [ PMC free article ] [ PubMed ] [ Google Scholar ]
Mendez 2012 {published data only}
- Mendez C, Jurkovich GJ, Wener MH, Garcia I, Mays M, Maier RV. Effects of supplemental dietary arginine, canola oil, and trace elements on cellular immune function in critically injured patients . Shock 1996; 6 ( 1 ):7‐12. [ PubMed ] [ Google Scholar ]
Mendoza 2004 {published data only}
- Mendoza C, Peerson JM, Brown KH, Lönnerdal B. Effect of a micronutrient fortificant mixture and 2 amounts of calcium on iron and zinc absorption from a processed food supplement . American Journal of Clinical Nutrition 2004; 79 ( 2 ):244‐50. [ PubMed ] [ Google Scholar ]
Mishaan 2004 {published data only}
- Mishaan AM, Zavaleta N, Griffin IJ, Hilmers DC, Hawthorne KM, Abrams SA. Bioavailability of iron and zinc from a multiple micronutrient‐fortified beverage . Journal of Pediatrics 2004; 145 ( 1 ):26‐30. [ PubMed ] [ Google Scholar ]
Mukhopadhyay 2007 {published data only}
- Mukhopadhyay K, Narnag A, Mahajan R. Effect of human milk fortification in appropriate for gestation and small for gestation preterm babies: a randomized controlled trial . Indian Pediatrics 2007; 44 ( 4 ):286. [ PubMed ] [ Google Scholar ]
Muthayya 2009 {published data only}
- Muthayya S, Eilander A, Transler C, Thomas T, Knaap HC, Srinivasan K, et al. Effect of fortification with multiple micronutrients and n–3 fatty acids on growth and cognitive performance in Indian schoolchildren: the CHAMPION (Children’s Health and Mental Performance Influenced by Optimal Nutrition) Study . American Journal of Clinical Nutrition 2009; 89 ( 6 ):1766‐75. [ PubMed ] [ Google Scholar ]
Osei 2010 {published data only}
- Osei AK, Rosenberg IH, Houser RF, Bulusu S, Mathews M, Hamer DH. Community‐level micronutrient fortification of school lunch meals improved vitamin A, folate, and iron status of schoolchildren in Himalayan villages of India . Journal of Nutrition 2010; 140 ( 6 ):1146‐54. [ PubMed ] [ Google Scholar ]
Ouédraogo 2010 {published data only}
- Ouédraogo HZ, Traoré T, Zèba AN, Dramaix‐Wilmet M, Hennart P, Donnen P. Effect of an improved local ingredient‐based complementary food fortified or not with iron and selected multiple micronutrients on Hb concentration . Public Health Nutrition 13; 11 :1923‐30. [ PubMed ] [ Google Scholar ]
Parker 2015 {published data only}
- Parker ME, Mosites E, Reider K, Ndayishimiye N, Waring M, Nyandimbane G, et al. A blinded, cluster‐randomized, placebo‐controlled school feeding trial in Burundi using rice fortified with iron, zinc, thiamine, and folic acid . Food and Nutrition Bulletin 2015; 36 ( 4 ):481‐92. [ PubMed ] [ Google Scholar ]
Pettifor 1989 {published data only}
- Pettifor JM, Rajah R, Venter A, Moodley GP, Opperman L, Cavaleros M, et al. Bone mineralization and mineral homeostasis in very low‐birth‐weight infants fed either human milk or fortified human milk . Journal of Pediatratic Gastroenterology and Nutrition 1989; 8 ( 2 ):217‐24. [ PubMed ] [ Google Scholar ]
Phu 2010 {published data only}
- Phu P, Hoan N, Salvignol B, Treche S, Wieringa FT, Khan NC, et al. Complementary foods fortified with micronutrients prevent iron deficiency and anemia in Vietnamese infants . Journal of Nutrition 2010; 140 ( 12 ):2241‐7. [ PubMed ] [ Google Scholar ]
- Phu PV, Hoan NV, Salvignol B, Treche S, Wieringa FT, Dijkhuizen MA, et al. A six‐month intervention with two different types of micronutrient‐fortified complementary foods had distinct short‐ and long‐term effects on linear and ponderal growth of Vietnamese infants–3 . Journal of Nutrition 2012; 142 ( 9 ):1735‐40. [ PubMed ] [ Google Scholar ]
Phuka 2008 {published data only}
- Phuka JC, Maleta K, Thakwalakwa C, Cheung YB, Briend A, Manary MJ, et al. Complementary feeding with fortified spread and incidence of severe stunting in 6‐to 18‐month‐old rural Malawians . Archives of Pediatrics & Adolescent Medicine 2008; 162 ( 7 ):619‐26. [ PMC free article ] [ PubMed ] [ Google Scholar ]
Phuka 2009 {published data only}
- Phuka J, Thakwalakwa C, Maleta K, Cheung YB, Briend A, Manary M, et al. Supplementary feeding with fortified spread among moderately underweight 6–18‐month‐old rural Malawian children . Maternal & Child Nutrition 2009; 5 ( 2 ):159‐170. [ PMC free article ] [ PubMed ] [ Google Scholar ]
Pullakhandam 2011 {published data only}
- Pullakhandam R, Nair KM, Pamini H, Punjal R. Bioavailability of iron and zinc from multiple micronutrient fortified beverage premixes in Caco‐2 cell model . Journal of Food Science 2011; 76 ( 2 ):H38‐42. [DOI: 10.1111/j.1750-3841.2010.01993.x] [ PubMed ] [ CrossRef ] [ Google Scholar ]
Ramakrishnan 2004 {published data only}
- Ramakrishnan U, Neufeld LM, González‐Cossío T, Villalpando S, García‐Guerra A, Rivera J, et al. Multiple micronutrient supplements during pregnancy do not reduce anemia or improve iron status compared to iron‐only supplements in Semirural Mexico . Journal of Nutrition 2004; 134 ( 4 ):898‐903. [ PubMed ] [ Google Scholar ]
Ramírez‐Silva 2013 {published data only}
- Ramírez‐Silva I, Rivera JA, Leroy JL, Neufeld LM. The Oportunidades program's fortified food supplement, but not improvements in the home diet, increased the intake of key micronutrients in rural Mexican children aged 12‐59 months . Journal of Nutrition 2013; 143 ( 5 ):656. [ PubMed ] [ Google Scholar ]
Rohner 2016 {published data only}
- Rohner F, Raso G, Aké‐Tano SO, Tschannen AB, Mascie‐Taylor CG, Northrop‐Clewes CA. The effects of an oil and wheat flour fortification program on pre‐school children and women of reproductive age living in Côte d'Ivoire, a malaria‐endemic area . Nutrients 2016; 8 ( 3 ):148. [DOI: 10.3390/nu8030148] [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
Rosado 2010 {published data only}
- Rosado JL, González KE, C Caamaño M, García OP, Preciado R, Odio M. Efficacy of different strategies to treat anemia in children: a randomized clinical trial . Nutrition Journal 2010; 9 ( 1 ):40. [ PMC free article ] [ PubMed ] [ Google Scholar ]
Schümann 2009 {published data only}
- Schümann K, Longfils P, Monchy D, Xylander S, Weinheimer H, Solomons NW. Efficacy and safety of twice‐weekly administration of three RDAs of iron and folic acid with and without complement of 14 essential micronutrients at one or two RDAs: a placebo‐controlled intervention trial in anemic Cambodian infants 6 to 24 months of age . European Journal of Clinical Nutrition 2009; 63 ( 3 ):355. [ PubMed ] [ Google Scholar ]
Seal 2008 {published data only}
- Seal A, Kafwembe E, Kassim IA, Hong M, Wesley A, Wood J, et al. Maize meal fortification is associated with improved vitamin A and iron status in adolescents and reduced childhood anaemia in a food aid‐dependent refugee population . Public Health Nutrition 2008; 11 ( 7 ):720‐8. [ PubMed ] [ Google Scholar ]
Semba 2011 {published data only}
- Semba RD, Moench‐Pfanner R, Sun K, Pee S, Akhter N, Rah JH, et al. Consumption of micronutrient‐fortified milk and noodles is associated with lower risk of stunting in preschool‐aged children in Indonesia . Food Nutrition Bulletin 2011; 32 ( 4 ):347‐53. [ PubMed ] [ Google Scholar ]
Shatrugna 2006 {published data only}
- Shatrugna V, Balakrishna N, Krishnaswamy K. Effect of micronutrient supplement on health and nutritional status of schoolchildren: bone health and body composition . Nutrition 2006; 22 ( 1 ):S33‐9. [ PubMed ] [ Google Scholar ]
Stuetz 2012 {published data only}
- Stuetz W, Carrara VI, McGready R, Lee SJ, Erhardt JG, Breuer J, et al. Micronutrient status in lactating mothers before and after introduction of fortified flour: cross‐sectional surveys in Maela refugee camp . European Journal of Nutrition 2012; 51 ( 4 ):425‐34. [DOI: 10.1007/s00394-011-0226-z] [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
Sun 2011 {published data only}
- Sun J, Dai Y, Zhang S, Huang J, Yang Z, Huo J, et al. Implementation of a programme to market a complementary food supplement (Ying Yang Bao) and impacts on anaemia and feeding practices in Shanxi, China . Maternal & Child Nutrition 2011; 7 Suppl 3 :96‐111. [ PMC free article ] [ PubMed ] [ Google Scholar ]
Tazhibayev 2008 {published data only}
- Tazhibayev S, Dolmatova O, Ganiyeva G, Khairov K, Ospanova F, Oyunchimeg D, et al. Evaluation of the potential effectiveness of wheat flour and salt fortification programs in five Central Asian countries and Mongolia, 2002‐2007 . Food and Nutrition Bulletin 2008; 29 ( 4 ):255‐65. [ PubMed ] [ Google Scholar ]
Thomas 2012 {published data only}
- Thomas T, Eilander A, Muthayya S, McKay S, Thankachan P, Theis W, et al. The effect of a 1‐year multiple micronutrient or n‐3 fatty acid fortified food intervention on morbidity in Indian school children . European Journal of Clinical Nutrition 2012; 66 ( 4 ):452. [ PubMed ] [ Google Scholar ]
Torrejón 2004 {published data only}
- Torrejón CS, Castillo‐Durán C, Hertrampf ED, Ruz M. Zinc and iron nutrition in Chilean children fed fortified milk provided by the Complementary National Food Program . Nutrition 2004; 20 ( 2 ):117‐80. [ PubMed ] [ Google Scholar ]
Troesch 2011 {published data only}
- Troesch B, Stuijvenberg ME, Smuts CM, Kruger HS, Biebinger R, Hurrell RF, et al. A micronutrient powder with low doses of highly absorbable iron and zinc reduces iron and zinc deficiency and improves weight‐for‐age Z‐scores in South African children . Journal of Nutrition 2011; 141 ( 2 ):237‐42. [ PubMed ] [ Google Scholar ]
Ueland 2007 {published data only}
- Ueland PM Clarke R. Homocysteine and cardiovascular risk: considering the evidence in the context of study design, folate fortification, and statistical power . Clinical Chemistry 2007; 53 ( 5 ):807‐9. [ PubMed ] [ Google Scholar ]
Unger 2017 {published data only}
- Unger SA, Drammeh S, Hasan J, Ceesay K, Sinjanka E, Beyai S, et al. Impact of fortified versus unfortified lipid‐based supplements on morbidity and nutritional status: A randomised double‐blind placebo‐controlled trial in ill Gambian children . PLoS Medicine 2017; 14 ( 8 ):e1002377. [ PMC free article ] [ PubMed ] [ Google Scholar ]
Van Stuijvenberg 2001 {published data only}
- Stuijvenberg ME, Dhansay MA, Smuts CM, Lombard CJ, Jogessar VB, Benadé AJ. Long‐term evaluation of a micronutrient‐fortified biscuit used for addressing micronutrient deficiencies in primary school children . Public Health Nutrition 2004; 4 ( 6 ):1201‐9. [ PubMed ] [ Google Scholar ]
Varea 2012 {published data only}
- Varea A, Malpeli A, Disalvo L, Apezteguía M, Falivene M, Ferrari G, et al. Evaluation of the impact of a food program on the micronutrient nutritional status of Argentinean lactating mothers . Biological Trace Element Research 2012; 150 ( 1‐3 ):103‐8. [ PubMed ] [ Google Scholar ]
Varma 2007 {published data only}
- Varma JL, Das S, Sankar R, Mannar MG, Levinson FJ, Hamer DH. Community‐level micronutrient fortification of a food supplement in India: a controlled trial in preschool children aged 36‐66 mo . American Journal of Clinical Nutrition 2007; 85 ( 4 ):1127. [ PubMed ] [ Google Scholar ]
Yeh 2013 {published data only}
- Yeh KY, Wang HM, Chang JW, Huang JS, Lai CH, Lan YJ, et al. Omega‐3 fatty acid‐, micronutrient‐, and probiotic‐enriched nutrition helps body weight stabilization in head and neck cancer cachexia . Oral Surgery, Oral Medicine, Oral Pathology, Oral Radiology, and Endodontics 2013; 116 ( 1 ):41‐8. [ PubMed ] [ Google Scholar ]
Zagré 2007 {published data only}
- Zagré NM, Desplats G, Adou P, Mamadoultaibou A, Aguayo VM. Prenatal multiple micronutrient supplementation has greater impact on birthweight than supplementation with iron and folic acid : a cluster‐randomized, double‐blind, controlled programmatic study in rural Niger . Food Nutrition Bulletin 2007; 28 ( 3 ):317. [ PubMed ] [ Google Scholar ]
Additional references
- Aaron GJ, Dror DK, Yang Z. Multiple‐micronutrient fortified non‐dairy beverage interventions reduce the risk of anemia and iron deficiency in school‐aged children in low‐middle income countries: a systematic review and meta‐analysis . Nutrients 2015; 7 ( 5 ):3847‐68. [ PMC free article ] [ PubMed ] [ Google Scholar ]
Akseer 2017
- Akseer N, Al‐Gashm S, Mehta S, Mokdad A, Bhutta ZA. Global and regional trends in the nutritional status of young people: a critical and neglected age group . Annals of the New York Academy of Sciences 2017; 1393 ( 1 ):3‐20. [ PubMed ] [ Google Scholar ]
- Allen LH, Benoist B, Dary O, Hurrell R. Guidelines on food fortification with micronutrients . World Health Organization and Food and Agriculture Organization of the United Nations 2006 2006; Vol. www.who.int/nutrition/publications/guide_food_fortification_micronutrients.pdf.
- Allen LH. Anemia and iron deficiency: effects on pregnancy outcome . American Journal of Clinical Nutrition 2008; 71 :1208S. [ PubMed ] [ Google Scholar ]
Bailey 2015
- Bailey RL, West KP Jr, Black RE. The epidemiology of global micronutrient deficiencies . Annals of Nutrition and Metabolism 2015; 66 ( 2 ):22‐33. [ PubMed ] [ Google Scholar ]
Benoist 2008
- Benoist B, McLean E, Egll I, Cogswell M. Worldwide Prevalence of Anaemia 1993‐2005: WHO Global Database on Anaemia . Geneva, Switzerland: World Health Organization, 2008. [ Google Scholar ]
- Best C, Neufingerl N, Del Rosso JM, Transler C, Van den Briel T, Osendarp S. Can multi‐micronutrient food fortification improve the micronutrient status, growth, health, and cognition of schoolchildren? A systematic review . Nutrition Reviews 2011; 69 ( 4 ):186‐204. [ PubMed ] [ Google Scholar ]
Bhutta 2008
- Bhutta ZA. Micronutrient needs of malnourished children . Current Opinion in Clinical Nutrition & Metabolic Care 2008; 11 ( 3 ):309. [ PubMed ] [ Google Scholar ]
Bhutta 2013
- Bhutta ZA, Das JK, Rizvi A, Gaffey MF, Walker N, Horton S, et al. Maternal and Child Nutrition Study Group. Evidence‐based interventions for improvement of maternal and child nutrition: what can be done and at what cost? . Lancet 2013; 382 ( 9890 ):452‐77. [ PubMed ] [ Google Scholar ]
- Black RE, Allen LH, Bhutta ZA, Caulfield LE, De Onis M, Ezzati M, et al. Maternal and child undernutrition: global and regional exposures and health consequences . Lancet 2008; 371 ( 9608 ):243‐62. [ PubMed ] [ Google Scholar ]
- Black RE, Victora CG, Walker SP, Bhutta ZA, Christian P, Onis M, et al. Maternal and child undernutrition and overweight in low‐income and middle‐income countries . Lancet 2013; 382 ( 9890 ):427‐51. [ PubMed ] [ Google Scholar ]
- Das JK, Salam RA, Kumar R, Bhutta ZA. Micronutrient fortification of food and its impact on woman and child health: a systematic review . Systematic Reviews 2013; 2 ( 1 ):1‐24. [ PMC free article ] [ PubMed ] [ Google Scholar ]
De Lourdes Samaniego‐Vaesken 2012
- Lourdes Samaniego‐Vaesken, M, Alonso‐Aperte E, Varela‐Moreiras G. Vitamin food fortification today . Food & Nutrition Research 2012; 56 ( 1 ):5459. [ PMC free article ] [ PubMed ] [ Google Scholar ]
De‐Regil 2011
- De‐Regil LM, Suchdev PS, Vist GE, Walleser S, Peña‐Rosas JP. Home fortification of foods with multiple micronutrient powders for health and nutrition in children under two years of age . Cochrane Database of Systematic Reviews 2011, Issue 9 . [DOI: 10.1002/14651858.CD008959.pub2] [ PubMed ] [ CrossRef ] [ Google Scholar ]
Dijkhuizen 2001
- Dijkhuizen MA, Wieringa FT, West CE, Muherdiyantiningsih, Muhilal. Concurrent micronutrient deficiencies in lactating mothers and their infants in Indonesia . American Journal of Clinical Nutrition 2001; 73 ( 4 ):786‐91. [ PubMed ] [ Google Scholar ]
Eichler 2012
- Eichler K, Wieser S, Rüthemann I, Brügger U. Effects of micronutrient fortified milk and cereal food for infants and children: a systematic review . BMC Public Health 2012; 12 :506. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Cochrane Effective Practice, Organisation of Care (EPOC). Suggested risk of bias criteria for EPOC reviews. EPOC Resources for review authors . epoc.cochrane.org/resources/epoc‐resources‐review‐authors 2017.
Fabbri 2018
- Fabbri A, Holland TJ, Bero LA. Food industry sponsorship of academic research: investigating commercial bias in the research agenda . Public Health Nutrition 2018; 21 ( 18 ):3422‐30. [ PMC free article ] [ PubMed ] [ Google Scholar ]
Gibson 2002
- Gibson RS, Hotz C. Dietary diversification/modification strategies to enhance micronutrient content and bioavailability of diets in developing countries . British Journal of Nutrition 2002; 85 :S159‐66. [ PubMed ] [ Google Scholar ]
Glenton 2010
- Glenton C, Santesso N, Rosenbaum S, Nilsen ES, Rader T, Ciapponi A, et al. Presenting the results of Cochrane Systematic Reviews to a consumer audience: a qualitative study . Medical Decision Making 2010; 30 ( 5 ):566‐77. [ PubMed ] [ Google Scholar ]
GRADEpro [Computer program]
- McMaster University, 2015 (developed by Evidence Prime, Inc.). Available from gradepro.org. GRADEpro GDT: GRADEpro Guideline Development Tool [Software] . McMaster University, 2015 (developed by Evidence Prime, Inc.). Available from gradepro.org, 2015.
