

Self Inflating Balloon: Baking Soda and Vinegar Balloon Experiment
This post may contain affiliate links.
Have you ever tried the baking soda and vinegar balloon experiment? This classic science experiment is really one of my favorites. It is an easy science experiment to do and it really is exciting to watch. It creates a self-inflating balloon that kids think is the coolest!
The reaction between the baking soda and vinegar cause the balloon to inflate all on its own! It is a show-stopper experiment for kids.
(See more of my STEM projects for kids.)

How to Do the Baking Soda and Vinegar Balloon Experiment:
Supplies you will need for this simple science experiment:.

Plastic empty water bottle or soda bottles, cleaned out Large Balloon White Vinegar (acetic acid) Baking Soda (or sodium bicarbonate) Small Funnel Spoon
TIP: Before starting the experiment, you will want to stretch out the balloon to make it more loose and easier to inflate.
Step 1- Pour 1-2 spoonfuls of baking soda into the opening of the balloon, using a funnel. You’ll need to shake it a bit to get it down into the base of the balloon.
Step 2- Use the funnel again and pour some vinegar into the empty plastic bottle until it is about an inch or two deep- maybe a few tablespoons of vinegar. Exact amounts do not matter. Add a few drops of food coloring to change it up if you want- totally unnecessary.

Step 3- Carefully stretch the opening of the balloon around the mouth of the bottle leaving it hanging down until you are ready for the reaction.
TIP: Don’t let any of the baking soda dump into the bottom of the bottle while attaching it.
Step 4- When you are ready to see the chemical reaction happen, lift up the balloon allowing the baking soda to fall down into the bottle.

This is when the fun starts! Baking soda and vinegar mix to create an awesome chemical reaction. The gas from combining the two will escape as bubbles of carbon dioxide gas that cause the balloon to inflate. It’s impressive. The more gas there is created, the larger the balloon will get.
Your kids, if they are anything like mine, will beg to do the experiment again, then 10 more times! This is really a perfect science project for kids to try on their own.

If you do repeat it, you will need fresh vinegar in the bottle. Once a reaction happens, it is not quite so strong the second time through. The balloon does not usually inflate again unless the vinegar is fresh.My kids were amazed and wanted to do it again and again and again. Stock up on baking soda and vinegar if you are planning this one! Luckily they are both quite inexpensive.
(It’s a good thing they are both so cheap!)
The Science Behind It: Why the Baking Soda and Vinegar Reaction Works?
When the baking soda and vinegar reaction happens, it is an acid-base reaction. Vinegar is the acid and baking soda is the base. This reaction between the two causes a gas called carbon dioxide to bubble and foam. This gas having nowhere else to go, expands the balloon making the self-inflating balloon happen.
Here is the chemical equation behind it: Baking soda + vinegar — yields carbon dioxide + water + sodium ion + acetate ion There is more to it than that, but that’s the basic explanation.
Try some variations to see if other reactions work:
Will baking powder work instead of baking soda?
Would lemon juice work instead of vinegar?
Could you do the same thing with an alka-seltzer tablet and soft drinks?
Use the scientific method to investigate different variations on this experiment to see how they work.
Want More Baking Soda and Vinegar Experiments?
We love the carbon dioxide reactions that these two substances create.
Check out these other ones we have done:
Easy Bottle Rocket Experiment
Bathtub Bottle Rocket
Film Canister Rocket Experiment
How to Make a Volcano experiment! (This one is fun because when the eruption occurs, the carbon dioxide bubbles pour over like lava!
Former school teacher turned homeschool mom of 4 kids. Loves creating awesome hands-on creative learning ideas to make learning engaging and memorable for all kids!
Similar Posts

Non-Toxic Static Slime

Compound Words Foldable and Puzzles
Letter of the Week: Preschool Letter G Activities

Water Striders: Insect Science

Firefly Craft: Sewn Circuits
Children’s music by mr. roberelli (giveaway).
What a great idea! I can’t believe I haven’t heard of this experiment. My girls are 12 and 11 and still love doing at home science projects. Although they use Time4Learning science curriculum it is always fun to do your own.
Thanks for the idea!
Awesome! I’m making a list of simple, fun experiments to do this summer, and I’m adding this one to it! We don’t seem to get to these types of experiments during the regular school year! Stopping by from HHH and new follower! Thanks for sharing your experiment!
My boys loved this experiment too. 🙂
- Pingback: Fizzing & Bubbling Science Experiments - Teach Beside Me
I do experiments with 4 year old grandson, he loves this one. We did the volcano as well and now he explains what happens to everyone he wants to show it too. Thank you for sharing, it is fun teaching when the things work as well as yours.
fantastic from a grammy
Nice , it is possible to send easy experiments for kids with the help of video
Leave a Reply Cancel reply
You must be logged in to post a comment.
Cool Science Experiments Headquarters
Making Science Fun, Easy to Teach and Exciting to Learn!
Science Experiments
Balloon Blow-up Science Experiment
Can you blow up a balloon without using your mouth? In this simple science experiment, we’re going to show you how to do it with only a few everyday items you probably already have in your home. It makes a great experiment for young children because the set-up is simple and it only takes a few minutes to get to the exciting finale.
In addition to a video demonstration and detailed printable instructions, we also have the scientific explanation of how this simple chemical reaction works making it perfect for older scientists too.

JUMP TO SECTION: Instructions | Video Tutorial | How it Works
Supplies Needed
- Small Soda Bottle
- Baking Soda
Balloon Blow-up Science Lab Kit – Only $5

Use our easy Balloon Blow-up Science Lab Kit to grab your students’ attention without the stress of planning!
It’s everything you need to make science easy for teachers and fun for students — using inexpensive materials you probably already have in your storage closet!
Balloon Blow Up Science Experiment Instructions
Step 1 – Start with some questions: How do you blow up a balloon? What if I told you that you couldn’t blow air into it, do you think you could still inflate (blow-up) the balloon? Then observe the supplies for the experiments. Do you think they can be use to blow up the balloon? If so how? Write down your hypothesis (prediction).

Step 2 – Using a funnel, pour about a third of a cup of vinegar into the bottle. We used Apple Cider Vinegar, but any type of vinegar will work.

Step 3 – Then insert another funnel into the mouth of the balloon. We recommend using two different funnels. One funnel for filling the bottle with vinegar and one for the balloon. However, you can do the experiment with only one funnel. Just make sure you completely wash and dry the funnel after you add the vinegar and before you put it into the balloon. This is very important.

Step 4 – Place two teaspoons of baking soda into the funnel so it falls into the balloon. When the balloon is filled with the baking soda, carefully remove it from the funnel.

Step 5 – Next, secure the mouth of the balloon over the mouth of the bottle. Take your time doing this and don’t let any of the baking soda fall out of the balloon and into the bottom of the bottle. Take a moment to make some observations. What will happen if we lift up the balloon? Write down your hypothesis (prediction) and then test to see if you were right!

Step 6 – While holding the bottle, lift the end of the balloon and allow the baking soda to drop into the bottle.

Step 7 – What happens to the balloon? Was your hypothesis correct? Wondering what caused the balloon to inflate? Find out the answer in the how does this experiment work section below.
Video Tutorial
How Does the Science Experiment Work?
When baking soda (a base) and vinegar (an acid) are mixed together they create a chemical reaction that results in the formation of carbon dioxide gas. Gases do not have a specific shape or volume, rather they expand rapidly filling their container. Gases expand rapidly because their particles move at high speeds in all directions. As the carbon dioxide gas fills the bottle, it has nowhere else to go so it begins to fill the balloon. As the carbon dioxide gas fills the balloon, the balloon inflates. The more gas that is created, the larger the balloon will inflate.
The baking soda and vinegar chemical reaction will continue to inflate the balloon as long as there is still baking soda and vinegar to react. Once the reaction between baking soda and vinegar has stopped, the balloon will slowly begin to deflate.
An acid is a substance that tastes bitter, reacts with metals and carbonates, and turns blue litmus paper red. A base is a substance that tastes bitter, feels slippery, and turns red litmus paper blue.
Other Ideas to Try
Does changing the amount of baking soda and vinegar change the size of the balloon when it inflates? What would happen if you used another acid like lemon juice instead of the vinegar? Would it react the same with the baking soda?
I hope you enjoyed the experiment. Here are some printable instructions:

Instructions
- Using a funnel, pour about a third of a cup of vinegar into the bottle. Tip: I used Apple Cider Vinegar, but any kind of vinegar will work.
- Then insert another funnel into the mouth of the balloon. Tip: It is best to have two funnels, one for filling the bottle with vinegar and one for the balloon. If you only have one funnel, it is important that you completely wash and dry the funnel after you add the vinegar and before you put it into the balloon.
- Place two teaspoons of baking soda into the funnel so it falls into the balloon. Then remove the balloon from the funnel.
- Next, secure the the mouth of the balloon over the top of the bottle. Tip: Don’t let any of the baking soda drop into the bottle…yet!
- While holding the bottle, lift the end of the balloon allowing the baking soda to drop into the bottle.
- Watch in amazement as the balloon magically inflates!

Reader Interactions
November 2, 2017 at 11:00 am
Yeah but don’t just eyeball the measurements of things because if you use to much baking soda it will make the baloon spring a leak and all sorts of stuff will fly out and make a big mess.
I speak form experience
Seriously, don’t do this
April 21, 2018 at 10:26 am
I did this experiment and it is perfect!
You need to hold properly the bottle when you mix the baking soda into vinegar.
May 22, 2019 at 8:57 am
We’re doing science experiments at school and this one is brilliant! I loved it a lot.
June 22, 2020 at 11:15 am
I love this experiment! My balloon grew 6 inches!
June 19, 2023 at 11:17 pm
I tried and it worked well – Exited to do such experiment
Leave a Reply Cancel reply
Your email address will not be published. Required fields are marked *
Save my name, email, and website in this browser for the next time I comment.

- Privacy Policy
- Disclosure Policy
Copyright © 2024 · Cool Science Experiments HQ
Get Your ALL ACCESS Shop Pass here →

Baking Soda and Vinegar Balloon Experiment
Combine a fizzing baking soda and vinegar reaction with balloon play with this easy-to-set-up balloon science experiment for kids . Find out how to blow up a balloon with baking soda and vinegar. Grab a few simple ingredients from the kitchen, and you have fantastic chemistry for kids.

BAKING SODA AND VINEGAR BALLOON EXPERIMENT
Don’t have vinegar for this experiment? Try a citric acid like lemon juice, and check out our citric acid and baking soda experiment here.
- Baking Soda
- Empty Water Bottles
- Measuring Spoons
- Funnel {optional but helpful)

BLOW-UP BALLOON EXPERIMENT SETUP:
Step 1. Blow up the balloon a bit to stretch it out some, and use the funnel and teaspoon to add baking soda to the balloon. We started with two teaspoons and added a teaspoon for each balloon.
Step 2. Fill the container with vinegar halfway.
Step 3. When your balloons are all made up, attach them to the containers making sure you have a good seal!
Step 4. Next, lift up the balloon to dump the baking soda into the container of vinegar. Watch your balloon blow up!
To get the most gas out of it, we swirled around the container to get it all going!

