Distillation Experiment: Distilling Water at Home (Minimal Materials)


Introduction: Distillation Experiment: Distilling Water at Home (Minimal Materials)

***Students, do not use a stove without your parents permission/supervision.***
In Grade 7, students learn about pure substances and mixtures. They learn about the particle theory of matter and how it explains the properties of these pure substances and mixtures. In particular, we learn about solutions. A solution is a mixture made up of parts with very similar properties and characteristics. Salt water, coffee, alloy metals are all solutions. The parts of a solution are very difficult to separate. Though not impossible!
We begin to learn about different types of filtration that can be used to separate solutions. The most common strategies involve making the solute or solvent change state. (ie. changing the solute from a solid to a liquid)
In this experiment students will learn about the filtration process of distillation. This experiment is designed for my students to do at home with as little materials as possible. There are some much better water distilling Instructables available, such as this one created by erbst . This instructbable is intended to be as simple as possible.
- 1 cup of water
- 1 Tablespoons of fine table salt
- One metal pot and lid
- One spatula (recommended)
- One water glass
Step 1: Create a Salt Water Solution

Fill a glass with 1 cup of water. Then add one table spoon of salt. To make a proper solution you should stir until the salt as been absorbed and is no longer visible. Give it a taste! Just make sure there is a sink nearby!
Step 2: Add to Pot and Boil

Add your salt water solution to a pot (I chose a large pot with a large surface area) with a lid. Close the lid. On high heat bring the water to a boil. Let your water boil, you should see some steam escaping, let it continue to boil for a minute or two. Then reduce the heat to low.
Step 3: Lift the Lid

**Be careful! The pot will be hot! If your pot has an exposed metal handle use over mitts or a tea towel**
Carefully lift the lid and turn it over in one smooth motion. You should see many water droplets have formed on the lid of the pot.
Step 4: Pour Water Into a Cup

Bring your lid over to a new glass. With a spatula, wipe the water droplets into the cup.
You may not get enough water on your first try. If not, return the lid to the pot and reheat. Repeat these steps until you have collected enough water to drink.
Step 5: Bottom's Up!

Enjoy the fruits of your labor!
Take a drink of the water you have collected, distilled rather!
What do you notice? How has the salt water solution separated?
Recommendations

Art and Sculpture Contest

Outdoor Life Contest

Microcontrollers Contest


- Science Notes Posts
- Contact Science Notes
- Todd Helmenstine Biography
- Anne Helmenstine Biography
- Free Printable Periodic Tables (PDF and PNG)
- Periodic Table Wallpapers
- Interactive Periodic Table
- Periodic Table Posters
- Science Experiments for Kids
- How to Grow Crystals
- Chemistry Projects
- Fire and Flames Projects
- Holiday Science
- Chemistry Problems With Answers
- Physics Problems
- Unit Conversion Example Problems
- Chemistry Worksheets
- Biology Worksheets
- Periodic Table Worksheets
- Physical Science Worksheets
- Science Lab Worksheets
- My Amazon Books
How to Make Distilled Water – 5 Easy Methods