Guyatt 2008
- Guyatt GH, Oxman AD, Vist G, Kunz R, Falck‐Ytter Y, Alonso‐Coello P, et al. Rating quality of evidence and strength of recommendations GRADE: an emerging consensus on rating quality of evidence and strength of recommendations . BMJ 2008; 336 :924‐6. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Haimi M, Lerner A. Nutritional deficiencies in the pediatric age group in a multicultural developed country . World Journal of Clinical Cases 2014; 2 ( 5 ):120‐5. [ PMC free article ] [ PubMed ] [ Google Scholar ]
Harrison 2010
- Harrison GG. Public health interventions to combat micronutrient deficiencies . Public Health Reviews 2010; 32 ( 1 ):256. [ Google Scholar ]
Hennessy 2013
- Hennessy Á, Walton J, Flynn A. The impact of voluntary food fortification on micronutrient intakes and status in European countries: a review . Proceedings of the Nutrition Society 2013; 72 ( 4 ):433‐40. [ PubMed ] [ Google Scholar ]
Higgins 2017
- Higgins JP, Altman DG, Sterne JA, editor(s). Chapter 8: Assessing risk of bias in included studies. In: Higgins JP, Churchill R, Chandler J, Cumpston MS, editor(s). Cochrane Handbook for Systematic Reviews of Interventions version 5.2.0 (updated June 2017), The Cochrane Collaboration, 2017 . Available from www.training.cochrane.org/handbook .
Higgins 2019
- Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA, editor(s). Cochrane Handbook for Systematic Reviews of Interventions version 6.0 (updated July 2019). The Cochrane Collaboration, 2019 . Available from www.training.cochrane.org/handbook .
Hurrell 1997
- Hurrell RF. Preventing iron deficiency through food fortification . Nutrition Reviews 1997; 55 ( 6 ):210‐22. [ PubMed ] [ Google Scholar ]
Ibrahim 2017
- Ibrahim MK, Zambruni M, Melby CL, Melby PC. Impact of childhood malnutrition on host defense and infection . Clinical Microbiology Reviews 2017; 30 ( 4 ):919‐71. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Lotfi M, Venkatesh Mannar MG, Merx RJ, Naber‐van den Heuvel P. Micronutrient fortification of foods: current practices, research, and opportunities, 1996 . https://idl‐bnc‐idrc.dspacedirect.org/bitstream/handle/10625/14002/104386.pdf?sequence=1 (accessed 25 November 2019).
McLean 2008
- McLean E, Benoist B, Allen LH. Review of the magnitude of folate and vitamin B12 deficiencies worldwide . Food and Nutrition Bulletin 2008; 29 ( 2 Suppl ):S38‐51. [ PubMed ] [ Google Scholar ]
O’Neill 2014
- O’Neill J, Tabish H, Welch V, Petticrew M, Pottie K, Clarke M, et al. Applying an equity lens to interventions: using PROGRESS ensures consideration of socially stratifying factors to illuminate inequities in health . Journal of Clinical Epidemiology 2014; 67 ( 1 ):56–64. [ PubMed ] [ Google Scholar ]
Ramakrishnan 2002
- Ramakrishnan U. Prevalence of micronutrient malnutrition worldwide . Nutrition Reviews 2002; 60 :S46‐52. [ PubMed ] [ Google Scholar ]
RevMan 2014 [Computer program]
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan) . Version 5.3. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
- Salam RA, MacPhail C, Das JK, Bhutta ZA. Effectiveness of micronutrient powders (MNP) in women and children . BMC Public Health 2013; 13(Suppl 3) :S22. [ PMC free article ] [ PubMed ] [ Google Scholar ]
Schoonees 2019
- Schoonees A, Lombard M, Musekiwa A, Nel E, Volmink J. Ready‐to‐use therapeutic food (RUTF) for home‐based nutritional rehabilitation of severe acute malnutrition in children from six months to five years of age . Cochrane Database of Systematic Reviews 2019, Issue 5 . [DOI: 10.1002/14651858.CD009000.pub3] [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
Serdula 2010a
- Serdula M. Maximizing the impact of flour fortification to improve vitamin and mineral nutrition in populations . Food and Nutrition Bulletin 2010; 31 ( 1 Suppl ):S86‐93. [ PubMed ] [ Google Scholar ]
Serdula 2010b
- Serdula M. The opportunity of flour fortification: building on the evidence to move forward . Food and Nutrition Bulletin 2010; 31 (1 Suppl) :S3. [ PubMed ] [ Google Scholar ]
Stanger 2009
- Stanger O, Fowler B, Piertzik K, Huemer M, Haschke‐Becher E, Semmler A, et al. Homocysteine, folate and vitamin B12 in neuropsychiatric diseases: review and treatment recommendations . Expert Review of Neurotherapeutics 2009; 9 ( 9 ):1393‐412. [ PubMed ] [ Google Scholar ]
Suchdev 2014
- Suchdev PS, Peña‐Rosas JP, De‐Regil LM. Multiple micronutrient powders for home (point‐of‐use) fortification of foods in pregnant women . Cochrane Database of Systematic Reviews 2015, Issue 6 . [DOI: 10.1002/14651858.CD011158.pub2] [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
Viteri 2002
- Viteri FE, Gonzalez H. Adverse outcomes of poor micronutrient status in childhood and adolescence . Nutrition Reviews 2002; 60 :S77‐83. [ PubMed ] [ Google Scholar ]
- Welch V, Petticrew M, Petkovic J, Moher D, Waters E, White H, et al. Extending the PRISMA statement to equity‐focused systematic reviews (PRISMA‐E 2012):explanation and elaboration . Journal of Clinical Epidemiology 2016; 70 :68–89. [ PubMed ] [ Google Scholar ]
- WHO. The World Health Report 2002 ‐ Reducing Risks, Promoting Healthy Life . Geneva, World Health Organization 2002. [ PubMed ]
- World Health Organization. Global prevalence of vitamin A deficiency in populations at risk 1995‐2005. WHO Global Database on Vitamin A Deficiency, 2009 . whqlibdoc.who.int/publications/2009/9789241598019_eng.pdf (accessed 18 November 2019).
- World Health Organization. Health for the world's adolescents: a second chance in the second decade: summary . apps.who.int/adolescent/second‐decade/ 2014 (accessed 11 November 2019).
Zlotkin 2005
- Zlotkin SH, Schauer C, Christofides A, Sharieff W, Tondeur MC, Hyder SM. Micronutrient sprinkles to control childhood anaemia . PLoS Medicine 2005; 2 ( 1 ):e1. [ PMC free article ] [ PubMed ] [ Google Scholar ]
References to other published versions of this review
- Das JK, Salam RA, Kumar R, Lassi ZS, Bhutta ZA. Food fortification with multiple micronutrients: impact on health outcomes . Cochrane Database of Systematic Reviews 2014, ( 11 ):DOI: 10.1002/14651858.CD011400. [ PMC free article ] [ PubMed ] [ Google Scholar ]
SYSTEMATIC REVIEW article
Impact of food-based fortification on nutritional outcomes and acceptability in older adults: systematic literature review.

- 1 Centre des Sciences du Goût et de l'Alimentation, CNRS, INRAE, Institut Agro, Université de Bourgogne, Dijon, France
- 2 CHU Dijon Bourgogne, Unité de recherche Pôle Personnes Âgées, Dijon, France
Background: “Do it yourself” (DIY) food-based fortification involves adding fortificants into everyday foods. It is a flexible solution that allows older people with reduced appetite to meet their nutritional needs.
Objectives: The aims of the systematic review are (a) to describe DIY fortified recipes, (b) to evaluate their acceptability, and (c) to evaluate whether they are effective levers to improve nutritional outcomes in older people.
Methods: A systematic search of 3 databases (Web of Science, PubMed, Scopus, last searched on January 2022) was undertaken. Main eligibility criteria include older adults aged ≥60 years living at home, in an institution or in hospital. Studies carried out for a specific medical condition or targeting only micronutrient fortification were excluded. After reviewing all titles/abstracts then full-text papers, key data were extracted and synthesized narratively. The quality of included studies was assessed using Kmet et al.
Results: Of 21,493 papers extracted, 44 original studies were included (3,384 participants), with 31 reporting nutritional outcomes, 3 reporting acceptability outcomes and 10 reporting both nutritional and acceptability outcomes. The review highlighted a wide variety of DIY fortified recipes, with additional energy ranging from 23 to 850 kcal/d ( M = 403; SE = 62) and/or protein ranging from 4 to 40 g/d ( M = 19; SE = 2). Compared to a standard diet, DIY fortification seems to be a valuable strategy for increasing energy and protein intake in older people. However, no strong evidence was observed on the nutritional status.
Implication for future: Further acceptability studies are crucial to ensure that DIY fortified foods are palatable and thus have a significant impact on the nutritional status. In addition, it would be useful for studies to better describe DIY recipes. This information would result in a better understanding of the factors that maximize the impact of DIY fortification on nutritional outcomes. Study registration: PROSPERO no. CRD42021244689.
Systematic review registration : PROSPERO: https://www.crd.york.ac.uk/prospero/display_record.php?ID=CRD42021244689 .
1. Introduction
Contrary to common beliefs, our nutritional needs decrease little with age and are sometimes higher in late adulthood than in early adulthood. With regard to caloric intake, the European Food Safety Authority ( 1 ) recommends a daily allowance from 2000 to 2,500 Kcal for people aged 50 to 59 and from 1800 to 2,300 Kcal for people aged 70 to 79. More recently Volkert et al. ( 2 ) established that recommended energy intake should reach 30 Kcal per kg of body weight per day. With regard to protein intake, recent works carried out by the PROT-AGE consortium ( 3 ) and by the European Society for Clinical Nutrition and Metabolism (EPSEN) ( 4 ) show that older people need to ingest more protein than younger people to stay healthy, to maintain their abilities and to fight infections. As a result, the daily protein intake should be 1 to 1.2 g protein per kg of body weight per day for a healthy person over 60 versus 0.8 to 1 g per kg of body weight in younger adults. The literature review by Shad et al. ( 5 ) highlighted the importance of a constant distribution of protein intake over the main meals of the day at amounts of 25–30 g/meal to avoid catabolic protein status [see also ( 3 , 6 )].
At the same time, a decline in appetite can appear with aging ( 7 ). Various studies have reported that 31 to 56% of the aged population are “small eaters” ( 8 – 10 ). Small eaters are characterized by a low consumption of every food category compared to the overall population – they eat foods in small or even very small amounts ( 8 – 11 ). A recent French survey carried out by CREDOC (“Centre de Recherche pour l’Observation et les Conditions de Vie”) showed that 87% of adults aged 18–54 met the recommendations for protein intake compared with only 56% of those over 65 ( 12 ). This situation is even worse when older adults are frail and dependent. In an aged population receiving a Home-Delivery Meal (HDM) service or living in nursing homes, Sulmont-Rossé and Van Wymelbeke ( 13 ) observed that 7–8 out of 10 people did not meet their energy and/or protein needs. This study also showed that 55% of home-delivery meal recipients and 46% of people living in nursing homes had energy and/or protein intake lower than 2/3 of the recommendations. In addition to age, many factors can be at the origin of this decline in appetite, such as physiological changes, sensory decline and eating/swallowing difficulties, which appear during aging. It also can be related to “life-breaking moments” (e.g., widowhood, illness, dependence) that can amplify iatrogenic factors correlated with medications and affect sociological/psychological aspects ( 13 ). Thus, poor appetite in older adults leads to a decrease in food and nutrient intake, which increases the risk of undernutrition ( 14 , 15 ). Undernutrition, a recognized pathology in the older population, corresponds to an imbalance between nutritional intake and the body’s needs. This imbalance leads to weight loss, a decrease in muscle reserves and an alteration of the body’s defences. In older people, undernutrition increases the risk of falls and therefore fractures. It contributes to the increase in infectious morbidity ( 16 ), nosocomial infections ( 17 ) and the appearance of pressure ulcers ( 18 ). If left untreated, undernutrition can induce or aggravate a state of fragility and dependence, which affects the quality of life and life expectancy of our elders ( 16 , 19 ).
Understanding the factors responsible for appetite decline is certainly important, but a major challenge is to get older people with reduced appetite to fulfill their nutritional needs in order to prevent undernutrition and the associated consequences. Food-based fortification, which consists in incorporating ingredients of nutritional interest (namely “fortificants”) in commonly consumed foods ( 20 ) in order to deliberately increasing the content of an essential nutrient in a diet without increasing (too much) the volume to be ingested, is acknowledged to be a relevant approach for older adults with reduced appetite ( 21 ). Fortificants can be: (a) regular food products (e.g., semolina, oils, butter, cream, pureed nuts, egg), or (b) macronutrients extracts (e.g., whey protein isolate, milk protein concentrate, caseinate, maltodextrin) ( 22 , 23 ). Besides the numerous fortified foods developed and marketed by the food industry, “do it yourself” (DIY) fortification recipes empower older adults and their carers to take a personalized approach to their nutrition and current diet. DIY fortification is a flexible strategy that may fit better with older people’s food habits and preferences: older people (or their carers) add fortificants to the food they usually eat, during the preparation of daily meals. This constitutes a significant advantage in the older population, which is often reluctant to change their consumption habits. However, DIY fortification remains largely unknown and underused by older adults as well as by caregivers and healthcare professionals although it is now known to be a relevant approach to counterbalance appetite decline and to adjust to nutritional needs ( 24 ).
The goal of the present study was to conduct a systematic review of all studies related to the nutritional and acceptability aspects of DIY food-based fortification in older people. The aims of this review are (a) to describe the DIY food-based fortification solutions and recipes that have been developed, (b) to evaluate the acceptability of these solutions in older people, and (c) to evaluate whether these solutions can be relevant and effective levers to preserve or improve nutritional outcomes in older people.
2. Materials and methods
The present systematic review followed the approach proposed by Xiao and Watson ( 25 ), which summarizes the evidence available on a topic to convey the breadth and depth of that topic. The protocol was written using the Preferred Reporting Items for Systematic Reviews and Meta-analysis Protocols (PRISMA-P, ( 26 ), see Supplementary material ). The protocol was deposited on the HAL website 1 and on PROSPERO with the registration number CRD42021244689. The PRISMA checklist is available on the Supplementary material .
2.1. Research question
The research question is: “What are the objectives, characteristics and results of existing research conducted on the nutritional issues and/or on acceptability among older people receiving DIY fortified foods?”
2.2. Inclusion and exclusion criteria
The PICOS (Population, Intervention, Comparator, Outcome, Study design) eligibility criteria was as follows ( 27 ):
Population: Any studies focusing on adults aged 60 years and older living either at home, in an institution or in hospital was eligible for inclusion. Older adults of all nutritional status, cognitive status and oral ability (e.g., chewing, swallowing) were eligible for inclusion. Studies carried out in the context of a specific medical condition (e.g., cardiac rehabilitation, renal failure, cancers, diabetes) were excluded.
Intervention: Any DIY food-based fortification intervention was eligible for inclusion (e.g., incorporating ingredients of nutritional interest in food products). Fortification in energy and/or macronutrients was eligible for inclusion. Studies without an intervention (e.g., observational studies) were relevant for inclusion. Were excluded from the review: (a) studies targeting only micronutrient fortification, non-food dietary supplement or bio-fortification (genetically modified crop), (b) studies using only fortified food developed and marketed by the Food Industry, and (c) interventions targeting artificial nutrition (e.g., tube feeding, parenteral feeding, enteral feeding).
Comparators: As the present review aimed at compiling DIY food-based fortification recipes and reporting their acceptability, any comparator was eligible for inclusion (e.g., studies comparing food-based fortification versus Oral Nutritional Supplements (ONS), or studies comparing two types of fortified food). In addition, studies without a comparator were eligible for inclusion.
Outcomes: Three categories of outcomes were considered: (a) characterization of the nutritional intake (e.g., dietary pattern, nutrient intake), (b) characterization of the nutritional status (e.g., body mass index (BMI), weight, undernutrition) and (c) characterization of the acceptability (e.g., liking, preference, pleasure).
Study design: All types of study design including interventional and observational design were eligible. All period of times and duration of follow-up were eligible.
Other: No restriction was set for the publication date. Only publications written in English were included because of the uncertainty surrounding the words used to refer to the concept of “DIY food-based fortification” in foreign languages. Narrative review, conference abstracts, editorials, and grey literature were excluded.
2.3. Information sources and search strategy
A search strategy with both thesaurus and free-text terms was developed – after repeated attempts and adjustments – to retrieve relevant articles in the following databases: Web of Science (WOS), PubMed and Scopus ( Supplementary material ). Separate title, abstract and keywords searches were conducted for older people, food-based fortification and outcomes in February 2021. An update was performed in January 2022. The results for the three separate search strings were combined to identify relevant articles. Afterwards, for further screening, references from selected articles and systematic reviews were checked manually in case they were not identified during the whole search process. After duplicates removal, titles and abstracts in the first step and full texts in a second step were screened by two independent reviewers (AG and MP) according to the agreed inclusion and exclusion criteria. For each screening level, a training exercise was conducted before the starting of the screening process on a random sample of 100 titles and abstracts and 10 full texts to ensure high inter-reviewer reliability. Disagreements between reviewers were resolved by consensus or by consulting a third reviewer (CSR or VVW). The reasons for exclusion were recorded at the full-text stage (the list of excluded studies at the full-text stage and the reasons of exclusion are presented on Supplementary material ).
2.4. Charting the data
A standardized data summarization form was developed a priori and revised, as needed, after the completion of a training exercise completed on a sample of 5 articles. All included studies were summarized by two reviewers (AG and MP), independently, with conflicts resolved by a third reviewer (CSR or VVW). The data summarization included the following items:
- Article identifiers (authors, year of publication)
- Study identifiers (objective, design, country)
- Population (age, gender, sample size, inclusion and exclusion criteria)
- Intervention (description of the DIY fortification recipes)
- Comparator (if applicable)
- Outcomes (endpoints, measurement method, main results)
2.5. Quality assessments
All included studies were independently assessed for quality by two reviewers (AG and MP); conflicts were resolved by consensus. The articles’ quality was assessed with the quality assessment criteria developed by Kmet et al. ( 28 ). The criteria are presented in Supplementary material . In addition, the description quality of the DIY fortification recipes (fortificants, food matrices, concentration) was assessed (but not included in the quality score).
2.6. Collating, summarizing and reporting the results
A descriptive numerical summary of the characteristics of the included studies was performed. Tables and graphs were created to reflect the number of studies included, study designs and settings, publication years, the characteristics of the study populations, the outcomes reported, and the countries where the studies were conducted. In line with systematic literature review guidelines, the quality of the included studies was assessed ( 25 , 29 ).
3.1. Characteristics of the included studies
On the 21,493 articles retrieved, 253 records were kept for full text screening and 49 studies were included in the systematic review: 44 original studies ( Figure 1 ; 3,384 participants) and 5 systematic literature reviews ( 21 – 24 , 30 ). The reasons for excluding papers were: no original research ( n = 18), wrong population ( n = 18), no DIY food-based fortification ( n = 135), fortification with micronutrients only ( n = 15), wrong outcomes ( n = 18). Wrong outcomes included functional outcomes (muscle strength), gastric emptying, glycemia, gut hormones, bone mineral density, quality of life. Two articles ( 31 , 32 ) were excluded because they did not provide enough information about the nutritional strategy used.