Optional Art: Go ahead and use a sharpie to draw emojis, shapes, or fun pictures on your balloons before filling them with baking soda.
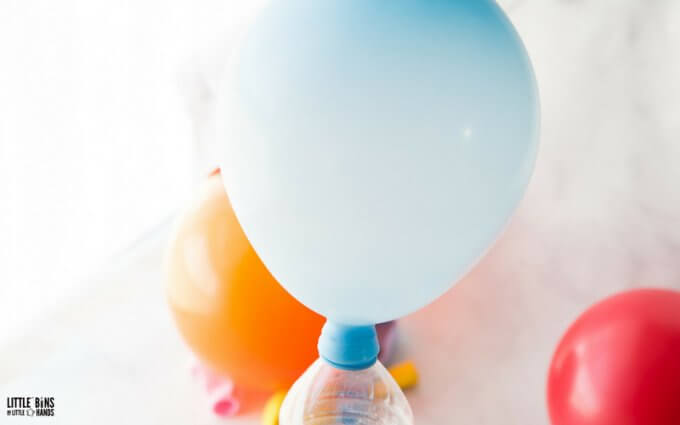
BALLOON EXPERIMENT TIPS
My son suggested we try different amounts of baking soda in our experiment to see what would happen. Also, will the balloon size grow bigger if more vinegar is in the bottle?
Always encourage your kids to ask questions and wonder what will happen if…
This is also a great way to encourage inquiry, observation, and critical thinking skills. You can read more about teaching the scientific method to kids here.
Make predictions! Ask questions! Share observations!

Be cautious with the amount of baking soda you add, as the reaction will get bigger each time. Safety goggles are always great for young scientists!
You could see the difference in the baking soda we put in the balloons! The red balloon with the least baking soda inflated the least. The blue balloon with the most inflated the most.
What else can you do with baking soda? Check out these unique baking soda experiments!
HOW DOES THE BALLOON EXPERIMENT WORK?
The science behind this baking soda and vinegar balloon science experiment is a chemical reaction between an acid and base . The base is the baking soda and the acid is vinegar. When the two ingredients mix, the balloon baking soda experiment gets its lift!
That lift is gas, carbon dioxide, or CO2. As the gas tries to leave the plastic container, it goes up into the balloon because of the tight seal you have created. Check out states of matter experiments !
The gas has nowhere to go and is pushing against the balloon it blows it up. Similar to how we exhale carbon dioxide when we blow up balloons ourselves.
We love exploring simple chemistry you can do at home or in the classroom. Science that isn’t too crazy but is still lots of fun for kids! You can check out more cool chemistry experiments .
Read more about the science behind baking soda and vinegar experiments .
WHAT IS THE SCIENTIFIC METHOD FOR KIDS?
The scientific method is a process or method of research. A problem is identified, information about the problem is gathered, a hypothesis or question is formulated from the information, and the hypothesis is tested with an experiment to prove or disprove its validity. Sounds heavy…
What in the world does that mean?!? The scientific method should be used as a guide to help lead the process. It’s not set in stone.
You don’t need to try and solve the world’s biggest science questions! The scientific method is all about studying and learning things right around you.
As kids develop practices that involve creating, gathering data evaluating, analyzing, and communicating, they can apply these critical thinking skills to any situation.
Learn more about the scientific method and how to use it.
Even though the scientific method feels like it is just for big kids…
This method can be used with kids of all ages! Have a casual conversation with younger kiddos or do a more formal notebook entry with older kiddos!
Click here to get your FREE Science Challenge Calendar

MORE SCIENCE EXPERIMENTS WITH BALLOONS
Have leftover balloons? Why not try one of these fun and easy balloon science experiments below!
- Explore physics with a balloon rocket
- Try this screaming balloon experiment
- Make a balloon-powered car
- Try a pop rocks and soda balloon experiment
- Learn about static electricity with a balloon and cornstarch experiment
- Bend water with a balloon.

Helpful Science Resources To Get You Started
Here are a few resources that will help you introduce science more effectively to your kiddos or students and feel confident yourself when presenting materials. You’ll find helpful free printables throughout.
- Best Science Practices (as it relates to the scientific method)
- Science Vocabulary
- 8 Science Books for Kids
- All About Scientists
- Free Science Worksheets
- Science Supplies List
- Science Tools for Kids
- Scientific Method for Kids
- Easy Science Fair Projects
- Citizen Science Guide
- Join us in the Club
Printable Science Projects For Kids
If you’re looking to grab all of our printable science projects in one convenient place plus exclusive worksheets and bonuses like a STEAM Project pack, our Science Project Pack is what you need! Over 300+ Pages!
- 90+ classic science activities with journal pages, supply lists, set up and process, and science information. NEW! Activity-specific observation pages!
- Best science practices posters and our original science method process folders for extra alternatives!
- Be a Collector activities pack introduces kids to the world of making collections through the eyes of a scientist. What will they collect first?
- Know the Words Science vocabulary pack includes flashcards, crosswords, and word searches that illuminate keywords in the experiments!
- My science journal writing prompts explore what it means to be a scientist!!
- Bonus STEAM Project Pack: Art meets science with doable projects!
- Bonus Quick Grab Packs for Biology, Earth Science, Chemistry, and Physics

42 Comments
Need more info on experiments. Thanks, Miranda
What information would you like?
thanks a lot very funny experiment
Your welcome!
(I was thinking that the pint bottle was going to blow up I got really scared first time I saw a science magic) but I can make smoke come out of my mouth it is very simple
I’m doing a Science Fair Project on this, but I don’t know and how to do the table and graphs, like the data and stuff. Can you help me?
And it’s due May 18, 2016 🙁
this is cool thanks you verry much
Your welcome! Try drawing on the balloons too!
Does the size of the container or size of balloon have any affect on how the balloon will blow up?
Yes, it will because of the space the gas has to fill once the baking soda and vinegar are combined. Great experiment to try different sizes using the same amounts of both vinegar and baking soda.
my team did the balloon inflating thing and it was fun
Is it safe for kids to do this experiment in school
I would think it would be as it is just baking soda and vinegar. You would need to use your best judgement of course. We have never had a balloon explode.
hi this is STEM project . can anyone explain how to connect – T technology E Engineering M mathematics through this experiment . thanks in advance
I will look into my information. Remember a STEM project does not need to contain each of the 4 pillars of STEM but at least two. I can tell you we used math {measuring} and science {chemical reaction}.
Definitely is cool
i love yo stuff
- Pingback: Tutors Only: Week 16 Science Experiment – ccricelake
If we wanted to use this for a science fair project what would the Question asking be?
How much baking soda/vinegar is needed to inflate balloon completely. Or, which acid is better vinegar or lemon juice? Do different shape balloons fill better?
We just did this experiment, but we only used one balloon. My kids are 2.5, 4 and 7 so we have a range of ability levels, but I wanted to add my kids’ favorite part! We took the balloon off the bottle and tied it shut, careful not to lose the gas. And then I blew a balloon up the same size, I asked them which one they thought would hit the ground first as I held them even in the air. Try it out!!
That’s awesome! We will def have to try that. What a great idea!
Where did you find your containers to hold the baking soda and vinegar?
- Pingback: Fun With Balloons
- Pingback: Grow Sugar Crystals for Edible Rock Candy Chemistry Experiment
- Pingback: Summer Science Camp for Young Scientists : 5 Days of Fun!
- Pingback: Exploding Science Experiments for Kids | My Home Based Life
- Pingback: Discrepant Event | Colleen Boyds teacher e-portfolio
- Pingback: 100+ STEM Projects for Kids (With Free Cheat Sheets)
- Pingback: Insanely Rad Pre K Science Projects for Your Curious Little Einsteins - MyVyllage
- Pingback: 30 Incredible Chemistry Experiments - 123 Homeschool 4 Me
- Pingback: Rainbow Science Experiments and STEM Ideas (St. Patricks Day)
- Pingback: 50 Simple Science Experiments with Supplies You Already Have
- Pingback: Science Experiments for Preschoolers - Round Rock Teravista
- Pingback: Science Experiments for Preschoolers - Sienna Plantation
- Pingback: M&M Candy Experiment For Kids | Little Bins for Little Hands
- Pingback: The BEST Very Simple Science Experiments for Kids to Try Anywhere
- Pingback: Kitchen Science Experiments and Activities for Kids
- Pingback: 60 Very Simple Science Experiments Your Kids Will Love
- Pingback: 25 Must Try Science Experiments For Kids | Little Bins for Little Hands
- Pingback: Bump Club And Beyond
Comments are closed.

Subscribe to receive a free 5-Day STEM Challenge Guide
~ projects to try now ~.

- Grades 6-12
- School Leaders
Have you seen our latest free teacher workshop?
Baking Soda and Vinegar Balloon Experiment (Plus Free Worksheet)
It’s like magic!

Kids and balloons go hand-in-hand so why not try a fun science experiment that incorporates a balloon or two? This experiment requires little more than what you already have in your kitchen cabinet. Grab a dash of baking soda, a splash of vinegar, and learn all about acids, bases, states of matter, and chemical reactions! Everyone will be amazed watching a balloon inflate without a single breath being blown.
Fill out the form on this page to grab your free printable recording sheet , and try the baking soda and vinegar balloon experiment with your little scientists!
How does the baking soda and vinegar balloon experiment work?
The baking soda and vinegar balloon experiment demonstrates a chemical reaction between an acid and a base. The baking soda acts as the base and the vinegar as the acid. When the two combine, carbon dioxide (CO2) escapes the container and causes the balloon to blow up.
What does this experiment teach?
This experiment teaches how different states of matter transform when combined. In this case, a solid (baking soda) and a liquid (vinegar) mix to produce a gas (CO2). Since carbon dioxide is the same gas that is released when humans breathe out, students will make the connection between human breath blowing up a balloon and the reaction of this experiment doing the same.
Is there a baking soda and vinegar balloon video?
This video shows how to do the baking soda and vinegar balloon experiment, using just a few ingredients.
Materials Needed
To do the baking soda and vinegar balloon experiment, you will need:
- Approximately 1/2 cup of vinegar
- Empty water bottle or similar container
- Baking soda
- Measuring spoon
- Deflated balloon
Our free recording sheet is also helpful— fill out the form on this page to get it.
Baking soda and balloon experiment steps:
1. blow up a balloon just enough to stretch it out a bit. then, use the funnel and measuring spoon to add about a teaspoon of baking soda inside the balloon..

2. Fill the water bottle or other container about halfway with vinegar.

3. Attach your filled balloon to the container with the vinegar. Make sure the seal is tight!

4. Once you’re all set up, hold the balloon up so the baking soda gets released into the vinegar.

5. Finally, watch the balloon blow up!

Grab our baking soda and vinegar balloon experiment worksheet!
Click the button below to get your worksheet. The worksheet asks kids to guess the correct order of the steps in the experiment. Next, kids must make a prediction about what they think will happen. They can use the provided spaces to draw what happens before and after they add the baking soda to the vinegar. Did their predictions come true?
Additional Reflection Questions
- What happened when the baking soda was added to the vinegar?
- Why do you think the balloon inflated?
- Why do you think the balloon eventually stops blowing up?
- What do you think would happen if we used more or less baking soda?
Can this experiment be done for a science fair?
Yes! If you want to do the baking soda and vinegar balloon experiment for a science fair, we recommend switching up some of the variables. For example: Does the amount of vinegar matter? What if you run two experiments side by side with different amounts of baking soda? Which balloon filled up faster? Form a hypothesis about how changing the variables will impact the experiment. Good luck!
Looking for more experiment ideas? Check out our big list of science experiments.
Plus, be sure to subscribe to our newsletters for more articles like this., you might also like.