Distilled water is water purified by condensing water vapor into liquid water. Usually, the distillation process involves boiling impure water and collected the condensed vapor in a fresh container. However, you can obtain distilled water from damp soil, plants, snow, and rain, too. You can distill water to make drinking water for emergencies or improve your tap water. Here are several methods for making distilled water yourself. Which one you choose depends on your situation and resources.
Distill Water Using a Stove or Fire
If you have a heat source, such as a stove or campfire, it’s better to distill impure water for drinking than to simply boil it. This is because boiling kills many pathogens, but doesn’t remove chemical impurities or kill certain bacterial spores. You can use this method with any water, including seawater, questionable tap water, or even water from a puddle. To distill water, you need a large container to hold the impure water, a smaller container that either floats in the larger one or can be propped up above the water level, and a rounded lid that fits the large container. The process goes much faster if you also have some ice.
- 5-gallon aluminum or stainless steel pot
- Rounded lid for the pot
- Metal or glass bowl that floats inside the pot
- Fill the large pot partially full of the impure water.
- Float the collection bowl on the water. The goal is to drip water from the inverted lid into this bowl, so make sure the bowl is large enough to catch the drips.
- Place the pot lid upside down on the pot. When you heat the water, water vapor will rise in the pot, condense into droplets on the lid, and fall into the collection bowl.
- Heat the pot. The process occurs more quickly if the water boils , but it’s okay if it only gets hot.
- If you have ice cubes, place them on top of the pot lid. The ice chills the lid of the pot and helps condense water vapor into liquid water.
- Use care when removing the lid from the pot so you don’t get burned by steam, the pot, or the hot water. Store the distilled water (the water in the collection bowl) in a clean container. Ideally, store the water in a sterile container (one immersed in boiling water) or dishwasher-cleaned container. Use a container meant for water storage so contaminants don’t leach into the clean water over time.
Alternative Collection Method
A better method is to collect the distilled water outside of the pot. Basically, this is a simple still. It is superior to the first method because it reduces the risk of contaminating the clean water with the “dirty” water and allows for continuous heating of the source water. One option is to place a funnel over the boiling water rather than a lid. Use plastic aquarium tubing or copper tubing to connect the end of the funnel to a collection bottle. Make sure the collection bottle is lower than the funnel so gravity can drain the water.
Distilled Water From Rain or Snow
Another way to get distilled water is to let Mother Nature do the work for you. Rain and snow are naturally distilled water. Water evaporates from the land, ocean, lakes, and rivers and condenses in the atmosphere to fall as precipitation. Precipitation does pick up particulates from the air, but it’s pure enough to drink except in highly polluted areas. Also, it’s important to collect rain or snow fresh from the sky and not off of trees or buildings.
Collect rain or snow in a clean container. Allow time for any sediment to fall to the bottom of the bowl. You can drink this water or further purify it by filtering it through a coffee filter or by boiling it.
Distill Water From Plants, Mud, or Urine
In a dire emergency, you may not have access to niceties like pots and fire. It’s still possible to distill water using a homemade solar still. This method of distillation uses the heat of the Sun to evaporate water that you can collect to drink. You can use any source of moisture, such as urine, dew, plants, damp soil, or sea water. However, be careful to avoid poisonous plants because volatile toxins may contaminate the distilled water. Cacti, ferns, and grasses are generally safe to use. The major disadvantage to this method is that it takes a long time to collect water.
- Dig a hole in the ground in a sunny location.
- Place a cup in the center of the bottom of the hole to collect distilled water.
- Pile damp non-toxic plants or moist soil around the outside of the cup.
- Cover the hole with a piece of plastic and secure it with rocks or soil. Try to seal the hole as well as you can to prevent moisture from escaping. The plastic traps the water and also traps heat via the greenhouse effect.
- Place a pebble or other small weight on the plastic right above the buried cup. As water evaporates, it condenses on the plastic and falls toward the depression, finally dripping into the cup.
- Don’t mess with your set-up except to drink water or add more plants or soil. Every time you unseal the plastic, you release moisture and slow down the process.

Use a Home Distillation Kit
It’s often cheaper to buy distilled water than make it yourself because it costs fuel or electricity to heat water. But, home distillation kits can be less expensive than bottled water, especially if you use sunlight (solar heat) to heat the water. Home distillation kits typically range in price from $100 to several hundred dollars. More expensive kits are used for labs or for processing large volumes of water.
Pros and Cons of Drinking Distilled Water
On the plus side, distilled water is safer to drink than contaminated water. It can save lives when the only available water is seawater, water from a river or stream, or a questionable public water supply. It also removes trace contaminants that are always present in a municipal water supply from the treatment process, including residual aluminum, chlorine, fluorine, and chloramines. Distillation removes radionuclides, heavy metals (including lead from some plumbing), and many organic compounds.
The high level of purification is also an argument against drinking distilled water, at least over the long term. Distillation demineralizes water, removing healthful minerals, such magnesium and calcium. These minerals are associated with positive health effects, especially for the cardiovascular system. If distilled water is the only source of drinking water, it’s important to get these minerals from other sources.
- Anjaneyulu, L.; Kumar, E. Arun; Sankannavar, Ravi; Rao, K. Kesava (13 June 2012). “Defluoridation of drinking water and rainwater harvesting using a solar still”. Industrial & Engineering Chemistry Research . 51 (23): 8040–8048. doi: 10.1021/ie201692q
- Fischetti, Mark (September 2007). “Fresh from the Sea”. Scientific American . 297 (3): 118–119. doi: 10.1038/scientificamerican0907-118
- Kozisek F. (1980). “Health risks from drinking demineralised water”. Nutrients in Drinking Water . World Health Organization. pp. 148–159. ISBN 92-4-159398-9.
- O’Meagher, Bert; Reid, Dennis; Harvey, Ross (2007). Aids to survival: a handbook on outback survival (25th ed.). Maylands, W.A.: Western Australia Police Academy. ISBN 978-0-646-36303-5.
- Taylor, F. Sherwood (1945). “The Evolution of the Still”. Annals of Science . 5 (3): 186. doi: 10.1080/00033794500201451
Related Posts
Using Distillation to Purify Water
Please enable JavaScript
(Earth Science for ages 5+)
Did you know that the Earth is 70% water? It’s true, that’s why it is called the “Blue Planet.” Most of this water (97%) is saltwater , which people cannot drink or even use in their everyday life.
In the video above, you can see how distillation occurs with just a bit of heat. Here’s what you’ll need:
When the sun is no longer shining directly on the bowl, this water vapor cools and condenses on the inside of the plastic wrap. Because the plastic wrap is sloped down toward the empty plastic cup, the water condensation drips slowly down into the cup, leaving you with freshwater in the cup and saltwater in the bowl.
This whole process is called distillation because the freshwater is distilled and purified from the saltwater.
Choose an Account to Log In