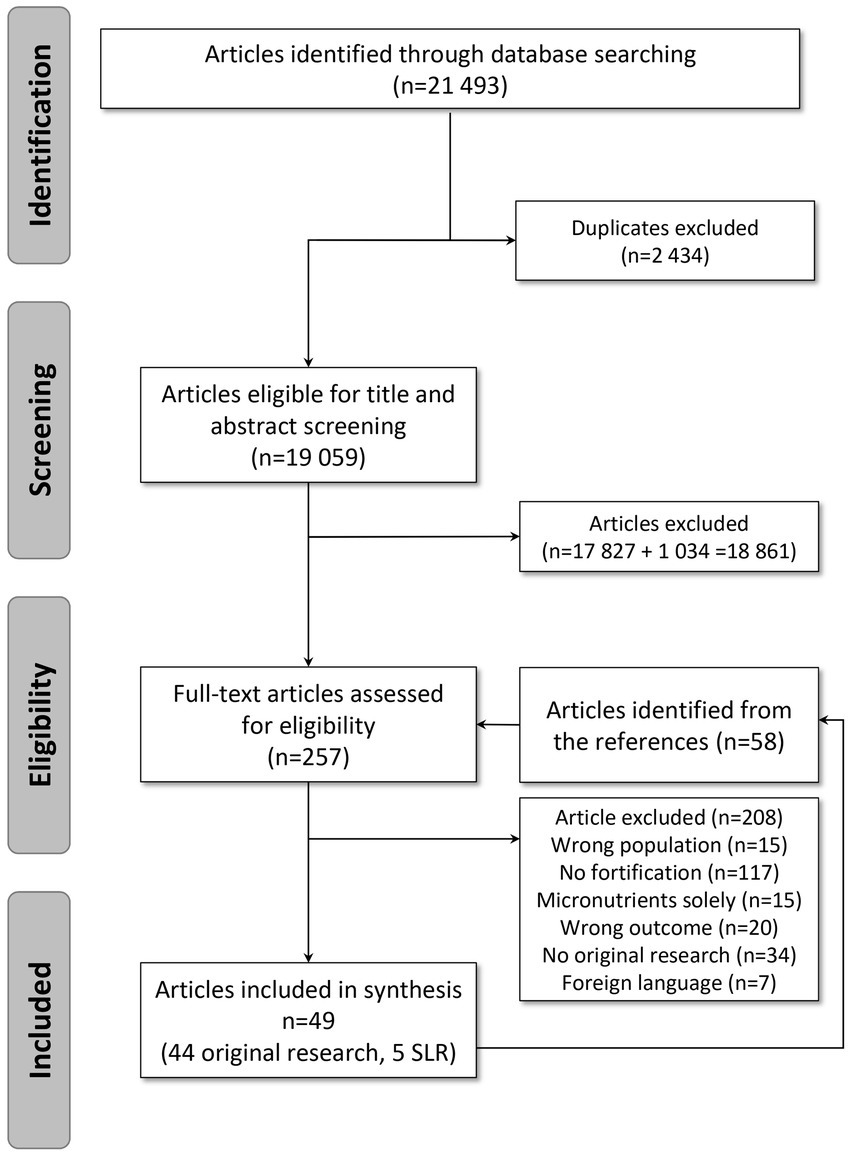
Figure 1 . PRISMA flow diagram.
The included articles were published between 1996 and 2021, and most were published after 2011 ( n = 34) ( Table 1 ). The studies mainly took place in Europe ( n = 33). The rest took place in Australia ( n = 4), North America ( n = 4) or Asia ( n = 3). The setting was most often the hospital ( n = 20) followed by nursing homes ( n = 13) and home setting ( n = 13). Twenty-seven studies of the selection were longitudinal with follow-up times between 10 days to 12 months and 16 studies were cross-sectional ( Table 1 ). In addition, 30 studies used a between-subject design while 13 studies used a within-subject design; only 1 study was observational. Finally, sample sizes varied (ranging from 7 to 320 participants), but most studies recruited 20 to 49 subjects ( n = 17).
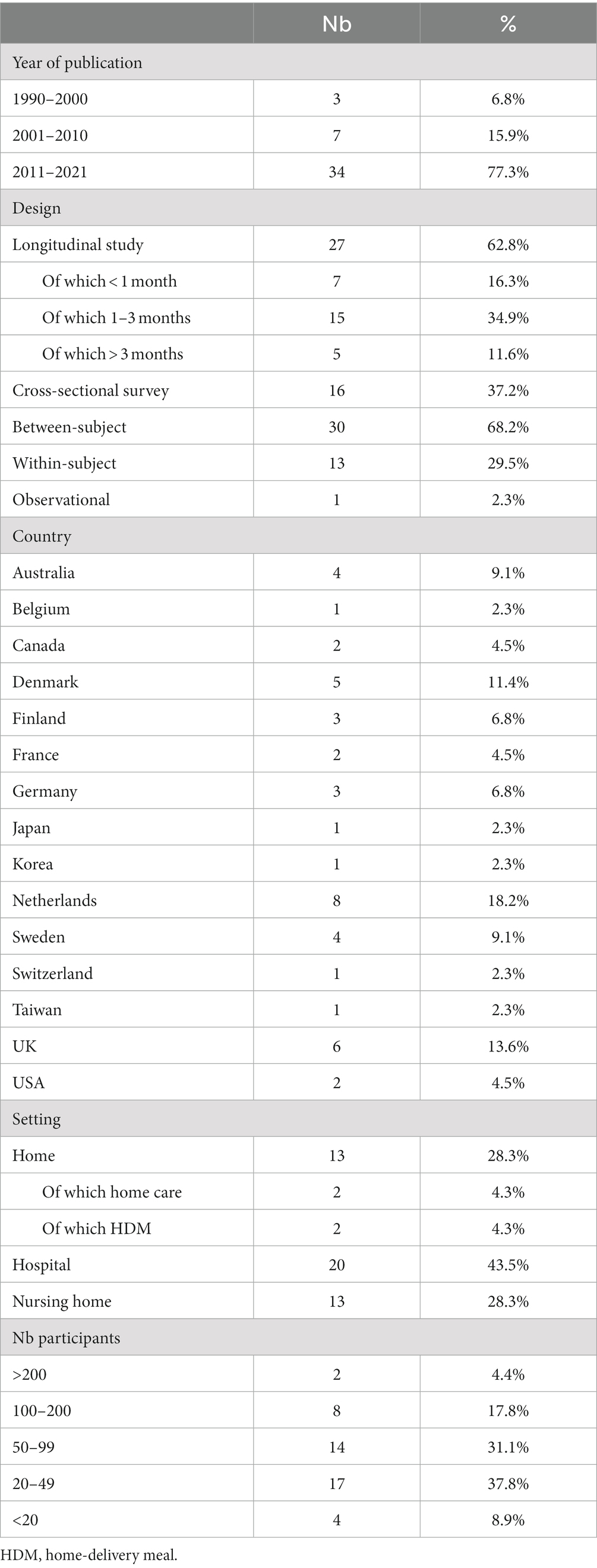
Table 1 . Characteristics of the systematic literature review articles.
Among the 44 original research studies, 3 were fully focused on the acceptability outcome ( 33 – 35 ). Among the 41 remaining articles, the majority ( n = 31) were entirely dedicated to nutritional outcomes. Finally, 10 articles were “mixed” and assessed both nutritional and acceptability outcomes.
A descriptive summary of the included studies yielded the four following topics:
- Description of DIY fortification recipes: which types of food are fortified? Which nutrients are added? In what form? At which concentration?
- Assessment of DIY fortified foods acceptability: to which extent do older people like fortified food? Do the sensory characteristics of fortified foods fulfil older people’ sensory expectations and preferences?
- Assessment of the nutritional impact of DIY food-based fortification: did older people who received fortified food improve their nutritional intake and nutritional status compared to a standard diet?
- Comparison of DIY food-based fortification with other alternatives (e.g., dietary counseling, Oral Nutritional Supplement – ONS): is fortified food more acceptable and/or does it provide a nutritional benefit compared to other alternatives?
3.2. Quality assessment
A quality assessment was performed for each outcome, i.e., nutritional outcome and acceptability outcome ( Supplementary material ). In fact, in mixed articles, different panels and designs were often used for nutritional and acceptability outcomes.
Regarding nutritional outcomes, the methodological quality of the studies was in general good with an average quality score of 0.92 (standard deviation: 0.09) ranging from 0.62 ( 36 ) to 1 ( 37 – 54 ) ( Supplementary material ). Overall, recruitment of participants was the variable that was the most poorly rated in the selected studies. This was because the majority of studies did not detail the recruitment procedure nor the precise localization where the study took place. Sample size and control for confounding factors were badly rated because a large number of studies did not reach an appropriate sample size or did not consider confounding variables (e.g., age, gender, Body Mass Index (BMI), weight, nutrition status) in data analysis. Study design and subject description factors were moderately rated due to insufficient/incoherent information preventing clear understanding of concerned articles.
The methodological quality of the 13 studies related to acceptability outcomes was on the whole lower than for the nutritional outcomes, with an average quality score of 0.75 (standard deviation: 0.23) ranging from 0.33 ( 37 ) to 1 ( 33 , 43 , 44 ) ( Supplementary material ). Usually, recruitment of participants, sample size, analytic methods and results were the lowest rated factors. As for the nutritional quality assessment, the majority of studies did not detail the recruitment procedure nor the precise localization where the study took place. Moreover, most studies did not clearly describe the analytic method used when it was mentioned. For 4 criteria (sample size, results, outcomes measures and study design) the poor quality is related to the fact that the acceptability measure was not the main outcome of the article.
Finally, the description of the DIY fortification recipes was also poorly rated: very few studies provided precise information about food matrices, fortificants and recipes.
3.3. Description of DIY fortified recipes
Table 2 shows the description of the DIY fortified recipes. On the whole, 7 articles implemented energy fortification, 18 implemented protein fortification and 19 implemented a combination of protein and energy fortification. It should be noted that 10 articles did not specify the nature of food matrices ( 38 , 44 , 55 , 58 , 61 , 64 , 65 , 67 , 72 , 74 ) and 5 articles did not specify the nature of the fortificants ( 50 , 53 , 56 , 57 , 74 ). Only 8 articles provided enough details about the recipes for them to be reproduced by a third party ( 33 – 35 , 39 , 46 , 49 , 54 , 73 ).
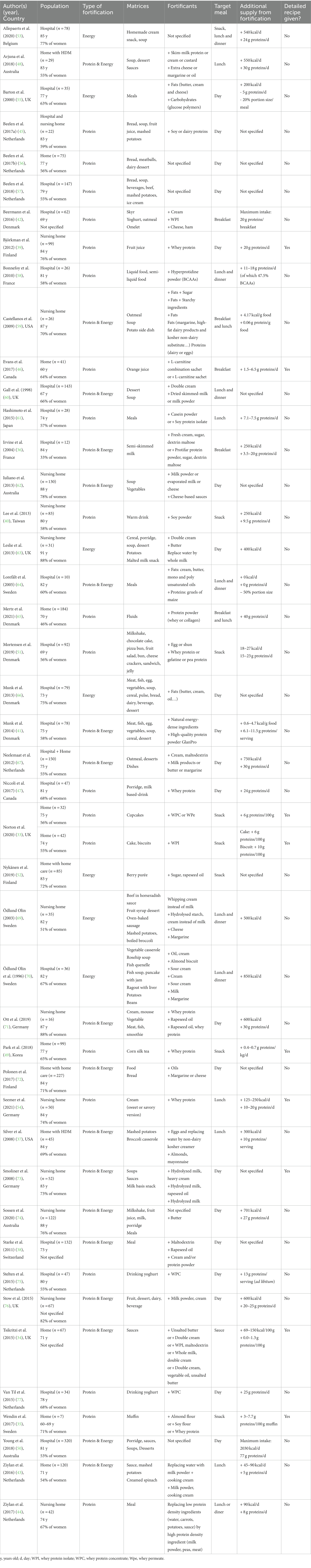
Table 2 . Description of DIY fortified recipes.
Overall, 137 DIY fortified recipes were listed: 75 savory and 62 sweet. Among these recipes, 64 were meant to be eaten cold and 67 were meant to be eaten hot (6 can be eaten cold or hot). The food matrices included desserts ( n = 20 articles; mousse, pie, muffin, cake, biscuit, ice-cream…), meat and fish dishes ( n = 18; meatball, chicken sticks, marinated duck, baked salmon…), side dishes ( n = 17; purée, sautéed vegetables), dairy products ( n = 17; milk, yoghurt, cream), soups ( n = 14), carbohydrate-based dishes ( n = 14; oatmeal, cereal, risotto, pancake), beverages ( n = 9; fruit juice, tea), sauces ( n = 9), breads ( n = 8), fruits ( n = 7; compote/purée, salad, smoothie), eggs dishes ( n = 3; omelet) and pulse-based dishes ( n = 1). It is interesting to note that food matrices included both liquids (milk, soup, fruit juice…), semi-liquid foods (purée, yoghurt…) and solid foods (cake, chicken sticks, bread). There was a large variability in the number of matrices used for fortification in the articles. Twelve articles used one only matrix category to be fortified ( 33 – 36 , 39 , 40 , 46 , 49 , 52 , 54 , 75 , 77 ). Munk et al. ( 66 ) developed 36 fortified dishes in collaboration with dietitians, chefs and patients from a hospital. These dishes covered a large range of different food types (soup, meat and fish dishes, vegetable dishes, bread, dessert, beverages).
Twenty different fortificants were identified across all the studies, including 10 regular food ingredients and 10 macronutrient isolates or concentrates. Four articles ( 38 , 45 , 59 , 66 ) did not provide enough details about fortificants (“high fat dairy food,” “dairy,” “non-dairy substitute,” “natural energy-dense ingredient,” “protein powder,” “soy origin”), thus they could not be classified. Seven fortificants targeted energy fortification, 8 targeted protein fortification and 5 targeted both. Most of the fortificants were powdered ( n = 11). Other fortificants were solid ( n = 4), semi-liquid ( n = 3) or liquid ( n = 2). Energy fortificants included cream ( n = 20 articles), butter/margarine ( n = 13), oils ( n = 10), carbohydrates ( n = 7), hydrolyzed starch ( n = 1), mayonnaise ( n = 1) and maize ( n = 1). Protein fortificants included whey protein ( n = 15 articles), protein concentrates/isolates ( n = 5; Protifar, Hyperprotidine, L-Carnitine…), soy ( n = 3), pea ( n = 2), meat ( n = 2), collagen ( n = 1), casein ( n = 1), and gelatine ( n = 1). Energy and protein fortificants included milk powder ( n = 10), cheese ( n = 7), milk ( n = 5), eggs ( n = 3) and almonds ( n = 3). Finally, Figures 2 , 3 illustrate the wide variability regarding the additional load of energy and protein provided by fortified food across the studies. This additional load varies from 23 to 850 kcal / day for energy (M = 403; SE = 62) and from 4 to 40 g / day for protein (M = 19; SE = 2).
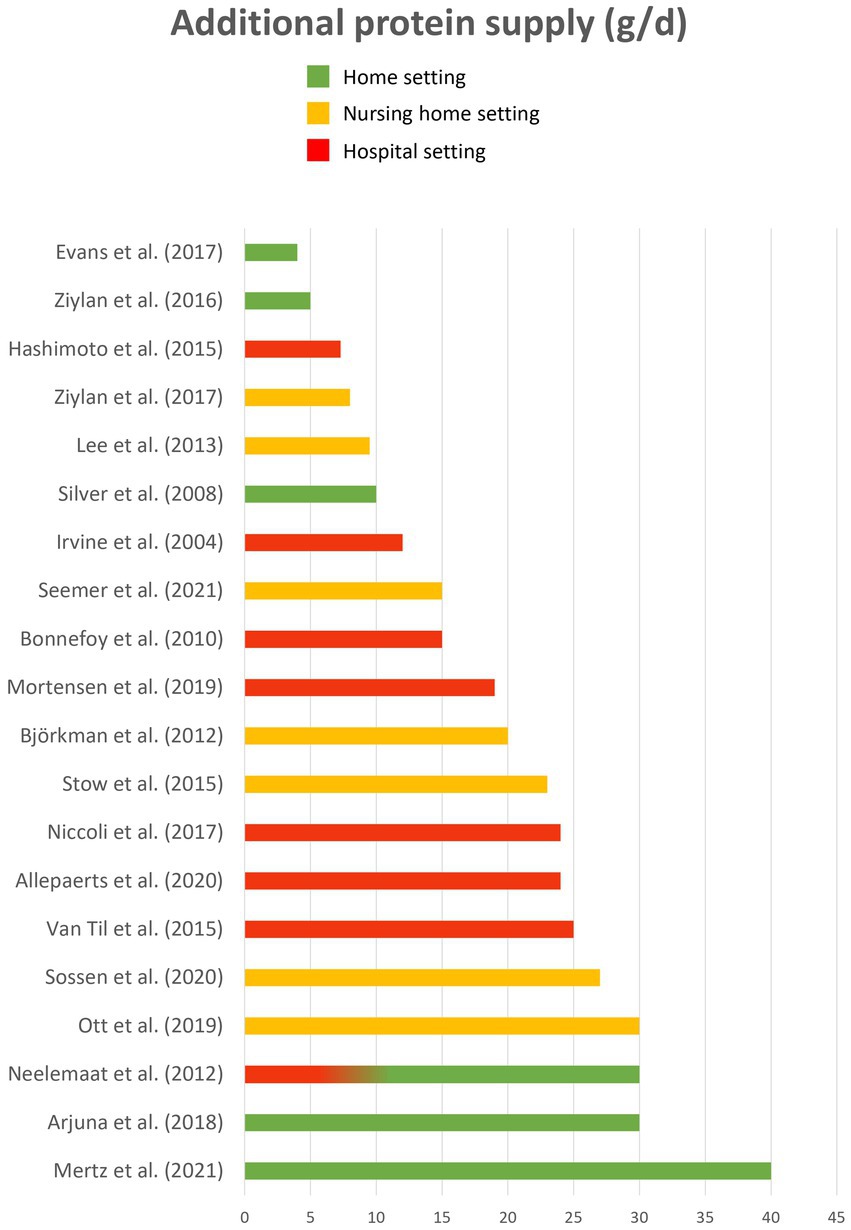
Figure 2 . Additional protein load (g/d).
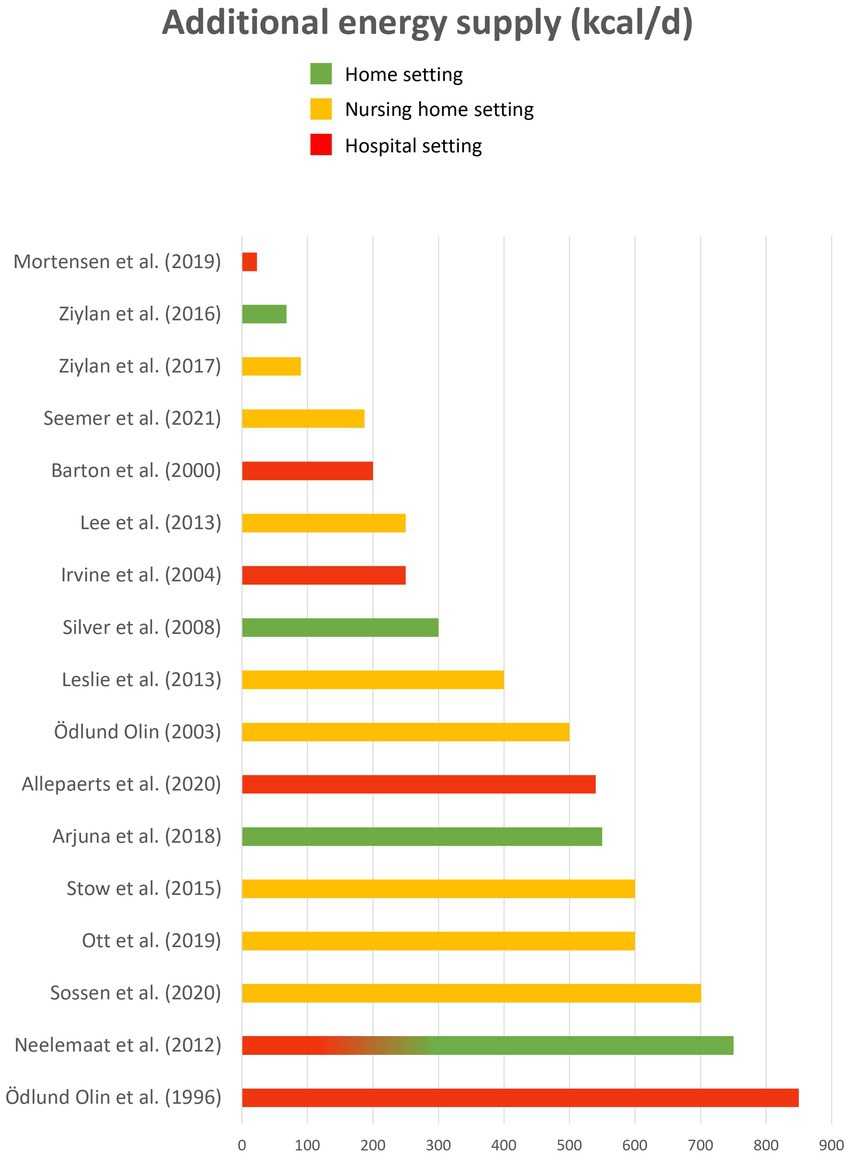
Figure 3 . Additional energy load (kcal/d).