Mentos and Coke Experiment: How-To Plus Free Worksheet
This explosive experiment teaches kids about physical reactions. Continue Reading
Copyright © 2024. All rights reserved. 5335 Gate Parkway, Jacksonville, FL 32256
Open today from 9:00AM - 4:00PM
- buy tickets

Balloon Blow-Up Science Experiment
- Written by Children's Museum Team
- Posted on Monday April 13, 2020

Blow-Up a Balloon Using Science! Ages 3+
Using items easily found at home, you can blow up a balloon without using your mouth or your own breath! This isn’t magic; it’s science!
This experiment demonstrates how states of matter can change – mixing a solid with a liquid to create gas! The science behind this balloon baking soda experiment is the chemical reaction between the base – baking soda – and the acid – vinegar. When the two ingredients mix together the balloon baking soda experiment gets its lift! The gas produced from the two ingredients is carbon dioxide or CO2.
Carbon dioxide is the same gas that is produced by the human lungs and is a biproduct of our respiratory system. We breathe in oxygen and breathe out carbon dioxide.
Vocabulary:
- Extra Credit Word: Exothermic (absorbs heat, so it feels cold)
Balloon Blow-Up Experiment Materials:
- Empty 12-16 oz soda bottle (or any bottle about that size with a small neck)
- Baking Soda
- Small funnel
- Small measuring cup
Balloon Blow-Up Experiment Directions:
- Have your children scoop the baking soda into the balloon using the funnel
- Help your children put the vinegar into the flask using a pipette or small measuring cup
- Next, attach the balloon to the top of the flask; make sure not to pour the baking soda into the vinegar!
- Ask your children what might happen, and why.
- Count to 3 and everyone holds up their balloon so the baking soda falls into the vinegar, creating a chemical reaction and blowing up their balloon.
- Let the kids know what will happen scientifically SCIENCE: When baking soda and vinegar are mixed together, it creates a gas called carbon dioxide. The gas begins to expand in the bottle and starts to inflate the balloon. The more gas that is created, the larger the balloon will inflate.
- Follow up experiment: ask your children if they think blowing a balloon up using their breath is faster or slower than with baking soda and vinegar. Why? Test it out!
Check out these other STEAM activities that are sure to engage, entertain, and educate!
Upcoming Events

Nature Explorers

Geared toward children 4 years and up, this hands-on program lets little ones experience and explore nature around the Museum with a California Naturalist.

Creation Station

This open studio time allows children of all ages to create open-ended art using all recycled and repurposed materials! Our Art Studio Specialist is there to facilitate the use of…

Big Thinkers

Join Nick and the Museum team for Big Thinkers, a special program designed to engage older children. Engage in unique experiences and experiments using scientific concepts, physics, engineering, and art through science.
(Geared toward children ages 5+)

Newsletter Signup
- First Name *
- Last Name *
- Email Address *

Learn about Reactions with This Vinegar and Baking Soda Experiment
Take a peek in your pantry. Do you have baking soda and vinegar? If so, you and your kids have the basic supplies for a bubbly science experiment!
These two products are staples in many households because they are essential cooking ingredients. , Baking soda helps baked goods rise, and a pinch or two will balance the acidity in dishes like tomato soup. Vinegar is a common ingredient in salad dressings and sauces, and a splash will elevate any dish in need of a bright, tangy flavor.
But can you mix vinegar and baking soda together? You sure can—and the result is a wonderful at-home science experiment. Vinegar and baking soda create a very effective cleaner for dishwashers, washing machines, clogged sinks, and even tile grout because of the chemical reaction that occurs when the two substances interact. You can harness this same reaction for an exciting chemistry activity!
The Science behind the Reaction
A baking soda and vinegar reaction is simple and safe for budding scientists of all ages. Older children may be curious about why the reaction occurs—here’s the rundown:
When combined, baking soda and vinegar undergo an acid-base reaction. Acids and bases are aqueous solutions (meaning they’re substances dissolved in water) that exist at opposite ends of the pH (potential of hydrogen) scale, which spans from 0 to 14. Acids measure between 0 and 7 and bases measure between 7 and 14, while a pH of 7 indicates a neutral substance. In simple terms, bases have the potential to gain hydrogen ions (an ion is a type of atom, one of the tiny building blocks that make up all matter), while acids have the potential to donate hydrogen ions to another substance.
Baking soda, or sodium bicarbonate, has a pH level of 9, making it a base. Vinegar, which is acetic acid dissolved in water, has a pH level of 2–3, making it an acid. , Baking soda is made up of sodium, hydrogen, carbon, and oxygen atoms. Vinegar is made up of hydrogen, carbon, and oxygen atoms.
When you mix baking soda and vinegar together, two hydrogen atoms move from the vinegar to the baking soda to create a salt called sodium acetate. The remaining atoms create a new acid—but it breaks down quickly into water and carbon dioxide gas.

It’s Time for an Experiment
Your kids can witness an acid-base chemical reaction right in your kitchen. This baking soda experiment for kids combines vinegar and baking soda, then uses the resulting carbon dioxide gas to inflate a balloon. This same reaction is behind science projects like fizzy potions and DIY volcanoes .
Children of all ages can participate in every step of this experiment, but younger kids may need help from an adult. You know your kid scientists’ abilities best!
Vinegar and Baking Soda Experiment
- ⅓ cup baking soda
- 1 cup white vinegar
- 1 empty plastic water or soda bottle
- 1 uninflated balloon
- 1 funnel
- Use the funnel to fill the uninflated balloon with baking soda. Kids can help pour the baking soda while an adult holds the funnel and balloon.
- Pour the vinegar into the empty plastic bottle. Fit the opening of the balloon over the mouth of the bottle, trying not to spill any baking soda out of the balloon.
- Once the balloon is securely attached to the bottle with no gaps for air to escape, lift the balloon and let the baking soda fall into the vinegar below.
- Watch as the baking soda and vinegar react—the mixture will bubble and fizz. As the reaction takes place, the balloon should begin to expand and fill with carbon dioxide gas!

Using the Scientific Method
The balloon experiment is an excellent way to show your children the wonders of chemistry from the comfort of home. You can build on their learning by teaching them to follow the scientific method. This process is what scientists around the world use to construct and test their hypotheses (what they think will happen in an experiment).
Help your children navigate the following steps:
- Identify a problem or question: Ask your kids, “What do you think will happen to the balloon when we combine vinegar and baking soda?”
- Form a hypothesis : Encourage your children to guess how the vinegar and baking soda will react—and how that reaction will affect the balloon. Help them frame their hypothesis using the “if _____, then _____” format. For example: If the baking soda and vinegar mix, then bubbles will form and the balloon will expand.
- Conduct the experiment : Follow the steps in the experiment above to test their hypotheses.
- Collect and analyze the results: Help your kids monitor how the experiment plays out. Older children can make notes, while young ones may want to take photos or draw pictures of the experiment.
- Provide a conclusion: Ask your kids if their hypotheses were correct. If they were, great! If not, ask them how the experiment differed from what they expected. Remember: Science is all about making wrong guesses and learning from them. Help your young chemists understand that an incorrect hypothesis is just as good as a correct one.
You and your kids can apply the scientific method to all kinds of at-home science experiments. There are plenty of family-friendly activities to grow your children’s interest in chemistry. For example, you could test whether a substance is acidic or basic using cabbage and water , or using carbon dioxide gas to create a miniature “snowstorm.”
If your kids have a passion for experiments, sign them up for a science-based subscription box from Little Passports. The Science Junior box , designed for five-to-eight-year-olds, helps early elementary schoolers explore the wonders of science firsthand. Science Expeditions teaches children ages eight and up about more advanced scientific topics, such as aerodynamics and solar energy. Each month you’ll receive a box full of activities and discoveries to help your budding scientists flourish and grow.
Get Free Activities and Exclusive Offers
Blog categories.
- Craft & DIY
- World Holidays
Watch the Video!
Recent Posts
- Celebrate Your Daughter with This Japanese Girls’ Day Craft
- Enjoy a Fun-Filled Day with 10 Non-Screen Activities for Kids
- Spinning Science: How to Make a Tornado in a Bottle
- These Cute Easter Bunny Crafts Are a Hopping Good Time
- 3 Adorable DIY Gifts for Your Kids’ Favorite Teachers
Choose an Account to Log In

Notifications
Science project, baking soda and vinegar balloon.

By now, you've probably heard of the explosive reaction that occurs when you mix baking soda and vinegar. That's old news. However, do you know what happens when you add a balloon to the mix? Discover more about chemical reactions with this fun and surprising science experiment.
Can you blow up a balloon with the reaction between baking soda and vinegar?
- 2 water bottles
- 8 ounces white vinegar
- 4 tablespoons baking soda
- Permanent marker
- Small funnel
- Mark your two balloons with permanent marker: "1" for room temperature, "2" for ice bath.
- Mark the water bottles the same way: "1" for room temperature, "2" for ice bath.
- Use the small funnel to put 2 tablespoons of baking soda in each balloon.
- Clean the funnel well with running water, then dry it.
- Use the small funnel to put 4 ounces of white vinegar in each water bottle.
- Place Bottle 2 in the bowl of ice.
- After three minutes place the balloon on top of the bottles, being careful not to spill the baking soda into the bottles yet.
- Set your timer.
- Tip both balloons upwards to drop the baking soda into the vinegar.
- Record your results.
Baking soda and vinegar should have been able to blow up the ballon every time. However, you should have observed that the balloon at room temperature blew up significantly faster than the cold balloon.
When vinegar and baking soda mix, they create the gas carbon dioxide and water. The carbon dioxide has no where to go, but into the balloon - blowing it up. But you should have found that the balloon at room temperature may have been slightly faster in blowing up your balloon. This is because the colder temperature causes the reaction to take place more slowly, releasing the gas less effectively.
With science, the learning never stops; you can always change an experiment a little bit and get a completely different result! What if you used hot water instead of an ice bath? What if you used lemon juice instead of vinegar? What if you changed the size of the balloons? Whatever you do, make sure you only make one change at a time and leave all other factors the same...then conduct your experiment and record your results!
Related learning resources
Add to collection, create new collection, new collection, new collection>, sign up to start collecting.
Bookmark this to easily find it later. Then send your curated collection to your children, or put together your own custom lesson plan.
- Renew Membership
Save Your Favorite AACT Resources! ×
Log in or join now to start building your personalized "My Favorites" page. Easily save all the resources you love by logging in and clicking on the star icon next to any resource title.
- AACT member benefits »
- Forgot User Name or Password?
Inflating a Balloon with Chemistry Mark as Favorite (28 Favorites)
DEMONSTRATION in Observations , Chemical Change , Chemical Change , Mixtures , Acids & Bases , Unlocked Resources , Kitchen Chemistry . Last updated September 11, 2020.
In this demonstration, the teacher will perform a reaction between acetic acid (vinegar) and sodium bicarbonate (baking soda) in order to inflate a balloon and to introduce the concept of a chemical reaction to students. Students will observe the reaction, and identify indicators of chemical change as well as discuss the different types of matter that are involved.
Grade Level
Elementary, middle or high school
NGSS Alignment
This demonstration will help prepare your students to meet the performance expectations in the following standards:
- 5-PS1-4: Conduct an investigation to determine whether the mixing of two or more substances results in new substances.
- MS-PS1-2: Analyze and interpret data on the properties of substances before and after the substances interact to determine if a chemical reaction has occurred.
- HS-PS1-2: Construct and revise an explanation for the outcome of a simple chemical reaction based on the outermost electron states of atoms, trends in the periodic table, and knowledge of the patterns of chemical properties.
- Analyzing and Interpreting Data
- Engaging in Argument from Evidence
By the end of this demonstration, students should be able to
- Define chemical reaction .
- Understand the meaning of reactant and product .
- Classify products and reactants by the appropriate state of matter in a reaction.
- Identify indicators of chemical change in a chemical reaction.
Chemistry Topics
This lesson supports students’ understanding of
- Chemical reactions
- Chemical Change
Observations
- States of matter
Teacher Preparation : 10 minutes
Lesson : 30 minutes
For each group
- Baking soda (sodium bicarbonate, NaHCO 3 )—1.5 tsp. or 8.75 grams
- Store bought vinegar (5% acetic acid solution. HC 2 H 3 O 2 )—10 tbsp., or ~150 ml
- Empty plastic water bottle—700 ml or larger
- Food coloring (optional)