Notifications
Science project, distilling water.
Grades Level: 7th - 9th; Type: Chemistry
Make fresh water from salt water by using the process of distillation.
Research Questions:
- What is an element?
- What is a mixture?
- How does a mixture differ from a compound?
- What is meant by boiling point?
- What is the boiling point of water?
- What is distillation, desalination, and desalinization?
- Why are we or should we be concerned about changing sea water into regular drinking water?
Is it possible to drink sea water? By itself, salt water is harmful to humans, but using a process known as distillation, salt water can become drinkable! In this experiment you will convert salt water into fresh water using distillation, which involves boiling a salt solution so that the water of the solution is turned into water vapor or water gas.
- Distilling flask
- Thermometer
- Liebig condenser
- 250ml beaker
- Rubber tubing
- Rubber stopper
- Bunsen burner or electric burner
- Paper towels (for cleanup)
Experimental Procedure:
- Gather all the materials you will need for this science project. You may wish to include a camera and take photos to include in your report and use on your display charts.
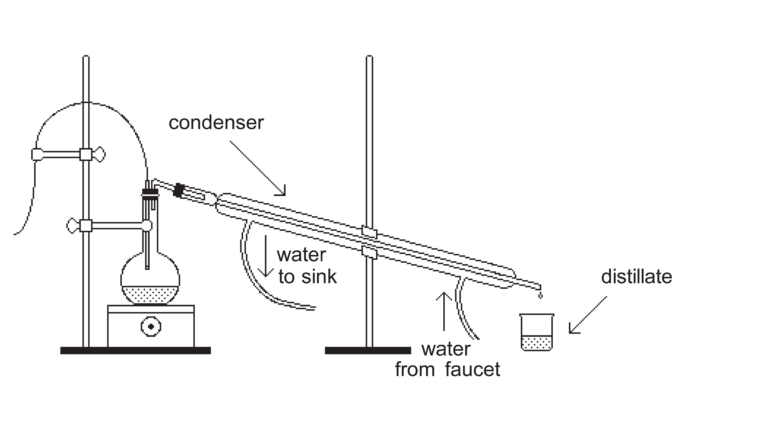
- Put on your safety equipment.
- Set up the distillation apparatus and shown in the diagram below. This diagram is provided by the free Wikipedia Encyclopedia. You are free to use it in your report.
- Start by filling you r beaker with tap water and add one tablespoon of salt. Mix well. Pour the salt water into the distilling flask
- Place sand in the basin and now place the distilling flask into the sand before you start heating it.
- Carefully place the burner under the flask.
- Connect the distilling flask to one end of the Liebig's condenser.
- Place the condenser so that it slopes downward and that it other end is directly above the beaker.
- Bring the salt solution to a boil. Keep an eye on that thermometer
- Collect the water vapor that is now turning into liquid water.
- Observe. You will find the water is tasteless and has no distinct odor.
- Write up your experiment. You may wish to include photos of the apparatus. Be certain to include your bibliography.

Terms/Concepts: elements; compounds; mixtures; solutions; boiling point; distillation; desalinization
References: Wikipedia's Distillation page
Related learning resources
Add to collection, create new collection, new collection, new collection>, sign up to start collecting.
Bookmark this to easily find it later. Then send your curated collection to your children, or put together your own custom lesson plan.

Simple Distillation Experiment
To separate a mixture of two miscible liquids by simple distillation.

What is a Simple Distillation Experiment?
Simple distillation separates components from their liquid mixtures based on their boiling point differences. In this method, the mixture to be separated is heated and then cooled using a water condenser. The condensed vapours are then collected from the outlet of the condenser tube.
In this way, different components are collected at different boiling temperatures. When the mixture’s components differ widely in their boiling points (more than 25 ° C), this method is most suitable for their separation. The liquid obtained after condensation is called distillate and can be easily collected in a beaker or conical flask.
Aim of Experiment:
Apparatus and material required:.
- Distillation Flask
- Laboratory Thermometer (-10 °C to 110 °C)
- Two Beakers (250 mL)
- Tripod Stand
- Single Bore Cork
A mixture of Water and Acetone
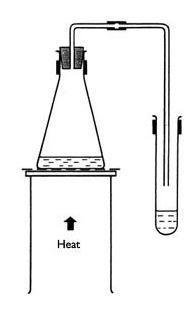
- Step 1: Place the tripod stand and the wire gauze over the burner.
- Step 2: The distillation flask should be kept on the tripod stand and clamped to an iron stand.
- Step 3: Now seal the flask using a single bored cork. Insert a laboratory thermometer into the bore of the cork to measure the temperature while boiling the liquid.
- Step 4: Insert the distillation flask arm into a condenser attached to an iron stand.
- Step 5: The inlets and outlets of the condenser should be properly attached to the water pipes.
- Step 6: Place an empty beaker below the open end of the condenser.
- Step 7: Heat the mixture of acetone and water slowly and carefully monitor the temperature rise.
- Step 8: Observe and note the temperature at which the first component of the mixture distils out; that is, the vapours get cooled and collected in a beaker kept at the other end of the condenser.
- Step 9: Continue heating and observe and note the temperature at which the second component distils out.
Precautions:
- The thermometer bulb should be at the arm of the distillation flask to prevent any erroneous reading.
- The distillation flask should be sealed tightly using the cork to prevent the escape of vapours.
- The condenser should be supplied with a continuous flow of cold water.
FAQs on Simple Distillation Experiment
Q: how do we define boiling point.
Answer: The temperature at which the vapour pressure of a liquid becomes equal to the external pressure surrounding the area of the liquid is known as the boiling point of that liquid.
Q: What is the use of porcelain pieces in the distillation flask?
Answer: While heating the distillation flask, the solution evaporates. Porcelain pieces are placed in the distillation flask to prevent the solution from bumping due to uneven heating.
Q: What is the difference between boiling and evaporation?
Answer: Evaporation is a natural process that occurs when the liquid changes into a gaseous form because of increased pressure or temperature. Evaporation does not produce any bubbles. Boiling is an unnatural process where the liquid gets heated up and vaporised due to continuous heating of the liquid. Boiling produces continuous bubbles during heating.
Q. What are the two processes in distillation?
Answer: Distillation is a two-step process that includes distillation and condensation reflux. The liquid boils at a high temperature, converts into gas, and then condenses. The gas-liquid two-phase flow over the countercurrent contact is commonly carried out in a distillation column.
Q. How can aniline and chloroform be separated?
Answer: The distillation technique can be used to separate aniline and chloroform due to the greater difference in boiling point. The aniline has the formula C6H5NH2. The organic compound chloroform has the formula CHCl3.