3.4. Assessment of DIY fortified foods acceptability
Thirteen studies have assessed consumer acceptability for DIY fortified foods ( Table 3 ). All these studies conducted the acceptability evaluation with older people except for one ( 71 ), who asked nursing home staff to provide feedback on product acceptance based on residents’ observation. Six articles ( 33 , 34 , 43 , 44 , 52 , 59 ) used liking scales to assess product acceptance while the others only collected qualitative data through interviews, focus groups or an acceptability survey. However, most of the articles do not provide enough information about the methodology used to assess acceptability and/or about the results. In most of the articles ( n = 10/13), acceptability was only a secondary outcome while nutrition was the first one. In these studies, acceptability tests were usually conducted with the same sample as the one recruited for nutritional assessment (the whole sample in 6 articles; a smaller sub-sample in 2 articles). Three articles ( 33 – 35 ) were dedicated to assessing acceptability of DIY fortified foods versus regular foods.
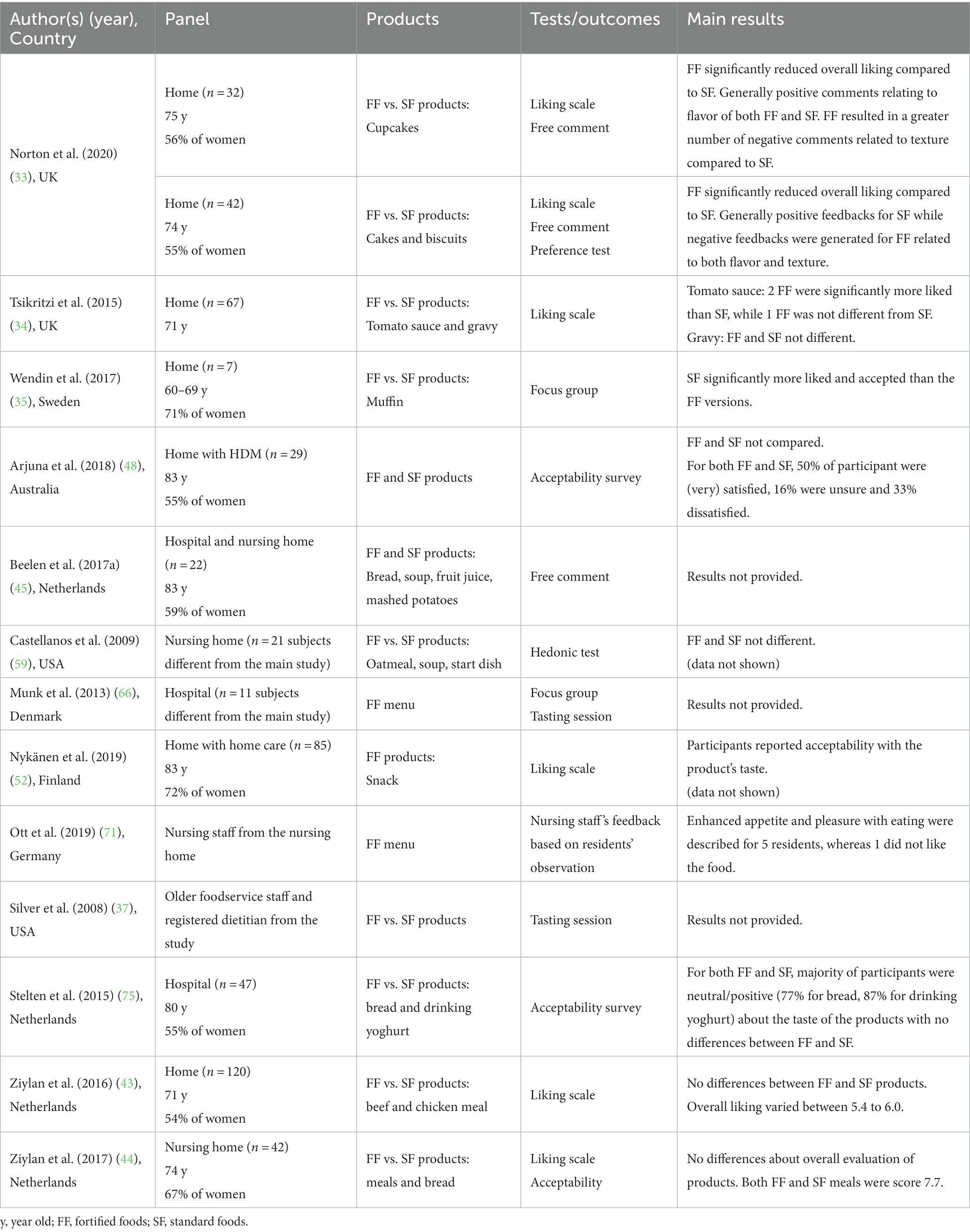
Table 3 . DIY fortified food acceptability assessment.
Seven articles provided results on comparison between DIY fortified and regular foods. Among them, 4 articles ( 37 , 43 , 44 , 59 , 75 ) reported no significant difference in acceptability when comparing fortified and regular foods while 2 articles ( 33 , 35 ) reported that fortified foods were less appreciated than regular food. Only one article reported that some fortified foods were more appreciated than regular food, but it depended on the nature of the fortificant added to the food ( 34 ). In fact, tomato sauce fortified with cream or with a mix of whey protein and maltodextrin were more liked than regular tomato sauce, but tomato sauce fortified with butter was less liked than regular tomato sauce. Wendin et al. ( 35 ) also showed some difference between foods fortified with different fortificants: the regular muffin was more liked than the muffin fortified with almond flour, which was more liked than the whey muffin, itself more liked than the soy muffin.
3.5. Assessment of the nutritional impact of DIY food-based fortification
Forty studies assessed the impact of diet enrichment including DIY food-based fortification on nutritional outcomes (food and/or nutrient intakes, nutritional status or body weight) compared to a standard diet ( Table 4 ). Among these studies, 3 combined DIY food-based and diet-based fortification (i.e., modifying the diet by adding nutritionally rich foods), 6 combined food-based fortification and fortified foods marketed by the Food Industry, 1 combined food-based fortification and Oral Nutritional Supplements (ONS), and 2 combined food-based fortification, diet-based fortification and ONS, while 27 studies assessed the impact of DIY food-based fortification alone. Nutritional intake was mainly measured by using dietary record. Nutritional status was mainly assessed by measuring body weight or BMI (20 studies), by using the Mini-Nutritional Assessment Questionnaire [MNA, 8 studies – ( 39 , 48 , 49 , 52 , 57 , 72 , 73 , 77 )] or by measuring muscle mass [4 studies – ( 39 , 46 , 49 , 58 )]. A few studies used other indicators such as the Subjective Global Assessment ( 74 ) or albumin and pre-albmin ( 40 , 47 , 52 , 58 , 72 ).

Table 4 . Comparison between DIY fortified diet and standard diet on nutritional outcomes.
When all the studies are considered, results highlight that provided protein-fortified foods led to a significant increase in protein intake (26 studies over 29) and that provided energy-fortified led to a significant increase in energy intake (15 studies over 20). Only a few studies showed a significant impact of DIY fortification on nutritional status compared to regular food offer: 3 out 8 observed a significant impact on MNA score, 7 out 20 observed a significant impact on body weight or BMI and 2 out 4 observed a significant impact on muscle mass. None observed a negative impact.
When only the studies which assessed the impact of DIY fortification alone are considered (in bold in the Table 4 ), results still highlight that provided protein-fortified foods led to a significant increase in protein intake (16 studies over 18) and that provided energy-fortified led to a significant increase in energy intake (9 studies over 13). Only a few studies showed a significant impact of DIY fortification on nutritional status compared to regular food offer: 1 out 5 observed a significant impact on MNA score, 4 out 13 observed a significant impact on body weight or BMI and 1 out 3 observed a significant impact on muscle mass.
3.6. Comparison of DIY food-based fortification with other alternatives
Seven studies evaluated two DIY food-based fortification strategies with either different energy/protein loads ( 36 , 49 , 59 ), different fortificants ( 46 , 61 , 65 ) or different portion sizes ( 43 ). Four studies compared DIY food-based fortification with another alternative such as ONS ( 76 ), ( 74 ), adding high-energy and/or high-protein food items to the menu ( 55 ), or increased staff assistance to older people during mealtime ( 50 ) ( Table 5 ). However, very few studies have produced statistics to compare the different options. Not surprisingly, higher energy/protein loads are associated with higher energy/protein intake ( 36 , 49 ). However, there was no significant difference between the 1.2 and the 1.5 g of protein / kg of body weight / day in the evolution of nutritional status and muscle mass over the 12-week intervention ( 49 ). In Ziylan et al. ( 43 ), the reduced-size enriched chicken meal led to a significantly higher energy intake than the normal-size meal. However, the difference in intake was rather small and no impact of portion size was observed for the enriched beef meals. In Evans et al. ( 46 ), a combination of three amino acids significantly improved muscle mass over 2 months while no change was observed when a single amino acid was used to fortify the orange juice. Stow et al. ( 76 ) observed no difference between food-based fortification and ONS while Sossen et al. ( 74 ) observed a slight advantage for DIY food-based fortification compared to ONS. Energy and protein intakes were higher with DIY fortification than with ONS, and body weight was stable with DIY fortification whereas it decreased with ONS during the 6 months of follow-up. Finally, providing DIY fortified food led to higher energy and protein intake when compared with improving staff assistance to older people during mealtime ( 50 ).
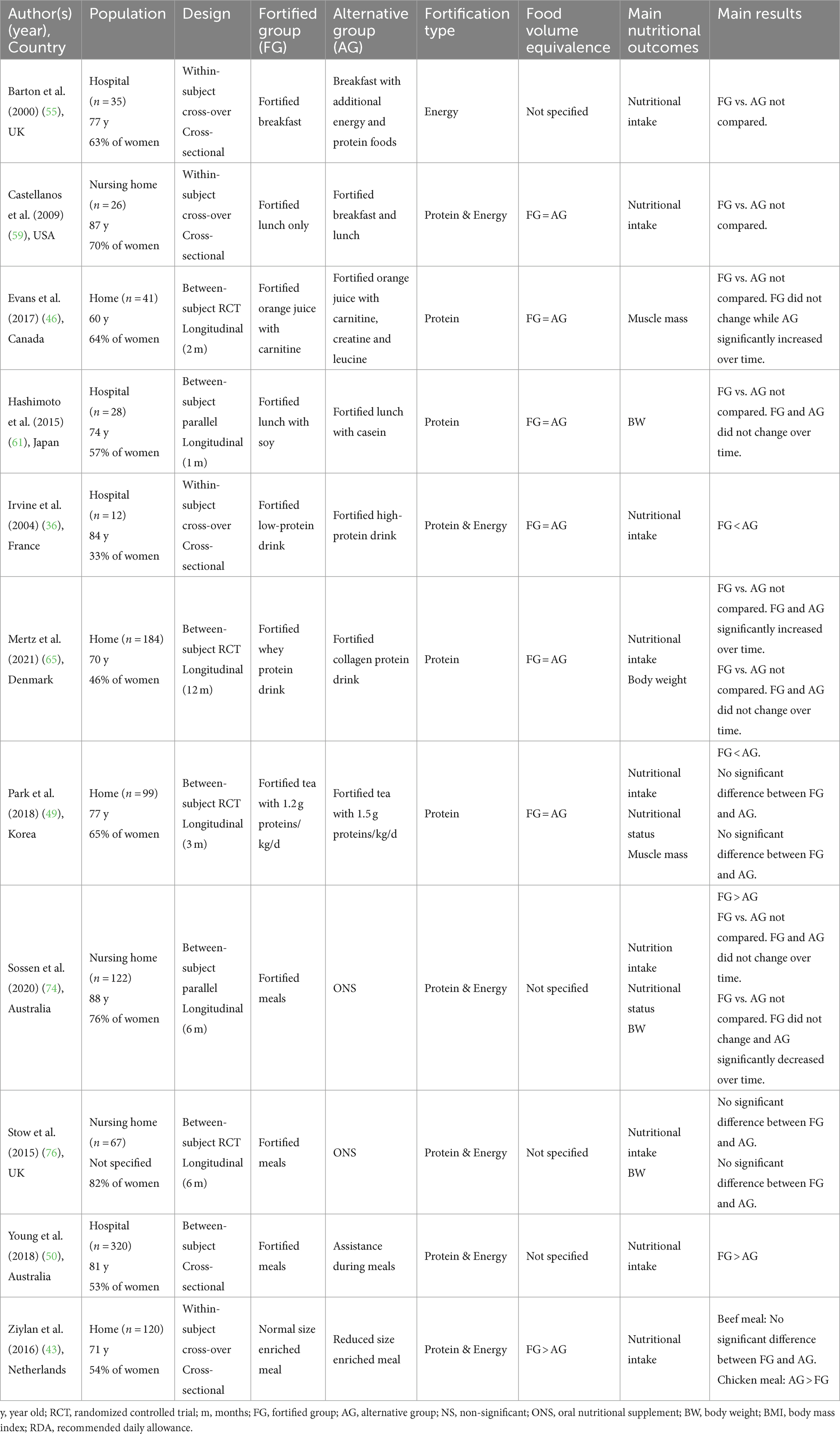
Table 5 . Comparison between DIY fortification and other alternatives.
4. Discussion
4.1. originality/value of the present review.
A survey of the literature allowed the identification of five systematic literature reviews close to the topic of the present review ( 21 – 24 , 30 ). Firstly, the systematic review of Trabal and Farran-Codina ( 23 ) investigated whether, compared to a standard diet, DIY food-based fortification with regular ingredients and/or powdered modules could improve energy and protein intake in older adults in hospital settings, long-care facilities or home settings. This review included 9 articles. The authors concluded that DIY fortification is a valid intervention for improving energy intake in older adults yet there was insufficient evidence for protein intake, nutritional status and body weight. Secondly, Morilla-Herrera et al. ( 21 ) targeted all studies related to DIY food-based fortification with macronutrients to prevent the risk of malnutrition in older patients receiving hospital services for acute or chronic disease, in older people living in nursing homes and in older people with home-care. This review encompassed 7 articles, and the meta-analysis highlighted that DIY food-based fortification yields positive results in the total amount of ingested calories and protein. Thirdly, Douglas et al. ( 22 ) aimed to evaluate the effect of DIY fortification with regular food ingredients (excluding protein powders) on energy and protein intake compared to standard diet among adults aged 60 and more in acute-care hospitals, long-term care settings or living at home. Ten articles were included. This review suggested that DIY fortification was effective in increasing energy and protein intake among older individuals. Fourthly, the systematic review by Mills et al. ( 24 ) explored the evidence for the use of energy and/or protein dense meals (DIY food-based fortification) or additional snacks (diet-based fortification) to increase the dietary energy and protein intake of adults older than 60 in hospital or rehabilitation facilities. Ten articles were identified. Authors reported that when compared with usual nutritional care, DIY fortification could be an effective, well-tolerated and cost-effective intervention to improve dietary intake among hospitalized patients. Finally, Sossen et al. ( 30 ) investigated the effect of food-based and diet-based fortification on energy and protein intake compared to any/no nutritional strategy in residents living in nursing homes. Sixteen articles were included. The results of the meta-analysis showed that fortified menus may significantly increase energy and protein intakes compared with standard menus.
The present review retrieved 44 articles that tested DIY food-based fortification in people over the age of 65. This review differs from previous reviews in the following respects. Firstly, we focused the review on DIY food-based fortification, i.e., the addition of regular food ingredients or macronutrient extracts into conventional food matrices to increase energy and protein content in the final dishes. Douglas et al. ( 22 ) considered only culinary ingredients. Mills et al. and Sossen et al. ( 24 , 30 ) considered both food-based fortification and diet-based fortification via the addition of supplementary conventional foods like snacks to participants’ diets. Second, we considered all living settings, i.e., at home, with or without assistance, institutions and hospitals [Morilla-Herrera et al. ( 21 ) only considered dependent older people]. Thirdly, we considered not only nutritional outcomes but also acceptability outcomes. In addition, we used a wide range of keywords to account for the lack of consensual terminology regarding the concept of DIY food-based fortification ( Supplementary material ). This allowed us to identify a much larger number of articles than in previous reviews.
4.2. Description of DIY fortified recipes
A wide variety of DIY fortified recipes were extracted from this review, including liquid (35% of the recipes), semi-solid (17%) and solid food matrices (48%). However, the quality evaluation of the articles highlighted the lack of information provided by the authors on the description of fortified recipes. Only 8 articles provided sufficient information for a third party to reproduce the same fortified recipes as used in the articles. In order to identify efficient DIY fortified solutions, it is essential that in future articles provide a detailed description of the fortified recipes, including the nature of food matrices and fortificants, final energy and protein concentration, additional nutrient load provided by the fortified food compared to the standard food, consumption time, and portion size. From the information collected, energy fortification is mainly achieved through the use of fats and dairy products (cream, butter, oil) while protein fortification is mainly achieved through protein extracts. Such products are usually in powder form (‘protein powders’) and proved to have varied applications and uses within food processing as well as high nutritional and functional value ( 68 ). The present review showed that the protein products used in fortified recipes were mainly derived from animal sources (85% of the recipes), especially from milk (67% of the recipes), and to a lesser extent from plant sources (15% of the recipes). Animal-derived proteins are more readily digestible and effective in muscle protein synthesis than plant derived proteins ( 78 ).
4.3. Evaluation of DIY food-based fortification solutions
Results suggest that food-based fortification is an effective strategy to improve energy and/or protein intake. This trend is observed whether all the studies – including the ones that combined DIY fortification with other strategies (i.e., providing ONS, additional food items, fortified foods from Food Industry) or whether only the studies which assessed the impact of DIY fortification alone are considered. In other words, DIY fortification seems to be an effective strategy to improve nutritional intake, whether used alone or combined with other enrichment strategies. However, no strong evidence is observed regarding the impact of DIY fortification to improve the nutritional status (e.g., MNA score, body weight, muscle mass).
It should be noted that providing fortified food was not necessarily enough to get participants to meet the recommended nutritional allowance ( 50 , 55 , 60 , 75 ). For instance, in Stelten et al. ( 75 ), 64% of the fortified group did not reach the threshold of 1.2 g protein/kg of body weight/day. This raises the question of the need for new fortification solutions with higher levels of energy and protein content. In addition, consuming fortified foods throughout the various meals of the day may be more efficient than consuming fortified foods only once per day. For instance, Castellanos et al. ( 59 ) reported higher energy intake when both breakfast and lunch were fortified than when only lunch was fortified, but they did not carry out statistical analysis to compare these two conditions.
Besides the relatively large number of studies that have tested the impact of DIY food-based fortification on nutritional outcomes, very few studies have looked at the acceptability of DIY fortified food. Only 10 of the 41 nutrition-related articles reported an evaluation of the acceptability of DIY fortified foods and only 3 of the 44 articles included in this review were completely devoted to the assessment of acceptability of DIY fortified food. Unsurprisingly, the quality of the acceptability studies is much better in the articles focused only on this outcome than in the articles that conducted an acceptability study alongside a nutritional study. In the latter, the sample size is often insufficient, the methods are often qualitative and the results are often imprecise and incomplete. In addition, the people who assess the acceptability of fortified food are sometimes different from the end-users [e.g., the fortified foods are tasted by the staff ( 37 )]. Overall, the results tend to show that DIY fortified foods are equally or less appreciated than standard foods – never more. However, before drawing any final conclusions, there is a need to carry out further acceptability studies with a higher quality, taking into account the good practices and the norms of sensory evaluation ( 79 , 80 ). Indeed, fortified foods should not only be good from a nutritional point of view, but also “good to eat” to ensure that they are actually consumed by the target population. Furthermore, it would be worthwhile to optimize the sensory quality of fortified foods by recruiting older adults in tasting panels. Fortification improvement based on older people’s feedback led to increased food intake in nursing homes ( 81 , 84 ).