- Always wear safety goggles when handling chemicals in the lab.
- Students should wear proper safety gear during chemistry demonstrations. Safety goggles and lab apron are required.
- Students should wash their hands thoroughly before leaving the lab.
Teacher Notes
- The reaction of sodium bicarbonate (baking soda) and acetic acid (vinegar) produces carbon dioxide gas, water and sodium acetate (soluble in water). The carbon dioxide gas can originally be seen as bubbles in the solution, but will quickly be released from the solution. The amount of carbon dioxide gas will exceed the space in the bottle, and will move into the deflated balloon, and will inflate it.
- Chemical reaction : A process where atoms of the reactant(s) will rearrange themselves to create a new arrangement of atoms, called the product(s).
- Reactant : A substance or substances present at the start of the reaction.
- Product : A resulting substance or substances formed by a chemical reaction.
- Chemical Change : Any change that result in the formation of a new substance or substances.
- Indicators of Chemical Change or Chemical Reaction : 1-Formation of gas; 2-Change of color; 3-Formation of a precipitate; 4-Formation of heat and light. Please note that these indicators do not always designate that a chemical change occurred, as there are many instances of these occurring without a resulting chemical change.
- The types of matter identified in chemical reactions are solids, liquids, gases and aqueous solutions. Liquids and aqueous solutions differ in that liquids are pure substances in the liquid form, whereas aqueous is a substance dissolved in water.
- Reactants: Baking Soda – Solid; Vinegar – Aqueous (this may be tricky to some, but by examining the vinegar container you will find that it is 5% acetic acid, and 95% water.)
- Products: Carbon Dioxide – Gas; Water- Liquid; Sodium Acetate – Aqueous (a clear solution will be left in the flask upon completion of the reaction, it is a solution of water and Sodium Acetate)

- Measure 1.5 tsp. or 8.75 grams of baking soda.
- Insert a funnel into the opening of a balloon and add the baking soda to the balloon through the funnel. See image to the right.
- Measure 10 tbsp. or approximately 150ml of vinegar.
- Add the vinegar to the empty bottle.
- If you wish to add food coloring to the vinegar, add 3-5 drops. The food coloring will help students to see the gas formation/bubbles during the demo.
- Secure the balloon around the opening of the bottle, but make sure that the baking soda remains in the balloon at this time. Allow the balloon to hang to the side once it is attached to the bottle until you are ready to complete the demo. See video for reference.
- Lift the balloon, straightening it out over the opening of the flask. This will allow the baking soda to drop out of the balloon and enter the flask.
- Hold the bottle at the base while the reaction occurs.
- The baking soda will quickly react with the vinegar in the flask, creating carbon dioxide gas as one of its products, causing the balloon to quickly inflate.
- After the reaction is complete the balloon will remain inflate. You can pop the balloon with a tack if you wish to confirm with the students that the balloon was filled with gas.
- Have students complete the provided student worksheet, before and after the demonstration. Allow students to feel the bottle, as there should be a decrease in temperature (endothermic reaction) during the reaction.
- Introduce the law of conservation of mass, as shown in this demonstration.
- Use this demonstration to lead into the concept of writing chemical equations and balancing chemical equations.
- This demonstration can be used as an introduction, followed by an opportunity for students to complete this lab themselves. Teachers may want to assign groups to reacting different amount of baking soda and vinegar to investigate the implications on the amount of carbon dioxide gas produced.
- High school teachers may want to extend this demonstration to a lab opportunity to teach Limiting Reactants & Percent Yield , or to focus on Acid-Base reactions and Mole Ratios.
For the Student
You will have the opportunity to observe a demonstration of a chemical reaction carried out by your teacher. First, attempt to answer all of the questions below to the best of your ability (it is okay if you aren’t completely sure about an answer!)
- What are some examples of things you expect to see when observing a chemical reaction?
- What is the difference between a “reactant” and a “product” in a chemical reaction?
- What types of matter can be involved in a chemical reaction?
- Proper safety gear should be worn during chemistry demonstrations. Safety goggles and lab apron are required.
- Wash your hands thoroughly before leaving the lab.
Record your observations for what happened during each portion of the experiment in the data table below.
Answer the following questions based on what you recorded in your data table.
- Describe the types of matter used to generate this chemical reaction.
- Describe the types of matter that were formed in this chemical reaction.
- How do you know that a chemical reaction occurred?
Blow-up balloon
If a chemical reaction produces a gas, you might not notice it, unless the gas has a colour or a smell. This activity will show how you can capture the gas produced in a chemical reaction in a visually exciting way.
Printable downloads
Follow these steps….

Think and talk about…
- What can you see happening in the bottle?
- What is making the balloon inflate?
- Is it blowing up faster or slower than when you use your mouth??
Investigate…
- What happens if you use more baking soda? Or more vinegar?
- Time how long it takes to inflate and then repeat the experiment. Were the times similar?
- Try using a different size balloon and see what effect it has.
- What happens if you use a bigger or smaller bottle?
Did you know?
Carbon dioxide is a greenhouse gas that contributes to global warming. Natural sources include volcanoes, decomposing vegetation and respiration from living organisms. Human sources include the burning of fossil fuels and deforestation.
What’s the science?

Because the balloon forms a seal around the bottle, the gas produced cannot escape, so it fills up the balloon.
Science in your world

Related resources

Oozing oobleck
Oobleck: solid or liquid?

Can you make milk move without touching it?

Can you make an egg bounce?

Instant Ice Cream
Explore states of matter in the tastiest way – by turning milk into ice cream instantly!

Classes for Curious minds
A place for children to learn, experiment, explore and play
For children aged 3-12 years
Blow Up a Balloon Using Just Bicarbonate of Soda and Vinegar
Blow Up a Balloon Using Just Bicarbonate of Soda and Vinegar
January 2019

Blow up a balloon using just bicarbonate of soda and vinegar in this simple science experiment
You will need.
● Clear bottle ● Vinegar ● Balloon ● Bicarbonate of soda ● Funnel

The Experiment
1. Pour 4 tablespoons of vinegar into the bottle. 2. Use the funnel to add 1 tablespoon of bicarbonate of soda into the balloon. 3. Pull the neck of the balloon over the neck of the bottle without releasing any bicarbonate of soda. 4. Lift the balloon so that the bicarbonate of soda falls from the balloon into the bottle and mixes with the vinegar. 5. Watch the balloon inflate on its own.
The Science
The bicarbonate of soda and vinegar react together to make an acid-base chemical reaction which produces carbon dioxide gas. Once the carbon dioxide fills the bottle it expands into the balloon, causing it to inflate.
Want More Epic Experiments?
If you enjoyed this experiment and want more fun, more science, and more epic experiments, enrol now in our science holiday camps and science classes where kids can learn, experiment, explore, and play!
Book your Holiday workshops and Term classes today!
Schools & Nurseries
Start a Science or Little Maths club to your school/nursery
We offer tailor-made tutoring to students of Chemistry, Physics, Biology and Maths.
Latest News

We published our first book: FULL OF BRAINS!

Holiday Science Camps
AGES 4-12 YEARS
find out more and enrol

Science Term Classes
AGES 3-4 YEARS AGES 4-7 YEARS AGES 8-12 YEARS

Schools & Nursery Clubs
AGES 3-4 YEARS AGES 4-12 YEARS
Our website uses cookies to give you a better customer experience. By continuing we will assume you are happy with receiving all cookies. ACCEPT You can get more information on cookies HERE
Self-Inflating Balloon Science Experiment
The self-inflating balloon science experiment is a true science experiment that would be perfect for science fairs or a science lesson. What kids learn in this lesson is that different chemical reactions and gasses can be used to inflate balloons. The real question is, will any of the balloons be able to float?

* This post may contain affiliate links for your convenience. Click here for my full disclosure.
What you’ll need for the self-inflating balloon experiment:
- Balloons (1 for each type of inflation material)
- Plastic squeeze bottles (1 for each type of inflation material)
- Baking soda
- Measuring cup
One thing to note before starting is that the yeast balloon takes some time to inflate. You’ll want to get that started before you dive into the other ones so you can compare them at the same time. We waited about 10 minutes after starting our yeast mixture to give it time to inflate before we did the other experiments.
What mixture will inflate a balloon the best?
Hypothesis:
The kids thought that the baking soda and vinegar would make the biggest balloon because they’ve made baking soda paint bombs before, and know the power of this reaction!

How to do the Self-Inflating Balloon Experiment:
To keep this scientific, add the same amount of inflation material into each bottle. We added about 3 tablespoons of hot water into one bottle, vinegar into another, and warm water into the third (for the yeast). We added 1 teaspoon of sugar along with half a yeast packet to the yeast bottle.

On top of each bottle, tape a balloon tightly around the spout so they can’t pop off.
Screw the lid on tightly to the yeast bottle and the hot water bottle.
Fill the cap with baking soda and quickly screw the lid onto the last bottle.

The baking soda and vinegar bottle will inflate the most and the fastest. In fact, ours nearly popped the lid off the bottle and made a huge mess, but we caught it in time. The yeast is a slow-inflating balloon, but it lasted the longest.

The hot water was barely enough air to help the balloon stand up straight.
What Kids Learn in the Self-Inflating Balloon Experiment
This experiment is a classic science experiment with a hypothesis, experiment, and results record. This experiment is simple, yet helps kids understand how the scientific process works. Additionally, who wouldn’t want to learn what the best replacement for helium in balloons is? Of course, some kids might be disappointed to learn that these balloons won’t actually float in the air, but that’s another lesson too! These balloons can’t float because none of the fillings are lighter than air!

Looking for more STEAM (Science, Technology, Engineering, Arts and Math) projects and inspiration?
Can’t get enough STEAM? We’ve got you covered. With the super affordable, super sweet STEAM Kids book! It has a Year’s Worth of Hands-on Science, Technology, Engineering, Art, & Math Activities for Kids. Plus a lot more…. And, you get a free STEAM Kids Valentine’s Day EBook with every purchase of the original STEAM Kids book.
FREE DOWNLOAD
Discover how to get siblings to get along even when all they do is annoy each other with the Sibling “Get Along” Poster Pack!
1 thought on “Self-Inflating Balloon Science Experiment”
This is much easier to do if you use 12-20 oz soda bottles and regular balloons instead of the water balloons pictured here.
Leave a Comment Cancel Reply
Your email address will not be published. Required fields are marked *
Save my name, email, and website in this browser for the next time I comment.

CONNECT WITH ME
Science Fun

Baking Soda Balloon Chemical Reaction Science Experiment
Chemical reaction science experiments using baking soda and vinegar are a lot of fun and are great learning opportunities. In this quick and easy experiment, we are going to use an endothermic chemical reaction and the resulting carbon dioxide caused by mixing baking soda and vinegar to inflate a balloon.
- Empty plastic or glass bottle
- 1 cup of vinegar
- 1/3 cup of baking soda
Instructions:
- Use the funnel to add the 1/3 cup of baking soda into the balloon.
- Twist the neck of the balloon a few times to keep the baking soda from spilling out and set the balloon aside.
- Rinse the funnel and then use it to add the 1 cup of vinegar to the bottle.
- Next, carefully stretch the mouth of the balloon over the bottle opening. Be sure to keep the neck of the balloon twisted to keep any of the baking soda from falling into the bottle and reacting with the vinegar.
- Once the balloon is securely attached to the bottle, allow the balloon the drape over to one side.
- When you are ready, lift the balloon directly over the opening of the bottle and untwist the balloon.
- Quickly shake out the baking soda.
- Step back and observe.
EXPLORE AWESOME SCIENCE EXPERIMENT VIDEOS!