Experiments Related to this Topic
Esterification reaction, how can we prepare soap in the lab, comparing the foaming capacity of soap samples, effect of mass on period of a simple pendulum, effect of amplitude on period of a simple pendulum, cleaning capacity of soap, oxidation reaction of alcohol with alkaline kmno4, study reaction of zinc with sulphuric acid, complete combustion of alcohol, phototropism and geotropism.

39 Insightful Publications

Embibe Is A Global Innovator

Innovator Of The Year Education Forever

Interpretable And Explainable AI

Revolutionizing Education Forever

Best AI Platform For Education

Enabling Teachers Everywhere

Decoding Performance

Leading AI Powered Learning Solution Provider

Auto Generation Of Tests

Disrupting Education In India

Problem Sequencing Using DKT

Help Students Ace India's Toughest Exams

Best Education AI Platform

Unlocking AI Through Saas

Fixing Student’s Behaviour With Data Analytics

Leveraging Intelligence To Deliver Results

Brave New World Of Applied AI

You Can Score Higher

Harnessing AI In Education

Personalized Ed-tech With AI

Exciting AI Platform, Personalizing Education

Disruptor Award For Maximum Business Impact

Top 20 AI Influencers In India

Proud Owner Of 9 Patents
Trending Searches
Distillation Of Water From an Aqueous Solution Using A Disposable Apparatus.
Introduction: (initial observation).
Distillation is a commonly used method of purification and separation of liquids. Many areas in the world have no access to drinking water, so they make their drinking water by distilling salt water from oceans. As time passes, water get more polluted and access to drinking water becomes more difficult. Bottling companies are vigorously purchasing water resources around the world and soon the only drinking water that we have access to will be expensive bottled water or soda drinks.

Distillation is also used in petrochemical and chemical industries as a means of extracting certain chemicals from mixtures. Natural oil for example is a mixture of many different hydrocarbons. Refineries use distillation to separate them to individual products.
Because of the importance of distillation, this project is an attempt to experiment distillation with a disposable apparatus that is easily available to everyone. Using a disposable apparatus is also a valuable experience for scientists because a successful scientist must be able to utilize everything available to him/her in conducting research in an efficient manner.
This project guide contains information that you need in order to start your project. If you have any questions or need more support about this project, click on the “Ask Question” button on the top of this page to send me a message.
If you are new in doing science project, click on “How to Start” in the main page. There you will find helpful links that describe different types of science projects, scientific method, variables, hypothesis, graph, abstract and all other general basics that you need to know.
Project advisor
Information Gathering:
Find out about distillation and its applications. Read books, magazines, or ask professionals who might know in order to learn about the structure of different distillation apparatuses. Keep track of where you got your information from.
Following are samples of information that you may find.
Although extracting a pure substance from a mixture is done by chemists in chemical factories, often physical properties of material are the key in such separations. Differences in physical properties such as boiling point, melting point, density and solubility can help us separate many substances from a mixture.
Distillation means vaporization of a liquid and subsequent condensation of the resultant gas back to liquid form. It is used to separate liquids from nonvolatile solids or solutes (e.g., alcoholic beverages from the fermented materials, water from other components of seawater) or to separate two or more liquids with different boiling points (e.g., gasoline, kerosene, and lubricating oil from crude oil). Many variations have been devised for industrial applications. An important one is fractional distillation, in which liquids with similar boiling points are repeatedly vaporized and condensed as they rise through an insulated vertical column. The most volatile of the liquids emerges first, nearly pure, from the top of the column, followed in turn by less and less volatile fractions of the original mixture. This method separates the mixture’s components far better than simple distillation does.
Although many people have a fair idea what “distillation” means, the important aspects that seem to be missed from the manufacturing point of view are that:
Source…
distillation is the most common separation technique
it consumes enormous amounts of energy, both in terms of cooling and heating requirements
Distillation is probably the most common technique for purifying liquids. In simple distillation, a liquid is boiled and the vapors work through the apparatus until they reach the condenser where they are cooled and re-liquified.