4.4. Limitations and strengths of the present SLR
The strength of this paper is its reliable literature search, with a complete overview of nutritional and acceptability issues for fortified food targeting older people. Given the lack of a consensual definition of the concept of food-based fortification, we have used a broad set of keywords to retrieve articles of interest. The limitations of the present literature review are the following: the literature search strategy did not include trial registries, nor grey literature, and it was restricted to English papers. There are two discrepancies between the present method and the one published before the review was carried out. In the published method, we considered including papers published in both English and French (the authors’ native language), but papers in French were ultimately excluded in order to avoid a language bias in the literature search. In addition, in the published method, we considered including papers related to micronutrients fortification, but ultimately focused the scope of the present review on macronutrient fortification, otherwise the scope of the review would have been too broad. Finally, a limitation lies in the fact that it was not always easy to determine whether the products used in the nutritional interventions were a DIY fortified food, a fortified food marketed by the Food Industry or an ONS. For instance, we excluded the studies where enrichment consisted of providing participants with a sachet of nutrient constituents to be dissolved in water [for instance ( 82 , 83 )]. Indeed, dissolving a sachet of powder in water is more like taking a drug than having a drink. Conversely, all the interventions consisting of adding a nutrient-dense ingredient to a food matrix were included, even when the fortificant was very specific [for example, branched chain amino acids powder ( 58 ), L-carnitine ( 46 )]. However, the question arises as to the accessibility of this type of fortificant to the end-user in real life.
5. Conclusion
The present systematic literature review highlighted that, compared to a standard diet, DIY food-based fortification – i.e., incorporating ingredients of nutritional interest into commonly consumed foods – is a valuable strategy for increasing energy and protein intake in older people. However, no strong evidence was observed regarding the impact of DIY fortification to improve the nutritional status (i.e., MNA score, body weight, muscle mass). In addition, further research is needed to better assess the acceptability of this strategy among end-users. Given the limitations of the studies included in this systematic review, we put forward four recommendations for future research. First, we emphasize the need to develop a consistent definition of DIY food-based fortification that clearly distinguishes this strategy from other enrichment strategies such as the consumption of ONS or fortified food from food industry. Second, it would be useful for studies to better describe the recipes used for DIY fortification. This information would result in a better understanding of the factors that maximize the impact of food-based fortification on nutritional outcomes. Third, it would be relevant to systematically assess the acceptability of DIY fortified foods in addition to the nutritional outcomes. This should be done by implementing consumer tests that respect the good practices and the recommendations defined in sensory evaluation for such tests (sample size, methods…). To achieve this, it is essential to encourage more pluri-disciplinary research projects involving experts in nutrition, sensory evaluation and food technology. Fourth, we encourage researchers to further compare the impact of food-based fortification with other enrichment strategies, and in particular ONS, in order to better decipher the impact of each of these strategies in tackling undernutrition in the older people. Finally, future research should also study how to promote DIY food fortification among the older people, their caregivers, as well as among catering and health professionals. Indeed, despite this strategy has proved effective in sustaining caloric and protein intake in older people, it remains largely unknown and underused. Several dissemination strategies could be considered. A first one could be the development and the diffusion of DIY fortified recipes booklets. Such booklets should indicate the amount of protein provided by each portion. These booklets would also need to be co-created with end-users, to ensure the feasibility and acceptability of the recipes in the field, considering various settings (home cooking, home-delivery meals, nursing home, hospital). A second dissemination strategy could be the organization of therapeutic workshops at hospital discharge or in day hospital, bringing together dieticians, chefs and older people to promote DIY food fortification. However, from a more global perspective, public policies are needed to raise awareness of the nutritional needs of the older people. These policies must combine information and tools to maintain adequate energy and protein intakes, in order to prevent undernutrition in the older population.
Data availability statement
The original contributions presented in the study are included in the article/ Supplementary material , further inquiries can be directed to the corresponding author/s.
Author contributions
AG: methodology, investigation, formal analysis, and writing – original draft. MP: methodology, investigation, formal analysis, and writing – review and editing. VW-D: conceptualization and writing – review and editing. CS-R: conceptualization, methodology, formal analysis, writing – original draft, and funding acquisition. All authors contributed to the article and approved the submitted version.
This work received funding by the French “Investissements d’Avenir” program, project ISITE-BFC (contract ANR-15-IDEX-0003) and from ANR (ANR-20-HDHL-0003 FORTIPHY), Research Council Norway (RCN 321819), BBSRC (BB/V018329/1) under the umbrella of the European Joint Programming Initiative “A Healthy Diet for a Healthy Life” (JPI HDHL) and of the ERA-NET Cofund ERA-HDHL (GA N°696295 of the EU Horizon 2020 Research and Innovation Programme).
Conflict of interest
During the past 36 months, CSGA and CHU Dijon received research grants from OGUST, SAVEURS et VIE, and INSTITUT NUTRITION. CS-R received consulting fees from BEL FOOD and author fees from Correspondances en Métabolismes Hormones Diabètes et Nutrition.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fnut.2023.1232502/full#supplementary-material
1. ^ https://hal.archives-ouvertes.fr/hal-03180038
1. European food safety authority (EFSA). Scientific opinion on dietary reference values for energy. EFSA J . (2013) 11:3005. doi: 10.2903/j.efsa.2013.3005
CrossRef Full Text | Google Scholar
2. Volkert, D, Beck, AM, Cederholm, T, Cruz-Jentoft, A, Goisser, S, Hooper, L, et al. ESPEN guideline on clinical nutrition and hydration in geriatrics. Clin Nutr . (2019) 38:10–47. doi: 10.1016/j.clnu.2018.05.024
PubMed Abstract | CrossRef Full Text | Google Scholar
3. Bauer, J, Biolo, G, Cederholm, T, Cesari, M, Cruz-Jentoft, AJ, Morley, JE, et al. Evidence-based recommendations for optimal dietary protein intake in older people: a position paper from the PROT-AGE study group. J Am Med Dir Assoc . (2013) 14:542–59. doi: 10.1016/j.jamda.2013.05.021
4. Deutz, NEP, Bauer, JM, Barazzoni, R, Biolo, G, Boirie, Y, Bosy-Westphal, A, et al. Protein intake and exercise for optimal muscle function with aging: recommendations from the ESPEN expert group. Clin Nutr . (2014) 33:929–36. doi: 10.1016/j.clnu.2014.04.007
5. Shad, BJ, Thompson, JL, and Breen, L. Does the muscle protein synthetic response to exercise and amino acid-based nutrition diminish with advancing age? A systematic review. Am J Physiol Endocrinol Metab . (2016) 311:E803–17. doi: 10.1152/ajpendo.00213.2016
6. Bollwein, J, Diekmann, R, Kaiser, MJ, Bauer, JM, Uter, W, Sieber, CC, et al. Distribution but not amount of protein intake is associated with frailty: a cross-sectional investigation in the region of Nürnberg. Nutr J . (2013) 12:109. doi: 10.1186/1475-2891-12-109
7. Giezenaar, C, Chapman, I, Luscombe-Marsh, N, Feinle-Bisset, C, Horowitz, M, and Soenen, S. Ageing is associated with decreases in appetite and energy intake—a meta-analysis in healthy adults. Nutrients . (2016) 8:10028. doi: 10.3390/nu8010028
8. Corrêa Leite, ML, Nicolosi, A, Cristina, S, Hauser, WA, Pugliese, P, and Nappi, G. Dietary and nutritional patterns in an elderly rural population in northern and southern Italy: (I). A cluster analysis of food consumption. Eur J Clin Nutr . (2003) 57:1514–21. doi: 10.1038/sj.ejcn.1601719
9. Samieri, C, Jutand, MA, Féart, C, Capuron, L, Letenneur, L, and Barberger-Gateau, P. Dietary patterns derived by hybrid clustering method in older people: association with cognition, mood, and self-rated health. J Am Diet Assoc . (2008) 108:1461–71. doi: 10.1016/j.jada.2008.06.437
10. Thorpe, MG, Milte, CM, Crawford, D, and McNaughton, SA. A comparison of the dietary patterns derived by principal component analysis and cluster analysis in older Australians. Int J Behav Nutr Phys Act . (2016) 13:30. doi: 10.1186/s12966-016-0353-2
11. Gazan, R, Béchaux, C, Crépet, A, Sirot, V, Drouillet-Pinard, P, Dubuisson, C, et al. Dietary patterns in the French adult population: a study from the second French national cross-sectional dietary survey (INCA2) (2006–2007). Br J Nutr . (2016) 116:300–15. doi: 10.1017/S0007114516001549
12. Perraud, S. (2020). Evolutions de la consommation de protéines par sources alimentaires entre 2010 et 2019 selon les profils de consommateurs. Available at: https://zenodo.org/record/4327397
Google Scholar
13. Sulmont-Rossé, C, and Van Wymelbeke, V. Les déterminants d’un apport protidique faible chez les personnes âgées dépendantes. Cahiers de Nutrition et de Diététique . (2019) 54:180–9. doi: 10.1016/j.cnd.2019.02.003
14. van der Pols-Vijlbrief, R, Wijnhoven, HAH, Schaap, LA, Terwee, CB, and Visser, M. Determinants of protein–energy malnutrition in community-dwelling older adults: a systematic review of observational studies. Ageing Res Rev . (2014) 18:112–31. doi: 10.1016/j.arr.2014.09.001
15. van der Meij, BS, Wijnhoven, HAH, Lee, JS, Houston, DK, Hue, T, Harris, TB, et al. Poor appetite and dietary intake in community-dwelling older adults. J Am Geriatr Soc . (2017) 65:2190–7. doi: 10.1111/jgs.15017
16. Hiesmayr, M, Schindler, K, Pernicka, E, Schuh, C, Schoeniger-Hekele, A, Bauer, P, et al. Decreased food intake is a risk factor for mortality in hospitalised patients: The NutritionDay survey 2006. Clin Nutr . (2009) 28:484–91. doi: 10.1016/j.clnu.2009.05.013
17. Schaible, UE, and Kaufmann, SHE. Malnutrition and infection: complex mechanisms and global impacts. PLoS Med . (2007) 4:7. doi: 10.1371/journal.pmed.0040115
18. Litchford, MD, Dorner, B, and Posthauer, ME. Malnutrition as a precursor of pressure ulcers. Adv Wound Care (New Rochelle) . (2014) 3:54–63. doi: 10.1089/wound.2012.0385
19. Ferry, M. 31 – Conséquences globales de la dénutrition In: M Ferry, D Mischlich, E Alix, P Brocker, T Constans, and B Lesourd, et al., editors. Nutrition De la Personne âgée (Quatrième Édition) [Internet] . Paris: Elsevier Masson (2012). 172–8.
20. Abeshu, MA, and Geleta, B. The role of fortification and supplementation in mitigating the hidden hunger. J Nutr Food Sci . (2016) 6. doi: 10.4172/2155-9600.1000459
21. Morilla-Herrera, JC, Martín-Santos, FJ, Caro-Bautista, J, Saucedo-Figueredo, C, García-Mayor, S, and Morales-Asencio, JM. Effectiveness of food-based fortification in older people a systematic review and meta-analysis. J Nutr Health Aging . (2016) 20:178–84. doi: 10.1007/s12603-015-0591-z
22. Douglas, JW, Lawrence, JC, and Knowlden, AP. The use of fortified foods to treat malnutrition among older adults: a systematic review. QAOA . (2017) 18:104–19. doi: 10.1108/QAOA-05-2016-0018
23. Trabal, J, and Farran-Codina, A. Effects of dietary enrichment with conventional foods on energy and protein intake in older adults: a systematic review. Nutr Rev . (2015) 73:624–33. doi: 10.1093/nutrit/nuv023
24. Mills, SR, Wilcox, CR, Ibrahim, K, and Roberts, HC. Can fortified foods and snacks increase the energy and protein intake of hospitalised older patients? A systematic review. J Hum Nutr Diet . (2018) 31:379–89. doi: 10.1111/jhn.12529
25. Xiao, Y, and Watson, M. Guidance on conducting a systematic literature review. J Plan Educ Res . (2019) 39:93–112. doi: 10.1177/0739456X17723971
26. Shamseer, L, Moher, D, Clarke, M, Ghersi, D, Liberati, A, Petticrew, M, et al. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015: Elaboration and explanation. Res Methods . (2015) 349:25. doi: 10.1136/bmj.g7647
27. Stone, PW. Popping the (PICO) question in research and evidence-based practice. Appl Nurs Res . (2002) 15:197–8. doi: 10.1053/apnr.2002.34181
28. Kmet, LM, Lee, RC, and Cook, LS. Alberta Heritage Foundation for Medical Research a, health technology assessment unit, University of Calgary, et al. standard quality assessment criteria for evaluating primary research papers from a variety of fields . Edmonton: Alberta Heritage Foundation for Medical Research (2004).
29. Higgins, J, Thomas, J, Chandler, J, Cumpston, M, Li, T, Page, M, et al. (2021). Cochrane handbook for systematic reviews of interventions (version 6.2) [internet]. Available at: www.training.cochrane.org/handbook
30. Sossen, L, Bonham, M, and Porter, J. Can fortified, nutrient-dense and enriched foods and drink-based nutrition interventions increase energy and protein intake in residential aged care residents? A systematic review with meta-analyses. Int J Nurs Stud . (2021) 124:104088. doi: 10.1016/j.ijnurstu.2021.104088
31. Beck, AM, Ovesen, L, and Schroll, M. Home-made oral supplement as nutritional support of old nursing home residents, who are undernourished or at risk of undernutrition based on the MNA. A pilot trial. Aging Clin Exp Res . (2002) 14:212–5. doi: 10.1007/BF03324439
32. Holyday, M, Daniells, S, Bare, M, Caplan, GA, Petocz, P, and Bolin, T. Malnutrition screening and early nutrition intervention in hospitalised patients in acute aged care: a randomised controlled trial. J Nutr Health Aging . (2012) 16:562–8. doi: 10.1007/s12603-012-0022-3
33. Norton, V, Lignou, S, Bull, SP, Gosney, MA, and Methven, L. Consistent effects of whey protein fortification on consumer perception and liking of solid food matrices (cakes and biscuits) regardless of age and saliva flow. Foods . (2020) 9. doi: 10.3390/foods9091328
34. Tsikritzi, R, Wang, J, Collins, VJ, Allen, VJ, Mavrommatis, Y, Moynihan, PJ, et al. The effect of nutrient fortification of sauces on product stability, sensory properties, and subsequent liking by older adults: fortified sauces for older patients…. J Food Sci . (2015) 80:S1100–10. doi: 10.1111/1750-3841.12850
35. Wendin, K, Höglund, E, Andersson, M, and Rothenberg, E. Protein enriched foods and healthy ageing effects of protein fortification on muffin characteristics. Agro Food Industry Hi Tech . (2017) 5:16–8. https://www.researchgate.net/publication/320858882
36. Irvine, P, Mouzet, JB, Marteau, C, Sallé, A, Genaitay, M, Favreau, AM, et al. Short-term effect of a protein load on appetite and food intake in diseased mildly undernourished elderly people. Clin Nutr . (2004) 23:1146–52. doi: 10.1016/j.clnu.2004.02.011
37. Silver, HJ, Dietrich, MS, and Castellanos, VH. Increased energy density of the home-delivered lunch meal improves 24-hour nutrient intakes in older adults. J Am Diet Assoc . (2008) 108:2084–9. doi: 10.1016/j.jada.2008.09.005
38. Starke, J, Schneider, H, Alteheld, B, Stehle, P, and Meier, R. Short-term individual nutritional care as part of routine clinical setting improves outcome and quality of life in malnourished medical patients. Clin Nutr . (2011) 30:194–201. doi: 10.1016/j.clnu.2010.07.021
39. Björkman, MP, Finne-Soveri, H, and Tilvis, RS. Whey protein supplementation in nursing home residents. A randomized controlled trial. Europ Geriat Med . (2012) 3:161–6. doi: 10.1016/j.eurger.2012.03.010
40. Lee, LC, Tsai, AC, Wang, JY, Hurng, BS, Hsu, HC, and Tsai, HJ. Need-based intervention is an effective strategy for improving the nutritional status of older people living in a nursing home: a randomized controlled trial. Int J Nurs Stud . (2013) 50:1580–8. doi: 10.1016/j.ijnurstu.2013.04.004
41. Munk, T, Beck, AM, Holst, M, Rosenbom, E, Rasmussen, HH, Nielsen, MA, et al. Positive effect of protein-supplemented hospital food on protein intake in patients at nutritional risk: a randomised controlled trial. J Hum Nutr Diet . (2014) 27:122–32. doi: 10.1111/jhn.12210
42. Beermann, T, Mortensen, MN, Skadhauge, LB, Høgsted, RH, Rasmussen, HH, and Holst, M. Protein and energy intake improved by breakfast intervention in hospital. Clin Nutr ESPEN . (2016) 13:e23–7. doi: 10.1016/j.clnesp.2016.02.097
43. Ziylan, C, Kremer, S, Eerens, J, Haveman-Nies, A, and de Groot, LCPGM. Effect of meal size reduction and protein enrichment on intake and satiety in vital community-dwelling older adults. Appetite . (2016) 105:242–8. doi: 10.1016/j.appet.2016.05.032
44. Ziylan, C, Haveman-Nies, A, Kremer, S, and de Groot, LCPGM. Protein-enriched bread and readymade meals increase community-dwelling older adults’ protein intake in a double-blind randomized controlled trial. J Am Med Dir Assoc . (2017) 18:145–51. doi: 10.1016/j.jamda.2016.08.018
45. Beelen, J, De Roos, NM, and De Groot, LCPGM. A: protein enrichment of familiar foods as an innovative strategy to increase protein intake in institutionalized elderly. J Nutr Health Aging . (2017) 21:173–9. doi: 10.1007/s12603-016-0733-y
46. Evans, M, Guthrie, N, Pezzullo, J, Sanli, T, Fielding, RA, and Bellamine, A. Efficacy of a novel formulation of L-carnitine, creatine, and leucine on lean body mass and functional muscle strength in healthy older adults: a randomized, double-blind placebo-controlled study. Nutr Metab (Lond) . (2017) 14. doi: 10.1186/s12986-016-0158-y
47. Niccoli, S, Kolobov, A, Bon, T, Rafilovich, S, Munro, H, Tanner, K, et al. Whey protein supplementation improves rehabilitation outcomes in hospitalized geriatric patients: a double blinded, randomized controlled trial. J Nutr Gerontol Geriatr . (2017) 36:149–65. doi: 10.1080/21551197.2017.1391732
48. Arjuna, T, Miller, M, Ueno, T, Visvanathan, R, Lange, K, Soenen, S, et al. Food services using energy- and protein-fortified meals to assist vulnerable community-residing older adults meet their dietary requirements and maintain good health and quality of life: findings from a pilot study. Geriatrics (Switzerland) . (2018) 3. doi: 10.3390/geriatrics3030060
49. Park, Y, Choi, JE, and Hwang, HS. Protein supplementation improves muscle mass and physical performance in undernourished prefrail and frail elderly subjects: a randomized, double-blind, placebo-controlled trial. Am J Clin Nutr . (2018) 108:1026–33. doi: 10.1093/ajcn/nqy214
50. Young, AM, Banks, MD, and Mudge, AM. Improving nutrition care and intake for older hospital patients through system-level dietary and mealtime interventions. Clin Nutr ESPEN . (2018) 24:140–7. doi: 10.1016/j.clnesp.2017.12.009
51. Mortensen, MN, Larsen, AK, Skadhauge, LB, Høgsted, RH, Beermann, T, Cook, ME, et al. Protein and energy intake improved by in-between meals: an intervention study in hospitalized patients. Clin Nutr ESPEN . (2019) 30:113–8. doi: 10.1016/j.clnesp.2019.01.007
52. Nykänen, I, Törrönen, R, and Schwab, U. Effect of dairy-based and energy-enriched berry-based snacks on quality of life in older people. Geriatr Gerontol Int . (2019) 19:960–1. doi: 10.1111/ggi.13674
53. Allepaerts, S, Buckinx, F, Bruyère, O, Reginster, JY, Paquot, N, and Gillain, S. Clinical impact of nutritional status and energy balance in elderly hospitalized patients. J Nutr Health Aging . (2020) 24:1073–9. doi: 10.1007/s12603-020-1527-9
54. Seemer, J, Kiesswetter, E, Fleckenstein-Sußmann, D, Gloning, M, Bader-Mittermaier, S, Sieber, CC, et al. Effects of an individualised nutritional intervention to tackle malnutrition in nursing homes: a pre-post study. Eur Geriatr Med . (2021). doi: 10.1007/s41999-021-00597-y
55. Barton, AD, Beigg, CL, Macdonald, IA, and Allison, SP. A recipe for improving food intakes in elderly hospitalized patients. Clin Nutr . (2000) 19:451–4. doi: 10.1054/clnu.2000.0149
56. Beelen, J, De Roos, NM, and De Groot, LCPGM. C: a 12-week intervention with protein-enriched foods and drinks improved protein intake but not physical performance of older patients during the first 6 months after hospital release: a randomised controlled trial. Br J Nutr . (2017) 117:1541–9. doi: 10.1017/S0007114517001477
57. Beelen, J, Vasse, E, Janssen, N, Janse, A, de Roos, NM, and de Groot, LCPGM. Protein-enriched familiar foods and drinks improve protein intake of hospitalized older patients: a randomized controlled trial. Clin Nutr . (2018) 37:1186–92. doi: 10.1016/j.clnu.2017.05.010
58. Bonnefoy, M, Laville, M, Ecochard, R, Jusot, JF, Normand, S, Maillot, S, et al. Effects of branched amino acids supplementation in malnourished elderly with catabolic status. J Nutr Health Aging . (2010) 14:579–84. doi: 10.1007/s12603-010-0090-1
59. Castellanos, VH, Marra, MV, and Johnson, P. Enhancement of select foods at breakfast and lunch increases energy intakes of nursing home residents with low meal intakes. J Am Diet Assoc . (2009) 109:445–51. doi: 10.1016/j.jada.2008.11.035
60. Gall, MJ, Grimble, GK, Reeve, NJ, and Thomas, SJ. Effect of providing fortified meals and between-meal snacks on energy and protein intake of hospital patients. Clin Nutr . (1998) 17:259–64. doi: 10.1016/S0261-5614(98)80317-8
61. Hashimoto, R, Sakai, A, Murayama, M, Ochi, A, Abe, T, Hirasaka, K, et al. Effects of dietary soy protein on skeletal muscle volume and strength in humans with various physical activities. J Med Investigat . (2015) 62:177–83. doi: 10.2152/jmi.62.177
62. Iuliano, S, Woods, J, and Robbins, J. Consuming two additional serves of dairy food a day significantly improves energy and nutrient intakes in ambulatory aged care residents: a feasibility study. J Nutr Health Aging . (2013) 17:509–13. doi: 10.1007/s12603-013-0025-8
63. Leslie, WS, Woodward, M, Lean, MEJ, Theobald, H, Watson, L, and Hankey, CR. Improving the dietary intake of under nourished older people in residential care homes using an energy-enriching food approach: a cluster randomised controlled study. J Hum Nutr Diet . (2013) 26:387–94. doi: 10.1111/jhn.12020
64. Lorefält, B, Wissing, U, and Unosson, M. Smaller but energy and protein-enriched meals improve energy and nutrient intakes in elderly patients. J Nutr Health Aging . (2005) 9:243–7.