How it Works:
Once the baking soda falls from the balloon into the vinegar, an endothermic chemical reaction will begin to occur. Carbon dioxide will be released that will create pressure and inflate the balloon.
Make This A Science Project:
Does the temperature of the vinegar effect the chemical reaction? Test different amounts of vinegar and baking soda. Try different sized balloons. Does the size and shape of the bottle effect the speed at which the balloon inflates. Does the addition of salt to the vinegar effect the chemical reaction and balloon inflation in any way?
EXPLORE TONS OF FUN AND EASY SCIENCE EXPERIMENTS!

SUBSCRIBE AND NEVER MISS A NEW SCIENCE FUN VIDEO!
previous experiment
Next experiment.
- Skip to primary navigation
- Skip to main content
- Skip to primary sidebar
Love to cook, bake, craft, create?
Join the e-club for the latest.
One Little Project
Cook, bake, craft, create, one little project at a time!
Baking Soda and Vinegar Balloons
These baking soda and vinegar balloons were so much fun! Mix everything together and watch as the reaction creates carbon dioxide and inflates the balloons! As far as science experiments go, this is a pretty simple one. I love that we had all of the materials needed for it at home already, and that it was quick and easy to put together! And on top of that, it was pretty darn cool to watch the balloon inflate all by itself!
My kids LOVE balloons. I swear, every time I give them a balloon, they find a way to entertain themselves with it for hours (or until it hits the popcorn ceiling and it pops – EEK!). Whenever I wrap presents (I keep the balloons with my ribbons and bows), they always sneak one out, and instantly put it in their mouths and start blowing. And when that fails – they are only 5 and 3 years old, so they definitely don’t have the lung power yet to blow them up by themselves – they hand me a wet balloon and say “Mommy, can you blow this up?”. Who doesn’t love blowing up a soggy balloon!? So we were all pretty excited that we could blow up balloons another way, without using our mouths!

Check out our video for how to inflate balloons using baking soda and vinegar:
Looking for even more awesome experiments to try with your kids?! You might want to try these out!
Snowstorm in a Jar
How to Make Slime without Borax
How to Make Crystal Stars
Shaving Cream Rain Clouds
How you do it:
- Use a funnel to add 1/3 cup baking soda to the inside of a balloon.
- Fill a plastic bottle with approximately 1 cup vinegar.
- Attach the balloon to the mouth of the plastic bottle, then lift the balloon upright so the baking soda falls and causes the reaction.
The science behind it:
So how does it work? The vinegar and the baking soda mix together to make an acid-base reaction. The reaction creates carbon dioxide gas that bubbles up from the mixture. The gas expands up and out of the bottle and inflates the balloon.
Another cool thing about these balloons is that carbon dioxide is heavier than air, so when you drop the balloon, you’ll notice that it falls to the ground faster than a regular balloon filled with air! (So no, these balloons definitely don’t float!)

Stretch the opening of the balloon over the end of the funnel. Pour about 1/3 cup of baking soda into the funnel and shake it around a bit until it all falls through the funnel and into the balloon. Just make sure you hang onto the balloon opening so it doesn’t fall off the funnel.

Rinse all the baking soda off the funnel (or you’ll get fizzing), and then use the funnel to pour the vinegar into a soda or water bottle. I didn’t measure the vinegar, but it was about 1/3 of the soda bottle full, or if you are using a smaller water bottle, fill it up half way. It was approximately 1 cup of vinegar.

- Gently stretch the opening of the balloon over the opening of the bottle. Make sure the balloon is draping down at the side to keep the baking soda from falling in.

- Lift the balloon so that it is completely upright allowing all of the baking soda to fall into the vinegar.

The balloon seemed to be on the bottle pretty snugly, but I’d still recommend pinching it onto the bottle opening the whole time. The last thing you want is for it to pop off!

As soon as the chemical reaction began, the balloon started to inflate! The more vinegar and baking soda you use, the bigger your balloon will get!

Here’s an animation of how it inflated. It was quick and easy!
My girls had a blast inflating these balloons! They aren’t strong enough to blow up balloons with their own lungs yet, so they thought it was pretty neat that they could inflate them another way!

My five year old’s balloon was the biggest one we made. Both of their little hands were too small to hold the balloons up from the bottom, so they had to hold the tops to keep them from falling over.

It was kind of cool, because all of the bubbles and foam rose up to the top of the bottle and half way up the balloon! The girls said it looked like the inside of the balloon was getting a bath, and it really did. It was like it was raining on the inside of the balloon.

We used 12″ balloons, but you can experiment with different balloon sizes. And you can also experiment with the quantities of vinegar and baking soda that you use. There’s really no need to measure, but you can try it with bigger amounts, to create more carbon dioxide gas, and therefore make the balloons grow to be even bigger! Just make sure you increase it a little at a time, or you’ll have a baking soda and vinegar volcano flying across your kitchen!

- 1 empty water bottle
- 1 Balloon
- 1 funnel
- 1/3 cup Baking soda
- 1 cup vinegar
Instructions
- Stretch the opening of the balloon over the end of the funnel. Pour about 1/3 cup of baking soda into the funnel and shake it around a bit until it all falls through the funnel and into the balloon.
- Rinse all the baking soda off the funnel, and then use the funnel to pour the vinegar into a water bottle.
- Watch the magic as your balloon begins to inflate!

These baking soda and vinegar balloons were a really simple experiment! We used household ingredients that we already had so it was super easy to put together. Both my 3 year old and 5 year old were able to do most of it all by themselves, which is always pretty awesome!
Here are even more fun experiments to try!

Our book Low-Mess Crafts for Kids is loaded with 72 fun and simple craft ideas for kids! The projects are fun, easy and most importantly low-mess, so the clean up is simple!

Where to buy:
You can purchase Low-Mess Crafts for Kids from Amazon , or wherever books are sold:
Amazon | Barnes and Noble | Books- A- Million | Indiebound | Indigo | Amazon Canada
More from One Little Project

About Debbie Chapman , the Author of this Post
I'm Debbie Chapman, founder of One Little Project and author of the book Low-Mess Crafts for Kids . I love creating fun and easy crafts and cooking up delicious recipes for my husband and 3 kids.

Low-Mess Crafts for Kids
72 Projects to Create Your Own Magical Worlds
Reader Interactions
Let us know what you think: cancel reply.
Your email address will not be published. Required fields are marked *
Save my name, email, and website in this browser for the next time I comment.
September 11, 2015 at 12:31 pm
Awesome post! I would love to invite you to my new link party The Beautifully Creative Inspired. The party launches every Fridays at 9AM eastern time on 5 BLOGS! It runs until Wednesday night 😀 Hope you can party with us!
January 20, 2017 at 5:12 pm
Heavier things, as long as the shape is equal, don’t fall faster.
February 24, 2018 at 6:37 pm
i going to try
September 1, 2019 at 8:56 am
Ok, Eric, so helium balloons don’t float in your world. 🙄 Please check back at your childhood science textbooks, or at least do a quick “.edu” Google search.
November 21, 2019 at 11:43 pm
No. Not helium. Its CO2
Bottle Balloon Blow-Up Experiment
Ron Levine/Getty Images
- Activities for Kids
- Chemical Laws
- Periodic Table
- Projects & Experiments
- Scientific Method
- Biochemistry
- Physical Chemistry
- Medical Chemistry
- Chemistry In Everyday Life
- Famous Chemists
- Abbreviations & Acronyms
- Weather & Climate
- University of Maine
If your child liked the Exploding Sandwich Bag Science Experiment or tried the Antacid Rocket Experiment , she’s really going to like Bottle Balloon Blow-Up experiments, although she might be a little disappointed when she finds out the only thing getting blown up is the balloon.
Once she realizes that none of the various forces used to blow up the balloons in these experiments require her to use air from her lungs, she’ll be intrigued.
Note: This experiment work best with latex balloons, but if any of your participants have using a different balloon will suffice.
What Your Child Will Learn (or Practice)
- The power of carbon dioxide gas
- The power of air pressure
Materials Needed:
- An empty water bottle
- A medium or large balloon
- Baking soda
Create a Hypothesis
This particular version of the experiment shows how the chemical reaction created by combining baking soda and vinegar is powerful enough to blow up a balloon. Talk with your child to see if she can predict what will happen when you combine baking soda and vinegar.
If she’s ever seen a science-fair volcano, remind her that these are the ingredients used in the volcano. Ask her to predict what will happen if you combine these ingredients when instead of leaving a hole in the top you cover the bottle with a balloon.
The Baking Soda Balloon Blow-Up Experiment
- Fill a water bottle one-third full of vinegar.
- Put a funnel in the neck of a balloon, and hold onto the balloon neck and funnel. Have your child pours in enough baking soda to fill the balloon halfway.
- Slide the funnel out of the balloon and have your child hold the portion of the balloon with the baking soda in it down and to the side. Stretch the neck of the balloon over the neck of the water bottle securely. Be careful not to let any of the baking soda fall into the bottle!
- Ask your child to slowly hold the balloon over the water bottle to let the baking soda pour inside.
- Continue to hold tight to the neck of the balloon, but move to the side listen and watch the bottle carefully. You should hear fizzing and crackling noises as the baking soda and vinegar solution activates. The balloon should begin to inflate.
What’s Going On:
When baking soda and vinegar are combined, the acetic acid in the vinegar breaks down the baking soda (calcium carbonate) into the basics of its chemical composition. The carbon combines with the oxygen in the bottle to create carbon dioxide gas. The gas rises, can’t escape from the bottle and goes into the balloon to blow it up.
Extend the Learning
- Experiment with different size bottles (half-size water bottles, liter bottles, or two-liter soda bottles, etc.) and balloons to see if the amount of oxygen in the bottle makes a difference in how fully the balloon expands. Does the size or weight of the balloon make a difference, too?
- Try varying the sizes of balloons and bottles and doing the experiment side by side with the variables changed. Which balloon blows up fuller? Which balloon fills up faster? What was the influencing factor?
- Use more vinegar or baking soda and see what happens. As a last experiment, you can also let go of the balloon when the baking soda drops into the vinegar. What happens? Does the balloon still blow up? Does it shoot across the room?
- Grade School Science Fair Project Ideas
- Middle School Science Experiments
- Kitchen Science Experiments for Kids
- How to Make a Mentos & Diet Soda Chemical Volcano Eruption
- Safe Science Experiments
- Mentos and Soda Project
- Fluorescent Light Science Experiment
- Science Experiments and Activities for Preschoolers
- Top Chemistry Projects for Bored Kids
- Dancing Ghost Halloween Science Magic Trick
- Does the Mentos and Soda Trick Work With Regular Coke?
- Have a Vinegar and Baking Soda Foam Fight
- Make Mood Ring Color Change Slime
- How to Make a Ketchup Packet Cartesian Diver
- How to Make a Yeast & Hydrogen Peroxide Volcano
- Mad Scientist Party Theme
Suggested keywords:
- Science Experiments
- DIY Experiments
- School Experiments
- Kids Experiments
- Lab Experiments
vinegar and baking soda experiment
The vinegar and baking soda experiment is a classic and exciting science activity that demonstrates a chemical reaction. Discover how to conduct this experiment step-by-step and understand the science behind it.
The vinegar and baking soda experiment is a classic and popular demonstration that showcases the fascinating chemical reaction that occurs when an acid and a base interact. By combining vinegar (acetic acid) and baking soda (sodium bicarbonate), we can observe an exciting display of bubbling and fizzing. This experiment not only captivates the imagination but also serves as a valuable educational tool, allowing us to explore the properties of acids and bases in an interactive and engaging manner.
To conduct this experiment, you will need a few basic materials: baking soda, vinegar, a clear plastic bottle or glass jar, a funnel (optional), and a balloon (optional).
Let's walk through the steps of the experiment:
- Begin by selecting a clear plastic bottle or glass jar to serve as the container for the reaction. It's helpful to use a clear vessel, as it allows for better observation of the reaction.
- If desired, use a funnel to carefully pour a small amount of baking soda into the bottle, filling it to about one-quarter of its capacity. The funnel helps to prevent any spillage or mess.
- Slowly pour vinegar into the bottle, being cautious not to fill it to the brim. Leave some space at the top to accommodate the reaction that will take place. As the vinegar comes into contact with the baking soda, the magic begins.
- Observe the reaction as the vinegar and baking soda interact. Almost immediately, you will witness a flurry of bubbles and fizzing. This effervescent display is a result of the chemical reaction occurring between the acetic acid in the vinegar and the sodium bicarbonate in the baking soda.
- For an added interactive element, you can stretch a balloon over the top of the bottle or jar before pouring in the vinegar. As the carbon dioxide gas is generated during the reaction, it will inflate the balloon, creating a visual representation of the gas production.
The chemical reaction that transpires between the vinegar and baking soda can be explained by the following equation:
CH3COOH (acetic acid) + NaHCO3 (sodium bicarbonate) → CO2 (carbon dioxide) + NaC2H3O2 (sodium acetate) + H2O (water)
The reaction between the acid (vinegar) and the base (baking soda) produces carbon dioxide gas, which manifests as bubbles. Simultaneously, sodium acetate and water are formed as byproducts of the chemical reaction.
This experiment serves as an excellent educational tool, particularly for teaching children about chemical reactions and the properties of acids and bases. It provides a hands-on experience that allows them to witness the exciting transformation and understand the principles at play.
By engaging in the vinegar and baking soda experiment, children can develop a deeper appreciation for the world of chemistry. They can learn about the concept of chemical reactions, how different substances interact, and the role of acids and bases in these processes. Furthermore, the visual effects of bubbling and the inflation of the balloon make the experiment even more captivating and memorable.
However, it is important to note that while this experiment is safe and enjoyable, caution should always be exercised. Adult supervision is advised, especially when working with young children. It's crucial to handle the materials responsibly, avoid ingestion or contact with eyes, and clean up any spills promptly.
In conclusion, the vinegar and baking soda experiment provides an exciting opportunity to explore the chemical reaction between an acid (vinegar) and a base (baking soda). Through the combination of these two substances, we witness a captivating display of bubbling and fizzing, accompanied by the production of carbon dioxide gas, sodium acetate, and water. This experiment serves as an interactive and educational tool, allowing children to gain insights into the world of chemistry, understand the properties of acids and
- Share this :
Mohamed Aslam
Related posts, water experiments, most popular, how to make waterproof bag, how to make slime at home, how to send a secret message like a secret agent, how to mix soft drink and baking soda: a refreshing experiment, to our newsletter.