The process is relatively simple: a) the water based liquid is heated to the boiling point and thus vaporizes b) (becomes steam), while other substances remain in solid state, in boiler. Steam is then directed into a cooler where it cools down and returns to liquid c) the end result is usually a purified liquid.
We can purchase a complete set of a distillation apparatus from a local laboratory supplier or we can setup a simple distillation apparatus as follows:
- Use a glass test tube or flask as a boiling unit.
- Use a glass tube as a condenser.
- Connect the boiling unit to the condenser via a rubber or plastic joint. It can be an elbow tube or any other rubber or plastic tube.
- Use stands and clamps or any other safe method to secure your setup like the following figure.
Heat source can be an alcohol burner, but you can also use Chafing Dish Fuel cans (sold in supermarkets and used to keep the food warm).
Condensed vapors can be collected in a beaker or glass cup.
Question/ Purpose:
What do you want to find out? Write a statement that describes what you want to do. Use your observations and questions to write the statement.
The purpose of this project is to design and construct a distillation device using readily available materials and use it to separate a colorless liquid from a common colored solution by distillation.
Question: Following are sample questions for this project.
- What is the rate of fuel consumption in your home made water distillatory? You want to know how much fuel is needed to produce one liter distilled water?
- How does the rate of production change in a home made distillatory system? You want to know if the production of distilled water start as soon as you start the heat and if it remains at a constant rate.
Note: You may use tea or soda as a colored solution.
Identify Variables:
When you think you know what variables may be involved, think about ways to change one at a time. If you change more than one at a time, you will not know what variable is causing your observation. Sometimes variables are linked and work together to cause something. At first, try to choose variables that you think act independently of each other.
For Question 1:
- Independent variable is the amount of consumed fuel.
- Dependent variable is the amount of produced distilled water.
- Constants are: Distillation time, the type of fuel and the heat source.
- Controlled variables are: Weather temperature, water temperature prior to distillation.
For Question 2:
- Independent variable is time
- Dependent variable is the rate of distillation (how many milliliters per minute)
Hypothesis:
Based on your gathered information, make an educated guess about what types of things affect the system you are working with. Identifying variables is necessary before you can make a hypothesis.
A simple distiller can be constructed using a soda can as a boiling vessel and aluminum foil as condenser.
I estimate the rate of fuel consumption to be about 1% to 5% in a home made distillation device.
Experiment Design:
Design an experiment to test each hypothesis. Make a step-by-step list of what you will do to answer each question. This list is called an experimental procedure. For an experiment to give answers you can trust, it must have a “control.” A control is an additional experimental trial or run. It is a separate experiment, done exactly like the others. The only difference is that no experimental variables are changed. A control is a neutral “reference point” for comparison that allows you to see what changing a variable does by comparing it to not changing anything. Dependable controls are sometimes very hard to develop. They can be the hardest part of a project. Without a control you cannot be sure that changing the variable causes your observations. A series of experiments that includes a control is called a “controlled experiment.”
Experiment 1: (For question 1)
Introduction: This experiment is particularly appropriate for middle school science classes or for a general or first-year course where scientific glassware is unavailable. A simple distillation is performed using a soda can and aluminum foil (or copper pipe) in place of traditional glassware. The experiment works sufficiently well to enable students to obtain a colorless liquid from a colored solution. Not only is the equipment inexpensive and readily available, but the entire apparatus is disposable.
- Rinse the soda can clean.
- Add the solution to be distilled until the can is l/3 to l/2 full. Boiling chips may be added if available, but are by no means necessary.
- Mount the soda can above the burner on a wire screen supported by an iron ring (attached to the ring stand). Mount the second iron ring around and near the top of the can to prevent it from tipping over.
- Insert the smaller glass jar into the larger one and surround liberally with an Ice-rich slush bath.
- Prepare an air-cooled condenser made of aluminum foil. This is best done by wrapping the foil lengthwise around a dowel rod or broom handle, taking care to seal the seam that runs the length of the foil tube by making several folds of foil neatly pressed back on itself. (Failure to do this will result in poor efficiency during distillation.)
- Fit one end of the condenser into the opening at the top of the soda can. Gently bend the other end down and insert it into the smaller glass jar which serves as a receiver flask for the distillation.
- Heat the soda can and its contents with a steady flame. As the solution boils, some vapor can be seen escaping from around the mouth of the can. Still, enough vapor makes its way through the air-cooled condenser so that condensation soon occurs in the chilled receiver flask.
- After pouring the mother liquor down the drain, the entire distillation apparatus may be disposed of with the solid waste. If desired, the jars may be saved for re-use. The aluminum cans could be recycled.
If alcohol burners are used, they should be filled when cold, only by the teacher. Adding common salt to the burner fuel makes it easier for students to see the flame and thus avoid possible burns. The aluminum foil condenser becomes quite hot during the distillation. Care should be taken to avoid touching it during collection of the distillate. Goggles must be worn throughout the experiment.
This is a sample setup similar to what is suggested in the above experiment. As you see, you can modify the design based on the material that may be available to you. In this experiment we used aluminum foil to build a condenser tube.