PubMed Abstract | Google Scholar
65. Mertz, KH, Reitelseder, S, Bechshoeft, R, Bulow, J, Højfeldt, G, Jensen, M, et al. The effect of daily protein supplementation, with or without resistance training for 1 year, on muscle size, strength, and function in healthy older adults: a randomized controlled trial. Am J Clin Nutr . (2021) 113:790–800. doi: 10.1093/ajcn/nqaa372
66. Munk, T, Seidelin, W, Rosenbom, E, Nielsen, AL, Klausen, TW, Nielsen, MA, et al. A 24-h a la carte food service as support for patients at nutritional risk: a pilot study. J Hum Nutr Diet . (2013) 26:268–75. doi: 10.1111/jhn.12017
67. Neelemaat, F, Lips, P, Bosmans, JE, Thijs, A, Seidell, JC, and Bokhorst-de van der Schueren, MAE Short-term Oral nutritional intervention with protein and vitamin D decreases falls in malnourished older adults. J Am Geriatr Soc . (2012);60:691–699. doi: 10.1111/j.1532-5415.2011.03888.x
68. Norton, V, Lignou, S, and Methven, L. Influence of age and individual differences on mouthfeel perception of whey protein-fortified products: a review. Foods . (2021) 10:433. doi: 10.3390/foods10020433
69. Ödlund Olin, A. Energy-dense meals improve energy intake in elderly residents in a nursing home. Clin Nutr . (2003) 22:125–31. doi: 10.1054/clnu.2002.0610
70. Ödlund Olin, A, Österberg, P, Hådell, K, Armyr, I, Jerström, S, and Ljungqvist, O. Energy-enriched hospital food to improve energy intake in elderly patients. J Parenter Enter Nutr . (1996) 20:93–7. doi: 10.1177/014860719602000293
71. Ott, A, Senger, M, Lötzbeyer, T, Gefeller, O, Sieber, CC, and Volkert, D. Effects of a texture-modified, enriched, and reshaped diet on dietary intake and body weight of nursing home residents with chewing and/or swallowing problems: an enable study. J Nutr Gerontol Geriatr . (2019) 38:361–76. doi: 10.1080/21551197.2019.1628158
72. Polonen, S, Tiihonen, M, Hartikainen, S, and Nykanen, I. Individually tailored dietary counseling among old home care clients – effects on nutritional status. J Nutr Health Aging . (2017) 21:567–72. doi: 10.1007/s12603-016-0815-x
73. Smoliner, C, Norman, K, Scheufele, R, Hartig, W, Pirlich, M, and Lochs, H. Effects of food fortification on nutritional and functional status in frail elderly nursing home residents at risk of malnutrition. Nutrition . (2008) 24:1139–44. doi: 10.1016/j.nut.2008.06.024
74. Sossen, L, Bonham, M, and Porter, J. Does a high-energy high-protein diet reduce unintentional weight loss in residential aged care residents? J Nutr Gerontol Geriatr . (2020) 39:56–68. doi: 10.1080/21551197.2019.1691108
75. Stelten, S, Dekker, IM, Ronday, EM, Thijs, A, Boelsma, E, Peppelenbos, HW, et al. Protein-enriched ‘regular products’ and their effect on protein intake in acute hospitalized older adults; a randomized controlled trial. Clin Nutr . (2015) 34:409–14. doi: 10.1016/j.clnu.2014.08.007
76. Stow, R, Ives, N, Smith, C, Rick, C, and Rushton, A. A cluster randomised feasibility trial evaluating nutritional interventions in the treatment of malnutrition in care home adult residents. Trials . (2015) 16:433. doi: 10.1186/s13063-015-0952-2
77. van Til, AJ, Naumann, E, Cox-Claessens, IJHM, Kremer, S, Boelsma, E, and de van der Schueren, MAE. Effects of the daily consumption of protein enriched bread and protein enriched drinking yoghurt on the total protein intake in older adults in a rehabilitation Centre: a single blind randomised controlled trial. J Nutr Health Aging . (2015) 19:525–30. doi: 10.1007/s12603-015-0471-6
78. Hoffman, JR, and Falvo, MJ. Protein – Which is Best? J Sports Sci Med . (2004) 3:118–30.
79. AFNOR. Méthodologie: Directives générales pour la réalisation d’épreuves hédoniques en laboratoire d’évaluation sensorielle ou en salle en conditions contrôlées impliquant des consommateurs . Paris – La Défense: AFNOR (2000). 23 p.
80. Maitre, I, Symoneaux, R, and Sulmont-Rossé, C. Sensory testing in new product development: working with older people In: J Delarue, JB Lawlor, and M Rogeaux, editors. Rapid sensory profiling techniques . Amsterdam: Elsevier (2015). 485–508.
81. Sulmont-Rossé, C, Symoneaux, R, Feyen, V, and Maître, I. Improving food sensory quality with and for elderly consumers In: G Ares and P Varela, editors. Methods in consumer research , vol. 2. Amsterdam: Elsevier (2018). 355–72.
82. Norton, C, Toomey, C, McCormack, WG, Francis, P, Saunders, J, Kerin, E, et al. Protein supplementation at breakfast and lunch for 24 weeks beyond habitual intakes increases whole-body Lean tissue mass in healthy older adults. J Nutr . (2016) 146:65–9. doi: 10.3945/jn.115.219022
83. Nilsson, MI, Mikhail, A, Lan, L, Carlo, AD, Hamilton, B, Barnard, K, et al. A five-ingredient nutritional supplement and home-based resistance exercise improve lean mass and strength in free-living elderly. Nutrients . (2020) 12:1–28. doi: 10.3390/nu12082391
84. Van Wymelbeke, V, Sulmont-Rossé, C, Feyen, V, Issanchou, S, Manckoundia, P, and Maître, I. Optimizing sensory quality and variety: an effective strategy for increasing meal enjoyment and food intake in older nursing home residents. Appetite . (2020) 153:104749. doi: 10.1016/j.appet.2020.104749
Keywords: elderly, enrichment, supplementation, food-first, malnutrition, intake, body weight, acceptability
Citation: Geny A, Petitjean M, Van Wymelbeke-Delannoy V and Sulmont-Rossé C (2023) Impact of food-based fortification on nutritional outcomes and acceptability in older adults: systematic literature review. Front. Nutr . 10:1232502. doi: 10.3389/fnut.2023.1232502
Received: 31 May 2023; Accepted: 02 October 2023; Published: 27 October 2023.
Reviewed by:
Copyright © 2023 Geny, Petitjean, Van Wymelbeke-Delannoy and Sulmont-Rossé. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY) . The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Claire Sulmont-Rossé, [email protected]
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Information
- Author Services
Initiatives
You are accessing a machine-readable page. In order to be human-readable, please install an RSS reader.
All articles published by MDPI are made immediately available worldwide under an open access license. No special permission is required to reuse all or part of the article published by MDPI, including figures and tables. For articles published under an open access Creative Common CC BY license, any part of the article may be reused without permission provided that the original article is clearly cited. For more information, please refer to https://www.mdpi.com/openaccess .
Feature papers represent the most advanced research with significant potential for high impact in the field. A Feature Paper should be a substantial original Article that involves several techniques or approaches, provides an outlook for future research directions and describes possible research applications.
Feature papers are submitted upon individual invitation or recommendation by the scientific editors and must receive positive feedback from the reviewers.
Editor’s Choice articles are based on recommendations by the scientific editors of MDPI journals from around the world. Editors select a small number of articles recently published in the journal that they believe will be particularly interesting to readers, or important in the respective research area. The aim is to provide a snapshot of some of the most exciting work published in the various research areas of the journal.
Original Submission Date Received: .
- Active Journals
- Find a Journal
- Journal Proposal
- Proceedings Series
- For Authors
- For Reviewers
- For Editors
- For Librarians
- For Publishers
- For Societies
- For Conference Organizers
- Open Access Policy
- Institutional Open Access Program
- Special Issues Guidelines
- Editorial Process
- Research and Publication Ethics
- Article Processing Charges
- Testimonials
- Preprints.org
- SciProfiles
- Encyclopedia

Article Menu

- Subscribe SciFeed
- Recommended Articles
- Google Scholar
- on Google Scholar
- Table of Contents
Find support for a specific problem in the support section of our website.
Please let us know what you think of our products and services.
Visit our dedicated information section to learn more about MDPI.
JSmol Viewer
Microgreens production: exploiting environmental and cultural factors for enhanced agronomical benefits.

1. Introduction
2. optimising growing conditions of microgreens, 2.1. seed density and quality, 2.2. substrate, 2.2.1. physicochemical properties of growing media, physical properties, chemical properties, 2.4.1. light quantity, 2.4.2. light quality, 2.5. temperature, 2.6. relative humidity (rh), 2.7. genetic traits influencing growth and yield, 2.8. fertiliser, 3. nutritional profile and sensory attributes, 4. agronomical benefits of microgreens, 4.1. short-growing time, 4.2. carbon footprint, 4.3. energy conservation, 4.4. higher productivity, 4.5. food security, 4.6. minimising waste, 5. future of microgreens, 5.1. comprehensive nutritional assessment, 5.2. increased demand and market growth, 5.3. advancements in cultivation techniques, 5.4. environmental impact and sustainability, 5.5. policy and regulatory frameworks, 6. conclusions, author contributions, data availability statement, conflicts of interest.
- Zaręba, A.; Krzemińska, A.; Kozik, R. Urban Vertical Farming as an Example of Nature-Based Solutions Supporting a Healthy Society Living in the Urban Environment. Resources 2021 , 10 , 109. [ Google Scholar ] [ CrossRef ]
- Misra, G.; Gibson, K.E. Characterization of Microgreen Growing Operations and Associated Food Safety Practices. Food Prot. Trends 2021 , 41 , 56–69. [ Google Scholar ] [ CrossRef ]
- Zhang, X.; Bian, Z.; Yuan, X.; Chen, X.; Lu, C. A review on the effects of light-emitting diode (LED) light on the nutrients of sprouts and microgreens. Trends Food Sci. Technol. 2020 , 99 , 203–216. [ Google Scholar ] [ CrossRef ]
- Treadwell, D.; Hochmuth, R.; Landrum, L.; Laughlin, W. Microgreens: A new specialty crop: HS1164, rev. 9/2020. Edis 2020 , 2020 . [ Google Scholar ] [ CrossRef ]
- Priti; Mishra, G.P.; Dikshit, H.K.; Tontang, M.T.; Stobdan, T.; Sangwan, S.; Aski, M.; Singh, A.; Kumar, R.R.; Tripathi, K.; et al. Diversity in Phytochemical Composition, Antioxidant Capacities, and Nutrient Contents Among Mungbean and Lentil Microgreens When Grown at Plain-Altitude Region (Delhi) and High-Altitude Region (Leh-Ladakh), India. Front. Plant Sci. 2021 , 12 , 710812. [ Google Scholar ] [ CrossRef ]
- Verlinden, S. Microgreens: Definitions, Product Types, and Production Practices. Hortic. Rev. 2020 , 47 , 85–124. [ Google Scholar ]
- Choe, U.; Yu, L.L.; Wang, T.T.Y. The Science behind Microgreens as an Exciting New Food for the 21st Century. J. Agric. Food Chem. 2018 , 66 , 11519–11530. [ Google Scholar ] [ CrossRef ]
- Bulgari, R.; Negri, M.; Santoro, P.; Ferrante, A. Quality Evaluation of Indoor-Grown Microgreens Cultivated on Three Different Substrates. Horticulturae 2021 , 7 , 96. [ Google Scholar ] [ CrossRef ]
- Xiao, Z.; Lester, G.E.; Luo, Y.; Wang, Q. Assessment of vitamin and carotenoid concentrations of emerging food products: Edible microgreens. J. Agric. Food Chem. 2012 , 60 , 7644–7651. [ Google Scholar ] [ CrossRef ]
- Dereje, B.; Jacquier, J.-C.; Elliott-Kingston, C.; Harty, M.; Harbourne, N. Brassicaceae Microgreens: Phytochemical Compositions, Influences of Growing Practices, Postharvest Technology, Health, and Food Applications. ACS Food Sci. Technol. 2023 , 3 , 981–998. [ Google Scholar ] [ CrossRef ]
- Lone, J.K.; Pandey, R. Microgreens on the rise: Expanding our horizons from farm to fork. Heliyon 2024 , 10 , e25870. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Teng, Z.; Luo, Y.; Pearlstein, D.J.; Wheeler, R.M.; Johnson, C.M.; Wang, Q.; Fonseca, J.M. Microgreens for Home, Commercial, and Space Farming: A Comprehensive Update of the Most Recent Developments. Annu. Rev. Food Sci. Technol. 2023 , 14 , 539–562. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Gitelson, I.; Terskov, I.; Kovrov, B.; Sidko, F.Y.; Lisovsky, G.; Okladnikov, Y.N.; Belyanin, V.; Trubachov, I.; Rerberg, M. Life support system with autonomous control employing plant photosynthesis. Acta Astronaut. 1976 , 3 , 633–650. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Amitrano, C.; Paglialunga, G.; Battistelli, A.; De Micco, V.; Del Bianco, M.; Liuzzi, G.; Moscatello, S.; Paradiso, R.; Proietti, S.; Rouphael, Y. Defining growth requirements of microgreens in space cultivation via biomass production, morpho-anatomical and nutritional traits analysis. Front. Plant Sci. 2023 , 14 , 1190945. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Boles, H.O.; Poulet, L.; Johnson, C.M.; Torres, J.J.; Koss, L.L.; Spencer, L.E.; Massa, G.D. Design, Build and Testing of Hardware to Safely Harvest Microgreens in Microgravity. Gravitational Space Res. 2023 , 11 , 1–14. [ Google Scholar ] [ CrossRef ]
- Bhaswant, M.; Shanmugam, D.K.; Miyazawa, T.; Abe, C.; Miyazawa, T. Microgreens—A comprehensive review of bioactive molecules and health benefits. Molecules 2023 , 28 , 867. [ Google Scholar ] [ CrossRef ]
- Jambor, T.; Knizatova, N.; Valkova, V.; Tirpak, F.; Greifova, H.; Kovacik, A.; Lukac, N. Microgreens as a Functional Component of the Human Diet: A Review. J. Microbiol. Biotechnol. Food Sci. 2022 , 12 , e5870. [ Google Scholar ] [ CrossRef ]
- Kowitcharoen, L.; Phornvillay, S.; Lekkham, P.; Pongprasert, N.; Srilaong, V. Bioactive composition and nutritional profile of microgreens cultivated in Thailand. Appl. Sci. 2021 , 11 , 7981. [ Google Scholar ] [ CrossRef ]
- Kyriacou, M.C.; Rouphael, Y.; Di Gioia, F.; Kyratzis, A.; Serio, F.; Renna, M.; De Pascale, S.; Santamaria, P. Micro-scale vegetable production and the rise of microgreens. Trends Food Sci. Technol. 2016 , 57 , 103–115. [ Google Scholar ] [ CrossRef ]
- Alrifai, O.; Hao, X.; Marcone, M.F.; Tsao, R. Current review of the modulatory effects of LED lights on photosynthesis of secondary metabolites and future perspectives of microgreen vegetables. J. Agric. Food Chem. 2019 , 67 , 6075–6090. [ Google Scholar ] [ CrossRef ]
- Mlinarić, S.; Gvozdić, V.; Vuković, A.; Varga, M.; Vlašiček, I.; Cesar, V.; Begović, L. The effect of light on antioxidant properties and metabolic profile of chia microgreens. Appl. Sci. 2020 , 10 , 5731. [ Google Scholar ] [ CrossRef ]
- Lanoue, J.; St. Louis, S.; Little, C.; Hao, X. Continuous lighting can improve yield and reduce energy costs while increasing or maintaining nutritional contents of microgreens. Front. Plant Sci. 2022 , 13 , 983222. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Lee, J.S.; Pill, W.G.; Cobb, B.B.; Olszewski, M. Seed treatments to advance greenhouse establishment of beet and chard microgreens. J. Hortic. Sci. Biotechnol. 2004 , 79 , 565–570. [ Google Scholar ] [ CrossRef ]
- Murphy, C.; Pill, W. Cultural practices to speed the growth of microgreen arugula (roquette; Eruca vesicaria subsp. sativa ). J. Hortic. Sci. Biotechnol. 2010 , 85 , 171–176. [ Google Scholar ] [ CrossRef ]
- Priti; Sangwan, S.; Kukreja, B.; Mishra, G.P.; Dikshit, H.K.; Singh, A.; Aski, M.; Kumar, A.; Taak, Y.; Stobdan, T.; et al. Yield optimization, microbial load analysis, and sensory evaluation of mungbean ( Vigna radiata L.), lentil ( Lens culinaris subsp. culinaris ), and Indian mustard ( Brassica juncea L.) microgreens grown under greenhouse conditions. PLoS ONE 2022 , 17 , e0268085. [ Google Scholar ] [ CrossRef ]
- Paradiso, V.M.; Castellino, M.; Renna, M.; Gattullo, C.E.; Calasso, M.; Terzano, R.; Allegretta, I.; Leoni, B.; Caponio, F.; Santamaria, P. Nutritional characterization and shelf-life of packaged microgreens. Food Funct. 2018 , 9 , 5629–5640. [ Google Scholar ] [ CrossRef ]
- Sharma, S.; Shree, B.; Sharma, D.; Kumar, S.; Kumar, V.; Sharma, R.; Saini, R. Vegetable microgreens: The gleam of next generation super foods, their genetic enhancement, health benefits and processing approaches. Food Res. Int. 2022 , 155 , 111038. [ Google Scholar ] [ CrossRef ]
- Poudel, P.; Connolly, E.L.; Kwasniewski, M.; Lambert, J.D.; Di Gioia, F. Zinc biofortification via fertigation using alternative zinc sources and concentration levels in pea, radish, and sunflower microgreens. Sci. Hortic. 2024 , 331 , 113098. [ Google Scholar ] [ CrossRef ]
- Di Gioia, F.; De Bellis, P.; Mininni, C.; Santamaria, P.; Serio, F. Physicochemical, agronomical and microbiological evaluation of alternative growing media for the production of rapini ( Brassica rapa L.) microgreens. J. Sci. Food Agric. 2017 , 97 , 1212–1219. [ Google Scholar ] [ CrossRef ]
- Saleh, R.; Gunupuru, L.R.; Lada, R.; Nams, V.; Thomas, R.H.; Abbey, L. Growth and biochemical composition of microgreens grown in different formulated soilless media. Plants 2022 , 11 , 3546. [ Google Scholar ] [ CrossRef ]
- Gunjal, M.; Singh, J.; Kaur, J.; Kaur, S.; Nanda, V.; Mehta, C.M.; Bhadariya, V.; Rasane, P. Comparative analysis of morphological, nutritional, and bioactive properties of selected microgreens in alternative growing medium. S. Afr. J. Bot. 2024 , 165 , 188–201. [ Google Scholar ] [ CrossRef ]
- Maluin, F.N.; Hussein, M.Z.; Nik Ibrahim, N.N.L.; Wayayok, A.; Hashim, N. Some emerging opportunities of nanotechnology development for soilless and microgreen farming. Agronomy 2021 , 11 , 1213. [ Google Scholar ] [ CrossRef ]