Baking Soda and Vinegar’s Reaction
An experiment for students who are blind or visually impaired using balloons to determine if different vinegars react differently with baking soda..
- Share on Twitter
- Share on Facebook
- Share on LinkedIn
- Share on Pinterest
This science project was done by Marina, who is a student at Texas School for the Blind and Visually Impaired (TSBVI).
What effect will different vinegars have on the baking soda/vinegar reaction? Will the balloon blow up more?
The white vinegar will blow up the balloon the most.
- apple cider vinegar
- white vinegar
- white wine vinegar
- balsamic vinegar
- rice vinegar (with seasoning)
- rice vinegar (no seasoning)
- 6 small mylar balloons
- baking soda
- measuring spoons
- ruler (in cm)
- 6 wine spouts
*Note in regard to procedure: I made changes to the procedure several times. The first time, the balloon didn’t blow up enough to measure, so I doubled the amount of vinegar and baking soda. Then, all of the balloons blew up completely so there was no difference between any of the balloons. I decided to increase the amount of baking soda and vinegar by only 50% and this worked.
- Measure 15 ml of baking soda using a measuring spoon. Pour the baking soda into the balloon using a funnel.
- Measure 45 ml of vinegar and pour it into a water bottle.
- Put the mouth of the balloon on the wine spout to keep the baking soda in the balloon. (The balloon will be flopped to one side.)
- Lift the balloon up and pour the baking soda into the bottle of vinegar.
- Observe for 1 minute
- Repeat for each type of vinegar.
- Measure the circumference of the balloon by wrapping string around the balloon and then measuring it with a piece of string.
- Record the data.
- Make the data into a graph.

Final trial on 5-12-15
Circumference of balloons:
- Rice vinegar with seasoning – 23 cm
- White vinegar – 23 cm
- Rice vinegar without seasoning – 12 cm
- Apple cider vinegar – 24 cm
- White wine vinegar – 24 cm
- Balsamic vinegar – 18.5 cm
My results did support my hypothesis because the white vinegar blew up the most.
Other vinegars could have also been tested.
NGSS Standards:
- Plan and conduct an investigation individually and collaboratively to produce data to serve as the basis for evidence, and in the design: decide on types, how much, and accuracy of data needed to produce reliable measurements and consider limitations on the precision of the data (e.g., number of trials, cost, risk, time), and refine the design accordingly. (HS-ESS2-5)
By Laura Hospitál

Return to Accessible Science main page .
Keep reading

How Different Vinegars Affect Reactions

Endothermic or Exothermic Reaction?

Sugar cookie recipe: Read by lines tech skill
- PRO Courses Guides New Tech Help Pro Expert Videos About wikiHow Pro Upgrade Sign In
- EDIT Edit this Article
- EXPLORE Tech Help Pro About Us Random Article Quizzes Request a New Article Community Dashboard This Or That Game Happiness Hub Popular Categories Arts and Entertainment Artwork Books Movies Computers and Electronics Computers Phone Skills Technology Hacks Health Men's Health Mental Health Women's Health Relationships Dating Love Relationship Issues Hobbies and Crafts Crafts Drawing Games Education & Communication Communication Skills Personal Development Studying Personal Care and Style Fashion Hair Care Personal Hygiene Youth Personal Care School Stuff Dating All Categories Arts and Entertainment Finance and Business Home and Garden Relationship Quizzes Cars & Other Vehicles Food and Entertaining Personal Care and Style Sports and Fitness Computers and Electronics Health Pets and Animals Travel Education & Communication Hobbies and Crafts Philosophy and Religion Work World Family Life Holidays and Traditions Relationships Youth
- Browse Articles
- Learn Something New
- Quizzes Hot
- Happiness Hub
- This Or That Game
- Train Your Brain
- Explore More
- Support wikiHow
- About wikiHow
- Log in / Sign up
- Education and Communications
- Science for Kids
How to Blow up a Balloon With Baking Soda and Vinegar
Last Updated: February 2, 2024 Fact Checked
wikiHow is a “wiki,” similar to Wikipedia, which means that many of our articles are co-written by multiple authors. To create this article, 50 people, some anonymous, worked to edit and improve it over time. This article has been fact-checked, ensuring the accuracy of any cited facts and confirming the authority of its sources. This article has been viewed 415,522 times. Learn more...
Learn how to inflate a balloon in this fun science experiment using these common kitchen ingredients! Balloons inflated this way are filled with carbon dioxide produced by the two ingredients reacting. They do not contain helium, so they will not float.
Inflating the Balloon

- You can try this with any kind of vinegar, but the inflation might take longer or require more vinegar to work. Other types of vinegar tend to be more expensive as well.
- Vinegar can damage metal containers, potentially adding an unpleasant taste to food and drink stored in that container. If you have no plastic bottles, use a high-quality stainless steel bottle to minimize the chance of this happening. Weakening the vinegar with an equal amount of water might also help, and won't prevent the balloon from inflating. [2] X Research source

- If you don't have a funnel, you can place a plastic straw into a pile of baking soda, put your finger over the top hole of the straw, then poke the straw into the balloon and lift your finger. Tap the straw to get the baking soda to fall out, and repeat until the balloon is at least 1/3 of the way full. [4] X Research source

- Shake the bottle gently to mix the two ingredients if there's not much fizzing.

- Don't go overboard. The bottle should never be more than about 1/3 full of vinegar.
Grasping how the Process Works

- Baking soda is another word for the molecule sodium bicarbonate .
- White vinegar is a mixture of acetic acid and water. Only the acetic acid reacts with the baking soda.

- Although the definition of acid and base can get complicated, you can compare the differences between the original substances and the "neutralized" result to see there are obvious changes. For instance, vinegar has a strong smell and can be used to dissolve grime and dirt. After being mixed with baking soda, it smells much less strongly and is no more effective at cleaning than water is.

- NaHCO 3 + HC 2 H 3 O 2 (aq) → NaC 2 H 3 O 2 (aq) + H 2 O(l) + CO 2 (g)
- The letters in parentheses show the state the chemicals are in during and after the reaction: (g)as, (l)iquid, or (aq)ueous. "Aqueous" means the chemical is dissolved in water.
Community Q&A
- This method can also be used in homemade cardboard or plastic rockets and you can make them go a long way if ingredients are out right. The reason it blows up is because the reaction creates gas, and the pressure builds up. Thanks Helpful 0 Not Helpful 0
- You can use lime juice instead of vinegar. Thanks Helpful 0 Not Helpful 0

- If the balloon is fully inflated and the liquid is still fizzing, the balloon might be about to explode. Decide whether you have time to pull off the balloon, or whether you should just cover your face before it gets spattered! Thanks Helpful 69 Not Helpful 25
Things You'll Need
- Baking Soda
- Bottle with narrow neck
- Funnel (optional)
You Might Also Like

- ↑ https://www.cityofsacramento.org/-/media/Corporate/Files/ParksandRec/4thR/4r-SAH2-BakingSodaVinegarBalloonExp.pdf?la=en
- ↑ https://www.exploratorium.edu/science_explorer/balloon_blowup.html
- ↑ http://www.education.com/science-fair/article/balloon-gas-chemical-reaction/
- ↑ https://www.education.com/science-fair/article/balloon-gas-chemical-reaction/
- ↑ https://www.pbs.org/parents/crafts-and-experiments/inflate-a-balloon-with-baking-soda-and-vinegar
- ↑ https://www.cmosc.org/balloon-blow-up-science-experiment/
About This Article
To blow up a balloon with baking soda and vinegar, pour 1–2 inches of white vinegar into a plastic bottle. Next, hold a balloon loosely by the neck, fit a funnel or plastic straw into it, and pour 2 tablespoons of baking soda through it into the balloon. Then, stretch the neck of the balloon over the top of the bottle before lifting the balloon up over the bottle. The baking soda will fall out of the balloon, through the neck of the bottle, and into the vinegar. The 2 ingredients will fizz and react to create carbon dioxide, which will then inflate your balloon! If you want to learn more about the chemical reaction that occurs, keep reading! Did this summary help you? Yes No
- Send fan mail to authors
Reader Success Stories
Akime Yashito
Jan 10, 2017
Did this article help you?
Jan 24, 2018
Coco Wright
Nov 29, 2016
Monserrath Garza
Oct 27, 2017
Emmanuel Nikoi
Nov 17, 2016

Featured Articles

Trending Articles

Watch Articles

- Terms of Use
- Privacy Policy
- Do Not Sell or Share My Info
- Not Selling Info
Get all the best how-tos!
Sign up for wikiHow's weekly email newsletter
Enter your email to download PDF and receive updates from OSMO
Scan to get started.
The Assessment App is available only on the Apple App Store . Please scan the QR code below with your iPhone device to download the app.