Measurements:
Weigh the alcohol burner before and after the experiment so you can calculate the amount of alcohol used in this process.
Weigh the can before and after the experiment so you can calculate the amount of distilled water.
Calculations:
Divide the amount of fuel used in process of distillation by the amount of distilled water to determine the rate of fuel consumption.
Additional Samples and Pictures:
Here we found a copper tube and matching copper elbow and used it as a condenser.
The problem was that it took a few minutes for milk to boil.
Adult supervision and safe experiment area is required in addition to safety goggles.

In this experiment we are using an electric heater instead of an alcohol burner.
The problem was that so much heat was being wasted because a soda can is small and the heater element is big.

Hear we used a propane torch as a heat source. Wire screen was necessary hear to distribute the flame and heat.

Repeat your tests and enter your data in a data table. For each experiment write how much fuel did you use and how much distilled water did you collect. Make sure to limit your distillation time and keep it constant. For example you may let the distillation continue for 15 minutes or 30 minutes. Whatever time length you choose, keep it the same every time you try the distillation process. The data table may look like this:
| (optional) | ||
| in grams | in dollars or cents | in milliliters (cubic centimeters) |
Use the above data table to calculate the average cost of producing distilled water with your home made apparatus.
Make a graph:
You can use a bar graph to visually present your results. Make a vertical bar for each trial. The height of bar will be the amount of distilled water you get on that trial.
If alcohol burners are used, they should be filled when cold, only by an adult. Adding common salt to the wick of the burner makes it easier for you to see the flame and thus avoid possible burns. All components become quite hot during the distillation. Care should be taken to avoid touching it during collection of the distillate. Goggles must be worn throughout the experiment.
Experiment 2: (For question 2)
How does the rate of production change in a home made distillatory system? In this experiment we measure and record the production of distilled water in each minute starting the moment we start the heat.
Prepare your distillation system and allow the distillate enter a plastic cup. On the top of every minute replace the cup with an empty cup. Number the cups you remove as soon as you remove them. Use a pipette or a graduated cylinder to measure the amount of distilled water in each cup. Record your results in a data table like this:
| Minute | Amount of distillate in milliliters |
| 1 | |
| 2 | |
| 3 | |
| 4 | |
| 5 | |
| 6 | |
| 7 | |
Does your distillatory apparatus have a constant rate of production? If not how does it change? Can you explain the changes?
Materials and Equipment:
Chemicals: crushed ice solution to be distilled–cranberry or apple juice, coke, orange soda, or colored aqueous solution Equipment: empty soda can–Pepsi, 7-Up, etc. 4 to 8-oz clear glass jar with narrow opening at top larger jar or other container to hold jar above 4-In x 12-in piece of aluminum foil Bunsen or alcohol burner ring stand iron rings wire screen Plastic cups (Small) Pipettes or small graduated cylinders
- A wide variety of common household solutions can be distilled in this experiment, including tea, fruit juices and strongly colored sodas.
- Highly colored inorganic chemical solutions (KMnO4, K2Cr2O7, CuSO4, etc.) should be avoided because they will react with the aluminum in the cans.
Results of Experiment (Observation):
Experiments are often done in series. A series of experiments can be done by changing one variable a different amount each time. A series of experiments is made up of separate experimental “runs.” During each run you make a measurement of how much the variable affected the system under study. For each run, a different amount of change in the variable is used. This produces a different amount of response in the system. You measure this response, or record data, in a table for this purpose. This is considered “raw data” since it has not been processed or interpreted yet. When raw data gets processed mathematically, for example, it becomes results.
In your experiment results, note the principles which allow distillation to be used as an effective purification tool (i.e., contaminants must be non-volatile). Be sure to compare the color of the starting material with that of the distillate.
Is your distilled water colorless?
Is your distilled water free of odors?
Is it free of salts?
Summary of Results:
Summarize what happened. This can be in the form of a table of processed numerical data, or graphs. It could also be a written statement of what occurred during experiments.
It is from calculations using recorded data that tables and graphs are made. Studying tables and graphs, we can see trends that tell us how different variables cause our observations. Based on these trends, we can draw conclusions about the system under study. These conclusions help us confirm or deny our original hypothesis. Often, mathematical equations can be made from graphs. These equations allow us to predict how a change will affect the system without the need to do additional experiments. Advanced levels of experimental science rely heavily on graphical and mathematical analysis of data. At this level, science becomes even more interesting and powerful.