- de la Fuente, B.; López-García, G.; Máñez, V.; Alegría, A.; Barberá, R.; Cilla, A. Evaluation of the bioaccessibility of antioxidant bioactive compounds and minerals of four genotypes of Brassicaceae microgreens. Foods 2019 , 8 , 250. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Renna, M.; Castellino, M.; Leoni, B.; Paradiso, V.M.; Santamaria, P. Microgreens Production with Low Potassium Content for Patients with Impaired Kidney Function. Nutrients 2018 , 10 , 675. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Kennard, N.; Stirling, R.; Prashar, A.; Lopez-Capel, E. Evaluation of Recycled Materials as Hydroponic Growing Media. Agronomy 2020 , 10 , 1092. [ Google Scholar ] [ CrossRef ]
- Bayineni, V.K.; Herur, N.K. Natural Synedrella Residues as a Growing Substrate Ingredient: An Eco-friendly Way to Improve Yield and Quality of Beet ( Beta vulgaris ) Microgreens. Eur. J. Agric. Food Sci. 2022 , 4 , 1–5. [ Google Scholar ] [ CrossRef ]
- Naik, B.P.K.; Sekhar, G.; Suryakumari, A.; Rajulu, G.S.G.; Harshini, K.; Deepika, L.A.S. Effect of growth and yield of mustard ( Brassica juncea ) microgreens on different growing medias in indoor condition. Int. J. Res. Agron. 2022 , 5 , 40–42. [ Google Scholar ] [ CrossRef ]
- Pathania, P.; Katoch, V.; Sandal, A.; Sharma, N. Anupama Sandal and Neelam Sharma. Role of Substrate Media in Growth and Development of Selected Microgreens. Biol. Forum-Int. J. 2022 , 14 , 1357–1361. [ Google Scholar ] [ CrossRef ]
- Kyriacou, M.C.; El-Nakhel, C.; Pannico, A.; Graziani, G.; Soteriou, G.A.; Giordano, M.; Palladino, M.; Ritieni, A.; De Pascale, S.; Rouphael, Y. Phenolic constitution, phytochemical and macronutrient content in three species of microgreens as modulated by natural fiber and synthetic substrates. Antioxidants 2020 , 9 , 252. [ Google Scholar ] [ CrossRef ]
- Sari, N.; Andansari, P. Microgreen quality of broccoli plants ( Brassica oleracea L.) and correlation between parameters. J. Phys. Conf. Ser. 2020 , 1569 , 042093. [ Google Scholar ]
- Weber, C.F. Broccoli Microgreens: A Mineral-Rich Crop That Can Diversify Food Systems. Front. Nutr. 2017 , 4 , 7. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Bantis, F.; Koukounaras, A. Microgreen vegetables’ production can be optimized by combining the substrate and nutrient solution in a PFAL. Sci. Hortic. 2024 , 333 , 113277. [ Google Scholar ] [ CrossRef ]
- Thuong, V.T.; Minh, H.G. Effects of growing substrates and seed density on yield and quality of radish ( Raphanus sativus ) microgreens. Res. Crops 2020 , 21 , 579–586. [ Google Scholar ] [ CrossRef ]
- Negri, M.; Bulgari, R.; Santoro, P.; Ferrante, A. Evaluation of different growing substrates for microgreens production. In Proceedings of the III International Symposium on Growing Media, Composting and Substrate Analysis 1305, Milan, Italy, 18 March 2021; pp. 109–114. [ Google Scholar ]
- D’Imperio, M.; Montesano, F.F.; Montemurro, N.; Parente, A. Posidonia natural residues as growing substrate component: An ecofriendly method to improve nutritional profile of brassica microgreens. Front. Plant Sci. 2021 , 12 , 580596. [ Google Scholar ] [ CrossRef ]
- Wieth, A.R.; Pinheiro, W.D.; Duarte, T.D.S. Purple cabbage microgreens grown in different substrates and nutritive solution concentrations. Rev. Caatinga 2019 , 32 , 976–985. [ Google Scholar ] [ CrossRef ]
- Li, T.; Lalk, G.T.; Arthur, J.D.; Johnson, M.H.; Bi, G. Shoot production and mineral nutrients of five microgreens as affected by hydroponic substrate type and post-emergent fertilization. Horticulturae 2021 , 7 , 129. [ Google Scholar ] [ CrossRef ]
- Kumari, V.; Junuthula, S.; Mandapaka, M.R.T. Microgreens for Nutritional Security ; National Institute of Agricultural Extension Management (MANAGE): Hyderabad, India, 2023; Volume 23. [ Google Scholar ]
- Hoang, G.M.; Vu, T.T. Selection of suitable growing substrates and quality assessment of Brassica microgreens cultivated in greenhouse. Acad. J. Biol. 2022 , 44 , 133–142. [ Google Scholar ] [ CrossRef ]
- DIN EN 13041:2012 ; Soil Improvers and Growing Media—Determination of Physical Properties—Dry Bulk Density, Air Volume, Water Volume, Shrinkage Value and Total Pore Space. Beuth: Berlin, Germany; Cologne, Germany, 2012.
- Poudel, P.; Duenas, A.E.; Di Gioia, F. Organic waste compost and spent mushroom compost as potential growing media components for the sustainable production of microgreens. Front. Plant Sci. 2023 , 14 , 1229157. [ Google Scholar ] [ CrossRef ]
- Goyal, M.R. Management of Drip/Trickle or Micro Irrigation ; CRC Press: Boca Raton, FL, USA, 2012. [ Google Scholar ]
- Rajan, P.; Lada, R.R.; MacDonald, M.T. Advancement in indoor vertical farming for microgreen production. Am. J. Plant Sci. 2019 , 10 , 1397. [ Google Scholar ] [ CrossRef ]
- Erekath, S.; Seidlitz, H.; Schreiner, M.; Dreyer, C. Food for future: Exploring cutting-edge technology and practices in vertical farm. Sustain. Cities Soc. 2024 , 106 , 105357. [ Google Scholar ] [ CrossRef ]
- Tavan, M.; Wee, B.; Brodie, G.; Fuentes, S.; Pang, A.; Gupta, D. Optimizing sensor-based irrigation management in a soilless vertical farm for growing microgreens. Front. Sustain. Food Syst. 2021 , 4 , 622720. [ Google Scholar ] [ CrossRef ]
- Xiao, Z.; Bauchan, G.; Nichols-Russell, L.; Luo, Y.; Wang, Q.; Nou, X. Proliferation of Escherichia coli 0157: H7 in Soil-Substitute and Hydroponic Microgreen Production Systems. J. Food Prot. 2015 , 78 , 1785–1790. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Painter, J.A.; Hoekstra, R.M.; Ayers, T.; Tauxe, R.V.; Braden, C.R.; Angulo, F.J.; Griffin, P.M. Attribution of foodborne illnesses, hospitalizations, and deaths to food commodities by using outbreak data, United States, 1998–2008. Emerg. Infect. Dis. 2013 , 19 , 407. [ Google Scholar ] [ CrossRef ]
- Riggio, G.M.; Wang, Q.; Kniel, K.E.; Gibson, K.E. Microgreens—A review of food safety considerations along the farm to fork continuum. Int. J. Food Microbiol. 2019 , 290 , 76–85. [ Google Scholar ] [ CrossRef ]
- Morales, G.; Llorente, I.; Montesinos, E.; Moragrega, C. Basis for a predictive model of Xanthomonas arboricola pv. pruni growth and infections in host plants. In Proceedings of the II International Workshop on Bacterial Diseases of Stone Fruits and Nuts, No. 1149, Izmir, Turkey, 24 November 2016; pp. 1–8. [ Google Scholar ]
- Paradiso, R.; Proietti, S. Light-quality manipulation to control plant growth and photomorphogenesis in greenhouse horticulture: The state of the art and the opportunities of modern LED systems. J. Plant Growth Regul. 2022 , 41 , 742–780. [ Google Scholar ] [ CrossRef ]
- Cheng, M.-C.; Kathare, P.K.; Paik, I.; Huq, E. Phytochrome signaling networks. Annu. Rev. Plant Biol. 2021 , 72 , 217–244. [ Google Scholar ] [ CrossRef ]
- Paik, I.; Huq, E. Plant photoreceptors: Multi-functional sensory proteins and their signaling networks. In Seminars in Cell & Developmental Biology ; Academic Press: Cambridge, MA, USA, 2019; pp. 114–121. [ Google Scholar ]
- Proietti, S.; Moscatello, S.; Riccio, F.; Downey, P.; Battistelli, A. Continuous lighting promotes plant growth, light conversion efficiency, and nutritional quality of Eruca vesicaria (L.) Cav. in controlled environment with minor effects due to light quality. Front. Plant Sci. 2021 , 12 , 730119. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Meas, S.; Luengwilai, K.; Thongket, T. Enhancing growth and phytochemicals of two amaranth microgreens by LEDs light irradiation. Sci. Hortic. 2020 , 265 , 109204. [ Google Scholar ] [ CrossRef ]
- Gao, M.; He, R.; Shi, R.; Zhang, Y.; Song, S.; Su, W.; Liu, H. Differential effects of low light intensity on broccoli microgreens growth and phytochemicals. Agronomy 2021 , 11 , 537. [ Google Scholar ] [ CrossRef ]
- Spencer, L.; Gooden, J.; Curry, A.; Romeyn, M.; Wheeler, R.; Sirmons, T. Novel Microgreen Crop Testing for Space. In Proceedings of the 52nd International Conference on Environmental Systems, Calgary, AB, Canada, 16–20 July 2023. [ Google Scholar ]
- Bian, Z.H.; Yang, Q.C.; Liu, W.K. Effects of light quality on the accumulation of phytochemicals in vegetables produced in controlled environments: A review. J. Sci. Food Agric. 2015 , 95 , 869–877. [ Google Scholar ] [ CrossRef ]
- Ilieva, I.; Ivanova, T.; Naydenov, Y.; Dandolov, I.; Stefanov, D. Plant experiments with light-emitting diode module in Svet space greenhouse. Adv. Space Res. 2010 , 46 , 840–845. [ Google Scholar ] [ CrossRef ]
- Qiao, J.; Li, Z.; Lv, Z.; Liu, S.; Chen, S.; Feng, Y. Effects of Different Combinations of Red and Blue Light on the Edible Organ Morphology and Quality of Buckwheat ( Fagopyrum esculentum Moench) Microgreens. Agronomy 2024 , 14 , 751. [ Google Scholar ] [ CrossRef ]
- Bantis, F. Light Spectrum Differentially Affects the Yield and Phytochemical Content of Microgreen Vegetables in a Plant Factory. Plants 2021 , 10 , 2182. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Zheng, L.; He, H.; Song, W. Application of light-emitting diodes and the effect of light quality on horticultural crops: A review. HortScience 2019 , 54 , 1656–1661. [ Google Scholar ] [ CrossRef ]
- Brazaitytė, A.; Miliauskienė, J.; Vaštakaitė-Kairienė, V.; Sutulienė, R.; Laužikė, K.; Duchovskis, P.; Małek, S. Effect of different ratios of blue and red led light on brassicaceae microgreens under a controlled environment. Plants 2021 , 10 , 801. [ Google Scholar ] [ CrossRef ]
- Samuoliene, G.; Virsile, A.; Brazaityte, A.; Jankauskiene, J.; Sakalauskiene, S.; Vastakaite, V.; Novickovas, A.; Viskeliene, A.; Sasnauskas, A.; Duchovskis, P. Blue light dosage affects carotenoids and tocopherols in microgreens. Food Chem. 2017 , 228 , 50–56. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Lobiuc, A.; Vasilache, V.; Pintilie, O.; Stoleru, T.; Burducea, M.; Oroian, M.; Zamfirache, M.-M. Blue and red LED illumination improves growth and bioactive compounds contents in acyanic and cyanic Ocimum basilicum L. microgreens. Molecules 2017 , 22 , 2111. [ Google Scholar ] [ CrossRef ]
- Palmitessa, O.D.; Gadaleta, A.; Leoni, B.; Renna, M.; Signore, A.; Paradiso, V.M.; Santamaria, P. Effects of greenhouse vs. growth chamber and different blue-light percentages on the growth performance and quality of broccoli microgreens. Agronomy 2022 , 12 , 1161. [ Google Scholar ] [ CrossRef ]
- Hatfield, J.L.; Prueger, J.H. Temperature extremes: Effect on plant growth and development. Weather Clim. Extrem. 2015 , 10 , 4–10. [ Google Scholar ] [ CrossRef ]
- Xiao, Z.; Luo, Y.; Lester, G.E.; Kou, L.; Yang, T.; Wang, Q. Postharvest quality and shelf life of radish microgreens as impacted by storage temperature, packaging film, and chlorine wash treatment. LWT Food Sci. Technol. 2014 , 55 , 551–558. [ Google Scholar ] [ CrossRef ]
- Dzaky, M.A.F.; Nugroho, A.P.; Prasetyatama, Y.D.; Sutiarso, L.; Falah, M.A.F.; Okayasu, T. Control of vapor pressure deficit (VPD) in micro-plant factory (McPF) to enhanced spinach microgreens growth. Sci. Hortic. 2024 , 332 , 113229. [ Google Scholar ] [ CrossRef ]
- Kong, Y.; Masabni, J.; Niu, G. Effect of temperature variation and blue and red LEDs on the elongation of arugula and mustard microgreens. Horticulturae 2023 , 9 , 608. [ Google Scholar ] [ CrossRef ]
- Dayarathna, N.N.; Gama-Arachchige, N.S.; Damunupola, J.W.; Xiao, Z.; Gamage, A.; Merah, O.; Madhujith, T. Effect of storage temperature on storage life and sensory attributes of packaged mustard microgreens. Life 2023 , 13 , 393. [ Google Scholar ] [ CrossRef ]
- Brazaitytė, A.; Viršilė, A.; Jankauskienė, J.; Sakalauskienė, S.; Samuolienė, G.; Sirtautas, R.; Novičkovas, A.; Dabašinskas, L.; Miliauskienė, J.; Vaštakaitė, V.; et al. Effect of supplemental UV-A irradiation in solid-state lighting on the growth and phytochemical content of microgreens. Int. Agrophys. 2015 , 29 , 13–22. [ Google Scholar ] [ CrossRef ]
- Ford, M.A.; Thorne, G.N. Effects of atmospheric humidity on plant growth. Ann. Bot. 1974 , 38 , 441–452. [ Google Scholar ] [ CrossRef ]
- Amitrano, C.; Rouphael, Y.; De Pascale, S.; De Micco, V. Modulating vapor pressure deficit in the plant micro-environment may enhance the bioactive value of lettuce. Horticulturae 2021 , 7 , 32. [ Google Scholar ] [ CrossRef ]
- Fanourakis, D.; Aliniaeifard, S.; Sellin, A.; Giday, H.; Körner, O.; Nejad, A.R.; Delis, C.; Bouranis, D.; Koubouris, G.; Kambourakis, E. Stomatal behavior following mid-or long-term exposure to high relative air humidity: A review. Plant Physiol. Biochem. 2020 , 153 , 92–105. [ Google Scholar ] [ CrossRef ]
- Yao, L.; Jiang, Z.; Wang, Y.; Wan, S.; Xin, X.-F. High air humidity dampens salicylic acid pathway and plant resistance via targeting of NPR1. bioRxiv 2022 , 2 , e113499. [ Google Scholar ]
- Jones-Baumgardt, C.; Llewellyn, D.; Zheng, Y. Different Microgreen Genotypes Have Unique Growth and Yield Responses to Intensity of Supplemental PAR from Light-emitting Diodes during Winter Greenhouse Production in Southern Ontario, Canada. HortScience 2020 , 55 , 156–163. [ Google Scholar ] [ CrossRef ]
- Di Bella, M.C.; Toscano, S.; Arena, D.; Moreno, D.A.; Romano, D.; Branca, F. Effects of growing cycle and genotype on the morphometric properties and glucosinolates amount and profile of sprouts, microgreens and baby leaves of broccoli ( Brassica oleracea L. var. italica Plenck) and Kale ( B. oleracea L. var. acephala DC.). Agronomy 2021 , 11 , 1685. [ Google Scholar ] [ CrossRef ]
- Cowden, R.J.; Ghaley, B.B.; Henriksen, C.B. Analysis of light recipe, seeding density, and fertilization effects on secondary metabolite accumulation and growth-defense responses in Brassicaceae microgreens. Food Biosci. 2024 , 59 , 104071. [ Google Scholar ] [ CrossRef ]
- Lobiuc, A.; Damian, C.; Costica, N.; Leahu, A. Morphological and biochemical parameters in chemically elicited rye sprouts. Life Sci. Ser. 2017 , 27 , 157–162. [ Google Scholar ]
- Di Gioia, F.; Hong, J.C.; Pisani, C.; Petropoulos, S.A.; Bai, J.; Rosskopf, E.N. Yield performance, mineral profile, and nitrate content in a selection of seventeen microgreen species. Front. Plant Sci. 2023 , 14 , 1220691. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Pill, W.; Collins, C.; Gregory, N.; Evans, T. Application method and rate of Trichoderma species as a biological control against Pythium aphanidermatum (Edson) Fitzp. in the production of microgreen table beets ( Beta vulgaris L.). Sci. Hortic. 2011 , 129 , 914–918. [ Google Scholar ] [ CrossRef ]
- Craver, J.K.; Gerovac, J.R.; Lopez, R.G.; Kopsell, D.A. Light intensity and light quality from sole-source light-emitting diodes impact phytochemical concentrations within Brassica microgreens. J. Am. Soc. Hortic. Sci. 2017 , 142 , 3–12. [ Google Scholar ] [ CrossRef ]
- Allred, J.; Mattson, N. Growing Better Greenhouse Microgreens in under Control: Tips for Controlled Environment Growing ; Greenhouse Product News: Sparta, MI, USA, 2018; pp. 10–13. [ Google Scholar ]
- Li, T.; Lalk, G.T.; Bi, G. Fertilization and pre-sowing seed soaking affect yield and mineral nutrients of ten microgreen species. Horticulturae 2021 , 7 , 14. [ Google Scholar ] [ CrossRef ]
- Li, T.; Arthur, J.D.; Bi, G. Shoot Yield and Mineral Nutrient Concentrations of Six Microgreens in the Brassicaceae Family Affected by Fertigation Rate. Horticulturae 2023 , 9 , 1217. [ Google Scholar ] [ CrossRef ]
- Nair, B.R. Microgreens: A Future Super Food. In Conservation and Sustainable Utilization of Bioresources ; Springer: Berlin/Heidelberg, Germany, 2023; pp. 103–122. [ Google Scholar ]
- Janovská, D.; Štočková, L.; Stehno, Z. Evaluation of buckwheat sprouts as microgreens. Acta Agric. Slov. 2010 , 95 , 157–162. [ Google Scholar ] [ CrossRef ]
- Sun, J.; Xiao, Z.; Lin, L.-Z.; Lester, G.E.; Wang, Q.; Harnly, J.M.; Chen, P. Profiling polyphenols in five Brassica species microgreens by UHPLC-PDA-ESI/HRMS n. J. Agric. Food Chem. 2013 , 61 , 10960–10970. [ Google Scholar ] [ CrossRef ]
- Huang, H.; Jiang, X.; Xiao, Z.; Yu, L.; Pham, Q.; Sun, J.; Chen, P.; Yokoyama, W.; Yu, L.L.; Luo, Y.S. Red cabbage microgreens lower circulating low-density lipoprotein (LDL), liver cholesterol, and inflammatory cytokines in mice fed a high-fat diet. J. Agric. Food Chem. 2016 , 64 , 9161–9171. [ Google Scholar ] [ CrossRef ]
- Singh, M.; Nara, U.; Rani, N.; Pathak, D.; Kaur, K.; Sangha, M.K. Comparison of Mineral Composition in Microgreens and Mature leaves of Celery ( Apium graveolens L.). Biol. Trace Elem. Res. 2023 , 201 , 4156–4166. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Pant, Y.; Lingwan, M.; Masakapalli, S.K. Metabolic, biochemical, mineral and fatty acid profiles of edible Brassicaceae microgreens establish them as promising functional food. Food Chem. Adv. 2023 , 3 , 100461. [ Google Scholar ] [ CrossRef ]
- Xiao, Z. Nutrition, Sensory, Quality and Safety Evaluation of A New Specialty Produce: Microgreens. Ph.D. Thesis, University of Maryland, College Park, MD, USA, 2013. [ Google Scholar ]
- Kou, L.; Yang, T.; Luo, Y.; Liu, X.; Huang, L.; Codling, E. Pre-harvest calcium application increases biomass and delays senescence of broccoli microgreens. Postharvest Biol. Technol. 2014 , 87 , 70–78. [ Google Scholar ] [ CrossRef ]
- Pasakdee, S.; Bañuelos, G.; Shennan, C.; Cheng, W. Organic N Fertilizers and Irrigation Influence Organic Broccoli Production in Two Regions of California. J. Veg. Sci. 2007 , 12 , 27–46. [ Google Scholar ] [ CrossRef ]