Baking Soda and Vinegar Balloon Experiment
What would you do to blow up a balloon? You must be blowing up balloons with your mouth or using air pumps. Do your children know how to blow up a balloon using baking soda and vinegar? If not, we are here to help you provide simple science experiments for kids to learn how baking soda and vinegar react to produce a gas responsible for blowing up a balloon. You can conduct a baking soda and vinegar balloon experiment using the materials available at home. This experiment will help little ones to observe the chemical reactions and learn the science behind how a balloon inflates using baking soda and vinegar.
Contents
- Easy Science Projects: Baking Soda and Vinegar Balloon Experiment
- Aim of the Project
- Materials Required
Benefits of Learning Baking Soda and Vinegar Balloon Experiment
Frequently asked questions on baking soda and vinegar balloon experiment.
Balloons are widely used as a decorative item in almost every celebration. Kids assist their parents and friends in blowing up balloons for the parties. Sometimes, they get tired of blowing so many balloons in a short time. Therefore, you can show a trick to kids on how to blow up a balloon effortlessly by conducting a small experiment. You can conduct this experiment for kids with two main ingredients, i.e. baking soda and vinegar easily found in the house. You can demonstrate this experiment to kids who are too young to perform, whereas little older children can perform this experiment under the supervision of elders. This is one of the best science experiments for toddlers , preschoolers and kindergarten kids.
Easy Science Projects: Baking Soda and Vinegar Balloon Experiment
Do your children know any other alternative to blow up a balloon instead of using the mouth or air pumps? You can conduct an easy experiment for kids to learn how to blow up a balloon using baking soda and vinegar. This is one of the DIY science project ideas for kids. They can perform this experiment at home or in the classroom to learn the chemical reactions between the acid and base. Most importantly, it will enable kids to observe how gas produced from the reaction inflates the balloon. Check out the baking soda and vinegar balloon experiment for kids in this article.
Aim of the Project
The aim of this experiment is to blow up a balloon using baking soda and vinegar under the supervision of elders.
Materials Required
- An empty bottle
- A cup of vinegar
- A tablespoon of baking soda
Procedure
- Take off the cap of an empty bottle.
- Pour a cup of vinegar into the bottle.
- Add a tablespoon of baking soda into the bottle.
- You can quickly place the mouth of the balloon on the bottle after adding the baking soda.
- Observe and record the changes.
In this experiment, kids observe that the balloon inflates due to the chemical reaction between baking soda and vinegar. The vinegar is acidic, whereas baking soda is the base. These two ingredients react to form water, sodium acetate and carbon dioxide. Therefore, carbon dioxide is the gas that forms bubbles inside the bottle enabling the balloons to inflate.
Also, explore how to make bubbles.
The benefits of learning how to blow up a balloon using baking soda and vinegar are mentioned below.
- It develops observational, analytical and practical thinking skills in children.
- It helps children learn chemical reactions between an acid and the base.
- It enables children to understand the scientific reason for blowing up a balloon using baking soda and vinegar.
- It creates interest among children to learn science and perform experiments.
- It increases children’s concentration and helps them stay focused throughout the experiment.
- It improves the academic performance of children.
To know more information, explore science games for kids , STEM activities for kids in the kids learning section at Osmo.
How to conduct a baking soda and vinegar balloon experiment?
You can teach kids how to blow up a balloon using baking soda and vinegar by following the procedure mentioned in this article. To conduct this experiment, you need a balloon, a tablespoon of baking soda, a cup of vinegar and an empty bottle.
What are the benefits of learning baking soda and vinegar balloon experiment?
The benefits of learning baking soda and vinegar balloon experiments for kids are that it helps them observe the chemical reactions between baking soda and vinegar to produce gas, which is responsible for inflating the balloon. Besides, it will help kids enhance their scientific knowledge and observational skills while performing this experiment.
Subscribe to Osmo & get
your first purchase

You’ve been subscribed with
Check the welcome mail to download the printables and avail your discount.
Explore our award-winning products for kids learning.
* Offer valid only for 7 days.
- Where To Buy
- Get Coupons & Tips
- Baking Soda
Easy Baking Soda Science Fair Projects for Kids
- Kids' Activities
Types of Baking Soda Science Experiments
There are thousands of science experiments to choose from, but ARM & HAMMER™ makes it easy with these simple and fun experiments for kids:
- Baking Soda Volcano Project
- Self-Inflating Balloon Project
- Rust Remover Project
- Bucket of Snow Project
- Bucket of Lava Project
Baking Soda Volcano Science Experiment
The baking soda volcano is a classic experiment that most adults remember from their childhood. But this science experiment being around for generations doesn’t lessen its wonder the first time a student sees the reaction, or decrease the fun and nostalgia for adults. Plus, you can add twists to the basic experiment to make it new and interesting all over again.
For the basic baking soda volcano experiment you need the following materials:
- Material to make the volcano out of. It can be a simple flour dough, papier mâché, air-hardening clay, or even playdoh
- A small cup, jar, vase, water bottle or plastic tube to go in the mouth of the volcano to hold the ingredients
- 2 tbsp ARM & HAMMER™ Baking Soda
- 4 oz. White vinegar
- Food coloring
- Cardboard (optional) to go in the cup, jar or vase if you are using multiple colors
- A pan, such as a brownie pan or casserole dish to catch the “lava”
- Build your volcano around the container you have chosen in a pan to cath the “lava”. Shape it like a mountain with a pointy peak and leave a hole at least 1.5” in diameter at the top for the lava to come out. If building with air-hardening clay, flour dough, or papier mâché, build the volcano 24 hours ahead of your experiment so that it is dry and hardened. You may choose to paint your volcano to make it look more realistic and topographical. Feel free to add small trees or a village at the base to add to the sense of impending disaster.
- Add about 2 tablespoons of baking soda to the container inside the volcano by pouring through the top. You can experiment with the amount of baking soda and vinegar you use for the best reaction.
- (optional step) If you want to make a multi-colored volcano, cut a piece of cardboard the diameter of your opening, and insert it fully into the tube, separating the baking soda into two wells.
- Add a few drops of food coloring into the tube on top of the baking soda. Try adding some red and some yellow to create an orange color, or go for a fantasy volcano by using any color you want. If you can find black food coloring, that makes a nice addition as well. If you are using multiple colors, add one food coloring to the left side, and one to the right.
- Add a squirt of dish soap on top of the baking soda and food coloring. The color of the dish soap does not matter.
- When you’re ready for the “explosion,” pour 4 oz. of vinegar into the top of the volcano and step back. In a few seconds, the colored “lava” will bubble up and out of the volcano, spilling down its sides.
Baking Soda Volcano Variants
While the original baking soda volcano is always fun and a great learning tool, there are many ways to experiment with the volcano by changing ingredients or the method. The changes below are an excellent way to teach kids about controls and variables in the scientific method.
- Baking Soda Ketchup Volcano: Instead of adding food coloring, add 2 tablespoons of ketchup instead. This will make a thicker, less runny (and of course, red) lava.
- Baking Soda Jack o’ Lantern: Instead of making a volcano, carve a pumpkin with a large mouth. Put the jar in the center of the carved-out pumpkin, where you would put a candle. Follow the procedure above, adding the vinegar last. The reaction will come out of the Jack o’ Lantern’s mouth (and sometimes also nose and eyes, depending on how “explosive” the reaction is). This is a fun experiment for a Halloween party!
- Baking Soda Cauldron: Instead of a volcano, use a pot or Halloween cauldron. Double your amounts of baking soda and vinegar from the standard recipe above to fill the larger volume of the pot/cauldron. Add the baking soda, food coloring, and 2-3 squirts of dish soap to the cauldron, then pour in the 8oz of vinegar. Green is a good color for a witches brew, or try multiple colors. Your cauldron will be bubbling in seconds. As with the volcano, put the pot or cauldron in a pan to help catch any overflow.
Baking Soda and Vinegar Balloon Experiment
This classic Self-Inflating Balloon experiment blows up a balloon thanks to carbon dioxide (CO 2 ) gas released when vinegar and baking soda are combined.
To create the reaction and blow up a balloon, you will need:
- A water bottle
- 2 tablespoons ARM & HAMMER™ Baking Soda
- 4 oz. white vinegar
- Small funnel
- 1 10- or 11-inch balloon
- Use the funnel to add the 4oz of vinegar to the water bottle.
- Rinse and dry the funnel.
- Use the funnel to add the 2 tbsp. of baking soda into the mouth of the balloon.
- Carefully attach the balloon opening over the bottle opening, taking care NOT to release the baking soda yet.
- Once balloon is firmly over the mouth, tip the balloon up to vertical over the bottle, dropping the baking soda into the bottle.
- As the two combine, they release the carbon dioxide gas, which will cause the balloon to expand.
Baking Soda & Vinegar Balloon Experiment: Rubber Glove Variant
If you want to forgo the funnel, you can also do this experiment with a wide mouthed jar or glass and a rubber glove instead of a balloon. The baking soda goes into the fingers of the glove, which will blow up into a puffy hand or a “chicken.” You might need to add a rubber band around the glove when it is over the jar, to ensure a tight seal.
Baking Soda & Vinegar Self-Inflating Balloon as a Science Fair Project
To make your self-inflating balloon a true science experiment, you’ll need to have a hypothesis and test to see whether it is correct. Now that you know what will happen when the baking soda and vinegar combine, you can change one of the variables to see how the reaction is affected and take some measurements. Add a digital timer to test how long it takes for the carbon dioxide to blow up the balloon, then see how the time is affected when you change the amount of vinegar or baking soda in the experiment. To uphold the integrity of the experiment, make sure you only change one variable at a time.
Here are some additional ways to change the variables and make this fun reaction into an easy and affordable science fair project (Adult Supervision Required)
What happens when the vinegar is heated or cooled vs. room temperature?
- You can accomplish this by using two bottles, adding room temperature vinegar to one as your control, then setting the second bottle into a bowl of ice for three minutes to cool it down. Make a hypothesis about which will fill faster: the room temperature vinegar or the cold vinegar. Repeat the experiment with both bottles and time how long it takes for the balloons to fill and which fills first. Record your results.
- Repeat the experiment with a bottle of room temperature vinegar and one with warm vinegar. Make a hypothesis about which will fill faster. Place one bottle with vinegar in a bowl of boiling or very hot water for three minutes to heat it. Conduct the experiment and time how long it takes for the balloons to fill and which fills first. Record your results.
- IMPORTANT: always use the same 4oz of vinegar and 2 tablespoons of baking soda. In a science experiment you should only change one variable at a time (in this case, the temperature of the vinegar) so that you know your changes in data are due to the specific change you made.
What if you use lemon juice instead of white vinegar?
- Repeat the procedure, except substitute 4oz of lemon juice for the 4oz of vinegar. Make a hypothesis about which you think will be faster, the lemon juice or the vinegar. Record your results and compare to the time data you already have for the baseline (room temperature) vinegar experiment.
What else could you change with this experiment?
- Size of the balloon
- Amount of baking soda
- Dilute the vinegar or lemon juice with water
- Size of the bottle
Other Easy Science Experiments Can You Do with Baking Soda?
There are many other science experiments that use baking soda. Here are a few we’ve compiled for easy, cheap, fun science fair projects for kids.
Baking Soda Rust Remover Experiment
Baking soda combined with lemon juice or white vinegar makes an effective DIY rust remover. The amount of baking soda and vinegar you’ll need depends on the size of the rusted object you need to refurbish. In general, the ratio is 2 parts baking soda to 1 part vinegar or lemon juice. You want to create a thick paste. To make:
- Combine 2 tablespoons of baking soda with 1 tablespoon of white vinegar or lemon juice in a small bowl.
- Stir together to create a thick paste. If it is too runny, add more baking soda, a little at a time.
- Apply the paste to the rusted areas on the object, such as a baking tray, a steel sink, or corroded metal fixture.
- Wait 20-30 minutes.
- Scrub, using a green scrubbing pad or a ball of aluminum foil. Repeat as necessary.
To make this into a science fair project, change the amount of time you leave the paste on the object, or try it with different scouring pads such as steel wool, a copper mesh scrubber, an abrasive sponge, or the aluminum foil. Make a hypothesis about which will work best and test it out!
A Bucket of “Snow” Experiment
For a fun science experiment, add about 8oz of baking soda to a bucket or cooler. For extra fun, place the baking soda in the freezer first, to give a cold effect. Very slowly add plain water, about 2 tablespoons at a time. There may be a small bubbling reaction but not a big fizz. Mix the baking soda with your hands or with a long handled spoon as you add water, and soon a lovely, packable “snow” will form. Now you can play, build snowmen or make snowballs!
After snowballs are made, you can set them in a bowl and slowly add vinegar (in a pipette or medicine dropper is ideal) to watch your snowballs fizz and melt.
A Bucket of Magic “Lava” Experiment
How about a bucket of fizzing, multi-color “lava”? Take a bucket and dribble different colors of food coloring in small distinct circles along the bottom. Optional: add 1 tablespoon of dish soap to the bottom. Cover the food coloring with about an inch of ARM & HAMMER Baking Soda.
Give kids a needleless syringe, pipette or medicine dropper of white vinegar and let them dribble it into the bucket a little at a time. The colors will begin to “magically” appear on the plain white surface. As more vinegar goes in, the surface will bubble up from the bottom and begin to fill the bucket with foam.
When finished, pour down your drain. Baking soda and vinegar make a great deodorizer and descaling agent for your pipes, and they are safe for septic systems and the environment.
More Information about Baking Soda for Science Fair Projects
ARM & HAMMER™ Baking Soda is the Standard of Purity. It only contains pure baking soda and its manufacturing process does not taint it with ammonia. You can be sure your Arm & Hammer Baking Soda will be consistent and react properly every time. Don’t skew your science fair project’s results by mixing brands of baking soda or settling for one that isn’t pure baking soda.
To get your supply of baking soda for these cool and easy science experiments try:
- ARM & HAMMER™8oz Orange Box
- ARM & HAMMER™3.5 lb. Pouch
Understand the differences between Baking Soda and Baking Powder with an article from our experts.
For more fun activities to do with your kids check out our DIY projects or our For Everything Soda activity page.
Trending Products
Arm & hammer™.
Baking Soda Box
Baking Soda Resealable Bag
Plus OxiClean™ Dirt Fighters Carpet Odor Eliminator, Pet Fresh
Clear Balance™ Pool Maintenance Tablets
Tips to freshen things up
Stinky-free sneakers.
Deodorize gym bags and sneakers by sprinkling in baking soda inside.
Clean Coffee Mug Stains
Clean coffee mug Stains using Arm & Hammer Baking Soda
3 of 227 How To
Video title
- Skip to primary navigation
- Skip to main content
- Skip to primary sidebar
- Skip to footer
From ABCs to ACTs
Baking Soda and Vinegar Balloon Experiment for Kids
Written by Amber
This post may contain affiliate links. For more information, please see my full disclosure policy .
Creating a reaction between baking soda and vinegar is a classic science experiment that kids of all ages love to watch.
We’ve actually used the mixture in a few different science experiments ourselves including our green themed experiment and our soda bottle speed boats .
But adding a balloon to the mix just ups the fun factor and makes it seem like a brand new experiment all over again.
Since balloons have added so much fun to our circus preschool theme already, we’ve figured we’d go ahead and throw in one more simple science experiment that the kids will love watching over and over again.