Conclusion:
Using the trends in your experimental data and your experimental observations, try to answer your original questions. Is your hypothesis correct? Now is the time to pull together what happened, and assess the experiments you did.
Related Questions & Answers:
What you have learned may allow you to answer other questions. Many questions are related. Several new questions may have occurred to you while doing experiments. You may now be able to understand or verify things that you discovered when gathering information for the project. Questions lead to more questions, which lead to additional hypothesis that need to be tested.
What would happen if the contaminants in water were volatile such as alcohol or acetic acid?
Possible Errors:
If you did not observe anything different than what happened with your control, the variable you changed may not affect the system you are investigating. If you did not observe a consistent, reproducible trend in your series of experimental runs there may be experimental errors affecting your results. The first thing to check is how you are making your measurements. Is the measurement method questionable or unreliable? Maybe you are reading a scale incorrectly, or maybe the measuring instrument is working erratically.
If you determine that experimental errors are influencing your results, carefully rethink the design of your experiments. Review each step of the procedure to find sources of potential errors. If possible, have a scientist review the procedure with you. Sometimes the designer of an experiment can miss the obvious.
For better sealing of the condenser tube, use one of the following procedures. The aluminum foil at the mouth of the can may be sealed with masking tape. Alternately, the condenser tube can be fitted carefully into corks or stoppers at the mouths of the can and the collection bottle; however, the system should not be completely sealed.
It is possible that a large portion of heat is being wasted. By using insulating material, you may probably reduce the rate of fuel consumption.
References:
Holtzclaw, H.F., Jr., Robinson, W.R., and Nebergall, W.R., College Chemistry with Qualitative Analysis, D.C. Heath and Company, Lexington, MA, 1984, p. 285. This work describes the theory of distillation. A similar discussion could be found in any college-level chemistry text.
Need Chemicals?
Attention Chemists, Schools, & Colleges ChemicalStore.com offers a large selection of chemicals for research and education at affordable price and convenience of online ordering. Visit ChemicalStore.com today.
It is always important for students, parents and teachers to know a good source for science related equipment and supplies they need for their science activities. Please note that many online stores for science supplies are managed by MiniScience.
Testimonials
" I called School Time and my husband and son came with me for the tour. We felt the magic immediately."
- Robby Robinson
" My husband and son came with me for the tour. We felt the magic immediately."
- Zoe Ranson
Contact Info
Our address, working hours.
Week Days: 07:00-19:00
Saturday: 09:00-15:00
Sunday: Closed
Science Project
Top Ten Projects
- Candle Race
- Home-Made Glue #1
- Soil Erosion
- Volcanic Gas
- Accelerate Rusting
- Vibrating Coin
- Mentos Soda Volcano
- Musical Bottles
- Human Battery Power
Latest Projects
- Sweet Erosion
- Your Planetary Age
- Exploding Ziploc
- Dehydrated Potato
- Homemade Windmill
Want to contribute?
Distillation of water.
To convert impure water into chemically pure water by distillation
Additional information
Distilled water finds its use in a wide range of applications where the natural dissolved salts that water normally contains are not desirable. Some of these are topping up lead acid batteries, preparing aseptic solutions in hospitals, automotive cooling systems, steam irons, etc. Distilled water is not however, considered to be suitable for human consumption on a regular basis simply because it lacks the natural beneficial minerals that ordinary drinking water contains. Besides, its bland taste is not very pleasant to the taste buds!
Sponsored Links
Required materials.
- Impure (muddy) water
- Distilling flask with thermometer
- Liebig’s condenser with stand
- Rubber cork / tubing
- Bunsen burner
- Tripod stand
- Stand with clamp
- Basin filled with sand
Estimated Experiment Time
Approximately 2 hours
Step-By-Step Procedure
- 1. Pour the muddy water into the distilling flask.
- 2. Use the stand to hold the flask in place, supported by the tripod stand.
- 3. Place the burner below this.
- 4. Connect the pout of the distilling flask to one end of the Liebig’s condenser.
- 5. Position the Liebig’s condenser using its stand so that it slopes downward slightly; its pout (other end) must open directly above the beaker.
- 6. Bring the muddy water to a boil and collect the condensed liquid for observation.
- Place the distilling flask in a sand basin before heating it – this will prevent vigorous boiling and damage thereof to the apparatus.
- Use the thermometer to monitor the temperature of the boiling liquid.
- The Liebig’s condenser is an integral part of the simple distillation process – it consists of two concentric layers of glass of which the outer layer has air vents that facilitate the cooling of the inner glass tube. This in turn allows condensation of vapors to take place within it.
Observation
The condensed liquid that gets collected in the beaker is clear as well as tasteless and odorless.
Pure and clear water can be obtained from an impure solution by simple distillation. In this process, the impure solution is heated so as to turn it into water vapor; this is later condensed in a Liebig’s condenser to form pure water. Hence distillation is one of the most complete methods of purifying water.
Take a moment to visit our table of Periodic Elements page where you can get an in-depth view of all the elements, complete with the industry first side-by-side element comparisons!
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||





