- Xiao, Z.; Codling, E.E.; Luo, Y.; Nou, X.; Lester, G.E.; Wang, Q. Microgreens of Brassicaceae: Mineral composition and content of 30 varieties. J. Food Compos. Anal. 2016 , 49 , 87–93. [ Google Scholar ] [ CrossRef ]
- Rusu, T.; Moraru, P.I.; Mintas, O.S. Influence of environmental and nutritional factors on the development of lettuce ( Lactuca sativa L.) microgreens grown in a hydroponic system: A review. Not. Bot. Horti Agrobot. Cluj-Napoca 2021 , 49 , 12427. [ Google Scholar ] [ CrossRef ]
- Carillo, P.; El-Nakhel, C.; De Micco, V.; Giordano, M.; Pannico, A.; De Pascale, S.; Graziani, G.; Ritieni, A.; Soteriou, G.A.; Kyriacou, M.C. Morpho-metric and specialized metabolites modulation of parsley microgreens through selective LED wavebands. Agronomy 2022 , 12 , 1502. [ Google Scholar ] [ CrossRef ]
- Tilman, D.; Balzer, C.; Hill, J.; Befort, B.L. Global food demand and the sustainable intensification of agriculture. Proc. Natl. Acad. Sci. USA 2011 , 108 , 20260–20264. [ Google Scholar ] [ CrossRef ]
- Ghosh, A.; Kumar, A.; Biswas, G. Exponential population growth and global food security: Challenges and alternatives. In Bioremediation of Emerging Contaminants from Soils ; Elsevier: Amsterdam, The Netherlands, 2024; pp. 1–20. [ Google Scholar ]
- Godfray, H.C.J.; Beddington, J.R.; Crute, I.R.; Haddad, L.; Lawrence, D.; Muir, J.F.; Pretty, J.; Robinson, S.; Thomas, S.M.; Toulmin, C. Food security: The challenge of feeding 9 billion people. Science 2010 , 327 , 812–818. [ Google Scholar ] [ CrossRef ]
- Premanandh, J. Factors affecting food security and contribution of modern technologies in food sustainability. J. Sci. Food Agric. 2011 , 91 , 2707–2714. [ Google Scholar ] [ CrossRef ]
- Strzepek, K.; Boehlert, B. Competition for water for the food system. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2010 , 365 , 2927–2940. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Delian, E.; Chira, A.; Bădulescu, L.; Chira, L. Insights into microgreens physiology. Horticulture 2015 , 59 , 447–454. [ Google Scholar ]
- Paraschivu, M.; Cotuna, O.; Sărățeanu, V.; Durău, C.C.; Păunescu, R.A. Microgreens-current status, global market trends and forward statements. Manag. Econ. Eng. Agric. Rural. Dev. 2021 , 21 , 633–639. [ Google Scholar ]
- Touliatos, D.; Dodd, I.C.; McAinsh, M. Vertical farming increases lettuce yield per unit area compared to conventional horizontal hydroponics. Food Energy Secur. 2016 , 5 , 184–191. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Bulgari, R.; Baldi, A.; Ferrante, A.; Lenzi, A. Yield and quality of basil, Swiss chard, and rocket microgreens grown in a hydroponic system. N. Z. J. Crop Hortic. Sci. 2016 , 45 , 119–129. [ Google Scholar ] [ CrossRef ]
- Tomas, M.; Zhang, L.; Zengin, G.; Rocchetti, G.; Capanoglu, E.; Lucini, L. Metabolomic insight into the profile, in vitro bioaccessibility and bioactive properties of polyphenols and glucosinolates from four Brassicaceae microgreens. Food Res. Int. 2021 , 140 , 110039. [ Google Scholar ] [ CrossRef ]
- Nájera, C.; Ros, M.; Moreno, D.A.; Hernández-Lara, A.; Pascual, J.A. Combined effect of an agro-industrial compost and light spectra composition on yield and phytochemical profile in mizuna and pak choi microgreens. Heliyon 2024 , 10 , e26390. [ Google Scholar ] [ CrossRef ]
- Kumar, A.; Singh, N. Embracing nutritional, physical, pasting, textural, sensory and phenolic profile of functional muffins prepared by partial incorporation of lyophilized wheatgrass, fenugreek and basil microgreens juice powder. J. Sci. Food Agric. 2024 , 104 , 4286–4295. [ Google Scholar ] [ CrossRef ]
- Astapova, M.; Saveliev, A.; Markov, Y. Method for monitoring growth of microgreens in containers using computer vision in infrared and visible ranges. In Proceedings of the Agriculture Digitalization and Organic Production: Proceedings of the First International Conference, ADOP 2021, St. Petersburg, Russia, 7–9 June 2021; pp. 383–394. [ Google Scholar ]
- Yang, M.; Liu, X.; Luo, Y.; Pearlstein, A.J.; Wang, S.; Dillow, H.; Reed, K.; Jia, Z.; Sharma, A.; Zhou, B. Machine learning-enabled non-destructive paper chromogenic array detection of multiplexed viable pathogens on food. Nat. Food 2021 , 2 , 110–117. [ Google Scholar ] [ CrossRef ]
- Misra, G. Food Safety Risk in an Indoor Microgreen Cultivation System ; University of Arkansas: Fayetteville, AR, USA, 2020. [ Google Scholar ]
- Hamilton, A.N.; Fraser, A.M.; Gibson, K.E. Barriers to implementing risk management practices in microgreens growing operations in the United States: Thematic analysis of interviews and survey data. Food Control 2023 , 152 , 109836. [ Google Scholar ] [ CrossRef ]
- Forcella, D.; Hudon, M. Green microfinance in Europe. J. Bus. Ethics 2016 , 135 , 445–459. [ Google Scholar ] [ CrossRef ]
- Torres, R.S.; Haddad, J.; Andrade, G.C.C.D.; Santos, A.H.M.; Rennó, P.L.P. Comparisons between Brazilian-Germany Legal Framework about in Mini and Microgeneration Regulatory Policies. Braz. Arch. Biol. Technol. 2022 , 66 , e23220413. [ Google Scholar ] [ CrossRef ]
Click here to enlarge figure
| Substrate | Crop | Observation/Challenges | Ref. |
|---|---|---|---|
| Natural Synedrella residues (aerial part of Synedrella nodiflora) | Beet | Better quality and yield of beet microgreens. | [ ] |
| Soil, vermicompost, cocopeat, farmyard manure (FYM) water | Mustard | Higher plant weight reported in vermicompost. | [ ] |
| Mixture of cocopeat, perlite, vermiculite and vermicompost | Kale, broccoli, lettuce, and turnip | Observed variation in the performance of different microgreens varieties. | [ ] |
| Coconut, jute fibre, vermiculite | Green basil, red basil, rocket | A significant effect on yield and dry matter percentage of microgreens. | [ ] |
| Agave fiber, capillary mat, cellulose sponge, coconut fiber, peat moss | Coriander, kohlrabi, Pak choi | The highest fresh and dry yield was obtained from peatmoss, although its dry matter and phenolic content were low. Nitrate and macro-nutrient levels rise when natural fibre substrates are used. | [ ] |
| Rockwool, cocopeat, Merang paper, Hydroton | Broccoli | Observed better growth of broccoli microgreens on cocopeat supplemented with coconut water. | [ ] |
| Peat, Sure to Grow textile fibers, jute-kenaf fibers | Rapini | Recycled textiles showed comparable fresh biomass yield as peat. | [ ] |
| Vermicompost, micro-mat hydroponic growing pads | Broccoli | Microgreens on compost showed greater elemental concentration than when grown hydroponically. | [ ] |
| Cannabis mat, coco coir and peat | Mustard, pea, and radish | Peat can be successfully replaced by coconut coir and cannabis mat for mustard microgreens. | [ ] |
| Sphagnum peat, coconut coir | Radish | In comparison to coconut coir, sphagnum peat requires less fertiliser to produce the same amount of microbial load within safe and legal limits. | [ ] |
| Coconut coir, vermiculite, jute | Green basil, rocket, and red basil | Both species and substrates affect yield and nutrition. For green and red basil, respectively, coconut fibre and vermiculite are the most effective. | [ ] |
| Posidonia natural residue and peat | Mizuna and rapini | Posidonia leaves or fibres were added to microgreens to raise their and B content without adversely impacting their production. | [ ] |
| Beifiur S10, Carolina Soil organic, Carolina Soil seedling, and CSC vermiculite | Purple cabbage | No impact of substrate type on shoot height at harvest or shoot fresh/dry matter yield. | [ ] |
| Hemp mat, jute mat, Micro-Mats (wood fiber), Biostrate (felt fibre) | Broccoli, cabbage, kale, mustard, and radish | Fresh, dry shoot weights and mineral nutrients in the microgreens under examination were affected by the kind of substrate. Microgreens in hemp had the highest weight, height, and K concentration, but the lowest N concentration. | [ ] |
| Species | Harvesting Day | Ref. |
|---|---|---|
| Radish ruby | Day 5 | [ ] |
| Cabbage Chinese | Day 6 | [ ] |
| Broccoli | Day 7 | [ ] |
| Red amaranth and leafy vegetable amaranth | Day 8 | [ ] |
| Arugula | Day 13 | [ ] |
| Lettuce | Day 15 | [ ] |
| Watercress | Day 17 | [ ] |
| Upland cress | Day 20 | [ ] |
| Parsley | Day 21 | [ ] |
| The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
Share and Cite
Dubey, S.; Harbourne, N.; Harty, M.; Hurley, D.; Elliott-Kingston, C. Microgreens Production: Exploiting Environmental and Cultural Factors for Enhanced Agronomical Benefits. Plants 2024 , 13 , 2631. https://doi.org/10.3390/plants13182631
Dubey S, Harbourne N, Harty M, Hurley D, Elliott-Kingston C. Microgreens Production: Exploiting Environmental and Cultural Factors for Enhanced Agronomical Benefits. Plants . 2024; 13(18):2631. https://doi.org/10.3390/plants13182631
Dubey, Shiva, Niamh Harbourne, Mary Harty, Daniel Hurley, and Caroline Elliott-Kingston. 2024. "Microgreens Production: Exploiting Environmental and Cultural Factors for Enhanced Agronomical Benefits" Plants 13, no. 18: 2631. https://doi.org/10.3390/plants13182631
Article Metrics
Article access statistics, further information, mdpi initiatives, follow mdpi.

Subscribe to receive issue release notifications and newsletters from MDPI journals

COMMENTS
Advantages and Disadvantages of Food Fortification. 3.1. Health. According to WHO mortality data, around 0.8 million deaths (1.5% of the total) can be attributed to iron deficiency each year, and a similar number to vitamin A deficiency [37], and contributes to a significant number of lives lost [38].
Introduction. Food f ortification (FF) is defined as the addition of. one or mo re e ssential nutrients t o a fo od, whet her or not it. is normall y contained in th e food, for t he pur pose of ...
Biofortification differs from food fortification in that the former involves the addition of nutrients to food crops prior to harvesting whilst the latter adds nutrients to foods during post-harvest processing (3, 81). Food fortification repeatedly adds nutrients to foods whilst biofortification of varieties of food crops occurs once (74, 81).
Abstract. Large-scale food fortification is an effective, sustainable, and scalable intervention to address vitamin and mineral deficiencies, however, pressing gaps exist globally around ensuring the quality of fortified foods. This paper summarizes the global challenges and gaps faced in monitoring the quality of fortified foods, the guidance ...
The main differences in the descriptions can be summarized as follows (Figure 1): . Type of strategy within which FtFF is placed: some describe it as synonymous with dietary diversification, others as a replacement for conventional fortification, and yet others as a method to improve existing and/or new food recipes/product formulation, or combinations of these.
Food Science & Nutrition is an author-friendly journal for the rapid dissemination of fundamental and applied research on all aspects of food science and nutrition. Abstract Food fortification is an important nutrition intervention to fight micronutrient deficiencies and to reduce their incidence in many low- and middle-income countries ...
Large-scale food fortification (LSFF) can increase dietary micronutrient intake and improve micronutrient status. Here we used food balance sheet data from the Food and Agriculture Organization of ...
Food fortification with micronutrients is widely implemented to reduce micronutrient deficiencies and related outcomes. Although many factors affect the success of fortification programs, high population coverage is needed to have a public health impact. We aimed to provide recent global coverage estimates of salt, wheat flour, vegetable oil, maize flour, rice, and sugar among countries with ...
This paper summarizes some of the literature on the cost effectiveness and cost benefit of food fortification with selected micronutrients most relevant for developing countries. Micronutrients covered include iron, iodine, vitamin A, and zinc. The main focus is on commercial fortification, although home fortification and biofortification are mentioned. Fortification with iron, vitamin A, and ...
Food Fortification in a Globalized World outlines experiences over the past 50 years—and future potential—for the application of food fortification across a variety of foods in the industrialized and developing world. The book captures recent science and applications trends in fortification, including emerging areas such as biofortification, nutraceuticals and new nutrient intake ...
Deficiencies in one or more micronutrients such as iron, zinc, and vitamin A are widespread in low- and middle-income countries and compromise the physical and cognitive capacity of millions of people. Food fortification is a cost-effective strategy with demonstrated health, economic and social benefits. Despite ongoing debates globally and in ...
This paper, authored by 2016 World Food Prize winner, Howarth Bouis and his colleague Amy Saltzman, both of the International Food Policy Research Institute, describes work under the HarvestPlus program on the global level on biofortification of staple foods to overcome micronutrient deficiencies.
Background: Vitamins and minerals are essential for growth and maintenance of a healthy body, and have a role in the functioning of almost every organ. Multiple interventions have been designed to improve micronutrient deficiency, and food fortification is one of them. Objectives: To assess the impact of food fortification with multiple micronutrients on health outcomes in the general ...
Food fortification is considered one of the most cost-effective and scientifically evidenced nutrition interventions that is readily available to ... One significant limitation of our review is that most of the included papers draw on research in HICs. Further research is needed in LMICs to ensure that evidence and key lessons on reformulation ...
Food fortification is a cost-effective strategy with demonstrated health, economic and social benefits. ... Feature papers represent the most advanced research with significant potential for high impact in the field. A Feature Paper should be a substantial original Article that involves several techniques or approaches, provides an outlook for ...
Feature papers represent the most advanced research with significant potential for high impact in the field. A Feature Paper should be a substantial original Article that involves several techniques or approaches, provides an outlook for future research directions and describes possible research applications. ... Food fortification is a public ...
reduced from 19 to 4% in children and from 18 to 10% in women; and in children, iron. deficiency was also reduced from 27 to 7% [ 40 ]. Despite the enormous benefits of food fortification ...
Food fortification is a sustainable, cost-effective approach to reducing vitamin and mineral deficiency. As the staple food for an estimated 3 billion people, rice has the potential to fill an obvious gap in current fortification programs. In recent years, new technologies have produced fortified rice kernels that are efficacious in reducing ...
Particularly, fortification is a method of incorporating nutrients or non-nutritive bioactive components into food products (Dwyer et al., 2015). Food fortification is one of the methods that has been applied increasingly and addressed to all age groups, being widely used to minimize micronutrient deficiency.
Fortification could be mass fortification (that is, adding micronutrients to foods that are commonly consumed, such as flour, salt, sugar and cooking oil), or point‐of‐use fortification, which involves adding single‐dose packets of vitamins and minerals in powder form that can be sprinkled onto any ready‐to‐eat food consumed at home ...
The reasons for excluding papers were: no original research (n = 18), wrong population (n = 18), no DIY food-based fortification (n = 135), fortification with micronutrients only (n = 15), wrong outcomes (n = 18). Wrong outcomes included functional outcomes (muscle strength), gastric emptying, glycemia, gut hormones, bone mineral density ...
Currently, food fortification encompasses a broader concept, and might be done for several reasons. The first is to restore nutrients lost during food processing, process known as enrichment. In this case, the amount of nutrients added is approximately equal to the natural content in the food before processing.
An exponential growth in global population is expected to reach nine billion by 2050, demanding a 70% increase in agriculture productivity, thus illustrating the impact of global crop production on the environment and the importance of achieving greater agricultural yields. Globally, the variety of high-quality microgreens is increasing through indoor farming at both small and large scales.
This work demonstrates how different programs in LMICs have successfully addressed challenges with food fortification and finds that these efforts are most successful when partnerships are formed that include the public and private sector as well as other parties that can provide support in key areas such as advocacy, management, capacity building, implementation and regulatory monitoring ...