If you’ve ever watched a baking soda and vinegar reaction with your kids, you know just how excited they get when they see all of the bubbles and hear the fizzing as the two parts come into contact with one another.
This experiment uses that reaction to blow up a balloon and the reaction you’ll get out of your preschooler will be even better!

To do this experiment, you’ll need:
- Plastic bottle
- Baking soda
- Either a small funnel or a piece of paper that you can roll into a cone

Carefully pour about a 1/4 cup of vinegar into your plastic bottle and use a small funnel to put a spoonful or two of baking soda into your balloon. Don’t have a funnel? That’s okay! Just roll a piece of paper into a cone shape so that there is an opening at both ends. Stick the smaller end into the balloon and you use it just as you would a funnel.

Once your balloon is full of baking soda, stretch the opening over the top of your plastic bottle making sure that you don’t spill any of the baking soda into the bottle just yet. You wouldn’t want it to react before you have the balloon all the way on :)

Once you’re sure that your balloon is on securely, have your child tip the balloon up and give it a little shake so that the baking soda falls into the bottle. Almost immediately you should see your balloon start to fill with air.
One of the coolest parts of this experiment is that the balloon is going to stay blown up for a while after the reaction is over. Your child will get to turn it around, take a good look at everything and, if they’re old enough, you can use this opportunity to talk about chemical reactions and why the reaction caused by these two ingredients was able to blow up a balloon.
Even if they’re not quite old enough to understand that quite yet, this is still a fun experiment that kids of all ages can enjoy!
Looking for more fun science experiments for kids? Here are a few of our favorites!

Amber is a former preschool teacher turned stay at home, homeschooling mom of 3 and the creator of From ABCs to ACTs. She loves sharing crafts, activities, and printables that encourage children to learn through play, creativity, and exploration. Learn More
- Kindergarten
- Middle School
- High School
- Alphabet Activities
- Digital Activities
- Dramatic Play Activities
- Theme Packs
- New? Start Here!
- Terms of Use
- Disclosure & Privacy

IMAGES
VIDEO
COMMENTS
Experimental Procedure. Using the funnel, add the baking soda to each balloon (two people may be needed for this; one person to hold the balloon open and the other person to put the baking soda inside of the balloon). Pour the vinegar into the bottle. Carefully fit the balloon over the bottle opening (be careful not to drop the baking soda into ...
Step 1- Pour 1-2 spoonfuls of baking soda into the opening of the balloon, using a funnel. You'll need to shake it a bit to get it down into the base of the balloon. Step 2- Use the funnel again and pour some vinegar into the empty plastic bottle until it is about an inch or two deep- maybe a few tablespoons of vinegar.
One funnel for filling the bottle with vinegar and one for the balloon. However, you can do the experiment with only one funnel. Just make sure you completely wash and dry the funnel after you add the vinegar and before you put it into the balloon. This is very important. Step 4 - Place two teaspoons of baking soda into the funnel so it falls ...
The science behind this baking soda and vinegar balloon science experiment is a chemical reaction between an acid and base. The base is the baking soda and the acid is vinegar. When the two ingredients mix, the balloon baking soda experiment gets its lift! That lift is gas, carbon dioxide, or CO2.
1. Blow up a balloon just enough to stretch it out a bit. Then, use the funnel and measuring spoon to add about a teaspoon of baking soda inside the balloon. 2. Fill the water bottle or other container about halfway with vinegar. 3. Attach your filled balloon to the container with the vinegar. Make sure the seal is tight!
Balloon Blow-Up Experiment Directions: Have your children scoop the baking soda into the balloon using the funnel. Help your children put the vinegar into the flask using a pipette or small measuring cup. Next, attach the balloon to the top of the flask; make sure not to pour the baking soda into the. vinegar!
This baking soda experiment for kids combines vinegar and baking soda, then uses the resulting carbon dioxide gas to inflate a balloon. This same reaction is behind science projects like fizzy potions and DIY volcanoes. Children of all ages can participate in every step of this experiment, but younger kids may need help from an adult.
Attach a balloon to the end of the funnel. Using the funnel, pour two level teaspoons (10 mL) of baking soda into the balloon (see photo below). (Make sure the funnel doesn't clog, and all the baking soda passes through the neck of the balloon.) Pour about 1/2 cup (120 mL) of vinegar into the bottle or flask.
Are you ready to learn about chemical reactions? In this experiment, we're going to learn how to blow up a balloon using baking soda and vinegar!👉 MORE: htt...
Use the small funnel to put 2 tablespoons of baking soda in each balloon. Clean the funnel well with running water, then dry it. Use the small funnel to put 4 ounces of white vinegar in each water bottle. Place Bottle 2 in the bowl of ice. After three minutes place the balloon on top of the bottles, being careful not to spill the baking soda ...
Insert a funnel into the opening of a balloon and add the baking soda to the balloon through the funnel. See image to the right. Measure 10 tbsp. or approximately 150ml of vinegar. Add the vinegar to the empty bottle. If you wish to add food coloring to the vinegar, add 3-5 drops.
Follow these steps…. Half fill the bottle with vinegar. Using a funnel, half fill the balloon with baking soda. It helps if you've pre-stretched the balloon by blowing it up. You can make a funnel by rolling up a piece of paper. Carefully place the balloon over the neck of the bottle and allow it to droop over to the side, making sure none ...
Pour 4 tablespoons of vinegar into the bottle. 2. Use the funnel to add 1 tablespoon of bicarbonate of soda into the balloon. 3. Pull the neck of the balloon over the neck of the bottle without releasing any bicarbonate of soda. 4. Lift the balloon so that the bicarbonate of soda falls from the balloon into the bottle and mixes with the vinegar.
On top of each bottle, tape a balloon tightly around the spout so they can't pop off. Screw the lid on tightly to the yeast bottle and the hot water bottle. Fill the cap with baking soda and quickly screw the lid onto the last bottle. The baking soda and vinegar bottle will inflate the most and the fastest. In fact, ours nearly popped the lid ...
Instructions: Use the funnel to add the 1/3 cup of baking soda into the balloon. Twist the neck of the balloon a few times to keep the baking soda from spilling out and set the balloon aside. Rinse the funnel and then use it to add the 1 cup of vinegar to the bottle. Next, carefully stretch the mouth of the balloon over the bottle opening.
Instructions. Stretch the opening of the balloon over the end of the funnel. Pour about 1/3 cup of baking soda into the funnel and shake it around a bit until it all falls through the funnel and into the balloon. Rinse all the baking soda off the funnel, and then use the funnel to pour the vinegar into a water bottle.
The Baking Soda Balloon Blow-Up Experiment. Fill a water bottle one-third full of vinegar. Put a funnel in the neck of a balloon, and hold onto the balloon neck and funnel. Have your child pours in enough baking soda to fill the balloon halfway. Slide the funnel out of the balloon and have your child hold the portion of the balloon with the ...
The vinegar and baking soda experiment is a classic science experiment that involves a reaction between vinegar (acid) and baking soda (base) to produce a fizzy ... a funnel (optional), and a balloon (optional). Let's walk through the steps of the experiment: Begin by selecting a clear plastic bottle or glass jar to serve as the container for ...
Pour the baking soda into the balloon using a funnel. Measure 45 ml of vinegar and pour it into a water bottle. Put the mouth of the balloon on the wine spout to keep the baking soda in the balloon. (The balloon will be flopped to one side.) Lift the balloon up and pour the baking soda into the bottle of vinegar. Repeat for each type of vinegar ...
X Research source. 3. Stretch the neck of the balloon over the top of the bottle. Be careful not to spill the baking soda while you do this. Hold the balloon's neck with both hands and stretch it over the top of the plastic bottle containing vinegar. Have a friend keep the bottle steady if the table or bottle is wobbly.
Procedure. Take off the cap of an empty bottle. Pour a cup of vinegar into the bottle. Add a tablespoon of baking soda into the bottle. You can quickly place the mouth of the balloon on the bottle after adding the baking soda. Observe and record the changes.
1 10- or 11-inch balloon. Procedure: Use the funnel to add the 4oz of vinegar to the water bottle. Rinse and dry the funnel. Use the funnel to add the 2 tbsp. of baking soda into the mouth of the balloon. Carefully attach the balloon opening over the bottle opening, taking care NOT to release the baking soda yet.
To do this experiment, you'll need: Plastic bottle. Balloon. Baking soda. Vinegar. Either a small funnel or a piece of paper that you can roll into a cone. Carefully pour about a 1/4 cup of vinegar into your plastic bottle and use a small funnel to put a spoonful or two of baking soda into your balloon.