IMAGES
COMMENTS
Investigate the separation of water from a coloured solution using this simple distillation video, including a step-by-step method, animation and suggested alternative method Chapter titles: 00:10 Introduction to distillation; 01:07 Carrying out the experiment; 03:44 Animation; 04:10 Alternative method: Quickfit apparatus; 04:52 Testing the ...
In this experiment students will learn about the filtration process of distillation. This experiment is designed for my students to do at home with as little materials as possible. There are some much better water distilling Instructables available, such asthis one created by erbst. This instructbable is intended to be as simple as possible.
Rounded lid for the pot. Metal or glass bowl that floats inside the pot. Ice cubes. Fill the large pot partially full of the impure water. Float the collection bowl on the water. The goal is to drip water from the inverted lid into this bowl, so make sure the bowl is large enough to catch the drips. Place the pot lid upside down on the pot.
We just learned two separation techniques, so let's learn one more! Distillation separates compounds by virtue of their differing boiling points. If two liqu...
The rate of distillation is the amount of distilled water produced per hour.Constants are heat source, the amount of test liquids, the type and the size of distillation apparatus. (So all experiments must be performed with the same or identical distillation devices).
Adult supervision. Procedure. Start by adding some saltwater to your large bowl. Mix 2 tablespoons of salt per cup of water. There should be about 2 inches of saltwater in your bowl, or enough to allow the cup to float on top of it. Set your bowl in a sunny spot, either outside or near a window, where it can stay for several hours undisturbed.
By itself, salt water is harmful to humans, but using a process known as distillation, salt water can become drinkable! In this experiment you will convert salt water into fresh water using distillation, which involves boiling a salt solution so that the water of the solution is turned into water vapor or water gas.
What is a Simple Distillation Experiment? Simple distillation separates components from their liquid mixtures based on their boiling point differences. In this method, the mixture to be separated is heated and then cooled using a water condenser. The condensed vapours are then collected from the outlet of the condenser tube. In this way, different components are collected at different boiling ...
Distillation and reflux are techniques used in multiple experiments, but the similarity of set-up can lead to students confusing the two. Distillation is a separation technique that students first encounter in simple experiments such as the separation of brine into salt and water. It can also be used to remove a solvent from a reaction product ...
Extra: Try to do this experiment again with household vinegar. Vinegar is a mixture of about 4-6% acetic acid and water. Can you separate these two liquids by distillation? How does your distillate taste in this case? Extra: You might know that the boiling temperature of pure water is 100ºC (212ºF) at normal atmospheric pressure. Adding a ...
A series of experiments that includes a control is called a "controlled experiment." Experiment 1: (For question 1) Introduction: This experiment is particularly appropriate for middle school science classes or for a general or first-year course where scientific glassware is unavailable. A simple distillation is performed using a soda can ...
This video discusses how the process of distillation can be used to separate the components of a salt water solution.#mrpauller#ScienceExperiments
Specified Practical Work: SP1B - Separation of liquids by distillation, eg ethanol from water, and by paper chromatography: Developing practical skills. ... A simple experiment looking at the three phases of water is a suitable activity, and can reinforce the difference between evaporation and boiling.
EXPERIMENT 7 - Distillation - Separation of a Mixture. Purpose: a) To purify a compound by separating it from a non-volatile or less-volatile material. ... Fig. 3 - The apparatus used in simple distillation Fig. 4 - The apparatus used in fractional distillation. out in water water. out in water water. Not all mixtures of liquids obey Raoult ...
K12 White label Content : https://www.k12mojo.comFree educational content videos for K-12Watch our Educational contents - Distillation is a process of separ...
1. Pour the muddy water into the distilling flask. 2. Use the stand to hold the flask in place, supported by the tripod stand. 3. Place the burner below this. 4. Connect the pout of the distilling flask to one end of the Liebig's condenser. 5.
Pour an extra cup of colored fruit juice in the bottom of the pot. (Your small ceramic plate or ceramic coffee cup will now be standing in the juice.) Together with your adult helper, turn on the ...
The liquid water is then collected at the lower end of the condenser. The non-volatile salt remains in the flask. In this experiment, the initial mixture you distill contains two volatile liquids: ethanol and water. In this distillation, both of the liquids will evaporate from the boiling solution. Ethanol and water have normal boiling ...
distillation, process involving the conversion of a liquid into vapour that is subsequently condensed back to liquid form. It is exemplified at its simplest when steam from a kettle becomes deposited as drops of distilled water on a cold surface. Distillation is used to separate liquids from nonvolatile solids, as in the separation of alcoholic ...
The apparatus required for evaporating and condensing water from copper (II) sulfate solution. Set up a Bunsen burner on the base of a stand placed on a heat resistant mat. Place a tripod and gauze above the burner. Clamp a flask and a test tube as shown in the diagram. Collect 20 cm 3 of copper sulfate solution and place it in the flask.
Hydrogen energy, as a clean, renewable, and high-calorific energy carrier, has garnered significant attention globally. Among various hydrogen production methods, the thermochemical iodine-sulfur (I-S) cycle is considered the most promising due to its high efficiency and adaptability for large-scale industrial applications. This study focuses on the distillation characteristics of the HIx ...